Note: Descriptions are shown in the official language in which they were submitted.
CA 02748998 2011-07-05
WO 2010/077676 PCT/US2009/067190
1
DELIVERY SYSTEM FOR INTRAVASCULAR DEVICE WITH NETTING
BACKGROUND
Many medical intravascular devices are currently being used either
temporarily or permanently inside the human body to address conditions
associated
with high blood pressure, diabetes, and stroke. One example of an
intravascular
device is a stent for use in, for instance, coronary angioplasty. Stents are
small
mechanical devices that can be implanted within a vascular structure, such as
a
blood vessel or an artery, and can be mechanically expanded to maintain an
open
lumen at a constricted location to permit a substantially clear flow path
therethrough.
A stent can also act to support a vessel wall in areas vulnerable to collapse.
The mechanical reopening of a constricted vessel can sometimes lead to
injuries of the tissues at the site of constriction or closure. Such injuries
can often
stimulate thrombus formation at such a site, as well as release of tissue
debris that
may subsequently act to block fluid flow within the vessel. Moreover, if
permitted to
proliferate, pronounced neointimal hyperplasia or restenosis can result.
Thrombus
production remains one of the most common post-stenting clinical problem, and
requires effective intervention or counter-measures to prevent and/or control
its
reoccurrence.
Currently, methods for preventing or controlling thrombus are specifically
aimed at influencing factors believed to be involved in the body's response to
external or internal tissue stimulants, such as angioplasty, stenting
procedures,
and/or viruses. Common countermeasures which have been used to prevent or
control restenosis generally fall into the one of several categories,
including (1)
mechanical atheroablative techniques, such as debulking, vascular filters, and
emboli-trapping devices, (2) ultrasound-initiated atheroablative techniques,
(3) light-
assisted procedures, predominantly excimer laser angioplasty, (4)
pharmacological
agents and gene therapy, (5) ultraviolet photophoresis, believed to be an
immune
modulator, (6) radiation therapy, such as external and endovascular
brachytherapy,
and (7) re-stenting.
CA 02748998 2013-09-12
2
In addition, modifications to stent designs and materials have been proposed
to prevent and/or control restenosis. In one approach, non-metallic,
biodegradable
stent materials, such as high molecular weight Poly-1-lactic acid (PLLA) is
used.
Numerous inorganic coatings and surface treatments have also been
developed to improve chemical inertness and biocompatibility of metallic
stents.
Some coatings, such as gold, however, yield a higher rate of in-stent
restenosis than
uncoated stents. Others, including silicon carbide and turbostatic carbon,
show
promise but additional studies must be done.
Organic coatings, including both synthetic and natural coatings, have also
been widely studied. Among the synthetic coatings studied are DacronTM,
polyester,
polyurethane, polytetrafluoroethylene (PTFE), polyethylacrylate/
polymethylmethacrylate, polyvinyl chloride, silicone, collagen, and iridium
oxide.
Results of studies, such as those with PTFE-coated stents, are disappointing
or
mixed at best, as there are high occurrences of late thrombo-occlusive events.
With
only a very few exceptions, the general consensus is that any favorable
outcome was
not associated with treatment of conventional in-stent restenosis using P'TFE-
coated
stents.
Intracoronary intervention have also been employed to reduce neointima
formation by reducing smooth muscle cell proliferation after balloon
angioplasty.
However, such intervention is often complicated by subacute and late
thrombosis.
Coronary thrombo-aspdrugiration and coronary pulsed-spray procedures, followed
by immediate endovascular therapy, have also been particularly helpful in
removing
thrombotic material associated with plaque.
In addition, pharmacotherapeutic agents have been used for the treatment of
some of the major post-angioplasty complications, including immunosuppresants,
anticoagulants and anti-inflammatory compounds, chemotherapy agents,
antibiotics,
antiallergenic drugs, cell cycle inhibitors, gene therapy compounds, and
ceramide
therapy compounds. Pharmacotherapeutic agents can be delivered either
systemically
or locally. Systemic treatment has shown limited success in reducing
restenosis
following stent implantation, a result believed to be due to inadequate
concentration
of the pharmacotherapeutic agents at the site of injury. Increased dose
administration, however, is constrained by possible systemic toxicity. It has
been
CA 02748998 2011-07-05
WO 2010/077676 PCT/US2009/067190
3
observed that local delivery of higher doses via drug eluting stents can
significantly
reduce adverse systemic effects. However, the local delivery of drugs via
stents may
be limited by the amount of surface area for drug elution.
