Note: Descriptions are shown in the official language in which they were submitted.
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
1
CATHETER
Field of the invention
The present invention relates to medical catheters and in particular to
neurosurgical catheters
for insertion directly into the brain parenchyma of a subject.
Background
There are many situations where there is a requirement to deliver therapeutic
agents directly
to specific targets within the brain parenchyma using implanted catheters.
Furthermore, many
of these therapeutic agents will cause unwanted side effects if delivered to
healthy parts of
the brain. Examples of treating abnormalities of brain function include the
acute infusion of
Gamma-amino-buturic-acid agonists into an epileptic focus or pathway to block
transmission,
and the chronic delivery of opiates or other analgesics to the peri-aqueductal
grey matter or to
thalamic targets for the treatment of intractable pain. Also, cytotoxic agents
can be delivered
directly into a brain tumour. Intraparenchymal infusion can also be used to
deliver
therapeutic agents to brain targets that can not be delivered systemically
because they will not
cross the blood-brain barrier. For example, the treatment of patients with
Parkinson's disease,
Alzheimer's disease, head injury, stroke and multiple sclerosis maybe carried
out by the
infusion of neurotrophic factors (e.g. Glial cell derived neurotrophic factor
(GDNF)) to
protect and repair failing or damaged nerve cells. Neurotrophins may also be
infused to
support neural grafts transplanted into damaged or malfunctioning areas of the
brain in order
to restore function.
A number of neurosurgical catheters have been developed previously that can be
guided (e.g.
using a stereo guide) to desired target sites within the brain parenchyma. For
example, it has
been described previously in W02003/077785 how a fine neurosurgical catheter
formed from
carbothane can be inserted into the brain using a guide tube arrangement of
the type
described in US6609020. In one embodiment described in W02003/077785, a guide
tube is
inserted into the brain along a guide wire using a stereotactic placement
technique. This
allows the distal end of the guide tube to be accurately located just short of
the desired brain
target. A fine neurosurgical catheter, reinforced by a fine tungsten guide
wire, is then inserted
into the implanted guide tube and passed along the guide tube until it reaches
the distal end
thereof. The catheter tip then exits the guide tube and catheter insertion is
continued until the
catheter tip reaches the desired target. The fine guide wire is then withdrawn
from the
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
2
catheter lumen leaving the catheter in situ. The use of fused silica catheters
for the delivery
of drugs in to the brain parenchyma has also been proposed previously. Fused
silica catheters
are, however, relatively brittle and tend to fracture if excessively bent.
This makes such
catheters unsuitable for long term implantation within a subject.
It would be advantageous to have a catheter that is stiff enough to allow it
to be inserted into
a target site within the brain, without the need for a guide wire.
Summary of the invention
The invention provides the use of zirconia or alumina for medical purposes,
especially for
the production of medical devices, especially neurosurgical devices. In
particular, the
invention provides the use of zirconia or alumina tubing for such purposes.
The invention
also provides neurosurgical tubing, especially catheters and guide tubes that
comprise
zirconia or aluminia, especially rigid forms of those ceramics, and
particularly rigid tubing
made from those ceramics. Zirconia and alumina may form or be used in
conjunction with
such devices described in W003/077784 and US6609020, both of which are
incorporated by
reference. The ceramic used is preferably zirconia.
The invention provides the use of a stiff or rigid zirconia or alumina tube as
an MR & CT
compatible tube, especially a guide tube to facilitate the implantation of a
neurological
instrument. Such a guide tube may be implanted just short of a desired target.
Following
implantation, a catheter is threaded through the guide tube's bore. Upon
completion of the
surgical procedure the guide tube and catheter may be removed.
In an alternative embodiment, the zirconia or alumina tube may be a stiff or
rigid MR & CT
compatible catheter, for delivery to a target, especially a target within the
brain. Such a
catheter may be used with a stereotactic system. The tube may be used to
deliver therapeutics
to the target site.
According to a first aspect of the present invention, there is provided a
delivery or sampling
device for insertion into a subject. The delivery or sampling device is
preferably a catheter
comprising a tube, substantially formed from, or comprising a rigid layer
substantially
formed from, zirconium dioxide or aluminium oxide.
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
3
The catheter is preferably a neurosurgical catheter, for insertion into the
brain parenchyma of
a subject. The catheter comprises a length of stiff tubing, the tip of which
can be accurately
located at a required target point or region within the brain. The catheter
may comprise one or
more lumens as required and, when implanted, may delivery any type of
therapeutic agent or
fluid directly to a target region within the brain.
A rigid catheter in accordance with the present invention has the advantage
that it can be
accurately guided to a target site within the brain parenchyma. In particular,
the catheter will
not be significantly deflected from the required insertion direction even when
passed through
virgin brain tissue or into tough matter such as brain tumours or similar
tisues. A catheter of
the present invention thus has the advantage of not requiring any additional
reinforcement
(e.g. using a stiffening wire or cannula) during implantation.
