Note: Descriptions are shown in the official language in which they were submitted.
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
HIGHLY VISCOUS DETERGENT EMULSION
Field Of The Invention
The invention relates to a high viscosity, phase stable alkaline cleaning
concentrate or composition characterized by a reduced water concentration (a
high
concentration of active materials such as alkalinity and surfactants) and to
methods
of their use and preparation. In industrial or institutional applications, the
materials
are phase stable, have very high viscosity yet remain dispensable from
automatic or
programmable dispensers to a use locus where they are easily mixed with water
in a
use locus to form an aqueous cleaner. The thickened emulsions are easily made
and
are effective in soil removal in laundry, ware washing, clean-in-place and
dairy
applications. The compositions provide improved or enhanced soil removal
properties because of high alkaline and surfactant contact.
Back2round Of The Invention
Cleaning compositions have been formulated in solid block, particulate and
liquid form. Solid forms provide high concentrations of actives, but must be
dissolved in water to form a cleaning liquid. Substantial attention in recent
years has
been directed to liquid detergent concentrates and in particular, liquid
detergents in
emulsion form. Such detergent concentrates typically are not as highly active
as
solids and are often greater than 50% water. Detergent emulsion concentrates
have
been employed as all-purpose cleaners, warewashing detergents and in
formulations
for cleaning hard surfaces by diluting the concentrate with water.
The typical emulsion liquid is less than 60% actives, less than 10%
surfactant less than 30-40% alkalinity. Many of the prior art emulsions are
not
sufficiently phase stable meaning that they do not remain globally homogenous
for
storage and use in a variety of applications, have reduced actives
concentration
(comprise greater than 50% water) or display reduced properties compared to
other
useful forms of detergent or are difficult to manufacture, dispense or store.
Many
prior art emulsions contain relatively low caustic content and relatively low
sequestrant and surfactant contents.
1
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
Substantial attention has been directed to concentrate materials having
substantially increased active content that can be manufactured as stable
liquids. A
need has existed to push the active concentrate of detergent components in the
emulsion to 60 to 65% in order to provide the efficacy and performance of
solids.
These liquids must have a stable viscosity and a handleable viscosity such
that the
liquid can be reliably dispensed from a source of the material to a use locus
such as a
laundry machine. We have found that, if the materials of the prior art are
simply
increased in concentration without the introduction of new technology, the
resulting
materials do not form simple solutions, do not form phase stable emulsions.
While
the prior art discloses a variety of liquid emulsion detergent compositions
that can be
used in a variety of forms, the prior art does not provide a highly alkaline,
highly
viscous, stable composition with a high active cleaning composition that is
easy to
manufacture, has acceptable cleaning properties in laundry, warewashing and
other
uses, and is dispensable due to its shear thinning characteristics in
specialized
detergent dispensers and are compatible with typical industrial or
institutional
cleaning equipment. We have filled a substantial need in improving composition
stability with desirable cleaning properties by improving formulations and
methods
of manufacture. A substantially improved thickened emulsion detergent
composition, methods of its use and methods of preparation have been
discovered
and are disclosed below
Summary Of The Invention
We have found an improved highly viscous, highly alkaline detergent
composition having high actives content. The composition comprises a
thickening
system compatible with the high alkaline formula resulting in a high viscosity
composition that exhibits shear thinning. The composition of the invention is
particularly novel in that its rheology is unique. The composition may be
defined as
a solid when no shear is applied but once shear is applied, the composition
begins to
flow. The composition comprises a source of alkalinity, nonionic
surfactant(s), a
water conditioning or sequestering agent, an optional alkyl polyglucoside
surfactant,
and a thickening system comprising a fatty acid and a polycarboxylic acid. The
resulting stable compositions are characterized by a low water content, high
actives
2
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
concentration (greater than 60 wt % based on the concentrate composition),
high
alkalinity, and a viscosity greater than about 250,000 centipoise at 20 C
using a
Bohlin CVO rheometer measured in rotating shear mode at the shear rate of 0.2
per
second. This improved detergent can be used for a variety of applications but
preferably is used in laundry applications. We have achieved cleaner
formulations
that comprise greater than 30 wt % of the combined alkaline source and the
surfactant load.
In laundry applications, soiled articles are contacted with an aqueous liquid
cleaning liquor comprising a major proportion of water and about 0.5 to 3.0
ppm of
the emulsion detergent. The clothes are contacted with the washing liquor at
an
elevated temperature of from about 25 C to about 80 C for a period of time to
remove soil. The soil and used liquor are then rinsed from the clothing in a
rinse
cycle. The improved detergents of the invention are made by a process that
comprises the steps of combining the nonionic surfactant or surfactant blend
with
anionic surfactants, chelating components, and various other textile detergent
components. To this blend, the sources of alkalinity are added followed by the
polyacrylic acid. Using standard mix tanks, all the components are briefly
mixed
until the product is homogenous, at which point it is filled and allowed to
attain its
end viscosity as described above. In applications other than laundry, slight
adjustments can be made to the formulation such as selecting a suitable
surfactant
system, to make it suitable for warewashing, or hard surface cleaning.
For the purpose of this patent application, the term "thickened emulsion"
connotes a substance that behaves as a solid until a sufficiently large load
or stress is
applied, at which point it flows like a fluid. Rheologically, a thickened
emulsion is a
viscoplastic material that behaves as a rigid body at low stresses but flows
as a
viscous fluid at high stress. Thickened emulsions of the invention comprise
two
immiscible liquids. For purposes of the invention, the continuous phase is
comprised substantially of caustic salts and other dissolved components in an
aqueous medium. However, since no additional water is added to compositions of
the invention, thickened emulsions of the invention result in a composition
having a
substantially low aqueous phase or low water content. For the purposes of this
3
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
invention, "emulsion" and "thickened emulsion" are used interchangeably.
However, it is noted that when used generally in the art the term "emulsion"
refers
to compositions having substantially lower viscosities than those of the
present
invention.
