Note: Descriptions are shown in the official language in which they were submitted.
CA 02752633 2011-09-19
1
1 Title of this Invention is:
2 Electret High Power: Battery / Generator, Safety Light, Refrigerator, Water
Distiller,
3 Insect Catcher
4 This invention refers to the study of several fields: Chemistry, Electricity
and Static
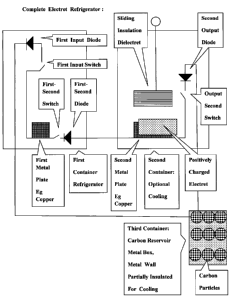
Electricity. Fig 1. Complete Electret Refrigerator :
6 This first section of the description is a lengthy overview of the
invention. It is followed by a
7 more detailed description and then a Review Section and finally an Internet
Reference
8 Section with copied text articles. This description is rather long because
Electret
9 Technology is historically relatively new compared to all the applications
of magnets and
motors, Fig 12. Fig 13.
11 There are devices which employ electrets but their voltage ratings are very
small. Electrets
12 can hold very high voltages for many decades but there are only holding
chucks which are
13 using high voltage charged Electrets. Fig 12.
14 Science reports neither Static Generators nor Static Motors that cannot
produce any useful
Work Energy. Most device or applications are designed to eliminate static
charges or for
16 low voltage Electret Microphones, Fig 13.
17 It is the intent of this invention to design applications to produce the
following: high
18 temperature cooling ranges and high output voltages and currents from
sources that have
19 abundant latent energy eg. air or gases, liquids, metals or other
materials. This means that
heat energy is directly converted to electrical energy without using
compressed gas heat
21 exchangers etc. so that any warm body eg. water or bulk material
containers, vehicles, any
22 building surface or any land surface can have its heat content directly
converted into useful
23 Electrical Energy, Fig 1.
24 This invention employs low power to move an Insulation Dielectric which
indirectly
produces much higher Voltages or larger cooling or heating temperature ranges.
The
26 following two devices: sound recording Electret Microphones and cooling /
heating Peltier
27 Thermo-Electric Effect Chips directly employ low Voltages, Fig 11. Electret
Mics , Fig 13,
28 vibrate to control electric circuits while Peltier Chips directly use low
DC Voltages to
29 produce lower temperature ranges of cooling or heating for many electronic
or lab
equipment.
CA 02752633 2011-09-19
2
1 The writer recommends that the Reference Section be reviewed because the
understanding
2 of static charges can become confusing. Static charges can be employed to
transfer moving
3 charges which can produce Useful Work. This invention is not a static
generator but uses
4 static charges to "Pull or Attract" and to "Push or Repel" High Energized
Free Electrons.
There are commercial applications related to the discharging or to eliminating
static
6 electricity to protect electronic components but this invention employs the
Principles of
7 "The Induction of Charges" to create the Flowing of High Energized Free
Electrons. This
8 invention does not employ energy to vibrate a Permanently Charged Electret
nor to vibrate
9 the Metal Plate as in an Electret Microphone, Fig 12, Fig 13.
This invention employs low power [ Current and Voltages] to move a sliding or
rotating
11 toothed Non Polarizing Insulation Dielectric which cannot be attracted to
any positive nor
12 negative charges ie. it cannot be Polarized. Eg. an electrician's
electrical insulated gloves.
13 Note that the First Container houses the following devices: the
Refrigeration Air or Gas,
14 the Wire from the Carbon Reservoir to the First Switch, the wire to the
First Metal Plate
and the wire to the First-Second Diode which is in the Second Container. A
First-Second
16 Switch is housed in either the First or in the Second Container and is used
to prevent Heat
17 Energy to travel on the wire by conduction when the device is not operating
or when the
18 frequency of the transfer of Energized Electrons is slow because the
sliding motion of the
19 Insulated Dielectric is slow.
Note that the Second Container houses the following devices: a First - Second
Diode, a
21 Second Metal Plate, a Second or Output Switch, a Second or Output Diode and
the wire
22 conductor to the Carbon Reservoir Metal Box or to a suitable Grounding
Terminal. The
23 parallel sliding Non Polarizing Insulation Dielectric [ see electrician's
glove or bonding
24 electrical tape] separates the parallel permanently positively charged
Electret from the
Parallel eg Copper Second Metal Plate, Fig 1.
26 Connecting electric conducting wires create a series circuit but this
circuit is controlled by
27 the First Input Switch and the Second Output Switch. These two switches do
not operate
28 simultaneously at the same time. The Carbon Reservoir acts as a type of
Input - Output
29 Switch Controlled Ground Loop.
This Sliding or Rotating Toothed Insulation Dielectric blocks the permanent
positive static
31 field of an Electret when it is placed between the Electret and the Second
Plate. When the
32 Insulation Dielectric is removed it will permit a stationary positive
static electric field
33 which is permanently stored in an Electret to pass and to attract
oppositely facing
34 negatively charged particles ie, electrons which are held in / on an
adjacent non-contact
CA 02752633 2011-09-19
3
1 Parallel Second Metal Plate. A Positively Charged Electret attracts Negative
Electrons in
2 an adjacent parallel non contact Second Metal Plate, Fig 1.
3 This invention focuses on employing a Positively Permanently Charged
Electret so that
4 Internet References contained in the Reference Section can be used to
explain the
principles of this invention. Electron Flow is more efficiently moved than the
movement of
6 Positive Ion Flow Carriers. Some applications can be similarly employed
using Negatively
7 Charged Electrets to be eg . attracting positively charged dust particles or
flying insects
8 etc...
9 Peltier Effect Chips employ Ion Carrier to transfer heat energy which moves
slower than
moving free electrons. Peltier Chips are limited to lower frequencies of
operation, low
11 powering voltages and to smaller limits of cooling or heating temperature
ranges. This
12 invention uses faster flowing Free Electrons for larger cooling or heating
temperature
13 ranges and much higher voltage charged Electrets.
14 This invention is designed to convert large volumes of Vibrating High
Energized Free
Electron Charges on a First Metal Plate to be converted and transferred into
Flowing
16 High Energized Free Electrons toward a Second Metal Plate which is partly
positively
17 charged. The First Container and its First Metal Plate undergoes a cooling
operation while
18 the wire connected Second Metal Plate in the Second Container is signal
controlled to
19 release these Free High Energized Electrons to produce useful Output
"Working" Energy /
Power. As the Second Metal Plate is constantly removing high energized
electrons from
21 the First Metal Plate then the Refrigeration Air or Gas Collisions continue
to lose Kinetic
22 Energy to the First Metal Plate and thus the Refrigeration Air or Gas
cools. See The ideal
23 Gas Law.
24 This invention is not a Static Generator. Static electric fields can
attract Free Electrons to
be turned into Flowing Currents. The info can be reviewed in the references
indicated
26 below. The text references are copied in the Reference Section of this
description.
27 Reference, Charging by... Induction:
28 http://www.physicsclassroom.com/class/estatics/u8l2b.cfm
29 The Electrophorus, The Electroscope,
Reference, Peltier Effect Chips:
31 http://www.tetech.com/FAQ-Technical-Information.html#1
32
CA 02752633 2011-09-19
4
1 A more Detailed Introduction to the Operating Principles of this Invention:
2 This invention operates with an Electret and uses non conductive electric,
non heat, non
3 static electric or non electric conductive insulated containers and a Non
Heat or Non
4 Electric Conductive Non Polarizing Moving/ Sliding or Rotating Toothed
Insulation
Dielectric, Fig 1.
6 These containers are the First "Input Cooling ", the Second "Controller
Power Charging
7 Capacitive Function " and the Third "Electron Carbon Particle Reservoir"
containers.
8 The "Electron Carbon Particle Reservoir" is housed in a partially insulated
Metal Box
9 which can dissipate heat energy. The Energized Free Electrons are received
and are
converted to Heat Energy in the Carbon Reservoir Metal Box. The "Energized
Electron
11 Equivalent Heat Energy" now in the Metal / Carbon Box Reservoir is expelled
to the
12 Ambient Air or to the Water Bath outside of the Refrigerator by the
conduction or
13 convection of heat energy by the Carbon Reservoir Metal Box Wall [s].
14 The Insulation Dielectric should be eg Ceramic or a suitable material so
that it does not
readily absorb or retain heat energy or have any electric capability to have
induced Eddy
16 Currents or Dielectric Static Hysteresis nor be able to be Polarized. The
Electret is selected
17 so that it does not readily absorb or retain heat energy.
18 A First Cooling Metal Plate is housed in the First "Input Cooling "
Container. The Kinetic
19 Energy of the collisions of the refrigerator "Air or Gas" is absorbed onto
the Surface of the
First Cooling Metal Plate. The First Cooling Metal Plate is connected by an
electric
21 conductive wire to a First-Second Diode and then to a Second Metal Plate.
