Note: Descriptions are shown in the official language in which they were submitted.
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
PROCESS FOR THE PRODUCTION OF CHEMICALS
FIELD OF THE INVENTION
The present invention relates to a process for producing chemicals. More
particularly, the
present invention relates to a process for producing chemicals using
bioelectrochemical
systems.
BACKGROUND
The global depletion of fossil fuel resources and the increasing awareness of
the possible
anthropogenic effect on climate change are leading to an increasing drive to
reduce
greenhouse gas emissions and to develop a more sustainable society. Besides
renewable
electricity, such a sustainable society also needs access to renewably
produced fuels and
chemicals. To be truly renewable these chemicals need to be produced from
renewable
raw materials such as biomass or from waste products such wastewater and/or
carbon
dioxide.
Recently, bioelectrochemical systems, such as microbial fuel cells and
microbial
electrolysis cells, have emerged as potentially interesting technology for the
production
of energy and products. Bioelectrochemical systems are based on the use of
electrochemically active microorganisms, which can either donate electrons to
an anode
or accept electrons from a cathode. If electrochemically active micro-
organisms are
electrochemically interacting with an anode, such an electrode is referred to
as a
biological anode, bioanode or microbial bioanode. In contrast, if
electrochemically active
micro-organisms are electrochemically interacting with a cathode, such an
electrode is
referred to as a biological cathode, biocathode or microbial biocathode.
Bioelectrochemical systems are generally regarded as a promising future
technology for
the production of energy from organic material present from aqueous waste
streams (e.g.,
wastewater). (Rozendal et al., Trends Biotechnol. 2008, 26, 450-459).
Industrial,
agricultural and domestic waste waters typically contain dissolved organics
that require
removal before discharge into the environment. Typically, these organic
pollutants are
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
2
removed by aerobic treatment, which can consume large amounts of electrical
energy for
aeration. Bioelectrochemical wastewater treatment can be accomplished by
electrically
coupling a microbial bioanode to a counter electrode (cathode) that performs a
reduction
reaction. As a result of this electrical connection between the anode and the
cathode, the
electrode reactions can occur and electrons can flow from the anode to the
cathode.
Moreover, the electrochemically active microorganisms at the anode transfer
electrons to
an electrode (anode) while they are oxidising (and thus removing) (in)organic
pollutants
in aqueous waste streams (e.g., wastewater). A bioelectrochemical system may
operate as
a fuel cell (in which case electrical energy is produced - e.g. Rabaey and
Verstraete,
Trends Biotechnol. 2005, 23, 291-298) or as an electrolysis cell (in which
case, electrical
energy is fed to the bioelectrochemical system - e.g., Patent W0200500598
1A2).
In 2003, Rozendal and Buisman patented biocatalysed electrolysis, which is a
bioelectrochemical system for the production of hydrogen gas from bio-
oxidisable
material (WO 2005005981, the entire contents of which are herein incorporated
by cross-
reference). The bio-oxisable material used for biocatalysed electrolysis can,
for example,
be dissolved organic material in wastewater. In their invention Rozendal and
Buisman
introduced bio-oxisable material into a reactor provided with an anode and a
cathode and
containing anodophilic bacteria in an aqueous medium, applied a potential
between the
anode and cathode of between 0.1 and 1.5 volt, while maintaining a pH of
between 3 and
9 in the aqueous medium and collected hydrogen gas from the cathode.
Although hydrogen is an interesting chemical to produce in a cathode, it would
be even
more interesting if chemicals with higher value could be produced, such as
fuels and
chemicals. Examples of such fuels and chemicals include alcohols such as
methanol.
ethanol, propanol, butanol, etc, carboxylic acids, such as formic acid, acetic
acid,
propionic acid, butyric acid, lactic acid, etc, biopolymers such as poly- P-
hydroxybutyrate
(PHB), etc., etc. However, to be able to catalyze these kind complex
production reactions
at a cathode, an advanced catalysis mechanism is required. It might be
possible to
develop chemical catalysts for this purpose, but these chemical catalysts are
likely to
become very complex and highly expensive as they likely necessitate the
application of
precious metals. Alternatively, cathodophilic microorganisms can be used for
catalyzing
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
3
cathodic reactions for the production of valuable chemicals. Cathodophilic
microorganisms are microorganisms that can interact with a cathode by
accepting
electrons or cathodic reaction products (such as hydrogen or reduced electron
mediators)
from the cathode and utilizing these for the production of valuable chemicals.
Such
electrode is referred to as a biological cathode, biocathode or microbial
biocathode.
