Note: Descriptions are shown in the official language in which they were submitted.
PF 62287 CA 02765430 2011-12-14
1
Method for producing formic acid
Description
The present invention relates to a process for preparing formic acid by
hydrogenation
of carbon dioxide in the presence of a catalyst comprising an element of group
8, 9 or
of the Periodic Table, a tertiary amine (I) and a polar solvent (III) at a
pressure of
from 0.2 to 30 MPa abs and a temperature of from 20 to 200 C to form two
liquid
phases, separation of the one liquid phase (A) enriched with the formic
acid/amine
10 adduct (II) from the other liquid phase (B) and recirculation of the liquid
phase (B) to the
hydrogenation reactor.
Adducts of formic acid and tertiary amines can be thermally dissociated into
free formic
acid and tertiary amine and therefore serve as intermediate in the preparation
of formic
acid. Formic acid is an important and versatile product. It is used, for
example, for
acidification in the production of animal feeds, as preservative, as
disinfectant, as
auxiliary in the textile and leather industry, as a mixture with its salts for
deicing aircraft
and runways and also as synthetic building block in the chemical industry.
The abovementioned adducts of formic acid and tertiary amines can be prepared
in
various ways, for example (i) by direct reaction of tertiary amine with formic
acid, (ii) by
hydrolysis of methyl formate to form formic acid in the presence of the
tertiary amine or
with subsequent extraction of the hydrolysis product with the tertiary amine
or (iii) by
catalytic hydration of carbon monoxide or hydrogenation of carbon dioxide to
form
formic acid in the presence of the tertiary amine. The latter process of
catalytic
hydrogenation of carbon dioxide has the particular attraction that carbon
dioxide is
available in large quantities and is flexible in terms of source.
WO 2008/116,799 discloses a process for the hydrogenation of carbon dioxide in
the
presence of a catalyst which comprises a transition metal of transition group
VIII
(groups 8, 9, 10) and is suspended or homogeneously dissolved in a solution, a
tertiary
amine having at least one hydroxyl group and a polar solvent to form an adduct
of
formic acid and the tertiary amine. The hydroxyl group(s) in the tertiary
amine enable
an increased carbon dioxide solubility compared to the triethylamine which is
usually
used to be achieved. As preferred homogeneous catalysts, mention may be made
of
RuH2L4 having monodentate phosphorus-based ligands L and RuH2(LL)2 having
bidentate phosphorus-based ligands LL and particularly preferably
RuH2[P(C6H5)3]4. As
polar solvents, mention may be made of alcohols, ethers, sulfolanes, dimethyl
sulfoxide
and amides whose boiling point at atmospheric pressure is at least 5 C above
that of
formic acid. The tertiary amines which are preferably to be used also have a
boiling
point above that of formic acid. Since no phase separation takes place, the
work-up of
the entire reaction product mixture is carried out by distillation, optionally
after prior
removal of the catalyst, in which the adduct of formic acid and the tertiary
amine which
PF 62287 CA 02765430 2011-12-14
2
is formed is thermally dissociated and the formic acid liberated is isolated
as overhead
product. The bottom product comprising tertiary amine, polar solvent and
catalyst is
optionally recirculated to the hydrogenation stage.
A disadvantage of this process is the introduction of the entire liquid
reaction product
mixture into the apparatus for thermal dissociation and distillation,
optionally after prior
specific removal of the homogeneous catalyst by means of a separate process
step, for
example an extraction, adsorption or ultrafiltration step. The apparatus for
the thermal
dissociation and distillation consequently has to be made larger and more
complex
both in terms of the higher liquid throughput and the more specific separation
properties, which is reflected, inter alia, in the capital costs (for example
via
engineering input, material, space requirement). In addition, the higher
liquid
throughput also results in a higher energy usage.
However, the fundamental work on the catalytic hydrogenation of carbon dioxide
to
form formic acid was carried out as early as the 1970s and 1980s. The
processes of
BP Chemicals Ltd. filed as the patents EP 0 095 321 A, EP 0 151 510 A and
EP 0 181 078 A may be considered to result therefrom. All three documents
describe
the hydrogenation of carbon dioxide in the presence of a homogeneous catalyst
comprising a transition metal of transition group VIII (groups 8, 9, 10), a
tertiary amine
and a polar solvent to form an adduct of formic acid and the tertiary amine.
As
preferred homogeneous catalysts, EP 0 095 321 A and EP 0 181 078 A in each
case
make mention of ruthenium-based carbonyl-, halide- and/or triphenylphosphine-
comprising complex catalysts and EP 0 151 510 A mentions rhodium-phosphine
complexes. Preferred tertiary amines are C1-C1o-trialkylamines, in particular
the short-
chain C1-C4-trialkylamines, and also cyclic and/or bridged amines such as
1,8-diazabicyclo[5.4.0]undec-7-ene, 1,4-diazabicyclo[2.2.2]octane, pyridine or
picolines. The hydrogenation is carried out at a carbon dioxide partial
pressure of up to
6 MPa (60 bar), a hydrogen partial pressure of up to 25 MPa (250 bar) and a
temperature from about room temperature to 200 C.
EP 0 095 321 A and EP 0 151 510 A teach the use of an alcohol as polar
solvent.
However, since primary alcohols tend to form formic esters (organic formates),
secondary alcohols, in particular isopropanol, are preferred. In addition, the
presence
of water is described as advantageous. According to the examples in EP 0 095
321 A,
the reaction product mixture is worked up by directly subsequent two-stage
distillation
in which the low boilers alcohol, water, tertiary amine are separated off in
the first stage
and the adduct of formic acid and the tertiary amine is separated off at the
top under
vacuum conditions in the second stage. EP 0 151 510 A likewise teaches a work-
up by
distillation, but with reference to EP 0 126 524 A with subsequent replacement
of the
tertiary amine in the adduct which has been separated off by distillation by a
weaker,
less volatile nitrogen base before thermal cleavage of the adduct in order to
aid or
PF 62287 CA 02765430 2011-12-14
3
make possible the subsequent thermal dissociation to produce free formic acid.
EP 0 181 078 A teaches the targeted choice of the polar solvent on the basis
of three
essential criteria which have to be fulfilled at the same time:
(i) the homogeneous catalyst has to be soluble in the polar solvent;
(ii) the polar solvent must not have an adverse effect on the hydrogenation;
and
(iii) the adduct of formic acid and the tertiary amine which is formed should
be able to
be readily separated off from the polar solvent.
As particularly suitable polar solvents, mention is made of various glycols
and
phenylpropanols.
According to the teaching of EP 0 181 078 A, the work-up of the reaction
product
mixture is carried out by firstly separating off the gaseous components (in
particular
unreacted starting materials hydrogen and carbon dioxide) at the top of an
evaporator
and separating off the homogeneous catalyst dissolved in the polar solvent at
the
bottom and recirculating them to the hydrogenation stage. The adduct of formic
acid
and the tertiary amine is subsequently separated off from the remaining liquid
phase
comprising the adduct of formic acid and the tertiary amine, free tertiary
amine and
possibly water and the remaining part of the liquid phase comprising the free
tertiary
amine and possibly water is recirculated to the hydrogenation stage. The
separation
can be effected by distillation or phase separation of the two-phase system
(decantation).
A further significant teaching of EP 0 181 078 A is the subsequent, absolutely
necessary replacement of the tertiary amine in the adduct which has been
separated
off by a weaker, less volatile nitrogen base before the adduct is thermally
dissociated in
order to aid or make possible the subsequent thermal dissociation to produce
free
formic acid. As particularly suitable weaker nitrogen bases, mention is made
of
imidazole derivatives such as 1-n-butylimidazole.
A disadvantage of the process of EP 0 181 078 A is the very complicated, four-
stage
work-up of the reaction product mixture by
(i) separating off the gaseous components and also the homogeneous catalyst
and
the polar solvent in an evaporator and recirculating them to the hydrogenation
stage;
(ii) separating off the adduct of formic acid and the tertiary amine in a
distillation
column or a phase separator and recirculating the remaining liquid stream to
the
hydrogenation stage;
(iii) replacing the tertiary amine in the adduct of formic acid and the
tertiary amine by
PF 62287 CA 02765430 2011-12-14
4
a weaker, less volatile nitrogen base in a reaction vessel having a superposed
distillation column and recirculating the tertiary amine liberated to the
hydrogenation stage; and
(iv) thermally dissociating the adduct of formic acid and the weaker nitrogen
base and
recirculating the weaker nitrogen base liberated to the base replacement
stage.
A further, important disadvantage of the process of EP 0 181 078 A and also of
the
processes of EP 0 095 321 A and EP 0 151 510 A is the fact that the adduct of
formic
acid and the tertiary amine partly redissociates into carbon dioxide and
hydrogen in the
presence of the homogeneous catalyst during the work-up in the evaporator. As
a
solution to this problem, EP 0 329 337 A proposes the addition of a
decomposition
inhibitor which reversibly inhibits the homogeneous catalyst. As preferred
decomposition inhibitors, mention is made of carbon monoxide and oxidants.
However,
disadvantages of this are the introduction of further substances into the
overall process
and the necessity of reactivating the inhibited homogeneous catalyst before it
is used
further.
EP 0 357 243 A, too, addresses the disadvantage of the partial redissociation
of the
adduct of formic acid and the tertiary amine in the process of EP 0 181 078 A
by joint
work-up of the reaction product mixture in the evaporator. The process
proposed in
EP 0 357 243 A teaches the use of a homogeneous catalyst comprising a
transition
metal of transition group VIII (groups 8, 9, 10), a tertiary amine and two
different
solvents, namely a nonpolar, inert solvent and a polar, inert solvent, which
form two
immiscible liquid phases in the catalytic hydrogenation of carbon dioxide to
form an
adduct of formic acid and tertiary amine. As nonpolar solvents, mention is
made of
aliphatic and aromatic hydrocarbons but also of phosphines having aliphatic
and/or
.aromatic hydrocarbon radicals. Polar solvents mentioned are water, glycerol,
alcohols,
polyols, sulfolanes and mixtures thereof, with water being preferred. The
homogeneous
catalyst dissolves in the nonpolar solvent and the adduct of formic acid and
tertiary
amine dissolves in the polar solvent. After the reaction is complete, the two
liquid
phases are separated, for example by decantation, and the nonpolar phase
comprising
the homogeneous catalyst and the nonpolar solvent is recirculated to the
hydrogenation stage. The polar phase comprising the adduct of formic acid and
tertiary
amine and the polar solvent is then subjected to an absolutely necessary
replacement
of the tertiary amine in the adduct by a weaker, less volatile nitrogen base
before
thermal dissociation of the adduct in order to aid or make possible the
subsequent
thermal dissociation to produce free formic acid. In a manner analogous to
EP 0 181 078 A, imidazole derivatives such as 1-n-butylimidazole are also
mentioned
here as particularly suitable weaker nitrogen bases.
A disadvantage of the process of EP 0 357 243 A is the very complicated, three-
stage
work-up of the reaction product mixture by
PF 62287
CA 02765430 2011-12-14
(i) separating the two liquid phases and recirculating the phase comprising
the
homogeneous catalyst and the nonpolar solvent to the hydrogenation stage;
(ii) replacing the tertiary amine in the adduct of formic acid and the
tertiary amine of
the other phase by a weaker, less volatile nitrogen base in a reaction vessel
with
5 superposed distillation column and recirculating the tertiary amine
liberated to the
hydrogenation stage; and
(iii) thermally dissociating the adduct of formic acid and the weaker nitrogen
base and
recirculating the weaker nitrogen base liberated to the base replacement
stage.
