Note: Descriptions are shown in the official language in which they were submitted.
CA 02743534 2011-06-13
FUSED AZABICYCLIC COMPOUNDS THAT INHIBIT VANILLOID RECEPTOR
SUBTYPE 1 (VRi) RECEPTOR
TECHNICAL BACKGROUND
The present invention relates to compounds of formula (1), which are useful
for
treating disorders caused by or exacerbated by vanilloid receptor activity,
pharmaceutical
compositions containing compounds of formula (I) and are useful in treating
pain, bladder
overactivity, and urinary incontinence.
BACKGROUND OF INVENTION
Nociceptors are primary sensory afferent (C and A6 fibers) neurons that are
activated
by a wide variety of noxious stimuli including chemical, mechanical, thermal,
and proton (pH
< 6) modalities. The lipophillic vanilloid, capsaicin., activates primary
sensory fibers via a
specific cell surface capsaicin receptor, cloned as VR1. The intradehrial
administration of
capsaicin is characterized by an initial burning or hot sensation followed by
a prolonged
period of analgesia. The analgesic component of VR.1 receptor activation is
thought to be
mediated by a capsaicin-induced desensitization of the primary sensory
afferent terminal.
Thus, the long lasting anti-nociceptive effects of capsaicin has prompted the
clinical use of
capsaicin analogs as analgesic agents. Further, capsazepinc, a capsaicin
receptor antagonist
can reduce inflammation-induced hyperalgesia in animal models. VR1 receptors
are also
localized on sensory afferents which innervate the bladder. Capsaicin or
resiniferatoxin has
been shown to ameliorate incontinence symptoms upon injection into the
bladder.
The VRl receptor has been called a "polymodal detector" of noxious stimuli
since it
can be activated in several ways. The receptor channel is activated by
capsaicin and other
vatulloids and thus is classified as a ligand-gated ion channel. VR1 receptor
activation by
capsaicin can be blocked by the competitive VRl receptor antagonist,
capsazepine. The
channel can also be activated by protons. Under mildly acidic conditions (pH 6-
7), the
affinity of capsaicin for the receptor is increased, whereas at pH <6, direct
activation of the
channel occurs. In addition, when membrane temperature reaches 43 C, the
channel is
opened. Thus heat can directly gate the channel in the absence of ligand. The
capsaicin
1
CA 02743534 2011-06-13
analog, capsazepine, which is a competitive antagonist of capsaicin, blocks
activation of the
channel in response to capsaicin, acid, or heat.
The channel is a nonspecific cation conductor. Both extracellular sodium and
calcium
enter through the channel pore, resulting in cell membrane depolarization.
This
depolarization increases neuronal excitability, leading to action potential
firing and
transmission of a noxious nerve impulse to the spinal cord. In addition,
depolarization of the
peripheral terminal can lead to release of inflammatory peptides such as, but
not limited to,
substance P and CGRP, leading to enhanced peripheral sensitization of tissue.
Recently, two groups have reported the generation of a "knock-out" mouse
lacking
the VRl receptor. Electrophysiological studies of sensory neurons (dorsal root
ganglia) from
these animals revealed a marked absence of responses evoked by noxious stimuli
including
capsaicin, heat, and reduced pH. These animals did not display any overt signs
of behavioral
impairment and showed no differences in responses to acute non-noxious thermal
and
mechanical stimulation relative to wild-type mice. The VR1 (-/-) mice also did
not show
reduced sensitivity to nerve injury-induced mechanical or thermal nociception.
However, the
VRl knock-out mice were insensitive to the noxious effects of intradermal
capsaicin,
exposure to intense heat (50-55 C), and failed to develop thermal hyperalgesia
following the
intradermal administration of carrageenan.
The compounds of the present invention are novel VR1 antagonists and have
utility in
treating pain, bladder overactivity, and urinary incontinence.
SUMMARY OF THE PRESENT INVENTION
The present invention discloses fused azabicyclic compounds, a method for
inhibiting
the VRl receptor in mammals using these compounds, a method for controlling
pain in
mammals, and pharmaceutical compositions including those compounds. More
particularly,
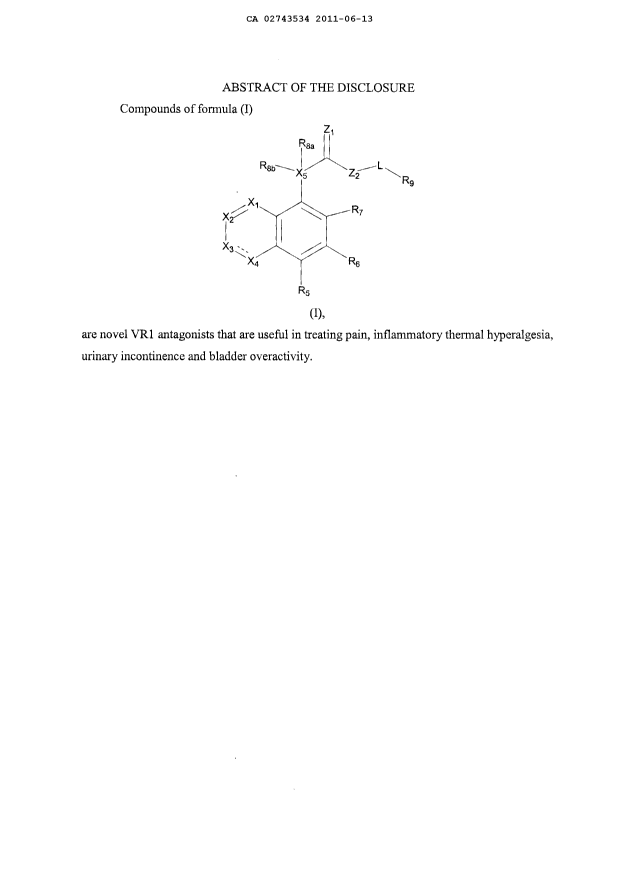
the present invention is directed to compounds of formula (I)
2
CA 02743534 2011-06-13
Z1
IR&a
Rsb-~ x5 Zz t
R9
X2 X, R7
I
X3~,
X4 Y ~ R6
R5
or a pharmaceutically acceptable salt or prodrug thereof, wherein
--- is absent or a single bond;
X1 is selected from the group consisting of N and CRS;
X2 is selected from the group consisting of N and CR_,;
X3 is selected from the group consisting of N, NR3, and CR3;
X4 is a bond or selected from the group consisting of N and CR4;
X5 is selected from the group consisting of N and C;
provided that at least one of X1, X2, X3, and X4 is N;
ZI is selected from the group consisting of 0, NH, and S;
Z2 is a bond or selected from the group consisting of NH and 0;
L is selected from the group consisting of alkenylene, alkylene, alkynylene,
Ry
cycloalkylene, - --~N- , -(CH2)mO(CH2)õ, and N(Ry), wherein the left end of
-(CH2)mO(CH2)n- is attached to Z2 and the right end is attached to R9;
in and n are each independently 0-6;
Ry is selected from the group consisting of hydrogen and alkyl;
R1, R3, R5, R6, and R7 are each independently selected from the group
consisting of
hydrogen, alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl,
alkyl, alkylcarbonyl, alkylcarbonylatkyl, alkylcarbonyloxy, alkylthio,
alkynyl, carboxy,
carboxyalkyl, cyano, cyanoalkyl, cycloalkyl, cycloalkylalkyl, formyl,
formylalkyl,
haloalkoxy, haloalkyl, haloalkylthio, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, nitro, (CF3)2(HO)C-, -NRAS(0)2RB, -S(O)2ORA, -S(O)2RB, -NZAZB,
3
CA 02743534 2011-06-13
(NZAZB)alkyl, (NZAZB)carbonyl, (NZAZB)carbonylalkyl and (NZAZB)sulfonyl,
wherein ZA
and ZB are each independently selected from the group consisting of hydrogen,
alkyl,
alkylcarbonyl, formyl, aryl, and arylalkyl;
R2 and R4 are each independently selected from the group consisting of
hydrogen,
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
allcoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonylalkyl, alkylcarbonyloxy, alkylthio, alkynyl,
carboxy,
carboxyalkyl, cyano, cyanoalkyl, cycloalkyl, cycloalkylalkyl, formyl,
formylalkyl,
haloalkoxy, haloalkyl, haloalkylthio, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoallyl, nitro, (CF3)2(HO)C-, -NRAS(O)2RB, -S(O)2ORA, -S(O)2RB, -NZAZB,
(NZAZB)alkyl, (NZAZB)alkylcarbonyl, (NZAZB)carbonyl, (NZAZB)carbonylalkyl,
(NZAZB)sulfonyl, (NZAZB)C(=NH)-, (NZAZB)C(=NCN)NH-, and (NZAZB)C(=NH)NH-;
RA is selected from the group consisting of hydrogen and alkyl;
RB is selected from the group consisting of alkyl, aryl, and arylalkyl;
Rga is selected from the group consisting of hydrogen and alkyl;
R8b is absent when X5 is N or Rnb is selected from the group consisting of
hydrogen,
alkoxy, alkoxycarbonylalkyl, alkyl, alkylcarbonyloxy, alkylsulfonyloxy,
halogen, and
hydroxy when X5 is C; and
R9 is selected from the group consisting of hydrogen, aryl, cycloalkyl, and
heterocycle.
DETAILED DESCRIPTION OF THE PRESENT INVENTION
In the principle embodiment, compounds of formula (I) are disclosed
Z,
R8a
R8b-_ I5 z2
R9
X~ Rr
X3
\4#R6
R5
m,
or a pharmaceutically acceptable salt or prodrug thereof, wherein
--- is absent or a single bond;
4
CA 02743534 2011-06-13
X, is selected from the group consisting of N and CR,;
X2 is selected from the group consisting of N and CR2;
X3 is selected 1 from the group consisting of N, NR3, and CR3;
X4 is a bond or selected from the group consisting of N and CR4;
X5 is selected from the group consisting of N and C;
provided that at least one of X,, X2, X3, and X4 is N;
Z, is selected from the group consisting of 0, N1=1, and S;
Z2 is a bond or selected from the group consisting of NH and 0;
L is selected from the group consisting of alkenylene, alkylene, allcynylene,
Ry
--N IAN-
cycloalkylene, "--" , -(CH2),,,0(CH2)n-, and N(Ry), wherein the left end of
-(CH2)mO(CH2)õ- is attached to Z2 and the right end is attached to R9i
m and a are each independently 0-6;
Ry is selected from the group consisting of hydrogen and alkyl;
R,, R3, R5, R6, and R7 are each independently selected from the group
consisting of
hydrogen, alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl,
alkyl, alkylcarbonyl, alkylcarbonylalkyl, alkylcarbonyloxy, alkylthio,
alkenyl, carboxy,
carboxyalkyl, cyano, cyanoalkyl, cycloalkyl, cycloalkylalkyl, formyl,
formylalkyl,
haloalkoxy, haloalkyl, haloalkylthio, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, nitro, (CF3)2(HO)C-,
-NRAS(O)2RB, -S(0)20RA, -S(0)2RB, -NZAZB, (NZAZB)alkyl, (NZAZB)carbonyl,
(NZAZB)carbonylalkyl and (NZAZB)sulfonyl, wherein ZA and ZB are each
independently
selected from the group consisting of hydrogen, alkyl, alkylcarbonyl, formyl,
aryl, and
arylalkyl;
R2 and R4 are each independently selected from the group consisting of
hydrogen,
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxyearbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonylalkyl, alkylcarbonyloxy, alkylthio, alkynyl,
carboxy,
carboxyalkyl, cyano, cyanoalkyl, cycloalkyl, cycloalkylallcyl, formyl,
formylalkyl,
haloalkoxy, haloalkyl, haloalkylthio, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, nitro, (CF3)2(HO)C-, -NRAS(0)2RB, -S(O)2ORA, -S(0)2RB, -NZAZB,
(NTZAZB)alkyl, (NZAZB)alkylcarbonyl, (NZAZB)carbonyl, (NZAZB)carbonylalkyl,
(NZAZB)sulfonyl, (NZAZB)C(=NH)-, (NZAZB)C(=NCN)NH-, and (NZAZB)C(=NH)NH-;
CA 02743534 2011-06-13
RA is selected from the group consisting of hydrogen and alkyl;
RB is selected from the group consisting of alkyl, aryl, and arylalkyl;
RSa is selected from the group consisting of hydrogen and alkyl;
R8b is absent when X5 is N or R8b is selected from the group consisting of
hydrogen,
alkoxy, alkoxycarbonylalkyl, alkyl, alkylcarbonyloxy, alkylsulfonyloxy,
halogen, and
hydroxy when X5 is C; and
R9 is selected from the group consisting of hydrogen, aryl, cycloallcyl, and
heterocycle.
. in another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; XI is CRI; X2 is CR2; X3 is N; X4 is
CR4; and R,, R2,
R4, R5, R6, R7, R8a, Rgb, R9, X5, Z1, Z2, and L are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein --- is a single bond; Xi is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; R.ab
is absent; ZI is 0; Z2 is NH; L is alkylene; R9 is aryl; and RI, R2, R4, R5,
R6, R7, and R8a are
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein -- is a single bond; XI is CR1; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; RI,
R6 and R7 are each hydrogen; R2 and R4 are independently selected from the
group consisting
of hydrogen, alkyl, halogen, hydroxy, and -NZAZB; R5 is selected from the
group consisting
of hydrogen and halogen; R8a is hydrogen; R8b is absent; ZI is 0; Z2 is NH; L
is alkylene; R9
is aryl wherein said aryl is phenyl optionally substituted with 1, 2, or 3
substituents
independently selected from the group consisting of alkoxy, alkyl,
alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-
pyrrolidinyl, 4-
thiomorpholinyl, and -NZCZD; and ZA, ZB, ZC, and ZD are independently selected
from the
group consisting of hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; Xi is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; RI,
R2, R4, R5, R6 and R7 are each hydrogen; Rab is absent; ZI is 0; Z2 is NH; L
is alkylene; R9 is
aryl wherein said aryl is substituted with aryloxy; and R8,, is as defined in
formula (1).
6
CA 02743534 2011-06-13
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein,--- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; R1,
R2, R1, R5, R6, R7, and R.g, are each hydrogen; R8b is absent; Z1 is 0; Z2 is
NH; L is alkylene;
R9 is aryl wherein said aryl is phenyl substituted with aryloxy wherein said
aryloxy is
phenoxy optionally substituted with 1, 2, or 3 substituents independently
selected from the
group consisting of alkoxy, alkyl, alkylsulfonyl, 2-azabicyclo[2.2.I]hept-2-
yl,
8-azabicyclo[3.2.1]oct-8-yl, I-azepanyl, 1-azocanyl, cyano, haloalkoxy,
haloalkyl,
haloalkylthio, halogen, methylenedioxy, 4-morpholinyl, 2,6,-dimethyl-4-
morpholinyl,
phenyl, I-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-pyrrolidinyl, 4-
thiomorpholinyl,
and -NZCZD; and Zc and ZD are independently selected from the group consisting
of
hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CR1; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; R1,
R2, R4, R5, R6, R7, and R8a are each hydrogen; R8b is absent; Z1 is 0; Z2 is
NH; L is alkylene;
and R9 is aryl wherein said aryl is napthyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is a single bond; X1 is CR1; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; R8b
is absent; Z1 is 0; Z2 is NH; L is alkylene; R9 is cycloalkyl; R1, R2, R4, R5,
R6, R7, and R8a is
as defined in formula (1).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CR1i X2 is CR2; X3 is N; X4 is
CR4; X5 is N; R1,
R6 and R7 are each hydrogen; R2 and R4 are independently selected from the
group consisting
of hydrogen, alkyl, halogen, hydroxy, and -NZAZB; R5 is selected from the
group consisting
of hydrogen and halogen; Rga is hydrogen;R8b is absent; Z1 is 0; Z2 is NH; L
is alkylene; R9
is cycloalkyl wherein said cyloalkyl is selected from the group consisting of
adamantanyl,
bicyclo[3.1.1jheptane, and cyclohexyl, wherein the cycloalkyl is optionally
substituted with 1
or 2 alkyl substituents; and ZA and ZB are independently selected from the
group consisting of
hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CR1; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; Rgb
is absent; Z1 is 0; Z2 is NH; L is alkylene; R9 is heterocycle; and R1, R2,
R4, R5, R6, R7, and
R8a are as defined in formula (1).
7
CA 02743534 2011-06-13
In another embodiment of the present invention, compounds of fonnula (I) are
disclosed wherein --- is a single bond; X1 is CR1; X2 is CR2; X3 is N; X4 is
CR4; Xs is N; RI,
R6 and R7 are each hydrogen; R2 and R4 are independently selected from the
group consisting
of hydrogen, alkyl, halogen, hydroxy, and -NZAZB; R5 is selected from the
group consisting
of hydrogen and halogen; R8a is hydrogen; R8b is absent; Z1 is 0; Z2 is NH; L
is alkylene; R9
is heterocycle wherein said heterocycle is pyridinyl optionally substituted
with 1, 2, or 3
substituents independently selected from the group consisting of alkoxy,
alkyl, alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkylthio, halogen, 4-morpholinyl, 2,6,-dimethyl-4-
morpholinyl,
phenyl, 1-piperidinyl, 4-methyl-I-piperidinyl, pyridinyl, 1 -pyrrolidinyl, 4-
thiomorpholinyl,
and -NZCZD; and ZA, ZB, Zc, and ZD are independently selected from the group
consisting of
hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) arc
disclosed wherein --- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; Z1 is
0; Z2 is NH; R8b is absent; R9 is hydrogen; and L, RI, R2, R4, R5, R6, R7, and
R8a are as
defined in formula (1).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; RI,
R2, R4, R5, R6, R7, and R8a are each hydrogen; R8b is absent; Z1 is 0; Z2 is
NH; L is alkylene;
and R9 is hydrogen.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CR1; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; Z1 is
0; Z2 is NH; L is cycloalkylene; R8b is absent; R9 is aryl; and RI, R2, R4,
R5, R6, R7, and R8a
are as defined in formula (1).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is a single bond; X1 is CRI; X2, is CR2; X3 is N; X4 is
CR4; X5 is N; RI,
R2, R4, R5, R6, R7, and R8a are each hydrogen; Rsb is absent; Z1 is 0; Z2 is
NH; L is
cycloalkylene; R9 is aryl wherein said aryl is phenyl optionally substituted
with 1, 2, or 3
substituents independently selected from the group consisting of alkoxy,
alkyl, alkylsulfonyl,
2-azabicyclo[2.2. 1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-
pyrrolidinyl, 4-
8
CA 02743534 2011-06-13
thiomorpholinyl, and -NZCZn; and Zc and ZD are independently selected from the
group
consisting of hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; XI is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; Z, is
0; Z2 is a bond; L is cycloalkylene; Rsb is absent; R9 is aryl; and RI, R2,
R4, R5, JZ6, R-1, and
R8a are as defined in formula (1).
In another embodiment of the present invention, compounds of fonnula (I) are
disclosed wherein --- is a single bond; Xi is CR1; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; R1,
R2, R4, R5, R6, R7, and R8a are each hydrogen; R8b is absent; Z1 is 0; Z2 is a
bond; L is
cycloalkylene; R9 is aryl wherein said aryl is phenyl optionally substituted
with 1, 2, or 3
substituents independently selected from the group consisting of alkoxy,
alkyl, alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-diunethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-1-piperidinyl, pyridinyl, 1-
pyrrolidinyl, 4-
thiomorpholinyl, and -NZCZn; and Zc and ZD are independently selected from the
group
consisting of hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X, is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; ZI is
0; Z2 is NH; L is -(CH2)mO(CH2)r,- wherein the left end is attached to Z2 and
the right end is
attached to R9i Rsb is absent; R9 is aryl; and in, n, RI, R2, R4, R5, R6, R7,
and R8a are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; XI is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; RI,
R2, R4, R5, R6, R7, and R8a are each hydrogen; R8b is absent; ZI is 0; Z2 is
NH; L is
-(C12)mO(CH2)n wherein the left end is attached to Z2 and the right end is
attached to R9; m
is 0-2; n is 0-2; R9 is aryl wherein said aryl is phenyl optionally
substituted with 1, 2, or 3
substituents independently selected from the group consisting-of alkoxy,
alkyl, alkylsulfonyl,
2-azabicyclo[2.2.I]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, I-azepanyl, 1-
azocanyl, cyan,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-
pyrrolidinyl, 4-
thiomorpholinyl, and -NZCZD; and Zc and ZD are independently selected from the
group
consisting of hydrogen and alkyl.
9
CA 02743534 2011-06-13
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CRI; X-, is CR2; X3 is N; X4 is
CR4; X5 is N; Z1 is
0; Z2 is NH; L is N(Ry); R8b is absent; R9 is aryl; and Ry, R1, R2, R4, R5,
R6, R7, and R8a are
as defined in formula (1).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; Ry,
R1, R2, R4, R5, R6, R7, and Rs, are each hydrogen; Rsv is absent; ZI is 0; Z2
is NH; L is
N(Ry); Rq is aryl wherein said aryl is phenyl optionally substituted with 1,
2, or 3 substituents
independently selected from the group consisting of alkoxy, alkyl,
alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, I -azepanyl, 1-
azocanyl, cyan,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, I -piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-
pyrrolidinyl, 4-
thiomorpholinyl, and -NZcZD; and Zc and ZD are independently selected from the
group
consisting of hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein --- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4;:X5 is N; Z1 is
0; Z2 is a bond;
Ry
+N I-`N-j-
L is \--J ; R8b is absent; R9 is aryl; and Ry, RI, R2, R4, R5, R6, R7, and R8a
are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; R1,
R5, R6, R7, and R8a are each hydrogen; R8b is absent; R2 is selected from the
group consisting
of hydrogen and alkyl; ZI is 0; Z2 is a bond;
Ry
--N r k N-
L is \-J ; R9 is aryl wherein said aryl is phenyl optionally substituted with
1, 2, or 3
substituents independently selected from the group consisting of alkoxy,
alkyl, alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl- I -piperidinyl, pyridinyl, 1-
pyrrolidinyl, 4-
CA 02743534 2011-06-13
thiomorpholinyl, and -NZCZD; and Ry, Zc, and ZD are independently selected
from the group
consisting of hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; Xi is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; RI,
R2, R4, R5 and R6 are each hydrogen; R7 is (CF3)2(HO)C-; Rgb is absent; ZI is
0; Z2 is NH; L
is alkylene; R9 is aryl wherein said aryl is phenyl optionally substituted
with 1, 2, or 3
substituents independently selected from the group consisting of alkoxy,
alkyl, alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dunethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-pip eridinyl, pyridinyl, 1-
pyrrolidinyl, 4-
thiomorpholinyl, and -NZCZD; and R8a, Zc, and ZD are independently selected
from the group
consisting of hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is a single bond; XI is CRI; X2 is CR2i X3 is N; X4 is
CR4; X5 is N; ZI is
0; Z2 is 0; L is allcylene; R8b is absent; R9 is aryl; RI, R2i R4, R5, R6, R7,
and Rga are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; XI is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; R1,
R2, R4, R5, R6, R7, and Rsa are each hydrogen; R81, is absent; ZI is 0; Z2 is
0; L is alkylene; R9
is aryl wherein said aryl is phenyl optionally substituted with 1, 2, or 3
substituents
independently selected from the group consisting of alkoxy, alkyl,
alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-
pyrrolidinyl, 4-
thiomorpholinyl, and -NZcZD; and Zc and ZD are independently selected from the
group
consisting of hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; XI is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; RI,
R2, R4, R5, R6 and R7 are each hydrogen; ZI is 0; Z2 is 0; L is alkylene; R8b
is absent; R9 is
aryl wherein said aryl is naphthyl; and Rsa is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X, is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; R8b
11
CA 02743534 2011-06-13
is absent; ZI is 0; Z2 is a bond; L is alkenylene; R9 is aryl; and R1, R2, R4,
R5, R6, R7, and R8a
are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is N; R1,
R6 and R7 are each hydrogen; R2 and R4 are independently selected from the
group consisting
of hydrogen, alkyl, halogen, hydroxy, and -NZAZB; R5 is selected from the
group consisting
of hydrogen and halogen; Rsa is hydrogen; Rgb is absent; Z1 is 0; Z2 is a
bond; L is
alkenylene; R9 is aryl wherein said aryl is phenyl optionally substituted with
1, 2, or 3
substituents independently selected from the group consisting of alkoxy,
alkyl, alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabieyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyan,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-
pyrrolidinyl, 4-
thiomorpholinyl, and -NZCZD; and ZA, ZB, Zc and ZD are independently selected
from the
group consisting of hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is C; ZI is
0; Z2 is NH; L is alkylene; R9 is heterocycle; and RI, R2, R4, R5, R6, R7,
R3a, and Rsb are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is a single bond; XI is CRi; X2 is CR2; X3 is N; X4 is
CR4; X5 is C; R1,
R6 and R7 are each hydrogen; R2 and R4 are independently selected from the
group consisting
of hydrogen, alkyl, halogen, hydroxy, and -NZAZB; R5 is selected from the
group consisting
of hydrogen and halogen; RSa and Rab are hydrogen; Z1 is 0; Z2 is NH; L is
alkylene; R9 is
heterocycle wherein said heterocycle is selected from the group consisting of
imidazolyl,
pyridinyl, pyrrolidinyl, and thienyl, wherein the heterocycle is optionally
substituted with 1
or 2 substituents independently selected from the group consisting of alkoxy,
alkyl,
alkylsulfonyl, 2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-
azepanyl, 1-
azocanyl, cyan, haloalkoxy, haloalkyl, haloalkylthio, halogen, oxo, 4-
morpholinyl, 2,6,-
dimethyl-4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl,
pyridinyl,
I -pyrrolidinyl, 4-thiomorpholinyl, and -NZCZD; and ZA, ZB, Zc and ZD are
independently
selected from the group consisting of hydrogen and alkyl.
12
CA 02743534 2011-06-13
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein --- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is C; Z1 is
0; Z2 is NH; L is -(CH2),,,O(CH2)õ wherein the left end is attached to Z2 and
the right end is
attached to R9i R9 is hydrogen; and in, n, R1, R2, R4, R5, R6, R7, Rg, and Rsb
are as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is C; R1,
R6 and R7 are each hydrogen; R2 and R4 are independently selected from the
group consisting
of hydrogen, alkyl, halogen, hydroxy, and -NZAZB; R5 is selected from the
group consisting
of hydrogen and halogen; Rga and Rsb are hydrogen; Z1 is 0; Z2 is NH; L is -
(CH2),,O(CH2)n-
wherein the left end is attached to Z2 and the right end is attached to R9; m
is 0-4; n is 0-4; R9
is hydrogen; and ZA and ZB are independently selected from the group
consisting of hydrogen
and alkyl.
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein --- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is C; Z1 is
0; Z2 is NH; L is alkylene; R9 is aryl; and R1, R2, R4, R5, R6, R7, Rga, and
Rsb are as defined
in formula (I).
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein --- is a single bond; X1 is CR1 i X2 is CR2; X3 is N; X4 is
CR4; X5 is C; R1,
R6, R7, Rga and Rib are each hydrogen; R2 and R4 are independently selected
from the group
consisting of hydrogen, alkyl, halogen, hydroxy, and -NZAZB; R5 is selected
from the group
consisting of hydrogen and halogen; Zi is 0; Z2 is NH; L is alkylene; R9 is
aryl wherein said
aryl is phenyl optionally substituted with 1, 2, or 3 substituents
independently selected from
the group consisting of alkoxy, alkyl, alkylsulfonyl, 2-azabicyclo[2.2.1]hept-
2-yl,
8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1 -azocanyl, cyano, haloalkoxy,
haloalkyl,
haloalkylthio, halogen, methylenedioxy, 4-morpholinyl, 2,6,-dimethyl-4-
morpholinyl,
phenyl, 1-piperidinyl, 4-methyl-1 -piperidinyl, pyridinyl, 1-pyrrolidinyl, 4-
fliiomorpholinyl,
and -NZCZD; and ZA, Z3, Zc and ZD are independently selected from the group
consisting of
hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is C; RI,
R6, and R7 are each hydrogen; R2 and R4 are independently selected from the
group
13
CA 02743534 2011-06-13
consisting of hydrogen, alkyl, halogen, hydroxy, and -NZAZB; R5 is selected
from the group
consisting of hydrogen and halogen; Rga is selected from the group consisting
of hydrogen
and alkyl; P1-8b is alkyl; ZI is 0; Z2 is NH; L is alkylene; R9 is aryl
wherein said aryl is phenyl
optionally substituted with 1, 2, or 3 substituents independently selected
from the group
consisting of alkoxy, alkyl, alkylsulfonyl, 2-azabicyclo[2.2.I Ihept-2-yl,
8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-azocanyl, cyano, haloalkoxy,
haloalkyl,
haloalkylthio, halogen, methylenedioxy, 4-morpholinyl, 2,6,-dimethyl-4-
morpholinyl,
phenyl, I-piperidinyl, 4-methyl-l-pip eridinyl, pyridinyl, 1-pyrrolidinyl, 4-
thiomorpholinyl,
and -NZCZD; and ZA, Zf3, Zc and ZD are independently selected from the group
consisting of
hydrogen and alkyl.
in another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is C; R1,
R6, and R7 and are each hydrogen; R2 and R4 are independently selected from
the group
consisting of hydrogen, alkyl, halogen, hydroxy, and -NZAZB; R5 is selected
from the group
consisting of hydrogen and halogen; R8a is hydrogen; R.gb is selected from the
group
consisting of alkoxy, alkoxycarbonylalkyl, alkylcarbonyloxy, alkylsulfonyl,
halogen, and
hydroxy; ZI is 0; Z2 is NH; L is alkylene; R9 is aryl wherein said aryl is
phenyl optionally
substituted with 1, 2, or 3 substituents independently selected from the group
consisting of
alkoxy, alkyl, alkylsulfonyl, 2-azabicyclo[2.2.1]hept-2-yl, 8-
azabicyclo[3.2.1]oct-8-yl, 1-
azepanyl, 1-azocanyl, cyano, haloalkoxy, haloalkyl, haloalkylthio, halogen,
methylenedioxy,
4-morpholinyl, 2,6,-dimethyl-4-morpholinyl, phenyl, l-piperidinyl, 4-methyl-l-
piperidinyl,
pyridinyl, I-pyrrolidinyl, 4-thiomorpholinyl, and -NZCZD; and ZA, ZB, Zc and
ZD are
independently selected from the group consisting of hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is a single bond; X1 is CRI; X2 is CR2; X3 is N; X4 is
CR4; X5 is C; R1,
R6, R7, and R7 are each hydrogen; R2 and R4 are independently selected from
the group
consisting of hydrogen, alkyl, halogen, hydroxy, and -NZAZB; R5 is selected
from the group
consisting of hydrogen and halogen; Rga is selected from the group consisting
of hydrogen
and alkyl; Rgb is selected from the group consisting of hydrogen,
alkoxycarbonylalkyl, alkyl,
and hydroxy; Z1 is 0; Z2 is 0; L is alkylene; R9 is hydrogen; and ZA and ZB
are independently
selected from the group consisting of hydrogen and alkyl.
14
CA 02743534 2011-06-13
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CR1; X2 is CR2; X3 is N; X4 is
N; and R1, R2, R5,
R6, R7, Rsa, R8b, R9, X5, Z1, Z2, and L are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; XI is CRI; X2 is CR2; X3 is N; X4 is
N; X5 is N; Rsb is
absent; Z1 is 0; Z2 is NH; L is alkylene; R9 is aryl; and R1, R2, R5, R6, R7,
Rsa, and Rsb are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CR1; X2 is CR2; X3 is N; X4 is
N; X5 is N; R1, R5,
R6 and R7 are each hydrogen; Rsb is absent; R2 is selected from the group
consisting of alkyl
and halogen; Z1 is 0; Z2 is NH; L is alkylene; R9 is aryl wherein said aryl is
phenyl optionally
substituted with 1, 2, or 3 substituents independently selected from the group
consisting of
alkoxy, alkyl, alkylsulfonyl, 2-azabicyclo[2.2.1]hept-2-yl, 8-
azabicyclo[3.2.1]oct-8-yl, 1-
azepanyl, t-azocanyl, cyano, haloalkoxy, haloalkyl, haloalkylthio, halogen,
methylenedioxy,
4-morpholinyl, 2,6,-dimethyl-4-morpholinyl, phenyl, I-piperidinyl, 4-methyl-l-
piperidinyl,
pyridinyl, 1-pyrrolidinyl, 4-thiomorpholinyl, and -NZcZD; and Rga, Zc, and ZD
are
independently selected from the group consisting of hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CR2; X2 is N; X3 is CR3; X4 is
CR4; and R1, R3,
R5, R6, R7, Rsa, Rsb, R9, Xs, Z1, Z2, and L are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is a single bond; X1 is CRI; X2 is N; X3 is CR3; X4 is
CR4; X5 is N; Rsb
is absent; Z1 is 0; Z2 is NH; L is alkylene; R9 is aryl; and R1, R3, R5, R6,
R7, and Rsa are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is a single bond; X1 is CRI; X2 is N; X3 is CR3; X4 is
CR4; X5 is N; R1,
R3, R4, R5, R6 and R7 are each hydrogen; Rsb is absent; Z1 is 0; Z2 is NH; L
is alkylene; R9 is
aryl wherein said aryl is phenyl optionally substituted with 1, 2, or 3
substituents
independently selected from the group consisting of alkoxy, alkyl,
alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkyltbio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-pipetdinyl, pyridinyl, 1-
pyrrolidinyl, 4-
CA 02743534 2011-06-13
thiomorpholinyl, and -NZCZD; and Rga, ZC, and ZD are independently selected
from the group
consisting of hydrogen and alkyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is absent; X1 is CR1i X2 is CR2; X3 is NR3; X1 is a
bond; and R1, R2, R3,
R5, R6, R7, Rga, R8b, R9, X5, Z1, Z2, and L are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is absent; X1 is CR1; X2 is CR2; X3 is NR3; X4 is a
bond; Xs is N; Rgb is
absent; Z1 is 0; Z2 is NH; L is alkylene; R9 is aryl; and R1, R2, R3, R5, R6,
R7, and Rga are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is absent; X1 is CR1 i X2 is CR2; X3 is N; X4 is a bond;
X5 is N; R1, R2,
R5, R6 and R7 are each hydrogen; Rgb is absent; Zl, is 0; Z2 is NH; L is
alkylene; R9 is aryl
wherein said aryl is phenyl optionally substituted with 1, 2, or 3
substituents independently
selected from the group consisting of alkoxy, alkyl, alkylsulfonyl, 2-
azabicyclo[2.2.1]hept-2-
yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-azocanyl, cyano, haloalkoxy,
haloalkyl,
haloalkylthio, halogen, methylenedioxy, 4-morpholinyl, 2,6,-dimethyl-4-
morpholinyl,
phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-pyrrolidinyl, 4-
thiomorpholinyl,
and -NZCZD; Rga, ZC, and ZD are independently selected from the group
consisting of
hydrogen and alkyl; and R3 is selected from the group consisting of hydrogen
and
alkoxycarbonyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is absent; X1 is CR1; X2 is CR2; X3 is NR3; X4 is a bond;
X5 is N; R1 and
R2 are each independently alkyl; R5, R6 and R7 are each hydrogen; Rgb is
absent; Zl is 0; Z2 is
NH; L is alkylene; R9 is aryl wherein said aryl is phenyl optionally
substituted with 1, 2, or 3
substituents independently selected from the group consisting of alkoxy,
alkyl, alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-
pyrrolidinyl, 4-
thiomorpholinyl, and -NZCZD; Rga, Zc, and ZD are independently selected from
the group
consisting of hydrogen and alkyl; and R3 is selected from the group consisting
of hydrogen
and alkoxycarbonyl.
16
CA 02743534 2011-06-13
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is absent; X1 is CRI; X2 is CR2; X3 is NR3; X4 is a
bond; Xs is N; Rsb is
absent; Z1 is 0; Z2 is 0; L is alkylene; R9 is aryl; and RI, R2, R3, R5, R6,
R7, R8a, and R9 are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is absent; X1 is CRI; X2 is CR2; X3 is NR3; X4 is a
bond; X5 is N; R8a,
RI, R2, R5, R6 and R7 are each hydrogen; Rgb is absent; Z1 is 0; Z2 is 0; L is
alkylene; R9 is
aryl wherein said aryl is phenyl optionally substituted with 1, 2, or 3
substituents
independently selected from the group consisting of allkoxy, alkyl,
alkylsulfonyl,
2-azabieyclo[2.2.1]hept-2-yl, 8-azabieyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, I -
pyrrolidinyl, 4-
thiomorpholinyl, and -NZcZD; Ria, Zc, and ZD are independently selected from
the group
consisting of hydrogen and alkyl; and R3 is selected from the group consisting
of hydrogen
and alkoxycarbonyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is absent; X1 is CRI; X2 is N; X3 is NR3; X4 is a bond;
and RI, R3, R5,
R6, R7, R8a, R.gb, R9, X5, ZI, Z2. and L are as defined in formula (1).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is absent; XI is CRI; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; Rib is
absent; Z1 is 0; Z2 is NH; L is alkylene; R9 is aryl; and RI, R3, R5, R6, R7,
and Rsa are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein -- is absent; XI is CRI; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; RI, R5,
R6 and R7 are each hydrogen;=Rib is absent; Z1 is 0; Z2 is NH; L is alkylene;
R9 is aryl
wherein said aryl is phenyl optionally substituted with 1, 2, or 3
substituents independently
selected from the group consisting of alkoxy, alkyl, alkylsulfonyl, 2-
azabicyclo[2.2. I ]hept-2-
yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-azocanyl, cyano, haloalkoxy,
haloalkyl,
haloalkylthio, halogen, methylenedioxy, 4-morpholinyl, 2,6,-dimethyl-4-
morpholinyl,
phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-pyrrolidinyl, 4-
thiomorpholinyl,
and -NZCZD; R8a, ZC, and ZD are independently selected from the group
consisting of
17
CA 02743534 2011-06-13
hydrogen and alkyl; and R3 is selected from the group consisting of hydrogen
and
alkoxycarbonyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is absent; Xl is CRI; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; RBa, R1,
R5, R6 and R7 are each hydrogen; R8b is absent; Z1 is 0; Z2 is NH; L is
alkylene wherein the
alkylene is -CH2-; R9 is aryl wherein said aryl is phenyl optionally
substituted with 2
substituents independently selected from the group consisting of 8-
azabicyclo[3.2.1]oct-8-yl,
trifluoromethyl, and -Cl; and R3 is selected from the group consisting of
hydrogen and
alkoxycarbonyl.
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein --- is absent; X1 is CRI; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; R8a, R1,
R5, R6 and R7 are each hydrogen; R8b is absent; Z1 is 0; Z2 is NH; L is
alkylene wherein the
alkylene is -CH2-; R9 is aryl wherein said aryl is 4-(8-azabicyclo[3.2.1]oct-8-
yl)-'3-
(trifluoromethyl)phenyl; and R3 is selected from the group consisting of
hydrogen and
alkoxycarbonyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is absent; XI is CRI; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; Rsa, R1,
R5, R6 and R7 are each hydrogen; R8b is absent; Z1 is 0; Z2 is NH; L is
alkylene wherein the
alkylene is -CH2-; R9 is aryl wherein said aryl is 4-(8-azabicyclo[3.2.1 ]oct-
8-yl)-2-
chlorophenyl; and R3 is selected from the group consisting of hydrogen and
alkoxycarbonyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is absent; XI is CRI; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; RI, R6
and R7 are each hydrogen; R5 is alkyl wherein a preferred alkyl is methyl or
ethyl; Rsb is
absent; Z1 is 0; Z2 is NH; L is alkylene; R9 is aryl wherein said aryl is
phenyl optionally
substituted with 1, 2, or 3 substituents independently selected from the group
consisting of
alkoxy, alkyl, alkylsulfonyl, 2-azabicyclo[2.2.l]hept-2-yl, 8-
azabicyclo[3.2.1]oct-8-yl, 1-
azepanyl, 1-azocanyl, cyano, haloalkoxy, haloalkyl, haloalkylthio, halogen,
methylenedioxy,
4-morpholinyl, 2,6,-dimethyl-4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-I-
piperidinyl,
pyridinyl, I -pyrrolidinyl, 4-thiomorpholinyl, and -NZcZD; Zc and ZD are
independently
selected from the group consisting of hydrogen and alkyl; and R8a and R3 are
as defined in
formula (I).
18
CA 02743534 2011-06-13
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein --- is absent; X1 is CRI; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; Rga, RI,
R6 and R7 are each hydrogen; R5 is alkyl wherein a preferred alkyl is methyl
or ethyl; Rnb is
absent; Z1 is 0; Z2 is NH; L is alkylene; R9 is aryl wherein said aryl is
phenyl optionally
substituted with 1, 2, or 3 substituents independently selected from the group
consisting of
alkoxy, alkyl, alkylsulfonyl, 2-azabicyclo[2.2.1Jhept-2-yl, 8-
azabicyclo[32.1Joct-8-yl, 1-
azepanyl, 1-azocanyl, cyano, haloalkoxy, haloalkyl, haloalkylthio, halogen,
methylenedioxy,
4-morpholinyl, 2,6,-dimethyl-4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-
piperidinyl,
pyridinyl, 1-pyrrolidinyl, 4-thiomorpholinyl, and -NZCZD; Zc and ZD are
independently
selected from the group consisting of hydrogen and alkyl; and R3 is selected
from the group
consisting of hydrogen and alkoxycarbonyl.
In another embodiment of the present invention, compounds of formula (1) aie
disclosed wherein --- is absent; XI is CRI; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; RI, R5,
R6 and R7 are each hydrogen; Rnb is absent; Z1 is 0; Z2 is NH; L is alkylene;
R9 is aryl
wherein said aryl is selected from the group consisting of naphthyl and
phenyl; and Rga and
R3 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein --- is absent; X1 is CRI; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; Rna, RI,
R5, R6 and R7 are each hydrogen; Rnb is absent; Z1 is 0; Z2 is NH; L is
alkylene; R9 is aryl
wherein said aryl is selected from the group consisting of naphthyl and
phenyl; and R3 is
selected from the group consisting of hydrogen and alkoxycarbonyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein -- is absent; X1 is CRI; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; RI, R5,
R6 and R7 are each hydrogen; R8b is absent; Z1 is 0; Z2 is NH; L is alkylene;
R9 is heterocycle
wherein said heterocycle is pyridinyl substituted with alkoxy, alkyl,
alkylsulfonyl, 2-
azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-diniethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-
pyrrolidinyl, 4-
thiomorpholinyl, and -NZCZD; and Zc, ZD, Rga, and R3 are as defined in formula
(I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is absent; X1 is CRI; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; X5 is N;
Rga, R1, R5, R6 and R7 are each hydrogen; R8b is absent; Z, is 0; Z2 is NH; L
is alkylene; R9 is
19
CA 02743534 2011-06-13
heterocycle wherein said heterocycle is pyridinyl substituted with alkoxy,
alkyl,
alkylsulfonyl, 2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-
azepanyl,
1-azocanyl, cyano, haloalkoxy, haloalkyl, haloalkylthio, halogen,
methylenedioxy,
4-morpholinyl, 2,6,-dimethyl-4-moipholinyl, phenyl, 1-piperidinyl, 4-methyl-l-
piperidinyl,
pyridinyl, 1-pyrrolidinyl, 4-thiomorpholinyl, and -NZCZD; Zo and ZD are
independently
selected from the group consisting of hydrogen and alkyl; and R3 is selected
from the group
consisting of hydrogen and alkoxycarbonyl.
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein --- is absent; Xi is CR1; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; REb is
absent; Z1 is 0; Z2 is NH; L is
Ry
\-/ ; R9 is heterocycle; and RBa, R1, R3, R5, R6 and R7 are as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein --- is absent; Xi is CRi; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; R1, R5,
R6 and R7 are each hydrogen; RBb is absent; Zi is 0; Z2 is NH; L is
Ry
rI1
--N -~N-.
R9 is heterocycle wherein said heterocycle is pyridinyl optionally substituted
with 1 or 2 substituents selected from the group consisting of alkoxy, alkyl,
alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyclo[3.2.1]oct-8-yl, 1-azepanyl, 1-
azocanyl, cyano,
haloalkoxy, haloalkyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, 1-piperidinyl, 4-methyl-l-piperidinyl, pyridinyl, 1-
pyrrolidinyl,
4-thiomorpholinyl, and -NZcZD; Zc and ZD are independently selected from the
group
consisting of hydrogen and alkyl; and RBa and R3 are as defined in formula
(I).
In another embodiment of the present invention, compounds of formula (1) are
disclosed wherein --- is absent; Xi is CR1; X2 is N; X3 is NR3; X4 is a bond;
X5 is N; RBa, R1,
R5, R6 and R7 are each hydrogen; RSb is absent; Zi is 0; Z2 is NH; L is
Ry
-- rk
N- .
N
R9 is heterocycle wherein said heterocycle is pyridinyl optionally substituted
with 1 or 2 substituents selected from the group consisting of alkoxy, alkyl,
alkylsulfonyl,
2-azabicyclo[2.2.1]hept-2-yl, 8-azabicyelo[3.2.1]oct-8-yl, I-azepanyl, 1-
azocanyl, cyano,
CA 02743534 2011-06-13
haloalkoxy, haloallcyl, haloalkylthio, halogen, methylenedioxy, 4-morpholinyl,
2,6,-dimethyl-
4-morpholinyl, phenyl, I-piperidi_nyl, 4-methyl-l-piperidinyl, pyridinyl, I-
pyrrolidinyl,
4-thiomorpholinyl, and -NZcZD; ZC and ZD are independently selected from the
group
consisting of hydrogen and alkyl; and R3 is selected from the group consisting
of hydrogen
and alkoxycarbonyl.
Another embodiment of the present invention relates to pharmaceutical
compositions
comprising a therapeutically effective amount of a compound of formula (I) or
a
pharmaceutically acceptable salt thereof.
Another embodiment of the present invention relates to a method of treating a
disorder wherein the disorder is ameliorated by inhibiting vanilloid receptor
subtype 1 (VRI.)
receptor in a host mammal in need of such treatment comprising administering a
therapeutically effective amount of a compound of formula (I) or a
pharmaceutically
acceptable salt thereof.
Another embodiment of the present invention relates to a method for
controlling pain
in a host mammal in need of such treatment comprising administering a
therapeutically
effective amount of a compound of formula (I) or a pharmaceutically acceptable
salt thereof.
Another embodiment of the present invention relates to a method of treating
urinary
incontinence in a host mammal in need of such treatment comprising
administering a
therapeutically effective amount of a compound of formula (I) or a
pharmaceutically
acceptable salt thereof.
Another embodiment of the present invention relates to a method of treating
bladder
overactivity in a host mammal in need of such treatment comprising
administering a
therapeutically effective amount of a compound of formula (I) or a
pharmaceutically
acceptable salt thereof.
Another embodiment of the present invention relates to a method of treating
inflammatory thermal hyperalgesia in a host mammal in need of such treatment
comprising
administering a therapeutically effective amount of a compound of formula (I)
or a
pharmaceutically acceptable salt thereof.
Definition of Terms
21
CA 02743534 2011-06-13
As used throughout this specification and the appended claims, the following
terms
have the following meanings:
The term "alkenyl" as used herein, means a straight or branched chain
hydrocarbon
containing from 2 to 10 carbons and containing at least one carbon-carbon
double bond
formed by the removal of two hydrogens. Representative examples of alkenyl
include, but
are not limited to, ethenyl, 2-propenyl, 2-methyl-2-propenyl, 3-butenyl, 4-
pentenyl, 5-
hexenyl, 2-heptenyl, 2-methyl-l-heptenyl, and 3-decenyl.
The term "alkenylene" means a divalent group derived from a straight or
branched
chain hydrocarbon of from 2 to 10 carbon atoms containing at least one double
bond.
Representative examples of alkenylene include, but are not limited to, -CH=CH-
,
-CH=CH2CH2-, and -CH=C(CH3)CH2-.
The term "alkoxy" as used herein, means an alkyl group, as defined herein,
appended
to the parent molecular moiety through an oxygen atom. Representative examples
of alkoxy
include, but are not limited to, methoxy, ethoxy, propoxy, 2-propoxy, butoxy,
tert-butoxy,
pentyloxy, and hexyloxy.
The term "alkoxyalkoxy" as used herein, means an alkoxy group, as defined
herein,
appended to the parent molecular moiety through an alkoxy group, as defined
herein.
Representative examples of alkoxyalkoxy include, but are not limited to,
methoxymethoxy,
ethoxymethoxy and 2-ethoxyethoxy.
The term "alkoxyalkyl" as used herein, means an alkoxy group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of alkoxyalkyl include, but are not limited to, tert-
butoxymethyl, 2-
ethoxyethyl, 2-methoxyethyl, and methoxymethyl.
The term. "alkoxycarbonyl" as used herein, means an alkoxy group, as defined
herein,
appended to the parent molecular moiety through a carbonyl group, as defined
herein.
Representative examples of alkoxycarbonyl include, but are not limited to,
methoxycarbonyl,
ethoxycarbonyl, and tert-butoxycarbonyl.
The term "alkoxycarbonylalkyl" as used herein, means an alkoxycarbonyl group,
as
defined herein, appended to the parent molecular moiety through an alkyl
group, as defined
herein. Representative examples of alkoxycarbonylalkyl include, but are not
limited to, 3-
methoxycarbonylpropyl, 4-ethoxycarbonylbutyl, and 2-tert-butoxycarbonylethyl.
22
CA 02743534 2011-06-13
The term "alkyl" as used herein, means a straight or branched chain
hydrocarbon
containing from 1 to 10 carbon atoms. Representative examples of alkyl
include, but are not
limited to, methyl. ethyl, n-propyl, iso-propyl, n-butyl, sec-butyl, iso-
butyl, tert-butyl, n-
pentyl, isopentyl, neopentyl, n-hexyl, 3-methylhexyl, 2,2-dimethylpentyl, 2,3-
diniethylpentyl,
n-heptyl, n-octyl, n-nonyl, and n-decyl.
The term "alkylcarbonyl" as used herein, means an alkyl group, as defined
herein,
appended to the parent molecular moiety through a carbonyl group, as defined
herein.
Representative examples of alkylcarbonyl include, but are not limited to,
acetyl, 1-oxopropyl,
2,2-dimethyl-l-oxopropyl, 1-oxobutyl, and I-oxopentyl.
The term "alkylcarbonylalkyl" as used herein, means an alkylcarbonyl group, as
defined herein, appended to the parent molecular moiety through an alkyl
group, as defined
herein. Representative examples of alkylcarbonylalkyl include, but are not
limited to, 2-
oxopropyl, 3,3-dimethyl-2-oxopropyl, 3-oxobutyl, and 3-oxopentyl.
The term "alkylcarbonyloxy" as used herein, means an alkylcarbonyl group, as
defined herein, appended to the parent molecular moiety through an oxygen
atom.
Representative examples of alkylcarbonyloxy include, but are not limited to,
acetyloxy,
etylcarbonyloxy, and tert-butylcarbonyloxy.
The term "alkylene" means a divalent group derived from a straight or branched
chain
hydrocarbon of from I to 10 carbon atoms. Representative examples of alkylene
include, but
are not limited to, -CH2-, -CH2CH2-, -CH2CH2CH2-, -CH2CH2CH2CH2-, -
CH2CII(CH3)CH2-,
and -(CH2)pCH(Rz)(CH2)q-, wherein p and q are independently 0-4 and Rz is
selected from
the group consisting of aryl, cycloalkyl, and hydroxy. A preferred aryl group
is phenyl.
The term "alkylsulfonyl" as used herein, means an alkyl group, as defined
herein,
appended to the parent molecular moiety through a sulfonyl group, as defined
herein.
Representative examples of alkylsulfonyl include, but are not limited to,
methylsulfonyl and
ethylsulfonyl.
The term "alkylthio" as used herein, means an alkyl group, as defined herein,
appended to the parent molecular moiety through a sulfur atom. Representative
examples of
alkylthio include, but are not limited, methylsulfanyl, ethylsulfanyl, tert-
butylsulfanyl, and
hexylsulfanyl.
The term "alkynyl" as used herein, means a straight or branched chain
hydrocarbon
group containing from 2 to 10 carbon atoms and containing at least one carbon-
carbon triple
23
CA 02743534 2011-06-13
bond. Representative examples of alkynyl include, but are not limited, to
acetylenyl, 1-
propynyl, 2-propynyl, 3-butynyl, 2-pentynyl, and 1-butynyl.
The term "alkynylene" means a divalent group derived from a straight or
branched
chain hydrocarbon of from 2 to 10 carbon atoms containing at least one triple
bond.
Representative examples of alkynylene include, but are not limited to, -C=C-,
-CH2C=C-, -CH(CH3)CH2C=C-, -C=CCH2-, and -C---CCH(CH3)CH2-.
The term "aryl" as used herein, means a phenyl group, or a bicyclic or a
tricyclic
fused ring system wherein one or more of the fused rings is a phenyl group.
Bicyclic fused
ring systems are exemplified by a phenyl group fused to a cycloalkyl group, as
defined
herein, or another phenyl group. Tricyclic fused ring systems are exemplified
by a bicyclic
fused ring system fused to a cycloalkyl group, as defined herein, or another
phenyl group.
Representative examples of aryl include, but are not limited to, anthracenyl,
azulenyl,
fluorenyl, indanyl, indenyl, naphthyl, phenyl and tetrahydronaphthyl.
The aryl groups of this invention can be substituted with 1, 2, 3, 4 or 5
substituents
independently selected from alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonylalkyl,
alkylcarbonyloxy,
alkylsulfonyl, alkylthio, alkynyl, carboxy, carboxyalkyl, cyano, cyanoalkyl,
cycloalkyl,
cycloalkylalkyl, ethylenedioxy, formyl, formylalkyl, haloalkoxy, haloalkyl,
haloalkylthio,
halogen, hydroxy, hydroxyalkyl, methylenedioxy, mercapto, mercaptoalkyl,
nitro, -NZcZD,
(NZcZD)alkyl, (NZCZD)carbonyl, (NZcZD)carbonylalkyl, (NZCZD)sulfonyl, -
NRAS(O)2RB,
-S(O)2ORA and -S(O)2RA wherein RA and RB are as defined herein. The aryl
groups of this
invention can be further substituted with any one of an additional aryl,
arylalkyl, aryloxy,
arylthio, heterocycle, heterocyclealkyl, heterocycleoxy, or heterocyclethio
group, as defined
herein, wherein the additional aryl, arylalkyl, aryloxy, arylthio,
heterocycle, heterocyclealkyl,
heterocycleoxy, and heterocyclethio group can be substituted with 1, 2, 3, 4,
or 5 substituents
independently selected from alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonylalkyl,
alkylcarbonyloxy,
alkylsulfonyl, alkylthio, allcynyl, carboxy, carboxyalkyl, cyano, cyanoalkyl,
cycloalkyl,
cycloalkylalkyl, formyl, formylalkyl, haloalkoxy, haloalkyl, haloalkylthio,
halogen, hydroxy,
hydroxyalkyl, mercapto, mercaptoalkyl, nitro, -NZCZD, (NZcZD)alkyl,
(NZcZD)carbonyl,
(NZCZD)carbonylalkyl, (NZcZD)sulfonyl, -NRAS(O)2RB, -S(O)2ORA and -S(O)2RA
wherein
RA and RB are as defined herein. Representative examples include, but are not
limited to, 4-
24
CA 02743534 2011-06-13
(2-azabicyclo[2.2.1 ]hept-2-yl)-2-(trifluoromethyl)phenyl, 4-(8-
azabicyelo[3.2.1 ]oct-8-yl)-2-
chlorophenyl, 4-(8-azabicyclo[3.2.1]oct-8-yl)-3-chlorophenyl, 4-(8-
azabicyclo[3.2.1]oct-8-
yl)phenyl, 4-(S-azabicyclo[3.2.1 ]oct-8-yl)-3-(trifluoromethyl)phenyl, 4-(8-
azabicyclo[3.2.1]oct-8-yl)-2-(trifluoromethyl)phenyl, 4-(8-
azabicyclo[3.2.I]oct-8-yl)-3-
fluorophenyl, 3-chloro-4-azepan-1-ylphenyl, 2-chloro-4-azepan-1-ylphenyl, 3,5-
difluoro-4-
azepan-1-ylphenyl, 4-(8-azabicyclo[3.2.1]oct-8-yl)-3,5-difluorophenyl, 4-
bromophenyl, 3-
chlorophenyl, 4-chlorophenyl, 3,4-dichlorophenyl, 2,3-dichlorophenyl, 2,4-
dichlorophenyl,
3,5-dichlorophenyl, 3,4-difluorophenyl, 4-bromo-2-fluorophenyl, 4-chloro-2-
fluorophenyl, 4-
(tert-butyl)phenyl), 4-cyanophenyl, 4-etylphenyl, 3-fluorophenyl, 2,4-
difluorophenyl, 4-
bromo-3-fluorophenyl, 2,3-difluoro-4-(trifluoromethyl)phenyl, 3-fluoro-4-
(trifluoromethyl)phenyl, 3-fluoro-5-(trifluoromethyl)phenyl, 3-
(trifluoromethyl)phenyl, 4-
(trifluoromethyl)phenyl, 4-(trifluoromethoxy)phenyl, 3-
(trifluoromethoxy)phenyl, 4-
[(trifluoromethyl)thio]phenyl, 4-azepan-1-yl-3-(trifluoromethyl)phenyl, 4-
azepan-l-yl-2-
(trifluoromethyl)phenyl, 3-methylphenyl, 3,4-dimethylphenyl, 2,4-
dimethylphenyl, 4-
isopropylphenyl, 4-methylphenyl, 4-bromo-3-methylphenyl, 4-fluoro-3-
(trifluoromethyl)phenyl, 3-chloro-4-fluorophenyl, 4-(1-pyrrolidinyl)phenyl, 4-
(1-
azepanyl)phenyl, 3-fluoro-4-(1-pyrrolidinyl)phenyl, 3-fluoro-4-(1-
azepanyl)phenyl, 4-(1-
azocanyl)phenyl, 4-(I-piperidinyl)phenyl, 3-fluoro-4-(l.-piperidinyl)phenyl, 4-
(2-
pyridinyl)phenyl, 1,1'-biphenyl, 3-fluoro-4-(4 methyl-l piperidinyl)phenyl, 4
(4 methyl 1
piperidinyl)phenyl, 4-(4-morpholinyl)phenyl, 4-(2,6-dimethyI-4-
morpholinyl)phenyl, 4-(4-
thiomorpholinyl)phenyl, 3,5-difluoro-4-(4-morpholinyl)phenyl, 3,5-
bis(trifluoromethyl)phenyl, and 2,5-bis(trifluoromethyl)phenyl.
The term "arylalkyl" as used herein, means an aryl group, as defined herein,
appended
to the parent molecular moiety through an alkyl group, as defined herein.
Representative
examples of arylalkyl include, but are not limited to, benzyl, 2-phenylethyl,
3-phenylpropyl,
and 2-naphth-2-ylethyl.
The term "aryloxy" as used herein, means an aryl group, as defined herein,
appended
to the parent molecular moiety through an oxygen atom. Representative examples
of aryloxy
include, but are not limited to, phenoxy, naphthyloxy, 3-bromophenoxy, 4-
chlorophenoxy, 4-
methylphenoxy, and 3,5-dimethoxyphenoxy.
The term "arylthio" as used herein, means an aryl group, as defined herein,
appended
to the parent molecular moiety through a sulfur atom. Representative examples
of arylthio
CA 02743534 2011-06-13
include, but are not limited to, phenylsulfanyl, naphth-2-ylsulfanyl, and 5-
phenyihexylsulfanyl.
The term "carbonyl" as used herein, means a -C(O)- group.
The term "carboxy" as used herein, means a -CO2H group.
The term "carboxyalkyl" as used herein, means a carboxy group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of carboxyalkyl include, but are not limited to,
carboxymethyl, 2-
carboxyethyl, and 3-carboxypropyl.
The term "cyan.o" as used herein, means a -CN group.
The term "cyanoalkyl" as used herein, means a cyano group, as defined herein,,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of cyanoalkyl include, but are not limited to,
cyanomethyl, 2-
cyanoyethyl, and 3-cyanopropyl.
The term "cycloalkyl" as used herein, means a monocyclic, bicyclic, or
tricyclic ring
system. Monocyclic ring systems are exemplified by a saturated cyclic
hydrocarbon group
containing from 3 to 8 carbon atoms. Examples of monocyclic ring systems
include
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl,
Bicyclic ring
systems are exemplified by a bridged monocyclic ring system in which two non-
adjacent
carbon atoms of the monocyclic ring are linked by an alkylene bridge of
between one and
three additional carbon atoms. Representative examples of bicyclic ring
systems include, but
are not limited to, bicyclo[3.1.1]heptane, bicyelo[2.2.1]heptane,
bicyclo[2.2.2]octane,
bicyclo[3.2.2]nonane, bicyclo[3.3.1]nonane, and bicyclo[4.2.1]nonane.
Tricyclic ring
systems are exemplified by a bicyclic ring system in which two non-adjacent
carbon atoms of
the bicyclic ring are linked by a bond or an alkylene bridge of between one
and three carbon
atoms. Representative examples of tricyclic-ring systems include, but are not
limited to,
tricyclo[3.3.1.03'7]nonane and tricyclo[3.3.1.13'7]decane (adamantyl).
The cycloalkyl groups of this invention can be substituted with 1, 2, 3, 4 or
5
substituents independently selected from alkenyl, alkoxy, alkoxyalkoxy,
alkoxyalkyl,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonylalkyl,
alkylcarbonyloxy, alkylsulfonyl, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano,
cyanoalkyl, formyl, haloalkoxy, haloalkyl, haloalkylthio, halogen, hydroxy,
hydroxyalkyl,
mercapto, mercaptoalkyl, nitro, -NZCZD, (NZcZD)alkyl, (NZcZD)carbonyl,
26
CA 02743534 2011-06-13
(NZcZD)carbonylalkyl, (NZcZn)sulfonyl, -NRAS(O)2RB, -S(O)20RA, and -S(O)2RA
wherein
RA and RB are as defined herein. Representative examples include, but are not
limited to,
6,6-dinethylbicyclo[3.1.1]heptyl, 6,6-dimethylbieyclo[3.1.1]hept-2-yl, 4-tcrt-
butylcyclohexyl, and 4-(trifluoromethyl)cyclohexyl.
The term "cycloalkylalkyl" as used herein, means a cycloalkyl group, as
defined
herein, appended to the parent molecular moiety through an alkyl group, as
defined herein.
Representative examples of cycloalkylalkyl include, but are not limited to,
cyclopropylmethyl, 2-cyclobutylethyl, cyclopentylmethyl, cyclohexyhnethyl, and
4-cycloheptylbutyl.
The term "cycloalkylene" as used herein, means a divalent group derived from a
cycloalkyl group, as defined herein. Representative examples of cycloalkylene
include, but
are not limited to
and
The term "ethylenedioxy" as used herein, means a -O(CH2)20- group wherein the
oxygen atoms of the ethylenedioxy group are attached to the parent molecular
moiety through
one carbon atom forming a 5 membered ring or the oxygen atoms of the
ethylenedioxy group
are attached to the parent molecular moiety through two adjacent carbon atoms
forming a six
membered ring.
The term "formyl" as used herein, means a -C(O)H group.
The term "formylalkyl" as used herein, means a formyl group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein. .
Representative examples of formylalkyl include, but are not limited to,
formylmethyl and 2-
formylethyl.
The term "halo" or "halogen" as used herein, means -Cl, -Br, -I or -F.
The term "haloalkoxy" as used herein, means at least one halogen, as defined
herein,
appended to the parent molecular moiety through an alkoxy group, as defined
herein.
Representative examples of haloalkoxy include, but are not limited to,
chloromethoxy, 2-
fluoroethoxy, trifluoromethoxy, 2-chloro-3-fluoropentyloxy, and
pentafluoroethoxy.
The term "haloalkyl" as used herein, means at least one halogen, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
27
CA 02743534 2011-06-13
Representative examples of haloalkyl include, but are not limited to,
chloromethyl, 2-
fluoroethyl, trifluoromethyl, pentafluoroethyl, and 2-chloro-3-fluoropentyl.
The term "haloalkylthio" as used herein, means at least one halogen, as
defined
herein, appended to the parent molecular moiety through an alkylthio group, as
defined
herein. Representative examples of haloalkylthio include, but are not limited
to,
trifluoromethylthio.
The term "heterocycle" or "heterocyclic" as used herein, means a monocyclic,
bicyclic, or tricyclic ring system. Monocyclic ring systems are exemplified by
any 3- or 4-
membered ring containing a heteroatom independently selected from oxygen,
nitrogen and
sulfur; or a 5-, 6- or 7-membered ring containing one, two or three
heteroatoms wherein the
heteroatoms are independently selected from nitrogen, oxygen and sulfur. The 5-
membered
ring has from 0-2 double bonds and the 6- and 7-membered ring have from 0-3
double bonds.
