Note: Descriptions are shown in the official language in which they were submitted.
CA 02756543 2011-10-26
HOLDING DEVICE FOR DRIED BIOLOGICAL FLUID SPOTTING MEMBRANE
AND RELATED METHODS
TECHNICAL FIELD
[0001] The present invention relates generally to dried biological fluid spot
testing. More
particularly, the present invention relates to devices and methods for holding
dried biological fluid
spotting membranes.
BACKGROUND
[0002] Dried biological fluid spot testing such as dried blood spot (DBS)
testing is becoming
increasingly popular for pharmaceutical companies in clinical trials.
Collection sites for clinical
trials may sample blood spots (or other types of biological fluid spots) in
the field, allow the spots to
dry, and then ship the spots at a lower cost than liquid samples due to the
non-biohazard status of
dried blood spots and the less rigorous requirements for temperature control.
Blood spotting is also
becoming useful in preclinical work as analytical chemists are required to
store samples for
Incurred Sample Reanalysis (ISR) studies, and dried blood spots have proven to
be an effective way
to stabilize the analytes and the matrix. Typically, DBS samples are prepared
by applying drops of
blood drawn from a finger or toe to an absorbent membrane or substrate (e.g.,
filter paper) of an
appropriate composition. The blood saturates the membrane and is air dried for
a period of time
(e.g., several hours) sufficient to form an array of circular dried blood
spots on the membrane. The
spot-containing membrane (which, as described below, is typically housed in a
cardboard holder)
may then be stored in a plastic container and transported as needed without
needing to be frozen.
The dried blood spots may thereafter be separated from the bulk membrane by
punching the dried
blood spots to create individual dried blood spot disks. Analytes such as
pharmaceutical
-1-
CA 02756543 2011-10-26
compounds may then be extracted from the dried blood spots by any number of
techniques and
subjected to analytical testing. Other types of biological fluid samples may
be dried and
subsequently processed in an analogous manner.
[0003] Typically, the absorbent membrane is housed in a membrane holder that
is made of
cardboard or chipboard. The absorbent membrane is sandwiched between a top
cardboard face and
a bottom cardboard face. The top face is secured to the bottom face by
adhesive. A user may
access the absorbent membrane via a window that passes through the top face
and/or the bottom
face. Conventional cardboard holders have many problems. For instance,
cardboard can bend or
warp easily (during testing, handling, shipping, etc.) which can impede the
uniformity of the
absorbent membrane. As another example, chemicals in the adhesives used to
secure the top
cardboard face to the bottom cardboard face may contaminate blood samples on
the absorbent
membrane. Also, conventional cardboard holders are generally not reusable.
[0004] In view of the foregoing, there is a need for providing devices and
methods specifically
designed for effectively holding dried biological fluid spotting membranes. In
particular, there is a
need for providing a holding device for a dried biological fluid spotting
membrane that is durable
and effective in holding its form or shape (and thus the form or shape of the
membrane) during
testing (including automated testing), handling, shipping, etc. In addition,
there is a need for
providing a holding device for a dried biological fluid spotting membrane that
minimizes (or
eliminates) the use of chemical adhesives. There is also a need for providing
a holding device for a
dried biological fluid spotting membrane that is capable of being reused.
-2-
CA 02756543 2011-10-26
SUMMARY
[0005] To address the foregoing problems, in whole or in part, and/or other
problems that may
have been observed by persons skilled in the art, the present disclosure
provides methods,
processes, systems, apparatus, instruments, and/or devices, as described by
way of example in
implementations set forth below.
[0006] According to one implementation, a holding device for a dried
biological fluid spotting
membrane includes a plastic top face, a plastic bottom face, and a pocket
disposed between the top
face and the bottom face. The plastic top face includes a window formed
therein, and the window
has a length and a height. The pocket has a length and a height, and is
configured for receiving a
dried biological fluid spotting membrane that is accessible through the window
by a user during
dried biological fluid spot testing.
[0007] In some implementations, the length of the window is less than the
length of the pocket,
and the height of the window is less than the height of the pocket.