Electrons and cathodic reaction products (such as hydrogen or reduced electron
mediators) are commonly referred to as reducing equivalents. Reducing
equivalents allow
reducing electron acceptors and can serve as electron donor for a microbial
metabolism.
Electron mediators are redox-active organic compounds and are known to person
skilled
in the art. They include compounds such as quinones, neutral red, methyl
viologen, etc.
Electron mediators shuttle in between the electrode and the microorganisms.
During this
shuttling the electron mediators are continuously reduced by the electrode and
subsequently oxidized again by the microorganism for the production of the
chemical
products.
Microbial biocathodes have already been demonstrated for oxygen reduction
(e.g.,
Rabaey et al., ISME J. 2008, 2, 1387-1396), nitrate reduction (e.g., Clauwaert
et al.,
Environ. Sci. Technol., 2007, 41, 7564-7569), dechlorination (e.g., Aulenta et
al.,
Environ. Sci. Technol., 2007, 41, 2554-2559), hydrogen production (e.g.,
Rozendal et al.,
Environ. Sci. Technol., 2008, 42, 629-634), and methane production (e.g.,
Clauwaert et
al., Water Sci. Technol., 2008, 57, 575-579), but have not been described for
the
production of more complex molecules such as those described above.
In general, mixed microbial cultures (i.e., multiple species) are unlikely to
produce
complex chemicals in high quantity, concentration or purity, because the
natural end
products in a mixed microbial culture are typically simple molecules such as
methane
under anaerobic conditions or carbon dioxide under aerobic conditions. In
practice,
complex molecules are therefore typically produced with a defined microbial
culture,
such as a pure microbial culture (i.e., single specie) or at least a well-
defined co-culture
(i.e., two or more carefully selected species). Therefore, unless methanogenic
activity can
be suppressed, a microbial biocathode capable of producing complex molecules
would
also require a defined microbial culture of cathodophilic microorganisms
(Rozendal et al.,
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
4
Trends Biotechnol. 2008, 26, 450-459), which are unlikely to be naturally
enriched from
complex inocula such as wastewater. A defined microbial culture of
cathodophilic
microorganisms should be a carefully selected or genetically engineered pure
culture, but
could also be a carefully selected co-culture of two or more carefully
selected or
genetically engineered pure cultures. These pure cultures or co-cultures
should consist of
microbial species that are capable of catalyzing the production reaction of
the desired
complex molecule.
A disadvantage of using a defined culture of cathodophilic microorganisms is
that these
cultures are susceptible to contamination with other microorganisms. So unless
the
activity of these other micro-organisms can be suppressed, these other
microorganisms
will break down the products produced by the defined culture of cathodophilic
microorganisms and consequently limit the product output of the
bioelectrochemical
system. Bioelectrochemical systems can prevent this contamination with other
micro-
organisms by applying an ion exchange membrane between the anode and the
cathode.
The application of an ion exchange membrane isolates the cathode from the rest
the
bioelectrochemical system and can make the defined culture of cathodophilic
microorganisms less susceptible to contamination. Even more so, because the
reducing
equivalents are essentially delivered to cathodophilic microorganisms sterile
in the form
of electrons delivered by the cathode and originally coming from the anode.
In the context of microbial fuel cells, Torres et al. (Torres et al., Environ.
Sci. Technol.,
2008, 42, 8773-8777) presented a method to decrease the pH difference between
the
anode and the cathode of a microbial fuel cell by having an anion exchange
membrane
between the cathode chamber and dosing carbon dioxide to an air cathode
(platinum
catalyst for oxygen reduction). This carbon dioxide reacts with the hydroxyl
ions and
forms carbonate species. While this decreases the pH of the cathode, the
carbonate
species also migrate across the anion exchange membrane from cathode to anode
and
increase pH in the latter. As a result, the pH difference between both is
decreased.
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
BRIEF DESCRIPTION OF THE INVENTION
In a first aspect, the present invention provides a process for producing one
or more
chemical compounds comprising the steps of providing a bioelectrochemical
system
5 having an anode and a cathode separated by a membrane, the anode and the
cathode
being electrically connected to each other, causing oxidation to occur at the
anode and
causing reduction to occur at the cathode to thereby produce reducing
equivalents at the
cathode, providing the reducing equivalents to a culture of microorganisms,
and
providing carbon dioxide to the culture of microorganisms, whereby the
microorganisms
produce the one or more chemical compounds, and recovering the one or chemical
compounds.
