Note: Descriptions are shown in the official language in which they were submitted.
CA 02744559 2016-01-15
WO 2010/062297
PCT/US2009/004482
REGENERATIVE TISSUE GRAFTS COMPRISING A SCAFFOLD
LOADED WITH MESENCHYMAL PROGENITOR CELLS
[0001] Background
[0002] Debridement of contaminated and devitalized tissue is the first step
in the surgical treatment of open extremity injuries. This event is often part
of a process comprising serial debridements over the span of several days to
fully assess the viability of the remaining tissue.
[0003] Penetrating trauma results in substantial bone and soft tissue loss
due to the primary injury and the debridement process. For example, as a
projectile or blast wave penetrates the skin, it transfers kinetic energy to
the
surrounding structures, which include bone, muscle, tendon, cartilage, and
fat. Jussila J. Measurement of kinetic energy dissipation with gelatine
fissure formation with special reference to gelatine validation. Forensic Sc!
Int. 2005, 150: 53-62. This energy is absorbed in the form of heat,
mechanical stress, and chemical stress, and it initiates a number of events,
including cell necrosis, apoptosis, and inflammation. Jussila J., Forensic Sc!
Int., 150: 53-62; and Jussila J, et al, Ballistic variables and tissue
devitalisation in penetrating injury¨establishing relationship through meta-
analysis of a number of pig tests. Injury. 2005, 36:282-92.
[0004] While much of the initial damage is largely the result of necrosis
and can be seen within the first twenty-four hours, delayed tissue death can
result from induced programmed cell death or vascular compromise and may
not be apparent for several days after the initial event. Williams AJ, et al,
Penetrating ballistic-like brain injury in the rat: differential time courses
of
hemorrhage, cell death, inflammation, and remote degeneration, J
Neurotrauma, 2006, 23:1828-46. Thus, the serial tissue debridement
protocol is necessary to avoid premature wound closure and to minimize the
amount of retained devitalized tissue.
[0005] After debridement, the tissues are reassessed and definitive
treatment is planned. The degree and nature of tissue loss determine the
need for tissue-grafting or tissue substitutes that are often derived from
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
allograft or synthetic sources. After fracture fixation and closure or
coverage
of open wounds, revision and reconstructive surgery is frequently required to
restore the function of the injured extremity. In many instances, this may
require bone and soft-tissue augmentation, lysis of adhesions (about joints
and along tendons), and/or ligament reconstruction. In most cases, revision
surgery stems from a need to repair or replace absent, damaged, or
deranged tissues such as articular cartilage, tendon, and/or bone with use of
autograft, allograft, bioengineered tissue replacement, or prosthetic
materials
and devices. Unfortunately, these options for tissue repair or replacement
are limited by the inability of the implant to fully integrate and
subsequently
remodel. In addition, the inferior structural, biomechanical, and biochemical
properties of the implant as compared with normal human tissue prevent full
restoration of the structure-function relationship.
[0006] An essential component of all tissue- engineering construct designs
is a readily available, viable, and plastic cell source. Many sources of
multipotent progenitor cells (e.g., bone marrow, trabecular bone, adipose
tissue, umbilical cord blood, and synovial tissue), which yield cells that
have
varying degrees of regenerative potential and that can be expanded in vitro,
have been described. Caterson EJ, et al, Human marrow-derived
mesenchymal progenitor cells: isolation, culture expansion, and analysis of
differentiation. Mol Biotechnol., 2002, 20:245-56; Noth U, et al, Multilineage
mesenchymal differentiation potential of human trabecular bone-derived
cells. J Orthop Res., 2002, 20:1060-9; Flynn A, et al, UC blood-derived
mesenchymal stromal cells: an overview. Cytotherapy. 2007, 9:717-26;
Boquest AC, et al, Epigenetic programming of mesenchymal stem cells from
human adipose tissue, Stem Cell Rev., 2006, 2:319-29; Koga H, et al,
Syno vial stem cells are regionally specified according to local
microenvironments after implantation for cartilage regeneration, Stem Cells,
2007, 25:689-96. However, these tissue types may not be readily available
as a source of autologous multipotent cells at the time of musculoskeletal
trauma.
- 2 -
CA 02744559 2016-01-15
WO 2010/062297
PCTIUS2009/004482
[0007] Adult stem cells are a useful clinical resource to enhance many
healing processes. One limitation, however, is the lack of availability of
one's own adult stem cells without invasive surgical procedures.
[0008] Peripheral nerve injury frequently accompanies musculoskeletal
trauma, which lengthens the recovery time and leads to significant
dysfunction. Current treatment of peripheral nerve injuries includes primary
repair, nerve autograft, or use of synthetic nerve tubes. The success of
nerve repair depends primarily on the speed of axonal growth and
myelination to bridge the damaged region and decrease the time to end
organ re-innervation. Lee SK, eta!, Peripheral nerve injury and repair, J Am
Aced Otthop Surg. 8, 243, 2000.
[0009] Conventional nerve tubes contain a single lumen to guide the
regenerating nerve from proximal to distal stump. Although increasing the
likelihood that some axons in the nerve will reconnect with the distal end,
many are unable to reconnect, and gaps larger than critical size defect are
likely never to regenerate.
[0010] Summary of the Invention
[0011] Mesenchymal progenitor cells (MPCs) are found in traumatized
tissue. MPCs share characteristic features of mesenchymal stem cells
(MSCs). Nesti LJ, et al. Differentiation potential of multipotent progenitor
cells derived from war-traumatized muscle, J. Bone Joint Surg. Am., 90
2390-98 (2008).
[0012] Based upon our further observation and work with MSCs, we have
discovered new methods for collecting and/or isolating MPCs from
musculoskeletal wound tissue; and we have devised various therapeutic
devices and methods for employing MPCs in regenerative medicine.
[0013] We present a method of harvesting mesenchymal progenitor cells
(MPCs) as an alternative source of cells from debrided muscle tissue
following orthopaedic trauma. MPCs offer advantages over MSCs of being
more plentiful (particularly in wound tissue), more easily obtained, and
- 3 -
CA 02744559 2016-01-15
WO 2010/062297
PCT/US2009/004482
capable of producing substantial quantity of various trophic factors,
including
neurotrophic factors, vasculotrophic factors, and osteotrophic factors. MPCs
have the added advantage of diminishing inflammation and scar formation.
[0014] Also disclosed herein is a new way of using MPCs in the tratment
of various tissue injury or disease states. By positioning MPCs near the
injury or defect, and in fluid contact with the injury or defect, the MPCs can
be used as an in situ or in vivo source of various biologically significant
and
therapeutically effective factors, e.g., growth factors and differentiation
inducing factors that promote regeneration and/or healing. The MPCs are
not consumed, nor are they completely differentiated in the process.
Jackson, W.M. et al., "Putative Heterotopic Ossification Progenitor Cells
Derived from Traumatized Muscle", J. Orthopaedic Res. (June 10, 2009)
(www.interscience.wiley.com; DOI 10.1002/jor.20924). Rather, they
substantially remain as MPCs and continue to secrete trophic factors and
promote differentiation of other cell types to regenerate the damaged tissue
for a considerable time. in vitro studies show that MPCs continue to secrete
trophic factors when the MPCs were at passage 2-3, which corresponds
roughly to 12-15 population doublings, or approximately 3-4 weeks after
harvest. MPCs likely continue to express trophic factors as long as they
remain in an undifferentiatied state.
[0015] The grafts of the present invention exploit unique properties of
MPCs, i.e., in a wound setting they remain in an undifferentiated state for a
prolonged period, and during that time secrete trophic factors that aid in the
regeneration of nerve, bone, vasculature and the like. The grafts comprise:
1) a structural element that may be referred to herein as a scaffold, and 2) a
therapeutic element. The scaffold may be structured in the form of a
conduit, wrap, patch, or the like. In some embodiments, the scaffold
includes at least a first component that is a porous matrix. The porous
matrix can be in the form of a woven or non-woven material, including
natural or synthetic fibers. Among other things, the matrix serves as a
reservoir or repository for a therapeutic component. As discussed more
below, the scaffold may further comprise a core of aligned fibers or a conduit
- 4 -
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
interior to and in fluid communication with the matrix, and/or a dam or
substantially fluid impermeable sheath exterior to the matrix. The
therapeutic component may include a cellular component such as MPCs.
Additionally, the therapeutic component may include other therapeutic
elements such as small molecule active agents commonly used to aid
healing or fight infection. The matrix retains the therapeutic component such
as the MPCs, but permits fluid flow within and through the matrix. The MPC-
seeded matrix may be placed proximate to the injury or defect, and in fluid
contact with the injury or defect.
