Note: Descriptions are shown in the official language in which they were submitted.
CA 02750088 2013-02-05
DESCRIPTION
OPERATION METHOD OF MIDDLE DISTILLATE HYDROTREATING REACTOR,
AND MIDDLE DISTILLATE HYDROTREATING REACTOR
[Technical Field]
[0001]
The present invention relates to an operation method of a middle distillate
hydrotreating reactor, and the middle distillate hydrotreating reactor, which
hydrotreat
and hydroisomerize a middle distillate including a component of a boiling
point range
equivalent to gas oil among hydrocarbon compounds synthesized by the Fisher-
Tropsch
synthesis reaction.
[Background Art]
[0002]
As one of methods for synthesizing liquid fuels from a natural gas, a GTL (Gas
To Liquids. liquid fuel synthesis) technique of reforming a natural gas to
produce a
synthesis gas containing a carbon monoxide gas (CO) and a hydrogen gas (H2) as
main
components, synthesizing hydrocarbon compounds (hereinafter referred to as "FT
synthesis hydrocarbons") using this synthesis gas as a source gas by the
Fischer-Tropsch
synthesis reaction (hereinafter referred to as an "FT synthesis reaction"),
and further
hydrotreating and fractionating the FT synthesis hydrocarbons to produce
liquid fuel
products, such as naphtha (raw gasoline), kerosene, gas oil (diesel fuel oil),
and wax, has
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
2
recently been developed.
[0003]
Here, since the liquid fuel products using the aforementioned FT synthesis
hydrocarbons as a feedstock have high paraffin content, and almost no sulfur
component,
for example, as shown in Patent Document 1, the liquid fuel products attract
attention as
an environmentally friendly fuel.
When the FT synthesis hydrocarbons are fractionated in a fractionator, a
middle
distillate including a component of a boiling point range equivalent to gas
oil is taken out
from a middle part of the fractionator. This middle distillate is used as a
feedstock of
gas oil. Additionally, a wax fraction with a large carbon number is taken out
from the
bottom of the fractionator. This wax fraction can be made light by
hydrocracking, and
can thereby be used as a feedstock of gas oil.
[0004]
Here, since a large amount of normal paraffins is included in the
aforementioned
middle distillate of the FT synthesis hydrocarbons, the freezing point
(freezing
temperature) tends to become high, and there is a possibility that the cold
properties of
gas oil obtained from this middle distillate used as a feedstock does not
satisfy demanded
specifications as a product. For this reason, as for the middle distillate
distilled from the
fractionator, when olefins and oxygen-containing compounds such as alcohols,
which are
produced as by-products in an FT synthesis reaction process, are converted
into saturated
hydrocarbons by hydrotreating, it is necessary to perform hydroisomerization
to convert
at least a portion of the normal paraffins into isoparaffins having a low
freezing point.
[Citation List]
[Patent Document]
[0005]
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
3
[Patent Document 1] Japanese Patent Unexamined Publication No.
2004-323626
[Summary of Invention]
[Technical Problem]
[0006]
If the progress of the hydroisomerization is insufficient in a middle
distillate
hydrotreating process which hydrotreats and hydroisomerizes the middle
distillate, a lot
of normal paraffins with a high freezing point remain in the obtained
hydrotreated middle
distillate, and the cold properties of the gas oil obtained from this middle
distillate used
as a feedstock are not sufficiently improved. On the other hand, in a case
where the
reaction conditions are selected so that hydroisomerization proceeds
excessively in the
middle distillate hydrotreating process, a decomposition reaction concurs to
lighten the
generated hydrocarbons, and there is a possibility that middle distillate may
not be
suitable as a feedstock of gas oil, and the yield of the gas oil as a product
may decrease.
For this reason, in order to obtain gas oil (diesel fuel oil) from the FT
synthesis
hydrocarbons, it is necessary to properly progress the hydroisomerization in
the
hydrotreating process of the middle distillate.
[0007]
The present invention was made in view of the aforementioned situation, and
the
object of the present invention is to provide an operation method of a middle
distillate
hydrotreating reactor, and the middle distillate hydrotreating reactor,
capable of properly
progressing hydroisomerization in a hydrotreating process of a middle
distillate of FT
synthesis hydrocarbons obtained by the FT synthesis reaction, producing a
hydrotreated
middle distillate with stable properties, and obtaining high-quality gas oil.
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
4
[Solution to Problem]
[0008]
In order to solve the above problem and achieve such an object, the present
invention suggests the following methods.
The operation method of a middle distillate hydrotreating reactor which
hydrotreats and hydroisomerizes a middle distillate including components of a
boiling
point range equivalent to gas oil among FT synthesis hydrocarbons synthesized
by the
Fisher-Tropsch synthesis reaction. The operation method comprising the steps
of:
bringing the middle distillate into contact with a catalyst to hydrotreat and
hydroisomerize the middle distillate to produce hydrotreated middle
distillate; measuring
the cloud point of the hydrotreated middle distillate flowing out from the
middle distillate
hydrotreating reactor; and controlling the operating conditions of the middle
distillate
hydrotreating reactor so that the cloud point comes to a predetermined target
value.
[0009]
In the operation method of a middle distillate hydrotreating reactor having
the
above configuration, the cloud point of the hydrotreated middle distillate
flowing out
from the middle distillate hydrotreating reactor, is measured, and the
operating conditions
of the middle distillate hydrotreating reactor are controlled so that the
cloud point
becomes a predetermined target value. Therefore, the cloud point of the
produced
hydrotreated middle distillate gets stable. In addition, the cloud point is
the
temperature when a component with a high freezing point (wax) precipitates as
a solid in
liquid hydrocarbons (middle distillate), and clouding occurs. As shown in JIS
K 2269,
for example, the cloud point can be measured by cooling a sample liquid at a
certain rate,
and measuring the liquid temperature when the clouding has occurred in the
sample
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
liquid.
Here, in a case where a large amount of normal paraffins is included in the
produced hydrotreated middle distillate, the cloud point of the hydrotreated
middle
distillate becomes high. On the other hand, in a case where the content of
normal
5 paraffins in the hydrotreated middle distillate is small, the cloud point
of the hydrotreated
middle distillate becomes low. That is, it is possible to understand the
degree of the
hydroisomerization in the middle distillate hydrotreating reactor by measuring
the cloud
point of the hydrotreated middle distillate.
[0010]
Accordingly, by measuring the cloud point of the hydrotreated middle
distillate,
and controlling the operating conditions of the middle distillate
hydrotreating reactor
based on this measurement value, it is possible to properly progress the
hydroisomerization in the reactor, to produce a hydrotreated middle distillate
with stable
properties, and to obtain high-quality gas oil.
In addition, in a case where gas oil as diesel fuel oil is used under cold
conditions, the normal paraffins precipitates as a wax component, and thus,
plugging may
occur in a filter installed in a fuel-oil supply system to a diesel engine.
Then, for the
purpose of preventing such a problem, generally gas oil products are managed
so as to
have a cloud point lower than or equal to a certain value. However, in a
reactor of a
producing process of a middle distillate used as a feedstock of the gas oil,
the cloud point
has not been used as an index of operation management conventionally.