Without being bound by theory, as previously mentioned, it is believed that
thickened emulsions of the invention are comprised of two limitedly miscible
liquid
phases. Compositions of the invention may comprise a third solid particulate
comprised substantially of the carboxymethylcellulose or optical
brightener(s), if
any is used, to formulate the thickened emulsions of the invention. If a third
phase
exists, this discrete phase is believed to include particles in the range of
between
about 1 micrometer up to about 100 microns; however, compositions of the
invention may include substantially larger particles including above about 100
microns
The insoluble or non-water soluble portion, typically a liquid nonionic
surfactant, forms dispersed droplets having a particle size less than about
10, less
than about 5 microns, preferably between about 0.5 and 8 microns. Phase stable
connotes that under typical manufacturing, storage and use conditions, the
dispersed
phase does not substantially lose its finely divided form and separate from
the
aqueous phase to a degree that the material becomes not useful in a laundry or
other
cleaning purpose. Some small amount of separation can be tolerated as long as
the
thickened emulsion retains greater than 98% of the discontinuous phase
(predominantly organic materials) in small emulsified form and provides
cleaning
activity.
The aqueous materials of the invention typically involve the emulsification
of a relatively insoluble, typically organic phase and an aqueous phase. The
organic
phase can contain one or more components such as surfactants, water
conditioning
agents, brighteners, etc. while the aqueous phase can contain, in an aqueous
medium, aqueous soluble components such as sodium hydroxide, water
conditioning
agents, brighteners, dyes and other components. The materials are typically
made by
dispersing the relatively "oily" organic insoluble phase in the aqueous phase
4
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
stabilized by an emulsion stabilizer composition with the application of
shear. In this
invention the emulsion stabilizer typically comprises the polycarboxylic acid
in
combination with a fatty acid at an amount that can promote a stable thickened
emulsion. We have found that the alkylpolyglucoside (APG) surfactants may be
added to enhance the stabilization of the emulsion. In another embodiment we
have
found that the emulsion stabilizers are polyacrylic acid in combination with
an
anionic surfactant that are sufficiently soluble in sodium hydroxide and
promote
small particle size formation in the typical organic phase used in the
thickened
emulsions of the invention. We have found that simple mixtures of aqueous
sodium
hydroxide and nonionic surfactant such as a alkyl ethoxylate without an
emulsion
stabilizer will rapidly separate into two separate phases. Such surfactants
have low
solubility in sodium hydroxide while sodium hydroxide is insoluble in this
organic
compound. The useful procedure for forming the dispersions of the invention
involves combining fatty alcohol ethoxylate(s) with alkyl polyglucoside,
carboxymethyl cellulose and tall oil. The APG can be added at this time and
the
contents of the vessel can be agitated strongly. Any optical brighteners are
added to
the described combination followed by adding aqueous caustic, typically 50 wt
%
aqueous caustic to a large metal vessel containing agitation apparatus.
Monoethanolamine is added to the combination followed by polycarboxylic acid
as
the final ingredient.
Although the main emphasis is on laundry detergents, this emulsion concept
could be applied elsewhere as well. This would include warewashing, clean in
place
cleaners and sanitizers, food and dairy formulations. In general, this
emulsion
concept could be used in any formulation where relatively insoluble nonionic
surfactants are mixed with caustic solutions to form a thickened emulsion with
properties balanced for the selected end use. The low foaming surfactants can
comprise nonionics such as linear alcohol ethoxylates (Lutensol available
from
BASF located in Ludwigshafen, Germany), particularly the fatty alcohol C13-C15
ethoxylates having 2 to 10 polyethylene oxide groups. Thickeners useful in the
invention include a combination of linear polycarboxylic acid such as Acusol
(Rohm & Haas) and a fatty acid such as tall oil.
5
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
Brief Description of the Figures
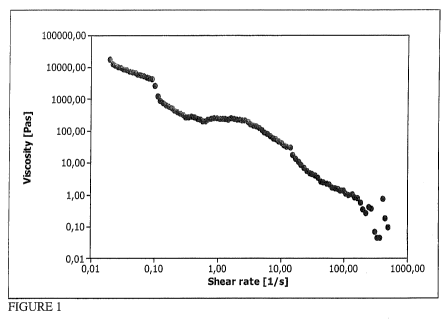
Figures 1-2 each have an x and y axis. The x axis in Fig. 1 is the shear rate,
in Fig. 2 the deviation of concentration from a standard mixture. The y axis
is
viscosity in both cases.
Figure 1 is a plot illustrating the decreasing viscosity of a composition of
the
invention with increasing shear rate.
Figure 2 is a plot illustrating the effect of concentration variation of some
of
the ingredients on viscosity.
Detailed Discussion Of The Invention
The term "surfactant" or "surface active agent" refers to an organic chemical
that when added to a liquid changes the properties of that liquid at a
surface.
The terms EO, PO, or EO/PO as used herein refer to ethylene oxide and
propylene oxide, respectively. EO/PO refers to ethylene oxide and propylene
oxide
groups.
The term "substantially free" may refer to any component that the
composition of the invention lacks or mostly lacks. When referring to
"substantially
free" it is intended that the component is not intentionally added to
compositions of
the invention. Use of the term "substantially free" of a component allows for
trace
amounts of that component to be included in compositions of the invention
because
they are present in another component. However, it is recognized that only
trace or
de minimus amounts of a component will be allowed when the composition is said
to be "substantially free" of that component.
All numeric values are herein assumed to be modified by the term "about,"
whether or not explicitly indicated. The term "about" generally refers to a
range of
numbers that one of skill in the art would consider equivalent to the recited
value
(i.e., having the same function or result). In many instances, the terms
"about" may
include numbers that are rounded to the nearest significant figure.
Weight percent, percent by weight, % by weight, and the like are synonyms
that refer to the concentration of a substance as the weight of that substance
divided
by the weight of the composition and multiplied by 100.
6
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
Unless otherwise stated, all weight percentages provided herein reflect the
weight percentage of the raw material as provided from the manufacturer. The
active weight percent of each component is easily determined from the provided
information by use of product data sheets as provided from the manufacturer.
Traditionally, emulsions have concerned systems of two isotropic,
substantially Newtonian liquids, one being dispersed in the other in the form
of
small droplets. The system is stabilized by absorbed amphiphiles which modify
interfacial properties. However, we have found that a large number of
emulsions act
in more than two phases. A discussion of emulsions and emulsion stability will
begin with the traditional two-phase system. An emulsion forms when two
immiscible liquids, usually water and oil, for example, are agitated so that
one liquid
forms droplets dispersed within the other liquid. Emulsions are stabilized by
a
compound adsorbed at the interface. This compound is termed an "emulsifier."
