Note: Descriptions are shown in the official language in which they were submitted.
CA 02756772 2015-02-23
IMPROVED AQUEOUS PHASE OXIDATION PROCESS
[0001]
[0002] A number of attempts have been made over the years to develop a
process that is
capable of effectively and cost efficiently oxidizing a variety of feed
materials. Many of these
processes were initially developed for use in smelting or the removal of metal
from ores. These
processes consumed large amounts of energy, emitted noxious gases, and rarely
achieved
complete recovery of all the metals entering the process. They were also
limited to very specific
uses related to smelting, which made them largely unsuitable for use with
other feed materials.
[0003] Other processes have also been developed to oxidize various feed
materials. One in
particular was an aqueous phase oxidation process that oxidized a feed
material in a solution of
nitric and sulfuric acid. The reaction occurred in a pressurized reactor that
was maintained at a
temperature no greater than about 210 C. Oxygen gas was added to reoxidize a
substantial
portion of the reduction products of nitric acid that were formed during
oxidation of the feed
materials.
1
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0004]
Although this process was a significant advance over conventional techniques
at the
time, it still suffered from a number of problems. For one, the process used a
significant amount
of oxygen gas to oxidize the reduction products of nitric acid. The oxygen gas
was initially
bubbled into the aqueous phase but quickly separated and collected in the
headspace of the
reactor where it was eventually removed. It was necessary to supply a large
amount of oxygen
gas to adequately oxygenate the aqueous phase.
[0005]
Another problem with this process concerned controlling the amount of oxygen
gas in
the aqueous phase. It was difficult to directly measure the amount of oxygen
gas in the aqueous
phase. However, it was relatively simple to measure the amount of oxygen gas
in the headspace.
Consequently, the amount of oxygen gas supplied to the reactor was controlled
based on this
measurement. Unfortunately, the amount of oxygen gas in the headspace bore a
tenuous
relationship to the amount of oxygen gas in the aqueous phase. It proved
difficult to precisely
control the amount of oxygen gas supplied to the aqueous phase.
[0006]
Other problems associated with this process were manifest when it was
attempted to
operate it continuously. The reactor was highly pressurized and the pressure
fluctuated
significantly over time. This made it difficult to introduce feed material
into the reactor at a
constant rate. The feed material had a tendency to enter in spurts and pauses,
which created
problems controlling the reaction. Each time a spurt of feed material entered
the reactor, a
number of parameters would have to be adjusted so that it could remain in the
reactor long
enough to completely oxidize.
[0007]
The process was further complicated by variations in the physical
characteristics of
the feed material, such as particle size, uniformity, moisture content, and
the like. These
problems were manifest by plugging and clogging at various points up to and
including entry
into the reactor, unpredictable residence times and reaction rates, process
control difficulties, and
the like. These problems resulted in oversizing the process equipment and
extending the
residence times to take into account the inconsistencies between the feed
materials.
2
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0008]
A. number of embodiments of an improved aqueous phase oxidation process are
described below. The improved process reduces or eliminates many of the
problems and
disadvantages associated with conventional aqueous phase oxidation processes.
SUMMARY
[0009]
Various embodiments of an improved process for oxidizing a feedstock are
described
herein. The process can be used to oxidize any suitable organic or inorganic
feedstock. In one
embodiment, the process is used to oxidize municipal and/or farm waste, e.g.,
dewatered sewage,
municipal sludge cake, or animal manure.
[0010]
The feedstock is oxidized in an aqueous reaction mixture by one or more
oxidizing
acids. In one embodiment, the oxidizing acid is regenerated in situ. Oxygen
gas may be
supplied to the reaction mixture to reoxidize the reduction products of the
oxidizing acid. The
reactor may be maintained at suitable pressures and temperatures to facilitate
regeneration of the
oxidizing acid. Suitable oxidizing acids that may be used in the process
include nitric acid and
sulfuric acid.
[0011]
In some embodiments, the feedstock may be processed before being fed to the
reactor
to give it uniform physical properties and to render it better suited to be
rapidly and efficiently
oxidized. This processing may include comminuting the feedstock so that the
particles have a
uniform size that allows the feedstock to easily enter the reactor, combining
the feedstock with
recycled effluent from the reactor, and/or combining the feedstock with one or
more oxidizing
acids before the feedstock enters the reactor.
[0012]
Once in the reactor, the feedstock is oxidized rapidly and efficiently. In
some
embodiments, gas from the headspace of the reactor, in particular, oxygen gas,
is dispersed into
the reaction mixture. This may be accomplished with a hollow impeller that
causes gas from the
headspace to flow through the impeller and into the reaction mixture as the
impeller rotates. The
result is that the composition of the gas in the reaction mixture is close to
or the same as the
composition of the gas in the headspace. In particular, the concentration of
oxygen gas in the
dissolved and undissolved gas portion of the reaction mixture is similar, if
not the same, as the
3
4762951...1.DOC
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
concentration of the oxygen gas in the heacispace. The reaction mixture may be
mixed
vigorously to increase the total amount of oxygen gas the enters the mixture.
[0013]
The gas in the reaction mixture may be removed as part of the liquid effluent
stream.
In other words, the gas that is dissolved and undissolved in the reaction
mixture is removed with
the reaction mixture effluent. A separate gas removal port on the reactor is
unnecessary, but may
be provided for other purposes.
[0014]
Once the effluent exits the reactor, it is cooled and the pressure is reduced
to allow
the gas to separate. The effluent may be vigorously agitated to speed up the
separation and make
it more complete. A portion of the effluent may be recycled back to the
beginning of the process
and combined with the feedstock as mentioned above.
100151
In one embodiment, the initial feedstock is combined with either or both of
the
effluent from the reactor or one or more oxidizing acids to form a primary
feedstock. The
primary feedstock is fed into the reactor where it is oxidized. The primary
feedstock is part of
the reaction mixture which also includes nitric acid and a secondary oxidizing
acid.
100161
in another embodiment, the initial feedstock is combined with either or both
of the
effluent from the reactor or one or more oxidizing acids to form the primary
feedstock. The
primary feedstock is fed into the reactor where it is oxidized. The primary
feedstock is part of
the reaction mixture which also includes nitric acid and oxygen gas. The
oxygen gas is supplied
to the reaction mixture in an amount that is sufficient to regenerate at least
a majority of the nitric
acid. The reaction mixture is maintained at a temperature that is no more than
approximately
210 C.
[0017]
In another embodiment, the initial feedstock is combined with either or both
of the
effluent from the reactor or one or more oxidizing acids to form the primary
feedstock. The
primary feedstock is oxidized in the reactor. The primary feedstock is part of
the reaction
mixture which also includes nitric acid. The pressure in the reactor is
maintained at at least
approximately 2070 kPa.
4
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0018]
In another embodiment, the initial feedstock is combined with either or both
of the
effluent from the reactor or one or more oxidizing acids to form the primary
feedstock. The
primary feedstock is oxidized in the reactor. The primary feedstock is part of
the reaction
mixture which also includes nitric acid, a secondary oxidizing acid, and
oxygen gas. The oxygen
gas is supplied to the reaction mixture in an amount that is sufficient to
regenerate at least a
majority of the nitric acid. The reaction mixture is maintained at a
temperature that is no more
than approximately 210 C. The pressure in the reactor is maintained at at
least approximately
2070 kPa.
100191
in another embodiment, the initial feedstock is comminuted to form the primary
feedstock where the largest dimension of at least approximately 95% of the
particles in the
primary feedstock is no more than 7 mm. The primary feedstock is oxidized in
the reactor where
it is part of the reaction mixture which also includes nitric acid and the
secondary oxidizing acid.
[0020]
In another embodiment, the initial feedstock is comminuted to form the primary
feedstock where the largest dimension of at least approximately 95% of the
particles in the
primary feedstock is no more than 7 mm. The primary feedstock is oxidized in
the reactor where
it is part of the reaction mixture which also includes nitric acid and oxygen
gas. The oxygen gas
is supplied to the reaction mixture in an amount that is sufficient to
regenerate at least a majority
of the nitric acid. The temperature of the reaction mixture is maintained at
no more than
approximately 210 C.
[0021]
In another embodiment, the initial feedstock is comminuted to form the primary
feedstock where the largest dimension of at least approximately 95% of the
particles in the
primary feedstock is no more than 7 mm. The primary feedstock is oxidized in
the reactor where
it is part of the reaction mixture which also includes nitric acid. The
pressure in the reactor is
maintained at at least approximately 2070 kPa.
[0022]
In another embodiment, the initial feedstock is comminuted to form the primary
feedstock where the largest dimension of at least approximately 95% of the
particles in the
primary feedstock is no more than 7 mm. The primary feedstock is fed into the
reactor at an
approximately constant feed rate and oxidized. The primary feedstock is part
of the reaction
4762951...1.DOC
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
mixture which also includes nitric acid, a secondary oxidizing acid, and
oxygen gas. The oxygen
gas is supplied to the reaction mixture in an amount that is sufficient to
regenerate at least a
majority of the nitric acid. The temperature of the reaction mixture is
maintained at no more
than approximately 210 "C. The pressure in the reactor is maintained at at
least approximately
2070 kPa.
[0023]
In another embodiment, the feedstock is fed into the reactor at a feed rate
that is
approximately constant. The feedstock is oxidized in the reactor where it is
part the reaction
mixture which also includes nitric acid and a secondary oxidizing acid. The
pressure in the
reactor is maintained at at least approximately 2070 kPa. The feed rate is
approximately constant
even though the pressure in the reactor may vary from approximately 2070 kPa
to 6,900 kPa.
[0024]
In another embodiment, the feedstock is fed into the reactor by a feeding
device that
is powered hydraulicly or by a gearmotor. The feedstock is oxidized in the
reactor where it is
part of the reaction mixture which also includes nitric acid and a secondary
oxidizing acid.
[0025]
In another embodiment, a first amount of the feedstock is fed into the
pressurized
reactor by the feeding device. The feeding device is isolated from the
pressurized reactor and
filled with a second amount of the feedstock. The second amount of the
feedstock is fed into the
pressurized reactor by the feeding device. The feedstock is oxidized in the
pressurized reactor
where it is part of the reaction mixture which also includes nitric acid and a
secondary oxidizing
acid. The pressure in the reactor is maintained at at least approximately 2070
kPa.
[0026]
In another embodiment, the feedstock is fed into the reactor at a feed rate
that is
approximately constant. The feedstock is oxidized in the reactor where it is
part of the reaction
mixture which also includes nitric acid, a secondary oxidizing acid, and
oxygen gas. The oxygen
gas is supplied to the reaction mixture in an amount that is sufficient to
regenerate at least a
majority of the nitric acid. The temperature of the reaction mixture is
maintained at no more
than approximately 210 'C. The pressure in the reactor is maintained at at
least approximately
2070 kPa. The feed rate is approximately constant even though the pressure in
the reactor may
vary from approximately 2070 kPa to 6,900 kPa.
6
4762951...1.DOC
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0027]
In another embodiment, the feedstock is fed into the reactor at a feed rate
that
fluctuates no more than approximately 10% per hour. The feedstock is oxidized
in a reactor
where it is part of the reaction mixture which also includes nitric acid. The
pressure in the
reactor is maintained at at least approximately 2070 kPa. The feed rate
fluctuates no more than
approximately 10% per hour even though the pressure in the reactor may vary
from
approximately 2070 kPa to 6,900 kPa.
