Note: Descriptions are shown in the official language in which they were submitted.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
1
Protease Inhibitors
Field of the invention
This invention relates to inhibitors of cysteine proteases, especially those
of the papain
superfamily. The invention provides novel compounds useful in the prophylaxis
or treatment of
disorders stemming from misbalance of physiological proteases such as
cathepsin K.
Description of the related art
The papain superfamily of cysteine proteases is widely distributed in diverse
species including
mammals, invertebrates, protozoa, plants and bacteria. A number of mammalian
cathepsin
enzymes, including cathepsins B, F, H, K, L, 0 and S, have been ascribed to
this superfamily,
and inappropriate regulation of their activity has been implicated in a number
of metabolic
disorders including arthritis, muscular dystrophy, inflammation,
glomerulonephritis and tumour
invasion. Pathogenic cathepsin like enzymes include the bacterial gingipains,
the malarial
falcipains I, II, III et seq and cysteine proteases from Pneumocystis carinii,
Trypanosoma
cruzei and brucei, Crithidia fusiculata, Schistosoma spp.
The inappropriate regulation of cathepsin K has been implicated in a number of
disorders
including osteoporosis, gingival diseases such as gingivitis and
periodontitis, Paget's disease,
hypercalcaemia of malignancy and metabolic bone disease. In view of its
elevated levels in
chondroclasts of osteoarthritic synovium, cathepsin K is implicated in
diseases characterised
by excessive cartilage or matrix degradation, such as osteoarthritis and
rheumatoid arthritis.
It is likely that treatment of bone and cartilage disorders such as
osteoarthritis and
osteoporosis will require life-long administration of a cathepsin K inhibitor,
often to a patient
population within or nearing the geriatric phase. This places unusually high
requirements on
the ease of administration of drugs intended for such disorders. For example
attempts are
underway to stretch the dosage regimes of the current osteoporosis drugs of
the
bisphosphonate class to weekly or longer administration regimes to aid
compliance. However,
even with improved dosing, other side effects of the bisphosphonates remain.
Bisphosphonates block bone turnover rather than attenuate it as a cathepsin K
inhibitor does.
For healthy bone it is important to maintain the remodelling process which
bisphosphonates
block completely. In addition, bis-phosphonates have a very long half-life in
bone so if effects
such as osteonecrosis of the jaw manifest themselves, it is impossible to
remove the
bisphosphonate from the bone. In contrast, cathepsin K inhibitors typically
have a fast onset
and off rate mode of action, which means that if a problem was to be
identified, dosing could
be halted and there would be no build up of the inhibitor in the bone matrix.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
2
There is thus a desire for alternative osteoporosis and osteoarthritis drugs
with superior
pharmacokinetic and/or pharmacodynamic properties.
International patent application no W02008/007107 discloses compounds of the
formula
Ra Rb
Rc 0
N
Rd H
O
where Rd is a substituted monocyclic ring, Rc is branched alkyl or cycloalkyl
and Ra and Rb
are a variety of groups including H, methyl, ethyl, ether, thioether, amine,
sulphonate etc. The
only compounds which are prepared have H or methoxy at this position.
There remains a need in the art for potent inhibitors of cathepsin K. Of
particular benefit are
inhibitors of cathepsin K which show selectivity for cathepsin K over other
cathepsins (e.g.
selectivity over cathepsin S and/or cathepsin L). Potent inhibitors of
cathepsin K which
demonstrate properties such as high permeability and/or advantageous metabolic
profiles may
be expected to be of great value in a clinical setting. Cathepsin K related
indications such as
osteoporosis or arthritis presuppose protracted periods of administration and
therefore it is
desirable that the compounds have minimal toxicity or genotoxicity.
Brief description of the invention
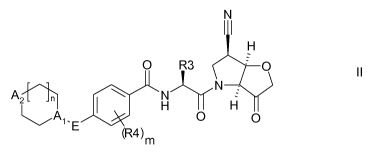
According to the present invention, there is provided a compound of formula
II:
N
H
O R3 = O
A2'[ 1 H H
Al-E O O II
(R4) m
wherein
R3 is C1-C3 alkyl or C3-C6 cycloalkyl, either of which is optionally
substituted with one or
two methyl and/or a fluoro, trifluoromethyl or methoxy, when R3 is C3-C6
cycloalkyl it
may alternatively be gem subsituted with fluoro;
R4 is methyl or fluoro; m is 0, 1 or 2;
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
3
E is a bond, or thiazolyl, optionally substituted with methyl or fluoro;
A, is CH or N,
A2 is CR6R' or NR6, provided at least one of A, and A2 comprises N;
R6 is H, C1-C4 alkyl, C1-C4 haloalkyl, C1-C3 alkyl-O-C,-C3 alkyl, or when A2
is C, R6 can
also be C1-C4 alkoxy or F;
R7 is H, C1-C4 alkyl or F
or a pharmaceutically acceptable salt, N-oxide or hydrate thereof.
It will be appreciated that the compounds of the invention can exist as
hydrates, such as those
of the partial formulae:
H H
O O
bN. N
H 4
0 HO OH
and the invention extends to all such alternative forms.
Suitably R3 is C1-C3 alkyl or C3-C6 cycloalkyl, either of which is optionally
substituted with one
or two methyl and/or a fluoro, trifluoromethyl or methoxy.
Representative values for cycloalkyl for R3 include cyclopropyl, cyclobutyl
and especially
cyclopentyl or cyclohexyl, any of which being substitued with fluoro or gem
fluoro. Gem-fluoro
at the 2 position of a cyclopropyl, the 3 position of cyclobutyl or
cyclopentyl or the 4 position of
cyclopropyl is often convenient. Gem-fluoro at the 4 position of cyclohexyl is
also often
convenient.
In one embodiment of the invention R3 represents the side chain of leucine. In
a second
embodiment of the invention R3 represents the side chain of isoleucine. In a
third embodiment
of the invention R3 represents the side chain of cyclohexylglycine. In a
fourth embodiment of
the invention R3 represents the side chain of cyclopentylglycine. In a fifth
embodiment of the
invention, R3 represents the side chain of of O-methylthreonine. In a fifth
embodiment of the
invention R3 represents the side chain of 4-fluoroleucine. In a sixth
embodiment of the
invention R3 represents the side chain of 3-methoxyvaline.
Currently preferred values of R3 include those embodied by the partial
structures:
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
4
and especially
In one embodiment of the invention m represents 2. Of particular interest are
compounds
wherein m represents 1. Still further embodiments of the invention have m as
0, especially
when the adjacent thiazolyl is substituted with Me or preferably F.
R4 suitably represents methyl or fluoro, especially fluoro. If m is 2, it is
currently preferred that
each R4 is the same.
When m represents 1, R4 is suitably positioned as shown by the partial
structure:
O
N
H
R4
E is conveniently a bond, that is the unsaturated nitrogen containing ring
bearing Al and A2 is
bonded directly to the para position of the phenyl ring. However, it is
currently preferred that E
is thiazolyl, which is optionally substituted with methyl or more preferably
fluoro. The preferred
orientation of the thiazolyl ring is:
A2'[ J1
Al N
I
S
R4) M
R5
where R5 is H, methyl or fluoro.
The ring containing A, and A2 is a saturated, nitrogen-containing ring of 5 or
6 ring atoms.
Thus n is 0 or 1 and suitably n is 1. Representative rings thus include
pyrrolidin-l-yl,
pyrrolidin-3-yl, piperazin-l-yl, piperidin-4-yl and piperidine-l-yl. The ring
is conveniently
substituted, for example with alkyl or haloalkyl, typically methyl or propyl
or trifluromethyl.
Alternatively the ring is substituted with an ether such as methoxymethyl- or
methoxyethyl-.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
When A2 is carbon, the ring can alternatively be substitued with alkoxy such
as methoxy, or
fluoro, especially gem-fluoro.
A favoured embodiment of the invention has the formula Ila:
N
H
O R3 O J~f N `-
N N
H O H O
R6-NN/ I (R4)m II a
S
R5
5 wherein
R3 is branched C2-C6 alkyl or C3-C6 cycloalkyl, either of which is substituted
with
halo or trifluoromethyl;
R4 is methyl or fluoro; m is 0 or 1 or 2;
R5 is H, methyl or fluoro;
R6 is C1-C4 alkyl;
or a pharmaceutically acceptable salt, N-oxide or hydrate thereof
(collectively referred to
herein as compounds of the invention).
R5 is preferably fluoro, especially when m is 0. The remaining preferments are
as defined
above in relation to Formula II. References to formula II below are understood
to include the
corresponding embodiments of formula Ila.
Representative embodiments of formula II include:
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-carbonyl)-3-methyl-butyl]-
4-[2-(4-
methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-yl)-1 -cyclohexyl-2-oxo-
ethyl]-4-[2-(4-
methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-yl)-1 -cycl open tyl-2-
oxo-eth yl]-4-[2-(4-
methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-carbonyl)-2-methyl-butyl]-
4-[2-(4-
methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-carbonyl)-3-methyl-butyl]-
4-[5-
methyl-2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
6
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-yl)-1 -cyclohexyl-2-oxo-
ethyl]-4-[5-
methyl-2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-yl)-1 -cyclohexyl-2-oxo-
ethyl]-4-[5-
methyl-2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-carbonyl)-2-methyl-butyl]-
4-5-methyl-
2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-carbonyl)-3-methyl-butyl]-
4-[5-fluoro-
2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-yl)-1 -cyclohexyl-2-oxo-
ethyl]-4-[5-
fluoro-2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-yl)-1 -cycl open tyl-2-
oxo-eth yl]-4-[5-
fluoro-2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-carbonyl)-2-methyl-butyl]-
4-[5-fluoro-
2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-carbonyl)-3-methyl-butyl]-
3-fluoro-4-
[2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-yl)-1 -cyclohexyl-2-oxo-
ethyl]-3-
fluoro-4-[2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-yl)-1 -cycl open tyl-2-
oxo-eth yl]-3-
fluoro-4-[2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide;
N-[1 -(6-nitrile-3-oxo-hexahydro-furo[3,2-b]pyrrol-4-carbonyl)-2-methyl-butyl]-
3-fluoro-4-
[2-(4-methyl-piperazin-1 -yl)-thiazol-4-yl]-benzamide
and pharmaceutically acceptable salts, N-oxides and hydrates thereof.
The C,-Cn alkyl definition of R6 or R7 is is meant to include both branched
and unbranched
alkyl moieties containing between one and n carbon atoms in total. Examples of
such R6
groups or R7 are methyl, ethyl, propyl (n-propyl and isopropyl), butyl (n-
butyl, isobutyl, tert-butyl
and sec-butyl). One R6 group of particular interest is methyl. A second R6
group of particular
interest is propyl (especially n-propyl). As R3 is optionally substituted with
one or two methyl
groups, this moiety may also define a branched alkyl chain of up to 5 C atoms.
In some embodiments of the invention A, is N.
Additional aspects of the invention include a pharmaceutical composition
comprising a
compound as defined above and a pharmaceutically acceptable carrier or diluent
therefor.
A further aspect of the invention is the use of a compound as defined above in
the
manufacture of a medicament for the treatment of disorders mediated by
cathepsin K, such as:
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
7
osteoporosis,
gingival diseases (such as gingivitis and periodontitis),
Paget's disease,
hypercalcaemia of malignancy,
metabolic bone disease,
diseases characterised by excessive cartilage or matrix degradation (such as
osteoarthritis and rheumatoid arthritis),
bone cancers including neoplasia,
pain (especially chronic pain).
Additionally provided is a method for the treatment or prevention of a
disorder mediated by
cathepsin K comprising the administration of a safe and effective amount of a
compound of the
invention for the purpose of treating or preventing said disorder which is
mediated by cathepsin
K.
Also provided is a compound of the invention for the treatment or prevention
of a disorder
mediated by cathepsin K.
Further, there is provided as an aspect of the invention novel intermediates
(as described
herein) which may be of use in the preparation of the compounds of the
invention.
