Note: Descriptions are shown in the official language in which they were submitted.
CA 02741443 2011-05-30
1
DECORATIVE LUMINARY
JhLd
The present invention relates to a decorative luminary for providing an
aesthetically
pleasing ambiance.
Baektroond
It is known in the art to utilize scented candles for providing a pleasing
ambiance.
However, scented candles can be messy and can also pose concerns related to
the utilization of
an open flame. The present invention overcomes these limitations. The
decorative luminary of
the present invention provides an aesthetically pleasing ambiance. It does not
utilize a flame,
hence eliminating concerns pertaining to the use of an open flame.
Furthermore, as the
decorative luminary is not waxed-based, there is no concern with the dripping
and messiness
that can be associated with scented candles. Yet further, the luminary of the
present invention
offers the user flexibility as the shade of the luminary is easily
interchangeable and disposable
thereby providing the user with choices as to scents and decorative styles.
This and other features; aspects, advantages, and variations of the present
invention will
become evident to those skilled in the art from a reading of the present
disclosure with the
appended claims and are covered within the scope of the claims.
Summary of the Invention
In one aspect, the present invention relates to a decorative luminary. The
decorative
luminary may comprise a base, a light source, and one or more disposable
shades. The shade
may be impregnated with a composition wherein from about 50% to about 100% is
comprised of
a volatile composition wherein the volatile composition includes at least one
ingredient which
has a Kovat's Index from about 600 to about 1800.
The disposable shade may be impregnated with a volatile composition comprising
perfume ingredients wherein the perfume ingredients are selected from a first
group of
ingredients having a boiling point of about 20 C to about 250 C and a ClogP
value from about -
2 to about 3; a second group of ingredients having a boiling point of about 20
C to about 250 C
and a ClogP value from about 3 to about 9; a third group of ingredients having
a boiling point of
about 250 C to about 400 C and a ClogP value from about -2 to about 3 ; a
fourth group of
CA 02741443 2011-05-30
2
ingredients having a boiling point of about 250 C to about 400 C and a ClogP
of about 3 to
about 9; or. a combination dtereof.
The disposable may include a volatile composition wherein the volatile
composition is
about 50% or more depleted from the shade within about twenty-four hours after
the shade is
exposed to air. The disposable shade may be comprised of a material having a
fluid holding
capacity of about 5 ml/ma to about 1000 mu ma and an average pore size of
about 0.1 microns to
about 100 microns.
In another aspect of the invention, there is provided a composition for a
decorative
luminary. The composition comprises a volatile composition wherein the
volatile composition
is provided in an amount capable of adding from about 60 milligrams to about
15 grams of the
volatile composition to a disposable shade. Prior to addition to the
disposable shade, the volatile
composition is contained in an ampoule, a pouch, a dropper bottle, a sachet, a
spray, a blow-fill
seal container, or a combination thereof. The volatile composition may have a
Kovat's Index of
about 600 to about 1800.
In yet another aspect of the invention, a decorative luminary, comprises a
base and a
disposable shade. The shade is associated with the base. The base includes a
connecting
element and the shade includes a reciprocal connecting element whereby the
connecting element
of the base contacts the reciprocal connecting element of the shade.
In another aspect of the invention, a disposable shade is provided wherein the
shade is
comprised of a material having a thickness of between about 0.008 mm and about
5 mm and
wherein the shade includes a volatile composition.
In a further aspect of the invention, a decorative luminary is provided
wherein the
decorative luminary comprises a disposable shade and changing indicia.
In an additional aspect of the invention, a method for making a decorative
luminary is
provided. The method comprises the steps of.
a) providing a base including a light source;
b) providing a disposable shade; and
c) associating the base with the disposable shade such that the base is in
communication
with the shade.
In another aspect of the invention, a method is provided for forming a
decorative
luminary including providing a disposable shade in a flat form or a
substantially flat form; and
expanding the disposable shade into a substantially non-flat form.
CA 02741443 2011-05-30
3
In a further aspect of the invention, a method is provided for a user to
customize the
components of a decorative luminary. The method comprises the steps of:
a) providing an interactive sample display which includes each option of each
decorative
luminary component;
b) providing a user access to the interactive sample display; and
c) allowing a user to select which option of each decorative luminary
component the user would
like so as to allow the user to view a sample of the decorative luminary
having incorporated
therein each option of each decorative luminary component the user has
selected.
Brief Deacrintion of the Drawings
It is believed that the present invention will be better understood from the
following
description taken in conjunction with the accompanying drawings in which:
FIG. 1 is a perspective view of an embodiment of a decorative luminary made in
accordance with the present invention.
FIG. 2 is a side view of the decorative luminary shown in FIG. I.
FIG. 3 is a perspective view of an embodiment of a decorative luminary made in
accordance with the present invention.
FIG. 4 is a side view of the decorative luminary shown in FIG. 3.
FIG. 5 is a perspective view of an embodiment of a decorative luminary made in
accordance with the present invention.
FIG. 6 is a side view of the decorative luminary shown in FIG. 5.
FIG. 7 is an exploded view of an embodiment of a base for a decorative
luminary made
in accordance with the present invention. -
FIG. 8 is an electrical schematic diagram of an embodiment of a light source
for a
decorative luminary made in accordance with the present invention.
. FIG..9 is a top sectional view of an embodiment of a substrate material made
in
accordance with the present invention.
FIG. 10 is a perspective view of an embodiment of a decorative luminary made
in
accordance with the present invention.
FIG. 11 is a perspective view of an embodiment of a decorative luminary made
in
accordance with the present invention.
FIG. 12 is the bottom view of the decorative luminary of FIG. 1 i.
CA 02741443 2011-05-30
4
FIG. 13 is an exploded view of an embodiment of a decorative luminary made in
accordance with the present invention.
FIG. 14 is a perspective view of the base of the decorative luminary of FIG.
13.
FIG. 15A is a side view of an embodiment of a decorative luminary.
FIG. 15B is a cross sectional view taken along line 15-15 of the decorative
luminary of
FIG. 15A.
FIG. 16A is a front view of an embodiment of a shade made in accordance with
the
present invention.
FIG. 16B is a top perspective view of the shade of FIG. 16 A.
FIG. 17 is a front view of an embodiment of a shade made in accordance with
the present
invention.
FIG. 18 is a front view of a shade of the shade of FIG. 17.
FIG. 19 is a perspective view of a shade of FIG. 17.
Detailed Description
Reference will now be made in detail to various embodiments of the present
invention,
examples of which are illustrated in the accompanying drawings wherein like
numerals indicate
the same elements throughout the views. All percentages, ratios and
proportions herein are on a
weight basis unless otherwise indicated.
Except as otherwise noted, all amounts including quantities, percentages,
portions, and
proportions, are understood to be modified by the word "about", and amounts
are not intended
to indicate significant digits.
Except as otherwise noted, the articles "a", "an", and "the" mean "one or
more".
As used herein, "comprising" means that other steps and other ingredients
which do not
affect the end result can be added. This term encompasses the terms
"consisting of" and
"consisting essentially of". The compositions and methods/processes of the
present invention
can comprise, consist of, and consist essentially of the essential elements
and limitations of the
invention described herein, as well as any of the additional or optional
ingredients, components,
steps, or limitations described herein.
As used herein, "disposable" refers to something which is discarded after a
few uses.
As used herein "durable" refers to something which can be used many times.
CA 02741443 2011-05-30
As used herein, "indicia" refers to any desired array that creates an image or
a pattern.
As used herein, "opacity" refers to an indication of how much light passes
through a
material. The higher the opacity, the less is the amount of light that passes
through the material.
Generally opacity is calculated from reflectance measurements of the material
with a black
backing and the same material with a white backing wherein:
%Opacity = (Ybt..k backing / Ywhite backing) X 100
wherein Y is the CIE tristimulus value of Y.
As used herein, "volatile materials" refers to a material that is vaporizable.
As used herein, "volatile dyes," refers to soluble or insoluble coloring
matter that is
vaporizable. The chemical composition can be a single component or mixture.
It should be understood that every maximum numerical limitation given
throughout this
specification includes every lower numerical limitation, as if such lower
numerical limitations
were expressly written herein. Every minimum numerical limitation given
throughout this
specification will include every higher numerical limitation, as if such
higher numerical
limitations were expressly written herein. Every numerical range given
throughout this
specification will include every narrower numerical range that falls within
such broader
numerical range, as if such narrower numerical ranges were all expressly
written herein.
Decorative Luminary
The decorative luminary of the present invention may comprise a base, a light,
and a
shade which encloses the light. The decorative luminary may also include a
volatile
composition which can be included separately and/or included with the base,
the shade, or a
combination thereof. The shade may be included with the base and/or with the
light
Alternatively, it may be provided separately as a stand-alone article. The
shade can include
indicia. In one non-limiting example the indicia can undergo a visual
transition during use.
A. Base
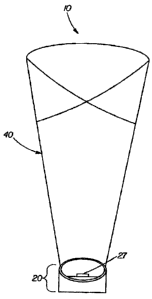
Referring to FIGS. I - 7, the decorative luminary 10 of the present invention
may
comprise a base 20. The base 20 may be sized and shaped to receive and support
the shade 40.
A light 27 may also be associated with the base. In the case where a light 27
is present a power
CA 02741443 2011-05-30
6
supply 26 may also be present in order to provide power to the light 27 and/or
switches which
turn the light on and off. Any power supply 26 may be used including but not
limited to
batteries, household current, solar power, or the like. In the Instance where
batteries are used it
may be desirable to optionally include an access panel such as a door so that
the batteries may be
easily accessed for replacement purposes. For instances, referring to FIG. 7,
the bottom 23 of
base 20 may serve as an access panel. Additionally, a volatile composition may
be associated
with the base 20. If desired, the base 20 may be decorated. Generally, the
base 20 should be
heavy enough to support the weight of the assembled decorative luminary 10
without tipping.
The base 20 may be formed in any number of ways familiar to those of ordinary
skill in the art,
non-limiting examples of which include injection molding, compression molding,
and
thermoforming. The base 20 may comprise one or more polymers, one non-limiting
example of
which is thermoplastic polymers. Typically, the light/light source 27 is
protected by utilizing a
cover 22. Cover 22 if desirable can also function as a light diffuser to help
diffuse the light from
light/light source 27. In one non-limiting embodiment the cover 22 may be a
thermoplastic
cover such as a transparent thermoplastic cover that is resistant to different
materials which may
come into contact with the base 20 such as components of the volatile
composition. One non-
limiting example of a suitable polymer is injection molded grades of impact
modified
acrylonitrile available under the name of BAREX and manufactured by Innovene
of Chicago,
Illinois. Other suitable materials include but are not limited to injection
molding and/or
thermoforming grades of styrene acrylonitrile ("SAN"), polypropylene,
polyethylene
terephthalate ("PET"), or combinations thereof.
Referring to FIGS. 14 and 15, in one non-limiting embodiment it may be
desirable to
include a step or ledge 293 around either cover 22 (as shown) or around base
20 (not shown) in
= order to provide a resting area for the bottom portion of a shade such as
shade 440.
Alternatively, one or more members (not shown), non-limiting examples of which
include pins
and protrusions, could be included with cover 22 and/or base 20 which could be
used to engage
with a shade in order to hold the shade (such as shade 40, shade 410, or shade
440 shown in
FIGS. 1- 6, FIGS. 10 -11, FIG. 13, and FIGS. 15 -19) in place on the cover 22
and/or base 20.
The members may be reciprocating or stationary, or a combination thereof. If
desired, the shade
may also include one or more reciprocating members which may be designed to
engage with the
reciprocating member(s) of the cover 22 and/or base 20. In addition to or
alternatively, if
desired, the reciprocating member included with cover 22, base 20, and/or the
shade 40 could
CA 02741443 2011-05-30
7
serve as a switch so that when the reciprocating member is engaged, the light
of the decorative
luminary is activated.
