Note: Descriptions are shown in the official language in which they were submitted.
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
-1-
CARBOXYLIC ACID ESTER COLOR-STABILIZED PHENOLIC BOUND ABRASIVE
PRODUCTS AND METHODS FOR MAKING SAME
RELATED APPLICATION(S)
This application claims the benefit of U.S. Provisional Application No.
61/199,472,
filed on November 17, 2008. The entire teachings of the above application are
incorporated
herein by reference.
BACKGROUND OF THE INVENTION
The term "phenolic resin" describes a wide variety of resin products that
result from
the reaction product of phenols and aldehydes. Phenols react with
formaldehydes under both
acidic and basic conditions. If a based-catalyzed mixture of a phenol and a
formaldehyde
contains one or more moles of formaldehyde per moles of phenol, it will
produce a
thermosetting (one-step) resin, or "resole." Common base compounds employed as
catalysts
for resole resins include the hydroxides of alkali metals, such as sodium,
potassium, or
lithium. While alkali metal hydroxide-catalyzed phenolic resins are
commercially useful,
they have an undesirable tendency to darken as they age, are heated or
otherwise cured. The
extent of darkening is known to be dependent on the curing or use temperature
of the resin
and the time of exposure to such temperature.
Alkali metal hydroxide-catalyzed phenolic resins are commonly used as a
component
of the bond system of abrasive products, such as coated, bonded, and three-
dimensional, low
density abrasive products. The resin darkening problem is particularly
pronounced in coated
abrasive and three-dimensional, low density abrasive products because of the
more visible
presence of the bond system. Also, since the darkening increases with
temperature and
exposure time, any variation in the temperature profile of the product results
in color
variation within the product itself. Color variation is particularly
noticeable for light-colored
products, causing such products to be unacceptable for aesthetic or other
reasons.
Furthermore, abrasive bond systems may comprise colorants to identify the
manufacturer, type of product, application, etc. The darkening of the resin
can interfere with
the desired coloration, causing the final product to have a different color
from the colorant
added. For example, a resin that normally turns yellow after curing will yield
a green colored
product when combined with a blue dye or pigment. On the other hand, if the
same yellow
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
-2-
resin is combined with a green dye or pigment, the resin will typically result
in simply
another shade of green.
One known method for imparting color stability in phenolic resoles include
adding
melamine formaldehyde resin into the formulation. While this achieves color
stability, it also
imparts brittleness, takes longer to cure, and results in mechanical weakness
and therefore
reduced grinding performance in the finished product.
Another proposed method includes the addition of an ammonium based salt to the
phenolic resole. However, this method is not sufficiently effective in
stabilizing the color of
phenolic resin products having certain colorants, such as light blue and
orange pigments or
dyes.
What is needed is an effective phenolic resin color-stabilizer that reduces
the
aforementioned problems without imparting undesirable properties in the
finished product.
SUMMARY OF THE INVENTION
In one aspect, the present invention is directed to a color-stable abrasive
article that
includes a phenolic resin binder; a color stabilizer, a colorant, and abrasive
grains. The color
stabilizer includes at least one carboxylic acid ester, ester, carboxylic
acid, or a dione or
acrylic group.
In another aspect, the present invention is directed to a method of making a
color-
stable abrasive article including the steps of blending a resole and a color
stabilizer to form a
resole composition; contacting a plurality of abrasive particles with the
resole composition;
and curing the resole composition to produce the color-stable abrasive
article. The color
stabilizer includes at least one carboxylic acid ester, ester, carboxylic
acid, or a dione or
acrylic group.
In yet another aspect, the present invention is directed to a method for
abrading a
work surface including applying color-stable abrasive article to a work
surface in an abrading
motion to remove a portion of the work surface. The abrasive product includes
a binder
having a phenolic resin; a color-stabilizer that includes at least one
includes at least one
carboxylic acid ester, ester, carboxylic acid, or a dione or acrylic group;
and abrasive grains.
Thus provided are color stabilized phenolic bound abrasives and a method for
making
such abrasives that resist color change over time and upon exposure to high
temperature and
maintain the mechanical strength of a phenolic resin.
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
-3-
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing will be apparent from the following more particular description
of
example embodiments of the invention, as illustrated in the accompanying
drawings in which
like reference characters refer to the same parts throughout the different
views. The drawings
are not necessarily to scale, emphasis instead being placed upon illustrating
embodiments of
the present invention.
