Note: Descriptions are shown in the official language in which they were submitted.
MULTI REACTOR ETHYLENE OLIGOMERIZATION PROCESS WITH RECYCLE
FIELD OF THE INVENTION
This invention relates to the oligomerization of ethylene using a Cr catalyst
having a so-called "bridged diphosphine ligand" in a process that uses at
least two
different types of reactors in series, namely at least one mixed reactor and
at least one
tubular reactor, with the provisio that part of the oligomer product that is
discharged
from the tubular reactor is recycled to the mixed reactor.
BACKGROUND OF THE INVENTION
Alpha olefins are commercially produced by the oligomerization of ethylene in
the presence of a simple alkyl aluminum catalyst (in the so called "chain
growth"
process) or alternatively, in the presence of an organometallic nickel
catalyst (in the so
' called Shell Higher Olefins, or "SHOP" process). Both of these processes
typically
produce a crude oligomer product having a broad distribution of alpha olefins
with an
even number of carbon atoms (i.e. butene-1, hexene-1, octene-1 etc.). The
various
alpha olefins in the crude oligomer product are then typically separated in a
series of
distillation columns. Butene-1 is generally the least valuable of these
olefins as it is
also produced in large quantities as a by-product in various cracking and
refining
processes. Hexene-1 and octene-1 often command comparatively high prices
because
these olefins are in high demand as comonomers for linear low density
polyethylene
(LLDPE).
Technology for the selective trimerization of ethylene to hexene-1 has been
recently put into commercial use in response to the demand for
hexene-1. The patent literature discloses catalysts which comprise a chromium
source
and a pyrrolide ligand as being useful for this process ¨ see, for example,
United States
.. Patent ("USP") 5,198,563 (Reagen et al., assigned to Phillips Petroleum).
1
\\chclients\IPGrounµRrnfficrscna,mi9no1Can docx
CA 2767615 2018-07-18
Another family of highly active trimerization catalysts is disclosed by Wass
et al.
in WO 02/04119 (now United States Patents 7,143,633 and 6,800,702). The
catalysts
disclosed by Wass et al. are formed from a chromium source and a bridged
diphosphine ligand and are described in further detail by Carter et al. (Chem.
Comm.
2002, p 858-9). As described in the Chem. Comm. paper, these catalysts
preferably
comprise a diphosphine ligand in which both phosphine atoms are bonded to two
phenyl groups that are each substituted with an ortho-methoxy group. Hexene-1
is
produced with high activity and high selectivity by these catalysts.
Similar diphosphine/tetraphenyl ligands are disclosed by Blann et al. in
.. W004/056478 and WO 04/056479 (now US 2006/0229480 and US 2006/0173226).
However, in comparison to the ligands of Wass et al., the
disphosphine/tetraphenyl
ligands disclosed by Blann et al. generally do not contain polar substituents
in ortho
positions. The "tetraphenyl" diphosphine ligands claimed in the '480
application must
not have ortho substituents (of any kind) on all four of the phenyl groups and
the
"tetraphenyl" diphosphine ligands claimed in '226 are characterized by having
a polar
substituent in a meta or para position. Both of these approaches are shown to
reduce
the amount of hexenes produced and increase the amount of octene (in
comparison to
the ligands of Wass et al.). Other bridged diphosphine ligands that are useful
for the
selective oligomerization of ethylene are disclosed in the literature. The
formation of
polymer as a by-product is a general problem with many of these ligands.
The oligomerization of ethylene is highly exothermic. The performance of the
Cr
bridged diphosphine catalysts is quite temperature dependent. Preferred
operating
temperatures are from 50 ¨ 150 C; especially from 60 ¨ 90 C. Sudden
temperature
changes (especially temperature drops) have been observed to lead to the
formation of
by-product polymer ¨ which is highly undesirable. In addition, the selectivity
of the
2
dm(
CA 2767615 2018-07-18
catalyst has been observed to change with temperature. Accordingly, good
temperature
control is highly desirable and an isothermal (as opposed to adiabatic)
reaction is
especially preferred.
It is also important to ensure that the ethylene feed and catalyst feed are
well
mixed. Ethylene concentration gradients (leading to localized high ethylene
concentrations) have been observed to lead to polymer fouling. Likewise,
localized
catalyst concentration gradients in a poorly mixed reactor are also believed
to lead to
polymer formation. It is also believed that the relative ethylene/catalyst
ratio is important
and that very low catalyst concentrations (at a given ethylene concentration)
lead to
excessive polymer formation.
Thus, a first preferred condition is to provide a well mixed reactor in order
to
minimize gradients in reactor temperature, ethylene concentration and catalyst
concentration.
Finally - and most interestingly - kinetic studies have shown the
oligomerization
reaction to be "mixed order" in ethylene concentration when using some bridged
diphosphine catalysts, particularly catalysts that enable selective
tetramerizations.
While not wishing to be bound by theory, we believe that two reactions are
taking place
simultaneously ¨ a selective trimerization reaction (which appears to be first
order in
ethylene) and a selective tetramerization reaction (which appears to be second
order in
ethylene). Thus, if it is desired to maximize octene production, a second
preferred
condition ¨ namely high ethylene concentration ¨ is desirable.
