Note: Descriptions are shown in the official language in which they were submitted.
( 1- 1
WO 2010/115907 PCT/EP2010/054558
1
Sample storage in microfluidics devices
Field of the invention
The invention relates to the field of microfluidics characterisation. More
particularly the
present invention relates to methods and devices for characterisation of
microliter amounts
of fluids.
Background of the invention
Characterisation of microfluidics is used in a wide variety of applications,
such as for example
in the field of biology, biotechnology, chemistry and for clinical and medical
purposes. One of
the requirements posed in the majority of these applications is the need for
high accuracy of
the analysis. Also, characterisation is often hampered by the limited amount
of sample that is
available.
One of the sources of inaccuracy when performing characterisation of
microfluidics,
especially when quantitative concentration determination is envisaged, is the
occurrence of
evaporation of the sample between the initial introduction of the sample in
the test device
and the actual measurement. One example of a known technique for determining
concentration of a component in a microfluidic sample using optical absorption
measurements, e.g. for determining the concentration of DNA molecules in a
microliter DNA
suspension, is as follows : a microfluidic sample is provided in an input
well. Some time later,
for example depending on the experience of the operator and/or the number of
channels
used in the characterisation device, the liquid is transported to a
measurement chamber for
performing the optical absorption measurement. Concentration is then
determined based on
the specific absorption of an irradiation beam by the sample. In the period
prior to the
measurement, evaporation of sample components takes place as long as the DNA
suspension
is in contact with the ambient air. Such an evaporation results in a decrease
of the absolute
amount of solvent or buffer in the DNA suspension, resulting in an increase of
the measured
concentration of DNA molecules. Such evaporation therefore may compromise the
accuracy
of the analysis, and assessment of the initial concentration is virtually
impossible. FIG. 1
illustrates an example of evaporation of different volumes of samples
dispensed in different
input wells. It can be seen that the volume drops significantly in a time
range of a couple of
minutes, which is typically the time that may lapse between initial
introduction of the sample
( 1- 1
WO 2010/115907 PCT/EP2010/054558
2
in the test device and the moment of actual measurement. The effect on the
quantitative
results obtained based thereon as function of the time lapsed between intial
introduction of
the sample in the test device and the moment of actual measurement is shown in
FIG. 2 for
the 1.5 l sample and for the 21A sample in one type of input well. It can be
seen that the
accuracy of the results obtained is significantly influenced by evaporation of
the sample.
Another disadvantage of evaporation of the sample is the reduction of the
volume of the
sample. As typically a minimal amount of sample is required to perform the
measurement,
evaporation results in a larger volume to be dispensed in order to be able to
perform the
measurements some time afterwards and thus in an increased use of sample. It
will be clear
for the skilled person that, as evaporation occurs independent of the amount
of sample
available, the problem becomes especially relevant when microliter samples
need to be
analysed. For small sample volumes, this may result in not sufficient sample
being available
for measurement.
One solution to at least partly counter evaporation is provided in European
patent
EP1255610, describing a covered microfluidic device wherein a lid cover is
provided to cover
the microchannel structures for minimising or preventing undesired evaporation
of liquids.
A further source of inaccuracy during characterisation of microfluidics can be
found in
improper introduction of sample in the characterisation device. Systems are
known, e.g. from
US application 2002/0114738 Al, wherein introduction of fluid in the system is
performed by
pressing the tip of a pipette tightly into a funnel shaped inlet port and
injecting fluid in a
filling chamber. These particular shapes of the inlet port may nevertheless
prevent
automated sample provision, especially when automated simultaneous sample
introduction
is envisaged in a multichannel characterisation device. For example, small
variations in the
distance between different channels in a multichannel pipetting device or
between the
multichannel characterisation device may lead to inaccurate positioning of the
pipette
positions with respect to the inlet ports, resulting in inaccurate
introduction of the sample in
the different channels and e.g. spilling of the sample. But even for single
channel dispensing
there is a limited accuracy in the positioning of the dispensing tip with
respect to the inlet
port due to the instrumental positioning accuracy of the tip and the
manufacturing
tolerances of the microfluidic device. In addition, tip release from the inlet
port after it has
( 1- 1
WO 2010/115907 PCT/EP2010/054558
3
been pressed into it, can lead to loss of the tip (in case of disposable tips)
or displacement of
the microfluidic device.
Summary of the invention
It is an object of embodiments of the present invention to provide good
devices and
methods for characterizing microliter amounts of fluids or assisting therein.
It is an advantage
of embodiments according to the present invention that a good or improved
accuracy can be
obtained in the characterisation of microliter samples.
The above objective is accomplished by a method and device according to the
present
invention.
The present invention relates to a microfluidics device comprising at least
one input
well for receiving an amount of liquid to be characterised in the
microfluidics device and a
storage chamber for, prior to said characterisation, storing the liquid e.g.
as inserted in the
input well, wherein the microfluidics device is adapted for, upon receipt of
the amount of
liquid in the input well, spontaneously transferring substantially all of said
amount of liquid to
said storage chamber. It is an advantage of embodiments of the present
invention that
substantially the complete fluid is removed automatically and quickly from the
input well to
the storage chamber, thus reducing and/or avoiding e.g. uncontrollable
evaporation of
liquids. Avoidance of uncontrollable evaporation of the liquid results in a
better accuracy of
the characterisation performed. It is an advantage of some embodiments
according to the
present invention that such reduction and/or control can be obtained without
hampering an
automated filling process, even for automated simultaneous filling in a multi-
channel system.
