Note: Descriptions are shown in the official language in which they were submitted.
CA 02760941 2016-10-03
SYSTEM AND METHOD FOR MONITORING CARDIOMYOCYTE
BEATING, VIABILITY AND MORPHOLOGY AND FOR SCREENING FOR
PHARMACOLOGICAL AGENTS WHICH MAY INDUCE
CARDIOTOXICITY OR MODULATE CARDIOMYOCYTE FUNCTION
15 TECHNICAL FIELD
This invention relates to the field of cell-based assays and more specifically
to
devices, methods and systems for performing extracellular recording of cells,
such as
cardiomyocytes, alone or in parallel with impedance monitoring.
BACKGROUND OF THE INVENTION
A. Electronic Analysis of Cells
Bioelectronics is a progressing interdisciplinary research field that involves
the
integration of biomaterials with electronic devices. Bioelectronics methods
have been
used for analyzing cells and assaying biological molecules and cells. In one
type of
application, cells are cultured on rriieroelectrodes and cell-electrode
impedance is
measured and determined to monitor cellular changes.
In PCT Application No. PCT/U S03/22557, titled "IMPEDANCE BASED DEVICES
AND METHODS FOR USE IN ASSAYS", filed on July 18, 2003, a device for
1
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
detecting cells and/or molecules on an electrode surface is disclosed. The
device
detects cells and/or molecules through measurement of impedance changes
resulting
from the attachment or binding of cells and/or molecules to the electrode
surfaces. A
number of embodiments of the device are disclosed, together with the
apparatuses,
and systems for using such devices to perform certain cell based assays.
B. Dynamic Monitoring of Cardiomyocyte Viability, morphology and Beating In
Vitro
To bring a new drug to the market it can take anywhere between 8 to 16 years,
and
average cost of developing a drug is now around $500m-$800m with the cost
expected to hit the $1bn mark within the next four years. Cardiotoxicity has
been
cited as the reason for 30 per cent of all failed drug compounds during
development
and is a major cause of compound attrition. The late detection of cardiotoxic
side
effects caused by pharmacological compounds can impede drug discovery and
development projects, and consequently increase their cost. Testing for the
potential
cardiotoxic side effects of compounds at an early stage of drug development
has
therefore been the goal of many pharmaceutical and biotechnology companies.
Cardiotoxicity itself can entail a number of short-term and long term cellular
events
including directly affecting the beating rate of cardiomyocytes, viability of
cardiomyocytes and morphology of cardiomyocytes as would occur in hypertrophy.
The core of the current issue in pharmacological safety assessment and drug
development is the lack of a reliable screening methodology capable of
monitoring
potential drug-mediated cardiotoxicity and distinguishing between different
modes of
cardiotoxicity. What is urgently needed in the field is a good cell-based
model system
as well as a monitoring system with a physiological and functional readout
that can
provide incisive information regarding potential cardiotoxic side effects of
drugs.
1. Cell-based Model System for Studying Cardiac Function
Traditionally, the drug discovery industry has undertaken two different
approaches for
toxicological assessment of drug candidate leads in cardiac function. The
first
approach involves isolation of cardiomyocytes directly from a mammalian
species
such as rats and dogs followed by electrophysiological and viability studies
on the
2
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
isolated cardiomyocytes. This approach is extremely labor-intensive, time
consuming
and costly and at the same time not very amenable to the high throughput
demands of
pharmaceutical industry.
An alternative method for prediction of cardiotoxicity of drug candidate leads
early in
the drug development process has involved utilizing cell-based assay models
which
heterologously express specific ion channels such as hERG channels or voltage-
gated
calcium channels. These cardiac ion channels have been envisioned as possible
molecular targets through which drugs could induce cardiotoxicity. These cell-
based
systems allow the assessment of drug-channel interaction by monitoring the
effect of
the drug on the currents produced by the different channels in cultured cells
using a
technique known as 'patch clamping', which isolates regions of the cell
membrane
containing channel proteins and measures changes in electrical potential
difference.
Use of this method in high throughput requires automation of patch clamping in
array
format, which even though is available in last several years but is not yet
widespread.
The other issue with this approach is that cardiac toxicity may occur by other
mechanisms which can easily be missed by this type of targeted approach. An
alternative to the in vitro ion-channel recording assays as well as the labor-
intensive
isolation of primary tissue is the differentiation of embryonic stem (ES)
cells into
cardiomyocytes as a starting material for functional assays.
The utility of ES cells as a treatment for various chronic diseases has
received much
attention in recent years. Mammalian ES cells are self renewing cells derived
from the
inner cell mass of a blastocyst stage embryo which can be differentiated into
multiple
different cell types. It has been demonstrated that the mouse ES cells as well
as
human ES cells can be differentiated into cardiomyocytes which retain the
ability to
beat in culture. The differentiation of ES cells first involves an
intermediate in vitro
developmental stage in which ES cells form compact cell structures known as
embryoid bodies. These embryoid bodies can induce the developmental program of
ES cell differentiation into multiple cell types including cardiomyocytes
which are
distinguished in culture by their ability to undergo spontaneous beating.
These ES
derived in vitro differentiated cardiomyocytes recapitulates the normal
development
of cardiomyocytes as evidenced by the stage-specific expression of
cardiomyocyte
specific genes. All the known transcription factors, ion channels and
structural
3
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
proteins that are part of normal heart development and function in vivo are
also
expressed in ES-derived cardiomyocyte.
Because ES cells are self renewing cells in culture they can serve as an
excellent
source for continuous production of cardiomyocytes. Therefore, these
cardiomyocytes
which behave in every way like normal cardiomyocytes isolated from the heart
tissue
itself addresses the ever important supply problem and for the first time
allows for
assessment of cardiac function and its modulation by lead candidate drugs and
compounds in relatively large scale in both viability assays, assessment of
morphology and in monitoring the beating function of cardiomyocytes.
Furthermore,
because the technology exists to selectively knockout or express trans-genes
in ES
cells, it provides an excellent model system to study the role of certain
genes in
cardiac development and function without having to be concerned about adverse
affects on overall embryonic development in transgenic animals. The ability to
express transgenes in ES cells has been utilized as a way to enrich for
preparation of
cardiomyocytes that are 100% pure. For example, the gene encoding GFP has been
cloned downstream of a cardiac-specific promoter and then introduced into ES
cells.
Embryoid cells which ultimately differentiate into cardiomyocytes will
therefore
express the GFP transgenes and these cells can be easily isolated by cell
sorting
techniques and therefore an enriched cardiomyocyte population can be obtained.
2. Technologies Used for Assessment of Cardiomyocyte Function In Vitro
Technologies designed to assess cardiomyocyte behavior and function and the
effect
of drugs and other manipulations in vitro can be divided into two different
approaches. One approach involves long term assessment of cardiomyocyte
viability
for example in response to certain compounds. Such assays are typically end
point
assays designed to measure a cellular component such as ATP which correlates
with
the degree of viability of the cells. The other approach involves studying
short term
effect of drugs and compounds on beating function of cardiomyocytes. High
throughput techniques for short term functional characterization of ion
channels and
other targets in cardiomyocytes has been rather challenging and limited. The
systems
such as automatic patch clamp instrumentation that are available can monitor a
single
cardiomyocyte at a time and with very limited throughput.
4
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
Accordingly, while there exists different approaches to assess cardiotoxicity
of
various compounds, each suffers from one or more drawbacks. Thus, there
remains a
need to develop improved devices and methods that have high throughput
capabilities
and improved accuracy and reliability with respect to measurements and
correlation
with cell phenotype.
SUMMARY OF THE INVENTION
The present invention discloses devices, systems and methods for performing
extracellular recording of excitable cells, such as cardiomyocytes, in vitro.
Specifically, the devices and methods provide improved characterization of
excitable
cells in response to compound administration to assess cardiotoxic effects,
while
increasing efficiency of throughput for potential drug candidates. The
invention
further provides improved characterization of cells during differentiation
processes
related to development of cardiomyocytes.
It is an object of the invention to provide extracellular recording
configurations,
which permit reproducible sampling across larger cell populations compared to
traditional approaches. It is another object of the invention to provide cell-
free zones
for positioning a reference electrode to prevent its direct interaction with a
cell
population loaded onto the device. It is another object of the invention to
provide a
combined device capable of both performing extracellular recording
measurements
and impedance monitoring, which may be selectively activated for measurement
and
thus provide a multi-faceted system for assessing or characterizing excitable
cells.
In one aspect of the present invention a device for performing extracellular
recording
of cells, such as excitable cells, cardiomyocytes, and cardiomyocyte precursor
cells is
provided. An exemplary device includes a) a nonconductive substrate forming or
provided as a base of one or more wells; b) a recording electrode positioned
on the
substrate within the well, wherein the recording electrode is accessible to
cells when a
cell sample is added to the device; and c) a reference electrode positioned
within the
5
CA 02760941 2016-10-03
well in a cell-free zone, the cell-free zone characterized as free from
contact with cells
when the cell sample is added to the device, thereby preventing contact
between cells
and the reference electrode. The cell-free zone prevents direct contact
between cells
from a cell sample and the reference electrode. In some embodiments, the
recording
electrode has a diameter from about 10 gm to about 200 gm. In other
embodiments,
the recording electrode comprises an electrode structure comprising a
plurality of
electrode elements.
In some embodiments, the cell-free zone is physically defined by the presence
of a
.. barrier, such as a wall, through which cells do not pass. In certain
embodiments, the
barrier could be in the form of a removable blockage material which is placed
directed
on the cell-free zone when cells are added to the well and which can be
removed after
cells have settled along the bottom of the well. Thus, the blocking structure
may be in
part removable. In other embodiments, the cell free zone is provided as a
platform or
surface positioned above the base substrate. In other embodiments, the cell
free zone
is positioned along an angled wall, which is angled upward from the bottom of
the
well. In other embodiments, the cell-free zone is positioned above the
substrate and
within the volume of the well such that an external electrode extends downward
into
sample media, optionally from a lid or cap for the well.
In some embodiments the extracellular recording electrode is a unitary or
unbranched
electrode and may be of a simple geometry such as a circle, a square and the
like. In
other embodiments, the extracellular recording electrode has a branched
configuration, which may permit extracellular recording over a larger cell
population.
.. Increasing the measured cell population for extracellular recording may
increase
measurement accuracy or reproducibility of the recorded signals. In one
exemplary
embodiment, a device for performing extracellular recordings of excitable
cells in
vitro includes a) a nonconductive substrate; b) one or more wells on the
substrate; c)
one or more electrode structures fabricated on the substrate, each electrode
structure
associated with one of the one or more wells; and d) one or more reference
electrodes
external to the substrate, each of which can be inserted into one of the one
or more
wells, wherein for each of the one or more wells, the corresponding electrode
structures and reference electrodes form an electrode pair. In further
embodiments
the substrate has a surface suitable for attachment of excitable cells,
wherein the
attachment of
6
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
excitable cells on the substrate can result in a detectable extracellular
recording
potential between each electrode pair. In a further embodiment, which is also
a
preferred embodiment, each electrode structure comprises multiple electrode
elements. In another preferred embodiment, each electrode structure comprises
multiple electrode elements, forming half of an interdigitated electrode
array. In
another preferred embodiment, each electrode structure comprises multiple
electrode
elements which have different electrode element shapes, including rectangular
shape,
or sinusoidal shape, or circle-on-line shape.
In another preferred embodiment of the device for performing extracellular
recording,
each electrode structure occupies a substantial percentage of surface area of
the well
that the electrode structure is associated with. In some preferred
embodiments,
percentage of surface area of the well at the bottom being occupied by the
electrode
structures is more than 1%. Preferably, percentage of surface area of the well
at the
bottom being occupied by the electrode structures is more than 5%. Preferably,
percentage of surface area of the well at the bottom being occupied by the
electrode
structures is more than 10%. More preferably, the percentage of surface area
of the
well at the bottom being occupied by the electrode structures is more than
20%.
More preferably, the percentage of surface area of the well at the bottom
being
occupied by the electrode structures is more than 30%. More preferably, the
percentage of surface area of the well at the bottom being occupied by the
electrode
structures is more than 50%. More preferably, the percentage of surface area
of the
well at the bottom being occupied by the electrode structures is more than
70%.
More preferably, the percentage of surface area of the well at the bottom
being
occupied by the electrode structures is more than 85%.
In another aspect of the present invention, a method of performing
extracellular
recording of excitable cells includes, providing a device of the present
invention,
adding excitable cells to the wells; providing an extra-cellular-recording
amplifier that
can measure and record voltage signals at microvolt levels, connecting
electrodes on
the devices to the extra-cellular-recording amplifier; monitoring and
recording
extracellular potentials at the electrodes of the devices. Preferably, the
method
further comprises analyzing recorded extracellualr potential waveforms. More
preferably, the method further comprises adding a compound to the well,
measuring
7
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
and recording extracellular potentials prior to and after the compound
addition; and
analyzing extracellular potential waveforms.
In still another aspect of the present invention, a device allowing for
parallel
extracellular recording and cell impedance monitoring of a cell population is
provided. The device includes a cell impedance monitoring means and an
extracellular recording means operably coupled through a same substrate. In a
further
embodiment a switching means switches operation of the device between
impedance
monitoring and extracellular recording.
In still another aspect of the present invention, a device allowing for
parallel
extracellular recording and cell impedance measurement comprises a
nonconductive
substrate, having a surface suitable for attachment of excitable cells, one or
more
wells on the substrate; for each well, a pair of impedance measurement
electrodes,
wherein the attachment of excitable cells on the substrate can result in a
detectable
impedance change between the pair of impedance measurement electrodes; for
each
well, a pair of extra-cellular recording electrodes, wherein the attachment of
excitable
cells on the substrate can result in a detectable extra-cellular recording
potentials
between the pair of extra-cellular recording electrodes. Preferably, the pair
of
impedance measurement electrodes is located on the substrate. More preferably,
for
the pair of extra-cellular recording electrodes, one recording electrode is
located on
the substrate and another electrode is located external to the substrate. In
other
preferred embodiments, the reference electrode may be isolated from cells by
positioning the reference electrode at a cell-free zone, such that it is a
cell-free
electrode. The reference electrode may or may not be located on the substrate.
The
device may also include or be operably connected to a switching means to
switch
from an impedance mode to an extracellular recording mode.
In another aspect of the invention, a device allowing for parallel
extracellular
recording and cell impedance measurement includes a nonconductive substrate
forming or provided as a base of one or more wells; at least two impedance
electrodes
capable of monitoring impedance of the cells, the at least two impedance
electrodes
positioned within a well and on the nonconductive substrate; and a reference
electrode, wherein a first impedance electrode from the at least two impedance
8
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
electrodes is electrically coupled to the reference electrode for performing
an
extracellular recording measurement at the first impedance electrode.
In still another aspect of the present invention, a method for parallel
measurement of
cell-substrate impedance and extra-cellular potentials includes a) providing a
device
allowing for parallel extracellular recording and cell impedance measurement;
b)
adding excitable cells to the wells of the device; c) providing an impedance
analyzer;
d) providing an extracellular potential amplifier; e) connecting impedance-
measurement electrodes of the devices to the the impedance analyzer; 0
connecting
extra-cellular recording electrodes of the devices to the the extra-cellular
potential
amplifier; g) performing parallel measurement of cell-substrate impedance and
extracellular potentials. Preferably, the method further comprises adding
compounds
to the cells and monitoring cell-substrate impedance and extracellular
potentials prior
to and after compound addition. Preferably, the method further comprises
analyzing
measured time dependent impedance responses. Still preferably, the method
further
comprising analyzing extracellular-recording wave forms.
In present invention, we describe label-free methods for monitoring
cardiomyocytes
in vitro. In one aspect of the present invention, extracellular-recording
measurement, or parallel impedance and extracellular recording measurement of
cardiomyocytes on microelectrodes is used to non-invasively monitor cardiac
cell
viability in vitro in long term experiments. This method will allow the
continuous
monitoring of cardiomyocyte viability overtime and can monitor the interaction
of
compounds which ultimately result in promoting loss of cardiomyocyte
viability.
In another aspect of the present invention, extracellular-recording
measurement or
parallel impedance and extracellular recording measurement of cardiomyocytes
on
microelectrodes is used to non-invasively monitor cardiomyocyte beating in
vitro.
The system is capable of continuously monitoring the excitation-contraction
coupling,
.. otherwise know as beating, of cardiomyocytes in a relatively high-
throughput manner
using either extracellular-recording measurement or parallel impedance and
extracellular recording measurement. It can be used for pharmacological safety
assessment, screening for novel compounds which modulate cardiomyocyte
function
in a specific manner and assessment of genes involved in cardiac function.
9
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
In another aspect of the present invention, extracellular recording
measurement or
parallel impedance and extracellular recording measurement can be used to
monitor
both short term beating whilst impedance measurement can be used to monitor
long
term viability status of cardiomyocytes in the same well. The ability to
monitor both
cardiomyocyte beating in conjunction with viability for the same population of
cardiomyocytes over time would be an added advantage. Certain manipulations
such
as drug treatment may not manifest their effect on cardiomyocyte beating
and/or
viability until at a later time period. Therefore, the time resolution as well
as the
capabilities of extracellular recording measurements together with impedance
measurements of the system of the present invention can be used to monitor
cardiomyocyte beating and viability over time.
In another aspect of the present invention, impedance readout can be used to
monitor
the morphological or differentiative behavior of cardiomyocytes in vitro.
Certain
treatments can induce changes in morphological behavior of cardiomyocytes,
such as
inducing hypertrophy which is associated with cardiomyocyte elongation and
expansion. Because impedance monitoring can detect changes in cell morphology,
it
can be used to for detection of hypertrophy in cardiomyocytes.
In another aspect of the present invention, direct optical monitoring of
cardiomyocytes is used to quantify and measure the beating of cardiomyocytes.
Still,
in another aspect of the present invention, extracellular recordings (i.e.
measurement
of electrical potentials of extracellular recording electrodes when
cardiomyocytes are
attached to the electrode surfaces) are used to quantify and measure the
beating of
cardiomyocytes. Still, in another aspect of the present invention, a physical
method
which can be used to monitor cell-substrate interaction is used to quantify
and
monitor beating of cardiomyocytes.
In another aspect of the present invention, the methods include real-time
impedance
monitoring and/or extracellular recording of cardiomyocyte in vitro. This
invention
will be able to monitor viability of cardiomyocytes, the field potential of
cardiomyocytes, or the excitation-contraction coupling or beating and
morphological
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
and differentiative aspects of cardiomyocytes in a label-free manner and real-
time
manner. Furthermore, the invention will be able to monitor cardiotoxicity and
the
effect of compounds and drugs for safety pharmacology purposes and can also
serve
as a screen for agents which can modulate cardiomyocyte function. This system
can
be used in conjunction with ES derived cardiomyocytes, adult stem-cell derived
cardiomyocytes as well as primary cardiomyocytes to study the role of
different genes
and proteins in cardiac function and development.
The system of the present invention having the capability of parallel
impedance-based
.. monitoring as well as extra-cellular recording based monitoring of
cardiomyocytes
fills a major technological gap in monitoring cardiomyocytes in vitro. At
present to
our knowledge there are only a few technologies that can monitor cardiomyocyte
population function in vitro, especially with regards to cardiotoxicity and
are limited
in their throughput. In addition to functional monitoring of cardiomyocyte
beating the
current invention offers several other major benefits which are worth
discussing.
