Note: Descriptions are shown in the official language in which they were submitted.
CA 02755266 2011-10-17
=
INTERMEDIATES FOR PREPARING MACROCYCLIC ANALOGS
= Background
The invention relates to pharmaceutically active macrolides. Halichondrin B is
a
potent anticancer agent originally isolated from the marine sponge
Halichondria okadai,
and subsequently found in Axinella sp., Phakellia carteri, and Lissondendryx
sp..
A total synthesis of Halichondrin B was published in 1992 (Aicher, T.D. et
al.,
J. Am. (hem. Soc. 114:3162-3164), Halichondrin B has demonstrated in vitro
inhibition of tubrilin polymerization, microtubnle assembly, betas-tubtilin
crosslinking,
GTP and vinblastine binding to tubulin, and tubulin-dependent GTP hydrolysis
and has
shown in vitro' and in vivo anti-cancer properties.
Summary of the Invention
'lire invention provides halichondrin analogs having pharmaceutical activity,
such as anticancer or arttimitotic (mitosis-blocking) activity. These
compounds are
substantially smaller than halichondriir B. The invention features a compound
having
. .25 the forinula-(I):
E y , =
= =
" ' =
Z co
A c 0
=
J'
= 0
LI' X =
=
Formula (I)
1
CA 02755266 2011-10-17 '
In formula (I), A is a C,, saturated or C26 unsaturated hydrocarbon skeleton,
the skeleton being unsubstituted or having between 1 and 13 substituents,
preferably
between 1 and 10 substituents, e.g., at least one substituent selected from
cyano, halo,
azido, Q,, and oxo. Each Q, is independently selected from OR,, SR,. S02121,
OSO,R,, NR2R,, NR2(CO)R,, NR2(C0)(CO)R,, NR4(CO)NR,R1. NR2(C0)0R,,
(C0)0R,, 0(CO)R1, (CO)NR2R,, and 0(CO)NR2R1. The number of substituents can
be, for example, between 1 and 6, I and 8, 2 and 5, or 1 and 4. Throughout the
disclosure, numerical ranges are understood to be inclusive.
Each of R,, R2, R4, R,, and R6 is independently selected from H, CI, alkyl, CI-
6.
haloalkyl, C1.6 hydroxyalkyl, C1.6 aminoalkyl, C6.10 aryl, C6_10 haloaryl
(e.g., p-
fluorophenyl or p-chlorophenyl), C,,, hydroxyaryl, C,, alkoxy-C6 aryl (e.g., p-
methoxyphenyl, 3,4,5-trimethoxyphenyl, p-ethoxyphenyl, or 3,5-diethoxyphenyl),
io aryl-C,õ alkyl (e.g., benzyl or phenethyl), C,õ alkyl-C6.10 aryl, C6.10
haloaryl-C,õ
alkyl, C,, haloaryl, (C,_3 alkoXy-C6 ary1)-C,.3 alkyl, C2., heterocyclic
radical, C2.9 heterocyclic radical-C,, alkyl, C2.9 heteroaryl, and C2
heteroaryl-C,õ
alkyl. There may be more than one R,, for example, if A is substituted with
two
different alkoxy (OR,) groups such as butoxy and 2-aminoethoxy.
Examples of A include 2,3-dihydtoxypropyl, 2-hydroxyethyl, 3-hydroxy-4-
µ-
perfluorobutyl, 2,4,5-trihydroxypentyl, 3-amino-2-hydroxypropyl, 1,2-
dihydroxyethyl,.2,3-dihydroxy-4-perfluOro¨butyl, 3-cyano-2-hydroxypropyl,
2-amino-l-hydroxyethyl, 3-azido-2-hydroxypropyl,
3,3-difluoro-2,4-dihydroxybutyl, 2,4-dihydroxybutyl,
2-hydroxy-2(p-fluorophenyI)-ethyl, -CH2(C0)(substituted or unsubstituted
aryl),
-CH2(C0)(alkyl or substituted alkyl, such as haloalkyl or hydroxyalkyl) and
3,3-
difluoro-2-hydroxypent-4-enyl.
Examples of Q1 include -NH(C0)(C0)-(heterocyclic radical or heteroaryl),
-0S02-(aryl or substituted aryl), -0(CO)NH-(aryl or substituted aryl),
aminoalkyl,
hydroxyalkyl, -NH(C0)(C0)-(aryl or substituted aryl), -
NH(C0)(alkyl)(heteroaryl or
heterocyclic radical), 0(substituted or unsubstituted alkyl)(substituted or
unsubstituted
.aryl), and ¨NH(C0)(alkyl)(aryl or substituted aryl).
Each of D and D' is independently selected from R3 and OR.,, wherein R3 is H,
C1.3 alkyl, or C1.3 haloalkyl. Examples of D and D' are methoxy, methyl,
ethoxy, and
ethyl. In some embodiments, one of D and D' is H.
The value for n is 1 or preferably 0, thereby forming either a six-membered or
five-membered ring. This ring can be unsubstituted or substituted, e.g., where
E is R5
or OR5, and can be a heterocyclic radical or a cycloalkyl, e.g. where G is S,
CH,, NR6,
or preferably 0.
CA 02755266 2011-10-17
Each of 3 and J is independently H, C1.6 alkoxy. or C14 alkyl: or J and taken
together are =CH; or ¨0-(straight or branched C1.5 alkylene or alkylidene)-0-,
such as
exocyclic methylidene, isopropylidene, methylene, or ethylene. Q is Ci4 alkyl.
and is
preferably methyl. T is ethylene or ethenylene, optionally substituted with
(C0)0117,
where R7 is H or CI.6 alkyl. Each of U and U' is independently H, C1.6 alkoxy,
or C1_6
alkyl; or U and U' taken together are =CH, or ¨0-(straight or branched Ci..5
alkylene or
alkylidene)-0-. X is H or C1.6 alkoxy. Each of Y and Y' is independently H or
CI,
alkoxy; or Y and Y' taken together are =0, =CH2, or ¨0-(straight or branched
C1.5
alkylene or alkylidene)-0-. Each of Z and Z' is independently H or Ci4 alkoxy;
or Z
and Z) taken together are =0, =CH2, or ¨04straight or branched C1.5 alkylene
or =
alkylidene)-0-.
In accordance with another aspect of the invention, there is provided the
= compound of the following structure:
MK),
HO?
0 - 1-1
_ 0 =
6õ
e
ilp =
and pharmaceutically acceptable salts thereof.
In accordance with another aspect of the invention, there is provided the
compound of the following structure: =
Mea,
. ,,,, , a
-
,
.... ."Me et.
and.pharmaceutically acceptable salts thereof.
3
CA 02755266 2011-10-17
In accordance with another aspect of the invention, there is provided a
method for identifying an agent that induces a sustained mitotic block in a
cell
after transient exposure of said cell to said agent, said method comprising
the
steps of:
(a) incubating a first cell sample with a predetermined concentration of a
test
compound for a time interval between that sufficient to empty the GI
population and that
equivalent to one cell cycle;
(b) substantially separating said test compound from said first cell sample:
(c) incubating said first sample in media free of said test compound for a
time
interval sufficient to allow at least 80% of the cells released from the
mitotic block
induced by a highly reversible mitotic inhibitor to complete mitosis and
return to the G/
phase; and
(d) measuring the percentage of transiently-exposed cells from step (c) that
have
completed mitosis and returned to the GI phase.
The invention features compounds of sufficient stability to be suitable for
pharmaceutical development. The invention also features pharmaceutically
acceptable
salts of disclosed compounds, disclosed novel synthetic intermediates,
pharmac. entical
compositions containing one or more disclosed compounds, methods of making the
disclosed compounds or intermediates, and methods of using the disclosed
compounds
or compositions. Methods of use include methods for reversibly or irreversibly
inhibiting mitosis in a cell, and for inhibiting cancer or tumor growth in
vitro, in vivo,
or in a patient. The invention also features methods for identifying an anti-
mitotic or
anti-cancer agent, such as a reversible or, preferably, an irreversible agent.
The effect of compound B1939 on mitosis was assessed using a mitotic
block assay. The minimum concentration of compound B1939 required for
complete mitotic block at 0 hour is 10 nM. The minimum concentration of
compound B1939 required for complete mitotic block at 10 hour (after washout)
is also 10 nM. The reversibility ratio is therefore I for compound B1939. The
percentage of viable cells at 5 days as a function of concentration of
compound
B1939 was also examined. Viability drops to very low levels at the same
concentration as the 10 hour mitotic block.
3a
CA 02755266 2011-10-17
The effect of compound B2042 on mitosis was assessed using a mitotic
block reversibility assay. The minimum concentration of compound B2042
required for complete mitotic block at 0 hour is 3 nM. The minimum
concentration of compound B2042 required for complete mitotic block at 10 hour
is 100 nM. The reversibility ratio for compound B2042 is therefore 33. The
percentage of viable cells at 5 days as a function of concentration of
compound
B2042 was also examined. Viability drops to very low levels at the same
concentration as the 10 hour mitotic block.
Detailed Description of the Invention =
= A. Definitions
B. Halichondrin Analogs
C. Synthesis of Halichondrin Analogs
D. Pharmacological Activity
E. Uses =
A. Definitions
The following terms are defined in part below and by their usage herein.
Hydrocarbon skeletons contain carbon and hydrogen atoms and may be linear,
= branched, or cyclic. Unsaturated hydrocarbons include one, two, three or
more C-C
= double bonds (sp2) or C-C triple bonds (sp). Examples of unsaturated
hydrocarbon
radicals include ethynyl, 2-propynyl, 1-propenyl, 2-butenyl, 1,3-butadienyl,
= pentenyl, vinyl (ethenyl), allyl, and isopropenyl. Examples of bivalent
unsaturated
hydrocarbon radicals include alkenylenes and alkylidenes such as methyfidyne,
ethylidene, ethylidyne, vinylidene, and isopropylidene. In general, compounds
of the
invention have hydrocarbon skeletons ("A" in formula (I)) that are
substituted, e.g.,
with hydroxy, amino, cyano, azido, heteroaryl, aryl, and other moieties
described
herein. Hydrocarbon skeletons may have two geminal hydrogen atoms replaced
with
oxo, a bivalent carbonyl oxygen atom (=0), or a ring-forming substituent, such
as -0-
(straight or branched alkylene or alkylidene)-0- to form an acetal or ketal.
Ci.6 alkyl includes linear, branched, and cyclic hydrocarbons, such as methyl,
-ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl,
isopentyl, sec-
pentyl. neo-pentyl, tert-pentyl, cyclopentyl, hexyl, isohexyl, sec-hexyl,
cyclohexyl, 2-
methylpentyl, tert-hexyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl, 1,3-
dimethylbutyl, and
2,3-dimethyl but-2-yl. Alkoxy (-OR), alkylthio (-SR), and other alkyl-derived
moieties
4
CA 02755266 2011-10-17
(substituted, unsaturated. or bivalent) are analogous to alkyl groups (R).
Alkyl
groups, and alkyl-derived groups such as the representative alkoxy, haloalkyl,
hydroxyalkyl, alkenyl. alkylidene, and alkylene groups, can be C2.6, C3.6,
C1.3, or C.N.
Alkyls substituted with halo, hydroxy, amino, cyano, azido, and so on can have
1, 2, 3, 4,5 or more substituents, which are independently selected (may or
may not be
the same) and may or may not be on the same carbon atom. For example,
haloalkyls
are alkyl groups with at least one substituent selected from fluoro, chloro,
bromo, and
iodo. Haloalkyls may have two or more halo substituents which may or may not
be the
same halogen and may or may not be on the same carbon atom. Examples include
1 0 chloromethyl, periodomethyl, 3,3-dichloropropyl, 1,3-difluorobutyl, and
1-bromo-2-
chloropropyl.
Heterocyclic radicals and heteroaryls include fury!, pyranyl, isobenzofuranyl,
chromenyl, xanthenyl, phenoxathienyl, 2H-pyrrolyl, pyrrolyl, imidazolyl (e.g.,
1-, 2-
or 4- imidazolyl), pyrazolyl, isothiazolyl, isoxazolyl, pyridyl (e.g., 1-, 2-,
or 3-
1 5 pyridyl), pyrazinyl, pyrimidinyl, pyridazinyl, indolizinyl, isoindolyl,
3H-indolyl,
indolyl (e.g., 1-, 2-, or 3-indoly1), indazolyl, purinyl, 411-quirtolizinyl,
isoquinolyl,
_
quinolyl, phthalazinyl, naphthyridinyl, quinoxalinyl, quinazolinyl,
cinnolinyl,
pteridinyl, pyrrolinyl, pyrrolidinyl, imidazolidinyl, pyrazolislinyl,
pyrazolinyl,
piperidyl, piperazinyl, indolinyl, isoindolinyl, and morpholinyl. Heterocyclic
radicals
20 and heteroaryls may be linked to the rest of the molecule at any
position along the ring.
Heterocyclic radicals and heteroaryls can be C2.9, or smaller, such as C3.6,
C2.5, or C3.7.
Aryl groups include phenyl, benzyl, naphthyl, tolyl, mesityl, xylyl, and
cumenyl.
It is understood that "heterocyclic radical", "aryl", and "heteroaryl" include
25 those having 1,2, 3, 4, or more substituents independently selected from
lower alkyl,
lower alkoxy, amino, halo, cyano, nitro, azido, and hydroxyl. Heterocyclic
radicals,
heteroaryls, and aryls may also be bivalent substituents of hydrocarbon
skeleton "A" in
formula (I).
30 B. Halichondrin Analogs
Referring to formula (I) in the Summary section, embodiments of the invention
include compounds wherein n is 0; wherein each of D and D' is independently
selected
from R3, C, alkoxy, and C1.3 haloalkyloxy; wherein R5 is selected from H, C24
alkyl,
35 C1.6 haloalkyl, CI, hydroxyalkyl, C1.6 aminoalkyl, C6.10 aryl, C6.10
haloaryl, C6.10
hydroxyaryl, C1.3 alkoxy-C6 aryl, C6.10 aryl-C1.6 alkyl, CI., alkyl-C640 aryl,
C6.10
haloaryl-C,õ alkyl. CI, alkyl-C6.10 haloaryl, (C1.3 alkoxy-C6 aryl)-C1.3
alkyl, C2.9
heterocyclic radical, C2,Q heterocyclic radical-C16 alkyl, C,, heteroaryl, and
C2.9
heteroaryl-C,.6 alkyl: and combinations thereof.
5
-
CA 02755266 2011-10-17
Other embodiments include compounds having one or more of the following
characteristics: (a) wherein A is a C1.6 saturated or C2.6 unsaturated
hydrocarbon
skeleton. the skeleton having at least one substituent selected from cyano,
halo. azido,
Q,, and oxo;_(l)) each Q, is independently selected from OR,, SR,, SO2R7,
OSO,R,,
NR2Ri, NR2(CO)RI, and 0(CO)NR2R1; (c) Z and Z' taken together are
=0 or =CH.,; (d) wherein each Q, is independently selected from ORI, SRI,
SO2R1,
OSO2R1, 11H(CO)R1, NH(C0)(CO)R1, and 0(CO)NHRI; (e) each R, is independently
selected from CI, alkyl, CI., haloalkyl, C, aryl, C6 haloaryl, C1.3 alkoxy-C6
aryl, C6
aryl-C1_3 alkyl, alkyl-C, aryl, C6 haloaryl-Cw alkyl, C1.3 alkyl-C6
haloaryl, (C1.3
1 0 alkoxy-C6 aryl)-C1.3 alkyl, C2.9 heterocyclic radical, C2.9 heteroaryl,
and C2.9 heteroaryl-
C,õ alkyl; (f) one of D and D' is methyl or methoxy and the other is H; (g) n
is 0; (h)G
is 0; (i) J and J' taken together are =CH,; (j) Q is methyl; (k) T is
ethylene; (I) U and U'
taken together are =CH, ; (m) X is H; (n) each of Y and Y' is H; and (o) Z and
Z' taken
together are =0. Examples of combinations are the combination of (h)-(m), the
1 5 combination of (a) and (b), the combination of (f) and (h), and the
combination of (h)
and where one of D and D' is methyl and the other is H. Two particularly
preferred
compounds are B1793 and B1939.
Another embodiment includes compounds wherein Q1 is independently selected
from ORI, SR,, SO2R1, and 0S02R1; and each RI is independently selected from
C1.6
20 alkyl, C1., haloalkyl, C6 aryl, C6haloaryl, C1.3 alkoxy-C6 aryl, C6 aryl-
C1.3 alkyl, C1.3
alkyl-C, aryl, C6haloaryl-C1.3alkyl, C1.3alkyl-C6haloaryl, and (C1.3 alkoxy-C6
aryl)-C1.
3 alkyl. Other embodiments include compounds wherein: one of D and D' is alkyl
or
alkoxy, where 'n is 1; (f) as above, where n is 1; E is alkoxy, where n is 1;
n is 0,
where one of D and D' is hydroxy and the other is H; and (f) as above, where n
is 1 and
25 . E is methyl.
The invention also features compounds wherein: (1) A has at least one
substituent selected from hydroxyl, amino, azido, halo, and oxo; (2) A is a
saturated
hydrocarbon skeleton having at least one substituent selected from hydroxyl,
amino,
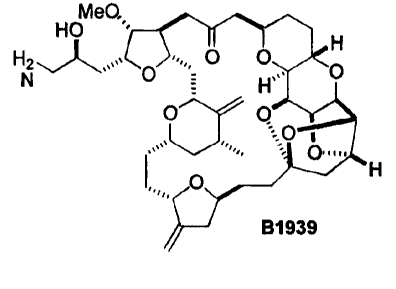
and azido (e.g.., B1793, B1939, B2042, B1794, and B1922); (3) A has at least
two
B2136); (4) A has at least two substituents independently selected from
hydroxyl and
amino (e.g., B2042 and B2090); (5) A has at least one hydroxyl substituent and
at least
one amino substituent (e.g., B1939 and B2136); (6) A has at least two hydroxyl
substituents (e.g., B1793 and B1794); (7) A is a C.2.4 hydrocarbon skeleton
that is
a C3 hydrocarbon skeleton that is substituted (e.g., B1793, B1920, B1984,
B1988,
B1939, B1940, B2014); (9) A has an (S)-hydroxyl alpha to the carbon atom
linking A
to the ring containing G (e.g., B1793, B1939 or BI920) or an (R)-hydroxyl
(e.g.
B2102, B2013, B2042); and (10) A is a Cf., saturated hydrocarbon skeleton
having at
6
=
CA 02755266 2011-10-17
least one substituent selected from hydroxyl and cyano (e.g.. B2013. B2037,
B2102,
B2086, and B2091). By (S)-hydroxyl is meant the configuration of the carbon
atom
having the hydroxyl group is (S). Embodiments of the invention also include
compounds which have at least two substituents on the carbon atoms (1) alpha
and
gamma, (2) beta and gamma, or preferably (3) alpha and beta to the carbon atom
linking
A to the ring containing G. The alpha, beta, and gamma carbon atoms can have
an (R)
or (S) configuration
The invention further provides preferred compounds having the formula (1) -A,
shown below, wherein the substituents are identical to those defined above.
D'
, Q
J 0
s=-k/'
'Q
U' X
Formula 1-A
The invention further features the following monosaccharide intermediate
having
formula (II):
OP3
OP2
OP1
0
Formula (H)
wherein R is methyl or methoxy, and each of PI, P2, and P3 is independently
selected
from H and primary alcohol protecting groups. Preferably, the diol sidechain
is below
= the plane of the page and 0P2 is above the plane of the page. Primary
alcohol
protecting groups include esters, ethers, silyl ethers, alkyl ethers. and
alkoxyalkyl
ethers.
7
CA 02755266 2011-10-17
Examples of esters include formates, acetates, carbonates, and sulfonates.
Specific examples include formate, benzoyl formate. chloroacetate,
trifluoroacetate,
methoxyacetate, triphenylmethoxyacetate, p-chlorophenoxyacetate, 3-
phenylpropionate, 4-oxopentanoate, 4,4-(ethylenedithio)pentanoate, pivaloate,
crotonate, 4-methoxy- crotonate, benzoate, p-phenylbenzoate,
2,4,64rimethylbenzoate,
carbonates such as methyl, 9-fluorenylmethyl, ethyl, 2,2,2-trichloroethyl, 2-
(trimethylsilyl)ethyl, 2-(phenylsulfonyl)ethyl, vinyl, allyl, and p-
nitrobenzyl.
Examples of sib?' ethers include trimethylsilyl, triethylsilyl, t-
butyldimethylsilyl,
t-butyldiphenylsilyl, triisopropylsilyl, and other trialkylsilyl ethers. Alkyl
ethers include
methyl, benzyl, p-methoxybenzyl, 3,4-dimethoxybenzyl, trityl, t-butyl, allyl,
and
allyloxycarbonyl ethers or derivatives. Alkoxyalkyl ethers include acetals
such as
methoxymethyl, methylthiomethyl, (2-methoxyethoxy)methyl, benzyloxymethyl,
beta-
(trimethylsilypethoxymethyl, and tetrahydropyranyl ethers., Examples of benzyl
ethers
include p-methoxybenzyl (MPM), 3,4-dimethoxyberizyl, o-nitrobenzyl, p-
nitrobenzyl,
p-halobenzyl, 2,6-dichlorobenzyl, p-cyanobenzyl, 2- and 4-picolyl. Preferably,
each of
PI and P2 are TBS and P3 is MPM (see alcohol 19 below). In one aspect, formula
(U) can be modified so the hydroxyethyl sidechain can also be a protected
hydroxyl, -
CH2CH,O-P4, wherein P4 is independently selected from values for Pl. A related
intermediate is alcohol 17, where the hydroxyethyl sidechain is a
hydroxymethyl
sidechain. A corresponding hydroxypropyl sidechain, or arninoalkyl side chain,
can be
similarly prepared.
PI and P2, taken together, can be a diol protecting group, such as cyclic
acetals
and ketals (methylene, ethylidene, benzylidenes, isopropylidene,
cyclohexylidene, and
cyclopentylidene), silylene derivatives such as di-t-butylsilylene and 1,1,3,3-
tetra-
isopropyldisiloxanylidene derivatives, cyclic carbonates, and cyclic
boronates.
Methods of adding and removing such hydroxyl protecting groups, and additional
protecting groups, are well-known in the art and available, for example, in
P.J.
Kocienski, Protecting Groups, Thieme, 1994, and in T.W. Greene and P.G.M.
Wuts,
Protective Groups in Organic Synthesis, 2" edition, John Wiley & Sons, 1992.
The following section provides representative syntheses of intermediates
of formula (II) and halichondrin analogs of formula (I).
C. Synthesis of Halichondrin Analogs
An overview is provided below, followed by synthetic schemes 1-16, and
several detailed protocols.
