Note: Descriptions are shown in the official language in which they were submitted.
CA 02768862 2012-01-20
WO 2011/011588 PCT/US2010/042880
FUEL COMPOSITION DERIVED FROM BIODIESEL
1. Field of the Invention
[0001] The present invention relates to a fuel composition and a method of
producing
the fuel composition. In particular, the fuel composition can be useful in
cold temperature
environments and as aviation fuel.
2. Background of the Invention
[0002] Global climate change is causing a shift in the sources of energy from
fossil fuels
to more sustainable and renewable resources, such as biodiesel. For ground
transportation, there
is a significant development effort to use electricity from non-fossil primary
fuel to power cars,
trucks and rail systems. However, in cold climates, such as in temperate or
polar regions of the
world (including a significant portion of the United States, Canada, northern
Europe and
northern Asia), biodiesel fuels tend to solidify rendering inoperable engines
that use it.
[0003] Furthermore, for aircraft, the energy densities available from
batteries, fuel cells
and other portable sources are not sufficient. Aviation fuel, such as jet
fuel, is generally a
specialized type of petroleum-based fuel used to power an aircraft and is
generally of a higher
quality than fuel used for ground transportation. Aviation fuel is designed to
remain liquid at
cold temperatures as found in the upper atmosphere where aircraft fly.
Aviation fuels can
include hydrocarbons, such as paraffins; olefins; naphthenes and aromatics;
antioxidants; and
metal deactivators. Known aviation fuels include jet fuels, such as JP-5, JP
8, Jet A, Jet A-1, and
Jet B. Aviation requires a high energy dense liquid fuel to achieve the speeds
and distances
airplanes can deliver today. Jet fuel has the highest volumetric energy
density of liquid fuels,
such as ethanol, butanol, bio-kerosene, and biodiesel.
[0004] There is a need in the art to develop a fuel composition that does not
solidify in
cold temperature environments for use as ground transportation fuel, that
satisfies the specified
standard requirements for use as aviation fuel, and is based on sustainable
and renewable
resources, such as biodiesel.
-1-
CA 02768862 2012-01-20
WO 2011/011588 PCT/US2010/042880
SUMMARY OF THE INVENTION
[0005] In one aspect, the present invention provides a fuel composition
including oil
derived from a biological source and alcohol. The fuel composition is at least
substantially free
of oxygen and oxygen-containing compounds.
[0006] The oil may be selected from the group of biological sources including
vegetable
oil, crop seed oil, animal oil, animal fat, and combinations thereof.
[0007] The alcohol may be selected from the group consisting of methanol,
ethanol,
propanol, isopropanol, butanol, and mixtures thereof.
[0008] The fuel composition may be oxygen free. In another embodiment, the
fuel
composition may have a level of oxygen that is less than the level of oxygen
in biodiesel. In yet
another embodiment, the fuel composition may have a level of oxygen that is
less than 2000
parts per million oxygen based on the fuel composition.
[0009] The fuel composition includes molecules and each of the molecules may
have a
carbon chain length of from 12 to 14 carbon atoms.
[0010] The fuel composition may be used for aviation fuel. In another
embodiment, the
fuel composition may be used for ground transportation fuel in a cold climate.
[0011] The fuel composition may include an alkane.
[0012] The fuel composition may further include a catalyst. The catalyst may
be
selected from the group consisting of calcium hydroxide, potassium hydroxide
and mixtures
thereof.
[0013] In another aspect, the present invention provides a method for
preparing a fuel
composition. The method includes reacting oil derived from a biological source
and alcohol to
produce an alkyl ester-containing product; and removing at least a portion of
oxygen from the
alkyl ester-containing product to produce a fuel composition which is at least
substantially free
of oxygen and oxygen-containing compounds.
[0014] In an embodiment, the alkyl ester-containing product may include an
alkyl ester
selected from the group consisting of methyl ester, ethyl ester, propyl ester,
isopropyl ester, butyl
ester and mixtures thereof.
[0015] In another embodiment, the alkyl ester-containing product may include
biodiesel.
[0016] In the method, the reacting step may be conducted in accordance with a
transesterification reaction.
-2-
CA 02768862 2012-01-20
WO 2011/011588 PCT/US2010/042880
[0017] In the method, the removing step may be conducted in accordance with a
nucleophilic acyl reaction.
