Note: Descriptions are shown in the official language in which they were submitted.
CA 02753127 2011-08-19
WO 2010/101571 PCT/US2009/036129
HYDROCARBON DEHYDROGENATION PROCESS
FIELD OF THE INVENTION
[0001] The field of art to which this invention pertains is the catalytic
dehydrogenation
of hydrocarbons. More specifically, the invention is a method for
dehydrogenating
hydrocarbons, including, paraffins and alkylaromatics.
BACKGROUND OF THE INVENTION
[0002] The revamp of an existing catalytic dehydrogenation facility having no
oxidative
reheating capability in order to increase capacity is limited by the reactor
internal velocities and
maximum temperatures of the reactor transfer lines. Because of these
limitations on an existing
facility, the maximum possible capacity expansion by simply increasing the
throughput to the
existing reactors is 35 percent.
[0003] The dehydrogenation of hydrocarbons is well known, with both acyclic
and
aromatic hydrocarbons being thereby converted to the corresponding less
saturated products.
For instance, dehydrogenation is performed commercially for the production of
styrene from
ethylbenzene. US 3,515,766 and US 3,409,689 disclose catalytic steam
dehydrogenation
processes for alkylaromatics including ethylbenzene. These references describe
the admixture
of superheated steam and the feed hydrocarbon and the admixture of additional
amounts of
superheated steam with the reactants between sequential beds of
dehydrogenation catalyst to
reheat the reactants.
[0004] The prior art also teaches to pass oxygen into a dehydrogenation zone
for the
purpose of reacting the oxygen with hydrogen released during the
dehydrogenation reaction to
thereby liberate heat and to consume hydrogen. The processes known to employ
this technique
utilize a hydrogen oxidation catalyst in an attempt to selectively oxidize the
hydrogen rather than
feed or product hydrocarbons also present in the dehydrogenation zone.
SUMMARY OF THE INVENTION
[0005] The present invention provides a means for increasing the capacity of
an existing
two reactor dehydrogenation process which is operated without oxidative
reheat. Accordingly,
the hydrocarbon feedstock is initially divided and a first portion of the
hydrocarbon feedstock is
-1-
CA 02753127 2011-08-19
WO 2010/101571 PCT/US2009/036129
introduced into a first dehydrogenation reaction zone which is operated
without oxidative reheat
and the resulting effluent is subsequently reheated and introduced into a
second dehydrogenation
reaction zone which is also operated without oxidative reheat. The resulting
effluent from the
second dehydrogenation reaction zone is introduced, along with a second
portion of the
hydrocarbon feedstock, into a third dehydrogenation reaction zone which is
operated with
oxidative reheat.
[0006] The portion of the feed which bypasses the first two dehydrogenation
reaction
zones is essentially the quantity of feed to achieve the desired capacity
increase. For example,
when 33 percent of the total hydrocarbon feed is bypassed, there is a
resulting 50 percent
increase in total plant capacity when starting with two reaction zones and
going to three reaction
zones. In performing the overall process in this manner, there is no change in
the steam flow
rate and temperature to the steam superheater and the combined feed to the
existing first
dehydrogenation reaction zone remains unchanged from the original design, so
that the critical
pieces of equipment i.e., the steam superheater and two existing reactors are
not directly affected
by the capacity expansion. Capacity increases of 50-60 percent are feasible
using the process of
the present invention.
BRIEF DESCRIPTION OF THE DRAWING
[0007] The drawing is a simplified process flow diagram of a preferred
embodiment of
the present invention. The drawing is intended to be schematically
illustrative of the present
invention and is not a limitation thereof.
