Note: Descriptions are shown in the official language in which they were submitted.
CA 02766034 2014-03-25
METHOD AND APPARATUS FOR
LIMITING EFFECTS OF REFRACTION IN CYTOMETRY
RELATED APPLICATIONS
[0001] This application is related to Canadian Patent Application 2,728,289.
FIELD OF THE INVENTION
[0002] The present invention relates, in general, to methods and apparatus for
performing cytometry and, in particular, to methods for mitigating and/or
eliminating the
effects of refraction in cytometry systems and apparatus using the method.
BACKGROUND
[0003] The term refraction refers to the change in direction of a wave due to
a change
in the speed of the wave. We encounter the effects of refraction in our day to
day life.
An object in a pool is not where it appears to be when one attempts to grasp
it, or a
straw in a glass of water, when observed from outside the glass, appears
disjoint. The
effects of refraction in these contexts may have little or no practical
consequence in
one's daily life. However, within the context of a system that analyzes and/or
measures
radiated waves (e.g., light, sound, etc.), the effects of refraction are
particularly
important.
[0004] A cytometer is one such instrument that analyzes and/or measures
radiated
waves. Cytometers analyze and/or measure various parameters of the waves to
count
and/or classify particles or cells. For simplicity, this specification will
hereafter use the
term "cell," though the principles taught and claimed herein may apply with
equal force
to other types of particulate matter or discrete bodies. Additionally, the
term "objective
lens" is used throughout the specification, in accordance with its ordinary
meaning, to
indicate a lens or combination of lenses that first receives the rays from an
object under
observation. Further, while the principles taught and claimed herein are
described with
respect to cytometers and, in particular, with respect to cytometers that
measure and/or
analyze light waves (i.e., electromagnetic waves with a wavelength between
approximately 10 nm and 100 um), these principles are applicable in any system
measuring or analyzing energy exhibiting wave transmission. Still further, the
following
detailed description describes embodiments utilizing one or more electronic
detectors,
the output of which a computer or other electronic means analyzes or measures.
However, the detector may be other than an electronic detector (e.g., a human
eye may
1
MGS-R)C/PCT-CDA
CA 02766034 2014-03-25
be a detector), and the analysis and/or measurement means need not be
electronic
(e.g., where a brain analyzes light detected by a human eye).
[0005] Cytometers analyze and/or measure light by collecting the light through
a
system of optical elements. The collected light may be light reflected,
transmitted,
and/or emitted by the object being observed. As just one example, an
illumination
source (e.g., an ultraviolet illumination source) may illuminate a cell,
causing the cell, or
a chemical or dye within the cell, to emit light of a different wavelength
(e.g., fluorescent
light). The optical elements may include lenses, mirrors, filters, and the
like, that
cooperate to form an optical path. The collected light follows the optical
path to a
detector (e.g., a photodiode, a human eye, etc.) where the light is analyzed
and/or
measured. In the example above, the detector may detect a peak in the received
light
for each cell in or passing through a detection/interrogation area, and a
computer may
count the peaks to determine the number of cells, Alternatively, or in
addition, the
detector may detect different amounts or types of light corresponding to
different cells,
and a computer may interpret or analyze signals received from one or more
detectors.
[0006] The various optical elements through which a cytometer collects light
typically
include a variety of materials (e.g., glass lenses, plastic filters,
crystalline materials,
metallic surfaces, etc.). Moreover, in traversing the entirety of the optical
path, from the
origin of the light to a detector, the light may pass through any number of
materials
and/or environments. For example, fluorescent light emitted from a stain
attached to
deoxyribonucleic acid (DNA) of a cell may pass through: various materials
within the
cell; a cell membrane; a buffer solution and/or cell medium in which the cell
is
suspended and/or bathed; a cover slip or other container material; a fixing
agent; water;
oil; air; a glass lens, etc. Each of these materials may have different
properties with
regard to the light waves incident upon the material, which properties may
affect the
speed of the light waves through the material and, ultimately, the path of the
light. In
short, refraction occurring at the interfaces of the various materials in the
optical path of .
a cytometer can alter the path of light collected to image the analyte. (Of
course,
refraction may also affect illumination light directed toward the analyte.)
The effect of
such alterations in the path of the collected (or transmitted) light may
include a reduction
in the peak power or intensity of incident or imaging light delivered to or
emitted from the
analyte in a focus series across the analyte. Similarly, power or intensity
profile in a
2
MGS-RIC/PCT-CDA
CA 02766034 2014-03-25
focus series may broaden, reflecting an increase in the effective focal volume
for the
system,
10007] One of the properties of a material is the refractive index. The
refractive index
is a number that indicates the speed of light in a given medium as either the
ratio of the
speed of light in a vacuum to that in the given material (i.e., an absolute
refractive index)
or the ratio of the speed of light in a specified medium to that in the given
medium (i.e., a
relative refractive index). Unless otherwise specified, refractive indices
within this
specification are absolute refractive indices.
[00 0 8] Solids and liquids generally have particularly large differences in
their
refractive indices. For example, the refractive index of water (which varies
by
temperature and wavelength) is in the range 1.331-1.345 at 20 C. Buffers for
use in
cytometry typically contain dissolved salts and other chemicals and have a
refractive
index similar to or higher than water alone. Such buffers are typically used
to contain
and/or transport cells that are the subject of the analysis (the 'analyte1).
Materials used
to construct elements of the optical path, such as an optical cell, include
glasses,
plastics, and crystalline materials, of which some examples may include
acrylic,
polycarbonate, quartz, sapphire glass, polystyrene, polypropylene, and/or
other
materials. Each of these solid materials typically has a refractive index
significantly
different from (and usually greater than) that of water.
10009] Well known to those skilled in the art of optical system design and
construction
are various approaches to ameliorating aberrations arising from the shape,
position, and
optical properties of the various elements of optical systems. Such systems
include, by
way of example but not exclusion, telescopic systems, microscope systems, and
imaging systems. Optical system design frequently involves the selection of
materials,
numbers and shapes of optical elements (where the figuring of optical elements
of
differing complexity is associated with different costs), and configurations,
where the
requirements of the system are assessed against the cost of achieving optical
performance that suffices to carry out the desired function. For example, a
telescope
that produces images for visual observation may perform satisfactorily despite
the
presence of chromatic aberrations induced by the different refraction angle of
light of
different wavelengths as it passes through the lenses of the system. However,
additional optical elements may be required to reduce or eliminate chromatic
aberration
in a similar telescope intended for precise astronomical photography.