[0018] In an embodiment, the cloud point of the fuel composition may be lower
than the
cloud point of the alkyl ester-containing product.
[0019] In the method, the removing step may further include removing a portion
of
carbon atoms from the alkyl ester-containing product.
[0020] In the method, the fuel composition includes molecules and each of the
molecules may have a carbon chain length of from 12 to 14 carbon atoms.
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] The invention as set forth in the claims will become more apparent from
the
following detailed description of certain preferred practices thereof
illustrated, by way of
example only, and the accompanying drawings, wherein
[0022] Figure 1 is a schematic showing a reaction process for reducing an
ethyl ester to
ethanol in accordance with an embodiment of the present invention; and
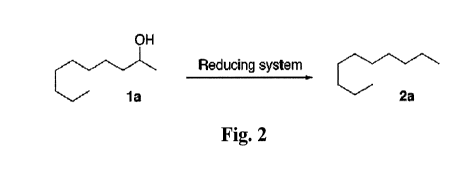
[0023] Figure 2 is a schematic showing a process for chemoselectively reducing
secondary and tertiary alcohols (e.g., 2-decanol) to alkanes (e.g., decane) in
accordance with an
embodiment of the present invention.
DETAILED DESCRIPTION OF THE INVENTION
[0024] The present invention relates to a fuel composition and method thereof.
The fuel
composition can be used in various applications. In particular, the fuel
composition can be
employed as a cold weather fuel for use in ground transportation vehicles,
such as trucks,
automobiles, railroads, and the like, and as an aviation fuel for use in
aircrafts, such as airplanes,
helicopters, and the like. Further, the fuel composition of the present
invention can be prepared
based on biodiesel. For example, biodiesel can be produced and converted to a
fuel composition
that is suitable for use as cold weather ground transportation and aviation
fuel. Biodiesel is
derived from plant oils, algae oils, and animal fats, and therefore, the
present invention provides
a fuel composition which is grown and produced using standard agricultural and
chemical
processing methods. The biodiesel can be converted to a fuel composition
including an alkane or
a mixture of alkanes.
-3-
CA 02768862 2012-01-20
WO 2011/011588 PCT/US2010/042880
[0025] Biodiesel has various characteristics and properties that make it
unattractive for
use in cold weather environments and as aviation fuel. For example, biodiesel
has an energy
density that is lower than required for aviation fuel. Further, at low
temperatures, certain
molecules within biodiesel begin to agglomerate into solid particles causing
the normally
translucent biodiesel to appear cloudy. The highest temperature at which the
biodiesel begins to
agglomerate or cloud is called the cloud point. The cloud point is an
important characteristic of
fuels used in internal combustion engines and jet engines because the presence
of solid or
agglomerated particles causes fuel pumps and injectors to clog rendering the
engines inoperable.
The cloud point for some common biodiesel products are as follows: 0 C for
canola; 1 C for
soybean; -6 C for safflower; 1 C for sunflower; -2 C for rapeseed; 13 C for
jatropha; and 15 C
for palm. The cloud points of various fossil fuels are as follows: 0 C for ULS
diesel; -40 C for
Jet A; -47 C for JP-8; and -40 C for ULS kerosene. Aviation fuels have very
low cloud points.
For aviation fuels, the low cloud point is important because the fuel must
remain liquid at high
altitude where temperatures are well below zero. For ground transportation
fuels, a low cloud
point is important because the fuel must remain liquid in cold weather
environments where
ground vehicles are used.
[0026] The cloud point of fuel is a function of its chemical composition. Most
fossil
fuels include numerous compounds in the form of linear or branched chains of
carbon atoms
with one or more oxygen and hydrogen atoms bound to each carbon atom. For
example, a
general composition of conventional aviation fuel is CmHn, where m is an
integer from 12-14,
and n is an integer from 20 to 30, and a general composition of conventional
biodiesel is
CjHkCO2CH3, where j is an integer from 14 to 16, and k is an integer from 26
to 33. Jet A and
synthetic aviation fuel are both composed of alkanes, which are compounds of
carbon and
hydrogen only. Biodiesel is a vegetable oil or animal fat-based diesel fuel
composed of long-
chain alkyl esters. The differences between aviation fuels and biodiesel
include (i) the size of the
molecules (biodiesel molecules are larger and include more carbon atoms per
molecule) and (ii)
the presence of oxygen (biodiesel contains oxygen, whereas aviation fuel is at
least substantially
oxygen free).
