Note: Descriptions are shown in the official language in which they were submitted.
CA 02764968 2016-09-07
Functionally Graded Coatings and Claddings for Corrosion and
High Temperature Protection
[moll This application claims priority to U.S. Patent Application No.
61/186,057, filed June
11, 2009, titled Functionally Graded Coatings and Claddings for Corrosion and
High
Temperature Protection.
[0002] A process for depositing functionally graded materials and structures
is described for
manufacturing materials that possess the high temperature and corrosion
resistant
performance of ceramics and glasses, while at the same time eliminating the
common
mismatches encountered when these are applied to structural metal or composite
substrates.
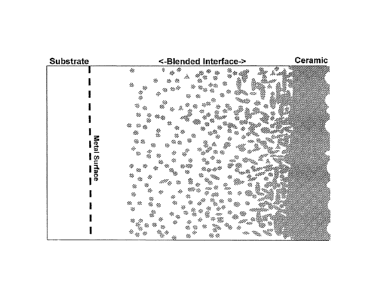
An example of the structure of a functionally graded coating is shown in FIG.
1. An example
of the functionally graded coating structure applied to a pipe is shown in
FIG. 2.
[0003] Electrolytic deposition describes the deposition of metal coatings onto
metal or other
conductive substrates and can be used to deposit metal and ceramic materials
via electrolytic
and electrophoretic methods. Electrodeposition which is a low-cost method for
forming a
dense coating on any conductive substrate and which can be used to deposit
organic primer
(i.e. "E-coat" technology) and ceramic coatings.
[0004] The embodiments described herein include methods and materials utilized
in
manufacturing functionally graded coatings or claddings for at least one of
corrosion,
tribological and high temperature protection of an underlying substrate. The
technology
described herein also is directed to articles which include a wear resistant,
corrosion resistant
and/or high temperature resistant coating including a functionally-graded
matrix.
[0005] One embodiment provides a method which will allow for the controlled
growth of a
functionally-graded matrix of metal and polymer or metal and ceramic on the
surface of a
substrate, which can corrode, or otherwise degrade, such as a metal.
[0006] Another embodiment provides a method which includes the electrophoretic
deposition
of controlled ratios of ceramic pre-polymer and atomic-scale expansion agents
to form a
ceramic (following pyrolysis). This form of electrophoretic deposition may
then be coupled
with electrolytic deposition to form a hybrid structure that is functionally
graded and changes
- 1 -
CA 02764968 2011-12-08
WO 2010/144145
PCT/US2010/001677
in concentration from metal (electrolytically deposited) to ceramic, polymer
or glass
(electrophoretically deposited).
[0007] Embodiments of the methods described here provide a high-density,
corrosion and/or
heat resistant material (e.g., ceramic, glass, polymer) that is deposited onto
the surface of a
substrate to form a functionally-graded polymer:metal, Ceramic:metal, or
glass:metal coating.
The result is a coating, of controlled density, composition, hardness, thermal
conductivity,
wear resistance and/or corrosion resistance, that has been grown directly onto
a surface.
[0008] The functionally-graded coating made according to the methods disclosed
hereinmay
be resistant to spallation due to mismatch in any of: coefficient of thermal
expansion,
hardness, ductility, toughness, elasticity or other property (together
"Interface Property"),
between the substrate and the ceramic, polymer, pre-ceramic polymer (with or
without fillers)
or glass (together "Inert Phase") as the coating incorporates a material at
the substrate
interface, which more closely matches the Interface Property of the substrate.
[0009] In general, coatings made according to methods described herein are
resistant to wear,
corrosion and/or heat due to the hard, abrasion-resistant, non-reactive and/or
heat-stable
nature of the Inert Phase.