DETAILED DESCRIPTION OF THE INVENTION
[0008] Processes for the dehydrogenation of aromatic hydrocarbons are in a
widespread
commercial use. For instance, large quantities of styrene are produced by the
dehydrogenation
of ethylbenzene. The resultant styrene may be polymerized with itself or it
may be
copolymerized with butadiene, isoprene, acrylonitrile, etc. Other hydrocarbons
which may be
dehydrogenated in much the same manner include diethylbenzene, ethyl toluene,
propylbenzene,
and isopropylbenzene. The subject process can also be applied to the
dehydrogenation of other
types of hydrocarbons including relatively pure or mixed streams of C2-C 16
paraffins. The
process can therefore be applied to the dehydrogenation of propane, butanes,
hexanes or
nonanes. However, since the great majority of the present commercial
dehydrogenation
-2-
CA 02753127 2011-08-19
WO 2010/101571 PCT/US2009/036129
processes are employed for the dehydrogenation of ethylbenzene, the following
description of
the subject invention will be presented primarily in terms of the
dehydrogenation of
ethylbenzene. This is not intended to exclude from the scope of the subject
invention those
alkylaromatic and acyclic hydrocarbons set out above or those having different
ring structures
including bicyclic compounds.
[0009] The dehydrogenation reaction is highly endothermic. Therefore, passing
the
reactants through a dehydrogenation catalyst bed results in a decrease in the
reactant
temperature. The endothermicity of the reaction is such that the temperature
decrease removes
the reactants from the desired temperature range. The reactants are actually
cooled to such an
extent that the desired reaction does not progress any further at a
commercially feasible rate.
The desired or commercially necessary per pass conversion therefore cannot be
achieved by
simply passing the reactants into contact with a single bed of dehydrogenation
catalyst. For this
reason, it has become standard commercial practice to in some manner perform
interstage
reheating. In interstage reheating the reactant effluent of a first bed of
catalyst is heated to the
desired inlet temperature of a second downstream bed of catalyst. This
reheating can be
performed through direct heat exchange as by the admixture of high temperature
steam into the
reactant stream emerging from the first catalyst bed.
[0010] A preferred method of interstage reheating comprises the use of
indirect heat
exchange. In this method, the effluent from a dehydrogenation zone is passed
through a heat
exchanger in which it is heated, and the reactants are then passed into the
subsequent
dehydrogenation zone. The high temperature fluid employed in this indirect
heat exchange
method may be high temperature steam, combustion gases, a high temperature
process stream or
other readily available high temperature fluids.
[0011] In accordance with the present invention, a first portion of the
dehydrogenatable
hydrocarbon feedstock is heated and introduced, preferably with steam, into a
first
dehydrogenation reaction zone operated without oxidative reheat to produce an
effluent stream
which is re-heated and introduced into a second dehydrogenation reaction zone.
The resulting
effluent from the second dehydrogenation reaction zone contains hydrogen
produced during
dehydrogenation which is then available for catalytic oxidation to produce
heat to reheat the
reactants before being passed into the downstream dehydrogenation catalyst.
The resulting
effluent from the second dehydrogenation reaction zone, a second portion of
the
-3-
CA 02753127 2011-08-19
WO 2010/101571 PCT/US2009/036129
dehydrogenatable hydrocarbon feedstock, oxygen, and optionally, steam are
reacted in a third
dehydrogenation reaction zone operated with oxidative reheat.
[0012] The second portion of the dehydrogenatable hydrocarbon feedstock,
oxygen and
steam, if present, are preferably admixed before entering the third
dehydrogenation reaction
zone in order to ensure a homogeneous feed to the oxidative reheat catalyst in
order to achieve
the desired reaction temperature before contacting the dehydrogenation
catalyst. This
homogeneity also eliminates the possibility of having explosive concentrations
of hydrocarbons.
[0013] The incentive for employing oxidative reheat is the recognition that
the
combustion of the hydrogen generated in the dehydrogenation reaction zones
performs two
functions which are beneficial in the dehydrogenation process. First, the
consumption of the
hydrogen is beneficial in shifting the equilibrium of the dehydrogenation
reaction to favor
increased amounts of dehydrogenation. Second, the selective combustion of the
hydrogen will
release heat sufficient to reheat the reactants to the desired dehydrogenation
conditions.
