Note: Descriptions are shown in the official language in which they were submitted.
CA 02752423 2011-09-15
OXIDATIVE REMEDIATION OF OIL SANDS DERIVED AQUEOUS
STREAMS
FIELD OF THE INVENTION
The present invention relates generally to remediation of industrial aqueous
streams, and particularly to oxidative remediation of aqueous streams derived
from oil sands operations.
BACKGROUND OF THE INVENTION
Control of the level of odor causing toxic species such as hydrogen sulphide
in oil sands derived streams and remediation of such streams has become of
increasing importance in view of the environmental regulations.
In chemical, mining, and mineral processes, examples of technologies used to
control odor causing species such as hydrogen sulphide in wastewater
include chemical scavenging techniques, oxidation, physical covers, and
scrubbing. Chemical scavenging refers to removing hydrogen sulphide from
wastewater before it volatizes to the atmosphere. Oxidation may involve the
use of hydrogen peroxide to treat wastewater by oxidation of the hydrogen
sulphide to elemental sulfur. Hydrogen peroxide has also been used to
prevent hydrogen sulphide formation by supplying dissolved oxygen. Physical
covers may be placed on the surface of the wastewater to reduce the release
of hydrogen sulphide to the atmosphere. Furthermore, physical, chemical
and/or biological scrubbing methods may be employed to remove the
hydrogen sulphide.
While there has been much attention devoted to the treatment of wastewater
derived from various industries, application and efficacy of such treatment
methods present issues for treating oil sands derived streams. Therefore,
there is a need for methods for controlling the level of odor causing
substances in streams derived from oil sands operations.
CA 02752423 2011-09-15
-2-
SUMMARY OF THE INVENTION
In accordance with one aspect of the invention, there is provided a method of
processing an oil sands derived aqueous stream having a concentration of
odor causing species. In various aspects, the oil sands derived aqueous
stream may comprise produced water, tailings water, SAGD-derived water,
and the odor causing species may comprise hydrogen sulphide.
The method involves monitoring the concentration of the odor causing species
in the oil sands derived aqueous stream, determining a concentration of an
oxidizer such as for example hydrogen peroxide to be added to the oil sands
derived aqueous stream to oxidize the odor causing species based on the
concentration of the odor causing species in the oil sands derived aqueous
stream, adding the determined concentration of the oxidizer to the oil sands
derived aqueous stream, and producing a treated aqueous stream depleted in
the odor causing species. In various aspects, the monitoring comprises a
titration of the odor causing species wherein the titration may be an on-line
titration, or the titration in combination with an oxidation reduction
potential
(ORP) analysis. In various aspects, the odor causing species may have a
concentration ranging from about 0 to about 200 ppm, the oxidizer may have
a concentration ranging from about 150 ppm to about 400 ppm.
In various aspects, the method may further involve modulating chemical
properties of the oil sands derived aqueous stream to convert the odor
causing species of interest to a desired chemical form. An example of
modulation of the chemical properties involves adjusting a pH of the oil sands
derived aqueous stream, which may be performed by adding KOH, caustic,
blending of one or more of the oil sands derived aqueous streams, or a
combination thereof.
In further aspects, the method may comprise analyzing the treated aqueous
stream for the presence of the oxidizer, the odor causing species or a
combination thereof. In various aspects, the treated aqueous stream may be
CA 02752423 2011-09-15
-3-
reused within the oil sands operations including steam assisted gravity
drainage. In various aspects, the treated aqueous stream may have a
concentration of the odor causing species ranging from about 0 ppm to about
200 ppm, and a concentration of the oxidizer ranging from about 0 to about
150 ppm, from about 150 ppm to about 400 ppm. In various aspects, where
for example a sufficient reduction in the odor causing species has not been
achieved, the treated aqueous stream may be further subjected to the
treatment as described in connection with the oil sands derived stream.
BRIEF DESCRIPTION OF THE DRAWINGS
In accompanying drawings which illustrate embodiments of the invention,
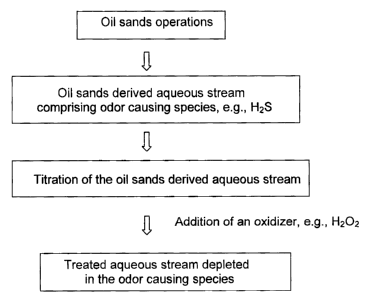
FIG. I illustrates a flow chart of the method according to an embodiment.
FIG. 2 illustrates results showing that inlet H2S (blue) does not correlate
with
ORP (black).
FIG. 3 illustrates peroxide flow for Plant 91 and shows the variation and
frequency of dosing adjustments.
FIG. 4 illustrates that H2S concentration does not correlate to ORP due to
interferences with sodium sulphite and reduced organics.
CA 02752423 2011-09-15
-4-
DETAILED DESCRIPTION
Reference will now be made in detail to implementations and embodiments of
various aspects and variations to the invention.
In various aspects, the present invention relates to methods for oxidative
remediation of aqueous streams derived from oil sands operations.
In various embodiments, the oxidative remediation of an oil sands derived
aqueous stream comprises treatment using an oxidizer under selected
conditions. In various embodiments, the treatment comprises monitoring a
concentration of the odor causing species in the oil sands derived aqueous
stream, optionally modulating the chemical properties of the oil sands derived
aqueous stream, which may for example entail converting the odor causing
species of interest to a desired chemical form, controlling the addition of
the
oxidizer to the oil sands derived aqueous stream based on results obtained
from the monitoring step such that the odor causing species react with the
oxidizer so as to lower a concentration of the odor causing species in the oil
sands derived aqueous stream to a desired target to form a treated aqueous
stream depleted in the odor causing species. A flow chart showing the
treatment of the oil sands derived stream according to an embodiment, by
way of example, is shown in Figure 1.
The various embodiments of the invention present several advantages. For
example, the process of the present invention results in production of the
treated aqueous stream depleted in the odor causing species, wherein the
treated aqueous stream meets environmental regulatory limits relating to
emissions stemming from the odor causing species. For example, the current
emission limit for hydrogen sulphide (H2S) is zero emission. The process the
present invention may further result in a reduction in exposure to hazardous
odor causing species (e.g., hydrogen sulphide) in the treated aqueous stream.