Furthermore, as
3
MGS-RICIPCT-CDA
CA 02766034 2014-03-25
another example, in telescopes and other optical systems, specially shaped
lenses may
be introduced to compensate for systematic aberrations introduced in the
imaging of the
object of study by the use of other elements that are ground to spherical
curves, an
aberration known as spherical aberration. Furthermore, in yet another example,
optical
systems may be designed that correct for specific and well-understood
aberrations that
occur outside of the lenses and other conventional components of the
constructed
optical system. For instance, water immersion type microscope compound
objective
lenses are now produced that correct for aberrations in the optical path in
imaging an
object lying beneath a cover slip and a layer of water, where the optical
system is
designed to correct for the refraction of light at both sides of the cover
slip. Such
corrective design may offer Improved focus arid resolution relative to optical
systems
that do not correct for systematic aberrations introduced by the properties of
the
materials through which the imaging light passes before entering the objective
lens.
[0010] The possibility of designing an optical system to compensate for
aberrations
that are internal to the optical system, or for aberrations that occur as a
result of
materials that are part of or near to the object being imaged, in no way
reduces the fact,
well understood to those skilled in the art, that it is desirable to reduce or
eliminate such
aberrations where possible. By way of example, oil immersion, where the space
between the objective lens of the microscope and the cover slip of the sample
is filled
= with an oil having a refractive index matching that of the coverslip
glass, is commonly
used in microscopy to reduce or eliminate the refraction that would occur at
the air-
coverglass interface in the absence of the oil. In practice, some aberrations
that are
introduced by materials and apparatus through which imaging light must be
collected
are not readily or affordably corrected in the design of an optical system. By
way of
example only, liquid jet-in-air cytometers feature a roughly cylindrical jet
of aqueous fluid
containing cells that are the object of study. Lenses to correct for
aberrations caused by
the interface of the aqueous cylinder with the surrounding air have not been
developed,
= since the expense and technical difficulty of designing such lenses is
high.
Nevertheless, those skilled in the art of cytometry will appreciate that
cytometers with
enclosed liquid streams featuring flat transparent walls through which imaging
light is
collected, may feature improved imaging, signal strength, focus, and/or
resolution by
virtue of reduced optical aberration.
4
MGS-RIC/PCT-CDA
CA 02766034 2014-03-25
[0011] Figs. 1 and 2 illustrate the problem that results from the boundaries
between
materials having different refractive indices. Fig. 1 depicts a typical
microscope
objective 10. The microscope objective 10 acts to focus light waves 12 passing
through
the microscope objective 10 to a nominal focal point (NFP) 14. Fig. 2 depicts
the same
microscope objective 10. A cover slip 16, such as a cover slip 16 that may be
used with
a microscope slide (not shown), is disposed in between the objective lens 10
and the
NFP 14. The cover slip 16 is a solid material (e.g., glass or plastic) having
a refractive
index higher than that of a medium 18 (e.g. air) on either side of the cover
slip 16. The
refractive index change occurring at an interface 20 of the cover slip 16 and
the medium
is, and the refractive index change occurring at an interface 22 of the cover
slip 16 and
the medium 18, result in a shift in the position of the NFP 14 away from the
lens 10.
The modified focal point is referred to as an Actual Focal Position (AFP) 24.
The AFP
24 is not a point but a region or volume in space, due to aberration induced
by the
refractive index changes in the media. The aberration may be characterized as
a point
spread function for the system and may be calculated numerically_ As a
consequence
of the aberration of the AFP 24, the peak intensity of light measured in a
focus series
(focusing through a sample located at a defined position) is reduced, and the
full-width
half maximum of the distribution is broadened.
[0012] In confocal microscopy there exists an alternative to using a specially
designed optical system to mitigate the effects of refraction. U.S. Patent No.
5,406,421
describes a coverslip for use in a confocal microscope. The coverslip is made
of a
transparent material having a refractive index which is lower or higher than
that of water
by 0.02 or less. In particular, the coverslip is made of a transparent
fluorocarbon resin
having a refractive index of approximately 1.34. When combined with a water-
dipping
objective lens, the use of such a coverslip can greatly decrease the
deterioration of
focusing accuracy of a confocal microscope. However, in cytometry, it may be
impractical to use a specially-designed objective fens_ For example, a
cytometer
requiring a specially-designed objective lens may prove too costly relative to
competing
devices or for a given application. Moreover, in some instances, particularly
in flow
cytometry, it may be impractical to use a coverslip, regardless of the
material from which
the coverslip is made, because, for example, a flat surface along any side of
the flow
path may detrimentally affect the orientation or the flow of the analyte
through the flow
path. Moreover, the use of a specially-designed coverslip, if possible, may
prove
6
MGS-RIC/PCT-CDA
CA 02766034 2014-03-25
insufficient to correct aberration in cytometry applications. For example, in
some
cytometer configurations, such as the flow cytometer described below with
respect to
Figs. 3 and 5, elements other than a coverslip, such as the walls of a flow
path, may
cause focal aberrations.
[0013] Regardless of the cause of the focal aberration, the loss of peak
intensity and
the dispersion of the focus causes a reduction in resolution and in the signal
to noise
ratio for the collected light or image. As a consequence, light or an image
may fail to be
resolved, or properties of them may be insufficiently distinct against the
background
noise of the system. An example of such a property is the fluorescence of a
fluorescent-
dye labeled cell. A reduction in the amount of light collected from such a
cell due to
aberration in the AFP 24 may raise the detection threshold for the measurement
of such
light, and may decrease the precision with which the fluorescent light is
measured. This
represents a reduction in the efficiency of the optical system as a whole and
has
practical implications for cytometry and for the design of a cytometry system.
By way of
example, the implications may require any or all of the following:
[0014] Increased observation time for the sample;
[0015] Reduced sample rate (analytes per unit time);
[0016] Incorporation of more and/or brighter fluorochromes (for fluorescent
samples);
(0017] More intense excitation light for (to cause fluorochrome excitation);
tool 8] More sensitive photodetector(s);
[001 9] Higher numerical aperture of objective lens(es); and
[0020] Lower optical and/or electronic background noise.
[0021] These requirements may have the effect of increasing the cost of a
cytometer,
and/or decreasing the throughput or analysis speed of the cytometer, and/or
changing
the type or expense of fluorochromes, samples, or other components that may be
used
for a specific purpose in a cytometer.
[0022] The situation illustrated in Fig. 2 represents a simple geometry having
flat
interfaces 20 and 22 between the medium 18 and the cover slip 16. Other
geometries
may be desirable in the design of a cytometer and may cause additional
aberration in
the AFP. For example, a flow path having a circular cross section provides
desired
benefits in a flow cytometer (I.e., a cytometer that measures an analyte as
the analyte
6
MGS-RIC/PCT-CDA
CA 02766034 2014-03-25
flows past or through a detection/interrogation region) and, in particular, in
a flow
cytometer used to sort mammalian sperm cells. Of course, curvature at a
boundary
between two media having different refractive indices (e.g., an aqueous
analyte-bearing
medium and a flow path in which the aqueous medium flows) will introduce
additional
aberration in the light path.
