Note: Descriptions are shown in the official language in which they were submitted.
CA 02749133 2013-02-06
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROAT
DISCOMFORT
BACKGROUND OF THE INVENTION
[0001] Throat discomfort, which includes throat pain and throat irritation, is
a
common symptom of viral and bacterial infections, allergies, breathing
polluted air, and
smoking. Throat lozenges constitute one category of over-the-counter
treatments for throat
discomfort. The most common active ingredient in such lozenges is menthol,
which is
sometimes used in combination with eucalyptus oil. Menthol exerts a
physiological cooling
effect that can soothe a sore throat. However, the use of menthol in throat
lozenges suffers
from two significant drawbacks. First, concentrations of menthol that are
effective for throat
soothing are also accompanied by a bitterness that many people find
objectionable. Second,
the physiological cooling effect of a menthol-containing lozenge is felt
primarily in the
mouth rather than the throat. There is therefore a desire for throat lozenge
compositions that
exhibit reduced bitterness and increased selectivity for cooling in the throat
rather than the
mouth.
BRIEF DESCRIPTION OF THE INVENTION
[0002] One embodiment is a method of treating throat discomfort, comprising
administering to a subject in need thereof an effective amount ofN-ethy1-2,2-
diisopropylbutanamide.
[0003] Another embodiment is a confection, comprising: at least 80 weight
percent
of a confectionery base; about 0.005 to about 0.5 weight percent of
monomenthyl glutarate,
N-ethyl-p-menthane-3-carboxamide, or a combination thereof; and about 0.03 to
about 0.4
weight percent otN-ethyl-2,2-diisopropylbutanamide; wherein all weight
percents are based
on the total weight of the confection.
[0004] These and other embodiments are described in detail below.
BRIEF DESCRIPTION OF THE FIGURE
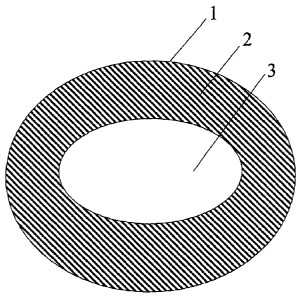
[0005] The FIGURE 1 is a side, cross-sectional view of a center-filled
confection.
DETAILED DESCRIPTION OF THE INVENTION
[0006] The present inventors have observed that N-ethyl-2,2-
diisopropylbutanamide
is a particularly effective for throat soothing. At therapeutically effective
doses it is much
less bitter than menthol. Moreover, it exhibits selectivity for throat cooling
over mouth
1
CA 02749133 2011-07-06
WO 2010/083133 PCT/US2010/020685
cooling. This selectivity for throat cooling is particularly surprising given
that a very close
chemical relative, N,2,3-trimethy1-2-isopropylbutanamide, exerts its cooling
effect primarily
in the mouth rather than the throat. The structures of N-ethyl-2,2-
diisopropylbutanamide and
N,2,3-trimethy1-2-isopropylbutanamide, which differ only by two methylene (-
CH2-) groups,
are shown below.
CH3
CH3 I 0 CH3 0
I CH2 I I CH3 I CH3 I I
I
CII C CH2 CH I C CH3
H3C C NH H3C C NH
I I
,CH ,CH
r,T__T T T ry
H3C k_A--13 ra3t._, CH3
N-ethyl-2,2-diisopropylbutanamide N,2,3-trimethy1-2-isopropylbutanamide
[0007] One embodiment is a method of treating throat discomfort, comprising
administering (e.g., via mouth) to a subject in need thereof an effective
amount of N-ethy1-
2,2-diisopropylbutanamide. N-Ethyl-2,2-diisopropylbutanamide is a known
compound,
having been described in Great Britain Patent No. 1,421,744 to Rowsell et al.
In that
reference, it is one of a family of acyclic carboxamides that is characterized
as exhibiting
physiological cooling activity. In particular, the family of compounds is
characterized as
"having a physiological cooling effect on the skin and on the mucous membranes
of the body,
particularly the mucous membranes of the nose and bronchial tract." GB
1,421,744 to
Rowsell et al., page 1, lines 12-15. The Rowsell patent includes a Table in
which each
compound's "cooling activity" is indicated by a scale of one to five
asterisks, but the
procedure by which cooling activity was evaluated is not provided, and there
is no specific
indication of throat-cooling activity.
[0008] For the treatment of throat discomfort, N-ethyl-2,2-
diisopropylbutanamide can
be administered by mouth in a variety of confectionery forms, including hard
candies, soft
candies, center-filled confections, jellies, and films.
[0009] In some embodiments, the effective amount of N-ethy1-2,2-
diisopropylbutanamide is administered in the form of a confection having a
mass of about 2
to about 10 grams and comprising about 0.03 to about 0.4 weight percent of N-
ethy1-2,2-
diisopropylbutanamide, based on the weight of the confection. Within the range
of about 2 to
about 10 grams, the confection weight can be about 3 to about 8 grams,
specifically about 4
2
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
to about 6 grams. Within the range of about 0.03 to about 0.4 weight percent,
the
concentration of N-ethyl-2,2-diisopropylbutanamide can be about 0.05 to about
0.25 weight
percent, specifically about 0.06 to about 0.12 weight percent.
[0010] In addition to the N-ethyl-2,2-diisopropylbutanamide, the confection
comprises a confectionery base. As used herein, the term "confectionery base"
includes any
ingredient or group of ingredients that represent the bulk of the
confectionery composition
and provide the confectionery composition with its structural integrity and to
which other
ingredients are added. Examples of confectionery bases include sucrose,
dextrose, maltose,
dextrin, xylose, ribose, glucose, mannose, galactose, fructose, lactose,
invert sugar,
fructooligosaccharide syrups, partially hydrolyzed starch, corn syrup solids
(e.g., in the form
of high fructose corn syrup), sorbitol, mannitol, maltitol, xylitol,
erythritol, polysaccharide
polyols, maltitol syrups, hydrogenated starch hydrolysates, polydextrose, and
combinations
thereof Still other examples can incorporate fats and hydrocolloids as in
bases that include
thin boiling starch mixed with sucrose, corn syrup and hydrogenated vegetable
oil or bases
that include pectin, sucrose, and corn syrup. The term "confectionery base"
does not include
the gum bases used in the formulation of chewing gums.
[0011] In the confection, the concentration of confectionery base can be at
least 80
weight percent, specifically at least 85 weight percent, more specifically at
least 90 weight
percent, still more specifically at least 95 weight percent, of the total
weight of the
confection.
[0012] In order to emphasize throat cooling over mouth cooling, N-ethy1-2,2-
diisopropylbutanamide can be used as the sole or primary physiological cooling
agent.
Alternatively, e.g., when a combination of mouth cooling and throat cooling is
desired,
N-ethyl-2,2-diisopropylbutanamide can be used in combination with other
physiological
cooling agents. In general, it should be noted that that the use of cooling
agents in gum and
confections pose different formulation challenges. In gum, release of the
cooling agents is
influenced by partitioning of the cooling agents between the gum base and the
essentially
aqueous environment of the mouth. In contrast, release of cooling agents from
confections is
controlled largely by the surface area and dissolution rate of the hard candy.
Therefore,
strategies to achieve sustained cooling sensation in gum by using multiple
cooling agents
with different water solubilities are unlikely to achieve the same effect in a
confection.
[0013] In the present confections, a desirable balance between mouth cooling
and
throat cooling can be attained when the confection comprises, based on the
total weight of the
confection, about 0.03 to about 0.4 weight percent N-ethyl-2,2-
diisopropylbutanamide and
3
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
about 0.005 to about 0.5 weight percent of monomenthyl glutarate, N-ethyl-p-
menthane-3-
carboxamide, or a combination thereof Within the range of about 0.005 to about
0.5 weight
percent, the amount of monomenthyl glutarate, N-ethyl-p-menthane-3-carboxamide
can be
about 0.02 to about 0.3 weight percent, specifically about 0.04 to about 0.2
weight percent,
more specifically about 0.08 to about 0.16 weight percent.