It is possible to satisfy both of the first preferred condition (i.e. a well
mixed
reactor, as noted above) and the second preferred condition ¨ namely high
ethylene
concentrations ¨ by operating a well mixed reactor (such as CSTR) at a high
ethylene
concentration and with low ethylene conversion. It will be appreciated by
those skilled in
3
t1rhrliontql1P(Irnim \crntt1SrAnsarl9012001Can.docx
CA 2767615 2018-07-18
. __________________________________________________ ¨
the art that the ethylene concentration in the discharge from a well mixed
CSTR will
correspond to the ethylene concentration in the bulk. Thus, a problem with the
use of a
CSTR in this process is that the product discharge will contain large amounts
of
unreacted ethylene.
It would appear that this problem might be mitigated by using a tubular
reactor.
In a tubular reactor, the ethylene is converted as the reaction mixture flows
through the
tube. Thus, the desired high ethylene concentrations are provided at the start
of the
tube and lower ethylene concentrations are present at the tube discharge.
However, it
is exceptionally difficult to provide well mixed conditions at the feed end
(or start) of a
reaction in a tube, particularly with a highly active catalyst and/or when
rapid mass
transfer is required. Both conditions are required with the present process
and the
resulting mixing problems could be expected to lead to gross polymer fouling.
The present invention mitigates these problems.
SUMMARY OF THE INVENTION
In one embodiment, the present invention provides:
A process for the oligomerization of ethylene in at least two reactors,
wherein
said process comprises
a) providing ethylene to a mixed reactor in the presence of an oligomerization
catalyst under oligomerization conditions, thereby producing an initial
oligomer
product;
b) discharging said initial oligomer product from said mixed reactor and
directing it
to a tubular reactor;
C) forming additional oligomer product in said tubular reactor;
d) discharging from said tubular reactor an oligomerization stream containing
said
initial oligomer product and said additional oligomer product; and
4
\t^h-"---"Dra--",e¨H`cren¨:"'012001Cartclocx
CA 2767615 2018-07-18
e) recycling a portion of said oligomerization stream from the discharge of
said
tubular reactor back to said mixed reactor;
with the provisio that said catalyst system comprises a chromium catalyst
having
a bridged diphosphine ligand.
For clarity, the present invention must include a recycle flow from the
discharge
of the tubular reactor to the mixed reactor. In this manner, a semi-batch
process is
enabled (whereby ethylene is added to the reactor on a continuous/semi
continuous
basis, but the oligomer product is only withdrawn in a batch or semi batch
manner).
It will also be appreciated that doing a batch/semi-batch operation, all of
the
discharge from the tubular reactor may be recycled back to the mixed reactor.
This
would continue until the reactor becomes full or the reactor is terminated for
a different
reason.
Conversely, in a continuous process, some of the oligomer product is withdrawn
during the reaction, as explained in more detail below. Thus, the term "a
portion" (when
referring to the oligomer product that is being recycled) is inclusive of
"some or all", as
the context requires.
The use of additional reactors is also contemplated. Most notably, the reactor
system may be fitted with two or more tubular reactors. This would facilitate
operating
the process at low rates (with one tubular reactor) and higher rates with the
additional
tubular reactor.
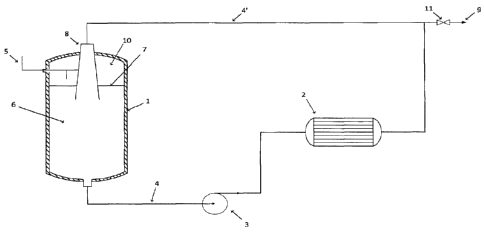
BRIEF DESCRIPTION OF THE DRAWING
Figure 1 is a process flow diagram that illustrates a preferred embodiment of
the
invention.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
5
\\chclients\IPGrouo\Scott\SCSoec12012001Can.docx
CA 2767615 2018-07-18
Figure 1 illustrates a process flow diagram of a preferred embodiment of the
present invention.
The mixed reactor 1 receives fresh ethylene via feed line 5 and catalyst feeds
via
feed line 6. It is especially preferred to also add hydrogen to mixed reactor
1. Thus,
hydrogen may be added in the ethylene feed line 5 or an alternative feed line.
Recycle
from the tubular reactor 2 is also added to the mixed reactor, via line 4.
In a preferred embodiment, mixed reactor '1 has a liquid 7 level that defines
a
gas space 10 above the liquid reaction mixture.
In one embodiment, mixed reactor 1 is a continuously stirred tank reactor
.. (CSTR) and mixing is provided by an agitator.
In an alternative (preferred) embodiment, mixed reactor I is equipped with a
gas/liquid ejector 8. The recycle liquid from reactor 2 is directed into
reactor 1 in the
form of a liquid jet through gas/liquid ejector 8 by way of recycle line 4'.
