Note: Descriptions are shown in the official language in which they were submitted.
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
1
Description
Stopper for sealing a compartment of a medicament container
The invention refers to a stopper for sealing a compartment of a medicament
container.
Medicament containers such as syringes or ampoules usually comprise a hollow
cylinder made of a pharmaceutical glass which is inert and chemically
resistant against
the drug stored inside, e.g. insulin. The container is sealed by a stopper or
bung at one
end of the cylinder which can be moved along the longitudinal axis of the
cylinder in
order to displace the drug and force it out of an outlet end which may be
sealed by a
pierceable membrane. The stopper and the pierceable membrane are
conventionally
made of an elastomere ensuring mechanical tightness under defined pressure
conditions and long term germ impermeability. Other important parameters
affecting the
dimensioning and choice of materials of the stopper and the pierceable
membrane are
the maximum force expected at the stopper and the number of allowable
piercings of
the pierceable membrane.
Before filling in the drug and sealing the container, the quality of the inner
surface of the
cylinder is improved by siliconization, so static and dynamic friction of the
stopper are
reduced.
DE 102 26 643 Al discloses a stopper for an injection arrangement, the stopper
comprising a stopper body, a stopper body support attached to a drive member
of the
injection arrangement and a sealing member for sealing a product container of
the
injection arrangement against the stopper body, wherein a membrane body is
arranged
in a cap-like manner at a proximal end of the stopper body wherein the sealing
member
is part of the membrane body. A sensor is provided for measuring a pressure
exerted by
the product on the proximal end of the membrane body. The sensor may be a
pressure
sensor.
US 2003/0125670 Al discloses a medicament cartridge comprising a cylinder and
a
displaceable plunger. The cartridge is provided with an electrical element
having
specified electrical properties located on an external face of the plunger.
The electrical
element can take the form of a conductive disk or two conductive rings joined
by a
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
2
resistive pad. The device may be equipped with electrical contacts for
contacting the
electrical element.
WO 01/56635 discloses a container for a substance, which container comprises a
coupling element for coupling the container with an administration unit for
the substance,
and a recognition element associated with the substance. The recognition
element may
be a bar code printed on a package, a chip card enclosed in the package or a
magnetic
card.
It is an object of the invention to provide an improved stopper for sealing a
compartment
of a medicament container.
The object is achieved by a stopper with the features of the independent claim
1 or 11.
Advantageous embodiments are given in the dependent claims.
According to one embodiment of the invention a microchip is embedded into a
stopper
for sealing a compartment of a medicament container. The microchip is arranged
for
storing data. The microchip comprises wireless communication means for
allowing the
stored data to be retrieved by an external wireless unit.
This allows for providing the medicament container with an ID upon
manufacturing in
order to make it tamper proof. For example an injector or pen, which the
medicament
container may be replaceably received in may be equipped with the external
wireless
unit in order to check the ID of the medicament container. Thus, medicament
containers,
authorized for usage may be distinguished from fake ones in order to avoid
injecting
poor quality medicaments, wrong medicaments or dangerous substances. The
injector
may be able to block movement of a plunger for advancing the stopper once an
error
such as a fake ID has been detected.
The data may be stored into the microchip upon production via the wireless
communication. The data may comprise time stamps for each manufacturing step
in
order to be able to retrace the production chain or the whole life cycle. The
injector may
thus detect whether the use-by date of the medicament is expired or not and
prevent an
expired medicament from being delivered to a patient.
The data may also comprise a country or regional or language information. This
allows
for automatically setting a display and menu language of a user interface of
the injector
depending on a destination market, the medicament container is intended for.
However,
the user may be given an option to overwrite the language setting.
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
3
The data may also comprise an information about the type of medicament stored
in the
medicament container thus allowing to use the injector for a variety of
medicaments.
The microchip may also store information on when and how often the medicament
container has been used for an injection in order retrace and/or control drug
compliance.
For example the injector may reject an injection once the daily dose has been
injected
already. Furthermore, the microchip may store information on a fill level of
the
medicament container and/or a number of doses already injected.
