Note: Descriptions are shown in the official language in which they were submitted.
CA 02773584 2014-07-30
PROCESS AND APPARATUS FOR PRODUCING
HYDROCARBON FUEL AND COMPOSITION
[0001]
FIELD OF THE INVENTION
[0002] The present invention relates to a process and apparatus for
preparing
hydrocarbon fuel by slurry hydrocracking (SHC) and solvent deasphalting (SDA).
DESCRIPTION OF RELATED ART
[0003] As the reserves of conventional crude oils decline, heavy oils must
be upgraded to
meet demands. In upgrading, the heavier materials are converted to lighter
fractions and most
of the sulfur, nitrogen and metals must be removed. Crude oil is typically
first processed in an
atmospheric crude distillation tower to provide fuel products including
naphtha, kerosene and
diesel. The atmospheric crude distillation tower bottoms stream is typically
taken to a
vacuum distillation tower to obtain vacuum gas oil (VGO) that can be feedstock
for an FCC
unit or other uses. VGO typically boils in a range between at or 300 C (572 F)
and at or
524 C (975 F).
[0004] SHC is used for the primary upgrading of heavy hydrocarbon
feedstocks obtained
from the distillation of crude oil, including hydrocarbon residues or gas oils
from atmospheric
column or vacuum column distillation. In SHC, these liquid feedstocks are
mixed with
hydrogen and solid catalyst particles, e.g., as a particulate metallic
compound such as a metal
sulfide, to provide a slurry phase. Representative SHC processes are
described, for example,
in US 5,755,955 and US 5,474,977. SHC produces naphtha, diesel, gas oil such
as VGO, and
a low-value, refractory pitch stream. The VGO streams are typically further
refined in
catalytic hydrocracking or fluid catalytic cracking (FCC) to provide saleable
products. To
prevent excessive coking in the SHC reactor, heavy VGO (HVGO) can be recycled
to the
SHC reactor.
- 1 -
CA 02773584 2014-07-30
[0005] SDA generally refers to refinery processes that upgrade
hydrocarbon fractions
such as mentioned above using extraction in the presence of a solvent. SDA
permits practical
recovery of heavier oil, at relatively low temperatures, without cracking or
degradation of
heavy hydrocarbons. SDA separates hydrocarbons according to their solubility
in a liquid
solvent, as opposed to volatility in distillation. Lower molecular weight and
more paraffinic
components are preferentially extracted. The least soluble materials are high
molecular
weight and most polar aromatic components.
[0006] Gas turbines have many uses including aviation propulsion,
power generation and
marine propulsion. As gas turbine material technology has evolved, the
combustion section
temperature has increased several hundred degrees, allowing for vast
efficiency
improvements in the Brayton cycle. The highest efficiency gas turbines can
have a hot
section operating at above 1093 C (2000 F) and therefore have cycle
efficiencies much
higher than older generation turbines. Higher efficiency gas turbines have
created a need for
tighter fuel specifications.
[0007] According to the article, Svensson, DNV APPROVES SIEMENS GAS TURBINE
FOR
HFO, 61 Royal Belgian Institute of Marine Engineers 55 (2007), a 17 MW Type
SGT-500
gas turbine successfully underwent a comprehensive test using a fuel oil
meeting IFO 180
specification and received DNV (Det Norske Veritas) approval from the
Norwegian
government for marine applications. At the time of the article, the IF0180
heavy fuel oil was
$US 200-250 cheaper than the medium distillate oil typically burned in
shipboard gas
turbines. The IFO 180 specification is also known as the RME 180 specification
applicable
to residual marine fuels used in non-turbine engines such as low-RPM diesel
engines
commonly found in marine systems.
[0008] There is a need for such fuel, because turbines are more
efficient than many other
power sources for generating electricity in small to medium-sized applications
such as for
peaking power for electric power grids, marine propulsion for fast ships such
as ferries,
military transport and other applications. Cogeneration facilities which
recover the waste
heat of the turbine to make steam or provide other low-level heat are other
examples of
systems which achieve high overall cycle efficiency but require fuel that is
suitable for the
turbine.
[0009] Many previous efforts have made a suitable gas turbine fuel from
a low value
hydrocarbon residue. One process involved hydroprocessing petroleum residue in
which the
- 2 -
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
conditions are adjusted to remove only a small portion of the sulfur and
nitrogen but most of
the metals over a demetallation catalyst in a "polishing process". An example
of this process
is known as GEFINERY of Japan Gasoline Corporation. The cost of this process
has been
considered unjustifiably high based on the limited upgrading margin.
[0010] Other processes propose to valorize residue from coal dissolution or
"solvent-
refined" coal products by hydroprocessing to produce a vacuum distillate.