Gene therapy have also been employed in the treatment of thrombus
production. The procedure is directed towards smooth muscle cells and involves
gene transfer via DNA, with or without integration of chromosomes, into
selected
cells. In transduction without integration, the gene is delivered to both
cytoplasm and
nucleus and is therefore non-selective. Gene transfer for integration employs
retrovirus to affect growth stimulators.
Antibiotics, likewise, has been used in the treatment of coronary artery
disease. It is known that antibiotics are effective in controlling
inflammation caused
by a variety of infectious agents found in fatty plaques blocking the
arteries. Results
of clinical investigation, such as with azithromycin, suggest a modest
antibiotic
benefits for heart patients.
Similarly, a phospholipid exhibiting immunosuppressive properties, has been
shown to block T-cell activation and proliferation, inhibit Taxol-induced cell
cycle
apoptosis, and activate protein kinase signal translation in malignant
myogenic cells.
Rapamycin and its analogs exhibit anti-tumor activities at relatively low dose
levels,
while inducing only mild side effects, an extremely important aspect of
patient care.
SUMMARY
The present invention provides, in one embodiment, an intravascular device,
such as a stent, for keeping open a previously constricted intravascular site
within a
vessel and for minimizing tissue debris from such a site from closing off the
vessel.
The device may also be used for local delivery of at least one
pharmacotherapeutic
agent to the intravascular site for the treatment or prevention of restenosis.
The intravascular device, in accordance with an embodiment of the
invention, includes an expandable substantially tubular body for placement
against a
vessel wall. The body of the device, in a particular configuration, may be
defined by
a framework having a plurality of openings. The device also includes a
flexible
netting system having a structural design for extending across each of the
openings.
Such a double stent design allows the netting system to expand along with each
opening in the framework to minimize occurrence of thrombus formation and
tissues
CA 02748998 2011-07-05
WO 2010/077676 PCT/US2009/067190
4
debris from closing the lumen of the vessel. The netting system can include a
plurality of pores to permit communication between fluid flow within the
vessel and
the vessel wall, and at least one pharmacotherapeutic agent for the treatment
or
prevention of certain conditions. In one embodiment, the netting system
includes a
plurality of extensible panels, each designed to be securely situated within
an
opening of the matrix. Alternatively, the netting system includes a mesh
disposed on
a substantially flexible matrix, such that the mesh can be placed
circumferentially
about the framework of the body. If desired, the flexible matrix can be
provided with
sufficient strength to permit the netting system to keep the lumen of the
vessel
temporarily open until the framework can be expanded. The device of the
present
invention, in an embodiment, can further include a second expandable
substantially
tubular framework concentrically positioned within the first framework of the
tubular body.
The present configuration also provides a method for the placement of such
an intravascular device within a vessel. The method includes initially
providing a
device having an expandable substantially tubular body defined by a framework
having a plurality of openings, and a plurality of netting panels situated
within each
of the openings. Next, the device may be advanced along a lumen of the vessel
to a
site of interest. Thereafter, the framework may be expanded at the site of
interest to
allow the lumen of the vessel to remain open. The device may subsequently act
to
elute at least one pharmacotherapeutic agent for treatment of a condition from
the
netting panels. The netting panels may also act to retain tissue debris
between the
netting panels and a vessel wall.
The present invention further provides another method for placement of an
intravascular device within a vessel. The method includes providing a device
having
an expandable substantially tubular body defined by an inner framework or
scaffolding having a plurality of openings, and an outer mesh or netting
disposed on
a substantially flexible matrix loosely positioned circumferentially about the
framework. Next the device may be advanced along a lumen of the vessel to a
site of
interest. Thereafter, the framework may be expanded at the site of interest,
and the
mesh on the flexible matrix be allowed to be secured between the framework and
a
vessel wall. In one embodiment, prior to expanding the framework, the flexible
CA 02748998 2013-09-12
matrix on which the mesh is disposed may be expanded. The device may
subsequently act to elute, from the mesh, at least one pharmaeotherapeutie
agent for
treatment of a condition. The mesh may also act to retain tissue debris
between the
netting panels and a vessel wall.
5 Conventional approaches suffer form the shortcoming that delivery of the
outer netting over the inner scaffolding is difficult to position such that
the balloon
catheter is positioned substantially centered and overlying the inner
scaffolding.