Compounds of general formula 4 can be prepared by the route outlined in
Scheme I. Key fragment F-2 exemplified by vinyl iodide compound X2 can be
prepared according to the procedure of Kishi, et al (Total synthesis of
halichondrin B
8
CA 02755266 2011-10-17
and norhalichondrin B. Aicher. T. D.: Buszek, K. R.; Fang, F. G.: Forsyth. C.
J.;
Jung. S. H.: Kishi. Y.; Matelich. M. C.,fgcola, P.M.; Spero, D. M.: Yoon. S.
K. J.
AM. Chem: Soc. 1992. 114. 3162-4).
MrsjT
"'"p0Pv
Vinyl Iodide X2
1v1e02 H-
O -
His'
OTBS
TBSO
TBSO
XF3
Key fragment F-3 can be obtained by DMALH reduction of the corresponding
methyl
ester, XF3, prepared according to the procedure of Stamos, et al (Scheme 2).
[Synthetic studies on halichondrins: a practical synthesis of the a1-C.13
segment.
Stamos, D. P.; Kishi, Y. Tetrahedron Lett. 1996, 37, 8643-8646]. Synthesis of
key
fragment F-1 exemplified by compound 20 can be synthesized as described in
Scheme
3 or Scheme 4.
Using B1793 as a representative example, coupling of the three key fragments
proceeded as outlined in Scheme 5: Nozaki-Hiyama-Kishi coupling of fragments
20 and
X2 followed by intramolecular Williamson ether formation furnished
tetrahydropyran
B2318. Protecting group modification as described in Scheme 5 or alternatively
in
Scheme 6 afforded primary iodide B2313. Halogen-metal exchange reaction and
coupling with key fragment F-3 furnished a mixture of diastereomeric alcohols
B2308. Additional protecting group manipulation and oxidation followed by an
intramolecular Nozaki-Hiyama-Kishi reaction afforded an intermediate, which
when
oxidized and treated with TBAF underwent intramolecular hetero-Michael ring
closure.
PPTs mediated acetal formation furnished B1793.
Aryl groups can be incorporated into the C32 sidechain (e.g. B2043) as
exemplified in Scheme 7. Intermediate B2318 was deprotected and the resulting
diol
9
CA 02755266 2011-10-17 '
oxidati vely cleaved to the corresponding aldehyde. Treatment with a Grignard
reagent
(e.g. p-F-PhMgBr). separation of the resulting diastereomers and silylation
furnished
204, which Was converted to final product in a manner similar to that
described in
Scheme 6.
Ether analogs can be prepared from B1793 by treatment with an appropriate
alkylating agent (e.g. Scheme 8). Similarly, sulfonates, esters. carbamates,
etc. can be
prepared from B1793 by treatment with an activated carbonyl component.
Oxidative
diol cleavage and reduction or selective hydroxyl group oxidation could
furnish
1 0 derivatives such as B2037 and B1934, respectively.
Alternatively, one or more hydroxyl groups could be converted to the
corresponding amino groups with subsequent coupling with an activated carbonyl
component (Scheme 9). Displacement of the sulfonyl intermediate (e.g. B1920)
by
1 5 carbon or heteroatom nucleophiles could also be readily accomplished
(Scheme 10).
C31 methyl analogs cart be prepared as outlined in Scheme 11. Indium
mediated coupling of an allyl bromide ester with 2,3-0-(3-isopropylidine)-D-
glyceraldehyde furnished lactone 103. Hetero Michael addition, lactone
reduction,
20 Wittig coupling and intramolecular Michael addition furnished
tetrahydrofuran 107.
Pummerer rearrangement, protecting group adjustment and DIBALH reduction
furnished key fragment F-1 (e.g., 114), which was converted to final compound
in a
manner analogous to that described in Scheme 6.
=
25 Fluorine atoms could be introduced as described in Schemes 12 - 14.
Beginning with the appropriate tetrahydrofuran intermediate, fluorinated key
fragment F-1
was obtained and carried to final compound in a manner analogous to that
illustrated in
Scheme 6.
30 Triol derivatives could be similarly.prepared from the
tetrahydrofuran
intermediate. For example, as outlined in Scheme 15 allyltributylstannane
addition to
aldehyde X32 furnished homoallylic alcoho1:33a that was carried to final
compound in a
similar manner to that described in Scheme 6. These triols could be further
modified as
exemplified in Scheme 6.
The 1,3 diol derivatives could be prepared from intermediates previously
described. For example, B2086 could be oxidatively cleaved and reduced to
afford
1,3-diol B2091 (Scheme 16).
CA 02755266 2011-10-17 '
Scheme 1
F-1 __________________________________________ 4
F-2 ___________________________ F-3 __
Ms* J' OHC0
= MPM 0
=
= =TBS
OPv TBSO
A CHO TBSO
U X
F-1 F-2 F-3
Y y'
D'
Z' =
A G 0
= J'
=
= =
=
. X
4, Formula (I)
II
CA 02755266 2011-10-17
Scheme 2
Me02C OHC
=
0 D1BALH
o. =
s. H"
TBSO ".H OTBS TBSO OTBS
TBSOTBSO
1
1
XF-3 F-3
12
CA 02755266 2011-10-17
Scheme 3
1) allyITMS,
V-1 Fl 1) TBDPSCI , Ac0 /0Ac BP3=Et20
HO
CHO 2) Ac20 OTBDPS 2) K2CO3
OH Ac 0 '
L-arabinose 2
HQ, /OH Me0,, H
1) separate isomers 1) PvCI
TBDPS '
./ 2) TBSCI .-i'--"'. 4."---nEl 2) BnBr
3) Mel
4a,4b 7
4) HCI
1) H2
2) TPAP
M ea fBn 1) AD MeQ,c_Ein 3) Tebbe
la,
__________________________ . ____________________________________ .
TBS ,,,, ,.õ,,,OPv
2) separate isomers 4) 9-BBN
3) TBSCI 5) Swem
9 11
6) Et3N
7) NaBH4
1) MPMOTCI
2) LAH
BSopMegc (¨OH 3) Swern Meg OMPM
TBSO,..õ...KõT _________________ ),õ,,,,,DPv .
4) Wittig TBSOA,õ..,õCHO
5) 9-BBN
15 20
6) Swem
13
CA 02755266 2011-10-17 .
Scheme 4
1) EtSH, ZnCl2 1) allyITMS,
OH OH H 2) TIPSCI Acq, spAc BF3=Et20
_
HO
CHOõ. ,,,TIPS
3) 12 2) K2CO3
OH 4) Ac20 Ac0
L-arabinose XX1 4
i.-10H 1) separate isomers Me0,, H 1) PvCI
_____________________________ . _____________________________ '
2) MPMOTCI
TIPS
---- 0 2) TBSCI ---- 0
3) Mel
XX15 XX16
4) TBAF
1) LAH
2) Swem
Mea.õ MPM 1) AD
,,,,,........41/3
,=,' _____________________ .
2) separate isomers Meg
TBSQ '
TBS : Bn 3) Wittig
--A)Pv 4) 9-BBN
3) TBSOTf 5) PvCI
XX17 XX18 6) DDO
1) Swem
2) Wittig
MeS H
TBSO 3) 9-BBN TBSO Meg OMPM
' -.
TBS
4) Swem TBS : .,õ,..CHO
5) Et3N
XX19 6) Nal3H4 XX20
7) MPMOTCI
8) LAH
9) Dess-Martin
14
-
CA 02755266 2011-10-17 ,
Scheme 5
Meg_cfC)MPM
MeOtfOMPM
TBS IBS
TBS ,,,õ =.õ,.,,CHO
TBS
20 1) NiC12/CrCl2
Mrsy, if 2) KHMOS
r.
Me
Me I io
...p.,......õ,
OPv
OPv
X2 132318 =
Me07---I
Ta
o
1) OM
2) separate isomers TBS
3) TsCI " t-
BuLL F3
4) LAH
5) MMTrCI
6) Nal ""; sT"'OMMTr
62313
MeQ
TBSO )
H 1) PPTs
2) Doss-Martin
3) NiC12/Cra2
0TBS ______________________________________________________________
.....õ.
Cli T
B1793
BSO
4) Dess-Martin
TBSO
6) PPTs
I
132307, B2308
_
_
CA 02755266 2011-10-17
Scheme 6
meg MPM
TBSO - Me MPM
TBSO -c
TBS ..,õCHO
0 TBS '
XX20 1) NiC12/CrCl2
Mrsi:X 2) KHMDS
.µ"Me
OPv
X2 BX2318
Meg 1
1) LAH TBSO '.
2) MMTrCI TBS ' õ,..
3) DDO 0 =
: t-Bulj, F3
:
4) MsCI 0:a-
5) separate isomers I Me
6) Nal .õ,5i0OMMTr
BX2313
TBSOMeS 1) PPTs
TBS= ' H 2) Dess-Martin
= ',... OH 0
H".. 3) NIC12/CrCl2
IPTBS B1794
TBSO
4) Doss-Martin
,atne TBSO `-,
I6) 5) TBAF PPTs
OMMTr I
BX2307, BX2308
16
CA 02755266 2011-10-17
Scheme 7
Meg. --OMPM Megd¨OMPM
T: = '
TBS 10M .c.1 õ,, 1) TBAF
2) Nal04
0 I
F
3) p-F-PhMgBr
4) separate isomers
i Me
5) TBSOTf
50.7õ.. I ....
132318 204
17
_
CA 02755266 2011-10-17
Scheme 8
Me0,
p-F-PhCH2Br Si =
0
Air
9 = .
111 B2014
Me0,
so Nyi)
PhNCO 0 -
Wier0
9
81793 B1984
Me0,,
1) Na104 HO" 0 0
H
2) NaBH4 C. = .
0õ
I.
B2037
Me0,
1) TBDPSCI 0 o
=
2) Dess-Martin,, 6.,
3) TBAF I Me
B1934
18
CA 02755266 2011-10-17
Scheme 9
Meg
H
c...,. = ,,,,, = , .
R = "
TsCI or MsCI = . H"'. = NaN3
:
B1793 ______
Q 0
B1920: R = Ts ,...(a:vie ' 6,
B2294: R = Ms 1,,,,, 0 H
Me , MeCt,
HO ' HO '
= .H H2N.J.,,,,..= .., 1 = H
ri H"' = Me3P . H"'.
= ! s
....:L)m
=== . 6, 6
Iõõ H Iõ,, o e I 10CMe ,
, = H
B1922 B1939
Me0,
= Iii H9 'in
PhCOCO2H 0 1 H".
a . = .
EDC...
===== ,,,,= : a
1 .
=
B1930
19
CA 02755266 2011-10-17
Scheme 10
Me()
HO
' 0
KCN
9 0
B2013
61920 ___
NSH
Me0
F.(
S..jõ,õ.=
0 =
HS' =
Q
0õ
L. 0
B2019
CA 02755266 2011-10-17 '
Scheme 11
---/-0 In Me
= 1) HSPh
Me.õ (¨SPh
0)
CHO 0===".-c-0
----(02Me _8 2) DIBALH
0
101 Br 106
102 103
Ac
1) Ph3P=CHCO2Et Me..4.0 r-SPh M a,(0. t'h
1) mCPBA
6
_______ _..,õ-0O2Et 02Et
2) DBU 2) Ac20
107 109
1) K2CO3 Me,c3 /---OH
1) MPMOTCI MeOMPM
_____ .
TBSO'-s"-_='''
2) NaB1-14 .- 2) Ts0H
3) TBSOTf TBS6
4) DIBALH
110 114
21
CA 02755266 2011-10-17 ,
-
Scheme 12
1) 9-88N 1) LAH
m ec)...dM PM MeVM PM
2) Swem 2) SWem
3) CF3TMS
TBSOy-N,..õ,µ
3) Wig
4) TBSOTf CF3 4) 9-BBN
302 306 5) PvCI
M eQ.,_1MPM Me0,r4 1) 9-BBN
1) DDQ 2) Sworn
TBSO.,,,-....,...,(0)",,..-opv 2) SwornTBSay.õ.,
0Pv 3) Et3N
0
CF3
3) CF3
.Tebbe 4) NaBH4
311 314
MeCker....01-i 1) MPMOTCI meg --OM PM
TBSOT-F-3,,, EtS ..õØ.õCHO
2) LAH T 0
3) [01 Ci-g¨:.-.
316 319
=
22 .
CA 02755266 2011-10-17
Scheme 13
M e 10,.... 0 H 1) MMTrCI MegIMPM 1) HCI
2) NaH, MPMCI.,' '', '''' (0),õ,..,OMMTr
2) PvCI
3) AD
7 X399
1) Natal
M eCtdMPM 2) CH2=CHCF2Br Me0dMPM 1) TBSOTt
In H9 õ 2) LAH
=.õ,.,,OPv .
0 3) 0s04
3) Sworn
X400 4) Na104 F F
402 4) Wittig
5) NaBEI4
1) 9-BBN
meg__ MPM
lBA,S,..Z..õ 2) PvCI TBS MeQ-(01
3) H2
F F 4) Swern F F
406 5) Tebbe 409
1) 9-BBN MeCti4-0H 1) MPMOTCI
2) Swem TBS 2) LAH
TBSO ="'Y'."'"--.0Pv
3) Et3N 3) separate isomers
4) NaBH4 F F 411 4) Doss-Martin
meg, MPM
TBS
F F
X412
23
CA 02755266 2011-10-17
Scheme 14
MeO OBn 1) [0] Me().)Bri
HO ' ______ 2) CF3TMS TBS 1) Swam
0 ' 3) TBSOTf F3 '' 0 - 3) Wittig
4) LAH TBSO 4) 9-BBN
10a EX11
MeRjBn 1) PVC(
TBS 2) H2, Pd(OH)2
ic-7 i----
F3
J) DeSS-Martin F3 "*.sV....*=-=''OPv
TBSO 4) Tebbe - TBSO
EX12 5) 9-BBN EX13
1) Dess-Martin Meg¨OH 1) MPMOTCI meg, MPM
-c7S.,
F3 TBS=''''Y.'"=OPv F3 .õ.= '''' .,CHO
2) Et3N 2) LAH
3) NaBlia TBS 3) [0] TBSO
EX14 EX15
24
CA 02755266 2011-10-17
Scheme 15
1) Na104
Meg OBn 2) NaBH4 Meg. H 1) TPAP
3) TBSCI TBSO""' 2) Tebbe
0 4) H2 3) 9-BBN
10a,10b 22
1) MPMOTCI
meg. ses¨OH 1) Swem Mec).--0H 2) LAH
2) Et3N TBSO--="..Y.'"--. Pv
3) Swem
3) NaBH4 4) Wittig
25 26 5) 9-BBN
MeC),./4--OMPM 1) PvCI MeV¨OMPM
TBSO--"" ''''' C00H 2) TBAF
3) Dess-Marlin
30 X32
Meg MPM
1).,.õ,....SnBu3
H9 = 1) TBS011
2) separate isomers '''' .""===---"OPv 2) AD
33a 3) separate isomers
1) TBSOTt
Meg_ MPM 2) LAH MeC)iOMPM
111 TBS TBSy
3) Dess-Martin TBS
35 a, majOr, upper C36-lsomer 38
35b, minor, lower C36-isomer
25
CA 02755266 2011-10-17
Scheme 16
Meg
H Hy
HO - ,..= 0 0
0
Fr. = = Na104
9 = A
1µ
82086
Meg
HO "
OHC,õ).õ.õ.= =,, 0 =
0 "!
NaB1-14
0=,
i=s".1%Ae
I= =
82068
Meg,
0 0
0 'F.
Fr
µ=.'s "'me
B2091
26
CA 02755266 2011-10-17
EXPERIMENTAL SECTION
Synthesis of Key Fragment F-3:
Me02C OHC
=
,0 01BALH
s, .
.511 FrõH
= SIBS *TBS.
TBS = TBS=
TBSO IBS
X F - 3 F-3
Key Fragment F-3. DIBALH (1 M in toluene, 3.86 mL) was added to a
solution of XF,3 (1,46 g, 1.93 mmol) in toluene (37 nth) at -78 C. After
stirring for
10 min, the reaction was quenched by careful addition of Me0H (0.46 mL) and
1120
= (0.21 mL), warmed to rt and stirred for 15 min. The white suspension was
filtered
thrcitugh CeliteTM with 1:1 CH2C12/Et20. The filtrate was concentrated and
purified by
column chromatography (IQ% Et0Ac-hexanes) to give key fragment F-3 (1.34 g,
96%) as an oil.
Synthesis of B1793:
20:
H9 9H TBDPSCI HQ frH
uO,cHO
HO
õ...OTEIDPS
0 =
L-arabinose 1
.. Trio! 1 A solution of TBDPSC1 (444 mL, 1.7 mol) in DMF (0.5 L) was
added in three portions to a suspension of L-arabinose (250.0 g, 1.66 mol),
imidazole
(231.4 g, 3.40 mol) and DMF (2.5 L). The addition of each portion took 1.5 h
with a '
min and a 15 h interval separating the second and third portions,
respectiVely. The
.resulting solution was stirred for 3 h, concentrated and purified by flash
30 chromatography (5% to 33% Et0Ac-he29.nes) to provide trio! 1(394 g,
61%).
27
CA 02755266 2011-10-17 .
HQ.. ,DH Acq, 10Ac
Ac20, pyridine
HO 0
).õ Ac0 0
TBDPS TBDPS
1 2
Triacetate 2 Acetic anhydride (6.06 mol) was added over 1.5 h to trio! 1
(1.01 mol) in pyridine (1.0 L) at 15 C. The solution was stirred for 1 h,
concentrated
and purified by flash chromatography (15% to 25% Et0Ac-hexanes) to afford
triacetate
2 (518 g, 97%).
Ac0 ,OAc BF3=Et20 Ac0.,4Ac
Ac0
0TBDPS
2 3
Diacetates 3 Allyltrimethylsilane (1.11 mol) followed by BF3.0Et, (1.11
mmol) was added over 1.5 h to triacetate 2 (164 g, 0.32 mol) in toluene (1.5
L) at 0 C.
The orange solution was stirred for 1 h at 0 C and for 2 h at rt. The mixture
was
slowly poured into saturated aqueous NaHCO3 (1.7 L) at 0 C and stirred for 30
min.
The separated aqueous layer was extracted with Et0Ac (3 x 600 rnL) and the
combined
organic layers were dried over Na,SO4, concentrated and purified by flash
chromatography (5% to 10% Et0Ac-hexanes) to furnish a mixture of diacetates 3
(108
g, 69%).
Acqe), Ac
./
OTBDPS K2CO3
________________________________ - HO.õ..r_..r
Me0H .-7.----'"ssY'''.--
-eTBDPS 1- ,...,V,. ,..H.,A. OTBDPS
3 4a 4b
Diol 4a Solid K2CO3 (72 mmol) was added to diacetates 3 (108 g, 218 mmol)
in Me0H (0.5 L) at rt. The suspension was stirred for 2.5 h and then
concentrated.
The orange residue was suspended in saturated aqueous NH4CI (150 mL),
extracted
with Et0Ac (3 x 150 mL) and the combined organic layers were dried over
Na2SO4,
concentrated and purified by flash chromatography (15% to 50% Et0Ac-hexanes)
to
afford alpha-isomer 4a (33.86 g, 37%), and beta-isomer 4b (58 g, 63%).
28
_
CA 02755266 2011-10-17
_,pH HO.õ. jTBS
TBSCI
4a 5
Alcohol 5 Imidazole (16.75 g, 246 mmol) and TBSC1 (16.08 g, 107 mmol)
were added to a solution of diol 4a (33.86 g, 82 mmol) in CH2C12 (250 mL) at 0
C.
After 18 h at 0 C and 5 h at it. the reaction mixture was diluted with
saturated aqueous
NaHCO3 (250 mL), stirred for 30 min and the layers were allowed to separate.
The
aqueous layer was extracted with Et0Ac (3 x 250 mL) and the combined organic
layers
were dried over Na.,SO4, concentrated and purified by flash chromatography (2%
to
50% Et0Ac-hexanes) to furnish alcohol 5 (36.0 g, 83%).
HO TBS Meg, il)TBS
Mel, NaH
5 6
Methyl ether 6 Iodomethane (16.5 mL, 265 mmol) and NaH (60% in
mineral oil, 5.28 g, 132 mmol) were added to a solution of alcohol 5(34.93 g,
66
mmol), THF (320 .mL) and D1V1F (80 mL) at 0 C. After 19 h at 0 C, the
reaction was
quenched with saturated aqueous N1140 and saturated aqueous Na,S203. The
resulting
mixture was stirred for 20 min and the layers were allowed to separate. The
aqueous
phase was extracted with Et0Ac (3 x 200 mL) and the combined organic layers
were
dried over Na,SO4, concentrated and purified by flash chromatography (3% Et0Ac-
hexanes) to afford methyl ether 6 (34.23 g, 96%).
MeQpTBS M eV)H
HCI
..... ).õ,õ,OTBDPS .....
0
6 7
Diol 7 HC1 (37% aqueous solution, 12.75 nth, 153 mmol) was added to a
solution of methyl ether 6 (32.93 g, 61 mmol) in Me0H (110 mL) at rt. After 17
h,
NaHCO3 (17 g) was added to the reaction mixture. The mixture was stirred for
30 min,
concentrated, suspended in Et0Ac and filtered. The filtrate was concentrated
and
29
CA 02755266 2011-10-17 '
purified by flash chromatography (50% Et0Ac-hexanes to Et0Ac) to give diol 7
(10.0
g, 87%).
M eCk , )0H Meq, )DH
. PvCI
7 8
Alcohol 8 A solution of pivaloyl chloride (8.4 mL, 67 mmol) in pyridine (50
mL) was added over 1.5 h to a solution of diol 7(12.24 g, 65 rnmol) in
pyridine (100
mL) at 0 C. After 1 h at 0 C and 18 h at rt, the mixture was diluted with
saturated
aqueous NH,CI and extracted with Et0Ac (3 x 800 mL). The combined organic
layers
were dried over Na2SO4, concentrated and purified by flash chromatography (50%
Et0Ac-hexanes) to furnish alcohol 8 (16.9 g, 96%).
Megõr_fi BnBr Meg, Bn
"µµsc)''."2C:IPv 0 ====.C)Pv
8 9
Olefin 9 Benzyl bromide (62 mL, 521 mmol) and Bu4NHS0, (10.6 g, 31
mmol) were added to a solution of alcohol 8 (16.9 g, 62 mmol) in CH,C12 (100
mL) at
0 C. A solution of NaOH (9.95 g, 248 mmol) in 1-120 (10 mL) was added to the
reaction mixture over 15 min. After 30 min at 0 C and 18 h at rt,-the
reaction mixture
was diluted with saturated aqueous NH,CI and extracted with CH,CI, (3 x 100
mL).
The combined organic layers were dried over Na,SO4, concentrated and purified
by
flash chromatography (hexanes to 30% Et0Ac-hexanes) to afford olefin 9 (22.1
g,
98%).