[0027] The presence of the oxygen in the biodiesel molecule causes it to have
a degree
of polarity (an electrostatic charge separation). This polarity results in an
attraction between
oxygen in one molecule to hydrogen atoms bound in an adjacent molecule through
van der
-4-
CA 02768862 2012-01-20
WO 2011/011588 PCT/US2010/042880
Waal's forces. This attractive force among biodiesel molecules causes
solidification at higher
temperatures than a similar molecule without oxygen. For example, Table 1
shows a
comparison of the solidification temperature for various alkanes, i.e., oxygen-
free molecules, and
corresponding alcohols, i.e., oxygen-containing molecules. As shown below, the
alcohols have a
single oxygen atom in addition to the corresponding alkane. The alcohol has a
higher
solidification point than the corresponding alkane. The presence of additional
oxygen atoms
would result in a greater difference in solidification points as compared to
the alkane.
Table 1
:llkane Composition Solidification (C) Alcohol Composition Solidification (C)
Methane CH4 -183 Methanol CH4O -97
Ethane C2H6 -183 Ethanol C2H60 -115
Propane C3H8 -190 Propanol C3H80 -127
Butane C4H1p -138 Butanol C4H10O -90
Hexane C6H14 -95 Hexanol C6H140 -47
Octane C8H18 -57 Octanol C8H18O -16
Dodecane C12H26 -10 Dodecanol C12H260 24
Eicosane C20H42 37 Eicosanol C20H420 66
[0028] Thus, it is contemplated by the present invention that the reduction in
or removal
of oxygen from the biodiesel, i.e., long-chain alkyl esters, will result in a
product, i.e., an alkane,
having a lower solidification temperature or cloud point.
[0029] In addition, it is contemplated by the present invention that reducing
the length of
the carbon chain in the biodiesel molecule from 16-18 carbons to 12-14 carbons
will further
reduce the solidification temperature or the cloud point of the resultant fuel
composition.
[0030] The reduction or removal of oxygen or oxygen-containing components from
biodiesel can be accomplished by employing a variety of chemical or thermal
processes that
result in a hydrocarbon, e.g., alkane, having less or no oxygen. The resulting
hydrocarbon (e.g.,
fuel) will have a lower solidification temperature or cloud point than the
starting biodiesel. The
process used for the reduction in or removal of oxygen from biodiesel can
include the reaction of
an ester group, e.g., alkyl ester, with other chemicals either catalytically
or electrically. In
addition to removing oxygen atoms from the ester group, this ester reaction
may also remove
carbon atoms such that the overall carbon chain length of the biodiesel
molecule is reduced. In
-5-
CA 02768862 2012-01-20
WO 2011/011588 PCT/US2010/042880
one embodiment, the fuel composition of the present invention is substantially
free of oxygen
and has a carbon chain length of from 12 to 14 carbon atoms. This embodiment
produces a fuel
composition that is essentially comparable to aviation fuel (and compatible
with aviation fuels to
produce a mixture thereof) and can be used as a fuel for ground transportation
vehicles in cold
climates. In another embodiment, the produced fuel contains a reduced oxygen
content and has a
carbon chain length of 12-14 atoms. In a further embodiment, the fuel
composition of the
present invention contains less than 2000 parts per million of oxygen based on
the fuel
composition.
[0031] Biodiesel can be produced by a variety of conventional processes that
are known
in the art. Biodiesel is an oil-based diesel fuel, wherein the oil is obtained
from a biological
source, such as, but not limited to, a vegetable oil or animal fat. Oils
suitable for use in
producing biodiesel can be oils obtained from a wide variety of biological
sources. Suitable oils
from a biological source can include, but are not limited to, crop seed oils,
vegetables oils,
animal oils, animal fats, and combinations thereof. The crop seed oils can be
isolated from
biological sources, such as, but not limited to, rapeseed oil, sunflower oil,
mustard oil, canola oil,
peanut oil, palm oil, coconut oil, soybean oil, and mixtures thereof.