A catheter of the present invention is particularly suited for use in
combination with guide
tube devices such as those described in W02003/077785 and US6609020. As
mentioned
above, W02003/077785 describes how a guide tube can be stereotactically
implanted in the
brain so that its distal end is just short of a desired target. A fine
flexible catheter, reinforced
by an even finer tungsten wire, is then inserted into the brain parenchyma
through the guide
tube. During catheter insertion, the catheter tip exits the distal end of the
guide tube and is
forced a short distance through virgin brain tissue to the desired target. It
has, however, been
found that in some instances the tip of the catheter described in
W02003/077785 can still
deviate from the axis of insertion defined by the longitudinal axis of the
guide tube during
such an implantation process. Even relatively small deviations from the
identified target site
are undesirable as they can significantly reduce treatment efficacy and may
cause unwanted
damage to sensitive regions of the brain. These deviations from the required
target have been
found to be a particular problem when the catheter has a small outside
diameter (thereby
requiring the use of a very thin tungsten wire) and/or when the tip has to be
inserted into
relatively tough tissue (such as a brain tumour or similar tissue). The
removal of the tungsten
guide wire after catheter implantation without disturbing catheter placement
can also prove
problematical. The present invention, through the provision of the stiff
catheter, overcomes
the need to use a reinforcing guide wire during catheter implantation whilst
also allowing
accurate guiding of the catheter tip to the required target. The present
invention thus avoids
certain problems that can arise when using catheters of the type described in
W02003/077785.
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
4
Alternatively, the catheter of the invention may be used without a guide tube,
it being stiff
enough to penetrate brain tissue without deviating from the desired axis of
insertion.
Accordingly, the device may be located stereotactically, using a stereotactic
guide or other
interface to direct the positioning of the device.
The catheter tube comprises a rigid layer of a solid ceramic, specifically
zirconium dioxide or
aluminium oxide. The rigid layer is preferably formed substantially from that
ceramic, the
layer comprising at least 95% by weight of the ceramic, preferably at least
97% by weight,
more preferably at least 99% by weight, more preferably 100% by weight.
Zirconium dioxide has been used in prior art devices, such as catheters
described in
EP1136085. In that application, zirconium dioxide was combined with a plastic
material to
provide a strengthened and radio-opaque wall. Alternatively, other prior art
devices have used
networks of braided ceramic fibres, as described in US20050163954, such
catheters being
strengthened by the braided fibres, but still remaining flexible.
The catheter of the present invention is rigid, unlike the prior art
catheters. Rigidity is
provided by the layer of ceramic in the catheter wall. The catheter wall may
also comprise
other layers, for example, the catheter may be a tube that is coated with the
ceramic layer. In
that case, the tube may be made of a flexible material, a rigid material or a
composite
material with flexible and rigid characteristics, such as coated fused silica.
The ceramic layer preferably covers at least 75%, more preferably at least
80%, more
preferably at least 85%, more preferably at least 90%, even more preferably
100% of
circumference of the catheter tube.
The ceramic layer is preferably substantially solid. If openings, holes or
apertures are
provided in the layer, they are preferably in fluid connection with the lumen
of the catheter.
The catheter may comprise a single fluid aperture at its distal end and/or one
or more
apertures may be provided in the sides of the catheter.
The catheter is preferably of an appropriate size for neurosurgical
implantation. For example,
the outer diameter of the catheter is preferably between 100 m and 1.5mm, more
preferably
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
between 200 m and 1.25mm, more preferably between 200 m and 500 m, preferably
between 220 m and 280 m, more preferably between 230 m and 250 m.
The inner diameter of the catheter is preferably between 70 m and 250 m,
preferably
between 80 m and 120 m, more preferably between 90 m and 110 m.
The walls of the catheter may be coated to improve elution, or to reduce
friction when the
catheter is inserted or removed.
The tip of the catheter at its distal end may be shaped to improve deliver of
fluids and to
reduce trauma when the catheter is inserted. For example, the tip may be
rounded in shape at
its end. Also, the end of the catheter may include a series of steps, reducing
the outer
diameter of the catheter in the region of the tip.
The interior or exterior walls, especially the exterior walls, may be shaped
or profiled, for
example provided with steps or grooves around the circumference of the wall or
longitudinally, along the length of the catheter. For example, the outer
diameter of the
catheter may be reduced towards the tip using one or more steps or by tapering
the walls.
Such profiling or shaping may be used to promote or discourage fluid movement
along the
catheter walls. The walls of the catheter may also be provided with markings
to indicate how
far the catheter has been inserted into a patient.
In certain embodiments, the rigid tube of the catheter may be connected to a
flexible tube at
the proximal end of rigid tube. This may aid in connecting the catheter to a
supply device
such as a hub or port. Alternatively, the catheter may be connected directly
to a supply tube
from that supply device.
For a long-term implantable embodiment, the proximal end of the catheter may
be connected
to a supply tube. The supply tube may be flexible and may have an outside
diameter that is
greater than the flexible tube emanating from the proximal end of the
catheter. The
connection between the flexible tube and the supply tube is conveniently
located outside of
the brain parenchyma and is preferably located outside the skull.
Advantageously, fixing
means are provided for securing the flexible tube of the catheter in place
(e.g. by fixing it to
the skull) after implantation; this ensures that the catheter tip does not
deviate from the
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
6
desired position within the brain parenchyma. The supply tube may, for
example, be
connected to the flexible tube by a connector or hub that is secured (e.g.
screwed) to the
outside of the skull and subcutaneously buried under the scalp.
The catheter may be designed for long term implantation and is thus preferably
fabricated
from materials that are suitable for long term implantation.
For a non-implantable embodiment, the supply tube & the flexible tube can be
replaced by
one continuous element, which may be connected to the rigid tubing as
required. This may be
preferable as it is not always desirable to leave a rigid device implanted
within the skull. In
such an embodiment, the supply tube may be connected prior or after insertion
of the
catheter. For example, the catheter may be inserted stereotactically either
with or without a
guide tube. If necessary, its position may be maintained by an external clamp.
The proximal
end of the catheter may then be temporarily connected to a supply tube
connecting it to a
delivery hub or pump.
It should also be noted that the catheter is preferably passively insertable
(i.e. it is preferably
not actively steerable).
