Note: Descriptions are shown in the official language in which they were submitted.
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
1
TOOL FOR ASSESSMENT OF SYMPTOMS OF IRRITABLE BOWEL SYNDROME
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This patent application claims the benefit of U.S. Provisional Patent
Application
No. 61/170,059, filed April 16, 2009, and U.S. Provisional Patent Application
No.
61/319,656, filed March 31, 2010, each of which is incorporated herein by
reference.
BACKGROUND OF THE INVENTION
[0002] About 10% to about 20% of adults and adolescents worldwide suffer from
irritable bowel syndrome (IBS). IBS exacts a disproportionate share of the US
health care
dollar. In US managed-care populations, for example, total direct costs
incurred by subjects
suffering from IBS have been measured to amount to about 50% higher than those
not
suffering from IBS. The negative impact of IBS on patients, their families,
and their friends
can be great, especially in moderate to severe IBS-D, which accounts for
extremely high
utilization of health care resources and impairment of quality-of-life.
[0003] According to the Rome III criteria, IBS is diagnosed with recurrent
abdominal
pain or discomfort at least three days per month in the last three months
associated with two
or more of the following: (1) improvement with defecation, (2) onset
associated with a
change in frequency of stool, and/or (3) onset associated with a change in
form (appearance)
of stool. It is also noted that these symptoms typically have had symptom
onset at least six
months prior to diagnosis. In addition, according to the Rome III criteria,
diarrhea-
predominant irritable bowel syndrome (IBS-D) is defined as loose mushy stool
at least 25%
of bowel movements and hard or lumpy stool in less than 25% of bowel
movements.
However, there currently is no well-validated symptom severity scales for IBS-
D.
[0004] Multidisciplinary approaches (including diet changes, over-the-counter
(OTC)
products, and prescription medications) for treating IBS patients are
frequent, and such drugs
and remedies are often prescribed by family practitioners. Current treatment
is mostly based
on pharmacological therapy and is palliative in nature. Only one prescription
medication -
Alosetron, a selective 5-HT3 antagonist - has been approved by the US Food and
Drug
Administration (FDA) for IBS-D, and it is only available under a restricted
access program
due to risks of severe side effects if taken by patients who suffer from any
other form of IBS
(e.g., constipation-predominant irritable bowel syndrome (IBS-C) and mixed
IBS).
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
2
[0005] An instrument (tool) developed and validated for the IBS patient
population,
ideally to assess symptom severity, would represent an advancement in the
treatment of IBS.
BRIEF SUMMARY OF THE INVENTION
[0006] The invention provides a tool for assessment of symptoms of irritable
bowel
syndrome in a human patient. The tool comprises a diary and/or event log,
suitable for
psychometric testing of symptoms of IBS. The symptom diary and event log can
be used
singly or in combination. The symptom diary and/or event log can be used to
assess the
progress of treatment of IBS in a patient.
[0007] In one aspect, the invention provides a method for diagnosing IBS
comprising (a)
providing a patient with an event log, wherein the patient is instructed to
record in the event
log the date and time of each bowel movement together with a description
thereof, and/or (b)
providing the patient with a symptom diary, wherein the patient is instructed
to complete the
symptom diary by selecting a characterization from a series of pre-determined
characterizations regarding the patient's subjective experience of events
associated with IBS;
(c) reviewing the event log and/or symptom diary completed by the patient; and
(d)
determining whether a diagnosis of irritable bowel syndrome is appropriate.
[0008] In another aspect, the invention provides a method for treating IBS
comprising (a)
administering to a patient having IBS an IBS treatment at a dosage and
frequency; (b)
providing the patient with an event log, wherein the patient is instructed to
record in the event
log the date and time of each bowel movement together with a description
thereof, and/or (c)
providing the patient with a symptom diary, wherein the patient is instructed
to complete the
symptom diary by selecting a characterization from a series of pre-determined
characterizations regarding the patient's subjective experience of events
associated with IBS;
(d) reviewing the event log and/or symptom diary completed by the patient to
assess changes
in symptoms; (e) revising the dosage and frequency of the IBS treatment, or,
optionally,
administering a different IBS treatment; and (f) repeating steps (a)-(e) until
the patient
achieves a clinically satisfactory improvement in IBS symptoms.