The liquid jet
flows through a zone of reduced cross sectional area, thereby forming a zone
of
especially fast liquid flow (which, in turn, produces low pressure). The
gas/liquid ejector
8 has an opening in the gas space 10 that communicates with the low pressure
zone of
the gas/liquid ejector 8 and this allows ethylene to be entrained in liquid
and mixed in
the jet flow. The flow from the ejector ¨ which consists of the reaction
liquid and
entrained/dissolved ethylene ¨ is directed into the liquid of mixed reactor 1.
Such
gas/liquid ejectors are known in the art and are also commonly referred to as
Venturi
mixers and/or jet ejectors (as well as gas/liquid ejectors). Reactors equipped
with such
an ejector are commonly referred to as "gas circulation" or "jet loop"
reactors.
It should be noted that the ejector 8 is only shown in a symbolic or
representative
manner (as are pump 3 and reactors 1 and 2) with much detail omitted. For
example,
the liquid flow channel for the jet and the gas flow channel (or channels)
which allow
6
st,i,,i;..f.uortr".,nm=-=-miQr`e---11012001Can.docx
CA 2767615 2018-07-18
gas to be entrained in the liquid are omitted. This type of detail will be
readily
understood by persons of ordinary skill in the art of ejector design. Such
detail is
available in the literature (see for example, Ullman's Encyclopedia of
Industrial
Chemistry, Fifth Edition, ed.: Elvers et at.; ISBN 0-89573-539-3; vol B4,
p.297). It
should also be noted that the Figure does illustrate the preferred down flow
of the liquid
jet.
It is preferred that the liquid flow through the jet is at least 10 meters per
second,
m/s (and especially at least 20m/s) in order to efficiently entrain the
ethylene.
It is preferred to use a pump 3 to provide the propulsion that is required to
circulate the flow through the jet; the mixed reactor 1 and the tubular
reactor 2. For
clarity: reaction liquid circulates from the discharge of mixed reactor 1,
through pump 3
and reactor 2 and then back to reactor 1. Thus, a combination of the
gas/liquid ejector 8
and the pump 3 providing mixing in reactor 1 is a preferred embodiment of this
invention.
In a preferred embodiment, the cooling system for the present invention is
provided as an external cooling system (i.e.: it is preferred to avoid the use
of internal
cooling coils).
It is especially preferred to provide an external cooling shell on the tubular
reactor and external cooling coils on the top of the well mixed reactor (to
provide
cooling for the gas space in mixed reactor 1.
As previously noted, it is difficult to mix high levels of ethylene and/or
catalyst in
a tubular reactor. Accordingly, it is especially preferred to add catalyst and
ethylene to
only the mixed reactor (i.e. to avoid adding fresh ethylene and/or catalyst to
the tubular
reactor). The term "tubular reactor" as used herein is meant to convey its
conventional
meaning, namely a reactor with a high length/diameter (or LID) ratio. A single
tube or
7
µkpi.,,li..,muorz,...nkc,ni+kQi'Q--,\1012001Can.docx
CA 2767615 2018-07-18
-----
multiple tube bundle may be suitably employed. Tubular reactors typically do
not have
internal agitators and that is the case in the present invention, with pump 3
preferably
providing the force to move the reaction liquid.
The ethylene is most preferably added as a gas although a portion of the
ethylene may be added as a liquid (thereby cooling the reactor as the liquid
ethylene
flashes to a gas). It is especially preferred to add hydrogen with the
ethylene. In one
embodiment, a portion of the ethylene may be added below the liquid level
(although
this is not necessary). In another embodiment, the ethylene is added to the
gas space
in reactor 1.
10 The process of the present invention has the additional advantage that
it
facilitates the start-up of the oligomerization. At start-up, the reactor
system will be
cleaned/purged according to good engineering practice. Start-up liquid and
(optionally)
hydrogen may then be added to the reactor. The amount of start-up liquid is
preferably
low (25-35% of the volume of the mixed reactor). The start-up liquid may be
any liquid
that facilitates the reaction (such as an aliphatic, an aromatic, or even
oligomer product
from a previous reaction). The pump preferably starts to circulate the liquid
through the
reactor system when adding the catalyst, thereby proving well mixed catalyst.
Ethylene is then gradually added to the mixed reactor to provide "light off'
(or
initiation of the reaction).
In one embodiment, the start-up liquid is a very good solvent for the catalyst
system (such as monochlorobenzene).
As the reaction progresses, the liquid level in the system increases as liquid
oligomer is produced. The liquid oligomer is then removed from the process.
This may
be done continuously (via a slip stream) or in a batch/semi-batch manner by
way of
product discharge line 9, through valve 11. The process may be operated with
8
mrhetiontquPrzr,..nlc,votmrq,,012001Can.docx
CA 2767615 2018-07-18
. _
additional solvent being added (for example, with the ethylene) or ¨
alternatively ¨ the
proces may be operated without additional solvent being provided after start
up.
Additional details are provided below.
PART A CATALYST SYSTEM
The preferred catalyst system used in the process of the present invention
must
contain three essential components, namely:
(i) a source of chromium;
(ii) a diphosphine ligand; and
(iii) an activator.
Preferred forms of each of these components are discussed below.