A further disadvantage of the process of EP 0 357 243 A is the use of two
solvents and
thus introduction of a further substance into the overall process.
As an alternative, EP 0 357 243 A also discloses the possibility of using only
one
solvent. In this case, the addition of the polar solvent in which the adduct
of formic acid
and the tertiary amine would otherwise dissolve is omitted. The sole solvent
used here
is the nonpolar solvent which dissolves the homogeneous catalyst. However,
this
alternative also has the disadvantage of the very complicated, three-stage
work-up as
described above.
DE 44 31 233 A likewise describes the hydrogenation of carbon dioxide in the
presence of a catalyst comprising a transition metal of transition group VIII
(groups 8,
9, 10), a tertiary amine and a polar solvent and water to form an adduct of
formic acid
and the tertiary amine, in which, however, the catalyst is present in
heterogeneous
form and the active component is applied to a inert support. Preferred
tertiary amines
are C1-C8-trialkylamines, polyamines having from 2 to 5 amino groups, aromatic
nitrogen heterocycles such as pyridine or N-methylimidazole and also cyclic
and/or
bridged amines such as N-methylpiperidine, 1,8-diazabicyclo[5.4.0]undec-7-ene
or
1,4-diazabicyclo[2.2.2]octane. As suitable polar solvents, mention is made of
the low-
boiling C1-C4-monoalcohols, and, in a manner analogous to EP 0 095 321 A,
secondary alcohols are preferred. The hydrogenation is carried out at a total
pressure
of from 4 to 20 MPa (from 40 to 200 bar) and a temperature of from 50 to 200
C. For
the work-up of the adduct of formic acid and tertiary amine which is formed,
DE 44 31 233 A teaches the use of known methods with explicit reference to the
work-
up with replacement of the tertiary amine in the adduct of formic acid and the
tertiary
amine by a weaker, less volatile nitrogen base as disclosed in EP 0 357 243 A.
In a
manner analogous to the process of EP 0 357 243 A, the process of DE 44 31 233
A
also has the disadvantage of the very complicated, three-stage work-up of the
reaction
product mixture.
It was an object of the present invention to discover a process for preparing
formic acid
by hydrogenation of carbon dioxide, which does not have the abovementioned
disadvantages of the prior art or suffers from them only to a significantly
reduced extent
PF 62287 CA 02765430 2011-12-14
6
and allows concentrated formic acid to be obtained in a high yield and high
purity.
Furthermore, the process should be able to be carried out in a simple manner
or at
least a simpler manner than is described in the prior art, for example by
means of a
different, simpler process concept, simpler process stages, a reduced number
of
process stages or simpler apparatuses. In addition, the process should also be
able to
be carried out with a reduced consumption of energy.
We have accordingly found a process for preparing formic acid by hydrogenation
of
carbon dioxide in the presence of a catalyst comprising an element of group 8,
9 or 10
of the Periodic Table, a tertiary amine (I) and a polar solvent (III) at a
pressure of from
0.2 to 30 MPa abs and a temperature of from 20 to 200 C to form two liquid
phases,
separation of the one liquid phase (A) enriched with the formic acid/amine
adduct (II)
from the other liquid phase (B) and recirculation of the liquid phase (B) to
the
hydrogenation reactor, wherein
(a) an amine which at a pressure of 1013 hPa abs has a boiling point which is
at least
5 C higher than that of formic acid and is present in enriched form in the
liquid
phase (B) is used as tertiary amine (I);
(b) a solvent whose electrostatic factor is >_ 200 - 10-30 Cm and which at a
pressure of
1013 hPa abs has a boiling point which is at least 5 C higher than that of
formic
acid and is present in enriched form in the liquid phase (A) is used as polar
solvent (III);
(c) the formic acid/amine adduct (II) of the liquid phase (A) which has been
separated
off is thermally dissociated into free formic acid and free tertiary amine (I)
in a
distillation unit;
(d) the free formic acid is removed by distillation; and
(e) the free tertiary amine (I) comprised in the bottoms from the distillation
unit and
the polar solvent (III) are recirculated to the hydrogenation reactor.
The catalyst to be used in the hydrogenation of carbon dioxide in the process
of the
invention can be heterogeneous or homogeneous in nature. It comprises an
element of
group 8, 9 or 10 of the Periodic Table, i.e. Fe, Co, Ni, Ru, Rh, Pd, Os, Ir
and/or Pt. The
catalyst preferably comprises Ru, Rh, Pd, Os, Ir and/or Pt, particularly
preferably Ru,
Rh and/or Pd, very particularly preferably Ru. The catalytically active
components can
be the metals themselves, for example in finely divided form, or complexes.
In the case of a heterogeneous catalyst, the elements mentioned are preferably
present as metals on an inert support. Inert support materials can be, for
example,
PF 62287 CA 02765430 2011-12-14
7
silicon dioxide, aluminum oxide, zirconium oxide or mixtures of these oxides
and also
graphite. As particularly preferred heterogeneous catalysts, mention may be
made of
Ru/aluminum oxide, Pd/graphite and triphenylphosphine complexes of Rh or Ru,
for
example bis(triphenylphosphine)ruthenium dichloride or
tris(triphenylphosphine)-
rhodium chloride, on silicon dioxide. The content of the elements mentioned is
generally from 0.1 to 10% by weight, based on the heterogeneous catalyst. The
heterogeneous catalysts can be used in various geometric shapes and sizes. In
the
case of a fixed-bed catalyst, use is made of, for example, pellets, cylinders,
hollow
cylinders, spheres, rods or extrudates. Their average particle diameter is
generally
from 2 to 5 mm. When heterogeneous catalysts are used, the amount of the
abovementioned metal component used is generally from 0.01 to 100% by weight,
based on the total weight of the catalyst, with all-active catalysts, for
example Raney
nickel or nanopalladium, being able to comprise up to 100% by weight of the
respective
metal. Suitable heterogeneous catalysts are commercially available or can be
obtained
by treatment of the support with solutions of the metal components and
subsequent
drying, heat treatment and/or calcination by known methods.
If a heterogeneous catalyst is used in the process of the invention, this
preferably
remains in the hydrogenation reactor. This is made possible, for example, by
it being
present in the form of a fixed-bed catalyst fixed in position in the reactor
or in the case
of a suspended catalyst being retained in the reactor by means of a suitable
screen or
a suitable filter.
In the case of a homogeneous catalyst, the abovementioned elements are
homogeneously dissolved in the form of complex-like compounds in the reaction
mixture. The homogeneous catalyst should be selected so that it is present in
enriched
form together with the tertiary amine (I) in the liquid phase (B). For the
present
purposes, "in enriched form" means a partition coefficient of the homogeneous
catalyst
P = [concentration of homogeneous catalyst in liquid phase (B)]/
[concentration of homogeneous catalyst in liquid phase (A)]
of > 1. The partition coefficient is preferably >_ 10 and particularly
preferably >_ 20. The
choice of the homogeneous catalyst is generally made by means of a simple test
in
which the partition coefficient of the desired homogeneous catalyst under the
planned
process conditions is determined experimentally.
Owing to their good solubility in tertiary amines (I), preference is given to
using metal-
organic complexes comprising an element of group 8, 9 or 10 of the Periodic
Table and
at least one phosphine group having at least one unbranched or branched,
acyclic or
cyclic, aliphatic radical having from 1 to 12 carbon atoms, where individual
carbon
atoms can also be substituted by >P-, as homogeneous catalysts in the process
of the
PF 62287 CA 02765430 2011-12-14
8
invention. Branched cyclic aliphatic radicals thus also include radicals such
as
-CH2-C6H11. Suitable radicals are, for example, methyl, ethyl, 1-propyl, 2-
propyl,
1-butyl, 2-butyl, 1-(2-methyl)propyl, 2-(2-methyl)propyl, 1-pentyl, 1-hexyl, 1-
heptyl,
1-octyl, 1-nonyl, 1-decyl, 1-undecyl, 1-dodecyl, cyclopentyl, cyclohexyl,
cycloheptyl and
cyclooctyl, methylcyclopentyl, methylcyclohexyl, 1-(2-methyl)pentyl 1-(2-
ethyl)hexyl,
1-(2-propyl)heptyl and norbonyl. The unbranched or branched, acyclic or
cyclic,
aliphatic radical preferably comprises at least 1 and preferably not more than
10 carbon
atoms. In the case of an exclusively cyclic radical in the abovementioned
sense, the
number of carbon atoms is from 3 to 12 and preferably at least 4 and also
preferably
not more than 8. Preferred radicals are 1-butyl, 1-octyl and cyclohexyl.
The phosphine group can comprise one, two or three of the abovementioned
unbranched or branched, acyclic or cyclic, aliphatic radicals. These can be
identical or
different. The phosphine group preferably comprises three of the
abovementioned
unbranched or branched, acyclic or cyclic, aliphatic radicals and particular
preference
is given to all three radicals being identical. Preferred phosphines are P(n-
CnH2n+1)3
where n is from 1 to 10, particularly preferably tri-n-butylphosphine, tri-
n-octylphosphine, very particularly preferably tri-n-butylphosphine and tri-
n-octylphosphine and in particular tri-n-butylphosphine.
As mentioned above, individual carbon atoms can also be substituted by >P- in
the
abovementioned unbranched or branched, acyclic or cyclic, aliphatic radicals.
Polydentate, for example bidentate or tridentate, phosphine ligands are thus
also
comprised. These preferably comprise the group >P-CH2CH2-P< or
I
>P-CH 2CH2 P-CH2CH2 P<
If the phosphine group comprises radicals other than the abovementioned
unbranched
or branched, acyclic or cyclic, aliphatic radicals, these generally correspond
to those
which are otherwise customarily used in phosphine ligands for metal-organic
complex
catalysts. Examples which may be mentioned are phenyl, tolyl and xylyl.
The metal-organic complex can comprise one or more, for example two, three or
four,
of the abovementioned phosphine groups having at least one unbranched or
branched,
acyclic or cyclic, aliphatic radical. The remaining ligands of the metal-
organic complex
can have various natures. Examples which may be mentioned are hydride,
fluoride,
chloride, bromide, iodide, formate, acetate, propionate, carboxylates,
acetylacetonate,
carbonyl, dimethyl sulfoxide, hydroxide, trialkylamine, alkoxides.
The homogeneous catalysts can either be used directly in their active form or
be
generated only under reaction conditions from customary standard complexes
such as
[M(p-cymene)C12]2, [M(benzene)CI2],,, [M(COD)(allyl)], [MCI3 x H201,
PF 62287 CA 02765430 2011-12-14
9
[M(acetylacetonate)3],= [M(DMSO)4CI2] where M is an element of group 8, 9 or
10 of the
Periodic Table by addition of the corresponding phosphine ligand or ligands.