[0011]
In the operation method of a middle distillate hydrotreating reactor of the
present invention, in the step of measuring the cloud point, the cloud point
may be
measured while cooling a obtained sample of the hydrotreated middle distillate
at a
CA 02750088 2011-07-19
OSP38125-38141(GTL0401)
6
cooling rate of 5.0 C/min or more and 15.0 C/min or less.
In this case, it is possible to measure the cloud point in a short time by
measuring the cloud point while cooling the sample at a cooling rate of 5.0
C/min or
more. As a result, the measurement result of the cloud point can be reflected
on the
control of the middle distillate hydrotreating reactor, without being
accompanied by a
large time lag after the taking of the sample of the hydrotreated middle
distillate.
Additionally, by measuring the cloud point while cooling the sample at a
cooling rate of
15.0 C/min or less, it is possible to accurately measure the cloud point, and
it is possible
to appropriately control the middle distillate hydrotreating reactor.
[0012]
Additionally, in the operation method of a middle distillate hydrotreating
reactor
of the present invention, in the step of measuring the cloud point, the cloud
point may be
measured while cooling the obtained sample at the cooling rate controlled by
an
electronic cooling unit using a Peltier device.
In this case, by cooling the hydrotreated middle distillate with the
electronic
cooling unit using the Peltier device, it is possible to accurately and easily
perform the
temperature control of the hydrotreated middle distillate, and it is possible
to accurately
measure the cloud point.
[0013]
Moreover, in the operation method of a middle distillate hydrotreating reactor
of
the present invention, in the step of controlling the operating conditions of
the middle
distillate hydrotreating reactor, at least one of hydrogen partial pressure,
reaction
temperature, and throughput of middle distillate per unit time may be
controlled.
In this case, it is possible to adjust the degree of the hydroisomerization by
controlling at least one of hydrogen partial pressure, reaction temperature,
and
CA 02750088 2011-07-19
OSP38125-38141(GTL0401)
7
throughput of middle distillate per unit time, which are the operation
conditions of the
middle distillate hydrotreating reactor. In addition, the throughput of middle
distillate
per unit time can be expressed by liquid hourly space velocity (LHSV(h-I)) as
the amount
of oil through the middle distillate hydrotreating reactor.
For example, if the cloud point exceeds the upper limit of an operation
control
target range, the hydroisomerization is promoted and the cloud point of the
hydrotreated
middle distillate can be lowered, by setting the conditions that the hydrogen
partial
pressure is raised, and/or the reaction temperature is raised, and/or the
throughput of
middle distillate per unit time (LHSV) is reduced. Additionally, if the cloud
point falls
below the lower limit of the operation control target range, the progress of
the
hydroisomerization is suppressed and the cloud point of the hydrotreated
middle distillate
can be elevated, by setting the conditions that the hydrogen partial pressure
is reduced,
and/or the reaction temperature is lowered, and/or the throughput of middle
distillate per
unit time (LHSV) is increased.
[0014]
The middle distillate hydrotreating reactor of the present invention is a
middle
distillate hydrotreating reactor which hydrotreats and hydroisomerizes a
middle distillate
including a component of a boiling point range equivalent to gas oil among FT
synthesis
hydrocarbons synthesized by the Fisher-Tropsch synthesis reaction. The reactor
includes: a sampling unit which takes a sample of the produced hydrotreated
middle
distillate; and a cloud point measuring unit which measures the cloud point of
the
obtained sample.
[0015]
According to the middle distillate hydrotreating reactor having this
configuration,
the cloud point of the hydrotreated middle distillate flowing out from the
middle distillate
CA 02750088 2011-07-19
OSP38125-38141(GTL0401)
8
hydrotreating reactor is rapidly measured. Then, by controlling the operating
conditions
based on the result, it is possible to appropriately adjust the degree of the
hydroisomerization, and to stabilize the properties of the produced
hydrotreated middle
distillate. This makes it possible to improve the quality of the gas oil
produced from the
middle distillate.
[0016]
In the middle distillate hydrotreating reactor of the present invention, the
sampling unit may be connected to the cloud point measuring unit by piping so
as to
automatically take the sample and transfer the sample to the cloud point
measuring unit,
and the cloud point measuring unit may automatically measure the cloud point
of the
transferred sample.
[0017]
Additionally, in the middle distillate hydrotreating reactor of the present
invention, the cloud point measuring unit may include a cooler capable of
cooling the
obtained sample at a cooling rate of 5.0 C/min or more and 15.0 C/min or
less.
In this case, as it is possible to cool the sample at the aforementioned
cooling
rate, the cloud point can be measured rapidly and accurately.
[0018]
Moreover, in the middle distillate hydrotreating reactor of the present
invention,
the cooler capable of cooling the sample at a cooling rate of 5.0 C/min or
more and 15.0
C/min or less provided at the cloud point measuring unit may be an electronic
cooling
unit using a Peltier device.
In this case, it is possible to accurately and easily perform the temperature
control of the sample, and the cloud point can be measured more accurately.
CA 02750088 2013-02-05
8a
According to an aspect, the invention provides for an operation method of a
middle distillate hydrotreating reactor, comprising the steps of: bringing a
middle distillate
into contact with a catalyst in the middle distillate hydrotreating reactor to
continuously
hydrotreat and hydroisomerize the middle distillate including components of a
boiling point
range equivalent to gas oil among FT synthesis hydrocarbons synthesized by the
Fisher-
Tropsch synthesis reaction to produce hydrotreated middle distillate;
measuring the cloud
point of the hydrotreated middle distillate flowing out from the middle
distillate
hydrotreating reactor; and controlling the operating conditions of the middle
distillate
hydrotreating reactor so that the cloud point comes to a predetermined target
value. In the
step of measuring the cloud point, the cloud point is measured while cooling a
sample of the
hydrotreated middle distillate at a cooling rate of 5.0 C/min or more and
15.0 C/min or
less.
According to another aspect, the invention provides for a middle distillate
hydrotreating reactor which continuously hydrotreats and hydroisomerizes a
middle distillate
including a component of a boiling point range equivalent to gas oil among FT
synthesis
hydrocarbons synthesized by the Fisher-Tropsch synthesis reaction, the reactor
comprising: a
sampling unit which takes a sample of the produced hydrotreated middle
distillate; and a
cloud point measuring unit which measures the cloud point of the obtained
sample. The
cloud point measuring unit includes a cooler adapted to cool the sample at a
cooling rate of
5.0 C/min or more and 15.0 C/min or less.
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
9
[Advantageous Effects of Invention]
[0019]
According to the present invention, it is possible to provide an operation
method
of a middle distillate hydrotreating reactor, and the middle distillate
hydrotreating reactor,
capable of properly controlling the progress of hydroisomerization in a
hydrotreating
process of a middle distillate of FT synthesis hydrocarbons obtained by the FT
synthesis
reaction, producing a hydrotreated middle distillate with stable properties,
and obtaining
high-quality gas oil (diesel fuel oil).
[Brief Description of Drawings]
[0020]
[FIG 1] FIG. 1 is a schematic diagram showing the overall configuration of a
liquid-fuel synthesizing system including a middle distillate hydrotreating
reactor
according to an embodiment of the present invention.