[0014] The oxidation is preferably accomplished in the presence of a catalyst
which
selectively promotes the oxidation of hydrogen as compared to the destructive
combustion or
oxidation of the more valuable feed and product hydrocarbons. The selective
combustion
method of interstage reheating presents a more economical dehydrogenation
process.
[0015] Despite the advances which have been achieved in the arts of catalysis
and
hydrocarbon conversion, the ultimate conversion which can be achieved during a
single passage
through a dehydrogenation zone is limited to an amount less than total
conversion. That is, it is
impossible to achieve a 100% conversion of a feed hydrocarbon to a
corresponding product
dehydrogenated hydrocarbon. A basic limitation in the degree of conversion
which may be
achieved in any dehydrogenation processes is the equilibrium concentration of
the various
reactants at the temperatures employed. The effluent stream of a catalytic
dehydrogenation zone
will therefore comprise a mixture of the feed hydrocarbon, the dehydrogenated
hydrocarbon
product, andhydrogen. Generally, it is necessary to separate and recover the
dehydrogenated
hydrocarbon product and to recycle the unconverted feed hydrocarbon. The
greater the rate of
conversion which is achieved in the dehydrogenation zone, the smaller the
amount of
unconverted material is realized which must be recycled. The separation of the
product and
unreacted hydrocarbons requires extensive capital equipment and consumes large
amounts of
utilities in the form of heat and electrical power. It is therefore desirable
to increase the
-4-
CA 02753127 2011-08-19
WO 2010/101571 PCT/US2009/036129
conversion which is achieved per pass in the dehydrogenation zone and to
thereby decrease the
amount of material which must be separated and recycled. A higher per passage
conversion will
also allow a smaller reaction zone to be employed in the process with the
associated reduction in
the cost of the reactors, catalyst and utilities cost of operating the
reaction zone. For these
reasons, it is highly desirable to achieve increased rates of total conversion
during the passage of
the dehydrogenation zone feed stream through a multibed dehydrogenation zone.
[0016] In the oxidative reheat process, an oxygen-containing gas stream is
preferably
admixed with the effluent of a preceding dehydrogenation reaction zone and the
resulting
admixture along with a portion of the dehydrogenatable hydrocarbon feedstock
is passed into a
bed of selective hydrogen oxidation catalyst. To achieve the optimum levels of
performance and
safety in this process, it is necessary to closely control the rate at which
oxygen is passed into the
process in this manner.
[00171 An insufficient amount of oxygen will result in a less than desired
consumption
of hydrogen and more importantly a less than desired reheating of the reactant
stream. The
result will be a decrease in the degree of dehydrogenation achieved during
passage through the
overall reaction zone. It is not normally desired to inject an excess amount
of oxygen into any
part of the dehydrogenation zone above that required to perform the desired
degree of hydrogen
combustion.
[0018] The passage of an excess amount of oxygen into the dehydrogenation zone
will
also have detrimental effects upon the long term operation of the process. For
instance, oxygen
will normally serve to deactivate or poison some commercially employed
dehydrogenation
catalyst. It is therefore undesirable to have residual oxygen emerging from
the oxidation catalyst
bed and thereupon contacting dehydrogenation catalyst. Operation of the
dehydrogenation zone
in a manner which does not result in the total consumption of the oxygen is
also undesirable
because of the obvious explosive nature of oxygen-hydrocarbon mixtures. The
explosive nature
of these mixtures can, however, be essentially negated by properly operating
the process to
avoid the presence of mixtures being within the explosive range, as through
the use of diluents
and intentionally low oxygen addition rates, and the presence of a sufficient
amount of solid
material to act as an explosion suppression means. Lastly, the presence of
oxygen is not
normally desired in vessels containing hydrocarbons as the oxygen may react
with the
hydrocarbons to form various undesired oxygenated compounds.