The reduction in concentration of hazardous odor causing species in various
embodiments also may decrease risks of corrosion and fouling to equipment
CA 02752423 2011-09-15
-5-
in oil sands operations which use, transport or store the treated aqueous
stream.
In various embodiments, the oil sands derived aqueous stream comprises
water derived from oil sands operations. In various embodiments, oil sands
operations in which oil sands derived water is used, produced, or transported
include for example bitumen mining and extraction, SAGD, tailings treatment
water. In various embodiments examples of water derived from oil sands
operations include for example produced water or other operational streams,
recycle water, wastewater, makeup water, make up well water, blowdown
streams, pond waters, water from deoiling operations, tailings water or a
combination thereof.
Oil sands derived aqueous streams such as oil sands derived water unlike
aqueous streams (e.g., wastestreams, produced water) derived from other
industrial operations present particular remediation challenges due to their
complex chemical nature. For example, the composition of the oil sands
derived water will vary depending on the type of oil sands operation from
which the water is derived. Therefore, even water derived from different
sources within the oil sands operations may present unique remediation
issues relating to that particular source or a combination of sources. For
example, the produced water tank in oil sands operations may receive a
combination of water influents having different contaminants, different pH,
temperature, which individually or synergistically may have different
downstream impacts. Therefore a remediation protocol for the particular oil
sands derived aqueous stream or a combination of streams may need to
accommodate the differences in the properties of the streams, which presents
unique processing challenges. Aqueous streams derived from oil sands
operations unlike water from other industrial operations has a complicated
contaminant profile which may include, for example, oil, odor causing species,
non-sulfur organic species, organo-sulfur species, organometallic species,
and inorganic species or a combination thereof which may be dissolved,
dispersed or bound within suspended solid material that may be present in the
CA 02752423 2011-09-15
-6-
oil sands derived aqueous streams depending on the source. Furthermore,
the oil sands water may comprise emulsions (e.g., hydrocarbon in water
emulsions) which may further complicate the remediation process for such
water.
For example, the produced water derived from oil sands operations may have
a high silica content, hydrogen sulphide, water soluble organics and oil from
bitumen, hardness causing species, as well as have a high temperature.
Other water sources derived from oil sand operations may have added caustic
or other pH modulating species, and therefore may have variable pH, salts,
sulphites, and dissolved and particulate iron.
In various embodiments, the odor causing species in the oil sands derived
aqueous stream include hydrogen sulphide (H2S), and other reduced sulphur
species. For example, hydrogen sulphide is a poisonous gas and is typically a
component of the produced water derived from the production wells.
Furthermore, use of sour diluent may also increase the concentration of
hydrogen sulphide in the produced water. At elevated pH, the sulphide may
be trapped in the produced water as an ion, however if the water is acidified,
it
would be released.
Remediation of the oil sand derived aqueous stream according to various
embodiments comprises treatment of the oil sands derived aqueous stream
with an oxidizer under selected conditions to reduce the concentration of or
eliminate the odor causing species from the oil sands derived aqueous
stream.
In various embodiments, the oxidizer is a compound or a chemical species
which through a reaction with the odor causing species reduces the
concentration of the odor causing species in the oil sands derived aqueous
stream to form a treated aqueous stream. In various embodiments, the
oxidizer may comprise, for example, fluorine, hydroxyl radical, sulfate
radical,
persulfate anion, hydrogen peroxide, permanganate, peroxysulfuric acid,
CA 02752423 2011-09-15
-7-
ozone, hypochlorite, chlorine dioxide or a combination thereof where
applicable. The choice of a suitable oxidizer or a combination of oxidizers
may be tailored to the particular properties of the oil sands derived aqueous
stream to be treated using the oxidizer, the particular treatment conditions,
desired targets to be achieved, or a combination thereof.
In various embodiments, the oxidizer or a combination of oxidizers may be
sequenced for treatment of the oil sands derived aqueous stream or one or
more treated aqueous streams to optimize the treatment for the particular
chemistry of the stream (e.g., different oxidizers may target different odor
causing species) in order to achieve the desired target. In various
embodiments, the oxidizer may be used in combination with other chemical
agents (e.g., activators, catalysts) to aid in optimizing the reaction between
the oxidizer and the odor causing species.
In various embodiments, the oxidizer may be supplied as a premixed solution
such as, for example, an aqueous hydrogen peroxide solution. In various
embodiments, the oxidizer may have various solution concentrations, for
example the concentration of hydrogen peroxide solution my range from
about 10% to about 30%, from about 30% to about 50%, from about 50% to
about 70%. In selected embodiments, the concentration of hydrogen peroxide
solution may be about 50 wt.%. In various embodiments, suitable
concentration of the oxidizer would depend on the type of oxidizer used,
operational conditions (e.g., temperature), the type and concentration of odor
causing species to be treated or a combination thereof. A suitable
concentration range of the oxidizer or a suitable oxidizer type for treating a
particular oil sands derived aqueous stream may be determined by treating a
sample of the oil sands derived aqueous stream and analyzing for a
concentration of the odor causing species in the treated aqueous stream.
Other options include calculating the required concentration, or using ORP
probes (oxidation/reduction potential probes), but these methods when used
alone were found not to work well for oil sands derived aqueous streams, and
in particular streams with variable composition.
CA 02752423 2011-09-15
-8-
In various embodiments, a suitable dose of the particular oxidizer for
treating
the oil sands derived aqueous stream may be determined from the
concentration of odor causing species in the oil sands derived stream and the
stoichiometry of the reaction. In various embodiments, an excess dose of the
oxidizer may be used having regard to potential side effects of using the
oxidizer in excess. For example, according reaction stoichiometrics, 4.25 kg
of
hydrogen peroxide is needed to oxidize 1 kg of sulphide in produced water
stream. In various embodiments, suitable dosing for a particular oxidizer and
for a particular composition of the oil sands derived aqueous stream is
determined by titration measurements for destruction of compounds alone or
in combination with oxidation reduction potential, calculation or a
combination
thereof. Excess of oxidizer may be required for treating the oil sands derived
aqueous stream comprising species that may consume or inhibit the oxidizer,
or in circumstances where decomposition of the oxidizer may have occurred
to some extent due to, for example, presence of oxygen or heat. The excess
oxidizer that is not used up in the reaction with the odor causing species may
be consumed by other species in the oil sands derived stream such as, for
example, water soluble organics.