B. Light
Referring to FIGS. I - 7, the present invention may also include a
light/source of light
27. Generally, the source of the light 27 will be associated with the base 20
though it could be
located in other areas including but not limited to the shade 40. Furthermore,
if desired there
could be more than one light/light source 27 such as shown in FIG. 7. Suitable
light sources
include but are not limited to light emitting diodes ("LEDs"), incandescent
sources of light
including but not limited to filament-based bulbs, and luminescent sources of
light including but
not limited to electroluminescent, chemiluminescent, cathodoluminescent,
triboluminescent, and
photoluminscent materials.
In one non-limiting embodiment the light source is one or more LEDs. The LED
can be
any number of colors including but not limited to yellow, white, red, green,
blue, pink, or a
combination thereof. One non-limiting example of an LED suitable for use with
the present
invention is part No. MV8305 (available from Fairchild Semiconductor of South
Portland,
Maine).
In one non-limiting embodiment of the present invention, the light 27 is
mounted on
mounting 25 present in the base 20 of the decorative luminary 10 as shown in
FIG. 7. It may be
designed such that the light 27 turns on automatically when the shade 40
contacts the base 20.
This may be accomplished in any number of ways, one non-limiting example of
which is
utilizing a surface mounted contact switch (not shown) that is engaged when
the shade 40
contacts the base 20. If desired, the light source could be connected to a
timer (not shown)
incorporated in the base 20 such that the light 27 automatically turns-off
after a predetermined
time period after the shade 40 is placed in contact with the base 20. In
another non-limiting
embodiment (now shown) the light source may be present in the shade 40. This
could be
accomplished in any number of ways. For instance, a surface mounted contact
switch (not
shown) could be mounted on the shade 40 such that when the shade 40 contacts
the base 20, a
light 27 turns on. Alternatively, electroluminescent and/or chemiluminescent
materials could be
used as the light source. In one non-limiting example, electroluminescent
materials are provided
either as part of the shade 40. as part of the base 20, or a combination
thereof. For instance, the
shade 40 and/or the base 20 could be made in whole or in part from
electroluminescent material.
CA 02741443 2011-05-30
8
One non-limiting example of electroluminescent material suitable for use with
the present
invention is EL available from Novatech Electroluminescent Incorporated of
Chino, California.
In yet another non-limiting embodiment, an absorbent and/or porous shade 40
could be
impregnated with phenyl oxalate ester with a fluorescent dye. A rupturable
pouch containing
hydrogen peroxide could be included with the shade 40t, A user would then
rupture the pouch
thereby allowing the hydrogen peroxide contact the phenyl oxalate ester/dye.
Contact of the
hydrogen peroxide contained in the pouch with the phenyl oxalate ester/dye
impregnated in the
shade 40 would provide light via the chemiluminescent reaction between the two
materials.
If desired, the light source may provide a light that is varying in intensity.
The light
source may be associated with a power source (non-limiting examples of which
include batteries
and/or an AC power source), a microcontroller, a switch, and one or more LEDs.
One non-
limiting example of a microcontroller suitable for use with the present
invention is Part. No.
MSP430F122, available from Texas Instruments of Dallas, Texas. The light
intensity may be
varied by the microcontroller using Pulse Width Modulation ("PWM"). PWM refers
to the
process of instantaneously controlling digitally the amount of power being
delivered to the
LEDs. FIG. 8 is illustrative of one non-limiting example of an electrical
schematic diagram
which depicts circuitry suitable for controlling the light source of the
present invention.
Referring to FIG. 8, microcontroller 200 may be programmed to execute an
algorithm to vary
the LED intensity. In essence, microcontroller 200 can be programmed to
closely mimic the
visual characteristics such as intensity and motion of the flame of a candle.
For example, a
typical candle flame generally has a substantial amount of left to right
motion which produces
variations in light intensity. Microcontroller 200 used to control the light
source of the present
invention may be programmed to capture the variations in light intensity. For
example, the left
to right motion may be captured by placing two LEDs side by side and using
pulse width
modulation to vary the intensity and motion. In an alternate non-limiting
example,
microcontroller 200 may be programmed to vary the intensity of the LEDs in a
random fashion.
In another example, microcontroller 200 may be programmed to increase or
decrease the
intensity of the LEDs over time.
C. Shade
Referring to FIGS. 1 - 7, the decorative luminary 10 of the present invention
also
comprises a shade 40. The shade 40 may be included in conjunction with the
base 20 and/or in
CA 02741443 2011-05-30
9
conjunction with the light source. Alternatively, the shade 40 may be provided
separately as a
stand-alone article. It is desirable that the shade 40 of the present
invention be disposable. The
shade 40 may be a single use disposable shade 40 or alternatively can be a
disposable shade 40
designed for more than one use. Alternatively as shown in FIGS. 13 - 15 the
decorative
luminary 10 may comprise a more durable outer shade 410 and a disposable inner
shade 440. In
one non-limiting example outer shade 410 could be a durable decorative shade
while inner shade
440 could be a disposable shade used to deliver scent without having to
dispose of outer shade
410. As inner shade 440 would be disposable, it could be easily interchanged
to allow a user to
experience different scents if desired.
Typically, when used in conjunction with the light source, the shade 40
surrounds a
substantial portion of the light source. For example, the shade 40 surrounds
at least about 900
around the light source, or at least about 180 around the light source, or
completely surrounds
the light source.
The shade 40 comprises a substrate. The substrate can be made of a single
material or a
combination of material. The shade 40 can be transparent, translucent, opaque,
or a combination
thereof. Generally, the shade 40 is made from any material or combination of
materials that will
let light pass through some portion of the substrate and that is stiff enough
to hold a shape. A
non-limiting list of suitable materials include cellulosic materials, non-
cellulosic materials, and
combinations thereof Non-limiting examples of these include thermoplastics
including but not
limited to foamed thermoplastics and polyolefinic-based materials including
but not limited to
polyethylene, polypropylene, and ethylene vinyl acetate ("EVA"); and
thermosets including but
not limited to polyurethanes; paper; vellum; parchment; leather; woven
materials; and non-
wovens. One suitable non-woven is SYNERGEX 6130 available from BBA/Fiberweb of
Simpsonville, South Carolina.
The shade 40 can be comprised of one or more layers of material 300 such as
shown in
FIG. 9. Each layer may comprise one or more types of materials. Each layer of
the shade
typically has a thickness of from about 0.008 mm to about 5 mm or fro m about
0.01 mm to
about 1 mm with an average total thickness for the shade of from about 0.02 mm
to about 5 mm
or from about 0.04 mm to about 1 mm as measured with a hand-held thickness
gauge such as
Model No. 22810 available from Mahr-Federal of Providence, Rhode Island.
CA 02741443 2011-05-30
The layers may be combined by any means known for creating layered flexible
structures, non-limiting examples of which include adhesively combining,
thermal or ultrasonic
bonding, extrusion coating, extrusion laminating, or combinations thereof.
In one non limiting embodiment, the shade 40 may include at least one layer of
an
absorbent material capable for example of holding a volatile composition
without dripping or
releasing due to gravity or capillary forces. Suitable absorbent materials
include but are not
limited to porous materials having a fluid holding capacity from about 5
nil/m2 of shade material
to about 1000 nil/m2 or from about 10 mI/m2 to about 400 ml/m2. Suitable
absorbent materials
include but are not limited to fibrous cellulose-based materials, fibrous
thermoplastic-based
materials including but not limited to spunbond polypropylene, spunbond
polyester, and the like,
and other fibrous materials including but not limited to fiberglass, wherein
the material has a
pore volume of about 10% to about 95% or a pore volume about 20% to about 90%
and an
average pore size of from about 0.1 microns to about 100 microns or from about
0.3 microns to
about 80 microns. Non-limiting examples of other suitable absorbent materials
include foams
made from thermoplastics one non-limiting example of which is open-celled
polyethylene foam
(such as those available from Sentinel Products Corporation of Hyannis.
Massachusets),
urethane, cellulose and/or starch, and microporous polymer films with at least
20% open area for
containing and releasing a volatile composition. Pore size distribution and
the % pore volume
can be measured, for example, by using a TRI autoporisimeter available from
TR7/Princeton of
Princeton, New Jersey. Examples of suitable absorbent materials include but
are not limited to
5YNERGEX 6130 manufactured by BBA/Fberweb of Simpsonville, South Carolina and
GRADE 7020 manufactured by Cellutissue Corporation of East Hartford,
Connecticut.
If desired, the absorbent material can be treated to be either hydrophilic.
hydrophobic,
oleophilic, or oleophobic, or a combination thereof so as to either aid in
releasing a volatile
composition or to aid in holding onto a volatile composition. If desired, all
or some portion of
the absorbent material could be treated. For instance, it may be desirable to
treat the entire
surface of the absorbent material or to just treat the edges so as to prevent
or limit the migration
of the volatile composition to the edges of the shade. For example, in one non-
limiting scenario,
treating about 5mm to about 20 mm along the edges of the shade with an
oleophobic coating
could potentially help prevent predominately oil based perfume from migrating
to the edges of
the shade.
CA 02741443 2011-05-30
11
If desired, the shade could be constructed in two layers, wherein the outer
layer consists
of a barrier material which is substantially impermeable to for example a
liquid volatile
composition impregnated in the inner layer. The outer layer could serve to
help minimize
contact between the user and the volatile composition impregnated in the inner
layer.
Alternatively, the shade 40 could be constructed in three layers as shown in
FIG. 9, wherein the
inner layer 310 could comprise an absorbent material impregnated with a
volatile composition,
the middle layer 320 could comprise a barrier material which is substantially
impermeable to the
volatile composition impregnated in the inner layer 310, and the outer layer
330 could comprise
a second material, non-limiting examples of which include porous material,
nonporous
material, or combinations thereof. Suitable barrier materials include but are
not limited to non-
porous films, such as low density polyethylene, high density polyethylene,
polypropylene,
polyester, ethylenevinyl alcohol ("EVOff ), aluminum oxide coated polyester,
silicon dioxide
coated polyester, metalized polyester a suitable example of which is PET, or
combinations
thereof. The barrier layer is typically from about 0.005 mm to about 1 mm in
thickness or from
about 0.01 mm to about 0.1 mm in thickness to maintain flexibility of the
shade 40. In another
variation of this embodiment, the middle barrier layer could be omitted.
In yet another embodiment (not shown), a volatile composition could be located
on a
discrete substrate that is separate from but connected to the shade 40. The
discrete substrate
may optionally be in the form of a patch or label. In one non limiting
embodiment, the discrete
substrate may be more absorbent and thicker than the shade 40 so as to allow
the discrete
substrate to contain a higher percentage of a volatile composition in a
smaller space or area. In
addition a thicker discrete substrate may also serve to provide support and
stability to the shade
for example by attaching it to the bottom of the shade 40. One example of a
suitable absorbent
material which can be used for the discrete substrate is Product No. 7620W
available from EMI
Specialty Papers of Redding, Connecticut.
The shade 40 should be stiff enough to stand under its own weight and to
easily fit over a
base 20 (when used). Generally it is desirable that the shade 40 have a
deflection force (i.e.;
force needed to deflect a sample 3 mm wherein the sample has the dimensions of
50 mm in
length, 12.7 mm in width and less than 1.6 mm in thickness as measured in
accordance with
ASTM D790-03 entitled "Flexural Properties of Unreinforced and Reinforced
Plastics and
Electrical Insulating Materials" using a span distance of 25.4 mm (as measured
on an Instron
Compression/Tensile Tester Model No. 550RC5451 manufactured by Instron
Corporation of
CA 02741443 2011-05-30
12
Norwood, Massachusetts) of between about 1 gram and about 200 grams or from
about 5 grams
to about 100 grams and a flexural modulus between about 0.1 gigapascals and
about 10
gigapascals ("GPa").