[0028]
In another embodiment, the feedstock is fed into the reactor with a feeding
device that
is powered hydraulicly or by a gearmotor. The feedstock is oxidized in the
reactor where it is
part of the reaction mixture which also includes nitric acid and oxygen gas.
The oxygen gas is
supplied to the reaction mixture in an amount that is sufficient to regenerate
at least a majority of
the nitric acid. The temperature of the reaction mixture is maintained at no
more than
approximately 210 C.
[0029]
In another embodiment, the feedstock is fed into the reactor with a feeding
device that
is powered hydraulicly or by a gearmotor. The feedstock is oxidized in the
reactor where it is
part of the reaction mixture which also includes nitric acid. The pressure in
the reactor is
maintained at at least approximately 2070 kPa,
[0030]
In another embodiment, the feedstock is fed into the reactor with a feeding
device that
is powered hydraulicly or by a gearmotor. The feedstock is oxidized in the
reactor where it is
part of the reaction mixture which also includes nitric acid, a secondary
oxidizing acid, and
oxygen gas. The oxygen gas is supplied to the reaction mixture in an amount
that is sufficient to
regenerate at least a majority of the nitric acid. The temperature of the
reaction mixture is
maintained at no more than approximately 210 C. The pressure of the reactor
is maintained at
at least approximately 2070 kPa,
100311
in another embodiment, a first amount of the feedstock is fed into a
pressurized
reactor by the feeding device. The feeding device is isolated from the
pressurized reactor and
filled with a second amount of the feedstock. The second amount of the
feedstock is fed into the
pressurized reactor by the feeding device. The feedstock is oxidized in the
pressurized reactor
where it is part of the reaction mixture that also includes nitric acid. The
pressure in the
7
4762951...1.DOC
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
pressurized reactor is maintained at at least approximately 2070 kPa. The
first amount of the
feedstock and the second amount of the feedstock are fed into the pressurized
reactor at a feed
rate that fluctuates no more than approximately 10% per hour.
[0032]
In another embodiment, a first amount of the feedstock is fed into the
pressurized
reactor by the feeding device. The feeding device is isolated from the
pressurized reactor and
filled with a second amount of the feedstock. The second amount of the
feedstock is fed into the
pressurized reactor by the feeding device. The feedstock is oxidized in the
pressurized reactor
where it is part of the reaction mixture that also includes nitric acid, a
secondary oxidizing acid,
and oxygen gas. The temperature of the reaction mixture is maintained at no
more than
approximately 210 'C. The pressure in the reactor is maintained at at least
approximately 2070
kPa. The first amount of the feedstock and the second amount of the feedstock
are fed into the
pressurized reactor at a feed rate that is approximately constant.
[0033]
In another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid and a secondary oxidizing
acid. The gas from
the headspace of the reactor is dispersed into the reaction mixture.
[0034]
In another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid and oxygen gas. The oxygen
gas is supplied to
the reactor and dispersed from the headspace into the reaction mixture in a
manner that is
sufficient to regenerate at least a majority of the nitric acid. The
temperature of the reaction
mixture is maintained at no more than approximately 210 'C.
[0035]
In another embodiment, the feedstock is oxidized in a reactor where it is part
of the
reaction mixture which also includes nitric acid and a secondary oxidizing
acid. The
concentration of dissolved and undissolved oxygen gas in the gaseous portion
of the reaction
mixture is maintained within approximately 25% of the concentration of oxygen
gas in a
headspace of the reactor.
[0036]
In another embodiment, the feedstock is oxidized in a reactor where it is part
of the
reaction mixture which also includes nitric acid, a secondary oxidizing acid,
and oxygen gas.
The oxygen gas is supplied to the reactor and dispersed from the headspace of
the reactor into
8
4762951...1.DOC
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Any. Dkt, No: 59858.0007
the reaction mixture in a manner that is sufficient to regenerate at least a
majority of the nitric
acid. The temperature of the reaction mixture was maintained at no more than
approximately
210 C. The pressure in the reactor was maintained at at least approximately
2070 kPa.
[0037]
In another embodiment, the feedstock is oxidized in a reactor where it is part
of the
reaction mixture which also includes nitric acid. Gas from the headspace of
the reactor is
dispersed into the reaction mixture. The pressure in the reactor is maintained
at at least
approximately 2070 kPa.
[0038]
In another embodiment., the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid and oxygen gas. The oxygen
gas is supplied to
the reactor mixture in an amount that is sufficient to regenerate at least a
majority of the nitric
acid. The concentration of dissolved and undissolved oxygen gas in the gaseous
portion of the
reaction mixture is maintained within approximately 25% of the concentration
of oxygen gas in
the headspace of the reactor. The temperature of the reaction mixture is
maintained at no more
than approximately 210 'C.
[0039]
In another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid. The concentration of
dissolved and undissolved
oxygen gas in the gaseous portion of the reaction mixture is maintained within
approximately
25% of the concentration of oxygen gas in the headspace of the reactor. The
pressure in the
reactor is maintained at at least approximately 2070 kPa.
[0040]
In another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid, a secondary oxidizing acid,
and oxygen gas.
The oxygen gas is supplied to the reaction mixture in an amount that is
sufficient to regenerate at
least a majority of the nitric acid. The concentration of dissolved and
undissolved oxygen gas in
the gaseous portion of the reaction mixture is maintained within approximately
25% of the
concentration of oxygen gas in the headspace of the reactor. The temperature
of the reaction
mixture is maintained at no more than approximately 210 C. The pressure in
the reactor is
maintained at at least approximately 2070 kPa.
9
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0041]
In another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid and a secondary oxidizing
acid. Gas is supplied
to the reactor, and reactor effluent is removed from the reactor. Also, at
least approximately 94
wt.% of the reaction mixture that exits the reactor does so in the reactor
effluent, and at least
approximately 94 wt.% of gas that exits the reactor does so in the reactor
effluent.
[0042]
In another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid and oxygen gas. Gas,
including oxygen gas, is
supplied to the reactor. The reactor effluent is removed from the reactor. The
temperature of the
reaction mixture is maintained at no more than approximately 210 C. The
oxygen gas is
supplied to the reaction mixture in an amount that is sufficient to regenerate
at least a majority of
the nitric acid Also, at least approximately 94 wt.% of the reaction mixture
that exits the reactor
does so in the reactor effluent, and at least approximately 94 wt.% of gas
that exits the reactor
does so in the reactor effluent,
[0043]
In another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid and a secondary oxidizing
acid. Oxygen gas is
supplied to the reactor, and the reactor effluent is removed from the reactor.
The amount of
oxygen gas in the reactor effluent is measured and the supply of oxygen gas to
the reactor is
adjusted based on the amount of oxygen gas measured in the reactor effluent.
[0044]
in another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid, a secondary oxidizing acid,
and oxygen gas.
Gas, including oxygen gas, is supplied to the reactor. Reactor effluent is
removed from the
reactor. The temperature of the reaction mixture is maintained at no more than
approximately
210 C. The pressure in the reactor is maintained at at least approximately
2070 kPa. The
oxygen gas is supplied to the reaction mixture in an amount that is sufficient
to regenerate at
least a majority of the nitric acid. Also, at least approximately 94 wt.% of
the reaction mixture
that exits the reactor does so in the reactor effluent, and at least
approximately 94 wt.% of gas
that exits the reactor does so in the reactor effluent.
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0045]
In another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid. Gas is supplied to the
reactor, and the reactor
effluent is removed from the reactor. The pressure in the reactor is
maintained at at least
approximately 2070 kPa. Also, at least approximately 94 wt.% of the reaction
mixture that exits
the reactor does so in the reactor effluent, at least approximately 94 wt.% of
gas that exits the
reactor does so in the reactor effluent.
[0046]
In another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid and oxygen gas. The oxygen
gas is supplied to
the reactor in an amount that is sufficient to regenerate at least a majority
of the nitric acid. The
reactor effluent is removed from the reactor. The amount of oxygen gas in the
reactor effluent is
measured and the supply of oxygen gas is adjusted accordingly, The temperature
of the reaction
mixture is maintained at no more than approximately 210 C.
[0047]
In another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid. Gas is supplied to the
reactor, and the reactor
effluent is removed from the reactor. The amount of oxygen gas in the reactor
effluent is
measured and the supply of oxygen gas is adjusted accordingly. The pressure in
the reactor is
maintained at at least approximately 2070 kPa.
[0048]
In another embodiment, the feedstock is oxidized in the reactor where it is
part of the
reaction mixture which also includes nitric acid, a secondary oxidizing acid,
and oxygen gas.
The oxygen gas is supplied to the reactor, and the reactor effluent is removed
from the reactor.
The amount of oxygen gas in the reactor effluent is measured and the supply of
oxygen gas is
adjusted accordingly. The temperature of the reaction mixture is maintained at
no more than
approximately 210 'C. The pressure in the reactor is maintained at at least
approximately 2070
kPa. The oxygen gas is supplied to the reaction mixture in an amount that is
sufficient to
regenerate at least a majority of the nitric acid.
[0049]
It should be appreciated that all pressures referred to herein are gauge
pressures
unless stated otherwise. Also, all references to molarity are given at
standard conditions for
temperature and pressure ¨ i.e., 0 C and 101.325 kPa ¨ unless stated
otherwise.
11
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0050] The foregoing and other features, utilities, and advantages of the
subject matter
described herein will be apparent from the following more particular
description of certain
embodiments as illustrated in the accompanying drawings,
DRAWINGS
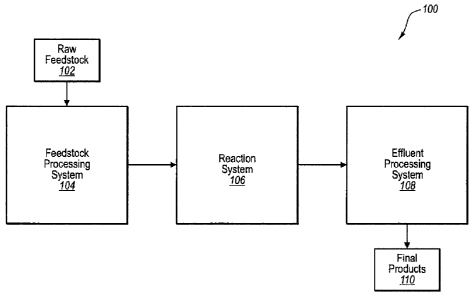
[0051] Figure 1 is a block diagram of an improved aqueous phase oxidation
process that
includes a feedstock processing system, a reaction system, and an effluent
processing system.
[0052] Figure 2 is a block diagram of one embodiment of the feedstock
processing system
from Figure 1.
[0053] Figure 3 is a block diagram of another embodiment of the feedstock
processing
system from Figure I.
[0054] Figure 4 is a block diagram of one embodiment of the reaction system
from Figure 1.
[0055] Figure 5 is a block diagram of one embodiment of the effluent
processing system
from Figure 1.
DETAILED DESCRIPTION
[0056] The improved oxidation process, in its various embodiments, can be
used to oxidize a
wide variety of materials. The process can be used to oxidize organic and/or
inorganic material
with very similar results in the sense that the feed material is completely or
nearly completely
oxidized, although the reaction products may be very different. Specific
materials that may be
oxidized using this process include, but are not limited to, municipal and
farm waste including
dewatered sewage, municipal sludge cake and animal manure; slaughter house
waste that
includes blood, bone, and flesh; petroleum based wastes such as plastics,
rubber, and paints;
tires; wood pulp; hazardous materials such as nerve gas, municipal garbage,
and metal ore such
as sulfide containing ores that are typically processed in smelters.