In various embodiments when the oil sands derived aqueous stream is
contacted with the oxidizer such as hydrogen peroxide, the odor causing
species (e.g., hydrogen sulphide) in the oil sands derived aqueous stream are
abated by oxidation. In various embodiments, to achieve the desired reactions
products, the chemical properties of the oil sands derived aqueous stream
may need to be modulated. For example, in the case of hydrogen peroxide as
the oxidizer, hydrogen peroxide is permanently destroyed and cannot be
released from the oil sands derived stream later through acidification when
contacted with the oil sands derived stream at alkaline pH which results in
the
production of a soluble reaction product (e.g., sulphate). In contrast, at
acidic
and neural pH, contacting the oil sands derived water comprising sulphide
with hydrogen peroxide would result in formation of elemental sulfur which is
undesirable because producing a solid reaction product can cause deposition
CA 02752423 2011-09-15
-9-
problems in the reaction vessel and downstream operation problems.
Examples of modulating the chemical properties of the oil sands derived
aqueous stream include, for example, pH adjustment (e.g., addition of caustic,
soda ash, KOH), catalyst addition, activator addition for activating the
oxidizer,
or a combination thereof. In various embodiments pH adjustment may be
used to adjust the pH of the oil sands derived aqueous stream to a pH ranging
from about 6 to about 8, about 8 to about 11.5, about 11.5 to about 12. In
various other embodiments, oil sands derived streams having different pH
values may be combined to achieve a desired pH. Such streams may be
mixed in a vessel or in pipe (e.g. T-mixing).
In various other embodiments, there may be no pH adjustment of the oil
sands derived aqueous stream. For example, hydrogen peroxide may be
added to the oil sands derived aqueous stream of any pH and still abate the
H2S, or the oil sands derived stream may have a suitable pH such that no pH
modulation is needed.
In various embodiments of the invention, caustic or other pH adjustment to the
oil sands derived aqueous stream may be performed, for example, in the
produced water tank, in pipe or in a reaction vessel. For example, the
addition
of the caustic may be batch wise or continuous depending on the chemical
properties of the oil sands derived stream. In various embodiments,
correlations between residual odor causing species, pH adjustment (e.g.
addition of caustic, soda ash, KOH), titration results relating to the
concentration of odor causing species in the oil sands derived aqueous
stream or a combination thereof may be calculated or obtained from
experimental results. In various embodiments, pH adjustment (e.g., addition of
caustic, soda ash, KOH), hydrogen peroxide addition or a combination thereof
may be based on the titration results relating to the odor causing species in
the oil sands derived aqueous stream. In various embodiments, the
monitoring of the concentration of the odor causing species, and therefore
determination of pH adjustment needed (e.g. addition of caustic, soda ash,
KOH), hydrogen peroxide addition or both may be performed on-line.
CA 02752423 2011-09-15
-10-
In various embodiments, pH adjustment such as for example caustic addition
may avoid silica precipitation for example in the evaporators. In various
other
embodiments, an anti-scale agent may be used since at basic pH calcium
carbonate scale may form. In various embodiments, a maximum pH may be
established for a particular oil sands derived aqueous stream at which
hydrogen peroxide may perform optimally and at which the tendency to form
calcium carbonate scale would be very low without using the anti-scale
agents.
In various embodiments, the need for treating the oil sands derived stream
may be determined by analyzing the stream that is to be used, produced or
transported within the oil sands operations for the presence of odor causing
species or by sampling the stream, the emissions relating to or arising from
the particular stream, or a combination thereof. For example, sampling
emissions from the produced water tank, evaporators, deareator, distillate
tanks or a combination thereof may provide an indication of whether or not the
oil sands derived stream in connection with these operations may need to be
treated according to the various embodiments of the present invention. The
emissions from the produced water tank, evaporators, or deareator and
distillate tanks may generate odor in the plant's surroundings, as well as
there
may be a potential health risk for the personnel that work in the plant area,
and therefore such streams would be suitable for treatment with the oxidizer.
In various embodiments a schedule for sampling the oil sands derived stream
or emissions arising from the stream may be implemented to determine the
need for treatment at various injection points. For example, a weekly sampling
and analyzing schedule in connection with the produced water from induced
static flotation (ISF) (to produced water tank) indicated that the maximum H2S
concentration in the water was about 56 ppm in the time duration studied. The
selected injection point for the hydrogen peroxide was the produced water
tank since it feeds the evaporators in this embodiment, and the oxidation
CA 02752423 2011-09-15
-11-
reaction would take place within the tank so as to mitigate emissions
downstream.
Further considerations for choosing a suitable injection point for the
oxidizer
include hazards associated with the injection of hydrogen peroxide at the
potential injection point (e.g., considerations regarding the presence of
caustic). Such hazards further differentiate the application of hydrogen
peroxide to oil sands derived aqueous stream from wastewater treatment
applications of the prior art. For example, if in various embodiments
unacceptable hazards are identified, then an alternate injection point for the
oxidizer should be adopted. For example, for the produced water tank one
option may be to inject the oxidizer through an internal pipe to the base of
the
tank, an alternative injection point may be to inject the oxidizer such as
hydrogen peroxide into a spare flange on top of the produced water tank. In
this option, the hydrogen peroxide would drip onto the produced water tank
liquid / air interface. This option may be acceptable because the tank's
outlet
nozzle is located near the bottom of the tank and it would take several hours
(i.e., the tank's residence time) for the hydrogen peroxide to reach the
bottom
nozzle, and the hydrogen peroxide should be well mixed by the time it is
processed by the evaporator pumps.