Megõ jiBn MeQ OBn
AD
0
9 10a
Diol 10a. 0s04 (0.1 M solution in toluene, 7.3 mL, 0.73 mmol) and a solution
of olefin 9 (24.9 g. 69 mmol) in t-BuOH (165 mL) were added to a solution of
K2CO3
CA 02755266 2011-10-17 '
(31.2 g, 161 mmol), K.,Fe(CN), (74.4 g. 161 mmol), (DHQ),PYR (1.33 g, 1.50
mmol), H20 (500 mL) and t-BuOH (330 mL) at 0 C. After 3 h at 0 'C. Na,S205 =
5
H20 (37.3 g 150 mmol) was added. The reaction mixture was warmed to rt,
stirred
for 1 h and extracted with Et0Ac (3 x 300 mL). The combined organic layers
were
dried over Na,SO4, concentrated and purified by flash chromatography (5%
isopropanol- CH2Cl2) to provide didl 10a (17.98 g, 75%).
MeQ OBn M eQ..gBn
HO ) TBSCI TBS
TBS 0 ..."Clipv
10a =11
Silyl ether 11 Imidazole (21 g, 308 mmol) and TBSC1 (26.5 g, 176 mmol)
were added to a solution of diol 10a (17.4 g, 44 nunol) in DMF (90 mL) at rt.
After 18
h, the reaction mixture was dilutedwith saturated aqueous NaHCO (250 mL),
stirred
for 1 h and extracted with CH,C12(3 x 100 rnL). The combined organic layers
were
dried over Na2SO4, concentrated and purified by flash chromatography (5% Et0Ac-
hexanes) to afford silyl ether 11(25.7 g, 94%).
Megen M eggH
2, F1)2
TBS9 TBS9 -
TBS0........A,µõ.=g 0 ..õ,.,DPv H Pd (0 0
OPV
1 1 1 2
Alcohol 12 A mixture of silyl ether 11(21.2 g, 33.8 mmol), Pd(OH)2 (20%,
4.7 g, 33.8 mmol) and Et0Ac (200 mL) was stirred at rt under 1 atm 112 for 3
h. The
mixture was filtered through Celite, concentrated and purified by flash
chromatography
(10% to 20% Et0Ac-hexanes) to afford alcohol 12 (17.4 g. 96%).
MeggH 1) TPAP MeQ
TBS ' TBS
TBS TBS
2) Tebbe 0
1 2 1 3
Olefin 13 4-Methylmorpholine N-oxide (7.66 g. 65 mmol) and TPAP (1.15
g. 3.26 mmol) was added in four portions over 20 min to a solution of alcohol
12
31
CA 02755266 2011-10-17
(17.4 g. 32.5 mmol) in CH,C12 (145 mL) at 0 C. After 20 min, the reaction
mixture
was diluted with Et20 (50 mL) and saturated aqueous Na2S205 (50 mL) and
filtered
TM
through Celite. The organic layer was separated. washed sequentially with
saturated
aqueous CuSO4-brine (1:1) and brine, dried over Na:.SO4. filtered through
Celiterm, and
concentrated to afford the desired crude ketone.
Tebbe reagent was prepared by stirring bis(cyclopentadienyl)titanium (11.36g.
45.6 mmol) and Me3A1 (2.0 M in toluene, 45.6 mL, 91.2 mmol) for 4 days at rt.
This
material was cooled to -25 C and a solution of crude ketone in THF (150 mL)
was =
added. The reaction mixture was warmed to 0 C, stirred for 30 min, quenched
by slow
addition of 0.1 N NaOH (3.5 mL), and then stirred for an additional 20 min at
n. The
mixture was diluted with Et,O, filtered through Centel-1nd concentrated. The
residue
was dissolved in CH2C12, filtered through basic A1203, concentrated and
purified by
flash chromatography (5% Et0Ac-hexanes) to give olefin 13 (12.8 g, 74% for two
steps).
MeQ 9-BBN MeQ
TBS9 T
TBS BS 0õ,, .õõOPv
1 3 1 4
=
Alcohol 14 9-BBN (0.5 M in THF, 165 mL, 83 mmol) was added to a
solution of olefin 13 (12.78 g, 24 mmol) in TIM (280 tn.1.) at 0 C. After
stirring for 5
h at rt, the reaction mixture was recooled to 0 C at which time H20 (200 mL),
THF
(100 mL) and NaB03=4 1120 (75 g) were added. The mixture was warmed to rt,
stirred
for 16 h and then concentrated. The aqueous residue was extracted with Et0Ac
(4 x
.300 mL) and the combined organic layers were dried over Na2SO4. Concentration
and
purification by flash chromatography (20% to 35% Et0Ac-hexanes) afforded
alcohol
14 (12.05 g, 91%).
MeQ 1) Swern MeQcfoy
TBS0S9
TB TBS.
÷ 0 2) Et3N TBS.....
OPV
3) NaBH4
14 15
Alcohol 15 DMS0 (9 mL, 127 mmol) was added to a solution of oxalyi
chloride (5.6 mL, 64 mmol) in CH2C12 (350 mL) at -78 C. After stirring for 15
min, a
32
CA 02755266 2011-10-17 '
solution of alcohol 14(11.7 g, 0.021 mmol) in CH2Cl2 (50 mL) was added and
stirring
was continued for 1 h, after which Et3N (26.7 mL. 192 mmol) was added. The
reaction mixture was warmed to 0 C, stirred for 15 min, diluted with
saturated aqueous
NH,CI, and extracted with CH2C12 (3 x 200 mL). The combined organic layers
were
dried over Na2S0, and concentrated to furnish the desired crude aldehyde.
This material was dissolved in CH,C12 (200 mL) and treated with Et,N (20 mL)
at rt. After stirring overnight, the reaction mixture was diluted with
saturated aqueous
NH,CI and extracted with CH2C12 (3 x 200 mL). The combined organic layers were
dried over Na,SO4, concentrated and filtered through a short SiO, column (20%
Et0Ac-
.
1 0 hexanes) to afford the crude epimerized product.
The aldehyde was dissolved in Et20-Et0H (1:1, 100 mL), cooled to 0 C and
treated with sodium borohydride (1.21 g, 32 mmol). The mixture was stirred for
20
min, carefully diluted with saturated aqueous NH,CI, stirred for 30 min at rt
and
extracted with CH,CI, (3 x 150 mL). The combined extracts were dried over
Na,SO4,
1 5 concentrated and purified by flash chromatography (20% Et0Ac-hexanes)
to afford
alcohol 15 (9.95 g, 85% for three Steps).
20 TSS
MeScioN Megcfompm
MPMOTCI
OPV
TBS TBS TBS ,, //opv
'
0
1 5 1 6
MPM-ether 16 BF3=0Et2 (0.1 M in CH2C12, 1.8 mL, 0.18 mmol) was
added to a solution of alcohol 15 (9.87 g, 18 mmol), MPM-trichloroimidate (4.9
mL,
27 mmol) and CH,Cl2 (175 mL) at 0 C. After 40 min, a second portion of
BF3.0Et,
25 (0.1 M in CH2CI,, 0.9 mL, 0.09 mmol) was added to the reaction mixture.
After 20
min, the reaction was quenched with saturated aqueous NH4C1, stirred for 1 h
at rt and
diluted with Etp (600 mL). The organic layer was separated and the aqueous
layer
was extracted with Etp (150 mL). The combined organic extracts were washed
sequentially with 0.1 N aqueous NaOH. saturated aqueous NaHCO:,, brine, dried
over
30 Na.:504, concentrated and purified by flash chromatography (20% Et0Ac-
hexanes) to
give MPM-ether 16 (10.20 g, 85%).
MeQ = OMPM LAH MeqcfOMPM
TBS
TBS 0 TBS
0
33
CA 02755266 2011-10-17 '
16 17
Alcohol 17 LAH (1 M in THE 22.5 mL. 22.5 mmol) was added to a
solution of MPM-ether 16 (10.05 g, 15 mmol) in Etp (1.0 L) at 0 C. After 30
min,
the reaction was cautiously quenched with 1-120 (1.3 mL), and I N aqueous NaOH
(1.3
mL). After stirring for 1 h at rt, the suspension was filtered through Celite,
concentrated and purified by flash chromatography (20% Et0Ac-hexanes) to
afford.
alcohol 17 (8.18 g, 93%).
Meg OMPM 1) Swem Me0c.--OMPM
TBS
TBS TBS
2) Wittig TBS
0
1 7 1 8
Olefin 18 DMSO (5.8 mL, 82.4 mmol) was added to a solution of oxalyl
1 5 chloride (3.6 mL, 41.2 mmol) in CH,C12 (100 mL) at -78 C. After 15
min, a solution
of alcohol 17 (7.94 g, 13.5 mmol) in CH2C12 (35 mL) was added to the reaction
mixture. After stirring for 1 h, Et3N (17 mL, 122 mmol) was added, the mixture
was
warmed to 0 C, stirred for 20 min, diluted with saturated aqueous NH4C1 and
then
extracted with CH2C12 (3 x 100 mL). The combined organic extracts were dried
over
Na,SO4, concentrated and filtered through a short SiO, column (20% Et0Ac-
hexanes)
to furnish the desired crude aldehyde.
n-BuLi (1.6 M, 20 mL, 30 mmol) was added dropwise to a solution of
CH3PPh3Br (10.1 g, 30 mmol) in TI-IF (350 mL) and DMSO (100 mL) at 0 C. After
1
h, a solution of the crude aldehyde in TI-IF (50 mL) was added. The reaction
mixture
was warmed to it and stirred for 3 h. Saturated aqueous NH4C1 was added and
the
mixture was extracted with Et0Ac (3 x 500 mL). The combined extracts were
washed
with brine, dried over Na2SO4, concentrated and purified by flash
chromatography (7%
Et0Ac-hexanes) to afford olefin 18 (5.57 g, 71% yield for 2 steps).
MeQ
OMPM 9-BBN MeQ OMPM
TBS TBS
TBS TBS
0 " .<0).-"-"-
µ'OH
1 8 1 9
34
CA 02755266 2011-10-17
Alcohol 19 9-BBN (0.5 M in TI-IF, 65 mL. 33 mmol) was added to a solution
of olefin 18 (5.56 g, 9.6 mmol) in TI-IF (85 mL) at 0 C. The mixture was
stirred for 5 h
at rt and then recooled to 0 C. H20 (200 mL). THF (100 mL), and NaB03=4 H20
(30g)
were sequentially added. After stirring overnight at rt, the organic volatiles
were removed
under reduced pressure. The aqueous residue was extracted with Et0Ac (3 x 200
mL)
and the combined organic layers were dried over Na-,SO4. Concentration and
purification
by flash chromatography (30% Et0Ac-hexanes) afforded alcohol 19 (12.05 g,
92%).
Me0
10:71( ¨OMPM Swern MeQ
TBS MPM
TBS
TBS
's 0 OH
1 9 2 0
Aldehyde 20. DMSO (1.36 mL, 19.2 mmol) was added dropwise over 4 min
to a solution of oxalyl chloride (1.26 mL., 14.4 mmol) in CHIC], (120 mL) at -
78 C. .
After stirring for 10 min, a solution of alcohol 19 (5.76 g, 9.61 mmol) in
CH2C12 (20
mL) was added via cannula. The transfer was completed by rinsing with
additional
CH2Cl2 (2 x 5 mL). After stirring for 20 min, the mixture was treated with
Et3N (5.36
mi., 38.4 mmol) and stirred for 10 mm at -78 C, 30 mm at 0 C and 10 min at
rt. The
reaction mixture was poured into saturated aqueous NaHCO3 (200 mL) and the
separated aqueous layer was extracted with CH2Cl2 (3x) followed by Et0Ac (100
mL).
The combined organic phases were dried over Na2SO4, concentrated and purified
by
column chromatography (10% to 20% Et0Ac-hexanes) to furnish aldehyde compound
20 (5.28 g, 92%) as an oil.
0
1) NiC12/CrC12.
MeQ., j¨OMPM X-2
TBS 1103X,sõ.( ,,, õ ,
0 2) KHMDS I Me
20 B2 3 1 8
B2318. 0.1% Nia/CrC12 (w/w, 3.21 2) and 1% NiC1,/CrCI, (w/w, 4.31 Er.)
was added to a solution of aldehyde 20 (3.73 g, 6.25 mmol), key fragment F-2
exemplified by vinyl iodide X2. (5.10 g, 9.16 mmol), 11-1F (85 mL) and DMF (21
CA 02755266 2011-10-17 =
mL) at rt in a glove box. The reaction mixture was stirred for 24 h. removed
from the
glove box, cooled to 0 C. diluted with Et0Ac (100 mL), quenched with
saturated
NH,C1 (200 mL) and stirred for 30 min. The separated aqueous phase was
extracted
with Et0Ac (6x) and the combined organic layers were dried over Na,SO4
concentrated
and purified by column chromatography (20% to 30%) to give B2318 (-3 g)
contaminated with close running impurities and the uncyclized intermediate
(4.61 g).
The latter (4.61 g, 4.48 mmol,) was dissolved in THE (150 mL), cooled to 0 C
and
treated with KHMDS (0.5 M in toluene, 14 mL, 7.0 mmol) over a 2 min period.
After
stirring at 0 C for 15 min, the reaction was quenched with saturated aqueous
NH4C1
(150 mL) and warmed to rt. The separated aqueous layer was extracted with
Et0Ac
(3x) and the combined organic phases were dried over Na,SO4, concentrated and
combined with the partially purified product obtained above. Column
chromatography
(10% Et0Ac-hexanes) afforded B2318 (3.17 g, 55%) as an inseparable -3:1
mixture
of C27 diastereomers.
meck_FOMPM M
TBS TBS
TBS ...III TBS
. 0 0
1) DIM
2) separate isomers
Me
.""pos0Pv
0 Pv
823I8 82317
B2317. DDQ (1.45 g, 6.42 mmol) was added portion-wise over 30 min to a
stirred solution of B2318 (3.12 g, 3.35 mmol) in CH,C1, (50 mL) and pH 7
phosphate
buffer (5 mL) at rt. The reaction was quenched with saturated aqueous NaHCO,
(50
rnL), stirred for 5 min, diluted with additional saturated aqueous NaHCO, (100
mL),
11,0 (200 mL) and extracted with Et,0 (5x). The combined organic phases were
dried
over Na2SO4, concentrated and purified by column chromatography (15% to 30%
Et0Ac-hexanes) to give recovered B2318 (1.40 g) and a mixture of the C27
isomeric
products. The recovered B2318 was resubmitted to the reaction conditions
described
above to afford additional product. Recovered starting material was again
cycled
through the deprotection conditions. All of the desired material was combined
and
separated by MPLC to afford B2317 (1.65 g, 61%).
36
CA 02755266 2011-10-17
aMe0K-OH M eV-OTs Tici,,3_sL -
TsCI
0..?...
''' 0Pv )
OPv
B23 1 7 B2 3 1 6
5 B2316. TsC1 (0.63 g, 3.30 mmol) was added to a solution of B2317
(1.60 g,
1.97 mmol) in CH,C12 (8 mL) and pyridine (2 mL) at rt. After stirring for 29
h, the
reaction was quenched with saturated aqueous NaHCO3 (30 mL) and H20 (10 mL).
The separated aqueous layer was extracted with Etp and the combined organic
layers
were dried over Na2SO4, concentrated and purified by column chromatography
(15% to
1 0 30% Et0Ac-hexanes) to give B2316 (2.01 g, 92%) as an oil along with
recovered
B2317 (92 mg, 5.8%).
MeRciOTs Meg., OTs
Tto.õ3A, =
Toa
E E
LAH
s.õ1.,..
I I Me
OH
B2316 B2315
- B2315. LAH (1 M in THF, 2.61 mL, 2.61 mmol) was added over 1
min to a
solution of B2316 (1.68 g, 1.74 mmol) in Et20 (80 mL) at 0 C. After stirring
for 7
min, the reaction was quenched by careful addition of Me0H (0.42 mL, 10.4
mmol)
and H20 (0.19 mL, 10 mmol), warmed to rt and stirred for 20 min. Filtration
through-
Celite with 1:1 CH2C12-Et20, concentration and purification by column
chromatography
. (30% to 40% Et0Ac-hexanes) gave B2315 (1.38 g, 90%) as an oil.
37
_
CA 02755266 2011-10-17
Me0,0Ts
TBS
TBS ¨ TBS
0
MMTrCI
I 0
r"-"-ThMMTr
B2 3 1 5 B2 3 1 4
B2314. MMTra. (0.70 g, 2.26 mmol) was added to a solution of B2315
(1.33 g, 1.51 mmol) in CH2C12 (25 mL) and iPr2NEt (0.79 mL, 4.53 mmol) at rt.
The
resulting mixture was stirred for 1 h and then poured into a mixture of
saturated
aqueous NaHCO, (20 mL), H20 (10 mL) and Et,0 (50 mL). The separated aqueous
layer was extracted with Et20 (3x). The combined organic phases were dried
over
Na,SO4, concentrated and purified by column chromatography (CH,C12 followed by
15% to 30% Et0Ac-hexanes) to give B2314 (1.65 g, 95%) as a solid foam.
Me0.ff-OTs MeOff¨I
TBS TBS
0
Nal
=
0 Me
Ior'¨'''''OMMTr
B2314 B2313
B2313. A mixture of B2314 (1.60 g, 1.39 mmol) and NaI (3.10g. 20.8
.mmol) in acetone (50 mL) Was heated under reflux for 13 h. After cooling to
rt, the
reaction mixture was diluted with Et0Ac, and concentrated. f120 (5 mL), brine
(20
mL) and Na,S203 (200 mg) were added and the resulting mixture was extracted
with
Et,0 (4x). The combined extracts were dried over Na,SO4, concentrated and
purified
by column chromatography (10% Et0Ac-hexanes) to give B2313 (1.50 g,µ97%) as an
oil.
38
CA 02755266 2011-10-17
Me0, I Meb,
TBS TBS '
TBS TBS
0 ===., OH 0
-
t-BuLi
TBSO
1..=
1="'(µ?''=-=Me
F-3 am TBSO IBSe
OMMTr
B 2 3 1 3 B2307, B2308
B2307 and B2308. Tert-BuLi (1.7 M in pentane, 1.00 mL, 1.7 mmol) was added
over 1
min to a solution of B2313 (0.90 g, 0.81 mmol) in Et,0 (14 mL) at ¨78 C.
After
stirring for 9 min, the mixture was transferred via cannula over 4 min to a
solution of
key fragment F-3 (0.83 g, 1.14 mmol) in Et20 (4 tnL) at -78 C. The transfer
was
completed by rinsing with additional Et,0 (2 mL). The resultant mixture was
stirred at -
78 C for 5 min and then at 0 C fOr 10 min, quenched with saturated aqueous
NaHCO3
1 0 (30 mL) and warmed to it. The separated aqueous layer was extracted
with Et,0 (3x)
and the combined organic phases were dried over Na,SQ, and concentrated. The
residue was combined with those of other two batches (corresponding to 0.11 g
and
0.44 g of B2313) and purified by column chromatography (10% to 20% Et0Ac-
hexanes) to give a mixture of B2307 and B2308 (1.86 g, 83%) as a solid foam.
1 5 Although the isomers could be separated by prep TLC (20% Et0Ac-
hexanes), they
were carried forward as a mixture.
39
CA 02755266 2011-10-17
Meg
lBs
TBS
0 OH 0
PPTs
O
TBSO TBS
TBSO
I 0
)0MMTr
Me0
B2307, B2308 TBS '
TBS õ=== OH =
O
TBSO TBS
sõ.= ,me TBSO
o)0H
B2305, B2306
B2305 and B2306. The mixture of 82307/82308 (1.80 g, 1.05 mmol)
was dissolved in Et0H (20 mL), treated with PPTS (10.0 mg, 0.04 mmol), stirred
at rt
for 11 h and then quenched with NaHCO3 (20.0 mg, 0.24 mmol). After stirring
for 15
min, the mixture was concentrated, azeotroped with toluene (15 mL), and
purified by
column chromatography (20% to 30% Et0Ac-hexanes) to give a mixture of 82305
and 82306 (1.22 g, 81%) as a solid foam. Although the isomers could be
separated
by prep TLC (30% Et0Ac-hexanes), they were carried forward as a mixture.
CA 02755266 2011-10-17
Mea,
cTla "
TBSOH 0
0 : .= =
H"
OTBS Des s-Martin
...=CaTBSO
me TBSO
OH
Me0,
TBS '
TBS
e
91
O
TBSO TBS
TBSO
B2305, B2306 B2304
B2304. A mixture of B2305/B2306 (1.16 g, 0.68 mmol), and Dess-Martin
periodinane (0.61 g, 1.44 mmol) in CH2C12 (35 rnL) was stirred at rt for 1 h.
Additional Dess-Martin periodinane (0.54 g, 1.27 mmol) was added to the
mixture and
stirring was continued for an additional 1 h. The mixture was diluted with
Et20 (100
mL), stirred for 20 min and filtered through Celite with Et,O. The colorless
filtrate was
washed with saturated aqueous NaHCO3 (100 rnL) and the separated aqueous layer
was
extracted with Et,0 (3X). The combined organic phases were dried over Na2SO4,
concentrated and purified by column chromatography (10% to 15% Et0Ac-hexanes)
to
give B2304 (0.98 g, 84%) as a solid foam.
Alternatively, B2304 may be prepared as follows and in fact the synthesis
described
below is superior to that given above.
TBS9
M MeR Ms
TBSOToaS
0 :
0
Ms20
1.="'U'Me
OPv
B2317 ER804025
41
CA 02755266 2011-10-17
To a solution of the alcohol, 2.4 g, in methylene chloride, 29 mL, was added
methanesulfonyl
anhydride, 770 ma. The mixture was stirred for 15 minutes, extracted with
saturated
sodium bicarbonate, dried and chromatographed to give 2.737 g, 100%.
MeR rOMs MeO,K-SPh
TB
TBS S TBS
0
PhSH
,,,,
Me
I Me
OPv
OPv
ER804025 ER804026
To a solution of the mesylate, 405 mg, in DMF, 0.06 mL, was added di-
isopropylethylamine, 0.130 mL, followed by benzenethiol, 0.061 mL. After 4
hours
and after 22 hours, additional amine, 0.03 mL, and benzenethiol, 0.015 mL,
were
added. After 24 hours, the mixture was diluted with 5% ethyl acetate/hexane, 1
mL and
chromatographed to give 409 mg.
Megr-SPh MeQc---SO2Ph
TBS
TBS 2 TBS TBS
TPAP
I
,,, Me NMO
1"
OPv
ER804026 ER804027
To a solution of the sulfide, 1.97 g, in acetonitrile, 16 mL, was added N-
methylmorpholine oxide (NMO), and then a solution of 1.02 g,
tetrapropylammonium
perruthenate(VI1), (TPAP), 38 mg, in acetonitrile, 1 mL. After 3.5 hours at
room
temperature, the mixture was heated to 40 C for 1 hour. The mixture was
cooled and
aqueous satd. Sodium thiosulfate was added and the mixture partitioned between
water
and ethyl acetate. The usual work-up gave 1.567 g of a brown oil.