Configurations herein are based, in part, on the observation that imprecise
centering
of the outer netting over the inner scaffolding may result in unequal balloon
catheter
expansion that may compromise stent placement. Accordingly, configurations
herein substantially overcome such shortcomings by providing a limiting ridge
for
defining advancement of the outer netting over the inner scaffolding, and
locking
clips integrated in the outer netting for engaging the scaffolding at the
insertion limit
to avoid shifting or movement from retracting of the delivery device. The
delivery
device further includes restricting ridges limiting advancement of the inner
netting to
overlie the inner scaffolding by a substantially equal portion on the
proximate and
distal ends such that balloon catheter expands first at the overlying portion
outside
the inner scaffolding to sealahly contact the lumen wall on each side of the
scaffolding to prevent contamination.
Alternate configurations of the invention include various arrangements for
delivering
a double stent apparatus.
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing and other objects, features and advantages of the invention
will be apparent from the following description of particular embodiments of
the
invention, as illustrated in the accompanying drawings in which like reference
characters refer to the same parts throughout the different views. The
drawings are
not necessarily to scale, emphasis instead being placed upon illustrating the
principles of the invention.
CA 02748998 2011-07-05
WO 2010/077676 PCT/US2009/067190
6
Fig. 1 shows a side view of a particular configuration of the double stent and
delivery device;
Fig. 2 shows a second configuration including an expandable balloon
catheter for insertion in the double stent;
Fig. 3 shows retainers of the delivery device around the balloon catheter of
Fig. 2;
Fig. 4 shows the inner scaffolding around the balloon catheter of Fig. 3;
Fig. 5 shows an extension region of the balloon catheter of Fig. 4;
Fig. 6 shows the outer netting disposed around the inner scaffolding;
Fig. 7 shows the outer netting installed by the retainers;
Fig. 8 shows an cross section of the axial plane of the balloon and extension
region adjacent the inner scaffolding of Fig. 5; and
Figs. 9 and 10 are a flowchart of general delivery of the double stent via the
delivery device.
DETAILED DESCRIPTION
Configurations described below depict various configurations of the double
stent and delivery and installation thereof, as in placement in a lumen of a
blood
vessel. In a particular configuration, the delivery system includes a balloon
catheter
with limiting (restricting ridges) at proximal and distal ends of balloon; a
scaffolding
stent placed circumferentially around the balloon between ridges; and self
expanding
netting with latching devices (hooks) retained in a delivery sleeve which is
placed
circumferentially over the body of the main balloon catheter and can be
advanced
and retracted along the axis of the catheter. The internal diameter of the
netting is
slightly larger than the outer diameter of ridges which allows the advancement
of the
netting on top of the scaffolding stent.
In operation, following the delivery of the scaffolding stent to the target
area
in the vessel, the sheet containing the self expanding netting is advanced
forward
until the proximal ridge on the catheter limits further advancement of the
sheet when
the inner edge of the delivery sheet at the proximal end of the netting.
Following
advancement of the netting over the scaffolding stent the locking (attaching)
hooks
(mechanism) engages the scaffolding stent. Next, the delivery sleeve for the
netting
is retracted while the netting remains in position due to its attachment to
the
CA 02748998 2011-07-05
WO 2010/077676 PCT/US2009/067190
7
scaffolding stent and expands as it is released from the sleeve.
In contrast to conventional mechanisms, the netting is delivered to the target
area in sequence (scaffolding first, netting on top second which eventually is
followed by expansion). This allows for greater flexibility of the device
compared to
a conventional preassembled double metal structure which will be much more
rigid
and difficult to deliver to the target area. Following the retraction of the
netting
delivery sleeve and expansion of the netting, the balloon is inflated and the
scaffolding stent is expanded to the desired diameter thus locking the netting
between the vessel wall and stent. The balloon is deflated and the entire
delivery
system is retracted.
