Note: Descriptions are shown in the official language in which they were submitted.
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
1
"RECLAIMING OF LEAD IN FORM OF HIGH PURITY LEAD
COMPOUND FROM RECOVERED ELECTRODE PASTE SLIME OF
DISMISSED LEAD BATTERIES AND/OR OF LEAD MINERALS"
FIELD OF THE INVENTION
This disclosure relates to techniques for reclaiming high purity lead compound
from impure mixtures, like recovered electrode paste slime of dismissed
batteries
or lead minerals.
BACKGROUND OF THE INVENTION
Departing from the long established processes for reclaiming valuable lead
content from recovered electrode paste of dismissed lead batteries or lead
minerals based on calcination, prior PCT patent application No.
PCT/IT2008/000022, in the name of the present applicant, the content of which
is
herein incorporated by express reference, disclosed a novel process almost
completely based on wet treatment of the recovered electrode paste or lead
minerals capable of producing highly purified lead carbonate at high yield
with
much reduced burden of disposing of noxious residues and efficient use of
reagents. Nevertheless, in order to use the reclaimed lead for making new
battery
paste, the lead carbonate has to be decomposed to lead oxide by heating the
carbonate to about 400-450 C. This is expensive in terms of energy required.
According to the process disclosed in the above-mentioned prior PCT patent
application, the starting material in the form of electrode paste slime or
finely
ground and eventually pre-treated lead mineral, is leached using an acid
different
from sulphuric, adding hydrogen peroxide or other reducing agent or lead
dioxide
that may be present in the starting material and sulphuric acid for converting
all
lead compounds to insoluble lead sulphate which is separated together with
other
insoluble substances and is thereafter selectively dissolved in an aqueous
solution
of a solubilizing compound. To the separated clear solution of lead sulphate
is
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
2
added a carbonate salt for precipitating carbonate/oxycarbonate of lead.
Besides the energy required for eventually converting the carbonate to lead
oxide,
the acid leaching step of the impure starting material implies certain costs
and
treatment plant complexities.
OBJECTIVES AND SUMMARY OF THE INVENTION
With the aim of reducing the lead reclaiming cost in terms of plant inventory
of
treatment vessels and related agitators, heaters and/or coolers, filters,
overall
treatment plant complexity, pumping and energy requirements, the applicant has
found that an outstandingly effective simplification of the all wet lead
reclaiming
process flow is not only viable but even more efficient.
The novel approach, unexpectedly found outstandingly efficient consists in
directly suspending the impure starting material in a lead sulphate dissolving
aqueous solution of an acetate salt and adding thereto either hydrogen
peroxide or
a sulphite or alternatively bubbling sulphurous anidride through it, in a
measure
adapted to reduce any lead dioxide expected to be present in the impure
starting
material to lead oxide, and sulphuric acid in a measure adapted to convert all
lead
oxide to lead sulphate that remains dissolved in the selected dissolving salt
solution.
A clear solution containing the dissolved lead sulphate may then be separated
from a solid phase residue that includes all undissolved impurities contained
in the
impure starting material.
Together with the solid phase of all insoluble substances, separated from the
lead
sulphate dissolving acetate salt solution, depending on the origin of the
impure
.starting material to be processed, there may be present certain compounds of
lead
such as oxysulphates or other oxides that could not be dissolved in the
acetate salt
solution. Even the lead of these compounds undissolved by the acetate salt
solution can eventually be reclaimed if considered economically or for other
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
3
reason desirable to do so. This can be carried out by suspending the separated
solid phase consisting of impurities and insoluble compounds of lead in a
concentrated solution of hydroxide of the same cation of the acetate salt for
decomposing and convert these compounds to soluble plumbites, that dissolve in
the hot hydroxide solution which may then be separated from the remaining
insoluble impurities. The hydroxide solution now containing the residual lead
stripped from the previously separated solid phase of impurities may be
introduced in the liquid acetate solution containing the lead sulphate in the
vessel
in which hydroxide of the same cation of the acetate salt is introduced for
precipitating the lead contained as lead sulphate in the liquid acetate
solution in
form of lead oxide or hydroxide.