[0016] As used herein, the term matrix is used to refer to a porous material
within which MPCs, and perhaps other cells or therapeutic materials, may be
infused and retained. The matrix material may be natural or synthetic, and
may be additionally treated with substances to enhance retention of cells,
e.g., treatment of polymeric material with hyaluronic acid. While it is
desirable to retain MPCs and the like within the matrix, it is to be
understood
that some migration will occur, both to the interior and exterior of the
various
grafts. The porosity and retention capacity of the matrix can be varied
according to known methods depending on the intended application, the
type of graft employed, the type of tissue under treatment, and the severity
of the injury or defect.
[0017] A second scaffold component may be added to the graft. One such
element provides structural support for tissue regeneration and/or isolation
and protection of the injury or defect. The second component can be a
structural element serving as a guide or framework on which the regenerated
tissue can form, or it can be a structural element that creates and/or retains
a void in or around the defect, or otherwise supports and/or retains the
damaged tissue as it is to be reformed so as to permit the tissue to
regenerate and return to its original dimension and shape. As used herein,
the term "tissue" includes nerve, bone, and vascular tissue.
[0018] These grafts may also comprise an element exterior to the matrix to
isolate and/or concentrate the MPCs and the various factors produced, and
to avoid loss or migration of other regenerative elements such as other cell
- 5 -
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
types. The exterior element can be fabricated to match the overall structure
of the scaffold, e.g., conduit, wrap, or patch. The material used in all of
the
foregoing elements may be the same or different, and may be fabricated to
afford differing levels of porosity. The exterior element can be fabricated to
be substantially or completely non-porous. The exterior element, which may
function as a dam or fluid retention device to protect and isolate therapeutic
components, can also be formed of biocompatible nanofibers, and created to
varying levels of porosity. Thus, for example, the dam can be made
substantially or entirely fluid impermeable; or it can be made to be
permeable but less so than the matrix.
[0019] MPCs express neurotrophic factors (e.g., BDNF, CTNF, NT-3),
which encourage axonal growth and nerve regeneration. Since nerve
damage frequently occurs in orthopaedic injury, the traumatized muscle-
derived progenitor cells are a readily available autologous cell source that
can be utilized to effect or improve nerve repair. MPCs may be used quite
effectively in a nerve graft.
[0020] We provide here a device for regenerating injured or damaged
nerve, and for enhancing the rate of axonal growth. One such device
incorporates a composite of nanofiber structures, and wherein the fibers are
used to construct two zones or chambers within the nerve graft conduit. In
the interior of the graft is a core of aligned fibers along the axis of
symmetry
through the longitudinal axis of symmetry of the graft. An exterior sheath
surrounding the core is fabricated of randomly non-aligned fibers to serve as
a matrix for seeding and supporting the MPCs, which promotes the activity of
the MPCs and other endogenous neuronal support cells (e.g., Schwann
Cells can migrate into the graft).
[0021] Also disclosed is a method of fabricating a novel peripheral nerve
graft to include a core of aligned nanometer-scale fibers, and an outer
sheath to support and retain the MPCs proximate to the wound or injury
following implantation of the device. The aligned fibers guide the
regeneration of individual neurons of the nerve.
- 6 -
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
[0022] In one embodiment, MPCs are loaded into a nerve graft in the form
of a tissue engineered peripheral nerve conduit. One such conduit includes
a porous matrix in the form of an outer sheath. MPCs are seeded within the
matrix. Trophic factors produced by MPCs are secreted within close
proximity of the injured nerve tissue thereby promoting healing and
differentiation of other cell types to regenerate the damaged tissue. The
outer sheath surrounds an interior area of the conduit. The interior area may
be a void within which the nerve regeneration is afforded space to reconnect
the proximal and distal ends of the nerve stub, or it may comprise a core of
aligned nanometer-scale fibers that guide the regeneration of individual
neurons of the nerve. The aligned fibers may be generally linear along an
axis of symmetry running longitudinally through the conduit. Trophic factors
produced by MPCs diffuse to the region of the aligned fibers, and promote
regeneration of the nerve along those fibers.
[0023] In one embodiment of the invention, there is a nerve graft, and
methods of making same, wherein the graft comprises a nerve tube having a
central region or sheath filled with aligned fibers (also referred to herein
as
"nanofibers"). As used herein, the term nanofibers refers to fibers of about
0.05-0.5 pm in diameter; or about 0.1-0.3 pm in diameter; or 0.2 pm in
diameter.
[0024] In one embodiment, the nerve tube comprises a composite of
electrospun fibers, both linear (e.g., at the inner sheath) and nonlinear
(e.g.,
at the outer sheath). The nerve tube device promotes axonal growth along
the linear, aligned fibers aided by neurotrophic factors secreted by support
cells seeded in the support matrix. In one embodiment, the nerve tube is
fabricated by a novel two step electrospinning process. The novel
electrospinning process can produce electrospun fibers on a non-conductive
mandrel.
[0025] Axonal nerve tubes have previously been used to guide the
regeneration of damaged nerve. Commercially available nerve tubes consist
of collagen, or a similar biological or biocompatible polymer, in the shape of
a hollow tube that is telescoped over the ends of the damaged nerve
- 7 -
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
stumps. The limitation of these devices is related to the speed of the axonal
growth through the hollow interior of the tube. If the axons do not reconnect
with the distal stump within several weeks, the nerve cannot be regenerated.
A device that increases rate of axonal growth will bridge longer nerve gaps
during axonal growth. The devices and methods disclosed herein facilitate
functional regeneration for a wide range of nerve injuries, both in terms of
length of damaged nerve and in speed of regeneration.
[0026] Also disclosed herein is a method of harvesting MPCs from
traumatized muscle; and isolated MPCs free of fat, fascia, bone, muscle
tissue, necrotic tissue, and other cell types associated with musculoskeletal
wounds. MPCs express neurotrophic factors (e.g., BDNF, CTNF, NT-3).
The neurotrophic factors stimulate and promote axonal growth and nerve
regeneration by increasing the rate of axonal outgrowth. Since nerve
damage frequently occurs in orthopaedic injury, the traumatized muscle-
derived progenitor cells are a readily available autologous cell source that
improve nerve repair. This can be achieved by loading MPCs into a tissue
engineered peripheral nerve conduit (nerve tube) as described elsewhere
herein.
[0027] Among other things, the present invention affords means and
devices for using a new cell type (MPC) found in traumatized muscle tissue.
MPCs possess potent regenerative properties. They can be harvested in
high numbers directly from traumatized muscle tissue, and can be employed
immediately in various therapies for effecting nerve regeneration. MPCs
afford an autologous resource for therapeutic material useful in various new
and known therapies. By affording an autologous resource, the risk of
allogenic response in a patient is dramatically reduced, if not eliminated.
[0028] Brief Description of the Drawings
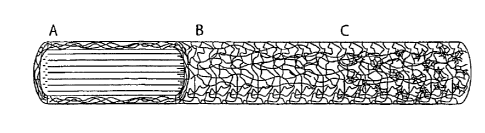
[0029] Figure 1: Is a schematic of the peripheral nerve guide. (A) Aligned
nanofibers for the core of the device guide and support the growing axons.
(B) A non-aligned sheath bundles the aligned fibers and provides support for
- 8 -
CA 02744559 2016-01-15
WO 2010/062297
PCT/US2009/004482
(C) mesenchymal progenitor cells, which provide biochemical factors to
enhance nerve regeneration.
[0030] Figure 2: One method of fabricating the device. (A) In an
electrospinning setup, polymer fibers are extruded onto a grounding plate.
(B) Two grounded axels are aligned end-on with the aligned nanofibers
between them. The entire assembly is rotated as a mandrel, and the
extruded nanofibers are attracted to the axels. As the extruded fibers
stochastically switch their target between the two targets, the aligned
nanofibers become encased in a non-aligned sheath.
[0031] Figure 3: Illustrates devices useful in MPC isolation in the form of
tubes having a tissue grinder for structural degradation of muscle tissue from
wound (Tube 1); and a tube having shear channels and a cell strainer (Tube
2).
[0032] Figure 4: Osteogenic gene expression profile. A: The differential
gene expression of 84 genes related to osteogenesis in MPCs compared to
MSCs cultured in growth medium.
Circles (0) represents RUNX2,
squares (s) represent ALP and diamonds (*) represent BGLAP
(Osteocalcin) expression. Genes differentially expressed significance
p<0.018 (Student's t-test with n=3) are drawn inside a box in the plot.
[0033] Figure 5: MSC gene expression profile. The differential gene
expression of 84 genes related to MSC Biology in MPCs compared to MSCs.