The substrate comprising the shade 40 may be transparent, translucent, opaque,
or a
combination thereof. Typically, the materials comprising the shade 40 are
selected so that at
least a portion of the shade 40 when assembled appears translucent,
transparent. or a
combination thereof. Additionally, the shade 40 may include portions wherein
the shade
material is removed one non-limiting example of which is where patterns are
die cut into the
shade 40 and the die cut portions of material removed from the shade 40. The
shade 40 may
have an opacity ranging from about 0% to about 100%, or from about 20% to
about 80%, or
from about 25% to about 70%. The opacity of the substrate may vary from area
to area and/or
within a given time frame. For example, the shade 40 when partially wetted
with a composition
(a non limiting example of which is a volatile composition such as a perfume).
may have a
particular opacity which will be different after evaporation of the
composition. One suitable
instrument which may be used for measuring opacity is the Hunter LabScan XE
manufactured
by Hunter Associates Laboratory of Reston, Virginia.
In one non-limiting embodiment the outwardly-facing side of the shade 40 is
made of a
material that has a natural appearance and is able to be printed or embossed
with a pattern or
image. In one embodiment a thin, flat material or combination of materials is
folded into a tube-
like shape and then bonded along one edge 340 to prevent the tube from
unrolling as shown in
FIGS. i - 7. If desired, the shade 40 can include creases to allow the shade
40 to be flattened.
For example, for purposes of packaging, it may be desirable to provide the
shade 40 to the
consumer in a flattened or substantially flattened form. The consumer upon
using can open the
shade 40 and expand it to a non flat form or substantially non-flat form, non-
limiting examples
of which include a cone, a tube, a sphere, a cube, a polygon, or any other
shape.
If desired, the shades may be packaged individually in a high barrier pouch
that will help
to prevent volatile components of the composition from escaping. One suitable
pouch material
available from Sonoco Flexible Packaging of Franklin, Ohio comprises a
lamination of
acrylonitrile sealant film extrusion '(sold under the tradename of BAREX)
laminated to an
aluminum foil barrier layer and a reverse printed PET outer layer. The BAREX
sealant layer is
desirable for its ability to minimize perfume absorption into the packaging
sealant film. Non-
limiting examples of other suitable sealant layers include but are not limited
to blends or mono-
CA 02741443 2011-05-30
13
layers of various polyolefins, EVOH, metalized polyester, and the like.
Alternatively, it is
possible to have multiple shades packaged into a high barrier recloseable
pouch. The pouch can
be made recloseable in any number of ways familiar to those of ordinary skill
in the art
including but not limited to utilizing a zip lock feature, adhesive tape, or a
combination thereof.
In another non-limiting example, it may be desirable to construct a shade
wherein the
shade and package are one unit as shown in FIGS. 17 - 19. This provides for an
integral air
freshening package without the need for separate packaging. As described
previously, the shade
may be constructed as a multi-layer system having an inner absorbent layer 540
and an outer
barrier layer 520 as shown in FIG. 17. If desired, an additional decorative
outer layer (not
shown) could be optionally added to the outside of barrier layer 520. Barrier
layer 520 may be
comprised of a material suitable for preventing the migration of perfume to
the outside as
previously described. In the present example, barrier layer 520 also becomes
the package layer
to minimize perfume loss throughout the shelf-life of the product. Referring
to FIGS. 18 and 19,
the multi-layer construction is rolled and sealed to itself along seal edge
555 by either a butt or
lap seam or other methods as known in the art. This tubular shape can then be
bonded on both
open ends along seal edge 550 as shown in FIG. 18 so as to contain the perfume
on the inside.
In doing so the shade is sealed on all sides to prevent perfume loss and the
shade becomes the
primary package. Sealed ends 550 of shadelpackage 570 can then be removed by
the user in any
number of ways known to those of ordinary skill in the art including but not
limited to cutting
them off with a scissors or utilizing perforations 540 such as shown in FIG.
17, peel tape, pull
strips, or any other convenient easy means of opening barrier pouches. The
shade/package 570
can then be placed into an open configuration by the user one non-limiting
example of which is
the cube form shown in FIG. 19. Once in the open configuration, perfume can
then be released
from the inside of the shade/package. Optionally, an element 560 can be used
to retain the shape
of the folded cube to keep it in the open cube form. Non-limiting examples of
such element 560
would be a piece of aluminum foil tape or a tin tie as shown in FIG. 18. A
further option (not
shown) could be a frame which would fit around the top of the cube form so as
to help retain the
shape of the cube.
When the inner absorbent layer is comprised of PET or other synthetic fibers
that are
difficult to tear it may be desirable to have the inner absorbent layer not
extend to the barrier
layer thus preventing the inner absorbent layer from being in the sealed end
portion of the
shade/package. It may be desirable for the integrated shade/package to be
comprised of a
CA 02741443 2011-05-30
14
transparent barrier layer, non-limiting examples of which are EVOH and/or
silicon dioxide so as
to allow light to pass through the luminary shade.
In another non-limiting embodiment, the shade may include multiple components
such
as an inner and outer shade both of which can be of similar shape and size or
could be a different
size and/or shape from one another. If desired, the outer or inner shade or
both could either be
scented, unscented, or one or the other scented. A non-limiting example of
this is shown in
FIGS.. 13 - 15. Referring to FIGS. 13 and 15, the outer shade 410 could be an
unscented
decorative shade while inner shade 440 could be scented. Inner shade 440 could
be a similar
shape but slightly smaller than outer shade 410 so that inner shade 440 could
be easily inserted
on base 20 and/or cover 22 without interfering with outer shade 410. Referring
to FIG. 15, (a
cross-sectional view taken along lines 13-13 of FIG. 13) outer shade 410 and
inner shade 440
are shown resting on base 20 and cover 22. Base 20 can optionally include a
ledge 293 so as to
help facilitate the centering of inner shade 440 within outer shade 410. Outer
shade 410 could
be made of a similar material as inner shade 440 or alternatively outer shade
410 could be made
from a more decorative and/or more expensive material. Inner shade 440 if
desired could be a
simpler form than outer shade 410 and could for example include a fragrance
thereby allowing
inner shade 440 to be changed more frequently while outer shade 410 is used
for repeated uses.
Outer shade 410 could be used once, several times, or could be a durable shade
meant to be used
as a decorative element for many uses. If desired, outer shade 410 could be
durable or semi-
durable while inner shade 440 could be disposable. Alternatively, outer shade
410 could be
disposable while inner shade 440 is durable or semi-durable. Yet further, if
desired, both outer
shade 410 and inner shade 440 could be durable or semi-durable or both outer
shade 410 and
inner shade 440 could be disposable. A more durable outer shade 410 could be
made from a
durable translucent material non-limiting examples of which include glass;
plastics such as
polycarbonate, CPET, polypropylene, or other plastics that can preferably be
injection molded;
vellum; cellulosic materials such as specialty papers; or various woven and
non woven
materials. A durable shade could also be made from a material that is not
translucent and has
openings to allow for light to pass through. Non-limiting examples of
materials which could
compose non-translucent durable sheets include thick/high caliper paper
structures, metal,
colored plastic materials, or combinations thereof. Utilizing separate inner
and outer shades
allows for more decorative materials to be utilized for the outer shade
without limitations related
to cost, perfume compatibility, and ink compatibility. Furthermore, utilizing
a separate outer
CA 02741443 2011-05-30
and inner shade allows a user to mix and match decorative shades with
different scented shades.
This could also potentially reduce the number of variations that a store would
need to maintain
in stock.
If desired, a scented shade 300 could be constructed to have one or more
handles a non-
limiting example of which are tabs 301 as shown in FIGS. 16A and 16B so as to
allow a user to
handle shade 300 without potentially getting perfume on their hands. Tabs 301
also provide an
easy way to grab shade 300 by squeezing tabs 301 inward so as to allow shade
300 to form a
shape such as the cube shape shown in FIG. 16B, Tabs 301 could also if desired
form the seal
area where layers forming shade 300 are combined such as by adhesively
combining, heat
sealing, or the like.
D. Volatile Composition
The decorative luminary 10 of the present invention may also comprise a
volatile
composition. As used herein, the terms "scent", "fragrance", "aroma", and
"perfume" are used
interchangeably. The volatile composition of the present invention can
encompass volatile
materials including but not limited to volatile dyes, fragrance compositions,
compositions that
function as insecticides, air fresheners, deodorants, aromacology.
aromatherapy, essential oils, or
any other material that acts to condition, modify, or otherwise emit into the
atmosphere or to
modify the environment. Deodorants or malodor control compositions may
comprise a material
chosen from: odor neutralizing materials a non-limiting example of which is
reactive aldehydes
(as disclosed in U.S. published application No. US 2005/0124512), odor
blocking materials,
odor masking materials, or sensory modifying materials, a non-limiting example
of which is
ionones (as disclosed in US 2005/0124512), and combinations thereof.
Typically the volatile composition is contained within the shade 40 and/or the
base 20.
The volatile composition may also be included in a separate sachet or pouch
(not shown). As
used herein, the terms "sachet" and "pouch" are used interchangeably.
Generally, the volatile
composition that is included in the shade 40, the base 20, separate sachet or
the like, or a
combination thereof, is included in an amount of about 60 milligrams to about
15 grams per
shade, base, separate sachet or combination thereof; or from about 120
milligrams to about 5
grams per shade 40, base 20, separate sachet or combination thereof, or from
about 250
milligrams to about 1 gram per shade 40, base 20, separate sachet or
combination thereof. In
one non-limiting embodiment the shade 40 is impregnated with abbut 0.1 gram to
about 2 grams
CA 02741443 2011-05-30
16
of the volatile composition or from about 0.3 grams to about 0.8 grams of the
volatile
composition. In one non-limiting embodiment, the volatile composition after
exposure to air is
about 50% or more depleted from the shade 40, the base 20, sachet, etc.,
within about twenty-
four hours, or within about twelve hours, or within about six hours assuming
an approximate
temperature of 21 C and an approximate 50% relative humidity of the air in
the location where
the volatile composition is located.
In one embodiment, the volatile composition could comprise a perfume
composition.
The perfume composition can provide a short-term scent experience. A number of
methods to
control the intensity of scent within the present invention are envisioned. In
some cases, this can
be a product of the perfume composition, or the design of the shade 40, base
20, or any surface
to which the perfume composition is added, or a combination thereof. For
example. the perfume
composition can be formulated so that it has characteristics that provide it
with a more rapid
release profile. Perfumes typically comprise one or more perfume ingredients.
Often, these
ingredients have different volatilities, boiling points, and odor detection
thresholds. When a
perfume composition volatilizes into the air, the ingredients with the higher
volatilities (referred
to as "top notes") will be the ingredients that will volatilize and be
detected by a person's sense
of smell more quickly than the ingredients with lower volatilities (referred
to as "middle notes")
and the ingredients with the lowest volatility (referred to as "bottom
notes"). This will cause the
character of the perfume to change over time since after the perfume is first
emitted, the overall
perfume character will contain fewer and fewer top notes and more bottom
notes.
