Note: Descriptions are shown in the official language in which they were submitted.
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
GAS LIQUID CONTACTOR AND EFFLUENT CLEANING SYSTEM AND METHOD
[0001] This application is a continuation-in-part of Application No.
12/012,568, entitled
"Two Phase Reactor," filed on February 4, 2008, which is a continuation of
U.S. Patent
Application No. 11/057,539, entitled "Two Phase Reactor," filed on February
14, 2005, now
Patent No. 7,379,487, which claims the benefit of U.S. Provisional Application
No. 61/100,564,
entitled "System for Gaseous Pollutant Removal," filed on September 26, 2008,
U.S. Provisional
Application No. 61/100,606, entitled "Liquid Gas Contactor System and Method,"
filed on
September 26, 2008, and U.S. Provisional Application No. 61/100,591, entitled
"Liquid Gas
Contactor and Effluent Cleaning System and Method," filed on September 26,
2008; all of the
disclosures set-forth above are herein specifically incorporated in their
entireties by this
reference.
BACKGROUND OF THE INVENTION
Field of the Invention
[0002] The invention relates to a gas liquid contactor and effluent cleaning
system and
method and more particularly to an array of nozzles configured to produce
uniformly spaced flat
liquid jets shaped to minimize disruption from a gas flow and maximize gas
flow and liquid flow
interactions while rapidly replenishing the liquid.
Discussion of the Related Art
[0003] The absorption of a gas into a liquid is a key process step in a
variety of gas liquid
contacting systems. Gas liquid contactors, also known as gas liquid reactors,
can be classified
into surface and volume reactors where the interfacial surface area between
the two phases is
created at the liquid surface and within the bulk liquid, respectively. There
are many examples
of surface gas liquid reactors such as rotating disks and liquid jet
contactors. Rotating disk
generators are disks (rotors) partially immersed in a liquid and exposed to a
stream of gas. A
thin film of liquid solution is formed on the rotor surface and is in contact
with a co-current
reagent gas stream. The disk is rotated to refresh the liquid reagent contact
with the gas. In a
volume gas liquid reactor, the gas phase is dispersed as small bubbles into
the bulk liquid. The
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
gas bubbles can be spherical or irregular in shape and are introduced into the
liquid by gas
spargers. The bubbles can be mechanically agitated to increase the mass
transfer.
[0004] In many gas liquid contacting systems, the rate of gas transport to the
liquid phase
is controlled by the liquid phase mass transfer coefficient, k, the
interfacial surface area, A, and
the concentration gradient, delta C, between the bulk fluid and the gas liquid
interface. A
practical form for the rate of gas absorption into the liquid is then:
ID =Oa =kGa(p-p,)=kLa(CL-CL)
where the variable c is the rate of gas absorption per unit volume of reactor
(mole/cm3); 0 is the
average rate of absorption per unit interfacial area (mole/cm); a is the gas
liquid interfacial area
per unit volume (cm2/cm3, or cm 1); p and p1 are the partial pressures (bar)
of reagent gas in the
bulk gas and at the interface, respectively; CL* is the liquid side
concentration (mole/cm3) that
would be in equilibrium with the existing gas phase concentration, pi; CL
(mole/cm 3) is the
average concentration of dissolved gas in the bulk liquid; and kG and kL are
gas side and liquid
side mass transfer coefficients (cm/s), respectively.
[0005] In the related art, there are many approaches to maximizing the mass
transfer and
specific surface area in gas contactor systems. The principal approaches
include gas-sparger,
wetted wall jet, and spray or atomization. The choice of gas liquid contactor
is dependent on
reaction conditions including gas/liquid flow, mass transfer, and the nature
of the chemical
reaction. Table 1 summarizes various mass transfer performance features of
some related art gas
liquid reactors. To optimize the gas absorption rate, the parameters kL, a,
and (CL* - C1) must be
maximized. In many gas liquid reaction systems the solubility of the CL* is
very low and control
of the concentration gradient, therefore, is limited. Thus, the primary
parameters to consider in
designing an efficient gas liquid flow reactor are mass transfer and the
interfacial surface area to
reactor volume ratio, which is also known as the specific surface area.
TABLE 1: COMPARISON OF CONVENTIONAL GAS LIQUID REACTOR
PERFORMANCE
Reactor Type kG kL a kLa
(%, gas (mole/cm2s (cm2s) (cm-) (S"1)
liquid atm) x 102 X102
volumetric x104
flow rate
ratio)
Packed Column 2-25 0.03 - 2 0.4 - 2 0.1 - 3.5 0.04-7.0
(counter-current)
2
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
Bubble Reactors 60 - 98 0.5 - 2 1 - 4 0.5 - 6 0.54 - 24
Spray Columns 2-20 0.5 - 2 0.7-1.5 0.1 - 1 0.07-1.5
Plate Column (Sieve 10 - 95 0.5 - 6 1-20 1 - 2 1.0 - 40
Plate)
[00061 There are various gas liquid contacting reactors whose performance is
dependent
on interfacial contact area. For example, the chemical oxygen iodine laser
(COIL) produces
laser energy from a chemical fuel consisting of chlorine gas (Cl2) and basic
hydrogen peroxide
(BHP). The product of this reaction is singlet delta oxygen, which powers the
COIL. The
present technology uses circular jets of liquid BHP mixed with CIz gas to
produce the singlet
delta oxygen. In a typical generator, the jets are on the order of 350 microns
in diameter or
smaller. To generate the jets, the liquid BHP is pushed under pressure through
a nozzle plate
containing a high density of holes. This produces a high interfacial surface
area for contacting
the CIz gas. The higher the surface area, the smaller the generator will be
and the higher the
yield of excited oxygen that can be delivered to the laser cavity. Smaller and
more densely
packed jets improve the specific surface area, but are prone to clogging and
breakup. Clogging
is a serious problem since the reaction between chlorine and basic hydrogen
peroxide produces
chlorine salts of the alkali metal hydroxide used to make the basic hydrogen
peroxide. Clogging
also limits the molarity range of the basic hydrogen peroxide, which reduces
singlet oxygen yield
and laser power. The heaviest element of the COIL system is this chemical
fuel. Problems
inherent in producing the fuel increase the weight and decrease the efficiency
of the COIL laser
as a whole. Thus, there exists a need for a COIL laser that has increased
efficiency and lower
weight than present designs.
[00071 In another example, gas liquid contactors are also used in aerobic
fermentation
processes. Oxygen is one of the most important reagents in aerobic
fermentation. Its solubility in
aqueous solutions is low but its demand is high to sustain culture growth.
Commercial
fermenters (>10,000 L) use agitated bubble dispersion to enhance the
volumetric mass transfer
coefficient kLa. The agitation helps move dissolved oxygen through the bulk
fluid, breaks up
bubble coalescence, and reduces the boundary layer surrounding the bubbles.
The interfacial area
in these systems is increased by increasing the number of bubbles in the
reactor and reducing the
size of the bubble diameter. However, oxygen mass transfer to the
microorganism is still
constrained by the relatively small interfacial surface area of the bubble and
the short bubble
residence times. Current sparger systems (bubble dispersion) show a relatively
small volumetric
3
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
mass transfer coefficient kL, (about 0.2/s); therefore, a new approach for
generating maximum
interfacial surface area is desired to overcome these mass transfer
limitations.
[00081 In designing systems for industrial applications, consideration must be
given to
both cost and efficiency. Conventional wisdom generally precludes that both
can be optimally
obtained simultaneously. In the case of gas liquid contactors, the
conventional wisdom is
generally maintained in industrial applications such as chemical processing,
industrial biological
applications, pollution control, or similar processes requiring reacting or
dissolving a gas phase
chemistry with a liquid phase in a dynamic flow system.
100091 In the example of pollution control, the standard methodology of
removing a
target compound or compounds in a wet process is a countercurrent flow system
utilizing fine
droplets of liquid phase falling through a flowing gas phase 180 in an
opposite direction.
Normally, gravity is used to draw the liquid phase to a capture sump at the
base of a column or
tower. The gas phase flows up through the same column or tower. This gas phase
is then
captured for further processing or released to the atmosphere.
100101 In order to accommodate for larger scale chemical processes, the column
or tower
must be scaled linearly with the size of the desired process either by length
or diameter. The
current logical methodology is to increase the scale of a single unit process
since capital costs of
a single unit process generally do not scale linearly with size.
100111 Another downside of standard countercurrent, gravitational or
aerosol/droplet gas
liquid contactors is that gas flows must be at a low enough velocity such that
gravity effects are
greater than the buoyancy of the droplets. Regardless, significant evaporation
of the liquid
reactant generally does occur since contact times are long, requiring
significant capture of that
vapor prior to secondary processing or release.
SUMMARY OF THE INVENTION
[0012] Accordingly, the invention is directed to a gas liquid contactor and
effluent
cleaning system and method that substantially obviates one or more of the
problems due to
limitations and disadvantages of the related art.
100131 An advantage of the invention is to provide large volumetric mass
transport
coefficients and resultant small size, low pressure sorbent operation
requiring minimal pumping
capability across the system.
100141 Another advantage of the invention is to provide a gas liquid contactor
with a
reduced system footprint as compared to the related art.
4
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
100151 Yet another advantage of the invention is to provide a gas liquid
contactor with a
module design.
100161 Still another advantage of the invention is to provide a gas liquid
contactor that
uses enhanced specific surface area of a flat jet (e.g., thin flat liquid jet)
to improve the
performance of gas liquid reactors.
100171 Another advantage of the invention is to provide a modular system that,
due to its
smaller size, footprint, factory build, and high contact area, has a
fractional cost and site impact
and potentially higher quality and unit to unit consistency as compared to
conventional systems
for the same reaction or scrubbing capacity.
100181 Additional features and advantages of the invention will be set forth
in the
description which follows, and in part will be apparent from the description,
or may be learned
by practice of the invention. The objectives and other advantages of the
invention will be
realized and attained by the structure particularly pointed out in the written
description and
claims hereof as well as the appended drawings.
100191 An embodiment of the invention is directed towards a gas liquid
contactor
module. The gas liquid contactor module includes a liquid inlet, gas inlet,
and gas outlet. The
contactor module also includes an array of nozzles in communication with the
liquid inlet and
the gas inlet. The array of nozzles is configured to produce uniformly spaced
flat liquid jets
shaped to minimize disruption from a gas. The gas liquid separator is capable
of allowing liquid
to pass through while substantially preventing gas from passing through. The
liquid outlet is in
fluid communication with the gas liquid separator.
100201 Another embodiment of the invention is directed towards a method of
processing
gas phase molecules with a gas liquid contactor. The method includes forming a
plurality of
essentially planar liquid jets, where each of said liquid jets includes a
planar sheet of liquid, and
where the plurality of liquid jets is arranged in substantially parallel
planes. Also, the method
includes providing gas with at least one reactive or soluble gas phase
molecule and removing or
reacting at least a portion of the gas phase molecule by a mass transfer
interaction between the
gas phase molecule and the liquid jets.
100211 Still another embodiment of the invention is directed towards a gas
liquid
contacting system. The gas liquid contactor system includes a reaction
chamber, a gas inlet, a
gas outlet, and a liquid plenum coupled to the reaction chamber. A nozzle
array is coupled to the
liquid plenum; the nozzle array is configured to provide essentially planar
liquid jets, where each
of said liquid jets includes a planar sheet of liquid, and where the plurality
of liquid jets is
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
arranged in substantially parallel planes. The system also includes a gas
fluid separator coupled
to the reaction chamber.
[0022] Yet another embodiment of the invention is directed towards a gas
liquid
contactor. The gas liquid contactor includes a fluid plenum configured to
provide a contacting
liquid and a contacting chamber in communication with the fluid plenum and
configured to
receive the contacting liquid from the fluid plenum. A gas inlet and outlet
are in communication
with the contacting chamber. The gas liquid contactor system is configured to
provide mass
transfer interaction having a volumetric mass transfer coefficient in a range
from about 5 sec-1 to
about 250 sec-1.
[0023] Still yet another embodiment of the invention is directed towards a gas
phase
molecule processing system. The gas phase molecule processing system includes
a plurality of
modular gas liquid contactors configured to be arranged in parallel or series
in order to be sized
as needed for gas phase molecule processing.
[0024] Another embodiment of the invention is directed towards a gas liquid
contacting
system that uses the enhanced specific surface area of a flat jet (e.g., thin
flat liquid jet) to
improve the performance of gas liquid flow reactors. In this embodiment, a
rigid nozzle plate
containing a plurality of orifices that generate very thin flat jets is used.
The flat jet orifice has in
one configuration a V-shaped chamber attached to the source of the liquid
reagent. The flat jet
orifice may have a pair of opposing planar walls attached to a vertex of the V-
shaped chamber.
The flat jet nozzle may have a conical nozzle attached to an opposite end of
the opposing planar
walls as the V-shaped chamber. In another configuration, the jet orifice may
have a circular
orifice attached to the liquid source chamber. The flat jet nozzle may have a
V-shaped groove
intersecting the circular orifice to create an oval shaped orifice. The flat
jet orifice may be
oriented perpendicularly, opposed or parallel to the inlet source of gas. A
smallest passage of the
flat jet nozzles may be larger than about 250 m. The nozzle may produce a
liquid flat jet that
has a width that is at least ten times its thickness. The flat jets may be
made as thin as about 10
pm or smaller and be separated by only 1 mm greater or smaller to generate
high packing jet
densities (8 = 0.01) and large specific surface areas of about 20 cm-1. This
is about 5 to about 10
times significant improvement over the specific surface area values listed in
Table 1. The thin
jet allows more of the liquid to be exposed to the gas flow generating a
higher yield of reaction
product per unit liquid mass flow than conventional contactors.
[0025] Another embodiment of the invention is directed towards providing a gas
liquid
contactor that generates a plurality of thin flat jet streams that are closely
and uniformly spaced,
6
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
that have high specific surface area, that have uniform jet velocity, that are
aerodynamically
shaped to minimize gas flow disruption of the liquid jets, that have orifices
free from salt
obstruction and clogging and that are operated within cross-flow, co-flow,
counter-flow and
parallel flow gas process streams.
[00261 Still another embodiment of the invention is directed towards an
improved COIL.
The COIL includes an excited oxygen generating chamber with an inlet for a
source of chlorine
and a flat jet nozzle for a source of BHP. The nozzle has a multitude of
orifices that have a
minimum dimension that is greater than about 600 m in length and it generates
thin flat jets of
high specific surface area. A photon generating chamber has a passage coupled
to the excited
oxygen generating chamber and an inlet for iodine. The BHP orifice may produce
a flat jet of
basic hydrogen peroxide that has a width that is at least ten times its
thickness. The source of
hydrogen peroxide may be a basic hydrogen peroxide which uses a single base or
a mixture of
bases. The single base may be potassium hydroxide or any of the alkali
hydroxides. The nozzle
may have a pair of parallel opposing plates having a second end attached to a
conical nozzle.
The nozzle may have a pair of V-shaped plates coupled to a first end of the
pair of parallel
opposing plates.
100271 Still another embodiment of the invention is directed towards an
improved COIL
that includes an excited oxygen generating chamber with an inlet for a source
of hydrogen
peroxide and a flat jet nozzle for a source of alkali (Li, Na, K) and alkaline
earth (Mg, Ca)
hypochlorite. In this embodiment, the hydrogen peroxide is a gas. The nozzle
has a multitude of
orifices that have a minimum dimension that is greater than about 600 M in
length and it
generates thin flat jets of high specific surface area. A photon generating
chamber has a passage
coupled to the excited oxygen generating chamber and an inlet for iodine.
100281 Yet another embodiment of the invention is directed towards an improved
fermentation reactor that includes an inlet source of oxygen, C02, or some
other nutrient or feed
gas and a nozzle containing a multitude of orifices for generating flat jets
of fermentation media.
100291 Another embodiment of the invention is to provide a high surface area
flat jet
generator for use in gas scrubbing processes where gases such as ammonia,
carbon dioxide, acid
gases, hydrogen sulfide or sulfur dioxide are separated from a gas by liquid
contact.
[00301 Still another embodiment of the invention is to provide a high surface
area
injector device for use in gas liquid jet combustor engines.
[00311 Yet another embodiment is directed towards a high performance gas
liquid
contactor. The gas liquid contactor includes a fluid plenum for providing a
contacting liquid.
7
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
The gas liquid contactor also includes a contacting chamber in communication
with the fluid
plenum and receiving the contacting liquid from the fluid plenum. The gas
liquid contactor also
includes a gas inlet in communication with the contacting chamber for
providing a gas and a gas
outlet in communication with the contacting chamber to carry away the gas.
Also, the gas liquid
contactor is characterized by a specific surface area in a range between about
1 cm -1 to about 50
cm"I and a gas pressure drop of less than about 5 Torr.
[00321 Another feature includes that the specific surface area is in a range
between about
cm -1 to about 20 cm 1. The gas pressure drop through the gas liquid contactor
in this
embodiment ranges from about 5 Torr to about 10 Torr. One feature of the
embodiments
includes that a reactor gas flow volume for a coal fired power plant output
can be greater than
about 2500 actual cubic feet per minute per molecular weight (MW) of plant
output through a
reactor volume of less than about 15 cubic feet, or gas flow rate to reaction
chamber volume
ratios in a range between 100 min- d and 1000 min- ~. Another feature includes
that a liquid
driving pressure for displacing the contacting liquid into the contacting
chamber is at a low
pressure, e.g., less than 50 pounds per square inch (psi). Another feature
includes that the liquid
driving pressure is less than about 20 pounds per square inch (psi). Yet
another feature includes
that about 99% of liquid entrainment is removed. Still another feature
includes that the
contacting liquid is displaced through a plurality of nozzles that produce
jets of flat liquid, and
the plurality of nozzles arranged such that the jets form a plurality of
parallel rows of jets.
Another feature includes that the gas flows in the contacting chamber parallel
to the rows of jets.
[00331 Another embodiment of the invention is directed towards a method of
contacting
a gas with a liquid. The method includes providing a gas liquid contactor
including a fluid
plenum for providing a contacting liquid, providing a contacting chamber in
communication with
the fluid plenum and receiving the contacting liquid from the fluid plenum.
The contacting
chamber includes a gas inlet in communication with the contacting chamber for
providing a gas
and a gas outlet in communication with the contacting chamber to carry away
the gas. The gas
liquid contactor is characterized by a specific surface area of between about
1 cm-1 to about 50
cm-1. The gas is driven at a pressure drop of less than about 0.05 psi per gas
flow lineal foot of
contactor.
[00341 Another feature includes a specific surface area in a range of about 10
cm"1 to
about 20 cm-1. Another feature is directed towards a method to drive the
contacting liquid into
the contacting chamber at a pressure less than about 20 pounds per square
inch. Another feature
includes that about 99% of liquid entrainment is removed. Another feature
includes that the
8
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
contacting liquid is displaced through a plurality of nozzles that produce
jets of flat liquid, the
plurality of nozzles arranged such that the nozzles form a plurality of
parallel rows of jets.
Another feature includes that the gas flows in the contacting chamber parallel
to the sheets of
jets.
[0035] Another embodiment of the invention is directed towards a gas liquid
contactor
including a plurality of essentially planar liquid jets, each of the liquid
jets comprising a planar
sheet of liquid, the plurality of liquid jets lying in parallel planes. A
contactor chamber housing
the planar liquid jets wherein the contactor chamber has an input and an
output defining a flow
of gas. One feature includes planar sheets having a thickness in a range of
about 10 m to about
1000 m. Another feature includes that the thickness is in a range of about 10
m to about 100
m. Another feature includes that the thickness is in a range of about 10 m to
about 50 m.
Another feature includes that each of the planar sheets of liquid is spaced
from the adjacent
planar sheet by a distance greater than about 10 m in a single row and less
than about 2 cm in
adjacent rows of nozzles. Another feature is that the gas liquid contactor
includes a plurality of
nozzles producing the plurality of liquid jets, however other geometric
configurations will work.
Another feature includes that each of the plurality of nozzles have an
approximately elliptical
exit. Another feature includes that the plurality of nozzles is arranged on a
plate. Another
feature includes that the plurality of nozzles is arranged on the plate such
that the flow of gas is
parallel to a flat face of the jets of flat liquid. Another feature includes
that the plurality of
nozzles is arranged in a plurality of rows forming an array of nozzles and
liquid jets. Another
feature is that the gas liquid contactor module includes an anti-splash grid.
Another feature
includes that a plurality of members of the anti-splash grid is angled to
assist in a flow of the
liquid after traveling through the contactor chamber. Another feature is that
the gas liquid
contactor module includes a mist eliminator.
[0036] Yet another embodiment of the invention is directed towards a nozzle
for creating
a flat liquid jet, the nozzle includes a plate for housing the nozzle. The
embodiment also
includes a fluid entrance aperture of the nozzle having a V-shape in cross-
section and a fluid exit
aperture of the nozzle having a conical exit in cross-section. One feature is
that a narrowest
aperture of the fluid entrance aperture meets a narrowest aperture of the
fluid exit aperture to
form a nozzle narrowest aperture. Another feature is that the nozzle narrowest
aperture is
greater than about 600 m. Another feature is that the base of the fluid exit
aperture is
approximately an oval.
9
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[00371 Still another embodiment of the invention is directed towards a
plurality of
nozzles for creating thin flat liquid jets including a channel, approximately
V-shaped, forming a
fluid entrance aperture for the plurality of nozzles. The embodiment also
includes a plurality of
fluid exit apertures in the channel, the fluid exit apertures having a conical
cross section.
Another feature is that the plurality of fluid exit apertures have an
elliptical shape. One feature is
that a narrowest aperture of the conical cross section and the plurality of
nozzles is greater than
about 600 m.
[00381 Yet another embodiment of the invention is directed towards an effluent
processing system that includes a plurality of nozzle plates for spraying a
solvent. Each of the
plurality of nozzle plates has a plurality of nozzles. The invention also
includes a scrubber unit,
for cleaning a flue gas, housing the plurality of nozzle plates. One feature
is that the plurality of
nozzles creates an array of flat liquid jets. Another feature is that the
plurality of flat liquid jets
is parallel to a flow of the flue gas. Another feature is that the plurality
of flat liquid jets is
arranged in rows. Another feature is that the system includes a flue gas
cooler. Another feature
is that the system includes a flue gas heater. Another feature is that the
system includes a second
scrubber unit. Another feature is that the scrubber unit includes a gas liquid
fluid separator.
Another feature is that the system includes a solvent pump for pumping the
solvent to the
scrubber unit and the plurality of nozzles. Another feature is that the system
includes a solvent
catch tank for collecting solvent passing through the scrubber unit. Another
feature is that the
plurality of nozzle plates is removable from the scrubber unit.
[00391 Another embodiment of the invention is directed towards an effluent
processing
system including a plurality of nozzles for spraying a solvent. The embodiment
also includes a
scrubber unit, for cleaning a flue gas, housing the plurality of nozzles. One
feature is that the
plurality of nozzles creates a plurality of flat liquid jets. Another feature
is that the plurality of
flat liquid jets is parallel to a flow of the gas. Another feature is that the
plurality of flat liquid
jets is arranged in rows.
[00401 Still another embodiment of the invention is directed towards a method
of
contacting a liquid with a gas. The method includes providing a contact
chamber having a liquid
entrance point, creating a plurality of flat liquid jets in the contact
chamber, and providing a flow
of gas parallel to the plurality of flat liquid jets. One feature is that the
liquid entrance point
includes a plate for housing a plurality of nozzles. Another feature is that
the plurality of nozzles
has a fluid entrance aperture having a U-shape in cross-section and a fluid
exit aperture of the
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
nozzle having a conical exit in cross-section. Another feature is that the
method further includes
arranging the plurality of liquid jets in a plurality of rows.
[00411 Yet another embodiment of the invention is directed towards a gas
liquid
contactor including a fluid plenum for providing a contacting liquid, a
contacting chamber in
communication with the fluid plenum for receiving the contacting liquid from
the fluid plenum, a
gas inlet in communication with the contacting chamber for providing a gas and
a gas outlet in
communication with the contacting chamber to carry away the gas. The specific
surface area for
the contacting chamber is in a range from about 10 cm"i to about 20 cm-i and a
liquid side mass
transfer coefficient for the contacting chamber is greater than about 0.02
cm/s. One feature may
be that the liquid side mass transfer coefficient is greater than about 0.1
cm/s. Another feature
may be that the liquid side mass transfer coefficient is greater than about 1
cm/s. Another feature
may be that the liquid side mass transfer coefficient is greater than about 10
cm/s. Another
feature may be that the liquid side mass transfer coefficient is greater than
about 25 cm/s.
Another feature may be that the liquid side mass transfer coefficient is less
than or equal to about
50 cm/s.
[00421 Another embodiment is directed towards a gas liquid contactor including
a fluid
plenum for providing a contacting liquid, a contacting chamber in
communication with the fluid
plenum for receiving the contacting liquid from the fluid plenum, a gas inlet
in communication
with the contacting chamber for providing a gas and a gas outlet is in
communication with the
contacting chamber to carry away the gas. A specific surface area is in a
range from about 10
cm i to about 20 cm-i and a volumetric mass transfer coefficient is greater
than about 0.2 sec-1.
One feature may be that the volumetric mass transfer coefficient is greater
than about 1 sec - i.
Another feature may be that the volumetric mass transfer coefficient is
greater than about 10 sec
1. Another feature may be that the volumetric mass transfer coefficient is
greater than 100 sec -1.
Another feature may be that the volumetric mass transfer coefficient is
greater than 1000 sec
Another feature may be that the volumetric mass transfer coefficient is less
than 2500 sec -1.
[00431 Yet another embodiment is directed towards a gas liquid contactor that
includes a
fluid plenum for providing a contacting liquid. The contactor also includes a
plurality of nozzles
in fluidic communication with the fluid plenum that produce a plurality of
flat liquid jets. The
contacting includes a chamber in communication with the fluid plenum and
receiving the
contacting liquid from the fluid plenum through the plurality of nozzles. A
gas inlet is in
communication with the contacting chamber for providing a gas, and a gas
outlet in
communication with the contacting chamber to carry away the gas. One feature
may be that a jet
11
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
length to jet width ratio is about 10:1. Another feature may be that a jet
length to jet width ratio
is greater than about 8:1 but less than about 12:1. Another feature may be
that a jet length to jet
width ratio is greater than about 10:1. Another feature is that each the
plurality of flat liquid jets
have a thickness of about 10 m to about 100 m. Another feature is that a jet
length of each of
the plurality of flat liquid jets is generally greater than about 5 cm but
less than about 30 cm.
Another feature is that jet velocities of the plurality of flat liquid jets is
about 10 m/s.
[0044] Still another embodiment is directed towards a high performance gas
liquid
contactor. The gas liquid contactor includes a fluid plenum for providing a
contacting liquid.
