Note: Descriptions are shown in the official language in which they were submitted.
CA 02771443 2012-02-17
WO 2011/020554
An Inhalation Device and a Method for Inhaling an Active Ingredient from a
Capsule
This invention relates to an inhalation device and a method for inhaling at
least one
active ingredient contained in a capsule.
Various so-called capsule-based inhalers for inhaling one or more active
ingredients
are generally known. The usually powdery active ingredient/s can be available
as such
individually and unbound or bound to a carrier substance. A capsule in general
contains one therapeutically required active ingredient dose each. The capsule
is
inserted in an inhaler where it is opened prior to inhalation. During the
inhalation, an
air flow passing in a flow direction longitudinally through the inhaler is
produced. At
least part of the active ingredient contained in a capsule can be transported
together
with this air flow into the lungs of a user.
When a powdery active ingredient is used for a pulmonary application, either
the
separately available active ingredient or the active ingredient bound to a
carrier
substance is separated from the carrier substance during the inhalation and
reaches the
lungs in a finely divided form. It is thus possible to ensure that the active
ingredients
in the air flow can reach their site of action in the lungs. The fine division
and/or
separation of the active ingredients or active ingredient is also referred to
as dispersion
or deagglomeration.
Known powder inhalers can be active or passive devices. When they are passive
devices, the inhalation is effected exclusively by a breath of an inhaling
person. In
contrast to this, active devices use in addition to a breath a mechanism which
in
response to an actuation of the inhaler supports a dispersion and/or
deagglomeration.
For example, a pressure reservoir can additionally be opened upon inhalation;
the
CA 02771443 2012-02-17
2
pressure released by it supports the deagglomeration. Active inhalation
devices
usually have a complex and thus expensive design.
A distinction is also made between so-called reservoir inhalation devices and
pre-
dosed systems.
Reservoir inhalation devices comprise a reservoir from which an individual
dose is
taken during an inhalation event and is supplied to a patient. Such devices
usually
have a complex design and make great demands on the so-called powder
technology
when an individual dose is separated, in particular since such reservoir
systems may
contain up to 200 individual doses and are used for a correspondingly long
period of
time. On the one hand, such a system must ensure that a given number of doses
can be
dispensed and, on the other hand, also that a dose is always dosed equally
irrespective
of the dispensing time. In addition, it must be evident to a patient whether
further
doses are available in such an inhalation device. Such reservoir devices are
also
accompanied by a complex development and testing of the formulation as well as
a
complicated design and therefore the production thereof involves high costs.
Furthermore, such reservoir devices often cannot be reused.
So-called pre-dosed inhalation devices already have a divided powder
formulation for
an application, for example as a capsule or in so-called blisters. A
distinction can here
be made between so-called multi-dose inhalers and so-called single-dose
inhalers.
Multi-dose inhalers comprise several separately provided individual doses,
e.g.
divided on a blister strip or blister disk. The blister is either integrated
into an
inhalation device or can be inserted, if necessary. Thus, these devices often
provide a
mechanism serving the further transport of the blister after the inhalation of
an
individual dose.
Such devices again have a complex design and make great demands on the
production
of the blister strips. The production also involves high costs. In addition,
such single-
dosed multi-dose systems usually cannot be reused.
CA 02771443 2012-02-17
3
Single-dose inhalers can take up precisely one single dose, e.g. in a capsule.
The user
inserts a single capsule in the inhalation device before the inhalation.
For example, EP 1 245 243 Al discloses a pin-like capsule inhaler having a
rotatable
cutting/perforation device for perforating or cutting a capsule. WO
2007/093149 Al
and US 5,651,359 disclose inhalation devices where a capsule is forced against
stationary knives to open it and the ends of the capsule are fully cut off. US
4,013,075
discloses an inhalation device where a capsule is opened by rotatable blades
and the
air flow is passed perpendicularly through the inhalation device.
Furthermore, EP 0 666 085 Al discloses an inhalation apparatus where a capsule
is
guided in a rotatable support past two knives and air is passed through the
opened
clamped capsule. US 4,889,114 discloses a tubular capsule inhaler through
which an
air flow is passed perpendicularly.
In addition, US 3,991,761 discloses a capsule inhaler where a capsule is
laterally
perforated by spring-loaded pins. Finally, WO 2007/098870 Al discloses a
capsule
inhalation device where an opening device movable with respect to the housing
is
provided at the mouthpiece.
All in all, it is often difficult to handle and clean the inhalation devices
known from
the prior art. They usually have a complex design including many components.