[0057] Although the process has a wide variety of uses, the following
description is provided
primarily in the context of oxidizing sewage and/or manure based feedstocks.
It should be
12
4762951_1.00C
CA 02756772 2015-02-23
appreciated, however, that the concepts and features described herein
generally apply to the
oxidation of other materials. Also, as each embodiment is described, it should
be understood that
the features, advantages, characteristics, etc., of one embodiment may be
applied to any other
embodiment to form one or more additional embodiments unless noted otherwise.
Furthermore,
the principles, features, characteristics, and parameters described in the
U.S. Patent No.
5,814,292 can be integrated into or substituted for various aspects of the
improved process.
[0058] Referring to Figure 1, a block diagram of an improved aqueous phase
oxidation
process 100 is shown. The process 100 includes a feedstock processing system
104, a reaction
system 106, and an effluent processing system108. The raw feedstock 102 enters
the feedstock
processing system 104 where it is modified and/or processed in a number of
ways to produce a
primary feedstock. The primary feedstock is fed to the reaction system 106
where it is oxidized.
The effluent from the reaction system 106 enters the effluent processing
system 108 where it is
separated and/or otherwise processed to produce final products 110. Each
system 104, 106, 108
is described in greater detail.
100591 The improved process is conceptually divided into the three systems
104, 106, 108
for purposes of description. It should be appreciated, however, that the
dividing line between
each system 104, 106, 108 is somewhat arbitrary and does not represent a hard
and fast
boundary. Indeed, various components of one system could just as easily be
considered part of a
different system. With this in mind, the three systems 104, 106, 108 should be
viewed as
nothing more than a conceptual framework from which to describe the overall
operation of the
process.
100601 As discussed above, the raw feedstock 102 may be any suitable
feedstock that is
capable of being oxidized in the manner described herein. In one embodiment,
the feedstock is a
sewage or manure based material that is approximately 3% to 20% solids (e.g.,
18% solids).
[0061] Figure 2 shows a block diagram of one embodiment of the feedstock
processing
system 200. The raw feedstock 102 is initially mixed with recycled effluent
204 to form an
intermediate feedstock. The grinder 206 comminutes the intermediate feedstock
thereby forming
13
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
a comminuted feedstock. Comminuting the intermediate feedstock reduces the
size of the
particles and makes the intermediate feedstock more uniform.
[0062]
The recycled effluent 204 may be combined with the raw feedstock 102 in the
grinder
206, as shown in Figure 2, or before entering the grinder 206. If they are
combined in the
grinder 206, the grinding action may serve to mix the two materials together.
If they are
combined before entering the grinder 206, the recycled effluent 204 and the
raw feedstock 102
may be mixed in a separate vessel.
[0063]
The recycled effluent 204 is added in an amount that is sufficient to create a
slurry
that doesn't plug or clog the grinder 206 and/or facilitates later processing
and transport. The
amount of recycled effluent 204 that is added may vary depending on the
characteristics of the
raw feedstock 102. Generally, larger quantities of the recycled effluent 204
are used if the raw
feedstock 102 is dry, while smaller quantities, or possibly none at all, are
used if the raw
feedstock 102 already includes a suitable amount of liquid. It is also
possible that certain
feedstocks may be so wet that they must be dewatered before entering the
process 100.
[0064]
In certain embodiments, particularly those where the raw feedstock 102 is
sewage or
manure based material, the volume ratio of the recycled effluent 204 to the
raw feedstock 102 in
the intermediate feedstock may be approximately 0.5 to 1.5 or, desirably,
approximately 0.75 to
1.25. In one embodiment, approximately equal parts by volume of the recycled
effluent 204 and
the raw feedstock 102 are combined to form the intermediate feedstock.
[0065]
The recycled effluent 204 may be supplied at an elevated temperature so that
it heats
the raw feedstock 102 when the two are mixed together. The resulting
intermediate feedstock
may be significantly above ambient temperature. The recycled effluent 204 may
be supplied at a
temperature of approximately 40 C to 90 C or, desirably, 50 C to 75 'C. For
example, the
intermediate feedstock may be approximately 37 C to 50 'C.
[0066]
As discussed in greater detail below, the effluent from reactor 402 (Figure 4)
is
heated by the exothermic oxidation of the feedstock. The recycled effluent 204
may be at an
elevated temperature simply because it has not cooled (either naturally or
actively cooled) after
leaving the reactor 402. The recycled effluent 204 may also be heated in a
heat exchanger to
14
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
keep it at an elevated temperature. In one example, described in greater
detail below, the
recycled effluent 204 is heated in a heat exchanger using heat from the
effluent that has just left
the reactor 402. The recycled effluent 204 may be stored in an insulated tank
or vessel before
being mixed with the raw feedstock 102 to maintain it at an elevated
temperature.
[0067]
The intermediate feedstock is comminuted to reduce the particle sizes, improve
the
uniformity of the feedstock, make the feedstock more amenable to evenly
controlled pumping,
and keep the solids suspended in the slurry. This makes it easier to feed the
feedstock into the
reactor 402, which is often operated at an elevated pressure, without plugging
the entry opening.
[0068]
The size and uniformity are also important because the reaction rate varies
significantly based on these factors, especially particle size. Larger
particles generally need
longer residence times to completely oxidize. If the feedstock has both large
and small particles,
the large particles tend to dictate the residence time. Thus, it is desirable
to create a feedstock
that generally has small, uniform particles. This is especially true when the
feedstock includes
organic matter such as sewage and/or manure.
[0069]
Increasing the reaction rate by comminuting the feedstock makes it possible to
reduce
the size of the reactor 402 and/or increase the feed rate of the feedstock
into the reactor 402.
Either adjustment has a beneficial effect on the economics of the process 100.
[0070]
In one embodiment, the largest dimension of at least approximately 95% of the
particles in the comminuted feedstock is no more than 7 mm, no more than 4 mm,
no more than
2.5 mm, desirably, no more than 1.5 mm, or., suitably, no more than 0.5 mm. In
another
embodiment, the largest dimension of at least approximately 98% of the
particles in the
comminuted feedstock is no more than 7 mm, no more than 4 mm, no more than 2.5
mm,
desirably, no more than 1.5 mm, or, suitably, no more than 0.5 mm. In yet
another embodiment,
the largest dimension of at least substantially all of the particles in the
comminuted feedstock is
no more than 7 mm, no more than 4 mm, no more than 2.5 mm, desirably, no more
than 1.5 mm,
or, suitably, no more than 0.5 mm.
[0071]
Returning to Figure 2, the comminuted feedstock moves from the grinder 206 to
a
mixing vessel 208 where it is combined with a primary oxidizing acid or first
acid 210 and a
15
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
secondary oxidizing acid or second acid 212 to form a primary feedstock.
Additional amounts of
the recycled effluent 204 may be combined in the vessel 208 to produce the
desired
concentration of the acids 210, 212 or to alter the consistency or other
properties of the
feedstock.
[0072]
It has been found that pre-treating the feedstock in this manner increases the
rate of
the redox reaction in the reactor 402, particularly for feedstock that
includes organic matter such
as sewage and/or manure. The acids 210, 212 initiate de-lignination of the
organic fibers and
other organic matter in the primary feedstock. De-lignination is beneficial
because it further
reduces the size of the particles in the feedstock and exposes them to
chemical attack in the
reaction system 106.
[0073]
In the embodiment shown in Figure 2, de-lignination begins when the recycled
effluent 204, which includes the acids 210, 212, is first combined with the
raw feedstock 102.
Thus, de-lignination is initiated when the recycled effluent 204 is combined
with the raw
feedstock 102 in the grinder 206 and accelerates when the additional acids
210, 212 are added in
the vessel 208.
[0074]
The primary oxidizing acid 210 and the secondary oxidizing acid 212 are added
until
the concentration of the acids 210, 212 in the primary feedstock, excluding
solids (i.e., the
concentration of the primary feedstock excluding the solids portion), is
approximately the same
as the concentration of the acids 210, 212, respectively, in the reactor 402
at start-up.
[0075]
The primary oxidizing acid 210 may be nitric acid, and the secondary oxidizing
acid
212 may be sulfuric acid. The nitric acid functions as the oxidizing agent to
oxidize the
feedstock. The nitric acid is included in an amount that is sufficient to
rapidly and completely
oxidize the feedstock.
[0076]
The sulfate ions of the sulfuric acid convert the salt forming reaction
products into
stable sulfate salts, thereby leaving the nitric acid in the acid state to
continue as the primary
oxidant. The sulfate reacts with nitrogen containing compounds to prevent the
formation of
ammonium nitrate, an explosive, and/or other undesirable reaction products.
Instead, the sulfate
reacts with nitrogen compounds to form ammonium sulfate. The sulfuric acid is
provided in an
16
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
amount that is sufficient to prevent the formation of ammonium nitrate, but
not enough to
precipitate sulfur or volatilize significant amounts of the sulfuric acid.
[0077]
In one embodiment, the nitric acid may be added to achieve a concentration in
the
primary feedstock, excluding solids, of at least approximately 0.08 mol/L,
desirably, at least
approximately 0.5 moUL, or, suitably, at least approximately 0.84 mol/L. In
another
embodiment, the nitric acid may be added to achieve a concentration in the
primary feedstock,
excluding solids, of no more than approximately 4.2 moUL, desirably, no more
than
approximately 3.3 mol/L, or, suitably, no more than approximately 2.5 moUL. In
yet another
embodiment, the nitric acid may be added to achieve a concentration in the
primary feedstock,
excluding solids, of approximately 0.08 mol/L to 4.2 mol/L, desirably,
approximately 0.5 moUL
to 3.3 mol/L, or, suitably, approximately 0.84 mol/L to 2.5 mol/L.
[0078]
On a weight basis, the nitric acid may be added to achieve a concentration in
the
primary feedstock, excluding solids, of at least approximately 0.5 wt.%,
desirably, at least
approximately 3 wt.%, or, suitably, at least approximately 5 wt.%. In another
embodiment, the
nitric acid may be added to achieve a concentration in the primary feedstock,
excluding solids, of
no more than approximately 25 wt.%, desirably, no more than approximately 20
wt.%, or,
suitably, no more than approximately 15 wt.%. In yet another embodiment, the
nitric acid may
be added to achieve a concentration in the primary feedstock, excluding
solids, of approximately
0.5 wt.% to 25 wt.%, desirably, approximately 3 wt.% to 20 wt.%, or, suitably,
approximately 5
wt.% to 15 wt.%.
[0079]
With regard to sulfuric acid, in one embodiment, the sulfuric acid may be
added to
achieve a concentration in the primary feedstock, excluding solids, of at
least approximately 0.1
mol/Lõ desirably, at least approximately 0.12 mol/Lõ or, suitably, at least
approximately 0.16
mol/L. In another embodiment, the sulfuric acid may be added to achieve a
concentration in the
primary feedstock, excluding solids, of no more than approximately 1 mol/L,
desirably, no more
than approximately 0.54 moUL, or, suitably, no more than approximately 0.32
mobil,. In yet
another embodiment, the sulfuric acid may be added to achieve a concentration
in the primary
17
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
feedstock, excluding solids, of approximately 0.1 mol/L to 1 mol/L, desirably,
approximately
0.12 mon to 0.54 mol/L, or, suitably, approximately 0.16 mat to 0.32 mol/L.