[FIG 2] FIG. 2 is a detailed explanatory view of the surroundings of the
middle
distillate hydrotreating reactor according to the embodiment of the present
invention.
[FIG 3] FIG. 3 is a schematic configuration diagram of a cloud point measuring
unit shown in FIG. 2.
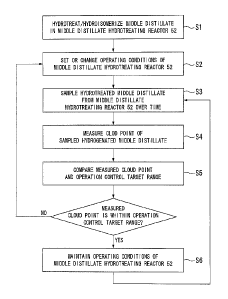
[FIG 4] FIG. 4 is a flow chart showing an operation method of the middle
distillate hydrotreating reactor according to the embodiment of the present
invention.
[FIG 5] FIG. 5 is a graph showing results of confirmatory experiments.
[Description of Embodiments]
[0021]
A preferred embodiment (hereinafter referred to as the "present embodiment")
of
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
the present invention will be described below with reference to the
accompanying
drawings.
First, with reference to FIG. 1, the overall configuration and process of a
liquid-fuel synthesizing system (hydrocarbon synthesis reaction system) where
an
5 operation method of a middle distillate hydrotreating reactor that is the
present
embodiment is used will be described.
[0022]
As shown in FIG. 1, the liquid-fuel synthesizing system 1 (hydrocarbon
synthesis reaction system) according to the present embodiment is a plant
facility which
10 carries out the GTL process which converts a hydrocarbon feedstock, such
as a natural
gas, into liquid fuels. This liquid-fuel synthesizing system 1 includes a
synthesis gas
production unit 3, an FT synthesis unit 5, and an upgrading unit 7.
The synthesis gas production unit 3 reforms a natural gas, which is a
hydrocarbon feedstock, to produce a synthesis gas including a carbon monoxide
gas and
a hydrogen gas.
The FT synthesis unit 5 produces liquid hydrocarbons by the FT synthesis
reaction from the synthesis gas produced in the synthesis gas production unit
3.
The upgrading unit 7 hydrotreats and fractionates the liquid hydrocarbons
produced by the FT synthesis reaction to produce liquid fuels (naphtha,
kerosene, gas oil,
wax, etc.). Hereinafter, components of these respective units will be
described.
[0023]
The synthesis gas production unit 3 mainly includes a desulfurization reactor
10,
a reformer 12, a waste heat boiler 14, vapor-liquid separators 16 and 18, a
CO2 removal
unit 20, and a hydrogen separator 26.
The desulfurization reactor 10 is composed of a hydrodesulferizer, etc., and
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
11
removes sulfur components from a natural gas that is a feedstock.
The reformer 12 reforms the natural gas supplied from the desulfurization
reactor 10, to produce a synthesis gas including a carbon monoxide gas (CO)
and a
hydrogen gas (H2) as main components.
The waste heat boiler 14 recovers waste heat of the synthesis gas produced in
the reformer 12, to produce a high-pressure steam.
The vapor-liquid separator 16 separates the water heated by the heat exchange
with the synthesis gas in the waste heat boiler 14 into gas (a high-pressure
steam) and
liquid.
The vapor-liquid separator 18 removes condensed components from the
synthesis gas cooled down in the waste heat boiler 14, and supplies gas
components to
the CO2 removal unit 20.
The CO2 removal unit 20 has an absorption tower 22 which removes carbon
dioxide gas by using an absorbent from the synthesis gas supplied from the
vapor-liquid
separator 18, and a regeneration tower 24 which diffuses the carbon dioxide
gas from the
absorbent including the carbon dioxide gas to regenerate the absorbent.
The hydrogen separator 26 separates a portion of the hydrogen gas included in
the synthesis gas, the carbon dioxide gas in which has been separated by the
CO2
removal unit 20.
It is to be noted herein that the above CO2 removal unit 20 is not necessarily
provided depending on the circumstances.
[0024]
The FT synthesis unit 5 mainly includes, for example, a bubble column reactor
(a bubble column type hydrocarbon synthesis reactor) 30, a vapor-liquid
separator 34, a
separator 36, a vapor-liquid separator 38, and a first fractionator 40.
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
12
The bubble column reactor 30, which is an example of a reactor which
synthesizes liquid hydrocarbons from a synthesis gas, functions as an FT
synthesis
reactor which synthesizes liquid hydrocarbons from the synthesis gas by the FT
synthesis
reaction. The bubble column reactor 30 is composed of, for example, a bubble
column
type slurry bed reactor in which a slurry having solid catalyst particles
suspended in
liquid hydrocarbons (product of the FT synthesis reaction) is contained inside
a column
type vessel. The bubble column reactor 30 makes the carbon monoxide gas and
hydrogen gas in the synthesis gas produced in the above synthesis gas
production unit 3
react with each other to synthesize liquid hydrocarbons.
The vapor-liquid separator 34 separates the water circulated and heated
through
a heat transfer pipe 32 disposed in the bubble column reactor 30 into a steam
(a
medium-pressure steam) and a liquid.
The separator 36 separates the catalyst particles and liquid hydrocarbons in
the
slurry contained inside the bubble column reactor 30.
The vapor-liquid separator 38 is connected to the top of the bubble column
reactor 30 to cool down the unreacted synthesis gas discharged from the bubble
column
reactor 30 and a product which is gaseous under the conditions of the bubble
column
reactor 30, and to separate a condensed liquid product from a gas component.
The first fractionator 40 fractionates the FT synthesis reaction product
including
the liquid hydrocarbons, which is supplied via the separator 36 and the vapor-
liquid
separator 38 from the bubble column reactor 30 as main components, into
individual
fractions.
[0025]
The upgrading unit 7 includes, for example, a wax fraction hydrocracking
reactor 50, a middle distillate hydrotreating reactor 52 according to the
present
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
13
embodiment, a naphtha fraction hydrotreating reactor 54, vapor-liquid
separators 56, 58,
and 60, a second fractionator 70, and a naphtha stabilizer 72.
The wax fraction hydrocracking reactor 50 is connected to the bottom of the
first
fractionator 40, and has the vapor-liquid separator 56 provided at the
downstream
thereof
The middle distillate hydrotreating reactor 52 is connected to a middle part
of
the first fractionator 40, and has the vapor-liquid separator 58 provided at
the
downstream thereof
The naphtha fraction hydrotreating reactor 54 is connected to top of the first
fractionator 40, and has the vapor-liquid separator 60 provided at the
downstream
thereof
The second fractionator 70 fractionates the liquid hydrocarbons supplied from
the vapor-liquid separators 56 and 58.
The naphtha stabilizer 72 rectifies liquid hydrocarbons of a naphtha fraction
supplied from the vapor-liquid separator 60 and the second fractionator 70, to
discharge
butane and components lighter than butane as flare gas, and to separate and
recover
hydrocarbon components having a carbon number of five or more as a naphtha
product.
[0026]
Next, a process (GTL process) of synthesizing liquid fuels from a natural gas
by
the liquid-fuel synthesizing system 1 configured as above will be described.