In
addition, known inhalation devices often fail to fully empty a capsule.
On this background, it is the object of this invention to provide an improved
inhalation
device which overcomes at least part of the above drawbacks.
These objectives are achieved by the subject matters of the independent
claims.
Preferred embodiments are indicated in the subclaims.
CA 02771443 2012-02-17
4
An inhalation device according to the invention can comprise a swirl chamber
for
receiving a capsule and for swirling the capsule content, at least one air
introduction
channel and at least one air discharge channel, wherein the air introduction
channel
and the swirl chamber and the air discharge channel are in fluid communication
and
wherein the air introduction channel and the air discharge channel extend at
least
partially in opposite directions. Sucked-in air can be introduced into the
swirl chamber
through the air introduction channel and can be discharged through the air
discharge
channel.
According to the invention, one or more air introduction channels and/or air
discharge
channels can generally be provided. However, it is preferred to only provide
for one
air introduction channel and one air discharge channel each since it is thus
possible to
obtain a defined and reproducible air flow by simple means. The arrangement
and
design of the air introduction channel and the air discharge channel, which
extend at
least partially in opposite directions, effects a particularly advantageous
air passage
since the air is passed circumferentially during an inhalation event.
The invention also provides a method for inhalation by means of an inhalation
device
according to the invention. The method comprises the steps of: introducing a
capsule
into a capsule support of an inhalation device, which is arranged and formed
in a swirl
chamber; opening the capsule by means of a cutting device, sucking in air
through a
mouthpiece via an air introduction channel and an air discharge channel, the
capsule
being caused to vibrate and rotate as a result of the air flow inside the
swirl chamber
so as to empty the capsule.
Further advantageous embodiments are indicated below.
The air introduction channel can comprise any geometries and can be formed at
least
partially in the lower part of the inhalation device. The air introduction
channel of the
inhalation device according to the invention can extend at least partially
tangentially
to the swirl chamber.
CA 02771443 2012-02-17
The inhalation device according to the invention can also comprise a
mouthpiece
which is in fluid communication with the air discharge channel.
Furthermore, an air inlet opening for the air introduction channel can be
developed in
the lower part at any position. The air introduction channel of the inhalation
device
according to the invention can also have an inlet which is arranged in an area
of the
inhalation device outside the areas provided for holding/gripping by a user
during the
inhalation, preferably adjacent to or near the mouthpiece.
When the external air is introduced from the direction of the mouthpiece -
i.e. coming
from the front side of the inhaler -, an inhaling patient usually cannot close
the air
inlet opening and thus the air introduction channel while gripping and holding
the
inhalation device in the ordinary way by his hand and/or individual fingers. A
perfect
functioning of the inhalation device is thus ensured.
The air flow can basically be introduced into the swirl chamber from any sides
and
positions. The air introduction channel of the inhalation device according to
the
invention can also have an outlet which opens into a lower part of the swirl
chamber
and/or laterally into the swirl chamber. Thus, air sucked in from the outside
can be
introduced into the swirl chamber from below and/or laterally. An advantageous
air
flow onto the capsule can be produced in this way. The air flow then acts upon
the in
particular lower end of a capsule accommodated in the swirl chamber, said end
facing
the air inlet.
The air flow partially flows in circles, also in extension of the air
introduction
channel, along a vertical inner curvature of the swirl chamber. The air flow
introduced
from below lifts the capsule, thus supplying energy from below and causes the
capsule
to vibrate and rotate. As a result, the capsule can be lifted and be carried
from below
by an air current eddy acting as an air cushion.
The air discharge channel of the inhalation device according to the invention
can also
have an inlet which is in fluid communication with an upper area of the swirl
chamber
CA 02771443 2012-02-17
6
and an outlet, and the air discharge channel can extend between the inlet and
the outlet
in the shape of an arch or S.
The transition from the swirl chamber into the air discharge channel can
initially
extend, directly next to the swirl chamber, perpendicularly upwards and then
in the
shape of an arch and/or S towards the mouthpiece. For example, the air flow
can
initially be passed and/or sucked off from the swirl chamber upwards during an
inhalation event. As a result, additional forces are released and the
turbulent flows are
enhanced. Thus, the capsule is advantageously sucked upwards with the air
flow, e.g.
like a whirlwind, and supported from below by the air flow, e.g. in the way of
an air
cushion.