[0014] Combinations of N-ethyl-2,2-diisopropylbutanamide with other
physiological
cooling agents are possible. Besides monomenthyl glutarate and N-ethyl-p-
menthane-3-
carboxamide, other physiological cooling agents that can be combined with N-
ethy1-2,2-
diisopropylbutanamide include N-(2-hydroxyethyl)-2-isopropy1-2,3-
dimethylbutanamide,
N-(3-ethoxypropy1)-2-isopropy1-2,3-dimethylbutanamide, N-(3-propoxypropy1)-2-
isopropy1-
2,3-dimethylbutanamide, N-(3-butoxypropy1)-2-isopropy1-2,3-dimethylbutanamide,
N-ethyl-
p-menthane-3-carboxamide (WS-3), the ethyl ester of N-[[5-methy1-2-(1-
methylethyl)cyclohexyl]carbonyl]glycine (ethyl 3-(p-menthane-3-
carboxamido)acetate; WS-
5), N-(1,1-dimethy1-2-hydroxyethyl)-2,2-diethylbutanamide, isopulegol,
3-(L-menthoxy)propane-1,2-diol, 3-(L-menthoxy)-2-methylpropane-1,2-diol,
menthane diols
such as p-menthane-2,3-diol and p-menthane-3,8-diol, 6-isopropy1-9-methy1-1,4-
dioxaspiro[4,5]decane-2-methanol, menthyl succinate and its alkaline earth
metal salts,
trimethylcyclohexanol, N-ethyl-2-isopropy1-5-methylcyclohexanecarboxamide,
Japanese
mint oil, peppermint oil, menthone, isomenthone, menthone glycerol ketals,
menthyl lactate,
3-(L-menthoxy)ethan-1-ol, 3-(L-menthoxy)propan-1-ol, 3-(L-menthoxy)butan-1-ol,
L-menthylacetic acid N-ethylamide, L-menthyl-4-hydroxypentanoate,
L-menthyl-3-hydroxybutyrate, N,2,3-trimethy1-2-(1-methylethyl)-butanamide, N-
ethyl-trans-
2-cis-6-nonadienamide, N,N-dimethyl menthyl succinamide, menthyl pyrrolidone
carboxylate, xylitol, erythritol, menthane, menthone ketals, substituted p-
menthanes, acyclic
carboxamides, substituted cyclohexanamides, substituted cyclohexane
carboxamides,
substituted menthanols, hydroxymethyl derivatives of p-menthane,
2-mercapto-cyclodecanone, 2-isopropy1-5-methylcyclohexanol, cyclohexanamides,
menthyl
acetate, menthyl salicylate, N,2,3-trimethy1-2-isopropylbutanamide (WS-23),
icilin, camphor,
borneol, eucalyptus oil, peppermint oil, bornyl acetate, lavender oil, wasabi
extracts,
horseradish extracts, 3,1-menthoxypropane 1,2-diol, and the like, and
combinations thereof
These and other suitable cooling agents are further described in, for example,
U.S. Patent
Nos. 4,032,661 and 4,230,688 of Rowsell et al., 4,459,425 to Amano et al.,
4,136,163 to
Watson et al., 5,266,592 to Grub et al., 6,627,233 to Wolf et al., and
7,030,273 to Sun.
4
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
[0015] The confection can, optionally, further comprise menthol in an amount
of
about 0.01 to about 0.2 weight percent, specifically about 0.02 to about 0.15
weight percent,
more specifically about 0.05 to about 0.13 weight percent, based on the total
weight of the
confection.
[0016] In some embodiments, the confection further comprises eucalyptus oil in
an
amount of about 0.001 to about 0.02 weight percent, specifically about 0.002
to about 0.015
weight percent, more specifically about 0.003 to about 0.01 weight percent,
based on the total
weight of the confection.
[0017] Another embodiment is a confection, comprising: at least 80 weight
percent
of a confectionery base; about 0.005 to about 0.5 weight percent of
monomenthyl glutarate,
N-ethyl-p-menthane-3-carboxamide, or a combination thereof; and about 0.03 to
about 0.4
weight percent of N-ethyl-2,2-diisopropylbutanamide; wherein all weight
percents are based
on the total weight of the confection. Within the limitation of at least 80
weight percent, the
amount of confectionery based can be at least 85 weight percent, specifically
at least 90
weight percent, more specifically at least 95 weight percent. Within the range
of about 0.005
to about 0.5 weight percent, the amount of monomenthyl glutarate and/or N-
ethyl-p-
menthane-3-carboxamide can be about 0.02 to about 0.3 weight percent,
specifically about
0.04 to about 0.2 weight percent, more specifically about 0.08 to about 0.16
weight percent.
Within the range of about 0.03 to about 0.4 weight percent, the amount of N-
ethy1-2,2-
diisopropylbutanamide can be about 0.05 to about 0.25, specifically about 0.06
to about 0.12
weight percent. The confection can be hard candy confection, a soft candy
confection, a
center-filled confection, a jelly, or a film. As used herein, the term "hard
candy" is
synonymous with "hard boiled candy" and "high boiled candy," and includes all
confectionery compositions wherein the saccharide component(s) are heated to
temperatures
high enough to remove most of their moisture or where saccharide component(s)
are blended
without cooking such that the finished product water content is less than or
equal to five
weight percent.
[0018] The confection can, optionally, further comprise menthol and/or
eucalyptus
oil. When present, the amount of menthol is about 0.01 to about 0.2 weight
percent,
specifically about 0.02 to about 0.15 weight percent, more specifically about
0.05 to about
0.13 weight percent. When present, the amount of eucalyptus oil is about 0.001
to about 0.02
weight percent, specifically about 0.002 to about 0.015 weight percent,
specifically about
0.003 to about 0.01 weight percent.
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
[0019] The confection can be a solid mass of uniform composition.
Alternatively, the
confection can comprise a center filling that is softer than the candy shell
that surrounds it.
For example the confection can be a center-filled confection comprising a
shell and a filling
partially or fully surrounded by the shell. A cross sectional view of a
representative
center-filled confection is presented in the Figure, where the center-filled
confection 1
comprises a shell 2 surrounding a center filling 3. In some embodiments, the
confection
comprises about 60 to about 95 weight percent, specifically about 70 to about
92 weight
percent, of the shell, and about 5 to about 40 weight percent, specifically
about 8 to about 30
weight percent, of the center filling. The N-ethyl-2,2-diisopropylbutanamide
can be included
in the shell, the center filling, or both.
[0020] Some embodiments are directed to center-filled products, such as center-
filled
candy. Such products generally include a center-fill region and a region that
at least partially
surrounds the center-fill region. The region that at least partially surrounds
the center-fill
region can be a hard candy or soft candy composition. Suitable candy, or
confectionery,
compositions are described above.
[0021] The center-fill region in some embodiments can be a liquid, solid or
semi-
solid, gas, or the like. For example, in some embodiments, the center-fill
region can be a
powdered confectionery composition. Center-fill compositions can include any
of the
sweeteners, flavors, cooling agents, coloring agents and the like described
above.
[0022] In some embodiments, the center-fill region can be substantially or
completely
filled with the liquid, solid, semi-solid or gaseous center-fill composition.
In some other
embodiments, the center-fill region can be only partially filled with the
liquid, solid, semi-
solid or gaseous center-fill composition.