[0027]
A natural gas (whose main component is CH4) as a hydrocarbon feedstock is
supplied to the liquid-fuel synthesizing system 1 from an external natural gas
supply
source (not shown), such as a natural gas field or a natural gas plant. The
above
synthesis gas production unit 3 reforms this natural gas to produce a
synthesis gas (mixed
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
14
gas including a carbon monoxide gas and a hydrogen gas as main components).
[0028]
First, the above natural gas is supplied to the desulfurization reactor 10
along
with the hydrogen gas separated by the hydrogen separator 26. In the
desulfurization
reactor 10, sulfur components included in a natural gas are converted into
hydrogen
sulfide by the action of a hydrodesulfurization catalyst under the existence
of a hydrogen
gas, and is adsorbed and removed by, for example, ZnO.
The desulfurized natural gas is supplied to the reformer 12 after the carbon
dioxide (CO2) gas supplied from a carbon-dioxide supply source (not shown) and
the
steam generated in the waste heat boiler 14 are mixed. The reformer 12 reforms
the
natural gas by the steam and carbon-dioxide-gas reforming method using a
carbon
dioxide and a steam to produce a high-temperature synthesis gas including a
carbon
monoxide gas and a hydrogen gas as main components.
[0029]
The high-temperature synthesis gas (for example, 900 C, 2.0 MPaG) produced
in the reformer 12 in this way is supplied to the waste heat boiler 14, and is
cooled down
by the heat exchange with the water which circulates through the waste heat
boiler 14
(for example, 400 C). Then, the water heated by the heat exchange becomes a
high-pressure steam, and consequently, the waste heat is recovered.
The synthesis gas cooled down in the waste heat boiler 14 is supplied to the
absorption tower 22 of the CO2 removal unit 20, or the bubble column reactor
30, after
condensate components are separated and removed from the synthesis gas in the
vapor-liquid separator 18. The absorption tower 22 absorbs a carbon dioxide
gas
included in the synthesis gas within the absorbent contained therein, to
separate the
carbon dioxide gas from the synthesis gas. The absorbent including the carbon
dioxide
CA 02750088 2011-07-19
OSP38125-38141(GTL0401)
gas within this absorption tower 22 is introduced into the regeneration tower
24, the
absorbent including the carbon dioxide gas is heated and subjected to a
stripping
treatment with, for example, a steam, and the resulting diffused carbon
dioxide gas is
supplied to the reformer 12 from the regeneration tower 24, and is reused for
the above
5 reforming reaction.
[0030]
The synthesis gas produced in the synthesis gas production unit 3 in this way
is
supplied to the bubble column reactor 30 of the above FT synthesis unit 5. At
this time,
the composition ratio of the synthesis gas supplied to the bubble tower
reactor 30 is
10 adjusted to a composition ratio (for example, I-12:C0=2:1 (molar ratio))
suitable for FT
synthesis reaction.
[0031]
Additionally, the hydrogen separator 26 separates the hydrogen gas included in
the synthesis gas, by the adsorption and desorption (hydrogen PSA) utilizing a
pressure
15 difference. This separated hydrogen gas is continuously supplied from a
gas holder (not
shown) or the like via a compressor (not shown) to various hydrogen-utilizing
reaction
devices (for example, the desulfurization reactor 10, the wax fraction
hydrocracking
reactor 50, the middle distillate hydrotreating reactor 52, the naphtha
fraction
hydrotreating reactor 54, etc.) which perform predetermined reactions
utilizing hydrogen
gas within the liquid fuel synthesizing system 1.
[0032]
Next, the above FT synthesis unit 5 synthesizes liquid hydrocarbons by the FT
synthesis reaction from the synthesis gas produced in the above synthesis gas
production
unit 3.
[0033]
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
16
The synthesis gas produced in the above synthesis gas production unit 3 flows
into the bottom of the bubble column reactor 30, and flows up in the slurry
contained in
the bubble column reactor 30. At this time, within the bubble column reactor
30, the
carbon monoxide and hydrogen gas which are included in the synthesis gas react
with
each other by the FT synthesis reaction, thereby synthesizing hydrocarbons.
The liquid
hydrocarbons synthesized in the bubble column reactor 30 are introduced into
the
separator 36 along with catalyst particles as slurry.
[0034]
The separator 36 separates the slurry into a solid component, such as catalyst
particles, and a liquid component including liquid hydrocarbons. A portion of
the
separated solid component, such as the catalyst particles, is returned to the
bubble
column reactor 30, and a liquid component is supplied to the first
fractionator 40.
Additionally, the unreacted synthesis gas, and the hydrocarbons which are
generated by
the FT synthesis reaction and are gaseous under the conditions within the
bubble column
reactor 30, are introduced into the vapor-liquid separator 38 from the top of
the bubble
column reactor 30. The vapor-liquid separator 38 cools down these gases to
separate
condensed liquid hydrocarbons to introduce them into the first fractionator
40.
Meanwhile, the gas component separated by the vapor-liquid separator 38, i.e.,
a mixed
gas including the unreacted synthesis gas (CO and H2), and hydrocarbon gas
with a low
carbon number (C4 or less) as main components are recycled to the bubble
column
reactor 30, and the unreacted synthesis gas included in the mixed gas is
subjected to the
FT synthesis reaction again. In addition, for the purpose of preventing
gaseous
hydrocarbons composed mainly of C4 or less from being accumulated at high
concentration within an FT synthesis reaction system due to the recycling of
the mixed
gas, some of the mixed gas is not recycled to the bubble column reactor 30,
but is
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
17
introduced into an external combustion facility (a flare stack (not shown)),
and is
combusted, and then emitted to the atmosphere.
[0035]
Next, the first fractionator 40 fractionates the FT synthesis reaction product
including the liquid hydrocarbons, which is supplied via the separator 36 and
the
vapor-liquid separator 38 from the bubble column reactor 30 as described
above, as main
components, into a naphtha fraction (whose boiling point is lower than about
150 C), a
middle distillate equivalent to kerosene and gas oil (whose boiling point is
about 150 to
350 C), and a wax fraction (whose boiling point exceeds about 350 C).
The wax fraction (mainly C21 or more) drawn from the bottom of the first
fractionator 40 is brought to the wax fraction hydrocracking reactor 50, the
middle
distillate (mainly C11 to C20) drawn from the middle part of the first
fractionator 40 is
brought to the middle distillate hydrotreating reactor 52, and the naphtha
fraction (mainly
C5 to C10) drawn from the upper part of the first fractionator 40 is brought
to the naphtha
fraction hydrotreating reactor 54.
[0036]
The wax fraction hydrocracking reactor 50 hydrocracks the wax fraction
(approximately C21 or more), which has been drawn from the bottom of the first
fractionator 40, by using the hydrogen gas supplied from the above hydrogen
separator
26, to convert the wax fraction into hydrocarbons of C20 or less. In this
hydrocracking
reaction, the wax fraction is converted into hydrocarbons with a small carbon
number by
cleaving C-C bonds of hydrocarbons with a large carbon number, using a
catalyst and
heat. A product including the liquid hydrocarbons hydrocracked in this wax
fraction
hydrocracking reactor 50 is separated into gas and liquid in the vapor-liquid
separator 56,
the liquid hydrocarbons of which are brought to the second fractionator 70,
and the gas
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
18
component (including a hydrogen gas) of which is brought to the middle
distillate
hydrotreating reactor 52 and the naphtha fraction hydrotreating reactor 54
where the
hydrogen gas is reused.