These are molecules that possess both polar and nonpolar regions and which
serve to
bridge the gap between the two immiscible liquids. For example, in an oil-and-
water
emulsion, the polar portion of an emulsifier is soluble in the water phase,
while the
nonpolar region is soluble in the oil phase. In general, formation of an
emulsion or
emulsification involves breaking large droplets into smaller ones due to shear
forces.
In order to discuss the stability of emulsions, it is necessary to first
discuss
how an emulsion fails. The initial step in emulsion failure is known as
flocculation,
in which individual droplets become attached to each other but are still
separated by
a thin film of the continuous phase. The next step is coalescence, in which
the thin
liquid film between the individual droplets destabilizes, allowing large
droplets to
form. As coalescence continues, the emulsion separates into an oil layer and
an
aqueous layer. In general, emulsions are stabilized by slowing the
destabilization or
flocculation process. This can be done either by reducing the droplet
mobility, by
increasing viscosity or by the insertion of an energy barrier between
droplets. In the
invention, the size of droplets or particles of the dispersed phase are less
than 10
microns, preferably less than 5 microns in diameter.
Rheological Characteristics
Compositions of the invention are particularly unique in the rheological
characteristics they exhibit. The inventors have characterized their invention
as a
7
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
"thickened emulsion" despite the fact that compositions of the invention
behave
differently than any presently known emulsions. The compositions are
characterized
as emulsions because they are comprised of at least two immiscible phases as
is
commonly known in the emulsion art. However, the thickened emulsions of the
invention exhibit characteristics of a solid when they are not under shear.
The
thickened emulsions of the invention exhibit a viscosity of greater than
1,000,000
centipoise at zero shear. As shear increases, the rheology of the invention
composition changes substantially until, when the compositions of the
invention are
under high shear, they exhibit properties of a liquid.
Compositions of the invention exhibit unusually high viscosity. In an
embodiment thickened emulsions of the invention exhibit a viscosity of greater
than
100,000 centipoise at 20 degrees centigrade when using a Bohlin CVO rheometer
measured in rotating shear mode at the shear rate of 0.2 per second. In other
embodiments, thickened emulsions of the invention exhibit a viscosity of
greater
than 300,000, greater than 500,000, greater than 700,000, greater than
900,000,
greater than 1,100,000, greater than 1,300,000, up to greater than 1.5 million
centipoise at 20 degrees centigrade when measured using a Bohlin CVO rheometer
measured in rotating shear mode at the shear rate of 0.2 per second. The
viscosity of
the invention compositions may be engineered to suit a specific use or
dispensing
system. Generally, as the amount of thickening agents such as fatty acid and
polycarboxylic acid are increased, the viscosity of the composition increases.
It has been discovered that fatty acids, polyacrylates positively influence
the
viscosity of the present invention. Compositions otherwise prepared according
to
the invention except they lack fatty acids are viscous fluids with viscosities
of about
10 Pas when taken at 0.2s-1. Increasing the content of fatty acids from 0% to
1%
results in a continuous increase of shear viscosity up to several hundreds of
Pas
along with the appearance changing from a flowing liquid to a solid gel or
paste.
Similar observations are made when modifying the concentration of
polycarboxylic
acid. As previously mentioned, without polycarboxylic acid the composition
appears as a viscous liquid with shear viscosity below 10 Pas (0.2s-1). With
the
concentration of polycarboxylic acid between 5 and 8 weight percent, the
8
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
composition turns into a paste resulting in viscosities in the range of 100-
1000 Pas
(0.2s-1).
Alkalinity Source
A source of alkalinity is needed to control the pH of the use detergent
solution. The alkalinity source is selected from the group consisting of
alkali metal
hydroxide, such as sodium hydroxide, potassium hydroxide or mixtures thereof;
an
alkali metal silicate such as sodium metasilicate and carbonate salts may also
be
used. The preferred source, which is the most cost-effective, is commercially
available sodium hydroxide which can be obtained in aqueous solutions in a
concentration of about 50 wt-% and in a variety of solid forms in varying
particle
sizes. The sodium hydroxide can be employed in the invention in either liquid
or
solid form or a mixture of both. Other sources of alkalinity are useful but
not limited
to the following: alkali metal carbonates, alkali metal bicarbonates, alkali
metal
sesquicarbonates, alkali metal borates and alkali metal silicate. In an
embodiment,
between about 40 to about 80 weight percent, between about 45 to about 75
weight
percent, and between about 50 to about 70 weight percent sodium hydroxide (50%
actives) is included in the composition of the invention.
Nonionic Surfactant
Conventional, nonionic detersive surfactants that can be used with the
invention include the alcohol ethoxylates. A combination of nonionic
surfactants is
preferred. These include linear and branched alcohol ethoxylates. Suitable
alcohol
ethoxylates include those having between about 5 and 10 EO, between about 6
and 8
EO and a linear alcohol ethoxylate having between about 1 and 6 EO, between
about
2 and 5 EO. Examples of these include the fatty alcohol C13-C15 ethoxylate
(7E0)
commercially available as Lutensol A07 and the fatty alcohol C13-C15
ethoxylate
(3E0) commercially available as Lutensol A03, both available from BASF.
Dehydol surfactants available from Cognis and Malipal surfactants available
from
Sasol are also useful in the present invention. Lutensol surfactants are
ethoxylates
of oxo-alcohols which are synthetic alcohols. Yet other examples of nonionic
surfactants suitable for use in the present invention include but are not
limited to
9
CA 02746854 2015-02-20
ethylene oxide/propylene oxide copolymers, ethylene oxide/propylene
oxide/ethylene oxide
copolymers, propylene oxide/ethylene oxide/propylene oxide copolymers (such as
those
available under the PluronicsTM mark and Pluronics R from BASF, those
surfactants sold
under the Plurafacs mark from BASF and those sold under the Polytergents mark
also from
BASF).
In an embodiment compositions of the invention include between about 5 and 35
weight percent nonionic surfactant or nonionic surfactant mixture, between
about 10 and 30
weight percent, and between about 15 and 25 weight percent. In another
embodiment, the
composition comprises between about 2 to about 25 weight percent, between
about 5 and
about 20 weight percent, and between about 8 and 17 weight percent of a first
nonionic
surfactant; and between about 0.5 and about 15 weight percent, between about 2
and 13
weight percent, and between about 5 and 10 weight percent of a second nonionic
surfactant
for a total of between 2.5 and 40 weight percent non-ionic surfactant in
total.