It is an advantage of embodiments according to the present invention that
evaporation can
be reduced, resulting in more accurate results, especially in the case of
small sample
volumes.
The present invention also relates to a microfluidics device comprising at
least one
input well for receiving an amount of liquid to be characterized in the
microfluidics device, a
storage chamber for storing, prior to said characterisation, the liquid, and a
measurement
chamber for receiving said liquid from said storage chamber at a later time
for
characterization, after said storing, wherein the storage chamber is a
capillary chamber and
has a volume adapted for, upon receipt of the amount of liquid in the input
well,
( 1- 1
WO 2010/115907 PCT/EP2010/054558
4
spontaneously transferring through capillary force substantially all of said
amount of liquid to
said storage chamber, the storage chamber being shaped so that, directly after
filling the
storage chamber, the interface area of the liquid with ambient gas in the
storage chamber is
smaller than the interface area of liquid with ambient gas in the input well.
The present invention also relates to a method for characterising a
microfluidic
sample, the method comprising receiving an amount of liquid to be
characterised in the
microfluidics device and spontaneously transferring substantially all of said
amount of liquid
to a storage room for storing the liquid prior to the characterisation.
The present invention furthermore relates to a characterisation system for
characterising microfluidic samples comprising a microfluidic structure as
described above.
Particular and advantageous aspects of some embodiments of the invention are
set
out in the accompanying dependent claims. Features from the dependent claims
may be
combined with features of the independent claims and with features of other
dependent
claims as appropriate and not merely as explicitly set out in the claims.
These and other aspects of the invention will be apparent from and elucidated
with
reference to the embodiment(s) described hereinafter. Embodiments of the
present
invention lead to improved characterisation of microfluidics.
Brief description of the drawings
FIG. 1 and FIG. 2 illustrate the effect of evaporation on the volume of a
sample and on
the determined concentration of a component in a sample respectively as
function of time
lapsed between initial introduction and measurement of the sample,
illustrating a problem
that can be solved by embodiments of the present invention.
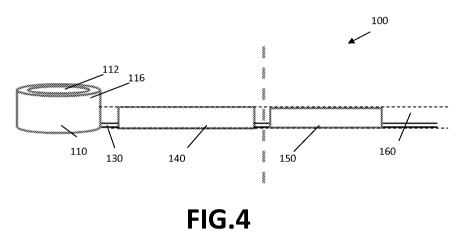
FIG. 3 and FIG. 4 illustrate a cross-section and side view of a microfluidic
device with
spontaneous filling of a storage chamber upon filling of the input well,
according to an
embodiment of the present invention.
FIG. 5A to FIG. 5G illustrate different ways for introducing a microfluidic
sample in an
input well of a microfluidic device according to an embodiment of the present
invention.
FIG. 6 illustrates two possible shapes of an input well as may be used
according to
embodiments of the present invention, whereby the bottom surface is formed by
the
( 1- 1
WO 2010/115907 PCT/EP2010/054558
throughput opening to the storage chamber (A) or whereby a small surface is
surrounding
the throughput opening to the storage chamber, the ensemble forming the bottom
surface
(B).
FIG. 7A to FIG. 7C illustrates a number of cornered shapes for a throughput
hole as
5 can be used in a microfluidic device according to an embodiment of the
present invention.
FIG. 8 illustrate a snake-like shape of a storage chamber as can be used in a
microfluidic device according to an embodiment of the present invention.
FIG. 9A to FIG. 9J shows different configurations for a microfluidic device
according to
embodiments of the present invention.
FIG. 10 illustrates an exemplary method for characterising a microfluidic
sample or
assisting therein, as can be performed according to an embodiment of the
present invention.
FIG. 11A to FIG. 11C illustrate the measured concentration and the amount of
evaporation as function of residence time for dsDNA in a hydrophilic coated
device,
illustrating features and advantages of embodiments of the present invention.
FIG. 12A to FIG. 12C illustrate the measured concentration and the amount of
evaporation as function of residence time for dNTP in a hydrophilic coated
device, illustrating
features and advantages of embodiments of the present invention.
FIG. 13A to FIG. 13C illustrate the measured concentration and the amount of
evaporation as function of residence time for BSA in a hydrophobic device
illustrating
features and advantages of embodiments of the present invention.
FIG. 14A to FIG. 14C illustrate the measured concentration and the amount of
evaporation as function of residence time for dsDNA in a hydrophobic device
illustrating
features and advantages of embodiments of the present invention.
FIG. 15A to FIG. 15C illustrate the measured concentration as function of the
residence time in the input well, illustrating problems as can be at least
partly solved by
embodiments according to the present invention.
The drawings are only schematic and are non-limiting. In the drawings, the
size of
some of the elements may be exaggerated and not drawn on scale for
illustrative purposes.
Any reference signs in the claims shall not be construed as limiting the
scope. In the different
drawings, the same reference signs refer to the same or analogous elements.
( 1- 1
WO 2010/115907 PCT/EP2010/054558
6
Detailed description of illustrative embodiments
While the invention will be illustrated and described in detail in the
drawings and foregoing
description, such illustration and description are to be considered
illustrative or exemplary
and not restrictive. The invention is not limited to the disclosed
embodiments. Other
variations to the disclosed embodiments can be understood and effected by
those skilled in
the art in practicing the claimed invention, from a study of the drawings, the
disclosure and
the appended claims. In the claims, the word "comprising" does not exclude
other elements
or steps, and the indefinite article "a" or "an" does not exclude a plurality.