-5-
CA 02753127 2011-08-19
WO 2010/101571 PCT/US2009/036129
[0019] The structure of the dehydrogenation reaction zones may be varied by
changing
the type of catalyst bed which is employed. For instance, radial flow through
annular catalyst
beds as well as vertical flow through cylindrical catalyst beds. It is to be
noted that with radial
flow, the beds of dehydrogenation catalyst and oxidation catalyst may be
concentrically located
at the same elevation within the vessel or vessels. Either the oxidation
catalyst or the
dehydrogenation catalyst may be located in the outer bed of this arrangement.
The gas flow
would then pass through cylindrical center pipe regions located in the middle
of the radial flow
catalyst beds and through annular gas collection and distribution void volumes
located between
the outer surface of the catalyst beds and the inner wall of the vessel.
Variation is also possible
in the number of beds of catalyst which may be employed. Suitable systems for
catalyst
deployment may be patterned after those presented in US 3,498,755; US
3,515,763; and
US 3,751,232.
[0020] Dehydrogenation catalysts generally consist of one or more metallic
components
selected from Groups VI and VIII of the Periodic Table. One typical catalyst
for the
dehydrogenation of alkylaromatics comprises 85 wt% ferric oxide, 2 wt%
chromia, 12 wt%
potassium hydroxide and 1 wt% sodium hydroxide. A second dehydrogenation
catalyst, which
is used commercially, consists of 87 to 90 wt% ferric oxide, 2 to 3 wt%
chromium oxide and
from 8 to 10 wt% potassium oxide. A third typical catalyst comprises 90 wt%
iron oxide, 4 wt%
chromia and 6 wt% potassium carbonate. Methods for preparing suitable
catalysts are well
known in the art. This is demonstrated by the teachings of US 3,387,053, which
describes the
manufacture of a catalytic composite of at least 35 wt% iron oxide as an
active catalytic agent,
from 1 to 8 wt% zinc or copper oxide, 0.5 to 50 wt% of an alkali promoter, and
from 1 to 5 wt%
chromic oxide as a stabilizer and a binding agent. US 4,467,046 also describes
a catalyst for the
dehydrogenation of ethylbenzene in the presence of steam. This catalyst
contains 15 to 30 wt%
potassium oxide, 2 to 8 wt% cerium oxide, 1.5 to 6 wt% molybdenum oxide, 1 to
4 wt%
calcium carbonate, with the balance being iron oxide.
[0021] Dehydrogenation conditions in general include a temperature of 500 to
750 C
and preferably 565 to 675 C. The temperature required for efficient operation
of any specific
dehydrogenation process will depend on the feed hydrocarbon and the activity
of the catalyst
employed. The pressure maintained within the dehydrogenation zone may range
from 100 to
750 mm Hg, with a preferred range of pressures being from 250 to 700 mm Hg.
The operating
pressure within the dehydrogenation zone is measured at the inlet, midsection,
and outlet of the
-6-
CA 02753127 2011-08-19
WO 2010/101571 PCT/US2009/036129
zone to thereby provide an average pressure. The feed stream is charged to the
dehydrogenation
zone at a liquid hourly space velocity from 0.1 to 2.0 hr -1, and preferably
from 0.1 to 1.0 hr
based on the total liquid hydrocarbon charged at 15.6 C.
[0022] The hydrocarbon feed to be dehydrogenated is preferably admixed with
superheated steam to counteract the temperature lowering effect of the
endothermic
dehydrogenation reaction. The presence of steam has also been described as
benefiting the
stability of the dehydrogenation catalyst by preventing the accumulation of
carbon deposits.
Preferably, the steam is admixed with the other components of the feed stream
at a rate of 0.5 to
1.5 pound of steam per pound of feed hydrocarbon. Other quantities of steam
may be added
after one or more subsequent dehydrogenation catalyst beds if desired.
However, the
dehydrogenation zone effluent stream should contain less than 3 pounds of
steam per pound of
product hydrocarbon and preferably less than 2 pounds of steam per pound of
product
hydrocarbon.
[0023] The vaporous effluent stream from the last dehydrogenation zone may be
heat
exchanged against a stream of steam, a reactant stream of this or another
process, or used as a
heat source for fractionation. Commercially, the effluent stream is often
passed through several
heat exchangers thereby heating a number of different streams and cooling the
effluent stream.