42
CA 02755266 2011-10-17
Me0 SO2Ph Meg, -
SO2Ph
oTa ___________________________________________________________ ,
TBS TBS
DIBAL
I Me
I Me
OPv
ER804027 ER804028
To a solution of the pivaloate ester, 1.567 g, in methylene chloride, 11.2 mL,
at - 78 C
in was added DIBAL, 2.5 mL of a 1 M solution in toluene. After 15 minutes,
additional DIBAL, 0.8 rnL, was added. After an additional 5 minutes, methanol,
0.46
mL, was slowly added followed by water, 0.2 mL. The mixture was filtered
through
Celite and chromatographed to give 1.386 g of an oil.
Me0 SO2Ph
0 0
*
TBSO IBS
I Me TBSO
1,=õ,põ.=
OPv
ER804028 F-3
02Ph
Me0
TBS
TBS
"0":
H'.µ =
OTBS
n-BuLiTBSO
ame TBSO
ER804029
To a solution of the sulfone, 36 mg, in DME, 1 mL, at -40 C was added n-
butyllithium, 2.8 equivalents. After 35 minutes, a solution of the aldehyde,
42 mg, in
DME, 0.5 mL) was added. After 40 minutes, saturated aqueous ammonium chloride
was added and the mixture extracted with ethyl acetate. The usual work-up,
followed
43
CA 02755266 2011-10-17
by chromatography gave 52 mg of an oil.
so2Ph
MeQ 1 SO2Ph
TBSO ' MeQ
HTBSO '
..õ of i 0 H
H
" 0 ": TB SO.,K.õ.= -.., =I =
= = . =
II 'MS H''
0-;'''' TBSO Dess-Martin OTBS
,.===(.,_7===me TBSO \
1õ... = I . . .
= =
11 = ..n_CH 0 1
ER804029 ER804030
To a solution of the alcohol, 42 mg, in methylene chloride, 2 mL, was added
the Dess
Martin reagent, 36.4 mg. The mixture was stirred for 30 minutes and ether was
added.
The mixture was filtered through Celite, washed with saturated sodium
bicarbonate,
with saturated sodium thiosulfate, worked up in the usual way and
chromatographed to
give 38 mg of an oil.
MeQ
02Ph ,
cl-BA
Q =
Me I H
T a , = TBS = =
I =E H"µ =
=
TE3S0 =
OTBS
H's e
i TBSO
TBS.
....= "Me TBSO .,,,,OTBS
SmI2 õ.,ame TBSO
I
-.... 0 5_1õ...-CHO 1
I.CHO I
ER804030 B2304
Preparation of SmI, Solution
A solution of 1,2-di-iodoethane in 10 mL of TI-LF was added to a suspension of
Sm,
0.16 g, in THF, 1 mL. The mixture was stirred for 1 hour.
. An aliquot of this solution, 0.03 mL, was added to a solution of the
sulfone in THF at
-78 C. After 5 minutes, additional SmI2 reagent, 0.05 mL, was added. After a
few
additional minutes, more reagent, 0.25 mL, was added. The cooling bath was
removed
and saturated aqueous sodium bicarbonate, 3 mL, was added. The mixture was
" partitioned between ether and water and the usual work-up gave 9.1 mg, 81%,
of an
oil.
44
_
CA 02755266 2011-10-17
M ea
oTa .'=
I H
TBS
"O": = Hµ0 0
: OTBS NiC12/CrCl2
TBSO
me TBSO ',..
10CHO I
M e0
TB S IZSI."=
=
0 ..."'- =.. a
H"
TB SO
..... '= me TBSO
OTBS
i 0
OH
B2304 B2302, B2303
B2302 and B2303. In a glove box, NiCI,/CrC12 (1% w/w, 1.09 g, 8.86
mmol) was added to a solution of B2304 (1.01 g, 0.70 mmol) in THF (600 mL) and
DMF
(150 mL) at rt. After stirring for 2 days the reaction mixture was taken out
of the glove
box, cooled to 0 C, quenched with saturated aqueous NH4C1 (300 mL) and
stirred at 0
C for 20 min. After addition of 1-LO (100 mL), the two layers were separated
and the
aqueous layer was extracted with Et0Ac (5x). The combined organic phases were
washed with brine, dried over Na2SO4, concentrated and purified by column
chromatography (15% Et0Ac-hexanes) to furnish a mixture of B2302 and B2303
(0.84 g, 92%) as a solid foam. Although the isomers could be separated by prep
TLC
(20% Et0Ac-hexanes), they were carried forward as a mixture.
_
CA 02755266 2011-10-17 ,
Me0,
H
TBS IZSI,. c
' 0 ": H'''s =
TBSO
"K-''Me TBSO "--. 1-BS
. Dess-Martin
-
1 4
'''' OH
Me0
c')N1175(
H
TBS
o 0
'i Fr.' =
TBSO
IS. -ve TBSO .77.'s
7...
õ, =
.
B2302, B2303 B2301
B2301. A mixture of B2302/82303 (0.79 g, 0.60 mmol) and Dess-Martin
periodinane (0.26 g, 0.60 mmol) in CH2C12 (30 mL) at rt was stirred for 30
min.
Additional Dess-Martin periodinane (0.26 g, 0.60 mmol) was added to the
mixture and
stirring was continued for additional 1.5 h. The mixture was then diluted with
Et20
(100 mL), stirred for 15 min and filtered through Celite. The filtrate was
washed with
saturated aqueous NaHCO3 (100 inL) and the separated aqueous layer was
extracted
with Et20 (3x). .The combined organic phases were dried over Na,SO4,
concentrated
and purified by column chromatography (10% to 15% Et0Ac-hexanes) to give B2301
(0.67 g, 85%) as an oil.
=
46
CA 02755266 2011-10-17
Me0,
TBS 1-0a µ,.. 0 0 =
' = -
H"' 1) TBAF
O
TBSO TBS
TBSO 2) PPTS
=
0
Met)
HOA,µ,.. 0 =-,õ
=
I
9 = ,
o
B 2 3 0 1 B1 7 9 3
B1793. TBAF (1 M in THF containing 0.5 M imidazole HC1, 4.60 mL, 4.60
mmol) was added over 2 min to a solution of B2301 (0.62 g, 0.48 mmol,) in THF
(29
mL) at rt and the resulting mixture was stirred for 18 h. After dilution with
hexanes
(10 mL), the reaction mixture was directly loaded onto a SiO2column packed
with 50%
Et0Ac-hexanes and eluted with 50% Et0Ac-hexanes (1 L) followed by 10%
MeOH/Et0Ac to collect a mixture of intermediates. After solvent removal, the
residue
was dissolved in CH,Cl2 (15 mL) and treated with PPTS (645 mg). After stirring
for 1
h at rt, additional PPTS (414 mg) was added and the resulting white suspension
was
stirred for 4.5 h. The reaction mixture was then directly loaded onto a SiO2
column
packed with 70% Et0Ac-hexanes and eluted with 70% Et0Adhexanes (0.5 L), Et0Ac
(1 L). Elution with 5% to 10% Me0H/Et0Ac furnished pure B1793 (181 mg) and
elution with 15% Me0H-Et0Ac gave additional semi-pure product, which after
purification by preparative TLC (10% Me0H-Et0Ac) provided additional pure
B1793
(42 mg). B1793 (total 223 mg, 64%) was obtained as a white solid. HRMS: calcd
for C4011580,2 + Na 753.3826. Found: 753.3808.
47
CA 02755266 2011-10-17
Synthesis of B1794:
cx,"H
CHO Me0,
OH HO '
HO e
0 "", S.
Q =
0
Arabinose B1 7 94
B1794. Except for stereochernical and protecting group differences (see
Schemes
4 and 6)arabinose was converted to B1794 in a manner similar to that described
for
B1793 (see schemes 3 and 5). HRMS: calcd for C4,115801, + Na 753.3826. Found:
753.3856.
Synthesis of Representative B1793 Analogs:
Me0,
HOAs, =
1-r*. TsCI
Q =
6õ
M e0,
R9
Ts0õ,_õ-k, ,== =
= = =
q =
t
0
B1793 B1920: R = H
B1921: R = Ts
48
CA 02755266 2011-10-17
B1920 and B1921 TsCI (9.9 mg, 0.052 mmol) was added to a solution of diol
B1793 (7.6 mg. 0.010 mmol) in CH2Cl2 (1 mL) and pyridine (0.1 mL) at it After
48
h, the reaction was quenched with a 1:4 mixture of saturated aqueous NaHCO3-
brine and
extracted with CH2Cl2 (4x). The combined extracts were dried over Na2SO4 and
concentrated. Purification by preparative TLC (80% Et0Ac-hexanes) afforded
monotosylate B1920 (6.0 mg, 67%), and ditosylate B1921 (1.8 mg, 18%).
MeQ
HO FA = -
MsCI
9 = =
L.
= H Me0.
0 H=,.µ.
µL) 6
B1793 B2294
B2294 MsC1 (0.3 M in CH2C12, 98 AL, 0.030 mmol) was added dropwise
over 40 min to a mixture 'of collidine (7 AL, 0.054 mmol), B1793 (20.8 mg,
0.028
rnmol) and CH2C1, (1 mL) at 0 C. After 76 h at 4 C, the reaction was
quenched with a
1:4 mixture of saturated aqueous NaHCO3-brine and extracted with CH2C12 (4x).
The
combined extracts were dried over Na2SO4 and concentrated. The crude product
was
dissolved in toluene (3 mL) concentrated and purified by preparative TLC (1.5%
Me0H-Et0Ac) to afford mesylate B2294 (21.4 mg, 95%).
49
CA 02755266 2011-10-17
Me0HO
.=== 0 0
0
Fr p-F-PhCH2Br
=
Q =
'Me Oõ Ag20
=
B1793
MegMeg
=
, H
0 HO
H''s = 0 - = =
µµ..(t 9 6,,
q = ,
=="(am
e
I Me
B2 0 1 4 B2 0 1 5
B2014 and B2015 A 0.016 M solution of 4-fluorobenzyl bromide in Et20
(800 itL, 13 Amol) and Ag20 (10 mg, 43 Arnol) were each added in three
portions at 1
h intervals to a rt solution of B1793 (1.7 mg, 2.3 Athol) in Et20 (1.2 mL).
The
mixture was protected from light, stirred for 7 h and then filtered through
Celite.
Concentration and purification by preparative TLC (Et0Ac) afforded primary
ether
B2014 (1.1 mg, 56%), and secondary ether B2015 (0.6 mg. 31%). HRMS (FAB):
calcd for C37H63F012 Na 861.4201. Found: for B2014 861.4178, for B2015
861.4160.
CA 02755266 2011-10-17
M e0,
HO
A.. 0 H"' ArNCO
B1793 q = _1
1.'" ."Me q===
Me0,
H 119
_ 0 =
0
Hs,. =
0
X 9 = A
B1984: X ''''=H ' 'Me 1/44,
B1990: X=C1 I, =
B1992: X=Me0
General. A mixture of B1793 (1 mg, 1.37 micromol), Et3N (10 microL, 72
micromol) and ArNCO (2 to 4 equiv.) in CH2C12 (0.2 mL) was stirred at rt for 4
h to
overnight until the reaction was judged to be complete by TLC. The reaction
mixture
was diluted with saturated NaHCO3 (3 mL), extracted with CH2C12 (3x) and Et0Ac
(2x), dried over Na,SO4 and purified by preparative TLC (5% Me0H-CH2C12) to
afford
the products:
B1984. (1.0 mg, 86%) FIRMS (FAB): calcd for C471-163N013 + Na
872.4197. Found: 872.4214.
B1990. (1.1 mg, 92%) FIRMS (FAB): calcd for C41H62C1N013 + Na
906.3807. Found: 906.3826.
B1992. (1.0 mg, 83%) FIRMS (FAB): calcd for C48H65N014 + Na
902.4303. Found: 902.4269.
51
CA 02755266 2011-10-17
Meg
HOA, 0
0
Mitsunobu
= ,
1,,,õ =
H meQ
HO
0 - 0
1-Issµ
0
0 ____________________________________________________________
I
I 0 Me
B2042
B 1793
B2042 DEAD (0.4 M in ether, 50 AL, 19 Amol) was added to a solution of
B1793 (2.0 mg, 2.7 Amol), triphenylphosphine (5 mg, 19 Amol), 4-nitrobenzoic
acid
(3.2 mg, 19 Amol) and Et20 (500 AL) at rt. After 22 h, the reaction mixture
was loaded
directly onto a preparative TLC plate and eluted with 60% Et0Ac-hexanes to
give the
intermediate diester (3.0 mg). This material was taken up in Me0H (300 AL) and
treated
with K,CO2 (approximately 1 mg). After stirring at rt for 30 min, the reaction
mixture
1 0 was diluted with brine and extracted with CH2C12 (5x). The combined
extracts were dried
over Na,SO4, concentrated and purified by preparative TLC (5% Me0H-Et0Ac) to
afford
B2042 (1.2 mg, 60% for two steps). FIRMS (FAB): calcd for CH58012 + Na
753.3826. Found: 753.3810.
5
CA 02755266 2011-10-17
Me0,,
=.õ, 0 0 H
= _
= NaB114
q =
=H MeO,
HO
'.= OH = H
0
Fr =
q. = ,
B1793 B1896, B1897
B1896 and B1897. NaBH, (3 mg, 0.08 mmol) was added to a solution of
B1793 (2.30 mg, 3.15 Arno') in 1:1 CH2C12-Me0H (0.2 mL) at rt. Concentration
of
the reaction mixture and purification by preparative TLC (8% Me0H-Et0Ac)
provided
B1896 (0.80 mg, 35%) and B1897 (2:1 mixture, 0.15 mg, 6.5%). HRMS (FAB)
for B1896: calcd for C40H60012 + Na 755.3983. Found: 753.3969.
MeO,
0 0 H
0 : Nal04
9 = .
MeO,
0 0 H
= - 14"..
Q
B1793 B1918
B1918. A mixture of B1793 (2.0 mg, 2.74 itinol), NaIO, (35 mg, 0.16
1 5 mmol), Me0H (0.8 mL) and H20 (0.2 mL) was stirred at rt for 40 min. The
reaction
53
CA 02755266 2011-10-17 ,
mixture was diluted with F1,0 (1 mL). extracted with CH,C12 (6x). dried over
Na:504,
concentrated and purified by column chromatography (5% Me0H-C1-1,C1z) to give
B1918 .(1.9 mg. 100%).
Me0,õ
=õ,, 0 0
0 - H"" = Na8H4
Q e
Me0,
HO" 0" =
CL Qs
=
B1918 B2037
B2037. A 0.034 M solution of NaBH, (0.1 mL, 3.4 itmol) in Et0H was added
portion-wise to a solution of B1918 (1.9 mg, 2.72 Amol) in Me0H (0.8 mL) and
CH2C12 (0.2 mL) at -78 C to it until the reaction was judged to be complete
by TLC.
The reaction was quenched with saturated aqueous NI-WI (2 mL) at -78 C,
warmed to
rt, extracted With CH2C12 (6x), dried over Na,SO4 and purified by preparative
TLC (5%
Me0H- CH2Cl2) to afford B2037 (1.7 mg, 89%). FIRMS (FAB): calcd for C39E156011
+ Na 723.3720. Found: 723.3749.
54
CA 02755266 2011-10-17
M e0,,,
0 ,, = H
e
".. = 1) Na104
H
Q i
1, "'Me
..'s '' 6,,
H 2) CH2=CHCF2Br
I 0 In
MeQ,
43.,,,,.., = = ,,,,, 1
= H
11'''' =
F F :
q =
z d
1 o
==.õ H
B1 7 9 3 B2 0 3 5
B2 0 3 5
Nal , (35 mg, 0.16 mmol) was added to a solution of B1793 (1.7 mg, 0.0023
rrunol), Me0H (800 AL) and H20 (200 tiL) and after 15 min, the mixture was
diluted
with H20 and extracted with CH2C12 (5x). The organic extracts were dried over
Na2SO4, concentrated and the intermediate aldehyde was immediately dissolved
in DMF
1 0 (300 pL). 3-Bromo-3,3-difluoropropene (3AL, 0.023 mmol) and
indium powder (3
mg, 0.23 mmol) were added and after 24 h additional 3-bromo-3,3-
difluoropropene (1
AL, 0.008 mmol) was added. After 18 h, H20 was added the mixture was extracted
with Et0Ac (3x). The combined organic extracts were washed successively with
H20
and brine, dried over Na.2504, concentrated and purified by preparative TLC
(80%
Et0Ac-hexanes) to provide B2035 (0.74 mg, 41% for 2 steps) as a mixture of
isomers. HRMS (FAB): calcd for C42H58F2011 + Na 799.3845. Found: 799.3883.
_ .
CA 02755266 2011-10-17
MeC1
HOA 0 1) NaBH4
H"'= 2) Na104
s
I 3) p-F-PhMgBr
I, = Me 0,
4) Dess-Martin
Meg
0 '
0 - =
Fr =
Q =
B 1793 B2011: X = H
B2008: X = F
B2008, B2011
NaBH, (2 mg, 0.05 mmol) was added to a solution of B1793 (2.2 mg, 0.003
mmol) in 1:1 CH2C12-Me0H (200 AL) at rt. After 15 min saturated aqueous NH4C1
and
H20 were added, and the mixture was extracted with CH2C12 (6x) and Et0Ac (2x).
The
combined organic extracts were dried over Na,SO4, concentrated and purified by
' 10 column chromatography (10% Me0H-Et0Ac) to provide an intermediate
triol, which
was dissolved in Me0H (800 AL) and H20 (200 AL). NaI04 (35 mg, 0.16 mmol) was
added and after 20 min, the mixture was diluted with H20 and extracted with
CH2Cl2
(6x). The organic extracts were dried over Na2SO4, concentrated and the
intermediate
aldehyde was immediately dissolved in THF (500 AL). 4-Fluorophenylmagnesium
bromide (2M in Et,O, 12 AL, 0.024 mmol) was added and after 20 min the
reaction was
quenched with saturated aqueous N1-140. The mixture was extracted with C1-
1,C12 (6x)
and the combined organic extracts were dried over Na,SO4 and concentrated.
Purification by preparative TLC (Et0Ac) provided the desired product as a
mixture of 4
isomers (1.32 mg, 55% for 3 steps).
Dess-Martin periodinane (-3 mg, 0.007 mmol) was added to a solution of the .
above product (0.95 mg, 0.0012 mmol) in CH2Cl2 (300 AL) and the mixture.was
stirred at rt for 20 min. Additional Dess-Martin periodinane (-3 mg, 0.007
mmol) and
CH2Cl2 (300 AL) were added and after another 40 min Et,O, saturated aqueous
NaHCO3(4 mL) and saturated aqueous Na,S203 (1 mL) were added. The mixture was
extracted with Et20 (3x) and the combined extracts were washed with brine,
dried over
Na2SO4, concentrated and purified by column chromatography (20% Et0Ac-hexanes)
56
CA 02755266 2011-10-17
to provide B2008 (0.58 mg. 61%). FIRMS (FAB): calcd for CõHõFOõ + Na
815.3783. Found: 815.3758.
B2011. In an analogous manner, B1793 (1.9 mg. 0.003 mmol) was
converted to B2011 (0.87 r112, 42% for 4 steps). HRMS (FAB): calcd for
C35H5801
+ Na 797.3877. Found: 797.3877.
Me0
H9 "-
H
Ts0-õ,..A.,õ... = ,õ,. 0 0 . =
H"µ KCN
Q =
0,
1 = H Mea
..õ, ,
NcAS. =
H
= 0 "- H".. .
9 = Oõ
1 ''''' = '''Me
H
B1920 B2013
B2013
A solution of B1920 (1.4 mg, 0.0016 mmol), KCN (1 mg, 0.016 mmol) and
DMSO ,
(500 AL) was heated at 60 C for 8 h. After cooling to rt, H20 was added and
the
mixture was extracted with Et0Ac (3x). The combined organic extracts were
washed
successively with 1120 and brine, dried over Na2S0õ concentrated and purified
by
preparative TLC (80% Et0Ac-hexanes) to provide B2013 (0.78 mg, 67%). HRMS
(FAB): calcd for C41H57N011 Na 762.3829. Found: 762.3848.
57
_
CA 02755266 2011-10-17
M e0,
TsOa
= = =- Nal
9.1õ
Me
I =
Me0,
0 o
H÷' e
4C( =
=
B 1 92 0 X1920
X1 9 20
A mixture of B1920 (1.3 mg, 1.47 /mop, NaI (30 mg, excess) and acetone (1 mL)
was stirred at 60 C for 3.5 h. After cooling to rt, the reaction mixture was
diluted with
saturated aqueous NaHCO3 (3 mL), extracted with CI-12C12 (5x) and Et0Ac, dried
over
Na2SO4 and purified by column chromatography (50% Et0Ac-CH2C12 to 80% Et0Ac-
hexanes) to give the iodide X1920 (1.3 mg, 100%).
Me0.,
LA
H"'s ArSH
=
.
I= M e0,
ArSA,õ, 0
0
X1920 '9 =
.==
1."' "'Me a=
B1998: Ar p-CI-Ph
B2010: Ar = p-Me0-Ph
B2019: Ar = 2- imidazole
58
CA 02755266 2011-10-17
General. A mixture of iodide X1920 (1.0 equiv.), iPr2EtN (11 to 22 equiv.),
ArSH (9 to 46 equiv.) and DMF (0.3 mL) was stirred at rt until the reaction
was judged
to be complete by TLC (typically 24 to 48 h). The reaction mixture was diluted
with
saturated aqueous NaHCO3 (2 mL), extracted with CH2C12 and Et0Ac, dried over
Na,SO4 and purified by preparative TLC (80% Et0Ac-hexanes or 5% Me0H-CH,C12)
to afford the sulfide products:
B1998. (1.3 mg gave 1.1 mg, 85%) FIRMS (FAB): calcd for C46H6ICI0IIS
+ Na 897.3521. Found: 897.3533.
B2010. (1.1 mg gave 0.7 mg, 59%). FIRMS (FAB): calcd for C47H6401.2S +
Na 875.4016. Found: 875.4057.
B2019. (1.1 mg gave 0.7 mg, 61%) MS (FAB): M + Na
H
ArS õ.. = ,,,, 0 0 _
w mCPBA
,,,,,,, = 6õ
m eC1
ArS02A 0 0 H
9 =
=,,me 0õ
B1998: Ar = p-CI-Ph B2016: Ar =p-a-Ph
B2010: Ar =p-Me0-Ph B2030: Ar =p-Me0-Ph
General. A 0.01 M solution of mCPBA (1.2 equiv.) in CH2C12 was added to
a solution of a sulfide (1.0 equiv.) in CI-12C12 (0.5 mL) at 0 C for 30 min.