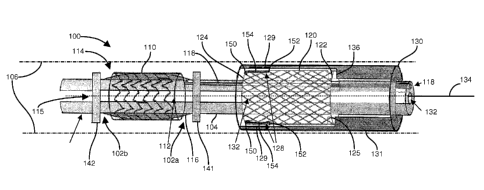
Fig. 1 shows a side view of a particular configuration of the double stent and
delivery device. Referring to Fig. 1, the double stent (stent) 100 includes an
inner
scaffolding 110 and an outer netting 120 adapted for slideable disposition via
a
catheter 104. A delivery device 130 advances the outer netting 120 over the
inner
scaffolding 110 to install the double stent 100, discussed further below. The
stent
100 includes restricting ridges 141 and 142, at proximate (141) and distal
(121) ends
from the delivery device 130, which align with proximate 125 and distal 124
opening in the outer netting 120 following advancement. A guide wire channel
132
retains a guide wire 134 in slideable communication with the delivery device
130 for
advancing the outer netting 120 over the inner scaffolding 110. A limiting
ridge 136
engages a proximate end 122 of the inner netting 120 for advancing the netting
120
over the scaffolding 110. A proximate restricting ridge 141 limits advancement
by
engaging the limiting ridge 136 at a point which the outer netting 120 is
substantially
centered over the inner scaffolding 110 such that overlying regions 102a and
102b
(102 generally) are defined between a proximate opening 112 and a distal
opening
114 and the corresponding proximate and distal restricting ridges 141, 142,
respectively. After full advancement, withdrawal of the delivery device 130
activates locking clips 128 such that anterior hooks 150 engage the inner
scaffolding
to secure the outer netting through integrated posterior hooks 152 attached
via an
arm 154.
A balloon 116 occupies a cylindrical cavity 115 in the inner scaffolding 110
and couples to an inflation channel 118 adjacent the guide wire 134. Following
CA 02748998 2011-07-05
WO 2010/077676 PCT/US2009/067190
8
delivery of the outer netting 120 over the inner scaffolding 110, inflation of
the
balloon 116 causes radial expansion of the inner scaffolding 110. As the
balloon
116 commences expansion, the overlying regions 102a and 102b define expansion
regions unrestrained by the inner scaffolding 110 such that the balloon 116
forms an
expansion bubble 119, discussed further below in Fig. 8, for sealably engaging
the
delivered outer netting 120 against a lumen wall 106 defined by the vessel
into
which the stent 100 is inserted, thus preventing contamination in the region
between
the inner scaffolding 110 and the lumen wall 106.
In a second configuration, the delivery system includes a balloon catheter
with retainers , a scaffolding stent placed on top of the balloon; and netting
placed
circumferentially around scaffolding stent which is held in place by the
retainers
which overly the proximal and distal end of the netting. The retainers are
flexible so
as to be withdrawn through the scaffolding following expansion of the stent
100.
Fig. 2 shows the second configuration including an expandable balloon
catheter 104 for insertion in the double stent of Fig. 1. Referring to Figs. 1
and 2,
the balloon 116 expands from compressed air or any suitable fluid delivered
via the
inflation channel 118. The balloon 116 expands radially to install the stent
100
including the inner scaffolding 110 and outer netting 120, now discussed in
further
detail.
Fig. 3 shows retainers 140, 143 of the delivery device around the balloon
catheter of Fig. 2. Referring to Figs. 1 and 3, a pair of retainers include a
proximate
retainer 140 and a distal retainer 143, the distal retainer 143 furthest
advanced along
the catheter 104. In a particular arrangement, the proximate retainer 140
includes
the limiting ridge 136 for advancing the inner netting 120, effectively
performing the
operations of the delivery device 130. Alternatively, a separate delivery
device 130
may be extended and retracted, as discussed below.
In operation, the balloon catheter 116 and stent 100 assembly is advanced
until the stent 100 reaches the target area. The balloon is inflated which
causes its
expansion initially at the proximal and distal ends which pushes the netting
ends out
from underneath the flexible (elastic) retainers and against the vessel wall.
As the
pressure in the balloon 116 builds up the scaffolding stent 110 and outer
netting 120
above it are also pushed against the vessel wall 106. The balloon 116 is
deflated and
CA 02748998 2011-07-05
WO 2010/077676 PCT/US2009/067190
9
the catheter is retracted. The delivered stent assembly 100 includes the outer
netting
120 over the inner scaffolding 110 stent assembly and the retainers 140, 143
which
keep the assembly together until delivery.
Following delivery and expansion of the stent assembly 100 including the
inner scaffolding 110 and outer netting 120, retraction includes retracting
the distal
retainer 143 and the proximate retainer 140 through the inner scaffolding 110
after
expansion and deflation of the balloon 116 leaving the outer netting 120
around the
inner scaffolding 110.
Fig. 4 shows the inner scaffolding 110 around the balloon catheter of Fig. 3.
