Note: Descriptions are shown in the official language in which they were submitted.
CA 02745736 2011-07-04
IMMUNOTHERAPY AND IMPROVED VACCINES
FIELD OF THE INVENTION
The present invention relates to immunotherapeutic
compositions and methods, and to improved protective and
therapeutic vaccines and improved methods for prophylactically
and/or therapeutically inducing immune responses against
antigens.
BACKGROUND OF THE INVENTION
Vaccines are useful to immunize individuals against
target antigens such as pathogen antigens or antigens
associated with cells involved in human diseases.
Antigens
associated with cells involved in human diseases include
cancer-associated tumor antigens and antigens associated with
cells involved in autoimmune diseases.
The overall objective of any immunization strategy
is to induce specific immune responses which protect the
immunized individual from a given pathogen over his or her
lifetime. One major challenge in meeting this goal is that the
correlates of protection from an individual pathogen vary from
one infectious agent to the next. Therefore, a more clinically
effective vaccine should elicit a more specific immune
responses against the targeted pathogen.
Immunization
strategies need to be designed which can be "focused"
according to the correlates of protection known for the
particular pathogen.
CA 02745736 2011-07-04
- 2 -
In designing such vaccines, it has been recognized
that vaccines which produce the target antigen in the cell of
the vaccinated individual are effective in inducing the
cellular arm of the immune system.
Specifically, live
attenuated vaccines, recombinant vaccines which use avirulent
vectors and DNA vaccines all lead to the production of
antigens in the cell of the vaccinated individual which
results induction of the cellular arm of the immune system.
On the other hand, sub-unit vaccines which comprise only
proteins and killed or inactivated vaccines, which do induce
a humoral response, do not induce good cellular immune
responses.
A cellular immune response is often necessary to
provide protection against pathogen infection and to provide
effective immune-mediated therapy for treatment of pathogen
infection, cancer or autoimmune diseases.
Accordingly,
vaccines which produce the target antigen in the cell of the
vaccinated individual such as live attenuated vaccines,
recombinant vaccines which use avirulent vectors and DNA
vaccines are preferred.
Nucleic acid immunization is a new vaccination
technique which delivers DNA constructs encoding for a
specific immunogen into the host. In addition to DNA vaccine's
ability to induce both antigen-specific cellular and humoral
immune responses, this technique has the potential to
manipulate the immune responses generated through the
co-delivery of immunologically important molecules.
While such vaccines are often effective to immunize
individuals prophylactically or therapeutically against
pathogen infection or human diseases, there is a need for
improved vaccines. There is a need for compositions and
methods which produce an enhanced immune response.
SUMMARY OF THE INVENTION
The present invention relates to gene constructs
which comprise nucleotide sequences that encode
immunomodulating proteins which can be administered to
CA 02745736 2011-07-04
- 3 -
individuals undergoing prophylactic or therapeutic vaccination
protocols or therapeutic immunodulation protocols.
The
immunodulating proteins include human proteins IL-12, GM-CSF,
IL-1, TNF-a, TNF-g, IL-2, IL-4, IL-5, IL-10, IL-15, IL-18, and
a novel molecule designated BL-1.
The present invention relates to gene constructs
that comprise: a nucletide sequence that encodes IL-12, GM-
CSF, IL-1, TNF-a, TNF-0, IL-2, IL-4, IL-5, IL-10, IL-15,
IL-18, or BL-1; or nucleotide sequences -that encode two or
more of IL-12, GM-CSF, IL-1, TNF-a, TNF-g, IL-2, IL-4, IL-5,
IL-10, IL-15, IL-18, or BL-1.
It is intended that gene
constructs can contain multiple copies of the same nucleotide
sequence.
The present invention relates to methods of
vaccinating an individual by administering a vaccine
composition to introduce an immunogen to the individual in
combination with the introduction of the gene constructs which
comprise nucleotide sequence(s) that encodes one or more
immunomodulating proteins which results in an enhanced and/or
more desirable immune response.
Moreover, the present
invention relates to methods of modulating the immune response
of an individual by administering a gene construct which
comprises nucleotide sequence(s) that encode one or more
immunomodulating proteins.
The modulation of the immune
response may be a step in a vaccination protocol in which the
patients immune response is switched from a primarily Th1 to
Th2 response or vice-versa by first co-administering a vaccine
composition with an immunomodulating protein that favors one
form of immune response and boosting the individual by co-
administering the vaccine composition with an immunomodulating
protein that favors the other form of immune response.
The vaccine compositions are preferably plasmids
which are directly introduced into the individual. Similarly,
the gene construct that comprises nucleotide sequence(s) that
encode one or more immunomodulating proteins is preferably a
plasmid.
CA 02745736 2011-07-04
- 4 -
The present invention relates to a plasmid which
comprises nucleotide sequences that encode human IL-12 protein
operably linked to regulatory elements necessary for
expression in eukaryotic cells and a nucleotide sequence that
encodes an immunogenic target antigen operably linked to
regulatory elements necessary for expression in eukaryotic
cells. In some preferred embodiments, the immunogenic target
antigen is a pathogen antigen, a cancer-associated antigen or
an antigen linked to cells associated with autoimmune
diseases. In
some embodiments, the plasmid comprises a
nucleotide sequence that encodes a single chain human IL-12
protein operably linked to regulatory elements necessary for
expression in eukaryotic cells.
The single chain IL-12
protein is a single protein which is encoded by a single
coding sequence and which includes a linker connecting the two
subunits.
The linker is sufficiently large enough and
flexible enough to allow the single protein to fold into the
biologically active conformation assumed by the functional,
native two-subunit IL-12 protein.
The present invention relates to a method of
inducing, in an individual, an immune response against an
antigen comprising the step of administering to an individual,
a plasmid which comprises a nucleotide sequence that encode
human IL-12 protein operably linked to regulatory elements
necessary for expression in cells of the individual, and a
nucleotide sequence that encodes a target antigen operably
linked to regulatory elements nedessary for expression in
cells of the individual. In some preferred embodiments, the
target antigen is a pathogen antigen, a cancer-associated
antigen or an antigen linked to cells associated with
autoimmune diseases. In preferred embodiments, the immune
response that is induced against the target antigen provides
a therapeutic benefit with respect to infections, diseases,
disorders and conditions associated with the proteins to which
the anti-antigen immune: response is directed and/or a
protective immune response is induced against pathogens or
cells having proteins that cross react to the immune response
CA 02745736 2011-07-04
- 5 -
generated against the antigen. In some embodiments, the human
IL-12 protein is a single chain IL-12 protein.
The present invention relates to a composition which
comprises a plurality of plasmids which collectively comprise
nucleotide sequences encoding both subunits of human IL-12 and
a target antigen, each coding sequence being operably linked
to regulatory elements necessary for gene expression. In some
embodiments, the composition includes two plasmids: a first
plasmid which comprises nucleotide sequences that encode IL-12
protein operably linked to regulatory elements necessary for
expression in eukaryotic cells and a second plasmid which
comprises a nucleotide sequence that encodes an immunogenic
target antigen operably linked to regulatory elements
necessary for expression in eukaryotic cells.
In some
embodiments, one plasmid contains a nucleotide sequence that
encodes the immunogenic target protein and one subunit of
human IL-12, and a second plasmid contains a nucleotide
sequence that encodes the other subunit of human IL-12. In
some embodiments, three different plasmids are provided: one
that contains a nucleotide sequence that encodes the
immunogenic target protein, one that encodes the p35 subunit
of human IL-12 and one that encodes the p40 subunit of human
IL-12.
In some embodiments, the composition includes two
plasmids: a first plasmid which comprises a nucleotide
sequence that encodes a single chain IL-12 protein operably
linked to regulatory elements necessary for expression in
eukaryotic cells and a second pIasmid which comprises a
nucleotide sequence that encodes an immunogenic target antigen
operably linked to regulatory elements necessary for
expression in eukaryotic cells.
The present invention relates to a method of
inducing, in an individual, an immune response against an
antigen comprising the step of administering to an individual,
a composition which comprises a plurality of plasmids which
collectively which collectively comprise nucleotide sequences
encoding both subunits of human IL-12 and a target antigen,
each coding sequence being operably linked to regulatory
CA 02745736 2011-07-04
- 6 -
elements necessary for gene expression. In some embodiments,
two or three plasmids as described above which together
comprise a nucleotide sequences that encode both subunits of
human IL-12 protein and an immunogenic target protein, the
nucleotide sequences that encode protein being operably linked
to regulatory elements necessary for expression in cells of
the individual. In preferred embodiments, the immune response
induced by the target antigen cross reacts to a pathogen
antigen, a cancer-associated antigen or an antigen linked to
cells associated with autoimmune diseases. The
present
invention relates to a method of immunizing an individual
against a pathogen, cancer or an autoimmune disease.
In
preferred embodiments, the target antigen is a pathogen
antigen, a cancer-associated antigen or an antigen linked to
cells associated with autoimmune diseases. In
some
embodiments, human IL-12 is a single chain IL-12 protein.
The present invention relates to a plasmid which
comprises nucleotide sequence(s) that encode one or more of
human GM-CSF, IL-la, TNF-a and TNF-A, IL-2, IL-15, IL-18,
IL-4, IL-5 and IL-10 operably linked to regulatory elements
necessary for expression in eukaryotic cells and a nucleotide
sequence that encodes an immunogenic target antigen operably
linked to regulatory elements necessary for expression in
eukaryotic cells.
In some preferred embodiments, the
immunogenic target antigen is a pathogen antigen, a cancer-
associated antigen or an antigen linked to cells associated
with autoimmune diseases.
The present invention relates to a method of
inducing, in an individual, an immune response against an
antigen comprising the step of administering to an individual,
a plasmid which comprises nucleotide sequence(s) that encode
one or more of human GM-CSF, IL-1a, TNF-a and TNF-A, IL-2,
IL-15, IL-18, IL-4, IL-5 or IL-10 protein operably linked to
regulatory elements necessary for expression in cells of the
individual, and a nucleotide sequence that encodes a target
antigen operably linked to regulatory elements necessary for
expression in cells of the individual. In some preferred
CA 02745736 2011-07-04
- 7 -
embodiments, the target antigen is a pathogen antigen, a
cancer-associated antigen or an antigen linked to cells
associated with autoimmune diseases.
In preferred
embodiments, the immune response that is induced against the
target antigen provides a therapeutic benefit with respect to
infections, diseases, disorders and conditions associated with
the proteins to which the anti-antigen immune response is
directed and/or a protective immune response is induced
against pathogens or cells having proteins that cross react
to the immune response generated against the antigen.
The present invention relates to a composition which
comprises a plurality of plasmids which includes two plasmids:
a first plasmid which comprises nucleotide sequence(s) that
encode one or more of human GM-CSF, IL-1a, TNF-a, TNF-g, IL-2,
IL-15, IL-18, IL-4, IL-5 or IL-10 protein operably linked to
regulatory elements necessary for expression in eukaryotic
cells and a second plasmid which comprises a nucleotide
sequence that encodes an immunogenic target antigen operably
linked to regulatory elements necessary for expression in
eukaryotic cells. In some embodiments, the composition
comprises three plamids including a third plasmid which
comprises nucleotide sequence(s) that encode one or more of
human GM-CSF, IL-la, TNF-a, TNF-g, IL-2, IL-15, IL-18, IL-4,
IL-5 or IL-10 protein operably linked to regulatory elements
necessary for expression in eukaryotic cells. In
some
embodiments, the composition comprises four plamids including
a third plasmid which comprises nutleotide sequence(s) that
encode one or more of human GM-CSF, IL-la, TNF-a, TNF-0, IL-2,
IL-15, IL-18, IL-4, IL-5 or IL-10 protein operably linked to
regulatory elements necessary for expression in eukaryotic
cells and a fourth plasmid
which comprises nucleotide
sequence(s) that encode one or more of human GM-CSF, IL-la,
TNF-a, TNF-g, IL-2, IL-15, IL-18, IL-4, IL-5 or IL-10 protein
operably linked to regulatory elements necessary for
expression in eukaryotic cells.
The present invention relates to a method of
inducing, in an individual, an immune response against an
CA 02745736 2011-07-04
- 8 -
antigen comprising the step of administering to an individual,
a composition which comprises a plurality of plasmids which
includes two plasmids: a first plasmid which comprises
nucleotide sequence(s) that encode one or more of human GM-
CSF, IL-1a, TNF-a and TNF-g, IL-2, IL-15, IL-18, IL-4, IL-5
or IL-10 protein operably linked to regulatory elements
necessary for gene expression and a second plasmid which
comprises a nucleotide sequence that encodes an immunogenic
target antigen operably linked to regulatory elements
necessary for expression. In
preferred embodiments, the
immune response induced by the target antigen cross reacts to
a pathogen antigen, a cancer-associated antigen or an antigen
linked to cells associated with autoimmune diseases. The
present invention relates to a method of immunizing an
individual against a pathogen, cancer or an autoimmune
disease. In preferred embodiments, the target antigen is a
pathogen antigen, a cancer-associated antigen or an antigen
linked to cells associated with autoimmune diseases. In some
embodiments, the method comprises administering a composition
that comprises three plamids including a third plasmid which
comprises nucleotide sequence(s) that encode one or more of
human GM-CSF, IL-1a, TNF-a, TNF-g, IL-2, IL-15, IL-18, IL-4,
IL-5 or IL-10 protein operably linked to regulatory elements
necessary for expression in eukaryotic cells.
In some
embodiments, the method comprises administering a composition
that comprises four plamids including a third plasmid which
comprises nucleotide sequence(s) that encode one or more of
human GM-CSF, IL-1a, TNF-a, TNF-g, IL-2, IL-15, IL-18, IL-4,
IL-5 or IL-10 protein operably linked to regulatory elements
necessary for expression in eukaryotic cells and a fourth
plasmid which comprises nucleotide sequence(s) that encode
one or more of human GM-CSF, IL-1a, TNF-a, TNF-0, IL-2, IL-15,
IL-18, IL-4, IL-5 or IL-10 protein operably linked to
regulatory elements necessary for expression in eukaryotic
cells.
=
The present invention relates to an improved
recombinant vaccine vector which comprises nucleotide
CA 02745736 2011-07-04
- 9 -
sequence(s) that encode one or more of human IL-12, GM-CSF,
IL-la, TNF-a and TNF-g, IL-2, IL-15, IL-18, IL-4, IL-5, IL-10
of BL-1 protein operably linked to regulatory elements
necessary for expression in eukaryotic cells and a nucleotide
sequence that encodes a target antigen operably linked to
regulatory elements necessary for expression in eukaryotic
cells.
In some embodiments, genes encoding human IL-12
protein encode IL-12 as a single chain protein. In preferred
embodiments, the target antigen is a pathogen antigen, a
cancer-associated antigen or an antigen linked to cells
associated with autoimmune diseases.
The present invention relates to a method of
immunizing an individual against a pathogen, cancer or an
autoimmune disease comprising the step of administering to an
individual, a recombinant vaccine vector which comprises
nucleotide sequence(s) that encode one or more of human IL-12,
GM-CSF, IL-la, TNF-a and TNF-g, IL-2, IL-15, IL-18, IL-4,
IL-5, IL-10 of BL-1 protein operably linked to regulatory
elements necessary for expression in cells of the individual,
and a nucleotide sequence that encodes a target antigen
operably linked to regulatory elements necessary for
expression in cells of the individual, wherein the target
antigen is a pathogen antigen, a cancer-associated antigen or
an antigen linked to cells associated with autoimmune
diseases.
The present invention relates to an improved live,
attenuated vaccine which comprises nucleotide sequence(s) that
encode one or more of human IL-12, GM-CSF, IL-la, TNF-a and
TNF-g, IL-2, IL-15, IL-18, IL-4, IL-5, IL-10 of BL-1 protein
or single chain IL-12 protein operably linked to regulatory
elements necessary for expression in eukaryotic cells.
The present invention relates to a method of
immunizing an individual against a pathogen, cancer or an
autoimmune disease comprising the step of administering to an
individual, an attenuated vaccine which comprises nucleotide
sequence(s) that encode one or more of human IL-12, GM-CSF,
IL-la, TNF-a and TNF-g, IL-2, IL-15, IL-18, IL-4, IL-5, IL-10
CA 02745736 2011-07-04
- 10 -
of BL-1 or single chain IL-12 protein operably linked to
regulatory elements necessary for expression in cells of the
individual.
The present invention relates to a plasmid which
comprises nucleotide sequence(s) that encode one or more of
human IL-12, GM-CSF, IL-la, TNF-a and TNF-g, IL-2, IL-15,
IL-18, IL-4, IL-5, IL-10 of BL-1 protein operably linked to
regulatory elements necessary for expression in eukaryotic
cells.
The present invention relates to a pair of plasmids
which one plasmid comprises a nucleotide sequence that encodes
human IL-12 protein p35 subunit operably linked to regulatory
elements necessary for expression in eukaryotic cells and the
other plasmid comprises a nucleotide sequence that encodes
human IL-12 protein p40 subunit operably linked to regulatory
elements necessary for expression in eukaryotic cells.
The present invention relates to a plasmid which
comprises a single nucleotide sequence that encodes a single
chain human IL-12 protein operably linked to regulatory
elements necessary for expression in eukaryotic cells wherein
the single chain human IL-12 protein is a single protein in
which the p35 and p40 subunits are connected to each other by
a linker sequences wherein when expressed the single chain
protein can form a biologically active IL-12 molecule.
The present invention relates to a pharmaceutical
composition which comprises a plasmid which comprises
nucleotide sequence(s) that encode one or more of IL-12, GM-
CSF, IL-la, TNF-a and TNF-g, IL-2, IL-15, IL-18, IL-4, IL-5,
IL-10 of BL-1 protein operably linked to regulatory elements
necessary for expression in eukaryotic cells and a
pharmaceutically acceptable carrier or diluent.
The present invention relates to a pharmaceutical
composition which comprises a pair of plasmids which one
plasmid comprises a nucleotide sequence that encodes human IL-
12 protein p35 subunit operably linked to regulatory elements
necessary for expression in eukaryotic cells and the other
plasmid comprises a nucleotide sequence that encodes human IL-
CA 02745736 2011-07-04
- 11 -
12 protein p40 subunit operably linked to regulatory elements
necessary for expression in eukaryotic cells and a
pharmaceutically acceptable carrier or diluent.
The present invention relates to a pharmaceutical
composition which comprises a plasmid which comprises a single
nucleotide sequence that encodes a single chain human IL-12
protein operably linked to regulatory elements necessary for
expression in eukaryotic cells wherein the single chain human
IL-12 protein is a single protein in which the p35 and p40
subunits are connected to each other by a linker sequences
wherein when expressed the single chain protein can form a
biologically active IL-12 molecule and a pharmaceutically
acceptable carrier or diluent.
The present invention relates to a method of
treating an individual who is suffering from an allergic
reaction, a pathogen infection, cancer or an autoimmune
disease comprising the step of administering to an individual,
a plasmid which comprises nucleotide sequences that encode IL-
12 protein operably linked to regulatory elements necessary
for expression in cells of the individual.
The present invention relates to a method of
treating an individual who is suffering from an allergic
reaction, a pathogen infection, cancer or an autoimmune
disease comprising the step of administering to an individual,
a pair of plasmids which one plasmid comprises a nucleotide
sequence that encodes human IL-12 protein p35 subunit operably
linked to regulatory elements necessary for expression in
eukaryotic cells and the other plasmid comprises a nucleotide
sequence that encodes human IL-12 protein p40 subunit operably
linked to regulatory elements necessary for expression in
eukaryotic cells.
The present invention relates to a method of
treating an individual who is suffering from an allergic
reaction, a pathogen infection, cancer or an autoimmune
disease comprising the step of administering to an individual,
a plasmid which comprises a single nucleotide sequence that
encodes a single chain human IL-12 protein operably linked to
CA 02745736 2011-07-04
- 12 -
regulatory elements necessary for expression in eukaryotic
cells wherein the single chain human IL-12 protein is a single
protein in which the p35 and p40 subunits are connected to
each other by a linker sequences wherein when expressed the
single chain protein can form a biologically active IL-12
molecule.
The present invention relates to a method of
enhancing or driving an immune response in an individual
toward a Thl type immune response comprising the step of
administering to an individual, a plasmid which comprises
nucleotide sequences that encode human IL-12 protein operably
linked to regulatory elements necessary for expression in
cells of the individual.
The present invention relates to a method of
enhancing a TH1 type immune response in an individual or
driving an immune response in an individual toward a Thl type
immune response comprising the step of administering to an
individual, a pair of plasmids which one plasmid comprises a
nucleotide sequence that encodes human IL-12 protein p35
subunit operably linked to regulatory elements necessary for
expression in eukaryotic cells and the other plasmid comprises
a nucleotide sequence that encodes human IL-12 protein p40
subunit operably linked to regulatory elements necessary for
expression in eukaryotic cells.
The present invention relates to a method of
enhancing a TH1 type immune response in an individual or
driving an immune response in an individual toward a Thi type
immune response comprising the step of administering to an
individual, a plasmid which comprises a single nucleotide
sequence that encodes a single chain human IL-12 protein
operably linked to regulatory elements necessary for
expression in eukaryotic cells wherein the single chain human
IL-12 protein is a single protein in which the p35 and p40
subunits are connected to each other by a linker sequences
wherein when expressed the single chain protein can form a
biologically active IL-12 molecule.
CA 02745736 2011-07-04
- 13 -
The present invention relates to a recombinant
vector which comprises nucleotide sequences that encode human
IL-12 protein operably linked to regulatory elements necessary
for expression in eukaryotic cells.
The present invention relates to a recombinant
vector which comprises a single nucleotide sequence that
encodes a single chain human IL-12 protein operably linked to
regulatory elements necessary for expression in eukaryotic
cells wherein the single chain human IL-12 protein is a single
protein in which the p35 and p40 subunits are connected to
each other by a linker sequences wherein when expressed the
single chain protein can form a biologically active IL-12
molecule.
The present invention relates to a pharmaceutical
composition which comprises a recombinant vector which
comprises nucleotide sequence(s) that encode one or more of
human IL-12, GM-CSF, IL-la, TNF-a and TNF-g, IL-2, IL-15,
IL-18, IL-4, IL-5, IL-10 of BL-1 protein operably linked to
regulatory elements necessary for expression in eukaryotic
cells and a pharmaceutically acceptable carrier or diluent.
The present invention relates to a pharmaceutical
composition which comprises a recombinant vector which
comprises a single nucleotide sequence that encodes a single
chain human IL-12 protein operably linked to regulatory
elements necessary for expression in eukaryotic cells wherein
the single chain human IL-12 protein is a single protein in
which the p35 and p40 subunits are connected to each other by
a linker sequences wherein when expressed the single chain
protein can form a biologically active IL-12 molecule and a
pharmaceutically acceptable carrier or diluent.
The present invention relates to a method of
treating an individual who is suffering from an allergic
reaction, a pathogen infection, cancer or an autoimmune
disease comprising the step of administering to an individual,
a recombinant vector which pomprises nucleotide sequences that
encode human IL-12 protein operably linked to regulatory
elements necessary for expression in eukaryotic cells.
CA 02745736 2011-07-04
- 14 -
The present invention relates to a method of
treating an individual who is suffering from an allergic
reaction, a pathogen infection, cancer or an autoimmune
disease comprising the step of administering to an individual,
a recombinant vector which comprises a single nucleotide
sequence that encodes a single chain human IL-12 protein
operably linked to regulatory elements necessary for
expression in eukaryotic cells wherein the single chain human
IL-12 protein is a single protein in which the p35 and p40
subunits are connected to each other by a linker sequences
wherein when expressed the single chain protein can form a
biologically active IL-12 molecule.
The present invention relates to a method of
enhancing a TH1 type immune response in an individual or
driving an immune response in an individual toward a Thl type
immune response comprising the step of administering to an
individual, a recombinant vector which comprises nucleotide
sequences that encode human IL-12 protein operably linked to
regulatory elements necessary for expression in eukaryotic
cells.
The present invention relates to a method of
enhancing a TH1 type immune response in an individual or
driving an immune response in an individual toward a Thl type
immune response comprising the step of administering to an
individual, a recombinant vector which comprises a single
nucleotide sequence that encodes a single chain human IL-12
protein operably linked to regulatdry elements necessary for
expression in eukaryotic cells wherein the single chain human
IL-12 protein is a single protein in which the p35 and p40
subunits are connected to each other by a linker sequences
wherein when expressed the single chain protein can form a
biologically active IL-12 molecule.