The reaction
mixture was diluted with saturated NaHCO, (2 mL), extracted with CH2C12 and
Et0Ac,
59
CA 02755266 2011-10-17
dried over Na,SO4 and purified by preparative TLC (80% Et0Ac-hexanes or Et0Ac)
to
afford the products:
B2016. (0.9 mg gave 0.7 mg, 74%)
B2030. (1.0 mg !nye 0.6 mg, 61%) HRMS (FAB): calcd for CõHõOuS +
Na 907.3914. Found: 907.3950.
MeQHO ,
' = '-
H"µµ = 1) TBDPSCI
cc(r-1 =
2) Dess-Martin
3) TBAF
=
M e0,
= ,
'µµs 0 =
9 =
B1793 B1934
B1934. TBDPSC1 (3.0 AL, 12 Amol) was added to a solution of B1793 (1.3
mg, 1.78 Amol), imidazole (10 mg, 166 Amol) and DMF (0.10 mL) at rt. After
stirring
for 1 h, the reaction mixture was diluted with saturated aqueous NaHCO3 (2
mL),
extracted with CH2C12 (3x) and Et0Ac (2x), dried over Na2SO4 and purified by
preparative TLC (5% Me0H-CH,C12) to give the intermediate silyl ether (1.3 mg,
77%).
This material was dissolved in CH.,C12 (0.5 mL) and treated with Dess-Martin
periodinane (10 mg, 24 Amol) for 1.5 h at rt, diluted with Et:0 and filtered
through
Celite. The filtrate was concentrated and purified by preparative TLC (50%
Et0Ac-
hexanes) to afford the diketone intermediate (1.0 mg, 77%), which was
dissolved in
THF (0.5 mL) and treated with 0.02 M TBAF containing 0.01 M imida7ole =
hydrochloride (THF solution, 75 AL, 1.5 Amol) at rt for 15 min. The reaction
mixture
was eluted through a SiO2 column (50% Et0Ac-hexanes to 5% Me0H-CH2C12) and the
desired product was further purified by preparative TLC (5% Me0H-CH2C12) to
afford
B1934 (0.75 mg, 100%). FIRMS (FAB): calcd for C401-15,012 + Na 751.3669.
Found: 751.3690.
_ .
CA 02755266 2011-10-17
Synthesis of B1939:
Meg
o H9 H
0 , 0
H' nBu4NN3
Me 0õ
Me0o=
= = =
B2294 B1922
B1922 Tetra-n-butylammonium azide (0.2 M in DMF, 0.5 ml., 0.10 mmol)
was added to a solution of mesylate B2294 (21.4 mg, 0.026 mmol) in DMF (2 InL)
at
rt. After stirring at 83 C for 3.5 h, the reaction mixture- was cooled to rt,
diluted with
toluene. concentrated and purified by preparative TLC (80% ethyl acetate-
hexanes) to
furnish B1922 (18 mg, 92%).
Meg.
0 II
0 = = Me3P
= .
0õ
I..õ,Me0
= H
1.A.,
H2N
= 0 :
H"'
=
.,(Cr $
Me
=
==.õ(
1 I
B1922 B1939
61
=
CA 02755266 2011-10-17
81939 Mey (1 M in THF) and H:0 (0.8 mL) were sequentially added to a
solution of azide B1922 (24.6 mg. 0.032 mmol) in THF (3.2 mL) at rt. The
mixture
was stirred for 22 h, diluted with toluene, concentrated and purified by flash
chromatography [step gradient. 10% Me0H-Et0Ac followed by Me0H-Et0Ac-30%
aqueous NH,OH (9:86:5)] to provide the desired primary amine (23.3 mg), which
by
IH-NMR contained ¨I% trimethylphosphine oxide. Lyophilization from benzene
and.
standing under high vacuum for 2 d furnished B1939 (20.3 mg, 87%).
Synthesis of Representative B1939 Analogs:
B1930, B1940, B1973, B1987, B1988, B1991, B2003, B2004
Me0,
H9 '
.=== ==,, 0 0 =
= = '- = = 1) Me3P
=
2) RCO2H, EDC
Me0..
B1922 H I= H
RyN
0
'd..
=
B1991 R= I 0
B1930 R=
81940 R= L. JJ Me*
82003 R=
Me0 411111
Me
B1973 R= =0
me.
me=
1
81987 R= \ 82004 R=
Me0
B1930 MO' (1 M in THF, 13 AL, 0.013 mmol) was added to a solution of
B1922 (1.6 mg, 2.1 /mon. THF (400 1.1.1.) and 1120 (100 AL) at it The mixture
was
62
CA 02755266 2011-10-17
stirred for 22 h. diluted with toluene. concentrated. and azeotropically dried
with toluene
(2x) to give the crude amine which was used directly in the next step.
EDC (0.06 M in CH2C12. 100 /IL. 11 Amon was added to a solution of the crude
amine. benzoylforrnic acid (0.8 Trig, 5.3 Amon and CH2C12 (200 AL) at n. After
30 min.
the reaction was quenched with a 1:4 mixture of saturated aqueous NaHCO3-brine
and
extracted with CH-.CI, (5x). The combined extracts were dried over Na.,SO4 and
concentrated and purified by preparative TLC (Et0Ac) to afford B1930 (1.5 mg.
83%
for two steps). HRMS (FAB): calcd for C481-163N013+ Na 884.4197. Found:
884.4166.
B1940 Using the procedure described above for 131930, B1922 was reduced,
coupled with 3-pyridylacetic acid hydrochloride and purified by preparative
TLC
f(Me0H-Et0Ac-30% aqueous NH4OH (9:86:5)) to afford B1940 (0.8 mg, 67 ck for
two
steps). HRMS (FAB): calcd for C41H64N2012+ Na 871.4357. Found: 871.4362.
B1973 Using the procedure described above. B1922 (0.9 mg, 1.2 limo!) was
reduced, coupled with phenylacetic acid and purified by preparative TLC (5%
Me0H-
Et0Ac) to afford B1973 (0.44 mg, 44 % for two steps). HRMS (FAB): calcd for
CõHõNOI, + Na 870.4404. Found: 870.4447.
B1987 Using the procedure described above, B1922 (0.9 mg, 1.2 ,umol) was
reduced. coupled with 3-indoleglyoxylic acid and purified by preparative TLC
(3%
Me0H-Et0Ac) to afford B1987 (0.8 mg, 75 % for two steps). HRMS (FAB): calcd
for
C50H64N2013 + Na 923.4306. Found: 923.4338.
B1991 Using the procedure described above, B1922 (1.0 mg. 1,3 urnol) was
reduced, coupled with 4-chlorobenzoic acid and purified by preparative TLC (3%
Me0H-
Et0Ac) to afford 131991 (0.8 mg, 70% for two steps). HRMS (FAB): calcd for
C471-162CINO1.. + Na 890.3858. Found: 890.3843.
B2003 Using the procedure described above, B1922 (1.0 mg, 1.3 Amol) was
reduced, coupled with 3,4,5-trimethoxybenzoylformic acid and purified by
preparative
TLC (Et0Ac) to afford B2003 (0.7 mg, 56 % for two steps). HRMS (FAB): calcd
for
C511-16NO,, + Na 974.4514. Found: 974.4525.
B2004 Using the procedure described above. B1922 (1.0 mg. 1.3 umol) was
reduced, coupled with 3,4,5-trimethoxybenzoic acid and purified by preparative
TLC (57(
Me0H-Et0Ac) to afford B2004 (0.7 mg, 58 % for two steps). HRMS (FAB): calcd
for C501-169N015 + Na 946.4565. Found: 946.4599.
63
CA 02755266 2011-10-17
Me0,
0 FjH,
" 0
1110 1-1"µ Dess-Martin
=
q =
1,'(=Me
I 0
Me0
= H
0
= 0 =
0
0
0
Me
B1 9 3 0 B1 9 8 8
B1988 Dess-Martin periodinane (1 mg, 2.3 Arno') was added to a solution of
B1930 (0.80 mg, 0.93 Amol) in CH2CI, (500 L) at rt. After 1 h, the reaction
was
diluted with Et20 and filtered through Cate. The filtrate was washed
sequentially with
a 1:9 mixture of saturated aqueous NaHCO3-Na2S203 and brine, dried over
Na,SO4,
concentrated and purified by preparative TLC (80% Et0Ac-hexanes) to afford
B1988
(0.45 mg, 56%). HRMS (FAB): calcd for C48H6IN0I1 + Na 882.4041. Found:
884.4012.
64
CA 02755266 2011-10-17
Synthesis of B2090:
In
CHO
CO2Me
+0
Br
1 0 1 102 103
Compound 103. Indium powder (1.35 g, 11.8 mmol) was added to a
solution of 102 (3.38 g, 17.6 mmol) in DIVIF (20 mL) at rt. After stirring for
30 min,
the reaction mixture was cooled to 0 C. Neat aldehyde 101 (3.72 g, 28.6 mmol)
was
then added and the mixture was stirred overnight while allowing the
temperature to
warm to it The reaction mixture was recooled to 0 C and then quenched
carefully with
saturated aqueous NII4C1 (100 mL). After stirring for 30 min, the resulting
mixture
was extracted with Et20 (3x), dried over Na2SO4, concentrated and purified by
column
chromatography (10% to 20% Et0Ac-hexanes) to give pure crystalline 103 (2.20
g,
59%).
Me e Me, fsph .¨SPh
HSPh
- 0 0 +
0-
103 104 105
Compound 104. Et1N (72pL, 0.51 pmol) was added to a solution of 103 (1.09 g,
5.13 mmol) and thiophenol (0.63 inL, 7.16 mmol) in CH2Cl2 and the resulting
mixture
was stirred at 0 C for 1 h. Filtration through SiO2 gave a mixture of 104 and
105,
which after MPLC (15% to 20% Et0Ac-hexanes) afforded 104 (0.53 g, 32%) and
105 (0.92 g, 56%).
CA 02755266 2011-10-17
Me SPh Me SPh
DIBALH
OH
6
1 0 4 1 0 6
Compound 106. DIBALH (I M in toluene, 3.28 mL, 3.28 mmol) was added
to a solution of 104 (0.53 g, 1.64 mmol) in toluene (10 mL) at -78 C and the
mixture
was stirred at
-78 C for 10 min. The reaction was quenched by careful addition of Me0H (0.40
mL,
9.84 mmol) and H20 (0.17 mL, 9.84 mmol). warmed tort and stirred for 20 min.
The
white suspension was filtered through a mixture of Celite and SiO, with 1:1
CH,C12-
Et20 and concentrated to give 106 (0.53 g, 100%) as an oil.
,
1) Ph3P=CHCO2Et Me. Ch
02Et
2) DBU
106 107
Compound 107. A mixture of 106 (0.53 g, 1.64 mmol) and ethyl
(triphenylphosphoranylidene)acetate (1.15 g. 3.29 mmol) in toluene (10 mL) was
heated to 80 C for 15 h. The mixture was cooled to rt and DBU (25 L. 0.16
mmol)
was introduced. The mixture was heated to 80 C for 1.5 h, cooled to rt.
concentrated
and purified by column chromatography (10% to 20% Et0Ac-hexanes) to give 107
(0.54 g, 83%) as an oil (3:1 ratio of a:13 isomers).
Me:Pch CPh
mCPBA
02Et 0 02Et."".
66
CA 02755266 2011-10-17
107 108
Compound 108. A solution of mCPBA (-55%. 450 mg in 4.5 mL CH:Clõ
1.44 mmol) was added to a solution of 107 (0.54 g, 1.36 mmol) in CH2C12 (10
mL) at
-78 C. The reaction mixture was diluted with saturated aqueous NaHCO3 (50
mL),
H,0 (10 mL), and Et,0 (60 mL) and then warmed to rt. The separated aqueous
layer
was extracted with Et0Ac (4x) and the combined organic phases were dried over
Na,SO4, concentrated and purified by column chromatography (50% Et0Ac-hexanes)
to give 108 (0.51 g, 92%) as an oil.
Ac
Ma ,COPh Mat5Ph
Ac20
CO2Et 02Et
0 0
6
108 109
Compound 109. A mixture of 108 (0.51 g, 1.24 mmol) and Na0Ac (1.00 g, 12.4
mmol) in Ac,0 (10 mL) was stirred at 140 C for 12 h, cooled to rt and then
concentrated. The residue was partitioned between saturated aqueous NaHCO3 (20
mL)
and Et20 (30 mL), and stirred vigorously at rt for 30 min. The separated
aqueous layer
was extracted with Et20 (2x), and the combined organic phases were dried over
Na,SO4, concentrated and purified by column chromatography (5% to 15% Et0Ac-
hexanes) to give 109 (0.41 g, 73%) as an oil.
Ac sph
1) K2003 Me OH
CO2Et
0
6 2) NaE3H4
109 110
Compound 110. A mixture of 109 (0.41 g, 0.91 mmol and K2CO3 (44.3 mg. 0.31
mmol) in Et0H (5 mL) was heated to 60-70 C for 1 d. After cooling to rt. the
reaction
mixture was concentrated and eluted through a SiO2 column (10% to 20% Et0Ac-
67
= - .
CA 02755266 2011-10-17
hexanes) to give the partially purified aldehyde intermediate. This' matenal
was
dissolved in Et0H (2.5 mL), treated with NaBH, (50 mg, 1.32 mmol) and stirred
at n
for 30 min. The mixture was concentrated and purified by column chromatography
(40% Et0Ac-hexanes) to give 110
(181 mg. 66%).
OHrOMPM
MPMOTC1
0 2
1 1 0 1 1 1
Compound 111. BF,(.0Et, (0.05 M in CH2Cl2, 175 AL, 8.75 Amol) was
added to a solution of 110 (181 mg, 0.60 mmol) and p-methoxybenzyl 2,2,2-
trichloroacetimidate (0.50 mL, 1.80 mmol) in CH2C12 (5 mL) at 0 C. The
resulting
mixture was stirred for 1.5 h at 0 C and for 2 h at rt until the reaction was
complete.
The mixture was quenched with saturated aqueous NaHCO3 (25 mL) and extracted
with
Et,0 (5x). The combined organic phases were dried over Na2SO4, concentrated
and
purified by column chromatography (CH2Cl2 and then 20% Et0Ac-hexanes) to give
semi-pure 111 (0.37 g, >100%) as an oil.
Me.õ rOMPM Ts0H MerOMPM
OH
111 112
Compound 112. A mixture of 111 (0.37 (2. max.= 0.60 mmol) and
Ts011.1120 (36 mg) in Et0H (5 mL) was stirred initially at rt overnight and
then at 60
C for 1 h. Additional Ts01-14120 (31 m2) was added at rt and the reaction
mixture was
stirred for! h at rt. The mixture was then concentrated, quenched with
saturated
aqueous NaHCO, and extracted with Et0Ac (5x). The combined organic phases were
68
CA 02755266 2011-10-17
dried over Na,SO4. concentrated and purified by column chromatography (20% to
50%
Et0Ac-hexanes and then 5cic Me0H-CH,C12) to give 112
(121 mg, 53%) as an oil along with recovered 111 (49 mg. 21%).
Me rOMPM Me ,¨OMPM
TBSOTf
,=
HO õ --"CO2Et
OH TBSO
112 113
Compound 113. TBSOTf (250 AL, 1.09 mmol) was added to a solution of
112 (121 mg, 0.32 mmol) and Et3N (176 AL, 1.26 mmol) in CH2Cl2 at 0 C and the
resulting mixture was stirred for 25 min. The reaction was quenched with
saturated
aqueous NaHCO3 (15 mL) and the separated aqueous layer was extracted with
ether
(3x). The combined organic phases were dried over Na2SO4, concentrated and
purified
by column chromatography (5% to 10% Et0Ac/hexanes) to give 113 (165 mg, 85%)
as an oil.
Me rOMPM Me ,¨OMPM
DIBALH
)'""=,-0O2Et
TBSO_"'s
TBSO TBSO
113 1 1 4
Compound 114. DIBALH (1 M in toluene, 0.54 mL, 0.54 mmol) was added
to a solution of 113 (165 mg, 0.27 mmol) in toluene (5 mL) at -78 C and the
resulting
mixture was stirred at -78 C for 10 min. The reaction was quenched by careful
addition of Me0H (65 AL, 0.81 mrnol) and 1-120 (29 p.L, 0.81 mmol), warmed
tort and
stirred for 25 min. The white suspension was filtered through Celite with 1:1
CH2C12-
Et,0. Concentration and purification by column chromatography (10% to 20%
Et0Ac-
hexanes) gave 114 (153 mg, 100%) as an oil.
69
CA 02755266 2011-10-17
Me OMPM
TBSO
=
0 =
Hs'
HO
Q =
A
uõ.
I 0
114 B2090
B2090. In a manner similar to that described in Scheme 6 for the synthesis of
B1794, intermediate 114 was converted to B2090. FIRMS (FAB): calcd for
C19H560õ+Na 723.3720. Found: 723.3731.
Me
0 = IH,s, 0
HO
=
6,
Me, "
õ 0 0
H2N----'sµ 0 '4.
OH 0
z 0
B2090 - B2136
B2136. In a manner analogous to that of B1939, B2090 was converted to
B2136. HRMS (FAB): calcd for C91-15õNO,0+ Na 722.3880. Found: 722.3907.
CA 02755266 2011-10-17
Synthesis of B2039/B2043:
Me(14,f-OMPM
TBDPS to.= Y HO (
TBAF
IIIXMe
OPv I,
".' o
X2318 201
Diol 201 TBAF (1 M in THF, 383 AL, 0.383 mmol) was added to a solution
of X2318 (350-LS-218 )(80.8 mg, 0.0765 mmol) in THF (7 mL) and stirred at n
for 16 h. After partial concentration, the residue was loaded directly onto a
SiO2
column packed using 30% Et0Ac-hexanes. Gradient elution (30% Et0Ac-hexanes to
Et0Ac) furnished diol 201 (49.7 mg, 92%).
meckõ_/-0MPM
Me04.¨OMPM
HO ,,, OHCõ,1J.õ
0
SXIIIXMe
Na104
is' Me
Pv
201 202
Aldehyde 202 A mixture of diol 201 (49.7 mg, 0.0707 mmol), NaIO, (100
mg, 0.47 mmol), Me0H (10 mL) and H20 (2.5 mL) was stirred at rt for 30 min.
H20
was added and the mixture was extracted with CH2Cl2 (4x). The combined organic
extracts were dried over Na,SO4. concentrated and purified by column
chromatography
(30% Et0Ac-hexanes) to provide aldehyde 202 (41.7 mg, 88%).
71
CA 02755266 2011-10-17
moqompm MeV-0MM
HO .
OHC.,õ,..( ==.,.
- p-F-PhMgBr F
(..,,
.yõ'
rs,ss ,..Me c Me
1õ,,,p0Pv
202 203
Alcohol 203 4-Fluorophenylmagnesium bromide (2 M in Et20, 155 AL,
0.31 mmol) was added to a solution of aldehyde 202 (41.7 mg, 0.062 mmol) in
THF
(6 mL). After 15 min at rt, the reaction was quenched with saturated aqueous
NH4C1
and extracted with CH2C12 (4x). The combined organic extracts were dried over
Na,SO4, concentrated and purified by preparative TLC (40% Et0Ac-hexanes) to
provide alcohol 203 (32.4 mg, 68%) as a 1:1 mixture of C34 isomers. The minor
undesired C27 isomer was separated at this stage and was also isolated as a
1:1 mixture
of C34 isomers (8.4 mg, 18%).
mead¨OMPM Mead¨OMPM
H. = TBS= '=
TBSOT1
F F
5
70Pv
203 204
Ether 204 Et3N (18 AL, 0.13 mmol) and TBSOTf (15 ItL, 0.063 mmol) were
added to a solution of alcohol 203 (32.4 mg, 0.042 mmol) in CH2C12 (5 mL) at 0
C.
After 20 min the reaction was quenched by the addition of saturated aqueous
NH4CI and
extracted with CH2Cl2 (3x). The combined organic extracts were dried over
Na2SO4.
concentrated and purified by column chromatography (20% Et0Ac-hexanes) to
provide
ether 204 (33.1 mg. 89c).
72
_
CA 02755266 2011-10-17
Meg Me0, rOMPM
TBS='= __ (MPM TBS= "-
LAH
0 = 0/ .'2.- 0 ' 0) 'i
F F
10"Me
I 0 I. 0 Me
OPv OH
204 205
Alcohol 205 LAH (1 M in THF, 113 AL, 0.113 mmol) was added dropwise
to a solution of ether 204 (33.1 mg, 0.0375 mmol) in Et,0 (10 mL) at 0 C.
After 20
mm, H20 and 1 M NaOH were added and the mixture was stirred at rt for 10 min.
Filtration through Celite, concentration and purification by column
chromatography
(40% Et0Ac-hexanes) furnished alcohol 205 (28.4 mg, 95%).
Me0 ¨, OMPM MeR OMPM
TBS= - TBS= =
0
MMTrCI
:
_ ..... ,_
F 7.,... - F
(,?.,,
10"s r 'Me
OH
1õ,,,5._õ...õ,..,,¨,
OMMTr
205 206
Ether 206 Diisopropylethylamine (31 ilL, 0.18 mmol) and MMTrCI (22 mg,
206 as a -1.5:1 mixture of C34 epimers (45 mg, quant), which contained a small
73
CA 02755266 2011-10-17
me0 (¨OH
TBS= - TBS=
DDQ
F
I.Me I Me
OMMTr OMMTr
206 207
Alcohol 207 DDQ (40 mg, 0.18 mmol) was added to a solution of ether 206
(37 mg, 0.034 mmol) in CH2C12 (4 mL) and a 1:10 mixture of /13u0H:pH 7
phosphate
buffer (2 mL) at 0 C. The mixture was stirred vigorously in the dark for 15
min.
Three additional portions of DDQ (40 mg, 0.18 mmol) were added at 10 inin
intervals,
then the reaction was diluted with saturated aqueous NaHCO3 and extracted with
CH2Cl2 (3x). The combined organic extracts were washed with brine, dried over
Na,SO4, concentrated and purified by preparative TLC (30% Et0Ac-hexanes) to
provide alcohol 207 (19.2 mg, 59%) as well as recovered ether 206 (9.7 mg,
26%).
Me00,---OHMeQ OMs
TBS= TBS=Z
msc,
I Me
OMMTr
207 208A and 208B
Mesylates 208A and 208B Et3N (19 AL, 0.13 mmol) and Ms20 (10 mg,
0.056 mmol) were sequentially added to a solution of alcohol 207 (21.3 mg,
0.022
mmol) in CH,C12 (6 mL) at 0 C. After 30 min, saturated aqueous NaHCO3 was
added
and the mixture was extracted with CH2C12 (3x). The combined extracts were
washed
with brine, dried over Na,SO4. concentrated and purified by preparative TLC
(30%
Et0Ac-hexanes) to provide mesylates 208A (11.7 mg, 51%) and 208B (6.5 mg,
28%) as single C34 isomers.