[00010] Polymer-derived ceramics that incorporate active fillers (e.g.,
TiN, Ti
disilicide, and others) to improve density, have shown promise as a way to
process a variety
of Inert Phases, which are more dense than polymer-derived ceramics which do
not
incorporate these fillers. Polymer-derived ceramic composites have been
demonstrated for
applications, including-oxidation resistance and thermal barriers, due to
their high density
and low open-pore volume (e.g., the ceramic has less than 1, 5, 10, 20, 30,
40, or 50 percent
voids based on volume). See, JD Torrey and RK Bordia, Journal of European
Ceramic
Society 28 (2008) 253-257. These polymer-derived ceramics can be
electrophoretically
deposited. Electrophoretic deposition is a two-step process. In a first step,
particles
suspended in a liquid are forced to move towards one of the electrodes by
applying an electric
field to the suspension (electrophoresis). In a second step (deposition), the
particles collect at
one of the electrodes and form a coherent deposit on it. Since the local
composition of the
deposit is directly related to the concentration and composition of the
suspension at the
moment of deposition, the electrophoretic process allows continuous processing
of
functionally graded materials. Polymer-derived ceramics is the method used in
commercial
production of Nicalon and Tyranno fibers.
-2-
CA 02764968 2011-12-08
WO 2010/144145
PCT/US2010/001677
[00011] In embodiments, the technology of this disclosure includes the use
of
electrochemical deposition processes to produce composition-controlled
functionally-graded
coating through chemical and electrochemical control of the initial
suspension. This
deposition process is referred to as Layered Electrophoretic and Faradaic
Depostion (LEAF).
By controlling the composition and current evolution during the deposition
process, LEAF
affords the means to engineer step-graded and continuously graded
compositions; see Figs.
and reference graphs that show dependence of Ni and Si as a function of
solution
chemistry and current density. Control of current evolution and direction of
the electric
field also offers the possibility to orient anisotropic powders allowing
intimate control of both
the density AND the morphology of the Inert Phase (e.g., the content and
organization of
added ceramic, polymer or glass materials incorporated into an
electrodeposited functionally-
graded coating). For example, in one embodiment by controlling current
evolution and the
direction of the electric field in a solution including pre-ceramic polymer,
the resulting
density of ceramic can be varied through the coatings to produce a varying
morphology of
ceramic/metal composition.
BRIEF DESCRIPTION OF THE DRAWINGS
[00012] FIG. 1. is an illustration of a functionally graded material.
[00013] FIG. 2. is an illustration of a pipe based on functionally graded
material shown
in FIG. I.
[00014] FIG. 3. is graph illustrating mass loss of a substrate per area
over time for
several materials exposed to concentrated sulfuric acid at 200 degrees C.
[00015] FIG. 4 illustrates Active Filler Controlled Pyrolysis.
[00016] FIG. 5. illustrates LEAF electrophoretic deposition process on a
fiber mat.
[00017] FIG. 6 illustrates the concentration of Si and nickel in deposits
found by
changing the current density. Si is the left most member of each bar graph
pair and nickel the
right most member of each bar graph pair measured at a specific current
density
[00018] FIG. 7 illustrates the concentration of Ni in the emulsion
increases from left to
right. . Si is the left most member of each bar graph pair and nickel the
right most member of
each bar graph pair prepared with the noted solution concentration of nickel.
DETAILED DESCRIPTION
[00019] Polymer-derived ceramics have shown promise as a novel way to
process low-
dimensional ceramics, including matrices, fibers and coatings. Polymer-derived
ceramic
-3-
CA 02764968 2011-12-08
WO 2010/144145
PCT/US2010/001677
composites have been demonstrated for applications including oxidation
barriers, due to their
high density and low open-pore volume. See, Torrey and RK Bordia, Journal of
European
Ceramic Society 28 (2008) 253-257.
[00020] The Active Filler Controlled Pyrolysis (AFCoP), polymer-derived
ceramics
offer many benefits over tradition ceramic processing methods including:
= Liquid form with low crosslinking temperature
= High purity reactants
= Tailorable composition, microstructure, nanostructures and properties
= Ability to produce crystalline and beta-SiC phases
[00021] Pure polymer-derived ceramics suffer from certain performance
limitations.
One such limitation is the occurrence of volume shrinkage ¨ up to 50%, upon
sintering. To
prevent this, and in order to increase the density of PDC matrices, the AFCoP
process is
employed, as shown in Figure 4.
