Note: Descriptions are shown in the official language in which they were submitted.
CA 02768359 2013-07-24
- 1 -
REMOVAL OF SULFUR COMPOUNDS FROM A GAS STREAM
FIELD OF THE INVENTION
The present invention relates to a method and apparatus for removing or
reducing certain
sulfur species from an incoming gas stream that also contains combustible
hydrocarbons and
H2S, and for further reducing or removing H2S from such incoming gas stream or
alternatively
readying said gas stream for being able to subsequently remove such H2S by a
known processes
thereby permitting such gas stream to be cleansed of such sulfur species.
BACKGROUND OF THE INVENTION
Many industrial gas streams are contaminated with sulfur compounds/species
such as
hydrogen sulphide (H2S), sulfur dioxide (SO2), carbonyl sulphide (COS),
mercaptans (ie "RSH",
where R represents an alkane, alkene, or other carbon-containing groups of
atoms), and/or
carbon disulphide (CS2). For environmental or regulatory reasons it is
typically mandated to
remove or reduce the levels of such sulphur sulfur species in such industrial
gas streams, such as
in pipelines proximate to human habitation.. It has been estimated that about
forty percent or
2600 Tcf of the world's natural gas reserves are in the form of sour gas where
H2S and CO2
compositions exceed 10% of the raw produced sour gas. Other gas streams also
contain sulfur
species: for examples, refinery gas, in situ combustion produced gas, and coal
and petroleum
gasifiers.
A number of prior art processes currently exist to remove specifically the H2S
from gas
streams.
For example, one approach is subject the gas stream to an acid gas removal
unit, which
removes substantial amounts of FI,S and CO2 from the fl,S containing stream.
The off-gas from
the acid-gas removal unit is mainly H25 and CO,. The sulfur from this off-gas
stream is usually
removed by the Claus reaction which produces saleable elemental sulfur.
Specifically, the Claus
process may be used for processing large volumes, and a liquid reduction-
oxidation processes
used for intermediate sour gas volumes, and H2S disposable scavengers for
small volumes.
However, these processes can be relatively expensive in capital and operating
costs.
CAL_LAW\ 1773685\3
CA 02768359 2012-02-17
- 2 -
Below is a review of prior art and sulfur species chemical reactions:
In the first step in the Claus process, about one third of the H2S present may
be oxidized
to SO2 In the second step, remaining H2S and SO2 are reacted in the presence
of a Claus catalyst
to form elemental sulfur in a series of Claus reactors according to Reaction
1:
la. H2S + 3/2 02 - SO2 H20
lb. 2H2S + SO2 2H20 + 3S Claus reaction
The Claus reaction is limited by thermodynamic equilibrium and only a portion
of the
sulfur can be produced. Therefore, multiple stages with sulfur condensation
between the stages
are used to increase the sulfur recovery factor. However, the effluent gas
from a series of reactors
in a Claus plant can contain varying amounts of different compounds including
sulfur vapour,
SO2, un-reacted H2S, COS, and CS2. Carbon disulphide is formed according to
Reaction 2:
2. CH4 + 4S ¨0 CS2 + 2H2S High temp. Claus furnace or
combustion reaction
Typically, this Claus plant effluent gas stream is burned with air to convert
all sulfur-
containing compounds in the stream to SO2 before discharge into the
atmosphere. As
environmental requirements are become stricter, the SO2 emission limit is
being lowered, giving
rise to the challenge of how to reduce or eliminate SO2 emissions.
Another process for the oxidation of H2S to elemental sulfur is described in
U.S. patent
4,197,277 by the following H2S Oxidation Reactions 3a and 3b:
3a. H2S + 0.502 -> S + H2O H2S Partial oxidation
3b. H2S + 1.502 4 SO2 + H20 H2S Complete oxidation
According to the '277 patent, the H2S-containing gas is passed with an oxygen-
containing gas over a catalyst which comprises iron oxide and vanadium oxide
as active
materials and aluminum oxide as a support material. The catalyst described in
the patent gives
rise to at least a partial Claus equilibrium, so that SO2 formation cannot be
prevented. Similarly,
U.S. patent 5,352,422 describes a process for oxidizing the un-reacted H2S in
the Claus tail gas
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 3 -
to elemental sulfur. The patent describes a catalyst prepared by impregnation
of an iron
containing solution or an iron/chromium-containing solution into several
carriers followed by
calcination in air at 500 C.
U.S. patent 4,818,740 discloses a catalyst for the H2S oxidation to elemental
sulfur, the
use of which is said to prevent the reverse Claus reaction to a large extent.
The catalyst
comprises a support of which the surface exposed to the gaseous phase does not
exhibit any
alkaline properties under the reaction conditions, while a catalytically
active material is applied
to this surface. A modification of the method disclosed in '740 is disclosed
in European patent
409,353. This patent relates to a catalyst for the selective oxidation of
sulfur-containing
compounds to elemental sulfur, comprising at least one catalytically active
material and
optionally a support. The described catalyst exhibits substantially no
activity towards the reverse
Claus reaction under the reaction conditions.
The direct oxidation of H2S to elemental sulfur is known to take place on a
wide range of
catalysts. However, many of the catalysts experience a rapid deactivation and
fouling due to a
high level of carbon and/or sulfur deposits and irreversible sulphation of the
catalyst surface.
Alumina-based catalysts are particularly susceptible in this regard. U.S.
patent application
2005/0100504 relates to a process for selective oxidation of H2S to elemental
sulfur in the
presence of an inert liquid medium to moderate the reaction temperature and to
remove the sulfur
from the reaction zone. The inert medium used in this application could be
water, produced
liquid sulfur, or any other liquid that is not substantially consumed under
the reaction conditions.
The oxidation reaction was carried out at a temperature in the range of from
120-160 C and a
high pressure preferably in the range of from 60-120 bars (absolute) in order
to maintain the
supplied liquid in the liquid form during the oxidation process to enable it
to remove the sulfur
from the reaction zone. Nevertheless, carrying the H2S oxidation reaction at
temperatures below
the sulfur dew point and high pressures can force the produced sulfur to
deposit inside the
catalyst pore structures.
The gas streams from different chemical processes may contain a range of
sulfur-
containing compounds such as, H2S, SO2, COS, CS2 and RSH. Gases from
combustion
A8124663W0\CAL_LAW \ 1773685\2
CA 02768359 2012-02-17
- 4 -
processes, such as in-situ combustion and coal or coke gasification may also
contain CO, CO2
and H2. In the direct oxidation process, represented by Reaction 3a, oxygen is
reacted with H2S
over a catalyst to convert it to elemental sulfur. Because SO2 and COS are not
altered in the
catalytic direct oxidation process, a pretreatment process of the gas feed
stream is conducted to
convert sulfur-containing compounds to H2S so that a higher sulfur removal
efficiency can be
achieved. U.S. patents 4,552,746 and 4,857,297 relate to a process for the
oxidation of H2S to
elemental sulfur in the presence of oxidation catalyst and a feed gas stream
comprising less than
vol % water. The feed stream is pretreated to convert the sulfur-containing
compounds to
H2S. The feed gas pretreatment could be accomplished in Reactions 4-7 by
using, for example a
10 dual hydrolysis/hydrogenation catalyst of cobalt or nickel/molybdenum on
alumina to convert
the undesirable components in the gas stream to H2S so that the stream would
become amenable
to direct oxidation:
Hydrogenation
4. RCH2SH + H2 4 H2S + RCH3 Mercaptan hydrogenation
5. SO2 + 3H2 4 H2S + 2H20 Sulfur dioxide hydrogenation
6. CS2 + 4H2 -> 2H2S + CH4 Carbon disufide hydrogenation
7. COS + 4H2 4 H25 +CH4 + H20 Carbonyl sulphide hydrogenation
The rapid deactivation of the H2S direct oxidation catalyst was addressed by
carrying out
the oxidation reaction at a temperature above the sulfur dew point at the
reaction conditions.