[00231 Further, the flow path itself may introduce more than two interfaces
between
materials with different refractive indices. Fig. 3 illustrates one
configuration for a flow
cytometer, in which configuration an objective lens 26 is oriented coaxially
with a flow 28
bearing an analyte 30. For reasons explained in greater detail in Canadian
Patent
Application 2,728,289, the configuration depicted in Fig. 3 may be preferable
over other
flow cytometer configurations, especially in situations in which the analyte
is a
mammalian sperm cell, the viability of which sperm cell must be maintained, As
Fig. 3
illustrates, the analyte 30 flows within a flow path 31 toward the objective
lens 26, and
through a nominal focal point 32, before being diverted by a transverse flow
34 into an
exit path 36. Walls 38 and 40 of the flow path 31 may be made of like or
dissimilar
materials, and typically have a refractive index different from an aqueous
solution (not
shown) bearing the analyte. The aberration of the AFP in such a situation will
be
complex due to the fact that rays between the NFP 32 and the objective lens 26
must
pass through the walls 38 and 40 of the flow path 31. Current cytometers may
avoid
this problem, in part, by situating the optical pathway and, in particular,
the NFP, such
that the walls 38 and 40 of the flow path 31 do not interfere with the optical
path.
[0024] The use of "water immersion" or "water dipping" objective lenses may,
in part,
correct aberration caused by the collection of light through a parallel-sided
wall or cover
slip and/or a fluid. Water immersion objective lenses correct for an optical
path that
passes through a liquid medium and a determined thickness of a medium of a
higher
refractive index, typically a glass cover slip. However, even variations in
cover slip
thickness smaller than the tolerances to which cover slips are typically
manufactured
can cause the AFP to vary from the NFP. Further, the determined cover slip
thickness
for which a water immersion objective lens is designed limits the design of
any optical
cell in which a flow path may be formed. Fig. 4 illustrates a water immersion
objective
lens 44 having a nominal focal point 32. A tip 45 of the water immersion
objective lens
44 sits in water 46 on a glass coverslip 47 of a determined thickness. An
aqueous
medium 49 (which may be water) having the same refractive index as the water
46 is
7
11110.S-141(1/PrIT-CnA
CA 02766034 2014-03-25
below the coverslip 47. Contrasted with water immersion objective lenses,
water
dipping objective lenses are fully corrected for imaging in water without an
intervening
cover slip.
[0025] Moreover, even a cytometer employing a corrected objective lens, such
as a
water dipping or a water immersion objective lens, remains subject to
refractive effects
in many instances. For example, Fig. 5 depicts a cytometer 50 in which glass
42 forms
the flow path 31. As will be appreciated, the cytometer 50 includes a number
of
interfaces between different materials, including a glass-immersion fluid
interface 51, an
Immersion fluid-cover material interface 53, a cover material-analyte medium
interface
55, and analyte medium-flow path wall interfaces 57 and 59. As in Fig. 4, the
tip 46 of
the water immersion objective lens 44 sits in water 46. Unlike Fig. 4, the
water 46 in
Fig. 5 sits on a top thickness 48 of the flow path 31, which top thickness 46
is the same
as that of the coverslip 47 depicted in Fig 4, and corresponds to the
thickness for which
the water immersion objective lens 44 is corrected. Accordingly, light 52
passing
between the water immersion objective lens 44 and the NFP 32 without
intersecting the
walls 38 and 40 of the flow path 31 remains in focus. However, the glass 42
forming the
walls 38 and 40 refracts light 54 that intersects the walls 38 and 40 at the
interfaces 57
and 59, causing aberration from the NFP.
[0026] It is an objective of the presently described methods and apparatus to
mitigate
and/or eliminate refractive effects in cytometric devices and methods.
SUMMARY
[00271 The present specification describes methods and apparatus for
performing cell
cytometry, which methods and apparatus mitigate or eliminate the effects of
refraction
that result from interfaces between materials having different refractive
Indices. In some
embodiments, a cytometer includes a flow path having an input, an output, and
a
detection region. An excitation energy source excites a molecule or molecules
in an
analyte and a detector detects the resulting energy. A processor, coupled to
the
detector and to a memory device interprets a signal from the detector. An
objective lens
has a focal point in the detection region. The focal point and the objective
lens define a
virtual conical volume. At least a portion of a component disposed wholly or
partially
within the virtual conical volume, or disposed at least partially within a
volume through
which light from the focal point passes between the focal point and the
optical focusing
8
Mr4R-RICIPCT-CDA
CA 02766034 2014-03-25
element, comprises a material having a refractive index in the range of 1.30
to 1.40
inclusive.
[0028] In some embodiments, the material with the refractive index in the
range of
1.30 to 1.40 inclusive is one of a perfluoroalkoxy polymer, an amorphous
fluoropolymer;
and an amorphous perfluoropolymer.
[0029] In some embodiments, the objective lens is a corrected objective lens
and, in
particular, is one of a water dipping objective lens, a water immersion
objective lens, or
an oil immersion objective lens.
[0030] In some embodiments, a volume defined by an objective lens and a focal
point
associated with an objective lens includes a material having a refractive
index between
1.30 and 1.40 inclusive. In some embodiments, the material forms at least a
portion of
one or more of the group consisting of an optical cell, a window, a cuvette, a
tube, a
passage, a chamber, a slide, a wall, and a boundary.
[0031] In alternate aspects, the cytometer is a scanning cytometer, an imaging
cytometer, or a flow cytometer.
[0032] In some embodiments, the objective lens is in contact with one of a
buffer
solution, a sheath fluid, a growth medium, and a fluid used to carry, suspend,
or bathe
the analyte. Similarly, in some embodiments, the material with the refractive
index
between 1.30 and 1.40 inclusive is in contact with one of a buffer solution, a
sheath
fluid, a growth medium, and a fluid used to carry, suspend, or bathe the
analyte.
[0033] In some embodiments, a method of performing cytometry includes
adjusting
the refractive index of a first material such that the difference between the
refractive
index of the first material and the refractive index of a second material is
less than 0.02.
In some embodiments, the first material is used to carry the analyte, suspend
the
analyte, or bathe the analyte. In particular, the first material may be one of
a buffer
solution, a sample fluid, a sheath fluid, a growth medium, and a lens
immersion fluid.
Further, in some embodiments, the second material is one of an optical cell, a
window, a
cuvette, a tube, a passage, a chamber, a slide, a wall, and a boundary.
[0034] In some embodiments, the second material has a refractive index between
1.30 and 1.40 inclusive and, in particular, the second material may be one of
a
perfluoroalkoxy polymer, an amorphous fluoropolymer; and an amorphous
perfluoropolymer.