Thus, a deagglomeration can further be improved and a complete emptying of the
capsule can be ensured. The air passage in the shape of an arch or S towards
the
mouthpiece enhances the above effects by additional flow fields. The particles
contained in the air flow are deflected in a way by approximately 180 , thus
colliding
with the lateral upper closure of the swirl chamber and with the defining
walls of the
air discharge channel, which in turn results in the application of a force
onto the
particles. This in turn further enhances swirling and deagglomeration.
The transition between swirl chamber and air discharge channel can be kept
free.
Also, a grid can be arranged in the inhalation device according to the
invention at the
start of the inlet of the air discharge channel. The grid prevents fragments
forming
when the capsule is cut from entering the air discharge channel and thus from
entering
a patient's lungs. Moreover, the active ingredients and carrier substances
partially
collide with the grid, which in turn advantageously supports the
deagglomeration.
The air discharge channel can comprise a uniform cross-sectional area. The air
discharge channel of the inhalation device according to the invention can also
have a
cross-section which becomes greater or smaller downstream of the swirl
chamber.
Thus, the air discharge channel tapers and/or widens through its cross-
sectional area.
CA 02771443 2012-02-17
7
The tapering of the cross-section serves for increasing the flow rate of the
air flow. A
widening of the cross-section serves for slowing down the flow rate of the air
flow.
For example, the flow cross-section widens advantageously towards the
mouthpiece to
provide a high volume flow having a comparatively low flow rate at the
transition to
the patient. When the air flow passes out of the swirl chamber and into the
air
discharge channel, the cross-section is tapered so that at this place high
flow rates and
an equal volume flow occur. The pulse effect is particularly great in this
area, which
in turn enhances the deagglomeration.
In addition, one or more recesses and/or protruding structures can be arranged
and/or
formed in the air introduction channel and/or in the air discharge channel
and/or in the
swirl chamber. The recesses and/or structures enhance a deagglomeration in the
air
introduction channel and/or in the air discharge channel and/or in the swirl
chamber.
Here, the recesses and/or protruding structures can be made e.g. as
dissipaters,
projections of any geometries and/or so-called baffles with which the active
ingredient/s and carrier substances collide through the air flow and are
deflected. Such
a design of the air introduction channel and/or air discharge channel and/or
the swirl
chamber advantageously enhances a swirling of the air flow and results in an
additional pulse effect on the active ingredients and carrier substances. This
in turn
improves the powder dispersion.
The inhalation device according to the invention can also comprise at least
one
additional channel at least partially surrounding the air discharge channel
for
generating an additional air flow. This additional channel can be arranged and
designed such that the additional air flow encloses the air flow containing
swirled
active ingredients. For this purpose, the air discharge channel and the at
least one
additional channel can extend at least partially parallel, the additional
channel passing
an air flow from above and/or laterally or circumferentially to the edge of
the air
discharge channel. It is thus possible to further improve the described
deagglomeration and swirling effects and reduce the active ingredient
deposition at
the oropharynx.
CA 02771443 2012-02-17
8
A capsule can only be inserted in the swirl chamber. This can be advantageous,
for
example, when the capsule was opened via an external cutting device or a
protection
film was peeled off.
The swirl chamber of the inhalation device according to the invention can also
comprise a support for receiving a capsule. The capsule support can have the
same
dimensions as a capsule or also be made larger than the capsule.
In addition, the support of the inhalation device according to the invention
can be
designed such that a capsule is held in positive and/or frictional engagement.
The
capsule is advantageously fixed in a defined position.
During an inhalation event, the air flow supplied through the air introduction
channel
moves an inserted sphere in the swirl chamber and causes the capsule to move
intensely in the form of a preponderant rotation and vibration. The swirl
chamber can
have any geometries. A freely movable capsule leads to markedly more turbulent
flow
fields than a resting capsule, for example. As a consequence, greater forces
occur
which support the emptying of the capsule, on the one hand, and the
deagglomeration
and/or dispersion of the active ingredient to be inhaled, on the other hand.
The air
passage thus supports an acceleration and movement of the capsule and also an
acceleration and movement of the active ingredient/s and the carrier
substance/s.
As described, a capsule can be inserted in the inhalation device according to
the
invention when it is already open, e.g. after removing a protection film or
opening it
by means of an external cutting mechanism.
Alternatively, it can be opened in the inhalation device. For this purpose,
the
inhalation device according to the invention can be provided with a cutting
apparatus
for opening a capsule. It can be arranged in the upper part. When the cutting
device is
integrated into the inhalation device, there are advantageously no transport
losses of
active ingredients from an open capsule.