[0008] In some implementations, the window of the top face is a first window
and the bottom
face comprises a second window formed therein. The second window has a length
that is
substantially the same as the length of the first window, and a height that is
substantially the same
as the height of the first window.
[0009] In some implementations, the holding device includes a plastic laminate
layer and a
plastic body. The plastic laminate layer includes the top face. The plastic
body includes the bottom
face and an opposing inner face. The laminate layer is adhered to the inner
face.
[0010] In some implementations, the holding device includes a plastic top
piece and a plastic
bottom piece. The plastic top piece includes the top face and a first inner
face opposing the top
face. The first inner face includes a plurality of male components extending
from the first inner
-3-
CA 02756543 2011-10-26
face. The plastic bottom piece includes the bottom face and a second inner
face opposing the
bottom face. The second inner face includes a plurality of female components
extending into the
second inner face. The female components are configured for detachably mating
with the male
components.
[0011] According to another implementation, a method is provided for
assembling a holding
device. A dried biological fluid spotting membrane is positioned within a
pocket of a plastic
membrane housing. The membrane is secured within the pocket, and is accessible
by a user via a
window formed in a top face of the plastic membrane housing.
[0012] In some implementations, securing the membrane within the pocket
includes
overmolding a top piece onto an inner face of the plastic membrane housing.
The inner face
includes the pocket formed therein, and the top piece includes the top face.
[0013] In some implementations, securing the membrane within the pocket
includes adhering a
plastic laminate layer to an inner face of the plastic membrane housing. The
plastic laminate layer
includes the top face.
[0014] In some implementations, securing the membrane within the pocket
includes detachably
mating male components extending from a first inner face of a top piece with
female components
extending into a second inner face of a bottom piece of the membrane housing.
The top piece
includes the top face and the first inner face opposing the top face. The
bottom piece includes a
bottom face and the second inner face opposing the bottom face. The second
inner face includes the
pocket formed therein.
[0015] According to another implementation, a method is provided for using a
holding device in
which a membrane is secured in a pocket of the holding device. A dried
biological fluid spot is
formed on the membrane by accessing the membrane via a window of the holding
device, applying
-4-
CA 02756543 2011-10-26
a drop of a biological fluid sample to the membrane to form a biological fluid
spot, and allowing the
biological fluid spot to dry.
[0016] In some implementations, the method includes accessing the dried
biological fluid spot
via the window and extracting a portion of the dried biological fluid spot
formed on the membrane.
[0017] In some implementations, the method includes removing the top piece
from the bottom
piece by detaching male components from female components. The membrane may be
removed
from the pocket. The removed membrane may be replaced with a second dried
biological fluid
spotting membrane by positioning the second membrane within the pocket. The
second membrane
may be secured within the pocket by detachably mating the male components and
the female
components.
[0018] Other devices, apparatus, systems, methods, features and advantages of
the invention
will be or will become apparent to one with skill in the art upon examination
of the following
figures and detailed description. It is intended that all such additional
systems, methods, features
and advantages be included within this description, be within the scope of the
invention, and be
protected by the accompanying claims.
BRIEF DESCRIPTION OF THE DRAWINGS
[0019] The invention can be better understood by referring to the following
figures. The
components in the figures are not necessarily to scale, emphasis instead being
placed upon
illustrating the principles of the invention. In the figures, like reference
numerals designate
corresponding parts throughout the different views.
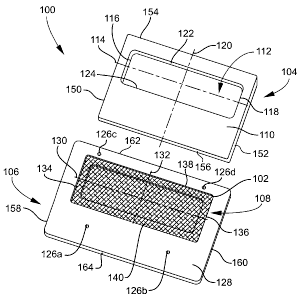
[0020] FIG. 1 is an exploded view of an example of a holding device for a
dried biological fluid
spotting membrane according to an implementation disclosed herein.
-5-
CA 02756543 2011-10-26
[0021] FIG. 2A is a plan view of a top face of a top piece of the holding
device illustrated in
FIG. 1.
[0022] FIG. 2B is a plan view of a first inner face opposite the top face
illustrated in FIG. 2A.