[0023] In some embodiments, the center-fill region can include two or more
center-
fill compositions. The two or more center-fill compositions can be the same or
different
forms. For example, some embodiments can contain a mixture of two or more
distinct
liquids. Similarly, some embodiments can contain two or more distinct solids,
semi-solids, or
gases in the center-fill region. Mixtures of different center-fill forms also
can be included in
some embodiments. For example, a liquid and a solid can be included in the
center-fill
region. The two or more liquids, solids, semi-solids and/or gases employed in
the center-fill
region can be included in the same or different amounts and can have similar
or distinct
characteristics. More specifically, in some embodiments, the two or more
center-fill
compositions can differ in a variety of characteristics, such as, viscosity,
color, flavor, taste,
texture, sensation, ingredient components, functional components, sweeteners,
or the like.
6
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
[0024] In some embodiments, the center-fill composition can include a
viscosity
modifier. Suitable viscosity modifiers include, for example, glycerin,
lecithin, medium chain
triglycerides, and mixtures thereof.
[0025] In some embodiments, the center-fill composition also can include non-
liquid
components, such as, for example, flavor beads, fruit particles, nut
particles, flavor particles,
gelatin beads or portions, and the like.
[0026] In some embodiments, the sensate composition including N-ethyl-2,2-
diisopropylbutanamide can be present in the center-fill region, the shell
region, which at least
partially surrounds the center-fill region, or both regions. Some embodiments
can include a
first cooling composition in the center-fill region and a second cooling
composition in the
candy or gum region. The second cooling composition can be the same as or
different from
the first.
[0027] Some center-fill embodiments optionally can include a third, or
coating,
region. In some embodiments, the coating also can be referred to as the
"outermost region" of
the product. The coating can at least partially surround the shell region. The
coating can be
any conventional sugar or sugarless coating, which forms an exterior surface
on the center-
filled product.
[0028] A variety of coating processes are known. In some embodiments, a hard
coating is applied in numerous thin layers of material in order to form an
appropriate uniform
coated and finished quality surface on the products. The coating material,
which can include
sugar, maltitol, sorbitol, or any other polyol, including those described
herein, and optionally
flavoring, is sprayed onto the candy pieces as they pass through a coating
mechanism or a
coating tunnel and are tumbled and rotated therein. In addition, conditioned
air is circulated
or forced into the coating tunnel or mechanism in order to dry each of the
successive coating
layers on the formed products. In some embodiments, the coating, or outermost
region, can
be formed by lamination, dual or multiple extrusion, or any other process that
creates an
outermost region.
[0029] In addition to hard coatings, other types of coatings can include
gumming or
glazing, soft coating, smoothing, frosting, sanding, and wet crystallization.
These coating
processes are described in more detail in U.S. Patent No. 5,527,542 to
Serpelloni et al.
Further, the exterior or outermost region can be a lipid material such as an
oil. This lipid
material can be applied to the product by any suitable means.
[0030] The center-fill can be a powder center filling. For example, in some
embodiments, the shell comprises a hard candy shell composition comprising,
based on the
7
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
total weight of the confection composition, at least 80 weight percent of a
confectionery base;
about 0.005 to about 0.5 weight percent of monomenthyl glutarate, N-ethyl-p-
menthane-3-
carboxamide, or a combination thereof; and about 0.03 to about 0.4 weight
percent of N-
ethy1-2,2-diisopropylbutanamide; and the center filling comprises a powder
center filling
composition comprising, based on the total weight of the fluid center filling
composition,
about 90 to about 99.99 weight percent of a powdered confectionery base; about
0.5 to about
25 weight percent of a powder flow agent selected from the group consisting of
powdered
cellulose, magnesium stearate, stearic acid, paraffin and microcrystalline
waxes, polyethylene
waxes, mineral and other lubricating oils, talc, silicone dioxide, lactose,
calcium citrate, and
mixtures thereof; about 0.005 to about 0.5 weight percent of monomenthyl
glutarate, N-ethyl-
p-menthane-3-carboxamide, or a combination thereof; and about 0.03 to about
0.4 weight
percent of N-ethyl-2,2-diisopropylbutanamide. In some embodiments, the hard
candy
composition, the fluid center filling composition, or both comprises
monomenthyl glutarate.
In some embodiments, the hard candy composition, the fluid center filling
composition, or
both comprise N-ethyl-p-menthane-3-carboxamide.
[0031] In some embodiments, the center-fill is a fluid center filling that is
softer than
the shell, and it can be soft enough to flow at room temperature. For example,
in some
embodiments, the fluid center filling has a viscosity of about 10 to about
200,000 centipoise,
specifically about 100 to about 50,000 centipoise, at 25 C. The fluid center
filling
composition typically has a water content of about 7 to 13 weight percent,
based on the
weight of the fluid center filling composition. The fluid center filling
composition also can
have about 5 to about 20 weight percent of a viscosity modifier, such as
glycerin, lecithin,
medium chain triglycerides, and mixtures thereof
[0032] In some embodiments of the center-filled confection, the shell
comprises a
hard candy shell composition comprising, based on the total weight of the
confection
composition, at least 80 weight percent (specifically at least 85 weight
percent, more
specifically at least 90 weight percent, still more specifically at least 95
weight percent) of a
confectionery base; about 0.005 to about 0.5 weight percent (specifically
about 0.02 to about
0.3 weight percent, more specifically about 0.04 to about 0.2 weight percent,
still more
specifically about 0.08 to about 0.16 weight percent) of monomenthyl
glutarate, N-ethyl-p-
menthane-3-carboxamide, or a combination thereof; and about 0.03 to about 0.4
weight
percent (specifically about 0.05 to about 0.25 weight percent, more
specifically about 0.06 to
about 0.12 weight percent) of N-ethyl-2,2-diisopropylbutanamide; and the
center filling
comprises a fluid center filling composition comprising, based on the total
weight of the fluid
8
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
center filling composition, about 70 to about 95 weight percent of a
confectionery base; about
to about 20 weight percent of a viscosity modifier selected from the group
consisting of
glycerin, lecithin, medium chain triglycerides, and mixtures thereof; about
0.005 to about 0.5
weight percent (specifically about 0.02 to about 0.3 weight percent, more
specifically about
0.04 to about 0.2 weight percent, even more specifically about 0.08 to about
0.16 weight
percent) of monomenthyl glutarate, N-ethyl-p-menthane-3-carboxamide, or a
combination
thereof; and about 0.03 to about 0.4 weight percent (specifically about 0.05
to about 0.25
weight percent, more specifically about 0.06 to about 0.12 weight percent) of
N-ethy1-2,2-
diisopropylbutanamide.
[0033] In some embodiments, the hard candy composition, the fluid center
filling
composition, or both comprises monomenthyl glutarate. In some embodiments, the
hard
candy composition, the fluid center filling composition, or both comprise N-
ethyl-p-
menthane-3 -carboxamide.
[0034] In a very specific embodiment of the center-filled confection, it
comprises,
based on the total weight of the center-filled confection, about 70 to about
95 weight percent
of the hard candy shell and about 5 to about 30 weight percent of the fluid
center filling; the
hard candy shell composition comprises, based on the total weight of the hard
candy shell
composition, about 90 to about 99.5 weight percent of the confectionery base,
about 0.08 to
about 0.16 weight percent monomenthyl glutarate, about 0.06 to about 0.12
weight percent
N-ethyl-2,2-diisopropylbutanamide, and about 0.05 to about 0.13 weight percent
menthol; the
fluid center filling comprises a fluid center filling composition comprising,
based on the total
weight of the fluid center filling, about 75 to about 90 weight percent
confectionery base,
about 10 to about 17 weight percent glycerin, about 0.08 to about 0.16 weight
percent
monomenthyl glutarate, about 0.06 to about 0.12 weight percent N-ethy1-2,2-
diisopropylbutanamide, and about 0.02 to about 0.07 weight percent menthol.
[0035] The confection can, optionally, further include a taste potentiator.