The present invention may also comprise a neurosurgical kit comprising; a
neurosurgical
catheter as described above and a neurosurgical guide tube device, wherein the
neurosurgical
guide tube device comprises a guide channel (e.g. formed by an elongate guide
tube) through
which the neurosurgical catheter can be passed. The neurosurgical guide tube
device is
preferably of the type described previously in US6609020 or W02003/077785.
Conveniently, the outer diameter of the catheter is less than the internal
diameter of the guide
channel and such relative diameters are preferably arranged so that the
catheter fits snugly
within the guide channel. The guide channel of the guide tube thus acts to
guide the catheter
to the desired target even after the distal end of the tip has exited the
guide channel. Based on
the teachings contained herein, a skilled person would thus be able to select
the relative
lengths of the catheter and the guide tube for the particular surgical
procedure being
performed; this selection would vary from subject to subject and would take
into account the
required proximity of the guide tube to the desired target and the depth of
the target within
the brain. It should also be noted that the guide tube and/or catheter may be
manufactured as
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
7
standard lengths and tailored (e.g. cut by the surgeon) to the required length
before or during
the surgical procedure. The kit may also include other components. For
example, a
subcutaneous drug delivery pump and/or additional fluid tubing may be
provided. A
stereoguide for implanting the guide tube device may also be provided as part
of the kit.
As described above, one of the primary uses of the delivery or sampling device
of the
invention is as a neurosurgical catheter. Also envisaged is the use of the
device as a biopsy
needle. In that case, the device preferably comprises a rigid tube formed from
zirconium
dioxide or aluminium oxide or comprising a rigid layer of such a ceramic, the
tube being
appropriately shaped for use as a biopsy needle. For example, the tip of the
tube may be
shaped to form a point.
Alternatively, the device may be used for the delivery of a solid agent, such
as a pellet of a
radio isotope. In that case, the device may be formed as a rigid rod or tube
made from or
comprising a rigid layer of zirconium dioxide or aluminium oxide. The rod or
tube may be
shaped to allow the solid agent to be mounted upon it or delivered through it.
Also, the device may be used to deliver an electrode to a site of interest.
Accordingly, the
device may be formed as a rigid rod or tube made from or comprising a rigid
layer of
zirconium dioxide or aluminium oxide and comprising an electrically conducting
material
extending along the length of the tube or rod and being exposed or
electrically connected to
an exposed area on the surface of the rod or tube. The rod or tube is
preferably arranged to
allow connection of the electrically conducting material to an electrical
supply.
Also provided by the invention is a rigid implantable device, such as a bone
implant, formed
from or including a ceramic, especially zirconium dioxide or aluminium oxide.
The devices of the invention are preferably formed from, or comprise zirconium
oxide.
According to another aspect of the invention, a method of manufacturing a
device comprises
the steps of extruding a rigid tube or rod of zirconium dioxide or aluminium
oxide or coating
a tube or rod with a rigid layer of zirconium dioxide or aluminium oxide (e.g.
a flame-
deposited ceramic coating).
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
8
Aspects of the shaping, profiling, marking and sizing, as discussed above in
relation to the
catheter may also be found on other devices according to the invention.
According to a fifth aspect of the invention, a method of delivering a
therapeutic substance to
a target with the brain parenchyma of a subject is provided. The method
comprises the steps
of (i) taking a delivery device, especially a catheter, according to the
invention and (ii)
inserting the device into a subject, especially into the brain parenchyma of
the subject.
Advantageously, step (ii) comprises inserting the catheter into the brain
parenchyma through
a previously implanted guide tube device. An initial step may thus be
performed of
implanting a guide tube device, such as a guide tube device of the type
described previously
in US6609020 or W02003/077785, in the brain parenchyma of a subject. During
the
implantation of the guide tube device, its distal end maybe located (just)
short of the required
target within the brain parenchyma. Advantageously, step (ii) comprises
passing the catheter
through the previously implanted guide tube device until the tip of the
catheter reaches the
desired target within the brain parenchyma. Conveniently, the tip may be
guided with the aid
of the guide tube device as it exits therefrom and is moved towards the
target.
Once implanted, the step (iii) maybe performed of delivering a therapeutic
substance to the
brain parenchyma via the implanted catheter. A catheter may be implanted
whenever delivery
of a therapeutic substance is required or it may advantageously remain
implanted for the
long term (e.g. for months or years).
When using a device according to the invention, or other implantable devices,
it may be
advantageous to be able to advance or retract the device without the surgeon
manually pulling
or pushing on the device. This is particularly important where the surgeon may
be acting
remotely. Accordingly, there is provided an implantable device, such as a
catheter or a guide
tube, comprising an advancement means for retracting or advancing a portion of
the device
along an axis of insertion into a subject, minimising tissue trauma. The means
may advance
or retract the device using any appropriate method, examples being a slide, a
piezo-electric
motor or a helical screw, such that when the means is turned, the device is
advanced or
retracted. The means may be used to advance or retract the device over a
length appropriate
to the device's use, but is preferably only used to advance or retract the
device short
distances, such as less than 10mm. One or both of the device and the
advancement means
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
9
may be provided with a scale to indicate how far the device has been advanced
or retracted.
In addition, one or both of the device and the advancement means may be
provided with a
stop to prevent movement beyond a certain maximum position. Such a stop may be
moveable
prior to use of the advancement means and then fixable in the desired
position.