Preferably, at least one sensor connected to the microchip may be embedded in
the
stopper, wherein the microchip is arranged for storing data comprising
measurement
data acquired by the sensor. The data may be acquired continuously or time-
discrete.
The sensor may preferably include the function of a light-sensitive sensor.
According to
other embodiments, the sensor may include the function of a temperature sensor
or a
pressure sensor. Combinations of said functions included in one sensor are
also
possible.
The stored data may comprise manufacturing data and data related to ambient
and
stocking conditions such as a manufacturing date and time, temperature data
acquired
by a temperature sensor, an ambient pressure acquired by a pressure sensor,
light
exposure data or data related to a haze of a medicament inside the compartment
acquired by a light-sensitive sensor or a dose volume of the medicament
container
which is sealed by the stopper. Thus the whole life cycle of the medicament
container
comprising production, filling, storage and injection may be retraced. By
means of a
temperature sensor it may be retraced whether the cold chain was interrupted
or not.
The temperature and/or pressure may be the temperature and/or pressure inside
the
compartment or an ambient temperature and/or pressure.
The stored data may also comprise a pH-value acquired by a pH sensor. The pH
sensor
may be a potentiometry or ISFET sensor or a hydrogel based sensor.
The stored data may also comprise a chemical composition of the substance held
in the
compartment acquired by a chemical composition sensor.
In general, the sensor may use physical or chemical measurement methods.
Preferably the wireless communication means is an RFID means.
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
4
In case that the sensor includes the function of a light-sensitive sensor, it
is preferred
that the sensor is adapted and arranged to receive only ambient light while
light
stemming from inside the stopper may have no or only a negligible effect on
the sensor.
Provided that the medicament is affected mainly through ambient conditions and
not
through internal conditions within the stopper, this may enhance the desired
sensitivity
of the sensor.
According to another embodiment, the stopper is free of any kind of light
source which
allows for omitting specific measures to suppress internal light, for example
an opaque
material or surface of the stopper, and helps to save electric power.
Furthermore, additional features may be included in the function of the light
sensitive
sensor.
For example, an optoelectronic sensor may be included which is adapted to
measure
the incident light which may be in the visible, in the infrared or in the
ultraviolet range in
order to establish a profile according to the storing conditions and in order
to identify the
deterioration of the medicament.
In addition, an optoelectronic sensor for conversion of light into electric
energy may be
included as a power supply for the microchip present in the stopper.
In addition, an optoelectronic sensor for receiving information from other
devices, for
example other drug delivery devices or blood glucose meters may be included.
In addition, an optoelectronic sensor for receiving information according to
the bottling
of the ampoule for storing process-related data may be included.
In addition, an optoelectronic sensor may be included for the identification
of the
location and the position, respectively of the stopper within the ampoule or
relative to
other components of the injection device, for example of a piston rod.
The sensor may be integrated in or connected to the microchip. A microchip is
a cheap
and tiny component which may be unremarkably embedded into the stopper. The
microchip may comprise an antenna, an analogue circuit for transmitting and
receiving,
a digital circuit for processing data and a permanent memory. An energy
source, e.g. a
button cell for powering the microchip may be embedded into the stopper. The
energy
may also be provided by a coil, embedded into the stopper, which may be
excited by an
external exciting field.
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
According to another embodiment of the invention the stopper for sealing a
compartment of a medicament container has at least one surface coated with a
sensitive material which changes its visual appearance upon a change of an
ambient
5 condition or a condition inside the compartment. The change of the visual
appearance
may be a change of colour.
In particular, the compartment contains a medicament that is sensitive to a
change of an
ambient condition or a condition inside the compartment which means that the
change
is changing the medical properties of the medicament. The material of the
coating is
chosen such that it is also sensitive to the change. The sensitivity of the
material is
represented as a change of its visual appearance upon the change of condition
once
the change of condition occurs. A user may recognize the change of visual
appearance
and thus conclude that the medicament has been affected in view of its medical
properties. On the other hand, the sensitivity of the medicament and possibly
of the
container is not represented as a change of its visual appearance, which means
that the
change leaves the visual appearance of both the medicament and the container
unchanged. The user thus cannot conclude any affect to the medication when
watching
the medicament or the container alone.