Representative examples of monocyclic ring systems include, but are not
limited to,
azetidinyl, azepanyl, aziridinyl, diazepinyl, 1,3-dioxolanyl, dioxanyl,
dithianyl, furyl,
imidazolyl, imidazolinyl, imidazolidinyl, isothiazolyl, isothiazolinyl,
isothiazolidinyl,
isoxazolyl, isoxazolinyl, isoxazolidinyl, morpholinyl, oxadiazolyl,
oxadiazolinyl,
oxadiazolidinyl, oxazolyl, oxazolinyl, oxazolidinyl, piperazinyl, piperidinyl,
pyranyl,
pyrazinyl, pyrazolyl, pyrazolinyl, pyrazolidinyl, pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl,
pyrrolinyl, pyrrolidinyl, tetrahydrofuranyl, tetrahydrothienyl, tetrazinyl,
tetrazolyl,
thiadiazolyl, thiadiazolinyl, thiadiazolidinyl, thiazolyl, thiazolinyl,
thiazolidinyl, thienyl,
thiomorpholinyl, 1,1-dioxidothiomorpholinyl (thiomorpholine sulfone),
thiopyranyl, triazinyl,
triazolyl, and trithianyl. Bicyclic ring systems are exemplified by any of the
above
monocyclic ring systems fused to an aryl group as defined herein, a cycloalkyl
group as
defined herein, or another monocyclic ring system. Additionally, bicyclic ring
systems are
exemplified by a bridged monocyclic ring system in which two non-adjacent
carbon atoms of
the monocyclic ring system are linked by an alkylene group. Representative
examples of
bicyclic ring systems include, but are not limited to, 2-
azabicyclo[2.2.1]heptyl,
8-azabicyclo[3.2.1]octyl, benzimidazolyl, benzodioxinyl, benzothiazolyl,
benzothienyl,
benzotriazolyl, benzoxazolyl, benzofuranyl, benzopyranyl, benzothiopyranyl,
cinnolinyl,
indazolyl, indolyl, 2,3-dihydroindolyl, indolizinyl, naphthyridinyl,
isobenzofuranyl,
isobenzothienyl, isoindolyl, isoquinolinyl, phthalazinyl, pyranopyridinyl,
quinolinyl,
quinolizinyl, quinoxalinyl, quinazolinyl, tetrahydroisoquinolinyl,
tetrahydroquinolinyl, and
28
CA 02743534 2011-06-13
thiopyranopyridinyl. Tricyclic rings systems are exemplified by any of the
above bicyclic
ring systems fused to an aryl group as defined herein, a cycloalkyl group as
defined herein, or
a monocyclic ring system. Representative examples of tricyclic ring systems
include, but are
not limited to, acridinyl, carbazolyl, carbolinyl, dibenzo[b,d]furanyl,
dibenzo[b,d]tluenyl,
naphtho[2,3-b]furan, naphtho[2,3-bjthienyl, phenazinyl, phenothiazinyl,
phenoxazinyl,
thianthrenyl, thioxanthenyl and xanthenyl.
The heterocycles of this invention can be substituted with 1, 2,or 3
substituents
independently selected from alkenyl, alkoxy, alkoxyalkoxy, a.lkoxyalkyl,
alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonylalkyl,
alkylcarbonyloxy,
alkylsulfonyl, alkylthio, alkenyl, arylalkyl, aryloxy, arylthio, carboxy,
carboxyalkyl, cyano,
cyanoalkyl, cycloalkyl, cycloalkylalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl,
haloalkylthio, halogen, hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, nitro,
oxo, -NZCZD,
(NZCZD)alkyl, (NZcZD)carbonyl, (NZcZD)carbonylalkyl, (NZcZD)sulfonyl, -
NRAS(0)2RB, -
S(O)20RA and -S(O)2RA wherein RA and RB are as defined herein. The
heterocycles of this
invention can be further substituted with any one of an additional aryl,
arylalkyl, aryloxy,
arylthio, heterocycle, heterocyclealkyl, heterocycleoxy, or heterocyclethio
group, as defined
herein, wherein the additional aryl, arylalkyl, aryloxy, arylthio,
heterocycle, heterocyclealkyl,
heterocycleoxy, and heterocyclethio group can be substituted with 1, 2, or 3
substituents
independently selected from alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonylalkyl,
alkylcarbonyloxy,
alkylsulfonyl, allcylthio, alkynyl, carboxy, carboxyalkyl, cyano, cyanoalkyl,
cycloalkyl,
cycloalkylalkyl, ethylenedioxy, formyl, formylalkyl, haloalkoxy, h.aloalkyl,
haloalkylthio,
halogen., hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, nitro, -NZCZD,
(NZCZD)alkyl,
(NZCZD)carbonyl, (NZCZD)carbonylalkyl, (NZCZD)sulfonyl, -NRAS(O)2RB, -S(O)20RA
and
-S(O)2RA wherein RA and RB are as defined herein. Representative examples
include, but are
not limited to, 2,6-dimethylmorpholinyl, 4-(3-chlorophenyl)-1-pip erazinyl, 4-
(3,4-
dimethylphenyl)-1-piperazinyl, 4-(4-chlorophenyl)-1-piperazinyl, 4-(4-
methylphenyl)-3-
methyl-l-piperazinyl, 4-(2,3-dimethylphenyl)-1-piperazinyl, 4-(2,3-
dichlorophenyl)-1-
piperazinyl, 4-(3,4-dichlorophenyl)-1-pip erazinyl, 4-[3-
(trifluoromethyl)phenyl]-l-
piperazinyl, 4-(4-bromophenyl)-1-piperazinyl, 4-[4-(trifluoromethyl)-2-
pyridinyl]-1-
piperazinyl, 2-oxo-l-pyrrolidinyl, 5-(trifluoromethyl)-2-pyridinyl, 6-
(trifluoromethyl)-3-
pyridinyl.
29
CA 02743534 2011-06-13
The term "heterocyclealkyl" as used herein, means a heterocycle, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of heterocyclealkyl include, but are not limited to,
pyridin-3-
ylmethyl and 2-pyrimidin-2-ylpropyl.
The term "heterocycleoxy" as used herein, means a heterocycle group, as
defined
herein, appended to the parent molecular moiety through an oxygen atom.
Representative
examples of heterocycleoxy include, but are not limited to, pyridin-3-yloxy
and quinolin-3-
yloxy.
The term "heterocyclethio" as used herein, means a heterocycle group, as
defined
herein, appended to the parent molecular moiety through a sulfur atom.
Representative
examples of heterocyclethio include, but are not limited to, pyridin-3-
ylsulfanyl and quinolin-
3-ylsulfanyl.
The teen "hydroxy" as used herein, means an -OH group.
The term "hydroxyalkyl" as used herein, means at least one hydroxy group, as
defined
herein, appended to the parent molecular moiety through an alkyl group, as
defined herein.
Representative examples of hydroxyalkyl include, but are not limited to,
hydroxymethyl, 2-
hydroxyethyl, 3-hydroxypropyl, 2,3-dihydroxypentyl, and 2-ethyl-4-
hydroxyheptyl.
The term "mercapto" as used herein, means a -SH group.
The term "mercaptoalkyl" as used herein, means a mercapto group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein,
Representative examples of mercaptoalkyl include, but are not limited to, 2-
mercaptoethyl
and 3-mercaptopropyl.
The term "methylenedioxy" as used herein, means a -OCHZO- group wherein the
oxygen atoms of the methylenedioxy are attached to the parent molecular moiety
through two
adjacent carbon atoms.
The term "nitro" as used herein, means a -NO2 group.
The term "-NZAZB" as used herein, means two groups, ZA and ZB, which are
appended to the parent molecular moiety through a nitrogen atom. ZA and ZB are
each
independently selected from hydrogen, alkyl, alkylcarbonyl, formyl, aryl and
arylalkyl.
Representative examples of -NZAZB include, but are not limited to, amino,
methylamino,
acetylamino, benzylamino, phenylamino, and acetylmethylamino.
CA 02743534 2011-06-13
The teen "(NZAZB)alkyl" as used herein, means a -NZAZB group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of (NZAZB)alkyl include, but are not limited to,
anninomethyI, 2-
(methylamino)ethyl, 2-(dimethylamino)ethyl and (ethylmethylarnino)methyl.
The term "(NZAZB)alkylcarbonyl" as used herein, means a (NZAZB)alkyl group, as
defined herein, appended to the parent molecular moiety through a carbonyl
group, as defined
herein. Representative examples of (NZAZB)alkylearbonyl include, but are not
limited to,
d.imethylaminomethylcarbonyl, 2-(dimethylamino)ethylcarbonyl, and
(ethylmethylamino)methylcarbonyl.
The term "(NZAZB)carbonyl" as used herein, means a -NZAZB group, as defined
herein, appended to the parent molecular moiety through a carbonyl group, as
defined herein.
Representative examples of (NZAZB)carbonyl include, but are not limited to,
aminocarbonyl,
(methylamino)carbonyl, (dim ethylam no)carbonyl and (ethylmethyl
amino)carbonyl.
The tern "(NZAZB)carbonylalkyl " as used herein, means a (NZAZB)carbonyl
group,
as defined herein, appended to the parent molecular moiety through an alkyl
group, as
defined herein. Representative examples of (NZAZB)carbonylalkyl include, but
are not
limited to, (aminocarbonyl)methyl, 2-((methylan-tino)carbonyI) ethyl and
((dimethylamino)carbonyl)methyl.
The term "( NZAZB)sulfonyl" as used herein, means a -NZAZB group, as defined
herein, appended to the parent molecular moiety through a sulfonyl group, as
defined herein.
Representative examples of (NZAZB)sulfonyl include, but are not limited to,
aminosulfonyl,
(methylainino)sulfonyl, (dimethylamino)sulfonyl and
(ethylmethylamino)sulfonyl.
The term "-NZAZB" as used herein, means two groups, ZA and ZB, which are
appended to the
parent molecular moiety through a nitrogen atom. ZA and ZB are each
independently selected
from hydrogen, alkyl, alkylcarbonyl, formyl, aryl and arylalkyl.
Representative examples of
-NZAZB include, but are not limited to, amino, methylamino, acetylamino,
benzylainino,
phenylamino, and acetylmethylamino.
The term "-NZCZD" as used herein, means two groups, ZC and ZD, which are
appended to the parent molecular moiety through a nitrogen atom. Zc and ZD are
each
independently selected from hydrogen, alkyl, alkylcarbonyl, formyl, aryl and
arylalkyl.
Representative examples of-NZcZD include, but are not limited to, amino,
methylamino,
acetylamino, benzylamino, phenylamino, and acetylmethylamino.
31
CA 02743534 2011-06-13
The term " (NZCZD)alkyl" as used herein, means a -NZcZD group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of (NZcZD)alkyl include, but are not limited to,
aminomethyl, 2-
(methylamino)ethyl, 2-(dimethylamino)ethyl and (ethylmethylamino)methyl.
The term "(NZcZD)carbonyl" as used herein, means a -NZcZD group, as defined
herein, appended to the parent molecular moiety through a carbonyl group, as
defined herein.
Representative examples of (NZcZD)carbonyl include, but are not limited to,
aminocarbonyl,
(methylamino)carbonyl, (dimethylamino)carbonyl and (ethylmethylamino)carbonyl.
The term "(NZcZD)carbonylalkyl " as used herein, means a (NZcZD)carbonyl
group,
as defined herein, appended to the parent molecular moiety through an alkyl
group, as
defined herein. Representative examples of (NZcZD)carbonylalkyl include, but
are not
limited to, (aminocarbonyl)methyl, 2-((methylamino)carbonyl.)ethyl and
((dimethylaniino)carbonyl)methyl.
The term "(NZcZD)sulfonyl" as used herein, means a -NZcZD group, as defined
herein, appended to the parent molecular moiety through a sulfonyl group, as
defined herein.
Representative examples of (NZcZD)sulfonyl include, but are not limited to,
aminosulfonyl,
(methylamino)sulfonyl, (dimethylamino)sulfonyl and (ethylmethylamino)sulfonyl.
The term "oxo" as used herein, means =O.
The term "sulfonyl" as used herein, means a -S(O)2- group.
In Vitro Data
Determination of Inhibition Potencies
Dulbecco's modified Eagle medium (D-MEM)(with 4.5 mg/mL glucose) and fetal
bovine serum were obtained from Hyclone Laboratories, Inc. (Logan, Utah).
Dulbecco's
phosphate-buffered saline (D-PBS)(with 1 mg/mL glucose and 3.6 mg/l Na
pyruvate)(without phenol red), L-glutamine, hygromycin B, and LipofectamineTM
were
obtained from Life Technologies (Grand Island, NY). G418 sulfate was obtained
from
Calbiochem-Novabiochem Corp. (San Diego, CA)_ Capsaicin (8-methyl-N-vanillyl-6-
nonenamide) was obtained from Sigma-Aldrich, Co. (St. Louis, MO). Fluo-4 AM (N-
[4-[6-
[(acetyloxy)methoxy]-2,7-difluoro-3-oxo-3H-xanthen-9-yl]-2-[2-[2-[bis[2-
[(acetyloxy)methoxy]-2-oxyethyl]amino]-5-methylphenoxy]ethoxy]phenyl]-N-[2-
[(acetyloxy)methoxy]-2-oxyethyl]-glycine, (acetyloxy)methyl ester) was
purchased from
Molecular Probes (Eugene, OR).
32
CA 02743534 2011-06-13
The cDNAs for the human VRl receptor were isolated by reverse transcriptase-
polymerase chain reaction (RT-PCR) from human small intestine poly A+RNA
supplied by
Clontech (Palo Alto, CA) using primers designed surrounding the initiation and
termination
codons identical to the published sequences (Hayes et al. Pain 88: 205-215,
2000). The
resulting cDNA PCR products were subcloncd into pCIneo mammalian expression
vector
(Promega `111) and fully sequenced using fluorescent dye-terminator reagents
(Prism, Perkin-
Elmer Applied Biosystems Division) and a Perkin-Elmer Applied Biosystems Model
373
DNA sequencer or Model 310 genetic analyzer. Expression plasniids encoding the
hVR1
eDNA were transfected individually into 1321N1 human astrocytoma cells using
LipofectamnineTM. Forty-eight hours after transfection, the neomycin-resistant
cells were
selected with growth medium containing 800 rg/ nL GeneucinTM (Gibco BRL).
Surviving
individual colonies were isolated. and screened for VR1 receptor activity.
Cells expressing
recombinant homomeric VRl receptors were maintained at 37 C in D-MEM
containing 4
mM L-glutamine, 300 g/mL G418 (Cal-biochem) and 10% fetal bovine serum under
a
humidified 5% CO2 atmosphere.
The functional activity of compounds at the VRI receptor was determined with a
Ca2+
influx assay and measurement of intracellular Ca2+ levels ([Ca2+]i). All
compounds were
tested over an I 1-point half-log concentration range. Compound solutions were
prepared in
D-PBS (4x final concentration), and diluted serially across 96-well v-bottom
tissue culture
plates using aBiomek 2000 robotic automation workstation (Beckman-Coulter,
Inc.,
Fullerton, CA). A 0.2 M solution of the VR1 agonist capsaicin was also
prepared in D-
PBS. The fluorescent Ca2+ chelating dye fluo-4 was used as an indicator of the
relative levels
of [Caz+]i in a 96-well format using a Fluorescence Imaging Plate Reader
(FLIPRINI)(Molecular
Devices, Sunnyvale, CA). Cells were grown to confluency in 96-well black-
walled tissue
culture plates. Then, prior to the assay, the cells were loaded with 100 L
per well of fluo-4
AM (2 M, in D-PBS) for 1-2 hours at 23 C. Washing of the cells was performed
to remove
extracellular fluo-4 AM (2 x 1 mL D-PBS per well), and afterward, the cells
were placed in
the reading chamber of the FLIPRTM instrument. 50 gL of the compound solutions
were added
to the cells at the 10 second time mark of the experimental run. Then, after a
3 minute time
delay, 50 L of the capsaicin solution was added at the 190 second time mark
(0.05 [.iM final
concentration)(final volume = 200 L) to challenge the VRI receptor. Time
length of the
experimental nun was 240 seconds. Fluorescence readings were made at 1 to 5
second
33
CA 02743534 2011-06-13
intervals over the course of the experimental run. The peak increase in
relative fluorescence
units (minus baseline) was calculated from the 190 second time mark to the end
of the
experimental run, and expressed as a percentage of the 0.05 M capsaicin
(control) response.
Curve-fits of the data were solved using a four-parameter logistic Hill
equation in GraphPad
Prism (GraphPad Software, Inc., San Diego, CA), and IC5o values were
calculated.
The compounds of the present invention were found to be antagonists of the
vanilloid
receptor subtype 1 (VR1) receptor with IC5o, from 1000 nM to 0.1 nM. In a
preferred range,
compounds tested had IC5o, from 500 nM to 0.1 nM. In a more preferred range,
compounds
tested had IC5o, from 50 nM to 0.1 nM.
In Vivo Data
Determination of Antinociceptive Effect
Experiments were performed on 400 adult male 129J mice (Jackson laboratories,
Bar
Harbor, ME), weighing 20-25 g. Mice were kept in a vivarium, maintained at 22
C, with a
12 hour alternating light-dark cycle with food and water available ad libitum.
All
experiments were performed during the light cycle. Animals were randomly
divided into
separate groups of 10 mice each. Each animal was used in one experiment only
and was
sacrificed immediately following the completion of the experiment. All animal
handling and
experimental procedures were approved by an IACUC Committee.
The antinociceptive test used was a modification of the abdominal constriction
assay
described in Collier, et at., Br. J. Pharmacol. Chemother. 32 (1968) 295-3 10.
Each animal
received an intraperitoneal (i.p.) injection of 0.3 mL of 0.6% acetic acid in
normal saline to
evoke writhing. Animals were placed separately under clear cylinders for the
observation
and quantification of abdominal constriction. Abdominal constriction was
defined as a mild
constriction and elongation passing caudally along the abdominal wall,
accompanied by a
slight twisting of the trunk and followed by bilateral extension of the hind
limbs. The total
number of abdominal constrictions was recorded from 5 to 20 minutes after
acetic acid
injection. The ED50, were determined based on the i.p. injection.
The compounds of the present invention tested were found to have
antinocieeptive
effects with ED505 from 1 mg/kg to 500 mg/kg.
The in vitro and in vivo data demonstrates that compounds of the present
invention
antagonize the VRI receptor and are useful for treating pain.
34
CA 02743534 2011-06-13
Compounds of the present invention, as VRI antagonists, are also useful for
ameliorating or preventing additional disorders that are affected by the VRI
receptors such
as, but not limited to, infammatory thermal hyperalgesia, bladder
overactivity, and urinary
incontinence.
Compounds of the present invention, including but not limited to those
specified in
the examples, can be used to treat pain as demonstrated by Nolano, M. et al.,
Pain 81 (1999)
135; Caterina, M.J. and Julius, D., Annu. Rev. Neurosci. 24, (2001) 487-517;
Caterina, M.J.
et al., Science 288 (2000) 306-313; Caterina, M.J. et al., Nature 389 (1997)
816-824.
Compounds of the present invention, including but not limited to those
specified in
the examples, can be used to treat bladder overactivity and/or urinary
incontinence as
demonstrated by Fowler, C. Urology 55 (2000) 60.
Compounds of the present invention, including but not limited to those
specified in
the examples, can be used to treat inflammatory thermal hyperalgesia as
demonstrated by
Davis, J. et al., Nature 405 (2000) 183-187.
The present invention also provides pharmaceutical compositions that comprise
compounds of the present invention. The pharmaceutical compositions comprise
compounds
of the present invention that may be formulated together with one or more non-
toxic
pharmaceutically acceptable carriers.
The pharmaceutical compositions of this invention can be administered to
humans
and other mammals orally, rectally, parenterally , intraeisternally,
iutravaginally,
intraperitoneally, topically (as by powders, ointments or drops), bucally or
as an oral or nasal
spray. The term "parenterally," as used herein, refers to modes of
administration which
include intravenous, intramuscular, intraperitoneal, intrastemal, subcutaneous
and
intraarticular injection and infusion.
The term "pharmaceutically acceptable carrier," as used herein, means a non-
toxic,
inert solid, semi-solid or liquid filler, diluent, encapsulating material or
formulation auxiliary
of any type. Some examples of materials which can serve as pharmaceutically
acceptable
carriers are sugars such as, but not limited to, lactose, glucose and sucrose;
starches such as,
but not limited to, corn starch and potato starch; cellulose and its
derivatives such as, but not
limited to, sodium carboxymethyl cellulose, ethyl cellulose and cellulose
acetate; powdered
tragacanth; malt; gelatin; talc; excipients such as, but not limited to, cocoa
butter and
suppository waxes; oils such as, but not limited to, peanut oil, cottonseed
oil, safflower oil,
CA 02743534 2011-06-13
sesame oil, olive oil, corn oil and soybean oil; glycols; such a propylene
glycol; esters such
as, but not limited to, ethyl oleate and ethyl laurate; agar; buffering agents
such as, but not
limited to, magnesium hydroxide and aluminum hydroxide; alginic acid; pyrogen-
free water;
isotonic saline; Ringer's solution; ethyl alcohol, and phosphate buffer
solutions, as well as
other non-toxic compatible lubricants such as, but not limited to, sodium
lauryl sulfate and
magnesium stearate, as well as coloring agents, releasing agents, coating
agents, sweetening,
flavoring and perfuming agents, preservatives and antioxidants can also be
present in the
composition, according to the judgment of the formulator.
Pharmaceutical compositions of this invention for parenteral injection
comprise
pharmaceutically acceptable sterile aqueous or nonaqueous solutions,
dispersions,
suspensions or emulsions as well as sterile powders for reconstitution into
sterile injectable
solutions or dispersions just prior to use. Examples of suitable aqueous and
nonaqueous
carriers, diluents, solvents or vehicles include water, ethanol, polyols (such
as glycerol,
propylene glycol, polyethylene glycol and the like), vegetable oils (such as
olive oil),
injectable organic esters (such as ethyl oleate) and suitable mixtures
thereof. Proper fluidity
can be maintained, for example, by the use of coating materials such as
lecithin, by the
maintenance of the required particle size in the case of dispersions and by
the use of
surfactants.
These compositions may also contain adjuvants such as preservatives, wetting
agents,
emulsifying agents and dispersing agents. Prevention of the action of
microorganisms can be
ensured by the inclusion of various antibacterial and antifungal agents, for
example, paraben,
chlorobutanol, phenol sorbic acid and the like. It may also be desirable to
include isotonic
agents such as sugars, sodium chloride and the like. Prolonged absorption of
the injectable
pharmaceutical form can be brought about by the inclusion of agents which
delay absorption
such as aluminum monostearate and gelatin.
In some cases, in order to prolong the effect of the drug, it is desirable to
slow the
absorption of the drug from subcutaneous or intramuscular injection. This can
be
accomplished by the use of a liquid suspension of crystalline or amorphous
material with
poor water solubility. The rate of absorption of the drug then depends upon
its rate of
dissolution which, in turn, may depend upon crystal size and crystalline form.
Alternatively,
delayed absorption of a parenterally administered drug form is accomplished by
dissolving or
suspending the drug in an oil vehicle.
36
CA 02743534 2011-06-13
Injectable depot forms are made by forming microencapsule matrices of the drug
in
biodegradable polymers such as polylactide-polyglycolide. Depending upon the
ratio of drug
to polymer and the nature of the particular polymer employed, the rate of drug
release can be
controlled. Examples of other biodegradable polymers include poly(orthoesters)
and
poly(anhydrides). Depot injectable formulations are also prepared by
entrapping the drug in
liposomes or microemulsions which are compatible with body tissues.
The injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter or by incorporating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile injectable
medium just prior to use.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders
and granules. In such solid dosage forms, the active compound may be mixed
with at least
one inert, pharmaceutically acceptable excipient or carrier, such as sodium
citrate or
dicalcium phosphate and/or a) fillers or extenders such as starches, lactose,
sucrose, glucose,
mannitol and silicic acid; b) binders such as carboxymethylcellulose,
alginates, gelatin,
polyvinylpyrrolidone, sucrose and acacia; c) humectants such as glycerol; d)
disintegrating
agents such as agar-agar, calcium carbonate, potato or tapioca starch, alginic
acid, certain
silicates and sodium carbonate; e) solution retarding agents such as paraffin;
f) absorption
accelerators such as quaternary armnonium compounds; g) wetting agents such as
cetyl
alcohol and glycerol monostearate; h) absorbents such as kaolin and bentonite
clay and i)
lubricants such as talc, calcium stearate, magnesium stearate, solid
polyethylene glycols,
sodium lauryl sulfate and mixtures thereof. In the case of capsules, tablets
and pills, the
dosage form may also comprise buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and hard-
filled gelatin capsules using such carriers as lactose or milk sugar as well
as high molecular
weight polyethylene glycols and the like.
The solid dosage forms of tablets, dragees, capsules, pills and granules can
be
prepared with coatings and shells such as enteric coatings and other coatings
well-known in
the pharmaceutical formulating art. They may optionally contain opacifying
agents and may
also be of a composition such that they release the active ingredient(s) only,
or preferentially,
in a certain part of the intestinal tract, optionally, in a delayed manner.
Examples of
embedding compositions which can be used include polymeric substances and
waxes.
37
CA 02743534 2011-06-13
The active compounds can also be in micro-encapsulated form, if appropriate,
with
one or more of the above-mentioned carriers.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, solutions, suspensions, syrups and elixirs. In addition to the
active compounds,
the liquid dosage forms may contain inert diluents commonly used in the art
such as, for
example, water or other solvents, solubilizing agents and emulsifiers such as
ethyl alcohol,
isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl
benzoate, propylene
glycol, 1,3-butylene glycol, dimethyl formamide, oils (in particular,
cottonseed, groundnut,
corn, germ, olive, castor and sesame oils), glycerol, tetrahydrofurfuryl
alcohol, polyethylene
glycols and fatty acid esters of sorbitan and mixtures thereof.
Besides inert diluents, the oral compositions may also include adjuvants such
as
wetting agents, emulsifying and suspending agents, sweetening, flavoring and
perfuming
agents.
Suspensions, in addition to the active compounds, may contain suspending
agents as,
for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and
sorbitan esters,
microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar,
tragacanth and
mixtures thereof.
Compositions for rectal or vaginal administration are preferably suppositories
which
can be prepared by mixing the compounds of this invention with suitable non-
irritating
carriers or carriers such as cocoa butter, polyethylene glycol or a
suppository wax which are
solid at room temperature but liquid at body temperature and therefore melt in
the rectum or
vaginal cavity and release the active compound.
Compounds of the present invention can also be administered in the form of
liposomes. As is known in the art, liposomes are generally derived from
phospholipids or
other lipid substances. Liposomes are formed by mono- or multi-lamellar
hydrated liquid
crystals which are dispersed in an aqueous medium. Any non-toxic,
physiologically
acceptable and metabolizable lipid capable of forming liposomes can be used.
The present
compositions in liposome form can contain, in addition to a compound of the
present
invention, stabilizers, preservatives, excipients and the like. The preferred
lipids are natural
and synthetic phospholipids and phosphatidyl cholines (lecithins) used
separately or together.
Methods to form liposomes are known in the art. See, for example, Prescott,
Ed.,
Methods in Cell Biology, Volume XIV, Academic Press, New York, N.Y. (1976), p.
33 et
38
CA 02743534 2011-06-13
seq.
Dosage forms for topical administration of a compound of this invention
include
powders, sprays, ointments and inhalants. The active compound may be mixed
under sterile
conditions with a pharmaceutically acceptable carrier and any needed
preservatives, buffers
or propellants which may be required. Opthalmic formulations, eye ointments,
powders and
solutions are also contemplated as being within the scope of this invention.
Actual dosage levels of active ingredients in the pharmaceutical compositions
of this
invention can be varied so as to obtain an amount of the active compound(s)
which is
effective to achieve the desired therapeutic response for a particular
patient, compositions and
mode of administration. The selected dosage level will depend upon the
activity of the
particular compound, the route of administration, the severity of the
condition being treated
and the condition and prior medical history of the patient being treated.
When used in the above or other treatments, a therapeutically effective amount
of one
of the compounds of the present invention can be employed in pure form or,
where such
forms exist, in pharmaceutically acceptable salt, ester or prodrug form. The
phrase
"therapeutically effective amount" of the compound of the invention means a
sufficient
amount of the compound to treat disorders, at a reasonable benefit/risk ratio
applicable to any
medical treatment. It will be understood, however, that the total daily usage
of the
compounds and compositions of the present invention will be decided by the
attending
physician within the scope of sound medical judgement. The specific
therapeutically
effective dose level for any particular patient will depend upon a variety of
factors including
the disorder being treated and the severity of the disorder; activity of the
specific compound
employed; the specific composition employed; the age, body weight, general
health, sex and
diet of the patient; the time of administration, route of administration, and
rate of excretion of
the specific compound employed; the duration of the treatment; drugs used in
combination or
coincidental with the specific compound employed; and like factors well known
in the
medical arts.
The compounds of the present invention can be used in the form of
pharmaceutically
acceptable salts derived from inorganic or organic acids. The phrase
"pharmaceutically
acceptable salt" means those salts which are, within the scope of sound
medical judgement,
suitable for use in contact with the tissues of humans and lower animals
without undue
toxicity, irritation, allergic response and the like and are commensurate with
a reasonable
39
CA 02743534 2011-06-13
benefit/risk ratio.
Pharmaceutically acceptable salts are well-known in the art. For example, S.
M.
Berge et al. describe pharmaceutically acceptable salts in detail in (J.
Pharmaceutical
Sciences, 1977, 66: 1 et seq). The salts can be prepared in situ during the
final isolation and
purification of the compounds of the invention or separately by reacting a
free base function
with a suitable organic acid. Representative acid addition salts include, but
are not limited to
acetate, adipate, alginate, citrate, aspartate, benzoate, benzenesulfonate,
bisulfate, butyrate,
camphorate, camphorsulfonate, digluconate, glycerophosphate, hemisulfate,
heptanoate,
hexanoate, fumarate, hydrochloride, hydrobromide, hydroiodide, 2-
hydroxyethansulfonate
(isothionate), lactate, maleate, methanesulfonate, nicotinate, 2-
naphthalenesulfonate, oxalate,
palmitoate, pectinate, persulfate, 3-phenylpropionate, picrate, pivalate,
propionate, succinate,
tartrate, thiocyanate, phosphate, glutamate, bicarbonate, p-toluenesulfonate
and undecanoate.
Also, the basic nitrogen-containing groups can be quaternized with such agents
as lower alkyl
halides such as, but not limited to, methyl, ethyl, propyl, and butyl
chlorides, bromides and
iodides; dialkyl sulfates like dimethyl, diethyl, dibutyl and diamyl sulfates;
long chain halides
such as, but not limited to, decyl, lauryl, myristyl and stearyl chlorides,
bromides and iodides;
arylallcyl halides like benzyl and phenethyl bromides and others. Water or oil-
soluble or
dispersible products are thereby obtained. Examples of acids which can be
employed to form
pharmaceutically acceptable acid addition salts include such inorganic acids
as hydrochloric
acid, hydrobromic acid, sulfuric acid, and phosphoric acid and such organic
acids as acetic
acid, fumaric acid, maleic acid, 4-methylbenzenesulfonic acid, succinic acid
and citric acid.
Basic addition salts can be prepared in situ during the final isolation and
purification
of compounds of this invention by reacting a carboxylic acid-containing moiety
with a
suitable base such as, but not limited to, the hydroxide, carbonate or
bicarbonate of a
pharmaceutically acceptable metal cation or with ammonia or an organic
primary, secondary
or tertiary amine. Pharmaceutically acceptable salts include, but are not
Limited to, cations
based on alkali metals or alkaline earth metals such as, but not limited to,
lithium, sodium,
potassium, calcium, magnesium and aluminum salts and the like and nontoxic
quaternary
ammonia and amine cations including ammonium, tetramethylammonium,
tetraethylammonium, methylamine, dimethylamine, trimethylamine, triethyl
amuie,
diethylamine, ethylamine and the like. Other representative organic amines
useful for the
CA 02743534 2011-06-13
formation of base addition salts include ethylenediamine, ethanolamine,
diethanolamine,
piperidine, piperazii e and the like.
The term "pharmaceutically acceptable prodrug" or "prodrug,"as used herein,
represents those prodrugs of the compounds of the present invention which are,
within the
scope of sound medical judgement, suitable for use in contact with the tissues
of humans and
lower animals without undue toxicity, irritation, allergic response, and the
like,
commensurate with a reasonable benefit/risk ratio, and effective for their
intended use.
Prodrugs of the present invention may be rapidly transformed in vivo to
compounds of
formula (I), for example, by hydrolysis in blood. Representative examples
include, but are
not limited to, methyl 4-{[({[4-(8-azabicyclo[3.2.I)oct-8-yl)-3-
(trifluoromethyl)phenyl]methyl} amino)carbonyl]amino } -1 H-indazole- l -
carboxylate,
ethyl 4-{[({[4-(8-azabicyclo[3.2.1]oct-8-yl)-3-
(trifluoromethyl)phenyl]methyl} ami.no)earbonyl]amino }-1 H-indazole- l-
carboxylate,
tert-butyl 4-[({[4-(8-azabicyclo[3.2.1 ]oct-8-yl)-3-
(trifluoromethyl)benzyl]amino}carbonyl)amino]-1H-indazole-l-carboxylate, tert-
butyl 4-
[({[4-(8-azabicyclo[3.2.1 ]oct-8-yl)-2-chlorobenzyl]amino} carbonyl)amino]-1 H-
indazole-l-
carboxylate, ethyl 4-[( {[4-(8-azabicyclo[3.2.1 ]oct-8-yl)-2-
chlorobenzyl]amino} carbonyl)amino]-IH-indazole-l -carboxylate, and methyl 4-
[({[4-(8-
azabicyclo[3.2.1 ]oct-8-yl)-2-chlorobenzyl]amino } carbonyl)amino]-1 H-
indazole- l -
carboxylate.
The present invention contemplates compounds of formula (I) formed by
synthetic
means or formed by in vivo biotransformation of a prodrug.
The compounds of the invention can exist in unsolvated as well as solvated
forms,
including hydrated forms, such as hemi-hydrates. In general, the solvated
forms, with
pharmaceutically acceptable solvents such as water and ethanol among others
are equivalent
to the unsolvated forms for the purposes of the invention.
The total daily dose of the compounds of this invention administered to a
human or
lower animal may range from about 0.01 to about 100 mg/kg/day. For purposes of
oral
administration, more preferable doses can be in the range of from about 0.1 to
about 25
mg/kg/day. If desired, the effective daily dose can be divided into multiple
doses for
purposes of administration; consequently, single dose compositions may contain
such
amounts or submultiples thereof to make up the daily dose.
41
CA 02743534 2011-06-13
Compounds of the present invention were named by ACD/ChemSketch version 5.0
(developed by Advanced Chemistry Development, Inc., Toronto, ON, Canada) or
were given
names which appeared to be consistent with ACD nomenclature.
Abbreviations
Abbreviations which have been used in the descriptions of the Schemes and the
Examples that follow are: dba for dibenzylideneacetone; DBU for 1,8-
diazabicyclo[5.4.0]undec-7-ene; BINAP for 2,2'-bis(diphenylphosphino)-1,1'-
binaphthyl;
DCC for 1,3-dicyclohexylcarbodiimide; DIEA for diisopropylethylam.ine; DMAP
for 4-
di nethylaminopyridine; DMF for N,N-dimethylformani.ide; DMSO for
dimethylsulfoxide;
EDCI or EDC for 1-ethyl-3-[3-(dimethylamino)propyl]-carbodiimide
hydrochloride; HMPA
for hexamethylphosphoramide; HPLC high pressure liquid chromatography; NBS for
N-
bromosuccinimide; Pd for palladium; Ph for phenyl; psi for pounds per square
inch; and THE
for tetrahydrofuran.
Preparation of Compounds of the Present Invention
The compounds and processes of the present invention will be better understood
in
connection with the following synthetic Schemes and Examples which illustrate
a means by
which the compounds of the present invention can be prepared.
42
CA 02743534 2011-06-13
Scheme I
0
RI NH2 R, HNA,CC13
R2 R7= O R2 R7
N, / *R6 + CV CC13 base Rs
R4 R5 R4 R5
(1) (2)
O H
R1 HNAN"L-R9
(2) + H2N=L.R9 DBU R2 -I Rr
(3) R6
R4 R5
(4)
O
R1 HN0 L,R9
O + HO-L.Rs DBU- R2 I R7
2 -^-
N
(5) R6
R4 R5
(6)
Ureas of general formula (4), wherein RI, R2, R4, R5, R6, R7, R0, and L are as
defined
in formula (I), may be prepared as described in Scheme 1. 5-Aminoisoquinolines
of general
formula (1), purchased commercially or prepared using standard chemistry known
to those in
the art, can be treated with trichloroacetyl chloride and a base such as, but
not limited to,
triethylamine in a solvent such as dichloromethane to provide
trichloroacetamides of general
formula (2). TrichloroacetanZides of general formula (2) can be treated with
amines of
general formula (3) and a non-nucleophilic base such as, but not limited to,
DBU in a solvent
such as, but not limited to, acetonitrile to provide ureas of general formula
(4).
Carbamates of general formula (6), wherein RI, R2, R4, R5, R6, R7, R9 and L
are as
defined in formula (I), may also be prepared as described in Scheme 1.
Trichioroacetamides
of general forniula (2) can be treated with alcohols of general formula (5)
and a non-
nucleophilic base such as, but not limited to, DBU in a solvent such as, but
not limited to,
acetonitrile to provide carbamates of general formula (6).
43
CA 02743534 2011-06-13
Scheme 2
H2N'L`R9 CIC(O)CI OCN R9
O
H
R1 NH2 R1 HNNR9
R2 R7
R2 R7 L` I
I
N + OCN' R9 N
Rs Rs
R4 R5 (g) R4 R5
(1) (4)
Ureas of general formula (4), wherein R1, R2, R4, R5, R6, R7, R9, and L are as
defined
in formula (I), may be prepared as described in Scheme 2. Amines of general
formula (3) can
be treated with phosgene or triphosgene and DMAP in a solvent such as, but not
limited to,
dichloromethane to provide isocyanates of general formula (8). 5-
Aminoisoquinolines of
general formula (1) can be treated with isocyanates of general formula (8) in
a solvent such
as, but not limited to, toluene or YElP or a combination thereof to provide
ureas of general
formula (4).
Scheme 3
R1 NH2 R1 NCO
R2 R7 CIC(O)CI R2 R7
N N
Rs R6
R4 R5 R4 R5
(1) (10)
O
~ L`
R1 HN N' R9
(10) + H2N.L,R9 R2 \ *R7
(3) Rs
R4 R5
(4)
Ureas of general formula (4), wherein R1, R2, R4, R5, R6, R7, R9, and L are as
defined
in formula (I), may be prepared as described in Scheme 3. 5-Aminoisoquinolines
of general
formula (1) can be treated with phosgene or triphosgene and DMAP in a solvent
such as, but
not limited to, dichloromethane to provide isocyanates of general formula
(10). Isocyanates
of general formula (10) can be treated with amines of general formula (3) in a
solvent such
44
CA 02743534 2011-06-13
as, but not limited to, toluene or THE or a combination thereof to provide
ureas of general
formula (4).
Scheme 4
O O
R1 NH2 R1 HNN R9 R1 HNO R9
R2 R7 R2 R7 R2 R7
I Schemes 1-3 I 1
NN Rs N N Rs N,N Rs
R5 R5 R5
(12) (13) (14)
O H O
R1 NH2 R1 HNAN L R9 R1 HNAO'L,R9
R7 R7 \ \ R~
N Schemes 1-3 N N
R3 R6 R3 R6 R3 R6
R4 R5 R4 R5 R4 R5
(15) (16) (17)
Ureas of general formula (13), wherein R1, R2, R5, R6, R7, R9, and L are as
defined in
formula (I), and carbamates of general formula (14), wherein R1, R2i R5, R6,
R7, R9 and L are
as defined in formula (1), may be prepared as described in Scheme 4. 5-
Aminocinnolines of
general formula (12), purchased commercially or prepared using standard
chemistry known
to those in the art, may be processed as described in Schemes 1-3 to provide
ureas of general
formula (13) and carbamates of general formula (14).
Ureas of general formula (16), wherein R1, R3, R4, R5, R6, R7, R9 and L are as
defined
in formula (1), and carbamates of general formula (17), wherein R1, R3, R4,
R5, R6, R7, R9 and
L are as defined in formula (1), may be prepared as described in Scheme 4.
8-Aminoisoquinolines of general formula (15), purchased commercially or
prepared using
standard chemistry known to those in the art, may be processed as described in
Schemes 1-3
to provide ureas of general formula (16) and carbamates of general formula
(17).
CA 02743534 2011-06-13
Scheme 5
I0' 0
R NH2 )H L,
R7 R, HN N~ R9 RZ HN~0R9
R Schemes 1-3 R7 R7
N R6 R2 N R2
R3 R5 , R6 I R6
(19) R3 R5 R3 R5
(20) (21)
O 0
NH2 ~H,
Ri HN N L, R9 R1 HN R9
N ~OrL N \ R7 Schemes 1-3 N R, R7 N R7
R6 N 'N
R3 R5 , R6 Rs
(22) R3 R5 R3 R5.
(23) (24)
Ureas of general formula (20), wherein R1, R2, R3, R5, R6, R7, R9, and L are
as defined
in formula (I), and carbamates of general formula (21), wherein R1, R2, R3,
R5, R6, R7, R9,
and L are as defined in formula (I), maybe prepared as described in Scheme 5.
4-Aminoindoles of general formula (19), purchased commercially or prepared
using standard
chemistry known to those in the art, may be processed as described in Schemes
1-3 to
provide ureas of general formula (20) and carbamates of general formula (21).
Ureas of general formula (23), wherein R1, R3, R5, R6, R7, R9 and L are as
defined in
formula (1), and carbamates of general formula (24), wherein R1, R3, R5, R6,
R7, R9, and L are
as defined in formula (I), may be prepared as described in Scheme 5. 4-
Aminoindazoles of
general formula (22), purchased commercially or prepared using standard
chemistry known
to those in the art, may be processed as described in Schemes 1-3 to provide
areas of general
formula (23) and carbamates of general formula (24).
46
CA 02743534 2011-06-13
Scheme 6
0
R1 NH2 R1 Br R1O OEt
R2 R7 R2 R7 R2 R7
N NBS (C(O)OEt)2
R6 R6 Rs
R4 R5 R4 R5 R4 R5
(1) (27) (28)
0 O O
ZRjOEt R10 OEt
Reducing R2 R7 R2 R7
Agent N CIC(O)CH3 N
(28) Rs R6
R4 R5 R4 R5
(29) (30)
O O
H
R1 oEt R1 N L'R9
10% PD/C R2 R7 H2N R9 R2 R7
TEA, H2 (3) N N
(30) / R6 R6
R4 R5 R4 R5
(31) (32)
O
R1 O- R9
HO~L'R9 R2 R7
(5) i
(31) N R6
R4 R5
(33)
Amides of general formula (32), wherein Rl, R2, R4, R5, R6, R7, R9, and L are
as
defined in formula (1), can be prepared as described in Scheme 6. Amines of
general formula
(1) can be treated with an acid such as, but not limited to, concentrated
sulfuric acid and
N-bromosuccinimide to provide bromides of general formula (27). Bromides of
general
formula (27) can be treated with an organolithium reagent such as, but not
limited to, n-
butyllithiurn and diethyl oxalate in a solvent such as, but not limited to,
THE to provide keto
esters of general formula (28). Keto esters of general formula (28) can be
treated with a
reducing agent such as, but not limited to, 10% Pd/C under a hydrogen
atmosphere (50 psi) in
a solvent such as, but not limited to, ethanol to provide hydroxy esters of
general formula
47
CA 02743534 2011-06-13
(29). Hydroxy esters of general formula (29) can be treated with an acid
chloride such as, but
not limited to, acetyl chloride in a solvent such as, but not limited to,
pyridine to provide
desters of general formula (30). Diesters of general formula (30) can be
treated with 10%
Pd/C and a base such as, but not limited to, triethylamine tinder a hydrogen
atmosphere (60
psi) in a solvent such as, but not limited to, ethanol to provide esters of
general formula (31).
Esters of general formula (31) can be treated with amines of general formula
(3) to provide
amides of general formula (32). Alternatively, esters of general formula (31)
can be treated
with aqueous base such as, but not limited to, aqueous sodium hydroxide or
aqueous
potassium hydroxide to provide the acids which can then be converted into
amides of general
formula (32) by treatment with amines of general formula (3) under standard
DCC or EDCI
coupling procedures that are well known in the art.
Esters of general formula (33), wherein RI, R2, R4, R5, R6, R7, R9 and L are
as defined
in formula (I), can be prepared as described in Scheme 6. Esters of general
formula (31) can
be treated with alcohols of general formula (5) under standard
transesterification conditions
well known to those of skill in the art to provide esters of general formula
(33).
48
CA 02743534 2011-06-13
Scheme 7
NO2 NO2 NO9
R7 NaNO 2 N~ R7 ROC(O)CI Nom` R7
H2N _R6 - - / .Ro fl R6
R5 H R R=alkyl R
O O 5
(35) (36) 1
R (37)
0
NH2 HN-~-N L^R9
PdIC R7 H
H N I 1) CIC(O)CI R7
N
N R6
R
(37) O/~O R5 2) H2N.L R9 6
R (38) (3) R O 5
(39)
O
HN,JI-N -L 'R9
H
MeOH/NaOH / R7
(39) N
N R6
H R5
(40)
Indazoles of general formula (39) and indazoles of general formula (40),
wherein L,
R5, R6, R7, and R9 are as defined in formula (1) and R is alkyl as defined
herein, can be
prepared as described in Scheme 7. Nitro anilines of general formula (35) can
be treated with
sodium nitrite and an acid including, but not limited to, acetic acid in water
to provide
indazoles of general formula (36). Indazoles of general formula (36) can be
treated with
chloroformates to provide indazoles of general formula (37). Indazoles of
general formula
(37) can be treated with a transition metal catalyst including, but not
limited to, palladium on
carbon under a hydrogen atmosphere (about I atm to about 60 atm) to provide
indazoles of
general formula (38). Indazoles of general formula (38) can be processed as
described in
Scheme 1-3 to provide indazoles of general formula (39). Indazoles of general
formula (39)
can be treated with a base including, but not limited to, sodium hydroxide or
potassium
hydroxide to provide indazoles of general formula (40).
49
CA 02743534 2011-06-13
Scheme 8
NO 2 NO2 NH2
Pd/C
N, R7 ROCOCI N I R7 H2 Nr R7
N - R6 DBU N - R6 'N R6
R5 O-111O R5 O O R5
(42) (43) 'R (44)
N
O
O
O O
HN~O
(44) + N"OYO,N R7
O O N R6
O~O R5
(45)
LR9 LR9
\--0
H2N R9 HN HN
(45) (3) Ni I R7 OH- Ni I R7
H
N R6 N / R6
O O R5 R5
R (46) (47)
Indazoles of general formula (46) and indazoles of general formula (47),
wherein L,
R5, R6, R7, and R9 are as defined in formula (1) and R is alkyl as defined
herein, can be
prepared as described in Scheme 8. Nitro indazoles of general formula (42),
purchased or
prepared using chemistry known in the art, can be treated with chloroformates
and a non-
nucleophilic base such as, but not limited to, DBU in a solvent such as, but
not limited to,
N,N-dimethylformamide to provide nitro indazoles of general formula (43).
Nitro indazoles
of general formula (43) can be treated with a transition metal catalyst
including but not
limited to, palladium on carbon under a hydrogen atmosphere (about 1 atm to
about 60 atm)
in a solvent including, but not limited to, methanol, ethanol, or ethyl
acetate to provide amino
indazoles of general formula (44). Amino indazoles of general formula (44) can
be treated
with N,N'-disuccinimidyl carbonate in a solvent including, but not limited to,
acetonitrile to
provide indazoles of general formula (45). Indazoles of general formula (45)
can be treated
with an amine of general formula (3) and a base including, but not limited to,
CA 02743534 2011-06-13
diisopropylethylamine or triethylamine in a solvent including, but not limited
to,
N,N-dimethylfonmainide to provide indazoles of general formula (46). hndazoles
of general
formula (46) can be treated with a hydroxide anion source including, but not
limited to,
sodium hydroxide or potassium hydroxide in a solvent including, but not
limited to,
acetonitrile, methanol, ethanol, aqueous acetonitrile, aqueous methanol, or
aqueous ethanol to
provide indazoles of general formula (47).
Amino indazoles of general formula (44) can be processed with phosgene as
described in Scheme 2 or Scheme 3 to provide indazoles of general formula
(46). Indazoles
of general formula (46) can then be treated with hydroxide anion to provide
indazoles of
general formula (47).
The following Examples are intended. as an illustration of and not a
limitation upon
the scope of the invention as defined in the appended claims.
Example 1
N-(2-(3 -fluorophenyl )ethyll-N'-iso g uinolin-5-ylurea
Example 1A
2,2,2-tri.chloro-N-isoguinolin-5-ylacetamide
A solution of 5-aminoisoquinoline (1.0 g, 6.9 mmol) in dichloromethane (40 mL)
and
Et3N (lmL) at 5 C was treated with tiichloroacetyl chloride (1.38 g, 7.6
mmol) dropwise.
The reaction mixture was stirred at ambient temperature for 14 hours,
concentrated, diluted
with ethyl acetate and washed with IN HCI. The aqueous layer was treated with
aqueous
NaHCO3 and extracted with ethyl acetate. The organic layer the was washed with
water and
concentrated. The solid residue was suspended in ethyl acetate (5 mL) and
filtered to obtain
1.3 g (65%) of the title compound as a tan solid. 'H NMR (300 MHz, d6-DMSO) 6
11.20
(broad s, 1H), 9.41, (s, 11-1), 8.60 (d, IH), 8.18 (ni, 1H), 7.77 (m, 2H),
7.66 (d, 1H); MS
(DCUNH3) m/z 289 (M+H)+.
Example lB
N-(2-(3 -fluorophenyl)ethyl -N-iso guinolin-5 -ylurea
51
CA 02743534 2011-06-13
The product from Example IA (0.65 g, 2.25 mmol), DBU (0.85 g, 5.6 mmol) and 2-
(3-fluorophenyl)ethylamine (0.35 g, 2.5 iumol) in acetonitrile (50 mL) were
refluxed for 10
hours. The mixture was cooled, concentrated, diluted with ethyl acetate,
washed twice with
aqueous ammonium chloride and concentrated to dryness. The solid obtained was
suspended
in ethyl acetate and filtered to obtain 0.45 g (65%) of the title compound as
a tan solid. 1H
NMR (300 MHz, d6-DMSO) S 9.27 (s, 1H), 8.63 (s, 1H), 8.51 (d, 1H), 8.26 (d,
1H), 7.89 (d,
1H), 7.71 (d, 1H), 7.59 (m, 1H), 7.35 (m, 1H), 7.18-7.0 (m, 3H), 6.60 (t, 11-
1), 3.42 (m, 2H),
2.72 (m, 2H); MS (DCI NH3) m/z 310 (M+11)'; Anal. Calcd. For C18H,6N3F0.
0.1H20: C
69.48; H 5.25; N 13.51. Found: C 69.31; 1- 15.25; N 13.46.
Example 2
N-f 2-(3-bromophenyl)ethyll-N-isoguinolin-5-ylurea
The title compound was prepared using 2-(3-bromophenyl)ethylamine, DBU, the
product from Example IA and the procedure described in Example I.B. 1H NMR
(300 MHz,
d6-DMSO) S 9.26 (s, 11-1), 8.63 (s, 1H), 8.51 (d, 11-1), 8.23 (d, 1H), 7.90
(d, 1H), 7.71 (d, 1H),
7.59 (m, 1I-1), 7.40 (m, 2H), 7.29 (m, 2H), 6.60 (t, I H), 3.42 (m, 2H), 2.80
(m, 2H); MS
(DCUNH3) m/z 370 (M+H)+; Anal. Calcd. For C,SHt6N3BrO: C 58.39; H 4.36; N
11.35.
Found: C 58.17; H 4.46; N 11.28.
Example 3
N-isoguinolin-5-yl-IN1'-14-(trifluoromethyl)benzyl)urea
The title compound was prepared using 4-(trifluorometlryl)benzylarnine, DBU,
the
product from Example IA and the procedure described in Example 1B. 'H NMR (300
MHz,
d6-DMSO) 9.26 (s, 1H), 8.82 (s, 1H), 8.52 (d, 1H), 8.26 (d, 1H), 7.94 (d, 1H),
7.71 (m, 3H),
7.58 (m, 3H), 7.20 (t, 1H), 4.48 (d, 2H); MS (DCI/NH3) m/z 346 (M+H)+; Anal.
Calcd. For
C1sH,4N3F30. 0.05H20: C 62.63; H 4.19; N 12.04. Found: C 62.41; H 4.58; N
11.44.
Example 4
N-i soguinolin-5-yl-N'-(4-phenoxyb enzyl)urea
The title compound was prepared using 4-phenoxybenzylamine, DBU, the product
from Example IA and the procedure described in Example 1B. 1H NMR (300 MHz, d6-
52
CA 02743534 2011-06-13
DMSO) 6 9.30 (s, 1H), 8.75 (s, 11-1), 8.58 (d, 1II), 8.31 (d, 111), 7.92 (d,
lii), 7.75 (d, IH),
7.60 (t, 1H), 7.40 (m, 4H), 7.18-6.95 (m, 6H), 4.38 (d, 2H); MS (DCUNH3) n3Jz
369 (M+H)+.
Example 5
N-(3-fluoro-5-(trifluoromethy)b 1 -N_isoquinolin-5-ylurea
The title compound was prepared using 3-fluoro-5-(trifluoromethyl)benzylimine,
DBU, the product from Example 1A and the procedure described in Example IB. 'H
NMR
(300 MHz, d6-DMSO) 6 9.28 (s, 1H), 8.88 (s, 1H), 8.53 (d, 111), 8.22 (d, IH),
7.90 (d, 1H),
7.77 (d, 1H), 7.55 (m, 4H), 7.20 t, 1H), 4.45 (d, 2H); MS (DCI/NH3) m/z 364
(M+H)+
Example 6
N-(2, 5-dichlorob enzyl)-N-i soquinolin-5-ylurea
The title compound was prepared using 2,5-dichlorobenzylatnine, DBU, the
product
from Example IA and the procedure described in Example 113. 'H NMR (300 MTHz,
d6-
DMSO) 6 9.30 (s, 111), 8.90 (broad s, IH), 8.55 (d, IH), 8.36 (d, 1H), 7.97
(d, 1H), 7.76 (d,
IH), 7.61-7.13 (m, 5H), 4.43 (d, 2H); MS (DCUNH3) m/z 345 (M+H)+; Anal. Calcd.
For
C17H13N3C120Ø2H20: C 58.07; H 3.90; N 11.95. Found: C 57.76; H 3.84; N
11.64.
Example 7
N-(1,3-benzodioxol-5-ylmethyl)-N'-isoquinolin-5-ylurea
The title compound was prepared using 1,3-benzodioxol-5-ylmethylamine, DBU,
the
product from Example IA and the procedure described in Example 1B. 1H NMR (300
MHz,
d6-DMSO) S 9.27 (s, 11-1), 8.85 (broad s, IH), 8.50 (d, 1H), 8.30 (d, 1H),
8.00 (d, 1H), 7.73
(d, 1H), 7.60 t, IIT), 7.15 (m, 2H), 6.89 (m, 2H), 6.00 (s, 2H), 428 (d, 2H);
MS (DCIINH3)
m/z 322 (M+H)+; Anal. Calcd. For C H,3N3O. 0.5H2OØ8NH4C1: C 57.94; H 5.19; N
14.26.
Found: C 57.63; H 5.14; N 14.41.
Example 8
N-[2-(4-fluorophenyl)ethyll-N-isoquinolin-5-ylurea
The title compound was prepared using 2-(4-fluorophenyl)ethylamine, DBU, the
product from Example IA and the procedure described in Example 1B. 'H NMR (300
MHz,
d6-DMSO) 6 9.25 (s, 1H), 8.70 (broad s, IH), 8.50 (d, 1H), 8.27 (d, IH), 7.93
(d, 1H), 7.71
53 1
CA 02743534 2011-06-13
(d, 1H), 7.60 (t, 1H), 7.30 (m, 211), 7.13 (m, 2H), 6.70 (t, 111), 3.40 (m,
2H), 2.80 (m, 2H);
MS (DCI/NH3) m/z 310 (M+H)+; Anal. Calcd. For C17H13N3FO. 0.1H20Ø2NH4CI: C
67.18;
H 5.32; N 13.93. Found: C 66.86; H 5.41; N 13.75.
Example 9
N-(3-bromobenzyl)-N' isoquinolin-5-ylurea
The title compound was prepared using 3-bromobenzylamine, DBU, the product
from
Example IA and the procedure described in Example 113. 'H NMR (300 MHz, d6-
DMSO) S
9.29 (s, 1H), 8.80 (broad s, 1H), 8.53 (d, 111), 8.25 (d, 1H), 7.93 (d, 1H),
7.77 (d, 1H), 7.58
(m, 2H), 7.48 (m, 1H), 7.30 (m, 2H), 7.10 (t, 1H), 4.39 (d, 211); MS (DCI/NH3)
m/z 356
(M+H)+; Anal. Calcd. For C17H,4N3BrO: C 57.32; H 3.96; N 11.80. Found: C
57.06; H 3.90;
N 11.45.
Example 10
N-(2-(3,4-dimethylphenyl)ethyll-N'-isoquinolin-5-yurea
The title compound was prepared using 2-(3,4-dimethylphenyl)ethylamine, DBU,
the
product from Example IA and the procedure described in Example I B. 'H NMR
(300 MHz,
d6-DMSO) S 9.25(s, 1H), 8.68 (broad s, IH), 8.50 (d, 1H), 8.28 (d, 1H), 7.90
(d, 1H), 7.70 (d,
1H), 7.57 (t, 1H), 7.00 (m, 3H), 6.60 (t, 111), 3.40 in, 2H), 2.71 (m, 2H),
2.19 (s, 311), 2.16 (s,
3H); MS (DCI/NH3) m/z 320 (M+H)}; Anal. Calcd. For C20H21N30Ø3H20: C 73.96;
H
6.70; N 12.94. Found: C 73.80; H 6.32; N 12..98.
Example 11
N-` 1-(4-bromophenyl)ethyll-N'-isoquinolin-5-ylurea
5-Aminoisoquinoline (0.64 g, 4.42 mmol) in dichloromethane (20 mL) was treated
with 1-bromo-4-(I-isocyanatoethyl)benzene (1.0 g, 4.42 mmol) in toluene (10
mL). The
mixture was stirred 14 hours at ambient temperature and filtered to obtain 1.2
g (74%) of the
product as light grey solid. 'H NMR (300 MHz, d6-DMSO) S 9.28 (s, 1H), 8.68
(broad s,
1H), 8.56 (d, 1H), 8.28 (d, 1H), 7.90 (d, 1H), 7.72 (d, 111), 7.59 (m, 2H),
7.35 (m, 2H), 7.10
(d, 1H), 4.85 (m, IH), 1.40 (d, 3H); MS (DCI/NH3) m/z 370 (M+H)+; Anal. Calcd.
For
C18H,6N3BrOØ1H20: C 58.11; H 4.39; N 11.29. Found: C 57.79; H 4.21; N 11.16.
54
CA 02743534 2011-06-13
Example 12
4-(trifluoromethyl)benzyl isoguinolin-5-ylcarbamate
The title compound was prepared using [4-(trifluoromethyl)phenyl]methanol,
DBU,
the product from Example IA and the procedure described in Example 113. 'H NMR
(300
Nfz, d6-DMSO) S 9.90 (broad s, 1H), 9.30 (s, 1H), 8.52 (d, 1H), 7.94 (m, 3H),
7.80 d, 2H),
7.70 (m, 3H), 5.30 (s, 2H); MS (DCUNH3) m/z 347 (M-i-H)+; Anal. Calcd. For
C18H,3N2O2F3:
C 62.43; H 3.78; N 8.09. Found: C 62.23; H 3.83; N 7.99.
Example 13
2-(3-bromophcnyl)ethyl isoguinolin-5-ylcarbamate
The title compound was prepared using 2-(3-bromophenyl)ethanol, DBU, the
product
from Example IA and the procedure described in Example 1B. 'H NMR (300 MHz, d6-
DMSO) S 9.70 (broad s, 1H), 9.30 (s, 1H), 8.50 (d, 1H), 7.88 (m, 3H), 7.64 (t,
1H), 7.56 (s,
1H), 7.45 (m, 1H), 7.30 (m, 2I-I), 4.34 (t, 2H), 3.00 (t, 2H); MS (DCUNH3) m/z
371 (M+H)+;
Anal. Calcd. For C18H15N2O2Br: C 58.24; H 4.07; N 7.55. Found: C 58.35; H
4.07; N 7.51.
Example 14
1-naphthyllmethyl isoguinolin-5-ylcarbamate
The title compound was prepared using 1-naphthylmethanol, DBU, the product
from
Example IA and the procedure described in Example 1B. 'H NMR (DMSO-d6) S 9.85
(s,
1H), 9.31 (s, 1H), 8.48 (d, 111), 8.15 (d, 1H), 8.04-7.91 (m, 5H), 7.72-7.52
(m, 5H), 5.69 (s,
2H); MS (ESI+) mlz 328 (M+H)+; Anal. Calcd. For C21H16N2O2: C 76.81, H 4.91, N
8.53;
Found: C 76.64, H 4.73, N 8.29.
Example 15
N-isoguinolin-5-yl-N'-[4-(trifluoromethoxy)benzyllurea
The title compound was prepared using 4-(trifluoromethoxy)benzylamine, DBU,
the
product from Example 1A and the procedure described in Example I.B. MS (ESI+)
mlz 362
(M+H)+; 'H NMR (DMSO-d6) S 4.41 (d, 2H), 7.14 (t, 1H), 7.35 (d, 2H), 7.48 (d,
2H), 7.60 (t,
1H), 7.75 (d, 1H), 7.95 (d, 111), 8.28 (d, 1H), 8.53 (d, IH), 8.79 (s, 1H),
9.27 (s, 1H).
Example 16
CA 02743534 2011-06-13
N 4-dichlorobenzyl)-N'-(3-methylcinnolin-5-y1)urea
Example 16A . .
2,2,2-tricliloro-N-(3-methylcinnolin-5-yl)acetalnide
The title compound was prepared using 3-methylcinnolin-5-amine (commercially
available, Maybridge), triethylamine, trichloroacetyl chloride and the
procedure described in
Example IA.
Example 16B
N-(3,4-dichlorobenzyl)-N-(3-methylcinnolin-5- ly )urea
The title compound was prepared using 3,4-dichlorobenzylamine, the product
from
Example 16A, DBU and the procedure described in Example 1B. MS (ESI+) iu/z 362
(11M+H)+; 'H NMR (DMSO-d6) 6 2.88 (s, 3H), 4.36 (d, 2H), 7.10 (t, 1H), 7.34
(dd, 1H), 7.59
(m, 2H), 7.76 (t, 1H), 8.04 (d, 2H), 8.19 (d, 1H), 8.93 (s, 1H).
Example 17
N-isoguinolin-5-yl-N'-(4-methylbenzyl)urea
The title compound was prepared using 4-methylbenzylamine, the product from
Example IA, DBU and the procedure described in Example 1B. MS (ESI+) m/z 292
(M+H)+; 1H NIVIR (DMSO-d6) 6 2.29 (s, 3H), 4.33 (d, 2H), 7.00 (t, 1H), 7.17
(d, 2H), 7.24 (d,
2M, 7.60 (t, 1H), 7.73 (d, 1I1), 7.93 (d, 1H), 8.30 (d, 1H); 8.53 (d, 1H),
8.70 (s, 1H), 9.26 (s,
IH).
426934 Example 18
N-(4-fluorobenzyl)-N'-isoguinolin-5-ylurea
The title compound was prepared using 4-fluorobenzylamine, the product from
Example 1A, DBU and the procedure described in Example 1B. MS (APCI+) m/z 296
(M+H)+; 1H NMR (DMSO-d6) S 4.37 (d, 2H), 7.07 (t, 111), 7.18 (t, 2H), 7.40
(dd, 2H), 7.60
(t, 1H), 7.74 (d, 1H), 7.94 (d, 1H), 8.28 (d, 11D, 8.54 (d, 1H), 8.74 (s, 1H),
9.27 (s, 1H).