The Second
22 Metal Plate is housed in the Second "Controller Power Charging Capacitive
Function"
23 Container. See: The Ideal Gas Law.
24 Review the references on "The Induction of Charges", Fig 7. When the Second
Metal Plate
has the equivalent of a Positively Charged Area on the Second Metal Plate it
can attract
26 Highly Energized Free Electrons from the First Metal Plate. The Second
Metal Plate has a
27 partial positive charge which attracts electrons from the First Metal
Plate. See the
CA 02752633 2011-09-19
1 references: The positively charged Electret attracts electrons nearest to
itself so that the
2 opposite side of the Second Metal Plate has a positive charge, Induction of
Charges, Fig 7.
3 This positive side of the Second Metal Plate attracts electrons through the
First - Second
4 Diode and then the electrons from the First Metal Plate. The First - Second
Diode only
5 permits electron flow to the Second Metal Plate from the First Metal Plate.
Fig 3. The
6 Second Metal Plate can only discharge electrons to the Electron Carbon
Reservoir Metal
7 Box. The Negative Charge on the Second Metal Plate when it discharges its
Electrons
8 must be at a higher Negative Charge than the Electrons in the Carbon
Reservoir Metal
9 Box. Electrons only flow to the Carbon Reservoir. The Second Diode prevents
electrons to
flow from the Carbon Reservoir to the Second Metal Plate.
11 The Second "Controller Power Charging Capacitive Function " Container is a
type of
12 Capacitive Electron Vacuum Collector. The Second Metal Plate alternatively
draws in
13 Highly Energized Free Electrons through the First-Second Diode which came
from the
14 First Cooling Metal Plate. Afterward, these Energized Electrons which are
now on the
Second Metal Plate will be expelled by their own repulsion to the Output which
is the
16 Electron Carbon Particle Reservoir or Grounding if grounding is used, Fig
1.
17 This repulsion occurs when the three following conditions or events exist:
the Insulation
18 Dielectric now is returned between the Positively Permanently Charged
Electret and the
19 Second Metal Plate ; the First-Second Diode blocks reverse electron flow to
the First
Metal Plate ; the First-Second Switch can be Opened to reduce the stress on
the First-
21 Second diode; the Output Switch is closed ; and the Output Diode only
permits Highly
22 Energized Electrons to go to the Carbon Reservoir Metal Box. See Capacitor
references.
23 Note that the Permanent Charge on the Electrets must be much larger than
any free
24 electron charges in the Electron Carbon Reservoir or any Grounding if
grounding is used.
More Steps are explained in the following paragraphs.
26 The Second Container, "Controller Power Charging Capacitive Function "
[Capacitive
27 Electron Vacuum] operates as follows: An insulated non heat / non
conductive electric /
28 non static electric field Dielectric separates the Positively Charged
Electret from the
CA 02752633 2011-09-19
6
1 parallel orientated electrical conductive Second Metal plate. When the
Dielectric is
2 removed from between the Electret and the Second Metal Plate, the Negatively
Charged
3 Energized Electrons are attracted and concentrated closer on the Second
Metal Plate and
4 facing to the Positively Charged Electret.
Note that the Electret and the Second Metal Plate are close and parallel but
never touch.
6 The First and the Second Metal Plates are electrically connected by a wire
so that
7 Energized Free Electrons on the First Metal Plate are drawn to the Second
Metal Plate
8 through the First-Second Diode, Fig 3.
9 There are in effect two circuits which are like two electron conveyor belt
systems such that
one electric belt is fed with low energy electrons and in turn this first
conveyor belt feeds
11 its cargo of Energized Free Electrons onto the second conveyor. The
electron cargo can
12 never flow backwards because of the conveyor diode gates. The warm electron
cargo is
13 emptied into a metal box and the low energy electrons return to the First
Conveyor.
14 This cargo analogy is similar to the gases in a compressed gas refrigerator
or in a Thermo-
Electric Peltier Chip. The important difference is that no direct DC
electricity is used. This
16 invention operates only by controlling the output field attraction of the
Electret to attract
17 electrons which are on the surface of the Second Metal Plate.
18 These electrons on the Second Metal Plate repel each other when the field
of attraction is
19 blocked and there is an electrical passage to a lower energy destination
Carbon Reservoir.
NOTE: A First-Second Switch should be placed in series between the First and
Second
21 Metal Plates to protect the "Controller Power Charging Capacitive Function
", Second
22 Container, from heating up by wire heat conduction when the invention is
not required to
23 operate. The Second Container and the Second Metal Plate should be kept as
cool as
24 possible so that the Second Metal Plate can attract more efficiently the
Energized Free
Electrons which have higher Kinetic Energy, Switches Fig 10.
26 The Surface Area of the Electret is very Highly Positively Voltage Charged.
The Surface
27 Area of the Electret and the Second Metal Plate are several times larger
than the Surface
CA 02752633 2011-09-19
7
1 Area of the First Metal Plate. This is done so that Capacitive Effect of
Surface Areas
2 draws Electrons effectively from the First Metal Plate toward the Second
Metal Plate. This
3 First-Second Diode separates the First and the Second Metal Plates such that
Energized
4 Electrons cannot ever return to the First Metal Plate, Fig 7.
When the Dielectric is returned to be between the Electret and the Second
Metal Plate,
6 then the Output Switch Closes. The crowded Electrons on the Second Metal
Plate repel
7 each other toward the Electron Carbon Reservoir or Ground if grounding is
used. The
8 Output Diode prevents Electrons to enter the Second Metal Plate from the
Electron
9 Carbon Reservoir, Fig 1.
The cycle repeats by the Output Switch Opening and the Dielectric is returned
to be placed
11 in parallel between the Permanently Charged Electret and the now low
uncharged Second
12 Metal Plate. The Input First Switch now closes to recharge the First Metal
Plate with Low
13 Energy Level Electrons from the Electron Carbon Reservoir. The Input First
Diode
14 prevents any Electrons from transferring from the First Metal Plate to the
Electron
Carbon Reservoir.
16 The other end of the First Metal Plate is controlled to receive Free
Electrons from a Low
17 Kinetic Energy Electron Carbon Particle Reservoir. The First Cooling Metal
Plate is also
18 connected to an Input Switch and Series Diode. Input First Switch and
Series Diode are
19 series connected to the Low Kinetic Energy Electron Carbon Particle
Reservoir.
This is a closed loop system. The Output Switch is used to conduct High Level
Kinetic
21 Energy Free Electrons which came from the First and then to the Second
Metal Plate and
22 finally to the Carbon Particle Reservoir Ground. An Input First Switch is
used to conduct
23 Low Kinetic Energy Electrons to the First Metal Plate.
24 First Container Gases collide with the First Metal Plate and transfer its
Kinetic Energy to
the First Metal Plate.
26 Review Section with Some Additional References and a Few Applications:
CA 02752633 2011-09-19
8
1 It refreshes the info with References to Capacitors or Metals Connected in
Series with
2 Different Charges and notes that the Second Container could possible need
wall air cooling
3 but without any induced static charges to operate more efficiently. There
are many
4 Dupont Xiameter silicon products which can very efficiently conduct heat
away and which
will not transfer humidity, static nor electric charges ,
https://www.xiameter.com/
6 1. Invention Parts
7 2. Comparison of Other Devices, eg. Peltier Thermoelectric Chips:
8 The Second Metal Plate in the separate Second Insulated Container holds a
Permanently
9 Charged Electret which is facing parallel to the Second Metal Plate. The
Electret and the
Second Metal Plate are separated by a Movable Insulation non Electric non heat
non static
11 Dielectric Gate. When the Gate is not between the two plates then the
Electret Charges
12 attract Free Electrons on the Second Metal Plate.
13 The First and the Second Metal Plates act as one Capacitor Plate. Electrons
on the Second
14 metal Plate are drawn away and concentrated so that a void is created on
the Second Metal
Plate. This void enables Higher Kinetic Energy Charged Electrons on the First
to be
16 transferred to the void areas on the Second Metal Plate, Fig 7. A properly
oriented Diode
17 prevents these Higher Kinetic Energy Charged Electrons to return to the
First Metal Plate.
18 The Second Metal Plate is also connected to an Output Second Switch and to
another in
19 Series Output Diode and finally to a Carbon Particle Electron Reservoir.
This Carbon
Particle Electron Reservoir is also the Source of the Recycled Low Kinetic
Energy
21 Electrons which resupplies the First Metal Plates through a Recycle Switch
and properly
22 oriented Recycle Input Diode. The Recycle Input Diode can only pass Low
Kinetic Energy
23 Electrons to the First Metal Plate. Thus the Input Diode cannot pass High
Level Charged
24 Kinetic Energy Electrons which are charged on the First Metal Plate to go
to the Carbon
Particle Electron Grounding Reservoir.
26 As the refrigerator "Air or Gas" keeps colliding and releasing Kinetic
Energy to the First
27 Metal Plate, the "First Cooling Container" starts to cool.
CA 02752633 2011-09-19
9
1 The Ideal Gas Law: http://hyperphysics.phy-
astr.gsu.edu/hbase/kinetic/idegas.html
2 Note that Peltier Chips operate at very low DC Voltages and low Amps.
Electrets can hold
3 100's of Volts. The Dielectric can move between the gap of the Electret and
the Second
4 Metal Plate. Since the Dielectric cannot be charged the only energy required
is very small
sliding or rotating energy. This invention uses the fact that an uncharged
Capacitor which
6 is connected in parallel with a charged Capacitor will distribute its
charges between
7 themselves or a highly charged rod will send charges to a rod which is less
charged, Fig 5.