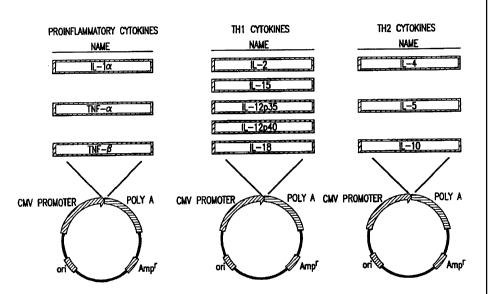
The present invention provides compositions that
comprise nucleic acid molecules that encode one or more human
proinflammatory cytokines, (IL-la, TNF-a and TNF-g), Thl
cytokines (IL-2, IL-15, and IL-18), and Th2 cytokines (IL-4,
IL-5 and IL-10) protein as a primary agent and to methods of
ak 02745736 2011-07-04
- 15 -
using the same to drive and direct an immune response by
administering such nucleic acid molecules to an individual.
The present invention relates to methods of enhancing
antigen-specific humoral response by the co-delivery of IL-5
or IL-18 with vaccines that introduce target immunogens such
as DNA vaccine constructs. The present invention relates to
methods of increasing antigen-specific T helper cell
proliferation by co-delivery of IL-2, IL-5, IL-10, IL-18, TNF-
u or TNF-g with vaccines that introduce target immunogens such
as DNA vaccine constructs. The present invention relates to
methods of enhancing the cytotoxic response with the
co-administration of TNF-u or IL-15 genes with vaccines that
introduce target immunogens such as DNA vaccine constructs.
The present invention provides compositions that
comprise nucleic acid molecules that encode human GM-CSF, and
to method of inducting and regulating immune responses by
delivering or co-delivering gene constructs that encode GM-
CSF. The present invention relates to methods of enhancing
antigen-specific antibody and T helper cell proliferation
responses by co-injection of GM-CSF genes with DNA vaccine
constructs.
The present invention relates to substantially pure
BL1 and immunomodulating fragments thereof.
The present invention relates to isolated nucleic
acid molecules that encode BL1 and immunomodulating fragment
thereof.
The present invention relates to nucleic acid probes
and primers specifically directed to nucleic acid molecules
that encode BL1, or immunomodulating fragments thereof.
The present invention relates to oligonucleotide
molecules that consist of a nucleotide sequence complementary
to a specific portion of the nucleotide sequence that encodes
BL1.
The present invention relates to vectors comprising
nucleic acid molecules encoding BL1 or immunomodulating
fragments thereof.
CA 02745736 2011-07-04
- 16 -
The present invention relates to recombinant
expression vectors that comprise nucleic acid sequences
that encode BL1 or immunomodulating fragments thereof.
The present invention relates to host cells that
comprise recombinant expression vectors which include
nucleic acid sequences that encode BL1 or immunomodulating
fragments thereof. The present invention relates to genetic
therapy vectors comprising nucleic acid molecules encoding
BL1 or immunomodulating fragments thereof.
The present invention relates to isolated antibody
which binds to a specific epitope on BL1.
The present invention is related to methods of making
BL1 and immunomodulating fragments thereof.
The present invention is related to methods of
modulating an immune response in an individual comprising
administering to the individual BL1 protein or an
immunomodulating fragment thereof, or a vector which
comprises a nucleotide sequence that encodes BL1 protein or
an immunomodulating fragment thereof. According to aspects
of the invention, the vector which comprises the BL1 coding
sequence is sufficient to modulate the immune response.
The present invention is related to methods of
enhancing and directing an immune response in an individual
comprising administering to the individual a vaccine
composition for delivery of an immunogen and a BL1 protein
or an immunomodulating fragment thereof, or a vector which
comprises a nucleotide sequence that encodes BL1 protein or
an immunomodulating fragment thereof. According to aspects
of the invention, the vector which comprises the BL1 coding
sequence is sufficient to modulate the immune response.
In one aspect, there is provided a plasmid comprising
a nucleotide sequence that encodes a) an immunomodulating
CA 02745736 2011-07-04
- 16a -
protein comprising: a) a nucleotide sequence that encodes
IL-12 p35 subunit operably linked to one regulatory
element; b) a nucleotide sequence that encodes IL-12 p40
subunit operably linked to a second regulatory element;
and c) a nucleotide sequence that encodes an immunogen.
In a further aspect, there is
provided a
pharmaceutical composition comprising a plasmid of the
invention and a pharmaceutically acceptable carrier.
In a further aspect, there is provided a use of a
plasmid of the invention for administration to an
individual for immunizing against a pathogen.
In a further aspect, there is provided a composition
comprising two or more plasmids including: a first plasmid
comprising a nucleotide sequence that encodes an
immunomodulating protein comprising: a) a
nucleotide
sequence that encodes IL-12 p35 subunit operably linked to
one regulatory element; b) a nucleotide sequence that
encodes IL-12 p40 subunit operably linked to a second
regulatory element; and a second plasmid comprising a
nucleotide sequence that encodes an
immunogen. Pharmaceutical compositions
comprising
this composition with a pharmaceutically acceptable carrier
are also provided. In a further aspect, there is provided a
use of this composition for administration to an individual
for immunizing against a pathogen.
In a further aspect, there is provided a recombinant
vaccine comprising a nucleotide sequence that encodes an
immunomodulating protein comprising: a) a
nucleotide
sequence that encodes IL-12 p35 subunit operably linked to
one regulatory element; b) a nucleotide sequence that
encodes IL-12 p40 subunit operably linked to a second
regulatory element; and c) a nucleotide sequence that
CA 02745736 2011-07-04
- 16b -
encodes an immunogen. Use of the recombinant vaccine of the
invention for administration to an individual for
immunizing against a pathogen is also provided.
BRIEF DESCRIPTION OF THE DRAWINGS
Figures 1A and 13 shows data from Example 4. In Figure
1A, 50 gg of respective cDNA expression cassettes were
administered intramuscularly at day 0. At 14 days post-
immunization, spleens harvested from all immunized animals
were weighed. The negative control animals were immunized.
CA 02745736 2011-07-04
- 17 -
the spleens from the mice injected with Gag/Pol alone and IL-
12 alone weighed similar to those from the unimmuniZed control
mice (about 100 mg). However, the spleens from mice injected
with Gag/Pol+IL-12 genes weighed almost three times as much
as the control spleens. In contrast, Gag/Pol+GM-CSF immunized
mouse spleens were not enlarged. In Figure 1B, the white
blood cells were prepared and purified from each spleen.
Corresponding directly to their spleen weight difference, the
number of cells from the Gag/Pol+IL-12 immunized spleens were
more than three the number derived from the control spleens.
Gag/Pol+GM-CSF immunized mouse spleens did not have any
significant increase in the number of lymphocytes above the
control spleen cell number.
In Figure 2, 50 g of respective cDNA expression
cassettes were administered intramuscularly at day 0. At 14
days post-immunization, spleens harvested and were
photographed. The visual size of the spleens corresponded
directly to the weights where the immunogen+IL-12 vaccinated
spleens were significantly larger than the unimmunized
control spleens. Groups: (-) unimmunized; IL-12 immunized;
Envelop+IL-12 immunized; Gag/Pol+IL-12 immunized.
In Figure 3, co-administration of each chain of IL-
12 was performed. 50 g of each plasmid was used. Both p35
and p40 chains as well as Gag/Pol were necessary for spleen
enlargement.
In Figure 4, 50 g of respective cDNA expression
cassettes were administered inttamuscularly at day 0.
Antisera from immunized mice were collected and analyzed for
specific antibody responses against HIV-1 antigens by ELISA.
The ELISA results from the samples collected at day 28 is
shown. At 1:100 dilution, sera from the group immunized with
Envelop+GM-CSF showed antibody response against HIV-1 gp120
protein which was greater than those of the group immunized
with Envelop only. On the other hand, the group immunized
with Envelop+IL-12 showed a significantly less humoral
response over the same period.
CA 02745736 2011-07-04
- 18 -
In Figure 5, activation and proliferation of T
helper lymphocytes play a critical role in inducing both
humoral immune response via expansion of antigen-activated B
cells and cellular immune response via expansion of CD8+
cytotoxic T cells. 50
gg of respective cDNA expression
cassettes were administered intramuscularly at day 0. The
harvested spleen cells were tested for T cell proliferation.
Recombinant p55 protein 20 fig/m1 was plated in each well to
stimulate proliferation of T cells. 10 gg/m1 of lectin PHA
was used as a polyclonal stimulator positive control
Stimulation index is the level of radioactivity detected from
the cells stimulated with specific protein divided by the
level detected from the cells in media. The stimulation index
of PHA stimulated control was 58.8.
In Figure 6, 50 Ag of respective cDNA expression
cassettes were administered intramuscularly at day 0. CTL
assay without in vitro stimulation as conducted using the
cells prepared from harvested spleens. The control group
immunized with only IL-12 gene cassette resulted in no
specific lysis of target cells above the background level.
In addition, low level (3=0 of specific lysis was observed
with Gag/Pal only immunization at the 50:1 effector:target
ratio.
In contrast, 62c.k. specific lysis was seen with
Gag/Pol+IL-12 co-administration samples at the 50:1
Effector:Target ratio and titered out to 9%; at the 12.5:1
Effector:Target ratio. Those immunized with Gag/Pol+GM-CSF
plasmids resulted in no detectable cm activity. The same CTL
assay conducted against targets prepared with irrelevant
antigen-expressing vaccinia did not result in any significant
CTL response.
In Figure 7, 50gg of respective cDNA expression
cassettes were administered intramuscularly at day 0. CTL
assays without in vitro stimulation was conducted using the
cells prepared from harvested spleens.
At 50:1
Effector:Target ratio, the :group immunized with Envelop only
and Envelop+GM-CSF resulted in low levels of specific CTL at
4% and 1%, respectively.
On the other hand, a dramatic
CA 02745736 2011-07-04
- 19 -
enhancement of CTL activity was seen from the Envelop+IL-12
group at 59%. The same CTL assay conducted against targets
prepared with irrelevant antigen-expressing vaccinia did not
result in any significant CTL response.
Figures 8A, 83 and 8C show plasmids useful in the
invention. Figure 8A shows a plasmid that includes a coding
sequence for IL-12 as a single chain protein. Figures 8B and
8C show plasmids each include two coding sequences for each
of the two subunits.
Figure 9 shows Table 3.
As shown in Figure 10, each cytokine gene was cloned
into expression plasmids under the control of a CMV promoter
and was transfected in vitro into RD cells. Expression of
each cytokine was verified using either immunoprecipitation
or cytokine ELISA.
Figures 11A-110 show results from experiments
determining MHC Class I-Restricted CTLs. As shown in Figures
11A-11E, two weeks after the first DNA co-injection with pCEnv
(50 Ag of each), the mice (four mice per group) were boosted
with same dosage. After 1 additional week, spleens were
collected from immunized mice and their lymphocytes were
isolated and tested for CTL response using target cells
prepared with envelope-specific peptide (RIHIGPGRAFYTTKN)
which has been reported to be MHC class I-restricted in balb/c
mice. As shown in Figures 11F-1101 two weeks after the first
DNA co-injection with pCGag/pol (50 Ag of each), the mice
(four mice per group) were boosted with same dosage. After
1 additional week, spleens were collected from immunized mice
and their lymphocytes were isolated and tested for CTL
response. A CTL assay was performed with the removal of CD8+
T cells by complement lysis. Effector cells were prepared as
described with the presence of CD8+ T cells (top) and the
removal of CD8+ T cells (bottom). These experiments have been
repeated two times with similar results.
Figure 12 show results from experiments evaluating
direct antigen-specific CTL (without in vitro stimulation of
effectors). Two weeks after the first DNA co-injection with
CA 02745736 2011-07-04
- 20 -
pCEnv (50 Ag of each), the mice (four mice per group) were
boosted with same dosage. After 1 additional week, spleens
were collected from immunized mice and their lymphocytes were
isolated and tested for CTL response using target cells
infected with specific (vMN462) and non-specific vaccinia
(vSC8). These experiments have been, repeated with similar
results.
Figure 13 shows a summary of the each cytokine
co-administration effects on antibody (y-axis), T helper
(x-axis), and cytotoxic T lymphocyte responses (z-axis). Each
cytokine is plotted on the 3-D axis according to its effects
on the three modes of immune response.
Figure 14 shows the nucleotide sequence (SEQ ID
NO:1) and the amino acid sequences encoded thereby (SEQ ID
NOS: 2-20).
.Figure 15 shows the ligation of BLI into PCR3
eukaryotic expression vector as well as the vector pBBKan.
Figure 16 shows results of ELISA assays comparing
anti-HIV antigen responses directed at the HIV antigen Nef
with and without con-administration of BL1.
Figure I7A, 17B, 17C and I7D shows results of assays
comparing anti-HIV antigen immune responses directed at the
HIV antigen Gag/Pol with and without co-administration of
BL1.
DETAILED DESCRIPTION OF THE INVENTION
As used herein, the term "immunomodulating protein"
is meant to refer to one of human IL-12, GM-CSF, IL-1, TNF-a,
TNF-g, IL-2, IL-15, IL-18, IL-4, IL-5 and IL-10, and a novel
molecule designated BL-1.
As used herein, the term "IL-12 genetic construct"
is meant to refer to plasmids which comprise coding sequences
that encode one or both human IL-12 protein subunits and/or
the immunogenic target protein, the coding sequences being
operably linked to regulatory elements required for expression
of the coding sequences :in eukaryotic cells. Regulatory
elements for DNA expression include a promoter and a
polyadenylation signal. In addition, other elements, such as
CA 02745736 2011-07-04
- 21 -
a Kozak region, may also be included in the genetic construct.
Initiation and termination signals are required regulatory
elements which are often considered part of the coding
sequence. The coding sequences of genetic constructs of the
invention include functional initiation and termination
signals.
As used herein, the term "desired IL-12 protein" is
meant to refer to one or both human IL-12 subunits including
single chain IL-12 proteins in which the two subunits are
encoded by a single coding sequence and expressed as a single
protein having a linker sequences connecting the two subunits.
As used herein, the term "desired protein" is meant
to refer to the immunogenic target protein encoded by the
coding sequence of a vaccine that comprises a nucleic acid
molecule that encodes an immunogenic target protein.
As used herein, the term "single chain protein" and
"single chain IL-12 protein" is meant to refer to a single
protein in which the IL-12 p35 and p40 subunits are connected
to each other by an amino acid linker that is sufficiently
long and flexible enough to allow the single protein to allow
the two subunit portions to interact with each other and
assume the conformation of the biologically active complex
that is IL-12.
Single chain IL-12 functions essentially
identical as IL-12 made up of p35 and p40.
The present
invention is meant to include the use of single chain IL-12
in all places where IL-12 is used. The single protein is
encoded by a single nucleotide sequence.
Interleukin-12 (IL-12), a heterodimeric cytokine
produced primarily by macrophages and B cells. IL-12 is
composed of two different subunits which are designated p35
and p40 (Podlaski, F.J. et a/. (1991) Arch. Biochem. Blophys.
294(1):230-237.
Different immune responses involve T cell
populations.
Specifically, there are two distinct types of
T cells, Type 1 T-helper cells (Th1) and Type 2 T-helper cells
(Th2), which differ from each other, among other things, in
their production of cytokines. IL-12 has been found to play
CA 02745736 2011-07-04
- 22 -
a critical role in Thl immune response mainly by inducing
production of Thl-associated cytokine interferon-gamma (IFN-
gamma). It activates natural killer (NK) and T cells through
induction and release of various cytokines including IFN-
gamma.
Aspects of the present invention relates to the use
of nucleic acid molecules that encode human IL-12 protein as
an immunomodulator. The nucleic acid molecules that encode
human IL-12 protein may be delivered as the primary active
agent, i.e. as a gene therapeutic, or as part of or in
conjunction with vaccine compositions such as vaccines which
comprise nucleic acid molecules that encode immunogenic target
proteins.
With regard to the use of nucleic acid molecules
that encode human IL-12 protein as a primary agent, the
present invention provides compositions and methods for
driving an immune response toward or enhancing a Thl immune
response by administering nucleic acid molecules that encode
human IL-12 protein to an individual. According to some
aspects of the invention, individuals suffering from allergy
disorders, pathogen infections, cancer or autoimmune diseases
can be treated by administering to such individuals, nucleic
acid molecules that include nucleotide sequences that encode
human IL-12 operably linked to regulatory elements such that
nucleic acid molecules are expressed in cells of the
individual. The nucleic acid molecules are taken up by the
cells and the nucleotide sequence that encodes human IL-12 is
expressed. The human IL-12 thus produced by the cell is
biologically active and its activity results in the induction
and/or enhancement of the immune response generated by the
individual. In some preferred embodiments, the nucleic acid
molecule that encodes human IL-12 protein is a plasmid.
Aspects of the invention include the use of nucleic
acid molecules that encode granulocyte-macrophage colony
stimulating factor (GM-CSF), GM-CSF is a hematopoietic growth
factor which stimulates neutrophil, monocyte/macrophage, and
eosinophil colony formation. It also induces proliferation
CA 02745736 2011-07-04
- 23 -
and differentiation of erythroid and megakaryocyte progenitor
cells. GM-CFS also increases the antibody-dependent cell-
mediated cytotoxicity of neutrophiles, eosinophils, and
macrophages but has not been reported to have a direct role
in the generation of CTL response in vivo. The
present
invention provides compositions and methods for driving an
immune response by administering gene constructs that include
nucleotide sequences that encode GM-CSF to an individual. The
nucleic acid molecules are taken up by the cells and the
nucleotide sequence that encodes GM-CSF is expressed and thus
produced by the cell. The GM-CSF is biologically active and
its activity results in the induction and/or enhancement of
the immune response generated by the individual. In some
preferred embodiments, the nucleic acid molecule that encodes
GM-CSF is a plasmid.
Aspects of the invention include the use of nucleic
acid molecules that encode human proinflammatory cytokines
(IL-1a, TNF-a and TNF-g), Thl cytokines (IL-2, IL-15, and
IL-18), and Th2 cytokines (IL-4, IL-5 and IL-10) protein as
a primary agent. The present invention provides compositions
and methods for driving an immune response toward or enhancing
an immune response by administering nucleic acid molecules
that encode human proinflammatory cytokines (IL-la, TNF-u and
TNF-0), Thl cytokines (IL-2, IL-15, and IL-18), and Th2
cytokines (IL-4, IL-5 and IL-10) to an individual. According
to some aspects of the invention, individuals are treated by
administering nucleic acid molecule's that include nucleotide
sequences that encode human proinflammatory cytokines (IL-la,
TNF-a and TNF-0), Thl cytokines (IL-2, IL-15, and IL-18), and
Th2 cytokines (IL-4, IL-5 and IL-10) operably linked to
regulatory elements such that nucleic acid molecules are
expressed in cells of the individual.
The nucleic acid
molecules are taken up by the cells and the nucleotide
sequence that encodes the protein is expressed. The human
protein thus produced by the cell is biologically active and
its activity results in the induction and/or enhancement of
CA 02745736 2011-07-04
- 24 -
the immune response generated by the individual. In some
preferred embodiments, the nucleic acid molecule is a plasmid.
With regard to the use of nucleic acid molecules that
encode human IL-12 protein as part of or in conjunction with
nucleic acid molecules that encode immunogenic target protein,
i.e. as part of a vaccine to induce an immune response against
the immunogenic protein, the nucleic acid molecules that encode
human IL-12 protein may be a component of a vaccine that
includes a nucleic acid molecules that encodes the immunogenic
target protein, a component of a vaccine that includes
immunogenic target, or a separate composition that is co-
administered with either a vaccine that includes a nucleic acid
molecules that encodes the immunogenic target protein or vaccine
that includes immunogenic target. In some preferred embodiments,
the nucleic acid molecule that encodes human IL-12 protein is a
plasmid and the vaccine is a DNA vaccine that comprises a
plasmid which encodes the immunogenic target protein. In some
preferred embodiments, the DNA vaccine comprises a plasmid which
encodes the immunogenic target protein and human IL-12 protein.
IL-12 is described in published PCT application WO
90/05147 published May 17, 1990.
Wolf, S.F. et al. 1991 J.
Immunol. 146 (9):3074-3081, discloses the nucleotide sequence of
cDNA that encodes IL-12 as well as the predicted amino acid
sequence of the IL-12 protein.
Native human IL-12 protein
consists of two subunits, p35 and p40. The two subunits form a
heterodimeric complex that is biologically active.
According to some embodiments of the invention, the
nucleotide sequences that encode each subunit of IL-12 are on a
single plasmid, non-plasmid nucleic acid molecule, or viral or
microbial genome, wherein the nucleotide sequence encoding each
subunit being under the control of its own set of regulatory
elements. In some preferred embodiments, coding sequences for
both subunits of IL-12 are on a single plasmid; each coding
sequence being operably linked to its own set of
CA 02745736 2011-07-04
- 25 -
regulatory elements. In some embodiments, the coding sequence
for a target immunogenic protein, operably linked to
regulatory elements, is on the same plasmid as the coding
sequences for both subunits. In some embodiments, the coding
sequence for a target immunogenic protein, operably linked to
regulatory elements, is on a separate plasmid from a plasmid
which contains the coding sequences for both subunits and the
two plasmids are delivered to an individual.
According to some embodiments of the invention, the
nucleotide sequence that encodes the p35 subunit is on a first
plasmid and the nucleotide sequence that encodes the p40
subunit is on a second plasmid and the two plasmids are co-
administered to the same site on an individual.
In some
embodiments, the coding sequence for a target immunogenic
protein, operably linked to regulatory elements, is on the
same plasmid as the coding sequences for the p35 subunit. In
some embodiments, the coding sequence for a target immunogenic
protein, operably linked to regulatory elements, is on the
same plasmid as the coding sequences for the p40 subunit. In
some embodiments, the coding sequence for a target immunogenic
protein, operably linked to regulatory elements, is on a
separate plasmid from either plasmid which contains the coding
sequences for respective subunits and the three plasmids are
delivered to an individual.
IL-12 protein, and the nucleotide sequence encoding
it, may be modified so that the two subunits are encoded by
a single nucleotide sequence and expressed as a single chain
(fusion) protein molecule. According to the invention, a
linker amino acid sequence is provided which essentially
connects the two subunits but which is flexible enough so that
a biologically active protein can form by the complexing of
different portions of the single chain protein. Figure BA
shows an example of a single chain protein in which the coding
sequence for the single chain protein is under the control of
a human cytomegalovirus promoter. The coding sequence of the
single chain protein includes, from 5' to 3', the coding
sequence of the p35 subunit, a coding sequence for a linker
CA 02745736 2011-07-04
- 26 -
and the coding sequence of the p40 subunit as a single coding
sequence.
It is contemplated that in an alternative
arrangement, the coding sequence of the single chain protein
includes the coding sequence of the p40 subunit, a coding
sequence for a linker and the coding sequence of the p35
subunit as a single coding sequence. The linker must be long
enough and flexible enough to allow the two parts of the
single protein to assume positions relative to each other such
that a biologically active complex is formed.
According to some embodiments of the invention, the
nucleotide sequences that encode single chain IL-12 proteins
in which the two subunits are joined by a linker to form a
single protein are incorporated into a plasmid, non-plasmid
nucleic acid molecule, or viral or microbial genome, and
operably linked to regulatory elements necessary for
expression in eukaryotic cells. In preferred embodiments, the
nucleotide sequences that encode the single chain proteins in
which the two subunits are joined by a linker to form a single
protein are incorporated into a plasmid. In some embodiments,
the coding sequence for a target immunogenic protein, operably
linked to regulatory elements, is on the same plasmid as the
coding sequences for the single chain IL-12 protein. In some
embodiments, the coding sequence for a target immunogenic
protein, operably linked to regulatory elements, is on a
separate plasmid from the plasmid which contains the coding
sequences for the single chain protein and the two plasmids
are delivered to an individual.
According to aspects of the present invention relate
to improved methods and compositions for vaccination,
particularly DNA vaccination in which DNA that encodes target
immunogens is administered into the individual in whom the DNA
is taken up and expressed and an immune response is generated
against the immunogen. According to aspects of the invention,
DNA that encodes immunomodulating proteins is co-delivered to
the individual and the expression of such DNA produces the
immunomodulating protein which controls the magnitude and
direction of the immune response in order to induce specific
CA 02745736 2011-07-04
- 27 -
immune responses to tailor the immunization more closely to
the correlates of protection which vary from disease to
disease.