This heat exchange is performed subject to the constraints set out above.
Preferably, the cooling
is sufficient to condense at least 95 mole percent of the C6+ hydrocarbons,
i.e. hydrocarbons
having 6 or more carbon atoms per molecule, and at least 95 mole percent of
the water vapor in
the dehydrogenation zone effluent stream. Essentially all of the
dehydrogenated hydrocarbon
product such as styrene, most water and other readily condensable compounds
present in the
effluent stream are thereby converted to liquids. This produces a mixed phase
stream which is
passed into a phase separation vessel. This procedure allows the facile crude
separation by
decantation of the hydrocarbons from the water and hydrogen present in the
effluent stream.
The dehydrogenated hydrocarbon product present in the dehydrogenation zone
effluent stream
becomes part of the hydrocarbon stream which is withdrawn from the separation
vessel and
transferred to the proper separation facilities. The dehydrogenated
hydrocarbon product is
preferably recovered from the hydrocarbon stream by using one of the several
fractionation
systems known in the art. This fractionation will preferably yield a
relatively pure stream of the
unconverted hydrocarbon feed such as ethylbenzene, which may be recycled for
improved
economics. An additional hydrocarbon stream comprising by-products of the
dehydrogenation
-7-
CA 02753127 2011-08-19
WO 2010/101571 PCT/US2009/036129
reaction may also be obtained during product fractionation. For example, in
the production of
styrene from ethylbenzene, benzene and toluene may be recovered, and may be
recycled in part
as taught in US 3,409,689 and GB 1,238,602 or entirely rejected from the
process. If desired,
methods other than fractionation may be used to recover the dehydrogenated
hydrocarbon
product. For instance, US 3,784,620 teaches the separation of styrene and
ethylbenzene through
the use of a polyamide permeation membrane such as nylon-6 and nylon 6, 10. US
3,513,213
teaches a separation method employing liquid-liquid extraction in which
anhydrous silver
fluoroborate is used as the solvent. Similar separation methods using cuprous
fluoroborates and
cuprous fluorophosphates are described in US 3,517,079; US 3,517,080; and US
3,517,081.
[0024] The oxygen supply stream to the process may be air but is preferably a
gas
having a higher oxygen content than air. It is preferred that the oxygen
supply stream has a
nitrogen content less than 10 mole percent, with the use of substantially pure
oxygen being
highly preferred if it is economically viable. The preferred oxygen
concentration in the oxygen
supply stream is primarily a matter of economics and may be determined by a
comparison of the
advantage of having pure oxygen to the cost of obtaining the oxygen. The basic
disadvantages
of the presence of nitrogen are the dilution of the hydrogen-containing gas
stream removed from
the product separation vessel and the fact that the nitrogen passes through
the dehydrogenation
zone thereby increasing the pressure drop through the catalyst bed and the
absolute pressure
being maintained within the dehydrogenation zone. On the other hand, the
presence of nitrogen
favorably affects the equilibrium conversion level by acting as a diluent.
[0025] The oxidation catalyst employed in the oxidative reheat or oxidation
zone to
promote the hydrogen oxidation may be any commercially suitable catalyst. The
oxidation
catalyst will have a different composition than the dehydrogenation catalyst.
Preferably, the
oxidation catalyst will have a high selectivity for the oxidation of hydrogen
with only small
amounts of the feed or product hydrocarbons being oxidized. A preferred
oxidation catalyst
comprises an IUPAC Group 7, 8, or 9 noble metal and at least one other metal
or metal cation
with both of these materials being present in small amounts on a refractory
solid support. The
preferred noble metals are platinum and palladium, but the use of ruthenium,
rhodium, osmium
and iridium is also contemplated. In an embodiment, the noble metal is present
in an amount
ranging from 0.01 to 5.0 wt% of the finished catalyst. The metal or metal
cation is preferably
chosen from IUPAC Groups I or 2 and is present in an amount ranging from 0.01
to 20 wt% of
the finished catalyst. The metal or metal cation may be selected from the
group consisting of
-8-
CA 02753127 2011-08-19
WO 2010/101571 PCT/US2009/036129
lithium, potassium, rubidium, and cesium. In an embodiment, the metal or metal
cation is
lithium or potassium. Another, optional, component of the oxidation catalyst
may be selected
from IUPAC Group 14.