Chromium Source ("Component (i)")
Any source of chromium that is soluble in the process solvent and which allows
the oligomerization process of the present invention to proceed may be used.
Preferred chromium sources include chromium trichloride; chromium (III) 2-
ethylhexanoate; chromium (III) acetylacetonate and chromium carbonyl complexes
such as chromium hexacarbonyl. It is preferred to use very high purity
chromium
compounds as these should generally be expected to minimize undesirable side
reactions. For example, chromium acetylacetonate having a purity of higher
than 99%
is commercially available (or may be readily produced from 97% purity material
¨ using
recrystallization techniques that are well known to those skilled in the art).
Liqand Used in the Oliqomerization Process ("Component (ii)")
In general, the ligand used in the oligomerization process of this invention
is
defined by the formula (R1)(R2)-P1-bridge-P2(R3)(R4) wherein R1, R2,R3 and R4
are
independently selected from the group consisting of hydrocarbyl and
heterohydrocarbyl
and the bridge is a moiety that is bonded to both phosphorus atoms.
9
= = = ¨ -.4r..'^-'2,0012001Can.docx
CA 2767615 2018-07-18
Another family of suitable ligands uses a fluorocarbyl oxide (especially the
aromatic group ¨ C6F5) for the R4 group ¨ with R1 to R3 being as defined
above.
The term hydrocarbyl as used herein is intended to convey its conventional
meaning ¨ i.e. a moiety that contains only carbon and hydrogen atoms. The
.. hydrocarbyl moiety may be a straight chain; it may be branched (and it will
be
recognized by those skilled in the art that branched groups are sometimes
referred to
as "substituted"); it may be saturated or contain unsaturation and it may be
cyclic.
Preferred hydrocarbyl groups contain from 1 to 20 carbon atoms. Aromatic
groups ¨
especially phenyl groups ¨ are especially preferred. The phenyl may be
unsubstituted
(i.e. a simple C6I-15 moiety) or contain substituents, particularly at an
ortho (or "o")
position.
Similarly, the term heterohydrocarbyl as used herein is intended to convey its
conventional meaning ¨ more particularly, a moiety that contains carbon,
hydrogen and
heteroatoms (such as 0, N, R and S). The heterohydrocarbyl groups may be
straight
.. chain, branched or cyclic structures. They may be saturated or contain
unsaturation.
Preferred heterohydrocarbyl groups contain a total of from 2 to 20 carbon +
heteroatoms (for clarity, a hypothetical group that contains 2 carbon atoms
and one
nitrogen atom has a total of 3 carbon + heteroatoms).
It is preferred that each of R1, R2, R3 and R4 is a phenyl group (with an
optional
substituent in an ortho position on one or more of the phenyl groups).
Highly preferred ligands are those in which R1 to R4 are independently
selected
from the group consisting of phenyl, o-methylphenyl (i.e. ortho-methylphenyl),
o-ethylphenyl, o-isopropylphenyl and o-fluorophenyl. It is especially
preferred that none
of R1 to R4 contains a polar substituent in an ortho position. The resulting
ligands are
useful for the selective tetramerization of ethylene to octene-1 with some co
product
mhaentqmpnrniirACrntfiSCAn0M2012001Can.docx
CA 2767615 2018-07-18
hexene also being produced. The term "bridge" as used herein with respect to
the
ligand refers to a moiety that is bonded to both of the phosphorus atoms in
the ligand ¨
in other words, the "bridge" forms a link between P1 and P2. Suitable groups
for the
bridge include hydrocarbyl and an inorganic moiety selected from the group
consisting
of N(CH3)-N(CH3)-, -B(R6)-, -Si(R6)2-, -P(R6)- or -N(R6)- where R6 is selected
from the
group consisting of hydrogen, hydrocarbyl and halogen.
It is especially preferred that the bridge is -N(R5)- wherein R5 is selected
from the
group consisting of hydrogen, alkyl, substituted alkyl, aryl, substituted
aryl, aryloxy,
substituted aryloxy, halogen, alkoxycarbonyl, carbonyloxy, alkoxy,
aminocarbonyl,
carbonylamino, dialkylamino, silyl groups or derivatives thereof and an aryl
group
substituted with any of these substituents. A highly preferred bridge is amino
isopropyl
(i.e. when R5 is isopropyl).
In one embodiment, two different types of ligands are used to alter the
relative
amounts of hexene and octene being produced. For clarity: the use of a ligand
that
produces predominantly hexene may be used in combination with a ligand that
produces predominantly octene.
Activator ("Component 'Hp")
The activator (component (iii)) may be any compound that generates an active
catalyst for ethylene oligomerization with components (i) and (ii). Mixtures
of activators
may also be used. Suitable compounds include organoaluminum compounds,
organoboron compounds and inorganic acids and salts, such as tetrafluoroboric
acid
etherate, silver tetrafluoroborate, sodium hexafluoroantimonate and the like.
