Note: Descriptions are shown in the official language in which they were submitted.
CA 02742033 2011-04-21
WO 2010/048533 PCT/US2009/061894
1
DUAL CYLINDER HYDROGEN GENERATOR SYSTEM
REFERENCE TO RELATED APPLICATIONS
This application claims an invention which was disclosed in Provisional
Application Number 61/108,062, filed October 24, 2008, entitled "DUAL CYLINDER
HYDROGEN GENERATOR". The benefit under 35 USC 119(e) and Article 8, Patent
Cooperation Treaty of the United States provisional application is hereby
claimed, and the
aforementioned application is hereby incorporated herein by reference.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR
DEVELOPMENT
Not applicable.
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
The present invention is directed to a water electrolysis apparatus; and in
particular, a water electrolysis apparatus for use in internal combustion
engines to increase
engine performance by the addition of hydrogen and oxygen generated by water
electrolysis to the air-fuel mixture.
DESCRIPTION OF RELATED ART
Internal combustion engines operating with fossil fuels helped build the
standard of
living we enjoy today. Just a few of the myriad tasks they perform include
transporting
people and cargo; pumping water, oil, and other liquids, mowing lawns, and
generating
electricity. But fossil-fueled engines have two major drawbacks. The first is
the
apparently limited supply of those fuels. The second is the pollution that is
produced from
the combustion process. Engines burning hydrocarbon fuels generate carbon
dioxide,
carbon monoxide, nitrous oxides, sulfur dioxide, and other noxious gases.
These products
CA 02742033 2011-04-21
WO 2010/048533 PCT/US2009/061894
2
are a result, in part, of imperfect combustion in the engine and the resultant
incomplete
burning of the fuel.
Pure hydrogen has long been considered as an alternative fuel source, as it
releases
almost three times the energy of fossil fuels per pound while producing only
water as a
combustion product. However, it is an explosive gas that is difficult to
store, especially in
vehicular applications. Moreover, hydrogen cannot be removed from the ground
and
refined like fossil fuels. Although it can readily be produced from water,
more energy
(often derived from fossil fuels) is required to separate hydrogen and oxygen
from water
than produced in the recombination (combustion) process.
However, mixing hydrogen and/or oxygen with gasoline or diesel fuel and air
has
been shown to improve engine efficiency and reduce pollutant emissions. In
fact, the
addition of hydrogen and/or oxygen as a fuel additive to an internal
combustion engine is
an art nearly as old as the internal combustion engine itself. Many attempts
have been
made by various practitioners in the prior art to improve combustion
efficiency and reduce
emissions through the introduction of hydrogen and/or oxygen to the fuel/air
mixture.
In particular, many attempts have been made in the prior art to use a basic
electrolysis reaction of various solutions (water or other chemical
compositions containing
hydrogen and oxygen) to produce elemental hydrogen and oxygen in gaseous form.
As
hydrogen is highly flammable, especially in the presence of the oxygen usually
also
produced during electrolysis, the current state of the art is to electrolyze
only enough water
to produce just enough hydrogen and/or oxygen gases to achieve the desired
results of
improved combustion, enhanced fuel economy and reduced emissions. Storage in
tanks,
as well as accumulation of excess amounts of hydrogen and oxygen gases, is
thereby
avoided. U.S. Pat. No. 4,111,160 (Talenti) provides a broad overview of prior
art attempts
and the use of the basic electrolysis reaction to achieve enhanced combustion.
Since storage or accumulation of hydrogen and oxygen is to be avoided, control
of
the electrolysis reaction is of key consideration. Producing hydrogen and
oxygen only in
the amounts immediately necessary avoids the requirement in earlier prior art
of venting
excess hydrogen and/or oxygen to the atmosphere, which wastes energy and
creates
additional safety concerns by requiring safe dissipation of these gases in
close proximity
CA 02742033 2011-04-21
WO 2010/048533 PCT/US2009/061894
3
with heat and ignition sources such as the host internal combustion engine,
associated
electrical components, or smoking occupants inside the vehicle.
Efficiency is also a major concern. Significant amounts of electrical energy
are
required to electrolyze water to produce hydrogen and oxygen. This energy is
supplied, in
the case of a vehicle, by the vehicle's alternator and electrical system. Care
must be taken
not to overload the electrical generation system or cause premature failure of
the alternator
or other components. Controlling the electrolysis reaction to generate only
the volumes of
hydrogen and oxygen required to improve combustion contributes to efficiency,
but design
of the system in general and the electrolysis cells in particular should
reflect this goal as
well.
Making the system rugged is an important consideration as well, especially in
mobile applications where temperature extremes and vibration are to be
expected. A long
service life reduces maintenance and maximizes the benefits of fuel economy
and
emissions reduction. U.S. Pat. No. 5,105,773 (Cunningham et al.) is an example
of a
design that attempts to take all of these desirable attributes into account.