In particular there is provided a compound of the formula:
N
H O
OMe
N 'H OMe
H
or an N-protected derivative thereof (e.g. Boc, CBz, or Fmoc-protected). Also
provided by the
invention is the corresponding 1,3-dioxolane protected analogue and N-
protected derivatives
thereof (e.g. Boc- CBz, or Fmoc protected). A further novel intermediate of
the invention is has
the formula:
O
Oz: H
OMe
N OMe
H
or the corresponding 1,3-dioxolane protected analogue, in each case wherein
the N function is
optionally protected with a conventional protecting group such as Boc, CBz or
Fmoc.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
8
The compounds of the invention can form salts which form an additional aspect
of the
invention. Appropriate pharmaceutically acceptable salts of the compounds of
Formula II
include salts of organic acids, especially carboxylic acids, including but not
limited to acetate,
trifluoroacetate, lactate, gluconate, citrate, tartrate, maleate, malate,
pantothenate, isethionate,
adipate, alginate, aspartate, benzoate, butyrate, digluconate, cyclopentanate,
glucoheptanate,
glycerophosphate, oxalate, heptanoate, hexanoate, fumarate, nicotinate,
palmoate, pectinate,
3-phenylpropionate, picrate, pivalate, proprionate, tartrate, lactobionate,
pivolate, camphorate,
undecanoate and succinate, organic sulphonic acids such as methanesulphonate,
ethanesulphonate, 2-hydroxyethane sulphonate, camphorsulphonate, 2-
napthalenesulphonate, benzenesulphonate, p-chlorobenzenesuIphonate and p-
toluenesulphonate; and inorganic acids such as hydrochloride, hydrobromide,
hydroiodide,
sulphate, bisulphate, hemisulphate, thiocyanate, persulphate, phosphoric and
sulphonic acids.
The compounds of the invention may in some cases be isolated as the hydrate.
Hydrates are
typically prepared by recrystallisation from an aqueous/organic solvent
mixture using organic
solvents such as dioxin, tetrahydrofuran or methanol. Hydrates can also be
generated in situ
by administration of the corresponding ketone to a patient.
The N-oxides of compounds of the invention can be prepared by methods known to
those of
ordinary skill in the art. For example, N-oxides can be prepared by treating
an unoxidized form
of the compound of the invention with an oxidizing agent (e.g.,
trifluoroperacetic acid,
permaleic acid, perbenzoic acid, peracetic acid, meta-chloroperoxybenzoic
acid, or the like) in
a suitable inert organic solvent (e.g., a halogenated hydrocarbon such as
dichloromethane) at
approximately 0 C. Alternatively, the N-oxides of the compounds of the
invention can be
prepared from the N-oxide of an appropriate starting material.
Examples of N-oxides of the invention include those with the partial
structures:
0 0
N 0 N
R4N N H R4-N N H
0 ~N~S R3 \N (/ I R3
R5 R5
Compounds of the invention in unoxidized form can be prepared from N-oxides of
the
corresponding compounds of the invention by treating with a reducing agent
(e.g., sulfur, sulfur
dioxide, triphenyl phosphine, lithium borohydride, sodium borohydride,
phosphorus bichloride,
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
9
tribromide, or the like) in an suitable inert organic solvent (e.g.,
acetonitrile, ethanol, aqueous
dioxane, or the like) at 0 to 80 C.
It should be noted that the radical positions on any molecular moiety used in
the definitions
may be anywhere on such moiety as long as it is chemically stable.
Radicals used in the definitions of the variables include all possible isomers
unless otherwise
indicated. For instance butyl includes t-butyl, i-butyl, n-butyl etc.
When any variable occurs more than one time in any constituent, each
definition is
independent.
Unless otherwise mentioned or indicated, the chemical designation of a
compound
encompasses the mixture of all possible stereochemically isomeric forms, which
said
compound may possess. Said mixture may contain all diastereomers and/or
enantiomers of
the basic molecular structure of said compound. All stereochemically isomeric
forms of the
compounds of the present invention both in pure form or mixed with each other
are intended to
be embraced within the scope of the present invention.
Pure stereoisomeric forms of the compounds and intermediates as mentioned
herein are
defined as isomers substantially free of other enantiomeric or diastereomeric
forms of the
same basic molecular structure of said compounds or intermediates. In
particular, the term
"stereoisomerically pure" concerns compounds or intermediates having a
stereoisomeric
excess of at least 80% (i.e. minimum 90% of one isomer and maximum 10% of the
other
possible isomers) up to a stereoisomeric excess of 100% (i.e. 100% of one
isomer and none of
the other), more in particular, compounds or intermediates having a
stereoisomeric excess of
90% up to 100%, even more in particular having a stereoisomeric excess of 94%
up to 100%
and most in particular having a stereoisomeric excess of 97% up to 100%. The
terms
"enantiomerically pure" and "diastereomerically pure" should be understood in
a similar way,
but then having regard to the enantiomeric excess, and the diastereomeric
excess,
respectively, of the mixture in question.
Compounds of the invention can be prepared as their individual stereoisomers
by reacting a
racemic mixture of the compound with an optically active resolving agent to
form a pair of
diastereoisomeric compounds, separating the diastereomers and recovering the
optically pure
enantiomer. While resolution of enantiomers can be carried out using covalent
diasteromeric
derivatives of compounds of Formula II, dissociable complexes are preferred
(e.g., crystalline;
diastereoisomeric salts). Diastereomers have distinct physical properties
(e.g., melting points,
boiling points, solubilities, reactivity, etc.) and can be readily separated
by taking advantage of
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
these dissimilarities. The diastereomers can be separated by chromatography,
for example
HPLC or, preferably, by separation/resolution techniques based upon
differences in solubility.
The optically pure enantiomer is then recovered, along with the resolving
agent, by any
practical means that would not result in racemization. A more detailed
description of the
5 techniques applicable to the resolution of stereoisomers of compounds from
their racemic
mixture can be found in Jean Jacques Andre Collet, Samuel H. Wilen,
Enantiomers,
Racemates and Resolutions, John Wiley & Sons, Inc. (1981).
The compounds of formula II or any subgroup of formula II as defined herein
include
radioisotopes or radiomarked compounds, wherein one or more of the atoms is
replaced by an
10 isotope of that atom, i.e. an atom having the same atomic number as, but an
atomic mass
different from, the one(s) typically found in nature. Examples of isotopes
that may be
incorporated into the compounds of formula I or any subgroup of formula I,
include but are not
limited to isotopes of hydrogen, such as 2H and 3H (also denoted D for
deuterium and T for
tritium respectively), carbon, such as 110 13C and 14C, nitrogen, such as 13N
and 15N, oxygen,
such as 150, 170 and 180, phosphorus, such as 31P and 32P, sulphur, such as
355, fluorine, such
as 18F, chlorine, such as 36CI, bromine such as 75Br, 76Br, 77Br and 82Br, and
iodine, such as
1231, 1241, 1251 and 1311
The choice of isotope included in an isotope-labelled compound will depend on
the specific
application of that compound. For example, for drug or substrate tissue
distribution assays,
compounds wherein a radioactive isotope such as 3H or 14C is incorporated will
generally be
most useful. For radio-imaging applications, for example positron emission
tomography (PET)
a positron emitting isotope such as 110 18F 13N or 150 will be useful. The
incorporation of a
heavier isotope, such as deuterium, i.e. 2H, may provide greater metabolic
stability to a
compound of formula I or any subgroup of formula 1, which may result in, for
example, an
increased in vivo half life of the compound or reduced dosage requirements.
For synthetic convenience it will generally be preferred that the compounds of
formula 11 are in
the natural isotopic state.
Isotopically labelled compounds of formula I or any subgroup of formula 11 can
be prepared by
processes analogous to those described in the Schemes and/or Examples herein
below by
using the appropriate isotopically labelled reagent or starting material
instead of the
corresponding non-isotopically labelled reagent or starting material, or by
conventional
techniques known to those skilled in the art.
It will be appreciated that the invention extends to prodrugs, solvates,
complexes and other
forms releasing a compound of the invention in vivo.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
11
While it is possible for the active agent to be administered alone, it is
preferable to present it as
part of a pharmaceutical formulation. Such a formulation will comprise the
above defined active
agent together with one or more acceptable carriers/excipients and optionally
other therapeutic
ingredients. The carrier(s) must be acceptable in the sense of being
compatible with the other
ingredients of the formulation and not deleterious to the recipient.
The formulations include those suitable for rectal, nasal, topical (including
buccal and
sublingual), vaginal or parenteral (including subcutaneous, intramuscular,
intravenous and
intradermal) administration, but preferably the formulation is an orally
administered formulation.
The formulations may conveniently be presented in unit dosage form, e.g.
tablets and
sustained release capsules, and may be prepared by any methods well known in
the art of
pharmacy.
Such methods include the step of bringing into association the above defined
active agent with
the carrier. In general, the formulations are prepared by uniformly and
intimately bringing into
association the active agent with liquid carriers or finely divided solid
carriers or both, and then
if necessary shaping the product. The invention extends to methods for
preparing a
pharmaceutical composition comprising bringing a compound of Formula II or its
pharmaceutically acceptable salt in conjunction or association with a
pharmaceutically
acceptable carrier or vehicle. If the manufacture of pharmaceutical
formulations involves
intimate mixing of pharmaceutical excipients and the active ingredient in salt
form, then it is
often preferred to use excipients which are non-basic in nature, i.e. either
acidic or neutral.
Formulations for oral administration in the present invention may be presented
as discrete
units such as capsules, cachets or tablets each containing a predetermined
amount of the
active agent; as a powder or granules; as a solution or a suspension of the
active agent in an
aqueous liquid or a non-aqueous liquid; or as an oil-in-water liquid emulsion
or a water in oil
liquid emulsion and as a bolus etc.
With regard to compositions for oral administration (e.g. tablets and
capsules), the term
suitable carrier includes vehicles such as common excipients e.g. binding
agents, for example
syrup, acacia, gelatin, sorbitol, tragacanth, polyvinylpyrrolidone (Povidone),
methylcellulose,
ethylcellulose, sodium carboxymethylcellulose, hydroxypropyl-methylcellulose,
sucrose and
starch; fillers and carriers, for example corn starch, gelatin, lactose,
sucrose, microcrystalline
cellulose, kaolin, mannitol, dicalcium phosphate, sodium chloride and alginic
acid; and
lubricants such as magnesium stearate, sodium stearate and other metallic
stearates, glycerol
stearate stearic acid, silicone fluid, talc waxes, oils and colloidal silica.
Flavouring agents such
as peppermint, oil of wintergreen, cherry flavouring or the like can also be
used. It may be
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
12
desirable to add a colouring agent to make the dosage form readily
identifiable. Tablets may
also be coated by methods well known in the art.
A tablet may be made by compression or moulding, optionally with one or more
accessory
ingredients. Compressed tablets may be prepared by compressing in a suitable
machine the
active agent in a free flowing form such as a powder or granules, optionally
mixed with a
binder, lubricant, inert diluent, preservative, surface-active or dispersing
agent. Moulded
tablets may be made by moulding in a suitable machine a mixture of the
powdered compound
moistened with an inert liquid diluent. The tablets may be optionally be
coated or scored and
may be formulated so as to provide slow or controlled release of the active
agent.
Other formulations suitable for oral administration include lozenges
comprising the active
agent in a flavoured base, usually sucrose and acacia or tragacanth; pastilles
comprising the
active agent in an inert base such as gelatin and glycerin, or sucrose and
acacia; and
mouthwashes comprising the active agent in a suitable liquid carrier.
The appropriate dosage for the compounds or formulations of the invention will
depend upon
the indication and the patient and is readily determined by conventional
animal trials. Dosages
providing intracellular (for inhibition of physiological proteases of the
papain superamily)
concentrations of the order 0.01-100 uM, more preferably 0.01-10 uM, such as
0.1-25 uM are
typically desirable and achievable.
Compounds of the invention are prepared by a variety of solution and solid
phase chemistries.
The compounds are typically prepared as building blocks reflecting the P1, P2
and P3 moieties
of the end product inhibitor. Without in any way wishing to be bound by
theory, or the
ascription of tentative binding modes for specific variables, the notional
concepts P1, P2 and
P3 as used herein are provided for convenience only and have substantially
their conventional
Schlecter & Berger meanings and denote those portions of the inhibitor
believed to fill the S1,
S2, and S3 subsites respectively of the enzyme, where S1 is adjacent the
cleavage site and
S3 remote from the cleavage site. Compounds defined by Formula II are intended
to be within
the scope of the invention, regardless of binding mode.
Broadly speaking the P1 building block will have the formula:
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
13
11 NI
O 0
PGN or PG'N
O \ Rb 0,Rc
wherein
R1 and R2 are as defined above, the two Rb groups define a ketal, such as the
bis methyl ketal
or together define a cyclic ketal such as 1,3-dioxolane;
and Rc is an hydroxy protecting group. Less commonly Rc is H or represents the
keto function
of the end-product inhibitor in cases where the P1 building block as the
ketone is elongated
with P2 and P3.