It has been discovered that the co-production of IL-
12 protein in cells of a vaccinated individual that are
expressing target antigens results in an surprisingly enhanced
immune response against the target antigen. By providing an
expressible form of nucleotide sequence that encodes IL-12
protein, vaccines which function by expressing target antigen
in the cells of the vaccinated individual, such as DNA
vaccines, recombinant vector vaccines and attenuated vaccines,
the vaccines are improved.
The co-production of IL-12 in cells producing
antigens results in enhanced cellular immunity against the
antigen. Accordingly, the present invention provides improved
vaccines by providing a nucleotide sequence that encodes IL-12
operably linked to necessary regulatory sequences for
expression in vaccinees as part of vaccines such as DNA
vaccines, avirulent recombinant vector vaccines and live
attenuated vaccines.
The present invention provides induction and
regulation of immune responses from the co-delivery of gene
constructs that encode GM-CSF. Co-injection of GM-CSF genes
with DNA vaccine constructs enhances antigen-specific antibody
and T helper cell proliferation responses.
The present invention provides induction and
regulation of immune responses froth the co-delivery of gene
constructs that encode proinflammatory cytokines (IL-la, TNF-a
and TNF-g), Thl cytokines (IL-2, IL-15, and IL-18), and Th2
cytokines (IL-4, IL-5 and IL-10).
Some aspects of the present invention provide a
significant enhancement of antigen-specific humoral response
by the co-delivery of IL-5 or IL-18. =
Some aspects of the present invention provide an
increase in antigen-specific T helper cell proliferation by
co-delivery of IL-2 IL-5, IL-10, IL-18, TNF-a or TNF-g.
CA 02745736 2011-07-04
=
- 28 -
Some aspects of the present invention provide an
enhancement of the cytotoxic response with the
co-administration of TNF-u or IL-15 genes.
Thus, in a case where T cell mediated response is
paramount, but the humoral response may not be needed or even
be harmful, IL-12 genes are preferred as the immune modulator
to be co-delivered with a specific DNA immunogen. On the other
hand, for building vaccines to target extracellular bacteria,
for example, IL-4, IL-5 or IL-10 genes could be co-injected.
Furthermore, in cases where both CD4+ T helper cells and
antibodies play more important roles in protection, GM-CSF as
well as IL-2 could be co-delivered. Lastly, in cases where all
three arms of immune responses are critical, TNF-a could be
co-injected to give a combined enhancement of antibody, T
helper cell, and CTL responses.
The nucleotide and amino acid sequences of human n-
ice are well known and set forth in Telford, et al. (1986)
Nucl. Acids Res. 14:9955-9963, Furutani, et al. (1985) Nucl.
Acids Res. 14:3167-3179, March, et al. (1985) Nature 315:641-
647, and accession code Swissprot P01583.
The nucleotide and amino acid sequences of human IL-
2 are well known and set forth in Holbrook, et al. (1984)
Proc. Natl. Acad. Sci. USA 81:1634-1638, Fujita, et al. (1983)
Proc. Natl. Acad. Sci. USA 80:7437-7441, Fuse, et al. (1984)
Nucl. Acids Res.
12:9323-9331, Taniguchi, et al. (1983)
Nature 302:305-310, Maeda, et al. '(1983) Biochem. Biophys.
Res. Comm. 115:1040-1047, Devos, et al. (1983) Nucl. Acids
Res. 11:4307-4323, and accession code Swissprot P01585.
The nucleotide and amino acid sequences of human IL-
4 are well known and set forth in Arai, et al. (1989) J.
Immunol. 142:274-282, Otsuka, et al. (1987) Nucl. Acids Res.
15:333-344, Yokota, et al. (1986) Proc. Natl. Acad. Sci. USA
83:5894-5898, Noma, et al. (1984) Nature 319:640-646, Lee, et
al. (1986) Proc. Natl. Acad. Sci. USA 83:2061-2063, and
accession code Swissprot 05112 (the accession code for murine
CA 02745736 2011-07-04
- 29 -
IL-4 is Swissprot 07750).
The nucleotide and amino acid sequences of human
IL-5 are well known and set forth in Campbell, et al.
(1987) Proc. Natl. Acad. Sci. USA 84:6629-6633, Tanabe, et
al. (1987) J. Biol. Chem. 262:16580-16584, Campbell, et
al. (1988) Eur. J. Biochem. 174:345-352, Azuma, et al.
(1986) Nucl. Acids Res. 14:9149-9158, Yokota, et al.
(1986) Proc. Natl. Acad. Sci. USA 84:7388-7392, and
accession code Swissprot P05113.
The nucleotide and amino acid sequences of human
IL-10 are well known and set forth in Viera, et al. (1991)
Proc. Natl. Acad. Sci. USA 88:1172-1176, =and accession
code Swissprot P22301.
The nucleotide and amino acid sequences of human
IL-15 are well known and set forth in Grabstein, et al.
(1994) Science 264:965-968, and accession code Swissprot
U03099.
The nucleotide and amino acid sequences of human
IL-18 are well known and set forth in Ushio, et al. (1996)
J. Immunol. 156:4274-4279, and accession code D49950.
The nucleotide and amino acid sequences of human
TNF-a are well known and set forth in Pennica, (1984)
Nature 312:724-729, and accession code Swissprot P01375.
The nucleotide and amino acid sequences of human
TNF-0 are well known and set forth in Gray, (1984) Nature
312:721-724, and accession code Swissprot P01374.
DNA vaccines are described in U.S. Patent No.
5,593,972, U.S. Patent No. 5,589,466, PCT/US90/01515,
PCT/US93/02338, PCT/US93/048131, and PCT/U594/00899, and
the priority applications cited therein each of the
patents and published patent applications. In addition to
the delivery protocols described in those applications,
alternative methods of
CD, 02745736 2011-07-04
- 30 -
delivering DNA are described in U.S. Patent Nos. 4,945,050 and
5,036,006.
An improvement of the present invention relates to
the inclusion of genetic material for the co-production "of an
immunomodulating protein in addition to the production of the
antigenic target encoded by nucleic acid sequences of the DNA
vaccines.
The present invention relates to methods of
introducing genetic material into the cells of an individual
in order to induce immune responses against proteins and
peptides which are encoded by the genetic material.
The
methods comprise the steps of administering to the tissue of
said individual, either a single nucleic acid molecule that
comprises a nucleotide sequence that encodes a target protein
and a nucleotide sequence that encodes an immunomodulating
protein, or a composition having two nucleic acid molecules,
one that comprises a nucleotide sequence that encodes a target
protein and one that comprises a nucleotide sequence that
encodes an immunomodulating protein.
The nucleic acid
molecule(s) may be provided as plasmid DNA, the nucleic acid
molecules of recombinant vectors or as part of the genetic
material provided in an attenuated vaccine.
According to the present invention, compositions and
methods are provided which prophylactically and/or
therapeutically immunize an individual against a pathogen or
abnormal, disease-related cell. The genetic material that
encodes a target protein, i.e. a =peptide or protein that
shares at least an epitope with an immunogenic protein found
on the pathogen or cells to be targeted, and genetic material
that encodes an immunomodulating protein. The
genetic
material is expressed by the individual's cells and serves as
an immunogenic target against which an immune response is
elicited.
The resulting immune response reacts with a
pathogen or cells to be targeted and is broad based: in
addition to a humoral immune response, both arms of the
= cellular immune response are elicited. The methods of the
present invention are useful for conferring prophylactic and
CA 02745736 2011-07-04
- 31 -
therapeutic immunity. Thus, a method of immunizing includes
both methods of protecting an individual from pathogen
challenge, or occurrence or proliferation of specific cells
as well as methods of treating an individual suffering from
pathogen infection, hyperproliferative disease or autoimmune
disease.
As used herein the term "target protein" is meant
to refer to peptides and protein encoded by gene constructs
of the present invention which act as target proteins for an
immune response. The
term "target protein" refers to a
protein against which an immune response can be elicited. The
target protein is an immunogenic protein which shares at least
an epitope with a protein from the pathogen or undesirable
cell-type such as a cancer cell or a cell involved in
autoimmune disease against which immunization is required.
The immune response directed against the target protein will
protect the individual against and treat the individual for
the specific infection or disease with which the target
protein is associated. The target protein does not need to
be identical to the protein against which an immune response
is desired. Rather, the target protein must be capable of
inducing an immune response that cross reacts to the protein
against which the immune response is desired.
The present invention is useful to elicit broad
immune responses against a target protein, i.e. proteins
specifically associated with pathogens or the individual's own
"abnormal" cells. The present invention is useful to immunize
individuals against pathogenic agents and organisms such that
an immune response against a pathogen protein provides
protective immunity against the pathogen. The
present
invention is useful to combat hyperproliferative diseases and
disorders such as cancer by eliciting an immune response
against a target protein that is specifically associated with
the hyperproliferative cells. The present invention is useful
to combat autoimmune diseases and disorders by eliciting an
immune response against a target protein that is specifically
associated with cells involved in the autoimmune condition.
CA 02745736 2011-07-04
- 32 -
According to the present invention, DNA or RNA that
encodes a target protein and an immunomodulating protein is
introduced into the cells of tissue of an individual where it
is expressed, thus producing the target protein. The DNA or
RNA sequences encoding the target protein and an
immunomodulating protein are linked to regulatory elements
necessary for expression in the cells of the individual.
Regulatory elements for DNA expression include a promoter and
a polyadenylation signal. In addition, other elements, such
as a Kozak region, may also be included in the genetic
construct.
As used herein, the term "expressible form" refers
to gene constructs which contain the necessary regulatory
elements operable linked to a coding sequence that encodes a
target protein or immunomodulating protein, such that when
present in the cell of the individual, the coding sequence
will be expressed.
As used herein, the term "sharing an epitope" refers
to proteins which comprise at least one epitope that is
identical to or substantially similar to an epitope of another
protein.
As used herein, the term "substantially similar
epitope" is meant to refer to an epitope that has a structure
which is not identical to an epitope of a protein but
nonetheless invokes an cellular or humoral immune response
which cross reacts to that protein.
Genetic constructs comprise a nucleotide sequence
that encodes a target protein and/or immunomodulating protein
operably linked to regulatory elements needed for gene
expression. According to the invention, combinations of gene
constructs which include one that comprises an expressible
form of the nucleotide sequence that encodes a target protein
and one that includes an expressible form of the nucleotide
sequence that encodes immunomodulating protein are provided.
Incorporation into a living cell of the DNA or RNA molecule(s)
which include the combination of gene constructs results in
the expression of the DNA or RNA and production of the target
CA 02745736 2011-07-04
- 33 -
protein and immunomodulating protein. An enhanced immune
response against the target protein results.
When taken up by a cell, the genetic construct(s)
may remain present in the cell as a functioning
extrachromosomal molecule and/or integrate into the cell's
chromosomal DNA. DNA may be introduced into cells where it
remains as separate genetic material in the form of a plasmid
or plasmids. Alternatively, linear DNA which can integrate
into the chromosome may be introduced into the cell. When
introducing DNA into the cell, reagents which promote DNA
integration into chromosomes may be added. DNA sequences
which are useful to promote integration may also be included
in the DNA molecule. Alternatively, RNA may be administered
to the cell. It is also contemplated to provide the genetic
construct as a linear minichromosome including a centromere,
telomeres and an origin of replication. Gene constructs may
remain part of the genetic material in attenuated live
microorganisms or recombinant microbial vectors which live in
cells. Gene constructs may be part of genomes of recombinant
viral vaccines where the genetic material either integrates
into the chromosome of the cell or remains extrachromosomal.
Genetic constructs include regulatory elements
necessary for gene expression of a nucleic acid molecule. The
elements include: a promoter, an initiation codon, a stop
codon, and a polyadenylation signal. In addition, enhancers
are often required for gene expression of the sequence that
encodes the target protein or the immunomodulating protein.
It is necessary that these elements be operable linked to the
sequence that encodes the desired proteins and that the
regulatory elements are operably in the individual to whom
they are administered.
Initiation codons and stop codon are generally
considered to be part of a nucleotide sequence that encodes
the desired protein. However, it is necessary that these
elements are functional in the individual to whom the gene
construct is administered. The initiation and termination
codons must be in frame with the coding sequence.
CA 02745736 2011-07-04
- 34 -
Promoters and polyadenylation signals used must be
functional within the cells of the individual.
Examples of promoters useful to practice the present
invention, especially in the production of a genetic vaccine
for humans, include but are not limited to promoters from
Simian Virus 40 (SV40), Mouse Mammary Tumor Virus (MMTV)
promoter, Human Immunodeficiency Virus (HIV) such as the HIV
Long Terminal Repeat (LTR) promoter, Moloney virus, ALV,
Cytomegalovirus (CMV) such as the CMV immediate early
promoter, Epstein Barr Virus (EBV), Rous Sarcoma Virus (RSV)
as well as promoters from human genes such as human Actin,
human Myosin, human Hemoglobin, human muscle creatine and
human metalothionein.
Examples of polyadenylation signals useful to
practice the present invention, especially in the production
of a genetic vaccine for humans, include but are not limited
to SV40 polyadenylation signals and LTR polyadenylation
signals. In particular, the SV40 polyadenylation signal which
is in pCEP4 plasmid (Invitrogen, San Diego CA), referred to
as the SV40 polyadenylation signal, is used.
In addition to the regulatory elements required for
DNA expression, other elements may also be included in the DNA
molecule. Such additional elements include enhancers. The
enhancer may be selected from the group including but not
limited to: human Actin, human Myosin, human Hemoglobin, human
muscle creatine and viral enhancers such as those from CMV,
RSV and EBV.
Genetic constructs can be provided with mammalian
origin of replication in order to maintain the construct
extrachromosomally and produce multiple copies of the
construct in the cell.
Plasmids pCEP4 and pREP4 from
Invitrogen (San Diego, CA) contain the Epstein Barr virus
origin of replication and nuclear antigen EBNA-1 coding region
which produces high copy episomal replication without
integration.
In some preferred embodiments related to
immunization applications, nucleic acid molecule(s) are
CA 02745736 2011-07-04
- 35 -
delivered which include nucleotide sequences that encode a
target protein, IL-12 protein and, additionally, genes for
proteins which further enhance the immune response against
such target proteins. Examples of such genes are those which
encode cytokines and lymphokines such as ce-Interferon, gamma-
interferon, platelet derived growth factor (PDGF), GC-SF, GM-
CSF, TNF, epidermal growth factor (EGF), IL-1, IL-2, IL-4, IL-
6, IL-8, IL-10 and E7.2. In some embodiments, it is preferred
that the gene for GM-CSF is included in genetic constructs
used in immunizing compositions.
An additional element may be added which serves as
a target for cell destruction if it is desirable to eliminate
cells receiving the genetic construct for any reason. A
herpes thymidine kinase (tk) gene in an expressible form can
be included in the genetic construct. The drug gangcyclovir
can be administered to the individual and that drug will cause
the selective killing of any cell producing tk, thus,
providing the means for the selective destruction of cells
with the genetic construct.
In order to maximize protein production, regulatory
sequences may be selected which are well suited for gene
expression in the cells the construct is administered into.
Moreover, codons may be selected which are most efficiently
transcribed in the cell. One having ordinary skill in the art
can produce DNA constructs which are functional in the cells.
Examples two types of backbones include one type for
use in two plasmid systems, and one type for use in single
plasmid systems. In two plasmid systems, one plasmid has an
expressible form of target coding sequence and one has an
expressible form of IL-12 coding sequence. In single plasmid
systems, the single plasmid contains expressible forms of both
target coding sequence and IL-12 coding sequence.
The method of the present invention comprises the
steps of administering nucleic acid molecules to tissue of the
individual. In some preferred embodiments, the nucleic acid
molecules are administered intramuscularly, intranasally,
intraperatoneally, subcutaneously,
intradermally,
CA 02745736 2011-07-04
- 36 -
intravenously, by aerosol administration to lung tissue or
topically or by lavage to mucosal tissue selected from the
group consisting of vaginal, rectal, urethral, buccal and
sublingual.
In some embodiments, the nucleic acid molecule is
delivered to the cells in conjunction with administration of
a facilitating agent. Facilitating agents are also referred
to as polynucleotide function enhancers or genetic vaccine
facilitator agents. Facilitating agents are described in
U.S. Patent No. 7,001,759 and U.S. Patent Number 5,593,972
issued January 14, 1997. In addition, facilitating agents
are described in U.S. Patent Nos. 5,962,428 and 6,127,170,
and U.S. Patent Application Publication No. 2005/0239204.
Facilitating agents which are administered in conjunction
with nucleic acid molecules may be administered as a mixture
with the nucleic acid molecule or administered separately
simultaneously, before or after administration of nucleic
acid molecules. In addition, other agents which may function
transfecting agents and/or replicating agents and/or
inflammatory agents and which may be co-administered with or
without a facilitating agent include growth factors,
cytokines and lymphokines such as a- interferon, gamma-
interferon, platelet derived growth factor (PDGF), GC-SF,
GM-CSF, TNF, epidermal growth factor (EGF), IL-1, IL-2, IL-
4, IL-6, IL-8, IL-10, IL-12 and 37.2 as well as fibroblast
growth factor, surface active agents such as immune-
stimulating complexes (ISCOMS), Freund's incomplete
adjuvant, LPS analog including monophosphoryl Lipid A (MPL),
muramyl peptides, quinone analogs and vesicles such as
squalene and squalene, and hyaluronic acid.
In some preferred embodiments, the genetic
constructs of the invention are formulated with or
administered in conjunction with a facilitator selected from
the group consisting of benzoic acid esters, anilides,
CA 02745736 2011-07-04
- 37 -
amidines, urethans and the hydrochloride salts thereof such
as those of the family of local anesthetics.
The facilitators in some preferred embodiments may
be a compound having one of the following formulae:
Ar _ R1 _ 0 _ R2 _ R3
or
Ar - N - R1 - R2 -
or
R4 _ N _ R5 _ R6
or
R4 - 0 - R1 - N- R'
wherein:
Pr is benzene, p-aminobenzene, m-aminobenzene, o-
aminobenzene, substituted benzene, substituted p-aminobenzene,
substituted m-aminobenzene, substituted o-aminobenzene,
wherein the amino group in the aminobenzene compounds can be
amino, C1-05 alkylamine, C1-05, C1-05 dialkylamine and
substitutions in substituted compounds are halogen, C1-05
alkyl and C1-05 alkoxy;
R1 is C=0;
R2 is C1-C10 alkyl including branched alkyls;
R3 is .hydrogen, amine, C1-05 alkylamine, CI-05, C1-05
dialkylamine;
R2 + R3 can form a cyclic alkyl, a C1-C10 alkyl
substituted cyclic alkyl, a cyclic aliphatic amine, a Cl-Clo
alkyl substituted cyclic aliphatic amine, a heterocycle, a C1-
C10 alkyl substituted heterocycle including a C1-C1.0 alkyl N-
substituted heterocycle;
R4 is Ar, R2 or C1-05 alkoxy, a cyclic alkyl,a
alkyl substituted cyclic alkyl, a cyclic aliphatic amine, a
C1-C10 alkyl substituted cyclic aliphatic amine, a heterocycle,
a C1-00 alkyl substituted heterocycle and a Cl-C10 alkoxy
substituted heterocycle including a C1-C alkyl N-substituted
heterocycle;
R5 is C=NH;
CA 02745736 2011-07-04
- 38 -
R6 is Ar, R' or C1-05 alkoxy, a cyclic alkyl,a C1-C10
alkyl substituted cyclic alkyl, a cyclic aliphatic amine, a
C1-C10 alkyl substituted cyclic aliphatic amine, a heterocycle,
a C1-C10 alkyl substituted heterocycle and a C fC 10 alkoxy
substituted heterocycle including a C1-00 alkyl N-substituted
heterocycle; and.
R7 is Ar, le or C1-05 alkoxy, a cyclic alkyl,a C1-C10
alkyl substituted cyclic alkyl, a cyclic aliphatic amine, a
C1-C10 alkyl substituted cyclic aliphatic amine, a heterocycle,
a C1-C10 alkyl substituted heterocycle and a C-c nalkoxy
substituted heterocycle including a C1-C10 alkyl N-substituted
heterocycle.
Examples of esters include: benzoic acid esters such
as piperocaine, meprylcaine and isobucaine; pera-aminobenzoic
acid esters such as procaine, tetracaine, butethamine,
propoxycaine and chloroprocaine; meta-aminobenzoic acid esters
including metabuthamine and primacaine; and para-ethoxybenzoic
acid esters such as parethoxycaine. Examples of anilides
include lidocaine, etidocaine, mepivacaine, bupivacaine,
pyrrocaine and prilocaine. Other examples of such compounds
include dibucaine, benzocaine, dyclonine, pramoxine,
proparacaine, butacaine, benoxinate, carbocaine, methyl
bupivacaine, butasin picrate, phenacaine, diothan, luccaine,
intracaine, nupercaine, met abutoxycaine,
piridocaine,
biphenamine and the botanically-derived bicyclics such as
cocaine, cinnamoylcocaine, truxilline and cocaethylene and all
such compounds complexed with hydrdchloride.
In preferred embodiments, the facilitator is
bupivacaine. The difference between bupivacaine and
mepivacaine is that bupivacaine has a N-butyl group in place
of an N-methyl group of mepivacaine. Compounds may have at
that N, C1-00. Compounds may be substituted by halogen such
as procaine and chloroprocaine. The anilides are preferred.
The facilitating agent is administered prior to,
simultaneously with or subsequent to the genetic construct.
The facilitating agent and the genetic construct may be
formulated in the same composition.
CA 02745736 2011-07-04
- 39 -
Bupivacaine-HC1 is chemically designated as 2-
piperidinecarboxamide, 1-
butyl-N-(2,6-dimethylpheny1)-
monohydrochloride, monohydrate and is widely available
commercially for pharmaceutical uses from many sources
including from Astra Pharmaceutical Products Inc. (Westboro,
MA) and Sanofi Winthrop Pharmaceuticals (New York, NY),
Eastman Kodak (Rochester, NY). Bupivacaing is commercially
formulated with and without methylparaben and with or without
epinephrine. Any such formulation may be used.
It is
commercially available for pharmaceutical use in concentration
of 0.25%, 0.5% and 0.75% which may be used on the invention.
Alternative concentrations, particularly those between 0.05% -
1.0% which *elicit desirable effects may be prepared if
desired. According to the present invention, about 250 g to
about 10 mg of bupivacaing is administered. In
some
embodiments, about 250 g to about 7.5 mg is administered. In
some embodiments, about 0.05 mg to about 5.0 mg is
administered. In some embodiments, about 0.5 mg to about 3.0
mg is administered. In some embodiments about 5 to 50 g is
administered. For example, in some embodiments about 50 gl
to about 2 ml, preferably 50 Al to about 1500 Al and more
preferably about 1 ml of 0.25-0.50% bupivacaing-HCl and 0.1%
methylparaben in an isotonic pharmaceutical carrier is
administered at the same site as the vaccine before,
simultaneously with or after the vaccine is administered.
Similarly, in some embodiments, about 50 gl to about 2 ml,
preferably 50 gl to about 1500 Al and more preferably about
1 ml of 0.25-0.50% bupivacaing-HC1 in an isotonic
pharmaceutical carrier is administered at the same site as the
vaccine before, simultaneously with or after the vaccine is
administered. Bupivacaine and any other similarly acting
compounds, particularly those of the related family of local
anesthetics may be administered at concentrations which
provide the desired facilitation of uptake of genetic
constructs by cells.
In some embodiments of the invention, the individual
is first subject to injection of the facilitator prior to
CA 02745736 2011-07-04
- 40 -
adminstration of the genetic construct. That is, up to, for
example, up to a about a week to ten days prior to
administration of the genetic construct, the individual is
first injected with the facilitator. In some embodiments, the
individual is injected with facilitator about 1 to 5 days, in
some embodiments 24 hours, before or after administration of
the genetic construct. Alternatively, if used at all, the
facilitator is administered simultaneously, minutes before or
after adminstration of the genetic construct. Accordingly,
the facilitator and the genetic construct may be combined to
form a single pharmaceutical compositions.
In some embodiments, the genetic constructs are
administered free of facilitating agents, that is in
formulations free from facilitating agents using adminstration
protocols in which the genetic constructions are not
administered in conjunction with the administration of
facilitating agents.
Nucleic acid molecules which are delivered to cells
according to the invention may serve as genetic templates for
proteins that function as prophylactic and/or therapeutic
immunizing agents. In preferred embodiments, the nucleic acid
molecules comprise the necessary regulatory sequences for
transcription and translation of the coding region in the
cells of the animal.