In embodiments where modulation of the chemical properties of the oil sands
derived aqueous stream (e.g., pH) is performed using, for example, addition
of caustic, soda ash, or KOH, may be injected into the evaporator feed recycle
piping inside the evaporator building. The recycle water / pH modulation
blend may be routed outside and redirected into the produced water tank.
The total piping distance between for example the caustic injection point and
the proposed hydrogen peroxide injection point may be, for example, about 75
feet or more. Such a method may mitigate any explosion risks associated
with contact of the peroxide with the caustic. In the embodiments comprising
pH adjustment such as for example caustic addition, the oil sands derived
aqueous stream may contain about 0.09 to about 0.11 wt.% caustic. In
various embodiments, the pH of the oil sands derived aqueous stream may
CA 02752423 2011-09-15
-12-
range from about 6 to about 8, about 8 to about 11.5, about 11.5 to about 12,
including embodiments in which no pH adjustment is performed or required.
In various embodiments the treated aqueous stream may be reused within the
oil sands operations. For example, the treated aqueous stream may be used
for production of steam or for use in SAGD.
In various embodiments, residual peroxide in the treated aqueous stream is
determined using a titration determination. In various embodiments, this type
of titration is advantageous as organic sulfur species should not pose
interference.
One of the surprising findings in connection with the various embodiments of
the invention relates to the finding that conventional solutions associated
with
wastewater treatment were not successful in the application to the oil sands
derived aqueous streams. Many treatment methods have been used for
treating wastewater from other industries, examples of which include
treatments comprising aeration, ozone, chlorine dioxide, sodium hypochlorite,
biological treatment, advanced oxidation, water soluble H2S scavengers, and
metal salt/sulphide precipitation. The treatment reagents used in such
wastewater treatments, when added to the oil sands derived aqueous stream,
which has particularly challenging chemical properties, are unsuitable
because they tend to increase the total dissolved solids content (TDS) in the
oil sands derived aqueous stream, and therefore the solids and potentially
other contaminants generated during the treatment would subsequently need
to be removed prior to using the treated aqueous stream in other oil sands
operations. Therefore, the prior art approaches are not operationally and
economically effective for application to the oil sands derived aqueous
stream.
Unlike the oxidants generally used in the prior art wastewater treatment
methods in other industries, the use of hydrogen peroxide in the treatment of
oil sands derived aqueous stream according to various embodiments of the
present invention does not increase a total dissolved solids content (TDS) in
the oil sands derived water, and the hydrogen peroxide degrades to oxygen
CA 02752423 2011-09-15
-13-
and water. Therefore, the methods of the present invention are particularly
advantageous for use in treating oil sands derived aqueous streams which
following treatment may be suitable for subsequent re-use within the oil sands
operations.
In various aspects, because of the particular chemical properties of the oil
sand derived water and the hazards associated with the use of hydrogen
peroxide, the methods of the present invention require appropriate dosing of
the hydrogen peroxide depending on the composition of the produced water.
Various methods in the prior art have been used to determine proper dosing
of a treatment agent for treating wastewater. One such method is oxidation
reduction potential (ORP). Oxidation-reduction potential (ORP) or "redox"
indicates the relative capability of a solution to oxidize or reduce.
Application
of ORP to oil sands derived aqueous stream was tested and found not be
successful if used alone because the oil sands derived aqueous streams have
particularly complex chemical properties, which negatively affect the results
that may be obtained with ORP. The ORP sensor was found to be unable to
provide a predictable indication of the demand for the oxidizer such as
hydrogen peroxide in the oil sands derived water to achieve effective control
of the concentration of odor causing species such as hydrogen sulphide in the
resultant treated aqueous stream. For example, the amounts of "reduced"
organics and "reduced" sulfur species in the oil sands derived aqueous
stream made it difficult to use ORP to distinguish the hydrogen sulphide from
other "reduced" species (e.g., sulfite, mercaptans, thiopenes, and organics)
in
the stream. Figures 2 and 4 show by way of example that H2S concentration
does not correlate to OPR measurements due to the makeup of the oil sands
derived water. Figure 3 shows by way of example variation and frequency of
dosing adjustments of the oxidizer.
Another prior art method tested for application to the oil sands derived
aqueous streams involved correlating peroxide residuals in the stream.
However, analytical measurements of residual oxidant species in connection
with this method were also unpredictable due to interferences from other
CA 02752423 2011-09-15
-14-
contaminants in the stream (e.g., in the produced water). Additionally, the
peroxide added was consumed rapidly even when dosed in excess. The
present method, in contrast to the prior art, involves determination of dosing
of
the oxidizer using titration and therefore allows controlled dosing of the
oxidant (e.g., hydrogen peroxide) such that a reduction in the concentration
of
the odor causing species in the oil sands derived aqueous stream may be
achieved in an efficient and economical manner.
Examples
In the examples below, in connection with selected embodiments, the oxidizer
is hydrogen peroxide and the odor causing species is hydrogen sulphide. For
example, a concentration of dissolved hydrogen sulphide present in the
produced water from deoiling processes may be controlled by the addition of
hydrogen peroxide to the produced water. Because levels of hydrogen
sulphide in the produced water may vary considerably with which wells are
being pumped and the amount of blowdown or make-up water blended with
the produced water, the corresponding hydrogen peroxide injection rate must
be continuously managed to prevent an under-dose or over-dose situations.
Use of ORP measurements alone was found to be an insufficient means of
control of hydrogen peroxide injection because of several chemical
interferences in the produced water which make the ORP measurements
unreliable. Therefore, a titration method was developed for controlled
peroxide dosing or injection rates. In various embodiments, the titration
method was found to be reliable for controlling hydrogen peroxide injection
rates and may be used alone or in combination with prior art methods such as
ORP to provide substantially reliable results relating to the concentration of
odor causing species in the oil sands derived aqueous stream and the
required dosing of the oxidant.