Examples of this
process are the SRC (solvent refined coal) process and Hypercoal process of
Japan New
Energy Development Organization. In another process, residual petroleum is
subjected to
SDA, wherein the yield of deasphalted oil (DAO) is kept relatively low to
avoid pulling any
organometallic compounds into the DAO. A last process combines SDA with
downstream
purification or hydroprocessing of the DAO to remove metals. These three
process examples
have been considered disadvantageous due to their limited ability to produce
suitable fuel
meeting applicable specifications.
[0011] The special fuel that is the subject of this invention would be
less expensive to
produce than the typical marine diesel oil or kerosene. Even accounting for
the need for
downstream pollution control to remove SOx and NOx from the exhaust, it would
be
advantageous to burn such fuel in turbines.
[0012] There is an ongoing need for hydrocarbon fuel compositions that
can be
inexpensively made and be used in gas turbines and in marine engines.
SUMMARY OF THE INVENTION
[0013] In an exemplary embodiment, the present invention involves a
process for making
hydrocarbon fuel comprising slurry hydrocracking a heavy feed to provide
slurry
hydrocracked products. The slurry hydrocracked products are separated to
provide a pitch
stream and a HVGO stream. At least a portion of the pitch stream is mixed with
a solvent to
dissolve a portion of the pitch in the solvent. The dissolved portion of the
pitch is blended
with at least a portion of the HVGO stream to provide a blended product. In
aspect, the
blended product comprises no more than 5 wppm sodium, no more than 50 wppm
vanadium
and at least 80 vol-% of the blended product boils at a temperature at or
above 426 C
(800 F).
[0014] In another exemplary embodiment, the present invention involves a
process for
making hydrocarbon fuel comprising slurry hydrocracking a heavy feed to
provide slurry
- 3 -
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
hydrocracked products. The slurry hydrocracked products are separated to
provide a pitch
stream and a HVGO stream. At least a portion of the pitch stream is solvent
deasphalted to
provide DAO. The DA0 is blended with at least a portion of the HVGO stream to
provide a
blended product.
[0015] In a further exemplary embodiment, the present invention involves an
apparatus
for making hydrocarbon fuel comprising a slurry hydrocracking reactor for
reacting heavy
feed and hydrogen over catalyst to produce slurry hydrocracked products. A
fractionation
section in communication with the slurry hydrocracking reactor fractionates at
least a portion
of the slurry hydrocracked products. The fractionation section has a side or
HVGO outlet for
emitting a HVGO stream and a bottom or pitch outlet for emitting a pitch
stream. An SDA
column in communication with the pitch outlet produces a DA0 stream emitted
from a DA0
outlet. A vessel or line in communication with the side outlet and the DA0
outlet blends at
least portions of the HVGO stream and the DA0 stream.
[0016] In a still further exemplary embodiment, the apparatus comprises
a separator for
separating hydrogen from slurry hydrocracked products in communication with
the SHC
reactor.
[0017] In an even additional exemplary embodiment, the fractionation
section of the
apparatus also comprises a side outlet for emitting a diesel stream and a side
outlet for
emitting a light VG0 (LVGO) stream.
[0018] In an even further exemplary embodiment the present invention
involves a
hydrocarbon composition comprising no less than 73 wt-% aromatics, no more
than 5 wt-%
heptane insolubles and no more than 50 wppm vanadium. At least 80 vol-% of the
composition boils at a temperature above 426 C (800 F). In other aspects, the
composition
may comprise no less than 75 wt-% aromatics, may comprise no more than 5 wt-%
hexane
insolubles or no more than 5 wt-% pentane insolubles. In another aspect, at
least 90 vol-% of
the composition boils at a temperature above 426 C. In another aspect, the
composition has
no more than 30 wppm or no more than 10 wppm vanadium. In a further aspect,
the
composition has a viscosity of no greater than 180 Cst at 50 C. In a still
further aspect, the
composition has no more than 5 wppm sodium.
[0019] These and other aspects and embodiments relating to the present
invention are
apparent from the Detailed Description.
- 4 -
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
DEFINITIONS
[0020] The term "aromatic" means a substance comprising a ring-
containing molecule as
determined by ASTM D 2549.
[0021] The term "communication" means that material flow is operatively
permitted
between enumerated components.
[0022] The term "downstream communication" means that at least a
portion of material
flowing to the subject in downstream communication may operatively flow from
the object
with which it communicates.
[0023] The term "upstream communication" means that at least a portion
of the material
flowing from the subject in upstream communication may operatively flow to the
object with
which it communicates.