CA 02771443 2012-02-17
9
To this end, the cutting device of the inhalation device according to the
invention can
comprise at least one movable cutting member. The use of two parallel cutting
members is of special advantage. For example, knives or blades can be used as
the
cutting members. They advantageously serve for avoiding the formation of
capsule
fragments and for achieving smooth cutting or stab edges as compared to pins.
The
drawbacks of other opening means are also prevented, such as the formation of
fragments and/or of a closure of a perforated capsule surface by fragments
and/or by
the active ingredient contained therein.
The cutting members can be designed so as to be inserted in the upper part of
the
inhalation device. They are preferably firmly fixed in the upper part, e.g.
adhered,
mechanically connected or formed integrally in the upper part during the
production
thereof. In the case of a mechanical connection, the knives are preferably
exchangeable.
In general, the at least one cutting member of the inhalation device according
to the
invention can be made from any metals or the alloys thereof but also from
other
suitable materials, such as ceramics. For example, the material used can be a
high-
alloy steel which is surface-treated or hardness-treated by means of a
nitriding
method, for example. The blades can be accurately cut using the spark erosion
technology, for example. The cutting members preferably consist of a steel
grade
admitted for pharmaceutical applications; e.g. AISI 316L.
The capsule ends are advantageously cut on both sides by means of two parallel
cutting members. For this purpose, the blades can be designed and arranged
such that
they do not cut off the capsule ends but only cut partially or fully into the
capsule
ends. As a result, irregular cutting edges and a trapping of capsule ends are
avoided.
Another advantage is that the air flow is not impeded by capsule ends which
were
possibly cut off. The powder contained in the capsule can be emptied into the
swirl
chamber by the cuts. Thus, the cutting course is also closely correlated with
the air
passage.
CA 02771443 2012-02-17
The knives can be equipped with any suitable blades. For example, the knives
can be
made with a rounded, one-sided double-chamfered blade having a radius of
curvature
of 1.28 cm. They are preferably made with a straight, two-sided, double-
chamfered
blade and a blade angle of 80 each or with a straight, one-sided, double-
chamfered
blade and a blade angle of 67 or with straight, double-chamfered blades and a
blade
angle of 0 . A straight, one-sided, single-chamfered or double-chamfered blade
having
a blade angle of 35 is particularly preferred.
The capsules in an inhalation device according to the invention can basically
be
moved to a cutting device. However, the cutting members are advantageously
forced
against the fixed and resting capsule. Since a capsule is fixed in the capsule
support, a
movement of the capsule is avoided during opening and the capsule is always in
the
same position. Thus, the cutting course and the cutting point at the capsule
can be
reproduced as desired and miscuts are avoided.
The at least one cutting member of the inhalation device according to the
invention
can also first cut the capsule with the tips of its blades. When the tips of
the cutting
member first contact the capsule surface, a deformation of the capsule can
advantageously be avoided and accurate cuts can be achieved. In order to open
a
capsule, the cutting members approach the capsule from above and the latter is
then
cut vertically from above. The cutting angle can be 0 - 90 to the
longitudinal axis of
the capsule. It is preferably 10 and more preferably 0 .
The capsules can basically consist of any materials. However, rather high
forces are
required for opening gelatin capsules, for example. In particular when the
humidity is
low, gelatin capsules additionally tend to become brittle and/or to split.
However,
when the humidity is high, gelatin capsules become soft and hence tend to
deform.
Capsules based on hydroxypropylmethyl cellulose have shown to be particularly
suited for an inhalation device according to the invention. This material
distinguishes
itself in that it can be opened particularly easily and hardly tends to form
fragments.
CA 02771443 2012-02-17
11
This material also retains its described properties over a wide range of
humidity so
that the risk of a capsule deformation or a fragment formation is low.
The inhalation device according to the invention can also comprise an
actuation
member for actuating the cutting member. The actuation member is
advantageously
arranged in the upper part of the inhalation device so as to be separate from
the air
passage. A positive engagement of all air-conducting components is thus
ensured so
that no leakages can occur and the cutting device has no disadvantageous
influence on
the air flow during the inhalation.
In addition, the inhalation device according to the invention can comprise a
spring
member to keep the cutting device biased in a pull-back position. The
actuation
member can be guided by at least one guide member. It ensures an accurate
guidance
of the actuation member and thus of the cutting member/s. The guide member can
be
designed as a guide rail, guide groove or any other suitable guide mechanism.