Homogeneous catalysts which are preferred in the process of the invention are
[RU(PnBU3)4(H)2], [Ru(P^OCtyl3)4(H)2], [RU(PnBU3)2(1,2-
bis(dicyclohexylphosphino)-
ethane)(H)2], [Ru(Pnoctyl3)2(1,2-bis(dicyclohexylphosphino)ethane)(H)2]. By
means of
these, TOF (turnover frequency) values of greater than 1000 h-1 can be
achieved in the
hydrogenation of carbon dioxide.
When homogeneous catalysts are used, the amount of the specified metal
component
in the metal-organic complex which is used is generally from 0.1 to 5000 ppm
by
weight, preferably from 1 to 800 ppm by weight and particularly preferably
from 5 to
500 ppm by weight, in each case based on the total liquid reaction mixture in
the
hydrogenation reactor.
In the process of the invention, preference is given to using a homogeneous
catalyst as
catalyst in the hydrogenation of carbon dioxide.
When a homogeneous catalyst is used, this is also present in enriched form in
the
liquid phase (B) and can thus be largely recirculated to the hydrogenation
reactor via
the liquid phase (B); thus, the liquid phase (A) enriched in the formic
acid/amine adduct
(II) generally still comprises valuable residual amounts of catalyst. These
can lead to a
backreaction with decomposition into carbon dioxide and hydrogen in the
thermal
dissociation, which equates to a decrease in the yield of formic acid. In
addition, the
homogeneous catalyst carried through the thermal dissociation can generally
not be
used in the hydrogenation step without renewed activation or work-up. It is
therefore
preferred, in the process of the invention, that in feature (e) the bottoms
from the
distillation unit are separated into a phase comprising the free tertiary
amine (I) and a
phase comprising the polar solvent (III) and the two phases are separately
recirculated
to the hydrogenation reactor, with the phase comprising the free tertiary
amine (I) being
recirculated via an extraction unit to the hydrogenation reactor and, in said
extraction
unit, extracting homogeneous catalyst from the liquid phase (A) which has been
separated off, before the formic acid/amine adduct (II) in the liquid phase
(A) which has
been separated off is, according to feature (c), thermally dissociated into
free formic
acid and free tertiary amine (I) in a distillation unit.
The extraction is generally carried out at a temperature of from 0 to 150 C
and a
pressure of from 0.1 to 8.0 MPa abs. The temperature is preferably at least 20
C and
particularly preferably at least 30 C and also preferably not more than 100 C
and
particularly preferably not more than 80 C. The pressure is preferably at
least
0.01 MPa abs. and particularly preferably at least 0.1 MPa abs. and also
preferably not
more than 10 MPa abs. and particularly preferably not more than 1 MPa abs.
PF 62287 CA 02765430 2011-12-14
The extraction unit comprises both a facility for extraction and a facility
for separating
the two liquid phases. The two parts can be integrated together in one
apparatus or be
divided over a plurality of apparatuses. In principle, the extraction
mentioned can be
carried out in any suitable apparatus known to those skilled in the art.
Preference is
5 given to using a countercurrent extraction column, a mixer-settler cascade
or a
combination of mixer-settlers with columns.
The additional extraction step is particularly advantageously used when the
liquid
phase (A) enriched in the formic acid/amine adduct (II) still comprises more
than
10 10 ppm by weight of catalyst metal. However, it is also advisable to use
the additional
extraction step even when more than 1 ppm by weight of catalyst metal is
present in
the liquid phase (A).
It can be advantageous to integrate an apparatus for the adsorption of traces
of the
homogeneous catalyst into the apparatus between the extraction unit and the
distillation unit in which the thermal dissociation occurs. Suitable
adsorbents are known
to those skilled in the art. Examples of suitable adsorbents are, for example,
polyacrylic
acid and salts thereof, sulfonated polystyrenes and salts thereof, activated
carbons,
montmorillonites, bentonites, silica gels and zeolites.
The tertiary amine (I) to be used in the hydrogenation of carbon dioxide in
the process
of the invention has a boiling point which is at least 5 C higher than that of
formic acid
at a pressure of 1013 hPa abs. The tertiary amine (I) is to be selected or
matched to
the polar solvent (III) so that the tertiary amine (I) is present in enriched
form in the
liquid phase (B). For the present purposes, "in enriched form" means a
proportion by
weight of > 50% of the free, i.e. not bound in the form of the formic
acid/amine adduct
(II), tertiary amine (I) in the liquid phase (B), based on the total amount of
free, tertiary
amine (I) in the two liquid phases (A) and (B). The proportion by weight is
preferably
> 90%, particularly preferably > 95% and very particularly preferably > 97%.
The
tertiary amine (I) is generally selected by means of a simple test in which
the solubility
of the desired tertiary amine (I) in the two liquid phases (A) and (B) under
the planned
process conditions is determined experimentally.
The tertiary amine (I) to be used preferably has a boiling point which is at
least 10 C
higher, particularly preferably at least 50 C higher and very particularly
preferably at
least 100 C higher, than that of formic acid. A restriction in terms of an
upper limit to
the boiling point is not necessary since a very low vapor pressure of the
tertiary amine
(I) is in principle an advantage for the process of the invention. In general,
the boiling
point of the tertiary amine (I) at a pressure of 1013 hPa abs, optionally at a
pressure
extrapolated by known methods from vacuum to 1013 hPa abs, is below 500 C.
The tertiary amine (I) which is preferably to be used in the process of the
invention is
PF 62287 CA 02765430 2011-12-14
11
an amine of the general formula (la)
NR1R2R3 (la),
where the radicals R1 to R3 are identical or different and are each,
independently of one
another, an unbranched or branched, acyclic or cyclic, aliphatic, araliphatic
or aromatic
radical having in each case from 1 to 16 carbon atoms, preferably from 1 to 12
carbon
atoms, where individual carbon atoms can also be substituted, independently of
one
another, by a hetero group selected from the group consisting of -0- and >N-
or two or
all three radicals can also be joined to one another to form a chain
comprising at least
four atoms in each case.
Examples of suitable amines are:
= Tri-n-propylamine (bp1ol3 hPa = 156 C), tri-n-butylamine, tri-n-pentylamine,
tri-
n-hexylamine, tri-n-heptylamine, tri-n-octylamine, tri-n-nonylamine, tri-n-
decylamine,
tri-n-undecylamine, tri-n-dodecylamine, tri-n-tridecylamine, tri-n-
tetradecylamine, tri-
n-pentadecylamine, tri-n-hexadecylamine, tri(2-ethylhexyl)amine.
= Dimethyldecylamine, dimethyldodecylamine, dimethyltetradecylamine, ethyldi(2-
propyl)amine (bp,013 hPa = 127 C), dioctylmethylamine, dihexylmethylamine.
= Tricyclopentylamine, tricyclohexylamine, tricycloheptylamine,
tricyclooctylamine and
derivatives thereof which are substituted by one or more methyl, ethyl, 1-
propyl,
2-propyl, 1-butyl, 2-butyl or 2-methyl-2-propyl groups.
= Dimethylcyclohexylamine, methyldicyclohexylamine, diethylcyclohexylamine,
ethyldicyclohexylamine, dimethylcyclopentylamine, methyldicyclopentylamine.
= Triphenylamine, methyldiphenylamine, ethyldiphenylamine,
propyldiphenylamine,
butyldiphenylamine, 2-ethylhexyldiphenylamine, dimethylphenylamine,
diethylphenylamine, dipropylphenylamine, dibutylphenylamine, bis(2-
ethylhexyl) phenyla mine, tribenzylamine, methyldibenzylamine,
ethyldibenzylamine
and derivatives thereof which are substituted by one or more methyl, ethyl, 1-
propyl,
2-propyl, 1-butyl, 2-butyl or 2-methyl-2-propyl groups.
= N-C,-C,2-alkylpiperidines, N,N-di-C,-C12-alkylpiperazines, N-C,-C12-
alkylpyrrolidines,
N-C,-C,2-alkylimidazoles and derivatives thereof which are substituted by one
or
more methyl, ethyl, 1-propyl, 2-propyl, 1-butyl, 2-butyl or 2-methyl-2-propyl
groups.
= 1,8-Diazabicyclo[5.4.0]undec-7-ene ("DBU"), 1,4-diazabicyclo[2.2.2]octane
("DABCO"), N-methyl-8-azabicyclo[3.2. 1 ]octane ("tropane"), N-methyl-
PF 62287
CA 02765430 2011-12-14
12
9-azabicyclo[3.3.I]nonane ("granatane"), 1-azabicyclo[2.2.2]octane
("quinuclidine").
It is naturally also possible to use mixtures of various tertiary amines (I)
in the process
of the invention.
Among the above-described tertiary amines of the general formula (Ia),
preference is
given to those in which the radicals R1 to R3 are identical or different and
are each,
independently of one another, an unbranched or branched, acyclic or cyclic,
aliphatic,
araliphatic or aromatic radical having in each case from 1 to 16 carbon atoms,
preferably from 1 to 12 carbon atoms, where individual carbon atoms can,
independently of one another, also be substituted by a hetero group selected
from the
group consisting of -0- and >N- or two or all three radicals can also be
joined to one
another to form a saturated chain comprising at least four atoms in each case.
Preference is given to at least one of the radicals bearing two hydrogen atoms
on the
alpha-carbon atom.
In the process of the invention, particular preference is given to using an
amine of the
general formula (Ia) in which the radicals R1 to R3 are selected independently
from the
group consisting of C,-C12-alkyl, C5-C8-cycloalkyl, benzyl and phenyl as
tertiary amine
(I).
Particular preference is given to using a saturated amine of the general
formula (la) as
tertiary amine (I) in the process of the invention.
Very particular preference is given to using an amine of the general formula
(Ia) in
which the radicals RI to R3 are selected independently from the group
consisting of
C5-C8-alkyl, in particular tri-n-pentylamine, tri-n-hexylamine, tri-n-
heptylamine, tri-
n-octylamine, dimethylcyclohexylamine, methyldicyclohexylamine,
dioctylmethylamine
and dimethyldecylamine, as tertiary amine (I) in the process of the invention.
The tertiary amine (I) is preferably present in liquid form in all process
stages of the
process of the invention. However, this is not absolutely necessary. It would
also be
sufficient for the tertiary amine (I) to be at least dissolved in suitable
solvents. Suitable
solvents are in principle those which are chemically inert in respect of the
hydrogenation of carbon dioxide and the thermal dissociation of the adduct and
in
which the tertiary amine (I) and, if a homogeneous catalyst is used, also the
latter
readily dissolve but do not readily dissolve the polar solvent (III) and the
formic
acid/amine adduct (II) and at a pressure of 1013 hPa abs have a boiling point
which is
at least 5 C higher than that of formic acid. Possibilities are therefore in
principle
chemically inert, nonpolar solvents such as aliphatic, aromatic or araliphatic
hydrocarbons, for example octane and higher alkanes, toluene, xylenes.
PF 62287 CA 02765430 2011-12-14
13
The amount of the tertiary amine (I) to be used in the process of the
invention is
generally from 5 to 95% by weight, preferably from 20 to 60% by weight, in
each case
based on the total liquid reaction mixture in the hydrogenation reactor.