Unfortunately, in
this design, the electrical connections to the electrodes in the electrolysis
cell are
continuously exposed to the electrolyte solution. The positive (anode) and
negative
(cathode) terminals will eventually corrode, and if one connection or the
other fails, the
gas production of the cell will fall to a level well below that required to
obtain the desired
benefits.
Finally, simple operation is a desired goal. Many commercially-available
hydrogen/oxygen cells require frequent adjustments by the user, who must
determine,
often without proper instrumentation and usually through trial and error, the
proper
settings to achieve the desired results. U.S. Pat. No. 6,332,434 (De Souza et
al.) attempts
to achieve this goal through extensive computerization, but the design of the
electrolysis
cell produces a limited gas output rate that requires multiple cells to
achieve a rate
sufficient to obtain the desired benefits of increased fuel efficiency and
reduced pollutant
emissions.
Nearly all of the electrolysis cells in the prior art referred to above have
electrodes
in a non-conductive container that does not contribute to the electrolysis
reaction. The use
CA 02742033 2011-04-21
WO 2010/048533 PCT/US2009/061894
4
of such insulating containers often results in heat buildup that can require
extraordinary
steps to dissipate the heat that is produced due to driving the electrolysis
cell with a higher
voltage than required for the electrolysis reaction. In addition, because of
the limited
electrode area provided in these cells, and because the electrodes must be
separated further
apart than optimal, large amounts of poisonous and corrosive acids or bases
such as
potassium hydroxide must be added to the water to enable the electrolysis
reaction to
proceed. U.S. Pat. No. 5,450,822 (Cunningham) attempts to overcome all of
these
limitations, but its design is difficult to build and requires insulating
materials to be used
within the cell, resulting in a reduction of both efficiency and ruggedness.
The additional
requirement of prolonged exposure to the hot and often corrosive electrolytic
fluid would
also require the insulating material to be carefully selected and expensive.
The present invention overcomes the limitations of the prior art by achieving
all of
the above goals in a simple, straightforward and readily adaptable manner.
SUMMARY OF THE INVENTION
The present invention is a self-contained, automatic system which generates
hydrogen and oxygen by the controlled electrolysis of water and feeds these
gases into an
internal combustion engine. The invention is comprised of a rugged
electrolysis cell
fabricated in a preferred embodiment completely of stainless steel, itself
comprised of two
half-cells that when fitted together provide a closed electrolysis chamber
with closely-
spaced electrodes alternatively charged positive and negative.
The invention also comprises a separate electrolyte tank, fabricated in a
preferred
embodiment of coated aluminum, that simultaneously stores excess electrolyte
to provide
a much longer operating range than the prior art, allows the separation of
gaseous
hydrogen and oxygen from the electrolyte, provides a small pressure chamber
for the
temporary storage of hydrogen and oxygen prior to introduction into the host
internal
combustion engine, and provides a heat sink for dissipating the excess heat
that is
produced by the electrolysis cell.
In addition, an electrolyte pump continually circulates the electrolyte
between the
electrolysis cell and the electrolyte tank, clearing the electrodes of formed
bubbles of
CA 02742033 2011-04-21
WO 2010/048533 PCT/US2009/061894
hydrogen and oxygen to prevent the bubbles from preventing contact of the
electrodes
with the electrolyte solution.
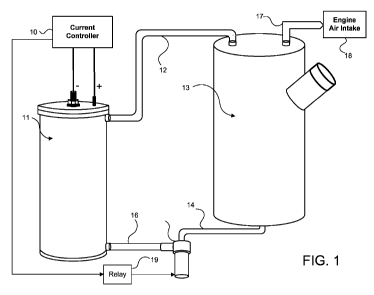
BRIEF DESCRIPTION OF THE DRAWING
Figure 1 shows the components of the electrolysis system and the mechanical,
fluid, and
5 electrical connections therebetween.
Figure 2 is a side elevation view of the upper (positively-charged) half of
the electrolysis
cell.
Figure 3 is a bottom plan view of the upper (positively-charged) half of the
electrolysis
cell.
Figure 4 is a vertical sectional view of the upper (positively-charged) half
of the
electrolysis cell along 4-4 of Figure 3.
Figure 5 is a side elevation view of the bottom (negatively-charged) half of
the electrolysis
cell.
Figure 6 is a top plan view of the bottom (negatively-charged) half of the
electrolysis cell.
Figure 7 is a vertical sectional view of the bottom (negatively-charged) half
of the
electrolysis cell along 7-7 of Figure 6.
Figure 8 is an exploded view of all the component parts of the electrolysis
cell.