Another aspect of the present invention provides an optical instrument for use
in surgery, the
instrument comprising a tube having at least one optical fibre arranged within
a bore region, a
wall of the tube comprising a rigid layer formed substantially from a ceramic
selected from
zirconium dioxide or aluminium oxide. Preferably, the at least one optical
fibre comprises a
plurality of optical fibres. It is also preferable that the at least one
optical fibre extends
between a distal end of the tube and a proximal end of the tube and is capable
of transporting
light between both ends. Embodiments of the optical instrument advantageously
provide a
rigid tube that is capable of transporting light into, and/or out of, a
patient. Due to the rigid
nature of the tube it can be accurately guided to a target site within the
patient and will resist
deflection by internal parts of the patient's body, such as, brain tissue or
brain tumours.
Preferably, the at least one optical fibre is further arranged to receive
light from outside the
tube at the distal end, and provide the received light at the proximal end to
an image
reproducing means for reproduction of an image present at the distal end. It
is also preferable
that at the distal end of the tube the at least one optical fibre is
terminated with a substantially
convex profile so that the instrument's field of view is large with respect to
an outer diameter
of the tube. These embodiments are capable of being inserted inside a patient
in order to
collect images therefrom. Further, the area imaged by these embodiments can be
large in
comparison to the area of the opening at the distal end of the tube through-
which images are
collected. Preferably, the optical instrument is further arranged to be
coupled to a light source
for delivering light to the distal end. It is an advantage of this embodiment
that bright and
detailed images can be obtained.
Preferably, the at least one optical fibre is further arranged to emit light
out of the tube from
the distal end. It is also preferable that the at least one optical fibre is
terminated at the distal
end with a profile for causing at least one of the following effects in the
emitted light:
a. diffusion which is wide with respect to an outer diameter of the tube;
b. focusing which is narrow with respect to an outer diameter of the tube; and
c. refraction at 90 to a central axis of the tube.
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
It is additionally preferable that the emitted light is received from a light
source coupled to
the proximal end. It is an advantage of these embodiments that the optical
instrument can be
used to deliver light to a target site inside a patient, for example, to
effect treatment of an
illness. It is a further advantage that the way in which light is delivered to
the patient from the
optical instrument can be adjusted in dependence on the type of treatment to
be administered.
A further aspect of the present invention provides a surgical probe comprising
a tube
terminated at a distal end by a tip, at least a wall of the tube comprising a
rigid layer formed
substantially from a ceramic selected from zirconium dioxide or aluminium
oxide, the probe
further comprising a first electrode housed within a bore region of the tube
and positioned
towards the distal end. Preferably, the first electrode is a disc electrode
which is coaxial with
the tube, positioned adjacent to the tip and in electrical communication with
a proximal end
of the tube. These embodiments advantageously provide a rigid mono-polar probe
which is
suitable for insertion inside a patient and for measuring the electrical
impedance of internal
parts of the patient's body. Due to the rigid nature of the probe it can be
accurately guided to
a target site within the patient and will resist deflection by internal parts
of the patient, such
as, brain tissue or brain tumours.
Preferably the surgical probe further comprises a second electrode housed
within the bore
region, positioned the proximal side of the first electrode, wherein both
electrodes are
electrically insulated from each other. It is additionally preferable that the
second electrode is
a disc electrode which is coaxial with the tube, positioned adjacent to the
first electrode, and
in electrical communication with the proximal end. These embodiments
advantageously
provide a rigid bi-polar version of the surgical probe.
Brief description of the drawings
The invention will now be described, by way of example only, with reference to
the
accompanying drawings in which;
Figure 1 illustrates a prior art neurosurgical catheter and guide tube
arrangement,
Figure 2 illustrates a catheter of the present invention, and
Figure 3 illustrates a catheter of the present invention inserted into an
implanted guide tube.
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
11
Figure 4 illustrates an advancement means according to the present invention,
on a rigid
guide tube of the present invention (A) and on a catheter (B) of the present
invention.
Figure 5 shows the advancement means of the present invention.
Figure 6 is an exploded view of the advancement means.
Figures 7 and 8 illustrate the optical instrument of the invention.
Figures 9 and 10 illustrate the surgical probe of the invention.
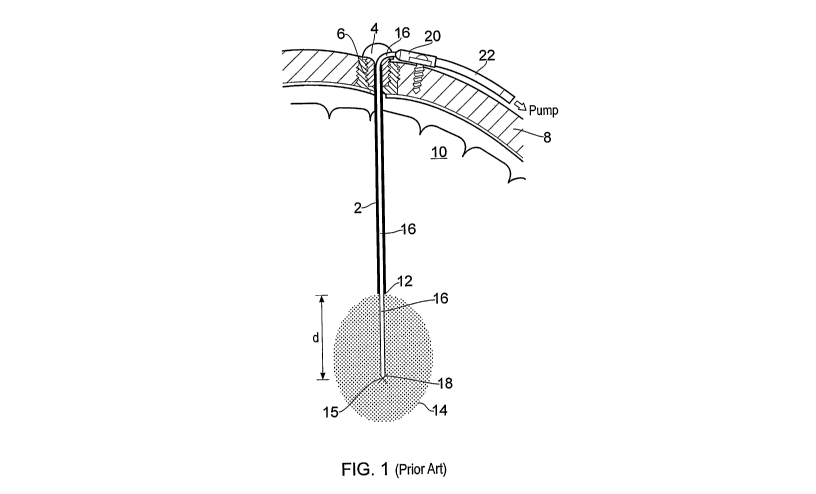
Referring to figure 1, a prior art implanted fluid delivery system of the type
described in
W02003/077785 is illustrated. The fluid delivery system comprises a guide tube
device
comprising an elongate guide tube 2 having a head portion 4 at its proximal
end. The head
portion 4 has an external thread 6 to allow attachment to a burr hole formed
in the skull bone
8 of a subject. The guide tube device is inserted stereotactically into the
brain parenchyma 10
using a stereoguide device. In particular, the guide tube device can be
accurately inserted in
the brain along a predefined axis of insertion such that its distal end 12 is
located just short
(by a distance d) of a target point 15. More details concerning accurate (e.g.
stereotactic)
insertion of the guide tube can be found elsewhere; for example, see
W02003/077784,
W02003/077785 and US6609020.
