Note: Descriptions are shown in the official language in which they were submitted.
CA 02742354 2011-04-29
1
DESCRIPTION
GALVANIZED STEEL SHEET AND METHOD FOR MANUFACTURING THE SAME
Technical Field
The present invention relates to a method for stably
manufacturing a galvanized steel sheet having a low sliding
resistance during press forming and excellent press formability
and a galvanized steel sheet having excellent press
formability.
Background Art
The galvanized steel sheet has been widely utilized in
wide ranging fields focusing on the application to automobile
bodies. A galvanized steel sheet in such application is press
formed for use. However, the galvanized steel sheet has a
disadvantage in that the press formability is inferior to that
of a cold-rolled steel sheet. This is because the sliding
resistance of the galvanized steel sheet in a press die is higher
than that of the cold-rolled steel sheet. More specifically,
the galvanized steel sheet becomes difficult to flow into a
press die in a portion where the sliding resistance between a
die and a bead, which easily causes fracture of the steel sheet.
Here, particularly a galvannealed steel sheet that has
been subjected to alloying treatment after hot dip galvanizing
treatment among galvanized steel sheets has more excellent
weldability and coatability than those of a hot-dip zinc-plated
steel sheet that has not been subjected to alloying treatment,
CA 02742354 2011-04-29
2
and thus has been more preferably used as automobile bodies.
The galvannealed steel sheet is one in which an Fe-Zn alloy
phase is formed by galvanizing a steel sheet, and heating the
same so that Fe in the steel sheet and Zn in a plating layer
are dispersed to cause an alloying reaction. The Fe-Zn alloy
phase is a coating film generally containing a F phase, a 131
phase, and a 4 phase and has a tendency that the hardness and
the melting point decrease with a reduction in the Fe
concentration, i.e., in the order of F phase -* 81 phase -*
phase. Therefore, a coating film having a high Fe concentration
in which the hardness is high, the melting point is high, and
adhesion is difficult occur is effective from the viewpoint of
slidability. A galvannealed steel sheet in which the press
formability is emphasized is manufactured in such a manner that
the average Fe concentration in the coating film is slightly
high.
However, the coating film having a high Fe concentration
has problems in that the F phase that is hard and brittle is
easily formed on the plated-steel sheet interface and a
phenomenon of separation from the interface during processing,
i.e., a so-called powdering, is likely to occur.
As methods for solving the problems, Patent Document 1
and Patent Document 2 disclose a technique of increasing the
weldability and the processability by subjecting the surface
of a galvanized steel sheet to electrolysis treatment,
immersion treatment, coating oxidation treatment, or =
heat-treatment to form an oxide film mainly containing ZnO.
CA 02742354 2011-04-29
3
However, when the techniques of Patent Document 1 and
Patent Document 2 are applied to a galvannealed steel sheet,
the surface reactivity becomes poor due to the presence of an
Al oxide and an effect of improving the press formability cannot
be stably obtained because the surface irregularities are large.
More specifically, since the surface reactivity is low, it is
difficult to form a given film on the surface even when the
electrolysis treatment, immersion treatment, coating
oxidation treatment, heat-treatment, or the like is performed
and the film thickness is small in a portion where the reactivity
is low, i.e., a portion in which the number of Al oxides is large.
Since the surface irregularities are large, the surface convex
portions directly contact a press die during press forming. The
sliding resistance in contact portions of thin portions of the
convex portions and the die becomes large, and thus an effect
of improving the press formability is not sufficiently
obtained.
Patent Document 3 discloses a technique of forming an
oxide layer on a plated surface layer by hot dip galvanizing
a steel sheet, alloying the same by heat treatment, subjecting
the resultant steel sheet to temper rolling, bringing the same
into contact with an acidic solution having pH buffer action,
holding the same for 1 to 30 seconds, and then washing with water.
Similarly, as a method for uniformly forming an oxide
layer on a surface flat portion of a hot dip galvanized steel
sheet that has not been subjected to alloying treatment, Patent
Document 4 discloses a method including bringing a hot dip
CA 02742354 2011-04-29
4
galvanized steel sheet after temper rolling into contact with
an acidic solution having pH buffer action, holding the same
for a given period of time in a state where a liquid film of
the acidic solution is formed on the surface of the steel sheet,
and then washing with water and drying the same.