Genes differentially expressed significance p<0.05 (Student's t-test with n=3)
are labeled.
[0034] Figure 6: lmmunophenotyping of MPCs. A: The MPCs were
positive for CD44, CD49e, CD73, CD90 and CD105 and negative for CD14,
CD31, CD34 and CD45. The fluorescence intensity of each marker (black
lines) compared to the isotype control (grey lines). B: The fluorescence
intensity of each cell-surface marker was normalized against the
fluorescence intensity of CD73. The CD105/CD73 ratio was significantly
greater for MPCs than bone-marrow derived MSCs (p = 0.01). tThe
- 9 -
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
CD90/CD73 ratios are shown scaled by a factor of 10 and the values
correspond to the right axis.
[0035] Figure 7: MPC Neurotrophic Factor Expression. A. RT-PCR
analysis of neurotrophic factor gene expression in traumatized muscle
MPCs. B. Protein level measurement of neurotrophic factor production in
traumatized muscle MPCs.
[0036] Figure 8: Gene expression of trophic factors secreted by MPCs
and MSCs.
[0037] Figure 9: Protein-level expression of VEGFA in MPCs and MSCs.
[0038] Figure 10: Protein-level activity of MMPs, via gelatin zymography,
in MPCs and MSCs.
[0039] Figure 11: Illustrates a conditioned media experiment, wherein
progenitor cells were cultured in either growth or neurotrophic induction
medium. For both media types, medium that was conditioned by the MPCs
resulted in a higher density of neurite extensions compared to the
corresponding no cell controls.
[0040] Figure 12: Endothelial cell proliferation in MPC vs. MSC
conditioned media.
[0041] Figure 13: Suppression of inflammatory response by MPC vs.
MSC secreted trophic factors. The data is represented as a percentage of
the T-cell proliferation in the positive control sample that was not
conditioned
by the MPCs factors. Bone marrow MSC lmmunosuppression is also
represented for comparison.
[0042] Figures 14: Peripheral Nerve Graft. Figure 14(a): Electron
rnicrographs of composite scaffold (1) interior core of aligned nanofibers,
(2)
surrounding non-aligned fibers, (3) non-aligned fibers seeded with MPCs;
14(b): Viability of seeded cells in graft in GM and NM; 14(c):BDNF
production of MPCs in GM and NM; and 14(d): Cross section of BDNF-
secreting MPCs in peripheral nerve graft.
- 10 -
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
[0043] Detailed Description
[0044] Debrided muscle contains multipotent cells useful in cell-based
tissue-engineering therapies and studies. Following a modified stem cell
isolation protocol on tissues obtained at surgery, we obtained viable cells
expressing markers characteristic of mesenchymal stem cells. See, e.g.,
Chamberlain G, et al, Concise review: mesenchymal stem cells: their
phenotype, differentiation capacity, immunological features, and potential for
homing, Stem Cells, 2007, 25:2739-49; Baksh D, et al, Comparison of
proliferative and multilineage differentiation potential of human mesenchymal
stem cells derived from umbilical cord and bone marrow, Stem Cells, 2007,
25:1384-92. We defined these cells as mesenchymal progenitor cells, or
MPCs.
[0045] These mesenchymal progenitor cells were culture-expanded, and
they exhibited multipotentiality (adipogenic, osteogenic, and chondrogenic)
on appropriate induction. These MPCs can be used in the initial reparative
process or in future reconstructive operations in combination with
appropriate tissue-engineering biomaterial scaffolds.
[0046] These studies are particularly significant in that they were
performed not on animal models but human tissues and cells derived from
traumatized muscle. Also, the histological evidence of differentiation was
compared with differentiated bone-marrow-derived mesenchymal stem cells,
a well-characterized cell type with known multiple differentiation potential.
Finally, multiple assays were performed to verify the multipotent
differentiation activities of the traumatized muscle-derived MPCs.
[0047] The differentiation assays were corroborated by the expression of
corresponding adipogenic, osteogenic, and chondrogenic lineage-specific
genes. On the basis of these findings, we have verified that muscle-derived
MPCs have the potential to differentiate into osteoblasts, adipocytes, and
chondrocytes.
[0048] MPCs derived from traumatized muscle require substantial
characterization in terms of their origin within the body and their
relationship
to better-characterized stem cell types. The MPCs may originally reside in
-11-
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
the nontraumatized muscle tissue in a quiescent state (i.e., as pericytes)
(Bianco P, et al, Mesenchymal stem cells: revisiting histoty, concepts, and
assays, Cell Stem Cell., 2008, 2:313-9), or they may have migrated from the
bone marrow to the site of injury in response to wound-healing signals.
Kumagai K, et al, Circulating cells with osteogenic potential are
physiologically mobilized into the fracture healing site in the parabiotic
mice
model. J Orthop Res. 2008;26:165-75; Friedenstein AJ, et al, Osteogenesis
in transplants of bone marrow cells. J Embryo! Exp Morphol. 1966,16:381-
90.
[0049] Initial plating of the MPCs yielded a greater number of tissue-
adherent cells than is typically reported for progenitor cell populations.
Without being bound by any theory, this might have been due to a lower
overall cellularity and a higher percentage of MPCs relative to other
erythroid
or mononuclear cell types in traumatized muscle compared with other
sources. General characteristics of the Mesenchymal progenitor cells, such
as the associated cell-surface markers, prolonged culture-expansion
capabilities, and multidifferentiation potential, are characteristic features
of
mesenchymal stem cells. Chamberlain G, et al., Concise review:
mesenchymal stem cells: their phenotype, differentiation capacity,
immunological features, and potential for homing. Stem Cells.
2007;25:2739-49; Dominici M, et al, Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular Therapy
position statement. Cytotherapy. 2006;8:315-7; Yoon YS, et al, Clonally
expanded novel multipotent stem cells from human bone marrow regenerate
myocardium after myocardial infarction. J Clin Invest. 2005;115:326-38;
Pang Y, et al, Quantitative study of tissue-engineered cartilage with human
bone marrow mesenchymal stem cells, Arch Facial Plast Surg., 2005, 7:7-
11.
[0050] One difference in the gene-expression profile of differentiated
MPCs has been noted in the present study. PPARy2, which is an indicator
of adipogenic differentiation in bone marrow-derived mesenchymal stem
cells, is also upregulated by osteogenic induction of traumatized muscle-
- 12-
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
derived mesenchymal progenitor cells. The effect of PPARy2 on the MPCs
does not appear to be anti-osteogenic as there is strong evidence from the
histological and gene-expression findings that cells underwent osteogenic
induction. In fact, the regulatory pathways governing PPARy2 activity can
modulate its anti-osteogenic function (Lecka-Czemik B, et al, Divergent
effects of selective peroxisome proliferator-activated receptor gamma 2
ligands on adipocyte versus osteoblast differentiation, Endocrinology, 2002,
143:2376-84) and may represent a tissue-specific feature of regenerative
cells that are present in muscle tissue.
[0051] Surgical debridement of open wounds is a medical and surgical
necessity. Although removal of tissue from wounds that are characterized
by substantial tissue loss is counterintuitive, it is essential for definitive
treatment, wound closure, and proper healing. Granick M, et al, Toward a
common language: surgical wound bed preparation and debridement.
Wound Repair Regen, 2006, 14 Suppl 1:S1-10; Gregory P, et al, The
management of severe fractures of the lower extremities. Clin Orthop Re/at
Res. 1995;318:95-105; Jacob E, et al, A retrospective analysis of open
fractures sustained by U.S. military personnel during Operation Just Cause.
Mil Med., 1992, /57:552-6. The results of the present study suggest that this
waste tissue may possess cellular building blocks that might be useful in
future treatment and tissue-regeneration strategies. Although MPCs have
been theorized to occupy traumatized muscle and their presence has been
demonstrated in animal models, to our knowledge, the present report is the
first to describe and characterize these cells in human tissues.
[0052] Qu-Petersen and colleagues described the presence of a
population of progenitor cells obtained from skeletal muscle in a mouse
model that exhibited characteristics similar to, but distinct from,
mesenchymal stem cells. Qu-Petersen Z, et al, Identification of a novel
population of muscle stem cells in mice: potential for muscle regeneration. J
Cell Biol. 2002, 157:851-64. They isolated a cell population by a preplating
technique, which selects for the least-adherent cell population after a series
of six serial platings. With this method, we were unable to obtain
-13-
CA 02744559 2016-01-15
WO 2010/062297
PCT/US2009/004482
multiprogenitor cells from human tissue samples, and the cells that were
obtained differed significantly from the multiprogenitor cells described here.