For example, the medicament container may be exposed to a temperature of above
40 C and below 60 C. In this case, the temperature may be high enough to
start the
medicament to decompose although the decomposition is too weak to represent as
a
visible change of the medicament which would however be the case if the
temperature
would rise above 60 C. By means of the sensitive material which changes its
appearance already at 40 C the user recognizes the affection of the
medicament.
Preferably, in order to enhance the handling comfort for the user, the
sensitive material
is arranged on the stopper in such a way that it is permanently visible.
The condition, upon which the visual appearance changes, may be a temperature,
a
pressure, a light exposure, e.g. a cumulative light exposure, a haze of a
substance, e.g.
medicament stored in the compartment or an exposure to a chemical composition
or pH
value of the medicament.
Both embodiments allow for monitoring and retracing manufacturing and stocking
conditions of the medicament container. This may help a patient or healthcare
professional to determine whether the shelf life of the medicament container
has been
expired or not.
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
6
Furthermore, the change of visual appearance of the coating could be triggered
by a
chemical reaction between the coating and some sort of humidity or oxygen from
the
ambient air in order to recognize leakage or in order to indicate the storage
time of the
ampoule. For example a stopper in a freshly filled container may appear in
green colour
while the same stopper may appear in red colour when the medicament is
subsided due
to humidity or oxygen impact.
Furthermore, the coating may be sensitive to electromagnetic fields.
Generally, the effect of thermochromism may be used for the coating.
Thermochromism
is the ability of certain substances to change colour due to a change in
temperature.
This process is reversible, i.e. after cooling down the substance reverts to
its initial
colour. As an example, temperature indicating films may be used. These films
may
comprise self-adhesive laminas which are coated with a temperature-sensitive
layer,
whose colour changes from bright to dark when a certain temperature has been
reached. The colour change is reversible. The relevant error range of such an
indicator
may lie between 1 to 2 % of the imprinted value. Such films can be used for
a
measuring range of between 40 to 250 C.
For example, the indicator bromothymol blue being is embedded in a pH-
dependent
polymer matrix, can have thermochromic properties under certain conditions.
The matrix
changes the pH-value with a change in temperature, thus effecting a color
change of
the indicator. The polymer matrix, which contains lithium chloride, is green
within a
temperature range of between -5 to +33 C and changes its colour to yellow
when the
maximum of the given temperature range is exceeded.
There are also thermochromic colours, whose colour change is irreversible. In
these
materials, exceeding a certain temperature results in a chemical change, e.g.
oxidation
prompted by oxygen, or a change in structure, e.g. melting.
Furthermore, temperature measuring colors may be used. These colours are also
known as thermochromatic colors or thermochromic colors and show a change in
temperature by a change in colour or a change in the colour tone.
The pigments of those colours are complex salt compounds. A change in
temperature
results for example in a colour change from white to green or from black to
turquoise.
Furthermore, photochromic colours may be used. Such colours show a reversible
change in the colour tone when they are exposed to ultraviolet light, e.g. sun
light or
black light. The ultraviolet light changes the chemical structure of the
colours and thus
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
7
their absorption properties. In contrast to ordinary pigments, which reflect
part of the
light, photochromic colors absorb part of the light and allow the rest of the
light to pass
through. The remaining light is reflected from the background, in particular a
bright,
preferably white, background, and the material appears coloured.
The basic materials for the production of photochromic colours are available
in the form
of powder, microcapsules, and nurdles. There are four basic colours available,
which
change their colour from white to purple, blue, yellow, or red.
The stopper according to either embodiment can comprise the same materials as
conventional stoppers, such as elastomeres. The primary packaging, i.e. the
glass
cylinder of the medicament container may remain unchanged. Design
modifications of
the ampoule or the injection device are not required.
