Note: Descriptions are shown in the official language in which they were submitted.
CA 02757180 2015-01-15
DEVICES, SYSTEMS, AND RELATED METHODS
FOR DELIVERY OF FLUID TO TISSUE
10
Field of the Invention
The present invention relates generally to needleless injection devices for
the
delivery of therapeutic fluids to a treatment site. Exemplary methods and
devices
can be used to treat tissue of the urinary tract (e.g., prostate tissue,
kidneys, ureters,
urethral tissue, bladder, etc.), but the methods and devices will also be
useful for
other treatment sites. Exemplary embodiments of devices can involve an
injector
body (or "shaft") having multiple orifices, one or more injection orifice as
well as
one or more opposing orifices for positioning the injection orifice against
the target
tissue, or multiple orifices for ejecting fluid in multiple directions. These
and other
embodiments can alternately or additionally be useful for injecting tissue at
a
shallow angle.
Background
Urinary tract health is an increasingly important health issue, e.g., based on
an aging population. Treatment of urinary tract conditions is an area of much
investigation. Many methods and devices have been proposed to deliver
therapeutic
materials such as therapeutic fluid to the urinary tract, e.g., kidneys,
ureters, and
lower urinary tract (urethra, prostate, bladder, bladder neck).
Much effort has been focused on treating prostate tissue. Prostate disease is
a significant health risk for males. Diseases of the prostate include
prostatitis,
1
CA 02757180 2015-01-15
benign prostatic hyperplasia (BPH, also known as benign prostatic
hypertrophy),
prostatic intraepithelial neoplasia (PIN), and prostatic carcinoma.
In addition to prostate conditions, other tissue of the urinary tract can be
affected by medical conditions that can be treated by delivery of various
therapeutic
materials in the form of fluids. Tissues of the bladder (which includes the
bladder
neck), ureter, kidneys, urethra, as well as the prostate, can be treated by
delivery of
drugs or other therapeutic agents.
Various treatments of the bladder that are currently used or proposed, such as
transurethral administration of an active pharmaceutical agent, involve
placement of
a therapeutic fluid into the bladder using a single needle located at the
distal end of a
rigid shaft inserted into the bladder through the urethra. These methods can
involve
various difficulties or undesired effects and can be difficult to perform.
Needleless devices and methods for treating tissue of the urinary tract are
discussed in U.S. Patent Application Publication No. 2009/0312696 (Copa et
al.),
and U.S. Patent Application Publication No. 2006/0129125 (Copa et al.) .
A wide variety of
medical treatments are at least partially performed through the delivery and
introduction of therapeutic compositions to a treatment location by way of
needless ,
injection. For example, diseases of the prostate such as prostatitis, benign
prostatic
hyperplasia, and prostatic carcinoma, are treated by injection. Surgical
methods
used to relieve the symptoms of BPH include methods of promoting necrosis of
tissue that blocks the urethra by chemical ablation (chemoablation). In one
chemical
ablation technique, absolute ethanol is injected transurethrally into the
prostate
tissue. This technique is known as transurethral ethanol ablation of the
prostate
(TEAP). The injected ethanol causes cells of the prostate to burst, killing
the cells.
The prostate shrinks as the neerosed cells are absorbed.
One way in which therapeutic fluids can be delivered internally is through
the use a tube-like device configured to provide a jet-injection of the
therapeutic
fluid at a desired treatment site. Generally, a needleless injector is to
deliver the
therapeutic fluid from an external reservoir located at a proximal end of the
tube-like
device with such administration occurring at a distal end of the tube-like
device.
Due to the relatively long travel length of the therapeutic fluid through the
tube-like
2
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
device, the remote injector must generally be capable of pressurizing the
therapeutic
fluid to pressures exceeding about 2,000 psi. To accommodate these pressures,
the
tube-like devices have been fabricated of alloys such as NiTi or stainless
steel or
with metal-reinforced polymers such as the braided tubes typically found in
catheters. While the use of alloys and metal reinforced polymers satisfy the
operational requirements related to burst pressure and distention strength,
they are
generally of limited flexibility making them difficult to navigate within the
tortuous
paths often found in the human body such as, for example, the urogenital
tract.
According to certain methods of injecting the prostate, a transuretheral
flexible endoscopic probe is directed to the area of interest. Because a
flexible
endoscope is rotated inside bends, the injection tube will tend to
uncontrollably
rotate inside the channel of the endoscope because it does not have equal
bending
stiffness in all degrees of movement. Moreover, the articulating section of
the
flexible endoscope can typically only bend on one direction making compound
bends impossible. This is a problem in the anatomy around the prostate.
Therefore
there is a need to fix the injection tube in a preselected orientation so as
to enable an
injection in the desired direction.
Furthermore, treatment is more efficiently performed if the injection orifice
is proximate the target tissue. As the injection catheter is directed through
the
channel to the target tissue, whether within the endoscope or independently,
it is
unacceptable to simply rely on luck for proper placement. Thus there is a
further
need to direct the injection orifice proximate the target tissue with the
minimum of
moving parts and complexity due to the space constraints.
Different practical challenges exist for performing injections of other types
of tissue. Some tissues, such as bladder tissues, are thin in their depth
dimension
(i.e., shallow), making injection a challenge. For these tissues, there is
ongoing need
to improve injections, such as by increasing uniform distribution of agents
within
the thin tissue, over a desired area of the tissue.
For any injection or injected tissue, therapeutic agents should be delivered
with minimized discomfort and procedure time, and with the best possible
degree of
accuracy of delivery location and delivery volume, and with uniform and
accurate
distribution of a fluid throughout injected tissue. As such, there exists
continuing
3
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
need to provide improved devices for delivering therapeutic fluids to
different
tissues including but not limited to locations of the urinary tract including
the
bladder, bladder neck, prostate, urethra, kidneys, ureters, etc.
Summary
The invention involves needleless fluid injection devices. These devices
allow for localized delivery of therapeutic fluids that include biologically
active
species and agents such as chemical and biochemical agents, at desired
anatomical
tissue locations including but not limited to locations in the male or female
urinary
tract, e.g., bladder, bladder neck, kidney, ureters, urethra, prostate, etc.
Exemplary
devices can be designed to delivery fluid at various tissue locations,
optionally also
multiple different therapeutic fluids or multiple different tissue locations.
The
devices can be capable of delivery of precise amounts of fluid for injection
at precise
locations, for improved treatment based on precision and accuracy of fluid
delivery.
Features of described devices and methods address certain practical problems
associated with delivering (injecting) fluid to tissue. For example, injection
of fluid
to bladder tissue by use of a single needle at a distal end of a rigid shaft
can require
specialized dexterity and experience of a doctor due to the cumbersome nature
of a
rigid shaft, with just one needle. Devices and methods described herein
overcome
some of the challenges involved in using past tissue injection methods.
Embodiments of the described invention involve a fluid delivery system with
an injector source and an access device. The access device can comprise a
minimally invasive, tubular delivery lumen such as a catheter or endoscope.
The
injector source can include a non-metal, polymeric tube-like device for
delivering a
therapeutic fluid to a treatment site within a patient. The tube-like device
can further
include one or more apposing jets that can be selectively fired to force the
injection
orifice of the tube-like device against the target tissue. Selective firing
can include a
continuous firing during the injection to improve the efficiency of the
treatment. It
is envisioned that the apposing jets can have an independent source of jet
fluid and
an independent driving force such as a pressurized tank, magnetohydrodynamic
power, expanding steam, gas power or similar methods of propulsion. The
apposing
jets can include nozzles or vanes to improve the ability of the operator to
selectively
fire the apposing jet for creating contact with the target tissue.
4
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
The non-metal, polymeric tube-like device can be fabricated using suitable
high strength polymers including, for example, polyimide, polyetherimide
available
from General Electric under the trade name Ulteme, and linear aromatic
polymers
such as PEEKTM, available from Victrex plc for transporting the treatment
fluid and
the apposing jet medium to the treatment area. In some embodiments, the non-
metal, polymeric tube-like device can be reinforced through the inclusion of
materials including nano-particles, clays and/or glass. In some presently
contemplated embodiments, the non-metal, polymeric tube-like device can be
reinforced with one or more polymers such as, for example, tubes braided with
Kevlar or other high-strength polymers. The non-metal, polymeric tube-like
device
can be fabricated so as to have a burst strength exceeding at least about
2,000 psi
and in some embodiments, having a burst strength within a range of about 2,000
psi
to about 5,000 psi. The non-metal, polymeric tube-like device can be
fabricated so
as to have distention properties, wherein one or more orifices or jet ports
located at a
distal end of the polymeric tube-like device retains its shape and/or size
without
suffering swelling that can have a detrimental impact on a fluid jet used to
deliver
the therapeutic fluid at the treatment site.
In various embodiments, devices as described can be useful for injecting
tissue at different tissue depths and in any desired direction (relative to a
surface of
the injected tissue), including relatively deep injection ("deep injection")
of fluid
into tissue of any size or depth, or for shallow injection of fluid into
tissue at a depth
near a tissue surface, such as if the tissue is of a limited depth. Depending
on the
desired injection depth, orifices can be oriented at different locations along
a length
of a shaft and at different directions or angles relative to the shaft.
For tissue of limited (shallow) depth, such as bladder tissue, treatment of
the
tissue by injection may require the injected fluid to pass only a short
distance
beneath the tissue surface. Previous needleless methods of injecting shallow
tissue
have been performed by injecting a fluid stream at large angles relative to a
tissue
surface, such as normal (orthogonal) to a tissue surface. Upon passage through
the
tissue surface, the stream might disperse. In other words, prior art injection
methods
may allow an injected fluid stream to become dispersed after entry of the
"stream" at
an angle that may be normal to the tissue surface at the location of the
injection.
5
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
Embodiments of presently described methods allow for shallow tissue injection,
for
example by injecting a fluid stream at an angle that is non-normal to a tissue
surface,
such as a shallow angle relative to a tissue surface.
Shallow tissue injection (e.g., at an orthogonal angle) can be difficult,
especially if injecting tissue that includes a membrane at a surface that must
be
traversed by a fluid stream prior to the fluid stream reaching desired tissue;
a fluid
stream would require sufficient velocity to penetrate the membrane while not
passing through the shallow tissue to exit the tissue on the opposite side of
the
tissue. Past methods can also be very sensitive to technique: an operator of
an
injection device must be aware of the amount or pressure exerted by the tip of
an
injection device on tissue being injected, because the amount of pressure can
affect
the degree of penetration of a fluid stream injected at a perpendicular angle.
According to certain methods described herein, challenges of shallow tissue
injection can be overcome by injecting shallow tissue with a fluid stream
directed at
the tissue surface at an angle that is not normal to the surface but that is
directed at
the surface at a shallow angle. For example, such problems can be avoided if
the
injection orifice that produces the jet (fluid stream) is not aimed normal to
the
general boundary of targeted tissue but is aimed at an angle that is non-
normal,
especially a relatively shallow angle relative to the boundary, e.g., parallel
to the
boundary, or approximately parallel to the boundary. An injection aimed
parallel to
the general tissue boundary effectively lengthens or "thickens" the target
tissue with
respect to jet penetration: the amount of distance allowed for injection
(effective
depth of the tissue) increases. In certain embodiments, the fluid stream can
be
ejected from the orifice at a location that is below the general surface of
the tissue,
while not penetrating the tissue surface.
Devices useful for shallow injection can include an injection orifice at a
location near an end of a shaft (e.g., a distal end tip) to inject tissue by
placing the
distal end in an orientation normal to tissue can sometimes be referred to as
"end-
fire" devices. End-fire devices can be used for shallow injection method and
also
for deep injection methods, depending for example on the angle between the
direction of the injection orifice and the longitudinal axis of the shaft.
6
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
Certain described methods and devices can be useful for relatively "deep"
injection, e.g., injection to a depth that is greater than a shallow
injection. Devices
designed for deep injection can include one or multiple injection orifices
placed at
any useful location along a length of a shaft to contact tissue for injection,
and at any
angle. The injection orifices can be located, for example, a distance from a
distal
end tip that allows the injection orifice to be oriented to inject a tissue
surface as the
shaft is oriented lengthwise along a surface of the tissue, e.g., so a length
of shaft
can contact the tissue surface, such as if the shaft the portion of a shaft
that includes
an injection orifice is oriented parallel to a tissue surface. These devices
are
sometimes referred to as "side-fire" device embodiments.
Certain devices as described can include design features that allow for
improved handling, placement, control, and accuracy of injected fluid in terms
of
location distribution, and volume of fluid delivery. For example, multiple
injection
orifices can be arranged along a length or a circumference of a shaft to cause
forces
produced by ejection of fluid to be balanced or otherwise controlled, relative
to the
shaft. In some embodiments a net force on the shaft created by the ejection of
fluid
from multiple orifices at a shaft distal end can be zero. In other
embodiments, a net
force on a shaft created by the ejection of fluid from multiple orifices may
create a
force used to control the distal end of a device. A net force may be created
by
ejected fluid, for example, to place an injection orifice in apposition to
tissue; i.e., a
net force can cause a shaft and an injection orifice to be pressed against a
tissue
surface, for secure engagement between the injection orifice, shaft, and
tissue
surface, during an injection.
Still referring to certain exemplary embodiments (e.g., that allow for
improved handling, placement, control, and accuracy of injected fluid in terms
of
location distribution, and volume of fluid delivery) an access device can
comprise a
minimally invasive, tubular delivery lumen such as a catheter or endoscope;
the
tube-like device can further include one or more apposing jets that are
selectively
fired to force the injection orifice of the tube-like device against the
target tissue;
selective firing can include a continuous firing during the injection to
improve the
efficiency of the treatment. It is envisioned that the apposing jets can have
an
independent source of jet fluid and an independent driving force such as a
7
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
pressurized tank, magnetohydrodynamic power, expanding steam, gas power or
similar methods of propulsion. The apposing jets can include nozzles or vanes
to
improve the ability of the operator to selectively fire the apposing jet for
creating
contact with the target tissue.