Canadian patent 2,318,734 relates to a process for passing a hydrogen sulphide-
containing gas
stream mixed with the oxygen-containing stream over a catalyst comprising
niobium oxide and a
promoter on a titanium dioxide carrier. The stability of the catalyst was
investigated in the
presence of water and carbon dioxide and at a temperature above the sulfur dew
point to slow the
deactivation of the oxidation catalyst due to the sulfur deposition. The H2S
conversion to
elemental sulfur was greater than 90% and sulfur selectivity was greater than
85%. Although the
presence of CO2 in the feed gas stream increases the possibility of the COS
formation (Reaction
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 5 -
11) during the H2S direct oxidation to elemental sulfur, the effluent gas was
not analyzed for
COS:
11. H2S + CO2 4 COS +H20
Furthermore, the inventors of CA 2,318,734 and those of U.S. patents 4,552,746
and
4,857,297 apparently did not evaluate the performance of the disclosed 112S
oxidation catalyst in
the presence of a feed stream containing carbon monoxide, which undergoes side
reactions
during the H2S direct oxidation process to form COS:
CO Reactions
8. CO + S 4 COS
9. CO + H2S 4 COS + H2
10. 3C0 + SO2 4 COS + 2CO2
X-Ray diffraction analysis of the disclosed catalyst showed a homogenous
mixture of the
oxides of Nb and Ti. The presence of Nb oxides in the catalyst increases the
number of the Lewis
acid sites on the Ti surface (Jih-Mirn Jehng and Israel E. Wachs, Catalysis
Today, 8, 1, 1990).
As a result, the catalyst could became inactive for the COS and/or CS2
hydrolysis even at high
temperature and in the presence of water (P. Grancher, C. Blanc, G. Guyot, M.
Mathieu, J.
Npugayrede, and J. Tessier, Inform. Chem., 199, 145, 1980).
Therefore, the concentration of COS and/or CS2 in the product gas effluent of
the above
patents is expected to be substantial, and a post-treatment process would be
required to convert
the produced COS to H2S, which in turn can be recycled to the H2S direct
oxidation reactor to
achieve a high sulfur removal efficiency. Such hydrolysis/hydrogenation pre-
and/or post-
treatment steps require additional capital and operating costs for the supply
of hydrogen, which
supply is lacking due to only providing, in accordance with the aforementioned
patents
(particularly U.S. patents 4,552,746 and 4,857,297) which stipulate that water
in the feed gas
stream comprise less than 10 vol % water.
A8124663W0\CALLAW\ 1773685\2
CA 02768359 2012-02-17
'
- 6 -
SUMMARY OF THE INVENTION
In order to overcome the above problems foreseen with the methods and
apparatus of
U.S. patents 4,552,746, 4,857,297 and CA 2,318,734, namely the failure to
eliminate
concentrations of COS and/or CS2 in the produced gas stream and the need for a
post-treatment
process to convert the COS to H2S, the possible fouling of the catalyst, and
the additional capital
and operating costs for the supply of hydrogen for any hydrogenation reactions
used in any pre-
treatment processes, in a broad aspect the present invention advantageously
provides a method
and apparatus for overcoming such problems economically, using only addition
of oxygen and
water (and thus no need to supply hydrogen gas) which method substantially
converts sulfur
species present in an incoming gas stream to elemental sulfur or alternatively
to SO2 which can
subsequently by means of a Claus reaction be easily then converted to
elemental sulfur.
As used herein, "sulphur species" refers to one or more sulphur-containing
compounds
which high quantities are undesirable in an effluent stream. Such compounds
include, but are not
limited to, hydrogen sulphide (H2S), sulphur dioxide (S02), carbonyl sulphide
(COS),
mercaptans (RSH), and carbon disulphide (CS2). As used herein, "sour gas"
refers to gas
containing more than 5.7 milligrams of H2S per cubic meter.
Specifically, the present invention, by utilizing water in the incoming stream
or supplying
additional quantities of water or steam to such incoming gas stream, is able
to utilize such water
or steam in the catalytic means provided to hydrolyze the water and
hydrogenate the sulfur
species present in the incoming gas stream to form H2S, and subsequently by
oxidation catalysts
oxidize such H2S and any pre-existing H2S in the incoming gas stream to
elemental sulfur or to
SO2 which can subsequently by means of a Claus reaction be easily then
converted to elemental
sulfur, thereby resulting in substantial elimination of such sulfur species
from the produced gas
stream. Typically, gas feed streams from sour gas wells contain sulfur species
in sufficient
quantities such that hydrogenation of such species to H2S requires that water
or steam (water
equivalent) be present in, or injected into such feed stream, such that the
total of such water or
steam (water equivalent) is in excess of 10% by volume of such feed stream. Of
course, the
amount of water or steam needed to be injected, if any, for the hydrolysis and
hydrogentation
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 7 -
reaction depends on the amount of water or steam originally present in the
feed stream, and the
amount of sulfur species needed to be hydrogenated.
Advantageously, such method avoids the need (and associated expense and
equipment) to
separately supply hydrogen gas for the hydrogenation of sulfur species in the
gas stream, as was
required in the prior art, including the pre-treatment method disclosed in
U.S. patents 4,552,746,
4,857,297.
Accordingly, in a broad aspect the present invention comprises a method of
reducing
the amount COS and/or CS2 in an incoming gas stream that also contains
combustible
hydrocarbons and H2S, comprising:
a.
injecting, if necessary, molecular oxygen to said incoming gas stream so that
the
stoichiometric ratio of molecular oxygen to H2S in said incoming gas stream is
at least
0.5 :1.0;
b.
injecting water or steam to said incoming gas stream, if necessary so that
the gas
stream contains water or steam greater than 10 vol % (water equivalent);
c.
providing a reaction zone comprising catalyst means comprising active sites
suitable for hydrogenation of said COS and/or CS2 to H2S via hydrolysis of
said injected
water or steam, and a reaction zone comprising oxidation catalyst means for
oxidation of
H2S to elemental sulfur or SO2, wherein the temperature of each reaction zone
is at or
above the elemental sulfur dew point at the reaction pressure; and
d. flowing said gas stream over said catalyst to produce an effluent
stream;
wherein the effluent stream comprises a reduced level of said COS and/or CS2
species
when compared to the incoming gas stream.