Thus,
the guidance of the knife and the cutting angles are advantageously
reproducible in
any way.
In general, the actuation member can be actuated via any suitable manipulating
mechanisms. For example, the actuation member can comprise a thread which
cooperates with a thread arranged in the upper part. For cutting into a
capsule, the
actuation member can be turned, i.e. screwed into the inhalation device, the
cutting
member being forced against the capsule and cutting into the ends thereof.
Furthermore, the at least one cutting member can be fully accommodated in the
upper
part in the pull-back position. A danger of injury by the knives is thus
advantageously
reduced. It is also ensured that the air flow is not impeded by the cutting
device during
the inhalation.
The actuation member can be actuated against the force of the spring member
into an
actuation position such that the at least one cutting member can be moved into
the
swirl chamber by means of the at least one guide member so as to open a
capsule
CA 02771443 2012-02-17
12
without touching the bottom part. All in all, the cutting device of the
inhalation device
according to the invention advantageously ensures a maximum of reproducibility
of
the cut by the cutting member/s.
Since the fragment formation and capsule deformation are avoided during the
cutting
step, the emptying step during the inhalation can also be reproduced. This in
turn
enables a uniform dose dispense. Furthermore, it is also ensured that the
active
ingredient is also almost fully emptied from the capsule. Only a minimum of
active
ingredient is left in the inhalation device. The cutting device also optimizes
the
dimensions and design of the air-conducting components and also the separation
of
the active ingredient from the carrier material and/or the dispersion of the
active
ingredient/s. This serves for avoiding dose fluctuations, such as dosage
failure or
insufficient dosage in the worst case.
The inhalation device according to the invention can also comprise an upper
part and
a lower part which are connected to each other. For this purpose, a snap-
action or
locking device or any other suitable mechanism can be used, for example.
Basically any opening mechanism can be provided. The upper part and the lower
part
of the inhalation device according to the invention can also be pivotably
connected,
preferably such that the upper part can be moved between an open position to
remove
or receive a capsule and a closed position for the inhalation. For this
purpose, e.g. a
hinge arrangement can be used. The inhalation device can be pivoted between a
defined open position to remove or receive a capsule and into a defined closed
position for the inhalation. The pivot positions can additionally be defined
and fixed
by nap-in connections, for example.
The inhalation device according to the invention can also comprise a safety
mechanism which can prevent an actuation of the actuation member in the open
position. It can be a locking pin, for example.
CA 02771443 2012-02-17
13
In addition or alternatively, the inhalation device according to the invention
can
comprise a further safety mechanism which can prevent an inhalation upon
actuation
of the actuation member. For this purpose, the actuation member can be
mechanically
linked to the safety mechanism, for example. An inappropriate dosage can be
advantageously avoided in this way.
An inhalation device according to the invention can also comprise any specific
device
resistances. The inhalation device according to the invention preferably
comprises a
specific device resistance of 0.027 to 0.050 kPa 5=L/min, resulting in flow
rates of 40
- 75 L/min with a pressure drop of 4 kPa via the inhalation device (1);
preferably of
0.031 to 0.044 kPa 5=L/min, resulting in flow rates of 45 - 65 L/min with a
pressure
drop of 4 kPa via the inhalation device (1); more preferably of 0.036 to 0.040
kPaO 5=L/min, resulting in flow rates of 50 - 55 L/min with a pressure drop of
4 kPa
via the inhalation device.
These middle to high device resistances are advantageously considered to be
more
pleasant by many patients, this having a positive effect on a patient
compliance. In
this range, it is also possible for all patient groups to obtain the flow
rates required for
the inhalation.
The thus achieved air flows are obtained compared to devices having low
resistances
in lower air velocities and longer residence times of the powder in the highly
turbulent
inhaler zones, which results in an improved separation of active ingredient
and carrier.
The capsule is also exposed to the turbulent air flow for a prolonged period
of time,
which has a positive effect on the emptying thereof. Thus, the drawbacks of
inhalers
which operate at low and very high resistances are advantageously overcome.
All in all, the preferred embodiments provide a flexible and compact
inhalation device
and an inhalation method. The inhalation device according to the invention is
made in
particular as a single-dose capsule inhaler.
CA 02771443 2012-02-17
14
An inhalation device according to the invention can easily be manipulated and
cleaned
advantageously by a patient. The inhalation device according to the invention
also
only comprises some few components and has a simple design so that it can be
produced inexpensively. In addition, it can be operated with commercially
available
capsules. These capsules advantageously make a comparatively low demand on the
powder technology and their production is inexpensive.