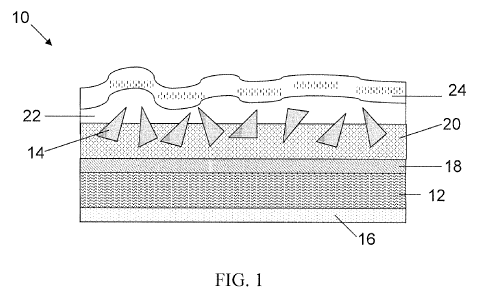
FIG. 1 is a schematic representation of a cross-sectional view of one
embodiment of
coated abrasive tools of the invention.
FIG. 2 is a schematic representation of a cross-sectional view of another
embodiment
of coated abrasive tools of the invention.
FIG. 3 contains photographs comparing cured phenolic resin samples containing
no
color stabilizer and color stabilizers of the present invention.
FIG. 4 contains photographs comparing the color change of an embodiment of the
present invention and a control over time during curing.
1.5 FIG, 5 is a graphical representation of the color change in the samples of
FIG. 4 over
time.
DETAILED DESCRIPTION OF THE INVENTION
The teachings of all patents, published applications and references cited
herein are
incorporated by reference in their entirety. In particular, U.S. Ser. No.
61/199,471, entitled,
"Acrylate Color-Stabilized Phenolic Bound Abrasive Products and Methods for
Making
Same," of Wijaya, which application is filed concurrently herewith, is
incorporated by
reference herein in its entirety.
The present invention relates to abrasive articles which include a phenolic
resin
binder that is color-stabilized by at least one carboxylic acid ester. The
color-stabilized
abrasive article further includes a colorant and abrasive grains. As used
herein, an abrasive
article or resin is considered to be "color-stable" if it has essentially the
same color after
about 8 hours of curing at about 235 OF as it does after about 2 hours of
curing at about 235
OF.
The term "phenolic resin" refers to any resinous reaction product of a phenol,
such as
phenol, resorcinol, alkyl-substituted phenol such as cresol, xylenol, p-tert-
butylphenol, and p-
phenylphenol and the like, with an aldehyde, such as formaldehyde,
acetaldehyde and
furfuraldehyde, and the like. "Color-stabilized alkali metal hydroxide
catalyzed phenolic
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
-4-
resin" refers to a cured alkali metal hydroxide phenolic resin which is color-
stabilized by a
color stabilizing agent.
The abrasive products are characterized by having a phenolic resin binder, one
or
more carboxylic acid ester color-stabilizers, one or more optional colorants,
abrasive grains, a
support member or backing, and can further include curing agents, non-reactive
thermoplastic resins, fillers, grinding aids, and other additives.
Structure and Methods of Making the Abrasive Article
In one embodiment, the color-stable abrasive article includes a phenolic resin
binder,
a color stabilizer, and an abrasive material. The color-stable abrasive
article can be either a
bonded, structured, or coated abrasive.
Coated abrasive tools of the invention can include a substrate, an abrasive
material
and at least one phenolic resin binder to hold the abrasive material to the
substrate. As used
herein, the term "coated abrasive tool" encompasses a nonwoven abrasive tool.
The abrasive
material, such as abrasive grains, particles or agglomerate thereof, can be
present in one layer
(e.g., resin-abrasive layer) or in two layers (e.g., make coat and size coat)
of the coated
abrasive tools. Examples of such coated abrasive tools that can be made by the
methods of
the invention are shown in FIGs. 1 and 2. Referring to FIG. 1, in coated
abrasive tool 10,
substrate 12 is treated with optional backsize coat 16 and optional presize
coat 18.
Overlaying the optional presize coat 18 is make coat 20 to which abrasive
material 14, such
as abrasive grains or particles, are applied. Size coat 22 is optionally
applied over make coat
20 and abrasive material 14. Overlaying size coat 22 is optional supersize
coat 24.
Depending upon their specific applications, coated abrasive tool 10 may or may
not include
backsize coat 16 and/or presize coat 18. Also, depending upon their specific
applications,
coated abrasive tools 10 may or may not include size coat 22 and/or supersize
coat 24.