The present invention may be used to immlnize an
individual against all pathogens such as viruses, prokaryote
and pathogenic eukaryotic organibms such as unicellular
pathogenic organisms and multicellular parasites. The present
invention is particularly useful to immunize an individual
against those pathogens which infect cells and which are not
encapsulated such as viruses, and prokaryote such as
gonorrhea, listeria and shigella. In addition, the present
invention is also useful to immunize an individual against
protozoan pathogens which include a stage in the life cycle
where they are intracellular pathogens. As used herein, the
term "intracellular pathogen" is meant to refer to a virus or
pathogenic organism that, at least part of its reproductive
CA 02745736 2011-07-04
- 41 -
or life cycle, exists within a host cell and therein produces
or causes to be produced, pathogen proteins. Table 1 provides
a listing of some of the viral families and genera for which
vaccines according to the present invention can be made. DNA
constructs that comprise DNA sequences which encode the
peptides that comprise at least an epitope identical or
substantially similar to an epitope displayed on a pathogen
antigen such as those antigens listed on the tables are useful
in vaccines. Moreover, the present invention is also useful
to immunize an individual against other pathogens including
prokaryotic and eukaryotic protozoan pathogens as well as
multicellular parasites such as those listed on Table 2.
In order to produce a genetic vaccine to protect
against pathogen infection, genetic material which encodes
immunogenic proteins against which a protective immune
response can be mounted must be included in a genetic
construct as the coding sequence for the target. Whether the
pathogen infects intracellularly, for which the present
invention is particularly useful, or extracellularly, it is
unlikely that all pathogen antigens will elicit a protective
response. Because DNA and RNA are both relatively small and
can be produced relatively easily, the present invention
provides the additional advantage of allowing for vaccination
with multiple pathogen antigens. The genetic construct used
in the genetic vaccine can include genetic material which
encodes many pathogen antigens. For example, several viral
genes may be included in a single construct thereby providing
multiple targets.
Tables 1 and 2 include lists of some of the
pathogenic agents and organisms for which genetic vaccines can
be prepared to protect an individual from infection by them.
In some preferred embodiments, the methods of immunizing an
individual against a pathogen are directed against HIV, HTLV
or HBV.
Another aspect of the present invention provides a
method of conferring a broad based protective immune response
against hyperproliferating cells that are characteristic in
CA 02745736 2011-07-04
- 42 -
hyperproliferative diseases and to a method of treating
individuals suffering from hyperproliferative diseases. As
used herein, the term "hyperproliferative diseases" is meant
to refer to those diseases and disorders characterized by
hyperproliferation of cells. Examples of hyperproliferative
diseases include all forms of cancer and psoriasis.
It has been discovered that introduction of a
genetic construct that includes a nucleotide sequence which
encodes an immunogenic "hyperproliferating cell"-associated
protein into the cells of an individual results in the
production of those proteins in the vaccinated cells of an
individual. As used herein, the term "hyperproliferative-
associated protein" is meant to refer to proteins that are
associated with a hyperproliferative disease. To immunize
against hyperproliferative diseases, a genetic construct that
includes a nucleotide sequence which encodes a protein that
is associated with a hyperproliferative disease is
administered to an individual.
In order for the hyperproliferative-associated
protein to be an effective immunogenic target, it must be a
protein that is produced exclusively or at higher levels in
hyperproliferative cells as compared to normal cells. Target
antigens include such proteins, fragments thereof and peptides
which comprise at least an epitope found on such proteins.
In some cases, a hyperproliferative-associated protein is the
product of a mutation of a gene that encodes a protein. The
mutated gene encodes a protein which is nearly identical to
the normal protein except it has a slightly different amino
acid sequence which results in a different epitope not found
on the normal protein. Such target proteins include those
which are proteins encoded by oncogenes such as myb, myc, fyn,
and the translocation gene bcr/abl, ras, src, P53, neu, trk
and EGRF.
In addition to oncogene products as target
antigens, target proteins for anti-cancer treatments and
protective regimens include variable regions of antibodies
made by B cell lymphomas and variable regions of T cell
receptors of T cell lymphomas which, in some embodiments, are
CA 02745736 2011-07-04
- 43 -
also used target antigens for autoimmune disease.
Other
tumor-associated proteins can be used as target proteins such
as proteins which are found at higher levels in tumor cells
including the protein recognized by monoclonal antibody 17-1A
and folate binding proteins.
While the present invention may be used to immunize
an individual against one or more of several forms of cancer,
the present invention is particularly useful to
prophylactically immunize an individual who is predisposed to
develop a particular cancer or who has had cancer and is
therefore susceptible to a relapse. Developments in genetics
and technology as well as epidemiology allow for the
determination of probability and risk assessment for the
development of cancer in individual. Using genetic screening
and/or family health histories, it is possible to predict the
probability a particular individual has for developing any one
of several types of cancer.
Similarly, those individuals who have already
developed cancer and who have been treated to remove the
cancer or are otherwise in remission are particularly
susceptible to relapse and reoccurrence.
As part of a
treatment regimen, such individuals can be immunized against
the cancer that they have been diagnosed as having had in
order to combat a recurrence. Thus, once it is known that an
individual has had a type of cancer and is at risk of a
relapse, they can be immunized in order to prepare their
immune system to combat any future 'appearance of the cancer.
The present invention provides a method of treating
individuals suffering from hyperproliferative diseases. In
such methods, the introduction of genetic constructs serves
as an immunotherapeutic, directing and promoting the immune
system of the individual to combat hyperproliferative cells
that produce the target protein.
The present invention provides a method of treating
individuals suffering from:autoimmune diseases and disorders
by conferring a broad based protective immune response against
CA 02745736 2011-07-04
- 44 -
targets that are associated with autoimmunity including cell
receptors and cells which produce "self"-directed antibodies.
T cell mediated autoimmune diseases include
Rheumatoid arthritis (RA), multiple sclerosis (MS), Sjogren's
syndrome, sarcoidosis, insulin dependent diabetes mellitus
(IDDM), autoimmune thyroiditis, reactive arthritis, ankylosing
spondylitis, scleroderma, polymyositis, dermatomyositis,
psoriasis, vasculitis, Wegener's granulomatosis, Crohn's
disease and ulcerative colitis. Each of these diseases is
characterized by T cell receptors that bind to endogenous
antigens and initiate the inflammatory cascade associated with
autoimmune diseases. Vaccination against the variable region
of the T cells would elicit an immune response including CTLs
to eliminate those T cells.
In RA, several specific variable regions of T cell
receptors (TCRs) which are involved in the disease have been
characterized. These TCRs include vg-3, vg-
17 and V-
17. Thus, Thus, vaccination with a DNA construct that encodes at
least one of these proteins will elicit an immune response
that will target T cells involved in RA. See: Howell, M.D.,
et al., 1991 Proc. Natl. Acad. Sci. USA 88:10921-10925;
Paliard, X., et a/., 1991 Science 253:325-329; Williams, W.V.,
et a/., 1992 J. Clin. Invest. 90:326-333.
In MS, several specific variable regions of TCRs
which are involved in the disease have been characterized.
These TCRs include v3-7 and Va-10. ' Thus, vaccination with a
DNA construct that encodes at least one of these proteins will
elicit an immune response that will target T cells involved
in MS. See: Wucherpfennig, K.W., et al., 1990 Science
248:1016-1019; Oksenberg, J.R., et al., 1990 Nature 345:344-
346.
In scleroderma, several specific variable regions
of TCRs which are involved in the disease have been
characterized. These TCRs: include v3-6, v3-6, vg-14 and Va-
16, Va-3C, Va-7, Va-14, Va-15, Va-16, Va-28 and Va-12. Thus,
vaccination with a DNA construct that encodes at least one of
CA 02745736 2011-07-04
- 45 -
these proteins will elicit an immune response that will target
T cells involved in scleroderma.
In order to treat patients suffering from a T cell
mediated autoimmune disease, particularly those for which the
variable region of the TCR has yet to be characterized, a
synovial biopsy can be performed. Samples of the T cells
present can be taken and the variable region of those TCRs
identified using standard techniques. Genetic vaccines can
be prepared using this information.
B cell mediated autoimmune diseases include Lupus
(SLE), Grave's disease, myasthenia gravis, autoimmune
hemolytic anemia, autoimmune thrombocytopenia, asthma,
cryoglobulinemia, primary biliary sclerosis and pernicious
anemia. Each of these diseases is characterized by antibodies
which bind to endogenous antigens and initiate the
inflammatory cascade associated with autoimmune diseases.
Vaccination against the variable region of antibodies would
elicit an immune response including CTLs to eliminate those
B cells that produce the antibody.
In order to treat patients suffering from a B cell
mediated autoimmune disease, the variable region of the
antibodies involved in the autoimmune activity must be
identified. A biopsy can be performed and samples of the
antibodies present at a site of inflammation can be taken.
The variable region of those antibodies can be identified
using standard techniques. Genetic vaccines can be prepared
using this information.
In the case of SLE, one antigen is believed to be
DNA. Thus, in patients to be immunized against SLE, their
sera can be screened for anti-DNA antibodies and a vaccine can
be prepared which includes DNA constructs that encode the
variable region of such anti-DNA antibodies found in the sera.
Common structural features among the variable
regions of both TCRs and antibodies are well known. The DNA
sequence encoding a particular TCR or antibody can generally
be found following well known methods such as those described
in Kabat, et al. 1987 Sequence of Proteins of Immunological
CA 02745736 2011-07-04
- 46 -
Interest U.S. Department of Health and Human Services, Bethesda
MD. In addition, a general method for cloning functional
variable regions from antibodies can be found in Chaudhary,
V.K., et a/., 1990 Proc. Natl. Acad. Sci. USA 87:1066.
In addition to using expressible forms of
immunomodulating protein coding sequences to improve genetic
vaccines, the present invention relates to improved attenuated
live vaccines and improved vaccines which use recombinant
vectors to deliver foreign genes that encode antigens. Examples
of attenuated live vaccines and those using recombinant vectors
to deliver foreign antigens are described in U.S. Patent Nos.:
4,722,848; 5,017,487; 5,077,044; 5,110,587; 5,112,749;
5,174,993; 5,223,424; 5,225,336; 5,240,703; 5,242,829;
5,294,441; 5,294,548; 5,310,668; 5,387,744; 5,389,368;
5,424,065; 5,451,499; 5,453,364; 5,462,734; 5,470,734; and
5,482,713. Gene constructs are provided which include the
nucleotide sequence that encodes an immunomodulating protein is
operably linked to regulatory sequences that can function in
the vaccinee to effect' expression. The gene constructs are
incorporated in the attenuated live vaccines and recombinant
vaccines to produce improved vaccines according to the
invention.
The present invention provides an improved method of
immunizing individuals that comprises the step of delivering
gene constructs to the cells of individuals as part of vaccine
compositions which include are provided which include DNA
vaccines, attenuated live vaccines and recombinant vaccines.
The gene constructs comprise a nucleotide sequence that encodes
an immunomodulating protein and that is operably linked to
regulatory sequences that can function in the vaccinee to
effect expression. The improved vaccines result in an enhanced
cellular immune response.
In some aspects of the invention, the nucleic acid
molecules encoding a human immunomodulating protein are
CA 02745736 2011-07-04
- 47 -
delivered as a gene therapeutic i.e. without co-administration
of an immunogenic target or nucleic acid molecule encoding an
immunogenic target protein. The gene therapy aspects arise
from function of the immunomodulator proteins which drives the
immune responses.
Genetic constructs that are delivered as gene
therapeutics are nucleic acid molecules that encode human IL-
12. Such genetic constructs are preferably plasmids. Also
contemplated are other nucleic acid based vectors such as
recombinant viruses, recombinant microorganisms and linear
nucleic acid molecules, all of which are well known to those
having ordinary skill in the art.
Plasmids useful for
adminstration to individuals in whose tissue the plasmid is
taken up and expressed are well known.
Contemplated
recombinant viruses include: recombinant vaccinia virus
vectors, recombinant adenovirus virus vectors, and recombinant
retroviral vectors. Contemplated recombinant organisms
include: recombinant ECG vectors, and recombinant Salmonella
vectors. Linear nucleic acid molecules as well as plasmid DNA
molecules may be encapsulated in microspheres and liposomes.
In some preferred embodiments, plasmids that encode
human an immunomodulating protein are those genetic constructs
described above.
Essentially, the same compositions .and
methods may be used for gene therapeutics that encode human
immunomodulating proteins as described for genetic
immunization compositions and methods that include gene
constructs which encode human immunomodulating protein except
the gene therapy compositions do not include coding sequences
for immune target proteins. This disclosure is intended to
describe gene therapeutics that encode human immunomodulating
proteins by referring to the above gene constructs. The gene
constructs described above which include nucleotide sequences
that encode human immunomodulating protein operably linked to
regulatory elements necessary for expression in an individual
are intended to describe gene therapeutics. Such constructs
may be administered to individuals in order to modify and/or
drive an immune response.
CD, 02745736 2011-07-04
- 48 -
For example, IL-12 may be delivered to treat an
individual suffering from an allergy, cancer, autoimmune
diseases or infections. Genetic constructs that encode IL-12
are administered with or without facilitators.
In some
embodiments, the genetic constructs are administered in
conjunction with one or more of the facilitating agents
described above. In some embodiments, the gene construct is
delivered free of any facilitating agent. In some preferred
embodiments, the gene encoding IL-12 is free of any infectious
agents. In
some embodiments, the gene construct is
administered using a needleless injection device. In some
embodiments, the gene encoding IL-12 is delivered using
microprojectiles. In some embodiments, the gene encoding IL-
12 is delivered free of any solid particles.
The pharmaceutical compositions according to the
present invention which are either genetic vaccines or gene
therapy compositions comprise about 1 nanogram to about 1000
micrograms of DNA.
In some preferred embodiments, the
compositions contain about 10 nanograms to about 800
micrograms of DNA. In
some preferred embodiments, the
compositions contain about 0.1 to about 500 micrograms of DNA.
In some preferred embodiments, the compositions contain about
1 to about 350 micrograms of DNA.
In some preferred
embodiments, the compositions contain about 25 to about 250
micrograms of DNA. In
some preferred embodiments, the
compositions contain about 100 micrograms DNA.
The pharmaceutical compositions according to the
present invention are formulated according to the mode of
administration to be used. One having ordinary skill in the
art can readily formulate a pharmaceutical composition that
comprises a genetic construct. In cases where intramuscular
injection is the chosen mode of administration, an isotonic
formulation is preferably used. Generally, additives for
isotonicity can include sodium chloride, dextrose, mannitol,
sorbitol and lactose. In some cases, isotonic solutions such
as phosphate buffered saline are preferred.
Stabilizers
include gelatin and albumin.
In some embodiments, a
CA 02745736 2011-07-04
- 49 -
vasoconstriction agent is added to the formulation.
The
pharmaceutical preparations according to the present invention
are provided sterile and pyrogen free.
The methods of the present invention are useful in
the fields of both human and veterinary medicine.
Accordingly, the present invention relates to genetic
immunization of mammals, birds and fish. The methods of the
present invention can be particularly useful for mammalian
species including human, bovine, ovine, porcine, equine,
canine and feline species.
A novel immunomodulator, human BL1, has been
discovered. The DNA and predicted amino acid sequences are
set forth in Figure 14. This protein and fragments thereof
enhance immune responses when co-administered with vaccine
compositions that can introduce an immunogen to the
individual.
As used herein, the term "immunomodulating
fragment" is meant to refer to a fragment of BL1 that is less
than the full length sequence shown in Figure 14 but which
retains the immunodulating activity of the full length
compound. According to the invention, delivery of the BL1
gene sequence results in immunomodulation.
In some
experiments, protein expression was not detected but imnmune
responses were nonetheless affected by co-delivery indicating
that the delivery of the BL1 DNA is the critical step to
modulating and directing immune responses. As set forth
herein, the disclosure is intended to disclose the use of BL1
DNA in immunodulating compositions and methods irrespectiuve
of protein production.
Accordingly, the disclosure is
intended to relate to methods of using BL1 DNA and vecotrs
comprising the same as immunomodulating agents and in
immunomodulaing compositions useful to manipulate and direct
immune responses either as primary active agents or as co-
agents administered in conjunction with immunogens or DNa that
encodes immunogens.
Protein encoded by BL1 DNa may be isolated and
purified; hybridomas which produce antibodies that bind to the
protein can be generated; cDNAs that encode this protein have
CA 02745736 2011-07-04
- 50 -
been isolated, sequenced, incorporated into vectors including
expression vector which were introduced into host cells that
then express the proteins recombinantly. Co-administration
of the vectors with vectors that encode immunogens resulted
in enhanced immune responses against the immunogen.
The discovery of BL1 provides the means to design
and utilize vaccination protocols which enhance, drive and
direct immune responses.
Isolated cDNA that encodes BL1 is useful as a
starting material in the construction of recombinant
expression vectors that can produce BL1 or immunomodulating
fragments thereof. The cDNA is incorporated into vectors
including expression vectors which are introduced into host
cells that then express the proteins recombinantly. Nucleic
acid molecules and fragments thereof may be used as probes to
detect the presence of the BL1 coding sequence. Such probes
hybridize specifically to BL1 coding sequences.
As used
herein, the term "specific BL1 sequence" is meant to refer to
those sequences which are unique to KA..
Nucleic acid
molecules which comprise a nucleotide sequence which are
complementary to specific fragments of the cDNA that encode
BL1 may be used as antisense molecules and primers to inhibit
translation of mRNA and amplify genetic sequences,
respectively.
BL1 is encoded by cDNA shown in Figure 14 and has
a predicted amino acid sequence shown in Figure 14. BL1
coding sequences can be synthesized toutinely and BL1 protein
can be produced by recombinant DNA methods or synthesized by
standard protein synthesis techniques.
Using standard techniques and readily available
starting materials, a nucleic acid molecule that encodes BL1
may be prepared. The present invention relates to an isolated
nucleic acid molecule that comprises a nucleotide sequence
that encodes BL1.
The present invention relates to an
isolated nucleic acid molecule that comprises a nucleotide
sequence that encodes an amino acid sequence of Figure 14, or
an immunomodulating fragment thereof. In some embodiments,
CA 02745736 2011-07-04
- 51 -
the nucleic acid molecules consist of a nucleotide sequence that
encodes BL1. In some embodiments, the nucleic acid molecules
comprise the nucleotide sequence that consists of the coding
sequence in Figure 14. In some embodiments, the nucleic acid
molecules consist of the nucleotide sequence set forth in Figure
14. The isolated nucleic acid molecules of the invention are
useful to prepare constructs and recombinant expression vectors.
The probes or primers that are specific for BL1 have
at least 16 nucleotides, preferably at least 24 nucleotides. The
probes or primers are used to screen the cDNA library using
standard hybridization techniques.
The cDNA that encodes BL1 may be used to design PCR
primers for amplifying nucleic acid sequences. PCR technology is
practiced routinely by those having ordinary skill in the art
and its uses in diagnostics are well known and accepted. Methods
for practicing PCR technology are disclosed in "PCR Protocols: A
Guide to Methods and Applications", Innis, M.A., et al. Eds.
Academic Press, Inc. San Diego, CA (1990). Applications of PCR
technology are disclosed in "Polymerase Chain Reaction" Erlich,
H.A., et al., Eds. Cold Spring Harbor Press, Cold Spring Harbor,
NY (1989). Some simple rules aid in the design of efficient
primers. Typical primers are 18-28 nucleotides in length having
50% to 60% g+c composition. The entire primer is preferably
complementary to the sequence it must hybridize to. Preferably,
primers generate PCR products 100 base pairs to 2000 base pairs.
However, it is possible to generate products of 50 base pairs to
up to 10 kb and more.
PCR technology allows for the rapid generation of
multiple copies of nucleotide sequences by providing 5' and 3'
primers that hybridize to sequences present in a nucleic acid
molecule, and further providing free nucleotides and an enzyme
which fills in the complementary bases to the nucleotide
sequence between the primers with the free nucleotides to
produce a complementary strand of DNA. The
CA 02745736 2011-07-04
- 52 -
enzyme will fill in the complementary sequences adjacent to
the primers. If both the 5' primer and 3' primer hybridize
to nucleotide sequences on the complementary strands of the
same fragment of nucleic acid, exponential amplification of
a specific double-stranded product results. If only a single
primer hybridizes to the nucleic acid molecule, linear
amplification produces single-stranded products of variable
length.
The present invention relates to a vector or a
recombinant expression vector that comprises a nucleotide
sequence that encodes BL1 that comprises an amino acid
sequence in Figure 14. As used herein, the term "recombinant
expression vector" is meant to refer to a plasmid, phage,
viral particle or other vector which, when introduced into an
appropriate host, contains the necessary genetic elements to
direct expression of the coding sequence that encodes BL1.
One having ordinary skill in the art can insert a nucleic acid
molecule that encodes BL1 into an expression vector using
standard techniques and readily available starting materials.
The coding sequence is operably linked to the necessary
regulatory sequences. Expression vectors are well known and
readily available. Examples of expression vectors include
plasmids, phages, viral vectors and other nucleic acid
molecules or nucleic acid molecule containing vehicles useful
to transform host cells and facilitate expression of coding
sequences. In some embodiments, the recombinant expresSion
vector comprises the nucleotide sequence set forth in Figure
14. The recombinant expression vectors of the invention are
preferably plasmids.
The present invention relates to a host cell that
comprises the recombinant expression vector that includes a
nucleotide sequence that encodes BL1. In some embodiments,
the host cell comprises a recombinant expression vector that
comprises the nucleotide sequence in Figure 14. Host cells
for use in well known recombinant expression systems for
production of proteins are well known and readily available.
Examples of host cells include bacteria cells such as E. coli,
CA 02745736 2011-07-04
- 53 -
yeast cells such as S. cerevisiae, insect cells such as S.
frugiperda, non-human mammalian tissue culture cells chinese
hamster ovary (CHO) cells and human tissue culture cells such
as HeLa cells.
In some embodiments, for example, one having
ordinary skill in the art can, using well known techniques,
insert DNA molecules into a commercially available expression
vector for use in well known expression systems. For example,
the commercially available plasmid pSE420 (Invitrogen, San
Diego, CA) may be used for production of BL1 in E. coli. The
commercially available plasmid pYES2 (Invitrogen, San Diego,
CA) may, for example, be used for production in S. cerevisiae
strains of yeast. The commercially available MAXBAC' complete
baculovirus expression system (Invitrogen, San Diego, CA) may,
for example, be used for production in insect cells. The
commercially available plasmid pcDNA I or pcDNA3 (Invitrogen,
San Diego, CA) may, for example, be used for production in
mammalian cells such as Chinese Hamster Ovary cells. One
having ordinary skill in the art can use these commercial
expression vectors and systems or others to produce hVIP
routine techniques and readily available starting materials.
(See e.g., Sambrook'et al., Molecular Cloning a Laboratory
Manual, Second Ed. Cold Spring Harbor Press (1989). Thus,
the desired proteins can be prepared in both prokaryotic
and eukaryotic systems, resulting in a spectrum of
processed forms of the protein.
One having ordinary skill in the art may use other
commercially available expression vectors and systems or
produce vectors using well known methods and readily available
starting materials.
Expression systems containing the
requisite control sequences, such as promoters and
polyadenylation signals, and preferably enhancers, are readily
available and known in the art for a variety of hosts. See
e.g., Sambrook et al., Molecular Cloning a Laboratory Manual,
Second Ed. Cold Spring Harbor Press (1989).
The expression vector including the DNA that encodes
BL1 is used to transform the compatible host which is then
CA 02745736 2011-07-04
- 54 -
cultured and maintained under conditions wherein expression
of the foreign DNA takes place. The protein of the present
invention thus produced is recovered from the culture, either
by lysing the cells or from the culture medium as appropriate
and known to those in the art. One having ordinary skill in
the art can, using well known techniques, isolate BL1 that is
produced using such expression systems.
The methods of
purifying BL1 from natural sources using antibodies which
specifically bind to BL1 as described above, may be equally
applied to purifying BL1 produced by recombinant DNA
methodology.
Examples of genetic constructs include the BL1
coding sequence operably linked to a promoter that is
functional in a human as set forth above or a cell line into
which the constructs are transfected.
Examples of
constitutive promoters include promoters from cytomegalovirus
or SV40.
Examples of inducible promoters include mouse
mammary leukemia virus or metallothionein promoters.