74
CA 02755266 2011-10-17
MeQ 1¨OMs
TBSO
F
I lo Me
Y\\OMMTr M ea,
He
= ===,,, 0
Oti,õ =
208A and 208B F
= A
'Me `-'==
B2039, B2043
B2039 and B2043. In a manner similar to that described in Scheme 6 for the
synthesis of B1794, both diastereomers 208A and 208B were independently
converted to B2039 and B2043. HRMS (FAB): calcd for CõHõFOõ + Na
817.3939. Found: for B2039 817.3896, B2043 817.3910.
Synthesis of B2086, B2088, B2091
MeQ.4, DBn. 1) Na104 Me0..e)Bn
HOA.õ= ¨Pv
2) NaBH4 HO =
10a, 10b X- 20
Alcohol X-20 Nal , (1.16g. 5.4 mmol) was added to a solution of diols 10
a, b (1.19 g, 3.0 mmol) in Me0H-Hp (4:1.75 rnL) at 0 C. The reaction mixture
was
allowed to warm to rt. After stirring for 40 min. the mixture was diluted with
Et0Ac.
filtered through Celite. concentrated. and partitioned between brine and
CH2C1z. The
separated aqueous layer was extracted with CH:C1.2 (2x). The combined orgahic
layers
were dried over Na,S03 and concentrated to furnish the crude aldehyde
intermediate.
NaBH, (228 mg, 6.0 mmol) was added to a solution of the aldehyde in Me0H-
Etp (1:1. 40 mL) at 0 C. The mixture was stirred for 30 min. carefully
quenched with
CA 02755266 2011-10-17
saturated aqueous NH,C1. stirred for 20 min at rt and extracted with CH,CI,
(3x). The
combined extracts were dried over Na,S0,. concentrated and purified by flash
chromatography (40% to 50% Et0Ac-hexanes) to afford alcohol X-20 (1.02 g93%
for two steps).
Meg, ,111Bn TBSCI Megi )011Bn
HO"'µ.c Pv TBSOc).'"" Pv
X-20 21
Silyl ether 21 Imidazole (0.94 g, 13.9 mmol) and TBSC1 (0.59 g, 3.89
mmol) were added sequentially to a solution of alcohol X-20 (1.02 g, 2.78
mmol) in
DMF (10 mL) at rt. After 14 h, the reaction mixture was diluted with saturated
aqueous
NH4C1 and extracted with Et0Ac (3x). The combined organic extracts were washed
with H20, brine, dried over Na,SO4, concentrated and purified by flash
chromatography (5% to 15% Et0Ac-hexanes) to afford silyl ether 21 (1.3 g,
98%).
MeQ Sri H2 Meg/ H
PO(OH)2/C
TBSO'¨'-="µ.(0 ..""-) Fav
21 22
Alcohol 22 A mixture of Pd(OH):,. (20%. 0.8 g), silyl ether 21(1.3 g, 2.70
mmol) and Et0Ac (30 mL) was stirred for 1 h under 1 atm 1-12 at rt, filtered
through
Celite, concentrated and purified by flash chromatography (20% to 40% Et0Ac-
hexanes) to afford alcohol 22 (0.96 g, 91%).
1) TPAP MeQ,
TBSCI"-'"=."(0)'''Pv 2) Tebbe
3) 9-BBN
22 25
76
CA 02755266 2011-10-17
Alcohol 25 4-Methylmorpholine N-oxide (980 mg. 8.4 mmol) and TPAP
(131 mg, 3.26 mmol) were added sequentially to a solution of alcohol 22 (1.78
g, 4.6
mmol) in CH,Cl2 (45 mL) at it A cold bath was necessary to control the
exotherm.
After 20 min, the reaction mixture was diluted with hexanes, filtered through
a short
Tebbe reagent (14.9 mL, 9.0 mmol) was added over 10 min to a solution of the
crude ketone in THF (60 rnL) at 0 C. After 20 min, the reaction mixture was
poured
into Et,0 (100 mL) that was precooled to -78 C, quenched by slow addition of
H20
(30 mL), warmed to it, stirred for 30 min and extracted with Et,0 (4x). The
combined
9-BBN (0.5 M in THF, 11.6 mL, 5.8 mmol) was added to a solution of the
olefin in THF (15 mL) at 0 C. The reaction mixture was allowed to warm to rt,
stirred
Meg õ¨OH 1) Swem Meg (¨OH
0,.õ.=(;),õõOPµf
TBS 2) Et3N TBSCrs'". çPv
3) Na BH4
25 26
Alcohol 26 Using the procedure previously described, alcohol 25 (604 mg,
1.49 mmol) was sequentially oxidized, isomerized, and reduced. Purification by
flash
Meg (-0F1 MPMOTCI Meg (---OMPM
TBSO-s'..c).""=-7 Pv
77
CA 02755266 2011-10-17
26 27
MPM-ether 27 BF,=0Et2 (0.05 M in CH2C12. 270 AL, 0.013 mmol) was
added to a solution of alcohol 26 (545 mg, 1.35 mmol) and MPM-trichloroimidate
(1.14 g, 4.0 mmol) in CH2C12 (40 mL) at 0 C. After 1 h. the reaction was
quenched
with saturated aqueous NaHCO3, extracted with CH2Cl2. dried over Na2SO4,
concentrated and purified by flash chromatography (10% to 15% Et0Ac-hexanes)
to
afford MPM-ether 27 (580 mg, 82%).
MeQd¨OMPM LAH MeQd¨OMPM
________________________________________ ..
TBSO"¨''s 0 'F1
27 28
Alcohol 28 LAH (1 M in THF, 1.9 mL, 1.9 mmol) was added to a solution
of MPM-ether 27 (580 mg, 1.11 mmol) in Et20 (100 mL) at 0 C. After 30 min, the
reaction was quenched carefully with H10 (0.5 mL), and 1 N aqueous NaOH (0.5
mL),
stirred for 1 h at rt, filtered through Celite, concentrated and purified by
flash
chromatography (30% to 50% Et0Ac-hexanes) to afford alcohol 28 (460 mg, 95%).
----
Meg, OMPM 1) Swern Me0,.,
r_OMPM
________________________________________ ¨
) .-,-
TBSO"'' 0 '...¨.- F-1 2) Wittig
28 29
Olefin 29 DMSO (441 AL, 6.23 mmol) was added to a solution of oxalyl
chloride (272 AL, 3.12 mmol) in CH2C12 (30 mL) at -78 C. After 15 min, a
solution of
alcohol 28 (458 mg, 1.04 mmol) in CH2C12 (15 mL) was added to the reaction
mixture.
After stirring for 1 h at -78 C, Et3N (1.3 mL, 9.35 mmol) was added. The
reaction
mixture was warmed to 0 C, stirred for 10 min, diluted with saturated aqueous
NH4C1
and extracted with CH2C12 (3x). The combined organic extracts were dried over
Na,SO4, concentrated and filtered through a short SiO2 column (20% to 30%
Et0Ac-
hexanes) to provide the crude aldehyde.
78
CA 02755266 2011-10-17
n-BuLi (1.63 M. 1.4 mL, 2.28 mmol) was added dropwise to a solution of
CH,PPhiBr (815 mg, 2.28 mmol), THF (20 mL) and DMSO (7.5 mL) at 0 C. After 1
h. a solution of the aldehyde in THF (10 mL) was added. The reaction mixture
was
warmed to rt and stirred for 3 h. Saturated aqueous 1\11140 was added and the
mixture
5 was extracted with Et0Ac (4x). The combined organic extracts were washed
with H20,
brine, dried over Na,SO4, concentrated and purified by flash chromatography
(10% to
15% Et0Ac-hexanes) to afford olefin 29 (380 mg, 95% yield for 2 steps).
MeR, OMPm 1) 9-BBN. MeR OMPM
f
10 --%
TBSO's 0 ''' 2) PvCI TBSO.-"(0)'""=-'--
'0Pv
29 31
Compound 31 9-BBN (0.5 M in THF, 6 mL, 3 mmol) was added to a
15 solution of olefin 29 (370 mg, 0.85 mmol) in THF (7 mL) at 0 C. The
mixture was
allowed to warm to rt and stirred for 1 h. After recooling to 0 C, H20 (30
mL), THF
(20 mL), and NaB03.4 1120 (2.8 g) were added. After stirring for 3 h at rt,
the THF
was removed under reduced pressure. The aqueous residue was extracted with
Et0Ac
(4x), dried over Na,SO4, concentrated and purified by flash chromatography
(25% to
20 50% Et0Ac-hexanes) to afford alcohol 30 which was used directly in the
next step.
Pivaloyl chloride (157 L, 1.27 mmol) was added to a solution of alcohol 30 in
CH2C12-pyridine (1:1 mixture, 10 mL) at rt. After 18 h, additional pivaloyl
chloride
(100 AL, 0.81 mmol) was added. After 1 h, the reaction mixture was cooled to 0
C,
quenched with Me0H (0.5 mL), concentrated, diluted with brine and extracted
with
25 CH,C12 (4x). The combined organic extracts were dried over Na,SO4,
concentrated and
purified by flash chromatography (10% to 15% Et0Ac-hexanes) to afford compound
31 (410 mg, 90% for two steps).
Meg_r_i¨OMPM TBAF MeQr_r-OMPM
31 32
79
. .
CA 02755266 2011-10-17
Alcohol 32 TBAF (1 M in THF, 1.14 mL, 1.14 mmol) was added to a
solution of 31 (410 mg. 0.761 mmol) in THE (5 mL) at rt. After 1.5 h. the
reaction
mixture was .concentrated and purified by flash chromatography (40% Et0Ac-
hexanes
to 100% Et0Ac) to afford alcohol 32 (320 mg, 100%).
Meg,d OMPM 1) Dess-Martin _
__________________________________________________ . H?
Mak,
OMPM
2)SnBu3
C34
BF3=Et20
32 33a: C34 a-OH
33b: C34 8-0H
Alcohols 33a and 33b Dess-Martin periodinane (925 mg, 2.18 mmol) was
added to a solution of alcohol 32 (309 mg, 0.727 mmol) in CH2C12 (19 mL) at
it. After
1 h, the reaction was diluted with Et-,O and filtered through Celite. The
filtrate was
washed sequentially with a 1:9 mixture of saturated aqueous NaHCO3-Na2S203 and
brine, dried over Na2SO4, concentrated and purified by flash chromatography
(20% to
30% Et0Ac-hexanes) to afford the desired aldehyde, which was taken immediately
through the next step.
BF3.0Et, (135 AL, 1.1 mmol) was added to a solution of the crude aldehyde,
tri-n-butylallyltin (337 AL, 1.08 mmol) and CH2Cl2 (16 mL) at -78 C. After 1
h, the
reaction was quenched with saturated aqueous NaHCO3 and extracted with CH2C12
(3x). The combined organic extracts were dried over Na,SO4, concentrated and
purified by MPLC (25% to 30% Et0Ac-hexanes) to afford the major, more polar
alcohol 33a (165 mg, 49% for two steps) and the minor less polar product 33b
(90
mg, 27% for two steps).
HQMea..r_r TBSQ OMPM TBSOTf MeCkr_rOMPM
= =
0) ' OPv
33a 34
Compound 34. TBSOTf (163 AL, 0.710 mmol) was added to a solution of alcohol
33a
(165 mg, 0.355 mmol). Et3N (247 pi, 1.78 mmol) and CH2C12 (5 mL) at 0 C.
After 25
min, the reaction was quenched with saturated aqueous NaHCO,, extracted with
CH2C12
_
CA 02755266 2011-10-17
(3x), dried over Na,SO4, concentrated and purified by flash chromatography
(15% to
20% Et0Ac-hexanes) to afford compound 34 (200 mg. 98%).
M eqr_r-OMPM AD MeRr_TOMPM
TBSO -
HO
34 35a and 35b
Diols 35a and 35b 0s04 (0.1 M solution in toluene, 32 AL, 3.2 Arno]) was added
to a solution of K2CO3 (168 mg, 1.22 mmol), K3Fe(CN)6 (400 mg, 1.22 mmol),
(DHQ)2PYR (11 mg, 12 mop, H20 (3.2 mL) and t-BuOH (2.2 mL) at 0 C. Then a
solution of olefin 34 (200 mg, 0.345 mmol) in t-BuOH (1 mL) was added to the
reaction mixture. After 5 h at 0 C, Na,S205=5 1-120 (200 mg) was added. The
reaction
mixture was warmed to rt, stirred for 30 min and extracted with CH2C12 (5x).
The
1 5 combined organic extracts were washed with brine, dried over Na,SO4,
concentrated
and purified by preparative TLC (70% Et0Ac- hexanes) to afford the major, less
polar
diol 35a (118 mg, 56%), and minor, more polar diastereomeric product 35b (74
mg,
35%). The individual diastereomers were each carried forward separately.
Megr_f---OMPM TBSOTf MeC),(--
OMPM
TBS TBS9 -
HO TBS
35a 36
Compound 36. TBSOTf (177 AL, 0.77 mmol) was added to a solution of diol
35a (118 mg. 0.192 mmol), Et3N (267 AL, 1.92 mmol) and CH2C12 (5 mL) at 0 C.
After 25 min, the reaction was quenched with saturated aqueous NaHCO3,
extracted with
CH2C12 (3x). dried over Na2SO4, concentrated and purified by flash
chromatography
(10% to 15% Et0Ac-hexanes) to afford compound 36(161 mg, 100%).
81
CA 02755266 2011-10-17
Meg ¨OMPM LAH M eRr4-
0 MPM
TBS TBS?
TBSOj TBS
0 OH
36 37
Alcohol 37 Using the procedure described previously for the preparation of
alcohol 28, compound 36 (161 mg, 0.192 mmol) afforded alcohol 37 (135 mg, 93%)
after purification by flash chromatography (20% to 40% Et0Ac-hexanes).
MeCZ,r4---OMPMMeg., j¨OMPM
Dess-Ma rtin
TBS TBS? TBS TBS? \-
0
37 38
Aldehyde 38 Dess-Martin periodinane (227 mg, 0.535 mmol) was added to
a solution of alcohol 37 (135 mg, 0.178 mmol) in CH,C12 (5 mL) at rt. After 1
h, the
reaction mixture was diluted with Et,0 and filtered through Celite. The
filtrate was
washed sequentially with a 1:9 mixture of saturated aqueous NaHCO3-Na2S203 and
brine, dried over Na,SO4, concentrated and purified by flash chromatography
(10% to
20% Et0Ac-hexanes) to afford aldehyde 38 (127 mg, 95%).
MeQ OMPM
TBS9 TBS? "?.
sco = CHO MeR
H9 }-101 =
..,õ = =
=
0
38 B2086, B2102
B2086, B2102. Each of the diastereomers obtained above were separately
carried to final product in a manner similar to that described in scheme 6 for
B1794.
Diastereomer 35a afforded B2086. Diastereomer 35b afforded B2102.
82
CA 02755266 2011-10-17
= Me0,
H9 H(?..
HO = = = Na104
H*".
Q =
Me0,
I 0 H HQ '
o 0
= 11".
0
0
: Oõ
I Me
B2086 B2088
112088 Nal , was added to a solution of B2086 (1 mg, 1.29 !mop in Me0H-
1-1,0 (4:1,
1 rnL) at rt. After 30 min, the reaction mixture was diluted with H2O,
extracted with
CH2C12 (6x), dried over Na2SO4, and concentrated to afford B2088 (1.2 mg).
Me0,
HQ "
0== = NaBH4
Ks
9 =
Me
I 0 H
õ,õ
O
-
E =
0
0
O.
I Me
B2088 B2091
B2091 NaBH, (0.013 M in Et0H, 20 AL, 0.27 mop was added to a solution
of B2088 (1 mg, 1.29 Imo!) in Me0H-CH2C12 (4:1Ø5 mL) at -78 C. Additional
NaBH, was periodically added with close monitoring of the reaction by TLC
(total of 220
83
-
CA 02755266 2011-10-17 .
AL of the NaBH, solution was required). The reaction mixture was quenched at 0
C
with saturated aqueous NH,C1. stirred for 20 min at rt and extracted with
CH2C1, (6x).
The combined extracts were dried over Na2SO4, concentrated and purified by
preparative
TLC (7% Me0H-Et0Ac) to furnish B2091 (0.40 mg, 50%).
Synthesis of B1933:
Me0MPM 9-BBN Meg , 1DMPM
" 0 =
302 303
Alcohol 303 9-BBN (0.5 M in THF, 23 mL, 0.012 mol) was added
dropwise over 30 min to a solution of alkene 302 (1.51 g, 0.00386 mol) in THF
(40
mL) at 0 C. After stirring at rt for 80 min, the mixture was cooled to 0 C
and H,0 (80
mL) was cautiously added followed by NaB03=4 H,0 (4.2 g, 0.027 mol). The
mixture
was stirred vigorously at rt for 2.3 h, then extracted with Et0Ac (3x). The
combined
organic extracts were washed with brine, dried over Na,SO4, concentrated and
purified
by column chromatography (50% Et0Ac-hexanes) to provide alcohol 303 (1.37 g,
87%).
Meg,MPM
,_. JO Swern Me0 MPM
_________________________________________ ,
HO.,.7".,.,õ.=(),õ,,,.....0Pv
OHC"--- ''''' 0 '13v
303 304
Aldehyde 304 Oxalyl chloride (88 AL, 1.00 mmol) was added dropwise to a
solution of DMSO (142 pL, 2.00 mmol) in CH,C12 (20 mL) at -78 C. After 30
min, a
solution of alcohol 303 (137 mg, 0.335 mmol) in CH2C1, (5 mL) was added and
stirred at -78 C for 1 h. BO (420 AL, 3.01 mmol) was added and after 10 min
the
reaction was stirred for 10 min at 0 C at which point saturated aqueous NH3C1
was
added and the resulting mixture was extracted with CH,C12 (3x). The combined
organic
extracts were washed with brine, dried over Na2SO4, concentrated and purified
by flash
chromatography (50% Et0Ac-hexanes) to provide intermediate aldehyde 304 (0.114
g,
84%) which was immediately used in the next step.
84
_ _
CA 02755266 2011-10-17
MeR,MPM 1) CF3TMS
,(
______________________________________ , Meg, JpMPM
OHC----=''s ..."'DPv 2) TBAF
HO
304 305
Alcohol 305 TBAF (1 M in THE, 5 ILL, 0.005 mmol) was added to a
solution of aldehyde 304 (0.114 g, 0.27 mmol) in CF3TMS (0.5 M in THE, 1.1
rnL,
0.54 mmol) at 0 C. After 20 min, a second portion of TBAF (1 M in THE, 100
AL,
0.1 mmol) was added and the mixture was stirred for 10 min at which point
excess
TBAF (1 M in THF, 270 AL, 0.27 mmol) was added idropwise to cleave the
intermediate silyl ether. After 30 min, the mixture was diluted with H20 and
extracted
with Et0Ac (3x). The organic extracts were washed with H20, brine, dried over
Na,SO4, concentrated and purified by column chromatography (50% Et0Ac-hexanes)
to provide alcohol 305 (123 mg, 95%) as an inseparable 1:1 mixture of isomers.
MeO,MPM
, JO
TBSOTf MeR, MPM
,,,, 0Pv
HO TBSO
305 306
Sily1 ether 306 TBSOTf (265 AL, 1.16 mmol) was added to a
solution of alcohol 305 (123 mg, 0.257 mmol) and Et3N (430 AL, 3.08 mmol) in
CH,C1, (8 mL) at 0 C. After stirring at rt for 20 h, saturated aqueous NaHCO,
was
added, and the mixture was extracted with CH2CI, (3x). The combined organic
extracts
were washed with brine, dried over Na,SO4, concentrated and purified by column
chromatography (20% Et0Ac-hexanes) to provide silyl ether 306 (148 mg, 97%).
Mt30,MPM
llfl
LAH
_____________________________________ . MeqJDMPM
TBSO TBSO
_
-
CA 02755266 2011-10-17
306 307
Alcohol 307 LAH (1 M in THE 220 AL, 0.22 mmol) was added dropwise
to a solution of silyl ether 306 (131 mg, 0.22 mmol) in Et20 (5 mL) at 0 C.
After 20
min. H20 and 1 M NaOH were cautiously added. The mixture was stirred at rt 30
min,
filtered through glass wool, concentrated and purified by column
chromatography (50%
Et0Ac-hexanes) to provide alcohol 307 (112 mg, quant.).
MeSMPM 1) Swern
JO Meg MPM
0 2) Wittig
TBSO TBSO
307 309
Alkene 309 Oxalyl chloride (58 AL, 0.66 mmol) was added dropwise to a
solution of DMSO (94 AL, 1.3 mmol) in CH2C12 (10 mL) at -78 C. After 30 min,
a
solution of alcohol 307 (112 mg, 0.22 mmol) in CH2C12 (3 mL) was added. After
1 h,
Et3N (276 AL, 1.98 mmol) was added, and after 10 min at -78 C the reaction
was
stirred at 0 C for 10 min. Saturated aqueous NH4C1 was added and the mixture
was
extracted with CH2C12 (3x). The combined organic extracts were washed with
brine,
dried over Na,SO4, concentrated and purified by flash chromatography (50%
Et0Ac-
hexanes) to provide aldehyde 308 (101 mg, 91%) which was immediately used in
the
next step. .
nBuLi (1.63 M in THF, 200 /IL, 0.33 mmol) was added dropwise to a solution
of CH31Th3Br (118 mg, 0.33 mmol) in THF (3 mL) and DMSO (1.2 mL) at 0 C.
After 70 min, a solution of aldehyde 308 (101 mg, 0.20 mmol) in THF (3 mL) was
added and after 10 min at
0 C, the reaction was stirred at rt for 1 h. Saturated aqueous NH4C1 was
added and the
mixture was extracted with Et0Ac (3x). The combined organic extracts were
washed
with brine, dried over Na,SO4, concentrated and purified by column
chromatography
(20% Et0Ac-hexanes) to provide alkene 309 (90.9 mg, 90%).
86
-
CA 02755266 2011-10-17
Meg, ,OMPM Meg., )0MPM
9-BBN
TBSO TBSO
309 310
Alcohol 310 9-BBN (0.5 M in Tiff, 17 mL, 8.45 mmol) was added
dropwise to a solution of alkene 309 (1.06 g, 2.11 mmol) in THF (30 mL) at 0
C.
After stirring for 2.5 h at rt, the reaction was cooled to 0 C and H20 (60
mL) followed
by NaB02=4 H10 (3.25 g, 21.1 mmol) were cautiously added. The mixture was
stirred
vigorously at it for 2 h, then diluted with Hp and extracted with Et0Ac (3x).
The
combined organic extracts were washed with brine, dried over Na2SO4,
concentrated
and purified by column chromatography (20% to 30% Et0Ac-hexanes) to provide
alcohol 310 (0.920 g, 84%).