In one aspect the invention relates to a needleless injection device that
includes a flexible shaft comprising a proximal end, a distal end, a distal
end tip, and
an injection lumen extending from the proximal end to ;the distal end. The
distal end
includes an injection orifice at a length-wise location of the distal end on a
proximal
side of the distal end tip. The injection orifice is in communication with the
injection lumen. The injection orifice is directed at an angle in the range
from 45 to
about 100 degrees relative to a longitudinal axis of the shaft at the length-
wise
location of the injection orifice. The shaft is capable of ejecting a fluid
stream from
the injection orifice, the fluid stream being capable of being injected into
tissue by
penetrating a tissue surface as a fluid stream at a non-normal angle relative
to the
tissue surface.
In another aspect the invention relates to a needleless injection device that
includes a flexible shaft comprising a proximal end, a distal end, a distal
end tip, and
an injection lumen extending from the proximal end to the distal end. The
distal end
includes an injection orifice at a length-wise location of the distal end on a
proximal
side of the distal end tip. The injection orifice is in communication with the
injection lumen. The injection orifice is directed at an angle in the range
from about
10 to about 170 degrees relative to a longitudinal axis of the shaft at the
length-wise
location of the injection orifice. At least one additional ejection orifice is
present at
the distal end. The device is capable of ejecting fluid from the injection
orifice in a
manner to produce an injection force on the distal end. And the device is
capable of
ejecting fluid from the at least one additional ejection orifice in a manner
to produce
an ejection force that at least partially opposes the injection force.
In another aspect the invention relates to a method of injecting tissue. The
method includes: providing a needleless injection device as described herein,
providing an injectate at the proximal end and in communication with the
injection
lumen, placing the injection orifice near a tissue surface without penetrating
a tissue
surface, and pressurizing the injectate to cause the injectate to be ejected
from the
8
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
injection orifice as a fluid stream that passes through the tissue surface and
disperses
as fluid particles in tissue below the tissue surface.
In another aspect the invention relates to a needleless method of injecting
tissue. The method includes providing a needleless injection device comprising
a
flexible shaft comprising a proximal end, a distal end, a distal end tip, and
an
injection lumen extending from the proximal end to the distal end. The distal
end
includes an injection orifice at a length-wise location of the distal end on a
proximal
side of the distal end tip. The injection orifice is in communication with the
injection lumen. The injection orifice is directed at an angle in the range
from about
45 to about 100 degrees relative to a longitudinal axis of the shaft at the
length-wise
location of the injection orifice. The method includes positioning the distal
end at a
location near a tissue surface and normal to the tissue surface, without the
injection
orifice penetrating the tissue surface, and ejecting a fluid stream from the
injection
orifice such that the fluid stream penetrates the tissue surface at a non-
normal angle
relative to the tissue surface.
In another aspect the invention relates to a needleless method of injecting
tissue. The method includes providing a needleless injection device
comprising: a
flexible shaft comprising a proximal end, a distal end, a distal end tip, an
injection
lumen extending from the proximal end to the distal end, and a control lumen
extending from the proximal end to the distal end. The distal end includes an
injection orifice at a length-wise location of the distal end on a proximal
side of the
distal end tip, the injection orifice in communication with the injection
lumen, the
injection orifice directed at an angle in the range from 10 to about 170
degrees
relative to a longitudinal axis of the shaft at the length-wise location of
the injection
orifice; and a control orifice. The method includes positioning the distal end
at a
location near a tissue surface with the injection orifice directed toward the
tissue
surface without the injection orifice penetrating the tissue surface, ejecting
a fluid
stream from the injection orifice such that the fluid stream penetrates the
tissue
surface, the ejection producing an injection force on the distal end, and
ejecting fluid
from the orifice to produce an control force to oppose the injection force.
9
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
In another aspect the invention relates to combinations of any two or more
components of a needleless injection system as described herein and selected
from: a
console, a connector member, an injection shaft, and a working shaft.
In another aspect, a combination can as indicated can be used by steps that
include: providing a needleless injection system comprising a console and
multiple
injection shafts; attaching a first injection shaft to the console and
ejecting a first
fluid to inject a first tissue of a first patient; detaching the first
injection shaft; and
attaching a second injection shaft to the console and ejecting a second fluid
to inject
a second tissue of a second patient. The combination can also include one or
more
connector member (e.g., detachable pressure chamber) that can also be changed
between injections.
The above summary of the various representative embodiments of the
invention is not intended to describe each illustrated embodiment or every
implementation of the invention. Rather, the embodiments are chosen and
described
so that others skilled in the art may appreciate and understand the principles
and
practices of the invention. The figures in the detailed description that
follows more
particularly exemplify these embodiments.
Brief Description of the Drawings
All drawings are exemplary and not to scale.
Figure 1 illustrates a side view of a past method of injecting shallow tissue.
Figures 2A, 2B, 2C, and 2D illustrate various features and details of
described methods of injecting tissue, such as shallow tissue.
Figures 3A, 3B, 3F, 3G, and 3H are side (3G) or side-sectional views of
distal ends of embodiments of injection shafts as described.
Figures 3C, 3D, and 3E illustrate distal ends of an embodiment of injection
shaft as described, and related methods.
Figures 31, 3J, 3K, and 3L are cross-sectional views of distal ends of
embodiments of injection shafts as described.
Figures 4A and 4B illustrate side-sectional views of distal ends of
embodiments of injection shafts as described.
Figures 4C is a cross-sectional view of a distal end of an embodiment of
injection shaft as described.
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
Figures 5A, 5B, and 5C illustrate cross sectional views of distal ends of
shafts as described, and related methods.
Figure 6 illustrates a side view of a distal end of a shaft as described, and
related method steps.
Figures 7A, 7B, 7C, 7D, 7E, and 7F illustrate side-sectional views of distal
ends of shafts as described.
Figures 8A, 8B, 8C, and 8D illustrate side-sectional and end views of distal
ends of shafts as described.
Figure 9 illustrates a side-sectional view of a distal end of a shaft as
described.
Figure 10 is a schematic of an injector system incorporating the present
invention.
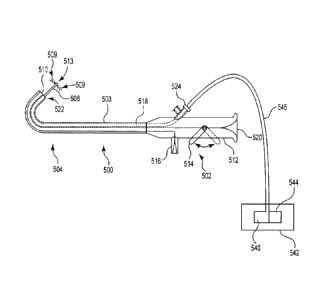
Figure 11 is a perspective view of an exemplary access device and injector
source.
Figure 12 is a cross sectional view of an exemplary injector source.
Figure 13 is a cross sectional view of an exemplary injector source relative
to
a treatment location with jets firing.
Figure 14 is cross sectional view of an exemplary injector source relative to
a
treatment location with jet firing and the injector firing.
Figure 15 is cross sectional view of an exemplary injector source.
Figure 16 illustrates a system as described.
Figure 17 illustrates options of combinations of systems as described.
Figures 18A, 18B, 18C, and 18D are side-sectional views of distal ends of
embodiments of injection shafts as described.
Figure 19 is a schematic view of an exemplary injection shaft of a high-
pressure injection system in accordance with the present invention.
Figure 20 is a cross-sectional view of an exemplary injection shaft of a high-
pressure injection system in accordance with the present invention.
Figure 21 is a side view of an exemplary configuration for the injection shaft
of Figure 20.
Figure 22 is a side view of another exemplary configuration for the injection
shaft of Figure 20.
11
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
Figure 23 is a side view of another exemplary injection shaft of a high-
pressure injection system in accordance with the present invention.
Figure 24 is a side view of another exemplary injection shaft of a high-
pressure injection system in accordance with the present invention.
Figure 25 is an end view of an exemplary configuration that can be used for
either of the injection shafts of Figures 23 and 24.
Figure 26 is another end view of an exemplary configuration that can be used
for either of the injection shafts of Figures 23 and 24.
Figure 27 is a partial schematic view of an exemplary deflector device in
accordance with the present invention.
Detailed Description
In the following detailed description, numerous specific details are set forth
to provide a thorough understanding of various aspects of the described
devices and
methods. It will be apparent to those of skill in the relevant arts that
described
features can be practiced without these specific details. In other instances,
well-
known methods, procedures, and components have not been described in detail so
as
to not unnecessarily obscure inventive aspects.
The invention relates to devices and methods useful for injecting fluid into
tissue for treatment. The fluid can be injected without the use of a needle
that would
include a needle structure to penetrate tissue, projecting through a tissue
surface to
place a needle opening within a tissue mass. A needleless injection orifice
delivers
fluid in the form of a stream of fluid (e.g., a "jet" or "fluid stream") at a
pressure,
velocity, and stream size, that allow the fluid stream to pass through a
tissue surface,
penetrate into the bulk of the tissue below the tissue surface, and become
dispersed
as fluid particles within the tissue, such as in the form of a cloud of
dispersed fluid
particles or droplets, without a needle structure passing into the tissue. The
type of
tissue injected for treatment can be any amenable tissue including but not
limited to
tissue at or near the urinary tract, e.g., tissue of the prostate, kidneys,
ureters,
urethral tissue, bladder (including the bladder neck), etc., or other tissues
such as
heart tissue, as desired.
Needleless devices as described generally include a distal end and a proximal
end. As used herein, the "distal end" refers to a portion of the device that
is located
12
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
internally within a patient's body during a treatment procedure, generally
including
the distal end of an elongate shaft. A distal end may include functional
features that
operate on fluid or tissue during use, such as one or more ejection orifice,
optional
delivery head (end effector, nozzle, etc.) to house one or more ejection
orifices,
optionally a frictional tissue holding tip, optionally a tissue tensioner,
optionally one
or more of a light, optical feature, steering feature, etc.
A "proximal end" of an exemplary needleless device can include an injector
body or "console" that remains external to the patient during use. An
exemplary
console can include a housing that connects to or is otherwise (directly or
indirectly)
in fluid communication with the shaft. The console can include fluid that can
be
pressurized by a pressure source to cause the fluid to flow through the shaft
for
injection into tissue at the distal end.
A device can eject fluid from one or multiple ejection orifices including at
least one injection orifice located at the distal end of the shaft.
Optionally, multiple
ejection orifices may be located at one or more locations along a length of or
about a
circumference of a shaft distal end. An ejection orifice can be of a type
designed to
eject fluid to be injected into tissue, i.e., an "injection orifice." Other
ejection
orifices can be designed to eject fluid to produce a control force at a distal
end of a
shaft during an injection, i.e., a "control orifice." In some embodiments, an
ejection
orifice may both eject fluid for injection, and function to produce a control
force,
e.g., such as occurs with multiple injection orifices arranged at different
locations
around a circumference of a shaft at a single length-wise location. A lumen
within a
shaft can connect an ejection orifice (injection orifice or control orifice)
at a distal
end with a fluid source at a proximal end of the device; a shaft may contain
one or
multiple such lumens.
A shaft may include any one or more control feature to control placement of
injected fluid by improving control of a distal end of a device at a location
of an
injection orifice. Examples of control features include the presence of
multiple
injection orifices directed to different tissue locations or in multiple
directions
around a circumference of a shaft; the use of non-injection orifices referred
to as
"control" orifices to offset forces produced by injected fluid; tissue
tensioners; a
13
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
distal end tissue holding tip that can be used to frictionally engage tissue;
a steerable
distal end; and combinations of these.
Devices, systems, and methods are provided that can be used to inject a fluid
(sometimes referred to as an "injectate" or "injection fluid" which may be any
type
of fluid such as a therapeutic fluid) into tissue in a needleless manner
whereby the
injectate passes as a pressurized fluid stream (or "jet") through a surface of
a tissue,
penetrating without the use of a needle through the tissue surface and into
the bulk
of the tissue, and dispersing as particles or droplets within the tissue below
the tissue
surface. This contrasts with injections performed using a needle, whereby a
hollow
needle structure penetrates tissue to locate a hollow end of the needle within
a tissue
mass, below the tissue surface, after which the needle carries fluid into the
bulk of
the tissue and delivers the fluid at a relatively low pressure to the tissue
in the form
of a body or pool of fluid known as a bolus.
A fluid stream or jet ejected for injection into tissue by a needleless
injection
system can be of a size (e.g., diameter), velocity, pressure, and volume to
allow the
fluid stream to penetrate directly through a tissue surface, then disperse
within the
tissue. The stream can be considered to be a relatively high velocity, high
pressure,
small diameter jet that after entry through a tissue surface, disperses within
the
tissue, preferably as a multi-directional collection of particles (e.g., a
"cloud") or
droplets within the bulk of the tissue. Exemplary pressures of a fluid at a
pressure
chamber can be at least 200 pounds per square inch (psi), e.g., from 300 to
5000
pounds per square inch. Without limiting the scope of the present description:
when
injecting bladder tissue a pressure of from 250 to 1000 psi can be effective,
measured at the pressure chamber; when injecting prostate tissue a pressure of
from
3500 to 5000 psi can be effective, measured at the pressure chamber.
Exemplary needleless devices may be used for treating various physical
ailments or conditions at any bodily tissue, for example to treat tissue that
contains
or is within reach of injection through a body cavity or body lumen, e.g., by
accessing tissue through a body lumen, vessel, or cavity, and injecting tissue
by
placing an injection orifice within the lumen, vessel, or cavity. Examples of
specific
tissues that can be treated by injection include tissue of the urinary tract
and nearby
tissues, e.g., tissue of the bladder or bladder neck, kidney, ureter, urethra,
prostate.
14
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
Other tissues can also be treated by injection using devices and methods as
described. Devices and methods as described can accommodate injection of
diverse
tissue types, including tissues at different locations or of different sizes,
including
tissues that exhibit a limited depth or thickness dimension that may be
difficult to
inject using other needleless (or needle-type) methods and devices. For
example,
certain embodiments of methods and devices can be particularly useful for
injecting
tissue that has a shallow "depth," by injecting the tissue laterally at a
shallow angle
relative to the tissue.
Exemplary devices and methods can perform shallow injection of fluid into
tissue by placing an injection orifice near a tissue surface and ejecting
fluid laterally
to penetrate the tissue surface and become dispersed within the tissue at a
location
near the tissue surface. Certain tissues are somewhat shallow in depth, such
as
bladder tissue. Shallow injection methods may be used to treat any type of
bodily
tissue, if desired. Yet certain tissues, due to a shallow depth, may not be
easily
treated using past needleless injection methods. For example, some types of
shallow
tissue may be susceptible of injected fluid being passed through a shallow
tissue
during injection, exiting the tissue on the side opposite of the injection,
possibly
negating the effect of a portion of the injected fluid or placing injected
fluid at an
undesired location. Such tissues may not have substantial depth, e.g., are not
at least
10 millimeters deep, e.g., measured between opposing tissue surfaces. Examples
of
tissues that can be treated using shallow injection methods as described
herein
include tissues that have a thickness dimension that is less than 10
millimeters, such
as tissues having a thickness in the range from 2 to 10 millimeters. Such
tissues
include bladder tissue (including the bladder neck).