[0009] In another aspect, the invention provides calculating an index value
based on the
patient's score in each category of the symptom diary and/or event log,
wherein the index
value indicates the relative severity of a patient's IBS symptoms as compared
to a healthy
individual.
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
3
[0010] The IBS can be any type of IBS, such as IBS-D. Also, the tool can be
used in
conjunction with therapy for IBS (e.g., dietary changes, medication, or both),
i.e., in which
the patent is undergoing treatment for IBS. In this sense, the event log,
symptom diary, or
both, can provide meaningful cognitive feedback concerning the severity of the
IBS
symptoms in response to therapy. In a preferred embodiment, the patient is
undergoing
treatment with a prescription medication approved for treating IBS or under
investigation for
such indication.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWING(S)
[0011] Figure 1A depicts page 1 of an exemplary 10-question symptom diary to
be
provided to a patient.
[0012] Figure 1 B depicts page 2 of an exemplary 10-question symptom diary to
be
provided to a patient.
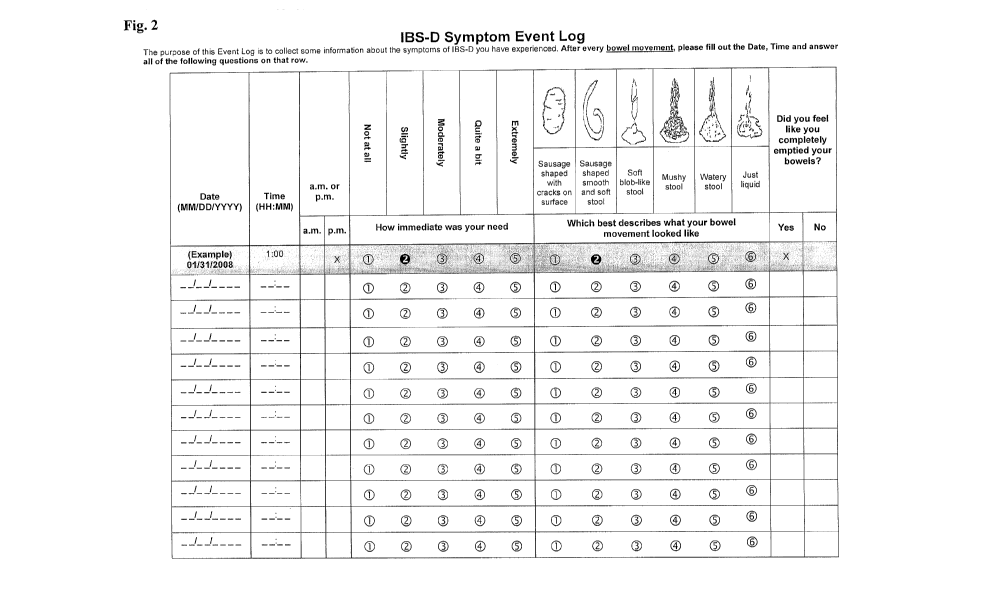
[0013] Figure 2 depicts an exemplary event log to be provided to a patient.
[0014] Figure 3A depicts page 1 of an exemplary 15-question symptom diary to
be
provided to a patient.
[0015] Figure 3B depicts page 2 of an exemplary 15-question symptom diary to
be
provided to a patient.
[0016] Figure 3C depicts page 3 of an exemplary 15-question symptom diary to
be
provided to a patient.
[0017] Figure 4 depicts an exemplary event log to be provided to a patient.
[0018] Figure 5A depicts page 1 of an exemplary 17-question symptom diary to
be
provided to a patient.
[0019] Figure 5B depicts page 2 of an exemplary 17-question symptom diary to
be
provided to a patient.
[0020] Figure 5C depicts page 3 of an exemplary 17-question symptom diary to
be
provided to a patient.
[0021] Figure 6 depicts an exemplary event log to be provided to a patient.
DETAILED DESCRIPTION OF THE INVENTION
[0022] The invention provides methods of using the symptom diary and/or event
log,
such as provided in Figure 1 and Figure 2, respectively, in treating or
diagnosing IBS, or in
assessing a patient's symptoms or response to IBS treatment.