[0037]
The middle distillate hydrotreating reactor 52 hydrotreats and hydroisomerizes
liquid hydrocarbons of the middle distillate (approximately Cii to C20), which
have been
brought out from the middle part of the first fractionator 40, by using the
hydrogen gas
supplied via the wax fraction hydrocracking reactor 50 from the hydrogen
separator 26.
A product including the hydrotreated liquid hydrocarbons is separated into
vapor and
liquid in the vapor-liquid separator 58, the liquid hydrocarbons of which are
brought to
the second fractionator 70, and the gas component (including hydrogen gas) of
which is
reused for the above hydrogenation reaction.
[0038]
The naphtha fraction hydrotreating reactor 54 hydrotreats liquid hydrocarbons
of
the naphtha fraction with a low carbon number (approximately C10 or less),
which have
been brought out from the top of the first fractionator 40, by using the
hydrogen gas
supplied via the wax fraction hydrocracking reactor 50 from the hydrogen
separator 26.
A product including the hydrotreated liquid hydrocarbons (hydrotreated
naphtha) is
separated into vapor and liquid in the vapor-liquid separator 60, the liquid
hydrocarbons
of which are brought to the naphtha stabilizer 72, and the gas component
(including
hydrogen gas) of which is reused for the above hydrogenation reaction.
[0039]
Next, the second fractionator 70 fractionates the liquid hydrocarbons, which
are
supplied from the wax fraction hydrocracking reactor 50 and the middle
distillate
hydrotreating reactor 52 as described above, into hydrocarbons of C10 or
less(whose
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
19
boiling point is lower than about 150 C), a kerosene fraction (whose boiling
point is
about 150 to 250 C), a gas oil fraction (whose boiling point is about 250 to
350 'V), and
a so-called uncracked wax fraction (whose boiling point exceeds about 350 C)
which is
not subjected to sufficient hydrocracking in the wax fraction hydrocracking
reactor 50.
The uncracked wax fraction is brought out from the bottom of the second
fractionator 70,
and this is recycled to the upstream of the wax fraction hydrocracking reactor
50 and is
supplied to the wax fraction hydrocracking reactor 50 again. The kerosene
fraction and
the gas oil fraction are brought out from the middle part of the second
fractionator 70.
Meanwhile, hydrocarbons of Cip or less is brought out from the top of the
second
fractionator 70, and is supplied to the naphtha stabilizer 72.
[0040]
Moreover, the naphtha stabilizer 72 rectifies the hydrocarbons of C10 or less,
which have been supplied from the above naphtha fraction hydrotreating reactor
54 and
the top of the second fractionator 70, and obtains high-purity naphtha (C5 to
CIO as a
product from the bottom. Meanwhile, a gas other than target products,
including
hydrocarbons of C4 or less as a main component, is discharged from the top of
the
naphtha stabilizer 72. Additionally, this gas is introduced into an external
combustion
facility (not shown), is combusted therein, and is then emitted to the
atmosphere.
[0041]
The process (GTL process) of the liquid-fuel synthesizing system 1 has been
described hitherto. By the GTL process concerned, a natural gas is converted
into liquid
fuels, such as high-purity naphtha (C5 to CIO, kerosene (C11 to C15), and gas
oil (C16 to
C20)=
[0042]
Next, the configuration and operation of the surroundings of the middle
distillate
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
hydrotreating reactor 52 will be described in detail with reference to FIG. 2.
This middle distillate hydrotreating reactor 52 includes a supply line 101
connected to the middle part of the first fractionator 40, a discharge line
102 which
discharges a middle distillate hydrotreated in the middle distillate
hydrotreating reactor
5 52, a sampling unit 103 which takes the sample of the hydrotreated middle
distillate from
the discharge line 102, a cloud point measuring unit 110 which measures the
cloud point
of the obtained sample of the hydrotreated middle distillate, and a control
unit 104 which
controls the operating conditions (hydrogen partial pressure/reaction
temperature/throughput of middle distillate per unit time (for example, LHSV))
of the
10 middle distillate hydrotreating reactor 52.
[0043]
A middle distillate hydrotreating process to which the operation method of the
middle distillate hydrotreating reactor of the present embodiment is applied
is a process
to hydrotreat and hydroisomerize the middle distillate obtained by the FT
synthesis
15 reaction. In the FT synthesis reaction, other than saturated
hydrocarbons which are a
main product, olefins and oxygen-containing compounds such as alcohols
including an
oxygen atom originated from a carbon monoxide, are produced as by-products,
and these
by-products are also included in a middle distillate obtained by fractionating
the FT
synthesis oil. The hydrotreating in the middle distillate hydrotreating
process mainly
20 includes a reaction in which the olefins are hydrogenated and are
converted into saturated
hydrocarbons (paraffin hydrocarbons), and a reaction in which the oxygen-
containing
compounds are hydrodeoxygenated, and are converted into saturated hydrocarbons
and
water. As a catalyst which is effective in this hydrotreating, a catalyst
including a metal
component having hydrogenation ability as an active site is used.
Meanwhile, the hydroisomerization in the middle distillate hydrotreating
process
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
21
is a reaction in which normal paraffins included in the middle distillate are
converted into
isoparaffins. As a catalyst which is effective in this hydroisomerization, a
catalyst
composed of a metal component having hydrogenation/dehydrogenation ability,
and a
solid acid component is used. First, a normal paraffin is dehydrogenated into
an olefin
by the action of a metal component, and this olefin is skeleton-isomerized by
the action
of a solid acid component, and is hydrogenated by the action of the metal
component,
and is converted into an isoparaffin.
In the middle distillate hydrotreating process, both a catalyst effective in
the
hydrotreating and a catalyst effective in the hydroisomerization may be used.
However,
since the catalyst effective in the hydroisomerization is generally effective
even in the
hydrotreating, it is efficient and preferable to use the catalyst effective in
the
hydroisomerization.
[0044]
Although the type of the middle distillate hydrotreating reactor according to
the
present invention is not limited, it is preferable that the reactor is a fixed-
bed continuous
flow type reactor. A single reactor may be adopted, and a plurality of
reactors which is
arranged in series or in parallel may be adopted. Additionally, a single
catalyst bed may
be provided within a reactor, and a plurality of divided catalyst beds may be
used.
[0045]
As a catalyst to be charged into the middle distillate hydrotreating reactor,
a
catalyst generally used for hydrotreating and/or hydroisomerization in
petroleum refining,
or the like, i.e., a catalyst in which an active metal having hydrogenation
(dehydrogenation) ability is carried in an inorganic support, can be used.
As active metals which constitute the catalyst, one or more kinds of metals
selected from a group consisting of metals belonging to the 6th group, the 8th
group, the
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
22
9th group, and the 10th group on the periodic table of elements, are used.