Previous needleless injection methods of shallow tissue have been performed
by injecting a fluid stream at large angles relative to a tissue surface, such
as
substantially normal (orthogonal) to a tissue surface, or approximately
orthogonal,
e.g., within 10 or 20 degrees from orthogonal. Figure 1, for example,
illustrates a
device and method of injecting shallow tissue, exemplary of previous methods.
Referring to figure 1, tissue 10 has a relatively shallow thickness "t," and
may be,
for example, bladder tissue, which may have a thickness in the range from
about 3 to
4 millimeters. Shaft 12 includes lumen 14 and orifice 20 passing through the
end of
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
shaft 12 in a direction along a longitudinal axis AL of shaft 12. Shaft 12 is
oriented
in an orthogonal attitude relative to tissue surface 18, at the location of
contact
between the end of shaft 12 and surface 18. To inject fluid 16 into tissue 10,
fluid
16 is ejected from orifice 20, passes through tissue surface 18, and enters
the bulk of
tissue 10. Fluid 16 is injected through surface 18 and into tissue 10 by
ejecting
stream of fluid 16 in an orientation that is relatively orthogonal to surface
18.
In contrast, according to certain methods and devices described herein, tissue
can be injected at a shallow angle relative to a tissue surface, to place
injectate
within a mass of tissue, near a tissue surface. The tissue may be shallow
tissue such
as bladder tissue. Alternately, the tissue may be non-shallow tissue such as
prostate
tissue or cardiac tissue, e.g., if desired to inject non-shallow tissue by
placing
injectate at a location near a tissue surface. Examples of shallow injection
involve
injecting a fluid stream into a tissue surface at a shallow angle to allow for
injection
and dispersal of fluid within tissue near a tissue surface, while reducing the
risk that
fluid passes through tissue, exiting on an opposite surface.
In many or most instances of placing a distal end tip of a shaft in contact
with tissue, at a normal (i.e., orthogonal) orientation, the distal end tip
will cause the
tissue to deflect or deform ("indent") due to the deformable nature of soft
tissue.
(As used herein, a "distal end tip" can be considered a location of a distal
end of a
shaft that is the farthest (most distal) feature of the distal end). The size
(area) of the
deformed tissue will depend on factors such as the amount of pressure exerted
on the
tissue, the size of the distal end tip, the nature (e.g., deformability) of
the tissue,
among others. When injecting tissue that can become deformed or indented by
pressure placed on the tissue by a distal end tip, at least a portion of the
distal end of
the shaft, potentially including one or more injection orifice, may become
located at
a position that is below a level of a surface of adjacent tissue. (See figures
2A
through 2D.) In these instances, the distal end tip does not necessarily
penetrate the
tissue surface but deforms the tissue surface so the distal end tip and
optionally one
or more injection orifice can become located at a position relative to nearby
tissue
that is "below" the tissue surface. As illustrated at figure 2C, for example,
distal end
tip 42, D02, and orifices 40, are located "below" line T, which intersects
tissue
surface 38. By this placement of a distal end tip and injection orifices, an
injection
16
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
orifice can access tissue laterally, and by ejecting fluid laterally can
inject fluid a
greater distance (i.e., a lateral distance) into the tissue.
Exemplary shallow injection methods can involve using a distal end of a
shaft by orienting the distal end in an orientation that is orthogonal to a
tissue
surface (meaning, for example, within 20 or 10 degrees of nonnal, preferably
within
5 degrees of normal), and placing a longitudinal (normal) force on the distal
end to
cause the distal end tip to exert pressure on the tissue surface. A shallow
injection
method can inject a fluid into tissue by injection of a fluid stream that is
non-normal
to the tissue surface, such as by orienting a fluid stream at an angle in the
range from
0 to 45 degrees below a tissue surface, e.g., from 0 to 10 degrees, or
approximately
(within 10 degrees from) parallel to a tissue surface.
For purposes of measuring angles of a fluid stream relative to a tissue
surface, a direction (i.e., a line) of a fluid stream can be considered to be
the same
direction (line) as a direction (line) defined by an axis (e.g., axis of flow)
of an
orifice that delivers the fluid stream. A direction (line) of a tissue surface
can be a
direction along a tissue surface (which surface is generally not planar, and
optionally
may be indented by a distal end tip of a shaft), the direction intersecting a
longitudinal axis of a shaft, so the direction of the tissue surface is
coplanar with the
longitudinal axis, the direction of the tissue surface also being coplanar
with the
direction of the fluid stream. By one exemplary measurement, the direction of
the
tissue surface can be taken as the direction of the tissue surface at the
location
(point) at which the fluid stream enters tissue. See, for example, figure 2B
and
related text. By another exemplary measurement, the direction of a tissue
surface
can be taken as a line that extends across a distance of tissue surface, a
distance
away from the shaft, optionally and preferably a distance away from any tissue
indented by a distal end tip of a shaft, the distance being, for example, the
lateral
distance to which fluid penetrates the tissue when injected at a shallow
angle. See,
for example, figure 2C and related text. A direction or line of the tissue
surface can
be assessed as an average location of surface tissue along a chosen distance.
Figures 2A through 2D illustrate an exemplary device and exemplary method
for injecting shallow tissue, at a shallow angle. (The illustrated device and
method
could alternately be useful to inject non-shallow tissue at a shallow angle).
17
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
Referring to figures 2A through 2D, tissue 30 has a relatively shallow
thickness "t"
and may be, for example, bladder tissue having an exemplary thickness in the
range
from about 3 to about 4 millimeters (mm). A portion of a distal end of shaft
(e.g.,
injection shaft) 32 includes lumen (e.g., injection lumen) 34, two injection
orifices
40, and distal end tip 42. Each injection orifice 40 is located within
millimeters of
distal end tip 42, and each is located at the same length-wise location along
the
length of shaft 32. Each orifice 40 is directed in a direction D0,1, D0,2, and
these
directions, as illustrated, are opposing directions that intersect
longitudinal axis AL
of shaft 32 and extend laterally at angle a, perpendicular to longitudinal
axis AL,
which is approximately 90 degrees. Shaft 32 is oriented in a substantially
orthogonal attitude relative to tissue surface 38, measured at the location of
contact
between distal end tip 42 and tissue surface 38 (i.e., a tangent of tissue
surface 38;
see figure 2D, showing angle a3, approximately 90 degrees, between AL, and
tissue
surface 38 (line S is tangent to surface 38 at the location at which tissue
surface 38
contacts distal end tip 42).
Still referring to figures 2A through 2D, fluid can be ejected from orifices
40
as a two opposing fluid streams (not shown), each of which can penetrate
tissue
surface 38 and become dispersed as particles or droplets within tissue 30. The
fluid
streams each pass through tissue surface 38 at the intersection of orifice
directions
D0,1 and D0,2, and the locations of tissue surface 38 immediately adjacent to
each
orifice 40. At figure 2B, lines T1 and T2 each represent a direction of tissue
surfaces
38 at points of entry 13E0 and PE,2. As also illustrated at figure 2B, angles
of entry
ctE,i and aE,2 (angles between a fluid stream and a tissue surface) may
generally be in
the range between approximately 20 degrees and 90 degrees, depending, e.g., on
the
depth to which distal end tip 42 indents into tissue surface 38.
Figure 2C illustrates an alternate embodiment of a method of injecting a fluid
stream at a shallow angle between a tissue surface and an injected fluid
stream (or
injection orifice). As shown at figure 2C, line T illustrates a surface of
tissue surface
38, determined as a line that intersects an average location of tissue surface
38 in a
direction that intersects axis AL, and that is coplanar with both D0,2 and AL.
As
illustrated, the angle between injection orifice 40 (as represented by line
D0,2) and
an average location of tissue surface below which fluid is injected by a fluid
stream
18
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
ejected from injection orifice 40 (as represented by line T), may be a shallow
angle,
such as from about 0 degrees (i.e., an angle at which the injection stream is
parallel
to tissue surface T) and 45 degrees, such as from 0 degrees to about 30
degrees.
Still referring to figures 2A through 2D, fluid becomes injected through
surface 38 and into tissue 30 by ejecting a fluid stream from each orifice 40,
in an
orientation that is at a shallow angle to surface 38, as measured at points of
entry
(PE,1 and PE,2) of a fluid stream into a tissue surface. Further, as described
with
reference to figure 2C, according to these methods and devices, the fluid is
also
injected at a shallow angle to surface 38 as a shallow angle is measured
between a
direction of a fluid stream (e.g., injection orifice) and a direction of
tissue below
which the fluid is injected.
As will be appreciated from the present description, shallow angle tissue
injection can be performed using various approaches and techniques. By certain
techniques, tissue can be indented by a distal end of a shaft (e.g., by a
distal end tip)
to different depths, and injection orifices can be located at various
positions on the
distal end, e.g., on a proximal side of the "distal end tip," but near the
distal end tip.
A device can be designed with various and useful different shapes and
geometries of
a distal end, especially near a "distal end tip," such as designs that can
result in
indentation of tissue. Also, different orifice geometries and different
orientations
(angles and length-wise and circumferential positions) of one or more orifice
relative
to a shaft can be used, as desired. Multiple orifices may be placed around the
circumference of a distal end tip, optionally in combination with a structure
near a
distal end tip that acts to indent or deflect tissue (e.g., a "tissue
indenter") to allow an
injection orifice to be located below a level of adjacent tissue. A tissue
indenter can
be a structure near or adjacent to a distal end tip that is designed to
deflect (depress,
indent, or deform) tissue to allow an injection orifice to become located at a
location
beneath a surface of adjacent tissue (non-indented tissue that is adjacent to
the
indented tissue), to allow the orifice to direct a fluid stream or jet of
ejected fluid in
a lateral direction to penetrate the adjacent tissue below the surface of the
adjacent
tissue. The injection orifice can be and aimed (directed) at a shallow angle
to (e.g.,
parallel to) to the general boundary or surface of target tissue away from the
indented tissue to allow shallow tissue injection.
19
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
Optionally, a distal end of a shaft designed for shallow tissue injection and
indentation of tissue, by an end-fire design, can include a feature that
provides
feedback to the user as to the depth to which the distal end tip is indenting
tissue, or
a feature that limits the depth to which an injection orifice located near a
distal end
tip can indent tissue. The feedback or depth-limiting structure can be a
substantially
lateral extension emanating from the shaft on a proximal side of the distal
end tip,
also on a proximal side of the injection orifice; various examples of suitable
structures include a "mane" or shoulder that extends laterally (e.g.,
approximately 90
degrees from a longitudinal axis of the shaft) around a circumference of the
shaft,
adjacent to and on a proximal side of the injection orifice, e.g., at a
distance less than
5 millimeters from distal end tip; graduations as described here to visually
(by an
optical function of a shaft) measure a depth of indentation of a shaft distal
end and
an injection orifice relative to a tissue surface; or any other structural
protrusion that
allows feedback for a level of indentation of the distal end tip, into tissue.
The
depth-limiting structure may be prepared of any material suitable for a shaft
or
injection shaft, such as most metals, strong polymers such as PEEK,
polycarbonate,
UltemTM, and others. The structure and a nearby distal end tip may be of any
size
and geometry to allow indentation of the distal end tip and optional feedback
or
depth-limiting functionality, and may be formed directly from the material of
the
injection shaft.
A shallow injection as described can be useful to inject a fluid to a location
that is a shallow distance beneath a tissue surface. This may be desirable for
tissue
that is of a shallow depth such as bladder tissue, or for other tissues such
as heart
tissue, even if the tissue is not of a shallow depth. A shallow injection may
allow
injection of fluid to a depth of up to about 10 millimeters below a tissue
surface.
In a device useful to perform a shallow injection method, an injection orifice
may be directed at an angle that is in the range from 45 degrees to about 135
degrees
relative to a longitudinal axis of a shaft (e.g., an injection shaft), for
example an
angle that is in the range from 70 degrees to about 110 degrees from the
longitudinal
axis of a shaft at the location of the injection orifice. The direction (line)
of the
injection orifice can be measured as an axis of an injection orifice (e.g.,
bore or
aperture) that intersects the longitudinal axis (or a tangent thereof) of the
shaft, that
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
is coplanar with the longitudinal axis (or a tangent thereof) of the shaft,
and that is
based on the longitudinal axis (or a tangent thereof) in a direction of the
distal end
tip being an angle of zero degrees and the longitudinal axis in a direction of
the
proximal end of a shaft being an angle of 180 degrees.
Also according to certain shallow injection methods, an injection orifice may
be located at a length-wise location along a length of a distal end of a
shaft, near a
distal end tip, to allow the distal end to be placed normal to a surface, and
to direct
an ejected fluid to enter tissue at a shallow angle relative to the surface.
In these
embodiments an injection orifice can be located relatively near a distal end
tip of a
distal end of a shaft so that as the distal end of the shaft is placed normal
to tissue
(optionally to indent or deflect the tissue), the injection orifice is located
at a
location near the tissue surface and directed to inject fluid into the tissue
at a shallow
angle relative to the tissue surface. A useful distance between an injection
orifice
(measured at a center or axis of the injection orifice) and a distal end tip
may be, for
example, less than 5 millimeters, such as in the range between about 3 to
about 1
millimeter (e.g., measured along a line that is parallel to the longitudinal
axis of the
shaft).
Figures 3A through 3D illustrate examples of distal ends of shafts that can be
considered end-fire devices having one or more injection orifice located at a
location
to inject fluid into tissue at a shallow angle, with the shaft distal end
positioned
against tissue in an approximately orthogonal or noiinal orientation.
Referring to figure 3A, shaft distal end 50, an injection shaft, in cross-
section, includes shaft sidewalls 52, injection lumen 54, and injection
orifices 56 and
57, directed in opposing directions 58 and 59. Directions 58 and 59 are
substantially
perpendicular to longitudinal axis AL. Distal end tip 60 is a surface
orthogonal to
longitudinal axis AL. Distance D between injection orifices 56 and 57, and a
plane
orthogonal to distal end tip 60, can be, e.g., shorter than 5 millimeters.