The contactor includes a contacting chamber in communication with the fluid
plenum and
receiving the contacting liquid from the fluid plenum. A gas inlet is in
communication with the
contacting chamber for providing a gas and a gas outlet is in communication
with the contacting
chamber to carry away the gas. The gas liquid contactor is characterized by an
enhanced specific
surface area in a range between about 1 cm -1 to about 50 cm -1 and a very low
gas pressure drop
of less than about 5 Torr or about 1 psig. Another feature is that the very
low gas pressure drop is
less than about 0.05 psi per lineal gas liquid contactor contact distance.
Another feature is that
the very low gas pressure drop is less than about 1 psi for the entire gas
liquid contact system
including gas heaters, gas chillers, and demisters. Another feature is that
the contacting liquid is
displaced through a plurality of nozzles that produce jets of flat liquid when
a liquid flows
through the plurality of nozzles, the plurality of nozzles arranged such that
the jets form a
plurality of parallel rows of jets. Another feature is that the gas flows in
the contacting chamber
parallel to the rows of jets.
[0045] Another embodiment is directed towards a high performance gas liquid
contactor.
The gas liquid contactor includes a fluid plenum for providing a contacting
liquid. The gas
liquid contactor includes a contacting chamber in communication with the fluid
plenum and
receiving the contacting liquid from the fluid plenum. A gas inlet is in
communication with the
contacting chamber for providing a gas, and a gas outlet is in communication
with the contacting
chamber for carrying away the gas. The gas liquid contactor is characterized
by an enhanced
specific surface area in a range of about 1 cm -1 to about 50 cm-1 and a
liquid driving pressure for
displacing the contacting liquid into the contacting chamber is less than
about 15 psi. One
feature is that the liquid driving pressure for displacing the contacting
liquid into the contacting
chamber is less than about 10 psi. Another feature is that the contacting
liquid is displaced
through a plurality of nozzles that produce jets of flat liquid when a liquid
flows through the
plurality of nozzles, the plurality of nozzles arranged such that the jets
form a plurality of parallel
12
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
rows of jets. Another feature is that the gas flows in the contacting chamber
parallel to the rows
of jets.
[00461 Still another embodiment of the invention is directed towards a high
performance
gas liquid contactor module. The module includes a fluid plenum for providing
a contacting
liquid and a contacting chamber in communication with the fluid plenum to
receive the
contacting liquid from the fluid plenum. A gas inlet is in communication with
the contacting
chamber for providing a gas and a gas outlet is in communication with the
contacting chamber
for carrying away the gas. The gas liquid contactor is characterized by a
pollutant removal
percentage of greater than about 80%. One feature is that the contactor volume
is less than about
0.5m3. Another feature is that the pollutant removal percentage is greater
than about 90%.
Another feature is that the pollutant removal percentage is greater than about
95%. Another
feature is that the pollutant removal percentage is about 99% or greater.
Another feature is that a
plurality of modular gas liquid contactors is designed to be arranged in
parallel in order for the
aggregate system to be sized as needed. Another feature may be that the
plurality of modular gas
liquid contactors is arranged vertically. Another feature may be that the
plurality of modular gas
liquid contactors is arranged horizontally. Another feature may be that the
plurality of modular
gas liquid contactors is arranged serially. Another feature may be that the
system parasitic load is
less than about 5%. Another feature is that the system parasitic load is less
than about 1 %.
Another feature is that a scrubbing removal percentage for a pollutant such as
SO2 is greater than
about 90%. Another feature is that a scrubbing removal percentage for a
pollutant such as about
SO2 is greater than about 95%. Another feature is that a scrubbing removal
percentage for a
pollutant such as SO2 is greater than about 99%.
[00471 Yet another embodiment is directed towards a gas liquid contactor
module
including a number of combined features. The module includes a liquid inlet to
provide a
reactive or solvent liquid to the contactor module. It also includes a gas
inlet and outlet which
provides a conduit for the reactive gas or gas solute or gas phase reactant to
pass through the
contactor module. The distribution of fluid through the contactor is provided
by an array of
nozzles in liquid communication with the liquid inlet where that array of
nozzles is configured to
produce uniformly spaced flat liquid jets shaped to minimize disruption from a
gas flowing
through the contactor. Across the contactor chamber from those liquid jet
nozzles is a gas liquid
separator capable of allowing liquid to pass through while substantially
preventing gas from
passing through, which in turn is in liquid contact with a liquid outlet.
13
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[0048] Another embodiment of the invention is directed towards a method of
processing
gas phase molecules with a gas liquid contactor. This method includes a
plurality of essentially
planar liquid jets where each of those liquid jets comprises a planar sheet of
liquid. The plurality
of liquid jets is arranged in substantially parallel planes. The method also
provides gas with at
least one reactive or soluble gas phase molecule. In this method, at least a
portion of the gas
phase molecule is removed by a mass transfer interaction between the gas phase
molecule and
the liquid jets.
[0049] Yet another embodiment of the invention is directed towards a gas
liquid
contacting system including a number of combined subsystems. Those combined
subsystems
include a reaction chamber, a gas inlet coupled to the reaction chamber, a gas
outlet coupled to
the reaction chamber, a liquid plenum coupled to the reaction chamber, a
nozzle array coupled to
the liquid plenum, and a gas fluid separator coupled to the reaction chamber.
With respect to the
nozzle array, the nozzle array is configured to provide essentially planar
liquid jets.
Furthermore, each liquid jet comprises a planar sheet of liquid and those jets
are arranged in a
plurality of liquid jets lying essentially in substantially parallel planes.
[0050] Another embodiment of the invention is directed towards a gas liquid
contactor
where a fluid plenum is configured to provide a contacting liquid to a
contacting chamber. A
second feature is that the contacting chamber is in communication with the
fluid plenum and
itself is configured to receive the contacting liquid from the fluid plenum.
Thirdly, the contactor
has a gas inlet and a gas outlet in communication with the contacting chamber.
Overall, the gas
liquid contactor system is configured to provide mass transfer interaction
having a volumetric
mass transfer coefficient in a range from about 5 sec- to about 250 sec" 1.
[0051] It is to be understood that both the foregoing general description and
the
following detailed description are exemplary and explanatory and are intended
to provide further
explanation of the invention as claimed.
BRIEF DESCRIPTION OF THE DRAWINGS
[0052] The accompanying drawings, which are included to provide a further
understanding of the invention and are incorporated in and constitute a part
of this specification,
illustrate embodiments of the invention and together with the description
serve to explain the
principles of the invention.
[0053] In the drawings:
14
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[0054] FIG. 1 is a block diagram of a system for producing a flat jet
according to an
embodiment of the invention;
[0055] FIG. 2 is a block diagram of a system for producing excited oxygen
according to
another embodiment of the invention;
[0056] FIG. 3 is a block diagram of an improved chemical oxygen iodine laser
according
to another embodiment of the invention;
[0057] FIG. 4 is a top right perspective view of a flat jet nozzle according
to another
embodiment of the invention;
[0058] FIG. 5 is a bottom left perspective view of the flat jet nozzle of FIG.
4;
[0059] FIG. 6 is cross-sectional view of a precursor to a nozzle bank
according to another
embodiment of the invention;
[0060] FIG. 7 is a side view of the precursor to the nozzle bank shown in FIG.
6;
[0061] FIG. 8 is a top view of a nozzle bank according to another embodiment
of the
invention;
[0062] FIG. 9 is a side view of the nozzle bank of FIG. 8;
[0063] FIG. 10 is a cross-sectional view of the nozzle bank of FIG. 8 along
cut B shown
in FIG. 9;
[0064] FIG. 11 is a detail view of the nozzle bank of FIG. 8 as defined by cut
A shown in
FIG. 9;
[0065] FIG. 12 is a perspective view of the nozzle bank of FIG. 8;
[0066] FIG. 13 is a perspective view of a plate into which nozzle banks are
welded;
[0067] FIG. 14 is a side view a nozzle plate according to another embodiment
of the
invention;
[0068] FIG. 15 is a top view of the nozzle plate of FIG. 14;
[0069] FIG. 16 is a perspective view of the nozzle plate of FIG. 14;
[0070] FIG. 17 is a detail blown up view of the nozzle plate of FIG. 14 along
cut line A
shown in FIG. 15;
[0071] FIG. 18 is a perspective view of an array of flat thin flat liquid jets
as produced by
the nozzle plate of FIG. 14;
[0072] FIG. 19 is a front view of the array of flat thin flat liquid jets
produced by the
nozzle plate of FIG. 14;
[0073] FIG. 20 is a side view of the array of flat thin flat liquid jets
produced by the
nozzle plate of FIG. 14;
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[00741 FIG. 21 is a fluid side exit view of a nozzle plate according to
another
embodiment of the invention;
[00751 FIG. 22 is the fluid entrance side view of the nozzle plate of FIG. 21;
[00761 FIG. 23 is a fluid side exit view of a nozzle plate in another
embodiment of the
invention;
100771 FIG. 24 is the fluid entrance side view of the nozzle plate of FIG. 23;
[00781 FIG. 25 is a fluid side exit view of a nozzle plate with a nozzle bank
removed;
[00791 FIG. 26 is the fluid entrance side view of the nozzle plate of FIG. 25;
[00801 FIG. 27 is a top view of a precursor for a nozzle bank;
[00811 FIG. 28 is a side view of the precursor of FIG. 27;
100821 FIG. 29 is a cut away view of a schematic of a gas liquid contactor
according to
another embodiment of the invention;
[00831 FIG. 30 depicts a schematic arrangement of a plurality of gas liquid
contactors
according to another embodiment of the invention;
100841 FIG. 31 is a schematic of a multi-contaminant removal system according
to
another embodiment of the invention;
[00851 FIG. 32 is a schematic of a multi-contaminant removal system according
to
another embodiment of the invention;
100861 FIG. 33 is a schematic of a general gas liquid contactor that enables
interaction
between gas and liquid phases according to another embodiment of the
invention;
[00871 FIG. 34 is a graph of absorbance vs. run time for a NO2 removal system;
[00881 FIG. 35 is a graph of the CO2 FTIR (Fourier Transform Infrared)
absorption
spectrum with the liquid aqueous ammonia jets on and off;
100891 FIG. 36 is a picture of a 2 MW prototype system;
[00901 FIG. 37 is a picture of a gas liquid contactor;
[00911 FIG. 38 is a picture of the solvent pumps of the system of FIG. 41;
[00921 FIG. 39 is a graph of SO2 scrubbing results using H2O, NaOH (0.1 wt%),
0.13
MW scale;
[00931 FIG. 40 is a graph of CO2 scrubbing tests using 19 wt% aqueous ammonia,
0.13
MW scale;
[00941 FIG. 41 is a graph of SO2 scrubbing results using H2O, NaOH (0.1 wt%),
2 MW
scale;
101001 FIG. 42 is a representation of 60 MW scrubbing unit and supporting
structures;
16
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
101011 FIG. 43 is a front view of one section 2 MW section of the scrubber
tower FIG.
47;
[01021 FIG. 44 is a side view of one section 2 MW section of the scrubber
tower of FIG.
47;
[01031 FIG. 45 shows the geometry of the entrance channel and jet pack zone;
[01041 FIG. 46 shows a representation of a jet pack zone with removable nozzle
plate;
101051 FIG. 47 shows the configuration of the nozzle plates in the jet pack
zone of FIG.
51;
[01061 FIG. 48 shows a seal system for the jet pack zone of FIG. 46;
101071 FIG. 49 is a process flow diagram for a pollutant removal system
according to
another embodiment of the invention; and
[01081 FIG. 50 is a process flow diagram from a pollutant removal system
according to
another embodiment.
DETAILED DESCRIPTION OF THE ILLUSTRATED EMBODIMENTS
101091 The invention relates to a gas liquid contactor and effluent cleaning
system and
method and more particularly to an array of nozzles configured to produce
uniformly spaced flat
liquid jets shaped to minimize disruption from a gas. Moreover, various
embodiments directly
provide a plurality of small single unit processes, aggregated into modules,
which, by their
design, overcome the shortcomings of conventional designs. Modularizing single
unit processes
allows for small systems which may be scaled by simply multiplying the module
by convenient
integers to accommodate the scale of the process.
[01101 Moreover, a single gas liquid contactor capable of producing a thin
flat liquid jet
can be readily multiplied and aggregated into a module or modules that perform
within a range
of gas flow rates in a very compact design, dramatically smaller than a
conventional
countercurrent reactor for an equivalent reaction yield. This aggregation into
a module or
modules may be conducted serially or in parallel.
101111 In the serial embodiment modules are incorporated one after another
with the gas
flowing sequentially through each module. Of course, some modules may be
bypassed or have
recirculation loops. Also, the modules can run the same liquid phase, or
different liquid phases
depending on the desired selectivity of the target reactions of gas molecule
capture and sequence.
17
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[0112] In a parallel embodiment modules are incorporated next to or on top of
each other
such that all processing or scrubbing the same gas feed, with each module
processing roughly
equivalent amounts of gas or gas molecules as an adjacent module. In general,
parallel modules
run identical liquid phases to each other since processing in each is
contemporaneous with
adjacent modules.
[01131 An embodiment of the invention is targeted to accommodate higher gas
flow rates
or smaller mass transfer coefficients than the design standard for this single
module, the module
itself may be multiplied in convenient integer units into a larger functional
module having longer
contact times without formally splitting the target process into redundant
systems. Moreover,
this design logic may extend to other sub-modules in the chemical processor,
such as the liquid
capture systems and liquid delivery systems, all accommodating a single gas
flow main plenum
and single liquid processing stage. The expensive capital equipment, such as
pumps and blowers
from the gas and/or liquid flow systems, can be linearly scaled to feed the
incremental modules;
these modules, by their unique designs, couple together to form a functionally
single process in a
very compact design.
[0114] In another embodiment of the invention, the modules may be designed to
force the
liquid phase at very high rates using liquid jets, e.g., thin flat jets,
thereby negating the reliance on
gravity or buoyancy to provide mass transport. The liquid may flow at very
high rates, the gas phase
can also flow at very high velocities transversely, along the same vector, or
in counter current flows.
Because all flows are at high velocities, the direction of flow can be chosen
by design convenience
rather than by gravity or thermal convection limitations. Moreover, the mass
transfer and
volumetric transfer coefficients can be very high and the contact length can
be, again, scaled
modularly to accommodate for both loading and reaction yield.
[0115] In another embodiment of the invention, a gas liquid contactor is
configured to
achieve selective and high mass transfer rates of gas reactants from high
volumetric gas flow
rates into continuously replenished liquids confined in small system volumes.
Also, in various
methods of the invention large dense packed arrays of high velocity stable
liquid jets, e.g., thin
flat liquid jets, are configured to interact with a high velocity gas flow.
Jet formation orifices
and densities may be optimized based on liquid sorbent or reactant
characteristics such as
viscosity and surface tension. In general terms, with no consideration for
chamber size or overall
processing scale, as liquid viscosity increases, liquid jet stability
increases. As such, nozzle
density in the nozzle array can increase and nozzle size can decrease.
However, this is not
required, but can be desirable to decrease jet to jet spacing, thus increasing
and optimizing
18
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
specific surface area of the contactor. In contrast, lower surface energies
tend to destabilize the
jets, leading to small droplet formation in some conditions, which is not
desired in this invention
and which is more typical of the current art. In the case of lower surface
energies, lower liquid
pressure and larger nozzle sizes might be indicated to optimize jet properties
for a given fluid.
[01161 An embodiment of the invention significantly increases the efficiency
of
processes for gas reactants and liquid reactants over conventional methods and
systems. The
efficiencies of the method and system are achieved from the large volumetric
mass transport
coefficients and resultant small size, low pressure sorbent operation
requiring minimal pumping
capability across the system due to the low resistance of the liquid jets and
the modular and
combinable nature of the design. Therefore, unexpected results of embodiments
of the invention
are achieved, e.g., roughly equivalent performance is achieved with reference
to conventional
reactors but with a footprint which can be at least ten times smaller and
capital cost less than at
least half of conventional gas liquid contactors.
[01171 An embodiment of the invention is directed towards a gas liquid
contactor module.
The gas liquid contactor module includes a liquid inlet and outlet and a gas
inlet and outlet. The
module also includes an array of nozzles in communication with the liquid
inlet and the gas inlet
where the array of nozzles is configured to produce uniformly spaced flat
liquid jets shaped to
minimize disruption from a gas. The module also includes a gas liquid
separator capable of
allowing liquid to pass through while substantially preventing gas from
passing through. The
module may be connected to other modules in series or in parallel.
[0118J The module may be manufactured from a plurality of different materials,
e.g.,
copper, nickel, chrome, steel, aluminum, coated metals, and combinations
thereof. In addition,
the module may include a plastic material, or at least one of structural
polymers, polyimides,
composites and combinations thereof.
[01191 The array of nozzles may be formed in a plurality of different
configurations, e.g., in
a staggered configuration. In one staggered configuration a first row of
nozzles, a second row of
nozzles and a third row of nozzles, are arranged such that the second row of
nozzles is offset and
positioned between the first and third row of nozzles.
[01201 The array of nozzles may also include a plurality of nozzles spaced
apart by a
predetermined dimension. The nozzles may include at least two nozzles
separated by a distance
greater than about 0.2 cm. The nozzles may include any number of rows and
columns. In a
preferred embodiment, at least three rows of nozzles are provided and
separated by a uniform
distance. The distance between nozzles may be in a range from about 0.1 cm to
about 5.0 cm.
19
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[01211 The nozzles may be formed from liquid channels having a number of
different
geometric shapes, e.g., a U-shaped channel, V-shaped, and the like. The
channel may be formed
using various methods including, but not limited to, machining or otherwise
forming of a metal,
composite, or ceramic plate, or by machining nozzle orifices in a tube or
portion of a tube.
When machining a single plate, a V or U-shaped channel is machined into the
liquid side of the
plate. These channels are then bisected on the process side of the plate by a
second V-shaped
groove, the depth of which penetrates into the liquid channel space. Depending
on the depth of
the second groove, the resulting hole or nozzle formed by the intersection of
the liquid channel
and the process-side channel can be different sizes.
[01221 A higher penetration intersection results in larger nozzles. That is,
the amount of
intersection of the cone into the V or U-shaped channel results in a larger
nozzle. When forming
nozzles in tubes, a tangential cut is made at about a 90 angle to the axis of
tube radius on the
outside of the radius (the process-side). Depending on the depth of this cut
and the radius of the
tube, both dimension (smallest to largest cross section) and size of the
resulting nozzle can be
changed; deeper cuts result in larger nozzles. The liquid channel feeding the
nozzle may have
depth greater than about 2 mm. In embodiments of the invention the channel may
have depth in
a range from about 2 mm to about 20 mm.
[01231 In another embodiment the shape of the nozzle is formed to be
substantially oval,
such that the nozzle includes a minor to major axis ratio of less than 0.5. In
other embodiments
the nozzle may have a projected cross sectional area in the range from about
0.25 mm2 to about
20 mm2. The projected cross sectional area is determined by evaluation of the
two dimensional
shape of the nozzle when viewed with a backlight projected onto a two-
dimensional surface,
although acknowledging that the actual shape is three dimensional and complex
depending both
on depth of cut and radius and/or shape of curvature of the channel.
[01241 Another embodiment of the invention is directed towards a method of
processing
gas phase molecules with a gas liquid contactor. This method includes forming
a plurality of
essentially planar liquid jets where each of those liquid jets, is formed into
a planar sheet of
liquid. The plurality of liquid jets is arranged in substantially parallel
planes. The method also
provides gas with at least one reactive or soluble gas phase molecule.
[01251 In this embodiment, at least a portion of the gas phase molecule is
removed by a
mass transfer interaction between the gas phase molecules and the liquid jets.
The gas phase
molecules may include effluent from an industrial process, e.g., coal fired
plants or other
industrial effluents, such as contaminants or pollutants may include SOX, NOX,
CO2, Hg, and
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
combinations of the same. Of course other gaseous molecules may also be
removed such as
acidic gases like HCl, HBr, HF, H2SO4, and HNO3, CO, H2S, amines (including
ammonia),
alkanolamines, urea, formamides, alcohols, carboxylates (like acetic acid),
combinations of the
same and a wide variety of other gas phase molecules. The limitation of the
invention is simply
the ability to provide a gas phase molecular reactant or solute and a liquid
phase within which it
is reactive or soluble, respectively. Although the main description in this
invention specification
focuses on aqueous systems, one skilled in the art will readily recognize the
applicability of this
gas liquid contactor invention to non-aqueous systems as well. Such as,
partial fluorination of
pharmaceutics or chlorination of petrochemical feed stocks as known in the
art.
[01261 In an embodiment of the invention, the liquid may be chosen to remove
contaminants in the gas as known in the art. An aqueous base solution may be
utilized for
removing SO2 and other flue gas constituents, such as, a solution containing
about 0.1 M to
about 1.0 of NaOH, NH4HCO3, Na2S03. As known in the art, the concentrations of
these liquid
reactants may be adjusted depending on the mass transfer of the gas liquid
interaction and the
preferred products.
[01271 In addition, some examples of liquid include a solution of at least one
of water,
ammonia, ammonium salts, amines, alkanolamines, alkali salts, alkaline earth
salts, peroxides,
hypochlorites, calcium salt, magnesium, and combinations of the same. Other
solutions may
include seawater, brine, combinations of the same and the like.
[01281 Seawater or brine can be used to scrub SO2 or C02, or both, depending
on pH
control and other engineering factors. Additionally, these liquids would also
be effective for
scrubbing other acid gases, like HCI or HE
[01291 The method forms at least one jet. The jet may be configured to have
various
physical dimensions. For example, the jet may have a length of in a range of 5
cm and 20 cm, jet
width of 1 cm to 15 cm, jet thickness between 10 m to 1000 m. Moreover, the
jet may have a
length to width ratio in a range of 0.3 to 20.
[01301 Another embodiment of the invention provides a gas liquid contacting
system that
includes a number of combined subsystems. Those combined subsystems include a
reaction
chamber, a gas inlet coupled to the reaction chamber, a gas outlet coupled to
the reaction
chamber, a liquid plenum coupled to the reaction chamber, a nozzle array
coupled to the liquid
plenum, and a gas fluid separator coupled to the reaction chamber. With
respect to the nozzle
array, the nozzle array is configured to provide essentially planar liquid
jets. Furthermore, each
21
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
liquid jet comprises a planar sheet of liquid and the plurality of liquid jets
is arranged so as to lie
essentially in substantially parallel planes.
[0131] Reference will now be made in detail to embodiments of the invention,
examples
of which are illustrated in the accompanying drawings.
[0132] FIG. 1 is a block diagram of a system for producing a flat jet
according to an
embodiment of the invention. Referring to FIG. 1, a system 0 includes an array
of flat jet
orifices for producing liquid jets, e.g., thin flat liquid jets, that are
highly dense and have a high
surface area. In this embodiment, a small segment of a nozzle array is
machined from a single
plate. This shows the V-shaped liquid channels, but in this example, the
process side orifice
intersects the V liquid channel with a cone as opposed to a groove. However,
the resulting
nozzle orifice still is elliptical. The nozzle array includes orifices
staggered such that the orifices
are separated by distance. The distance may range from about 0.1 cm to about 5
cm in the x
direction and 0.1 cm to about 2 mm in the y direction. In a preferred
embodiment, the distance is
2 cm in the x direction and 2 mm in the y direction. Of course, the distance
between orifices
does not need to be constant throughout the array of orifices.
[0133] The orifice has a V-shaped entrance 1 and a conical exit 2 channel for
jet
development. The intersection of entrance 1 and exit 2 channels creates the
orifice. A cross
sectional view of the nozzle plate 3 shows contours of the entrance 4 and exit
5 channels. An
approximate representation of the jet exiting the orifice is shown as 7. A
cross sectional close up
of the entrance 8 and exit 9 channels are provided. The thin flat liquid jets
may be formed with a
variable length, e.g., the jet length to jet width ratio may is about 10:1
where the jet has a
thickness in a range from about 10 m to about 100 m. The jet length may be
in a range from
about 5 cm to about 20 cm. The jet width may be in a range from about 0.5 cm
to about 20 cm.
[0134] FIG. 2 is a block diagram of a system for producing excited oxygen
according to
another embodiment of the invention. The COIL is more efficient, weighs less
and is smaller
than previous designs as it uses a nozzle array according to one embodiment of
the invention that
is capable of creating a large specific area of liquid, e.g., basic hydrogen
peroxide. Referring to
FIG. 2 the COIL is represented by reference number 10. The COIL 10 is used for
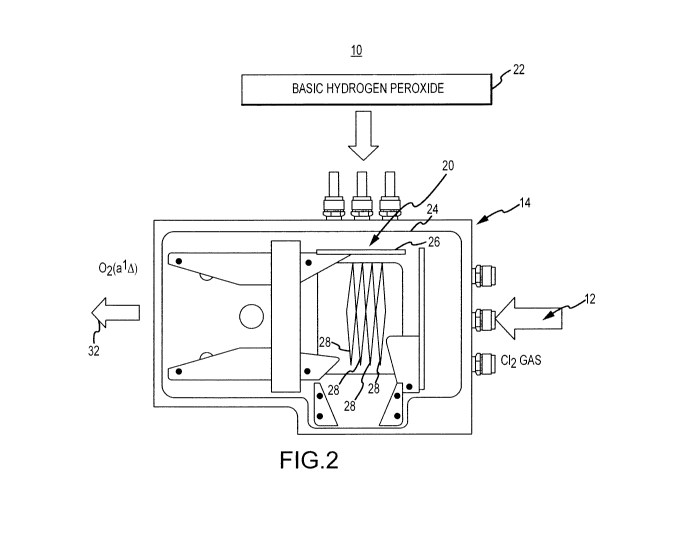
producing
excited oxygen. The COIL 10 includes a gas reactant source 12, e.g., chlorine
gas, attached to a
manifold 14. The manifold 14 has a number of openings, e.g., holes (not
shown), configured to
allow gas jets to enter an excited oxygen generating chamber 20. The COIL 10
also has a source
of liquid reactant 22, e.g., basic hydrogen peroxide formed with a single
base. In one
embodiment, the single base is potassium hydroxide (KOH). The basic hydrogen
peroxide 22
22
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
source is coupled by a piping 24 to a plurality of nozzles 26. The nozzles 26
are configured to
create thin flat jets 28 of the liquid basic hydrogen peroxide. The thin flat
jets 28 of hydrogen
peroxide 22 react with the chlorine gas jets to produce excited oxygen 32. The
COIL 10 may
also include a method collecting the basic hydrogen peroxide for reuse, e.g.,
recycle loop.
[01351 The use of the liquid jets increases the specific surface area of
hydrogen peroxide
22, thereby increasing the efficiency of the reaction with the chlorine gas
12. Tests have shown
that the specific surface area of the thin flat liquid jets is more than three
times greater than that
for related art circular jets. In addition to increasing the surface area of
the hydrogen peroxide,
the flat jets do not require the small throats required by previous nozzles.
