Note: Descriptions are shown in the official language in which they were submitted.
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
PROCESS FOR UPGRADING NATURAL GAS WITH A HIGH HYDROGEN
SULFIDE CONTENT
The present invention relates to a process for the
upgrading of natural gas with a high hydrogen sulfide
content.
More specifically, the present invention relates to
a process for the upgrading of natural gas containing
hydrogen sulfide in concentrations higher than or equal
to 60% by volume.
Even more specifically, the present invention
relates to a process for the upgrading of natural gas
containing hydrogen sulfide in concentrations higher
than or equal to 60o by volume by the recovery of
hydrogen from both the sulfurized compound and
hydrocarbon phase present.
As is known, natural gas essentially consists of
methane, but, in addition to significant traces of
higher C2-C7+ hydrocarbons, it can also contain variable
quantities of inert gases or pollutants, for example
carbon dioxide or nitrogen, whose presence must be
eliminated or reduced to satisfy the specifications of
use.
Among the pollutants of natural gas, there is also
hydrogen sulfide, which contrary to nitrogen and carbon
-1-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
dioxide, must be completely eliminated, before
introducing the gas into the supply system, as it is an
extremely harmful product.
Fields/reservoirs of natural gas with a high
hydrogen sulfide content can be found all over the
world, for example Bearberry fields or Panther River in
Canada are natural gas reservoirs containing
approximately 90% by volume and 68% by volume of H2S,
respectively. In the United States there are fields
such as Black Creek and Cox (Missisipi) which contain
approximately 78o and 65% of H2S, respectively. "Super-
sour" gas fields having large dimensions also exist,
such as the Zhaolazhuang-Hebei field in China which
comprises 19 wells which produce a natural gas whose
concentration of H2S varies from 60 to 90% by volume.
These superacid gas reservoirs, also known as
"super-sour" gas reservoirs, either remain unused, as
the recovery of natural gas (methane) is too onerous,
or, as in the case of the Zholazhuang field, they are
used for the production of sulphur by means of the
Claus process, sending the gaseous stream,
substantially as it leaves the production well,
directly to the combustion reactor where the partial
oxidation of the hydrogen sulfide to SO2, takes place.
In this way, the methane present is burnt and this is
-2-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
an economic loss which becomes increasingly more
significant, the higher the methane content in the
natural gas.
As the request for sulfur on a worldwide scale is
stationary and does not seem to be increasing, most of
the "super-sour" natural gas fields at present have
remained unused.
The Applicant has now found a process for upgrading
"super-sour" natural gas containing concentrations of
H2S higher than or equal to 60o by volume which allows
the hydrogen contained in both the methane molecule and
in that of the hydrogen sulfide to be recovered.
Hydrogen is a raw material which is extremely
requested in refineries, for example, for all
hydrotreatment processes such as hydrocracking and
hydrodesulfurization, and it would therefore be
extremely desirable to be able to obtain it from a
source with zero value such as current "super-sour"
natural gas reservoirs.
A method which allows hydrogen to be recovered from
natural gas, strongly acid due to hydrogen sulfide, is
the reforming reaction with methane according to the
reaction:
2H2S + CH4 = CS2 + 4H2 (1)
wherein the molar ratios necessary for satisfying the
-3-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
stoichiometry of the reaction are substantially
guaranteed by the composition itself of natural gas.
This reaction can be carried out with high
conversions only at high temperatures (for example
higher than 900 C) and is strongly endothermic (LH298=
232 kJ/mole), consequently energy must be supplied
externally by burning a fuel with a considerable
increase in production costs.
The Applicant has also found that it is possible to
use the CS2, produced by the reforming reaction between
methane and H2S, as fuel for sustaining the reaction
(1). By exploiting, in fact, the large quantity of
reaction heat which is developed in the combustion of
CS2, (LH298= -1032 kJ/mole), the previous endothermic
reforming reaction of H2S with methane can be sustained
without having to burn any high-quality fuel. The SO2
produced by the combustion can be upgraded downstream
as intermediate for specific synthesis reactions, for
example to produce sulfuric acid, or it can be disposed
of by injection into the subsoil.
The possible presence of carbon dioxide in the
super-sour gas does not represent a disadvantage. Under
the reaction conditions, object of the present
invention, the equilibrium is in fact established
between the carbon dioxide present and the hydrogen
-4-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
formed according to:
CO2+H2=CO+H20
A further preferred aspect of this reaction is the
use of the mixture thus formed of carbon monoxide and
hydrogen, after the separation of the water, for the
production of methanol according to:
CO + 2 H2 = CH3OH
In the case of the presence of carbon dioxide in
the super-sour gas, it is therefore possible to
directly produce synthesis gas without having a further
reforming unit.