W005/066180 describes the preparation of intermediates towards the above P1
building
blocks, including:
HO 0 .110 BnO 0
N
0-1( N //OH
(D~0~O 6 0,O 58
The first stage in the synthesis of compounds of the invention is typically
the preparation in
solution of a functionalized P1 building block. Scheme 1 illustrates a route
to a convenient 6-
aldehyde intermediate.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
14
O H O O H O HO H
0
iii, iv
CC/ Me
Me N
H OH ) H Me bocH Me
O O O 0 1c
1a 1b
OH 0
H O H O H O
V, vi vii viii
Me Me Me
N H Me N H Me N Me
boc boc b H
oc
1d le if
i) Dess-Martin Periodinane, DCM; ii) Trimethylorthoformate, pTs, MeOH; iii)
Pd(OH)2, H2, MeOH; iv)
Boc20, 10 % Na2C03, v) Dess-Martin Periodinane, DCM; vi) 1) CH3PPh3Br, KOtBu,
THF;
vii) 1) 9-BBN-H, THF, 2) NaBO31 H2O, THF; viii) Dess-Martin Periodinane, DCM.
Scheme 1
The starting bicyclic alcohol (1 a) can be prepared as described in
WO05/066180. Oxidation of
the hydroxy function for example with Dess-Martin periodinane followed by
transformation of
the afforded keto function into a dimethyl ketal effected by treatment with
trimethyl
orthoformate in the presence of an acid like p-toluenesulphonic acid provides
the ketal (1 b).
Removal of the Cbz and benzyl protecting groups effected for instance by
hydrogenolysis
using a catalyst like Pd(OH)2 or the like, followed by boc protection of the
afforded free amine
provides the alcohol (1 c). Oxidation of the afforded free alcohol using for
instance Dess-Martin
periodinane in a solvent like dichloromethane followed by a Wittig reaction
using methyl
triphenylphosphinium bromide in the presence of KOt.Bu or the like provides
the olefin (1 d).
Hydroxylation of the double bond effected for example by treatment with 9-BBN-
H, provides
the primary alcohol (1e) which subsequently can be oxidized to the
corresponding aldehyde
(1f) using any suitable oxidation method such as treatment with Dess-Martin
periodinane or the
like.
Scheme 2 illustrates a typical procedure for a 6-nitrile P1 building block
commencing from the
6-aldehyde intermediate of Scheme 1.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
H H 0 HO, N H H O
O
0
N H i\ N iH 0\ ii N H 00
if 1g 1h
is NH2OH.HCl, NaOAc
ii: Tf20, Et3N
Conversion of the aldehyde if (Scheme 2) to the corresponding desired nitrile
1 h (Scheme 2)
proceeds via dehydration of oxime 1 g (Scheme 2). Hence the aldehyde in an
ethanol/water
mixture can be treated with NH2OH and sodium acetate, for example overnight at
room
5 temperature. TLC can be used to monitor that the starting material had been
consumed.
Conventional work-up provides the crude oxime 1g (E and Z isomers) used
without further
purification. The crude oxime 1g may be taken up in dichloromethane and
triethylamine added
at -78 C. Trifluoromethanesulfonic anhydride in an organic solvent such as
dichloromethane
can be added, typically while allowing the reaction to warm up to room
temperature. Once the
10 reaction appears to be complete, extraction and chromatography of the
residue, for example
on silica gel affords a typical nitrile building block 1 h, generally in good
yield. Typically only one
isomer of the nitrile 1 h, eg that with C-6S stereochemistry is isolated as
the reaction usually
proceeds without loss of stereochemical integrity. Numerous examples in the
literature show
this conversion from an aldehyde to the corresponding nitrile to proceed with
a similar retention
15 of stereochemistry eg. Hutt et al, Journal of Organic Chemistry, 72(26),
10130, 2007.
Typically to get to the final compound, the P1 building block such as 1h above
is N-
deprotected in a conventional fashion, such as treatment with acetyl chloride
in methanol to
remove an N-Boc protecting group. With the subsequent free amine, the P2
residue is
introduced, eg via BocP2-OH using standard coupling conditions such as HATU,
DIPEA in
DMF. The terminal Boc protection is again removed with acetyl chloride in
methanol and the
P3 residue introduced via P3-OH using standard coupling conditions such as
HATU, DIPEA in
DMF. Finally the dimethylketal protection is removed with TFA to afford the
required final
compound.
Elongation is typically carried out in the presence of a suitable coupling
agent e.g.,
benzotriazole-1-yloxytrispyrrolidinophosphonium hexafluorophosphate (PyBOP), 0-
benzotriazol-I-yl-N,N,N',N'-tetramethyl-uronium hexafluorophosphate (HBTU), O-
(7-
azabenzotriazol-1-yl)-1,1,3,3-tetramethyl-uronium hexafluorophosphate (HATU),
1-(3-
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
16
dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), or 1,3-
dicyclohexyl
carbodiimide (DCC), optionally in the presence of I-hydroxybenzotriazole
(HOBT), and a base
such as N,N-diisopropylethylamine, triethylamine, N-methylmorpholine, and the
like. The
reaction is typically carried out at 20 to 30 C, preferably at about 25 C,
and requires 2 to 24 h
to complete. Suitable reaction solvents are inert organic solvents such as
halogenated organic
solvents (e.g., methylene chloride, chloroform, and the like), acetonitrile,
N,N-
dimethylformamide, ethereal solvents such as tetrahydrofuran, dioxane, and the
like.
Alternatively, the above elongation coupling step can be carried out by first
converting the
P3/P2 building block into an active acid derivative such as succinimide ester
and then reacting
it with the P1 amine. The reaction typically requires 2 to 3 h to complete.
The conditions
utilized in this reaction depend on the nature of the active acid derivative.
For example, if it is
an acid chloride derivative, the reaction is carried out in the presence of a
suitable base (e.g.
triethylamine, diisopropylethylamine, pyridine, and the like). Suitable
reaction solvents are
polar organic solvents such as acetonitrile, N,N-dimethylformamide,
dichloromethane, or any
suitable mixtures thereof.
The P2 building block is typically an N-protected amino acid such as L-
leucine, L-isoleucine,
O-methyl-L-threonine, L-3-hydroxyvaline, 4-fluoroleucine, L-
cyclopentylglycine or L-
cyclohexylglycine, and P3 typically comprises a capping group such as a
benzoic acid
derivative with, eg, the N-alkyl-piperazinyl-E moiety already introduced or
provided with a
synthon therefor in the para position.
The suitably protected individual building blocks can first be prepared and
subsequently
coupled together, preferably in the sequence P2+P1- P2-P1 followed by N-
alkylpiperazinyl-E-
benzoic acid*+P2-P1_ N-alkylpiperazinyl-E-benzoate-P2-P1, where * denotes an
activated
form, in order to minimise racemisation at P2.
Coupling between two amino acids, an amino acid and a peptide, or two peptide
fragments
can be carried out using standard coupling procedures such as the azide
method, mixed
carbonic-carboxylic acid anhydride (isobutyl chloroformate) method,
carbodiimide
(dicyclohexylcarbodiimide, diisopropylcarbodiimide, or water-soluble
carbodiimide) method,
active ester (p-nitrophenyl ester, N-hydroxysuccinic imido ester) method,
Woodward reagent
K-method, carbonyldiimidazole method, phosphorus reagents or oxidation-
reduction methods.
Some of these methods (especially the carbodiimide method) can be enhanced by
adding 1-
hydroxybenzotriazole or 4-DMAP. These coupling reactions can be performed in
either
solution (liquid phase) or solid phase.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
17
More explicitly, the coupling step involves the dehydrative coupling of a free
carboxyl of one
reactant with the free amino group of the other reactant in the present of a
coupling agent to
form a linking amide bond. Descriptions of such coupling agents are found in
general
textbooks on peptide chemistry, for example, M. Bodanszky, "Peptide
Chemistry", 2nd rev ed.,
Springer-Verlag, Berlin, Germany, (1993) hereafter simply referred to as
Bodanszky, the
contents of which are hereby incorporated by reference. Examples of suitable
coupling agents
are N,N'-dicyclohexylcarbodiimide, 1-hydroxybenzotriazole in the presence of
N,N'-
dicyclohexylcarbodiimide or N-ethyl-N'-[ (3-dimethylamino) propyl]
carbodiimide. A practical
and useful coupling agent is the commercially available (benzotriazol-1-
yloxy)tris-
(dimethylamino) phosphonium hexafluorophosphate, either by itself or in the
present of 1-
hydroxybenzotriazole or 4-DMAP. Another practical and useful coupling agent is
commercially
available 2-(IH-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium
tetrafluoroborate. Still another
practical and useful coupling agent is commercially available O-(7-
azabenzotriazol-1-yl)-
N,N,N',N'-tetramethyluronium hexafluorophosphate.
The coupling reaction is conducted in an inert solvent, e. g. dichloromethane,
acetonitrile or
dimethylformamide. An excess of a tertiary amine, e. g. diisopropylethylamine,
N-
methylmorpholine, N-methylpyrrolidine or 4-DMAP is added to maintain the
reaction mixture at
a pH of about 8. The reaction temperature usually ranges between 0 C and 50
C and the
reaction time usually ranges between 15 min and 24 h.
The functional groups of the constituent non-natural amino acids generally
must be protected
during the coupling reactions to avoid formation of undesired bonds. The
protecting groups
that can be used are listed in Greene, "Protective Groups in Organic
Chemistry", John Wiley &
Sons, New York (1981) and "The Peptides: Analysis, Synthesis, Biology", Vol.
3, Academic
Press, New York (1981), hereafter referred to simply as Greene, the
disclosures of which are
hereby incorporated by reference.
The alpha-carboxyl group of the C-terminal residue is usually protected as an
ester that can be
cleaved to give the carboxylic acid. Protecting groups that can be used
include 1) alkyl esters
such as methyl, trimethylsilyl and t-butyl, 2) aralkyl esters such as benzyl
and substituted
benzyl, or 3) esters that can be cleaved by mild base or mild reductive means
such as
trichloroethyl and phenacyl esters.
The alpha-amino group of each amino acid to be coupled is typically N-
protected. Any
protecting group known in the art can be used. Examples of such groups
include: 1) acyl
groups such as formyl, trifluoroacetyl, phthalyl, and p-toluenesulfonyl; 2)
aromatic carbamate
groups such as benzyloxycarbonyl (Cbz or Z) and substituted
benzyloxycarbonyls, and 9-
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
18
fluorenylmethyloxycarbonyl (Fmoc); 3) aliphatic carbamate groups such as
tertbutyloxycarbonyl (Boc), ethoxycarbonyl, diisopropylmethoxy-carbonyl, and
allyloxycarbonyl;
4) cyclic alkyl carbamate groups such as cyclopentyloxycarbonyl and
adamantyloxycarbonyl;
5) alkyl groups such as triphenylmethyl and benzyl; 6) trialkylsilyl such as
trimethylsilyl; and 7)
thiol containing groups such as phenylthiocarbonyl and dithiasuccinoyl. The
preferred alpha-
amino protecting group is either Boc or Fmoc. Many amino acid derivatives
suitably protected
for peptide synthesis are commercially available.
The alpha-amino protecting group is typically cleaved prior to the next
coupling step. When the
Boc group is used, the methods of choice are trifluoroacetic acid, neat or in
dichloromethane,
or HCI in dioxane or in ethyl acetate. The resulting ammonium salt is then
neutralized either
prior to the coupling or in situ with basic solutions such as aqueous buffers,
or tertiary amines
in dichloromethane or acetonitrile or dimethylformamide. When the Fmoc group
is used, the
reagents of choice are piperidine or substituted piperidine in
dimethylformamide, but any
secondary amine can be used. The deprotection is carried out at a temperature
between 0 C
and room temperature usually 20-22 C.
Once the inhibitor sequence is completed any remaining protecting groups are
removed in
whatever manner is dictated by the choice of protecting groups. These
procedures are well
known to those skilled in the art.
P2 building blocks in the form of N-protected L-amino acids are readily
available commercially,
for example L-leucine, L-isoleucine, L-cyclohexylglycine, O-methyl-L threonine
and others are
available commercially with a number of protecting group variants such as CBz,
Boc or Fmoc.