More particularly,
previous nozzles have a throat size in a range from about 150 m to about 350
m. The nozzles
26 can use a throat that is greater than about 250 m, or more preferably,
larger than 600 ms.
Therefore, the nozzles 26 are unlikely to clog due to contaminants, e.g.,
salts formed by the
reaction of the hydrogen peroxide and the chlorine gas. In addition, this
allows the system 10 to
use a higher starting molarity of basic hydrogen peroxide solution, e.g.,
molarities as high as
about ten moles/L may be used. Previous systems are generally limited to a
starting molarity of
five moles/L due to the contaminants clogging the system, e.g., formation of
clogging salts.
Most systems reuse the hydrogen peroxide, however once the molarity drops to
about 2.5
moles/L the system's performance is seriously degraded. As a result, most
previous systems are
limited to a delta molarity in a range from about 2.5 moles/L to about 5
moles/L while this
embodiment allows a delta molarity to be in a range from about 2.5 moles/L to
about 10
moles/L. Therefore, the apparatus can carry one third as much basic hydrogen
peroxide or have
three times the capacity of previous systems.
[01361 In another embodiment, a COIL includes an excited oxygen generating
chamber
with an inlet for a source of hydrogen peroxide and a flat jet nozzle for a
source of alkali (Li, Na,
K) and alkaline earth (Mg, Ca) hypochlorite. The hydrogen peroxide is a gas.
The nozzle has a
multitude of orifices with a minimum dimension that is greater than about 300
m in length
capable of generating thin flat jets of high specific surface area. A photon
generating chamber
having a passage coupled to the excited oxygen generating chamber and an inlet
for iodine.
[0137) FIG. 3 is a block diagram of an improved COIL in accordance with
another
embodiment of the invention. Referring to FIG. 3, the improved COIL is
generally represented
as reference number 50. The COIL 50 has a source of gas 52, e.g., chlorine gas
physically
coupled by a conduit or pipe 54 through a number of inlets to an excited
oxygen generating
chamber 56. A source of liquid reactant 58, e.g., basic hydrogen peroxide 58
is transported by a
23
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
pipe 60 to an array of flat jet nozzles 62. The nozzles 62 allow the liquid
basic hydrogen
peroxide 58 to mix with the chlorine gas 52. The reaction produces excited
oxygen 64, including
singlet delta oxygen. The excited oxygen 64 is transported to a photon
generating chamber 66.
A source of iodine 68 is coupled to an inlet 70 of the photon generating
chamber 66. The iodine
68 results in the excited oxygen 64 decaying and releasing photons. The photon
generating
chamber 66 has mirrors that allow lasing 72 with an output perpendicular to
the flow of the
excited oxygen. Spent oxygen 74 exits the photon generating chamber 66. The
laser 50 may
include a system for reclaiming the basic hydrogen peroxide for reuse. The
COIL 50 uses the
array of nozzles 62 to increase the surface area of the hydrogen peroxide and
allow for a higher
starting molarity of basic hydrogen peroxide. As a result, the COIL 50 is more
efficient allowing
for either a smaller size and weight than previous systems or greater laser
firing capacity.
[01381 FIG. 4 is a top right perspective view of one embodiment of a flat jet
nozzle
according to an embodiment of the invention. Referring to FIG. 4, a nozzle 80
has a V-shaped
chamber 82 that attaches at a vertex 83 to a first end 84 of a pair of
opposing plates 86. A
second end 88 of the opposing plates 86 is attached to a conical nozzle 90.
The liquid, e.g., basic
hydrogen peroxide flows into the V-shaped liquid delivery channels or chambers
82 and is
forced through the passage 92 between the opposing plates 86 and out the
nozzle 90 and creates
a flat liquid jet 94. Depending on nozzle area, jet flow rate and velocity,
the jet thickness 96 can
be in a range from about 5 pm to about 100 pm and the width 98 can be in a
range from 1 cm to
about 5 cm.
[01391 In this embodiment, the width to thickness ratio is significantly
greater than a
factor of ten. For example, for jet velocities of about 10 m/s, the length of
the flat jet stream may
be about fifteen or more centimeters. The narrowest passage 100 is where the
conical nozzle 90
meets the opposing planar plates 86 is greater than about 600 m. This nozzle
80 allows for a
large surface area of liquid, e.g., basic hydrogen peroxide, which
significantly increases the
efficiency of the reaction between the basic hydrogen peroxide and the
chlorine. Further, due to
large jet surface area and small jet thickness this nozzle 80 produces a very
large specific surface
area ranging from about 10 cm- to about 20 cm- , which enables a smaller
generator volume and
higher yields of excited oxygen delivered to the laser cavity. In addition,
the nozzle 80 does not
require a small throat or passage that is likely to clog with contaminants,
e.g., salts that result
from the reaction of the chlorine and basic hydrogen peroxide, thereby
allowing the system to
have a much higher starting molarity for the basic hydrogen peroxide.
24
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[01401 FIG. 5 is a bottom left perspective view the flat jet nozzle of FIG. 4.
Referring to
FIG. 5, the flat jet nozzle 80 includes a number of conical nozzles that may
be attached to the
second end 88 of the opposing planar plates 86. Note that the only exit from
the second end 88
of the opposing planar plates 86 is through the conical nozzles. It is noted
that while the
description has focused on the application of a COIL, these embodiments are
also applicable to
any two phase reactor or contacting system. The use of this two phase reactor
system
significantly increases the interaction between the gas phase reactant and the
liquid phase
reactant. As a result, the reaction is significantly more efficient than
previous two phase reactor
designs allow.
101411 Thus there has been described a COIL that is lighter, smaller and more
efficient
than similar capacity previous COIL lasers. This allows the laser to be used
with smaller
transport systems or increases the capacity of present transport systems.
[01421 Effluent Contacting System and Method
[01431 As described above, system 0 provides an array of nozzles for producing
thin,
highly dense flat jets of high surface area. System 0 is described in relation
to usage with COIL.
In an alternative embodiment, many of the principles of the system 0 may be
applied to a system
and method of pollutant mitigation. In one embodiment the system and method of
pollutant
mitigation includes a gas liquid contactor. The gas liquid contactor includes
a plurality of nozzle
plates. In this embodiment, each nozzle plate includes a plurality of nozzles
as described in
FIGS. 6-17, the nozzles 1010 form an array of nozzles.
[01441 The system and method of pollutant mitigation will be described from a
bottom
up perspective, concentrating first on the unique and innovative nozzle 1010
used, then the
nozzle plate and the unique arrangement of the nozzles, then the configuration
of liquid gas
reactor, then the arrangement of the entire system and method of pollutant
mitigation, and
followed by the implementation of the system in respect to various pollutants.
The sub-
components of the system and method of pollutant mitigation has numerous
applications beyond
that related to the system and method of pollutant mitigation, as will be
clear from the following
description.
[01451 Nozzle
101461 As presented above, in relation to system 0, orifices are described as
delivering a
flat jet of hydrogen peroxide. The orifice has a V-shaped entrance 1 and a
conical exit 2 channel
for jet development. The intersection of entrance 1 and exit 2 channels
creates the orifice. Cross
section views of the nozzle plate show contours of the entrance 3 and exit 4
channels. An
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
approximate representation of the jet exiting the orifice is shown as 5. A
cross sectional close up
of the entrance 6 and exit 7 channels is provided. The jet length to jet width
ratio is about 10:1
with a thickness ranging from about 10 m to about 100 m.
[0147] In addition to increasing the surface area of the reactant or sorbent,
the flat jets do
not require the small throats required by nozzles in the related art. As
previously explained,
related art nozzles have a throat size ranging from about 150 m to about 350
m in their largest
dimension. By way of contrast, embodiments of invention are configured such
that flat jet
nozzles can have a throat that is about 250 m or larger in a smaller
dimension of the nozzle.
For example, flat jet nozzles can have a throat in a range from about 250 m
to 2000 m.
Therefore, the nozzles of embodiments of the invention are unlikely to clog
due to contaminants,
e.g., salts formed by the reaction of the liquid sorbents and gases, thereby
making systems of the
invention robust. Also, allowing systems to use a higher starting molarity of
reactants or even
fine sorbent slurries. Molarities as high as about 10 moles/L may be used,
while previous
systems are generally limited to a starting molarity of about 5 moles/L due to
the formation of
clogging salts and/or solid byproducts or precipitates. Most systems reuse the
liquid sorbents or
reactants, however once the molarity drops significantly the system's
performance may be
seriously degraded. In embodiments of the invention, the sorbent or reactant
liquid is easily
replenished through simple monitoring of concentrations and titration of
reactants appropriately
into the liquid systems.
[0148] The nozzle 1010, as shown in FIGS. 8-13 is similar to the above
described conical
nozzle 90. For example, the similarity being that the resulting nozzle cut
could be described as
an intersection of an approximate cone with the U-shaped channel, although it
is manufactured in
different way than using a cone shaped machine tool bit. Nozzle 1010 creates a
flat jet when
liquid flows through and may be configured to produce uniformly spaced flat
liquid jets shaped
to minimize disruption from a gas in a gas liquid contractor system. A flat
jet with plug flow
characteristics may also be created. The flat jet created initially has very
low turbulence
characteristics, enabling the flat jet to retain its characteristics for a
significant length.
[0149] FIG. 6 is a cross-sectional view of one embodiment of a precursor to a
nozzle
bank. FIG. 7 is a side view of the precursor to the nozzle bank shown in FIG.
6. Referring to
FIGS. 6-8, a precursor rod 1012 is used to form the nozzle 1010 and the nozzle
bank 1011. The
rod 1012 may have various dimensions and generally resembles half of a pipe
that has been
flattened. The precursor material may take the form of a number of different
geometric shapes,
such as, ellipsoidal, oval, and semicircular.
26
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[0150] The rod 1012 has a shell rod thickness 1015, straight rod height 1016
and a total
rod height 1017. In embodiments of the invention the shell rod thickness 1015
may range from
about 0.015 inches to about 0.055 inches, the straight rod height 1016 may
range from about
0.05 inches to about .75 inches and a total rod height 1017 may range from
about 0.25 inches to
about 0.95 inches. In a preferred embodiment, the rod has a shell rod
thickness 1015 of about
0.035 inches, a rod width 1014 with a maxiumum measurement of about 0.323
inches, a straight
rod height 1016 of about 0.10 inches, a total rod height 1017 of about 0.31
inches, and a rod
length 1018 of about 7.470 inches. The total width 1014 is about 0.323 inches
and the nozzle
edge 1019 starts at about 0.035 inches from the edge of the nozzle bank 1011
as shown in FIG. 9.
[0151] In this embodiment, the procedure for creating the nozzle bank 1011 is
as follows.
The nozzle bank 1011 is created using a progressive die. The first stage of
the die cuts create a
rectangular piece of metal of the proper size to be formed. The material used
for the die can be
single metal or alloy, e.g., stainless steel. In addition, the metal selection
may depend on the liquid
chemistry being used and its corrosivity or reactivity, therefore, other
metals can be chosen
including copper, nickel, chrome, aluminum, or alloys including these metals.
[0152] In the second stage, the specific geometry of rod 1012 in FIGS. 6 and 7
is created.
Preferably, the rod 1012 is de-burred to eliminate sharp edges or corners.
Next, a plurality of
nozzles 1010 is formed by machining the nozzles 1010 into the rod 1012. In a
preferred
embodiment, the nozzles 1010 are formed with a wire electrical discharge
machining (EDM)
machine. For example, the rod 1012 is mounted to a fixture and put into a
production EDM
machine and the nozzles are formed into the rod 1012, e.g., as shown in FIGS.
8-11.
[0153] As shown in FIG. 12, end caps 1023 are welded to the nozzle bank 1011
and the
nozzle bank 1011 is welded into a plate as shown in FIG. 13. The welding may
be conducted as
known in the art, e.g., laser welding. In FIGS. 8-13 the nozzle row 1011 is
shown with
completed nozzles 1010. The nozzles 1010 are cuts with about a 90 degree angle
as can be seen
in FIG. 11. The depth of the nozzle cut may be in a range from about 1 mm to
about 2.5 mm. In
a preferred embodiment, the nozzles 1010 are cut to a nozzle depth 1020 of
about 0.058 inches.
The depth of the channel may be in the range from about 2 mm to 20 mm.
[0154] The nozzles may be formed to have a uniform or non-uniform distance
between
the centers 1021. In embodiments, of the invention the distance between the
centers may range
from about 0.1 cm to about 5 cm. In a preferred embodiment, the distance
between nozzle
centers 1021 is about 0.158 inches. In addition, there may be any number of
nozzles in a nozzle
bank 1011. In a preferred embodiment, 45 nozzles are formed into a nozzle bank
1011. In
27
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
addition, an end space 1022 is formed at both ends of the nozzle bank 1011. In
a preferred
embodiment, the end space 1022 is formed to about 0.235 inches. FIG. 12 shows
where the
nozzle end caps 1023 are welded. FIG. 13 shows how nozzle bank 1011 is welded
into a plate
1024 along the channel seam 1025. The configuration of this embodiment is
advantageous as it
is able to provide large surface area as compared to volume of liquid and
furthermore provide a
large number of jets in a low volume container at normal atmospheric pressure.
In another
embodiment, the channels may also be machined directly into a plate rather
than welding as
described herein. In addition, the nozzles may be configured to have a narrow
approximately
oval slit could also be produced having a smallest dimension less than about
0.5 mm, but greater
than about 50 mm in length. Although this nozzle would have undesired high
liquid flow
volume as compared to the preferred embodiment, it does form a thin flat
liquid sheet of high
surface area.
[01551 Nozzle Plates
[01561 The arrangement of the nozzles 1010 on the nozzle banks 1011 or plate
1024
allow the liquid jets produced by the nozzles to be packed tightly in a small
volume. The
predictable nature of the fluid flow allows the jets to be closely packed
without interference and
causing turbulence. In a preferred embodiment, the fluid flow is configured
such that the
incoming liquid flows at about 90 to the direction of the liquid nozzle feed
channel. This has
been found to produce the best liquid jet properties with aqueous fluids;
fluid flow along or
parallel to the liquid feed channel can lead to deflection of the resulting
jet in the direction of
fluid flow, an undesirable effect. In contrast, the laminar flow of the jets
created by nozzle
plates 1020 produce closely packed jets without intersection of the streams in
adjacent rows.
Therefore, little turbulence is created and the distribution of the fluid
remains uniform.
[01571 FIG. 14 is a side view a nozzle plate according to another embodiment
of the
invention. FIG. 15 is a top view of the nozzle plate of FIG. 14. FIG. 16 is a
perspective view of
the nozzle plate of FIG. 14. FIG. 17 is a detail blown up view of the nozzle
plate of FIG. 14
along cut line A shown in FIG. 15.
[01581 Referring now to FIGS. 14-17, a nozzle plate is generally depicted as
reference
number 1020. In this embodiment, each individual nozzle 1010 is cut into a
formed channel
1015 creating a row of nozzles in the channel 1015. Several channels are
created in a plate 1020
to form the orifice plate or nozzle jet array. The channels 1015 may be nozzle
banks 1011 that
are welded into a plate as described above. Alternatively, the channels and
nozzles may be
formed from a single plate by machining, a typical result as shown in FIGS. 21-
24.
28
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
101591 In this embodiment, the nozzles 1010 are precisely spaced to maximize
the
volume filled with the produced jets, such that the jets fill the intended
volume but do not
intersect with each other. Spacing of the jets too closely may lead to jet
collision and breakup
into small droplets in contrast to cohesive flat jets, an undesirable result
which decreases the
effectiveness of this embodiment. Also, spacing of the jets too far apart
which may lead to a
lower specific area able to react with gas phase molecules, also reducing the
effectiveness of the
embodiment. Optimal spacing is mainly a combined function of nozzle design and
dimension,
reaction effectiveness (or mass transfer), fluid viscosity, and fluid surface
energy.
101601 FIG. 18 is a perspective view of an array of flat thin flat liquid jets
as produced by
the nozzle plate of FIG. 14. FIG. 19 is a front view of the array of flat thin
flat liquid jets
produced by the nozzle plate of FIG. 14. FIG. 20 is a side view of the array
of flat thin flat liquid
jets produced by the nozzle plate of FIG. 14.
101611 Referring, to FIGS. 18-20, illustrate the array or matrix of flat jets
formed when
the liquid is forced through the nozzles. In this embodiment, each nozzle 1010
is configured to
create a flat, stable jet 1050. In a preferred embodiment, the jet is
configured to be about 2 cm
wide, about 25 cm long, and about 0.1 mm thick. Of course, other dimensions
may be utilized.
Each row of nozzles 1055 produces a row of jets 1060 and the rows are ordered
to produce the
matrix or array of flat liquid jets 1065. This plate is configured to produce
24 rows 1055 of jets.
Of course, the number of rows may be increased or decreased. The preferred
number of rows of
jets may be prescribed by the size of the gas liquid contactor application and
the practical aspects
of manufacturing nozzle arrays or jet plates and ancillary fluid handling
hardware. However,
there is no fundamental size limit on the upper side. For a very small
reactor, e.g., sized for
research applications, the practical number of rows is three to provide two
liquid channels (and
half a channel on each of the edges due to one half being the reactor wall).
In operation the gas
is configured to flow between the flat jets, parallel to the flat side of the
jets, thereby creating a
very large surface area for intimate contact.
101621 FIG. 21 is a fluid side exit view of a nozzle plate according to
another
embodiment of the invention. FIG. 22 is the fluid entrance side view of the
nozzle plate of FIG.
21.
101631 Referring to FIGS. 21-22, a nozzle plate is generally depicted as
reference number
1101. The nozzle plate 1101 includes nozzles 1010 in an offset or staggered
configuration. In
one embodiment, as shown in FIG. 18, the gas may be configured to flow
parallel to the flat
surface created by the jets. The staggered or offset configuration of the
nozzles 1010 may allow
29
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
for slightly increased flow as compared to a non-staggered configuration
because the staggered
configuration blocks gas cross flow channels than may cause turbulence.
[01641 FIG. 23 is a fluid side exit view of a nozzle plate in another
embodiment of the
invention. FIG. 24 is the fluid entrance side view of the nozzle plate of FIG.
23.
[01651 Referring to FIGS. 23-24, a nozzle plate is generally depicted as
reference number
1110. As shown the fluid exits the face shown in FIG. 23 and the gas may be
configured to flow
parallel to the flat surfaces of the jets. FIG. 24 shows the reverse side of
nozzle plate 1110. The
nozzle plate includes a plurality of nozzles 1010, are set in nozzle arrays
1112 (see nozzle banks
1011) described above. In an alternative embodiment, the nozzle arrays 1112
may be configured
to be removable. The ability to remove the nozzle array provides an ability to
service the array
in the case of nozzle erosion or a requirement to change nozzle dimension
(e.g., in the case of
using a different fluid with different viscosity).
[01661 FIG. 25 is a fluid side exit view of one embodiment of a nozzle plate
with a
nozzle bank removed. FIG. 26 is the fluid entrance side view of the nozzle
plate of FIG. 25.
Referring to FIGS. 25-26, it is shown that nozzle rows 1113 are removable from
nozzle array
assembly 1120 the nozzle bank 1113 is removed. The ability to remove the
nozzle banks 1113
may aid in the ability of a user to clean the nozzle plate 1120 or replace
broken nozzle banks
1113, without having to replace a whole plate. In addition, removable nozzle
banks 1113 may
aid in the manufacturing processes as contactor specific area can be adjusted;
e.g., gas phase
molecules with very high mass transfer may not require as much specific area
to meet capture or
reaction yield. As such, nozzle banks can potentially be removed to reduce
total liquid flow rates
in an existing system.
[01671 For example, in one embodiment, the nozzle banks or rows 1113 shown in
FIGS.
25 and 26 are cut from a flattened tube 1130 (shown in FIGS. 27 and 28). The
tube 1130 is cut
lengthwise from an appropriate tube and flattened slightly or, alternatively,
is formed from a flat
sheet over a mandrel. A plurality of nozzles 1010 are cut into the tube 1130.
This is an
alternative method of nozzle bank formation. Tube 1130 is shown flattened and
with wire EDM
grooves formed for orifice fabrication. After tube 1130 is cut lengthwise and
the un-flattened
ends are removed, the groove piece 1113 is substantially complete and ready
for fitting into a
nozzle plate 1120.
[01681 Gas Liquid Contactor
[01691 FIG. 29 is a cut away view of a schematic of a gas liquid contactor
according to
another embodiment of the invention. The gas liquid contactor increases the
efficiency and
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
reduces the entrainment of the gas liquid contactor for the COIL (as described
herein). Nozzles
in the gas liquid contactor are configured to create stable, planar jets of
liquid that hold their
shape in the gas stream.
[01701 These nozzles can be manufactured into a nozzle plate (as described
herein) in an
array that creates a close packed, parallel matrix of the planar liquid jets.
The flat jet arrays are
aerodynamically shaped and provide stable jet formation at relatively high gas
flow. That is, an
array of nozzles is configured to produce uniformly spaced flat liquid jets
shaped to minimize
disruption from a gas. Moreover, the array of nozzles produces liquid sheets
that are parallel to
the gas flow, providing very high contact area and low gas side pressure drop.
The gas flow can
be across the liquid jets (cross flow), counter current, or co-current.
[01711 The liquid pressure drop required to create the jets with the nozzles
is also low,
resulting in low pumping cost on both the liquid and gas sides. The liquid
pressure drop across
the main restrictive orifice, e.g., nozzle array. For example, the liquid
pressure range in which
this embodiment functions is between 2 psi and 50 psi, with the best range
being between 3 psi
and 15 psi. Also, liquid pressures lower than 2 psi can still provide for thin
flat jets (depending
on nozzle dimensions), however, the liquid velocity may become low leading to
significant
deflection by high velocity gas flows. Likewise, pressures above 50 psi can
produce excellent
thin flat jets, but the energy required to provide for this hydraulic pressure
becomes high, which
adds to parasitic energy losses of the system.
[01721 In addition to these advantages, since the nozzles are not atomizing
the liquid,
liquid entrainment in the gas is greatly reduced as compared to systems that
atomize the liquid.
The gas liquid contactor has a very high specific area, e.g., 20 cm 1, which
results in high contact
efficiency and a small footprint, e.g., less than the equivalent of 100 ft2/MW
for the contactor
and supporting pumps. Also, the specific area and other parameters of the gas
liquid contactor
are shown in Table 2.
[01731 Referring to FIG. 29, the gas liquid contactor is generally depicted as
reference
number 1600. In this embodiment, a cross flow configuration is utilized, the
gas flows from left
to right through the contactor 1600. Liquid enters the top 1610 of the
contactor 1600 through
inlet plenum 1630 and is forced through the nozzle plates 1640 at the top of
the contact chamber
1650. Flat liquid jets are formed by the nozzles and flow down through the
chamber. The gas
flows from left to right in the system depicted in FIG. 29 between the
parallel jets, where the
mass transfer takes place, then through the low pressure drop mist eliminator
1660, and on to the
exit 1670. The liquid is collected through an anti splash grid 1680 at the
bottom of the contactor,
31
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
treated as necessary, and possibly recycled. The anti splash grid submodule
1680 is a grid with
apertures shaped to receive the flat jets. The anti splash guard or gas fluid
separator is also
configured to substantially minimize back-splash of liquid in operation. The
apertures of the anti
splash grid 1680 may be angled slightly towards the exits 1700 and/or 1690 of
the liquid capture
outlet plenum 1620 to aid in the exit of the fluid without the application of
pressure to the fluid.
[0174] The following Tables 2 and 3 compare the contact efficiency and
advantages /
disadvantages of several gas liquid contactors, including the current
invention.
TABLE 2: OPERATING CHARACTERISTICS OF COMMON GAS LIQUID
CONTACTORS
Contactor Type Specific Surface Area, a Liquid Side Mass Volumetric Mass
(contact area/contactor Transfer Coefficient, Transfer
volume), in cm 1 kL, in cm/s Coefficient, kL*a, in
S-1
Packed Column 0.1 -3.5 0.004-0.02 0.004-0.07
(counter current)
Bubble Column 1-20 0.003-0.04 0.003-0.08
(agitated)
Membrane 15-70 0.02-0.06 0.3-4
Spray column 0.1 - 5 0.007-0.15 0.0007 - 0.075
Venturi Ejector 0.05-0.10 0.08-2.5
Current Invention 1 - 50 0.02 - 50 0.2 - 2500
(flat jet)
TABLE 3: COMPARISONS OF COMMON GAS LIQUID CONTACTORS
Contactor Type Advantages Disadvantages
Packed Column = Long contact time = Liquid flooding
= Large contact area = Liquid entrainment
= Good mixing = High gas pressure drop
= Counter current for multi = Clogging in packed bed
stage effect
Bubble Column = Simple construction = Gas bubbles coalesce
(agitated) = Low operating cost = Back mixing of the liquid phase
= Power consumption for agitation
= Single stage
Membrane = High mass transfer = Expensive construction
coefficient = High gas pressure drop
= No flooding = Membrane resistance
= Reduced liquid entrainment = Membranes are fragile
32
CA 02737637 2011-03-18
WO 2010/036436 PCT/1JS2009/049707
. .....
C rn be cu,,,-r n for ?-a,,is rfcox Ãai i
mitki stage NO,
- ---- -------- ----
l:.t??` t . # 3ai i o-\:##'i'~ # E..3Seit f. S.? Ã z C~:~S
= 1:i;rk o'pe"ztii 3y; Cost f = 1b) liquid punipi ?:N asst due s
= Ã.; a.ta be ?iiEFt t .;'iii Is?; high rz . S '4 dror. all d ià z3
m ii su.4 ? efl t S 2t i T?"i#iip n--I > i 3 '
?9 is L md iai# alaiS e-w
9 `Fit >ta <t. i? " at.E~ i'a 6gh' = Wry high liquid "it ? >? =ti
i>i wd slat 'a e a-roa = ho n :owaii ' Ãiaaa
....................... ............::.. ,.....................