Instead, we selected the most adherent cells two hours after initial plating
and expanded the isolated cells in a culture medium identical to that used for
bone marrow derived mesenchymal stem cells. The MDSCs identified by
Qu-Peterson et al. were identified primarily on their ability to undergo
myogenic differentiation and they express myogenic specific markers, e.g.,
MYOD, MCAM, desmin, etc. In contrast, MPCs do not undergo myogenic
differentiation, nor do they express myogenic specific markers. MPCs and
MSCs can be differentiated from each other based on their gene expression
profiles. Jackson, W.M. et al., "Putative Heterotopic Ossification Progenitor
Cells Derived from Traumatized Muscle," J. Orthopaedic Res. (June 10,
2009) (www.interscience.wiley.com; DOI 10.1002/jor.20924). For example,
relative to MSCs, MPCs express significantly greater levels of TGFB3; MPCs
continue to proliferate while being induced to differentiate into osteoblasts,
and express lower levels of osteocalcin, an osteoblastic gene that is
expressed during later stages of osteogenic differentiation. Additionally,
there are differences in the osteogenic gene expression profile between the
MPCs and MSCs, which may reflect the tissue of origin for both cell types.
MPCs express higher levels of COL15A1, a gene associated with muscle
tissue development, and GDF10, shown to be a negative regulator of
osteogenesis, whereas the bone-marrow derived MSCs express higher
levels of genes associated with bone physiology and maintenance: VEGFA
17, VCAM1 18 and IGF2 19. These differences may also reflect the fact that
traumatized muscle-derived MPCs are harvested from an active wound bed,
where they likely participate in the process of muscle tissue repair. During
osteogenic differentiation, COL15A1 and GDF10 are substantially down-
regulated, while VEGFA, VCAM1 and IGF2 are similarly up-regulated,
suggesting that the MPCs can assume the role of osteoprogenitors under
the appropriate biological environment, in a manner similar to other
populations of MSCs.
- 14 -
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
[0053] The traumatized muscle-derived MPCs are of particular benefit in
blast trauma-induced injuries given the high prevalence of heterotopic
ossification associated with such injuries. E.g., Potter BK, et al.,
Heterotopic
ossification following traumatic and combat-related amputations. Prevalence,
risk factors, and preliminary results of excision, J Bone Joint Surg Am. 2007,
89:476-86. The osteogenic potential of these cells suggests their possible
role in pathological processes that result in ectopic bone formation. Granick
M, et al, Toward a common language: surgical wound bed preparation and
debridement, Wound Repair Regen. 2006, 14 Suppl 1:S1-10.
[0054] Traumatized muscle tissue contains MPCs that can be harvested
and expanded in vitro. This cell type may be used in reconstructive efforts or
in cell-based tissue-engineered constructs for bone, tendon, cartilage, and
fat. Multipotent adult stem cells are already being employed for orthopaedic
reconstructive procedures; for example, bone-marrow aspirates have been
used to augment bone defects, and intraoperative isolation systems have
been used to augment fracture fixation and spine fusions with additional
mesenchymal stem cells. Kumagai K, et al, Circulating cells with osteogenic
potential are physiologically mobilized into the fracture healing site in the
parabiotic mice model, J Orthop Res. 2008;26:165-75; Muschler GF, etal.
Selective retention of bone marrow-derived cells to enhance spinal fusion.
Clin Orthop Relat Res. 2005;432:242-51; Sen MK, et al., Autologous iliac
crest bone graft: should it still be the gold standard for treating nonunions?
Injury, 2007, 38 Suppl 1:S75-80.
[0055] In one embodiment, a method for isolating MPCs comprises:
structural degradation or destruction (e.g., mincing or chopping) of a muscle
sample from a wound (e.g., a debrided wound); suspension of the sample in
a digestion medium; culturing and/or incubating the sample; and isolating
MPCs.
[0056] In another embodiment, a method for isolating MPCs comprises:
removal of unhealthy tissue (e.g., fat, fascia, damaged connective tissue,
and necrotic tissue) from a healthy margin of muscle in a wound; isolation of
a sample of the muscle; washing the muscle sample; structural degradation
-15-
CA 02744559 2016-01-15
WO 2010/062297
PCT/US2009/004482
or destruction (e.g., mincing or chopping) of the muscle sample to create a
tissue suspension; washing the tissue suspension of the sample in a
digestion medium; and culturing and/or incubating the sample; and isolating
MPCs. In one embodiment, the tissue is repeatedly washed in a salt
solution (e.g., Hanks' Balanced Salt Solution (Gibco, Carlsbad, California)).
[0057] In another embodiment, the tissue sample is chopped or minced in
digestion medium (e.g., Dulbecco's Modified Eagle Medium (Gibco)), to
which antibiotic may be added (e.g., penicillin/streptomycin/FungizoneTM
(Gibco)). Mincing or chopping of the sample is carried out until an
appropriate particle size is achieved, e.g., about 5 mm3 or less, or about 1
mm3, or such that the product can pass through a pipette). The minced
tissue can then be transferred to digestion medium, e.g., containing
Dulbecco's Modified Eagle Medium, 3X penicillin/streptomycin/Fungizone
and 0.5 mg/mL collagenase type 2 (Worthington Biochemical, Lakewood,
New Jersey)). The tissue slurry can then be cultured and/or incubated. In
one embodiment, the tissue slurry is agitated at about 37 C for two hours.
The resulting digest may then be filtered (e.g., through a 40-prn cell
strainer
(Falcon)). The resulting digest may also be subjected to centrifugation. A
pellet resulting from a centrifugation step may be resuspended (e.g., in
growth medium such as Dulbecco's Modified Eagle Medium with 10% fetal
bovine serum; Gibco) and 5X penicillin/streptomycin/Fungizone).
[0058] The resulting digest and/or the resuspended pellet may be plated
onto tissue culture and incubated. In one embodiment, incubation is carried
out at elevated temperature (e.g., >25 C; or >30 C; or about 37 C); and may
be carried out in a CO2-hurnidified environment (e.g., 1-10% CO2; or about
5% COP). The incubation may be performed in a cell incubator (e.g., for at
least about one-half hour; or for about two hours). Following incubation, the
culture may be washed with a biologically compatible medium (.e.g., with
Hanks' Balanced Salt Solution). As a next step, a fresh growth medium may
be added with additional antibiotic (e.g., 3X penicillin, streptomycin, &
Fungizoner. In one embodiment, when the multiprogenitor cell colony
forming units are observed, the concentration of penicillin, streptomycin, &
-16-
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
Fungizone is lowered to about 1X. In another embodiment, the cell cultures
may be routinely passaged at 80% to 90% confluence and split (e.g., 1:4).
[0059] In another embodiment, the present invention provides methods
and devices to harvest MPCs from wound tissue. One such method deploys
two chambers or tubes. The chambers may be configured in shape and size
as a conventional centrifuge tube. See Figure 3. As such, a first tube is
outfitted with a tissue grinding device, optionally near an opening of a tube.
The grinder chops or minces MPC-containing tissue (e.g., to about 5 mm3 or
less, or about 1 mm3 or less). Interior to the grinder within the chamber is a
reservoir of a medium containing enzymes, growth factors, antibiotics, and
other suitable cell-sustaining agents. Optionally, those agents are
suspended in a sterile saline solution, and the tissue fragments are
enzymatically digested. The digestion may occur over an incubation period,
optionally at elevated temperature (e.g., >25 C; or ¨37 C). Following
incubation, the digested tissue is processed through another chamber,
device or tube. Here, the second chamber is outfitted with a series of shear
channels, which cause the digested tissue fragments to disperse. The
resulting tissue fragments then are processed through a cell strainer, which
isolates the MPCs. The isolated MPCs are then suitable for implantation
and use in the various methods and devices disclosed elsewhere herein,
and in other methods and therapies as would occur to one of ordinary skill in
the art. Such methods and therapies can be readily adapted to be
performed at the point-of-care.
[0060] Progenitor cells (MPCs) harvested from traumatized muscle have
several characteristics of MSC: 1) similar morphology, proliferation rate,
cell
surface markers and gene expression profile; 2) differentiation into
osteoblasts, adipocytes and chondrocytes; and 3) immunosuppressive, pro-
angiogenic, anti-fibrotic properties. Additionally, the differentiation
potential
of MPC populations is uniform between patients; and traumatized muscle-
derived MPCs could be harvested clinically for use in regenerative medicine
applications, e.g., cellular therapy and tissue engineering.