Figure 3B shows another example of a similar shaft, this one modified to
include tissue holding tip 61, adjacent to distal end tip 60 that includes a
frictional
extension capable of frictionally engaging tissue when distal end 50 is placed
at an
orientation orthogonal to a tissue surface.
21
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
The frictional extension may be designed to frictionally engage tissue to
prevent movement of distal end 50 and injection orifices 56 and 57, upon
ejection of
fluid from the injection orifices. Additionally or alternately, a frictional
extension
can be used to allow a user to place a shaft distal end at an orientation
normal to a
tissue surface. In some anatomical locations, a surface of a tissue may not be
sufficiently accessible to allow a long injection shaft to approach a tissue
surface
from a normal orientation. In such instances, a shaft distal end (50) having a
frictional extension or tissue holding tip 61, can approach a tissue surface
(53) at a
more shallow angle, e.g., from 10 to 80 degrees relative to a tissue surface,
or from
20 to 70 degrees. See figure 3C. As shown at figure 3D, the tissue holding tip
(61)
can frictionally engage (without necessarily penetrating, but optionally
merely
indenting) tissue surface 53 at a non-normal angle. An operator can then
manipulate
flexible shaft distal end 50 using pressure and movement, e.g., by creating a
curve
(49) at flexible shaft distal end 50, while tissue holding tip 61 remains
frictionally
engaged with tissue surface 53, to place at least a portion of shaft distal
end 50 near
tissue surface 53, at an orientation normal to tissue surface 53. See figure
3E.
Figure 3F shows another example of a similar shaft, this one modified to
include a tissue holding tip 61, adjacent to distal end tip 60 that includes a
frictional
extension capable of frictionally engaging tissue when distal end 50 is placed
at an
orientation orthogonal to a tissue surface. Additionally, orifices 56 and 57
are
directed in directions 58 and 59, respectively, angled to longitudinal axis
AL. The
angle between directions 58 and 59 of orifices 56 and 57, and longitudinal
axis AL,
can be, e.g., from about 60 to 30 degrees.
Figure 30 (side view) shows another example of a similar shaft, this one
modified to include graduations 63, which are markings on an outside surface
of
shaft sidewalls 52. Graduations 63 can be markings or other indications that
indicate a distance from an orifice, e.g. 56, so that a degree of deflection
of tissue
can be measured by comparison of a tissue surface to graduations 63, using an
optical feature of a working shaft. For example, a graduation can be used by
placing
shaft distal end 50 normal to tissue and placing nonual pressure onto the
shaft such
that distal end tip 60 becomes located below a general tissue surface, due to
indenting or deflecting of the tissue. Using an optical functionality such as
that of
22
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
an endoscope, cystoscope, or other working shaft or medical device shaft, the
distance to which orifice 56 becomes located below a general surface of
tissue, due
to indentation of the tissue, can be measured according to graduations 63.
Upon a
desired degree of indentation, an injection can be made. Graduations 63 can be
any
markings, and can indicate any measure of distance, generally a small distance
such
as a millimeter or fraction of an inch.
Figure 3H shows another variation of a distal end 50 that includes only a
single injection orifice 58, at an angle about 45 degrees from longitudinal
axis AL.
Shaft distal ends 50 as illustrated at figures 3A through 3H are exemplary,
for example are illustrated to include one or two injection orifices. Any of
these
distal ends could be further modified as described herein, such as to include
additional injection lumens, additional injection orifices, one or more
control
orifices, etc. As illustrated at figures 31, 3J, 3K, and 3L, (in cross-section
at a
length-wise location of multiple injection lumens along a length of the shaft)
multiple injection orifices can be placed around a circumference of an
injection
shaft, at any desired angle or angles relative to a longitudinal axis (e.g.,
perpendicular to the axis, or at an angle directed distally). As shown at
figures 31,
3J, 3K, and 3L, injection shaft 50 includes sidewalls 52, injection lumen 54,
and
injection orifices 58 (bores or apertures in sidewalls 52). Fluid streams 64
are being
ejected from injection orifices 58. Advantages of multiple injection orifices
at a
single length-wise location along a length of a distal end, e.g., distributed
at
equidistant locations around a shaft circumference, can balanced injection
forces and
improved uniformity of injection of tissue around the perimeter of the
injection
shaft. As illustrated, injection orifices are in the form of apertures or
bores formed
directly in shaft sidewalls; alternately, orifices can be part of a nozzle,
end effector,
injection head, etc.
Embodiments of the invention also allow for "deep" injection of fluid into
tissue having substantial depth by placing an injection orifice near a tissue
surface
and ejecting fluid from the injection orifice into tissue, substantially into
the tissue
below the surface and not merely near a tissue surface as with shallow
injection
methods. Description of an injection as a "deep" injection is relative,
referring to an
injection that can be relatively deeper into tissue compared to a shallow
injection, as
23
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
discussed. Deep injection methods can be used to inject tissue to cause
injectate to
penetrate past a tissue surface, for example to a depth that is at least about
7
millimeters below a tissue surface, e.g., to a depth in the range from about
10 to 30
millimeters below a tissue surface. A fluid stream may be directed
substantially
perpendicular to a tissue surface, or at any angle.
According to exemplary deep injection methods, one or more injection
orifice need not be (but at least one may be) located near a distal end tip;
one or
more injection orifice may be on a proximal side of a distal end tip at a
location that
allows the injection orifice and adjacent injection shaft sidewall to contact
a tissue
surface as a longitudinal axis of a shaft that contains the injection orifice
is
positioned in an orientation that is parallel to the tissue surface. These
device
embodiments are sometimes referred to as "side-fire" devices, herein.
In certain embodiments of "side-fire" devices, an injection orifice can be
located a distance away from a distal end tip, on a proximal side of the
distal end tip,
so the injection orifice is located to contact tissue for injection by placing
the shaft
sidewall in contact with tissue. The injection orifice can be located at a
location
along a length of the distal end of a shaft a distance away from a distal end
tip, so
that when a sidewall of the distal end of the shaft is placed to contact
tissue, such as
from within a body lumen, the injection orifice is located in position to
inject fluid
into the tissue. Examples of injection orifice locations for these embodiments
can be
locations along a distal end of a shaft that are in the range from about 1 to
about 40
millimeters from the distal end tip, on a proximal side of the distal end tip,
e.g., such
as a distance in the range from about 1 to about 25 millimeters from the
distal end
tip.
Examples of tissue that can be treated using a side-fire device for a deep
injection method can include tissues that have a depth dimension that is at
least 10
centimeters, optionally also tissue that is accessible through a body lumen or
cavity.
Such tissues include prostate tissue, which may be injected by passing
injectate
through a urethra, i.e., an injection can be initiated from an injection
orifice located
within a urethral lumen, the fluid stream penetrates urethra tissue, traverses
the
urethra tissue, and enters and penetrates prostate tissue.
24
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
A fluid stream for deep tissue injection may be directed at any angle relative
to a longitudinal axis of a shaft. The angle may differ depending on the type
of
tissue being injected and the location of the injection orifice along a distal
end of a
shaft. Useful angles may generally between 5 degrees to 175 degrees relative
to a
longitudinal axis of a shaft (based on the longitudinal axis in a direction of
the distal
end tip being an angle of zero degrees and the longitudinal axis in a
direction of the
proximal end of a shaft being an angle of 180 degrees). Exemplary angles can
include angles in the range from 20 to 160 degrees, such as angled in the
range from
45 degrees to 135, or from 70 to 110 degrees.
Figures 4A, 4B, and 4C illustrate examples of distal ends of shaft devices
that can be considered side-fire devices having one or more injection orifice
located
at a location along a length of the distal end of a shaft a distance away from
a distal
end tip, so that when a sidewall of the distal end of the shaft is placed to
contact
tissue, such as from within a body lumen, the injection orifice is located
adjacent to
a tissue surface in position to inject fluid into the tissue, through the
tissue surface.
Referring to figure 4A, shaft distal end 70, an injection shaft, in length-
wise
cross-section, includes shaft sidewalls 72, injection lumen 74, injection
orifices 76
and 77, directed laterally in directions 78 and 79, which are substantially
perpendicular to longitudinal axis AL. Distal end tip 60 is a surface
orthogonal to
longitudinal axis AL. Distance D between injection orifices 76 and 77, and a
plane
that contains distal end tip 60 (i.e. orthogonal to longitudinal axis AL at
the location
of distal end tip 60), can be e.g., in a range between 1 and about 40
millimeters.
Figure 4B is a variation of the shaft distal end of figure 4A. Shaft distal
end
70 of figure 4B includes control orifices 80 and 82 that are directed in
directions 81
and 83, opposite of directions 78 and 79. Control orifices 80 and 82 are
connected
to control lumen 84, which communicates with a proximal end of a needleless
injection device. Control fluid can flow under pressure from the proximal end,
through control lumen 84, and be ejected from each of control orifices 80 and
82.
Ejection of a control fluid through each of control orifices 80 and 82, during
an
ejection of fluid from injection orifices 76 and 77, can produce an ejection
force that
opposes an injection force created by the ejection of injectate from injection
orifices
76 and 77.
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
As illustrated at figures 4A, 4B, and 4C, a single control orifice opposes
each
injection orifice. In alternate embodiments, more than one control orifice
could be
used to oppose an injection force associated with each injection orifice.
Also, as
illustrated, each of the two control orifices 80 and 82 is connected to the
same
control lumen, 84; in alternate embodiments each control orifice may be
connected
to a separate, different control lumen. Figure 4C shows a cross-section end
view of
distal end 70 from a length-wise location at orifices 77 and 82. Also, as
illustrated,
injection and control orifices are in the form of apertures or bores formed
directly in
shaft sidewalls; alternately, orifices can be part of a nozzle, end effector,
injection
head, etc. Directions 78, 79, 81 and 83 are all shown to be substantially
perpendicular to longitudinal axis AL, but may alternately be angled relative
to
longitudinal axis AL, such as in a direction toward distal end tip 60, or
alternately,
toward a proximal shaft.
According dngeteop injection
certain embodiments
methods
mboedimes or
shallow
of athlleow injection
m
methodsdeiscreibcteidethds
ona, useful efulevices,
involving either
methods can involve controlling the placement of or movement of (e.g.,
reducing or
preventing movement of) an injection orifice (and structure that supports the
injection orifice such as a shaft, nozzle or nozzle head, end effector,
injection shaft,
or other component of a shaft located near the injection orifice) relative to
tissue,
during ejection of fluid from the injection orifice. Control of the placement
of an
injection orifice relative to tissue, and prevention of movement during an
injection,
can improve placement and therefore efficacy of injected fluid.
When fluid is ejected as a fluid stream from an orifice such as an injection
orifice, especially at high velocity, the ejected fluid produces a force
("ejection
force") on the orifice and structure supporting the orifice at a location of
the
ejection. The ejection force is in a direction opposite of the direction of
the ejected
fluid jet. (If the ejection is from an injection orifice, the ejection force
can be
referred to as an "injection force"). If unopposed, an ejection force (e.g.,
injection
force) can cause movement of an ejection orifice (e.g., injection orifice) and
nearby
supporting structure during the ejection (e.g., injection) and, consequently,
movement of the ejection orifice and structure that supports or contains the
ejection
orifice in the direction of the ejection force. An injection force can be
sufficient to
26
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
cause an injection orifice to be moved during an injection and alter or
misdirect the
direction of ejected fluid (injectate).
According to certain described methods and devices, an injection force can
be opposed to prevent movement of an injection orifice that would be cause by
an
injection force produced during injection. An opposing force can be at a
location
that is at the same length-wise location of a shaft as an injection force,
that is in the
opposite direction of the injection force, and that is preferably equal to or
greater in
magnitude than the injection force.
According to exemplary methods, fluid can be ejected from one or more
control orifice or injection orifice to produce an ejection force that opposes
an
injection force produced by fluid ejected from an injection orifice. An
opposing
force may be produced by a single orifice or a combination of two or more
orifices
that combine to produce a resultant force that opposes an injection force;
e.g., two or
more opposing forces can be used to produce a single resultant force that
opposes an
injection force. An opposing force from any particular ejection orifice may be
less
,than, greater than, or equal to the injection force, to produce a combined
resultant
opposing force that is preferably equal to or greater than an injection force
in
magnitude, and in an opposite direction. In some embodiments, a resultant
force
that opposes an injection force may be equal in magnitude to the injection
force. In
other embodiments a resultant force that opposes an injection force may be
greater
in magnitude than and opposite in direction relative to an injection force, to
result in
a net force on the shaft at a length-wise location of the injection orifice
that places
pressure between an injection orifice and tissue to be injected.
As illustrated and described, ejection orifices can take the form of an
aperture in a shaft, shaft sidewall, injection head, end effector, nozzle, or
the like.
Ejection orifices can be directed in any useful direction, as measured as an
angle
relative to a longitudinal axis of a shaft that contains the ejection orifice.
Exemplary
control orifices can be in the form of an aperture or bore having an axis
along the
direction of flow of fluid through the control orifice, and that intersects a
= 30 longitudinal axis of a shaft; intersection of an axis of an
ejection orifice and a
longitudinal axis of a shaft can avoid forces being placed on the shaft that
may tent
to produce twisting or rotational movement or unbalanced pressure on the
shaft.
27
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
Certain embodiments of methods and devices involve controlling, including
preventing, movement of an injection orifice during ejection of a fluid from
an
injection orifice. Certain exemplary methods can be useful with shallow
injection
methods and devices that include injection orifices placed near a distal end
tip, such
as end-fire devices. For example, a needleless injector can involve multiple
ejection
orifices arranged around a circumference of a shaft, optionally each at the
same
length-wise location along the length of the shaft, to cause a net force on a
shaft (a
device shaft or a component of a device shaft such as an injection shaft,
injection
head, nozzle, etc.) to be balanced to produce no net force on the shaft. E.g.,
an
injection force can be opposed or balanced by the use of multiple ejection
orifices
located around a circumference of a shaft. Multiple fluid streams can be
ejected
from the multiple ejection orifices at once, simultaneously, preferably each
producing a force of equal magnitude (such as by ejecting equal flows of fluid
at
equal velocities), each producing a separate ejection force. Each force may be
the
same magnitude or different magnitudes, but the resultant force of the
combined
multiple ejection forces on a shaft can be balanced to prevent a net force on
the shaft
that would cause movement of the shaft during injection.
