Note: Descriptions are shown in the official language in which they were submitted.
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
Process for converting biomass to coal-like material using
hydrothermal carbonisation
FIELD OF THE INVENTION
The present invention relates generally to a process for
the preparation of coal-like material from biomass, more
particularly to an improved and quicker process yielding
the coal-like material in enhanced space-time yield, and
which, moreover, allows for enhanced quality control of the
final product, as well as improved reproducibility. Also,
the process of the invention is cheaper in terms of the
necessary equipment, and it is a very safe process.
BACKGROUND
In the past, many efforts have been made to imitate the
natural coalification of biomass, which takes place on a
time scale of some hundred (peat) to hundred million (black
coal) years. Besides the formation of charcoal by pyrolysis
of dry biomass, the so-called hydrothermal carbonization
(HTC) process for the manufacture of coal or coal-like
materials has recently attracted increasing attention. The
first experiments were carried out already in 1913 by
Bergius, who described the hydrothermal transformation of
cellulose into coal-like materials. More systematic
investigations were later performed by E. Berl et al. (Ann.
Chem. 493 (1932), pp. 97-123; Angew. Chemie 45 (1932), pp.
517-519) and by J.P. Schumacher et al. (Fuel, 39 (1960),
pp. 223-234). Recently, the hydrothermal carbonization has
seen a renaissance starting with reports on the low
temperature hydrothermal synthesis of carbon spheres using
sugar or glucose as precursors (Q. Wang et al., Carbon 39
(2001), pp. 2211-2214 and X. Sun and Y. Li, Angew. Chem.
Int. Ed. 43 (2004), pp. 597-601). Furthermore, metal/carbon
1
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
hybrid nanostructures, such as nanocables prepared by a
hydrothermal carbonization co-reduction process using
starch and noble metal salts such as AgN03 as starting
materials were described by S.H. Yu in Adv. Mater 16
(2004), pp. 1636-1640. H.S. Qian et al., in Chem. Mater 18
(2006), pp. 2102-2108 reported the synthesis of Te@carbon-
rich composite nanocables and carbonaceous nanofibers by
the hydrothermal carbonization of glucose.
M.M.Titirici et al., in New J. Chem., 31 (2007), pp. 787-
789 described the catalyzed HTC as an attractive
alternative for the sequestration of carbon from biomass to
treat the C02 problem. According to the paper, the optimum
reaction conditions involve a heating of a biomass
dispersion under weakly acidic conditions in a closed
reaction vessel for 4 - 24 h to temperatures of around
200 C.
Generally, the HTC of biomass to afford carbon-like
products was carried out as a one-step process.
US 2008/0006518 Al relates to a process for reforming a
biomass by heating the biomass in pressurized hot water to
carbonize the biomass. According to a specific embodiment,
the process comprises performing a primary heating of the
biomass gradually at a temperature ranging from 200 to
260 C, and then performing a secondary heating of the
biomass at a temperature ranging from 270 to 330 C. The
products of the process are referred to as carbides. As
mentioned in the document, the obtained carbides contain
approximately 75 wt% of carbon. Owing to the temperatures
close to the critical range which are used in the U.S.
patent application, the process is complex and requires
elaborate and thus expensive equipment to be carried out
with safety.
2
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
The known processes for the preparation of coal-like
materials left still room for optimization in terms of
yield, efficiency and quality control of the final coal-
like material. Accordingly, it is an object of the present
invention to provide a process for the preparation of coal-
like material from biomass which is quicker, more efficient
and gives a higher yield of product, and which moreover
allows an enhanced control and reproducibility of the
quality of the final product in comparison to the methods
of the prior art. A still further object resides in a
process which requires less elaborate and expensive
equipment and can nevertheless be carried out with high
safety.
SUMMARY OF THE INVENTION
it has been surprisingly found by the present inventor that
the above objects can be attained by a process for the
preparation of coal-like material from biomass as recited
in Claim 1, which is a process comprising a first
activation step, and a second polymerization step, with the
second step being initiated by the addition of a
polymerization initiator to the reaction mixture obtained
in the first, i.e. the activation step.
Preferred embodiments are subject of the dependent claims.
BRIEF DESCRIPTION OF THE DRAWINGS
The appended Fig. 1 provides a schematic flow diagram
showing a preferred mode of carrying out the process of the
invention in a continuous mode.