Suitable
organoaluminium compounds include compounds of the formula AIR3, where each R
is
independently Ci ¨C12 alkyl, oxygen or halide, and compounds such as LiAIH4
and the
like. Examples include trimethylaluminium (TMA), triethylaluminium (TEA), tri-
ll
"^h^,"^`^^^-^",012001Can docx
_ _ _ _ _
CA 2767615 2018-07-18
isobutylaluminium (TIBA), tri-n-octylaluminium, methylaluminium dichloride,
ethylaluminium dichloride, dimethylaluminium chloride, diethylaluminium
chloride,
ethylaluminiumsesquichloride, methylaluminiumsesquichloride, and alumoxanes.
Alumoxanes are well known in the art as typically oligomeric compounds which
can be
prepared by the controlled addition of water to an alkylaluminium compound,
for
example trimethylaluminium. Such compounds can be linear, cyclic, cages or
mixtures
thereof. Commercially available alumoxanes are generally believed to be
mixtures of
linear and cyclic compounds. The cyclic alumoxanes can be represented by the
formula [R6A10]s and the linear alumoxanes by the formula R7(R8A10)s wherein s
is a
number from about 2 to 50, and wherein R6, R7, and R8 represent hydrocarbyl
groups,
preferably Ci to Ce alkyl groups, for example methyl, ethyl or butyl groups.
Alkylalumoxanes especially methylalumoxane (MAO) are preferred.
It will be recognized by those skilled in the art that commercially available
alkylalumoxanes may contain a proportion of trialkylaluminium. For instance,
commercial MAO usually contains approximately 10 wt % trimethylaluminium
(TMA),
and commercial "modified MAO" (or "MMAO") contains both TMA and TIBA.
Quantities
of alkylalumoxane are generally quoted herein on a molar basis of aluminium
(and
include such "free" trialkylaluminium).
Examples of suitable organoboron compounds are boroxines, NaBF14,
trimethylboron, triethylboron, dimethylphenylammoniumtetra(phenyl)borate,
trityltetra(phenyl)borate, triphenylboron, dimethylphenylammonium
tetra(pentafluorophenyl)borate, sodium tetrakispis-3,5-
trifluoromethyl)phenyliborate,
trityltetra(pentafluorophenyl)borate and tris(pentafluorophenyl) boron.
12
mtutuantsmPnmiln\srnitncsnPrA9012001Can.docx
CA 2767615 2018-07-18
- ¨
Activator compound (iii) may also be or contain a compound that acts as a
reducing or oxidizing agent, such as sodium or zinc metal and the like, or
oxygen and
the like.
In the preparation of the catalyst systems used in the present invention, the
quantity of activating compound to be employed is easily determined by simple
testing,
for example, by the preparation of small test samples which can be used to
oligimerize
small quantities of ethylene and thus to determine the activity of the
produced catalyst.
It is generally found that the quantity employed is sufficient to provide 0.5
to 1000 moles
of aluminium (or boron) per mole of chromium. MAO is the presently preferred
activator. Molar Al/Cr ratios of from 1/1 to 1500/1, especially 300/1 to 900/1
are
preferred.
PART B PROCESS CONDITIONS
The chromium (component (i)) and ligand (component (ii)) may be present in any
molar ratio which produces oligomer, preferably between 100:1 and 1:100, and
most
preferably from 10:1 to 1:10, particularly 3:1 to 1:3. Generally the amounts
of (i) and (ii)
are approximately equal, i.e. a ratio of between 2:1 and 1:2.
Components (i)-(iii) of the catalyst system utilized in the present invention
may
be added together simultaneously or sequentially, in any order, and in the
presence or
absence of ethylene in any suitable solvent, so as to give an active catalyst.
For
example, components (i), (ii) and (iii) and ethylene may be contacted together
simultaneously, or components (i), (ii) and (iii) may be added together
simultaneously or
sequentially in any order and then contacted with ethylene, or components (i)
and (ii)
may be added together to form an isolable metal-ligand complex and then added
to
component (iii) and contacted with ethylene, or components (i), (ii) and (iii)
may be
added together to form an isolable metal-ligand complex and then contacted
with
13
docx
CA 2767615 2018-07-18
ethylene. Suitable solvents for contacting the components of the catalyst or
catalyst
system include, but are not limited to, hydrocarbon solvents such as heptane,
toluene,
1-hexene and the like, and polar solvents such as diethyl ether,
tetrahydrofuran,
acetonitrile, dichloromethane, chloroform, chlorobenzene, acetone and the
like. A
preferred solvent is the oligomer product that is produced by the present
process or
some fraction thereof ¨ such as hexene, octene or a mixture of the two.
For further clarity: the catalyst components may be mixed together in the
oligomerization reactor, or ¨ alternatively ¨ some or all of the catalyst
components may
be mixed together outside of the oligomerization reactor. In general, it is
preferred to
mix the catalyst components outside of the reactor (due to comparative ease of
control)
then add the catalyst to the reactor shortly thereafter (because "aged"
catalyst may
suffer from some loss of activity). This method of catalyst synthesis is
illustrated in the
examples. The solvent that is used to prepare the catalyst is preferably the
olefinic
product that is produced by the reactor (or some portion thereof). We have
found that
the use of octene generally works well. However, some catalyst components have
comparatively low solubility in octene. For example, MAO that is made solely
with
trimethylaluminum (as opposed to "modified MAO" which also contains some
higher
alkyl aluminum, such as triisobutyl aluminum) is less soluble in octene than
in some
cyclic hydrocarbons such as xylene or tetralin. Accordingly, when one or more
catalyst
components are mixed together outside of the oligomerization reactor, the use
of
toluene, xylene chlorobenzene, or tetralin as the solvent may be preferred.