Meg, õ C1 MPM
JO Meg )0MPM
Pv
F3õ00= ,, ----,
= 0 -- OH
Cy" "OPv
TBSO TBSO
310 311
Pivaloate 311 A mixture of alcohol 310 (65.8 mg. 0.0126 mmol), pyridine
(61 AL, 0.76 mmol) and Pva (23 AL, 0.189 mmol) in CH2Cl2 (3 mL) was stirred at
rt
for 5 h. A second reaction utilizing alcohol 310 (0.92 g, 1.76 mmol) was run
under
similar conditions and both reactions were combined during the work-up:
saturated
aqueous NH4C1 was added and the mixture was extracted with CH2C12 (3x). The
combined organic extracts were washed with brine, dried over Na2SO4,
concentrated
and purified by column chromatography (20% Et0Ac-hexanes) to provide pivaloate
311 (1.08 g, quant).
MegMPM Meg., H
DDQ
F3C)-="µ*(0).."'=OPv _________________ - F3 ,., =
C's* .OPv
TBSO TBSO
311 312
87
_ _
CA 02755266 2011-10-17 '
Alcohol 312 A mixture of ether 311 (0.811 a. 133 mmol). DDQ (6.1 g, 27
mmol) and 10:1 tBuOH: pH 7 phosphate buffer (42 mL) in CH2Cl2 (84 ml.) was
stirred
vigorously in the dark at rt for 1.5 h, at which point additional DDQ (1.0g.
4.4 mmol)
was added. After 1 h, saturated aqueous NaHCO3 was added and the mixture was
extracted with CH2CI, (4x). The combined organic extracts were washed
successively
with saturated aqueous NaHCO3 and brine, dried over Na.,SO4, concentrated and
purified by column chromatography (20% Et0Ac-hexanes) to provide alcohol 312
(0.56 g, 87%) as well as recovered starting material 311 (97 mg, 12%).
MeO H MeQ
Swern
,õ=
= o OPv F3 = .
TBSO TBSO
312 313
Ketone 313 Oxalyl chloride (21 AL, 0.12 mmol) was added dropwise to a
solution of DMSO (34 AL, 0.48 mmol) in CH2C12 (3 mL) at -78 C. After 1 h, a
solution of alcohol 312 (39.4 mg, 0.081 mmol) in CH2C1, (1.5 mL) was added and
the
mixture was stirred for 1.5 h. Et3N (100 AL, 0.73 mmol) was added, and after
10 min
the mixture was warmed to 0 C. Saturated aqueous NH4C1 was added and the
mixture
was extracted with CH2Cl2(3x). The combined organic extracts were washed with
brine, dried over Na,SO4, concentrated and purified by flash chromatography
(30%
Et0Ac-hexanes) to provide ketone 313 (36.6 mg, 93%) which was used immediately
in the next step.
Tebbe
= (0) OPv - F3 =
TBSO TBSO
313 314
Alkene 314 Tebbe reagent (-0.65 M in toluene, 720 AL, 0.47 mmol) was
added dropwise to a solution of ketone 313 (151 mg, 0.31 mmol) in THF (5 mL)
at 0
C. After 15 min. H20 was cautiously added and the mixture was extracted with
Et0Ac
(3x). The combined organic extracts were washed with brine, dried over Na2SO4,
88
_
_
CA 02755266 2011-10-17
concentrated and purified by column chromatography (10% Et0Ac-hexanes) to
provide
alkene 314 (139 mg, 93%).
Meg, j, MeQ
õ=
9-BBN
õ= ),õ
== (-) ".-0Pv
TBSO TBSO
314 315
Alcohol 315 9-BBN (0.5 M in THF, 6.0 mL, 2.9 mmol) was added
dropwise to a solution of alkene 314 (468 mg, 0.97 nunol) in THF (10 mL) at 0
C.
The mixture was stirred at rt for 2 h at which point additional 9-BBN (0.5 M
in THF,
500 AL, 0.25 mmol) was added. After 2.5 h, the mixture was cooled to 0 C and
11,0
(10 mL) followed by NaB03=4 1120 (1.5 g, 9.7 mmol) were cautiously added. The
mixture was stirred vigorously at It for 5 h, diluted with H,0 and extracted
with Et0Ac
(3x). The combined organic extracts were washed with brine, dried over Na,SO4,
concentrated and purified by column chromatography (gradient 20% to 30% Et0Ac-
hexanes) to provide alcohol 315 (0.47 g, 97%).
MeQ, OH 1) Swern MeQ,,_/-0H
.==
2) Et3N
0 OPv
315 316
Alcohol 316 Oxalyl chloride (246 AL, 2.82 mmol) was added dropwise to a
solution of DMSO (400 AL, 5.64 mmol) in CH,C), (40 mL) at -78 C. After 1 h, a
solution of alcohol 315 (0.47 g, 0.94 mmol) in CH2C12 (10 mL) was added and
the
mixture was stirred for 1 h. Et3N (1.2 mL, 8.5 mmol) was added, and after 10
min the
mixture was warmed to 0 C and stirred for 10 min. Saturated aqueous N1-14C1
was
added and the mixture was extracted with CH,C12 (3x). The combined organic
extracts
were washed with brine, dried over Na2SO4 and concentrated. The crude aldehyde
was
stirred in CH,C1, (20 mL) and Et3N (2 mL) at It overnight. Saturated aqueous
NH4C1
was added and the mixture was extracted with CH,C12 (3x). The combined organic
extracts were washed with brine, dried over NaõSO4, concentrated and purified
by flash
89
CA 02755266 2011-10-17 ,
chromatography (30% Et0Ac-hexanes) provided the epimerized aldehyde which was
immediately dissolved in 1:1 Etp:Et0H (10 mL) and cooled to 0 C. NaBH, (35 mg.
0.94 mho]) was added and after 10 min the reaction was quenched with saturated
aqueous NH4C1. The mixture was extracted with Et0Ac (3x) and the combined
organic
extracts were washed with brine, dried over Na2SO4. concentrated and purified
by
column chromatography (30% Et0Ac-hexanes) to provide alcohol 316 (0.410 g, 87%
yield for 3 steps).
Meq, OH M ea ., rOMPM
s' c '' OPvf
MPMOTCI ,
TBSO TBSO
316 317
Ether 317 Alcohol 316 (60.7 mg, 0.12 mmol) and MPMOTCI (0.10 g, 0.36
mmol) were combined, azeotroped from toluene (3x) and dried under high vacuum
overnight. CH2C12 (3 rriL) was added and the mixture was cooled to 0 C.
BF3.0Et,
(approx. I AL, 0.01 mmol) was added and after stirring for 10 mm the reaction
was
quenched with saturated aqueous NHICI. The mixture was extracted with CH,C12
(3x)
and the combined extracts were washed with brine, dried over Na,SO4,
concentrated
and purified by preparative TLC (30% Et0Ac-hexanes) to provide ether 317 (55.4
mg,
74%).
Me0--OMPM
MeRcfOMPM
LAH
TBSO TBSO
317 318
Alcohol 318 LAH (1 M in THF, 104 AL, 0.104 mmol) was added dropwise
to a solution of ether 317 (54 mg, 0.087 mmol) in Et20 (5 mL) at 0 C. After
30 min,
1120 and 1 M NaOH were cautiously added. The mixture was stirred at rt for 10
min,
filtered through glass wool, concentrated and purified by column
chromatography
(30%-50% Et0Ac-hexanes) to provide alcohol 318 (45.5 mg. 98%).
_ _
CA 02755266 2011-10-17
Meg,. f-OMPM MeV¨OMPM
Swern
F3 =(
0 ______________________________________ -
TBSO TBSO
318 319
Aldehyde 319 Oxalyl chloride (11 tiL, 0.13 mmol) was added dropwise to a
solution of DMSO (18 AL, 0.25 mmol) in CH2C1, (2 mL) at -78 C. After 1.8 h, a
solution of alcohol 318 (22.6 mg, 0.042 mmol) in CH2C12 (1 mL) was added and
the
1 0 mixture was stirred for 1 h. Et3N (53 ILL, 0.38 mmol) was added and
after 10 min, the
reaction was warmed to 0 C and stirred 10 min. Saturated aqueous NILCI was
added
and the mixture was extracted with CH,Cl2 (3x). The combined organic extracts
were
washed with brine, dried over Na,S03, concentrated and purified by flash
chromatography (20% Et0Ac-hexanes) to provide aldehyde 319 (21.7 mg, 97%).
MeCt¨OMPM
õsUo F3C ,õCHO
,,y"......0 Me0
TBSO
="0
0
HO
Q
. 0.,
s
319 B1 9 3 3
B1933. In a manner similar to that described in Scheme 6 for the synthesis of
B1794, intermediate 319 was converted to B1933. HRMS (FAB): calcd for
C4,1157F30õ + H 783.3931. Found: 783.3940.
91
_ _
CA 02755266 2011-10-17
Me0
HO OH 0
1) Na104
' =
= = 2) CF3TMS
Me 3) TBAF
4) Dess-Marlin
Meg
0 '
F3CA''' 0
B1896, B1897
'Me =
B 1942
B1942. A mixture of B1896/B1897 (2 mg, 2.73 mop. NaI04 (35 mg, 0.16 mmol),
Me0H (0.8 mL) and H,0 (0.2 mL) was stirred at rt for 30 min. The reaction
mixture
was then diluted with H,0 (3 niL) and extracted with CH2Cl2 (6x) and Et0Ac
(2x).
The combined organic phases were dried over Na,SO4 and purified by column
chromatography (5% Me0H- CH,Cl2) to give the desired aldehyde.
This material was dissolved in THF (0.1 mL), cooled to at 0 C and treated
with
0.5 M CF3TMS in TI-IF (30 AL, 15 mmol) followed by 0.05 M TBAF in THE (5 mL,
0.025 mmol). After stirring for 30 min, the reaction mixture was diluted with
saturated
aqueous NaHC03 (2 mL) and 1120 (1 mL), extracted with Et0Ac (6x), dried over
Na,SO4, filtered and concentrated to give the crude bis-TMS ether.
This material was dissolved in MT (0.5 mL) and treated with 1 M TBAF in
1 5 THF containing 0.5 M imidazole hydrochloride (8 AL, 8 Arno') at rt for
30 min. The
reaction mixture was eluted through a SiO, column (50% Et0Ac-hexanes to Et0Ac)
to
afford the diol intermediate.
A mixture of this product and Dess-Martin periodinane (10 mg, 24 mmol) in
CH2CI, (0.5 niL) was stirred at rt for 1 h, diluted with Et20 (5 mL) and
filtered through
Celite. The filtrate was concentrated and purified by preparative TLC (50%
Et0Ac-
hexanes) to furnish B1942 (1.5 mg, 72% for 5 steps). HRMS (FAB): calcd for
C40H53F3011 + H 767.3516. Found: 767.3542
Synthesis of B2070/B2073:
92
CA 02755266 2011-10-17
Meg, ,IDOMPM 1) Na104 MeR, jIMPM
2) CH2=-CHCF2Br
In F F
X 4 0 0 401
Alcohol 401 A mixture of NaI04 (375 mg, 1.74 mmol), X400 (674 mg,
1.58 mmol), Me0H (16 mL) and ILO (4 mL) was stirred at rt for 1 h. After
dilution
with H2O, the mixture was extracted with CH-.C1, (4x) and the combined organic
extracts were dried over Na2SO4, concentrated and purified by flash
chromatography
(30% Et0Ac-hexanes) to provide the intermediate aldehyde (570 mg), which was
immediately dissolved in DMF (15 mL). Indium
(275 mg, 2.4 mmol) and 3-bromo-3,3-difluoropropene (240 AL, 2.4 mmol) were
added and after stirring at rt for 17 h, Hp and 0.1 M HO were added. The
mixture
was extracted with Et0Ac (3x) and the combined organic extracts were washed
successively with 11,0 and brine, dried over Na,SO4, concentrated and purified
by
column chromatography (20% to 30% Et0Ac-hexanes) to provide alcohol 401 as a
1:1
mixture of C34 isomers (605 mg, 81% for 2 steps).
MeR, MPM 1) 0s04 Meq. FIMPM
.=== =====( )"===. Pv
' 0 2) Na104 0
F F 3) NaBH4 F F
401 402
Diol 402 A mixture of 0s04 (1 xstal), alcohol 401 (605 mg, 1.28 mmol), 4-
methyl-morpholine N-oxide (0.45 g, 3.84 mmol), acetone (30 mL) and H20 (6 mL)
was stirred at rt for 29 h. Additional 0s04 (3 xtals) and 4-methylmorpholine N-
oxide
(0.1 g, 0.8 mmol) were added and after 2 days saturated aqueous Na,S,O, was
added.
The mixture was extracted with C1-LC12 (6x) and the combined organic extracts
were
dried over Na2SO4 and concentrated. The crude intermediate triol was
immediately
dissolved in 4:1::MeOH:H10 (25 mL) and Nal , (0.41 g, 1.9 mmol) was added.
After
stirring vigorously at rt for 2 h, the mixture was diluted with H20, extracted
with
CH2C12 (3x) and the combined organic extracts were dried over Na2SO4 and
concentrated to provide the intermediate aldehyde which was immediately
dissolved in
1:1 Et0H-Et20 (30 mL) and cooled to 0 C. NaBH, (48 mg, 1.3 mmol) was added
and
93
CA 02755266 2011-10-17
after 20 min the reaction was quenched with H20 and extracted with CH,C12
(4x). The
combined or2anic extracts were dried over Na2SO4. concentrated and purified by
column chromatography (50% Et0Ac-hexanes) to provide diol 402 (485 m2. 80% for
3 steps).
MeQ OMPM MeQ OMPM
TBSOTi TBS?
0 .'". P
F F F F
402 403
Silyl ether 403 TBSOTf (2.3 raL, 10 mmol) was added dropwise to a
mixture of diol 402 (485 mg, 1.0 mmol), Et3N (2.8 mL, 20 mmol) and CH,C12 (30
mL) at 0 C. After stirring for 1 h at n, saturated aqueous INTH4C1 was added
and the
mixture was extracted with CH2C12.(3x). The combined organic extracts were
washed
1 5 with brine, dried over No:SO4, concentrated and purified by column
chromatography
(20% Et0Ac-hexanes) to provide silyl ether 403 (668 mg, 95%).
MeQaMPM M eRdMPM
TBS LAH
-
=
TBSO Co) TBSO 0
F F F F
403
404
Alcohol 404 LAH (1 M in THF, 2.8 mL, 2.8 mmol) was added dropwise to
a solution of silyl ether 403 (668 mg, 0.948 mmol) in Et20 (60 mL) at 0 C.
After 15
min, 1120 and 1 M NaOH were cautiously added. The mixture was stirred at it
for 20
min, filtered through glass wool, concentrated and purified by column
chromatography
(30% Et0Ac-hexanes) to provide alcohol 404 (500 mg, 85%).
MeadMPM Met), QMPM
õ_.1)TBS(1,,, Swern TBS? =
TBSO 0 TBSO---"'.µ 0 ...CHO
F F F F
404 405
94
CA 02755266 2011-10-17
Aldehyde 405 Oxalyi chloride (210 ttL, 2.42 mmol) was added dropwise to
a solution of DMSO (345 //L. 4.84 mmol) in CH2Cl2 (30 mL) at -78 'C. After I
h, a
solution of alcohol 404 (500 mg. 0.806 mmol) in CH2Cl2 (10 mL) was added.
After
40 min. Et,N (1.0 mL, 7.2 mmol) was added. After stirring at -78 C for 10
min, the
reaction mixture was warmed to 0 C and stirred for an additional 10 min.
Saturated
aqueous NH4C1 was added and the mixture was extracted with CH2Cl2 (3x). The
combined organic extracts were washed successively with H.O. brine, dried over
Na_SO, and concentrated. Purification by flash chromatography (30% Et0Ac-
hexanes)
provided aldehyde 405 (486 mg, 98%) which was immediately used in the next
step.
M eqMPM (TBS MeRdMPM ? = Wittig
-
TBSO". 0 CHO TBSO 0
F F F F
405 406
Alkene 406 nBuLi (1.63 M, 860 L, 1.4 mmol) was added dropwise to a
solution of C1-J3PPh2Br (500 mg, 1.4 mmol) in TIM (15 mL) and DMSO (6 mL) at 0
C. After 1 h, a solution of aldehyde 405 (486 mg) in THF (15 mL) was added.
The
reaction mixture was warmed to rt and stirred for 30 min. Saturated aqueous
NH4C1
was added, the mixture was extracted with Et0Ac (3x) and the combined extracts
were
washed successively with H20 and brine, dried over Na,SO4, concentrated and
purified
by column chromatography (20% Et0Ac-hexanes) to provide alkene 406 (450 mg,
93%).
MeQ, PMPM 1) 9-BBN MeQ.,c)MPM
TBS - TBS
TBSO 0 2) PvCI
F F F F
=
406 407
Ester 407 9-BBN (0.5 M in THE 9.0 mL, 4.5 mmol) was added dropwise
to a solution of alkene 406 (0.460 g. 0.746 mmol) in THF (10 mL).at 0 C. After
warming to n, the mixture was stirred for 3 h and two additional portions of 9-
BBN
CA 02755266 2011-10-17
(0.5 M in THF, 3.0 mL. 1.5 mmol) were added at 30 min intervals. The reaction
mixture was recooled to 0 CC. whereupon THE
(10 mL), H20 (10 mL) and NaB03=4 H,0 (1.72 g. 11.2 mmol) were cautiously
added.
The mixture was stirred vigorously at it for 1.5 h. and additional NaB03=4
1120 (1.0 g,
6.5 mmol) was added. After 2 h the mixture was diluted with H,0 and extracted
with
Et0Ac (3x). The combined extracts were washed with brine, dried over Na,SO4,
concentrated and purified by column chromatography (20% to 30% Et0Ac-hexanes)
to
provide the intermediate alcohol (509 mg) which was immediately dissolved in
CH,C12
(10 mL) and treated with pyridine (600 AL, 7.5 mmol) and PvC1 (275 AL, 2.2
mmol).
After 6 h, saturated aqueous was added and the mixture was extracted with
CH,C1, (3x). The combined organic extracts were washed with brine, dried over
Na-,SO4, concentrated and purified by column chromatography (20% to 30% Et0Ac-
hexanes) to provide ester 407 (423 mg, 79% for 2 steps).
Meg DMPM H2 eg(
µ,.1,1,;BS(Z.,, = TBS
TBSO TBSO 0
F F F F
407 408
Alcohol 408 A mixture of ester 407 (11 mg. 0.015 mmol) and Pd(OH)2/C
(10 mg) in Et0Ac (500 ILL) was stirred vigorously under a H2 atmosphere at it
for 6 h.
The mixture was filtered through Celite, concentrated and purified by column
chromatography (30% Et0Ac-hexanes) to provide alcohol 408 (9.4 mg, quant).
megdHMeg
1) Sw ern
TBSI? - TBS
2) Tebbe TBSO "O
F F F F
408 409
Alkene 409 Oxalyl chloride (7 AL, 0.075 mmol) was added dropwise to a
solution of DMSO (11 AL. 0.15 mmol) in CH2C1, (2 mL) at -78 C under N2. After
4()
min, a solution of alcohol 408 (15.2 mg, 0.025 mmol) in CH2C1, (1 mL) was
added
and the reaction was stirred at -78 C for 1 h. Et,N (31 /IL. 0.22 mmol) was
added.
96
CA 02755266 2011-10-17
and after stirring for 10 min the mixture was warmed to 0 C. After 10 min.
the reaction
mixture was quenched with saturated aqueous NH4C1 and extracted with CH2Cl2
(3x).
The combined extracts were washed successively with Hz0 and brine, dried over
Na,S03 and concentrated. After flash chromatography (30% Et0Ac-hexanes), the
intermediate ketone (13 mg) was immediately dissolved in THF (500 AL) and
treated
with Tebbe reagent (-0.65 M in toluene. 62 jiL, 0.040 mmol) at 0 C. After 1.5
h
additional Tebbe reagent (-0.65 M in toluene. 62 AL, 0.040 mmol) was added and
after 10 min H20 and then brine were cautiously added. The mixture was
extracted
with Et0Ac (3x) and the combined organic extracts were washed with brine,
dried over
1 0 Na2SO4, concentrated and purified by column chromatography (10% Et0Ac-
hexanes)
to provide alkene 409 (11.9 mg, 80% for 2 steps).
M ep_ 9-BBN MeQ ..-0H
TBS 2 / _______ TBS? 2 C
TBSO "sss(No
F F F F
409 410
Alcohol 410 9-BBN (0.5 M in THF, 1.5 nth, 0.72 mmol) was added
dropwise to a solution of alkene 409 (0.144 g, 0.242 mmol) in THF (2 mL) at 0
C.
After warming to rt, the mixture was stirred for 3 h. The reaction mixture was
recooled
to 0 C, whereupon THF (2 mL), H20 (2 mL) and NaB03=4 H20 (0.38 g. 2.4 mmol)
were cautiously added. The mixture was stirred vigorously at rt for 4 h.
diluted with
H20 and extracted with Et0Ac (3x). The combined extracts were washed with
brine,
dried over Na2SO4, concentrated and purified by column chromatography (20%
Et0Ac-hexanes) to provide alcohol 410 (0.140 g, 94%).
Meg õ--OH M eg OH
1) Swern
TBS 2
2) Et3N TBSOOQpv
''''
F F 3) NaBH4 F F
410 4 1 1
Alcohol 411 Oxalyl chloride (26 L. 0.30 mL) was added dropwise to a
solution of DMSO (43 AL 0.60 mmol) in CH2Cl2 (4 mL) at -78 C. After 1 h. a
solution of alcohol 410
97
CA 02755266 2011-10-17
(57 mg, 0.093 mmol) in CH2C13 (2 mL) was added. After 45 min.-Et3N (125-4-90
mmol) was added. After stirring_ at -78 C for 10 min. the reaction mixture
was warmed
to 0 C and stirred for an additional 10 min. Saturated aqueous NH,C1 was
added and
the mixture was extracted with CH,C1, (3x). The combined organic extracts were
washed with brine, dried over Na,SOõ and concentrated. The crude product was
dissolved in CH2C12 (4 mL). treated with Et3N (400 ILL) and stirred at it for
15 h.
Saturated aqueous NH,Cl was added and the mixture was extracted with CH2C1,
(3x).
The combined organic extracts were washed with brine, dried over Na2SO4,
concentrated and purified by flash chromatography (30% Et0Ac-hexanes) to
provide
the intermediate aldehyde (48 mg), which was immediately dissolved in 1:1 Et,0-
Et0H
(4 mL), cooled to 0 C and treated with solid NaBH, (-4 mg, 0.09 mmol). After
stirring for 15 min, saturated aqueous NH4C1 was cautiously added and the
mixture was
extracted with Et0Ac (3x). The combined extracts were washed with brine, dried
over
Na,SO4, concentrated and purified by column chromatography (20% to 30% Et0Ac-
hexanes) to provide alcohol 411 (45.6 mg, 80% for 3 steps).