DETAILED DESCRIPTION OF THE INVENTION
The process for the preparation of coal-like material
according to the present invention can be referred to as a
3
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
hydrothermal process, in particular as a hydrothermal
carbonisation process. This terminology is intended to show
that the process involves the heating of a reaction mixture
comprising water and will yield carbonized coal-like
material.
The term "biomass" as used herein is broadly understood as
encompassing all kinds of plant and animal material and
material derived from the same. According to a preferred
embodiment, biomass as meant in the present specification
shall not include petroleum or petroleum derived products.
The biomass for use in the present invention may comprise
macromolecular compounds, examples of which are lignin and
polysaccharides, such as starch, cellulose, and glycogen.
As used herein, the term "cellulose" is intended to
encompass hemicelluloses commonly also referred to as
polyoses.
As will be appreciated, certain kinds of biomass may
include both, plant and animal-derived material. As
examples, manure (dung), night soil and sewage sludge can
be mentioned. While the biomass for use in the present
invention is preferably plant biomass, i.e. biomass of or
derived from plants, certain contents of animal biomass
(i.e. biomass of or derived from animals) may be present
therein. For instance, the biomass may contain up to 30 %
of animal biomass.
According to a preferred embodiment, the biomass for use in
the present invention, which is preferably plant biomass,
contains more than 70 wt%, most preferably > 90 wt%, of
polysaccharides and lignines in terms of the solid contents
of the biomass.
4
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
For instance, the plant biomass may be agricultural plant
material (e.g. agricultural wastes) or all kinds of wood
material.
Without limitation, examples of biomass are crop,
agricultural food and waste, feed crop residues, wood (such
as wood flour, wood waste, scrap wood, sawdust, chips and
discards), straw (including rice straw), grass, leaves,
chaff, and bagasse. Furthermore, industrial and municipal
wastes, including waste paper can be exemplified.
The term "biomass" as used herein preferably also includes
monosaccharides such as glucose, ribose, xylose, arabinose,
mannose, galactose, fructose, sorbose, fucose and rhamnose,
as well as oligosaccharides.
As is known to one of average skill in the art, "coal-like
material", as used herein, refers to a material, which is
similar to natural coal in terms of property and texture.
Owing to the method of the preparation thereof, it may also
be referred to as hydrothermal coal. It is a product, more
precisely a carbonized product that is obtained or
obtainable by the hydrothermal carbonization process of the
invention.
As such, the coal-like material can be distinguished from
synthetic resins, including those synthetic resins, which
have been prepared using monomers obtained from
lignocellulosic material, such as phenolic resins, and in
particular novolac-type phenolic resins. Such synthetic
resins are preferably not encompassed by the expression
"coal-like material" as used herein.
The coal-like material obtained in the process of the
present invention typically comprises, without limitation,
< 70 wt% of carbon, for example 60 to 65 wt% of carbon.
Also, the coal-like material obtained in the process of the
5
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
invention can have a carbon content of > 50 wt% of carbon,
which is significantly above the carbon content of dried
(raw) biomass, which is in the order of 40 wt%. For
instance, the coal-like material of the invention may
comprise 65 to 72 wt% of carbon. As will be appreciated,
the above carbon contents refer to the dry mass of the
coal-like materials. In the present invention, the carbon
content of the coal-like materials can be determined by
elemental analysis (combustion). For instance, the carbon.
content of the materials can be measured using a Vario
Micro analyzer (Elementar Analysensysteme, Hanau, Germany),
Moreover, as can be ascertained by way of solid state 13C-
NMR spectroscopy, the coal-like material as meant herein is
more aliphatic than e.g. the product presumably obtained in
US 2008/0006518, which is more aromatic.
The coal-like material obtained in the hydrothermal
carbonisation process of the present invention has a high
calorific value, for instance > 23 MJ/kg, preferably 24 to
38 MJ/kg, and more preferably 24 to 32 MJ/kg. The calorific
value of the coal-like material can be determined by
standard calorimetry. More specifically, in the present
invention, the calorific values of the materials can be
determined in accordance with DIN 51900 or BGS RAL-GZ 724.
As will be appreciated, the above calorific values of the
coal-like material are expressed in terms of the dry mass
of the coal-like material (in kg).