[0024] As used herein, the term "boiling point temperature" means
atmospheric
equivalent boiling point (AEBP) as calculated from the observed boiling
temperature and the
distillation pressure, as calculated using the equations furnished in ASTM
D1160 appendix
A7 entitled "Practice for Converting Observed Vapor Temperatures to
Atmospheric
Equivalent Temperatures".
[0025] As used herein, "pitch" means the hydrocarbon material boiling
above 538 C
(975 F) AEBP as determined by any standard gas chromatographic simulated
distillation
method such as ASTM D2887, D6352 or D7169, all of which are used by the
petroleum
industry.
[0026] As used herein, "pitch conversion" means the conversion of
materials boiling
above 524 C (975 F) converting to material boiling at or below 524 C (975 F).
[0027] As used herein, "heavy vacuum gas oil" means the hydrocarbon
material boiling
in the range between 427 C (800 F) and 538 C (975 F) AEBP as determined by any
standard
gas chromatographic simulated distillation method such as ASTM D2887, D6352 or
D7169,
all of which are used by the petroleum industry.
[0028] As used herein, solvent "insolubles" means materials not
dissolving in the solvent
named.
[0029] The term "liquid hourly space velocity" means the volumetric
flow rate of liquid
feed per reactor volume, wherein the volume is referenced to a standard
temperature of 16 C.
- 5 -
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
BRIEF DESCRIPTION OF THE DRAWING
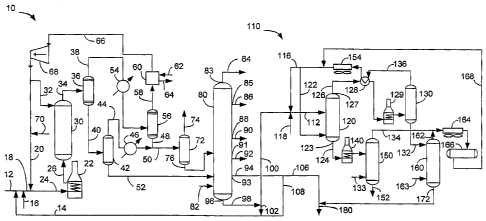
[0030] The FIGURE is a schematic view of a process and apparatus of the
present
invention.
DETAILED DESCRIPTION
[0031] Slurry hydrocracking enables conversion of up to 80-95 wt-% of many
low value
vacuum bottoms streams to 524 C (975 F) and lighter distillate and a small
quantity of pitch.
The toluene soluble portion of SHC product that boils at 524 C (975 F) or
higher has
relatively low molecular weight, such as 700-900 as measured by vapor pressure
osmometry
per ASTM D 2503, and is contaminated with some nickel and vanadium. Slurry
hydrocracking over iron-based catalysts at pressures below 20.7 MPa (3000
psig) has limited
ability to open metalloporphyrinic rings. Surprisingly, it was learned that
the pentane soluble
portion of the pitch residue boiling over 524 C from slurry hydrocracking over
iron-based
catalyst at conversions above 80 wt-% contains very low concentrations of
nickel and
vanadium. This is in contrast to solvent-deasphalted straight run oils which
contain
substantial amounts of soluble organometallic nickel and vanadium and would
not be
possible to run in the latest generation turbines. These metals-laden fuels
could only be
possibly run in cooler turbines using certain techniques such as metal
passivating additives
and offline water wash to remove blade deposits.
[0032] Also, it was learned that the heaviest portions of the vacuum
gas oil distillate
boiling in the range of 426-524 C (800-975 F) atmospheric equivalent boiling
point known
as HVGO produced by slurry hydrocracking 524+ C residue over iron-based
catalyst at
conversions above 80 wt-% contains no measureable nickel and vanadium. This
material also
contains some paraffins in the C30-C45 range as well as multi-ring aromatics
and
heteroatomic material. This material has excellent fuel properties and is
pourable at room
temperature. The lighter portion of the vacuum gas oil distillate boiling in
the range of
343-426 C (650-800 F) atmospheric equivalent boiling point known as LVGO from
slurry
hydrocracking are suitable for direct burning as turbine fuel, but often it
will be desired to
upgrade this oil in further processing to naphtha and diesel to better
valorize the stream.
[0033] Accordingly, HVGO and solvent-deasphalted pitch obtained from
SHC may be
blended together to provide a hydrocarbon fuel that meets the RME 180 and the
IFO 180 fuel
- 6 -
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
specification. Hence, the hydrocarbon fuel may be burned in gas turbines and
in marine
engines without need of further upgrading. The special composition of
hydrocarbon fuel
made by the process and apparatus of this invention may be used as-such or in
blends with
other fuels either in bulk or blended at the point of use.
[0034] Embodiments of the invention relate to slurry hydrocracking a heavy
hydrocarbon
feedstock for primary upgrading into fuel. According to one embodiment, for
example, the
heavy hydrocarbon feedstock comprises a vacuum column residue. Representative
further
components of the heavy hydrocarbon feedstock include residual oils boiling
above 566 C
(1050 F), tars, bitumen, coal oils, and shale oils. Bitumen is also known as
natural asphalt, tar
sands or oil sands. Bitumen has been defined as rock containing hydrocarbons
more viscous
than 10,000 Cst or such hydrocarbons that may be extracted from mined or
quarried rock.