[00022] To produce fully-dense ceramic matrices, the active-filler additive
can be
occluded into the liquid polymer prior to casting and sintering. During
sintering, this additive
acts as an expansion agent, resulting in a fully dense part with near zero
volume loss (e.g.,
there are no voids present). Active fillers include Si, Al, Ti and other
metals, which on
pyrolysis form SiC, A1203 or TiSi2, for example. One of the limitations of
this process, as it
is practiced currently, is the limited reactivity of the fillers. In many
cases, due to kinetic
limitations, even for the finest available powders, the filler conversion is
incomplete. As will
be shown in the processes described herein, the reactive "filler" and the
polymer will mixed
at molecular scale leading to highly efficient conversion of the filler to the
product phase.
[00023] Polymer-derived ceramics and in particular, AFCoP ceramics, have
shown
promise as a novel way to process a variety of ceramics forms, including
matrices, fibers and
coatings. Polymer-derived ceramic composites have been demonstrated for
applications,
including-oxidation resistance and thermal barriers, due to their high density
and low open-
pore volume. See, JD Torrey and RK Bordia, Journal of European Ceramic Society
28
(2008) 253-257. In the some embodiments of this disclosure the AFCoP concept
and the
LEAF deposition process are combined to enable a manufacturing capability
which can
produce tailorable, low-cost, ultra-high-performance SiCf/SiC composites and
parts.
[00024] The Layered Electrophoretic And Faradaic (LEAF) production process
employed herein enables the low-cost production of tailored ceramic matrices.
A schematic
of one embodiment of that process described in Scheme A.
Scheme A
-4-
CA 02764968 2011-12-08
WO 2010/144145
PCT/US2010/001677
Machine or Weave +.41 Place in 1 I Run Plating
--I,- Rinse HO.
Preform I Plating Tank F41 Routine
I Nondestructive ____________________
¨+ Remove part 11. Assemble
Inspection I
[00025] Starting from SiC powders and fiber, a first portion of the LEAF
process
consists in depositing either direct SiC powders, pre-ceramic polymer
emulsions (including
active fillers) or a combination of these onto the SiC fiber. Electrophoretic
deposition is a
two-step process. In a first step, particles suspended in a liquid are forced
to move towards
one of the electrodes by applying an electric field to the suspension
(electrophoresis). In a
second step (deposition), the particles collect at one of the electrodes and
form a coherent
deposit on it. Since the local composition of the deposit is directly related
to the concentration
and composition of the suspension at the moment of deposition, the
electrophoretic process
allows continuous continuous processing of functionally graded materials.
[00026] A variety of substrates may be employed to prepare the compositions
described herein. In one embodiment, the compositions are prepared by the LEAF
electrophoretic deposition process outlined above on fiber mat as illustrated
in Figure 5.
[00027] The LEAF process offers the ability to reliably produce composition-
controlled "green" (not yet sintered) ceramic through chemical and
electrochemical control of
the initial suspension. By shaping the starting fiber, which serves as a
mandrel, LEAF
provides a means to manufacture free standing parts of complex geometry, and
hybrid,
strength-tailored materials.
[00028] By controlling the composition and current evolution during
deposition
process, LEAF affords the means to engineer step-graded and continuously
graded
compositions. Control of current evolution and direction of the electric field
also offers the
possibility to orient anisotropic powders allowing intimate control of both
the density AND
the morphology of the ceramic deposit.
[00029] Layer thickness can be controlled by, among other things, the
application of
current in the electrodeposition process. In some embodiments current density
may be varied
within the range between 0.5 and 2000 mA/cm2. Other ranges for current
densities are also
possible, for example, a current density may be varied within the range
between: about 1 and
20 mA/cm2; about 5 and 50 mA/cm2; about 30 and 70 mA/cm2; 0.5 and 500 mAkm2;
100
and 2000 mA/cm2; greater than about 500 mA/cm2; and about 15 and 40 mA/cm2
base on the
-5-
CA 02764968 2011-12-08
WO 2010/144145
PCT/US2010/001677
surface area of the substrate or mandrel to be coated. In some embodiments the
frequency of
the wave forms may be from about 0.01 Hz to about 50 Hz. In other embodiments
the
frequency can be from: about 0.5 to aboutl 0 Hz; 0.02 to about 1Hz or from
about 2 to 20Hz;
or from about 1 to about 5 Hz.