Patent Document 1: Japanese Unexamined Patent
Application Publication No. 53-60332
Patent Document 2: Japanese Unexamined Patent
Application Publication No. 2-190483
Patent Document 3: Japanese Unexamined Patent
Application Publication No. 2003-306781
Patent Document 4: Japanese Unexamined Patent
Application Publication No. 2004-3004
When the techniques disclosed in Patent Document 3 and
Patent Document 4 are applied, favorable press formability can
be obtained under former manufacturing conditions. However,
in recent years, the development of a manufacturing method for
generating a thicker oxide film in a shorter period of time has
been demanded in order to increase the productivity. When
performed under such conditions, a sufficient oxide film is not
formed and favorable press formability is not obtained in some
cases in the techniques disclosed in Patent Document 3 and
Patent Document 4.
In view of such circumstance, it is an object of the
present invention to provide a method capable of stably
manufacturing a galvanized steel sheet having excellent press
formability even in a short time and a galvanized steel sheet
CA 02742354 2011-04-29
having excellent press formability.
Disclosure of Invention
The present inventors have repeatedly conducted
extensive research in order to solve the problems. As a result,
the following findings have been obtained.
The acidic solution for use in the techniques of Patent
Document 3 and Patent Document 4 has pH buffer action in order
to promote the dissolution of zinc. Therefore, it is considered
that an increase in the pH is delayed, and thus the formation
of an oxide layer is delayed. In order to compensate zinc for
forming an oxide layer with zinc eluting from a plated coating
film, an elution time of zinc is included in a generation time
of the oxide film. As a result, it is considered that generating
a thick oxide film in a short time becomes difficult.
Then, the present inventors have devised a technique of
generating an oxide film in a shorter time by omitting an elution
time of zinc by blending zinc ion in an aqueous solution for
generating an oxide film beforehand. However, the formation
of an oxide film has not been promoted merely by blending zinc
ion in an aqueous solution beforehand. Particularly in the case
where the pH is 2 described in Examples of Patent Document 3
and Patent Document 4, even when zinc is blended in a treatment
liquid, the formation of an oxide film has not been promoted.
This is considered to be because, according to the
techniques of Patent Document 3 and Patent Document 4, an
environment is established in which a zinc oxide is likely to
CA 02742354 2011-04-29
6
generate because the pH near the surface increases due to the
reduction of hydrogen ion occurring simultaneously with the
elution of zinc, but the pH near the surface does not increase
merely by blending zinc ion in an aqueous solution, and thus
an environment is not established in which a zinc oxide is likely
to generate.
Then, the present inventors have devised a technique of
setting the pH of an aqueous solution to 4 to 6, the pH at which
a zinc oxide is likely to generate. Then, the present inventors
have found that, by setting the pH of a treatment liquid to 4
to 6, zinc is generated as a hydroxide due to a slight increase
in the surface pH caused by slight elution of zinc of a plated
coating film.
The present invention has been accomplished based on the
findings, and the gist is as follows.
[1] A method for manufacturing a galvanized steel sheet,
includes galvanizing a steel sheet, bringing the steel sheet
into contact with an aqueous solution, holding the steel sheet
for 1 to 60 seconds after the termination of the contact
treatment, and then washing with water and drying the steel
sheet to thereby form an oxide layer on the surface of the steel
sheet, in which the aqueous solution for use in the contact
treatment of the steel sheet contains zinc ion in the range of
to 100 g/1 as the zinc ion concentration, has a pH of 4 to
6, and has a liquid temperature of 20 to 70 C.
[2] The method for manufacturing a galvanized steel sheet
according to [1] above, in which the aqueous solution contains
CA 02742354 2013-05-28
7
zinc sulfate.
[3] The method for manufacturing a galvanized steel sheet
according to [1] or [2] above, in which a liquid film to be
formed on the surface of the steel sheet after the steel sheet
contacts the aqueous solution is 5 to 30 g/m2.
[4] The method for manufacturing a galvanized steel sheet
according to any one of [1] to [3] above, in which the oxide
layer contains a metal component that is at least 50% zinc and
is formed on the surface of the steel sheet in such a manner as
to have an average thickness of from 10 nm to 100 nm.
In the invention, the galvanized steel sheet is a plated
steel sheet having a coating film containing zinc as the main
component formed on the surface and includes a hot dip
galvanized steel sheet (abbreviated as a GI steel sheet), a
galvannealed Steel Sheet (abbreviated as a GA steel sheet), an
electrogalvanized steel sheet (abbreviated as an EG steel
sheet), a vapor deposition galvanized steel sheet, an alloy
galvanized steel sheet containing an alloy element of Fe, Al,
Ni, MgCo, or the like, etc.