Both, the carbon content and the calorific value of the
coal-like material are indicative of the high degree of
carbonization of the coal-like material of the invention
that can be achieved in the process of the present
invention. According to a preferred embodiment, the coal-
like material of the invention that is obtained by
polymerization of the activated biomass initiated by the
addition of a polymerization initiator in step (ii), has a
6
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
carbon content of > 50 wt% (in terms of the dry mass of the
coal-like material) and a calorific value of > 23 MJ/kg (in
terms of the dry mass of the coal-like material). As meant
herein, the "dry mass" of the coal-like material refers to
the mass thereof after drying under such drying conditions
that no further loss of water is observed. For instance,
the dry mass can refer to the mass of the coal-like
material after drying at a temperature of 80 C for at least
24 h.
in the step (i), i.e. the first step, the biomass is
activated for the subsequent polymerization to coal-like
material in the second step.
Without wishing to be bound to theory, the mechanism of the
activation in step (i) is assumed to be as follows. Upon
heating, the macromolecular species, e.g. the
polysaccharides contained in or constituting the biomass
may be molten or dissolved in water. For instance,
cellulose contained in the biomass, which is a crystalline
material, will be molten in water. Moreover, the
polysaccharides can be disintegrated or broken down to
smaller fragments, such as monosaccharides and
oligosaccharides. Those fragments will undergo consecutive
rapid dehydration to more reactive intermediates, which are
capable of undergoing rapid conversion to coal-like
material, i.e. coalification, in the second step. Due to
this capability, the reactive intermediates can also be
referred to as "coal monomers". The dehydration of glucose
to hydroxymethylfurfural is an example for such a
dehydration reaction. These "coal monomers" are typically
characterized by increased chemical reactivity towards
intermolecular reactions, as compared to the raw biomass,
e.g. via vinylic subunits, reactive aldehyde side groups,
or activated hydroxymethyl groups onto furane moieties. For
this reason, the first step of the hydrothermal
carbonization process of the invention can also be referred
7
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
to as the activation step, and the intermediate product of
that step as activated biomass.
The water being present in the reaction mixture of the
first step may be water adhering or bound to the original
biomass, which can also be referred to as "raw" biomass. In
this specification, the biomass to be subjected to step (i)
is also referred to as raw biomass. As meant herein, raw
biomass may for instance be the biomass obtained as waste
(e.g. wood, agricultural, municipal waste) from the
provider, without further treatment, or as collected from
natural sources. In the case of wood, the raw wood biomass
may be the wood collected in the forest (as the natural
source), or sawdust from the wood processing industry. The
water content of raw biomass may for instance be up to 80
wt%. As will be appreciated from the above, the raw biomass
can be used as such and with water contents as mentioned
above. Though drying is not excluded, e.g. in order to
reduce the weight and consequently the transportation
costs, the (raw) biomass to be subjected to the process of
the invention is preferably not dried. Consequently, the
present invention allows avoiding the energy-consuming
drying of the biomass.
The presence of water in the process of the invention
distinguishes this from e.g. pyrolytic processes for the
conversion of biomass to coal-like materials by simple
heating, typically in the absence of oxygen
(carbonization).
In addition to the water present in, e.g. bound to the raw
biomass such as obtained from natural sources, water may be
added to the wet or dry biomass to adjust the water content
in the reaction mixture of step (i). The total amount of
water, i.e. the water bound to or contained in the
as-obtained biomass and the additional water is not
specifically limited. Preferably, the weight ratio of water
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
to biomass (water/biomass) in the reaction mixture of the
first step is in the range of 0.3 to 10. For the ease of
transportability, especially in a continuous process, the
solid contents of the reaction mixture to be subjected to
the step (i) is preferably 5 to 35%, more preferably 10 to
30%, especially 15 to 25% by weight. The reaction mixture
having such solid contents is preferably in the form of a
slurry.
The reaction mixture comprising water and biomass to be
subjected to heating in the first step may comprise,
without limitation, further ingredients as long as these
will not inhibit the activation of the biomass.
The hydrothermal carbonisation process of the present
invention can be carried out in water alone. Organic
solvents such as ketones are unnecessary, and they are
preferably omitted. According to a preferred embodiment,
the reaction mixture of step (i) contains water as a single
solvent, with other solvents such as ethanol only
incidentially brought in by the biomass, e.g. by
fermentation. Consequently, preferably at least 95 wto,
more preferably at least 98 wta of the solvent present in
the reaction mixture of the first step is water.
The present inventor found that an acidic pH in step (i) is
advantageous. The pH is preferably in the range of 3 to 7,
more preferably 4 to G. By adjusting the pH to the acidic
range, the disintegration, in particular of polymeric
compounds in the biomass, e.g. by hydrolysis can be
accelerated, and the yield of activated biomass, e.g.
smaller fragments can be increased. There are kinds of
biomass, which are more difficult to activate than others.