Precipitation of high purity lead compound from the clear lead sulphate
solution
may then by effected either by adding to the solution carbonate of the same
cation
of the acetate salt used for selectively dissolved lead sulphate for
precipitating
insoluble carbonate/oxycarbonate of lead, as contemplated in the process
disclosed in said prior PCT patent application No. PCT/IT2008/000022, or, more
preferably, according to an alternative embodiment, instead of a carbonate
salt, to
the clear solution of lead sulphate is added hydroxide of the same cation of
the
acetate salt used for selectively dissolved lead sulphate for causing
precipitation
of either oxide or hydroxide of lead, depending from the temperature of the
precipitation bath, thus eliminating even the burden of eventually having to
convert the reclaimed lead carbonate to lead oxide by heating the carbonate in
an
oven.
The applicant has found that whether a carbonate salt or a hydroxide is used
for
causing it, precipitation of all lead in the solution as highly pure lead
compound is
practically complete. Therefore, separation of a solid phase of the highly
pure lead
compound from the acetate solution is carried out from the same acetate
solution
in which the impure starting material had been suspended.
Though the clear solution of acetate salt becomes progressively enriched of
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
4
sulphate of the same cation of the acetate salt used for selectively
dissolving lead
sulphate, it may be recycled to the suspension step of the impure starting
material
for as long as the sodium sulphate concentration remains below saturation.
When
the sulphate concentration in the clear acetate salt solution approaches
saturation,
the solution may be cooled to about 10 C for selectively crystallizing and
precipitating solid phase constituted by sulphate of the cation of the acetate
salt
used, which is recovered by filtering. The clear acetate salt solution freed
of the
sulphate salt may then be recycled to the suspension bath of the impure
starting
material.
Preferably, before cooling it for selectively crystallizing and precipitating
solid
phase constituted by sulphate of the cation of the acetate salt used, the
acetate
solution is percolated through a column filled with chelating resin for
sequestering
any residual lead ions in the solution, before cooling the solution in order
to
produce lead-free sulphate salt, as a by-product, of broader market
acceptance.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a flow sheet of the main steps of the process of this disclosure
for
reclaiming high purity lead oxide from dismissed battery electrode slime or
lead
minerals, alternatively for producing pure lead carbonate/oxycarbonate or pure
lead oxide/hydroxide.
Figure 2 is a simplified diagram of a plant for reclaiming highly pure lead
compound from dismissed battery electrode slime or lead minerals,
alternatively
as pure lead carbonate/oxycarbonate or pure lead oxide/hydroxide, according to
the alternative process flows of Figure 1.
DESCRIPTION OF EMBODIMENTS OF THE INVENTION
Referring to the flow sheet of FIG. 1, according to a first embodiment of the
process of this invention, particularly suited for treating raw electrode
paste slime
recovered from crushed dismissed batteries, but usable, with eventual trivial
adaptations, also with finely ground lead minerals, for example galena
commonly
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
roasted for converting lead sulphite to lead sulphate, anglesite and lesser
common
minerals, the impure starting solid material is suspended in an aqueous
solution of
a salt capable of dissolving lead sulphate, such as for example an acetate
salt of
sodium, ammonium, potassium, urea, mono-, di- or tri-ethanolamine.
To this suspension, sulphuric acid in an amount necessary to convert all
oxides
present in the starting material to sulphate, and a reducing agent chosen
among
hydrogen peroxide, a sulphite salt and sulphurous anhydride, is gradually
added or
bubbled through the suspension bath in an amount necessary to reduce any lead
dioxide that may be contained in the starting material (as would be the case
with
slime from discarded lead batteries) to lead oxide.
Therefore, in this first Step (1) of the process flow sheet of FIG. 1, in the
suspension bath of the starting material take place the chemical reactions
that are
formally described herein below in view of the fact that they take place
simultaneously, causing the conversion of all lead compounds to sulphate that
dissolves in the aqueous solution containing the specific dissolving salt
mentioned
above.
The below described reactions make clearly recognizable the existence of a
synergical action that results in a sensible increment of the speed of the
chemical
conversion process, in view of the fact that dissolution of lead sulphate
frees the
lead oxide to which (for the case of dismissed battery electrode paste slime)
it was
originally intimately tied in the electrode paste, to form compounds of the
type
3PbOPbSO4 and 4PbOPbSO4, in consequence of which the oxide quickly reacts,
transforming itself to sulphate, which in turn dissolves in the acetate salt
solution
that, in the considered embodiment, is sodium acetate, while the lead dioxide
physically embedded in concretions of the above-identified oxysulphates,
become
more easily reached by the reacting species and therefore is more quickly
reduced
to lead oxide.
