Note: Descriptions are shown in the official language in which they were submitted.
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
A DIAGNOSTIC DEVICE FOR IDENTIFYING
RUPTURE OF MEMBRANE DURING PREGNANCY
Field of the invention
The present invention relates to diagnostic methods and devices. More
particularly, the present invention relates to diagnostic test and devices for
identification of membrane rupture during pregnancy.
Background of the invention
Labor is different for every woman, and it can be difficult to pinpoint the
moment when it begins. Rather than a single event, it is an entire process,
with several physiological changes and events occurring in the body that
combine to eventually deliver a baby. One of those changes or events is the
rupture of the membrane surrounding the baby in the uterus; which is usually
a clear indication that labor is imminent. The rupture of the membrane
happens sometimes abruptly and obviously, with a copious flow of amniotic
fluid. But often, a pregnant woman may have a ruptured membrane and be
very uncertain or unaware whether the membrane has been really ruptured.
There are two main reasons for this. First, the baby's head may act as
a cork at the opening of the uterus and so, instead of gushing out, the
amniotic fluid will only be slowly released. Moreover, amniotic fluid may leak
out drop by drop from a tiny opening of the uterus, and the pregnant woman
may not feel the first contractions until hours later. Secondly, in late
pregnancy, women often have difficulty controlling their urination. Because of
this, a gradual flow of amniotic fluid from the vagina will not be noticed
when
the woman is accustomed to having small amounts of urine escape
involuntarily from time to time.
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
2
Wrong and untimely diagnosis of amniotic fluid leakage, especially in
high-risk pregnancies, can result in failure to implement proper treatment,
which increases the risk to the pregnant women and her fetus. Risks of
neonatal consequences of unnoticed amniotic fluid leakage include fetal
distress, infection and preterm delivery and can lead to very dangerous
sequels for both the mother and fetus.
Currently, the only available way for a pregnant woman to detect at
home amniotic fluid is achieved by using an expensive panty liner self-testing
diagnostic kit. Thus, almost all pregnant women choose to visit their
1o physicians or the emergency room in a hospital. At the hospital, the tests
available today for the identification of amniotic fluid are invasive,
indecisive,
or expensive and in any case lead to discomfort for the patient. There is a
need to greatly reduce the likelihood of membrane rupture being unnoticed at
home by using an accurate, fast and inexpensive device that can act also as a
tool for the physician to manage the test at the clinic/hospital.
Prior art
Among several conventional methods available today by physicians, a
few examples are described herein. The most widespread method for urine
composition analysis is by using a technique known as a "dip-and-read test
strip" or "dipstick". A dipstick is an assay strip that is made up of chemical
reagents bonded to a reagent carrier matrix (pad) on a strip. Usually, the
strip
itself is formed from polymeric materials such as polyethylene, polycarbonate
or polystyrene. Each carrier matrix has a different reagent in it. This
dipstick is
dipped into a urine sample and removed. Upon contact between the reagents
imbedded in the matrices and the urine sample, color reactions occur. A
dipstick can be designed either as a single pad test strip (for the assay of
one
analyte) or as a multiple pad test strip (for the assay of several different
3o analytes all together). Dipstick can be used manually or with the
appropriate
chemistry analyzer. Multiple profile reagent strip for simultaneously or
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
3
sequentially performing multiple analyses of analyte is disclosed in U.S. Pat.
Nos. 4,595,439, 4,526,753, 4,160,008 3,123,443, 3,212,855, 3,814,668,
4,038,485, 3,531,254.
Several such test devices are available in the market. The following is a
partial list of dipstick trademarks CLINISTIX, MULTISTIX, KETOSTIX, N-
MULTISTIX, DIASTIX, DEXTROSTIX, AUTION STICKS, CHEMSTRIP.
The reagent carrier matrix is usually an absorbent material which
allows the liquid sample to move through the matrix. This movement of liquid
sample is in response to capillary forces formed in the matrix. During the
io movement of the liquid through the matrix, it contacts the chemical reagent
composition impregnate in the matrix. Thereafter, detectable and measurable
color transition occurs. If the dipstick can measure several analytes
simultaneously, the color change in each reagent carrier matrix can be
correlated to the amount of different analyte in the liquid sample. Manual
is analysis of the results requires comparison of the color development of the
test on the dipstick to a color chart.
The reagent carrier matrix material can be of any substance that can
incorporate the chemical reagents necessary to carry out the assay of
interest. The preferable matrix should be inert with respect to the chemical
20 reagents and should not alter the sample or the test results. Reagent
carrier
matrices can be made of many materials, some of these materials are: fiber-
containing papers such as filter papers, woven and nonwoven fabrics,
synthetic or modified natural polymers, sponge materials, cellulose, glass
fiber, microporous membranes, and wood. The reagent matrix can also differ
25 in regards to roughness and smoothness together with softness and
hardness. The following list of patents describe the use of different
matrices:
U.S. Pat. Nos. 3,846,247, 3,552,928, 3,802,842, 3,418,083.
Sewell DL, et al. discusses, among other things, the cost of using the
dipstick as a screening method for urinalysis in a scientific paper published
in
30 the American Journal of Clinical Pathology Vol. 83 (6) pages 740-743, 1985.