9
nAGA-Rinipm--cria
CA 02766034 2015-04-07
[0034a] In some embodiments, there is provided a flow cytometer comprising a
flow
path having an input, an output, and a detection region; an excitation energy
source; a
detector; a processor communicatively coupled to the detector and to a memory
device;
a sorting mechanism; and an optical focusing element having a focal point in
the
detection region. The boundaries of the optical focusing element cooperate
with the
focal point to define a virtual conical volume; wherein the axis of the
virtual conical
volume is coaxial with respect to the flow axis of the flow path; wherein a
component of
the flow cytometer disposed at least partially within the virtual conical
volume, or
disposed at least partially within a volume through which light from the focal
point
passes between the focal point and the optical focusing element, comprises a
material
having a refractive index between 1.30 and 1.40 inclusive.
[0034b] In some embodiments, there is provided a cytometer comprising a
conical
volume defined by (1) an objective lens focusing for a detector radiation from
particles in
a flow path and (2) a focal point associated with the objective lens. The
volume has an
axis coaxial with respect to a flow axis of the flow path passing through the
focal point,
and includes a solid material having a refractive index between 1.30 and 1.40
inclusive,
wherein the cytometer is a sorting flow cytometer.
[0034c] In some embodiments, there is provided a method of performing
cytometry of
an analyte. The method comprises adjusting the refractive index of a first
material
comprising a fluid in an optical path between the analyte and a detector such
that the
difference between the refractive index of the first material and the
refractive index of a
second, solid material comprising a portion of a body holding the analyte is
less than
0.02, wherein performing cytometery comprises performing flow cytometry and
wherein
the second material comprises one or more of the group consisting of: a
perfluoroalkoxy
polymer; an amorphous fiuoropolymer; and an amorphous perfluoropolymer.
[0034d] In some embodiments, there is provided a cytometer comprising a
conical
volume defined in part by a focal point associated with an optical element,
the volume
including a material having a refractive index between 1.30 and 1.40
inclusive, wherein
the cytometer is a sorting flow cytometer, wherein the axis of the volume is
coaxial with
respect to a flow axis passing through the focal point, and wherein the
material
comprises one of the group consisting of: a perfluoroalkoxy polymer; an
amorphous
fluoropolymer; arid an amorphous perfluoropolymer,
MGS=RIC/PCT-CDA
CA 02766034 2014-03-25
[0034e] In some embodiments, there is provided a cytometer comprising a volume
defined by an objective lens and a focal point associated with the objective
lens, the
volume including a material having a refractive index between 1.30 and 1.40
inclusive;
and a water immersion objective lens or a water dipping objective lens;
wherein the
cytometer is a flow cytometer, and wherein the objective lens is in contact
with one of
the group consisting of: a buffer solution; a sheath fluid; a growth medium;
and a fluid
used to carry, suspend, or bathe the analyte.
BRIEF DESCRIPTION OF THE FIGURES
[0036] Fig. 1 depicts a nominal focal point of an objective lens;
[0036] Fig. 2 depicts focal distortion caused by the placement in the optical
path of a
two interfaces between materials of different refractive indices;
[0037] Fig. 3 depicts an embodiment of a flow cytometer in accordance with the
presently described methods and apparatus;
[0038] Fig. 4 depicts a water immersion objective lens in accordance with the
presently described methods and apparatus;
[0039] Fig. 5 illustrates the effects of refraction on the focal volume of a
known flow
cytometer;
[0040] Fig. 6A illustrates an advantage of a flow cytometer implementing the
presently described methods and apparatus over the flow cytometer of Fig. 5A;
[0041] Fig. 613 illustrates an advantage of a flow cytometer implementing the
presenting described methods and apparatus over the flow cytometer of Fig. 5A;
[0042] Fig. 7 depicts an embodiment of a flow cytometer in accordance with the
described methods and apparatus;
[0043] Fig. 8 depicts an embodiment of a conical volume between a focal point
and
an objective lens, and including materials of different refractive indices, in
accordance
With the described methods and apparatus;
[0044] Fig. 9 depicts an embodiment of a flow cytometer, according to the
described
methods and apparatus, in which the cells flow through a tube;
[0045] Fig. 10 depicts an alternate embodiment of the flow cytometer of Fig.
9;
11
MOS-RIC/PCT-CDA
CA 02766034 2014-03-25
[00461 Fig. 11 depicts another alternate embodiment of the flow cytometer
of Fig. 9;
[0047] Fig. 12 depicts yet another alternate embodiment of the flow cytometer
of Fig.
9;
[0048] Fig. 13 depicts an embodiment of a flow cytometer, according to the
described
methods and apparatus, in which the cells flow through a tube formed in a
cuboid;
[0049] Fig. 14 depicts an alternate embodiment of the flow cytometer of Fig.
13;
[0050] Fig. 15 depicts an embodiment of a flow cytometer, according to the
described
methods and apparatus, in which the analyte flows through a flow path formed
in a
body;
[0051] Fig. 16 depicts an alternate embodiment of the flow cytometer of Fig.
15;
[0052] Fig, 17 depicts another alternate embodiment of the flow cytometer of
Fig. 15;
[0053] Fig. 18 depicts yet another alternate embodiment of the flow cytometer
of Fig.
15;
[0064] Fig. 19 is a perspective view of a body for use in a flow cytometer
according to
the described methods and apparatus;
[0065] Fig. 20 is a cross-sectional view of the body of Fig. 19;
[0056] Fig. 21 is a perspective view of a body and transparent boundary
material for
use in a flow cytometer according to the described methods and apparatus;
[0067] Fig. 22 is a cross-sectional view of the body of Hg. 21;
[0058] Fig. 23 is a perspective view of a body for use in a flow cytometer,
the body
having a window, insert, or opening in accordance with the described methods
and
apparatus;
[0059] Fig. 24 is a cross-sectional view of the body of Fig. 23;
[0060] Fig. 25 is a perspective view of a body for use in a flow cytometer,
the body
having an insert in accordance with the described methods and apparatus;
[0061] Fig. 26 is a cross-sectional view of the body of Fig. 25;
[0062] Fig. 27 is a perspective view of an alternate embodiment of a body for
use in a
flow cytometer in accordance with the described methods and apparatus;
12
MGS-RIC/PCT-CDA
CA 02766034 2014-03-25
[0063] Fig. 28 is a perspective view of alternate embodiment of the body
depicted in
Fig_ 27;
[0064] Fig. 29 is a perspective view of another alternate embodiment of the
body
depicted in Fig. 27 with an associated transparent boundary material; and
[0065] Fig. 30 is a cross-sectional view of yet another alternate embodiment
of the
body depicted in Fig. 28, in which an objective lens functions as a wall of
the flow path.
DETAILED DESCRIPTION
[0066] The present specification describes methods and apparatus for
performing
cytometry and, in particular, methods and apparatus that minimize or eliminate
the
effects of refraction in cytometry systems and apparatus using the methods.