[0080]
On a weight basis, the sulfuric acid may be added to achieve a concentration
in the
primary feedstock, excluding solids, of at least approximately 0.9 wt.%,
desirably, at least
approximately 1.1 wt.%, or, suitably, at least approximately 1.5 wt.%. In
another embodiment,
the sulfuric acid may be added to achieve a concentration in the primary
feedstock, excluding
solids, of no more than approximately 10 wt.%, desirably, no more than
approximately 5 wt.%,
or, suitably, no more than approximately 3 wt.%. In yet another embodiment,
the sulfuric acid
may be added to achieve a concentration in the primary feedstock, excluding
solids, of
approximately 0.9 wt.% to 10 wt.%, desirably, approximately 1.1 wt.% to 5
wt.%, or, suitably,
approximately 1.5 wt.% to 3 wt.%.
[0081]
The mixing vessel 208 may be any suitable tank, pipe, or other vessel that is
capable
of holding and/or mixing the materials. The mixing vessel 208 should be made
of a material that
is chemically resistant to the acids 210, 212. Suitable materials include
plastic, stainless steel,
titanium, or the like. In an alternate embodiment, the grinder 206 and the
mixing vessel 208 may
be combined together so that everything is comminuted and/or mixed in the same
vessel,
[0082]
As shown in Figure 2, the primary feedstock exits the mixing vessel 208 and is
stored
in a storage vessel or tank 214 before it is fed into the reactor 402. In one
embodiment, the
storage vessel 214 may be insulated to maintain the temperature of the primary
feedstock and
conserve energy. It should be noted that it is generally not desirable to
store the primary
feedstock for a long period of time before feeding it into the reactor 402.
The presence of the
acids 210, 212 may cause the primary feedstock to separate and the texture to
change in a way
that can make it difficult to feed into the reactor 402.
[0083]
The primary feedstock is now prepared to be fed into the reactor 402. This is
accomplished using one or more feeding devices 216. In one embodiment, the
primary feedstock
is transferred to the feeding device 216 via a low pressure pump and a
combination of vacuum
and gravity flow. It should be appreciated, however, that any suitable method
may be used to
transfer the primary feedstock to the feeding device 216.
18
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0084]
The feeding device 216 is used to feed the primary feedstock into the reactor
402 at a
steady rate. It has been discovered that relatively minor fluctuations in the
feed rate can cause
large fluctuations in the redox reaction. If the feed rate drops, the reactor
402 is starved and if
the feed rate climbs, the reactor 402 is overfed.
[0085]
The redox reaction is much more sensitive to feed rate fluctuations than it is
to other
parameters such as temperature and pressure. For this reason, it is desirable
to tightly control the
feed rate. However, this is not a simple matter since the reactor 402
experiences relatively large
fluctuations in pressure and temperature. The pressure swings make it
particularly difficult to
feed the primary feedstock into the reactor 402 at a steady rate.
[0086]
The feeding device 216 may have any suitable configuration that allows it to
feed the
primary feedstock at a steady rate. In one embodiment, the feeding device 216
is actuated or
powered hydraulicly. For example, the feeding device 216 may include one or
more hydraulic
rams that dispense or force the primary feedstock into the reactor 402. One
example of a suitable
hydraulicly powered feeding device is a cycling ram pump.
[0087]
In another embodiment, the feeding device 216 is actuated or powered by a
gearmotor. For example, the feeding device 216 includes a gearmotor that turns
a screw which,
in turn, feeds the primary feedstock into the reactor 402. The feeding device
216 may be
configured so that pressure fluctuations in the reactor 402, even up to the
reactor's safe operating
pressure limit of approximately 13,800 kI)a, do not significantly change the
feed rate.
[0088]
In one embodiment, the feeding device 216 is an extruder andlor injector that
is
hydraulicly or gear actuated. Multiple feeding devices 216 may be used to
provide an
uninterrupted supply of the primary feedstock to the reactor 402. The multiple
feeding devices
216 may be sequentially activated and refilled. When one feeding device 216 is
injecting the
feedstock into the reactor 402, another feeding 216 may be refilled with the
primary feedstock.
Also, the use of multiple feeding devices 216 is advantageous because it
allows one or more
devices 216 to be offline for maintenance or repairs while the remainder of
the devices 216
provide a continuous supply of feedstock to the reactor 402.
19
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0089]
The feeding device 216 may feed the primary feedstock into the reactor at a
rate that
fluctuates no more than approximately 10% per hour, desirably, no more than
approximately 5%
per hour, or, suitably no more than approximately 2% per hour. In another
embodiment, the
feeding device 216 feeds the primary feedstock into the reactor at a feed rate
that is
approximately constant. The feeding device 216 is capable of maintaining these
feed rates even
though the pressure in the reactor 402 may vary from approximately 2070 Oa to
6,900 Oa.
[0090]
The feeding device 216 is exposed to the high pressure of the reactor 402 when
it is
feeding the primary feedstock into the reactor 402. However, the feeding
device 216 is at a low
pressure when it is filled with the primary feedstock from the storage vessel
214. The valves
218, 220 may be used to selectively isolate the feeding device 216 from the
reactor 402 during
feeding and refilling operations. The valve 218 is closed and the valve 220 is
open when the
feeding device 216 injects the primary feedstock into the reactor 402. The
valve 220 is closed
and the valve 218 is open when the feeding device 216 is refilled with the
primary feedstock.
[0091]
The valves 218, 220 may also be used to isolate the feeding device 216 so that
it can
be repaired while the reactor 402 remains in operation. Moreover, the valves
218, 220 can also
prevent backflow from the reactor 402 into the feedstock processing system 104
during an
overpressure event. It should be appreciated that although the valves 218, 220
are depicted as
being separate from the feeding device 216, the valves 218, 220 may be
provided as integral
components of the feeding device 216.
[0092]
A pressure release system 222 may be provided that allows the feeding device
216 to
transition from a high pressure state to a low pressure state without causing
undue wear on the
components and/or blowback into the mixing vessel 208 when the valve 218 is
opened. In on
embodiment, the pressure release system may include a tank that is capable of
absorbing excess
pressure.
[0093]
It should be appreciated that the feedstock processing system 104 may be
configured
in a number of other ways besides what is shown in Figure 2. For example,
Figure 3 shows a
block diagram of another embodiment of a feedstock processing system 300. This
embodiment
is similar to the feedstock processing system 200 except that the raw
feedstock does not enter a
20
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
grinder before entering the mixing vessel 208. Also, the primary feedstock is
not stored in a
separate storage vessel.
[0094]
The feedstock processing system 300 may be suitable for situations where the
raw
feedstock 102 does not need to be comminuted. For example, the raw feedstock
102 may
already be uniform with small particles. Also, the mixing vessel 208 may
function as a storage
vessel so that the primary feedstock is drawn from the mixing vessel 208 into
the reactor 402.
Numerous other changes to the feedstock processing system 104 are also
contemplated.
[0095]
Referring to Figure 4, a block diagram is shown of one embodiment of a
reaction
system 400. The reaction system 400 includes the reactor 402, which receives
the processed
feedstock from the feedstock processing system 104. The reactor 402 is in
fluid communication
with a make-up acid source 404, an oxygen gas source 406, a control gas source
408, and a
recycled gas source 410. The reactor 402 includes one or more sensors 412 and
an impeller or
dispersion device 414. The temperature of the reactor 402 may be controlled by
an energy
control system 416.
[0096]
At start-up, the reactor 402 is initially charged with an initial reaction
mixture that
includes an aqueous solution of the primary oxidizing acid and the secondary
oxidizing acid. In
one embodiment, the primary oxidizing acid is nitric acid and the secondary
oxidizing acid is
sulfuric acid. The reactor 402 may be initially charged with an aqueous
mixture of nitric and
sulfuric acid having any of the concentrations described above. For example,
equal volumes of
approximately 3.35 molVL nitric acid and 0.4 mol/L sulfuric acid may be
combined in the reactor
402 to form the initial reaction mixture,
[0097]
The reactor 402 may be filled to any suitable level with the initial reaction
mixture.
In one embodiment, the initial reaction mixture occupies at least
approximately 25% of the
volume of the reactor 402 or, suitably, at least approximately 35% of the
volume of the reactor.
In another embodiment, the initial reaction mixture occupies no more than
approximately 80% of
the volume of the reactor 402 or, suitably, no more than approximately 70% of
the volume of the
reactor 402. In yet another embodiment, the initial reaction mixture occupies
approximately
25% to 80% of the volume of the reactor 402 or, suitably, approximately 35% to
70% of the
21
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
volume of the reactor 402. Preferably, the initial reaction mixture occupies
approximately 50%
of the volume of the reactor 402. In any of these embodiments, the remainder
of the volume of
the reactor 402, i.e., the headspace, is occupied by gases.
[0098]
The headspace is initially charged with oxygen gas and/or one or more other
gases,
preferably inert gases. The oxygen gas is used to regenerate the nitric acid
in the reaction
mixture as described in greater detail below. The oxygen gas 406 may be
supplied from any
suitable source. For example, the oxygen source may be air, pure oxygen, or
even a product of
another reaction.
100991
In one embodiment, the gas in the headspace at start-up includes at least
approximately 2 volume percent oxygen gas, desirably, at least approximately 5
volume percent,
or, suitably, at least approximately 8 volume percent. In another embodiment,
the gas in the
headspace at start-up includes no more than approximately 60 volume percent
oxygen gas,
desirably, no more than approximately 45 volume percent oxygen gas, or,
suitably, no more than
approximately 35 volume percent oxygen gas. In yet another embodiment, the gas
in the
headspace at start-up includes approximately 2 to 60 volume percent oxygen
gas, desirably, 5 to
45 volume percent oxygen gas, or, suitably, 8 to 35 volume percent oxygen gas.
[0100]
The headspace may also be charged with other gases that are inert or otherwise
unable to adversely affect the redox reaction. Suitable gases include
nitrogen, argon, and the
like. These gases are supplied as the control gas 408 in Figure 4.
[0101]
At start-up, the temperature and pressure are increased together until
operating
conditions are reached. For example, when the temperature reaches 60 C, the
pressure is
increased (by adding gas to the headspace) to approximately 1035 kPa. At 150
C, the pressure
is increased to approximately 2070 kPa. Once the mixture reaches operating
temperature, the
pressure is increased to approximately 3450 kPa. It should be appreciated,
that the temperature
and pressure may fluctuate substantially from the initial levels during
processing.
[0102]
The initial reaction mixture is heated by the energy control system to at
least 150 C
as the impeller 414 vigorously mixes or agitates the reaction mixture. The
reactor 402 may be
heated using a heat exchanger in the energy control system 416 that is in
fluid communication
22
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
with a heating jacket on the outside of the reactor 402. It should be
appreciated that in most
situations the reactor 402 only needs to be heated at start-up. Once the redox
reaction begins, it
is sufficiently exothermic that it is unnecessary to continue heating the
reactor 402 during
operation. Instead, the reactor 402 may include an internal cooling coil that
is used to maintain
the temperature of the reaction mixture below a maximum threshold. It should
be appreciated
that the same coil may be used to heat and cool the reactor 402, if desired.