-17-
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
[0061] We also provide various grafts that can be used to promote healing
of an injury or defect in nerve, bone, or vascular tissue. We have discovered
that MPCs produce various bioactive agents that promote healing and
regeneration, such as growth factors or differentiation factors, and/or
promote the migration of other healing influences to the site of the injury or
defect such as other cell types (e.g., Schwann cells), or that promote the
actual differentiation of other cell types into lines needed to regenerate or
restore injured or damaged tissue. The grafts are configured to place MPCs
in proximity to the injury or defect, and in fluid contact with the injury or
defect such that the agents produced and/or secreted by MPCs produce the
intended effect at or within the injury. The grafts differ from other grafts
incorporating other multiprogenitor cells such as stem cells in that the
instant
grafts avoid or diminish access or contact of the MPCs, per se, to the site of
the injury or defect. That is, the wound, injury, or defect is not treated by
applying the MPCs within the injury or defect with the idea that those cells
will differentiate into the cell types needed to fill the gap or eliminate the
defect. Rather, they are placed proximate, but outside, the actual defect,
and in fluid communication with the defect, such that the biologically active
agents and factors can migrate to the defect and promote the body's natural
healing and/or regeneration processes. By promoting those processes,
injuries or defects are healed more promptly, and injuries that might not
otherwise be able to heal at all are effectively treated.
[0062] The scaffold component of these grafts generally has a structure
including a matrix material that is porous in which the MPCs are seeded.
The matrix material is made of nonaligned fibers that form many discrete
interstices or voids, which, due to the complex topology of the matrix serve
to retain the MPCs. The MPCs can be implanted within the matrix by
passive infusion, by mechanical injection or introduction into the interior of
the matrix, or by cultivating the cells to grow within the matrix, or some
combination thereof. Additionally, the cells can be fixed more stringently
within the matrix by chemical modification of the matrix to increase the
coefficient of friction between the matrix and the cells, or by increasing the
- 18-
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
chemical attraction or bonding (e.g., covalent or ionic) between the matrix
and the MPCs.
[0063] The structure of the matrix is highly adaptable, and can be modified
to whatever shape and dimension is best suited to put the MPCs proximate
and in fluid contact with the injury or defect. Thus, the MPC-seeded matrix
can be in the form of a patch, wrap, conduit, or the like. Additionally,
patches and wraps can be further modified and configured to fit the contours
and dimension of the wound as in any bandage or wound treatment. The
matrix can be further modified in accordance with known principles to be
used internally or externally.
[0064] The shape and dimension of the matrix can be further modified to
complement other structural features of the graft. For example, and as
discussed elsewhere herein, the matrix can be made to serve as an outer
sheath of a conduit, wrapping around, enveloping, or overlaying structural
features that might serve as scaffolding for tissue growth.
[0065] Also provided is a graft comprising a porous matrix of non-aligned
fibers forming interstices; the interstices consisting of a cellular component
and a non-cellular component, and wherein the majority of the cellular
component is MPCs; a conduit internal to the matrix; and wherein the interior
of the conduit is in fluid communication with the matrix. In one embodiment,
there will be a level of fluid communication between matrix and the interior
of
the conduit. It is contemplated that the permeability will permit migration of
various factors secreted by MPCs into the conduit, but generally retain a flow
of fluid within and through the conduit.
[0066] The matrix, aligned fibers, conduit, or other elements of the grafts
disclosed herein can be formed of any known and adaptable biocompatible
structural material that lends itself to fabrication according to the demands
of
the end use. In some embodiments, the material is a synthetic structural
material such as a biocompatible polymer. Biodegradable polymers are also
desirable. Those materials can be selected such that they are dissolved or
resorbed by the body without the need for surgical removal procedures.
Biocornpatible, biodegradable materials useful in the grafts disclosed herein
- 19-
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
include polyglycolic acid (PGA), type 1 collagen, Poly-DL-lactide--
caprolactone (PCL), laminin, gelatin, and the like.
[0067] The graft may further comprise a liquid impermeable exterior liner
overlaying the matrix. In one embodiment, the impermeable exterior liner
isolates the fluid and cells of the interior of the conduit with the external
media. Impermeable generally refers to fluid impermeable, though it also
contemplates a less than perfectly fluid impermeable membrane.
[0068] Among other things, the exterior liner can be used to seal the
interior of the graft. This will be advantageous in vascular grafts. The
exterior liner can also be configured to include additional structural
elements
such as flanges, sleeves, connectors, and other sealing means. Those
additional structural elements can be configured to create a connection or,
alternatively, a seal between the biological conduits of the vasculature and
the conduit of the graft, and to isolate the contents of the two from external
biological media. Connecting elements used to effect a seal or connection
between the graft and vasculature (or other conduit) can be fabricated from
the same material as the impermeable exterior liner, or may be fabricated of
another material, and may be part of yet another layer or structural element
altogether.
[0069] We have also devised a novel nerve graft device or a nerve tube.
The nerve tube comprises several chambers or sheaths (e.g., two or more).
The tube may comprise two sheaths, which may in turn be surrounded or
encased within a conduit wall, which may optionally provide a fluid-
impermeable exterior wall thus isolating the nerve tube and its contents from
the exterior environment. The inner sheath comprises aligned fibers
generally parallel to a longitudinal axis of symmetry through the tube. The
fibers may be nanofibers. The sheath of aligned fibers is surrounded by
another sheath containing random or non-aligned and/or non-linear fibers.
The inner and outer sheaths may be in fluid communication. The exterior
wall may extend in length beyond the inner and/or outer sheaths. In such an
embodiment, the tube may be placed around a proximal and/or distal nerve
stump, optionally enclosing and isolating a space between the stumps.
- 20 -
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
[0070] In one embodiment, the nanofibers are oriented within an inner
sheath at the core of a generally tubular structure having an inner sheath
and an outer sheath. The tubular structure may be circular or elliptical, or
any shape necessary to align the nerve tube with the injured or damaged
nerves needing repair or regeneration.
[0071] The inner sheath constitutes aligned fibers paralleling an axis of
symmetry through the center of a tube. The aligned fibers may be linear,
parallel fibers less than about 100 pm, or less than about 10 pm, and
running the length of the tube. The nanofibers create sub-micron sized
scaffolding that provides a structural framework supporting the growth of
nerve regeneration elements, e.g., axons, along the length of the nerve tube
or graft. As such, the aligned fibers provide three dimensional support and
directional orientation for the growing axon. The aligned fibers may be made
of a biocompatible, biodegradable material that is resorbed or dissolved
within the body avoiding the need for surgical removal.
[0072] The nerve tube or graft device further includes a three-dimensional
matrix in an outer sheath of the graft. The outer sheath of the nerve tube or
graft device surrounds the aligned nanofibers, and may permit fluid
exchange between the two. The outer sheath may share an axis of
symmetry with the nerve tube, and may be generally concentric with the
bundle of aligned fibers of the inner sheath. By "aligned fibers" is meant
that
the fibers are generally aligned along a longitudinal axis of the inner sheath
or tube. The fibers are not necessarily strictly parallel, and some deviation
in
direction and linearity is contemplated.
[0073] The material of the outer sheath provides a support matrix for
multipotent cells, particularly MPCs, within the outer sheath. The multipotent
cells can be MPCs alone or in combination with MSCs and/or other
regenerative cells.
[0074] In one embodiment, the nerve tube comprises a tube comprising
two zones or sheaths. The zones or sheaths may be coaxial. The inner
sheath may comprise aligned fibers generally parallel to an axis of symmetry
along the length of the nerve tube. The ends of the aligned fibers are
- 21 -
CA 02744559 2016-01-15
WO 2010/062297
PCT/1iS2009/004482
exposed to the exterior of the tube permitting axonal growth along the fibers
through the tube.
[0075] Surrounding the inner sheath, and optionally in fluid contact
therewith, is an outer sheath comprising non-aligned and/or non-linear fibers.
The fibers of the two sheaths may be of the same or different material, and
may or may not be of the same size, shape, diameter, and dimension.
Either or both of the sheaths of the nerve tube may further comprise
biologically active agents in addition to those secreted by MSCs and/or
MPCs, e.g., hormones, steroids, anti-inflammatories, analgesics, antivirals,
immunosuppressives, anticoagulants, muscle relaxants and antispasmodics,
antibiotics and/or antimicrobials, growth factors, colony stimulating factors,
nutrients such as vitamins, peptides, small drug molecules, gene therapy
agents, e.g., plasmids, retrovirals, and combinations thereof; and other
pharmacologically acceptable excipients, diluents, buffers, preservatives,
and the like. Either or both of the sheaths may further comprise growth
media and/or nutrients, antibiotics, preservatives, buffers, and the like to
maintain viability of the MSCs and/or MPCs. Other muftipotent cells may
also be included.