[0023] FIG. 2C is a side cross-sectional elevation view of a male component
illustrated in FIG.
2B.
[0024] FIG. 3A is a plan view of a second inner face of a bottom piece
illustrated in FIG. 1,
shown without the dried biological fluid spotting membrane.
[0025] FIG. 3B is a side cross-sectional elevation view of the bottom piece
illustrated in FIG.
3A.
[0026] FIG. 4 is an exploded view of another example of a holding device for
the dried
biological fluid spotting membrane according to an implementation disclosed
herein.
[0027] FIG. 5 is a perspective view of another example of a holding device for
the dried
biological fluid spotting membrane according to the present invention.
DETAILED DESCRIPTION
[0028] In the context of the present disclosure, the term "fluid" refers
generally to liquid-phase
materials and gas-phase materials, unless a liquid-phase material or a gas-
phase material is
specifically indicated. The terms "liquid-phase" and "liquid," and "gas-phase"
and "gas," are used
interchangeably. A liquid-phase material or liquid may be any liquid, such as
a solution,
suspension, slurry, multi-phase mixture or the like, and may include gaseous
components (e.g.,
bubbles) and/or solid components (e.g., particles). A gas-phase material or
gas may be any gas or
vapor, and may include liquid components (e.g., droplets) and/or solid
components (e.g., particles).
-6-
CA 02756543 2011-10-26
A "dried fluid sample" or a "dried fluid spot" refers generally to a material
that was initially
provided in the liquid phase and was thereafter dried, such as by air drying.
[0029] In the context of the present disclosure, the term "analyte" refers
generally to any sample
molecule of interest-that is, a molecule on which an analysis is desired such
as, for example, a
chromatographic analysis.
[0030] Examples of implementations of the subject matter disclosed herein will
now be
described in more detail with reference to FIGS. 1-5.
[0031] FIG. 1 is an exploded view of an example of a holding device (or
"membrane housing")
100 for a dried biological fluid spotting membrane (or "substrate," or "card")
102 according to an
implementation of the present teachings. Examples of biological fluids that
may be formed into
dried biological fluid spots using the membrane 102 include, but are not
limited to, blood-based
samples such as whole blood, plasma or serum. In these cases, the spot-
containing membrane 102
is often termed a dried blood spotting card. It will be appreciated, however,
that biological fluids
formable into dried spots are not limited to blood-based samples. The membrane
102 may be made
of any composition suitable for use as a spotting card, non-limiting examples
of which include
various types of cellulosic filter papers, glass fiber/cellulose composites,
cellulose-free glass fiber
paper, polyamides (e.g., nylon), propylene, nitrocellulose, polyethersulfone,
etc. Further examples
of membrane compositions are described in U.S. Patent Application No.
12/860,669, titled DRIED
BLOOD SPOTTING PAPER DEVICE AND METHOD, filed August 20, 2010, which is
incorporated by reference herein in its entirety. Preferably, the membrane 102
material is able to
uniformly absorb a biological fluid sample to form a homogeneous circular
spot. Indicia such as
dashed circles may be provided on the membrane 102 for assisting in placement
of multiple
-7-
CA 02756543 2011-10-26
biological fluid samples when it is desired to fonn an array of dried
biological fluid spots. The
indicia may include printed matter, perforations and/or scoring.