A single unit may
fulfill the functions of several items recited in the claims. The foregoing
description details
certain embodiments of the invention. It will be appreciated, however, that no
matter how
detailed the foregoing appears in text, the invention may be practiced in many
ways, and is
therefore not limited to the embodiments disclosed. It should be noted that
the use of
particular terminology when describing certain features or aspects of the
invention should
not be taken to imply that the terminology is being re-defined herein to be
restricted to
include any specific characteristics of the features or aspects of the
invention with which that
terminology is associated.
Where in embodiments according to the present invention reference is made to
an interface
liquid / ambient air, the latter may refer to the meniscus defining the edge
between liquid
and ambient air.
Where in embodiments according to the present invention reference is made to
"directly
after filling the storage chamber", reference is made to instantaneously after
filling the
storage chamber. The latter also may be referred to as the moment directly
after the
spontaneous transfer from the input well to the storage chamber has occurred
or as soon as
the spontaneous transfer from the input well to the storage chamber has
occurred or as soon
as the storage chamber has been filled.
Where in embodiments according to the present invention reference is made to
substantially
all liquid, reference is made to all liquid, except for e.g. a small part of
the fluid e.g. sticking to
the walls of the input well. Such a small part may for example be less than
20%,
advantageously less than 10%, more advantageously less than 5%.
In a first aspect, the present invention relates to a microfluidics device for
characterising
microfluidics. Embodiments of the present invention are especially suitable
for
( 1- 1
WO 2010/115907 PCT/EP2010/054558
7
characterisation of microfluidic samples of which only a small volume is
available. The
embodiments may be especially suitable for sample volumes between 0.211I and
711I,
advantageously between 11A and 51A, more advantageously between 111I and
3.511I.
Characterisation of microfluidic samples may comprise detection of the
presence of certain
components, determination of concentration of certain components,
determination of
certain reactions occurring, etc. Such characterisation may include for
example applications
in the field of biology, biotechnology, chemistry, the clinical field and/or
the medical field.
The microfluidic device according to embodiments of the present invention
comprises an
input well for receiving an amount of liquid to be characterised and a storage
chamber for
storing, prior to the characterisation, the liquid. The liquid may be stored
as it was inserted in
the input well. In embodiments of the present invention, the microfluidic
device thereby is
adapted for, upon receipt of the amount of liquid in the input well,
spontaneously
transferring substantially all of said amount of liquid to the storage
chamber. With
transferring substantially all of the amount of liquid, there is meant at
least 80% of the liquid
received in the input well, more advantageously at least 90% of the liquid
received in the
input well, still more advantageously at least 95% of the liquid received in
the input well.
Spontaneous thereby is defined as of its own motion, e.g. under gravity force
or more
advantageously via capillary forces, i.e. without the need for forces induced
by an external
source. According to embodiments of the present invention, "upon receipt of
the amount of
liquid in the input well" means that such spontaneous transfer may occur
within a time span
sufficiently short so that substantially no evaporation has occurred in the
input well, e.g. a
time span with an upper limit of 120 seconds from the moment the liquid is
introduced in the
input well, or more advantageously a timespan with an upper limit of 60
seconds from the
moment the liquid is introduced in the input well, still more advantageously a
timespan with
an upper limit of 30 seconds from the moment the liquid is introduced in the
input well.
The microfluidic device may be a multichannel device, comprising a plurality
of channels in
which characterisation can be performed independently. The microfluidic device
may for
example comprise at least 8 channels, at least 16 channels, at least 32
channels, at least 96
channels or at least 384 channels. Further aspects and advantages will further
be described
with reference to FIG. 3 and FIG. 4, indicating standard and optional
components of an
exemplary microfluidic device according to embodiments of the present
invention. FIG. 3
( 1- 1
WO 2010/115907 PCT/EP2010/054558
8
illustrates a schematic representation of a microfluidic device in cross-
section, whereas FIG. 4
illustrates a schematic representation of a microfluidic device in side view.
As indicated above, the microfluidic device 100 comprises at least one input
well 110. The
volume of the input well 110 may be selected so that it can receive the sample
volume to be
measured, without the sample expelling over the edges of the input well 110.
The surface
area of the top surface 112 (also referred to as receiving surface) and the
bottom surface 114
of the input well 110 may be determined by the available space on the
microfluidic device
100. The available space may be limited as also the storage chamber 140, the
measurement
chamber 150, interconnection channels such as the throughput channel 130
connecting the
input well 110 and the storage chamber 140, a throughput opening 120 and
alignment holes
require space on the device 100. The available space is of course even more
limited in multi-
channel characterisation devices 100, as the overall size of the
characterisation device 100
will be limited, preferably adapted to conventional sizes such as defined in
the microtiter
plate standard. In one example, the available space per channel is between 10
and 100 mm2.
In embodiments according to the present invention, the receiving surface 112
of the input
well 110, i.e. the receiving opening, is significantly larger than the average
tip of a fluid
introduction means, e.g. a pipette, used in microfluidics for introducing
liquid, so that
variation of the tip in size or position does not hamper accurate introduction
of the liquid in
the input well 110. In some embodiments according to the present invention,
the input well
110 comprises upstanding walls 116 extending above a plate-shaped portion 160
of the
microfluidics device 100 comprising the storage chamber 140, measurement
chamber 150
and interconnection channels 130. The upstanding walls may extend e.g. between
0.2mm
and 50mm above the plate-shaped portion. It is an advantage of such
embodiments that the
input well 110 is clearly visible for the user and/or that - in a multi-
channel system - the
upstanding walls 116 form a physical barrier for avoiding cross-contamination
between
neighbouring channels. Upstanding walls 116 extending above the plate-shaped
portion 160
also have the advantage that the volume of the input well 110 can be selected
significantly
large while the input well 110 still has a limited footprint. It is
furthermore an advantage of
upstanding walls 116 extending above the plate-shaped portion 160 that these
walls 116 can
be used for releasing the liquid from the pipette tip. Upstanding walls also
may lead to less
convection of ambient air above the inlet port and thus lower evaporation.