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
4
[0023] In one aspect, the invention provides a method for diagnosing IBS
comprising (a)
providing a patient with an event log, wherein the patient is instructed to
record in the event
log the date and time of each bowel movement together with a description
thereof; and/or (b)
providing the patient with a symptom diary, wherein the patient is instructed
to complete the
symptom diary by selecting a characterization from a series of pre-determined
characterizations regarding the patient's subjective experience of events
associated with IBS;
(c) reviewing the event log and/or symptom diary completed by the patient; and
(d)
determining whether a diagnosis of irritable bowel syndrome is appropriate. In
preferred
embodiments, the patient is provided with both an event log and a symptom
diary. However,
in some embodiments, the patient can be provided with an event log and not a
symptom
diary, or a symptom diary and not an event log.
[0024] In another aspect, the invention provides a method for treating IBS
comprising (a)
administering to a patient having IBS an IBS treatment at a dosage and
frequency; (b)
providing the patient with an event log, wherein the patient is instructed to
record in the event
log the date and time of each bowel movement together with a description
thereof; and/or (c)
providing the patient with a symptom diary, wherein the patient is instructed
to complete the
symptom diary by selecting a characterization from a series of pre-determined
characterizations regarding the patient's subjective experience of events
associated with IBS;
(d) reviewing the event log and/or symptom diary completed by the patient; (e)
revising the
dosage and frequency of the IBS treatment, or, optionally, administering a
different IBS
treatment; and (f) repeating steps (a)-(e) until the patient achieves a
clinically satisfactory
improvement in IBS symptoms. In preferred embodiments, the patient is provided
with both
an event log and a symptom diary. However, in some embodiments, the patient
can be
provided with an event log and not a symptom diary, or a symptom diary and not
an event
log.
[0025] In embodiments in which the patient is provided with an event log, the
patient can
be instructed to record bowel movements over a specified time period. For
example, the
patient can be instructed to record bowel movements over one, two, three, or
four weeks.
Alternatively, the patient can be instructed to record bowel movements over
one or multiple
months. In some embodiments, such as when the patient is undergoing
investigational drug
therapy, it may be preferred that the patient records bowel movements for the
full duration of
the investigational drug therapy.
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
[0026] The event log can have any form useful to the clinician. However, in
preferred
embodiments, the event log includes one or more of the questions provided in
Table 4 of
Example 1, along with pre-determined characterizations from which the patient
can select. In
a more preferred embodiment, the event log includes each question provided in
Table 4. In a
most preferred embodiment, the event log comprises the form of Figure 2.
[0027] In embodiments in which the patient is provided with a symptom diary,
the patient
can be instructed to select a characterization from a series of pre-determined
characterizations
regarding the patient's subjective experience of events associated with IBS.
The patient can
be instructed to complete the symptom diary using characterizations of the
patient's
experience of events associated with IBS on a single day. Alternatively, the
patient can be
instructed to complete multiple symptom diaries over a specified time period,
wherein each
symptom diary is completed using characterizations of the patient's experience
of events
associated with IBS on a different day. For example, the patient can be
instructed to
complete symptom diaries each day for one, two, three, or four weeks.
Alternatively, the
patient can be instructed to complete daily symptom diaries over one or
multiple months. In
some embodiments, such as when the patient is undergoing investigational drug
therapy, it
may be preferred that the patient completes daily symptom diaries for the full
duration of the
investigational drug therapy.
[0028] The symptom diary can have any form useful to the clinician. However,
in
preferred embodiments, the symptom diary includes one or more of the questions
provided in
Table 3 of Example 1, along with pre-determined characterizations from which
the patient
can select. In preferred embodiments, the symptom diary comprises each
question provided
in Table 3. In a most preferred embodiment, the symptom diary comprises the
form provided
in Figure 1 (l A and 1 B).
[0029] It will be understood that the event log and symptom diary are intended
to record
a patient's experience. Therefore, any suitable format providing any number of
pre-
determined characterizations can be provided for the patient's answers. In
some
embodiments, it will be useful to provide pre-determined characterizations for
the symptom
diary and event log as shown in Tables 3 and 4, respectively. However, in
general, the pre-
determined characterizations can be based on a 5-point scale (Likert scale),
an 11-point scale
(0-10), or a positive/negative scale (yes/no). In responding to questions
regarding the
appearance of the bowel movement, the event log desirably provides visual
and/or text
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
6
characterizations based on the Adapted Bristol Stool Form Scale, which, for
IBS-D, can be
modified to include forms relevant to IBS-D (e.g., excluding very hard stool
forms).