After the guide device has been implanted, a catheter is inserted through the
head portion 4
and into the guide tube 2. The catheter comprises a length of flexible fine
tubing 16. The
tubing has an outside diameter of lmm or less. During implantation, the fine
tubing 16 is
inserted into the guide tube 2 and advanced therethrough until the distal end
18 of the fine
tube 16 protrudes a distance "d" from the distal end 12 of the guide tube 2
and thereby
reaches the target point 15.
As described in W02003/077785, when a flexible catheter is used, it is
typically reinforced
by a guide wire (not shown) during implantation to prevent the catheter
significantly
deviating from the required axis of insertion as it exits the distal end 12 of
the guide tube 2
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
12
and is driven towards target point 15. Once implanted, the guide wire is
withdrawn from the
catheter leaving the catheter in situ.
The fine tube 16 of the catheter is connected to a hub 20 that is screwed to
the outside of the
skull 8. A supply tube 22 is in fluid communication with the fine tube 16 via
a channel
formed in the hub 20. The supply tube 22 may receive fluid from an implanted
drug pump,
the fluid then being routed along the fine tube 16 to the target volume 14.
The catheter and
guide tube device are arranged to be long term implantable thereby allowing
drug delivery,
either continuously or intermittently, over prolonged periods of time.
Although the prior art neurosurgical catheter system described above with
reference to figure
1 typically enables accurate catheter placement, it has been found by the
present inventors
that it can sometimes suffer from a number of problems. For example, the use
of a fine tube
16 (e.g. having an outer diameter of lmm or less) means that only a relatively
small diameter
guide wire can be used to stiffen the catheter during insertion. This means
that the distal end
18 of the catheter can still wander off course during implantation, especially
when insertion
into tough tissue (such a brain tumour or cyst) is required. It has also been
found that the
process of removing a fine guide wire from the fine tubing 16 can prove
difficult to perform
in a surgical environment and in particular that the process of guide wire
removal can
sometimes reduce the accuracy with which the distal end 18 is located relative
to the target
point 15.
Referring to figure 2, an improved catheter 30 according to the present
invention is shown.
The catheter 30 comprises a length of rigid tube 32. The tube is formed from
or comprises a
layer of a rigid ceramic, especially zirconium dioxide and has an outside
diameter of around 1
min or less.
The tip 34 of the catheter is shaped. The distal edge of the catheter is
rounded to reduce
trauma on insertion. The external wall is provided with a series of steps 38
to gradually
reduce the external diameter of the catheter. The external wall may also be
provided with
grooves or channels (not shown). The catheter may be coated with, for example,
polyimide.
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
13
The catheter may be provided with an advancement means 36 to allow automatic
advancement or retraction of the catheter. The advancement means is shown in
more details
in figures 4 to 6. The external surface of the catheter is provided with a
scale to indicate how
far the catheter has been moved. Such an advancement means could be used with
any other
catheter or implantable device that is to be advanced or retracted along an
axis.
In this embodiment of the invention, a single lumen is provided through the
catheter and that
fluid will exit the catheter through a single aperture located at the distal
end of the tube 32. It
should, however, be noted that multiple lumen variants of the catheter maybe
provided.
Furthermore, the fluid aperture may be located in a different position to that
shown in figure
2; for example, an aperture may be provided on the side of the tube. If
necessary, more than
one fluid aperture may also be provided.
In other embodiments, not shown, the device of the invention comprises a rigid
rod, needle or
implant, formed from or comprising a rigid ceramic especially zirconium
dioxide or
aluminium oxide.
The catheter 30 can be fabricated using any one of a number of techniques. In
a preferred
embodiment, the catheter 30 is fabricated by coating a long fused silica tube
with the required
ceramic. Alternatively, the ceramic may be extruded to form the rigid tube 32
and then
sintered. Other devices of the invention may be fabricated in similar manners,
either by
coating a support such as a rod with the ceramic, or by extruding the ceramic
to form the
device.
Referring to figure 3, implantation of a catheter of the present invention in
a subject will be
described. In common with prior art arrangements of the type described with
reference to
figure 1, a guide tube device comprising a guide tube 102 and a head portion
104 is firstly
implanted in a subject (e.g. a person or an animal) using known stereotactic
techniques. The
guide tube 102 may thus define the axis of insertion to a target point 115 for
delivery of a
therapeutic agent to a target volume 114 within the brain parenchyma 10. A
thread 106
provided on the head portion 104 firmly anchors the guide device to the skull
bone 8 of the
subject. The catheter 30 of the present invention is inserted into the guide
tube 102 through
the head portion 104. The tip 34 of the catheter is then fed along the guide
tube 102 towards
the target volume 114. The catheter 30 is inserted into the guide device until
the distal end of
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
14
the catheter tip 34 extends a distance d from the distal end of the guide tube
102. This
distance d can be set by providing a mark or other indicator (e.g. a graticule
or scale) on the
rigid tube 32 and a corresponding mark on the head portion 104; alignment of
these marks
indicates that the distal end of the catheter has advanced the required
distance d from the
distal end of the guide tube 102. Imaging techniques may also or alternatively
be used during
implantation to identify catheter tip position.