[0026] In a preferred embodiment, the refractory solid support of the
oxidation catalyst
is alumina having a surface area between 1 and 300 m2/g; an apparent bulk
density between 0.2
and 1.5 g/cc; and an average pore size greater than 20 angstroms. The metal-
containing
components are preferably impregnated into solid particles of the solid
support by immersion in
an aqueous solution followed by drying and calcination at a temperature
ranging from 500 C to
1200 C in air. The support may be in the form of spheres, pellets or
extrudates. The total
amount of oxidation catalyst present within the dehydrogenation zone is
preferably less than 30
wt% of the total amount of dehydrogenation catalyst and more preferably is
between 5 and 15
wt% of this total amount of dehydrogenation catalyst.
[0027] The conditions utilized during the contacting of the reactant stream
with the bed
of oxidation catalyst will be set to a large extent by the previously
described dehydrogenation
conditions. The preferred outlet temperature of the oxidation catalyst is the
preferred inlet of the
downstream bed of dehydrogenation catalyst. The temperature rise across the
oxidation catalyst
is preferably adjusted by the amount of hydrogen conversion across the
oxidation catalyst. The
liquid hourly space velocity, based on the liquid hydrocarbon charge at 15.6
C, is preferably
between 2 and 20 hr-1.
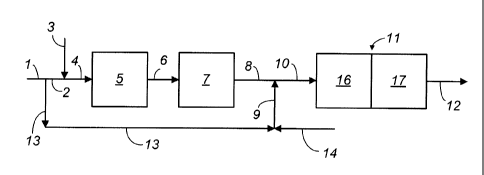
DETAILED DESCRIPTION OF THE DRAWING
[0028] In the drawing, the process of the present invention is illustrated by
means of a
simplified schematic flow diagram in which such details as pumps,
instrumentation, heat-
exchange and heat-recovery circuits, compressors and similar hardware have
been deleted as
being non-essential to an understanding of the techniques involved. The use of
such
miscellaneous equipment is well within the purview of one skilled in the art.
[0029] Referring now to the drawing, a hydrocarbon feed stream comprising a
C3+ feed
hydrocarbon, i.e. a hydrocarbon having 3 or more carbon atoms per molecule, is
introduced into
the process via line 1 and bifurcated to provide a first portion and a second
portion. The first
portion of the feed stream is carried via line 2 and is combined with steam
provided by line 3
and the resulting mixture is carried via line 4 and introduced into
dehydrogenation reaction
-9-
CA 02753127 2011-08-19
WO 2010/101571 PCT/US2009/036129
zone 5. Dehydrogenation zone 5 is operated without oxidative reheating and the
resulting
effluent is carried via line 6, heated via indirect heat exchange (not shown)
and introduced into
dehydrogenation reaction zone 7. Dehydrogenation zone 7 is operated without
oxidative
reheating and the resulting effluent is transported via line 8 and is combined
with the second
portion of the feed stream which is carried via lines 13 and 9. The resulting
effluent from
dehydrogenation zone 7 carried via line 8 is also combined with an oxygen and
steam mixture
provided via lines 14 and 9. This resulting mixture is carried via line 10 and
introduced into
dehydrogenation reaction zone 11 which is conducted with oxidative reheating.
Dehydrogenation reaction zone 11 contains an oxidation zone 16 and a
dehydrogenation
zone 17. The resulting effluent from dehydrogenation reaction zone 11
comprises the product
dehydrogenated hydrocarbon which is carried via line 12 and recovered.
-10-