The xylene
may be a mixture of ortho, meta and para isomers ¨ i.e. it is not necessary to
use a
pure isomer.
A variety of methods are known to purify solvents used in the oligomerization
process including use of molecular sieves (3A), adsorbent alumina and
supported
14
1\chcliants IPGrounlScott\SCSopc12012001Can docx
CA 2767615 2018-07-18
_
de-oxo copper catalyst. Several configurations for the purifier system are
known and
depend on the nature of the impurities to be removed, the purification
efficiency
required and the compatibility of the purifier material and the process
solvent. In some
configurations, the process solvent is first contacted with molecular sieves,
followed by
adsorbent alumina, then followed by supported de-oxo copper catalyst and
finally
followed by molecular sieves. In other configurations, the process solvent is
first
contacted with molecular sieves, followed by adsorbent alumina and finally
followed by
molecular sieves. In yet another configuration, the process solvent is
contacted with
adsorbent alumina. When alpha olefinic solvents are used in the process, the
preferred
purifier system consists of molecular sieves, followed by adsorbent alumina
and finally
followed by another set of molecular sieves.
The catalyst components (i), (ii) and (iii) utilized in the present invention
can be
unsupported or supported on a support material, for example, silica, alumina,
MgC12 or
zirconia, or on a polymer, for example polyethylene, polypropylene,
polystyrene, or
poly(aminostyrene). If desired the catalysts can be formed in situ in the
presence of the
support material, or the support material can be pre-impregnated or premixed,
simultaneously or sequentially, with one or more of the catalyst components.
The
quantity of support material employed can vary widely, for example from
100,000 to 1
gram per gram of metal present in the transition metal compound. In some
cases, the
support material can also act as or as a component of the activator compound
(iii).
Examples include supports containing alumoxane moieties.
Oligomerization reactions can generally be conducted under solution phase,
slurry phase, gas phase or bulk phase conditions. Suitable temperatures range
from
10 C to +300 C preferably from 10 C to 150 C, especially from 20 to 80 C.
Suitable
\ ehrliantellPnrni \ Crrift \ Cr.Cnor17012001Can.docx
CA 2767615 2018-07-18
pressures are from atmospheric to 800 atmospheres (gauge) preferably from 5
atmospheres to 150 atmospheres, especially from 10 to 100 atmospheres.
Irrespective of the process conditions employed, the oligomerization is
typically
carried out under conditions that substantially exclude oxygen, water, and
other
materials that act as catalyst poisons. In addition, the reactor is preferably
purged with
a nonreactive gas (such as nitrogen or argon) prior to the introduction of
catalyst. A
purge with a solution of MAO and/or aluminum alkyl may also be employed to
lower the
initial level of catalyst poisons. Also, oligomerizations can be carried out
in the
presence of additives to control selectivity, enhance activity and reduce the
amount of
polymer formed in oligomerization processes. Potentially suitable additives
include, but
are not limited to, hydrogen or a halide source (especially the halide sources
disclosed
in U.S. patent 7,786,336, Zhang et al.). Other (optional) additives include
antistatic
agents (such as the polysulfone polymer sold under the trademark Stadise)
and/or
fluorocarbons to mitigate reaction fouling; or amines to alter the
hexene/octene ratio of
the product oligomer (as disclosed in U.S. application 20090118117, Elowe et
al.). The
use of hydrogen is especially preferred because it has been observed to reduce
the
amount of polymer that is formed. It is within the scope of this invention
that an
oligomerization product might also serve as a solvent or diluent. The
preferred
catalysts of this invention predominantly produce hexene and octene (as shown
in the
examples) but smaller quantities of butene and Cio+ olefins are also produced.
The
crude product stream may be separated into various fractions using, for
example, a
conventional distillation system. Mixtures of inert diluents or solvents also
could be
employed. The preferred diluents or solvents are aliphatic and aromatic
hydrocarbons
and halogenated hydrocarbons such as, for example, isobutane, pentane,
toluene,
xylene, ethylbenzene, cumene, mesitylene, heptane, cyclohexane,
methylcyclohexane,
16
11,=h,l012001Can.docx
CA 2767615 2018-07-18
1-hexene, 1-octene, chlorobenzene, dichlorobenzene, and the like, and mixtures
such
as lsoparTM.
Techniques for varying the distribution of products from the oligomerization
reactions include controlling process conditions (e.g. concentration of
components (i)-
(iii), reaction temperature, pressure, residence time) and properly selecting
the design
of the process and are well known to those skilled in the art.
In another embodiment, a catalyst that produces ethylene homopolymer is
deliberately added to the reactor in an amount sufficient to convert from 1 to
5 weight%
of the ethylene feed to an ethylene homopolymer. This catalyst is preferably
supported.
