Note: Descriptions are shown in the official language in which they were submitted.
CA 02773795 2012-04-11
=
. , .
Title: METHODS AND APPARATUS FOR IMAGE PROCESSING IN WIRELESS
CAPSULE ENDOSCOPY
Field
[1] The described embodiments relate to methods and apparatus for image
processing and, in particular, to image processing suitable for use in images
having
a dominant color, such as those produced in wireless capsule-based endoscopy.
Background
[2] In the United States alone, over 3 million people suffer from
gastrointestinal
(GI) diseases annually. The cause of these diseases can be difficult to
diagnose, and
is never found in over one-third of cases. Endoscopy is a significant medical
diagnostic technique that can assist in detecting the cause of GI disease,
however
conventional endoscopy involves traversing portions of the GI tract with a
wired
device, which can be uncomfortable and unpleasant for the patient.
Summary
[3] In a first broad aspect, there is provided a method of processing an
image
captured using a color image sensor, the image comprising a plurality of
samples in
a plurality of color channels, the method comprising: generating a luma
channel
based on the plurality of color channels; generating a first chroma channel
based on
a difference between the luma channel and a first color channel in the
plurality of
color channels; generating a second chroma channel based on a difference
between
the luma channel and a second color channel in the plurality of color
channels;
generating, using a proce¨ssor, a plurality of predicted sample values for the
luma
channel and the first and second chroma channels using a lossless predictive
coding
mode; computing a plurality of difference values between the plurality of
predicted
sample values and the respective generated sample values; and variable length
coding the plurality of difference values to produce a processed image.
[4] Each sample value of the luma channel may be generatable using
(e.g.,
generated using only) addition and shift operations. Each sample value of the
first
and second chroma channels may be generatable using (e.g., generated using
only)
addition, negation and shift operations.
¨ 1 ¨
CA 02773795 2012-04-11
. . .
[5] Each sample value of the luma channel may be generated based on a
summation of corresponding sample values in the plurality of color channels.
The
summation may be of: a first color channel sample value bitshifted once to
divide by
two; a second color channel sample value bitshifted twice to divide by four;
and a
remaining color channel sample value bitshifted twice to divide by four.
[6] The difference between the luma channel and the first color channel may
be
computed based on: the luma sample value bitshifted once to divide by two; and
the
first color channel sample value bitshifted once to divide by two.
[7] The difference between the luma channel and the second color channel
may
be computed by: computing a sum of the first color channel sample value and
the
remaining color channel sample value, bitshifting the sum three times to
divide by
eight, and subtracting from the bitshifted sum the second color channel sample
value
bitshifted twice to divide by four.
[8] The image may comprise a dominant color, and the first and second color
channels may correspond to colors other than the dominant color.
[9] The color image sensor may be an RGB sensor, and the first color
channel
may be a green color channel and the second color channel may be a blue color
channel.
[10] The method may further comprise subsampling the first chroma channel
relative to the luma channel prior to generating the plurality of predicted
sample
values.
[11] The method may further comprise subsampling the second chroma channel
relative to the luma channel prior to generating the plurality of predicted
sample
values.
[12] The method may further comprise clipping at least one portion of the
image
prior to generating the plurality of predicted sample values.
[13] The lossless predictive coding mode may be a JPEG lossless predictive
coding mode. The JPEG lossless predictive coding mode may be left pixel
prediction.
[14] The plurality of difference values may be variable length coded using
Golomb-
Rice coding.
[15] In another broad aspect, there is provided a method of generating an
endoscopic image for wireless transmission, the method comprising:
illuminating a
diagnostic area using at least one light source; capturing, using a color
image
¨2¨
CA 02773795 2012-04-11
sensor, an image of the diagnostic area under illumination, the image
comprising a
plurality of samples in a plurality of color channels; and processing the
image
according to the described methods to produce the endoscopic image for
wireless
transmission.