[0076] Also provided is a method of forming a nerve graft in a patient
suffering an injury to a nerve comprising: seeding a nerve guide or tube with
MPCs; and implanting the nerve guide between a proximal nerve stump and
a distal nerve stump. The implant may be removed following regeneration of
the nerve; or the implant may be made of a resorbable material eliminating
the need of a surgical removal process. The nerve guide is constructed
and/or implanted such that it resides proximate to the nerve stumps, and
bridging the gap between the nerve stumps. Thus, each end of the nerve
guide or tube connects the nerve.
[0077] The nerve guide or tube is maintained in position relative to the
nerve stumps for a period sufficient to guide axonal growth and restoration
between the respective nerve stumps. As used herein, the term "nerve
stump" refers to the one or more residual portions of a nerve following injury
or damage to the nerve resulting in a loss of function, e.g., as by severed
- 22 -
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
axon. Nerve injury may be in the form of traumatic injury, surgery, the result
of disease, or other cause. In one embodiment, the nerve guide is a
peripheral nerve guide as disclosed elsewhere herein.
[0078] We have also developed a peripheral nerve guide. The guide can
be used to bridge a critical sized defect in a peripheral nerve following
traumatic injury.
[0079] In one embodiment, the device has at least two components (see,
e.g., Figure 1). First, is a cellular scaffold. The scaffold can be fabricated
using polycapralactone, or other suitable biocompatible and/or
biodegradable polymer such as polylactic acid, collagen, laminin, gelatin,
and the like. The polymer is formed into nano-meter scale fibers (e.g., about
50-500 nm diameter; or about 100-300 nm diameter; or about 200 nm
diameter). In one embodiment, the scaffold is formed by extrusion of
polymer using an electrospinning process. However, it will be appreciated
by those skilled in the art that other methods of fabricating such fibers are
available, and thus are contemplated herein.
[0080] The scaffold contains a core of aligned nanofibers, which guide and
support growing axons to bridge the defect in the nerve. The aligned fibers
may be surrounded by a sheath of non-aligned, randomly oriented fibers.
MPCs may be seeded in the sheath of non-aligned fibers. Among other
things, MPCs can be cultured to secrete biochemical factors. Those factors
enhance the growth rate of axons, and recruit native neuronal support cells
into the device. MPCs can be used as the only active growth promoting
agent within the scaffold, or in combination with other agents, including
other
multipotent cells such as stem cells, including MSCs. Alternatively, other
agents and/or multipotent cells can be used in the place of MPCs, and those
too can be employed alone or in combination with other biologically active
agents, including those fostering and/or promoting the growth of bone and/or
other tissue. Thus, the instant scaffold or grafting device is not limited to
nerve grafts, but may be used to repair various injuries including bone, blood
vessel, muscle, ligament, and tendon.
- 23 -
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
[0081] Among other things, the grafts disclosed herein assist nerve
regeneration. They provide a protective environment for axonal sprouting
and improve the chances that the damaged axons will reach the distal nerve
stump. Biocompatible, biodegradable materials useful in the grafts disclosed
herein include polyglycolic acid (PGA), type 1 collagen, Poly-DL-lactide--
caprolactone (PCL).
[0082] The instant grafts have the particular advantage of providing
individual axon guidance with nanofiber filaments, and facilitate access and
activity of axonal support cells such as Schwann cells or MSCs. Both of
these features constitute improvements over currently available devices, and
enhance the speed of axonal growth. The speed and accuracy of axonal
growth determines the success of reinnervation. The instant devices provide
improved regeneration of peripheral nerves compared to the currently
available alternatives. Furthermore, by increasing the speed of axonal
growth, the instant nerve guides and methods expand the versatility and
range of nerve regeneration; and enable regeneration of nerves suffering
defects of substantially greater size than has been possible under prior
therapies.
[0083] In one embodiment, the first device comprises a solid core of
aligned nanofibers. The nanofibers support growth of regenerating axons.
Although the use of aligned fibers has been shown to accelerate the rate of
axonal growth, such use of fibers has been limited. A technique used to
fabricate a sheet of aligned fibers has been reported, and previous
investigators have cut strips of these aligned fibers and pulled them through
the lumen of a conventional nerve guide. Those strips or sheets of aligned
fibers afford only a two dimensional framework, and diminishes migration of
Schwann cells into the lumen. That has the effect of diminishing growth of
the axon through the lumen, and less effective regeneration of the nerve.
While the axons that reach one of the strips of aligned nanofibers appear to
grow faster and reach the distal nerve stump, this improved regeneration
only accounts for a fraction of the total number of axons in the nerve. Those
devices also suffer because the fibrin bridges that form naturally in an empty
- 24 -
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
conduit are inhibited by the aligned sheets of fibers. Without the fibrin
bridges, the Schwann cells are limited in their migration into the void
therefore there is limited chemotaxis for axons to bridge the gap. Our device
is designed to obviate the need for fibrin bridging as the scaffold for axon
growth is already in place, and the MPCs seeded within the graft provide or
enhance chemotaxis.
[0084] In at least one embodiment of the present invention, all of the
regenerating axons have access to aligned nanofibers. This can be effected
by surgically implanting the device so that it is contact with the two nerve
stumps.
[0085] In one embodiment, the device is formed by electrospinning the
non-aligned sheath of nanofibers that surround the aligned core (Figure 2).
In one embodiment, electrospinning is performed by passing a polymer
solution through a blunt-tipped needle that contains a high electric charge
(e.g., greater than about 15kV). As the solution passes through the needle,
part of the charge is transferred to the polymers, which are extruded out of
the needle towards a grounding plate. In one embodiment of the present
invention, the grounding plate is replaced with two axels that are aligned
end-on. In between the axels, there is a strip of aligned nanofibers, and the
entire assembly is rotated as a mandrel. As the polymers are extruded from
the charged needle, the resulting nanofibers are attracted to ground on one
of the two axels, and the target axel can switch stochastically. As the
extruded polymer fiber alternates between the two targets, the fiber is
stretched over the strip of aligned nanofibers. Eventually, the aligned
nanofibers become completely encased by the sheath of non-aligned fibers.
This novel electrospinning process facilitates the formation of a device
having an aligned core of fibers surrounded by non-aligned fibers to support
the neural support cells.
[0086] In another embodiment, there is provided a method of treating a
patient suffering an injury to tissue and requiring restoration and/or
regeneration of the tissue comprising applying to the injury a graft
comprising a porous matrix of non-aligned fibers forming interstices, the
-25-
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
interstices consisting of a cellular component and a non-cellular component,
and wherein the majority of the cellular component is mesenchymal
progenitor cells. As used herein, the term interstices refers to voids among
the fibers. The voids can be seeded with, or infused with, MPCs and other
cell types, cell culturing components, and non-cellular components such as
other active ingredients. Use of the term majority means greater than 50%.
[0087] In such methods, MPCs can be seeded within the graft such that
the majority of MPCs are not in direct contact with the tissue requiring
repair
or regeneration. The treatment method contemplates that the MPCs are
placed in proximity to the injury or damage site, but are not positioned or
administered in such a way that they are used to pack a gap or defect in the
tissue. That is, the MPC seeded matrix will not form a structural element
restoring or regenerating the damaged or missing structural or function
elements of the wounded tissue, but will be part of a separate matrix that is
not ultimately incorporated into the gap or defect. Generally, the MPC-
seeded matrixes described herein will be a biocompatible, biodegradable
material that is dissolved or resorbed by the body, but preferably not until
the
gap or defect in the tissue is healed or sufficiently diminished that the
body's
natural healing capacity can take over and complete the healing and/or
regeneration process.
[0088] Among other things, the methods and materials disclosed herein
can be used to: prevent fibrosis; augment muscle regeneration; improve
fracture healing. Devices can also be seeded with MPCs for tissue
engineering, e.g., peripheral nerve; bone; blood vessel; tendons and
ligaments.
[0089] EXAMPLES
[0090] Example 1: Muscle-derived Mesenchymal Progenitor Cell Isolation
[0091] Mesenchymal Progenitor cells (MPCs) were harvested from
traumatized human muscle debridements using a previously established
procedure. Nesti LJ, et al. Differentiation potential of multipotent
progenitor
-26-
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
cells derived from war-traumatized muscle, J. Bone Joint Surg. Am., 90,
2390-98 (2008). MSCs were obtained from human bone marrow.
