Note: Descriptions are shown in the official language in which they were submitted.
CA 02761360 2011-12-08
1
A CATHETER DEVICE
This application is a divisional of Canadian Application No. 2,451,356 filed
June 28, 2002
Field of the Invention
The present Invention relates to an elongated tubular catheter member for
draining bodily
fluids, e.g. from the bladder.
Background of the Invention
Catheters for draining the bladder are increasingly used.for Intermittent as
well as
Indwelling or permanent catheterisation. Typically catheters are used by
patients suffering
from urinary Incontinence or by disabled individuals like para- or
tetrapleglcs who may
have no control permitting voluntary urination and for whom catheterisation
may be the
way of urinating.
.Catheterisation is thus Increasingly. becoming a daily-life procedure
significantly. Improving
quality of life for a large group of patients.
Typically, catheters are designed for one-time use and accordingly the costs
for producing,
packing and sterilising a catheter Is an Important Issue. Existing catheters
are made from a
single piece of a continuous catheter tube. Typically, the thickness of the
catheter tube Is
constant throughout its length.
The length of the catheter enables Insertion of a certain length Into the
urethra until urine
starts to flow. At this point, a certain over-length of the catheter should be
available. The
over-length supports for the user to firmly hold the catheter and to guide the
urine to a
place of disposal and to withdraw the catheter safely and without any risk of
the catheter
disappearing Into the urethra.
Existing catheters are designed to minimise the risk of sores in the mucous
membrane and
to give substantially no sensation of pain during Insertion. Accordingly known
catheters are
typically provided with a smooth and slippery surface optimised for safe and
comfortable
insertion Into the urethra. Therefore, it may often be difficult, not least
for the disabled
user, to handle the catheter by manipulation of the slippery over-length.
It Is Important that the tubular member does not collapse or kink and thereby
blocks the
passage for the urine to drain through the catheter. Existing catheters are
therefore
typically made from a form stabile and relatively hard but still bendable tube
e.g. made
from PVC, PU or PE. Since the hardness of the tubes is selected relatively
high with the
view to avoid kinking, the catheters may collapse if they are bend with a too
small radius
of curvature.
CA 02761360 2011-12-08
2
Accordingly, existing catheters not only have a considerable length but they
are also
typically packed in an elongate condition. Therefore the existing catheters
may be
troublesome to handle and to bring along, not least for the individuals for
whom
catheterisation Is a daily-life procedure, wherein catheterisation takes place
several times
a day and wherein the used catheters must be disposed via the garbage
collection.
Description of the invention
It is an object of the present invention to overcome the above described
disadvantages of
the known catheters by providing a kit for preparing a catheter for draining a
human
bladder, the kit comprising at least two catheter sections defining a passage
therein, the
sections being adapted to be arranged in such a mutual configuration that the
passages
are joined into one passage and the sections together constitute a catheter of
a length
larger than the length of each individual section and having such a rigidity
that the entire
catheter is manipulatable by manipulation of at least one of the Individual
sections. It is a
further object of the invention to provide an improved method for producing a
urinary
catheter.
Accordingly a catheter is provided which may be foldable, collapsible,
bendable, separable
or in any other way adapted at least for one configuration wherein the
catheter can be
Inserted into urethra or into an artificial urinary canal and one
configuration wherein the
length of the catheter is reduced. As an example, the length may be reduced to
a length in
the range of one half, one third, one fourth or even to one fifth of the
normal required
length including the required over-length for manipulation of the catheter.
The catheter section could be provided in the form of oblong tubular, hollow
sections
wherein the passage is defined Inside the sections or the sections may
comprise an oblong
solid kernel with one or more vanes extending radially from the kernel and
along the entire
length thereof. The vanes thus defines a number of draining passages for
draining urine
between the kernel and a bodily draining passage, e.g. the urethra. The
advantage of
using a passage defined between a solid kernel and a wall of the urethra is
that the flow of
bodily fluid cleans the urethra and thus reduces the risk of Infection
compared with a
traditional catheter, wherein the bodily fluid is drained inside the catheter
Isolated from the
body canal.
A rigidity of substantially the full length of the catheter allows for
manipulation of the
catheter as one uniform catheter tube. Thereby, insertion of the proximal end
of the
catheter may be performed without touching the part of the catheter which is
going to be
CA 02761360 2011-12-08
3
inserted into the urethra. Preferably the catheter Is provided with a bending
moment
defined as the product between E-modulus and moment of inertia of at least 1
MPamm4.
Since the proximal (inserted) end of the catheter, for male individuals, must
pass prostate
In a curved passage, the proximal end portion of the catheter, e.g. the first
10-50 mm.,
such as 20-40 mm., such as 25-35 mm, such as the first 30 mm. of the catheter
may be
provided with an even lower bending moment defined as the product between E-
modulus
and moment of inertia of less than e.g. 0,6 MPamm4 or even less than 0,3
MPamm4. Other
parts of the catheter, e.g. a distal end portion where the urine Is drained
into the lavatory,
a bag or similar place of disposal, may similarly be provided with a reduced
bending
moment.
The cross-sectional flow area or the hydraulic radius defined as the ratio of
the cross-
sectional flow area to the wetted perimeter, may be selected independently
upon the
length, e.g. on the-basis-of-the-size of the urethra, which size depends on
the-individual
using the catheter. Each of the sections may have either the same cross-
sectional flow
area or hydraulic radius or each section may have individual cross-sectional
flow areas or
hydraulic radiuses. However, at least one part of one section should have a
cross-sectional
shape and size adapted for the size of urethra or an artificial urinary canal.