In addition to producing BL1 by recombinant
techniques, automated peptide synthesizers may also be
employed to produce BL1. Such techniques are well known to
those having ordinary skill in the art and are useful if
derivatives which have substitutions not provided for in DNA-
encoded protein production.
Nucleic acid molecules that encode BL1 may be
delivered using any one of a variety of delivery components,
such as direct plasmid administration, recombinant viral
expression vectors or other suitable delivery means, so as to
affect their introduction and expression in an individual or
compatible host cells. In general, viral vectors may be DNA
viruses such as recombinant adenoviruses and recombinant
vaccinia viruses or RNA viruses such as recombinant
retroviruses. Other recombinant vectors include recombinant
prokaryotes which can infect cells and express recombinant
genes. In addition to recombinant vectors, other delivery
components are also contemplated such as encapsulation in
liposomes, transferrin-mediated transfection and other
CA 02745736 2011-07-04
- 55 -
receptor-mediated means. The invention is intended to include
such other forms of expression vectors and other suitable
delivery means which serve equivalent functions and which become
known in the art subsequently hereto.
The present invention also relates to a transgenic
non-human mammal that comprises the recombinant expression
vector that comprises a nucleic acid sequence that encodes BL1.
Transgenic non-human mammals useful to produce recombinant
proteins are well known as are the expression vectors necessary
and the techniques for generating transgenic animals. Generally,
the transgenic animal comprises a recombinant expression vector
in which the nucleotide sequence that encodes BL1 is operably
linked to a mammary cell specific promoter whereby the coding
sequence is only expressed in mammary cells and the recombinant
protein so expressed is recovered from the animal's milk. One
having ordinary skill in the art using standard techniques, such
as those taught in U.S. Patent No. 4,873,191 issued October 10,
1989 to Wagner and U.S. Patent No. 4,736,866 issued April 12,
1988 to Leder, can produce transgenic animals which produce BL1.
Preferred animals are goats, sheep or rodents, particularly rats
and mice.
BL1 protein or expression vectors for producing the
same can be formulated into pharmaceutical compositions.
Pharmaceutical compositions according to the invention
include delivery components in combination with nucleic acid
molecules which further comprise a pharmaceutically acceptable
carriers or vehicles, such as, for example, saline. Any medium
may be used which allows for successful delivery of the nucleic
acid. One skilled in the art would readily comprehend the
multitude of pharmaceutically acceptable media that may be used
in the present invention. Suitable pharmaceutical carriers are
described in Remington's Pharmaceutical Sciences, A. Osol, a
standard reference text in this field. The pharmaceutical
compositions of the present invention may be
CA 02745736 2011-07-04
- 56 -
administered by any means that enables the active agent to
reach the targeted cells. Because peptides are subject to
being digested when administered orally, parenteral
administration, i.e., intravenous, subcutaneous, transdermal,
intramuscular, would ordinarily be used to optimize
absorption. Intravenous administration may be accomplished
with the aid of an infusion pump.
The pharmaceutical
compositions of the present invention may be formulated as an
emulsion. Alternatively, they may be formulated as aerosol
medicaments for intranasal or inhalation administration. In
some cases, topical administration may be desirable.
The dosage administered varies depending upon
factors such as: pharmacodynamic characteristics; its mode and
route of administration; age, health, and weight of the
recipient; nature and extent of symptoms; kind of concurrent
treatment; and frequency of treatment. Usually, the dosage
of peptide can be about 1 to 3000 milligrams per 50 kilograms
of body weight; preferably 10 to 1000 milligrams per 50
kilograms of body weight; more preferably 25 to 800 milligrams
per 50 kilograms of body weight.
Ordinarily 8 to 800
milligrams are administered to an individual per day in
divided doses 1 to 6 times a day or in sustained release form
is effective to obtain desired results. Formulations for
topical administration may include transdermal patches,
ointments, lotions, creams, gels, drops, suppositories,
sprays, liquids and powders. Conventional pharmaceutical
carriers, aqueous, powder or oily bases, thickeners and the
like may be necessary or desirable. Compositions for oral
administration include powders or granules, suspensions or
solutions in water or non-aqueous media, capsules, sachets or
tablets. Thickeners, flavoring agents, diluents, emulsifiers,
dispersing aids or binders may be desirable. Compositions for
parenteral, intravenous, intrathecal or intraventricular
administration may include sterile aqueous solutions which may
also contain buffers, diluents and other suitable additives
and are preferably sterile and pyrogen free. Pharmaceutical
CA 02745736 2011-07-04
- 57 -
compositions which are suitable for intravenous administration
according to the invention are sterile and pyrogen free.
For parenteral administration, the peptides of the
invention can be, for example, formulated as a solution,
suspension, emulsion or lyophilized powder in association with
a pharmaceutically acceptable parenteral vehicle. Examples
of such vehicles are water, saline, Ringer's solution,
dextrose solution, and 5% human serum albumin. Liposomes and
nonaqueous vehicles such as fixed oils may also be used. The
vehicle or lyophilized powder may contain additives that
maintain isotonicity (e.g., sodium chloride, mannitol) and
chemical stability (e.g., buffers and preservatives). The
formulation is sterilized by commonly used techniques. For
example, a parenteral composition suitable for administration
by injection is prepared by dissolving 1.5% by weight of
active ingredient in 0.9% sodium chloride solution.
The pharmaceutical compositions of the present
invention may be administered by any means that enables the
active agent to reach the agent's site of action in the body
of a mammal. The pharmaceutical compositions of the present
invention may be administered in a number of ways depending
upon whether local or systemic treatment is desired and upon
the area to be treated.
Administration may be topical
(including ophthalmic, vaginal, rectal, intranasal,
transdermal), oral or parenteral. Parenteral administration
includes intravenous drip, subcutaneous, intraperitoneal or
intramuscular injection, pulmonary administration, e.g., by
inhalation or insufflation, or intrathecal or intraventricular
administration.
Hybridomas which produce antibodies that bind to
BL1, and the antibodies themselves, are useful in the
isolation and purification of BL1 and protein complexes that
include BL1. In addition, antibodies are specific inhibitors
of BL1 activity. Antibodies which specifically bind to hVIP
may be used to purify the protein from natural sources using
well known techniques and readily available starting
materials. Such antibodies may also be used to purify the
CA 02745736 2013-09-26
- 58 -
protein from material present when producing the protein by
recombinant DNA methodology.
As used herein, the term "antibody" is meant to
refer to complete, intact antibodies, and Fab fragments and
F(ab)2 fragments thereof. Complete, intact antibodies include
monoclonal antibodies such as murine monoclonal antibodies,
chimeric antibodies and humanized antibodies. Antibodies that
bind to an epitope is useful to isolate and purify that
protein from both natural sources or recombinant expression
systems using well known techniques such as affinity
chromatography, i.e. the antibodies do not cross react with
other proteins.
Such antibodies are useful to detect the
presence of such protein in a sample and to determine if cells
are expressing the protein.
The production of antibodies and the protein
structures of complete, intact antibodies, Fab fragments and
F(ab), fragments and the organization of the genetic sequences
that encode such molecules are well known and are described,
for example, in Harlow, E. and D. Lane (1988) ANTIBODIES: A
Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring
Harbor, NY.
Briefly, for example, ELI, or an immunogenic fragment thereof,
is injected into mice. The spleen of the mouse is removed,
the spleen cells are isolated and fused with immortalized
]5 mouse cells. The hybrid cells, or hybridomas, are cultured
and those cells which secrete antibodies are selected. The
antibodies are analyzed and, if found to specifically bind to
BL1, the hybridoma which produces them is cultured to produce
a continuous supply of antibodies.
The Examples set out below include representative
'examples of aspects of the present invention. The Examples
are not meant to limit but rather serve exemplary purposes.
In addition, various aspects of the invention can be
summarized by the following description.
However, this
description is not meant to limit but rather to highlight
various aspects of the invention.
One having ordinary
skill in the art can readily
CA 02745736 2011-07-04
- 59 -
appreciate additional aspects and embodiments of the invention.
EXAMPLES
Example 1
The following is a list of constructs which may be
used in the methods of the present invention. The vector
pBabe.puro, which is used as a starting material to produce
many of the below listed constructs, was originally constructed
and reported by Morgenstern, J.P. and H. Land, 1990 Nucl. Acids
Res. 18(12):3587-3596. The pBabe.puro plasmid is particularly
useful for expression of exogenous genes in mammalian cells.
DNA sequences to be expressed are inserted at cloning sites
under the control of the Moloney murine leukemia virus (Mo
MuLV) long terminal repeat (LTR) promoter. The plasmid contains
the selectable marker for puromycin resistance.
PlasmidpBa.Va3-IL-12 is a plasmid that contains a 2.7
kb EcoRI genomic fragment encoding the T cell receptor Va3
region containing the L, V and J segments cloned into the EcoRI
site of pBabe.puro and the IL-12 coding sequence operably
linked to the CMV promoter and SV40 polyadenylation sequence.
The T cell receptor-derived target protein is useful in the
immunization against and treatment of T cell mediated
autoimmune disease and clonotypic T cell lymphoma and leukemia.
Plasmid pBa.gagpo/-vpr-IL-12 is a plasmid that
contains the gag/poi and vif genes from HIV/MN cloned into
pBabe.puro. The vpr gene is deleted. The plasmid which contains
these HIV viral genes, which encode HIV target proteins, and
the IL-12 coding sequence operably linked to the CMV promoter
and SV40 polyadenylation sequence is useful in the immunization
against and treatment of HIV infection and AIDS. The HIV DNA
sequence is published in Reiz, M.S., 1992 AIDS Res. Human
Retro. 8:1549. The sequence is accessible from Genbank No.:
M17449.
CA 02745736 2011-07-04
- 60 -
Plasmid pM160 is a plasmid that contains the 2.3 kb PCR
fragment encoding the HIV-I/3B envelope protein and rev/tat
genes cloned into pMAMneoBlue. The nef region is deleted. The
plasmid which contains these HIV viral genes, which encode
HIV target proteins, and the IL-12 coding sequence operably
linked to the CMV promoter and SV40 polyadenylation sequence
is useful in the immunization against and treatment of HIV
infection and AIDS. The DNA sequence of HIV-1/3B is published
in Fisher, A., 1985 Nature 316:262. The sequence is
accessible from Genbank No.: K03455.
Plasmid pBa.VL-IL-12 is a plasmid that contains PCR
fragment encoding the VL region of an anti-DNA antibody
cloned into pBabe.puro at the XbaI and EcoRI sites and the
IL-12 coding sequence operably linked to the CMV promoter and
SV40 polyadenylation sequence. The antibody-derived target
protein is an example of a target protein useful in the
immunization against and treatment of B cell mediated
autoimmune disease and clonotypic B cell lymphoma and
leukemia. A general method for cloning functional variable
regions from antibodies can be found in Chaudhary, V.K., et
al., 1990 Proc. Nat/. Acad. Sci. USA 87:1066.
Plasmid pOspA.B-IL-12 is a plasmid which contains
the coding regions encoding the OspA and OspB antigens of the
Borrelia burgdorferi, the spirochete responsible for Lyme's
disease cloned into pBabe.puro at the BamHI and Sail sites
and the IL-12 coding sequence operably linked to the CMV
promoter and SV40 polyadenylation sequence. See: Williams,
W.V., et al. 1992 DNA and Cell.Biol. 11(3):207. The plasmid
which contains these pathogen genes, which encode target
proteins, is useful in the immunization against Lyme's
disease.
Plasmid pBa.Rb-G-IL-12 is a plasmid which contains
a PCR generated fragment encoding the rabies G protein cloned
into pBabe.puro at the BamHI site and the IL-12 coding
CA 02745736 2011-07-04
- 61 -
sequence operably linked to the CMV promoter and SV40
polyadenylation sequence. The plasmid which contains this
pathogen gene, which encodes the rabies G protein, is useful
in the immunization against Rabies. The DNA sequence is
disclosed in Genebank No.: M32751. See also: Anilionis, A.,
et al., 1981 Nature 294:275.
Plasmid pBa.HPV-L1 is a plasmid which contains a PCR
generated fragment encoding the Li caps it protein of the
human papillomavirus (HPV) including HPV strains 16, 18, 31
and 33 cloned into pBabe.puro at the BamHI and EcoRI sites
and the IL-12 coding sequence operably linked to the CMV
promoter and SV40 polyadenylation sequence. The plasmid is
useful in the immunization against HPV infection and the
cancer caused thereby. The DNA sequence is disclosed in
Genebank No. :M15781. See also: Howley, P., 1990 Fields
Virology, Volume 2, Eds.: Channock, R.M. et al. Chapter
58:1625; and Shah, K. and P. Howley, 1990 Fields Virology,
Volume 2, Eds.: Channock, R.M. et a/. Chapter 59.
Plasmid pBa.HPV-L2-IL-12 is a plasmid which
contains a PCR generated fragment encoding the L2 caps it
protein of the human papillomavirus (HPV) including HPV
strains 16, 18, 31 and 33 cloned into pBabe.puro at the BamHI
and EcoRI sites and the IL-12 coding sequence operably linked
to the CMV promoter and SV40 polyadenylation sequence. The
plasmid is useful in the immunization against HPV infection
and the cancer caused thereby. The DNA sequence is disclosed
in Genebank No. :M15781. See also: Howley, P., 1990 Fields
Virology, Volume 2, Eds.: Channock, R.M. et al. Chapter
58:1625; and Shah, K. and P. Howley, 1990 Fields Virology,
Volume 2, Eds.: Channock, R.M. et a/. Chapter 59.
Plasmid pBa.MNp7-IL-12 is a plasmid which contains
a PCR generated fragment encoding the p7 coding region
including the HIV MN gag (core protein) sequence cloned into
pBabe.puro at the BamHI site and the IL-12 coding sequence
CA 02745736 2011-07-04
- 62 -
operably linked to the CMV promoter and SV40 polyadenylation
sequence. The plasmid which contains these HIV viral genes,
which encode HIV target proteins, is useful in the
immunization against and treatment of HIV infection and AIDS.
Reiz, M.S., 1992 AIDS Res. Human Retro. 8:1549. The sequence
is accessible from Genbank No. :M17449.
Plasmid pGA733-2-IL-12 is a plasmid that contains
the GA733-2 tumor surface antigen cloned from the colorectal
carcinoma cell line 5W948 into pCDM8 vector (Seed, B. and A.
Aruffo, 1987 Proc. Natl. Acad. Sci. USA 84:3365) at BstXI
site and the IL-12 coding sequence operably linked to the CMV
promoter and SV40 polyadenylation sequence. The tumor-
associated target protein is an example of a target protein
useful in the immunization against and treatment of
hyperproliferative disease such as cancer. The GA733-2
antigen is a useful target antigen against colon cancer. The
GA733 antigen is reported in Szala, S. et a/., 1990 Proc.
Nat/. Acad. Sci. USA 87:3542-3546.
Plasmid pT4-pMV7-IL-12 is a plasmid that contains
cDNA which encodes human CD4 receptor cloned into pMV7 vector
at the EcoRI site and the IL-12 coding sequence operably
linked to the CMV promoter and SV40 polyadenylation sequence.
The CD4 target protein is useful in the immunization against
and treatment of T cell lymphoma. Plasmid pT4-pMV7 is
available from the AIDS Repository, Catalog No. 158.
Plasmid pDJGA733-IL-12 is a plasmid that contains
the GA733 tumor surface antigen cloned into pBabe.puro at the
BamHI site and the IL-12 coding sequence operably linked to
the CMV promoter and SV40 polyadenylation sequence. The
tumor-associated target protein is an example of a target
protein useful in the immunization against and treatment of
hyperproliferative disease such as cancer. The GA733 antigen
is a useful target antigen against colon cancer.
CA 02745736 2011-07-04
- 63 -
Plasmid pBa.RAS-IL-12 is a plasmid that contains
the ras coding region that was first subcloned from
pZIPneoRAS and cloned into pBabe.puro at the BamHI site and
the IL-12 coding sequence operably linked to the CMV promoter
and SV40 polyadenylation sequence. The ras target protein is
an example of a cytoplasmic signaling molecule. The method of
cloning ras is reported in Weinberg 1984 mol.. Cell. Biol.
4:1577. Ras encoding plasmid are useful for the immunization
against and treatment of hyperproliferative disease such as
cancer; in particular, ras related cancer such as bladder,
muscle, lung, brain and bone cancer.
Plasmid pBa.MNp55-IL-12 is a plasmid which contains
a PCR generated fragment encoding the p55 coding region
including the HIV MN gag precursor (core protein) sequence
cloned into pBabe.puro at the BamHI site and the IL-12 coding
sequence operably linked to the CMV promoter and SV40
polyadenylation sequence. The plasmid which contains these
HIV viral genes, which encode HIV target proteins, is useful
in the immunization against and treatment of HIV infection
and AIDS. Reiz, M.S., 1992 AIDS Res. Human Retro. 8:1549. The
sequence is accessible from Genbank No. :M17449.
Plasmid pBa.MNp24-IL-12 is a plasmid which contains
a PCR generated fragment from the pMN-SF1 template encoding
the p24 coding region including the whole HIV MN gag coding
region cloned into pBabe.puro at the BamHI and EcoRI sites
and the IL-12 coding sequence operably linked to the CMV
promoter and 5V40 polyadenylation sequence. The plasmid which
contains these HIV viral genes, which encode HIV target
proteins, is useful in the immunization against and treatment
of HIV infection and AIDS. Reiz, M.S., 1992 AIDS Res. Human
Retro. 8:1549. The sequence is accessible from Genbank No.:
M17449.
Plasmid pBa.MNp17-IL-12 is a plasmid which contains
a PCR generated fragment encoding the p17 coding region
CA 02745736 2011-07-04
- 64 -
including the HIV MN gag (core protein) sequence cloned into
pBabe.puro at the BamHI and EcoRI sites and the IL-12 coding
sequence operably linked to the CMV promoter and SV40
polyadenylation sequence. The plasmid which contains these
HIV viral genes, which encode HIV target proteins, is useful
in the immunization against and treatment of HIV infection
and AIDS. Reiz, M.S., 1992 AIDS Res. Human Retro. 8:1549. The
sequence is accessible from Genbank No.: M17449.
Plasmid pBa.SIVenv-IL-12 is a plasmid which
contains a 2.71 PCR generated fragment amplified from a
construct containing SIV 239 in pBR322 cloned into pBabe.puro
at the BamHI and EcoRI sites and the IL-12 coding sequence
operably linked to the CMV promoter and SV40 polyadenylation
sequence. The plasmid is available from the AIDS Research and
Reference Reagent Program; Catalog No. 210.
Plasmid pcTSP/ATK.env-IL-12 is a plasmid which
contains a PCR generated fragment encoding the complete HTLV
envelope coding region from HTLV-1/TSP and /ATK isolates
subcloned into the pcDNAl/neo vector and the IL-12 coding
sequence operably linked to the CMV promoter and SV40
polyadenylation sequence. Plasmid pcTSP/ATK.env is reported
in 1988 Proc. Natl. Acad. Sci. USA 85:3599. The HTLV env
target protein is useful in the immunization against and
treatment of infection by HTLV and T cell lymphoma.
Plasmid pBa.MNgp160-IL-12 is a plasmid which
contains a 2.8 kb PCR generated fragment amplified from a
construct containing MNenv in pSP72 and cloned into
pBabe.puro at the BamHI and EcoRI sites and the IL-12 coding
sequence operably linked to the CMV promoter and SV40
polyadenylation sequence. Reiz, M.S., 1992 AIDS Res. Human
Retro. 8:1549. The sequence is accessible from Genbank
No. :M17449. The plasmid which contains these HIV viral genes,
which encode HIV target proteins, is useful in the
immunization against and treatment of HIV infection and AIDS.
CA 02745736 2011-07-04
- 65 -
Plasmid pC.MNp55-IL-12 is a plasmid which contains
a 1.4 kb PCR generated fragment amplified from the gag region
of MN isolate and cloned into the pCEP4 vector. The plasmid
which contains these HIV viral genes, which encode HIV target
proteins and the IL-12 coding sequence operably linked to the
CMV promoter and SV40 polyadenylation sequence is useful in
the immunization against and treatment of HIV infection and
AIDS. Reiz, M.S., 1992 AIDS Res. Human Retro. 8:1549. The
sequence is accessible from Genbank No.: M17449.
Plasmid pC.Neu-IL-12 is a plasmid that contains a
3.8 kb DNA fragment containing the human neu oncogene coding
region that was cut out from the LTR-2/erbB-2 construct and
subcloned into the pCEP4 vector and the IL-12 coding sequence
operably linked to the CMV promoter and SV40 polyadenylation
sequence. The pC.Neu plasmid is reported in DiFiore 1987
Science 237:178. The neu oncogene target protein is an
example of a growth factor receptor useful as a target
protein for the immunization against and treatment of
hyperproliferative disease such as cancer; in particular,
colon, breast, lung and brain cancer.
Plasmid pC.RAS-IL-12 is a plasmid that contains a
1.4 kb DNA fragment containing the ras oncogene coding region
that was first subcloned from pZIPneoRAS and subcloned into
pCEP4 at the BamHI site and the IL-12 coding sequence
operably linked to the CMV promoter and SV40 polyadenylation
sequence. The pC.RAS plasmid is reported in Weinberg 1984
Mel.. Cell. Biol. 4:1577. The ras target protein is an
example of a cytoplasmic signalling molecule. Ras encoding .
plasmid are useful for the immunization against and treatment
of hyperproliferative disease such as cancer; in particular,
ras related cancer such as bladder, muscle, lung, brain and
bone cancer.
Plasmid pNLpuro-IL-12 is a plasmid which contains
HIV gag/pol and SV40-puro insertion. The plasmid which
CA 02745736 2011-07-04
- 66 -
contains these HIV viral genes, which encode HIV target
proteins and the IL-12 coding sequence operably linked to the
CMV promoter and SV40 polyadenylation sequence, is useful in
the immunization against and treatment of HIV infection and
AIDS.
Example 2
Plasmid pCSIL-12 contains the IL-12 coding
sequences operably linked to CMV promoters and SV40
polyadenylat ion sequences.
Example 3
Compositions according to some embodiments of the
invention may be prepared by combining plasmid pCSIL-12 with
any one of the following plasmids.
PlasmidpBa.Va3 is a 7.8 kb plasmid that contains a
2.7 kb EcoRI genomic fragment encoding the T cell receptor
Va3 region containing the L, V and J segments cloned into the
EcoRI site of pBabe.puro. The T cell receptor-derived target
protein is useful in the immunization against and treatment
of T cell mediated autoimmune disease and clonotypic T cell
lymphoma and leukemia.
PlasmidpBa.gagpo/-vpr is a 9.88 kb plasmid that
contains the gag/poi and vif genes from HIV/MN cloned into
pBabe.puro. The vpr gene is deleted. The plasmid which
contains these HIV viral genes, which encode HIV target
proteins, is useful in the immunization against and treatment
of HIV infection and AIDS. The HIV DNA sequence is published
in Reiz, M.S., 1992 AIDS Res. Human Retro. 8:1549. The
sequence is accessible from Genbank No.: M17449.
Plasmid pM160 is an 11.0 kb plasmid that contains
the 2.3 kb PCR fragment encoding the HIV-I/35 envelope
protein and rev/tat genes cloned into pMAMneoBlue. The nef
region is deleted. The plasmid which contains these HIV viral
genes, which encode HIV target proteins, is useful in the
immunization against and treatment of HIV infection and AIDS.
CA 02745736 2011-07-04
- 67 -
The DNA sequence of HIV-1/3B is published in Fisher, A., 1985
Nature 316:262. The sequence is accessible from Genbank No.:
K03455.
Plasmid pBa.VL is a 5.4 kb plasmid that contains
PCR fragment encoding the VL region of an anti-DNA antibody
cloned into pBabe.puro at the XbaI and EcoRI sites. The
antibodyderived target protein is an example of a target
protein useful in the immunization against and treatment of B
cell mediated autoimmune disease and clonotypic B cell
lymphoma and leukemia. A general method for cloning
functional variable regions from antibodies can be found in
Chaudhary, V.K., et al., 1990 Proc. Natl. Acad. Sci. USA
87:1066.
Plasmid pOspA.B is a 6.84 kb plasmid which contains
the coding regions encoding the OspA and OspB antigens of the
Borrelia burgdorferi, the spirochete responsible for Lyme's
disease cloned into pBabe.puro at the BamHI and Sail sites.