Taste
potentiators are substances capable of reducing or eliminating undesirable
tastes in edible
substances. Taste potentiators can also serve to enhance desirable tastes in
edible substances
such as sweetness potentiators that increase sweetness intensity. In the
context of cooling
agents, taste potentiators can be effective to reduce or eliminate bitterness,
undesired
mintiness, or other undesired taste. The taste potentiator compositions can
have controlled-
release properties. The taste potentiator can work synergistically with the
cooling agent to
enhance the perception of the cooling agent. In some embodiments, delivery of
a sweetener
in combination with a taste potentiator can enhance the sweet taste upon
consumption of the
9
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
composition. The incorporation of the potentiator, therefore, can allow for
reduced amounts
of cooling agent and/or sweetener without compromising the levels of cooling
and sweetness
provided by the composition.
[0036] Any of a variety of substances that function as taste potentiators can
be
employed. For instance, suitable taste potentiators include water-soluble
taste potentiators,
such as neohesperidin dihydrochalcone, chlorogenic acid, alapyridaine,
cynarin, miraculin,
glupyridaine, pyridinium-betain compounds, glutamates, such as monosodium
glutamate and
monopotassium glutamate, neotame, thaumatin, tagatose, trehalose, salts, such
as sodium
chloride, monoammonium glycyrrhizinate, vanilla extract (in ethyl alcohol),
water-soluble
sugar acids, potassium chloride, sodium acid sulfate, water-soluble hydrolyzed
vegetable
proteins, water-soluble hydrolyzed animal proteins, water-soluble yeast
extracts, adenosine
monophosphate (AMP), glutathione, water-soluble nucleotides, such as inosine
monophosphate, disodium inosinate, xanthosine monophosphate, guanylate
monophosphate,
alapyridaine (N-(1-carboxyethyl)-6-(hydroxymethyl)pyridinium-3-ol inner salt,
sugar beet
extract (alcoholic extract), sugarcane leaf essence (alcoholic extract),
curculin, strogin,
mabinlin, gymnemic acid, 2-hydroxybenzoic acid (2-HB), 3-hydroxybenzoic acid
(3-HB),
4-hydroxybenzoic acid (4-HB), 2,3-dihydroxybenzoic acid (2,3-DHB), 2,4-
dihydroxybenzoic
acid (2,4-DHB), 2,5-dihydroxybenzoic acid (2,5-DHB), 2,6-dihydroxybenzoic acid
(2,6-
DHB), 3,4-dihydroxybenzoic acid (3,4-DHB), 3,5-dihydroxybenzoic acid (3,5-
DHB), 2,3,4-
trihydroxybenzoic acid (2,3,4-THB), 2,4,6-trihydroxybenzoic acid (2,4,6-THB),
3,4,5-
trihydroxybenzoic acid (3,4,5-THB), 4-hydroxyphenylacetic acid, 2-
hydroxyisocaproic acid,
3-hydroxycinnamic acid, 3-aminobenzoic acid, 4-aminobenzoic acid, 4-
methoxysalicylic
acid, and combinations thereof
[0037] Other suitable taste potentiators are substantially or completely
insoluble in
water, such as citrus aurantium, vanilla oleoresin, water insoluble sugar
acids, water insoluble
hydrolyzed vegetable proteins, water insoluble hydrolyzed animal proteins,
water insoluble
yeast extracts, insoluble nucleotides, sugarcane leaf essence. and
combinations thereof
Some other suitable taste potentiators include substances that are slightly
soluble in water,
such as maltol, ethyl maltol, vanillin, slightly water-soluble sugar acids,
slightly water-
soluble hydrolyzed vegetable proteins, slightly water-soluble hydrolyzed
animal proteins,
slightly water-soluble yeast extracts, slightly water-soluble nucleotides and
combinations
thereof
[0038] As mentioned above, sweetener potentiators, which are a type of taste
potentiator, enhance the taste of sweetness. Exemplary sweetener potentiators
include
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
monoammonium glycyrrhizinate, licorice glycyrrhizinates, citrus aurantium,
alapyridaine,
alapyridaine (N-(1-carboxyethyl)-6-(hydroxymethyl)pyridinium-3-ol) inner salt,
miraculin,
curculin, strogin, mabinlin, gymnemic acid, cynarin, glupyridaine, pyridinium-
betain
compounds, sugar beet extract, neotame, thaumatin, neohesperidin
dihydrochalcone, tagatose,
trehalose, maltol, ethyl maltol, vanilla extract, vanilla oleoresin, vanillin,
sugar beet extract
(alcoholic extract), sugarcane leaf essence (alcoholic extract), compounds
that respond to G-
protein coupled receptors (T2Rs and T1Rs, 2-hydroxybenzoic acid (2-HB), 3-
hydroxybenzoic acid (3-HB), 4-hydroxybenzoic acid (4-HB), 2,3-dihydroxybenzoic
acid
(2,3-DHB), 2,4-dihydroxybenzoic acid (2,4-DHB), 2,5-dihydroxybenzoic acid (2,5-
DHB),
2,6-dihydroxybenzoic acid (2,6-DHB), 3,4-dihydroxybenzoic acid (3,4-DHB),
3,5-dihydroxybenzoic acid (3,5-DHB), 2,3,4-trihydroxybenzoic acid (2,3,4-THB),
2,4,6-
trihydroxybenzoic acid (2,4,6-THB), 3,4,5-trihydroxybenzoic acid (3,4,5-THB),
4-
hydroxyphenylacetic acid, 2-hydroxyisocaproic acid, 3-hydroxycinnamic acid,
3-aminobenzoic acid, 4-aminobenzoic acid, 4-methoxysalicylic acid and
combinations
thereof
[0039] The sensation of warming or cooling effects can also be prolonged with
the
use of a hydrophobic sweetener as described in U.S. Patent Publication No.
2003/0072842
Al of Johnson et al. For example, such hydrophobic sweeteners include those of
the
formulae I-XI as set forth below:
(DI 1
. x
0
OH
ZY
wherein X, Y and Z are independently at each occurrence selected from the
group consisting
of CH2, 0 and S;
ci)I "
. x
OH
Y
11
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
wherein X and Y are independently at each occurrence selected from the group
consisting of
S and 0;
In
R
R2 I. X
ZY le
R1
wherein X is S or 0; Y is 0 or CH2; Z is CH2, SO2 or S; R is OCH3, OH or H; Rl
is SH or
OH and R2 is H or OH;
0 OH IV
0
ll
R1
wherein X is C or S; R is OH or H and Rl is OCH3 or OH;
v
o
R1
R R2
WI OH
R3 o
wherein R, R2 and R3 are OH or H and Rl is H or COOH;
12
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
VI
0
R .
401 X OH
wherein X is 0 or CH2 and R is COOH or H;
0 OH vii
0
SIR
wherein R is CH3CH2, OH, N (CH3)2 or Cl;
o VIII
O*
01 /
OH
0 ;
IX
0 OH 0
0 0
I. o
,
13
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
X
0 OH
0
0 ; and
0 ONa XI
0
I.
[0040] Perillartine also can be added as described in U.S. Patent No.
6,159,509 to
Johnson et al.
[0041] Any of the above-listed taste potentiators can be used alone or in
combination.
[0042] Some embodiments include two or more taste potentiators that act
synergistically with one another. For instance, in some embodiments, a
sweetener potentiator
composition can be provided, which includes two or more sweetener potentiators
that act
synergistically with one another. The sweetener potentiator composition can
enhance the
sweetness of products into which it is incorporated by reducing the amount of
sucrose needed
to provide a sweetness intensity equivalent to sucrose. The sweetness
enhancing effect of the
combination of sweetener potentiators can be greater than the effect of either
compound used
individually.
[0043] Additional taste potentiators include those described, for example, in
U.S.
Patent Nos. 5,631,038 and 6,008,250 to Kurtz et al. In some embodiments, the
taste
potentiator can comprise 3-hydroxybenzoic acid and a dihydroxybenzoic acid
selected from
the group consisting of 2,4-dihydroxybenzoic acid, 3,4-dihydroxybenzoic acid,
and
combinations thereof Comestible salts, such as sodium, potassium salts,
calcium,
magnesium, and ammonium salts, can be substituted for the free acids in these
potentiator
combinations.