Example 19
N-isoguinolin-5-yl-N-((trans)-2-phenylcyclopropyl}urea
56
CA 02743534 2011-06-13
The title compound was prepared using trans 2-phenylcyclopropylamine
hydrochloride, the product from Example IA, DBU and the procedure described in
Example
lB. MS (ESI+) m/z 304 (M+I4)+; 'H NMR (DMSO-d6) 6 1.21 (m, 2H), 2.05 (in, 1H),
2.82
(m, 1H), 7.00 (d, 1H), 7.17 (t, 311), 7.27 (t, 2H), 7.60 (t, 1H), 7.74 (d, 11-
1), 7.88 (d, 11-1), 8.26
(d, 11-1), 8.53 (d, 1H), 8.57 (s, 114), 927 (s, 1H).
Example 20
N-[2-(3,4-dichlorophenyl)ethyl]-N'-isoquinolin-5-ylurea
The title compound was prepared using 2-(3,4-dichlorophenyl)ethylamine, the
product from Example 1A, DBU and the procedure described in Example I.B. MS
(ESI+)
m/z 361 (M+H)+; 'H NMR (DMSO-d6) 6 2.82 (t, 2H), 3.43 (q, 2H), 6.63 (t, 1H),
7.29 (dd,
114), 7.59 (m, 311), 7.73 (d, 114), 7.88 (d, 1H), 8.23 (d, 1H), 8.52 (d, 1H),
8.65 (s, 111), 9.26 (s,
114).
Example 21
N-[2-(3 ,5-dimethoxyphenyl)ethyll-N'-isoquino lin-5-ylurea
The title compound was prepared using 2-(3,5-dimethoxyphenyl)ethylamine, the
product from Example IA, DBU and the procedure described in Example IB. MS
(ESI+)
m/z 352 (M+H)+; 'H NMR (DMSO-d6) 6 2.74 (t, 214), 3.42 (q, 21I), 3.73 (s, 6H),
6.36 (t, l H),
6.44 (d, 2H), 6.59 (t, 1H), 7.59 (t, 114), 7.72 (d, 1H), 7.91 (d, 11-1), 8.27
(d, 1H), 8.52 (d, 1H),
8.67 (s, 11-1), 9.26 (s, 1H).
Example 22
N-(4-ehlorobenzyl)-N'-isoquinolin-5-ylurea
The title compound was prepared using 4-chlorobenzylamine, the product from
Example 1A, DBU and the procedure described in Example 1B. MS (ESI+) m/z 313
(M+I4)+;'H NMR (DMSO-d6) 6 4.37 (d, 2H), 7.14 (t, IH), 7.40 (q, 4H), 7.60 (t,
111), 7.74 (d,
1H), 7.95 (d, 11-1), 8.28 (dd, IH), 8.53 (d, 1H), 8.80 (s, 1H), 9.27 (s, 111).
Example 23
N-i soq uinolin-5-yl-N'- {2-(3 -(trifluoromethyl)phenyll ethyl } urea
57
CA 02743534 2011-06-13
The title compound Iwas prepared using 2-[3-
(trifluoromethyl)phenyl]ethylamine, the
product from Example IA, DBU and the procedure described in Example 1B. MS
(ESI+)
m/z 360 (M+H)+; 'H NMR (DMSO-d6) S 2.91 (t, 2H), 3.46 (q, 2H), 6.62 (t, 1H),
7.59 (m,
4H), 7.64 (s, IH), 7.73 (d, 1H), 7.87 (d, 1H), 8.23 (d, 1H), 8.51 (d, IH),
8.64 (s, 1H), 9.26 (s,
1H)_
Example 24
N-[2-(2,6-dichlorophenyl)ethyl]-N'-isoq uinolin-5-ylurea
The title compound was prepared using 2-(2,6-dichlorophenyl)ethylamine, the
product from Example 1A, DBU and the procedure described in Example 1B. MS
(ESI+)
m/z 361 (M+H)+; 'H NMR (DMSO-d6) S 3.12 (t, 2H), 3.40 (q, 2H), 6.72 (t, 1H),
7.28 (t, IH),
7.46 (d, 2I3), 7.58 (t, 113), 7.72 (d, 1H), 7.87 (d, 1H), 8.19 (d, IH), 8.51
(d, 1H), 8.60 (s, 1H),
9.25 (s, 1H).
Example 25
N-[2-(2,3-dichlorophenyl)ethyl-N'-isoquinolin-5-ylurea
The title compound was prepared using 2-(2,3-dichlorophenyl)ethylamine, the
product from Example 1A, DBU and the procedure described in Example 1B. MS
(ESI+)
m/z 361 (M+H)+; 'H NMR (DMSO-d6) S 3.01 (t, 2H), 3.46 (q, 2H), 6.67 (t, 1H),
7.34 (t, IH),
7.38 (dd, 1H), 7.53 (dd, IH), 7.59 (t, IM, 7.74 (d, 1H), 7.87 (d, 111), 8.21
(d, 1H), 8.52 (d,
1H), 8.64 (s, 1H), 9.26 (s, 1H).
Example 26
N-isoquinolin-5-yl-N'-[3-(trifluoromethoxy)benzyllurea
The title compound was prepared using 3-(trifluoromethoxy)benzylamine, the
product
from Example 1A, DBU and the procedure described in Example 113. MS (ESI+) m/z
362
(M+H)+; 'H NMR (DMSO-d6) 6 4.44 (d, 2H), 7.15 (t, 1H), 7.26 (d, 1H), 7.34 (s,
1H), 7.40 (d,
1H), 7.50 (t, 1H), 7.61 (t, 1H), 7.76 (d, 1H), 7.95 (d, IH), 8.25 (d, 1H),
8.53 (d, IH), 8.80 (s,
1H), 9.28 (s, IH).
Example 27
N-[2-(4-ethoxy-3-methoxypheny_1)ethyl]-N'-isoquinolin-5-ylurea
58
CA 02743534 2011-06-13
The title compound was prepared using 2-(4-ethoxy-3-methoxyphenyl)ethylamuie,
the product from Example IA, DBU and the procedure described in Example 113.
MS (ESI+)
m/z 366 (M+H)}; 'H NMR (DMSO-d6) 8 1.31 (t, 3H), 2.73 (t, 2H), 3.40 (q, 2H),
3.76 (s, 3H),
3.97 (q, 2H), 6.62 (t, 1H), 6.76 (dd, 1H), 6.87 (d, 2H), 7.59 (t, 1H), 7.72
(d, 1H), 7.93 (d, 11-1),
8.28 (d, 1H), 8.52 (d, 1H), 8.69 (s, 1H), 9.26 (s, 1H).
Example 28
N-(2-(2,4-dichlorophenyl)ethyll-N'-isoquinolin-5-ylurea
The title compound was prepared using 2-(2,4-dichlorophenyl)ethylamine, the
product from Example IA, DBU and the procedure described in Example 113. 'H
NMR
(DMSO-d6) 5 9.26 (s, 114); 8.62 (s, 111); 8.53 (d, 1I1); 8.22 (dd, 1H); 7.88
(d, 11-1); 7.74 (d,
I11); 7.61 (m, 1H); 7.57 (d, 111); 7.42 (m, 2H); 6.64 (t, 1H); 3.43 (q, 2H);
2.93 (t, 2H).
Example 29
N-(3-bromo-4-fluorobenzyl)-N'-isoquinolin-5-ylurea
The title compound was prepared using 3-bromo-4-fluorobenzylamine, the product
from Example IA, DBU and the procedure described in Example 113. MS (ESI+) m/z
376
(M+H)+; 'H NMR (DMSO-d6) 6 9.55 (s, 11-1); 9.06 (s, IM; 8.64 (d, 1 H); 8.42
(d, 11-1); 8.25
(d, 1H); 7.95 (d, 1H); 7.76 (t, 111); 7.70 (dd, 1I-1); 7.38 (m, 2H); 7.15 (m,
2H); 4.35 (d, 2H).
Example 30
N-(3,4-dimethylbenzyl)-N-isoquinolin-5-ylurea
The title compound was prepared using 3,4-dimethylbenzylamine, the product
from
Example IA, DBU and the procedure described in Example 113. MS (ESI+) m/z 307
M+H)+;
'H NMR (DMSO-d(,) 5 9.55 (s, 1H); 8.98 (s, 111); 8.62 (d, 1H); 8.46 (d, 1H);
8.25 (d, 1H);
7.94 (d, 1H); 7.78 (t, 111); 7.08 (m, 3H); 6.95 (m, 211); 4.30 (d, 2H); 2.20
(s, 311); 2.18 (s,
3H).
Example 31
N-isoquinolin-5-yl-N-(3-phenylpropyl)urea
The title compound was prepared using 3-phenylpropylamine, the product from
Example IA, DBU and the procedure described in Example 1B. MS (ESI+) m/z 306
59
CA 02743534 2011-06-13
(1\4+H)+; 'H NMR (DMSO-d6) 8 9.61 (s, 1H); 9.05 (s, 111); 8.65 (d, 111); 8.50
(d, I H); 8.40
(d, 1H); 7.96 (d, 1H); 7.80 (t, 1H); 7.21 (m, 6H); 6.92 (t, 1H); 3.18 (q, 2H);
2.65 (t, 2I-I); 1.78
(m, 2H).
Example 32
N-(3 , 5-dichlorob enzyl)-N'-isoguinolin-5 -ylurea
The title compound was prepared using 3,5-dichlorobenzylamine, the product
from
Example I A, DBU and the procedure described in Example 1B. MS (ESI+) m/z 347
(M+I-I)+; 'H NMR (DMSO-d6) 8 9.60 (s, 1H); 9.18 (s, IH); 8.65 (d, 1H); 8.44
(d, 111); 8.35
(d,.1H); 7.96 (d, 1H); 7.80 (t, 1H); 7.43 (dt, 1H); 7.40 (m, 2H); 7.35 (m,
1H); 725 (d, 1ITJ;
4.40 (d, 2H).
432465 Example 33
N-(3-chloro-4-methylbenzyl)-N-isoquinolin-5-ylurea
The title compound was prepared using 3-chloro-4-methylbenzylamine, the
product
from Example IA, DBU and the procedure described in Example 1B. MS (ESI+) m/z
326
(M+H)+; 'H NMR (DMSO-d6) 6 9.65 (s, 1H); 9.20 (s, 1H); 8.65 (d, 1H); 8.50 (d,
1H); 8.40
(d, 11-1); 8.00 (d, 1H); 7.80.(t, 1H); 7.30 (m, 5H); 4.35 (d, 2H); 2.30 (s,
3H).
Example 34
N-isoquinolin-5-yl-N-(2-phenoxyethyl)urea
The title compound was prepared using 2-phenoxyethylamine, the product from
Example IA, DBU and the procedure described in Example lB. MS (ESI+) m/z 308
(M+H)+; 'H NMR (DMSO-d6) 6 9.50 (s, 111); 8.98 (s, 111); 8.61 (d, 1H); 8.45
(d, 1H); 8.20
(d, IH); 7.90 (d, 1H); 7.75 (t, 1H); 7.26 (m, 3H); 6.95 (m, 41); 4.00 (t, 2H);
3.50 (m, 2H).
Example 35
N-(3,4-dichlorobenzyl)-N'-isoquinolin-5-ylurea
The title compound was prepared using 3,4-dichlorobenzylamine, the product
from
Example IA, DBU and the procedure described in Example 1B. MS (ESI-) m/z 344
(M-H) ;
'H NMR (300 MHz, DMSO-d6) 8 9.27 (s, 111), 8.82 (bs, 1H), 8.54 (d, 1H), 8.25
(m, IM,
CA 02743534 2011-06-13
7.94 (d, 1H), 7.76 (d, 1H), 7.56-7.65 (m, 31-1), 7.35 (m, 1H), 7.15 (t, 1H),
4.38 (d, 211); Anal.
Calcd for C17H13C12N30: C, 58.98; H, 3.78; N, 12.14. Found: C, 59.02; H, 3.70;
N, 12.10.
Example 36
N-(3-fluorobenzyl)-N'-isoquinolin-5-ylurea
The title compound was prepared using 3-flu orobenzylamine, the product from
Example IA, DBU and the procedure described in Example 1B. MS (ESI-) m/z 294
(M-H)-;
'H NMR (300 MHz, DMSO-d6) 6 9.28 (s, 1I-1), 8.80 (bs, 1H), 8.54 (d, 1H), 8.28
(ni, 1H),
7.95 (d, 1H), 7.76 (d, 1H), 7.60 (t, 1H), 7.35-7.45 (m, 1H), 7.05-7.15 (m,
4H), 4.40 (d, 2H);
Anal. Calcd for C17H14FN30: C, 69.14; H, 4.78; N, 14.23. Found: C, 68.98; H,
4.83; N,
14.27.
Example 37
N-(4-tert-butylbenzyl)-N'-isoquinolin-5-ylurea
The title compound was prepared using 4-tert-butylbenzylamine, the product
from
Example IA, DBU and the procedure described in Example 1B. MS (ESI+) m/z 334
(M+H)+; 1H NMR (300 MHz, DMSO-d6) 6 9.26 (s, 1H), 8.70 (bs, 1H), 8.53 (d, 1H),
8.31
(dd, 1H), 7.92 (d, 1H), 7.73 (d, 1H), 7.60 (t, 1H), 7.38 (m, 2H), 7.28 (m,
2H), 7.01 (t, 1H),
4.32 (d, 2H), 1.27 (s, 9H). Anal. Calcd for C21H23N30Ø3 H2O: C, 74.44; H,
7.02; N, 12.40.
Found: C, 74.19; H, 6.88; N, 12.33.
Example 38
N-isoquinolin-5-yl-N'-[2-(3-methylphenyl)ethyl]urea
The title compound was prepared using 2-(3-methylphenyl)ethylamine, the
product
from Example IA, DBU and the procedure described in Example 113. MS (ESI+) m/z
306
(M+H)+; 1H NMR (300 MHz, DMSO-d6) 6 9.26 (m, 1H), 8.66 (bs, 1H), 8.52 (d,
III), S28
(dd, 1H), 7.90 (d, IH), 7.72 (d, 1H), 7.59 (t, 1H), 7.21 (t, 1H), 7.00-7.11
(m, 3H), 6.60 (t,
1H), 3.41 (m, 2H), 2.76 (t, 2H), 2.30 (s, 3H); Anal. Calcd for C19H19N30Ø1
H20: C, 74.29;
H, 6.30; N, 13.68. Found: C, 74.06; H, 6.43; N, 13.76.
Example 39
N-isoquinolin-5-yl-N'-[2-(4-methylphenyl)ethyllurea
61
CA 02743534 2011-06-13
The title compound was prepared using 2-(3-rethylphenyl)ethylamine, the
product
from Example IA, DBU and the procedure described in Example 1B. MS (ESI+) m/z
306
(M+H)+; 1H NMR (300 MHz, DMSO-d6) 6 9.26 (s, 1H), 8.66 (bs, 11-1), 8.52 (d,
1H), 8.28 (m,
114), 7.90 (d, 1H), 7.72 (d, 1H), 7.59 (t, IH), 7.10-7.20 (in, 4H), 6.58 (t,
1H), 3.40 (in, 2H),
2.75 (t, 2H), 2.28 (s, 3H); Anal. Calcd for C,9H,9N30Ø2 H20: C, 73.86; H,
6.33; N, 13.60.
Found: C, 73.69; H, 6.53; N, 13.51.
Example 40
N-[2-(2,4-dimethylphenyl)ethyl]-N'-isoguinolin-5-ylurea
The title compound was prepared using 2-(2,4-dimethylphenyl)ethylamine, the
product from Example IA, DBU and the procedure described in Example 113. MS
(ESI+)
m/z 320 (M+H)+; 'H NMR (300 MHz, DMSO-d6) S 9.26 (s, 1H), 8.66 (bs, 1H), 8.53
(d, 1H),
8.28 (m, 1H), 7.90 (d, 1H), 7.73 (d, 1H), 7.59 (t, 1H), 7.08 (d, 1H), 6.92-
7.02 (m, 2H), 6.63 (t,
111), 3.34 (m, 2H), 2.75 (t, 2H), 2.29 (s, 3H), 2.24 (s, 3H); Anal. Caled for
C20H21N30Ø45
H20: C, 73.35; H, 6.74; N, 12.83. Found: C, 73.70; H, 6.53; N, 12.45.
Example 41
N-isoguinolin-5-yl-N'-[2-(2-methylphenyl)ethyllurea
The title compound was prepared using 2-(2-methylphenyl)ethylanaine, the
product
from Example 1A, DBU and the procedure described in Example 1B. MS (ESI-) m/z
324
(M-H)-; 'H NMR (300 MHz, DMSO-d6) S 9.26 (s, 1H), 8.64 (bs, 1H), 8.53 (d, IH),
8.25 (m,
1H), 7.89 (d, 1H), 7.73 (d, 1H), 7.59 (t, 1H), 7.46 (dd, 1H), 7.40 (dd, 1H),
7.23-7.36 (m, 2H),
6.67 (t, IH), 3.44 (m, 2H), 2.94 (t, 2H); Anal. Calcd for C18H16CIN30: C,
66.36; H, 4.95; N,
12.90. Found: C, 66.19; H, 4.87; N, 12.91..
Example 42
N-isoguinolin-5-yl-N- f4-[(trifluoromethyl)thiolbenzyl}urea
The title compound was prepared using 4-[(trifluoromethyl)thio]benzylamine,
the
product from Example 1A, DBU and the procedure described in Example 1B. MS
(ESI-) m/z
376 (M-H)-; 1H NMR (300 MHz, DMSO-d6) 5 9.27 (s, 1H), 8.82 (bs, 1H), 8.54 (d,
1H), 8.27
(dd, IH), 7.95 (d, 1H), 7.68-7.78 (m, 3H), 7.60 (t, 1H), 7.51 (d, 2H), 7.17
(t, 1H), 4.45 (d,
62
CA 02743534 2011-06-13
2H); Anal. Calcd for C18H14F3N3OS: C, 57.29; H, 3.74; N, 11.13. Found: C,
57.00; H, 3.73;
N, 11.04.
Example 42
N-isoquinolin-5-yl-N'-(3-(trifluoromethyl)benzyl lure a
The title compound was prepared using 3-(trifluoromethyl)benzylamine, the
product
from Example IA, DBU and the procedure described in Example 1B. MS (ESI-) m/z
344
(M-I-I)-; 1H NMR (300 MHz, DMSO-d6) 8 9.27 (s, 1H), 8.82 (bs, 1H), 8.53 (d, I
IT), 8.25 (dd,
1H), 7.94 (d, 1H), 7.55-7.79 (m, 6H), 7.18 (t, 1H), 4.47 (d, 2H); Anal. Calcd
for
CisH14F3N3O: C, 62.61; H, 4.09; N, 12.17. Found: C, 62.39; H, 3.87; N, 12.28.
Example 43
N-isoquinolin-5-yl-N'-(4-m ethox),benzyl)urea
The title compound was prepared using 4-methoxybenzylamine, the product from
Example IA, DBU and the procedure described in Example lB. MS (ESI-) m/z 306
(hM-H)-;
iH NIvlR (300 MHz, DMSO-d6) 6 9.26 (s, 1H), 8.70 (bs, 1H), 8.53 (d, 1H), 8.31
(dd, 1H),
7.92 (d, 1H), 7.73 (d, 114), 7.60 (t, IH), 7.29 (m, 2II), 6.88-7.03 (m, 3H),
4.30 (d, 2H), 3.74
(s, 3H); Anal. Calcd for CjsH17N302: C, 70.34; H, 5.58; N, 13.67. Found: C,
70.21;1-1, 5.47;
N, 13.46.
Example 44
N-(4-chloro-3-(trifluoromethyl)benzyll-N'-isoquinolin-5-ylurea
The title compound was prepared using 4-chloro-3-(trifluoromethyl)benzylamine,
the
product from Example IA, DBU and the procedure described in Example 113. MS
(ESI-) m/z
378 (M-H)-; 'H NMR (300 MHz, DMSO-d6) 6 9.73 (s, 11-1), 9.53 (s, 1H), 8.69 (d,
1H), 8.61
(d, 1H), 8.54 (d, 1H), 8.07 (d, 1H), 7.82-7.92 (m, 2H), 7.63-7.75 (m, 3H),
4.47 (d, 2H); Anal.
Calcd for C18H13C1F3N30.1.2 HCI: C, 51.05; H, 3.38; N, 9.92. Found: C, 51.26;
H, 3.68; N,
9.50.
Example 45
N-(3 , 5-dimethylbenzyl)-N'-isoquinolin-5-ylurea
63
CA 02743534 2011-06-13
The title compound was prepared using 3,4-dimethylbenzylamine, the product
from
Example IA, DBU and the procedure described in Example 1B. MS (ESI-) m/z 304
(M-H)-;
1H NMR (300 MHz, DMSO-d6) S 9.74 (s, 1H), 9.41 (bs, 1H), 8.69 (d, 1H), 8.62
(d, 2H),
8.05 (d, 1H), 7.88 (t, IH), 7.44 (t, 1H), 6.96 (bs, 2H), 6.89 (bs, 1H), 4.31
(d, 2H), 2.26 (s,
6H); Anal. Calcd for C19H,9N30.1.l HCI: C, 66.05; H, 5.86; N, 12.16. Found: C,
66.09; H,
5.83; N, 12.14.
Example 46
N-(3,5-difluorobenzyl)-N'-isoquinolin-5-ylurea
The title compound was prepared using 3,5-difluorobenzylamine, the product
from
Example IA, DBU and the procedure described in Example 1B. MS (ESI+) m/z 312
(M-H)
'H NMR (300 MHz, DMSO-d6) 6 9'76 (s, 1H), 9.66 (bs, 1H), 8.65-8.79 (m, 2H),
8.60 (d,
111), 8.08 (d, 1H), 7.89 (t, 1H), 7.77 (t, 1H), 7.02-7.18 (m, 311), 4.43 (d,
2H); Anal. Calcd for
C17H,3F2N30=HCl-0.3 H20: C, 57.49; H, 4.14; N, 11.83. Found: C, 57.76; H,
4.59; N,
11.76.
Example 47
N-hexyl-N'-isoguinolin-5-ylurea
The title compound was prepared using hexylamine, the product from Example IA,
DBU and the procedure described in Example I.B. MS (ESI-) m/z 270 (N4-M-; 'H
NMR
(DMSO-d6) 6 9.25 (s, 1H), 8.60 (s, 11-1), 8.55 (d, 1H), 8.39 (d, 1H), 7.93 (d,
IH), 7.71 (d, 1H),
7.59 (t, 1H), 6.60 (t, 1H), 3.15 (q, 2H), 1.49 (m, 2H), 1.32 (m, 6H),.90 (m,
3H).
Example 48
N-(4-bromobenzyl)-N'-isogiiinolin-5-ylurea
The title compound was prepared using 4-bromobenzylamine, the product from
Example IA, DBU and the procedure described in Example 1B. MS (ESI-) m/z 355
(M-H)-;
'H NMR (DMSO-d6) S 9.27 (s, 1H), 8.78 (s, 1H), 8.53 (d, 1H), 8.27 (d, 1H),
7.93 (d, 1H),
7.74 (d, 1H), 7.61 (d, 1H), 7.55 (d, 2H), 7.42 (d, 2H) 7.10 (t, 1H); Anal.
Calcd for
C17H14BrN3O: C, 57.32; H, 3.96; N, 11.80. Found C, 57.05; H, 3.79; N, 11.64.
Example 49
64
CA 02743534 2011-06-13
N-(3, 5-dimethoxybenzyl)-N'-isoguinolin-5 -ylurea
The title compound was prepared using 3,5-dimethoxybenzylamine, the product
from
Example 1A, DBU and the procedure described in Example 113. MS (ESI-) nilz 336
(M-II)-;
'I-I NMR (DMSO-d6) 6 9.70 (s, 1H), 9.32 (s, 11-1), 8.69 (d, IH), S.55 (dd,
2H), 8.10 (ci, 111),
7.85 (t, 11=I), 7.39 (t, 111), 6.54 (s, 2H), 6.41 (s, 1H) 4.35 (d, 211), 3.75
(s, 6H); Anal. Calcd for
C19H19N303 1.25 HCI C, 59.59; H, 5.33; N, 10.97. Found C, 59.22; H, 5.41; N,
10.84.
Example 50
N-isoguinolin-5-yl.-N'-(3,4,5-trimethoxybenzyl)urea
The title compound was prepared using 3,4,5-trimethoxybenzylamine, the product
from Example IA, DBU and the procedure described in Example 1B. MS (ESI-) m/z
366
(M-H) 'H NMR (DMSO-d6) 6 9.79 (s, 1H), 9.50 (s, 1H), 8.69 (d, 1H), 8.80 (d,
1H), 8.65
(dd, 2H), 8.08 (d, 1H), 7.90 (d, 1H), 7.68 (m, IH), 6.71 (s, 2H), 4.53 (d, 2H)
3.79 (s, 6H),
3.53 (s, 3H). Anal. Calcd for C20H21N3041.3 HCI: C, 57.91; H, 5.42; N, 10.13.
Found C,
57.65; H, 5.60; N, 10.09.
Example 51
N-isoguinolin-5-yl-N'-[4-(methyl sulfonyl)benzyl]ure
The title compound was prepared using 4-(methylsulfonyl)benzylamine, the
product
from Example IA, DBU and the procedure described in Example IB. MS (ESI-) m/z
354
(M-H);'H NMR (DMSO-d6) 6 9.65 (s, 1H), 9.30 (s, 1H), 8.65 (d, 1H), 8.49 (d,
1H), 8.42 (d,
1H), 8.00 (d, 1H), 7.91 (d, 2H), 7.82 (t, 1H), 7.61 (d, 2H), 7.47 (t, 1H),
4.50 (d, 2H), 3.20 (s,
3H); Anal. Calcd for C20H21N304 1.0 HCI: C, 55.17; H, 4.63; N, 10.72. Found C,
54.92; H,
4.54; N, 10.42.
Example 52
N-(3,4-dimethoxybenzyl)-N'-isoguinolin-5-ylurea
The title compound was prepared using 3,4-dimethoxybenzylamine, the product
from
Example 1A, DBU and the procedure described in Example 1B. MS (ESI-) m/z (M-
H)" 336;
'H NMR (DMSO-d6) S 9.78 (s, 1H), 9.50 (s, IH), 8.70 (s, 211), 8.62 (d, 1H),
8.05 (d, 1H),
7.87 (t, 1H), 7.51 (t, IH), 6.99 (s, 11f), 6.79 (ds, 2H), 4.32 (d, 2H), 3.75
(s, 3H), 3.71 (s. 3H);
CA 02743534 2011-06-13
Anal. Calcd for C1911, 9N303 1.0 HCl: C, 61.04; H, 5.39; N, 11.24. Found C,
60.82; H, 5.38;
N, 11.19.
Example 53
N-isoguinolin-5-yl-N'-(3-phenoxybnzI)urea
The title compound was prepared using 3,4-dimethoxybenzylamine, the product
from
Example IA, DBU and the procedure described in Example 113. MS (ESI-) m/z 368
(M-H)-;
'H NMR (DMSO-d6) S 9.65 (s, 1H), 9.25 (s, 1H), 8.65 (d, 1H), 8.52 (d 1H), 8.48
(d, IH),
8.03 (d, 1H), 7.82 (t, 1H), 7.35 (m, 4H), 7.15 (d, 2H), 7.05 (s, 2H), 7.00 (s,
1H), 6.84 (d, 1H),
2.37 (d, 2H); Anal. Calcd for C23HI9N302 1.25 HCI: C, 66.57; H, 4.92; N, 10.
13. Found C,
66.49; H, 5.02; N, 10.14.
Example 54
N-iso guino lin-5-yl-N'-(1-naphthylmethyl )urea
The title compound was prepared using 1-naphthyhnethylamine, the product from
Example IA, DBU and the procedure described in Example 1B. MS (ESI+) m/z 328
(M+H)+; FIRMS (FAB): Calculated for C21H18N30 328.1450; observed 328.1438
(M+H)+;'H
NMR (DMSO-d6) 5 9.25 (s, 1H), 8.48, (d, 1H), 8.39 (d, 114), 8.22 (d, 1H), 8.19
(d, 1H), 7.97
(d, 1H), 7.87 (d, 1H), 7.78-7.71 (m, 2H), 7.63-7.49 (m, 6H), 4.85 (d, 2H).
Example 55
N-(2,4-dimethylbenzyl)-N'-isoguinolin-5-ylurea
The title compound was prepared using 2,4-dimethylbenzylamine, the product
from
Example IA, DBU and the procedure described in Example 113. MS (ESI+) m/z 306
(M+H)+;'H NMR (DMSO-d6) 5 9.26 (s, 1H), 8.67 (s, 1H), 8.53 (d, 1H), 8.32 (d,
1H), 7.92
(d, I H), 7.72 (d, I H), 7.60 (t, I H), 7.19 (d, 111), 7.03-6.95 (m, 2H), 9.90
(t, IM, 4.31 (d, 2H),
2.30 (s, 3H), 2.26 (s, 3H); Anal. Calcd for C19H19N30Ø2H20: C, 73.86, H
6.33, N 13.60.
Found: C 73.75, H 6.49, N 13.49.
Example 56
N-(4-(dimethylamino)benzy l-N'-isoguinolin-5-ylurea
66
CA 02743534 2011-06-13
The title compound was prepared using 4-(aminomethyl)-N,N-dinlethylaniluie,
the
product from Example 1 A, DBU and the procedure described in Example 1B. MS
(ESI+)
miz 321 (M+H)*; 'H NMR (DMSO-d6) 6 9.26 (s, 1H), 8.71 (s, 1H), 8.52 (d, 1H),
8.32 (d,
IH), 7.93 (d, 1H), 7.72 (d, IH), 7.59 (t, 1H), 7.18 (d, 2H), 6.96 (t, IH),
6.71 (d, 21-1), 4.23 (d,
2H), 2.86 (s, 6H); Anal. Calcd for Ct9H2oN40Ø7H20: C, 68.53, H 6.48, N
16.82. Found: C
68.59, H 6.48, N 16.60.
Example 57
N-isoguinolin-8-yl-N-J4-(trifluoromethyl)benzyl)urea
Example 57A
5-bromoisoguinoline
Concentrated H2SO4 (260 mL) was cooled to -25 C while stirring with a
mechanical
stirrer. Isoquinoline (30 mL, 0.25 mol) was added slowly so the temperature
did not exceed 0
C. After the addition was complete. the red solution was recooled to -25 C
and treated with
N-bromosuccinimide (55.49 g, 0.31 mol) in small portions so that the
temperature did not
exceed -20 C. The reaction mixture was stirred for 5 hours keeping the
temperature between
-30 C and -18 C. The reaction mixture was then allowed to warm to -10 C and
was poured
carefully over 600 g of ice. The resulting slurry was adjusted to pH 10 using
25% NH4OH.
The mixture was then extracted with diethyl ether (3 x 600 mL). The ether
fractions were
combined, filtered through a CeliteTM plug and the filtrate concentrated under
reduced pressure.
The residue was suspended in hot heptane (600 mL). The heptane was decanted.
This
procedure was repeated with hexane (2 x 200 mL). The combined heptane and
hexane
fractions were concentrated under reduced pressure to give a mustard yellow
solid. The title
compound was obtained by recrystallization from heptanc (26.37 g, 50%). nip 78
-80 C;
NIS (ESI+) u7/z 209 (vI+H)k; IH NMR (DMSO, 300 MHz) S 7.65 (t, J 7.9, 1H),
7.94 (d, J
8.1, 1H), 8.17 (dd, J 1.0, 7.4, 1H), 8.22 (d, J 9. 1, 1H), 8.68 (d, 3 6.1,
1H), 9.37 (s, IH); Anal.
Calcd for C9H6BrN: C, 51.96; H, 2.91; N, 6.73; Br, 38.41. Found: C, 51.24; H,
2.79; N, 6.52;
Br, 38.81.
Example 57B
5-bromo-8-nitroisoguinoline
67
CA 02743534 2011-06-13
The diethyl ether solution from Example 57A was treated with potassium nitrate
(10.1
g, 100 mmol). After stirring for one hour, The mixture was poured onto ice and
neutralized
with concentrated ammonium hydroxide (-300 nil). The crude product was
collected by
filtration, dried, and recrystalization from methanol to provide the title
compound (8.83 g).
Example 57C
isoguinolin-8-amine
The product from Example 57B was treated with Pd/C under a hydrogen atmosphere
to provide the title compound.
Example 57D
2,2,2-trichloro-N-isoguinolin-8-ylacetamiide
The product from Example 57C and trichloroacetylchloride were processed as
described in Example IA to provide the title compound.
Example 57E
N-isoguinoLin-8-y]-N-r4-(trifluoromethyl)benzyI lure
The title compound was prepared using 4-(trifluoromethyl)benzylamine, the
product
from Example 57D, DBU and the procedure described in Example I.B. MS (ESI+)
m/z 346
(M+H)+; 1H NMR (DMSO-d6) S 9.58 (s, 111), 9.10 (s, I H), 8.49 (d, I H), 8.12
(d, I H), 7.81-
7.54 (m, 7H), 7.20 (t, 1H), 4.47 (d, 214); Anal. Calcd for C18H14F3N30Ø2
H2O: C, 61.96, H
4.16, N 12.04. Found: C 62.06, H 4.23, N 11.91.
Example 58
N-(4-bromobenzyl)-N-isoguinolin-8-ylurea
The title compound was prepared using 4-bromobenzylamine, the product from
Example 57D, DBU and the procedure described in Example 1B. MS (ESI+) mlz 356
(M+H)+;'H NMR (DMSO-d6) S 9.52 (s, IH), 9.15 (s, IH), 8.49 (d, 1H), 8.11 (d,
111), 7.77
(d, 1H), 7.67 (t, 1H), 7.55 (m, 3H) 7.32 (d, 2H), 7.25 (t, 1H), 4.34 (d, 2H);
Anal. Calcd for
C17H14BrN30Ø25 H20Ø16 MeOH: C 56.34, H 4.17, N 11.49. Found C, 56.32, H
4.45, N
11.70.
68
CA 02743534 2011-06-13
Example 60
N-(4-bromobenzyl)-N'-(3-chloroisoquinolin-5- 1 urea
Example 60A
isoquinoline-1,3(211,411)-dione
2-(Carboxyrnethyl)benzoic acid (10 g, 55.6 mmol) was dissolved in concentrated
NH4OH (15 mL) and then was evaporated to dryness under reduced pressure. The
process
was repeated with additional NI-140H (5 mL). The resulting residue was treated
with 1,2-
dichlorobenzene (20 rL) and heated with stirring at 200 C without a condenser
allowing the
solvent to evaporate. The concentrated mixture was allowed to cool to room
temperature,
diluted with methanol (20 mL), and allowed to stand overnight. The precipitate
was collected
by filtration, washed with methanol, and dried under reduced pressure to
provide the title
compound as tan needles (6.6 g, 74%).
Example 60B
1,3-dichloroisoquinoline
The product from Example 60A (6.5 g, 40.4 mmol) was treated with
phenylphosphonic dichloride (11.5 mL, 81.1 mmol) and heated at 160 C for 3
hours. The
reaction was allowed to cool to room temperature and stand overnight. The
resulting waxy
orange material was dissolved in tetrahydrofuran (200 mL), treated with water
(60 mL), and
then concentrated under reduced to remove the tetrahydrofuran. The remaining
aqueous
material was neutralized with concentrated NH4OH and extracted with ethyl
acetate. The
ethyl acetate phases were combined, washed with water, brine, dried over
Na2SO4 and
concentrated under reduced pressure to provide the title compound as yellow
flakes (6.92 g,
74%).
Example 60C
3-chloroisoguinoline
The product from Example 60B (6.73 g, 33.8 mmol) was suspended in glacial
acetic
acid (37 mL) and concentrated HCl (13 mL), treated with tin powder (12.1 g,
101.9 mmol),
and heated at 55-60 C for 3 hours with stirring. The mixture was allowed to
cool to room
temperature and the precipitated tin salts were removed by filtration through
Celite. The
69
CA 02743534 2011-06-13
fi
filtrate was basified to pH 9 with concentrated NH4OH and then extracted with
ethyl acetate.
The organic extracts were combined, washed with saturated NaHCO3 solution,
dried over
Na2SO4, and concentrated under reduced pressure to provide the title compound
as a gummy
yellow residue (1.28 g, 23%).
Example 60D
3-chloro-5-nitroisoguinoline
The product from Example 60C (1.28 g, 7.85 mmol) in concentrated H2S04 (30 mL)
at 0 C was treated with a solution of KNO3 (0.84 g, 8.32 mmol) in
concentrated H2S04 (5
mL) dropwise over 5 minutes. The mixture was stirred at 0 C for 10 minutes,
allowed to
warm to room temperature, and stirred overnight. The mixture was poured onto
65 g of ice
and the precipitated yellow solid was collected by filtration. The solid was
slurried in water,
collected by filtration, washed with water, and allowed to air-dry to provide
the title
compound as a pale yellow solid (0.45 g, 28%).
Example 60E
3-chloroisoguinolin-5-amine
The product from Example 60D (0.45 g, 2.16 mmol) was suspended in glacial
acetic
acid (4 mL) and warmed to 60 C while adding water (4 mL). The heated mixture
was
treated with powdered iron (0.33g, 5.91 mmol) in three portions over about 2
minutes. The
reaction mixture stirred at 60 C for 2 hours, allowed to cool to room
temperature and stir
overnight. The mixture was basified with 25% aqueous NaOH, diluted with a
little water,
and the brown precipitate was collected by filtration and dried overnight at
50 C in a vacuum
oven. The filter cake was then broken up and extracted with boiling ethyl
acetate. The
extracts were combined, dried over Na2SO4, filtered, and the filtrate was
concentrated under
reduced pressure to provide the title compound as a gold-orange solid (200 mg,
52%).
Example 60F
N-(4-bromobenzyl)-N'-(3-ehloroisoguinolin-5-yl)urea
The product from Example 60E (250 mg, 1.4 mmol) and 1-bromo-4-
(isocyanatomethyl)benzene (0.22 mL, 1.57 mmol) were heated in toluene (5 mL)
at 80 C for
3 hours. The mixture was allowed to cool to room temperature, filtered, the
filter cake was
CA 02743534 2011-06-13
.washed with toluene, and air-dried to provide the title compound (335 mg,
61%). 'H NMR
(300 MHz, DMSO-d6) 6 9.18 (s, 111), 8.81 (s, 1H), 8.32 (dd, J=7.8Hz, 0.7 Hz,
1H), 8.09 (s,
1ff), 7.80 (d, J=8.2 Hz, 111), 7.53-7.65 (m, 31-1), 7.32 (m, 2H), 7.05 (t,
J=5.7 Hz, Ili), 4.35 (d,
JJ5.7 Hz, 2H); MS (EST') m/z 391/393 (111+f, 35C1/37CI).
Example 61
4-cyanobenzyl isoguinolin-S-ylcarbamate
Example 61A
5-isocyanatoisoguinoline
Phosgene (20 ml, 20% in toluene from Fluka) in CH2C12 (300 mL) at 0 C was
treated
with DMAP (10 g) in CH2C12 (100 mL) slowly. After complete addition, the
mixture was
treated with 5-aminoisoquinoline (5 g) in CH2CI2 (100 mL) dropwise. The
mixture was
allowed to warm to room temperature and then stirred overnight. The solvent
was removed
under reduced pressure. The solid residue was extracted with diethyl ether
(400 mL). The
diethyl ether was filtered to provide the title compound in diethyl ether as a
pale yellow
solution. The diethyl ether solution was used in subsequent reactions without
further
purification.
Example 61B
4-cyanobenzyl isoquinolin-5-ylcarbamate
4-Cyanobenzyl alcohol (150 mg, 1.13 mmol) in diethyl ether (10 mL) was treated
with the product from Example 61 A as an ethereal solution. The mixture was
stirred for 2
hours, filtered, and the filter cake was washed with diethyl ether to provide
the title
compound as an off-white solid (150 mg, 44%). 'H NMR (300 MHz, DMSO-d,,) 8
9.95 (s,
1H), 9.32 (d, J=1.0 Hz, IH), 8.52 (d, J=6.1 Hz, IH), 7.88-7.99 (m, 5H), 7.65-
7.70 (m, 3H),
5.31 (s, 2H); MS (ES)*) m/z 304 (M+H)+.
Example 62
N-[(4-cyanophenyl)methyl]-N-isoguinolin-5-ylurea
N, N-bis(tert-butoxycarbonyl)-4-cyanobenzyl amine (0.75 g, 2.25 mmol, prepared
according to Synth. Comm. (1998) 28, 4419) in CH2C12 (1S mL) was treated with
71
CA 02743534 2011-06-13
trifluoroacetic acid (8 mL), and the resulting mixture was stirred at room
temperature for 3
hours. The mixture was concentrated under reduced pressure and then azeotroped
with
diethyl ether. The residue was taken up in diethyl ether (10 mL) and treated
with N,N-
diisopropylethylamine (5 mL) and the product from Example 61A. After stirring
for 1 hour,
the mixture was filtered and the filter was purified by chromatography (95:5
CH2C12-MeOH)
to provide the title compound as a white solid (65 mg). The corresponding
hydrochloride salt
was prepared using methanolic HCI. 'H NMR (300 MHz, DMSO-d6) 8 9.75 (s, 1H),
9.62 (s,
111), 8.69 (s, 2H), 8.58 (dd, J=7.8 Hz, 1.0 Hz, 1H), 8.07 (d, J=7.4 Hz, 1H),
7.90 (d, J=8.1 Hz,
1H), 7.81-7.85 (m, 2H), 7.74 (t, J=6.1 Hz, 1H), 7.54-7.57 (m, 2H), 4.48 (d,
J=6.1 Hz, 2H);
MS (ESI) m/z 303 (M+H)*.
Example 63
N-((4-bromophenyl)methyll-N'-(3-methylisoguinolin-5-yl)urea
Example 63A
3-methylisoguinolin-5-amine
3-Methylisoquinoline was processed as described in Examples 60D and 60E to
provide the title compound.
Example 63B
N-((4-bromophenyl)methyll-N'-(3-methylisoguinolin-5-A)urea
The product from Example 63A (500 mg, 3.1 mmol) in toluene (10 mL) was treated
with I-bromo-4-(isocyanatomethyl)benzene (0.5 mL, 3.57 mmol) with stirring and
then the
mixture was heated at 80 C overnight. The mixture was allowed to cool to room
temperature, filtered, the filter cake was washed with toluene, and allowed to
air-dry to
provide the title compound. The corresponding hydrochloride salt was prepared
using
methanolic HCl to afford a tan solid (919 mg, 73%). 'H NMR (300 MHz, DMSO-d6)
8 9.70
(s, 1H), 9.54 (s, 1H), 8.63 (s, 1H), 8.57 (dd, J=7.8 Hz, 1.0 Hz, IH), 8.02 (d,
J=8.2 Hz, 11-1),
7.78-7.83 (m, 1H), 7.67-7.71 (m, 1H), 7.52-7.57 (rn, 21=I), 7.30-7.35 (m, 2H),
4.36 (d, J=5.7
Hz, 214), 2.78 (s, 3H); MS (ES1) m/z 370/372 (M+H, 79Br/81Br).
Example 64
72
CA 02743534 2011-06-13
N-[(4-bromophenyl)methyl1-N'-(1-chloroisoquinolin-5-yl)urea
Example 64A
I -chloroisoquinolin-5-amine
1-Chloroisoquinoline was processed as described in Examples 60D and 60E to
provide the title compound.
Example 64B
N-[(4-bromophenyl)methyl)-N'-(1-chloroisoquinolin-5-yl)urea
The product from Example 64A (520 mg, 2.91 ninol) in toluene (8 mL) was
treated
with 1-bromo-4-(isocyanatomethyl)benzene (0.41 mL, 2.93 mmol) with stirring
and then the
mixture was heated at 90 C for 2 hours. The mixture was allowed to cool to
room
temperature, filtered, the filter cake washed with toluene, and air-dried to
provide the title
compound as an off-white solid (717 mg, 63%). 'H NMR (300 MHz, DMSO-d6) S 8.89
(s,
1H), 8.34-8.37 (m, 2H), 8.00 (dd, J=6.1 Hz, 0.7 Hz, 1H), 7.92-7.95 (m, 1H),
7.73 (t, J=8.1,
IH), 7.53-7.56 (m, 2H), 7.30-7.33 (m, 2H), 7.12 (t, J=5.8Hz, 1H), 4.35 (d,
J=5.8 Hz, 2H); MS
(ESI) m/z 390/392 (M+H+, 35C1/37C1).
Example 65
N-f(4-bromophenyl)methyl}-N'-(1-methylisoquinolin-5-yl urea
Example 65A
1-methylisoquinolin-5 -amine
1-Methylisoquinoline was processed as described in Examples 60D and 60E to
provide the title compound.
Example 65B
N-[(4-bromophenyl)methyll-N'-(1-methylisoquinolin-5-yl)urea
The product from Example 65A (480 mg, 3.04 mmol) in toluene (9 mL) was treated
with 1-bromo-4-(isocyanatomethyl)benzene (0.43, 3.07 mmol) with stirring.
After heating
the mixture at 90 C for 1 hour, the mixture was allowed to cool to room
temperature,
filtered, and the filter cake washed with toluene to provide the title
compound. The
73
CA 02743534 2011-06-13
corresponding di-hydrochloride salt was prepared using methanolic HCI (680 mg,
50%). 1H
NMR (300 MHz, DMSO-d6) 6 8.74 (s, 114), 8.38 (d, J=6.1 Hz, 1H), 8.25 (d, J=7.8
Hz, 1H),
7.78-7.85 (m, 2H), 7.53-7.61 (m, 3I-I), 7.32 (d, J=8.5 Hz, 2H), 7.11 (t, J=6.1
Hz, 1H), 4.34 (d,
J=6.1 Hz, 2H), 2.88 (s, 3H); MS (ESl+) m/7,370/372 (M+H+, 79Br/81Br).
Example 66
N-isoguinolin-5-yl-N'-((4-morpholur-4-ylphenyl)methyllurea
Example 66A
4-morpholin-4-ylbenzonitrile
4-Fluorobeuzonitrile (1 g, 8.26 mmol) and morpholine (2.2 mL, 25.2 mmol) were
combined in DMSO (25 mL) and heated at 100 C for 2.5 hours. The mixture was
allowed to
cool to room temperature, poured into water, and extracted with diethyl ether.
The organic
extracts were combined, washed with water and brine, dried over Na_2SO4, and
concentrated
under reduced pressure to provide the title compound as a white solid (1.24 g,
80%).
Example 66B
(4-morpholin-4-ylphenyl)methylamine
The product from Example 66A (1.24 g, 6.6 mrnol) in THE (25 mL) was treated
with
LiAIH4 (2.5 g, 65.9 mmol) at 0 C. The mixture was allowed to warm to room
temperature
and then refluxed for 1 hour. The mixture was allowed to cool to room
temperature and then
treated with IN NaOH carefully followed by water. The mixture was concentrated
under
reduced pressure and the resulting aqueous mixture was extracted with diethyl
ether. The
organic extracts were combined, washed with saturated NaHCO3 solution, dried
over
Na2SO4, filtered, and the filtrate concentrated under reduced pressure to
provide the title
compound as a yellow oil (286 mg, 23%).
Example 66C
N-isoguinolin-5-yl-N'-[(4-morpholin-4-ylphenyl)methyllurea
The product from Example 66B (285 mg, 1.48 mmol) in diethyl ether (10 m.L) was
treated with the product from Example 61A. The mixture was filtered and the
filter cake
purified by chromatography (95:5 CH2CI2-MeOH, eluant) to provide that title
compound as a
74
CA 02743534 2011-06-13
white solid. The corresponding di-hydrochloride salt was prepared using
methanolic HC1 to
afford a yellow solid (505 mg, 78%). iH NMR (300 MHz, DMSO-d6) 8 9.26 (s, 1H),
8.67 (s,
1H), 8.52-8.55 (m, 1H), 8.32 (dd, J=7.8 Hz, 1.1 Hz, 1H), 792 (d, J-6.1 Hz,
1H), 7.73 (d,
J=8.2 Hz, 111), 7.60 (m, 11-I), 7.23 (d, J=8.8 Hz, 2H), 6.92-6.96 (m, 3H),
4.26 (d, 5.4 Hz, 2H),
3.72-3.75 (in, 4H), 3.06-3.12 (m, 4H); MS (ESI) m/z 363 (M+H)+.
Example 67
N- (4-(2,6-dimethylmorpholin-4-yl)phenyllmethyl}-N'-isoguinoiin-5-ylurea
Example 67A
[4-(2, 6-dimethylmorpholin-4-yl)phenyl]methylamine
4-Fluorobenzonitrile and 2,6-dimethylmorpholine were processed as described in
Examples 66A and 66B to provide the title compound.
Example 67B
N- { (4-(2,6-dimethylmorpholin-4-yl)phenyllmethyl} -N'-isoguinolin-5-ylurea
The product from Example 67A and the product from Example 61A were processed
as described in Example 66C to provide a waxy material which was purified by
chromatography (95:5 CH2CI2-MeOH, eluant) to provide the title compound as a
white solid.
The corresponding di-hydrochloride salt was prepared using methanolic HCI. 1H
NIvIR (300
MHz, DMSO-d6) 6 9.26 (s, 1H), 8.67 (s, 1H), 8.53 (d, J=6.1 Hz, 1H), 8.31 (dd,
J=7.6 Hz, 1.1
Hz, 1H), 7.92 (d, J=6.1 Hz, 1H), 7.73 (d, J=8.1 Hz, 1H), 7.57-7.62 (m, 1H),
7.22 (d, J=8.8
Hz, 2H), 6.92-6.95 (nz, 3H), 4.26 (d, J=5.7 Hz, 2H), 3.68 (m, 2H), 3.54-3.57
(m, 211), 2.21
(m, 2H), 1.16 (s, 3H), 1.14 (s, 313); MS (ESI+) m/z 391 (M+H).
Example 68
N-isoquinolin-5-yl-N'-{(4-thiomorpholin-4-ylphenyl )m ethyl] urea
Example 68A
(4-thiomorpholin-4-ylphenyl)methylamine
4-Fluorobenzonitrile and thiomorpholine were processed as described in
Examples
66A and 66B to provide the title compound.
CA 02743534 2011-06-13
Example 68B
N-isoguinolin-5-y1-N-((4-thiomorpholin-4-ylphenyl)methy1 uurea
The product from Example 68A and the product from Example 61A were processed
as described in Example 66C to provide the title compound. The free base was
treated with
methanolic HCl to form the corresponding di-hydrochloride salt. 'H NMR (300
MHz,
DMSO-d-6) S 9.26 (s, 1H), 8.67 (s, IH), 8.53 (d, J=6.1 Hz, IH), 8.32 (dd,
J=7.8 Hz, 1.1 Hz,
1H), 7.92 (d, J=6.1 Hz, IM, 7.73 (d, J=8.2 Hz, IH), 7.60 (m, 1H), 7.20-7.23
(m, 2H), 6.90-
6.96 (m, 31-1), 4.25 (d, J=5.8 Hz, 2H), 3.45-3.51 (m, 411), 2.64-2.67 (m, 4H);
MS (EST) m/z
379 (M+H)+.
Example 69
4-(3,4-dichlorophenyl)-N-isoguinolin-5-ylpiiperazine- l -carboxainide
1-(3,4-Dichlorophenyl)piperazine (1280 mg, 5.55 mmol) in diethyl ether (30 mL)
was
treated with the product from Example 61A (-40 mL). The mixture was filtered,
the filter
cake washed with diethyl ether, and dried under reduced pressure to provide
the title
compound as a white solid (1.78 g, 80%). 'H NMR (300 MHz, DMSO-d6) S 9.29 (d,
J=1.0
Hz, 111), 8.84 (s, 1H), 8.49 (d, J=5.8 Hz, 11-1), 7.92 (d, J=7.8 Hz, 1H), 7.78
(m, 1H), 7.61-7.71
(in, 2H), 7.44 (d, J=8.8 Hz, 1H), 7.22 (d, J=3.1 Hz, 111), 7.01 (dd, J=9.1,
2.7 Bz, 1IT), 3.68
(m, 4H), 3.30 (m, 4H); MS (EST) m/z 401/403 (M+H+, 35C1/37C1).
Example 70
2-isoguinolin-5-yl-N-(4-(trifluoromethyl)benzyllacetamide
Example 70A
ethyl isoguinolin-5-yl(oxo)acetate
The product from Example 57A (11.80 g, 56.6 mmol) in THE (200 mL) at -78 C
was
treated with n-butyllithium (30 mL, 75.0 mmol, 2.5M in hexanes) dropwise.
After 30
minutes, the mixture was treated with diethyl oxalate (25.0 mL, 184 mmol).
After 20
minutes, the solution was allowed to warm to room temperature and was treated
with
saturated NH4C1(150 mL). The mixture was conentrated under reduced pressure.
The
residue was treated with dichloromethane (100 mL) filtered, and the filtrate
concentrated
76
CA 02743534 2011-06-13
under reduced pressure. The residue was purified by column chromatography (20%
ethyl
acetate/hexanes) to provide the title compound as light brown oil (4.57 g,
35%). MS (ESI+)
m/z 248 (100), 230 (M+H)+, (EST-) m/z 200 (M-Et)-; 'H NMR (DMSO-d,6, 300 MHz)
rotomers 8 1.26 (t, J 7.1, 0.6H), 1.37 (t, J 7.1, 2.411), 4.21 (q, J 7.1,
0.411), 4.47 (q, J 7.1,
1.611), 7.89 (t, J 7.5, 111), 8.41 (dd, J 1.0, 7.5, 1H), 8.57 (d, J S. 1,
111), 8.64 (d, J 5.7, 1H),
8.73 (d, J 6.3, 111), 9.50 (s, lfl).
Example 70B
ethyl hydroxy(isoguinolin-5-yl)acetate
The product of Example 70A (1.11 g, 4.83 mmol) in absolute ethanol (20 mL) was
added to 10% Pd/C (115.5 mg) under an argon atmosphere. The reaction mixture
was stirred
under H2 (50 psi) for 5 hours at which time an additional 105.9 mg of catalyst
was added as a
suspension in ethanol. After 3 additional hours, the reaction mixture was
filtered though a
nylon membrane and the filtrate concentrated under reduced pressure to provide
the title
compound as dark brown oil (1.02 g, 91%). MS (ESI+) m/z 232 (M+H)+, (ESI-) m/z
202
(M-Et)-; 'H NMR (DMSO-d6, 300 MHz) 8 1.05 (t, J 7.1, 3H), 4.07 (m, 211), 5.77
(d, J 4.7,
111), 6.36 (d, J 4.7, 111), 7.68 (dd, J.7.3, 8.1, 111), 7.85 (d, J 7.0, 111),
8.09 (t, J 7.5, 2H), 8.53
(d, J 6.2, 1H), 9.33 (s, 1H).
Example 70C
ethyl (acetyloxy)(isoguinolin-5-yl)acetate
The product of Example 70B (1.0202g, 4.41 mmol) in pyridine (15 nL) was
treated
with acetyl chloride (0.35 mL, 4.92 mmol) dropwise. The solution was stirred
at room
temperature for 4 hours and concentrated under reduced pressure. The residue
was purified
by column chromatography (2% methanol/CH2C12) to provide the title compound as
yellow
oil (0.8100 g, 67%). MS (ESI+) m/z 274 (M+H)+; 'H NMR (DMSO-d6, 300 MHz) 6
1.07 (t,
J 7.1, 311), 2.17 (s, 311), 4.13 (m, 2H), 6.62 (s, 111), 7.74 (m, 111), 7.94
(d, J 7.1, 1H), 8.03 (d,
J 6.1, 111), 8.22 (d, J 7.6, 111), 8.60 (d, J 5.7, 1H), 9.39 (s, 111).
Example 70D
ethyl isoguinolin-5-ylacetate
77
CA 02743534 2011-06-13
The product of Example 70C (1.43 g, 5.23 mmol) in absolute ethanol (200 mL)
was
treated with dry 10% Pd/C (0.122 g) and triethylamine (10.4 mL). The reaction
mixture was
stirred at 60 C for 6 hours under H2 (60 psi), filtered and the filtrate
concentrated under
reduced pressure. The residue was purified by column chromatography (5%
methanol/CH2C12) to provide the title compound as light brown oil (0.93 g,
67%). MS (ESI+)
m/z 216 (M+H)+, (ESI-) m/z 214 (M-H)-; 1H NMR (DMSO-d6, 300 MHz) 6 1.17 (t, J
7.1,
3H), 4.09 (q, J 7.1, 211), 4.17 (s, 2H), 7.64 (m, 1H), 7.72 (d, J 6.2, 111),
7.81 (d, J 5.7, IM,
8.07 (d, J 7.9, 1H), 8.54 (d, J 6.1, 1H), 9.33 (s, IH).
Example 70E
2-isoguinolin-5-yl-N-[4-(trifluoromethyl)benzyll acetanii de
The product from Example 70D (0.207 g, 0.96 mmol) in dichloromethane (10 rnL)
was treated with trimethylaluminum (1 mL, 2.0 mmol, 2M in toluene) dropwise.
After 30
minutes, the mixture was teated with 4-(trifluoromethyl)benzylamine (0.350 g,
2.0 mmol) in
dichloromethane (2 mL) and then refluxed for 16 hours. The reaction mixture
was allowed to
cool to room temperature, treated with IM HCI (3 mL), basified to between pH 9
and 10 with
concentrated NH. OH, treated with water and CH2C12 and the phases separated.
The organic
layer was washed with water (1 x 10 mL), brine (1 x 10 mL), dried (MgSO4), and
the filtrate
was concentrated under reduced pressure. The residue was purified by column
chromatography (5% methanol/CH2C12) to provide a yellow residue which was
triturated
with diethyl ether to provide the title compound as a white solid (0.122 g,
37%). MS (ESI+)
m/z 345 (M+H)+; MS (ESI-) m/z 343 (M-H)-; 1H NMR (DMSO, 300 MHz) S 4.00 (s,
2H),
4.37 (d, J 5.7, 2H), 7.46 (d, J 7.8, 2H), 7.67 (m, 411), 7.93 (d, J 5.4, 1M,
8.03 (d, J 7.8, 1 H),
8.52 (d, J 5.8, 1H), 8.80 (t, J 5.7, 1H), 9.31 (s, IM; Anal. Calcd for
C19H15F3N20: C, 66.28;
H, 4.39; N, 8.14. Found: C, 66.16; H, 4.27; N, 7.96.
Example 71
methyl 5-({ {(4-bromobenzyl)aminolcarbonyl} amino)isoguinoline-3-carboxylate
Example 71A
methyl 5-nitroisoguinoline-3-carboxylate
78
CA 02743534 2011-06-13
Methyl isoquinoline-3-carboxylate (9.58 g, 51.2 rnmol) in concentrated H2S04
(100
mL) at 0 C was treated with sodium nitrate (4.79 g, 56.4 mmol) in small
portions such that
the temperature was maintained below 5 C. Ten minutes after addition was
complete, the
reaction mixture was allowed to warm to room temperature and stirred for 2
hours. The
mixture was poured over ice and adjusted to pH between 7 and 8 and filtered to
afford the
title compound as a bright yellow solid (11.44 g, 96%). MS (ESI+) m/z 233
(M+H)+; 1H
NMR (DMSO, 300 MHz) S 3.97 (s, 3H), 8.06 (t, J 8.2, 1H), 8.72 (dt, J 1.0, 8.2,
1H), 8.78 (dd,
J 1.0, 7.8, 1H), 9.11 (s, 1H), 9.65 (s, IH).
Example 71B
methyl 5-aminoisoguinoline-3-carboxylat
The product of Example 71A (10.33 g, 44.5 mmol) in acetic acid/water (3/1)
(320
mL) was treated with iron powder (5.06 g, 90.7 mmol). After stirring for 16
hours at room
temperature, the reaction mixture was filtered the filtrate concentrated under
reduced pressure
to approximately half the original volume. The mixture was then extracted with
dichloromethane (3 x 200 mL). The organic fractions were combined, dried
(MgSO4), and
the filtrate concentrated under reduced pressure to afford crude material. A
precipitate
formed in the aqueous phase after sitting for several hours. This was filtered
to afford
additional crude material. The crude material was purfidied by column
chromatography (2%
methanol/CH2C12) to provide the title compound. MS (ESI+) mlz 203 (M+H)+; MS
(ESI-)
m/z 201 (M-H) 'H NMR (DMSO-d6, 300 MHz) S 3.92 (s, 311), 6.34 (s, 2H), 6.96
(dd, J 1.0,
7.8, IM, 7.31 (d, J 8.1, IH), 7.51 (t, J 7.9, 1H), 8.82 (s, 1H), 9.15 (s, 1H);
Anal. Calcd for
C11HION202: C, 65.34; H, 4.99; N, 13.85. Found: C, 65.03; H, 4.95; N, 13.65.
Example 71C
methyl 5-(f f (4-bromobenzyl)aminolcarbonyl} amino)isoguinoline-3-carboxylate
The product of Example 71B (0.156 g, 0.77 mmol) in THF:toluene (10 ml., 1:1)
was
treated with a solution of 1-bromo-4-(isocyanatomethyl)benzene (0.201 g, 0.95
mmol) in
THE (1.0 mL). After stirring for 16 hours at room temperature, the reaction
mixture was
concentrated under reduced pressure and the residue was triturated with
diethyl ether to
provide the title compound as a tan solid (0.272 g, 85%). MS (ESI+) m/z 415
(M+1-1)+; MS
(ESI-) m/z 413 (M-H)-; 'H NMR (DMSO-d6, 300 AEz) S 3.95 (s, 3H), 4.36 (d, J
5.6, 21-1),
79
CA 02743534 2011-06-13
7.23 (t, J 5.6, 1H), 7.33 (m, 2H), 7.56 (m, 2H), 7.76 (t, J 7.8, 1H), 7.85 (d,
J 8.3, 1H), 8.41
(dd, J 1.5, 7.8, 1H), 8.82 (s, 1H), 9.06 (s, 1H), 9.35 (s, IH); Anal. Calcd
for C19Hi6BrN3O3:
C, 55.09; H, 3.89; N, 10.14. Found: C, 55.06; H, 3.56; N, 9.84.
Example 72
methyl 5-({((2,4-dichlorobenzyl)amino]carbonyl} amino)isoguinoline-3-
carboxylate
The product of Example 71B (0.156 g, 0.77 mmol) in THFAoluene (10 niL, 1:1)
was
treated with a solution of 2,4-dichloro-l-(isocyanatomethyl)benzene (0.195 g,
0.97 mmol) in
THE (1.0 mL). After stirring for 16 hours at room temperature, the reaction
mixture was
concentrated under reduced pressure and the residue was triturated with
diethyl ether to
provide the title compound as a tan solid (0.226 g, 73%). MS (ESI+) m!z 404 (-
%4+H)'-; MS
(ESI-) m/z 402 (M-H)-; 'H NMR (DMSO-d6, 300 MHz) 8 3.96 (s, 3H), 4.44 (d, J
6.0, 2H),
7.29 (m, 1H), 7.48 (m, 1H), 7.65 (d, J 1.7, 1H), 7.76 (t, J 7.8, 1H), 7.86 (d,
J 7.8, 1H), 8.41
(dd, J 1.0, 7.8, 1H), 8.84 (s, 1H), 9.15 (s, 1H), 9.35 (s, IH); Anal. Calcd
for C19H1SC12N303:
C, 56.45; H, 3.74; N, 10.39. Found: C, 56.08; H, 3.67; N, 10.03.
Example 73
N-(8-bromoisoguino1in-5-yl)-N'-(2,4-dichlorob enzyl)urea
Example 73A
8-bromoisoguinolin-5-amine
5-Ami.noisoquinoline (5.50 g, 38.1 mmol) and aluminium trichloride (15.1 g,
113
nnnol) were combined and heated at 80 C in a 3-necked flask equipped with a
dropping
funnel, stirrer bar, needle and sintered glass tube. Bromine (3.04 g, 19.05
mmol) was dripped
onto the sintered glass funnel and the vapour diffused onto the complex over a
period of 2
hours. Heating was continued for 2 hours. The suspension was added portionwise
to crushed
ice and the solution basified with concentrated NaOH solution. The aqueous
layer was
extracted with ethyl acetate (4 x 100 mL) and the layers were separated. The
organic layers
were combined, dried (Na2SO4), filtered and the filtrate was concentrated to
give a grey solid.