The following reactions relate to an exemplary embodiment of reclaiming high
purity lead compound from electrode paste slime of dismissed batteries in
which
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
6
lead sulphate is present as measure component (close to about 60% by weight)
in
the impure starting material and dissolved in the aqueous solution of sodium
acetate.
Reaction 1: dissolution of lead sulphate
PbSO4 (insoluble) + 4 CH3COONa - soluble complex of PbSO4
Reaction 2: dissolution of lead oxide
2CH3COONa + H2SO4 - 2CH3COOH + Na2SO4
PbO (insoluble) + 2CH3COOH - Pb(CH3COOH)2 (soluble)+ H2O
Pb(CH3COOH)2 + Na2SO4 - PbSO4 (insoluble)+ 2CH3COONa
PbSO4 (insoluble) + 4 CH3COONa 4 soluble complex of PbSO4
Reaction 3: reduction of lead dioxide and dissolution
2CH3000Na + H2SO4 4 2CH3COOH + Na2SO4
Pb02 (insoluble)+ H202 - PbO (insoluble) + H2O + %z 02
PbO (insoluble) + 2CH3COOH 4 Pb(CH3COOH)2 (soluble)+ H2O
Pb(CH3COOH)2 + Na2SO4 - PbSO4 (insoluble)+ 2CH3COONa
PbSO4 (insoluble) + 4 CH3COONa 4 soluble complex of PbSO4
(Optional) Reaction 4= solubilization of insoluble lead compounds possibly
present in the impure starting material separated together with impurities for
reclaiming of such a minor amount of lead
4PbO*PbSO4 (insoluble) +12NaOH 4 5Na2PbO2(soluble) + Na2SO4 + 6H20
By way of example, in order to process electrode paste slime recovered from
crushed discarded lead batteries, an aqueous solution of tri-hydrated sodium
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
7
acetate dissolved in water in a concentration comprised between 37,5 and 54,5%
by weight can be satisfactorily used. Sulphuric acid is added in an amount
corresponding to or just exceeding the stoichiometric requirement for
converting
all lead oxides to lead sulphate as pre-evaluated for the impure starting
material to
be processed. Preferably, after having added the required amount sulphuric
acid,
to the suspension bath is added hydrogen peroxide the amount of which may also
be pre_calculated in terms of the stoichiometric requirement for reducing the
lead
dioxide contained in the starting material.
The amount of electrode paste that can be treated in a certain volume of
solution,
depends on the solubilizing capacity of the lead sulphate in the solution of
the
selected acetate salt and of the added amount of sulphuric acid. The ability
of
dissolve lead sulphate of the acetate salt solution depends from its salt
concentration. By way of example, one liter of aqueous solution with a
concentration of 37,5% by weight of sodium acetate is able to dissolve 100 g
of
lead sulphate. By increasing the concentration of the acetate salt, the amount
of
lead sulphate that can be dissolved increases proportionally. The temperature
at
which the above described reactions can be carried out in the suspension bath
may
be comprised between about 10 C up to boiling point. The suspension may be
stirred with a pails or turbine mixer in order to favor breaking down of lead
compound aggregates.
The combination of the chosen operating conditions (type and fineness of the
starting solid material, type and concentration of the lead sulphate
dissolving salt
solution, eventual lead dioxide reducing agent addition, temperature, stirring
.mode) will influence the time needed for completing this first step (1) of
the all-
wet reclaiming process. The sulphuric acid used for the sulphation of all the
lead
oxide in the solution should preferably have a high concentration in order not
to
excessively dilute the lead sulphate dissolving solution.
Once the reaction time that, depending on the combination of the numerous
parameters may generally range between 6 and 15 minutes, a limpid acetate
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
8
solution containing lead sulphate may be separated from the solid phase
residues,
for example by filtration. All insoluble impurities and substances are
therefore
separated from the solution (Step 2 of the flow sheet of FIG. 1).
The subsequent reaction conducted in the limpid acetate solution containing
substantially all the lead content of the starting material in form of lead
sulphate
by adding to the solution a hydroxide of the same cation of the selected
acetate
salt (i.e. either sodium, potassium or ammonium), according to the preferred
alternative contemplated for the Step 3 of the flow sheet of FIG. 1, at a
temperature sufficiently high to ensure precipitation of all the lead in
solution in
form of PbO (of yellow aspect) instead of in form of lead hydroxide (of white
aspect) produces a selective precipitation of lead oxide because of its much
lower
solubility than that of lead sulphate. Generally, the critical temperature is
in the
vicinity of 70 C, therefore the precipitation may be carried out at about 72-
73 C
(unless for some reason one should prefer to recover a highly purified lead
hydroxide, thermally convertible to lead oxide eventually).