Figure 9 is a cross-sectional view of the assembled electrolysis cell.
Figure 10 is a longitudinal sectional view of the assembled electrolysis cell.
DETAILED DESCRIPTION OF THE INVENTION
A preferred embodiment of a hydrogen generator system of the present invention
is
illustrated in Figure 1. When the hydrogen generator system shown in Figure 1
is
activated, preferably when the engine to which the hydrogen generator system
is attached
is started, current is supplied from current controller 10 to electrolysis
cell 11 and from
CA 02742033 2011-04-21
WO 2010/048533 PCT/US2009/061894
6
relay 19 to pump 15. Pump 15 then pumps electrolyte stored in electrolyte tank
13
through hoses 14 and 16 into the lower opening of the electrolysis cell. This
forced
electrolyte flow dislodges hydrogen and oxygen bubbles forming on the
electrodes inside
electrolysis cell 11 and pushes them, along with excess electrolyte, through
hose 12 back
into electrolyte tank 13. In electrolyte tank 13, the gas bubbles separate
from the
electrolyte. The increase in pressure caused by the increased volume of the
generated
gases naturally forces them from electrolyte tank 13 through hose 17 into
engine air intake
18 for mixing with outside air and subsequent introduction, along with
hydrocarbon fuel
(diesel or gasoline) into the combustion chambers of the host engine.
The design of current controller 10 is important to the proper operation of
the
hydrogen generation system. Most modem motor vehicles operate with 12 volt
direct
current electrical systems. However, the electrolysis of water only requires
1.23 volts.
Some of the overvoltage is necessary to force current to flow through the
electrolytic fluid,
but the remainder of the overvoltage is wasted, being converted to heat. Prior
art systems
usually limit the average current by pulsing 12 volts direct current into one
or more
electrolysis cells. More or less average current is provided by changing the
duty cycle of
the applied pulse, thus turning on the current for a longer period of time.
This pulse-width
power switching allows varying the average current, but during the period of
time the
current is switched on, very high current flows, which places a heavy burden
on the motor
vehicle's alternator, battery, and electrical system. In addition, the
overvoltage is still
converted to heat, just at a lower average rate.
In a preferred embodiment, current controller 10 employs a DC-DC converter
such
as described in U.S. Patent 6,209,493 (Ross). This reduces the input current
required from
the vehicle's electrical system by decreasing the output voltage. Such
converters are well-
known in the electronic arts, and can be designed to provide current (at a
lower voltage)
limited to a selected design value, such as 40-60 amperes. If the electrolyte
concentration
is high enough that this current is achieved at a voltage of 6 volts, then
only about 20-30
amperes at 12 volts will be required from the vehicle's electrical system.
Even lower
voltages are possible, with a corresponding decrease in the current required
from the
vehicle's electrical system.
CA 02742033 2011-04-21
WO 2010/048533 PCT/US2009/061894
7
In a preferred embodiment, pump 15 is a readily-available auxiliary coolant
pump
commonly used in European cars. Bosch part number G3050-12429 is
representative of a
suitable component for this purpose. When power is applied to current
controller 10, it is
also applied to pump 15, which continuously circulates electrolytic fluid from
electrolyte
tank 13 into the bottom of electrolysis cell 11. Pump 15 is constructed of
high-
temperature plastic to easily handle the hot electrolytic fluid. Operation of
pump 15
during electrolysis improves gas production by sweeping insulating bubbles of
hydrogen
and oxygen from the electrodes. In addition, the temperature of electrolysis
cell 11 is
reduced by moving electrolyte (heated by overvoltage and power loss occurring
in
electrolysis cell 11) into electrolyte tank 13, which then dissipates some of
the heat from
the electrolyte prior to its return to electrolysis cell 11.
In a preferred embodiment, electrolysis cell 11 is fabricated entirely of 316L
stainless steel, making the cell highly resistant to vibration, extremes in
temperature and
the corrosive effects of the electrolyte solution. Electrolysis cell 11 is
comprised of two
parts. Figure 2 illustrates the upper portion 20. Top plate 21 is made of 3/8"
thick stainless
steel plate and is 5" in diameter. Center hole 22, 5/8" in diameter and
unthreaded, is
drilled in the center of top plate 21. Figure 3 is a plan view looking from
the bottom
toward the top of upper portion 20. Four stainless steel tubes 25, 26, 27, and
28 with
outside diameters approximately 1", 2", 3", and 4", respectively, are
concentrically welded
to the lower side of top plate 20 around center hole 22. The wall thicknesses
of stainless
steel tubes 25, 26, 27, and 28 are nominally 0.064" to strike a balance
between cost and
service life. Figure 4 is a sectional view of upper portion 20. Each of tubes
25, 26, and 27
and 28 have four half-circle cutouts 33 disposed 90 apart near the point
where the tubes
are welded to top plate 21 to allow the flow of electrolytic fluid and
generated gases
during cell operation. Groove or fillet welding may be used to attach tubes
25, 26, 27, and
28 to top plate 21, but Heliarc (TIG) welding is necessary due to the
materials used.