Stabilizers
Alkyl Polyglucoside Surfactant
We have found that the thickened emulsions of the invention are stabilized
using a
soap and polycarboxylic acid and alkyl polyglucosides optionally improve
consistency,
stability, and homogeneity of the thickened emulsions of the invention. Such
surfactants
have a strongly hydrophobic alkyl group with a strongly hydrophilic glycoside
group that
can have its hydrophilicity modified by the presence of ethylene oxide groups.
We have
found these materials are effective emulsion stabilizers when the material is
soluble in the
aqueous phase and can promote small particle size emulsions. The alkyl
polyglucoside,
GlucoponTM 215 contains a hydrophobic group with an alkyl straight chain of C8
to C10.
This material has good solubility in sodium hydroxide solutions. There are
other
commercially available alkyl polyglucosides with different alkyl groups and
DP's. The
general class of alkyl polyglucosides produces low interfacial tension between
mineral oil
and water. Low interfacial tension is probably responsible for the success of
these
surfactants in stabilizing the thickened emulsion. The system that is being
used is different
than the typical emulsion. The oil phase is the surfactant (fatty alcohol
ethoxylate) while the
aqueous phase is the sodium hydroxide solution along with other materials.
Without being
bound by theory, it is believed that the
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
surfactant droplets are stabilized by the presence of a surfactant at the
interface and
the unusually high viscosity of the system. The surfactant (fatty alcohol
ethoxylate)
has essentially no solubility in the sodium hydroxide solution and the sodium
hydroxide has essentially no solubility in the surfactant phase.
The alkyl polyglucosides is a surfactant useful as an emulsifier and it is
known from the literature that it reduces the interfacial tension between the
sodium
hydroxide solution phase and surfactant phase. With this general concept it
can be
envisioned that other surfactants can be used and would stabilize the
thickened
emulsion in these systems if they reduced the interfacial tension of sodium
hydroxide solution with a surfactant. In an embodiment, compositions of the
invention optionally comprise between about 0.01 to about 10 weight percent
alkyl
polyglucoside, between about 0.5 to between about 8 weight percent, and
between
about 1 and about 5 weight percent (50% active).
It has been found that alkylpolyglucosides ("APGs") have a synergistic
thickening, stabilizing, and homogenizing effect when used in combination with
certain other thickening agents. In one embodiment APG is used in combination
with a fatty acid and/or a polycarboxylic acid. In another embodiment APG is
used
in combination with a polyacrylate copolymer. In yet another embodiment APG is
used in combination with an alcohol ethoxylate and/or an acrylic acid polymer
and/or a polycarboxylic acid. Each of these thickening agents is discussed in
more
detail below.
Thickening agents
Fatty Acid
It has surprisingly been found that the combination of fatty acid with
polycarboxylic acid and optionally alkylpolyglucoside in the present
formulation
provides a composition having a sufficiently high viscosity, improved
homogeneity
and stability even at the high alkalinity required. At the elevated pH and
high
caustic content provided in the formulation of the invention, it was found
that other
thickening agents widely known in the industry such as CMC, guar gums, xanthan
gums, polyethylene glycol to name a few break down and are therefore
ineffective at
creating a high viscosity formulation. It is unusual for a composition having
such a
11
CA 02746854 2014-06-27
,
high caustic content and alkalinity to achieve a high viscosity as exhibited
by compositions
of the present invention.
A fatty acid of choice in compositions of the invention comprises tall oil.
Tall oil is
also referred to as liquid resin and is a resinous yellow-black oily liquid
composed of a
mixture of rosins, fatty acids, sterols, high-molecular alcohols, and other
alkyl chain
materials. The crude tall oil is distilled into tall oil rosin (having the
rosin content of 10 -
35%), further refinery gives to tall oil fatty acid (the rosin content of 1 -
10%). Tall oil fatty
acid is particularly preferred in compositions of the invention due to its low
cost and
flowability. Unlike many other fatty acids, tall oil remains a liquid at
ambient temperature
making it easier to handle than other fatty acids. Tall oil is an unsaturated
fatty acid
containing primarily a combination of oleic and linolic acids and in lesser
amounts palmitic
and stearic acids among others. In embodiments of the invention tall oil fatty
acid
comprises from about 0.01 to about 5 weight percent, from about 0.1 to about 4
weight
percent, and from about 0.2 to about 3 weight percent.
Polycarboxylic acids
Polycarboxylic acids are also useful as thickening agents in compositions of
the invention.
ACUS OLS 445 is a partially neutralized, liquid detergent polymer. In the
presently most
preferred embodiment of the invention, the polyacrylate comprises ACUSOL 445,
a
polyacrylic acid with a molecular weight of 4500 manufactured by Rohm and
Haas,
Philadelphia, Pennsylvania. Other polyacrylic acids of molecular weight 4500
(CRITERIONTm 2005) and 8000 (CRITERION 2108) can be purchased from Kemira
Chemicals, Kennesaw, Georgia. It is believed that other suitable examples
include
SoakalanTM CP5 available from BASF, CoatexTM DE185, or IsolTM Dispersant HN44.
In embodiments of the invention polycarboxylic acid comprises from about 1 to
about 30 weight percent, from about 5 to about 20 weight percent, and from
about 10 to
about 15 weight percent.
Alcohol Ethoxylates and Acrylic Acid Polymers
Alcohol ethoxylates are also useful as thickening agents in compositions of
the
invention. Isotridecanol ethoxylate is a nonionic surfactant available as
Lutensole TO 8
from BASF that has been found effective at providing thickening,
12
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
stabilizing, and homogeneity in compositions of the invention. Alcohol
ethoxylates
such as isotridecanol ethoxylate have improved performance as a thickening
agent
when combined with either a polycarboxylic acid such as Accusol 445
discussed
above, and/or an acrylic acid polymer such as Carbopol ETD 2691 available
from
Lubrizol Corporation. Carbopol is a lightly crosslinked polyacrylic acid
polymer.
In an embodiment of the invention alcohol ethoxylates are present in an
amount from about 0.5 to about 10 weight percent, from about 0.75 to about 9.5
weight percent, and from about 0.9 to about 9.0 weight percent.
Polyacrylate copolymer
Polyacrylate copolymers are also useful thickening, stabilizing, and
homogenizing agents when incorporated into compositions of the invention. An
example of such a copolymer is Cosmedia available from Cognis. Cosmedia is
an emulsifying copolymer consisting of methacrylic acid and acryclic acid
ethyl and
butyl ester.