The features of the embodiments may be combined with each other, i.e. the
plunger
may comprise both the microchip and the sensitive coating.
E.g. the stopper may be applied in an insulin pen injector, both for faster or
slower
reacting drugs. Preferably, the use of the stopper is restricted to the
purpose of testing
the injector or the injection device.
The injector may be a mechanical injector equipped with display means for
allowing a
user to check information encoded in the stored data. Preferably the injector
is an
electromechanical injector which may process the data and take appropriate
action
such as blocking an injection or restricting a dose depending on the data.
The term "medicament" and the term "drug", as used herein, preferably means a
pharmaceutical formulation containing at least one pharmaceutically active
compound,
wherein in one embodiment the pharmaceutically active compound has a molecular
weight up to 1500 Da and/or is a peptide, a proteine, a polysaccharide, a
vaccine, a
DNA, a RNA, an enzyme, an antibody, a hormone or an oligonucleotide, or a
mixture of
the above-mentioned pharmaceutically active compound,
wherein in a further embodiment the pharmaceutically active compound is useful
for the
treatment and/or prophylaxis of diabetes mellitus or complications associated
with
diabetes mellitus such as diabetic retinopathy, thromboembolism disorders such
as
deep vein or pulmonary thromboembolism, acute coronary syndrome (ACS), angina,
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
8
myocardial infarction, cancer, macular degeneration, inflammation, hay fever,
atherosclerosis and/or rheumatoid arthritis,
wherein in a further embodiment the pharmaceutically active compound comprises
at
least one peptide for the treatment and/or prophylaxis of diabetes mellitus or
complications associated with diabetes mellitus such as diabetic retinopathy,
wherein in a further embodiment the pharmaceutically active compound comprises
at
least one human insulin or a human insulin analogue or derivative, glucagon-
like
peptide (GLP-1) or an analogue or derivative thereof, or exedin-3 or exedin-4
or an
analogue or derivative of exedin-3 or exedin-4.
Insulin analogues are for example Gly(A21), Arg(B31), Arg(B32) human insulin;
Lys(B3),
Glu(B29) human insulin; Lys(B28), Pro(B29) human insulin; Asp(B28) human
insulin;
human insulin, wherein proline in position B28 is replaced by Asp, Lys, Leu,
Val or Ala
and wherein in position B29 Lys may be replaced by Pro; Ala(B26) human
insulin;
Des(B28-B30) human insulin; Des(B27) human insulin and Des(B30) human insulin.
Insulin derivates are for example B29-N-myristoyl-des(B30) human insulin; B29-
N-
palmitoyl-des(B30) human insulin; B29-N-myristoyl human insulin; B29-N-
palmitoyl
human insulin; B28-N-myristoyl LysB28ProB29 human insulin; B28-N-palmitoyl-
LysB28ProB29 human insulin; B30-N-myristoyl-ThrB29LysB30 human insulin; B30-N-
palmitoyl- ThrB29LysB30 human insulin; B29-N-(N-palmitoyl-Y-glutamyl)-des(B30)
human insulin; B29-N-(N-lithocholyl-Y-glutamyl)-des(B30) human insulin; B29-N-
(w-
carboxyheptadecanoyl)-des(B30) human insulin and B29-N-(w-
carboxyheptadecanoyl)
human insulin.
Exendin-4 for example means Exendin-4(1-39), a peptide of the sequence H-His-
Gly-
Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-
Phe-
Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-N H2.