Among these include the impedance system described here can monitor
cardiomyocytes for short durations, milliseconds to minutes and long
durations,
several hours to days. Therefore, both short term and long term effects of
drugs not
only on cardiomyocyte beating, but viability and changes in morphology and
adhesion
can also be assessed. This feature is especially important because certain
compounds
such as f3-2 adrenergic receptor agonists, well known and characterized
modulators of
heart function in vivo and in vitro, can induce long term hypertrophic
responses in
cardiomyocytes, which is associated with elongated morphology of the cells. In
addition, the systems of the present invention can also record cardiomyocyte
action
potentials or field potentials, allowing detailed electrophysiological
analysis and
assessment of cardiomyocytes for relatively short duration at a time.
The method of the present invention is to devise a label-free cell-based assay
system
for continuous monitoring of cardiomyocyte viability, the rhythmic beating of
.. cardiomyocytes and cardiomyocyte morphology and differentiation and to
screen for
pharmacological agents which may modulate these processes and induce
cardiotoxicity. In one aspect, viability is monitored based on long term
impedance
monitoring of cardiomyocytes seeded in microelectronic plates (E-PLATES), and
in
11
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
parallel, the field potential generated by the excitation of the
cardiomyocytes or other
excitable cells is monitored using the extracellular potential recording
electrodes
associated with the wells of the E-PLATES. Viable cells will continue to
generate
impedance signal and any changes in viability, especially due to cytotoxic or
cardiotoxic drugs will be reflected by changes in impedance. In one respect,
the
method includes providing a device for measuring cell-substrate impedance and
extracellular potentials operably connected to an impedance analyzer and
extracellular
recording potential amplifier, wherein the device includes at least one well
optionally
coated with fibronectin or other appropriate extracellular matrix proteins
(e.g. gelatin)
to expedite attachment; adding cells to the at least one well, where the cells
can be
mouse or human or other mammalian ES cells or adult stem cells destined to
differentiate into cardiomyocytes or primary cardiomyocytes isolated directly
from
the heart of an experimental system including mice, rats, rabbits or dog;
monitoring
impedance of the at least one well over a period of time and optionally
determining
cell index from impedance values; and monitoring the extracellular potentials
using
recording electrodes associated with the wells of the E-PLATES.
In another aspect, the method includes providing a device for measuring cell-
substrate
impedance and for performing extracellular recording operably connected to an
impedance analyzer and extracellular recording potential amplifier, wherein
the
device includes at least two wells optionally coated with fibronectin (or
other
extracellular matrix proteins such as gelatin) to expedite attachment; adding
cells to
the at least two wells, where the cells can be mouse or human or other
mammalian ES
cells or adult stem cells destined to differentiate into cardiomyocytes or
primary
cardiomyocytes isolated directly from the heart of an experimental system
including
mice, rats, rabbits or dog; monitoring impedance and monitoring extracellular
potentials of the at least two wells over a period of time. Treating at least
one well
with an agent; where the agent could include but is not limited to a compound,
peptide, protein, antibody, siRNA, shRNA, lipid or any combination of thereof
and
the other well is treated with an appropriate control; continue monitoring of
control
and treated well over a period of time preoptimized for the experiment of
interest;
concluding that the factor may affect cell viability if the impedance of the
treated well
is significantly different than the impedance of the treated well and/or
concluding that
12
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
the factor may affect cell excitation if the extracellular potential of the
treated well is
significantly different from that of the treated well.
Dynamic monitoring of cardiomyocyte beating is based on quantification in real
time
of the rhythmic changes in cardiomyocyte morphology as a result of the
excitation
contraction coupling of the electrically excitable cardiomyocytes growing on
microelectrodes' surface in ACEA E-PLATES. The quantification of the rhythmic
changes in cardiomyocyte morphology is achieved via the fast (down to
milliseconds
level, for example, at 40 milli-second, 30 milli-second, 20 milli-second, 10
millisecond or 5 milli-second time resolutions) and continuous measurement of
electrode impedance. The method essentially provides a cellular cardio-gram
which
can provide incisive information about the status of cardiomyocytes especially
upon
treatment with pharmacological agents. In addition, the method allows for
parallel
measurement of extracellular potentials of beating cardiomyocytes, providing
important electro-physiological property information of the cells. The method
includes providing a device for measuring cell-substrate impedance and for
monitoring extracellular potential operably connected to an impedance analyzer
and
an extracellular potential amplifier, wherein the device includes at least one
well
optionally coated with fibronectin (or other suitable extracellular matrix
proteins) to
expedite attachment; adding cells to the at least one well, where the cells
can be
mouse or human or other mammalian ES cells or adult stem cells destined to
differentiate into cardiomyocytes or primary cardiomyocytes isolated directly
from
the heart of an experimental system including mice, rats, rabbits or dog;
monitoring
impedance of the at least one well at time intervals over a period of time and
optionally determining cell indices from impedance values and monitoring
extracellular potentials from the at least one well for a time period (for
example, 10
seconds) at certain time intervals (for example, every 10 minutes); optionally
calculating average rate of beats per unit time, average amplitude intensity
in a unit
time as well as the average length of time between the beats.
In another aspect, the present invention is directed to method to screen for
potential
agents that modulate ES- derived cardiomyocyte beating, adult stem cell-
derived
cardiomyocyte beating or primary cardiomyocyte beating by monitoring and
measuring the excitation-contraction coupling of cardiomyocyte and its
modulation
13
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
by the agent. The agent may include but is not limited to compounds, drugs,
peptides,
proteins, antibodies, siRNA, shRNA, miRNA, cDNA, lipids and any combination
thereof. The method includes providing a device for measuring cell-substrate
impedance and for monitoring extracellular potentials operably connected to an
impedance analyzer and to an extracellular potential amplifier, wherein the
device
includes at least two wells; adding ES cells, adult stem-cell derived
cardiomyocytes or
primary cardiomyocytes to at least two wells; monitoring impedance of the at
least
two wells at different or similar time intervals over a period of time and
optionally
determining cell indices from impedance values and monitoring and recording
extracellular potentials of the at least two wells for a time window at time
intervals
over a period of time; generating an impedance-based curve or optionally a
cell index
curve for each of the at least one known factor and the control; comparing the
impedance-based curves or optionally the cell index curves between the at
least one
known biologically active agent well and the control well; the impedance-based
curves could be direct measurement of cardiomyocyte excitation-contraction
coupling
and if significantly different, concluding that the biologically active agent
modulates
cardiomyocyte function, and/or comparing the extracellular potential waveforms
from
the active agent well and the control well and, if significantly different,
concluding
that the biologically active agent modulates the excitation property of the
cardiomyocytes. Optionally, impedance-based curves or optionally cell index
curves
are used to calculate average rate of beats of cardiomyocytes per unit time,
average
amplitude intensity in a unit time as well as the average length of time
between the
beats, comparison of these optionally derived parameters is made between the
at least
one known biologically active agent well and the control well, and if
siginificant
differences exist, one may optionally conclude that the biologically active
agents
modulate cardiomyocyte functions.
It is well established that certain pharmacological treatments and disease
conditions
can result in cardiac hypertrophy or atrophy culminating in changes in the
morphology of cardiomyocyte. Cell substrate impedance can be used to precisely
measure and quantify these changes in cell morphology and shape. Certain
treatments
can also affect the differentiative process of ES cells to cardiomyocytes
which may
involve specific morphological and adhesive changes. In another aspect, the
present
invention is directed to method to screen for potential agents that may
modulate the
14
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
morphology of ES- derived cardiomyocyte, adult stem cell-derived cardiomyocyte
or
primary cardiomyocyte or its differentiation. The agent may include but is not
limited
to compounds, drugs, peptides, proteins, antibodies, siRNA, shRNA, miRNA,
cDNA,
lipids and any combination thereof. The method includes providing a device for
.. measuring cell-substrate impedance operably connected to an impedance
analyzer,
wherein the device includes at least two wells; adding ES cells, adult stem-
cell
derived cardiomyocytes or primary cardiomyocytes to at least two wells;
monitoring
impedance of the at least two wells at different or similar time intervals
over a period
of time and optionally determining cell indices from impedance values;
generating an
impedance-based curve or optionally a cell index curve for each of the at
least one
known factor and the control; comparing the impedance-based curves or
optionally
the cell index curves between the at least one known biologically active agent
well
and the control well; the impedance-based curves could be direct measurement
of
changes in cell morphology and if significantly different, concluding that the
biologically active agent modulates cardiomyocyte function. Preferably, the
method
further comprises providing the device of the present invention operably
connected to
an extracellular potential amplifier, monitoring extracellular potentials of
cells from
one biologically active agent well and one control well. Optionally, impedance-
based
curves or optionally cell index curves are used to calculate the compound dose-
dependent changes in cardiomyocyte morphology and generate an EC-50 value for
the potency of the compound. In addition, extracellular recording permits
comparisons with cells at various developmental stages to assess development.
In another aspect, the present invention is directed to method to establish an
assay to
assess the effect of gene knockout or transgene expression in ES cells
differentiated to
cardiomyocytes and functionally monitored by the system of the present
invention.
The method includes providing a device for measuring cell-substrate impedance
and
for monitoring extracellular potential operably connected to an impedance
analyzer
and extracellular potential amplifier, wherein the device includes at least
two wells;
adding vvildtype ES cells as control to at least 1 well and ES cells with a
gene
knockout or a transgene in at least 1 other well; monitoring impedance of the
at least
two wells at time intervals over a period of time and optionally determining
cell
indices from impedance values; comparing the impedance-based curves or
optionally
the cell index curves between the control well and the well containing the ES
cells
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
harboring a kockout of a specific gene or expressing a specific transgene; and
if
significantly different, concluding that the gene knockout or the transgene
can affect
either cardiomyocyte viability, morphology from ES cells or cardiomyocyte
function
as monitored by observing the excitation-contraction coupling, and
alternatively,
monitoring and recording extracellular potentials from the at least two wells
for a time
period, comparing the extracellular potential waveforms between the control
well and
the well containing the ES cells harboring a kockout of a specific gene or
expressing a
specific transgene; and if significantly different, concluding that the gene
knockout or
the transgene can affect cardiomyocyte electrophysiological property of the
cardiomyocytes.
In another aspect of the present invention, direct optical monitoring of
cardiomyocytes is used to quantify and measure the beating of cardiomyocytes.
The
method includes providing a device for optically monitoring cells and
monitoring cell
morphology operably connected to an optical measurement system, where the
device
includes at least two wells optionally coated with fibronectin (or other
suitable
extracellular matrix proteins) to expedite attachment; adding cells to the at
least two
wells, where the cells can be mouse or human or other mammalian ES cells
destined
to differentiate into cardiomyocytes or primary cardiomyocytes isolated
directly from
the heart of an experimental system including mice, rats, rabbits or dog;
optically
monitoring the cells of at least two wells at time intervals over a period of
time via the
optical measurement system; optionally calculating average rate of beats per
unit
time, average amplitude intensity in a unit time as well as the average length
of time
between the beats. The device for such optical measurement of cells may
include
microtiter plates. The optical system may include optical magnification
instrument
such as microscope, optical CCD camera, optical-signal processing algorithm to
quantify cell beating and to derive cell-beating parameters (such as
calculating
average rate of beats per unit time, average amplitude intensity in a unit
time as well
as the average length of time between the beats) based on cell morphology
images.
In another aspect of the present invention, extracellular recordings (i.e.
measurement
of electrical potentials of extracellular recording electrodes when
cardiomyocytes are
attached to the electrode surfaces) are used to quantify and measure the
beating of
cardiomyocytes. The method includes providing a device with microelectrodes
for
16
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
extracellular recording of multiple cells operably connected to an
extracellular
potential recording system, where the device includes at least two wells
optionally
coated with fibronectin (or other extracellular matrix proteins) to expedite
attachment;
adding cells to the at least two wells, where the cells can be mouse or human
or other
mammalian ES cells destined to differentiate into cardiomyocytes or primary
cardiomyocytes isolated directly from the heart of an experimental system
including
mice, rats, rabbits or dog; performing extracellular recording of the cells of
at least
two wells via the extracellular recording system; optionally calculating
average rate of
beats per unit time, average amplitude intensity in a unit time as well as the
average
length of time between the beats. The device for such extracellular recording
system
includes microelectrode arrays or structures inside wells of microtiter
plates. The
microelectrode array may comprise two electrode structures having the
substantially
same surface area and may be located along the majority of the surface of the
wells.
The extracellular recording system may include electronic measurement circuits
capable of recording small extracellular potentials induced on
microelectrodes, where
cells are cultured. The signals from the extracellular recording can be used
to derive
the average rate of beats of cardiomyocytes per unit time, average amplitude
intensity
in a unit time as well as the average length of time between the beats, or
other
electrophysiologically-associated parameters.
Still, in another aspect of the present invention, a physical method which can
be used
to monitor cell-substrate interaction is used to quantify and monitor beating
of
cardiomyocytes. The method includes providing a device for physically
monitoring
cells and monitoring cell morphology operably connected to a physical
measurement
system, where the device includes at least two wells optionally coated with
fibronectin (or other extracellular matrix proteins) to expedite attachment;
adding
cells to the at least two wells, where the cells can be mouse or human or
other
mammalian ES cells destined to differentiate into cardiomyocytes or primary
cardiomyocytes isolated directly from the heart of an experimental system
including
mice, rats, rabbits or dog; optically monitoring the cells of at least two
wells at time
intervals over a period of time via the physical measurement system;
optionally
calculating average rate of beats per unit time, average amplitude intensity
in a unit
time as well as the average length of time between the beats. The device for
such
physical measurement of cells shall have such property that the interaction
between
17
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
cells and substrate surfaces can be monitored. Depending which physical method
is
used for monitoring cell-substrate interaction, different devices may be used.
For
example, the device can be an optical sensor, which can be used to detect and
measure
biological reactions. One example of optical sensor is resonant waveguide,
which
consists of a substrate with an optical grating and a coating with a high
refraction
index. This grating forms an optical waveguide, a tiny channel through which a
specific wavelength of light propagates. Only this specific wavelength that is
resonant with the waveguide grating structure is strongly reflected. In this
case, the
physical measurement system can detect changes in refractive index upon cell-
substrate interaction events or upon intracellular events. Examples of cell-
substrate
interaction events or intracellular events may include those taking place in
cultured
cells stimulated by a compound treatment.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows the cell index curves measured on RT-CES system for 4 different
seeding densities (3750, 7500, 15,000 and 30,000 cells per well) of mouse ES-
derived
cardiomyocytes. Cell index is a dimensionless parameter derived in RT-CES
system.
Figure 2A shows the time dependent, noimalized cell index for embryonic stem
cell
derived cardiomyocytes in different wells treated with different
concentrations of
sodium dichromate dehydrate. Figure 2B shows the dose dependent normalized
cell
index values at 24 hrs after treatment of the cells with dichromate dehydrate
as a
function of the concentration of dichromate dehydrate.
Figure 3A shows that the time dependent, normalized cell index for embryonic
stem
cell derived cardiomyocytes in different wells treated with different
concentrations of
isoproteranol. Figure 3B shows the dose dependent, normalized cell index at
several
hours after treatment of the cells with isoproteranol as a function of the
concentration
of isoproteranol.
Figure 4 shows the cell index curves of ES-derived cardiomyocytes measured on
RT-
CES system for 4 individual wells, with cell culture medium as background
starting
from cell seeding to about 86 hrs after cell seeding.
18
CA 02760941 2016-10-03
Figure 5 shows the detection of cardiomyocyte beating using the ACEA RT-CES
system at different time periods along the growth and clifferentiative curve
of ES-
derived cardiomyocytes seeded in ACEA E-PLATES. Each well of the E-PLATE
comprises an impedance measurement electrode array, comprising two circle-on-
line
electrode structures. Figure 5A, 5B and 5C show the cell index curves at time
resolution of 40 milli-seconds for time points at 24, 48 and 72 hrs after cell
seeding,
respectively. Figure 5D shows the cell index curves monitored at 1 hr time
resolution.
Figure 6A and 6B show the cell index curve for ES-derived cardiomyocytcs and
corresponding beat rate as analyzed using Fourier transform.
Figure 7 shows the detection of cardiomyocyte beating using the ACEA RT-CES
system for the mouse ES-derived cardiomyocytes treated with 4.4 M sotalol.
Figure 7A and 7B show the beating rate and amplitude, respectively, for the
cells
before and after the treatment. Figure 7C shows overall cell index curves
whilst
Figure 7D shows the cell-index based beating curve for a short time period.
Note
that one second displayed in Figure 7C and 7D is equivalent to 40 milli-
seconds for
real time measurement. The time resolution of the RT-CES measurement for
Figures
7C and 7D is 40 milli-seconds.
Figure 8A and 8B show the detection of cardiomyocyte beating using the ACEA RT-
CES system to assess the effect of chemical compound carbachol (333 nM, 8A)
and
isoproternaol (4.4 M, 8B) on the beating of cardiomyocyte. Note that one
second
displayed in Figure 8A and 8B is equivalent to 40 milli-seconds for real time
measurement. The time resolution of the RT-CES measurement for Figures 8A and
8B is 40 milli-seconds.
Figures 9A-S show the detection of cardiomyocyte beating using the ACEA RT-CES
system to assess the effect of various chemical compounds on the beating of
mouse
ES-derived cardiomyocytes: (9A) 33.3 pIVI sotalol with 0.033% DMSO; (9B) 400
nM
Astemizole with 0.004% DMSO; (9C) 200 nM terfenadine with 0.065% DMSO; (9D)
13.3 u.M Erythromycin with 0.13% DMSO; (9E) 20 04 moxifloxacin with 0.3%
19
CA 02760941 2016-10-03
DMSO; (9F) 20 tiM pentamidine with 0.2% DMSO; (9G) 4.4 tiM amitripyline with
0.04% DMSO; (9H) 130 nM verapamil with 0.0013% DMSO; (91) 13.3 tiM
rosiglitazone with 0.13% DMSO; (9J) 13.3 JIM rofecoxib; (9K) 4.4 JIM
celecoxib;
(9L) 40 1,1A4 doxorubicin with 0.4% DMSO; (9M) 13.3 jiM cyclosporine with
0.13%
DMSO; (9N) 4.4 tAM propranolol with 0.04% DMSO; (90) 9.1 nM E4031; (9P) 8
1AM DDT with 0.08% DMSO; (9Q) 8p.M PCB with 0.08% DMSO; (9R) 81.tM
endosulfan with 0.08% DMSO; (9S) 0.13% DMSO. Figure 9S shows the 0.13%
DMSO control, indicating that DMSO at such a concentration does not produce a
significant change on the beating of the cardiomyocytes. Note that one second
displayed in Figures 9A ¨ 9S is equivalent to 40 milli-seconds for real time
measurement. The time resolution of the measurement for Figures 9A ¨ 9S is 40
milli-seconds.
Figure 10 shows the change of the beating pattern of mouse ES-derived
cardiomyocytes as a result of treatment with different compounds as monitored
by
ACEA RT-CES system. The compounds being tested here include 130 nM
verapamil, 40 nM astemizole, 20 1.04 sotalol; 200 nM trefenadine; 4 1.1.M
propranolol;
4 ttM celexocib; 150 nM carbachol and 82 nM E4031.
Figure 11 shows the change of the beating pattern of mouse ES-derived
cardiomyocytes as a result of treatment with compound Aztemizole as monitored
by
ACEA RT-CES system. The compounds concentrations being tested here include
400 nM, 40 nM, 13.3 nM, 1.33 nM, 0.133 nM and 40 pM.