( 1- 1
WO 2010/115907 PCT/EP2010/054558
9
By way of illustration, a number of examples for dispensing of a drop of
liquid 510 to be
characterised in the input well 110 using a liquid dispensing means 520 like a
pipette are
illustrated in FIG. 5A to FIG. 5G. The dispensed liquid may make contact with
one or both of
the upstanding walls, or bottom and may be either centered above the
throughput hole or
not centered with respect thereto.
The shape of the input well 110 is selected so that the capillary upward
forces on the liquid in
the input well 110 and/or the pinning of the liquid in the input well 110
around the
throughput opening 120 to the storage chamber 140, hindering the fluid from
transferring to
the storage chamber 140, are reduced. By way of example, FIG. 6 illustrates
cross-sections of
two possible shapes for such an input well, i.e. an input well whereby the
bottom surface of
the input well 110 is reduced to the throughput opening 120 towards the
storage chamber
(FIG. 6A) and an input well 110 having a small surface surrounding the
throughput opening to
the storage chamber (FIG. 6B), whereby the surrounding surface area is
advantageously
smaller than 50% of the receiving opening of the input well, more
advantageously smaller
than 35% of the receiving opening of the input well and still more
advantageously smaller
than 20% of the receiving opening 112 of the input well 110. Upon pinning at
the throughput
opening 120 in the surrounding surface area, only a small portion of the
liquid will not be
transferred, leaving only a small film on the upstanding walls of the input
well after the
remaining liquid has been transferred to the storage chamber 140.
Alternatively, the
throughput opening 120 also may be provided at the bottom of a side wall.
In advantageous embodiments the throughput opening 120 and throughput channel
130, if
present, may be hydrophilic.
The portion of sample liquid left in the input well 110 after transfer to the
storage chamber
140 is significantly small so that still substantially all of the liquid is
transferred to the storage
chamber. The upstanding walls 116, or at least a part of the upstanding walls
closest to the
bottom surface, advantageously are tilted with respect to the bottom 114 or
the receiving
surface 112 of the input well 110 so that capillary forces counteracting the
transfer of the
liquid to the storage chamber 140 are small, i.e. at least smaller than the
pulling force in the
capillary storage chamber. In one example, at least part of the upstanding
walls 116 makes
an angle c of at least 20 , advantageously at least 30 , more advantageously
at least 40 with
the normal to the bottom surface. The input well 110 may at least partly have
a conical
( 1- 1
WO 2010/115907 PCT/EP2010/054558
shape. The inner surfaces of the input well 110 advantageously may be made as
smooth as
possible, in order to prevent pinning of the liquid. The production process of
the microfluidic
device 100, e.g. spray casting using different malls, may introduce burrs
which may be the
source of unwanted pinning of the fluid in the device. Such burrs may be
removed by
5 mechanical drilling, punching, laser ablation, etc. Furthermore, also during
the production
process measures may be taken to reduce the effect of burrs on pinning, e.g.
by selecting
different portions of the device to be formed by different malls, resulting in
the burrs
occurring in a different direction. In one embodiment, the walls 116 of the
input well 110
also may be provided with grooves so that small air bubbles can be avoided in
the
10 throughput channel, if present, and the storage chamber 140.
In order to prevent pinning at the throughput opening 120 in the input well
110, the
throughput opening 120 may have a cornered shape such as a polygonal shape. By
way of
illustration, a number of cornered shapes for the throughput opening 120 are
illustrated in
FIG. 7A to FIG. 7C indicating a top view of the throughput opening 120 being
the start portion
of the throughput channel 130. An advantage of providing such structures thus
is the
increase of capillary forces on the liquid. FIG. 7A illustrates an input well
whereby the
throughput opening has a triangular shape. FIG. 7B illustrates a similar input
well whereby
the bottom of the throughput channel near the throughput opening has a
triangular
cornered shape for avoiding pinning. FIG. 7C shows a similar cornered shape as
in FIG. 7B but
not centered on the throughput opening 120.
One or more of the above described properties further may assist in leaving
none or only
small amounts of liquid in the input well and thus further may reduce the
possibility of
evaporation of the liquid provided in the input well and may lead to improved
accuracy.
The microfluidic device 100 also comprises a storage chamber 140. The storage
chamber 140
may be positioned between the input well 110 and a measurement chamber 150. It
may be
connected to the input well 110 via a throughput opening 120 and a throughput
channel 130.
The throughput channel, if present, also may be considered as part of the
storage chamber
140. The storage chamber 140 may have a volume adapted for receiving
substantially the full
amount of sample received in the input well 110. The latter allows that, upon
filling of the
storage chamber 140, substantially no fluid remains in the input well 110, so
that the
problem of evaporation thereby is reduced. In one embodiment, the volume of
the storage
( 1- 1
WO 2010/115907 PCT/EP2010/054558
11
chamber 140 thus may correspond with at least the volume of the input well
110. In some
examples, the volume of the storage chamber 140 may be between 0.211I and
711I. In one
particular embodiment, the storage chamber 140 may have a minimum capacity of
3.511I. The
storage chamber 140 furthermore advantageously may be adapted in shape so that
after
transfer of the liquid to the storage chamber, the free interface between the
liquid and
ambient air (upstream) in the storage chamber 140 is substantially smaller
than the
corresponding interface in the input well 110. In other words, the free
surface at which
evaporation can occur directly after transfer of the liquid to the storage
chamber 140
advantageously is substantially smaller than for the liquid in the input well
110 so that
evaporation can be significantly reduced.