Referring, for example, to figures 31, 3J, 3K, and 3L, these show cross
sections of shaft distal ends, at a length-wise location of a shaft, that can
exhibit
balanced forces produced by multiple injection orifices. In each figure,
multiple
fluid streams are ejected from injection orifices. Each stream is directed
along a line
that intersects longitudinal axis AL. When all of the fluid streams ejected
from
multiple injection orifices of the illustrated devices produce equal injection
forces,
the injection forces produce a balanced force (net zero force) on the shaft.
In alternate embodiments that result in a balanced force on a shaft, one or
more ejection orifices can eject a fluid that does not become injected into
tissue, but
that opposes (i.e., at least in part), balances, or overcomes an injection
force; the
fluid can be referred to as a control fluid and the orifice can be referred to
as a
control orifice.
Embodiments of devices and methods can involve controlling placement of
an injection orifice adjacent to desired tissue by use of an ejection force in
the form
of a control force. During ejection of an injectate, for instance by a deep
injection
28
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
method, an injection orifice may desirably be placed adjacent to or against a
tissue
surface, e.g., in close contact with the tissue surface, to cause a jet of
ejected fluid
(injectate) to penetrate the tissue surface and become dispersed beneath the
tissue
surface, within the tissue. To improve the nature of the injection, the
injection orifice
may preferably be held in close contact with the tissue surface, such as with
a force
that causes the injection orifice (or adjacent shaft sidewall, ejection head
surface,
etc.) to be pressed against the tissue surface.
According to various embodiments of devices and methods, a device may
include multiple ejection orifices arranged around a circumference of a shaft,
optionally but not necessarily each at the same length-wise location along the
length
of the shaft, to cause a net force on a shaft to cause an injection orifice
(or nearby
shaft surface, or the like) to contact and to be placed with pressure against
tissue to
be injected. For example one or multiple control orifice can be directed to
produce a
net force ("control force") in a direction opposite of an injection force. The
net
ejection force from the control orifices can be in an opposite direction
relative to an
injection force, and of a greater magnitude than the injection force. The
magnitude
of the control force can be sufficiently greater than the magnitude of the
injection
force to cause the injection orifice to maintain contact with a surface of
tissue during
an injection. The control force can be applied during the injection, but also
prior to
the injection. Without limiting the present disclosure, generally, an force
can be any
amount, but may generally be no greater than about 0.5 pound-force.
Figures 5A, 5B, and 5C illustrate examples (in cross-section) of distal ends
of injection shafts that involve an injection force that is opposed by a
control force
produced by ejection of control fluid from multiple control orifices; the
control
orifices create an apposition force (e.g., opposing force) against an
injection force, to
cause apposition of the injection orifice against an injection site, to allow
injection
of fluid into tissue in an effective manner. Generally, methods and devices
that
involve control orifices for apposition (the placement of pressure of an
injection
orifice against tissue) during injection can involve ejection of any gaseous
or liquid
fluid from one or multiple control orifices, to create a control force that
opposes an
injection force. The control force can be opposite in direction and greater in
magnitude, relative to the magnitude and direction of the injection force. A
single
29
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
control orifice may produce a useful control force, or multiple control
orifices can be
located in any desired arrangement circumferentially or axially, each
producing a
force, the combination of the individual forces being a resultant control
force that is
opposite in direction and greater in magnitude relative to the injection
force. By
exemplary methods, a distal end of a shaft may be placed near tissue that is
to be
injected; a control force may be created to cause apposition of the injection
orifice,
i.e., to pressure the shaft and injection orifice against tissue; with the
control force in
place, the injection may be performed by ejection fluid from the injection
orifice
placed in apposition to the tissue; after injection the control force may be
removed.
Referring, for example, to figures 5A through 5C, these show cross sections
of shaft distal end 90, at a length-wise location of a shaft. Shaft distal end
90
includes sidewall 92, injection lumen 94, injection orifice 96, control
orifices 102,
and control lumen 104. Shaft distal end 90 is shown to be located within lumen
106,
which may be any body lumen such as a urethra passing through a prostate.
At figure 5A, distal end 90 is placed within lumen 106 (e.g., a urethral
lumen), which is adjacent to tissue 108 (e.g., prostate tissue). Figure 5B
shows
distal end 90 within lumen 106, with control fluid (e.g., gas or liquid) being
ejected
from control orifices 102. A resultant control force is illustrated as vector
Fc.
Control force Fc presses injection orifice 96 and adjacent sidewalls of distal
end 90,
against an internal surface of lumen 106. Figure 5C shows distal end 90 within
lumen 106, with control fluid (e.g., gas or liquid) being ejected from control
orifices
102, and also with injection fluid being ejected from injection orifice 96.
The
injection force is illustrated as vector F1. Control force Fc, which is
greater in
magnitude and opposite in direction relative to injection force F1, continues
to press
injection orifice 96 and adjacent sidewalls of distal end 90, against an
internal
surface of lumen 106, during the injection. After the injection is completed,
the
control force can be removed by stopping the ejection of control fluid from
control
orifices 102.
As illustrated, both the injection orifices and the control orifices are in
the
form of apertures or bores formed directly in shaft sidewalls. Alternately, if
desired,
these ejection orifices can be part of a nozzle, end effector, injection head,
etc. Also,
figures 5A through 5C show only a single length-wise location along a length
of a
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
shaft distal end, and, therefore, identify only a single set of injection
orifice and
control orifices; not illustrated is that the shaft distal end can optionally
include one
or more additional injection orifice and control orifices at other length-wise
positions along the shaft distal end.
Advantages of a distal end of an injection shaft that includes control
orifices
for use to control the placement of the distal end, including one or more
injection
orifice, can be a reduced cross-sectional size, i.e., a low profile, of the
distal end,
compared to similar alternate distal ends that may include other mechanisms
(e.g., a
balloon) to position the distal end during injection. Exemplary distal ends
that use
control orifices may exhibit a profile that is sufficiently reduced to allow
the distal
end to be easily contained by a lumen of a larger shaft, such as a working
lumen of a
flexible endoscope, cystoscope, or catheter. The distal end may be capable of
being
loosely contained in a working lumen with room to be easily moved and rotated
(moved longitudinally and circumferentially) within the working lumen.
Additionally, a distal end of such an injection shaft can be constructed to
include no
moving parts, and can be of an essentially one-piece construction.
Figure 6 illustrates an alternate embodiment of a device and method for deep
injection of tissue, e.g., transurethral injection of prostate. At figure 6,
distal end 90
is placed within lumen 106 (e.g., a urethral lumen), which is adjacent to
tissue 108
(e.g., prostate tissue). Nozzle (or "end effector" or "injection head") 95
includes
injection orifice 96 directed normal to tissue surface 101. Injection lumen 94
connects injection orifice 96 to a proximal end of a needleless injection
device.
Injection orifice 96 is positioned at an oblique angle, such as an angle in a
range
from about 45 to 70 degrees relative to longitudinal axis AL of shaft 97 and
nozzle
95. Flexible shaft 97 may have flexibility, steerability, and optic features
of a shaft
as described herein, such as a shaft of an endo scope or cystoscope. Flexible
shaft 97
allows nozzle 95 to be oriented as shown, within body lumen 97 (e.g.,
urethra), and,
combined with injection orifice 96 being oriented at the illustrated oblique
angle, a
fluid stream can be ejected from orifice 96 in direction D, normal to tissue
101
within lumen 106.
Figures 19-26 illustrate additional examples (in side cross-section) of distal
ends of injection shafts that involve an injection force that is opposed by a
control
31
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
force in use. The control force can be produced by inflation of a balloon (or
other
apposition device or mechanism), ejection of control fluid from one or more
control
orifice such as described herein, or both.
At figure 19, injection shaft 250 having distal end 252 is shown positioned
within body lumen 254, which is adjacent to body tissue 256. Injection fluid
is
shown being ejected from injection orifices 258. A resultant generally
opposing
control force (to the ejection force) is preferably provided by balloon 260,
as shown,
and which includes fluid supply lumen 259. Other apposition devices can be
used in
addition to or in place of balloon 260 including one or more control orifice
such as
those described with respect to the embodiments described herein. As shown,
injection orifices 258 are longitudinally spaced apart along the length of
injection
shaft 250 and may be positioned relative to injection shaft 250 in any desired
manner such as described herein including being spaced around the
circumference of
injection shaft 250 and at any desired angle relative to the longitudinal axis
of
injection shaft 250.
At figure 20 a cross-sectional view of another exemplary injection shaft 262
is shown wherein injection orifices 264 and 266 are spaced around the
circumference of injection shaft 262 so injection orifices 264 and 266 are
radially
spaced apart at a predetermined angle. Injection orifices 264 and 266 may be
positioned at the same location along the length of injection shaft 262 such
as shown
in figure 21 or may be spaced apart along the length of injection shaft 262 as
shown
in figure 22.
At figure 23 a cross-sectional view of another exemplary injection shaft 268
having distal end 270 is shown. First and second injection orifices 271 and
272 are
in fluid communication with lumen 269, spaced apart along the length of
injection
shaft 268, and each directed at an angle relative to (or otherwise oriented
relative to)
the central axis of injection shaft 268 that provides diverging streams of
injection
fluid. The term diverging, as used herein, means that the streams of injection
fluid
provided by injection orifices 271 and 272 are directed in a manner that
causes the
streams of injection fluid provided by injection orifices 271 and 272 to move
further
away from each other as the streams move away from injection shaft 268.
Preferably, the angle between each of injection orifices 271 and 272 and the
central
32
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
axis of injection shaft 268, as measured in the plane that contains the
injection
orifice and the central axis, is not normal (not 90 degrees, non-normal, non-
right, or
non-orthogonal). It is contemplated, however, that one or both of injection
orifices
271 and 272 may be provided at angle normal to the central axis of injection
shaft
268. The streams of injection fluid provided by injection orifices 271 and 272
do
not need to lie in the same plane (such as is shown in figure 25). That is,
injection
orifices 271 and 272 may be spaced apart around the circumference of injection
shaft 268 such as is described herein with respect to other embodiments of the
present invention and as shown in figure 26. The angle between each of
injection
orifices 271 and 272 may be the same or may be different as measured in
consistent
frames of reference. Any number, geometrical arrangement, or positioning of
injection orifices can be used as described herein.
Balloon 273 is provided generally opposite from orifices 271 and 272 as
shown. Other apposition devices can be used in addition to or in place of
balloon
273 including one or more control orifice such as those described with respect
to the
embodiments described herein.
At figure 24 a cross-sectional view of another exemplary injection shaft 274
having distal end 276 is shown wherein injection orifices 277 and 278 are in
fluid
communication with lumen 275, spaced apart along the length of injection shaft
274,
and each directed at an angle relative to the central axis of injection shaft
274 that
provides converging streams of injection fluid. The term converging, as used
herein,
means that the streams of injection fluid provided by injection orifices 277
and 278
are directed in a manner that causes the streams of injection fluid provided
by
injection orifices 277 and 278 to move closer to each other as the streams
move
away from injection shaft 274. It is not required that the streams of
injection fluid
provided by injection orifices 277 and 278 cross or otherwise intersect to be
considered converging streams. The streams of injection fluid provided by
injection
orifices 277 and 278 do not need to lie in the same plane (such as is shown in
figure
25). That is, injection orifices 277 and 278 may be spaced apart around the
circumference of injection shaft 274 such as is described herein with respect
to other
embodiments of the present invention and as shown in figure 26. Preferably,
the
angle between each of injection orifices 277 and 278 and the central axis of
injection
33
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
shaft 274, as measured in the plane that contains the injection orifice and
the central
axis, is not normal (not 90 degrees, non-normal, non-right, or non-
orthogonal). It is
contemplated, however, that one or both of injection orifices 277 and 278 may
be
provided at an angle nonnal to the central axis of injection shaft 274. The
angle
between each of injection orifices 277 and 278 may be the same or may be
different
as measured in consistent frames of reference. Any number, geometrical
arrangement, or positioning of injection orifices can be used.
Balloon 279 is provided generally opposite from orifices 277 and 278. Other
apposition devices can be used in addition to or in place of balloon 279
including
one or more control orifice such as those described with respect to the
embodiments
described herein. Any of injection orifices 271, 272, 277, and 278 may be
aligned
along the length of the injection shaft as shown in figure 25 or radially
spaced
around the circumference of the injection shaft such as shown in figure 26.
The diverging and converging arrangements of injection fluid functions to
reduce the force from the injection fluid that pushes the injection shaft away
from
tissue and can also provide tissue tensioning at the injection site.
According to certain embodiments of devices and methods, a distal end of a
shaft, e.g., adjacent to a distal end tip, can include a structure that can
mechanically
hold a distal end in contact with a tissue location during an injection. In
particular,
for end-fire devices that place a distal end normal to a tissue surface for
shallow
angle injection, a frictional structure located adjacent to a distal end tip
may
frictionally contact and hold or grasp tissue during an injection, to oppose
an
injection force and prevent the distal end and the injection orifice from
moving in
response to an injection force. A useful engagement between a frictional
tissue
holding tip, and tissue, can be sufficient to engage tissue and oppose an
injection
force, also optionally to allow a flexible distal end to bond to a position or
portion of
a distal end in an orthogonal orientation relative to a tissue surface, as
illustrated at
figures 3C, 3D, and 3E. The structure required for sufficient engagement may
vary
depending on factors such as the magnitude of the injection force, the amount
of
normal force that can be applied to the shaft distal end and between a distal
end tip
and a tissue surface, and the nature of the tissue. Certain types of tissue,
such as
bladder tissue, may be deformable, low friction (e.g., slick or slippery), or
both of
34
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
these. A tissue holding tip may include a frictional surface that can create a
frictional force between the tissue holding tip and adjacent tissue, even
slick
deformable tissue, to prevent movement of the tip relative to the tissue
surface,
during an injection, or to allow placement of a shaft distal end at a normal
orientation relative to a tissue surface by the tissue holding tip engaging
tissue at a
non-normal angle, followed by bending of the distal shaft end.
Embodiments of useful tissue holding tips may include one or more
projections that are pointed, either to a dull or a relatively sharp point, as
desired.