The polar solvent (III) to be used in the hydrogenation of carbon dioxide in
the process
of the invention has an electrostatic factor, also referred to as EF for
short, of
>_ 200. 10-30 cm and at a pressure of 1013 hPa abs has a boiling point which
is at least
5 C higher than that of formic acid. The polar solvent (III) is to be selected
or matched
to the tertiary amine (I) so that the polar solvent (III) is present in
enriched form in the
liquid phase (A). For the present purposes, "in enriched form" means a
proportion by
weight of > 50% of the polar solvent (III) in the liquid phase (A) based on
the total
amount of polar solvent (III) in the two liquid phases (A) and (B). The
proportion by
weight is preferably > 90%, particularly preferably > 95% and very
particularly
preferably > 97%. The polar solvent (III) is generally selected by means of a
simple test
in which the solubility of the desired polar solvent (III) in the two liquid
phases (A) and
(B) under the planned process conditions is determined experimentally.
The electrostatic factor EF is defined as the product of the relative
dielectric constant Er
and the dipole moment p (see, for example, C. Reichardt, "Solvents and Solvent
Effects in Organic Chemistry", 3rd edition, Wiley-VCH Verlag GmbH & Co KGaA,
Weinheim 2003, chapter 3.2, page 67 bottom to page 68 top). The abovementioned
minimum value of the electrostatic factor ensures that the polar solvent (III)
has a
certain minimum polarity and the formic acid/amine adduct (II) preferably
dissolves
therein.
The polar solvent (III) to be used preferably has a boiling point which is at
least 10 C
higher, particularly preferably at least 50 C higher and very particularly
preferably at
least 85 C higher, than that of formic acid. A restriction in terms of an
upper limit to the
boiling point is not necessary since a very low vapor pressure of the polar
solvent (III)
is in principle an advantage for the process of the invention. In general, the
boiling point
of the polar solvent (III) at a pressure of 1013 hPa abs, optionally at a
pressure
extrapolated by known methods from vacuum to 1013 hPa abs, is below 500 C.
As classes of substances suitable as polar solvents (III), preference is given
to diols
and also the formic esters thereof, polyols and the formic esters thereof,
sulfones,
sulfoxides, open-chain or cyclic amides and also mixtures of the classes of
substance
mentioned.
As suitable diols and polyols, mention may be made by way of example of
ethylene
glycol (EF = 290.3 = 10-30 cm), diethylene glycol (EF = 244.0 = 10.30 cm),
triethylene
glycol, polyethylene glycol, 1,3-propanediol (EF = 285.6 = 10-30 cm), 2-methyl-
1,3-propanediol, 1,4-butanediol (EF = 262.7. 10-30 cm), dipropylene glycol,
PF 62287 CA 02765430 2011-12-14
14
1,5-pentanediol (EF = 212.5. 10-30 cm), 1,6-hexanediol and glycerol. Diols and
polyols
can, due to their OH groups, be esterified in the presence of formic acid. In
the process
of the invention, this occurs particularly in the thermal dissociation of the
formic
acid/amine adduct (II) in the abovementioned distillation unit. Since the
formic esters
formed display a very similar phase behavior, they are likewise well suited as
polar
solvent. The water formed in the esterification, which can be an amount of up
to 5% by
weight based on the total hydrogenation mixture, also does not interfere
either in the
hydrogenation or in the thermal dissociation. Accumulation of water in
continuous
operation of the process of the invention does not occur since water in these
small
amounts can be separated off in the formic acid-high-boiler azeotrope via a
side offtake
in the distillation unit for thermal dissociation. This may even be
advantageous, when
using diols or polyols, to add additional water in order to shift the
equilibrium between
the OH groups and the formic ester groups in the direction of the OH groups.
In the
case of an addition of water, the amount of water added is generally from 0.1
to 20%
by weight based on the total liquid reaction mixture in the hydrogenation
reactor.
Suitable sulfoxides are, for example, dialkyl sulfoxides, preferably C,-C6-
dialkyl
sulfoxides, in particular dimethyl sulfoxide (EF = 627.1 = 10-30 cm).
Suitable open-chain or cyclic amides are, for example, formamide (EF = 1243.2
10-30 cm), N-methylformamide (EF = 2352.9 .10-30 cm), N,N-dimethylformamide
(EF =
396.5 = 10-30 cm), N-methylpyrrolidone (EF = 437.9. 10-30 cm), acetamide and
N-methylcaprolactam.
In the process of the invention, preference is given to using an aliphatic,
saturated
hydrocarbon having from 2 to 5 OH groups or a formic ester thereof as polar
solvent
(III). Particularly preferred diols and polyols are ethylene glycol, 1,4-
butanediol,
2-methyl-1,3-propanediol and formic esters thereof.
The molar ratio of the polar solvent (III) to be used in the process of the
invention to the
tertiary amine (I) used is generally from 0.5 to 30 and preferably from 2 to
20.
It is generally known that compounds comprising OH groups accelerate the
hydrogenation of carbon dioxide. Thus, for instance, EP 0 095 321 A, EP 0 151
510 A,
EP 0 181 078 A, EP 0 357 234 A and DE 44 31 233 A teach the addition of water.
In
the present process of the invention, too, the use of compounds comprising OH
groups
as promoter for the hydrogenation of carbon dioxide is preferred in principle.
However,
since the abovementioned diols and polyols likewise exercise such a positive
effect, in
the case of these the addition of further compounds comprising OH groups is
generally
not necessary. On the other hand, when other polar solvents (III) which do not
comprise any OH groups are used, for example formic esters, sulfones,
sulfoxides or
open-chain or cyclic amides, the situation is different. In these cases, it is
PF 62287 CA 02765430 2011-12-14
advantageous to add water and/or alcohols, for example aliphatic, saturated
monoalcohols. In general, an addition of from 1 to 500 mmol per mg of the
group 8 to
10 metal catalyst is sufficient.
5 The carbon dioxide to be used in the hydrogenation of carbon dioxide can be
used in
solid, liquid or gaseous form. It is also possible to use industrially
available gas
mixtures comprising carbon dioxide as long as these are largely free of carbon
monoxide. The hydrogen to be used in the hydrogenation of carbon dioxide is
generally
gaseous. Carbon dioxide and hydrogen can also comprise inert gases such as
nitrogen
10 or noble gases. However, the content of these is advantageously below 10
mol%
based on the total amount of carbon dioxide and hydrogen in the hydrogenation
reactor. Although larger amounts may likewise be tolerable, they generally
require the
use of a higher pressure in the reactor which in turn makes further
compression energy
necessary.
The hydrogenation of carbon dioxide is carried out in the liquid phase at a
temperature
of from 20 to 200 C and a total pressure of from 0.2 to 30 MPa abs. The
temperature is
preferably at least 30 C and particularly preferably at least 40 C and also
preferably
not more than 150 C, particularly preferably not more than 120 C and very
particularly
preferably not more than 80 C. The total pressure is preferably at least 1 MPa
abs and
particularly preferably at least 5 MPa abs and also preferably not more than
20 MPa
abs, particularly preferably not more than 15 MPa abs and especially not more
than
10 MPa abs.
The partial pressure of carbon dioxide is generally at least 0.5 MPa and
preferably at
least 2 MPa and also generally not more than 6 MPa and preferably not more
than
5 MPa. The partial pressure of hydrogen is generally at least 0.5 MPa and
preferably at
least 1 MPa and also generally not more than 250 MPa and preferably not more
than
10 MPa.
The molar ratio of hydrogen to carbon dioxide in the feed to the hydrogenation
reactor
is preferably from 0.1 to 10 and particularly preferably from 1 to 3.
The molar ratio of carbon dioxide to tertiary amine (I) in the feed to the
hydrogenation
reactor is generally from 0.1 to 10 and preferably from 0.5 to 3.
As hydrogenation reactors, it is in principle possible to use all reactors
which are
suitable in principle for gas/liquid reactions at the given temperature and
the given
pressure. Suitable standard reactors for liquid-liquid reaction systems are
indicated, for
example, in K.D. Henkel, "Reactor Types and Their Industrial Applications", in
Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH Verlag GmbH &
Co.
KGaA, DOI: 10.1002/14356007.b04_087, chapter 3.3 "Reactors for gas-liquid
PF 62287 CA 02765430 2011-12-14
16
reactions". Examples which may be mentioned are stirred tank reactors, tube
reactors
or bubble column reactors.
The hydrogenation of carbon dioxide in the process of the invention can be
carried out
batchwise or continuously. In the case of batch operation, the reactor is
charged with
the desired liquid and optionally solid starting materials and auxiliaries and
carbon
dioxide and hydrogen are subsequently introduced to the desired pressure at
the
desired temperature. After the reaction is complete, the reactor is generally
depressurized and the two liquid phases (A) and (B) which are formed are
separated
from one another. In the continuous mode of operation, the starting materials
and
auxiliaries including the carbon dioxide and hydrogen are introduced
continuously.
However, any heterogeneous fixed-bed catalyst to be used is present beforehand
in
fixed form in the reactor. Accordingly, the liquid phase is continuously
discharged from
the reactor so that the average liquid level in the reactor remains constant.
Preference
is given to the continuous hydrogenation of carbon dioxide.
The average residence time in the reactor is generally from 10 minutes to 5
hours.
The formic acid/amine adduct (II) formed in the hydrogenation of carbon
dioxide in the
presence of the catalyst to be used and the tertiary amine (I) usually has the
general
formula (Ila)
NR1R2R3 = x HCOOH (Ila)
where the radicals R1 to R3 are the radicals described for the tertiary amine
(Ia) and x is
from 0.5 to 5, preferably from 0.7 to 1.5. The factor x can be determined, for
example,
by titration with an alcoholic KOH solution against phenolphthalein. The
precise
composition of the formic acid/amine adduct (II) depends on many parameters,
for
example the prevailing concentrations of formic acid and tertiary amine (I),
pressure,
temperature or the presence and nature of further components, in particular a
polar
solvent (III). The composition of the formic acid/amine adduct (II) can
therefore also
change over the individual process steps in which the formic acid/amine adduct
(II) is in
each case referred to in the present patent application. The composition of
the formic
acid/amine adduct (II) can easily be determined in each process step by
determining
the formic acid content by acid-base titration and determining the amine
content by gas
chromatography.
Two liquid phases are formed in the hydrogenation of carbon dioxide by the
process of
the invention. Liquid phase (A) is enriched with the formic acid/amine adduct
(II) and
the polar solvent (III). With regard to the formic acid/amine adduct (II),
"enriched"
means a partition coefficient of the formic acid/amine adduct (II)
PF 62287 CA 02765430 2011-12-14
17
P = [concentration of formic acid/amine adduct (II) in liquid phase (A)]/
[concentration of formic acid/amine adduct (II) in liquid phase (B)]
of > 1. The partition coefficient is preferably >_ 2 and particularly
preferably >_ 5. The
liquid phase (B) is enriched with the tertiary amine (I). If a homogeneous
catalyst is
used, this is likewise present in enriched form in the liquid phase (B).
The two liquid phases (A) and (B) formed are, in the process of the invention,
separated from one another and the liquid phase (B) is recirculated to the
hydrogenation reactor. Recirculation of a further liquid phase comprising
unreacted
carbon dioxide present in addition to the two abovementioned liquid phases and
also of
a gas phase comprising unreacted carbon dioxide and/or unreacted hydrogen to
the
hydrogenation reactor may also be advantageous. It may also be desirable, for
example to discharge undesirable by-products or impurities, to discharge part
of the
liquid phase (B) and/or part of the carbon dioxide or liquid or gaseous phases
comprising carbon dioxide and hydrogen from the process.