An object of the present invention therefore
relates to a process for upgrading superacid natural
gas, with a content of hydrogen sulfide higher than or
equal to 609,; by volume, with the production of
hydrogen, which comprises.
a. feeding the superacid natural gas to a reforming
reactor operating at a temperature ranging from 900
to 1500 C and at atmospheric pressure, or slightly
lower than atmospheric pressure, for example ranging
from 0.08 to 0.1 MPa, to produce a mixture
essentially consisting of carbon disulfide (CS2) and
hydrogen (H2) ;
b. cooling the reaction products, separating the carbon
disulfide from the remaining reaction mixture
-5-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
containing hydrogen and recovering the hydrogen;
c. burning the carbon disulfide with a gas containing
oxygen to produce a gaseous mixture, essentially
consisting of CO2 and SO2, at a high temperature;
d. feeding at least a part of the hot gases of the
combustion of carbon disulfide to the reforming
step, as heat source for maintaining the endothermic
reaction of step (a); and
e. providing the combustion gases of carbon disulfide,
also coming from step (d), as intermediates for
chemical syntheses downstream or for their disposal
by injection into specific geological structures.
At the end of the reforming, the reaction products
can be cooled to a temperature which is optimum for the
subsequent operations, for example to a temperature
lower than 50 C, in order to recover the carbon
disulfide, which is liquid at those temperatures, from
the gaseous phase essentially consisting of hydrogen,
residual H2S and possible reaction by-products and/or
hydrocarbons. The gaseous phase can then be treated
with conventional methods, for example by means of the
selective adsorption technology of the PSA type or
treatment with membranes, for the recovery of the
hydrogen. The possible H2S in excess can be recovered by
the traditional techniques, for example by absorption
-6-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
with amines.
The cooling phase preferably takes place in heat
exchangers where the cooling liquid is water, which can
be transformed into vapour at a temperature of 100-
150 C and a pressure of 0.2-10 MPa. The vapour can then
be used to produce electric energy or as a heat source
to be destined for the running of other plants.
Alternatively, before being cooled in the heat
exchanger, the reaction products can pre-heat the
reagents which are to be fed to the reforming reactor.
The carbon disulfide recovered in the liquid state,
is burnt in a specific reactor, with air or air
enriched in oxygen as comburent.
The combustion gases leaving the reactor at a
temperature of 1000-2500 C, are fed, either partially
or totally, to a further system of heat exchangers to
bring the reagents, possibly pre-heated, to the correct
temperature, before being introduced into the reforming
reactor.
In this way, from the integrated cycle described in
the process, object of the present invention, the
production of hydrogen can be obtained from a stream
containing hydrogen sulfide without having to use any
external high-quality fuel and a stream containing SO2
is also obtained as by-product, which can be
-7-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
advantageously used as raw material for chemical
syntheses such as, for example, the production of
sulfuric acid. Alternatively, if convenient, the stream
containing SO2 can be injected into adequate geological
structures.
An alternative embodiment of the process, object of
the present invention envisages that only a part of the
CS2 produced is burnt as energy source, whereas the
remaining part is separated and destined for
commercialization.
The balance between the aliquot of burnt CS2 and
that separated and destined for sale depends on the
quantity of heat which is to be produced to sustain the
endothermic reforming reaction of methane with H2S. The
sufficient quantity of CS2 to be destined for combustion
to sustain the reforming of methane with H2S is equal to
at least 556 by weight of the total CS2 produced.
Depending on the demands, the remaining part of CS2 can
be separated and sold or burnt to provide heat energy
for also sustaining other process equipment, for
example for producing high-pressure vapour which can be
used in this or other plants.
Another innovative aspect of the process, object of
the present invention, consists of the fact that the
sulfur present in the H2S molecule is transformed
-8-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
directly into SO2, which can in turn be transformed, by
reactions and processes well-known in literature, for
example, to sulfuric acid, requested by the chemical
industry, rather than into elemental sulfur as in the
S conventional Claus process. Elemental sulfur does in
fact have considerable environmental storage problems
and many nations in which there are natural gas
reservoirs impose heavy economic sanctions for the
storage of sulfur. By transforming sulfur to sulfuric
acid, on the other hand, a liquid product is obtained,
which can be easily transported and sold as such.
The process for upgrading superacid natural gas
with the production of hydrogen, object of the present
invention, can be better understood with reference to
the scheme of the enclosed figure which represents an
illustrative and non-limiting embodiment.