[00030] In some embodiments the electrical potential employed to prepare
the coatings
is in the range of 5V and 5000 V. In other embodiments the electrical
potential is within a
range selected from 5 and 200 V; about 50 and 500 V; about 100 and 1000 V; 250
and 2500
V; 500 and 3000 V; 1,000 and 4,000 V; and 2000 and 5000 V.
[00031] In addition to direct electrophoretic deposition of SiC pre-
polymers onto SiC
fibers, studies have also demonstrated the co-deposition of densification
additives. This is
similar to the AFCoP process described above. These active-filler additives
allow low-
temperature densification without any detrimental effects on the fibers, as
many densification
additives can be sintered well below the re-crystallization temperature of the
SiCf. See,A.R.
Boccaccini et al., Journal of European Ceramic Society 17 (1997) 1545-1550. By
combining
these additives into the LEAF process, it is possible to produce high density
and density
graded ceramic matrices.
[00032] Density gradation allows for the design and development of a highly
optimized SiC-fiber:SiC-matrix interface. Density gradation provides a means
for balancing
the optimization of the interface strength, while still maintaining a high
density, and in some
embodiments gas impermeable and hermetically sealed matrix. Gas impermeability
is
especially important in corrosion protection where a high level of gas
diffusion through the
coating may result in substrate attack. The LEAF process enables control and
gradation of
density such that a high density region near the substrate may protect the
substrate from
attack while a low density region near the surface may reduce the thermal
conductivity of the
coating.
[00033] It is believed to be possible to join non oxide ceramics using
preceramic
polymers with active fillers based on the work of Borida. See, JD Torrey and
RK Bordia,
Journal of European Ceramic Society 28 (2008) 253-257. In regard t the
embodiments
described herein, refinement of the microstructure of ceramics joined by the
LEAF processes
leads to higher bond strengths In one embodiment of the technology, a sample
composition
can be controlled by controlling the voltage. Specifically, by slowly
transitioning from a low
voltage electrolytic deposition regime to a high voltage electrophoretic
deposition regime it
may be possible to create a functionally-graded material that gradually
changes from metal to
-6-
CA 02764968 2011-12-08
WO 2010/144145
PCT/US2010/001677
ceramic or polymer. The same could be achieved by controlling the current to
selectively
deposit ionic (metal) species and/or charged particle (Inert Phase) species.
To create a
metal:ceramic functionally graded SiC composite material would significantly
increase the
corrosion-resistance, wear-resistance, toughness, durability and temperature
stability of a
ceramic-coated structure.
[00034] In another embodiment, the coating composition can be fimctionally-
graded
by modifying the metal concentration in the electrolyte solution during
electrochemical
deposition. This approach affords an additional means to control the
composition of the
functionally-graded coating, and allows for deposition to occur at relatively
lower current
densities and voltages, which produced a better quality in the deposited
composites. The
standard cathodic emulsion system, where the emulsion particles comprise
polymer, pre-
ceramic polymer, ceramic or a combination thereof, can be adjusted by adding
increasing
amounts of nickel to the solution. This embodiment is described in Example #3.
[00035] In other embodiments, this disclosure provides a corrosion
resistant coating,
which changes in composition throughout its depth, from a high metal
concentration at the
interface with the substrate to which it is applied to an Inert Phase at the
surface.
[00036] In another embodiment, the present disclosure provides a heat
resistant
coating, which changes in composition throughout its depth, from a high metal
concentration
at the interface with the substrate to which it is applied to an Inert Phase
at the surface.
[00037] As used herein "Inert Phase" means any polymer, ceramic, pre-
ceramic
polymer (with or without fillers) or glass, which can be electrophoretically
deposited. This
Inert Phase may include A1203, Si02, TiN, BN, Fe203, MgO, and Ti02, SiC, TiO,
TiN, silane
polymers, polyhydriromethylsilazane and others.