Shown in FIG. 2 is an example of coated abrasive tools that can be formed by
the methods of
the invention, where coated abrasive tool 30 includes a single layer of an
abrasive material
and adhesive(s) (binder-abrasive layer 32) and optionally backsize coat 16.
Optionally,
presize coat 18, size coat 22 and supersize coat 24, as shown in FIG. 1, can
be included in
coated abrasive tool 30. The coated abrasive article can include a color
stable phenolic resin
binder in at least one layer selected from the group consisting of a binder-
abrasive layer, a
backsize coat, a presize coat, a make coat, a size coat, and a supersize coat.
In embodiments including size coats and supersize coats, such as that shown in
FIG.
1, abrasive materials can be applied separately by gravity, electrostatic
deposition, air stream,
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
-5-
or as a slurry together with the polyurethane adhesive compositions. The make
coat 20
adheres the abrasive material to the surface of the substrate, and can be
formed by
impregnating the support substrate with the phenolic resin binder without
abrasive grains.
In the embodiment of FIG. 2, the support substrate may be impregnated with a
resin/abrasive slurry that includes an abrasive material and a resin
composition including a
phenolic resin binder and a color-stabilizer, to form a binder/abrasive layer
32.
In one embodiment, a method of making a color-stable abrasive article
includes:
blending a resole and a color-stabilizer comprising at least one carboxylic
acid ester to form a
resole composition; contacting a plurality of abrasive particles with the
resole composition;
and curing the resole composition to produce the color-stable abrasive
product. In addition to
the coated abrasives described above, color-stable abrasive articles formed by
this method
include, for example, structured abrasives and bonded abrasives.
With respect to structured abrasives, the article is formed by any of those
techniques
known in the art in which abrasive structures are shaped prior to curing. Such
techniques
include, for example, embossing techniques. In one embodiment, for instance, a
mixture
including a phenolic resin binder, at least one compound selected from the
group consisting
of a carboxylic acid ester, an ester, a carboxylic acid, a compound including
a dione, a
compound including an acrylic group, and combinations of thereof, optional
colorants, and
abrasive grains, can be contacted with a backing and a production tool wherein
the mixture
adheres to one surface of the backing. Abrasive structures are thus formed
that have the
shape of an inside surface of the production tool.
A bonded abrasive article can be formed by preparing an agglomerate that
includes
the phenolic resin binder, at least one compound selected from the group
consisting of a
carboxylic acid ester, an ester, a carboxylic acid, a compound including a
dione group, a
compound including an acrylic group and combinations of thereof, optional
colorants, and
abrasive grains. The agglomerate is then shaped using any of the techniques
known in the art
for preparing a bonded abrasive. Suitable techniques for preparing bonded
abrasives are
further described, for example, in U.S. Patent Nos. 5,738,696 of Wu; 5,738,697
of Wu, et al.;
and 6,679,758 of Bright, et al.; and U.S. Patent Publication No. 2003/0192258
Al of Simon,
the entire contents of each of which are incorporated herein by reference.
A work surface is abraded by applying the color-stable abrasive article to a
work
surface in an abrading motion to remove a portion of the work surface.
Phenolic Resin Binder
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
-6-
Typical phenolic resins employed in the present invention are resoles, which
result
from the alkali metal hydroxide catalyzed reaction of phenol and formaldehyde
in a mole
ratio of phenol: formaldehyde of about 1:1 to about 1:3 moles and a mole ratio
of
phenol:alkali metal hydroxide of about 1:1 to about 100:1. The color of such
resole, or base-
catalyzed phenolic resin is stabilized by the addition of one or more
carboxylic esters. Durez
Varcum Resin No. 94908, manufactured by Durez Corporation is one example of a
water-
based, single-staged liquid phenolic resin that can be used as the binder.
Color- Stabilizer
The color-stabilizers employed in the present invention include carboxylic
acid esters,
esters, carboxylic acids, compounds containing a dione group, compounds
containing an
acrylic group, and combinations of thereof. For example, suitable compounds
include but are
not limited to methyl lactate, ethyl lactate, n-propyl lactate, butyl lactate,
2-ethylhexyl lactate,
heptanoic acid, lactic acid, ethyl acetoacetate, 2,2,5-trimethyl-1-3-dioxane-4-
6-dione, and 2-
(dimethylamino) ethylmethacrylate.