In another aspect, the present invention provides a process for producing one
or more
chemical compounds comprising the steps of providing a bioelectrochemical
system
having an anode and a cathode separated by a membrane, the anode and the
cathode
being electrically connected to each other, the system having a cathode
compartment and
the cathode compartment being provided with microorganisms that form the one
or more
chemical compounds in the cathode compartment, causing oxidation to occur at
the anode
and causing reduction to occur at the cathode, wherein carbon dioxide is
supplied to the
cathode compartment, and the microorganisms produce the one or more chemical
compounds, and recovering the one more chemicals from the cathode compartment.
In some embodiments, the system further includes a power supply in the
electrical circuit.
The power supply may comprise a DC power supply, such as a battery or a DC to
AC
converter.
The power supply can be used to apply a voltage on the system, which increases
chemical
production rates. The voltage applied with a power supply between the anode
and the
cathode may be between 0 and 10 V, preferably between 0 and 1.5 V, more
preferably
between 0 and 1.0 V. This may result in a volumetric current density in the
bioelectrochemical cell of between 0 and 10,000 A/m3 of bioelectrochemical
cell,
preferably between 10 and 5,000 A/m3 of bioelectrochemical cell, more
preferably
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
6
between 100 and 2500 A/m3 of bioelectrochemical cell and/or an area specific
current
density of between 0 and 1,000 A/m2 membrane surface area, preferably between
1 and
100 A/m2 membrane surface area, more preferably between 2 and 25 A/m2 membrane
surface area.
In embodiments of the present invention, the microorganisms present in the
cathode
compartment or receiving reducing equivalents from the cathode compartment
utilise
reducing equivalents produced at the cathode and carbon dioxide to make
organic
molecules. Therefore, the carbon dioxide acts as a carbon-containing feed
material to the
microorganisms that receive reducing equivalents from the cathode or are
present in the
cathode compartment. Indeed, the carbon dioxide can be the only carbon-
containing feed
component supplied to the microorganisms. In other embodiments, the carbon
dioxide is
used in conjunction with other organic materials by the microorganisms to
produce the
desired chemicals. Examples of suitable microorganisms in this regard include
chemolithoautotrophic bacteria. For example, in butanol formation,
chemolithoautotrophic bacteria at the cathode would proceed according to:
4 CO2 + 24 H+ + 24 e" 4 C4H9OH + 7 H2O (2)
It will be appreciated that utilising carbon dioxide as a carbon-containing
material for
conversion into the desired chemical products has the desirable effect of
reducing carbon
dioxide emissions (and hence reducing greenhouse gas emissions).
In one embodiment, the microorganisms provided to the cathode compartment or
receiving reducing equivalents from the cathode compartment comprise a defined
microbial culture containing one or more selected microbial species. In one
embodiment,
the defined microbial culture comprises a pure microbial culture containing a
single
species of microorganisms. In another embodiment, the defined microbial
culture
comprises a co-culture of two or more carefully selected microbial species.
The microbial
species are selected such that the one or more chemical compounds are produced
by the
microbial species. Suitably, the microbial species do not form methane in
notable
quantities when grown in the cathode.
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
7
The defined microbial culture containing one or more selected microbial
species may be
formed or selected by any technique known to be suitable to persons skilled in
the art.
In this embodiment, an essentially "pure" microbial culture is provided
(either in the
cathode compartment or to receive reducing equivalents from the cathode). The
essentially "pure" microbial culture is selected such that the microbial
culture produces
the one or more desired chemical compounds. For example, Clostridium
carboxidivorans
sp. nov. could be selected for the production of acetate, ethanol, butyrate
and butanol
from carbon dioxide and cathodically produced hydrogen (Liou et al., Int. J.
Syst. Evol.
Microbiol., 2005, 55, 2085-2091). In order to ensure that the essentially pure
microbial
culture remains essentially pure, any feed streams to the culture of
microorganisms
should be essentially free of other microorganisms. For example, the carbon
dioxide
stream fed to the culture of microorganisms should also free of contaminating
microorganisms.
The carbon dioxide stream being fed to be cathode compartment may be derived
from an
offgas stream or a flue gas stream from a burner or a boiler. It will be
appreciated that
such offgas streams or flue gas streams exit the burner or boiler at a very
high
temperature and, as a result, will be essentially sterile (in that they will
not contain any
contaminating microorganisms). These streams may simply be cooled and then
used as a
carbon dioxide containing feed stream to the cathode compartment. If the
offgas stream
or flue gas stream contains other material that may be toxic to the
microorganisms in the
cathode compartment, that other material should be removed therefrom prior to
feeding to
the cathode compartment. It will be appreciated that only part of the offgas
stream or flue
gas stream may be fed to the cathode compartment.