The authors state that using a dipstick procedure "cost approximately $0.76
for reagents". Various devices are described in the literature for the
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
4
determination of particular urinary analytes with the use of reagent carrier
matrices (filter paper, microcapsules, dipstick, etc.). The following list of
assay
devices utilizing prior art includes dry tablets, dipsticks, or other
techniques for
the analysis urinary constituents. U.S. patent no. 4,147,514 describe the
detection of ketone bodies; U.S. Pat. no. 3,146,070 discloses chemical
compositions in dry form on a carrier (dipstick) impregnated with a pH
indicator for the determination of pH. Methods, composition, and test device
for determining the ionic strength or specific gravity of a test sample such
as
urine are disclosed in the following U.S. patents: 4,318,709, 5,403,744.
Jaffe method is a widely known method for the determination of
creatinine. This method involves formation of orange-red color with an
alkaline
picrate solution. U.S. pat. no. 6,001,656 discloses a device for the assay of
creatinine in fluid test samples. The improvement in this patent involves the
inclusion of one or more selected quinolines in the reagent formulation.
Another method for creatinine determination is described by Benedict and
Behre in the Journal of Biological Chemistry (1936) which involves the
reaction of 3,5-dinitrobenzoic acid with creatinine in an alkaline medium.
Other
methods, composition, and test devices for determining creatinine in a liquid
sample such as urine are disclosed in the following U.S. patents: 4,215,197
(using an enzymatic composition), 5,662,867, 5,733,787. Suitable materials
for the detection of creatinine include picric acid, 3,5-dinitrobenzoic acid,
3,4-
dinitrobenzoic acid, 2,4-dinitrobenzene sulfonic acid, (3,5-dinitrobenz)yl
alcohol, (3,5-dinitrobenzo)-nitrile, (3,5-dinitrobenz)amide and N,N-diethyl-
(3,5-
dinitrobenz)amide.
Different methods for protein determination in fluid have been reported.
These methods include the Biuret method, Lowry method, Kjeldahl method,
dyestuff combination method, fluorometric method and UV method. Of these
methods, the Kingbury-Clark method; reported in J. Lab. Clin. Med., 11, 981
(1926) and the Meulemans method; reported in Clin. Chim. Acta, 5, 757
(1960) and the Coomassie brilliant blue method; reported in Anal. Biochem.
72, 248 (1967) are widely used. The Bradford dye assay for protein
determination, U.S. Pat. no. 4,023,933, is also used routinely in almost every
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
biochemical laboratory. Generally, protein interacts with substances,
principally with dyes such as coomassie brilliant blue, bromphenol blue
(tetrabromophenol blue), and eosine as well as metal ions such as copper (II),
lead (II) zinc (11)and silver (I). The addition of protein-containing solution
to the
5 reaction between a dye and a metal ion gives a spectral change to a dye-
metal ion solution. More protein indicators include those described as well as
the merocyanine and nitro or nitroso substituted polyhalogenated
phenolsulfonephthaleins disclosed in U.S. Pat. no. 5,279,790. Other protein
indicators are Fast Green FCF, Light Green SF, pyrogallol red and
io pyrocatechol violet, bromochlorophenol blue (3',3"-dibromo-5',5"-
dichlorophenolsulfonephthalein), basic fuchsin, basic violet, martius yellow,
phloxine B, methyl yellow, congo red, methyl orange and ethyl orange (4-(4-
diethylaminophenylazo)benzenesulfonic acid). The following list of U.S.
patents concern with measurement of protein in solution, such as urine, using
reagent systems usable in the dipstick method: 5,424,215, 5,593,895,
6,815,210, 4,960,710, 3,485,587, 5,087,575, 4,023,933.
Various dipsticks used for urine testing contain tests for urobilinogen.
CHEMSTRIP of Roch diagnostics, and MULTISTIX of Bayer diagnostics are
typical examples of such products which include tests for urobilinogen. The
classical urobilinogen test, developed by Paul Ehrlich in 1901, employs
paradimethylaminobenzaldehyde as a test for which in strongly acid medium
produces a brown-orange-red color with Ehrlich's reagent. More background
on urobilinogen, Ehrlich reaction and urobilinogen testing is described in,
Tietz, Textbook of Clinical Chemistry, W. B. Saunders Company. Examples of
U.S. patents and dipsticks for the determination of urobilinogen based on
Ehrlich's reaction and the diazonium coupling reaction are: US Pat. nos.
3,853,466, 3,630,680, 4,665,038, 4,290,771, 3,989,462, 3,814,586. More U.S.
patents concern with measurement of urobilinogen in solution, such as urine,
using reagent systems usable in the dipstick method are: 4,158,546 and
3,447,905.
The earliest method of alkaline phosphatase test was introduced by
Kay in 1930. Later, a popular assay method for the determination of alkaline
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
6
phosphatase using p-nitrophenyl phosphate introduced in 1946 by Bessey,
Lowry and Brock. This method relays on the fact that after exposure to fluids
containing alkaline phosphatase, the colorless p-nitrophenyl phosphate is
catalytically hydrolyzed into a yellow colored product p-nitrophenol (and
phosphate). Thus, the concentration of the enzyme is determined by following
the increased intensity of the yellow color of the reaction's product.