Additional examples of
suitable oils include, but are not limited to, waste vegetable oil; animal
fats, including tallow,
lard, yellow grease, chicken fat, by-products of the production of Omega-3
fatty acids from fish
oil, and mixtures thereof; algae; oil from halophytes, such as salicornia
bigelovii; and mixtures
thereof. In alternative embodiments, the oil from a biological source can be
distilled, separated,
or at least partially purified to increase or decrease the content of a
particular component of the
oil, such as, but not limited to, triglycerides, diglycerides, monoglycerides,
saturated fatty acids,
unsaturated fatty acids, trilaurin, erucic acid, lauric acid, oleic acid,
linoleic acid, linolenic acid,
stearic acid, palmitic acid, and mixtures thereof.
[0032] In an embodiment, the oil from a biological source can be hydrocracked
in
accordance with conventional processes known in the art to yield smaller
molecular weight
species.
[0033] Biodiesel includes long-chain alkyl esters, such as, but not limited
to, methyl
ester, ethyl ester, propyl ester, isopropyl ester, butyl ester, and mixtures
thereof. Biodiesel can
be prepared by a transesterification process, wherein lipids are chemically
reacted with alcohol.
Suitable lipids include the oils derived from biological sources (such as, but
not limited to,
-6-
CA 02768862 2012-01-20
WO 2011/011588 PCT/US2010/042880
vegetable oils and animal fats) described herein. Suitable alcohols include,
but are not limited to,
ethanol, methanol, propanol, isopropanol, butanol, and mixtures thereof. In
addition, the
transesterification process can include the presence of catalyst. The catalyst
can be selected from
a wide variety of materials known in the art to facilitate the reaction
between lipids and alcohols.
Suitable catalysts include, but are not limited to, calcium hydroxide,
potassium hydroxide, and
mixtures thereof. The transesterification reaction can be carried out using a
variety of
conventional processes known in the art. Suitable processes include, but are
not limited to,
common batch processes, supercritical processes, and ultrasonic methods. In
general, the
transesterification reaction converts base oil in the vegetable or animal
starting materials to the
desired esters, e.g., alkyl esters, in the biodiesel product. The
transesterification process can
produce by-products. For example, free fatty acids present in the base oil are
typically converted
to soap and removed from the process or they are esterified using an acidic
catalyst. Further,
glycerol can be produced as a by-product of the transesterification process.
Typically, this crude
glycerol is purified by employing a conventional purification process known in
the art, such as,
but not limited to, vacuum distillation. The refined, purified glycerol then
can be utilized
directly or converted into other products.
[0034] In one embodiment, biodiesel is made by reacting animal fat or
vegetable oil
with methanol by transesterification. The process yields two products: (i)
methyl esters, i.e.,
biodiesel, and (ii) glycerin, i.e., a by-product that can be used for the
production of soap. In
alternate embodiments, this process can be conducted on any scale, e.g., in a
mason jar or in a
large-scale production facility.
[0035] In another embodiment, vegetable oil or animal fat reacts with ethanol
or
methanol or mixtures thereof, and a catalyst, such as calcium hydroxide or
potassium hydroxide
or a mixture thereof. In a further embodiment, the initial oil or fat is
relatively low in free fatty
acids in order to reduce or prevent the formation of soap. The reactants are
mixed thoroughly for
about an hour at room temperature or slightly-elevated temperature. In one
embodiment, the
temperature is from about 20 C to about 50 C. After about one hour of mixing,
the solution is
allowed to settle.
[0036] During the settling time, the heavy glycerin (glycerol) settles to the
bottom of the
solution and biodiesel is formed on top. The glycerin is then separated by,
for example, draining
it from the bottom of a settling tank. In an alternate embodiment, separation
can be
-7-
CA 02768862 2012-01-20
WO 2011/011588 PCT/US2010/042880
accomplished by using a centrifuge which is typically employed in large scale
production of
biodiesel to reduce the time needed to carry out the process.
[0037] The final steps in the process include washing and drying the biodiesel
using
conventional methods known in the art. In one embodiment, a fine mist of water
is applied to the
biodiesel to absorb any trace amounts of the catalyst remaining in the
biodiesel. This is
separated from the biodiesel, for example, in the same manner as the glycerin
separation. A
subsequent bubbling of air through the biodiesel removes any remaining water
to ensure a high
purity biodiesel product for use in diesel engines.
[0038] In another embodiment, wherein the starting material contains a
significant
amount of free fatty acids (as is typical in vegetable oil after it is used
for cooking or animal fat),
a pretreatment of the free fatty acid can be conducted using conventional
methods known in the
art. For example, the vegetable oil or animal fat can be reacted with
hydrochloric acid and
methanol. This pretreatment converts the free fatty acids into biodiesel.