The other alternative contemplated for the Step 3 of the flow chart of FIG. 1,
consists in adding to the liquid acetate solution containing substantially all
the
lead content of the starting material in the form of lead sulphate, instead of
a
hydroxide, a carbonate of the same cation of the selected acetate salt (i.e.
either
sodium, potassium or ammonium), which produces a selective precipitation of
= lead carbonate or a mixture of lead carbonate and oxycarbonate, because of
their
much lower solubility than that of lead sulphate. In this alternative
embodiment,
precipitation may be conducted at any temperature comprised between ambient
temperature up to boiling point.
Once the reaction of Step 3 is complete, the precipitated lead oxide or
hydroxide
or carbonate/oxycarbonate is separated by filtration (Step 4 of the flow chart
of
FIG. 1) from the solution while sulphate of the cation of the hydroxide or
carbonate used for precipitating the lead as insoluble oxide (or hydroxide) or
carbonate (and/or oxycarbonate) remains in the solution.
CA 02769175 2012-01-25
WO 2011/013149 PCTlIT2009/000344
9
The limpid acetate solution, now containing also the sulphate of the same
cation
of the acetate salt, can be integrally recycled to the suspension bath of
selective
dissolution of the lead sulphate (Step 1) of the process, for as long as the
content
of sulphate remains below saturation (this limit depends primarily on the type
of
dissolving salt solution of the lead sulphate and processing conditions).
Of course, precipitation of excess sulphate salt together with the lead oxide
or
hydroxide or carbonate/oxycarbonate must be prevented. Therefore, excess
sulphate salt must be eventually eliminated from the solution, well before
approaching the saturation limit (Step 8 of the flow sheet of FIG. 1). This
may be
easily done by exploiting the different solubilities at different temperatures
of the
sulphate salt (i.e. of sodium, potassium or ammonium sulphate) from that of
the
corresponding acetate salt for selectively crystallizing the sulphate and
separating
it from the acetate solution.
The concentration of the aqueous solubilizing salt solution and the
temperature at
which lead sulphate dissolution in it is carried out are not essential
parameters
because they simply influence the time necessary for completing the reactions
discussed above and the quantity of lead sulphate that may be dissolved in the
solution. In practice, if the solubilizing solution, after precipitation of
the
-dissolved lead as oxide or hydroxide or carbonate/oxycarbonate, is recycled
back
and therefore one's operates with a recycled solution, more and more recycles
may
be necessary to complete dissolution of a given quantity of lead sulphate.
The novel approach of this disclosure has proved itself outstandingly suitable
to
process electrode paste slimes where the amounts of the three main lead
compounds, namely lead sulphate, lead oxide and lead dioxide, oscillate in the
vicinity of a mean value by a range of variability of about 2% by weight and
this
may in practice impede to calculate exactly the quantity of sulphuric acid
solution
for converting to sulphate all lead oxides present in the impure starting
material.
Nevertheless, if in conducting the novel process of this disclosure sulphuric
acid
happens to be added in excess of the stoichiometrically necessary amount,
after
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
having precipitated the pure lead compound by adding a hydroxide or a
carbonate
of the same cation of the selected acetate salt, an excess of sulphate of the
cation
of the added compound forms compared to the amount strictly relative to the
precipitation of lead sulphate, because of the presence in the solution of
free
sulphuric acid. Vice versa, if sulphuric acid happens to be added in defect of
the
stoichiometrically necessary amount, incomplete conversion of oxides to
sulphate
occurs, thus a residual amount of oxide remains undissolved in the acetate
solution when separating the solid impurities. Should this accidentally occur,
the
separated solid phase may be simply reintroduced in the suspension bath to be
eventually converted by introducing an excess of sulphuric acid.
Continuously or intermittently, whenever the sulphate concentration in the
clear
acetate salt solution approaches saturation, the solution is preferably
percolated
through a column filled with chelating resin for sequestering any residual
lead
ions in the solution (Step 5 of the flow sheet of FIG. 1), before cooling the
solution to about 10 C for precipitating a crystalline solid phase (Step 6 of
the
flow sheet of FIG. 1), constituted by sulphate of the cation of the acetate
salt
used, which is recovered by filtering (Step 7 of the flow sheet of FIG. 1).