Some natural bitumens are solids, such as gilsonite, grahamite, and ozokerite,
which are
distinguished by streak, fusibility, and solubility. Other asphaltene-
containing materials may
also be used as components processed by SHC. In addition to asphaltenes, these
further
possible components of the heavy hydrocarbon feedstock, among other
attributes, generally
also contain significant metallic contaminants, e.g., nickel, iron and
vanadium, a high content
of organic sulfur and nitrogen compounds, and a high Conradson carbon residue.
The metals
content of such components, for example, may be in the range of 100 ppm to
1,000 ppm by
weight, the total sulfur content may range from 1 to 7 wt-%, and the API
gravity may range
from -5 to 35 . The Conradson carbon residue of such components is generally
at least 5
wt-%, and is often from 10 to 30 wt-%.
[0035] As shown in the FIGURE, the present invention for converting
heavy
hydrocarbons to hydrocarbon fuels is exemplified by a SHC unit 10 and a
solvent
deasphalting unit110.
[0036] The heavy feed stream in line 12 is presented as feed to the SHC
unit 10 as shown
in the FIGURE. A heavy product recycle in line 14 may be mixed with the heavy
feed
stream 12. A coke-inhibiting additive or catalyst of particulate material in
line 16 is mixed
together with the feed stream in line 12 to form a homogenous slurry. A
variety of solid
catalyst particles can be used as the particulate material. Particularly
useful catalyst particles
are those described in US 4,963,247. Thus, the particles are typically ferrous
sulfate having
particle sizes less than 45 [tm and with a major portion, i.e. at least 50% by
weight, in an
aspect, having particle sizes of less than 10 lam. Iron sulfate monohydrate is
a preferred
- 7 -
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
catalyst. Bauxite catalyst may also be preferred. In an aspect, 0.01 to 4.0 wt-
% of coke-
inhibiting catalyst particles based on fresh feedstock are added to the feed
mixture. Oil
soluble coke-inhibiting additives may be used alternatively or additionally.
Oil soluble
additives include metal naphthenate or metal octanoate, in the range of 50 to
1000 wppm
based on fresh feedstock with molybdenum, tungsten, ruthenium, nickel, cobalt
or iron.
[0037] This slurry of catalyst and heavy hydrocarbon feed in line 18
may be mixed with
hydrogen in line 20 and transferred into a fired heater 22 via line 24. The
combined feed is
heated in the heater 22 flows through an inlet line 26 into an inlet in the
bottom of the tubular
SHC reactor 30. In the heater 22, iron-based catalyst particles newly added
from line 16
typically convert to forms of iron sulfide which are catalytically active.
Some of the
decomposition will take place in the SHC reactor 30. For example, iron sulfate
monohydrate
will convert to ferrous sulfide and have a particle size less than 0.1 or even
0.01 [tm upon
leaving heater 22. The SHC reactor 30 may take the form of a three-phase,
e.g., solid-liquid-
gas, reactor without a stationary solid bed through which catalyst, hydrogen
and oil feed are
moving in a net upward motion with some degree of back mixing. Many other
mixing and
pumping arrangements may be suitable to deliver the feed, hydrogen and
catalyst to the
reactor 30.
[0038] In the SHC reactor 30, heavy feed and hydrogen react in the
presence of the
aforementioned catalyst to produce slurry hydrocracked products. The SHC
reactor 30 can
be operated at quite moderate pressure, in the range of 3.5 to 24 MPa, without
formation of
coke. The reactor temperature is typically in the range of 350 to 600 C with
a temperature of
400 to 500 C being preferred. The LHSV is typically below 4 hr-1 on a fresh
feed basis, with
a range of 0.1 to 3 hr-1 being preferred and a range of 0.2 to 1 hr-1 being
particularly
preferred. The pitch conversion may be at least 80 wt-%, suitably at least 85
wt-% and
preferably at least 90 wt-%. The hydrogen feed rate is 674 to 3370 Nm3/m3
(4000 to 20,000
SCF/bbl) oil. SHC is particularly well suited to a tubular reactor through
which feed and gas
move upwardly. Hence, the outlet from SHC reactor 30 is above the inlet.
Although only one
is shown in the FIGURE, one or more SHC reactors 30 may be utilized in
parallel or in
series. Because of the elevated gas velocities, foaming may occur in the SHC
reactor 30. An
antifoaming agent may also be added to the SHC reactor 30 to reduce the
tendency to
generate foam. Suitable antifoaming agents include silicones as disclosed in
US 4,969,988.