In an embodiment of the invention polyacrylate copolymers are present in an
amount from about 0.5 to about 10 weight percent, from about 0.75 to about 9.5
weight percent, and from about 0.9 to about 9.0 weight percent.
Water Conditioners
Compositions of the invention preferably include water conditioning agents.
The thickened emulsion compositions of the invention can comprise a polyvalent
metal complexing, sequestering or chelating agent that aids in metal compound
soil
removal and in reducing harmful effects of hardness components in service
water.
Typically, a polyvalent metal cation or compound such as a calcium, a
magnesium,
an iron, a manganese, a molybdenum, etc. cation or compound, or mixtures
thereof,
can be present in service water and in complex soils. Such compounds or
cations can
comprise a stubborn soil or can interfere with the action of either washing
compositions or rinsing compositions during a cleaning regimen. A chelating
agent
can effectively complex and remove such compounds or cations from soiled
surfaces
and can reduce or eliminate the inappropriate interaction with active
ingredients
including the nonionic surfactants and anionic surfactants of the invention.
Both
13
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
organic and inorganic chelating agents are common and can be used. Inorganic
chelating agents include such compounds as sodium tripolyphosphate and other
higher linear and cyclic polyphosphates species. Organic chelating agents
include
both polymeric and small molecule chelating agents. Organic small molecule
chelating agents are typically organocarboxylate compounds or organophosphate
chelating agents. Polymeric chelating agents commonly comprise polyanionic
compositions such as polyacrylic acid compounds. Small molecule organic
chelating
agents include sodium gluconate, sodium glucoheptonate, N-
hydroxyethylenediaminetriacetic acid (HEDTA), ethylenediaminetetraacetic acid
(EDTA), nitrilotriaacetic acid (NTA), diethylenetriaminepentaacetic acid
(DTPA),
ethylenediaminetetraproprionic acid, triethylenetetraaminehexaacetic acid
(TTHA),
and the respective alkali metal, ammonium and substituted ammonium salts
thereof,
ethylenediaminetetraacetic acid tetrasodium salt (EDTA), nitrilotriacetic acid
trisodium salt (NTA), ethanoldiglycine disodium salt (EDG), diethanolglycine
sodium-salt (DEG), and 1,3-propylenediaminetetraacetic acid (PDTA),
dicarboxymethyl glutamic acid tetrasodium salt (GLDA), methylglycine-N-N-
diacetic acid trisodium salt (MGDA), and iminodisuccinate sodium salt (IDS).
All
of these are known and commercially available. Small molecule organic
chelating
agents also include biodegradable sequestrants having combinations of
chelating and
hydrotroping functionalities from EDG, MGDA and GLDA-type molecules.
Preferred sequestrants include ethanoldiglycine disodium salt (EDG),
dicarboxymethyl glutamic acid tetrasodium salt (GLDA), and methylglycine-N-N-
diacetic acid trisodium salt (MGDA), due to their biodegradability and their
ability
to bind easily with hydrotropes to form ultra-compact concentrates. Yet other
sequestrants that are suitable to include in compositions of the invention
include
anionic polyelectrolytes such as polyacrylates and acrylic acid copolymers,
itaconic
acid copolymers such as an acrylic/itaconic acid copolymer, maleates,
sulfonates
and their copolymers, alkali metal gluconates. Also suitable chelating agents
are
organic phosphonates such as 1-hydroxyethylidene-1,1-diphosphonic acid, amino
tri(methylene phosphonic acid), hexamethylene diamine tetra(methylene
phosphonic
acid), diethylene triamine penta(methylene phosphonic acid), and 2-
phosphonobutane-1,2,4-tricarboxylic acid and other commercially available
organic
14
CA 02746854 2011-06-13
WO 2010/076595
.. PCT/1B2008/055571
phosphonates water conditioning agents. Most conventional agents appear to
work
since they are compatible in either the continuous phase or the droplet phase.
In an embodiment, a chelating agent is present in the composition
concentrate in an amount of between about 0.05 and about 15 weight percent,
more
preferably about 1 to about 10 wt%, most preferably 3 to about 8 wt%.
Dequest 2010 may be included in compositions of the invention as the
sequestering or chelating agent. Dequest 2010 is 1-Hydroxyethylidene -1,1,-
diphosphonic acid, acts as a sequestrant in the present invention, and is a
commercially available from ClearTech Industries, Inc. located in Saskatoon,
Saskatchewan, Canada. In an embodiment compositions of the invention comprise
between about 0.01 to about 10 weight percent sequestrant, between about 0.5
to
between about 8 weight percent, and between about 1 and about 5 weight percent
(60% active).
Baypure CX100 may be included in compositions of the invention as a
chelating agent. Baypure CX100 is a medium-strength complexing agent with low
remobilization of heavy metals. It is effective at complexing iron, copper and
calcium ions and is biodegradable. If Baypure CX100 is included in
compositions
of the invention it is included in amount from about 1 up to about 30 weight
percent,
from about 2 up to about 20 weight percent, and from about 3 up to about 10
weight
percent for a 34% actives solution of Baypure .
As one skilled in the art will recognize, either one or a combination of
sequestrants may be included in the invention.
Water
The composition of the invention does not generally include additional
water. While it is recognized that certain ingredients contain some amount of
water
because they are provided as a solution in water, additional water is not
generally
added to compositions of the invention. This is important in particular to
maintain
the high viscosity required of compositions of the invention. Compositions of
the
invention can be formulated without any additional water or can be provided
with a
relatively small amount of water in order to reduce the expense of
transporting the
composition.
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
The water of dilution that can be used to dilute the composition to form a use
composition is preferably soft water. That is, it is preferable that water of
dilution
be substantially free of hardness. Water can be characterized as hard water
when it
includes at least 1 grain hardness.
Minor Ingredients
Detergents typically contain a number of conventional, important but minor
ingredients. These can include optical brighteners, soil antiredeposition
agents,
antifoam agents, low foaming surfactants, defoaming surfactants, pigments and
dyes, which are used in these formulas. The compositions can also include
chlorine
and oxygen bleaches, which are not currently used in these formulas. Such
materials
can be formulated with the other ingredients or added during cleaning
operations.