Alkaline
phosphatase activity is naturally present in raw milk, whereas after
pasteurization, the enzyme is denatured. So, alkaline phosphatase activity is
used as an indicator for proper milk pasteurization. One such dry test of
io alkaline phosphatase activity in milk is PHOSPHATESMO MI, manufactured
by MACHEREY-NAGEL GmbH & Co.
The presence of hemoglobin in urine is called hemoglobinuria, such a
condition can occur as a result of lysis of red blood cells (RBS) in the
urinary
tract. The term hematuria is used when intact RBS are present in the urine.
This condition can occur in bleeding in the renal or genitourinary systems.
The
most widely used tests for the detection of blood in urine or feces depend on
the fact that the heme proteins can act as peroxidases. This reaction requires
a hydrogen donor molecule. Typical examples of products that include tests
for blood detection are MULTISTIX 10SG, HEMOCCULT II, and AUTION
STICKS.
More recently, United States Patent 4,357,945, issued on Nov. 9, 1982
to Janco for DEVICE FOR TESTING AND RUPTURING AMNIOTIC
MEMBRANE, describes a finger-engaging device provided with a pH
indicator. Upon exposure to fluids, the indicator changes color if the
amniotic
membrane has ruptured. United States Patent 5,425,377, issued on Jun. 20,
1995 to Caillouette for PH MEASUREMENT OF BODY FLUID, describes a
swabbing structure on a stick, provided with a pH indicator for the
measurement of vaginal fluid pH.
Several other methods are provided in the following: United States
Patent 5,554,504 issued on Sep. 10, 1996 to Rutanen for DIAGNOSTIC
METHOD FOR DETECTING THE RUPTURE OF FETAL MEMBRANES,
describes the detection of insulin-like growth factor binding protein 1 in a
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
7
vaginal secretion sample. United States Patent 5,281,522, issued on Jan. 25,
1994 to Senyei et al. for REAGENTS AND KITS FOR DETERMINATION OF
FETAL FIBRONECTIN IN A VAGINAL SAMPLE, describes kits for detection
of rupture of membranes by sampling from the vaginal cavity and exposing it
to antibodies such as anti-fetal fibronectin antibody and an anti-fibronectin
antibody. United States Patent 5,096,830, issued on Mar. 17, 1992 to Senyei
et al. for PRETERM LABOR AND MEMBRANE RUPTURE TEST describes a
method for determining fetal membrane rupture by removing a sample from
the vaginal cavity and contacting it with an insoluble support to which anti-
fetal
io antigen antibody is adhered, and the fetal antigen binding to the support
is
determined.
Several devices involving panty shields or tampons with pH indicators
are known. Following here are examples of some of these patents: United
States Patent 6,149,590 issued on Nov. 21, 2000 to Smith et al. for SYSTEM
FOR IDENTIFYING PREMATURE RUPTURE OF MEMBRANE DURING
PREGNANCY describes a pad having an upper outer layer, a lower outer
layer, and an intermediate pH-responsive component. United States Patents
6,921,647 and 6,627,394 issued to Kritzman et al. for SECRETION-
MONITORING ARTICLE and DIAGNOSTIC PAD describes pads with pH
sensitive indicators for the detection of amniotic fluid leakage. United
States
Patent 5,217,444 issued to Schoenfeld for ABSORBENT TAMPON
demonstrates an absorbent material containing a pH indicator material
indicating by a color change the acidity or alkalinity of a liquid coming into
contact with it.
It should be mentioned that tests that measure only the pH of the
vaginal secretion for the differentiation of amniotic fluid from urine are not
accurate due to the following: 1) The pH of urine may vary from 4.5 to 8
depending on the kidneys homeostatic activity and water intake, and 2) The
pH of amniotic fluid ranges from 6.9 to 7.15 in late pregnancy. This
overlapping range of pH can lead to false diagnosis that may cause medical
complications.
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
8
Immunochromatographic tests which are based on antibodies detecting
specific proteins in the amniotic fluid or urine, are not commonly available
to
the general public mainly because of high price due to the
monoclonal/polyclonal antibodies which they contains. Generally, Enzymatic
methods are also expensive due to the high cost of enzyme production.
Summary of the present invention
It is an object of the present invention to provide unique devices and
methods for differentiating of amniotic fluid from urine that are based on
analyzing more then one analyte using dry chemical reactions without the
need of enzymatic or immunology methods.
It is another object of the present invention to provide unique devices
and methods for differentiating of amniotic fluid from urine that are simple
and
are of money and time-saving nature that ultimately help solve the dilemma of
whether labor may soon begin or not. A woman or a medical caretaker will be
able to observe immediately after the first drop of leaking liquid meets the
device, whether it contains amniotic fluid or urine.
It is yet another object of the present invention to provide unique
devices and methods for differentiating of amniotic fluid from urine that can
be
used with pregnant female subjects either human or non-human. Determining
the onset of labor in non-human females can be even more unpredictable due
to the inability of the animal to discuss its condition.
In addition, another object of the present invention is the point of care
practice of rapid chemical analysis of biological fluids such as urine,
saliva,
sweat, cerebrospinal fluid (CSF), milk or fluids from other sources.