The carbon dioxide stream being fed to the cathode compartment may also be
biogas,
containing a mixture of methane and carbon dioxide (and potentially other
gases)
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
8
The carbon dioxide being fed to the cathode may also be derived from a coal
seam or
layer, in which carbonate rich fluid is pumped from the coal seam through the
cathode
compartment.
In another embodiment, the microorganisms provided to the cathode compartment
comprised a mixed, non-selected culture and the process further comprises the
steps of
producing the one or more chemicals in the cathode compartment and recovering
the one
or more chemicals from the cathode compartment whilst suppressing formation of
methane in the cathode compartment. Persons skilled in the art will understand
that
mixed, non-selected cultures typically contain methanogenic organisms and, if
the
cathode compartment is operated without special precautions, the final product
from the
cathode compartment is likely to be methane. Therefore, in this embodiment,
the cathode
compartment is operated such that methane formation is suppressed. Methane
formation
may be suppressed by one or more of the following:
= Adding one or more chemicals to the cathode compartment that suppress the
formation of methane or suppress the activity of the methanogenic organisms.
For
example, 2-bromoethane sulfonate (BES) is known to suppress the activity of
methanogenic organisms. Other chemicals that suppress the activity of
methanogenic organisms may also be used.
= Operate the cathode compartment such that a low residence time is used in
the
cathode compartment. In this regard, most methanogenic organisms are slow
growing and utilising a low residence time in the cathode compartment will
suppress the formation or growth of a significant number of methanogenic
organisms in the cathode compartment because they simply will not have
sufficient time to grow in any great number. In this embodiment, fresh cathode
compartment liquid may be frequently or continuously provided to the cathode
compartment.
= Operate the cathode compartment at low pH, such as below 5.5, preferably
below
pH 5.
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
9
= Periodically expose the cathode compartment to air or oxygen.
In one embodiment, the CO2 is provided to the cathode compartment via
diffusion or
transport from the anode of the bioelectrochemical system. This transport can
occur either
through the membrane separating anode and cathode, or via an additional
conduit.
In one embodiment, the present invention may be operated with a bioanode and a
biocathode. In embodiments where the anode is operated as a bioanode, one of
the
products likely to be formed in the bioanode compartment is carbon dioxide.
This carbon
dioxide may be used as a feed to the cathode compartment. This carbon dioxide
can for
example separated from the anode effluent and subsequently transported to the
cathode.
In embodiments where the anode is operated as a bioanode, a waste stream, such
as a
wastewater stream, may be used as a feed material to the anode. The anode
reaction is
then catalyzed by microorganisms, such as electrochemically active
microorganisms, and
generates electrons (e-) and protons and/or carbon dioxide and/or other
oxidation products
(e.g. sulfur):
(In)organic materials - x HCO3- + y H+ + z e + other products (3)
The anode may be located in an anode compartment, with the anode compartment
being
separated from the cathode compartment by a membrane. In the anode
compartment,
organic and/or inorganic components in the waste stream are oxidised to
liberate
electrons which, in turn, flow through the electrical connection to the
cathode.
In one embodiment, an anion exchange membrane separates the anode compartment
from
the cathode compartment. Anion exchange membranes are known to the person
skilled in
the art and include membranes such as AMI-7001 (Membranes International),
Neosepta
AMX (ASTOM Corporation), and fumasep FAA (fumatech).
In some embodiments, bicarbonate ions form in the cathode compartment and
subsequently move through the anion exchange membrane to the anode
compartment.
This may be advantageous in that pH control in the cathode compartment is also
obtained
by adding carbon dioxide to the cathode compartment. In this embodiment, the
carbon
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
dioxide acts as a feed material as a building block for producing the chemical
compounds
and also acts to control pH in the cathode compartment. It will be understood
that
hydroxyl ions can be generated by the reactions occurring at the cathode. The
hydroxyl
ions can react with the carbon dioxide to form bicarbonate ions and the
bicarbonate ions
5 can subsequently pass through the anion exchange membrane. In this fashion,
an
undesirable increase in pH in the cathode compartment (which has the potential
to kill the
culture of microorganisms) can be avoided and homeostatic conditions can be
maintained
in the cathode compartment. Carbon dioxide dosing does not significantly
increase salt
concentrations in the cathode compartment. This is advantageous as it will
mean that a
10 homeostatic situation can be achieved in the bioelectrochemical system.