After washing and
drying the hydrochloric acid from the resulting solution using a conventional
method known in
the art, the remaining vegetable oil can be converted to biodiesel using the
transesterification
process described herein.
[0039] The following table gives approximate amounts of the reactants needed
to
produce one gallon of biodiesel in accordance with an embodiment of the
invention. The
amounts can depend on the level of free fatty acid and water in the feedstock
vegetable oil or
animal fat.
-8-
CA 02768862 2012-01-20
WO 2011/011588 PCT/US2010/042880
Table 2
Feedstock (veg. oil or animal fat) 1 gal
Methanol 0.1667 gal
Potassium hydroxide 0.0435 kg
Hydrochloric Acid 0.001 Gal
Water 0.4 Gal
Electric Power 0.6667 kW/Gal/hr
[0040] In one embodiment of the present invention, multiple reactions may be
necessary
to reduce or remove the oxygen and/or oxygen-containing compounds from
biodiesel to produce
a fuel having a low solidification temperature or cloud point for use in cold
climates or as
aviation fuel. For example, an ester, such as an alkyl ester in biodiesel, can
be reduced to two
alcohols through a reaction with lithium aluminum hydride (LiA1H4). This
nucleophilic acyl
substitution reaction is generally conducted as follows:
OH
LiffiW + Ct H- C-= ' + =_ '0-H
[0041] Figure 1 shows a four-step reaction process for reducing an ethyl ester
(I) to
ethanol (V). In Figure 1, the nucleophilic hydrogen atom (H) from the hydride
reagent (LiA1H4)
adds to the electrophilic carbon (C) in the polar carbonyl group of the ester
(I). Electrons from
the carbon and oxygen double bond (C=O) move to the electronegative oxygen
atom (0)
creating an intermediate metal alkoxide complex (II).
[0042] The tetrahedral intermediate collapses and displaces the alcohol
portion of the
ester as a leaving group, which produces a ketone as an intermediate (III).
[0043] The nucleophilic H from the hydride reagent adds to the electrophilic C
in the
polar carbonyl group of the aldehyde. Electrons from the C=O move to the
electronegative 0
creating an intermediate metal alkoxide complex (IV).
[0044] The final step is a simple acid/base reaction. Protonation (H+) of the
alkoxide
oxygen creates the primary alcohol product (V) from the intermediate complex.
[0045] Reduction of the resultant alcohol to alkane can be accomplished by a
variety of
chemical processes. Figure 2 shows a process for chemoselectively reducing
secondary and
-9-
CA 02768862 2012-01-20
WO 2011/011588 PCT/US2010/042880
tertiary alcohols to alkanes. This direct pathway shows selective reduction of
the hydroxyl (OH)
moiety without affecting other functional groups. In Figure 2, 2-decanol
(compound 1 a) is
reduced to decane (compound 2a) using this process. The reducing system in
Figure 2 includes
dissolving 2-decanol in a CH2C1CH2C1 solvent with hydrosilane and indium
chloride (InCl3)
catalyst, at a temperature of about 80 C for about 4 hours.
[0046] The fuel composition of the present invention can provide at least one
of the
following benefits:
- Reduced solidification temperature or cloud point; and
- Agricultural-based starting materials.
[0047] In one embodiment, wherein the fuel composition of the present
invention
includes the conversion of biodiesel fuel to ground transportation fuel or
aviation fuel, the cloud
point of the fuel composition is less than the cloud point of the biodiesel.
In another
embodiment, wherein the fuel composition of the present invention is used as
aviation fuel, the
cloud point can be less than or equal to about -40 C. In another embodiment,
wherein the fuel
composition of the present invention is used as ground transportation fuel in
cold temperature
conditions, the cloud point can be less than about -20 C. Cold climate
conditions can vary and
in one embodiment, cold climate temperatures can be less than or equal to
about 0 C.
[0048] While specific embodiments of the invention have been described in
detail, it
will be appreciated by those skilled in the art that various modifications and
alternatives to those
details could be developed in light of the overall teachings of the
disclosure. Accordingly, the
particular embodiments disclosed are meant to be illustrative only and not
limiting as to the
scope of the invention, which is to be given the breadth of the appended
claims and any and all
equivalents thereof.
-10-