Advantageously, by providing water in excess of 10%vol. (water equivalent)
when such
water is mixed with the incoming gas stream, such water is thereby able to
provide sufficient
hydrogen to allow the catalyst means to effectively hydrogenate of the
quantities of sulfur
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 8 -
species typically present in hydrocarbon gas streams to produce H2S through
one or more of the
following reactions:
CS2 + 4H2 4 2H2S + Cl-I4 Carbon disufide hydrogenation
COS + 4H2 4 H2S +CH4 + H20 Carbonyl sulphide hydrogenation
Thereafter, the H2S may then, depending on the quantum of oxygen and water
supplied in the
process, be able to be directly oxidized to elemental sulfur through H2S
partial oxidation
(reaction 3a above-H2S + 0.502 4 S + H20), or via a complete oxidation to SO2
(via reaction 3b
above- H2S + 1.502 4 SO2 + H20).
The step in the above method of partial oxidiation of H2S to elemental sulfur
results in
elimination of all sulfur species from the incoming stream. Specifically,
wherein the incoming
gas stream contains additional sulfur species, and wherein said water or steam
is injected in
sufficient quantity, and a flow rate through the reaction zone, and the
quantity of said catalyst
means is sufficient, to permit hydrogenation of substantially all of said COS
and/or CS2 to H2S
via said catalyst means, and thereafter oxidize substantially all of said H2S
to elemental sulfur
upon injection of molecular oxygen if required, all such sulfur species can be
removed.
In some conditions, however, depending on the concentration of H2S and COS
and/or
CS2 in the incoming stream, it may be more desirable to fully oxidize in the
oxidation step the
H2S to SO2, which will allow a subsequent Claus reaction to thereafter convert
the so-formed
SO2 and remaining H2S (which advantageously in the present method can be
adjusted in the
process to the preferred stochiometric ratio of 2 H2S : 1 SO2 for the
subsequent Claus reaction) to
elemental sulfur and water, via the following reaction, namely:
2H2S + SO2 -4 2H20 + 3S
Such last step may be done via Claus, CrystaSulf, or other redox sulfur
removal units.
This may reduce or eliminate the need for an amine scrubber or H2S burner.
In one embodiment of the above method of the present invention, the reaction
zone for
the hydrogenation and the reaction zone for oxidation are one and the same
reaction zone.
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
'
- 9 -
In a further embodiment such method comprises first treating the incoming gas
stream
via a catalyst to hydrogenate COS and/or CS2 to H2S, and subsequently
subjecting the resulting
stream to a direct oxidation catalyst to oxidize H2S to elemental sulphur or
SO2.
In a further refinement of the above method, water is introduced in liquid
form, and gas
to liquid water ratio in the incoming gas stream is in the range of from 10 to
5000 NL of gas per
kg of water.
In a further refinement, water is introduced as steam, and gas to steam ratio
in the
incoming gas stream is in the range of from 10 to 5000 NL of gas per kg of
steam (water
equivalent).
In a further embodiment the water/oxygen levels in the incoming gas stream are
adjusted
such that effluent stream from the reaction zone comprising oxidation catalyst
means comprises
H2S and SO2 in a ratio of from about 1:1 to about 3:1, and more preferably in
the optimum
stochiometric ratio for a subsequent Claus reaction, namely a ratio of about
2:1 .
The catalysts employed comprise a metal oxide and/or sulphide deposited or
mixed with
one or more refractory metal oxides. The present process may employ any
catalysts suitable for
the hydrolysis of COS and/or CS2 to H2S, and for the oxidation of H2S to
elemental sulfur or
SO2. The oxidation catalyst may comprise an oxide and/or sulphide form of one
or more metals
deposited or mixed with one or more refractory metal oxides. The metal oxides
and/or sulphides
include, but are not limited to, oxides and/or sulphides of V, Cr, Mn, Fe, Co,
Ni, Cu, Nb, Mo, Tc,
Ru, Rh, Pd, Hf, Ta, W, Re, Os, Ir, Pt, Au, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb,
Dy, Ho, Er, Tm,
Yb, Lu, Bi, and any suitable combinations thereof. The refractory metal oxides
include, but are
not limited to, Al, Ti, Si, Zr, and any suitable combinations thereof. The
first catalyst may
include any oxidation catalyst suitable for the selective oxidation of H2S to
elemental sulfur.
Such oxidation catalysts are known in the art. For example, they may comprise
an oxide and/or
sulphide form of one or more metals. The second catalyst may be a hydrolysis
catalyst suitable
for the hydrolysis of COS and/or CS2 to H25. The second catalyst may be of a
higher basicity
than the first catalyst and may comprise one or more metal oxides. Examples of
such catalysts
include, but are not limited to, A1203, Ti02, Cu/A1203, Co&Mo/A1203, and
suitable combinations
A8124663W0\CAL _LAW\ 1773685\2
CA 02768359 2013-07-24
thereof. The first catalyst and second catalyst may be utilized in the
presence of water or water
vapour under the same reaction conditions.
As noted above, the hydrogenation and oxidization reactions are carried out
above the
dew point of elemental sulfur, to avoid fouling the catalysts, to allow the
sulfur to be carried
5 away. At atmospheric pressure, the dew point of sulfur is approximately
150 C. Thus in
embodiments where the pressures are atmospheric or higher, the e temperature
of the reaction
zone is in the range of from about 150 C to about 400 C, such temperatures, of
course, being
above the dew point temperature of sulfur for the pressure being used, which
results in a pressure
in the reaction zone is in the range of from about atmospheric pressure to
about 500 psig.
10 In a preferred embodiment, the incoming gas stream is flowed over the
oxidation catalyst
at a gas hourly space velocity of 100 to 10,000 hr-1, which allows sufficient
time for the
hydrogenation and oxidative reactions to occur.
Because the hydrogenation Reactions 4-7 which take place in the method of the
present
invention form quantities of WS, and considering additional quantities of H2S
quantities may
be originally present in the feed stock, typically greater quantities of
oxidation catalyst than
hydrogenation/hydrolysis catalyst will be needed in the method of the present
invention. In
preferred embodiments, the ratio of the oxidation catalyst to the hydrolysis
catalyst is in the
range of from about 0.5 to about 10 to 1, depending on the quantity of H2S
originally present in
the feed stock, the amount of sulfur species, and which of CS2 and/or COS and
their respective
quantities are originally present in the feed stock.
The reaction zone may comprise a first catalytic zone for hydrogenation and a
second
catalytic zone comprises an H2S oxidation catalyst. The reaction zone may be a
vertical reaction
zone, having a top catalytic zone and a bottom catalytic zone, the top zone
comprising the
oxidation catalyst and the bottom zone comprising the hydrolysis catalyst, and
the feed stream
enter firstly the bottom, and exit via the top. Alternatively for a vertical
reaction zone, the top
zone may comprise the hydrogenation catalyst and the bottom zone comprising
the oxidation
catalyst, and the feed stream enter firstly the top, and exit via the bottom.
44446644Vg4CALJAVV\1773685\43.
CA 02768359 2012-02-17
- 11 -
Alternatively, the reaction zone may comprises a catalytic zone(s) comprising
a mixture
of a first catalyst and a second catalyst.
In the preferred embodiment, the reaction conditions within the first
catalytic zone where
hydrogenation occurs, and the second catalytic zone occurs are each carried
out at a temperature
in the range of from ambient to 400 C, a pressure in the range of from
atmospheric to 500 psig,
and at a gas hourly space velocity in the range from 100 to 10,000 hr-1.
Alternatively, only one
of the first catalytic zone or second catalytic zones may be carried out under
these conditions.