In certain embodiments, the color-stabilizer is present in an amount between
about I
% and about 40 % by weight of the phenolic resin. In other embodiments, the
color-stabilizer
is present in an amount between about 4 % and about 10% by weight of the
phenolic resin.
Ethyl lactate has been found to be particularly effective. For example, one
embodiment of the invention includes:
Ingredient %
Durez 94-908 PF resin 53.89
Ethyl Lactate 2.72
Tamol 165A 0.8
Nalco 2341 0.29
Blue Dye / Pigment 4.81
Syn. Cryolite 37.3
Cab-O-Sil 0.19
Heptanoic acid, lactic acid, ethyl acetoacetate, 2,2,5 -trimethyl- 1 -3 -
dioxane-4-6-dione,
2-(dimethylamino)ethylmethacrylate have demonstrated to be effective color
stabilizer agents
when added in amounts as little as 5% or 10% of the total weight of the resin
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
-7-
Colorant
The abrasive product can include a colorant, for example, dyes or pigments.
Generally, a portion of the colorant can be visible through the cured resin.
In some
embodiments, a portion of the colorant is included in the cured resin, in an
optional support
substrate, and/or in a coating between the optional support substrate and the
cured resin. In
particular embodiments, the colorant can include organic polycyclic dyes,
organic monoazo
dyes, organic diazo dyes, organometal complexes, inorganic pigments such as
metal oxides
or complexes. Dyes can be perinone, anthraquinone, azo dye complexes and
thioindigoid.
A fluorescent colorant is a dye or pigment containing a fluorescent organic
molecule.
Detailed descriptions of fluorescent colorants can be found in Zollinger, H.,
"Color
Chemistry: Synthesis, Properties, and Applications of Organic Dyes and
Pigments", 2rid Ed.,
VCH, New York, 1991, the entire teachings of which are incorporated herein by
reference.
As used herein, a fluorescent colorant can be, for example, a xanthene,
thioxanthene, fluorene
(e.g., fluoresceins, rhodamines, eosines, phloxines, uranines, succineins,
sacchareins,
rosamines, and rhodols), napthylamine, naphthylimide, naphtholactam,
azalactone, methine,
oxazine, thiazine, benzopyran, coumarin, aminoketone, anthraquinone,
isoviolanthrone,
anthrapyridone, pyranine, pyrazolone, benzothiazene, perylene, or
thioindigoid. More
preferably, a fluorescent colorant is selected from the group consisting of
xanthenes,
thioxanthenes, benzopyrans, coumarins, aminoketones, anthraquinones,
isoviolanthrones,
anthrapyridones, pyranines, pyrazolones, benzothiazenes, thioindigoids and
fluorenes. Most
preferably, the fluorescent colorant is a thioxanthene or thioxanthene.
One skilled in the art understands that, for many, commercially available
colorants,
the specific chemical structure of individual derivatives within a class,
e.g., thioxanthene
derivatives, may not be publicly available. Thus, specific fluorescent
colorants are typically
referred to by Colour Index (C.I.) name, as defined in "Colour Index
International", 4th Ed.
American Association of Textile Chemists and Colorists, Research Triangle
Park, NC, 2002.
The Colour Index is also available online at www.colour-index.org. The entire
teachings of
the Colour Index are incorporated herein by reference.
Examples of preferred fluorescent colorants include C.I. Solvent Orange 63
(Hostasol
Red GG, Hoechst AG, Frankfurt, Germany), C.I. Solvent Yellow 98 (Hostasol
Yellow 3G,
Hoechst AG, Frankfurt, Germany), and C.I. Solvent Orange 118 (FL Orange SFR,
Keystone
Aniline Corporation, Chicago, Illinois).
The amount of colorant that can be employed depends on the particulars of the
intended use, the characteristics of the colorant, the other components in the
composition, and
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
-8-
the like. One skilled in the art will know how to judge these details to
determine the amount
of colorant for a particular use. Typically, the amount of colorant will be a
weight fraction of
the total composition of between about 0.01 and about 2%, more preferably
between about
0.05 and about 0.5%, and most preferably, about 0.2%.