Brief Description of Drawings
Fig. 1 is a view of a principal part of an oxide layer
formation treatment facility used in Examples.
Fig. 2 is a schematic front view showing a friction
coefficient measuring device.
Fig. 3 is a schematic perspective view showing the shape
CA 02742354 2011-04-29
8
and the size of a bead in Fig. 2.
Fig. 4 is a schematic perspective view showing the shape
and the size of the bead in Fig. 2.
Fig. 5 is a view showing influence of the zinc ion
concentration on the oxide film thickness.
Best Modes for Carrying Out the Invention
In the invention, when forming an oxide layer on the
surface of a steel sheet by galvanizing a steel sheet, bringing
the steel sheet into contact with an aqueous solution, holding
the steel sheet for 1 to 60 seconds after the termination of
the contact treatment, and then washing with water and drying
the steel sheet, the aqueous solution contains zinc ion in the
range of 5 to 100 g/1 as the zinc ion concentration, the pH is
4 to 6, and the liquid temperature is 20 to 70 C. To prepare
an aqueous solution containing zinc ion in a given concentration
and having a specified pH and a specified liquid temperature
as described above as the aqueous solution for use in the contact
treatment of the steel sheet is an important requirement and
a feature in the invention. Thus, an oxide layer sufficient
for securing favorable press formability can be formed in a
short time.
The "after the termination of the contact treatment"
refers to "after the termination of an immersion process" in
the case of immersion treatment, "after the termination of a
spraying process" in the case of spraying treatment, and "after
the termination of a coating process" in the case of roll
CA 02742354 2011-04-29
9
coating.
The use of an aqueous solution containing zinc ion as the
aqueous solution for use in the contact treatment of the steel
sheet allows omission of an elution time of zinc. In this case,
the zinc ion is in the range of 5 to 100 g/1 as the zinc ion
concentration. When the zinc ion concentration is lower than
g/l, sufficient zinc is not supplied, resulting in a failure
of the formation of an oxide layer. In contrast, when the zinc
ion concentration exceeds 100 g/l, the concentration of
sulfuric acid contained in the oxide layer to be formed becomes
high, resulting in concern about contamination of a treatment
liquid when the oxide dissolves in chemical conversion
treatment to be carried out thereafter.
In order to form a stable zinc compound as an oxide layer,
it is preferable to add zinc ion as a sulfate. It is considered
that when zinc ion is added as a sulfate, sulfuric acid ion is
taken into an oxide layer to be formed to thereby produce an
effect of stabilizing the oxide layer.
As described above, the formation of an oxide film is not
promoted merely by blending zinc ion in a treatment liquid
beforehand. Then, in the invention, the pH needs to be set to
4 to 6, at which a zinc oxide easily generates. When the pH
of a treatment liquid is set to 4 to 6, zinc generates as a
hydroxide due to a slight increase in the surface pH caused by
slight elution of zinc of a plated coating film. As a result
thereof, the zinc elution time can be omitted and the generation
of a zinc oxide can be achieved. When the pH exceeds 6, zinc
CA 02742354 2011-04-29
ion precipitates in the aqueous solution (formation of a
hydroxide) and is not formed as an oxide on the surface of the
steel sheet. When the pH is lower than 4, the formation of the
oxide layer is hindered due to the delay of an increase in the
pH as described above.
The temperature of the aqueous solution is 20 to 70 C.
Since the oxide layer formation reaction occurs when holding
the steel sheet in a given period of time after contacting the
aqueous solution, it is effective to control the sheet
temperature during holding in the range of 20 to 70 C. When
the sheet temperature is lower than 20 C, a long period of time
is required for the oxide layer generation reaction, resulting
in a reduction in the productivity. In contrast, when the sheet
temperature exceeds 70 C, a reaction relatively quickly
proceeds but treatment unevenness is likely to occur on the
surface of the steel sheet.
The aqueous solution used in Patent Document 3 and Patent
Document 4 has a feature in that the aqueous solution is acidic
and has pH buffer action. In the invention, however, since an
aqueous solution containing zinc ion is used, a sufficient oxide
layer can be formed even when the dissolution of zinc is not
caused by increasing the pH of the aqueous solution. A prompt
increase in the pH is considered to be advantageous for the
formation of an oxide. Therefore, the pH buffer action is not
necessarily indispensable.