Other variants of R3 are easily prepared from commercially available starting
materials. For
example compounds wherein R3 is -C(CH3)20CH3 can be prepared by reacting CBz
protected
(S)-(+)-2-amino-3-hydroxy-3-methylbutanoic acid with 3,3-dimethoxy-hexahydro-
furo(3,2b)pyrrole to form the desired P2-P1 unit. The P2 side chain alcohol
can now be
methylated using methyliodide under conventional sodium hydride, imidazole,
THE conditions
to obtain the desired P2 without substantial racemisation of the alpha centre.
This P2-P1
moiety can now be carried through the synthesis as described herein, namely
CBz removal
and coupling.
W005/565299 describes the preparation of a gamma-fluoroleucine P2 building
block. An
alternative synthsis of Fmoc and N-Boc-gammafluoroleucine building blocks is
shown in
Truong et al Syn. Lett. 2005 no 8 1278-1280.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
19
The preparation of P3 building blocks are described in W005/066180,
W008/007114 or
readily prepared by analogous methods. For example, Scheme E below shows the
preparation
of a P3 building block wherein E is a fluoro-substituted thiazolyl:
0 0 0
Br F
~
H3CO / H3CO H3CO I/
O 0 0
F F
O s s
F I N ~ N \ - - / N - N / > - - N V _ / N -
I V HO I /
H 3 C 0 Br IV H3CO
Y
O 0 0
i. HOAc, Br2, RT, 2h, 55% yield; ii. KF, 18-crown-6, CH3CN, 90 C, 16 h, 31%
yield; iii. HOAc,
Br2, 45 C, 4 h, 100% yield ; iv. 4-methylpiperazine-1-carbothioamide,
ethanol, 70 C, 2 h, 74%
yield, v. LiOH, THF, H2O, RT, 16 h, 79 % yield.
Scheme E
Synthesis of 4-[5-fluoro-2-(4-methyl-piperazin-1-yl)-thiazol-4-yl]-benzoic
acid
The starting material, methyl 4-acetylbenzoate, is commercially available.
Bromination at the a-
position to the ketone is achieved with bromine in acetic acid to provide the
desired 4-(2-
bromo-acetyl)-benzoic acid methyl ester. Subsequent treatment of 4-(2-bromo-
acetyl)-benzoic
acid methyl ester with potassium fluoride in the presence of 18-crown-6 at 90
C, provides 4-
(2-fluoro-acetyl)-benzoic acid methyl ester after column chromatography.
Repeated
bromination at the a-position to the ketone is achieved with bromine in acetic
acid to provide
the desired 4-(2-bromo-2-fluoro-acetyl)-benzoic acid methyl ester. Formation
of the thiazole is
typically carried out by heating 4-(2-bromo-2-fluoro-acetyl)-benzoic acid
methyl ester with 4-
methylpiperazine-1 -carbothioamide at 70 C for 2 hours. On cooling, the
desired 4-[5-fluoro-2-
(4-methyl-piperazin-1-yl)-thiazol-4-yl]-benzoic acid methyl ester precipitates
out. Deprotection
of the methyl ester is carried out using a lithium hydroxide solution and the
desired acid, 4-[5-
fluoro-2-(4-methyl-piperazin-1-yl)-thiazol-4-yl]-benzoic acid is generally
obtained in good yield
as the dihydrochloride salt on workup with hydrochloric acid.
The term "N-protecting group" or "N-protected" as used herein refers to those
groups intended
to protect the N-terminus of an amino acid or peptide or to protect an amino
group against
undesirable reactions during synthetic procedures. Commonly used N-protecting
groups are
disclosed in Greene, "Protective Groups in Organic Synthesis" (John Wiley &
Sons, New York,
1981), which is hereby incorporated by reference. N-protecting groups include
acyl groups
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
such as formyl, acetyl, propionyl, pivaloyl, t-butylacetyl, 2-chloroacetyl, 2-
bromoacetyl,
trifluoracetyl, trichloroacetyl, phthalyl, o-nitrophenoxyacetyl, a-
chlorobutyryl, benzoyl, 4-
chlorobenzoyl, 4-bromobenzoyl, 4-nitrobenzoyl, and the like; sulfonyl groups
such as
benzenesulfonyl, p-toluenesulfonyl, and the like, carbamate forming groups
such as
5 benzyloxycarbonyl, p-chlorobenzyloxycarbonyl, p-methoxybenzyloxycarbonyl,
p-n itrobenzyloxycarbonyl, 2-n itrobenzyloxycarbonyl, p-
bromobenzyloxycarbonyl,
3,4-d imethoxybenzyloxycarbonyl, 4-methoxybenzyloxycarbonyl,
2-nitro-4,5-d imethoxybenzyloxycarbonyl, 3,4,5-trimethoxybenzyloxycarbonyl,
1-(p-biphenylyl)-1-methylethoxycarbonyl, a,a-dimethyl-3,5-d
imethoxybenzyloxycarbonyl,
10 benzhydryloxycarbonyl, t-butoxycarbonyl, diisopropylmethoxycarbonyl,
isopropyloxycarbonyl,
ethoxycarbonyl, methoxycarbonyl, allyloxycarbonyl, 2,2,2-
trichloroethoxycarbonyl,
phenoxycarbonyl, 4-n itrophenoxycarbonyl, fluorenyl-9-methoxycarbonyl,
cyclopentyloxycarbonyl, adamantyloxycarbonyl, cyclohexyloxycarbonyl,
phenylthiocarbonyl,
and the like; alkyl groups such as benzyl, triphenylmethyl, benzyloxymethyl
and the like; and
15 silyl groups such as trimethylsilyl and the like. Favoured N-protecting
groups include formyl,
acetyl, benzoyl, pivaloyl, t-butylacetyl, phenylsulfonyl, benzyl (bz), t-
butoxycarbonyl (BOC) and
benzyloxycarbonyl (Cbz).
Hydroxy and/or carboxy protecting groups are also extensively reviewed in
Greene ibid and
include ethers such as methyl, substituted methyl ethers such as
methoxymethyl,
20 methylthiomethyl, benzyloxymethyl, t-butoxymethyl, 2-methoxyethoxymethyl
and the like, silyl
ethers such as trimethylsilyl (TMS), t-butyldimethylsilyl (TBDMS)
tribenzylsilyl, triphenylsilyl, t-
butyldiphenylsilyl triisopropyl silyl and the like, substituted ethyl ethers
such as 1-ethoxymethyl,
1-methyl-1-methoxyethyl, t-butyl, allyl, benzyl, p-methoxybenzyl,
dipehenylmethyl,
triphenylmethyl and the like, aralkyl groups such as trityl, and pixyl (9-
hydroxy-9-
phenylxanthene derivatives, especially the chloride). Ester hydroxy protecting
groups include
esters such as formate, benzylformate, chloroacetate, methoxyacetate,
phenoxyacetate,
pivaloate, adamantoate, mesitoate, benzoate and the like. Carbonate hydroxy
protecting
groups include methyl vinyl, allyl, cinnamyl, benzyl and the like.
Detailed Description of the Embodiments
Various embodiments of the invention will now be described by way of
illustration only with
reference to the following Examples.
Reference Example 1
A P3 building block
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
21
Step a)4-Cyanopropiophenone
QN
O
As described for the preparation of 4-cyanoacetophenone (Synth. Commun 1994,
887-890), a
mixture of 4-bromopropiophenone (5.65 g, 26.4 mmol), Zn(CN)2 (1.80 g, 15.3
mmol), and
Pd(PPh3)4 (2.95 g, 2.6 mmol) was refluxed at 80 C in deoxygenated DMF (35 mL,
stored over
4 A molecular sieves, bubbled with Ar before use) for 18 h. The mixture was
partitioned
between toluene (100 mL) and 2N NH4OH (100 mL). The organic phase was
extracted with 2N
NH4OH (100 mL), washed with saturated aqueous NaCl (2 x 100 mL), dried, and
evaporated.
A 10 mmol scale reaction was done similarly and the crude products were
combined. Flash
chromatography (330 g silica, 6/1 petroleum ether - EtOAc) gave white solids
(5.17 g, 89%).
1 H NMR (CDCI3) 6 ppm: 1.22 (t, 3H, J = 7.2 Hz), 3.00 (q, 2H, J = 7.3 Hz),
7.75 (d, 2H, J = 8.8
Hz), 8.03 (d, 2H, J = 8.4 Hz)
13C NMR (CDCI3) 6 ppm: 7.8, 32.1, 116.1, 117.9, 128.3, 132.4, 139.7, 199.2
step b) 4-Propionylbenzoic acid
\ 15
4-Cyanopropiophenone (4.67 g, 29.3 mmol) was refluxed with 2N NaOH (90 mL, 180
mmol)
and dioxane (90 mL) at 95 C overnight. The mixture was diluted with water
(150 mL), washed
with ether (75 mL), acidified to pH 2 with concentrated HCI, and extracted
with ether (3 x 75
mL). The organic phase was washed with saturated aqueous NaCl (3 x 75 mL),
dried, and
evaporated to give yellow solids (5.12 g, 98%).
1H NMR (CDCI3+ CD3OD) 6 ppm: 1.18 (t, 3H, J = 7.2 Hz), 2.99, (q, 2H, J = 7.1
Hz), 7.95 (d,
2H, J = 8.4 Hz), 8.08 (d, 2H, J = 8.8 Hz)
13C NMR (CDCI3) 6 ppm: 7.9, 32.1, 127.7, 130.0, 134.0, 140.0, 168.0, 200.8
Step c)Methyl 4-propionylbenzoate
)__(__C00Me
0
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
22
The benzoic acid above (890 mg, 5 mmol), NaHCO3 (1.26 g, 15 mmol) and
iodomethane (935
L, 15 mmol) in DMF (10 ml-) were stirred at RT overnight. The mixture was
diluted with
saturated aqueous NaCl (50 ml-) and extracted with ether (3 x 50 mL). The
organic phase was
washed with water (50 mL), dried, and evaporated. Flash chromatography (90 g
silica, 2/1
petroleum ether - EtOAc) gave white solids (744 mg, 77%).
1 H NMR (CDCI3) 6 ppm: 1.24 (t, 3H, J = 7 Hz), 3.03 (q, 2H, J = 7 Hz), 3.95
(s, 3H), 8.0 and
8.12 (ABq, 4H)
Step d) Methyl 4-(2-bromopropionyl)benzoate
Br a COOMe
0
Methyl 4-propionylbenzoate (744 mg, 3.87 mmol), pyrrolidone hydrotribromide
(1.98 g), and 2-
pyrrolidinone (380 mg, 4.5 mmol) in THE (38 ml-) were heated at 50 C under
nitrogen for 3 h.
The mixture was cooled, filtered, concentrated, and then redissolved in ether
(50 mL). The
ether solution was washed successively with water (20 mL), saturated aqueous
Na2S2O5 (20
mL), saturated aqueous NaCl (20 mL), and water (20mL), dried and evaporated to
give a
yellow oil (1.025 g) that was used directly in the Hantzsch coupling. This
material contained
91 % of the desired bromoketone, 5% starting ketone, and 4% 4-bromo-1-butanol,
as
determined by 1 H NMR.
1 H NMR (CDCI3) 6 ppm: 1.92 (d, 3H, J = 7 Hz), 3.96 (s, 3H), 5.28 (q, 1 H, J =
7 Hz), 8.07 and
8.14 (ABq, 4H)
Step e)4-[2-(4-tent-Butoxycarbonylpiperazin-1-yl)-5-methylthiazol-4-yllbenzoic
acid methyl
ester
~--\ s
BocN N4
N
COOMe
All of the a-bromoketone above and 4-thionocarbonylpiperazine-1-carboxylic
acid tent-butyl
ester (J. Med. Chem., 1998, 5037-5054, 917 mg, 3.73 mmol) were refluxed in 36
mL THE at
70 C for 2 h, under N2. The precipitate was filtered and the filtrate
evaporated to give yellow
solids. Flash column chromatography (silica, 5/1 petroleum ether - EtOAc) gave
624 mg of
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
23
light yellow solids. Chromatography of the precicpitate (silica, 2/1 petroleum
ether - EtOAc)
gave 32 mg more of compound. Total yield is 44%.
1 H NMR (CDC13) 6 ppm: 1.46 (s, 9H), 2.43 (s, 3H), 3.42, (m, 4H), 3.54 (m,
4H), 3.90 (s, 3H),
7.68 and 8.04 (ABq, 4H).