The grey solid was subjected to column chromatography (hexanes:ethyl acetate,
3:1) to
provide the title compound (2.96 g, 35%). MS (ESI+) m/z 225 (M+H)+; MS (ESI-)
m/z 223
(M-H)-; 'H NMR (CDC13, 300 MHz) S 4.22 (br s, 2H), 6.83 (d, J 8.1, 1H), 7.25
(s, 1H), 7.54
(d, J 5.8, 1H), 7.61 (d, J 8.1, 1H), 8.59 (d, J 5.8, 1H), 9.56 (s, 111).
CA 02743534 2011-06-13
~E -
Example 73B
N-(8-bromoisoguinolin-5-yl)-N'-(2,4-dichlorobenzyl)urea
The product from Example 73A (120 mg, 0.52 mmol) in THF:toluene (1:4, 5 mL)
was treated with a solution of 2,4-dichloro-l-(isocyanatomethyl)benzene (108
mg, 0.52
mmol) in THE (0.5 mL). After stirring for 16 hours at room temperature, the
mixture was
filtered and the filter cake dried under reduced pressure to provide the title
compound as a
white solid (178 mg, 78%). The hydrochloride salt was obtained by dissolving
the product in
hot THE and adding HCl in diethyl ether (2M). The yellow precipitate was
collected by
filtration and dried under reduced pressure. MS (ESI+) m/z 426 (M+H)+; MS (ESI-
) m/z 424
(M-M-; 'H NMR. (DMSO-d6, 300 MHz) 6 4.42 (d, 5.8, 2H), 7.22 (t, J 5.8, 1H),
7.65 (ni, IH),
7.91 (d, J 8.5, 1H), 8.02 (d. J 6.1, 1H), 8.22 (d, J 8.5, 1H), 8.69 (d, J 5.8,
1H), 9.01 (s, 1II),
9.44 (s, 1H); Anal. Calcd for C17H12BrC12N3O HCI 0.25EtOH: C, 44.41; H, 3.14;
N, 8.88.
Found: C, 44.80; H, 2.76; N, 8.84.
Example 74
N-(8-bromoi soguinolin-5-yl)-N'-(4-fluorob enzyl)urea
The title compound was prepared using 1-fluoro-4-(isocyanatomethyl)benzene,
the
product of Example 73A and the procedure described in Example 73B (white
solid, 108 mg,
65%). MS (ESI+) m/z 376 (M+H)+; MS (ESI-) m/z 374 (M-H)"; 'H NMR (DMSO-d6, 300
MHz) 6 4.35 (d, 5.8, 2H), 7.12 (m, 1H), 7.18 (m, 2H), 7.40 (m, 1H), 7.91 (d, J
8.5, 1H), 7.99
(d, J 6.1, 1H), 8.24 (d, J 8.5, IH), 8.69 (d, J 5.8, 1H), 8.88 (s, IH), 9.44
(s, 1H); Anal. Calcd
for C17H13BrFN3O: C, 54.56; H, 3.50; N, 11.23. Found: C, 54.61; H, 3.35; N,
11.14.
Example 75
N-(8-bromoisoguinolin-5-yl)-N'-(3-fluorobenzyl)urea
The title compound was prepared using 1-fluoro-3-(isocyanatomethyl)benzene,
the
product of Example 73A and the procedure described in Example 73 (white solid,
108 mg,
65%). MS (ESI+) ni/z 376 (M+H)+; MS (ESI-) m/z 374 (M-M-; 'H NMR (DMSO-d6, 300
MHz) 6 4.39 (d, 5.8, 2H), 7.09 (m, 1H), 7.17 (m, 2H), 7.40 (m, 1H), 7.91 (d, J
8.5, 1H), 8.01
(d, J 6.1, 1H), 8.23 (d, J 8.5, 1H), 8.69 (d, 3 5.8, 1H), 8.93 (s, 1H), 9.44
(s, 1H); Anal. Calcd
for C17H13BrFN3O: C, 54.56; H, 3.50; N, 11.23. Found: C, 54.64; H, 3.33; N,
11.19.
81
CA 02743534 2011-06-13
Example 76
N-f 1-(4-chlorophenyl)-1-methylethyll-N'-isoguinolin-5-ylurea
Example 76A
2-(4-chlorophenyl)-2-meth lpropanoyl chloride
2-(4-Chlorophenyl)-2-methylpropanoic acid (3.85 g, 19.4 mmol) in toluene (S
mL)
was treated with thionyl chloride (5.00g, 3.1 mL) and heated at 80 C for 2
hours. The
cooled solution was concentrated under reduced pressure to provide a yellow
oil containing a
crystalline residue. The mixture was dissolved in hexane, filtered and the
filtrate
concentrated to provide the compound as a pale yellow oil (4.10 g, 98%).
Example 76B
1-chloro-4-(1-isocy anato-l-methylethyl)benzene
The product of Example 76A (4.00 g, 19.4 mmol) in acetone (9 mL) at 0 C was
treated with a solution of sodium azide (1.27 g) in water (9 mL) dropwise over
15 minutes.
After stirring for 30 minutes at 0 C, the mixture was extracted with toluene
(20 mL). The
organic extract was dried with MgSO4, filtered, and the filtrate heated at
reflux for 1 hour.
The mixture was allowed to cool to room temperature and was concentrated under
reduced
pressure to provide the title compound as a pale yellow oil (3.45 g, 96%).
Example 76C
N-f 1-(4-chlorophenyl)-1-methylethyll-N'-isoguinolin-5-ylurea
The title compound was prepared using 5-anainoisoquinoline, the product of
Example
76B and the procedure described in Example 73B except that THE was used as
solvent. The
product was recrystallized from ethyl acetate to provide the title compound as
a white solid
(840 mg, 34%). MS (ESI+) m/z 355 (M+H)+; MS (ESI-) m/z 353 (M-H)-; 'H NMR
(DMSO-
d6, 300 MHz) S 1.63 (s, 6H), 7.23 (s, IH), 7.37 (d, J 8.8, 2H), 7.47 (d, J
8.8, 2I-i), 7.73 (t, J
9.2, 1H), 7.93 (d, J 8.1, 1H), 8.25 (d, J 6.4, 1H), 8.39 (d, J 8.1, 111), 8.67
(d, J 6.4, 1H), 8.87
(s, 111), 9.58 (s, 1H); Anal. Calcd for C19H1SC1N30 HC10.25EtOH: C, 60.40; H,
5.33; N,
10.54. Found: C, 60.82; H, 5.23; N, 10.45.
82
CA 02743534 2011-06-13
Example 77
N-(4-bromobenzyl)-N'- f 6-[2,2,2-trifluoro-l-hydroxy-1-
(trifluoromethyl)ethyl]isoquinolin-5-
1 urea
Example 77A
2-(5-aminoisoquinolin-6-yl)-1,1,1,3,3,3-hex afluoroprol an-2-o l
5-Aminoisoquinoline (288 mg, 2.00 mmol) and p-toluenesulfonic acid (5 mg) were
combined and treated with hexafluoroacetone hexahydrate (0.29 mL, 462 mg, 2.10
nunol).
The mixture was stirred in a sealed pressure tube and heated to 150 C for 18
hours. The
reaction was allowed to cool to room temperature and partitioned between
CH2C12 (20 mL)
and water (10 mL). The organic layer was passed thru Na-2SO4 and then filtered
through
activated charcoal. The charcoal was washed with methanol (3 x 10 mL) and the
filtrate and
washuigswere collected and concentrated under reduced pressure to provide the
title
compound (130 mg, 30%) as a yellow solid. MS (ESI+) m/z 311 (M+H)+; MS (ESI-)
m/z
309 (M-H)-; 1H NMR (DMSO, 300 MHz) S 6.64 (br s, 2H), 7.30 (d, J 8.7, 1H),
7.40 (d, J 8.7,
IH), 8.09 (d, J 6.1, 1H), 9.49 (d, J 6.1, 1H), 9.14 (s, IH); 13C NMR (DMSO,
100 MIHIz) S
107.02, 110.60, 113.95 (1), 115.46 (1), 122.03, 124.92, 124.92, 1.25.94,
126.98 (1), 128.17,
142.43 (1), 144.82, 151.85 (1).
Example 77B
N-(4-bromobenzyl)-N- f 6-[2,2,2-trifluoro-l-hydroxy-l-
(trifluoromethyl)ethyl]isoquinolin-5-
Iurea
The title compound was prepared using 1-bromo-4-(isocyanatomethyl)benzen.e,
the
product of Example 77A'and the procedure described in Example 73B except that
THE was
used as solvent (white solid, 840 mg, 34%). MS (ESI+) m/z 376 (M+H)+; MS (ESI-
) m/z 374
(1\2-H)-; 'H NMR (DMSO-d6, 300 MHz) S 4.35 (d, 5.8, 2H), 7.12 (m, 1H), 7.18
(m, 2H), 7.40
(m, IH), 7.91 (d, J 8.5, 1H), 7.99 (d, J 6.1, 1H), 8.24 (d, J 8.5, 1H), 8.69
(d, J 5.8, 1H), 8.88
(s, 1H), 9.44 (s, 1H); Anal. Calcd for C20H14BrF6N3O2: C, 46.00; H, 3.50; N,
11.23. Found:
C, 54.61; H, 3.35; N, 11.14.
Example 78
N-(4-bromobenzyl)-N-1 H-indol-4-ylurea
83
CA 02743534 2011-06-13
4-aminoindole (0.13 g, I mmol) in THE (3 mL) was treated with 1-bromo-4-
(isocyanatomethyl.)benzene (0.23 g, 1.1 mmol) for 3 hours at ambient
temperature. Hexane
was added to the reaction mixture to precipitate 0. 26 g of the title compound
as a tan solid.
mp 198 C; 'H NMR (300 MHz, DMSO-d6) S 4.30 (d, 2H), 6.51 (t, lii), 6.89 (t,
1H), 6.95 (d,
2H), 7.29 (t, IH), 7.31 (d, 2H), 7.55 (d, 2H), 7.62 (dd, 1H), 8.3 (s, 111),
11.04 (s, 11-1); MS
(DCI+) m/z 346 (M+H); Anal. Calcd. For C16H14N3BrO: C, 55.83; H, 4.10; N,
12.21. Found:
C, 55.71, H, 4.12; N, 12.01.
Example 79
N-(3 ,4-dichl orob enzyl)-N'-1 H-indol-4-y lurea
4-Aminoindole (0.13 g, I mmol) in THE (3 mL) was treated with 1,2-dichloro-4-
(isocyanatomethyl)benzene (0.22 g, 1.1 mmol) for 3 h at ambient temperature.
Hexane was
added to the reaction mixture to precipitate 0. 25 g of the title compound as
a tan solid. mp
201 C; 'H NMR (300 MHz, DMSO-d6) S 4.23 (d, 2 H), 6.36 (s, 1H), 6.54 (t, 1H),
7. 0 (dd, 1
H), 7.25 (m, 2H), 7.30 (d, 2H), 7.45 (d, 1H), 7.6 (m, 21-1),8.31 (s, IH),
10.87 (s, 1H) MS
(DCI+) m/z 336 (M+H); Anal. Calcd. For C16H13N3C12O: C, 57.50; H, 3.92; N,
12.57. Found:
C, 56.94, H, 3.68; N, 11.97.
Example 80
N- I H-indol-4-yl-N'-(4-(trifluoromethyl)benzyljurea
Example 80A
4-isocyanato-1 H-indole
4-Aminoindole (0.5 g, 3.78 mmol) in toluene (50 mL) was treated with
triphosgene
(0.4 g, 1.35 mmol) and heated at reflux for 5 hours. The reaction mixture was
allowed to
cool to room temperature and concentrated under reduced pressure. The residue
was taken
up in diethyl ether, filtered, and the filtrate was concentrated under reduced
pressure to
provide title compound as yellow oil (0.4 g). 1H NMR (300 MHz, CDC13-d6) S
6.62 (m, 1H),
6.84 (d, 1H), 7.1 (t, 1H), 7.23 (m, 2H), 8.3 (s, IH).
Example 80B
N- 1 H-indol-4-yl-N'-[4-(trifluoromethyl )benzyljurea
84
CA 02743534 2011-06-13
The product of Example 80A (0.16 g, I mmol) in THE (3 mL) was treated with 4-
(trifluoromethyl)benzylamine (0.19 g, 1.1 mmol) at ambient temperature. After
stirring for 3
hours, hexane was added to the reaction mixture to precipitate the title
compound as a solid.
mp 178 C. 'H NMR (300 MHz, DMSO-d6) 8 4.43 (d, 2H), 6.53 (t, 1H), (6.98 m,
3H), 7.26
(t, 111, 7.57 (d, 2H), 7.62 (d, 1H), 7.71 (d, 2H), 8.37 (s, 1H), 11.04 (s,
IH); MS (DCI+) nn/z
334 (M+H); Anal. Calcd. For C17H14N3F30: C, 61.26; H, 4.23; N, 12.61. Found:
C, 61.28, H,
3.83; N, 12.31.
Example 81
N-IH-indol-4-yl-N'-r4-(tiifluoromethoxy)ben7-yI urea
4-(Trifluoromethoxy)benzylamine (0.21 g, 1.1 mmol) and the product of Example
80A
(0.16 g, 1 mmol) were treated as described in Example SOB to provide the title
compound
(0.23 g). nip 177 C; 'H NMR (300 MHz, DMSO-d6) 6 4.36 (d, 2 H), 6.52 (m, 1H),
6.95 (m,
3H), 7.24 (t, 1 H), 7.36 (d, 2H), 7.48 (d, 2H), 7. 63 (dd, 1H), 8.32 (1H),
11.06 (s, 1H); MS
(DCI+) m/z 349.9 (M+H)+; Anal. Calcd. For C17H14N3F302: C, 58.63, H, 4.34, N,
12.07.
Found: C, 58.51, H, 3.98, N, 12.03.
Example 82
N-j3-fluoro-4-(trifluoromethyl)benzyll-N'-1 H-indol-4-ylurea
3-Fluoro-4-(trifluoromethyl)benzylamine (0.22g, 1.1 mmol) and the product of
Example 80A (0.16 g, 1 mmol) were treated as described in Example 80B to
provide the title
compound (0.24 g). mp 198 C; 1H NMR (300 MHz, DMSO-d6) 8 4.43 (d, 2H), 6.52
(m,
1H), 6.98 (m, 3 H), 7.26 (m, 111, 7.39 (m, 2 H), 7.57 (dd, 1H), 7.77 (t, 1H),
8.40 (s, 1H),
11.05 (s, 1H); MS (DCI+) m/z 349.9 (M+H)+. Anal. Calcd. for C17H13N3F40: C,
58.12; H,
3.73; N, 11.96. Found C, 58.52; H, 3.99; N, 11.55.
Example 83
1 -(4-Chloro-3-trifluoromethyI-benzyl)-3-(1 H-indol-4-yl)-urea
4-Chloro-3-(trifluoromethyl)benzylamine (0.27g, 1.1 mmol) and the product of
Example 80A (0.16 g, 1 mmol) were treated as described in Example 80B to
provide the title
compound. mp 197 C;'H NMR (300 MHz, DMSO-d6) 6 4.42 (d, 2H), 6.52 (m, 1H),
6.96
(m, 3H), 7.25 (m, 1H), 7.56 (dd, 11-1), 7.67 (dd, 111), 7.70 (t, 1H), 7.81 (s,
11-1), 8.37 (s, 1H),
CA 02743534 2011-06-13
11.06 (s, 1H); MS (DCI+) m/z 368 (1VI+H). Anal. Calcd. for C17H13N3C1F30:.C,
55.52, H,
3.56; N, 11.43. Found C, 55.46; H, 3.65; N, 11.58.
Example 84
1-(4-Chloro-3-trifluoromethyl)-3-(1H-indol-4-yl)-urea
4-Chlorobenzylamine (0.2-,1.4 mmol) and the product of Example 80A (0.2 g,
1.27
mmol) were treated as described in Example 80B to provide the title compound.
mp 205 C.
'H NMR (300 MHz, DMSO-d6) 5 4.32 (d, 2H), 6.52 (m, I H), 6.87 (m, I H), 6.97
(m, 2H),
7.25 (m, 1H), 7.37 (m, 4H), 7.6 (m, IM, 8.30 (s, 111), 11.06 (s, 1H). MS
(DCI+) m/z 300
(M+H). Anal. Calcd. for C16H14N3C130: C, 64.11; H, 4.71; N, 14.02. Found: C,
63.99; H,
4.70; N, 13.77.
Example 85
N-[2-(2,4-dichlorophenyl)ethyl)-N-1 H-indol-4-ylurea
2-(2,4-Dichlorophenyl)ethylamine (0.21 g, 1.1 mmol) and the product of Example
80A (0.16 g, 1. mmol) were treated as described in Example 80B to provide the
title
compound. mp 170 C; 1H NMR (300 MHz, DMSO-d6) 8 2.90 (m, 2H), 3.31 (m, 214),
6.47
(m, 2H), 6.93 (m, 2H), 7.23 (m, 1H), 7.40 (m, 2H), 7.60 (m, 2H), 8.15 (s, 1H),
11.02 (s, 114).
MS (DCI+) m/z 347 (M+H). Anal. Calcd. for C17H15N3C120: C, 58.63; H, 4.34; N,
12.07.
Found: C, 58.49; H, 4.49; N, 12.38.
Example 86
4-(trifluoromethyl)benzyl 1H-indol-4-ylcarbamate
[4-(Trifluoromethyl)phenyl]methanol (0.09 g, 0.55 mmol) and the product of
Example 80A (0.08 g, 0.5 mmol) in THE (5 mL) were heated at reflux for 16
hours with a
catalytic amount of triethylamine. The reaction mixture was concentrated under
reduced
pressure and the residue was purified by chromatography on silica gel eluting
with 50%
hexane:ethylacetate to provide the title compound as an oil (0.09 g). 1H NMR
(300 MHz,
DMSO-d6) 6 5.32 (s, 2H), 6.73 (s, IH), 7.0 (t, 1H), 7.11 (d, 1H), 7.23 (t,
1H), 7.38 (d, III),
7.66 (d, 2H), 7.78 (d, 2H), 9.52 (s, 1H), 11.08 (s, 1H). Anal. Calcd. for
C17H13N2F3O2: C,
61.08; H, 3.92; N, 8.38. Found: C, 60.97; H, 4.21; N, 8.17.
86
CA 02743534 2011-06-13
Example 87
4-(trifluoromethoxy)benzyl 1 H-indol-4-ylcarbamate
[4-(Trifluoromethoxy)phenyl]methanol (0.13 g, 0.7 mmol) and the product of
Example 80A (0.1 g, 0.63 mmol) in THE (5 mL) were heated at reflux for 16
hours with a
catalytic amount of triethylamine. The reaction mixture was concentrated under
reduced
pressure and the residue was triturated with diethyl ether/hexane to provide
the title
compound as tan crystals (0.12 g). 'H NMR (300 MHz, DMSO-d6) 8 5.21 (s, 2H),
6.73 (s,
11-1), 7.0 (t, IH), 7.1 (d, 1H), 7.23 (t, IH), 7.38 (dd, 1H), 7.4 (d, 2H), 7.6
(d, 211), 9.5 (s, 111),
11.06 (s, 1H). ). Anal. Calcd. for C17H13N2F303Ø25 H2O: C, 57.55; H, 3.84;
N, 7.90. Found:
C, 57.42; H, 3.81; N, 7.32.
Example 88
N- (4-bromobenzyl)-N-(2,3-dunethyl-lH-indol-4-yl)urea
2,3-Diunethyl-4-aminoindole (0.11 g, 0.7 mmol) in THE (3 mL) was treated with
1-
bromo-4-(isocyanatometlryl)benzene (0.17 g, 0.8 nunol) at ambient temperature.
After
stirring for 3 hours at ambient temperature, hexane was added to the reaction
mixture to
precipitate the title compound as a tan solid (0.12 g). mp 190 C 'H NMR (300
MHz,
DMSO-d6) 6 2.24 (s, 31.1), 2.25 (s, 311), 4.25 (d, 2H), 6.51 (t, 1H), 6.82 (t,
1H), 6.85 (d, 2H),
6.95 (m, 2H), 7.25 (d, 2H), 7.53 (d, 2H), 7.78 (s, 111), 11.04 (s, 1H); MS
(DCI+) m/z 346
(M+H)+; Anal. Calcd. for C13Hi8N3BrO: C, 58.08; H, 4.87; N, 11.29. Found: C,
57.97, H,
4.92; N, 11.30.
Example 89
N-(4-bromobenzyl)-N'-1 H-indazol-4-ylurea
Example 89A
1 H-indazol-4-amine
4-Nitro-IH-indazole (1.63 g, 10 mmol) in ethanol (100 mL) was treated with
BiC13
(3.46 g, 11 mmol) followed by a portionwise addition of NaBH4. The reaction
mixture was
stirred at ambient temperature for 20 minutes and filtered through Celite. The
filtrate was
evaporated under reduced pressure and the residue was partitioned between
ethyl
acetate/dilute NaHCO3 solution. The organic layer was dried over MgSO4,
filtered, and the
87
CA 02743534 2011-06-13
filtrate concentrated under reduced pressure to provide the title compound as
a tan solid (1.0
g) 'H NMR (300 MHz, DMSO-d6) S 5.64 (s, 2H), 6.1 (d, 1H), 6.6 (d, 1H), 6.98
(t, 1H), 8.03
(s, 1H), 12.6 (s, 1H).
Example 89B
N-(4-bromobenzyl)-N'-1H-indazol-4-ylurea hydrochloride salt
The product of Example 89A (0.16 g, 1.2 mmol) in THE (10 mL) was treated with
1-
bromo-4-(isocyanatomethyl)benzene (0.52 g, 2.4 mmol) at room temperature.
After stirring
for 16 hours, the reaction mixture was concentrated and the residue was
treated with
methanol (20 mL) and 3N HCl (10 mL) and heated at reflux for 3 hours. The
reaction
mixture was allowed to cool to room temperature, evaporated under reduced
pressure, and the
residue was treated with water and the pH adjusted to 5. The obtained compound
was
purified by chromatography eluting with 5% of ethanol:methylene chloride and
converted to
HCl salt mp 126 C. 'H NMR (300 MHz, DMSO-d6) S 4.32 (d, 2H), 7.0 (t, 111),
7.05 (d,
1 H), 7.18 (t, 111), 7.3 (d, 2H), 7.55 (d, 2H), 7.61 (d, 111), 8.16 (s, 111),
8.92 (s, 111); Analysis
Caled for C15H13N4BrO HCI: C, 47.21; H, 3.70; N, 14.68. Found C, 46.99; H,
4.08; N, 14.13.
Example 90
N-(3 ,4-dichlorob enzyl)-N'-1 H-indazol-4-ylurea
Example 90A
methyl 4-nitro-lH-indazole-l-carboxylate
Sodium hydride (0.3 g, 12.5 mmol) suspended in DMF (5 mL) at 0 C was treated
with 4-nitro-lH-indazole (1.33 g, 10 mmol). After stirring at room temperature
for 1 hour, the
mixture was treated with methylchloroformate (0.9 mL). After stirring at room
temperature
for 3 hours, the mixture was carefully treated with water and filtered to
provide the title
compound (1.2 g). 1H NMR (300 MHz, DMSO-d6) S 4.19 (s, 3H), 7.9 (t, 1H), 8.38
(d, 1H),
8.62 (d, 1H), 8.85 (s, 111).
Example 90B
methyl 4-amino-1 H-indazole- l -carboxylate
88
CA 02743534 2011-06-13
The product of Example 90A (1.66 g, 7.5 mmol) in ethanol (20 mL) was treated
with
BiC13 (8.2 g, 2.6 mmol) followed by the addition of NaBH4 (1.13 g, 30.5 mmol).
The
reaction mixture was stirred at room temperature for 20 minutes, filtered
through Celite, and
the filtrate was evaporated under reduced pressure. The residue was
partitioned between
ethyl acetate/dilute NaHCO3 solution. The organic phase was separated, dried
over MgSO4,
filtered and the filtrate concentrated under reduced pressure to provide the
title compound
(1.2 g). 'H NMR (300 MHz, DMSO-d6) 6 6.1 (s, 2H), 6.41 (dd, II-I), 7.21 (m,
2H), 8.42 (s,
1H).
Example 90C
methyl 4-(f ((3,4-dichlorobenzyl)aminolcarbonyl} amino)- 1H-indazole- l -carbo
y1ate
The product of Example 90B (0.19 g, 1 mmol) in TIC' (3 mL) was treated with
1,2-
dichloro-4-(isocyanatomethyl)benzene (0.22 g, 1.1 mmol) at ambient
temperature. After
stirring for 3 hours, hexane was added to the reaction mixture to precipitate
the title
compound as a tan solid (0. 25 g). 'H NMR (300 MHz, DMSO-d6) 6 4.38 (d, 2H),
6.97 (t,
IM, 7.36 (dd, I H), 7.48 (t, 1H), 7.6 (m, 2H), 7.7 (d, I H), 7.8 (d, 114),
8.45 (s, 1 H), 9.16 (s,
1H).
Example 90D
N-(3,4-dichlorobenzyl)-N'-1 H-indazol-4-ylurea
The product of Example 90C (0.25 g, 0.6 mmol) was heated at reflux in methanol
(5
mL) and 0.5N KOH (1 mL) for 0.5 hours. The reaction mixture was allowed to
cool to
ambient temperature, pH was adjusted to 5, and volume was reduced under
reduced pressure.
Methylene chloride and water was added, the phases were separated, and the
organic phase
concentrated under reduced pressure to provide the title compound. 'H NMR (300
MHz,
DMSO-d6) S 4.38 (d, 2H), 6.9 (t, 1H), 7.05 (d, 1H), 7.19 (t, 1H), 7.35 (dd,
IH), 7.6 (m, 2 H),
8.06 (s, 1H), 8.82 (s, 1H). MS (DCI+) m/z 336 (M+H)+; Anal. Calcd. For
C15H13N4C120: C,
53.75; H, 3.62; N, 16.72. Found: C, 53.84, H, 3.44; N, 16.88.
Example 97
N-(1,1'-biphenyl-4-ylmethyl)-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example IB
using
1,1'-biphenyl-4-ylmethylamine instead of 2-(3-fluorophenyl)ethylamine. NMR
(DMSO-d6) 6
89
CA 02743534 2011-06-13
9.78 (s, 1H), 9.57 (s, 1H), 8.69 (s, 2H), 8.53 (d, 1H), 8.11 (d, 1H), 7.87 (t,
1H), 7.64 (m, 5Ii),
7.45 (m, 4H), 7.35 (m, 1H), 4.43 (d, 2H); MS (ESI) (M+H)+ 354.
Example 98
N-(3-fluoro-4-(trifluoromethyl)benzyl]-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 113
using
3-fluoro-4-(trifluoromethyl)benzylamine instead of 2-(3-
fluorophenyl)ethylamine. NMR
(DMSO-d6) 6 9.78 (s, 1H), 9.74 (s, 11-I), 8.77 (d, 1H), 8.71 (d, IH), 8.61 (d,
1H), 8.08 (d, 1H),
7.87 (m, 2H), 7.78 (d, 1H), 7.43 (m, 2H), 4.49 (d, 2H); MS (ESI) (M+H)+ 364.
Example 99
N-5-isoguinolinyl-N'-(3-methylbenzyl)urea
The title compound was prepared using the procedure described in Example 113
using
3-methylbenzylaminc instead of 2-(3-fluorophenyl)ethylainine. NMR (DMSO-d6) 6
9.68 (s,
1H), 9.18 (s, 11-1), 9.23 (s, 1H), 8.66 (d, 1H), 8.37 (d, IH), 8.48 (d, 1H),
8.04 (d, 1H), 7.85 (t,
1H), 7.35 (t, 1H), 7.23 (t, 1H), 7.7.26 (m, 11-1), 7.06 (m, 1H),4.28 (d, 21-
1), 2.31 (s, 3H); MS
(ESI) (M+H)+ 291.
Example 100
N-(4-fluoro-3-(trifluoromethyl)benzyl]-N'-5-isoq uinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 4-fluoro-3-(trifluoromethyl)benzylamine instead of 4-cyanobenzyl
alcohol. NMR
(DMSO-d6) 6 9.31 (s, 111), 8.84 (s, 111), 8.65 (d, 2H), 7.95 (d, 2H), 7.86 (m,
2H), 7.60 (t,
1H), 7.50 (d, 1H), 7.17 (t, 1H), 4.43 (d, 2H); MS (ESI) (M+H)+ 364.
Example 101
N-(3 -chl oro-4-fluorobenzyl)-N'-5 -isoq uino linylurea
The title compound was prepared using the procedure described in Example 61B
using 3-chloro-4-fluorobenzylamine instead of 4-cyanobenzyl alcohol. NMR (DMSO-
d6) 6
9.72 (s, 1H), 9.42 (s, 1H), 8.68 (d, 1H), 8.58 (d, 2H), 8.05 (d, 1H), 7.88 (t,
1H), 7.67 (m, 2H),
7.20 (m, 2H), 4.38 (d, 2H); MS (ESI) (M+H)+ 330.
CA 02743534 2011-06-13
Example 102
N-5-isoquinolinyl-N'-pentyl urea
The title compound was prepared using the procedure described in Example 60F
using 1-isocyanatopentane and 5-isoquinolinamine instead of the product from
Example 60E
and 1-bromo-4-(isocyanatomethyl)benzene. NMR (DMSO-d6) 6 9.70 (s, 1H), 9.19
(s, 1H),
8.64 (d, 1H), 8.57 (m, 2H), 8.01 (d, 11I), 7.84 (d, 111), 7.85 (t, 1H), 6.95
(in, 111), 3.17 (m,
2H), 2.48 (m, 2H), 1.23 (m, 4H), 0.86 (M, 3H); MS (ESI) (M+II)+ 339.
Example 103
N-5-isoquinolinyl-N'-octylurea
The title compound was prepared using the procedure described in Example 60F
using 1-isocyanatooctane and 5-isoquinolinamine instead of the product from
Example 60E
and 1-bromo-4-(isocyanatomethyl)benzene. NMR (DMSO-d6) 6 9.53 (s, 11-1), 9.23
(s, 111),
8.65 (d, I H), 8.99 (d, 111), 8.05 (d, 1H), 7.86 (t, 111), 7.01 (m, I H), 3.15
(m, 211), 1.51 (in,
2H), 1.28 (m, 5H), 0.83 (in, 3H); MS (ESI) (M+H)+ 300.
Example 104
N-(1-adamantylmethyl)-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 1-(1-adamantyl)methanamine instead of 4-cyanobenzyl alcohol. NTI (DMSO-
d6) 6
9.68 (s, 11-1), 9.20 (s, 1H), 8.64 (d, 2H), 8.60 (d, 111), 8.65 (in, 1H), 8.00
(d, 1H), 7.83 (t, 1H),
6.95 (in, 1H), 2.90 (d, 211), 1.99 (m, 2H), 1.64 (m, 5H), 1.53 (m, 5H); MS
(ESI) (M+H)+ 336.
Example 105
N-(cyclohexylmethyl)-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 1-eyclohexylmethanamine instead of 4-cyanobenzyl alcohol. NMR (DMSO-d6)
6 9.70
(s, IH), 9.18 (s, 1H), 8.67 (d, 2H), 8.57 (m, 3H), 8.00 (d, 1H), 7.84 (t, 1H),
7.00 (in, 1H), 3.06
(m,2H), 1.70 (m, 5H), 1.43(m, 111), 1.21 (m, 3H). 0.97 (m, 2H); MS (ESI)
(M+H)+ 284.
619946 Example 107
N-((6, 6-dimethylbicyclo [ 3.1. I lhept-2-yl)methyll-N'-5-iso g uino linylurea
91
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 61B
using [6,6-dimethylbicyclo[3.1.1]hept-2-yl]methylamine instead of 4-
cyanobenzyl alcohol.
NMR (DMSO-d6) S 9.74 (s, 1H), 9.28 (s, 113), 8.64 (d, 11-1), 8.60 (m, 211),
8.03 (s, 1H), 7.85
(t, IM, 7.08 (m, 1H), 3.17 (m, 2H), 2.38 (m, 1H), 2.18 (m,3H), 2.00 (m, IH),
1.88 (in, 5H),
1.20 (s, 3H), 1.03 (s, 3H); MS (ESI) (M+H)+ 324.
Example 108
N-5-isoguinolinyl-N'-14-(1-pyrrolidinyl)benzyllurea
The title compound was prepared using the procedure described in Example 61B
using 4-(1-pyrrolidinyl)benzylamine instead of 4-cyanobenzyl alcohol. NMR
(DMSO-d6) S
9.81 (s, 1H), 9.58 (s, 111), 8.80 (d, 1H), 8.71 (m, 2H), 8.11 (d, 1H), 7.93
(t, 1H), 7.48 (bs,
1H), 7.20 (m, 2H), 6.65 (m, 2H), 4.43 (d,2H), 3.13 (m, 4H), 1.97 (m, 4H); MS
(ESI) (M+H)+
347.
Example 109
N-[4-(1-azepanyl)benzyll-N-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 4-(1-azepanyl)benzylamine instead of 4-cyanobenzyl alcohol. NMR (DMSO-
d6) 6
9.80 (s, 1H), 9.58 (s, 1H), 8.79 (m, 1H), 8.71 (m, 2H), 8.11 (d, 1H), 7.95 (t,
111), 7.48 (bs,
1H), 7.20 (m, 2H), 6.85 (bs, 2H), 4.23 (d,2H), 3.45 (in, 4H), 1.69 (bs, 4H),
1.50 (bs, 4H); MS
(ESI) (M+H)+ 375.
Example 110
N- f3-fluoro-4-(1-pyrrolidinyl)benzyll-N-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 3-fluoro-4-(1-pyrrolidinyl)benzylamine instead of 4-cyanobenzyl alcohol.
NMR
(DMSO-d6) 6 9.82 (s, 1H), 9.72 (s, 1H), 8.85 (d, 111), 8.70 (m, 2H), 8.14 (d,
1H), 7.95 (t,
1H), 7.64 (bs, 1H), 7.03 (m, 2H), 6.75 (t, 1H), 4.25 (d,2H), 3.30 (m, 4H),
1.74 (m, 4H); MS
(ESI) (M+H)+ 365.
Example 111
N-14-(1-azepanyl)-3-fluorobenzyll-N-5-isoguinolinylurea
92
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 61B
using 4-(1-azepanyl)-3-fluorobenzylamine instead of 4-cyanobenzyl alcohol. NMR
(DMSO-
df) 8 9.85 (s, 1H), 9.77 (s, 1H), 8.71 (m, 2H), 8.13 (d, 111), 7.94 (t, IH),
7.77 (bs, M), 7.64
(bs, 1H), 7.10-6.90 (m, 2H), 4.28 (d,2H), 3.35 (m, 41I), 1.77 (m, 4H), 1.58
(in, 4H); MS (ESI)
(h2+H)+ 393.
Example 112
N-[4-(I-azocanyl)benzyl]-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 4-(l-azocanyl)benzylamine instead of 4-cyanobenzyl alcohol. NMR (DMSO-
d6) 6
9.85 (s, 1H), 9.67 (s, 1H), 8.70 (s, 1H), 8.77 (m, 211), 8.13 (s, 111), 7.95
(t, 1H), 7.45 (bs, 11-I),
7.17(d, 2H), 6.63 (d, 214), 4.23 (d,2H), 3.43 (m, 6H), 1.68 (m, 3H), 1.44 (m,
5H); MS (ESI)
(M+H)+ 389.
Example 114
N-1 H-indazol-4-y1-N'-[4-(1-piperidinyl)benzyl]urea
The title compound was prepared using the procedure described in Example 89B
using ]-[4-(isocyanatomethyl)phenyl]piperidine instead of 1-bromo-4-
(isocyanatomethyl)benzene. NMR (DMSO-d6) 6 9.23 (s, 1H), 9.30 (s, 1H), 7.78
(d, 2H),
7.64 (d, 1H), 7.63 (d, 211), 7.53 (s, 1H), 7.38 (bs, 1H), 7.19 (t, 1H), 7.06
(d, 1H), 4.39 (d,2H),
3.53 (m, 4H), 1.97 (bs, 411), 1.64 (bs, 2H); MS (ESI) (M+H)+ 350.
Example 115
N-[3-fluoro-4-(1-pip eridinyl)benzyl]-N'-1 H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 89B
using 1-[2-fluoro-4-(isocyanatomethyl)phenyl]piperi dine instead of 1-bromo-4-
(isocyanatomethyl)benzene. NMR (DMSO-d6) 8 9.17 (s, 1H), 8.28 (s, 111), 7.63
(d, 1H),
7.40-7.15 (m, 6H), 7.05 (d, 1H), 4.37 (s,2H), 3.17 (m, 4H), 1.77 (m, 4H), 1.58
(m, 2H).4H),
1.64 (bs, 2H); MS (ESI) (M+H)+ 368.
Example 116
N-I H-indazol-4-y1-N'-[4-(1-pyrrolidinyl)benzyl]urea
93
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 89B
using 1-[4-(isocyanatomethyl)phenyl]pyrrolidine instead of 1-bromo-4-
(isocyanatomethyl)benzene. NMR (DMSO-d6) b 8.83 (s, 1H), 8.15 (s, 1H), 8.01
(bs, 1H),
7.63 (d, 1H), 7.21 (m, 3H), 7.04 (d, 1H), 6.70 (bs, 1H), 6.63 (m, 1H), 6.56
(d, 1H), 4.12
(d,2H), 3.13 (m, 4H), 1.97 (m, 4H); MS (ESI) (M+H)+ 336.
Example 117
N- [3-fluoro-4-(1-pyrrolidinyl)benzyl -N'-1H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 89B
using 1-[2-fluoro-4-(isocyanatomethyl)phenyl]pyrrolidine instead of 1-bromo-4-
(isoeyanatomethyl)benzene. NMR (DMSO-d6) S 9.89 (s, 1H), 8.17 (s, 1H), 7.63
(d, 1H),
7.19 (t, 1H), 7.07 (m, 1H), 7.02 (d, III), 6.99(s, 113), 6.93 (bs, 2H), 6.74
(t, 1H), 4.23 (s,2H),
3.29 (m, 4H), 1.87 (m, 4H); MS (ESI) (M+H)+ 354.
Example 118
N-14-( 1-azepanyl)benzyll-N'-1 H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 89B
using 1-[4-(isocyanatomethyl)phenyl]azepane instead of 1-bromo-4-
(isocyanatomethyl)benzene. NMR (DMSO-d6) S 8.86 (s, 1H), 8.17 (s, 1H), 8.00
(bs, 1H),
7.64 (d, 1H), 7.20 (m, 3H), 7.02 (d, 1H), 6.25 (bs, 2H), 6.70 (d, 1H), 4.21
(s,2H), 1.88 (m,
6H), 1.47 (m, 6H); MS (ESI) (M+H)+ 364.
764293 Example 119
N-[4-( 1-azepanyl)-3-fluorobenzyll-N-1 H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 89B
using 1-[2-fluoro-4-(isocyanatomethyl)phenyl]azepane instead of 1-bromo-4-
(isocyanatomethyl)benzene. NMR (DMSO-d6) S 9.04 (s, 1H), 8.13 (s, 1H), 7.63
(d, IH),
7.19 (t, 1H), 7.10 (s, IH), 7.02 (d, 4H), 4.23 (s,2H), 3.37 (m, 4H), 1.79 (m,
4H), 1.57 (m,
4H); MS (ESI) (M+H)+ 382.
Example 120
N-(1-methyl-I H-indazol-4-yl)-N'-[4-(1-piperidinyl)benzyl]urea
94
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 89B
using l-[4-(isocyanatomethyl)phenyl]piperidine and 1-methyl-IH-indazol-4-amine
instead of
1-bromo-4-(isocyanatomethyl)benzene and the product from Example 89A. NMR
(DMSO-
d6) 8 9.43 (s, 1H), 8.37 (s, 111), 7.82 (d, 2H), 7.69 (d, 1H), 7.63 (m, 3H),
7.22 (t, 1H), 7.11 (t,
1Hn, 4.40 (d,2H), 3.99 (s, 3H), 3.50 (m, 4H), 1.98 (m, 4H), 1.67 (m. 2H); MS
(ESI) (M+H)+
364.
Example 121
N-[3-fluoro-4-(1-piperidinyl)benzyll-N'-(1-methyl-I H-indazol-4-yl)urea
The title compound was prepared using the procedure described in Example 89B
using 1-[2-fluoro-4-(isocyanatomethyl)phenyl]piperidine and 1-methyl-iH-
indazol-4-amine
instead of 1-bromo-4-(isocyanatomethyl)benzene and the product from-Example
89A. NMR
(DMSO-d6) 6 9.19 (s, 1H), 8.22 (s, 1H), 7.25 (m, 4H), 7.18 (d, 2H), 4.31
(s,2H), 4.00 (s, 3H),
3.15 (m, 4H), 1.77 (m, 4H), 1.66 (m, 2H); MS (ESI) (M+H)+ 382.
Example 122
N-(1-methyl- 1H-indazol-4-yl)-N'-[4-(I -pyrrolidinyl)benzyllurea
The title compound was prepared using the procedure described in Example 89B
using 1-[4-(isocyanatomethyl)phenyl]pyrrolidine and 1-methyl-1H-indazol-4-
amine instead
of 1-bromo-4-(isocyanatomethyl)benzene and the product from Example 89A. NMR
(DMSO-d6) 6 8.98 (s, 1H), 8.16 (s, 1H), 7.63 (d, 1H), 7.13 (m, 3H), 7.12 (d,
1H), 6.94 (m,
IH),6.73 (bs, 2H), 4.23 (s, 211), 3.99 (s, 31-1), 3.24 (m, 41-i), 1.98 (m,
4H); MS (ESI) (M+H)+
350.
764300 Example 123
N-[3-fluoro-4--l -pyrrolidinyl)benzyll-N'-(1-methyl-1H-indazol-4-yl)urea
The title compound was prepared using the procedure described in Example 89B
using 1-[2-fluoro-4-(isocyanatomethyl)phenyl]pyrrolidine and 1-methyl-IH-
indazol-4-amine
instead of 1-bromo-4-(isocyanatomethyl)benzene and the product from Example
89A. NMR
(DMSO-d6) S 8.98 (s, 1H), 8.18 (s, 1H), 7.63 (d, IH), 7.12 (t, IH), 7.10 (m,
21-1), 7.01 (m,
2H), 6.75 (t, 1H), 4.22 (s, 2H), 3.99 (s, 3H), 3.30 (m, 4H), 1.89 (m, 4H); MS
(ESI) (M+H)+
368.
CA 02743534 2011-06-13
ps
Example 124
N-[4-(1-azepanyl)benzyl]-N'-(1-methyl-1 H-indazol-4-yl)urea
The title compound was prepared using the procedure described in Example 89B
using 1-[4-(isocyanatomethyl)phenyl]azepane and 1-methyl-lH-indazol-4-amine
instead of
1-bromo-4-(isocyanatomethyl)benzene and the product from Example 89A. NMR
(DMSO-
d6) 8 8.97(s, 1H), 8.18 (s, 1H), 7.65 (d, 1H), 7.14 (m, 4H), 7.11 (d, 1H),
6.95 (bs, 2H), 4.23
(s, 2H), 3.99 (s, 3H), 3.27 (m, 4H), 1.90 (m, 4H), 1.53 (m, 41-1); MS (ESI)
(M+H)+ 378.
Example 125
N-[4-(1-azepanyl)-3 -fluorobenzyl]-N'-(l -methyl- I H-indazol-4-yl)urea
The title compound was prepared using the procedure described in Example 89B
using 1-[2-fluoro-4-(isocyanatomethyl)phenyl]azepane and 1-methyl-IH-indazol-4-
amine
instead of 1-bromo-4-(isocyanatomethyl)benzene and the product from Example
89A. NMR
(DMSO-d6) S 9.03(s, 1H), 8.19 (s, 1H), 7.67 (d, 1H), 7.24 (t, 1H), 7.12-6.95
(m, 5H), 4.22 (s,
2H), 3.99 (s, 3H), 3.35 (m, 4H), 1.78 (m, 4H), 1.55 (m, 4H); MS (ESI) (M+H)+
396.
Example 126
4-methylbenzyI 5-isoguinolinylcarbamate
The title compound was prepared using the procedure described in Example lB
using
4-methylbenzyl alcohol instead of 2-(3-fluorophenyl)ethylamine. 1H NMR (300
A41-1z, d6-
DMSO) 9.82 (s, 1H), 9.31 (s, 111), 8.50 (d, 111), 7.93 (m, 3H), 7.68 (t, I H),
7.37 (d, 2H), 7.25
(d, 2H), 5.19 (s, 2H), 2.32 (s, 3H); MS (DCUNH3) m/e 293 (M+H)+.
Example 127
N-5-isoguinolinyl-2-[4-(trifluoromethyl)phenyllhydrazinecarboxamide
The title compound was prepared using the procedure described in Example 61B
using 4-tiifluoromethylphenyl hydrazine instead of 4-cyanobenzyl alcohol. ]H
NMR (300
MHz, d6-DMSO) 9.80 (m, 2H), 9.10 (broad s, 1H), 8.90-8.43 (m, 314), 8.40
(broad s, 1H),
8.20 (d, 1H), 7.93 (t, 1H), 7.58 (d, 2H), 6.96 (d, 2H); MS (DCI/NH3) m/e 347
(M+H)+; Anal.
Calcd. For C17H13N4OF3. 1.0 HCl 0.1 H20: C 53.09; H 3.72; N 14.57. Found: C
52.80; H
3.81; N 14.51.
96
CA 02743534 2011-06-13
Example 128
4-bromobenzyl 5-isoguinolinylcarbamate
The title compound was prepared using the procedure described in Example 1B
using
4-bromobenzyl alcohol instead of 2-(3-fluorophenyl)ethylamine. 1H NMR (300
MHz, d6-
DMSO) 10.23 (s, IH), 9.86 (s, 1H), 8.69 (d, 111), 8.50 (d, 1H), 8.30 (d, 2IT),
7.98 (t, 111), 7.60
(m, 211), 7.44 (d, 2H), 5.20 (s, 211); MS (DCI/NH3) m/e 357 (M+H)+; Anal.
Calcd. For
C17H13N2O2Br. 1.0 HCI: C 51.87; H 3.58; N 7.12. Found: C 51.95; H 3.45; N
7.03.
Example 129
N-benzhydryl-N'-5-isoguinolin_ylurea
The title compound was prepared using the procedure described in Example 61B
using benzhydrylamine instead of 4-cyanobenzyl alcohol. 'H NMR (300 MBz, d6-
DMSO)
9.26 (s, 1IT), 8.78 (s, 111), 8.57 (d, 1H), 8.31 (m, 1H), 7.94 (d, 1H), 7.70
(d, 1H), 7.60 (m,
2H), 7.38 (m, 8H), 7.27 (m, 211), 6.02 (d, 1H); MS (DCI/NH3) m/e 354 (M+H)+;
Anal. Calcd.
For C23H19N30. 0.1 H20: C 77.77; H 5.45; N 11.83. Found: C 77.52; H 5.30; N
11.98.
Example 130
N-r(1 S)-1-(4-bromophenyl)ethyll-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using (1S)-1-(4-bromophenyl)ethanamine instead of 4-cyanobenzyl alcohol. 1H
NMR (300
MHz, d6-DMSO) 9.78 (s, 1H), 9.46 (s, 1H), 8.70 (s, 2H), 8.59 (d, 1H), 8.04 (d,
11-1), 7.84 (t,
111), 7.75 (d, 1H), 7.58 (d, 2H), 7.40 (d, 2H), 4.85 (m, 1H), 1.40 (d, 311);
MS (DCI/NH3) m/e
370 (4+H)+.
Anal. Calcd. For C18H16N3OBr. 1.2 HCl: C 52.22; H 4.19; N 10.15. Found: C
51.86; H 4.28;
N 9.78.
Example 131
N-{(1 R)-1-(4-bromophenyl)ethyll-N'-5 -i soguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using (1R)-1-(4-bromophenyl)ethanamine instead of 4-cyanobenzyl alcohol. 'H
NMR (300
97
CA 02743534 2011-06-13
MHz, d6-DMSO) 9.65 (s, 1H), 9.46 (s, 1H), 8.71 (s, 2H), 8.60 (d, 111), 8.04
(d, 1H), 7.84 (t,
1H), 7.78 (d, 1H), 7.58 (d, 211), 7.38 (d, 2H), 4.87 (m, 11-1), 1.40 (d, 3H);
MS (DCUNH3) m/e
370 (M+H)+; Anal. Calcd. For C1sH16N3OBr. 1.1 HC1: C 52.69; H 4.20; N 10.24.
Found: C
52.52; H 4.28; N 10.00.
Example 132
N-(4-bromobenzyl)-2-(3 -methyl-5-isoguinolinyl)acetanude
Example 132A
5-allyl-3-methylisoguinoline
3-Methyl-5-bromoisoquinoline (1.0 g, 4.5 mmol), tributylallyltin (1.6 mL, 5.0
mmol),
and dichlrobis(tri-o-tolylphosphine)palladium (11) were combined in toluene
(100 mL) and
refluxed for 14 hours. The mixture was cooled, diluted with ethyl acetate, and
washed twice
with aqueous NH4C1. The organic phase was separated, concentrated, and the
residue was
purified by chromatography (ethyl acetate:hexanes, 30:70) to provide the title
compound. 'H
NMR (300 MHz, d6-DMSO) 9.21 (s, 111), 8.00 (d, 1H), 7.63 (m, 2H), 7.58 (m,
1H), 4.18 (s,
211), 3.62 (s, 3H), 2.62 (s, 3H). MS (DCI/NH3) m/e 216 (M+H)
Example 132B
methyl (3-methyl-5-isoguinolinyl)acetate
The product from Example 132A (0.8 g, 4.37 mmol) in CH2CL2 (40 mL) and 2.5
MNaOH in MeOH (9 mL, 22 mmol, 5 eq.) was ozonized at -78 C for 3 hours. The
mixture
was diluted with diethyl ether and washed with aqueous NH4C1. The organic
phase was
separated, concentrated, and the residue was purified by chromatography (ethyl
acetate:hexanes, 40:60) to provide the title compound. 'H NMR (300 MHz, d6-
DMSO) 9.20
(s, 1H), 7.92 (d, 1H), 7.73 (s, 1H), 7.55 (m, 21D, 6.08 (m, 111), 5.15-5.04
(m, 2H), 3.80 (d,
21'), 2.63 (s, 3H); MS (DCI/NH3) m/e 184 (M+H)+
Example 132C
N-(4-bromobenzyl)-2-(3-methyl-5-isoguinolinyl)acetamide
4-Bromobenzylamine (3.06 mmol) in CH2C12 (30 mL) was treated with 2M Me3AI
(1.53 mL, 3.06 mmol) in toluene. After 30 minutes, the mixture was treated
with the product
98
CA 02743534 2011-06-13
from Example 132B (0.33 g, 1.53 mmol) and refluxed for 16 hours. The mixture
was cooled,
quenched with IN HCI, diluted with ethyl acetate, and washed with water,
aqueous NaHCO3
and aqueous NH4CI. The organic phase was evaporated and the residue dissolved
in
CH2C12:MeOH and 1M HCI (3 mL) in diethyl ether. After stirring for 2 hours,
the mixture
was concentrated under reduced pressure to provide the title compound. 1 H NMR
(300 MHz,
d6-DMSO) 9.75 (s, 1H), 8.92 (m, 1H), 8.30 (m, 2H), 8.00 (d, 11-1), 7.82 (m,
1H), 7.60 (d, 2H),
7.20 (d, 2H), 4.22 (d, 2H), 4.08 (s, 2H), 2.78 (s, 3H); MS (DCUNF13) m/e 369
(M-I-H)+; Anal.
Calcd. For C19H17N2OBr. 2.0 HCI. 1.7 H2O: C 48.27; H 4.78; N 5.92. Found: C
47.89; H
4.21; N 6.32.
Example 133
N-(4-bromobenzyl)-2-(5-isoguinolinyI)acetamide
Example 133A
5-allylisoguinoline
The title compound was prepared using the procedure described in Example 132A
using 5-bromoisoquinoline instead of 3-methyl-5-bromoisoquinoline.
Example 133B
methyl 5-isoguinolinylacetate
The title compound was prepared using the procedure described in Example 132B
using the product from Example 133A instead of the product from Example 132A.
Example 133C
N-(4-bromobenzyl)-2 (5-isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 132C
using the product from Example 133B instead of the product from Example 132B.
'H NMR
(300 MHz, d6-DMSO) 9.78 (s, 1H), 8.85 (m, 1H), 8.68 (d, 1H), 8.42 (d, 1H),
7.90 (d, 1H),
8.01 (d, I H), 7.94 (m, 111), 7.52 (d, 2H), 7.20 (d, 2H), 4.22 (d, 2H), 4.10
(s, 211); MS
(DCI/NH3) m/e 355 (M+H){; Anal. Calcd. For C1SH15N2OBr. 1.0 HCl. 0.3 H2O: C
54.44; H
4.21; N 7.05. Found: C 54.11; H 4.18; N 6.86.
99
CA 02743534 2011-06-13
{ Example 134
N-(1-(4-bromophenyl)ethyll-2-(5-isogwinolinyl)acetamide
The title compound was prepared using the procedure described in Example 132C
using the product from Example 133B and 1-(4-bromophenyl)ethanamine instead of
the
product from Example 132B and 4-bromobenzylanline. 1H NMR (300 MHz, d6-DMSO)
9.81
(s, 1H), 9.00 (d, 1H), 8.70 (d, 1H), 8.48 (d, 1H), 8.40 (d, 111), 8.04 (d,
1H), 7.92 (m, 1H),
7.51 (d, 2H), 7.23 (d, 2H), 4.84 (m, 1H), 4.10 (s, 2H), 1.35 (d, 3H). MS
(DCI/NH3) mle 369
(M+H)+; Anal. Calcd. For C19H17N2OBr. 1.0 HCI. 1.0 H2O: C 53.86; H 4.76; N
6.61. Found:
C 53.47; H 4.53; N 6.76.
Example 135
N-41-(4-bromophenyl)ethyl}-2-(3-methyl-5-isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 132C
using 1-(4-bromophenyl)ethanamine instead of 4-bromobenzylamine. 'H NMR (300
MHz,
d6-DMSO) 9.68 (s, 1H), 8.84 (d, 1H), 8.24 (m, 2H), 7.92 (d, 1H), 7.80 (m, 1H),
7.50 (d, 2H),
7.28 (d, 2H), 4.02 (s, 21-1), 2.75 (s, 311), 1.38 (s, 311); MS (DCI/NH3) mle
383 (M+H)+; Anal.
Caled. For C20H19N2OBr. 0.9 HCI: C 57.73; H 4.82; N 6.73. Found: C 57.69; H
4.80; N 6.07.
Example 136
N-5 -isog uinolinyl-N- { 1-(4-(trifluoromethyl)phenyI{ ethyl } urea
Example 136A
1-r4-(trifluoromethyl)phenyllethanone oxime
4-Trifluoromethylacetophenone (13.6 g, 72.3 mmol) and O-methylhydroxylamine
hydrochloride were combined in pyridine (100 mL) and stirred at ambient
temperature for 16
hours. The mixture was concentrated under reduced pressure and the residue was
suspended
in diethyl ether. The suspension was filtered and the filter cake was washed
with diethyl
ether. The filtrate was washed with water, 1N HCI, and water. The organic
phase was
concentrated to provide the title compound. 'H NMR (300 MHz, d6-DMSO) 7.90-
7.68 (m,
4H), 3.97 and 3.78 (2S, 1H), 2.20 and 2.17 (2s, 31-1); MS (DCI/NH3) m/e 218
(M+H)+.
Example 136B
1- [4-(tri fluorom ethyl)phenyl l eth an am i n e
100
CA 02743534 2011-06-13
The product from Example 136A (21.0 g, 100 mmol) in MeOH (220 mL) and
ammonia (30 mL) was treated with 10% PdIC tinder 60 psi of hydrogen gas for 2
hours. The
mixture was filtered and the filtrate was concentrated to provide the title
compound. 'H
NMR (300 MHz, d6-DMSO) 7.60 (q, 4H), 4.07 (q, 1H), 3.28 (broad s, 2H), 1.24
(d, 314); MS
(DCI/NH3) m/e 190 (M+H)+
Example 1.36C
N-5-isoquinolinyl-N'-Il-F4-(trifluoromethyl)phenyllethyl urea
The title compound was prepared using the procedure described in Example 61B
using the product from Example 136B instead of 4-cyanobenzyl alcohol. 'H NMR
(300
MHz, d6-DMSO) 9.80 (s, 1H), 9.75 (s, 1H), 8.90 (d, 1H), 8.73 (d, 1H), 8.63 (d,
1H), 8.08 (m,
2H), 7.90 (t, 1H), 7.77 (d, 211), 7.64 (d, 211), 4.95 (in, 1H), 1.41 (d, 3H);
MS (DCI/NH3) m/e
360 (M+H)+; Anal. Calcd. For C19H16N30F3. 1.0 HCI. 0.3 H2O: C 56.88; H 4.42; N
10.47.
Found: C 56.61; H 4.49; N 10.28.
Example 138
O N-5-isoquinolinyl-N-{(1S) 1-[4-(trifluoromethyl)phenyllethyl}urea
Example 138A
(1.R)-2-oxo-I-phenyl-2-({1-[4-(trifluoromethyll)phenyl]eth)ll}a.lnino)ethyl
acetate
1-[4-(Trifluoromethyl)phenyl]ethanamine (37.5 g, 198.4 mmol) and (R)-
acetylmandelic acid (40.4 g, 208.3 mmol, 1.05 eq.) were combined in DMAP (0.7
g, 5.7
nunol) and treated with DCC (45.0 g, 218 mmol). After stirring overnight at
ambient
temperature, the mixture was filtered through a plug of silica. The filtrate
was concentrated
and the residue was purified by chromatography on Biotage Flash 75 column
(ethyl
acetate:hexanes, 25:75) to provide a faster running diastereomer and a slower
running
diastereomer. (fast diastereomer)'H NMR (300 MHz, CDCI3) 7.58 (d, 2H), 7.39
(m, 711),
6.30 broad (d, 111), 6.08 (s, 1H), 5.18 (m, 1H), 2.20 (s, 3H), 1.29 (d, 3H);
MS (DCI/NH3) m/e
366 (M+H)+. (slow diastereomer)'H NMR (300 MHz, CDC13) 7.58 (d, 2H), 7.40 (m,
5H),
7.31 (d, 2H), 6.21 (broad d, IH), 6.06 (s, 1H), 5.18 (m, 1H), 2.20 (s, 311),
1.50 (d, 3H); MS
(DCI/NH3) m/e 366 (M+H)+.
Example 138B
(-) 1-[4-(trifluoromethyl)phenyllethanamine
101
CA 02743534 2011-06-13
the raster running diastereomer from Example 138A (29.2 g, 80 mmol) was
treated
with 48% aqueous HBr (350 nzL) and water (50 nil-) and was refluxed for 16
hours. The
mixture was cooled and extracted with diethyl ether. The aqueous phase was
basified with
2N NaOH (pH 12-13) and extracted with diethyl ether. The organic phase was
concentrated
to provide the title compound. 94% ee (by Mosher amide NMR). [a]D -19.1 (c
1.15;
McOH);'H NMR (300 MHz, CDCI3) 7.60 (d, 2H), 7.47 (d, 2H), 4.20 (m, 1H), 1.65
(br s,
2H), 1.40 (s, 3H); MS (DCI/NH3) m/e 190 (M+I'+.
Example 138C
(+) 1-[4-(trifluoromethyl)phenyllethanamine
The slower running diastereomer from Example 138A (29.2 g, 80 mmol) was
treated
with 48% aqueous HBr (350 mL) and water (50 mL) and was refluxed for 16 hours.
The
mixture was cooled and extracted with diethyl ether. The aqueous phase was
basified with
2N NaOH (pH 12-13) and extracted with diethyl ether. The organic phase was
concentrated
to provide the title compound. [a]D +20.5 (c 1.47; McOH). 94% ee (Mosher
amide NMR);
'H NMR (300 IVIRz, CDCI3) 7.60 (d, 2H), 7.47 (d, 2H), 4.20 (m, 1H), 1.60 (br
s, 2H), 1.40 (s,
3H); MS (DCI/NH3) m/e 190 (M+H)+
Example 138D
(-) N-5-isoguinolinyl-N'-{(1S)-1-[4-(trifluorometh_yl)phenyl)ethyl}urea
The title compound was prepared using the procedure described in Example 61B
using the product from Example 138B instead of 4-cyanobenzyl alcohol. 'H NMR
(300
MHz, d6-DMSO) 9.90 (s, 1H), 9.83 (s, 1H), 9.00 (d, 1H), 8.72 (d, 1H), 8.66 (d,
1H), 8.23 (d,
1H), 8.10 (d, 1H), 7.90 (t, 1H), 7.72 (d, 2H), 7.64 (d, 2H), 4.98 (m, 111),
1.43 (d, 314); MS
(DCI/NH3) m/e 360 (M+H)+; [all) -18.4 (c 1.24; MeOH); Anal. Caled. For
C19H16N30F3.
1.0 HCIØ7 H2O: C 55.88; H 4.54; N 10.29. Found: C 55.70; H 4.40; N 10.12.
Example 139
(+} N-5-isoguinolinyl-N-{(1S)-1-[4-(trifluoromethyl)phenyllethyl}ure
The title compound was prepared using the procedure described in Example 61B
using the product from Example 138C instead of 4-cyanobenzyl alcohol. 'H NMR
(300
MHz, d6-DMSO) 9.90 (s, 2H), 8.98 (d, 1H), 8.72 (d, 1H), 8.66 (d, 1H), 8.19 (d,
1H), 8.10 (d,
102
CA 02743534 2011-06-13
1H), 7.90 (t, IH), 7.72 (d, 2H), 7.64 (d, 2H), 4.98 (m, 11), 1.43 (d, 311); MS
(DCIJNH3) m/e
360 (M+H)+. [(X ID +17.0 (c 1.55; MeOH); Anal. Calcd. For C19H16N30F3. 1.0
HCI. 0.4 H2O:
C 56.63; H 4.45; N 10.43. Found: C 56.43; 114.52; N 10.24.
Example 140
N-[ 1-(4-tert-butylphenyl)ethyl l-N'-5-isoquinolinylurea
Example 140A
1-(4-tert-butylphenyl)ethanamuie
The title compound was prepared using 1-(4-tert-butylphenyl)ethanone and the
procedures described in Examples 136A and 136B
Example 140B
N-[ l -(4-tert-butylphenyl)ethyll-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using the product from Example 140A instead of 4-cyanobenzyl alcohol. 'H NMR
(300
MHz, d6-DMSO) 9.88 (s, 1I1), 9.72 (broad s, IH), 8.90 (d, 1H), 8.70 (d, IH),
8.64 (d, II-I),
8.07 (d, 1H), 7.87 (t, 111), 7.78 (d, 1H), 7.38 (m, 4H), 4.94 (in, 1H), 1.42
(d, 311), 1.27 (s,
9H); MS (DCUNH3) m/e 348 (M+H)+; Anal. Calcd. For C22H25N30. 1.0 HCI. 0.6 H20:
C
66.94; H 6.96; N 10.65. Found: C 66.69; H 6.92; N 10.52.
Example 141
N- {cyclopropyl[4-(trifluoromethyl)phenyllmethyl} -N'-5-isoguinolinylurea
Example 141A
N-methoxy-N-methyl-4-(trifluoromethyl)benzamide
4-(Trifluoromethyl)benzoyl chloride (5.0 g, 23.9 minol) and N,O-
dimethyihydroxylamine hydrochloride (2.55 g, 26.3 mmol, 1.1 eq.) were combined
in CH2CI2
(200 mL) at 0 C and treated with pyridine (4.3 mL, 52.6 mrnol). After
stirring for 2 hours,
the mixture was allowed to attain ambient temperature, diluted with diethyl
ether and washed
with water, aqueous HCI, and water. the organic phase was separated and
concentrated to
103
CA 02743534 2011-06-13
provide the title compound which was used directly in the next step. 'H NMR
(300 MHz, d6-
DMSO) 7.90 (m, 4H), 3.52 (s, 3H), 3.28 (s, 3H); MS (DCUNH3) m/e 234 (M+H)+.
Example 141B
cyclopropyl(4-(trifluoromethyl)phenyl1methanone
The product from Example 141A (1.02 g, 4.38 mmol) in THE (50 mL) at 0 C was
treated with 0.8M solution of cyclopropylmagnesium bromide (7.1 mL, 5.7 mmol,
1.3 eq) in
THF. After stirring for 1 hour, the mixture was treated with water (51nL), 3N
HCl (0.5 mL),
diluted with diethyl ether, and washed with water. The organic phase was
separated,
evaporated, and and the residue was purified by chromatography (ethyl
acetate:hexanes,
5:95) to provide the title compound. 1H NMR (300 MHz, d6-DMSO) 8.24 (d, 2H),
7.92 (d,
2H), 2.92 (m, 1H), 1.10 (m, 4H).