The two liquid phases (A) and (B) are generally separated by gravimetric phase
separation. This may be carried out using, for example, standard apparatuses
and
standard methods which are described, for example, in E. Muller et al.,
"Liquid-Liquid
Extraction", in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-
VCH Verlag
GmbH & Co. KGaA, DOI:10.1002/14356007.b03_06, chapter 3 "Apparatus". In
general, the liquid phase (A) enriched with the formic acid/amine adduct (II)
and the
polar solvent (III) is heavier and forms the lower phase.
The phase separation can be effected, for example, by depressurization,
preferably to
about or close to atmospheric pressure, and cooling of the liquid reaction
mixture, for
example to about or close to ambient temperature. However, there is a risk
that at least
part of the gas dissolved in the liquid phases at the higher reaction
pressure, in
particular carbon dioxide, will degas during the depressurization and have to
be
compressed separately as a gas stream and recirculated to the hydrogenation
reactor.
Likewise, the liquid phase (B) also has to be compressed separately before
recirculation to the hydrogenation reactor. Each of the two compressor stages
for the
gas and liquid phases to be recirculated requires a suitable compressor
designed
appropriately for the pressure difference to be overcome and consumes
additional
energy in operation.
In the context of the present invention, it has surprisingly been found that
in the case of
the present system, i.e. a liquid phase (A) enriched with the formic
acid/amine adduct
(II) and the polar solvent (III) and a liquid phase (B) enriched with the
tertiary amine (I)
and in the case of the use of a homogeneous catalyst also with this, the two
liquid
phases separate very well from one another even at a significantly elevated
pressure.
CA 02765430 2011-12-14
PF 62287
18
The separation of the one liquid phase (A) enriched with the formic acid/amine
adduct
(II) and the polar solvent (III) from the other liquid phase (B) enriched with
the tertiary
amine (I) and the recirculation of the liquid phase (B) to the hydrogenation
reactor are
therefore preferably carried out at a pressure of from 1 to 30 MPa abs in the
process of
the invention. Depending on the total pressure in the hydrogenation reactor,
the
pressure is preferably not more than 15 MPa abs and particularly preferably
not more
than 10 MPa abs. It is even possible to separate the two liquid phases from
one
another without prior depressurization and recirculate the liquid phase (B) to
the
hydrogenation reactor without an appreciable pressure increase. In this case,
and also
in the case of an only slight depressurization, it is possible to entirely
dispense with
recirculation of any gas phase. Whether this omission is possible for the
respective
specific system should be determined beforehand in the case of doubt by simple
experimental examples.
The phase separation is particularly preferably carried out at a pressure of
at least
50%, very particularly preferably at least 90% and in particular at least 95%,
of the
reaction pressure. The pressure in the phase separation is particularly
preferably not
more than 105% and very particularly preferably not more than 100% of the
reaction
pressure.
It has surprisingly also been found that in the case of the present system the
two liquid
phases separate very readily from one another even at an elevated temperature
corresponding to the reaction temperature. In this case, no cooling is
necessary for the
phase separation and no subsequent heating of the liquid phase (B) to be
recirculated
is required, which likewise saves energy.
Established experience with phase separation under elevated pressure and at
elevated
temperature is surpassed by the liquid phase (B) of the system according to
the
invention having a particularly high absorption capacity for carbon dioxide
under
superatmospheric pressure. This means that any excess carbon dioxide which has
not
reacted in the hydrogenation reaction is highly preferentially present in the
liquid phase
(B) and can thus be recirculated without problems as liquid to the reactor.
The formic acid/amine adduct (II) in the liquid phase (A) which has been
separated off
is then thermally dissociated into free formic acid and free tertiary amine
(I) in a
distillation unit, with the free formic acid formed being removed by
distillation and the
free tertiary amine (I) present in the bottoms from the distillation unit and
also the polar
solvent (III) being recirculated to the hydrogenation reactor. The formic acid
liberated
can be taken off, for example, (i) at the top, (ii) at the top and as side
offtake stream or
(iii) only as side offtake stream. If formic acid is taken off at the top, a
formic acid purity
of up to 99.9% by weight is possible. When formic acid is taken off as side
offtake
stream, aqueous formic acid is obtained, with a mixture comprising about 85%
by
PF 62287 CA 02765430 2011-12-14
19
weight of formic acid being of particular importance here in industrial
practice.
Depending on the water content of the feed to the distillation unit, the
majority of the
formic acid is taken off as overhead product or as side product. If necessary,
it is even
possible to take off formic acid only as side product, in which case the
required amount
of water may be deliberately added. The thermal dissociation of the formic
acid/amine
adduct (II) is generally carried out under the process parameters known from
the prior
art in respect of pressure, temperature and configuration of the apparatus.
Thus, for
example, reference may be made to the descriptions in EP 0 181 078 A or
WO 2006/021,411. The distillation unit to be used generally comprises a
distillation
column which generally comprises random packing elements, ordered packings
and/or
bubble cap trays.
The bottom product taken off from the distillation unit can still comprise
small residual
amounts of formic acid, but the molar ratio of formic acid to tertiary amine
(I) is
preferably <_ 0.1 and particularly preferably s 0.05.
In general, the temperature at the bottom of the distillation column is at
least 130 C,
preferably at least 150 C and particularly preferably at least 170 C, and
generally not
more than 210 C, preferably not more than 190 C and particularly preferably
not more
than 185 C. The pressure is generally at least 1 hPa abs, preferably at least
50 hPa
abs and particularly preferably at least 100 hPa abs, and generally not more
than
500 hPa abs, preferably not more than 300 hPa abs and particularly preferably
not
more than 250 hPa abs.
A water-comprising stream of formic acid is optionally taken off as side
product. In the
case of addition of water, for example to promote the hydrogenation, this is
even
particularly advantageous.
DE 34 28 319 A has described the thermal dissociation of an adduct of formic
acid and
a tertiary amine having C6-C14-alkyl radicals in a dissociation column.
Likewise,
WO 2006/021,411 also describes the thermal dissociation of an adduct of formic
acid
and a tertiary amine having a boiling point at atmospheric pressure of from
105 to
175 C in a dissociation column. EP 0 563 831 A similarly discloses the thermal
dissociation of an adduct of formic acid and a tertiary amine having a boiling
point
higher than that of formic acid, with added formamide being said to give a
particularly
color-stable formic acid.
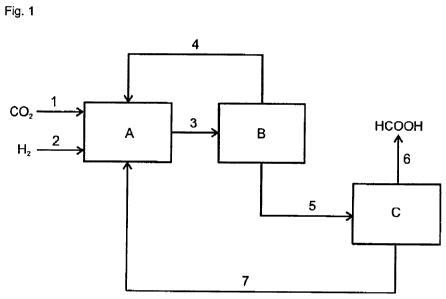
Figure 1 shows a schematic block diagram of a possible embodiment of the
process of
the invention. Here, the individual letters have the following meanings:
A = hydrogenation reactor
B = phase separator
PF 62287 CA 02765430 2011-12-14
C = distillation unit
Carbon dioxide and hydrogen are fed into the hydrogenation reactor "A". In
this reactor,
the carbon dioxide and hydrogen are reacted in the presence of a catalyst
comprising
5 an element of group 8, 9 or 10 of the Periodic Table, a tertiary amine (I)
and a polar
solvent (III) to form a formic acid/amine adduct (II). The two liquid phases
(A) and (B)
are formed. Liquid phase (A) is enriched with the formic acid/amine adduct
(II) and the
polar solvent (III), while liquid phase (B) is enriched with the tertiary
amine (I) and in the
case of the use of a homogeneous catalyst also with the latter. The two liquid
phases
10 are fed to a phase separator "B" and separated from one another. Liquid
phase (B),
which is generally the upper phase, is recirculated to the hydrogenation
reactor "A".
Liquid phase (A) is fed to a distillation unit "C" and the formic acid/amine
adduct (II)
formed in the hydrogenation reactor "A" is thermally dissociated therein into
free formic
acid and tertiary amine (I). The free formic acid is, for example, removed as
overhead
15 product. The bottoms from the distillation unit "C" are recirculated to the
hydrogenation
reactor "A".
It is of course possible to supplement the process of the invention by further
process
steps or inflows or outflows of streams if required. A nonlimiting example
which may be
20 mentioned is, for instance, the introduction of auxiliaries such as
tertiary amine (I),
polar solvent (III), homogeneous catalyst or water to maintain their
concentrations in
the process.
Figure 2 shows a schematic block diagram of a preferred embodiment of the
process of
the invention. Here, the individual letters have the following meanings:
A = hydrogenation reactor
B = phase separator
C = distillation column
D = phase separator
E = extraction apparatus
F = phase separator
As regards the carrying out of the process in the apparatuses "A" and "B",
what has
been said in respect of Figure 1 applies. In the preferred process as per
Figure 2, the
liquid phase (B), which is generally the upper phase, is also recirculated to
the
hydrogenation reactor "A". However, in this case the liquid phase (A) is
firstly subjected
to an extraction with tertiary amine (I) to be recirculated in order to
separate off further
homogeneous catalyst before being fed to the distillation column "C". This
extraction is
carried out in the extraction apparatus "E" with subsequent phase separation
in the
phase separator "F". In the latter, the two liquid phases (C) and (D)
separate. The liquid
phase (C), which is the amine phase enriched in the homogeneous catalyst and
PF 62287 CA 02765430 2011-12-14
21
generally represents the upper phase, is fed to the hydrogenation reactor "A".
The
liquid phase (D) which is enriched with the formic acid/amine adduct (II) and
the polar
solvent (III) is fed to the distillation column "C" and the formic acid/amine
adduct (II) is
thermally dissociated therein into free formic acid and tertiary amine (I).
The free formic
acid is, for example, removed as overhead product. The bottoms from the
distillation
column "C" are separated in the phase separator "D" into two liquid phases (E)
and (F).
The liquid phase (E), which comprises mainly the tertiary amine (I) and
generally
represents the upper phase, is fed to the extraction apparatus "E". The liquid
phase (F),
which comprises mainly the polar solvent (III), is recirculated to the
hydrogenation
reactor "A".
The process of the invention makes it possible to obtain concentrated formic
acid in
high yield and high purity by hydrogenation of carbon dioxide. In particular,
it provides a
particularly simple and elegant mode of operation which compared to the prior
art has a
simpler process concept, simpler process stages, a smaller number of process
stages
and simpler apparatuses. Thus, for example, if the tertiary amine (I) and the
polar
solvent are appropriately selected in the case of the use of a homogeneous
catalyst,
the latter is separated off from the formic acid/amine adduct (II) by phase
separation
and recirculated without further work-up steps to the hydrogenation reactor.
The
prompt separation of the catalyst from the formic acid/amine adduct (II)
formed
suppresses a backreaction with decomposition into carbon dioxide and hydrogen.
In
addition, losses of catalyst and thus losses of noble metal are minimized by
the
retention or removal of the catalyst as a result of the formation of two
liquid phases.