In various embodiments, laboratory titration can be performed by an on-line
process analyzer. For example, the titration may require about 2-3 hours each
CA 02752423 2011-09-15
-15-
day to complete and provides a substantially accurate indication of hydrogen
peroxide injection rates required per day. In various embodiments, an
automated titration (produced water in and treated produced water out) may
be used to measure hydrogen sulphide and thus provide control over the
injection of hydrogen peroxide to reduce the concentration of or eliminate the
hydrogen sulphide in the oil sands derived water.
For example, in various embodiments, the on-line process analyzer may be
mounted near the produced water in and produced water out sample points.
In various embodiments, the analyzer may be configured to collect and
analyze a sample on an hourly basis or other selected frequency. To process
a sample, the analyzer may open a valve which allows sample to fill an
internal sample holder. An internal pump may then add a buffering agent to
the sample, an electrode in the sample holder may measure the signal as
titrant is pumped in to the sample holder. The analyzer detects the ending
point of the titration and sends out an analog signal corresponding to the
measured hydrogen sulphide concentration.
In various embodiments, the analyzer may be installed in the produced water
tank "IN" sample point, and the resulting hourly hydrogen sulphide
concentrations (or concentrations measured at other time intervals) could be
automatically stored and made available for adjustment of hydrogen peroxide
injection rates. The analyzer may also perform a produced water tank "OUT"
stream sample analysis. The titration method may be performed off line or,
preferably, on line to account for any changes in the oil sands derived stream
or combinations of streams.
In various embodiments, control over dosing of the hydrogen peroxide is
based on results obtained from monitoring of the concentration of odorous
sulfur species. Proper dosing of the hydrogen peroxide is important because
of the associated risks with use of this oxidizer. The purpose of adding
hydrogen peroxide to the oil sands derived aqueous stream (e.g. produced
water tank) is to oxidize the hydrogen sulphide. In various embodiments, the
CA 02752423 2011-09-15
-16-
peroxide may be injected into caustic adjusted oil sands derived aqueous
stream. At a high pH, the dissolved hydrogen sulphide in the produced water
will be converted to sulfate (Formula 1) when the hydrogen peroxide is added.
Conversely, if the hydrogen peroxide is added to low pH produced water, the
sulphide would be changed to elemental sulphur (Formula 2), which is not
desirable. Accordingly, in various embodiments, the chemical properties of
the oil sands derived aqueous stream need to be monitored and may be
modulated prior to dosing with the oxidizer (e.g., hydrogen peroxide).
S2'(aq) + 4 H2O2 (aq) = S042" (aq) + 4 H20(I) (Formula 1)
8 H2S(g) + 8 H202(aq) = S8(s) + 16 H20(I) (Formula 2)
In selected embodiments, a Metter Toledo T90 titration analyzer was used to
manually measure the concentration of hydrogen sulphide in the oil sands
derived water stream, and the amount of peroxide that would be sufficient to
oxidize substantially all of the hydrogen sulphide was calculated. The
titration
method, unlike the methods of the prior art such as ORP, provides a
substantially accurate representation of the concentration of the odor causing
species or of other undesirable species in the oil sands derived water, and
therefore a controlled means of oxidizer dosing based on the odor causing
species concentration.
In various embodiments, the odor causing species (e.g., hydrogen sulphide)
titration may be performed continuously when a sample of the oil sands
derived water is passed through the analyzer, diverted to the analyzer, or by
extracting a sample.
In various other embodiments, a TOC (total organic content) analyzer may be
used in addition to a titration analyzer to measure organics in the oil sand
derived water. Including the TOC analyzer may help further optimize the
dosing control by anticipating the organic consumption of hydrogen peroxide.
CA 02752423 2011-09-15
-17-
In yet other embodiments, dissolved oxygen measurements may also be
performed. For example, an online dissolved oxygen meter may be installed
after the oil sands derived aqueous stream has been oxidized by the
hydrogen peroxide. Readings obtained using the dissolved oxygen meter will
provide an indication of whether or not an overdose of the hydrogen peroxide
to the point that oxygen is present in the water has taken place. Having such
measurements would reduce the risk of gross overdose of the hydrogen
peroxide, which provides economical benefits.
In various embodiments, the oxidizer (e.g., hydrogen peroxide) injection rate
may be continuously adjusted to ensure proper dosage particularly when the
oil sands derived stream has a variable composition. Overdosing can result in
wastage and corrosion issues. Insufficient dosage may not provide effective
suppression of the odor causing species or other undesirable target species.
While the injection rate can be readily adjusted by altering pump speed, the
control input may require measurements of disposal water flow rate, and other
variables such as for example pH, temperature, oil content or a combination
thereof in the reaction vessel.
The flow rate of the oil sands derived aqueous stream may be measured. The
higher the flow rate of the oil sands derived aqueous stream having a given
odor causing species (e.g., H2S) concentration, the more oxidant (e.g., H202)
would be required.
Examples of Dosing Regimes of Hydrogen Peroxide and Results Using
Prior Art ORP
The examples below illustrate various embodiments relating to treating oil
sands derived aqueous streams by using a suitable dosing regime of
hydrogen peroxide to reduce the concentration of H2S in the stream and to
produce treated aqueous stream suitable for use within oil sands operations,
including evaporator vents, SAGD or a combination thereof.
CA 02752423 2011-09-15
-18-
In various embodiments, the oil sands derived aqueous stream such as
produced water (e.g. water from produced water tank of Firebag Plant 92)
contains hydrogen sulphide. When this water was treated, for example, in the
evaporators to make boiler feed water, on average about 55 kg/d of H2S was
being released to the atmosphere, which accounted for the majority of the 61
kg/d of H2S emitted site wide.
In various embodiments, as described below, controlled peroxide dosing and
addition to the oil sands derived aqueous stream was effected at various
addition or injection points, such as for example, in the recycle line to the
produced water tank. In various embodiments, the peroxide addition was
performed after the pH of the oil sands derived aqueous stream was raised
which prevented formation of elemental sulphur. For example, the addition of
hydrogen peroxide to the produced water tank of Firebag Plant 92 has been
successful at reducing the H2S emissions from the evaporators to 0 kg/d. The
byproduct of the treatment process was sulphate which remained dissolved in
the evaporator blowdown and may be disposed, for example, into a brine
formation.