The perfume compositions can include components that are suitably used in
volatile .
composition emitting devices such as the present invention. The components are
not limited but
can be selected based on their Kovat's Index ("KI") (as determined on 5%
phenyl-
methylpolysiloxane as non-polar silicone stationary phase). The KI places the
volatility
attributes of an analyte (e.g. component of a volatile composition) on a gas
chromatography
column in relation to the volatility characteristics of an n-alkane (normal
alkane) series on that
column. A typical gas chromatograph ("OC") column is a DB-5 column available
from Agilent
Technologies of Palo Alto, California. By this definition, the KI of a normal
alkane is set to
100n, where n is the number of carbon atoms in the n-alkane. The KI of an
analyte, x, eluting at
time t', between two n-alkanes with number of carbon atoms 'h' and "N' having
corrected
retention times t', and ttN respectively, will then be calculated as:
CA 02741443 2011-05-30
17
KI=IOO(n+ loglogy"
log t'., - log t'"
On a non-polar to slightly polar GC stationary phase, KI of analytes are
correlated with
their relative volatility. For example, analytes with smaller Kis tend to be
more volatile than
those with larger KIs. Ranking analytes with their corresponding KI values
gives a good
comparison of analyte evaporation rates in liquid gas partitioning systems.
The volatile
composition according to the present invention can have at least one
ingredient with a KI value
of about 600 to about 1800, or about 800 to about 1700, or about 900 to about
1600. The
volatile composition can comprise about 50% to about 100%, or about 70% to
about 100%, or
about 80% to about 100% of one or more ingredients having these ICI values.
. Rather than, or in addition to Kovat's Index, the volatile composition
components can be
selected based on their boiling point (or "B.P.") and their octanol/water
partition coefficient (or
"P"). The boiling point referred to herein Is measured under normal standard
pressure of 760
mm Hg. The boiling points of many perfume ingredients, at standard 760 mm Hg
can be found
in "Perfume and Flavor Chemicals (Aroma Chemicals)," written and published by
Steffen
Arctander, 1969.
The octanol/water partition coefficient of a perfume ingredient is the ratio
between its
equilibrium concentrations in octanol and in water. The partition dents of the
perfume
ingredients used in the volatile composition may be more conveniently given in
the form of their
logarithm to the base 10, logP. The loge values of many perfume ingredients
have been
reported; see for example., the Pomona92 database, available from Daylight
Chemical
Information Systems, Inc. (Daylight CIS) of Irvine. California. However, the
logP values are
most conveniently calculated by the "CLOGP" program, also available from
Daylight CIS. This
program also lists experimental loge values when they are available in the
Pomona92 database.
The calculated loge ("ClogP") is determined by the fragment approach of Hansch
and Leo (A.
Leo, in Comprehensive Medicinal Chemistry, Vol. 4, C. Rauch, P. G. Sammens, I.
B. Taylor
and C. A. Ramsden. Eds., page 295, Pergamon Press, 1990). The fragment
approach is based on
the chemical structure of each perfume ingredient, ind takes into account the
numbers and types
of atoms, the atom connectivity, and chemical bonding. The ClogP values, which
are the most
reliable and widely used estimates for this physicochemical property, are
typically used instead
CA 02741443 2011-05-30
1$
of the experimental logP values in the selection of perfume ingredients for
the volatile
composition.
The perfume composition might comprise perfume ingredients selected from one
or
more groups of ingredients. A first group of ingredients may comprise perfume
ingredients that
have a boiling point of about 20 C to about 250 C, or a boiling point of about
25 C to about
240 C, or a boiling point of about 30 C to about 235 C. The first group of
ingredients may
comprise perfume ingredients that have a CIogP value from about -2 to about 3
or from about -1
to about 2.5. In certain embodiments, perfume ingredients selected from the
first group of
perfume ingredients when present may be present at a level of from about 20%
to about 100%
by weight of the perfume composition, or from about 40% to about 100% by
weight of the
perfume composition, or from about 50% to about 100% by weight of the perfume
composition.
A second group of ingredients might comprise perfume ingredients that have a
boiling
point of about 20 C to about 250 C, or a boiling point of about 25 C to about
240 C, or a
boiling point of about 30 C to about 235 C. The second group of ingredients
may comprise
perfume ingredients that have a ClogP value from about 3 to about 9 or from
about more or
about 3.5 to about 7. In certain embodiments, the second perfume ingredient
when present may
be present at a level of from about 20% to about 100% by weight of the perfume
composition, or
from about 40% to about 100% by weight of the perfume composition, or from
about 50% to
about 100% by weight of the perfume composition.
A third group of ingredients might comprise perfume ingredients that have a
boiling
point of about 250 C to about 400 C. or about 260 C to abut 375 C. The third
group of
ingredients may comprise perfume ingredients that have a ClogP value of about
from about -2 to
about 3 or from about -1 to about 2.5. In certain embodiments, perfume
ingredients selected
from the third group of perfume ingredients when present may be present at a
level of about
0.5% to about 90% by weight of the perfume composition or about 1% to about
80% by weight
of the perfume composition.
A fourth group of ingredients might comprise perfume ingredients that have a
boiling
point of about 250 C to about 400 C, or about 260 C to abut 375 C. The fourth
group of
ingredients may comprise perfume ingredients that have a ClogP value from
about 3 to about 9
or from about more or about 3.5 to about 7. In certain embodiments, perfume
ingredients
selected from the fourth group of perfume ingredients when present may be
present at a level of
CA 02741443 2011-05-30
19
about 0.5% to about 90% by weight of the perfume composition or about 1% to
about 80% by
weight of the perfume composition.
When formulating perfume compositions, some perfume ingredients may also have
other
functionality such as functioning as a solvent, diluent, extender, fixative,
or the like. Non-
limiting examples of these materials are ethyl alcohol, carbitol, diethylene
glycol, dipropylene
glycol, diethyl phthalate, triethyl citrate, isopropyl myristate, and benzyl
benzoate.
In addition to methods described above for delivering the volatile
compositions of the
present invention, other non-limiting suitable methods for delivering these
compositions are
discussed below. In one non-limiting embodiment a volatile composition may be
delivered by
utilizing a pouch with a semi-permeable membrane on at least one side of the
pouch. The pouch
may be attached to the shade 40. The membrane may be selected from several
known materials
which can allow perfume vapor to pass without allowing significant liquid
leakage. Examples
of suitable membrane materials include but are not limited to polyethylene and
microporous
polyethylene, ethylene vinyl acetate, microporous polytetraflouralethylene
("PTFE"), and other
microporous membranes as known in the art. These membranes allow volatile
actives to be
released by either rapid diffusion through the membrane material such as with
a thin
polyethylene film or by evaporation through porous regions of the material
such as microporous
PTFE.
The scent intensity of the shade 40 can optionally be varied by varying the
size of the
shade 40 in width and/or height as well as by changing the pore size of the
material and/or the
material properties. Shades 40 at different heights yet same width can be
provided to deliver
different levels of scent intensity as well as to effectively communicate to
the end user which
shade 40 to use for the room or occasion. For example, a scented shade 40 of
the present
invention with a 20 cm circumference and 22 cm height may be an appropriate
size for a living
room or family room and a scented shade 40 with a 20 cm circumference and a 10
cm height
may be a size appropriate for a bathroom or smaller space. Typically the
shades 40 are of a
similar circumference such that the shade 40 will fit over the same base.
The release rate of the volatile composition from a substrate could also be
controlled by
providing layers wherein the release rate can be customized to last different
periods of time. For
instance, the substrate could include one or more layers. = An individual
layer could be an
intermittent or continuous layer that is designed to release the perfume at
various time intervals.
The layer can be activated with UV light, oxygen, moisture change, or other
stimuli.
CA 02741443 2011-05-30
In another non-limiting embodiment, the release of the volatile composition
could be
changed by varying the material properties of the shade 40. While not wishing
to be limited by
theory, in general, volatile compositions impregnated in a polymer such as a
thermoplastic will
release slower than volatile compositions loaded in an absorbent or porous
material. Porous
materials exposed to the atmosphere will allow volatile compositions to
evaporate at the surface
whereas volatile compositions impregnated in a polymer generally require the
volatile
composition to diffuse through the polymer. For example, a thermoplastic
material such as an
EVA or polyolyefin can be impregnated with a perfume and the diffusion
properties and/or
thickness of the thermoplastic can be tailored to release the perfume at
varying rates.
Incorporating open or closed cells in the thermoplastic will also have a big
effect on the
diffusion properties and these cells can change the effective level of perfume
that is saturated or
absorbed into the material. While not wishing to be limited by theory, in
general, open
cells/pores will release volatile compositions faster than closed cells since
the open cells allow
for the volatile composition to be exposed directly to the atmosphere to
evaporate and therefore
minimizes the need for diffusion of the composition through the cell walls. It
is known in the art
that various foaming agents and/or gases can be incorporated in the process of
extruding or
injecting the thermoplastic to create these pores or cells in the plastic
wall. Engelhard
Corporation of Iselin, New Jersey is one suitable manufacturer of foaming
agents that can be
incorporated in thermoplastic extrusion processes. Trexel Incorporated of
Woburn,
Massachusetts is a provider of technology for injecting gases in thermoplastic
create closed cell
foam-like materials suitable for use with the present invention. Non-limiting
examples of open
celled thermoplastic foams suitable for use with the present invention are
CELLECT foams
available from Sentinel Products Corporation of Hyannis, Massaehussets.
Another means of controlling the release of the volatile composition is by
encapsulating
the volatile composition in the form of capsules non-limiting examples of
which include
microeapsules, or starch encapsulates. There are a number of means in which
the capsules can
be designed to release the scent. For example the scent can be released by
either rupturing the
capsules or by diffusion through the capsule wall. In another example the
scent is released from
the microcapsule when moisture in the air causes the capsule wall to rupture.
Alternatively, the
capsules can be ruptured by peeling off an adhesive layer that causes the
microcapsule to tear or
rupture. The capsule size and material properties can also be adjusted to
control the diffusion.
CA 02741443 2011-05-30
21
In another non-Limiting embodiment, when the perfume is located inside of the
shade 40,
the release of the perfume could be controlled by varying the air flow through
the inside of the
shade 40 so as to change the evaporation rate of the perfume. While not
wishing to be limited
by theory, this could be thought of as a chimney effect except that the vapors
from the perfume
may flow upward or downward depending upon the perfume and the shade design.
It is believed
that the perfume will naturally cool the air when it evaporates and hence the
air flow is naturally
downward due to the cooler air being heavier. Typically most perfumes are
naturally heavier
than air and hence there is a tendency for the perfume to "sink" or flow
downward. To
maximize scent release, it is desirable that the shade 40 be fully open on the
top and bottom to
maximize air flow. Furthermore, the larger the shade 40 opening is on the top
and the bottom,
the more the air flow will be through this opening. This can be especially
desirable with
perfumes which comprise higher molecular weights or higher KI values which
tend to have
slower evaporation rates. To minimize or stop scent release, it is desirable
that the top and
bottom openings of the shade 40 be substantially closed from the environment.
For instance, the
top and/or bottom of the shade 40 could have vents 220 such as those shown in
FIG. 10.
Additionally, or alternatively, referring to FIGS. 11 and 12, the base 20
could also include vents
220. If desired the vents could be designed such that they that can be closed
or opened (to some
degree) to regulate/adjust the perfume evaporation rate. There are a number of
non-limiting
ways in which the top of the shade 40 and/or the base 20 could be made to
adjust air flow. For
instance, in one non-limiting example, an adjustable slide (not shown) could
be included in the
bottom of the base 20 which would allow a user to rotate or slide an element
that opens or closes
the openings that allow air flow through the inside of the shade 40. In
another non-limiting
example, the top of the shade 40 could be adjusted to control air flow through
the top. One non-
limiting example is to use a frame (not shown) that fits on top of the shade
40 and has an
integral slide element that can be rotated or slid open to open or close the
top vent. Another
non-limiting means of doing this would be to utilize a material which upon
folding retains the
folded shape. For instance, the top of the shade 40 could be comprised of a
material such as a
thin metal which upon applying pressure to both sides of the top of the shade
40, the two sides
would come together so as to close the opening thereby limiting air flow
through the shade 40
opening. In another non-limiting example, the top opening of the shade 40
could be restricted
by using a draw string, a thin metal wire tie, or the like.