Met), ,¨OH 1) MPMOTCI MeQ_,c
TBS 7 ? TBS
TBS 2) LAH TBSO 0
F F 3) separate isomers F F
411 412A, 412B
412A and 412B Alcohol 411 (120 mg, 0.196 mmol) and MPMOTCI (0.17
g, 0.59 mmol) were combined. azeotroped from toluene (3x) and dried under high
vacuum for 1 h. CH2C12 (9 mL) was added and the mixture was cooled to 0 C.
BF3=0Et, (0.016 M in CH2C12, 125 AL, 0.002 mmol) was added dropwise and after
stirring for 20 min, the reaction was quenched with saturated aqueous NH4C1.
The
mixture was extracted with CH2C1, (3x) and the combined extracts were washed
with
brine, dried over Na,SO4, concentrated and purified by preparative TLC (20%
Et0Ac-
hexanes) to provide the intermediate MPM ether which contained some close-
running
impurities. This material was immediately dissolved in Et20 (10 mL) and
treated with
LAH (IM in THE, 300 AL, 0.300 mmol) at 0 C. After 10 min. H20 and 1 M NaOH
were added, and after stirring for 10 min at it, the mixture was filtered
through Celite,
concentrated and purified by preparative TLC (35% Et0Ac-hexanes) to provide
412A
(49 mg, 39% for 2 steps) as a single C34 isomer and 412B (46 mg, 36% for 2
steps)
as a -9:1 mixture of C34 isomers.
98
CA 02755266 2011-10-17
MeQ
TBS /"-
TBSO " OH Me0,
0 = = 0
F F
'Me
0
==.õ
412A, 412B B2070, B2073
B2070 and B2073. In a manner similar to that described in Schemes 4 and 6
for the synthesis of B1794, intermediates 412A and 4128 were converted to
B2070
and B2073, respectively. For B2070: HRMS (FAB): calcd for C411-158F2012+ Na
803.3794. Found: 803.3801. For B2073: HRMS (FAB): calcd for C411-138F2012+
Na 803.3793. Found: 803.3781
Synthesis of B1963:
MeQ OBn MeQ Bn
HO 1) [0)
H
2) CF3TMS
HO
10a
501
Diol 501 (64) Saturated aqueous NaHCO3 (21 mL) and KBr (89 mg, 0.75
mmol) were added to a solution of diol 10a (1.35 g. 3.4 mmol) in C1-1202 (34
mL). The
mixture was cooled to 0 C, and 4-methoxy-2,2,6,6-tetramethyl-l-piperidinyloxy
(0.05
M in CH2Cl,, 7.45 mL, 0.37 mmol) and Na0C1 (0.07 M in 1-120, 5.6 0.39 mmol)
99
CA 02755266 2011-10-17
were sequentially added. After 1 h, the reaction mixture was quenched with
saturated
aqueous Na,S20õ. diluted with saturated aqueous NaHCO, and extracted with
CH2C1,
(3x). The combined extracts were dried over Na2SO4, concentrated and dissolved
in
THF (21 mL).
After cooling to 0 C, CF3TMS (1.5 g, 10.5 mmol) and TBAF (0.1 M in THF,
680 AL. 0.068 Imo!) were sequentially added. After stirring for 40 min,
additional
TBAF (1 M in THF, 8.3 inL, 8.3 nunol) was added. After 30 min. the reaction
was
quenched with 11,0 and extracted with Et0Ac (3x). The combined organic
extracts were
washed with brine, dried over Na,SO4, concentrated and purified by flash
chromatography (30%, 40%, 50% Et0Ac-hexanes followed by Et0Ac) to afford a 2:1
mixture of diols (553 mg, 35%). Separation by MPLC (1.5% Me0H- CH2C12) gave
the
major, more polar isomer 501 (64) (340 mg, 22%) and the minor, less polar
isomer
(152 mg, 10%).
M eCk jBn
F3 1-4,
" 0 Me0,
HO H9
501 F3 ',., 0 0
0 - =
H"µ
HO
Q. =
O.,
= Me
81963
B1963. In a manner similar to that described in Schemes 4 and 6 for the
synthesis of B1794, intermediate 501 was converted to B1963.
_20
Synthesis of B2320 and Related Analogs
100
CA 02755266 2011-10-17
Me0 Me0
HO "=
Ms0õ.,K
==. = =
R 'N
0 "":
= or RR RA
'NH =
Q .
: 9 0
..... ''me
o I=,õ
82294
These compounds are made by treating B2294 with an appropriate amine in a
solvent
such as methanol for a period of a few hours to several days. Progress of the
reaction
may be monitored by thin layer chromatography. A standard work-up procedure,
well
known to those of skill in the art, provides the desired compounds. The
procedure
below is to prepare ER803868; however this procedure is general and can be
used to
prepare any desired analog.
Synthesis of ER803868
To a solution of B2294, 1.2 mg, in methanol, 0.5 mL, was added morpholine,
0.012
mL. The mixture was stirred for 10 days with additional morpholine, 0.012 mL,
being
added on days 1,2,3, 4 and 8. The mixture was then chromatographed to give 1.4
mg
of the desired compound.
Me0
s 0 - o=
Hs
Q = ,
'
Me
B2320 R = N.N-dimethylamino
B2330 R = N-isopropylamino
B2336 R = N-methylarnino
B2339 R = N-t-butylamino
B2417 R = N-2-hydroxyethylamino
B2418 R = N-piperazinyl
B2489 R = N,N-bis-(2-hydroxyethypamino
101
CA 02755266 2011-10-17
B2490 R = N-1,3-dihydroxy-2-propylamino
B249I R = N-benzylamino
ER803834 R = N-piperidinyl
ER803835 R = N-pyrrolidinyl
ER803836 R = N-3-(R)-hydroxypyrrolidinyl
ER803843 R = N-homopiperidinyl
ER803845 R = N-para-methoxybenzylamino
ER803846 R = N-phenethylamino
ER803851 R = N-2-(S-
hydroxymethyl)pyrrolidinyl
ER803852 R = N-2-(R-
hydroxymethyl)pyrrolidinyl
ER803868 R = N-morpholinyl
ER803869 R = N-ethylamino
ER803870 R = N-imidazoyl
ER803883 R = N,N-diethylamino
ER803884 R = N-para-chlorobenzylamino
D. Pharmacological Activity
Many of the individually disclosed drugs were tested for in vitro and in vivo
activity (see Table 1, below). Screening methods included a standard in vitro
cell
growth inhibition assay using DLD-1 human colon cancer cells (ATCC accession
number CCL 221) in a 96-well microtiter plate format (Finlay, G.J. et al
Analytical
Biochemistry 139:272-277, 1984), a U937 (ATCC accession number CRL 1593)
mitotic block reversibility assay (described below), and in some cases, a LOX
human
melanoma tumor xenograft in vivo growth inhibition assay (see Table 1).
Chemical
stability to esterase degradation was also examined.
U937 Mitotic Block Reversibility Assay
U937 human histiocytic lymphoma cells were added to 75 cm tissue culture
flasks as 2.5 x 106 cells in 22.5 rriL of RPMI Medium 1640 containing 10%
Fetal
1 5 Bovine Serum. Cells were allowed to adapt to the culture during 36 h of
incubation at
37 C in a humidified atmosphere containing 5% CO2. Each test drug was then
added to
a flask as 2.5 mL of 10x final concentration. Final concentrations achieved
were 0.1-
1000 nM, in half log-increments, for a total of 10 concentration steps
including a drug-
free control flask which received 2.5 InL of media. Cells were incubated with
drug for
12 h pretreatment period at 37 C in a humidified atmosphere containing 5%
CO2.
102
CA 02755266 2011-10-17
The contents were removed from each flask and centrifuged at 300 x g for 10
min at room temperature. after which drug-containing media was removed from
cell
pellet. Cells were resuspended in 25 mL of warm drug-free media and
centrifuged at
300 x g for 10 min at room temperature. After removing media from cell pellet,
cells
were resuspended in 35 rriL of warm drug-free media, transferred to fresh
flasks, and a
mL sample of cells immediately removed from each flask, immediately processed
as
described below and stored for later cell cycle analysis (0 hours of drug
washout).
Incubation of the remaining 25 mL of cells continued in drug-free media for
another 10 h. A 10 mL sample of cells was removed from each flask, immediately
1 0 processed and stored for later cell cycle analysis (10 hours of drug
washout) and 10 mL
fresh replacement media was added to each incubation flask. Incubation of
cells in
drug-free media continued for 5 days. At day two, 20 mL of media and cells was
removed from each flask and replaced with 20 mL fresh media. Viability of
cells was
quantified after 5 days by trypan blue exclusion techniques using
hemacytometer
1 5 counting.
Cells were processed for cell cycle analysis using modifications of the method
published in Becton Dickinson Immunocytometry Systems source book section 1.11
(Preparation of Alcohol-Fixed Whole Cells From Suspensions For DNA Analysis).
Briefly, each 10 mL sample of cells removed from the flasks at 0 and 10 hours
of drug
washout was separately centrifuged at 300 x g for 10 min. After removing the
media
from the cell pellet, cells were resuspended in 3 mL cold saline. Seven
milliliters cold
100 % ethanol was slowly added with vigorous vortexing. Ethanol treated cell
samples
from 0 hour and 10 hour periods of compound washout were stored overnight at 4
C.
Ethanol treated cells were centrifuged 300 x g for 10 min, ethanol removed and
cells
then washed in 10 mL Phosphate Buffered Saline (PBS). Cells were resuspended
in
0.5 mL of 0.2 mg/mL Ribonuclease A (Sigma No. R-5503) in PBS and incubated in
37
C water bath for 30 min.
Cells were transferred to appropriate flow cytometry tubes and 0.5 mL of 10
mg/mL propidium iodide (PI) (Sigma No. P4170) in PBS was added to each tube.
Cells were incubated with PI at room temperature in the dark for at least 15
min prior to
analysis with a flow cytometer (Becton Dickinson FACScan flow cytometer or
equivalent). Cells should be analyzed within an hour and kept in the dark at 4
C until
ready. Cell cycle analysis was performed on 0 hour and 10 hour cells using
flow
cytometric measurement of the intensity of cellular fluorescence. The
intensity of
propidium iodide fluorescence for each cell was measured on a linear
amplification scale
with doublet events ignored using doublet discrimination. The results obtained
from
analyzing 15,000 cells were presented as a histogram with increasing.
fluorescence
103
CA 02755266 2011-10-17
intensity on the x-axis and the number of cells at a particular intensity
level on the y-
axis.
The intensity of PI staining is dependent on the amount of DNA in the cell so
it
is possible to identify cells in various phases of the cell cycle, such as
cells that have not
yet synthesized DNA since the last mitosis (G1 phase), cells that are in
intermediate
stages of DNA synthesis (S phase), and cells that have doubled their
complement of
DNA and are ready to divide (G2 phase). Cells that are blocked in the mitosis
phase of
the cell cycle also have double the amount of DNA compared to GI phase cells.
If all
cells are blocked in mitosis there are no G, phase cells, but if the block is
removed
1 0 when compound is removed, cells complete mitosis and reappear in the GI
phase. The
number of cells so reappearing in the G1 or Sphase is thus a measure of the
number of
cells which have recently completed mitosis. For each sample at 0 and 10 hours
after
compound removal, the percentage of cells completing mitosis was quantified
(as the
number of cells reappearing in the GI phase) and plotted as a function of the
initial
1 5 concentration of compound used during the 12 hour pretreatment period.
The
percentage of cells still viable 5 days after drug washout was superimposed on
the same
graph, see, for example FIG. 1 and FIG. 2. A ratio can be determined between
the
compound concentration required to completely block all cells in mitosis at 0
hour and
the concentration required to maintain the block 10 hours after compound
removal.
20 This was taken as a measure of a compound's reversibility, with ratios
close to or equal
to one indicating likely potent in vivo anti-tumor compounds (see Table 1,
columns 4-
6, and FIGS. 3 and 4).
Table I
In Vitro Inhibition and Reversibility Data
DLD-1* Complete Mitotic Block
Reversibility
compound mean 1050. nm SE 0 hour. nM** 10
hour, nMI Ratio
B1793 0.93 0.04 3 44 14.7
B1794 12.20 0.72
B1918 1.27 0.12
BI920 2.00 0.15
B1921 24.00 1.15
B1922 0.53 0.01 3 30 10.0
B1930 0.87 0.03
BI933 0.79 0.16
B1934 1.05 0.21 3 30 10.0
B1939 19.34 2.36 12 12 1.0
104
=
CA 02755266 2011-10-17
- .---
B1940 5.43 0.62 -
-
B1942 0.60 0.03 3 30 10.0
. .
B1963 0.56 0.04 3 20 6.7
B1973 1.15 0.24 _
B1984 1.01 0.15
-
B1987 1.82 0.21
B1988 2.67 1.02
- _
B1990 1.30 0.06
-
B1991 0.69 0.03
B1992 0.86 0.07
B1998 1.23 0.13
B2003 1.21 0.12 .
B2004 0.63 ' 0.04
B2008 2.63 0.63
B2010 . 0.71 0.12
B2011 1.81 0.52 _
-
B2013 0.49 0.07 2 30 15.0
,
B2014 0.87 - 0.09
. .
B2015 2.78 0.23
B2016 0.66 0.06
-
B2019 0.82 0.07 -
B2034 0.74 ' 0.03 _
B2035 0.76 0.09 -
B2037 0.66 ' 0.11 -
-
B2039 0.91 0.08
B2042 1.93 0.11 ' 3 100 33.3 _
B2043 ' 1.70 0.06 '
,
B2070 0.64 0.09 3 30 10.0
,
B2073 0.89 0.15 '
B2086 11.17 1.96 '
. ,
B2088 1.23 0.12
B2090 0.52 0.04 2 10 5.0
B2091 1.36 0.07 3 30 10.0
_ -
B2102 3.47 0.22
-
B2136 5.23 1.04 3 3 1.0
'
B2294 0.80 0.01
- ,
B2320 1.20 0.17 1 10 10.0
,
B2330 4.40 0.42 7 7 1.0
_
105
CA 02755266 2011-10-17
B2336 3.33 0.09 10 10
1.0
B2339 4.30 0.21 3 3
1.0
'
B2417 12.67 0.33 10 10
1.0
_
B2418 3.63 0.17 10 10
1.0
B2489 14.67 2.03 10 10
1.0
B2490 35.67 3.33 100 100
1.0
B249I 0.92 0.14 i
_ 10
6.7
ER803834 0.47 0.08 1 10
10.0
'
ER803835 15.33 0.33 10 10
1.0 _
,
ER803836 1.97 0.12 3 10
3.3
ER803843 0.49 0.05 1 10
10.0
ER803845 1.50 0.20 3 10
3.3
ER803846 1.16 0.10 1 10
10.0
ER803851 3.33 0.26 3 3
1.0
ER803852 3.03 0.50 3 10
3.3
ER803868 0.43 0.03 3 10
3.3
_
ER803869 4.13 0.64 3 3
1.0
ER803870 1.27 0.12 3 30
10.0
ER803883 ' 1.02 0.04 1 10
10.0
ER803884 0.59 0.03 1 10
10.0
* = in vitro cell growth inhibition ** = before washout $ :--- after washout
The invention also features a method for identifying an agent that induces a
sustained mitotic block in a cell after transient exposure of the cell to the
agent. The
invention features determining the relative reversibility of the test compound
by relating
5 the measurement of step (d) and the measurement of step (f). as described
below. This
determination may be a ratio, or an arithmetic difference, for example. In one
aspect,
the method includes:
(a) incubating a first cell sample with a predetermined concentration of a
test compound
1 0 for a time interval between that sufficient to empty the G1 population
and that equivalent
to one cell cycle (e.g., typically, 8 -16 hours, or about 12 hours);
(b) substantially separating the test compound from said first cell sample
(e.g. by
washing or changing media);
(c) incubating said first sample in media free of the test compound for a time
interval
sufficient to allow at least 80% (e.g., 85%, 90%. and preferably 95%, 98%, or
99%)
of the cells released from the mitotic block induced by a highly reversible
mitotic
106
_ .
CA 02755266 2011-10-17
inhibitor to complete mitosis and return to the G, phase (e.g., typically 6-14
hours, or
about 10 hours after separation step (b)): and
(d) measuring the percentage of transiently-exposed cells from step (c) that
have
completed mitosis and returned to the G, phase (e.g., measuring a cell cycle
marker,
such as DNA-dependent PI fluorescence).
One aspect of this screening method include the further steps of:
(e) incubating a second sample of cells with a concentration of the test
compound less
than or equal to that used in step (a) for a time interval between that
sufficient to empty
the G, population and that equivalent to one cell cycle;
(f) measuring the percentage of cells from step (e) that have completed
mitosis and have
1 5 returned to the G, phase; and
(g) determining a reversibility ratio of the test compound.
In one embodiment of the method, the first and second cell samples are
suspension culture cells selected from, for example, human leukemia, human
lymphoma, murine leukemia, and murine lymphoma cells. The first and second
cell
samples may be incubated simultaneously (steps (a) and (e)) or in separate
portions.
Other embodiments further include before step (a), the step (i) of estimating
a desirable
time interval for incubating said first cell sample with a reversible
mititotic blocking
agent (or, alternatively, said test compound) to provide a satisfactory
majority of cells
collected at mitotic block; and wherein the incubation of step (a) is for the
time interval
estimated in step (i). Another embodiment of the method further includes
before step
(c), the step (ii) of estimating a desirable time interval for the test
compound-free
incubation of step (c), said step (ii) comprising determining the time
interval after which
at least 80 % of the cells pretreated with a highly reversible antimitotic
agent complete
mitosis and reenter G, phase; and wherein the incubation of step (c) is for
the time
interval determined in step (ii). Another embodiment of the method utilizes
non-
suspension culture cells from, for example, adherent human or murine cancer
cells.
harvested by any suitable means for detaching them from tissue culture flasks.
One aspect of the method further includes repeating steps (a) ¨ (f) using a
range
of relative concentrations of test compound to determine what two
substantially
minimum concentrations of the test compound provide substantially complete
mitotic
block in step (d) and in step (f), respectively. The ratio of these minimum
sufficient
concentrations is an index of reversibility (see detailed U937 protocol for
preparation of
107
CA 02755266 2011-10-17
exemplary dose-response curves). These concentrations may be determined by
extrapolating curves of the percentage of cells (from steps (d) and (f)) as a
function of
concentraticin (e.g., by testing only a few concentrations. such as 3 or
fewer), or by
empirically testing a full range of concentrations.
The above methods are useful for identifying an agent (test compound) that
inhibits mitosis, for identifying a mitotic blocking agent which substantially
retained its
mitosis blocking effectiveness after its removal, and for predicting. for
example, the
IC50 or the IC95 of a mitotic blocking agent. When compared with relatively
reversible
antimitotic agents, substantially irreversible antimitotic agents, in other
words, agents
which continue to block mitosis in a cell which has been only transiently
exposed to the
agent, are likely to be more effective in vivo where natural processes,
including multi-
drug resistance (MDR) pumps and metabolic or other degradative pathways,
prevent
prolonged exposure. The effectiveness of relatively reversible antimitotic
agents may
depend upon a period of sustained exposure.
In view of the cost of developing pharmaceuticals, the economic advantages of
determining reversibility ratios, as described above, are considerable. The
above
methods can be used, for example, to predict whether a test compound with good
in
vitro activity will be effective in vivo, such as in a clinical trial.
Relatively reversible
agents would not be expected to perform as well as irreversible agents. This
is shown,
for example, by contrasting the data for two known compounds, the relatively
irreversible antimitotic agent vincristine and the highly reversible
antimitotic agent
vinblastine.
108
CA 02755266 2011-10-17
Table 2
Reversibility Characteristics of Vinblastine and Vincristine
Drug concentration required for
complete mitotic block, nM
0 hour 10 hour Reversibility Interpretation
Compound (before washout) (after washout) ratio
Vinblastine 10 600 60 Highly
Reversible
Vincristine 10 10 1 Irreversible
Analyses of the antimitotic drugs vinblastine and vincristine in the U937
Mitotic
Block Reversibility Assay indicate that despite identical potencies to induce
initial
mitotic blocks (0 hour values), the abilities of the two drugs to induce
mitotic blocks
which are sustained 10 hour after drug washout (10 hour values) are very
different:
vincristine induces irreversible mitotic blocks, while those induced by
vinblastine are
highly reversible.
Analyses of in vivo anticancer activities of the antimitotic drugs vinblastine
and
vincristine against COLO 205 human colon cancer xenografts grown sub-
cutaneously in
immunocompromised (nude) mice indicate that at equivalent doses of 1 mg/1<g,
vincristine shows substantial cancer growth inhibitory activity while
vinblastine is
inactive (FIG. 5). At the lower dose of 0.3 mg/kg, vincristine still produces
moderate
growth inhibition, while vinblastine is again inactive. The greater in vivo
activity of
vincristine correlates with its irreversibility relative to vinblastine's high
reversibility.
E. Use
The disclosed compounds have pharmacological activity, including anti-tumor
and anti-mitotic activity as demonstrated in section D above. Examples of
tumors
include melanoma, fibrosarcoma, monocytic leukemia, colon carcinoma, ovarian
carcinoma, breast carcinoma, osteosarcoma, prostate carcinoma, lung carcinoma
and
ras-transformed fibroblasts.
The invention features pharmaceutical compositions which include a compound
of formula (I) and a pharmaceutically-acceptable carrier. Compositions can
also
109
CA 02755266 2013-05-17
include a combination of disclosed compounds, or a combination of one or more
disclosed compounds and other pharmaceutically-active agents. such as an anti-
tumor
agent, an immune-stimulating agent, an interferon, a cytokine, an anti-MDR
agent or
an anti-angiogenesis agent. Compositions can be formulated for oral, topical,
parenteral, intravenous, or intramuscular administration, or administration by
injection
or inhalation. Formulations can also be prepared for controlled-release,
including
transderrnal patches.
A method for inhibiting tumor growth in a patient includes the step of
administering to the patient an effective, anti-tumor amount of a disclosed
compound
1 0 or composition. The invention also contemplates combination therapies,
including
methods of co-administering a compound of formula (I) before, during, or after
administering another pharmaceutically active agent. The methods of
administration
may be the same or different. Inhibition of tumor growth includes a growth of
the cell
or tissue exposed to the test compound that is at least 20% less, and
preferably 30%,
50%, or 75% less than the growth of the control (absence of known inhibitor or
test
compound).
Other Embodiments
While particular embodiments of the present invention have been illustrated
and
described, the scope of the claims should not be limited by the preferred
embodiments set
forth in the examples, but should be given the broadest interpretation
consistent with the
description as a whole.
110