Wood is an example of biomass, which is quite difficult to
activate. In the case of biomass, which is more difficult
to activate, the pH is adjusted to lie within the acidic
range with particular benefit.
9
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
The desired pH of the reaction mixture in the first step
can be controlled to lie within the above ranges by adding
suitable acids, which do not interfere with the activation
of the biomass. The acid is preferably a strong acid, e.g.
having a pKa of less than 4.5. Both, anorganic acids, e.g.
mineral acids, and organic acids can be used. An example of
a suitable mineral acid is phosphoric acid. Citric acid,
lactic acid and pyruvic acid are examples of (strong)
organic acids. According to a preferred embodiment, an acid
such as those exemplified above is added to the reaction
mixture prior to or during step (i) for the adjustment of
the pH of the reaction mixture to lie in the range of 3 to
7, especially 4 to 6.
The reaction mixture to be subjected to the step (i), which
may e.g. comprise an acid in addition to the (raw) biomass
and water, can be prepared in a suitable mixer.
Dependent on which type of biomass is used as a starting
material, the particular reaction conditions in step (i)
may be selected appropriately. In particular, for biomass
which can be activated relatively easily in step (i), such
as monosaccharides, the duration of the activation step may
be shorter and the pH less acidic than for polymeric
biomass starting material.
The heating temperature (or the reaction temperature) in
step (i) is not particularly limited, as long as it is
sufficient to convert at least larger parts of the (raw)
biomass subjected to the process to activated biomass as
defined herein. Preferably, the heating temperature is such
that at least 80 wt% of the raw biomass are converted to
activated biomass. The heating temperature (or the reaction
temperature) may be in the range of 190 to 270 C, and it
preferably is 210 to 250 CC, more preferably 230 to 240 CC.
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
In this specification, the reaction temperature in step (i)
is occasionally denoted T1.
According to a particularly preferred embodiment, the
temperature is 210 to 250 C, and the pH value is acidic,
especially 3 to 7.
In the process of the invention, in particular under the
above preferred reaction conditions in terms of temperature
and pH, the reaction time of step (i) (i.e. until at least
80 wt% of the biomass have been converted to activated
biomass as meant herein) can be reduced to 5 to 15 minutes,
preferably 5 to 10 minutes. As will be appreciated, the
duration of the first step will depend on the kind of
biomass used.
The (raw) biomass to be subjected to the first step may be
used in any form. Preferably, however it is divided into an
appropriate particle size prior to use, e.g. in the range
of 0.1 to 20 mm, more preferably 0.3 to 10 mm, especially
0.5 to 5 mm. Suitable particle sizes such as those
exemplified above can be obtained by methods such as
grinding, chopping or sawing.
The activated biomass present in the reaction mixture
obtained in step (i) comprises the products of the
disintegration and/or dehydration of the starting "raw"
biomass as detailed above, collectively referred to as
"activated biomass" in the present specification.
In step (ii), the activated biomass is subjected to
polymerization to give coal-like material as defined above.
To account for the fact that the "activated biomass"
obtained in step (i) will be polymerized in step (ii), the
"activated biomass" may in the alternative be referred to
as "polymerizable biomass".
11
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
The polymerization in the second step is initiated by
addition a polymerization initiator to the reaction
mixture. The "polymerization" which takes place in the
second step (i.e, in step (ii)) is to be construed broadly
and means any reaction of molecules of the activated
biomass resulting in the built-up of larger molecules
eventually yielding coal-like material. The polymerization
may include chain-growth of the monomers and inter-chain
crosslinking. In the process of the invention, the
polymerization will yield coal-like material, which
preferably has, in terms of the dry mass thereof, a carbon
content of > 50 wt% and a calorific value of > 23 MJ/kg. In
the present invention, the polymerization initiator is
added in step (ii) as a reactant to initiate the
polymerization resulting in coal-like material, which
preferably has a carbon content and calorific value as
indicated above.
According to a particularly preferred embodiment, the
reaction mixture obtained in step (i) is directly subjected
to step (ii), i.e. without any intermediate treatment.
However, the reaction mixture obtained in step (i) may be
cooled by allowing to stand or by active cooling, prior to
adding the polymerization initiator to start step (ii).