Unless
otherwise defined, all technical and scientific terms used herein have the
same meaning
as commonly understood by one of ordinary skill in the art to which the
claimed
inventions belong.
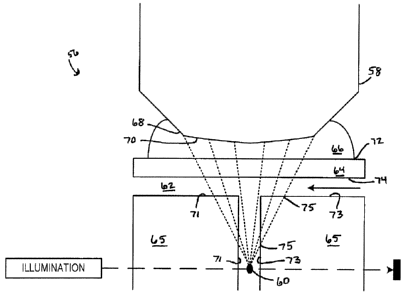
[0067] Fig. 6A depicts a portion 56 of an embodiment of a cytometer according
to the
described apparatus and/or employing the described methods. An objective lens
58 has
a nominal focal point 60 in an analyte medium 62 bearing an analyte, (not
shown) such
as a population of biological cells, disposed at or around the nominal focal
point 60. The
analyte may flow past the nominal focal point 60 (as in the flow cytometer
illustrated in
Fig. 6A) or may be disposed on a surface (as in scanning and/or imaging
cytometry) or
in suspension such that the analyte is generally in the same focal plane as
the nominal
focal point 60. A solid cover medium 64, illustrated as a top wall of a flow
path in the
portion 56, sits between the objective lens 58 and the nominal focal point 60.
The solid
cover medium 64 may be, by way of example and not limitation, a cover slip,
the top of a
Petri dish, a top surface of a flow path, a top surface of an optical cell
(e.g., a cuvette) in
which a flow path is formed, a window in a material forming a flow path, a
chamber, a
microscope slide, a transparent boundary material, etc. A lens immersion
medium 66
may be disposed between the objective lens 58 and the solid cover medium 64
such
that a tip 68 of the objective lens 58 is in contact with the lens immersion
medium 66.
Thus, there exists in Fig. 6A at least four physical interfaces between
differing materials.
A first interface 70 exists between the objective lens 58 and the lens
immersion medium
66, a second interface 72 exists between the lens immersion medium 66 and the
solid
cover medium 64, a third interface 74 exists between the solid cover medium 64
and the
13
MCS-RIC/PCT-CDA
CA 02766034 2014-03-25
analyte medium 62, and additional interfaces 75 may exist between the analyte
medium
62 and other portions of the cytometer, such as flow path walls 71 and 73,
which may be
formed of a medium 65, which may, in some embodiments, be the same as the
medium
64. Each of the interfaces 70, 72, 74, and 75 presents an opportunity for
refraction to
occur (and thus an opportunity to mitigate such refraction) if the respective
refractive
indices of the materials forming the interface 70, 72, 74, or 75 differ.
[0068] Referring still to Fig. 6A, the solid cover medium 64 and the medium 65
forming the flow path walls 71 and 73 are formed of a material having a
refractive index
between 1.30 and 1.40 inclusive, and one or both of the immersion medium 66
and the
analyte medium 62 has a refractive index between 1.30 and 1.40 inclusive. For
example, the analyte medium 62 and/or the immersion medium 66 may be water or
other similar fluid having a refractive index in the range of 1.33 to 1.35. In
particular, the
analyte medium 62 may be any liquid used to carry the analyte, suspend the
analyte, or
bathe the analyte including, but not limited to: a buffer solution, a sample
fluid, a sheath
fluid, or a growth medium. The solid cover medium 64, meanwhile, may be formed
of a
perfluoroalkoxy polymer, an amorphous fluoropolymer, an amorphous
perfluoropolymer,
or other such materials having a refractive index between 1.30 and 1A0
inclusive. By
way of example and not limitation, the solid cover medium 64 may be formed of
CytopTm, manufactured by Asahi Glass Ca, Ltd., Teflon AF, manufactured by
DuPontTM, or Teflon PFA, also manufactured by DuPontTM, which have
refractive
indices of approximately 1.34, 1.31-1.33, and 1.34-1.35, respectively.
[0069] Of course, one need not achieve an exact match between the materials
forming one of the interfaces 70, 72, 74, and 75. For example, at the
interface 74, the
analyte medium 62 may have a refractive index around 1.35 (e.g., water), while
the
cover medium 64 may have a refractive index around 1.34 (e.g., Cytoprm). In
such
instance, depicted in Fig. 6B, the relatively small differences between the
media 62 and
64 forming the interface 74, the media 64 and 66 forming the interface 72, and
the
media 62 and 65 forming the interfaces 75, provides a marked improvement over
the
prior art, in which the cover medium 64 generally is formed of glass having a
refractive
index in the range of 1.47 (Pyrex glass) to 2.04 (arsenic trisulfide glass)
or plastic
having a refractive index in the range of 1.46 to 1.55. Moreover, one need not
improve
the match of the refractive indices of the materials at each of the interfaces
70, 72, 74,
14
MGS-RIC/PCT-CDA
CA 02766034 2014-03-25
and 75, as improving the match of the refractive indices of the materials at
even one of
the interfaces 72 and 74 will improve the performance of the cytometer.
[0070] Further, in instances in which the refractive index of the material
forming the
cover medium 64 does not precisely match the refractive index of the analyte
medium
62 (or of the immersion medium 66), one may adjust the refractive index of the
analyte
medium 62 (or of the immersion medium 66). As just one example, if the cover
medium
64 is formed of Cytop TM with a refractive index of 1.34, and the analyte
medium 62 is
water with a refractive index of 1.33, one may adjust the refractive index of
the water
(e.g., by adding salts) to better match the refractive index of the Cytop TM .
Though not
required, it is preferable to adjust the refractive index of the analyte
medium 62 (or of the
immersion medium 66) to be within 0.02 of the refractive index of the cover
medium 64.
[0071] Fig. 7 depicts a flow cytometer 76 in accordance with one or more of
the
described methods and apparatus. The cytometer 76 includes a flow path 78 that
passes, at least partially, through a cuvette 80. An interrogation region 82
includes a
portion of the flow path 78, which portion of the flow path 78 includes a
nominal focal
point 84. Of course, the interrogation region 82 may include a portion of a
transverse
flow 86 in the flow path 78. An objective lens 88 focuses light or other
energy 90
collected from the nominal focal point 84, resulting in focused energy 92. The
focused
energy 92 may interact with one or more optical elements, such as a filter 94,
before
arriving at a detector 96. The detector 96 detects the focused energy 92, and
may send
a signal representative of the energy 92 over a connection 98 to a controller
100. The
controller 100 may include, for example, a processor 102 and a memory 104.
[0072] As generally known in the art, one or more lens elements 106 (e.g., a
hemispherical front lens, a meniscus lens, etc.) act to create the nominal
focal point 84.