The projections may be in the form of a dome, a spike, a cleat, a pyramid, a
cone,
etc., and may be sufficiently pointed (sharp) to slightly penetrate through
the tissue
surface, or alternately may be not sufficiently sharp to penetrate into tissue
but to
instead merely deflect or indent tissue.
A tissue holding tip may include a single extension that may include a
longitudinal axis that is shared with a longitudinal axis of a shaft.
Alternately, a
single or multiple extensions may each have a longitudinal axis parallel to
but offset
from a longitudinal axis of a shaft. Or, a single or multiple extensions may
each
have a longitudinal axis that is angled from, and may or may not intercept, a
longitudinal axis of a shaft. An extension can be curved or straight or bent
at an
angle, as desired, such as curved or bent toward or away from a longitudinal
axis of
a shaft.
Examples of tissue holding tips are illustrated at figures 7A through 7F and
Figures 18A through 18D. In these figures, shown in length-wise cross-section,
distal ends 110 (of injection shafts) include sidewalls 112, injection lumens
114,
injection orifices 116, tissue holding tips 118, and distal end tips 120. As
illustrated,
a tissue holding tip may be a single conical or pyramidal point (figure 7A and
18A);
multiple (two or more) conical or pyramidal points (figure 7B); a single,
straight,
elongate spike having a longitudinal axis along the longitudinal axis of the
shaft
(figure 7C); multiple elongate spikes parallel with a longitudinal axis of the
shaft,
optionally curved at their tips (inward (as illustrated) or outward relative
to a
longitudinal axis of the shaft) (figure 7D, 18B, 18C, and 18D); multiple (3,
4, 5, or
more) short spines located around a perimeter of a shaft and angled away from
a
longitudinal axis of a shaft (figure 7E); and multiple (e.g., 5, 10, or more)
short
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
pyramidal or conical points, or elongate spikes, with axes parallel to a
longitudinal
axis of the shaft (figure 7F).
Exemplary needleless fluid delivery devices or systems can include a
proximal end that includes a console, and an elongate shaft extending from a
proximal end in communication with the console, to a distal end. One or more
injection orifice at the distal end can be in fluid communication with the
console.
A console generally can include a housing, a pressure chamber, and a
pressure source. A console can be of any configuration, size, or design,
ranging
from a small, hand-held design to a relatively larger floor or table-mounted
console.
Optionally a console can include separate or separable components such as a
pressure chamber that can be attached, used for an injection procedure, and
detached
and optionally discarded. A shaft can also be attached to a console or a
pressure
chamber in a manner to allow separation and optional re-attachment or
disposal.
With separable components, a shaft or pressure chamber can be attached to a
console housing and used to inject a first patient or a first injectate; the
shaft or
pressure chamber can then be discarded or sterilized. A second shaft or
pressure
chamber can be attached to the console to treat a second patient or the first
patient
with second injectate or another amount of the first injectate. The second
patient or
injectate can involve injection and treatment of the same type of tissue as
the first
patient or injectate, or of a new type of tissue (e.g., prostate or bladder).
In this
manner, separable and optionally disposable shaft or pressure chamber
components
of a needleless injection system can allow a console housing to be used
multiple
times to inject the same or different injectates, to the same or different
patients, and
to the same or different types of body tissue.
A console can include actuating features to control distal end features, e.g.,
for steering a steerable distal end of a steerable shaft, to actuate ejection
of fluid
(control fluid or injection fluid), to move a moveable or extendable injection
shaft or
one or more injection orifice or control orifice relative to another shaft
component
such as a working shaft, optional ports to connect a console housing to
auxiliary
devices, electronics such as controls, and optic features such as a lens,
fiber optic, or
electronic viewing mechanism to allow viewing through an optical feature (to
view a
location of delivery). One or more attachment ports can optionally attach a
console
36
CA 02757180 2015-01-15
to an external and optionally remote component such as an external or remote
pressure source,
vacuum source, or an external or remote fluid reservoir to supply injectate or
control fluid. For
example, a console housing may have a fluid port that attaches to a source of
a fluid (injectate or
control fluid), to supply the fluid to the console housing, such as to a
permanent or detachable
pressure chamber. The console can include a removable or permanent pressure
chamber and a
pressure source capable of pressurizing a fluid contained in the pressure
chamber to cause the
fluid to flow from the console, through a lumen in the shaft, and then through
an ejection orifice
as either injectate or a control fluid.
In embodiments of devices that involve the use of a control fluid, a
pressurized control
fluid can be produced by a console using any useful technique and mechanism,
e.g., pressure
source, such as any pressurized fluid source, magnetohydrodynamic power,
expanding steam or
gas power, etc., with any available and useful control fluid, which may be a
liquid or a gas.
Examples of consoles, console features and combinations of console features
that can be
useful according to the present description are identified at U.S. Patent
Publication No. 2006-
0129125, filed on July 21, 2005, by Copa et al., entitled NEEDLELESS DELIVERY
SYSTEMS; U.S. Patent Publication No. 2009-0312696, filed June 27, 2008, by
Copa et at.,
entitled DEVICES, SYSTEMS, AND RELATED METHODS FOR DELIVERY OF FLUID TO
TISSUE; International Application No. PCT/US2009/006384, filed December 4,
2009, by AMS
Research Corporation, entitled NEEDLESS INJECTION DEVICE COMPONENTS,
SYSTEMS, AND METHODS; International Application No. PCT/US2009/006383, filed
December 4, 2009, by AMS Research Corporation, entitled METHOD AND APPARATUS
FOR
COMPENSATING FOR INJECTION MEDIA VISCOSITY IN A PRESSURIZED DRUG
INJECTION SYSTEM; International Application No. PCT/US2009/006381, filed
December 4,
2009, by AMS Research Corporation, entitled DEVICES, SYSTEMS AND METHODS FOR
DELIVERING FLUID TO TISSUE; International Application No. PCT/US2009/006390,
filed
December 4, 2009, by AMS Research Corporation, entitled DEVICES, SYSTEMS AND
RELATED METHODS FOR DELIVERING OF FLUID TO TISSUE.
37
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
A fluid chamber can be a space (volume) at a proximal end of a device such
as at a console housing, useful to contain pressurized or non-pressurized
fluid, such
as control fluid or injectate. Examples of specific types of fluid chambers
include
fluid reservoirs and pressure chambers. Optionally a proximal end of a device
may
include one or multiple fluid reservoirs and pressure chambers, optionally for
one or
more different fluids including one or more injectate, one or more control
fluid, or
combinations of these.
A fluid reservoir is generally a type of fluid chamber that can contain a
fluid
for a purpose of containing, transferring, holding, or storing a fluid, such
as a fixed
volume fluid chamber, and may be included as a permanent or removable
(attachable and detachable) component of a console.
A pressure chamber can be a type of fluid chamber for containing fluid (e.g.,
control fluid or injectate) for a purpose of placing the fluid under pressure
to deliver
the fluid through a lumen to a distal end of a shaft for ejection from an
ejection
orifice. Examples of pressure chambers include a syringe chamber and other
variable volume spaces that can be used to contain and pressurize a fluid.
Examples
of variable volume pressure chambers include spaces that can exhibit a
variable
volume based, e.g., on a plunger, piston, bellows, or other mechanism for
increasing
or decreasing the volume (and correspondingly decreasing or increasing
pressure)
within the variable volume chamber space. A pressure chamber can be
pressurized
by a pressure source attached to the plunger, bellows, or piston, etc., such
that fluid
contained in the pressure chamber is ejected under pressure, e.g., for priming
a
device, or for ejecting fluid from an ejection orifice for injection or to
produce a
control force. A pressure source may be any source of energy (e.g.,
mechanical,
electrical, hydraulically derived, pneumatically derived, etc.) such as a
spring,
solenoid, compressed air, manual syringe, electric power, hydraulic, pneumatic
pressure sources, etc. A pressure chamber may be a permanent or removable
(attachable and detachable) component of a console or console housing.
In communication with a proximal end of a device is an elongate shaft that
extends from the proximal end (i.e., from a proximal shaft end), that is
optionally
removably connected to the console (or a component of the console such as a
removable pressure chamber), to a distal end that can be placed in a patient
during
38
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
an injection procedure. A shaft can be of various designs, minimally including
an
injection lumen to carry injectate from a proximal end of the device to a
distal end of
the shaft. A useful shaft may optionally include at least one separate lumen
for
carrying control fluid ("control fluid lumen") to a distal end.
An injection shaft minimally includes an injection lumen in communication
with an injection orifice. The injection shaft can include structure such as
sidewalls
that define the injection lumen, the sidewalls being of sufficient strength to
withstand operating pressures sufficient to deliver injectate from the
injection orifice
at an elevated pressure sufficient to cause the injectate to be ejected from
the
injection orifice to penetrate a tissue surface and become injected and into
and
dispersed below the tissue surface, as described herein. Exemplary elevated
pressures ("injection pressures") may be 200 pounds per square inch or
greater, e.g.,
as measured at the distal end of the injection lumen, at the pressure chamber.
The
pressure that will be required for any particular treatment can depend on
factors such
as the type of tissue being injected, the volume of injectate, etc. An
injection shaft
may be of a flexible material (e.g., a metal or polymeric tube) that can
withstand
such injection pressure, and may be prepared from exemplary materials capable
of
withstanding pressure of an injection, e.g., nitinol, stainless steel,
reinforced (e.g.,
braided) polymer, as also described elsewhere herein.
A basic version of a useful shaft of a device as described can be an
"injection
shaft" that includes a proximal end, a distal and, a sidewall that defines an
internal
lumen ("injection lumen"), and at least one injection orifice at the distal
end in
connection with the injection lumen. An injection shaft can optionally include
multiple injection orifices, optionally one or more control orifice at the
distal end,
and optionally a control lumen extending from the proximal end to the optional
control orifice.
An injection shaft can be any elongate structure capable of delivering fluid
to
a distal end of a shaft at a pressure suitable to inject tissue, as described.
Exemplary
injection shaft structures include relatively flexible hollow bodies having a
distal
end, a proximal end, sidewalls extending between the ends, an internal lumen
defined by interior surfaces of the sidewall. The injection lumen is in
communication with one or more injection orifice at the distal end; the
injection
39
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
orifice may be as described herein, such as an aperture or bore in an
injection shaft
sidewall, an aperture or bore in a nozzle, end effector, injection head, or
other
structure in communication with the injection lumen.
An exemplary injection shaft can be in the form of a non-metal, polymeric
tube-like device and can be fabricated using suitable high strength polymers
including, for example, polyimide, polyetherimide available from General
Electric
under the trade name Ultem and linear aromatic polymers such as PEEKTM
available from Victrex plc for transporting the treatment fluid and the
apposing jet
medium to the treatment area. In some embodiments, the non-metal, polymeric
tube-like device can be reinforced through the inclusion of materials
including nano-
particles, clays and/or glass. In some presently contemplated embodiments, the
non-
metal, polymeric tube-like device can be reinforced with one or more polymers
such
as, for example, tubes braided with Kevlar or other high-strength polymers.
The
non-metal, polymeric tube-like device can be fabricated so as to have a burst
strength exceeding at least about 200 psi, such as at least 1,000 psi, or
2,000 psi, and
in some embodiments, having a burst strength within a range of about 2,000 psi
to
about 5,000 psi (depending in the treatment application, and, e.g., the type
of tissue
being injected). The non-metal, polymeric tube-like device can be fabricated
so as
to have distention properties, wherein one or more orifices or jet ports
located at a
distal end of the polymeric tube-like device retains its shape and/or size
without
suffering swelling that can have a detrimental impact on a fluid jet used to
deliver
the therapeutic fluid at the treatment site. See, e.g., U.S. Pat. Publ. No.
2008/0119823
An exemplary injection shaft can include a sidewall that defines an outer
shaft surface and an inner injector lumen, these being of continuous and
relatively
uniform dimensions of inner diameter, outer diameter, and wall thickness,
along an
entire length of the injection shaft. Alternately, an injection shaft,
injector lumen, or
sidewall, may change dimensions (e.g., wall thickness) along the length of the
injection shaft, with a larger wall thickness (e.g., greater outer diameter)
at a
proximal end and a thinner wall thickness (e.g., reduced outer diameter) at
the distal
end. An example of an inner diameter of an injection shaft (i.e., a diameter
of an
injection lumen) can be greater than 0.020 inches, e.g., from 0.022 to 0.030
inches
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
(for a lumen made of polyetheretherketone, or "PEEK"); exemplary outer
diameters
for the same exemplary injection shaft may be at least 0.032 inches e.g., from
0.034
to 0.045 inches. A length of an injection shaft can be any length that
functions to
place a proximal end at a console and a distal end at a desired tissue
location;
exemplary lengths can be from as little as 15 inches if the console is a hand-
held
console, to as long as 100 inches if the console is floor based or table
based.
An injection shaft can be an only component of a shaft of a useful needleless
injection device or system or may be a component of a larger shaft structure.
Other
shaft components may include additional elongate shaft structures with desired
functionality, a single example being a device referred to herein as "medical
device
shaft" or a "working shaft," which can be used to securely or moveably support
or
house an injection shaft. For instance, an injection shaft can be incorporated
permanently or movably (e.g., removably) against (alongside) or within (in a
"working lumen" of) a working shaft. In exemplary embodiments an injection
shaft
can be loosely contained in a working lumen of a working shaft to allow
movement
of the injection shaft length-wise and rotationally relative to the working
shaft; an
injection shaft may be capable of moving longitudinally within a working lumen
to
allow the injection lumen to be extended distally from an open end of a
working
lumen at a distal end of the working shaft.
An example of a "working shaft" or "medical device shaft" can be a shaft
that is useful in conjunction with an injection shaft, to manipulate and place
the
injection orifice of an injection shaft at a desired location for treatment of
tissue. A
"working shaft" or "medical device shaft" can function to support the
injection shaft,
and can optionally and preferably include any of a variety of optional
functionalities
such as steerability, an optical function, a tissue tensioner, or combinations
of these,
in addition to supporting the injection shaft.