According to embodiments of the present invention, the microfluidic device 100
also is
adapted so that spontaneous transfer of substantially all of the liquid sample
provided in the
input well 110 occurs to the storage chamber 140. In one embodiment, for
obtaining quick
and spontaneous transfer, the shape of the storage chamber 140 is adapted so
that a large
capillarity effect occurs pulling the sample liquid from the input well 110 to
the storage
chamber 140. As capillarity is higher for chambers having a large ratio of
circumference to
cross-section for contact angles smaller than 90 between the fluid meniscus
and the wall ,
the storage chamber 140 may be selected to be small and long. By inducing
strong capillarity,
the spontaneous filling of the storage chamber 140 may be very liable, such
that
spontaneous fluid flow is not obstructed by dust particles or small rough
features in the
storage chamber. Selection of the cross-section of the storage chamber 140 may
be
performed, taking into account the available space, the minimal volume
required, the design
and the capillary forces required. The cross-section of the storage chamber
may vary along
the length of the storage chamber 140.By way of illustration, an exemplary top
view of a
design for a storage room 140 is shown in FIG. 8. By providing a snake like
design, the
required space for the storage chamber 140 can be significantly small and fit
e.g. a 96 or 384
channel microfluidic device according to the microtiterplate standard. In one
embodiment, a
fixed cross-section over the full length of the storage chamber 140 is
preferred, whereas in
other embodiments, it may be preferred to have a storage chamber 140 wherein
the cross-
section reduces at positions in the storage chamber 140 further away from the
input well
110. In one example, the storage chamber 140 has a cross section of 0.2mm by
0.4mm and a
( 1- 1
WO 2010/115907 PCT/EP2010/054558
12
length of 44mm, resulting in a storage capacity of about 3.511I. The walls of
the channel may
be slightly sloped in order to easily remove the device after moulding.
In one embodiment, for obtaining capillary forces, the walls or some of the
walls of the input
well 110, the storage chamber 140 and the throughput channel 130 may be
hydrophilised.
Applying a hydrophilic coating assists in obtaining a contact angle smaller
than 90 . In some
embodiments, the hydrophilic coating may be selected so that a contact angle
between 80
and 0 is obtained. Other coatings also may be applied. Additional features for
supplementary
control of the flow, such as for example anti-wicking structures, also may be
used. The
application of the hydrophilic coating may be spatially limited. The
hydrophilic coating may
only be applied to the input well 110, the storage chamber 140 and the
connection 120, 130
between the input well and the storage chamber. Examples of techniques for
applying a
hydrophilic coating are vacuum plasma coating whereby particles from an
ionized suitable
mixture of gas or atmospheric plasma coating whereby through flow particles of
a plasma
torch are moved towards the surface to be treated. By controlling the amount
of movement,
the portions of the microfluidic structure for which deposition is performed
can be selected.
Wet chemical coating is another example of a method for applying a hydrophilic
coating,
whereby a chemical substance is introduced in the microfluidic structure and
whereby after
drying a hydrophilic coating remains present at the walls of the microfluidic
structure 100.
The hydrophilic coating may be provided according to a predetermined pattern.
The
hydrophilic coating does not need to be constant over the walls of the storage
chamber. As in
some applications, the spatial distribution of the deposition of the
hydrophilic coating may
be difficult to control, the storage chamber may be slightly oversized so that
the spatial
distribution of the application of the hydrophilic coating becomes less
sensitive. It is an
advantage of methods whereby local application of a hydrophilic coating is
applied, that
unwanted condensation, e.g. on windows in a measurement chamber, can be
reduced or
avoided. The presence of a hydrophilic coating in the storage chamber 140 has
as major
advantage in that it further assists in the spontaneous transfer of
substantially all of the
sample fluid from the input well to the storage chamber 140. Another advantage
of the
hydrophilic coating is that its application to the input well increases the
ease with which the
sample fluid is released from the fluid introduction means, i.e. the pipette.
( 1- 1
WO 2010/115907 PCT/EP2010/054558
13
It has surprisingly been found that applying a hydrophilic coating results has
an advantageous
effect on quantification of the sample. Although, albeit at a substantially
smaller rate,
evaporation still occurs in the storage chamber channel, in devices having a
hydrophilic
coating in the storage channel, the measured concentration is substantially
independent of
the amount of sample that was evaporated in the storage chamber channel as
long as the
remaining sample is sufficient to fill the measurement chamber. The latter
allows for
accurate quantification, less dependent or independent on the time between
input of the
sample in the input well and measurement of the sample in the measurement
chamber.
As indicated above, the microfluidic device typically may comprise further
channels and
chambers, such as for example a measurement chamber 150. The measurement
chamber
may be adapted to the characterisation technique used for characterizing the
sample liquid.
In one embodiment, the microfluidic device may be adapted for characterisation
using
absorption measurements, and windows may be provided so that an illumination
beam can
be guided through the measurement chamber. Other features of the measurement
chamber
may be as known in the art. Other components in the microfluidic device may be
a mixing
chamber, a metering or dosing chamber, a reaction chamber, etc. These and
other optional
features may be as known in the art.