[0030] In some embodiments, the patient is undergoing a treatment for IBS. The
treatment can be any IBS treatment, such as a pharmaceutical or biologic
treatment, or a
dietary therapy, or other therapy. In some embodiments, the therapy is a
prescription
medication approved or under investigational use for treatment of IBS.
[0031] In another aspect, the invention provides calculating an index value
based on the
patient's score in each category of the symptom diary and event log, wherein
the index value
indicates the relative severity of a patient's IBS symptoms as compared to a
healthy
individual or to a previous baseline. The index value can also be used to
determine whether a
patient has achieved clinically sastisfactory improvement in IBS symptoms. The
index value
can be calculated by any method known to one of skill in the art and/or deemed
to be useful
in applying the methods of the present invention. However, it is not necessary
to calculate a
single index value in order to determine whether a patient has achieved
improvement in IBS
symptoms. If the patient shows improvement based on the total score of six or
more items of
the symptom diary, in general, such improvement will be considered clinically
satisfactory
improvement in IBS symptoms.
[0032] Additionally, the symptom diary and event log can be used to assess
primary and
secondary endpoints for diagnosing patients as having IBS or for evaluating
the progress of
treatment of IBS patients. For IBS-D, diarrhea (stool frequency and stool
consistency) and
pain are the most frequent and most bothersome symptoms of IBS-D, and these
can serve as
co-primary endpoints. For example, a patient reporting a weekly average
abdominal pain
severity over a 24 hour period of a threshold value (e.g., 3 or higher, 4 or
higher, 5 or higher,
etc. on a 0-10 point scale) and a weekly average stool consistency of a
threshold value (for
example, 4 or above according to the scale presented in Figure 2) could on
that basis be
diagnosed as having IBS-D. To assess a patient's response to therapy for IBS-
D, a patient
can be considered responding if, for example, the patient experiences a
threshold decrease
(such as, for example, 25% or greater, 30% or greater, 35% or greater) in the
weekly average
of abdominal pain severity over a 24 hour period. Successful response to IBS-D
treatment
also can be indicated by a patient who experiences a decreased weekly average
stool
consistency relative to a baseline measurement, or a weekly average stool
consistency below
a threshold value (for example, 3 or below according to the scale presented in
Figure 2).
Preferably, improvement in both abdominal pain severity and stool consistency
are
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
7
considered. Further, for IBS-D, immediate need to have a bowel movement,
bloating,
pressure, gas, incomplete evacuation, and rectal symptoms can serve as
secondary endpoints,
and improvement in these categories can also be employed to assess an IBS-D
patient's
response to treatment.
[0033] The following examples further illustrate the invention but, of course,
should not
be construed as in any way limiting its scope.
EXAMPLE 1
[0034] This example demonstrates a method for developing a tool for assessment
of
symptoms of Irritable Bowel Disease.
[0035] Two sets of concept elicitation focus groups were conducted, with
patients in each
set meeting the ROME III inclusion and exclusion criteria for IBS-D. In the
first (Set 1), 34
patients were interviewed in 8 concept elicitation focus groups having 2-9
subjects per group.
In the second (Set 2), 32 patients under 70 years of age were interviewed in
four gender-
specific concept elicitation focus groups (n=3 female, n=1 male). Demographic
and health
information for each patient was recorded and is presented in Table 1.