[00038] In some embodiments, ceramic particles may include of one or more
metal
oxides that can be selected from Zrx0õ, Yt0õ, Alx0õ, Si0õ, FeO, Ti0õ, MgO
where x=1-4,
and include mixed metal oxides with the structure MAY, where M is a metal and
Y is Zrx0õ,
Yt0x, Alx0x, SiOx, FeO, TiOx, MgO. In another embodiment, M is selected from
Li, Sr, La,
W, Ta, Hf, Cr, Ca, Na, Al, Ti, Zr, Cs, Ru, and Pb.
[00039] As used herein, "metal" means any metal, metal alloy or other
composite
containing a metal. These metals may comprise one or more of Ni, Zn, Fe, Cu,
Au, Ag, Pd,
Sn, Mn, Co, Pb, Al, Ti, Mg and Cr. In embodiments where metals are deposited,
the
percentage of each metal may independently be selected. Individual metals may
be present at
about 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 15, 20, 25, 30, 30, 35,
40, 45, 50, 55, 60, 65,
-7-
CA 02764968 2011-12-08
WO 2010/144145
PCT/US2010/001677
70, 75, 80, 85, 90, 95, 98, 99, 99.9, 99.99, 99.999 or 100 percent of the
electrodeposited
species/composition.
[00040] In other embodiments, the coating can have a coating thickness
that varies
according to properties of the material that is to be protected by the
coating, or according to
the environment that the coating is subjected to. In some embodiments, the
coating can range
from 0.2 and 250 millimeters, and in other embodiments the range can vary from
0.2 to 25
millimeters, 25 to 250 millimeters, or be greater than about 25 millimeter and
less than about
250 millimeters. In still other embodiments, the coating thickness can range
from 0.5 to 5
= millimeters, 1 to 10 millimeters, 5 to 15 millimeters, 10 to 20
millimeters, and 15 to 25
millimeters. In still other embodiments, the overall thickness of the
functionally-graded
coating can vary greatly as, for example, between 2 micron and 6.5 millimeters
or more. In
some embodiments the overall thickness of the functionally-graded coating can
also be
between 2 nanometers and 10,000 nanometers, 4 nanometers and 400 nanometers,
50
nanometers and 500 nanometers, 100 nanometers and 1,000 nanometers, 1 micron
to 10
microns, 5 microns to 50 microns, 20 microns to 200 microns, 200 microns to 2
millimeters
(mm), 400 microns to 4 mm, 200 microns to 5 mm, 1 mm to 6.5 mm, 5 mm to 12.5
mm, 10
mm to 20 mm, 15 mm to 30 mm.
[00041] The functionally graded coatings described herein are suitable for
coating a
variety of substrates that are susceptible to wear and corrosion. In one
embodiment the
substrates are particularly suited for coating substrates made of materials
that can corrode and
wear such as iron, steel, aluminum, nickel, cobalt, iron, manganese, copper,
titanium, alloys
thereof, reinforced composites and the like.
[00042] The functionally graded coatings described herein may be employed
to protect
against numerous types of corrosion, including, but not limited to corrosion
caused by
oxidation, reduction. stress (stress corrosion), dissolution, dezincification,
acid, base,
sulfidation and the like.
[00043] The functionally graded coatings described herein may be employed
to protect
against thermal degradation. In one embodiment, the coatings will have a lower
thermal
conductivity than the substrates (e.g., metal surfaces) to which they are
applied.
[00044] The coatings described herein may be employed to protect against
numerous
types of corrosion, including, but not limited to corrosion caused by
oxidation, reduction.
stress (stress corrosion), dissolution, dezincification, acid, base,
sulfidation and the like. In
-8-
CA 02764968 2011-12-08
WO 2010/144145
PCT/US2010/001677
one embodiment, the coatings are resistant to the action of strong mineral
acid, such as
sulfuric, nitric, and hydrochloric acids.