The microfluidic device may be made in any suitable way, such as for example
by spray
casting, milling, moulding, laminating, sealing with a foil, etc. or a
combination thereof. The
microfluidic device may be made of any suitable material, such as for example
polymers,
glass, quartz, silicon, gels, plastics, resins, carbon, metals, etc.
By way of illustration, a number of configurations for the microfluidics
devices are illustrated
in FIG. 9A to FIG. 9J. FIG. 9A and FIG. 9B illustrate microfluidic devices
having an input well
110 with rather steep walls and a flat bottom portion surrounding the
throughput opening. In
FIG. 9A the cross section of the full storage chamber is equal to the cross-
section of the
throughput opening. In FIG. 9B, portions of the storage chamber have a larger
cross-section
than the throughput opening. FIG. 9C and FIG. 9D show similar structures as
FIG. 9A and FIG.
9B respectively, but an input well with walls tilted 45 from the normal to
the bottom surface
of the input well is provided. FIG. 9E and FIG. 9F indicate similar structures
as FIG. 9C and FIG.
9D, but without the presence of a flat bottom portion surrounding the
throughput opening.
FIG. 9G and FIG. 9H show similar strutures as FIG. 9E and FIG. 9F, but without
the presence of
( 1- 1
WO 2010/115907 PCT/EP2010/054558
14
a vertical throughput channel. FIG. 91 and FIG. 9J show similar structures as
FIG. 9E and FIG.
9F but part of the upstanding walls are vertically oriented, i.e. parallel
with the normal to the
plate-shaped portion of the microfluidic device. In these drawings, only a
portion of the
storage chamber is shown. The storage chamber may become more narrow further
downstream (not shown), as discussed above.
In embodiments of the present invention, further measures can be taken to
decrease sample
evaporation, e.g. sample evaporation in the storage channel, by changing
storage
temperature and humidity, and/or inhibiting convection of ambient air.
It is an advantage of some embodiments according to the present invention that
a number of
liquids can be subsequently dispensed in the input well and that these liquids
can all be
stored in the storage chamber.
Different embodiments for microfluidic devices are described above, providing
amongst
others the advantage of reducing evaporation of liquid before characterization
and
consequently improving measurement accuracy.
The solution of using a storage chamber as provided in these embodiments is
advantageous
over e.g. a solution wherein a hard cover lid or a foil is used to cover the
input well, as these
latter require additional steps to be performed through human intervention,
leading to
additional costs and increased risk for errors.
It is an advantage of some embodiments according to the present invention that
the use of a
storage chamber reduces considerably the evaporation while still allowing a
reference
measurement. Such reference measurement typically is a blank measurement on an
empty
measurement chamber allowing to compensate for intrinsic absorption in the
microfluidic
device. It is an advantage of embodiments according to the present invention
that the use of
a storage chamber does not hamper the use of a further mixing or reaction
chamber.
It is an advantage of embodiments according to the present invention that it
does not make
use of compensation for evaporation by adding additional sample or solvent, as
this may
require additional sample fluid andor may influence the analysis results.
It is an advantage of embodiments according to the present invention that
substantially all of
the liquid received in the input well is spontaneously transferred to the
storage chamber
allowing characterisation of small amounts of liquids.
( 1- 1
WO 2010/115907 PCT/EP2010/054558
Devices and methods according to embodiments of the present invention are
especially
suitable for performing characterisation by optical absorption measurements.
In a further aspect, the present invention also relates to a method for
characterizing a
microfluidic sample or for assisting therein. The method may be especially
suitable for
5 characterizing a microfluidic sample by absorption measurements, although
the invention is
not limited thereto. The method comprises receiving an amount of liquid to be
characterized
in the microfluidics device and spontaneously transferring substantially all
of said amount of
liquid to a storage chamber for storing the liquid prior to said
characterisation. In one
embodiment, receiving an amount of liquid may be performed by introducing an
amount of
10 liquid by releasing the liquid at an upstanding wall of an input well of a
characterization
device, the upstanding wall extending outside a plate-shaped portion of the
characterization
device comprising the storage room. By way of illustration, the present
invention not being
limited thereto, an exemplary method according to an embodiment of this aspect
is
described and standard and optional steps are shown in FIG. 10. The method
1100 for
15 characterizing a microfluidic sample comprises the step of dispensing 1110
the microfluidic
sample to be characterized in an input well of the microfluidic device. The
latter is
substantially immediately, i.e. within a time span wherein evaporation can be
neglected, e.g.
a time span of less than 120 seconds, preferably less than 60 seconds, even
more preferably
less than 30 seconds, spontaneously transferred to a storage chamber in the
microfluidic
device. The latter may for example be based on capillary forces, although the
invention is not
limited thereto. The microfluidic sample thus is stored in the storage chamber
and can be
kept there for a longer period, as substantially less or even no significant
evaporation occurs
in the storage chamber. At a moment chosen by the user, the microfluidic
sample than may
be transferred 1130 to the measurement chamber and characterization of the
microfluidic
sample may be performed 1140. Methods according to embodiments of the present
invention furthermore may comprise steps expressing the functionality of the
different
components or parts thereof of a microfluidic device as described according to
the first
aspect. Such methods may result in similar advantages.
In still another aspect, the present invention relates to a characterization
system for
characterising a microfluidic sample, the system comprising a microfluidic
device as
described in embodiments of the first aspect of the present invention and a
detector for
( 1- 1
WO 2010/115907 PCT/EP2010/054558
16
detecting a property of the microfluidic sample in a measurement chamber. The
system may
further comprise conventional and optional components of a microfluidic
characterization
system as known in the prior art, such as for example a controller, an
irradiation source, a
pumping unit, a fluid introducing means, a processor, etc.