One of the advantages of this process relates to downstream systems
eliminating issues of H2S from utility steam and condensate. The process was
also found to present economic benefits that are complementary to the
environmental benefits achieved (e.g., emissions reductions achieved through
energy conservation).
Example of Experimentation with Prior Art ORP Method
In selected embodiments, H202 was used to prevent downstream release of
H2S from oil sands-derived water (e.g., produced water treatment process).
Initially, it was thought that control of peroxide dosing and treatment of the
oil
sands derived water could be achieved using oxidation-reduction potential
(ORP) because ORP has been used in treating wastewater in various
CA 02752423 2011-09-15
-19-
industrial applications. ORP indicates the relative capability of a solution
to
oxidize or reduce. ORP sensors measure the electrochemical potential
between the solution and a reference electrode. ORP meters measure in
millivolts and positive readings indicate increased oxidizing potential and
negative readings show increased reduction. ORP sensors do indicate
concentrations of compounds but rather only relative changes in oxidation or
reduction.
The purpose of adding hydrogen peroxide (i.e., the oxidant) to the produced
water tank was to oxidize the H2S. For example, peroxide was injected at a
high pH in the recycle line after the caustic injection. At a high pH, the
dissolved H2S in the produced water was converted to sulfate, which is the
desired product when the hydrogen peroxide is added. Conversely, if the
peroxide were added to low pH produced water, the sulphide would be
changed to elemental sulfur which, in some embodiments, may not be
desirable as elemental sulfur could cause turbidity problems.
The ORP measurement was then used to determine the peroxide dose. ORP
sensor technology was found to be inadequate when applied to oil sands
derived aqueous streams because it was found not to predictably indicate the
demand for peroxide to ensure control of H2S removal from the stream. For
example, although this method allowed periodic water tests for sulphide to
determine dosing of H202, this method did not account for changes between
tests and thus control of H202 dosing to achieve a suitable removal of H2S.
For example, runs were performed where the peroxide rate was decreased
from about 2100 ml/min to about 1900 ml/min even though the ORP readings
were indicating that more peroxide was needed. Based on the incoming H2S
readings, only an amount of about 550 ml/min of peroxide was required.
The ORP method was found not to provide a suitable indicator of peroxide
feed requirements because other water quality factors in the oil sands derived
water such as iron content, sulphides, sulfites, and organics content appear
to
have affected the ORP values. The unreliable ORP readings that were
CA 02752423 2011-09-15
-20-
obtained in the produced water tank were likely due to the organics from the
make-up oil sands-derived water. For example, the readings obtained in the
produced water tank "PWT IN ORP" were more positive then the readings
obtained in "PWT OUT ORP". These results were counter-intuitive because
adding peroxide should increase the ORP thereby making the "OUT" more
positive than the "IN".
These results therefore indicate that ORP commonly used in the prior art in
wastewater treatment applications in other industries is not a suitable method
for application to oil sands-derived water because the ORP readings do not
appear to trend very well with H2S concentration in this type of application.
It
was observed that the ORP electrodes become coated with materials in the
oil sands derived aqueous stream and do not respond, and as a result would
have to be cleaned and calibrated often which is not a practical solution for
the present application.
Example of Application of Online H2S Analyzer Technology for
Analyzing Oil Sands-Derived Water to Determine Proper Dosing of
Hydrogen Peroxide
Lead acetate analyzer, UV photometric analyzer, and online titration were
tested to measure H2S concentration in oil sands-derived aqueous stream to
evaluate suitability for applications to the stream for measuring trace H2S in
such stream to achieve a reliable online means of dosing peroxide for
efficiency and safety regarding H2S control.
Lead Acetate Analyzer
The lead acetate analyzer tested was equipped with a pre-designed sample
system to strip the H2S from the oil sands-derived water through heating. The
system was equipped with a membrane filter to prevent any water carryover
to the lead acetate tape. This type of analyzer was found not to be suitable
for
analyzing oil sands-derived water for the following reasons:
CA 02752423 2011-09-15
-21-
1. Due to the composition of the oil sands-derived water, problems with
plugging were encountered;
2. Sparging (heating) was found not to be effective at removing substantially
all of the H2S from the oil sands-derived water. Consequently, the
obtained results are not reliable and errors were difficult to quantify; and
3. This technology was found to generate hazardous waste that needed to be
disposed of.
UV Photometric
A UV photometric analyzer was evaluated for application to the oil sands-
derived water. This type of analyzer measures the oil sands-derived water
directly through the UV absorption of entrained H2S. This type of analyzer was
found not to be suitable for analyzing oil sands-derived water for the
following
reasons:
1. Coating of the optics windows of the analyzer as a result of the presence
of any entrained oil or hydrocarbon components in the oil sands-derived
water. Consequently, the obtained results would not be reliable.
Example of Online Titration
Online titration type analyzers were evaluated for application to the oil
sands-
derived aqueous stream. This type of analyzer measures the oil sands-
derived aqueous stream directly through online titration by the addition of
reagents. The detection method to be used (i.e., titration, ionic or
colorimetric) may vary depending on the specific design of the analyzer.
Various analyzers of this type may be used, for example, Applikon Analytical
(WJF Instrumentation Ltd., NEXTchem Process Analyzers (Capital H2O
Engineering), or Galvanic Tytronics (Galvanic Applied Sciences Inc.).
The oil sands derived aqueous streams sampled were the produced water
that comes from the oil treatment area, specifically, before entering the
produced water tank (and before H202 was injected to oxidize the H2S). The
analyzer could be configured to collect and analyze a sample at a selected
CA 02752423 2011-09-15
-22-
interval (e.g. on an hourly basis), and this frequency could be changed as
required.
In various embodiments, the analyzer results may be sent to the distributive
control system (DCS) which controls the whole plant for further use on the
process monitoring and control.