Exendin-4 derivatives are for example selected from the following list of
compounds:
H-(Lys)4-des Pro36, des Pro37 Exendin-4(1-39)-NH2,
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
9
H-(Lys)5-des Pro36, des Pro37 Exendin-4(1-39)-NH2,
des Pro36 [Asp28] Exendin-4(1-39),
des Pro36 [IsoAsp28] Exendin-4(1-39),
des Pro36 [Met(O)14, Asp28] Exendin-4(1-39),
des Pro36 [Met(O)14, IsoAsp28] Exendin-4(1-39),
des Pro36 [Trp(02)25, Asp28] Exendin-4(1-39),
des Pro36 [Trp(02)25, IsoAsp28] Exendin-4(1-39),
des Pro36 [Met(O)14 Trp(02)25, Asp28] Exendin-4(1-39),
des Pro36 [Met(O)14 Trp(02)25, IsoAsp28] Exendin-4(1-39); or
des Pro36 [Asp28] Exendin-4(1-39),
des Pro36 [IsoAsp28] Exendin-4(1-39),
des Pro36 [Met(O)14, Asp28] Exendin-4(1-39),
des Pro36 [Met(O)14, IsoAsp28] Exendin-4(1-39),
des Pro36 [Trp(02)25, Asp28] Exendin-4(1-39),
des Pro36 [Trp(02)25, IsoAsp28] Exendin-4(1-39),
des Pro36 [Met(O)14 Trp(02)25, Asp28] Exendin-4(1-39),
des Pro36 [Met(O)14 Trp(02)25, IsoAsp28] Exendin-4(1-39),
wherein the group -Lys6-NH2 may be bound to the C-terminus of the Exendin-4
derivative;
or an Exendin-4 derivative of the sequence
H-(Lys)6-des Pro36 [Asp28] Exendin-4(1-39)-Lys6-NH2,
des Asp28 Pro36, Pro37, Pro38Exendin-4(1-39)-NH2,
H-(Lys)6-des Pro36, Pro38 [Asp28] Exendin-4(1-39)-NH2,
H-Asn-(GIu)5des Pro36, Pro37, Pro38 [Asp28] Exendin-4(1-39)-NH2,
des Pro36, Pro37, Pro38 [Asp28] Exendin-4(1-39)-(Lys)6-NH2,
H-(Lys)6-des Pro36, Pro37, Pro38 [Asp28] Exendin-4(1-39)-(Lys)6-NH2,
H-Asn-(GIu)5-des Pro36, Pro37, Pro38 [Asp28] Exendin-4(1-39)-(Lys)6-NH2,
H-(Lys)6-des Pro36 [Trp(02)25, Asp28] Exendin-4(1-39)-Lys6-NH2,
H-des Asp28 Pro36, Pro37, Pro38 [Trp(02)25] Exendin-4(1-39)-NH2,
H-(Lys)6-des Pro36, Pro37, Pro38 [Trp(02)25, Asp28] Exendin-4(1-39)-NH2,
H-Asn-(GIu)5-des Pro36, Pro37, Pro38 [Trp(02)25, Asp28] Exendin-4(1-39)-NH2,
des Pro36, Pro37, Pro38 [Trp(02)25, Asp28] Exendin-4(1-39)-(Lys)6-NH2,
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
H-(Lys)6-des Pro36, Pro37, Pro38 [Trp(02)25, Asp28] Exendin-4(1-39)-(Lys)6-
NH2,
H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Trp(02)25, Asp28] Exendin-4(1-39)-(Lys)6-
NH2,
H-(Lys)6-des Pro36 [Met(O)14, Asp28] Exendin-4(1-39)-Lys6-NH2,
des Met(O)14 Asp28 Pro36, Pro37, Pro38 Exendin-4(1-39)-NH2,
5 H-(Lys)6-desPro36, Pro37, Pro38 [Met(O)14, Asp28] Exendin-4(1-39)-NH2,
H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Met(O)14, Asp28] Exendin-4(1-39)-NH2,
des Pro36, Pro37, Pro38 [Met(O)14, Asp28] Exendin-4(1-39)-(Lys)6-NH2,
H-(Lys)6-des Pro36, Pro37, Pro38 [Met(O)14, Asp28] Exendin-4(1-39)-(Lys)6-NH2,
H-Asn-(Glu)5 des Pro36, Pro37, Pro38 [Met(O)14, Asp28] Exendin-4(1-39)-(Lys)6-
NH2,
10 H-Lys6-des Pro36 [Met(O)14, Trp(02)25, Asp28] Exendin-4(1-39)-Lys6-NH2,
H-des Asp28 Pro36, Pro37, Pro38 [Met(O)14, Trp(02)25] Exendin-4(1-39)-NH2,
H-(Lys)6-des Pro36, Pro37, Pro38 [Met(O)14, Asp28] Exendin-4(1-39)-NH2,
H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Met(O)14, Trp(02)25, Asp28] Exendin-4(1-
39)-
NH2,
des Pro36, Pro37, Pro38 [Met(O)14, Trp(02)25, Asp28] Exendin-4(1-39)-(Lys)6-
NH2,
H-(Lys)6-des Pro36, Pro37, Pro38 [Met(O)14, Trp(02)25, Asp28] Exendin-4(S1-39)-
(Lys)6-NH2,
H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Met(O)14, Trp(02)25, Asp28] Exendin-4(1-
39)-
(Lys)6-NH2;
or a pharmaceutically acceptable salt or solvate of any one of the afore-
mentioned
Exedin-4 derivative.