[0044] The confectionery base typically contributes sweetness to the
confection.
Additional sweeteners, specifically high-intensity sweeteners, can also be
used. High-
14
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
intensity sweeteners include, for example, the potassium salt of
3,4-dihydro-6-methy1-1,2,3-oxathiazine-4-one-2,2-dioxide, L-aspartyl-L-
phenylalanine
methyl ester, L-alpha-aspartyl-N-(2,2,4,4-tetramethy1-3-thietany1)-D-
alaninamide hydrate,
N4N-(3,3-dimethylbuty1)-L-aspartyl]-L-phenylalanine 1-methyl ester,
chlorinated derivatives
of sucrose, thaumatin, monatin, mogrosides, rebaudiosides (including
Rebaudioside A), and
combinations thereof
[0045] The confection can, optionally, further include a flavorant. Suitable
flavorants
include artificial or natural flavors known in the art, for example synthetic
flavor oils, natural
flavoring aromatics and/or oils, oleoresins, extracts derived from plants,
leaves, flowers,
fruits, and the like, and combinations thereof Nonlimiting representative
flavors include oils
such as spearmint oil, cinnamon oil, oil of wintergreen (methyl salicylate),
peppermint oil,
clove oil, bay oil, anise oil, eucalyptus oil, thyme oil, cedar leaf oil, oil
of nutmeg, allspice,
oil of sage, mace, oil of bitter almonds, cassia oil, citrus oils including
lemon, orange, lime,
grapefruit, vanilla, fruit essences, including apple, pear, peach, grape,
strawberry, raspberry,
blackberry, cherry, plum, pineapple, apricot, banana, melon, tropical fruit,
mango,
mangosteen, pomegranate, papaya, and honey lemon essences, and the like, and
combinations
thereof Other types of flavorants can include various aldehydes and esters
such as cinnamyl
acetate, cinnamaldehyde, citral diethylacetal, dihydrocarvyl acetate, eugenyl
formate,
p-methylamisol, acetaldehyde (apple), benzaldehyde (cherry, almond), anisic
aldehyde
(licorice, anise), cinnamic aldehyde (cinnamon), citral, i.e., alpha-citral
(lemon, lime), neral,
i.e., beta-citral (lemon, lime), decanal (orange, lemon), ethyl vanillin
(vanilla, cream),
heliotrope, i.e., piperonal (vanilla, cream), vanillin (vanilla, cream), alpha-
amyl
cinnamaldehyde (spicy fruity flavors), butyraldehyde (butter, cheese),
valeraldehyde (butter,
cheese), citronellal (modifies, many types), decanal (citrus fruits), aldehyde
C-8 (citrus
fruits), aldehyde C-9 (citrus fruits), aldehyde C-12 (citrus fruits), 2-ethyl
butyraldehyde
(berry fruits), hexenal, i.e., trans-2-hexenal (berry fruits), tolyl aldehyde
(cherry, almond),
veratraldehyde (vanilla), 2,6-dimethy1-5-heptenal, i.e., melonal (melon), 2,6-
dimethyloctanal
(green fruit), and 2-dodecenal (citrus, mandarin). Flavorants can be used in
liquid or solid
form. When used in solid (dry) form, suitable drying means such as spray
drying the oil can
be used.
[0046] In the confection, the physiological cooling agent(s) can, optionally,
be
combined with a warming agent. Warming agents include a wide variety of
compounds
known to provide the sensory signal of warming to the user. These compounds
offer the
perceived sensation of warmth, particularly in the oral cavity, and often
enhance the
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
perception of flavors, sweeteners and other organoleptic components. Suitable
warming
agents include vanillyl alcohol n-butylether (TK-1000) supplied by Takasago
Perfumary
Company Limited, Tokyo, Japan, vanillyl alcohol n-propylether, vanillyl
alcohol
isopropylether, vanillyl alcohol isobutylether, vanillyl alcohol n-aminoether,
vanillyl alcohol
isoamyl ether, vanillyl alcohol n-hexyl ether, vanillyl alcohol methyl ether,
vanillyl alcohol
ethyl ether, gingerol, shogaol, paradol, zingerone, capsaicin,
dihydrocapsaicin,
nordihydrocapsaicin, homocapsaicin, homodihydrocapsaicin, ethanol, isopropyl
alcohol,
isoamyl alcohol, benzyl alcohol, glycerine, and combinations thereof In some
embodiments,
a warming agent and a cooling agent can be incorporated into spatially
distinct regions of the
confection.
[0047] The confection can, optionally, further include a breath freshener.
Breath
fresheners can include zinc citrate, zinc acetate, zinc fluoride, zinc
ammonium sulfate, zinc
bromide, zinc iodide, zinc chloride, zinc nitrate, zinc fluorosilicate, zinc
gluconate, zinc
tartarate, zinc succinate, zinc formate, zinc chromate, zinc phenol sulfonate,
zinc dithionate,
zinc sulfate, silver nitrate, zinc salicylate, zinc glycerophosphate, copper
nitrate, chlorophyll,
copper chlorophyll, chlorophyllin, hydrogenated cottonseed oil, chlorine
dioxide, beta
cyclodextrin, zeolite, silica-based material, carbon-based material, enzymes
such as laccase,
and combinations thereof Breath fresheners can include essential oils as well
as various
aldehydes and alcohols. Essential oils used as breath fresheners can include
oils of
spearmint, peppermint, wintergreen, sassafras, chlorophyll, citral, geraniol,
cardamom, clove,
sage, carvacrol, eucalyptus, cardamom, magnolia bark extract, marjoram,
cinnamon, lemon,
lime, grapefruit, orange, and combinations thereof Aldehydes such as cinnamic
aldehyde
and salicylaldehyde can be used. Additionally, chemicals such as carvone, iso-
garrigol, and
anethole can function as breath fresheners.
[0048] The confection can, optionally, further include a mouth moistener.
Suitable
mouth moisteners can include saliva stimulators such as acids and salts
including acetic acid,
adipic acid, ascorbic acid, butyric acid, citric acid, formic acid, fumaric
acid, glyconic acid,
lactic acid, phosphoric acid, malic acid, oxalic acid, succinic acid, and
tartaric acid. Mouth
moisteners can also include hydrocolloid materials that hydrate and can adhere
to oral surface
to provide a sensation of mouth moistening. Hydrocolloid materials can include
naturally
occurring materials such as plant exudates, seed gums, and seaweed extracts or
they can be
chemically modified materials such as cellulose, starch, or natural gum
derivatives.
Furthermore, hydrocolloid materials can include pectin, gum arabic, acacia
gum, alginates,
agar, carageenans, guar gum, xanthan gum, locust bean gum, gelatin, gellan
gum,
16
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
galactomannans, tragacanth gum, karaya gum, curdlan, konjac, chitosan,
xyloglucan, beta
glucan, furcellaran, gum ghatti, tamarin, and bacterial gums. Mouth moisteners
can include
modified natural gums such as propylene glycol alginate, carboxymethyl locust
bean gum,
low methoxyl pectin, and combinations thereof Modified celluloses can be
included such as
microcrystalline cellulose, carboxymethylcellulose (CMC), methylcellulose
(MC),
hydroxypropylmethylcellulose (HPCM), hydroxypropylcellulose (HPC), and
combinations
thereof
[0049] Coloring agents can be used to produce a desired color for the
confection.