It may be noted that some polymerization of components
contained in the activated biomass may take place already
in step (i). The polymerization step (ii) as such is
initiated by adding the polymerization initiator.
The polymerization initiator for use in the present
invention is not specifically limited in kind, as long as
it is suitable to initiate the polymerization of the
activated biomass to the carbon-like material in the second
step of the hydrothermal carbonization process of the
present invention. At the reaction conditions of the second
step, the polymerization initiator is usually capable of
12
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
generating radicals which will start the polymerization of
the activated biomass to coal-like material.
Useful polymerization initiators are for instance azo
compounds, peroxides, oxygen and redox initiators, as well
as mixtures thereof. The azo compound may be
azobisisobutyronitrile. Useful peroxides are inorganic
peroxides, e.g. persulfates such as potassium persulfate
and ammonium persulfate; metal peroxides such as
(C2H5)2BOOC2H5 and compounds obtained by replacing the
boron atom of (C2H5)2BOOC2H5 with Al or Zn; organic
peroxides, e.g., aryl peroxides such as benzoyl peroxide,
alkyl peroxides such as t-butyl peroxide and cumyl
peroxide, peroxy acid esters such as t-butyl peroxalate, or
hydrogen peroxide. As the redox initiator, there may be
used hydrogen peroxide-Fe2+ (Fenton's reagent), persulfate
and sulfite and cumene hydroperoxide-amine-based compounds.
In addition, copper salts such as CuC12 can be used. In the
alternative and most preferably, FeCl3 and H202 are used as
the redox initiator.
More specifically, polymerization initiators known to be
useful for the hardening of unsaturated polyesters are also
useful to initiate the polymerization in the second step of
the present process.
One specific type of polymerization initiators useful in
the present invention is commonly referred to as warm
hardeners. Warm hardeners are typically peroxides which
will be decomposed at their decomposition temperature to
form a radical which will start the polymerization, i.e.
carbonization, to yield the target coal-like material.
Examples of such peroxides are benzoyl peroxide,
cumolhydroperoxide, methylisobutylketonperoxide, and tert.-
butylperoxybenzoate. As will be appreciated, the reaction
mixture in the second step is preferably heated to or above
the decomposition temperature of the peroxide to generate
13
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
radicals, when warm hardeners are used as polymerization
initiators.
The second type of polymerization initiators which are
likewise useful in the process of the present invention are
often referred to as cold hardeners, e.g. in the field of
unsaturated polyesters. Cold hardeners representing
suitable polymerization initiators for use in the present
invention generally comprise an accelerator compound and a
peroxide, the peroxide being added with preference after
the accelerator compound. Examples of the accelerator
compound are iron salts. Suitable peroxides are e.g.
acetylacetone peroxide, methylethylketone peroxide and
cyclohexanone peroxide. Further examples of the cold
hardeners are amine-based accelerators, such as dimethyl
aniline and diethylenaminetetracetate, in combination with
benzoyl peroxide. As suggested by the name, cold hardeners
do not require any heating to form radicals to start the
polymerization in the second step.
In order to avoid any contamination of the product, i.e.
the coal-like material obtained in step (ii), the
polymerization initiator does preferably not contain any
metal. If the initiator contains any metal, the metal
content in the coal-like material is adjusted to preferably
not more than 0.5 wt%, more preferably not more than
0.1 wt%.
Due to the addition of the polymerization initiator, the
reaction temperature can be kept much lower in the second
step of the process of the invention in comparison to the
prior art such as US 2008/0006518 Al. In particular, the
reaction temperature in the second step can be lower than
in the first step. This allows for carrying out the process
in less elaborate equipment, e.g. in a simple autoclave and
has also significant benefits in terms of the
reproducibility of the process and the quality control of
14
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
the end product. Furthermore, in comparison to the one-step
processes of the prior art, the polymerization, i.e.
carbonization to the coal-like material will proceed much
quicker, e.g. by a factor of 3 to 10.
For instance, the reaction temperature in the second step
(occasionally denoted T2 in this specification) can be in
the range of 140 to 220 C, preferably it is 170 to 210 C,
and more preferably 180 to 200 C. According to a
particularly preferred embodiment, the temperature in the
second step is below the temperature in the first step. For
instance, the reaction mixture may be heated to a
temperature in the range of 210 to 250 C in the first
step, and to a temperature in the range of 170 to below
(i.e, not including) 210 C, especially to 180 to 200 C in
the second step. This is a particularly preferred
embodiment. According to another preferred embodiment, the
reaction temperature in the first step may be 220 to
250 C, and in the second step to 170 to 210 C.
Without limitation, the polymerization in the second step
may be finished for instance within 1 to 3 hours. That is,
within that time frame, a coal-like material of
reproducible quality can be obtained. Of course, the
reaction can be carried out longer or shorter if desired.