8 http://www.techlearner.com/DCPages/DCCap.htm
9 This invention utilized several existing Scientific Laws, Principles of
Component
Operations, existing Electronic Devices and How Strong Static Charges repel or
attract
11 Weaker Charges on other objects which affects the Levels of Kinetic Energy
of these Free
12 Electrons on the adjacent parallel facing Second Conductive Metal Plate.
13 This is a closed loop electric circuit system which recycles Low Level Free
Electrons to the
14 First Metal Plate to be again energized to higher energy levels of Kinetic
Energy by the
collisions of ambient or refrigerator gases against this First Metal Plate .
This invention is
16 not using compressed gases nor Low Power Peltier Effect Chip Electronics.
17 Depending on the applications then Capacitor Principles draws away the
induced charges
18 of Higher Levels of Kinetic Energy of Free Electrons which are on a First
Metal Plate.
19 Through switches, timing and Diode Circuitry the Metal Plate is
alternatively replenished
of Low Kinetic Energy Free Electrons and then these higher Level Energized
Free
21 Electrons are removed by Capacitive Effects and Grounding Principles.
22 This example was a refrigerator gas which collides with a Metal Plate and
transfer Heat
23 Energy to the Metal Plate. The Free Electrons on the Metal Plate then have
higher Kinetic
24 Energy. By continuously removing the more active Electrons on the Metal
Plate thus the
gas or air cools the Refrigerator Container. This invention operation is
similar but very
26 different than the Thermo-Electric Peltier Effect Semiconductors because no
PN Peltier
27 Chips nor very low DC Voltages and Currents are used. A moving Insulation
Dialectric
CA 02752633 2011-09-19
1 Plate controls or screens the attractive effects of the very high permanent
static charges on
2 the Electret Plate, Fig 7.
3 This invention uses Scientific Topics and Electronic Devices which involves
High Voltage
4 Permanent Static Charged Electrets. The Permanent Charge Field on the
Electret are used
5 to Direct the Free Electrons of a Metal to produce useful work.
6 This invention does not function as an Electret Microphone because the
Electret is not
7 powered to vibrate toward or away from a Conductive Metal Plate. The
Electret is acting
8 more as stationary object with an active Static Field. Depending on the
application it is
9 used to attract or to repel charges in another material, eg electrons in a
Metal Plate or to
10 attract dust or flying insects. Note that only the Dielectric moves under
low energy to
11 control a high energy electric field.
12 Electrets can be charged to hold permanently 100's of Volts but this
invention does not
13 move the Electret Disk to induce charges in another metal but rather a
Parallel Moving
14 Insulation Disk alternatively blocks and permits the passage of a Static
Charged Disk or
Plate. This moving disk or plate is a very strong Dielectric Insulator which
cannot conduct
16 electricity and cannot be polarized so that it does not interact with the
Electret, the Metal
17 Plate nor generate any electric or magnetic fields. The Moving Plate only
operates as a
18 Static Field Gate, eg as insulated glove or mat which protects a worker or
an electronic
19 component from charged surfaces.
This invention references: The Principles of the Ideal Gas Law, Kinetic Energy
of Free
21 Electrons in a Conductive Metal, Capacitor Formulas, Electret Permanent
Static Charges,
22 Diodes and Circuits, Grounding and Dielectrics which are Non-Polarized
Insulations.
23 The Electron Carbon Reservoir serves two functions. A Copper Box with a
side exposed to
24 the ambient air can cool efficiently because of the quantity of Free
Electrons. The Carbon
Powder eg Slate Carbon Powders from Printing Toners, is also a good conductor
of heat
26 and electricity because of the much larger quantity of Free Electrons.
CA 02752633 2011-09-19
11
1 NOTE: This is very important: A First-Second Switch should be placed in
series between
2 the First and Second Metal Plates to protect the "Controller Power Charging
Capacitive
3 Function ", Second Container, from heating up by wire conduction when the
invention is
4 not required to operate. The Second Container and the Second Metal Plate
should be kept
cooler so that it attracts Energized Free Electrons which have higher Kinetic
Energy. This
6 is why the Second Container is insulated from the warm ambient air.
Alternatively, the
7 Second Container could be enclosed in a conductive heat material and be
placed in a water
8 bath which will absorb heat which forms in the Second Metal Plate,
especially for High
9 Power Applications which require much more cooling to operate more
efficiently.
Note the references below because Peltier Cooling Chips use Ion Carriers to
conduct Heat
11 Energy but this invention employs the Free Electrons which have higher
Kinetic Energy
12 and this energy transfer is much faster because of the smaller bulk and do
not transfer heat
13 energy to each other electron as compared to Ions to Ion Heat Transfer in
Peltier
14 Thermoelectric Cooling. This invention uses fast electronic switching, fast
moving Devices
to move the Insulation Dielectric and efficient cooling of the Second
Container so that the
16 Second Metal Plate is kept cool as possible and to permit the High Energy
Electrons to be
17 sent to the Output, Electron Carbon Reservoir or Grounding if grounding is
employed.
18 ----------------------------------------------------------------------------
-----------------------------------------
19 Brief Reference on Metals:
http://resources schoolscience.co.uk/corus/16plus/steelchlpg2.html
Ionic vibrations
"If a metal is heated, the positive metal ions vibrate more vigorously. These
ions collide
with neighbouring ions and make them vibrate more vigorously too. In this way,
the
energy is passed, or conducted, through the metal.
... metals are particularly good conductors of heat. In general, they are
better than ionic
compounds which also have strong bonds. ... it is their free electrons."
21
CA 02752633 2011-09-19
12
The electrons at the hot end will speed up - they gain kinetic energy from the
vigorously
vibrating ions. "
"In effect, the electrons have carried the vibration energy from the hot end
to the cold
end.... they are free to move through the lattice, they are able to do this
more quickly
than the bonds between the ions in the lattice."
1 -----------------------------------------------------------------------------
-------------------------------------
2 Several Other Invention Applications for Higher Output Electric Energy and
Cooling:
3 1. An insect LED Catcher.
4 The designs of an Electret Microphone can easily power a tiny Mic or a tiny
buzzer in a
small toy and higher power ones to collect dust. Since this design can use
Electrets and
6 Dielectric at 100's of Volts then this invention can be used to power a
small LED to attract
7 flying insects into a cone wire trap and also be electrified and become fish
farm food. The
8 LED is placed in series with the Output Circuit. The First Metal Plate can
be exposed to
9 the Warm Air so that it is charged more and does not operate as a
refrigerator. This First
Container can have a funnel screen door and an interior LED Light which
flashes to
11 attract the flying insects. A bare wire section could electrocute the
insects.
12 http://home.howstuffworks.com/bug-zapper.htm
13 To obtain higher output voltages:
14 As above, the First Metal Plate could be exposed to the Warm or Hot Ambient
Air or Solar
Warmed Water or a Salt Water Brine. The device operates to extract heat energy
from the
16 warm air or warm water and produce a higher Output Pulsed Voltage. The
Output can
17 employ a transformer with a rectifier- filter circuit to power an inverter
or to charge DC
18 Batteries or directly to a cooking device or to an LED light. The power to
the moving
19 Dialectric can be Mechanical, Manual, Wind or Solar Powered. Energy moves
from a high
energized state to a lower energy state. When the Second Metal Plate is highly
charged
CA 02752633 2011-09-19
13
1 relative to the Carbon Reservoir or Grounding then electrons will flow
toward the Carbon
2 Reservoir or Ground.
3 To Distill Water by Higher Output Voltages:
4 As above, the First Metal Plate can be exposed to warmer ambient air or warm
water so
that more energy is extracted. The high output voltage / energy can be used to
distill water
6 and smaller refrigerator section can be used to condense the evaporated
distilled water.
7 Thus low energy from wind or Solar can be used to operate the Dielectric and
operate
8 this invention as a higher temperature heat pump which pumps highly charged
electrons
9 rather than compressed refrigeration gases.
Large Scale Cooling, Heating, Output Voltages:
11 This invention is capable of converting large scale quantities of Heat
Energy to Electrical
12 Energy when the invention employs: 1. higher Voltage Permanently Charged
Electrets, 2.
13 Larger Size Electrets, 3. Larger Size Metal Plates, 4. corresponding
Insulation Dielectric, 5.
14 suitable Powered Devices [ manual, mechanical, Solar Electric, Wind
Powered,... ] to
perform the sliding Motion of the Dielectric, 6. Larger Electron Carbon
Reservoir or
16 suitable Grounding Conditions, eg conductive soils with abundant free
electrons ie. normal
17 standard Grounding according to Building Codes, Charging Battery Fig 2, Fig
6.