The nominal focal point 84 defines the apex of a generally conical volume 108
between
the nominal focal point 84 and an outer element 110 of the objective lens 88
forming a
base 112 of the conical volume 108. The conical volume 108 may be a right
circular
conical volume, but may also be an oblique conical volume. Further, the
conical volume
108 may be formed of sections 114A, 114B, and 1140 of multiple cones 116, 118,
and
120 joined together, as illustrated in Fig. 8, such as is the case where one
or more
interfaces 122 and 124 are formed of materials 126, 128, and 130 having
differing
refractive indices. Moreover, the volume 108 need not be precisely conical,
but may
MR-R ie.:Jr:Fr:Tx:met
CA 02766034 2014-03-25
generally include the volume through which energy passes between the focal
point 84
(or an actual focal point) and the objective lens 88.
[0073] While Fig. 7 depicts the flow cytometer 76 as having the objective lens
88
generally coaxially aligned with the flow path 78 in the Interrogation region
82, one could
employ the presently described methods and apparatus in flow cytometers having
other
configurations. For example, the presently described methods and apparatus may
be
employed in a flow cytometer 76 in which the objective lens 88 is generally
perpendicular to the flow path 78 in the interrogation region 82, or a flow
cytometer 76 in
which the objective lens 88 is at an oblique angle to the flow path 78 in the
interrogation
region 82. Moreover, in the flow cytometer 76, the flow path 78 need not pass
through
the cuvette 80, but may instead or additionally pass through an optical cell,
a tube, a
passage, a chamber, etc., any of which may be formed of a material having a
refractive
index between 1_30 and 1.40 inclusive.
[0074] Figs. 9-14 show various embodiments of flow cytometers using the
methods
and apparatus described herein. In each, an objective lens 144 operates to
focus light
from an analyte (not shown). As described above, the methods and apparatus may
mitigate and/or eliminate refraction due to interfaces between materials
having different
refractive indices in various configurations. The objective lens 144 observes
the analyte
(not shown) flowing through a tube 170 that forms a flow path. An analyte
fluid 150,
suspending or carrying the analyte, may flow through the tube 170. Of course,
the tube
170, while depicted in Figs_ 9-13 as a right circular cylinder, need not have
a circular
cross-section and, in fact, need not be cylindrical at all. Instead, the tube
170 may have
a rectangular cross-section, as depicted in Hg. 14. In accordance with the
methods and
apparatus described, the tube 170 may be formed of a material having a
refractive index
similar to or the same as either or both of a fluid (not shown) flowing
through the tube
170, an immersion fluid 172 in which the objective lens 144 is Immersed (Fig.
10), or a
dipping fluid 160 in which the objective lens 144 is dipped (Fig. 11). In
particular, the
tube 170 may be formed from one of a perfluoroalkoxy polymer, an amorphous
fluoropolymer, or an amorphous perfluoropolymer, or other such material having
a
refractive index between 1.30 and 1.40 inclusive.
[0075j Fig. 9 depicts an embodiment in which the objective lens 144 does not
contact
the tube 170. Instead, a volume of air 152 exists between the objective lens
144 and
the tube 170. Accordingly, in the embodiment depicted in Fig. 9 there remains
at least
16
IV18-RIC/PCT-CDA
CA 02766034 2014-03-25
an interface 154, between the objective lens 144 and the air 152, and an
interface 156,
between the air 152 and the tube 170. Thus, while refraction may still affect
the optical
system, the system operates to reduce the refractive effects because the
refractive
indices of the analyte fluid 150 and the tube 170 may be the same or
approximately the
same (e_g., within 0.02).
[0076] If the objective lens 144 depicted in Fig. 9 was in contact with the
tube 170,
both of the interfaces 154 and 156 could be eliminated. In one aspect of the
embodiment, light passing between the analyte fluid 150 and the objective lens
144
passes through media having identical (or at least similar) refractive
indices. For
example, and without limitation, the analyte fluid 150 may have a refractive
index close
to or equal to that of water (e.g., 1.33) and the tube 170 may be formed of
CytopT", with
a refractive index of 1.34. Moreover, in accordance with the methods described
herein,
the refractive index of the analyte fluid 150 may be adjusted to match the
refractive
index of the tube 170 such that the refractive indices of both the analyte
fluid 150 and
the tube 170 are about 1.34.
[0077] In the depiction of Fig. 10, the objective lens 144 is a water (or
oil) immersion
objective lens, in contact with the immersion fluid 172 (e.g., water or oil)
disposed
between the objective lens 144 and the tube 170. In instances where the
immersion
fluid 172 has the same or similar refractive index as the tube 170 and/or the
analyte fluid
150, the refractive effects may be minimized. For example, and without
limitation, the
immersion material 172 and/or the analyte fluid 150 may be water or other
fluids having
(or adjusted to have) a refractive index of 1.34, and the tube 170 may be
formed of
CytopTM also having a refractive index of 1.34.
[0078] The embodiment depicted in Fig. 11 substitutes for the objective lens
144 a
water dipping objective, and substitutes a dipping fluid 160 (e.g., water) for
the
immersion fluid 172. Of course, either or both of the dipping fluid 160 and
the
immersion fluid 172 may be the same as the analyte fluid 150. For example, in
one
embodiment, the analyte fluid 150 may be a buffer solution, and may also be
the same
fluid used as the immersion fluid 172 or the dipping fluid 160.
[0079] In some embodiments, a tip 171 of the objective lens 144 forms a
portion of
the tube 170 (Fig. 12), eliminating at least the interface between the tube
wall and the
analyte fluid 150, and the interface between the tube wall and the objective
lens 144. In
17
RAC4S-RIC/PCT-CDA
CA 02766034 2014-03-25
some embodiments, the tube 170 may be formed or embedded in a cuboid, a
cylinder,
or other generally prismatic shape. Figs. 13 and 14, respectively, depict a
cylindrical
tube 170A and a rectangular tube 1708 embedded or formed within a cuboid 173.
[0080] As will be appreciated, the embodiments depicted in Figs. 9-14 mitigate
or
eliminate the effects of refraction as light passes through the various
materials between
the analyte arid the objective lens 144. For example, these embodiments, as
well as
others, may improve greatly flow cytometry systems employing optical elements
oriented orthogonally, or at approximately right angles, or at oblique angles
with respect
to the axis of flow of cells, in addition to flow cytometry systems employing
coaxial
detection (as depicted in Fig. 3). It is well known that, with respect to the
flow of cells
through the flow path of a flow cytometer, a flow path with a curvilinear
cross-section
(such as a cylinder) may be preferable over a flow path with a rectangular
cross-section
in some applications. However, it is likewise well known that, in flow
cytometers
employing orthogonal detection, the curvilinear flow path walls of such a flow
path
introduce focal aberration due to refraction occurring at least at the
interface of the
medium carrying the cells and the flow path wall. The described methods and
apparatus mitigate the refractive effects of at least that interface, and
possibly others, by
matching the refractive indices of the various materials at the interface.