Concrete
examples of such metals include noble metals, such as platinum, palladium,
rhodium,
ruthenium, iridium, and osmium, or cobalt, nickel, molybdenum, tungsten, iron,
or the
like, preferably includes platinum, palladium, nickel, cobalt, molybdenum, and
tungsten,
and more preferably includes platinum and palladium. Additionally, such metals
are
preferably used by combining two or more kinds thereof. Preferable
combinations in
that case include platinum-palladium, cobalt-molybdenum, nickel-molybdenum,
nickel-cobalt-molybdenum, nickel-tungsten, or the like. Additionally, in a
case where
combinations, such as cobalt-molybdenum, nickel-molybdenum, nickel-cobalt
molybdenum, nickel-tungsten, or the like, are used as the active metals, these
combinations may be sulfurated by a sulfur compound before a catalyst is
provided to the
hydrotreating. In addition, the periodic table of elements means the long
period type
periodic table of elements based on the regulations of IUPAC (International
Union of
Pure and Applied Chemistry).
[0046]
The inorganic support which constitutes the catalyst includes, for example,
metal oxides, such as alumina, silica, titania, zirconia, and boria. These
metal oxides
may be one kind of metal or two kinds of mixtures, or may be composite metal
oxides,
such as silica-alumina, silica-zirconia, alumina-zirconia, and alumina-boria.
The
inorganic support is preferably composite metal oxides having solid acidity,
such as
silica-alumina, silica-zirconia, alumina-zirconia, and alumina-boria, from the
viewpoint
that the hydroisomerization of the normal paraffins efficiently proceeds
simultaneously
with the hydrotreating. Additionally, a small amount of zeolite may also be
included in
the inorganic support. Moreover, a binder may be blended with the inorganic
support
for the purpose of improvement of the moldability and mechanical strength of
the support.
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
23
Preferable binders include alumina, silica, magnesia, or the like.
[0047]
In a case where the active metal is the above noble metals, the content of the
active metal in the catalyst is preferably about 0.1 to 3 mass % based on the
mass of a
support as metal atoms. Additionally, in a case where the active metal is a
metal other
than the above noble metals, the content of the active metal in the catalyst
is preferably
about 2 to 50 mass % based on the mass of a support as a metal oxide. In a
case where
the content of the active metal is less than the lower limit, the
hydrotreating and the
hydroisomerization tend to proceed insufficiently. On the other hand, in a
case where
the content of the active metal exceeds the upper limit, the dispersion of the
active metal
tends to deteriorate, and thus, the activity of the catalyst tends to
decrease.
Consequently, the catalyst cost increases.
[0048]
The reaction temperature in the middle distillate hydrotreating reactor 52 in
present embodiment is 180 to 400 C, preferably 280 to 350 C, and more
preferably, 300
to 340 C. Here, the reaction temperature means the mean temperature of a
catalyst bed
in the middle distillate hydrotreating reactor 52. If the reaction temperature
is higher
than or equal to the lower limit temperature, the middle distillate is
sufficiently
hydrotreated and hydroisomerized, and if the reaction temperature is lower
than or equal
to the upper limit temperature, the concurrence of the decomposition reaction
of the
middle distillate can be suppressed, and a decrease in the lifetime of the
catalyst is
suppressed.
[0049]
The pressure (hydrogen partial pressure) in the middle distillate
hydrotreating
reactor 52 is preferably 0.5 to 12 MPa, and more preferably 1 to 5 MPa. If the
pressure
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
24
of the hydrotreating reactor is higher than or equal to 0.5 MPa, a raw middle
distillate
fraction is sufficiently hydrotreated, and if the pressure is lower than or
equal to 12 MPa,
the facility cost for increasing the resistance to pressure of the facility
can be suppressed.
[0050]
The liquid hourly space velocity (LHSV) in the middle distillate hydrotreating
reactor 52 is preferably 0.1 to 10 and more preferably 0.3 to 3.5 h-1. If
LHSV is
greater than or equal to 0.1 h, it is not necessary to make the volume of a
reactor
excessively large, and if the LHSV is equal to and less than 10h-1, the middle
distillate is
efficiently hydrotreated and hydroisomerized.
[0051]
The hydrogen gas/oil ratio in the middle distillate hydrotreating reactor 52
is
preferably 50 to 1000 NL/L, and more preferably 70 to 800 NL/L. Here, "NL"
means
the hydrogen volume (L) in the standard condition (0 C and 101325 Pa). If the
hydrogen gas/oil ratio is greater than or equal to 50 NL/L, the middle
distillate is
sufficiently hydrotreated and hydroisomerized, and if the hydrogen gas/oil
ratio is less
than or equal to 1000 NL/L, a facility for supplying a large amount of
hydrogen gas
becomes unnecessary, and an increase in operation cost can be suppressed.
[0052]
In addition, the above reaction conditions in the middle distillate
hydrotreating
reactor 52 are determined based on a measured cloud point of the hydrotreated
middle
distillate which flows out of the reactor.
[0053]
In the sampling unit 103, a sample of the hydrotreated middle distillate may
be
manually put into a container, the obtained sample may be carried to an
independent
cloud point measuring unit, and measurement of the cloud point may be manually
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
performed. In that case, the sampling unit 103 can be composed, for example,
by
doubly installing manual valves at piping which have branched off from the
discharge
line 102.
[0054]
5 On the other hand, the sampling unit 103 and the cloud point measuring
unit 110
may have the configurations in which taking of the sample and the measurement
of the
cloud point are performed not manually but automatically, respectively. The
sampling
unit 103 in that case, for example, is composed of small-diameter piping which
branches
off from the discharge line 102 and returns to the discharge line 102 again, a
plurality of
10 valves which is installed along the piping to switch a flow line whose
opening and
closing are controlled based on time, and a control mechanism which controls
the
opening and closing of the valves based on time. A small amount of newly
produced
hydrotreated middle distillate always flows through the inside of the sampling
unit 103,
and a fixed amount of the sample is periodically taken by switching the
valves. The
15 sampling unit 103 and the cloud point measuring unit 110 are connected
together by
piping, and the sample obtained by the sampling unit 103 is automatically
transferred to
the cloud point measuring unit 110. Then, the cloud point measuring unit 110
automatically measures the cloud point of the transferred sample by
interlocking the
control of the valve of the sampling unit 103 with the control of the cloud
point
20 measuring unit 110. When the measurement of the cloud point is
completed, the
measurement result is displayed on a display device, for example, provided in
a control
console of the control unit 104 which controls the operation of the middle
distillate
hydrotreating reactor 52. Additionally, in the cloud point measuring unit 110,
the
sample of the hydrotreated middle distillate whose measurement is completed is
25 automatically discharged, and preparation of the next measurement is
performed.
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
26
[0055]
As shown in FIG 3, the cloud point measuring unit 110 includes a vessel body
111 in a bottomed cylindrical shape made of aluminum, a lid portion 112 which
closes up
an opening of the vessel body 111, a cooler 113 which cools the vessel body
111, a vessel
temperature sensor 114 which measures the temperature of the vessel body 111,
a liquid
temperature sensor 115 which measures the temperature of the sample charged
into the
vessel body 111, and a cloud detector 116 which detects the cloud of the
sample charged
into the vessel body 111.
The cooler 113, which is an electronic cooling unit using a Peltier device
(not
shown), has the configurations to be able to control a cooling rate.