Williams, W.V., et a/. 1992 DNA and Cell. Biol. 11(3) :207.
The plasmid which contains these pathogen genes, which encode
target proteins, is useful in the immunization against Lyme's
disease.
Plasmid pBa.Rb-G is a 7.10 kb plasmid which
contains a PCR generated fragment encoding the rabies G
protein cloned into pBabe.puro at the BamHI site. The plasmid
which contains this pathogen gene, which encodes the rabies G
protein, is useful in the immunization against Rabies. The
DNA sequence is disclosed in Genebank No. :M32751. See also:
Anilionis, A., et al., 1981 Nature 294:275.
Plasmid pBa.1-IPV-L1 is a 6.80 kb plasmid which
contains a PCR generated fragment encoding the Ll caps it
protein of the human papillomavirus (HPV) including HPV
strains 16, 18, 31 and 33 cloned into pBabe.puro at the BamHI
and EcoRI sites. The plasmid is useful in the immunization
against HPV infection and the cancer caused thereby. The DNA
CA 02745736 2011-07-04
- 68 -
sequence is disclosed in Genebank No. :M15781. See also:
Howley, P., 1990 Fields Virology, Volume 2, Eds.: Channock,
R.M. et al. Chapter 58:1625; and Shah, K. and P. Howley, 1990
Fields Virology, Volume 2, Eds.: Channock, R.M. et a/.
Chapter 59.
Plasmid pBa.HPV-L2 is a 6.80 kb plasmid which
contains a PCR generated fragment encoding the L2 capsid
protein of the human papillomavirus (HPV) including HPV
strains 16, 18, 31 and 33 cloned into pBabe.puro at the BamHI
and EcoRI sites. The plasmid is useful in the immunization
against HPV infection and the cancer caused thereby. The DNA
sequence is disclosed in Genebank No. :M15781. See also:
Howley, P., 1990 Fields Virology, Volume 2, Eds.: Channock,
R.M. et al. Chapter 58:1625; and Shah, K. and P. Howley, 1990
Fields Virology, Volume 2, Eds.: Channock, R.M. et al.
Chapter 59.
Plasmid pBa.MNp7 is a 5.24 kb plasmid which
contains a PCR generated fragment encoding the p7 coding
region including the HIV MN gag (core protein) sequence
cloned into pBabe.puro at the BamHI site. The plasmid which
contains these HIV viral genes, which encode HIV target
proteins, is useful in the immunization against and treatment
of HIV infection and AIDS. Reiz, M.S., 1992 AIDS Res. Human
Retro. 8:1549. The sequence is accessible from Genbank
No. :M17449.
Plasmid pGA733-2 is a 6.3 kb plasmid that contains
the GA733-2 tumor surface antigen cloned from the colorectal
carcinoma cell line SW948 into pCDM8 vector (Seed, B. and A.
Aruffo, 1987 Proc. Natl. Acad. Sci. USA 84:3365) at BstXI
site. The tumor-associated target protein is an example of a
target protein useful in the immunization against and
treatment of hyperproliferative disease such as cancer. The
GA733-2 antigen is a useful target antigen against colon
CA 02745736 2011-07-04
- 69 -
cancer. The GA733 antigen is reported in Szala, S. et al.,
1990 Proc. Natl. Acad. Sci. USA 87:3542-3546.
Plasmid pT4-pMV7 is a 11.15 kb plasmid that
contains cDNA which encodes human CD4 receptor cloned into
pMV7 vector at the EcoRI site. The CD4 target protein is
useful in the immunization against and treatment of T cell
lymphoma. Plasmid pT4-pMV7 is available from the AIDS
Repository, Catalog No. 158.
Plasmid pDJGA733 is a 5.1 kb plasmid that contains
the GA733 tumor surface antigen cloned into pBabe.puro at the
BamHI site. The tumor-associated target protein is an example
of a target protein useful in the immunization against and
treatment of hyperproliferative disease such as cancer. The
GA733 antigen is a useful target antigen against colon
cancer.
Plasmid pBa.RAS is a 6.8 kb plasmid that contains
the ras coding region that was first subcloned from
pZIPneoRAS and cloned into pBabe.puro at the BamHI site. The
ras target protein is an example of a cytoplasmic signalling
molecule. The method of cloning ras is reported in Weinberg
1984 Mol.. Cell. Biol. 4:1577. Ras encoding plasmid are
useful for the immunization against and treatment of
hyperproliferative disease such as cancer; in particular, ras
related cancer such as bladder, muscle, lung, brain and bone
cancer.
Plasmid pBa.MNp5S is a 6.38 kb plasmid which
contains a PCR generated fragment encoding the p55 coding
region including the HIV MN gag precursor (core protein)
sequence cloned into pBabe.puro at the BamHI site. The
plasmid which contains these HIV viral genes, which encode
HIV target proteins, is useful in the immunization against
and treatment of HIV infection and AIDS. Reiz, M.S., 1992
AIDS Res. Human Retro. 8:1549. The sequence is accessible
from Genbank No. :M17449.
CA 02745736 2011-07-04
- 70 -
Plasmid pBa.MNp24 is a 5.78 kb plasmid which
contains a PCR generated fragment from the pMN-SF1 template
encoding the p24 coding region including the whole HIV MN gag
coding region cloned into pBabe.puro at the BamHI and EcoRI
sites. The plasmid which contains these HIV viral genes,
which encode HIV target proteins, is useful in the
immunization against and treatment of HIV infection and AIDS.
Reiz, M.S., 1992 AIDS Res. Human Retro. 8:1549. The sequence
is accessible from Genbank No.: M17449.
Plasmid pBa.MNp17 is a 5.5 kb plasmid which
contains a PCR generated fragment encoding the p17 coding
region including the HIV MN gag (core protein) sequence
cloned into pBabe.puro at the BamHI and EcoRI sites. The
plasmid which contains these HIV viral genes, which encode
HIV target proteins, is useful in the immunization against
and treatment of HIV infection and AIDS. Reiz, M.S., 1992
AIDS Res. Human Retro. 8:1549. The sequence is accessible
from Genbank No.: M17449.
Plasmid pBa.SIVenv is a 7.8 kb plasmid which
contains a 2.71 PCR generated fragment amplified from a
construct containing SIV 239 in pBR322 cloned into pBabe.puro
at the BamHI and EcoRI sites. The plasmid is available from
the AIDS Research and Reference Reagent Program; Catalog No.
210.
Plasmid pcTSP/ATK.env is a 8.92 kb plasmid which
contains a PCR generated fragment encoding the complete HTLV
envelope coding region from HTLV-1/TSP and /ATK isolates
subcloned into the pcDNAl/neo vector. Plasmid pcTSP/ATK.env
is reported in 1988 Proc. Natl. Acad. Sci. USA 85:3599. The
HTLV env target protein is useful in the immunization against
and treatment of infection by HTLV and T cell lymphoma.
Plasmid pBa.MNgp160 is a 7.9 kb plasmid which
contains a 2.8 kb PCT generated fragment amplified from a
construct containing MNenv in pSP72 and cloned into
CA 02745736 2011-07-04
- 71 -
pBabe.puro at the BamHI and EcoRI sites. Reiz, M.S., 1992
AIDS Res. Human Retro. 8:1549. The sequence is accessible
from Genbank No. :M17449. The plasmid which contains these HIV
viral genes, which encode HIV target proteins, is useful in
the immunization against and treatment of HIV infection and
AIDS.
PlasmidpC.MNp55 is a 11.8 kb plasmid which contains
a 1.4 kb PCR generated fragment amplified from the gag region
of MN isolate and cloned into the pCEP4 vector. The plasmid
which contains these HIV viral genes, which encode HIV target
proteins, is useful in the immunization against and treatment
of HIV infection and AIDS. Reiz, M.S., 1992 AIDS Res. Human
Retro. 8:1549. The sequence is accessible from Genbank No.:
M17449.
Plasmid pC.Neu is a 14.2 kb plasmid that contains a
3.8 kb DNA fragment containing the human neu oncogene coding
region that was cut out from the LTR-2/erbB-2 construct and
subcloned into the pCEP4 vector. The pC.Neu plasmid is
reported in DiFiore 1987 Science 237:178. The neu oncogene
target protein is an example of a growth factor receptor
useful as a target protein for the immunization against and
treatment of hyperproliferative disease such as cancer; in
particular, colon, breast, lung and brain cancer.
Plasmid pC.RAS is a 11.7 kb plasmid that contains a
1.4 kb DNA fragment containing the ras oncogene coding region
that was first subcloned from pZIPneoRAS and subcloned into
pCEP4 at the BamHI site. The pC.RAS plasmid is reported in
Weinberg 1984 Mai.. Cell. Biol. 4:1577. The ras target
protein is an example of a cytoplasmic signalling molecule.
Ras encoding plasmid are useful for the immunization against
and treatment of hyperproliferative disease such as cancer;
in particular, ras related cancer such as bladder, muscle,
lung, brain and bone cancer.
CA 02745736 2011-07-04
- 72 -
Plasmid pNLpuro is a 15 kb plasmid which contains
HIV gag/poi and SV40-puro insertion. The plasmid which
contains these HIV viral genes, which encode HIV target
proteins, is useful in the immunization against and treatment
of HIV infection and AIDS.
Example 4
It may be important to specifically direct immune
responses in order to improve vaccines. The type of immune
response (Thl vs Th2) has been reported to be important in a
variety of disease models including infectious diseases,
autoimmune diseases, and allergies. To induce strong and
stable cell mediated immune response against HIV infection,
the use of immunologic adjuvants and immune modulators such
as cytokines in conjunction with immunization could enhance
cellular immune response and direct antigen-dependent immune
response from Th2 to Thl type.
In order to engineer the immune response in
vivo,human cytokine genes, either IL-12 genes or GM-CSF
genes, were co-delivered along with HIV constructs. Immnune
responses induced by co-delivery of these genes with HIV-1
DNA vaccines were examined and the induction of cellular
immunity, specifically antiviral CTL responses was studied.
The genes for IL-12 and GM-CSF were individually
cloned into expression vectors under control of a CMV
promoter. The gene plasmid expression cassettes were then
injected into mice along with DNA vaccine cassettes for HIV-1
which have been referred to above or in U.S. Patent No.
6,143,527. The immunological effects of the co-immunization
with these genetic adjuvant cassettes on the magnitude of
antigen-specific immune responses was analyzed. A reduction
in humoral response was seen with IL-12 co-delivery while a
mild enhancement of humoral response was seen with IL-12
coimmunization. An increase in antigen-specific T helper cell
proliferation was seen with co-immunization with either IL-12
CA 02745736 2011-07-04
- 73 -
or GM-CSF. Importantly, a significant CTL induction with co-
administration of genes for IL-12 using a direct CTL assay was
observed. In contrast, almost no effect on CTL induction was
observed with the genes for GM-CSF in these studies. These
results demonstrate the utility of DNA vaccines for the
tailored production of specific immune responses. They also
demonstrate the utility of this approach to elucidate basic
immunological functions in a molecule-specific fashion.
MATERIALS AND METHODS
Mice
Balb/c female mice, aged 6-8 weeks were purchased
from Harlan Sprague Dawley, Inc., (Indianapolis, Indiana).
The mice were housed in a temperature controlled, light-cycled
room.
Their care were under the guidelines of National
Institute of Health and University of Pennsylvania.
Reagents
DNA vaccine formulations pCMN160, pCGN160,
pCGag/Pol, were prepared. IL-12 and GM-CSF genes were cloned
and inserted into an expression vector with CMV promoter.
Recombinant vaccinia (vMN462, vVK1, VV:gag, and vSC8) were
obtained from the NIH AIDS Research and Reference Reagent
Program.
DNA inoculation
A facilitated DNA inoculation protocol which results
in increased in vivo protein expression levels from plasmid
delivered genes in vivo was utilized.
Specifically, the
quadriceps muscles of BALB/c mice were injected with 100 41
of solution containing 0.25k bupivacaine-HCI (Sigma, St.
Louis) using a 27-gauge needle. Two days later, 50 g of the
DNA construct of interest in phosphate-saline buffer was
injected into the same region of the muscle as the bupivacaine
injection.
Co-administration of various gene expression
cassettes involved mixing the chose plasmids prior to
injection.
FACS Analysis =
Cells (1x105) were washed 3x with FACS buffer (PBS
containing 1%. BSA and 0.1% sodium azide) and incubated with
CA 02745736 2011-07-04
=
- 74 -
FITC and/or PE-conjugated mAbs at a saturating conditions for
30 minutes on ice. After being washed 3x with FACS buffer,
cells were analyzed using a FACScan (Becton Dickinson).
ELISA
Two gg/ml of gp120 or gp41 (Intracel Corp.
Cambridge, MA) in 0.1M carbonate-bicarbonate buffer (pH 9.5)
was adsorbed onto microtiter wells overnight at 4 C as
previously described. The plate was washed with PBS-0.05%
Tween4-20 blocked with 3% BSA in PBS with 0.05% Tween-20 for
1 hour at 37 C, then incubated with a manufacturer suggested
dilution of HRP-conjugated goat anti-mouse IgG or IgA (Sigma,
St Louis, MO). The plate was washed and developed with TM
blue buffer (sigma). The OD 450 nm was read on a DynatecE
MR5000 plate reader.
T cell Proliferation Assay
Lymphocytes from harvested mouse spleens were
prepared. The isolated cell suspensions were resuspended to
a concentration of 1x106 cells/mL. A
100 gL aliquot
containing 1x10 cells was immediately added to each well of
a 96 well microtiter flat bottom plate. Ten gl of protein was
added to wells in triplicate of 20 gg/mL. The cells were
incubated at 37 C in 5% CO2 for three days.
One gCi of
tritiated thymidine was added to each well and the cells were
incubated for 12-18 hours at 37 C. The plate was harvested
and the amount of incorporated tritiated thymidine was
measured in a Beta Plate reader (Wallac, Turku, Finland). To
assure that cells were healthy, 10 g/m1 of PHA was used as a
polyclonal stimulator positive control.
Cytotoxic T Lymphocyte Assay
A 5 hour chromium' release CTL assay was performed.
Lymphocytes were harvested from spleens and prepared as the
effector cells by removing the erythrocytes and by washing
several times with fresh media. Vaccinia infected targets
were prepared by infecting 3x106 p815 cells for 16 hours at
37 C. The target cells were labeled with 100 gCi/m1 Na251Cr04
for 90 min. and used to incubate the stimulated splenocytes
for 4-6 hours at 37 C. CTL was tested at effector:target
CA 02745736 2011-07-04
- 75 -
(E:T) ratio ranging from 50:1 to 12.5:1. Supernatants were
harvested and counted on a LKB CliniGamma gamma-counter.
Percent specific lysis was determined from the formula:
100 xlexperimental release - spontaneous release
maximum release - spontaneous release
Maximum release was determined by lysis of target cells in 1%
Triton X-100 containing medium. An assay was not considered
valid if the value for the 'spontaneous release' counts are
in excess of 20t of the 'maximum release'.
RESULTS
Phenotyping of Mouse Spleens
Following co-inoculation, it was observed that
spleens collected from individual experimental groups appeared
different. Accordingly, spleens collected from all immunized
animals were weighed and visually examined. The
spleen
weights of these animals are shown in Figure 1A. Whereas the
spleens from the mice injected with single formulation
controls weighed similar to those of the unimmunized control
mice (about 100 mg), the spleens from mince injected with
Gag/Pol+IL-12 genes weighed about three times as much as the
control spleens. On the other hand, Gag/Pol+GM-CSF immunized
mouse- spleens were not enlarged. It is interesting to note
that those immunized with Gag/Pol only or IL-12 alone did not
result in significantly enlarged spleens.
Only when the
antigen and IL-12 gene cassettes were co-injected did the
splenomegaly result, suggesting that this was a combined
effect of both gene products. Furthermore, as shown in Figure
1B, the number of lymphocytes derived from the Gag/Pol+IL-12
spleens were more than three times the number derived from the
control spleens. Again, the Gag/Pol and GM-CSF immunized
mouse spleens did not have any significant increase in the
number of lymphocytes above the control spleen cell number.
The photograph of representative spleens are shown in Figure
2. Corresponding to their weights, the antigen+IL-12 spleens
were observed to be several times larger than other spleens.
It has been reported that p35 chain of IL-12 is constitutively
CA 02745736 2011-07-04
- 76 -
expressed in many cell types. Therefore it would be possible
that only a single p40 chain and DNA immunogen were
responsible for the effects. To test this, each of the two
IL-12 heterodimer genes (p35 and p40) was co-administered with
Gag/Pol. As shown in Figure 3, no enlargement of spleen size
was observed in either case. These data indicate that the co-
injection with DNA vaccine and both p35 and p40 IL-12 genes
resulted in the increased size of spleen and corresponding
augmentation of the number of splenic cells. These data
support that the plasmids entered the same cells in vivo and
coordinated transcription of all three components, p35 chain,
p40 chain, and the specific antigen, to induce the biological
changes observed.
FACS Analysis
To further characterize the cellular composition of
the enlarged spleens, FACS analysis was performed. Table 3
shows the FACS results from the double-staining of the
splenocytes with antibodies for CD3 with antibodies for B220,
CD4, and CD8. As shown, a slight reduction in the percentage
of B220 positive B cell population in the groups immunized
with Envelop+IL-12 or Gag/Pol+IL-12 constructs (17.46% and
21.62%, respectively) from the percentage of B cell in
unimmunized control spleens (25.43%) was observed.
In
addition, there was a moderate increase in the percentage of
CD8+ T cells in the groups immunized with Envelop+IL-12 or
Gag/Pol+IL-12 constructs (21.72% and 16.88%, respectively)
versus the percentage in the unimmmtnized group (13.69%).
Humoral Response
Antisera from immunized mice were collected and
analyzed for specific antibody responses against HIV-1
antigens by ELISA. Figure 4 shows the ELISA results from the
samples collected at 28 days post-immunization. At 1:100
dilution, sera from the group immunized with pCEnv+pCGM-CSF
showed antibody response against HIV-1 gp120 protein which was
greater than those of the-group immunized with only pCEnv.
On the other hand, the group immunized with pCEnv+pCIL-12
showed a significantly less humoral response over the same
CA 02745736 2011-07-04
- 77 -
period. In repeated experiments, IL-12 generally suppressed
specific antibody responses by 1-020.% while GM-CSF appeared
to have the opposite effect. This humoral effect could be
related to the observed change in B cell number in the
splenocytes as identified on FACS analysis.
T Cell Proliferation
Activation and proliferation of T helper lymphocytes
play a critical role in inducing both humoral immune response
via expansion of antigen-activated B cells and cellular immune
response via expansion of CD8+ cytotoxic T lymphocytes. Two
weeks after DNA immunization, spleens were collected from
immunized mice and their lymphocytes were isolated. These
cells were then tested for T cell proliferation as described
above. Figure 5 shows the proliferation assay results for the
mice immunized with DNA vaccine encoding for HIV-1 gag/poi
(pCGag/Pol) and those mice co-immunized with pCGag/Pol and IL-
12 or GM-CSF. Recombinant p55 protein 20 g/m1 of lectin PHA
was used as a polyclonal stimulator positive control. As
shown, low background level of proliferation was observed from
control group from naive mouse spleens with a stimulation
index of 1.2 at 1:2 dilution and a moderate level of
proliferation was observed from the group immunized with
pCGag/Pol alone with a stimulation index of 9.2 at 1:2
dilution. A dramatic boosting in the proliferation was seen
from the group co-immunized with pCGag/Pol and IL-12 genes
with a stimulation index of 17.1 and the group co-immunized
with pCGag/Pol and GM-CSF genes with a stimulation index of
15.6.
CTL Assay Without In Vitro Stimulation
To further investigate the enhancement of the
cellular activity, direct CTL assays were conducted. The CTL
assay was performed on spleen cells harvested from immunized
mice as described above with no in vitro stimulation induced
on the splenocytes. The assay was conducted on the day of
spleen harvest measuring tile chromium release from specific
and non-specific vaccinia infected targets. A
dramatic
increase in specific CTL activity from Gag/Pol+IL-12 immunized
CA 02745736 2011-07-04
- 78 -
splenocytes (Figure 6) was observed.
The control group
immunized with only IL-12 gene cassette resulted in no
specific lysis of target cells above the background level.
In addition, low level (3%) of specific lysis was observed
with Gag/Pol only immunization of the 50:1 effector: target
ratio.
In contrast, 62% specific lysis was seen with
Gag/Pol+IL-12 co-administration samples at the 50:1
effector:target ratio and titered out to 9% at the 12.5:1
effector:target ratio. On the other hand, those immunized
with Gag/Pol and GM-CSF plasmids resulted in no detectable CTL
activity. Similar results were observed from the mice co-
immunized with the HIV-1 envelop construct and cytokine genes
(Figure 7).
The group immunized with Envelop alone and
Envelop+GM-CSF resulted in low levels of specific CTL at 4%
and 1%, respectively. On
the other hand, a dramatically
enhancement of CTL activity was observed in the Envelop+IL-12
group at 59% lysis.
In both Gag/Pol and Envelope co-
immunizations (Figures 6 and 7), the same CTL assay conducted
against targets prepared with irrelevant antigen-expressing
vaccinia did not result in significant CTL lysis. Therefore,
the dramatic enhancement of CTL activity from antigen and IL-
12 DNA immunization results were not due to NK activity as the
results were antigen specific.
DISCUSSION
The generation of immune responses in vivo using DNA
inoculation was reported using different therapeutic targets
and delivery techniques.
Induction of cell-mediated immunity may be an
important feature for many vaccines. For example, during
natural infection, anti-HIV-1 CTL responses appear very early
and temporarily appear to correlate with the establishment of
the viral set point. CTL T cells play a critical role in
viral clearance by targeting and destroying virus-infected
cells. Directing immune responses against viral proteins
through the development of specific CTL responses would allow
induction of a more broad immune response against multiple
antigenic targets within the virus. The CTL activity against
CA 02745736 2011-07-04
- 79 -
the virus is more readily measured in healthy infected
patients as compared with AIDS patients, and specific CTL's
have been reported to decrease as disease pathogenesis
increases clearly linking CTL responses with preferred
clinical status. In this regard, a cynomolgous macaque with
high specific CTL and low antibody responses was protected
against a chimeric SIV/HIV (SHIV) challenge while the animals
with low CTL and high antibody responses controlled viral
replication but were not completely protected. Specific CTL
responses appear to contribute to the maintenance of the
asymptomatic phase of HIV-1 infection. Thus the induction of
strong HIV-1 specific CTL's in vivo through DNA immunization
may play a crucial role in the ultimate protection of the host
from the progression of HIV infection.
The potential enhancement of immune responses,
especially the CTL response, from DNA vaccines for HIV-1 via
co-delivery IL-12 and GM-CSF genes as genetic adjuvants was
investigated. The genes for IL-12 and GM-CSF were cloned into
expression vectors and injected them into mice along with DNA
vaccine cassettes for HIV-1. Co-immunization of plasmids
encoding for IL-12 with DNA vaccine for HIV-1 resulted in a
dramatic increase
antigen specific CTL response. GM-CSF
co-delivery appeared to increase humoral responses while IL-12
co-delivery suppressed humoral response by about 209E.
There are many significant immunological effects of
co-delivering cytokine genetic adjuvants with DNA vaccines for
HIV-1.
First, the size and weight of spleens from mice
injected with DNA vaccine and IL-12 genes weighed almost three
times as much as the control spleens. In addition, the number
of white blood cells from these spleens were more than twice
the number of cells from the control spleens. These results
agree with previous findings that in vivo administration of
recombinant IL-12 in mice caused splenomegaly. Car et al.
found that IL-12 injection resulted in a fivefold increase of
spleen weight in wild-type :mice. These IL-12 induced changes
in wild-type mice were associated with markedly increased IFN-
gamma serum levels.
However, IL-12 administration also
CA 02745736 2011-07-04
- 80 -
induced a qualitatively similar (to 2 x normal) increase in
spleen size in IFN-gamma receptor deficient mice. These
earlier studies reported splenomegaly following injection of
IL-12 protein. A small amount of IL-12 genes delivered in
vivo to induce splenomegaly to the level comparable to those
published works with recombinant IL-12 proteins. It has been
reported that in vivo injection of recombinant IL-12 into mice
could have a degree of toxic effects on injected mice such as
weight reduction and even death. It is important to note that
co-administration of IL-12 genes induced enlarged spleens
without any visible adverse changes in the injected mice.