18 Metals often are used to build land or water or air vehicles, transformers,
computer server
19 rooms, motor installations, storage containers, building walls and roofs
and interior
supports frames. This invention can be employed to replace or to compliment
the cost of
21 AC-E , Air Conditioning-Equipment or the cost of maintaining such AC
Equipment or to
22 convert heat energy to electricity from the finished products , eg melted
formed plastics /
23 metals / ceramics / glass, food cooking, welding operations, laundry
cleaning, agricultural
24 poultry / cattle / pig / fish ventilation.
This invention can be used for energy generation eg Solar Salt Water Ponds,
without using
26 coils which are costly to operate and to clean from crystals which coat the
coils. Induction
CA 02752633 2011-09-19
14
1 Heating Operations or Geothermal Springs or warm soils under homes or under
roads can
2 recover converted heat to electrical energy.
3 Building Cooling or Transfer Converted Electrical Energy to Heating While
Producing
4 Higher Output Voltages:
Examples: Transformers, Kitchens, Vehicles, Greenhouses , Storage of Water,
Grains,
6 Warehouse Materials Fig 2, Fig 6.
7 Reference:
8 A heated metal to very high temperatures can emit many more electrons if the
Destination
9 Metal Plate has a Positive Charge,
eg. The Edison Effect.
A
T"' T
Electron flow
A
11 No current
12 Internet Reference Section:
13 The Ideal Gas Law: http://hyperphysics.phy-
astr.gsu.edu/hbase/kinetic/ide2as.html
CA 02752633 2011-09-19
1 "Ideal Gas Law
2 An ideal gas is defined as one in which all collisions between atoms or
molecules are
3 perfectly eleastic and in which there are no intermolecular attractive
forces. One can
4 visualize it as a collection of perfectly hard spheres which collide but
which otherwise do
5 not interact with each other. In such a gas, all the internal energy is in
the form of kinetic
6 energy and any change in internal energy is accompanied by a change in
temperature.
7 An ideal gas can be characterized by three state variables: absolute
pressure (P), volume
8 (V), and absolute temperature (T). The relationship between them may be
deduced from
9 kinetic theory and is called the
Ideal gas law: PV_ .PT= NkT
11 = n = number of moles
12 = R = universal gas constant = 8.3145 J/mol K
13 = N = number of molecules
14 k = Boltzmann constant = 1.38066 x 1023 J/K = 8.617385 x 10-5 eV/K
= k = R/NA
16 NA = Avogadro's number = 6.0221 x 1023/mol
17 The ideal gas law can be viewed as arising from the kinetic pressure of gas
molecules
18 colliding with the walls of a container in accordance with Newton's laws.
But there is also a
19 statistical element in the determination of the average kinetic energy of
those molecules.
The temperature is taken to be proportional to this average kinetic energy;
this invokes the
21 idea of kinetic temperature. One mole of an ideal gas at STP occupies 22.4
liters.
22 Metal Heat and Electric Conduction:
23 http://resources.schoolscience.co.uk/corus/16plus/steelchlpg2.html
Why are metals good conductors of heat and electricity?
CA 02752633 2011-09-19
16
Metallic bonds are made from a lattice of ions in a 'cloud' of free electrons.
These free
electrons are responsible for the ability of metals to
1. conduct electricity
2. conduct heat especially well.
1. Electrical conductivity
Electric current is the flow of electrons in a wire. In metals, the outer
electrons of the
atoms belong to a `cloud' of delocalised electrons. They are no longer firmly
held by a
specific atom, but instead they can move freely through the lattice of
positive metal ions.
Normally they move randomly. However, when the wire is connected to a cell,
they are
pushed away from the negative terminal and drawn to the positive one.
The cloud of electrons drifts through the wire. The drift velocity of the
cloud is about
3 mm s-1. The electrons within the cloud are still moving randomly (at much
higher
speeds) - rather like a swarm of bees leaving a hive.
2. Thermal conducivity
Metals are good conductors of heat. There are two reasons for this:
= the close packing of the metal ions in the lattice
= the delocalised electrons can carry kinetic energy through the lattice.
Ionic vibrations
The positive metal ions in a metal structure are packed closely together in a
symmetrical
geometric arrangement. They don't move from their position in the lattice but
they are
constantly vibrating. If a metal is heated, the positive metal ions vibrate
more vigorously.
These ions collide with neighbouring ions and make them vibrate more
vigorously too. In
this way, the energy is passed, or conducted, through the metal.
However, metals are particularly good conductors of heat. In general, they are
better
than ionic compounds which also have strong bonds. So we need another
mechanism to
explain their especially good conductivity. It is their free electrons.
Free electrons
The ions in the lattice are vibrating. The ions at the hot end of a piece of
metal vibrate
more. [Note the electrons have been left out of picture 1.5 to keep it clear.]
Let's look at just a few electrons.
CA 02752633 2011-09-19
17
= The electrons at the hot end will speed up - they gain kinetic energy from
the
vigorously vibrating ions.
= Some of them will move down to the cooler end and collide with ions that are
vibrating less vigorously than those at the hot end.
= In these collisions, the electrons will lose kinetic energy and make the
ions vibrate
more vigorously.
In effect, the electrons have carried the vibrational energy from the hot end
to the cold
end. And, because they are free to move through the lattice, they are able to
do this more
quickly than the bonds between the ions in the lattice.
1
2 http://resources.schoolseience.co.uk/Corus/14-16/heat/psch3pg2.html
3
"Hot to cold
A hot object will always transfer energy to a cooler object by heating it. If
they are in contact with each other, then the energy is transferred by
conduction. In conduction, the moving particles pass energy between each
other. Let's see how this happens.
4 "
Metals and conduction
Metals are good conductors and so is carbon. Carbon, being a refractory, can
be used at
the extremely high temperatures in furnaces. Good conductors are useful in
situations
where we need to keep something cool by conducting the heat away. For example:
= to stop the hearth of a blast furnace overheating
= cooling the steel in the mould and rollers of a continuous casting mill
Metals and carbon are such good conductors because their electrons are free to
move. As
one side of a slab gets hot, the electrons speed up and move through the
lattice, bashing
into ions and making them vibrate. The free electrons carry energy across the
block
much faster than the vibrations in an insulator.
http://www.physlink.com/education/askexperts/ae432.cfm
CA 02752633 2011-09-19
18
1 Question
2
3 Is there a relationship between electrical conductivity and thermal
conductivity?
4
Asked by: Darell Hayes
6
7 Answer
8
9 There is a relationship for metals and it is known as the Wiedemann-Franz
law. Metals are
good electrical conductors because there are lots of free charges in them. The
free charges
11 are usually negative electrons, but in some metals, e.g., tungsten, they
are positive 'holes.'
12 For purposes of discussion, let's assume we have free electron charges.
13
14 When a voltage difference exists between two points in a metal, it creates
an electric field
which causes the electrons to move, i.e., it causes a current. Of course, the
electrons bump
16 into some of the stationary atoms (actually, 'ion cores') of the metal and
this frictional
17 'resistance' tends to slow them down. The resistance depends on the
specific type of metal
18 we're dealing with. E.g., the friction in silver is much less than it is in
iron. The greater the
19 distance an electron can travel without bumping into an ion core, the
smaller is the
resistance, i.e., the greater is the electrical conductivity. The average
distance an electron
21 can travel without colliding is called the 'mean free path.' But there's
another factor at
22 work too. The electrons which are free to respond to the electric field
have a thermal speed
23 a sizable percentage of the speed of light, but since they travel randomly
with this high
24 speed, they go nowhere on average, i.e., this thermal speed itself doesn't
create any current.
26 The thermal conductivity of this metal is, like electrical conductivity,
determined largely by
27 the free electrons. Suppose now that the metal has different temperatures
at its ends. The
28 electrons are moving slightly faster at the hot end and slower at the cool
end. The faster
29 electrons transmit energy to the cooler, slower ones by colliding with
them, and just as for
electrical conductivity, the longer the mean free path, the faster the energy
can be
31 transmitted, i.e., the greater the thermal conductivity. But the rate is
also determined by
CA 02752633 2011-09-19
19
1 the very high thermal speed-the higher the speed, the more rapidly does heat
energy
2 flow(i.e., the more rapidly collisions occur). In fact, the thermal
conductivity is directly
3 proportional to the product of the mean free path and thermal speed.
4
Both thermal and electrical conductivity depend in the same way on not just
the mean free
6 path, but also on other properties such as electron mass and even the number
of free
7 electrons per unit volume. But as we have seen, they depend differently on
the thermal
8 speed of the electrons-electrical conductivity is inversely proportional to
it and thermal
9 conductivity is directly proportional to it. The upshot is that the ratio of
thermal to
electrical conductivity depends primarily on the square of the thermal speed.
But this
11 square is proportional to the temperature, with the result that the ratio
depends on
12 temperature, T, and two physical constants: Boltzmann's constant, k, and
the electron
13 charge, e. Boltzmann's constant is, in this context, a measure of how much
kinetic energy
14 an electron has per degree of temperature.
16 Putting it all together, the ratio of thermal to electrical conductivity
is:
17
18 ( 7T2 / 3 ) * ( (k/e)2 ) * T
19
the value of the constant multiplying T being: 2.45x10"8 W-ohm-K-squared.