Hormones are for example hypophysis hormones or hypothalamus hormones or
regulatory active peptides and their antagonists as listed in Rote Liste, ed.
2008,
Chapter 50, such as Gonadotropine (Follitropin, Lutropin, Choriongonadotropin,
Menotropin), Somatropine (Somatropin), Desmopressin, Terlipressin,
Gonadorelin,
Triptorelin, Leuprorelin, Buserelin, Nafarelin, Goserelin.
A polysaccharide is for example a glucosaminoglycane, a hyaluronic acid, a
heparin, a
low molecular weight heparin or an ultra low molecular weight heparin or a
derivative
thereof, or a sulphated, e.g. a poly-sulphated form of the above-mentioned
polysaccharides, and/or a pharmaceutically acceptable salt thereof. An example
of a
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
11
pharmaceutically acceptable salt of a poly-sulphated low molecular weight
heparin is
enoxaparin sodium.
Pharmaceutically acceptable salts are for example acid addition salts and
basic salts.
Acid addition salts are e.g. HCI or HBr salts. Basic salts are e.g. salts
having a cation
selected from alkali or alkaline, e.g. Na+, or K+, or Ca2+, or an ammonium ion
N+(R1)(R2)(R3)(R4), wherein R1 to R4 independently of each other mean:
hydrogen,
an optionally substituted C1-C6-alkyl group, an optionally substituted C2-C6-
alkenyl
group, an optionally substituted C6-C10-aryl group, or an optionally
substituted C6-C10-
heteroaryl group. Further examples of pharmaceutically acceptable salts are
described
in "Remington's Pharmaceutical Sciences" 17. ed. Alfonso R. Gennaro (Ed.),
Mark
Publishing Company, Easton, Pa., U.S.A., 1985 and in Encyclopedia of
Pharmaceutical
Technology.
Pharmaceutically acceptable solvates are for example hydrates.
Further scope of applicability of the present invention will become apparent
from the
detailed description given hereinafter. However, it should be understood that
the
detailed description and specific examples, while indicating preferred
embodiments of
the invention, are given by way of illustration only, since various changes
and
modifications within the spirit and scope of the invention will become
apparent to those
skilled in the art from this detailed description.
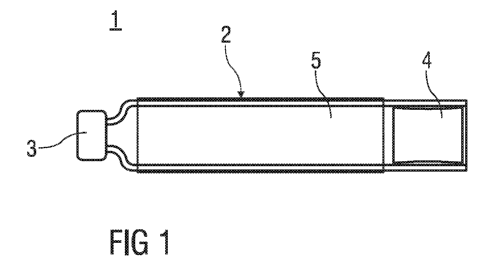
Figure 1 is a conventional art medicament container with a glass cylinder
sealed by
a pierceable membrane and a stopper,
Figure 2 is a stopper with a microchip and sensor according to one embodiment
of
the invention, and
Figure 3 is a stopper coated with sensitive materials according to another
embodiment of the invention.
Figure 1 is a conventional art medicament container 1 with a hollow cylinder 2
sealed by
a pierceable membrane 3 and a stopper 4. The pierceable membrane 3 and the
stopper
4 define a compartment 5 between for holding a substance, e.g. a medicament.