Figure 12 shows a pair of extracellular recording electrodes, comprising a
recording
electrode structure on the substrate and a reference electrode external to the
substrate.
Figure 13 shows examples of electrode structures having multiple electrode
elements
that can be used for extracellular recording and/or impedance measurement
where
13A, 13B, 13C, 13D, 13E and 13F are the rectangular shape, circle-on-line
shape, a
complete interdigitated electrode array, sinuosoidal shape, castellated shapes
and a
complete circle-on-line electrode array, respectively.
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
Figures14A and 14B show two examples of extracellular recording devices where
each well comprises one recording electrode structure comprising multiple
electrode
elements and a reference electrode having large surface area.
Figures 15 shows an example of an extacellular recording device where each
well of
the device comprises a simple, circle-shaped recording electrode and a
reference
electrode (1501) having large surface area (1502).
Figure 16 shows an example of a device of the present invention where in one
well, a
pair of impedance measurement electrodes is located on the substrate and one
of the
impedance measurement electrode structures is used as an extracellular
recording
electrode, together with an externally applied reference electrode.
Figure 17 shows a multi-electrode chip comprising an array of 30 electrodes
where
the electrode diameter is 50 lint and the interval between each two neighbor
electrode
tips is 50 um.
Figure 18 shows the extracellular field potential (FP) recorded for mouse ES-
derived
cardiomyocytes on day 2 and day 3 after cell plating using electrode array
shown in
Figure 17.
Figure 19 shows the extracellular field potential (FP) recorded for mouse ES-
derived
cardiomyocytes on day 3 after cell plating using electrode array shown in
Figure 17.
The cells are treated with compound E4031 at different concentrations ranging
form 0
nM (control), 75 nM, 150 nM, 250 nM and 750 nM respectively.
Figure 20 shows the dependency of field potential duration in milli-seconds on
the
concentration of E4031.
Figure 21 shows the "anti-arrhythmic" effect of compound E4031 at 75 nM and
150
nM on field potential frequency for the mouse ES-cell derived cardiomyocytes.
Figure 22 shows extra-cellular field potentials recorded for mouse stem-cell
derived
cardiomyocytes obtained using a device of the present invention, where the
recording
21
CA 02760941 2016-10-03
electrode is a circle-on-line electrode structure and the reference electrode
is a gold
wire electrode that is introduced into the well after cell seeding. Figure
22A, 22B,
22C and 22D shows the recorded waveforms before treatment, ¨ 18 seconds, ¨ 3
minutes and ¨ 5.5 minutes after treatment with E4031, respectively.
Figure 23 shows extra-cellular field potentials recorded for mouse stem-cell
derived
cardiomyocytes obtained using a device of the present invention, where the
recording
electrode is a circle-on-line electrode structure and the reference electrode
is a gold
wire electrode that is introduced into the well after cell seeding. Figure
23A, 23B,
23C, 23D and 23E show the recorded waveforms before treatment, 10 seconds, 50
seconds, 3 minutes and 9 minutes after treatment with 3 1.1M Quinidine,
respectively.
Figure 24 shows parallel impedance monitoring of beating of mouse stem-cell
derived cardiomyocytes obtained using a device of the present invention, where
the
impedance is monitored using an electrode array comprising two electrode
structures
each of which comprises multiple circle-on-line electrode elements. Figure
24A,
24B, 24C, and 240 show the recorded impedance beating signals before
treatment,
1.5 minute, ¨ 3 minute and ¨ 11 minute after treatment with 3 ytM Quinidine,
respectively.
Figure 25 shows extra-cellular field potentials recorded for primary
cardiomyocytes
obtained using a device of the present invention, where the recording
electrode is a
circle-on-line electrode structure and the reference electrode is a gold wire
electrode
that is introduced into the well after cell seeding.
Figure 26 shows a device of the present invention wherein the well (2605) of
the
device comprises a recording electrode (2601) for extra-cellular recording, an
interdigitated electrode array (2602) comprising two electrode structures
(2603 and
2606) each of which comprises multiple electrode elements. The electrodes are
positioned on non-conductive substrate 2604. Extracellular recording of field
potentials can be achieved by amplifying and recording voltage signals between
the
recording electrode 2601 and one electrode structure 2603 (or 2606). The
impedance measurement can be conducted by monitoring impedance between
electrode structures 2603 and 2606.
22
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
Figure 27 shows the design of an electrode array used for combined cell-
impedance
measurement and extra-cellular recording.
Figure 28 shows the impedance beating result obtained for the Cor. At cells
(mouse
ES derived cardiomyocytes) for ¨ 12 seconds with time-solution about 28.8 ms
per
data point. The impedance data is represented with cell index, a dimension
less
parameter.
Figure 29 shows the extracellular field potential signals obtained for the
Cor. At cells
(mouse ES derived cardiomyocytes) using the electrode array in Figure 27 for
three
different recording modes, including: (1) ECR electrode versus impedance
electrode-
structure, (2) ECR electrode versus external, gold, reference electrode and
(3)
impedance electrode-structure versus external, gold, reference electrode.
Figure 30 shows the design of another electrode array used for combined cell-
impedance measurement and extra-cellular recording.
Figure 31 shows the impedance beating result obtained for the Cor. At cells
(mouse
ES derived cardiomyocytes) for ¨ 12 seconds with time-solution about 28.8 ms
per
data point. The impedance data is represented with cell index, a dimension
less
parameter.
Figure 32 shows the extracellular field potential signals obtained for the
Cor. At cells
(mouse ES derived cardiomyocytes) using the electrode array in Figure 20 for
three
different recording modes, including: (1) ECR electrode versus impedance
electrode-
structure, (2) ECR electrode versus external, gold, reference electrode and
(3)
impedance electrode-structure versus external, gold, reference electrode.
Figure 33 shows top view and cross-sectional view of a device of the present
invention.
23
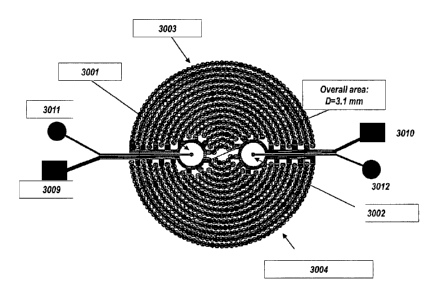
Figure 34 shows a device of the present invention where the well of the device
3405
comprises a recording electrode 3401 and an interdigitated electrode array
3406 on
the substrate 3404, and an external, reference electrode 3402.
DETAILED DESCRIPTION OF THE INVENTION
A. Definitions
For clarity of disclosure, and not by way of limitation, the detailed
description of the
invention is divided into the subsections that follow. Further, unless defined
otherwise, all technical and scientific terms used herein have the same
meaning as is
commonly understood by one of ordinary skill in the art to which this
invention
belongs. If a definition set forth in this section is contrary to or otherwise
inconsistent
with a definition set forth in the patents, applications, published
applications and other
publications, the definition set forth in this section prevails over the
definition that is
in the patents, applications, published applications and other publications.
As used herein, "biocompatible membrane" means a membrane that does not have
deleterious effects on cells, including the viability, attachment, spreading,
motility,
growth, cell division or cell beating.
A "biomolecular coating" or a "biological molecule coating" is a coating on a
surface
that comprises a molecule that is a naturally occurring biological molecule or
biochemical, or a biochemical derived from or based on one or more naturally
occurring biomolecules or biochemicals. For example, a biological molecule
coating
can include an extracellular matrix component (e.g., fibronectin, collagens),
or a
derivative thereof, or can comprise a biochemical such as polylysine or
polyornithine,
which are polymeric molecules based on the naturally occurring biochemicals
lysine
and ornithine. Polymeric molecules based on naturally occurring biochemicals
such
as amino acids can use isomers or enantiomers of the naturally-occuring
biochemicals.
24
CA 2760941 2017-12-15
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
A "cell-free zone" is a region of the device where cells, when added to the
device, are
not physically present. The "cell-free zone" may be a region on the substrate,
which
is blocked from cell access. The "cell-free zone" may be a spatial position
free from
contact with the substrate such as a spatial position above a substrate base
and within
the volume of the well. The "cell-free zone" should permit the passage of
electrical
current such as through a conductive medium. For example, a reference
electrode or
external electrode may be suspended in a cell-free zone, free from contact
with the
substrate, yet remain electrically coupled to an extracellular recording
electrode
disposed on the substrate.
A "cell-free electrode" is an electrode that is free from contact with all
cells present in
the device.
The Willi "free from contact" or "free from direct contact" refers to two
individuals,
whether cells or apparatus components that lack contacting surfaces. A cell
suspended
in medium, in which only the medium contacts an electrode is "free from
contact" or
"free from direct contact" with the electrode.
An "organic compound coating" is a coating on a surface that includes an
organic
compound. For example an organic compound may include a natural ligand or an
agonist or an antagonist for a cell surface receptor.
An "extracellular matrix component" is a molecule that occurs in the
extracellular
matrix of an animal. It can be a component of an extracellular matrix from any
species
and from any tissue type. Nonlimiting examples of extracellular matrix
components
include laminins, collagens fibronectins, other glycoproteins, peptides,
glycosaminoglycans, proteoglycans, etc. Extracellular matrix components can
also
include growth factors.
An "electrode" is a structure having a high electrical conductivity, that is,
an electrical
conductivity much higher than the electrical conductivity of the surrounding
materials, which in the present invention are typically nonconductive. An
"extracellular recording electrode" or "recording electrode" or "ECR
electrode" is
such a structure used to detect electrical signal corresponding to
extracellular field
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
potential of the cell or cell population. For instance, a "recording
electrode" may be
used to monitor the extracellular field potential of a cardiomyocyte during
the
generation of membrane action potentials. A "reference electrode" is the
complementary structure used to complete the electrical circuit during
extracellular
recording. An "impedance electrode" or an "impedance measurement electrode" or
"impedance measurement electrode structure" is a structure, such as an
electrode,
used for impedance monitoring. An "impedance electrode" may also operate as an
extracellular recording electrode and thus may provide both impedance
monitoring
and extracellular recording measurements, albeit at different time points.
As used herein, an "electrode structure" refers to a single electrode,
particularly one
with a complex structure (as, for example, a spiral electrode structure), or a
collection
of at least two electrode elements that are electrically connected together.
All the
electrode elements within an "electrode structure" are electrically connected.
As used herein, "electrode element" refers to a single structural feature of
an electrode
structure, such as, for example, a fingerlike or branched projection of an
interdigitated
electrode structure. An electrode structure may have a plurality of electrode
elements.
As used herein, a "unitary electrode structure" refers to a single electrode
that is
unbranched. That is, a "unitary electrode structure" does not include a
plurality of
electrode elements. For example, an unitary electrode structure may be of a
circle, a
square or other geometry.
As used herein, an "electrode array" or "electrode structure unit" is two or
more
electrode structures that are constructed to have dimensions and spacing such
that
they can, when connected to a signal source, operate as a unit to generate an
electrical
field in the region of spaces around the electrode structures. Preferred
electrode
structure units of the present invention can measure impedance changes due to
cell
attachment to an electrode surface. Non-limiting examples of electrode
structure units
are interdigitated electrode structure units and concentric electrode
structure units.
An "electrode bus" is a portion of an electrode that connects individual
electrode
elements or substructures. An electrode bus provides a common conduction path
from
26
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
individual electrode elements or individual electrode substructures to another
electrical connection. In the devices of the present invention, an electrode
bus can
contact each electrode element of an electrode structure and provide an
electrical
connection path to electrical traces that lead to a connection pad.
"Electrode traces" or "electrically conductive traces" or "electrical traces",
are
electrically conductive paths that extend from electrodes or electrode
elements or
electrode structures toward one end or boundary of a device or apparatus for
connecting the electrodes or electrode elements or electrode structures to an
analyzer
or amplifier, such as an impedance amplifier, a voltage amplifier and the
like. Both
impedance electrodes and extracellular recording electrodes may connect to an
"electrode trace." The end or boundary of a device may correspond to the
connection
pads on the device or apparatus.
A "connection pad" is an area on an apparatus or a device of the present
invention
which is electrically connected to at least one electrode or all electrode
elements
within at least one electrode structure on an apparatus or a device and which
can be
operatively connected to external electrical circuits (e.g., an impedance
measurement
circuit or a signal source or an extracellular voltage signal amplifier). The
electrical
connection between a connection pad and an impedance measurement circuit, an
extracellular recording circuit or a signal source can be direct or indirect,
through any
appropriate electrical conduction means such as leads or wires. Such
electrical
conduction means may also go through electrode or electrical conduction paths
located on other regions of the apparatus or device.
"Interdigitated" means having projections coming one direction that interlace
with
projections coming from a different direction in the manner of the fingers of
folded
hands (with the caveat that interdigitated electrode elements preferably do
not contact
one another).
As used herein, a "high probability of contacting an electrode element" means
that, if
a cell is randomly positioned within the sensor area of a device or apparatus
of the
present invention, the probability of a cell (or particle) contacting an
electrode
element, calculated from the average diameter of a cell used on or in a device
or
27
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
apparatus of the present invention, the sizes of the electrode elements, and
the size of
the gaps between electrode elements, is greater than about 50%, more
preferably
greater than about 60%, yet more preferably greater than about 70%, and even
more
preferably greater than about 80%, greater than about 90%, or greater than
about 95%.
As used herein, "at least two electrodes fabricated on the substrate" means
that the at
least two electrodes are fabricated or made or produced on the substrate. The
at least
two electrodes can be on the same side of the substrate or on the different
side of the
substrate. The substrate may have multiple layers, the at least two electrodes
can be
either on the same or on the different layers of the substrate.
As used herein, "at least two electrodes fabricated to a same plane of the
substrate"
means that, if the nonconducting substrate has multiple layers, the at least
two
electrodes are fabricated to the same layer of the substrate.
As used herein, "an electrode positioned on a different plane" refers to the
positioning
of an electrode, typically an external electrode or reference electrode,
above, below or
along a different surface angle than that which it is compared. An "electrode
positioned on a different plane" may be parallel to that of the first.
As used herein, "the. . . electrodes (or electrode structures) have
substantially the
same surface area" means that the surface areas of the electrodes referred to
are not
substantially different from each other, so that the impedance change due to
cell
attachment or growth on any one of the electrodes (or electrode structures)
referred to
will contribute to the overall detectable change in impedance to a same or
similar
degree as the impedance change due to cell attachment or growth on any other
of the
electrodes (or electrode structures) referred to. In other words, where
electrodes (or
electrode structures) have substantially the same surface area, any one of the
electrodes can contribute to overall change in impedance upon cell attachment
or
growth on the electrode. In most cases, the ratio of surface area between the
largest
electrode and the smallest electrode that have "substantially the same surface
area" is
less than 10. Preferably, the ratio of surface area between the largest
electrode and
the smallest electrode of an electrode array is less than 5, 4, 3, 2, 1.5, 1.2
or 1.1. More
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
preferably, the at least two electrodes of an electrode structure have nearly
identical or
identical surface area.
As used herein, "the device has a surface suitable for cell attachment or
growth"
means that the electrode and/or non-electrode area of the apparatus has
appropriate
physical, chemical or biological properties such that cells of interest can
viably attach
on the surface and new cells can continue to attach, while the cell culture
grows, on
the surface of the apparatus. However, it is not necessary that the device, or
the
surface thereof, contain substances necessary for cell viability or growth.
These
necessary substances, e.g., nutrients or growth factors, can be supplied in a
medium.
Preferably, when a suspension of viable cardiomyocytes, neuron cells, muscle
cells or
other excitable cells or other adherent cells such as epithelial cells or
endothelial cells
is added to the "surface suitable for cell attachment" when at least 50% of
the cells are
adhering to the surface within twelve hours. More preferably, a surface that
is
suitable for cell attachment has surface properties so that at least 70% of
the cells are
adhering to the surface within twelve hours of plating (i.e., adding cells to
the
chamber or well that comprises the said device). Even more preferably, the
surface
properties of a surface that is suitable for cell attachment results in at
least 90% of the
cells adhering to the surface within twelve hours of plating. Most preferably,
the
surface properties of a surface that is suitable for cell attachment results
in at least
90% of the cells adhering to the surface within eight, six, four, two hours of
plating.
As used herein, "detectable change in impedance between or among said
electrodes"
(or "detectable change in impedance between or among the electrode
structures")
means that the impedance between or among the electrodes (or electrode
structures)
would have a significant change that can be detected by an impedance analyzer
or
impedance measurement circuit when cells attach on the electrode surfaces. The
impedance change refers to the difference in impedance values when cells are
attached to the electrode surface and when cells are not attached to the
electrode
surface, or when the number, type, activity, adhesiveness, or morphology of
cells
attached to the electrode-comprising surface of the apparatus changes. In most
cases,
the change in impedance is larger than 0.1% to be detectable. Preferably, the
detectable change in impedance is larger than 1%, 2%, 5%, or 8%. More
preferably,
the detectable change in impedance is larger than 10%. Impedance between or
among
29
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
electrodes is typically a function of the frequency of the applied electric
field for
measurement. "Detectable change in impedance between or among the electrodes"
does not require the impedance change at all frequencies being detectable.
"Detectable change in impedance between or among said electrodes" only
requires a
detectable change in impedance at any single frequency (or multiple
frequencies). In
addition, impedance has two components, resistance and reactance (reactance
can be
divided into two categories, capacitive reactance and inductive reactance).
"Detectable change in impedance between or among said electrodes" requires
only
that either one of resistance and reactance has a detectable change at any
single
frequency or multiple frequencies. In the present application, impedance is
the
electrical or electronic impedance. The method for the measurement of such
impedance is achieved by, (1) applying a voltage between or among the
electrodes at
a given frequency (or multiple frequencies, or having specific voltage
waveform) and
monitoring the electrical current through said electrodes at the frequency (or
multiple
frequencies, or having specific waveform), dividing the voltage amplitude
value by
the current amplitude value to derive the impedance value; (2) applying an
electric
current of a single frequency component (or multiple frequencies or having
specific
current wave form) through said electrodes and monitoring the voltage resulted
between or among said electrodes at the frequency (or multiple frequencies, or
having
specific waveform), dividing the voltage amplitude value by the current
amplitude
value to derive the impedance value; (3) other methods that can measure or
determine
electric impedance. Note that in the description above of "dividing the
voltage
amplitude value by the current amplitude value to derive the impedance value",
the
"division" is done for the values of current amplitude and voltage amplitude
at same
frequencies. Measurement of such electric impedance is an electronic or
electrical
process that does not involve the use of any reagents.
As used herein, "multiple pairs of electrodes or electrode structures
spatially arranged
according to wells of a multi-well microplate" means that the multiple pairs
of
electrodes or electrode structures of a device or apparatus are spatially
arranged to
match the spatial configuration of wells of a multi-well microplate so that,
when
desirable, the device can be inserted into, joined with, or attached to a
multiwell plate
(for example, a bottomless multiwell plate) such that multiple wells of the
multi-well
microplate will comprise electrodes or electrode structures.
CA 02760941 2011-11-03
WO 2010/129725 PCT/US2010/033801
As used herein, "arranged in a row-column configuration" means that, in terms
of
electric connection, the position of an electrode, an electrode array or a
switching
circuit is identified by both a row position number and a column position
number.