Suitable coloring agents include pigments and natural food colors and dyes
suitable for food,
drug, and cosmetic applications. Suitable food colors include annatto extract
(E160b), bixin,
norbixin, astaxanthin, dehydrated beets (beet powder), beetroot red/betanin
(E162),
ultramarine blue, canthaxanthin (El 61g), cryptoxanthin (E16 1 c), rubixanthin
(E161d),
violanxanthin (E 161e), rhodoxanthin (E161f), caramel (E150(a-d)), beta-apo-8-
carotenal
(E160e), carotene (E160a), alpha carotene, gamma carotene, ethyl ester of beta-
apo-8-
carotenal (E 160f), flavoxanthin (E161a), lutein (E161b), cochineal extract
(E120), carmine
(E132), carmoisine/azorubine (E 122), sodium copper chlorophyllin (E 141),
chlorophyll
(E140), toasted partially defatted cooked cottonseed flour, ferrous gluconate,
ferrous lactate,
grape color extract, grape skin extract (enocianina), anthocyanins (E163),
haematococcus
algae meal, synthetic iron oxide, iron oxides and hydroxides (E172), fruit
juice, vegetable
juice, dried algae meal, tagetes (Aztec marigold) meal and extract, carrot
oil, corn endosperm
oil, paprika, paprika oleoresin, phaffia yeast, riboflavin (E101), saffron,
titanium dioxide,
turmeric (E100), turmeric oleoresin, amaranth (E123), capsanthin/capsorbin
(E160c),
lycopene (E160d), FD&C blue #1, FD&C blue #2, FD&C green #3, FD&C red #3, FD&C
red #40, FD&C yellow #5 and FD&C yellow #6, tartrazine (E102), quinoline
yellow (E104),
sunset yellow (E110), ponceau (E124), erythrosine (E127), patent blue V
(E131), titanium
dioxide (E171), aluminum (E173), silver (E174), gold (E175), pigment
rubine/lithol rubine
BK (E180), calcium carbonate (E170), carbon black (E153), black PN/brilliant
black BN
(E151), green S/acid brilliant green BS (E142), and combinations thereof In
some
embodiments, certified colors can include FD&C aluminum lakes, and
combinations thereof
[0050] The confection can, optionally, further include an acidulant. Suitable
acidulants include acetic, citric, fumaric, hydrochloric, lactic, and nitric
acids as well as
sodium citrate, sodium bicarbonate and carbonate, sodium or potassium
phosphate and
magnesium oxide, potassium metaphosphate, sodium acetate, and combinations
thereof
17
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
[0051] The confection can, optionally, further include a buffering agent.
Exemplary
buffering agents can include sodium bicarbonate, sodium phosphate, sodium
hydroxide,
ammonium hydroxide, potassium hydroxide, sodium stannate, triethanolamine,
citric acid,
hydrochloric acid, sodium citrate, and combinations thereof
[0052] The confection can, optionally, further include an antioxidant.
Antioxidants
include butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), propyl
gallate,
and combinations thereof
[0053] The confection can, optionally, further include a nutraceutical.
Suitable
nutraceuticals include herbs and botanicals such as aloe, bilberry, bloodroot,
calendula,
capsicum, chamomile, cat's claw, echinacea, garlic, ginger, ginkgo,
goldenseal, various
ginseng, green tea, guarana, kava kava, lutein, nettle, passionflower,
rosemary, saw palmetto,
St. John's wort, thyme, and valerian. Also included are mineral supplements
such as calcium,
copper, iodine, iron, magnesium, manganese, molybdenum, phosphorous, zinc, and
selenium.
Other nutraceuticals can include fructooligosaccharides, glucosamine,
grapeseed extract, cola
extract, guarana, ephedra, inulin, phytosterols, phytochemicals, catechins,
epicatechin,
epicatechin gallate, epigallocatechin, epigallocatechin gallate, isoflavones,
lecithin, lycopene,
oligofructose, polyphenols, flavanoids, flavanols, flavonols, and psyllium as
well as weight
loss agents such as chromium picolinate and phenylpropanolamine. Exemplary
vitamins and
co-enzymes include water or fat soluble vitamins such as thiamin, riboflavin,
nicotinic acid,
pyridoxine, pantothenic acid, biotin, folic acid, flavin, choline, inositol
and para-
aminobenzoic acid, carnitine, vitamin C, vitamin D and its analogs, vitamin A
and the
carotenoids, retinoic acid, vitamin E, vitamin K, vitamin B6, and vitamin B12.
Combinations
of the foregoing nutraceuticals can be used.
[0054] The confection can, optionally, further include a medicament. Suitable
medicaments can include oral care agents, throat care agents, allergy relief
agents, and
general medical care agents.
[0055] Suitable oral care agents include breath fresheners, tooth whiteners,
antimicrobial agents, tooth mineralizers, tooth decay inhibitors, topical
anesthetics,
mucoprotectants, stain removers, oral cleaning, bleaching agents,
desensitizing agents, dental
remineralization agents, antibacterial agents, anticaries agents, plaque acid
buffering agents,
surfactants, and anticalculus agents, and combinations thereof Examples of
such ingredients
include hydrolytic agents such as proteolytic enzymes, abrasives such as
hydrated silica,
calcium carbonate, sodium bicarbonate and alumina, other active stain-removing
components
such as surface-active agents, including anionic surfactants such as sodium
stearate, sodium
18
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
palmitate, sulfated butyl oleate, sodium oleate, salts of fumarie acid,
glycerol, hydroxylated
lecithin, sodium lauryl sulfate and chelators such as polyphosphates, which
are typically
employed as tartar control ingredients. Oral care agents also include
tetrasodium
pyrophosphate and sodium tri-polyphosphate, sodium bicarbonate, sodium acid
pyrophosphate, sodium tripolyphosphate, xylitol, sodium hexametaphosphate,
peroxides such
as carbamide peroxide, calcium peroxide, magnesium peroxide, sodium peroxide,
hydrogen
peroxide, and peroxydiphospate.
[0056] In addition, oral care ingredients also include antibacterial agents
comprising
triclosan, chlorhexidine, zinc citrate, silver nitrate, copper, limonene,
cetyl pyridinium
chloride, and combinations thereof
[0057] Anticaries agents include fluoride ions, fluorine-providing components
(e.g.,
inorganic fluoride salts), soluble alkali metal salts (e.g., sodium fluoride,
potassium fluoride,
sodium fluorosilicate, ammonium fluorosilicate, potassium fluoride, sodium
monofluorophosphate), and tin fluorides, (e.g., stannous fluoride and stannous
chloride,
potassium stannous fluoride (SnF2-KF), sodium hexafluorostannate, stannous
chlorofluoride),
and combinations thereof
[0058] Lubricants also can be added in some embodiments to improve the
smoothness of the comestible, such as, for example hard candy embodiments.
Smoothness
also is a characteristic that leads to an increased perception of mouth-
moistening upon
consumption. Suitable lubricants include, for example, fats, oils, aloe vera,
pectin, and the
like, and combinations thereof
[0059] Similarly, in some embodiments, the comestible can have smooth edges.
In
such embodiments, the comestible can have any shape, such as square, circular
or diamond-
shaped, however, the edges are rounded to provide a smooth comestible. Another
manner of
lending smoothness to the comestibles is to deposit the comestible composition
into molds
during the manufacturing process. Accordingly, in some embodiments, the
comestible is
deposited, as described in more detail below.
[0060] Hard candy confectionery compositions can be prepared using
conventional
methods and equipments, such as fire cookers, vacuum cookers, and scraped-
surface cookers
(also referred to as high speed atmospheric cookers). When using a fire
cooker, the desired
quantity of confectionery base is dissolved in water by heating the agent in a
kettle until the
confectionery base dissolves. Additional confectionery base can then be added
and cooking
continued until a final temperature of, for example, 145 to 156 C is
achieved. The batch is
19
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
then cooled and worked as a plastic-like mass to incorporate additives
separately or in the
form of one or more concentrates or pre-mixes.
[0061] Center filling compositions can be prepared by blending the component,
optionally in the presence of heat, to form an intimate blend.
[0062] Methods of forming confections of uniform composition are known in the
art
and include forming a rope of the above-described plastic-like mass, and
dividing the rope
into individual confections of desire shape and weight.