Step f) 4-[2-(4-tent-Butoxycarbonylpiperazin-1-yl)-5-methylthiazol-4-
yllbenzoic acid
~-- s
BocN N-/,,
N
COOH
The above methyl ester (564 mg, 1.35 mmol) was heated with 1.35 mL 2N NaOH, 5
mL THF,
and 3.65 mL water at 60 C for 4 h. The reaction mixture was evaporated,
poured into 20 mL
saturated aqueous NaCl and 20 mL CH2C12, and then acidified to pH 3 with 5%
citric acid, in
an ice bath. The layers were separated and the organic phase was extracted
further with 2 x
10 mL CH2C12. The organic phases were combined, washed with water (10 mL),
dried, and
evaporated to give light yellow solids (537 mg, 98%).
1 H NMR (CDC13) 6 ppm: 1.48 (s, 9H), 2.47 (s, 3H), 3.47 (m, 4H), 3.57 (m, 4H),
7.74 and 8.12
(ABq, 4H).
13C NMR (CDC13) 6 ppm: 12.6, 28.3, 42.8, 48.1, 80.3, 119.1, 127.8, 128.2,
130.1, 140.5,
145.6, 154.6, 167.2, 171.4.
LC MS: (M + H)+ 404, (M - H)- 402.
Step g)4-[5-methyl-2-(4-methyl-piperazin-1-yl)-thiazol-4-yllbenzoic acid
/--\ s
-N N -/,,N
COOH
4-[4-(4-Carboxy-phenyl)-5-methyl-thiazol-2-yl]-piperazine-1-carboxylic
acid tert-butyl ester (0.421 mmol) was dissolved in 4M HCI in 1,4-dioxane, and
stirred at room
temperature for 1 h. The solvent was then removed under vacuum, and the
residue 4-(5-
methyl-2-piperazin-1-yl-thiazol-4-yl)-benzoic acid was suspended in methanol
(10 ml) and
treated with AcOH/AcONa buffer (pH -5.5, 5 ml), and formaldehyde (0.547 mmol).
The
reaction mixture was stirred at room temperature for 1 h, then treated with
NaCNBH3 (0.547
mmol) and stirred at room temperature overnight. The solvent was then removed
under
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
24
vacuum, and the residue was purified by column chromatography to afford the
title compound
(0.403 mmol, 95%).
MS(ES) m/z 318 (100%, [M+H]+).
Reference Example 2
An alternative P3 building block
3-Fluoro- 4-[2-(4-methylpiperazin-1-yl)-thiazol-4-yllbenzoic acid HCI salt
TMSCHN2 Zn, Ac20, TFA
MeOH-PhMe 0 allyl CI, CoBr2
O
Br -W OH 0 deg to rt Br \ McCN 0 ~-WoMe
OH 95% OMe 36% F F F
0 -N~\N~ S
CN HBr3 v-" NH2 0
0 0 EtOH, 70 C N~ OMe
OMe N_N/ I ,
2-pyrrolidinone Br 72% over 2 steps
THF, 60-65 C F S F
0
6M HCI CI OH
quant NN
N--~/S _Ir
F
Step a)Methyl 4-bromo-3-fluorobenzoate
4-Bromo-3-fluorobenzoic acid (2.46 g, 11.2 mmol) was dissolved in MeOH (9 mL)
and toluene
(4 mL) and cooled in an ice bath. (Trimethylsilyl)diazomethane (11 mL, 2.0 M
in hexanes, 22
mmol) was added dropwise until the yellow color persisted. The solution was
stirred at room
temperature for 40 mins and then concentrated in vacuo. A second batch of
carboxylic acid
(2.43 g) was treated similarly. The crude product from both batches were
combined and
subjected to flash chromatography (silica, 5/1 pentane - EtOAc) to give the
methyl ester as
white solids (4.92 g, 95% yield).
1H NMR (400 MHz, CDCI3) delta ppm 7.77 (m, 1 H), 7.71 (m, 1 H), 7.64 (m, 1 H),
3.93 (s, 3H).
Step b)Methyl 4-acetoxy-3-fluorobenzoate
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
Allyl chloride (105 pL, 1.28 mmol) and TFA (20 pL, 0.26 mmol) were added to a
suspension of
zinc dust (480 mg, 7.34 mmol) and anhydrous cobalt(II) bromide (96.6 mg, 0.44
mmol) in
MeCN (4 mL), under inert gas. After stirring at room temperature for 10 min,
the aryl bromide
(1.003 g, 4.30 mmol dissolved in 5 mL MeCN) from (a) was added, followed by
acetic
5 anhydride (0.45 mL, 4.79 mmol) and more MeCN (1 mL). The mixture was stirred
overnight,
quenched with 1 M HCI (20 mL), and then extracted with EtOAc (3 x 20 mL). The
organic
phase was washed successively with saturated aqueous NaHCO3 (20 mL) and
saturated NaCl
(2 x 20 mL), dried (Na2SO4), and concentrated. Flash chromatography (silica,
6/1 to 4/1
petroleum ether - EtOAc gave recovered bromide (161.1 mg, 16%) and the desired
ketone
10 (white solids, 305.5 mg, 36%).
NMR (CDC13) b ppm: 1H (400 MHz) 7.94-7.86 (m, 2H), 7.80 (dd, 1H, J = 11.2, 1.6
Hz), 3.95 (s,
3H), 2.67 (d, 3H, J = 4.4 Hz); 19F (376 MHz) -109.2 (m); 13C (100 MHz) 195.4
(d , J = 3.7 Hz),
165.1 (d, J = 2.2 Hz), 161.6 (d, J = 255 Hz), 135.8 (d, J = 8.1 Hz), 130.7 (d,
J = 2.9 Hz), 129.0
d, J = 14 Hz), 125.2 (d, J = 3.6 Hz), 117.9 (d, J = 26 Hz), 52.7 (s), 31.4 (d,
J = 7.3 Hz).
15 Step c)Methyl 4-(2-bromoacetoxy)-3-fluorobenzoate
THE (10 mL) and 2-pyrrolidinone (91 pL, 1.20 mmol) were added to a mixture of
the ketone
from b) (198 mg, 1.01 mmol) and pyrrolidone hydrotribromide (532 mg, 1.07
mmol). After
heating at 60-65 C for 2 h, the mixture was concentrated under vacuum and
then partitioned
between EtOAc (20 mL) and saturated Na2S2O3 (10 mL). The aqueous phase was
extracted
20 with EtOAc (10 mL). The organic phases were combined, washed with saturated
NaCl (2 x 10
mL), dried (Na2SO4), and concentrated. Flash chromatography (silica, 7/1
petroleum ether-
EtOAc) gave white solids (0.2634 g) containing 84% of the desired bromide (as
determined by
integration of 19F NMR peaks).
NMR (CDC13) b ppm: 1H (400 MHz) 7.93 (m, 1 H), 7.88 (m, 1H), 7.79 (dd, 1H, J =
11.2, 1.6 Hz),
25 4.50 (d, 2H, J = 2.4 Hz), 3.94 (s, 3H); 19F (376 MHz) -108.4 (m).
Step d)Methyl 3-fluoro- 4-[2-(4-methylpiperazin-1-yl)-thiazol-4-yllbenzoate
EtOH (5.0 mL) was added to the bromoketone above (193 mg, 0.70 mmol) and 4-
methyl-
piperazine-1-carbothioic acid amide (113 mg, 0.71 mmol) and the mixture was
heated at 70 C
for 2h 15 min. The precipitates were filtered, washed with cold EtOH, and
dried under vacuum
and characterized. The procedure was repeated in a larger scale for 1.75 g
bromoketone (6.36
mmol).
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
26
NMR (1/1 CDC13- CD3OD) b ppm: 1H (400 MHz) 8.20 (m, 1H), 7.86 (dd, 1H, J =
8.4, 1.6 Hz),
7.76 (dd, 1 H, J = 11.4, 1.8 Hz), 7.38 (d, 1 H, J = 2.4 Hz), 4.23 (br, 2H),
3.95, (s, 3H), 3.65 (br,
4H), 3.32 (br, 2H), 2.98 (s, 3H); 19F (376 MHz) -114.0 (m). LCMS [M+H]+ = 336.
The precipitates from both preparations were combined and suspended in
saturated NaHCO3
(50 mL). The mixture was extracted with EtOAc. The organic phase was washed
with water,
dried (Na2SO4), and evaporated to give the title compound as cream solids
(1.76 g).
Step e)3-fluoro- 4-[2-(4-methylpiperazin-1-yl)-thiazol-4-yllbenzoic acid HCI
salt
The methyl ester (1.76 g, 5.25 mmol) from (d) was heated at 80 C with 6M HCI
(40 mL) for 5.5
h. More 6M HCI (10 mL) was added and the mixture was heated at 90 C for 1 h
15 min. After
cooling, the mixture was then evaporated under vacuum and freeze-dried from
water to give
the final product as cream solids in quantitative yield.
NMR (DMSO-d6) b ppm: 1H (400 MHz) 11.60 (br, 1 H), 8.18 (t, 1 H, J = 8.0 Hz),
7.82 (dd, 1 H, J
= 8.4, 1.6 Hz), 7.72 (dd, 1 H, J = 12.0, 1.6 Hz), 7.48 (d, 1 H, J = 2.8 Hz),
4.11 (m, 2H), 3.58 (m,
2H), 3.49 (m, 2H), 3.19 (m, 2H), 2.80 (d, 3H, J = 4.4 Hz); 19F (376 MHz) -
113.5 (m); 13C (100
MHz) 168.9, 166.0, 159.0 (d, J = 250 Hz), 143.4, 131.4 (d, J = 8 Hz), 129.8,
125.8 (d, J = 11
Hz), 125.6, 116.6 (d, J = 24 Hz), 111.1 (J = 15 Hz), 51.1, 45.0, 41.9. LCMS
[M+H]+ = 322.
Reference Example 3
6-aldehyde- intermediate
0
H
O
N
O
0 0 H
6-Formyl-3,3-dimethoxy-hexahydro-furo[3,2-b]pyrrole-4-carboxylic acid tert-
butyl ester
Step a
O
H
OuN ` O
O H
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
27
(3as, 6aS)-6R-benzyloxy-3-oxo-hexahydro-furo[3,2-blpvrrole-4-carboxylic acid
benzyl ester
Dess-Martin reagent (12.5 g, 30 mmol) was dissolved in DCM (250 mL). 6-
Benzyloxy-3-
hydroxy-hexahydro-furo[3,2-b]pyrrole-4-carboxylic acid benzyl ester (prepared
as described in
W005/066180) (7.4 g, 20 mmol) in DCM (50 mL) was added to a stirred solution
of oxidant at
rt under a nitrogen atmosphere over 45 min. After an additional 90 min
stirring the reaction was
deemed to be complete by TLC. Aqueous 10% Na2S2O3 (200 mL) was added and the
mixture
was stirred at rt for another 15 minutes. The two phase system was transferred
into a
separation funnel and extracted twice with EtOAc (200 mL and 100 mL
respectively). The
combined organic phases were washed once with aqueous saturated NaHCO3 (100
mL) and
brine (100 mL), dried over Na2SO4, filtered and the solvent removed in vacuo,
yielding the
crude product title compound as a clear oil (7.69 g,); ESI+, m/z: 368 (M+ +1).
Step b
O
H
0"-"0 N 0
I0 H
-0 0-
(3aS,6aS)-6R-benzyloxy-3,3-dimethoxy-hexahydro-furo[3,2-blpvrrole-4-carboxylic
acid benzyl
ester
The keto derivative of step a) (7.6 g) was dissolved in dry methanol (100 mL).
Trimethyl
orthoformate (30 mL) and pTsOH (0.2 g) was added at rt under a nitrogen
atmosphere. The
mixture was heated at 60 C for 8 hours. When the reaction was deemed to have
reached
completion according to TLC, it was cooled to rt and concentrated in vacuo.
The crude product
was purified by column chromatography over silica gel eluting with ethyl
acetate-heptane (1:4)
which gave the title compound as a clear oil (5.9 g, 71 % over 2 steps); ESI+,
m/z: 382 (M+ -
OMe).