It is therefore provided in accordance with a preferred embodiment of
the present invention a dry diagnostic device for distinguishing between
amniotic fluid and urine in female secretion, the dry diagnostic device
comprising:
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
9
a base layer;
at least two inert carrier matrices provided on said base layer;
dry reagents provided on said at least two inert carrier
matrices wherein said dry reagents are capable of forming a
chemical reaction with substances in the female secretion so as to
visually distinguishing between the amniotic fluid and urine wherein
said dry reagents in each of said at least two carrier matrices is
capable of reacting with different substance of said substances.
Furthermore and in accordance with yet another preferred embodiment
io of the present invention, said at least two carrier matrices are made of
absorbent material selected from a group of materials such as fiber-containing
papers, woven and non-woven fabrics, synthetic or modified natural polymers,
sponge materials, cellulose, glass fiber, micro-porous membranes, wood,
micro porous polymer materials such as styrene based copolymer, latex
based, cellulose based or cotton based matrices.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said dry reagents are capable of reacting with
substances present in the amniotic fluid or in the urine, wherein the
substances have concentration markedly higher in one of the amniotic fluid or
urine than their concentration in the former.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said substances include substances such as
creatinine, alkaline phosphatase, total protein, urea, urobilinogen and blood.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, one, of the dry reagents in one of said at least two
carrier matrices is capable of reacting with creatinine.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said one of the carrier matrices comprises two
reagent layers; one of which contains creatinine sensitive dye fixed with a
dye
fixing agent and a second one containing a buffer capable of keeping said one
of the test zones in a relatively high pH value.
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said one of the carrier matrices comprises a unique
layer containing a creatinine sensitive dye, buffer, and dye fixing agent.
Furthermore and in accordance with yet another preferred embodiment
5 of the present invention, said creatinine sensitive dye is selected from a
group
of dinitro derivatives such as 3'5'-dinitrobenzoic acid, 2'4'-dinitrobenzoic
acid,
3'5'- dinitrobenzotrifluoride, 3'5'-d initrobenzamide, 3'5'-dinitrobenzoyl-
phenyl
glycine, 3,5-dinitrohydroxyphenylpropionic acid.
Furthermore and in accordance with yet another preferred embodiment
to of the present invention, said dye fixing agent selected from polymerized
quaternary ammonium cations (quats) such as
polydiallyldimethylammoniumchloride,
polymonoallyltrimethylammoniumchloride,
polytrimethylaminoethylmethacrylatechioride,
polyvinylbenzyltrimethylammoniumchloride, polyvinylm(3thylpyridine- chloride.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said creatinine sensitive dye and said dye fixing
agent are buffered so as to keep a stable pH in a range of about 9 to 13.5.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, a buffer is selected from a group of sodium
metasilicate, sodium hydroxide, potassium hydroxide, calcium hydroxide,
sodium hydroxide-potassium chloride, potassium carbonate, glycine - sodium
hydroxide, and sodium borate.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, one of the dry reagents in one of the carrier
matrices
is capable of reacting with total protein.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said one of the dry reagents is a dye selected from
a
group of 3',3",5',5"-Tetrabromophenolsulfonephthalein, coomassie brilliant
3o blue, Fast Green, Light Green, Pyrogallolsulfonephthalein (pyrogallol red),
Pyrocatecholsulfonphthalein (Pyrocatechol Violet), 3',3"-Dibromo-5',5"-
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
11
dichlorophenolsulfonephthalein, Fuchsin acid, 2,4-Dinitro-1-naphthol (martius
yellow) phloxine B, Congo red, ethyl orange and methyl orange.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, the device further comprising a buffer such as
potassium citrate, potassium chloride, potassium sulfate, potassium iodate or
potassium phosphate.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, the device further comprising metal ion selected
from
Copper, lead, Zink, Silver.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, one of the dry reagents in one of the carrier
matrices
is capable of reacting with alkaline phosphatase.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said one of the dry reagents Alkaline phosphates'
substrate selected from p-nitrophenyl phosphate, indoxyl phosphate, 4-
methylumbelliferyl phosphate and alpha-naphthyl-phosphate.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, one of said dry reagents is a pH sensitive reagent.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said dry reagents cannot exit the carrier matrices.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said at least two carrier matrices are covered by a
protective layer.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said protective layer is transparent.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said protective layer can be made of a thin "one-way
structure" membrane permeable to liquids flowing to said at least two carrier
matrices and prevents flow of reagents outwardly from the device.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, a sticky backing layer is provided beneath said base
layer.
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
12
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said sticky backing layer is adjacently provided
with
an outer protective layer.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said at least two carrier reagent matrices are
organized in substantially parallel lines.
Furthermore and in accordance with yet another preferred embodiment
of the present invention, said at least two reagent carrier matrices are
organized in concentric lines.
In addition and in accordance with yet another preferred embodiment of
the present invention, said at least two reagent carrier matrices are
surrounded by adhesive material so as to allow adhering the diagnostic device
when said at least two test zones are opposite a vaginal canal of a female
animal.
Brief description of the figures
Some embodiments of the invention are herein described, by way of
example only, with reference to the accompanying drawings. With specific
reference now to the drawings in detail, it is stressed that the particulars
shown
are by way of example and for purposes of illustrative discussion of the
preferred embodiments of the present invention only, and are presented in the
cause of providing what is believed to be the most useful and readily
understood description of the principles and conceptual aspects of the
invention. In this regard, no attempt is made to show structural details of
the
invention in more detail than is necessary for a fundamental understanding of
the invention, the description taken with the drawings making apparent to
those
skilled in the art how the several forms of the invention may be embodied in
practice.