As used herein, "each well contains substantially same number. . . of cells"
means
that the lowest number of cells in a well is at least 50% of the highest
number of cells
in a well. Preferably, the lowest number of cells in a well is at least 60%,
70%, 80%,
90%, 95% or 99% of the highest number of cells in a well. More preferably,
each
well contains an identical number of cells.
As used herein, "each well contains. . .same type of cells" means that, for
the
intended purpose, each well contains same type of cells; it is not necessary
that each
well contains exactly identical type of cells. For example, if the intended
purpose is
that each well contains mammalian cells, it is permissible if each well
contains same
type of mammalian cells, e.g., human cells, or different mammalian cells,
e.g., human
cells as well as other non-human mammalian cells such as mice, goat or monkey
cells,
etc.
As used herein, "each well contains. . . serially different concentration of a
test
compound" means that each well contains a test compound with a serially
diluted
concentrations, e.g., an one-tenth serially diluted concentrations of 1 M, 0.1
M, 0.01
M, etc.
As used herein, "dose-response curve" means the dependent relationship of
response
of cells on the dose concentration of a test compound. The response of cells
can be
measured by many different parameters. For example, a test compound is
suspected
to have cytotoxicity and cause cell death. Then the response of cells can be
measured
by percentage of non-viable (or viable) cells after the cells are treated by
the test
compound. Plotting this percentage of non-viable (or viable) cells as a
function of the
dose concentration of the test compound constructs a dose response curve. In
the
present application, the percentage of non-viable (or viable) cells can be
expressed in
terms of measured impedance values, or in terms of cell index derived from
impedance measurement, or in terms of cell change indexes. For example, for a
give
31
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
cell type and under specific cellular physiological condition (e.g., a
particular cell
culture medium), cell index can be shown to have a linear correlation or
positive
correlation with the number of viable cells in a well from which cell index
was
derived from the impedance measurement. Thus, in the present application, one
can
plot cell index as a function of the dose concentration of the test compound
to
construct a "dose-response curve". Note that, generally, cell index not only
correlate
with the number of viable cells in the wells but also relate to the cell
morphology and
cell attachment. Thus plotting cell index versus dose concentration provides
information not only about number of cells but also about their physiological
status
(e.g. cell morphology and cell adhesion). Furthermore, an important advantage
offered by the system and devices of the present invention is that in a single
experiment, one can obtain "dose-response curves" at multiple time points
since the
system allows for the continuous monitoring of cells and provides impedance
measurement at many time points over a time range as short as a few minutes to
as
long as days or weeks.
As used herein, "microelectrode strip or electrode strip" means that a non-
conducting
substrate strip on which electrodes or electrode structure units are
fabricated or
incorporated. The non-limiting examples of the non-conducting substrate strips
include polymer membrane, glass, plastic sheets, ceramics, insulator-on-
semiconductor, fiber glass (like those for manufacturing printed-circuits-
board).
Electrode structure units having different geometries can be fabricated or
made on the
substrate strip by any suitable microfabrication, micromachining, or other
methods.
Non-limiting examples of electrode geometries include interdigitated
electrodes,
circle-on-line electrodes, diamond-on-line electrodes, castellated electrodes,
spiral
electrodes or sinusoidal electrodes. Characteristic dimensions of these
electrode
geometries may vary from as small as less than 5 micron, or less than 10
micron, to as
large as over 200 micron, over 500 micron, over 1 mm. The characteristic
dimensions
of the electrode geometries refer to the smallest width of the electrode
elements, or
smallest gaps between the adjacent electrode elements, or size of a repeating
feature
on the electrode geometries. The microelectrode strip can be of any geometry
for the
present invention. One exemplary geometry for the microelectrode strips is
rectangular shape ¨ having the width of the strip between less than 50 micron
to over
10 mm, and having the length of the strip between less than 60 micron to over
15 mm.
32
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
An exemplary geometry of the microelectrode strips may have a geometry having
a
width of 200 micron and a length of 20 mm. A single microelectrode strip may
have
two electrodes serving as a measurement unit, or multiple such two-electrodes
serving
as multiple measurement units, or a single electrode structure unit as a
measurement
unit, or multiple electrode structure units serving as multiple electrode
structure units.
In one exemplary embodiment, when multiple electrode structure units are
fabricated
on a single microelectrode strip, these electrode structure units are
positioned along
the length direction of the strip. The electrode structure units may be of
squared-
shape, or rectangular-shape, or circle shapes. Each of electrode structure
units may
occupy size from less than 50 micron by 50 micron, to larger than 2 mm x 2mm.
As used herein, "sample" refers to anything which may contain a moiety to be
isolated, manipulated, measured, quantified, detected or analyzed using
apparatuses,
microplates or methods in the present application. The sample may be a
biological
sample, such as a biological fluid or a biological tissue. Examples of
biological fluids
include suspension of cells in a medium such as cell culture medium, urine,
blood,
plasma, serum, saliva, semen, stool, sputum, cerebral spinal fluid, tears,
mucus,
amniotic fluid or the like. Biological tissues are aggregates of cells,
usually of a
particular kind together with their intercellular substance that form one of
the
structural materials of a human, animal, plant, bacterial, fungal or viral
structure,
including connective, epithelium, muscle and nerve tissues. Examples of
biological
tissues also include organs, tumors, lymph nodes, arteries and individual
cell(s). The
biological samples may further include cell suspensions, solutions containing
biological molecules (e.g. proteins, enzymes, nucleic acids, carbohydrates,
chemical
molecules binding to biological molecules) .
As used herein, a "liquid (fluid) sample" refers to a sample that naturally
exists as a
liquid or fluid, e.g., a biological fluid. A "liquid sample" also refers to a
sample that
naturally exists in a non-liquid status, e.g., solid or gas, but is prepared
as a liquid,
fluid, solution or suspension containing the solid or gas sample material. For
example, a liquid sample can encompass a liquid, fluid, solution or suspension
containing a biological tissue.
33
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
A "compound" or "test compound" is any compound whose activity or direct or
indirect effect or effects on cells is investigated in any assay. A test
compound can be
any compound, including, but not limited to, a small molecule, a large
molecule, a
molecular complex, an organic molecule, an inorganic molecule, a biomolecule
or
biological molecule such as but not limited to a lipid, a steroid, a
carbohydrate, a fatty
acid, an amino acid, a peptide, a protein, a nucleic acid, or any combination
thereof. A
test compound can be a synthetic compound, a naturally occurring compound, a
derivative of a naturally-occurring compound, etc. The structure of a test
compound
can be known or unknown. In one application of the present invention, a
compound
is capable of, or is suspected of, effecting cell adhesion or cell spreading.
In another
application of present invention, a compound is capable of, or is suspected
of,
stimulating or inhibiting cell adhesion or cell spreading. In still another
application, a
compound is capable of, or is suspected of, interacting with cells (for
example,
binding to cell surface receptor, or inhibiting certain intracellular signal
transduction
pathway, or activating cells).
A "known compound" is a compound for which at least one activity is known. In
the
present invention, a known compound preferably is a compound for which one or
more direct or indirect effects on cells is known. Preferably, the structure
of a known
compound is known, but this need not be the case. Preferably, the mechanism of
action of a known compound on cells is known, for example, the effect or
effects of a
known compound on cells can be, as nonlimiting examples, effects on cell
beating,
cell viability, cell adhesion, apoptosis, cell differentiation, cell
proliferation, cell
morphology, cell cycle, IgE-mediated cell activation or stimulation, receptor-
ligand
binding, cell number, cell quality, cell cycling, cell adhesion, cell
spreading, etc.
An "impedance value" is the impedance measured for electrodes in a well with
or
without cells present. Impedance is generally a function of the frequency,
i.e.,
impedance values depend on frequencies at which the measurement was conducted.
For the present application, impedance value refers to impedance measured at
either
single frequency or multiple frequencies. Furthermore, impedance has two
components, one resistance component and one reactance component. Impedance
value in the present application refers to resistance component, or reactance
component, or both resistance and reactance component. Thus, when "impedance
34
CA 02760941 2016-10-03
value" was measured or monitored, we are referring to that, resistance, or
reactance,
or both resistance and reactance were measured or monitored. In many
embodiments
of the methods of the present application, impedance values also refer to
parameter
values that are derived from raw, measured impedance data. For example, cell
index,
or normalized cell index, or delta cell index could be used to represent
impedance
values.
A "Cell Index" or "CI" is a parameter that can be derived from measured
impedance
values and that can be used to reflect the change in impedance values. There
are a
number of methods to derive or calculate Cell Index. The details of the method
for
calculating Cell Index, Normalized Cell Index, Delta Cell Index and cell
change index
can be found in United States patent application No. 10/705,447, filed on
November
10, 2003; United States patent application No. 10/705,615, filed on November
10,
2003; United States patent application No. 10/987,73, filed on Nov 12, 2004;
United
States patent application No. 11/055,639, filed on Feb 9 2005; United States
patent
application No. 11/198,831, filed on Aug 4, 2005; United States patent
application
No. 11/197,994, filed on Aug 4, 2005; United States patent application No.
11/235,938, filed on Sept 27, 2005.
A "Normalized Cell Index" at a given time point is calculated by dividing the
Cell
Index at the time point by the Cell Index at a reference time point. Thus, the
Normalized Cell Index is 1 at the reference time point. Generally, for an
assay
involving treatment of the cells with compounds or with other bio-manipulation
of the
cells, the reference time point is the last time point for impedance
measurement before
the treatment of the cells.
A "delta cell index" at a given time point is calculated by subtracting the
cell index at
a standard time point from the cell index at the given time point. Thus, the
delta cell
index is the absolute change in the cell index from an initial time (the
standard time
point) to the measurement time.
A "Cell Change Index" or "CCI" is a parameter derived from Cell Index and
"CCI" at
a time point is equal to the 1'" order derive of the Cell Index with respect
to time,
divided by the Cell Index at the time point. In other words, CCI is calculated
as
CA 02760941 2011-11-03
WO 2010/129725 PCT/US2010/033801
CCI(t)= dCI(t)
CI(t) = dt
The term "extracellular recording" refers to measuring, monitoring and/or
recording
of electric potential difference between two electrodes typically caused by
ionic
movement or ionic current through the media or solution due to charge
fluctuations
across ion channels in a cell or in a group of cells. The cells are in the
media or the
solution. In contrast to intracellular recording where the recording
electrodes are
placed inside a cell through the cell membrane, the extracellular recording
electrodes
.. are located outside of the cells.
B. Method to assess and quantify cardiomyocyte viability in vitro using cell
sensor
impedance technology
The methods and devices of the present invention may assess or quantify the
viability
of cardiomyocyte or cardiomyocyte precursor cells in addition to performing
extracellular recording assays. Such assessment may be performed prior to
performing an extracellular recording assay to assess the initial viability or
characteristics of cells or may be performed periodically throughout the
extracellular
recording process to continually monitor the viability or characteristics of
cells, or
may be performed in parallel with extracellular recording.
Isolated primary cardiomyocytes, ES-derived cardiomyocytes and adult stem cell-
derived cardiomyocytes can be maintained in culture. These cells provide an
excellent
model system to study the effect of drugs and other factors on cardiomyocyte
viability.
Exemplary steps involved in using an impedance-monitoring system for
measurement
of cardiomyocte viability include:
(1) Provide a single-well or multi-well device that comprise
microelectrode arrays in well(s) of the device, which can be
used for monitoring cell-substrate impedance.
36
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
(2) Optionally coat wells of the device with either fibronectin or
other matrix proteins.
(3) Seed either embryonic stem cells (ES cells) of mammalian
origin, mammalian adult stem cell-derived cardiomyocytes
or primary cardiomyocytes isolated directly from
mammalian heart tissue at specific seeding densities to the
wells of the device.
(4) Allow the cells to attach and spread.
(5) Monitor cardiomyocyte viability over time using the
impedance-monitoring system to monitor electrode
impedance at pre-specified intervals of time (non-limiting
examples of the time include 5, 15, 30 minutes, 1 hr and 2
hrs) for specified length of time such as 12, 24, 48, 72 hours
or longer.
One example of the impedance measurement system is ACEA Bioscicnces' RT-CES
system, where the device is ACEA E-PLA IE in the form of microtiter plates
whose
wells comprise microelectrode structures. Thus in a related embodiment,
exemplary
steps involved in using the RT-CES system for measurement of cardiomyocte
viability include:
(1) Optionally coat E-PLATES with either fibronectin or other matrix
proteins.
(2) Seed either embryonic stem cells (ES cells) of mammalian origin,
mammalian adult stem cell-derived cardiomyocytes or primary
cardiomyocytes isolated directly from mammalian heart tissue at
specific seeding densities to the wells of the device.
(3) Allow the cells to attach and spread.
(4) Monitor cardiomyocyte viability over time using the impedance-
monitoring system to monitor electrode impedance at pre-specified
intervals of time (non-limiting examples of the time include 5, 15, 30
minutes, lhr and 2 hrs) for specified length of time such as 12, 24, 35,
48, 72 hours or longer.
37
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
As an example, we describe here the use of the ACEA RT-CES system to measure
and monitor the attachment, growth and viability of mouse ES-derived
cardiomyocytes which were seeded at different seeding densities. Mouse ES
cells
were seeded at a density ranging from 3000 cells to 50,000 cells per well in
ACEA E-
PLATES that had been precoated with fibronectin. The attachment, growth and
viability of the cells were monitored on the RT-CES system measuring impedance
signal in the form of cell index every 30 minutes for 48 hours. At about 48
hrs after
cell seeding, the growth of the cells had ceased and the appearance of beating
cardiomyocytes were evident as judged by looking at the cells inside the E-
PLATE
under the microscope. As such, these cells would be suitable for use, for
example, in
extracellular recording experiments to determine the effect of external
stimuli, such as
changes in cardiomoycte beating after administration of a test compound, or in
the
experiments to monitor cardiomyocyte beating through impedance measurement at
milli-second time resolution. Figure 1 shows the cell index curves measured on
RT-
CES system for 4 different seeding densities (3750, 7500, 15,000 and 30,000
cells per
well) of mouse ES-derived cardiomyocytes. For such long ternt measurement,
cell
electrode impedance and corresponding cell indices were measured at about 15
minute intervals. Based on the cell index growth and viability curves, it is
evident
that the extent of the impedance signal correlates well with the seeding
density of
viable ES-derived cardiomyocytes.
C. Method to assess and quantify modulation of cardiomyocyte viability in
vitro
using cell sensor impedance technology
Certain cardiotoxic drugs can directly affect the viability of cardiomyocytes.
Accordingly, continuing to monitor the viability of cardiomyoytes over time
may be
used to supplement data obtained from extracellular recording of cells.
Exemplary
steps involved in using an impedance-measurement system for measurement of
loss
of viability of cardiomyocyte include:
(1) Optionally coat E-PLATES with either fibronectin or other matrix
proteins.
(2) Seed either embryonic stem cells (ES cells) of mammalian origin,
mammalian adult stem cell-derived cardiomyocytes or primary
38
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
cardiomyocytes isolated directly from mammalian heart tissue at
specific seeding densities to the wells of the device.
(3) Allow the cells to attach and spread.
(4) Monitor cardiomyocyte viability over time using the impedance-
monitoring system to monitor electrode impedance at pre-specified
intervals of time for specified length of time such as 12, 24, 37, 48, 72
hours or longer
(5) At certain time after cell seeding, treat the cell with cytotoxic agent
at one or more concentration; using the vehicle that the agent is
dissolved in as a control.
(6) Continue monitoring the cardiomyocytes at pre-specified intervals
of time for specified length of time such as additional 12, 24, 48, 72
hours or longer
(7) Quantify the extent of cardiotoxicity by normalizing the cell index
values immediately prior to agent addition and determine the
normalized cell index at a given time point after agent addition;
alternatively the rate of cytotoxicity can also be quantified for a given
time period after compound addition for a given agent concentration or
a group of concentrations. The extent of cytotoxicity can be expressed
as IC-50 value which quantifies the activity of the agent with respect to
the cardiomyocytes.
As an example, we describe here the use of the ACEA RT-CES system to measure
and monitor the attachment and growth of mouse ES cells derived cardiomyocytes
and subsequently treated with a cytotoxic agent (Figure 2). Mouse ES-derived
cardiomyocytes were seeded at a density of 25,000 cells per well in ACEA E-
Plates
that had been precoated with fibronectin. The attachment and growth of the
cells were
monitored on RT-CES system for 72 hours and then treated with increasing doses
of
the compound of sodium dichromate dehydrate which is known to induce
cytotoxicity. According to Figure 2A of the plot of normalized cell index for
cells in
different wells treated with different concentrations of sodium dichromate
dehydrate
(SDD), SDD causes a concentration dependent decrease in viability of ES-
derived
cardiomyocytes. To quantify the extent of sodium dichromate dehydrate activity
against the cardiomyocytes, the normalized cell indices at 24 hrs after
compound
39
CA 02760941 2016-10-03
treatment were plotted against the log of the corresponding sodium dichromate
dehydrate concentrations. From the sigmoidal curve, shown in Figure 2B, half
maximal activity or IC-50 value of 1.48 jiM was derived for the compound.
D. Method to assess and quantify modulation of cardiomyocyte morphology in
vitro
using cell sensor impedance technology
Certain cardiotoxic drugs can illicit their effect by affecting the
morphological aspects
of cardiomyocyte morphology. For example, it is well known that compounds such
as
.. b-2 adrenergic receptor agonists can induce morphological changes resulting
in an
elongated cardiomyocyte morphology, otherwise known as hypertrophy.
Morphological changes can occur immediately in the order of minutes as with
certain
GPCR agonists or can be of longer duration detectable over several days. The
time
resolution of the RT-CES system can be used to distinguish between different
kinds
of morphological effects. The steps involved in using an impedance-monitoring
system on the RT-CES system for measurement of morphological modulation of
cardiomyocytes include:
(1) Optionally coat E-PLATES with either fibronectin or other matrix
proteins.
(2) Seed either embryonic stem cells (ES cells) of mammalian origin,
mammalian adult stem cell-derived cardiomyocytes or primary
cardiomyocytes isolated directly from mammalian heart tissue at
specific seeding densities to the wells of the device.
(3) Allow the cells to attach and spread.
(4) Monitor cardiomyocyte viability over time using the impedance-
monitoring system at prespecified intervals of time for 12, 24, 48, 72
hours or longer.
(5) At certain time after cell seeding, treat the cell with agents that may
cause morphology changes at one or more concentration; using the
vehicle that the agent is dissolved in as a control.
(6) Continue monitoring the cardiomyocytes at 1 minute intervals for
at least 1-2 hours to capture any immediate morphological changes and
continue to monitor at 30 minutes intervals of time for additional 12,
24, 48, 72 hours or longer to detect long term morphological changes
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
(7) Quantify the extent of morphological change by normalizing the
cell index values immediately prior to agent addition and determine the
normalized cell index at a given time point after agent addition; The
extent of morphological change can be expressed as EC-50 value
which quantifies the activity of the agent with respect to the
cardiomyocyte shape changes.