Z. ,:Ã sltF: on * .: iE 1 i a ? in #.lir' I:3
iid
liquid pe`.isu drop `1R `ros:` lo"< is g
Low gw, &q)
l v xis ti;#` = $.C'war,.uiiieat , n #g..i# ti?u
be ,
~~. 4?taatZ~Ã i; li.i'r F', #at
1 75 1 i Q h 3ai at i..:.a<lf:3a Zi i1 o i ai <'#. ' used Or lea i tai taal
'., i . ai<lf in ;a COIL
laser, but aid ..t E? >ic adons are à of ,o HOW The a > t. be in a it,til Or
of
dif i"ront ai~. x#+?ia z Si> any a t t''Zi `. h`, frb ti,]LZ', ~#i ;IN. s
:. ~i F.c ~ .:wa,... , ~f'~: co s.< ?i'a:.d.
t:it,iti's?nt<a ; set emi is and z.i '.}:id i t sbe . Some .xampàs Wh hem
l?ia,IF} w2: `ii
is arld 1zluid am MAY < ~ : z 3 -?s tt i i~ , as cooling a ;s ~a ,i~>< tr Ãiz
t . 'raeh as
?E3 R?.fl<ii#:i4 Huai a Prat: as w am. eherniia. I~ t :.tc?#a b >vien a
b<Jpbd and ;a r s, i h as dii~
Ã. 3.#. app iQabo>a_ and b#t33t 43'ic.?suc.i3 as avuebi a\`e t n Mina Ma,
can e u can b can aaplaha b eunne :d p e?#`:lacttu s 4 series and. p aip$?i
0e tit ?.Eai from
the and. of to . ?lxiisti: ,'..ta t t;i >.iL lS.?# to the ,: of of the "i.nt
upstream en o t t `k` .
3 aGti. i . ~ .li?a"?.ba>
tan di :3d:ren h.ii i#Fd ,<i' rta,~ or ;Z;.i.l.tf>> can be a~lnii ed ii2sEC:-
pm,r, fly to Z 3;[
a. c ,1i tai #il
Z.ont,,i~ aor ; inst.ahe. Waft to ?.n for two s . ` no tnun gas
flow. .,z ibodi> ms oft the gas ia,a.i?i c Frame' ai p#adi c' : ;a ` as bq,A
er > iph
4t?.>:IEI>CÃ>i' 2a'>iÃs eI cuef iiin t. ban ? r ?i are d 3 b' ..ai?iit?
:ivy. i$'L drop,
'eiv ad
si"..a. f< size, < Al<"fn a to t c gg #me, kiar hgrihd tÃ< T tii i#1 at at id
up..ibap Z, s:t.
i }., e :f~rilpi:: shed lad a an t..i i L a aÃe ; E`t?.. h plat ;1.: S i.
`t:t herein) Midi
..}ilia an .:fÃ"ta` of
special nozzles t h at create a matrix ?t fla: `table .jets at i.iu.i~.E
that are ekne
pacd i to each tbe_rand p<ai.a. k to he s Ma tI)n i,ia o.i#itae ai tar a smak
stage gas
33
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
liquid contactor is described below. The gas flow rate and the number of jet
rows determine the
contact time for this single stage.
[01761 In this embodiment, the jet plate is housed in the gas liquid contactor
1600. Gas
enters from the left 1672, proceeds through the flat liquid jets of contact
chamber 1650, through
the mist eliminator 1660, and out the gas exit 1670. The liquid enters the
inlet plenum 1630, is
forced down through the series of nozzle plates 1640 to form the flat liquid
jets, then falls
through the gas liquid separator 1680 and into the liquid collection chamber
1620 at the bottom
of the contactor. The liquid then proceeds to exits 1690 and 1700 to be
processed and/or
recycled.
[01771 FIG. 30 depicts a schematic arrangement of a plurality of gas liquid
contactors
according to another embodiment of the invention. Referring to FIG. 30, a
multistage cross-flow
device contactor is generally depicted as reference number 3000 and is
connected in series. The
multistage cross-flow device contactor 3000 includes a first gas liquid
contactor 3002, a second
gas liquid contactor 3004, and third gas liquid contactor 3006. Of course,
there may be more
than three gas liquid contactors, e.g., the number of gas liquid contactors
can be determined for
the application. That is, the number of contactors used is a function of the
ultimate capture or
reaction yield required by the specific chemistry. Sequential contactors may
be roughly
compared to a sequential chemical extraction well known in the art. Gas flows
through each
contactor in turn and the liquid flows cross flow from the downstream end 3008
of the train to
the upstream end 3010.
[01781 Liquid pumps between each stage (not shown) provide the liquid to each
of the
contactors. Optionally, a single liquid delivery plenum could service all
serially installed gas
liquid contactor modules, requiring only a single liquid pump to deliver that
liquid to a single
serial liquid delivery plenum or in parallel from a single pump plenum.
[01791 The gas liquid contactors may be made from a variety of different
materials. For
example, the contactor may be made from stainless steel. The material may also
be chosen based
on the liquid and/or gas chemistry and its corrosivity or reactivity, e.g.,
copper, nickel, chrome,
aluminum, and alloys thereof. In addition, coated components or piping
materials may also be
used, e.g., glass lined, epoxy or powder coated, etc. Alternatively, some of
the structural and/or
fluid handling parts of the contactor can be fabricated from plastics or
polymers, fiber reinforced
epoxies or polymers, structural polymers, polyimides, and composites and
combinations thereof.
[01801 Aqueous Ammonia Pollutant Removal
34
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[0181] Embodiments of the invention as described herein can be used for
pollutant
removal in effluent through the usage of ammonia. A significant cost driver
for pollutant
removal is the low partial pressure of the pollutants in the flue gas and slow
gas absorption rates.
For example, reaction rates are generally a function of reactant initial
concentrations; higher
concentration corresponds to faster reactions. However, with low initial
concentrations, mass
transfer becomes the limiting variable in reacting or removing gas or liquid
molecules. In
embodiments of this invention, low mass transfer coefficients are offset by a
high relative
specific area and high flow rates.
[0182] In the related art, flue gas desulphurization (FGD) systems were
developed and
installed in power generating plants to address the contribution of SO2 and
SO3 to acid rain and
air pollution. Most FGD systems contact the flue gas with wet limestone to
absorb the SO2 as
CaSO3, which is then oxidized to CaSO4 (gypsum), precipitated, and either sold
or placed in a
land fill. A shortfall of lime or limestone based FGD is that it cannot
address various pollutants,
e.g., NOR, Hg, or CO2. Another disadvantage is the large footprint and capital
investment
required for a FGD system, e.g., spray tower and oxidizing tank.
[0183] The preferred sorbent for gas absorption and removal are those systems
that
demonstrate high liquid jet performance, high gas loading capacity, high
oxidative stability, low
heat of reaction, low sorbent cost, low corrosivity and a salable product
stream. An exemplary
sorbent is aqueous ammonia. Ammonia, ammonia salts, and urea are injected into
the boiler or
flue gas to reduce NOR via selective catalytic reduction (SCR) or selective
non-catalytic
reduction (SNCR). Ammonia and its salts may control SOX and multiple
pollutants.
[0184] Also, in the related art multi-pollutant control using an aqueous
absorbent requires
that NO, the major component of NOR in flue gas, be either reduced to N2 via
selective catalytic
reduction (SCR) or selective non-catalytic reduction (SNCR), or be oxidized to
NO2 because NO
has a very low solubility in water. If oxidized to NO2, then the NOR can be
absorbed with any
basic solution or nitric acid. When ammonia based systems are used, valuable
by-products are
produced. Ammonium nitrate and ammonium sulfate can be used for fertilizer.
Ammonia is
even more efficient at capturing CO2 than monoethanol amine (MEA) or
diethanolamine (DEA)
and the CO2 can be used for enhanced oil field recovery.
[0185] Embodiments of the invention can capture several pollutants of
interest, such as
but not limited to, acidic gases, ammonia, VOCs, SOX, NOR, C02, Hg, and
combinations of the
same. Moreover, some embodiments of the invention are configured to have a
single, small
footprint, system and the production of valuable by-products. In addition,
embodiments do not
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
contact with slurry and thus avoid associated material handling difficulties.
Not using a slurry
avoids the heat required to complete a phase change in the ammonia regenerator
(or CO2
stripper).
[01861 In embodiments of the invention, flue gas is cleaned of fly ash in the
bag house or
electrostatic precipitator (ESP), then cooled as necessary for the first wet
contact. The SO2 and
NO in the flue gas may then be oxidized with gaseous hydrogen peroxide, or
oxidized in the first
scrubber with aqueous hydrogen peroxide. The scrubbers are highly efficient,
small footprint,
horizontal cross flow gas liquid contactors as described herein. The scrubbers
scrub the flue gas
with basic aqueous ammonium sulfate to remove the acid gases, e.g., SO2, SO3,
NO2, HCI, HF.
Make-up ammonia is added to control the pH and provide hydroxide ions to react
with the
hydrogen ions produced by the hydrolyzed gases. This converts the gases to
soluble ammonium
salts and reduces their vapor pressure to near zero. Better than about 99%
absorption of the SOx
can be achieved. Mercury may also be removed through the oxidation and/or
absorption
processes, e.g., HgOx is much more soluble than elemental Hg. Some reaction
mechanisms for
pollutant removal in embodiments of the invention include:
NH3 Hydrolization:
NH3 + H2O H NH4+ + OH" (1)
SO2 Capture:
H2O + SO2 -~ H+ + HS03" (2)
'/2 02+ HS03" -) HS04" (3)
2NH3 + HSO4" + H2O -) (NH4)2SO4 + OH" (ammonium sulfate) (4)
NO, Capture:
NH3 + H2O H NH4+ + OH" (5)
H202 + OW -~ H02" + H2O (6)
H02" + NO -~ NO2 + OH" (7)
2NO2 + H202 -~ 2HN03 (8)
NH3 + HNO3 -~ NH4NO3 (ammonium nitrate) (9)
Hg Capture:
H202 + Hg -) Hg(II) + products (10)
H2S Capture:
H2S (aq) -)HS" + H+ (11)
HS" + NH3 + H+ -)NH4HS (12)
36
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[01871 After the sulfur and nitrogen oxides are captured as salts (NH4)NO3 and
(NH4)2SO4, the contacting solution may be concentrated and precipitated for
sale or disposal.
Heavy metals (Hg) and halides (Cl and F) may be precipitated separately in a
pH adjustment
step. The lean contacting solution is recycled to the scrubber.
[01881 The flue gas after those processes is now better than about 95% clean
of all
pollutants and is ready for partial CO2 removal. The second scrubber uses an
aqueous ammonia
and/or ammonia salt combination for the liquid. The CO2 is absorbed and reacts
with
ammonium carbonate and water to form ammonium bicarbonate. Low temperature and
high pH
favor the absorption of CO2. Make-up ammonia controls the pH and level of free
ammonia in
the scrubbing solution. Higher concentrations of ammonia raise the pH and
increase the
absorption of CO2 and the CO2 loading, but also increase the vapor pressure of
the ammonia.
Some simplified reaction mechanisms for CO2 capture according to embodiments
of invention
include:
2NH3 + H2O + CO2 H (NH4)2-CO3 (ammonium carbonate) (13)
(NH4)2 CO3 + CO2 + H2O H 2NH4HCO3 (ammonium bicarbonate) (14)
[01891 The rich liquid from the contactor is sent to a CO2 stripper where the
temperature
is raised to reverse the reaction and release gaseous CO2 and produce ammonium
carbonate.
High temperature and low pH favor the evolution of CO2. The low pH favors the
absorption of
ammonia, so a low pH helps evolve the C02, but keeps the ammonia in solution.
The CO2 is
separated and compressed and the ammonium carbonate is returned to the
scrubber.
[01901 A common problem with related art ammonia based systems is ammonia
slip,
where ammonia dissolved in the absorbing liquid returns to the gas phase and
is carried up the
stack in the flue gas. This can cause a visible plume if the ammonia reacts
with a constituent in
the flue gas to precipitate a solid. In addition, ammonia slip greatly
increases reagent cost.
[01911 In an embodiment, a plurality of gas liquid contactors as shown in FIG.
30 is used
for pollutant removal. In this embodiment, each gas liquid may be configured
for different
purposes. For example, the gas liquid contactor 3006 may be specifically
designed to capture
any ammonia that might slip through the first two contactors 3002, 3004,
respectively. In this
embodiment, the optimum pH for absorbing acid gases (SO, NON, and C02) is high
above 7,
because the vapor pressure of those gasses is lowest at high pH, but the vapor
pressure of
ammonia is highest at high pH. Under optimum conditions the first gas liquid
contactor 3002
can capture SO2 at better than about 99%. While the third contactor 3006
allows the first gas
liquid contactor 3002 and second gas liquid contactor 3004 to operate under
optimum conditions
37
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
for absorbing the acid gases, with high ammonia slip, as the third contactor
3006 is run under
optimum conditions to capture the ammonia. This captured ammonia is returned
to the first two
gas liquid contactors. The high efficiency and small size of the gas liquid
contactors means a
third gas liquid contactor can be affordable and allow very high capture
efficiencies.
[0192] A number of efficiencies are created by this embodiment, including:
greater
efficiencies for removal of multiple contaminants from flue gas by reducing
the energy
consumption and cost of the removal system; greater efficiencies for removal
of multiple
contaminants from flue gas by minimizing the size of the removal system;
greater efficiencies for
removal of multiple contaminants from flue gas by creating modular systems
that can be factory
produced and combined in parallel in a way that provides the necessary level
of flue gas
processing capability; greater efficiencies for removal of multiple
contaminants from flue gas by
creating modular systems that can be combined in parallel to adapt to a
variety of facility sizes;
greater efficiencies for removal of multiple contaminants from flue gas by
creating modular
systems with very low flow resistance (pressure drop) that can be combined
serially for selective
and sequential removal of contaminants; greater efficiencies for removal of
multiple
contaminants from flue gas by creating modular systems that are combined to
provide
redundancy (high availability) and maintainability (selective access for
periodic maintenance or
in event of unit failure); greater efficiencies for removal of multiple
contaminants from flue gas
by creating modular systems that can be mass produced in an assembly line
process; and greater
efficiencies for removal of multiple contaminants from effluent gases from a
variety of types and
sizes of power generating and chemical processing facilities.
[0193] Moreover, the embodiment can be described as a method and system for
achieving selective and high mass transfer rates of flue gas contaminants from
high volumetric
flue gas flow rates into continuously replenished liquids confined in small
system volumes. In
the method and system, large dense packed arrays of high velocity, very broad,
thin, long and
stable jets, interact with a high velocity flue gas flow. Jet formation
orifices are optimized based
on liquid sorbent characteristics such as viscosity and surface tension. Cross-
flow and counter-
flow designs represent two different embodiments.
[0194] The efficiencies of the method and system are achieved from the large
volumetric
mass transfer and resultant small size, low pressure sorbent operation
requiring minimal
pumping capability, and low pressure flue gas pressure drop across the system
due to the low
resistance of the aerodynamically shaped jets and the modular and combinable
nature of the
design. See also Tables 3-4. This significantly increases the efficiency of
processes for flue gas
38
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
contaminant removal and makes economically feasible the removal of
contaminants such as
CO2, SON, NON, and Hg.
[01951 In another embodiment, a small scale version is readily adapted to the
exhaust of
large commercial vehicles for contaminant removal. In still another embodiment
the volatile
organic compounds from a chemical plant can be removed from the exhaust. In
yet another
embodiment very dry air streams can be achieved by use of cryogenic liquid
flows. In another
embodiment a gas may be humidified or dehumidified and particulate matter
removed.
[01961 FIG. 31 is a schematic for one embodiment of a multi-contaminant
removal
system. Referring to FIG. 31, a multi-contaminant removal system is generally
illustrated by
reference number 2100. The system 2100 may be configured to capture SON, NON,
C02, Hg,
HC1, and HE In this embodiment, flue gas 2120 from boiler 2110 is first
cleaned of particles,
e.g., fly ash at particular removal point 2130 (for example a settling chamber
or a net filter) and
cooled at cooling station 2140 as necessary. At this point 2150 the flue gas
contains mostly N2,
H2O, C02, S02, NO, Hg, HC1, and HE This of course depends on the processing of
the boiler
2110. The flue gas is then contacted in a high efficiency gas liquid contactor
2160 as described
in herein with aqueous ammonia and dissolved ammonium salts. The dissolved
ammonium salts
are from the recycle stream 2170 and from the supernatant 2210 from the
precipitation step 2190
and includes ammonium sulfite (SO3), sulfate (S04), nitrate (NO3), chloride
(Cl), fluoride (F),
and in some cases a small amount of carbonate (C03) and bicarbonate (HC03).
The ammonium
carbonate and bicarbonate may be kept to a minimum by using an approximately
stoichiometric
amount of make-up ammonia.
[01971 In step 2165, oxidation takes place in the liquid phase in the gas
liquid contactor
as described in embodiments of the invention. Of course, several oxidizers may
be used to
convert the NO to N02 for better absorption. The S03- is also oxidized to SO42-
in the liquid
phase. A bleed stream 2220 of the liquid is sent to the precipitators to
remove the heavy metals
and ammonia salts. In the first step 2230 a pH adjustment will precipitate
2210 the heavy metals
such as Hg. In the second step 2190 the liquid is concentrated and ammonia
salts are
precipitated. The heavy metal solids from the precipitation step may be
disposed properly 2240
and the ammonium salt solids sold as fertilizer 2250. If the ammonia salts can
be sold as
fertilizer (with the Hg removed) in concentrated liquid form, the second
precipitation may be
eliminated.
[01981 Next, the flue gas 2120, containing only N2, H2O, and C02, is contacted
in
another high efficiency gas liquid contactor 2260 as described herein with
ammonia and
39
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
dissolved ammonium carbonate/bicarbonate. Again, the ammonia is a make-up
stream and the
dissolved salts come from the recycle stream 2270. Ammonia is added to target
the pH of the
contacting liquid to optimum. The CO2 is absorbed as ammonium bicarbonate in
the liquid
which is sent to the CO2 stripper 2280. Here, the temperature is raised (and
pH adjusted if
necessary) to drive the reaction in reverse and release the CO2 as a gas 2290,
leaving ammonium
carbonate in the liquid phase 2300 to be recycled to the CO2 absorber. The CO2
may then be
compressed 2310 and sold or sequestered 2320. Sequestration involves, e.g.,
injection into
depleted natural gas wells, secondary oil recovery, and other methods which
will not be
described here in detail because they are out of scope of this invention.
[01991 After the CO2 absorption step, the flue gas is contacted with water in
a third high
efficiency contactor 2330 as described herein to strip out any ammonia that
may slip from the
previous contactors. The pH of the contact liquid (water) is adjusted as
necessary to ensure
complete absorption of the ammonia. The bleed stream 2340 may be sent to the
CO2 stripper
2300 or the SO, absorber.
[02001 Finally the clean flue gas 2350, consisting of only nitrogen, water,
some oxygen,
and any unabsorbed CO2 is heated 2360 to reduce condensation and sent to the
ID fan 2370 and
the stack. The flue gas heater 2360 and cooler 2140 are interconnected with a
liquid heat carrier
to economize the process. Cool liquid is contacted with the hot flue gas in a
gas liquid heat
exchanger 2140. The cool flue gas proceeds to the first absorber 2160. The now
hot liquid is
sent downstream to the flue gas heater 2360 where it contacts cool flue gas
2350 from the last
absorber 2330. This gas liquid heat exchanger 2360 cools the liquid to be sent
back to the cooler
2140 and heats the flue gas 2350 in preparation for exhaust into the
atmosphere. The hot liquid
may also be used as heat input in the CO2 stripper 2300.
[02011 Optionally, waste heat from the industrial process can be used as a
heat source for
stripping CO2 or re-heating the waste gas to avoid moisture condensation. For
example, in a
power plant this could come from the fly ash bag house.
[02021 Optionally, the process can been modified to eliminate the CO2 capture
if desired.
That is, the system focus is to capture SO,, NON, Hg, HCI, and HF and produce
ammonium
sulfate and nitrate as fertilizer.
[02031 FIG. 32 is a schematic of a multi-contaminant removal system according
to
another embodiment of the invention. Referring to FIG. 32, the process can be
simplified to
capture only SON, HCI, and HE Process 2400 is designed to capture only those
acid gasses most
easily absorbed. The flue gas 2120 from boiler 2110 is first cleaned of fly
ash at particular
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
removal point 2130 (for example a settling chamber or a net filter) and cooled
at cooling station
2140 as necessary. At this point 2150 the flue gas contains mostly N2, H2O,
C02, SO2, NO, Hg,
HCI, and HE
[0204] The flue gas 2150 is then contacted in a high efficiency gas liquid
contactor 2410
as described herein with sodium hydroxide and sulfate/sulfite salts from the
recycle stream 2420.
The oxidation step 2430 takes place in the liquid phase in the gas liquid
contactor. The sulfite
(S032-) is oxidized to sulfate (S042-) in the liquid phase using oxygen from
air or from the flue
gas. A bleed stream 2440 of the liquid is sent to the precipitators 2450 to
remove the heavy
metals and sulfate salts. In the first step a pH adjustment 2460 will
precipitate the heavy metals
such as Hg. In the second step calcium hydroxide 2470 is added to precipitate
calcium sulfate,
which may be separated, dried, and removed at the precipitator 2480. The
supernatant 2490
from this precipitator is returned to the recycle stream. The heavy metal
solids from the
precipitation step may be disposed properly 2510 and the calcium sulfate sold
as gypsum 2520.
[0205] Finally the clean flue gas 2530, consisting of only nitrogen, water,
NON, and CO2
is heated to reduce condensation at the heater 2360 and sent to the ID fan and
the stack 2370.
The flue gas heater 2360 and cooler 2140 are interconnected with a liquid heat
carrier to
economize the process as described above.
[0206] Elimination of SO2
[0207] Various performance areas for enhancing the SO2 capture capability
include
reducing reactor vessel size, pressure drop and using efficient mass transfer
sorbent systems with
salable byproducts. Achieving these target performances requires innovative
design approaches
that couple high SO2 absorption kinetics and value-added product streams.
[0208] Gas liquid mass transfer operations take place across the gas liquid
interface. The
absorption rate of a gas into a liquid sorbent is controlled by the liquid
phase mass transfer
coefficient, kL, the specific surface area (gas liquid interfacial surface
area to volume ratio), a,
and the concentration gradient between the bulk fluid, CL and the gas liquid
interface, CL*. In
many gas liquid reaction systems the solubility of the CL* is low and control
of the concentration
gradient is limited. To enhance the gas absorption rate, gas liquid contactor
embodiments
increase mass transfer kinetics, gas liquid mixing and/or interfacial surface
area to volume ratio.
[0209] In embodiments of the invention, to efficiently capture SO2, the
contactor can be
used with a wide variety of aqueous-based sorbents including but not limited
to limestone/lime
(CaCO3), sodium carbonate (Na2C03)/sodium hydroxide (NaOH), ammonium hydroxide
(commonly called aqueous ammonia and abbreviated AA), double alkali (sodium
hydroxide,
41
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
NaOH, plus lime), magnesium oxide (MgO) and zinc oxide (ZnO). The addition of
oxidizing
agents (OX) enhances the oxidation of SO2, which facilitates the formation of
sulfate, S042 In a
preferred embodiment, the OX agent is hydrogen peroxide (H202). Combining
aqueous
ammonia and hydrogen peroxide is especially beneficial since a salable,
revenue generating
byproduct stream such as ammonium sulfate (a fertilizer) can be produced. In
addition, H202
decomposition products (water and oxygen) are environmentally and equipment
friendly.
102101 It is thought that the likely chemical steps to SO2 oxidation in the
presence of
aqueous ammonium hydroxide and hydrogen peroxide are as follows:
NH3 + H2O + SO2 - NH4+ + HS03" (1)
NH4+ + HS03" + NH3 - 2NH42+ +S03 2- (2)
H202 + S032" - H2O + S042- (3)
2NH4+ + S042- - NH4SO4 (ammonium sulfate) (4)
102111 In embodiments of the invention, the gas effluent cleaning process
allows for the
removal of sulfur dioxide with high efficiency. The system in this embodiment
includes an array
of nozzles with an orifice plate (or nozzle plate as described herein)
reshaping and fluid
composition engineering for adapting to a wide range of fluids and operating
conditions. The
removal of SO2 is performed by passing the gas through a high surface to
volume gas liquid
contactor unit as described above. The gas effluent is passed horizontally
(referred to as cross-
flow) through the gas liquid contactor having substantially reduced contactor
volume and gas
flow pressure drop as compared to the related art. Intersecting the cross flow
gas flow are a
plurality of low pressure, vertically oriented flat jet arrays composed of an
aqueous based sorbent
and of substantial surface area. The flat jet arrays are aerodynamically
shaped so as to provide
stable jet flow with low liquid particle entrainment at relatively high gas
velocity.
102121 In a preferred embodiment, the sorbent for sulfur dioxide absorption
and removal
are those systems that demonstrate high SO2 capacity, high oxidative
stability, low heat of
reaction, low sorbent cost, low corrosivity and a salable product stream. An
exemplary sorbent
for effective SO2 removal is 28 wt% ammonia in water. In order to optimize the
contactor, from
a fluid and jet performance standpoint, about 1% to about 2% polymer or
suspension is added to
the aqueous ammonia solutions to enhance contactor performance. An example of
an additive is
one that is neither reactive toward aqueous ammonia or interferes with the
mass transfer process.
A polymer or suspension may allow tailoring the sorbent properties, e.g.,
viscosity, for achieving
maximum jet performance (jet width, length, thickness, surface area) at
minimum liquid side
42
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
pressure drop. An exemplary polymer additive is diethylene glycol. Other
polymer additives
include polyethylene oxide or polyvinyl alcohol. An exemplary inorganic
additive is bentonite.
[02131 Additional chemical compounds are preferred to aid in the rate of SO2
oxidation
and thus the mass transfer kinetics. An exemplary additive to the preferred
sorbent system is
hydrogen peroxide. To avoid excessive hydrogen peroxide decomposition at high
pH, a stabilizer
is added to the sorbent mixture. An exemplary hydrogen peroxide stabilizer at
high pH is poly(a-
hydroxy acrylic acid). Hydrogen peroxide oxidation capability may be further
enhanced by the
addition of hydrogen peroxide catalysts. An exemplary hydrogen peroxide
catalyst is iron (III)
tetra-amido macrocyclic ligand (TAML).
[02141 FIG. 33 is a schematic of a general gas liquid contactor system design
which
enables interaction between gas and liquid phases according to another
embodiment of the
invention. The gas liquid contactor system includes a gas inlet 2600 coupled
to a gas distribution
unit 2605 to provide gas to the gas liquid contactor 2645. The system also
includes a liquid
reagent tank 2610 coupled to a pump 2615 and to a liquid catch tank 2620. The
catch tank 2620
is coupled to the gas liquid contactor 2645 for collecting liquid from the gas
liquid contactor
2645. The catch tank 260 is optionally coupled to a liquid recirculation pump
2625. The liquid
recirculation pump 2625 enables a method of liquid recirculation. A flow
control valve 2630 is
coupled to a liquid plenum 2635 for controlling liquid into the liquid plenum
2635. An array of
nozzles 2640 for forming a liquid jets is coupled to the liquid plenum and the
gas liquid
contactor 2645. The gas liquid contactor 2645 includes a liquid gas jet
contact zone. A gas
liquid separator 2650 to separating gas from liquid sorbent jets is arranged
in the gas liquid
contactor 2645. A demister 2660 capable of removing small gas droplets from
the exit gas is
positioned near a gas outlet 2655.