21
22 Answered by: Frank Munley, Ph.D., Associate Professor, Physics, Roanoke
College
23
24 http://www2 ph ed ac uk/teaching/course-notes/documents/63/247-chapterl.pdf
http://www bbc co uk/schools/gcsebitesize/science/aga/energy/heatrevl.shtml
26 Heat transfer by conduction and convection
27 Heat is thermal energy. It can be transferred from one place to another by
conduction,
28 convection and radiation. Conduction and convection involve particles, but
radiation
29 involves electromagnetic waves.
CA 02752633 2011-09-19
1 Conduction
2
3 Thermogram of a pan being heated on a stove
4 Heat energy can move through a substance by conduction. Metals are good
conductors of
5 heat, but non-metals and gases are usually poor conductors of heat. Poor
conductors of
6 heat are called insulators. Heat energy is conducted from the hot end of an
object to the
7 cold end.
8 The electrons in piece of metal can leave their atoms and move about in the
metal as free
9 electrons. The parts of the metal atoms left behind are now charged metal
ions. The ions
10 are packed closely together and they vibrate continually. The hotter the
metal, the more
11 kinetic energy these vibrations have. This kinetic energy is transferred
from hot parts of
12 the metal to cooler parts by the free electrons. These move through the
structure of the
13 metal, colliding with ions as they go.
14 Heat transfer by conduction
S WP Metat Atom
Heat
16 Convection
17 Liquids and gases are fluids. The particles in these fluids can move from
place to place.
18 Convection occurs when particles with a lot of heat energy in a liquid or
gas move and take
19 the place of particles with less heat energy. Heat energy is transferred
from hot places to
cooler places by convection.
CA 02752633 2011-09-19
21
1 Liquids and gases expand when they are heated. This is because the particles
in liquids and
2 gases move faster when they are heated than they do when they are cold. As a
result, the
3 particles take up more volume. This is because the gap between particles
widens, while the
4 particles themselves stay the same size.
The liquid or gas in hot areas is less dense than the liquid or gas in cold
areas, so it rises
6 into the cold areas. The denser cold liquid or gas falls into the warm
areas. In this way,
7 convection currents that transfer heat from place to place are set up.
8 Comparison of surfaces abilities to reflect and absorb radiation
.m?u ~. , i. .~=r ., der
I TT
co
n. ,=x ili 14 epiU*~ r m diatiiab It rb thet
ilk" _6i dark dull or matt good good
light shiny poor poor
9 If two objects made from the same material have identical volumes, a thin,
flat object will
radiate heat energy faster than a fat object. This is one reason why domestic
radiators are
11 thin and flat. Radiators are often painted with white gloss paint. They
would be better at
12 radiating heat if they were painted with black matt paint, but in fact,
despite their name,
13 radiators transfer most of their heat to a room by convection.
14
http://www.physicsclassroom.com/class/estatics/u8l2b.cfm
16 The Electrophorus
17 "A commonly used lab activity that demonstrates the induction charging
method is the
18 Electrophorus Lab. In this lab, a flat plate of foam is rubbed with animal
fur in order to
19 impart a negative charge to the foam. Electrons are transferred from the
animal fur to the
more electron-loving foam (Diagram i.). An aluminum pie plate is taped to a
Styrofoam
21 cup; the aluminum is a conductor and the Styrofoam serves as an insulating
handle. As the
22 aluminum plate is brought near, electrons within the aluminum are repelled
by the
23 negatively charged foam plate. There is a mass migration of electrons to
the rim of the
24 aluminum pie plate. At this point, the aluminum pie plate is polarized,
with the negative
charge located along the upper rim farthest from the foam plate (Diagram ii.).
The rim of
26 the plate is then touched, providing a pathway from the aluminum plate to
the ground.
27 Electrons along the rim are not only repelled by the negative foam plate,
they are also
CA 02752633 2011-09-19
22
1 repelled by each other. So once touched, there is a mass migration of
electrons from the rim
2 to the person touching the rim (Diagram iii.). Being of much greater size
than the
3 aluminum pie plate, the person provides more space for the mutually
repulsive electrons.
4 The moment that electrons depart from the aluminum plate, the aluminum can
be
considered a charged object. Having lost electrons, the aluminum possesses
more protons
6 than electrons and is therefore positively charged. Once the foam plate is
removed, the
7 excess positive charge becomes distributed about the surface of the aluminum
plate in
8 order to minimize the overall repulsive forces between them (Diagram iv.)."
Charging an Aluminum Pie Plate by Induction
Diagram i. Diagram ii. Diagram m. Diagram iv. Diagram v.
A foam plate is An ahnmrun plate What tourhed om the The ahnmrt.n Ranaininge-
nfbedwithimZ isbra tnearthe mn,e-movethra plate,havn,glast moveam+nd
ad givaa a - foam, ard~e- the had to the groud. e-,nowhas a + +ntil the + chaz
9 rho. movaneat to mn. charge. redistributed.
"The Electrophorus Lab further illustrates that when charging a neutral object
by
11 induction, the charge imparted to the object is opposite that of the object
used to induce the
12 charge. In this case, the foam plate was negatively charged and the
aluminum plate became
13 positively charged. The lab also illustrates that there is never a transfer
of electrons
14 between the foam plate and the aluminum plate. The aluminum plate becomes
charged by
a transfer of electrons to the ground. Finally, one might note that the role
of the charged
16 object in induction charging is to simply polarize the object being
charged. This
17 polarization occurs as the negative foam plate repels electrons from the
near side, inducing
18 them to move to the opposite side of the aluminum plate. The presence of
the positive
19 charge on the bottom of the aluminum plate is the result of the departure
of electrons from
that location. Protons did not move downwards through the aluminum. The
protons were
21 always there from the beginning; it's just that they have lost their
electron partners. Protons
22 are fixed in place and incapable of moving in any electrostatic
experiment."
23
CA 02752633 2011-09-19
23
1 The Electroscope
2 "Another common lab experience that illustrates the induction charging
method is the
3 Electroscope Lab. In the Electroscope Lab, a positively charged object such
as an
4 aluminum pie plate is used to charge an electroscope by induction. An
electroscope is a
device that is capable of detecting the presence of a charged object. It is
often used in
6 electrostatic experiments and demonstrations in order to test for charge and
to deduce the
7 type of charge present on an object. There are all kinds of varieties and
brands of
8 electroscope from the gold leaf electroscope to the needle electroscope."
9 "While there are different types of electroscopes, the basic operation of
each is the same.
The electroscope typically consists of a conducting plate or knob, a
conducting base and
11 either a pair of conducting leaves or a conducting needle. Since the
operating parts of an
12 electroscope are all conducting, electrons are capable of moving from the
plate or knob on
13 the top of the electroscope to the needle or leaves in the bottom of the
electroscope. Objects
14 are typically touched to or held nearby the plate or knob, thus inducing
the movement of
electrons into the needle or the leaves (or from the needle/leaves to the
plate/knob). The
16 gold leaves or needle of the electroscope are the only mobile parts. Once
an excess of
17 electrons (or a deficiency of electrons) is present in the needle or the
gold leaves, there will
18 be a repulsive affect between like charges causing the leaves to repel each
other or the
19 needle to be repelled by the base that it rests upon. Whenever this
movement of the
leaves/needle is observed, one can deduce that an excess of charge - either
positive or
21 negative - is present there. It is important to note that the movement of
the leaves and
22 needle never directly indicate the type of charge on the electroscope; it
only indicates that
23 the electroscope is detecting a charge."
Gold Leaf Electroscope Needle Electroscope
Lai ID
Relaxed L ve5 Deflected Lxeve Relaxed Needle Derv; ed Needle
24 Neutral CIS Neil Charged
CA 02752633 2011-09-19
24
1
2 "Suppose a needle electroscope is used to demonstrate induction charging. An
aluminum
3 pie plate is first charged positively by the process of induction (see
discussion above). The
4 aluminum plate is then held above the plate of the electroscope. Since the
aluminum pie
plate is not touched to the electroscope, the charge on the aluminum plate is
NOT
6 conducted to the electroscope. Nonetheless, the aluminum pie plate does have
an affect
7 upon the electrons in the electroscope. The pie plate induces electrons
within the
8 electroscope to move. Since opposites attract, a countless number of
negatively charged
9 electrons are drawn upwards towards the top of the electroscope. Having lost
numerous
electrons, the bottom of the electroscope has a temporarily induced positive
charge. Having
11 gained electrons, the top of the electroscope has a temporarily induced
negative charge
12 (Diagram ii. below). At this point the electroscope is polarized; however,
the overall charge
13 of the electroscope is neutral. The charging step then occurs as the bottom
of the
14 electroscope is touched to the ground. Upon touching the bottom of the
electroscope,
electrons enter the electroscope from the ground. One explanation of their
entry is that
16 they are drawn into the bottom of the electroscope by the presence of the
positive charge at
17 the bottom of the electroscope. Since opposites attract, electrons are
drawn towards the
18 bottom of the electroscope (Diagram iii.). As electrons enter, the needle
of the electroscope
19 is observed to return to the neutral position. This needle movement is the
result of negative
electrons neutralizing the previously positively charged needle at the bottom
of the
21 electroscope. At this point, the electroscope has an overall negative
charge. The needle does
22 not indicate this charge because the excess of electrons is still
concentrated in the top plate
23 of the electroscope; they are attracted to the positively charged aluminum
pie plate that is
24 held above the electroscope (Diagram iv.). Once the aluminum pie plate is
pulled away, the
excess of electrons in the electroscope redistribute themselves about the
conducting parts of
26 the electroscope. As they do, numerous excess electrons enter the needle
and the base upon
27 which the needle rests. The presence of excess negative charged in the
needle and the base
28 causes the needle to deflect, indicating that the electroscope has been
charged (Diagram
29 v.)."