In a preferred embodiment of the above method, effluent from the first
catalytic zone is cooled to
below the dew point of sulfur, and preferably cooled to room temperature, to
allow separation of
sulfur therefrom before said effluent is subsequently supplied to the second
catalytic zone
comprising the H2S oxidation catalyst.
In a further preferred embodiment, the stream produced from the first
catalytic zone
where hydrogenation occurs is mixed with molecular oxygen in the form of pure
oxygen (or less
preferably air which undesirably contains small amounts of CO2) before it is
supplied to the
second catalytic zone where direct oxidation of H2S occurs.
It should be emphasized that the reaction zone may be in a single reactor,
with oxygen
and water being injected into the reaction zone where hydrogenation and
oxidation occur.
Alternatively, the reactions of hydrogenation and oxidation may take place in
separate
catalytic beds or even separate reactors. Accordingly, in such alternative
embodiment, the
method of the present invention comprises a method of reducing the amount COS
and/or CS2 in
an incoming gas stream that also contains combustible hydrocarbons and H2S,
and COS and/or
CS2, the method comprising:
a. injecting water or steam to said incoming gas stream, if necessary, so
that the gas
stream contains sufficient water or steam to allow hydrolysis of said water or
steam and
hydrogenation of said amounts of COS and/or CS2 to H2S;
b. providing catalyst means comprising active sites suitable for said
hydrolysis of
water and hydrogenation of said COS and/or CS2 to H2S, and passing said
incoming
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 12 -
stream through said catalyst means, at a temperature at or above the elemental
sulfur dew
point at the reaction pressure, thereby forming a treated stream;
c.
injecting, if necessary, molecular oxygen to said treated stream so that
the
stoichiometric ratio of molecular oxygen to H2S in said treated stream is at
least 0.5:1.0;
d.
providing a reaction zone having oxidation catalyst means comprising active
sites
suitable for oxidation of H2S to elemental sulfur or SO2, wherein the
temperature of the
reaction zone is at or above the elemental sulfur dew point at the reaction
pressure; and
e.
passing said treated stream through said reaction zone to form an effluent
stream;
and
wherein said effluent stream comprises a reduced level of said COS and/or CS2
species when
compared to the incoming gas stream.
In most embodiments, due to the concentration of sulfur species in the feed
stream, water
or steam is present in said incoming stream provided to said catalyst means at
a volume in excess
of 10% vol. (water equivalent).
In the above method, the oxygen is preferably added to said treated stream at
a
stoichiometric ratio of molecular oxygen to H2S equal to or greater than 1.5:
1Ø
Again, the injection rates of said water and/or oxygen are adjusted such that
the effluent
stream from the reaction zone comprises H2S/S02 in a ratio of about 2:1 .
Similarly, where the oxygen and water injection rates are adjusted so that the
oxidation
catalytic reaction is a full oxidation of H2S to SO2, in a preferred
embodiment the so-formed
SO2 and remaining unoxidized H2S are further reacted in the presence of a
Claus catalyst to
form elemental sulfur.
In another aspect of the invention, the invention comprises an apparatus for
carrying out the
above method. More particularly, in another aspect of the invention, an
apparatus for the
removal of COS and/or CS2 from a hydrocarbon-containing feed stream comprising
H2S, and
COS and/or CS2, is provided, comprising:
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 13 -
(i) reactor means, comprising a hydrogenation catalyst for catalyzing the
hydrogenation of said COS and/or CS2 to H2S, and an oxidation catalyst capable
of
catalyzing the oxidation of the H2S to elemental sulfur or SO2;
(ii) an incoming feed stream inlet, in fluid communication with said reactor
means, adapted for conveying said feed stream to the reactor means;
(iii) a water inlet, adapted to allow water to be introduced into said feed
stream
entering said reactor means;
(iv) an outlet from said reactor, adapted for conveying an effluent gas stream
away from the reactor.
In a preferred embodiment of the apparatus of the present invention, such
apparatus
comprises molecular oxygen injection means, for injecting said molecular
oxygen in the form of
oxygen gas, or less preferably air, into said reactor prior to said incoming
gas stream flowing to
said oxidizing catalyst.
To be able to adjust the rate of oxygen injected relative to the rate of feed
gas entering, or
the rate of water being injected relative to the rate of feed gas entering the
reaction zone(s), mass
flow controllers may be provided, capable of controlling the flow of any one
of (i) the rate of
water injection; (2) the rate of oxygen injection; and/or (3) the feed stream
flow rate.
In a preferred embodiment, the apparatus further comprises a heat exchanger
capable of
heating the incoming gas stream.
In a still further embodiment, the apparatus further comprises a gas
chromatograph for
analyzing the effluent gas stream.
In a yet still further embodiment, the oxidation catalyst comprises metal
compounds in
the oxide and/or sulfide form.
This summary does not necessarily describe the entire scope of the present
invention.
Other aspects, features and advantages of the invention will be apparent to
those of ordinary skill
in the art upon review of the following description of specific embodiments of
the invention.
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
,
- 14 -
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic of an apparatus for direct oxidation of H2S.
Figure 2a is a schematic of an apparatus utilizing a single reactor apparatus
for direct oxidation
of H2S comprising a pump suitable for adding water.
Figure 2b is a schematic of an apparatus utilizing a dual reactor apparatus
for direct oxidation of
H2S comprising pumps suitable for adding water.
Figure 3 is a graph showing the water injection rate to percent reduction in
COS for a process
according to an embodiment of the present disclosure.
Figure 4 is a graph showing water addition rate to the amount of COS in a
produced gas for a
process according to an embodiment of the present disclosure.
DETAILED DESCRIPTION OF THE INVENTION
The present disclosure provides embodiments of a process for reducing the
amount of
sulfur species in a gas stream, which does not entail addition of hydrogen
gas, but rather makes
use of water (or steam) in the feed stream which through catalysts is
hydrolyzed and such
catalyst further acts to hydrogenate various sulfur species in the feed stream
to H2S. Oxygen, and
oxidizing catalysts are subsequently used to produce elemental sulfur which
can be removed, or
an exhaust gas containing an H2S/S02 ratio of about 2:1, which is can be fed
to Claus,
CrystaSulf, or other redox sulfur removal units.
H2S-containing streams suitable for the present processes include, but are not
limited to,
light hydrocarbons such as methane, ethane, or propane; natural gas;
associated gas from oil
production; gases produced from oil sand refining, e.g. coker gas; gases
produced from the Toe-
to-Heel-Air-Injection process (THAITm), or other in-situ combustion processes;
coal or oil
gasification processes; inert gases such as nitrogen, helium or carbon
dioxide, and oxidizable
gases such as, carbon monoxide, hydrogen and any combinations thereof.
In certain processes according to the present disclosure a suitable amount of
air or a
molecular oxygen-containing gas is introduced into an H2S-containing gas
stream along with
A8124663W0\CALLAW\ 1773685\2
CA 02768359 2012-02-17
- 15 -
water in the vapour or liquid state, and the resulting mixture directed to an
H2S direct oxidation
reactor. The water, or the water equivalent in the case of steam, need
typically be greater than 10
vol % of the total feed gas volume for typical incoming feed streams, in order
to provide the
necessary hydrogen for the hydrogenation of the sulfur species within the feed
stream to be
hydrogenated to H2S. The concentration of the oxygen in the molecular oxygen-
containing
stream may be adjusted depending on the concentration of the H2S in the H2S-
containing stream.