[02151 The gas inlet may include a plurality of different gases. For example,
it may
include industrial effluents, such as contaminants or pollutants may include
SO, NON, C02, Hg,
and combinations of the same. Of course other gaseous molecules may also be
removed such as
acidic gases like HCI, HBr, HF, H2SO4, and HN03, CO, H2S, amines (including
ammonia),
alkanolamines, urea, formamides, alcohols, carboxylates (like acetic acid),
combinations of the
same and a wide variety of other gas phase molecules. The limitation of the
invention is simply
the ability to provide a gas phase molecular reactant or solute and a liquid
phase within which it is
reactive or soluble, respectively. Although the main description in this
invention specification
focuses on aqueous systems, one skilled in the art will readily recognize the
applicability of this
gas liquid contactor invention to non-aqueous systems as well.
43
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[0216] In this embodiment, injection of a gas effluent containing SO2 into the
gas liquid
chamber is described. A gas plenum distributes the gas flow evenly across the
liquid flat jets.
Liquid jets are created by pumping the sorbent into a liquid plenum that
distributes the sorbent
evenly across the nozzle orifices. The created jets flow vertically downward
into the contactor
chamber and through a gas liquid separator into a catch tank. In the gas
liquid chamber the
vertical flowing sorbent intersects the gas cross-flow. Sulfur dioxide is
absorbed into the sorbent
liquid and removed from the gas effluent stream. Clean gas effluent is
discharged at the exit of
the contactor chamber. The sorbent is recirculated for continuous SO2 removal
from the effluent
gas stream.
[0217] The performance of the gas liquid contactor was demonstrated on a
small, sub-
scale test bed as illustrated in FIG. 33. Table 4 summarizes the geometric
parameters for the
example.
TABLE 4: GAS LIQUID CONTACTOR GEOMETRIC DIMENSIONS
Parameter No. Jet Jet Single GLC GLC Channel Specific Contactor
Orifices Packing Jet Channel Channel Length Surface Volume
Density Surface Width Height (cm) Area (cm)
(jets/cm2) Area (cm) (cm) (cm-1)
(cm2)
Value 96 4 22 15 25 30 5-10 11,250
[0218] The jet orifice geometry used in this example is described above in
relation to the
nozzle plates and gas liquid contactor. Prior to operation the liquid jet
surface area was
optimized for jet length, width and thickness by varying the pump backing
pressure to the jet
orifice plate. Further optimization with respect to jet surface area (length
and width) can be
obtained using additives (for example, diethylene glycol) to enhance the
sorbent
viscosity/surface tension properties or by reshaping the orifice nozzle.
[0219] An example of the gas liquid contactor operating conditions and
performance is
presented in Table 5. A sorbent system containing about 28 wt% aqueous ammonia
was tested.
No viscosity or oxidant additives were added to the sorbent mixture. The
effluent gas consisted
of N2 mixed with SO2 at 500 ppmv. The gas mixture was injected into the
contactor under
ambient temperature and pressure conditions and measured using calibrated mass
flow
controllers. The liquid volumetric flow rate was determined by recording the
amount of the
liquid jet discharge into a calibrated receiving vessel over a measured time
interval. The test
44
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
results for SO2 absorption under the described test conditions show 95% SO2
removal without an
oxidative enhancer (i.e., H202).
TABLE 5: GAS LIQUID CONTACTOR OPERATING CONDITIONS AND SO2
ABSORPTION RESULTS
Sorbent Contactor Total Total Liquid Inlet Inlet Inlet % SO2
Press. Gas Liquid Jet SO2 Gas Liquid Removed
(Tory) Flow Jet Backing Conc. Temp. Temp.
Rate Flow Pressure (ppm) (K) (K)
(LPM) Rate (psi)
(LPM)
AA 609 7.5 14 11 500 293 293 >95
(28 wt%)
[02201 NO, Capture Device
[02211 Another embodiment of the invention is directed towards utilizing the
gas liquid
contactor to capture NON. NOX is a primary pollutant consisting mainly of
nitric oxide (NO) and
nitrogen dioxide (NO2). Depending on the combustion process, more than 90% of
the NOX is
nitric oxide. NOX is produced from the reaction of nitrogen and oxygen at high
combustion
temperatures (>2700 F) as well as oxidation of nitrogen in the fuel. Various
performance areas
for enhancing the NOX capture capability include reducing reactor vessel size,
reducing pressure
drop and using efficient mass transfer sorbent systems.
[02221 Gas liquid mass transfer operations take place across the gas liquid
interface. The
absorption rate of a gas into a liquid sorbent is controlled by the liquid
phase mass transfer
coefficient, kL, the specific surface area (gas liquid interfacial surface
area to volume ratio), a,
and the concentration gradient between the bulk fluid, CL and the gas liquid
interface, CL *. In
many gas liquid reaction systems the solubility of the CL* is low and control
of the concentration
gradient is limited. To enhance the gas absorption rate, gas liquid contactor
designs should
demonstrate increased mass transfer kinetics, gas liquid mixing and
interfacial surface area to
volume ratio.
[02231 An embodiment of the invention includes a high performance gas liquid
contactor
(as described herein, e.g., FIG. 33). The system is based on an array of high
density, high
surface area, aerodynamically shaped thin flat jets that improve the overall
mass transfer and
contactor performance. The gas liquid contactor is characterized by enhanced
specific surface
area, ranging from about 1 cm 2 to about 50 cm 2, a generator volume of about
1/10th the volume
of related art packed towers, low gas pressure drop across the contactor of
less than 5 torr/lineal
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
ft, a liquid jet driving pressure less than 50 psi and more preferably less
than 20 psi, and minimal
liquid entrainment in the gas flow.
102241 In a preferred embodiment, the system includes a specific surface area
in a range
from about 10 cm- to about 20 cm" t, a generator volume of about 1/10th the
volume of related
art packed towers, a gas pressure drop of less than about 1 Torr per lineal
foot of contactor, and a
jet driving pressure ranging from about 5-10 psi, and minimal liquid
entrainment in the gas flow.
102251 To efficiently capture NOR, the gas liquid contactor can be used with a
wide
variety of aqueous-based sorbents including but not limited to ammonium
hydroxide (commonly
called aqueous ammonia and abbreviated AA), metal chelates or urea. The
addition of oxidizing
agents (OX) enhances NO oxidation to NO2, which increases the sorbent
absorption rate.
Various OX agents include sodium chlorite (NaC1O2), sodium hypochlorite
(NaOCI) sodium
hydroxide-potassium permanganate (KOH-KMnO4), and hydrogen peroxide (H202). In
preferred embodiments, the contactor utilizes aqueous ammonia and hydrogen
peroxide as the
decomposition products of H202 are environmentally and equipment friendly
(water and
oxygen), that is, neither are corrosive to normal materials of construction
and ammonium nitrate
is produced, which can be sold as a crop fertilizer to reduce operating costs.
102261 It is believed the that chemical mechanism to NO and NO2 oxidation in
the
presence of ammonium hydroxide and hydrogen peroxide are:
NH3 + H2O - NH4+ + OH" (1)
H202 + OH" - H02" + H2O (2)
HO2 + NO - NO2 + OH" (3)
NO2 + NO2 - N204 (4)
N204 + H2O - HNO2 + HNO3 (5)
HNO2 + H202 - HNO3 + H2O (6)
HN03 (aqueous) - H+ + N03" (7)
NH4+ + N03" - NH4NO3 (ammonium nitrate) (8)
102271 In an embodiment, a gas effluent cleaning process for removing nitrogen
oxide
with high efficiency is used. The system includes an array of nozzles. The
array of nozzles
includes an orifice plate (nozzle plate) reshaping and fluid composition
engineering for adapting
to a wide range of fluids and operating conditions. According to the
embodiment, the removal of
NO, is performed by passing the gas through a high surface to volume gas
liquid contactor unit
as described herein. The gas effluent is passed horizontally (referred to as
cross-flow) through
the gas liquid contactor having substantially reduced contactor volume and gas
flow pressure
46
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
drop. Intersecting the cross flow gas flow are a plurality of low pressure,
vertically oriented flat
jet arrays composed of an aqueous based sorbent and of substantial surface
area. The array of
nozzles is configured to produce flat jet arrays that are aerodynamically
shaped so as to provide
stable jet flow with low liquid particle entrainment at relatively high gas
velocity.
102281 In embodiments of the invention, a sorbent for nitrogen oxide
absorption and
removal may include those systems that demonstrate high NOX capacity, high
oxidative stability,
low heat of reaction, low sorbent cost, low corrosivity and a salable product
stream. In a
preferred embodiment, an exemplary sorbent for effective NOX removal is about
28 wt%
ammonia in water. The nozzle plate (described herein) may be optimized from a
fluid and jet
performance standpoint by adding about I% to about 2% polymer or suspension to
the aqueous
ammonia solutions to enhance contactor performance. A preferred additive is
such that it is
neither reactive toward aqueous ammonia or interferes with the mass transfer
process. A
polymer or suspension that allows tailoring the sorbent properties (for
example, viscosity) for
achieving maximum jet performance (jet width, length, thickness, surface area)
at minimum
liquid side pressure drop may be used. An exemplary polymer additive is
diethylene glycol.
Other polymer additives include polyethylene oxide or polyvinyl alcohol. An
exemplary
inorganic additive is bentonite. Additional chemical compounds are preferred
to aid in the rate
of NO oxidation and thus the mass transfer kinetics. An exemplary additive to
the preferred
sorbent system is hydrogen peroxide. To avoid excessive hydrogen peroxide
decomposition at
high pH, a stabilizer is added to the sorbent mixture. An exemplary hydrogen
peroxide stabilizer
at high pH is poly(a-hydroxy acrylic acid). Hydrogen peroxide oxidation
capability may be
further enhanced by the addition of hydrogen peroxide catalysts. An exemplary
hydrogen
peroxide catalyst is Iron(III) tetra-amido macrocyclic ligand (TAML).
102291 As discussed with regard to FIG. 33 the system can be utilized for a
NOx capture.
The process is described by the injection of a gas effluent containing NOX
into the gas liquid
chamber 2645. A gas plenum 2605 distributes the gas flow evenly across the
liquid flat jets.
Liquid jets are created by pumping the sorbent into a liquid plenum 2635 that
distributes the
sorbent evenly across the jet orifices. The created jets flow vertically
downward into the
contactor chamber and through a gas liquid separator into a catch tank 2620.
In the gas liquid
chamber 2645 the vertical flowing sorbent intersects the gas cross-flow.
Nitrogen oxide is
absorbed into the sorbent liquid and removed from the gas effluent stream.
Clean gas effluent
2655 is discharged at the exit of the contactor chamber. The sorbent is
recirculated for
continuous NOX removal from the effluent gas stream. The performance of the
gas liquid
47
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
contactor was demonstrated on a small, sub-scale test bed as illustrated in
Figure 33. Table 6
summarizes the geometric parameters for the example.
TABLE 6: GAS LIQUID CONTACTOR GEOMETRIC DIMENSIONS
Parameter No. Jet Jet Single GLC GLC GLC Specific Contactor
Orifices Packing Jet Channel Channel Channel Surface Volume
Density Surface Width Height Length Area (cm)
(jets/cm2) Area (cm) (cm) (cm) (cm2)
(cm2)
Value 96 4 22 25 25 30 5-10 11,250
[02301 The jet orifice geometry used in this example is described above. Prior
to
operation the liquid jet surface area was optimized for jet length, width and
thickness by varying
the pump backing pressure to the jet orifice plate. Further optimization with
respect to jet surface
area (length and width) can be obtained using additives (for example,
diethylene glycol) to
enhance the sorbent viscosity/surface tension properties or by reshaping the
orifice nozzle.
[02311 An example of the gas liquid contactor operating conditions and
performance is
presented in Table 7. A sorbent system containing about 28 wt% aqueous ammonia
was tested
under the given operating conditions in Table 2. The sorbent did not contain
an oxidizer (Ox) or
viscosity additive to enhance the removal of NO2. The effluent gas consisted
of nitrogen (N2)
mixed with NO2 at 500 ppmv. The gas mixture was injected into the contactor
under ambient
temperature and pressure conditions and measured using calibrated mass flow
controllers. The
liquid volumetric flow rate was determined by recording the amount of the
liquid jet discharge
into a calibrated receiving vessel over a measured time interval. The
reduction of NO2
concentration leaving the contactor was determined by measuring the optical
absorbance of NO2
at 400 rim. Background NO2 concentrations were recorded prior to each run. A
stable flow of
N02/N2 was first generated and the absorbance recorded without jet flow, Aoff.
The jet flow (28
wt% AA) was then injected into the reactor chamber and the absorbance
recorded. The
amount of NO2 reduced (absorbed) is expressed as a percentage using:
% NO2 Reduction = 100x(Aoff-Aon)/Aoff (1)
[02321 FIG. 34 is a graph of absorbance vs. run time for a NO2 removal system.
Referring to FIG. 34, a representative NO2 absorption spectrum with the liquid
aqueous
ammonia jets on and off is shown. The y-axis represents the absorbance at 400
rim and the x-
axis represents time in seconds. As shown in this example, a short lived
absorbance spike
just after the start of the jet flow is attributed to a flow perturbation in
the chamber. An
average of four test runs was performed for each test result. The test results
for NO2
48
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
absorption under the described test conditions show adequate NO2 removal (-
35%) even
without an oxidation enhancer (i.e., H202).
[02331 Hg Capture Device
[02341 Another embodiment of the invention is directed towards utilizing the
gas liquid
contactor to capture Hg. Gas liquid mass transfer operations take place across
the gas liquid
interface. The absorption rate of a gas into a liquid sorbent is controlled by
the liquid phase mass
transfer coefficient, kL, the specific surface area (gas liquid interfacial
surface area to volume
ratio), a, and the concentration gradient between the bulk fluid, CL and the
gas liquid interface,
CL*. In many gas liquid reaction systems the solubility of the CL* is low and
control of the
concentration gradient is limited. Therefore to improve the gas absorption
rate, enhancement of the
mass transfer kinetics and the interfacial surface area to volume ratio is
required.
[02351 An embodiment of the invention, includes a high performance gas liquid
contactor
(as described herein, e.g., FIG. 33). The system is based on an array of high
density, high surface
area, aerodynamically shaped thin flat jets that improve the overall mass
transfer and contactor
performance. The gas liquid contactor is characterized by enhanced specific
surface area ranging
from about 1 to about 50 cm 2, a generator volume of about 1/10th the volume
of related art packed
towers, low gas pressure drop across the contactor of less than about 5
Torr/lineal ft, a liquid jet
driving pressure less than about 50 psi and more preferably less than about 20
psi, and minimal
liquid entrainment in the gas flow.
[02361 In a preferred embodiment, the system includes a specific surface area
in a range
from about 10 cm- to about 20 cm", a generator volume of about 1/10th the
volume of related art
packed towers, a gas pressure drop of less than about 1 Torr, and a jet
driving pressure of about 5
psi, and minimal liquid entrainment in the gas flow.
[02371 The gas liquid contactor can be used with a variety of aqueous-based
sorbents that
oxidize elemental mercury (Hg ) to Hg(II). Once in the Hg(II) state, mercury
becomes soluble in
aqueous solutions and Hg(II) can catalytically remove elemental mercury (Hg )
from the flue gas
stream. The oxidants (OX) include but are not limited to sodium hypochlorite
(NaOCI), and
hydrogen peroxide (H202). The preferred oxidant to be used in the contactor is
hydrogen
peroxide (H202) with a catalytic (Cat) additive to enhance Hg oxidation
rates. An exemplary
additive is HgC12, TAML (Iron(III) tetraamido macrocyclic ligand), catalase or
peroxidase.
[02381 It is believed that the likely chemical mechanisms to Hg oxidation in
the presence
of aqueous hydrogen peroxide are:
H202 + Hg - Hg(II) + products (1)
49
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
H202 + Cat + Hg --> Hg(II) + products (2)
[0239] In this embodiment a gas effluent cleaning process for removing mercury
with
high efficiency utilizes the high efficiency gas liquid contactor. The system
includes an array of
nozzles, e.g., orifice plate (as described above in relation to the nozzle
plate) reshaping and fluid
composition engineering for adapting to a wide range of fluids and operating
conditions. The
removal of Hg is performed by passing the gas through a high surface to volume
gas liquid
contactor unit as described in U.S. Patent No. 7,379,487, which is hereby
incorporated by
reference. The gas effluent is passed horizontally (referred to as cross-flow)
through the gas
liquid contactor having substantially reduced contactor volume and gas flow
pressure drop.
Intersecting the cross flow gas flow is a plurality of low pressure,
vertically oriented flat jet
arrays composed of an aqueous based sorbent and of substantial surface area.
The flat jet arrays
are aerodynamically shaped so as to provide stable jet flow with low liquid
particle entrainment
at relatively high gas velocity.
[0240] In a preferred embodiment, sorbent for mercury absorption and removal
are those
systems that demonstrate high Hg capacity, high oxidative stability, low heat
of reaction, low
sorbent cost, low corrosivity and a salable product stream. An exemplary
sorbent is aqueous
with hydrogen peroxide, about 10 wt%, with a catalyst, about 0.1 wt%, to
enhance the oxidation
of elemental Hg to Hg(II). The nozzle plate configuration may be optimized by
adding about
I% to about 2% suspension to the aqueous hydrogen peroxide solution to enhance
contactor
performance. The additive may be designed such that it is neither reactive
toward aqueous
hydrogen peroxide or interferes with the mass transfer process. The additive
may allow tailoring
the sorbent properties (for example, viscosity) for achieving maximum jet
performance (jet
width, length, thickness, surface area) at minimum liquid side pressure drop.
An exemplary
additive is bentonite.
[0241] Additional chemical compounds are preferred to aid in the rate of Hg
oxidation
and thus the mass transfer kinetics. An exemplary additive to the preferred
sorbent system is
hydrogen peroxide. To avoid excessive hydrogen peroxide decomposition at high
pH, a
stabilizer is added to the sorbent mixture. An exemplary hydrogen peroxide
stabilizer at high pH
is poly(a-hydroxy acrylic acid). Hydrogen peroxide oxidation capability is
further enhanced by
the addition of hydrogen peroxide catalysts. An exemplary hydrogen peroxide
catalyst is
Iron(III) tetra-amido macrocyclic ligand (TAML).
[0242] As discussed with regard to FIG. 33 the system can be utilized for Hg
capture.
The process is described by the injection of a gas effluent containing Hg into
the gas liquid
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
chamber 2645. A gas plenum 2605 distributes the gas flow evenly across the
liquid flat jets.
Liquid jets are created by pumping the sorbent into a liquid plenum 2635 that
distributes the
sorbent evenly across the jet orifices. The created jets flow vertically
downward into the
contactor chamber and through a gas liquid separator into a catch tank 2620.
In the gas liquid
chamber 2645 the vertical flowing sorbent intersects the gas cross-flow.
Mercury is absorbed
into the sorbent liquid and removed from the gas effluent stream. Clean gas
effluent 2655 is
discharged at the exit of the contactor chamber. The sorbent is re-circulated
for continuous Hg
removal from the effluent gas stream.
[0243] H2S Capture Device
[0244] Another embodiment of the invention is directed towards utilizing the
gas liquid
contactor to capture H2S. Hydrogen sulfide is a highly toxic, flammable and
noxiously smelling
gas. It is considered a broad-spectrum poison; however, the central nervous
system is primarily
affected. The sources for anthropogenic hydrogen sulfide stem primarily from
processing natural
gas and high sulfur content crude oils. Natural gas can contain concentrations
of H2S up to about
28%. Manmade emissions account for about 10% of total global H2S emissions.
Petroleum
refineries contribute the largest portion of industrial H2S emission through
hydrodesulfurization
processes. Other industrial sources for H2S include coke ovens, paper mills
and tanneries.
[0245] Environmental concerns over refinery H2S emissions and high sulfur
containing
fuel products (gasoline and diesel) have led to stringent government controls.
These regulations
have resulted in significant cost increases for natural gas and oil refinery
operations. Numerous
technologies for removing H2S have been demonstrated.
[0246] The most prevalent approach is the Claus process as known in the art,
which
converts H2S through oxygen combustion into elemental sulfur. One problem with
the Claus
process is that CO2 present in the feedstock reacts with H2S to form carbonyl
sulfide and carbon
disulfide. Another is that due to equilibrium considerations some unreacted
H2S becomes
entrained in the elemental sulfur product. Other processes for H2S removal
include reaction with
alkanolamines (monoethanolamine, diethanolamine and methyldiethanolamine),
iron
oxide/sodium carbonate, thiosarsenate, quinine and vanadium metal processes.
However, there
is no single commercial approach that demonstrates high capability and cost
efficiency for
removing H2S from flue gas effluents. The significant cost drivers (excluding
labor and
construction equipment) for H2S capture are reagent cost, handling and waste
processing,
hardware (absorber vessel, flue gas handling and ductwork) and installation
space constraints.
51
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[02471 Achieving a hydrogen sulfide removal capability that is efficient and
cost
effective is a major technical challenge. Various performance areas for
enhancing the H2S
capture capability include reducing reactor vessel size, reducing pressure
drop and using efficient
mass transfer sorbent systems with salable byproducts. An embodiment of the
invention is
directed to achieving these target performances with innovative design
approaches that couple
high H2S absorption kinetics and value-added product streams.
[02481 Flat Jet Spray Contactor
[02491 Gas liquid mass transfer operations take place across the gas liquid
interface. The
absorption rate of a gas into a liquid sorbent is controlled by the liquid
phase mass transfer
coefficient, kL, the specific surface area (gas liquid interfacial surface
area to volume ratio), a,
and the concentration gradient between the bulk fluid, CL, and the gas liquid
interface, CL *. In
many gas liquid reaction systems the solubility of the CL * is low and control
of the concentration
gradient is limited. Therefore to improve the gas absorption rate, enhancement
of the mass
transfer kinetics and the interfacial surface area to volume ratio is
required.
[02501 An embodiment of the invention includes a high performance gas liquid
contactor
(as described herein, e.g., FIG. 33). The system is based on an array of high
density, high surface
area, aerodynamically shaped thin flat jets that improve the overall mass
transfer and contactor
performance. The gas liquid contactor is characterized by enhanced specific
surface area ranging
from about 1 em-2 to about 50 cm"2, a generator volume of about 1/10th the
volume of related art
packed towers, low gas pressure drop across the contactor of less than about 5
torr/lineal ft, a
liquid jet driving pressure less than 50 psi and more preferably less than
about 20 psi, and minimal
liquid entrainment in the gas flow.
[02511 In a preferred embodiment, the system includes a specific surface area
in a range
from about 10 cm"i to about 20 cm- , a generator volume of about 1/10th the
volume of related
art packed towers, a gas pressure drop of less than about 1 Torr, a jet
driving pressure of about 5
psi, and minimal liquid entrainment in the gas flow.
[02521 The contactor can be used with a variety of conventional liquid
(aqueous-based)
sorbents that oxidize H2S and other sulfur based compounds. The oxidants (OX)
include but are
not limited to aqueous ammonia, alkanolamines (monoethanolamine,
diethanolamine and
methyldiethanolamine), iron oxide/sodium carbonate, thiosarsenate, quinine,
vanadium metal
processes, sodium hypochlorite (NaOCI), and hydrogen peroxide (H202). The
preferred oxidant
to be used in the contactor is a basic (pH > 7) hydrogen peroxide (H202)
solution with a catalytic
(Cat) additive to enhance the oxidation rate and a stabilizer to control
hydrogen peroxide
52
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
decomposition. An exemplary catalytic additive is TAML (Iron(111) tetraamido
macrocyclic
ligand ). An exemplary stabilizer is poly alpha hydroxyacrylic acid, sodium
silicate or
dimethylene triaminepentaacetic acid.
[02531 It is believed that the likely chemical mechanisms to H2S oxidation in
the
presence of aqueous basic hydrogen peroxide are:
HzS + OH" - HS"+ H2O (1)
4H202 + HS" - S042" + H++4H20 (2)
[02541 Process for H2S Removal
[02551 This embodiment is directed towards a gas effluent cleaning process for
removing
hydrogen sulfide with high efficiency. The invention includes an array of
nozzles including a
nozzle orifice plate (see the description of the nozzle plate above) reshaping
and fluid
composition engineering for adapting to a wide range of fluids and operating
conditions. The
removal of H2S is performed by passing the gas through a high surface to
volume gas liquid
contactor unit as described above. The gas effluent is passed horizontally
(referred to as cross-
flow) through the gas liquid contactor having substantially reduced contactor
volume and gas
flow pressure drop. Intersecting the cross flow gas flow is a plurality of low
pressure, vertically
oriented flat jet arrays composed of an aqueous based sorbent and of
substantial surface area.
[02561 The flat jet arrays are aerodynamically shaped so as to provide stable
jet flow with
low liquid particle entrainment at relatively high gas velocity. Sorbents for
hydrogen sulfide
absorption and removal are those that demonstrate high H2S capacity, high
oxidative stability,
low heat of reaction, low sorbent cost, low corrosivity and a salable product
stream. An
exemplary sorbent is aqueous with hydrogen peroxide, about 10 wt%, with a
catalyst, about 0.1
wt%, to enhance the oxidation of H2S. In order to optimize the contactor,
about a 1% to about
2% suspension may be added to the aqueous hydrogen peroxide solution to
enhance contactor
performance. An example of an additive is such that it is neither reactive
toward aqueous
hydrogen peroxide or interferes with the mass transfer process. An example of
an additive allows
tailoring the sorbent properties (for example, viscosity) for achieving
maximum jet performance
(jet width, length, thickness, surface area) at minimum liquid side pressure
drop. An exemplary
additive is bentonite.
[02571 In a preferred embodiment, additional chemical compounds are used to
aid in the
rate of H2S oxidation and thus the mass transfer kinetics. An exemplary
additive to the preferred
sorbent system is hydrogen peroxide. To avoid excessive hydrogen peroxide
decomposition at
high pH, a stabilizer may be added to the sorbent mixture. An exemplary
hydrogen peroxide
53
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
stabilizer at high pH is poly(a-hydroxy acrylic acid). Hydrogen peroxide
oxidation capability
may be further enhanced by the addition of hydrogen peroxide catalysts. An
exemplary hydrogen
peroxide catalyst is Iron(III) tetra-amido macrocyclic ligand (TAML).
[02581 As discussed with regard to FIG. 33 the system can be utilized for H2S
removal.
The process is described by the injection of a gas effluent containing H2S
into the gas liquid
chamber 2645. A gas plenum 2605 distributes the gas flow evenly across the
liquid flat jets.