[16] The diagnostic area may be illuminated with a wide spectrum light
source.or
at least one narrow band light source.
[17] The at least one light source may comprise a wide spectrum light source
and
at least one narrow band light source, and the method may further comprise
switching between the wide spectrum light source and the at least one narrow
band
light source.
[18] The at least one light source may comprise a wide spectrum light source,
and
the method may further comprise switching between a wide spectrum imaging mode
and a narrow band imaging mode.
[19] The at least one narrow band light source may comprise a green light
source
or a blue light source.
[20] In another broad aspect, there is provided an apparatus for processing an
image captured using a color image sensor, the image comprising a plurality of
samples in a plurality of color channels, the apparatus comprising: a color
conversion module configured to: generate a luma channel based on the
plurality of
color channels; generate a first chroma channel based on a difference between
the
luma channel and a first color channel in the plurality of color channels; and
generate
a second chroma channel based on a difference between the luma channel and a
second color channel in the plurality of color channels; a predictive encoder
configured to: generate a plurality of predicted sample values for the luma
channel
and the first and second chroma channels using a lossless predictive coding
mode;
and compute a plurality of difference values between the plurality of
predicted
sample values and the respective generated sample values; and a variable
length
coder configured to encode the plurality of difference values to produce a
processed
image.
[21] Each sample value of the luma channel may be generatable using (e.g.,
generated using only) addition and shift operations. Each sample value of the
first
and second chroma channels may be generatable using (e.g., generated using
only)
addition, negation and shift operations.
¨3¨
CA 02773795 2012-04-11
[22] Each sample value of the luma channel may be generated based on a
summation of corresponding sample values in the plurality of color channels.
The
summation may be of: a first color channel sample value bitshifted once to
divide by
two; a second color channel sample value bitshifted twice to divide by four;
and a
remaining color channel sample value bitshifted twice to divide by four.
[23] The difference between the luma channel and the first color channel may
be
computed based on: the luma sample value bitshifted once to divide by two; and
the
first color channel sample value bitshifted once to divide by two.
[24] The difference between the luma channel and the second color channel may
be computed by: computing a sum of the first color channel sample value and
the
remaining color channel sample value, bitshifting the sum three times to
divide by
eight, and subtracting from the bitshifted sum the second color channel sample
value
bitshifted twice to divide by four.
[25] The image may comprise a dominant color, and the first and second color
channels may correspond to colors other than the dominant color.
[26] The color image sensor may be an RGB sensor, and the first color channel
may be a green color channel and the second color channel may be a blue color
channel.
[27] The apparatus may further comprise a subsampler configured to subsample
the first chroma channel relative to the luma channel prior to generating the
plurality
of predicted sample values.
[28] The subsampler may be configured to subsample the second chroma channel
relative to the luma channel prior to generating the plurality of predicted
sample
values.
[29] The apparatus may further comprise a clipper configured to clip at least
one
portion of the image prior to generating the plurality of predicted sample
values.
[30] The lossless predictive coding mode may be a JPEG lossless predictive
coding mode. The JPEG lossless predictive coding mode may be left pixel
prediction.
[31] The plurality of difference values may be variable length coded using
Golomb-
Rice coding.
[32] In another broad aspect, there is provided an apparatus for generating an
endoscopic image for wireless transmission, the apparatus comprising: at least
one
light source configured to illuminate a diagnostic area; a color image sensor
¨4¨
CA 02773795 2012-04-11
=
configured to capture an image of the diagnostic area under illumination, the
image
comprising a plurality of samples in a plurality of color channels; and the
image
processing apparatus as described herein, configured to generate the
endoscopic
image for wireless transmission.
[33] The at least one light source may comprise a wide spectrum light source
or at
least one narrow band light source.
[34] The at least one light source may comprise a wide spectrum light source
and
at least one narrow band light source, and the apparatus may comprise a switch
for
selecting between the wide spectrum light source and the at least one narrow
band
light source.