An example of a particularly preferred working shaft can include features of
a typical cystoscope, endoscope, ureteroscope, choledoscope, hysteroscope,
catheter
(e.g., urinary catheter), or the like, or other similar type of medical device
shaft,
including one or more feature of flexibility, an optical function, a steerable
distal
shaft end, and a working lumen. The working lumen can be sized to loosely
house
or contain the injection shaft, preferably in a manner to allow the injection
shaft to
41
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
be moved lengthwise and rotationally within the working lumen, relative to the
working lumen, such as to allow the injection lumen to be extended from an
opening
of the working shaft at a distal end of the working shaft. A typical diameter
(or
other dimension) of a working lumen, extending along a length of a distal end
of a
working shaft, can be in the range from about 1 to about 3 millimeters. A
typical
length of working shaft for placement of a distal end at a location of the
urinary tract
can be, e.g., from 15 to 25 centimeters.
As used herein, the term "flexible shaft" refers to a shaft (e.g., an
injection
shaft or a working shaft) that is sufficiently pliable to allow bending and
flexing that
allow the shaft to be inserted through the meatus or an external incision,
into the
urethra or another body lumen, and to allow a portion of a distal end of the
shaft to
be guided into a body lumen such as a urethra and optionally the bladder neck
or
bladder, as can be done with a Foley catheter. A flexible shaft can be
sufficiently
soft and pliable to conform or partially conform to a patient's anatomy, such
as
would a Foley-type catheter. A "steerable" shaft is a type of a flexible shaft
having
a distal end that can be maneuvered directionally (e.g., bent or curved) from
a
proximal end; steerable shaft distal ends are sometimes features of endoscopes
and
other medical device shafts.
Optionally, a shaft of a device as described may also be malleable, or
"shapeable," meaning that a shaft distal end, or portion thereof, can be of a
material
capable of being shaped to a form, and to remain in that form during use, such
as for
insertion into a body lumen, until re-formed. A shaft or a shaft component,
such as a
working shaft or an injection shaft, can include a malleable component such as
a
bendable metal wire, coil, ribbon, tube, or the like, capable of being shaped,
used
without substantial deformation, and re-shaped. A malleable distal end can
allow a
distal end to be shaped by a user to assist in placement of the distal end
through a
body lumen such as a urinary tract, at a desired location. In some methods of
treatment, there may be difficulties or challenges in passing a shaft distal
end
through a body lumen, or to place the distal end in contact with tissue for
injection.
A malleable shaft distal end, e.g., of an injection shaft in particular, e.g.,
used in
conjunction with a working shaft within which the malleable injection shaft
distal
end is moveably disposed, may assist in overcoming such potential
difficulties. The
42
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
malleable distal end tip may be formed by a user to a desired curve or bend,
before
or after placement in a working channel; the working shaft may be inserted
into a
body lumen such as a urethra, and the formed, malleably injection shaft distal
end
may be extended from the working shaft with a shape that improves the ability
to
position the injection shaft or ejection orifices thereof, at tissue for
injection. A
shapeable portion may vary in stiffness, length, resilience, material,
radiopacity, etc.,
and may be of any malleable material such as a polymer, metal, or polymer-
metal
composite.
Figure 9 illustrates an example of a malleable distal end. Shaft distal end 70
(e.g., injector shaft distal end) of figure 9 includes sidewall 72, injection
orifice 76
and control orifice 80, directed in opposing directions, distal end tip 60,
and
cylindrical tissue holding tip proximal to distal end tip 60. Control orifice
80 is
connected to control lumen 84, which communicates with a proximal end of a
needleless injection device. Injection orifice 76 is connected to injection
lumen 74,
which communicates with a proximal end of a needleless injection device.
Within
sidewall 72 is malleable elongate member 75, which can be a metal, polymer, or
metal-polymer composite, as described.
A distal end of a shaft (e.g., an injection shaft or a working shaft) includes
one or multiple ejection orifices for ejecting fluid within a body of a
patient,
including at least one injection orifice. An ejection orifice may be for
injecting fluid
into tissue, in which case the ejection orifice is referred to as an
"injection orifice";
alternate ejection orifices may be for ejection of non-therapeutic fluids,
such as
ejection of control fluid, in which case the ejection orifice may be referred
to as a
"control orifice." An injection orifice or a control orifice can be any form
of
opening, aperture, or orifice, such as an aperture or bore in an injection
shaft
sidewall or other shaft sidewall, or an aperture or bore in a nozzle, end
effector,
injection head, or other structure in communication with an injection lumen or
control lumen, as desired.
Embodiments of devices as described can include multiple ejection orifices
at a distal end. The orifices can be located at relative locations and
orientations
along a length or circumference of a shaft distal end to result in ejection
and
distribution of ejected fluid in different directions (e.g., circumferentially
relative to
43
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
the shaft), optionally or alternately at different distances along the length
of the
shaft. An ejection orifices can be directed at any angle relative to a
longitudinal axis
of a shaft, such as perpendicular, angled toward a distal end, or angled
toward a
proximal end.
An injection orifice may have any useful size (e.g., length and diameter) to
produce a fluid stream of ejected fluid that can penetrate a tissue surface to
become
injected into tissue. Examples of a useful range of diameter of an injection
orifice
may be from about 0.001 to 0.05 inches, e.g., from 0.001 to 0.010 inches,
depending
on factors such as desired injection parameters (injection depth, volume,
pressure,
exit velocity, etc.) and the type and size (e.g., depth) of tissue being
injected. An
injection orifice may be larger or smaller than an injection lumen leading to
the
injection orifice, if desired, to affect the exit velocity of the jet of
injectate from the
injection orifice. Examples of useful orifice shapes may include features such
as a
venturi, a continuous uniform diameter along the length of an orifice, a
funnel-
shape, etc. These dimension and shape features can also apply to control
orifice.
Ejection orifices, as indicated, can be of various structures and designs,
such
as a simple bore in a shaft, or a bore or aperture of a connected structure
such as a
nozzle, end effector, injection head, or other structure that can be connected
to a
shaft to allow communication between a lumen within the shaft, and an ejection
orifice. Examples of alternate forms of ejection orifices are shown at figures
8A,
8B, 8C, and 8D.
An example of one type of nozzle is show at figures 8A and 8B. Referring to
figure 8A, shaft distal end 50, an injection shaft, in cross-section, includes
shaft
sidewall 52, injection lumen 54, and is attached to and in fluid communication
with
nozzle 61. Nozzle 61 includes multiple injection orifices 56, directed (as
illustrated), parallel with longitudinal axis AL. Distal end tip 60 is
coextensive with
a surface 63 of nozzle 61, orthogonal to longitudinal axis AL; in this
embodiment,
injection orifices 56 are at the same length-wise location as distal end tip
60 (the
farthest most location of the distal end, which includes structure of nozzle
61).
The nozzle of figures 8A (cross-sectional side view) and 8B (end vies) may
be referred to as a "shower-head" nozzle, and can deliver various injectates
(e.g.,
drugs) to a tissue surface, such as a surface of a shallow tissue, e.g.,
bladder tissue,
44
CA 02757180 2011 09 29
WO 2011/011382 PCT/US2010/042554
through a multi-orifice, end-firing device shaft. Alternately, the nozzle may
be
useful for deep injections of other tissues. The shower-head nozzle is an
attachment
to shaft sidewall 52, secured and in communication communicate with single
injection lumen 54 (alternately, multiple lumens), and includes multiple
injection
orifices placed at a surface of the nozzle, resulting in ejection of fluid in
a direction
along the longitudinal axis of the nozzle.
This angle of ejection of the injection orifices of figures 8A and 8B, i.e.,
the
angle between the direction of the ejection orifices and the longitudinal axis
of the
nozzle, is shown to be zero, but may be any other angle, such as an angle
directed at
least partially away from the longitudinal axis. For example, nozzle 61 of
figures
8C (cross-sectional and 8D (end view) includes injection apertures 56 directed
at
approximately 90 degrees relative to longitudinal axis AL of nozzle 61. Nozzle
61
of figures 8C and 8D includes multiple injection orifices placed at a
circumference
of the nozzle, resulting in balanced ejection forces and distribution of
injectate in a =
circumferential pattern, allowing for good dose distribution. The angle
between the
ejection orifices and the longitudinal axis is shown to be perpendicular, but
may be
angled more toward tissue, or away from tissue, as desired. As illustrated,
nozzle 61
includes multiple frictional spikes 65, for engaging a tissue surface when
nozzle 61
is place normally against a tissue surface; spikes 65 assist in preventing
movement
of nozzle 61 during an injection.
The control fluid may be supplied by a lumen or may be taken from the local
environment of the catheter (irrigation fluid, urine, blood, etc.). A
structure that
defines the fluid stream of control fluid can be a control orifice, and may be
in the
form of an aperture or bore located in a sidewall of an injection shaft, or
other
structure such as a separate nozzle, vane, end effector, etc., that is placed
at the shaft
distal end.
A needleless fluid delivery system 200 is illustrated generally in figure 10.
Needleless fluid delivery system 200 can comprise console (injector) 202 and
shaft
(applicator lumen) 204. Console 202 can be as simple as a manually activated
syringe, or console 202 can comprise an automated console 203 including a user
interface 206 and a connector member (e.g., in the form of a detachable
pressure
chamber) 208. Connector member 208 can include a surface opening 209 and a
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
therapeutic fluid supply 210. User interface 206 can comprise an input means
for
selectively delivering a pressurized fluid through shaft 208. Representative
input
means can include foot pedal 207, switches, buttons or a touch-screen capable
of
receiving touch commands as well as displaying system information including a
mode of operation as well as operating parameters.
As seen in figure 10, shaft 204 generally attaches to connector member 208.
Shaft 204 is generally continuously defined from a (proximal) supply end 211
to a
(distal) delivery end 212. Shaft 204 can comprise a variety of configurations
including, for example, an endoscope or catheter configuration. In some
embodiments, shaft 204 can comprise a flexible tube 214 to allow for easy
positioning of the delivery end 212. Supply end 211 is generally configured to
attach to the connector member 208 and can include a quick-connect style
connector
216. Delivery end 212 can comprise a variety of configurations depending upon
the
style of the shaft 204 and a specified treatment location in a patient's body
such as,
for example, a rectal treatment location, a gastrointestinal treatment
location, a nasal
treatment location, a bronchial treatment location, or an esophageal treatment
location. Various distal end configurations, such as end-fire and side-fire
configurations, either with or without resultant balanced injection forces or
control
forces on a shaft 204, are useful.
In some embodiments, shaft 204 can include an application specific
applicator 218 having a fluid administration port 220 (control orifice). A jet
system
400, providing a fluid source (control fluid) and control system, can be
connected to
the applicator 218. It is envisioned that the jet system 400 includes an
independent
source of jet (control) fluid and an independent driving force such as a
pressurized
tank, magneto-hydrodynamic power, expanding steam, gas power or similar
methods of propulsion. It is also envisioned that the jet system 400 can be
incorporated into the injector 202.
As seen in figure lithe shaft 204 of the needleless fluid delivery system 200
is inserted into the working channel 302 of a flexible endoscope or cystoscope
300.
Applicator lumen (shaft) or injection lumen (injection shaft) 204 has a distal
treatment end 222 proximate with a fluid administration port (injection
orifice) 220
is disposed and at least one jet port (control orifice) 224 is disposed on an
opposing
46
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
side. The fluid administration port (injection orifice) 220 is fluidly
connected to the
injection source. The jet port (control orifice) 224 is fluidly connected to a
jet fluid
source or to the injection source.
Generally, flexible cystoscope 300 can comprise a length of polymeric
tubing 304 having a distal treatment end 308. In some embodiments, flexible
cystoscope 300 can deliver a therapeutic fluid to the treatment location
through the
polymeric tubing 304 or alternatively, the polymeric tubing can be use to
provide
access for medical instruments and/or tools such as, for example, a fiber
optic scope
and/or light to assist in diagnosing and/or treating tissue.
In positioning the flexible cystoscope 300 at a treatment location, it will be
understood that a medical professional frequently employs a medical imaging
system such as, for example, computer axial tomography (CAT), magnetic
resonance imaging (MRI), or in the case of treatment of a prostate gland, the
preferred imaging means is transrectal ultrasound (TRUS) so as to achieve the
desired position of the distal treatment end 308. Through the use of a medical
imaging system, or an optical component of a working shaft, a medical
professional
can verify that the distal treatment end 308 is properly positioned for
delivering
therapy at the treatment location.
Referring to figure 10, a flexible cystoscope 300 of the present invention can
comprise a length of polymeric tubing 304 having a working channel (working
lumen) 302 and one or more treatment tools such as, for example, fiber optic
lights
306 and an objective lens 310. Located within working channel (working lumen)
302 can be one or more components of an injection device 200 that can include
one
or more injection shaft 204. Injection device 200 is configured so as to have
a cross-
sectional profile that does not fully occupy the working channel (working
lumen)
302 so as to define an open channel 313. Injection device 200 simultaneously
contacts the polymeric tubing 304 at a plurality of contact locations so as to
maintain
a desired orientation within the working channel 302 and to provide lateral
support
to the flexible cystoscope 300.
Polymeric tubing 204 and injection device 200 are preferably fabricated of
medical grade polymers and copolymers. In some embodiments, polymeric tubing
204 and injection device 200 can be molded of the same polymer so as to
promote
47
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
maximum compatibility and similar performance characteristics. Depending upon
the treatment application, polymeric tubing 204 and/or injection device 200
can be
fabricated with high strength polymers including, for example, polyimide,
polyetherimide available from General Electric under the trade name Ultem and
linear aromatic polymers such as PEEKTM available from Victrex plc. In some
embodiments, the polymeric tubing 204 and/or the injection device 200 can be
reinforced through the inclusion of materials including nano-particles, clays
and/or
glass within the polymer. Alternatively, polymeric tubing 204 and injection
device
200 can be reinforced with one or more polymers such as, for example, tubes
braided with Kevlar or other high-strength polymers. In some embodiments, the
polymeric tubing 204 and/or injection device 200 can be fabricated so as to
have a
burst strength exceeding at least about 200psi, e.g., 2,000 psi, and in some
embodiments, having a burst strength within a range of about 2,000 psi to
about
5,000 psi.