The oxygen-containing gas may be, but is not limited to, air, oxygen, or
mixtures thereof. In
embodiments of the present process the ratio of molecular 02 to H2S may be
slightly above the
stoichiometric ratio of 0.5 Preferably the ratio is in the range of from about
0.5 to about 2, such
as from about 0.6 to about 1. If desired, an excess molecular 02 can be
adjusted to produce SO2,
so that the exhaust effluent contains an H2S/S02 ratio suitable for feeding to
downstream redox
reactors. For example, the H2S/S02 ratio may be about 2:1.
The addition of water to the feed stream of an H2S direct oxidation reactor
may have
various functionality:
a. Water may hydrolyze COS and CS2 that can be present in the feed gas
stream
and/or formed during the direct oxidation of H2S to elemental sulfur and/or
SO2 inside the
reactor (Reactions 13 and 14):
hydrolysis reactions
13. COS + H20 4 H2S + CO2 COS hydrolysis (reverse of Reaction 10)
14. CS2 + 2H20 2H2S + CO2 CS2 hydrolysis
b. Water in the presence of H2S in the gas stream may reduce
sulphation of the H2S
oxidation catalyst surface, in particular alumina and titania based catalysts
[R.K. Kerr,
H.G. Paskall and N. Ballash, Energy Process, 40 (1977), and Z. M. George, Can.
J.
Chem. Eng., 56, 711 (1977)]. The sulphation of alumina and titania based
catalysts can
increase the strength of the Lewis acid sites on the catalysts surface and
consequently
reduce their activities toward COS and/or CS2 hydrolysis and reduce the
efficiency of
H2S direct oxidation to elemental sulphur;
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
,
- 16 -
c. Water can reduce the condensation of the produced sulfur such as in the
catalyst
pores which may improve the durability of the catalyst; and
d. Water may reduces the sulfur contact time on the catalytic bed and
consequently
eliminate certain side reactions, such as Reactions 8 and 15:
15. S + 02 4 SO2
In embodiments of the present process, the I-12S direct oxidation (Reactions
3a and 3b)
and COS and CS2 hydrolysis reactions (Reactions 13 and 14) take place in the
presence of water
and at a temperature higher than the sulfur dew point under the reaction
conditions.
The present disclosure provides an apparatus for implementing a process as
described
herein.
The apparatus comprises a gas-stream inlet, a reactor, and an outlet. The
inlet is suitable
for conveying a gas stream comprising a sulfur species to the reactor. The
reactor is suitable for
the oxidation of a sulfur species to elemental sulfur and contains an
oxidation catalyst capable of
catalyzing the oxidation of the sulfur species to elemental sulfur. The oxygen
and water may be
introduced to the reactor via the inlet or may be introduced though
alternative means. The outlet
is adapted for conveying an effluent gas stream wherein the effluent gas
stream comprising less
of the sulfur species than the inlet gas stream.
In an embodiment of the present process, an H2S-containing gas stream and air
or
molecular oxygen are supplied to a reaction zone comprising an oxidation
catalyst in the
presence of water. The oxidation catalyst comprises active sites suitable for
H2S oxidation to
elemental sulfur and for COS and/or CS2 hydrolysis to H2S. The H25 oxidation
reaction and/or
hydrolysis of COS and/or CS2 can be conducted in a down flow reactor, a slurry
bubble column,
or any other suitable reactor operates at a temperature in the range of from
ambient to about
400 C, a pressure of up to about 1500 psig, and a gas hourly space velocity in
the range of from
about 100 to about 10,000 hr-I.
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 17 -
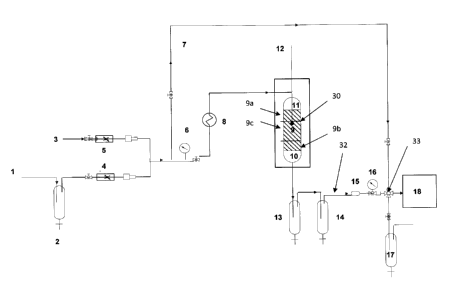
This embodiment may be implemented in an apparatus according to Figure 1,
which
shows a schematic of a pilot process and an embodiment of the method of the
present invention.
The direct oxidation may conducted in a down-flow reactor 30. The reactor may
comprise a
single reaction zone 9 containing an appropriate amount oxidation catalyst
such as 10.4 litres of
an alumina supported bismuth and copper catalyst. The catalyst may be enclosed
between two
inert-particle zones 10 and 11 respectively. The apparatus has an inlet feed
stream 1. Water may
be added to the stream by any appropriate means (not shown). In this apparatus
the stream 1
passes through a liquid collector 2 to separate liquid hydrocarbon droplets
from the feed gas. The
flow of the feed stream 1 may be controlled by a mass flow controller 4. The
temperature and
pressure of the stream may also be controlled as appropriate. For example,
when used in an
exemplary process for the direct oxidation of H2S the temperature of the
stream was at ambient
and the pressure at 18 psig. Air 3 may be mixed with the feed stream 1 prior
to the heat
exchanger 8, and its flow may be controlled via a mass flow controller 5. The
feed stream 1 may
enter a heat exchanger 8 to bring the feed stream temperature to the desired
reaction temperature.
The pressure of the inlet feed stream 1 may be monitored by a pressure gauge
6, and the
combined inlet and air feed stream bmay be analyzed for the H2S/02 ratio. The
temperature of
the oxidation reactor 30 can be sensed and recorded by a thermocouple 12. The
produced fluid
may be cooled to separate the produced sulfur from the gas phase in separators
13 and 14. A
micro-filter 15 can be mounted on the outlet effluent line 32 to prevent the
deposition of sulfur in
the back pressure control valve 16. The composition of the produced gas may be
analyzed
periodically (e.g. at 30 minute intervals). This may be done by a gas
chromatograph 18 which
can be equipped with an automated stream selection valve 33. The produced
stream may be
passed through a sodium hydroxide solution 17 to absorb un-reacted H2S.
Water supplied to the reaction zone 9 may serve as an internal coolant and can
be in the
vapour or liquid form, preferably in vapour form. Water absorbs heat released
by exothermic
oxidation reaction and may maintain the reaction temperature fairly constant
and suitable for
COS and/or CS2 hydrolysis. If water is supplied in vapour form, the water
vapour content in the
feed stream is preferably adjusted to produce a gas mixture containing water
vapour greater than
10 vol % of the total volume of the feed stream. If the water is present in
liquid form, the volume
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
,
- 18 -
of the feed gas to the weight of the supplied water is preferably in the range
of from about 10 to
about 5000 NL of gas/kg of water. The H2S-containing stream and water can be
supplied to the
reaction zone 9 separately or co-mixed.
The amount of molecular oxygen entering via line 3 may be adjusted to achieve
the
highest conversion of H2S to elemental sulfur and to produce a gas effluent
comprising a suitable
H2S/S02 ratio such as about 2:1. An effluent comprising gas, water and sulfur
can be removed
from the reaction zone and then cooled to room temperature. Liquid water
entraining fine
dispersed sulfur particles may then separated from a gaseous stream. A gaseous
stream
comprising H2S/S02, preferably in a ratio of about 2:1, can be supplied to a
Claus or other redox
type process for further oxidation of H2S to elemental sulfur if desired.