By way of illustration, embodiments of the present invention not being limited
thereto, a set
of concentration determination experiments are described. For these
experiments a plastic
disposable chip with 16 identical structures was used, each containing an
inlet suitable for
receiving samples up to a few microliter, a hydrophilic capillary channel for
storing the
sample right after dispensing and two measurement chambers on top of each
other suitable
for characterizing the samples using optical absorption measurements over an
optical path
length 0.2mm and/or over an optical path length of 1mm (combined 0.2mm and
0.8mm).
The chips were suitable for optical absorption measurements in a custom device
containing
a multi-channel UV-Vis spectrophotometer. Two optical measurements were
performed on
each sample: a first one with only chamber 1 filled with sample (0.2mm optical
path length),
and a second one with both chambers filled (1.0mm accumulated optical path
length).
The experiments were performed on different sample types to illustrate that
the results are
not limited to a particular class of samples only. In all cases the measured
concentrations
remain constant within the accuracy range of the optical measurement system
despite the
slowed down evaporation in the storage chamber that can become substantial
after a
prolonged time. This illustrates that not only evaporation is reduced by the
storage chamber,
but also that, surprisingly, in case a hydrophillic coating is used, the
remaining evaporation
occurring in the storage channel does not influence the measured
concentration. In a first
set of experiments, a sample comprising ds DNA was tested. The sample
comprised 3.011L of
purified dsDNA (Calf Thymus dsDNA, Invitrogen) solution. Series of 15
identical samples were
dispensed in the plastic chips. The 16th sample was a 3.OuL buffer solution
only (no analyte),
serving as blank for the other samples. After some time (= residence time) the
solution was
transported by external pressure to the measurement chambers positioned after
the
storage channel, where the sample was probed with an optical beam and the
concentration
was calculated from the absorption spectrum. Experiments were performed for
two dsDNA
concentration, one with a nominal concentration of approximately 116 ng/uL
(concentration
Cl) and one with a nominal concentration of approximately 492 ng/uL
(concentration C2).
( 1- 1
WO 2010/115907 PCT/EP2010/054558
17
Optical measurements were performed at two distinct path lengths: in the first
case a 0.2mm
optical path length (Path length PL1 ) is realized in single measurement
chamber, containing
approximately 0.3uL. In the second case a 1.0mm optical path length (path
length PL2) is
realized by two measurement chambers on top of each other, consuming
approximately
2.1uL all together. The evaporation was estimated by analysing the length of
the liquid
volumes in the storage chamber for different residence times. The depicted
evaporated
volumes are averaged over both concentrations. FIG. 11A to FIG. 11C illustrate
the measured
concentration and the evaporated volume as function of the residence time in
the storage
channel. FIG. 11A and FIG. 11B illustrate the concentration and evaporation
behaviour for
initial concentrations C1 and C2 respectively for a measurement path length
PL1, whereas
FIG. 11C illustrate the concentration and evaporation behaviour for initial
concentrations C1
for a measurement path length PL2. The transmission for a solution with
initial concentration
C2 and a measurement path length PL2 was too low to provide accurate
information In the
graphs each data point represents a chip with 15 identical samples and 1
blank. The depicted
concentration is the average of the successful measurements on that same chip.
A successful
measurement is defined as any measurement that was performed on one or more
fully filled
measurement chambers. This implicates a minimal amount of sample still being
present in
the storage channel right before filling the measurement chamber(s). Due to
pipetting
tolerances (approximately 15% variation) and accuracy of the optical
measurement
(approximately 1% to 5%, depending on the concentration) some spread can be
expected in
the results for the individual wells.
From these experiments it can be seen that the measured concentrations remain
constant
(within the accuracy range of the optical measurement system) for at least 3
hours, while
more than 30% of the sample in the hydrophilic storage channel has evaporated
within that
time. For PL1 all experiments were successful for at least 3 hours as
sufficient sample was
available to fill the measurement chamber. For PL2 all measurements were
successful up to 2
hours. Longer residence times lead to measurement failures as the chambers
cannot always
be completely filled. The longer the residence time, the more failures occur.
A second set of experiments was performed with a solution of dNTP
(deoxyribonucleotide
triphosphate, Promega), following a similar procedure as described above. The
dNTP samples
had a nominal concentration of approximately 68 ng/uL (concentration Cl) and
335 ng/uL
( 1- 1
WO 2010/115907 PCT/EP2010/054558
18
(concentration C2). FIG. 12A to FIG. 12C illustrate the measured concentration
and the
evaporated volume as function of the residence time in the storage channel.
The depicted
evaporated volumes are averaged over both concentrations. FIG. 12A and FIG.
12B illustrate
the concentration and evaporation behaviour for initial concentrations C1 and
C2
respectively for a measurement path length PL1, whereas FIG. 12C illustrate
the
concentration and evaporation behaviour for initial concentration C1 for a
measurement
path length PL2. The transmission for a solution with initial concentration C2
and a
measurement path length PL2 was too low to provide accurate information.
Overall, similar
conclusions can be drawn as for dsDNA solutions, i.e. while evaporation
occurs, this has no
influence on the concentration values determined.
A third set of experiments was performed with a solution of BSA (BSA Fraction
V,Invitrogen),
following a similar procedure as described above. The BSA samples had a
nominal
concentration of approximately 0.94 mg/mL (concentration Cl) and 3.88 mg/mL
(concentration C2). FIG. 13A to FIG. 13C illustrate the measured concentration
and the
evaporated volume as function of the residence time in the storage channel.