Similarly one
section should preferably have a length selected on the basis of the length of
the urethra
or the urinary canal. Thereby it may be achieved that only one section Is to
be inserted
and therefore no transition between sections needs to be Inserted. However,
especially for
male individuals where urethra is particularly long, a catheter having an
Inserted length
divided In two sections or more may be provided. In this specific case it will
be appropriate
to provide a transition between the sections which at least on the outer
surface of the
catheter have substantially no recess or sharp edge.
Preferably, at least one of the catheter sections Is provided in a length in
the range of 50-
90 mm., such as in the range of 55-85 mm., such as In the range of 60-80 mm.
such as
with a length in the size of 70 mm. which length has been found to be a
suitable Insertable
length for most female Individuals. For male Individuals, catheter sections
may preferably
be provided In a length in the range of 180-250 mm., such as In the range of
190-240
mm., such as in the range of 200-230 mm. such as in the size of 220 mm. For
the male
individuals it may further be preferred to provide at least a part of the
inserted end of the
catheter in a material or in dimensions so that a the tube becomes very
flexible, without
kinking. This will easy the passage of the catheter past prostate.
The outer cross-sectional shape of at least one of the sections should
preferably be
substantially circular with a cross-sectional area in the range of 0,5 mm2 -
30 mm2.
CA 02761360 2011-12-08
4
Even more preferred is to provide at least one of the sections with a
hydraulic radius
("cross-sectional area"/"circumferential length") In the size of 0,2-1,5 mm..
Alternatively,
at least one of the sections should have a cross-sectional shape matching the
shape of
urethra or an artificial urinary canal, still with a cross-sectional area in
the range of 0,5
mm2 - 30 mmZ. or a hydraulic radius in the size of 0,2-1,5 mm. However, the
other of the
sections does not necessarily have to have the same cross-sectional shape, nor
the same
hydraulic radius. The wall thickness of the catheter should preferably be in
the range
between 0,5-1,5 mm.
The catheter or at least a part of the catheter could be made from a
thermoplastic
elatomeric material, other thermoplastic materials, curable elastomeric
materials,
polyamide resins or elastomers or any mixture thereof, i.e. the group may
comprise
materials like, PVC, PU, PE, latex, and/or KratonTm.
According to a preferred embodiment, the present invention relates to a
urinary catheter
divided Into completely separated catheter sections. Each catheter section has
at least one
end provided with means for connecting the section with another section
corresponding to
an adjacent part of the catheter. As an example the catheter may be divided
Into two
tubular connectable pieces connected by connecting means.
Preferably, the connecting means are provided with a rigidity allowing for
manipulation of
at least one of the catheter sections by manipulation of one of the other
catheter sections.
At least the connection between each of the pieces should provide sufficient
rigidity to
allow one proximal section to be inserted into the urethra by manipulation of
one of the
other sections. Therefore, the connection is preferably provided so that at
least the part of
the catheter extending the connection zone, has a bending moment defined as
the product
between E-modulus and moment of inertia of at least 0,6 MPamm4 such as at
least 1
MPamm4. In order not to have the Individual sections failing apart during use,
the
connection should preferably be adapted to take up an axial force of at least
0,5 Newton or
at least to take up an axial force larger than the axial force required for
withdrawal of the
catheter from the urethra or artificial urinary canal.
The pieces may be connected e.g. telescopically or via a hinge enabling one of
two sections
to rotate in relation to the other of the two sections. It Is appreciated that
the sections are
in fixed engagement so that they do not disconnect during use of the catheter,
while urine
Is drained through the catheter. However, since the urine is always drained in
one
direction, the connection does not necessarily have to be liquid-tight. As an
example a
telescopic connection may be established by inserting the section adapted for
insertion into
urethra into a distal section. The flow direction of the urine will at least
substantially
CA 02761360 2011-12-08
prevent the connection from leaking even though the connection as such is not
completely
liquid tight. However, a completely sealed connection may provide an even
safer catheter
with a reduced risk of contaminating hands etc.
5 In one embodiment wherein the two catheter sections are arranged in a
telescopic fashion,
a first one of the catheter sections may be intended for Insertion into the
human urethra,
whereas a second one of the catheter sections is usually intended for forming
a
prolongation of the catheter outside the human urethra during use of the
catheter. In use,
that is in the first mutual configuration of the two catheter sections, the
second catheter
section preferably coextends with the first catheter section away from a
distal end of the
first catheter section. In the second mutual configuration, which usually is
the
configuration in which the telescopic kit is stored and transported, at least
a portion of the
first catheter section may be surrounded by the second catheter section. In
the second
mutual configuration, a tubular protective member may be provided between an
outer
surface of the first catheter section and an inner wall of the second catheter
section. The
dimensions of the tubular protective member and the catheter sections may be
such that,
in the second mutual configuration, a substantially annular and longitudinally
extending
cavity is formed between an outer surface of the first catheter section and an
Inner wall of
the second catheter section. The first catheter section may have a hydrophilic
surface, and
a liquid swelling medium may be provided in the annular cavity, so as to swell
the
hydrophilic surface of the first catheter section, whereby the first catheter
section being
encapsulated in the tightly sealed annular cavity may be preserved In its wet,
swelled
condition for a period of 1-5 years, such as 3-5 years, or more. A tight
sealing of the
annular cavity is desired for all kinds of catheter surfaces, Including
hydrophilic and
hydrophobic catheter surfaces, in order to prevent contamination to enter into
the cavity.
Thus, in the second mutual condition, the telescopical joint may serve to
define a liquid
and contamination tight seal between the second catheter section and an
ambient
atmosphere.