In another embodiment, the membrane separating the anode and the cathode
comprises a
porous membrane. The porous membrane may allow liquid and ions to pass
therethrough
but prevent microorganisms from passing therethrough. In one embodiment, the
anode
may be operated as a bioanode, and a waste stream, such as a wastewater stream
may be
used as a feed material to the anode. As mentioned above, the pore size in the
porous
membrane may be small enough to prevent microorganisms from passing through
the
membrane. These membranes are known to the person skilled in the art and
include
microfiltration and ultrafiltration membranes. During normal operation liquid
passes
through the porous membrane from the anode into the cathode chamber. The
protons that
are generated in the anode reaction equation (3) are transported through the
membrane to
the cathode compartment and react with the hydroxyl ions generated in the
cathode
reaction in accordance with equation (4):
H+ + OH" - H2O (4)
As a result, an undesirable increase in the pH in the cathode compartment is
avoided and
no acid needs to be dosed in the cathode compartment. The pH and salt
concentration in
the cathode chamber remain stable and homeostasis is maintained. Dissolved or
gaseous
CO2 can be transferred from the anode to the cathode alongside with the fluid.
In yet another one embodiment, the present invention may be operated with a
biocathode
only. In this embodiment, the anode may comprise an essentially conventional
anode. In
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
11
this embodiment an acid solution (e.g. sulfuric acid) may be provided to the
anode
compartment and the anode reaction may be a proton generating reaction (e.g.
oxygen
generation from water). The membrane may comprise a cation exchange membrane.
Cation exchange membranes are known to the person skilled in the art and
include
membranes such as CMI-7000 (Membranes International), Neosepta CMX (ASTOM
Corporation), fumasep FKB (Fumatech), and Nafion (DuPont). In this embodiment
protons migrate through the cation exchange membrane and react with the
hydroxyl ions
generated in the cathode reaction. As a result, no acid needs to be dosed in
the cathode
compartment the pH and salt concentration in the cathode chamber remain stable
and
homeostasis is maintained.
In yet another embodiment, the electrical current used to provide the
reduction in the
cathode is derived from a photo-anode. Photo-anodes are known to persons
skilled in the
art and capture sunlight and transfers the reducing power to the electrical
circuit.
In yet another embodiment, the electrical current provided to drive the
biochemicals
production is derived from a renewable power source such as solar power, hydro-
power,
or others as known to a person skilled in the art.
In yet another embodiment, the membrane separating the anode and the cathode
comprises a bipolar membrane. Bipolar membranes are known to persons skilled
in the
art and include membranes such as NEOSEPTA BP-1E (ASTOM Corporation) and
fumasep FBM (Fumasep). Bipolar membranes are composed of a cation exchange
layer
on top of an anion exchange layer and rely on the principle of water splitting
into protons
and hydroxyl ions in between the ion exchange layers of the membrane,
according to
equation (5):
H2O 4 H+ + OH- (5)
In embodiments where a bipolar membrane is used as the membrane in the
bioelectrochemical system, the anion exchange layer is directed towards the
anode
chamber and the cation exchange layer is directed towards the cathode chamber.
When
electrical current flows, water diffuses in between the ion exchange layers of
the bipolar
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
12
membrane and is split into protons and hydroxyl ions. The hydroxyl ions
migrate through
the anion exchange layer into the anode chamber, where they compensate for the
proton
production in the anode reaction equation (3) and the protons migrate through
the cation
exchange layer into the cathode chamber where they compensate for the hydroxyl
ion
production (or proton consumption) in the cathode reaction. As a result, no
acid needs to
be dosed in the cathode compartment and the pH and salt concentration in the
cathode
chamber remain stable, maintaining homeostasis.
In another embodiment of the present invention, the effluent of the anode may
be sent to,
for example, a stripping column or membrane unit to recover gaseous carbon
dioxide.
This carbon dioxide can be provided to the cathode as a gas.
In a variation of the above embodiment effluent from the anode can be passed
through a
membrane unit to allow separation of carbon dioxide from the anode effluent,
the
membrane unit having a liquid flow on the other side of the membrane. In this
manner,
the separated carbon dioxide can go into solution in the fluid on the other
side of the
membrane. The carbon dioxide can be provided to the cathode in dissolved form.
The
fluid passing through the membrane unit on the other side of the anode fluid
can be
cathode fluid.