[0032] As illustrated in FIG. 1, the holding device 100 may include a plastic
top piece 104, a
plastic bottom piece 106, and a pocket 108 configured for receiving the
membrane 102. The top
piece 104 and the bottom piece 106 may generally be made of any suitable
plastic material capable
of being injection molded or formable by any other suitable fabrication
technique. Examples of
suitable plastic materials include, but are not limited to, polypropylene,
polyethylene, other
polyolefins, polyamide, polyacrylate, and the like. As injection molding of
plastic materials is
widely known to those of skill in the art, a detailed discussion of the
injection molding process will
not be discussed in detail in the present disclosure. The top piece 104 may
include a top face 110, a
first inner face (see FIG. 2B, element 220) opposing the top face 110 and a
first window 112 formed
between the top face 110 and the first inner face 220. As used herein, the
phrase "a first window
112 formed between the top face 110 and the first inner face 220" is not
intended to limit the
method by which the first window 112, the top face 110, or the first inner
face 220 is formed. For
example, in the injection molding process, a mold for the top piece 104 may be
designed such that
when the plastic material is injected into the mold, a protrusion in the mold
may form the first
window 112 between the top face 110 and the first inner face 220. As another
example, after the
injection molding process, appropriate tools may be used to cut the first
window 112 between the
top face 1.10 and the first inner face 220. It will be understood that the
present disclosure
encompasses various components formed into a surface or between two or more
surfaces, as well as
a surface or surfaces formed so as to provide various components therein or
therethrough.
[0033] The first window 112 has a length and a height. To more easily describe
dimensions of
various components of the present invention, lengths and heights of the
various components will be
-8-
CA 02756543 2011-10-26
described in relation to horizontal and vertical axes. Although the cross-
sections of the holding
devices 100 (and holding devices 400 and 500 discussed below in conjunction
with FIGS. 4-5)
described herein are rectangular in shape, it will be understood that the
cross-sections of the holding
devices 100, 400 and 500 (as well as the shapes of windows described herein)
may be any suitable
shape, such as circular, polygonal, elliptical, etc. The length of the first
window 112 is measured
along a horizontal axis 114, and is the distance between a first side wall 116
and a second side wall
118 of the first window 112. The height of the first window 112 is measured
along a vertical axis
120, and is the distance between a top wall 122 and a bottom wall 124 of the
first window 112. The
first window 112 allows a user to access the membrane 102 when the top piece
104 is secured to the
bottom piece 106.
[00341 FIG. 2A is a plan view of the top face 110 of the holding device 100
illustrated in
FIG. 1. FIG. 2B is a plan view of the first inner face 220, which is opposite
the top face 110
illustrated in FIG. 2A. The first inner face 220 may include a plurality of
male components 226a -
226d extending from the first inner face 220. The male components 226a - 226d
are configured for
detachably mating with corresponding female components (see FIGS. I and 3A-3B,
elements 126a
- 126d) extending into a second inner face 128. In some implementations, the
male components
226a - 226d may not be visible from the top face 110. The broken lines formed
into circles on the
top face 110 in FIG. 2A illustrate where the male components 226a - 226d are
positioned on the
first inner face 220 relative to the top face 110. Although the holding device
100 in the present
implementation includes four male components 226a - 226d and four
corresponding female
components 126a - 126d, it will be understood that the holding device 100 may
include any suitable
number of male components 226a - 226d and corresponding female components 126a
- 126d, and
the male components 226a - 226d and female components 126a - 126d may be
positioned in any
-9-
CA 02756543 2011-10-26
suitable place on the first inner face 220 and the second inner face 128,
respectively. In some
implementations, the first inner face 220 may include female components 126a -
126d extending
into the first inner face 220, and the second inner face 128 may include male
components 226a -
226d extending from the second inner face 128.
[0035] FIG. 2C is a side cross-sectional elevation view of the male component
226c illustrated
in FIG. 2B. As shown in FIG. 2C, the male component 226c may include a
compressible portion
230 such as a collar that is configured for compressing as the male component
2260 is pressed or
otherwise detachably secured within the corresponding female component 126c.
For example, as
the male component 226c is positioned within the female component 126c, the
compressible portion
230 may be compressed by force imparted by the inner wall(s) of the female
component 126c,
which may result in an interference fit, friction fit, press fit, or the like
between the male component
226c and the corresponding female component 126c. As shown in FIG. 2C, in some
implementations the compressible portion 230 may include two or more segments
separated by a
gap that are movable relative to each other when force is imparted to one or
more of the segments.
The other male components 126a, 126b and 126d may be configured substantially
identical to male
component 226c.
[0036] Returning to FIG. 1, the bottom piece 106 of the holding device 100
includes the second
inner face 128 and a bottom face (see FIG. 3B, element 340). As discussed
above, the second inner
face 128 may include the female components 126a - 126d extending into the
second inner face 128.