Color Stabilizing Agent
Compositions of the invention optionally include a component to inhibit
discoloration or browning of the formulation otherwise referred to as a color
stabilizing agent. Such a color stabilizer is optional; however, due to the
off-putting
nature of discolored laundry detergent it is a desirable option to include. In
compositions of the invention this color stabilizer is monoethanolamine. In an
embodiment of the invention, a color stabilizing agent comprises from about
0.01 to
about 5 weight percent, from about 0.05 to about 3 weight percent, and from
about
0.10 to about 2 weight percent.
Optical Brighteners
Optical brighteners may also be optionally added to compositions of the
invention. Brighteners are commonly added to laundry detergents to replace
whitening agents removed during washing and to make the clothes appear
cleaner.
Optical brighteners are commonly dyes that absorb light in the ultraviolet and
violet
region (usually 340-370nm) of the electromagnetic spectrum, and re-emit light
in the
blue region (typically 420-470nm). These additives are often used to enhance
the
appearance of color of fabric, causing a perceived "whitening" effect, making
materials look less yellow by increasing the overall amount of blue light
reflected.
16
CA 02746854 2014-06-27
Optical brighteners may or may not be a desirable addition to compositions of
the
invention. Whether or not it is desirable to include optical brighteners is
dependent upon the
user. For instance, a side effect of optical whitening is to make the treated
fabrics more
visible with night vision devices than non-treated ones. For military or other
applications
such an effect would likely be undesirable.
Examples of class types of optical brighteners include triazine-stilbenes (di-
, tetra-
or hexa- sulfonated), coumarins, imidazolines, diazoles, triazoles,
benzoxazolines and
biphenyl-stilbenes to name a few. A single optical brightener or combinations
of optical
brighteners may be useful in compositions of the invention. In an embodiment
of
compositions of the invention optical brighteners comprise from about 0.1 to
about 5
weight percent, from about 0.15 to about 3 weight percent, and from about 0.2
to about 2
weight percent. Examples of commercially available optical brighteners
suitable for use in
compositions of the invention include but are not limited to DMS-XTm and CBS-
XTM, a
distyryl biphenyl derivative, both available from Vesta- Intracon BV.
Soil Antiredeposition Agents
Compositions of the invention may further include antiredeposition agents.
Antiredeposition agents may be made from complex cellulosic materials such as
carboxymethylcellulose (CMC), or synthetic materials such as polyethylene
glycol and
polyacrylates. They aid in preventing loosened soil from redepositing onto
cleaned fabrics.
Polyphosphate builders also help in reducing redeposition.
Colorants
Colorants are optionally added to compositions of the invention. Colorants may
be
in the form of a pigment or dye and may be added to provide a certain color to
the
composition. Additionally, blue colorants may be added to provide a bluing
that imparts a
desirable blue/white color to white fabrics.
Fragrances
17
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
Fragrances are optionally added to compositions of the invention.
Fragrances generally provide three functions, regardless of the scent used.
They
cover the chemical odor of the detergent and the odor of soils in the washing
solution and they impart a pleasant scent to fabrics, thus reinforcing the
clean
performance. Additionally, a fragrance may contribute to the character of the
product. In an alternative embodiment, compositions of the invention may omit
a
fragrance in order to provide an unscented version thereby appealing to
consumers
who prefer low or no scent on laundry or to those whose skin is sensitive to
fragrance ingredients.
The following table includes exemplary ingredients and amounts to prepare
compositions of the invention:
Ingredient % by % by % by % by % by
weight weight weight weight weight
Sodium hydroxide 40-80 45-75 50-70 30-50 30-60
(50%)
Fatty alcohol C13- 4-20 9-15 10-14 4-20
C15 Ethoxylate
(7E0)
Fatty alcohol C13- 7-15 6-12 4-10
C15 Ethoxylate
(3E0)
C10 Alcohol 0.5-10
ethoxylate
Fatty alcohol 0.5-10
(C12-C14) EOPO
Isotridecanol 2-20
ethoxylate
Polycarboxylic 2-25 4-20 5-15 5-15
acid (20%)
Monoethanol- 0.01-5 0.05-3 0.1-2.0
18
CA 02746854 2011-06-13
WO 2010/076595 PCT/1B2008/055571
amine
Alkyl 0.1-7 0.5-5 1.0-3.0 0.1-5 0.1-5
Polyglucoside
Polyacrylate 0.5-10
copolymer
Acrylic Acid 0.01-2
Polymer
Hydroxyethylidene 0.1-7 0.5-5 1.0-3.0 0.5-5 0.5-5
diphosphonic acid
(60%)
Iminodisuccinate, 3-15 3-15
sodium salt (34%)
GLDA 0.5-10
Carboxymethyl 0.1-7 0.5-5 1.0-3.0 0.5-5 0.5-5
cellulose
Distyryl Biphenyl 0.1-5 0.25-3 0.5-2.0 0.1-5 0.1-5
derivative
Fatty Acid 0.1-5 0.15-3 0.25-2.0
Polycarboxylic 2-30 5-25 8-20 5-25
acid (20%)
Water 10-30 0-10
Optical brightener 0-5 0-2 0-1 0-1 0-1
Defoamer 0-3 0-3
Preparing Compositions of the Invention
Compositions of the invention are prepared by combining a nonionic
surfactant, an alkyl polyglucoside composition, CMC and tall oil. The APG may
be
added at this time along with strong agitation. Any optical brighteners are
added to
the combination followed by addition of an aqueous base, the aqueous base
comprising 50 wt. % active aqueous sodium hydroxide, to form an alkaline
surfactant blend. Monoethanolamine is added to the combination followed by
19
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
polycarboxylic acid as the final ingredient. The final mixture is exposed to
high
shear to form a stable thickened emulsion characterized by a viscosity of
greater
than about 250,000 centipoise at 20 degrees C using Bohlin CVO rheometer with
air
bearing and thermostat. Measurement is taken using two parallel plates each
having
20mm diameter with a distance of 2mm between the plates. The sample is
confined
in the gap between the two plates at a constant temperature of 20 degrees C.
The
measurement is performed in such way that the upper plate is rotating at a
certain
speed (shear rate) and the lower plate is fixed, thereby shearing the sample
between
the plates. The torque that is required to maintain a certain shear rate is
measured.
The shear stress on the sample is calculated from the torque and the viscosity
results
as the ratio of shear stress and shear rate.