The
cylinder 2 may consist of glass. The stopper 4 can be moved along a
longitudinal axis of
the cylinder 2 in order to displace the medicament and force it out of an
outlet provided
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
12
the pierceable membrane 3 is pierced. The stopper 4 and the pierceable
membrane 3
may be made of an elastomere. The medicament container may have a label
indicating
its content, e.g. insulin.
Figure 2 is a stopper 4 with an embedded microchip 6 according to one
embodiment of
the invention. The microchip 6 comprises at least one sensor (not shown). The
microchip 6 is arranged for storing data comprising measurement data acquired
by the
sensor. The data may be acquired continuously or time-discrete. The microchip
6
further comprises wireless communication means (not shown) for allowing the
stored
data to be retrieved by an external wireless unit (not shown). The external
wireless unit
may be arranged in an injection device where the medicament container 1 may be
received as a replaceable cartridge.
The stored data may comprise manufacturing data and data related to ambient
and
stocking conditions such as a manufacturing date and time, temperature data
acquired
by a temperature sensor, an ambient pressure acquired by a pressure sensor,
light
exposure data or data related to a haze of a medicament inside the compartment
acquired by a light-sensitive sensor or a dose volume of the medicament
container 1
which is sealed by the stopper 4.
The temperature and/or pressure may be the temperature and/or pressure inside
the
compartment 5 or an ambient temperature and/or pressure.
The stored data may also comprise a pH-value acquired by a pH sensor. The pH
sensor
may be a potentiometry or ISFET sensor or a hydrogel based sensor.
The stored data may also comprise a chemical composition of the substance held
in the
compartment 5 acquired by a chemical composition sensor.
In general, the sensor may use physical or chemical measurement methods.
Preferably the wireless communication means is an RFID means.
The stopper 4 may have an embedded microchip 6 without a sensor just for
storing data.
The data may comprise an ID and/or time stamps for different production or
life cycle
steps.
The data may also comprise a country or regional or language information.
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
13
Furthermore the data may comprise information about the type of medicament
stored in
the medicament container 1.
An energy source, e.g. a button cell for powering the microchip 6 may be
embedded
into the stopper 4. The energy may also be provided by a coil embedded into
the
stopper 4, which may be excited by an external exciting field.
The microchip 6 may also store information on when and how often the
medicament
container 1 has been used for an injection. Furthermore, the microchip 6 may
store
information on a fill level of the medicament container 1 and/or a number of
doses
already injected.
Figure 3 shows a stopper 4, whose surfaces 7.1, 7.2, 7.3 are coated with
sensitive
materials according to another embodiment of the invention. The sensitive
material
changes its visual appearance upon a change of an ambient condition or a
condition
inside the compartment 5. The change of the visual appearance may be a change
of
colour.
The condition, upon which the visual appearance changes, may be a temperature,
a
pressure, a light exposure, e.g. a cumulative light exposure, a haze of a
substance, e.g.
medicament stored in the compartment 5 or an exposure to a chemical
composition or
pH value of the medicament.
The stopper 4 may be applied in an insulin pen injector or in another
injection device,
e.g. for injecting one of an analgetic, an anticoagulant, insulin, an insulin
derivate,
heparin, Lovenox, a vaccine, a growth hormone, a peptide hormone, a proteine
and
complex carbohydrates.
There may be more than one compartment 5 and more than one stopper 4 in a
medicament container 1, e.g. in an injector where two or more substances have
to be
stored separately but mixed prior to use.
The features of the embodiments of figures 2 and 3 may be combined with each
other,
i.e. the stopper 4 may comprise both the microchip 6 and the sensitive
coating.
CA 02772582 2012-02-28
WO 2011/032956 PCT/EP2010/063510
14
Reference numerals
1 Medicament container
2 Cylinder
3 Pierceable membrane
4 Stopper
5 Compartment
6 Microchip
7.1, 7.2, 7.3 surface