Furthermore, no complicated separate base replacement is required in the
process of
the invention, so that the formic acid/amine adduct (II) formed in the
hydrogenation
reactor can be used directly for the thermal dissociation. The tertiary amine
(I) liberated
here is recirculated to the hydrogenation reactor. This phase separation can
even be
carried out under superatmospheric pressure. The simpler process concept makes
it
possible for the production plant required for carrying out the process of the
invention
to be made more compact in the sense of a smaller space requirement and the
use of
fewer apparatuses compared to the prior art. It has a lower capital cost
requirement
and a lower energy consumption.
Further removal of the catalyst by means of the tertiary amine (I) to be
recirculated can
be effected by additional extraction of the stream comprising formic
acid/amine adduct
(II) before it is fed to the thermal dissociation. Owing to the resulting
still lower content
of homogeneous catalyst in the formic acid/amine adduct (II) to be
dissociated, a
possible backreaction in the dissociation with decomposition into carbon
dioxide and
hydrogen is suppressed even better. In addition, further introduction of
homogeneous
catalyst in the form of fresh catalyst is reduced further by the resulting
increased
recirculation factor of the homogeneous catalyst.
PF 62287 CA 02765430 2011-12-14
22
Examples
Unless indicated otherwise, the trialkylamines mentioned are in each case the
corresponding tri-n-alkylamines.
Examples A-1 to A-27 (hydrogenation and phase separation)
Unless indicated otherwise, a 250 ml autoclave made of Hastelloy C and
provided with
a magnetic stirrer bar was charged under inert conditions with tertiary amine,
polar
solvent and homogeneous catalyst to give, a two-phase mixture (upper phase:
amine
and catalyst; lower phase: solvent). The autoclave was subsequently closed and
CO2
was injected at room temperature. H2 was then injected and the reactor was
heated
while stirring (700 rpm). After the desired reaction time, the autoclave was
cooled and
the reaction mixture was depressurized. Unless indicated otherwise, a two-
phase
product mixture was obtained, with the upper phase being enriched with the
still free
tertiary amine and the homogeneous catalyst and the lower phase being enriched
with
the polar solvent and the formic acid/amine adduct formed. In some examples,
the
partition coefficient K of ruthenium
K = [Ru concentration in the upper phase] / [Ru concentration in the lower
phase]
was determined by means of atomic adsorption spectrometry (AAS). The total
content
of formic acid in the formic acid/amine adduct was determined by titration
with 0.1 N
KOH in 2-propanol against phenolphthalein with subtraction of the blank. The
TON, the
TOF and the reaction rate were calculated therefrom. The parameters and
results of
the individual experiments are shown in tables 1.1 to 1.7.
The blank results from small amounts of carbon dioxide also dissolving in the
phase
comprising formic acid/amine adduct and being able to be titrated by KOH
against
phenolphthalein. The blank was determined separately for each example by means
of
a fully analogous blank experiment in which only the homogeneous catalyst was
omitted and the emulsified total sample was titrated as described above at the
end.
Examples A-1 to A-27 show that high to very high reaction rates of even up to
above
1 mol kg-1 h-1 can be achieved in the process of the invention even with
variation of the
tertiary amine, the polar solvent, the catalyst in respect of the ligands and
the metal
component, the amount of catalyst and also with additional addition of water.
All
systems examined formed two phases, with the upper phase in each case being
enriched with the still free tertiary amine and the homogeneous catalyst and
the lower
phase in each case being enriched with the polar solvent and the formic
acid/amine
adduct formed.
PF 62287 CA 02765430 2011-12-14
23
Examples B-1 to B-12 (Phase behavior and C02 solubility under pressure)
In these examples, the phase behavior of mixtures comprising tertiary amine,
polar
solvent and formic acid/amine adduct in respect of the specific solubility of
C02 under
superatmospheric pressure was examined. For this purpose, 0.5 g of formic acid
was
in each case added to a two-phase mixture comprising 10.0 g of polar solvent
and
10.0 g of tertiary amine while stirring vigorously. The formic acid in each
case reacted
with the tertiary amine to form the corresponding formic acid/amine adduct
which in
each case dissolved in the phase of the polar solvent. 4 ml of the emulsion
obtained in
each case were introduced into a high-pressure sight cell and separation into
two
phases was awaited. The volume levels of the two liquid phases were
subsequently
marked and C02 was injected to 6.5 MPa abs at 20 C. Further C02 was in each
case
introduced until a constant pressure of 6.5 MPa abs was established. The
volume
levels of the two liquid phases were marked again after 15 minutes and
compared with
the original levels before injection of the C02. An increase in the phase
volume is
attributable to dissolution of CO2. The parameters and results of the
individual
experiments are shown in tables 2.1 to 2.3.
The examples firstly show that the two phases are retained in many systems
even at
high pressure and phase separation is thus still possible without problems
even at
6.5 MPa abs. Secondly, the volume increase of the upper liquid phase (B)
enriched
with the tertiary amine in the examples shows that many of the tertiary amines
tested
have a high CO2 absorption capacity.
In the preferred embodiment of phase separation and recirculation of the
liquid phase
(B) to the hydrogenation reactor under superatmospheric pressure, this is
particularly
advantageous since the CO2 can be recirculated in dissolved form. In addition,
a high
CO2 solubility in the liquid phase (B) is particularly advantageous for the
hydrogenation
when a homogeneous catalyst is used, since the homogeneous catalyst is present
in
the liquid phase (B) and a high concentration of dissolved C02 is thus present
as
starting material in its environment.
Examples C-1 to C-34 (distribution of the homogeneous catalysts)
In these examples, the distribution of various homogeneous catalysts in
various two-
phase mixtures comprising an upper phase comprising free tertiary amine and a
lower
phase comprising polar solvent and formic acid/amine adduct was examined. In
experiments C-1 to C-26, 5 g of the formic acid-trihexylamine adduct (prepared
from
formic acid and trihexylamine in a molar ratio of amine to formic acid of 1:2)
were in
each case dissolved in 5 g of the polar solvent at room temperature while
stirring and
admixed with 5 g of trihexylamine and 10 mg of the homogeneous Ru catalyst.
The
two-phase system obtained was stirred vigorously at room temperature for 30
minutes.
PF 62287 CA 02765430 2011-12-14
24
In experiments C-27 to C-34, 2.5 g of formic acid were in each case mixed with
20 g of
the respective amine and stirred at room temperature for 30 minutes. After the
reaction,
g of the lower phase were separated off or, in the case of single-phase
mixtures, the
reaction mixture was used and added to a mixture of 10 mg of [Ru(PnBu3)4(H)2],
5 g of
5 the respective amine and 5 g of ethylene glycol were added and the two-phase
system
obtained was stirred vigorously at room temperature for 10 minutes. After
separation of
the two phases in experiments C-1 to C-34, the partition coefficient K of
ruthenium
K = [Ru concentration in the upper phase]/[Ru concentration in the lower
phase]
was subsequently determined by means of atomic adsorption spectrometry (AAS).
The parameters and results of the individual experiments are shown in Tables
3.1 to
3.2.
The examples show that the partition coefficient K of ruthenium was
significantly above
1 in all systems tested, in some systems even significantly above 10. The
various
homogeneous Ru catalysts were enriched in the upper, amine-containing phase in
all
systems tested (various polar solvents, various tertiary amines).
PF 62287 CA 02765430 2011-12-14
Examples D-1 to D-2 (hydrogenation, phase -separation, extraction and catalyst
recirculation)
To examine the continuous hydrogenation, phase separation, extraction and
catalyst
5 recirculation, a laboratory plant as shown in Fig. 3 was used. Here, the
individual letters
have the following meanings:
A = hydrogenation reactor (270 ml autoclave made of Hastelloy C with blade
stirrer)
B = phase separator
E = extraction vessel (350 ml stirred glass vessel with glass blade stirrer)
10 F = phase separator
K = solvent pump
L = depressurization valve
M = recirculation pump for tertiary amine (I) and homogeneous catalyst
N = product stream pump
15 0 = amine pump
P = recirculation pump for tertiary amine (I) and extracted homogeneous
catalyst
Furthermore:
Solvent = solvent
20 Off-gas = off-gas
Amine (I) = tertiary amine (I)
Under inert conditions, an emulsion was produced by dissolving the homogeneous
catalyst in tertiary amine (I) and subsequently adding the polar solvent while
stirring.
25 The parameters of the individual experiments are shown in Tables 4.1 and
4.2. The
hydrogenation reactor "A" and the phase separator "B" were then charged with
the
emulsion produced. The extraction vessel "E" and the phase separator "F" were
charged with a mixture of tertiary amine (I) and polar solvent. The
hydrogenation
reactor "A" was then heated while stirring and, after setting an initial H2
pressure, CO2
was injected. Further H2 was subsequently injected and the hydrogenation
reactor "A"
was then left under the conditions set with depressurization valve "L" closed
for the
stated initial reaction time. The depressurization valve "L" was subsequently
opened
and the plant was operated continuously. Here, polar solvent as stream 7a, CO2
as
stream 1 and H2 as stream 2 were fed in continuously. Product mixture went via
the
opened depressurization valve "L" into the phase separator "B" and was
separated into
two phases. The upper phase was recirculated as stream 4 to the hydrogenation
reactor "A". The lower phase was conveyed as stream 5 to the extraction vessel
"E". In
this, fresh tertiary amine (I) was introduced as stream 10a for extraction of
the residual
homogeneous catalyst. The tertiary amine (I) was in each case introduced in an
amount corresponding to the amount discharged via stream 8a. In the subsequent
phase separator "F", the output from the extraction vessel "E" was separated
into two
phases. The upper phase was recirculated as stream 12 to the hydrogenation
reactor
PF 62287 CA 02765430 2011-12-14
26
"A". The lower phase was discharged as stream 8a. Gas which accumulated in the
phase separator "B" and extraction vessel "E" was removed as off-gas. The
amount of
formic acid formed was determined in stream 8a by titration with OA N KOH in
2-propanol against Phenolphthalein. The TON, the TOF and the reaction rate
were
calculated therefrom. The ruthenium contents were determined by means of
atomic
adsorption spectrometry (AAS). Under these conditions, the plant was in each
case
operated for a number of hours. The results of the individual experiments are
likewise
shown in Table 4.2.
Examples D-1 and D-2 show that very good removal of the homogeneous catalyst
with
subsequent recirculation to the hydrogenation reactor is also possible via the
phase
separation when the plant is operated continuously. Thus, the upper phase to
be
recirculated from the phase separator "B" in each case comprised, with 175 ppm
by
weight (D-1) or 390 ppm by weight (D-2), very significant enrichment of the
homogeneous catalyst compared to only 15 ppm by weight (D-1) or 26 ppm by
weight
(D-2) in the lower phase (product stream). The preferred extraction using
tertiary amine
(I) in the extraction vessel "E" with subsequent phase separation in the phase
separator "F" enabled the product stream to be depleted further in homogeneous
catalyst, in example D-1 from 15 ppm by weight to 10 ppm by weight and in
example
D-2 from 26 ppm by weight to 11 ppm by weight, which corresponds to a further
depletion to 67% and 42%, respectively. The upper phase in the phase separator
"F"
comprised 46 ppm by weight (D-1) or 160 ppm by weight (D-2), which could in
each
case be recirculated to the hydrogenation reactor. It is thus possible to
separate off a
higher proportion of the homogeneous catalyst directly and reuse it in the
hydrogenation.