In specific embodiments, the colorant is a red, orange, yellow, green, blue,
indigo, or
violet colorant. In specific embodiments, the colorant is fluorescent, for
example, a
fluorescent red, fluorescent orange (blaze orange), fluorescent yellow,
fluorescent green, or
the like.
Examples of suitable colorants include Elcoment Orange GS (Blaze); Elcoment
Green
Nort Liq.; and especially Elcoment Blue RS, all of which are available from
Greenville
Colorants located in Greenville, SC; and Akrosperse E5137, which is available
from
Akrochem Corp. located in Akron, OR
The colorant can be employed to identify the abrasive product, e.g., for
commercial
branding, for usage indication such as wet, dry, wood, metal, or the like, or
for identification
of grit size, or the like.
Abrasive Materials
Abrasive grains can include of any one or a combination of grains, including,
but not
limited to, silica, alumina (fused or sintered), zirconia, zirconia/alumina
oxides, silicon
carbide, garnet, diamond, cubic boron nitride (CBN), silicon nitride, ceria,
titanium dioxide,
titanium diboride, boron carbide, tin oxide, tungsten carbide, titanium
carbide, iron oxide,
chromia, flint, and emery. For example, the abrasive grains may be selected
from a group
consisting of silica, alumina, zirconia, silicon carbide, silicon nitride,
boron nitride, garnet,
diamond, cofused alumina zirconia, ceria, titanium diboride, boron carbide,
flint, emery,
alumina nitride, and a blend thereof. In some instances, dense abrasive grains
comprised
principally of alpha-alumina and/or gamma alumina can be used.
The abrasive grains can also include abrasive agglomerate grains, also known
as
agglomerated abrasive grains. Abrasive agglomerate grains include abrasive
particles
adhered together by a particle binder material. The abrasive particles present
in abrasive
agglomerate grains can include one or more of the abrasives known for use in
abrasive tools
such as, for example, silica, alumina (fused or sintered), zirconia,
zirconia/alumina oxides,
silicon carbide, garnet, diamond, cubic boron nitride (CBN), silicon nitride,
ceria, titanium
dioxide, titanium diboride, boron carbide, tin oxide, tungsten carbide,
titanium carbide, iron
oxide, chromia, flint, emery, and combinations thereof. The abrasive particles
can be of any
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
-9-
size or shape. The abrasive agglomerate grains can be adhered together by a
particle binder
material such as, for example, a metallic, organic, or vitreous material, or a
combination of
such materials. Abrasive agglomerate grains suitable for use in the present
invention are
further described in U.S. Patent No. 6,797,023, to Knapp, et al., the entire
contents of which
are incorporated herein by reference.
The abrasive grains can have one or more particular shapes. Example of such
particular shapes include rods, triangles, pyramids, cones, solid spheres,
hollow spheres and
the like. Alternatively, the abrasive grains can be randomly shaped.
Typically, the abrasive grains have an average grain size not greater than
2000
microns such as, for example, not greater than about 1500 microns. In another
example, the
abrasive grain size is not greater than about 750 microns, such as not greater
than about 350
microns. In some embodiments, the abrasive grain size may be at least 0.1
microns, such as
from about 0.1 microns to about 1500 microns, and, more typically, from about
0.1 microns
to about 200 microns or from about 1 micron to about 100 microns. The grain
size of the
abrasive grains is typically specified to be the longest dimension of the
abrasive grain.
Generally, there is a range distribution of grain sizes. In some instances,
the grain size
distribution is tightly controlled.
Support Member/ Backing
The abrasive articles can include a support member, or backing. The backing
can be
flexible or rigid. The backing can be made of any number of various materials
including
those conventionally used as backings in the manufacture of coated abrasives.
Suitable
backings can include polymeric films (for example, a primed film), such as
polyolefin films
(e.g., polypropylene including biaxially oriented polypropylene), polyester
films (e.g.,
polyethylene terephthalate), polyamide films, or cellulose ester films; metal
foils; meshes;
foams (e.g., natural sponge material or polyurethane foam); cloth (e.g.,
woven, non-woven,
fleeced, stitch bonded, or quilted, or cloth made from fibers or yams
comprising polyester,
nylon, silk, cotton, poly-cotton or rayon); paper; vulcanized paper;
vulcanized rubber;
vulcanized fiber; nonwoven materials; a treated backing thereof; or any
combination thereof.