The nominal flowing conditions in this example were about 290 kPag at about
80 C. A separate analyzer may be positioned at each injection or testing
point. For example, there were two plants tested with two separate analyzers
at each of the plants. In one of the plants, the produced water pH ranged
between about 11 - 11.5 with oil contents nominally below 20 ppm (which in
other embodiments could be higher if upsets in the oil treatment area). The
H2S concentration in this oil sand derived water was 15 ppm average and
could be as high as about 60 ppm. On the other plant, the pH was lower but
was adjusted before entering the tank.
The titration tests indicated that proper dosing of the H202 into the produced
water tank to maintain a concentration of H2S concentration substantially at
zero on the outlet of the produced water tank may be achieved using this
method.
In various embodiments, the titration was used for determining dosing and
monitoring, and optionally ORP was used to obtain additional information. The
concentration (ppm) of H2S in the oil sands derived water was determined, for
example, by titration with AgCI. As H202 also reacts with other compounds in
the water (mercaptans, organics, other reducing agents), the peroxide
concentration was overdosed by at least about 200mUmin to maintain a
'buffer'. This also allows for changes in the H2S concentration between
measurements as the titration for this experiment was performed once per
day. In other embodiments, the need for overdosing may be reduced, if the
titration is performed more frequently.
CA 02752423 2011-09-15
-23-
An example is illustrated below. Initial H2S results showed about 33 ppm H2S
in the test stream. The addition of 1 mL 3% H202 with a reaction time of about
minutes brought the H2S down to about 2.5 ppm with a sample size of
about 50 mL. This would be the equivalent of treating a 410 m3/hr flow with an
5 injection of about 50% H202 at a rate of about 0.007 m3/hr or about 0.18
m3/day. Increasing the 3% H202 from about 1 mL to about 10 mL decreased
the H2S residual to about 0.9 ppm.
Additional trials were run with various amounts of about 3% H202 ranging
10 from about 1 mL to about 5 mL, about 10 mL and about 50 mL.
The "blank" was pure water. Potential effects of H202 on the baseline were
determined by running a pure water sample with a H202 volume similar to use
and see if it affects the baseline at all.
In embodiments where the pH is about 10, free sulfur should not be a concern
at the levels that may be present and sulfphate is the predominant species.
One of the parameters that may be modulated is contact time/residency time.
Typical treatment times are in a range of about 15 to 30 minutes, however
depending on temperature, the reaction may occur within seconds. Factors
such as temperature, mixing, reaction time are important. The metallic
centers do act as catalysts for this reaction, and therefore it is not
uncommon
to add small amounts or metal salts to the oil sands derived aqueous stream.
Example of Improved Control of H2S in Oil Sands-Derived Aqueuous
Stream (e.g., produced water) using an On-Line H2S Process Analyzer
In this example, dissolved hydrogen sulphide (H2S) present in the produced
water from deoiling processes is controlled by the addition of hydrogen
peroxide (H202) to the Produced Water Tank (PWT) at Suncor's Plant 91 and
Plant 92.
CA 02752423 2011-09-15
-24-
One of the challenges arises due to the levels of H2S in the produced water
varying considerably with which wells are being pumped and the amount of
blowdown or make-up water blended with the produced water. Therefore, the
corresponding H202 injection rate must be continuously managed to prevent
an under-dose or over-dose situation.
If a prior art method is used, the H202 injection rate may be controlled
manually by the unit operators who routinely (e.g., about every 6 hours) may
measure the oxidation reduction potential (ORP) of the feed water flowing out
of the PWT (PWT OUT). However, use of ORP results was found to be an
insufficient means of control when applied to the oil sands derived aqueous
stream due to several chemical interferences in the stream (e.g., in the
produced water) which make the ORP results unreliable.
The H2S concentrations in the oil sands derived stream such as for example
the produced water can change considerably over the course of a few hours.
Therefore, in various aspects, present invention provides for controlling the
H202 injection rates by automated means of measuring H2S in the oil sands-
derived aqueous stream such as produced water (PWT IN) or the treated
produced water (PWT OUT).
It was found that the use of an in-line ORP probe cannot provide the level of
control required due to chemical interferences present in the oil sands-
derived
aqueous stream, which is different from wastewater applications in other
industries in which ORP is generally applied.
In one aspect, an on-line process analyzer for H2S may be used. For
example, the on-line process analyzer would be mounted near the current
PWT IN or PWT OUT sample points located in Plant 91 or 92. The analyzer
may be configured to collect and analyze a sample on an hourly basis or other
frequency.
CA 02752423 2011-09-15
-25-
Although continuous operation may be applicable in some embodiments, the
process may be operated in a non-continuous manner since the analyzer
requires the use of chemical titration reagents and because water conditions
typically do not change significantly within a short time interval e.g., an
hour.
Tests were conducted to determine a hydrogen peroxide demand for various
oil sands-derived aqueous streams. For example, deoiled produced water
combined with make-up water was used as the oil sands-derived stream and
titration was used for measuring the concentration of the odor causing species
and suitable dosing of the oxidant. The peroxide demand due to H2S was
determined to be about 1420 mL/min, while the calculated demand due to
SO3 was about 1082 mUmin.
Water Sources
Water sources feeding the produced water tank include produced water from
for example upstream deoiling operations, makeup water, makeup well water,
blowdown, retention pond water or a combination thereof.
In various embodiments, the produced water may be hot having a skim tank
temperature of about 70 C to 88 C, 88 C to 90 C. The produced water may
comprise high silica content, H2S, water soluble organics and trace oil which
are derived from bitumen, and some hardness species. In certain
embodiments, the produced water may comprise a large amount of oil from a
deoiling upset, for example, about 2 ppm to about 100 ppm oil and other
species such as silica which may range, for example, from about 50 ppm to
about 300 ppm.
Blowdown (e.g., low pressure blowdown) may be derived from a hot water
source and have a high pH and alkalinity. In various embodiments, such an
influent into the produced water tank may impact pH control.