The capsule can basically be opened for the purpose of emptying at any
positions.
However, when the capsule is open at the ends, the capsule openings are
advantageously found at the site of maximum centrifugal forces in the case of
a
rotating capsule. As a result of such a combination of the opening position
with the
movement of the capsule, the forces are markedly enhanced compared to systems
having a resting capsule and other opening positions. The forces support
and/or
maximize the emptying of the capsule and the deagglomeration of the active
ingredient or an active ingredient dispersion to be inhaled. All in all, an
almost full
emptying of the capsule can advantageously be achieved. The active ingredient
is also
swirled such that only some few powder residues are left in the air-conducting
parts of
the inhalation device and/or the capsule and the active ingredient and the
carrier
material are separated as extensively as possible.
The deagglomeration and/or dispersion are also correlated with a capsule
opening and
the dimensions and the design of the air-conducting components. All these
factors are
optimized according to the invention. Both the intense movement of the capsule
and
the dimensioning of the air-conducting parts ensure a maximum of turbulence in
the
inhalation device according to the invention. This, in turn, advantageously
effects
reproducible capsule emptying via a defined air flow.
The invention is now explained by means of preferred embodiments with
reference to
the enclosed exemplary figures. Members which correspond in the different
figures
bear the same reference numerals.
CA 02771443 2012-02-17
Figure I shows a longitudinal section of an inhalation device according to the
invention;
Figure 2a a longitudinal section of the upper part of the inhalation device of
figure
l;
Figure 2b shows a top view of the upper part of figure 2a from below;
Figure 3a shows a cross-sectional view of the lower part of the inhalation
device
of figure 1;
Figure 3b shows a top view of the lower part of figure 3a from above;
Figure 4 shows a cross-sectional view of the inhalation device according to
the
invention; and
Figure 5 shows a longitudinal section through a lower part of a further
inhalation
device according to the invention.
Positional designations used hereinafter, such as above, below, front, rear,
right and
left, relate to the view of the observer onto an inhalation device arranged in
front of
him, the mouthpiece pointing to the user and the upper part upwards.
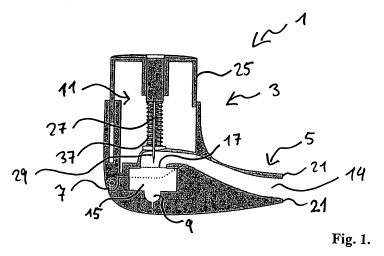
Figure 1 shows an inhalation device I according to the invention, which is
designed as
a capsule-based powder inhaler. The inhalation device 1 comprises an upper
part 3
and a lower part 5; both are connected to each other via a hinge arrangement
7. The
upper part 3 can be pivoted with respect to the lower part 5 between an open
position
(not shown) and a closed position.
In the open position, a user can insert a capsule in a swirl chamber 15 or
remove an
emptied capsule therefrom. The capsule contains an active ingredient or active
ingredients to be inhaled which can be combined with a carrier substance or
carrier
CA 02771443 2012-02-17
16
substances. An inserted capsule moves in the swirl chamber 15 via the air flow
caused
during the inhalation and rotates and/or vibrates. In the open position, all
components
are easily accessible and therefore it is easy to clean the inhalation device.
Figure 1 shows the inhalation device in its closed position. The upper part 3
comprises
a housing 23 in which a cutting device 11 is arranged. Prior to the
inhalation, the user
opens an inserted capsule by means of the cutting device 11 (see figures 2a,
2b and 3).
For this purpose, the cutting device 11 comprises an actuation member 25 which
is
here made as a pin-like button as well as two parallel cutting members 27
which are
made as knives in the embodiment as shown. The button 25 is linked with the
upper
part 3 via an attachment means, e.g. a screw or a pin. In addition, the
cutting device II
is provided with a spring member 37 which biases the button 25 and thus the
knives
27 into an initial position. The spring member can be any spring, e.g. a
compression or
spiral spring. In the initial position, the knives 27 are arranged within the
housing. 23.
In order to prevent the danger of injury in the open state, the upper part 3
can be
designed so as not to be fully pivotable but only to such an extent that a
removal or an
insertion of a capsule and/or a cleaning of the inhalation apparatus I is
easily possible.
Alternatively or additionally, a safety mechanism (not shown) can also be
provided
which prevents an actuation of a cutting apparatus 11 in the open position.