CA 02752633 2011-09-19
Charging an Electroscope by Induction
Diagram i. Diapm u. Diagram 711. Diagam iv. Diagram v.
e'
Aneud cal The + aluminum plate Whim touched, e' The a-scope has a - The excess
electroscope. attracts e' from the eater from grol.d chazW- this - rho
elecheus
bottom of the a-scope to the tonwrtmlize the + is still located in "'te
1 top plate of the a-scope. chi imneedle. the top plate. themselves.
2
3 "The above discussion provides one more illustration of the fundamental
principles
4 regarding induction charging. These fundamental principles have been
illustrated in each
5 example of induction charging discussed on this page. The principles are:
6 The charged object is never touched to the object being charged by
7 induction.
8 The charged object does not transfer electrons to or receive electrons from
9 the object being charged.
10 The charged object serves to polarize the object being charged.
11 The object being charged is touched by a ground; electrons are transferred
12 between the ground and the object being charged (either into the object or
out of
13 it).
14 The object being charged ultimately receives a charge that is opposite that
15 of the charged object that is used to polarize it."
16 -------- ________
17 http://www.practicalphysics.org/go/Guidance 134.html
CA 02752633 2011-09-19
26
1 Electron guns
2 "When a piece of metal is heated, electrons escape from its surface. These
free electrons
3 can be accelerated in a vacuum, producing a beam. The hot metal surface and
the
4 accelerating plates are sometimes called an `electron gun'.
6 In an electron gun, the metal plate is heated by a small filament wire
connected to a low
7 voltage. Some electrons (the conduction electrons) are free to move in the
metal - they are
8 not bound to ions in the lattice. As the lattice is heated, the electrons
gain kinetic energy.
9 Some of them gain enough kinetic energy to escape from the metal surface. We
sometimes
say that they are `boiled off the surface or `evaporate' from it. Although
they do not form
11 a gas in the strictest sense, these are good descriptions.
12
13 If the hot metal plate is in a vacuum, then the evaporated electrons are
free to move. The
14 electrons can be pulled away from the hot surface of the plate by putting a
positive
electrode (anode) nearby. The anode is created by connecting an electrode to
the positive
16 terminal of a power supply, and the hot plate is connected to its negative
terminal. The hot
17 plate is then the cathode.
18
19 As soon as the electrons evaporate from the surface of the hot plate, they
are pulled
towards the anode. They accelerate and crash into the anode. However, if there
is a small
21 hole in the anode, some electrons will pass through, forming a beam of
electrons that came
22 from the cathode or a cathode ray.
23
24 This cathode ray can be focused and deflected and can carry small currents.
This is the
basis of the important experiments carried out by J J Thomson and others. It
is also the
26 basis of early electronic devices.
27
28 You could explain the operation of an electron gun thus:
29
= At one end of the tube there is a little rocket shaped 'gun. In that gun a
starting plate is
31 heated by a tiny electric grill. The plate has a special surface that lets
electrons loose rather
CA 02752633 2011-09-19
27
1 easily. Electrons come off that plate. They are speeded up in the gun by a
large potential
2 difference between that starting plate ('negative cathode) and the gun
muzzle (`positive
3 anode').
4
cathode anode
ter.
6=3V HT
AC 100.120V
6
7 = Electrons come out at high speed through a tiny hole in the cone-shaped
muzzle.
8
9 = The electrons continue at that constant speed through the vacuum because
there is nothing
for them to collide with - until they hit a fluorescent screen, where they
make a bright spot.
11
12 = The glass globe of the tube has been pumped out to a very good vacuum,
removing air which
13 would soon slow down electrons by collisions. But then a very little helium
(or hydrogen) gas
14 is let in. Because the helium atoms give out a green glow when hit by
electron, you can see the
path of the electrons made visible as a thin line of glow. (Hydrogen glows
blue.)
16
17 = Look at the thin glowing line carefully. You are seeing the path of
electrons flying through
18 thin helium (or hydrogen), almost a vacuum, all by themselves, with no
wires there.
19
Focusing
21
22 "The fine beam tube is improved by adding a small conical electrode - often
connected to
23 the anode. This produces a converging electric field which focuses the
electrons and
CA 02752633 2011-09-19
28
1 produces a tighter beam and sharper spot on the fluorescent screen. "
2
3 Updated 5 May 2009
4 Related Content
6 http://hyperphysics.phy-astr.Ilsu.edu/hbase/electric/ohmmic.html#c2
7 http://hyperphysics.phy-astr.gsu.edu/hbase/solids/fermi.html#cl
8 " Fermi Level
9 "Fermi level" is the term used to describe the top of the collection of
electron energy levels
at absolute zero temperature. This concept comes from Fermi-Dirac statistics.
Electrons
11 are fermions and by the Pauli exclusion principle cannot exist in identical
energy states. So
12 at absolute zero they pack into the lowest available energy states and
build up a "Fermi
13 sea" of electron energy states. The Fermi level is the surface of that sea
at absolute zero
14 where no electrons will have enough energy to rise above the surface. The
concept of the
Fermi energy is a crucially important concept for the understanding of the
electrical and
16 thermal properties of solids. Both ordinary electrical and thermal
processes involve
17 energies of a small fraction of an electron volt. But the Fermi energies of
metals are on the
18 order of electron volts. This implies that the vast majority of the
electrons cannot receive
19 energy from those processes because there are no available energy states
for them to go to
within a fraction of an electron volt of their present energy. Limited to a
tiny depth of
21 energy, these interactions are limited to "ripples on the Fermi sea".
CA 02752633 2011-09-19
29
At higher temperatures a certain fraction, characterized by the
Fermi function, will exist above the Fermi level. The Fermi
level plays an important role in the band theory of solids. In
doped semiconductors, p-type and n- e, the Fermi level is
shifted by the impurities, illustrated by their band gaps. The
Fermi level is referred to as the electron chemical potential in
other contexts.
Conduction
In metals, the Fermi energy gives us information about the Band
velocities of the electrons which participate in ordinary Egap
At absolute
electrical conduction. The amount of energy which can be Fermi zero OK Egap
given to an electron in such conduction processes is on the Level 2
order of micro-electron volts (see copper wire example), so only (E)
Valenm Band
those electrons very close to the Fermi energy can participate.
The Fermi velocity of these conduction electrons can be Context of Fermi IeveJ
calculated from the Fermi energy. for a semiconductor
vF = Ã2Ex Table
V' M
This speed is a part of the microscopic Ohm's Law for
electrical conduction. For a metal, the density of conduction
electrons can be implied from the Fermi energy. "
1 The Fermi energy also plays an important role in understanding the mystery
of why
2 electrons do not contribute significantly to the specific heat of solids at
ordinary
3 temperatures, while they are dominant contributors to thermal conductivity
and electrical
4 conductivity. Since only a tiny fraction of the electrons in a metal are
within the thermal
energy kT of the Fermi energy, they are "frozen out" of the heat capacity by
the Pauli
6 principle. At very low temperatures, the electron specific heat becomes
significant.
7
CA 02752633 2011-09-19
Microscopic View of Ohm's Law
When electric current in a material is
The electron moves at the proportional to the voltage across it, the
Fermi speed, and has only material is said to be "ohmic", or to
a bny drift ve" sups sed obey Ohm's law. A microscopic view
by the applied electric field.
suggests that this proportionality comes
' .r from the fact that an applied electric
field superimposes a small drift
n' velocity on the free electrons in a
rr.P .ak)
tz-' ' ~'r { metal. For ordinary currents, this drift
velocity is on the order of millimeters
per second in contrast to the speeds of
Electric the electrons themselves which are on
field E
the order of a million meters per
second. Even the electron speeds are
themselves small compared to the
speed of transmission of an electrical
signal down a wire, which is on the Index
order of the speed of light, 300 million
meters per second.
The current density (electric current per unit area, J=I/A) can be expressed
in
terms of the free electron density as
r =friee electron density
.l = -t~'Vd n vd drift velocity
The number of atoms per unit volume (and the number free electrons for
atoms like copper that have one free electron per atom) is
Avogadro's number Density
ri (Naa'toms 1 mole)(p kg / m)
A(kg 1 male)
Atomic mass
From the standard form of Ohm's law and resistance in terms of resistivity:
CA 02752633 2011-09-19
31
R-
J-
R A-
V _ V _EL_E_~.E
RA PLA-1 P
1
The next step is to relate the drift velocity to the electron speed, which can
be
approximated by the Fermi speed:
VF - ( L Table
The drift speed can be expressed in terms of the accelerating electric field
E,
the electron mass, and the characteristic time between collisions.