In another embodiment, the anode effluent can be sent through a membrane unit
to allow
carbon dioxide together with organic constituents of the anode effluent to
pass to a
second liquid. For example, effluents from fermentation reactors can be sent
through an
anode, the effluent of the anode can be sent to a membrane unit where aside
from the
carbon dioxide fatty acids such as propionate, butyrate and others as known to
a person
skilled in the art pass through the membrane and become captured in a second
fluid. This
fluid can be sent to the cathode where reduction of the organics can occur. In
this
embodiment, both carbon dioxide and the other organic materials provide feed
material
for the microorganisms to convert into the desired chemical products.
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
13
In one embodiment, the cathode is also provided with organic molecules to
assist in the
production of the biochemicals. Examples of such organic molecules are
glycerol,
glucose, lactate, butyrate, and others known to a person skilled in the art.
These
compounds can be added to provide the microorganisms with a source for
adenosyl
triphosphate (ATP) formation, which facilitates microbial growth and product
formation.
In the case of glycerol addition, the product formation may include 1,3-
propanediol or
butanol. Glycerol may be added to the cathode compartment, to the anode
compartment
or to both. Glycerol can also be (partially) converted to propionate prior to
entry in the
bioelectrochemical system, and subsequently be added to the cathode as a
mixture of
glycerol and propionate.
In one embodiment, the microorganisms in the cathode compartment are
genetically
engineered to receive electrons from the cathode. Examples of modifications
include the
addition of hydrogenases, cytochromes, sortases and other enzyme complexes to
the cell.
Alternatively, the cathode can be provided with conductive structures, such as
nanowires,
to electrically connect microorganisms with the cathode.
In another embodiment, redox mediators can be added to the cathode fluid,
allowing
transport of electrons from the cathode to the microorganism. Examples of
redox
mediators are methyl viologen, neutral red, phenazine carboxamide, amido black
and
others as known to a person skilled in the art. The redox shuttles allow in
certain
embodiments to increase the ratio NADH/NAD+ inside the microbial cell, which
drives
the production of reduced molecules.
In some embodiments of the process of the present invention, a mixture of
desirable
chemicals may be formed in the cathode compartment. In such embodiments, the
present
invention further comprises the steps of removing a mixture of chemical
compounds from
the cathode compartment and separating the mixture of chemical compounds into
two or
more streams. The mixture of chemical compounds may be separated using known
separation techniques, such as ion exchange, liquid-liquid extraction,
absorption,
absorption, gas stripping, distillation, reverse osmosis, membrane separation,
cryogenic
separation, or indeed any other separation technique known to be suitable to a
person
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
14
skilled in the art. In some embodiments, one or more of the chemical compounds
may be
reacted to form another chemical compound that is more susceptible to removal
from the
remaining chemical compounds. In some embodiments, one or more of the chemical
compounds formed in the cathode compartment may comprise a solid compound. In
such
embodiments, any suitable solid/liquid separation technique may be used,
including
centrifugation, filtration, settling, clarification, flotation, or the like.
In other
embodiments, one or more of the chemical compounds formed in the cathode
compartment may comprise a gaseous compound. In such embodiments, product can
conveniently be collected with a gas collection device, such as a gas-liquid
separator, as
known to a person skilled in the art.
In some embodiments of the present invention, the cathode compartment is
filled with the
microbial culture. The microbial culture is typically part of -an aqueous
mixture in the
cathode compartment. In other embodiments of the present invention, the
microbial
culture grows on the electrode surface. In yet other embodiments of the
present invention,
the cathode compartment is filled with part of the microbial culture and
another part of
the microbial culture grows on the electrode surface.
In some embodiments of the present invention, the cathode compartment may
comprise a
first compartment housing the cathode, the first compartment including a redox
shuttle,
and a second compartment containing one or more microorganisms, wherein the
redox
shuttle is reduced in the first compartment and a reduced redox shuttle is
provided to the
second compartment, the second compartment containing microorganisms that use
the
reduced redox shuttle as an electron donor to facilitate formation of the one
more
chemicals. The reduced redox shuttle is converted to an oxidised redox shuttle
in the
second compartment. The oxidised redox shuttle may be returned to the first
compartment.
Examples of chemical compounds that can be formed using the present invention
include:
- alcohols such as methanol, ethanol, propanol, butanol, isobutanol etc.
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
- carboxylic acids, such as formic acid, acetic acid, propionic acid, butyric
acid, lactic
acid, etc.
- diols such as 1,3-propanediol and 1,2-propanediol
- biopolymers such as poly-(3-hydroxybutyrate (PHB), etc,
5 - any other organic chemical that can be produced by micro-organisms from
carbon
dioxide and reducing equivalents, in the presence or absence of organic
chemicals.