In the drawings:
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
13
Figure 1 illustrates a cross sectional view of the diagnostic device in
accordance with a preferred embodiment of the present invention.
Figures 2-5 illustrate diagnostic devices for adhering onto women panties in
accordance with preferred embodiments of the present invention.
Figures 6a-d illustrate diagnostic pads in accordance with preferred
embodiments of the present invention.
Figure 7 illustrates a veterinary diagnostic pad in accordance with another
preferred embodiment of the present invention.
Detailed description of the invention
Before explaining at least one embodiment of the invention in detail, it is
to be understood that the invention is not necessarily limited in its
application
to the details set forth in the following description or exemplified by the
Examples. The invention is capable of other embodiments or of being
practiced or carried out in various ways.
The terms "comprises", "comprising", "includes", "including", and
"having" together with their conjugates mean "including but not limited to"
The term "consisting of' has the same meaning as "including and
limited to".
The term "consisting essentially of' means that the composition,
method or structure may include additional ingredients, steps and/or parts,
but
only if the additional ingredients, steps and/or parts do not materially alter
the
basic and novel characteristics of the claimed composition, method or
structure.
As used herein, the singular form "a", "an" and "the" include plural
references unless the context clearly dictates otherwise. For example, the
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
14
term "a compound" or "at least one compound" may include a plurality of
compounds, including mixtures thereof.
Throughout this application, various embodiments of this invention may
be presented in a range format. It should be understood that the description
in
range format is merely for convenience and brevity and should not be
construed as an inflexible limitation on the scope of the invention.
Accordingly, the description of a range should be considered to have
specifically disclosed all the possible sub-ranges as well as individual
numerical values within that range.
It is appreciated that certain features of the invention, which are, for
clarity, described in the context of separate embodiments, may also be
provided in combination in a single embodiment. Conversely, various features
of the invention, which are, for brevity, described in the context of a single
embodiment, may also be provided separately or in any suitable sub-
1s combination or as suitable in any other described embodiment of the
invention. Certain features described in the context of various embodiments
are not to be considered essential features of those embodiments, unless the
embodiment is inoperative without those elements.
The present invention provides unique and novel devices to distinguish
amniotic fluids from urine in a simple and fast manner so as to allow a
pregnant woman to know her pregnancy condition. According to one aspect of
the present invention, a device that resembles an 'adhesive plaster', which is
a disposable strip containing sensitive chemical indicators, is used during
last
months of pregnancy. The strip is relatively small in its dimensions and
comprises at least two layers; an adhesive backing and an absorbing material
containing the indicators. The woman using the device simply adheres it to
her panties with the test zones facing up towards the body. In case the
woman uses panty liner, she can place the strip on top of it. The rapid
chemical reactions in the test zones of the device cause a distinct color
change in the case of amniotic fluid leakage and a clearly different color
change when urine has contacted the test zones. The device of the present
invention acts as a diagnostic tool to allow the customer to detect whether
the
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
secretion contains amniotic fluid or only urine, according to a known color
index supplied with the device.
According to a second aspect of the present invention, a device
resembling a pad containing sensitive indicators is used independently of
5 contacting the woman body. This device can be used by pressing it against
wet underwear or a wet panty liner after leakage of fluids has been noticed.
This will cause the reagents in the reagent carrier matrices to come in
contact
with the body fluids and almost immediately after the leaking liquid meets the
diagnostic device, chemical reactions occur in the reagent carrier matrices
1o and cause a color change. Again, the woman (or caregiver) using this device
will be able to see whether the liquid contains amniotic fluid or only urine,
according to a known color index supplied with the device.
In accordance with a third aspect of the present invention, a device that
resembles a panty-liner is containing the sensitive indicators that allow the
15 distinction between amniotic fluid and urine.. The pregnant woman will
attach
this product to her underwear and will get on with her day. The indicator
reagent areas should be positioned directly opposite the vagina. This way, the
sensitive panty-liner will be in a close contact with the woman body fluids.
In all aspects of the invention, if storage is needed following collection,
the sample can be transferred to a suitable container for storage.
Alternatively, immediate processing of the sample can be performed. If used,
the sample is placed directly on the reagent carrier matrices of the device
and
testing is performed within minutes of sample collection.
In accordance with another aspect of the present invention, a
veterinary device is provided. The device containing the sensitive reagents
will
be directly attached to a non-human female vagina opening. This may be
achieved simply by a bandage design in which the adhesive zone is around or
on the sides of the reagent carrier matrices test zone. Thus, a close contact
with the animal body fluids will be achieved. Using a device without direct
contact with the animal body can also be achieved by obtaining a fluid sample
with a swab having a fibrous tip or by suction or lavage device, and applying
it
to the indicators areas on the device.