In the present example, the female components 126a - 126d have hexagonal cross-
sections. It will
be understood that the female components 126a - 126d may include any suitable
cross-sectional
shape, so long as the female components 126a - 126d are configured for
detachably receiving the
corresponding male components 226a - 226d. In some implementations, the female
components
-10-
CA 02756543 2011-10-26
126a - 126d may extend into the second inner face 128 a selected depth. In
some implementations,
the female components 126a - 126d may extend through the bottom piece 106,
i.e., from the second
inner face 128 to the bottom face 340.
[00371 As shown in FIG. 1, the pocket 108 may be formed in the second inner
face 128, and is
configured for receiving the membrane 102. The pocket 108 is shown more
clearly in FIG. 3A.
FIG. 3A is a plan view of the second inner face 128 illustrated in FIG. 1,
shown without the dried
biological fluid spotting membrane 102. The pocket 108 has a length and a
height. The length of
the pocket 108 is measured along a horizontal axis 130, and is the distance
between a first side wall
134 and a second side wall 136 of the pocket 108. The height of the pocket 108
is measured along a
vertical axis 132, and is the distance between a top wall 138 and a bottom
wall 140 of the pocket
108. The length of the first window 112 may be less than the length of the
pocket 108, and the
height of the first window 112 may be less than the height of the pocket 108,
such that the
membrane 102 may be securely held between the top piece 104 and the bottom
piece 106 when the
top piece 104 is secured to the bottom piece 106 (i.e., when the male
components 226a - 226d are
detachably mated with the corresponding female components 126a - 126d).
[00381 FIG. 3B is a side cross-sectional elevation view of the bottom piece
106 illustrated in
FIG. 3A. In some implementations, the first window 112 (FIGS. 1, 2A and 2B) is
the only window
of the holding device 100, and the bottom face 340 serves as a solid backing
for the membrane 102
and thus may serve as a disposable punching surface. In other implementations,
as illustrated in
FIGS. 3A-3B, the bottom face 340 may include a second window 312 formed
therein. The second
window 312 allows a user to access the membrane 102 via the bottom face 340 of
the holding
device 100. The second window 312 has a length and a height. The length of the
second window
312 is measured along the horizontal axis 130, and is the distance between a
first side wall 320 and
-11-
CA 02756543 2011-10-26
a second side wall 322 of the second window 312. The height of the second
window 312 is
measured along the vertical axis 132, and is the distance between a top wall
324 and a bottom wall
326 of the second window 312. As with the first window 112, the length of the
second window 312
may be less than the length of the pocket 108, and the height of the second
window 312 maybe less
than the height of the pocket 108. In some implementations, the length of the
second window 312
may be substantially the same as the length of the first window 112, and the
height of the second
window 312 may be substantially the same as the height of the first window
112.
[0039] Returning to FIG. 1, the top piece 104 has a length and a height. The
length of the top
piece 104 is measured along the horizontal axis 114, and is the distance
between a first outer side
wall 150 and a second outer side wall 152 of the top piece 104. The height of
the top piece 104 is
measured along the vertical axis 120, and is the distance between an outer top
wall 154 and an outer
bottom wall 156 of the top piece 104. Similarly, the bottom piece 106 has a
length and a height.
The length of the bottom piece 106 is measured along the horizontal axis 130,
and is the distance
between a first outer side wall 158 and a second outer side wall 160 of the
bottom piece 106. The
height of the bottom piece 106 is measured along the vertical axis 132, and is
the distance between
an outer top wall 162 and an outer bottom wall 164 of the bottom piece 106. In
some
implementations, the length of the top piece 104 and the length of the bottom
piece 106 may range
from about 1 inch to about 6 inches, and the height of the top piece 104 and
the height of the bottom
piece 106 may range from about 1 inch to about 3 inches. In some
implementations, the length
and/or height of the top piece 104 may be less than the respective length
and/or height of the bottom
piece 106.