The knives 27 can be equipped with any blades 29, e.g. with a rounded, one-
sided,
double-chamfered blade having a radius of curvature of 1.28 cm; preferably
with a
straight, two-sided, double-chamfered blade and a blade angle of 80 each or
with a
straight, one-sided, double-chamfered blade and a blade angle of 67 or with a
straight, double-chamfered blade and a blade angle of 0 . Knives 27 including
a
straight, one-sided, single-chamfered or double-chamfered blade and a blade
angle of
35 have shown to be particularly suitable.
The knives 27 can also have any dimension. Knives having a width of 3 mm, a
thickness of 0.6 mm and a length of 35 mm have shown to be particularly
suitable.
CA 02771443 2012-02-17
17
In order to open a capsule, a user pushes the actuation button 25 and thus the
knives
27 against the spring force vertically downwards towards the lower part 5 and
thus to
the capsule into an operating position. For this purpose, two of the openings
adapted
to the blade size are formed in the bottom side of the upper part 3 through
which the
knives 27 move into the lower part 5 to a capsule inserted in the capsule
support 15.
The tips 33 of the blades 29 first strike the capsule surface of a capsule and
then cut
into and through both capsule ends.
The actuation button 25 is slidably guided by two guiding members 39 arranged
on
both sides. Any known guide mechanisms can be provided as the guidance. In the
embodiment shown herein (cf. figures 1 and 2a), the guide members are made as
slide
rails 39 between which the actuation button 25 is vertically slidable. Several
slide rails
39 can also be mounted on certain circumferential positions of the upper part
3. In the
embodiment shown herein, the slide rails 39 are formed circumferentially
around the
upper part 3. In this connection, the sleeve-like section of the actuation
button 25 is
enclosed by an outer slide rail 39 from outside by an inner slide rail 39 from
inside.
The slide rails 39 ensure an accurate guidance and prevent jamming of the
actuation
button 25. This in turn ensures a uniform accurate guidance of the anchored
knives 27.
Alternatively, the actuation button 25 can also comprise a means, such as a
spring,
which meshes with a spring guidance like a tongue and groove system.
The cutting angle is defined via the arrangement of the knives 27 and can be
made as
desired. Cutting angles of 90 , preferably 10 , more preferably 0 , based on
the
longitudinal axis of the capsule, are particularly preferred. While cutting
into a
capsule, the knives 27 move through the capsule beyond the lower end of the
capsule
and into two recesses 35 formed in the bottom side of the capsule support 9.
The
recesses 35 correspond to the knife size or can be made somewhat larger and
prevent a
contact of the tips 33 of the blades 29 with the lower part 5.
Having opened a capsule, the user releases the actuation button 25. The spring
37
exerts a force on the actuation button 25and moves the latter and thus the
knives 27
into the initial position. The knives 27 are then fully retracted into the
housing 23.
CA 02771443 2012-02-17
18
In addition, a further safety mechanism (not shown) can also be provided which
prevents an inhalation while the actuation button 25 is pressed down, e.g. a
blockage
of the air channels. An incorrect use of the inhalation device I or an
inappropriate
dosage can thus be avoided.
When the inhalation device I is closed, part of the bottom side of the upper
part 3
closes both an upper closure of the swirl chamber 15 and also partially the
top side of
an air discharge channel 14 in the mouthpiece 21 by means of a positive fit
with the
lower part 5. Since the rest of the air introduction channel 13 and/or the air
discharge
channel 14 are formed in the lower part 5, a seal of the air-conducting
channels 13 and
14 is ensured when the inhalation device 1 is closed. When the inhalation
device I is
closed, a connection between the swirl chamber 15 and the air discharge
channel 14 is
thus established.
In addition, another safety mechanism, e.g. a snap-action device (not shown)
can
secure the positive fit between the upper part 3 and the lower part 5. It also
prevents
an opening of the inhalation device I during an inhalation.
Alternatively, the air introduction channel 13 and/or the air discharge
channel 14 can
also fully be arranged and designed in the lower part 5 so that there is no
cover by the
upper part 3. The channels 13, 14 are then cleaned through the mouthpiece 21,
for
example.