The
clear acetate salt solution freed of the sulphate salt may then be recycled to
the
suspension bath of the impure starting material while the lead-free sulphate
salt
constitutes a marketable by-product.
Herein below several examples are reported solely for illustrating different
possible embodiments of the process of this invention without in any manner
-meaning to exclude other possible embodiments.
Example 1
80 g of recovered dried electrode paste having a lead content, expressed as
metal
equivalent, of 72% was treated under stirring with 1000 ml aqueous solution of
=tri-hydrated sodium acetate at 37,5 % by weight, with the addition of g 12,2
of
concentrated sulphuric acid at 94-96% by weight, at the temperature of 83 C.
Successively, hydrogen peroxide at 32% by weight was slowly added to the
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
11
suspension (dropwise for about 10 minutes) until no further clarification of
the
suspension was observed.
The hot suspension was then filtered and the separated solid phase was
constituted
by insoluble lead compounds and lead compound concretions, electrode grid
fragments and various additives used for making the electrode paste such as
carbon black, barium sulphate, fibers, etc. and impurities such as sand,
plastic
materials, etc.. The amount of this dark grey solid phase was about 4-12 % by
weight of the solid mass of the dry electrode paste.
The filtered limpid solution containing lead sulphate was stirred at 83 C
adding
thereto sodium hydroxide until reaching a practically complete precipitation
of the
lead in the form of lead oxide. The suspension was thereafter filtered
separating
the precipitate from the solubilizing solution of sodium acetate now enriched
of
sodium sulphate that was recycled to the stirred lead sulphate dissolution
vessel
for as long as the content of sodium sulphate in the solution remained below
'saturation.
When the content of sodium sulphate in the sodium acetate solution became
close
to the saturation limit, the solution was percolated through a column filled
with
chelating resins, for example of the commercial type denominated Chelex- 100
or
Dowex A- 1, though any other equivalent resin may be used. The resin filler
sequestered almost completely the surprisingly small quantity of lead ions
residually present in the sulphate solution.
Subsequently the purified sulphate solution (practically lead-free) was slowly
cooled to 10 C, under slow stirring, for precipitating a crystalline solid
phase
.constituted by sodium sulphate that was then recovered by filtering the
suspension, while the clear solution was recycled to the dissolution vessel of
the
lead sulphate.
The filtered lead oxide accurately rinsed with de-ionized water was dried at
160 C
for as long as reaching constancy of weight.
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
12
The separated dark grey solid phase was suspended in sodium hydroxide at 40%
by weight, at 50 C for 15 minutes. The separated limpid liquid phase was
introduced into the limpid acetate solution containing also the lead sulphate,
as
part of the required amount of sodium hydroxide for precipitating all lead in
solution as lead oxide or hydroxide (according to a preferred embodiment) in
consideration of the fact that also the lead present in the solution as sodium
plumbite converts itself to lead oxide (or hydroxide).
At the end of the tests, the following mass balance was recorded.
In 80 g amount of recovered electrode paste used in an experiment, there were
4 g
of insoluble substances of dark grey color containing metallic lead and
extraneous
substances such as sand, carbon black, barium sulphate and other substances in
minor amounts.
The calculated maximum quantity of recoverable lead oxide was of 62,05 g while
the quantity of lead oxide effectively recovered was of 62,03 g for a recovery
yield of 99,96%.
Chemical analysis of the recovered solid product confirmed that it was
constituted
exclusively by PbO at 99.99% purity, while the sodium sulphate that was
eventually recovered had a purity of about 99.90%.
The following table summarizes relevant conditions, peculiarities and results
of
other four exemplary embodiments of the process of Example 1, described in
detail above, but with the indicated alternative conditions and the results
that were
obtained, always using as starting material electrode paste of the same lot
recovered from crushed dismissed batteries.
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
13
n. Lead sulphate Reaction time Precipitating Obtained Yield
dissolving and temperature compound purified lead %
solution compound
2 l 000ml 10 minutes NaOH Pb(OH)2 99.94
Sodium 65 C
acetate,
@37.5%
3 1000ml 8 minutes NaOH PbO 99.95
Sodium 90 C
acetate,
40.0%
4 1000ml 12 minutes Na2C03 PbC03 99.91
Sodium 45 C
acetate,
@42.5%
The lead oxide (whether directly produced by the all-wet process or obtained
by
heating lead carbonate/oxycarbonate produced by the all-wet process) is
perfectly
suitable for preparing electrode pastes for new batteries.