[0040] The user of the holding device 100 may easily attach the top piece 104
to the bottom
piece 106 by pressing the male components 226a - 226d into the corresponding
female components
-12-
CA 02756543 2011-10-26
126a - 126b. The user of the holding device 100 may easily detach the top
piece 104 from the
bottom piece 106 by removing the male components 226a - 226d from the female
components 126a
- 126b (e.g., pulling the top piece 104 away from the bottom piece 106). The
membrane 102 may
be positioned in the pocket 108, and the top piece 104 may then be secured to
the bottom piece 106.
One or more dried biological fluid spots may be formed on the membrane 102.
For example, a user
(or an automated testing apparatus) may access the membrane 102 via the first
window 112 (or, in
some implementations, via the second window 312), and apply a drop(s) of a
biological fluid
sample to the membrane to form a biological fluid spot. The biological fluid
spot may be allowed
to dry over a period of time. A user may form a dried biological fluid spot on
the membrane 102
without actually handling the membrane 102, which may decrease the likelihood
of contamination
of the biological fluid sample. A user may extract a portion of the dried
biological fluid spot (for
example, by using a punch device, or any other suitable extraction apparatus)
for assaying. A
portion of the dried biological fluid spot may be extracted (e.g., punched)
via the first window 112,
for example. The membrane 102 does not have to be handled by a user in order
to extract a portion
of the dried biological fluid spot. The user may detach the top piece 104 from
the bottom piece 106,
and remove the membrane 102 from the pocket 108. In some implementations, the
top piece 104
and bottom piece 106 may be sterilized after the membrane 102 is removed. The
user may position
a second membrane within the pocket 108. The user may secure the second
membrane within the
pocket 108 by detachably mating the male components 226a - 226d and the
corresponding female
components 126a - 126d. As may be seen from the present disclosure, the
holding device 100 may
be reused. The holding device 100 does not require the use of adhesives. Since
the holding device
100 is made of injection molded plastic, the holding device 100 is a durable
alternative to
-13-
CA 02756543 2011-10-26
conventional cardboard holders. For example, the plastic holding device 100
may be less likely to
bend or deform during shipping, handling, automated testing, etc.
[0041] FIG. 4 is an exploded view of another example of a holding device 400
for the dried
biological fluid spotting membrane 102 according to an implementation
disclosed herein. The
holding device 400 includes a plastic body 402. The body 402 may be composed
of any suitable
polymer such as described by example above, and may be formed by any suitable
fabrication
technique, such as injection molding. The plastic body 402 includes an inner
face 436, a bottom
face (not shown) opposing the inner face 404 and a pocket 406 formed in the
inner face 436. The
pocket 406 is configured for receiving the membrane 102. The pocket 406 has a
length and a
height. The length of the pocket 406 is measured along a horizontal axis 408,
and is the distance
between a first side wall 410 and a second side wall 412 of the pocket 406.
The height of the
pocket 406 is measured along a vertical axis 414, and is the distance between
a top wall 416 and a
bottom wall 418 of the pocket 406. The holding device 400 includes a plastic
laminate layer 420
that may be adhered to the inner face 436. The plastic laminate layer 420 may
be made of any
suitable plastic material, and may be adhered to the inner face 436 by any
suitable adhesive known
to those skilled in the art. The laminate layer 420 includes a top face 404.
The top face 404
includes a first window 422 therein, which allows a user to access the
membrane 102 when the
laminate layer 420 is adhered to the inner face 436. The first window 422 has
a length and a height.
The length of the first window 422 is measured along a horizontal axis 424,
and is the distance
between a first side wall 426 and a second side wall 428 of the first window
422. The height of the
first window 422 is measured along a vertical axis 430, and is the distance
between a top wall 432
and a bottom wall 434 of the first window 422. The length of the first window
422 may be less than
the length of the pocket 406, and the height of the first window 422 may be
less than the height of
-14-
CA 02756543 2011-10-26
the pocket 406, such that the membrane 102 may be securely held within the
pocket 406 when the
laminate layer 420 is adhered to the inner face 436. In some implementations,
the bottom face of
the plastic body 402 may include a second window (not shown) therein, which
allows a user to
access the membrane 102 via the bottom face. The second window may be
configured similarly to
the second window 312 discussed above in conjunction with FIGS. 1- 3B.