V ~eEe'E d
M m VF
The conductivity of the material can be expressed in terms of the Fermi speed
and the mean free path of an electron in the metal.
6 tie2d Table
Numerical example for copper. Table of resistivities
Table of free electron densities
Go Back
HyperPhysics***** Electricity and Magnetism R Nave
1
2
3
4
CA 02752633 2011-09-19
32
Microscopic View of Copper Wire
As an example of the microscopic view of Ohm's law, the parameters for
copper will be examined. With one free electron per atom in its metallic
state,
the electron density of copper can be calculated from its bulk density and its
atomic mass.
n _ (6.02x102'laton:s 1 mole)(8.92x103kg / m) = 8.46x10"'1 m3
63.5x10- k,g / prole
The Fermi energy for copper is about 7 eV, so the Fermi speed is
VF = C 2EF = 3x10xm / 2x7eV =1.57x10{'m /.v
rnc` I T I 10OOe V
The measured conductivity of copper at 20 C is
Cr 5.9x107/Urn
Index
The mean free path of an electron in copper under these conditions can be
calculated from
d= Orr2VF - (5,9x1 071' f2na)(9.11x10-{ kkk)(1.57x106r Is) =3.9xI O-$rn
r (8.46A 0281 rri3)(I.6x10-49)2
The drift speed depends upon the electric field applied. For example, a copper
wire of diameter l mm and length 1 meter which has one volt applied to it
yields the following results.
R = L = lm 0.0216
caA (5.9x10 /S2m)(ir.0005`,r:`)
For 1 volt applied this gives a current of 46.3 Amperes and a current density
J=5.9xI07A/mrm.2
This corresponds to a drift speed of only millimeters per second, in contrast
to
the high Fermi speed of the electrons.
CA 02752633 2011-09-19
33
V 5.9'x1f&7 A / m1 = 0.0043m / s
! n
e 8.46x 10 /m ' )(l .6x 11 C}
Caution! Do not try this at home! Dr. Beihai Ma of Argonne National
Laboratory wrote to point out that the current density of 5900 A/cm2 in this
example is over ten times the current density of 500 A/cm2 that copper can
normally withstand at 40 F. So doing this in the laboratory might be too
exciting. Thanks for the sanity check Dr. Ma.
(If you scale down the voltage applied so that the current is just 3 Amperes,
current density 382 A/cm2, so that the copper wire will remain intact, the
calculated drift velocity is just 0.00028 m/s. This would be more typical for
working conditions in this wire. )
Go Back
HyperPhysics***** Electricity and Magnetism R Nave
1
2
3
4
CA 02752633 2011-09-19
34
Free Electron Density in a Metal
The free electron density in a metal is a factor in determining its electrical
conductivity. It is involved in the Ohm's law behavior of metals on a
microscopic scale. Because electrons are fermions and obey the Pauli exclusion
principle, then at 0 K temperature the electrons fill all available energy
levels
up to the Fermi level. Therefore the free electron density of a metal is
related to
the Fermi level and can be calculated from
01t),1m3''` 2 ~t2 _ tirl-jr( lZC2 ~I- 2 E 3/2 show
h-' F ) - ht. 3 k 3
A metal with Fermi energy EF = eV
will have free electron density n = r X10
/m3.
Index
Table of Fermi Energies
Alternatively, if you can identify the number of electrons per atom that
participate in conduction, then the free electron density can just be implied
from the atomic mass and mass density of the material. The number of
atoms per unit volume can be implied from the atomic mass and bulk
density of the material:
Avogadro'-., number Density
tt' . (N, atoms / rnole)(p kg / rn3)
A(k,g I mole)
Atomic mass
Periodic Table of Elements
A metal with bulk density = kg/m3
and atomic mass A = x 10-3 kg/mole
CA 02752633 2011-09-19
will have a number of atoms per unit volume n' = x l 0 /m3.
The number of atoms per unit volume multiplied by the number of free
electrons per atom should agree with the free electron density above.
While these two approaches should be in agreement, it may be instructive to
examine both for self-consistency.
Consider the element zinc with a tabulated Fermi energy of 9.47 eV. This leads
to a free electron density of
Ip - 8J2;r(.511 ~hfev) ' (2(947V)3/2 1321 r3m3 = 1,32x1429 / n3
(1240 eV n,~n 3 }
From the Periodic Table, the density of zinc is 7140 kg/m3 and its atomic mass
is 65.38 gm/mole. The number of atoms per unit volume is then
1 N4 p - (6.O2x1023nt(ams I rnol)(7140kg / ml) = 2s
n _ - 6.57x10 a~'r~m s 1 3
.
_ A 65.38x14 -,kg/m /
The number of free electrons per zinc atom to make these consistent is
n 1.2a 1 _ 2.01 free electrons per atom
n 6.57x14' s
This number is what we would expect from the electron configuration of zinc,
(Ar)3d104s2 , so these two approaches to the free electron density in a metal
are
consistent.
Go Back
HyperPhysics***** Electricity and Magnetism R Nave
1
2 Reference, Peltier Effect Chips:
3 http://www.tetech.com/FAQ-Technical-Information.html#l
CA 02752633 2011-09-19
36
1 Frequently Asked Questions on Thermoelectrics
2
3 1. How does a thermoelectric module work?
4
"Thermoelectric modules are solid-state heat pumps that operate on the Peltier
effect (see
6 definitions). A thermoelectric module consists of an array of p- and n-type
semiconductor
7 elements that are heavily doped with electrical carriers. The elements are
arranged into
8 array that is electrically connected in series but thermally connected in
parallel. This array
9 is then affixed to two ceramic substrates, one on each side of the elements
(see figure
below). Let's examine how the heat transfer occurs as electrons flow through
one pair of p-
11 and n-type elements (often referred to as a "couple") within the
thermoelectric module:
Schematic of a Thermoelectric Cooler
ceramic
substrate heat absorbed
++ (+
F + + .1 + electrons
copper
conductor P-type N-type
holes
+ + ' ) +1
heat rejected
ceramic
heat sink direct current
12 sub str ate
CA 02752633 2011-09-19
37
1 The p-type semiconductor is doped with certain atoms that have fewer
electrons than
2 necessary to complete the atomic bonds within the crystal lattice. When a
voltage is
3 applied, there is a tendency for conduction electrons to complete the atomic
bonds. When
4 conduction electrons do this, they leave "holes" which essentially are atoms
within the
crystal lattice that now have local positive charges. Electrons are then
continually dropping
6 in and being bumped out of the holes and moving on to the next available
hole. In effect, it
7 is the holes that are acting as the electrical carriers.
8 Now, electrons move much more easily in the copper conductors but not so
easily in the
9 semiconductors. When electrons leave the p-type and enter into the copper on
the cold-side,
holes are created in the p-type as the electrons jump out to a higher energy
level to match
11 the energy level of the electrons already moving in the copper. The extra
energy to create
12 these holes comes by absorbing heat. Meanwhile, the newly created holes
travel downwards
13 to the copper on the hot side. Electrons from the hot-side copper move into
the p-type and
14 drop into the holes, releasing the excess energy in the form of heat.
The n-type semiconductor is doped with atoms that provide more electrons than
necessary
16 to complete the atomic bonds within the crystal lattice. When a voltage is
applied, these
17 extra electrons are easily moved into the conduction band. However,
additional energy is
18 required to get the n-type electrons to match the energy level of the
incoming electrons
19 from the cold-side copper. The extra energy comes by absorbing heat.
Finally, when the
electrons leave the hot-side of the n-type, they once again can move freely in
the copper.
21 They drop down to a lower energy level, and release heat in the process.
22 The above explanation is imprecise as it does not cover all the details,
but it serves to
23 explain in words what are otherwise very complex physical interactions. The
main point is
24 that heat is always absorbed at the cold side of the n- and p- type
elements, and heat is
always released at the hot side of thermoelectric element. The heat pumping
capacity of a
26 module is proportional to the current and is dependent on the element
geometry, number
27 of couples, and material properties.
28
29
CA 02752633 2011-09-19
38
Back to the top
2
3 2. What is the mathematical equation for describing the operation of a
thermoelectric
4 module?
QC
Tc
A = cross-
sectional
L area
n P
Qh
I
(+) (-)
D.C. source
6
The figure above represents a thermoelectric couple. It shows some terms used
in the
mathematical equation:
L = element height A = cross-sectional area Qc = heat load
Tc = cold-side temperature Th = hot-side temperature I = applied current
Additionally, there is the following:
S = Seebeck coefficient R = electrical resistivity K = thermal conductivity
V = voltage N = number of couples
Here are the basic equations:
Qc=2*N*IS*I*Tc-1/2*I^2*R*L/A-K*A/L*(Th-Tc)]
CA 02752633 2011-09-19
39
V=2*N*[S*(Th-Tc)+I*R*L/A)
1
2 The first Qc term, S*I*Tc, is the peltier cooling effect. The second
term,1/2*I112*R*L/A,
3 represents the Joule heating effect associated with passing an electrical
current through a
4 resistance. The Joule heat is distributed throughout the element, so 1/2 the
heat goes
towards the cold side, and 1/2 the heat goes towards the hot side. The last
term,
6 K*A/L*(Th-Tc), represents the Fourier effect in which heat conducts from a
higher
7 temperature to a lower temperature. So, the peltier cooling is reduced by
the losses
8 associated with electrical resistance and thermal conductance.