The practice of the process of this invention using as starting material a
mineral or
a mixture of minerals of lead may be substantially similar to the above
described
embodiments, an essential pre-step being that of converting as much as
possible
any different salt of lead present in the mineral to either lead sulphate or
to lead
oxide. For example, in case of galena, the most common lead mineral, the
mineral
should be heated in air, according to common roasting techniques, until
oxidizing
the lead sulphite to sulphate. The other common mineral anglesite does not
need
any prior treatment being itself already constituted by lead sulphate. Of
course the
mineral(s) should be finely ground for facilitating their processing.
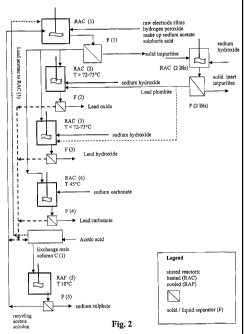
FIG.2 is a schematic diagram of possible embodiments for an industrial plant
for
reclaiming variable lead in form of high purity compounds from recovered
electrode paste of dismissed lead batteries and/or finally ground led
minerals,
eventually pretreated for converting as much of the lead compounds to lead
sulphate.
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
14
The scheme of FIG. 2 provides a multi-embodiment illustration of the discussed
processing alternatives (though sodium is indicated as the exemplary selected
cation of both the acetate salt and of the alternatively added compounds for
precipitating the desired pure compound of lead, according to the various
alternatives).
In practice, the plant requires essentially three stirred and temperature-
controlled
reactors. To a first reactor RAC (1) in which the impure material is suspended
in
an aqueous acetate salt solution, is associated a first solid-liquid separator
F(l) for
separating the lead sulphate containing solution from the solid phase
constituted
by insoluble impurities of the impure starting material.
To a second reactor for precipitating the desired lead compound of high
purity,
that in the multiple alternative scheme of FIG. 2 is anyone of the reactors
RAC
(2), RAC (3) and RAC (4), is associated a second solid-liquid separator, that
is the
related one F(2), F(3) and F(4).
The third and last reactor RAC (5) and associated third and last solid-liquid
separator F(5) are required for at least periodically (or more preferably
continuously) treating the recycling acetate salt solution and to recycle it
to the
first sulphate dissolving reactor RAC (1). The treatment consists of
selectively
crystallizing by virtue of the significantly different solubilities of the
acetate salt
and of the sulphate of the same cation of the acetate salt, introduced in the
second
reactor for precipitating the desired lead compound, and removing it from the
system. This step must be performed (continuously or intermittently) in order
to
prevent saturating the recycling acetate salt solution with the sulphate of
the same
cation, which if let to occur would cause co-precipitation of this salt
together with
the lead sulphate (making vain the purification process).
According to the preferred embodiment, in order to ensure that the "by-
product"
sulphate salt (e.g. sodium sulphate) be substantially lead-free and thus
economically marketable, the plant may include an exchange resin column C(l)
filled with an appropriate chelating resin, through which the solution, when
CA 02769175 2012-01-25
WO 2011/013149 PCT/IT2009/000344
directed to the selective sulphate crystallization reactor RAC (5) (whether
continuously or periodically) passes, for sequestering residual lead ions that
may
be present in the solution.
Of course, the chelating resin filler will gradually loose its activity and
periodically a stripping of sequestered lead ions must be carried out by
circulating
through the column (Cl) acetic acid for a certain period of time. The lead
ridden
stripping solution of acetic acid used for this periodical re-activation of
the
exchange resin, now containing lead acetate, may be "disposed of' by simply
introducing it into the first reactor RAC (1), as shown by the relative line.
The multi-embodiment plant diagram of FIG. 2, illustrates also the optional
reactor RAC (2bis) in which, if desirable, in consideration of the composition
of
the impure starting material to be processed, residual amount of lead that may
remain associated with the separated solid phase of impurities, in form of
compounds or concretions that could not be dissolved during the treatment of
the
impure material in the first reactor RAC (1). The separated solid phase is
suspended in hot concentrated solution of hydroxide of the same cation of the
selected acetate salt for dissolving also these compounds of lead or
concretions
'thereof. The associated liquid-solid separator F (2bis) permits to separate
all non-
lead impurities from a liquor of hydroxide containing sodium plumbite
dissolved
in it which may be conveniently used as part of hydroxide addition in a second
reactor RAC (2) or RAC (3) that may be used for precipitating all the lead in
the
solution as lead oxide or lead hydroxide, according to a preferred embodiment.