[0092] With institutional review board approval from Walter Reed Army
Medical Center and informed patient consent, tissue specimens were
obtained from patients who had sustained traumatic extremity injury during
Operation Iraqi Freedom and Operation Enduring Freedom. These patients
presented to Walter Reed Army Medical Center approximately three to
seven days after the injury and underwent serial debridement and irrigation
procedures until the wounds were determined to be clinically acceptable for
definitive orthopaedic treatment. The amount and nature of debrided tissue
was surgeon dependent and was based on trauma surgery principles of
circumferential removal of all grossly contaminated, apparently necrotic, and
nonviable tissue along with a thin margin of healthy-appearing tissue. This
procedure was repeated at each surgical encounter until only healthy tissue
remained, and cells typically were harvested from muscle tissue obtained
during the second or third serial debridement.
[0093] The protocol for extracting muscle-derived multiprogenitor cells was
based on a modification of previous work in isolating mesenchymal stem
cells that was performed in our laboratory. Caterson EJ, Human marrow-
derived mesenchymal progenitor cells: isolation, culture expansion, and
analysis of differentiation. Mol Biotechnol. 2002;20:245-56. Fat, fascia,
other connective tissue, and necrotic tissue were dissected away from the
healthy margin of the debrided muscle sample. Approximately 0.5 cc of the
remaining muscle tissue was processed for cell extraction. The tissue was
washed three times in Hanks' Balanced Salt Solution (Gibco, Carlsbad,
California) and then was extensively minced in a 10-cm culture dish
containing Dulbecco's Modified Eagle Medium (Gibco) and 3X
penicillin/streptomycin/Fungizone (Gibco) until it could pass through the tip
of
a 25-mL serological pipette (Falcon; BD Biosciences, San Jose, California).
The minced tissue was transferred to a 50-mL conical vial with digestion
medium containing Dulbecco's Modified Eagle Medium, 3X
penicillin/streptomycin/Fungizone, and 0.5 mg/mL collagenase type 2
-27-
CA 02744559 2016-01-15
WO 2010/062297 PCUUS2009/004482
(Worthington Biochemical, Lakewood, New Jersey). The tissue slurry was
agitated gently at 37 C for two hours, and the resulting digest was filtered
through a 40-pm cell strainer (Falcon), pelleted by means of centrifugation,
resuspended in growth medium (Dulbecco's Modified Eagle Medium with
10% fetal bovine serum; Gibco) and 5X penicillin/streptomycin/Fungizone,
and then plated onto tissue culture polystyrene (150-cm2 flask; Falcon). The
cells were incubated at 37 C in a 5% CO2-humidified cell incubator for two
hours and then were extensively washed with Hanks' Balanced Salt Solution
before fresh growth medium was added with 3X
penicillin/streptomycin/Fungizone. Once multiprogenitor cell colony forming
units were observed, the concentration of penicillin/streptomycin/Fungizone
was lowered to 1X. Cell confluence was obtained after approximately two
weeks. The cell cultures were routinely passaged at 80% to 90% confluence
and split 1:4.
(00941 Adult human bone marrow-derived mesenchymal stem cells were
isolated as described previously (Caterson EJ, Human marrow-derived
mesenchymal progenitor cells: isolation, culture expansion, and analysis of
differentiation. Mol Biotechnol. 2002;20:245-56) with use of bone marrow
obtained from the medullary canal of long bones from patients undergoing
elective total hip replacement. The cells were then washed and plated onto
tissue culture polystyrene.
[0095] Example 2: Differences between MPCs and MSCs
[0096] A. Gene expression profile differences (ostecioenicl
(0097] Significant differences were noted between the traumatized muscle-
derived MPCs and bone marrow-derived MSCs (Figure 4 and Table 1). First, the
MPCs
continue to proliferate while being induced to differentiate into osteoblasts.
There is evidence supporting that the entire population of MPCs is slow to
shift from the proliferative state to differentiation, since histological
evidence
of differentiation appears homogeneous throughout the MPG cultures
undergoing osteogenesis. These cells also express lower levels of
osteocalcin, an osteoblastic gene that is expressed during later stages of
- 28 -
CA 02744559 2016-01-15
Table 1
Gene Fold Difference
COL15A1 64.2
GDF10 18.1
COL11A1 11.5
COL1A1 6.3
COL4A3 5.9
COMP 5.4
COL12A1 5.3
COL1A2 4.9
AMELY 4.6
CD36 4.3
ALPL 4.2
TGFBR2 4.2
SMAD4 -4.8
SOX9 -7.0
VEGFA -46.5
VCAM1 -58.8
IGF2 -200.0
-28 a-
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
osteogenic differentiation. Second, there are differences in the osteogenic
gene expression profile between the MPCs and MSCs cultured under growth
conditions, which may reflect the tissue of origin for both cell types. MPCs
express higher levels of COL15A1, a gene associated with muscle tissue
development, and GDF10, shown to be a negative regulator of
osteogenesis, whereas the bone-marrow derived MSCs express higher
levels of genes associated with bone physiology and maintenance: VEGFA,
VCAM1 and IGF2. These differences may also reflect the fact that
traumatized muscle-derived MPCs are harvested from an active wound bed,
where they likely participate in the process of muscle tissue repair. During
osteogenic differentiation, COL15A1 and GDF10 are substantially, albeit
non-significantly, down-regulated, while VEGFA, VCAM1 and IGF2 are
similarly up-regulated, suggesting that the MPCs can assume the role of
osteoprogenitors under the appropriate biological environment, in a manner
similar to other populations of MSCs.
[0098] B. Gene expression profile differences (MSC Biology)
[0099] Three specific genes associated with MSC were differentially
regulated between MPCs and MSCs (Figure 5). FGF10 is a gene
associated with development and the initiation of wound healing, GDF6
codes for a cytokine that works in concert with bone Morphogenic proteins,
and VCAM1 provides a molecular adhesion to vascular structures. The
VCAM1 result corroborates the finding from the previous experiment. These
genes may play a role in the ability of MSCs to reside in the bone marrow
and detect damage to the bone.
[0100] C. Differences in cell surface epitope profiles of MSCs and MPCs
lmmunophenotyping
[0101] Mouse anti-CD29 monoclonal IgG (clone Ha2/5), mouse anti-CD44
monoclonal IgG (clone IM7), mouse anti-CD105 monoclonal IgG (clone 35),
and mouse anti-CD146 monoclonal IgG (clone P1H12) antibodies and
phytoerythrin-conjugated mouse anti-CD45 monoclonal IgG (clone TU116),
-29 -
CA 02744559 2016-01-15
WO 2010/062297
PCT/US2009/004482
mouse anti-CD73 monoclonal IgG (clone AD2), mouse anti-CD90
monoclonal IgG (clone 5E10), and mouse anti-CD105 monoclonal IgG
(done 35) antibodies were obtained from BD Biosciences (San Jose,
California). All antibodies were reactive against human antigens. Donkey
anti-mouse IgG conjugated with fluorescein isothiocyanate were obtained
from Jackson ImmunoResearch (West Grove, Pennsylvania). Testing with
negative and positive controls confirmed the specificity of these antibodies.
[0102] Cells used for staining were cultured in growth medium on glass
coverslips for fourteen days during the second or third passage. They were
washed once with Hanks' Balanced Salt Solution and then were fixed in 3%
phosphate-buffered paraformaldehyde for twenty minutes. Fixed cells were
first blocked in 2% bovine serum albumin (Sigma-Aldrich, St. Louis,
Missouri) for thirty minutes and then were incubated with the respective
primary antibodies in phosphate-buffered saline solution (diluted 1:100) with
1% whole donkey IgG for two hours at room temperature or overnight at 4 C
and then with fluorescein isothiocyanate-conjugated secondary antibodies
(diluted 1:100) and DAPI (4',6-diamidino-2-phenylindole; Invitrogen,
Carlsbad, California; diluted 1:10,000) in phosphate-buffered saline solution
for thirty minutes. The coverslips were then mounted to slides with
VECTASHIELL(Vector Laboratories, Burlingame, California) and viewed
with a Zeiss 510 Metaconfocal laser scanning microscope (Carl Zeiss
Microimaging, Thornwood, New York).
[0103] During the second passage, approximately 250,000 cells were
plated in a 150-cm2 cell-culture flask for flow cytometric analysis. When the
cultures were approximately 80% confluent, the cells were rinsed once with
Hanks' Balanced Salt Solution and then were lifted off the surface with
0.25% trypsin and were transferred to a 50-mL centrifuge tube. The tube
was centrifuged for five minutes at 200 g, the supernatant was aspirated,
and the pellet was resuspended in (fluorescence activated cell-sorting) buffer
(0.1% bovine serum albumin and 0.01% sodium azide in Hanks' Balanced
Salt Solution). Next, 100 pL of the cell suspension was aliquoted into
fluorescence activated cell-sorting tubes, and the phytoerythrin- conjugated
- 30 -
CA 02744559 2016-01-15
WO 2010/062297
PCT/US2009/004482
antibodies (CD14, CD73, CD90, CD105, and an isotype control; BD
Biosciences) were added to each tube at a 1:50 dilution.