[0042] FIG. 5 is a perspective view of another example of a holding device 500
for the dried
biological fluid spotting membrane 102 according to the present invention. The
holding device 500
may include a bottom face (not shown), a top face 502, and a pocket 504
disposed between the
bottom face and the top face 502, where the pocket 504 includes the membrane
102 positioned
therein. A bottom piece of the holding device 500 may include an inner face
(not shown) and the
bottom face. The inner face may include the pocket 504 formed therein. The
pocket 504 has a
length and a height. The length of the pocket 504 is measured along a
horizontal axis 506, and is
the distance between a first side wall 508 and a second side wall 510 of the
pocket 504. The height
of the pocket 504 is measured along a vertical axis 512, and is the distance
between a top wall 514
and a bottom wall 516 of the pocket 504. The top face 502 includes a first
window 518 therein,
which allows a user to access the membrane 102 during dried blood spot
testing, for example. The
first window 518 has a length and a height. The length of the first window 518
is measured along
the horizontal axis 506, and is the distance between a first side wall 520 and
a second side wall 522
of the first window 518. The height of the first window 518 is measured along
the vertical axis 512,
and is the distance between a top wall 524 and a bottom wall 526 of the first
window 518. The
length of the first window 518 may be less than the length of the pocket 504,
and the height of the
first window 518 may be less than the height of the pocket 504, such that the
membrane 102 maybe
more securely held within the pocket 504. In some implementations, the bottom
face may include a
-15-
CA 02756543 2011-10-26
second window (not shown) therein, which allows a user to access the membrane
102 via the
bottom face. The second window may be configured similarly to the second
window 312 discussed
above in conjunction with FIGS. 1- 3B.
[0043] The holding device 500 may be made according to the steps presented
below. Unless
expressly provided, the following steps arc presented in no particular order.
A bottom piece (not
shown) may be injection molded. The membrane 102 may be positioned within the
pocket 504.
The top face 502 maybe injection molded (or otherwise formed by a suitable
fabrication technique)
to the bottom piece (e.g., via overmolding, a process known by those skilled
in the art).
[0044] It will be understood that various methods are intended to be within
the scope of the
present disclosure, including, but not limited to: methods for holding the
dried biological fluid
spotting membrane 102 during dried biological fluid spot testing; methods for
assembling and
disassembling the holding device 100, 400 or 500; methods for preparing the
dried biological fluid
spotting membrane 102 for dried biological fluid spot testing; and methods for
using the holding
device 100, 400 or 500 in the context of dried biological fluid spot testing.
In conjunction with
holding and/or using the holding device 100, 400 or 500, a window of the
holding device 100, 400
or 500 may be utilized to access the membrane 102 for various purposes. For
example, one or more
drops of a biological fluid sample may be applied to the membrane 102 and
allowed to dry (e.g., air
dry) to create one or more dried biological fluid spots. As another example, a
dried biological fluid
spot or a portion thereof may be extracted from the membrane 102 via the
window by any suitable
means.
[0045] As one non-limiting example of extracting a dried biological fluid spot
or portion thereof
from the membrane 102, a punch device (or punch tool) may be operated to punch
out a dried
biological fluid sample unit (i.e., a portion of the membrane 102 containing
the dried biological
-16-
CA 02756543 2011-10-26
fluid spot or a portion of the dried biological fluid spot) from the membrane
102. For this purpose,
the holding device 100, 400 or 500 may be placed or mounted on a suitable
support surface, and the
punch device may then be thrust through the window and through the membrane
102 where the
target dried biological fluid spot is located. The punch device is typically
cylindrical and thus the
as-formed sample unit is typically disk-shaped. Once the sample unit has been
formed it may be
transported to an analytical device or any other desired destination.