Figures 3a, 3b and 4 show further details of the lower part 5. The air
introduction
channel 13, the capsule support 9 and the lower part of the swirl chamber 15
are
arranged and formed in the lower part 5. The dimensions of the swirl chamber
15 can
be made as desired. For example, a height of 7 to 9 mm has shown to be
suitable as
dimensions of the swirl chamber 15; with 8 mm being particularly suitable. The
capsule support 9 is formed in the bottom of the swirl chamber 15. The
dimensions of
the capsule support 9 are adapted to a capsule size such that the capsule can
be fixed
CA 02771443 2012-02-17
19
therein. For example, a capsule cannot move or slip during cutting and
therefore a
maximum reproducibility is ensured for the cut by the knives 27.
The air introduction channel 13 extends from the front side of the inhalation
device 1
laterally into the swirl chamber 15. Suitable cross-sectional areas of the air
introduction channel 13 are 11.8 mm2, for example. 17.7 mm2 or in particular
23.6
mm2 have shown to be particularly suitable.
The air inlet of the air introduction channel 13 can be arranged and designed
at any
positions in the area between the mouthpiece 21 and the capsule support 9 in
the lower
part. In the embodiment as shown, the air inlet of the air introduction
channel 13 is
formed between mouthpiece 21 and upper part 3. An arrangement coming from the
front side of the inhaler has the advantage that it is impossible for an
inhaling person
to close the air introduction channel 13 when the inhalation device 1 is
gripped and
held as usual.
In the case of an inhalation event, the air flow is introduced through the air
introduction channel 13 via an air inlet laterally into the lower region of
the round
swirl chamber 15. The air flow partially acts on the in particular lower end
of a
capsule which faces the air inlet and partially flows in circular fashion in
extension of
the air introduction channel 13 along the vertical outer curvature of the
swirl chamber
15. The air flow lifts a capsule and conveys it out of the capsule support 9
to the upper
area of the swirl chamber 15 where the air flow causes the capsule to vibrate
and
rotate. The clearance for the capsule in the capsule support 9 is large such
that it
cannot clamp or jam in any other way. The resulting suction ensures via the
rotary
motion and the vibration of the capsule the emptying of the powder into the
surrounding swirl chamber 15.
A grid 17 is arranged at the transition between swirl chamber 15 and air
discharge
channel 14. The grid 17 supports a powder fragmentation, on the one hand, and
hinders fragments which might form when the capsule is cut from entering the
air
discharge channel 14, on the other hand. The grid can have any dimensions. A
free
CA 02771443 2012-02-17
area of 51 mm2, more preferably 36 mm2, most preferably 21 mm2, and a ridge
width
of 1.30 mm, more preferably 0.86 mm, most suitably 0.42 mm, have proven
suitable.
The air discharge channel 14 leading to the mouthpiece 21 is arranged and
formed
such that it starts from the grid 17. The air discharge channel 14 extends
downstream
of the swirl chamber 15 vertically upwards. An arch-shaped transition area
follows
which extends to the mouthpiece 21. The air introduction channel 13 and the
air
discharge channel 14 thus extend at least partially parallel and in opposite
directions.
The air flow introduced through the air introduction channel 13 is sucked off
upwards
into the air discharge channel l4after flowing through the swirl chamber 15.
This is
where the air flow is deflected by almost 180 and is thus guided at least
partially
opposite to the air passage in the air introduction channel 13 through an arch-
shaped
area of the air discharge channel 14. The air flow here conveys an active
ingredient or
several active ingredients to the mouthpiece 21 where the air flow leaves the
inhalation device I and can be taken up in the lungs of a patient. The
vibrating and
rotating capsule is sucked upwards by the air flow and is lifted like an air
cushion
from below.
Moreover, the longitudinal area of the air discharge channel 14 tapers or
widens with
respect to its cross-section. At the transition from the swirl chamber 15 to
the air
discharge channel 14, the flow cross-section tapers so that there is an equal
volume
flow having a high flow rate during the inhalation. The cross-section widens
towards
the mouthpiece 21 so that the outflowing air flow comprises a high volume flow
with
low flow rate.
Figure 5 shows a longitudinal section through a lower part 5 of a further
inhalation
device I according to the invention with additional channels 41. The
additional
channels 41 partially extend parallel to the air discharge channel 14 and pass
an air
current from above circumferentially to the edge of the air discharge channel
14.
During an inhalation event, an additional air flow can be produced via the
additional
channels 41, said flow enclosing the air flow containing the swirled active
ingredients.
CA 02771443 2012-02-17
21
Thus, the described deagglomeration and swirling effects can even be improved
and
the active ingredient deposition at the oropharynx can be reduced.
Further embodiments and variations of the present invention follow for a
person
skilled in the art from the below claims.