BRIEF DESCRIPTION OF THE DRAWINGS
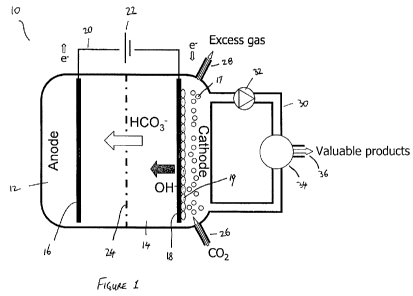
Figure 1 shows a schematic view of a bioelectrochemical system suitable for
use in
embodiments of the present invention;
10 Figure 2 shows a schematic diagram of apparatus suitable for use in another
embodiment
of the present invention;
Figure 3 shows a schematic diagram of apparatus suitable for use in a further
embodiment of the present invention; and
Figure 4 shows a schematic diagram of apparatus suitable for use in a further
15 embodiment of the present invention.
DETAILED DESCRIPTION OF THE DRAWINGS
It will be appreciated that the drawings have been provided for the purpose of
illustrating
preferred embodiments of the present invention. Therefore, it will be
understood that the
present invention should not be considered to be limited to the features are
shown in the
drawings.
The bioelectrochemical system 10 shown in figure 1 includes an anode
compartment 12
and a cathode compartment 14. The anode compartment 12 includes an anode 16.
The
cathode compartment 14 includes a cathode 18. The anode and the cathode are in
electrical connection with each other via an electrical circuit 20 that
contains a power
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
16
supply 22. The anode compartment 12 is separated from the cathode compartment
14 by
an anion exchange membrane 24.
The cathode compartment 14 contains a microbial culture (shown schematically
in Figure
I as ovals or circles 17 and 19). In the embodiment shown in figure 1, the
microbial
culture comprises a defined culture. A defined microbial culture of
cathodophilic
microorganisms should be a carefully selected or genetically engineered pure
culture, but
could also be a carefully selected co-culture of two or more carefully
selected or
genetically engineered pure cultures. These pure cultures or co-cultures
should consist of
microbial species that are capable of catalyzing the production reaction of
the desired
complex molecule. The microbial culture in the cathode compartment 14 is
contained in
an aqueous medium (see reference numeral 17) and/or attached to the cathode
(see
reference numeral 19).
The cathode compartment 14 is provided with a gas inlet 26 and a gas outlet
28. A carbon
dioxide containing stream is fed into the gas in 26 and excess gas is removed
through gas
outlet 28. The carbon dioxide containing stream that is fed to the cathode
compartment is
a sterile carbon dioxide containing stream in that it contains no
contaminating
microorganisms. One possible source of such a carbon dioxide containing stream
is an
offgas stream from a boiler or a furnace. Such an offgas stream leaves the
boiler or
furnace that elevated temperatures and therefore contained no microorganisms
(and
microorganisms would be killed by the high temperatures encountered in the
offgas
stream). The offgas stream may be cooled (in a manner which does not introduce
any
contaminating bacteria into the gas stream, such as by using indirect heat
exchange) and
subsequently be fed to the cathode compartment 14.
Liquid from the cathode compartment 14 circulates through liquid line 30. Pump
32 is
used to maintain this liquid circulation. A separator 34 is used to separate
valuable
product from the liquid and the valuable product is recovered at 36. The
nature of the
separator will be determined by the valuable product(s) to be separated from
the liquid.
The person skilled in the art will readily appreciate how to design an
appropriate
separator for each product being formed.
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
17
Figure 2 shows a schematic view of an alternative apparatus suitable for use
in the
present invention. The apparatus shown in figure 2 includes an anode
compartment 112
that contains an anode 116. The apparatus also includes a cathode compartment
114 that
contains a cathode 118. A membrane 124 separates the cathode compartment from
the
anode compartment. An electrical circuit 120 that includes a power supply 122
(in the
form of a DC power supply, such as a battery or an AC to DC converter)
electrically
connects the anode 116 to cathode 118.
The apparatus also includes a separate vessel 130. The vessel 130 has an inlet
132 in
which carbon dioxide and oxygen or an oxygen containing gas can be supplied.
The
oxygen and carbon dioxide can be transferred via line 134 to compartment 114.
Line 136
returns fluid and excess gas to the vessel 130. The vessel 130 may also be
provided with
an aqueous medium and the carbon dioxide and oxygen may dissolve into the
aqueous
medium, with the aqueous medium containing dissolved carbon dioxide and oxygen
being transferred to the cathode compartment 114.