The guide tube 102 is arranged to have an internal diameter that is only
slightly larger than
the outside diameter of the rigid tube 32 of the catheter. In this manner, the
stiff tube 32 is
guided along the axis of insertion defined by the guide tube 102 and,
importantly, such
guidance is still provided even when the distal end of the catheter 30 exits
the guide tube 102.
The inherent stiffness of the catheter thus accurately guides the tip to the
target point 115
without the need to use any kind of wire or cannula to reinforce the catheter.
The problems
associated with using, and removing, a guide wire are thus mitigated thereby
making the
catheter implantation process simpler and quicker whilst providing high
targeting accuracy.
Furthermore, a catheter of the present invention can be primed before
insertion thereby
preventing the introduction of air in to the brain. In order to allow the
connection of the
catheter to a hub or other device, the catheter may be connected to a flexible
tube 38. Once
the distal end of the catheter 30 has been placed at the target point 115, the
flexible tube 38
can be bent either within or above the head portion 104 of the guide device.
The flexible tube
is sufficiently bendable to be routed (without fracturing) through a right
angle in the vicinity
of the skull bone (e.g. within the head portion 104 of the guide tube device)
to allow
subcutaneous burying of the catheter. It should be noted that it is the
flexible tube 38 that is
bent and there is no need to bend the stiff tube 32.
In the present embodiment, the proximal end of the flexible tube 38 is
attached to a hub 120
that may be screwed to the skull bone 8 of the patient thereby securing the
catheter in place if
the catheter is for long term implantation or may be clamped above the head,
especially if the
catheter is only for short term implantation. A supply tube 122 for supplying
fluid from an
associated (e.g. implanted) drug pump is also connected to the flexible tube
38 via the hub
120. It should, however, be noted that the hub 120 and supply tube 122 are not
essential parts
of the invention and merely provide a convenient means for routing fluid to
the catheter for
onward delivery to the target volume 114. The proximal end of the rigid tube
could be
connected, permanently or whenever required, to any (e.g. implanted or
external) fluid source
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
when fluid delivery through the catheter is required. The length of the
flexible tube 38 and/or
tube 122 may thus be selected to permit the required fluid connections.
It should also be noted that the catheter of the present invention can also
allow the distance d
between the distal end 112 of the guide tube 102 and the required target point
115 to be
increased if required without significantly degrading targeting accuracy.
Increasing this
distance can reduce the amount of damage to brain tissue and can also reduce
fluid reflux
along the interface between the brain tissue and the guide tube. The tip
length and/or the
distance d between the distal end 112 of the guide tube 102 and the target
point 115 can thus
be varied as required on a patient-to-patient basis to provide the optimum
treatment regimen.
It is also important to note that the catheter of the present invention can be
used with a
different type of guide tube than that described above and may even be used
without any kind
of guide tube device. For example, a catheter or other appropriate device of
the present
invention may be inserted directly into the brain parenchyma, without any
guide tube. In such
an instance, the device, especially a catheter, is inserted stereotactically
into the brain
parenchyma using a stereoguide device. In particular, the device can be
accurately inserted in
the brain along a predefined axis of insertion such that its distal end is
located at a target
point. Details of stereotactic implantation of devices are described in
W02003/077784,
W02003/077785 and US6609020, which are incorporated by reference herein.
Also described above is the implantation of a device that comprises a flexible
tube for
connection of the device to a supply device. A device according to the
invention may not
comprise such a flexible tube and may simply comprise a rigid portion of
tubing. The tubing
may be directly connectable to a supply device, if needed.
It should also be noted that although the above examples refer to delivery of
therapeutic
agents (e.g. drugs, viruses etc) through the catheter, it would also be
possible to collect a fluid
using the catheter. The above described catheter is particularly suited for
use in neurosurgical
applications where catheter insertion directly into the brain parenchyma
through a hole in the
skull is required. The catheter can, however, also be used for other medical
applications. For
example, it may be used in applications where fluid needs to be delivered to
an accurately
defined target within an organ (e.g. to the liver, kidneys etc). The skilled
person would thus
be aware of the numerous applications for the catheter described herein.
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
16
Referring now to figures 4 to 6, an implantable device may be provided with an
advancement
means. As shown in figure 4, the advancement means 130 comprises a controller
132 and an
actuator means 134. Activation, in this case turning, of the controller
results in advancement
or retraction of the instrument to which the advancement means is attached. As
shown in
figure 6, the actuator means may be a linear actuator which translates
rotational movement of
the controller. Alternatively, the actuator means may be another mechanical,
electromechanical or piezoelectric actuator. A variety of controllers may also
be used.
As shown in figure 4, the advancement means may control a catheter 136 or
infusion tube
within a guide tube 138. Alternatively the catheter or tube may be used
without the guide
tube. In the latter case, the catheter or tube may be provided with an endstop
140, to prevent
further advancement (or retraction) of the catheter.
A device bearing the advancement means may be implanted into a subject's
brain, using
stereotactic techniques as described previously. The device may be implanted
such that the
tip of the device is short of the target site. The device, or a portion of the
device may then be
advanced using the advancement means such that it reaches the target site. For
example, the
device may comprise a guide tube that is inserted short of the target site.
The device may
further comprise a fine infusion tube within the guide tube. The advancement
means may
then be used to advance the infusion tube out of the guide tube and towards
the target site,
until the tip of the infusion tube reaches the target. The advancement means
may also be used
to retract the device away from the target. This may be done to remove the
device. The
device might also be advanced or retracted during infusion of an agent to
increase the target
area.