[0103]
It should be appreciated that the energy control system 416 can be viewed as a
collection of any number, type, or configuration of heat exchangers, heat
sources, heat sinks and
other energy transfer devices and components that can be used to add and/or
extract heat from
various streams, reactors, etc. For example, the energy control system 416 may
include a
supplemental heat source that is used to supply and/or remove heat from the
heat exchanger
using one or more heat exchange coils. Numerous other examples are also
contemplated.
[0104]
The impeller 414 is used to thoroughly and vigorously mix the reaction mixture
and
disperse the gas from the headspace into the reaction mixture. The impeller
414 may have any
suitable design or configuration so long as it is capable of adequately doing
these things. In one
embodiment, the impeller may be a gas entrainment impeller. The gas is
dispersed by impeller
blades attached to a hollow shaft through which gases are continuously
recirculated from the
headspace of the reactor 402. The gas enters openings near the top of the
shaft and is expelled
through dispersion ports located at the tips of the impeller blades. The high
speed rotation of the
impeller blades creates a low pressure area at the tip. The pressure at the
tip of the blades drops
as the speed of the impeller 414 increases, thereby increasing the rate at
which gas is dispersed
from the headspace through the reaction mixture.
[0105]
The reactor 402 may also include one or more baffles that enhance dispersion
of the
headspace gas as well as the general stirring of the reaction mixture. The
transfer of gas is
governed by the relative speed of the tips of the impeller 414 to the liquid
phase, which reduces
the pressure at the tips (i.e., creates a vacuum) of the impeller 414 and
thereby draws gas into the
reaction mixture. A baffle may be used to impede rotation of the liquid
reaction mixture relative
to the impeller 414. This may enhance the operation of the impeller 414. A
baffle designed
23
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
specifically for this purpose may be placed in the reactor 402. Alternatively,
the cooling coil
andlor other structures that are integral or added to the reactor 402 may
function as a baffle. In
one embodiment, the cooling coil may have a serpentine shape.
[0106]
The sensors 412 may measure one or more of the following parameters:
temperature,
pressure, or liquid level. The sensors 412 may be used to implement an
automated control
system or simply provide the operator with information about the status of the
reactor 402. The
reactor 402 may have an emergency blowdown system as well as a gas out port.
[0107]
The emergency blowdown system includes a large-diameter, high pressure pipe
that
runs from the reactor 402 to an emergency blowdown containment vessel. In the
event of an
emergency overheat/overpressure situation, the pipe will quickly empty the
reactor 402 into the
emergency blowdown containment vessel. The vessel will receive all the
contents of the reactor
402 without leaking anything to the surrounding environment.
[0108]
The gas out port is not ordinarily used to remove the gas from the reactor
402.
Instead, the gas is primarily removed in the reactor effluent. The reactor 402
may be any
suitable size that is capable of accommodating the desired throughput.
[0109]
Once the reactor 402 reaches its start-up parameters, it is ready to begin
receiving and
oxidizing the primary feedstock. Shortly after the primary feedstock enters
the reactor 402, the
redox reaction reaches a steady operating state. At this point, the reaction
mixture includes the
primary feedstock, the initial start-up oxidizing acids, water, dissolved and
undissolved gases as
well as various reaction products. The redox reaction can be indefinitely
sustained at a steady
state. Although conditions in the reactor 402 may vary significantly over
time, they do not vary
so much that the reaction is adversely affected.
[0110]
In some respects, the start-up parameters of the reactor 402, such as the
oxygen gas
concentration in the headspace and the volume occupied by the reaction
mixture, are maintained
during operation. For example, the oxygen gas concentrations are maintained at
the levels
described above during operation. Also, the reaction mixture may occupy the
same volume of
the reactor 402 as the initial reaction mixture. Thus, the volume amounts
described above in
24
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
connection with the initial reaction mixture apply equally to the reaction
mixture during
operation.
[0111]
The pressure in the reactor 402 is maintained at a level that is sufficient to
keep the
reaction products of nitric acid in solution so that they can react with the
oxygen to regenerate
the nitric acid. In one embodiment, the pressure in the reactor 402 is
maintained at at least
approximately 2070 kPa, desirably, at least approximately 2410 kPa, or,
suitably, at least
approximately 2800 kPa. In another embodiment, the pressure in the reactor 402
is maintained
at no more than approximately 6900 kPa, desirably, no more than approximately
6200 kPa, or,
suitably, no more than approximately 5515 kPa. In yet another embodiment, the
pressure in the
reactor 402 is maintained at approximately 2070 kPa to 6900 kPa, desirably,
approximately 2410
kPa to 6200 Oa, or, suitably, approximately 2800 kPa to 5515 kPa.
[0112]
The pressure in the reactor 402 may be maintained by selectively adding the
oxygen
gas 406, the control gas 408, or the recycled gas 410. If the concentration of
oxygen gas 406 is
low, then oxygen gas 406 is added to increase the pressure. However, if
additional oxygen gas
406 is not needed, then either the control gas 408 or the recycled gas 410 are
added to increase
the pressure. It should be understood that the redox reaction generates gas
that also contributes
to the pressure inside the reactor 402. Due to the high operating pressure of
the reactor 402, the
oxygen gas 406, the control gas 408, and/or the recycled gas 410 may be
supplied at pressures
greater than 6900 kPa so that they will flow into the reactor 402.
[0113]
The temperature of the reaction mixture is maintained at a level that prevents
the
nitric acid from decomposing, but encourages the rapid oxidation of the
feedstock. The
temperature is controlled with the energy control system 416 as described
above. In one
embodiment, the temperature of the reaction mixture is maintained at no more
than 210 C or,
desirably, no more than 205 'C. In another embodiment, the temperature of the
reaction mixture
is maintained at at least approximately 150 C or, desirably, approximately
160 C. In yet
another embodiment, the temperature of the reaction mixture is maintained at
approximately 150
C to 210 C or, desirably, approximately 160 C to 205 C.
25
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0114]
During operation, the impeller 414 is configured to disperse a sufficient
amount of the
oxygen gas from the headspace into the reaction mixture to regenerate the
nitric acid. The
oxygen reacts with the nitric acid reduction products to form nitric acid
without any processing
outside of the reactor. The amount of the nitric acid that is regenerated can
vary. In one
embodiment, at least a majority of the nitric acid is regenerated, desirably,
at least 75% of the
nitric acid is regenerated, or, suitably at least 90% of the nitric acid is
regenerated.
[0115]
The impeller 414 circulates the gas from the headspace through the reaction
mixture
so that the concentration of the gases in the reaction mixture is very
similar, if not the same, as
the concentration of the gases that are dissolved or undissolved in the
reaction mixture. The
advantage of this is that the amount of oxygen gas supplied to the reaction
mixture can be closely
controlled based on oxygen gas measurements taken in the headspace. In one
embodiment,
concentration of dissolved and undissolved oxygen gas in the gaseous portion
of the reaction
mixture is within approximately 25% of the concentration of oxygen gas in the
headspace,
desirably, within approximately 10% of the concentration of oxygen gas in the
headspace, or,
suitably, within approximately 5% of the concentration of oxygen gas in the
headspace.
10116]
The composition of the gas in the headspace may be adjusted to control the
reaction
products produced by the redox reaction. Preferably, the desired reaction
products are
maximized when the composition of gases inside the reactor meet the following
parameters:
oxygen has the concentration given above, carbon dioxide 5% - 25% by volume;
carbon
monoxide 2% - 10% by volume; nitrous oxide (N20) 2% - 5% by volume with the
remainder
being Argon and/or Nitrogen as well as minor amounts of NO and SO, as trace
impurities.
[0117]
The concentration of the oxidizing acids in the reaction mixture may be the
same or
similar to the concentration at start-up. Additional acid is added from the
make-up acid source
404 as needed.
[0118]
Inside the reactor 402, the feedstock undergoes a complex, exothermic, redox
process. The nitrogen compounds in the reaction mixture are altered so that
the nitrogen
compounds are reduced to gaseous nitrogen and/or nitrous oxide (N20). Except
those already
listed, no compounds of the NO type are produced in the reaction mixture at
more than trace
26
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
levels, A portion of the nitrogen compounds in the reaction mixture is
incorporated into the
complex hydrocarbons noted below.
[0119]
A substantial portion of the carbon in the feedstock is oxidized to carbon
dioxide
and/or carbon monoxide. That portion of the carbon in the feedstock that is
not oxidized to
either carbon dioxide or carbon monoxide is incorporated into heavier
hydrocarbon molecules.
In situations where the oxidation potential was held to a sustained low level,
a portion of the
carbon in the feedstock was reduced to furanones, and furandiones, as well as
other complex
hydrocarbons such as paraffins.
[0120]
The hydrogen in the reaction mixture is oxidized primary to water. However, in
certain conditions, the hydrogen may be incorporated into complex hydrocarbons
such as organic
hydrofluorides of the type amine-dihydrofluoride. Other minor/trace components
such as
phosphorous, potassium, ammonia, iron, and the like, form sulfates, nitrates,
and other more
complex salts.
[0121]
A reactor effluent may be continually extracted from the reactor 402. The
reactor
effluent primarily includes salty, acidic water (and in some embodiments,
minor levels of
complex hydrocarbons as noted above) since that is all that is left when the
reaction is complete.
In one embodiment, most, if not all, of the gas that is removed from the
reactor 402 exits with the
reactor effluent. The gas that exits with the effluent is the dissolved and
undissolved gas in the
reaction mixture-- i.e., the gaseous portion of the reaction mixture.
[0122]
In one embodiment, at least approximately 94 wt% of the reaction mixture that
exits
the reactor 402 does so in the reactor effluent, and at least approximately 94
wt.% of the gas that
exits the reactor does so in the reactor effluent. In another embodiment, at
least approximately
98 wt.% of the reaction mixture that exits the reactor 402 does so in the
reactor effluent, and at
least approximately 98 wt.% of the gas that exits the reactor does so in the
reactor effluent. In
yet another embodiment, at least substantially all of the reaction mixture
that exits the reactor
402 does so in the reactor effluent, and at least approximately substantially
all of the gas that
exits the reactor does so in the reactor effluent.
27
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0123]
Upon exiting the reactor 402, the effluent may enter the energy control system
416.
The energy control system 416 serves two primary functions: to extract energy
from the process
and to maintain the operating temperature of the reactor 402. Energy can be
extracted by
allowing the effluent to flow to a slightly reduced pressure heat exchanger
which transfers
energy to harness it for productive ends. The second function is accomplished
as described
above.
[0124]
It should be noted that any unspent nitric acid in the reactor effluent may be
removed
by flashing it off before it is cooled below the boiling point of nitric acid.
Also, any excess water
may be flashed off in the energy control system. The need to flash or
otherwise separate water
from the effluent may be reduced by restricting the amount of water that is
added to the
feedstock.