Threaded rod 29 is welded to the upper side of top plate 21 to facilitate
connection of an
electrical cable to the positive terminal of current controller 10.
Figure 5 illustrates the lower portion 30 of electrolysis cell 11. Bottom
plate 31 is
made of 3/8" thick stainless steel plate and is 4 1/2" in diameter. Center
hole 32, 5/8" in
diameter, is drilled in the center of bottom plate 31 and threaded to securely
accept a
CA 02742033 2011-04-21
WO 2010/048533 PCT/US2009/061894
8
threaded rod when electrolysis cell 11 is completely assembled as described in
further
detail below. Figure 6 is a plan view looking from the top toward the bottom
of lower
portion 30. Using the same welding techniques described previously, four
stainless steel
tubes 35, 36, 37, and 38 with outside diameters approximately 1 1/2", 2 1/2",
3 1/2", and 4
1/2", respectively, are concentrically welded to one side of bottom plate 30
around center
hole 31. Tubes 35, 36 and 37 extend for 11 7/8" from bottom plate 31 and are
nominally
0.064" in thickness, while tube 38 extends a full 12" from bottom plate 31 and
is
nominally .125" in thickness to facilitate welding of stainless steel ring 34
at the opposite
end of tube 38 as discussed below.
Figure 7 is a longitudinal sectional view of lower portion 30. Each of tubes
35, 36,
and 37 have four half-circle cutouts 33 disposed 90 apart near the point
where the tubes
are welded to bottom plate 30 to permit the flow of electrolytic fluid during
cell operation.
A 1/4" thick, 3/8" tall stainless steel ring 34 is welded to the opposite end
of tube 38 from
its connection point to bottom plate 31 in order to create a sealing surface
when assembled
with upper portion 20. Gas fittings 41 and 42 are welded to opposite ends of
tube 38 to
permit electrolyte and gases to flow into and out of electrolysis cell 11.
Figure 8 is an exploded view of the component parts of electrolysis cell 11.
Upper
portion 20 fits into lower portion 30. Gasket 40, made from silicone rubber,
electrically
and mechanically separates top plate 21 from stainless steel ring 33, also
serving to
prevent leakage of electrolyte and generated gases. Insulator 23 is inserted
into center hole
22, then rod 24 is inserted through insulator 23 until contact is made with
bottom plate 31.
Rod 24, the bottom portion of which is threaded to match center hole 31, is
then rotated to
tightly seal center hole 31. Upper portion 20 is then secured to lower portion
30 by
placing a stainless steel washer 43 on top of insulator 23 and rod 24, then
tightening
stainless steel nut 44 to fasten the two portions together. Stainless steel
nut 44 also
facilitates connection of an electrical cable to the negative terminal of
current controller
10.
Figure 9 is a cross-sectional view of the assembled electrolysis cell 11.
Tubes 25,
26, 27, and 28 fit inside tubes 35, 36, 37, and 38, respectively. Tubes 25,
26, 27, and 28
alternate with rod 24 and tubes 35, 36, 37, and 38 to form eight circular
chambers 51, 52,
CA 02742033 2011-04-21
WO 2010/048533 PCT/US2009/061894
9
53, 54, 55, 56, 57, and 58 containing an anode on one side, a cathode on the
other, and
electrolytic fluid within. The alternating electrode design maximizes the
surface area of
the electrodes and therefore the contact of the electrodes with the
electrolytic fluid.
Fabricating electrolysis cell 11 with an overall length of 12" results in a
total electrode
surface area of over 600 square inches, all of which is in continuous contact
with the
electrolytic fluid.
Figure 10 is a longitudinal sectional view of the assembled and operating
electrolysis cell. As the electrolytic fluid 59 is forced by the pump through
bottom fitting
42 into electrolysis chambers 51, 52, 53, 54, 55, 56, 57 and 58, it sweeps off
bubbles 60 of
hydrogen and oxygen that are forming on the electrodes, causing them to be
removed from
electrolysis cell 11 much more quickly than would occur through the action of
gravity
alone. The combined electrolytic fluid and bubbles of gas 61 continue to push
up through
the electrolysis cell and out through top fitting 41.
It is to be understood that the embodiments of the invention herein described
are
merely illustrative of the application of the principles of the invention.
Reference herein to
details of the illustrated embodiments is not intended to limit the scope of
the claims,
which themselves recite those features regarded as essential to the invention.