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
28
Step c
HO H
O
N
O
H H O-
(3aS,6aS)-3,3-dimethoxv-hexahvdro-furo[3,2-b1 pyrrol-6R-ol
A solution of the compound of step b) (2.5 g, 6.4 mmol) in methanol (60 mL)
and Pd(OH)2 (0.7
g) was stirred at rt under H2 atmosphere for 48 hours. When the reaction was
deemed to have
reached completion according to TLC, the mixture was filtered and concentrated
in vacuo to
yield the crude title compound as a brownish oil (1.15 g); ESI+, m/z: 190 (M+
+1).
Step d
HO H
O
N
O
O H O-
0
(3aS, 6aS)-6R-hydroxy-3,3-dimethoxv-hexahvdro-furo[3,2-blpyrrole-4-carboxylic
acid tert-butyl
ester
3,3-Dimethoxy-hexahydro-furo[3,2-b] pyrrol-6-ol from step c) (2.80 g, 14.8
mmol) was
dissolved in 75 mL of a mixture of dioxan/water (2:1). A solution of 10%
Na2CO3 (25 mL) was
added drop wise to pH 9-9.5. The mixture was cooled to 0 C in an ice-water
bath and Boc
anhydride was added in one portion. The reaction was stirred at rt overnight
and the pH of the
mixture was maintained at 9-9.5 by addition of more 10% solution of Na2CO3 if
necessary. The
reaction was monitored by TLC (50:50 ethyl acetate:isohexane). Once completed,
the mixture
was filtered to eliminate the salts formed and the solvent was evaporated in
vacuo. The
aqueous mixture was extracted with 3 x 100 mL EtOAc, the combined organic
phases were
washed with 100 mL of water and 100 mL brine, dried over Na2SO4, filtered and
the solvent
was evaporated in vacuo to afford 3.79 g of the title carbamate as a clear oil
(89%), 94% pure
(HPLC), ESI+, m/z: 312 (M++Na).
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
29
Step e
0 H
O
N
O
O H O-
O
3,3-Dimethoxy-6-oxo-hexahydro-furo[3,2-blpvrrole-4-carboxylic acid tert-butyl
ester
To the alcohol from step e) (3.674 g, 12.70 mmol) dissolved in DCM (80 mL) was
added Dess-
Martin Periodinane (7.00 g, 16.5 mmol) and the solution was stirred for 3 h at
room
temperature. The reaction was then quenched by the addition of 10% Na2S203
(aq) (150 mL)
and the resulting slurry was stirred for 15 minutes. The mixture was
transferred to a separation
funnel and the phases were separated. The aqueous phase was extracted trice
with DCM and
the combined organic phases were subsequently washed twice with sat. NaHCO3
solution and
were the dried, filtered, and concentrated. The crude material was purified by
flash column
chromatography (toluene/ethyl acetate 3:1) which gave the title compound
(2.882 g, 79%).
Step f
H
O
N
I O
O H 0-
0
3,3-Dimethoxy-6-methylene-hexahydro-furo[3,2-blpvrrole-4-carboxylic acid tert-
butyl ester
The keto compound from step e (1.10 g, 3.83 mmol) was dissolved in dry THE (30
mL) and the
solution was cooled to 0 C. A solution of methyl triphenylphosphonium bromide
(4.0 g, 11.2
mmol) and KOtBu (1.17 g, 10.5 mmol) in dry THE (40 mL) was added in 3 aliquots
with 2
hours interval. After 6 hrs the solution was poured into a separatory funnel
with diethyl ether
(70 mL) and extracted with 10 % citric acid (aq)(2*40 mL). The organic phase
was washed with
saturated aqueous NaHCO3 (40 mL), dried with Na2SO4, filtered and the solvent
was
evaporated in vacuo. The crude product was purified by flash chromatography
(heptane: ethyl
acetate 4:1) which gave the title compound (524 mg, 48%)
1H NMR (CDC13, 400 MHz) b 1.48 (s, 9H), 3.27 (s, 3H), 3.40 (d, 3H, J = 16.6),
3.57- 3.64 (m,
1 H), 3.84 (d, 1 H, J = 9.5), 3.92 (d, 1 H, J = 16.3), 4.07- 4.25 (m, 1 H),
4.35- 4.49 (m, 1 H), 4.98
(bs, 1 H), 5.22 (d, 1 H, J = 16.4), 5.34 (s, 1 H).
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
Step q
OH
H
O
N
J H O
O O-
O
6-Hydroxymethyl-3,3-dimethoxy-hexahydro-furo[3,2-b]pyrrole-4-carboxylic acid
tert-butyl ester
5 The olefin from step f) (524 mg, 1.84 mmol) was dissolved in dry THE (70
mL). 9-BBN-H (0.5
M in THF) (7.34 mL, 3.67 mmol) was added and the solution was stirred over
night. The
solvent was removed by rotary evaporation and redissolved in THE (20 mL). MeOH
(10 mL)
was slowly added to the solution and when the gas evolution had ceased, H2O
(20 mL) was
added to the solution followed by NaBO3. The solution was filtered after it
had been stirred for
10 18 hrs and the filtrate was diluted with EtOAc (70 mL) and washed with
brine (2*50 mL). The
organic phase was dried with Na2SO4, filtered and the solvent was evaporated
in vacuo. The
crude product was purified by flash chromatography (heptane: ethyl acetate
2:1) which gave
the title compound (477 mg, 86%).
1H NMR (CDC13, 400 MHz) 61.47 (s, 9H), 2.09- 2.25 (m, 2H), 3.02- 3.20 (m, 1
H), 3.29 (s, 3H),
15 3.39 (s, 3H), 3.65- 3.93 (m, 4H), 4.44 (d, 1 H, J = 5.7), 4.70- 4.84 (m, 1
H).
Step h
0
H
O
N
J H O
O
O
6-Formyl-3,3-dimethoxy-hexahydro-furo[3,2-blpyrrole-4-carboxylic acid tert-
butyl ester
To a solution of the alcohol of step g) 1 g (370 mg, 1.22 mmol) dissolved in
dry DCM (10 mL)
was added Dess Martin periodinane (673 mg, 1.59 mmol). The reaction was
stirred for 40
minutes and then quenched by addition of 10 mL of 10% Na2S203: NaHC03(sat)
1:1. The
solution was diluted with DCM (50 mL) and extracted with a 1:1 mixture of 10%
Na2S203:
NaHCO3(sat) (50 mL). The organic phase was dried with Na2SO4, filtered and
evaporated. The
crude product was purified by flash chromatography (heptane: ethyl acetate
(2:1) which gave
the title compound (290 mg, 79%).
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
31
1H NMR (CDC13, 400 MHz) 61.47 (s, 9H), 2.90- 3.06 (m, 1 H), 3.29 (s, 3H), 3.38
(s, 3H), 3.67-
3.85 (m, 2H), 3.88- 4.55 (m, 3H), 4.93- 5.19 (m, 1 H), 9.64* and 9.80* (s, 1
H). * Two peaks due
to rota m ers.
Reference Example 4
6-nitrile P1 building block
N
H O
N
H
O~O
Step a)
NOH
H
O
N
boc H O O
\
515 mg (1.71 mmol) of 6-Formyl-3,3-dimethoxy-hexahydro-furo[3,2-b]pyrrole-4-
carboxylic acid
tert-butyl ester from reference example 3,_130.6 mg (1.88 mmol) of
hydroxylamine
hydrochloride and 168 mg (2.05 mmol) of sodium acetate were stirred in ethanol-
water mixtute
(10/15 ml) at room temperature overnight. The mixture was evaporated and
distributed
between water and ethyl acetate phases. The organic phase was washed with
brine, dried
over sodium sulfate, evaporated and purified on silica (EtOAc-hexane 1:1) to
give the mixture
of cis and trans-isomers of the above depicted. Rf 0.43 and 0.48 (EtOAc-hexane
1:1). Yield
395 mg (73%)
NC
H
O
N
boc H 00
The oxime of step a) (114 mg, 0.36 mmol) and triethylamine (105 L, 0.757
mmol) were
dissolved in 1,5 ml of dichloromethane and cooled till -78 C.
Trifluoromethanesulfonic
anhydride (61 L, 0.36 mmol) in 600 .tL of dichloromethane was added dropwise
over 7 min.
The reaction mixture was allowed to warm up till room temperature and was
stirred for 2 hours.
The mixture was diluted with 15 ml of DCM, washed with precooled 5% citric
acid, sodium
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
32
bicarbonate, brine, dried over sodium sulfate, evaporated and purified on
silica (gradient
EtOAc-hexane 1:3 to 1:1). Rf 0.65 (EtOAc-hexane 1:1). Yield 65 mg (61%)
Reference Example 5
A typical P1/P2 deprotection and coupling
/N
O H
~ON N O
H H:
O
%
The nitrile building block of reference example 4 (95 mg, 0.032 mmol) was
dissolved in 5 ml of
methanol cooled down to 0 C and 0.5 ml of acetyl chloride was added dropwise.
The resulting
mixture was stirred at r.t. for 4h, then evaporated. The residue was dissolved
in 2.5 ml of DMF,
80 mg (0.32 mmol) of Boc-Leu-OH was added, followed by addition of 0.5 ml of
diisopropylethylamine. The resulting mixture was cooled down till 0 C and 160
mg (0.42 mmol)
of HATU (O-(7-Azabenzotruazol-1-yl)-N,N,N',N'-tetramethyluronium
hexafluorophsphate) was
added. The reaction mixture was allowed to warm up till room temperature and
stirred for 1.5
h. Mixture was evaporated, distributed between water and ethyl acetate phases,
organic phase
was washed with water, brine, dried over sodium sulfate, evaporated and
purified on silica
(EtOAc Rf 0.35) to give 82 mg of the title compound. Yield 82 mg (62%) for 2
steps.
Reference Example 6
An alternative P3 building block
o O O
0i03 F OCH3
iii.
O O
F I~ OCH3 F I~ OCH3 F OH iv. Br /
O S S
yN yN
\N) \N)
i. AcOH, bromine, RT, 2 h, 55 % yield; ii. KF, acetonitrile, 18-crown-6, 90
C, 16 h; 31 % yield;
iii. AcOH, bromine, 45 C, 4 h, 100 % yield; iv. 4-Methyl-piperazine-1-
carbothioic acid amide,
A, 2 h, 74 % yield; LiOH, RT, 16 h, 100 % yield.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
33
Availability of starting materials -
Methyl 4-acetylbenzoate is available from Aldrich; 4-methyl-piperazine-1-
carbothioic acid
amide - 11 suppliers found in SciFinder (perhaps Chem Pur Products Ltd in
Germany most
convienient).
Step a) 4-(2-Bromo-acetyl)-benzoic acid methyl ester
O
Br I OMe
O
To a solution of 4-acetyl-benzoic acid methyl ester (8.4 mmol) in acetic acid
(20 ml-) was
added bromine (8.4 mmol). The reaction was stirred at RT for 2 h over which
time the red
colour disappeared and an off white precipitate formed. The product was
collected by filtration
and washed with cold methanol/water (200 mL 1:1) to yield a white powder (55
%). 1 H NMR
(400MHz, CDC13) 3.98 (3H, s), 4.20 (2H, s), 8.02 (2H, d, J = 8Hz), 8.18 (2H,
d, J = 8Hz).
Step b) 4-(2-Fluoro-acetyl)-benzoic acid methyl ester
O
F ,e We
i
To a suspension of potassium fluoride (3.11 mmol) in acetonitrile (1 ml-) was
added 18-crown-
6 (0.1 mmol) and the reaction was heated at 90 C for 30 mins. 4-(2-Bromo-
acetyl)-benzoic
acid (1.56 mmol) was added and the reaction heated at 90 C for 16 h. The
reaction was
diluted with water (10 ml-) and extracted with ethyl acetate (3 x 20 mL). The
product was
purified on silica eluting with 5-15 % ethyl acetate in iso-hexane to yield on
concentration in
vacuo of the desired fractions, the title product as a white solid (31 %). 1H
NMR (400MHz,
CDC13) 3.98 (3H, s), 5.55 (2H, d, J = 50Hz), 7.95 (2H, d, J = 8Hz), 8.18 (2H,
d, J = 8Hz).
Step c) 4-(2-Bromo-2-fluoro-acetyl)-benzoic acid methyl ester
O
F I OMe
Br
O
To a suspension of 4-(2-fluoro-acetyl)-benzoic acid (1.19 mmol) in acetic acid
(5 ml-) was
added bromine (1.19 mmol). The reaction was heated at 45 C for 4 h over which
time a green
solution formed. The reaction was concentrated in vacuo and azeotroped twice
with toluene to
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
34
yield the title compound as a green solid (100 %). The product was used crude
in the next
step. 1 H NMR (400MHz, CDC13) 3.98 (3H, s), 7.04 (1 H, s), 8.05 - 8.10 (4H,
m).