[0125]
Turning to Figure 5, a block diagram of one embodiment of an effluent
processing
system 500 is shown. The effluent processing system 500 receives the effluent
after it exits the
energy control system 416. A number of sensors 506 are used to measure
parameters such as pH
and conductivity of the cooled effluent. This information may be used to
control the amount of
the acids 210, 212 that are added to the mixing vessel 208. For example, the
lower the pH of the
effluent, the less acid that needs to be added to the mixing vessel 208.
[0126]
The cooled effluent flows to the gas separation system 502 where the pressure
is
allowed to drop to ambient inside the separation equipment. At this point, the
effluent is
vigorously agitated to drive off the dissolved and undissolved gases. From the
gas separation
system 502, the liquid/solids stream is split with a portion of the stream
going to a mixing area
510 and a portion going to the solids separation system 504. From solids
separation system 504,
the stream is split with part going to the mixing area 510 and the remainder
going to the waste
water treatment 514. From the mixing area 510, the effluent is recycled back
to the feedstock
processing system 104. As shown in Figure 4, the recycled effluent may be
heated in the energy
control system 416 before it reaches the feedstock processing system 104. The
solids recovered
from the solids separation system 504 are sent to post processing for refining
into final solid
products, which can are then stored, packaged, shipped and/or disposed.
28
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0127]
The gases move from the gas separation system 502 through sensors 508 and on
to
either be recycled back to the reactor 402 or to the gas processing system
516. The different
gases are separated in the gas processing system 516. From the gas processing
system 516, the
oxygen, plus the amounts of argon/nitrogen needed for the reactor 402 are
pumped into a
pressured holding tank. At the gas processing system, the gases not required
for the reactor 402
are processed and moved to the final gas products 518 for storing, packaging,
shipping and/or
disposal.
Illustrative Embodiments
[0128]
Reference is made in the following to a number of illustrative embodiments of
the
subject matter described herein. The following embodiments illustrate only a
few selected
embodiments that may include the various features, characteristics, and
advantages of the subject
matter as presently described. Accordingly, the following embodiments should
not be considered
as being comprehensive of all of the possible embodiments. Also, features and
characteristics of
one embodiment may and should be interpreted to equally apply to other
embodiments or be
used in combination with any number of other features from the various
embodiments to provide
further additional embodiments, which may describe subject matter having a
scope that varies
(e.g., broader, etc.) from the particular embodiments explained below.
Accordingly, any
combination of any of the subject matter described herein is contemplated.
[0129]
According to one embodiment, a method comprises: combining an initial
feedstock
and effluent from a reactor to form a primary feedstock; and oxidizing the
primary feedstock in
the reactor, the primary feedstock being part of a reaction mixture that also
includes nitric acid
and a secondary oxidizing acid. The method may comprise comminuting the
primary feedstock.
The method may comprise combining the initial feedstock, the effluent, and an
oxidizing acid to
form the primary feedstock. The method may comprise combining the initial
feedstock, the
effluent, nitric acid, and the secondary oxidizing acid to form the primary
feedstock, and wherein
the concentration of nitric acid and the secondary oxidizing acid in the
primary feedstock,
excluding solids, may be approximately the same as the concentration of the
nitric acid and the
secondary oxidizing acid, respectively, in the reactor at start-up. The
primary feedstock may
29
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
include particles where the largest dimension of at least approximately 95% of
the particles in
the primary feedstock is no more than 4 mm. The effluent may be at a
temperature that is
elevated relative to the ambient temperature. The effluent may be acidic.
[0130]
According to another embodiment, a method comprises: combining an initial
feedstock and effluent from a reactor to form a primary feedstock; oxidizing
the primary
feedstock in the reactor, the primary feedstock being part of a reaction
mixture that also includes
nitric acid and oxygen gas; supplying the oxygen gas to the reaction mixture
in an amount that is
sufficient to regenerate at least a majority of the nitric acid; and
maintaining the reaction mixture
at a temperature that is no more than approximately 210 C. The method may
comprise
comminuting the primary feedstock. The method may comprise combining the
initial feedstock,
the effluent, and an oxidizing acid to form the primary feedstock. The method
may comprise
combining the initial feedstock, the effluent, nitric acid, and a secondary
oxidizing acid to form
the primary feedstock, and wherein the concentration of nitric acid and the
secondary oxidizing
acid in the primary feedstock, excluding solids, may be approximately the same
as the
concentration of the nitric acid and the secondary oxidizing acid,
respectively, in the reactor at
start-up. The primary feedstock may includes particles where the largest
dimension of at least
approximately 95% of the particles in the primary feedstock is no more than 4
mm. The effluent
may be at a temperature that is elevated relative to the ambient temperature.
[0131]
According to another embodiment, a method comprises: combining an initial
feedstock and an oxidizing acid to form a primary feedstock; oxidizing the
primary feedstock in
a reactor, the primary feedstock being part of a reaction mixture that also
includes nitric acid and
oxygen gas; supplying the oxygen gas to the reaction mixture in an amount that
is sufficient to
regenerate at least a majority of the nitric acid; and maintaining the
reaction mixture at a
temperature that is no more than approximately 210 C. The method may comprise
comminuting the primary feedstock. The oxidizing acid may be a primary
oxidizing acid, and
the method may comprise combining the initial feedstock, the primary oxidizing
acid, and a
secondary oxidizing acid to form the primary feedstock. The concentration of
the primary
oxidizing acid and the secondary oxidizing acid in the primary feedstock,
excluding solids, may
30
4762951...1.DOC
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
be approximately the same as the concentration of the nitric acid and the
secondary oxidizing
acid, respectively, in the reactor at start-up. The primary feedstock may
include particles where
the largest dimension of at least approximately 95% of the particles in the
primary feedstock is
no more than 4 mm. The oxidizing acid may include nitric acid.
[0132]
According to another embodiment, a method comprises: combining an initial
feedstock and effluent from a reactor to form a primary feedstock; oxidizing
the primary
feedstock in the reactor, the primary feedstock being part of a reaction
mixture that also includes
nitric acid; and maintaining a pressure in the reactor of at least
approximately 2070 kPa. The
method may comprise comminuting the primary feedstock. The method may comprise
combining the initial feedstock, the effluent, and an oxidizing acid to form
the primary
feedstock. The method may comprise combining the initial feedstock, the
effluent, nitric acid,
and a secondary oxidizing acid to form the primary feedstock, and wherein the
concentration of
nitric acid and the secondary oxidizing acid in the primary feedstock,
excluding solids, may be
approximately the same as the concentration of the nitric acid and the
secondary oxidizing acid,
respectively, in the reactor at start-up. The primary feedstock may include
particles where the
largest dimension of at least approximately 95% of the particles in the
primary feedstock is no
more than 4 mm. The pressure in the reactor may be at least 2800 kPa.
[0133]
According to another embodiment, a method comprises: combining an initial
feedstock and an oxidizing acid to form a primary feedstock; and oxidizing the
primary
feedstock in a reactor, the primary feedstock being part of a reaction mixture
that also includes
nitric acid and a secondary oxidizing acid.
[0134]
According to another embodiment, a method comprises: combining an initial
feedstock and an oxidizing acid to form a primary feedstock; oxidizing the
primary feedstock in
a reactor, the primary feedstock being part of a reaction mixture that also
includes nitric acid;
and maintaining a pressure in the reactor of at least approximately 2070 kPa.
[0135]
According to another embodiment, a method comprises: combining an initial
feedstock and effluent from a reactor to form a primary feedstock; oxidizing
the primary
feedstock in the reactor, the primary feedstock being part of a reaction
mixture that also includes
31
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
nitric acid, a secondary oxidizing acid, and oxygen gas; supplying the oxygen
gas to the reaction
mixture in an amount that is sufficient to regenerate at least a majority of
the nitric acid;
maintaining the reaction mixture at a temperature that is no more than
approximately 210 C;
and maintaining a pressure in the reactor of at least approximately 2070 kI)a.
101361
According to another embodiment, a method comprises: combining an initial
feedstock and an oxidizing acid to form a primary feedstock; oxidizing the
primary feedstock in
a reactor, the primary feedstock being part of a reaction mixture that also
includes nitric acid, a
secondary oxidizing acid, and oxygen gas; supplying the oxygen gas to the
reaction mixture in an
amount that is sufficient to regenerate at least a majority of the nitric
acid; maintaining the
reaction mixture at a temperature that is no more than approximately 210 C;
and maintaining a
pressure in the reactor of at least approximately 2070 Oa.
[01371
According to another embodiment, a method comprises: comminuting an initial
feedstock to form a primary feedstock that includes particles where the
largest dimension of at
least approximately 95% of the particles in the primary feedstock is no more
than 7 mm;
oxidizing the primary feedstock in a reactor, the primary feedstock being part
of a reaction
mixture that also includes nitric acid and a secondary oxidizing acid. The
method may comprise
feeding the primary feedstock into the reactor at a feed rate that is
approximately constant. The
method may comprises combining an oxidizing acid with the initial feedstock
and/or the primary
feedstock before the primary feedstock enters the reactor. The method may
comprise combining
effluent from. the reactor with the initial feedstock and/or the primary
feedstock before the
primary feedstock enters the reactor. The initial feedstock may include
effluent from the reactor.
The largest dimension of at least approximately 95% of the particles in the
primary feedstock
may be no more than 4 mm. The largest dimension of at least approximately 95%
of the
particles in the primary feedstock may be no more than 2.5 mm. The largest
dimension of at
least approximately 95% of the particles in the primary feedstock may be no
more than 1.5 mm.
The largest dimension of at least approximately 95% of the particles in the
primary feedstock
may be no more than 0.5 mm.
32
4762951...1.DOC
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0138]
According to another embodiment, a method comprises: comminuting an initial
feedstock to form a primary feedstock that includes particles where the
largest dimension of at
least approximately 95% of the particles in the primary feedstock is no more
than 7 mm;
oxidizing the primary feedstock in a reactor, the primary feedstock being part
of a reaction
mixture that also includes nitric acid and oxygen gas; supplying the oxygen
gas to the reaction
mixture in an amount that is sufficient to regenerate at least a majority of
the nitric acid; and
maintaining the reaction mixture at a temperature that is no more than
approximately 210 C.
The method may comprise combining nitric acid with the initial feedstock
and/or the primary
feedstock before the primary feedstock enters the reactor. The method may
comprise combining
effluent from the reactor with the initial feedstock and/or the primary
feedstock before the
primary feedstock enters the reactor. The initial feedstock may include
effluent from the reactor.
The reaction mixture may include a secondary oxidizing acid. The largest
dimension of at least
approximately 95% of the particles in the primary feedstock may be no more
than 2.5 mm.
[0139]
According to another embodiment, a method comprises: comminuting an initial
feedstock to form a primary feedstock that includes particles where the
largest dimension of at
least approximately 95% of the particles in the primary feedstock is no more
than 7 mm;
oxidizing the primary feedstock in a reactor, the primary feedstock being part
of a reaction
mixture that also includes nitric acid; and maintaining a pressure in the
reactor of at least
approximately 2070 kPa. The method may comprise combining nitric acid and a
secondary
oxidizing acid with the initial feedstock and/or the primary feedstock before
the primary
feedstock enters the reactor. The method may comprise combining effluent from
the reactor
with the initial feedstock and/or the primary feedstock before the primary
feedstock enters the
reactor. The initial feedstock may include effluent from the reactor. The
largest dimension of at
least approximately 95% of the particles in the primary feedstock may be no
more than 2.5 mm.