Referring to Figs. 3, 4 and 5, the inner scaffolding 110 includes a plurality
of flexible
members 110' in an interconnected matrix for permitting radial expansion in
response to balloon 116 inflation. An extension region 102a, 102b denotes an
area
where the balloon 116 extends beyond the ends 112, 114 of the inner
scaffolding
110. The extension regions 102a and 102b are substantially equal from
centering of
the inner scaffolding 110 over the balloon 116. Fig. 5 shows the extension
region
102a of the balloon catheter of Fig. 4, which allows the balloon 116 to
inflate
unrestrained by the inner scaffolding 110, thus permitting sealable engagement
with
the lumen wall 106 and the outer netting 120.
Fig. 6 shows the outer netting 120 disposed around the inner scaffolding 110.
The inner scaffolding 110 is disposed centered between the ends 122, 124 of
the
outer netting 122 such that the extension region 102a is defined by the
portion of the
balloon 116 underlying the outer netting 120 and unconstrained from the inner
scaffolding 110.
Fig. 7 shows the outer netting 120 installed by the retainers 140, 143. The
proximate retainer 140 may define the delivery device 130 by including the
cylindrical cavity 131 for containing the outer netting and limiting ridge 136
for
positioning the outer netting 120 substantially centered over the inner
scaffolding
110 between the proximate and distal restricting ridges 141 and 142,
respectively.
Fig. 8 shows an cross section of the axial plane of the balloon 116 and
extension region 102a adjacent the inner scaffolding 110 of Fig. 5. Referring
to
Figs. 1, 5 and 8, the restricting ridges 141 and 142 define the location of
the
openings 122, 124 of the outer netting 120 following delivery. Such delivery
CA 02748998 2011-07-05
WO 2010/077676 PCT/US2009/067190
disposes the outer netting 120 substantially centered over the inner
scaffolding 110
such that the extension regions 102a, 102b are formed by the netting 120
portion
overlying the scaffolding 110 and concentric with the balloon 116. Balloon 116
inflation results in expansion bubbles 119 forming along the ends 122, 124 and
5 adjacent to the restricting ridges 141 and 142 unconstrained by the inner
scaffolding
110. The expansion bubble 119 seals the lumen wall 106 against the balloon 116
and outer netting 120 to restrict debris before the remainder of the balloon
116
radially expands the inner scaffolding 110 against the netting 120 and lumen
wall
106 to complete installation.
10 Therefore, the body of the catheter 104 facilitates the expansion
bubble 119
in the extension region 102a and 102b from the ring-like restricting ridges
141 and
142 and the proximal and distal ends of the balloon 116 which have an external
diameter equal to the external diameter of the inner scaffolding 110 as it is
crimped
on top of the balloon 116. The restricting ridges 141 and 142 prevent movement
of
the inner scaffolding 110 during introduction of the outer netting 120 such
that
friction between the resilient (springy) locking clips 128 does not dispose
the
scaffolding 110 forward and also to insure exact placement of the netting 120
above
the scaffolding 110 by engaging the limiting ridge 136 of the delivery device
130.
Figs. 9 and 10 are a flowchart of general delivery of the particular
configuration of the double stent via the delivery device 130 during an
example
delivery and installation of the stent 100. Referring to Figs 1, 9 and 10, the
method
for delivering the stent 100 device includes inserting a guide wire 134 to an
afflicted
area, such that the guide wire 134 is adapted for slideable insertion of a
stent and
catheter to an afflicted area, as depicted at step 200. The method involves
delivering
the inner scaffolding along the guide wire 134, such that the inner
scaffolding 110
defines a cylindrical volume 115 around the catheter 104 having distal 142 and
proximate 141 ridges and a balloon 116 catheter therebetween, in which the
inner
scaffolding 110 has flexible members 110' expandable radially and responsive
to the
balloon catheter 104, as shown at step 201.
The catheter 104 disposes, via a delivery device 130, an outer netting 120, in
which the outer netting 120 defines a cylindrical shape having ends defining
distal
124 and proximate 125 openings for disposing concentrically around the inner
CA 02748998 2011-07-05
WO 2010/077676 PCT/US2009/067190
11
scaffolding 110, in which the outer netting 120 has a diameter larger than the
diameter of the inner scaffolding 110, and the delivery device 130 has
cylindrical
body 131 and a limiting ridge 136 for engaging the outer netting 120 at the
proximate opening 125 for slideable insertion around the inner scaffolding
110, such
that the limiting ridge 136 is defined by an outer diameter larger than the
outer
netting 120 and an inner diameter smaller than the proximate restricting ridge
141 on
the proximate end of the inner scaffolding 110, as depicted at step 202. In
particular
configurations, such as in Fig. 3, the stent 100 assembly further comprises a
distal
retainer 143 at the distal end of the inner scaffolding 110, such that the
distal retainer
143 performs a similar function as the distal limiting ridge 142, as depicted
at step
203.