(0081] Figs. 15-18 depict embodiments similar to those depicted by Figs. 9-12
and,
like the Figs. 9-12, are adapted for use with a flow cytometer. In Figs. 15-
18, the
objective lens 144 observes an analyte (not shown) flowing through a body 174
in which
a flow path 176 is formed. While the figures depict the flow path 176 as a "T"
intersection, the flow path 176 may, instead, form an inverted "L" as
depicted) for
example, in Figs. 19 and 20. In accordance with the methods and apparatus
described,
the body 174 may be formed of a material having a refractive index similar to
or the
same as either or both of a fluid (not shown) flowing through the flow path
176, a fluid
172 in which the objective lens 144 is immersed (Fig. 16), or a fluid 160 in
which the
objective lens 144 is dipped (Fig. 17). Alternatively, the objective lens 144
may form a
portion of the flow path 176, protruding into the body 174 through an opening
175, as
depicted in Fig. 18. In this manner, the embodiments depicted In Figs. 15-18
mitigate or
eliminate the effects of refraction as light passes through the various
materials between
the analyte and the objective lens 144.
18
11Mt:Q_P rtirte4T-ettlA
CA 02766034 2014-03-25
[0082] Figs. 19 and 20 illustrate a perspective view and a cross-sectional
view,
respectively, of a body 178 in which a flow path 180 may be formed. The flow
path 180
creates an inverted "L" shape within the body 178. The flow path 180 has an
entrance
flow section 182 and an exit flow section 184. While the figures illustrate
the entrance
flow section 182 as a right circular cylinder and the exit flow section 184 as
a channel,
the respective sections 182 and 184 may have any desired cross-sectional
shape. In
accordance with the methods and apparatus described, the body 178 may be
formed of
a material having a refractive index similar to or the same as either or both
of a fluid (not
shown) flowing through the flow path 180, or a fluid (not shown) in which an
objective
lens (not shown) is immersed or dipped. In this mariner, the embodiments
depicted in
Figs. 19 and 20 mitigate or eliminate the effects of refraction as light
passes through the
various materials between the analyte and the objective lens.
[0083] Figs. 21 and 22 depict a perspective view and a cross-sectional view,
respectively, of a body 186 in which a flow path 188 may be formed. The flow
path 188
includes an entrance flow section 190 and an exit flow section 192, and may
optionally
include a transverse flow entrance section 194 (shown as a broken line). In
contrast to
the embodiment depicted in Figs. 19 and 20, the exit flow section 192 (and the
transverse flow entrance section 194) may be formed as a channel 195 having
edges
196 that are generally coplanar with a top surface 197 of the body 186. An
optional
transparent boundary material 198 may be disposed between the body 186 and an
objective lens (not shown). In accordance with the methods and apparatus
described,
the body 186 and/or the transparent boundary material 198 may be formed of a
material
having a refractive index similar to or the same as either or both of a fluid
(not shown)
flowing through the flow path 188, or a fluid (not shown) in which the
objective lens is
immersed or dipped. In this manner, the embodiments depicted in Figs. 21 and
22
mitigate or eliminate the effects of refraction as light passes through the
various
materials between the analyte and the objective lens.
[0084] Figs. 23 and 24 illustrate a perspective view and a cross-sectional
view,
respectively, of an embodiment according the methods and apparatus described,
in
which a flow path 202 is formed in a body 200. The flow path 202, while
illustrated as
forming an inverted "L" shape, may also form a "T" shape as depicted in Figs.
15-18. In
the embodiment illustrated in Figs. 23 and 24, the body 200 may or may not be
formed
of a material having a refractive index similar to or the same as either or
both of a fluid
19
mns.RIC/PCT.CIDA
CA 02766034 2014-03-25
(not shown) flowing through the flow path 202, or a fluid (not shown) in which
an
objective lens (not shown) is immersed or dipped. An insert, window, or
opening 204
allows the objective lens to view a section 206 of the flow path 202. The
insert, window,
or opening 204 may be a negative space, open to the flow path 202, may be a
window
covering a negative space over the flow path 202, or may be an insert disposed
within
the body such that a surface of the insert is in contact with the flow path
202 and/or a
fluid (not shown) in the flow path 202. Further, where the body includes the
insert 204,
the insert 204 may extend into an area 208 (shown as a broken line) along one
side of
the flow path 202. While the insert, window, or opening 204 is depicted in
Figs. 23 and
24 as circular, the insert, window, or opening 204 may be any desired shape.
In
accordance with the methods and apparatus described, where the body 200
includes
the insert or window 204, the insert or window 204 may be formed of a material
having a
refractive index similar to or the same as either or both of the fluid flowing
through the
flow path 202, or the fluid in which the objective lens is immersed or dipped.
In this
manner, the embodiments depicted in Figs. 23 and 24 mitigate or eliminate the
effects
of refraction as light passes through the various materials between the
analyte and the
objective lens,
[0085] In some embodiments, illustrated in Figs. 25 and 26 in perspective and
cross-
sectional views, respectively, a body 210, such as the bodies 174, 178, 186,
and 200,
formed at least in part by a material having a refractive index similar to or
the same as
either or both of the fluid flowing through the flow path or the fluid in
which the objective
lens is immersed or dipped, may be inset into a larger body 212 in which a
flow path 214
is formed. A portion 216 of the flow path 214 passes through the body 210.
Openings
218 at the ends 220 of the portion 216 align with portions 222 of the flow
path 214 in the
larger body 212. While Figs. 25 and 26 depict the body 210 as a rectangular
cuboid, the
body 210 may be any desired shape and, in particular, may be cylindrical.
[0086] In still other embodiments, such as that depicted in Fig. 27, a body
224
includes a reservoir 226 formed at an intersection 228 of a first flow path
portion 230
and a second flow path portion 232. For example, the first flow path portion
230 may
intersect the reservoir 226 at a generally planar bottom surface 234 that is
generally
parallel to a surface 236 of the body 224. Two parts 238A and 238B of the
second flow
path portion 232 may connect to the reservoir 226 at opposing surfaces of the
reservoir
226, which may generally have the shape of a flattened cylinder. Of course,
the
nArt ore=trit¨r rinn
CA 02766034 2014-03-25
reservoir 226 could be any desirable shape including, by way of example, a
flattened
cuboid. Further, there is no requirement that the second flow path portion 232
include
both the parts 238A and 238B. That is, the flow path 232 need not include a
transverse
flow but, instead, could include only the outlet portion 238A. In accordance
with the
methods and apparatus described, the body 224 may be formed of a material
having a
refractive index similar to or the same as either or both of a fluid (not
shown) flowing
through the flow path portions 230 and 232 and the reservoir 226, or a fluid
(not shown)
En which the objective lens (not shown) is immersed or dipped. In this manner,
the
embodiments depicted in Fig. 27 mitigate or eliminate the effects of
refraction as light
passes through the various materials between the analyte and the objective
lens.