Referring now to figure 7, an optical instrument 150 is shown comprising a
hollow
cylindrical tube 152 having a distal end 154 and a proximal end 156. A central
bore of the
tube 152 houses a cylindrical fibre optic bundle 158 comprising a plurality of
fibre optic
strands 160. Each of the fibre optic strands 160 extends between the distal
end 154 and the
proximal end 156 and is capable of transporting light between both ends. Each
of the fibre
optic strands 160 is terminated at the distal end so that the bundle 158
terminates with a
substantially convex profile. More specifically, a radially outermost layer of
fibre optic
strands 160 of the bundle 158 terminate such that they are flush with the end
of the distal
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
17
portion 154. A layer of fibre optic strands 160 which are immediately adjacent
to, and
radially inward of, the radially outermost layer extend just beyond the
radially outermost
layer. Each subsequent radially inner layer of fibre optic strands 160 extends
just beyond an
immediately adjacent and radially outer layer of fibre optic strands 160.
Accordingly, fibre
optic strands 160 which are at a centre of the bundle 158 extend the greatest
distance beyond
the distal end 154.
The proximal end 156 of the optical instrument 150 is arranged to be coupled
to an image
reproducing means (not shown). The image reproducing means is capable of
reproducing an
image from light received at the distal end 154 and transported by the bundle
158 to the
proximal end 156. Accordingly, the image reproducing means when combined with
the
optical instrument 150 is capable of reproducing an image present at the
distal end 154 of the
optical instrument 150. Additional lighting means (not shown) can also be
provided to
illuminate the area at the distal end 154 and thereby increase the quantity of
light received by
the optical instrument 150 and the quality of the image provided by the image
reproducing
means, as is well known in the art. Suitable image reproducing means will be
apparent to the
skilled person and include, for example, an eye piece or a charge coupled
device (CCD)
camera. Also, suitable methods of coupling the tube 152 and the bundle 158 to
the image
reproducing means will be apparent to the skilled person and are outside the
scope of the
present embodiment.
The profile with which the bundle 158 terminates at the distal end 154 defines
how the image
present at the distal end 154 is transmitted and refracted before it is
transported to the
proximal end 156. Moreover, the termination of the bundle 158 at the distal
end 154 acts as a
lens between the image at the distal end 154 and the proximal end 156.
Further, the profile of
the termination defines the properties of the lens, i.e. how the image at the
distal end 154 is
transmitted and refracted before it is provided at the proximal end 156. As
discussed above,
the profile in figure 7 is substantially convex and therefore, it acts as a
substantially `fish-eye'
shape lens. An advantage of a convex profile is that it provides a large field
of view with
respect to an outer diameter of the tube 152. A convex profile also distorts
the image in order
to provide a large field of view however, the distortion can be compensated
for in order to
provide an image having a large field of view and minimal distortion. For
example, the
optical instrument 150 together with an image reproducing means can be used to
view a
calibrated artefact in order to obtain an optical error-map. The optical error-
map allows any
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
18
subsequent image provided by the arrangement to be mapped and real-time
corrected, as is
well known in the art. Accordingly, the arrangement is capable of providing
highly anaclastic
optical performance with a large field of view from the relatively narrow
diameter tube 154.
The optical instrument 150 is suitable for being inserted inside a patient as
part of a surgical
procedure. For example, the optical instrument 150 can be inserted through a
patient's skull
and inside the patient's brain as part of a neurosurgical procedure. The
optical instrument 150
is particularly well suited to neurosurgical applications by virtue of its
construction. More
specifically, the tube 152 comprises a rigid layer formed substantially from a
ceramic
selected from zirconium dioxide or aluminium oxide which gives the tube 154 a
rigid
material property. A rigid instrument is advantageous because the instrument
can be
accurately guided to a target site within the brain parenchyma. In particular,
the instrument
will not be significantly deflected from the required insertion direction even
when passed
through virgin brain tissue or into tough matter such as brain tumours or
similar tissues. The
instrument therefore has the further advantage of not requiring any additional
reinforcement
during insertion. Additionally, the optical instrument 150 is suitable for
operating within a
magnetic resonance environment by virtue of the fact it is not constructed
from materials
which are influenced by a magnetic field.
Various modifications can be made to the embodiment of figure 7, such as, for
example,
rather than having an independent light source the optical instrument itself
can be provided
with a light source which is capable of providing illumination to an image
located at the distal
end 154.
Thus far the optical instrument of figure 7 has been described for use as an
endoscope, and in
particular, a neuro-endoscope. However, it is within the scope of appended
claims that the
optical instrument of figure 7 is suitable for use as a fibre-optic delivery
instrument.
In order to function as a fibre-optic delivery instrument, the proximal end
156 is coupled to a
light source (not shown) according to a method which will be apparent to the
skilled person
and therefore is outside the scope of the appended claims. According to this
arrangement,
light from the light source is received at the proximal end 156 by the bundle
158 and is
transported to the distal end 154 via the bundle 158. On reaching the end of
the bundle 158 at
the distal end 154 the light is emitted out of, and away from, the optical
instrument 150. As
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
19
discussed above, the termination of the bundle 158 acts as a lens which
transmits and refracts
the light according to the profile of the termination. However, in contrast to
the above, the
light is emitted from the distal end 154 rather than received at the distal
end 154. The bundle
158 terminates with a convex profile and therefore, light is emitted from the
distal end 154
with a wide-angle of dispersion. Such an arrangement is particularly suitable
for surgical
procedures, such as, for example, photodynamic therapy (PDT) wherein it is
desirable to have
light dispersed over a wide area to improve treatment effectiveness.
As seen more particularly on figure 8, an alternative fibre-optic delivery
tube 164 comprises
the bundle 158 terminating with a substantially concave profile at the distal
end 154.