In the invention, an oxide layer excellent in slidability
can be stably formed when zinc is contained in the aqueous
CA 02742354 2011-04-29
11
solution contacting the surface of the steel sheet. Therefore,
even when other metal ions, inorganic compounds, and the like
are contained as impurities or intentionally contained in the
aqueous solution, the effects of the invention are not impaired.
Even when N, P, B, Cl, Na, Mn, Ca, Mg, Ba, Sr, Si, and the like
are taken into the oxide layer, it can be applied insofar as
the effects of the invention are not impaired.
Preferably, after bringing a galvanized steel sheet into
contact with the aqueous solution containing the above, the
aqueous solution is present on the surface of the steel sheet
in the form of a thin liquid film. This is because when the
amount of the aqueous solution present on the surface of the
steel sheet is large, the pH of the aqueous solution is hard
to increase even when the dissolution of zinc occurs, and a long
period of time is required for the formation of the oxide layer.
From this viewpoint, it is preferable and effective to adjust
the amount of an aqueous solution film to be formed on the surface
of the steel sheet to 30 g/m2 or lower. In order to prevent
the liquid film from drying, the amount of the liquid film of
g/m2 or more is suitable. As described above, the liquid film
to be formed on the surface of the steel sheet after contacting
the aqueous solution is preferably 5 to 30 g/m2. The adjustment
of the amount of the aqueous solution film can be performed by
a squeeze roll, air wiping, or the like.
The time (retention time before washing with water)
before washing with water after immersion in the aqueous
solution is 1 to 60 seconds. When the time before washing with
CA 02742354 2011-04-29
12
water is lower than 1 second, the aqueous solution is washed
away before a sufficient oxide layer is formed, and thus an
effect of improving the slidability is not obtained. In
contrast, when the time before washing with water exceeds 60
seconds, the productivity decreases. Since the object of the
invention is to stably manufacture a galvanized steel sheet even
in a short time, the retention time is 60 seconds or lower for
sufficiently demonstrating the effects of the invention.
As described above, on the surface of the plated steel
sheet of the invention, an oxide layer mainly containing zinc
as a metal component and having an average thickness of 10 nm
or more is obtained.
The "mainly containing zinc" refers to containing zinc
in a proportion of 50% by mass or more as a metal component.
The oxide layer in the invention refers to a layer
containing an oxide and/or a hydroxide mainly containing zinc
as a metal component. The average thickness of the oxide layer
is required to be 10 nm or more. When the average thickness
of the oxide layer is small, e.g., lower than 10 nm, an effect
of reducing sliding resistance becomes insufficient. In
contrast, when the average thickness of the oxide layer
containing zinc as an essential ingredient exceeds 100 nm, there
is a tendency that the coating film breaks during press
processing, the sliding resistance increases, and the
weldability decreases. Thus, such a thickness is not
preferable.
Methods for bringing the galvanized steel sheet into
CA 02742354 2011-04-29
13
contact with the aqueous solution containing zinc are not
particularly limited. For example, a method for immersing the
plated steel sheet in the aqueous solution, a method for
spraying the aqueous solution to the plated steel sheet, a
method for applying the aqueous solution to the plated steel
sheet with a coating roll, and the like are mentioned. It is
preferable for the aqueous solution to be finally present on
the surface of the steel sheet in the form of a thin liquid film.
For manufacturing the galvannealed steel sheet according
to the invention, Al needs to be added into a plating bath but
additional element ingredients other than Al are not
particularly limited. More specifically, even when Pb, Sb, Si,
Sn, Mg, Mn, Ni, Ti, Li, Cu, and the like other than Al are
contained or added, the plating bath can be applied insofar as
the effects of the invention are not impaired.
EXAMPLES
Next, the invention will be described in more detail with
reference to Examples.
A GI steel sheet was produced by performing hot dip
galvanizing in which the deposit amount per surface was 45 g/m2
and the Al concentration was 0.20% by mass on a cold-rolled steel
sheet having a sheet thickness of 0.8 mm, and then performing
temper rolling. A GA steel sheet was obtained by forming a
plated coating film in which the deposit amount per surface was
45 g/m2, the Fe concentration was 10% by mass, and the Al
concentration was 0.20% by mass on a cold-rolled steel sheet
CA 02742354 2011-04-29
14
having a sheet thickness of 0.8 mm by a standard galvannealing
method, and further performing temper rolling. An EG steel
sheet was produced by having a plated coating film having a
deposit amount per surface of 30 g/m2 on a cold-rolled steel
sheet having a sheet thickness of 0.8 mm by a standard
electrogalvanizing method.