[0063] Methods of forming center-filled confections are known in the art and
include
the co-deposition technique described in U.S. Patent No. 4,517,205 to Aldrich.
[0064] The invention is further illustrated by the following non-limiting
examples.
EXAMPLES 1 and 2
[0065] These experiments illustrate the surprising differences in
regioselectivity of
cooling exhibited by N-ethyl-2,2-diisopropylbutanamide and N,2,3-rimethy1-2-
isopropylbutanamide. These differences are particularly surprising given the
small difference
in the respective chemical structures, as noted above.
[0066] The cooling agents N-ethyl-2,2-diisopropylbutanamide and
N,2,3-trimethy1-2-isopropylbutanamide were evaluated in fondant at
concentrations of 0.03,
0.06, and 0.09 weight percent. The fondant base consisted of 85 weight percent
6X fondant
sugar and 15 weight percent deionized water.
[0067] Sensory evaluations of the cooling agent-containing fondants were
conducted
by six evaluators, who observed the location, intensity, and onset speed of
cooling sensation
as they tasted approximately 0.5 gram of fondant by allowing it to dissolve in
the mouth.
Results are summarized in Table 1. The results show that, relative to N,2,3-
trimethy1-2-
isopropylbutanamide, N-ethyl-2,2-diisopropylbutanamide provides superior and
unexpected
throat cooling when used in a candy system.
Table 1
Example 1 (N-ethyl-2,2- Example 2
diisopropylbutanamide) (N,2,3-trimethy1-2-
isopropylbutanamide)
Predominant mouth cooling No Yes
Long-lasting mouth cooling No No
Predominant throat cooling Yes No
Long-lasting throat cooling Yes No
CA 02749133 2011-07-06
WO 2010/083133 PCT/US2010/020685
EXAMPLES 3-6
[0068] These examples illustrate formulations of center-filled confections for
throat
soothing. The confections consist of a hard candy shell and a fluid center
filling.
[0069] Representative compositions for two hard candy shell compositions are
provided in Table 2, where all component amounts are expressed in weight
percent based on
the total hard candy shell composition.
[0070] Hard candy shell compositions are prepared by combining sweeteners
(e.g.,
bulk sweetener) as well as a solvent (e.g., water), in a mixing vessel for
about four to about
ten minutes to form a slurry. The slurry is heated to about 70 C to 120 C to
dissolve any
sweetener crystals or particles and form an aqueous solution. Once dissolved,
heat at
temperatures of about 135 C to 160 C and vacuum are applied to cook the batch
and boil off
water until a residual moisture of less than about 4% is achieved. The batch
changes from a
crystalline to an amorphous phase. The flavor agent, food-grade acid
composition and
optionally cooling agent(s) are then admixed in the batch by mechanical mixing
operations,
along with any other optional additives, such as coloring agents.
[0071] The batch is then cooled to about 100 C to 20 C to attain a semi-solid
or
plastic-like consistency. Once the candy mass has been properly tempered, it
ready for
forming into desired hard candy shell shapes having the correct weight and
dimensions.
[0072] The sucrose/glucose mixture illustrated by Examples 3 and 4 can be
partially
or completely replaced by hydrogenated isomaltulose, maltitol, hydrogenated
starch
hydrolysate, and mixtures thereof
Table 2 Hard Candy Shell Compositions
Component Example 3 Example 4
Sucrose 45 - 65 45 - 65
Glucose 40 - 55 40 - 55
Lemon Flavor 0.25 0
Strawberry Flavor 0 0.325
Menthol 0.089 0.089
Eucalyptus oil 0.0066 0.0066
Monomenthyl glutarate 0.125 0.125
N-Ethyl-2,2-diisopropylbutanamide 0.09 0.09
Lubricants 0.9998 0.9998
2 weight % aqueous solution of beta-carotene 0.15 0
FD&C Red 40 0 0.0019
50 weight % aqueous solution of citric acid 1.0 0.36
50 weight % aqueous solution of potassium citrate 0.336 0.12
monohydrate
Water 0.5-5 0.5-5
21
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
[0073] Representative compositions for two fluid center filling compositions
are
provided in Table 3, where all component amounts are expressed in weight
percent based on
the total fluid center filling composition. The fluid center filling
compositions are prepared
by blending all the components to form an intimate blend.
Table 3. Fluid Center Filling Compositions
Component Example 5 Example 6
High fructose glucose syrup 80-95 80-95
Glycerin 2-20 2-20
Sucrose 2-10 2-10
Lemon flavor 0.192 0
Strawberry flavor 0 0.25
Menthol 0.06 0.045
Monomenthyl glutarate 0.125 0.125
N-Ethyl-2,2-diisopropylbutanamide 0.09 0.09
[0074] The finished center-filled confections consist of about 83 to about 93
weight
percent of a hard candy shell according to one of the compositions of Table 2,
and about 7 to
about 17 weight percent of a fluid center filling according to one of the
compositions of Table
3.
EXAMPLES 7-21
[0075] These examples illustrate various center-fill compositions with fat
carriers
(Table 4; Examples 7-10), shell compositions (Table 5; Examples 11-17), and
coating
compositions (Table 6; Examples 18-21). Collectively, these examples can be
used to form
multi-region confectioner compositions. In Tables 4-6, component amounts are
expressed in
weight percent based on the total weight of the composition.
[0076] Multi-region chewy confectionery compositions including a filling
region, a
shell region, and optionally a coating region are prepared according to the
compositions in
Tables 4-6 with each region according to the corresponding components for
Examples 5-19.
[0077] To prepare the filling compositions, the powdered ingredients are
combined in
a mixing vessel and mixed until homogeneous. Next, the fat and emulsifier are
melted
together. The N-ethyl-2,2-diisopropylbutanamide and any other cooling
compounds are
solubilized in the flavor and added along with the acid to the melted
fat/emulsifier blend.
Once the fat mixture is homogeneously mixed, the powders are mixed in until
homogeneously dispersed.
22
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
[0078] To prepare the shell region, the sucrose with corn syrups and water or
polyol
(maltitol) and water are mixed to create a homogeneous mixture and the mixture
is cooked to
about 130 C. Separately, the gelatin is hydrated by mixing with a small amount
of water.
When the cooked mixture reaches about 90 C, the hydrated gelatin is added
along with fat,
emulsifier, color, high intensity sweetener(s) and acid and mixed thoroughly.
The mixture is
then placed on a cooling table where flavor (including N-ethyl-2,2-
diisopropylbutanamide
and any other cooling compounds solubilized in the flavor) and acid are added.
The mixture
is then pulled to a desired consistency before being fed into a process for
forming a multi-
region chewy confectionery product.
[0079] The shell and filling compositions are then extruded together and
formed into
any desired shape configuration. An optional coating composition as shown in
Table 6 can
be applied as described above in the section describing the coating region.
The confectionery
pieces can each have a total weight of approximately 2 to 10 grams. In the
final
confectionery pieces, the confectionery region is about 40-65% by weight, the
filling is about
5-45% by weight, and the coating is about 0-50% by weight.