The pressure in the reactor may be at least 2800 kPa.
[0140]
According to another embodiment, a method comprises: comminuting an initial
feedstock to form a primary feedstock that includes particles where the
largest dimension of at
least approximately 95% of the particles in the primary feedstock is no more
than 7 mm; feeding
33
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
the primary feedstock into a reactor at an approximately constant feed rate;
oxidizing the primary
feedstock in the reactor, the primary feedstock being part of a reaction
mixture that also includes
nitric acid, a secondary oxidizing acid, and oxygen gas; supplying the oxygen
gas to the reaction
mixture in an amount that is sufficient to regenerate at least a majority of
the nitric acid;
maintaining the reaction mixture at a temperature that is no more than
approximately 210 C;
and maintaining a pressure in the reactor of at least approximately 2070 kPa.
The largest
dimension of at least approximately 95% of the particles in the primary
feedstock may be no
more than 2.5 mm. The initial feedstock may include effluent from the reactor.
The may
comprise combining nitric acid and the secondary oxidizing acid with the
initial feedstock and/or
the primary feedstock before the primary feedstock enters the reactor. The
method may
comprise: combining effluent from the reactor with the initial feedstock to
form an intermediate
feedstock; comrninuting the intermediate feedstock to form a comminuted
feedstock; and
combining the comminuted feedstock, nitric acid, and the secondary oxidizing
acid to form the
primary feedstock.
[0141]
According to another embodiment, a method comprises: feeding a feedstock into
a
reactor at a feed rate that is approximately constant; oxidizing the feedstock
in the reactor, the
feedstock being part of a reaction mixture that also includes nitric acid and
a secondary oxidizing
acid; and maintaining a pressure in the reactor of at least approximately 2070
kPa; wherein the
feed rate is approximately constant even though the pressure in the reactor
may vary from
approximately 2070 kPa to 6,900 kPa. The feedstock may be a slurry. The slurry
may include
nitric acid andlor the secondary oxidizing acid. The method may comprise a
plurality of feeding
devices that are sequentially activated and refilled to feed the feedstock
into the reactor. The
feedstock may include particles where the largest dimension of at least
approximately 95% of the
particles in the feedstock is no more than 4 mm. The feedstock may be fed into
the reactor with
a feeding device that is hydraulicly powered. The feedstock may be fed into
the reactor with a
feeding device that is powered by a gearmotor.
[0142]
According to another embodiment, a method comprises: feeding a feedstock into
a
reactor with a feeding device that is powered hydraulicly or by a gearmotor;
and oxidizing the
34
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
feedstock in the reactor, the feedstock being part of a reaction mixture that
also includes nitric
acid and a secondary oxidizing acid. The method may comprise maintaining a
pressure in the
reactor of at least approximately 2070 kPa, The feedstock may include
particles where the
largest dimension of at least approximately 95% of the particles in the
feedstock is no more than
4 mm. The method may comprise a plurality of the feeding devices that are
sequentially
activated and refilled to feed the feedstock into the reactor. The feedstock
may include effluent
from the reactor and/or an oxidizing acid.
[0143]
According to another embodiment, a method comprises: feeding a first amount of
a
feedstock into a pressurized reactor with a feeding device; isolating the
feeding device from the
pressurized reactor; filling the feeding device with a second amount of the
feedstock; feeding the
second amount of the feedstock into the pressurized reactor with the feeding
device; oxidizing
the feedstock in the pressurized reactor, the feedstock being part of a
reaction mixture that also
includes nitric acid and a secondary oxidizing acid; and maintaining a
pressure in the reactor of
at least approximately 2070 kPa. The method may comprise a valve that isolates
the feeding
device from the pressurized reactor. The first amount of the feedstock and the
second amount of
the feedstock may be fed into the pressurized reactor at a feed rate that is
approximately
constant. Filling the feeding device with the second amount of the feedstock
may be done at a
pressure that is greatly reduced from the pressure of the pressurized reactor.
The pressure in the
reactor may be at least 2800 kPa.
[0144]
According to another embodiment, a method comprises: feeding a feedstock into
a
reactor at a feed rate that is approximately constant; oxidizing the feedstock
in the reactor, the
feedstock being part of a reaction mixture that also includes nitric acid, a
secondary oxidizing
acid, and oxygen gas; supplying the oxygen gas to the reaction mixture in an
amount that is
sufficient to regenerate at least a majority of the nitric acid; maintaining
the reaction mixture at a
temperature that is no more than approximately 210 C; and maintaining a
pressure in the reactor
of at least approximately 2070 kPa; wherein the feed rate is approximately
constant even though
the pressure in the reactor may vary from approximately 2070 kPa to 6,900 kPa.
The feedstock
may include particles where the largest dimension of at least approximately
95% of the particles
35
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
in the feedstock is no more than 4 mm. The method may comprise a plurality of
feeding devices
that are sequentially activated and refilled to feed the feedstock into the
reactor. The feedstock
may include effluent from the reactor and/or an oxidizing acid.
[0145]
According to another embodiment, a method comprises: feeding a feedstock into
a
reactor at a feed rate that fluctuates no more than approximately 10% per
hour; oxidizing the
feedstock in a reactor, the feedstock being part of a reaction mixture that
also includes nitric
acid; and maintaining a pressure in the reactor of at least approximately 2070
kPa; wherein the
feed rate fluctuates no more than approximately 10% per hour even though the
pressure in the
reactor may vary from approximately 2070 kPa to 6,900 kPa.
[0146]
According to another embodiment, a method comprises: feeding a feedstock into
a
reactor with a feeding device that is powered hydraulicly or by a gearmotor;
oxidizing the
feedstock in the reactor, the feedstock being part of a reaction mixture that
also includes nitric
acid and oxygen gas; supplying the oxygen gas to the reaction mixture in an
amount that is
sufficient to regenerate at least a majority of the nitric acid; and
maintaining the reaction mixture
at a temperature that is no more than approximately 210 C.
[0147]
According to another embodiment, a method comprises: feeding a feedstock into
a
reactor with a feeding device that is powered hydraulicly or by a gearmotor;
oxidizing the
feedstock in the reactor, the feedstock being part of a reaction mixture that
also includes nitric
acid; and maintaining a pressure in the reactor of at least approximately 2070
kPa.
[0148]
According to another embodiment, a method comprises: feeding a feedstock into
a
reactor with a feeding device that is powered hydraulicly or by a gearmotor;
oxidizing the
feedstock in the reactor, the feedstock being part of a reaction mixture that
also includes nitric
acid, a secondary oxidizing acid, and oxygen gas; supplying the oxygen gas to
the reaction
mixture in an amount that is sufficient to regenerate at least a majority of
the nitric acid;
maintaining the reaction mixture at a temperature that is no more than
approximately 210 C;
and maintaining a pressure in the reactor of at least approximately 2070 kPa.
[0149]
According to another embodiment, a method comprises: feeding a first amount of
a
feedstock into a pressurized reactor with a feeding device; isolating the
feeding device from the
36
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
pressurized reactor; filling the feeding device with a second amount of the
feedstock; feeding the
second amount of the feedstock into the pressurized reactor with the feeding
device; oxidizing
the feedstock in the pressurized reactor, the feedstock being part of a
reaction mixture that also
includes nitric acid; and maintaining a pressure in the pressurized reactor of
at least
approximately 2070 kPa; wherein the first amount of the feedstock and the
second amount of the
feedstock are fed into the pressurized reactor at a feed rate that fluctuates
no more than
approximately 10% per hour;
[0150]
According to another embodiment, a method comprises: feeding a first amount of
a
feedstock into a pressurized reactor with a feeding device; isolating the
feeding device from the
pressurized reactor; filling the feeding device with a second amount of the
feedstock; feeding the
second amount of the feedstock into the pressurized reactor with the feeding
device; oxidizing
the feedstock in the pressurized reactor, the feedstock being part of a
reaction mixture that also
includes nitric acid, a secondary oxidizing acid, and oxygen gas; supplying
the oxygen gas to the
reaction mixture in an amount that is sufficient to regenerate at least a
majority of the nitric acid;
maintaining the reaction mixture at a temperature that is no more than
approximately 210 C;
and maintaining a pressure in the reactor of at least approximately 2070 kPa;
wherein the first
amount of the feedstock and the second amount of the feedstock are fed into
the pressurized
reactor at a feed rate that is approximately constant;
[0151]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid and a
secondary oxidizing acid; and dispersing gas from a headspace of the reactor
into the reaction
mixture. The method may comprise dispersing the gas from the headspace into
the reaction
mixture with an impeller that is hollow and causes gas from the headspace to
flow through the
impeller into the reaction mixture. The method may comprise dispersing the gas
from the
headspace into the reaction mixture with a gas entrainment impeller. The
method may comprise
supplying oxygen gas to the reactor and dispersing the oxygen gas from the
headspace of the
reactor into the reaction mixture. The method may comprise a baffle positioned
in the reaction
mixture to enhance the dispersion of the gas from the headspace into the
reaction mixture. The
37
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
method may comprise maintaining a pressure in the reactor of at least
approximately 2070 kPa.
The gas in the headspace may include 2 to 60 volume percent oxygen gas.
[0152]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid and oxygen
gas; supplying the oxygen gas to the reactor; dispersing the oxygen gas from a
headspace of the
reactor into the reaction mixture in a manner that is sufficient to regenerate
at least a majority of
the nitric acid; and maintaining the reaction mixture at a temperature that is
no more than
approximately 210 'C. The gas in the headspace may include 2 to 60 volume
percent oxygen
gas. The method may comprise dispersing the gas from the headspace into the
reaction mixture
with an impeller that is hollow and causes gas from the headspace to flow
through the impeller
into the reaction mixture. The method may comprise dispersing the gas from the
headspace into
the reaction mixture with a gas entrainment impeller. The method may comprise
maintaining a
pressure in the reactor of at least approximately 2070 kPa.
[0153]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid and a
secondary oxidizing acid; and maintaining the concentration of dissolved and
undissolved
oxygen gas in the gaseous portion of the reaction mixture within approximately
25% of the
concentration of oxygen gas in a headspace of the reactor. The method may
comprise
maintaining the concentration of dissolved and undissolved oxygen gas in the
gaseous portion of
the reaction mixture within approximately 10% of the concentration of oxygen
gas in the
headspace of the reactor. The method may comprise maintaining the
concentration of dissolved
and undissolved oxygen gas in the gaseous portion of the reaction mixture
within approximately
5% of the concentration of oxygen gas in the headspace of the reactor. The
method may
comprise dispersing the oxygen gas from the headspace into the reaction
mixture with a gas
entrainment impeller. The headspace may include 2 to 60 volume percent oxygen
gas. The
headspace may include 5 to 45 volume percent oxygen gas.