Additionally, the
cloud detector 116 is composed of an optical sensor including a photo-
transmitter and a
photo-receiver.
[0056]
A cloud point measuring method in this cloud point measuring unit 110 will be
described.
First, a obtained sample of the hydrotreated middle distillate is introduced
into
the vessel 111. Then, while the temperature is measured by the vessel
temperature
sensor 114 and the liquid temperature sensor 115, the hydrotreated middle
distillate is
cooled at a predetermined cooling rate by the cooler 113, and the liquid
temperature
when the generation of cloud has been detected by the cloud detector 116 is
determined
as the cloud point.
Here, it is preferable that the cloud point is measured while the obtained
sample
of the hydrotreated middle distillate is being cooled by the cooler 113 under
the condition
of a cooling rate of 5.0 C/min or more and 15.0 C/min or less. In the
present
embodiment, the cooling rate is set to 9.5 C/min.
CA 02750088 2011-07-19
OSP38125-38141(GTL0401)
27
[0057]
Next, the operation method of the middle distillate hydrotreating reactor 52
will
be described using the flow chart of FIG. 4.
A raw middle distillate distilled from the middle part of the first
fractionator 40
is supplied to the middle distillate hydrotreating reactor 52 through the
supply line 101,
and is hydrotreated and hydroisomerized in the reactor (S1).
The initial operating conditions of the reactor are set at the starting of the
middle
distillate hydrotreating reactor 52. Additionally, during normal operations,
the
operating conditions of the reactor are changed if the measured cloud point of
the
hydrotreated middle distillate in the subsequent steps is out of a target
range (S2).
[0058]
The hydrotreated middle distillate flowing the discharge line 102 from the
middle distillate hydrotreating reactor 52 is sampled (S3).
The cloud point of the sampled hydrotreated middle distillate is measured by
the
above-mentioned cloud point measuring unit 110 (S4).
Then, the measured cloud point, and an operation control target value are
compared with each other, and whether or not the measurement value is within
an
operation control target range is judged (S5).
If the cloud point is within the operation control target range, the operating
conditions of the middle distillate hydrotreating reactor 52 are maintained
(S6). In
addition, even in a case where the cloud point is within the operation control
target range,
minute changes in the operating conditions may be performed, for example, for
the
purpose of bringing the cloud point close to an operation control target
center value.
Then, confirmation/maintenance of a stable state becomes possible by
performing sampling of the hydrotreated middle distillate again after a
predetermined
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
28
period of time (returns to S3), and repeating the subsequent steps.
On the other hand, if the cloud point is out of the operation control target
range,
in the control unit 104, the operating conditions (hydrogen partial
pressure/reaction
temperature/throughput of middle distillate per unit time (for example, LHSV))
of the
middle distillate hydrotreating reactor 52 are changed (returns to S2).
Then, sampling of the hydrotreated middle distillate is performed again after
predetermined time (S3), and the subsequent steps are repeated. This makes it
possible
to confirm the effects of changes in the operating conditions of the middle
distillate
hydrotreating reactor 52 in S2.
[0059]
As for changes in the operating conditions of the middle distillate
hydrotreating
reactor 52 in S2, specifically, if the cloud point exceeds the upper limit of
the operation
control target range, the hydroisomerization is promoted and the cloud point
of the
hydrotreated middle distillate is lowered, by setting the conditions wherein
the hydrogen
partial pressure is raised, and/or the reaction temperature is raised, and/or
the throughput
of the middle distillate per unit time (LHSV) is reduced. Additionally, if the
cloud point
falls below the lower limit of the operation control target range, the
hydroisomerization is
suppressed and the cloud point of the hydrotreated middle distillate is
elevated by setting
the conditions wherein the hydrogen partial pressure is reduced, and/or the
reaction
temperature is lowered, and/or the throughput of the middle distillate per
unit time
(LHSV) is increased. Especially, by changing the reaction temperature, the
cloud point
of the hydrotreated middle distillate can be effectively changed.
In addition, the control unit which controls the operating conditions of the
middle distillate hydrogenation reactor 52, such as hydrogen partial pressure,
reaction
temperature, and throughput of the middle distillate per unit time, may be the
one which
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
29
performs the operation control of a general reactor.
[0060]
If the cloud point of the hydrotreated middle distillate which flows out of
the
middle distillate hydrotreating reactor 52 is out of the operation control
target range, the
operating conditions of the reactor are changed so that the cloud point falls
within the
operation control target range. Then, the handling of a hydrotreated middle
distillate
which flows out until it is confirmed that the cloud point of the hydrotreated
middle
distillate which flows out has fallen within the operation control target
range is not
particularly limited.
If the cloud point of the hydrotreating middle distillate which flows out of
the
middle distillate hydrotreating reactor 52 falls below the lower limit of the
operation
control target range, even if there is a problem in terms of efficiency that
the yield of the
middle distillate is lowered due to an increase in a light component caused by
a
concurrent decomposition reaction, there is a possibility that a middle
distillate product
obtained through the fractionation in the second fractionator 70 satisfies
product
specifications. Thus, the hydrotreated middle distillate may be brought to the
second
fractionator 70, and may be taken out as a product.
On the other hand, if the cloud point of the hydrotreated middle distillate
which
flows out of the middle distillate hydrotreating reactor 52 exceeds the upper
limit of the
operation control target range, the hydrotreated middle distillate may not be
brought to
the second fractionator 70, but may be brought to a slop tank. Otherwise,
after the
hydrotreated middle distillate is temporarily stored in another storage
facility, the middle
distillate is returned to and reprocessed separately in the middle distillate
hydrogenation
reactor 52. Then, after it is confirmed that the cloud point has fallen within
the
operation control target range, the hydrotreated middle distillate may be
brought to the
CA 02750088 2011-07-19
OSP38125-38141(GTL0401)
second fractionator 70 and may be taken out as a product.
Additionally, not only the hydrotreated middle distillate from the middle
distillate hydrotreating reactor 52 but a hydrocracked product from the wax
fraction
hydrocracking reactor 50 is supplied to the second fractionator 70. Hence,
even in a
5 case where the cloud point of the hydrotreated middle distillate which
flows out of the
middle distillate hydrotreating reactor 52 exceeds the upper limit of the
operation control
target range, the cloud point of a middle distillate product obtained from the
second
fractionator 70 may satisfy the product specifications. Accordingly, if the
hydrotreated
middle distillate is brought to and fractionated in the second fractionator
20, and if it is
10 expected that the middle distillate obtained satisfies the product
specifications, this may
be taken out as a product. If the cloud point of the product exceeds the upper
limit of
the range of the product specifications, the product may be returned to and
reprocessed in
the middle distillate hydrotreating reactor 52.
[0061]
15 According to the middle distillate hydrotreating reactor 52 and the
operation
method of the middle distillate hydrotreating reactor 52 constructed as
described above,
which are the present embodiment, the cloud point of the hydrotreated middle
distillate
flowing out from the middle distillate hydrotreating reactor 52 is measured,
and the
operating conditions of the middle distillate hydrotreating reactor 52 are
controlled based
20 on this cloud point. Thus, the degree of the hydroisomerization in the
middle distillate
hydrotreating reactor 52 is constantly maintained. Hence, the properties of
the
produced hydrotreated middle distillate become stable, and thus, the quality
of gas oil
(diesel fuel oil) produced using this hydrotreated middle distillate as a
feedstock can be
significantly improved.