9
For the voltage, the first term, S*(Th-Tc) represents the Seebeck voltage. The
second term,
11 I*R*L/A represents the voltage related by Ohm's law.
12
13 These equations are very simplified and are meant to show the basic idea
behind the
14 calculations that are involved. The actual differential equations do not
have a closed-form
solution because S, R, and K are temperature dependent. Unfortunately,
assuming constant
16 properties can lead to significant errors.
17
18 TE Technology uses special, proprietary modeling software which takes into
account the
19 temperature dependency of the thermoelectric material properties as well as
all the
relevant design aspects of the overall system. The software uses material
property data
21 from actual test results on thermoelectric modules, so it yields highly
accurate results.
22 When we build a custom cooler for your application, that high accuracy
means you
23 generally only need one prototype to verify cooling performance.
24
26
27
CA 02752633 2011-09-19
1 3. What are the advantages of a thermoelectric unit over a compressor?
2
3 Thermoelectric modules have no moving parts and do not require the use of
4 chlorofluorocarbons. Therefore they are safe for the environment, inherently
reliable, and
5 virtually maintenance free. They can be operated in any orientation and are
ideal for
6 cooling devices that might be sensitive to mechanical vibration. Their
compact size also
7 makes them ideal for applications that are size or weight limited where even
the smallest
8 compressor would have excess capacity. Their ability to heat and cool by a
simple reversal
9 of current flow is useful for applications where both heating and cooling is
necessary or
10 where precise temperature control is critical.
11 Fig 12. Electrostatic Chucks:
http://www.oxfordplasma.de/technols/e_chuck.htm
Wafer.,Conductive Substrate
orolectnc
L V
5000V 500V V
0-111 D 0
'D' Electrodes Concentric Rings
12 (Thick Film) (Thin Film)
13 Fig 13. Electret Mic, Microphone:
14 http://electronics wups lviv ua/KREM
literatura/hyperphysics/hbase/audio/mic2.html
10 -
0
0
Electrical
connections
Membrane made Insulator
of permanently
charged elect ret Air cavity
15 material.
CA 02752633 2011-09-19
41
1 Electret Condenser Microphone
Electret condenser microphones are not to be compared with
the studio standard condenser microphones which have such
0 excellent frequency response characteristics. The electret
class of microphones are condenser microphones which use
Elec#rical a permanently polarized electret material for their
canneations diaphragms, thus avoiding the necessity for the biasing DC
voltage required for the conventional condenser. They can be
Membrane made
of permanently Insulator made very inexpensively and are the typical
microphones on
charged el ectret Air cavity portable tape recorders. Better quality electret
condensers
material. incorporate a field-effect transistor (FET) preamplifier to
match their extremely high impedance and boost the signal.
2 Electret Microphone Diaphragm
The electret material of the diaphragm may be less than a
thousandth of an inch thick. Even so, it is polarized enough
to produce an active capacitive change in the voltage
Q Electrical between the membrane and the back plate when it is moved
connections by the pressure of a sound wave. The polarization is
achieved by a combination of heat and high voltage during
Membrane made Insulator
of permanently manufacture. Electron bombardment may also be used
charged electret Air cavity (Rossing). The diaphragm is backed by an
evaporated metal
material. film.
3 http://www.coilgun.info/theorycapacitors/capacitors2.htm
4 http://www.techlearner.com/DCPaies/DCCap.htm
Capacitance and Capacitors
6 I. Elementary Characteristics 'rn~9>aib
7 In its most elementary state a capacitor
8 consists of two metal plates separated by
9 a certain distance d, in between the plates
CA 02752633 2011-09-19
42
1 lies a dielectric material with dielectric
2 constant c , where c, is the dielectric of air.
3
4 The dielectric material allows for charge to accumulate between the
capacitor plates. Air
(actually vacuum)
6
7 has the lowest dielectric value of EQ = 8.854 * 1012 Farads/meter where the
Farad is the unit for
8 capacitance.
9
All other materials have higher dielectric values, since they are higher in
density and can
11 therefore accumulate
12
13 more charge.
14
Capacitance is defined to be the amount of charge Q stored in between the two
plates for a
16 potential difference
17
18 or voltage V existing across the plates. In other words:
19
The capacitance C is given by C = QN (electrical definition)
21
22 The Physical meaning of capacitance can be seen by relating it to the
physical characteristics of
23 the two plates,
24
so that, the capacitance is related to the dielectric of the material in
between the plates, the square
26 area of a
27
28 plate and the distance between the plates by the formula:
29
C = Eoc Aid (physical definition)
31
32 Clearly, the larger the area of the plate the more charge can be
accumulated and hence the larger
33 the
34
capacitance. Also, note that as the distance d increases the Capacitance
decreases since the
36 charge cannot be
37
38 contained as 'densely' as before.
39
Both definitions of Capacitance are compatible, although for our purposes we
will be referring
41 mostly to the
42
43 electrical definition.
44
CA 02752633 2011-09-19
43
1 Fig 14. Electronic Insect Zapper: http://home.howstuffworks.com/bug-
zapper.htm
t ;
Housing Top ---V"
Transformer --- -~.-
Light -----
Housing Frame
Wire Grids
2 02001 aW [tifPAUrks
3
4
6
7
8
9
11
12
13
14
CA 02752633 2011-09-19
44
1 In the drawings which form a part of this specification,
2 Fig 1. Complete Electret Refrigerator :
3 Fig 2. 12 Volt Rechargeable DC Battery:
4 Fig 3. Diode:
Fig 4. Square Wave Inverter :
6 Fig 5. Capacitor:
7 Fig 6. Diode Full Wave Bridge Circuit:
8 Fig 7. Induction of Charges Charges:
9 http://www.physicsclassroom.com/class/estatics/u8l2b.cfm
The Electrophorus, The Electroscope:
11 Fig 8. Solar Panels and Devices:
12 Fig 9. Sine Wave that is a Rectified Full Wave with Filtering:
13 Fig 10. Electrical Switches or PLC Logic Switches / Limit switches:
14 Fig 11. Thermo-Electric Peltier Effect Cooling Chip:
Fig 12. Electrostatic Chucks: http://www.oxfordplasma.de/technols/e_chuck.htm
16 Fig 13. Electret Mic, Microphone:
17
http://electronics.wups.lviv.ua/KREM_literatura/hyperphysics/hbase/audio/mic2.h
tml
18 Fig 14. Electronic Insect Zapper: http://home.howstuffworks.com/bug-
zapper.htm
CA 02752633 2011-09-19
In the drawings which form a part of this specification,
Fig 1. Complete Electret Refrigerator :
Fig 2. 12 Volt Rechargeable DC Battery:
Fig 3. Diode:
Fig 4. Square Wave Inverter :
Fig 5. Capacitor:
Fig 6. Diode Full Wave Bridge Circuit:
Fig 7. Induction of Charges Charges: The Electrophorus, The Electroscope:
http://www.physicsclassroom.com/class/estatics/u8l2b.cfm
Fig 8. Solar Panels and Devices:
Fig 9. Sine Wave that is a Rectified Full Wave with Filtering:
Fig 10. Electrical Switches or PLC Logic Switches / Limit switches:
Fig 11. Thermo-Electric Peltier Effect Cooling Chip:
Fig 12. Electrostatic Chucks: http://www.oxfordplasma.de/technols/e_chuck.htm
Fig 13. Electret Mic, Microphone:
http://electronics.wups.lviv.ua/KREM_literatura/hyperphysics/hbase/audio/mic2.h
tml
Fig 14. Electronic Insect Zapper:
http://home.howstuffworks.com/bug-zapper.htm
CA 02752633 2011-09-19
8
1 In the drawings which form a part of this specification,
2 Fig 1. Complete Electret Refrigerator :
3 Fig 2. 12 Volt Rechargeable DC Battery:
4 Fig 3. Diode:
Fig 4. Square Wave Inverter :
6 Fig 5. Capacitor:
7 Fig 6. Diode Full Wave Bridge Circuit:
8 Fig 7. Induction of Charges Charges: The Electrophorus, The Electroscope:
9 http://www.physicsclassroom.com/class/estatics/u8l2b.cfm
Fig 8. Solar Panels and Devices:
11 Fig 9. Sine Wave that is a Rectified Full Wave with Filtering:
12 Fig 10. Electrical Switches or PLC Logic Switches / Limit switches:
13 Fig 11. Thermo-Electric Peltier Effect Cooling Chip:
14 Fig 12. Electrostatic Chucks:
http://www.oxfordplasma.de/technols/e_chuck.htm
Fig 13. Electret Mic, Microphone:
16 Fig 14. Electronic Insect Zapper: http://home.howstuffworks.com/bug-
zapper.html