Liquid jets are created by pumping the sorbent into a liquid plenum 2635 that
distributes the
sorbent evenly across the jet orifices. The created jets flow vertically
downward into the
contactor chamber and through a gas liquid separator into a catch tank 2620.
In the gas liquid
chamber 2645 the vertical flowing sorbent intersects the gas cross-flow.
Hydrogen sulfide is
absorbed into the sorbent liquid and removed from the gas effluent stream.
Clean gas effluent
2655 is discharged at the exit of the contactor chamber. The sorbent is
recirculated for
continuous H2S removal from the effluent gas stream.
[02591 CO2 Capture Device Flat Jet Spray Contactor
[02601 Another embodiment is directed towards utilizing the gas liquid
contactor to
capture CO2. Gas liquid mass transfer operations take place across a gas
liquid interface. The
absorption rate of a gas into a liquid sorbent is controlled by the liquid
phase mass transfer
coefficient, kL, the specific surface area (gas liquid interfacial surface
area to volume ratio), a,
and the concentration gradient between the bulk fluid, CL, and the gas liquid
interface, CL*. In
many gas liquid reaction systems the solubility of the CL * is low and control
of the concentration
gradient is limited. To enhance the gas absorption rate, gas liquid contactor
designs should
demonstrate increased mass transfer kinetics, gas liquid mixing and
interfacial surface area to
volume ratio.
[02611 An embodiment of the invention is directed towards a high performance
gas
liquid contactor as described above and is based on an array of high density,
high surface area,
aerodynamically shaped thin flat jets that improve the overall mass transfer
and contactor
performance.
[02621 An embodiment of the invention includes a high performance gas liquid
contactor
(as described herein, e.g., FIG. 33). The system is based on an array of high
density, high
surface area, aerodynamically shaped thin flat jets that improve the overall
mass transfer and
contactor performance. The gas liquid contactor is characterized by enhanced
specific surface
area ranging from about 1 to about 50 cm-2, a generator volume of about 1/10th
the volume of
related art packed towers, a low gas pressure drop across the contactor of
less than about 5
54
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
torr/lineal ft, a liquid jet driving pressure less than about 50 psi and more
preferably less than
about 20 psi, and minimal liquid entrainment in the gas flow.
[0263] In a preferred embodiment, the system includes a specific surface area
in a range
from about 10 cm i to about 20 cm i, a generator volume of about 1/10th the
volume of related
art packed towers, a gas pressure drop of less than about 1 Torr, a jet
driving pressure of about 5
psi, and minimal liquid entrainment in the gas flow.
[0264] To efficiently capture C02, the contactor can be used with a wide
variety of
aqueous-based sorbents including but not limited to monoethanolamine (MEA),
hindered amines
such as methylaminopropanol (AMP) and piperazine (PZ), potassium carbonate
(K2CO3) and
ammonium hydroxide (commonly called aqueous ammonia and abbreviated AA). Using
the
contactor with aqueous ammonia is especially beneficial since ammonium
bicarbonate is created,
which can be converted to urea (a fertilizer) or sold as chemical feedstock to
reduce operating
costs. It is believed the likely chemical mechanisms to CO2 capture and
byproduct
generation in aqueous ammonia are:
2NH3 + H2O + CO2 - (NH4)2CO3 (ammonium carbonate) (1)
(NH4)2 CO3 + CO2 + H2O - 2NH4HCO3 (ammonium bicarbonate) (2)
NH4HCO3 + heat, pressure - (NH2)2CO (urea) (3)
[0265] Process for CO2 Removal
[0266] This embodiment is a gas effluent cleaning process for removing carbon
dioxide
with high efficiency via a gas liquid contactor of an embodiment of the
invention. The system
includes an array of nozzles including a nozzle orifice plate (the nozzle
plate as described above)
reshaping and fluid composition engineering for adapting to a wide range of
fluids and operating
conditions. The removal of CO2 is performed by passing the gas through a high
surface to
volume gas liquid contactor unit as described above. The gas effluent is
passed horizontally
(referred to as cross-flow) through the gas liquid contactor having
substantially reduced
contactor volume and gas flow pressure drop. Intersecting the cross flow gas
flow is a plurality
of low pressure, vertically oriented flat jet arrays composed of an aqueous
based sorbent and of
substantial surface area. The flat jet arrays are aerodynamically shaped so as
to provide stable jet
flow with low liquid particle entrainment at relatively high gas velocity. A
sorbent for carbon
dioxide absorption and removal may be those that demonstrate high carbon
dioxide capacity,
high oxidative stability, low heat of reaction, low sorbent cost, low
corrosivity and a salable
product stream. An exemplary sorbent for effective CO2 removal is about 28 wt%
ammonia in
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
water. In order to optimize the gas liquid contactor, about a 1% to about 2%
polymer or
suspension is added to the aqueous ammonia solutions to enhance contactor
performance.
[02671 An example of an additive is one that is neither reactive toward
aqueous ammonia
or interferes with the mass transfer process. The preferred polymer or
suspension allows
tailoring the sorbent properties (for example, viscosity) for achieving
maximum jet performance
(jet width, length, thickness, surface area) at minimum liquid side pressure
drop. An exemplary
polymer additive is diethylene glycol. Other polymer additives include
polyethylene oxide or
polyvinyl alcohol. An exemplary inorganic additive is bentonite.
[02681 As discussed with regard to FIG. 33 the system can be utilized for CO2
removal.
The process is described by the injection of a gas effluent containing CO2
into the gas liquid
chamber 2645. A gas plenum 2605 distributes the gas flow evenly across the
liquid flat jets.
Liquid jets are created by pumping the sorbent into a liquid plenum 2635 that
distributes the
sorbent evenly across the jet orifices. The created jets flow vertically
downward into the
contactor chamber and through a gas liquid separator into a catch tank 2620.
In the gas liquid
chamber 2645 the vertical flowing sorbent intersects the gas cross-flow.
Carbon dioxide is
absorbed into the sorbent liquid and removed from the gas effluent stream.
Clean gas effluent
2655 is discharged at the exit of the contactor chamber. The sorbent is re-
circulated for
continuous CO2 removal from the effluent gas stream.
[02691 The performance of the gas liquid contactor was demonstrated on a
small, sub-
scale test bed as illustrated in FIG. 33. Table 7 summarizes the geometric
parameters for the
example.
TABLE 7: GAS LIQUID CONTACTOR GEOMETRIC DIMENSIONS
Parameter No. Jet Jet Single Channel Channel Channel Specific Contactor
Orifices Packing Jet Width Height Length Surface Volume
Density Surface (cm) (cm) (cm) Area (cm3)
(jets/cm2) Area (cm2)
(cm2)
Value 96 4 22 15 25 30 5-10 11,250
[02701 The jet orifice geometry used in this example is described above. Prior
to
operation the liquid jet surface area was optimized for jet length, width and
thickness by varying
the pump backing pressure to the jet orifice plate. Further optimization with
respect to jet surface
area (length and width) can be obtained using additives (for example,
diethylene glycol or
bentonite) to enhance the sorbent viscosity/surface tension properties or by
reshaping the orifice
nozzle.
56
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
102711 An example of the gas liquid contactor operating conditions and
performance is
presented in Table 8. Two sorbent systems, aqueous ammonia and MEA, were
tested under the
given operating conditions. No viscosity additives were added to the sorbent
mixture. The effluent
gas consisted of air mixed with CO2 at a typical C02: air dilution ratio of
1:9. The gas mixture was
injected into the contactor under ambient temperature and pressure conditions
and measured using
calibrated mass flow controllers. The liquid volumetric flow rate was
determined by recording the
amount of the liquid jet discharge into a calibrated receiving vessel over a
measured time interval.
The amount of CO2 reduced (absorbed) is expressed as a percentage using:
% CO2 Reduction = 100 x (C1 -Cout)/C1n (1)
where C;,, and Cout is the concentration of CO2 entering the contactor and
exiting the contactor,
respectively. The relative amounts of CO2 entering and leaving the contactor
were determined
by integrating the fundamental absorption band of CO2 near 4.2 m with Fourier
Transform
Infrared (FTIR) spectrometry.
102721 FIG. 35 is a graph of the CO2 FTIR (Fourier Transform Infrared)
absorption
spectrum with the liquid aqueous ammonia jets on and off. Referring to FIG.
35, an average of
four test runs was performed for each test result. Background CO2
concentrations were recorded
prior to each run. Test results for CO2 absorption under the described test
conditions show
greater than 90% CO2 removal. The graph illustrates light absorbance of the
CO2 molecule in the
optical range of its fundamental optical absorption region from 2400 cm -1 to
2250 cm"'. The
graph clearly illustrates a reduction of absorbing species in this fundamental
CO2 region,
indicative of efficient removal. Performing standard mathematical analysis to
these spectra
provides the concentrations providing these levels of absorbance, which are
then examined by
ratio to determine percent removal.
TABLE 8: GAS LIQUID CONTACTOR OPERATING CONDITIONS AND SORBENT
TOTAL LIQUID JET FLOW RATE (LPM) CO2 ABSORPTION RESULTS
Sorbent Contactor Total Total Liquid Inlet Inlet Inlet % CO2
Press. Gas Liquid Jet CO2 Gas Liquid Removed
(Torr) Flow Jet Backing Conc. Temp. Temp.
Rate Flow Pressure (%) (K) (K)
(LPM) Rate (psi)
(LPM)
AA 609 1.8 14 11 9.5 293 293 96
(28 wt%)
MEA 609 1.8 14 11 9.5 293 293 91
(30 wt%)
57
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[02731 System for Gaseous Pollutant Removal Flat Jet Spray Contactor
[02741 In embodiments of the invention pollutants may be eliminated in gas
streams by a
gas liquid contactor. The system transfers mass from one phase (gas) to
another (liquid). In this
process a gas stream passes through or is contacted with a sorbent in the form
of a liquid spray or
pool. Since the gas pollutant is soluble in the sorbent, it is dissolved or
absorbed into the liquid
sorbent and removed from the gas stream. The extent of the absorption process
is governed by
mass transfer operations, which include gas and liquid diffusion, solubility
and chemical
reactivity.
[02751 Gas liquid mass transfer operations take place across the gas liquid
interface. The
absorption rate of a gas into a liquid sorbent is controlled by the liquid
phase mass transfer
coefficient, kL, the specific surface area (gas liquid interfacial surface
area to volume ratio), a,
and the concentration gradient between the bulk fluid, CL, and the gas liquid
interface, CL*. In
many gas liquid reaction systems the solubility of the CL* is low and control
of the concentration
gradient is limited. To enhance the gas absorption rate, gas liquid contactor
designs should
demonstrate increased mass transfer kinetics, gas liquid mixing and
interfacial surface area to
volume ratio.
[02761 An embodiment of the invention includes a high performance gas liquid
contactor
(as described herein). The system is based on an array of high density, high
surface area, and
aerodynamically shaped thin flat jets that improve the overall mass transfer
and contactor
performance. The gas liquid contactor is characterized by enhanced specific
surface area ranging
from about 1 to about 50 cm 2, a generator volume of about 1/10th the volume
of related art
packed towers, a low gas pressure drop across the contactor of less than about
5 torr/lineal ft, a
liquid jet driving pressure less than about 50 psi and more preferably less
than about 20 psi, and
minimal liquid entrainment in the gas flow.
[02771 In a preferred embodiment, the system includes a specific surface area
in a range
from about 10 cm -1 to about 20 cm 1, a generator volume of about 1/10th the
volume of related
art packed towers, a gas pressure drop of less than about 1 Torr, a jet
driving pressure of about 5
psi, and minimal liquid entrainment in the gas flow.
[02781 Efficient capture of gas pollutants is obtained using a wide variety of
aqueous-
based sorbents in combination with a polymer additive to enhance jet surface
area. Acid gases
such as H2S and CO2 are typically removed with alkanolamines, monoethanolamine
(MEA) and
diethanolamine (DEA). Baseline SO2 and NOx sorbents include calcium carbonate
mixtures
(limestone/lime) and ammonium hydroxide (aqueous ammonia), respectively.
Customizing the
58
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
sorbent system to enable an all in one pollutant capture system is preferred
since it simplifies and
reduces the size of the pollution control contactor. The all in one system may
be configured in
series or in parallel. Moreover, the all in one system utilizes a gas liquid
contactor as described
herein.
[0279] In a preferred embodiment, additives to enhance jet surface area are
polyvinyl
alcohol, polyvinyl oxide, ethylene glycol or diethylene glycol. Inorganic
suspensions such as
bentonite are also preferred as a treatment to enhance jet surface area.
Aqueous ammonia is the
preferred sorbent since it has the capability to remove C02, SO2, NOX and H2S.
The addition of
an oxidizing agent such as hydrogen peroxide helps oxidize NO and Hg, which
are otherwise
difficult to absorb in aqueous solutions. A hydrogen peroxide activation
catalyst for operating at
high pH is Iron(III) tetra-amido macrocyclic ligand (TAML). A preferred
hydrogen peroxide
stabilizer at high pH is poly(a-hydroxy acrylic acid). Aqueous ammonia is a
particularly
preferred sorbent since ammonium bicarbonate, ammonium nitrate and ammonium
sulfate are
byproducts of NOX and SO2 reaction with aqueous ammonia. These products can be
sold as
fertilizer to reduce plant operating costs. The basic chemistry for an all in
one pollutant capture
and byproduct generation system is:
SO2 Capture:
NH3 + H2O + S02 -> NH4+ + HS03" (1)
NH4+ + HSO3 + NH3 -> 2(NH4) + S032" (2)
2H202 + S032" -~ H2O + H2SO4 (sulfuric acid) (3)
H2SO4 + H2O -~ 2H+ + S042" + H2O (4)
2NH4++ S042" -~ (NH4)2SO4 (ammonium sulfate) (5)
NOX Capture:
NH3 + H2O -> NH4+ + OH" (1)
H202 + OH" ->HO2" + H2O (2)
H02" + NO -> NO2 + OH" (3)
NO2 + NO2 -> N2O4 (4)
N2O4 + H2O ->HNO2 + HNO3 (5)
HNO2 + H2O2 -> HNO3 + H2O (6)
HNO3 (aqueous) H+ + NO3 (7)
NH4+ + NO3 -> (NH4)N03 (ammonium nitrate) (8)
Hg Capture:
H202 + Hg -- Hg(II) + products (1)
H2S Capture:
H2S + H2O -> HS" + H3O+ (1)
HS" + NH3 + H2O -> NH4HS + OH" (2)
59
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[02801 System Process for Gaseous Pollutant Removal
[02811 An embodiment of the invention is directed towards a system including
an array
of nozzles for gaseous pollutant removal. The array of nozzles includes a
nozzle orifice plate
reshaping and fluid composition engineering for adapting to a wide range of
fluids and operating
conditions. The removal of pollutant gases is performed by passing the gas
through a high
surface to volume gas liquid contactor unit as described above. The gas
effluent is passed
horizontally (referred to as cross-flow) through the gas liquid contactor
having substantially
reduced contactor volume and gas flow pressure drop. Intersecting the cross
flow gas flow is a
plurality of low pressure, vertically oriented flat jet arrays composed of an
aqueous based sorbent
and of substantial surface area. The flat jet arrays are aerodynamically
shaped so as to provide
stable jet flow with low liquid particle entrainment at relatively high gas
velocity.
[02821 In a preferred embodiment, the preferred sorbent for gas absorption and
removal
are those systems that demonstrate high liquid jet performance, high gas
loading capacity, high
oxidative stability, low heat of reaction, low sorbent cost, low corrosivity
and a salable product
stream. The jet nozzle plate configuration (as described above) may be
optimized in one
embodiment by including about a 12% polymer or suspension added to the sorbent
solution to
enhance contactor performance.
[02831 The preferred additive is such that it is neither reactive toward the
sorbent or
interferes with the mass transfer process. The preferred polymer or suspension
allows tailoring
the sorbent properties (for example, viscosity) for achieving maximum jet
performance (jet
width, length, thickness, surface area) at minimum liquid side pressure drop.
An exemplary
sorbent is aqueous ammonia, about a 28 wt%, with a polymer additive or
suspension to enhance
liquid viscosity for optimum jet width, length and thickness at minimum
driving pressure. An
exemplary polymer additive is diethylene glycol. An exemplary inorganic
suspension is
bentonite.
[02841 Other additives may be used to aid in the rate of pollutant oxidation
and thus the
mass transfer kinetics. An exemplary additive to enhance the oxidation of
pollutant molecules
including but not limited to Hg and SO2 is hydrogen peroxide. To avoid
excessive hydrogen
peroxide decomposition at high pH, a stabilizer is added to the sorbent
mixture. An exemplary
hydrogen peroxide stabilizer at high pH is poly(a-hydroxy acrylic acid).
Hydrogen peroxide
oxidation capability is further enhanced by the addition of hydrogen peroxide
catalysts. An
exemplary hydrogen peroxide catalyst is Iron(III) tetra-amido macrocyclic
ligand (TAML).
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[02851 In one embodiment, a gas liquid contactor as described in FIG. 33 could
be
utilized for gaseous pollutant removal. The process is described by the
injection of a gas effluent
2600 into the gas liquid chamber 2645. A gas plenum 2605 distributes the gas
flow evenly across
the liquid flat jets. Liquid jets are created by pumping the sorbent into a
liquid plenum 2635 that
distributes the sorbent evenly across the jet orifices. The created jets flow
vertically downward
into the contactor chamber 2645 and through a gas liquid separator 2650 into a
catch tank 2620.
In the gas liquid chamber 2645 the vertical flowing sorbent intersects the gas
cross-flow. The
gas pollutants are absorbed into the sorbent liquid and removed from the gas
effluent stream.
Clean gas effluent 2655 is discharged at the exit of the contactor chamber.
The sorbent is re-
circulated for continuous pollutant removal from the effluent gas stream.
Table 9 summarizes a
preferred embodiment of the geometric parameters for this embodiment.
TABLE 9: GAS LIQUID CONTACTOR GEOMETRIC DIMENSIONS
Parameter No. Jet Jet Single Channel Channel Channel Specific Contactor
Orifices Packing Jet Width Height Length Surface Volume
Density Surface (cm) (cm) (cm) Area (cm3)
(jets/cm2) Area (cm2)
(cm2)
Value 96 4 22 15 25 30 10 11,250
[02861 Prior to operation the liquid jet surface area was optimized for jet
length, width
and thickness by varying the pump backing pressure to the jet orifice plate.
Further optimization
with respect to jet surface area (length and width) can be obtained using
preferred additives to
enhance the sorbent viscosity/surface tension or by reshaping the orifice
nozzle. However, for
these tests no polymeric additives were added to the liquid sorbents.
[02871 An example of the gas liquid contactor operating conditions and
performance is
presented in Table 10. Two sorbent systems, aqueous ammonia and MEA, were
tested under the
given operating conditions. No viscosity or oxidant additives were added to
the sorbent mixture.
The effluent gas consisted of air mixed with CO2 at a typical C02:air dilution
ratio of 1:9. The
gas mixture was injected into the contactor under ambient temperature and
pressure conditions
and measured using calibrated mass flow controllers. The liquid volumetric
flow rate was
determined by recording the amount of the liquid jet discharge into a
calibrated receiving vessel
over a measured time interval. The amount of CO2 reduced (absorbed) is
expressed as a
percentage using:
% CO2 Reduction = 100 * (Cin-Cout)/Cin (1)
61
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
where C;,, and Cout is the concentration of CO2 entering the contactor and
exiting the contactor,
respectively. The relative amounts of CO2 entering and leaving the contactor
were determined by
integrating the fundamental absorption band of CO2 near 4.2 m with Fourier
Transform Infrared
(FTIR) spectrometry. A representative CO2 FTIR absorption spectrum with the
liquid aqueous
ammonia thin flat liquid jets on and off is shown in FIG. 39. An average of
four test runs was
performed for each test result. Background CO2 concentrations were recorded
prior to each run.
Test results for CO2 absorption under the described test conditions show
greater than 90% CO2
removal.
TABLE 10: GAS LIQUID CONTACTOR OPERATING CONDITIONS AND CO2
ABSORPTION RESULTS
Sorbent Contactor Total Total Liquid Inlet Inlet Inlet % CO?
Press. Gas Liquid Jet CO2 Gas Liquid Removed
(Torr) Flow Jet Backing Conc. Temp. Temp.
Rate Flow Pressure (%) (K) (K)
(LPM) Rate (psi)
(LPM)
AA 609 1.8 14 11 9.5 293 293 96
(28 wt%)
MEA 609 1.8 14 11 9.5 293 293 91
(30 wt%)
[02881 Pilot Testing at a Coal Fired Power Plant
[02891 In this experiment, a trailer mounted, 2 MW unit (10,000 ACFM gas flow)
was
designed and fabricated for pilot testing at a coal fired power plant. The
device system consists
of a gas plenum, a flue gas blower, a heat exchanger submodule, a gas liquid
contactor module
including a liquid capture and anti-splash submodule, a demister submodule,
nozzle array
assemblies, sorbent pumps, liquid handling sub-module, diagnostics and other
ancillary
components. The system is designed to run in a closed loop steady state or
batch configuration
and meet power plant priority pollutant waste water discharge requirements.
The initial pilot
tests were conducted at a 0.13 MW scale (nominally 650 ACFM flue gas) on a
slipstream to
reduce development time and risk. A 650 ACFM slipstream was diverted to the
scrubber using
two six inch steel pipes. The flue gas velocity in the contactor was matched
to the power plant
effluent duct velocity (56 ft/s, 17 m/s) using an entrance channel area of 0.2
ft2. The gas
residence time in the contactor was about 0.04 seconds. The system was
operated with a 5 psi
liquid side pressure drop and a minimal flue gas pressure drop of
approximately 0.1 psi was
62
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
observed. The flue gas emissions for SO2, NO, NO2, CO, and CO2 were measured
using an
Environmental Protection Agency (EPA) performance verified flue gas analyzer.
The
slipstream entered the unit at a temperature and pressure of 150F and 11.2
psiA, respectively. A
0.1 wt% NaOH solution was circulated through the system to scrub the SO2.
[0290] FIG. 36 is a picture of a 2 MW prototype system. FIG. 37 is a picture
of a gas
liquid contactor. FIG. 38 is a picture of the solvent pumps of the system of
FIG. 36.
[0291] Referring to FIG. 36, a gas liquid contactor 3210 contains a flat
liquid jet
contacting system as described herein. A solvent feed plenum 3220 provides
solvent for
contacting to the contactor 3210. In FIG. 37 the flue gas enters at flue gas
entry point 3230 and
proceeds to contactor 3210. The flue gas exits at flue gas exit point 3250. In
FIG. 38, the
solvent pumps 3260 are shown. Figures 39-40 are graphs showing target
contaminant
concentrations in flue gas from a coal fired power plant on the Y-axis without
and with the gas
liquid contactor engaged versus time on the X-axis. A TESTO 335
electrochemical analyzer was
used for all three analytical measurements. FIG 39 shows the concentration of
SO2 in the first
small scale test using the contactor, at an equivalent flue gas draw of 0.13
MW. As the flue gas
was turned on, concentrations of SO2 reached approximate steady state at near
200 ppm.
Engaging the contactor system immediately reduced these SO2 emission levels to
near
instrument detection limits, again reaching steady state. The TESTO instrument
remained
sampling the system as the contactor was disengaged, showing an immediate rise
in SO2
concentrations towards the original contaminant levels. FIG. 40 is a figure
depicting CO2 levels
in the same mechanical system, but using a different sorbent. The TESTO
analyzer clearly
shows a reduction in CO2 levels over a period of 4 minutes, reaching steady
state at that level.
[0292] Also, deep SO2 removal efficiencies (> 99%) may be required for meeting
emission requirements and pre-treating the flue gas for efficient CO2
pollutant removal. Using
the 0.13 MW scrubber, an SO2 removal efficiency of about 99.5%, with an
average of about 99%
was achieved as seen in FIG. 39 was achieved. A scoping test for multi-
pollutant removal using
about 19 wt% aqueous ammonia was also performed as shown FIG. 40. Although the
system
was not optimized for CO2 absorption (low as and short residence time), the
unit absorbed more
than 50% of the slipstream CO2 under these conditions. In addition, more than
99.5% SO2 and
more than 80% NO, were simultaneously removed with aqueous ammonia. Through
jet and
solvent optimization, it is projected that only two units are needed to
achieve 90% CO2 removal
efficiency.
63
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[0293] Results were rapidly scaled and successfully used to demonstrate the
operation of
about 2 MW (8400 ACFM) modular pilot scrubber comprising parallel gas liquid
contactor
modules on the same power plant. The gas liquid contactor entrance channel
area was about 3.9
ft2, which provided a matching flow velocity to the power plant effluent of
about 58 ft/s (18 m/s)
and a residence time of about 0.07 seconds. Solvent flow rates were 2800 GPM,
giving an L/G
of about 330 GPM/1000 ACFM. The liquid pressure drop across the jets was about
6 psi. The
entire gas side pressure drop for a total of about 2 MW contactor stage
including mist eliminator
sub-module and jet pack sub-module was 0.4 psi where the gas pressure drop was
about 0.1 psi
across about 3.3 ft of jet pack (0.03 psi/ft). The input and output flue gas
temperatures were
about 250 F and about 115 F, respectively. A twenty four hour test operated
in steady state
(with solvent discharge) was performed with an average SO2 scrubbing
efficiency greater than
99%.
[0294] FIG 41 provides a view of a scaled up test for SO2 capture with
approximately 2
MW of equivalent flue gas flow through a larger contactor. Multiple on/off
cycles were
performed to confirm operational consistency. Referring to FIG. 41, a graph of
SO2 scrubbing
results using H2O, NaOH (0.1 wt%), 2 MW scale is shown. The graph includes on
the x-axis
time in hours and concentration is on the y-axis in ppm. As shown flue gas was
being emitted at
more than 350 ppm SO2 as a contaminant molecule. The operation of the gas
liquid contactor
module removed virtually all of it SO2. More particularly, to test whether
this was reproducible,
the contactor liquid jets were shut off, whereupon the SO2 concentration again
went above 350
ppm as shown in FIG. 41. Engaging the liquid jet module reduced the SO2
concentration to near
baseline. Repeating this consistently produces the same results as shown in
FIG. 41. Also,
recent follow up tests confirmed the SO2 removal efficiency. In addition, a
simulated waste
water treatment experiment using the spent solvent is being performed in the
laboratory to
demonstrate precipitation of calcium sulfate.
[0295] Embodiments of the invention are directed towards a modular gas liquid
contactor
or gas liquid contactor including post-combustion technology for removing
multiple flue gas
pollutants (SO,, NO,, CO2 and particles) over a wide range of flue gas
conditions. Wet scrubber
systems are susceptible to operational shutdowns due to mechanical or emission
compliance
failure. The gas liquid contactor scrubber systems are designed as small
footprint packages to
meet continuous on-line operation with performance, flexibility,
serviceability and reliability.