A distal end of the second catheter section Is preferably provided with a
tight seal which
may be tight to both liquids and contamination, and which may be removable, so
that
when a distal end of the second catheter section is Inserted into, e.g., a
urine collection
bag, a passage for urine is formed at the location from which the seal has
been removed.
The tubular protective member is preferably removable when the first and
second
catheter sections are In the first mutual position, so that, when the tubular
protective
member Is removed, the proximal end portion of the first catheter section is
exposed and
ready for insertion into the human urethra. The distal end of the second
catheter section
may as an alternative be provided into one piece with a collection bag. As an
example, the
CA 02761360 2011-12-08
6
second catheter section may be provided with a plastic welding-flange for
adhesively
bonding a plastic collection bag to the second catheter section.
According to another preferred embodiment, the catheter may comprise at least
two
sections not being separated but being divided by a bendable zone. The
bendable zone
could e.g. be a bellow shaped section or the zone could be an area wherein the
thickness
of the tubular material is smaller and wherein the zone accordingly has a
lower bending
moment. The zone could e.g. be provided in a more resilient or flexible
material allowing
for bending the catheter tube without kinking or damaging the tube.
In general, the problems of introducing a catheter into urethra depend not
only of the size
of the introduced part of the catheter but also on the slipperiness of the
introduced part.
The catheter section or at least a part of the catheter section or sections
adapted for
insertion into urethra or an artificial urinary canal- may provide a surface
slipperiness for
easy and safe Insertion. However, it has been found that lubricated or
slippery surfaces,
are difficult to handle, not least for a user having reduced dexterity. It is
therefore an
object of the present invention to provide a catheter with an inserted part
being treated so
as to provide a slippery surface and another part not being treated, so as to
provide a
surface which may easily be handled. The division of the catheter Into one
part being
treated and one part not being treated may preferably follow the
aforementioned division
of the catheter with the purpose of making the catheter collapsible or
separable. According
to an alternative embodiment, the parts may be provided In the form of one
part being
smooth and another part being provided with a rough surface.
According to a preferred embodiment, at least one of the sections is provided
with gripping
means easing a firm grip In the catheter. Not least for the disabled user, the
gripping
means will improve the value of the catheter considerably. Gripping means may
be
provided as a radially extending flange or flanges or as a zone having a large
outer cross
sectional diameter. The catheter, or at least one of the catheter sections,
may also be
provided with means for engaging an external handle. As an example, one of the
tubular
catheter tubes may be provided with a ring-shaped bulge for attaching a
handle. The ring-
shaped bulge could be provided as a short tubular piece of plastic with a
larger radial size
than the catheter, the catheter being inserted and glued into the short piece
of plastic.
A section provided with a hydrophilic surface treated with a liquid swelling
medium may
provide an excellent lubrication for the Insertion and also provide
compatibility with the
body tissue. It is therefore a further preferred embodiment of the invention
to provide at
least one of the sections with a hydrophilic surface layer.
CA 02761360 2011-12-08
7
One of the catheter sections could be used as a sterile package for the other
sections, e.g.
by arranging the sections in a telescopic manner Inside one section, dosing
and sealing
that section In both ends, e.g. by a peelable and optionally a metallised foil
e.g. made from
a thermoplastic elatomeric material, other thermoplastic materials, curable
elastomeric
materials, polyamide resins or elastomers or any mixture thereof, i.e. the
group may
comprise materials like, PVC, PU, PE, latex, and/or Kratonm, thereby allowing
for sterilising
the assembly by radiation.
The liquid swelling medium for the hydrophilic surface may be provided in the
package for
initiation of the low friction character already when the catheter is being
packed. The liquid
swelling medium may simply be a saline solution, a bactericidal solution
capable of
swelling the hydrophilic surface and capable of keeping the surface in a
sterile condition or
it may be pure water. The swelling may also be initiated already before
packaging of the
catheter, the catheter then being packed in a substantially gas impermeable
package for
conservation of the moistened surface. Furthermore, the liquid swelling medium
may be
provided In a capsule or container packed together with the catheter for
swelling of the
hydrophilic material immediately prior to the insertion.
According to a second aspect the present invention relates to a bendable
urinary catheter
for draining a human bladder comprising:
- a flexible elongated tube with an inner cross-sectional shape and size
defining a
first conduit for draining urine, said tube having an insertion end and a
discharge end, and
- a supporting member being introduced into the first conduit and provided
with
an outer cross-sectional shape and radial size substantially equal to the
inner
cross-sectional shape and size of the elongate tube so as to support said tube
against collapsing during bending of the tube, the supporting member having a
flexibility allowing curling.
The flexible elongated tube could have the shape of a regular catheter of the
known kind.
Preferably, the tube or at least a part of the tube is made from a
thermoplastic elatomeric
material, other thermoplastic materials, curable elastomeric materials,
polyamide resins or
elastomers or any mixture thereof, i.e. the group may comprise materials like,
PVC, PU,
PE, latex, and/or KratonTM.
The supporting member supports the catheter to avoid collapsing when the
catheter is
bend, e.g. for the purpose of packing the catheter in user friendly short
packages. The
supporting member may be either solid or the supporting member may be hollow
and thus
CA 02761360 2011-12-08
8
defining a second conduit. The solid supporting member should be adapted for
removal
prior to draining of the bladder, whereas a hollow supporting member may
remain Inside
the tube while the bladder is emptied through the first and second conduit.
The supporting member may as an example be glued inside the elongated tube or
the
supporting member may even be moulded Into the tube during the process of
producing
the tube. The supporting member may even be completely integrated in the
elongated
tube.