- 8 -
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
Additionally, hydrogen quench from line 32 may be injected into the top of the
SHC reactor
30 to cool the slurry hydrocracked product as it is leaving the reactor.
[0039] A slurry hydrocracked product stream comprising a gas-liquid
mixture is
withdrawn from the top of the SHC reactor 30 through line 34. The slurry
hydrocracked
stream consists of several products including VG0 and pitch that can be
separated in a
number of different ways. The slurry hydrocracked effluent from the top of the
SHC reactor
30 is in an aspect, separated in a hot, high-pressure separator 36 kept at a
separation
temperature between 200 and 470 C (392 and 878 F), and in an aspect, at the
pressure of
the SHC reaction. The hot, high pressure separator is in downstream
communication with the
SHC reactor 30. The optional quench in line 32 may assist in quenching the
reaction products
to the desired temperature in the hot high-pressure separator 36. In the hot
high pressure
separator 36, the effluent from the SHC reactor 30 in line 34 is separated
into a gaseous
stream comprising hydrogen with vaporized products and a liquid stream
comprising liquid
slurry hydrocracked products. The gaseous stream is the flash vaporization
product at the
temperature and pressure of the hot high pressure separator. Likewise, the
liquid stream is the
flash liquid at the temperature and pressure of the hot high pressure
separator 36. The gaseous
stream is removed overhead from the hot high pressure separator 36 through
line 38 while the
liquid fraction is withdrawn at the bottom of the hot high pressure separator
36 through
line 40.
[0040] The liquid fraction in line 40 is delivered to a hot flash drum 42
at the same
temperature as in the hot high pressure separator 36 but at a pressure of 690
to 3,447 kPa
(100 to 500 psig). The vapor overhead in line 44 is cooled in cooler 46 and is
combined with
the liquid bottoms from a cold high pressure separator in line 48 and enters
line 50. A liquid
fraction leaves the hot flash drum in line 52.
[0041] The overhead stream from the hot high pressure separator 36 in line
38 is cooled
in one or more coolers represented by cooler 54 to a lower temperature. A
water wash (not
shown) on line 38 is typically used to wash out salts such as ammonium
bisulfide or
ammonium chloride. The water wash would remove almost all of the ammonia and
some of
the hydrogen sulfide from the stream in line 38. The stream in line 38 is
transported to a cold,
high pressure separator 56 in downstream communication with the SHC reactor 30
and the
hot high pressure separator 36. In an aspect, the cold high pressure separator
56 is operated at
lower temperature than the hot high pressure separator 36 but at the same
pressure. The cold
- 9 -
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
high pressure separator 56 is kept at a temperature between 100 and 93 C (50
and 200 F)
and at the pressure of the SHC reactor 30. In the cold high pressure separator
56, the
overhead of the hot high pressure separator 36 is separated into a gaseous
stream comprising
hydrogen in line 58 and a liquid stream comprising slurry hydrocracked
products in line 48.
The gaseous stream is the flash vaporization fraction at the temperature and
pressure of the
cold high pressure separator 56. Likewise, the liquid stream is the flash
liquid product at the
temperature and pressure of the cold high pressure separator 56. By using this
type of
separator, the outlet gaseous stream obtained contains mostly hydrogen with
some impurities
such as hydrogen sulfide, ammonia and light hydrocarbon gases.
[0042] The hydrogen-rich stream in line 58 may be passed through a packed
scrubbing
tower 60 where it is scrubbed by means of a scrubbing liquid in line 62 to
remove hydrogen
sulfide and ammonia. The spent scrubbing liquid in line 64 may be regenerated
and recycled
and is usually an amine. The scrubbed hydrogen-rich stream emerges from the
scrubber via
line 66 and is recycled through a recycle gas compressor 68 and line 20 back
to the SHC
reactor 30. The recycle hydrogen gas may be combined with fresh make-up
hydrogen added
through line 70.
[0043] The liquid fraction in line 48 carries liquid product to adjoin
cooled hot flash drum
overhead in line 44 leaving cooler 46 to produce line 50 which feeds a cold
flash drum 72 at
the same temperature as in the cold high pressure separator 56 and a lower
pressure of 690 to
3,447 kPa (100 to 500 psig) as in the hot flash drum 42. The overhead gas in
line 74 may be a
fuel gas comprising C4- material that may be recovered and utilized. The
liquid bottoms in
line 76 from the cold flash drum 72 and the bottoms line 52 from the hot flash
drum 42 each
flow into the fractionation section 80.