The backing can have at least one of a saturant, a presize layer or a backsize
layer.
The purpose of these layers typically is to seal the backing or to protect
yarn or fibers in the
backing. If the backing is a cloth material, at least one of these layers
typically is used. The
addition of the presize layer or backsize layer may additionally result in a
"smoother" surface
on either the front or the back side of the backing. Other optional layers
known in the art can
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
- 10-
also be used (for example, a tie layer; see U.S. Patent No. 5,700,302 of
Stoetzel, et al., the
entire contents of which are incorporated herein by reference).
In some embodiments, the abrasive articles are intended for use as fine
grinding
materials and hence a very smooth surface can be preferred. Examples of such
smooth
surfaced backings include finely calendered papers, plastic films or fabrics
with smooth
surface coatings.
The backing can have antistatic properties. The addition of an antistatic
material can
reduce the tendency of the abrasive article to accumulate static electricity
when sanding
wood or wood-like materials. Additional details regarding antistatic backings
and backing
treatments can be found in, for example, U.S. Patent Nos. 5,108,463 of
Buchanan, et al.;
5,137,542 of Buchanan, et al.; 5,328,716 of Buchanan; and 5,560,753 of
Buchanan, et al., the
entire contents of which are incorporated herein by reference.
The backing can include a fibrous reinforced thermoplastic such as described,
for
example, in U.S. Patent No. 5,417,726 of Stout, et al., or an endless
spliceless belt, as
described, for example, in U.S. Patent No. 5,573,619 of Benedict, et al., the
entire contents of
which are incorporated herein by reference. Likewise, the backing can include
a polymeric
substrate having hooking stems projecting therefrom such as that described,
for example, in
U.S. Patent No. 5,505,747 of Chesley, et al., the entire contents of which are
incorporated
herein by reference. Similarly, the backing can include a loop fabric such as
that described,
for example, in U.S. Patent No. 5,565,011 of Follett, et al., the entire
contents of which are
incorporated herein by reference.
Other Components
The abrasive articles of the present invention can also include various other
components, such as curing additives, non-reactive thermoplastic resins,
fillers, grinding aids;
and other additives.
In some embodiments, the abrasive article includes a curing additive, such as
a
photoinitiator, which generates free radicals when exposed to radiation, e.g.,
UV radiation.
Free-radical generators can include organic peroxides, azo compounds,
quinones,
benzophenones, nitroso compounds, acryl halides, hydrozones, mercapto
compounds,
pyrylium compounds, triacrylimidazoles, bisimidazoles, chloroalkyltriazines,
benzoin ethers,
benzil ketals, thioxanthones and acetophenones, including derivatives of such
compounds.
Among these the most commonly employed photoinitiators are the benzil ketals
such as 2,2-
dimethoxy-2-phenyl acetophenone (available from Ciba Specialty Chemicals under
the
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
-11-
trademark IRGACURE 651) and acetophenone derivatives such as 2,2-
diethoxyacetophenone ("DEAP", which is commercially available from First
Chemical
Corporation), 2-hydroxy-2-methyl- l -phenyl-propan- l -one ("HMPP," which is
commercially
available from Ciba Specialty Chemicals under the trademark DAROCUR" 1173), 2-
benzyl-
2-N,N-dimethylamino- l -(4-morpholinophenyl)-1-butanone, (which is
commercially
available from Ciba Specialty Chemicals under the trademark IRGACURE 369);
and 2-
methyl-l-(4-(methylthio)phenyl)-2-morpholinopropan-l-one, (available from Ciba
Specialty
Chemicals under the trademark IRGACURE 907).
The abrasive articles can include a non-reactive thermoplastic resin such as,
for
example, polypropylene glycol, polyethylene glycol, and polyoxypropylene-
polyoxyethene
block copolymer.
Fillers include organic fillers, inorganic fillers, and nano-fillers. Examples
of suitable
fillers include, but are not limited to, metal carbonates such as calcium
carbonate and sodium
carbonate; silicas such as quartz, glass beads, glass bubbles; silicates such
as talc, clays,
calcium metasilicate; metal sulfate such as barium sulfate, calcium sulfate,
aluminum sulfate;
metal oxides such as calcium oxide, aluminum oxide; aluminum trihydrate, and
combinations
thereof.