The supporting member could be made from any suitable material such as e.g.
plastic,
steel, aluminium, a thermoplastic elatomeric material, other thermoplastic
materials,
curable elastomeric materials, polyamide resins or elastomers or any mixture
thereof. As
an example, the supporting member may be a helical spring provided in a length
in the
range of 20-60 mm, such as. In the, range of 30-50 mm., such asin .the range
of 35-45
mm. The spring should be positioned inside the elongated tube in the zone
where it is
desired to bend the catheter, e.g. midway along the longitudinal axis of the
elongated
tube. During use, the urine is drained through the first conduit of the
elongated tube and
past the supporting member through the second conduit.
According to a preferred embodiment, the supporting member is provided in a
length in
the range of 60-120 mm, such as in the range of 70-110 mm., such as In the
range of 80-
100 mm. and the supporting member may even be extending out of the discharge
end of
the elongated tube. This will enable the user to remove the supporting member
during the
process of inserting the catheter into urethra.
According to a further preferred embodiment, the supporting member is provided
with
gripping means for easing withdrawal of the supporting member from the
discharge end
during insertion of the catheter.
In a third aspect, the present Invention provides a kit for preparing a
catheter for draining
a human bladder, the kit comprising at least two catheter sections defining a
longitudinally
extending passage therein, the sections being arranged in a coextending
fashion with a
tubular protective member surrounding a first, proximal one of said catheter
sections, the
kit further comprising a joint for interconnecting the first and the second
catheter section,
the joint defining a liquid tight seal at a proximal end of a substantially
annular and
longitudinally extending cavity provided between the proximal end portion of
the first
catheter section and an inner wall of the tubular protective member, the
tubular protective
member being removably connected to the joint and/or to the second catheter
section, so
CA 02761360 2011-12-08
9
that, when the tubular protective member is removed, the proximal end portion
of the first
catheter section Is exposed and ready for insertion into the human urethra.
The passage may be defined either inside the hollow, tubular catheter sections
or between
a solid kernel and the wall of the urethra or similar bodily channel.
The discussion set forth above in connection with the first aspect of the
invention of
features of embodiments wherein the first and second catheter sections are
arranged in a
telescopic fashion also apply to the kit of the third aspect of the Invention.
Thus,
embodiments of the kit according to the third aspect of the Invention may be
regarded as
modifications of telescopic embodiments of the kit according to the first
aspect of the
Invention, the modification comprising that a longitudinal movement of the two
catheter
sections relative to each other Is usually not Intended and that only one
mutual
configuration is usually intended. Further, all elements and features
discussed above In
connection with the. first aspect of the invention may be provided in the kit
according to
the third aspect of the Invention, to the extent that such features and
elements are
appropriate In the catheter according to the third aspect of the invention.
In particular, the catheter according to the third aspect may be provided so
that the
sections are adapted to be moved between at least two positions with respect
to each
other. One position being a position wherein the second section surrounds the
first section
and the other position being a position wherein the second section forms an
extension for
the first section.
Preferably, the joint between the first section and the second section is a
telescopical joint
providing a liquid tight seal between the sections while the they are moved
between the
first position and the second position. As an example, the first section may
be provided
with a piston seal adapted to slide along the Inner surface of the second
section while the
first section is being pulled out of second section between the first and
second position.
In order to allow the user to insert the first section into a body canal, it
will be appreciated
to have a locking arrangement of the first and/or the second catheter section
for locking
the position of the first section with respect to the second section, when the
sections are In
the second position, i.e. when the catheter Is in a configuration ready for
insertion into the
body canal.
In order to allow the user to pull the first catheter section out of the
second catheter
section without touching the insertable part of the catheter, the tubular
protective member
may preferably be provided to engage the first catheter section in a locking
engagement.
Thereby, it will be allowed to use the tubular protective member to pull the
first catheter
section out of the second catheter section.
CA 02761360 2011-12-08
When the first catheter section has been pulled out of the first catheter
section, i.e. when
the sections are in the second position, i.e. In the position wherein the
second catheter
section forms an extension for the first catheter section, the tubular
protective member
5 should be allowed to disengage the first catheter section. When the tubular
protective
member has been removed, the catheter is in a "ready to Insert" state.
In order to use the second catheter section as a sealing envelope or package
for the first
catheter section, i.e. for the Insertable catheter section, the distal end of
the first catheter
10 section may preferably be adapted to seal an opening in a distal end of the
second
catheter section while the sections are In the first position and not to seal
set opening
when the sections are in the second position. When the sections are brought
Into the
second position, i.e. when the catheter is "ready for insertion", the opening
In the distal
end of the second section may be used for draining the bodily liquids, e.g.
urine out of the
catheter.
In order to allow the annular cavity to be used e.g. for carrying a frictional
reducing
substance, e.g. a water or saline solution for a hydrophilic catheter, a
hydrogel or similar
lubricating substance, the kit may preferably be provided with a sealing
engagement
between the tubular protective member and the first catheter section when the
tubular
protective member is engaging the first catheter section. When the tubular
protective
member is disengaged from the first catheter section, i.e. after the catheter
has reached
its "ready for Insertion state", the the annular cavity is open to the ambient
atmosphere
thus exposing the insertable tip of the first catheter section and allowing
the user to drain
surplus friction reducing substances.
According to a preferred embodiment, the first catheter section is provided
with a
hydrophilic surface and the friction reducing substance provided in the
annular cavity is a
liquid swelling medium, e.g. water or a saline solution.