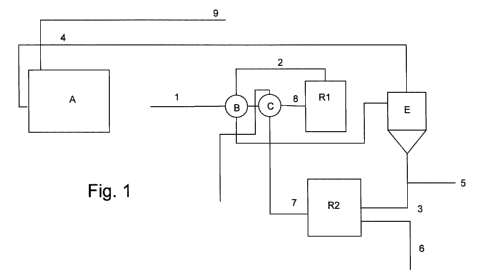
With reference to the figure, A is a conventional
plant using amines for the recovery of hydrogen sulfide
in excess with respect to the stoichiometric value of
the reforming reaction, B is a heat exchanger system
for heat recovery, C is a heat exchanger system which
can be used for providing the energy necessary for the
reforming, R1 is the methane/H2S reforming reactor and
can be, for example, a fixed bed or fluid bed catalytic
reactor, E is a condensation and collection system of
-9-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
the CS2 produced in the reactor R1, R2 is the combustion
reactor of CS2.
A stream of superacid gas (1) essentially
consisting of methane and a fraction of H2S equal, for
example, to 60-652,5 by volume, is provided. The stream
(1) is preheated in B, brought to the reforming
reaction temperature in C and then fed to the reactor
R1.
The hot reaction products (2) are recovered from
the reactor R1, at a temperature of about 900-15000C.
These hot gases are fed to B, for preheating the
reagents, and are then fed to the condenser E where the
carbon disulfide CS2, in the liquid state, stream (3),
is separated from the gaseous phase essentially
consisting of H2, non-reacted H2S/in excess and possibly
methane and/or other hydrocarbons and/or reaction by-
products, stream (4). A part of the carbon disulfide
produced, stream (5), can be deviated from the cycle,
object of the present invention, and destined for other
purposes.
The carbon disulfide (3) and comburent air (6) are
fed to the combustion reactor R2. The combustion gases,
stream (7), which comprise CO2 and SO2, leave the
reactor at a temperature of about 1000-2500 C and are
fed directly to the heat exchanger C where they heat
-10-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
the reagent gases (CH4 e H2S) bringing them to a
temperature of about 900-1500 C, or slightly higher.
The stream of heated reagent gases (8) is fed to the
reactor R1, in which the catalyst is positioned,
consisting for example of one or more sulfides of
metals of groups VIB, VIIB and VIIIB of the periodic
system. Among these metallic sulfides, chromium,
tungsten, molybdenum, iron, cobalt and nickel sulfides
are particularly preferred, used alone or in a mixture
with each other. The temperature inside Rl is kept
uniform at about 900-1500 C.
After bringing the reagents of the reforming
reaction to the reaction temperature, the combustion
gases of carbon disulfide can be further cooled, in
specific equipment not illustrated in the scheme of the
enclosed figure, and then used for further chemical
processes, for example the synthesis of sulfuric acid,
or they can be disposed of by injecting them into the
subsoil or into deep seawater.
The products of the reforming reaction which, after
cooling in E, are in gas phase, stream (4), are fed to
the plant A for recovery of the hydrogen sulfide. At
the end, the stream (9) essentially consisting of
hydrogen, is discharged from the plant A.
An illustrative and non-limiting example is
-11-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
provided for a better understanding of the present
invention and for its practical embodiment.
EXAMPLE
This is a stream of 1000 Nm3/h of a mixture of
methane + hydrogen sulfide with a concentration of H2S
equal to 65% in moles, 35o consisting of methane.
The mixture thus obtained with an overall flow-rate
of 780 Nm3/h, after being heated (to T=10500C) in a
system of two consecutive heat exchangers, is fed to
the reforming reactor, consisting in the case of this
example of a fixed bed reactor, filled with a catalyst
consisting of Cr2S3 supported on silica, operating at
T=950 C.
The outgoing fumes, essentially consisting of CS2,
hydrogen and non-reacted H2S, are sent to a condenser
from which the CS2 is separated as liquid, whereas the
gaseous stream comprising hydrogen and H2S, is sent to
an amine plant.
The CS2 obtained (149.6 1/h) is fed to the
combustion plant together with air, obtaining a stream
consisting of CO2 and SO2 (at T=1100 C) . This stream is
used for heating the reagents to be fed to the
reforming reactor. After transferring its heat to the
reagents of the reforming reaction, it can then be
subsequently fed to a plant for the production of
-12-
CA 02766037 2011-12-19
WO 2010/150063 PCT/IB2010/001344
sulfuric acid or disposed of by injection into the subsoil.
-13-