This suggests that the likely natural processing and sustained
low level delivery through plasmid inoculation may be
clinically beneficial. A DNA delivery strategy for inducing
significant systemic immune responses is demoinstrated here
without toxicity.
Aside from the induction of splenomegaly, the
specific immune response from Th2 to Thl can be manipulated
via co-administration of IL-12 genes with DNA vaccines. In
this regard, the co-delivery of IL-12 genes with DNA vaccine
resulted in the reduction of specific antibody response, while
the co-injection of GM-CSF genes resulted in the enhancement
of specific antibody response. These results agree with the
earlier reportings that IL-12 is a key cytokine in directing
the immune response from Th2 to Thl type response. These
antibody results were also in agreement with the spleen cell
FACS data where the reduction in the percentage of B220+ B
cells were observed with the mice immunized with immunogen
(HIV-1 envelop or Gag/Pol) and IL-12 genes. In addition, a
significant antigen-specific stimulation of T cells with
cytokine co-delivery was observed.
The antigen-specific
proliferation is a good indicator of CD4 helper T cell
induction which appears to be a feature of both cytokines.
To further elucidate the T cell response to the DNA
co-immunization, direct ,CTL assays without in vitro
stimulation of the harvested splenocytes were conducted. An
enhancement of CTL response is a key evidence in demonstrating
CA 02745736 2011-07-04
- 81 -
the ability to direct immune responses resulting from DNA
immunogens from the Th2 to Thl type response. A dramatic
increase in specific CTL response was observed from the group
co-immunized with DNA vaccine and IL-12 genes. In summary,
genes encoding immunogens and IL-12, a key cytokine
responsible producing the Thl type immune response, were co-
adminstered in vivo and shown to enhance the cellular immune
responses measured by the T cell proliferation and CTL assay.
Co-delivering genes for immunologically important
molecules to help direct and manipulate the type and direction
of immune responses, for example to direct responses from Th2
to Thl type, may be used to elicit more clinically efficacious
immune responses. By co-administering IL-12 genes with DNA
immunogen, humoral responses were moderately suppressed and
CTL responses were dramatically increased. In
addition
splenomegaly, which is characteristic trait in the in vivo
administration of recombinant proteins IL-12 in mice, was
induced. Thus, the power of DNA delivery in vivo for both the
production of a new generation of more effective vaccines as
well as an analytical tool for the molecular dissection of the
mechanisms of immune function was demonstrated.
Example 5
To further direct the immune response in vivo, the
induction and regulation of immune responses from the
co-delivery of a broad panel of cytokine genes along with
HIV-1 DNA immunogen constructs was investigated. Cytokine
gene co-delivery was chosen because tytokines play a critical
regulatory and signaling role in immunity. Although cytokines
are produced and released by many cells in addition to those
of the immune system, cytokines produced by lymphocytes are
of a special interest because of their role in regulating
cells of the immune system. For instance, the presence IL-2,
IFN-T, and IL-12 activates the Th0 precursor cell to become a
Thl inflammatory T cells. On the other hand, the release of
IL-4, IL-5, or IL-10 results in a Th0 precursor becoming an
armed Th2 helper cell. In addition, proinflammatory cytokines
CA 02745736 2011-07-04
- 82 -
such as IL-1, TNF-a and TNF-g play active roles in the
initiation of inflammatory responses.
The role of co-delivery of proinflammatory cytokines
(IL-la, TNF-a, and TNF-g), Thl cytokines (IL-2, IL-15, and
IL-18), and Th2 cytokines (IL-4, IL-5 and IL-10) was
investigated. Specifically, genes for these proinflammatory,
Thl, and Th2 cytokines were individually cloned into
expression vectors under control of a cytomegalovirus (CMV)
promoter. The gene plasmid expression cassettes were then
injected into mice along with DNA vaccine cassettes for HIV-1
which have been described previously.
The immunological
effects of the co-injection with these genetic adjuvant
cassettes on the direction and magnitude of antigen-specific
immune responses were analyzed.
Antigen specific immune responses could be modulated
by the co-injection of cytokine genes with DNA immunogen
cassettes. More generally, the power of this strategy of
co-delivering immunologically important genes as a vehicle for
the development of the next generation of DNA vaccines with
enhanced potential for clinical efficacy and utility was
demonstrated.
MATERIALS AND METHODS
DNA Plasmids
DNA vaccine constructs expressing HIV-1 envelope
protein (pCEnv) and gag/pal protein (pCGag/Pol) were prepared
using standard techniques and readily available starting
materials.
The genes for human IL-1a, IL-2, IL-5, IL-10,
IL-15, TNF-a, TNF-P and mouse IL-4 and IL-18 were cloned into
the pCDNA3 expression vector (Invitrogen, Inc., San Diego, CA)
using standard techniques and readily available starting
materials. Human IL-1a, IL-2, IL-5, IL-10, IL-15, TNF-a, and
TNF-P have been reported to be active in mouse cells. Plasmid
DNA was produced in bacteria and purified using Qiagen Maxi
Prep kits (Qiagen, Santa Clara, CA).
Reagents and Cell lines
Human rhabdomyosarcoma (RD) and mouse mastocytoma
P815 cell lines were obtained from ATCC (Rockville, MD).
CA 02745736 2011-07-04
- 83 -
Recombinant vaccinia expressing HIV-1 envelope (vMN462),
gag/pol (vVK1), and (-galactosidase (vSC8) were obtained from
the NIH AIDS Research and Reference Reagent Program. HIV-1
envelope peptide (RIHIGPGRAFYTTKN) was synthesized using
standard techniques and readily available starting materials.
Recombinant Pr55 or gp120 protein were obtained from Quality
Biological (Gaithersburg, MD). Recombinant p24 protein was
purchased from Intracell (Cambridge, MA).
Antibodies to
IL-la, IL-2, IL-5, IL-10, IL-15, TNF-a, and TNF-g were
obtained from R&D Systems (Minneapolis, MN).
Expression of cytokine gene constructs
Expression of cytokine constructs were verified by
immunoprecipitation or cytokine ELISA following transfection
into RD cells. The cells were washed twice with PBS, starved
for one hour in DMEM lacking serum, methionine and cysteine,
and then labeled with 200 yCi/m1 (1,200 Ci/mmole) of 'S
Protein labeling mix (NEN/DuPont). Labeled cells were lysed
in 0.5 ml of RIPA buffer (50 mM TrisHC1 pH7.6; 150 mM NaCl;
0.2%. Triton X-100; 0.296- Deoxycholic acid; 0.1% SDKs and 1 mM
PMSF) on ice and then clarified by centrifugation at 15000
r.p.m. for 10 min. The clarified lysates were incubated with
relevant antibodies (R&D System) for 90 min. on ice. Protein
A sepharose was added to antigen-antibody complexes and mixed
by shaking at 41C for 90 min.
The protein pellet was
resuspended in 50 yl of lx sample buffer and heated at 100'C
for 3-5 min. after extensive washing in buffers containing
high salt and BSA. A fraction of the protein sample was
analyzed by SDS 12%-PAGE. For fluorography, gels were soaked
in 1M sodium salicylate containing 10%- glycerol for 15 min.,
dried, and autoradiographed using Kodak X-oma-AR film.
Supernatants from the transfected RD cells were collected and
tested for expression using cytokine ELISA kits (Pharmingen,
San Diego, CA and R&D System).
DNA inoculation of mice
The quadriceps muscles of 6 to 8 weeks old balb/c
mice (Harlan Sprague Dawley, Inc., Indianapolis, IN) were
injected with 50iig of each DNA construct of interest
CA 02745736 2011-07-04
- 84 -
formulated in phosphate buffered saline (PBS) and 0.25%
bupivacaine-HC1 (Sigma, St. Louis, MO). Co-administration of
various gene expression cassettes involved mixing the chosen
plasmids prior to injection for a total of 100 Ag per
injection.
ELISA
Fifty Al of p24 or gp120 protein diluted in 0.1M
carbonate-bicarbonate buffer (pH 9.5) to 2 Ag/ml concentration
was adsorbed onto microtiter wells overnight at 4 C. The
plates were washed with PBS-0.05% Tween-20 and blocked with
3% BSA in PBS with 0.05% Tween-20 for one hour at 37 C. Mouse
antisera was diluted with 0.05% Tween-20 and incubated for one
hour at 37 C, then incubated with HRP-conjugated goat
anti-mouse IgG (Sigma, St. Louis, MO). The plates were washed
and developed with 3'3'5'5' TMB (Sigma) buffer solution. The
plates were read on a Dynatech MR5000 plate reader with the
optical density at 450 nm.
T helper cell proliferation assay
Lymphocytes were harvested from spleens and prepared
as the effector cells by removing the erythrocytes and by
washing several times with fresh media. The isolated cell
suspensions were resuspended to a concentration of 5 x 106
cells/ml. A 100 Al aliquot containing 5 x 10 cells was
immediately added to each well of a 96 well microtiter flat
bottom plate. Recombinant Pr55 or gp120 protein at the final
concentration of 5 Ag/ml and 1 Ag/ml was added to wells in
triplicate. The cells were incubated at 37 C in 5% CO, for
three days. One ACi of tritiated thymidine was added to each
well and the cells were incubated for 12 to 18 hours at 37 C.
The plate was harvested and the amount of incorporated
tritiated thymidine was measured in a Beta Plate reader
(Wallac, Turku, Finland). Stimulation Index was determined
from the formula:
Stimulation Index (SI) := (experimental count/spontaneous
count)
CA 02745736 2011-07-04
- 85 -
Spontaneous count wells include 10% fetal calf serum which
serves as irrelevant protein control. In addition, pCEnv or
control immunized animals routinely have SI of 1 against Pr55
protein. Similarly, pCGag/pol or control routinely have SI of
1 against gp120 protein. To assure that cells were healthy,
PHA or con A (Sigma) was used as a polyclonal stimulator
positive control. The PHA or con A control samples had a SI
of 20-40.
Cytotoxic T lymphocyte assay
A five hour "-Cr release CTL assay was performed
using vaccinia infected targets or peptide treated targets.
The assay was performed both with and without in vitro
effector stimulation. In the in vitro stimulated assay, the
effectors were stimulated with relevant vaccinia-infected
cells (vMN462 for envelope and vVK1 for gag/pol), and which
were fixed with 0.1% glutaraldehyde or with envelope-specific
peptides (RIHIGPGRAFYTTKN) at a 1 (M concentration for four
to five days in CTL culture media at 5 x 106 cells per ml.
CTL culture media consists of 1:1 ratio of Iscove's Modified
Dulbecco Media (Gibco-BRL, Grand Island, NY) and Hanks'
Balanced Salt Solution (Gibco-BRL) with 10% fetal calf serum
1640 (Gibco-BRL) and 10% RAT-T-STIM without Con A (Becton
Dickinson Labware, Bedford, MA). Vaccinia infected targets
were prepared by infecting 3 x 106 P815 cells at the
multiplicity of infection (MOI) of 10-20 for five to twelve
hours at 37 C. Peptide treated targets were prepared by
incubating P815 cells with 1gM conc'entration of the peptide.
A standard Chromium release assay was performed in which the
target cells were labeled with 100 pCi/m1 Na251Cr04 for 60 to
120 minutes and used to incubate with the stimulated effector
splenocytes for four to six hours at 37 C. CTL lysis was
determined at effector:target (E:T) ratios ranging from 50:1
to 12.5:1. Supernatants were harvested and counted on a LKB
CliniGamma gamma-counter.
Percent specific lysis was
determined from the formula:
100 x (experimental release - spontaneous
release)
CA 02745736 2011-07-04
- 86 -
maximum release - spontaneous release
Maximum release was determined by lysis of target cells in 1%
Triton X-100 containing medium. An assay was not considered
valid if the value for the 'spontaneous release' counts are
in excess of 20% of the 'maximum release'.
Complement lysis of CD8+ T cells
CDS+ T cells were removed from the splenocytes by a treatment
with anti-CD8 monoclonal antibody (Pharmingen, San Diego, CA)
followed by incubation with rabbit complement (Sigma) for 45
min. at 37 C.
RESULTS
Expression of cytokine gene cassettes
The cytokine genes were individually cloned into
pCDNA3 plasmid expression vectors (Figure 10). These cytokine
expression cassettes were verified by a sequencing analysis
of the entire insert (on both 5' and 3' sides). In addition,
these cytokine genes were transfected in vitro into RD cells
and the expression of these constructs were verified by
immunoprecipitation using relevant antibodies or by cytokine
ELISA as described in the Materials and Methods section.
Humoral response following co-injection with cytokine genes
Antisera from pCGag/pol immunized mice were
collected and analyzed for specific antibody responses against
H I V - 1 antigens by ELISA.
In these experiments, 50 g of each DNA was
co-administered intramuscularly at days 0 and 14. Prior to
injection and at 28 days following injection, the mice (four
mice per group) were bled and the sera were collected. The
serial dilutions were 1:100, 1:200, 1:400, 1:800,1:1600, and
1:3200. The background optical density level for ELISA was
<0.015. These experiments have been repeated with similar
results. As shown in the data, the endpoint antibody titers
for these immunized groups were determined using the ELISA
against p24 gag protein, and the endpoint antibody titers for
these immunized groups were determined using the ELISA against
gp120 envelope protein. The following data was generated for
CA 02745736 2011-07-04
- 87 -
gag/pol-specific antibody titer from sera collected at 28 days
post-DNA immunization:
Anti-D24 Antibody Titer
pCGag/pol Only 400
pCGag/pol + IL-lalpha 1600
pCGag/pol + TNF-alpha 1600
pCGag/pol + TNF-beta 1600
pCGag/pol + IL-2 3200
pCGag/pol + IL-15 800
pCGag/pol + IL-18 3200
pCGag/pol + IL-4 3200
pCGag/pol + IL-5 3200
pCGag/pol + IL-10 1600
The highest level of end point titer was observed with sera
from the IL-2, IL-4, IL-5, and IL-18 co-injected groups. A
dramatic enhancement of humoral response was also observed
with the group co-injected with IL-1a, TNF-a, TNF-g, and IL-10
over the group immunized with pCGag/pol alone. A similar
result was seen with the groups immunized with pCEnv.
Anti-cm120 Antibody Titer
pCEnv Only 200
pCEnv + IL-lalpha 800
pCEnv + TNF-alpha 800
pCEnv + TNF-beta 400
pCEnv + IL-2 800
pCEnv + IL-15 400
pCEnv + IL-18 800
pCEnv + IL-4 1600
pCEnv + IL-5 1600
pCEnv + IL-10 1600
Again, the highest level of end point titer was observed with
sera from the IL-4, IL-5, and IL-10 co-injected groups.
Generation of T helper cells
Activation and proliferation of T helper lymphocytes
play a critical role in inducing both a humoral immune
response via B cells and cellular immune response via CD8+
cytotoxic T cells. Mice received two DNA immunization (50 mg
each) separated by two weeks. At one week after the boost
injection, the mice were sacrificed, the spleens were
harvested, and the lymphocytes were isolated and tested for
T helper cell proliferation.
Proinflammatory cytokine co-injection
CA 02745736 2011-07-04
- 88 -
Proliferation assays were conducted with splenocytes
from mice co-injected with pCEnv or pCGag/pol and
proinflammatory cytokines IL-1a, TNF-a, and TNF-0.
In the experiments evaluating T helper cell
proliferation responses following co-injection with
proinflammatory cytokines, IL-1a, TNF-a, and TNF-g the methods
were as follows. Two weeks after the first DNA immunization
with pCEnv (50 gg of each), the mice (four mice per group)
were boosted with same dosage. After 1 additional week,
spleens were collected from immunized mice and their
lymphocytes were isolated and tested against recombinant gp120
protein (5 and 1 gg/ml final concentrations). Two weeks after
the first DNA co-injection with pCGag/pol (50 gg of each), the
mice (four mice per group) were boosted with same dosage.
After 1 additional week, spleens were collected from immunized
mice and their lymphocytes were isolated and tested for T cell
proliferative response against Pr55 protein (5 and 1 gg/m1
final concentrations). These experiments have been repeated
two times with similar results. The following data was
generated:
Antigen Specific T Cell Proliferative Response
gp120 protein
5aci/m1 1gq/m1
pCEnv Only 2.2 1.3
pCEnv + IL-1 alpha 2.3 0.1
pCEnv + TNF-alpha 6.1 2.7
pCEnv + TNF-beta 3.1 1.8
Control 0.5 0.2
p24 protein
5ua/m1 1ua/m1
pCGag/pol Only 2.4 0.8
pCGag/pol + IL-1 alpha 2.8 1.4
pCGag/pol + TNF-alpha 12.4 2.2
pCGag/pol + TNF-beta 4.0 1.9
Control 0.6 0.8
These data show that a background level of proliferation was
observed in the control group and a moderate level of
proliferation was observed:in the group immunized with pCEnv
or pCGag/pol alone. Even though the group co-injected with
IL-la did not result in any increase in T helper cell
CA 02745736 2011-07-04
- 89 -
proliferation in either pCEnv or pCGag/pol immunization, the
groups co-injected with pCEnv+TNF-g resulted in a significant
enhancement of T helper cell proliferation with the
stimulation index of 3.1 at 5 pg/ml gp120 protein
concentration. Similarly, pCGag/pol+TNF-0 resulted in a
stimulation index of 4.0 at 5 pg/m1 Pr55 protein
concentration. Even higher levels of T helper cell
proliferation were observed with pCEnv+TNF-a and
pCGag/pol+TNF-a co-injections with stimulation indexes of 6.1
and 12.4, respectively (at 5 pg/m1 of each protein
concentration).
Thl cytokine co-injection
The effects of co-delivering Thl cytokines IL-2,
IL-15, and IL-18 was investigated. pCEnv+IL-18 and
pCGag/pol+IL-18 co-injection groups resulted in a stimulation
indexes of 4.4 and 10.0, respectively (at 5 pg/ml of each
protein concentration). In experiments evaluating T helper
cell proliferation responses following co-injection with IFN-1,
inducing Thl cytokines, IL-12 and IL-18 the following methods
were followed. Two weeks after the first DNA immunization
with pCEnv (50 pg of each), the mice (four mice per group)
were boosted with same dosage. After 1 additional week,
spleens were collected from immunized mice and their
lymphocytes were isolated and tested against recombinant gp120
protein (5 and 1 pg/m1 final concentrations). Two weeks after
the first DNA co-injection with pCGag/pol (50 pg of each), the
mice (four mice per group) were boosted with same dosage.
After 1 additional week, spleens were collected from immunized
mice and their lymphocytes were isolated and tested for T cell
proliferative response against Pr55 protein (5 and 1 pg/ml
final concentrations). These experiments have been repeated
two times with similar results. The following data was
generated
gp120 protein
5pg/m1 lpg/ml
pCEnv Only 2.2 1.3
pCEnv + IL-18 4.4 0.8
pCEnv + IL-12 7.8 3.8
Control 0.5 0.2
CA 02745736 2011-07-04
- 90 -
p24 protein
5,1q/m1 1uq/m1
pCGag/pol Only 2.4 0.8
pCGag/pol + IL-18 10.0 2.1
pCGag/pol + IL-12 12.0 3.8
Control 0.6 0.8
In addition, pCEnv+IL-2 and pCGag/pol+IL-2 co-injections
resulted in stimulation indexes of 6.0 and 12.0, respectively
(at 5 I.I.g/m1 of each protein concentration). Co-delivery of
IL-15, however, resulted in a more moderate increase in T
helper cell proliferation. In experiments evaluating T helper
cell proliferation responses following co-injection with IL-2
receptor dependent Th1 cytokines, IL-2 and IL-15õ the
following methods were used. Two weeks after the first DNA
immunization with pCEnv (50 jig of each), the mice (four mice
per group) were boosted with same dosage. After 1 additional
week, spleens were collected from immunized mice and their
lymphocytes were isolated and tested against recombinant gp120
protein (5 and 1 jig/ml final concentrations). Two weeks after
the first DNA co-injection with pCGag/pol (50 jig of each), the
mice (four mice per group) were boosted with same dosage.
After 1 additional week, spleens were collected from immunized
mice and their lymphocytes were isolated and tested for T cell
proliferative response against Pr55 protein (5 and 1 jig/m1
final concentrations). These experiments have been repeated
two times with similar results. The following dfat were
generated.
gp120 protein
51/g/m1 lug/m1
pCEnv Only 2.2 1.3
pCEnv + IL-2 6.0 2.1
pCEnv + IL-15 2.3 2.0
Control 0.5 0.2
p24 protein
5thq/m1 1gq/m1
pCGag/pol Only 2.4 0.8
pCGag/pol + IL-2 12.0 2.5
pCGag/pol + IL-15 2.6 0.6
Control 0.6 0.8
Th2 cytokine co-injection
CA 02745736 2011-07-04
- 91 -
In addition to the examination of proinflammatory
and Thl cytokine co-injection, the effects of co-delivering
Th2 cytokines IL-4, IL-5 and IL-10 with pCEnv and pCGag/pol
was also investigated. Both the groups co-injected with IL-4
or IL-5 showed a moderate increases in T helper cell
proliferation over those of pCEnv or pCGag/pol immunization
alone. In experiments evaluating T helper cell proliferation
responses following co-injection with Th2 cytokines, IL-5 and
IL-10, the following methods were used. Two weeks after the
first DNA immunization with pCEnv (50 mg of each), the mice
(four mice per group) were boosted with same dosage. After
1 additional week, spleens were collected from immunized mice
and their lymphocytes were isolated and tested against
recombinant gp120 protein (5 and 1 mg/m1 final
concentrations). Two weeks after the first DNA co-injection
with pCGag/pol (50 mg of each), the mice (four mice per group)
were boosted with same dosage. After 1 additional week,
spleens were collected from immunized mice and their
lymphocytes were isolated and tested for T cell proliferative
response against Pr55 protein (5 and 1 mg/ml final
concentrations). These experiments have been repeated two
times with similar results. The following data was generated:
gp120 protein
5g/ml 1mq/m1
pCEnv Only 2.2 1.3
pCEnv + IL-4 3.8 2.9
pCEnv + IL-5 2.4 1.8
pCEnv + IL-10 4.5 2.3
Control ' 0.5 0.2
p24 protein
5uq/m1 Ifict/m1
pCGag/pol Only 2.4 0.8
pCGag/pol + IL-4 5.0 3.1
pCGag/pol + IL-5 3.1 2.1
pCGag/pol + IL-10 8.0 2.4
Control 0.6 0.8
Co-delivery of IL-10 resulted in a more dramatic enhancement
of T helper cell proliferation with stimulation indexes of 4.5
and 8.0, respectively (at 5 mg/ml of each protein
concentration).
Generation of Cytotoxic T Lymphocytes
CA 02745736 2011-07-04
- 92 -
To further investigate the enhancement of cellular
immunity, cytotoxic T lymphocyte (CTL) assays were performed
using splenocytes of mice immunized with pCEnv and pCGag/pol.
Mice received two DNA immunization (50 itg each) separated by
two weeks. At one week after the boost injection, the mice
sacrificed, the spleens harvested, and the lymphocytes were
isolated and tested for CTL response. The assay was performed
both with and without in vitro stimulation of effector
splenocytes prior to measuring chromium release from specific
and non-specific vaccinia infected or peptide treated targets.
To calculate specific lysis of targets, the percent lysis of
non-specific (vSC8 infected) targets was subtracted from the
percent lysis of specific (vMN462 or vVK1 infected) targets.