PF 62287
CA 02765430 2011-12-14
27
N
C
>, O 0 -
O N N (U cu .0 -0 a) 70
(U rC- 70 Y j (U a y C
X O C N (U N ~. X C CD
Ei (U r O d O CL O a E L Y
õ~ o x ri LO 2 r E C M 0) M (M o
X o a o 0 C 0 cQi cv) E
w m 0) o 0) ' Y 2 o
0 0 O o 0 C 0 CO
0) r F- F- Q C o
0 LO LO to U-) E
C) U m
o =_
E O O N N D
r
C? a -cn a) d cu .0 m .0
Q >, 0 0 m m U (U " c
m x C
cu E
CD 0
ll 2 0 N L
CL L N O. d
a)
E ~`. a
(U o NO O 1- a) c4 E
x O 0) co O CO
.. 0
w O ~O O CO F- F- C O
M
0
CU 0 0 = n
C
cu N N
04 E O ZT ^, (U -0
Q 7 d (U (U C
>. C C m
1
o
fl 5, o f n o a o, E
2 2 0 ; (D U) 0 0
m m m o o O rn > E
x o 1- w - rn rn N-
w 0 0 0 O 0) O 0 0
CT 0) 0) F- F- C O
O O
N CV O
O O '~
N (U
E N N N
- . L
N
IM d (B m
m U m` 7 0)
Q axi a 0) a o a:, CO
~- L Y
j 7 (U CV O o N (h N O
cl) CU w r N m 0 0) 0 CO E
x O - CO CO
w CD 0 0 cm o cm o V l- F- o
(y o r
0
(D
0
_ n, C N
c c U 4- s E a) E a?
(a, > O O U +_' >, U =~ N
O +' .C C C C C C
N O 0 p) 0 m 0 O
~+ C ..
N cU 0 =0 N '~ := 0
a .- U N 0 (U N 0 f ` Z LL fU
cu 0 F- F- M U d Z d a o F-
PF 62287
CA 02765430 2011-12-14
28
O U (CO Z L
>+ U v a3 O U)
co E 0) N co L - L
Q a) O O d (0 U
X C EC-p (0 V (0 i. m
a)
CL -5~ a) m E- d co a o a: E s
CU - - O o y oo M O a) E
E
,(D Cl - N F- ao I.
X
0 11
W O O ' 0 0 0
O O 0) 0) co E- F- 0
(n O .- d
l() 0
N
M 2 (a m
v cu (n U)
co ca m .0 a) I_-
ig '5, 75 0 m as c 7
v m m
co d 5 E r
) x a) a) a a o co IL v 0 0 co
Y
E m o Ci (n L u? co co
o rn o o o rn a) E
X
00
w o) 0) o Co o ' O~ 0 o
0 0 0 :. C o
M (- Q
0 o
a) U)
c c; 2 -0
E (U U)
Q as o a m CU a) L
T c
x (u cu
a)
c E OJ a ) a a 0 E s
CL a)
E ` N ca U-)
O ' N O L i O co 0 E
-
_ 2 .r.- C6 CY)
W O O O 0 0 O O co
O) O) O N H C p
It (0
O
r
L
In (U >, O O O O C
L5 ca x w co g U co m
CL a- I co g o 2 o; E It s
E L N o 7 N O m L M 0 O
Co w O p O `- p m E
O O Q. r- co
LLJ O O O O 0 O O cv)
C O O O
LO LO
O
a) a)
N 0)
C C_ _ c a)
O E w
0 0 U
C C C C 'C C C
0
r" ~' U) N (B O O 7 0 a3 p a) O
CU 75 U)
U) z U-
(6 a) O m a) =a) a) 0 (D a3 O O a)
I- I- 0 w CL d
0.. 0 F- H
PF 62287
CA 02765430 2011-12-14
29
a)
CU
N ~+ O (O L L t
m w O d ca (O
a) O (O p n" O n- 00 p Y
N Q
2 LO L i 0 0) O
0 0)
M CL 0 T-
r-: c6
X O p ' N
W 0) p M O O O O LO
0 (o 0) N F- I- C O
(o N co
O
N
C o = -Q
E 2, c0 O N
r fO 0) (O .a L
> O O O CO
0 c m CO O /(,O C 7
O 0 d N n' p LL 7 E r = Y
E ~ r- :3 c,4 0
I o N O Lo . N E
W w - F- o6 O O 0) 0 O O M
0) 0) m F- I- o
Z o cf)
Z (f) o
N
A= U)
CO
C ' Q
O E >, o 3
(O a O 0 Q (O (O
O c m 0) Q O E a CU 0 CU
CL 0 a- :3 E
Q- O L L Y
In
O
E õ O C0 O p T7 0 O E
c
w 0 p O (,. 0 0 q co C6
0 0 0 LU
0 L' O E O F- F- C O
LO -0 co
O Q
- CA
o Z .a
(n N
Q O a CO CO > c m ca C) (O L 0)
a) X (D & cY) d o n- o LO 4. Y
E =3 t M m Lo m 0 N co O
c 0 T7 0 LO M E
X 0 ao F-
W r: 'IT
O 0) 0) OR F- F- O
O p - CO
LO O
a)
N
C
S U 2 a) 0 3 a)
E a) 4-0 0 4- Q r> O - E -E
a) N
(h CO
, .` a) C C C
N N O o o 5.0 m o 0
(O (L3 +O U O O e
CO () 0 CO a). =O a) a) 2 N a) (O 0 O 0 a)
F- F- a 0 M C =C 2 d W d a. Y F- F- Of
PF 62287
CA 02765430 2011-12-14
>+ (Oj . ~ cn
co N ?, fU 0) V)
a) 0)
cu -0 E E a a (U m C
ca CU
0 Q- :3
E Z N
(a p N O r p 04 M N O
u< O - 0 0) H O co
0 rn f- F- 0
(n c0 O
e- In (D
.-'
i = L
Lo E (6
= CU .L.. o' (a a 'p
Q ?, v a) p (a (a N L
O C 01 fU C) (a ` C
d) 0 (a c
7 CD
E N 0 a N 2 C i O C%j L Y CO C-q
cU .C O O O co a) co W O O) Ch 4- E 0) 0 O O
O ) O O F- C c`)
(n (V (6 O
O
N
C - 2 .II
(U t O d f6 (Lp N L
a O
N E C m .~ (U U ca
.O N Q a N a 0 a- 7 = L- 4- O (h In -5
L i O L Y
r O
(U O a o N O co
W O m (Y) 0 " op 6 TS N
a O p
co (O r v) ti f - F- tt
(V ((')
O
0 fn
Q a) M a) rL- a iL co N 0 (U b
E 5, E C m ca U (a ` C
a z cV CU a a s o-
0 0 0 = C,~ LO o z: '
cu 0
W 0) 0 o c'? O co h- cy)
- O
uo ~ O O O
p r O (O C3 F- 0
12 C O
O
C
O N
0 N ai
C E
E (D
_~ (a ? _ O O O UO O CU
2>1 0
Q) (n mm O OC 0) L C .C C
(U N O (U O
4) M ca U L 4 Z LL
CD -a,
CL -T a- cr n _ 0 0 0
PF 62287
CA 02765430 2011-12-14
31
C4
c N
= m _
O E >. o co .n - s
cN co w a d co N
< >, o a) U co 0 co ` 7 0)
m aXi E m 0 M C- o a- E O
O L N O~ crj 2 LO L t to
O O
E 0 o cl O Lo E
X o o a 0) 0 0 04
W m O cr) 0 O H 0 c
o O L6
M
O
a) _ 0
v a)
cn U) _0 C _
0) co -0 m
ca .L. O t n. a) co a) L
Q X E C 0- a o co U) C
a) L N O v O O ' O 00 L Y
O N In L - c) co 0
E 0 a 0 W 0 co a) r E
X 0 0) M 0 O~ H O 0
O
W 0) O Q) co F- F0 C O
t~ r U)
1
O
N
i a) _
L N 7 a. co co C
(1) ca co 22 a) 0 co 7 m
Z N L' a . 2 L (D O O O
j N N
E 0 O- cr- 0 O O O N E
co -0 X O U N~ ti - co
w 7+ O 0 O 0 0 LO
co 0 m O rn F- F- c o
g N
L O
a) O 2 Q
r: (o N 0 _0
C4 CL O co c L
(
m co 0 co L 0)
o o a) LO E o E
X 0 c6 co
W O 0) 0H 0 0
3 co o
ry
c 0
c
a) a)
a
C
a)
C 0 2 E E
q E > w o o o "_ Y U
L
` O C C C C
=- ~+ U) ... N fo p p j O a) O t: Z LL CC
m ate) o c aa) .0 4)) aoi 2 a) a0) m O O
H F- d 0 d 2 CL w 4. d o F- F- LY
PF 62287
CA 02765430 2011-12-14
32
C C7 0 0
E ^ fa V) N
N ca O 0 CO Cu Cu x a 2 ca U co b)
a) a)
.c w o (L o o N- cY) t x
m m
N Q a- I Ch U') 0 M cM O
E 0 O o tl0 O H M M E
W O) 0 a O N F0 F_o6 (D
0 M
O O 0 6
L O
O
() N r~ U)
p = X . a)
C O Cl) Cu to cn
t _
r (a v
M E -' x ~-, 0 C ca U 0 0 t s
N L O 0 = V Cu cu co L)
OL
-R F-
>, CO a) (L o (L U 0) 7 0)
x
a t E a g 0 g o 0 > co CO Y
E N a N Ca U N co p O t C c CO CO O
ca 46 O N Cl O F_ X 0 V O E
W 0) o) 0 0 0 0 0) Cl C
M
- F-
LO c) 0 0) E O
U) ca
CV O
N
0
a) w r) x = X -0 co a) E s U) co v) U) 3 CU
N co N N -
< A L-0 C m ~ ~- cu cu cu a ca (~ ca ~= L CL a) 0 o L E c Y d o 2 o E E >' o
O t 7 0)
'c
w
E N C2 N L ch O 0 It O N CA
W O p C 0 0 0 0) ~ Y 0 o
O
O O O O 0) O O co a E
E
LO lf) O 0 0) U Cu
ch O
Cl)
() w Cu a)
(Y) x = . 3 N
co (n U) m
E ' m -0 .0 m a)
N ca >, 0 0 m co j t n. _L
X L p C m ca co `- X N O)
a) 2
0 E c Y a ~ rn g o 2 o E Z >, m s
co N LO c n o
CEa w o o o O U) Cm) v E
x 0 O Q U 4 ca a) -- LO
w o) o 0 0 0 . Q C LO
ocn n 0 0)
O o U m
ri
Cu a)
0) 0
Cl)
E U= r E aEFE
)
C C C 0 C
0 N C
N CD O C
0 C o cB 0 a) 0
Cu 75 -0 0 (n
a) 0 ca u .p N o t a a) ) N Q Q N
F- F- U d = d W Q)
d d 0 F-
PF 62287
CA 02765430 2011-12-14
33
1 (D
= W
.^~. X
c c1 ~ = () .0
t a)
ti O C m
N m >, O m CO a m C L
Q X - 16 c U N N m m V
Q t E a C m (o ( +O, 0 i O M O
E N d t U N O ch E
Co w 0 Q O ~--
W 0
0) 0) 0 0 0 0 0 m 0) 0 0 co
N F-
04 LO N - E C O
O It 0
O O CD
O
n' ~+ U)
(D o m
'C 7 .C U) (/)
N E o a L~ L
m >, 0 7 v t m Co
Q m a i 5 a m O m
L -Y
N E C d C i Lr) 0- 0 CL Uo 00 co 04
E N a- o o o o
m w w O n a 0 H -~ E
X o o a w w U .. ao
W 0) 0) 0 0 c 0 LO
0) 0 0 co
O O O O Q H H O
Ln LO 0)
O
0
N
aa) M 2 -0
1 W
C r v U)
t M -0 .0 (N m m a m m
E a) w a a O 7 0)
a z a) E a ~ 0 =3 O ``) Y
E p p 64 Oa o Ci 2 LO L6 ,I-
r,: 0 (O r- co lql E
F- N
W C;) O 0 Q "-' - co r,~ co
C)
LO o 0 0) 0
rn 0) O F- H 0
O
U') LO
O
0
N a) c c U~ 2 Co a)
f` m 0 0 U .... ', 0 (d
O "- L c c a) c E c
U) p 0 O p ~ 0
a) (0 O U N
m O o a) Z LL U
(D a) cu cu
a) a)
H H CL U d 2
0 OC d d H H w
PF 62287
CA 02765430 2011-12-14
34
a)
o
N
N 0 0)
X O a3 O C
O U 0
a) O a 0
Q T C7 C c
E O F- a)
E
0
X : T
W v) 0
z
E E
Z N
0
a) N 0 a)
a) O
E co
s
co E o
a) f0 Q O a) c O 0
L L
C M O O N
C
E
CU (1) ~ Q OL
X Q a) ? '>
W w O a) O Z
E Q a
N
N a)
O 0)
N a) 2)
cu C)
) ca
a
O
0
E C U 0
Q O a) O. O a)
E x E 0 F- a) P
W CV Q D O >
M O 0
> 0
z
N
o u 0) O 0)
C ' cu c:
T- a)
~- CO
E >' U O U
(0 Q C
Q a)
E >' E LO CD
X O cV 7 O N
W C`? > (D
Ci) 0
Z
a)
L O C O 0)
a .C .+
O co Cr (n L)
Q O
a) 6 :3 c
O E ca
(D 0 0) 0
> O CO
? 0 N 0 O C
O c6 L- M Q M O N
w L E L O> 0
75 F '- a O z o m 0 0 m a v0 ) a
PF 62287
CA 02765430 2011-12-14
a) o
C
c N O rn
ao a Q) w ca
OL 0 O 0
E ; O a)
E O i
E rn
X 00 O >
W O O
Z E > 6 Z
N
N
N
O 0)
>, a) g caO O C
m V w 0 0 t
_O L E C O 0 U
O_ O a5 CU c:
c 0 N i
E X ~ a)
cu E
X (D
W _ 4 O >
Z r O (n 0
> z
>, O N a)
cfl o 0 0)
cu U C
m N a p r
a) .C C C O 0
cu 0
N
cu E O (B . ~ O 4 :3
X
W 6 N j
O N O
2 > z
N a)
m C O c
C
a) N O L
O U
Co
CL ->>, cu 3 C
F- a) :-.