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
16
In accordance with a preferred embodiment of the present invention,
the determination whether amniotic fluid or urine is present in the woman's
secretion relies upon several non-enzymatic and non-immunological
separated reactions that can be determined as associated with either urine or
amniotic fluid by their distinguished color. In most of the tests available
today
for the identification of the cause of wetness during pregnancy, concentration
difference of only one analyte in urine or amniotic fluid is measured. In the
present invention, the existence of at least two of the following substances:
protein, creatinine, urea, urobilinogen, blood, and alkaline phosphatase are
1o identified as well as pH. Basically, it was identified that protein and
alkaline
phosphatase are present in higher concentrations in amniotic fluid relative to
urine while creatinine, urea and urobilinogen are present in higher
concentrations in urine relative to amniotic fluid. Blood may exist in
amniotic
fluid in higher concentration than in normal urine. Chemicals that can be used
15. in order to distinguish between the fluids in accordance with a preferred
embodiment of the present invention are ones that upon binding or reacting
with one of the substances indicated herein or act upon a certain environment
undergo a change in spectroscopic properties.
Therefore, diagnostic device that comprises reagent carrier matrices
20 provided with chemical indicators according to the present invention is
capable of detecting at least two of the following substances: protein,
creatinine, urobilinogen, urea, blood, and alkaline phosphatase as well as pH
value. The method of the present invention is based on the following facts 1)
The concentration of total protein in amniotic fluid is normally substantially
15
25 times higher than its concentration in urine, 2) The concentration of
creatinine
and urobilinogen in urine is. normally about 10 times more than their
concentration in amniotic fluid, and 3) The concentration of urea in urine is
normally more than 2 times its concentration in amniotic fluid, 4) The
concentration of alkaline phosphatase in amniotic fluid is normally more than
7
30 times higher than the concentration in urine, 5) During rupture of the
fetal
membranes, the amniotic fluid coming out may contain blood, which is in
contrast to urine of healthy woman. Therefore, even if a small amount of
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
17
amniotic fluid is present in vaginal secretion and comes in contact with a pad
of the present invention, the pad allows the diagnostic of fetal membrane
rupture with an extremely high accuracy. Other substances with similar
concentration differences may also be detected.
The present invention is further illustrated by the following examples of
devices provided with sensitive indicators for the detection and distinction
of
urine or amniotic fluid leakage.
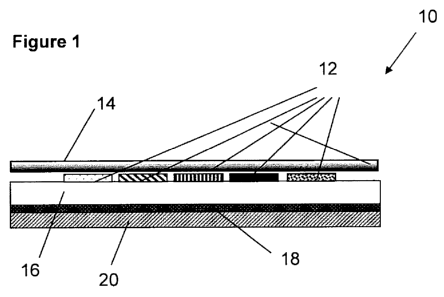
Reference is made to Figure 1 illustrating a diagnostic device in
accordance with a preferred embodiment of the present invention, in an upper
io view and cross sectional view, respectively. A device 10 has at least two,
and
preferably several reagent carrier matrices 12. Figure 1 illustrates a device
that is adapted to be attached to the woman's body and therefore covered
with a first layer 14 of soft and comfortable material that does not irritate
skin
upon contact so it can be worn in a woman panty. First layer 14 prevents
direct contact between reagents that are provided in within device 10 and the
adjacent skin.
It should be noted that first layer 14 may be transparent so as to allow
easy visual distinction of the colors formed in layers beneath it.
Device 10 is further comprises with a supportive base layer 16 that can
support reagent carrier matrices 12 capable of carrying out the assays of
interest. Reagent carrier matrices 12 are preferably an absorbent material
that
allows the liquid sample to move through the matrix. Reagent carrier matrices
12 should be inert with respect to the chemical reagents and should not alter
the sample or the test results.
Optionally, reagent carrier matrices 12 can be made of many materials,
for example: fiber-containing papers such as filter papers, woven and
nonwoven fabrics, synthetic or modified natural polymers, sponge materials,
cellulose, glass fiber, micro-porous membranes, and wood. Additional
materials can be micro porous polymer materials such as styrene based
copolymer, latex based, cellulose based or cotton based matrices.
The reagent carrier matrices can. also be different in characteristics
such as roughness, smoothness, softness, and hardness.
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
18
It should be noted that in the manufacturing process, the reagent
carrier matrices can be made from several layers, some of which carry
different reagents in different areas of the test zone. Any combination of the
supportive base layer and additional matrices carrying the indicators are
covered by the scope of the present invention and by no means limit the
scope of the present invention.
Beneath supportive base layer 16, a sticky backing layer 18 is provided
and an adjacent outer protective layer 20 is also provided. Those two layers
are similar in nature to the layers that are provided in protection panty
shields.
Reagent carrier matrices 12 are organized preferably in groups wherein
each group is provided with indicators capable of indicating one of the
substances that were listed herein before. Following are examples of chemical
reagents and methods of preparing the test zone for each:
Examples
Creatinine test zone preparation
Creatinine concentration in urine is normally about 10 times higher than its
concentration in amniotic fluid. Creatinine dry test is made in two optional
ways:
= Two reagent layers system that contains fixed creatinine sensitive dye
that is placed on one matrix and a buffer that keeps the system in a
relatively high pH value is placed on a second matrix.
= One reagent layer system that contains a creatinine sensitive dye,
buffer, fixing agents all placed in one matrix.