Produced solid sulfur
may be separated from the liquid water via, for example, a phase separation.
Alternatively, the
process may be operated to minimize the total sulfur compounds in the
effluent, which can then
be burned.
In a variation of the present process, the reaction zone 9 can comprise a
mixture of a first
catalyst and a second catalyst. The first catalyst may include any oxidation
catalyst suitable for
the selective oxidation of H2S to elemental sulfur. Such oxidation catalysts
are known in the art.
For example, they may comprise an oxide and/or sulphide form of one or more
metals. The
second catalyst may be a hydrolysis catalyst suitable for the
hydrolysis/hydrogenation of COS
and/or CS2 to H2S. The second catalyst may be of a higher basicity than the
first catalyst and
may comprise one or more metal oxides. Examples of such catalysts include, but
are not limited
to, A1203, Ti02, Cu/A1203, Co&Mo/A1203, and suitable combinations thereof. The
first catalyst
and second catalyst may be utilized in the presence of water or water vapour
under the same
reaction conditions.
In a variation of the present process, the reaction zone 9 can comprise a top
catalytic zone
9a and a bottom catalytic zone 9b in series. The use of two catalytic zones
9a, 9b in series can
be useful in the case of a feed stream containing high concentrations of COS
and/or CS2, such as
streams from combustion processes. The top catalytic zone 9a may comprise a
catalyst that has
activity for COS and/or CS2 hydrolysis. It may be possible to transfer the
effluent from the top
A8124663W0\CALLAW\ 1773685\2
CA 02768359 2012-02-17
- 19 -
catalytic zone 9a to the bottom catalytic zone 9b without any interstage
cooling. The bottom
catalytic zone 9b may comprise an oxidation catalyst suitable for the
oxidation of H2S to
elemental sulfur. The bottom zone 9b may comprise further catalysts such as
those suitable for
the hydrolysis of COS and/or CS2 to H2S.
In a variation of the present process, the reaction zone 9 can comprise a top
catalytic zone
9a and a bottom catalytic zone 9b in series, wherein the top catalytic zone 9a
comprises an
oxidation catalyst suitable for H2S oxidation to elemental sulfur and SO2 and
the bottom catalytic
zone 9b comprises a hydrolysis catalyst suitable for the hydrolysis of COS
and/or CS2 to H2S. In
a variation of the present process, the reaction zone 9 can comprise a top
catalytic zone 9a, a
middle catalytic zone 9c, and a bottom catalytic zone 9b. The use of multi-
catalytic zones can be
useful in the case of a feed stream containing high concentrations of H2S, COS
and/or CS2. The
top catalytic zone 9a can comprise a COS and/or CS2 hydrolysis catalyst to
hydrolyze COS
and/or CS2 to H2S. The middle catalytic zone 9c may comprise an H2S oxidation
catalyst to
oxidize H2S to elemental sulfur and SO2. The bottom catalytic zone 9b may
exclusively
comprise a COS and/or CS2 hydrolysis catalyst to hydrolyze the formed COS
and/or CS2 to H2S.
The effluent from the top catalytic zone 9a may be sent to the middle
catalytic zone 9c without
interstage cooling. The effluent from the middle catalytic zone 9c may be
transferred to the
bottom zone 9b without interstage cooling.
While the catalytic zones 9a, 9b, 9c may be contained in a single reactor, in
a variation of
the present process the H2S partial oxidation and COS and/or CS2 hydrolysis
reactions are
carried out in two or more separate reactors containing mono or multi
catalytic zones.
In a variation of the present invention shown in Figure 2b, such invention
relates to a
process for recovering sulfur from an H2S containing stream is conducted in a
first reactor and a
second reactor. The process may include contacting an H2S containing stream,
molecular
oxygen, and water with one or more catalysts contained in a first reactor
comprising mono or
multi catalytic zones. The molecular oxygen concentration in the feed stream
may be adjusted in
order to produce the desired (e.g. minimal) amount of SO2 in the produced
effluent from the first
reactor. Gas effluent may be removed from the first reactor and can be cooled
to condense water
A8124663W0\CALLAW\ 1773685\2
CA 02768359 2012-02-17
,
- 20 -
and sulfur. Liquid water comprising fine sulfur particles may be separated
from a gaseous stream
comprising un-reacted H2S, COS and/or CS2 and trace SO2. A second feed stream
comprising a
gas mixture of the first reactor product gas mixture, molecular oxygen, and
water may be
contacted with one or more catalysts contained in a second reactor comprising
mono or multi
catalytic zones. Gas effluent can be removed from the second reactor and may
be cooled to
separate water and sulfur. The molecular oxygen concentration in the second
feed stream may be
adjusted to achieve the desired (e.g. highest) conversion of H2S to elemental
sulfur. It can be
desirable to produce a gas effluent from the second reactor comprising an
H2S/S02 ratio of about
2:1. The catalysts contained in the first and second reactors may have
activity for catalyzing the
H2S oxidation to elemental sulfur and the hydrolysis of COS and/or CS2 to H2S.
The H2S partial oxidation and COS/CS2 hydrolysis reactions may be conducted in
one or
more reactors comprising mono or multi catalytic zones at temperatures above
the dew point of
the sulfur, a pressure up to about 1000 psig, a gas hourly space velocity in
the range of from
about 100-1000 hr-1, and in the presence of water in the form of liquid or
vapour.
The present processes can be utilized on any suitable H25-containing stream
without the
necessity of any pre or post hydrogenation and/or hydrolysis of COS and/or
CS2. The produced
effluent contains low COS and/or CS2 content. Preferably the H2S/S02 ratio in
the effluent is
about 2:1 and is thus ready for redox-type processing for further
desulfurization of the H2S
containing streams.
Figure 2a shows a schematic of the laboratory equipment used in direct H2S
oxidation
Examples 3 to 4. The catalytic oxidation of H2S to elemental sulfur was
performed in a fixed bed
laboratory reactor 9 with and without the presence of water 1. The system
consisted of a gas
feeding section 2 (H2, N2, CH4, CO2 and CO mixture), 3 (H25/N2) and 4 (air),
and a downflow
reactor containing a single catalytic zone 9, an oven, 11, a gas pre-heating
zone 8, and two-stage
gas-liquid separators (12 and 13). The downflow reactor 9 was a thick-walled
316 stainless steel
tube of 25 mm internal diameter and 255 mm length. The upper and bottom zones
of the
downflow reactor 9 were packed with inert particles and the H2S oxidation
catalyst was enclosed
between the zones of inert particles. The temperature of the oxidation reactor
9 was recorded by
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 21 -
a thermocouple 10. The reaction system included mass flow controllers 5 for
supplying the
various gases and an HPLC pump 1 for optionally adding water. The outlet
stream passed
through a micro-filter 14 and the pressure of the reactor 9 was controlled by
a back-pressure
control valve 15 mounted on the outlet gas stream. The produced fluid was then
cooled to
separate the produced sulfur from the gas phase in separators (12 and 13). The
composition of
the inlet 7 and outlet gas streams were monitored by a gas chromatograph 17
equipped with 4
channels. The product gas was passed through a sodium hydroxide solution 16 to
absorb the un-
reacted H2S.