CA 02741443 2011-05-30
22
In another non-limiting embodiment for controlling the evaporation rate of the
perfume,
a small amount of heat could be used to help drive air flow. While not wishing
to be limited by
theory, it is believed that a temperature rise of about 0.2 C to about 1 C
combined with
appropriate venting can potentially result in higher air flow through the
inside of the shade 40.
This chimney effect with a small temperature rise can be obtained in a number
of different ways
including but not limited to via the heat output of lights, circuitry, a small
heater such as a
battery powered heater, or via heat producing reactive chemistries that can be
incorporated in the
shade 40 and/or the base 20 such as iron oxide. In one non-limiting method,
iron oxide particles
can be coated or encapsulated with a fragrance and applied to shade 40. Shade
40 could then be
placed in a nitrogen flushed foil pouch that will prevent the oxidation of the
iron oxide until the
pouch is opened. The nitrogen flushed pouch can also be used to prevent oxygen
from oxidizing
the fragrance as well as prevents the fragrance from evaporating prior to use
by the consumer.
When the pouch is opened by the user the iron oxide encapsulated with perfume
begins to heat
up and results in a higher evaporation rate of perfume. This will allow low
volatility perfumes
to be more easily dispersed in a room providing superior scent quality with an
evaporative scent
release system.
The shade 40 could have multiple scents and/or scents that release at
different time
intervals. In some instances it may be desirable for a particular scent to be
released for the first
4 hours and a different scent begins to be released for the next 4 hours. This
can be done by
treating one side of the shade 40 with one technology as described above and a
different
technology (e.g.; different material, coating, thickness, or other) could be
used on the other side.
This provides the benefit of either extending the scent over a longer period
of time or providing
alternating scents so as to help reduce acclimation to the scent by the user.
The shade 40 could also be dosed or refilled with a volatile composition
including but
not limited to a perfume by the user using ampoules, pouches, dropper bottle,
sachets, a spray, or
any other means of delivering a fluid to the shade 40. A non-limiting example
would be the use
of individually contained pouches containing about 0.5 ml to about 5 ml of a
perfume
composition that a user could apply by tearing off a comer of a pouch and
squeezing the
contents onto the shade 40. In another non-limiting example, about' 0.5 ml to
about 5 ml of
perfume could be contained in either a small individual or- multi=use unit
dosed blow-fill seal
container similar to those unit dose containers used as contact eye solution
containers. One
suitable manufacturer of equipment to make such unit dose packages is Rommelag
USA
CA 02741443 2011-05-30
23
Incorporated of Edison, New Jersey. Alternatively, the perfume could be
encapsulated and
loaded onto the shade 40 and an occasional spray of water by the user could be
used to release
the volatile composition.
In another non-limiting embodiment, it may be desirable to treat the outside
of shade 40
with a deodorizing ingredient Non-limiting examples of such ingredients would
include
titanium dioxide, cyclodextrin, metal oxides, polyoxometalates, and the like.
While not wishing
to be limited by theory, it is believed that having deodorizing ingredients on
the inside or outside
of shade 40 would provide a means for delivering odor elimination benefits
beyond masking the
odors with fragrance or using reactive fragrance ingredients such as reactive
aldehydes as
mentioned previously. It is believed that the deodorizing ingredients are
ideally placed in a
position where air flow moves across the deodorizing ingredients prior to the
air evaporating the
perfume. In this way the deodorizing ingredients are not removing the perfume
from the air.
Non-limiting examples of locations could include along the edge of shade 40
where air flows
into the shade.
E. Optional Additional Components
The present invention may also include other optional components, a non-
limiting
example of which is a signal that communicates the status of the decorative
luminary 10 to a
user. For example, there may be a signal which indicates when the process has
commenced
and/or concluded. Non-limiting examples of signals which may be used include
color, sound,
and/or olfactory signals.
In some instances it may be desirable that the light source recognize the
shade 40 so as to
adjust the light pattern, intensity, or duration based on the age of the shade
40, design of the
shade 40, scent type, and/or intensity of the scent release. This would allow
for a smart device
that could deliver superior light 27, scent, and/or a dynamic indicia pattern
experience (i.e.;
where the indicia appearance changes during use). Alternatively, this could
also be used to
recognize when a shade is depleted of its scent and ready for replacement.
One non-limiting means by which this can be done is to incorporate a small
microchip
such as a Radio Frequency Identification ("RFID") chip) that communicates with
the base 20.
For example, an RFID chip could be incorporated into the shade 40 while an
RFII) sensor
capable of detecting the RFII) chip could be incorporated into the base 20. In
one non-limiting
example, the RFID sensor could detect whether the shade 40 is present by
detecting the RFID
CA 02741443 2011-05-30
24
chip in the shade 40 in order to send a signal to turn the light 27 on or in
another non-limiting
example the RFID sensor could detect whether the shade 40 with the RFID chip
has been
depleted of volatile composition. RFID chips and sensors suitable for use with
the present
invention are available from Texas Instruments of Dallas, Texas.
Another means to accomplish this would be to provide a color coded region on
the shade
40 that is detected by a light sensor such as a photo-eye located within the
base 20. One non-
limiting example of this is where the shade 40 would have a particular color
in a given region
that could be detected by the photo eye in the base 20 which in turn could
activate a light 27
upon sensing this color. This color coded region can also be designed to
change color with time
so as to communicate with the base 20 as to the age of the shade 40 and/or
communicate when
the shade 40 needs to be replaced.
Optionally, the shade 40 may function in combination with the base 20 to
provide a time
indicator. For instance, the time indicator could provide a signal that the
scent of the shade 40 is
depleted. For example, the light 27 could be made to dim or not function after
the shade 40 has
been used for a period of time. One non-limiting means of accomplishing this
is to incorporate a
surface mounted fuse such as those manufactured by Littelfuse Corporation of
Des Plaines,
Illinois into the shade 40 so that it is in electrical contact with the
control circuitry of the light
source. In this case, an electrical spike from the light source
microcontroller could cause the
fuse to open after a set time period corresponding to the scent duration. If
the base 20 senses an
open fuse on the shade 40, the light will not function or will dim. Another
approach is to have a
wet region of the shade 40 or a region of the shade 40 that contains an
electrolyte such that
electricity is conducted through this region when wet and no electricity is
conducted when the
region is dry. This in turn can be used as a communication signal to the light
source that the
shade 40 is fully used and needs to be replaced. Another approach is to
incorporate a thin
electrically conducting metal in the bottom portion of the shade 40. While not
wishing to be
bound by theory, it is believed that the resistance of the metal will increase
over time. The light
source would then measure the resistance and based on this would be able to
determine the age
of the shade 40.
In another non-limiting example the shade 40 may include a color changing dye.
The
color changing dye could be detected by a light sensor such as a photo-eye
located within the
light base. For instance, when the shade 40 is activated, for example by a
user opening the
package containing the shade, a color change on the bottom of the shade 40
begins to occur.
CA 02741443 2011-05-30
The color change could occur for example as a result of utilizing an oxidative
ink and/or an ink
that reacts with carbon dioxide present In the air. This color change can be
designed to take a
number of hours or days and can be used to communicate with the light base to
send a signal as
to whether this is a new shade or an old shade that needs to be replaced.. The
color change can
also potentially communicate the scent and desired intensity and adjust the
light to vary the
intensity, duration, or flickering pattern of the light. The color change
could correspond to a
predetermined code for the shade 40 such that the base would know what type of
shade 40 is
present. For instance a black dot could be associated with one scent
experience while a blue dot
could be associated with a different scent experience.
These fuses and/or circuit connectors within the shade 40 have the added
benefit of also
preventing the base 20 from working property unless a new shade of the right
design is placed
on the base 20. In yet another non-limiting example, the shade 40 and/or the
base 20 may
include indicia. The indicia may be static or it may be dynamic. In one non-
limiting example,
the base 20 could include an extended regiontsleeve that includes indicia. The
indicia could be
visible or non-visible through the use of light. Non limiting examples of
which include light
provided by the use of flat panel displays or fiber optics. The indicia may be
in the form of a
decorative pattern or image to provide an aesthetically pleasing article. In
addition to or
alternatively, the ipdicia can be used to provide a signal to a user such as
when the shade 40
needs to be changed and/or when the scent is depleted. The shade 40 can be
printed with inks
that are stable (i.e.; inks that do not bleed, dissolve, or rub-off easily)
while in contact with a
perfume or oil. In one non-limiting example, the indicia may be printed by
utilizing an inkjet
iM
printer. One non-limiting example of a suitable inkjet printer Is a DESKJET
9500 inkjet printer
equipped with ink cartridges Part Nos. 51645A and C6578D, available from
Hewlett Packard
Corporation of Palo Alto, California. The indica can also be printed by any
other known
printing method including but not limited to flexographic printing, screen
printing, gravure,
offset printing, air knife, roll coating, blade coating, and the like.
The shade 40 may include indicia that undergo a visual change following
activation by
the user. In one non-limiting example the shade 40 is imprinted with indicia
that undergo a
visual change following activation by the user. Alternatively or in addition
to the above, the
entire shade 40, or some portion of the shade 40 may undergo a visual change
including but not
limited to an opacity change following activation. Activation may include, for
example, removal
of the shade 20 from a secondary package, rupturing of a pouch, removal of a
film, addition of a
CA 02741443 2011-05-30
26
film, addition of a liquid, or other action that results in a stimuli to
affect the visual change.
Possible stimuli include changes in oxygen, carbon dioxide, or humidity of the
surrounding
atmosphere, changes in the amount of some other vapor in the surrounding
atmosphere, changes
in pH or temperature, exposure to ultraviolet or infrared radiation, or some
other stimuli. The
visual change may be in the form of a decorative pattern that appears,
disappears, changes color,
changes intensity, changes opacity, or any combination of these effects. The
visual change will
generally occur during the first 8 hours after activation, or during the first
4 hours after
activation, or during the first 2 hours after activation.
The visual change may be triggered by a variety of means. For example, the
decorative
pattern may appear, disappear, or change color as a result of a change in the
air composition
(i.e.; oxygen, carbon dioxide, nitrogen, and the like) of the atmosphere
surrounding the shade.
In one non-limiting embodiment, an oxygen indicator may be used, wherein the
shade is printed
with an oxygen indicator and is placed in a high barrier (e.g.; metalized PET
or foil) pouch that
is flushed with nitrogen or a different inert gas and then sealed to prevent
oxygen from entering
the pouch. Upon activation, e.g.; removal from the pouch, a color change would
occur as
exposure to oxygen in the atmosphere triggers the oxidation of the indicators.
Suitable oxygen
indicators and methods of preparing oxygen indicators are disclosed, for
example, in U.S. Patent
Nos. 4,349,504, 4,526,752, 4,912,053, and 6,703,245. Alternatively, another
non-limiting
example would be the visual change which may be triggered by a change in the
concentration of
carbon dioxide in the air in the presence of the shade 40.
The decorative pattern may appear, disappear, or change color for example as a
result of
a change in pH. Suitable indicators that change color with a change in pH
include but are not
limited to : phenolphthalein, thymolphthalein, m-Nitrophenol, ethyl red, and
Congo red just to
name a few. These acid base indicators can be found in the CRC Handbook 60th
Edition
published in 1979 on pages D150 - D153. Other widely know indicators include
fluorescent
indicators such as salicylic acid, acridine, and others as described in the
CRC Handbook.
Alternatively a volatile acid or base could be incorporated into the shade and
kept stable with
either barrier peelable layers or high barrier packaging that completely
surrounds the shades and
prevents the volatile components from evaporating or moving. Upon peeling of a
layer or
opening of a barrier package the volatile components are allowed to evaporate
causing a pH
change and hence a color change on the shade 40 where these components are
present. In one
non-limiting example, the pH change may result from a change in carbon dioxide
content of the
CA 02741443 2011-05-30
27
atmosphere surrounding the shade 40. For example, the shade 40 may be packaged
in a barrier
package substantially impermeable to carbon dioxide under an atmosphere richer
or poorer in
carbon dioxide than the general atmosphere.