The depicted
evaporated volumes are averaged over both concentrations. FIG. 13A illustrates
the
concentration and evaporation behaviour for initial concentration C2 for a
measurement
path length PL1, whereas FIG. 13B and FIG. 13C illustrate the concentration
and evaporation
behaviour for initial concentrations C1 and C2 respectively for a measurement
path length
PL2. The transmission for a solution with initial concentration C1 and a
measurement path
length PL1 was too high to provide accurate information.
Overall, similar conclusions can be drawn as for dsDNA solutions, i.e. while
evaporation
occurs, this has no influence on the concentration values determined. The main
difference
was that all PL2 measurements were successful up to 1.5 hours, then some
measurements
started to fail due to insufficient sample availability. BSA samples tend to
stick more to the
pipettor tips at dispensing, and this leads to a reduced dispensing volume in
the inlet wells.
This observation explains the reduced residence time for a successful
measurement.
In yet other experiments a hydrophobic instead of a hydrophilic channel is
used. As no
capillary forces are present, filling of the channel with sample requires
external means in
these examples. It has been found that it is not possible to keep the measured
concentrations constant within the same period of time.
( 1- 1
WO 2010/115907 PCT/EP2010/054558
19
For these experiments, a system with storage channel as described above was
used, wherein
the storage channel had hydrophobic walls instead of hydrophilic walls.
External pressure
was used to push dsDNA samples in the hydrophobic channels.
After some time (= residence time) the solution was further transported by
external pressure
to the measurement chambers right after the storage channel. Again two dsDNA
concentrations were measured in this way, with a nominal concentration of
approximately
120 ng/uL (concentration Cl) and of approximately 540 ng/uL (concentration
C2). Also in this
case, optical measurements were performed at two distinct path lengths: in the
first case a
0.2mm optical path length (Path length PL1 ) was realized in single
measurement chamber,
containing approximately 0.3uL. In the second case a 1.0mm optical path length
(path length
PL2) was realized by two measurement chambers on top of each other, consuming
approximately 2.1uL all together. The evaporation was again estimated by
analyzing the
length of the liquid volume in the storage chamber for different residence
times. FIG. 14A to
FIG. 14C illustrate the measured concentration and the evaporated volume as
function of the
residence time in the storage channel. FIG. 14A and FIG. 14B illustrate the
concentration and
evaporation behaviour for initial concentrations C1 and C2 respectively for a
measurement
path length PL1, whereas FIG. 14C illustrates the concentration and
evaporation behaviour
for initial concentration C1 for a measurement path length PL2. The
transmission for initial
concentration C2 with a measurement path length of PL2 was too low to provide
accurate
measurements.
From these experiments it can be seen that, in contrast to use of a
hydrophilic coating, the
measured concentration will change with the residence time. In case of a
measurement at
the short path length, only a small portion of the sample in the storage
channel is used for
the measurement and the measured concentration does not change a lot for a
period of 2.5
hours. After that time a substantial increase is measured . When the larger
path length is
used, more sample from the storage channel is used for filling both
measurement chambers.
In this case a substantial change in concentration is already observed after 1
hour. Similar as
for the above examples, for PL1 all experiments were successful for at least 3
hours as
sufficient sample was available to fill the measurement chamber. For PL2 all
measurements
were successful up to 2 hours. Longer residence times lead to measurement
failures as the
( 1- 1
WO 2010/115907 PCT/EP2010/054558
chambers cannot always be fully filled. The longer the residence time, the
more failures
occur.
For comparison yet another set of experiments was performed .
In these experiments, a system with storage channel as described above was
used, wherein
5 the storage channel had hydrophobic walls instead of hydrophilic walls. This
time no external
pressure was used to push dsDNA samples in the hydrophobic channels, instead
the samples
remained in the inlet wells.
After some time (= residence time) the solution was further transported by
external pressure
to the measurement chambers through the storage channel. Again two dsDNA
10 concentrations were measured in this way, with a nominal concentration of
approximately
116 ng/uL (concentration Cl) and of approximately 531 ng/uL (concentration
C2). Also in this
case, optical measurements were performed at two distinct path lengths: in the
first case a
0.2mm optical path length (Path length PL1 ) was realized in single
measurement chamber,
containing approximately 0.3uL. In the second case a 1.0mm optical path length
(path length
15 PL2) was realized by two measurement chambers on top of each other,
consuming
approximately 2.1uL all together. This time it was not possible to calculate
the evaporated
volumes in function of residence times.
FIG. 15A to FIG. 15C illustrate the measured concentration as function of the
residence time
in the input well. FIG. 15A and FIG. 15B illustrate the concentration
behaviour for initial
20 concentrations C1 and C2 respectively for a measurement path length PL1,
whereas FIG. 15C
illustrates the concentration behaviour for initial concentration C1 for a
measurement path
length PL2. The transmission for initial concentration C2 with a measurement
path length of
PL2 was too low to provide accurate measurements. From these experiments it
can be seen
that, in contrast to use of a hydrophilic coated storage channel, the measured
concentration
changes very rapidly with the residence time in the inlet well due to rapid
evaporation. Note
the reduced time scale in the graphs with respect to the previous experiments.
It was not possible anymore to obtain successful measurements after 1 hour for
PI-1 and
after 15 minutes for PL2 measurements. This clearly demonstrates the reduced
evaporation
rates that can be obtained in the storage channel.