25 [0062]
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
31
Additionally, since the sampled hydrotreated middle distillate is cooled by
the
cooler 113 composed of an electronic cooling unit using the Peltier device in
the cloud
point measurement, it is possible to control the cooling rate of the
hydrotreated middle
distillate accurately and easily. Also, in the present embodiment, the cooling
rate is set
to 5.0 C/min or more and 15.0 C/min or less, and more specifically, the
cooling rate is
set to 9.5 C/min. Thus, it is possible to accurately and rapidly measure the
cloud point.
This makes it possible to control the operating conditions by the control unit
104 with
suitable timing, and to stabilize the operation of the middle distillate
hydrotreating
reactor 52.
[0063]
Although the embodiments of the present invention have been described hitherto
in detail with reference to the drawings, concrete configurations are not
limited to the
embodiments, and the present invention also includes design changes which do
not
depart from the spirit of the present invention. For example, although the
cloud point
measuring unit has been described as one including the cooler composed of the
electronic
cooling unit using the Peltier device, the invention is not limited thereto.
For example,
as shown in JIS K 2269, the cloud point may be measured by gradually cooling
the
hydrotreated middle distillate using a cooling bath.
Additionally, the operating conditions of the upgrading unit, or the like, are
not
limited to the ranges described in the embodiment, and may be suitably changed
according to situations.
Moreover, the configurations of the synthesis gas production unit 3, FT
synthesis unit 5, and upgrading unit 7 are not limited to those described in
the present
embodiment, and it is only necessary to supply the middle distillate of the FT
synthesis
hydrocarbons to the middle distillate hydrotreating reactor 52.
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
32
[Embodiments]
[0064]
The results of a confirmatory experiment performed to confirm the effects of
the
present invention will be described.
[0065]
(Relationship between cloud point and degree of hydroisomerization)
A confirmatory experiment was performed on the relationship between the
degree of the hydroisomerization in the middle distillate hydrotreating
reactor, and the
cloud point (CP) of a produced hydrotreated middle distillate. The operating
conditions
20 [0066]
As shown in FIG 5, a strong correlation is observed between the cloud points
of
the hydrotreated middle distillates, and n-C19F content. Here, n-C19+ content
is an index
showing the degree of hydroisomeraization. It was confirmed from this that the
degree
of the hydroisomeraization in the middle distillate hydrotreating reactor can
be
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
33
[0067]
(Measuring method of cloud point)
Next, confirmation was performed with regard to the precision and measuring
time in the cloud point measurement when the measurement is performed by the
measuring method based on JIS K 2269 and the measuring method shown in the
present
embodiment in which the hydrotreated middle distillate sample is cooled by the
electronic cooling unit using the Peltier device at increased cooling rates.
Three kinds of samples (samples 1 to 3) of liquid hydrocarbons having a
boiling
point range equivalent to gas oil were prepared, and cloud points of these
samples were
measured by a measuring method based on JIS K 2269 using an automatic pour
point/cloud point/cold filter plugging point tester RPCF-03CML (Trade name),
made by
Rigo Co., Ltd., and were referred to as Examples 1 to 3.
Additionally, the cloud points of the aforementioned samples were measured in
conditions of cooling rates of 5.0 C/min, 7.0 C/min, and 9.5 C/min,
respectively, by
the cloud point measuring unit (specifically, an automatic pour point/cloud
point tester
MPC-102 Type made by Tanaka Scientific Ltd.) which is the above embodiment,
and
were referred to as Examples 4 to 12. In addition, in Examples Ito 12, the
identical
cloud point measurement was repeated four times. The results are shown in
Table 1.
[0068]
0SP38125-38141(GTL0401)
34
1
,
,
[Table 1]
Example Example Example Example Example Example Example Example Example
Example Example Example
1 2 3 4 5 6 7 8
9 10 11 12
(1) (2) (3) (1) , (2) _ (3) (1) . (2)
(3)_ (1) (2) (3)
Method 2269 2269 2269
C/min C/min C/min
. .
Measurement -9 -18 -30 -8 -17 -30 -7 -17
-30 -7 -17 -30
Value (1)
Measurement -8 -17 -29 -8 -17 -30 -8 -17
-30 -7 -17 -30
Value (2) _
Measurement -7 -17 -29 -8 -17 -30 -7 -17
-30 -7 -17 -30 0
Value (3)
,
0
Measurement -8 -18 -30 -7 -18 -30 -7 -17
-30 -6 -17 -30 iv
-.3
Value (4)
co
0
Measuring 60 min 70 min 90 min 8 min 12 min 21 min 7
min 10 min 19 min 6 min 8 min 17 min 0
co
co
Time
I\)
0
H
I7
0
.-.1
I
H
l0
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
[0069]
As shown in Table 1, the cloud points measured by JIS K 2269, and the cloud
points measured in conditions of cooling rates of 5.0 C/min, 7.0 C/min, and
9.5 C/min,
respectively, coincide with each other with an error within 2 C for the
identical sample.
5 Here, the error in JIS K 2269 is defined within 2 C for the identical
testing device and is
defined within 4 C for different testing devices. Then, the error within 2 C
between
Examples 1 to 3 and Examples 4 to 12 is within a range permitted even in JIS K
2269.
It was confirmed that even Examples 4 to 12, measuring in conditions of
cooling rates of
5.0 C/min, 7.0 C/min, and 9.5 C/min, respectively, can measure the cloud
points with
10 the precision equivalent to JIS K 2269.
[0070]
Additionally, the measuring time was 60 to 90 minutes in Examples 1 to 3 based
on JIS K 2269, whereas the measuring time was 6 to 21 minutes, during which
the cloud
points could be measured in a short time, in Examples 4 to 12 measured in
conditions of
15 cooling rates of 5.0 C/min, 7.0 C/min, and 9.5 C/min, respectively.
Accordingly, it was confirmed that the control of the middle distillate
hydrotreating reactor can be reliably and more rapidly performed by measuring
the cloud
points as in Examples 4 to 12.
20 [Industrial Applicability]
[0071]
According to the operation method of the middle distillate hydrotreating
reactor,
and the middle distillate hydrotreating reactor in the present invention, it
is possible to
properly hydroisomerize the FT synthesis hydrocarbons obtained by the FT
synthesis
CA 02750088 2011-07-19
0SP38125-38141(GTL0401)
36
reaction in the hydrotreating process of the middle distillate, to produce a
hydrotreated
middle distillate with stable properties, and to obtain high-quality gas oil.
[Description of Reference Numerals]
[0072]
1: LIQUID-FUEL SYNTHESIZING SYSTEM
7: UPGRADING UNIT
40: FIRST FRACTIONATOR
52: MIDDLE DISTILLATE HYDROTREATING REACTOR
103: SAMPLING UNIT
104: CONTROL UNIT
110: CLOUD POINT MEASURING UNIT
113: COOLER
116: CLOUD DETECTOR