Methods of Using Compositions of the Invention
The present invention also includes methods of use. Such methods include
contacting soiled laundry items with a wash liquor comprising a major
proportion of
water and about 0.5 to 3.0 ppm of a liquid cleaner concentrate composition in
the
form of an aqueous emulsion having a continuous phase and a dispersed phase,
the
thickened emulsion having a stable viscosity and dispersed phase particle
size, the
composition comprising a phase stable thickened emulsion comprising a
continuous
phase; an effective soil removing amount comprising about 15 to about 50 wt %
of a
source of alkalinity; an effective soil removing amount comprising about 10 to
about
wt % of a nonionic surfactant; an effective water conditioning or sequestering
amount about 0.1 to about 20 wt % of a water conditioning or sequestering
agent; an
effective soil removing and emulsion stabilizing amount comprising up to about
5
25 wt % of an alkyl polyglucoside surfactant; an effective thickening
amount of
polycarboxylic acid comprising about 1 to 20 wt %; and an effective thickening
amount of fatty acid comprising about 0.1 to 5 wt %; wherein the dispersed
phase
comprises at least a portion of the nonionic surfactant and the thickened
emulsion
concentrate has a viscosity of greater than 250,000 cps at 20 C using a
Bohlin CVO
30 rheometer measured in rotating shear mode at the shear rate of 0.2 per
second and
exhibits shear thinning thereby permitting dispensing during manufacture and
use to
form a washed laundry; and rinsing the washed laundry with an aqueous rinse.
CA 02746854 2011-06-13
WO 2010/076595 PCT/1B2008/055571
EXAMPLES
Example I
Compositions were prepared according to the invention except that they
lacked fatty acids. These comparative compositions were viscous fluids with
viscosities of about 10 Pas when taken at 0.2s-1. Increasing the content of
fatty acids
from 0% to 1% resulted in a continuous increase of shear viscosity with the
appearance changing from a flowing liquid to a solid gel or paste.
Compositions were prepared according to the invention except that they
lacked polycarboxylic acid. Without polycarboxylic acid the composition
appears
as a viscous liquid with shear viscosity below 10 Pas (0.2s-1). With the
concentration of polycarboxylic acid between 5 and 8 weight percent, the
composition turns into a paste resulting in viscosities in the range of 100-
1000 Pas
(0.2s-1).
Compositions were prepared according to the following tables.
1A 3A 5A 6A 8A
Fatty alcohol C13-15 15% 15% 15% 15% 15%
ethoxylate (7E0)
Fatty alcohol C13-15 4% 4% 4% 4% 4%
ethoxylate (3E0)
Alkyl Polyglycoside 2% 2% 2% 2% 2%
(50%)
Carboxymethyl cellulose 2% 2% 2% 2% 2%
Tall Oil fatty acids 0% 0.4% 0.65% 0.75% 1%
Optical Brightener DMS- 0.5% 0.5% 0.5% 0.5% 0.5%
X
Distyryl Biphenyl 0.25% 0.25% 0.25% 0.25% 0.25%
Derivative
Hydroxyethylidene 2% 2% 2% 2% 2%
Diphoyphonic Acid
21
CA 02746854 2011-06-13
WO 2010/076595 PCT/1B2008/055571
(60%)
Sodium Hydroxide 61% 60.6% 60.35 60.25 60%
Solution (50%) % %
Monoethanol amine 0.25% 0.25% 0.25% 0.25% 0.25%
Polycarboxylic acid 13% 13% 13% 13% 13%
(48%)
1B 3B 8B
Fatty alcohol C13-15 15% 15% 15%
ethoxylate (7E0)
Fatty alcohol C13-15 4% 4% 4%
ethoxylate (3E0)
Alkyl Polyglycoside 2% 2% 2%
(50%)
Carboxymethyl cellulose 2% 2% 2%
Tall Oil fatty acids 0.75% 0.75% 0.75%
Optical Brightener DMS- 0.5% 0.5% 0.5%
X
Distyryl Biphenyl 0.25% 0.25% 0.25%
Derivative
Hydroxyethylidene 2% 2% 2%
Diphoyphonic Acid
(60%)
Sodium Hydroxide 73.25 69.25 59.25
Solution (50%) % % %
Monoethanol amine 0.25% 0.25% 0.25%
Polycarboxylic acid 0% 4% 14%
(48%)
Viscosity was measured using a parallel plate system with each plate having
20 mm diameter with a 2 mm gap distance between the plates. Measurements were
taken at 20 degrees C. Each sample was confined in the gap between the two
plates
22
CA 02746854 2015-02-20
and the measurement was performed in such way that the upper plate was
rotating at
a certain speed (shear rate) and the lower plate was fixed, thereby shearing
the
sample between the plates. The torque that was required to maintain a certain
shear
rate was measured. The shear stress on the sample is calculated from the
torque and
the viscosity results as the ratio of shear stress and shear rate.
Results are provided in the tables below:
1A 3A 5A 6A 8A
Tall Oil 0 0.4 0.65 0.75 1
conc. (wt
%)
Viscosity 12 28 118 711 928
(0.2s"
')(Pas)
1B 3B 8B
Polycarboxylic 0 4 14
acid conc. (%)
Viscosity (0.2s" 4 80 215
1)(Pas)
The results show that with increasing tall oil concentration and increasing
polyearboxylic acid concentration, the viscosity increases.
Example H
This Comparative Example demonstrates that not all thickening polymers are
useful in the present invention. Compositions were prepared using Sokalan'AT
10
from BASF, CarbopolTMEZ3 and EZ 4 from Noveon and Acusorm 805S from Kohut &
Haas but the .resultant tnixtures were not stable and separated into two
viscous liquid
phases. Compositions were prepared according to the following table:
Ingredient IC 2C 3C 4C
Water 10,95% 10.95% 10.95% 10.95%
KOH, 50% 25.00% 25.00% 25.00% 25.00%
DMS-X, Stilbene Disulfonic Acid 0.30% 0.30% 0.30% 0.30%
23
CA 02746854 2011-06-13
WO 2010/076595
PCT/1B2008/055571
CBS-X, Distyryl Biphenyl
Derivative 0.10% 0.10% 0.10% 0.10%
Hydroxyethylene Diphosphonic
Acid (60%) 2.50% 2.50% 2.50% 2.50%
Sodium silicate (40%) 10.00% 10.00% 10.00% 10.00%
Iminodisuccinate Sodium Salt
(34%) 14.75% 14.75% 14.75% 14.75%
Methyl gylcine diacetic acid,
trisodium salt (40%) 7.50% 7.50% 7.50% 7.50%
Polyacrylic Acid Copolymer 7.50% 7.50% 7.50% 7.50%
Silicone Emulsion 0.20% 0.20% 0.20% 0.20%
Fatty alcohol ethoxylate blend 5.00% 5.00% 5.00% 5.00%
Alkyl benzene sulfonate 0.50% 0.50% 0.50% 0.50%
Fatty alcohol (C12-14)E0P0 9.50% 9.50% 9.50% 9.50%
Ethoxylated C10 (iso) longchain
alcohol polymer 5.00% 5.00% 5.00% 5.00%
Perfume 0.20% 0.20% 0.20% 0.20%
1% 1% 1% 1%
Carbopol Carbopol Sokalan Acusol
Thickener EZ3 EZ4 AT10 805S
Viscosities of each of the comparative compositions was taken as described
in Comparative Example I. The results are shown in the table below:
Formulation 1C 2C 3C 4C
Viscosity 205 Pas 152 Pas 0.09 Pas 88 Pas
The viscosity was measured at a shear rate of 0.1s-1. As all of these systems
separate
fast, the given values are only approximate.