The creatinine sensitive dye can be one of dinitro derivatives such as:
3'5'-dinitrobenzoic acid, 2'4'-dinitrobenzoic acid, 3'5'-
dinitrobenzotrifluoride,
3'5'-d initrobenzamide, 3'5'-dinitrobenzoy- phenyl glycine,
3,5-dinitrohydroxyphenylpropionic acid.
The sensitive dye fixing agent preferably includes polymerized
quaternary ammonium cations (quats) such as:
polydiallyldimethylammoniumchloride (Poly DADMAC),
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
19
polymonoallyltrimethylammoniumchloride,
polytrimethylaminoethylmethacrylatechloride,
polyvinylbenzyltrimethylammoniumchloride, polyvinylmpthylpyridine- chloride.
The buffer should be a strong base capable of keeping a stable pH in a
range of about 9 to 13.5. examples for such buffers are sodium metasilicate,
sodium hydroxide, potassium hydroxide, calcium . hydroxide, sodium
hydroxide-potassium chloride, potassium carbonate, glycine - sodium
hydroxide, and sodium borate.
Optionally, non volatile solid reagents are added to the sensitive dye in
1o order to improve the reaction.
Two reagent layers system; Example 1
Creatinine test indicator consisting of two reagent carrier matrices was
mounted on the diagnostic device base layer. The first matrix (can be
Whatman filter paper or napkin paper) was impregnated with creatinine
sensitive dye, 3'5'-dinitrobenzoic acid (dBA), and poly DADMAC (optional) as
a fixing agent that were dissolved in water. The dBA stock solution was mixed
in sodium carbonate buffer. A second reagent matrix was impregnated with
Sodium Metasilicate buffer. After both reagent matrices were dry, the first
reagent matrix was laid on the second reagent matrix that was impregnated
with Sodium Metasilicate buffer. Both dry reagent matrices were tightened on
the base layer of the device.
After a drop of vaginal secretions meet the dry reagent carrier matrix, a
distinct color change will identify the liquid and indicate whether it
contains
amniotic fluid or urine.
Two reagent layers system; Example 2
Two reagent absorbent matrices were impregnated each in one solution,
dried and were tightened on a base layer of the device. The first reagent
matrix was impregnated with solution 1 that consist of dBA (stock solution was
dissolved in Sodium Hydroxide) as a creatinine sensitive dye, non volatile
solid reagent and Poly DADMAC (optional) as a fixing agent. The second
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
reagent matrix was impregnated with solution 2 that include Sodium
Hydroxide. Both reagent matrices were dried. Dry reagent matrix with solution
1 was placed above dry reagent matrix with solution 2 and both of them were
tighten on the base layer of the device.
5
One reagent layers system; Example 1
As mentioned before, "one reagent layers system" has the same rational
as the "two reagent layers system" but contains all the chemicals on one
reagent carrier matrix. In both cases, the goal is differentiation between
urine
io and amniotic fluid using creatinine concentration.
Creatinine test device was prepared from the same absorbent and
support carriers as in the two reagent layers system. The reagent carrier
matrix consists of a creatinine sensitive dye, non volatile solid reagent, a
fixing
agent (optional) and a buffer that was dried and mounted on the carrier
matrix.
15 There was a preferable use of 3'5'-dinitrobenzoic acid (stock solution was
made in acetonitrile) as a sensitive dye, Poly DADMAC as a fixing agent
(optional) and Potassium Hydroxide (in ethyl alcohol solution) as a buffer.
The
reagents were dried all together and were ready for urine or amniotic fluid
sample test.
One reagent layers system; Example 2
An emulsified solution of 3'5'-dinitrobenzoic acid, Poly DADMAC
(optional), Styrene Acrylic acid, Sodium Metasilicate and a non volatile solid
reagent was prepared. A thin layer of the emulsion was spread on a latex
based matrix, and dried. Dry matrices were tightening on the support base
layer polymer of the device.
Total Protein test zone preparation
The concentration of total protein in amniotic fluid is normally about 15
times more than its concentration in urine. Interaction of proteins with
substances, principally dyes and metal ions, causes a spectral change to a
dye-metal ion solution. In implementing the device of the present invention, a
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
21
group of dyes and metal ions such as: 31,31',51,5"-
Tetrabromophenolsulfonephthalein, coomassie brilliant blue, Fast Green,
Light Green, Pyrogallolsulfonephthalein (pyrogallol red),
Pyrocatecholsulfonphthalein (Pyrocatechol Violet), 3',3"-Dibromo-5',5"-
dichlorophenolsulfonephthalein, Fuchsin acid, 2,4-Dinitro-1-naphthol (martius
yellow), Copper, lead, Zink, Silver, phioxine B, congo red, ethyl orange and
methyl orange can be used.
The reagent carrier matrix of the total protein test device is an absorbent
carrier that can be one of the matrices already mentioned, for example micro
to porous polymer material such as styrene based copolymer, latex based,
cellulose based or cotton based matrices. The reagent carrier matrix can be
polymerized urethane-based compound (as described in U.S. Patent no.
5,124,266) incorporating an indicator reagent compound capable of
interacting with proteins to produce a visually detectable response.
In order to reduce the variability due to urine density differences, the test
solution may include a low pH potassium salt based buffer such as potassium
citrate, potassium chloride, potassium sulfate, potassium iodate or potassium
phosphate.