The process according to the present invention is
preferably carried out in a pressure resistant reactor,
e.g. an autoclave or an extruder. Due to the water in the
reaction mixture, there will be a pressure increase upon
heating. As the hydrothermal carbonization reaction is
exothermic, external heating may no longer be necessary,
once the reaction, e.g. in the first step, has started,
provided the thermal insulation of the reactor or reactors
is sufficient.
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
For the purpose of this specification, the reaction
temperature is meant to refer to the temperature, more
specifically the average temperature, inside the reaction
mixture, which can be measured with a thermocouple.
Consequently, it is readily possible to control the
reaction temperature to lie within the desired range by
heating or cooling the reactor, as appropriate.
Subsequent to the second step, the solid phase comprised or
consisting of the coal-like material can be separated from
the reaction mixture, e.g. by filtration or decantation,
preferably by filtration, while a liquid phase will remain.
Without restriction, this separation, in particular the
filtration can take place at the elevated temperatures of
the second reactor, thus allowing an advantageous heat
management of the reaction system. Then, the coal-like
material can be dried.
As the present inventor found out, the residual liquid
phase obtained in the separation subsequent to step (ii)
can be reused in the hydrothermal carbonisation process of
the invention with particular benefit. For instance, the
above liquid phase, which preferably contains > 80 wt%,
more preferably > 90 wt% of water can be oxidized to obtain
an oxidized liquid phase. This can be done with any
oxidizing agent, as long as this has a suitable oxidation
potential to effect the oxidations as outlined hereinafter,
and as long as the oxidizing agent or the reaction products
thereof does not interfere with the further uses of the
(oxidized) liquid phase as detailed below. Examples of
useful oxidizing agents are, without limitation, oxygen,
hydrogen peroxide, percarbonate, and percarbonic acids.
Preferably, the oxidizing agent is an oxygen-containing
gas, which is preferably air. In the case of the oxygen-
containing gas, such as air, the oxidation of the liquid
phase can be effected by bubbling the gas through the
liquid phase, stirring the liquid phase in an atmosphere of
16
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
the gas or by allowing the liquid phase to stand in the
presence of the gas.
According to an alternative embodiment, the reaction
mixture obtained in the second step which contains the
liquid (aqueous) phase, rather than the (separated) liquid
phase as such is oxidized, and the oxidized liquid phase is
subsequently separated from the solid phase of the coal-
like material in the reaction mixture.
The present inventor has discovered that the liquid phase
in the mixture obtained in the second step, as a result of
the hydrothermal carbonization process, contains e.g.
ethers and ketones (such as laevulic acid), which can be
converted into the corresponding peroxides through the
oxidation. Examples of the peroxides are ketone peroxides,
such as hydroxyacetone peroxides. These (hydro)peroxides
can be recycled to the second step of the reaction, and be
used as a polymerization initiator.
Moreover, it was found out by the present inventor that
strong organic acids such as lactic acid or pyruvic acid
will be generated during the hydrothermal carbonization
process of the present invention. Hence the liquid phase
obtained in the second step can also be recycled to the
first step, where these acids will be effective in
accelerating the activation, in particular the
disintegration as detailed above. If desired, the liquid
phase obtained in the second step can be subjected to
oxidation, as explained above, prior to recycling to the
first step.
As meant herein, the recycling of the liquid phase to the
first step also covers the embodiment where it is recycled
to the mixing unit (to be further explained hereinafter)
from which it will be transferred to the reactor in which
the step (i) is to be carried out.
17
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
With an eye on the heat management, the optionally oxidized
liquid phase preferably is still hot (e.g. it has a
temperature of e.g. 50 - 220 C, preferably 90 -220 C,
most preferably 140 - 180 C) when it is introduced in the
reaction mixture of the first step and/or the second step.
Using the separated liquid (aqueous) phase from the second
step (with or without oxidation) to admix it to the biomass
prior to or during the first step of the hydrothermal
carbonization process of the invention has a number of
benefits. For instance, the water content (and hence also
the viscosity) of the reaction mixture in the first step
can be optimally adjusted. Moreover, the heat included in
the separated liquid phase can be reused in the process.