The delivery device 130 advances the outer netting 120 over the inner
scaffolding 110 from slideable movement of delivery device 130 along the guide
wire 134 and catheter 104 to engage the limiting ridge 136 with the proximate
ridge
141 of the inner scaffolding 120, as disclosed at step 204. The smaller inside
diameter of the limiting ridge 136 stops forward advancement at a point where
the
outer netting 120 is substantially centered over the inner scaffolding 110.
The outer
netting 120 is slightly longer than the inner scaffolding 110, as depicted at
step 205.
The distal retainer 143 has a diameter larger then the unexpanded inner
scaffolding
110, such that the distal retainer 143 is disposed such that a portion of the
netting
overlies the balloon 116 beyond the distal end 114 of the scaffolding 110, as
shown
at step 206. The overlying portion defines the extension region 102b; a
symmetrical
relation defines the extension region 102a on the proximate end.
The catheter 104 is employed in retracting the delivery device 130, thus
leaving the outer netting 120 around the inner scaffolding 110. The inner
netting
120 has locking clips disposed at the distal end 124, such that the locking
clips 128
are adapted to engage the outer netting 120 to the inner scaffolding 110, as
depicted
at step 207. The locking clips 128 are responsive to withdrawal from the
delivery
device 130 via the guide wire 134 such that withdrawal of the catheter 104
slideably
engages the locking clips 128 for securing the outer netting 120 over the
scaffold
110 from the removal motion, as shown at step 208. In particular arrangements,
removal engages the locking clips in a recess 129 on the internal cylindrical
surface
CA 02748998 2011-07-05
WO 2010/077676 PCT/US2009/067190
12
of the insertion cylinder 131, such that the recess is aligned for slideably
engaging
the integrated locking clips 128, as depicted at step 209. In alternate
configurations,
the distal retainer 143 is retracted through the inner scaffolding 110 as
balloon
expansion of the inner scaffolding 110 and outer netting 120 has radially
expanded
the stent 100 assembly. The outer netting 120 is biased so as to axially
compress
and radially expand upon slideably disengaging from the cylinder 131 of the
delivery
cylinder, such that the delivery cylinder radially compresses the netting 120
so as to
be insertable between the scaffolding 110 and the lumen wall 106, as depicted
at step
210.
In the example arrangement, the locking clips 128 each having an anterior
hook 150, an arm 154, and a posterior hook 152, such that the anterior hooks
150 are
adapted to engage the distal end 114 of the inner scaffolding 110, providing
securement from the arm 154 extending along the netting 120 to the posterior
hook
152, in which the posterior hook 152 is integrated in the netting 120 for
securing the
arm 154 thereto, disclosed at step 211.
Following forward advancement of the outer netting 120, installation
includes expanding the balloon 116 catheter to dispose the inner scaffolding
110
radially outward to the outer netting 120, such that the outer netting 110
limits
expansion within a lumen of a blood vessel in which the stent 100 is disposed,
as
discussed at step 212. The restricting ridges 141, 142 ensure that upon
expanding
the balloon 116 along the length of the outer netting 120, such that the outer
netting
120 extends beyond the proximate 112 and distal 114 ends of the inner
scaffolding
110, the balloon 116 expands first in the extension regions 102a, 102b
extending
beyond the ends 112, 114 of the inner scaffolding 110 such that the outer
netting 120
is in communication with a vessel wall 106 prior to expansion of the inner
scaffolding 110, as depicted at step 213. This preliminary expansion seals and
secures the netting 120 and scaffolding 110 from emergence of the expansion
bubbles 119 prior to the remainder of the stent 100, as discussed above.
Those skilled in the art should readily appreciate that the programs and
methods for stent delivery and installation may be augmented by those skilled
in the
art without departing from the principles described above for advancing the
double
stent 100, withdrawing the delivery device 130 to secure the scaffolding 110
and
CA 02748998 2011-07-05
WO 2010/077676
PCT/US2009/067190
13
netting 120 via locking clips 128, and selectively expanding first in the
extension
region define by the scaffolding 110 and netting. It will therefore be
understood by
those skilled in the art that various changes in form and details may be made
therein
without departing from the scope of the invention encompassed by the appended
claims.