[0296] Although the actual performance metrics (for example, removal of SO2)
are
directly comparable with conventional methods and equipment designs, the
designs, methods,
64
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
and systems presented in this invention which provide that process equivalency
are very
surprising; the size and cost to produce these results can be more than ten
times smaller at less
than half the capital cost, respectively, of conventional systems.
[02971 A modular design approach is used for manufacture and scaling of the
modular
gas liquid contactor scrubber unit. Scrubber modules are added in parallel or
serially to achieve
the necessary pollutant removal performance. This is enabled by the low
pressure drop, e.g.,
about a 0.4 psi pressure drop and the low parasitic power requirements, e.g.,
less than about 0.8%
per stage. This approach standardizes the manufacturing yet allows
customization of a scrubber
unit per site requirements. The modular gas liquid contactor is factory built
in an assembly line
production process.
[02981 Gas Liquid Contactor Modules
[02991 FIG. 42 is a representation of a 60 MW scrubbing unit and supporting
structures.
FIG. 43 is a front view of one section 2 MW section of the scrubber tower of
FIG. 42. FIG. 44 is
a side view of one section 2 MW section of the scrubber tower of FIG. 42. FIG.
45 shows the
geometry of the entrance channel and jet pack zone. In this embodiment, the
system is
configured to be less than about 600 lbs and have dimensions of about 5 ft x
10 ft x 10 ft. These
units can also handle more than about 85,000 cfm of flue gas flow and may be
scaled up or down
as needed.
[03001 The units are modules that are designed to be stacked in parallel and
sized as
needed for power plants. In one parallel configuration the modules are either
on top of or next to
(side by side) each other. Incoming gas stream is split, e.g., equally,
amongst the parallel
modules, each module providing equivalent processing. In one embodiment a 20
MW composite
module is created (85,000 cfm) by vertically stacking ten 2 MW base modules.
Three 20 MW
modules are then horizontally coupled to produce a 60 MW system with the
incoming gas stream
split equally between the three 20 MW modules including the 60 MW system.
[03011 In this embodiment and as shown in FIG. 42, sorbent is fed from sorbent
storage
tank 3315 to the scrubbing system at solvent feed plenum 3305 where it is
pumped through a
plurality of nozzles configured in a nozzle array. The nozzle array is
configured to provide
essentially planar liquid jets, each of said liquid jets comprising a planar
sheet of liquid, said
plurality of liquid jets lying in substantially parallel planes. The flat
liquid jets are formed in
scrubber tower 3345, where a flow of gas 3320 is passed parallel to the flat
surfaces of the jets.
After the sorbent falls to the bottom of the tower a heat exchanger 3340
captures heat that has
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
been absorbed by the sorbent in the contacting process. The sorbent then flows
in conduit 3335
to a pump house 3330 where it is subsequently pumped to a water treatment
system 3325.
[03021 The water treatment system depicted at 3325 is for schematic reference
only in
that, depending on secondary or tertiary treatment of the liquid, this segment
3325 of the
contactor system can be small or large. For example, a small system might only
include a heat
exchanger for dissolution of the captured gas phase molecule and might fit
into the "box"
depicted in FIG 42. A large system might include precipitation tanks, settling
tanks, and solid
press subsystems which could be large, depending on the chemistry and
applications being
applied.
[03031 The pump house depicted in 3330 would be an appropriately sized liquid
pump to
deliver an appropriate volume of liquid to the contactor system as known in
the art. The block
depicted in 3330 is optional, but would depend on the site environment and
choice of pump and
whether that pump choice needs to be protected from rain or snow.
[03041 Now referring to FIGS. 43-45 illustrating a geometry of the spray pack
base units.
The base units or base module in this embodiment consists of about 25 cm x 130
cm entrance
channel and about a 1.7 m2spray pack area that contains approximately 5
sprays/cm2 based on a
total of 3400 nozzles (40 rows of 85 nozzles) in the spray pack. FIG. 43 shows
an effluent
entrance 3360, an effluent exit 3350, and a jet pack zone 3355. The jet pack
zone 3355 is the
actual contactor volume where liquid jets and gas molecules contact one
another. In FIG. 45 a
jet scrubbing pack 3365 is shown as a side cutaway, followed by spray or mist
eliminator 3370,
which eliminates entrained fluid from the effluent stream. Although the
velocity of the liquid jets
is high, some liquid still is entrained in the gas flow, particularly as gas
velocities become higher.
This entrainment includes small droplets; e.g., an aerosol or mist. The mist
eliminator provides a
small zone where the small mist droplets move through a zone with elements as
represented by
1660 in FIG. 29, condensing on the surface of those elements and flowing back
into the liquid
sump system. In this particular embodiment, these elements are vertical rods,
but any design
which provides a small pressure drop and causes turbulent gas flow combined
with a
condensation/coalescence surface can be envisioned, including but not limited
to mesh, heat
exchanger elements, or aerodynamic plate or baffles.
[03051 FIG. 46 shows a representation of a jet pack zone with removable nozzle
plate
according to another embodiment of the invention. FIG. 47 shows the
configuration of the
nozzle plates in the jet pack zone of FIG. 46. FIG. 48 shows a seal system for
the jet pack zone
of FIG. 46. As shown, the modular gas liquid contactor is designed for
serviceability,
66
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
accessibility and reliability. The system, e.g., gas liquid contactor or
scrubbing unit may use a
snap-fit design approach for the array of nozzles including an orifice plate
so that worn or
clogged orifices can be replaced without ceasing operations. The system may
also be designed
with redundancy into the mechanical equipment and support systems. For
example, large plant
installations include about a 20% spare concept so parallel units can be
serviced if necessary
without disrupting plant operations.
Now referring to FIG. 46, a removable plate 3410 is shown in a partially
removed
position. The removable plate 3410 includes a plurality of nozzle plates
having a plurality of
rows of nozzles for creating a plurality of parallel flat liquid jets. FIG. 47
shows an entire
section of removable plates 3410. Removable plate 3415 is shown in set
position and removable
plate 3410 is shown being removed. FIG. 48 shows a sealing mechanism 3440
designed to seal
jet plate 3425 onto sealing surface 3435 with an elastomeric seal 3430. That
is, as shown a side
view to a small section of the edge of the jet plate 3425, a small
magnification of the edge of a
standard jet plate, e.g., 3410 or 3415. In this embodiment, the jet plate is
installed as per 3415,
the edge of 3415 having a series of small angled grooves in which pin 3440
attached to the frame
3435 fits into the groove 3445. The angle of the groove 3445 is such that
torque applied in the
direction to seal causes the angle of the groove to act as a cam against the
pin 3440, resulting in
pressure against the elastomeric seal 3430. Although this is one specific
embodiment, one
skilled in the art of mechanical systems and sealing of hydraulic surfaces
could well envision
alternatives that would be equally functional.
[0306] Modular Gas Liquid Contactor for SO,, NO,, and Particulates Process
[0307] Another embodiment is directed towards a design that has been analyzed
for deep
flue gas SO2 removal using a Na/Ca dual alkali process that incorporates the
compact, high
performance, low cost, low water usage and highly energy efficient gas liquid
contactor system
or scrubber system with advanced waste water and product stream processing.
The design
requirements for this process are provided in Table 11.
TABLE 11: DESIGN REQUIREMENTS FOR GLC SO2 REMOVAL
Parameter Requirement
SO2 Scrubbing >99%
NO, Scrubbing >80%
Particulate Matter Scrubbing >99%
Sulfate Removal >99%
System Parasitic Load <1%
Removal of Liquid Entrainment (mist) >99%
67
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
Solvent Loop pH 6
Solvent Loop Temperature 107 F
Solvent Loop Flow Rate 28000 GPM
Water Make-up to Scrubber 12 GPM
Caustic Capacity 7 days
Bleed Stream to Absorber Loop Flow Ratio < 1/500
Brine Stream to Recycled Solvent Flow Ratio <1/40
Continuous operation 24 hrs/day, 7 days/week
[0308] Flue Gas Conditioning System
[0309] In an application where flue gas is processed using an embodiment of
this
invention, the methodologies employed can be described in general terms. These
generalities
can be customized depending on site and application requirements but can
roughly be broken
into four sections. FIG. 49 is a process flow diagram for a pollutant removal
system. Referring
to FIG. 49, the baseline process flow diagram for the gas liquid contactor
system shows major
system components and key stream points. The four sections include: Section 1.
flue gas or
process gas section, Section 2. a scrubber or reactor section, Section 3.
sorbent or reactant input,
and Section 4. reaction product processing and sorbent recycle (or release).
Although, for the
purposes of this embodiment, these four sections will be described in greater
detail for the
application of one instance of a flue gas desulfurization application, one
skilled in the art would
recognize that these processes could be further modified to address a large
number of various
processes that could benefit from high efficiency gas liquid contacting
systems.
[0310] Referring to Section 1, flue gas 5402 is generated and released from an
industrial
process, e.g., coal fired plant. The flue gas enters Section 2 at process
point 1 (PP 1), the gas
flows through and is processed in Section 2 past PP2, the gas is then heated
in the optional flue
gas heater 5404 (if required), then flows to a fan or blower at PP3, which
forces the flue gas at
PP4 into the flue gas stack 5406 for release. Flue gas can contain a number of
contaminants
depending on the source of the fuel and the efficiency of the bum. In this
embodiment it is
assumed that only SON, NOR, H20, C02, HCI, and HF are formed in the boiler
feeding the reactor
system described in FIG. 49. In between generation and release of the flue gas
5402, a
slipstream of that flue gas stream (some or all) is redirected into a
pollution abatement or
scrubber system, e.g., Sections 2-4.
[0311] Section 2 includes a gas liquid contactor modular assembly 5408 which
performs
as the scrubber or reactor according to various embodiments of the invention.
This section
68
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
removes SO2, HCI, HF, and some NO2 from the flue gas when using the chemistry
described in
FIG. 49. The removal efficiency of CO2 from this flue gas stream and chemistry
described in
FIG. 49 can be marginal; it can be improved dramatically depending on the
chemistry used and
pH of the sorbent, but for this embodiment, it will be assumed that CO2 is not
captured to a large
extent. The contactor 5408 captures SO2, HCI, and HF. The fluid comprising the
liquid of the
liquid jet is an aqueous solution. That solution, with gases entrained, is
captured in a catch tank
5410. In Section 2, the capture fluid is recirculated using a recirculation
pump through PP6. A
slipstream of this recirculated fluid is drawn off through process valve 5418
for secondary
processing in Section 4.
[0312] Section 3 is the sorbent and solvent make up fluid section which is
configured to
adjust chemical activity, liquid pH, or replenish reactants. Any liquid lost
through evaporation is
replenished with a local water source 5412 through PP7. The liquid is
established with relatively
high pH (above 7), and is maintained at that level by a NaOH source 5414
through PP8 or, once
concentrations of soluble sulfite reach a more steady state, pH is maintained
by addition of lime
5416 in Section 4.
[0313] Section 4 is the secondary processing section where dissolved or
reacted gas
phase molecules are chemically converted or mineralized to solid products,
e.g., CaS04, solid
waste, e.g., CaS03, or other useful products, e.g., fertilizer/NH4SO4 or
NH4NO3. Process liquid
is drawn from Section 2 through PP12 into a precipitation tank 5420 where
lime/Ca(OH)2 5416
is added at PP 15 both to increase pH and to provide Ca 2+ for
reaction/precipitation with 5032- (or
in fully oxidized mode, SO42-). The resulting mix of CaSO3 flows through PP16
into a settling
tank 5422 and is stored after settling in a slurry holding tank 5424. Once a
sufficient amount of
CaS03 has been captured, it is moved to a filter press 5426 to remove the
liquid, which moves
through PP29 to a brine holding tank 5428. The solids 5430 from the filter
press 5426 are
disposed of either by landfill or sale for tertiary process, e.g., CaS04 for
gypsum/sheetrock.
Liquid from the brine tank 5428 is moved through PP26 for recycle and
regeneration in a
softening step 5432 to remove excess Ca2+ and replenish Na+ through addition
of soda ash
(Na2CO3) 5434. The regenerated sorbent is then sent back through PP33 to
Section 2 via a
second process valve 5436.
[0314] In the specific embodiment shown in FIG. 49 a 20 MW system takes a
portion of
the flue gas from a 140 MW coal fired power plant. The flue gas is generated
by burning low
sulfur coal, e.g., coal from Powder River Basin (Wyoming). Specific attributes
considered in
this embodiment are shown in Table 12. The coal burn produces roughly about
350 to about 400
69
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
ppm of SO2 in the flue gas, a contaminant which is the principal target for
removal in this system
example. Other contaminants include HC1, HF, NOR, and some Hg. This is the
front side of
Section 1, with gas flow rates in this slipstream at roughly 84,000 ACFM as
shown in Process
Point 1 (PP1). The temperature of the incoming flue gas at PP1 is in range
from about 250 F to
about 300 F and comes from the power plant fly ash bag house. The fly ash bag
house removes
the bulk of the fly ash produced by coal burning and also serves to reduce the
temperature to a
consistent range as stated. The concentration of water in this flue gas is in
a range from about
6% to about 7% by mass.
[03151 At PP1, the flue gas slipstream enters into Section 2, the scrubber
section. The
flue gas flows through the gas liquid contactor 5404 at a gas velocity at
roughly 10 m-sec"i. The
gas liquid contactor is described herein. The sorbent liquid being used is
sodium sulfite formed
by the initial start up reaction of NaOH with SO2. A 50% by weight sodium
hydroxide solution
is added to water in the sorbent loop to initially develop and maintain a pH
of roughly 6.5. The
NaOH can be used during continuous operation to maintain this pH at about 6.5,
if necessary. At
start up, water can absorb some SO2, but this quickly leads to a drop in pH
and an acidic
solution. Therefore, the NaOH serves to maintain the roughly neutral pH and
provide Na+ as a
counter ion for S032 The equations below describe the main reactions of
interest in this system,
which results in removal of SO2.
SO2 + H2O - 2H+ + S032" (1)
2NaOH + 2H+ + S032" - 2Na+ + S032" + 2H20 (2)
In steady state operation, it is the sodium sulfite solution which effectively
reacts with SO2,
building up to approximately 0.5M concentration, reacting to form sodium
bisulfite in water.
The chemical equation describing the overall reaction is:
Na2SO3 + SO2 + H2O - 2NaHSO3 (1)
[03161 The flue gas exiting the contactor has been evaporatively cooled
dramatically to a
temperature in the range of about 100 F to about 125 F. A mist eliminator
downstream of the
contactor but inside the module removes excess water. Once the flue gas exits
the gas liquid
contactor region depleted of SO2 (PP2), it is optionally heated with a flue
gas heater 5404 (to
increase temperature well above dew point), passed through an ID Fan 5440 and
released to the
flue gas exhaust stack 5406. Other options for flue gas conditioning include
using a gas to gas
heat exchanger, converting to wet stack configuration, or using power plant
waste steam for
reheating. In some cases, waste heat from the overall sorbent processing
system can be used as it
would be favorable to reheat the flue gas using the hot desorbed gases in a
thermal desorption
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
step coming off the stripper. Continuous operation of the gas liquid contactor
5408 would lead
to a build up of sodium bisulfite and a reduction in SO2 absorption efficiency
unless the SO2
reaction products are removed. As such, a slipstream (PP 12) of the liquid
sorbent recirculation
system in Section 2 is withdrawn on a continuous basis into a secondary
chemical processing
system.
[03171 In that secondary processing system shown in Section 4, SO2 is fully
mineralized,
forming a solid product of calcium sulfite (PP16). The calcium sulfite is
subsequently filtered
(PP18), removing excess water, and appropriately disposed of, e.g., in a
landfill (PP24). Lime
(Ca(OH)2) is used to mineralize the sulfite to a solid precipitate (PP 15),
and also serves to further
maintain pH at appropriate levels as a substitute for further NaOH additions
into the sorbent
loop. The reactions performed in secondary processing are:
2Ca(OH)2 + 4NaHSO3 - (CaS03)2H20 + 2Na2SO3 + 3H2O
Calcium scale issues are avoided by then removing excess calcium in a
"softening" step (PP26)
using standard ion exchange processes utilizing sodium carbonate (water
soluble - PP3 1) to form
calcium carbonate (insoluble at this pH) which is diverted to the filter press
process (PP32). In
this same step, sodium thiosulfate can be added (PP34) to help inhibit
oxidation of the sulfite
(S032) to sulfate (S042-) . The process also serves to reproduce the primary
SO2 capture reagent,
Na2SO3:
CaS03 + Na2CO3 - CaCO3 + Na2SO3
Subsequent to softening and regeneration of the active sorbent chemistry, this
is recycled back to
the main contactor process loop (PP33).
[03181 SO2 and Process Module Removal System
[03191 The process analysis and sizing of the gas liquid contactor scrubber
absorber
system is based on the design parameters in Table 12.
TABLE 12: DESIGN VARIABLES FOR THE 20 MW GLC ABSORBER SYSTEM
Parameters Value
Flue Gas (a)
Flow Rate 84,084 ACFM
Velocity 30-50 ft/s
Residence Time 0.07-0.10 s
Temperature 250-300 OF
Pressure -10 inches w.c.
Ambient Pressure 810 mBar
SOX 400 ppm
71
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
Dew Point S03/H2SO4 179 OF
Liquid Flow
Flow Rate 28,000 gpm
Temperature 107 F
Sorbent SON: dilute NaOH/Na2SO3
Salt Load 14.4%(wt) Na2SO4 H .0 M)
pH 6.5
Pressure Drop
Gas Side < 0.03 psi-ft-1
Liquid Side < 8 psi
Emission Removal Requirements
SON >99%
Absorber Specific Surface Area > 8 cm -1
L/G <330 gal/1000 CFM
[03201 The gas liquid contactor (absorber) is operated in a continuous, steady
state mode
using flat jet spray nozzles in a cross-flow configuration. The absorber
demonstrates high
overall volumetric mass transfer (Kca - 64 s"I) that maximizes SO2 removal
efficiency with
minimal use of water, reactor volume, pressure drop and contact time.
[03211 The absorber exhibits low gas and liquid side pressure drops which
translate to
low power consumption. The pressure drop across the liquid jet orifices is
less than about 10 psi,
greatly reducing hydraulic power requirements for operation. A 28,000 gpm
liquid pump for
circulating the solvent constitutes the bulk of the power consumption in the
absorber loop. The
power draw, P (kW) = [.75 x Flow rate (gpm) x AP]/[1714 x pump efficiency)] is
about 150kW
(or about power draw at 20 MWe) for about 8 psi pressure drop and pump
efficiency of about
65% at about 28,000 gpm. Gas side pressure drop in the flat jet system is
small at about 0.1 psi
(2.7 in w.c.) across about 3.3 ft of jet pack.
[03221 In comparison, an average pressure drop for a packed tower of the
related art is
about 1.0 inch H2O per foot of packing, or about 10 inches H2O for a typical
10 foot absorber
bed. There is reduced power consumption of the gas liquid contactor as
compared to
conventional technology, the absorber can be run with higher L/G ratios (330
gal/1000 ACFM)
and thus higher removal efficiencies than conventional absorbers (L/G 90 -
130).
[03231 The absorber system captures not only targeted pollutants from the flue
gas, such
as SOX, NON, and particulate matter, but also heavy metals, chlorides, and
fluorides. The
particulate matter is mostly fly ash carried over from the bag house that is
2.5 m or less. The
metals and halides derive from the coal and will depend on the particular coal
burned. All these
72
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
constituents are removed from the absorption loop in the solvent processing
system. The molar
flow rate of all constituents from the flue gas into the absorber loop is
equal to the molar flow
rate of these pollutants out of the absorber loop in the solvent process
system, and then out in the
solids and brine streams. The concentrations of all constituents in the
absorber loop reach steady
state.
[0324] Gas solubility, solvent temperature and pH also play a key role in
pollutant
absorption. The system of this embodiment operates at relatively low liquid
temperatures, e.g.,
100 F to about 125 F for optimizing gas solubility and minimizing solvent
evaporation. The
equilibrium solubility (300 K) between a pollutant in the gas phase, pa, and
in the aqueous
phase, Ca, is governed by Henry's coefficient, KH = Ca/Pa-
S02 (g) 4 H2SO3, KH = 1.4M/atm (1)
H2SO3 4 H+ + HS03 KI = 0.014 M (2)
HS03" 4 H+ + S032" , K2 = 7.1 x 10-8 M (3)
HS03" +'/202 - SO42" + H+ , k > 106 M-1 s-1 (4)
[0325] For discussion purposes, S(IV) is the sum of all forms of sulfur in the
+4
oxidation state, [S(IV)]tot = [SO2] + [HS03"] + [S032"], and S(VI) is the sum
of all forms of sulfur
in the +6 oxidation state, [S(VI)]tot = [SO3] + [HS04"] + [S042"]. As S02 gas
is dissolved into the
water, the solute is transformed to bisulfite (HS03") and sulfite (S032")
products according to the
equilibria governed by KH, KI and K2. The process is pH dependent given the H+
product
formation. As more H+ is formed (lowering the pH) the equilibrium shifts back
to reactant
formation. At pH values less than 3.5, a significant amount of SO2 off-gases
from the solvent.
To optimize S02 removal efficiency and cost, a steady state addition of sodium
hydroxide or
other chemical base is injected into the solvent loop to keep the pH of the
system near about 6-7:
S02(g) +'/202(g) + 2NaOH(aq) 4 Na2SO4(aq) + H2O (1)
[0326] Sulfite Oxidation System
[0327] The forced oxidation of sulfite to sulfate can be accomplished with a
simple air
sparger (using a 14kW air compressor) in a separate tank. Although a sparger
is simple, the gas
liquid contact efficiency is low so the flow rate of air is set to about 3
times the stoichiometric
amount required to complete oxidation. Assuming 100% oxidation, about 533
lb/hr of sulfite is
oxidized to sulfate. For a full scale system, an additional high efficiency
gas liquid contactor to
replace the sparger may be cost effective.
[0328] Solvent Processing System
73
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[03291 If running the system in a fully oxidized mode, that is with the
majority of sulfur
as sulfate (S042"), the design criteria for sulfate precipitation and removal
are presented in Table
13. The system is designed for <50 ppm HS03 and 14.4% S042" in the liquid in
steady state
operation. The flow rate of the solvent process stream depends directly on the
steady state loop
concentration of SOX and determines the size requirements for the design of
the solvent process
system.
TABLE 13: DESIGN CRITERIA FOR SULFATE REMOVAL
Parameter Value
Reagent Addition Rate Ca(OH)2 at 233 lb/hr
pH >10
Percent SO2 Processed >99%
S02 Process Rate 3.1 lb-mol/hr
Brine Stream Flow Rate <0.7 GPM
[03301 Advanced Design, Sorbent and Process Options.
[03311 Alternate sorbents are shown in Table 14 and include sodium hydroxide,
ammonia, sodium carbonate, magnesium hydroxide, calcium hydroxide, limestone
(calcium
carbonate), and possibly fly ash. Each sorbent requires a particular sorbent
processing system
and each power generating site may have particular solid and liquid disposal
requirements.
TABLE 14: ADVANCED DESIGN, SOLVENT AND PROCESS OPTIONS
Option Approach Benefit
Design - NH3/Ca dual loop with in situ - Possible enhanced absorption of NOx
oxidation of sulfites using air - Expensive NH3 is recycled
and/or chemical oxidizers with
gas liquid contactor - 100% separation of NH3 and Ca in precipitation
step.
- NH3 is onsite for CO2 absorption
Solvent - NH3 for SOx, NOx capture
- CaO/Ca(OH)2 for SOX capture - > 3X reduction in solvent cost
Process - Operate absorber close to S(IV) - Reduced water usage
solubility limits
-On site, inexpensive additive for sulfate
- Use fly ash as precipitating precipitation
agent for sulfate
- Reduced water usage, use inexpensive CaO or
- Use dual loop approach for fly ash to precipitate sulfates, generate gypsum
74
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
S(VI) precipitation product stream
[03321 Choosing a sorbent / sorbent processing / disposal system is driven by
performance, reagent cost and site byproduct disposal requirements. A
comparison of the most
commonly used reagents for S02 removal in packed towers with respect to
reactivity and
operating cost are shown in Table 15.
TABLE 15: COMPARISON OF REACTANT REACTIVITY AND COST
Reactant Reactivity Cost
Caustic (e.g., NaOH, KOH) Highest Highest
Ammonia (NH3) Highest Highest
Soda Ash (Na2CO3) Very Moderate
Magnesium Hydroxide (MgO) Moderate Moderate
Lime (CaO/Ca(OH)2) Less Low
Limestone (CaCO3) Least Lowest
[03331 Caustic and ammonia based systems offer the highest reactivity and
potential for
deep SO2 removal but at the detriment of higher cost. The cost of these
reactants can be
significantly offset by dual loop operation where the solvent is recycled back
to the absorber. Of
all the possibilities the most promising are the NaOHICa(OH), NaCO/Ca(OH),
NH/Ca(OH) dual
loops. The fly ash option presents the possibility of zero reagent cost but
also presents the
highest risk.
[03341 Although the Na/Ca dual loop can be preferable, the NHICa dual loop is
also a
viable alternative. It retains the advantages of a very reactive and soluble
sorbent, clear
contacting solution, and a sorbent processing loop that recycles the
relatively expensive
ammonia. The advantage of ammonia over sodium is that the precipitation step
separates the
ammonia as a gas, so the return loop contains no calcium or other contaminants
that may scale
the absorber. In addition, if the gas liquid contactor system of embodiments
of the invention is
combined with a CO2 absorption system, the ammonia may be used for both
because ammonia is
volatile and an additional (small) scrubber unit is placed in the flue gas
vent line to prevent
ammonia slip to the stack.
[03351 Fly ash shows promise as an FGD absorption agent because of its
alkaline nature
and ready availability. Table 16 lists the typical composition of Class C fly
ash (sub-
bituminous). Early attempts at combining FGD with fly ash capture, however,
were met with
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
difficulty because of fouling downstream and handling characteristics of the
FGD slurry in Gas
purification, Kohl, et al., Gulf Professional Publishing, 5 ed., (1997), which
is hereby
incorporated by reference. However, careful preparation of the fly ash (e.g.,
at optimized SO2-
CaO/MgO stoichiometric levels) to avoid cementitious reactions inside the gas
liquid contactor
itself may enable the operation of the contactor at conditions where the fly
ash does not foul
scrubber operation. Alternatively, these same reactions with Ca/Mg can be
desired, and as such
can be used to produce cementitious material in the sorbent processing area as
a commercial
product. Additionally, if commercial gypsum is a desired byproduct then a
solvent processing
system and disposal scheme for separating fly ash from gypsum is required.