According to a preferred embodiment, the liquid phase of
the reaction mixture in the second step is divided into two
parts, one of which is recycled (with or without oxidation)
to the first step for pH management, and the second of
which is recycled (after oxidation) to the second step to
make up for consumed polymerization initiator. As will be
appreciated, the liquid phase from the second step may be
subjected to the oxidation prior to dividing this into the
above two parts. in addition, the caloric heat can be
handled efficiently, when the respective parts of the
liquid phase are recycled when still hot. As will be
appreciated, the above recycling is particularly
advantageous in a continuous flow system. Generally
speaking, the above recycling allows for a minimization of
side products and an optimization of the carbon yield in
the hydrothermal carbonization process of the invention.
Using the aqueous liquid phase of the second step for
process reasons (both, as a carrier of heat and of
chemically active components) is also beneficial from the
point of view of sustainability. Side products in the water
18
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
phase (i.e. the aqueous liquid phase) only cumulate up to
their solubility limit while afterwards the equilibrium
reactions under HTC conditions (in particular in the second
step) ensure that the yield of coal-like material is
increased. For these reasons, bringing the reaction water
(i.e. the aqueous liquid phase) into the reaction multiple
times has a beneficial influence on both the reaction
kinetics as well as the carbonization yield, i.e. the yield
of the coal-like material.
In relation to the one-step hydrothermal carbonization
processes of the prior art, the two-step process of the
present invention, in which the first step is preferably
characterized by higher temperature, shorter residence
time, higher reaction viscosity (due to the raw biomass),
and higher technical demands, and in which the second step
is preferably characterized by comparably longer residence
time, lower temperatures and pressures, and a more passive
reaction handling also exhibits many processing advantages.
Specifically, the reactor, in which the first step is to
take place, which step, due to the preferably higher
temperature and pressure, is more demanding from a
process engineering point of view than the second, can be
kept rather small, which is safer and moreover gives better
heat transfer.
For instance, the reactor for carrying out the first step
can be an extruder. Extruders allow very high pressures of
up to 700 bars, temperatures of up to 400 C, are made for
viscous starting materials, are regarded as very reliable
and safe. Moreover, extruders allow effective and rapid
heating and the injection of additional reactants at
desired positions.
In a most preferable version, the temperature of the
reaction mixture containing the activated biomass is
19
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
reduced downstream, e.g. at the end of the extruder to the
desired reaction temperature of the second step, making use
of an efficient heat exchanger, and the heat is used for
the preheating of the biomass to be subjected to the first
step.
As lower temperature and pressure can be employed in the
second step due to the addition of the polymerization
initiator, less demanding and safer constructions of the
reactor(s) to be used in the second step are possible in
the hydrothermal carbonization process of the invention. As
heat is produced throughout the hydrothermal carbonization
process (it is a highly exothermic process) the reactor(s)
for use in the second step - sufficient insulation provided
- may not require further external heating and may be
heated by recirculation of the cooling liquid of the first
step.
Without restriction of generality, the second reaction step
can be carried out in a cascade of smaller reactors, which
improves the residence time within the second step and
gives an improved coal-like material.
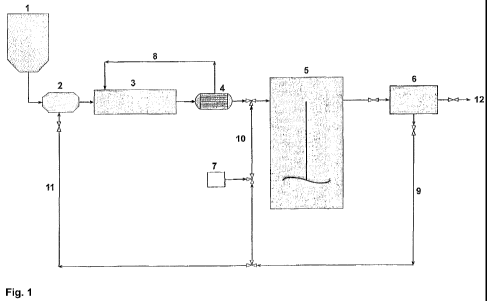
By reference to the appended Fig. 1, a preferred mode of
carrying out the process for the preparation of coal-like
material according to the present invention will be
illustrated. As can be seen, the process schematically
shown in the flow diagram provided in the figure is a
continuous process.
Reference numeral 1 denotes a (raw) biomass storage vessel.
Preferably, the biomass contained in the vessel 1 has a
suitable particle size, e.g. in the range of 0.1 to 20 mm.