In a fourth aspect, the Invention provides a method for producing a urinary
catheter
comprising a proximal insertion section defining an inner elongated passage
for urine, and
at least one opening near a proximal end of the proximal Insertion section for
allowing
urine to pass from the human bladder into the inner elongated passage, the
method
comprising the steps of:
- providing a mould, defining the shape of at least the proximal Insertion
section,
- forming the proximal insertion section by injection moulding,
- removing the proximal insertion section from the mould.
CA 02761360 2011-12-08
11
Whereas longitudinally extending catheters made from plastics materials have
hitherto
been manufactured by a relatively costly process involving extruding the
catheter body,
forming a rounded tip thereof by heat treatment, cutting transversely
extending passages
for urine near the tip of the catheter by means of a cutting tool, and
rounding edges of the
transversely extending passages by heat treatment, the method according to the
fourth
aspect of the invention has the advantage that it allows for a more efficient
and more
accurate controllable manufacturing process with less waste of material and
fewer
production steps.
The catheter may further comprise a connector part for connecting the proximal
Insertion
section to a further catheter section or to a urinary collection bag. The
connector part may
be made from the same material as the proximal insertion section, whereby, at
the step of
forming the proximal insertion section, the proximal Insertion section and the
connector
part may be formed substantially simultaneously. Alternatively, the connector
part may be
made from a material different from the material of the proximal insertion
section,
whereby the connector part and the proximal insertion section are formed in
distinct
process steps, for example in a multi-component Injection moulding process.
Brief description of the drawings
Preferred embodiments of the Invention will now be described in details with
reference to
the drawing In which:
Fig. 1 shows a catheter kit according to the present invention,
Fig. 2 shows the catheter kit of Fig. 1, assembled Into a configuration for
use,
Fig. 3 shows a "Swiss-knife" embodiment of a catheter kit according to the
present
Invention,
Fig. 4 shows the catheter kit of Fig. 3, unfolded and arranged In a
configuration for use,
Fig. 5 shows a collapsed catheter provided with a reinforcement sleeve,
Fig. 6 shows the catheter kit of Fig. 5, unfolded and in a configuration for
use,
Fig. 7 shows an embodiment of the kit, wherein one catheter part is Inserted
for storage
into another of the catheter parts thus substituting a catheter package,
CA 02761360 2011-12-08
12
Fig. 8 shows the embodiment of Fig. 7, wherein the inserted catheter part is
partially
withdrawn from one end of the package,
Fig. 9 shows the embodiment of Figs. 7 and 8, wherein the inserted catheter
part is
completely withdrawn from the package and then attached to the other end of
the
package, the package thus functions as a handle for manipulation of the
catheter.
Fig. 10 shows a folded telescopic catheter kit,
Fig. 11 shows the catheter kit of Fig. 10, in an extended configuration,
Fig. 12 shows the catheter kit of Fig. 10, unfolded and after withdrawal of
the combined
closure and withdrawal cap,
Fig. 13, shows a preferred embodiment of a combined closure and withdrawal cap
for the
kit shown in Figs. 11 and 12,
Fig. 14 shows yet another preferred embodiment of a combined closure and
withdrawal
cap for the kit shown in Figs. 11 and 12,
Figs. 15-18 illustrate an embodiment of a kit according to the invention,
wherein the
catheter sections are arranged in a telescopic fashion,
Figs. 19-22 show a further embodiment, wherein the catheter sections are
arranged in a
telescopic fashion,
Fig. 23 shows a kit wherein a distal part of the catheter is curled over
inserted part of the
catheter so as to protect the inserted part of the catheter.
Fig. 24 shows a bendable catheter with a supporting member,
Fig. 25 shows a catheter part provided with gripping means for easing the
handling of the
catheter,
Fig. 26 shows a preferred cross-sectional shape of a catheter part adapted for
insertion
into the urethra,
Fig. 27 shows a catheter produced by the method according to the invention,
and
CA 02761360 2011-12-08
13
Fig. 28, 29 and 30 shows an embodiment of a catheter section wherein the
passage is
defined between a solid kernel and the wall of a bodily channel such as the
urethra.
Detailed description of the drawings
S
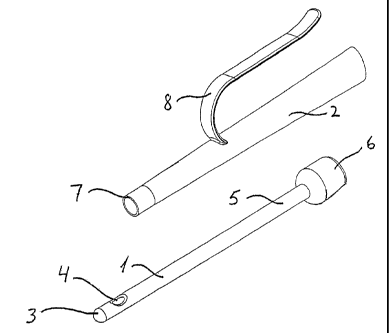
Referring to Fig. 1, a catheter kit according to the. present invention
comprises a first
elongate tubular catheter section 1 adapted for insertion into urethra or an
artificial urinary
canal and a second elongate tubular catheter section 2 adapted for
manipulation of the
catheter. At the proximal end 3, the tubular catheter section is provided with
holes 4
enabling urine to drain into the tubular member. In order to protect the
mucous
membrane, the holes may preferably be provided on the side of the tubular
member.
Alternatively, a tubular member may be provided with a hole In the tip. It Is
important that
the edge of the hole is rounded smoothly or that the material, for at least
this part of the
-tubular-mem ber-,4s--selected_with=the-. view-not.-to..cut-or-damage-urethra,-
i..e.-e..g.-a.soft
resilient rubber material.
At the distal end 5, the tubular member is provided with connecting means 6
for
connecting the catheter section to mating connecting means 7 of the second
tubular
catheter section. Preferably, the first and the second section is made from a
thermoplastic
elatomeric material, other thermoplastic materials, curable elastomeric
materials,
polyarrmide resins or elastomers or any mixture thereof, i:e. the group may
comprise
materials like, PVC, PU,.EE,1'atex, Kratbnm, PTFE (TefloriTM), FEP, Sil'pxane
(silicone rubber),
and/or FEP.