Makeup water such as for example oilsands makeup water (OSMU) may be a
reverse osmosis reject water from the oilsands facility. The feed rate of the
CA 02752423 2011-09-15
-26-
OSMU to the produced water tank may vary for example from about 0 m3/h to
more than about 100 m3/h. In various embodiments, OSMU may be a cold
stream having a temperature of about 18 C to about 20 C, and colder where
the temperature may be as low as about 5 C. The warm lime softener
downstream of the produced water tank may be upset by changes in
temperature, however blending OSMU in the tank instead of direct addition to
the warm lime softener helps equalize temperature and minimize swings. In
various embodiments, the OSMU may have variable pH. Caustic may be
added upstream to reduce pipeline corrosion potential. The pH target set point
for the OSMU may be from about 8.0 to about 8.5 pH, however the actual pH
value may range from about 6.5 to about 9.5 pH. Changes in OSMU pH and
influent rate may affect pH control in the produced water tank.
In various embodiments, the OSMU may be high in alkalinity. For example,
total alkalinity as CaCO3 may be about 770 ppm. Alkalinity, in some
embodiments, may help to reduce the warm lime softener soda ash demand
in the warm lime softener. In various embodiments, OSMU may comprise
sulphite, which may be added upstream to eliminate chlorine and scavenge
oxygen to help reduce pipeline corrosion potential. Sulphite represents a
demand for the hydrogen peroxide addition according to the various
embodiments for H2S abatement. OSMU may also comprise iron which may
include dissolved iron, particulate iron or a combination thereof.
In various embodiments, make-up well water may be introduced into the
produced water tank. The make-up well water may have a temperature range
from about 5 C to about 10 C. The pH of the make-up well water may be
lower than other streams and may range from about 7.0 to about 7.5 pH.
When the make-up well water is used, the pH may have an impact on overall
produced water tank pH control, and therefore, in some embodiments, the
caustic feed rate may need to be increased. The make-up well water may
comprise contaminants such as chemical species giving rise to hardness at a
concentration of about 200 ppm, iron at a concentration of about 10 ppm,
CA 02752423 2011-09-15
-27-
suspended solids at a concentration of about 74 ppm or a combination
thereof.
Hydrogen sulphide (H2S) is a poisonous gas and may be found in the
produced water from the production well. Operations where a sour diluent was
used may result in an increased concentration of hydrogen sulphide in
produced water. At elevated pH, the sulphide may be trapped in the produced
water as an ion; however, it may be released when the water is acidified.
According to the various embodiments, the hydrogen sulphide in the produced
water may be abated, for example, at the produced water tank to minimize
safety risks and meet environmental regulations.
According to an embodiment of the invention, for example at Firebag,
hydrogen sulphide may be abated by oxidation using hydrogen peroxide
(H202). According to the methods of the invention, the hydrogen sulphide is
permanently destroyed and cannot be released from the stream later though
acidification. According to an embodiment, about 50% H202 may be injected
into the produced water tank though an inlet to the produced water tank.
In various embodiments, the hydrogen peroxide solution used is a strong
oxidizer and may present some handling hazards when in contact with the oil
sands derived aqueous stream (e.g., produced water), which has a particular
chemical makeup. According to an embodiment of the invention, peroxide
feed is introduced in a continuous manner immediately before the produced
water tank. In an embodiment of the invention, dosage control may be
achieved based on daily sulphide analysis results relating to the produced
water influent stream and the hydrogen sulphide content of the effluent
stream. Based on these measurements, the rate of addition of a selected
hydrogen peroxide concentration may be adjusted. The hydrogen peroxide
may be added in a stochiometric amount or in a slight excess ranging for
example from about 5% to about 10% excess such that any species in the
produced water aside from hydrogen sulphide which may consume hydrogen
peroxide may be accounted for.
CA 02752423 2011-09-15
-28-
The reaction product of hydrogen sulphide and hydrogen peroxide is
dependent upon the pH of the water in which the reaction takes place. In
acidic and neutral solution, reaction yield will comprise elemental sulfur. In
some embodiments, this may be undesirable as producing a solid reaction
product can cause deposition problems in the tanks and downstream. Raising
the pH of the water changes the reaction path to produce a soluble reaction
product (e.g., sulphate S04 2-).
In various embodiments, to avoid formation of elemental sulfur as a result of
contact with hydrogen peroxide and in view of the variable composition of the
produced water, the tank may be equipped with a pH control system. Injection
of a suitable amount of caustic into the produced water tank may keep the pH
outside of the range in which elemental sulfur is likely to be precipitated.
Alternatively, various streams may be combined to synergistically give a pH in
the range of about 6 to about 11 (e.g., a pH of about 8.5 to about 9.5).
Upon contact of the produced water with a properly dosed hydrogen peroxide
under suitable conditions, there should be no residual peroxide in the
effluent
from the produced water tank (i.e., the treated aqueous stream). Other
constituents in the produced water compete with the hydrogen sulphide for
peroxide and will consume reasonable excess. Examples of such constituents
include water soluble organics.
The produced water tank may be equipped with a vent collection scrubber
system to remove volatile organic compounds, including hydrogen peroxide
as some hydrogen peroxide may be present in the headspace of the tank if
there is a problem with the hydrogen peroxide feed.
Contamination of the peroxide or of the peroxide tank may accelerate
decomposition of hydrogen peroxide, and if pressure builds up, there may be
a risk of explosion, rupture, or fire.
CA 02752423 2011-09-15
-29-
In some embodiments, the produced water in the produced water tank may
comprise oil as a result of process upsets. Detection for oil should be
performed to modify operating parameters. The produced water may be
further tested for silica concentration which also should be monitored so that
operating parameters may be adjusted.
Although specific embodiments of the invention have been described and
illustrated, such embodiments should not to be construed in a limiting sense.
Various modifications of form, arrangement of components, steps, details and
order of operations of the embodiments illustrated, as well as other
embodiments of the invention, will be apparent to persons skilled in the art
upon reference to this description. It is therefore contemplated that the
appended claims will cover such modifications and embodiments as fall within
the true scope of the invention. In the specification including the claims,
numeric ranges are inclusive of the numbers defining the range. Citation of
references herein shall not be construed as an admission that such
references are prior art to the present invention.