Subsequently, an oxide layer was formed using a treatment
facility having a structure shown in Fig. 1. First, steel
sheets S, such as the GI steel sheet, the GA steel sheet, and
the EG steel sheet obtained above were immersed in aqueous
solutions in which the treatment liquid composition, the
temperature, and the pH were different from each other as shown
in Tables 1-1 and 1-2 in a solution bath 2. Subsequently, the
amount of liquid films on the surface of the steel sheets was
adjusted with a squeeze roll 3. The adjustment of the amount
of liquid films was performed by changing the pressure of the
squeeze roll. Subsequently, the steel sheets were made to pass
through a washing bath 5 and a washing bath 6 without being
treated, hot water of 50 C was sprayed to the steel sheets in
a washing bath 7 for washing, and the steel sheets were dried
with a drier 8, so that an oxide layer is formed on the plated
surface. A washing bath 1 can be provided before the solution
bath 2.
As the aqueous solution for use in the immersion treatment
in the solution bath 2, an aqueous solution was used to which
a given amount of zinc sulfate heptahydrate was added in order
to add zinc ion. For comparison, a solution containing 20 g/L
CA 02742354 2011-04-29
of sodium acetate whose pH was adjusted with sulfuric acid was
also used in some cases.
The retention time before washing with water was the time
before washing in the washing bath 7 was started after adjusting
the amount of liquid films with the squeeze roll 3 and was
adjusted by changing the line speed. Some of the steel sheets
were produced by washing immediately after squeezing using a
shower washing device 4 at the exit side of the squeeze roll
3.
Next, the steel sheets produced as described above were
judged whether or not they have an appearance sufficient as an
exterior panel for automobiles, and also the measurement of a
friction coefficient as a method for simply evaluating the press
formability and a spherical head bulging test was carried out
in order to simulate the actual formability in detail were
carried out. The measurement methods are as follows.
(1) Press formability evaluation test (Friction coefficient
measurement test)
In order to evaluate the press formability, the friction
coefficient of each test piece was measured as follows.
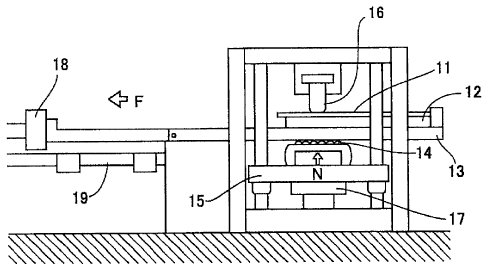
Fig. 2 is a schematic front view showing a friction
coefficient measuring device. As shown in Fig. 2, a friction
coefficient measuring sample 11 extracted from the test piece
is fixed to a sample stand 12. The sample stand 12 is fixed
to the upper surface of a horizontally movable slide table 13.
On the lower surface of the slide table 13, a vertically movable
slide table support stand 15 having a roller 14 contacting the
CA 02742354 2013-05-28
16
lower surface of the slide table 13. By pressing up the same,
a first load cell 17 for measuring a pressing load N to the
friction coefficient measuring sample 11 by a bead 16 is
attached to the slide table support stand 15. In order to
measure a sliding resistance F for horizontally moving the slide
table 13 along a rail 19 in the state where the pressing force
was made to act, a second load cell 18 is attached to one end
of the slide table 13. As a lubricant, a cleaning oil for
pressing, Pre-tonTM R352L manufactured by Sugimura Chemical
Industrial Co., Ltd., was applied onto the surface of the
friction coefficient measuring sample 11, and thus a test was
carried out.
Figs. 3 and 4 are schematic perspective view showing the
shape and the size of the used bead. The bead 16 slides while
the lower surface of the bead 16 being pressed against the
surface of the sample 11. In the bead 16 shown in FIG. 3, the
width is 10 mm, the length in the sliding direction of the sample
is 12 mm, and each end in the sliding direction of the lower
surface of the bead 16 is curved with a curvature of 4.5 mmR.
The lower surface of the bead 16 against which the sample is
pressed has a plane with a width of 10 mm and a length in the
sliding direction of 3mm. In the bead 16 shown in FIG. 4, the
width is 10 mm, the length in the sliding direction of the sample
is 69 mm, and each end in the sliding direction of the lower
surface of the bead 16 is curved with a curvature of 4.5 mmR.