[0044] The fractionation section 80 is in downstream communication with
the SHC
reactor 30 for fractionating at least a portion of said slurry hydrocracked
products. The
fractionation section 80 may comprise one or several vessels although it is
shown only as one
vessel in the FIGURE. The fractionation section 80 may comprise an atmospheric
stripping
fractionation column and a vacuum flash drum column but in an aspect is just a
single
vacuum column. In an aspect, inert gas such as medium pressure steam may be
fed near the
bottom of the fractionation section 80 in line 82 to strip lighter components
from heavier
components. The fractionation section 80 produces an overhead gas product
emitting from an
overhead outlet 83 in line 84, a naphtha product stream emitting from a side
outlet 85 in line
- 10-
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
86, a diesel product stream emitting from a side outlet 88 in line 90, a LVGO
stream emitting
from a side outlet 91 in line 92, a HVGO stream emitting from a side outlet 93
in line 94 and
a pitch stream emitting from a bottom outlet 96 in bottoms line 98.
[0045] The SHC pitch product stream in bottoms line 98 from bottom
outlet 96 will be
heavily aromatic and contain SHC catalyst. The pitch will typically boil at
above 524 C
(975 F). The pitch in line 98 is split between line 100 which enters the SDA
unit 110 and
line 102 for recycle back to the SHC reactor 30. The HVGO product stream in
line 94 from
the side outlet is split between line 106 for blending and line 108 for
recycle back to the SHC
reactor 30. Streams in lines 102 and 108 may be combined in line 14. The HVGO
product
stream will boil at above 427 C (800 F) and less than the boiling range for
pitch. At least 80
wt-% of the HVGO stream will boil at above 427 C. In an additional aspect, at
least 80 wt-%
of the HVGO stream will boil below 524 C (975 F). Line 106 carries at least a
portion of the
HVGO stream from line 94.
[0046] The pitch stream in line 100 enters into the SDA unit 110. In
the SDA process, the
pitch feed stream in line 100 is pumped and admixed with a recycled solvent in
line 116 and a
make-up solvent in line 118 before entering into a first extraction column 120
as feed in line
112. Additional solvent, for example, recycled solvent, may be added to a
lower end of the
extraction column 120 via line 122. The light paraffinic solvent, typically
propane, butane,
pentane, hexane, heptane or mixtures thereof dissolves a portion of the pitch
in the solvent.
The pitch solubilized in the solvent rises to an overhead of the column 120.
The determining
quality for solvency of a light hydrocarbon solvent is its density, so
equivalent solvents to a
particular solvent will have an equivalent density. For example, in an
embodiment, heptane
is the densest solvent that can be used without lifting high concentrations of
vanadium in the
DAO. Solvents with lower densities than heptane would also be suitable for
lifting lower
concentrations of vanadium in the DAO. Specifically, the solvent solubilizes
the paraffinic
and less polar aromatic compounds in the pitch feed. N-pentane is a suitable
solvent. The
heavier portions of the feed stream 112 are insoluble and settle down as an
asphaltene or
pitch stream from pitch outlet 123 in line 124 and a first DAO stream is
extracted in an
extract emitted in line 126 from DAO outlet 127. The DAO stream in line 126 is
the
dissolved portion of the pitch. The extraction column 120 will typically
operate at 93 to
204 C (200 to 400 F) and 3.8 to 5.6 MPa (550 to 850 psi). The temperature and
pressure of
the extraction column 120 are typically below the critical point of the
solvent but can be
-11-
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
above or below the critical point as long as the density is well controlled.
The DA0 stream
in line 126 has a lower concentration of metals than in the feed stream in
line 112. The first
DA0 stream is heated to supercritical temperature for the solvent by indirect
heat exchange
with heated solvent in the solvent recycle line 136 in heat exchanger 128 and
in fired heater
129 or other additional heat exchanger. The supercritically heated solvent
separates from the
DA0 in the DA0 separator column 130 which is in downstream communication with
an
overhead of the first extraction column 120. A solvent recycle stream exits
the DA0
separator column 130 in the solvent recycle line 136. The solvent recycle
stream is condensed
by indirect heat exchange in heat exchanger 128 with the extract in line 126
and condenser
154. The DA0 separator column 130 will typically operate at 177 to 287 C (350
to 550 F)
and 3.8 MPa to 5.2 MPa (550 to 750 psi). The extractor bottoms stream in line
124 contains a
greater concentration of metals than in the feed in line 112. The bottoms
stream in line 124 is
heated in fired heater 140 or by other means of heat exchange and stripped in
a pitch stripper
column 150 to yield a solvent-lean pitch stream in bottoms line 152 and a
first solvent
recovery stream in line 134. Steam from line 133 may be used as stripping
fluid in the pitch
stripper column 150. The pitch stripper column 150 is in downstream
communication with a
pitch outlet 123 from said solvent deasphalting column 120 for separating
solvent from pitch.