CTL Response with in vitro stimulation of effectors
Data was generated to evaluate cytotoxic T
lymphocyte responses following co-injection with various
cytokines. The following data was generated:
Antigen Specific CTL Response
50:1 25:1 12.5:1
pCEnv 10% 49,1 3%
pCEnv + IL-1 alpha 14% 10% 6%
pCEnv + TNF-alpha 30% 23% 18%
pCEnv + TNF-beta 20% 16% 13%
pCEnv + IL-2 22% 16% 4%
pCEnv + IL-15 46% 28% 10%
pCEnv + IL-12 35% 24% 19% .
pCEnv + IL-18 22% 16% 13%
pCEnv + IL-4 4% 4% 7%
pCEnv + IL-5 13% 11% 9%
pCEnv + IL-10 13% 8%. 3%
Control 396 2.0% 3.5%
50:1 25:1 12.5:1
pCGag/pol 12% 11% 7%
pCGag/pol + IL-1a 16% 8%. 1%
pCGag/pol + TNF-a 29% 20% 7%
pCGag/pol + TNF-g 12% 13% 3%
pCGag/pol + IL-2 14% 11% 10%
pCGag/pol + IL-15 30% 21% 7%
pCGag/pol + IL-12 38% 24% 15%
pCGag/pol + IL-18 19% 15% 4%
pCGag/pol + IL-4 2% 2% 2%
pCGag/pol + IL-5 '0% ' 3% 2%
pCGag/pol + IL-10 6% 0%
Control 3.3% 2.8% 4.5%
CA 02745736 2011-07-04
- 93 -
In experiments performed evaluating cytotoxic T
lymphocyte responses following co-injection with
proinflammatory cytokines, IL-1a, TNF-a, and TNF-g, two weeks
after the first DNA co-injection with pCGag/pol (50 Ag of
each), the mice (four mice per group) were boosted with same
dosage. After 1 additional week, spleens were collected from
immunized mice and their lymphocytes were isolated and tested
for CTL response using target cells infected with specific
(vVKl) and non-specific vaccinia (vSC8). Two weeks after the
first DNA co-injection with pCEnv (50 g of each), the mice
(four mice per group) were boosted with same dosage. After
1 additional week, spleens were collected from immunized mice
and their lymphocytes were isolated and tested for CTL
response using target cells infected with specific (vMN462)
and non-specific vaccinia (vSC8). These experiments have been
repeated two times with similar results.
In experiments performed evaluating cytotoxic T
lymphocyte responses following co-injection with IFN-T
inducing Th1 cytokines, IL-12 and IL-18, two weeks after the
first DNA co-injection with pCGag/pol (50 gg of each), the
mice (four mice per group) were boosted with same dosage.
After 1 additional week, spleens were collected from immunized
mice and their lymphocytes were isolated and tested for CTL
response using target cells infected with specific (vVKl) and
non-specific vaccinia (vSC8). Two weeks after the first DNA
co-injection with pCEnv (50 gg of each), the mice (four mice
per group) were boosted with same dosage. After 1 additional
week, spleens were collected from immunized mice and their
lymphocytes were isolated and tested for CTL response using
target cells infected with specific (vMN462) and non-specific
vaccinia (vSC8). These experiments have been repeated two
times with similar results.
In experiments performed evaluating cytotoxic T
lymphocyte responses following co-injection with IL-2 receptor
dependent Th1 cytokines, ILL-2 and IL-15, two weeks after the
first DNA co-injection with pCGag/pol (50 lig of each), the
mice (four mice per group) were boosted with same dosage.
CA 02745736 2011-07-04
- 94 -
After 1 additional week, spleens were collected from immunized
mice and their lymphocytes were isolated and tested for CTL
response using target cells infected with specific (vVKl) and
non-specific vaccinia (vSC8). Two weeks after the first DNA
co-injection with pCEnv (50 gg of each), the mice (four mice
per group) were boosted with same dosage. After 1 additional
week, spleens were collected from immunized mice and their
lymphocytes were isolated and tested for CTL response using
target cells infected with specific (vMN462) and non-specific
vaccinia (vSC8). These experiments have been repeated two
times with similar results.
In experiments performed evaluating cytotoxic T
lymphocyte responses following co-injection with Th2
cytokines, IL-5 and IL-10, two weeks after the first DNA
co-injection with pCGag/pol (50 gg of each), the mice (four
mice per group) were boosted with same dosage. After 1
additional week, spleens were collected from immunized mice
and their lymphocytes were isolated and tested for CTL
response using target cells infected with specific (vVKl) and
non-specific vaccinia (vSC8). Two weeks after the first DNA
co-injection with pCEnv (50 gg of each), the mice (four mice
per group) were boosted with same dosage. After 1 additional
week, spleens were collected from immunized mice and their
lymphocytes were isolated and tested for CTL response using
target cells infected with specific (vMN462) and non-specific
vaccinia (vSC8). These experiments have been repeated two
times with similar results.
Proinflammatory cytokine co-injection
With regard to data from the CTL assay results for
the mice co-injected with pCEnv or pCGag/pol and
proinflammatory cytokines IL-la, TNF-a, and TNF-g, a
background level of specific killing was observed from the
control animals, whereas the animals immunized with pCEnv
alone showed a small level of CTL response. Co-injection with
pCEnv+IL-la or pCEnv+TNF-g resulted in a moderate increase in
CTL activity. On the other hand, a more dramatic increase in
the specific killing of targets infected with vaccinia
CA 02745736 2011-07-04
- 95 -
(vMN462) expressing HIV-1 envelope was observed after
co-injection with pCEnv+TNP-a. Greater than 30% specific
lysis of target cells was observed after co-injection with
pCEnv+TNF-a at a 50:1 effector to target (E:T) ratio.
Similarly, the mice immunized with pCGag/pol+TNF-a resulted
in a significant enhancement of antigen-specific CTL lysis of
targets infected with vaccinia (vVY1) expressing HIV-1 gag/pol
(29% lysis at E:T ratio of 50:1) while co-injection with
pCGag/pol+IL-la or pCGag/pol+TNF-P resulted in a small
increase in CTL response.
Th1 cytokine co-injection
The effects of co-delivering Thl cytokines with DNA
vaccine constructs was investigated. With regard to CTL assay
results for the mice immunized with pCEnv and those mice
co-injected with Thl cytokines IL-12 and IL-18. Unlike IL-12
co-administration, IL-18 co-injection resulted in a more
moderate increase in CTL response. Co-administration of IL-2
also resulted in a moderate increase in CTL response. On the
other hand, a more dramatic increase in CTL response at 46%
specific lysis was observed after pCEnv+IL-15 immunization.
Similarly, the mice injected with pCGag/pol+IL-15 resulted in
a significant enhancement of antigen-specific CTL lysis (at
30%).
Th2 cytokine co-injection
In addition to investigating the effects from the
co-delivery of proinflammatory and Thl cytokines, the effects
of co-injections with Th2 cytokines IL-4, IL-5 and IL-10 on
the level of CTL response were also studied. Although the
co-injections with these cytokines resulted in the increase
in T helper cell proliferative responses, the co-injections
of IL-4, IL-5 or IL-10 with either pCEnv or pCGag/pol did not
result in any specific increase in CTL response.
Determination of MHC class I restriction in CTL response
To determine whether the increases in CTL response
via co-injection with TNF-,a and IL-15 was due to a CD8+ MHC
class I restricted stimulus, CTL assays were performed using
a HIV-1 envelope peptide (RIHIGPGRAFYTTKN) which has been
CA 02745736 2011-07-04
- 96 -
shown to be a specific epitope for MHC class I-restricted CTL
for balb/c mice. Mice received two immunizations of 50 Ag of
each DNA construct separated by two weeks and their spleens
were harvested one week after the second immunization. The
CTL assay was performed on the splenocytes following in vitro
stimulation with envelope-specific peptides. A significant
enhancement of CTL response was observed after both
co-injection (Figures 11A-11E) with IL-15 and TNF-u at 25% and
32% specific killing at an E:T ratio of 50:1, respectively.
This observation was verified by measuring CTL activity after
the removal of 0D8+ T cells from the effector cell population
by complement lysis. Mice received two immunizations of 50
yg of each plasmid at the same interval as above. A CTL assay
was performed in which one group of effector cells was treated
as before and CD8+ T cells from the second group were removed.
As shown in Figures 11F-110, the removal of CD8+ T cells
resulted in the suppression of antigen-specific CTL
enhancement observed after co-injections with IL-15 and TNF-u.
These results indicate that the enhancement of cytolytic
activity was antigen-specific, class I-restricted and CD8+ T
cell dependent.
Direct CTL response (without in vitro stimulation of
effectors)
The level of direct CTL response induced by
co-injection with TNF-a or IL-15 was investigated because a
high and consistent level of CTL response (with in vitro
stimulation) was observed from these two co-administration
groups. The chromium release assay was performed on the same
day the splenocytes were isolated. Unlike seeing direct CTL
with IL-12 co-injection, the induction of direct CTL activity
after co-injection with pCEnv and TNF-a or IL-15 (Figure 12)
was not observed.
DISCUSSION
The overall aim of any immunization strategy is to
induce potent and durable pathogen-specific immune responses
using the least number of immunizations.
However, the
correlates of protection from may vary from one pathogen to
CA 02745736 2011-07-04
- 97 -
the next and improvements in results can be achieved by
directing the immune response. For example, high levels of
specific antibody response are thought to be important for
protection from infection with hepatitis B virus while
protection from lymphocytic choriomeningitis virus (LCMV)
infection is mediated primarily through T cell-mediated
responses. The design of DNA vaccination strategies can be
improved by tailoring the direction and magnitude of induced
immune responses to fit the correlates of protection for each
target pathogen.
As a new immunization strategy, nucleic acid
immunization has been demonstrated to elicit both
antigen-specific humoral and cellular immune responses in vivo
in a variety of animal models. More clinically efficacious
vaccines may be produced using a strategy of controlling the
direction and magnitude of immune responses.
The finer
control in generating specific types and directions of the
immune responses from vaccine and immune therapies can be
accomplished by the co-delivery of genes for immunologically
important molecules such as cytokines and costimulatory
molecules.
Cytokines play important roles in the immune and
inflammatory responses as the initiators and regulators of the
immune network. Based upon their specific function in the
immune system these cytokines could be further grouped as
proinflammatory, Th1, and Th2 cytokines.
Proinflammatory
cytokines IL-1, TNF-a and TNF-g play important role as the
initiator of the host responses to injury and infection. IL-1
indirectly activates T cells by inducing the production of
IL-2 and up-regulating the IL-2R on these cells. IL-1 also
influences B cells by inducing their differentiation, growth,
and synthesis of IgGs. At least two forms of IL-1, designated
as IL-1a and IL-1g, exist and exhibit similar activities.
TNF-a and TNF-g are closely related proteins (about 301,- amino
acid residue homology) which bind to the same cell surface
receptors. TNF-a is produced by activated macrophages and
monocytes neutrophils, activated lymphocytes, and NK cells
CA 02745736 2011-07-04
- 98 -
whereas TNF-g is produced by lymphocytes. TNF-a and TNF-g are
also implicated in septic shock following infection by
Gram-negative bacteria and in rheumatoid arthritis.
Furthermore, TNF-a has been suggested to play a pivotal role
in regulating the synthesis of other proinflammatory
cytokines.
Thl cytokines regulate the cellular or T
cell-mediated arm of the immune response.
IFN-y, a
prototypical Thl-type cytokine, is produced by Thl, CD8+, and
NK cells, and has been shown to have antiviral effects as well
as immunomodulatory effects such as the up-regulation of MHC
class I and II antigens. A new cytokine IFN-y-inducing factor
(IGIF) or IL-18 has been found to enhance the production of
IFN-y while inhibiting the production of IL-10 in stimulated
PBMC. IL-18 also augments natural killer (NK) cell activity
in cultures of human peripheral blood mononuclear cells
(PBMC), similar to the structurally unrelated cytokine IL-12.
IL-2 is produced primarily by T cells activated by external
stimulation; it is critical for the proliferation and clonal
expansion of antigen-specific T cells. IL-2
serves this
pivotal role in T cell activation by its interaction with a
receptor system consisting of three chains, (21, g, and yc
chains . IL-15, a newly identified homologue of IL-2, is a
pleiotropic cytokine which possesses T cell stimulatory
activities similar to IL-2.
Th2 cytokines regulate the humoral or
antibody-mediated arm of the immune response.
IL-5 is a
dimeric cytokine that controls the differentiation of B cells
into antibody producing plasma cells. IL-5 has been shown to
induce antigen-specific IgA production by murine and human B
cell.
In addition, IL-5 also promotes the growth and
proliferation of eosinophils. Although IL-10 has been shown
initially to be produced by Th2 T cell clones, it is also
produced by B cells and monocytes. Touted as a prototypical
Th2-type cytokine, IL-10 has been shown to inhibit the
production of such cytokines as IL-la, IL-6, IL-8, and TNF-a
by mitogen-activated monocytes as well as to inhibit the
CA 02745736 2011-07-04
- 99 -
macrophage activating effects of IFN-y. A possible role of
IL-10 in HIV-1 infection has also been reported; IL-10 mRNA
was up-regulated and increased levels of IL-10 were observed
from PBMC from asymptomatic HIV-positive individuals compared
with PBMC from uninfected individuals. In addition, IL-10 has
been shown to decrease in vitro viral replication in human
macrophages.
Expression cassettes for proinflammatory, Th1, and
Th2 cytokines were developed in an effort to analyze their
ability to function as in vivo modulators of the immune
responses induced by DNA vaccines. Cytokine genes were co-
delivered along with DNA immunogen constructs into mice
intramuscularly and analyzed their effects in the direction
and magnitude of induced immune responses. A
dramatic
increase in the antibody response was observed with
co-injection with IL-2, IL-4, IL-5, IL-10, and IL-18.
Co-injection with TNF-a, TNF-0, IL-21 IL-10, and IL-18
resulted in a dramatic enhancement of T helper proliferation
response while co-injection with IL-5 and IL-15 resulted in
a more moderate increase in T helper proliferation.
Furthermore, among all co-injection combinations, only TNF-a
and IL-15 co-injections resulted in a level of CTL enhancement
(greater than 30% specific lysis) similar to that of IL-12
co-injection.
Co-injection with TNF-g, IL-2, and IL-18
resulted in a more moderate increase in CTL response over
those groups immunized with only DNA immunogen. As observed
with IL-12 or CD86 co-injection, the enhancement of CTL
responses observed from the co-injections with TNF-a and IL-15
were restricted by MHC Class I and CD8+ T cells.
IL-18 has been reported to share similar activities
of IL-12. For instance, IL-18 augments natural killer (NK)
cell activity in cultures of human peripheral blood
mononuclear cells (PBMC), similarly to the structurally
unrelated cytokine IL-12. IL-18 also enhances the production
of IFN-y while inhibiting the production of IL-10 in
concanavalin A (Con A)-stimulated PBMC. IL-18 has been
observed to induce IFN-( through IL-12-independent pathway.
CA 02745736 2011-07-04
- 100 -
Even though a significant increase in the level of T cell
mediated responses and a moderate reduction in humoral
response with IL-12 co-delivery, similar effects from IL-18
co-administration was not observed. Instead, a significant
increase in the antibody titers with IL-18 co-administration
was seen. In addition, unlike IL-12 co-delivery which induced
a dramatic enhancement of CTL, but IL-18 co-injection did not
induce a similar level of CTL enhancement. The
immunomodulatory characteristics of IL-18 appeared to be
similar to that of IL-10.
In addition to the differential in vivo effects of
IL-12 and IL-18 co-administrations, co-injection with IL-2 and
IL-15 also resulted in different direction and magnitude of
immune responses. IL-2 and IL-15 have been reported to have
similar bioactivities which include the sharing of the y chain
of the IL-2 receptor and signaling machinery for T cell
stimulation. IL-2 co-administration resulted in a dramatic
increase in antibody and T helper cell proliferative responses
while the IL-15 co-injection resulted in a significant
enhancement of CTL responses. Such
differences may be
explained by the pleiotropic nature of IL-15. For example,
IL-15 has been reported to induce significant TNF-a production
in rheumatoid arthritis through activation of synovial T
cells. On the other hand, IL-2 induced significantly lower
level of TNF-a. This in vivo data suggest that signaling
machinery is differentially activated by engagement of these
two molecules.
Th2 cytokines could be used to improve Th2-type
immune responses without affecting the level of T
cell-mediated responses. IL-
4, IL-5 and IL-10 have been
reported to be potent Th2 cytokines. A significant increase
in the level of antibody response as well as the level of T
helper proliferation with IL-4, IL-5 and IL-10 co-delivery.
On the other hand, no increase in the level of CTL response
was observed.
These results demonstrate that a Th2-type
response could be engineered with IL-4, IL-5, or IL-10
co-administration.
CA 02745736 2011-07-04
- 101 -
A dramatic observation was the role of TNF-a and
IL-15 as multi-functional immune modulators.
The role of various immunologically important
cytokines on the induction of host immune responses from DNA
immunization was investigated. As summarized in Figure 13,
the induction of specific arms of immune responses could be
engineered using the strategy of co-administration of cytokine
genes. This cytokine gene adjuvant network underscores a new
level of control in the induction of specific immune responses
to tailor the vaccination programs more closely to the
correlates of protection which vary from disease to disease.
This type of fine control of vaccine and immune therapies was
previously unattainable.
As a result, controlling the
magnitude and direction of the immune response could be
advantageous in a wide variety of vaccine strategies. For
instance, in a case where T cell mediated response is
paramount, but the humoral response may not be needed or even
be harmful, IL-12 genes could be chosen as the immune
modulator to be co-delivered with a specific DNA immunogen.
On the other hand, for building vaccines to target
extracellular bacteria, for example, IL-4, IL-5 or IL-10 genes
could be co-injected. Furthermore, in cases where both CD4+
T helper cells and antibodies play more important roles in
protection, GM-CSF as well as IL-2 could be co-delivered.
Lastly, in cases where all three arms of immune responses are
critical, TNF-a could be co-injected to give a combined
enhancement of antibody, T helper dell, and CTL responses.
Example 6
Using PCR reactions an insert, designated as BL1 and
shown in Figure 14, was cloned and ligated it into PCR3
eukaryotic expression vector as well as the vector pBBKan
using the appropriate restriction enzymes as shown in Figure
15. The BL1 construct was co-admministered with different HIV-
1 antigens and measured the,immunostimulatory effects in mice.
The results, which are presented in Figures 16 and 17A, 17B,
17C and 17D indicate that the DNA fragment in BL1 enhances
CA 02745736 2011-07-04
- 102 -
immune responses when coimmunized with HIV-1 antigens. The
different observed effects are the increase in spleen size,
and an increase in antibody and CTL response.
=
CA 02745736 2011-07-04
=
- 103 -
Table 1
Picornavirus Family
Genera: Rhinoviruses: (Medical) responsible for
- 50% cases of the common cold.
Etheroviruses: (Medical) includes
pol iovi ruses ,
coxsackievi ruses ,
echoviruses, and human enteroviruses such
as hepatitis A virus.
Apthoviruses: (Veterinary) these are the
foot and mouth disease viruses.
Target antigens: VP1, VP2, VP3, VP4, VPG
Calcivirus Family
Genera: Norwalk Group of Viruses: (Medical) these
viruses are an important causative agent
of epidemic gastroenteritis.
Togavirus Family
Genera: Alphaviruses: (Medical and Veterinary)
examples include Senilis viruses,
RossRiver virus and Eastern & Western
Equine encephalitis.
Reovirus: (Medical) Rubella virus.
Flariviridue Family
Examples include: (Medical) dengue,
yellow fever, Japanese encephalitis, St.
Louis encephalitis and tick borne
encephalitis viruses.
Hepatitis C Virus: (Medical) these viruses are not placed in
a family yet but are.believed to be either a togavirus or a
flavivirus. Most similarity is with togavirus family.
Coronavirus Family: (Medical and Veterinary)
Infectious bronchitis virus (poultry)
Porcine transmissible gastroenteric virus
(pig)
Porcine hemagglutinating
encephalomyelitis virus (pig)
Feline infectious peritonitis virus
(cats)
Feline enteric coronavirus (cat)
Canine coronavirus (dog)
The human respiratory coronaviruses cause
-40 cases of common cold. EX. 224E, 0C43
Note - coronaviruses may cause non-A, B
or C hepatitis
Target antigens:
El - also called M or matrix protein
E2 - also called S or Spike protein
E3 - also called HE or hemagglutin-
elterose glycoprotein (not present
in all coronaviruses)
N - nucleocapsid
CA 02745736 2011-07-04
- 104 -
Rhabdovirus Family
Genera: Vesiliovirus
Lyssavirus: (medical and veterinary)
rabies
Target antigen:
G protein
N protein
Filoviridue Family: (Medical)
Hemorrhagic fever viruses such as Marburg
and Ebola virus
Paramyxovirus Family:
Genera: Paramyxovirus: (Medical and Veterinary)
Mumps virus, New Castle disease virus
(important pathogen in chickens)
Morbillivirus: (Medical and Veterinary)
Measles, canine distemper
Pneuminvirus: (Medical and Veterinary)
Respiratory syncytial virus
Orthomyxovirus Family (Medical)
The Influenza virus
Bungavirus Family
Genera: Bungavirus: (Medical) California
encephalitis, LA Crosse
Phlebovirus: (Medical) Rift Valley Fever
Hantavirus: Puremala is a hemahagin fever
virus
Nairvirus (Veterinary) Nairobi sheep
disease
Also many unassigned bungaviruses
Arenavirus Family (Medical)
LCM, Lassa fever virus
Reovirus Family
Genera: Reovirus: a possible human pathogen
Rotavirus: acute gastroenteritis in
children
Orbiviruses: (Medical and Veterinary)
Colorado Tick fever, Lebombo (humans)
equine encephalosis, blue tongue
Retrovirus Family
Sub-Family:
Oncorivirinal: (Veterinary) (Medical)
feline leukemia virus, HTLVI and HTLVII
Lentivirinal: (Medical and Veterinary)
HIV, feline immunodeficiency virus,
equine, infections, anemia virus
Spumavirinal
Papovavirus Family
Sub-Family:
CA 02745736 2011-07-04
- 105 -
Polyomaviruses: (Medical) BKU and JCU
viruses
Sub-Family:
Papillomavirus: (Medical) many viral
types associated with cancers or
malignant progression of papilloma
Adenovirus (Medical)
EX AD7, ARD., O.B. - cause respiratory disease
- some adenoviruses such as 275 cause
enteritis
Parvovirus Family (Veterinary)
Feline parvovirus: causes feline enteritis
Feline panleucopeniavirus
Canine parvovirus
Porcine parvovirus
Herpesvirus Family
Sub-Family: alphaherpesviridue
Genera: Simplexvirus (Medical)
HSVI, HSVII
Varicellovirus: (Medical - Veterinary)
pseudorabies - varicella zoster
Sub-Family - betaherpesviridue
Genera: Cytomegalovirus (Medical)
HCMV
Muromegalovirus
Sub-Family: Gammaherpesviridue
Genera: Lymphocryptovirus (Medical)
EBV - (Burkitts lympho)
Rhadinovirus
Poxvirus Family
Sub-Family: Chordopoxviridue (Medical - Veterinary)
Genera: Variola (Smallpox)
Vaccinia (Cowpox)
Parapoxivirus - Veterinary
Auipoxvirus - Veterinary
Capripoxvirus
Leporipoxvirus
Suipoxvirus
Sub-Family: Entemopoxviridue
Hepadnavirus Family
Hepatitis B virus
Unclassified
Hepatitis delta virus
CA 02745736 2011-07-04
- 106 -
Table 2
Bacterial pathogens
Pathogenic gram-positive cocci
include:
pneumococcal; staphylococcal; and streptococcal.
Pathogenic gram-negative cocci include:
meningococcal; and gonococcal.
Pathogenic enteric gram-negative bacilli include:
enterobacteriaceae; pseudomonas, acinetobacteria
and eikenella; melioidosis;
salmonella;
shigellosis; hemophilus; chancroid; brucellosis;
tularemia; yersinia (pasteurella); streptobacillus
moniliformis and spirillum
listeria
monocytogenes; erysipelothrix rhusiopathiae;
diphtheria; cholera; anthrax;
donovanosis
(granuloma inguinale); and bartonellosis.
Pathogenic anaerobic bacteria include: tetanus;
botulism; other clostridia; tuberculosis; leprosy;
and other mycobacteria.
Pathogenic spirochetal
diseases include: syphilis; treponematoses: yaws,
pinta and endemic syphilis; and leptospirosis.
Other infections caused by higher pathogen bacteria
and pathogenic fungi include: actinomycosis;
nocardiosis; cryptococcosis,
blastomycosis,
histoplasmosis and coccidioidomycosis; candidiasis,
aspergillosis, and mucormycosis; sporotrichosis;
paracoc c i di odomyco s s ,
petriellidiosis,
torulopsosis, mycetoma and chromomycosis; and
dermatophytosis.
Rickettsial infections include rickettsial and
rickettsioses.
Examples of mycoplasma and chlamydial infections
include: mycoplasma pneumoniae; lymphogranuloma
venereum; psittacosis; and perinatal chlamydial
infections.
=
Pathogenic eukaryotes
Pathogenic protozoans and helminths and infections
thereby include: amebiasis; malaria; leishmaniasis;
trypanosomiasis; toxoplasmosis; pneumocystis
carinii; babesiosis; giardiasis; trichinosis;
filariasis; schistosomiasis; nematodes; trematodes
or flukes; and cestode (tapeworm) infections.
=