E
W a) E .2
4 =3 N
o > Mn 0
z
N
a) N 6 in
OO C C v rn
O 'C +J
O O C a) 5 a) O. -O
C O -c (0
U "' - U D
(6 O O C >
N c> .O O
p a- O O N
c\l (n a)
a) cB a) > Q > O C ,co
(o E O t E^ L CO >
U
-6 =3
CO H Z M O m m a 0 m
PF 62287
CA 02765430 2011-12-14
36
m o
E ()
04 a) caa O
a 0 c
O O N 4- O . -c
m
` 2 U U
O CL Q 0
C 0 )
N r
E
0
X >+ 0 O >
W > t O V) 0
> z
E
Z
N
c O aD
(B O O U c
>, (D E wc
w
c
O
a- ~. 3 M Co ('
0. a) a
Em
W N _ O O
Q Z
- O O c)
O
r, 0 -6 cu O C
m 0 a) c U 0 0
N 3
0 C 0 a) i
Q - E m F a) =
ca E
0 O X >
W a) O N 0
Z > 'a z
1 -
C O vOi O 0)
c
C6 c a) >, C N ~O co
c 0 3 c 0 a~
E E a) E 2 H (1) :..
7
CO
'd c . j 0 >
W Z r. 0 N cl)
0
Z > z
0)
0) .N U
N C = 0. -0
O 'c co s E'0
U -
O 0 c0
.~ - 0. a) 0 D
a) 4
cY) caa > O Q O Lf E N" Q C
04 L ca
0 N CO O U a .O g
> j a) a)
cu a)
a)
ca a) 0 0
F- h- a Z a .~ m caa m a u0) a.
PF 62287
CA 02765430 2011-12-14
37
0
Y E
O C 'C
,r CO to 'IT r-': 04 O M M O M M O I~ CO 0 CO O
T) co r cc) M CO CO O O
M 'U L M M N CO M M N r r N r N N N
0
U
N N N N N N N N N N
_ -
U) y
Ca m U m U m U m U m U m U m U m V m U m U
U c Q c Q c O O O O c Q c Q c Q c O
CL o n. CL n_ c (_ M c a. c n. n. c n_ c
0 a)
+. U)
E
0
a) co
'= a) 0 O O O O O O O O O O O O O O O O O O O O
C N N N N N N N N N N N N N CV N N N N N N
x x
O p a)
cc Co
LL E
m
O O O o
cn U)
0 0 O O C O O U U O O
C E E 0 0 'a -0 L >, >, -p 'p Q Q
a) a) a) a) a) m m >, >, a) a) N D 5 a) a) O) O p p
> C C "0 '0
m co -- c cm ca 0 m cma W 0 a s
O ) C C a) a) m co
o o - - am) ao om
a) a)
75 -5 0 L- "
O L GL n i i >, >, Q d n. d
N N
n_ a) a) L L M M to to 2 2 L>
E E a) a) r r r r :LD : r r a a.
a) a) D V U
E E N N
) m W a) a) a) a) m a) a) a) m a) a) a) m a) m a) a) a)
C C C C C C C C_ C c C C C C c c C C C C
Q E E E E E E E E E E E E E E E E .E E .E E
m m m m m m co m ca m m m m m m m m m m m
_m x x x x x x x x x x x x x x x x x x x x
L L L L t L L L r- L L L L L L t a L
a) C 'C 'C 'C C 'C 'C 'C 'C 'C 'C 'C 'C 'C =C 'C 'C
C6 fl O ~- N M qt LO CO I- M O O
N E r N M O M O r r r r r r r r r r N
X 0 U U V V U V V V V V V V U V U U U U ~..)
W
PF 62287
CA 02765430 2011-12-14
38
0
c
E
U 0
EF LO c0 ^ O O O
C co to O l! ) In 0 r- O C N
'IT M cp d O N N L
O
N N N _
i V C4 N N N N N N N N
= d 2 a = _ = Z = = 2 = _
(/) a v v M , e a < v v v v
0 - > > - 0 7 O 7 7 7 7
+.~'e m U m U m U m m m m m co co co
,V c Q c O c O c c c c c C C C
c Q. c Q. c Q_ Q. Q. Q. Q. a. Q_ a.
IL IL
U
U =
E =-= 0
0,00
O c0 c c c c c c c c
C x O O O O O O L 0) N N
N N (V CV N N
x c d) O a O 0 0 0 0
O ;0 C 0 0 0 0 0 0 0 0
U U E C C C C C C C C
cu c0 (O
U-
(D w c) 4)
(~ (O c c:
E E O O N (D
L- " 0 0 >, a) >,
C O O A T a (O c- m
N 0 d 0) ) N 0 E 2 E
N (O N (O Q a 0) 0) 0) 0) 0) 0) 0) 0)
(On U U E E M M w N N N O (D a) a) m
O O - V - V C C C C C C C C
(O 0) m. 4- w , O N N N O N O N
Q c c Li Qi L A L j, L L L L L L t
v v_ rn a, a) m a) a) Cl) a) a) a) L N N
a) n)
C C
E a) E
c (O a) C (O (D (D
c c c c c c U C C , E L .E a .s c
E
m (Ea a 0a 9 w x >, 2 9 c
m E x Q
x x x x x x - r V s N o
O c Q Z E 2 Z O =p
C L t t c Cl) ~ O
E
Z U
Z Z
N N
co Q r- N M LO (o ti w m O N co 't
Cl) E N N N N N N N N N M M M M M
(LO X U U U U U U U U O U U U U U
F- W
PF 62287 CA 02765430 2011-12-14
39
C;a
a) c ? z ~' 0
w a) E
O Q
N m >, o m s C N a C) cc
>,
a) X a) a) a a 0 a) c co o U m a
c la 0 3 5> o- o a 0 o
CL s E
E .`. N OL 0) a) 0) O a cA
O` L L 4) U ` cO T 4- co
W O o O C- O c') \ =` A L crj O
O O O p (~ ~, T +~ O 0
- ch to O H
OR O N- 3 T
a) ' x a
c ~'? a) m w a) E
Q, to
T L c 0 E
U >+ O U L c N -0 p L ti (Q
1~ a)
O C C) a3
W X '~ m U N E
N a) a) a U ns 0 0 CO
a t m C ~, n o N L 2 0
E N a N-c fl 0) O` LO T L
_ T 0 r- 3:
C) co
W O O O O 0 0 M ~j O
O O L O >, 0
p p Q U) C T O O
O M M o N
0 ~0 3
a)
E
a) a) cn 0 N c
C C G z N o = O
E a) E a) N> Ca > a 0 O
N
) 2 O O 0
N ( N
as CO ca 0 a) cu
H d U a) a a) c -c c
co
0 O cA
a)
f` a)
B .
C C.
O N a) N LL
c6 a) `
C C rn O
0 a) ca a) W a
N y O 0 -ca :3
3 "0 'a X_ U) 0. IL
co E c a)
H W L co > (n (A
PF 62287
CA 02765430 2011-12-14
L .Q .Q L L L L L
(II (u (If (o O O O O
W L L a s n. a`.. 3 3 3
o
_ o
00
E M M N 00 O O O r E E
cu LO x E E Q
.
W O O (N a a '
U U
0 0 0 0 O O
o o rn co
U) 0 N N N M r r
cn Cl) Cl) U) ~, .r rr
(Q (Q (II (B a) O) a)
(0 (Q (0 (6 a) Q)
cL d- a. a- N L cS ~} L .X ~, a > A
CO O O r r r 0 p L O L L
N N O 6 0 E E E
x N M Q a Q Q
w U U U U v
0 `n ti
0 0 0 0 m
LO Ul) N N r r r
N N a) Vd)
ca CO w LL m
c t cu co
2 Q a a a
C u) C C C C
11..
C (a > cu N m N N L.L (n
ca co ca L
p N O N C Q O Q O Q O O
L) ca cu M
O N co N U (p N N z u co cc
cl, O X L O O O O N a O N a a,
v=a=n wIL F- -J v~D (n.~ (nom us
O
C
c
a
0 o
U
O (p E
C
O C V N
3 E L
F- 0 i
L