In use, flexible cystoscope 300 can be positioned for treatment as previously
described with the cystoscope of the prior art. As the injection device 200 is
slidably introduced into the working channel 302, the cross-section of the
injection
device 200 and more specifically, the injection lumen (injection shaft) 204,
contacts
locations that constrain the orientation and positioning of the injection
lumen
(injection shaft) 204 such that the injection lumen (injection shaft) 204
cannot
buckle within the working channel 302. As the injection lumen (injection
shaft) 204
cannot buckle within the working channel 302, open channel 313 remains
unobstructed so as to accommodate irrigant flow to a treatment location. As
injection lumen (injection shaft) 204 is advanced through the working channel
302,
the injection lumen (injection shaft) 204 can be oriented such that the
preferred axis
of bending for the injection lumen (injection shaft) 204 matches the preferred
axis of
bending of the cystoscope 300 so as to resist twisting of the injection lumen
(injection shaft) 204 and to maintain the desired orientation of the injection
device
200.
Once the distal treatment end 308, and more specifically, the administration
orifice or fluid injection port 220 is positioned with respect to the
treatment location,
the injector 200 can be actuated so as to begin delivery of a therapeutic
fluid. If the
48
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
fluid injection port 220 is not in contact with the treatment location, the
jet system
400 can be activated to propel the injection lumen 204 toward the treatment
location.
As the jet fluid reaches distal treatment end 222, the jet fluid is rapidly
accelerated
through the jet orifice 224 to propel the lumen 204 toward the treatment area.
Meanwhile, as the therapeutic fluid reaches distal treatment end 222, the
therapeutic
fluid is rapidly accelerated through the administration orifice (injection
orifice) 220
to form a fluid jet that contacts the treatment area. Therapeutic fluid can be
controllably dispensed directly at the treatment location so as to reduce the
potential
for exposure to other non-desired areas. The jet control system 400 should be
able
to compensate for the activation of the needless injection system 200.
Figure 12 illustrates the cross section of the injection lumen 204 proximate
the jet ports (control orifice) 224 and injection port (injection orifice) 220
within a
body cavity 500. An injection lumen 230 is fluidly connected to the injector
202.
Therapeutic fluid is advanced through the injection lumen 230 to the injection
nozzle 232 and out the injection port (injection orifice) 220. The jet ports
(control
orifices) 224 are each fluidly connected to a jet system 400 by way of
apposition
lumen (control lumen) 404. The jet or apposition fluid (control fluid) is
advanced
through the apposition lumen (control lumen) 404 to the apposition nozzles 406
and
out the jet port (control orifice) 224.
In operation, as illustrated in figures 13 and 14, the jet system 400 is
activated to create jets 410 (of control fluid) that direct lumen (injection
shaft) 204
to the treatment location 412. While jets 410 continue firing, the therapeutic
fluid
advances through the injection lumen 230 to the injection nozzle 232. An
injection
jet 240 is then delivered to treatment area 412.
It is envisioned that alternate embodiments, such as illustrated in figure 15,
can be used for the jet system 400. For example, there can be multiple
apposition
lumens (control lumens) 404 each fluidly connected to a separate jetport 224.
The
apposition nozzles (control nozzle) 406 can be circular, crescent shaped,
slits or any
suitable shape. The apposition nozzles (control nozzles) can also be located
circumferentially or axially about the lumen 204. While the above description
makes repeated reference to a liquid jet, the system can operate by utilizing
a
49
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
compressed gas for the jets. It is further envisioned that an apposition lumen
may
not be necessary as the gas or liquid can be supplied from the catheter
environment.
While the invention is amenable to various modifications and alternative
forms, specifics thereof have been shown by way of example in the drawings and
will be described in detail. It should be understood, however, that the
intention is
not to limit the invention to the particular embodiments described. On the
contrary,
the intention is to cover all modifications, equivalents, and alternatives.
Another exemplary embodiment of a needleless injection system according
to the present description is illustrated at figure 16. Device 500 includes a
handle
502 and working shaft distal end 504 of working shaft 503, which includes
injection
shaft 508 disposed within working lumen 518. The proximal end of the devices
includes handle 502 of a scope that connects to working shaft 503 (e.g., of a
cystoscope, endoscope, catheter, or other medical device shaft), including
features
useful for manipulating or operating features at distal end 504. Handle 502
includes:
fiber optic light source 516; steering actuator 514, which can be manipulated
to
cause the steerable distal end of device 500 to move in at two or more
dimensions);
viewing lens 520 that allows viewing through fiber optic cable 510; and port
524,
which allows for connection of a fluid source to handle 502. Articulation for
steering of distal end 504 is indicated in dashed lines.
Still referring to figure 16, body 512 connects to working shaft 503, which
includes lumens and mechanisms that connect features of proximal end handle
502
to distal end 504. Working lumen 518 is a hollow lumen or channel that extends
within working shaft 503 and supports and contains injection shaft 508 in a
manner
that allows injection shaft 508 to move longitudinally along the length of
working
shaft 503, to allow the distal end of injection shaft 508 to extend from end
opening
522 of working lumen 518. Working shaft 503 also includes fiber optic 510 and
a
steering mechanism (not shown) that allows steering (deflecting) of distal end
504
by movement of actuator 514. Light source 516 transmits light to distal end
504 by
fiber optic 510.
Distal end 504 includes end opening 522 of working lumen 518 from which
can be extended injection shaft 508, which includes at least one injection
orifice (not
shown). Also distal end 504 can be steered to allow movement of the tip of
working
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
shaft distal end 504, in coordination with extension of injection shaft 508,
based on
viewing through fiber optic 510, to deliver a fluid with accurate placement at
a
desired tissue location. The distal end of injection shaft 508 can be any
design as
described herein, e.g., can include multiple ejection orifices at different
length-wise
or circumferential locations, optional control orifices, optional tissue
holding tip, etc.
As illustrated, fluid streams 509 are shown being ejected from injection
orifice on
opposite sides of the injection shaft distal end, on a proximal side of distal
end tip
513 of injection shaft 508.
Also illustrated at figure 16 is shaft 546 extending between port 524 of
handle 502 and console 542. Console 542 includes pressure chamber 540 and
pressure source 544.
Referring to Figure 27 distal end 550 of another embodiment of an injection
device in accordance with the present invention is shown. Distal end 550 of
the
injection device is shown and includes working shaft 555, working channel 560,
fluid delivery lumen 565 (which includes jet orifices as described herein, not
shown), and tip deflector 570. As shown, working channel 560 extends through
working shaft 555 and tip deflector 570. Fluid delivery lumen 565 is
operatively
positioned within working channel 560, as shown. Tip deflector 570 is
preferably
designed to facilitate guiding, shaping, and directional positioning of fluid
delivery
lumen 565 to provide functional control of injection direction. For example,
tip
deflector 570 can be configured to provide lateral injection such as can be
achieved
with end-firing devices described herein. Tip deflector 570 can be configured
as
desired for use with any desired target tissue. Tip deflector 570 can be
integrated
with distal end 550 or may be provided as a separate device that is
selectively
attachable to distal end 550. In use, fluid delivery lumen 565 and tip
deflector 570
are positioned within a body lumen of a patient. The tip deflector 570 and/or
the
fluid delivery lumen is then pushed into the wall of the body lumen to provide
apposition of jet orifices of the fluid delivery lumen 565 and fluid delivery
can be
provided as described herein.
With any of the above features of fluid delivery devices, a device could
include an electronic process control system that can be programmed to make
fluid
deliveries having various locations, volumes, and other injection properties
such as
51
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
depth and degree (e.g., shape and distance) of dispersion and size of
particles of
fluid.
A needleless injection system can be use to perform treatment methods by
steps that include one or more of the following: providing a needleless
injection
device substantially as described herein; inserting a distal end of a shaft of
the fluid
delivery device into a patient, e.g., through the meatus and into the urethra;
navigating the distal end until an injection orifice at the distal end of the
shaft is
positioned at a desired delivery site. Optionally depending on the type of
treatment
and tissue being treated the shaft distal end can be positioned in an
orthogonal
(normal) orientation relative to a tissue surface (optionally by assistance of
a tissue
holding tip and with pressure to cause bending of flexible injection shaft),
such as if
the tissue is bladder tissue; in these embodiments, longitudinal pressure may
be
placed on the distal end to cause a distal end tip to indent a tissue surface,
optionally
causing an injection orifice to become positioned at a level below a tissue
surface,
not by penetrating the surface but because the injection orifice is located at
the
indented tissue. In alternate methods a distal end can be positioned with a
sidewall
in contact with tissue, with a longitudinal axis of the shaft in line with
(e.g., parallel
to) tissue; a sidewall of the shaft distal end can be optionally be pressed
against the
tissue surface to cause an injection orifice to contact the tissue surface for
injection,
such as by the use of one or more control orifice to produce a control force.
By any of the described methods, multiple ejection orifices can provide the
ability to place one or more different fluids at multiple locations of the
urethra,
prostate, bladder, or bladder neck, or other tissue, etc. Features of devices
described
herein, such as optical features, steerable shafts, extendable or moveable
fluid
delivery orifices, and the ability to deliver multiple different types of
fluid, allow for
improved control over the location of injection or instillation of a fluid.
Exemplary methods of treatment can include one or multiple discrete steps
relating to insertion of a fluid delivery device as described herein;
positioning of the
device to place one or more fluid delivery orifices at desired locations
within the
bladder or bladder neck or other location of the urinary tract; optionally,
use of an
optic device; optional extension of a needle or a needleless delivery orifice
extension
from the shaft of the device to contact tissue of the bladder or bladder neck,
etc.;
52
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
delivery of one or more biologically active fluid or agent from a delivery
orifice
(needle or needleless delivery orifice) to either contact or penetrate tissue
of the
bladder or bladder neck, etc.; optionally, one or multiple steps of re-
positioning one
or more fluid delivery orifices; optionally, one or more additional delivery
steps that
involve the same or different delivery orifices.
According to fluid delivery procedures of the invention, fluid such as ethanol
or a biologically active agent can be delivered to the bladder, urethra,
urethra, or
bladder neck, etc., in a manner that causes the fluid to be injected into the
tissue
using a needleless delivery orifice.
Devices of the present description can be useful to treat of various tissues,
including of the urinary tract, in females or males. For example, devices as
described may be useful to inject the bladder, bladder neck, the urethral
tissue itself
or the external sphincter, or for transurethral injection of the prostate in a
male.
Other treatment locations can include a rectal treatment location, a
gastrointestinal
treatment location, a nasal treatment location, a bronchial treatment
location, and an
esophageal treatment location. In other embodiments, a fluid may be injected
into
tissue of the urinary tract (e.g., bladder, urethra, kidneys, ureters,
prostate, etc.) such
as individual or combination treatments using drugs or other therapeutic
agents, e.g.,
botulinum toxin ("botox"), an antiandrogen, among others as will be
understood.
One advantage of injection of an active pharmaceutical agent at a location of
use is
the placement of the agent to avoid systemic side effects. Specific examples
of
active pharmaceutical agents that may be injected include Botulinum toxin
types A
through G; 5-alpha reductase inhibitors such as dutasteride and finasteride;
alpha
blockers such as alfuzosin, doxazosin, prazosin, tamsulosin hydrochloride,
terazosin,
ethanol, to treat BPH; or any of various antibiotics (e.g., to treat
prostatitis) and
analgesics.
The invention also contemplates needleless injection systems that include
any combination of components as described, including one or more console
(e.g., a
housing with one or more removable pressure chamber); one or more additional
pressure chamber for dispensing one or a variety of different fluids to a
single
patient or to multiple patients; one or multiple different injection shaft
attachments
for dispensing the same or different fluids to one or multiple patients; and
one or
53
CA 02757180 2011 09 29
WO 2011/011382
PCT/US2010/042554
multiple working shaft. As an example, a combination of the invention may
include
multiple different injection shaft attachments, each having a proximal end
that can
be attached and removed from a console, e.g., a removable pressure chamber.
Each
injection shaft attachment can be the same or different, e.g., for treating
bladder
tissue (e.g., having an end-fire distal end, optionally balanced control
orifices,
optionally also a tissue holding tip), for treating prostate tissue (e.g.,
having a side-
fire distal end, optionally one or more control orifice). One or multiple
working
shafts may also be suited to different treatments, e.g., one to treat prostate
tissue, one
to treat bladder tissue.
Figure 17 illustrates components of combination 620 of the invention. Any
different combination of components can be included in a system or set. The
components include console 600, optional "connector member" or external,
removable pressure chamber 602, multiple varieties of injection shaft
attachments (i)
through (v) that can be separately attached to console 600 or removable
pressure
chamber 602, and a single working shaft 610 including handle 612. Console or
console housing 600 can be as described, and includes at least a pressure
source.
Port 601 allows connection to optional removable pressure chamber 602, which
can
be connect at a proximal end to port 601, and has distal end 605 that can be
connected to a proximal end of an injection shaft attachment. Optional port
603 of
pressure chamber 602 can be used to insert fluid into pressure chamber 602.
Each of
injection shaft attachments (i), (ii), (iii), (iv), and (v), are exemplary and
for
purposes of illustration of exemplary combinations. Each includes a proximal
end
(611) that can removably attach to console or console housing 600, optionally
by
removably attaching to connector member 602 at distal end 605. Each injection
shaft attachment also includes one or more injection orifice 606 at a distal
end 604,
connected through an inflation lumen (not shown) to the proximal end. Each
injection orifice as illustrated is on a proximal side of a distal end tip
607.
An optional component of combination 620 is working shaft 610, which may
be as described herein, e.g., including handle 612, port 622 suitable to
introduce an
injection shaft into working lumen 616 of working shaft 614, optional
steerable
distal end 618, and an optional optical feature (not shown).
54
CA 02757180 2015-01-15
A combination can include any one or combination of injection shaft
attachments as
shown or otherwise described herein. An exemplary injection shaft attachment
can include any
one or more of. a side-fire distal end with no control orifice (i), e.g., for
deep injection treatment
of prostate tissue; a side- fire distal end with a malleable distal end
feature (not shown) (ii), e.g.,
for deep injection treatment of prostate tissue; an end-fire distal end with
balanced injection
orifices and a tissue holding tip (iii), e.g., for shallow injection treatment
of bladder tissue; an
end-fire distal end with balanced injection orifices and no tissue holding tip
and optional control
orifices (not shown) (iv), e.g., for shallow injection treatment of bladder
tissue; or a side-fire
distal end with multiple injection orifices along a length of a distal end and
multiple opposed
control orifices (v), e.g., for deep injection treatment of prostate tissue.
The scope of the claims should not be limited by the preferred embodiments set
forth in
the examples, but should be given the broadest purposive construction
consistent with the
description as a whole.