From the storage vessel 1, the biomass is fed to a mixing
unit 2 where it is mixed with further ingredients such as
water and acid to give a reaction mixture. A stream 11 of
the (aqueous) liquid phase separated in the separating unit
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
6 is recycled to the mixing unit 2 to provide at least part
of the water and acid. According to a preferred embodiment,
the stream of the aqueous liquid 11 (comprising acids) is
mixed with the biomass in the mixing unit 2 without adding
any further ingredients. Moreover, the separated liquid
phase 11 preferably has a temperature of 140 to 220 C. The
reaction mixture obtained in the mixing unit 2 is then
transferred to the reactor 3 in which the step (i) is
carried out. The reactor 3 is preferably an extruder as
detailed above. The reaction temperature T1 may be between
190 and 270 C. The reaction mixture comprising activated
biomass obtained in the reactor 3 is then transported
through a heat exchanger 4, from which the heat energy 8
can be recycled to the reactor 3. The reaction mixture
comprising activated biomass leaving the heat exchanger 4
is transferred to the reactor 5 for carrying out the
polymerization step (ii). The reactor 5 is a pressure
vessel, e.g. an autoclave. Preferably, it is provided with
stirring means, which are schematically shown in the
figure. The internal temperature in the reactor 5 may be
between 140 and 220 C. To the reactor 5, a polymerization
initiator may be added from the external (not shown). In
the alternative or in addition, oxidized aqueous liquid
phase 10 separated in the separating unit 6 and containing
suitable peroxides is fed along with the reaction mixture
comprising activating biomass to the reactor 5. The
reaction mixture comprising coal-like material obtained in
the step (ii) is transferred from the reactor 5 to the
separating unit (e.g. filtration unit) 6, in which it is
separated into a solid phase of the coal-like material 12,
and an (aqueous) liquid phase 9. In the continuous process
illustrated in the figure, the liquid phase 9 is split into
two parts. The first part is recycled as stream 11
(preferably having a temperature between 140 and 220 C) to
the mixing unit 2. The second part is oxidized by adding a
suitable oxidizing agent from the oxidizing unit 7 to
obtain species in the oxidized liquid phase 10 which can
21
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
act as a polymerization initiator and can as such be
recycled to the reactor 5 as detailed above.
While the above description was focussed on the
hydrothermal carbonization process, the present invention,
according to a preferred embodiment, relates to the coal-
like material obtained or obtainable by the process of the
invention as such which is preferably characterized by the
physical characteristics, in particular the carbon content
and the calorific value as detailed above.
The present invention will be further understood from the
following examples, which are given by way of illustration
and must not be construed in a limiting sense. In the
examples, the carbon content of the materials was
determined with a Vario Micro analyzer (Elementar
Analysensysteme, Hanau, Germany), and the calorific value
of the materials was measured in accordance with BGS RAL-
GZ 724.
EXAMPLES
EXAMPLE 1
6.0 g glucose is dissolved in 24.0 g water, and citric acid
is added to adjust the pH to 5. A 40 ml stainless steel
autoclave is charged with the mixture and heated to 230 C
for 5 min. After cooling, the autoclave is opened, and
mg benzoyl peroxide is added under stirring. Then, the
30 autoclave is sealed again, and heated to 180 C. After 90
min, the reaction is terminated by quenching. 2.9 g of
hydrothermal coal containing 64 wt% carbon is obtained. The
calorific value of the coal is 26 kJ/g.
22
CA 02737596 2011-03-15
WO 2009/127727 PCT/EP2009/054602
EXAMPLE 2
200 g wood flour (sawdust) and 600 g water are formed into
a slurry, and phosphoric acid is added to adjust the pH to
4. A 1 1 stainless steel autoclave (manufactured by Paar,
Germany), equipped with an internal thermoelement, is
charged with the mixture and heated to 250 C. After
exceeding the internal temperature of 240 C, the reaction
is quenched after 10 min. Subsequently, 100 mg FeC13 = 6
H2O and 200 mg 30% aqueous H202 are added at a temperature
of about 100 C. The reaction mixture is heated again to
195 C, and further reacted for 3 h. After cooling and
opening the autoclave, the product is separated into a
solid phase (containing the hydrothermal coal) and a liquid
phase. There is obtained 76 g of hydrothermal coal which
contains 68 wt% carbon and can be pulverized by hand. The
calorific value of the coal is 25 kJ/g.
EXAMPLE 3
The liquid phase separated from the reaction mixture in
Example 2, while still warm (60 C), is bubbled with air to
obtain an oxidized liquid phase. Then, Example 2 is
repeated except for using 200 ml of the thus-obtained
oxidized liquid phase in place of the FeC13 = 6 H2O and
H202. A product is obtained which is, within the
measurement accuracy, the same as that obtained in Example
2.
23