The decorative pattern may appear, disappear, or change color or intensity as
a result of
capillary/wicking action. This may be accomplished in one non-limiting
embodiment by
providing a source of fluid contained in a discrete pouch or.reservoir
attached to the shade 40.
Upon rupture of the discrete pouch, the fluid may wick through the shade 40 as
a result of
capillary action. A water activated dye or pH activated dye can be printed on
the shade 40 such
that it is invisible when dry but appears when wet. Alternatively the fluid
may contain a dye, a
pH changing ingredient that would change the pH of the fluid, a volatile
composition including
but not limited to perfume, or a combination thereof. Optionally, the user of
the product can add
water to the shade 40 by either pouring or spraying water over the material or
by adding water to
a holding reservoir that comes in contact with the shade 40 to allow wicking
of the fluid up the
shade 40. The holding reservoir can be incorporated within the base 20, can be
attached to the
shade 40, can be a separate piece, or a combination thereof.
In another non-limiting embodiment, the decorative pattern may appear,
disappear, or
change color as a result of exposure to ultraviolet or infrared radiation. The
base 20 may include
for example a source of ultraviolet or infrared radiation to affect the
change. For example, the
base 20 may include light 27 such as one or more LEDs that emits light at
wavelengths below
about 410 tun. An example of a suitable LED is Part No. SSL-LX5093SUVC
available from
Lumex Incorporated of Palatine, Illinois. In conjunction with the ultravoiolet
or infrared LED
containing light source, the shade 40 may include indicia containing
photochromic dyes such as
those known in the art. Non-limiting examples of suitable photochromic dyes
are those
provided by Chromatic Technologies Incorporated of Colorado Springs, Colorado
and available
under the tradename DYNACOLOR PHOTOCHROMIC INK The ultraviolet or infrared LED
is positioned to affect a visual change in the indicia printed with the
photochromic ink. The
decorative pattern may disappear, or change color or intensity as a result of
evaporation of a
volatile dye. Non-limiting examples of suitable volatile dyes include
guaiazulene (1,4-dimethyl-
7-(1-methyletheylazulene, CAS# 489-84-9) and azulene
(Bicyclo[5.3.0]decapentaene,
CAS#275-51-4). The volatile dye may be dissolved in a solvent such as
methanol, ethanol,
acetone, isopropanol or other volatile solvent, or may be formulated into an
ink by combination
with other ingredients such as binders as is known in the art.
CA 02741443 2011-05-30
28
Another non-limiting means of creating a visual change is by designing the
shade 40 so
that at least a portion of the shade 40 undergoes an opacity change after
activation. One way to
affect the opacity change is by evaporation of a volatile composition from a
porous material that
makes up at least a portion of the shade 40. Porous shade materials suitable
for use with this
invention when wetted with water and/or a volatile composition such as a
perfume will typically
have an opacity decrease of about 5% to about 30% depending upon the thickness
and type of
material. For example, a polyester non-woven, one suitable non-limiting
example of which is
SYNERGEX 6130 available from BBA/ rberweb, with a basis weight of about 100
grams/m2
may have an opacity of about 63% when dry but drops to about 43% when wetted
with a volatile
fragrance. As the fragrance evaporates over approximately a 1 hour time period
the opacity can
change from about 43% slowly back to about the original 63%. This change in
opacity also will
effect how light from the light source is diffused and hence can be used to
provide a visual
signal to a consumer that the shade 40 is changing. In another non-limiting
example a KIM
WIPES tissue, manufactured by Kimberly Clark Corporation of Neenah,
Wisconsin, may have
an opacity of about 48% when dry and an opacity of about 25% when wetted with
a liquid
volatile composition such as a perfume.
If the opacity change is large enough, the changing opacity of the shade 40
can also be
used to expose or hide a graphic that is printed on the shade 40. For example
materials which
include micropores having a mean pore size typically between about 0.01
microns and about 15
microns are opaque when dry but become semi-transparent when wetted with a
fluid. Suitable
materials for this purpose include but are not limited to microporous
polyethylene,
polypropylene, nitrocellulose, and polyester, having a pore volume of about
50% volume of
pores/volume of total material ("v/v") to about 99% v/v. Examples of suitable
materials include
those available under the tradename SOLUPOR, grades 7P03A. SPO9B, and 1OP05A
available
from DSM Solutech of Heerlen, Netherlands. In one non-limiting example, these
microporous
materials can have an opacity decrease of about 20% to about 70% depending
upon the
thickness, material type, and pore size. For instance, SOLUPOR 7P03A with a
nominal basis
weight of abort 7 g/m2 has an opacity of about 95% when dry and about 45% when
wetted. A
graphic or image can be printed on one side of the sheet and as the fluid
evaporates the sheet
slowly becomes opaque and eventually will let little or no light through.
Initially the graphic
can be seen from the other side especially with a back-light such as from a
light source. As the
CA 02741443 2011-05-30
29
shade dries it become more opaque letting less light pass through the shade 40
as well as making
the graphic no longer visible.
The pore size of the shade material is typically between about 0.01 microns
and about 50
microns or between about 0.05 microns and about 5 microns to provide the
highest opacity when
dry while still providing transparency when wetted. The average pore size
diameter to absorb
the volatile composition is typically between about 0.1 micron to about 100
microns or between
about 1 micron to about 50 microns average pore size diameter. Hence, an
average pore size
diameter for a material that is both opaque and absorbent without the use of
pigments could be
between about 0.1 micron and about 50 microns. This allows for a single
material to be able to
hold a fluid such as a perfume and provide a semi-opaque shade when dry and
semi-transparent
shade when wetted with a liquid composition. Alternatively a multi-layer
structure could be
used for the shade whereby one layer serves as an opaque changing layer with a
pore size
between about 0.01 microns and about 50 microns and an additional layer is
designed to hold
onto a volatile composition with an average pore size between about 0.1 micron
and about 100
microns.
In another non-limiting embodiment it may be desirable to have the shade 40
with a
unique geometry/shape that corresponds to a similar/reciprocal geometry in the
base 20 so as to
ensure proper alignment and/or recognition between the base 20 and the shade
40. Suitable non-
limiting examples include a tab located on the shade 40 with a corresponding
indentation on the
base to accommodate the tab. Another non-limiting example could be a pin/hole
configuration.
Kit
The components of the decorative luminary of the present invention including
but not
limited to the shade 40, the base 20, the light source/light 27, the sealed
pouch to hold shade 40,
and the volatile composition, may be provided as a kit. Alternatively, one or
more of the
components of the decorative luminary may be provided separately. For
instance, in one non-
limiting embodiment the shade 40 of the decorative luminary may be provided
with one or more
of the other components as part of a kit or may be provided and/or sold
separately. In another
non-limiting example, the volatile composition may be provided as part of a
kit, for instance it
may be incorporated into the shade or provided in a separate sachet, pouch,
ampoule, bottle, or
the like. In yet another non-limiting embodiment, the volatile composition may
be provided
separately such as part of a dosing container or refill unit which may be
purchased separately by
CA 02741443 2011-05-30
a user. In an additional non-limiting embodiment, the base and/or the light
source may be
provided in conjunction with the other components of the decorative luminary,
may each be
provided as one unit, or may each be provided and/or sold separately. For
instance, it may be
desirable for a user to have a choice of the type of light source that is to
be used or to have the
flexibility to interchange the light(s) and/or the base.
It may also be desirable for a user to have the ability to choose the
individual
components comprising the decorative luminary 10 so as to customize the
decorative luminary
10 in accordance with the individual's preferences. For instance, an
interactive sample display
could be provided to allow the user to choose/customize the components (e.g.;
shads 40, base
20, light source/light 27, volatile composition) that he or she would want the
decorative
luminary 10 to be comprised of. The interactive sample display could be
manually controlled by
the user, computer controlled by the user, or a combination thereof. This
interactive sample
display could be provided electronically for Instance through an
internet(virtual web site or it
could be located at a physical site such as at a store location, or delivered
to the user's home such
as through the parcel post and/or via a newspaper/magazine insert. In one non-
Iimiting example
of a manual interactive sample display, each option of each decorative
luminary component is
provided on a moveable wheel located along a single row of the sample display
so as to overlay
one component on top of the next such that the user can view what the
decorative luminary 10
would look like based on the options chosen for each component. As an
alternative or in
addition to the above, different samples of the volatile composition, for
instance a perfume,
could also be overlaid with the components for example in a scratch and sniff
type of
configuration so that the user can have the benefit of holistically
customizing/choosing the
decorative luminary 10 which best meets the user's individual preferences
prior to making a final
purchase commitment.
Self-Instructing Article of Commerce .
The present invention also encompasses articles of commerce comprising 1) the
decorative luminary 10 of the present invention, and 2) a set of instructions
directing the user
how to utilize the decorative luminary 10.
In one embodiment, the article of commerce comprises the decorative luminary
10 of the
present invention in association with a set of instructions, wherein the
instructions direct the
user to follow the method of utilizing the decorative luminary 10. The
instructions may be in
CA 02741443 2011-05-30
31
the form of written words, pictorials, symbols/icons, and the like, as well as
combinations
thereof. In one embodiment, such instructions would direct the user to 1)
place the decorative
luminary 10 on a surface; and 2) activate the decorative luminary 10 for
instance by removing
the shade 40 from the package and/or by placing it in the base 20.
Herein, "in association with", when referring to such instructions, means the
instructions
are either directly printed on the decorative luminary 10; directly printed on
the packaging for
the decorative luminary 10; printed on a label attached to the decorative
luminary 10 or the
packaging for the decorative luminary 10; or presented in a different manner
including, but not
limited to, a brochure, print advertisement, electronic advertisement,
broadcast or internet
advertisements, and/or other media, so as to communicate the set of
instructions to a consumer
of the decorative luminary.
Exam es
Non-limiting examples relating to the instant invention are disclosed below.
Example 1
A wicking shade is made by incorporating a rupturing pouch in the base of the
shade.
The rupturing pouch is made with a high barrier metalized PET laminate
manufactured by
Peehiney Plastic Packaging of Neenah, Wisconsin with a SURLYN sealant
available from
DuPont of Wilmington, Delaware. The pouch is sealed with a lower temperature
on One side
(approximately 93 C versus approximately 149 C for the other two sides of the
pouch) to create
a frangible seal that will burst with less than about 5 pounds (2.3 kilograms)
of force when
pressed. The pouch is filled with about 3 ml of water, 3 drops of a green food
color dye, and
about 1 ml of a pine scent perfume oil. The shade is constructed by taking a 6
inch x 11 inch (15
cm x 28 cm) piece of absorbent paper (non-limiting examples of which are paper
towel, facial
tissue, and bath tissue) with a basis weight of 50 g/m2 and laminating it to a
1 mil (0.0254 mm)
layer of polyethylene film such that one side is absorbent paper and the other
side is
polyethylene film. The paper side is then covered with a template in the shape
of a tree and a
lacquer coating is sprayed onto the paper to create porous and non-porous
regions in the paper
for controlled wicking of a fluid. A rupturable pouch is then placed on the
paper side of the
shade material and the bottom edge is folded over the pouch and sealed to
prevent the pouch
from coming loose and moving within the shade. The shade is then folded in the
form of a tube
CA 02741443 2011-05-30
32
with a 0.25 inch (6.35 mm) overlap and sealed along the 6 inch (15 cm) edge to
create a tube
approximately 9 inches (23 cm) tall with a circumference of 10.5 inches (27
cm) due to the
overlap. The shade is folded such that the polyethylene side is on the outside
of the tube and the
paper is on the inside. The rupturable pouch is located at the base of the
tree such that when
ruptured the fluid would release near the base and slowly wick up the tree
based on the capillary
properties of the paper and paper/film lamination. The shade is then placed in
an 8 Inch x 5 inch
(20 cm x 13 cm) foil pouch and sealed.