Table 4. Filling Compositions with Fat Carriers
Ex. 7 Ex. 8 Ex. 9 Ex. 10
Dextrose powder 20-80 0 10-40 0
Erythritol powder 0 20-80 10-40 10-40
Xylitol powder 0 0 0 10-40
Hydrogenated vegetable fat 20-80 20-80 20-80 0
Cocoa fat 0 0 0 20-80
Emulsifier 0-5 0-5 0-5 0-5
Food acid 0-5 0-5 0-5 0-5
Flavor 0-5 0-5 0-5 0-5
N-Ethyl-2,2-diisopropylbutanamide 0.02-0.5 0.02-0.5 0.02-0.5 0.02-0.5
Color 0-0.5 0-0.5 0-0.5 0-0.5
Table 5. Confectionery Shell Compositions
Ex. 11 Ex. 12 Ex. 13 Ex. 14
62 DE* Corn Syrup 35-60 0 17-30 0-20
43 DE Corn Syrup 0 35-60 17-30 35-60
Maltitol 0 0 0 0
Isomalt 0 0 0 0
Sorbitol 0 0 0 0
Hydrogenated Starch Hydrolysates 0 0 0 0
Water 0-25 0-25 0-25 0-25
Hydrogenated vegetable fat 1-10 1-10 1-10 1-10
Emulsifier 0-1 0-1 0-1 0-1
Sucrose 25-65 25-65 25-65 25-65
23
CA 02749133 2011-07-06
WO 2010/083133
PCT/US2010/020685
Fondant (6X or 10X) sugar 0-10 0-10 0-10 0-10
Gelatin 1-10 1-10 1-10 0
Acesulfame K 0 0 0 0
Sucralose 0 0 0 0
Aspartame 0 0 0 0
N-Ethyl-2,2-diisopropylbutanamide 0.02-0.5 0.02-0.5 0.02-0.5 0.02-0.5
Other Cooling Compounds 0-0.5 0-0.5 0-0.5 0-0.5
Food acid 0-5 0-5 0-5 0-5
Flavor 0-5 0-5 0-5 0-5
Color 0-0.5 0-0.5 0-0.5 0-0.5
*DE = Dextrose Equivalent
Table 5. Confectionery Shell Compositions (cont.)
Ex. 15 Ex. 16 Ex. 17
62 DE Corn Syrup 0 0 0
43 DE Corn Syrup 0 0 0
Maltitol 0 10-90 0
Isomalt 10-90 0 0
Sorbitol 0 0 10-90
Hydrogenated Starch Hydrolysates 0-30 0-30 0-30
Water 0-25 0-25 0-25
Hydrogenated vegetable fat 1-10 1-10 1-10
Emulsifier 0-1 0-1 0-1
Sucrose 0 0 0
Fondant (6X or 10X) sugar 0 0 0
Gelatin 1-10 1-10 1-10
Acesulfame K 0.001-1.5 0.001-1.5 0.001-1.5
Sucralose 0.001-1.5 0.001-1.5 0.001-1.5
Aspartame 0.001-1.5 0.001-1.5 0.001-1.5
N-Ethyl-2,2-diisopropylbutanamide 0.02-0.5 0.02-0.5 0.02-0.5
Other Cooling Compounds 0-0.5 0-0.5 0-0.5
Food acid 0-5 0-5 0-5
Flavor 0-5 0-5 0-5
Color 0-0.5 0-0.5 0-0.5
Table 6. Confectionery Coating Compositions
Ex. 18 Ex. 19 Ex. 20 Ex. 21
Sucrose 90-99.9 0 0 0
Dextrose 0 90-99.9 0 0
Maltitol 0 0 0 90-99.9
Isomalt 0 0 90-99.9 0
Gum Arabic 0-5 0-5 0-5 0-5
Starch 0-5 0-5 0-5 0-5
N-Ethyl-2,2-diisopropylbutanamide 0.02-0.5 0.02-0.5 0.02-0.5 0.02-0.5
Other Cooling Compounds 0-0.5 0-0.5 0-0.5 0-0.5
Flavor 0-5 0-5 0-5 0-5
Color 0-0.5 0-0.5 0-0.5 0-0.5
24
CA 02749133 2011-07-06
WO 2010/083133 PCT/US2010/020685
EXAMPLES 22-25
[0080] These examples illustrate powder filling compositions comprising N-
ethy1-
2,2-diisopropylbutanamide. Powder filling compositions are summarized in Table
7, where
all amounts are in parts by weight based on the total weight of the powder
filling
compositions. To prepare the filling compositions, the powdered ingredients
are combined in
a mixing vessel and mixed until homogeneous. The powder filling compositions
can be
combined with the confectionery shell compositions of Table 5, and,
optionally, the
confectionery coating compositions of Table 6 to form center-filled
confections. In the final
confectionery pieces, the shell region is about 40-65% by weight, the filling
is about 5-45%
by weight, and the coating is about 0-50% by weight.
Table 7. Filling compositions with Particulate Materials
Ex. 22 Ex. 23 Ex. 24 Ex. 25
Dextrose powder 90-100 0 45-50 0
Erythritol powder 0 90-100 45-50 45-50
Xylitol powder 0 0 0 45-50
Food acid 0-5 0-5 0-5 0-5
Flavor 0-5 0-5 0-5 0-5
N-Ethyl-2,2-diisopropylbutanamide 0.02-0.5 0.02-0.5 0.02-0.5 0.02-0.5
Color 0-0.5 0-0.5 0-0.5 0-0.5
Bulk density 0.5-0.6 0.2-0.3 0.3-0.4 0.3-0.4
Tap density 0.6-0.7 0.5-0.6 0.6-0.7 0.6-0.7
EXAMPLES 26-29
[0081] These examples illustrate center-fill compositions comprising N-ethy1-
2,2-
diisopropylbutanamide and aqueous carriers. The center-fill compositions are
summarized in
Table 8, where all amounts are in parts by weight based on the total weight of
the powder
filling compositions. To prepare the center-fill compositions, the
hydrogenated starch
hydrolysates and/or corn syrup(s) are mixed together with any glycerin until
homogeneous.
Then the powdered ingredients, flavor, acid, color, cooling agent, and
emulsifiers are
combined into the liquid blend and mixed until homogeneous. The powder filling
compositions can be combined with the confectionery shell compositions of
Table 5, and,
optionally, the confectionery coating compositions of Table 6 to form center-
filled
confections. In the final confectionery pieces, the shell region is about 40-
65% by weight,
the filling is about 5-45% by weight, and the coating is about 0-50% by
weight.
CA 02749133 2013-02-06
Table 8. Filling compositions with Aqueous Carriers
Ex. 26 Ex. 27 Ex. 28 Ex. 29
Dextrose powder 15-35 0 15-35 0
Erythritol powder 15-35 35-65 15-35 15-35
Xylitol powder 0 0 0 15-35
Hydrogenated Starch Hydrolysate 0 0 15-75 15-75
Glycerin 0-20 0-20 0-20 0-20
High Fructose Corn Syrup 5-15 5-15 0 0
High Maltose Corn Syrup 0-10 0-10 0 0
Invert Sugar 10-30 10-30 0 0
Carboxymethyl Cellulose 0-0.2 0-0.2 0 0
Emulsifier 0-5 0-5 0-5 0-5
Food acid 0-5 0-5 0-5 0-5
Flavor 0-5 0-5 0-5 0-5
N-Ethyl-2,2-diisopropylbutanamide 0.02-0.5 0.02-0.5 0.02-0.5 0.02-0.5
Color 0-0.5 0-0.5 0-0.5 0-0.5
[0082] This written description uses examples to disclose the invention,
including the
best mode, and also to enable any person skilled in the art to make and use
the invention.
The patentable scope of the invention is defined by the claims, and may
include other
examples that occur to those skilled in the art. Such other examples are
intended to be within
the scope of the claims if they have structural elements that do not differ
from the literal
language of the claims, or if they include equivalent structural elements with
insubstantial
differences from the literal language of the claims.
[0083] All ranges disclosed herein are inclusive of the endpoints, and the
endpoints
are independently combinable with each other.
[0084] The use of the terms "a" and "an" and "the" and similar referents in
the
context of describing the invention (especially in the context of the
following claims) are to
be construed to cover both the singular and the plural, unless otherwise
indicated herein or
clearly contradicted by context. Further, it should further be noted that the
terms "first,"
"second," and the like herein do not denote any order, quantity, or
importance, but rather are
used to distinguish one element from another. The modifier "about" used in
connection with
a quantity is inclusive of the stated value and has the meaning dictated by
the context (e.g., it
includes the degree of error associated with measurement of the particular
quantity).
26