Example III
Increasing shear was applied to a sample prepared according to the
formulation of sample 6A. Figure 1 shows shear thinning of a sample 6A of the
24
CA 02746854 2015-02-20
invention. As shear is increased, viscosity decreases by about five orders of
magnitude. As can be seen from the graph of Figure 1, the viscosity varies
over
more than 5 orders of magnitude in a shear rate range between 0.01 and 500s-1.
Example IV
This Example demonstrates that both tall oil and polycarboxylic acid have
positive influences upon viscosity of compositions of the invention.
Compositions
were prepared according to the following table:
Ingredient % by weight
Fatty alcohol C13-15 ethoxylate (7E0) 10-14%
Fatty alcohol C13-15 ethoxylate (3E0) 5-9%
Alkyl Polyglycoside (50%) 2%
Carboxymethyl cellulose 0.5-2%
Tall Oil fatty acids 0.25%4/75%
Optical Brightener DMS-X 0.5%
Distyryl Biphenyl Derivative 0.25%
Hydroxyethylidene Diphoyphonic Acid (60%) 2%
Sodium Hydroxide Solution (50%) 40-60%
Monoethanol amine 0.25%
Polycarboxylic acid (48%) 11.7-14.3%
This experiment was performed on the basis of a statistical method named
DOE (Design of Experiment) ¨ Mixture Design. Some ingredients were kept at a
constant concentration whereas the content of others was varied within the
limits
given in the above table. Evaluation of the measured viscosities with
statistical
methods resulted in the graph shown In Figure 2 which demonstrates the effect
of
the varied ingredient.s A-F on viscosity when their concentrations are
increased.
The viscosity of each composition from the above table was taken according
to the protocol provided in Example I. The graph of Figure 2 illustrates the
results.
As can be seen, both tall oil and polycarboxylic acid (Rheosolve or Acusol)
have a
strong positive effect on viscosity, NaOH and Lutensol A()3 (fatty alcohol
CA 02746854 2015-02-20
ethoxylate) have a negative effect and Lutensol A07 (fatty alcohol ethoxylate)
and
CMC do not largely influence the viscosity.
Example V
This Example demonstrates that the thickened emulsion of the invention
performed well in cleaning a variety of stains. A composition of the invention
was
prepared according to the following table:
Ingredient Manufacturer % by weight
,utensol A07 BASF 15.00
Lutensol A03 BASF 4.00
Glucopon 215 UP Cognis /.00
CMC Hercules 2.00
Tall Oil Mosselman 0.50
DMS-X Ciba 1.00
CBS-X Hebei 0.25
Dequest 2010 Thermphos 2.00
Na011 (50%) BASF 60.00
Monethanolamine BASF 0.25
AccusolTm 445 Rohm & Haas 13.00
The pH of a 1% solution of the above-identified 'formulation was in the range
Of 11.1 to 11.9.
Standard test stains which are commercially available from WFK
I() (Krefeld/Germany) were used for this Example. The test stains were
produced in a
standardized manner and were placed on test strips. These were included in a
washing machine together with 6.5kg of polyester textiles. 1:.,ach procedure
was
repeated 4 times in 4 different washing machines, the values in the tables
below are
the averages of the 4 results for each procedure. The above-prepared
composition
was included in a dosage of 1.5g/1 along with 1m1/1 of a bleaching agent
(Ozonit
super available from Ecolab, Inc.) was added in each test. The washing time
was 10
minutes, temperature 70 C, bath ratio 1:5; soft water was used. After
finalizing the
26
CA 02746854 2015-02-20
wash test, the stain removal from the test strips was measured with an optical
tnethocl, the light transmission at a fixed wave length is measured with the
stain
before and after washing. The results are the remission values shown in the
tables.
The tables below compare lab wash results of the thickened emulsion of the
invention with a commercially available detergent. The top two tables show the
results of wash performance with fatipigment stains, the bottom table shows
enzymatic stains. The results demonstrate that the thickened emulsion of the
invention pertbnyied as well or better than the commercially available
detergent in
most situations.
Pigment/ Pigment/ Pigment/ Pigment/ Soot/Min Soot/Min
Lanolin Lanolin on Sebum on Sebum on
Oil on Oil on
on Cotton Polyester/ Cotton Polyester/ Cotton PES/CO
Cotton Cotton
Commercially 62.2 45.7 60.4 62.6 34.3 37.3
available
detergent
Thickened 63.2 49.9 62.7 68 35 38.7
Emulsion
Used Motor Used Motor Oil Make up on Make up on
Oil on on Cotton Polyester/Cotton
Cotton Polyester/Cotton
Commercially 44.6 34.2 75 80.3
available
detergent
Thickened 44.4 34.5 753 81.9
liniuision
Blood Blood Blood/Milk/ Milk Blood/Milk/ Egg Soot
aged unaged Ink on cocoa Soot on on C
cotton unaged cotton
Commercially 87.4 85.4 32.9 43.9 53.7 48
available
detergent
Thickened 87 86.1 40 52 69.3 58
Emulsion
The invention has been described with reference to various specific and
preferred embodiments and techniques. However, the scope of the claims should
not
be limited by the preferred embodiments set forth in the examples, but should
be given
the broadest interpretation consistent with the description as a whole.