EXAMPLES
[00045] Example 1. Preparation of a functionally graded coating comprising
a Inert
Phase and a metal formed utilizing a combination of electrolytic (faradaic)
and
electrophoretic deposition includes the following steps:
1. Acquire the desired substrate material and cut it to its appropriate size
2. Sand the substrate on a circular sander using three steps to achieve a 600
Grit finish
a. 120 Grit
b. 420 Grit
c. 600 Grit
3. Attrition Mill TiSi2 powder for 10 or more hours.
a. Add isopropanol to the TiSi2 powder to aid in grinding
b. The longer the time period the smaller the particle size
c. Rinse with isopropanol
d. Dry at 100 C for 8 hours
4. Mix the Pre-ceramic Polymer with the solvent
a. Pre-ceramic Polymer, Polyhydridomethysilazane (PHMS): 5.25g
b. Add to Solvent, n-Octane: 6.25 mL
c. Add an electrodepositable metal species (e.g. Ni) to the slurry
d. The total Volume ratio of slurry : n-Octane is 3:5
5. Mix TiSi2 powder at 30% volume with PHMS from step 4 to create slurry
6. Ball mill slurry for 4 hours with 200, 5/32" diameter glass beads
7. Dissolve the Ru3(C0)12 catalyst in n-Octane
a. Ru3(CO)12: 2.63 mg
b. n-Octane: 6.25 mL
c. Combine the mixture with the slurry
8. Ball mill for the entire slurry from step 7 for 30 minutes
9. Dip-coat the slurry onto the prepared substrate
a. Dip substrate into slurry
b. Apply a current to affect electrolytic deposition of the metal content
of the
coating
c. Increase the current to affect electrophoretic deposition of the ceramic
content
of the coating
d. Attach the substrate to the Instron head
e. Optionally dip into the substrate into the slurry and remove it at a
rate of 50
cm/min
10. Cross-link the samples in humid air
-9-
CA 02764968 2011-12-08
WO 2010/144145
PCT/US2010/001677
a. Hang the dipped substrates in ajar filled 1/5 with water
b. Temperature: 150C
c. Time: 2 hours
11. Pyrolyze the dipped samples with flowing air
a. Hang the samples from a ceramic stand and place them in the oven
b. Ramp rate: 2C/min
c. Hold temperature: 800C
d. Hold Time: 2 hours
e. Ramp down: 2C/min
12. Remove the completed sample from the oven.
[00046] The resistance of a TiSi2 filled and an unfilled coating to
degradation by 200
degree C concentrated sulfuric acid is shown in Figure 3. A standard of Alloy
20 and 316
stainless steel are provide for reference. The filled coating showed the least
loss of weight.
[00047] Example 2. Toughness Improvements Employing LEAF Processes To
Incorporate a Low-Content of Metal Binder Into Composites
[00048] In order to improve toughness, the LEAF processes a low-content of
a metal
binder (e.g., nickel in this Example) may be incorporated into composites. As
shown in
figure 6, the concentration of nickel in deposits can be controlled by
changing the current
density employed.
[00049] Example 3. A Functionally Graded Coating
[00050] In order to create a functionally-graded coating, a standard nickel
plating bath
was added to the polymer emulsion in 1% increments by volume up to 10%.
[00051] Samples were subsequently exposed to a DC current for a fixed
period. The
bath was stirred and agitated at the conclusion of each test in order to
ensure proper solution
mixing and suspension.
[00052] The observations attained from the optical image of the samples
were
confirmed by the EDX compositional analysis. The Ni composition of the coating
was
increasing as the Ni concentration in solution increased. These results once
again
demonstrate the feasibility of creating a functionally graded ceramic:metal
composite
material by controlling the concentration of metal and Inert Phases in the
electrolyte during
the deposition process.
[00053] In addition, the data demonstrated that the silicon content in the
deposit
remain constant over time. This result is to be expected as a result of the
voltage driven
nature of electrophoretic deposition, and a constant current density and
similar voltages were
-10-
CA 02764968 2011-12-08
WO 2010/144145
PCT/US2010/001677
used for the samples. The nickel emulsion system can be optimized through
concentration
alteration and current and voltage modulation to create a structural material
suitable for
corrosion resistant, wear resistant, heat resistant and other applications.