Step d)4-[5-Fluoro-2-(4-methyl-piperazin-1-yl)-thiazol-4-yl]-benzoic acid
methyl ester
0
F I OMe
s
)=N
N)
NJ
/
4-(2-Bromo-2-fluoro-acetyl)-benzoic acid methyl ester (1.18 mmol) and 4-methyl-
piperazine-1-
carbothioic acid amide (1.18 mmol) were dissolved in ethanol (10 mL). The
reaction was
heated at reflux for 2 h. The reaction was cooled to RT causing the product to
precipitate. The
product was collected by filtration and washed with cold ethanol. The product
was given an
aqueous sodium bicarbonate work up to yield the title compound as a colourless
oil (74 %).
MS (ES+) 337 (M+H, 100%).
Step f)
4-[5-Fluoro-2-(4-methyl-piperazin-1-yl)-thiazol-4-yl]-benzoic acid di-
hydrochloride
O
F I OH
~, N
0 N>
NJ
/
To a solution of 4-[5-fluoro-2-(4-methyl-piperazin-1-yl)-thiazol-4-yl]-benzoic
acid methyl ester
(0.43 mmol) in tetrahydrofuran/water (2.5 mL, 4:1) was added lithium hydroxide
(0.5 mmol).
The reaction was stirred at RT for 16 h. The reaction was concentrated in
vacuo and
hydrochloric acid (2N, 3 ml-) was added causing the product to precipitate as
a white solid.
The product was collected by filtration to yield the title product as a white
solid (79 %). MS
(ES+) 322 (M+H, 100%).
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
Example 1
N
O H
\ N N O
N N / 0 H,.
NI o
S
N41-(6-cyano-3-oxo-hexahyd ro-fu rof3,2-blpyrrole-4-carbonyl)-3-methyl-butyll-
4-f2-(4-methyl-
piperazin-1-yl)thiazol-4-yll-benzamide
5
Step a)
/N
/
O H
\ N N O
N/~ N I / H O H,,
N I i0 0
S
The P1/P2 building block of reference example 5 (60 mg, 0.147 mmol) was
dissolved in 3 ml of
methanol, cooled down to 0 C and 0.4 mL of acetyl chloride was added
dropwise. The
10 resulting mixture was stirred at r.t. for 4h, then evaporated. The residue
was dissolved in 2.5
mL of DMF, 52 mg (0.147 mmol) of the P3 building block (prepared as in
W00566180 as the
acid was added, followed by addition of 0.5 mL of diisopropylethylamine. The
resulting mixture
was cooled down till 0 C and 70 mg (0.184 mmol) of HATU (O-(7-azabenzotriazol-
l-yl)-
N,N,N',N'-tetramethyluronium hexafluorophsphate) was added. The reaction
mixture was
15 allowed to warm up till room temperature and stirred for 1.5 h. The mixture
was evaporated,
distributed between water and ethyl acetate phases, and the organic phase
washed with
water, brine, dried over sodium sulfate, evaporated and purified on silica (5%
MeOH in EtOAc).
The desired fractions were combined and concentrated in vacuo to afford the
desired material
in a yield of 56 mg (64 %), LC/MS 597 (M+1)
Step b)
N
O H
\ N N O
N I / H 0 H,,
N I 0
S
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
36
The ketal of step a) (56 mg, 0.094 mmol) was treated with 3 mL of TFA-water
mixture (2.5%
water in TFA) for 4 h. The reaction was monitored by LC/MS. The reaction
mixture was
evaporated, dissolved in acetonitrile (5 mL), stirred with solid sodium
carbonate for 1 h, then
solids were filtered off, the mother liquor was concentrated in vacuo, and
purified by
preparative HPLC (NH4OAc buffer, 30-80 system (MeCN-water) to give 25 mg of
desired
product (yield 45 %). LC/MS M+1 551, M+19 569 (hydrate form)
Example 2
NC
NC H NC ~/ N~T YNC N~-
HO /\) N O Ac CI N HATU-coupling O N O 0 TFA/ H2O 0 H O McOH H O H N 0 HN O /
H N O O H
boc
HCI I ,'N F
N \ N F
(2) ~N/ S r-' N
~S
(3) (4)
The protected P1-P2 building block of reference example 5 (1) (33 mg,
0.08mmol) was
dissolved in 3 ml of methanol cooled down to 0 C and 0.4 ml of acetyl chloride
was added
dropwise acid was added. The resulting mixture was stirred at r.t. for 4h,
then evaporated. The
residue was dissolved in 2.5 ml of DMF, 29 mg (0.08 mmol) of the P3 acid of
reference
example 2 (as a HCI salt) was added, followed by addition of 0.5 ml of
diisopropylethylamine.
The resulting mixture was cooled down till 0 C and 39 mg (0.101 mmol) of HATU
(O-(7-
azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate) was
added. The
reaction mixture was allowed to warmed up till room temperature and stirred
for 1.5 h. Mixture
was evaporated, distributed between water and ethyl acetate phases, organic
phase was
washed with water, brine, dried over sodium sulfate, evaporated and purified
on silica (5%
MeOH in EtOAc). Yield of (3) 32mg (67%), LC/MS 615 (M+1)
Ketal (3) (32 mg, 0.054mmol) was treated with 3 ml of TFA-water mixture (2,5%
water in TFA)
for 4 h. The reaction was monitored by LC/MS. Reaction mixture was evaporated,
dissolved in
acetonitrile (5 ml), stirred with solid sodium carbonate for 1 h, then solids
were filtered off,
mother liquor was concentrated i vacuo, and purified on prep. LC/MS purified
by prep. HPLC
(NH4OAc buffer, 30_80 system (MeCN-water) to give 13 mg of product (yield
42%). LC/MS
M+1 569, M+19 587 (hydrate form)
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
37
Example 3
NC
NC H NC H O NCN~=
NN N N O AcCI HATU-coupling N O H O TFA HzOO H O McOH O H N O HN O / H ,N O H
boc N
HCI r'N~S
F N
(1) (2) -N J 'N /' S F
(5)
(6)
The protected P1 P2 bilding block of reference example 5 (1) (33 mg, 0.08mmol)
was dissolved
in 3 ml of methanol cooled down to 0 C and 0.4 ml of acetyl chloride was added
dropwise acid
was added. The resulting mixture was stirred at r.t. for 4h, then evaporated.
The residue was
dissolved in 2.5 ml of DMF, 29 mg (0.08 mmol) of the P3 acid of reference
example 6, as a
HCI salt) was added, followed by addition of 0.5 ml of diisopropylethylamine.
The resulting
mixture was cooled down till 0 C and 39 mg (0.101 mmol) of HATU (O-(7-
azabenzotriazol-1-
yl)-N,N,N',N'-tetramethyluronium hexafluorophsphate) was added. The reaction
mixture was
allowed to warm up till room temperature and stirred for 1.5 h. Mixture was
evaporated,
distributed between water and ethyl acetate phases, organic phase was washed
with water,
brine, dried over sodium sulfate, evaporated and purified on silica (5% MeOH
in EtOAc). Yield
of (5) 30mg (63%), LC/MS 615 (M+1)
Ketal (5) (30 mg, 0.051 mmol) was treated with 3 ml of TFA-water mixture (2,5%
water in TFA)
for 4 h. The reaction was monitored by LC/MS. Reaction mixture was evaporated,
dissolved in
acetonitrile (5 ml), stirred with solid sodium carbonate for 1 h, then solids
were filtered off,
mother liquor was concentrated i vacuo, and purified on prep. LC/MS purified
by prep. HPLC
(NH4OAc buffer, 30_80 system (MeCN-water) to give 11 mg of product (yield
38%). LC/MS
M+1 569, M+19 587 (hydrate form)
Biological Examples
Determination of cathepsin K of cathepsin K proteolic catalytic
activitycatalic activity
Convenient assays for cathepsin K are carried out using human recombinant
enzyme, such as
that described in PDB.
ID BC016058 standard; mRNA; HUM; 1699 BP.
DE Homo sapiens cathepsin K (pycnodysostosis), mRNA (cDNA clone MGC:23107
RX MEDLINE;. RX PUBMED; 12477932.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
38
DR RZPD; IRALp962G1234.
DR SWISS-PROT; P43235;
The recombinant cathepsin K can be expressed in a variety of commercially
available
expression systems including E coli, Pichia and Baculovirus systems. The
purified enzyme is
activated by removal of the prosequence by conventional methods.
Standard assay conditions for the determination of kinetic constants used a
fluorogenic peptide
substrate, typically H-D-Ala-Leu-Lys-AMC, and were determined in either 100 mM
Mes/Tris,
pH 7.0 containing 1 mM EDTA and 10 mM 2-mercaptoethanol or100mMNa phosphate,
imM
EDTA, 0.1 %PEG4000 pH 6.5 or 100 mM Na acetate, pH 5.5 containing 5 mM EDTA
and 20
mM cysteine, in each case optionally with 1 M DTT as stabiliser. The enzyme
concentration
used was 5 nM. The stock substrate solution was prepared at 10 mM in DMSO.
Screens were
carried out at a fixed substrate concentration of 60 pM and detailed kinetic
studies with
doubling dilutions of substrate from 250 pM. The total DMSO concentration in
the assay was
kept below 3%. All assays were conducted at ambient temperature. Product
fluorescence
(excitation at 390 nm, emission at 460 nm) was monitored with a Labsystems
Fluoroskan
Ascent fluorescent plate reader. Product progress curves were generated over
15 minutes
following generation of AMC product.
Cathepsin S Ki determination
The assay uses baculovirus-expressed human cathepsin S and the boc-Val-Leu-Lys-
AMC
fluorescent substrate available from Bachem in a 384 well plate format, in
which 7 test
compounds can be tested in parallel with a positive control comprising a known
cathepsin S
inhibitor comparator.
Substrate dilutions
280p1/well of 12.5% DMSO are added to rows B - H of two columns of a 96 deep
well
polypropylene plate. 70p1/well of substrate is added to row A. 2 x 250p1/well
of assay buffer
(100mM Na phosphate, 100mM NaCl, pH 6.5) is added to row A, mixed, and double
diluted
down the plate to row H.
Inhibitor dilutions
100 p1/well of assay buffer is added to columns 2-5 and 7-12 of 4 rows of a 96
well V bottom
polypropylene plate. 200p1/well of assay buffer is added to columns 1 and 6.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
39
The first test compound prepared in DMSO is added to column 1 of the top row,
typically at a
volume to provide between 10 and 30 times the initially determined rough K.
The rough Ki is
calculated from a preliminary run in which 10 pl/well of 1mM boc-VLK-AMC (1/10
dilution of 10
mM stock in DMSO diluted into assay buffer) is dispensed to rows B to H and 20
pl/well to row
A of a 96 well Microfluor TM plate. 2 pl of each 10mM test compound is added
to a separate
well on row A, columns 1-10. Add 90 pl assay buffer containing 1 mM DTT and 2
nM cathepsin
S to each well of rows B-H and 180 pl to row A.Mix row A using a multichannel
pipette and
double dilute to row G. Mix row H and read in the fluorescent
spectrophotometer. The
readings are Prism data fitted to the competitive inhibition equation, setting
S = 100 pM and KM
= 100 pM to obtain an estimate of the K;, up to a maximum of 100 pM.
The second test compound is added to column 6 of the top row, the third to
column 1 of the
second row etc. Add 1 pl of comparator to column 6 of the bottom row. Mix
column 1 and
double dilute to column 5. Mix column 6 and double dilute to column 10.
Using an 8-channel multistepping pipette set to 5 x 10 pl, distribute 10
pl/well of substrate to
the 384 well assay plate. Distribute the first column of the substrate
dilution plate to all
columns of the assay plate starting at row A. The tip spacing of the
multichannel pipette will
correctly skip alternate rows. Distribute the second column to all columns
starting at row B.
Using a 12-channel multistepping pipette set to 4 x 1 Opl, distribute 1
Opl/well of inhibitor to the
384 well assay plate. Distribute the first row of the inhibitor dilution plate
to alternate rows of
the assay plate starting at Al. The tip spacing of the multichannel pipette
will correctly skip
alternate columns. Similarly, distribute the second, third and fourth rows to
alternate rows and
columns starting at A2, B1 and B2 respectively.