The pitch stripper 150 will typically operate at 204 to 260 C (400 to 500 F)
and 344 kPa to
1,034 kPa (50 to 150 psi). A solvent-lean DA0 steam exits the DA0 separator
column 130 in
line 132 and enters DA0 stripper column 160 in downstream communication with a
bottom
of the DA0 separator column 130 and said DA0 outlet 127. The DA0 stripper
column 160
further separates a second solvent recovery stream 162 from the DA0 stream 132
by
stripping DA0 from the entrained solvent at low pressure. Steam from line 163
may be used
as stripping fluid in the DA0 stripper column 160. The DA0 stripper column 160
will
typically operate at 149 to 260 C (300 to 500 F) and 344 kPa to 1,034 kPa
(50 to 150 psi).
The second solvent recovery stream leaves in line 162 and joins the first
solvent recovery
stream in line 134 before being condensed by cooler 164 and stored in solvent
reservoir 166.
Recovered solvent is recycled from the reservoir 166 as necessary through line
168 to
supplement the solvent in line 136 to be mixed with pitch stream in line 100.
Essentially
solvent-free, DAO, which is at least a portion of the DA0 emitted from the DA0
outlet 127,
is provided in line 172.
- 12 -
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
[0047] DAO, which is the dissolved portion of the pitch, in line 172 is
blended with the
HVGO in line 106 in a vessel or a line 180, as shown in the FIGURE, to provide
a blended
product having a hydrocarbon composition comprising no less than 73 wt-%
aromatics and
preferably no less than 75 wt-% aromatics. Line 180 or unshown vessel is in
downstream
communication with the HVGO side outlet 93, the pitch outlet 96 and with the
DA0 outlet
127. The composition may have no more than 5 wt-% heptane insolubles and no
more than
50 wppm vanadium. In a further embodiment, the hydrocarbon composition may
have no
more than 5 wt-% hexane insolubles and no more than 30 wppm vanadium. In a
still further
embodiment, the hydrocarbon composition may have no more than 5 wt-% pentane
insolubles and no more than 10 wppm vanadium. At least 80 vol-%, preferably 90
vol-%, of
the composition boils at a temperature at or above 426 C (800 F). In an
embodiment, the
hydrocarbon composition comprises no more than 3.5 wt-% sulfur, suitably no
more than 1.0
wt-% sulfur and preferably no more than 0.5 wt-% sulfur. In a further
embodiment, the
blended hydrocarbon composition has a viscosity of no more than 180 cSt at 50
C and an
average molecular weight of no more than 500. In an embodiment, the
hydrocarbon
composition has no more than 5 wppm of sodium and preferably no more than 2
wppm, so it
can be a suitable turbine fuel.
EXAMPLES
[0048] The following examples were conducted to demonstrate the utility
of the
invention.
EXAMPLE 1
[0049] An SHC reactor was used to convert vacuum residue of bitumen
from the Peace
River formation of Alberta, Canada at a pitch conversion levels of 80 and 90
wt-%.
Respective SHC products were separated to provide a pitch product and a HVGO
product.
Aromatic concentrations were determined for SHC product fractions by ASTM
D2549-
02(2007) Standard Test Method for Separation of Representative Aromatics and
Nonaromatics Fractions of High-Boiling Oils by Elution Chromatography. Pitch
that leaves
the SHC reactor is comfortably assumed to be 100% aromatic molecules at all
conversion
levels above 80 wt-%. Aromatic concentrations that were determined for each
HVGO cut are
given in Table I.
- 13 -
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
TABLE I
SHC Conversion, Boiling Aromatics,
Product wt-% Range, C wt-%
HVGO 80 425-524 71.3
HVGO 90 425-524 70.8
Pitch all 524+ 100
EXAMPLE 2
[0050] An SHC reactor was used to convert the vacuum residue of bitumen
from the
Peace River formation of Alberta, Canada at a pitch conversion level of 87 wt-
%. The SHC
product was separated to provide a pitch product and a HVGO product. The pitch
product
was then subjected to solvent separation using a normal pentane solvent to
extract DAO. A
blending calculation was conducted to determine properties of a blend of a
hydrocarbon
composition with selected proportions of the HVGO product and pentane-
extracted DAO.
The properties of the blended hydrocarbon composition with comparison to the
RME180/IF0180 specification are shown in Table II. The RME180/IF180
specification is
taken from ISO standard 8217:2005(E) Table 2: Requirements for Marine Residual
Oils.