Example 141 C
1 -cyclopropyl- l -T4-(trifluoromethyl)phenyl lmethanamine
The title compound was prepared using the product from Example 141B and the
procedures described in Examples 136A and 136B. 1H NMR (300 MHz, d6-DMSO) 7.92
(m,
4H), 3.24 (d, 1H), 1.92 (broad s, 2H), 0.93 (m, 1H), 0.50-0.27 (m, 4H); MS
(DCIINH3) m/e
216 (M+H)+.
Example 141D
N- fcyclopropyl[4-(trifluoromethyl)phenyllmethyl}-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using the product from Example 141C instead of 4-cyanobenzyl alcohol. 'H NMR
(300 MHz,
d6-DMSO) 9.78 (s, 1H), 9.63 (s, 1H), 8.80 (d, 1H), 8.70 (d, 1H), 8.60 (d, 1H),
8.07 (m, 21),
7.86 (t, 1H), 7.73 (d, 2H), 7.63 (d, 2H), 4.37 (t, 1H), 1.10 (m, 1H), 0.60-
0.40 (m, 4H); MS
(DCI NH3) m/e 386 (M+H)+; Anal. Calcd. For C21H1sN30F3. 1.0 HCL 0.25 H2O: C
59.16; H
4.81; N 9.86. Found: C 58.81; H 4.76; N 9.62.
Example 142
(2E)-N-5-isoguinolinyl-3-[4-(trifluoromethyI)phenyl}-2-butenamide
104
CA 02743534 2011-06-13
Example 142A
ethyl (2E)-3-[4-(trifluoromethyl)phenyll-2-butenoate
A suspension of 98% Nall (0.81 g, 33.7 mmol) in THE (100 mL) at ambient
temperature was treated with triethyl phosphonate (6.9 g, 31 mmol) dropwise
and the
resulting mixture was stirred for 15 minutes. The mixture was treated with I -
[4-
(trifluoromethyl)phenyllethanone (5.0 g, 26.6 nunol) portion wise and refluxed
for 6 hours.
After cooling to ambient temperature, the mixture was quenched with aqueous
NH4C1,
diluted with diethyl ether, and washed with water and aqueous N L1C1. The
organic phase
was separated, concentrated, and the residue purified by chromatography (ethyl
acetate:hexanes, 2:98) to provide the (E) isomer (3.4 g, 50%) and the (Z)
isomer (1.3 g, 19
%). Geometry of the double bond was established by NOE studies. (E) isomer:
III NMR for
(300 MHz, d6-DMSO) 7.78 (m, 41-1), 6.25 (in, 1H), 4.19, (q, 2H), 2.51, s, 3H),
1.22 (t, 3H);
MS (DCI/NH3) role 259 (M+H)+.
Example 142B
ethyl (2Z)-3-[4-(trifluoromethyl)phenyll-2-butenoate
The title compound was isolated from the chromatography described in Example
142A. (Z) isomer: 'H NMR (300 MHz, d6-DMSO) 7.71 (d, 2H), 7.42 (d, 21-1), 6.03
(m, 1H),
3.90 (q, 2H), 2.18 (d, 3H), 1.00 (t, 3II); MS (DCI/NH3) m/e 259 (M+1)+.
Example 142C
(2E)-3-[4-(trifluoromethyl)phenyll-2-buteiioic acid
The product from Example 142A (3.5 g, 13.5 mmol) in EtOH (80 mL) was treated
with aqueous IM NaOH (40 mL) and stirred for 16 hours at ambient temperature.
The
reaction mixture was neutralized with IN HCl (40 mL), diluted with brine, and
extracted with
diethyl ether to provide the title compound. NMR (CDC13) 2.60 (s, 31), 6.82
(s, IH), 7.58
(d, 2H), 7.65 (d, 2H).
Example 142D
(2E)-N-5-isoguinolinyl-3-[4-(trifluoromethyl)phenyll-2-butenamide
The product from Example 142C (0.23 g, 1.00 mmol) in CH2CI2 (5 mL) was treated
with oxalyl chloride (0.15 g, 1.2 mmol), 1 drop of DMF, and stirred at ambient
temperature
105
CA 02743534 2011-06-13
for 45 minutes. The mixture was treated with a solution of 5-aminoisoquinoline
(0.14 g, 1.0
mmol) and 98% NaH (0.048 g, 1.2 mmol) in DMF (5 mL) prepared separately by
stirring for
45 minutes. The resulting mixture was stirred for 15 minutes, poured into
water, and
extracted with CH2CI2. The organic phase was dried (MgSO4), evaporated, and
the residue
triturated with diethyl ether. The solid was dried under reduced pressure to
provide the title
compound. 'H NMR (300 MHz, d6-DMSO) 2.61 (s, 311), 2.73 (s, 0.45H, DMF)), 2.89
(s,
0.45H (DMF)), 6.82 (br s, 1H), 7.70 (t, 1H), 7.83 (s, 4H), 7.95 (d, 1H), 8.04
(d, IH), 8.21 (d,
IH), 8.56 (d, 1H), 9.33 (s, 1H), 10.20 (s, IM; MS (ESI+) 357 (M+H)+;
Elemental: Calculated
for C20H15N2OF3-HC1-0.l5C3H7NO: C66.87, H4.40, N8.20; Found: C66.83, H4.20,
N8.27.
Example 143
N-5-isoguinolinyl-3- f4-. trifluoromethyl)phenyll-3-butenamide
The title compound was isolated from the procedure described Example 142D as a
side-product. 'H NMR (300 MHz, d6-DMSO) 3.83 (s, 2H), 5.49 (s, 1H), 5.74 (s,
1H), 7.64 (t,
1H), 7.77 (m, 4H), 7.93 (m, 2H), 8.49 (d, 1H), 9.30 (s, 1H), 10.18 (s, 111);
MS (ESI+) 357
(M+H)+; Elemental: Calculated for C20H15N2OF3Ø6H20: C65.43, H4.45, N7.63;
Found:
C65.49, H4.08, N7.93.
Example 144
(2Z)-N-5-isoguinolinyl-3-f 4-(trifluoromethyl)phenyll-2-butenamide
Example 144A
(2Z)-3-f4-(trifluorometh_y1)phenyll-2-butenoic acid
The title compound was prepared using the procedure described in Example 142C
using the product from Example 142B instead of the product from Example 142A.
Example 144B
(2Z)-N-5-isoguinolinyl-3-(4-(trifluoromethyl)phen ll-2-butenamide
The title compound was prepared using the procedure described in Example 142D
using the product from Example 144A instead of the product from Example 142C.
'H NMR
(300 MHz, d6-DMSO) 2.21 (s, 3H), 6.48 (s, 1H), 7.50 (d, 2H), 7.60 (t, 1H),
7.67 (d, 2H), 7.90
(d, 1H), 7.95 (d, 1H), 8.44 (d, 1H), 9.27 (s, 1H), 10.03 (s, 1H); MS (ESI+)
357 (M+H)+;
106
CA 02743534 2011-06-13
Elemental: Calculated for CZOH15NZOF3: C67.41, H424, N7.86; Found: C67.16,
H4.15,
N7.59.
Example 145
(2E)-3- [3-fluoro-4-(trifluoromethyl)phenyl-N-5-isoguinolinyl-2-butenamide
Example 145A
(2E)-3-[3-fluoro-4-(trifluoromethyl)phenyl]-2-butenoic acid
The title compound was prepared using 1-[3-fluoro-4-
(trifluoromethyl)phenyljethanone and the procedures described in Examples 142A
and 142C.
Example 145B
(2E)-3-{3-fluoro-4-(trifluoromethyl)phenyll-2-butenoic acid
The title compound was prepared using the procedure described in 142D using
the
product from Example 145A instead of the product from Example 142C. 1H NMR
(300
MHz, d6-DMSO) 2.59 (s, 3H), 6.92 (s, 1H), 7.68 (d, 1H), 7.78 (d, 1H), 7.93 (m,
2H), 8.25 (d,
1H), 8.44 (d, 1H), 8.49 (d, 111), 8.70 (d, 11-1), 9.76 (s, 111), 10.59 (s,
1H); MS (ESI+) 375
(M+H)+; Elemental: Calculated for C201:114N2OF4.1.6HC1: C55.52, H3.63, N6.47;
Found:
C55.60, H3.80, N6.09.
Example 146
3-{3-fluoro-4-(trifluoromethyl)phenyl)-N-5-isoguinolinyl-3-butenamide
The title compound was isolated from the procedure described in Example 145B
as a
side-product. 1H NMR (300 MHz, d6-DMSO) 3.88 (s, 2H), 5.57 (s, 1H), 5.86 (s, 1
H), 7.60-
7.88 (m, 4H), 8.18 (m, 3H), 8.64 (d, 1H), 9.65 (s, 1H), 10.50 (s, 1H); MS
(.ESI+) 375
(M+H)+; Elemental: Calculated for C20H14N2OF4=HCl=0.2NH4Cl: C56.99, H3.78,
N7.3 1;
Found: C56.73, H3.69, N7.43.
768062 Example 147
(2E)-N-5-isoguinolinyl-3-[4-(1-piperidinyl)phenyl)-2-butenamde
Example 147A
107
CA 02743534 2011-06-13
(2E)-3-[4-(1-piperidinyl)phenyl]-2-butenoic acid
The title compound was prepared using 1-[4-(1-piperidinyl)phenyl]ethanone and
the
procedures described in Examples 142A and 142C.
Example 147B
(2E)-N-5-isoquinolinyl-3-[4-(1-piperidinyl)phenyl)-2-butenamide
The title compound was prepared using the procedure described in 142D using
the
product from Example 147A instead of the product from Example 142C. 1H NMR
(300
MHz, d6-DMSO) 10.50 (s, 111), 9.82 (s, 1H), 8.71 (d, 1H), 8.58 (d, 111), 8.47
(d, 111), 8.26 (d,
lH), 7.95 (m, 2H), 7.62 (m, 2II), 6.80 (s, 1H), 3.20 (m, 4H), 2.58 (s, 3II),
1.90-1.56 (m, 6H);
MS (DCI/NH3) m/e 372 (M+H)+; Anal. Calcd. For C24H25N30. 2.0 HCI. 2.0 H2O. 0.3
DMF:
C 59.24; H 6.69; N 9.27. Found: C 59.44; H 6.83; N 9.24.
Example 148
N-(3-fluorobenzyl)-N-(3 methyl-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 60F
using 1-fluoro-3-(isocyanatomethyl)benzene and 3-methyl-5-isoquinolinamine
instead of the
product from Example 60E and 1-bromo-4-(isocyanatornethyl)benzene. 1H NMR (300
MIIz,
DMSO-d6) S 9.18 (s, 1H), 8.69 (bs, iH), 8.87 (bs, 11-1), 8.20 (d, 1H, J=6.9
Hz), 7.76 (s, lH),
7.70 (d, IN, J=7.8 Hz), 7.50 (t, 1H, J=7.8 Hz), 7.41 (m, 1H), 7.23-7.05 (m,
3H), 4.39 (d, 2H,
J=6 Hz), 2.65 (s, 3H). MS (ESI) 310 (M+H)+. Anal. Calcd for C18H16FN30: C,
69.89; H,
5.21; N, 13.58. Found: C, 69.86; H, 5.24; N, 13.56.
Example 149
N-(4-bromo-3-fluorobenzyl)-N-5-isoquinolinylurea
The title compound was prepared using the procedure described in Example I B
using
4-bromo-3-fluorobenzylanline instead of 2-(3-fluorophenyl)ethylamine. 1H NMR
(300 MHz,
DMSO-d6) 8 9.74 (s, 1H), 9.55 (s, 1H), 8.67 (m, 2H), 8.57 (dd, IH, J=7.8, 1.5
Hz), 8.06 (d,
I H, J=7.8 Hz), 7.8 8 (t, 1 H, J=7.8 Hz), 7.67 (m, 2H), 7.3 5 (dd, I H, J=9.6,
2.4 Hz), 7.17 (dd,
1H, J=8.7,1.8 Hz), 4.39 (d, 2H, J=6.3 Hz). MS (ESI) 374/376 (M+H)+. Anal.
Calcd for
C17H13BrFN30=HCI: C, 49.72; H, 3.44; N, 10.23. Found: C, 50.04; H, 3.50; N,
10.25
108
CA 02743534 2011-06-13
Example 150
N-(3-amino-5 -isoguinolin I -N'- 4- 1- eeridinyl)benzy1)urea
Example 1SOA
N-(3-amino-5-isoguinolinyl)-2,2,2-trichloroacetamide
The title compound was prepared using the procedure described in Example IA
using
3,5-isoquinolinediamine instead of 5-arninoisoquinoline.
Example 150B
N-(3-amino-5-isoguinolinyl)-N-[4-(1-piperidinyl)benzyllurea
The title compound was prepared using the procedure described in Example IB
using
4-(1-piperidinyl)benzylamine and the product from Example 150A instead of 2-(3-
iluorophenyl)ethylarnine and the product from Example IA. 'H NMR (300 MHz,
DMSO-d6)
6 8.77 (s, 1H), 8.22 (s, 1H), 7.87 (d, 1H, J=8 Hz), 7.46 (d, 1H, J=8 Hz), 7.16
(d, 2H, J=8.4
Hz), 7.07 (t, 1H, J=8 Hz), 6.91 (d, 2H, J=8.4 Hz), 6.82 (t, 1H, J=6 Hz), 6.70
(s, IH), 5.91 (s,
2H), 4.22 (d, 2H, J=6 Hz), 3.10 (m, 4H), 1.70-1.45 (m, 6II). NIS (ESI) 376
(M+H)+. Anal.
Caled for C22H25N50Ø1H2O: C, 70.04; H, 6.73; N, 18.56. Found: C, 69.66; H,
6.50; N,
18.55.
Example 151
N-(3-amino-5-isoguinolinyl)-N'-[4-(I -azepanyl)benzyllurea
The title compound was prepared using the procedure described in Example lB
using
4-(1-azepanyl)benzylamine and the product from Example 150A instead of 2-(3-
fluorophenyl)ethylamine and the product from Example IA. 'H NMR (300 MHz, DMSO-
d6)
6 8.77 (s, 1H), 8.19 (s, 111), 7.88 (d, 1H, J=8.7 Hz), 7.45 (d, 1H, J=8.7 Hz),
7.09 (m, 3H),
6.76 (t, 1H, J=5.4 Hz), 6.66 (m, 3H), 5.90 (s, 2H), 4.17 (d, 2H, J=5.4 Hz),
3.24 (m, 4H), 1.71
(m, 4H), 1.44 (m, 4H); MS (ESI) 390 (M+H)+; Anal. Calcd for C23H27N50Ø4H20:
C,
69.64; H, 7.06; N, 17.65. Found: C, 69.53; H, 6.81; N, 17.38.
Example 152
N-(1,1'-biphenyl-3-ylmethyl)-N-5-isoguinolinylurea
109
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 61B
using 1,1'-biphenyl-3-ylmethylamine instead of 4-cyanobenzyl alcohol. 'H NMIZ
(300 MHz,
DMSO-d6) 8 9.73 (s, 1H), 9.47 (s, 111), 8.64 (m, 3H), 8.05 (d, 1H, J=9 Hz),
7.87 (t, IH, J=9
Hz), 7.68 (m, 3H), 7.58 (m, 2H), 7.47 (m, 3H), 7.37 (m, 2H), 4.48 (d, 2H, J=6
Hz); MS (ESI)
354 (M+H)+. Anal. Calcd for C23Hj9N30&HC1: C, 70.86; H, 5.17; N, 10.78. Found:
C,
70.77; H, 5.16; N, 10.74.
Example 153
N-5-isoguinolinyl-N-(4-(2-pyridinyl)benzyl}urea
The title compound was prepared using the procedure described in Example 61B
using 4-(2-pyridinyl)benzylamine instead of 4-cyanobenzyl alcohol. 'H NMR (300
MHz,
DMSO-d6) 8 9.83 (s, 1H), 9.81 (s, 1H), 8.88 (d, 1H, J=6.3 Hz), 8.72 (m, 3H),
8.10 (m, 5H),
7.92 (m, 2H), 7.56 (m, 3H), 4.49 (d, 2H, J=5.4 Hz); MS (EST.) 355 (M+H)+;
Anal. Calcd for
C22H,8N40=l.8HC1: C, 62.91; H, 4.75; N, 13.34. Found: C, 62.95; H, 4.99; N,
13.27.
Example 154
N-(4-bromo-3-fluorob enzyl)-N'-(3 -methyl-5-isog uinolinyl)urea
N-CEO
H3C , \
N
Example 154A
5-isocyanato-3-methylisoguinoline
The title compound was prepared using the procedure described in Example 61A
using 3-methyl-5-isoquinolinamine instead of 5-aminoisoquinoline.
Example 154B
N-(4-bromo-3-fluorobenzyl)-N-(3-methyl-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 61B
using 4-bromo-3-fluorobenzylamine and the product from Example 154A instead of
4-
cyanobenzyl alcohol and the product from Example 61A. 'H NMR (300 MHz, DMSO-
d6) 6
9.68 (s, I H), 9.46 (s, I H), 8.51 (m, 2H), 8.01 (d, I H, J=7.8 Hz), 7.80 (t,
1H, J=7.8 Hz), 7.67
(m, 2H), 7.36 (dd, 1H, J=9, 1.5 Hz), 7.18 (dd, 1H, J=9, 1 Hz), 4.39 (d, 2H,
J=6 Hz), 2.77 (s,
110
CA 02743534 2011-06-13
3H); MS (ESI) 388/390 (M+H)t; Anal. Calcd for C18H15BrFN3O-HCl: C, 50.91; H,
3.80; N,
9.89. Found: C, 50.81; H, 3.74; N, 9.87
Example 155
N-f 3-fluoro-4-(4-methyl-l- piperidinyl)benzyll-N'-(3-methyl-5-
isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 61B
using 3-fluoro-4-(4-methyl-l-piperidinyl)benzylamine and the product from
Example 154A
instead of 4-cyanobenzyl alcohol and the product from Example 61A. 1H NMR (300
MHz,
DMSO-d6) S 9.74 (s, 1H), 9.52 (s, 111), 8.66 (s, 1H), 8.59 (d, IH, J=8.4 Hz),
8.04 (d, 1H,
J=8.4 Hz), 7.83 (t, 1H, J=8.4 Hz), 7.62 (t, 1H, J=6 Hz), 7.10 (m, 3H), 4.32
(d, 2H, J=6 Hz),
3.31 (m, 2H), 2.79 (s, 31-1), 2.69 (m, 2H), 1.71 (m, 2H), 1.49 (m, 1H), 1.32
(m, 2H), 0.95 (d,
3H, J=6 Hz). MS (ESI) 407 (M+H)+; Anal. Calcd for C24H27FN40.2.3HC1: C, 58.79;
II,
6.02; N, 11.43. Found: C, 58.73; H, 6.18; N, 11.19.
Example 156
N-(3-methyl-5-isoguinolinyl)-N'-[4-(4-methyl-l -piperidinyl)benzyl lure
The title compound was prepared using the procedure described in Example 61B
using 4-(4-methyl-l-piperidinyl)benzylamine and the product from Example 154A
instead of
4-cyanobenzyl alcohol and the product from Example 61A. 'H NMR (300 MHz, DMSO-
d6)
8 9.70 (s, 111), 9.66 (s, 1 H), 8.74 (s, I H), 8.59 (d, IH, J=8.7 Hz), 8.01
(d, I H, J=8.7 Hz), 7.82
(m, 2H), 7.65 (m, 2H), 7.48 (m, 2H), 4.40 (d, 2H, J=6 Hz), 3.54 (m, 41T), 2.78
(s, 3H), 1.90-
1.50 (m, 5H), 0.98 (d, 3H, J=6 Hz); MS (ESI) 389 (M+H)+; Anal. Calcd for
C24H28N40.2.6HC1: C, 59.64; H, 6.38; N, 11.59. Found: C, 59.31; H, 6.39; N,
11.19.
Example 157
N-[3-fluoro-4-(1-piperidinyl)benzyll-N'-(3-methyl-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 61B
using 3-fluoro-4-(1-piperidinyl)benzylamine and the product from Example 154A
instead of
4-cyanobenzyl alcohol and the product from Example 61A. 1H NMR (300 MHz, DMSO-
d6)
S 9.73 (s, 1H), 9.47 (s, 111), 8.62 (s, 1H), 8.58 (d, 1H, J=8.4 Hz), 8.04 (d,
1H, J=8.4 Hz), 7.83
(t, 1H, J=8.4 Hz), 7.57 (t, 1H), 7.10 (m, 3H), 4.32 (d, 2H, J=6 Hz), 2.98 (m,
4H), 2.79 (s, 3H),
111
CA 02743534 2011-06-13
1.67 (m, 4H), 1.53 (m, 2H); MS (ESI) 393 (M+H)+; Anal. Calcd for C23I-
125FN40.1.5HC1: C,
61.78; H, 5.97; N, 12.53. Found: C, 61.40; H, 6.04; N, 12.18.
Example 158
N-(3-methyl-5-isoguinolinyl)-N'-(4-(l -piperidinyl)benzyllurea
The title compound was prepared using the procedure described in Example 61B
using 4-(1-piperidinyl)benzylatnine and the product from Example 154A instead
of 4-
cyanobenzyl alcohol and the product from Example 61A. 'H NMR (300 MHz, DMSO-
d6) 6
9.69 (s, 111), 9.60 (s, 1H), 8.68 (s, 1H), 8.57 (d, III, J=7.5 Hz), 8.00 (d,
IH, J=7.5 Hz), 7.85-
7.55 (m, 4H), 7.43 (m, 211), 4.40 (d, 2H, J=6 Hz), 3.44 (m, 4H), 2.77 (s, 3H),
1.90 (m, 4.H),
1.65 (m, 2H); MS (ESI) 375 (M+H)+; Anal. Calcd for C23H2GN40.2.4HC1: C, 59.80;
H,
6.20; N, 12.13. Found: C, 59.91; H, 6.45; N, 11.78
Example 159
N-L4-(1-azepanyl)benzyll-N'-(3-methyl-5-isoguinolinyrl)urea
The title compound was prepared using the procedure described in Example 61B
using 4-(1-azepanyl)benzylamine and the product from Example 154A instead of 4-
cyanobenzyl alcohol and the product from Example 61A. 'H NMR (300 NIHz, DMSO-
d6) 8
9.16 (s, 1H), 8.53 (s, 1H), 8.28 (d, 1H, J=8 Hz), 7.74 (s, 1H), 7.67 (d, IH,
J=8 Hz), 7.50 (t,
1 H, J=8 Hz), 7.14 (d, 2H, J=9 Hz), 6.84 (t, 1 H, J=6 Hz), 6.66 (d, 2H, J=9
Hz), 4.20 (d, 2H,
J=6 Hz), 3.44 (m, 4H), 2.63 (s, 3H), 1.71 (m, 414), 1.45 (m, 4H). MS (ESI) 389
(M+H)+;
Anal. Calcd for C24H28N40Ø31120: C, 73.18; H, 7.32; N, 14.22. Found: C,
73.08; H, 7.38;
N, 14.22.
Example 160
N-(3-meth),l-5-isoguinolin__yl)-N r4-(1-pyrrolidinyl)benzyllurea
The title compound was prepared using the procedure described in Example 61B
using 4-(1-pyrrolidinyl)benzylamine and the product from Example 154A instead
of 4-
cyanobenzyl alcohol and the product from Example 61A. 'H NMR (300 MHz, DMSO-
d6) 8
9.15 (s, 1H), 8.54 (s, 1H), 8.27 (d, 1H, J=7.5 Hz), 7.73 (s, IM, 7.67 (d, 1H,
J=7.5 Hz), 7.49
(t, IH, J=7.5 Hz), 7.16 (d, 2H, J=9 Hz), 6.84 (t, 1H, J=6 Hz), 6.53 (d, 2H,
J=9 Hz), 4.22 (d,
112
CA 02743534 2011-06-13
2H, J=6 Hz), 3.20 (m, 4H), 2.63 (s, 3H), 1.94 (in, 4H); MS (ESI) 361 (M+IFI)i-
. Anal. Calcd
for C22H24N40-0.2H20: C, 72.58; H, 6.76; N, 15.39. Found: C, 72.33; H, 6.64;
N, 1.522.
Example 161
N-f3-fluoro-4-(1-pyrrolidinyl)benzyll-N'-(3-methyl-5-isp uinolinyl)urea
The title compound was prepared using the procedure described in Example 61 B
using 3-fluoro-4-(1-pyrrolidinyl)benzylamine and the product from Example 154A
instead of
4-cyanobenzyl alcohol and the product from Example 61A. 'H NMR (300 MHz, DMSO-
d6)
6 9.17 (s, ITT), 8.59 (s, 1H), 8.22 (d, 1H, J=7.5 Hz), 7.73 (s, 1H), 7.69 (d,
IH, J=7.5 Hz), 7.50
(t, IH, J=7.5 Hz), 7.03 (in, 2H), 6.93 (t, 1H, J=6 Hz), 6.72 (m, 1H), 4.24 (d,
2H, J=6 Hz),
3.28 (m, 4H), 2.64 (s, 3H), 1.88 (m, 4H); MS (EST) 379 (M+H)+; Anal. Calcd for
C22H23FN40: C, 69.82; H, 6.13; N, 14.80. Found: C, 69.76; H, 6.06; N, 14.69.
Example 162
N-(4-(1-azepanyl)-3-fluorobenzyll-N'-(3-methyl-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 61 B
using 4-(1-azepanyl)-3-fluorobenzylamine and the product from Example 154A
instead of 4-
cyanobenzyl alcohol and the product from Example 61A. 'H NMR (300 MHz, DMSO-
d6) 6
9.74 (s, 1H), 8.50 (s, 1H), 8.67 (s, 1H), 8.60 (d, 1H, J=8.1 Hz), 8.14 (d, 1H,
J=8.1 Hz), 7.83
(t, 111, J=8.1 Hz), 7.56 (t, 1H), 7.04 (m, 2H), 6.90 (m, 1H), 4.26 (d, 2H, J=6
Hz), 3.32 (m,
4H), 2.79 (s, 3H), 1.75 (m, 4H), 1.55 (m, 4H); MS (ESI) 407 (M+H)+; Anal.
Calcd for
C24H27FN40.2HCI: C, 60.13; H, 6.10; N, 11.69. Found: C, 60.09; H, 6.35; N,
11.47.
Example 163
N-[4-(1 -azoeanyl)b enzyl]-N'-(3 -methyl-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 61B
using 4-(1-azocanyl)benzylamine and the product from Example 154A instead of 4-
cyanobenzyl alcohol and the product from Example 61A. 'H NMR (300 MHz, DMSO-
d6) S
9.15 (s, 111), 8.53 (s, IM, 8.27 (d, 1H, J=7.5 Hz), 7.73 (s, 111), 7.67 (d,
1H, J=7.5 Hz), 7.50
(t, 1H, J=7.5 Hz), 7.15 (m, 21-1), 6.83 (t, 1H, J=5.4 Hz), 6.63 (in, 2H), 4.20
(d, 2H, J=5.4 Hz),
3.43 (in, 4H), 2.63 (s, 3H), 1.67 (m, 4H), 1.48 (m, 6H); MS (EST) 403 (M+H)+;
Anal. Calcd
for C25H30N40: C, 74.60; H, 7.51; N, 13.92. Found: C, 74.26; H, 7.48; N,
13.64.
113
CA 02743534 2011-06-13
Example 164
N-[4-(1-azocanyl)-3-fluorobenz Iy 1-N'-(3-methyl-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 61B
using 4-(1-azocanyl)-3-fluorobenzylamine and the product from Example 154A
instead of 4-
cyanobenzyl alcohol and the product from Example 61A. 'H NMR (300 MHz, DMSO-
d6) 8
9.70 (s, 1H), 9.37 (s, 1H), 8.56 (m, 21-1), 8.01 (d, 1H, J=8.4 Hz), 7.81 (t,
1H, J=8.4 Hz), 7.45
(t, 1H), 7.02 (nl, 2H), 6.90 (in, 1H), 4.25 (d, 2H, J=6 Hz), 3.35 (m, 4H),
2.77 (s, 3H), 1.67 (m,
4H), 1.54 (m, 6H); MS (ESI) 421 (M+H)*; Anal. Calcd for C25H29FN40=HC1: C,
65.71; 11,
6.62; N, 12.26. Found: C, 65.44; H, 6.49; N, 12.15.
Example 165
N-[(1 S)-1-(4-bromophenyl)ethyll-N-(3-methyl-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 61B
using (iS)-1-(4-bromophenyl)ethanamine and the product from Example 154A
instead of 4-
cyanobenzyl alcohol and the product from Example 61A.
Example 166
N- {(1 S)-1-44-(1-azepanyl)phenyllethyl}-N'-(3-methyl-5-isoguinolinyl)urea.
The product from Example 165 (568 mg, 1.48 mmol, hexamethyleneimine (834 L,
7.39 mmol), Pd2dba3 (271 mg, 0.30 mmol), BINAP (460 mg, 0.74 mmol), and sodium
tert-
butoxide (1.42 g, 14.8 mmol) were combined in 1,4-dioxane (20 mL) and heated
to reflux.
After 16 hours, the reaction was cooled.to ambient temperature and
concentrated in vacuo.
The residue was purified by flash chromatography (I% to 5% CH3OH/CH2CI2) to
provide the
title compound. 'H NMR (300 MHz, DMSO-d6) b 9.15 (s, 111), 8.48 (s, 11D, 8.28
(d, 1H,
J=8.4 Hz), 7.72 (s, 1 H), 7.64 (d, 1 H, J=8.4 Hz), 7.47 (t, 1H, J=8.4 Hz),
7.16 (m, 2H), 6.90 (d,
1 H, J=7.5 Hz), 6.66 (m, 2H), 4.74 (m, 1H), 3.43 (m, 4H), 2.64 (s, 3H), 1.71
(m, 4H), 1.44 (m,
71-i). MS (ESI) 403 (M+H)}. Anal. Calcd for C25H30N40Ø2CH30H: C, 74.01; H,
7.59; N,
13.70. Found: C, 74.39; H, 7.60; N, 13.32.
Example 167
N-benzyl-N-(3-chloro-5-isoguinolinyl)urea
114
CA 02743534 2011-06-13
The product from Example 60E (250 mg, 1.4 mmol) and 1-bromo-4-
(isocyanatomethyl)benzene (0.22 mL, 1.57 mmol) were heated in toluene (5 mL)
at 80 C for
3 hours. The mixture was cooled to room, temperature and the precipitated
solid was
collected by filtration, washed with toluene, and air-dried to provide the
title compound. 1H
NMR (300 MHz, DMSO-d6) S 9.18 (s, 1H), 8.81 (s, IM, 8.32 (dd, J=7.8Hz, 0.7 Hz,
IH),
8.09 (s, 1H), 7.80 (d, J=8.2 Hz, 1H), 7.53-7.65 (m, 3H), 7.32 (m, 2H), 7.05
(t, J=5.7 Hz, 1H),
4.35 (d, J=5.7 Hz, 2H); MS (ESI) m/z 391/393 (M+H, 35CU37C1).
Example 168
N-(4-bromobenzyl)-N'-(1-chloro-5-isoguinolinyl)urea
Example 168A
1-chloro-5-isoguinolinamine
The title compound was prepared using the procedures described in Examples 60D
and 60E using I -chloroisoquinoline instead of the product from Example 60C.
Example 168B
N-(4-bromobenzyl)-N'-(l -chloro-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 60F
using the product from Example 168A instead of the product from Example 60E.
'H NMR
(300 MHz, DMSO-d6) S 8.89 (s, 1H), 8.34-8.37 (m, 2H), 8.00 (dd, J=6.1 Hz, 0.7
Hz, 1H),
7.92-7.95 (nz, 1H), 7.73 (t, J=8.1, 1H), 7.53-7.56 (m, 211), 7.30-7.33 (m, 21-
1), 7.12 (t,
J=5.8Hz. 1H), 4.35 (d, J=5.8 Hz, 2H); MS (ESr) m/z 390/392 (M+H, 3SC1/37CI).
Example 169
N-(4-cyanobenzyl)-N'-5-isoguinolinylurea
Example 169A
4-(aminomethyl)benzonitrile
A solution of N, N-bis(tert-butoxycarbonyl)-4-eyanobenzylamine (0.75 g, 2.25
mmol,
prepared according to the literature described in Synthetic Communications
4419:28 (1998),
in CH2CI2 (15 mL) was treated with trifluoroacetic acid (8 mL). After stirring
at room
115
CA 02743534 2011-06-13
temperature for 3 hours, the mixuture was concentrated under reduced pressure
and the
residue was azeotroped with diethyl ether.
Example 169B
N-(4-cyanobenzyl)-N-5-isoquinolinylurea
The title compound was prepared using the procedure described in Example 61 B
using the product from Example 169A instead of 4-cyanobenzyl alcohol.
Purification was by
chromatography (95:5 CI12C12:MeOH) to provide the title compound. 1H NMR (300
MHz,
DMSO-d6) 6 9.75 (s, 1 I3), 9.62 (s, I H), 8.69 (s, 2H), 8.58 (dd, J=7.8 Hz,
1.0 Hz, IH), 8.07 (d,
J=7.4 Hz, 1H), 7.90 (d, J=8.1 Hz, 111), 7.81-7.85 (m, 21.1), 7.74 (t, J=6.1
Hz, 1H), 7.54-7.57
(m, 2H), 4.48 (d, J=6.1 Hz, 2H); MS (ES1') m/z 303 (M+H)+.
Example 170
N-(4-bromobenzyl)-N-(3-methyl-5-isoguinolinyl)urea
The product from Example 63A (500 mg, 3.1 mmol) and 1-bromo-4-
(isocyanatomethyl)benzene (0.5 mL, 3.57 mmol) were stirred in toluene (10 mL)
at 80
overnight. The mixture was cooled to room temperature, and the resulting
precipitate was
collected by filtration, washed with toluene, and allowed to air-dry. The
corresponding
hydrochloride salt was prepared using methanolic HCI. to afford a tan solid.
1H NMR (300
MHz, DMSO-d6) 6 9.70 (s, 1H), 9.54 (s, 1H), 8.63 (s, 1H), 8.57 (dd, J=7.8 Hz,
1.0 Hz, 111),
8.02 (d, J=8.2 Hz, 1H), 7.78-7.83 (m, 1H), 7.67-7.71 (m, 1H), 7.52-7.57 (m,
2H), 7.30-735
(m, 2H), 4.36 (d, J=5.7 Hz, 2H), 2.78 (s, 3H); MS (EST m/z 370/372 (M+H,
79Br/81Br)+.
Example 171
N-(4-bromobenzyl)-N-(1-methyl-5-isoguinolinyl)urea
Example 171A
1-methyl-5-isoguinolinamine
The title compound was prepared using the procedures described in Examples 60D
and 60E using 1-methylisoquinoline instead of the product from Example 60C.
Example 171B
116
CA 02743534 2011-06-13
N-(4-bromobenzyl)-N'-(l-methyl-5-isoquinolin 1)urea
The product from Example 171A (480 mg, 3.04 mmol) and 1-bromo-4-
(isocyanatomethyl)benzene (0.43, 3.07 mmol) were stirred in toluene (9 mL) at
90 for I
hour, then the mixture was cooled to room temperature. The precipitate was
collected by
filtration and washed with toluene. The corresponding di-hydrochloride salt
was prepared
using methanolic HC1. IH NMR (300 MHz, DMSO-d6) fi 8.74 (s, 1H), 8.38 (d,
J=6.1 Hz,
1H), 8.25 (d, J=7.8 Hz, 1H), 7.78-7.85 (m, 2H), 7.53-7.61 (m, 311), 7.32 (d,
J=8.5 Hz, 2H),
7.11 (t, J=6.1 Hz, 1H), 4.34 (d, J=6.1 Hz, 2H), 2.88 (s, 3H); MS (ESI+) m/z
370/372 (M+H,
79Br/81Br)+.
Example 172
N-5-isoguinolinyl-N'-[4-(4-morpholinyl)benzyllurea
Example 172A
4-(4-iiorpho linyl)b enzoni trile
4-Fluorobenzonitrile (1 g, 8.26 nunol) and morpholine (2.2 mL, 25.2 mmol) were
stirred in DMSO (25 mL) at 100 C for 2.5 hours, cooled to room temperature,
poured into
H2O, and extracted with diethyl ether. The combined organic extracts were
washed with H2O
and brine, dried over Na2SO4, and evaporated in vacuo to provide the title
compound.
Example 172B
4-(4-morpholinyl)benzylamine
4-(4-Morpholinyl)benzonitrile (1.24 g, 6.6 mrol) in THE (25 mL) at 0 C was
treated
with LiAIH4 (2.5 g, 65.9 mmol) and refluxed for 1 hour. The mixture was cooled
to room
temperature and quenched by careful addition of IN NaOH and then H2O. The
mixture was
concentrated, extracted with diethyl ether. The combined ethereal extracts
were washed with
saturated NaHCO3 solution, dried over Na2SO4, and evaporated in vacuo to
provide the title
compound which was dried over MgSO4 as a THF:diethylether solution before the
next step.
Example 172C
N-5-isoguinolinyl-N'-[4-(4-morpholinyl)benzyllurea
The product from Example 172B (285 mg, 1.48 mmol) in diethyl ether (10 mL) was
treated with an ethereal solution of 5-isocyanatoisoquinoline, causing a white
precipitate to
117
CA 02743534 2011-06-13
form. This precipitate was collected by filtration and purified by
chromatography (95:5
CH2C12-MeOH, eluant) to provide the title compound. The corresponding di-
hydrochloride
salt was prepared using methanolic HO to afford a yellow solid. 1H NMR (300
MHz,
DMSO-d6) S 9.26 (s, 111), 8.67 (s, lH), 8.52-8.55 (m, 111), 8.32 (dd, J=7.8
Hz, 1.1 Hz, 1H),
7.92 (d, J=6.1 Hz, 1H), 7.73 (d, J=8.2 Hz, 1H), 7.60 (m, 1H), 7.23 (d, J=8.8
Hz, 2H), 6.92-
6.96 (m, 3H), 4.26 (d, 5.4 Hz, 2H), 3.72-3.75 (m, 4H), 3.06-3.12 (m, 4H); MS
(ESI+) m/z 363
(M+H)+.
Example 173
N- 4- 2,6-dimethyl-4-morpholinyl)benzyll-N'-5-isoguinolinylurea
Example 173A
4-(2,6-dimethyl-4-morpholinyl)benzylamine
The title compound was prepared using the procedures described in Examples
172A
and 172B using 2,6-dimethylmorpholine instead of morpholine.
Example 173B
N-f 4-(2,6-dimethyl-4-morpholinyl)benzyll-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 172C
using the product from Example 173A instead of the product from Example 172B.
1H NMR
(300 MHz, DMSO-d6) 6 9.26 (s, 1H), 8.67 (s, 1H), 8.53 (d, J=6.1 Hz, 111), 8.31
(dd, J=7.6
Hz, 1.1 Hz, 1H), 7.92 (d, J=6.1 Hz, 1H), 7.73 (d, J=8.1 Hz, 1H), 7.57-7.62 (m,
1H), 7.22 (d,
J=8.8 Hz, 2H), 6.92-6.95 (m, 3H), 4.26 (d, J=5.7 Hz, 2H), 3.68 (m, 2H), 3.54-
3.57 (m, 2H),
2.21 (m, 2H), 1.16 (s, 3H), 1.14 (s, 3H); MS (ESI) m/z 391 (M+H).
Example 174
N-5-isoguinolinyl-N'-(4-(4-thiomorpholinyl)benzyllurea
Example 174A
4-(4-thiomorpholinyl)benzylamine
The title compound was prepared using the procedures described in Examples
172A
and 172B using thiomorpholine instead of morpholine.
118
CA 02743534 2011-06-13
Example 174B
N-5-isoquinolinyl-N'-(4-(4-thiomorphholinyl)b enzyl )urea
The title compound was prepared using the procedure described in Example 172C
using the product from Example 174A instead of the product from Example 172B.
1H NMR
(300 MHz, DMSO-d6) 6 9.26 (s, 1H), 8.67 (s, IH), 8.53 (d, J=6.1 Hz, 1H), 8.32
(dd, .1=7.8
Hz, 1.1. Hz, 1H), 7.92 (d, J=6.1 Hz, 1H), 7.73 (d, J=8.2 Hz, 1H), 7.60 (m,
1H), 7.20-7.23 (m,
2H), 6.90-6.96 (in, 3H), 4.25 (d, J=5.8 Hz, 2H), 3.45-3.51 (m, 4H), 2.64-2.67
(m, 4H); MS
(ESI) m/z 379 (M+H).
Example 175
N-(4-brornobenzyl)-N'-(3-fluoro-5-isoq uinolinyl)urea
Example 175A
3-fluoro-5-isoquinolinamine
The title compound was prepared using the procedures described in Examples 60D
and 60E using 3-fluoroisoquinoline, prepared according to the procedure
described in J. Am.
Chem. Soc., 687:73 (1951), instead of the product from Example 60C.
Example 175B
N-(4-bromob enzyl_)-N'-(3-fluoro-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 60F
using the product from Example 175A instead of the product from Example 60E.
'H NMR
(300 MHz, DMSO-d6) 6 9.09 (s, 1H), 8.74 (s, IH), 8.28 (d, IH, J=7.8 Hz), 7.83
(d, 1H, J=8.4
Hz), 7.66 (s, 1H), 7.55 (m, 3H), 7.32 (d, 2H, J=8.5 Hz), 7.03 (t, 1H, J=5.9
Hz), 4.35 (d, 2H,
J=6.1 Hz); MS (ESI) m/z 373/375 (M+H, 79Br/81Br).
Example 176
N-(3-chloro-5-isoguinolinyl)-N'-(4-(4-morpholinyl)benzylJurea
Example 176A
3-chloro-5-isocyanatoisoquinoline
119
CA 02743534 2011-06-13
5-Amino-3-chloroisoquinoline (740 mg, 4.15 mmol) was suspended in toluene (20
mL) and treated with 20% w/v phosgene solution in toluene (9 mL) and
triethylamine (5 mL).
The mixture was refluxed overnight and was then concentrated in vacuo and used
in the next
step without further purification.
Example 176B
N-(3-chloro-5-isoguinolinyl)-N-(4-(4-morpholinyl)benzyliurea
The product from Example 176A in diethyl ether (40 mL) was treated with the
product from Example 172B (300 mg, 1.56 mmol) and triethylamine (3 mL) in 1:1
diethyl
ether:CH3CN (10 mL). After stirring for 3 hours, the mixture was filtered, and
the collected
solid was washed with diethyl ether. The solid was purified by silica gel
chromatography
(95:5 CH2C12:MeOH) to'provide the title compound. 1H NMR (300 MHz, DMSO-d6) 6
9.18
(s, 1H), 8.71 (s, IH), 8.37 (d, iH, J=6.7 Hz), 8.08 (s, 1I1), 7.79 (d, 2H,
J=8.2 Hz), 7.63 (t, 1H,
J=8.0 Hz), 7.23 (d, 2H, J=8.7 Hz), 6.94 (d, 2H, J=8.4 Hz), 6.91 (t, 1H, 5.5
Hz), 4.26 (d, 2H,
5.7 Hz), 3.73 (m, 4H), 3.07 (m, 4H); MS (ESI) m/z 397/399 (M+H, 35C1/37C1)_
Example 177
N-(3, 5-difluoro-4-(4-morpholinyl)benzyll-N'-5-isoquinolinylurea
Example 177A
3,5-difluoro-4-(4-morpholinyl)benzylamine
The title compound was prepared using the procedures described in Examples
172A
and 172B using 3,4,5-trifluorobenzonitrile instead of 4-fluorobenzonitrile.
Example 177B
N-(3,5-difluoro-4-(4-morpholinyl)benzyll-N-5-isoguinolinylurea
The product from Example 177A (500 mg, 2.19 mmol) in diethyl ether (5 mL) was
treated with an ethereal solution of 5-isocyanatoisoquinoline. The resulting
waxy precipitate
was collected by filtration and air-dried to provide the title compound. 1H
NMR (300 MHz,
DMSO-d6) 6 9.27 (s, 1H), 8.79 (s, 1H), 8.54 (d, 1H, J=6.1 Hz), 8.26 (dd, IH,
J=7.8 Hz, 1.0
Hz), 7.94 (d, 1H, 6.1 Hz), 7.76 (d, 1H, 8.2 Hz), 7.60 (t, 3H, J=7.6 Hz), 7.10
(t, 1H, J=6.0 Hz),
7.03 (m, 2H), 4.31 (d, 2H), 3.68 (m, 4H), 3.07 (m, 4H); MS (ESI+) mlz 399
(M+H).
120
CA 02743534 2011-06-13
Example 178
N-(4-bromobcnzyl)-N'-(1,3-dimethyl-5-isoguinolinyl)urea
Example 178A
1,3-dimethyl-5-isoquinolinamine
The title compound was prepared using the procedures described in Examples 60D
and 60E using 1,3-dimethylisoquinoline, prepared according to the procedure
described in
Helv. Chim. Acta 1627:75 (1992), instead of the product from Example 60C.
Example 178B
N-(4-bromobenzyl)-N'-(1,3-dimethyl-5-isoguinolinyl)urea
The product from Example 178A(375 mg, 22 mmol) in toluene (7 mL) was treated
with 1-bromo-4-(isocyanatomethyl)benzene (0.31 mL, 2.2 mmol). After stirring
at 85-90 C
for 3 hours, the mixture was cooled to room temperature and filtered. The
filter cake was
treated with methanolic HCl to provide the title compound as the hydrochloride
salt. 'H
NMR (300 MHz, DMSO-d5) S 8.62 (s, 1H), 8.17 (d, 1H, J=7.8 Hz), 7.80 (d, 1H,
J=8.5 Hz),
7.45-7.60 (m, 4H), 7.32 (d, 2H, J=8.1 Hz), 7.06 (t, 1H, 5.7 Hz), 4.34 (d, 2H,
5.8 Hz), 2.84 (s,
3H), 2.75 (s, 311); MS (ESI) m/z 383/385 (M+H, 79Br/s'Br).
Example 179
N-(3,4-dimethylbenzyl)-N'-(3-methyl-5-isoguinolinyl urea
3,4-Dimethylbenzylamine (0.3 mL, 2.1 mmol) in toluene (11 mL) was added
carefully to a 20% w/v solution of phosgene in toluene (4.5 mL). The mixture
was refluxed
overnight and was then concentrated in vacuo. The residue was then taken up in
toluene (10
mL) and treated with DIEA (1.5 mL, 8.63 mmol) and 5-amino-3-methylisoquinoline
(155
mg, 1.08 mmol). The reaction mixture was stirred at 80 for 2 h and was then
cooled to room
temperature. The precipitated solid was collected by filtration and was
chromatographed on
silica gel (97:3 CH2CI2-CH3OH to 9:1 CH2C12-CH3OH, eluant gradient) to afford
the desired
product, A-473191. Treatment of this solid with methanolic HCl yielded the
corresponding
hydrochloride salt. 'H NMR (300 MHz, DMSO-d6) 8 9.16 (s, 1H), 8.59 (s, 1H),
8.24 (d, 1H,
J=7.8 Hz), 7.74 (s, 111), 7.68 (d, 1H, J=8.2 Hz), 7.50 (t, 1H, J=7.9 Hz), 7.08-
7.12 (m, 3H),
121
CA 02743534 2011-06-13
6.95 (m, 1H), 4.28 (d, 2H, 5.8 Hz), 2.64 (s, 3H), 2.22 (s, 3H), 2.20 (s, 3H);
MS (ESI) m/z
320 (M+H).
Example 180
N-f3,5-bis trifluoromethyl)benzyl7-N-(3-methyl-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 179
using
3,5-bis(trifluoromethyl)benzylamine instead of 3,4-dimethylbenzylamine. 'H NMR
(300
MHz, DMSO-d6) S 9.18 (s, 1H), 8.79 (s, 1H), 8.01-8.13 (in, 4M, 7.73 (m, 2H),
7.51 (t, 1H,
J=8.0 Hz), 7.23 (t, 1H, J=6.0 Hz), 4.55 (d, 2H, J=6.1 Hz), 2.64 (s, 3H); MS
(ESI) m/z 428
(M+H).
Example 181
N-(3 -amino-5-isoguinolinyl)-N-(4-bromobenzyl)urea
Example 181A
N-3-isoguinolinylaeetamide
3-Aminoisoquinoline (495 mg, 3.44 mmol) was stirred in Ac20 (9 mL) at 60 for
16
hours. The mixture was cooled to room temperature and concentrated in vacuo to
provide the
title compound which was used in the next step without further purification.
Example 181B
3, 5 -isoguinolinedi amine
The title compound was prepared using the pr9cedures described in Examples 60D
and 60E using the product from Example 181A instead of the product from
Example 60C.
Example 181 C
N-(3-amino-5-isoguinolinyl)-N'-(4-bromobenzyl)urea
The title compound was prepared using the procedure described in Example 179
using
4-bromobenzylamine and the product from Example 181B instead of 3,4-
dirnethylbenzylamine and 5-amino-3-methylisoquinoline. The corresponding
hydrochloride
salt was formed by treatment of the free base with methanolic HCI. 'H NMR (300
MHz,
DMSO-d6) S 8.78 (s, 1H), 8.33 (s, 1H), 7.82 (d, 1H, J=7.5 Hz), 7.47-7.56 (m,
3H), 7.29 (d,
122
CA 02743534 2011-06-13
2H, J=8.1 Hz), 7.08 (t, 1H, J=7.8 Hz), 6.99 (m, 1H), 6.71 (s, 1H), 5.94 (br s,
21-I), 4.31 (d, 2H,
J=6.1 Hz); MS (ESI+) m/z 370/372 (M+H, 79Br/s"Br).
Example 182
N-(3-methyl-5-isoguinolinyl)-N'-[4-(trifluoromethy1 benzyl urea
4-(Trifluoromethyl)benzylarnine (1 mL, 7.02 mmol) in toluene (4 mt) was
treated
with 20% w/v phosgene solution in toluene (5 mL), and the whole mixture was
refluxed
overnight. After this time, the mixture was concentrated in vacuo, then was
taken up again in
toluene (8 mL). To this was added 5-amino-3-methylisoquinoline (340 mg, 2.15
mmol) and
DIEA (4 mL) in toluene (8 mL). The reaction was allowed to stir at 80 for 3 h
and then was
cooled to room temperature. The precipitate was collected by filtration and
purified by
chromatography on silica gel (97:3 CH2C12-CH3OH to 95:5 CH2C12-CH3OH, eluant
gradient)
to afford A-638488 as a white solid. Treatment with methanolic HCl yielded the
corresponding hydrochloride salt. 'H NMR (300 MHz, DMSO-d6) S 9.18 (s, 1H),
8.73 (s,
1H), 8.20 (d, 1H, J=7.3 Hz), 7.69-7.75 (m, 4H), 7.58 (d, 2H, J=8.2 Hz), 7.50
(t, 1H, 7.8 Hz),
7.16 (t, 1H, J=5.9 Hz), 4.47 (d, 2H, J=6.1 Hz), 2.65 (s, 3H); MS (ESI}) m/z
360 (M+H).
Example 183
N-(4-ter-butylbenzyl)-N'-(3-methyl-5-isoguinolinyl)urea
The title compound was prepard using the procedure described in Example 182
using
4-tert-butylbenzylanune instead of 4-(trifluoromethyl)benzylamine. The
corresponding
hydrochloride salt was obtained after treatment of the free base with
methanolic HC1. 1H
NMR (300 MHz, DMSO-d6) S 9.16 (s, 1H), 8.60 (s, 1H), 8.24 (dd, 1H, J=7.8 Hz,
1.1 Hz),
7.74 (s, 1H), 7.68 (d, 2H, J=8.2 Hz), 7.50 (t, 1 H, J=7.9 Hz), 7.38 (m, 2H),
7.29 (m, 2H), 6.99
(t, 1H, J=5.8 Hz), 4.32 (d, 2H, J=5.8 Hz), 2.64 (s, 3H), 1.28 (s, 9H); MS
(EST) mlz 348
(M+H).
Example 184
N-(4-tert-butylbenzyl)-N-(1,3-dimethyl-5-isoguinolinyl)urea
Example 184A
1-(isocyanatomethyl)-4-(trifluoromethyl)benzene
123
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 61A
using 4-(trifluoromethyl)benzylamuie instead of 5-arninoisoquinoline.
Example 184B
N-(4-tert-butylbenzyl)-N'-(1,3-dunethyl-5-isoguinolinyl)urea
The product from Example 184A (3.16 mmol) in toluene (12 mL) was treated with
the product from Example 178A (273 mg, 1.59 mmol) and DIEA (5 mL). The mixture
was
heated at 80 for 3 hours before being cooled to room temperature and
filtered. The
precipitate thus obtained was purified by silica gel chromatography (97:3
CH2CI2-CH3OH to
95:5 CH2C12-CH3OH, eluant gradient) to provide the title compound. The
corresponding
hydrochloride salt was prepared by treatment with methanolic HCI. 'H NMR (300
MHz,
DMSO-d6) S 8.68 (s, 1H), 8.16 (d, 1H, J=7.5 Hz), 7.80 (d, 1H, J=8.1 Hz), 7.73
(d, 21-1, J=8.2
Hz), 7.56-7.61 (m, 3H), 7.48 (t, 1H, J=8.1 Hz), 7.15 (t, 1H, J=5.7 Hz), 4.46
(d, 2H, J=5.7 Hz),
2.84 (s, 3H), 2.58 (s, 3H); MS (ESO m/z 374 (M+H).
Example 185
4-(3-chlorophenyl)-N-5-isoguinolinyl-l -piperazinecarboxamide
1-(3-Chlorophenyl)piperazine (206 mg, 1.05 mmol) in diethyl ether (20 mL) was
treated with an ethereal solution of 5-isocyanatoisoquinoline. The precipitate
that formed
was collected by filtration, washed with diethyl ether and air-dried to
provide the title
compound. 1H NMR (300 MHz, DMSO-d6) 8 9.30 (s, 1H), 8.84 (s, 1H), 8.49 (d, 1H,
J=7.1
Hz), 7.92 (d, 1H, J=7.8 Hz), 7.78 (d, 1H, 6.8 Hz), 7.61-7.72 (m, 2H), 7.25 (t,
1H, J=8.1 Hz),
6.96-7.04 (m, 2H), 6.81-6.84 (m, 1H), 3.68 (m, 4H), 3.29 (m, 4H); MS (ESI+)
m/z 367
(M+H).
Example 186
N-(4-tert-butylbenzyl)-N'-(1,3 -dimethyl-5-isoquinolinyl)urea
Example 186A
1-tert-butyl-4-(isocyanatomethyl)benzene
The title compound was prepared using the procedure described in Example 61A
using 4-tert-butylbenzylamine instead of 5-aminoisoquinoline.
124
CA 02743534 2011-06-13
Example 186B
N-(4-tert-butylbenzy N'-(1,3-dimethyl-5-i soquinolinyl)urea
The product from Example 186A (3.42 mmol) in toluene (12 mL) was treated with
5-
amino-l,3-dimethylisoquino line (245 mg, 1.42 mmol) and DIEA (5 mL). The
mixture was
heated at 80 for 3 hours, cooled to room tempoerature, and filtered. The
precipitate thus
obtained was purified by silica gel chromatography (97:3 CH2CI2:CH3OH to 95:5
CH2CI2:CH3OH) to provide the title compound. The corresponding hydrochloride
salt was
prepared by treatment with methanolic HCI. 'H NMR (300 MHz, DMSO-d6) 6 8.55
(s, 1H),
8.21 (d, 1H, J=7.1 Hz), 7.78 (d, 111, 8.5 Hz), 7.59 (s, 114), 7.48 (t, 1H,
J=8.0 Hz), 7.36-7.40
(m, 2H), 7.27-7.29 (m, 2H), 6.98 (m, 1H), 4.31 (d, 2H, J=5.8 Hz), 2.84 (s,
3H), 2.58 (s, 3H),
1.28 (s, 9H); MS (ESI) m/z 362 (M+II).
Example 187
4-(3,4-dimethylphenyl)-N-5-isoguinolinyl- l -piperazinec arboxaniide
1-(3,4-Dimethylphenyl)piperazine (194 mg, 1.02 mmol) in diethyl ether (20 mL)
was
treated with an ethereal solution of 5-isocyanatoisoquinoline. The precipitate
that formed
was collected by filtration, washed with diethyl ether, and air-dried to
provide the title
compound. 1H NMR (300 MHz, DMSO-d6) 8 9.29 (d, 1H, J=0.7 Hz), 8.82 (s, 1H),
8.49 (d,
J=6.2 Hz, 111), 7.90-7.93 (m, 1H), 7.76-7.79 (m, 1H), 7.61-7.71 (m, 2H), 7.00
(d, 1H, 8.5
Hz), 6.83 (d, 1H, J=2.4 Hz), 6.73 (dd, 1H, J=8.3 Hz, 2.5 Hz), 3.67 (m, 4H),
3.15 (m, 4H),
2.19 (s, 3H), 2.13 (s, 311); MS (ESI) m/z 361 (hZ+H).
Example 188
4-(4-chlorophenyl)-N-5-isoquinolinyl-l -piperazinecarboxamide
1-(4-Cblorophenyl)piperazine (197 mg, 1.01 mmol) in diethyl ether (20 mL) was
treated with an ethereal solution of 5-isocyanatoisoquinoline. The precipitate
that formed
was collected by filtration, washed with diethyl ether, and air-dried to
provide the title
compound. 'H NMR (300 MT-Iz, DMSO-d6) S 9.29 (d, 1H, J=1.0 Hz), 8.83 (s, 111),
8.49 (d,
1H, 6.1 Hz), 7.93 (d, 1H, J=7.8 Hz), 7.77 (in, 1H), 7.61-7.72 (m, 2H), 7.26-
7.29 (m, 2H),
7.01-7.04 (in, 2H), 3.68 (m, 4H), 3.23 (m, 4H); MS (ESt) mlz 367 (M+H).
125
CA 02743534 2011-06-13
Example 189
N S isoquinolinyl 3 methyl 4 4-methylphenyl)_l1iperazinecarboxamide
The title compound was prepared using the procedure described in Example 188
using
2-methyl-l-(4-niethylphenyl)piperazine instead of 1-(4-
chlorophenyl)piperazine. 1H NMR
(300 MHz, DMSO-d6) S 9.29 (d, 1H, J=0.6 Hz), 8.77 (d, 1H, J=5.1 Hz), 8.49 (d,
1H, J=5.7
Hz), 7.92 (d, 1H, 7.5 Hz), 7.61-7.77 (m, 311), 7.06 (d, 2H, 8.2 Hz), 6.86-
6.91. (in, 2H), 3.61
and 4.53 (2m, IM, 4.09 (m, 1H), 3.93 (m, 1H), 3.49 (m, 1H), 3.39 (m, IH), 2.62-
3.24 (m,
2H), 2.22 (s, 3H), 1.35 and 0.98 (2d, 3H, J=6.4 and 6.1 Hz); MS (ESI+) m/z 361
(M+H).
Example 190
4-(2,3-dimethylphenyl)-N-5-isoquinolinyl-l -piperazinecarboxamide
The title compound was prepared using the procedure described in Example 188
using
1-(2,3-dimethylphenyl)piperazine instead of 1-(4-chlorophenyl)piperazine. 1H
NMR (300
MHz, DMSO-d6) 6 9.29 (d, 1H, J=1.0 Hz), 8.80 (s, 1H), 8.50 (d, 1H, J=5.7 Hz),
7.92 (d, 1H,
J=8.2 Hz), 7.79 (dd, lH, J=6.lHz, 1.0 Hz), 7.61-7.73 (m, 2H), 7.07 (m, 1HO,
6.90-6.96 (m,
21-1), 3.70 (m, 4H), 2.87 (m, 4H), 2.23 (s, 311), 2.23 (s, 31); MS (ESI) m/z
361 (M+H).
Example 191
4-(2,3_dichlorophenyl)-N-5-isoquinolinyl-l -piperazinecarboxamide
The title compound was prepared using the procedure described in Example 188
using
1-(2,3-dichlorophenyl)piperazine instead of 1-(4-chlorophenyl)piperazine. 1H
NMR (300
MHz, DMSO-d6) S 9.30 (d, 1H, J=0.7 Hz), 8.83 (s, 1H), 8.50 (d, 1H, J=5.8 Hz),
7.92 (d, 1H,
J=7.8 Hz), 7.79 (dd, 1H, J=5.1 Hz, 1.0 Hz), 7.62-7.73 (m, 2H), 7.33-7.36 (m,
2H), 7.21-7.24
(m, 1H), 3.71 (m, 4H), 3.07 (m, 4H); MS (ESI+) m/z 401/403 (M+H, 35C1/37C1).
Example 192
N-[3-fluoro-4-(trifluoromethyl)benzyl]-N`-(3-methyl-5-isoguinolinyl)urea
Example 192A
2-fluoro-4-(isocyanatomethyl)-1-(trifluorometbyl)benzene
The title compound was prepared using the procedure described in Example 61A
using 3-fluoro-4-(trifluoromethyl)benzylamine instead of 5-aminoisoquinoline.
126
CA 02743534 2011-06-13
Example 192B
N-(3-fluoro-4-(trifluoromethyl)benzyll-N'-(3-methyl-5-isoguinolinyI urea
The product from Example 192A (4.4 mmol) in toluene (10 mL) was treated with 5-
amino-3-methylisoquinoline (460 mg, 2.9 mmol) and DIEA (3 mL). The mixture was
heated
at 80 for 1.5 hours, cooled to room temperature, and filtered. The
precipitate thus obtained
was purified by silica gel chromatography (97:3 CH2CI2:CH3OH to 95:5
CH2CI2:CH3OH) to
provide the title compound. The corresponding hydrochloride salt was prepared
by treatment
with methanolic HCI. 'H NMR (300 MHz, DMSO-d6) 8 9.18 (s, 1H), 8.77 (s, IH),
8.17 (dd,
1H, J=7.8 Hz, 1.0 Hz), 7.70-7.81 (m, 3H), 7.38-7.53 (m, 3H), 7.19 (t, 1H, 6.1
Hz), 4.47 (d,
2H. J=5.8 Hz), 2.65 (s, 3H); MS (ESI) m/z 378 (M+H).
Example 193
N-[ 1 -(4-b romophenyl) ethyl]-N'-(3 -methyl-5 -is o g ui no linyl) urea
Example 193A
1-bromo-4-(1-isocyanatoethyl)benzene
The title compound was prepared using the procedure described in Example 61A
using 1-(4-bromophenyl)ethylamine instead of 5-aminoisoquino]ine.
Example 193B
N-[ 1-(4-bromophenyl)ethyl)-N'-(3-methyl-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example I92B
using the product from Example 193A instead of the product from Example 192A.
The
corresponding hydrochloride salt was prepared by treatment with methanolic
HCI. TI-I NMR
(300 MHz, DMSO-d6) 8 9.16 (s, 1H), 8.56 (s, 11), 8.20 (dd, 1H, J=7.8 Hz, 1.0
Hz), 7.72 (s,
IH), 7.67 (d, 1H, J=8.2 Hz), 7.56 (m, 2H), 7.47 (t, III, J=7.8 Hz), 7.35 (m,
2H), 7.12 (d, 1H,
J=7.4 Hz), 4.85 (m, 1H), 2.65 (s, 3H), 1.43 (d, 3H, J=7.1 Hz); MS (EST") m/z
384/386 (M+H,
79Br/81Br).
Example 194
N-(3,4-dichlorobenzyl)-N'-(3-methyl-5-isoguinolinyl)urea
127
CA 02743534 2011-06-13
Example 194A
1,2-dichloro-4-(isocyanatomethyl)benzene
The title compound was prepared using the procedure described in Example 61A
using 3,4-dichlorobenzylamine instead of 5-aminoisoquinoline.
Example 194B
N-(3 ,4-dichlorob enzyl)-N-(3 -methyl-5-isoguinolinyl)urea
5-Amino-3-methylisoquinoline (390 mg, 2.47 mmol) and the product from Example
194A (0.36 mL, 2.45 mmol) were heated in toluene (10 mL) at 80 for 2.5 hours.
Upon
cooling to room temperature, a precipitate formed, which was collected by
filtration, washed
with toluene, and air-dried. Remaining impurities were removed by slurrying
the solid in 9:1
CH2CI2:CH3OH and then filtering the mixture to provide the title compound. The
corresponding hydrochloride salt was formed by treatment of the free base with
methanolic
HCI. 'H NMR (300 MHz, DMSO-d6) S 9.17 (s, IH), 8.27 (s, 1H), 8.17 (dd, 1H,
J=7.8 Hz,
1.0 Hz), 7.74 (s, 1H), 7.71 (d, 1H, J=8.1 Hz), 7.61 (m, 2H), 7.50 (t, 1H,
J=8.0 Hz), 7.35 (dd,
1H, J=8.3 Hz, 2.2 Hz), 7.12 (t, 1H, 5.9 Hz), 4.37 (d, 2H, J=6.1 Hz), 2.65 (s,
3H); MS (ESI*
mlz 360/362 (M+H, 35CU37Cl).