Mix 20 ml assay buffer and 20 pl 1 M DTT. Add sufficient cathepsin S to give 2
nM final
concentration.
Using the a distributor such as a Multidrop 384, add 30 pl/well to all wells
of the assay plate
and read in fluorescent spectrophotomoter such as an Ascent.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
Fluorescent readings, (excitation and emission wavelengths 390nm and 460nm
respectively,
set using bandpass filters) reflecting the extent of enzyme cleavage of the
fluorescent
substrate, notwithstanding the inhibitor, are linear rate fitted for each
well.
Fitted rates for all wells for each inhibitor are fitted to the competitive
inhibition equation using
5 SigmaPlot 2000 to determine V, Km and Ki values.
Cathepsin L Ki
The procedure above with the following amendments is used for the
determination of Ki for
cathepsin L.
The enzyme is commercially available human cathepsin L (for example
Calbiochem). The
10 substrate is H-D-Val-Leu-Lys-AMC available from Bahcem. The assay buffer is
100mM sodium
acetate 1 mM EDTA, pH5.5) The DMSO stock (10mM in 100%DMSO) is diluted to 10%
in
assay buffer. Enzyme is prepared at 5nM concentration in assay buffer plus 1
mM dithiothreitol
just before use. 2u1 of 10mM inhibitor made up in 100% DMSO is dispensed into
row A. 1 Opl of
pM substrate (=1/200 dilution of 10 mM stock in DMSO,diluted in assay buffer)
15 Inhibition Studies
Potential inhibitors are screened using the above assay with variable
concentrations of test
compound. Reactions were initiated by addition of enzyme to buffered solutions
of substrate
and inhibitor. K; values were calculated according to equation 1.
VS
vo =
KM I+ I +S (1)
Ki
where vo is the velocity of the reaction, V is the maximal velocity, S is the
concentration of
20 substrate with Michaelis constant of KM, and I is the concentration of
inhibitor.
Results are presented as:
A under 50 nanomolar
B 50-500 nanomolar
C 501-1000 nanomolar
25 D 1001 - 5000 nanomolar
E 5001 - 10 000 nanomolar
F in excess of 10 000 nanomolar
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
41
TABLE 1
Example Test Number Ki cathepsin K Ki cathepsin S Ki cathepsin L
1 Test 1 A D B
1 Test2 3.0nM 3300nM 530nM
The compounds of formula II are thus potent inhibitors of cathepsin K and yet
selective over
the closely related cathepsin S and L.
Metabolic Stability
Compounds of the invention and the indicated comparative examples were tested
for
metabolic stability in a cytosol assay in which the compounds were incubated
with
commercially available human hepatic cytosol fractions and the disappearance
of the
compound monitored by HPLC or LC/MS. Pooled human liver cytosol fractions are
less likely
to represent outlier individuals than blood from a single individual and can
be stored frozen,
unlike whole blood. The cytosol assay thus provides a consistent assay testbed
as a guide
to the stability of a compound in the in vivo environment, such as when
exposed to whole
blood.
In short, test compounds (2 pM) are incubated in pooled human liver cytosol
(Xenotech LLC
Lenexa US, 1 mg/mL protein in 0.1 M phosphate buffer, pH 7.4) at 37
centigrade over a one
hour period. The incubations are initiated by the addition of 1 mM NADPH co-
factor. Timed
sub-samples were taken at 0, 20, 40 and 60 minutes and "crash precipitated" by
the addition of
3 volumes of ice-cold acetonitrile. The samples were centrifuged at reduced
temperature and
the supernatants were separated and analyzed by LC-MS-MS.
Alternatively, an analogous stability assay is carried out in human or monkey
whole blood
and/or commerically available liver microsomes.
TABLE 2
Example Structure CLint CLint
whole blood HLM
uI/min/mg uI/min/mg
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
42
comparative 9 49
H
example O O
N
r
~N \ N I/ H O H
S
Example 1 INI NA NA
H
O O
/~ N
_-N \ N I/ O H H O
H
/N-_~
s
The Comparative Example represents a compound bearing a carbon-carbon bond at
the 6
position within the scope of W02008/007107 cited above. It was prepared in a
facile manner
from compound 1 d (scheme 1). Hence with the exocyclic alkene 1 d in hand,
stereoselective
hydrogenation of the alkene with Adams' catalyst (platinum dioxide) in ethyl
acetate under a
hydrogen atmosphere, proceeded with syn addition of hydrogen. This
hydrogenation afforded
essentially one product, namely the C-6 methyl isomer (LCMS [M+H] = 288 found)
with R-
stereochemistry in good yield. The facial selectivity seen here for the
hydrogenation step, is
similar to that reported previously in the literature for a closely related
bicyclic structure
(Srinivas et al, Synlett, 1999, 555-556). The thus prepared building block was
deprotected,
elongated and oxidised to the active keto form as for the compopunds of the
invention
exemplified above.
Improved stability in vivo allows for a better distribution of the compound in
the body
throughout the day, notwithstanding QD or BID dosing. This is particularly
important for
indications such as osteoporosis where diurnal variation is significant.
Permeability
This experiment measures transport of inhibitors through the cells of the
human gastroenteric
canal. The assay uses the well known Caco-2 cells with a passage number
between 40 and
60.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
43
Apical to basolateral transport
Generally every compound will be tested in 2-4 wells. The basolateral and the
apical wells will
contain 1.5 mL and 0.4 mL transport buffer (TB), respectively, and the
standard concentration
of the tested substances is 10 M. Furthermore all test solutions and buffers
will contain 1 %
DMSO. Prior to the experiment the transport plates are pre-coated with culture
medium
containing 10% serum for 30 minutes to avoid nonspecific binding to plastic
material. After 21
to 28 days in culture on filter supports the cells are ready for permeability
experiments.
Transport plate no 1 comprises 3 rows of 4 wells each. Row 1 is denoted Wash,
row 2 "30
minutes" and row 3 "60 minutes". Transport plate no 2 comprises 3 rows of 4
wells, one
denoted row 4 "90 minutes", row 5 "120 minutes and the remaining row
unassigned.
The culture medium from the apical wells is removed and the inserts are
transferred to a wash
row (No. 1) in a transport plate (plate no.1) out of 2 plates without inserts,
which have already
been prepared with 1.5 mL transport buffer (HBSS, 25 mM HEPES, pH 7.4) in rows
1 to 5. In
A-B screening the TB in basolateral well also contains 1 % Bovine Serum
Albumin.
0.5 mL transport buffer (HBSS, 25 mM MES, pH 6.5) is added to the inserts and
the cell
monolayers equilibrated in the transport buffer system for 30 minutes at 37 C
in a polymix
shaker. After being equilibrated to the buffer system the Transepithelial
electrical resistance
value (TEER) is measured in each well by an EVOM chop stick instrument. The
TEER values
are usually between 400 to 1000 S2 per well (depends on passage number used).
The transport buffer (TB, pH 6.5) is removed from the apical side and the
insert is transferred
to the 30 minutes row (No. 2) and fresh 425 .tL TB (pH 6.5), including the
test substance is
added to the apical (donor) well. The plates are incubated in a polymix shaker
at 37 C with a
low shaking velocity of approximately 150 to 300 rpm.
After 30 minutes incubation in row 2 the inserts will be moved to new pre-
warmed basolateral
(receiver) wells every 30 minutes; row 3 (60 minutes), 4 (90 minutes) and 5
(120 minutes).
25 .tL samples will be taken from the apical solution after -2 minutes and at
the end of the
experiment. These samples represent donor samples from the start and the end
of the
experiment.
300 .tL will be taken from the basolateral (receiver) wells at each scheduled
time point and the
post value of TEER is measured at the end the experiment. To all collected
samples
acetonitrile will be added to a final concentration of 50% in the samples. The
collected samples
will be stored at -20 C until analysis by HPLC or LC-MS.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
44
Basolateral to apical transport
Generally every compound will be tested in 2-4 wells. The basolateral and the
apical wells will
contain 1.55 mL and 0.4 mL TB, respectively, and the standard concentration of
the tested
substances is 10 .tM. Furthermore all test solutions and buffers will contain
1 % DMSO. Prior to
the experiment the transport plates are precoated with culture medium
containing 10% serum
for 30 minutes to avoid nonspecific binding to plastic material.
After 21 to 28 days in culture on filter supports the cells are ready for
permeability experiments.
The culture medium from the apical wells are removed and the inserts are
transferred to a
wash row (No.1) in a new plate without inserts (Transport plate).
The transport plate comprises 3 rows of 4 wells. Row 1 is denoted "wash" and
row 3 is the
"experimental row". The transport plate has previously been prepared with 1.5
mL TB (pH 7.4)
in wash row No. 1 and with 1.55 mL TB (pH 7.4), including the test substance,
in experimental
row No. 3 (donor side).
0.5 mL transport buffer (HBSS, 25 mM MES, pH 6.5) is added to the inserts in
row No. 1 and
the cell monolayers are equilibrated in the transport buffer system for 30
minutes, 37 C in a
polymix shaker. After being equilibrated to the buffer system the TEER value
is measured in
each well by an EVOM chop stick instrument.
The transport buffer (TB, pH 6.5) is removed from the apical side and the
insert is transferred
to row 3 and 400 .tL fresh TB, pH 6.5 is added to the inserts. After 30
minutes 250 .tL is
withdrawn from the apical (receiver) well and replaced by fresh transport
buffer. Thereafter 250
L samples will be withdrawn and replaced by fresh transport buffer every 30
minutes until the
end of the experiment at 120 minutes, and finally a post value of TEER is
measured at the end
of the experiment. A 25 .tL samples will be taken from the basolateral (donor)
compartment
after -2 minutes and at the end of the experiment. These samples represent
donor samples
from the start and the end of the experiment.
To all collected samples acetonitrile will be added to a final concentration
of 50% in the
samples. The collected samples will be stored at -20 C until analysis by HPLC
or LC-MS.
Calculation
Determination of the cumulative fraction absorbed, FAcum, versus time. FAcum
is calculated
from:
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
Y CRI
FACUM
CDI
Where CRi is the receiver concentration at the end of the interval i and CDi
is the donor
concentration at the beginning of interval i. A linear relationship should be
obtained.
The determination of permeability coefficients (Papp, cm/s) are calculated
from:
(k - VR)
5 Papp = (A . 60)
where k is the transport rate (min-1) defined as the slope obtained by linear
regression of
cumulative fraction absorbed (FAcum ) as a function of time (min), VR is the
volume in the
receiver chamber (mL), and A is the area of the filter (cm2).
Reference compounds
Category of absorption in Markers % absorption in man
man
PASSIVE TRANSPORT
Low (0-20%) Mannitol 16
Methotrexate 20
Moderate (21-75%) Acyclovir 30
High (76-100%) Propranolol 90
Caffeine 100
ACTIVE TRANSPORT
Amino acid transporter L-Phenylalanine 100
ACTIVE EFFLUX
PGP-MDR1 Digoxin 30
Greater permeability through the gastrointestinal tissue is advantageous in
that it allows for the
use of a smaller dose to achieve similar levels of exposure to a less
permeable compound
administered in a higher dose. A low dose is advantageous in that minimises
the cost of goods
for a daily dose, which is a crucial parameter in a drug which is taken for
protracted time
periods.
CA 02738025 2011-03-21
WO 2010/034789 PCT/EP2009/062407
46
Mutagenicity
The mutagenic potential of compounds is conveniently tested in the Ames Test,
typically
carried out in a variety of bacterial strains such as Salmonella typhimurium
TA100, TA102, TA
1535, TA 1537 with and without liver S9 fraction activation, for example at
30, 300 and 3000
ug/plate concentrations.
Ames testing is readily available at a number of CROs around the world.
Abbreviations
DMF dimethylformamide DCM dichloromethane
TBDMS tert-butyldimethylsilyl RT room temperature
THE tetrahydrofuran Ac acetyl
TLC thin layer chromatography DMAP dimethylaminopyridine
EtOAc ethyl acetate um micromolar
All references referred to in this application, including patents and patent
applications, are
incorporated herein by reference to the fullest extent possible.
Throughout the specification and the claims which follow, unless the context
requires
otherwise, the word `comprise', and variations such as `comprises' and
`comprising', will be
understood to imply the inclusion of a stated integer, step, group of integers
or group of steps
but not to the exclusion of any other integer, step, group of integers or
group of steps.