Table 1- Summary of Socio-Demographic and Health Information
Criteria Set I Set 2 Overall
Number 34 32 64
Female (%) 22 (64.71) 24 (75.00) 46 (71.88)
Age Mean SD (Range) 44.65 15.5 (23, 69) 45 10.84 (21, 68) 45.02 f 13.35
(21, 69)
Ethnicity
Asian 3 (8.82%) N/A 3 (4.69%)
Black/African American 4 (11.76%) 4(12.50%) 8 (12.50%)
Hispanic/Latino (of any race) 1 (2.94%) 1(3.13%) 2 (3.13%)
White/Caucasian 26 (76.47%) 26(81.25%) 52 (81.25%)
Multiracial N/A 1 (3.13%) l (l.56%)
Level of education (%)
High school diploma or GED 5 (14.71%) 6 (18.75%) 11(17.19%)
Some college 6 (17.65%) 13 (40.63%) 19 (29.69%)
Vocational school or certificate 2 (5.88%) 5 (15.63%) 7 (10.94%)
program
College or university degree (2- 16 (47.06%) 6 (18.75%) 22 (34.38%)
or 4- year)
Graduate degree 5(14.71%) 2 (6.25%) 7 (10.94%)
Work status (%)
Working full-time 15 (44.12) NE NE
Working part-time 7 (20.59) NE NE
Homemaker 3 (8.82) NE NE
Student 2 (5.88) NE NE
Retired 8 (23.53) NE NE
Unemployed 1 (2.94) NE NE
Health in general/health status
Excellent 4 (11.76%) N/A 4 (6.25%)
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
8
Very good 9 (26.47%) 6 (18.75%) 15 (23.44%)
Good 14 (41.18%) 20(62.50%) -4(53.13%)
Fair 5(14.71%) 6(18.75%) 1](17.19%)
Poor 2 (5.88%) N/A 2(3.13%)
Severity of IBS-D (%)
Very mild 4(11.76%) 1(3.13%) 5(7.81%)
Mild 8 (23.53%) 4 (12.50%) 12 (18.75%)
Moderate 19 (55.88%) 18 (56.25%) 37(57.81%)
Severe 3 (8.82%) 8 (25.00%) 11(17.19%)
Very severe (%) 0 1 (3.13%) ](1.5.6%)
GED: General Educational Development; IBS-D: diarrhea-predominant irritable
bowel
syndrome ; N/A: not applicable; NE: not evaluated
[0036] Grounded theory data collection and analysis methods were used,
including
constant comparison of quotations by participants, to determine whether
concepts were
simple or complex; to determine the grouping of sub-concepts; to determine
concepts and
domains; to determine the recall period; to determine the response options
(e.g., a severity or
frequency item); to determine whether the items emphasizing patients' exact
words were
appropriate; and to develop the conceptual framework.
[0037] In Set 1, thirty-six different concepts were elicited, with 29 concepts
saturated.
Responses from Set 2 were compared with those of Set 1, and determined to be
similar.
Ethnicity and other demographic data did not impact the reporting of the
symptoms by the
patients. In particular, educational level did not impact how the symptoms
were reported by
patients. For example, all patients tend to describe the occurrence of
diarrhea (in relation to
eating), and description of diarrhea ("the runs") similarly, as illustrated in
Table 2.
Table 2
Level of Education Sample Quotes
High school diploma or GED = ... overeating. Sometimes like a holiday -
Thanksgiving ... I'll get the diarrhea, got to empty
my system.
= ...I got the runs...
Vocational school or certificate = ...maybe the food that I was eating.. .the
runs.
program = ...I get the runs...
At least some college, college = ... as soon as you eat something...
immediately
degree or higher have to go to the restroom ... like every time I eat
something.
= Just got the runs.. .had the runs when I drank
coffee.
[0038] Concepts elicited that were mentioned spontaneously and that were
saturated were
included in a draft symptom diary (Figure 5) and a draft event log (Figure 6).
However,
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
9
some concepts that met these criteria were excluded due to reasons such as a
lack of clinical
relevance or specificity to IBS-D, such as vomiting, nausea, heartburn, and
sweating. More
specifically, concepts included in the draft symptom diary and draft event log
were limited to
lower GI symptoms in accordance with the ROME III Criteria and clinical
diagnosis of IBS-
D.
[0039] The concepts that were retained for use in the draft symptom diary and
event log
were: Diarrhea, immediate need (urgency), bloating/pressure, frequency of
bowel movement,
cramps, abdominal/stomach pain, gas, complete emptying of bowels/incomplete
evacuation,
accident, bubbling in intestines/bowel sounds, rectal burning, stool
consistency, rectal spasm,
and pain while wiping.
[0040] The draft symptom diary was developed to capture IBS-D symptoms over a
given
day while the draft event log was developed to report information regarding
individual bowel
movements.