Example 195
N-(2,4-dichlorobenzyl)-N-(3 -methyl-5-isoguinolinyl)urea
Example 195A
2,4-dichloro-l-(isocyanatomethyl)benzene
The title compound was prepared using the procedure described in Example 61A
using 2,4-dichlorobenzylamine instead of 5-aminoisoquinoline.
Example 195B
N-(2,4-dichlorobenzyl)-N-(3-methyl-5-isoguinolinyl)urea
5-Amino-3-methylisoquinoline (390 mg, 2.47 mmol) and the product from Example
195A (0.36 mL, 2.47 mmol) were heated in toluene (10 mL) at 80 for 2.5 hours.
Upon
cooling to room temperature, a precipitate formed, which was collected by
filtration, washed
128
CA 02743534 2011-06-13
with toluene, and air-dried. Remaining impurities were removed by slurrying
the solid in 9:1
CH2C12:CH3OH and then filtering the mixture to provide the title Compound. The
corresponding hydrochloride salt was formed by treatment of the free base with
methanolic
HCI. 'H NMR (300 MHz, DMSO-d6) S 9.17 (s, 1H), 8.78 (s, 1H), 8.21 (dd, 1H,
J=7.4 Hz,
1.0 Hz), 7.77 (s, 111), 7.70 (d, 1 H, J=8.1 Hz), 7.64 (m, 1H), 7.44-7.52 (m,
3H), 7.14 (t, I H,
J=6.1 Hz), 4.38 (d, 211., J=6.0 Hz), 2.65 (s, 31-1); MS (ESF1) mlz 360/362
(M+H, 35Cl/37C1).
Example 196
N-(3-chlorob enzyl)-N'-(3 -methyl-5 -isoguino linyl)urea
3-chlorobenzylamine (141 mg, 1.0 mmol) in ether (20 mL) was treated with an
ethereal solution of 5-isocyanato-3-methylisoquinoline. The precipitate that
formed was
collected by filtration, washed with diethyl ether, and air-dried to provide
the title compound.
'H NMR (300 MHz, DMSO-d6) 6 9.18 (s, 1H), 8.69 (s, 1H), 8.20 (d, 1 H, J=7.S
Hz), 7.75 (s,
1H), 7.70 (d, 1H, J=8.2 Hz), 7.51 (t, 1H, J=7.8 Hz), 7.31-7.43 (m, 4H), 7.10
(m, 111), 4.38 (d,
2H, J=5.7 Hz), 2.65 (s, 3H); MS (ESI') m/z 326/328 (M+H, 35C1137C1)_
Example 197
N-(3 -methyl-5-isoguinolinyl)-N'-(4-(trifluoromethoxy)benzyllurea
The title compound was prepared using the procedure described in Example 196
using
4-(trifluoromethoxy)benzylamine instead of 3-chlorobenzylamine. 'H NMR
(3001VMz,
DMSO-d6) b 9.17 (s, 1H), 8.68 (s, 1H), 8.21 (d, 1H, J=7.8 Hz), 7.75 (s, 1H),
7.70 (d, 1H,
J=8.1 Hz), 7.46-7.53 (m, 3H), 7.35-7.37 (m, 2H), 7.10 (t, 1H, 5.9 Hz), 4.40
(d, 2H, J=5.7 Hz),
2.64 (s, 3H); MS (ESI+) m/z 376 (M+H).
Example 198
N-[2-(3,4-dichlorophenyl)ethyl]-N'-(3-methyl-5-isoguinolinyl)urea
The title compound was prepared using the procedure described in Example 196
using
2-(3,4-dichlorophenyl)ethylamine instead of 3-chlorobenzylamine. 'H NMR (300
MHz,
DMSO-d6) S 9.16 (s, 1H), 8.54 (s, IH), 8.17 (d, 1H, J=7.5 Hz), 7.67-7.70 (m,
2H), 7.57-7.60
(m, 2H), 7.49 (t, 1H, 7.8 Hz), 7.29 (dd, 1H, J=8.1 Hz, 2.0 Hz), 6.57 (t, 1H,
J=5.7 Hz), 3.43
(m, 2H), 2.82 (m, 2H), 2.64 (s, 3H); MS (ESI) mlz 374/376 (M+H, 35C1/37C1).
129
CA 02743534 2011-06-13
Example 199
N-(4-ethylbenzyl)-N'-(3-methyl-5 -i soquinolinyl )urea
The title compound was prepared using the procedure described in Example 196
using
4-ethylbenzylamine instead of 3-chlorobenzylamine. 'H NMR (300 MHz, DMSO-d6) 5
9.17
(s, IH), 8.61 (s, 1H), 8.24 (d,.1H, J=7.8 Hz), 7.74 (s, 111), 7.68 (d, 1H,
J=7.8 Hz), 7.50 (t, 1H,
J=7.8 Hz), 7.19-7.29 (m, 4H), 6.99 (m, 1H), 4.32 (d, 2H, J=5.7 Hz), 2.64 (s,
3H), 2.59 (q, 2H,
J=7.6 Hz), 1.17 (t, 3H, J=7.6 Hz); MS (ESI+) m/z 320 (M+H).
Example 200
N-(3-methyl-5-isoguinolinyl)-N'- {2-[4-(trifluoromethyl)phenyllethyl} urea
The title compound was prepared using the procedure described in Example 196
using
2-[4-(trifluoromethyl)phenyl]ethylamine instead of 3-chlorobenzylamine. 'H NMR
(300
MHz, DMSO-d6) S 9.16 (s, 1H), 8.55 (s, 1H), 8.20 (d, 1H, J=7.8 Hz), 7.67-7.70
(m, 4H),
7.46-7.53 (m, 3H), 6.60 (t, IH, J=5.6 Hz), 3.46 (m, 2H), 2.91 (m, 2H), 2.64
(s, 3H); MS
(ESI+) m/z 374 (M+H).
Example 201
N-(3-methyl-5-isoquinolinyl)-N- {4-((trifltloromethyl)thiolbenzyl}urea
The title compound was prepared using the procedure described in Example 196
using
4-[(trifluoromethyl)thio]benzylamine instead of 3-chlorobenzylamine. III NMR
(300 MHz,
DMSO-d6) 8 9.18 (s, 111), 8.72 (s, 1H), 8.21 (d, 1H, J=7.8 Hz), 7.69-7.76 (m,
4H), 7.50-7.53
(m, 3H), 7.15 (m, 1H), 4.44 (d, 2H, J=6.1 Hz), 2.65 (s, 3H); MS (ESI+) m/z 392
(M+H).
Example 202
N-(4-chlorobenzyl)-N-(3-methyl-5-isoquinolinyl)urea
The title compound was prepared using the procedure described in Example 196
using
4-chlorobenzylamine instead of 3-chlorobenzylamine. 1H NMR (300 MHz, DMSO-d6)
6
9.17 (s, 1H), 8.67 (s, 1H), 8.21 (m, 1H), 7.66-7.74 (m, 2H), 7.37-7.53 (m,
5H), 7.08 (m, 1H),
4.36 (d, 2H, J=5.8 Hz), 2.64 (s, 311); MS (ESI{) m/z 326/328 (M+H, 35C1/37C1).
Example 203
4-(3,4-dichlorophenyl)-N-(3-methyl-5-isoguinolinyl)-1-piperazinecarboxamide
130
CA 02743534 2011-06-13
The title compound was prepared'using the procedure described in Example 196
using
1-(3,4-dichlorophenyl)piperazine instead of 3-chlorobenzylamine. 'II NMR (300
MHz,
DMSO-d6) S 9.20 (s, 1H), 8.74 (s, 1H), 7.86 (d, 1H, J=8.1 Hz), 7.51-7.66 (m,
3H), 7.43 (d,
1H, J=8.8 Hz), 7.22 (d, 1H, J=3.1 Hz), 7.01 (dd, 1H, J=9.1 Hz, 3.1 Hz), 3.67
(m, 411), 328
(m, 4H), 2.62 (s, 3H); MS (ESI) m/z 415/417 (M+H, 35C1/37C1).
Example 204
N-(2,4-di fluorobenzyl)-N'-(3-methyl-5-isoguuiolinyl)urea
The title compound was prepared using the procedure described in Example 196
using
2,4-difluorobenzylatnine instead of 3-chlorobenzylamine. 'H NA4R (300 MIIz,
DMSO-d6) S
9.17 (s, 1H), 8.67 (s, 1H), 8.21 (dd, 1H, J=7.5 Hz, 1.0 Hz), 7.74 (s, IH),
7.70 (d, 1H, J=8.1
Hz), 7.47-7.52 (m, 2H), 7.05-7.29 (m, 3H), 4.38 (d, 2H, J=5.7 Hz), 2.64 (s,
3H); MS (ESI)
m/z 328 (M+H).
Example 205
N-(1,3-dimethyl-5-isoguuiolinyl)-M-(3-fluoro-4-(trifluoromethyl)benzyl)ure
Example 205A
2-fluoro-4-(isocyanatomethyl)-1-(trifluoromethyl)benzene
The title compound was prepared using the procedure described in Example 61 A
using 3-fluoro-4-(trifluoromethyl)benzylamin.e instead of 5-aminoisoquinoline.
Example 205B
N-(1,3-dimethyl-5-isoguinolinyl)-N'-(3-fluoro-4-(trifluoromethyl)benzyl1urea
The product from Example 205A (4.4 mmol) in toluene (10 mL) was treated with
1,3-
dimethyl-5-isoquinolinamine (375 mg, 2.18 mmol) and DIEA (3.5 mL). The mixture
was
heated at 80 overnight. After cooling to room temperature, the precipitated
solids were
collected by filtration and chromatographed on silica gel (98:2 CH2CI2:CH3OH
to 95:5
CH2C12:CH3OH) to provide the title compound. The corresponding hydrochloride
salt was
prepared by treatment with methanolic HCI. iH NMR (300 MHz, DMSO-d6) S 8.72
(s, 1H),
8.13 (d, 1H, J=7.8 Hz), 7.75-7.83 (m, 2H), 7.61 (s, 1H), 7.38-7.51 (m, 3H),
7.18 (t, 1H, J=6.1
Hz), 4.46 (d, 211, J=5.8 Hz), 2.84 (s, 311), 2.59 (s, 311); MS (ES1{) m/z 392
(M+H).
131
CA 02743534 2011-06-13
Example 206
N-5-i soguinolinyl-4-(3-(trifluoromethyl)phenyll- l -piperazinecarboxamide
The title compound was prepared using the procedure described in Example 188
using
1-[3-(trifluoromethyl)phenyl]piperazine instead of 1-(4-
chlorophenyl)piperazine. 'H NMR
(300 MHz, DMSO-d6) 8 9.30 (d, 1H, J=1.0 Hz), 8.85 (s, 1H), 8.49 (d, 1H, J=5.7
Hz), 7.93 (d,
lH, J=7.7 Hz), 7.78 (m, 1H), 7.61-7.72 (m, 2H), 7.46 (m, 1H), 7.26-7.31 (m,
2H), 7.12 (d,
1H, J=7.5 Hz), 3.70 (m, 4H), 3.35 (in, 4H); MS (ESI*) m/z 401 (M+H).
Example 207
4-(4-bromophenyl)-N-5-isoguinolinyl- l -piperazinecarboxami de
The title compound was prepared using the procedure described in Example 188
using
1-(4-bromophenyl)piperazine instead of 1-(4-chlorophenyl)piperazine. The
precipitate that
formed was collected by filtration, washed with diethyl ether, and air-dried.
Purification by
silica gel chromatography provided the title compound. 1H NMR (300 MHz, DMSO-
d6) S
9.29 (d, 1H, J=1.0 Hz), 8.83 (s, 1H), 8.49 (d, 1H, J=6.1 Hz), 7.92 (d, 1H,
J=7.8 Hz), 7.77 (in,
1H), 7.61-7.71 (in, 211), 7.37-7.40 (m, 2H), 6.96-6.99 (m, 21D, 3.68 (m, 4H),
3.23 (m, 411);
MS (ESI+) m/z 411/413 (M+H, 79Br/81Br).
Example 208
N-(4-isopropylben 1)-N'-(3-methyl-5-isoguinolinyl)urea
4-Isopropylbenzylamine (748 mg, 5.02 mmol) in toluene (20 mL) was refluxed
with
20% w/v phosgene solution in toluene (3 mL) overnight. The mixture was cooled
to room
temperature and concentrated in vacuo. The residue was taken up in toluene (20
niL) and
was treated with DIEA (4 mL) and 5-amino-3-methylisoquinoline (500 mg, 3.16
mmol). The
reaction mixture was stirred was at 80 C for 6 hours. After cooling to room
temperature, a
precipitate formed which was collected by filtration and purified by silica
gel
chromatography (98:2 CH2C12:CH3OH) to provide the title compound. The
corresponding
hydrochloride salt was formed by treatment with methanolic HCI. 1H NMR (300
MHz,
DMSO-d6) S 9.16 (s, 1H), 8.60 (s, 1H), 8.24 (dd, 1H, J=7.5 Hz, 1.0 Hz), 7.74
(s, 1H), 7.68 (d,
1H, J=8.2 Hz), 7.50 (t, 1H, J=8.0 Hz), 7.22-7.30 (m, 4H), 6.99 (t, 111, 5.6
Hz), 4.32 (d, 2H,
J=7.8 Hz), 2.88 (m, 1H), 2.64 (s, 3H), 1.20 (d, 6H, J=6.8 Hz); MS (ESI') m/z
334 (M+H).
132
CA 02743534 2011-06-13
Example 209
N_[4-fluoro-3-(trifluoromethyl)benzyl]-N'-(3-methyl- 5-isoogj inoluiyl urea
4-Fluoro-3-(trifluoromethyl)benzylanvne (0.8 g, 4.15 mmol) in toluene (20 mL)
was
refluxed with 20% w/v phosgene solution in toluene (2.1 mL) overnight. The
mixture was
cooled to room temperature and concentrated in vacuo. The residue was again
taken up in
toluene (25 mL) and was stirred overnight at 80 C with DIEA (2 mL, 11.5 mmol)
and 5-
amino-3-methylisoquinoline (500 mg, 3.16 nimol). The mixture was cooled to
room
temperature, concentrated in vacuo, and the residue was purified by silica gel
chromatography (97:3 CH2C12:CH3OH, eluant) to provide the title compound. The
corresponding hydrochloride salt was prepared by treatment with methanolic
HCI. 1H NMR
(300 MHz, DMSO-d6) 6 9.18 (s, 1H), 8.72 (s, 1H), 8.16 (d, 1I1, J=7.8 Hz), 7.70-
7.77 (m, 4H),
7.48-7.54 (m, 3H), 7.14 (t, 1H, J=5.9 Hz), 4.42 (d, 2H, J=6.1 Hz), 2.64 (s,
3H); MS (E Sr)
mlz 378 (M+H).
Example 210
N-(3-amino-5-isoquinolinyl)-N'- { 1-[4-(trifluoromethyl)phenyll ethyl[ urea
1-(1-Isocyanatoethyl)-4-(trifluoromethyl)benzene (1.64 mmol) in toluene (8
inL) was
treated with N-(5-amino-3-isoquinolinyl)acetamide (220 mg, 1.09 mmol) and DIEA
(1.4
mL). The mixture was heated at 80 C for 6 hours, cooled to room temperature,
and the
precipitate was collected by filtration. The solid was triturated with 97:3
CH2CI2:CH3OH and
stirred as a suspension in 48% aqueous HBr (8 mL) at 60 C for 4 hours. After
cooling to
room temperature, the mixture was poured into concentrated NH4OH (20 mL) and
filtered.
The solid was washed with water and air-dried to provide the title compound.
The
corresponding hydrochloride salt was prepared by treatment with methanolic
HCI. 1H NMR
(300 MHz, DMSO-d6) 6 9.05 (s, 1H), 8.64 (s, 111), 7.91 (d, 1H), 7.58-7.74 (m,
4H), 7.22-7.36
(m, 3H), 7.14-7.18 (m, 2H), 6.97 (s, 1H), 4.94 (m, 111), 1.44 (d, 3H, J=6.8
Hz); MS (ESF )
mlz 375 (M+H).
Example 211
N-(3-amino-5-isoquinolinyl)-N'-[3-fluoro-4-(trifluoromethyl)benzyllurea
133
CA 02743534 2011-06-13
2-Fluoro-4-(isocyanatomethyl)-1-(trifluoramethyl)ben.zene (2.59 mmol) in
toluene
(10 mL) was treated with N-(5-amino-3-isoquinolinyl)acetamide (400 mg, 1.99
rninol) and
DIEA (1.8 mL). The mixture was heated at 80 C for 5 hours, cooled to room
temperature,
and filtered. The solid was triturated with 97:3 CH2CI2:CH3OH and stirred as a
suspension in
48% aqueous HBr (8 mL) at 60 C for 2 hours. After cooling to room
temperature, the
mixture was poured into concentrated NH4OH (20 mL). The solid was washed with
water
and air-dried to provide the title compound. The corresponding hydrochloride
salt was
prepared by treatment with methanolic HCl. 'H NMR (300 MHz, DMSO-d6) S 8.81
(s, 1H),
8.68 (s, 1 H), 7.94 (s, IH), 7.76 (t, 1 H, J=7.9 Hz), 7.3 5-7.49 (m, 311),
7.26 (s, I H), 7.09 (s,
IH), 6.92 (s, 1H), 6.84 (t, 1H, J=6.0 Hz), 6.62 (s, 11-1), 4.41 (d, 2H, J=6.1
Hz).
Example 212
N-f(2,4-dichlorobenzyl)oxy]-N'-5-isoquinolinylurea
The title compound was prepared using the procedure described in Example 61B
using O-(2,5-dicblorobenzyl)hydroxylamuie instead of 4-cyanobenzyl alcohol. MS
(ESI)
m./z: 361.96 (M+H)+; 'H NMR (DMSO-d6) 8 5.03 (s, 2H), 7.52 (dd, 1H), 7.69 (m,
4H), 7.88
(d, 1H), 7.93 (d, 1H), 8.52 (d, 1H), 9.00 (s, 1H), 9.31 (s, 1H), 9.77 (s, 1H).
Example 213
N-(5-bromo-2-fluorobenzyl)-M-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 5-bromo-2-fluorobenzylamine instead of 4-cyanobenzyl alcohol. MS (ESI)
m/z:
373.93 (M+H)+; 'H NMR (DMSO-d6) S 4.42 (d, 2H), 7.22 (t, IH), 7.54 (m, 2H),
7.60 (dd,
IH), 7.86 (t, IH), 8.05 (d, 1H), 8.56 (t, 2H), 8.69 (d, 1H), 9.45 (s, 1H),
9.72 (s, IM.
Example 214
N (4..chloro-2-fluorobenzyl)-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 4-chloro-2-fluorobenzylamine instead of 4-cyanobenzyl alcohol. MS (ESI)
mlz:
329.99 (M+fff ;'H NMR (DMSO-d6) 6 4.41 (d, 2H), 7.31 (dd, 1H), 7.47 (m, 3H),
7.85 (t,
iH), 8.04.(d, 1H), 8.56 (d, 2H), 8.68 (d, 1H), 9.42 (s, IH), 9.71 (s, 111).
134
CA 02743534 2011-06-13
Example 215
2-(4-chlorophenyl)ethyl 5-isoguinolinylcarbamate
The title compound was prepared using the procedure described in Example 61B
using 2-(4-chlorophenyl)ethanol instead of 4-cyanobenzyl alcohol. MS (ES1) n-
/z: 327.04
(M+H)+; 'H NMR (DMSO-d6) 6 2.99 (t, 2H), 4.37 (t, 2H), 7.36 (q, 4H), 7.89 (t,
1H), 8.12 (d,
IH), 8.20 (d, 1H), 8.30 (d, IH), 8.63 (d, IH), 9.72 (s, 111), 9.97 (s, 111).
Example 216
2- 2-(trifluoromethyl)phenyllethyl 5-isoquinolinylearbamate
The title compound was prepared using the procedure described in Example 61B
using 2-[2-(trifluoromethyl)phenyl]ethanol instead of 4-cyanobenzyl alcohol.
MS (ESI) m/z:
361.06 (M+H)+; 'H NMR (DMSO-d6) 6 3.18 (t, 2H), 4.42 (t, 2H), 7.48 (t, 1H),
7.63 (m, 2H),
7.72 (d, 1H), 7.90 (t, 1H), 8.13 (d, 1H), 8.20 (d, 1H), 8.30 (d, 1H), 8.63 (d,
1H), 9.72 (s, 1H),
10.01 (s, 1H).
Example 217
N-(4-tert-butylbenzyl)-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 4(-tert-butyl)benzylamine instead of 4-cyanobenzyl alcohol. MS (ES1)
m/z: 333
(M+H)+; 'H NMR (DMSO-d6) S 1.27 (s, 9H), 2.80 (t, 2H), 2.95 (t, 2H), 7.22 (d,
2H), 7.33 (d,
2H), 7.67 (t, 1I-1), 7.80 (d, 1H), 7.96 (t, 2H), 8.48 (d, 1H), 9.33 (s, 1H),
9.99 (s, IH).
Example 218
N-r(4-tert-butyleyclohexyl)methyll-M-5-isoquinolinylure
The title compound was prepared using the procedure described in Example 61 B
using (4-tert-butylcyclohexyI)methylamine instead of 4-cyanobenzyl alcohol. MS
(ESI) m/z:
340.18 (M+H)+; 'H NMR (DMSO-d6) 6 0.82 (d, 9H), 0.93 (d, 4H), 1.09-1.50 (m,
2H), 1.74
(d, 2H), 1.82 (d, 2H), 3.01 & 3.19 (t & dd, 2H), 7.19 & 7.24 (t & t, 1H), 7.87
(t, 1H), 8.03 (d,
1H), 8.63 (dd, 1H), 8.67 (d, 1H), 8.76 (dd, 1H), 9.47 (d, 1H), 9.74 (s, 1H).
Example 219
N-(3,4-difluorobenzyl)-N'-5-isoguinolinylurea
135
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 61B
using 3,4-difluorobenzylamine instead of 4-cyanobenzyl alcohol. MS (ESI) m/z:
314.07
(M+H)+; 1H NMR (DMSO-d6) S 4.36 (d, 2H), 7.12 (t, IH), 7.20 (in, IH), 7.40 (t,
2H), 7.60 (t,
I H), 7.75 (d, IH), 7.94 (d, I FD, 8.26 (dd, I H), 8.54 (d, l II), 8.79 (s, I
H), 9.27 (s, I H).
Example 220
N-5-isoquinolinyl-N'- f[4-(trifluoromethyl)cyclohexyl)methyl) urea
The title compound was prepared using the procedure described in Example 61B
using [4-(trifluoromethyl)cyclohexyl]methylamine instead of 4-cyanobenzyl
alcohol. MS
(ESI) n-i1z: 352.07 (M+H)+; 'H NMR (CDC13) 5 1.05 & 1.27 (q & q, IH), 1.58
(in, 2H), 1.66
(m, 2H), 1.70 (m, 21-1), 1.94 (m, 2H), 2.08 (m, 1H), 3.21 & 3.34 (d & d, 2H),
7.16 (br, IH),
7.84 (s, 2H), 8.35 (s, 1H), 8.82 (d, 1H), 9.12 (d, 1H), 9.36 (s, 111), 9.49
(s, 1H).
Example 221
ethyl 5-isoquinolinylacetate
5-Bromoisquinoline (7.19 g, 34.5 mmol) in toluene (80 mL) was treated with
dichlorobis(tri-o-tolylphosphine)palladium(II) (5 mol%, 1.3639 g, 1.7 mmol)
and
tributylstannanylacetic acid ethyl ester in toluene (20 mL). This mixture was
heated at 125
C overnight, cooled, diluted with ethyl acetate (1001nL), washed with water (2
x 50 mL),
dried (MgSO4), and the filtrate was concentrated under reduced pressure. The
residue was
pruified by column chromatography (20% ethyl acetate in hexanes to 50% ethyl
acetate in
hexanes) to provide the title compound. MS (ESI+) m/z 216 (M+H)+, (ESI-) m/z
214 (M-H)-
;'H NMR (DMSO, 300 MHz) S 1.17 (t, J 7.1, 3H), 4.09 (q, J 7.1, 2H), 4.17 (s,
2H), 7.64 (m,
111), 7.72 (d, J 6.2, 1H), 7.81 (d, J 5.7, 1H), 8.07 (d, J 7.9, 1H), 8.54 (d,
J 6.1, 1H), 9.33 (s,
1H); Anal. Caled for C13H13NO2 - 0.6 H20: C, 69.07; H, 6.33; N, 6.2. Found: C,
59.4; H,
6.09; N, 5.89.
Example 222
2-(5-isoguinolinyl)-N-f4-(rifluoromethoxy)benzyl)acetamide
Example 222A
5-isoguinolinylacetic acid
136
CA 02743534 2011-06-13
Ethyl 5-isoquinolinylacetate (1.15 g, 5.34 mmol) was dissolved in concentrated
H2SO4 (12 mL) and heated at 100 C for 2 hours. The reaction mixture was
poured into ice
(20g) and the pH was adjusted to 6 with 50% NaOH/H2O. The mixture was allowed
to set of
several hours, filtered, and the filter cake was rinsed with water to provide
the title
compound. MS (ESI+) m/z 188 (M+H)i; 'H NMR (DMSO, 300 MHz) d 4.07 (s, 2II),
7.67
(m, 211), 7.83 (d, J 5.7, IH), 8.05 (d, J 8.1, IH), 8.53 (d, J 6.1, 1H), 9.32
(s, IH), 12.50 (s,
1H); 13C NMR (DMSO, 75 N1Hz) S 37.6 (CH2CO), 117.1 (CH, C4), 126.8, 127.0 (CH,
C7 &
C8),128.4 (C), 131.1 (C), 132.0 (CH, C6), 134.4 (C), 143.0 (CH, C3), 152.7
(CH, Cl), 172.3
(CO); Anal. Calcd for C11H9N02: C, 70.58; H, 4.85; N, 7.48. Found: C, 70.42;
H, 4.93; N,
7.34.
Example 222B
2-(5-isoguino luryl)-N-(4-(trifluoronrethoxy)benzyl) acetami de
Polymer supported 1,3-dicyclohexylcarbodiunide (0.845 g) in dichloromethane (5
mL) was treated with 5-isoquinolinylacetic acid (0.075 g, 0.40 mmol) in
dichloromethane (1
mL), 1-hydroxy-7-azabenzotriazole (0.049 g), and triethylamine (0.080 g) in
dichloromethane (1 mL). After stirring for 5 minutes, the mixture was treated
with
4-(trifluoromethoxy)benzylaiuine (0.40 mmol). After stirring for 16 hours, the
mixture was
treated with MP-Carbonate resin (0.310 g), stirred for 5 minutes, and filters-
d. The filtrate
was diluted with dichloromethane (40 mL), washed with water (4x20 mL), brine
(1 x20 mL)
dried (NaSO4), filtered, and the filtrate was concentrated under reduced
pressure to provide
the title compound which was purified by forming the hydrochloride salt and
triturating the
solid with.hot ethyl acetate. MS (ESI+) mlz 361 (M+H)+; MS (ESI-) rnlz 359 (M-
H) 111
NUR (DMSO, 300 MHz) 6 3.99 (s, 2H), 4.31 (d, J 5.7,2H), 7.31 (d, J 8.8, 2H),
7.36 (d, J
6.4, 2H), 7.66 (n, 2H), 7.93 (d, J 6.1, 1H), 8.03 (d, J 8.2, 1H), 8.51 (d, J
6.1, 1H), 8.74 (t, J
6.1, IH), 9.31 (s, 114); Anal. Calcd for C,9H15F3N202 + 1 HCI: C, 57.51; H,
4.06; N, 7.06.
Found: C, 57.42; H, 3.98; N, 6.72.
Example 223
N-(4-tert-butylbenzyl)-2-(5-isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 222B
using 4-(tert-butyl)benzylamine instead of4-(trifluoromethoxy)benzylamine. MS
(ESI+) m/z
137
CA 02743534 2011-06-13
.i.i.3 (1V1+Y1) ; 1V1b (t 1-) rn/Z jj 1 (M-H)-; 1H NMR (DMSO, 300 MHz) 6 1.26
(s, 9H), 3.96
(s, 2H), 4.24 (d, J 6.1, 21-1),7.17 (d, J 8.5,2M, 7.32 (d, 1 6.4, 2H), 7.66
(m, 2H), 7.83 (d, j
6.1, 1H), 8.03 (d, J 8.1, 1H), 8.51 (d, J 6.1, 1H), 8.65 (t, J 5.8, IH), 9.30
(s, 111); Anal. Calcd
for C22H24N20 + 1.15 HCI: C, 70.58; H, 6.77; N, 7.48. Found: C, 70.56; H,
6.80; N, 7.39.
Example 224
N-[3-fluoro-4-(trifluoromethyl)benzyll-2-(5-isoguinolinyl)acetunide
The title compound was prepared using the procedure described in Example 222B
using 3-fluoro-4-(trifluoromethyl)benzylarnine instead of 4-
(trifluoromethoxy)benzylamine.
MS (ESI+) m/z 363 (M+H)+; MS (ESI-) rn/z 361 (M-H)-; 'H NMR (DMSO, 300 MIIz) S
4.21 (s, 2H), 4.38 (d, J 6.1,2H), 7.32 (m, 2H), 7.73 (t, 3 7.8, 1H), 7.98 (t,
J 8.1, 1H), 8.13 (d, J
7.1, 1H), 8.44 (d, J 8.4, 1H), 8.72 (d, J 6.8, IH), 9.07 (t, J 6.1, IH), 9.88
(s, IH); Anal. Calcd
for C19H14F4N20 + 1.15 HCl: C, 56.45; H, 3.78; N, 6.93. Found: C, 56.57; H,
3.69; N, 6.88.
Example 225
N- { 1-[3-fluoro-4-(trifluoromethyI)phenyl]ethyl)-2-(5-isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 222B
using 1-[3-fluoro-4-(trifluoromethyl)phenyl]ethylamine instead of
4-(trifluoromethoxy)benzylamine. MS (ESI+) m/z 377 (M+H)+; MS (ESI-) m/z 375,
411 (NM-
H)-; 'H NMR (DMSO, 300 MHz) ,5 1.41 (d, 3 7.1, 3H), 4.17 (s, 2H), 4.93 (q, J
7.4, 1H), 7.39
(m, 2H), 7.72 (t, J 7.8, 1 H), 7.96 (t, J 8.1, 1 I-1), 8.10 (d, 3 6.4, 1 H),
8.42 (d, J 8.2, 1 H), 8.55 (d,
J 6.8, 1H), 8.71 (d, J 6.8, 1H), 9.07 (d, J 7.5, IH), 9.86 (s, 1H); Anal.
Cased for C20H16F4N20
+ 1.55 HCI: C, 55.50; H, 4.18; N, 6.52. Found: C, 55.49; H, 4.09; N,6.47.
Example 226
N- f I -[3-fluoro-4-(trifluoromethyl)phenyllpropyll -2-(5-
isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 222B
using 1-[3-fluoro-4-(trifluoromethyl)phenyl)propylamine instead of
4-(trifluorometho),)benzylamine. MS (ESI+) m/z 391 (M+H)+; MS (ESI-) rn/z 389,
425 (M-
H)'; 'H NMR (DMSO, 300 MHz) 61.06 (t, J 6.8, 3H), 3.44 (q, J 7.1, 2H), 4.20
(s, 2H), 4.73
(q, J 7.5, 1H), 7.41 (m, 2H), 7.72 (t, J 7.8, 1H), 7.97 (t, J 8.2, 1H), 8.12
(d, J 7.1, 1H), 8.44 (d,
J 8.1, 1H), 8.59 (d, J 6.7, 111), 8.72 (d, J 6.8, 1H), 9.10 (d, J 8.2, 1H),
9.88 (s, 1H); Anal.
138
CA 02743534 2011-06-13
Lalcd for L21HISr4N2(J + 1.:3 1-.L1: C, 57.46; H, 4.70; N, 6.51. Found: C,
57.62; I-1, 4.44; N,
6.40.
Example 227
2-(3-methyl-5-isouinolinyl)-N44- trifluoromethyl)benzyllacetalnide
Example 227A
ethyl (3-methyl-5-isoquinolinyl)acetate
The title compound was prepared using the procedure described in Example 221
using
5-bromo-3-methylisquinoline instead of 5-brornoisoquinoline. MS (EST+) Inlz
230 (M+H)};
MS (ESI-) m/z 228 (M-H)-;'H NMR (DMSO, 300 MHz) 5 1.18 (t, J=7.1, 3H), 2.63
(s, 3H),
4.10 (m, 5H), 7.54 (t, J=7.1, 1H), 7.65 (m, 2H), 8.01 (d, J 8.1, 114), 9.22
(s, 1H).
Example 227B
(3-methyl-5-isoguinolinyl)acetie acid
The title compound was prepared using the procedure described in Example 222A
using ethyl (3-methyl-5-isoquinolinyl)acetate instead of ethyl 5-
isoquinolinylacetate. MS
(ESI+) in/z 202 (M+H)+; MS (ESI-) m/z 200, 156 (M-H)-; 'H NMR (DMSO, 300 MHz)
8
2.62 (s, 3H), 4.03 (s, 2H), 7.58 (t, J 8.2, 1H), 7.64 (m, 21T), 7.99 (d, J 8.
1, 1H), 9.21 (s, IH),
12.46 (s, 1H); Anal. Calcd for C12H11NO2: C, 71.63; H, 5.51; N, 6.96. Found:
C, 71.00; H,
5.42; N, 6.79.
Example 227C
2-(3-methyl-5-isoguinolinyl)-N-(4-(trifluoromethyl)benzyll acetamide
The title compound was prepared using the procedure described in Example 222B
using (3-methyl-5-isoquinolinyl)acetic acid and 4-(trifluoromethyl)benzylamine
instead of 5-
isoquinolinylacetic acid and 4-(trifluoromethoxy)benzylamine. MS (ESI+) m/z
359 (M+H)+;
MS (ESI-) m/z 357 (M-H)-; 'H NMR (DMSO, 3001\*Iz) 8 2.77 (s, 3H), 4.12 (s,
211), 4.37
(d, J 6.1, 2H), 7.47 (d, J 7.8, 2H), 7.68 (d, J S. 1, 2H), 7.86 (t, J 7.4,
1H), 8.03 (d, J 6.4, IH),
8.36 (m, 2I1), 9.03 (t, J 5.8, 1H), 9.77 (s, 1H); Anal. Calcd for C20H17F3N20
+ 1.85 HC1: C,
56.44; H, 4.57. Found: C, 56.41; H, 4.46.
139
CA 02743534 2011-06-13
Example 28
N-[3-fluoro-4-(trifluoromethyl)benzyl}- -methyl-5-isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 222B
using (3-methyl-5-isoquinolinyl)acetic acid and 3-fluoro-4-
(trifluoromethyl)benzylamine
instead of 5-isoquinolinylacetic acid and 4-(trifluoromethoxy)benzylamine. MS
(ESI+) m/z
377 (M+H)+; MS (ESI-) mlz 375 (M-H)-;1H NMR (DMSO, 300 MHz) 6 2.77 (s, 3H),
4.14
(s, 2H), 4.38 (d, J 6.1, 21-1), 7.33 (m, 21-1), 7.72 (t, J 7.8, 1H), 7.86 (t,
J 7.5, 1H), 8.04 (d, J 6.8,
1H), 8.36 (m, 2H), 9.07 (t, J 6.1, 1H), 9.77 (s, 1H); Anal. Calcd for
C20H16F4N2O + 1.2 HCl +
0.3 DMF: C, 56.62; H, 4.20; N, 7.48. Found: C, 56.79; H, 4.40; N, 7.29.
Example 229
2-(5-isoguinolinyl)-N- f 2-[3-(trifluoromethyl)phenyllethyl}acetamide
The title compound was prepared using the procedure described in Example 222B
using 2-[3-(trifluoromethyl)phenyl]ethylamine instead of 4-
(trifluoromethoxy)benzylamine.
MS (ESI+) mlz 359 (M+H)+; MS (ESI-) m/z 357, 393 (M-H)-; 1H NMR (DMSO, 300
MHz)
S 2.83 (t, J 7.1, 2H), 3.35 (q, J 6.8, 2H), 4.03 (s, 2H), 7.50 (m, 4H), 7.98
(m, 2H), 8.47 (m,
311), 8.68 (d, J 6.8, 1H), 9.89 (s, 111); Anal. Calcd for C2oH17F3N20 + 1.55
HCI: C, 57.94; H,
4.64; N, 6.73. Found: C, 57.90; H, 4.51; N, 6.75.
Example 230
N-(3,3-diphenylpropyl)-2-(5-isoguinolinyl)acetami de
The title compound was prepared using the procedure described in Example 222B
using 3,3-diphenylpropylamine instead of 4-(trifluoromethoxy)benzylamine. MS
(ESI+) m/z
381 (M+H)+; MS (ESI-) m/z 379 (M-H)-; 1H NMR (DMSO, 300 MHz) 6 2.17 (q, J
7.8,21D,
2.96 (q, J 5.8, 2H), 3.99 (s, 2H), 7.16 (m, 21D, 7.25 (m, 9H), 7.84 (t, J 7.5,
1H), 7.93 (d, J 6.5,
1H), 8.29 (m, 3H), 8.63 (d, J 6.5, 111), 9.64 (s, 1B); Anal. Calcd for
C26H24N20 + 1-HCl +
0.45 H2O: C, 73.47; H, 6.14; N, 6.59. Found: C, 73.84; H, 6.17; N, 6.07.
Example 231
N-(3-butoxypropyl)-2-(5 -isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 222B
using 3-butoxypropylamine instead of 4-(trifluoromethoxy)benzylamine. MS
(ESI+) m/z 301
140
CA 02743534 2011-06-13
(M+H)+; MS (ESI-) m/z 299 (M-1-1)'; 111 NMR (DMSO, 300 MHz) b 0.85 (t, J 7.5,
3H), 1.28
(in, 2H), 1.43 (ni, 2H), 1.63 (m, 2H), 3.11 (in, 2H), 3.32 (m, 4H), 3.97 (s,
211), 7.81 (t, J 7 .2,
1H), 7.89 (d, J 6.8, 1H), 8.22 (in, 3H), 8.63 (d, J 5.9, 1H), 9.59 (s, 1H).
Example 232
2-(5-isoguino linyl)-N-(3-phenylpropyl~ac etamide
The title compound was prepared using the procedure described in Example 222B
using 3-phenylpropylamine instead of 4-(trifluoromethoxy)benzylamine. MS
(ESI+) m/z 305
(M+H)+; MS (ESI-) in/z 303 (M-H)-;'H NMR (DMSO, 300 MHz) 6 1.70 (p, J 7.1,
2H), 2.55
(t, J 7.1, 2H), 3.07 (q, J 6.8, 21-1), 4.05 (s, 2H), 7.21 (m, 5H), 7.92 (t, J
7.5, 1H), 8.04 (d, J 6.4,
1H), 8.38 (m, 2H), 8.48 (d, J 6.5, 1H), 8.69 (d, J 6.5, 1H), 9.79 (s, IH);
Anal. Calcd for
C2oH20N20 + 1.5 HCI: C, 66.97; H, 6.18; N, 8.06. Found: C, 66.90; H, 6.04; N,
7.80.
Example 233
2-(5 -i soguinolinyl)-N-(2-(2-thienyl)ethyll acetam ide
The title compound was prepared using the procedure described in Example 222B
using 2-(2-thienyl)ethylamine instead of 4-(trifluoromethoxy)benzylamine. MS
(ESI+) m/z
297 (M+H)+; MS (ESI-) mlz 295 (M-H)'; 'H NMR (DMSO, 300 MHz) S 2.93 (t, J 6.8,
2H),
3.32 (q, J 6.9,2H), 3.96 (s, 2H), 6.83 (d, J 2.5, 1H), 6.93 (q, J 3.4, 1H),
7.31 (t, J 3.7, IH),
7.77 (t, J 8.1, 1H), 7.82 (d, J 7.2, 1H), 8.14 (d, J 6.2, 1H), 8.18 (d, J 8.1,
IH), 8.35 (t, J 6.1,
1H), 8.59 (d, J 6.2, 1H), 9.53 (s, 1H).
Example 234
N-(3 -(l H-imidazol-1-yl)propyll-2-(5-isoquinolinyl)acetamide
The title compound was prepared using the procedure described in Example 222B
using 3-(1H-imidazol-1-yl)propylamine instead of 4-
(trifluoromethoxy)benzylamine. MS
(ESI+) m/z 295 (M+H)+; MS (ESI-) m/z 293 (M-M-; 'H NMR (DMSO, 300 MHz) S 1.96
(m,
2H), 3.07 (q, J 6.9, 211), 3.97 (s, 2H), 4.19 (t, J 6.8, 2H), 7.73 (m, 4H),
8.09 (d, J 5.9, 11),
8.14 (d, J 8.1, 1H), 8.32 (t, J 5.3, 1H), 8.58 (d, J 5.9, IH), 9.07 (s, 1H),
9.46 (s, 1H).
Example 235
2-(5-isoguinolinyl)-N-[3-(2-oxo-l -pyrrolidinyl)propyl)acetamide
141
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 222B
using 1-(3-aminopropyl)-2-pyrrolidinone instead of 4-
(trifluorornethoxy)benzylamine. MS
(ESI+) m/z 312 (M+H)+; MS (ESI-) m/z 310 (M-H)-;'H NMR (DMSO, 300 MHz) S 1.59
(p,
J 7.5, 15.3, 2H), 1.89 (p, J 7.2, 14.0, 211), 2.19 (t, J 8.2, 2H), 3.04 (q, J
5.9, 2H), 3.15 (t, J 7.1,
2H), 3.28 (t, J 7.2, 2H), 3.99 (s, 2H), 7.81 (t, J 7.2, 1H), 7.99 (d, J 6.9,
1H), 8.23 (m, 3H),
8.63 (d, J 6.3, 1H), 9.60 (s, 1H).
Example 236
N-(2,2-diphenyl ethyl)-2 -(5-isoguinolinyl)acetami de
The title compound was prepared using the procedure described in Example 222B
using 2,2-diphenylethylamine instead of 4-(trifluoromethoxy)benzylamine. MS
(ESI+) m/z
367 (M+H)+; MS (ESI-) m/z 365 (M-H)-; 'H NMR (DMSO, 300 MHz) S 3.73 (q, J 6.0,
2H),
3.83 (s, 2H), 4.18 (t, J 8.1, 1H), 7.18 (m, 2H), 7.25 (m, 9H), 7.60 (m, 2H),
7.81 (d, J 6.5, 1 ),
8.06 (d, J 8.1, 1H), 8.25 (t, J 4.7, 114), 8.43 (d, J 5.6, IH), 9.37 (s, 1H).
Example 237
N-benzyl-2-(5-isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 222B
using 2,2-diphenylethylamine instead of 4-(trifluoromethoxy)benzylamine. MS
(ESI+) m/z
277 (M+H)+; MS (ESI-) rn/z 275 (1\4-M-; 'H NMR (DMSO, 300 MHz) 6 4.05 (s, 2H),
4.29
(d, J 5.9,2H), 7.23 (t, J 5.3, 3H), 7.30 (t, J 3.4, 2H), 7.78 (t, J 7.8, 1H),
7.88 (d, J 6.9, 1H),
8.20 (t, J 7.8, 2H), 8.60 (d, J 6.3, 1 H), 8.72 (t, J 5.3, 1 H), 9.54 (s, 1
H).
Example 238
2-(5-isoguinolinyl)-N-{4-((trifluoromethyl)thio]benzyll acetamide
The title compound was prepared using the procedure described in Example 222B
using 4-[(trifluoromethyl)thio]benzylamine instead of 4-
(trifluoromethoxy)benzylamine. MS
(ESI+) m/z 377 (M+H)+; MS (ESI-) m/z 375 (M-M-; IN NMR (DMSO, 300 MHz) S 4.06
(s,
2H), 4.35 (d, J 5.9, 2H), 7.40 (d, J 8.1, 2H), 7.66 (d, 1 8.2, 2H), 7.75 (t, J
7.5, 11-I), 7.85 (d, J
6.6, 1H), 8.16 (m, 2H), 8.59 (d, J 5.9, 1H), 8.79 (t, J 5.9, IH), 9.50 (s,
IH).
Example 239
142
CA 02743534 2011-06-13
2-(5-isoguinolinyl -N-(2-phenylethyl)acetalnide
The title compound was prepared using the procedure described in Example 222B
using 2-phenylethylamine instead of 4 (trifluoromethoxy)benzylamine. MS (ESI+)
mlz 291
(M+H){; MS (ESI-) m/z 289 'H NMR (DMSO, 300 MHz) S 2.71 (t, J 7.1, 2H), 3.31
(q, J 7.1, 2H), 3.94 (s, 2H), 7.17 (m, 311), 7.25 (t, J 7.5, 2H), 7.77 (t, J
7.5, 1H), 7.82 (d, J 6.6,
1H), 8.15 (d, J 6.2, 1H), 8.19 (d, J 7.8, 1H), 8.27 (t, J 5.3, 1H), 8.59 (d, J
5.0, 11-1), 9.55 (s,
1H).
Example 240
2-(5-isoguinolinyl)-N-[2-(3-pyridinyl)ethyllacetaroide
The title compound was prepared using the procedure described in Example 222B
using 2-(2-pyridinyl)ethylamine instead of 4-(trifluoromethoxy)benzylamine. MS
(ESI+)
rn/z 292 (M+H)+; MS (ESI-) m/z 290 (M-H)-;'H NMR (DMSO, 300 MHz) 8 2.84 (t, J
6.9,
2H), 3.39 (q, J 6.5, 2H), 3.92 (s, 2H), 7.58 (t, J 5.3, IM, 7.75 (m, 2H), 7.97
(d, J 7.8, 1H),
8.05 (d, J 5.9, 1H), 8.16 (d, J 7.8, 1H), 8.29 (t, J 5.6, 1H), 8.57 (m, 3H),
9.51 (s, 1H).
Example 241
N-{ 1-[3-fluoro-4-(trifluoromethyl)phenyllethyl; -N-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 1-[3-fluoro-4-(trifluoromethyl)phenyl]ethylamine instead of 4-
cyanobenzyl alcohol.
MS (ESI+) m/z 378 (M+H)+; MS (ESI-) m/z 376 (M-H)-; 'H NMR (DMSO, 300 MHz) S
1.46 (d, J 7.1, 3H), 4.97 (p, J 7.1, 1H), 7.51 (m, 2H), 7.77 (t, J 7.8, 1H),
7.86 (t, J 8.2, 1H),
7.99 (d, J 7.1, 1H), 8.06 (d, J 8.1, 1H), 8.58 (d, J 6.8, IH), 8.71 (d, J 6.8,
1H), 8.78 (d, J 6.8,
IH), 9.62 (s, IH), 9.76 (s, 1H); Anal. Calcd for C19H15F4N30 + 1 HCI: C,
54.93; H, 3.99; N,
10.09. Found: C, 55.15; H, 3.90; N, 10.15.
Example 242
N- { 1-[3-fluoro-4-(trifluoromethyl)phenyllpropyl} -N'-5-isoquinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 1-[3-fluoro-4-(trifluoromethyl)phenyl]propylamine instead of 4-
cyanobenzyl alcohol.
MS (ESI+) m/z 392 (M+H)+; MS (ESI-) m/z 390 (M-H)-; 'H NMR (DMSO, 300 MHz) S
0.94 (t, J 7.4, 311), 1.78 (m, 2H), 4.80 (q, J 7.5, IH), 7.59 (m, 2H), 7.77
(t, J 8.1, 1H), 7.84 (t,
143
CA 02743534 2011-06-13
J 8.2, 1H), 7.96 (d, J 8.2, 1H), 8.04 (d, J 8.1, 1H), 8.56 (d, J 7.1, 1H),
8.73 (m, 2H), 9.59 (s,
1H), 9.73 (s, 11-1); Anal. Calcd for C2oH17F4N3O + 1 HCI: C, 56.1.0; H, 4.26;
N, 9.81. Found:
C, 56.15; H, 4.24; N, 9.82.
Example 243
N-[3-bromo-4-(trifluoromethyl)benzyll-2-(5-isoguinolinyl)acetamide
Example 243A
3-bromo-4-trifluoromethylbenzoic acid
3-Amino-4-trifluoromethylbenzoic acid (8.20 g, 40.0 mmol), prepared according
to
Astrid Giencke and Helmut Lackner, Liebigs Ann. Chem., 569-579:6 (1990), in
48% HBr (20
mL) and H2O (67 mL) at 0 C was treated with NaNO2 (2.99 g) in small portions
over 15
minutes. After stirring for 30 minutes, the mixture was treated with urea
(0.250 g) and then
the mixture was added dropwise to a solution of CuBr (10.0 g) in 48% HBr (40
mL) and H2O
(100 mL). The reaction mixture was heated at 75 C, stirred for 2 hours,
cooled to room
temperature, and stirred overnight. The mixture was treated with with 20% NaOH
until the
pH > 10. The resulting blue copper salts were removed by filtration through
Celite. The
mixture was acidified to pH 1 with HCI, extracted with CH2CI2 (3 x 200 mL),
dried over
Na2SO4, filtered, and the filtrate was concentrated under reduced pressure to
provide the title
compound.
Example 243B
3-bromo-4-(trifluoromethyl)benzamide
The product from Example 243A (4.00 g, 14.9 mmol) in thionyl chloride (20 mL)
was
heated at 80 C for 2 hours. The mixture was concentrated under reduced
pressure and the
residue was dissolved in MeOH (30 mL) and cooled to -60 C. The mixture was
treated with
ammonium hydroxide(10 mL) and allowed to reach room temperature over 3 hours.
The
solvent was removed to give crude 3-bromo-4-trifluoromethylbenzamide. mp 148-
150 C.
Example 243C
3-bromo-4-(trifluoromethyl)benzylarnine
144
CA 02743534 2011-06-13
LiAIH4 (0.906 g, 23.9 imnol) was suspended in 60 mL of dry THE and cooled to 0
C.
The mixture was treated with the product from Example 243B (3.2 g, 11.9 mmol)
in THE (10
mL) dropwise with stirring. After 20 minutes, the mixture was warmed to room
temperature
12 hours and treated in succession with ethyl acetate (2 mL), NaOH (50%, 5
mL), and diethyl
ether (100 mL). The organic phase decanted, dried (Na2SO4), filtered and the
filtrate was
concentrated under reduced pressure to provide the title compound-
Example 243D
N-[3 -bromo-4-(trifluoromethyl)benzyll-2-(5-isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 222B
using 3-bromo-4-(trifluoromethyl)benzylaniine instead of 4-
(trifluoromethoxy)benzvlamine.
MS (ESI+) m/z 425, 423 (M+H)+; MS (ESI-) mlz 423, 421 (M-H)-; 'H NMR (DMSO,
300
MHz) 5 4.09 (s, 2H), 4.36 (d, J 6.1, 2H), 7.43 (d, J 7.2, 2H), 7.67 (s, 1H),
7.79 (in, 2H), 7.90
(d, J 7.9, 1H), 8.22 (m, 2H), 8.61 (d, J 6.4, 1H), 8.86 (t, J 6.8, 1H), 9.56
(s, 1H); Anal. Calcd
for C29H14BrF3N2O + 0.9 TFA: C, 47.51; H, 2.86; N, 5.33. Found: C, 47.53; H,
2.92; N, 522.
Example 244
N-(4-bromo-3-methylbenzyl)-2-(5-isog uinolinyl)acetami de
Example 244A
4-bromo-3-methylbenzyl Amin e
LiAIH4 (0.68 g) in diethyl ether (30 mL) was treated with 4-bromo-3-
methylbenzonitrile (15 mmol) and refluxed for 2 hours. The mixture was, cooled
to 0 C and
treated in succession with water (0.7 mL), 20% NaOH (0.5 mL), and water (2.5
mL). The
mixture was filtered through a celite pad and the filter cake was washed
several times with
diethyl ether. The filtrate was dried over Na2SO4i filtered, and the filtrate
was concentrated
under reduced pressure to provide the title compound. MS (ESI+) rn/z 194 (M+1-
1)+; MS
(ESI-) m/z 192 (M-H)-; 'H NMR (DMSO, 300 MHz) S 3.97 (s, 2H), 7.30 (m, 1H),
7.46 in,
2H).
Example 244B
N-(4-bromo-3-methylbenz)rl)-2-(5-isoguinolinyl)acetamide
145
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 222B
using 4-bromo-3-methylbenzylamine instead of 4-(trifluoromethoxy)benzylamine.
MS
(ESI+) m/z 371, 369 (M+H)F; MS (ESI-) m/z 369, 367 (M-H)-; 'H NMR (DMSO, 300
MHz)
S 2.28 (s, 3H), 4.13 (s, 2H), 4.22 (d, J 6.1, 2H), 7.00 (d, J 8.1, 1H), 7.10
(s, 1H), 7.50 (d, J 8.1,
1H), 7.92 (m, 1H), 8.05 (d, J 7.1, IH), 8.38 (d, J 8.1, 1H), 8.48 (d, J 6.8,
1H), 8.70 (d, J 6.8,
1H), 8.86 (t, J 6.8, 1H), 9.80 (s, 1H); Anal. Calcd for C19H17BrNZO + 1.1 HC1:
C, 55.75; H,
4.46; N, 6.84. Found: C, 55.76; H, 4.23; N, 6.93.
Example 245
N-r2,4-bis(trifluorometbyl)benzyll-2-(5-isoguinolinyl)acetamid e
The title compound was prepared using the procedure described in Example 222B
using 2,4-bis(trifluoromethyI)benzylanvne instead of 4-
(trifluoromethoxy)benzylamine. MS
(BSI+) m/z 413 (M+H)+; MS (ESI-) m/z 411 (M-H) 1H NMR (DMSO, 300 MHz) rotamers
6 4.22 (s, 2H), 4.53 (d, J 6.1, 2H), 5.97 (d, J 6.4, 1H), 7.57-8.48 (m, 6H),
8.72 (d, J 6.4, 1I-I),
8.84 (m, 1H), 9.12 (t, J 6.8, 111), 9.73 (s, 1H), 9.82 (s, 1H); Anal. Calcd
for C20H14F6N20 1.2
HCI: C, 52.67; H, 3.36; N, 6.14. Found: C, 52.67; H, 3.21; N, 6.09.
Example 246
N-(2-clrloro-4-(trifluoromethyl)bent:yl l-2-(5-isoguinolinyl)aeetamide
Example 246A
2-chloro-4-(trifluoromethyl)benzylamine
The title compound was prepared using the procedure described in Example 244A
using 2-chloro-4-(trifluoromethyl)benzonitrile instead of 4-bromo-3-
methylbenzonitrile. MS
(ESI+) m/z 209 (M+H)+; 'H NMR (DMSO, 300 MHz) 5 3.97 (s, 2H), 7.50-7.70 (m,
3H).
Example 246B
N_ j2-chloro-4-(trifluoromethyl)benzyll-2-(5-isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 222B
using 2-ehloro-4-(trifluoromethyl)benzylamine instead of 4-
(trifluoromethoxy)benzylamine.
MS (ESI+) m/z 379 (M+H)+; MS (ESI-) m/z 377 (M-H)"; 'H NMR (DMSO, 300 MHz) 6
4.19 (s, 2H), 4.41 (d, J 6.1, 2H), 7.56 (d, J 8.1, IH), 7.70 (d, J 8.1, 1H),
7.83 (s, 111), 7.92 (m,
1H), 8.06 (d, J 7.1, 1H), 8.37 (d, J 8.1, 1H), 8.45 (d, J 6.8, 1H), 8.70 (d, J
6.8, 1H), 8.97 (t, J
146
CA 02743534 2011-06-13
6.8, 1H), 9.77 (s, 1H); Anal. Calcd for C19H14CIF3N2O + 1 HCI: C, 54.96; H,
3.64; N, 6.75.
Found: C, 54.75; H, 3.47; N, 6.90.
Example 247
N-[2,3-difluoro-4-(trifluoromethyl)benzyl]-2 5-isoguuiolinyl)a letaiiudc
Example 247A
2, 3 -difluoro-4-(trifluoromethyl)b enzylamine
The title compound was prepared using the procedure described in Example 244A
using 2,3-difluoro-4-(trifluoromethyl)benzonitrile instead of 4-bromo-3-
inethylbenzonitrile.
Example 247B
N-(2,3-difluoro-4-(trifluoromethyl)benzyll-2-(5-isoguinolinyl)acetamid.e
The title compound was prepared using the procedure described in Example 222B
using 2,3-difluoro-4-(trifluoromethyl.)benzylamine instead of
4-(trifluoromethoxy)benzylamine. MS (ESI+) m/z 381 (M+H)+; MS (ESI-) m/z 379
(M-H) ;
1H NMR (DMSO, 300 MHz) rotamers 8 4.16 (s, 2H), 4.42 (d, J 6.1, 21-1), 7.35
(m, 1H), 7.59
(m, 111), 7.93 (m, 1H), 8.07 (in, I H), 8.39 (d, J 7.1, I H), 8.46 (m, 1H),
8.70 (d, J 6.8, 11I),
9.05 (t, J 6.8, 1H), 9.81 (s, 1H); Anal. Calcd for C19H14C1F3N20 + I HCI: C,
54.96; H, 3.64;
N, 6.75. Found: C, 54.75; H, 3.47; N, 6.90.
Example 248
ethyl 2-(5-isoquinolinyl)propanoate
Lithium diisopropylamide (12.75 mL, 2M, 25.5 mmol) in THE (160 mL) at -78 C
under nitrogen was treated with ethyl 5-isoquinolinylacetate (5.00 g, 23.2
mmol) in THE (5
mL). After stirring for 30 minutes at -78 C, the mixture was treated with
HMPA (5.2 mL)
and methyl iodide (1.62 mL, 25.5 mmol). After stirring for 30 minutes at -78
C, the mixture
was warmed to 0 C over 1 hour and quenched by addition of saturated NH4CI
solution. The
mixture was concentrated under reduced pressure to a volume of -10 mL, diluted
with ethyl
acetate (200 mL), washed with water (100 mL x 5), washed with brine, dried
with anhydrous
MgSO4, filtered, and the filtrate was concentrated under reduced pressure to
provide the title
compound. MS (ESI+) mlz 230 (M+H)+; MS (ESI-) m/z 228 (M-H)-; 1H NMR (DMSO,
300
147
CA 02743534 2011-06-13
MHz) 5 1.53 (d, J 7.1, 3H), 4.35 (d, J 6.1, 2H), 4.47 (q, J 7.1, IH), 7.18 (m,
2H), 7.70 (m,
3H), 8.05 (m, 2H), 8.53 (d, J 6.1, 1H), 8.68 (t, J 6. 8, 1H), 9.32 (s, 1H);
Anal. Calcd for
C2oH16F4N20 + 1.25 HC1: C, 56.93; H, 4.12; N, 6.64. Found: C, 56.72; H, 4.45;
N, 7.03.
Example 249
N-(3-fluoro-4-(trifluoromethyl)bepall-2-(5-isoquinolinyl)propanamide
Example 249A
245-isoguinolinyl)propanoic acid
Ethyl 2-(5-isoquinolinyl)propanoate (1.00 g, 4.36 mmol) was heated at 85 C in
NaOH (25%, 20 mL) for 1 hour. The mixture was allowed to coot to room
temperature,
acidified to around pH 1 with HCI, and concentrated to a dry residue. The
solid was
extracted with CHC13:isopropyl alcohol (3:1, 50 mL x 4). The extracts were
combined,
filtered, and the filtrate concentrated under reduced pressure to provide the
title compound.
MS (ESI+) m/z 202 (M+W; MS (ESI-) m/z 200 (M-D-; 'H NMR (D11IS0, 300 MHz) b
1.42 (d, J 7.1, 3H), 4.01 (q, J 7.1, 1H), 7.58 (t, 3 8.1, 1H), 7.63 (d, J 7.5,
1H), 7.86 (d, J 8.1,
1H), 8.19 (d, J 6.8, 1H), 8.43 (d, J 6.8, 111), 9.22 (s, iN); Anal. Calcd for
C12HI0N02Na+ 0.9
1120: C, 60.20; H, 4.97; N, 5.85. Found: C, 60.45; H, 5.26; N, 5.46.
Example 249B
N-(3 -fluoro-4-(trifluoromethyl)benzyll-2-(5-isoguinolinyl)propanamide
The title compound was prepared using the procedure described in Example 222B
using 3-fluoro-4-(trifluoromethyl)benzylamiine and 2-(5-
isoquinolinyl)propanoic acid instead
of 4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+)
m/z 377
(M+H)}; MS (ESI-) m/z 375 (M-H)-; 'H NMR (DMSO, 300 MHz) b 1.53 (d, J 7.1,
3H), 4.35
(d, J 6.1, 2H), 4.47 (q, J 7.1, 1H), 7.18 (m, 2H), 7.70 (m, 3H), 8.05 (m, 2H),
8.53 (d, J 6.1,
1H), 8.68 (t, J 6.8, 111), 9.32 (s, IH); Anal. Calcd for C2oH,6F4N20 + 1.25
HC1: C, 56.93; H,
4.12; N, 6.64. Found: C, 56.72; H, 4.45; N, 7.03.
Example 250
2-(5-isoguinolinyl)-N-f 4-(lnfluoromethyl)benzyllpropanamide
148
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 222B
using 4-(trifluoromethyl)benzylamine and 2-(5-isoquinolinyl)propanoic acid
instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m/z
359
(M+H)+; MS (ESI-) m/z 357 (M-H) 'II NMR (DMSO, 300 MHz) b 1.59 (d, J 7.1, 3H),
4.35
(d, J 6.1, 2H), 4.69 (q, J 7.1, 1H), 7.40 (d, J 8.1, 1H), 7.65 (d, J 8.1,
1FI), 7.78 (in, 2H), 8.03
(t, 1H), 8.20 (d, J 7.1, 1H), 8.47 (d, J 7.8, 1H), 8.65 (br s, 1H), 8.75 (s,
1H), 9.05 (t, J 5.8,
ll-f), 9.93 (s, 1H); Anal. Calcd for C20H17F3N2O + 1.6 HCl + 1.3 H2O: C,
54.58; H, 4.86.
Found: C, 54.70; H, 5.10.
Example 251
2-(5-isoguinolinyl)-N- [3-(trifluoromethyl)benzyllprop anamide
The title compound was prepared using the procedure described in Example 222B
using 3-(trifluoromethyl)benzylamine and 2-(5-isoquinolinyl)propanoic acid
instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylaeetic acid. MS (ESI+) rn/z
359
(M+H)+; MS (ESI-) m/z 357 (M-H)-; 1H NMR (DMSO, 300 MHz) 6 1.54 (d, J 7.1,
3H), 4.28
(d, J 6.1, 2H), 4.50 (q, J 7.1, 1H), 7.41 (s, 1H), 7.49 (m, 2H), 7.56 (m, 1H),
7.80 (t, J 7.8, 1H),
7.95 (d, J 7.2, 1H), 8.21 (d, J 8.1, 1H), 8.32 (d, J 6.2, 1H), 8.60 (d, J 6.8,
1H), 8.72 (t, J 5.8,
1H), 9.56 (s, 1H).
Example 252
2-(5-isoguinolinyl)-N-14-f (trifluoromethyl)thio)benzyl, propanamide
The title compound was prepared using the procedure described in Example 222B
using 4-[(trifluoromethyl)thio]bcnzylamine and 2-(5-isoquinolinyl)propanoic
acid instead of
4-(trifluoromethoxy)benzylaniine and 5-isoquinolinylacetic acid. MS (ESI+) m/z
391
(M+H)+; MS (ESI-) m/z 389 (N4-M-; 1H NMR (DMSO, 300 MHz) S 1.57 (d, J 7.1,
3H), 4.33
(d, J 6.1, 2H), 4.65 (q, J 7.1, 1H), 7.33 (d, J 8.1, 1H), 7.65 (m, 2H), 7.76
(m, 1H), 7.98 (m,
2H), 8.19 (d, J 7.1, IH), 8.42 (d, J 7.8, 1H), 8.61 (br s, 1H), 8.62 (d, J
6.8, 1H), 8.73 (d, J 6.8,
1H), 8.96 (t, J 5.8, i1), 9.86 (s, 1H); Anal. Calcd for C20H17F3N2OS + 2.1
HCI: C, 51.44; H,
4.12. Found: C, 51.35; H, 3.91.
Example 253
N-(4-bromobenzyl)-2-(5-isoguinolinyl)propanamide
149
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 222E
using 4-bromobenzylamine and 2-(5-isoquinolinyl)propanoic acid instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m/z
371, 369
(M+H)+; MS (ESI-) m/z 369, 367 (M-H) 'H NMR (DMSO, 300 MHz) S 1.57 (d, J 7.1,
3H),
4.23 (d, J 6.1, 2H), 4.63 (q, J 7.1, 111), 7.14 (m, 2H), 7.47 (m, 2H), 7.76
(m, 1H), 7.98 (t, J
7.5, 1H), 8.17 (d, J 7.1, 1H), 8.43 (d, J 7.8, 1H), 8.69 (br s, 1H), 8.74 (d,
J 6.8, 1H), 8.92 (t, J
5.8, 1H), 9.88 (s, IM; Anal. Calcd for C29HI7BrN;O + 1.4 HCI: C, 54.30; H,
4.41; N, 6.66.