TABLE 16: CLASS C FLY ASH COMPOSITION (ASTM SPECIFICATION C 618)
Component Weight %
SO3 0.23 - 3
CaO 17 - 32
MgO 4 - 12.5
Si02 25 - 42
A1203 14 - 21
Fe203 5-10
Available Alkalies 0- 8
[03361 System Summary
[03371 The Na/Ca dual alkali contactor process and system offers advantages
over
conventional systems for its high techno-economic performance. Table 17
summarizes the key
baseline performance parameters for the contactor system in this embodiment
for the 20MW
case and a generalized (per MW) system.
TABLE 17: SUMMARY OF THE KEY OPERATIONAL PARAMETERS AND VALUES
Parameter 20 MW Contactor Value Generalized Contactor
(per MW)
SOX Removal Efficiency 99+% 99+%
S(IV) Concentration in incoming flue gas 400 ppm 200-4000 ppm
Solvent Loop pH 6 6
Solvent Loop Temperature 107 F 107 F
Solvent Loop Flow Rate 28000 GPM 1400 GPM/MW
Water Input to Scrubber 12 GPM 0.6 GPM/MW
76
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
50 wt% NaOH Input to Scrubber 0.07 GPM 0.003 GPM/MW
0.66 TPD 0.033 TPD/MW
Ca(OH)2 (solid) input to precipitator 2.80 TPD 0.14 TPD/MW
Waste Water Treatment Stream 40 GPM 2 GPM/MW
Precipitate Supernatant to Scrubber 39 GPM 2 GPM/MW
Solid Gypsum (CaS04 ' 2H2O) Waste 6.5 TPD 0.32 TPD/MW
Brine Stream <1 GPM <0.05 GPM/MW
Parasitic Power 1.3% 0.8%
[03381 Deep SO2 removal requires rapid and efficient mass transfer kinetics
and is met
by the use of NaOH. Although calcium hydroxide/carbonate systems are low cost
solvents, they
are also low reactivity solvents. To enhance reactivity the lime/limestone
based absorber is run
with slurries (solids). However, solids are prone to scaling absorber surfaces
(via calcium
sulfite/sulfate formation) and in some instances may even hinder mass
transfer. Significant cost
reductions using the Na/Ca approach are achieved since sodium (NaOH) is
reclaimed in the
solvent processing loop. The operating cost for a dual loop system is equal to
or less than for a
single loop limestone system, particularly for high sulfur fuels as in Gas
purification, Kohl, et
al., Gulf Professional Publishing, 5 ed., (1997), which is hereby incorporated
by reference as if
fully setforth herein. The least expensive reagent is calcium (Ca(OH)2) and is
used to precipitate
gypsum, a commercial byproduct. The process advantages of the dual loop system
are that it can
handle higher sulfur loads, the contact liquid is non-erosive, and it can
enable operation of a
much more efficient gas liquid contactor. The draw backs are increased
complexity and the
requirement for two reagents. By contrast, in a single loop wet limestone FGD
process all the
required steps are carried out in a single vessel, the required steps being
dissolving the limestone
as calcium carbonate, gas liquid contact to absorb the SO2, the reaction with
calcium, oxidation,
and precipitation. This results in a single, relatively simple system. The
drawback is the
corrosive/abrasive nature of the slurry requires exotic nozzle material and
the poor efficiency of
the spray tower requires a large contact area and thus a large tower.
[03391 The parasitic power for the major equipment components for a 20 MW and
greater than 200 MW contactor systems described here are summarized in Table
18. In these
embodiments, it is assumed that the flue gas exhaust blowers (ID fans) are
already in place and
thus not counted. The bulk of the power draws are tied to the solvent
recirculation pumps. The
contactor system operates at low liquid side hydraulic and mechanical power
due to the large
77
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
(>1OX conventional spray nozzle) flat jet orifice area. In this embodiment,
the 20 MW liquid
pumps (28,000 GPM) described draw considerable power from the system due to
moderate
pump efficiency (-65%). Larger liquid pumps (> 100,000 GPM) that can be used
in full scale
operations (>200MW) would be significantly more efficient (-85%) and thus
enable
considerably (1.7X) lower parasitic power loads.
TABLE 18: PARASITIC POWER LOADS FOR GLC SYSTEM
Equipment Parasitic Power 20 Parasitic Power
Component MW Unit >200 MW System (MWp/MWe)
(MWp/MWe)
Liquid Pumps 0.012 0.007
Solvent Supply 0.0001 0.0001
Pumps
Waste Processing 0.0003 (est.) 0.0003 (est.)
Brine stream dryer 0.001 0.001
% Parasitic Power 1.3 0.8
[03401 Gas liquid Contactor for CO2 Process
[03411 These advantages describe an advanced cost and energy savings process
incorporating the compact low cost, low pressure drop and highly energy
efficient scrubber
system to meet environmental goals of CO2 removal efficiency (>90%) and cost
of energy
(<20%). The three possible absorption/regeneration reactions using ammonia
solutions are
given in Fuel Processing Technology, Yeh, et al., Vol. 86, Issues 14-15, pp.
1533-1546, October
2005, which is hereby incorporated by reference.
2NH3 (aq)+ CO2(g)+ H2O 4 (NH4)2CO3(q, delta H,=-24.1 kcal/mole (-986 BTU/lb
C02) (1)
NH3(1) + C02(g) + H2O 4 NH4HCO3(q, delta H, = -15.3 kcal/mole (-622 BTU/lb
C02) (2)
(NH4)2C03(1)+ C02+H20 -2NH4HCO3(q, deltaHr = -6.4 kcal/mol (-262 BTU/lb C02)
(3)
[03421 The reactions are written for absorption and are thus exothermic. The
most
energy efficient route to CO2 capture and solvent regeneration is the
carbonate/bicarbonate
reaction in Equation (1). Since the absorption reaction is favored at low
temperature the
scrubber liquor is chilled to 90 F and the stripper liquor is heated to 140
F to release the CO2
gas at 1 atm. The ammonium carbonate/bicarbonate chemistry offers the
potential for significant
lower operating costs compared to alkanolamine-based solvents because its
regeneration energy
is less than half that of MEA.
[03431 Gas Liquid Contactor Process Flow Diagram and Analysis For CO2
78
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[0344] FIG. 50 is a process flow diagram from a pollutant removal system
according to
another embodiment. The flue gas first enters the contactor to remove
pollutants, e.g., SOX, NO,,
and particulate matter. It then enters the CO2 absorber where it is contacted
with, for example,
chilled ammonium carbonate solution or piperazine and a significant portion of
the CO2 is
captured as ammonium bicarbonate or as the carbamate of piperazine,
respectively. Other
amines, alkanolamines, and/or bases (e.g., KOH, NaOH, etc.) can also be
utilized in this loop. If
the chemistry system used is ammonium hydroxide/ammonium carbonate system is
used, some
ammonia enters the flue gas as ammonia slip, is carried to the ammonia
scrubber and removed.
[0345] The clean flue gas continues to the condensing heat exchanger to be
heated about
40 F above its dew point and then exits through the stack. The ammonium
carbonate/bicarbonate absorber stream is re-circulated back through a chiller
and heat pump
before returning to the scrubber. A side stream of the absorber solution is
removed, heated, and
sent to the stripper to release the captured CO2. The lean solution returns to
the absorber loop.
The stripped CO2 carries some water vapor and ammonia, which are removed in
the condensing
heat exchanger and during compression. The water and ammonia is returned to
the absorber
loop. The clean CO2 is sent to the compressor train and sequestered. A key
energy saving
process step is using a heat pump to cool the ammonium carbonate absorber
solution and reject
that heat to the stripper liquid to raise its temperature for separating the
CO2. Using a heat pump
to capture the energy in the absorber stream and transfer it to the stripper
stream saves about
10% parasitic power.
[0346] The design criteria for sizing and analyzing the system are shown in
Table 20.
The process is divided into six primary process Sections as shown in FIG. 50
including Section 1
the flue gas conditioning system, Section 2 the CO2 absorber loop, Section 3
the CO2 stripper
loop, Section 4 the ammonia or amine slip absorber loop, 5 the chiller system,
and Section 6 the
CO2 compressor train. All flow rates and heat loads are calculated for a 20 MW
demonstration
system.
TABLE 19: DESIGN CRITERIA FOR A 20 MW CO2 GAS LIQUID CONTACTOR
Parameters Value
Flue Gas
Flow Rate 84,084 ACFM
Velocity 58.8 ft/s
Residence Time .07s
Temperature 110F
Pressure -10 inches w.c.
79
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
Ambient Pressure 810 mBar
CO2 10-15%
Liquid Flow
Flow Rate 28,000 gpm
Temperature 90F
Sorbent 3M Ammonium Carbonate
Solids Loading none
pH 9
Pressure Drop
Gas Side <.03/ft psi
Liquid Side < 8 psi
Emission Removal Requirements
CO2 >90%
Absorber Specific Surface Area > 8 cm -1
L/G 330 gal/1000 CF
Parasitic Power < 20 %
Commercial By- Product CO2
[0347] Flue Gas Conditioning System
[0348] In this embodiment as shown in Fig. 50, the flue gas conditioning
system includes
an inlet 5002 of flue gas into an optional heat exchanger/chiller 5004 and an
outlet 5006. The
flue gas conditioning system receives an inlet gas which may have already seen
some processing,
e.g., processing to remove acid gases such as SO2, HCI, and the like. The heat
exchanger/chiller
5004 is optional as it depends on the inlet gas constituents and absorber
chemistry as known in
the art, e.g., ammonia/ammonia carbonate would require a chiller. The flue gas
has been cooled
and scrubbed of SO2, e.g., from a contactor system (not shown) according to an
embodiment of
the invention. In this embodiment, the flue gas 5002 contains contaminates
such as C02, N2,
H2O, 02 and other trace gases.
[0349] CO2 Absorber Loop
[0350] Referring to Section 2, the absorber loop includes gas liquid contactor
5008 and a
catch tank 5010. A heat exchanger/chiller 5012 of section 5 is an optional
component. Again,
the heat exchanger/chiller 5010 is optional as it depends on the inlet gas
constituents and
absorber chemistry as would be known to one of skill in the art. In this
embodiment, the gas
liquid contactor is coupled to an outlet 5006 of the Section 1. The gas liquid
contactor 5008 is
coupled to a catch tank 5010 and to a heat exchanger/chiller 5012 (Section 5)
as part of recycle
loop.
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[03511 In operation, the flue gas containing CO2 is directed through an inlet
5014 of the
gas liquid contactor 5008 and stripped of a portion of the CO2. The CO2
absorber loop of section
2 includes various values and pumps as required for appropriate flow and
recycle of the liquid
and operation as known in the art. After contacting the gas in the contactor
5008, the absorber
solution now carries an additional an amount of CO2 to catch tank 5010 as part
of the
recirculation loop.
[03521 In this embodiment, the energy requirements of the CO2 absorber loop
are
handled by the heat exchanger/chiller 5012 of Section 5. The general chemistry
described here,
using ammonium carbonate, amines, or alkanol amines, absorbs CO2 more
preferably when
chilled below the flue gas temperatures seen in typical systems. Therefore, if
required, the
Section 5 heat exchanger/chiller 5012 provides that cooling capacity to
maintain optimal
operating conditions of the absorber solution.
[03531 CO2 Stripper Loop
[03541 Referring to Section 3 includes an inlet 5016 coupled to a heat
exchanger/chiller
5018 having an outlet 5020. The outlet 5020 is coupled to a gas liquid
contactor 5022. The gas
liquid contactor 5022 has an outlet 5024 coupled to a catch tank 5023 with a
recycle loop. The
gas liquid contactor 5022 is configured to remove the captured CO2 from the
absorber solution.
CO2 stripping can be accomplished by a number of means, including pressure
swings, pH
adjustment, or by heating the C02/absorber solution. The components of Section
3 can vary
depending on the methodology chosen. In any case, it is advantageous to
capture the absorber
subsequent to release of the CO2 and recirculate that absorber liquid back to
the main absorber
loop in Section 2. An output of Section 3 is sent to stack 5034.
[03551 NH31PZAbsorber Loop
[03561 The ammonia or amineabsorber loop, Section 4, is designed to capture
the ammonia
or amine slip in the flue gas after it leaves the CO2 absorber 5008. This may
be a particular issue
with NH3, depending on the temperature of the absorber solution (colder leads
to less slip). The
NH31PZAbsorber Loop includes a gas liquid contactor 5026 coupled to the inlet
5024. The gas
liquid contactor 5026 includes an outlet 5028 coupled to catch tank 5030,
recycle loop, and an
outlet 5032. If an amine, such as piperazine or alkanol amines, are used as
the absorber solution,
there is less need for Section 4, thus implementation of such a section would
be determined
through examination of the overall process requirements and temperatures. This
Section 4 is
optional as smaller molecular weight amines may slip and it may be
advantageous to capture while
81
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
higher molecular weight amines may not slip and therefore, they may not need
to be processed.
The output 5032 may directed to a flue gas stack 5034.
10357] CO2 Compressor Train
10358] Section 6 describes the process area for stripping CO2 from the
absorber solution.
The CO2 Compressor Train a compressor 5036 coupled to the gas liquid contactor
5022. This
section is configured capture and pressurize pure CO2 coming of the gas liquid
contactor 5022.
That is, subsequent to that section, in order to be transportable in a
convenient manner for
secondary industrial uses, Enhanced Oil Recovery (EOR), or sequestration,
secondary steps are
desired. One of these options might include that the CO2 is condensed in a
compressor train and
sequestered at super critical pressure forming liquid CO2 which can be
transported by truck or
pipeline to its end application.
10359] Other Solvent Systems for CO2 Capture
10360] In Sections 2 and 3 of FIG. 50 various sorbents may be used for CO2
capture
and/or stripping. The sorbents may include ammonia carbonate based solvent
selected as the
baseline solvent. However, the system can also be designed to operate with a
wide variety of
post combustion wet scrubbing solvents including amines such as ammonia,
diethanolamine
(DEA), and monoethanolamine (MEA), and advanced solvent systems such as
promoted
carbonates, piperazine, tertiary and hindered amines like methyldiethanolamine
(MDEA) and 2-
aminomethylpropanolamine (AMP), metal organic frameworks and molecular
encapsulation.
10361] Embodiments of the CO2 system presents several strengths and advantages
for
post combustion CO2 capture. Very large contact surface area is available in a
small contactor
volume. This translates into economic savings in two areas, the small
footprint required
translates to small capital cost and low gas and liquid side pressure drops
mean low operating
cost. Low capital and operating cost increase the range of possible CO2
sorbents. For instance,
if an inexpensive sorbent has a slow reaction rate and requires a large
contact area, such as
seawater or deep brine aquifers, it may still be economically feasible in the
CO2 gas liquid
contactor system whereas it might not be normally considered in a standard gas
liquid contactor
such as a bubble column or spray tower.
10362] In the most mature CO2 capture systems for flue gas, alkanolamines and
ammonium carbonate/bicarbonate (AC/ABC), the largest energy consumption is
related to the
heat of reaction. The energy associated with the AC/ABC reaction consumes
almost 70% of the
total energy required to absorb and desorb the CO2. The ABC solution must be
cooled to 55 F
to absorb the CO2 and heated to 265 F to release it in the stripper. Options
to reduce the energy
82
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
required are twofold: find a system with smaller heat of reaction to absorb
and desorb CO2 or
transfer the heat released during absorption to the desorption reaction.
Ammonium
carbonate/bicarbonate is currently the lowest energy chemical sorbent.
Physical solvents require
almost no energy for regeneration, but operate best at high pressure. Membrane
systems transfer
the energy of absorption to the desorption process, but are currently applied
only to small
systems. The following paragraphs describe the classification and description
of alternate
processes that could be applied to the capture of CO2 from flue gas.
[03631 In exploring alternate CO2 absorption systems, the sorbent must be
matched to the
technology. Three different post combustion absorption technologies are being
developed: gas
liquid contactor, dry contact systems, and membrane contact systems. The gas
liquid contactor
system requires a liquid sorbent. A gas liquid system separates the absorption
and desorption
into two separate process steps in two different vessels at different
pressures and temperatures.
This system generally requires expenditure of energy at both steps, cooling
for the exothermic
absorption, and heating for the endothermic desorption. The process requires a
temperature
and/or pressure swing, which is energy intensive. The dry regenerable sorbent
system using
sodium bicarbonate operates the same as the gas liquid contactor, except the
phase of the sorbent
is solid.
[03641 Membrane systems, however, are fundamentally different. In a membrane
system
a membrane is a very thin wall of permeable material that separates the two
streams and can be a
solid or a liquid held in a sponge-like material. Membranes are designed to
select for the gas to
be separated. Whether the membrane material is solid or liquid, the membrane
absorbs CO2 on
the concentrated side and transports it to the dilute side where the CO2 is
desorbed. The driving
force is the concentration gradient of CO2 across the very thin membrane. The
beauty of the
membrane system is that absorption and desorption are carried out in the same
vessel, at the
same temperature, at almost the same pressure. Because absorption and
desorption are carried
out within a few microns of each other in a very thin membrane, the energy of
absorption is
transferred to the desorption reaction at constant temperature. Thus the
change in entropy is zero
and the net energy required is zero. The liquid in the membrane can be
tailored to selectively
transport CO2. The drawbacks are manufacturing cost, membrane life, the
requirement for very
clean flue gas, and the enormous contact area required (almost a million
square meters for a full-
up commercial system). Several hundred thousand ACFM of flue gas traveling in
a duct of up to
100m2 cross section must be channeled into billions of fibers with cross
sections of each fiber of
60 nm2.
83
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[0365] Alternate solvent systems applicable to post combustion absorption in a
gas liquid
contactor are chemical and physical. Wet chemical sorbents include amines,
carbonates,
promoters, hybrid, and pH swing. Wet physical sorbents include metal organic
frameworks,
ionic liquids, seawater, and saline groundwater. Glycols are not discussed
because they are high
pressure systems and more applicable to pre-combustion absorption. Selexol is
an example of a
commercialized glycol system currently used to clean natural gas, a high
pressure process. Each
wet sorbent is discussed below as it applies to the CO2 gas liquid contactor.
[0366] Aqueous amines are the current state-of-the-art technology for CO2
capture for
power plants as recognized by those skilled in the art. Amine sorbents include
ammonia (NH3),
monoethanolamine (MEA), methyldiethanolamine (MDEA), 2-minomethylpropanolamine
(AMP), PZ piperazine (PZ), and others. All react with CO2 initially to form
the amine carbamate
(CO2 + 2RNH2 H RNH2COO H RNHCOOH). In addition amine and water can react with
CO2
to produce the amine bicarbonate (RNH2 + H2O + CO2 H RNH3+ + HC03").
Absorption/desorption can make use of the lowest energy reaction, bicarbonate
H carbonate.
All amine systems require a gas liquid contactor and a stripper. The benefit
of the system
described here is the very efficient gas liquid contactor. Although it would
benefit any amine
system, the ammonium carbonate/bicarbonate sorbent was chosen because ammonia
is less
expensive and the reaction energy for ammonia is less than alkonalamines like
MEA.
[0367] Alkali carbonates include Na, K, and Ca, carbonates/bicarbonates.
Although
alkali carbonates were used extensively in the early 1900s for ambient
temperature and pressure
absorption of C02, they have been replaced by more efficient alkanolamines.
Since the rate of
absorption of CO2 into aqueous solutions is normally slow, promoters
(catalysts or enzymes) are
often added to increase the rate.
[0368] Examples of promoters are formaldehyde, MEA, DEA, glycine, and carbonic
anhydrase in Gas purification, Kohl, et al., Gulf Professional Publishing, 5
ed., (1997), which is
hereby incorporated by reference. The fastest catalyst available for
absorption of CO2 is the
enzyme carbonic anhydrase as described in MC Trachtenberg, L Bao, SL Goldman.,
2004,
Seventh Int. Conf. On Greenhouse Gas Control Technologies (GHGT-7), Vancouver,
BC, which
is hereby incorporated by reference. Amino acids will also promote the
absorption of CO2 as
well or better than MEA or DEA as described in C02 capture from flue gas using
amino acid
salt solutions, Jacco van Holst, Patricia. P. Politiek, John P. M. Niederer,
Geert F. Versteeg,
Proceedings of 8th International Conference on Greenhouse Gas Control
Technologies, 2006,
which is hereby incorporated by reference. Although enzymes and catalysts do
not change the
84
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
energy of a reaction or its equilibrium point, they do lower the activation
energy and can increase
the reaction rate by several orders of magnitude. The hydrolysis of CO2 in
water and subsequent
reaction to bicarbonate is quite slow. The effect of rate increase is to
decrease residence time
required for contact, and thus decrease required contact area. However,
because it is a biological
enzyme, CA is sensitive to temperature and not applicable to a temperature
swing process with a
high desorption temperature. That means a pressure swing process is required.
Since the partial
pressure of CO2 in flue gas is about 0.15 atm, the desorption total pressure
must be substantially
less than 0.1 atm if pure CO2 is to be captured. The other alternative for CA
is to pressurize all
the flue gas before contact. Other promoters, such as DEA, are currently used
at high
temperature and pressure in the hot potassium carbonate process.
[03691 Hybrid sorbents use a combination of sorbentsand carbonate plus an
amine. A
current example is K2CO3 / PZ, an aqueous potassium carbonate solution
promoted by piperazine
(PZ) that is expected to use less energy than MEA. This system is currently
being investigated at
the University of Texas at Austin. They found the rate of absorption and
loading of CO2 are
significantly higher for K2CO3 / PZ than for MEA. In addition, loss and
degradation of PZ are
also significantly less than for MEA as described in Plasynski, et al., Carbon
Dioxide Capture by
Absorption with Potassium Carbonate, Carbon Sequestration, Project Facts,
USDOE, NETL,
April, (2008). The main contribution of the GLC absorber to this system would
be increased
contact efficiency, smaller footprint, and smaller pressure drops resulting in
lower capital and
operating costs.
[03701 The last chemical absorption/desorption system, pH swing, is not
normally
mentioned because the energy cost is very high. CO2 is absorbed with a base
like NaOH and
released with an acid like HCI. The resulting salt is then electrolyzed to
regenerate the acid and
base. The energy input goes into electrochemistry instead of pressure or
temperature swings.
The calculated energy required is much higher than other processes. This
process has been
commercialized, however, for SO2 absorption. Physical sorbents include
glycols, metal organic
frameworks (MOF), ionic liquids, seawater and saline ground water. These do
not depend on a
chemical reaction with the sorbent, but physical absorption. Although there is
no energy
associated with a chemical reaction, the desorption process does require a
change in pressure.
[03711 Glycols work best at high pressure in a pressure swing process, like
the Selexol
process proposed for pre-combustion separation of CO2 in the synthesis gas at
700 psi. The gas
liquid contactor system is not compatible with high pressure absorption
processes.
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
[03721 Metal organic framework (MOF) is a molecular "cage" that can enclose a
tiny
bubble of CO2 gas. MOFs have high selectivity, good absorption/desorption
rates, and high CO2
capacity. They are applicable to gas liquid contactors and liquid membranes.
The risk is high
reagent cost and that they have not been demonstrated in gas liquid
contactors.
[03731 Ionic liquids are organic salts that are liquid at room temperature.
They are not
aqueous solutions. Ionic liquids can absorb both CO2 and SO2 and so have high
potential for flue
gas cleaning. They are applicable to gas liquid contactors and liquid
membranes. Like MOFs,
ionic liquids have been synthesized only at laboratory scale and reagent cost
could be very high.
They have also not been tested in gas liquid contactors.
[03741 Saline ground water or Seawater - Because a favored CO2 sequestration
method
is injection into deep saline aquifers, a possible method for both capture and
sequestration is to
absorb the CO2 with naturally alkaline saline ground water and re-inject it as
a solution. This
entirely avoids the energy required to desorb and compress the CO2 for
injection as a gas.
Depending on the alkalinity of the ground water, the absorption rate may be
slow and require a
large contact area. The very high specific area of the GLC contactor would be
a perfect solution.
In addition, even though many naturally occurring saline aquifers are
naturally abundant in Ca
and/or Mg, the alkalinity of the ground water could be enhanced with lime for
an optimum
tradeoff between capital and operating costs.
[03751 This process is similar to seawater absorption of either/both CO2 or
SO2 which
makes use of essentially infinite availability of absorbent and disposal (site
dependent, of
course). Additionally, seawater has some level of natural abundance of Ca and
Mg which can
readily form solid precipitates as carbonates or sulfates. This or other
desired ratios can also be
produced artificially using various magnesium and/or calcium salts including
nitrates,
hydroxides, sulfates, carbonates, or halides. Solubilities of these salts vary
dramatically
depending on the starting compound, pH, and temperature of the target
solutions, and would
need to be considered by one skilled in the art regarding what their goals and
target compounds
might be.
[03761 The following table compares the advantages, disadvantages, and
estimated cost
of various CO2 sorbent systems that are applicable to the gas liquid
contactor.
86
CA 02737637 2011-03-18
WO 2010/036436 PCT/US2009/049707
TABLE 20: COMPARISON OF CO2 SORBENT SYSTEMS
Sorbent Advantages Disadvantages Reaction Reagent Absorption
Energy, Cost, Cost,
BTU/lb $/lbmol kWh/ Kg
CO2 CO2
MEA Mature, Expensive +703 for $40 0.53-0.78
commercial carbomate
technology -* MEA +
COQ
Ammonium Under NH3 is hazardous +262 for $5 for N}13 17.3
carbonate / development material 2NH4HCO3
bicarbonate -* CO2 +
(AB/ABC) H2O +
(NH4)2C03
Sodium Simple chemistry sodium recycle +-260 for $16 for 0.41-1.1
Carbonate / costs 2NaHCO3 NaOH
bicarbonate -* CO2 +
H2O +
Na2CO3
Carbonic Increases Insufficient -H-116 for tbd High
Anhydrase absorption rate amount available, HC03" + H+
Temperature -* CO2 +
sensitive, H2O
demonstrated in
membrane only
K2C03 / PZ High absorption Expensive reagent +259 for $40 for < MEA
rate, high loading, (PZ) 2KHCO3 -* K2C03,
Low degradation CO2 + H2O $300 for
+ K2CO3 PZ
pH swing Simple chemistry, Very high energy, +1,083 for $16 for High
Simple stripping, Regeneration of H2O -*H+ + NaOH
Regeneration of acid and base OW $10 For
acid and base is require another HCl
commercialized process island, not
demonstrated,
MOF and High absorption Lab scale only, Tbd high tbd
Ionic Liquids rate, high loading, High risk
Low degradation
Saline No stripping, no Not demonstrated, n/a -0 Potentially
Ground compression site dependent very low
Water or
Seawater
[0377] It will be apparent to those skilled in the art that various
modifications and
variation can be made in the present invention without departing from the
spirit or scope of the
invention. Thus, it is intended that the present invention cover the
modifications and variations
of this invention provided they come within the scope of the appended claims
and their
equivalents.
87