The lower surface of the bead 16 against which the sample is
pressed has a plane with a width of 10 mm and a length in the
CA 02742354 2011-04-29
17
sliding direction of 60 mm.
The friction coefficient measurement test was carried out
under two conditions shown below.
[Condition 1]
The bead shown in Fig. 3 was used, the pressing load N
was 400 kgf, and the sample drawing rate (horizontal movement
rate of the slide table 13) was 100 cm/min.
[Condition 2]
The bead shown in Fig. 4 was used, the pressing load N
was 400 kgf, and the sample drawing rate (horizontal movement
rate of the slide table 13) was 20 cm/min.
The friction coefficient between the test piece and the
bead was calculated based on Equation: = F/N.
(2) Spherical head bulging test
A test piece having a size of 200 x 200 mm was subjected
to bulge forming using a 150 mm(1) punch by a liquid pressure bulge
testing machine. Then, the maximum forming height when the test
piece was broken was measured. During the test, a wrinkle
pressing force of 100 Ton was applied in order to prevent inflow
of materials, and a lubricating oil was applied only to the
surface which the punch contacted. The used lubricating oil
is the same as that of the friction coefficient measurement test
described above.
(3) Measurement of thickness of oxide layer (oxide film
thickness)
An Si wafer on which a thermal oxidation Si02 film having
a film thickness of 96 nm was formed was used as a reference
CA 02742354 2011-04-29
18
substance, and the average thickness of the oxide layer in terms
of Si02 was determined by measuring the 0=Ka=X rays by an x-ray
fluorescence spectrometer. The analysis area is 30 mm.
The test results obtained in the above are shown in Tables
1-1 and 1-2.
CA 02742354 2011-04-29
19
Table 1-1
Time
Liquid Oxide Maxium
Used solution Solution film before film Friction
coefficient forming Steel sheet
Test water
pH amount thickness height
piece washing
No. pH buffer Zn
Temperatur (g/m2) (second) (nm) Conditio Condition (mm) appearance Remarks
concentratio e n 1 2 _
1 GA - - - - 8 0.180 0.223 35.0 0
Comparative example 1
2 Sodium - 2.0 35 C 10 10 15 0.149 0.190
36.5 0 Comparative example 2
3 acetate sulfuric acid 10 30 30 0.128 0.165 38.1
0 Comparative example 3
4 (20 g/L) added 10 60 42 0.120 0.163 39.3
0 Comparative example 4
5.0 35 C 10 10 8 0.183 0.219 35.6 0
Comparative example 5
6 sulfuric acid 10 30 8 0.179 0.221 35.9
0 Comparative example 6
7 added 10 60 8 0.180 0.217 35.9
0 Comparative example 7
8 - 2.5g/I 5.6 35 C 10 10 12 0.148 0.200 36.5
0 Comparative example 8
9 10 30 25 0.140 0.174 37.9
0 Comparative example 9
10 60 32 0.132 0.163 38.9 0 Comparative example 10
11 5g/I 5.5 35 C 10 10 18 0.138 0.187 37.6
0 Example of present invention 1
12 10 30 32 0.123 0.166 39.1
0 Example of present invention 2
13 10 60 43 0.122 0.163 39.5
0 Example of present invention 3
14 10g/I 5.2 35 C 10 10 25 0.134 0.174 39.0
0 Example of present invention 4
10 30 35 0.128 0.164 39.4 0 Example of present
invention 5
16 10 60 45 0.124 0.163 40.1
0 Example of present invention 6
17 50g/I 5.0 35 C 10 0 12 0.160 0.205 37.2
0 Comparative example 11
18 10 1 15 0.145 0.200 38.1
0 Example of present invention 7
19 10 5 24 0.137 0.173 39.0
0 Example of present invention 8
10 10 32 0.127 0.165 38.9 0 Example of present
invention 9
21 10 30 40 0.127 0.159 39.9
0 Example of present invention 10
22 10 60 50 0.125 0.160 40.8
0 Example of present invention 11
23 15 C 10 10 14 0.145 0.198 37.1
0 Comparative example 12
24 10 30 27 0.134 0.169 38.1