[00054] Example 4. Nickel, a siloxane-based pre-ceramic polymer particles
and
ceramic SiC particles are added to an organic electrolyte Note that in this
case, the polymer
is not deposited as an emulsion, but rather directly as a lacquer. A cathode
and an anode
were connected to a power supply. The substrate was connected to the cathode
and inert
anodes were connected to the anode. A potential was applied across the anodes
and cathode,
which potential ramped from a low voltage (around 5-100V) to a high voltage
(about 100-
1000V). The high voltage was held for a period of time. In an SEM of the
resulting
structure, where gray masses are the SiC fibers the darker gray areas are a
mixed matrix of
SiOC and SiC. SiOC is present due to the heat treatment in an environment in
which oxygen
was present. The white areas are where the nickel was able to infiltrate into
the cracks and
reinforce the structure of the material.
[00055] The addition of the SiC filler particles into the pre-ceramic
polymer led to the
densification and, strengthening of the specimen by reducing shrinkage on
formation. The
sub-micron size of the filler particles facilitated the flow and migration of
the matrix around
the SiC fibers. The upper-right corner of the image contains a zoomed in view
of the
interface around a fiber. Any gaps present were filled and strengthened by the
nickel metal
deposition.
[00056] Fiber break analysis was performed on a selection of samples that
contained
the functionally graded metal:SiC structure to determine the toughness and
fracture
characteristics of various SiC bundles. The toughness of the fiber matrix can
be determined
through the visual inspection of fiber pull-out during fracture. This is
observed in SEM
images of the fracture surface of a dipped coated ceramic bundle cross-linked
at 500 F for 2
hours.
[00057] The above descriptions of embodiments of methods and compositions
are
illustrative of the present technology. Because of variations which will be
apparent to those
skilled in the art, however, the technology is not intended to be limited to
the particular
embodiments described above.
-II-
WO 2010/144145 CA 02764968 2011-12-08
PCT/US2010/001677
, ,, ¨ ' = ' '- 7 ---- ,,,,
I
'..,4' ' -
- i 1 , -
' - - - 1
, = , -, ,.,
-.-, ,.-*õ ,../._.__: i; - , ,
,,,, _________I
õ-. =... - - -:-- -:,`-__( -- - -,- ' '
L - , , 1,; /1-----/_. ----=-----7,7,----,;;;-:,-10,-. t.T 1
-i '''''. 'Th C!1 CL'24µ '''. 'õ, - - - r.2..-`,..,
. +"' I WL-_. ¨ -"¨ -- -. -
/...i.'µ':'', ' \ - \ -i
..' ' . : -,. ' ,- '---.) ' . . 1, /¨" -
S S
1 s , , -,,, ' s + , ',,,, = - -
,
- -: -
.. . t3.õ ,. ,
I, , - ' , ' *--L' ' ''.:,- , , , .: = (
1 ') ' Y
,.
i
, = ''' ' -. ' - i '.1 ii
-) I '^. -,µ
h ' (, = * ' ,õ : '' ; ' ' "- 6A ' ri
i
'---, - - -- 3 - r% ,, / i
\
. ' , ... = -, s - ,,. -..---
, - ,r .. ,v, ' j - , , ;
.,-,,,' ,' ,. ,. ' - ,F,- r- , µ.
r .
- , , , , s (- , = r
.
, , - . ., - µ, , , 1
' '-:-::,- -',..: 1, ., -)- , 1
I, f . - -;.' sk:L-1:" _ - 1," , ' t= . .. = = : = -
, , 1' 0-- - t...:-..gn
ci
. = ' - \ D 1 0.1 m m
.1- ' - ' ''' ' - ' ___L...-----
X400 W
F .,,,--, .-` ..'., , ..'' -1_2,-- ''-'-'-'-
nmPO 30.0kV
0 SEM C-
UW
ic polymer¨SEM
eram
SIC fibers SEM
with 1
S.0 filler on -
Pre-Ceramic
r slurry w
Nickel /Siloxane Based of the pre-ceramic
polymer
(FIRST SEM IMAGE)
12
CA 02764968 2011-12-08
WO 2010/144145
PCT/US2010/001677
---- ;.-..;- -,>-xki. = ...,,-. = = ;
-
,,,,: .