The abrasive articles can include a grinding aid to increase the grinding
efficiency and
cut rate. Useful grinding aids can be inorganic, such as halide salts, e.g.,
sodium cryolite and
potassium tetrafluoroborate; or organic based, such as chlorinated waxes,
e.g., polyvinyl
chloride. In one particular embodiment, the abrasive article includes cryolite
and potassium
tetrafluoroborate with particle size ranging from about 1 micron to about 80
microns, most
typically from about 5 microns to about 30 microns. The concentration of
grinding aid in a
make coat is generally not greater than about 50 wt%, for example, the
concentration of
grinding aid is often about 0.1 wt% to 50 wt%, and most typically about 10 wt%
to 30 wt%
(all wt% based on make coat weight including abrasive grains).
Examples of additional additives include coupling agents, such as silane
coupling
agents, e.g., A-174 and A-1100 available from Osi Specialties, Inc., titanate,
and
zircoalurminates; anti-static agents, such as graphite, carbon black, and the
like; suspending
agent, such as fumed silica, e.g., Cab-O-Sil M5, Aerosil 200; anti-loading
agents such as zinc
stearate and calcium stearate; lubricants such as wax, PTFE powder,
polyethylene glycol,
polypropylene glycol, and polysiloxanes; wetting agents; pigments;
dispersants; and
defoamers.
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
-12-
EXEMPLIFICATION
Example 1
The invention will now be further and specifically described by the following
examples which are not intended to be limiting.
The following compounds were tested for performance as color stabilizer
agents:
heptanoic acid, lactic acid, ethyl acetoacetate, 2,2,5-trimethyl-1-3-dioxane-4-
6-dione, and 2-
(dimethylamino)ethylmethacrylate. Formulations containing neat phenolic resin
and the
color stabilizer agent (between 4% and 10% per resin weight) were prepared and
cured at
180 C for 12 hours; heptanoic acid was cured at 250 F for 6 hours. The cured
samples are
shown in Fig. 3.
Example 2
Ethyl lactate was tested as a color stabilizer agent in a phenolic resin size
coat
formulation and compared to a control formulation that did not includes ethyl
lactate. The
formulations are described below:
Control Size Formulation (considered "non-color stable")
Ingredient %
Durez 94-908 PF resin 55.39
Tamol 165A 0.81
Nalco 2341 0.32
Blue Dye / Pigment 4.94
Syn. Cryolite 38.34
Cab-O-Sil 0.20
Color Stable Size Formulation (includes Ethyl lactate)
Ingredient %
Durez 94-908 PF resin 53.89
Ethyl Lactate 2.72
Tamol 165A 0.8
Nalco 2341 0.29
Blue Dye / Pigment 4.81
Syn. Cryolite 37.3
Cab-O-Sil 0.19
CA 02743858 2011-05-16
WO 2010/057075 PCT/US2009/064546
- 13 -
Both of these formulations were applied to a pre-made coated abrasive sample.
The
samples included a backing on which a make coat and grain had been coated and
cured to the
normal extent before a typical size coat would be applied during a typical
manufacturing
process.
After the two size coats had been applied to the pre-made coated abrasive and
given a
standard size cure , 5 samples of each were placed into an oven at 235 F and a
single sample
was pulled from the oven after 2, 4, 6, 8 and 24 hours of dwell time to
emulate a "post cure"
process. The visual comparison of the color shift is shown in Fig. 4.
In addition to this qualitative comparison of color shift, a more quantitative
measurement was made using a MiniScan XE Plus Colorimeter supplied by
HunterLab, the
results of which are provided in Fig. 5.
Fig. 5 shows the reduction in total color shift, delta E, with the use of
ethyl lactate.
Typical processing conditions of post cure would be between 4 and 8 hours. In
this case, the
observable delta E for the non-color stable size during this time period
increased from 11.14
to 17.18, yielding a difference of 6.04. With the use of ethyl lactate at 5%
by weight of the
resin, the delta E increased only from 6.12 to 8.31, yielding a 2.19
difference, thus showing a
significant reduction in color shift.
Equivalents
While this invention has been particularly shown and described with references
to
example embodiments thereof, it will be understood by those skilled in the art
that various
changes in form and details may be made therein without departing from the
scope of the
invention encompassed by the appended claims.