0 Comparative example 13
10 60 37 0.125 0.166 39.2 0 Comparative example 14
26 25 C 10 10 19 0.138 0.189 38.5
0 Example of present invention 12
27 10 30 32 0.129 0.166 40.3
0 Example of present invention 13
28 10 60 43 0.127 0.162 40.0
0 Example of present invention 14
29 65 C 10 10 35 0.128 0.164 40.5
0 Example of present invention 15
10 30 44 0.125 0.162 41.6 0 Example of present
invention 16
31 10 60 51 0.123 0.160 41.8
0 Example of present invention 17
32 75 C 10 10 35 0.124 0.161 40.6
X Comparative example 15
33 10 30 45 0.120 0.163 40.5
x Comparative example 16
34 10 60 52 0.121 0.158 41.0
X Comparative example 17
35 C 30 10 20 0.143 0.185 38.5 0 Example of present
invention 18
36 30 30 34 0.128 0.163 39.1
0 Example of present invention 19
37 30 60 49 0.127 0.161 40.4
0 Example of present invention 20
38 35 C 40 10 13 0.155 0.202 37.4
0 Example of present invention 21
39 40 30 28 0.132 0.167 39.0
0 Example of present invention 22
40 60 42 0.127 0.163 39.7 0 Example of present
invention 23
41 3.5 35 C 10 10 14 0.149 0.201 36.0
0 Comparative example 18
42 sulfuric acid 10 30 27 0.130 0.165 39.2
0 Comparative example 19
43 added 10 60 40 0.124 0.162 41.0
0 Comparative example 20
44 100g/I 4.9 35 C 10 10 34 0.125 0.166 40.5
0 Example of present invention 24
10 30 41 0.123 0.164 40.9 0 Example of present
invention 25
46 10 60 50 0.121 0.163 40.8
0 Example of present invention 26
CA 02742354 2011-04-29
Table 1-2
Time
Liquid Maxium
before Oxide film
Used solution Solution film Friction coefficient
forming Steel sheet
Test water thickness
pH amount height
piece washing
No pH buffer Zn Temperature (02) (second)
(nm) Conditio Conditio (mm) appearance Remarks
concentration n 1 n 2
47 GI - - - 7 0.175 0.215 35.7 0
Comparative example 21
48 Sodium - 2.0 35 C 10 10 13 0.147
0.187 36.7 0 Comparative example 22
49 acetate sulfuric acid 10 30 27 0.125 0.164
38.6 0 Comparative example 23
50 (20 g/L) added 10 60 39 0.119 , 0.160 39.6 0
Comparative example 24
51 - 2.5g/1 5.6 35 C 10 10 11 0.161
0.198 36.3 0 Comparative example 25
52 10 30 24 0.13 0.168 38 0
Comparative example 26
53 10 60 34 0.121 0.163 39.1 0
Comparative example 27
54 10g/1 5.2 35 C 10 10 19
0.138 0.177 37.1 0 Example of present invention 27
55 10 30 32 0.124 0.161 38.5
0 Example of present invention 28
56 10 60 42 0.121 0.161 39.5
0 Example of present invention 29
57 50g/1 5.0 35 C 10 10 26
0.127 0.166 38.4 0 Example of present invention 30
58 10 30 36 0.122 0.163 39
0 Example of present invention 31
59 10 60 45 , 0.12 0.159 39.7
0 Example of present invention 32
60 EG - - 9 0.146 0.289 33.7 0
Comparative example 28
61 Sodium - 2.0 35 C 10 10 11 0.136
0.241 34.0 0 Comparative example 29
62 acetate sulfuric acid 10 30 23 0.135 0.195
36.6 0 Comparative example 30
63 (20 g/L) added 10 60 36 0.128 0.173 37.9 0
Comparative example 31
64 - 2.5g/I 5.6 35 C 10 10 12 0.140
0.199 37.0 0 Comparative example 32
65 10 30 23 0.131 0.181 37.2 0
Comparative example 33
66 10 60 31 0.131 0.159 37.5 0
Comparative example 34
67 10g/1 5.2 35 C 10 10 20
0.139 0.221 35.4 0 Example of present invention 33
68 10 30 35 0.132 0.195 36.9
0 Example of present invention 34
69 10 60 45 0.129 0.160 37.6
0 Example of present invention 35
70 50g/1 5.0 35 C 10 10 22
0.136 0.192 37.6 0 Example of present invention 36
71 10 30 37 0.131 0.153 39.3
0 Example of present invention 37
72 10 60 46 0.125 0.156 38.9
0 Example of present invention 38
CA 02742354 2011-04-29
21
The following items were clarified from the test results shown
in Tables 1-1 and 1-2.