As an example, we describe here the use of the ACEA RT-CES system to measure
and monitor the attachment and growth of mouse ES cells derived cardiomyocytes
and subsequently treated with isoproteranol, a 132 adrenergic receptor agonist
known
to induce hypertrophy (Figure 3A). Mouse ES-derived cardiomyocytes were seeded
at a density of 25,000 cells per well in ACEA E-PLATES that had been precoated
with fibronectin. The attachment and growth of the cells were monitored on RT-
CES
system for 72 hours and then treated with increasing doses of the compound
isoproteranol. According to Figure 3A, isoproteranol causes a concentration
dependent change in cell index readings. The timing of the cell index change
is
consistent with a change in the morphology of the cells which we have shown
previously for other GPCR agonists in primary cells (Yu et al (2006) Real-time
monitoring of morphological changes in living cells by electronic cell sensor
arrays:
an approach to study G protein-coupled receptors; Analytical Chemistry, Vol
78,
pages 35-43). To quantify the extent of isoproteranol-induced morphological
changes
in mouse ES-derived cardiomyocytes, the nolinalized cell indices were plotted
against
the log of the corresponding isoproteranol concentrations (Figure 3B). From
the
sigmoidal curve generated a half maximal activity or IC-50 value of 3.1 nM was
derived for the compound.
E. Method to assess and quantify cardiomyocyte beating in vitro using cell
sensor
impedance technology
Isolated primary cardiomyocytes as well as ES-derived cardiomyocytes retain
the
ability to beat in culture. These cells provide an excellent model system to
study
41
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
cardiomyocyte function in vitro, especially with regards to cardiotoxicity. A
number
of cardiotoxic drugs are known to affect certain heart channels, such as the
ERG
channels, that are involved in excitation-contraction coupling of
cardiomyocytes.
Cardiomyocytes have an innate ability to undergo mechanotransduction, that is
that
the spontaneous force generation of the beating cardiomyocyte is translated to
intracellular biochemical signals. Membrane receptors such as integrins, ion
channels
and other proteins have been shown to play a crucial role in cardiac
mechanotransduction and lead to a continuous and rhythmic dynamics of the
cardiac
actin cytoskeleton and morphology. Because the impedance-based technology and
-- system can sensitively and precisely detect transient changes in morphology
and
adhesive capacity of the cells, it can be used to monitor cardiomyocyte
beating in
vitro.
The steps involved in using an impedance-measurement system for measurement of
-- cardiomyocte beating include:
(1) Provide a single-well or multi-well device that comprise
microelectrode arrays in well(s) of the device, which can be used for
monitoring cell-substrate impedance.
(2) Optionally coat wells of the device with either fibronectin or other
matrix proteins.
(3) Seed either embryonic stem cells (ES cells) of mammalian origin or
primary cardiomyocytes at specific seeding densities to the wells of the
device.
(4) Allow the cells to attach and spread.
(5) After a specified period of time unique to ES-derived
cardiomyocytes or primary cardiomyocytes, monitor cardiomyocyte
beating using the impedance-monitoring system to monitor electrode
impedance by using milli-second kinetic readout to resolve the
individual beat cycles of the cells.
The milli-second kinetic readout requires that the impedance measurement
system can
provide impedance measurement data at milli-second time resolution. In other
words,
the time difference between two consecutive impedance measurement for a well
shall
be in the range of milli-seconds (e.g., less than 500 milli-second, less than
300 milli-
42
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
second, less than 100 milli-second, less than 40 milli-second, less than 30
milli-
second, less than 20 milli-second, less than 10 milliseond, or less than 1
millisecond
or faster). The milli-second kinetic readout is required to resolve the
individual beat
cycles of the cells. Thus, the time resolution for the impedance measurement
should
allow the system to perform measurement at least two time points for each beat
cycle,
or at more than two points for each beat cycle. These milli-second kinetic
readouts
may be performed within extracellular recording experiments by switching
between
an extracellular recording mode and impedance mode via a switching means. The
fast
kinetic readouts associated with such an impedance based system permits the
user to
obtain multiple impedance measurements to obtain the impedance data for one or
more beating cycle of the cardiomyocytes between extracellular recording
measurements.
One example of the impedance measurement systems is an improved fast-impedance-
measurement system from ACEA Biosciences, where the device is the E-PLATE in
the form of mierotiter plates whose wells comprise microelectrode structures.
An
important aspect of the present invention is that the impedance measurement
circuitry
together electronic switching circuitry and the associated is capable of
measuring
electronic impedances of the one or more wells at milli-second time
resolution, that is
to say, the time difference between two adjacent impedance measurements for
each
well is in the range of milli-seconds. This requirement is important,
especially when
the system comprises a device having multiple wells such as 4 wells, 8 wells,
16 wells
or 96 wells. For example, if the system comprises a device having 96 wells,
the
system hardware and software should be capable of measuring the impedances of
all
the 96 wells with milli-second time resolution between two adjacent impedance
measurement points for each and every well. Preferably, the time difference
between
two adjacent measurement points for each and every given well is less than 500
milliseconds. More preferably, the time difference between two adjacent
measurement points for each and every given well is less than 300
milliseconds.
More preferably, the time difference between two adjacent measurement points
for
each and every given well is less than 200 milliseconds. Still more
preferably, the
time difference between two adjacent measurement points for each and every
given
well is less than 100 milliseconds. Still more preferably, the time difference
between
two adjacent measurement points for each and every given well is less than 40
43
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
milliseconds. Still more preferably, the time difference between two adjacent
measurement points for each and every given well is less than 20 milliseconds.
Still
more preferably, the time difference between two adjacent measurement points
for
each and every given well is less than 10 milliseconds.
A number of improvements in the impedance measurement circuitry, electronic
switching circuitry, communication between impedance measurement circuitry and
software can be used to achieve such milli-second time resolution. One aspect
of
improvements includes the use of fast processing electronic chips for analogue-
to-
digital conversion, for parallel digital signal processing and data
calculation with
field-programmable gate array (FPGA) and for fast communication between the
impedance measurement circuitry and software. Another aspect of improvements
includes the use of multiple analogue-to-digital (AD) conversion channels so
that
analog electronic signals from multiple channels can be converted to digital
signals
simultaneously. Such parallel AD conversion is important, particular for the
system
having multiple wells, each of which's measurement time resolution is required
to be
in the milli-second resolution. Another very important aspect of improvements
is to
replace typical working mode of "measurement of one-well's impedance at a
time"
with a mode of "measurement of multiple-wells' impedance at a time". In "one-
well
at a time" mode, when the software issue a command for measuring one well's
impedance, the measurement circuitry would perform the measurement for one
well
including signal generation to the well, converting the voltage signal and the
electric
current signal for the well to digital signal, digitally processing the
signals to do
impedance calculation and sending the well's impedance data to the computer
over
the communication line between the impedance measurement circuitry and the
computer. The system will not perform any measurement for another well until
the
completion of the measurement of this well and until receiving another command
for
the measurement of another well. In "multiple-wells at a time" mode, the
software
would issue a command for measuring multiple wells' impedance. The measurement
circuitry would simultaneously or nearly simultaneously pert-aim signal
conversion,
signal processing and impedance calculation for multiple wells. The multiple
impedance data for the multiple wells would be sent over the communication
lines to
the computer sequentially with one well's data at time or simultaneously with
more
than one well's data being sent at a time. In this "measurement of multiple-
wells'
44
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
impedance at a time" mode, the system may be performing multiple tasks
simultaneously, for example, while one well's impedance data is being measured
and
calculated, another well's impedance data may be communicated and sent over
the
communication lines to the computer.
The steps involved in using the system for measurement of cardiomyocte
function
may include:
(1) Optionally coat E-PLATES with either fibronectin or other matrix
proteins.
(2) Seed either ES cells of mammalian origin or primary
cardiomyocytes at specific seeding densities.
(3) Allow the cells to attach and spread.
(4) After a specified period of time unique to ES-derived
cardiomyocytes or primary cardiomyocytes, monitor cardiomyocyte
beating using the RT-CES system by using milli-second kinetic
readout to resolve the individual beat cycles of the cells.
As an example, we describe here the use of the ACEA RT-CES system to measure
and monitor the beating of cardiomocytes using fast kinetic software. Mouse ES
cells
were seeded at a density of between 3,000 to 50,000 cells per well in ACEA E-
PLATES that had been precoated with fibronectin. The attachment and growth of
the
cells were monitored on RT-CES system. Figure 4 shows the cell index curves
measured on RT-CES system for 4 individual wells, with cell culture medium as
background starting from cell seeding to about 86 hrs after cell seeding. For
such
long term measurement, cell electrode impedance and corresponding cell indices
were
measured at about 15 minute intervals. As evidenced on these plots, the cell
index
curves were rather smooth upto about 44-48 hrs, after which there were
"noises" or
"small-spikes" on the cell index curves. Such spikes were most evident after
about 60
hrs. Such spikes in the impedance or cell index readout are associated with
the
beating of the cells. During the synchronized beating of the cells, the cell
morphology
and cell adhesion/attachment to the electrodes change regularly in synchrony
with the
cell beating. Such regular or periodic changes in cell morphology and cell
adhesion/attachment are then reflected in the changes in cell-electrode or
cell-
substrate impedances.
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
In order to monitor beating of cardiomyocytes, a specially designed software
and
measurement circuit hardware that are capable of millisecond impedance data
acquisition (e.g., typically with time resolution between consecutive
impedance
measurement for a same well being less than 40 milli-seconds) was used to
monitor
the quick rhythmic beating of the cardiomyocytes. For such measurement, the
background electrode impedance is measured with the cells inside the wells
(note, this
is in contrast with Figures 1 and 4, where the background measurement is
performed
using cell culture media). The software, together with specially designed
hardware
circuits was used to measure cardiomyocyte beating at distinct stages
throughout its
attachment and growth phases (Figures 5). For a baseline reference, the
impedance
measurement was done on the ES cells at 24 hours where the cells had not fully
spread and formed a tight monolayer and even though the cells appear to beat
when
visualized under a microscope, they do so asynchronously and as a result no
net
beating signal is detected (Figure 5A). For plot, impedance readout has been
converted into dimensionless cell indices. Figure 5B and 5C shows that ES
cells that
had fully spread and formed tight junctions with neighboring cells at 48 hours
and 72
hours respectively, show regular impedance-spikes which correlate with the
beating
frequency of cardiomyocytes as judged by microscopic observation.
To use the measured cell index curves, it is important to farther derive
various
physiologically relevant parameters. Several important parameters may include,
the
beating rate of the cardiomyocytes (i.e., how many times the cells beat within
a unit of
time for example, a minute), the beating amplitude (i.e. the magnitude of the
beating
of cells in terms of impedance change) the average amplitude intensity in a
unit time
as well as the average length of time between the beats, time of rise for a
beat, time of
decay for a beat. Because of the unique and complex nature of the impedance
readout
signals (smaller signal amplitude, sampling time resolution may be limited by
the
hardware and the software used), appropriate methods or techniques are
required for
analyzing cell index curves to derive the above mentioned parameters.
For deriving the beating rates of cardiomyocytes, one method may be by
counting
how many peaks there are within a given time frame (for example, one minute).
For
this approach to work, the sampling time resolution has to be sufficiently
high so that
46
CA 02760941 2016-10-03
the each beat of the cells does show a peak on the recorded cell index curves.
In
addition, determining a peak "automatically" also require some algorithm. For
example, each peak would have to have one "rise" in cell index and also one
"decay"
in cell decay. Each "rise"-and-"decay" pair forms a single peak. The algorithm
needs
to determine such "rise" and "decay" portions of the curves and then counts a
peak.
Another method to derive or count the beating rates of cradiomyocytes is to
perform a
detailed signal analysis to derive the frequency components of the cell index
curves
and to derive the magnitude of each frequency components. One method of signal
analysis is Fourier transform of the cell index curve (of the time domain).
Like above
method, the sampling time-resolution needs to be sufficiently high so that
each beat of
the cells has at least three time points being measured. After performing
Fourier
transform, we would look for the frequency components having the largest
magnitude
and such frequency would correspond to, or be very close to, the beating
frequency.
In addition, for such analysis, giving a fixed sampling time resolution, the
more time
points sampled for analysis, the more accurate it is for the analyzed beating
frequency. Figure 6A and Figure 6B shows a pair of cell index curves and the
corresponding beat rate based the above described Fourier transform. In Figure
6A,
the cell index curves last from time zero to time 42 seconds. In Figure 6B,
the
beating frequency for the traces in Figure 6A is analyzed using the method
described
here, i.e., Fourier transform followed by picking up the highest-magnitude
frequency
component. For each derived frequency data at one time moment in Figure 6B,
cell
index data from multiple time points (starting from previous 98 time points
plus the
time moment of interest) is used for analysis. Thus, the time axis in Figure
6B starts
from about 20 seconds to 42 seconds.
Another method deriving the beating rates of cardiomyocytes is to first
determine the
time length (AT in seconds) between two consecutive peaks and then calculate
the
beating rate according to the formula of "beats per minute =60/ AT". Thus for
each
two-consecutive peaks, one can calculate one beating rate. Furthermore, one
can plot
this beating rate as a function of the time (of the first of the two
consecutive peaks) to
obtain the time dependency of the beating rates. Figure 7A shows an example of
the
time-dependent beat rates derived using this method, for mouse ES-derived
cardiomycoytes treated with compound sotalol at a concentration of 4.4 M.
47
CA 02760941 2016-10-03
Corresponding cell index data is shown in Figures 7C and 7D, where the time
resolution between two adjacent points is 40 milli-seconds. In other words, a
second
in Figures 7C and 7D is equivalent to 40-milli-second.
For deriving the amplitude of the beating of the cardiomyocytes, there may
also be
different methods. One method is to analyze each peak and finding the peak
maximum and the peak minimum. The amplitude is calculated by subtracting peak
maximum by the peak minimum. Then, one can plot the peak amplitude as a
function
of the time of the peak to obtain the time dependency of the peak amplitude.
Figure
7B shows an example of the time-dependent peak amplitudes derived using this
method, for mouse ES-derived cardiomycoytes treated with compound sotalol at a
concentration of 4.4 M. Another approach may also be to use Fourier transform
described above. Then based on derived Fourier coefficients, one can re-
simulate
time domain cell index curves and look for the peak magnitude from the
simulated
curves.
For deriving the averaged length of time between the beats, there may also be
different methods. For each identified peak, one can first determine a
starting point
of the peak. Then the time difference between two consecutive peaks at the two
starting points of the peak can be used for the length of time between the
beats.
With the method of determining each peak, one can also calculate the time-of-
rise of
the peak and the time-decay-of the peak.
F. Method to assess the effect of known pharmacological agents, proteins,
peptides,
antibodies which modulate cardiac beating using the impedance-measurement
system
The method described in section E above offers a convenient label-free, real-
time and
quantitative method for screening of modulators of cardiac function using a
fast
impedance-measurement system capable of milli-second measurement time
resolution. The steps involved in screening for modulators of cardiomyocyte
beating
using an impedance-measurement system in vitro include:
48
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
(1) Provide a single-well or multi-well device that comprise
microelectrode arrays in well(s) of the device, which can be used
for monitoring cell-substrate impedance.
(2) Optionally coat wells of the device with either fibronectin or other
matrix proteins.
(3) Seed either embryonic stem cells (ES cells) of mammalian origin,
mammalian adult stem cell-derived cardiomyocytes or primary
cardiomyocytes at specific seeding densities to the wells of the
device.
(4) Allow the cells to attach and spread.
(5) After a specified period of time unique to ES-derived
cardiomyocytes or primary cardiomyocytes, monitor
cardiomyocyte beating using the impedance-monitoring system to
monitor electrode impedance by using milli-second kinetic readout
to resolve the individual beat cycles of the cells. This step should
be done immediately prior to addition of pharmacological agent, in
order to obtain a baseline of the cardiomyocyte beating frequency
using fast measurement software and hardware to ensure milli-
second kinectic readout signals.
(6) Addition of the pharmacological agents at one or more doses and
continue monitoring the cardiomyocyte beating frequency.
Similar to Section E, the milli-second kinetic readout requires that the
impedance
measurement system can provide impedance measurement data at milli-second time
resolution. In other words, the time difference between two consecutive
impedance
measurement for a well shall be in the range of milli-seconds (e.g., less than
500
milli-second, less than 300 milli-second, less than 100 milli-second, less
than 10
milliseond, or less than 1 millisecond or faster). The milli-second kinetic
readout is
required to resolve the individual beat cycles of the cells. Thus, the time
resolution
for the impedance measurement should allow the system to perform measurement
at
least two time points for each beat cycle, or at more than two points for each
beat
cycle.
49
CA 02760941 2016-10-03
One example of the impedance measurement systems is a fast impedance-
measurement system from ACEA Biosciences, where the device is ACEA E-PLATE
in the form of microtiter plates whose wells comprise microelectrode
structures. The
steps involved in screening for modulators of cardiomyocyte beating using the
fast
impedance-measurement system in vitro include:
(1) Seeding cardiomyocytes, in E-Plates exactly as described in steps 1-4 in
section E above for using the system for measurement of cardiomyocytes
beating.
(2) Prior to addition of pharmacological agent, obtaining a baseline of the
cardiomyocyte beating frequency using the fast kinetic RT-CES hardware and
software.
(3) Addition of the pharmacological agents at one or more doses and continue
monitoring the cardiomyocyte beating frequency.
In order to demonstrate the utility of the millisecond kinetic measurements,
we first
used two pharmacological agents, one known to suppress the heart rate and
consequently cardiomyocyte beating and the other known to increase the heart
rate
and consequently the rate of cardiomyocyte beating. As Mouse ES cells were
seeded
in FN-coated E-PLATES and monitored for about 72 hours when the cells
differentiated into beating cardiomyocytes, as described in Section E. A
baseline of
the cardiomyocyte was taken for approximately 40 seconds using the specially
designed fast kinetic data acquisition hardware and software which is capable
of
millisecond data acquisition and display. An agonist of muscarinic receptors,
carbachol, which is known to slow down the heart rate was added to one well at
a
final concentration of 333 nM and cardiomyocyte beating was monitored for 10
minutes (Figure 8A). The data clearly shows that carbachol significantly slows
down
the rate of cardiomyocyte beating from ¨ 80 beats/min prior to carbachol
addition to
60 beats/min after carbachol addition (Table I). Alternatively, addition of
isoproteranol at a final concentration of 4.4 1.1M significantly increased the
rate of
cardiomyocyte beating from ¨ 65 beats/min to 115-135 beats/min (Figure 8B and
Table I). These data clearly show that the readout system and the fast kinetic
software
are sufficiently robust and sensitive to detect these changes in rate of
cardiomyocyte
beating even at very low, compound concentrations. Similar to the cell index
plot
shown in Figures 7C and 7D, the time resolution between two adjacent points in
CA 02760941 2016-10-03
Figures 8A and 8B is 40 milli-seconds. In other words, a second in Figures 8A
and
8B is equivalent to 40-milli-second.
Table I.
Concentration Beat Pattern
Compound Mechanism Rate Amplitude Change
333 nM From -
Muscarnic receptor 80 to - From 0.07 Beating rate
Carbachol agonist 60 to 0.06 decreased
4.4 p..M From -
b2 Adrenergic 65 to From 0.19 Beating rate
lsoproteranol receptor agonist 115-136 to 0.16 increased
To further demonstrate the capabilities of the impedance-based monitoring of
cardiomyocyte beating in detecting drugs which may adversely affect heart
function, a
number of drugs which have been pulled out of the market due to cardiotoxic
side
effects such as ERG channel inhibition and OT elongation were compiled and
tested
in a dose-dependent manner. The list of these compounds, their mechanism and
adverse side affects are shown in Table II. For these tests, mouse ES derived
cardiomyocytes were seeded at a final density of 25,000 cells in ACEA E-PLATES
and continually monitored by the improved impedance-measurement system.