[0041] Eleven cognitive interviews were conducted to determine the
comprehensiveness,
understanding, appropriateness, and readability of the draft symptom diary and
draft event
log. Patients were asked to complete the 17-item draft symptom diary (Figure
5) and draft
event log (Figure 6), and to provide their feedback using the "think aloud"
method. Patients
were asked to identify words, terms, or concepts that they did not understand
or interpreted
differently than had been intended. They were also asked if revisions should
be made to the
17-item draft symptom diary or event log to make them more appropriate,
comprehensive, or
interpretable. After the cognitive interviews, two questions based on concepts
listed above
were deleted from the 17-item draft symptom diary: "In the past 24 hours, how
would you
rate your immediate need to use the bathroom to have a bowel movement?" and
"In the past
24 hours, how often did you feel full before finishing a normal size meal?"
[0042] Five additional questions were deleted from the resulting 15-item
symptom diary
(Figure 3) in order to avoid redundancy and optimize clarity. The remaining 10
questions of
the symptom diary are presented in Table 3. A complete version of the symptom
diary is
presented in Figure 1.
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
.l0
Table 3 - Symptom Diary
Question Provided responses
1. In the past 24 hours, on a scale of 0 to 10, A range of 0-10, with 0
characterized as "No
how would you rate the severity of your stomach pain" and 10 characterized as
stomach pain? "Worst stomach pain."
2. In the past 24 hours, on a scale of 0 to 10, A range of 0-10, with 0
characterized as "No
how would you rate the severity of the abdominal pressure" and 10
characterized as
pressure you felt in your abdomen? "Worst abdominal pressure."
3. In the past 24 hours, on a scale of 0 to 10, A range of 0-10, with 0
characterized as "Not
how bloated did you feel? bloated" and 10 characterized as "Extremely
bloated."
4. In the past 24 hours, on a scale of 0 to 10, A range of 0-10, with 0
characterized as "No
how would you rate the severity of your abdominal cramps" and 10 characterized
as
abdominal cramps? "Worst abdominal cramps."
5. In the past 24 hours, on a scale of 0 to 10, A range of 0-10, with 0
characterized as "No
how would you rate the severity of your rectal spasms" and 10 characterized as
rectal spasms? "Worst rectal spasms."
6. In the past 24 hours, on a scale of 0 to 10, A range of 0-10, with 0
characterized as "No
how would you rate the severity of the burning sensation" and 10 characterized
as
burning sensation you experienced after a "Worst burning sensation."
bowel movement?
7. In the past 24 hours, on a scale of 0 to 10, A range of 0-10, with 0
characterized as "No
how would you rate the severity of your abdominal pain" and 10 characterized
as
abdominal pain? "Worst abdominal pain."
8. In the past 24 hours, how often did you A 5-point Likert scale: (1) None of
the time;
have gas? (2) A little of the time; (3) Some of the time;
(4) Most of the time; (5) All of the time.
9. In the past 24 hours, how often were you A 5-point Likert scale: (1) None
of the time;
able to hear sounds coming from your (2) A little of the time; (3) Some of the
time;
abdomen that signaled that you might have (4) Most of the time; (5) All of the
time.
diarrhea?
10. In the past 24 hours did you have any Yes or No.
accidents (lose control of your bowels)?
[0043] The draft event log (Figure 4) was also modified after additional
analysis,
although it continued to require patients to note the date and time of each
episode, along with
the consistency thereof. For example, a question explicitly asking patients to
rate the severity
of their diarrhea was deleted on the basis that such conclusion is best drawn
from the number
and type of diarrhea events. Additionally, a new question was added having a
positive/negative response: "Did you feel like you completely emptied your
bowels?"
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
11
[0044] The resulting event log is shown in Figure 2. The questions provided
therein are
provided in Table 4.
Table 4 - Event Log
Question Provided responses
Which best describes what your bowel 6 pictures according to the Adapted
Bristol
movement looked like? Stool Form Scale.
Did you feel like you completely emptied Yes or No
your bowels?
How immediate was your need to have a A 5-point Likert scale: (1) Not at all;
(2)
bowel movement? Slightly; (3) Moderately; (4) Quite a bit; (5)
Extremely.
[0045] The final event log and symptom diary (Figures 1 and 2) reflect a
highly
descriptive yet compact tool for assessing a patient's symptoms of IBS-D.
EXAMPLE 2
[0046] This example demonstrates the use of the event diary and symptom log to
diagnose IBS-D in a patient.