Found: C, 54.49; H, 4.28; N, 6.75.
Example 254
N-(4-tert-butylbenzyl)-2-(5-isoguinolinyl)propanamide
The title compound was prepared using the procedure described in Example 222B
using 4-(tert-butyl)benzylamine and 2-(5-isoquinolinyl)propanoic acid instead
of
4-(trifluoromethoxy)benzylamine and 5-isoquinol nylacetic acid. MS (ESI+) mlz
347
(M+H)+; MS (ESI-) m/z 345 (M-H)-;'H NMR (DMSO, 300 MHz) S 1.56 (d, J 7.1, 3H),
4.22
(d,J6.1,2I1),4.57(q,J7.1, 1H),7.10(d,J8.5,2H),7.29(d,J8.5,211),7.98(t,17.5,
1H),
8.13 (d, J 7.1, 11-1), 8.34 (d, J 7.8, 1H), 8.56 (d, J 6.8, IH), 8.69 (m,
211), 9.78 (s, III); Anal.
Calcd for C23H26N20 + 1.1 HCI: C, 71.46; H, 7.07; N, 7.25. Found: C, 71.13; H,
7.17; N,
7.02.
Example 255
N-(3-fluoro-5-(trifluoromethyl)benzyll-2-(5-isoguinolinyl)propanamide
The title compound was prepared using the procedure described in Example 222B
using 3-fluoro-5-(trifluoromethyl)benzylamine and 2-(5-isoquinolinyl)propanoic
acid instead
of 4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+)
m/z 377
(M+H)+; MS (ESI-) m/z 375 (M-H)";'H NMR (DMSO, 300 MHz) 5 1.55 (d, J 7.1, 3H),
4.36
(m, 2H), 4.53 (q, 7 7.1, 1H), 7.29 (m, 2H), 7.49 (d, J 8.7, 1H), 7.80 (t, J
7.8, 11-1), 7.93 (d, J
6.5, 1H), 8.21 (d, J 8.1, 1H), 8.31 (d, 1 6.4, 1H), 8.60 (d, J 6.2, IH), 8.73
(t, J 5.8, 1H), 9.56
(s, 1H).
Example 256
2-(5-i sog uinolinyl)-N-14-(trifluoromethoxy)benzyl lpropanami d e
150
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 222B
using 2-(5-isoquinolinyl)propanoie acid instead of 5-isoquinolinylacetic acid.
MS (ESI+)
rn/z 375 (M+H)-"; MS (ESI-) rnlz 373 (M-H)-;'H NMR (DMSO, 300 MHz) 8 1.54 (d,
J 7.1,
311), 4.28 (d, J 6.1, 2H), 4.50 (q, J 7.1, 1H), 7.28 (q, J 8.5, 4H), 7.79 (t,
J 7.8, 111), 7.93 (d, J
7.2, 11-1), 8.18 (d, J S. 1, IH), 8.26 (d, J 6.2, 1H), 8.59 (d, J 6.8, 1H),
8.65 (t, J 5.8, 1H), 9.53
(s, 1H).
Example 257
2-(5 -isog uino linyl)-N- (3 -(trifluoromethoxy)benzyl lpropanamide
The title compound was prepared using the procedure described in Example 222B
using 3-(trifluoromethoxy)benzylamine and 2-(5-isoquinolinyl)propanoic acid
instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m/z
375
(hM+H)+; MS (EST-) m/z 373 'H NMR (DMSO, 300 MHz) S 1.54 (d, J 7.1, 311), 4.28
(d, J 6.1, 211), 4.50 (q, J 7.1, 1H), 7.05 (s, 1H), 7.20 (m, 2H), 7.40 (m,
1H), 7.81 (t, J 7.8, 1H),
7.96 (d, J 7.2, 1H), 8.21 (d, J 8.1, 1H), 8.32 (d, J 6.2, IH), 8.61 (d, J 6.8,
111), 8.70 (t, J 5.8,
111), 9.57 (s, 1H).
Example 258
N-( ,4-dimethylbenz),l)-2-(5-isoguinolinyl)propanamide
The title compound was prepared using the procedure described in Example 222B
using 2,4-dimethylbenzylamine and 2-(5-isoquinolinyl)propanoic acid instead of
4-(trifluoromethoxy)benzylamirne and 5-isoquinolinylacetic acid. MS (ESI+) m/z
319
(M+H)+; MS (ESI-) mlz 317 (M-H)-l- 'H NMR (DMSO, 300 MHz) 6 1.52 (d, J 7.1, 31-
1), 2.13
(s, 3H), 2.21 (s, 3H), 4.20 (m, 2H), 4.51 (q, J 7.1, 1H), 6.88 (d, J 7.5, 1H),
6.94 (s, 1H), 6.98
(d, J 7.5, 1H), 7.82 (t, J 7.8, 1 H), 7.99 (d, J 6.5, 1H), 8.21 (d, J 8.1,
1H), 8.35 (d, J 6.4, 1H),
8.44 (t, J 5.8, 1H), 8.62 (d, J 6.2, 1H), 9.57 (s, IH).
Example 259
N-(2, 5-dimethylbenzyl)-2-(5-isoguinolinyl)propanamide
The title compound was prepared using the procedure described in Example 222B
using 2,5-dimethylbenzylamine and 2-(5-isoquinolinyl)propanoic acid instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m/z
319
151
CA 02743534 2011-06-13
(M+H)+; MS (ESI-) m/z 317 (M-H)-; 'H NMR (DMSO, 300 MHz) 6 1.54 (d, J 7.1,
3H), 2.09
(s, 3I1), 2.12 (s, 3H), 4.20 (m, 211), 4.53 (q, J 7.1, 1H), 6.79 (s, li-i),
6.91 (d, 3 7.8, 1I1), 6.98
(d, J 7.8, I H), 7.83 (t, J 7.8, I H), 8.00 (d, 3 6.5, I H), 8.21 (d, J 8.1,
111), 8.37 (d, J 6.4, IM,
8.46 (t, J 5.8, 1H), 8.62 (d, J 6.2, IH), 9.57 (s, 1H).
Example 260
N-(2,3-dichlorobenzyl)-2-(5-isoguinolinyl)propanamide
The title compound was prepared using the procedure described in Example 222B
using 2,3-dichlorobenzylamine and 2-(5-isoquinolinyl)propanoic acid instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) mlz
359
(M+H)+; MS (ESI-) m/z 357 (M-H)"; 1H NMR (DMSO, 300 MHz) 8 1.54 (d, J 7.1,
3H), 4.20
(m, 2H), 4.53 (q, J 7.1, IH), 7.17 (d, J 7.8, 1H), 7.26 (t, J 7.8, IH), 7.52
(d, J 8.1, 1H), 7.78 (t,
J 7.8, 1H), 7.91 (d, J 6.5, 1H), 8.16 (d, J 8.1, 1H), 8.24 (d, J 6.4, 1H),
8.59 (d, J 6.2, 111), 8.66
(t, J 5.8, 1H), 9.50 (s, 1H).
Example 261
N-(2, -dichlorobenzyl)-2-(5-isoguinolinyl)propanamide
The title compound was prepared using the procedure described in Example 222B
using 2,4-dichlorobenzylanline and 2-(5-isoquinolinyl)propanoic acid instead
of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) mlz
359
(M+H)+; MS (ESI-) m/z 357 (M-H) ; 'H NMR (DMSO, 300 MHz) S 1.54 (d, J 7.1,
3H), 4.20
(m, 2H), 4.53 (q, J 7.1, 1H), 7.22 (d, 7 8.4, 1H), 7.33 (m, 2H), 7.56 (s,
111), 7.78 (t, J 7.8, 1H),
7.90 (d, J 6.5, 1H), 8.17 (d, J 8.1, 1H), 8.25 (d, J 6.4, IH), 8.62 (m, 2H),
9.51 (s, 1H).
Example 262
N-(2,5-dichlorobenzyl)-2-(5-isoguinolinyl)propanamide
The title compound was prepared using the procedure described in Example 222B
using 2,5-dichlorobenzylamine and 2-(5-isoquinolinyl)propanoic acid instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m/z
359
(M+H)+; MS (ESI-) m/z 357 (M-H)";'H NMR (DMSO, 300 MHz) S 1.54 (d, J 7.1, 3H),
4.20
(m, 2H), 4.53 (q, J 7.1, 1H), 7.06 (s, lH), 7.32 (d, J 8.4, 1H), 7.44 (d, J
8.4, 1H), 7.51 (s, 1H),
152
CA 02743534 2011-06-13
7.78 (t, J 7.8, IH), 7.90 (d, J 6.5, lII), 8.16 (d, J 8.1, 1H), 8.26 (d, J
6.4, IH), 8.60 (d, J 6.2,
111), 8.65 (t, J 5.8, I H), 9.49 (s, IH).
Example 263
N-(3,4-dichlorobenzyl)-2-(5-isoguinolinyl)prop anamide
The title compound was prepared using the procedure described in Example 222B
using 3,4-dichlorobenzylamine and 2-(5-isoquinolinyl)propanoic acid instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m/z
359
(117+H)+; MS (ESI-) m/z 357 (M-H) 'H NMR (DMSO, 300 MHz) S 1.54 (d, J 7.1,
3H), 4.20
(m, 2H), 4.53 (q, J 7.1, 1H), 7.16 (d, J 8.4, 1H), 7.31 (s, IH), 7.52 (d, J
8.4, 1H), 7.77 (t, J 7.8,
111), 7.89 (d, J 6.5, 1H), 8.16 (d, J 8.1, lH), 8.22 (d, J 6.4, 1H), 8.59 (d,
J 6.2, 1H), 8.64 (t, J
5.8, 111), 9.49 (s, 1H).
Example 264
N-(3,5-dichlorobenzyl)-2-(5-isoguinolinyl propanamide
The title compound was prepared using the procedure described in Example 222B
using 3,5-dichlorobenzylamine and 2-(5-isoquinolinyl)propanoic acid instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m/z
359
(M+H)4; MS (ESI-) m/z 357 (M-H)-;'H NMR (DMSO, 300 MHz) S 1.54 (d, J 7.1, 3H),
4.20
(m, 2H), 4.53 (q, J 7.1, 11-1), 7.13 (s, 2H), 7.42 (s, 11-1), 7.78 (t, J 7.8,
II1), 7.89 (d, J 6.5, 11-1),
8.17 (d, J 8.1, 1H), 8.23 (d, J 6.4, 1H), 8.59 (d, J 6.2, 1H), 8.64 (t, J 5.8,
IM, 9.51 (s, 11-1).
Example 265
N-[4-(1-azepanyl)-3-fluorobenzyll-2-(5-isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 222B
using 4-(1-azepanyl)-3-fluorobenzylamine instead of 4-
(trifluoromethoxy)benzylamine. MS
(ESI+) m/z 392 (M+H)+; MS (ESI-) m/z 390 (M-H)-; 'H NMR (DMSO, 300 MHz) S 1.53
(m,
4H), 1.72 (m, 4H), 3.32 (m, 4H), 3.96 (s, 2H), 4.18 (d, J 6.1, 2H), 6.86 (m,
3H), 7.69 (m, 2H),
7.94 (d, J 7.5, 1H), 8.03 (d, J 7.1, 11-1), 8.50 (d, J 7.8, 1H), 8.62 (t, J
5.8, 1H), 9.30 (s, 111);
Anal. Calcd for C24H26FN3O + 0.3 H2O: C, 72.63; H, 6.76; N, 10.59. Found: C,
72.78; H,
7.05; N, 10.80.
153
CA 02743534 2011-06-13
Example 266
N-[4-(1-azepanyl)benzyl]-2-(5-isoguinolinyl)propanaxnide
The title compound was prepared using the procedure described in Example 222B
using 4-(l-azepanyl)benzylamine and 2-(5-isoquinolinyl)propanoic acid instead
of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m!z
388
(M+H)+; MS (EST-) m/z 366 (M-H) 'H NMR (DMSO, 300 MHz) S 1.53 (m, 7H), 1.72
(m,
4H), 3.85 (s, 2H), 4.03 (q, J 7.1, 1H), 6.76 (m, 3H), 7.26 (m, 211), 7.58 (m,
111), 7.71 (m, 1H),
8.10 (m, 2H), 8.72 (t, J 5.8, 1H), 9.91 (s, 1H); Anal. Calcd for C25H29N3O +
2.15 HCI + 2
H2O: C, 59.82; H, 7.06. Found: C, 59.59; H, 7.28.
Example 267
N-[4-(1-azepanyl)-3-fluorobenzyll-2-(5-isoquinolinyl)propanainide
The title compound was prepared using the procedure described in Example 222B
using 4-(I-azepanyl)-3-fluorobenzylamine and 2-(5-isoquinolinyl)propanoic acid
instead of
4-(trifluorometho3.y)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m/z
406
(M+H)+; MS (ESI-) m/z 404 (M-H) 'H NMR (DMSO, 300 MHz) S 1.53 (m, 711), 1.72
(m,
41), 3.32 (m, 4H), 3.65 (s, 2H), 4.18 (q, J 7.1, 1H), 6.86 (m, 3H), 7.58 (m,
211), 7.74 (m, 1H),
8.13 (m, 2H), 8.52 (d, J 7.8, 1H), 9.30 (s, 1H); Anal. Calcd for C25H2sFN30 +
3.25 HCI: C,
57.30; H, 6.01. Found: C, 57.26; H, 5.98.
Example 268
ethyl 2-(5-isoguinolinyl)butanoate
The title compound was prepared using the procedure described in Example 248
using
ethyl iodide instead of methyl iodide. MS (ESI+) m/z 244 (M+H)+; MS (ESI-) m/z
242 (M-
H) 'H NMR (DMSO, 300 MHz) S 1.53 (d, J 7.1, 3H), 4.35 (d, J 6.1, 2H), 4.47 (q,
J 7.1, 1H),
7.18 (m, 21-1),7.70 (m, 3H), 8.05 (m, 2H), 8.53 (d, J 6.1, 1H), 8.68 (t, J
6.8, 1H), 9.32 (s, 1H);
Anal. Calcd for C,5H17NO2 + 0.4 H2O: C, 71.92; H, 7.16; N, 5.59. Found: C,
72.23; H, 7.32;
N, 5.31.
Example 269
N-[3-fluoro-4-(trifluoromethyl)benzyll-2-(5-isoquinolinyl)butanamide
154
CA 02743534 2011-06-13
Example 269A
2-(5-isoquinolinyl)butanoic acid
The title compound was prepared using the procedure described in Example 249A
using ethyl 2-(5-isoquinolinyl)butanoate instead of ethyl 2-(5-
isoquinolinyl)propanoate.
Example 269B
N-[3-fluoro-4-(trifluoromethyl)benzyll-2-(5-isoquinolinyl)butanami de
The title compound was prepared using the procedure described in Example 222B
using 3-fluoro-4-(trifluoromethyl)benzylamine and 2-(5-isoquinolinyl)butanoic
acid instead
of 4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+)
m!z 391
(M+H)+; MS (ESI-) m/z 389 (M-H)-;'H NMR (DMSO, 300 MHz) 6 0.91 (t, J 7.5, 3H),
1.81
(m, 1H), 2.17 (m, IH), 4.35 (m, 2H), 7.17 (m, 2H), 7.69 (t, J 7.8, 1H), 7.86
(t, J 7.8, 1H), 8.04
(d, J 7.1, 1H), 8.23 (d, J 8.1, 1H), 8.65 (d, 16. 8, IH), 8.83 (t, J 6.8, 11-
1), 9.60 (s, IH); Anal.
Calcd for C21H1sF4N20 + I HCI: C, 54.77; H, 3.80; N, 5.55. Found: C, 54.62; H,
3.57; N,
5.50.
Example 270
2-(5-isoquinolinyl)-N-r4-(trifluoromethyl)benzyl]butanamide
The title compound was prepared using the procedure described in Example 222B
using 4-(trifluoromethyl)benzylamine and 2-(5-isoquinolinyl)butanoic acid
instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m!z
391
(M+H)+; MS (ESI-) m!z 371 (M-H)-; 'H NMR (DMSO, 300 MHz) 6 0.91 (t, J 7.5,
311), 1.91
(m. IH), 2.19 (m, 1H), 4.38 (m, 2H), 7.38 (d, J 8.5, 2H), 7.64 (d, J 8.5, 2H),
7.96 (t, J 7.8,
1H), 8.20 (d, 1 7.1, 1H), 8.39 (d, J 8.1, 1H), 8.72 (s, IM, 9.02 (t, J 6.8,
1H), 9.81 (s, 1H),
10.12 (br s, 11-1); Anal. Calcd for C21H19F3N2O: C, 67.73; H, 5.14; N, 7.52.
Found: C, 67.46;
H, 4.90; N, 7.90.
Example 271
(4brornobenzyl)-2-(5-isoquinolinyl)butanamide
The title compound was prepared using the procedure described in Example 222B
using 4-bromobenzylamine and 2-(5-isoquinolinyl)butanoic acid instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m/z
385, 383
155
CA 02743534 2011-06-13
(M+H)+; MS (ESI-) m/z 383, 381 (M-H)-;'H NMR (DMSO, 300 MHz) 6 0.91 (t, J 7.5,
3H),
1.81 (m, 1H), 2.19 (m, IB), 3.39 (m, IH), 4.22 (m, 2M, 7.13 (d, J 8.5, 2H),
7.44 (m, 3H),
7.57 (t, J 7.8, 1H), 8.00 (in, 1H), 8.21 (d, J 7.1, 111), 8.41 (d, J 8.1, 1H),
8.72 (s, 1I-1), 8.93 (t, J
6.8, 1H), 9.81 (s, 1H), 10.16 (br s, IH); Anal. Calcd for C20H19BrN2O: C,
62.67; H, 5.00.
Found: C, 62.52; H, 4.95.
Example 272
2-(5-isoguinolinyl)-N- {4-((trifluoromethyl)thiolbenzyl}butanamide
The title compound was prepared using the procedure described in Example 222B
using 4-[(trifluoromethyl)thio]benzylamine and 2-(5-isoquinolinyl)butanoic
acid instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m/z
405
(M+H)+; MS (ESI-) m/z 403 (M-H) 'H NMR (DMSO, 300 MHz) S 0.91 (t, J 7.5, 3H),
1.81
(m, 11.1), 2.21 (in, 1H), 3.39 (m, IM, 4.34 (m, 2H), 7.31 (in, 2H), 7.58 (m,
2H), 7.62 (d, J 7.8,
1H), 8.00 (m, 2H), 8.22 (d, J 7.1, 1H), 8.45 (m, 1H), 8.77 (m, 1H), 8.82 (m,
1H), 9.06 (t, J
6.8, 1H), 9.87 (s, 1H), 10.30 (br s, 1H); Anal. Calcd for C21H19F3N20S + 0.65
HCI: C, 58.91;
H, 4.63; N, 6.54. Found: C, 59.24; H, 4.30; N, 6.60.
Example 273
N-(4-(1-azepanyl)-3-fluorobenzyll-2-(5-iso uinolinyl)butanamide
The title compound was prepared using the procedure described in Example 222B
using 4-(1-azepanyl)-3-fluorobenzylamine and 2-(5-isoquinolinyl)butanoic acid
instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS (ESI+) m!z
420
(M+H)+; MS (ESI-) m/z 418 (M-H)-; 1H NMR (DMSO, 300 MHz) S 0.90 (t, J 7.5,
3H), 8
1.55 (m, 411), 1.76 (in, 6H), 2.20 (m, 1H), 3.34 (m, 5H), 4.18 (m, 2H), 6.81
(m, 1H), 7.62 (m,
1H), 8.00 (m, 2H), 8.27 (d, J 7.1, 1H), 8.45 (d, 1H), 8.77 (d, 1H), 8.82 (m,
1H), 9.90 (s, IH),
10.18 (br s, 1H); Anal. Calcd for C26H3oFN30 + 0.45 H2O: C, 73.02; H, 7.28.
Found: C,
73.05; H, 7.20.
Example 274
ethyl 2-(5-isoguinolinyl)-2-methylpropanoate
The title compound was prepared using the procedure described in Example 248
using
ethyl 2-(5-isoquinolinyl)propanoate instead of ethyl 5-isoquinolinylacetate.
MS (ESI+) m/z
156
CA 02743534 2011-06-13
244 (11M+H)+; MS (ESI-) m/z 242 (M-H)-; 'H NMR (DMSO, 300 MHz) rotalners 6
0.98, 1.08
(t, J 7.1, 3H), 1.67 (s, 6H), 4.58 (q, J 7.1, 1H), 7.53 (m, 1H), 7.82 (ni,
1H), 7.97 (m, IH), 8.05
(m, 111), S.55, 8.50 (d, J 6.1, 11-1), 9.33 (s, 1H).
Example 275
2-(5-isoquinolinyl)-2-methyl-N- f 4-f (trifluoromethyl)thiolbenzyl propanamide
Example 275A
22- 5-isoquinolinyl)-2-methylpropanoic acid
The title compound was prepared using the procedure described in Example 249A
using ethyl 2-(5-isoquinolinyl)-2-methylpropanoate instead of ethyl 2-(5-
isoquinolinyl)propanoate.
Example 275B
2-(5-isoquinolinyl)-2-methyl-N- {4-((trifluoromethyl)thiolbenzyl}propanamide
The title compound was prepared using the procedure described in Example 222B
using 4-[(trifluoromethyl)thio]benzylainme and 2-(5-isoquinolinyl)-2-
methylpropanoic acid
instead of 4-(trifluorometboxy)benzylamine and 5-isoquinolinyl acetic acid. MS
(ESI+) m/z
405 (M+H)+; NIS (ESI-) m/z 403 (M-H)-; 'H NMVIR (DMSO, 300 MHz) 6 1.57 (s,
3H), 1.64
(s, 3H), 4.33 (d, J 6.1, 2H), 6.57 (s, 1H), 7.18 (m, 1H), 7.33 (m, 1H), 7.52
(m, 2H), 7.82 (m,
111), 8.11 (m, 1H), 8.36 (d, J 7.8, 1H), 8.57 (m, 1H), 9.42 (s, 1H), 10.08 (s,
1H); Anal. Calcd
for C21H19F3N20S + 2 HCI: C, 52.84; H, 4.43. Found: C, 52.66; H, 4.39.
Example 276
ethyl hydroxy(5-isoguinolinyl)acetate
The title compound was prepared using the procedure described in Example 248
using
(S) camphorsulfonyloxaziridine (2 equivalents) instead of methyl iodide.
Example 277
N-(4-tert-butylbenzyl)-2-hydroxy-2-(5-isoguinolinyl)acetamide
Example 277A
157
CA 02743534 2011-06-13
hydroxy(5-isoguinolinyl)acetic acid
The title compound was prepared using the procedure described in Example 249A
using ethyl hydroxy(5-isoquinolin yl)acetate instead of ethyl 2-(5-
isoquinolinyl)propanoate.
MS (ESI+) m/z 204 (M+H)+; MS (ESI-) m/z 202 (M-H)
'H NMR (DMSO, 300 MHz) S 4.97 (d, J 3.1, 1H), 5.34 (d, J 3.3, 1H), 7.55 (m,
1H), 7.68 (d,
J 7.5, 1H), 7.90 (d, J 8.1, IH), 8.21 (d, J 6.8, 1H), 8.40 (d, J 6.8, 1H),
9.22 (s, IH);
Anal. Calcd for C1!H9N03 + 1.9 HCI: C, 51.96; H, 5.00. Found: C, 51.89; H,
5.25.
Example 277B
N-(4-tert-butylbenzyl)-2-hydroxy-2-(5 -i soguinolinyl)acetamid e
The title compound was prepared using the procedure described in Example 222B
using 4-(tert-butyl)benzylamine and hydroxy(5-isoquinolinyl)acetic acid
instead of
4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. [aI22D -47.2
(c 0.7,
McOH); MS (ESI+) m/z 349 (M+H)+; MS (ESI-) m/z 347 (M-1-1)-; 'H NMR (DMSO, 300
MHz) S 1.26 (s, 9H), 3.69 (s, 1H), 4.27 (d, J 6.1, 2H), 5.62 (s, 111), 6.52
(br s, IH), 7.17 (d, J
8.1, 211), 7.29 (m, 3H), 7.67 (t, J 8.1, 1H), 7.82 (d, J 7.1, 1H), 8.10 (m,
2H), 8.44 (d, J 5.8,
111), 8.71 (t, J 6.1, 111), 9.30 (s, IH); Anal. Calcd for C72H24N202 + 0.25
H20: C, 74.87; H,
7.00; N, 7.94. Found: C, 75.22; H, 7.40; N, 7.80.
Example 278
N-(4-tert-butyl-3-fluorobenzyl)-2-hydroxy-2-(5-isoguinolinyl)acetamide
The title compound was prepared using the procedure described in Example 222B
using 3-fluoro-4-(trifluoromethyl)benzylannine and hydroxy(5-
isoquinolinyl)acetic acid
instead of 4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS
(ESI+) m/z
379 (M+H)+; MS (ESI-) m/z 377 (M-H) 'H NMR (DMSO, 300 MHz) 6 4.37 (d, J 6.1,
211),
5.65 (d, 1H), 6.63 (d, 1H), 7.27 (m, 211), 7.29 (m, 3H), 7.67 (m, 2H), 7.82
(d, J 7.1, 1H), 8.15
(m, 2H), 8.44 (d, J 5.8, 1H), 8.96 (t, 1 6.1, 111), 9.30 (s, 1H); Anal. Calcd
for C22H24N202 +
0.25 H2O: C, 74.87; H, 7.00; N, 7.94. Found: C, 75.22; H, 7.40; N, 7.80.
Example 279
4-tert-butyl 1-ethyl 2-(5-isoguinolinyl)succinate
158
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 248
using
tert-butyl bromoacetate instead of methyl iodide. MS (ESI+) mlz 330 (M-I-II)4;
MS (ESI-)
m/z 328 (M-H)-; 1H NMR (DMSO, 300 MHz) 6 1.06 (t, J 7.1, 3H), 1.30 (s, 911),
2.76 (dd, J1
16.7, J2 6.1, 1H), 3.14 (dd, J1 9.5, J2 6.1, 1H), 4.12 (q, J 7.1, 1H), 4.76
(dd, J1 16.7, J2 9.5,
1H), 7.70 (m, 2I1), 8.05 (m, 2I1), 8.58 (d, J 6.1, 1H), 9.34 (s, IH); Anal.
Calcd for C19I123N04
+ 1 H2O: C, 65.69; H, 7.25; N, 4.03. Found: C, 65.37; H, 6.91; N, 3.67.
Example 280
tert-butyl 4-f (4-tert-butylbenzyl)aminol-3-(5-isoguinolinyl)-4-oxobutanoate
Example 280A
4-terl-butoxy-2-(5-isoguinolinyl)-4-oxobutanoic acid
4-Tert-butyl 1-ethyl 2-(5-isoquinolinyl)succinate (1.00 g, 3.04 mmol) and LiOH
(0.29
g) were stirred in McOH:H20 (3:1, 20 mL) at room temperature for 5 hours. The
solution
was poured into aqueous H3PO4 (0.1M, 30 mL) and extracted with CHC13:IPA (3:1,
30 mL x
3). The extracts were combined, dried (MgSO4), filtered, and the filtrate was
concentrated
under reduced pressure to provide the title compound. MS (ESI+) mlz 302 (M+H)-
"; MS
(ESI-) m/z 300 (N4-M-; 1H NMR (DMSO, 300 MHz) 6 1.28 (s, 91-1), 2.74 (dd, J1
16.7, J2 6.1,
Ili), 3.10 (dd, J1 9.5, J2 6.1, 111), 4.68 (dd, J1 16.7, J2 9.5, 111), 7.70
(m, 2H), 8.05 (m, 21-1),
8.57 (d, J 6.1, IM, 9.33 (s, 1H); Anal. Calcd for C17H19NO4 + 1.25 H2O: C,
63.05; H, 6.69.
Found: C, 63.27; H, 6.95.
Example 280B
tert-butyl 4-[(4-tert-butylbenzyl)amino]-3 -(5-isoguinolinyl)-4-oxobutano ate
The title compound was prepared using the procedure described in Example 222B
using 4-(tert-but),l)benzylamine and 4-tert-butoxy-2-(5-isoquinolinyl)-4-
oxobutanoic acid
instead of 4-(trifluoromethoxy)benzylamine and 5-isoquinolinylacetic acid. MS
(ESI+) mlz
447 (N1+11)+; 'H NMR. (DMSO, 300 MHz) S 1.23 (s, 9H), 1.25 (s, 9H), 2.71 (dd,
1H), 3.02
(dd, 1H), 4.22 (m, 211), 4.71 (m, 1H), 6.57 (s, 111), 7.08 (d, 3 8.5, 2H),
7.24 (d, J 8.5, 2H),
7.67 (m, 1H), 7.78 (m, 1H), 8.03 (d, 3 7.8, 1H), 8.13 (d, J 7.1, 111), 8.55
(d, J 6.8, 1H), 8.63
(m, 1H), 9.31 (s, 1H); Anal. Calcd for C2sH34N203 + 1 CH3CN + 0.8 H2O: C,
71.77; H, 7.75;
N, 8.37. Found: C, 71.64; H, 7.38; N, 8.16.
159
CA 02743534 2011-06-13
Exam lp e 281
2-[(4-tert-butylbenzyl)amino-1-(5-isoguinohliyj)-2-oxoethyl acetate
The product from Example 277B (100 mg, 0.287 mmol) and DMAP (59 mg, 0.480
uunol) in CH2CI2 (1 mL) was treated with acetic anhydride (38 [LL). After
stirring for 30
minutes, the mixture was treated with CH2C12 (5 mL) and the phases separated.
The organic
layer was washed with water (10 mL x 3), dried (Na2SO4), filtered, and the
filtrate was
concentrated to provide the title compound. MS (ESI+) m/z 391 (M+M+; MS (ESI-)
m/z 389
(M-H)-;'H NMR (DMSO, 300 MHz) 6 1.25 (s, 911), 4.27 (dq, J, 14.9, J2 6.1, 2H),
6.56 (s,
1H), 7.09 (d, J 8.5, 2M, 7.28 (d, J 8.5, 2H), 7.72 (t, J 7.1, 1H), 7.90 (d, J
6.1, 1H), 8.07 (d, J
6.1, 1H), 8.17 (d, J 8.1, 1H), 8.53 (d, J 6. 1, 1H), 8.86 (t, J 6.1, 1H), 9.35
(s, 1H);Anal. Calcd
for C24H26N203 + 0.8 H2O: C, 71.19; H, 6.87; N, 6.92. Found: C, 70.87; H,
6.47; N, 6.92.
Example 282
2-((4-tert-butylbenz)l)aminol-l-(5-isoguinolinyl)-2-oxoethyl methanesulfonate
The product from Example 277B (1.00 g, 2.87 mmol) in pyridine (5 mL) was
treated
with methanesulfonyl chloride (5.56 L, 7.17 mmol). After stirring for 30
minutes, the
mixture was concentrated under reduced pressure and diluted with CH2C12 (50
ML). The
organic layer was washed with water (50 mL x 3), dried (Na2SO4), filtered, and
the filtrate
concentrated under reduce pressure to provide the title compound. MS (ESI+)
m/z 427
(M+H)+; MS (ESI-) m/z 425 (M-H) 'H NMR (DMSO, 300 MHz) 5 1.27 (s, 9H), 2.37
(s,
3H), 4.27 (dq, J, 14.9, J2 6.1, 2H), 6.84 (s, 1H), 7.17 (d, J 8.5, 2H), 7.38
(d, 1 8.5, 2I-1), 8.07
(m, 2H), 8.37 (d, J S. 1, 11-1), 8.60 (d, J 6.1, 1H), 8.97 (m, 1H), 9.21 (t, J
6.1, 111), 9.96 (s, 1H).
Example 283
N-(4-tert-butylbenzyl)-2-(5-isoguinolinyl)-2-methoxyacetamide
The product from Example 277B (100 mg, 0.287 mm.ol) in THE (2 mL) was treated
with NaH (95%, 8.7 mg, 0.344 mmol). After stirring at room temperature for 20
minutes, the
mixture was treated with methyl iodide (1.2 eq, 21.4 L) and stirred for 1
hour. The mixture
was concentrated under reduced pressure and CH2C12 (10 mL) was added. The
organic layer
was washed with water (5 mL x 3), dried (Na2S04), filtered, and the filtrate
concentrated
under reduced pressure to provide the title compound. MS (ESI+) m/z 363
(M+H)+; MS
160
CA 02743534 2011-06-13
(ESI-) m/z 361 (M-H)-; 'H NMR (DMSO, 300 MHz) rotamers 5 1.25 (s, 9H), 3.32
(s, 3H),
4.27 (d, J 6.1, 2H), 5.37 (s, IH), 7.18 (d, J 8.5, 2H), 7.32 (d, J 8.5, 2H),
7.70 (t, J 7.1, 1H),
7.83 (d, J 6. 1, 1H), 8.07 (m, 2H), 8.43 (d, J 6.1, 1H), 8.80 (t, J 6.1, 1H),
9.35 (s, IH); Anal.
Caled for C23H26N202 + 0.3 H2O: C, 75.09; H, 7.29; N, 7.61. Found: C, 75.02;
IT, 7.34; N,
7.43.
Example 284
N 4-tent butylbenz 1~ }-2 chloro 2 (5-isoquinolinyl)acetamide
The product from Example 182 (300 mg, 0.704 mmol) in toluene (10 mL) was
treated
with Bu.;NCI (458 mg, 1.408 mmol) and heated at 100 C for 12 hours. The
mixture was
concentrated under reduced pressure and diluted with CH2C12 (50 mL). The
organic layer
was washed with water (50 mL x 3), dried (Na2SO4), filtered, and the filtrate
concentrated
under reduced pressure. MS (ESI+) m/z 367 (M+H)+; MS (ESI-) m/z 365 (M-H)-; 1H
NMR
(DMSO, 300 MHz) S 1.26 (s, 911), 4.11 (d, J 5.1, 2H), 6.59 (s, 1H), 7.16 (d, J
8.1, 211), 7.36
(d, J 8.1, 2H), 7.97 (d, J 8.1, 2H), 8.30 (d, J 7.5, 1H), 8.48 (d, J 8.1, IH),
8.56 (d, J 6.8, IH),
8.73 (d, J 6.8, 1H), 8.97 (m, 111), 9.18 (t, J 6.1, 1H), 9.81 (s, 1H); Anal.
Calcd for
C22H23C1N20 + I HC1 + 1.5 CH3OH: C, 62.53; H, 6.70; N, 6.21. Found: C, 62.75;
H, 6.87; N,
6.11.
Example 285
N-5 -i sog uinolinyl-3 - [4-(trifluoromethyl )phenyl ] acry l amid e
5-Aminoisoquinoline (0.50 g, 3.47 mmol) and 3-[4-
(trifluoromethyl)phenyl]acrylic
acid (3.47 inmol) were combined in a sealed tube and heated at 175 C for 16
hours with
stirring. The mixture was cooled to room temperature, diluted with MeOH,
transferred to a
flask, and concentrated under reduced pressure. The residue was triturated
with ethyl acetate
and filtered to provide the title compound. MS (ESI+) m/z 343 (M+H)+; MS (EST-
) m/z 341
(M-H)-; 'H NMR (DMSO, 300 MHz) rotamers 8 6.68 (d, J 15.9, 1H), 7.29 (d, J
15.9, 1H),
7.60 (d, J 15.9, 1H), 7.80 (m, 1H), 8.25 (d, J 6.8, IH), 8.57 (d, J 5.8, 1H),
9.35 (s, IH), 10.36
(s, 1H); Anal. Calcd for C19H13F3N20 + 2 HCI + 0.3 H2O: C, 54.25; H, 3.74; N,
6.66. Found:
C, 53.90; H, 3.94; N, 7.20.
Example 286
161
CA 02743534 2011-06-13
i-4-3-isoguinoiinyt-3-[3-(tritluoromethyl)phM llacrylamide
The title compound was prepared using the procedure described in Example 285
using
3-[3-(trifluoromethyl)phenyl]acrylic acid instead of 3-[4-
(trifluoromethyl)phenyl]acrylic acid.
MS (ESI+) m/z 343 (M+H)+; MS (ESI-) m/z 341 (M-H)-; 'H NMR (DMSO, 300 MHz)
rotamers S 6.72 (d, J 15.9, 1H), 6.87 (d, J 7.4, 1H), 7.23 (d, J 8.1, 1H),
7.36 (t, J 7.8, IH),
7.70 (m, 2H), 7.93 (d, J 6.1, 1H), 8.10 (m, 2H), 8.35 (d, J 5.8, 1H), 9.09 (s,
IH); Anal. Calcd
for C19H13F3N2O + 2.15 HCI: C, 54.24; H, 3.63; N, 6.66. Found: C, 53.96; H,
3.93; N, 6.93.
Example 287
3-(4-isopropylphenyl)-N-5-isoguinolinylacrylarnide
The title compound was prepared using the procedure described in Example 285
using
3-(4-isopropylphenyl)aciylic acid instead of 3-[4-
(trifluoromethyl)phenyl]acrylic acid. MS
(ESI+) m/z 317 (M+H)+; MS (ESI-) m/z 315 (M-H) 'H NMR (DMSO, 300 MHz) S 1.24
(d,
J 6.8, 3H), 2.94 (sept, J 6.8, 1H), 7.10 (d, J 15.6, 11-1), 7.35 (d, J 7.4,
2H), 7.61 (d, J 8.1, 211),
7.63 (d, J 15.6, IH), 7.84 (t, J 7.8, 1H), 8.12 (d, J 7.8, 1H), 8.26 (d, J
6.4, 1H), 8.35 (d, J 7.1,
1H), 8.64 (d, J 6.8, 111), 9.56 (s, 1H), 10.35 (s, 1H); Anal. Calcd for
C21H20N20 + 0.35 TFA:
C, 73.15; H, 5.76; N, 7.86. Found: C, 73.02; H, 5.50; N, 7.88.
Example 288
N-5-isoguinolinyl-2-phenylcyclopropanecarboxamide
The title compound was prepared using the procedure described in Example 285
using
2-phenylcyclopropanecarboxylic acid instead of 3-[4-
(trifluoromethyl)phenyl]acrylic acid.
MS (ESI+) mJz 289 (M H)+; MS (ESI-) m/z 287 (M-H) 1H NMR (DMSO, 300 MHz) 8
1.46 (in, 1H), 1.56 (m, IH), 2.46 (m, 2H), 7.24 (m, 3H), 7.32 (m, 2H), 7.82
(t, J 7.8, lH),
8.10 (d, J 7.8, 1H), 8.28 (d, 7 6.4, 1H), 8.62 (d, J 6.8, 1H), 9.58 (s, 1H),
10.46 (s, lH); Anal.
Calcd for C19H16N2O + 0.65 TFA: C, 67.27; H, 4.63; N, 7.73. Found: C, 67.27;
H, 4.31; N,
7.52.
Example 289
3-(3,4-dichlorophenyl)-N-5-isoguinolinylacrylamide
The title compound was prepared using the procedure described in Example 285
using
3-(3,4-dichlorophenyl)acrylic acid instead of 3-[4-
(trifluoromethyl)phenyl]acrylic acid. MS
162
CA 02743534 2011-06-13
(ESI+) in/z 344 (M+H)*; MS (ESI-) m/7- 342 (M-H)";'H NMR (DMSO, 300 MHz) 6
7.20 (d,
3 15.6, 1H), 7.67 (rn, 3H), 7.84 (d, J 15.6, 111), 7.97 (d, J 1.7, IH), 8.10
(d, J 7.8, IH), 8.22 (d,
J 6.4, 111), 8.35 (d, J 7.1, 1H), 8.64 (d, J 6.8, 1H), 9.53 (s, 1H), 10.37 (s,
1H); Anal. Calcd for
Ci8H12C12N2O + 0.75 TFA: C, 54.63; H, 3.00; N, 6.53. Found: C, 54.43; H, 2.92;
N, 6.39.
Example 290
3-(1,1'-biphenyl-4-yl)-N-5-isoguinoluiylacrylamide
The title compound was prepared using the procedure described in Example 285
using
3-(1,1'-biphenyl-4-yl)acrylic acid instead of 3-[4-
(trifluoromethyl)phenyl]acrylic acid. MS
(ESI+) m/z 351 (M+H)+; MS (ESI-) m/z 349 (M-H) ; 'H NMR (DMSO, 300 MBz) S 7.21
(d,
J 15.6, 111), 7.39-7.79 (m, 10H), 7.9.7 (d, J 7.8, 1H), 8.08 (d, J 6.4, 11-1),
8.29 (d, J 7.1, 1H),
8.58 (d, J 6.8, 1H), 9.34 (s, IH); Anal. Calcd for C24H,8N2O + 0.85 HCI: C,
75.58; H, 4.98.
Found: C, 75.69; H, 4.69.
Example 291
3-(3-bromo-4-fluorophhenyl)-N-5-isoguinolinylacrylamide
The title compound was prepared using the procedure described in Example 285
using
3-(3-bromo-4-fluorophenyl)acrylic acid instead of 3-[4-
(trifluoromethyl)phenyl]acrylic acid.
MS (ESI+) m/z 374, 372 (M+H)}; MS (ESI-) m/z 372, 370 (M-H)-; 'H N (DMSO, 300
MHz) 6 7.14 (d, J 15.6, 1H), 7.50 (t, J 8.8, 1H), 7.65 (d, J 15.6, 1H), 7.76
(m, 1H), 7.83 (t, J
7.8, 111), 8.05 (dd, 3, 6.8, J2 2. 1, 111), 8.11 (d, J 7.8, IH), 8.24 (d, J
6.4, 1H), 8.36 (d, J 7.1,
1H), 8.64 (d, J 6.8, 1H), 9.55 (s, 1H), 10.35 (s, 1H); Anal. Calcd for
C18Hl2BrFN2O + 1 TFA:
C, 49.51; H, 2.70; N, 5.77. Found: C, 49.78; H, 2.71; N, 5.768.
Example 292
3 -(4-tert-butyllphenyl)-N-5 -isoguino linyl acrylami de
The title compound was prepared using the procedure described in Example 285
using
3-(4-tert-butylphenyl)acrylic acid instead of 3-[4-
(trifluoromethyl)phenyl]acrylie acid. MS
(ESI+) m/z 331 (M+H)+; MS (ESI-) m/z 329 (M-H)'; 'H NMR (DMSO, 300 MHz) 8 1.31
(s,
9H), 7.10 (d, J 15.6, 1H), 7.51 (d, J 8.5,2H), 7.62 (d, J 8.5,2H), 7.67 (d, J
15.6, 1H), 7.86 (t,
J 8.2, 1H), 8.14 (d, J 6.1, IH), 8.31 (d, J 8.2, 1H), 8.40 (d, J 6.1, 1H),
8.66 (d, J 6.1, 1H), 9.60
163
CA 02743534 2011-06-13
(s, 1H), 10.39 (s, 1H); Anal. Calcd for C22H22N20 + I TFA: C, 64.86; H, 5.22;
N, 6.30.
Found: C, 64.54; H, 5.13; N, 6.18.
Example 293
3-[3-fluoro-4-(trifluoromethyl)phenyll-N-5-isoguinolinylacrylamide
The title compound was prepared using the procedure described in Example 285
using
3-[3-fluoro-4-(trrifluoromethyl)phenyl]acrylic acid instead of 3-[4-
(trifluoromethyl)phenyl]acrylic acid. MS (ESI+) m/z 361 (M+H)+; MS (ESI-) m/z
359 (M-
H)-;'H NMR (DMSO, 300 MHz) S 7.30 (d, J 15.6, 1H), 7.72-7.85 (m, 4H), 7.91 (t,
J 8.2,
1H), 8.13 (d, J 6. 1, 1H), 8.24 (d, J 8.2, 1H), 8.35 (d, J 6. 1, 1H), 8.65 (d,
J 6.1, 111), 9.56 (s,
1H), 10.50 (s, 1H); Anal. Calcd for Cj9Hi2F4N2O + 0.8 TFA: C, 54.80; H, 2.86;
N, 6.20.
Found: C, 54.59; H, 2.82; N, 6.06.
Example 294
N-(8-bromo-5-isoguinolinyl)-N'-(2,4-dichlorobenzyl)ure
Example 294A
8-bromo-5-isoguinolinamine
6, 8-dbromo-5-i soguinolinamine
5-Aminoisoquinoline (5.50 g, 38.1 mmol) and aluminium trichloride (15.1 g, 113
mmol) were combined and heated at 80 C in a 3-necked flask equipped with a
dropping
funnel, stirrer bar, needle and sintered glass tube. The mixture was treated
with bromine
(3.04 g, 19.05 mmol) via the sintered glass funnel dropwise. After stirring at
80 C for 2
hours, the suspension was treated with crushed ice in small portions and the
solution was
basified with concentrated sodium hydroxide solution. The aqueous layer was
extracted with
ethyl acetate (4 x 100 mL). The organic layers were combined, dried (Na2SO4),
filtered, and
the filtrate was concentrated under reduced pressure. The residue was purified
by column
chromatography (hexanes:ethyl acetate, 3:1) to provide the separate title
compounds.
Monobromo: MS (ESI+) m/z 225 (M+H)+; MS (ESI-) m/z 223 (M-H)-;
'H NMR (CDC13, 300 MHz) 8 4.22 (br s, 2H), 6.83 (d, J 8.1, 1H), 7.25 (s, 1H),
7.54 (d, J 5.8,
111), 7.61 (d, J 8.1, 1H), 8.59 (d, J 5.8, 1H), 9.56 (s, 1H); Dibromo: MS
(ESI+) m/z 303
164
CA 02743534 2011-06-13
(M+H)+; MS (ESI-) m/z 301 (M-D-; 'H NMR (DMSO, 300 MHz) S 6.41 (hr s, 2H),
7.92 (s,
1H), 8.18 (d, J 6.1, 1H), 8.59 (d, J 6.1, 1H), 9.30 (s, 1H).
Example 294B
N-(8-bromo-5-isoquinolinyl) N' (2,4-dichlorobenzyl)urea
8-Bromo-5-isoquinolinamine (120 mg, 0.52 mmol) in THF:toluene (5 niL, 1:4) was
treated with 2,4-dichloro-l-(isocyanatomethyl)benzene (108 mg, 0.52 mrnol) in
THE (0.5
niL). After stirring for 16 hours at room temperature, the mixture was
filtered and the filter
cake was dried under reduced pressure to provide the title compound. MS (ESI+)
m/z 426
(M+H)+; MS (ESI-) mlz 424 (M-M 'H NMR (DMSO, 300 MHz) 3 4.42 (d, 5.8, 21=1),
7.22
(t, J 5.8, 11-1), 7.65 (m, 1H), 7.91 (d, J 8.5, 1H), 8.02 (d, J 6.1, 1H), 8.22
(d, J 8.5, 1H), 8.69
(d, J 5.8, 1H), 9.01 (s, 1I-1), 9.44 (s, 1H); Anal. Calcd for
C17H12BrC12N3O.HC1 + 0.25EtOH:
C, 44.41; H, 3.14; N, 8.88. Found: C, 44.80; H, 2.76; N, 8.84.
Example 295
N-(8-bromo-5-isoquinolinyl)-N'-(4-fluorob enzyl)urea
The title compound was prepaid using the procedure described in Example 294B
using 1-fluoro-4-(isocyanatomethyl)benzene instead of2,4-dichloro-l-
(isocyanatomethyl)benzene. MS (ESI+) m/z 376 (M+H)+; MS (ESI-) .m/z 374 (M-H)
'H NMR (DMSO, 300 MHz) S 4.35 (d, 5.8, 2H), 7.12 (m, 1H), 7.18 (m, 2H), 7.40
(m, 1H),
7.91 (d, J 8.5, 1H), 7.99 (d, J 6.1, 1H), 8.24 (d, J 8.5, 1H), 8.69 (d, J 5.8,
IH), 8.88 (s, IH),
9.44 (s, 1H); Anal. Calcd for Ci7H13BrFN3O: C, 54.56; H, 3.50; N, 11.23.
Found: C, 54.61;
H, 3.35; N, 11.14.
Example 296
N-(8-bromo-5-isoquinolinyl)-N'-(3-fluorobenzyl)ure
The title compound was prepard using the procedure described in Example 294B
using 1-fluoro-4-(isocyanatomethyl)benzene instead of 2,4-dichloro-l-
(isocyaiiatolnethyl)benzene. MS (ESI+) m/z 376 (M+H){; MS (ESI-) m/z 374 (M-
H);'H
NMR (DMSO, 300 MHz) S 4.39 (d, 5.8, 2H), 7.09 (m, 1H), 7.17 (m, 211), 7.40 (m,
1H), 7.91
(d, J 8.5, IM, 8.01 (d, J 6.1, 1H), 8,23 (d, J 8.5, 1H), 8.69 (d, J 5.8, 1H),
8.93 (s, 11f), 9.44 (s,
165
CA 02743534 2011-06-13
11I); Anal. Calcd for C H13BrFN3O: C, 54.56; H, 3.50; N, 11.23. Found: C,
54.64; H, 3.33;
N, 11.19.
Example 297
Nj1 -(4-chlorophenyl)-1-methylethyll-N'-5-isoguinolinyhu-ea
Example 297A
2-(4-chlorophenyl)-2-methylpropanoyl chloride
2-(4-Chlorophenyl)-2-methylpropanoic acid (3.85 g, 19.4 mmol) in toluene (5
mL)
and thionyl chloride (5.008, 3.1 mL) was heated at 80 C for 2 hours. The
mixture was
cooled and concentrated under reduced pressure to provide the title compound.
Example 297B
1-chloro-4-(1-isocyanato- I -methylethyl)benzene
The product from Example 297A (4.00 g, 19.4 mmol) in acetone (9 mL) at 0 C ws
treated with sodium azide (1.27 g) in water (9 mL) dropwise over 15 minutes.
after stirring
for 30 minutes at 0 C, the mixture was extracted with toluene (20 mL). The
toluene solution
was dried over MgSO4, filtered, and the filtrate was heated to reflux for 1
hour. The cooled
solution was concentrated under reduced pressure to provide the title
compound.
Example 297C
Nil -(4-chlorophenyl)-1-methylethyll-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 60F
using 1-chloro-4-(1-isocyanato-l-methylethyl)benzene and 5-isoquinolinamine
instead of the
product from Example 60E and 1-bromo-4-(isocyanatomethyl)benzene. MS (ESI+)
m/z 355
(M+H)}; MS (ESt) m/z 353 (M-H)-; 'H NMR (DMSO, 300 MHz) 6 1.63 (s, 6H), 7.23
(s,
1H), 7.37 (d, J 8.8, 2H), 7.47 (d, J 8.8, 2H), 7.73 (t, J 9.2, 1H), 7.93 (d, J
8.1, 1H), 8.25 (d, J
6.4, 1H), 8.39 (d, J 8.1, 1H), 8.67 (d, 3 6.4, 1H), 8.87 (s, 1H), 9.58 (s,
1H); Anal. Calcd for
C19H18CIN30.HCI + 0.25EtOH: C, 60.40; H, 5.33; N, 10.54. Found: C, 60.82; H,
5.23; N,
10.45.
Example 298
N(4-bromo-3-methylbenzyl)-N'-5-isoguinolinylurea
166
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 61B
using 4-bromo-3-methylbenzylamine instead of4-cyanobenzyl alcohol. MS (EST+)
m/z 372,
370 (M+H)+; MS (ESI-) m/z 370, 368 (M-IHI) 1H NMR (DMSO, 300 MHz) 6 2.34 (s,
3H),
4.31 (s, 2H), 4.09 (s, 21-1), 7.13 (d, J 7.2, 2H), 7.34 (s, 11I), 7.55 (m,
2H), 7.82 (d, J 7.9, 11-1),
7.90 (m, 1H), 8.09 (d, IH), 8.65 (m, 2H), 8.80 (d, J 6.4, 1H), 9.68 (s, 1H),
9.79 (s, 1H); Anal.
Calcd for C18H16BrN3O + 1.05 HCI: C, 52.66; H, 4.86; N, 10.24. Found: C,
53.00; H, 4.27;
N, 10.37.
Example 299
N-[2-fluoro-4-(trifluoromethyl)benzyl]-N'-5-i soquinolinylurea
Example 299A
2-fluoro-4-(trifluoromethyl)benzylamine
The title compound was prepared using the procedure described in Example 172B
using 2-fluoro-4-(trifluoromethyl)benzonitrile instead of 4-(4-
morpholinyl)benzonitrile. MS
(ESI+) m/z 194 (M+H)+; MS (ESI-) m/z 192 (M-H)-; 'H NMR (DMSO, 300 MHz) 6 3.97
(s,
2H), 7.30 (m, 1H), 7.46 m, 2H).
Example 299B
N-f 2-fluoro-4-(trifluoromethyl)benzyll-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61..B
using 2-fluoro-4-(trifluoromethyl)benzylamine instead of 4-cyanobenzyl
alcohol. AIS (ESI+)
m/z 364 (M+H)+; MS (ESI-) m/z 362 (M-H)-; 1H NMR (DMSO, 300 MHz) S 4.51 (d, J
5.8,
2H), 7.65 (m, 4H), 7.90 (t, J 8.1, 114), 8.09 (d, J 7.8, 1H), 8.59 (d, J 7.8,
1H), 8.71 (s, 2H),
9.66 (s, 1H), 9.76 (s, 111); Anal. Calcd for C18H13F4N3O + 1 HC1: C, 54.08; H,
3.53; N, 10.51.
Found: C, 54.40; H, 3.60; N, 10.61.
Example 300
N-(4-bromobenzyl)-N'-(3-hydroxy-5-isoguinolinyl)urea
Example 300A
5-nitro-3-isoguinolinol
167
CA 02743534 2011-06-13
3-Hydroxyisoquinoline (1.09 g, 7.53 mmol) in concentrated H2S04 (20 mL) at 0
C
was treated with NaNO3 (0.71 g, 8.34 mmol) in concentrated H2SO4 (5 mL)
dropwise over 15
minutes. After stirring for 90 minutes, the mixture was allowed to warn to
room
temperature, stir for 2 hours, poured over an ice-NH4C1 mixture, and the pH
was adjusted to
7-8 with 50% NaOH solution. The mixture was filtered and the filter cake dried
to provide
the title compound. Structure analysis determined a 2:1 mixture of the 5-nitro
and 7-nitro
isomers which were not separated. MS (ESI+) mlz 191 (M+H)+; MS (ESI-) m/z 189
(M-H
'iT NMR (DMSO, 300 MHz) 6 4.60 (s, 1H), 7.48 (t, J 8.0, IH), 7.57 (s, 1H),
8.42 (d, 1 8.0,
1H), 8.57 (d, J 7.7, lii), 9.19 (s, 1H).
Example 300B
5-nitro-3-isoguinolinyl acetate
5-Nitro-3-isoquinolinol (3.40 g, 17.9 nunol) in acetic anhydride (40 mL) was
treated
with acetic acid (5 n-LL) and pyridine (5 mL) and heated at 120 C for 2
hours. The mixture
was cooled to room temperature and concentrated under reduced pressure to
provide the title
compound which was used in the next step without further purification. MS
(ESI+) m/z 233
(M+H)+; MS (ESI-) m/z 231 (M-H)-; 'H NMR (DMSO, 300 MHz) isomers S 2.39 (s,
3H),
7.88 (m, 1H), 8.27 (m, 1H), 8.50 (m, IH), 8.65, 8.74 (d, J 7.8, 1H), 9.47,
9.55 (s, 1H).
Example 300C
5-amino-3-isoquinolinyl acetate
5-Nitro-3-isoquinolinyl acetate (50 mg, 0.21 mmol) in 1,4-dioxane (20 rnL) was
treated with RancyTM-nickel powder (85 mg) and exposed to a hydrogen
atmosphere via a
balloon for 16 hours. The mixture was filtered through a plug of Celite and
the filtrate was
concentrated under reduced pressure to provide the title compound which was
used without
further purification.
Example 3001)
N-(4-bromobenzyl)-N'-(3-hydroxy-5-isoquinolinyl urea
5-Amino-3-isoquinolinyl acetate in toluene:THF (5:1, 5 m.L) was treated with
1-bromo-4-(isocyanatomethyl)benzene (105 mg). After stirring for 1 hour, the
mixture was
concentrated under reduced pressure and the residue dissolved in MeOH (20 mL)
and treated
168
CA 02743534 2011-06-13
with K2C03 (4 eq) and stirred for 16 hours. The mixture was concentrated under
reduced
pressure and partitioned between CH2C12 and water. The aqueous phase was
separated and
the pH was adjusted to approximately 6 with HCI. The acidified solution was
filtered, and
the filter cake was dried. The solid was purified by reverse-phase
chromatography (using
TFA as eluent) to provide the title compound. MS (ESI+) m/z 374, 372 (M+1-1)+;
MS (ESI-)
m/z 372, 370 (M-H)-; 'H NMR (DMSO, 300 MHz) 6 4.33 (d, J 5.8, 2H), 7.06 (m,
1H), 7.29
(m, 3H), 7.57 (m, 31-1), 8.07 (d, J 7.8, 1H), 8.48 (s, lH), 8.80 (s, 1H), 8.87
(s, 1H); Anal.
Calcd for C17H14BrN3O2 + 0.2 TFA: C, 53.08; H, 3.42; N, 10.43. Found: C,
52.91; H, 3.62;
N, 10.43.
Example 301
N-5-isoquinolinyl-N- t ~5-(trifluoromethyl)-2-pyridinyl}methyl} urea
Example 301A
5-(trifluoromethyl)-2-pyridinecarbonitrile
Copper (1) cyanide (14.1 g) and 2-bromo-5-trifluoromethylpyridine (3.00 g,
13.3
mmol) in dry DMSO (70 mL) were combined and heated at 180 C for 2 hours,
cooled, and
poured into NH4OH (3M). The mixture was then extracted with ethyl acetate (3 x
500 mL),
washed with water (1 x 200 mL), dried (MgSO4), filtered and the filtrate
concentrated under
reduced pressure to provide the title compound. 1H NMR (DMSO, 300 MHz) 6 8.22
(m, 11-1),
8.42 (m, 1I.1), 9.01 (s, 1H).
Example 301B
f 5-(trifluoromethyl)-2-pyridinyl}methylaniine
The title compound was prepared using the procedure described in Example 172B
using 5-(trifluoromethyl)-2-pyridinecarbonitrile instead of 4-(4-
morpholinyl)benzonitrile.
Example 301C
N-5-isoguinolinyl-N'- f L5-(trifluoromethyl)-2-pyridinyl]methyl}urea
The title compound was prepared using the procedure described in Example 61B
using {5-(trifluoromethyl)-2-pyridinyl]methylamine instead of 4-cyanobenzyl
alcohol. 1H
NMR (DMSO, 300 MHz) S 4.51 (m, 2H), 7.97 (m, 2H), 8.12 (m, 1H), 8.47 (d, J
7.8, 1H),
169
CA 02743534 2011-06-13
8.72 (m, 3H), 9.13 (d, J 6.8, 1H), 9.78 (m, 2H), 10.80 (s, I H); Anal. Calcd
for C]7H,3F3N4O +
0.8 HCI + 0.7 CH3OH: C, 53.43; H, 4.20; N, 14.08. Found: C, 53.41; H, 4.31; N,
14.11.
Example 302
N-[3-bromo-4-(trifluoromethyl)benzyl]-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 3-bromo-4-(trifluoromethyl)benzylamine instead of 4-cyanobenzyl alcohol.
MS (ESI+)
m/z 426, 424 (M+H)+; MS (EST-) m/z 424, 422 (M-Td)-; 'H NMR (DMSO, 300 MHz) S
4.46
(d, J 5.8, 2H), 7.26 (t, J 6.1, IH), 7.56 (d, J 8.8, 1H), 7.90 (m, 2H), 7.97
(d, J 8.1, 1H), 8.21
(d, J 6.4, 1H), 8.39 (d, J 8.8, 1H), 8.64 (d, f 6.4, 1H), 9.08 (s, IH), 9.57
(s, 1H); Anal. Calcd
for C,5H13BrF3N3O + 0.9 TFA: C, 45.14; H, 2.66; N, 7.98. Found: C, 45.18; H,
2.64; N, 7.86.
Example 303
N f2,4-bis(trifluoromethyl)benzyl)-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 2,4-bis(trifluoromethyl)benzylamine instead of 4-cyanobenzyl alcohol. MS
(ESI+) m/z
414 (M+H)+; MS (ESI-) m/z 412 (M-H)-; 'H NMR (DMSO, 300 MHz) rotamers b 4.63
(d, J
5.8,2M, 7.70-8.20 (m, 6H), 8.60 (m, 3H), 9.60 (m, 2H); Anal. Calcd for
C19H13F6N30 + I
HCI: C, 50.74; H, 3.14; N, 9.34. Found: C, 50.88; H, 3.08; N, 9.10.
Example 304
N-(2,3-difluoro-4- trifluoromethyl)benzyl]-N-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 2,3-difluoro-4-(trifluoromethyl)benzylamine instead of 4-cyanobenzyl
alcohol. MS
(ESI+) m/z 382 (M+H)+; MS (ESI-) m/z 380 (M-H)-; 'H NMR (DMSO, 300 MHz)
rotamers
S 4.55 (d, J 5.8,2H), 7.45 (m, 1H), 7.63 (t, J 6.1, 113), 7.82 (m, 1H),
8.05(d, J 8.1, 1H), 8.56
(m, 2H), 8.69 (d, J 6.4, IH), 9.51 (s, 1H), 9.70 (s, IR); Anal. Calcd for
C15H12F5N3O + 0.8
HCl: C, 52.67; H, 3.14; N, 10.24. Found: C, 52.53; H, 3.38; N, 10.22.
Example 305
N-[2-chloro-4-(trifluoromethyl)benzyl]-N-S-isoguinolinylurea
170
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 61B
using 2-chloro-4-(trifluoromethyl)benzylarnine instead of 4-cyanobenzyl
alcohol. MS (ESI+)
m/z 380 (M+H)+; MS (ESI-) m/z 378 (M-H) 1H NMR (DMSO, 300 MHz) S 4.53 (d, J
5.8,
2H), 7.69 (m, 2H), 7.87 (m, 1H), 8.06 (d, J 8.1, 1H), 8.56 (d, J 7.8, IH),
8.63 (d, J 6.8, IH),
8.70 (d, J 6.8, 1H), 9.59 (s, 1H), 9.72 (s, 1H); Anal. Caled for C18H13CIF3N3O
+ 1.3 HCI: C,
50.61; H, 3.37; N, 9.84. Found: C, 50.60; H, 3.42; N, 9.61.
Example 306
N-S-isoquinolinyl-N'-f 1-methyl-l-[4-(trifluorometlyl)phenyl eth 1 urea
The title compound was prepared using the procedure described in Example 61B
using 2-[4-(trifluoromethy])phenyl]-2-propanamine instead of 4-cyanobenzyl
alcohol. MS
(ESI+) m/z 374 (M+H)+; MS (ESI-) miz 372 (M-H)-; 'H NMR (DMSO, 300 MHz) 6 1.67
(s,
6H), 7.67 (s, 4H), 7.82 (t, J 8.1, 1H), 8.02 (d, J 8.1, IH), 8.54 (d, J 7.8,
1H), 8.72 (d, J 6.8,
IH), 8.87 (d, J 6.8, 1H), 9.65 (s, 1H), 9.77 (s, IH); Anal. Calcd for
C20H18F3N30 + 1 HCI: C,
58.61; H, 4.67. Found: C, 58.62; H, 4.65.
Example 307
N-[2-(4-bromophenyl)-2-hydroxyethyll-N'-5-isoguinolinylurea
The title compound was prepared using the procedure described in Example 61B
using 2-amino-l-(4-bromophenyl)ethanol instead of 4-cyanobenzyl alcohol.