The purpose is to facilitate the removal of by-product polyethylene.
The ethylene feedstock for the oligomerization may be substantially pure or
may
contain other olefinic impurities and/or ethane. One embodiment of the process
of the
invention comprises the oligomerization of ethylene-containing waste streams
from
other chemical processes or a crude ethylene/ethane mixture from a cracker as
more
fully described in co-pending Canadian patent application 2,708,011 (Krzywicki
et al.).
The feedstock is preferably treated to remove catalyst poisons (such as
oxygen,
water and polar species) using techniques that are well known to those skilled
in the
art. The technology used to treat feedstocks for polymerizations is suitable
for use in
the present invention and includes the molecular sieves, alumina and de-oxo
catalysts
.. described above for analogous treatment of the process solvent.
Reactor Control
The control systems required for the operation of mixed reactors and tubular
reactors are well known to those skilled in the art and do not represent a
novel feature
of the present invention. In general, temperature, pressure and flow rate
readings will
provide the basis for most conventional control operations. The increase in
process
17
CA 2767615 2018-07-18
temperature (together with reactor flow rates and the known enthalpy of
reaction) may
be used to monitor ethylene conversion rates. The amount of catalyst may be
increased to increase the ethylene conversion (or decreased to decrease
ethylene
conversion) within desired ranges. Thus, basic process control may be derived
from
simple measurements of temperature, pressure and flow rates using conventional
thermocouples, pressure meters and flow meters. Advanced process control (for
example, for the purpose of monitoring product selectivity or for the purpose
of
monitoring process fouling factors) may be undertaken by monitoring additional
process
parameters with more advanced instrumentation. Known/existing instrumentation
that
may be employed include in-line/on-line instruments such as NIR infrared,
Fourier
Transform Infrared (FTIR), Raman, mid-infrared, ultra violet (UV)
spectrometry, gas
chromatography (GC) analyzer, refractive index, on-line densitometer or
viscometer.
The use of NIR or GC to measure the composition of the oligomerization reactor
and
final product composition is especially preferred.
The measurement may be used to monitor and control the reaction to achieve
the targeted stream properties including but not limited to concentration,
viscosity,
temperature, pressure, flows, flow ratios, density, chemical composition,
phase and
phase transition, degree of reaction, polymer content, selectivity.
The control method may include the use of the measurement to calculate a new
control set point. The control of the process will include the use of any
process control
algorithms, which include, but are not limited to the use of PID, neural
networks,
feedback loop control, forward loop control and adaptive control.
Catalyst Deactivation, Catalyst Removal and Polymer Removal
In general, the oligomerization catalyst is preferably deactivated immediately
downstream of the reactor as the product exits the reaction system. This is to
prevent
18
"^";,..+-"Dr,"¨oe^^000,"c^-.1-00120010an.docx
CA 2767615 2018-07-18
polymer formation and potential build up downstream of the reactor and to
prevent
isomerisation of the 1-olefin product to the undesired internal olefins. It is
generally
preferred to flash and recover unreacted ethylene before deactivation.
However, the
option of deactivating the reactor contents prior to flashing and recovering
ethylene is
also acceptable. The flashing of ethylene is endothermic and may be used as a
cooling
source.
In general, many polar compounds (such as water, alcohols and carboxylic
acids) will deactivate the catalyst. The use of alcohols and/or carboxylic
acids is
preferred ¨ and combinations of both are contemplated. It is generally found
that the
quantity employed to deactivate the catalyst is sufficient to provide
deactivator to metal
(from catalyst + activator) mole ratio between about 0.1 to about 4,
especially from 1 to
2 (thus, when MAO is the activator, the deactivator is provided on a ratio
based on
moles of Cr + to moles of Al).
The deactivator may be added to the oligomerization product stream before or
after the volatile unreacted reagents/diluents and product components are
separated.
In the event of a runaway reaction (e.g. rapid temperature rise) the
deactivator can be
immediately fed to the oligomerization reactor to terminate the reaction. The
deactivation system may also include a basic compound (such as sodium
hydroxide) to
minimize isomerization of the products (as activator conditions may facilitate
the
isomerization of desirable alpha olefins to undesired internal olefins).
Polymer removal (and, optionally, catalyst removal) preferably follows
catalyst
deactivation. Two "types" of polymer may exist, namely polymer that is
dissolved in the
process solvent and non-dissolved polymer that is present as a solid or
"slurry".
Solid/non-dissolved polymer may be separated using one or more of the
following types of equipment: centrifuge; cyclone (or hydrocyclone), a
decanter
19
CA 2767615 2018-07-18
equipped with a skimmer or a filter. Preferred equipment include so called
"self
cleaning filters" sold under the name V-auto strainers, self cleaning screens
such as
those sold by Johnson Screens Inc. of New Brighton, Minnesota and centrifuges
such
as those sold by Alfa Laval Inc. of Richmond, VA (including those sold under
the trade
name Sharpies).