Fig. 2 shows a view of the assembled catheter. The second catheter section is
adapted to
elongate the first catheter section so that the first and the second sections
together form a
rigid catheter having sufficient' length to enable catheterisation. The
rigidity of the-first
section should be sufficient to allow the section to be inserted into urethra
without
collapsing the section. The second section and the connection 6,7 - as shown
in Fig. 1 -
between the second section and first section is provided with a rigidity that
allows the
insertion of the first section by manipulation of the second section. As seen
in Figs. 1 and
2, the catheter may preferably have gripping means 8 for easing a firm grip
and
manipulation of the catheter. In the embodiments of Figs 1 and 2, the kit may
preferably
be packed in a sterile package.
As Indicated in Fig. 2, the kit may comprise one handle section and a number
of catheter
sections adapted for insertion or the kit may alternatively be packed in two
packages - one
containing a handle for multiple use and another separately steriliseable
package
containing one or more sections adapted for insertion and for one-time use.
The sections
CA 02761360 2011-12-08
14
may as an example be packed in manner similar to cartridges in a revolver or
in a
cartridge belt, i.e. Interconnected to form a long row or tube of sections.
Fig. 3 shows a "Swiss-knife" embodiment of the catheter kit. The first
catheter section 10
is folded into a slid 11 in the second catheter section 12. The first catheter
sections being
rotatably hinged to the second catheter section in the hinge connection 13.
Fig. 4 shows the "Swiss-knife" embodiment unfolded. The slid 11 could as an
example be
covered with a thin latex foil, so as to seal the second catheter section.
When the catheter
is folded, the first catheter section will simply fold the latex foil radially
Inwardly Into the
second catheter section. As the catheter is unfolded, the elasticity and a
slight pretension
of the foil will lift the foil out of the slit and thereby provide free
passage for urine to drain
through the second catheter section. The latex foil is not shown in the Figs.
3 and 4.
Fig. 5 shows an embodiment of the invention wherein a catheter is simply bend,
whereby
the catheter Is divided into a first catheter section 14 and a second catheter
section 15 by
a collapsed catheter part 16. The catheter is provided with a reinforcement
sleeve 17. The
connector 18 enables connection of the catheter e.g. to a bag for collecting
the urine.
Fig. 6 shows the unfolded catheter of Fig. 5. The sleeve 17 has now been
displaced along
the catheter so as now to support the catheter around the collapsed part 16 of
the
catheter.
Fig. 7 shows an embodiment of the catheter kit according to the present
invention,
wherein the first catheter section, not shown in Fig. 7, is sterilely packed
inside the second
catheter section 21, the second catheter section being seated In both ends
with sealing
caps or foils 22,23.
Preferably the First section Is coated with a hydrophilic coating, providing a
low friction
surface of the first catheter section when treated with a liquid swelling
medium. The
coating could be of the kind which sustains being activated with the liquid
swelling medium
for longer time, e.g. for several month. Thereby the liquid swelling medium
could be
provided in the catheter package from the time of packaging so as to provide a
ready-to-
use catheter. Hydrophilic coatings are known per se, see e.g. the published
patent
applications WO 98/58988, WO 98/58989, WO 98/58990 or EP 0570370. For this
purpose,
the sealing caps or foils should preferably be provided in a gas impermeable
material for
conservation of the humidity and thus the lubricity of the catheter for longer
time, e.g. for
several month. As an example, the second catheter section and/or the sealing
caps may be
made from a thermoplastic elatomeric material, other thermoplastic materials,
curable
CA 02761360 2011-12-08
elastomeric materials, polyamide resins or elastomers or any mixture thereof,
i.e. the
group may comprise materials like, PVC, PU, PE, latex, and/or KratonT. The
caps may be
provided with a thickness allowing for sufficient gas impermeability. As an
alternative, they
may be made from metallised foils.
5
As seen in Fig. 8, the first catheter section is easily withdrawn from the
second catheter
section by pulling the cap or foil 23 which cap or foil engages the distal end
of the first
catheter section.
10 Fig. 9 shows the assembled catheter after the first catheter section has
been attached to
the second catheter section. The foil or cap 23 can either be removed
completely as shown
in Fig. 9 or can at least be penetrated by the connecting means 24 of the
second catheter
section.
15 Fig. 10 shows an embodiment of the catheter kit wherein the first and the
second catheter
sections are connected telescopically. The first catheter section is sterilely
packed Inside
the second catheter section 26. The second catheter section being sealed by a
first sealing
closure 27 and a second sealing closure 28. Prior to use, the first sealing
closure is
removed. If the first catheter section is provided with a hydrophilic surface
layer, and if the
catheter section is packed with a liquid swelling medium, the liquid medium
may be
emptied through the passage opened by the first sealing closure. As best seen
In Fig. 11,
the second sealing closure engages the first catheter section 30 for easy
withdrawal of the
first catheter section. When the first catheter section has been completely
withdrawn, the
distal part of the first catheter section engages the proximal end of the
second catheter
section in the connecting zone 31 and the second sealing closure easily
disengages the
first catheter section. The catheter is then in a configuration for use.
Fig. 13 shows a preferred embodiment of the second sealing closure 28, wherein
the
closure is provided with internal and radially inwardly extending projections
33 adapted for
engaging the hole 32 shown in Fig. 12.
Fig. 14 shows another embodiment of the second sealing closure 28, wherein
flexible
gripping flanges 34 softly grips the proximal (inserted) end of the first
catheter section for
easy withdrawal of the first catheter section from the second catheter section
upon
removal of the second sealing closure.