Approximately, 72 hours after cell seeding the fast impedance-measurement
system
including fast kinetic software and fast measurement hardware circuitry was
used to
establish a baseline reading of cardiomyocyte beating for each well for about
40
seconds. Subsequently, the cells in each well were treated with the indicated
drug and
dose shown in Figure 9. Similar to the cell index plot shown in Figures 7C and
7D,
the time resolution between two adjacent points in all the figures in Figure 9
is 40
milli-seconds. In other words, a second in a figure in Figure 9 is equivalent
to 40-
milli-second.
Table II shows a summary of the results, clearly demonstrating that compounds
which have been shown to affect ERG channels do affect various aspects of
cardiomyocyte beating and function such as frequency of beating, magnitude of
beating. Furthermore, as shown in Figure 10 some of these compounds can lead
to
qualitatively different or similar patterns. For example, the compounds E4031,
Astezimole and dofetilide which are ERG channel inhibitors do contain patterns
with
51
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
similar features. In summary these results clearly indicate that using the
fast kinetic
software along with the RT-CES system do lead to a sensitive and robust
readout for
cardiomyocyte beating that can also detect drugs which are known to be
cardiotoxic.
Similar to the cell index plot shown in Figures 7C and 7D, the time resolution
between two adjacent points in all the figures in Figure 10 is 40 milli-
seconds. In
other words, a second in a figure in Figure 10 is equivalent to 40-milli-
second.
52
CA 02760941 2016-10-03
Table II.
Compound Mechanism Concentration Beat Rate Amplitude
Pattern Change
From 68-72 bpm to
irregular beating to
Astemizole anti-histamine 400 nM 0 <0.01 beating stopped
From 71-78 bpm to
irregular beating to
Terfenadine anti-histamine 200 nM 0 <0.01 beating
stopped
From - 60 to
Erythromycin anti-biotic 13.3 B..M - 80 0.09 to 0.07 No
pattern change
From - 80 to From 0.055 to
Moxifloxacin anti-biotic 20 iii.M -73 0.060 No pattern
change
From 71-78 From 0.24 to
= Pentamidine anti-infective 20 1.1,M to -71 0.21
No pattern change
Serotonergic From -70 to From 0.22 to
Amitriptyline Inhibitor 4.4 IA M - 90 0.17 No pattern
change
From - 65 bpm to
Ca channel From - 65 to From 0.13 to only
occasional
Verpamil blocker 130 nM 0 0.06 single beating.
From -79 to From 0.22 to
Rosglitazone PPAR agonist 13.31AM -75 0.17 No pattern
change
From - 80 to From 0.09 to Pattern changed,
Dontlite 500 nM - 180 0.02 much faster
COX-2 From - 68 to From 0.2 to
Rofecoxib Inhibitor 13.3 ttM -60 0.19 No pattern change
Pattern change, no
beating after initial
COX-2 From - 62 to From 0.16 to treatment,
then
Rofecoxib Inhibitor 40 jaM - 60 0.10 recovers
COX-2 From - 60 to From -0.2 to
Celecoxib Inhibitor 4.4 1.1.M (-20 - - 50) -0.12 Pattern
change
No pattern change
(initially). Beating
pattern changes
Doximbicin Anthracycline 40 ILIM -70 -0.16 after 2 hrs.
Calcineurin From -70 to From 0.18 to
Cyclosporin A inhibitor 13.3 tiM - 80 0.15 No pattern
change
p-adrenergie Pattern changed,
receptor From -70 to From 0.25 to much faster
and
. Propalanol antagonist 4.4 1.1M over 150 0.025
irregular
From - 80 to From 0.27 to Much faster,
Socalol 13.3 1.1,M - 160 0.07 pattern changed
K channel From 80 to From 0.09 to Pattern changed,
E4031 inhibitor 120 nM 160 0.03 much faster
From 80 to Initially become
140, then to - From 0.25 to faster, later
DDT Pesticide 8 B.M 80 0.02 irregular beating
Organic From - 65 to Beating stopped
PCB toxicant 8 1.1.M o From 0.2 to 0
From - 75 to Beating stopped
Endosulfan insecticide 8 1AM o From 0.2 to 0
53
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
To further demonstrate the capabilities of the impedance-based monitoring of
cardiomyocyte beating in detecting drugs which may adversely affect heart
function,
compounds at different concentrations were tested to demonstrate the dose-
dependent
effects of these compounds on cardiomyocytes. Approximately, 72 hours after
cell
seeding the impedance-measurement system including software and hardware
capable
of milli-second measurement time resolution was used to establish a baseline
reading
of cardiomyocyte beating for each well for about 40 seconds. Subsequently, the
cells
in each well were treated with drugs at different dose concentrations. Figure
11
shows an example of dose dependent effects of Astemizole on cardiomyocytes
beating at different concentrations. At high concentration of 400 nM,
Astemizole
had such a strong effect on the beating of cardiomyocytes that the beating
almost
stopped. The effect of Astemizole on the beating of the cardiomyocytes is
clearly
does-dependent. At low concentration of 40 pM, its effect on the beating of
the
cardiomyocytes is small that the cardiomyocyte beating rate was not affected.
Similar
to the cell index plot shown in Figures 7C and 7D, the time resolution between
two
adjacent points in all the figures in Figure 11 is 40 milli-seconds. In other
words, a
second in a figure in Figure 11 is equivalent to 40-milli-second.
G. Method to assess the developmental and functional consequence of specific
gene
knockout and transgene expression in ES-derived cardiomyocytes
The ES cells offer a suitable experimental model system that is amenable to
genetic
manipulation. Therefore, specific genes can be targeted in knockout experiment
as
well as genes can be expressed in a developmental or stage specific manner
under the
control of special promoters. The impedance-based measurement system can be
used
to evaluate the role of these genes in cardiac viability, morphology,
development and
or beating function. The steps involved in assessing the developmental and
functional
effect of gene knockout or transgene expression include
(1) Obtain ES cells harboring specific knockout of genes or which expresses a
particular transgene.
(2) Provide a single-well or multi-well device that comprise microelectrode
arrays in well(s) of the device, which can be used for monitoring cell-
substrate impedance.
54
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
(3) Optionally coat wells of the device with either fibronectin or other
matrix
proteins.
(4) Seed the embryonic stem cells (ES cells) of mammalian origin or adult stem
cells of mammalian origin at specific seeding densities to the wells of the
device.
(5) Allow the cells to attach and spread and monitor the growth and viability
of
the cells using an impedance measurement system.
(6) After a specified period of time unique to ES-derived cardiomyocytes or
primary cardiomyocytes, monitor cardiomyocyte beating using the
impedance-monitoring system to monitor electrode impedance by using
milli-second kinetic readout to resolve the individual beat cycles of the
cells.
(7) If a particular gene is required for development of cardiomyocytes from ES
cells, it is likely that the knockout of that gene will either affect the
viability
of the cells or block or delay the differentiation of ES cells to
cardiomyocytes. Since the impedance-based measurement system is capable
of functional monitoring of cardiomyocyte both in long term assays and
short teim assays, it can be used as a specific way to monitor the effect of
either gene knockout or transgene expression on cardiomyocyte function.
(8) Alternatively, the ES cells can be transfected with specific siRNA to
"knockdown" the product of a particular transcript and then monitor
cardiomyocyte viability, differentiation and function in vitro using the
impedance-based measurement system.
Similar to Section E, the milli-second kinetic readout requires that the
impedance
measurement system can provide impedance measurement data at milli-second time
resolution. In other words, the time difference between two consecutive
impedance
measurement for a well shall be in the range of milli-seconds (e.g., less than
500
milli-second, less than 300 milli-second, less than 100 milli-second, less
than 10
milliseond, or less than 1 millisecond or faster). The milli-second kinetic
readout is
required to resolve the individual beat cycles of the cells. Thus, the time
resolution
for the impedance measurement should allow the system to perform measurement
at
at least two time points for each beat cycle, or at more than two points for
each beat
cycle.
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
One example of the impedance measurement systems is an improved, fast
impedance-
measurement system from ACEA Biosciences, where the device is ACEA E-PLATE
in the form of microtiter plates whose wells comprise microelectrode
structures. The
steps involved in assessing the developmental and functional effect of gene
knockout
or transgene expression using the fast impedance-measurement system in vitro
include:
(1) Obtain ES cells harboring specific knockout of genes or which expresses a
particular transgene.
(2) Follow steps 1-4 of Section D for using the fast impedance-measurement
system for measurement of cradiomyocytes function.
(3) If a particular gene is required for development of cardiomyocytes from ES
cells, it is likely that the knockout of that gene will either block or delay
the
differentiation of ES cells to cardiomyocytes. Since the system is capable of
functional monitoring of cardiomyocyte, it can be used as a specific way to
monitor the effect of either gene knockout or transgene expression on
cardiomyocyte function.
(4) Alternatively, the ES cells can be transfected with specific siRNA to
"knockdown" the product of a particular transcript and then monitor
cardiomyocyte differentiation and function in vitro using the fast impedance-
measurement system.
H. Devices and method for extracellular recording
Devices and systems of the present invention permit extracellular recording of
cell
.. populations using a variety of technical approaches. Extracellular
recording may be
performed alone or together with impedance monitoring of cells. As discussed
in prior
sections, while the present device is suitable for performing extracellular
recording of
various cells and/or tissues, the present invention is particularly useful for
the
extracellular recording of cardiomyocyte cells, cardiomyoctye precursor cells,
as well
as tissues that contains such cells.
The devices and systems of the present invention provide structural
configurations
that functionally establish cell-free zones to prevent direct interaction
between cells
and reference electrodes, provide enhanced measurement reliability and
accuracy, and
56
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
bridge the current gap between impedance monitoring and extracellular
recording of
cells.
For instance, in a first approach, the devices and systems provide a cell-free
zone for
the positioning of a reference electrode. Accordingly, while traditional
systems often
require stringent physical parameters for the reference electrode for enhanced
measurement, such as stringent tolerances related to surface area, resistance,
impedance and the like, present invention provides an alternative solution.
Specifically, in one embodiment, a device for performing extracellular
recording of
cells, such as cardiomyocytes, is provided which includes a nonconductive
substrate
forming or provided as a base of one or more wells; an extracellular recording
electrode positioned on the substrate within the well, wherein the recording
electrode
is accessible to cells when a cell sample is added to the device; and a
reference
electrode positioned within the well in a cell-free zone, the cell-free zone
is
.. characterized as free from contact with cells when the cell sample is added
to the
device, thereby preventing contact between cells and the reference electrode.
That is,
the reference electrode is a cell-free electrode during operation.
Extracellular recording is conducted by amplifying and recording electrical
voltage
signals between the recording electrodes and reference electrodes. Such
electrical
voltages are induced on the electrodes as a result of ionic current or
movement
through cell culture media or solution supporting the cells during the
experiment as a
result of opening and/or closing of different ion channels across cell
membrane during
the action potential duration. Generally, it is desirable and it is recognized
for the
reference electrodes to have small electrode impedances. The small electrode
impedance is achieved by using reference electrodes with large effective
surface areas
by increasing the ratio of the surface area of the reference electrodes to
that of
recording electrode by a factor of a hundred, even thousands of times. Figure
15
shows a schematic representation of such electrode pairs placed on a non-
conductive
substrate, including a small area recording electrode 1501 and a large area
reference
electrode 1502. For clarity, the connection pads on the substrate for
connecting the
recording electrode and reference electrode to external circuitry are not
shown in
Figure 15. For example, in previous extracellular recording devices, the
recording
electrodes and reference electrodes are all incorporated onto a substrate
surface within
57
CA 02760941 2016-10-03
a fluid-container well where the recording electrodes arc of simple electrode
geometry
(e.g. circle, or square) having a diameter of 10 ¨ 100 microns in diameter
whereas the
reference electrodes would have much larger surface areas.
For the present invention, it is recognized that the extracellular recorded
voltage
signals are recorded as the difference in the electrical potentials between
the recording
electrode and reference electrode. In order to achieve improved consistency
and
reproducibility of the recorded voltage signals, it is desirable to minimize
the
contribution of any electrical signal from the reference electrode to the
recorded
voltage signals and to ensure that the majority, if not all, of the recording
voltage
signals are derived from that on the recording electrode. As mentioned above,
the
previous extracellular recording devices are designed to this goal by reducing
the
electrode impedance of the reference electrode through using reference
electrode of a
large surface area and so minimizing the voltage signals on the reference
electrode.
In one aspect, the present invention is directed to a device for extracellular
recording
of cells, the device comprising: a nonconductive substrate forming a base of
one or
more wells; a recording electrode positioned on the substrate within the well,
wherein
the recording electrode is accessible to cells when a cell sample is added to
the
device; and a reference electrode positioned within the well in a cell-free
zone, the
cell-free zone characterized as free from contact with cells when the cell
sample is
added to the device, thereby preventing contact between cells and the
reference
electrode. In one embodiment, the recording electrode has a diameter from
about 10
ni to about 2001.im. In another embodiment, the recording electrode comprises
an
electrode structure comprising a plurality of electrode elements. In preferred
embodiments, the reference electrode is positioned on the substrate in the
cell-free
zone.
Now with reference to particular configurations, the device for extracellular
recording
of the cells is designed and constructed so that the reference electrode is
positioned in
the well in a cell-free zone so that when the cells are added to the device,
the cells
would not be in contact with the reference electrode. In comparison with the
situation where the cells are in contact with the reference electrode, no-cell-
contact
58
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
with the reference electrode would significantly reduce the contribution of
the
electrical signals from the reference electrode so that the amplified and
recorded
voltage signals would mainly come from the recording electrode. Consider the
following. When the cells are in contact with the reference electrodes, the
ionic
current and ionic movement in the regions close to the cells due to the
opening and/or
closing of ion channels (and/or other ion transporters) in the cell membrane
would
directly cause an electrical potential signal on the close-by reference
electrode since
these reference electrodes are in direct contact with the cells. On the other
hand,
when there is no cell in direct contact with the reference electrode, only the
ionic
current and ionic movement due to the opening and/or closing of the ion
channels in
the membranes of other cells may result in small, if any, electrical potential
on the
reference electrode since these cells are not in contact with the reference
electrode.
The induced electrical potential on the reference electrode due to such no-
contact-
cells located at other regions of the wells would be significantly smaller
that that
induced by the cells in direct contact with reference electrode, because the
ionic
movement/current in the media would be much smaller in the reference electrode
region which is away from the cells.
There are different approaches to position the reference electrode within the
well in a
cell-free zone to prevent contact between cells and the reference electrodes.
In one
embodiment, the reference electrode is positioned on the substrate forming or
provided as the base of the wells to which the cells are added. The reference
electrode is located in a region that is separated by a barrier from the
region
containing the recording electrode. Preferably, for the device of the present
.. invention, the reference electrode may be separated by a barrier from the
recording
electrode so that when the cells are added to the wells containing the
recording
electrode, the cells will be prevented from landing on and contacting with the
reference electrode. When the cells are added to the recording electrode
region, the
cells are prevented from moving to the reference electrode region and
prevented from
contacting the reference electrode. Example of this embodiment is shown in
Figure
33 where both top view and cross-sectional view of a device of the present
invention
are shown. Here, the recording electrode 3301 and reference electrode 3302 are
incorporated on the substrate 3304. The barrier 3303 is placed inside the well
3305,
separating the recording electrode and reference electrode. In use, the cell
culture
59
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
media (or appropriate solutions for supporting the cells during the
experiment)
containing the cells is added first to the left half of the well 3305 to the
recording
electrode region, ensuring that the solution would not overflow above the
barrier to
the reference electrode. After all the cells settle down to the substrate
(which may
take some minutes to typically less than an hour), the culture media (or
appropriate
solutions for supporting the cells during the experiment) not-containing the
cells can
be added to the right half of the well and sufficient media (or or appropriate
solutions
for supporting the cells during the experiment) is added so that the media
would
overflow above the barrier, the final media height in the well would be above
the
barrier and there would be electrical connection path between the recording
electrode
and reference electrode through the media. Note that for simplicity of the
figure, the
electrical connection traces for the reference electrode and the recording
electrode to
the connection pads and the connection pads on the substrate are not shown in
Figure
33. In a related embodiment, the barrier could be provided in a configuration
having
a series of permeable apertures that selectively permit flow of media while
selectively
preventing the migration or movement of cells into the cell-free zone;
however, such a
configuration may lend to clogging of the apertures by blocking cells. In an
exemplary embodiment of this approach, the reference electrode may be located
under
the permeable aperture structure so that the media would flow to the reference
electrodes whilst the cells would not.
In another embodiment, the barrier is in a form of a plug that is made of
biocompatible materials and can be placed directly above and on the reference
electrodes. When the cells are added to the well, the cells are prevented by
such a
plug to be in contact with the reference electrode and the cells would land on
other
regions of the substrate, including the recording electrode region. After the
cells
settle down and adhere to the substrate of the well, the plug is removed and
the media
or solution in the well would move into the reference electrode region. A
complete
circuit is formed between the recording electrode and the reference electrode
through
the media or solution in the well.
Barriers may take alternative forms. For example, barriers may include ledges,
walls,
troughs and the like to act as a structural or physical barrier to cells. In
some
instances, a cell repulsive gel is applied to the substrate to define the cell-
free zone.
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
The barrier should however permit the medium to contact the reference
electrode such
that the recording and reference electrode are operably linked through a
conductive
media. Barriers may be permanently fixed to the device or may be removable as
the
configuration permits. Similarly, the barrier may be formed from any suitable
material and have any suitable dimensions consistent with isolating the
reference
electrode from cells, while obtaining extracellular recording measurements. In
some
embodiments the barrier is constructed from the same material as the
nonconductive
substrate.
In another embodiment, the reference electrode is positioned on a different
plane from
the recording electrode, ensuring that the cells added to the recording
electrode region
would not be in contact with the reference electrode. In such an embodiment
the cell-
free zone may be a raised surface to prevent cell migration or spreading onto
the cell-
free zone. In other embodiments an angled surface prevents migration upward
along
the angled surface and thus provides a suitable cell-free zone towards the top
of a
slope. In another embodiment, the cell-free zone is a region encapsulated by a
porous covering, which permits passage of medium while preventing access by
cells.
In still another embodiment, the reference electrode is an external electrode
free from
contact with the substrate to which the recording electrode is positioned. For
instance, a cell-free zone may be spatially positioned remote from the
substrate but
within a volume of the well. The schematic representation of such a device is
shown
in Figure 34. A recording electrode 3401 is positioned on the substrate 3404.
The
reference electrode is an external wire electrode (e.g. gold wire, or Silver-
silver
chloride wire electrode if they are placed into the well for very short time,
not causing
cytotoxic effects) 3402. In Figure 34, an impedance electrode array 3406 is
also
positioned on the substrate 3404. The electrode array 3406 is of straight-
line,
interdigitated electrode geometry and comprises two electrode structures, each
of
which comprise multiple electrode elements. In use, when the cells are added
to the
well 3405 containing the recording electrode 3401 and impedance measurement
electrode array 3406, the external reference electrode 3402 can be outside of
the well.
After the cells are added to the wells and are settled down to the bottom, the
external
reference electrode 3402 can be added to the well manually or automatically
and the
reference electrode would not be in contact with the substrate of the well and
not be in
contact with the cells on the substrate. In some embodiments, the external
reference
61
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
electrode 3402 is fabricated to a lid or cover which may be placed over top of
the
well.
Thus, a cell-free zone may be spatially positioned away from the substrate. In
such a
configuration the cell-free zone may occupy a volume of the well that does not
contact the substrate, yet does contact medium when added to the device.