[0104] The cells were incubated in the dark at 4 C for forty minutes,
washed once in fluorescence-activated cell-sorting buffer, and resuspended
in 100 pL of fresh fluorescence-activated cell sorting buffer. The fluorescent
intensity profiles of the cells were analyzed by means of fluorescence-
activated cell-sorting with use of a FACSCalibuTrmflow cytometer (BD
Biosciences).
[0105] The MPCs were positive for CD44, CD49e, CD73, CD90 and
CD105 and negative for CD14, CD31, CD34 and CD45. The CD105/CD73
ratio was significantly greater for MPCs than bone-marrow derived MSCs (p
= 0.01) (Figure 6). This experiment demonstrates that even though the
MPCs are positive for cell surface markers that are also present on MSCs,
they are expressed at different levels on the cell surface.
[0106] Example 3: MPC in vitro expression of trophic factors
[0107] A. Neurotrophic Factor Gene Expression
[0108] In 2-0 culture, the progenitor cells derived from traumatized muscle
were exposed to defined glial-induction media. Conditions to optimize the
neurotrophic potential of MSCs and muscle-derived progenitor cells were
determined using ELISAs to measure the concentration of secreted
neurotrophic factors (i.e., BDNF: Brain Derived Neurotrophic Factor, NGF:
Nerve Growth Factor, GDNF: Glial Derived Neurotrophic Factor, etc,).
[0109] The cells were capable of producing substantial amounts of
neurotrophic factors, even without neuroglial induction. After 7 days in
defined conditions for glial differentiation, the progenitor cells began to
produce neurotrophic factors. In particular, the progenitor cells could
produce substantial amounts of BDNF when cultured under optimal induction
conditions. Evidence also suggests that they expressed Glial Fibullary Acid
Protein, a glial cell specific marker. The amount of CNTF produced by the
progenitor cells was unaffected by the neuroglial induction media. MPCs
may also express nestin following neurotrophic induction, and the percent of
-31-
CA 02744559 2016-01-15
WO 2010/062297
PCT/US2009/004482
nestin positive cells appears to increase following neurotrophic induction.
Pre-treatment with retinoic acid (RA) and p-mercaptoethanol (BME)
significantly increases the production of BDNF in progenitor cells cultured in
GM, but not as much as the cells in the optimal neuroglial/neurotrophic
induction medium. Pre-treatment with RA and BME had no effect on the
cells in neuroglial differentiation media.
[0110] The final system of neurotrophic induction includes pre-treatment
with RA, followed by 7 days in the optimized neurotrophic induction media.
This system increased the production of BDNF and two other neurotrophic
factors important to peripheral nerve regeneration: CNTF and NT-3 (Figure
7).
Table 2: Formulations for the Neuroglial-Induction Media
Media Formulation
GM DMEM with 10% FBS
NMO Neurobasal Medium with 2%
627 Supplement
NM1 Neurobasal Medium with 2%
827 Supplement, 5 1tM cAMP, 5
MM IBMX, 2.51.1.g/mt_ Insulin and
25 ng/mi. NGF
NM2 Neurobasal Medium with 2%
B27 Supplement, 10 ng/mL
bFGF, 20 ng/mt_ EGF and 10
ng/mL of LIF
NM3 aMEM with 10% FBS, 5% Horse
Serum, 5011M Hydrocortisone
and 0.1 um Dexamethasone
NM4 DMEM/HAMS F12 with 2% B27
Supplement, 2% FBS, 20 M
Retinoic Acid and 10 ng/ml.
bFGF
-32-
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
[0111] B. MPC vs. MSC Expression of other specific trophic factors and
cvtokines
Despite the specific differences in gene expression and cell surface epitope
profiles that suggest functional differences between these two cell types,
many of the trophic factors appear to be expressed at similar levels (Figure
8), which indicates there is some overlap in the trophic functions of MPCs
and MSCs. FGF2 and TGFB3 are somewhat general cytokines that promote
growth and scarless wound healing, respectively. HGF, LIF and IL10
promote immunosuppression. VEGFA promotes vascular regeneration, and
BMP2, BMP4 and BMP6 have been shown to promote bone regeneration.
[0112] The MPCs produced a greater amount of VEGFA at days 1, 2 and
4 than MSCs than MSCs, as observed with western blots (Figure 9).
[0113] MMPs play a role in promoting endothelial cell migration and
infiltration. Similar to MSCs, MPCs express MMP-2 and MMP-9.
Comparison of the two cell types indicates they express similar levels.
However, MPCs begin expressing higher levels of MMP-2 and MMP-9
earlier, i.e. by Day 1 (Figure 10).
[0114] Example 4: Biological Performance of MPCs
[0115] A. MPC-enhanced axon qrowth in vitro
[0116] This experiment was performed in two ways. First, in a conditioned
media experiment, we cultured the progenitor cells for three days in either
growth or neurotrophic induction medium. Then, we transferred the media to
the DRG cultures for an additional three days. For both media types,
medium that was conditioned by the MPCs resulted in a higher density of
neurite extensions compared to the corresponding no cell controls. Figure
11. We further quantified this finding by counting the number of extended
neu rites under each condition and found that the factors secreted by the
progenitor cells resulted in a significant increase in the number of neurites
that extended beyond the minimum neurite length.
- 33 -
CA 02744559 2011-05-25
WO 2010/062297
PCT/US2009/004482
[0117] We also performed a co-culture assay, where the progenitor cells
were cultured together with the DRGs in a transwell system that allowed
soluble factor communication between the two cultures. We found the
results of these experiments to be similar to the conditioned media
experiment, but one notable difference was that soluble factor
communication appeared to enhance the neurotrophic potential of the
progenitor cells.
[0118] B. MPC trophic factor-induced endothelial cell proliferation
[0119] MPCs were allowed to secrete their trophic factors into conditioned
medium for three days. This medium was then added to fresh media and
transferred to endothelial cell culture. Substantial increase in the
proliferation of the endothelial cells was observed, as a result of the
trophic
factors secreted by the MPCs (Figure 12).
[0120] C. MPC trophic factor suppression of inflammatory response
[0121] A mixed lymphocyte reaction was performed to evaluate the
immunosuppressive properties of the MPCs. The proliferation to T cells was
measured following stimulation with an antigen. The factors secreted by
MPCs significantly decreased the T-cell proliferation in a dose-dependent
manner (Figure 13).
[0122] Example 5: Peripheral Nerve Graft
[0123] A novel, composite electropsun nanofiber scaffold was fabricated
and seeded with the MPC cell population to produce a peripheral nerve
graft.
[0124] The composite scaffold was fabricated by electrospinning
poly(E)caprolactone into nanometer-scale fibers (Figures 1 and 2). There
are three important features of the nerve graft: (1) the interior core of the
scaffold is filled with aligned nanofibers (Figure 14a), which are designed to
guide axon growth along the interior of the scaffold structure; (2) the
aligned
fibers of the scaffold are surrounded by a core of non-aligned fibers that
- 34 -
CA 02744559 2011-05-25
WO 2010/062297 PCT/US2009/004482
support the seeded progenitor cells; and (3) these cells will secrete their
neurotrophic factors into the interior core of the scaffold to augment the
nerve regeneration process.
[0125] Assays were performed to determine the biological performance of
the MPCs in the 3-D environment of the peripheral nerve graft. Viability of
the cells in the graft was assessed using a calcein/ethidium bromide
fluorescence assay. Within 24 hours, 98% of the seeded cells remained
viable and continued to proliferate on the graft at approximately the same
rate (Figure 14b). After one week in culture, the nerve graft was fixed and
prepared for immunohistochemical evaluation of the neurotrophic factor
distribution in the construct. The seeded cells produced BDNF while in the
graft in both growth media and neutrophic induction media, with the MPCs
cultured in the neurotrophic induction medium producing more BDNF on a
per cell basis (Figure 14c). Figure 14d shows cross sections through the
graft, with the dotted line indicating the interface between the non-aligned
exterior of the graft and the aligned interior core, and with the aligned
fibers
running in and out of the figure. We found most of the BDNF was localized
near the MPCs in the non-aligned region of the graft. In grafts cultured in
the
neurotrophic induction medium, the secreted factors also enriched the
aligned fibers.
[0126] The MPCs demonstrated the ability to remain viable and functional
after seeding onto the composite nanofibrous scaffold of the peripheral
nerve graft. The neurotrophic factors they produced will enhance the
neuroconductivity of the aligned nanofibers.
- 35 -