The telescopic embodiment of the catheter kit, disclosed in Figs. 10-14,
should preferably
be provided so that the internal diameter of the second catheter section Is
slightly larger
than the external diameter of the first catheter section. This Is an
advantage, e.g. in the
CA 02761360 2011-12-08
16
case where the first catheter section is coated with a hydrophilic surface
coating and in
order not to scrape of the coating when sliding the first catheter section out
of the second
catheter section. On the other hand, it Is an Important aspect to provide a
connecting zone
wherein the first catheter section and the second catheter section firmly
engages. Thereby,
insertion and orientation of the first section is possible merely by
manipulation of the
second section and without the sections mutually sliding in the telescopic
connection. It is
furthermore important to assure that the first catheter section does not slip
out of the
second section in which case the first catheter section might disappear into
the urethra.
For this purpose, the distal end (opposite the inserted end) of the first
catheter section
may be provided with a radially outwardly extending flange disallowing the
first catheter
section to slip out of the second catheter section.
Figs. 15-18 illustrate a second embodiment of a catheter kit wherein the first
and second
sections 42, 44 are telescopically interconnected. A tubular protective member
46
surrounds a portion of the first catheter section 42 and forms a substantially
annular cavity
48 around the first catheter section. In the second mutual configuration,
shown in Fig. 16,
in which the kit is intended to be stored and shipped, the first catheter
section 42 and the
tubular protective member 46 are inserted as far as possible into the second
catheter
section 44. A hydrophilic swelling medium, such as water, may be provided in
the cavity
48, so that a hydrophilic surface coating optionally provided at the surface
of the first
catheter section 48 is stored in Its swelled, i.e. wet condition. A surplus of
hydrophilic
swelling medium may be present in the cavity 48 In order to prevent the
hydrophilic
surface coating from drying out. A liquid-tight seal 50 is provided at the
distal end of the
first catheter section 42. A liquid-tight closing member 52 closes the distal
end of the
second catheter section 44. In one embodiment, the closing member 52 is
removable so
that a passage Is provided between the second catheter section 44 and a urine
collection
bag, or another device for accumulating or conveying urine, mounted to the
distal end of
the section catheter section 44, when the closing member 52 Is removed. In
another
embodiment, the closing member 52 is an integrated part of the second catheter
section
44, in which case a wall 53 of the closing member 52 may be perforated in
order to
provide a passage between the second catheter section 44 and a urine
collection bag, or
other device for accumulating or conveying urine, mounted to the distal end of
the section
catheter section 44. In yet another embodiment, the closing member 52 may be
substituted by a perforated end wall, e.g. a wall made from a central plate
connected to
the outer wall of the second catheter section 44 at its distal end by means of
radially
extending ribs or spokes. In such an embodiment, the first catheter section 42
and the
seal 50 may be formed as a single, integrated piece.
CA 02761360 2011-12-08
17
As shown In Fig. 16, an outer wail of the second catheter section 44 forms a
handle, the
tubular protective member 46 being arranged so that It extends out of the
handle at the
proximal end thereof. The tubular protective member 46 may form a flange at
Its proximal
end, so as to facilitate a user's extraction of the first catheter section 42
and the tubular
protective member 46 out of the handle/second catheter section 44. When
extracted, the
tubular protective member 46 and thus the first catheter section 42 surrounded
thereby
coextend with the handle or second catheter section 44, as illustrated in Fig.
17. A
protrusion 47 at the distal end of the tubular protective member 46 releasably
secures the
tubular protective member 46 to the seal 50, see Figs. 15, 17 and 18. The seal
50 may be
designed so that it engages the proximal end portion of the second catheter
section 44 by
a snap action once the seal 50 and the tubular protective member 46 have
reached the
fully extracted position shown in Fig. 17. In the example shown In Fig. 15;
the seal 50 has
a groove 51 which, in the extracted position shown In Figs. 17 and 18 engages
a flange 45
at the proximal end of the second catheter section 44. Immediately prior to
use of the
catheter, the tubular protective member 46 is removed, so that the first
catheter section
42 Is exposed, as shown in Fig. 18.
Figs. 19-22 illustrate a further embodiment of a catheter kit, wherein the
catheter sections
are arranged In a telescopic fashion. As shown in the exploded view in Fig.
22, the kit
comprises the following parts: a first catheter section 62, a second catheter
section 64
with one or more inner flange portions 65, a guide member 66 with protrusions
67, a joint
69 with a collar portion 70 and slits 71 for the guide member 66, as well as a
distal closure
member 72 and a proximal closure member 73. The kit is stored and transported
in the
configuration shown in Fig. 19, wherein the second catheter section surrounds
the first
catheter section 62 and the guide member 66. Prior to use of the catheter, the
distal
closure member 72 is removed, and the guide member 66 is extracted, as shown
in Fig.
20. The guide member 66 is extracted as far as possible, i.e. until the
protrusions 67, due
to their elasticity, engage respective grooves (not shown) provided in the
slits 71 of the
joint 69, see Fig. 22. The joint 69 is secured from sliding out of the second
catheter section
64 by means of the Inner flange portions 65 of the second catheter section 64.
The
proximal closure member 73 is also removed. Next, the guide member 66 is
pushed back
Into the second catheter section 64. As the guide member engages the joint
which is firmly
connected to the distal end of the first catheter section, the joint 69 and
the first catheter
section 62 are pushed out of the distal end of the second catheter section 64
as the guide
member 66 is pushed in the second catheter section 62. When the collar portion
70 of the
joint 69 engages an Inner flange or protrusion provided at the proximal end of
the second
catheter section 64, the kit is ready for use, and the first catheter section
62 may be
Introduced into the urethra of a human. A urine collection bag or other means
for
CA 02761360 2011-12-08
18
accumulating or conveying urine may be mounted to the proximal end of the
second
catheter section. 64.