[0154]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid, a secondary
38
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
oxidizing acid, and oxygen gas; supplying oxygen gas to the reactor;
dispersing oxygen gas from
a headspace of the reactor into the reaction mixture in a manner that is
sufficient to regenerate at
least a majority of the nitric acid; maintaining the reaction mixture at a
temperature that is no
more than approximately 210 C; and maintaining a pressure in the reactor of
at least
approximately 2070 kPa. The method may comprise maintaining the concentration
of dissolved
and undissolved oxygen gas in the gaseous portion of the reaction mixture
within approximately
10% of the concentration of oxygen gas in the headspace of the reactor. The
method may
comprise dispersing the oxygen gas from the headspace into the reaction
mixture with an
impeller that is hollow and causes the oxygen gas from the headspace to flow
through the
impeller into the reaction mixture. The method may comprise dispersing the
oxygen gas from
the headspace into the reaction mixture with a gas entrainment impeller.
[0155]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid; dispersing
gas from a headspace of the reactor into the reaction mixture; and maintaining
a pressure in the
reactor of at least approximately 2070 kPa.
[0156]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid and oxygen
gas; supplying the oxygen gas to the reactor mixture in an amount that is
sufficient to regenerate
at least a majority of the nitric acid; maintaining the concentration of
dissolved and undissolved
oxygen gas in the gaseous portion of the reaction mixture within approximately
25% of the
concentration of oxygen gas in a headspace of the reactor; and maintaining the
reaction mixture
at a temperature that is no more than approximately 210 C.
[0157]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid; maintaining
the concentration of dissolved and undissolved oxygen gas in the gaseous
portion of the reaction
mixture within approximately 25% of the concentration of oxygen gas in a
headspace of the
reactor; and maintaining a pressure in the reactor of at least approximately
2070 kPa.
39
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
[0158]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid, a secondary
oxidizing acid, and oxygen gas; supplying the oxygen gas to the reaction
mixture in an amount
that is sufficient to regenerate at least a majority of the nitric acid;
maintaining the concentration
of dissolved and undissolved oxygen gas in the gaseous portion of the reaction
mixture within
approximately 25% of the concentration of oxygen gas in a headspace of the
reactor; maintaining
the reaction mixture at a temperature that is no more than approximately 210
C; and
maintaining a pressure in the reactor of at least approximately 2070 kPa.
[0159]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid and a
secondary oxidizing acid; supplying gas to the reactor; and removing a reactor
effluent from the
reactor; wherein at least approximately 94 wt% of the reaction mixture that
exits the reactor
does so in the reactor effluent; and wherein at least approximately 94 wt.% of
gas that exits the
reactor does so in the reactor effluent. The method may comprise dispersing
gas from a
headspace of the reactor into the reaction mixture. The method wherein at
least approximately
98 wt.% of the reaction mixture that exits the reactor does so in the reactor
effluent; and wherein
at least approximately 98 wt.% of gas that exits the reactor does so in the
reactor effluent. The
method may comprise separating the gas from the reactor effluent. The method
may comprise
combining the feedstock with at least a portion of the reactor effluent. A
headspace of the
reactor may include 5 to 45 volume percent oxygen gas.
[0160]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid and oxygen
gas; supplying gas to the reactor, the supplied gas including the oxygen gas;
removing a reactor
effluent from the reactor; and maintaining the reaction mixture at a
temperature that is no more
than approximately 210 C; wherein the oxygen gas is supplied to the reaction
mixture in an
amount that is sufficient to regenerate at least a majority of the nitric
acid; wherein at least
approximately 94 wt% of the reaction mixture that exits the reactor does so in
the reactor
effluent; and wherein at least approximately 94 wt.% of gas that exits the
reactor does so in the
40
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
reactor effluent. The method may comprise dispersing the oxygen gas from a
headspace of the
reactor into the reaction mixture. A headspace of the reactor may include 5 to
45 volume percent
oxygen gas. The method may comprise combining the feedstock with at least a
portion of the
reactor effluent.
[0161]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid and a
secondary oxidizing acid; supplying oxygen gas to the reactor; removing a
reactor effluent from
the reactor; measuring the amount of oxygen gas in the reactor effluent; and
adjusting the supply
of oxygen gas to the reactor based on the amount of oxygen gas measured in the
reactor effluent.
The method may comprise dispersing the oxygen gas from a headspace of the
reactor into the
reaction mixture. The method may comprise maintaining a pressure in the
reactor of at least
approximately 2070 kPa. The method may comprise supplying inert gas to the
reactor to
maintain the pressure of at least approximately 2070 kPa. A headspace of the
reactor may
include 2 to 60 volume percent oxygen gas. A headspace of the reactor may
include 5 to 45
volume percent oxygen gas,
[0162]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid, a secondary
oxidizing acid, and oxygen gas; supplying gas to the reactor, the supplied gas
including the
oxygen gas; removing a reactor effluent from the reactor; maintaining the
reaction mixture at a
temperature that is no more than approximately 210 C; and maintaining a
pressure in the reactor
of at least approximately 2070 kPa; wherein the oxygen gas is supplied to the
reaction mixture in
an amount that is sufficient to regenerate at least a majority of the nitric
acid; wherein at least
approximately 94 wt.% of the reaction mixture that exits the reactor does so
in the reactor
effluent; and wherein at least approximately 94 wt.% of gas that exits the
reactor does so in the
reactor effluent. The method may comprise dispersing the oxygen gas from a
headspace of the
reactor into the reaction mixture. The method may comprise cooling the reactor
effluent; and
separating the gas from the reactor effluent. The reactor effluent may be
vigorously mixed at
41
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
low pressure to separate the gas. A headspace of the reactor may include 5 to
45 volume percent
oxygen gas.
[0163]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid; supplying gas
to the reactor; removing a reactor effluent from the reactor; and maintaining
a pressure in the
reactor of at least approximately 2070 kPa; wherein at least approximately 94
wt.% of the
reaction mixture that exits the reactor does so in the reactor effluent; and
wherein at least
approximately 94 wt.% of gas that exits the reactor does so in the reactor
effluent.
[0164]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid and oxygen
gas; supplying the oxygen gas to the reactor in an amount that is sufficient
to regenerate at least a
majority of the nitric acid; removing a reactor effluent from the reactor;
measuring the amount of
oxygen gas in the reactor effluent; adjusting the supply of oxygen gas to the
reactor based on the
amount of oxygen gas measured in the reactor effluent; and maintaining the
reaction mixture at a
temperature that is no more than approximately 210 C.
[0165]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid; supplying
oxygen gas to the reactor; removing a reactor effluent from the reactor;
measuring the amount of
oxygen gas in the reactor effluent; adjusting the supply of oxygen gas to the
reactor based on the
amount of oxygen gas measured in the reactor effluent; and maintaining a
pressure in the reactor
of at least approximately 2070 kPa.
[0166]
According to another embodiment, a method comprises: oxidizing a feedstock in
a
reactor, the feedstock being part of a reaction mixture that also includes
nitric acid, a secondary
oxidizing acid, and oxygen gas; supplying oxygen gas to the reactor; removing
a reactor effluent
from the reactor; measuring the amount of oxygen gas in the reactor effluent;
adjusting the
supply of oxygen gas to the reactor based on the amount of oxygen gas measured
in the reactor
effluent; maintaining the reaction mixture at a temperature that is no more
than approximately
210 C; and maintaining a pressure in the reactor of at least approximately
2070 kPa; wherein the
42
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
oxygen gas is supplied to the reaction mixture in an amount that is sufficient
to regenerate at
least a majority of the nitric acid.
[0167]
The terms recited in the claims should be given their ordinary and customary
meaning
as determined by reference to relevant entries (e.g., definition of "plane" as
a carpenter's tool
would not be relevant to the use of the term "plane" when used to refer to an
airplane, etc.) in
dictionaries (e.g., widely used general reference dictionaries and/or relevant
technical
dictionaries), commonly understood meanings by those in the art, etc., with
the understanding
that the broadest meaning imparted by any one or combination of these sources
should be given
to the claim terms (e.g., two or more relevant dictionary entries should be
combined to provide
the broadest meaning of the combination of entries, etc.) subject only to the
following
exceptions: (a) if a term is used herein in a manner more expansive than its
ordinary and
customary meaning, the term should be given its ordinary and customary meaning
plus the
additional expansive meaning, or (b) if a term has been explicitly defined to
have a different
meaning by reciting the term followed by the phrase "as used herein shall
mean" or similar
language (e.g., "herein this term means," "as defined herein," "for the
purposes of this disclosure
[the term] shall mean," etc.). References to specific examples, use of "i.e.,"
use of the word
"invention," etc., are not meant to invoke exception (b) or otherwise restrict
the scope of the
recited claim terms. Other than situations where exception (b) applies,
nothing contained herein
should be considered a disclaimer or disavowal of claim scope. The subject
matter recited in the
claims is not coextensive with and should not be interpreted to be coextensive
with any particular
embodiment, feature, or combination of features shown herein. This is true
even if only a single
embodiment of the particular feature or combination of features is illustrated
and described
herein. Thus, the appended claims should be read to be given their broadest
interpretation in
view of the prior art and the ordinary meaning of the claim terms.
[0168]
As used herein, spatial or directional terms, such as "left," "right,"
"front," "back,"
and the like, relate to the subject matter as it is shown in the drawing FIGS.
However, it is to be
understood that the subject matter described herein may assume various
alternative orientations
and, accordingly, such terms are not to be considered as limiting.
Furthermore, as used herein
43
4762951_1.00C
CA 02756772 2011.09.26
WO 2010/120450 PCT/US2010/028340
Atty. Dkt, No: 59858.0007
(i.e., in the claims and the specification), articles such as "the," "a," and
"an" can connote the
singular or plural. Also, as used herein, the word "or" when used without a
preceding "either"
(or other similar language indicating that "or" is unequivocally meant to be
exclusive ¨ e.g., only
one of x or y, etc.) shall be interpreted to be inclusive (e.g., "x or y"
means one or both x or y).
Likewise, as used herein, the term "and/or" shall also be interpreted to be
inclusive (e.g., "x
and/or y" means one or both x or y). In situations where "and/or" or "or" are
used as a
conjunction for a group of three or more items, the group should be
interpreted to include one
item alone, all of the items together, or any combination or number of the
items. Moreover,
terms used in the specification and claims such as have, having, include, and
including should be
construed to be synonymous with the terms comprise and comprising.
[0169.1
Unless otherwise indicated, all numbers or expressions, such as those
expressing
dimensions, physical characteristics, etc. used in the specification (other
than the claims) are
understood as modified in all instances by the term "approximately." At the
very least, and not
as an attempt to limit the application of the doctrine of equivalents to the
claims, each numerical
parameter recited in the specification or claims which is modified by the term
"approximately"
should at least be construed in light of the number of recited significant
digits and by applying
ordinary rounding techniques. Moreover, all ranges disclosed herein are to be
understood to
encompass and provide support for claims that recite any and all subranges or
any and all
individual values subsumed therein. For example, a stated range of 1 to 10
should be considered
to include and provide support for claims that recite any and all subranges or
individual values
that are between and/or inclusive of the minimum value of 1 and the maximum
value of 10; that
is, all subranges beginning with a minimum value of 1 or more and ending with
a maximum
value of 10 or less (e.g., 5.5 to 10, 2.34 to 3.56, and so forth) or any
values from 1 to 10 (e.g., 3,
5.8, 9.9994, and so forth).
44
4762951_1.00C