Total protein test; Example
The total protein solution test comprises a combination of two solutions:
the dye reagent solution and the buffer. For the dye solution, 3',3",5',5"
Tetrabromophenolsulfonephthalein was used and was dissolved in a weak
organic acid such as citric acid. The buffer, potassium citrate, was tittered
with
the same weak organic acid to a low pH value of around 3.5.
The dye solution was then diluted with the buffer solution in a wide ratio
scale. After the dilution, a very thin layer of the resulting solution was
spread
on a reagent carrier matrix and dried. While a urine and amniotic fluid comes
in contact with the reagents imbedded in the device, a distinct color reaction
can differentiate between them.
pH test zone preparation
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
22
As mentioned herein before, the preparation is placed on an absorbent
reagent carrier matrix of any type. The reagent matrix is impregnated with a
pH indicator for the measurement of fluid pH. The urine pH may vary from 4.5
to 8 while the amniotic fluid pH ranges from 6.9 to 7.15 in late pregnancy.
This
pH range can be check with one indicator or two different pH indicators; a low
pH indicator and a middle pH indicator.
According to their useful pH range, for the low pH, Methyl yellow, Methyl
orange, Methyl red, Bromofhenol Blue, Tetrabromphenol blue or Bromcresol
green can be used. For the middle pH range, Cresol Red, Nitrizine,
io Bromthymol blue, Neutral red, Rosolic acid, a-Naphtholphthalein or phenol
red can be used.
pH test; Example
Cresol Red was dissolved in water and was impregnated on 3M paper
(blotting paper ra- reeve angel). After the material is fully impregnated, the
matrix is dried.
Alkaline phosphatase test zone preparation
Alkaline phosphatase activity in amniotic fluid is much higher then in
normal urine. Therefore, this activity can be used to differentiate and
identify
amniotic fluid from urine in vaginal secretion by using a dry test by which
direct contact with such secretions is resulted by a distinct and different
color
formation for the secretions. When drop of vaginal secretion meets the dry
matrix reaction zone, a distinct color change will identify the secretion
content
and indicate whether it contains amniotic fluid.or urine.
Alkaline phosphatase test; Example
The composition of the reaction reagent matrix for the detection of
Alkaline phosphatase contained dry buffered solution of Alkaline phosphates'
substrate such as p-nitrophenyl phosphate, indoxyl phosphate, 4-
methylumbelliferyl phosphate and alpha-naphthyl-phosphate and may also
contain sensitive indicators such as bromocresol green.
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
23
Reference is now made to Figures 2 - 5 illustrating diagnostic device for
adhering onto women panties in accordance with preferred embodiments of
the present invention. All diagnostic devices shown in Figures 2-5 comprises a
base layer 22 that includes layers similar to the layers that are shown in
Figure 1 that allows the device to be used in the panty of a women, adhered
directly to the woman's panty or adhered onto a panty shield while the layer
with the test zone, which will be explained herein after, is directly
positioned
beneath the vaginal canal of the woman. In order to view the device, the
protective layer is removed from the drawings. Figure 2 illustrates a
diagnostic
io device 20 having test zones that are divided into five reagent carrier
matrices
24-32 wherein each of the reagent carrier matrices is provided with diagnostic
indicator that is capable of identifying a specific substance as elaborated
herein before - e.g. creatinine test, protein test, alkaline phospatase test
ect.
Figure 3 illustrates a diagnostic device 40 having three distinct test
zones 12. Each zone is divided into five different reagent carrier matrices
wherein each one is provided with different indicator capable of
distinguishing
between urine and amniotic fluid.
Figure 4 illustrates a diagnostic device 50 similar to device 20 wherein
different indicators are in 4 reagent carrier matrices 54-58.
Figure 5 illustrates a diagnostic device 60 having test zones 12, each
having a reagent carrier matrices 62-68 provided with different indicators so
as to distinguish between urine and amniotic fluid.
As mentioned herein before, according to the present invention, the
device may contain two or more reagent carrier matrices in lines, circles or
any other formation so at least two indicators are changed in their color so
as
to be able to clearly distinguish between the urine and the amniotic fluid.
Reference is now made to Figures 6a-d illustrating diagnostic pads in
accordance with preferred embodiments of the present invention. As
mentioned herein before, a pad can be used in a way that it is pressed against
3o a regular wet panty shield or wet panties, as an example, so as to prevent
contact between the skin and the pad. Basically, the pad is built similarly to
the adhered one, however, there is no adherence layer on the pad.
CA 02745309 2010-04-16
WO 2009/050711 PCT/IL2008/001371
24
Figure 6a illustrates a pad 70 having a base layer 72 onto which
different reagent carrier matrices 74-79 provided with different indicators.
Each reagent carrier matrices is capable of identifying a certain substance as
explained herein before.
Figure 6b illustrates a pad with base layer 82 onto which reagent carrier
matrices 94-98 or .104-106 are arranged in different arrangement. Figures 6c
and 6d illustrate additional embodiments of pads 90 and 100 provided with
less reagent carrier matrices zones, respectively.
Reference is now made to Figure 7 illustrating a veterinary diagnostic
1o pad in accordance with another preferred embodiment of the present
invention. Veterinary device 120 is basically similar to the diagnostic device
used for humans however, the figure shows an upper view of pad 120 in
which the adhesive zone 122 is around the test zones 124.