Fig. 23 shows an embodiment of the catheter kit wherein a second catheter
section
surrounding a first proximal catheter section can be turned inside out thereby
the second
catheter section protects the first catheter section prior to use. By
provision of sealing
foils or caps in both ends, the first catheter section may even-be kept in a
sterile condition
inside the second section. Before use, the second catheter section is turned
inside out by
rolling or curling, whereby the catheter is brought into a configuration- for
use.
Referring to Fig. 24, one aspect of the present Invention relates to a
bendable catheter.
The catheter Is provided e.g. as a soft and flexible plastic hose 35, e.g. at
least partly
made from a thermoplastic elatomeric material, other thermoplastic materials,
curable
._el.astamericinateri.a.ls.,,.poly_amide..resins_or__elastomer-s...or__any-
mixtur-e..there.of.,_i.e...the-_._
1S group may comprise materials like, PVC, PU, PE, latex, Kratonr h, PTFE
(Teflon TM ), FEP1
Slioxane (silicone rubber), and/or FEP. The catheter is provided with a zone
36 allowing
the catheter to bend. The zone may as an example be formed as a below shaped
part of
the catheter. If the catheter is relatively long and if a fairly large part of
the catheter is to
be inserted Into the urethra, which- is commonly the case for male users, it
may be an
advantage-to provide a catheter which has a bendable zone which on the outside
is so
smooth that it may be inserted Into the urethra. For this purpose the
invention relates to a
catheter having a supporting member Inserted into at least the. bendable
zone.. The
supporting. member may be.a piece of an elongate helical spring provided with
a conduit
for draining the urine. The spring will easily provide support for the
catheter so that the
catheter does not collapse. The spring. should be provided with an outer.
diameter as close
to the inner diameter of the catheter hose as possible. As an example, the
supporting
member may be provided as a small piece of a spring, glued inside the catheter
in the
zone adapted to be bend. As another example, -the supporting member may be
provided as
a longer spring 37, extending out through the opening of the catheter In the
distal end
(opposite the inserted end) of the catheter. The supporting member may thereby
be
removed prior to the insertion of the catheter Into the urethra or even
simultaneously with
the insertion of the catheter into the urethra. For this purpose the
supporting member may
be provided with a handle 38.
Fig. 25 shows a handle 40 for easy manipulation of the catheter. The handle
may be highly
appreciated not least for disabled users of the catheter e.g. for people
having a reduced
dexterity.
CA 02761360 2011-12-08
19
Fig. 26 shows a preferred cross-sectional shape of the insertable part of the
catheter. As
the inserted part has an oval cross-sectional shape, the bending moment around
the x-axis
(indicated In Fig. 26) will be different from the bending moment around the y-
axis. The
relatively low bending moment around the y -axis will enhance the ability of
the catheter
to bend in one direction, and thereby easy the insertion of the catheter past
prostate. The
relatively high bending moment around the x - axis will enhance the general
stiffness of
the catheter thereby easing manipulation of the inserted part of the catheter
from the part
of the catheter not being inserted.
Fig. 27 illustrates a catheter produced by the method according to the
Invention, the
catheter having a proximal catheter section 60, at least part of which is
adapted for
Insertion into the human urethra. The catheter section 60 forms one or more
transversal
passages 62, through which urine may flow once a proximal end of the catheter
section 60
Is inserted into the bladder. The section 60 is further provided with a
rounded proximal tip
64 ensuring that the section can be inserted without damaging the membrane of
the
urethra. The catheter section 60 is formed in one single piece by injection
moulding. The
connector part 66 is formed integrally with the catheter section 60 during the
same
moulding operation. The connector part 66 may be adapted for connection of the
section to
a handle section or to a urine bag.
Fig. 28 shows a perspective view of an embodiment of the catheter or a
catheter section,
comprising a solid kernel 281 with one or more vanes 282 extending radially
from the
kernel and along the entire length thereof. The vanes thus defines a number of
draining
passages 283 for draining urine between the kernel and a bodily draining
passage, e.g. the
urethra. The advantage of using a passage defined between a solid kernel and a
wall of the
urethra is, that the flow of bodily fluid cleans the urethra and thus reduces
the risk of
infection.
Fig. 29 shows a side view of the catheter section shown In Fig. 28. Fig. 30
shows a top
view of the catheter shown in Figs. 28 and 29. Fig. 30 shows the solid part
281 connected
to a number of vanes 282. The vanes are again connected to a connector 302 via
a
number of spokes 301. A number of openings 303 are formed between the solid
catheter
part 281 and the connector 302. Except from the fact that the passage Is
defined between
a solid core and the wall of the bodily channel and not inside a hollow
tubular body, the
catheter section of Figs. 28-30 corresponds to the catheter section 42 of ig.
15. The
openings 303 correspond to the perforations of the catheter section of Fig.
15. The
openings are provided In order to allow a friction reducing substance to drain
out of the
cavity 48 defined between the first and second catheter section, c.f. Fig. 15.
The spokes
301 may preferably be formed with due regard to fluid dynamic aspects in order
to allow
CA 02761360 2011-12-08
fluid to drain passed the spokes without causing turbulence and without
spreading the
fluid. Spokes with a cross-sectional shape of a rhombus arranged with the
longest leg
parallel to the flow-direction, will support a substantially undisturbed flow
passed the
spokes.