(1) Since Nos. 1, 47, and 60 were not treated with a
solution, an oxide film sufficient for increasing the
slidability was not formed on the flat portion. Thus, the
friction coefficient is high.
(2) Nos. 2 to 4, Nos. 48 to 50, and Nos. 61 to 63 are
comparative examples using an acidic solution having pH buffer
action. In the case of the treatment of 30 seconds or more,
the friction coefficient is low and the maximum forming height
is large but in the case of the treatment of 10 seconds, a
sufficient reduction in the friction coefficient and an
increase in the maximum forming height are not satisfied.
(3) Nos. 5 to 7 are comparative examples using an acidic
solution having pH buffer action. High friction coefficients
are exhibited.
(4) Nos. 8 to 10, Nos. 51 to 53, and Nos. 64 to 66 are
comparative examples in which zinc ion is contained but the
amount is smaller than the range of the invention. In the case
of the treatment of 30 seconds or more, the friction coefficient
is low and the maximum forming height is large but in the case
of the treatment of 10 seconds, a sufficient reduction in the
friction coefficient and an increase in the maximum forming
height are not satisfied.
(5) Nos. 11 to 13, Nos. 54 to 56, and Nos. 67 to 69 are
examples of the invention that were treated with a solution
containing zinc ion, in which the friction coefficient
CA 02742354 2011-04-29
22
decreases and also the maximum forming height increases. Nos.
14 to 16 and Nos. 44 to 46 are examples of the invention in which
the treatment conditions were the same as those of Nos. 11 to
13 and the zinc ion concentration in the liquid was increased.
The friction coefficient becomes stable at lower levels and also
the maximum forming height further increases. Similarly, Nos.
57 to 59 and Nos. 70 to 72 are examples of the invention in which
the treatment conditions were the same as those of Nos. 54 to
56 and the zinc ion concentration in the liquid was increased.
The friction coefficient becomes stable at lower levels and also
the maximum forming height further increases.
(6) Nos. 17 to 22 are examples in which a solution film
was formed on the surface of the steel sheets and the time until
washing with water was carried out was changed. In No. 17 which
was washed with water without being held, the friction
coefficient merely slightly decreases. In contrast, in Nos.
18 to 22 in which the retention time was 1 second or more, the
friction coefficient decreases and also the bulging properties
stably increase.
(7) Nos. 23 to 40 are examples in which the treatment
liquid temperature was changed. In Nos. 23 to 25 having a low
treatment liquid temperature, effects of improving the friction
coefficient and the maximum forming height are not sufficient
as compared with the other examples. In contrast, Nos. 32 to
34 are examples having a high treatment liquid temperature and
effects of improving the friction coefficient or the maximum
forming height were sufficient but treatment unevenness was
CA 02742354 2011-04-29
23
observed in many portions and thus the appearance was not
favorable as an exterior panel for automobiles.
(8) Nos. 35 to 40 are examples of the invention in which
the liquid film formation amount was changed relative to Nos.
20 to 22. A comparison between the samples in which the
retention time until washing with water was carried out is the
same shows that when the liquid film amount was large, a
sufficient reduction in the friction coefficient and an
increase in the maximum forming height are achieved but the
friction coefficient was slightly high and also the maximum
forming height was small as compared with the samples in which
the liquid film amount was small.
(9) Nos. 41 to 43 are comparative examples using a
treatment liquid in which pH is lower than the range of the
invention. The effect of reducing the friction coefficient is
not observed and also an increase in the maximum forming height
is not observed as compared with Nos. 20 to 22.
Fig. 5 is a view showing the influence of the zinc ion
concentration on the oxide film thickness using Nos. 8 to 22
and Nos. 44 to 46 of Tables 1-1 and 1-2. Fig. 5 shows that the
oxide film has a sufficient thickness even when the retention
time is short (e.g., 10 seconds) in the case where the zinc
concentration is 5 g/1 or more, and the problem of the invention
that the oxide film thickness becomes small when the retention
time is short is solved.
Industrial Applicability
CA 02742354 2011-04-29
24
According to the present invention, a galvanized steel
sheet having a low sliding resistance during press forming and
excellent press formability can be stably manufactured at a
saved space even under short-time manufacturing conditions.
For example, even when a high strength galvanized steel sheet
which has a high forming load and is likely to cause die galling,
the sliding resistance during press forming is low and excellent
press formability can be achieved. Since the press formability
is excellent, the invention can be applied to wide ranging
fields focusing on the application to automobile bodies.