The consumer uses the shade by opening the foil pouch. This is done by the
consumer
tearing off the sealed region along one edge of the foil pouch. The shade is
removed and placed
over a battery powered light base. The scent/colored dye in the rupturable
pouch is activated by
squeezing the shade and light base in the region where the rupturable pouch is
located at the
bottom of the shade. In doing so the pouch is ruptured which allows for the
release of
approximately 3 cc to about 4 cc of fluid. This fluid then comes into contact
with the shade.
The outside of the shade and the bottom of the shade do not become wet as the
film side of the
shade keeps the bottom surface and the outside of the shade dry. In
approximately 30 minutes
the fluid wicks up about 2 inches (5 cm) of the shade. Within 120 minutes the
fluid wicks up the
full 6 inch (15 cm) height of the shade. In this particular example, a tree
pattern appears as the
wicking occurs since the colored dye will only wick in the porous regions of
the paper. The
wicking fluid also delivers scent such that there is no initial scent
delivered upon bursting the
pouch but rather the scent is slowly released as the scent/oil mixture in the
pouch wick up the
paper and evaporate.
CA 02741443 2011-05-30
33
Example 2
A shade comprising three layers, including a translucent paper sold by CFI
Paper USA
of Sun Prairie Wisconsin under the name "Parchment White (llama Natural" with
a nominal
basis weight of 110g/m2, a low density polyethylene film having a thickness of
about 0.03 mm,
and a creped tissue paper having a nominal basis weight of about 23 g/m2, sold
by Cellutissue
Incorporated as grade 7020, are arranged so that the low density polyethylene
is in between the
two paper layers. The three layers are then thermally bonded using a thermal
roll laminator set
to sufficient temperature and laminating pressure to prevent the layers from
being easily
separated.
The 3-layer laminate described above is folded and cut to form a tube having a
circumference of 190 mm and a height of 220 mm. The tube is formed so that the
creped tissue
paper is located on the inside of the cylinder. The seam of the tube is
secured by applying
transfer adhesive tape (sold as 9471LE available from 3M Corporation of St.
Paul, Minnesota),
to an approximate 0.5 inch (1.3 cm) overlap. The tube is then creased to form
a flat structure.
Approximately 0.7g of a volatile composition having the composition shown in
(Table 1)
(wherein greater than 90% by weight of the volatile composition's ingredients
have a KI value
of less than about 1500) is applied to the creped tissue paper layer of the 3-
layer laminate tube
using a transfer pipette. The 3-layer laminate tube is then promptly placed
into a metalized poly
bag (available from ULine Corporation of Waukegan, Illinois as Part No. S-
6176) and heat
sealed.
After approximately 16 hours the 3-layer laminate tube is removed from the
metalized
bag and opened into the tube shape and placed into a room having a temperature
of
approximately 70 F (21 C). After about 6 hours, about 50% of the perfume
originally in the
shade at the time of removal from the metalized bag is evaporated from the
shade. After about
24 hours, more than about 70% w/w of the perfume originally in the shade at
the time of
removal from the metalized bag is evaporated from the shade.
CA 02741443 2011-05-30
34
Table 1
Composition of Volatile Composition of Example 2
Allyl ir+a (cas # 123-68-2) 1083 13 1 190
Ethyl Acetate (cas 0141-78-6) 610 3 77 14 Benzaldehyde (cas # 100-52-7) 9714 2
179
Prenyl Acetate (cas # 1191-16-8) 919 8 152
Benzyl Acetate (cas # 140-11-4) 1173 15 214
Ethyl-2-methyl Butyrate (cas #
7452-79-1) 850 8 132
Amyl Acetate (cas # 628-63-7) 912 3 149
as 3 Hexenyl Acetate (cas # 3681-
71-8) 1009 3 166
Ligustral (cas # 27939-60-2) 1094 5 177
Melonal (can # 106-72-9) 1060 1 116
Hexyl Acetate (cas #142-92-7) 1016 8 146
Fruitate (cas # 8065,7-64-3) 1470 10 243
Verdox (cas#88-41-5) 1319 10 221
Flor Acetate (cas # 5413-60-5) 1442 11 175
Orange Terpenes (cas # 68917-57-
7) 1040 10 176
Example 3.
A shade is prepared as in Example 2. except that the volatile composition is
that which is
described in Table 2 (wherein greater than 80% by weight of the volatile
composition's
ingredients have a IU value of less than about 1700). After about 6 hours,
about 20% w/w of the
perfume originally in the shade at the time of removal from the metalized bag
is evaporated from
the shade.
CA 02741443 2011-05-30
Table 2
Composition of Volatile Composition of Example 3
+ .'.ail' _ r`_t .~.`=~ :.ss. ': a;,y?= ~ _ ~ ~ ~i '". t?h .... i~' t
Aurantiol (cos # 89-43-0) 2294 2 413
Benzyl Salicylate (cas # 118-58-1) 2139 3 320
.:.......... ....
Coumarin (cas # 91-64-5) 1463 1 300
Ethyl Vanillin (cas # 121-32-4) 1652 4 285
Hexyl Cinnamic Aldehyde (cas # 1770
101-86-0) 3 334
Iso E Super (cas # 54464-57-2) 1706 5 307
Lanalool (cas # 78-70-6) 1243 7 195
Lanalyl Acetate (cas # 115-95-7) 1262 3 220
Methyl Dihydro Jasmonate (cas # 1668
24851-98-7) 9 300
Polysantol (cas # 107898-54-4) 1703 2 295
Silvanone Ci (mixture)' 1984 4 300
Vanillin (cas # 121-33-5) 1589 3 285
Dipropylene Glycol (cas # 110-98-5) 1152 3 231
Dipropylene Glycol Methyl Ether 997
(cas #34590-94-8) 50 190
Silvanone Ci is available from Quest International of Mount Olive, New Jersey.
Example 4
A shade is prepared using SYNERGEX 6130 manufacatured by BBA/Fiberweb of
Simpsonville, South Carolina. The SYNERGEX material is comprised of calendared
polyester
fibers with a basis weight of 102 g/m2 and a thickness of 0.3 mm. The material
is folded and cut
to forma tube having a circumference of 190 mm and a height of 190 mra. The
seam of the tube
CA 02741443 2011-05-30
36
is secured by applying transfer adhesive tape (sold as 9471LE available from
3M Corporation of
St. Paul, Minnesota) to an approximate 0.5 inch (1.3 cm) overlap. The tube is
then creased to
form a flat structure.
About 0.7 g of the volatile composition described in Table 3 (wherein only
about 62% by
weight of the volatile composition's ingredients have a KI value of less than
about 1700) is
applied to the tubular shade. The tubular shade is then promptly placed into a
metalized 2.2 mil
poly bag (0.055 mm) (available as Part No. S-6176 from U-Line Corporation) and
heat sealed.
After approximately 4 hours the tubular shade is removed from the metalized
poly bag
and opened to a tube shape and placed into a room having a temperature of
approximately 70 F
(21 C). After about 5 hours, about 20% w/w of the perfume originally in the
shade at the time
of removal from the metalized bag is evaporated from the shade. After about 24
hours, about
30% w/w of the perfume originally in the shade at the time of removal from the
metalized bag is
evaporated from the shade.
Table 3
Composition of Volatile Composition of Example 4
r'~..j
I1+fa Nine t p~? ` 3R t j' (~ y a
Aurantiol (cas # 89-43-0) 2294 3.800 260
BeazyI Salicylate (cas # 118-58-1) 2139 6.830 300
Coumarin (cas # 91-64-5) 1463 1780 300
Ethyl Vanillin (pas # 121-32-4) 1652 7.590 285
Hexyl Cinnamic Aldehyde (cas # 1770
101-86-0) 6.070 305
Iso E Super (cas # 54464-57-2) 1706 10.120 230
Linalool (cas # 78-70-6) 1243 14.930 195
Linalyl Acetate (cas # 115-95-7) 1262 5.110 220
Methyl Dihydro Jasmonate (cas # 1668
24851-98-7) 18.980 300
Polysantol (cas # 107898-54-4) 1703 3.800 295
Silvanone Ci (mixture)' 1984 7.590 300
CA 02741443 2011-05-30
37
Vanillin (cas # 121-33-5) 1589 6.070 285
Dipropylene Glycol (cas # 110-98- 1152
5) 6.330 231
Silvanone Ci is available from Quest International of Mount Olive, New Jersey.
Example 5
A shade is prepared using SYNERGEX 6130 manufactured by BBA/Fiberweb of
Simpsonville, South Carolina. The SYNERGEX material is comprised of calendared
polyester
fibers having a basis weight of 102 gsm and a thickness of 0.3 mm. The
material is folded and
cut to form a tube having a circumference of 190 mm and a height of 220 mm.
The seam of the
tube is secured by applying transfer adhesive tape (sold as 9471LE and
available from 3M
Corporation of St. Paul. Minnesota) to an approximate 0.5 inch (1.3 cm)
overlap. The tube is
then creased to form a flat structure.
0.7 g of the volatile composition described in Table 2 is applied to the
tubular shade.
The tubular shade is then promptly placed into a metalized 2.2 mil (0.055 nun)
poly bag
(available as Part No. S-6176 from U-Line Corporation) and heat sealed.
After approximately 24 hours the tubular shade is removed, from the metalized
poly bag
and opened into a tube shape and placed into a room having a temperature of
approximately
70 F (21 C). After about 5 hours, more than about 50% w/w of the perfume
originally in the
shade at the time of removal from the metalized bag is evaporated from the
shade. After about
24 hours, more than about 65% w/w of the perfume originally in the shade at
the time of
removal from the metalized bag is evaporated from the shade.
Example 6
A shade is prepared using SYNERGEX 6130 manufacatured by BBA/Fiberweb of
Simpsonville, South Carolina. The SYNERGEX material is comprised of calendared
polyester
fibers with a basis weight of 102 gsm and a thickness of 03 mm. The material
is folded and cut
to form a tube having a circumference of 190 mm and a height of 220 mm. The
seam of the tube
is secured by applying transfer adhesive tape (sold as 9471LE available from
3M Corporation of
St. Paul, Minnesota) to an approximate 0.5 inch (1.3 cm) overlap. The tube is
then creased to
form a flat structure.
CA 02741443 2011-05-30
38
0.7 g of the volatile composition described in Table 1 is applied to the
tubular shade.
The tubular shade is then immediately placed into a metalized 2.2 mil (0.055
mm) thickness
poly bag available as Part No. S-6176 from U-Line Corporation and heat sealed.
After approximately 24 hours the tubular shade is removed from the metalized
poly bag
and opened to a tube shape and placed into a room having a temperature of
approximately 70 F
(21 C). After about 5 hours, about 80% w/w of the perfume originally in the
shade at the time
of removal from the metalized bag is evaporated from the shade. After about 24
hours, more
than about 85% w/w of the perfume originally in the shade at the time of
removal from the
metalized bag is evaporated from the shade.
Example 7
A translucent paper sold by CTI Paper USA, of Sun Prairie Wisconsin, under the
name
"Parchment White Glama Natural" having a nominal basis weight of 110 g/m2 is
folded and cut
to form a tube having a circumference of 190 mm and a height of 220 mm. The
seam of the tube
is secured by applying transfer adhesive tape (sold as 9471LE and available
from 3M
Corporation of St. Paul, Minnesota) to an approximate 0.5 inch (1.3 cm)
overlap. The tube is
then creased to form a flat structure.