MS (ESI+) m/z 388, 386 (M+H)+; MS (ESI-) m/z 386, 384(M-H)-;
1H NMR (DMSO, 300 MHz) rotamers 6 3.27 (in, 1H), 3.42 (m, 1Ii), 4.70 (in,
111), 6.82 (t, J
5.0, 2H), 7.38 (d, J 8.5, lH), 7.56 (d, J 8.5, 1H), 7.81 (t, 3 7.8, IH), 7.98
(d, 3 8.7, 11-1), 8.29
(d, J 7.5, I H), 8.50 (d, J 5.9, I W, 8.67 (d, J 6.4, Ili), 9.01 (s, 111),
9.64 (s, I H);
Anal. Caled for C1sH16BrN3O2 + 2.35 TFA: C, 41.68; H, 2.83; N, 6.42. Found: C,
41.69; H,
2.86; N, 6.43.
Example 309
methyl 4-(f [(1-naphthyl.methyl)aminolcarbonyl}amino)-1H-indazole-l-
carboxylate
Example 309A
4-nitro- I H-indazole
171
CA 02743534 2011-06-13
2-Methyl-3-rv.troaniline (20 g) in acetic acid (-200 mL) was treated with
NaNO2 (20
g) in water (50 mL) at 4 C (mechanical stirring). The reaction mixture was
allowed to warm
to room temperature and stir overnight. The solvent was removed under reduced
pressure.
The residue was treated with water (700 mL) and the mixture was filtered. The
solid was
dried at 45 C in a vacuum oven overnight to provide the title compound. 'H
NMR (DMSO-
d6) 6 13.91 (s, 1 H), 8.55 (s, 1 H), 8.18 (d, 1 H), 8.10 (d, I H), 7.61 (dd, 1
H); MS (ESI) m/z 164
(M+H)
Example 309B
methyl 4-nitro-lH-indazole- l -carboxylate
NaH (0.3 g, 12.5 mmol) in DMF (5 mL) was treated with 4-nitro-IH-indazole
(1.33
g, 10 mmol) at 0 C. The reaction mixture was allowed to warm to room
temperature and stir
for 1 hour. The mixture was treated with methyl chloroformate (0.9 mL) and
stirred at room
temperature for 3 hours. The mixture was treated with water and filtered to
provide the title
compound as a solid. 'H NMR (300 MHz, DMSO-d6) 6 4.1 9 (s, 3H), 7.9 (t, 1H),
8.38 (d,
I H), 8.62 (d, 1H), 8.85 (s, 1H).
Example 309C
methyl 4-amino-IH-indazole-l -carboxylate
Methyl 4-nitro-IH-indazole-l-carboxylate 1.66 g, 7.5 mmol) and 10% PdIC were
combined in ethanol (20 mL) and exposed to a hydrogen atmosphere. The reaction
mixture
was heated at 80 C for 20 minutes, allowed to cool to room temperature, and
filtered through
Celite. The filtrate was evaporated to provide title compound. 'H NMR (300
MHz, DMSO-
d6) S 6.1 (s, 2H), 6.41 (dd, 1IT), 7.21 (m, 2H), 8.42 (s, 1H).
Example 309D
methyl 4-({ f.(2,5-dioxo-1 -pyrrolidinyl)oxylearbonyll amino)-1 H-indazole-l -
carboxylate
Methyl 4-amino-lH-indazole-l-carboxylate (1.9 g, 10 mmol) and 1-({[(2,5-dioxo-
l-
pyrrolidinyl)oxy]carbonyl}oxy)-2,5-pyrrolidinedione (2.8 g, 11 mmol) were
combined in
acetonitrile (100 mL), stirred for 48 hours at ambient temperature, and
filtered. The filter
cake was washed with acetonitrile (10 mL) and dried under reduced pressure at
ambient
temperature to provide the title compound.
172
CA 02743534 2011-06-13
.u,
Example 309E
methyl 4-({ [(1-naphthylmethyl)aminolcarbonyl} amino)-1H-indazole-l-
carboxylate
1-Naphthylmethylamine (2.1 mmol) and diisopropylethylamine (2 mrnol, 0.26 g)
were combined in DMF (6 mL) and treated with methyl 4-({[(2,5-dioxo-l-
pyrrolidinyl)oxy]carbonyl}amino)-IH-indazole-l-carboxylate (6.6 g, 2 mmol) at
ambient
temperature. After stirring for 30 minutes, the reaction mixture was diluted
with water (6 ml)
and filtered. The filter cake was washed with water:acetoniirile (1:1) and
dried to provide the
title compound. 'H NMR (DMSO-d6) S 4.02 (s, 3 H); 4.81 (d, 2 H))- 6.85 (in, 1
H); 7.42-7.64
(in, 5 H); 7.67 (d, 1 H); 7.87 (in, 2 H); 7.97 (d, 1 H); 8.15 (d, 1 H); 8.38
(s, 1 H); 8.99 (s, 1
H).
Example 310
methyl 4-({ [(l, l'-biphenyl-3-yllmethyl)aminolcarbonyl} amino)-1 H-indazole-
l -carboxylate
The title compound was prepared using the procedure described in Example 309E
except using 1,1'-biphenyl-3-ylmethylamine instead of 1-naphthylmethylamine.
'H NMR
(DMSO-D6) 6 4.02 (S, 3 H); 4.43 (D, 2 H); 6.89 (M, 1 H); 7.36 (M, 2 H); 7.42-
7.51 (M, 4
H); 7.56 (1t4, 1 H), 7.60-7.72 (M, 4 H); 7.82 (M, I H); 8.42 (S, 1 H); 9.04
(S, 1 H).
Example 311
methyl 4-({[(2-chlorobenzyl)aminolcarbonyl} amino)-1H-indazole- l-carboxylate
The title compound was prepared using the procedure described in Example 309E
except using 2-chlorobenzylamine instead of 1-naphthylmethylamine. 1H NMR
(DMSO-D6)
6 4.02 (S, 3 H); 4.43 (D, 2 H); 6.89 (M, 1 H); 7.25-7.39 (1\4, 2 H); 7.41-7.52
(M, 3 H); 7.68
(D, 1 H); 7.81 (D, 1 H); 8.44 (S, 1 H); 9.14 (S, 1 H).
EXAMPLE 312
methyl 4-[({ [2-fluoro- 5-(t dfluoromethyl)b enzyll amino } carbonyl)aminol- l
H-indazol e- l -
carboxylate
The title compound was prepared using the procedure described in Example 309E
except using 2-chloro-5-(trifluoromethyl)benzylamine instead of 1-
naphthylmethylaznine.'H
173
CA 02743534 2011-06-13
NMR (6, DMSO-d6) 6 4.02 (s, 3 H); 4.471 (d, 2 H); 6.97 (m, 1 H); 7.41-7.52 (m,
2 H); 7.69
(d, 1 H); 7.71-7.80 (in, 3 H); 7.97 (d, 1 H); 8.42 (s, I H); 9.14 (s, I H).
Example 313
N-1 H-indazol-4-yl-N'-(1-naphthylmethyl)urea
Methyl 4-( {{(l -naphthyhnethy1)aminocarbony1} amino)-1 H-indazole-l-
carboxylate
in methanol was treated with 5M sodium hydroxide (8 equivalents) (prepared by
dissolution
of 1 gram of sodium hydroxide in 20 mL of methanol). After stirring for 30
minutes, the
mixture was diluted with water (10 mL) and filtered. The filter cake was
washed with water
(10 mL), water:niethanol (1:1), and dried under reduced pressure to provide
the title
compound. MS (M+H)+ 317.
Example 314
N-(1,1'-biphenyl-3-yhnethyl)-N'-1H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 313
except using methyl 4-({[(1,1'-biphenyl-3-yhnethyl)amino]carbonyl} amino)-1H-
indazole-l-
carboxylate instead of methyl 4-({[(1-naphthylmethyl)amino]carbonyl} amino)-1H-
indazole-
1-carboxylate. MS (M+H)+ 343.
Example 315
N-(2-chlorobenzyl)-M-1 H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 313
except using methyl 4-( { [(2-chlorobenzyl)amino]carbonyl} amino)-1 H-indazole-
l -
carboxylate instead of methyl 4-(([(1-naphthyhnethyl)amino]carbonyl}amino)-1H-
indazole-
1-carboxylate. MS (M+H)+ 301.
Example 316
N-[2-fluoro-5-(trifluoromethyl)benzyll-N'-1H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 313
except using methyl 4-[({[2-fluoro-5-(trifluoromethyl)benzyl]amino}
carbonyl)amino]-1H-
indazole-1-carboxylate instead of methyl 4-({[(1-
naphthylmethyl)amino]carbonyl}amino)-
IH-indazole-l-carboxylate. MS (M+H)+354.
174
CA 02743534 2011-06-13
Example 317
N- 1 H-indazol-4-yl-N'-(3 -phenylpropyl)urea
Methyl 4-amino-IH-indazole-l-carboxylate (0.46 g, 2.4 mmol) and phosgene (20%
in
toluene, 2.4 ml, 4.8 mmol) were combined in toluene (80 nil) and heated at
reflux for 3 hours.
The mixture was allowed to cool to ambient temperature and the solvent was
removed under
reduced pressure. The residue was treated with diethyl ether (80 ml) and
triethyl amine (2
ml) and then filtered. The filtrate was treated with 3-phenylpropylamine (2.4
mrnol, 324 mg)
and stirred for 16 hours at ambient temperature. The solvent was concentrated
to half volume
under reduced pressure and filtered. The filter cake was washed with diethyl
ether:hexanes
(1:1). The obtained solid in methanol (10 ml) was treated with a 5M solution
of sodium
hydroxide in methanol (4 ml, 20 mmol) and stirred for 30 minutes. The reaction
mixture was
diluted with water and extracted with ethyl acetate (2 X 25 mL). The organics
were
combined, washed with water (2 X 25m1), brine, dried over Mg2SO4, filtered and
the filtrate
was concentrated under reduced pressure. The residue was treated with
ethanolic HCI to
provide the title compound as the HCI salt. 1H NMR (DMSO-d6) S 8.81 (s, 1H),
8.18 (s, 1H),
7.62 (d, 1H), 7.22 (m, 611), 7.03 (d, 1H), 3.17 (t, 2H), 2.62 (t, 2H), 1.78
(m, 2H); MS (ESI)
m/z 295 (M+H)+.
Example 318
N- f 2-(2,4-dimethylphenyl)ethyll-N'-1 H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 2-(2,4-dimethylphenyl)ethylamine instead of 3-phenylpropylamine.
1H NMR
(DMSO-d6) S 8.80 (s, 1H), 8.16 (s, 1H), 7.63 (d, 1H), 7.18 (t, IH), 7.05 (t,
2H), 6.88 (in, 2H),
3.30 (t, 2H), 2.74 (t, 2H), 2.25 (s, 3H), 2.22 (s, 3H); MS (ESI) m/z 309
(M+H)+
Example 319
N-f2-(3 4-dichlorophenyl)ethyll-N'-1H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 2-(3,4-dichlorophenyl)ethylamine instead of 3-phenylpropylamine.
'H NMR
(DMSO-d6) 6 8.82 (s, 1H), 8.17 (s, 11-1), 7.58 (m, 4H), 7.27 (dd, 1H), 7.18
(t, 1H), 7.03 (d
2H), 6.58 (bs, 1H), 3.40 (t, 2H), 2.80 (t, 2H); MS (ESI) m/z 349 (M+H)
175
CA 02743534 2011-06-13
Example 320
N-1H-indazol-4-y1N'-[2-(4-metl ylphenyl)ethyl urea
The title compound was prepared using the procedure described in Example 317
except using 2-(4-methylphenyl)ethylamine instead of 3-phenylpropylamine. 1H
NMR
(DMSO-d6) S 8.77 (s, IH), 8.20 (s, IN), 7.62 (d, 1H), 7.28 (m, 211), 7.15(m,
5H), 3.38 (t,
2H), 2.73 (t, 211); MS (ESI) mlz 395, (M+H)+.
Example 321
N-[4-azep an- l -yl-3-(trifluoromethyl)benzyl]-N'-1 H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 4-azepan-1-yl-3-(trifluoromethyl)benzylamine instead of 3-
phenylpropylamine.
'HNMR (DMSO-d6) S 8.98 (s, 1H), 8.18 (s, 1H), 7.60 (m, 3f1), 7.48 (d, 1H),
7.19(t, 1H),
7.06, (d, 211), 4.37 (d, 2H), 3.00 (m, 4H), 2.64 (s, 8H); MS (ESI) m/z 432,
(M+If+.
Example 322
N-[4-azepan-1-yl-2-(trifluoromethyl)benzyllN'-IH-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 4-azepan-1-yl-2-(trifluoromethyl)benzylamine instead of 3-
phenylpropylamine.
'H NMR (DMSO-d6) 6 9.00 (s, 1H), 8.19 (s, 11), 7.64 (d, 1H), 7.41 (d, 1H),
7.19 (t, 1 H),
7.03, (d, 1H), 6.91 (m, 4H), 4.37 (s, 2H), 3.43 (t, 4I-1), 1.71 (s, 413), 1.43
(i, 4H); MS (ESI)
mlz 432, (M+H)+.
Example 323
N- [4-(2-azabicyclo [2.2.1lhept-2-yl)-2-(trifluoromethyl)benzyl]-N'- I H-
indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 4-(2-azabicyclo[2.2. I ]hept-2-yl)-2-(trifluoromethyl)benzylamine
instead of
3-phenylpropylamine. 1H NMR (DMSO-d6) S 9.00 (s, 1H), 8.19 (s, 1H), 7.64 (d,
1H), 7.40
(d, 1H), 7.19 (t, 1H), 7.04 (d, 1H), 6.91 (bs, 111), 6.80 (dd, IH), 6.70 (d,
1H), 4.38 (s, 2H),
4.21 (s, 1H), 3.43 (m, 2H), 2.71 (d, 111), 2.60 (s, 1H), 1.1.65 (m, 3H), 1.50
(m, 111), 1.28 (m,
111); MS (ESI) m/z 430, (M+H)+.
Example 324
176
CA 02743534 2011-06-13
N-[4-(8-azabicyclo[3.2.lloct-8-yl)-2-(trifluoromethyl)benzyl]-N'-1H-indazo l-4-
ylurca
The title compound was prepared using the procedure described in Example 317
except using 4-(8-azabicyclo[3.2.1]oct-8-yl)-2-(trifluoromethyl)benzylamine
instead of
3-phenylpropylamine. 'H NMR (DMSO-d6) 8 9.02 (s, 1H), 8.20 (s, IH), 7.63 (d,
1H), 7.44
(d, 1H), 7.19 (t, 1H), 7.05 (m, 2H), 7.00 (m, 2H), 4.38 (s, 2H), 4.23 (s, 2H),
2.01 (m, 2H),
1.80 (m, 5H), 1.41 (m, 114), 1.26 (m, 2H); MS (ESI) m/z 444 (M- -H)+.
Example 325
N-f4-(8-azabicyclo[3.2.1 ]oct-8-yl)-3-fluorobenzyl]-N'-1H-indazol-4-ylurca
Example 325A
4-(8-azabicyclo[3.2.1 ]oct-8-yl)-3-fluorobenzonitrile
3,4-Difluorobenzonitrile (1.75g; 25.1mmol), 8-aza-bicyclo[3.2.1]octane
hydrochloride (2.1g; 13.8 mmol), and diisopropylethylamine (3.2g; 25.1 mmol),
were
combined in DMSO (30 mL) and heated at 120 C overnight. The mixture was
allowed to
cool to ambient temperature, poured into brine (75 mL), and extracted with
diethyl ether (2 X
50 mL). The organics were combined, dried over MgSO4, filtered, and the
filtrate was
concentrated under reduced pressure. The residue was filtered through a pad of
silica gel
(ethyl acetate as eluent) to provide the title compound. 'H NMR (DMSO-d6) 8
7.69 (dd, 1H),
7.42 (dd, 1H), 7.15 (t, 1H), 4.41 (s, 2H), 1.98 (m, 2H), 1.62-1.86 (m, 5H),
1.41 (m, 31-1); MS
(ESI) m/z 231, (M+H)+.
Example 325B
4-(8-azabicyclo[3.2.1 ]oct-8-yl)-3-fluorobenzylamine
Lithium aluminum hydride (1.6 g; 43.2 mmol) in THE was treated with
4-(8-azabicyclo[3.2.1]oet-8-yl)-3-fluorobenzonitrile (2.48 g; 10.8 mmol)
dropwise. After
complete addition, the slurry was refluxed for 2 hours, allowed to cool to
ambient
temperature, and quenched with sodium sulfate decabydrate. The mixture was
filtered and
the filter cake was washed with THE (2 x 50 mL). The organics were combined
and
concentrated under reduced pressure. The residue was chromatographed (Si02; 5%
methanol
in methylene chloride) to provide the title compound. 'H NMR (DMSO-d6) 8 7.03
(dd, 1H),
177
CA 02743534 2011-06-13
7.92 (dd, 1H), 7.85 (t, 1H), 4.21 (s, 2H), 3.60 (s, 2H), 1.93 (m, 2H), 1.77
(n7, 5H), 1.42 (m,
1H), 1.32 (m, 2H); MS (ESI) m/z 235, (M+H)+.
N-(4-(8-azabicyclo {3.2. l loct-8-_yl)-3-fluorobenzyll-N'-1H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 4-(8-azabicyclo[3.2.1 ]oet-8-yl)-3-fluorobenzylamine instead of
3-phenylpropylamine. 'H NMR (DMSO-d6) 6 9.00 (s, 1H), 8.20 (s, 111), 7.64 (d,
1H), 7.18
(t, 1H), 7.03 (m, 5H), 4.24 (s, 2H), 4.17 (s, 2H), 1.95 (m, 2H), 1.80 (m, 6H),
1.41 (in, 2H);
MS (ESI) m/z 394 (M+H)+
Example 326
N-(3-chloro-4-azepan-1-ylbenzyl)-N-1H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 3-chloro-4-azepan-1-ylbenzylamine instead of 3-phenylpropylamine.
'H NMR
(DMSO-d5) 6 8.90 (s, 1H), 8.19 (s, 1H), 7.62 (d, 1H), 7.36 (d, 1H), 7.20 (m,
4H), 7.03, (d,
1H), 6.98 (bs, 1H), 4.26 (s, 2H), 3.18 (m, 4H), 1.78 (m, 4H), 1.62 (in, 4H);
MS (ESI) m/z
398, (M+H)+.
Example 327
N-1H-indazol- -yl N'-{[6-(trifluoromethyl)-3-pyridinyllmethyl}urea
The title compound was prepared using the procedure described in Example 317
except using [6-(trifluoromethyl)-3-pyridinyl]methylamine instead of 3-
phenylpropylamine.
'H NMR (DMSO-d6) 6 9.09 (s, 1H), 8.78 (s, 1H), 8.20 (s, 1H), 8.03 (d, 1H),
7.90 (d, IH),
7.60, (d, 1H), 7.22 (m, 2H), 7.05 (d, 1H), 4.49 (dd, 2H); MS (ESI) m/z 336
(M+H)+.
Example 328
N-((1 S)-1-(4-bromophenyl)ethyll-N-lH-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using (1 S)-1-(4-bromophenyl)ethylamine instead of 3-phenylpropylamine.
'H NMR
(DMSO-d6) 6 8.82 (s, 1H), 8.16 (s, 1H), 7.57 (m, 3H), 7.35 (d, 2H), 7.17 (t,
1H), 7.03, (d,
2H), 4.82 (m, 1H), 1.41 (d, 3H); MS (ESI) m/z 336 (M+H)+.
178
CA 02743534 2011-06-13
Example 329
N- 3-bromo-4-fltiorobenzyl)-N'-I,H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 3-bromo-4-fluorobenzylamine instead of 3-phenylpropylamine. 1H
NMR
(DMSO-d6) S 9.02 (s, 1H), 8.20 (s, 1H), 7.63 (m, 21-1), 7.38 (m, 2H), 7.19 (t,
1H), 7.14 (bs,
1H), 7.03, (d, 1f ), 4.35 (m, 1H); MS (EST) m/z 364, (M+H).
Example 330
N-(2,4-dimethylbenzyl)-N'-1 H-indazol-4 lurea
The title compound was prepared using the procedure described in Example 317
except using 2,4-dimethylbenzylamine instead of 3-phenylpropylamine. 'H NMR
(DMSO-
d6) b 8.88 (s, 1H), 8.17 (s, 1H), 7.63 (d, 2H), 7.19 (m, 2H), 7.01 (m, 4H),
6.83 (bs, 1H), 4.28
(s, 2H), 2.20 (s, 3H), 2.16 (s, 3H); MS (ESI) m/z 295 (M+H)+.
Example 331
N-(4-chlorobenzyl)-N-1 H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 4-chlorobenzylamine instead of 3-phenylpropylamine. 1H NMR (DMSO-
d6) 6
8.96 (s, 1H), 8.18(s, 1H), 7.62 (d, 1H), 7.39 (m, 4H), 7.19 (t, 1H), 7.06 (d,
2H), 4.36 (d, 2H);
MS (ESI) m/z 301 (NI+H)+.
Example 332
N-[3-fluoro-4-(trifluoromethyl)benzyl]-N'-1H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 3-fluoro-4-(trifluoromethyl)benzylamine instead of 3-
phenylpropylamine. 1H
NMR (DMSO-d6) 8 9.17 (s, 1H), 8.22 (s, 1H), 7.77 (t, 1H), 7.60 (d, 1H), 7.41
(m, 2H), 7.28
(bs, 114), 7.19 (t, 2H), 7.05 (d, 111), 4.47 (d, 2H); MS (ESI) m/z 353, (M
ff)+.
Example 333
N- 1 H-indazol-4-yl-N'-(4-methylb enzyl)urea
The title compound was prepared using the procedure described in Example 317
except using 4-methylbenzylamine instead of 3-phenylpropylamine. 1H NMR (DMSO-
d6) S
179
CA 02743534 2011-06-13
9.02 (s, 1H), 8.21 (s, 1H), 7.64 (d, 1H), 7.19 (m, 6H), 7.05 (d, 1H), 4.30 (s,
1H); MS (ESI)
m/z 281, (I\M+H)+.
Example 334
N-1H-indazol-4-yl-N'-(3-(trifluoromethoxy)ben ,yl urea
The title compound was prepared using the procedure described in Example 317
except using 3-(trifluoromethoxy)benzylamine instead of 3-phenylpropylamine.
'H NMR
(DMSO-d6) S 9.07 (s, 1H), 8.22 (s, IH), 7.48 (t, 1H), 7.39 (d, 1H), 7.32 (s,
111), 7.20 (m, 3H),
7.03 (d, 1H), 4.20 (s, 1H); MS (ESI) m/z 351 (M+H)+.
Example 335
N-(3-chloro-4-fluorobenzyl)-N'-1 H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 3-chloro-4-fluorobenzylamine instead of 3-phenylpropylamine. 'H
NMR
(DMSO-d6) S 9.20 (s, 1H), 8.29 (s, 1H), 7.63 (d, 1H), 7.35 (dd, 1H), 7.38 (m,
3H), 7.20 (t,
1H), 7.03 (d, 1H), 4.38 (s, 1H); MS (ESI) m/z 353, (M+I-I)+
Example 336
N-(3,4-dimethylbenzyl)-N'-1H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 3,4-dimethylbenzylamine instead of 3-phenylpropylamine. 'H NMR
(DMSO-
d6); 9.00 (s, 1H), 8.21 (s, 1H), 7.64 (d, 1H), 7.20 (t, 1H), 7.07 (m, 5H),
4.23 (s, 1I-1), 2.21 (s,
3H), 1.98 (s, 3H); MS (ES1) m/z 295, (M+H)+.
Example 337
N-(3 -fluoro-5 -(trifluoromethyl)benzyl)-N'-1 H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 3-fluoro-5-(trifluoromethyl)benzylamine instead of 3-
phenylpropylamine. 'H
NMR (DMSO-d6) S 9.25 (s, 1H), 8.30 (s, 1H), 7.57 (m, 5H), 7.43 (bs, IH), 7.20
(t, IH), 7.05
(d, IH), 4.42 (s, 2H); MS (ESI) m/z 351 (M-H)".
Example 338
I80
CA 02743534 2011-06-13
N-(2-chloro-4-azepan- I -ylbenzyl)-N'-1 H-indazol-4-yl4rea
The title compound was prepared using the procedure described in Example 317
except using 2-chloro-4-azepan-1-ylbenzylamine instead of 3-phenylpropylamine.
'H NMR
(DMSO-d6) 6 9.00 (s, 1H), 8.20 (s, IH), 7.63 (d, 1H), 7.23 (d, IFI), 7.19 (t,
1H), 7.03 (d, 111),
6.98 (bs, 1H), 6.64 (m, 3H), 4.25 (s, 2H), 3.42 (m, 4H), 1.70 (m, 4H), 1.43
(m, 4H); MS (EST)
m/z 398 (M+H)+.
Example 339
N-(2,3-dichlorobenzyl)-N'- 1-i-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 2,3-dichlorobenzylamine instead of 3-phenylpropylamine. 'H NMR
(DMSO-
d6) S 9.06 (s, 1H), 8.18 (s, 1H), 7.61 (d, 1H), 7.58 (d, 1H), 7.40 (m, 2H),
7.19 (m, 1H), 7.12
(t, 1H), 7.06 (d, 1H), 4.25 (s, 2H), 4.23 (d, 2H); MS (ESI) m/z 336 (M+H)+.
Example 340
N-1 H-indazol-4-yl-N'- {4- f (trifluoromethyl)thiolbe 1 urea
The title compound was prepared using the procedure described in Example 317
except using 4-[(trifluoroinethyl)thio)benzylamine instead of 3-
phenylpropylamine. 'H NMR
(DMSO-d6) 6 9.06 (s, 111), 8.21 (s, 1H), 7.71 (d, 2H), 7.62 (d, 1H), 7.50 (d,
2H),
7.18 (m, 2H), 7.05 (d, 111), 4.21 (s, 2H); MS (ESI) m/z 367 (1\M+H)+.
Example 341
N-1H-indazol-4-yl-N'-f 3-(trifluoromethyl)benzyllurea
The title compound was prepared using the procedure described in Example 317
except using 3-(trifluoromethyl)benzylarnine instead of 3-phenylpropylamine.
'H NMR
(DMSO-d6) 6 9.17 (s, 1H), 8.24 (s, 1H), 7.63 (m, 6H), 7.30 (bs, 1H), 7.19 (t,
III),
7.16 (d, IH), 4.43 (s, IH), 421; MS (ESI) m/z 335 (M+H)+.
Example 342
N-(3,5-difluoro-4-azepan-1-ylbenzyl)-N'-1 H-indazol-4-ylurea
Example 342A
181
CA 02743534 2011-06-13
3,5-difluoro-4-azepan- l -ylbenzonitrile
The title compound was prepared using the procedure described in Example 325A
except using 3,4,5-trifluorobenzonitrile and azepane instead of 3,4-
difluorobenzonitrile and
8-aza-bicyclo[3.2. 1 ]octane hydrochloride. 'H NMR (DMSO-d6) S 7.62 (d, 2H),
3.39 (m,
4H), 1.73 (m, 4H), 1.61 (m, 4H); MS (ESI) m/z 237 (M+I1)+.
Example 342B
3,5-difluoro-4-azepan-1-ylbenzylamine
The title compound was prepared using the procedure described in Example 325B
except using 3,5-difluoro-4-azepan-1-ylbenzonitrile instead of4-(8-
azabicyclo[3.2.1]oct-8-
yl)-3-fluorobenzonitrile. 'H NMR (DMSO-d6) S 6.97 (d, 2H), 3.62 (s, 2H), 3.27
(m, 4H),
1.63 (m, 8H); MS (ESI) rn/z 241 (M+H)+
Example 342C
N-(3,5-difluoro-4-azepan-1-ylbenzyl)-N-1 H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 3,5-difluoro-4-azepan-1-ylbenzylanline instead of 3-
phenylpropylamine. 'H
NMR (DMSO-d6) 5 9.00 (s, 1H), 8.20 (s, 1H), 7.62 (d, 1H), 7.20 (t, 1H), 7.06
(d, 2H), 6.98
(d, 2H), 4.26 (s, 2H), 3.18 (m, 4H), 1.62 (m, 8H); MS (ESI) m/z 400 (M+H)+.
Example 343
N-[4-(8-azabicyclo[3.2.1loct-8-yl)-3,5-difluorobenzyll-N'-1H-indazol-4-ylurea
Example 343A
4-(8-azabicyclo[3.2.11oet-8-yl)-3,5-difluorobenzonitrile
The title compound was prepared using the procedure described in Example 325A
except using 3,4,5-trifluorobenzonitrile instead of 3,4-difluorobenzonitrile.
'H NMR
(DMSO-d6) 6 7.58 (dd, 211), 4.34 (s 211), 1.95 (m, 2H), 1.78 (m, 5H), 1.46 (m,
3H); MS (ESI)
m/z 249 (M+H)+.
Example 343B
4-(8-azabicyclo 3.2.1loct-8-yl)-3,5-difluorobenzylamine
182
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 325B
except using 4-(8-azabicyclo[3.2.1]oct-8-y1)-3,5-difluorobenzonitrile instead
of'4-(8-
aza.bicyclo[3.2.1 ]oct-8-yl)-3-fluorobenzonitrile. 1H NMR (DMSO-d6) S 6.97 (d,
2H), 4.00 (s,
21-1), 3.59 (in, 211), 1.91 (m, 21-1), 1.76 (m, 5H), 1.42 (m, 3H); MS (ESI)
M/7,253 (M+H)+
Example 343C
N-[4-(8-azabicyclo[3.2.1loct-8-yl) 3,5-difluorobenzyll-N'-IH-indazo1-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 4-(S-azabicyclo[32.1 ]oct-8-yl)-3,5-difluorobenzylamine instead
of
3-phenylpropylamine. 1H NMR (DMSO-d6) S 8.98 (s, I1), 8.20 (s, 1H), 7.62 (d, I
H), 7.19
(t, 1H), 7.06 ( d, 2H), 6.93 (d, 2H), 4.23 (s, 2H), 4.06 (s, 2H), 1.91 (m,
2H), 1.74 (m, 5H.),
1.41 (m, 3H); MS (ES1) m/z 412, (M+H)+.
Example 344
N-(4-chlorobenzyl)-N'-(1-methyl-lH-indazol-4- =1 urea
Example 344A
1-methyl--4-nitro-lH-indazole
A suspension of NaH (1.1 g, 32.2 mmol; 60% dispersion in mineral oil) in DIM
(40
mL) was treated with 4-nitro-IH-indazole (5 g, 30.6 mmol) in DMF (40 mL) at 0
C. After
stirring for 30 minutes, the mixture was treated with methyl iodide (4.6 g,
32.18 nunol) in
DMF (20mI) drop wise. The mixture was allowed to gradually warm to ambient
temperature
and stir overnight. The mixture was poured into water (250 ml) and extracted
with ethyl
acetate (2 x 100 mL). The organics were combined, washed with water, brine,
dried over
Na2SO4, filtered, and the filtrate was concentrated under reduced pressure.
The residue was
chromatographed (Si02, ethyl acetate/hexanes) to provide the title compound.
111 NMR
(DMSO-d6) b 8.50 (s, 1H), 8.24 (d, 1H), 8.19 (d, 1H), 7.65 (t, 111).
Example 344B
1-methyl-I H-indazol-4-amine
1-Methyl-4-nitro-1H-indazole (6.1g; 35.4 mmol) and 10% Pd/carbon (500 mg) were
combined in ethanol and hydrogenated in a Parr apparatus at 60 PSI hydrogen at
50 C for 1
183
CA 02743534 2011-06-13
hour. The mixture was allowed to cool to ambient temperature, filtered through
Celite, and
concentrated under reduced pressure to provide the title compound. 1H NMR
(DMSO-d6) 6
8.02 (s, IM, 7.02 (t, 111), 6.62 (d, IM, 6.14 (d, J H), 5.75 (s, 2H), 3.90 (s,
2H).
Example 344C
N-(4-chlorobenzyl)-N'-(1-methyl-lH-indazol-4-yl)urea
1-Methyl-lH-indazol-4-amine (1.00 g, 6.8 mmol) in toluene (225 mL) was treated
with phosgene (20% in toluene, 7 nil, 13.2 mmol). The mixture was heated at
reflux for 3
hours, cooled, and the solvent removed under reduced pressure. The residue was
taken in
diethyl ether (100 ml) and triethyl amine (6 ml), and filtered. The filtrate
was treated with 4-
chlorobenzylamine (963 mg, 6.8 mmol). After stirring at ambient temperature
for 16 hours,
the solvent was reduced to half volume under reduced pressure, filtered, and
the filter cake
washed with diethyl ether:hexanes (1:1) to provide the title compound. The
title compound
was treated with HCI/ethanol and evaporated to dryness to provide the
hydrochloride. 1H
NMR (DMSO-d6) 6 9.25 (s, 1H), 8.25 (s, 1H), 7.68 (d, 1H), 7.39 (m, 5H), 7.24
(t, 1H), 7.13
(d, 1H), 4.34 (s, 2H), 3.99 (s, 3H); MS (ESI) m/z 315 (M+H)+.
Example 345
methyl 4-((f (4-(8-azabicyclo[3.2.lloct-8-yl)-2-
chlorobenzyllamino}carbonyl)aminol-lH-
indazole- l -carboxylate
Example 345A
8-azabicyclo[3.2.lloctane
8-Methyl-8-azabicyclo[3.2.1]octane in dichloroethane (400 mL) was treated with
1-
chloroethylchloroformate (29.3 g, 205 mmol) in dichloroethane (75 mL) dropwise
via
addition funnel at 0 C. After complete addition, the mixture was heated at
reflux for four
hours. The mixture was allowed to cool to ambient temperature and was
concentrated under
reduced pressure. The residue was filtered through a silica gel plug eluting
with 1:1 ethyl
ether:hexane and the filtrate was concentrated under reduced pressure. The
residue was taken
up in 250 mL methanol, heated at reflux for 1 hour, allowed to cool to ambient
temperature,
and concentrated under reduced pressure. The residue was triturated with
diethyl ether and
filtered. The filter cake was washed with diethyl ether and dried under
reduced pressure at 60
184
CA 02743534 2011-06-13
C to provide the title compound as a solid. 1H NNIR (300 MHz, DMSO-d6) 6 8.90
(bs, 2H),
3.87 (in, 2H), 2.02-1.76 (in, 61-1), 1.74-1.40 (in, 4H); MS (DCI) 112 (M+H)+.
Example 345B
4-(8-azabicyclo[3.2.1 ]oct-8-yl)-2-chlorobenzonitrile
2-Chloro-4-fluorobenzonitrile (0.97 g, 6.2 mmol), 8-aza-bicyclo[32.1]octane
hydrochloride (0.92 g, 6.2 mmol), and N,N-diisopropylethylamine (1.6 g, 12
mmol) were
combined in DMSO (15 mL) and heated at 120 C for 16 hours. The mixture was
allowed to
cool to ambient temperature and partitioned between diethyl ether and
saturated NaHCO3
solution. The aqueous phase was separated. and extracted with diethyl ether.
The organic
layers were combined, washed with water, brine, dried (Na2SO4), filtered, and
the filtrate was
concentrated under reduced pressure to provide the title compound which was
used in the
next step without further purification.
Example 345C
4-(8-azabicyclo [ 3.2.11oct- 8-yl)-2-chl orobenzylamine
4-(8-Azabicyclo[3.2.1]oct-8-yl)-2-chlorobenzonitrile in THE (50 mL) was
treated
with solid LAH (0.47 g, 12 mmol) at 0 C portionwise. The mixture was heated
at reflux for
1 hour, allowed to cool to 0 C, and quenched by addition of (Na2SO4 10H20).
The mixture
was stirred for 30 minutes, filtered, and the filtrate concentrated under
reduced pressure. The
residue was purified by flash chromatography eluting with 5% to 10%
McOH/CH2CI2 to
provide the title compound. 1H NMR (300 MHz, DMSO-d6) S 7.27 (d, 1H, J=8.6
Hz), 6.74
(d, 1H, J=2.4 Hz), 6.70 (dd, 1H, J=8.6, 2.4 Hz), 4.16 (bs, 2H), 3.65 (s, 2H),
2.05 (bs, 2H),
1.97 (m, 2H), 1.88-1.65 (m, 5H), 1.40 (m, 1H), 1.23 (m, 2H); MS (ESI) 234 (M-
NH2)+.
Example 345D
methyl 4-[({[4-(8-azabicyclo[3.2.11oct-8-yl)-2-chlorobenzyllamino}
earbonyl)aminol-11-I-
indazole-1-carboxylate
A suspension of methyl 4-amino-lH-indazole-l-carboxylate (554 mg, 2.90 mmol)
in
toluene (100 mL) was treated with phosgene in toluene (2.90 mL, approx. 20%
w/w) via
syringe. The mixture was heated at reflux for 3.5 hours, allowed to cool to
ambient
temperature, and concentrated under reduced pressure. The residue was taken up
in diethyl
185
CA 02743534 2011-06-13
ether and concentrated under reduced pressure. The residue was again taken up
in diethyl
ether (100 rnL) and treated with triethylamine (3 mL). After stirring for 10
minutes, the
mixture was filtered. The filtrate was treated with 4-(8-azabicyclo[3.2.1]oet-
8-yl)-2-
chlorobenzylamine (484 mg, 1.93 mmol) in THE (10 mL). After stirring for 2
hours, the
mixture was concentrated under reduced pressure. The residue was purified by
flash
chromatography eluting with 2% to 5% McOHICH2C12 to provide the title compound
as a
solid. 'H NMR (300 MHz, DMSO-d6) 6 9.00 (s, 1H), 8.41 (s, 111), 7.85 (d, 1H,
J=7.8 Hz),
7.68 (d, 1 H, J=8.5 Hz), 7.48 (t, I H, J=8.1 Hz), 7.25 (d, I H, J=8.5 Hz),
6.82 (d, 1H, J=2.4 Hz),
6.73 (dd, IH, J=8.5, 2.4 Hz), 6.68 (t, IH, J=5.4 Hz), 4.30 (d, 2H, J=5.4 Hz),
4.19 (m, 2H),
4.03 (s, 3H), 2.05-1.65 (m, 7H), 1.40 (m, 1H), 1.25 (m, 2H); MS (ESI) 468
(M+H)+.
Example 346
N-[4-(8-azabicyclo [3.2.1loct-8-yl)-2-chlorobenzyll-N'-1H-indazol-4-ylurea
Methyl 4-[({ [4-(8-azabicyclo [3.2. 1 ]oct-8-yl)-2-chlorobenzyl] amino)
carbonyl)amino]-
1H-indazole-l-carboxylate (803 mg, 1.72 mmol) in methanol (40 mL) was treated
with 1.2N
NaOH in McOH (20 mL). After stirring for 30 minutes, the mixture was
concentrated under
reduced pressure. The residue was partitioned between ethyl acetate and
saturated NaHCO3
solution. The separated aqueous phase was extracted with ethyl acetate. The
organic layers
were combined, washed with brine, dried (Na2SO4), filtered, and the filtrate
was concentrated
under reduced pressure. The residue was purified by flash chromatography (7%
to 10%
McOHJCH2CI2) to provide the title compound as a solid. The obtained solid was
treated with
ethanolic HC1 followed by precipitation with diethyl ether to provide the
hydrochloride salt.
'H NMR (300 MHz, DMSO-d6) S 8.91 (s, 1H), 8.16 (s, 1H), 7.65 (d, 1H, f=7.5
Hz), 7.27 (d,
IH, J=8.5 Hz), 7.19 (t, IH, J=8.1 Hz), 7.05 (d, 1H, J=8.5 Hz), 6.87 (m, 2H),
6.79 (m, 1H),
4.29 (m, 2H), 4.21 (m, 2H), 2.05-1.65 (m, 7H), 1.40 (m, 1H), 1.26 (m, 2H); MS
(ESI) 410
(M+H)+; Anal. Calcd for C22H24C1N50.1.6HCI: C, 56.43; H, 5.51; N, 14.96.
Found: C,
56.30; H, 5.29; N, 14.81.
Example 347
methyl 4-[({[4-(8-azabicyclo[3.2.Ijoct-8-y1)-3-
(trifluoromethyl)benzyllamino) carbonyl)aminol-IH-indazole-l-carboxylate
186
CA 02743534 2011-06-13
Example 347A
4-(8-azabieyclo(3.2.1oct-8-yl)-3-(trifluoromethyl)benzonitrile
4-Fluoro-3-(trifluoromethyl)benzonitrile (1.35 g, 7.14 mmol), 8-aza-
bieyelo[3.2.1]octane hydrochloride (1.26 g, 8.57 mmol), and N,N-
diisopropyleth.ylamine
(1.79 g, 13.8 rnmol) were combined in DMSO (15 mL) and heated at 120 C for 24
hours.
The mixture was allowed to cool to ambient temperature and partitioned between
diethyl
ether and saturated NaHCO3 solution. The separated aqueous phase was extracted
with
diethyl ether and the combined organic layers were washed with water, brine,
dried (Na2SO4),
filtered, and the filtrate was concentrated under reduced pressure to provide
the title
compound which was used in the next step without further purification.
Example 347B
4-(8-azabicyclo[3.2. l loct-8-yl)-3-(trifluoromethyl)benzylamine
4-(8-Azabicyclo[3.2.1]oct-8-y1)-3-(trifluoromethyl)benzonitrile in THE (50 mL)
was
treated with solid LAH (0.68 g, 18 mmol) at 0 C portionwise. The mixture was
heated at
reflux I hour, allowed to cool to 0 C, and quenched by addition of (Na2SO4 I
OH2O). The
mixture was stirred 30 minutes, filtered, and the filtrate was concentrated
under reduced
pressure. The residue was purified by flash chromatography eluting with 5% to
10%
McOI-I/CH2C12 to provide the title compound. 'H NMR (300 MHz, DMSO-d6) S 7.57
(d, IH,
J=2.0 Hz), 7.42 (dd, IH, J=8.1, 2.0 Hz), 7.08 (d, 1H, J=8.4 Hz), 3.658 (m,
4H), 2.15 (bs, 2H),
1.92 (m, 2H), 1.88-1.55 (m, 5H), 1.48 (in, 3H); MS (ESI) 268 (M-NH2)}.
Example 347C
methyl 4-[( {[4-(8-azabicyclo(3.2.1)oct-8--yl)-3-
(ttrifluoromethyl)benzyllamino } carbonyl)aminol-1 H-indazole- l -carboxylat
Methyl 4-amino-lH-indazole-l-carboxylate (1.72 g, 9.00 rnmol) in toluene (300
mL)
was treated with phosgene in toluene (9.00 mL, approx. 20% w/w) via syringe.
The mixture
was heated at reflux for 3.5 hours, allowed to cool to ambient temperature,
and concentrated
under reduced pressure. The residue was taken up in diethyl ether and
concentrated under
reduced pressure. The residue was again taken up in diethyl ether (325 mL)
followed by
addition of triethylamine (10 mL). The mixture was stirred briefly and then
filtered. An
aliquot of the filtrate (150 mL, 4.14 mmol) was treated with 4-(8-azabicyclo[3
2.l]oct-8-yl)-
187
CA 02743534 2011-06-13
3-(trifluoromethyl)benzylamine (1.03 g, 3.62 mmol) in THE (10 mL). After
stirring for 2
hours, the mixture was concentrated under reduced pressure to approximately 30
mL and
filtered. The filter cake was washed with diethyl ether:hexane (1:1) and dried
under reduced
pressure to provide the title compound. 'H NMR (300 MHz, DMSO-d6) 6 9.07 (s,
111), 8.43
(s, 111), 7.80 (d, 1H, J=7.8 Hz), 7.69 (d, 1H, J=8.1 Hz), 7.58 (t, 1H, J=2.0
Hz), 7.47 (m, 2H),
7.14 (d, 1H, J=8.1 Hz), 6.86 (t, 1H, J=5.8 Hz), 4.32 (d, 2H, J=5.8 Hz), 4.03
(s, 3M, 3.72 (nl,
211), 1.93 (m, 2H), 1.88-1.55 (m, 5H), 1.49 (m, 311); MS (ESI) 502 (M+H)+.
Example 348
N-[4-(8-azabicyclo(3.2.1loct-8-yl)-3-(trifluoromethyl)benzyll-N'-1 H-indazol-4-
ylurea
A suspension of methyl 4-[({[4-(8-azabicyclo[3.2.1]oct-8-yl)-3-
(trifluoromethyl)benzyl]amino}carbonyl)amino]-1H-indazole-l-carboxylate (1.65
g, 3.29
mmol) in methanol (30 mL) was treated with 1.2 N NaOH in MeOH (10 mL). The
mixture
was stirred for 30 minutes and concentrated under reduced pressure. The
residue was
partitioned between ethyl acetate and saturated NaHCO3 solution. The separated
aqueous
phase was extracted with ethyl acetate and the combined organic layers were
washed with
brine, dried (Na2SO4), filtered, and the filtrate was concentrated under
reduced pressure. The
residue was purified by flash chromatography (7% to 10% McOHICH2C12) to
provide the
title compound as a solid. The obtained solid was treated with ethanolic HCl
followed by
precipitation with diethyl ether to provide the hydrochloride salt. 'H NMR
(300 MHz,
DMSO-d6) S 8.99 (s, 1H), 8.19 (s, 1H), 7.62 (d, 1H, J=7.5 Hz), 7.57 (d, IH,
J=2.0 Hz), 7.46
(dd, 111, J=8.5, 2.0 Hz), 7.19 (t, 1H, J=8.1 Hz), 7.10 (m, 3H), 4.31 (m, 2H),
3.72 (m, 2H),
1.93 (m, 2H), 1.87-1.56 (m, 51), 1.48 (m, 3H); MS (ESI) 444 (4+H)+; Anal.
Calcd for
C23H24F3N5O.1.6HCl: C, 55.05; H, 5.14; N, 13.96. Found: C, 54.95; H, 4.66; N,
13.53.
Example 349
N-[4-(8-azabicyclo[3.2.1 loct-8-yl)-3-chlorobenzyll-N-1 H-indazol-4-ylurea
Example 349A
4-(8-azabicyclo[3.2.11oct-8-yl)-3 -chlorobenzonitrile
188
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 347A
except using 4-fluoro-3-cblorobenzonitrile instead of 4-fluoro-3-
(trifluoromethyl)b enzonitrile.
Example 349B
4-(8-azabicyclo[3.2.11oct-8-yl)-3-chlorobenzylamine
The title compound was prepared using the procedure described in Example 347B
except using 4-(8-azabicyclo[3.2.1 ]oct-8-yl)-3-chlorobenzonitrile instead of
4-(8-
azabicyclo[3 2.1 ]oct-8-yl)-3-(tri.fluoromethyl)benzonitrile.
Example 349C
N-[4-(8-azabicyclo [3.2.1 lost-8-y1)-3 -chlorobenzyll-N'-1H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 4-(8-azabicyclo[3.2.1 ]oet-8-yl)-3-chlorobenzylamine instead of
3-phenylpropylaniine. 1H NMR (300 MHz, DMSO-d6) 8 8.84 (s, 1H), 8.13 (s, 111),
7.62 (d,
11-1, J=7.5 Hz), 7.35 (d, 1H, J=2.0 Hz), 7.16 (m, 2H), 7.03 (m, 2H), 6.91 (m,
1H), 4.25 (d, 2H,
J=4.4 Hz), 3.91 (m, 2H), 1.84 (m, 4H), 1.67 (m, 3H), 1.50 (m, 3H); MS (ESI)
410 (M+H)+;
Anal. Calcd for C22H24C1NT50Ø8HC1: C, 60.18; H, 5.69; N, 15.95. Found: C,
60.09; H,
5.37; N, 15.64.
Example 350
N-[4-(8-azabicyclo[3.2.1 lost-8-yl)bnzyll-N'-1 H-indazol-4-ylure
Example 350A
4-(8-azabicyclo [3.2. l feet-8-yl)benzonitrile
The title compound was prepared using the procedure described in Example 347A
except using 4-fluorohenzonitrile instead of 4-fluoro-3-
(trifluoromethyl)benzonitrile.
Example 350B
4-(8-azabicyclo[3.2.Iloct-8-yl)benzylamine
189
CA 02743534 2011-06-13
The title compound was prepared using the procedure described in Example 347B
except using 4-(8-azabieyclo[32.1]oct-8-yl)benzonitrile instead of4-(8-
azabicyclo[3.2.lJoct-
8-yl)-3-(trifluoromethyl)benzonitrile.
Example 350C
N-[4-(8-azabicyclo[3.2.1)oct-8-yl)benzyll-N'-1H-indazol-4-ylurea
The title compound was prepared using the procedure described in Example 317
except using 4-(8-azabicyclo[3.2.1 ]oct-8-yl)benzylamine instead of 3-
phenylpropylamine.
111 NMR (300 MHz, DMSO-d6) S 8.85 (m, 1H), 8.15 (s, 1H), 7.65 (d, IH, J=7.8
Hz), 7.25
(m, 2H), 7.19 (t, 1 H, J=8.0 Hz), 7.05 (d, 1 H, J=8.1 Hz), 6.87 (m, 2f), 4.26
(m, 4H), 2.10-
1.65 (m, 7H), 1.60-1.15 (m, 3H); MS (ESI) 376 (M+H)+; Anal. Calcd for
C22H25N5O.1.1HC1:
C, 63.58; H, 6.33; N, 16.85. Found: C, 63.36; H, 6.05; N, 16.57.
Example 351
N-(4-tert-butylbenzyl)-N-(1-methyl-lH-indazol-4-yl)urea
4-tert-Butylbenzylamine (0.46 mL, 2.62 mmol) in toluene (8 mL) was treated
with
20% phosgene solution (1.4 mL) and refluxed for 3 hours. The mixture was
allowed to cool
to ambient temperature and concentrated under reduced pressure. The residue
was then taken
up in toluene (10 mL) and treated with diisopropylamine (3 niL) and 1-methyl-
IH-indazol-4-
amine (prepared as described in J. Med. Chem. 45:742 (2002); 200 mg, 1.36
mmol). The
reaction mixture was heated at 80 C for 3 hours, allowed to cool to ambient
temperature, and
concentrated under reduced pressure. The residue was purified by flash
chromatography
(98:2 CH-2C12:CH3OH to 95:5 CH2C12:CH3OH, eluant gradient) to provide the
title
compound. The corresponding hydrochloride salt was prepared with methanolic
HCI. 'H
NMR (300 MHz, d6-DMSO) 8 8.72 (s, 1H), 8.03 (d, J=0.7 Hz, 1H), 7.66 (dd, J=7.8
Hz, 0.6
Hz, 1H), 7.37 (m, 2H), 7.27 (m, 2H), 7.24 (m, 1H), 7.13 (m, 1H), 6.74 (m, 1H),
4.30 (d, J=5.8
Hz, 21-1), 3.99 (s, 3H), 1.27 (s, 9H); MS (ESI+
) mlz 337 (M+H)}.
Example 352
N-[3-fluoro-4-(trifluoromethyl)benzyll-N'-(1-methyl- I H-indazol-4-yl)urea
The title compound was prepared using the procedure described in Example 351
except using 3-fluoro-4-(trifluoromethyl)benzylamine instead of 4-tert-
butylbenzylamine. 'H
190
CA 02743534 2011-06-13
NMR (300 MHz, d6-DMSO) 6 8.92 (s, 1H), 8.08 (d, J=1.1 Hz, 1H), 7.77 (t, J=8.0
Hz, 1H),
7.62 (dd, J=7.3 Hz, 0.7 Hz, 1H), 7.41 (m, 11I), 7.25 (m, 114), 7.15 (m., 1H),
6.98 (t, J=6.1 Hz,
114), 4.45 (d, J=6.1 Hz, 2H), 4.00 (s, 3H); MS (ESI+) m/z 367 (M+H)+.
Example 353
a
N-[4-chloro-3-(trifluoromethyl benzyl}-N'-(1-methyl-lH-indazol-4-y1)ure
The title compound was prepared using the procedure described in Example 351
except using 4-chloro-3-(trifluoromethyl)benzylan3ine instead of 4-tent-
butylbenzylamine. 'H
NMR (300 MHz, d6-DMSO) S 8.89 (s, 1H), 8.06 (d, J=1.0 Hz, 1H), 7.82 (s, 1I1),
7.60-7.70
(m, 3H), 7.22 (m, 1H), 7.17 (m, 1H), 6.92 (m, 1H), 4.42 (d, J=5.8 Hz, 211),
3.99 (s, 3M-, MS
(ESI) m/z 383/385 (M+H, 35C1/37C1)+
Example 354
N-(1-meth)rl-lH-indazol-4-yl)-4-[4-(trifluoromethyl)-2-pyridinyll-1-
piperazineearboxamide
1-Methyl-lH-indazol-4-amine (560 mg, 3.81 mmol) in toluene (20 mL) was treated
with 20% phosgene solution (2.5 mL) and refluxed overnight. The mixture was
allowed to
cool to ambient temperature and was concentrated under reduced pressure. The
residue was
taken up in THE (20 mL) and treated with diisopropylam.ine (5 mL) and
1-[4-(trifluoromethyI)-2-pyridinyl)piperazine (450 mg, 1.95 mmol). The mixture
was
refluxed overnight, allowed to cool to ambient temperature, and concentrated
under reduced
pressure. The residue was purified by flash chromatography (97:3 CH2C12:CH3OH
to 95:5
CH2CI2:CH3OH) to provide the title compound. The corresponding hydrochloride
salt was
prepared by treatment with methanolic HCI. 'H NMR (300 MHz, d6-DMSO) b 8.78
(s, 1H),
8.44 (m, 1H), 8.07 (d, J=1.0 Hz, 1H), 7.83 (m, 1H), 7.19-7.31 (m, 3H), 7.02
(d, 9.2 Hz, 1H),
4.00 (s, 3H), 3.74 (m, 4H), 3.63 (m, 4H); MS (ESI) m/z 405 (M+H)+.
Example 355
N-(3,4-dichlorob enzyl)-N-(1-methyl- I H-indazol-4-yl)ure a
1-Methyl-lH-indazol-4-amine (390 mg, 2.65 mmol) and 3,4-dichlorobenzyl
isocyanate (0.39 mL, 2.65 mmol) were combined in toluene (20 mL) and heated
overnight at
80 C. The mixture was allowed to cool to ambient temperature, filtered, and
the filter cake
191
CA 02743534 2011-06-13
was allowed to air-dry to provide the title compound. The corresponding
hydrochloride salt
was prepared by treatment with methanolic HC1. 'H NMR (300 MHz, d6-DMSO) 6
8.86 (s,
111), 8.06 (d, J=1.0 Hz, IM, 7.59-7.64 (in, 3H), 7.33 (m, 111), 7.25 (in,
111), 7.15 (m, III),
6.91 (t, J=6.0 Hz), 4.35 (d, J=5.8 Hz, 2H), 3.99 (s, 3H); MS (ESI) m/z 349/351
(M+H,
35C1/37C1)+
Example 356
N-(2,4-dichlorob enzyl)-N'-(l -methyl-1 H-indazol-4-yl)urea
1-Methyl-lH-indazol-4-amine (310 mg, 2.1 mmol) and 2,4-dichlorobenzyl
isocyanate
(0.3 mL, 2.06 mmol) were combined in toluene (10 mL) and heated for 2 hours at
80 C. The
mixture was then allowed to cool to ambient temperature, filtered, and the
filter cake was
allowed to air-dry to provide the title compound. The corresponding
hydrochloride salt was
prepared by treatment with methanolic HCI. 1H NMR (300 MHz, d6-DMSO) 6 9.22
(s, 111),
8.21 (d, J=1.0 Hz, 1H), 7.62-7.67 (m, 2H), 7.43-7.46 (m, 2H), 7.21-7.27 (m,
2H), 7.12 (in,
1H), 4.40 (d, J=5.5 Hz, 2H), 3.99 (s, 3H); MS (ESI') m/z 349/351 (M+H,
35Cl/37C1)+.
Example 357
N-(4-ethylbenzyl)-N'-(1-methyl- I H-indazol-4-yl)urea
The title compound was prepared using the procedure described in Example 354
except using 4-ethylbenzylamine instead of 1-[4-(trifluoromethyl)-2-
pyridinyl]piperazine. 'H
NMR (300 MHz, d6-DMSO) S 8.73 (s, 1H), 8.03 (d, 1H, J=0.7 Hz), 7.66 (d, J=7.4
Hz, 1H),
7.12-7.28 (m, 6H), 6.75 (t, J=5.8 Hz, 1H), 3.99 (s, 3H), 2.59 (q, J=7.6 Hz,
2H), 1.16 (t, J=7.5
Hz, 3H); MS (ESI) m/z 309 (M+H)+.
Example 358
N-(2-chlorobenzyl)-N'-(1-methyl-1 H-indazol-4-yl)urea
The title compound was prepared using the procedure described in Example 355
except using 2-chlorobenzyl isocyanate instead of 3,4-dichlorobenzyl
isocyanate. 'H NMR
(300 MHz, d6-DMSO) S 8.88 (s, 1H), 8.06 (d, J~=0.7 Hz, 1H), 7.65 (dd, J=7.4
Hz, 0.7 Hz,
1H), 7.44-7.49 (m, 2H), 7.28-7.39 (m, 2H), 7.25 (m, 1H), 7.14 (m, 1H), 6.87
(t, J=6.0 Hz,
111), 4.43 (d, J=6.1 Hz, 2H), 4.00 (s, 3H); MS (ESI) m/z 315/317 (M+H,
35C1/37C1)+.
192
CA 02743534 2011-06-13
Example 359
N-(4-fluorobenzyl)-N'-(1-methyl-1 H-indazol-4-yl)urea
The title compound was prepared using the procedure described in Example 355
except using 4-fluorobenzyl isocyanate instead of 3,4-dichlorobenzyl
isocyanate. 'II NMR
(300 MHz, d6-DMSO) 8 8.78 (s, 1H), 8.05 (d, J=1.0 Hz, 1II), 7.65 (m, 1H), 7.36-
7.41 (m,
2H), 7.12-7.28 (m, 4H), 6.82 (t, J=5.9 Hz, 1H), 4.33 (d, J=5.8 Hz, 2H), 3.99
(s, 3H); MS
(ESF') m/z 299 (M+H)+.
Example 360
N-(2-fluorobenzyl)-N'-(1-methyl-1 H-indazol-4-yl)urea
The title compound was prepared using the procedure described in Example 355
except using 2-fluorobenzyl isocyanate instead of 3,4-dichlorobenzyl
isocyanate. 'H NMR
(300 MHz, d6-DMSO) S 8.83 (s, 1H), 8.06 (d, J=1.0 Hz, 1H), 7.65 (m, 1H), 7.40
(m, 1H),
7.05-7.28 (m, 4H), 6.89 (t, J=5.9 Hz, 1H), 4.37 (d, J=5.8 Hz-, 2H), 3.99 (s,
3H); MS (ESl+)
mlz 299 (M+H)+.
Example 361
N-(l -(4-bromophenyl)ethyll-N'-(1-methyl-1 H-indazol-4-yl)urea
The title compound was prepared using the procedure described in Example 355
except using 1-bromo-4-(1-isocyanatoethyl)benzene instead of 3,4-
dichlorobenzyl
isocyanate. 'H NUR (300 MHz, d6-DMSO) 6 8.66 (s, l1), 8.02 (s, 1H), 7.61 (d,
J=7.4 Hz,
11-1), 7.54 (m, 2H), 7.33 (m, 2H), 7.23 (m, 1H), 7.12 (d, J=8.5 Hz, 1H), 6.85
(d, J=7.4 Hz,
1H), 4.83 (quintet, J=7.0 Hz, 1H), 3.99 (s, 3H), 1.42 (d, J=6.8 Hz, 31-1); MS
(ES[) m/z
373/375 (M+H, 79Br/I'Br)+.
Example 362
N-(1-methyl-IH-indazol-4-yl)-N'- j4 [(trifluoromethyl)thio}benzyl}urea
The title compound was prepared using the procedure described in Example 355
except using 4-[(trifluoromethyl)thio]benzylamine instead of 4-tert-
butylbenzylamine. 1H
NMR (300 MHz, d6-DMSO) S 8.86 (s, 1H), 8.06 (d, J=0.7 Hz, 1H), 7.70 (d, J=8.2
Hz, 2H),
7.65 (d, J=7.5 Hz, 1H), 7.50 (d, J=8.2 Hz, 2H), 7.13-7.28 (m, 3H), 6.92 (t,
J=5.9 Hz, 1H),
4.43 (d, J=6.1 Hz, 2H), 4.00 (s, 3H); MS (ESI) m/z 381 (M+H)+.
193
CA 02743534 2011-06-13
Example 363
N-(4-tert-butylbenz 1)-N'-(7-methyl-lH-indazol-4-yl)urea
Example 363A
2,2,2-trichloro-N-(7-methyl-lH-indazol-4-yl)acetamide
7-Methyl-lH-indazol-4-amine (J. Chem. Soc. 1955, 2412; 550 mg, 3.74 mmol) and
triethylamine (1.6 mL, 11.5 mmol) were combined in CH2C12 (22 rnL) and treated
with
trichloroacetyl chloride (0.54 mL, 4.84 mmol) dropwise at 0 C. The mixture was
allowed to
gradually warm to ambient temperature and stir overnight. The mixture was
concentrated
and the residue was purified by flash chromatography (98:2, CH2C12:CH3OH), to
provide the
title compound.
Example 363B
N-(4-tert-butylbenzy1)-N'-(7-methyl- l H-indazol-4-y1)urea
2,2,2-Trichloro-N-(7-methyl-lH-indazol-4-yl)acetamide (72 mg, 0.25 mmol), 4-
tert-
butylbenzylamine (55 mg, 0.34 mmol), and 1,8-diazabicyclo[5.4.0]undec-7-ene
(DBU) (0.09
mL, 0.60 mmol) were combined in CH3CN (6 mL) and refluxed overnight. The
mixture was
allowed to cool to ambient temperature and was concentrated under reduced
pressure. The
residue was taken up in ethyl acetate and washed twice with saturated aqueous
NH4C1
solution. The organic layer was dried (Na2SO4), filtered, and the filtrate was
concentrated
under reduced pressure. The residue was triturated with ethyl acetate to
provide the title
compound. The corresponding hydrochloride salt-was prepared with
methanolic_HCI. 'H
NMR (300 MHz, d6-DMSO) 6 12.90 (br s, 1H), 7.94 (d, J=1.3 Hz, lH), 7.91 (s,
1H), 7.47 (m,
2H), 7.37 (m, 2H), 7.25 (m, 2H), 6.84 (t, J=5.8 Hz, 11), 4.26 (d, J=5.7 Hz,
2H), 2.35 (s, 3H),
1.27 (s, 9H); MS (ESI) m/z 337 (M+11)+.
Example 364
N-(7-methyl1 H-indazol-4-yl)-N'-(4-(trifluoromethyl)benzyllurea
The title compound was prepared using the procedure described in Example 363B
except using 4-(trifluoromethyl)benzylamine instead of 4-tert-
butylbenzylamine. 'H NMR
(300 MHz, d6-DMSO) S 12.93 (s, 1H), 8.01 (s, 1H), 7.95 (d, J=1.4 Hz, 1H), 7.72
(d, J=8.2
194
CA 02743534 2011-06-13
Hz, 2H), 7.53 (d, J=8.2 Hz, 2H), 7.43 (m, 2H), 6.96 (m, 1H), 4.40 (d, J=5.8
Hz, 2H), 2.36 (s,
3H); MS (ESI+) m/z 349 (4+H) + .
Example 365
N-(7-methyl-lH-indazol-4-yl)-N'-{4-((trifluoromethyl)thio)benz 1 urea
The title compound was prepared using the procedure described in Example 363B
except using 4-[(trifluoromethyl)thio]benzylamine instead of 4-tert-
butylbenzylamine. 1H
NMR (300 MHz, d6-DMSO) 6 12.93 (s, 1H), 8.01 (s, 1H), 7.95 (d, J=1.4 Hz, IH),
7.70 (d,
J=8.1 Hz, 2H), 7.43-7.49 (m, 4H), 6.94 (m, 1H), 4.37 (d, J=6.1 Hz, 2H), 2.36
(s, 3H); MS
(ESI) m/z 381 (M+H)+.
It is understood that the foregoing detailed description and accompanying
examples
are merely illustrative and are not to be taken as limitations upon the scope
of the invention,
which is defined solely by the appended claims and their equivalents. Various
changes and
modifications to the disclosed embodiments will be apparent to those skilled
in the art. Such
changes and modifications, including without limitation those relating to the
chemical
structures, substituents, derivatives, intermediates, syntheses, formulations
and/or methods of
use of the invention, may be made without departing from the spirit and scope
thereof.
195