[0087] Fig. 28 depicts a related embodiment in which a top edge 240 of the
reservoir
226 is coplanar with the surface 236 of the body 224. A water-dipping
objective lens
(not shown) may extend into the reservoir 226 and, in doing so, may be in
contact with
fluid flowing through the flow paths 230 and 232 and the reservoir 226, which
may
mitigate and/or eliminate any interfaces between materials of differing
refractive indices.
[0088] Fig. 29 depicts yet another related embodiment, in which a transparent
boundary material 242 is placed over the exposed reservoir 226 depicted in the
embodiment of Fig. 28.
[0089] Fig. 30 depicts still another related embodiment, in which an objective
lens
240 (which may be a water dipping objective lens) protrudes through an opening
242
into the body 224 to form a boundary of the flow path 232 and, in particular,
to form a
boundary of the reservoir 226.
(0090] Each of the bodies 174, 178, 186, 200, 212, and 234 may be formed from
one
of a perfluoroalkoxy polymer, an amorphous fluoropolynner, or an amorphous
perfluoropolymer, particularly in applications in which the analyte is
suspended in,
carried in, or bathed by a medium having a refractive index close to that of
water.
Further, each of the bodies 174, 178, 186, 200, and 234 may be integral to a
flow
cytometer in accordance with the described methods and apparatus, or may be a
separable (i.e., removable, replaceable, etc.) component of the flow
cytometer. In some
embodiments, one of the bodies 174, 178, 186, 200, and 234 may be part of a
cartridge,
installed in the flow cytometer according to the application or according to
the analyte.
In some embodiments the cartridge may be reusable and/or amenable to
sterilization.
21
ARFIC_011,I1DrT_I" nit
CA 02766034 2014-08-22
The bodies 174, 178, 186, 200, and 234 and, in particular, respective flow
paths therein,
need not comprise the entire flow path of the flow cytometer and, accordingly,
may
connect to other flow path portions in the flow cytometer.
[0091] Although the foregoing text sets forth a detailed description of
numerous
different embodiments, it should be understood that the scope of protection is
defined by
the words of the claims to follow. The detailed description is to be construed
as
exemplary only and does not describe every possible embodiment because
describing
every possible embodiment would be impractical, if not impossible. One could
implement numerous alternative embodiments using either current technology or
technology developed after the filing date of this patent, which embodiments
would still
fall within the scope of the claims.
[0092] Thus, many modifications and variations may be made in the techniques
and
structures described and illustrated herein without departing from the scope
of the
present claims. Accordingly, it should be understood that the methods and
apparatus
described herein are illustrative only and are not limiting upon the scope of
the claims.
The specification above describes at least the following aspects:
[0093] 1. A flow cytometer comprising:
[0094] a flow path having an input, an output, and a detection region;
[0095] an excitation energy source;
[0096] a detector;
[0097] a processor communicatively coupled to the detector and to a memory
device;
[0098] an optical focusing element having a focal point in the detection
region, the
boundaries of the optical focusing element cooperating with the focal point to
define a
virtual conical volume;
[0099] wherein a component of the flow cytometer disposed at least partially
within
the virtual conical volume, or disposed at least partially within a volume
through which
light from the focal point passes between the focal point and the optical
focusing
element, comprises a material having a refractive index between 1.30 and 1.40
inclusive.
22
CA 02766034 2014-03-25
[0100] 2. The flow cytometer of aspect 1, wherein the component comprises one
or
more of the group consisting of: a perfluoroalkoxy polymer; an amorphous
fluoropolymer; and an amorphous perfluoropolymer.
[0101] 3. The flow cytometer of aspect 1 or aspect 2, wherein the optical
focusing
element is an objective lens.
[0102] 4. The flow cytometer of aspect 3, wherein the objective lens is a
corrected
objective lens.
[0103] 5. The flow cytometer of aspect 4, wherein the objective lens is either
a water-
dipping objective lens or a water-immersion objective lens.
[0104] 6, A cytometer comprising a volume defined by an objective lens and a
focal
point associated with the objective lens, the volume including a material
having a
refractive index between 1.30 and 1.40 inclusive.
[0105] 7. The cytometer of aspect 6, wherein the material forms at least a
portion of
one or more of the group consisting of: an optical cell; a window; a cuvette;
a tube; a
passage; a chamber; a slide; a wall; and a boundary.
[0106] 8. The cytometer of aspect 6 or aspect 7, wherein the material
comprises
one of the group consisting of: a perfluoroalkoxy polymer; an amorphous
fluoropoiymer;
and an amorphous perfluoropolymer,
[0107] 9. The cytometer of any of aspects 6 to 8, wherein the cytometer is a
flow
cytometer.
O108] 10. The cytometer of aspect 9, further comprising a flow path having a
curvilinear cross-section.
[0109] 11. The cytometer of any of aspects 6 to 10, further comprising either
a water
immersion objective lens or a water dipping objective lens.
[0110] 12. The cytometer of aspect 11, wherein the objective lens is in
contact with
one of the group consisting of: a buffer solution; a sheath fluid; a growth
medium; and a
fluid used to carry, suspend, or bathe the analyte.
[0111] 13. The cytometer of aspect 11, wherein the objective lens is in
direct contact
with the material having a refractive index between 1.30 and 1.40 inclusive.
23
MGS-RIC/PCT-CDA
CA 02766034 2014-03-25
[0112] 14. The cytometer of aspect 13, wherein the material having a
refractive index
between 1.30 and 1A0 inclusive, is also in contact with one of the group
consisting of: a
buffer solution; a sheath fluid; a growth medium; and a fluid used to carry,
suspend, or
bathe the analyte.
[0113] 15. A method of performing cytometry of an analyte, the method
comprising
adjusting the refractive index of a first material such that the difference
between the
refractive index of the first material and the refractive index of a second
material is less
than 0.02.
[0114] 16. The method of aspect 15, wherein the first material is used to
carry the
analyte, suspend the analyte, or bathe the analyte.
[0115] 17. The method of aspect 15, wherein the first material is one of the
group
consisting of: a buffer solution; a sample fluid; a sheath fluid; a growth
medium; and a
lens immersion fluid.
[0116] 18. The method of any of aspects 15 to 17, wherein the second material
is one
of the group consisting of: an optical cell; a window; a cuvette; a tube; a
passage; a
chamber; a slide; a wall; and a boundary.
[0117] 19. The method of any of aspects 15 to 18, wherein the second material
has a
refractive index between 1.30 and 1.40 inclusive.
[0118] 20. The method of any of aspects 15 to 19, wherein the second material
comprises one or more of the group consisting of: a perfluoroalkoxy polymer;
an
amorphous fluoropolymer; and an amorphous perfluoropolymer.
24
AM35-RIC/PCT-CDA