Soluble polymer may be separated from the final product by two distinct
operations. Firstly, low molecular weight polymer that remains soluble in the
heaviest
product fraction (C2o+) may be left in that fraction. This fraction will be
recovered as
"bottoms" from the distillation operations (described below). This solution
may be used
as a fuel for a power generation system.
An alternative polymer separation comprises polymer precipitation caused by
the
removal of the solvent from the solution, followed by recovery of the
precipitated
polymer using a conventional extruder. The technology required for such
separation/recovery is well known to those skilled in the art of solution
polymerization
and is widely disclosed in the literature.
In another embodiment, the residual catalyst is treated with an additive that
causes some or all of the catalyst to precipitate. The precipitated catalyst
is preferably
removed from the product at the same time as by-product polymer is removed
(and
using the same equipment). Many of the catalyst deactivators listed above will
also
cause catalyst precipitation. In a preferred embodiment, a solid sorbent (such
as clay,
silica or alumina) is added to the deactivation operation to facilitate
removal of the
deactivated catalyst by filtration or centrifugation.
Reactor fouling (caused by deposition of polymer and/or catalyst residue) can,
if
severe enough, cause the process to be shut down for cleaning. The deposits
may be
removed by known means, especially the use of high pressure water jets or the
use of
rhnhants \ I PGmi \ Srtritt1SC:Sne.r1701200ican.doex
CA 2767615 2018-07-18
a hot solvent flush. The use of an aromatic solvent (such as toluene or
xylene) for
solvent flushing is generally preferred because they are good solvents for
polyethylene.
The use of the heat exchanger that provides heat to the present process may
also be
used during cleaning operations to heat the cleaning solvent.
6 Distillation
In one embodiment of the present invention, the oligomerization product
produced from this invention is added to a product stream from another alpha
olefins
manufacturing process for separation into different alpha olefins. As
previously
discussed, "conventional alpha olefin plants" (wherein the term includes i)
those
processes which produce alpha olefins by a chain growth process using an
aluminum
alkyl catalyst, ii) the aforementioned "SHOP" process and iii) the production
of olefins
from synthesis gas using the so called Lurgi process) have a series of
distillation
columns to separate the "crude alpha product" (i.e. a mixture of alpha
olefins) into alpha
olefins (such as butene-1, hexene-1 and octene-1). The mixed hexene-octene
product
16 .. which is preferably produced in accordance with the present invention is
highly suitable
for addition/mixing with a crude alpha olefin product from an existing alpha
olefin plant
(or a "cut" or fraction of the product from such a plant) because the mixed
hexene-
octene product produced in accordance with the present invention can have very
low
levels of internal olefins. Thus, the hexene-octene product of the present
invention can
be readily separated in the existing distillation columns of alpha olefin
plants (without
causing the large burden on the operation of these distillation columns which
would
otherwise exist if the present hexene-octene product stream contained large
quantities
of internal olefins). As used herein, the term "liquid product" is meant to
refer to the
oligomers produced by the process of the present invention which have from 4
to
26 (about) 20 carbon atoms.
21
CA 2767615 2018-07-18
In another embodiment, the distillation operation for the oligomerization
product
is integrated with the distillation system of a solution polymerization plant
(as disclosed
in Canadian patent application no. 2,708,011, Krzywicki et al.).
If toluene is present in the process fluid (for example, as a solvent for a
MAO
activator), it is preferable to add water to the "liquid product" prior to
distillation to form a
water/toluene azeotrope with a boiling point between that of hexene and
octene.
The liquid product from the oligomerization process of the present invention
preferably consists of from 20 to 80 weight% octenes (especially from 35 to 75
weight%) octenes and from 15 to 50 weight% (especially from 20 to 40 weight%)
hexenes (where all of the weight% are calculated on the basis of the liquid
product by
100%.
The preferred oligomerization process of this invention is also characterized
by
producing very low levels of internal olefins (i.e. low levels of hexene-2,
hexene-3,
octene-2, octene-3 etc.), with preferred levels of less than 10 weight%
(especially less
than 5 weight%) of the hexenes and octenes being internal olefins.
In-Situ Polymerization
One embodiment of the present invention encompasses the use of components
(i) (ii) and (iii) in conjunction with one or more types of olefin
polymerization catalyst
system (iv) to oligomerize ethylene and subsequently incorporate a portion of
the
trimerisation product(s) into a higher polymer.
Component (iv) may be one or more suitable polymerization catalyst system(s),
examples of which include, but are not limited to, conventional Ziegler-Natta
catalysts,
metallocene catalysts, monocyclopentadienyl or "constrained geometry"
catalysts,
phosphinimine catalysts, heat activated supported chromium oxide catalysts
(e.g.
"Phillips"-type catalysts), late transition metal polymerization catalysts
(e.g. diimine,
22
11,11rhantel I DrInsun1Crntil crcn.Alni)nnt r n do cx
c -
CA 2767615 2018-07-18
diphosphine and salicylaldimine nickel/palladium catalysts, iron and cobalt
pyridyldiimine catalysts and the like) and other so-called "single site
catalysts" (SSC's).
23
Ilek,i,,,õ4,,ion"õ,,,µc,,aocro÷.,m17not Can docx
CA 2767615 2018-07-18