Accordingly, while cells do not contact the cell free zone, an electrically
conductive
media may still ensure operable contact between an extracellular recording
electrode
and a reference electrode.
For extracellular recording, the recording electrode is generally of simple
geometry
and consists of a single electrode element such as a circle, a square or some
other
geometry. The size of such recording electrode typically ranges from about 10-
30 to
about 100 micron in a diameter. Such small electrode geometry has advantages
of
recording the electrical potential generated by a small number of the cells
located on
the recording electrodes. Action potentials from such a small number of the
cells
tend to be synchronized or nearly synchronized, allowing for a better time
resolution
for recording extracellular potential and for resolving different features of
the
recorded potential. However, one limitation of such extra-cellular recording
is that
due to small area of such electrodes, there tends to be large variations in
recorded
signals between different recorded electrodes of the same geometry in the same
wells
(if multiple recording electrodes are positioned inside a single well) or
different wells.
In particular, if insufficient number of the cells is added to the well to
cover all the
recording electrodes, it is possible that some recording electrodes may not
show any
recorded signal or only show recorded signals of very small magnitude.
Furthermore, depending on exact distribution or locations of the cells on the
recording
electrodes, different recording electrodes may show significantly different
extracellular potential waveforms. For this reason, many existing devices for
extracellular recording comprise multiple small-area recording electrodes.
Extra-
cellular potentials from each such recording electrode are amplified and
recorded
separately. The user would pick and choose appropriate signal wavefoims
recorded
for some individual recording electrodes for data analysis.
62
CA 02760941 2016-10-03
To overcome such a limitation, in some embodiments of the present invention,
electrode structures having larger surface areas and including multiple
electrode
elements are used as recording electrodes. The advantage of recording
extracellular
potential with such electrode structures is that the extracellular potential
is an
integration of the potentials from all the cells on the electrode array and
would be
more reproducible between different wells. The variation in recorded signals
between difference electrode structures from different wells would be smaller
than
that recorded with small, single recording electrode elements. For such
devices, each
well may comprise a single electrode structure, or two electrode structures.
In
preferred embodiment, each well comprises a single electrode structure
consisting of =
multiple electrode elements.
Thus, the extracellular recording electrode may be provided in a variety of
configurations. In some instances the recording electrode includes a unitary,
circular
or unbranched configuration. In other embodiments the extracellular recording
includes an electrode structure, which itself may include a plurality of
electrode
elements. Exemplary electrode structure and electrode element configurations
may be
found in US patent nos US 7,470,533; US 4,459,303; US 7,192,752; US 7,560,269;
and US 7,468,255. However, the majority of the above listed patents refer to
arrays
of electrodes having at least two complementary electrode structures. Thus,
when
considering the configurations for use as an extracellular recording electrode
structure
or element, the skilled artisan would typically provide one half of the array
as set forth
in the patents. =
Figure 13 shows more specific examples of such electrode structures that could
be
used as recording electrode for recording extracellular potentials. In Figure
13A, a
single electrode structure is shown, comprising multiple rectangular electrode
elements 1301 that are connected together with an electrode bus 1303.
Similarly,
Figure 13B comprises a single electrode structure, comprising multiple circle-
on-line
electrode elements 1311 that are connected together with an electrode bus
1313. For
Figure 13C, two electrode structures are shown, one electrode structure
comprising
multiple electrode elements 1331 and having electrode bus 1333, and the other
electrode structure comprising electrode elements 1332 and having electrode
bus
1335. The extracellular recording can be achieved using either electrode
structure or
63
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
both electrode structures connected together. Figures 22 and 23 show example
of
recording extracellular potentials using circle-on-line electrode structures
as recording
electrode and external wire electrode as reference electrode. Whilst the
recorded
waveform is different from those obtained with small recording electrode, the
recorded signals are very reproducible between different wells.
By way of yet another exampleõ a device for performing extracellular
recordings of
excitable cells in vitro, may include, a) a nonconductive substrate; b) one or
more
wells on the substrate; c) one or more electrode structures fabricated on the
substrate,
each electrode structure is associated with one of the one or more wells; and
d) one or
more reference electrodes external to the substrate or free from contact with
the
substrate, each of which can be inserted into one of the one or more wells,
wherein for
each of the one or wells, the corresponding electrode structure and reference
electrode
form an electrode pair. In further embodiments, the substrate has a surface
suitable
for attachment of excitable cells, wherein the attachment of excitable cells
on the
substrate can result in detectable extracellular recording potentials between
each of
electrode pair. Figure 12 shows another illustrate example of such electrode
pair,
comprising a recording electrode structure on the substrate and a reference
electrode
external to the substrate. In a particularly preferred embodiment, each
electrode
structure comprises multiple electrode elements. Use of such a configuration
is
believed to increase the sampling size and thus minimize or reduce variation
between
cell populations. In another preferred embodiment, each electrode structure
comprises multiple electrode elements, forming half of an interdigitated
electrode
array (Figure 13A). In another preferred embodiment, each electrode structure
comprises multiple electrode elements so that these electrode elements form a
complete interdigitated electrode array (13C) wherein during extracellular
recording
the electrode elements within entire interdigitated electrode array are
connected
together. In another preferred embodiment, each electrode structure comprises
multiple electrode elements which have different electrode element shapes,
including
rectangular shape, or sinusoidal shape, spiral or circle-on-line shape. Figure
13
shows examples of electrode structures having multiple electrode elements that
can be
used for extracellular recording where 13A, 13B, 13C, 13D,13E and 13F are the
rectangular shape, circle-on-line shape, a complete interdigitated electrode
array,
sinuosoidal shape, castellated shapes and a complete circle-on-line electrode
array,
64
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
respectively. Figure 14 shows the examples of such electrode structures for
extracellular recording where a recording electrode structure and a reference
electrode
are incorporated on the substrate to form a pair of electrodes. Preferably,
the
reference electrode is separated from the recording electrode by a barrier to
the cells
so that the reference electrode is in a cell-free zone when the cells are
added to the
electrodes. Clearly, these electrode structures are significantly different
from
microelectrode arrays formed by simple electrodes such as circle-shaped
electrodes
and/or square-shaped electrodes currently used in the field of extracellular
recording.
In another preferred embodiment of the device for perfouning extracellular
recording,
each electrode structure occupies a substantial percentage of surface area of
the well
that the electrode structure is associated with. In some preferred
embodiments,
percentage of surface area of the well at the bottom being occupied by the
electrode
structures is more than 5%. Preferably, percentage of surface area of the well
at the
bottom being occupied by the electrode structures is more than 10%. More
preferably, the percentage of surface area of the well at the bottom being
occupied by
the electrode structures is more than 20%. More preferably, the percentage of
surface
area of the well at the bottom being occupied by the electrode structures is
more than
30%. More preferably, the percentage of surface area of the well at the bottom
being
occupied by the electrode structures is more than 50%. More preferably, the
percentage of surface area of the well at the bottom being occupied by the
electrode
structures is more than 70%. More preferably, the percentage of surface area
of the
well at the bottom being occupied by the electrode structures is more than
85%.
Clearly, such large-surface-area electrodes are significantly different from
microelectrode arrays currently used in the field of extracellular recording,
where
recording electrodes typically have a round disc shape or a square shape of
smaller
size with typical dimension of 10 to 100 micron in diameter..
In another aspect of the present invention, a method performing extracellular
recording of excitable cells comprises, providing a device of the present
invention,
adding excitable cells to the wells; providing an extra-cellular-recording
amplifier that
can measure and record voltage signals at microvolt levels, connecting
electrodes on
the devices to the extra-cellular-recording amplifier; monitoring and
recording
extracellular potentials at the electrodes of the devices. Preferably, the
method
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
further comprises analyzing recorded extracellualr potential waveforms. More
preferably, the method further comprises adding a compound to the well,
measuring
and recording extracellular potentials prior to and after the compound
addition;
analyzing extracellular potential waveforms.
I. Devices and method for parallel extracellular recording and cell impedance
measurement
In still another aspect of the present invention, a device allowing for
parallel
extracellular recording and cell impedance measurement is provided. Thus, a
single
device or system is capable of performing both impedance monitoring of cells
and
extracellular recording. Thus, the device may include a means for cell
impedance
measuring and a means for extracellular recording operably coupled to the same
substrate. In some instances, impedance monitoring occurs before extracellular
recording measurements. Such an approach may permit monitoring, determining or
confirming desired cell viability, morphology and the like as set forth in
prior sections
prior to initiating extracellular recording assays. Thus, impedance monitoring
permits
the user to confirm cellular phenotype or attributes prior to initiating
extracellular
recording. In other applications, the impedance measurement permits the
monitoring
of the beating of cardiomyocytes, the monitoring of the change to cell
morphology,
cell adhesion, cell viability or other properties after a compound treatment.
Further,
impedance monitoring may be conducted while performing extracellular recording
or
after extracellular recording. Impedance monitoring may be used to monitor
cell
beating, viability, morphology and the like. While impedance monitoring and
extracellular recording may be performed over the same time period, the actual
measurements may be obtained at different time points. That is, a switching
means
may permit the device to switch from an impedance monitoring mode to an
extracellular recording mode. The combined approach is facilitated in part due
to the
increased impedance based time resolution. That is, impedance monitoring with
millisecond time resolution permits such measurements to be conducted
throughout a
variety of time windows, without adversely affecting extracellular recording
measurements.
66
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
The skilled artisan will appreciate parallel extracellular recording may be
performed
using a variety of configurations provided herein. In a first example,
parallel
recording is performed using a device that includes both a pair of electrodes
for
impedance monitoring and a pair of electrodes for extracellular recording,
though
.. pairs of electrodes may include a third or more electrode. Each pair is
selectively
operated via a switching means able to activate either an impedance monitoring
mode,
which allows for the connection of the impedance electrodes to external
impedance
measurement circuitry; or extracellular recording mode, which allows for the
connection of the extracellular recording electrode and reference electrode to
external
voltage amplifier and signal measurement and recording circuits. In preferred
embodiments, the switch is a programmed switch with adjustable parameters
through
a computer interface. Thus, in one example, a device for parallel impedance
monitoring and extracellular recording of cells is provided, wherein the
device
includes: a nonconductive substrate forming or provided as a base of one or
more
.. wells; at least two impedance electrodes capable of monitoring impedance of
the
cells, the at least two impedance electrodes positioned within a well and on
the
nonconductive substrate, wherein the at least two impedance electrodes are
accessible
to cells when a cell sample is added to the device; an extracellular recording
electrode
positioned on the substrate within the well, wherein the recording electrode
is
accessible to cells when the cell sample is added to the device; and a
reference
electrode positioned within the well. In the preferred mode, the reference
electrode is
positioned in a cell-free zone, which is characterized as free from contact
with cells
when the cell sample is added to the device. When in impedance mode, the pair
of
impedance electrodes permits impedance monitoring of cells. When in
extracellular
recording mode the extracellular recording electrode and reference electrode
form a
pair of electrodes for extracellular recording measurements. In a related
embodiment,
a device is provided that includes a nonconductive substrate, having a surface
suitable
for attachment of excitable cells, one or more wells on the substrate; for
each well, a
pair of impedance measurement electrodes, wherein the attachment of excitable
cells
on the substrate can result in a detectable impedance change; for each well, a
pair of
extra-cellular recording electrodes comprising a recording electrode and a
reference
electrode, wherein the attachment of excitable cells on the substrate can
result in a
detectable extra-cellular recording potentials between the recording-electrode
and
reference-electrode pair. Preferably, the pair of impedance measurement
electrodes
67
CA 02760941 2016-10-03
=
is located on the substrate. Preferably the extracellular recording electrodes
are
located on the substrate and in the same plane as the impedance electrodes.
Also,
preferably in such a configuration, the reference electrode is provided in a
cell-free
zone. The cell-free zone prevents direct contact between cells and the
reference
electrode. In some instances, a physical barrier prevents contact between
cells and the
reference electrode. In other instances, the cell-free zone is provided on a
surface that
is not planar with the impedance monitoring electrodes such that the reference
electrode is free from contact with cells added to the device. In still
further instances,
the cell-free zone is spatially positioned within the well but does not
contact the
substrate. In such instances, one recording electrode may be located on the
substrate
and another electrode may be located external to the substrate, but within a
volume of
the well. Positioning the reference electrode in the volume of the well free
from
contact with cells and the substrate permits electrically conductive medium to
complete an electrical circuit between the recording electrode and reference
electrode;
however direct contact between cells and the reference electrode may be
avoided.
In some embodiments, the recording electrode has a diameter from about 10 tun
to
about 200 1.tm. In other embodiments, the recording electrode comprises an
electrode
structure comprising a plurality of electrode elements.
In other configurations, one of the impedance monitoring electrodes is
provided as an
extracellular recording electrode. Figure 16 shows an example of such device
where
in one well, a pair of impedance measurement electrodes is located on the
substrate
and one of the impedance measurement electrode structures is used as an
extracellular
recording electrode, together with an externally applied reference electrode.
In such a
configuration a switching means may selectively switch between coupling signal
between the two impedance electrodes in an impedance mode and between the
impedance electrode and a reference electrode in an extracellular recording
mode.
More particularly, in one embodiment the device for parallel extracellular
recording
includes a nonconductive substrate forming or provided as a base of one or
more
wells; at least two impedance electrodes capable of monitoring impedance of
the
cells, the at least two impedance electrodes positioned within a well and on
the
nonconductive substrate; and a reference electrode, wherein a first impedance
electrode from the at least two impedance electrodes is electrically coupled
to the
68
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
reference electrode for performing an extracellular recording measurement at
the first
impedance electrode. In such configurations, preferably the at least two
impedance
electrodes each have the same surface area and each comprise an electrode
structure
comprising a plurality of electrode elements. While in some embodiments the
reference electrode is positioned on the substrate in other embodiments the
reference
electrode is an external electrode free from contact with the non-conductive
substrate.
In still another aspect of the present invention a device for parallel
extracellular
recording includes a nonconductive substrate forming or provided as a base of
one or
more wells; at least two impedance electrodes capable of monitoring impedance
of the
cells, the at least two impedance electrodes positioned within a well and on
the
nonconductive substrate; and an extracellular recording electrode, wherein a
first
impedance electrode from the at least two impedance electrodes is electrically
coupled to the extracellular recording electrode to act as a reference
electrode for
performing an extracellular recording measurement. That is, the impedance
electrode
may also operate as a reference electrode for the extracellular recording
electrode. In
such configurations, preferably, the at least two impedance electrodes each
have the
same surface area and each comprise an electrode structure comprising a
plurality of
electrode elements. In one specific embodiment, the recording electrode for
extracellular recording is in the fowi of a unitary electrode structure and
the reference
electrode is one impedance electrode comprising multiple electrode elements.
For
the devices of such configuration, the cells would contact the impedance
electrodes
and contact the reference electrode. This is different from other embodiments
of the
devices used for extracellular recording described above. The advantage of
this
approach is that the electrode design for parallel impedance monitoring and
extracellular recording is simplified. Instead of using four electrodes with
two
electrodes for impedance monitoring and two electrodes for extracellular
recording,
three electrodes are used with one impedance electrode being used for
impedance
monitoring and used as a reference electrode for extracellular recording. For
such
configuration, the extracellular recorded voltage signals would have the
contributions
from extracellular potential from both recording electrode and the reference
electrode.
The skilled artisan will recognize the device may be operable linked to a
system
including an impedance analyzer, extracellular recording amplifier, electronic
69
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
switches, electrical noise filtering circuits, switching means capable of
switching
measurement between the at least two impedance electrodes and the first
impedance
electrode and the reference electrode and the like. The skilled artisan will
also
recognize the system may include software to instruct the desired measurements
or to
set desired parameters for testing or operation.
In still another aspect of the present invention, the method for parallel
measurement of
cell-substrate impedance and extra-cellular potentials comprises a) providing
a device
allowing for parallel extracellular recording and cell impedance measurement;
b)
adding excitable cells to the wells of the device; c) providing an impedance
analyzer;
d) providing an extracellular potential amplifier; e) connecting electrodes of
the
devices to the the impedance analyzer; f) connecting electrodes of the devices
to the
the extra-cellular potential amplifier; g) performing parallel measurement of
cell-
substrate impedance and extracellular potentials. Preferably, the method
further
comprises adding compounds to the cells and monitoring cell-substrate
impedance
and extracellular potentials prior to and after compound addition. Preferably,
the
method further comprises analyzing measured time dependent impedance
responses.
Still preferably, the method further comprising analyzing extracellular-
recording
wave forms.
J. Devices and method for monitoring cardiomyocyte beating using label-
free
method
In another aspect of the present invention, a label-free method is used to
monitor cell-
substrate interaction for quantifying and measuring the beating of
cardiomyocytes.
The label-free method refers to any method that can be monitor or measure the
cells
without the need of using labeling reagents or molecules. Cell impedance
measurement method is one example of label-free approaches. Another label-free
method includes the use of Resonant Waveguide Grating (RWG) bio sensor (see
reference, Y. Yang, Label-Free Cell-Based Assays with Optical Biosensors in
Drug
Discovery, in ASSAY and Drug Development Technologies, Volume 4, Number 5,
2006, 583-595; Y. Yang et al., Label-Free Cell-Based Assays for GPCR
Screening, in
Combinatorial Chemistry & High Throughput Screening, 2008, 11, 357-369; Y.
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
Yang, Non-invasive Optical Biosensor for Probing Cell Signaling, in Sensors,
2007,
Volume 7, 2316-2329).
An RWG biosensor consists of a substrate (e.g., glass), a waveguide thin film
with an
embedded grating structure, and a cell layer. The RWG biosensor utilizes the
resonant
coupling of light into a waveguide by means of a diffraction grating, leading
to total
internal reflection at the solution-surface interface. This type of biosensor
can be
used in cell-based assays to monitor changes in cell morphology, cell adhesion
or
other cell status parameters. The system for using such RWG biosensors can be
divided into those based on angle-shift or wavelength-shift measurements. In a
wavelength-shift measurement, polarized light covering a range of incident
wavelengths with a constant angle is used to illuminate the waveguide; light
at
specific wavelengths is coupled into and propagates along the waveguide.
Alternatively, in angle-shift instruments, the sensor is illuminated with
monochromatic light and the angle at which light is resonantly coupled is
measured.
The resonance conditions are influenced by the physical properties of the cell
layer
that contacts with the surface of a biosensor (e.g., cell confluency, adhesion
and status
such as proliferating or quiescent states).
Such optical biosensor in the form of resonant waveguide, consisting of a
substrate
with an optical grating and a coating with a high refraction index, may be
used as
label-free means to monitor the beating of cardiomyocytes by monitoring the
change
in refractive index upon cell-substrate interaction during cardiomyocyte
contraction
and relaxation cycle (i.e. beating cycle). The method for such label-free
monitoring
of beating of cardiomyocytes includes providing a RWG biosensor for monitoring
cell
status operably connected to angle-shift or wavelength shift measurement
system,
where the device includes at least two wells optionally coated with
fibronectin (or
other extracellular matrix proteins) to expedite attachment; adding cells to
the at least
two wells, where the cells can be mouse or human or other mammalian ES cells
destined to differentiate into cardiomyocytes or primary cardiomyocytes
isolated
directly from the heart of an experimental system including mice, rats,
rabbits or dog;
optically monitoring the cells of at least two wells at time intervals over a
period of
time via the measurement of wavelength shift or angle-shift, optionally
calculating
71
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
average rate of beats per unit time, average amplitude intensity in a unit
time as well
as the average length of time between the beats.