Aromatic concentrations of the blends in Table II were determined as a weight
average of the
aromatic concentration in the HVGO and the pitch cuts from Table I.
- 14 -
CA 02773584 2014-07-30
TABLE II
Pitch
extract Micro
in carbon
Pour Vis- Aro-
HVGO pentane Density residue Ash S
V Ni point cosity matics
in
blend in blend wt- Cst @
wt-% wt-%
g/cc wt-% wt-% % ppm ppm C 50 C wt-%
0.79 0.21 0.9988 6.95 0.02 3.7 2.7 2.4 <30 306.9 77.15
0.80 0.20 0.9979 6.64 0.02 3.7 2.6 2.4 <30 261.3 76.80
0.82 0.18 0.9961 6.03 0.02 3.7 2.6 2.3 <30 210.8 76.22
0.85 0.15 0.9935 5.11 0.03 3.7 2.6 2.1 <30 149.7 75.35
0.86 0.14 0.9926 4.80 0.03 3.6 2.6 2.0 <30 131.2 75.06
0.88 0.12 0.9909 4.19 0.03 3.6 2.6 1.9 <30 108.5 74.48
RME 180/
IFO 180
specification <0.9909 <15 <0.1 <4.5 <200 n/a <30 <180.0 n/a
[0051] All blends are expected to have a pour point less than 30 C
based on their
physical properties according to Procedure 2B8.1 of the API Petroleum Refining
Technical
Handbook, vol. 1 (1987). The blend with the ratio of HVGO to pentane soluble
pitch equal
to 79:21is calculated to have a viscosity of 1201Cst, and the blend with the
ratio of HVGO to
pentane soluble pitch equal to 88:12 is calculated to have a viscosity of 349
Cst at a
temperature of 30 C according to Procedure 2B2.1 and 2B2.3 in the API
Petroleum Refining
Handbook, vol. 1 (1987). Therefore, all compositions in the table are expected
to pour at
less than 30 C.
[0052] The blend with the ratio of HVGO to pentane soluble pitch equal to
79:21 is the
as-produced composition of SHC products. The blend with the ratio of HVGO to
pentane
soluble pitch equal to 85:15 has a composition that meets the viscosity
specification at 50 C
but is slightly too dense to meet the density specification. The blend with
the ratio of HVGO
to pentane soluble pitch equal to 88:12 has a composition that meets all of
the
RME180/IF180 specifications.
[0053] The blend with the ratio of HVGO to pentane soluble pitch equal
to 88:12 was
measured to have less than 2 wppm sodium. It was expected that all of the
blends had a
sodium concentration of less than 2 wppm.
- 15 -
CA 02773584 2012-03-07
WO 2011/071705
PCT/US2010/058152
EXAMPLE 3
[0054] An SHC reactor was used to convert vacuum residue of bitumen from
Peace
River, Alberta, Canada at a pitch conversion level of 87 wt-%. The SHC product
was
separated to provide a pitch product. The pitch product had the properties
given in Table III.
TABLE III
Pitch Density, g/cc 1.185
Nickel, wppm 120
Vanadium, wppm 109
[0055] The pitch product was then subjected to solvent separation using
a several
solvents to extract DAO. The concentration of metals and density of the pitch
lifted by
different solvents was examined and shown in Table IV.
TABLE IV
Solvent Extracted .Nickel + Extracted
Nickel, Vanadium,
Solvent Density, oil ' Vanadium, oil density,
wppm wppm
g/cc wt-% wppm g/cc
pentane 0.6312 15.7 7.0 3.0 10.0 1.074
hexane 0.6640 25.1 20.7 14.5 35.2 1.079
heptane 0.6882 32.4 31.6 22.5 54.1 1.082
toluene 0.8719 81.5 99.0 93.0 192.0 1.057
[0056] In this experiment, the nickel and vanadium concentrations in the
extracted oil
were found to be linear with either solvent density or wt-% yield. Hexane was
not actually
tested but properties were therefore interpolated between pentane and heptane
based on
solvent densities. It was surprising that such little nickel and vanadium was
present in the oil
extracted from pitch.
[0057] Without further elaboration, it is believed that one skilled in the
art can, using the
preceding description, utilize the present invention to its fullest extent.
The preceding
preferred specific embodiments are, therefore, to be construed as merely
illustrative, and not
limitative of the remainder of the disclosure in any way whatsoever.
- 16-
CA 02773584 2014-07-30
[0058] In the foregoing, all temperatures are set forth in degrees
Celsius and, all parts and
percentages are by weight, unless otherwise indicated.
[0059] The scope of the claims should not be limited by the preferred
embodiments set forth
in the examples, but should be given the broadest interpretation consistent
with the description as
a whole.
-17-