Figure 2b shows a schematic of the laboratory equipment used in the direct
oxidation
reaction according to Example 6. The H2S catalytic oxidation was performed in
a fixed bed
reactor 9 in the presence of water 1. The experimental set-up of the first
reactor is similar to that
employed in Example 3 and 4. However, the outlet fluids from reactor 9 entered
a first sulfur
separator 12 to remove sulfur from the effluent tail gas. The outlet gas from
the sulfur separator
12 was mixed with water 13 and air 14 and then supplied to a second pre-
heating zone 15. The
outlet stream from the pre-heating zone 15 was then fed to a second oxidation
reactor 16 and
then to a second sulfur separator 17. The temperature of the second oxidation
reactor was
recorded by a thermocouple 22. The outlet stream from the second sulfur
separator 17 passed
through a micro-filter 18 and then through a back-pressure control valve 19.
The composition of
the outlet stream was analyzed every 30 minutes by a gas chromatograph 21 and
the product gas
was passed through a sodium hydroxide solution 20 to absorb un-reacted H2S.
Figure 3 shows the effect of pressure and water concentration on the quantity
of COS
produced with the laboratory equipment of Figure 2. Compared with the absence
of injected
water, where A represents the percentage reduction in COS produced at 100 psig
for water, B at
80 psig, and C at 60 psig for additions of 0.1, 0.2, 0.3 and 0.4 ml/minute.
Figure 4 shows the reduction of effluent COS by water addition. The number 1
indicates
the ppm COS concentration in the effluent and the number 2 the ml/min of water
added.
A8124663W0\CALLAW\ 1773685\2
CA 02768359 2012-02-17
- 22 -
It is contemplated that any embodiment, aspect, example, method, composition,
or
element discussed in this specification may be implemented or combined in any
suitable manner
with any other embodiment, aspect, example, method, composition, or element.
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as is commonly understood by one of ordinary skill in the art to which
this invention
belongs. Unless otherwise specified, all patents, applications, published
applications and other
publications referred to herein are incorporated by reference in their
entirety. If a definition set
forth in this section is contrary to or otherwise inconsistent with a
definition set forth in the
patents, applications, published applications and other publications that are
herein incorporated
by reference, the definition set forth in this section prevails over the
definition that is
incorporated herein by reference. Citation of references herein is not to be
construed nor
considered as an admission that such references are prior art to the present
invention.
The invention includes all embodiments, modifications and variations
substantially as
hereinbefore described and with reference to the examples and figures. It will
be apparent to
persons skilled in the art that a number of variations and modifications can
be made without
departing from the scope of the invention as defined in the claims. Examples
of such
modifications include the substitution of known equivalents for any aspect of
the invention in
order to achieve the same result in substantially the same way.
Embodiments of the invention are illustrated, in part, by the following non-
limiting
methods and examples
Example 1 (comparative)
This Example used the apparatus shown in Figure 1. No water was added to the
gas
stream and the gas produced had the composition given in Table 1.
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
'
,
- 23 -
Table 1. THAITm Process gas composition (in situ petroleum combustion process)
Component % by volume
I-12 3.60
02 0.00
N2 74.41
CH4 4.31
CO 1.50
CO2 14.94
Ethane 0.55
H2S 0.40
Propane 0.28
In order to operate the downflow reactor 9 isothermally, the temperature of
the outer surface of
the reactor 9 was controlled with electrical heating elements and the reaction
temperature was
maintained at ¨230 C. The gas hourly space velocity of the feed gas was about
1500 hr-1 and the
02/H2S ratio was 0.5 and varied based on the H2S concentration in the feed
gas. The average
pressure of the inlet gas was 18 psig, while the average pressure of the
outlet gas was 4 psig. The
compositions of the feed and produced gases were monitored via a micro gas
chromatograph
equipped with 4 channels . As shown in Figure 4, after 2 weeks on stream, the
average H2S
conversion was 60% of the total inlet H2S and sulfur selectivity was
approximately 45%. The
activity of the catalyst toward H2S oxidation to elemental sulfur decreased
rapidly due to the
carbon and sulfur deposits. The amounts of carbon and sulfur deposited on the
catalyst surface
were 4.5% and 8.3%, respectively
Example 2
The fouled catalyst from Example 1 was used in this Example. Approximately 20
ml of
the fouled catalyst collected from Example 1 were used in this Example without
regeneration or
any other pre-treatment. The fouled catalyst was loaded into a downflow
laboratory reactor 9
(Figure 2a) and then placed in an oven. Two different gas streams shown below
were used to
prepare a synthetic direct oxidation raw inlet feed gas of the composition
shown in Table 2,
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 24 -
which is similar to that used in the THAITm Process direct oxidation field
pilot of Figure 1 and
Example 1:
(H2S/N2); CO2, CO, 112 and N2). Air was added to the synthetic
mixture.
Table 2. Synthetic feed gas composition.
Component % by volume
H2 3.03
02 0.00
N2 77.86
CH4 4.31
CO 1.07
CO2 13.24
H2S 0.49
The temperature of the catalyst bed was increased slowly to 220 C at a
constant flow of
the dry gas mixture and at atmospheric pressure. At a temperature of 220 C,
the pressure of the
reactor 9 was increased to the reaction pressure of 100 psig and the gas
hourly space velocity of
the inlet feed stream was set at 1000 hr-1. The out-flow lines were maintained
at 150 C to avoid
the sulfur plugging in the lines. The produced fluids entered a hot separator
where sulfur was
collected. A secondary cold separator was employed to separate the sulfur from
the gas phase,
and a 21A, stainless steel filter was used to capture the trace of the sulfur
before the back pressure
control valve. Gaseous and liquid effluents were continuously removed from the
reactor 9. After
96 hours of operation the H2S conversion and sulfur selectivity without water
addition were
calculated and are shown in Table 3 as Example 2a. Water was then mixed with
the feed gas in
the pre-heating zone at a rate of 0.4 gm/min and the results are also shown in
Table 3 as Example
2b. Comparing Example 2a and Example 2b it can be seen that there are
advantages to adding
water in the direct oxidation of H2S to elemental sulfur with fouled catalyst.
The presence of
water, with all other conditions unchanged, increased sulfur yield from 45 %
to 62.6 % and
reduced problematic COS from 1600 ppm to 245 ppm.
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 25 -
The addition of water to the H2S direct oxidation reactor comprising a fouled
alumina
supported bismuth and copper catalyst hydrolyzed a good deal of the formed COS
to H2S and
decreased its concentration in the produced effluent substantially. The
molecular oxygen
supplied to the oxidation reaction was consequently adjusted to achieve a
higher conversion of
HS to elemental sulfur and to produce a gas effluent comprising H2S/S02 in a
ratio of about 2:1.
While not wishing to be bound by theory it is believed that the oxygen reacted
selectively with
the H2S and, therefore, increased the sulfur selectivity from 52.3 % to 78.5%
upon adding water
to the oxidation reaction.
The above H2S direct oxidation experiments revealed that upon using a dry H2S-
containing stream as a feed gas, CO side reactions were present and produced
undesirable high
level of COS.
To test the effect of water level, water rates were set at 0.3, 0.2 and 0.1
ml/min.
A8124663W0\CALLAW\ 1773685\2
CA 02768359 2012-02-17
- 26 -
Table 3. Wet and dry Direct Oxidation of H2S.