[0047] A patient suspected of having IBS-D is provided by a clinician with an
event log
as shown in Figure 2. The patient is instructed to record all bowel movements
over a set
period, e.g., one week or two weeks, as well as to answer the questions
provided regarding
urgency, emptying of the bowel, and type of bowel movement according to the
Adapted
Bristol Stool Form Scale.
[0048] The clinician also provides the patient with a symptom diary as shown
in Figure 1.
The patient is instructed to complete a copy of the symptom diary each day for
a set period,
e.g., one week or two weeks.
[0049] Once the patient completes the event log and symptom diaries for the
prescribed
period, the clinician reviews them. If the patient shows a pattern of response
consistent with
IBS-D, the patient can be diagnosed with IBS-D.
[0050] Accordingly, the event diary and symptom log can be used to diagnose
IBS-D in a
patient.
EXAMPLE 3
[0051] This example demonstrates the use of the event diary and symptom log in
treating
IBS-D in a patient.
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
12
[0052] A patient undergoing a treatment for IBS-D is provided by a clinician
with an
event log as shown in Figure 2. The patient is instructed to record all bowel
movements over
a set period, e.g., one week or two weeks, as well as to answer the questions
provided
regarding urgency, emptying of the bowel, and type of bowel movement according
to the
Adapted Bristol Stool Form Scale.
[0053] The clinician also provides the patient with a symptom diary as shown
in Figure 1.
The patient is instructed to complete a copy of the symptom diary each day for
a set period,
e.g., one week or two weeks.
[0054] Once the patient completes the event log and symptom diaries for the
prescribed
period, the clinician reviews them. If the patient shows improvement based on
category score
or the total score of six or more items of the symptom diary, then the
clinician deems the
patient to have improved in IBS symptoms. Alternatively, improvement in the
patient's
response to two primary endpoints (abdominal pain severity and stool
consistency) is
considered a favorable response to the IBS-D therapy.
[0055] If the patient does not show adequate improvement in IBS symptoms, the
clinician
revises the dosage and frequency of the IBS treatment, or, optionally,
administers a different
IBS treatment.
[0056] The process of recording IBS events and symptoms in the symptom diary
and
event log, and evaluating such responses may be repeated until the patient
achieves a
clinically satisfactory improvement in IBS symptoms.
[0057] Accordingly, the event diary and symptom log can be used to evaluate a
IBS-D
patient's condition and modify treatment accordingly.
[0058] All references, including publications, patent applications, and
patents, cited
herein are hereby incorporated by reference to the same extent as if each
reference were
individually and specifically indicated to be incorporated by reference and
were set forth in
its entirety herein.
[0059] The use of the terms "a" and "an" and "the" and similar referents in
the context of
describing the invention (especially in the context of the following claims)
are to be
construed to cover both the singular and the plural, unless otherwise
indicated herein or
clearly contradicted by context. The terms "comprising," "having,"
"including," and
"containing" are to be construed as open-ended terms (i.e., meaning
"including, but not
limited to,") unless otherwise noted. Recitation of ranges of values herein
are merely
intended to serve as a shorthand method of referring individually to each
separate value
CA 02758969 2011-10-14
WO 2010/121209 PCT/US2010/031502
13
falling within the range, unless otherwise indicated herein, and each separate
value is
incorporated into the specification as if it were individually recited herein.
All methods
described herein can be performed in any suitable order unless otherwise
indicated herein or
otherwise clearly contradicted by context. The use of any and all examples, or
exemplary
language (e.g., "such as") provided herein, is intended merely to better
illuminate the
invention and does not pose a limitation on the scope of the invention unless
otherwise
claimed. No language in the specification should be construed as indicating
any non-claimed
element as essential to the practice of the invention.
[0060] Preferred embodiments of this invention are described herein, including
the best
mode known to the inventors for carrying out the invention. Variations of
those preferred
embodiments may become apparent to those of ordinary skill in the art upon
reading the
foregoing description. The inventors expect skilled artisans to employ such
variations as
appropriate, and the inventors intend for the invention to be practiced
otherwise than as
specifically described herein. Accordingly, this invention includes all
modifications and
equivalents of the subject matter recited in the claims appended hereto as
permitted by
applicable law. Moreover, any combination of the above-described elements in
all possible
variations thereof is encompassed by the invention unless otherwise indicated
herein or
otherwise clearly contradicted by context.