Examples: Extra-cellular Recording and Cell-substrate Impedance Measurement of
Stem-cell derived Cardiomyocytes
EXAMPLE 1: Extra-cellular Recording on a disc-shaped microelectrode array
Medium and reagents
The standard cell culture medium was Cor.Ate Culture Medium (AXIOGENESIS
AG, Cologne, Germany) supplemented with 5% of fetal bovine scrum ( Hyclone,
Logan, USA ), 100 ,g,/m1 of puromycin. Fibronectin from bovine plasma (1mg/mL
solution, Sigma, St. Louis, USA) was used as coating (4 hrs or overnight)
material
before plating the mouse embryonic stem cell. E4031, a class III
antiarrhythmic drug
which is a specific antagonist for hERG and EAG like channels was obtained
from
Sigma (St. Louis, USA).
Cardiomyocyte preparation
Cardiomyocytes (Cor.AtO, AXIOGENESIS AG, Cologne, Germany) are derived
from transgenic mouse embryonic stem cells. Each vial contains 1 million
viable
Cor.Ate cardiomyocytes (>99.9% pure). These highly purified cardiomyocyte
maintain the phenotype of adult mouse cardiomyocyte and express cardiac-
specific
connexin-43, which is an indication of the ability for excitation-contraction
coupling
of differentiated cardiomyocyte in vitro.
The stem cells were thawed and the suspension drop of cell density is around
106-7/ml.
The thawed stem cells were plated onto fibronectin coated multi-electrodes
chip
which is embed in PP dish (-130 1 volume) as the bottom. The cell plated
chips were
placed in 37 C incubator (with 5% CO2, 95% humidity). The cell culture medium
was changed once a day. The plated Cor.AtO cell line can be grown up to 21
days
post thaw with medium changes every other day.
72
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
Multichannel recording
After three to four days growing in 37 C incubator (with 5% CO2, 95%
humidity),
the differentiated cells formed a monolayer onto the multi-electrode chip with
some
congregated cardiomyocyte showing rhythmically self-beating. The earliest
onset of
self beating was found on the second day after cell plating. The multichannel
extracellular recording was carried out on day 3 to day 5 after cell plating
using WPI
ISO-DAM8A (eight channel module) amplifier (World Precision Instruments,
Sarasota, USA) and Dataq DI-0720 data acquisition interface (Dataq Instrument
Inc.,
Akron, USA). The multi-electrode chip contains an array of 30 electrodes
(Figure
17), and the diameter of the electrode tip is 50 !Am and the interval between
each two
neighbor electrode tips is 50 [tm. The signals were collected via the 4
electrodes in
center which were arranged in a rectangle shape (A2, B2, C2 and D2 as shown in
Figure 17). For extracellular field potential, the field potential is measured
between
each of the 4-electrodes and an electrical connection that connects to all the
other 26
electrodes. The extracellular field potential (FP) data were collected with
following
parameters being used: Low cut filter: 5 Hz; High cut filter: 1 K Hz; Sampling
rate: 5
K Hz; Display sensitivity: 10 K.
Results
General features of field potential
The FP signal is relatively small; the peak to peak amplitude is < 1 mV.
During the
.. early process (day 2 to day 4 after cell plating) of growing and
differentiating, the self
beating frequency of cardiomyocyte cluster was increased from 60 to 90 /min on
day
2 (n=4) to 160 to 220 / min on day 4 (n=4), and the beating frequency remain
nearly-
constant after day4. In the meantime, the duration of FP was reduced and the
depolarizing velocity which reflecting the upstroke of the FP was shortened
while the
amplitude of the FP was increased (Figure 18). As shown in Figure 18, the
electrophysiological features were changed as the mouse derived stem cell
grows and
develops on day 2 and day 3. The FP amplitude and frequency were increased
while
the duration and depolarizing velocity were shortened.
73
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
Since the extracellular FP is the negative reflection of the intracellular
voltage
fluctuation, the standard shape of FP waveform consists downward
depolarization
peak followed by upward repolarization peak. In actual recording, we observed
an
initial upward peak and downward peak followed by an upward peak (Figure 18).
Effect of E4031 on FP
E4031, antiarrhythmic agent, is a specific antagonist for hERG and EAG channel
family which participate repolarization during action potential phase 3. So In
present
study, we picked FP duration as our main target index, and it directly
correlated to the
"QT interval" in clinical ECG. We found E4031 concentration-dependently
delayed
the depolarizing velocity and prolonged FP duration in mouse cardiomyocyte
(Figure
19) with IC50 of 405 nM (Figure 20). The on-site action of E 4031 on field
potential
is a slightly slow and it possibly due relatively the low binding affinity of
E4031 to its
receptor. At a concentration of 75 to 150 nM, E4031 exerted "anti-arrhythmic"
action on field potential frequency in the mouse-stem-cell derived
cardiomyocytes
(Figure 21).
EXAMPLE 2: Extra-cellular Recording Using one electrode structure on the
substrate and one external reference electrode
Figure 22 shows an extra-cellular field potential recording of mouse stem-cell
derived cardiomyocytes obtained using a device of the present invention. The
circle-
on-line electrode array (with circle diameter 90 micron, line width 30 micron,
the
electrode gap distance 20 micron, see Figure 13F for representation of circle-
on-line
electrodes) consisting of two electrode structures are fabricated on a glass
substrate.
During extra-cellular field potential recording, one circle-on-line electrode
is used as a
recording electrode and an external wire electrode is inserted into the well
serving as a
reference electrode. Cell preparation, reagents, cell culture and
extracellular
recording setup are the same as those described in to example 1.
74
CA 02760941 2016-10-03
Figure 22A shows a field potential (after an amplification of 10,000) before
treatment
for a 3-day cardiomyocyte culture in a well containing circle-on-line
electrodes.
Cardiomyocytes were beating at a rate of ¨ 91 beats per minute. At the middle
point
of the Figure 22A, E4031 was added to the well. No immediate effect was
observed
after the treatment with E4031. However, with time, the field potential
gradually
became irregular. The irregular field potentials at ¨ 18 seconds and ¨ 3
minutes after
treatment were shown on Figure 22B and 22C. At ¨ 5.5 minutes after treatment,
the
field potential magnitude was significantly reduced and later on, no field
potential
peaks could be detected.
EXAMPLE 3. Parallel cell-impedance measurement and extra-cellular recording
for
cardiomyocytes prior to and after Quinidine treatment
Figure 23A through 23E show another example of field potential change for
cardiomyocytes at different time points before and after the treatment with 3
u.M
Quinidine, as recorded from a 3-day cardiomyocyte culture in a well containing
circle-on-electrodes. Similar to the configuration for data in Figure 22, an
external
wire electrode was inserted to the well serving as a reference electrode.
Before
treatment, cardiomyocytes were beating at a rate of ¨ 72 beats per minute. As
shown in Figure 23 B through D, corresponding to the field potential recorded
at 10
seconds, 50 seconds, 3 minutes and 9 minutes after treatment with 3 tiM
Quinidine,
Quinidine has a dramatic effect on the field potential of the cardiomyocytes.
In parallel, impedance of the cardiomyocytes in such a well is monitored.
Figure
24A shows the impedance spikes as monitored on the circle-on-line electrode
array
before treatment. As shown in Figure 24B through D, corresponding to the
impedance spike pattern measured at ¨ 1.5 minute, ¨ 3 minute and ¨ 11 minute
after
treatment with 3 1Ø4 Quinidine, impedance-based monitoring can readily
detect the
change in cardiomyocyte beating function as a result of Quinidine treatment.
Notably, at ¨ 11 minutes after Quinidine treatment, the amplitude of impedance
spikes significantly reduced and the impedance spiking also becomes very
irregular.
EXAMPLE 4. Extra-cellular recording for primary cardiomyocytes between a
circle-
on-line electrode structure and an externally applied reference electrode
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
Primary cardiomyocytes were prepared from by perfusing sacrificed mice' heart.
The primary cardiomyocytes were added to a well containing a circle-on-line
electrode array (with circle diameter 90 micron, line width 30 micron, the
electrode
gap distance 20 micron) on the well bottom. The electrode array is fabricated
on a
glass substrate. Before seeding the cells, the well was pre-coated with
fibronectin.
Each well was seeded with about 40,000 cells and was measured between 48 and
72
hrs after seeding. During extra-cellular field potential recording, one circle-
on-line
electrode structure is used as a recording electrode and an external wire
electrode is
inserted into the well serving as a reference electrode. The setting for extra-
cellular
recording is the same as those used in Example 1. Figure 25 shows a typical
field
potential from such a primary cardiomyocyte culture.
EXAMPLE 5: Parallel cell-impedance measurement and extra-cellular recording
for
cardiomyocytes
Figure 27 shows the design of an electrode array used for combined cell-
impedance
measurement and extra-cellular recording. For this design, the electrode array
consists of two extracellular-recording electrodes 2701 and 2702 with circle
geometry
having diameters of 60 microns, located on either left or right side of, and
in the
middle section the array. Note that the recording electrode diameter could be
varied
from being as small as 5 micron to as large as 0.5 mm, even 1 mm, or even
multiple
millimeters. Electrode array further consists of two electrode structures 2703
and
2704, each comprising multiple electrode elements. Electrode elements within
each
electrode structure are of circle-on-line geometry and comprise interconnected
circles
having a diameter of 90 microns positioned along a straight line of 30 micron
width
(the electrode gap distance 20 micron, see Figure 13F for representation of
circle-on-
line electrodes). Electrode element 2705 is the first element counting from
top for
electrode structure 2703. Electrode element 2706 is the first element counting
from
top for electrode structure 2704. Electrode elements on electrode structure
2703 are
connected together through electrode bus 2707, having a width of 0.2 mm, to a
connection pad 2709 which is located on the left side of Figure 27. Similarly,
electrode elements of the electrode structure 2704 are connected together
through
electrode bus 2708, having a width of 0.2 mm, to a connection pad 2710 which
is
76
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
located on the right side of Figure 27. The overall electrode area excluding
the
regions consisting of electrode buses 2707 and 2708 has a diameter of 3.1 mm.
The electrode array of Figure 27 is fabricated on glass substrate using
photolithography methods for pattern-generation of thin gold electrode arrays
(gold
film thickness of ¨ 100 nrn on top of a Cr adhesion layer of 10 nm). A
bottomless
well plate having a well-diameter of 5 mm is assembled to the electrodes-
containing
glass slides, with the electrode array shown on Figure 27 being positioned at
the
central region of the well.
For cell impedance measurement, the impedance between two electrode structures
2703 and 2704 is measured by connecting an ACEA real-time-cell-analysis
impedance analyzer capable of millisecond time resolution (e.g., 15
milliseconds or
less impedance data update rate for measuring 96 wells simultaneously) to the
two
connection pads 2709 and 2710, connected to responding electrode buses 2707
and
2708, respectively. For extracellular recording (ECR), various recording modes
can
be used.
(1) ECR mode 1 is used to for monitoring electrode electrical
voltage
signals between an ECR electrode 2701 (or 2702) and one
(impedance-measurement) electrode structure 2703 (or 2704) by
connecting an ECR connection pad (2711 or 2712) and an
impedance-measurement electrode connection pad (2709 or 2710)
to a voltage amplifier. For this mode, extracellular signals from
ECR electrodes are measured with the impedance-measurement
electrode structure 2703 or 2704 used as "reference electrode".
The impedance-measurement electrode structure 2703 (or 2704)
has a much smaller electrode-impedance due to its much larger
surface area (over 100 times larger) than ECR electrodes 2701 (or
2702).
(2) ECR mode 2 is used for monitoring electrode electrical voltage
signals between an ECR electrode 2701 (or 2702) and an
externally applied gold reference electrode (not shown on Figure
27) inserted into the well by connecting an ECR connection pad
(2711 or 2712) and the gold external electrode to a voltage
77
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
amplifier. For this mode, extracellular signals from ECR
electrodes are measured with the external gold electrode used as
"reference electrode". Such external gold electrode will not have
any cardiomyocytes attached and would serve as cell-free
reference electrodes.
(3) ECR mode 3 is used for monitoring electrical voltage signals
between impedance-measurement electrode structure 2703 (or
2704) and an externally applied gold reference electrode (not
shown on Figure 27) inserted into the well by connecting an
impedance-electrode connection pad (2709 or 2710) and the
external gold electrode to a voltage amplifier. For this mode,
extracellular signals from impedance-measurement electrodes are
measured with the external gold electrode used as "reference
electrode". Such external gold electrode will not have any
cardiomyocytes attached and would serve as cell-free reference
electrodes.
Figures 28 and 29 are examples of impedance measurement and extracellular
recording of Cor. At cardiomyocytes. Cor. At cells were prepared and cultured
using
the same method as those described in Example 1. The data on Figures 28 and 29
is
for the Cor. At cells on day 5 after cell seeding into the electrodes-
containing wells.
Figure 28 shows the impedance beating result obtained for the Cor. At cells
for ¨ 12
seconds with time-solution about 28.8 ms per data point. The impedance data is
represented with cell index, a dimension less parameter. Figure 29 shows the
ECR
voltage signals for the three different recording modes described above,
including
ECR electrode vs impedance electrode-structures, ECR electrode vs external
gold
electrode and impedance electrode-structure vs external gold electrode. The
voltage
amplifier and other ECR details are the same as those discussed in Example 1.
In
brief, the multichannel extracellular recording was carried out using WPI ISO-
DAM8A (eight channel module) amplifier (World Precision Instruments, Sarasota,
USA) and Dataq DI-0720 data acquisition interface (Dataq Instrument Inc.,
Akron,
USA). The extracellular field potential data were collected with following
parameters
being used: voltage signal gain: 10,000; Low cut filter: 1 Hz; High cut
filter: 1 K Hz;
Sampling rate: 2-5 K Hz.
78
CA 02760941 2011-11-03
WO 2010/129725
PCT/US2010/033801
EXAMPLE 6: Parallel cell-impedance measurement and extra-cellular recording
for
cardiomyocytes
Figure 30 shows the design of another electrode array used for combined cell-
impedance measurement and extra-cellular recording. For this design, the
electrode
array consists of two extracellular-recording electrodes 3001 and 3002 with
circle
geometry having diameters of 60 microns, located in the middle section the
array.
Note that the recording electrode diameter could be varied from being as small
as 5
micron to as large as 0.5 mm, even 1 mm, or even multiple millimeters. ECR
electrodes 3001 and 3002 are connected to the connection pads 3011 and 3012,
respectively. The electrode array further consists of two electrode structures
3003 and
3004, each comprising multiple electrode elements. Electrode elements within
each
electrode structure are of circle-on-arc (curved line) geometry and comprise
interconnected circles having a diameter of 90 microns positioned along curve
lines of
30 micron width (the electrode gap distance 20 micron, see Figure 13F for
representation of circle-on-line electrodes). The first electrode element of
the
electrode structure 3003, counting from top of the electrode array, has many
circles
aligned-on-curved-line, forming a semi-circle arc. The first electrode element
of the
.. electrode structure 3004, counting from bottom of the electrode array, has
many
circles aligned-on-curved line, forming a semi-circle arc. Electrode elements
on
electrode structure 3005 are connected together through horizontal lines
located in the
middle of the electrode array to the connection pad 3009 which is located on
the left
side of the electrode array. Electrode elements on electrode structure 3006
are
connected together through horizontal lines located in the middle of the
electrode
array to the connection pad 3010 which is located on the right side of the
electrode
array.
The electrode array of Figure 28 is fabricated on glass substrate using
photolithography methods for pattern-generation of thin gold electrode arrays
(gold
film thickness of 100 nm on top of a Cr adhesion layer of 10 nm). A bottomless
well plate having a well-diameter of 5 mm is assembled to the electrodes-
containing
glass slides, with the electrode array shown on Figure 30 being positioned at
the
central region of the well.
79
CA 02760941 2011-11-03
WO 2010/129725 PCT/US2010/033801
For cell impedance measurement, the impedance between two electrode structures
3003 and 3004 is measured by connecting an ACEA real-time-cell-analysis
impedance analyzer capable of millisecond time resolution (e.g., 15
milliseconds or
less impedance data update rate for measuring 96 wells simultaneously) to the
two
connection pads 3009 and 3010, connected to responding electrode structures
3003
and 3004, respectively. For extracellular recording (ECR), various recording
modes
can be used.
(1) ECR mode 1 is used to for monitoring electrode electrical voltage
signals between an ECR electrode 3001 (or 3002) and one (impedance-
measurement) electrode structure 3003 (or 3004) by connecting an ECR
connection pad (3011 or 3012) and an impedance-measurement electrode
connection pad (3009 or 3010) to a voltage amplifier. For this mode,
extracellular signals from ECR electrodes are measured with the impedance-
measurement electrode structure 3003 or 3004 used as "reference electrode".
The impedance-measurement electrode structure 3003 (or 3004) has a much
smaller electrode-impedance due to its much larger surface area (over 100
times larger) than ECR electrodes 3001 (or 3002).
(2) ECR mode 2 is used for monitoring electrode electrical voltage signals
between an ECR electrode 3001 (or 3002) and an externally applied gold
reference electrode (not shown on Figure 30) inserted into the well by
connecting an ECR connection pad (3011 or 3012) and the gold external
electrode to a voltage amplifier. For this mode, extracellular signals from
ECR electrodes are measured with the external gold electrode used as
"reference electrode". Such external gold electrode will not have any
cardiomyocytes attached and would serve as cell-free reference electrodes.
(3) ECR mode 3 is used for monitoring electrical voltage signals between
impedance-measurement electrode structure 3003 (or 3004) and an externally
applied gold reference electrode (not shown on Figure 30) inserted into the
well by connecting an impedance-electrode connection pad (3009 or 3010)
and the external gold electrode to a voltage amplifier. For this mode,
extracellular signals from impedance-measurement electrodes are measured
with the external gold electrode used as "reference electrode". Such external
CA 02760941 2011-11-03
WO 2010/129725 PCT/US2010/033801
gold electrode will not have any cardiomyocytes attached and would serve as
cell-free reference electrodes.
Figures 31 and 32 are examples of impedance measurement and extracellular
.. recording of Cor. At cardiomyocytes. Cor. At cells were prepared and
cultured using
the same method as those described in Example 1. The data on Figures 31 and 32
is
for the Cor. At cells on day 5 after cell seeding into the electrodes-
containing wells.
Figure 31 shows the impedance beating result obtained for the Cor. At cells
for ¨ 12
seconds with time-solution about 28.8 ms per data point. The impedance data is
represented with cell index, a dimension less parameter. Figure 32 shows the
ECR
voltage signals for the three different recording modes described above,
including
ECR electrode vs impedance electrode-structures, ECR electrode vs external
gold
electrode and impedance electrode-structure vs external gold electrode. The
voltage
amplifier and other ECR details are the same as those discussed in Example 1.
In
.. brief, the multichannel extracellular recording was carried out using WPI
ISO-
DAM8A (eight channel module) amplifier (World Precision Instruments, Sarasota,
USA) and Dataq DI-0720 data acquisition interface (Dataq Instrument Inc.,
Akron,
USA). The extracellular field potential data were collected with following
parameters
being used: voltage signal gain: 10,000; Low cut filter: 1 Hz; High cut
filter: 1 K Hz;
Sampling rate: 2-5 K Hz.
81