According to the embodiment of figure 8, light is emitted from the distal end
154 with a
narrow angle of dispersion and therefore, the light emitted from the distal
end 154 is focused
on a particular point or region. Further, the area of the point or region is
sized and shaped in
dependence on the precise shape of the concave profiling and therefore, the
area of the point
or region can be changed by altering the shape of the concave profile. A
concave profile is
particularly suitable for surgical procedures, such as, for example, tissue
ablation, wherein it
is desirable to have a highly focussed beam of light which can be directed
towards a
predefined area to administer treatment most effectively.
It is also within the scope of the appended claims that the bundle is
terminated with any
profile other than a convex or concave profile. Moreover, the profile could be
shaped to
generate a particular effect in the light either entering or exiting the
distal end. For example,
when the optical instrument is used as a fibre-optic delivery instrument, it
is often desirable
for light exiting the distal end to be emitted substantially perpendicularly
to the axis of the
tube of the optical instrument. Such an arrangement is particularly
advantageous in some
PDT or tissue ablation applications, wherein the instrument can be rotated
and/or moved
axially once it has been located in position within a patient to project light
onto tissue which
is radially outward from the distal end.
Additionally, it is within the scope of the appended claims that the outer
surface of the optical
instrument can be encoded with an absolute scale so that the instrument's
axial position
inside a patient can be quickly and easily determined. Accordingly, the
optical instrument can
be reliably guided to the correct depth within a patient.
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
Referring now to figure 9, wherein a cross section of a surgical probe 170 is
shown. The
probe 170 comprises a hollow cylindrical tube 172 having a central bore region
174. The tube
172 is open at a proximal end 176 and is terminated by a hemispherical tip 178
at a distal end
180. A disc electrode 182 is positioned inside the bore region 174 and towards
the distal end
180. Preferably, the disc electrode 182 is coaxial with the tube 172 and is
positioned
immediately behind the tip 178. The disc electrode 182 is conducted to the
proximal end 176
by electrical conductor 184, such as, an electrical lead, which is housed
within the bore
region 174.
The probe 170 is suitable for being inserted inside a patient as part of a
surgical procedure.
For example, the probe 170 can be inserted through the patient's skull and
inside the patient's
brain as part of a neurosurgical procedure. The probe 170 is particularly well
suited to
neurosurgical applications by virtue of its construction. More specifically,
the tube 172 and
the tip 178 comprise a rigid layer formed substantially from a ceramic
selected from
zirconium dioxide or aluminium oxide which gives the tube and tip a rigid
material property.
A rigid probe is advantageous because it can be accurately guided to a target
site within the
brain parenchyma. In particular, the probe 170 will not be significantly
deflected from the
required insertion direction even when passed through virgin brain tissue or
into tough matter
such as brain tumours or similar tissues. The probe 170 therefore has the
further advantage of
not requiring any additional reinforcement during insertion.
The probe 170 is capable of measuring electrical impedance using the disc
electrode 182 and
therefore, it is suitable for use during surgical procedures as part of a
medical imaging
system. More specifically, when used as part of a medical imaging system the
proximal end
176 of the probe 170 is coupled to a medical imaging system (not shown). The
medical
imaging system is capable of receiving an impedance measurement relating to an
aspect of a
patient from the probe 170 and using the impedance measurement to generate an
image of the
aspect. The probe 170 comprises a single electrode 182 and so the probe 170
provides a
mono-polar impedance probe. To enable the mono-polar probe 170 to calculate
the electrical
impedance of an aspect of a patient a second conductor is required and usually
comprises the
patient's body, as is well known in the art.
Figure 10 shows an alternative surgical probe 190 which provides a bi-polar
impedance
probe. The probe 190 differs in construction from the probe 170 of figure 9 in
the following
CA 02739173 2011-03-31
WO 2010/040970 PCT/GB2009/000737
21
ways. A second disc electrode 192 is positioned within the bore region 174 and
between the
disc electrode 182 and the proximal end 176. Preferably, the disc electrode
192 is coaxial
with the tube 172 and is positioned adjacent to the electrode 182. The second
disc electrode
192 is conducted to the proximal end 176 by the electrical conductor 184. The
probe 190 also
differs from the probe 170 by the presence of an electrically insulating
portion 194 which is
positioned in-between the electrode 182 and the second electrode 192. The
insulating portion
194 functions to electrically insulate the electrodes 182 and 192 from each
other.
Additionally, it is within the scope of the appended claims that the outer
surface of the probe
170 and the probe 190 is encoded with an absolute scale so that the probe's
axial position
inside a patient can be quickly and easily determined. Accordingly, each probe
can be
reliably guided to the correct depth within a patient.
It is within the scope of the present invention that the various embodiments
described above
are suitable for use with robotic equipment, preferably, robotic equipment for
use in medical
applications. For example, the above-described embodiments are suitable for
use with tele-
manipulator robotic equipment. A tele-manipulator provides a hand-like robotic
mechanism
which is capable of being controlled by a human operator to perform surgical
operations. For
example, a surgeon can remotely guide a tele-manipulator robot into a
patient's central
nervous system and thereby deliver to a target site within the patient an
embodiment of the
present invention, such as a catheter according to the present invention. The
use of robotic
equipment with embodiments of the present invention can be advantageous for a
number of
reasons. When access to a patient is restricted, for example when the patient
is inside an
magnetic resonance imaging (MRI) machine, robotic equipment can be built to
operate
within a space which is too constricted for a human to operate in effectively.
Also, gearing in
robotic equipment can provide improved dexterity during delivery of an
embodiment
according to the present invention when compared to manual delivery by a
human.