A strip of absorbent material (sold as product No. 7620W available from EMI
Specialty
Papers of Redding, Connecticut) is cut to about 1.7 cm by 19 cm and secured to
the inside of the
paper tube very near the end of the tube using a double sided tape (sold as
product No. 9500PC
available from 3M Corporation).
Approximately 0.45g of a volatile composition having the composition shown in
Table 1
(wherein greater than 90% by weight of the volatile composition's ingredients
have a KI value
of less than about 1500) is applied to the absorbent strip of material using a
transfer pipette. The
tube is then placed into a metalized poly bag (available from ULine
Corporation as Part No. S-
6176) and heat sealed.
After approximately 48 hours the tube is removed from the metalized poly bag
and
opened into a tube shape and placed into a room having a temperature of
approximately 70 F
(21 C). The tube is placed vertically on a table with the absorbent strip at
the top of the tube.
After about 6 hours, about 50% w/w of the perfume in the shade at the time of
removal from the
metalized bag is evaporated from the shade. After about 24. hours, about 65%
w/w of the
CA 02741443 2011-05-30
39
perfume originally in the shade at the time of removal from the metalized bag
is evaporated from
the shade.
Example 8
A shade is prepared using SYNERGEX 6130 manufacatured by BBA/F'iberweb of
Simpsonville, S.C. The SYNERGEX material is comprised of calendared polyester
fibers with
a basis weight of 102 g/m2 and a thickness of 0.3 mm. The average pore size
diameter of this
material is approximately 34 microns with a range of about 10 microns to about
75 microns.
The material is printed with a graphical pattern using. conventional inks with
an inkjet printer.
The SYNERGEX material is then cut to a dimension of 19 cm by 20 cm and rolled
into a tube
and sealed along the 19 cm length with a 1 cm overlap. The SYNERGEX material
is heat sealed
to itself using a VERTROD brand impulse heat sealer. The opacity of the shade
when dry prior
to perfume addition is measured to be 65% dry using a Hunter Labscan XE. The
shade is loaded
with 0.7 grams of the perfume composition shown in Table 1. Promptly after
loading the shade
with the perfume the opacity is measured and is found to be 43%. Four hours
after application
of the perfume composition to the shade 50% of the perfume is evaporated and
the opacity is
increased to 55%.
Example 9
A shade is prepared as in Example 2. Before sealing into the metalized poly
bag, a
decorative design in the shape of a flower is drawn on the outside of the
shade using a
"Disappearing Ink Marking Pen" available from Pyrm-Dritz Corporation of
Spartanburg, South
Carolina. The decorative design disappears from the shade within about 24
hours after removal
from the metalized bag.
Example 10
A flexible sheet article is constructed by adhesive laminating a layer of
translucent paper
sold by CTI Paper USA, of Sun Prairie WI, under the name "Parchment White
Glama Natural"
with a nominal basis weight of 110 g/m2 to a layer of microporous high
molecular weight
polyethylene sold by DSM Solutech of Heerlen, Netherlands under the name
SOLUPOR 7P03A
with a nominal basis weight of 7g/m2. The translucent paper is printed with
decorative graphics
using an HP inlsjet printer. The laminated structure is then cut to 200 mm a
185 mm and rolled
CA 02741443 2011-05-30
along the 200 nun width with a 10 mm overlap to create an oval shaped tube
with circumference
of 190 mm with a 185 mm height. The flexible article is rolled such that the
microporous
polyethylene is on the outside and the translucent paper with graphics printed
is on the inside.
A layer of double sided tape from 3M Corporation (available as 3M 947 ME) is
used in the
overlap region to keep the tube from unrolling.
Approximately 2 ml of a volatile composition having the composition described
in Table
1 is applied to the flexible tube article. The flexible article is then placed
in a high barrier pouch
and sealed to keep the article from losing the volatile composition until
ready to be used. The
flexible tube article can be used as a standalone air freshener or can be
placed on a base to help
hold the article upright. Alternatively the article can also be used as a
luminary shade when
placed over a light base.
Samples of the laminated structure are cut and the opacity is measured before
and after
wetting with the volatile composition. The dry structure has an opacity of 95%
and the wetted
structure has an opacity of 45%. When dry the tube article or luminary shade
is white in
appearance due to the opacity of the microporous polyethylene. When wet the
microporous
polyethylene is semi-transparent and the graphics on the inside can be seen
very clearly both
with and without illumination on the inside of the tube. As the volatile
composition evaporates
over a 10 hour time period the shade becomes more opaque and within 2 days is
back to the
original 95% opacity with very little graphic visible. This change in
appearance helps
communicate to a user that the fragrance has been substantially depleted and
can now be
discarded and replaced with a new shade.
Example 11
A flexible article is constructed with the same materials as described in
Example 10 but
the microporous polyethylene is printed with graphics instead of the
translucent paper and the
laminated structure is rolled into a tube such that the printed microporous PE
is on the inside
with the printed surface of the tube and the unprinted translucent paper is on
the outside.
The flexible article is again wetted with the same volatile composition as
used in Table 1
but now the volatile composition is on the inside of the tube. The opacity of
the article went
from 95% when dry to 45% when wet and again the graphics were highly visible
when wet but
were hidden due to the opacity change of the microporous polyethylene
material. With this
example the release rate of the volatile composition is slower due to the
wetted microporous
CA 02741443 2011-05-30
41
polyethylene being on the inside versus the outside of the oval tube shape.
The perfume is
released over a period of 5 days.
Example 12
Three shades are prepared using a multi-layer structure. The venting of the
shades is
adjusted to determine the effect of perfume evaporation rate. The shades are
constructed of
three layers. The outer layer is a 60 gsm heat sealable paper from Ahlstrom
Windsor Locks
LLC of Windsor Locks, Connecticut. The middle barrier layer is comprised of a
blown
PEIEVOH/PE 2.5 mil thick film available from Printpak Incorporated of Atlanta.
Georgia. The
inner layer is comprised of a 102 gsm spun bond polyester nonwoven with the
tradename of
SYNERGEX 6130 available from BBA/Fiberweb os Simpsonville, South Carolina. The
SYNERGEX material is comprised of calendared polyester fibers having a
thickness of 0.3 mm.
The three layers are laminated together using a hot roll laminator with a roll
pressure of 60 psi, a
roll temperature of 360 F (182 C) and a speed setting of 20. The tri-layer
sheet is then die cut
to the dimensions of 185 mm X 200 mm using a hydraulic press and a steel rule
die. The 185
mm X 200 mm sheet is then folded around a folding board that is 185 mm long by
95 mm wide
with the SYNERGEX absorbent material on the inside. The shade is then sealed
along the 185
mm length at the overlap using a lap seam. This creates a flattened tube that
is 185 min long
with a circumference of approximately 192 mm (having an 8 mm overlap). The
sealing is done
with an impulse beat sealer model No. 24LABMod SIN:V-49093 with a bar pressure
of 50 psi, a
heat pulse dwell of 8 seconds, and a cool dwell of 16 seconds. The power
setting to heat the
impulse metal ribbon is set at 27.5 on the dial.
The Inside (SYNERGEX layer) of the formed shades are dosed with 0.5 grains of
a
cucumber and melon perfume available from International Flavors and Fragrances
of Hazlet,
New Jersey. The shades are then placed on an oval shaped base to hold the
shade upright with
the shade tube opening facing upward and the other opening fitting snuggly in
the oval base.
Two different types of thermoformed bases are used to hold the shades. One
base has a
closed bottom such that the shade fits tightly into the base with little to no
possibility for air to
move through the base and/or between the interface between the base and the
shade. This is
designated the "no venting base". The other base has two 0.75 inch (19 rum)
diameter holes in
the center of the base with an equivalent 1 square inch (6.45 cma) area
openings on the sides of
CA 02741443 2011-05-30
42
the base. This is designated the "venting base" with an equivalent of 1 square
inch (6.45 cm2) of
area for air to flow through the base (sidewalls and top) and hence the bottom
of the shade.
The top of the shades are adjusted to be either open or closed using a paper
clip to close
them shut and a 35 mm diameter thin ring to open the shade. Optionally a foil
tape can be
applied on the top of the shade to help the shade stay opened or closed to a
desired shape. The
three shades are tested under three different conditions: 1) closed top and
closed base; 2) open
top and closed base; and 3) open top of shade and open base. The perfume
evaporation rate is
measured by weighing the shades at different time intervals and taking the
difference over the
time interval to calculate the average weight loss/hour. The results which are
shown in Table 4
below illustrate how the open top with the closed bottom increases the
evaporation and how the
evaporation is further increased when the vented base is used in conjunction
with the top and
bottom of the shade being vented.
Table 4
Perfume Release Rate over 6.7 Hours
(Average mg of Perfume Released per Hour of Time Elapsed)
los Vented
=~K - - 6 1 .iS + h.' Bas Ba a sand
~rG.', _y. '.rJ : =~ ,' lam' q q l l r
U g.3
x.- , y '4' 1 v yam. =^~,M
= :~ ~ i #rt. yir2 `'-~.~ Y +'..l
Tine Elapsed (Houma) fi ) nuug/hour)
.a_
0-1 3.0 rt 18.8 103.8
1 - 2.7 3.0 21.4 59.4
2.7 - 3.2 2.0 17.1 40.0
3.2-3.7 3.0 18.8 40.0
3.7-5.2 Z.0 19.0 27.9
5.2-6.7 3.0 9.8 32.6
Example 13
CA 02741443 2011-05-30
. 43
An integral shade and package are made to demonstrate how a low cost all-in-
one
scented product and package can be made. The shade is made by taking a bi-
laminate
substrate comprising an absorbent non-woven laminated to a barrier film.
SYNERGEX
6125 PET non-woven described previously is used for the inner absorbent layer.
The
outer barrier film is a film supplied by Printpak of Atlanta, Georgia
consisting of
PET/PE/EVOH/PE. The barrier film is thermally laminated to the SYNERGEX non-
woven in a similar manner as described in Example 12 with the exception that
the
SYNERGEX is 0.5 inches (1.3 cm) narrower on the top and bottom (see FIG. 20)
leaving
regions with no absorbent material present. This narrower width of the non-
woven is
done so the non-woven is not in the seal region. The non-woven is then loaded
with 1
gram of perfume as described in Table 1 of Example 2. The bi-laminate is then
folded on
itself with the perfumed non-woven on the inside and the barrier film facing
outward.
The barrier film is then heat sealed to itself on the 3 open ends. The sealed
package can
be opened by using scissors and cutting off the seal region on two ends.
Alternatively or
in addition, the barrier film can be laser scored to aid in easily tearing off
the ends. The
opened package can be formed into a cube shape and inserted on a base to allow
scent
release. This style of all-ib-one package and shade all in one can be made
using a vertical
or horizontal form/fill/seal machine such as manufactured by Hayssen Packaging
Technologies of Duncan, South Carolina.
Example 14
An integral shade and package are made in the same manner as described in
Example 13 except the absorbent material is product No. 7620W available from
EMI
Specialty Papers of Redding, Connecticut instead of the SYNERGEX 6125. This
thicker
more absorbent material is then dosed with 7 ml of perfume and sealed in the
same
manner as described in Example 13. This shade is opened by removing the sealed
ends
and opening to form a cube-like shape. This example illustrates how a longer
lasting low
cost air freshener could be produced. To provide a faster scent release the
cube can be
placed on some small blocks to allow air to flow through the cube.
While particular embodiments of the present invention have been illustrated
and
described, it would be obvious to those skilled in the art that various other
changes and
CA 02741443 2011-05-30
44
modifications can be made without departing from the spirit and scope of the
invention.
It is therefore intended to cover in the appended claims all such changes and
modifications that are within the scope of this invention. All documents cited
herein are
not to be
construed as an admission that it is prior art with respect to the present
invention.