,
.. =
,
-
. ___.
....7 .,:: :_..- ¨ . -.-- .
,_ 1:,47'2Y''..Cf7:!;;7;.,.a41r¨ ---7-__,- ..-4; ,- ,
. , .71
, -- --- - - - , - - -
. ---, '.:...itzr=: -',..-",-,--v,-÷-v'"''''µ,,,,1 7 µ-.-
' --'11 .-.-- c.... - ;
Pe..1, ., _:...7.-.., - =....L.j..,,,,....:4., ,--.-,-;sw.der,----ci-
',.4'''". r.- -.,..--- *:- 's^ = N.1
,, , . ' - .--..L....-.. e,õ......._ .!...-. -,---:,?-- '!-
µ,...........----$,----- ="' F"'"A',,.=
..........___, - = ' --õii...:., ' r- - -----:===4"": '''1G7----77-
; ' ...-..-' '-'41.Ntif -
1.....-__õ..._. ___________________________ ,,,...!. X.:1...r,- , -7.-- _ . -,
-, 'µ,..7`--'-= ,Afip, s --,,.. ..., -7: µ"."1- ^s-' ¨ -...- ,- ...' 14
'
;,, .,,=;'....;::.f."7.- ,..4.-it r...;,.,',"- .., .^,.':, It
' - -v,-,:,-,,:,..,""t- . ...' ,- " = -.-- -. '' õ õ elai. , ": ' , _.
= =Iitiok .,...N...........t
--x-----,,, -TA-4 -..,:' õ:" ' - - ...!, , t /, 1 ,...,,,,,t, , -',- - =---
.----,õ:::_--- -,1 is..õ. '7:44 ,õ-- 7- ,- = == ,-...
1.1"") = ''.7"---,67. 1 "------ 2. ...--,..--.---` i,..."-t, -: \ ." ---- e
, ,. ' ,-- ,--- ' ' ' = - ' 1.1,...mr---, -- .., .."'" ".....:1-,
. :: Zi)
.7.7,C;,- `-...^,õ.õ. __ , _ = , ,,..,''. , _*=,-..--
lik
% jõ,. 7,--. _,',;õ`,..t,,/ , ,..; ., --,-= it:, ;4,-,P.1"'" .'"'='=-=
'''''"' 1---77,..7 - . , .s. `pl---- = r1õ'-----",,t,
' N6
,,-:-
=-=--,"--======,,---'7.....:-...-...
t- , - , . '---,' - - - i'.. ' " ,-,-, ' .,--
- ------ = - - - ' vs . Ø = -. - , ...e.,f----r 7" õ ., -,. -.?
v .' . -- % vo, (1.F., õ.õ ,, r, l 4. ,_.^:,-,... 6-:_-
^ ----: j,,,,, _ __*,_ .... ....4õ..::::_.:=_.,:õ....õ õ _ , _.'...:.- ¨
4"..,- 1 7 s ..^-, )
*.- µ2,';': "Z..:,1..1.;;;: -1,:, ^=,,, ,:-., rii, ; - ::` ,' _ _ ' ''',...-
: --""',!.",;õ' ''''''' '''"."- '-','""4.;..---. - ,..;-õ..t --,:.771-2::::õ,-
.?..:,....'f.,:::__:_õ,_---
, = - A. 4 ..,,' ' = ' ' N `" - ` -r , - r -,,,----
,... ------ - ' ''',,t '''' '-'-
'
..=-=,...,.....s.......: `1 , ',..- - .- i,µ:r- ,--41::?.. -, 4 ' ...,-,..-.
:;. -Zs-- -"..- ,-.4 '1.4 ¨....-,
õ...1 ' = * - ' :,-",.. ' , , . ..,-. 3- , -- -
.-- ^ õs
F:, - . , -..., ..6,,,,,..IM,,,,,,,,,,..41¾...., ..g...."............
'.......'''......'..
U W 0 SEM SEI 15.0kV X160 WD 10.0mm 1004um
An SEM image of the fracture surface of a dipped coated ceramic bundle cross-
linked at
500 F for 2 hours.
(FIRST SEM IMAGE)
13