Figure 3 shows a schematic view of another apparatus suitable for use in
embodiments of
the present invention. The apparatus shown in figure 3 includes an anode
compartment
212 that contains an anode 216. The apparatus also includes a cathode
compartment 214
that contains a cathode 218. A membrane 224 separates the cathode compartment
from
the anode compartment. An electrical circuit 220 that includes a power supply
222 (in the
form of a DC power supply, such as a battery or an AC to DC converter)
electrically
connects the anode 216 to the cathode 218.
The apparatus shown in figure 3 also includes a further vessel 230. The vessel
230 has an
inlet 232 for admitting carbon dioxide to the vessel 230. In the embodiment
shown in
figure 3, a redox shuttle is reduced in the cathode compartment 214. The
reduced redox
shuttle is supplied via line 236 to the external compartment 230. A culture of
microorganisms in the vessel 230 uses the reduced redox shuttle as an electron
donor for
the reduction of carbon dioxide. The oxidised redox shuttle is then returned
to the cathode
compartment 214 via line 238.
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
18
Figure 4 shows a schematic view of another apparatus suitable for use in
embodiments of
the present invention. The apparatus shown in figure 4 includes an anode
compartment
312 that contains an anode 316. The apparatus also includes a cathode
compartment 314
that contains a cathode 318. A membrane 324 separates the cathode compartment
from
the anode compartment. An electrical circuit 320 that includes a power supply
322
electrically connects the anode 316 to cathode 318.
The apparatus also includes a vessel 330 that has an inlet 332 for supplying
oxygen (and
additional carbon dioxide, if required) thereto. Line 350 transfers oxygen and
carbon
dioxide to the cathode compartment 314 and line 352 returns excess oxygen and
carbon
dioxide to vessel 330. The oxygen and carbon dioxide may be transferred as
gaseous
streams or dissolved in liquid streams.
In the embodiment shown in figure 4, the reaction is taking place at the anode
produce
carbon dioxide in the anode compartment 312. An outlet 340 from the anode
compartment removes aqueous liquid containing carbon dioxide from the anode
compartment and passes it to a stripping column 342. In the stripping column,
carbon
dioxide is separated from the aqueous liquid. The aqueous liquid is returned
to the anode
compartment 312 via line 344. The stripped carbon dioxide is transferred via
line 346 to
inlet 334 of vessel 330. The carbon dioxide and oxygen in the vessel 330 is
transferred to
cathode compartment 314, where a selected culture of microorganisms converts
the
carbon dioxide into other chemical compounds. This embodiment is advantageous
in that
carbon dioxide that forms at the anode is captured and used as a feed to the
cathode
compartment, thereby reducing carbon dioxide emissions.
Examples
Example 1: Biopolymer production
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
19
In this example, which uses the apparatus as shown in Figure 2, bacteria in
the cathode
chamber use carbon dioxide and electrons from the cathode as energy and carbon
source,
in which case they can produce biopolymer under the form of poly-(3-
hydroxybutyrate
(PHB). The CO2 is provided in a way that a pure culture or a defined mixture
of bacteria
can be maintained. Oxygen is supplied to support the PHB synthesis. The
electrons reach
the bacteria either directly or indirectly through e.g. the production of
hydrogen at the
cathode. An external power source can provide the required additional reducing
power at
the cathode, if required
Example organism in the cathode: Cupriavidus necator (formerly Alcaligenes
eutrophus
or Ralstonia eutropha)
Example 2: Indirect provision of reducing power to biochemicals producing
organisms
In this example the apparatus as shown in figure 3 is used and a redox shuttle
is reduced
in the cathode compartment. The reduced redox shuttle is brought to the
external
compartment (possibly through a permeable membrane) where micro-organisms use
the
reduced redox shuttle as electron donor for the reduction of an electron
acceptor, being
CO2, and the production chemicals from this CO2. An external power source can
provide
the required additional reducing power at the cathode, if required
Example 3. Reuse of CO2 produced at the anode to drive the cathodic reaction.
This example is conducted in the apparatus as shown in Figure 4. The anode
contains
micro-organisms that oxidize a carbon source. The CO2 produced is stripped in
situ, or in
an external stripping reactor, and hence brought to the cathode compartment in
such way
that the cathode compartment can contain a well defined culture or mixed
culture of
micro-organisms to form the desired chemicals.
The present invention presents a cathode system for producing complex
molecules using
microbial biocathodes prevents the abovementioned problems associated with the
CA 02747212 2011-06-16
WO 2010/068994 PCT/AU2009/001645
contamination of unwanted micro-organisms and/or cathode chamber pH increase
and/or
salinity increase.