Depending on the design of the
punch device, the as-formed sample unit may be captured in the punch device
and transported
therewith and/or the punch device may be utilized to perform certain sample
preparation procedures
such as, for example, solid phase extraction, sample clean-up, etc.
[0046] In some implementations, after forming the spot-containing sample unit,
the sample unit
may be exposed to a flow of one or more elution solvents (e.g., methanol,
aectonitrile, ethanol, ethyl
acetate, methyl tert-butyl ether, dichlororethane, chloroform, water, etc.,
with or without buffers or
other additives) to create an analyte-inclusive liquid sample matrix. The
analyte-inclusive liquid
sample matrix may then be processed in any desired manner for separating,
concentrating,
purifying, and/or analyzing the analytes (i.e., subsequent analytical
techniques) eluted from the
sample unit. Examples of subsequent analytical techniques include, but are not
limited to, protein
precipitation, fraction collection, centrifugation, spectrophotometry, nuclear
magnetic resonance
(NMR) spectrometry, various types of SPE (e.g., normal-phase, reversed-phase,
ion-exchange, etc.),
and various types of chromatography (e.g., preparative chromatography, liquid
chromatography
(LC), gas chromatography (GC), etc.) as well as hyphenated techniques
entailing mass spectrometry
(LC/MS", GC/MS etc.). Other subsequent analytical techniques include the
testing or processing
of genetic material (i.e., "genetic testing") such as ribonucleic acid (RNA)
or deoxyribonucleic acid
(DNA). Examples of genetic testing include, but are not limited to, polymerase
chain reaction
-17-
CA 02756543 2011-10-26
(PCR), reverse transcriptase PCR (RT-PCR), ligase chain reaction (LCR),
hybridization, genomic
sequencing, labeling, assaying, etc. Hence, for example, in the case of sample
units formed from
dried blood spots, the eluents from these sample units may be tested for
pharmaceutical compounds,
other drug-related compounds, or other chemistries, or high molecular weight
(HMW) molecules
such as DNA, RNA, proteins or other polymers.
[0047] Some examples of punch devices and related devices that may be utilized
in conjunction
with the holding device 100, 400 or 500 include those disclosed in U.S. Patent
Application Serial
No. 12/916,834, titled DRIED BIOLOGICAL FLUID SPOT PUNCH DEVICE AND RELATED
METHODS, filed November 1, 2010; and U.S. Patent Application Serial No.
12/917,138, titled
APPARATUS FOR PUNCHING AND SOLID PHASE EXTRACTION OF DRIED
BIOLOGICAL FLUID SPOT AND RELATED METHODS, filed November 1, 2010, both of
which are incorporated herein by reference in their entireties.
[0048] As may be seen from the present disclosure, the holding devices 100,
400 and 500
described herein provide effective means for holding the membrane 102 during
dried biological
fluid spot testing, such as dried blood spot testing, including automated
dried blood spot testing. In
addition, the holding devices 100, 400 and 500 described herein provide
effective means for holding
the membrane 102 during shipping and handling. The holding devices 100, 400
and 500 may be
held or manipulated in a manner that avoids having to contact the membrane 102
and the dried
biological fluid spots contained thereon, thereby avoiding contamination of
the dried biological
fluid spots. In addition, the holding devices 100, 400 and 500 are effective
in maintaining the form
and/or shape of the membrane 102 during testing, thus facilitating reliable
test results.
[0049] In general, terms such as "communicate" and "in communication with"
(for example, a
first component "communicates with" or "is in communication with" a second
component) are used
-18-
CA 02756543 2011-10-26
herein to indicate a structural, functional, mechanical, electrical, signal,
optical, magnetic,
electromagnetic, ionic or fluidic relationship between two or more components
or elements. As
such, the fact that one component is said to communicate with a second
component is not intended
to exclude the possibility that additional components may be present between,
and/or operatively
associated or engaged with, the first and second components.
[0050] It will be understood that various aspects or details of the invention
may be changed
without departing from the scope of the invention. Furthermore, the foregoing
description is for the
purpose of illustration only, and not for the purpose of limitation-the
invention being defined by
the claims.
-19-