Single reactor, Single reactor, Two
reactors,
Reaction parameters
example 2a* Example 2b Example 5
Reactor A: Fouled
alumina supported
bismuth
and
Fouled alumina Fouled alumina
copper
Catalyst supported bismuth supported bismuth
and copper and copper Reactor B: Fresh
alumina supported
bismuth
and
copper
Reaction temperature, C 220 220 220
Reaction pressure, psig 100 100 100
Water rate, ml/min dry 0.4 0.4
Feed GHSV,11-1 1000 1000 1000 (Reactor A)
Total 02/H25 0.76 0.88 0.98
Total H2S conversion, % 86 79.7 90.4
Average SO2 produced, ppm 440 520 287
Average COS produced, ppm 1600 245 316
Sulfur yield, % 45 62.6 78.1
H2S/S02 ratio 2.0 2.0 2.0
*Dry Direct Oxidation of H2S:
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
,
- 27 -
Example 3
The reaction conditions used in Example 3 were similar to the reaction
conditions employed in
Example 2b, except that the pressure of the reaction was reduced to a pressure
of 80 psig.
Example 4
The reaction conditions used in Example 4 were similar to the reaction
conditions employed in
Example 2, except that the pressure of the reaction was reduced to a pressure
of 60 psig.
As shown in Table 4, it was found that higher operating pressures can be
beneficial in terms of
higher sulfur yields.
Table 4. The effect of pressure on the Direct Oxidation of H2S to elemental
sulfur
Reaction parameters Example 2b Example 3 Example 4
Catalyst Fouled alumina supported bismuth and copper
Reaction temp., C 220
Feed GHSV, fil 1000
Water rate, ml/min 0.4
Reaction pressure, psig 100 80 60
Total 02/H2S 0.88 0.94 1.1
Total H2S conversion, % 79.7 72.9 70.8
Average SO2 prod., ppm 520 565 765
Average COS prod., ppm 245 231 275
Sulfur yield, % 62.6 56 49.2
1-12S/S02 ratio 2.0 2.0 2.0
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 28 -
Considering Figure 4, at 100 psig, when there was no water addition, produced
COS was
at a maximum of 1910 ppm. When water was added at 0.1 ml/min, the COS rapidly
decreased to
480 ppm and then decreased further to a minimum of 230 ppm when water was
increased to 0.4
ml/min. When the pressure was decreased to 80 psig with 0.1 ml/min water
addition, the
concentration of produced COS increased, compared with the 100 psig result,
indicating that
COS removal is moderately benefited by higher pressures. Again at 80 psig, the
COS
concentration was reduced by increasing the level of added water.
Effect of water concentration
While not wishing to be bound by theory it is believed that the reactions of
CO with the
H2S present in the feed stream, and with the sulfur produced from the
oxidation reaction,
substantially reduced the sulfur yield. Therefore, an excess amount of water
was required to
simultaneously hydrolyze the produced COS to H2S according to Reaction 12.
From Figure 3,
the reduction of COS increases with higher water concentrations. As shown in
Reaction 1, the
stoichiometric conversion of pure H2S to elemental sulfur requires only 0.5
moles of oxygen.
However, in the presence of oxidizable carbon molecules such as carbon
monoxide and methane,
higher quantities of oxygen are needed. With a dry feed gas, excess oxygen
produces excess
quantities of SO2 (Reaction 2) and of COS (Reaction 9). Because water co-
injected with the feed
gas hydrolyzes COS (reverse of Reaction 12), the molecular oxygen level
supplied to the H2S
direct oxidation reactor can safely be increased above the stoichiometric
quantity, thereby
achieving a higher sulfur yield while still controlling COS formation.
Example 5
As noted from Example 2, mixing water at a rate of 0.4 ml/min with the H2S
containing
stream in the pre-heating zone prior to the oxidation reactor decreased the
COS level from 1600
ppm (using a dry feed stream) to 245 ppm. Using an 02/H2S ratio of 0.88, the
total H2S
conversion was 79.7%, sulfur yield was 62.6%, unconverted H2S was 1034 ppm,
and SO2
concentration was 520 ppm, giving the desired H2S/S02 ratio of about 2. In
order to further
improve the total H2S conversion, the 02/H2S ratio was increased from 0.88 to
1. However, the
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
- 29 -
SO2 concentration in the produced gas increased and accordingly, COS
concentration increased
via Reaction 9.
Example 5 demonstrates a variation on Example 2 by increasing the H2S
conversion and
sulfur yield through the use of higher 02/H2S ratios without increasing the
COS production. Two
identical fixed bed reactors (Reactor A and Reactor B) were employed for
oxidizing H2S to
elemental sulfur and hydrolyzing formed COS to 112S, simultaneously. Reactor A
and reactor B
are identical to the reactor employed in Example 2. Both of Reactor A and
reactor B were placed
in an oven at a temperature of 220 C. Reactor A was loaded with 20 ml of the
fouled alumina
supported bismuth and copper catalyst, whereas, Reactor B was loaded with 20
ml of a fresh
alumina supported bismuth and copper catalyst. Reactor A was operated at
reaction conditions
similar to the reaction conditions employed in Example 2, except that the
molecular oxygen
concentration was adjusted to achieve a low conversion of H2S to COS and SO2.
Water at a rate
of 0.4 ml/min was mixed with molecular oxygen and an H2S containing gas stream
of a
composition similar to the composition of the feed stream employed in Example
2. The inlet
fluid mixture was pre-heated to the desired reaction temperature in the pre-
heating zone prior to
Reactor A and the total gas hourly space velocity available for Reactor A was
1000 hfl. The H2S
oxidation reaction was conducted at 220 C and a pressure of 100 psig. The
fluid produced from
Reactor A entered a cold separator to condense and separate water and sulfur
from the gaseous
phase before it was supplied to Reactor B. The composition of the gas effluent
produced from
Reactor A was analyzed and the total H2S conversion was 60.8%. The low H2S
conversion may
be attributed to the low oxygen level in the feed gas. The sulfur selectivity
was 88.6%, COS
produced was 210 ppm and SO2 produced was 130 ppm.
The gas effluent produced from Reactor A was then mixed with molecular oxygen
containing stream (air) and water at a rate of 0.4 ml/min in the pre-heating
zone prior to Reactor
B. Molecular oxygen level was adjusted to achieve a high H2S conversion to
elemental sulfur.
The gas effluent produced from Reactor B was cooled to separate water and
sulfur from the
gaseous phase. Subsequently, the composition of the gas phase from Reactor B
was analyzed.
A8124663W0\CAL_LAW\ 1773685\2
CA 02768359 2012-02-17
,
- 30 -
Table 3 shows the results of the H2S direct oxidation to elemental sulfur and
COS
hydrolysis to H2S processes according to Example 2 and Example 5 utilizing a
single reactor and
two reactors. Utilizing two oxidation reactors in the presence of an excessive
amount of water
increased the sulfur yield from 62.6% (according to Example 2d) to 78.1
(according to Example
5), while still maintaining an H2S/S02 ratio of 2.
Use of examples in the specification, including examples of terms, is for
illustrative
purposes only and is not intended to limit the scope and meaning of the
embodiments of the
invention herein. Numeric ranges are inclusive of the numbers defining the
range. In the
specification, the word "comprising" is used as an open-ended term,
substantially equivalent to
the phrase "including, but not limited to," and the word "comprises" has a
corresponding
meaning.
A8124663W0\CAL_LAW\ 1773685\2