[35] The at least one light source may comprise a wide spectrum light source,
and
the apparatus may further comprise at least one narrow band color filter.
[36] The at least one narrow band light source may comprise a green light
source
or a blue light source.
Brief Description of the Drawings
[37] A preferred embodiment of the present invention will now be described in
detail with reference to the drawings, in which:
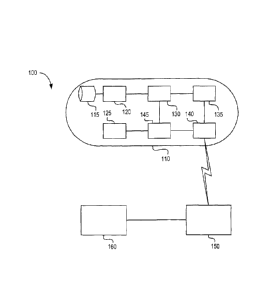
FIG. 1 illustrates an exemplary WOE system in accordance with at least some
embodiments;
FIGS. 2A and 2B are 3D plots of RGB component values (i.e., red, green and
blue) for the pixel positions of an image of both WBI and NBI endoscopic
images;
FIGS. 20 and 2D illustrate corresponding 3D plots of the same image of
FIGS. 2A and 2B, with the equivalent YEF component values;
FIGS. 3A and 3B are histograms for a WBI and an NBI endoscopic image,
respectively, following conversion into the YEF color space;
FIG. 4 illustrates a YEF812 subsampling scheme;
FIG. 5 illustrates the change in dX for an endoscopic image, in all three YEF
components;
FIG. 6 is a simplified block diagram of an exemplary DPCM encoder;
FIGS. 7A and 7B are histograms of dY, dE and dF of exemplary WBI and NBI
endoscopic images, respectively;
FIG. 8 is a plot showing the length of Golomb-Rice codes for various integer
values;
¨5¨
CA 02773795 2012-04-11
. , .
FIG. 9 illustrates an example of an image with corner clipping applied;
FIG. 10 is a simplified block diagram of an exemplary image processor in
accordance with at least some embodiments;
FIG. 11 is a flow diagram for an exemplary method of processing an image
captured using a color image sensor;
FIG. 12 is a flow diagram for an exemplary method of generating an
endoscopic image for wireless transmission; and
FIG. 13 is a flow diagram for another exemplary method of generating an
endoscopic image for wireless transmission.
Description of Exemplary Embodiments
[38] It will be appreciated that for simplicity and clarity of
illustration, where
considered appropriate, reference numerals may be repeated among the figures
to
indicate corresponding or analogous elements or steps. In addition, numerous
specific details are set forth in order to provide a thorough understanding of
the
exemplary embodiments described herein. However, it will be understood by
those
of ordinary skill in the art that the embodiments described herein may be
practiced
without these specific details. In other instances, well-known methods,
procedures
and components have not been described in detail since these are known to
those
skilled in the art. Furthermore, it should be noted that this description is
not intended
to limit the scope of the embodiments described herein, but rather as merely
describing one or more exemplary implementations.
[39] Recently, wireless capsule endoscopy (WOE) has been developed to capture
images of the gastrointestinal tract for medical diagnostic purposes. In WOE,
a
capsule comprising an imaging device and a wireless transmitter is swallowed
or
ingested by the patient, whereupon the capsule transmits images periodically
as the
capsule passes through the patient's gastrointestinal tract. The transmitted
images
may be captured by an external device, for review by a medical practitioner.
WOE is
generally more comfortable for patients than wired endoscopy. Moreover, in
contrast
to wired endoscopy, a complete examination of the entire GI tract, including
the
small intestine, can be performed using this technique, which may be more
difficult
or even impossible with wired endoscopy techniques. WOE can be used to detect
many diseases of the GI tract, such as bleeding, lesions, ulcers and tumours.
¨6¨
CA 02773795 2012-04-11
=
. . .
[40] Referring now to FIG. 1, there is illustrated an exemplary WCE system.
WCE
system 100 comprises a WCE capsule 110, which itself generally comprises a
digital
image sensor 120 (optionally provided with a lens 115), a light source 125 for
illuminating the GI tract so that image sensor 120 can capture images, an
image
processor 130, a battery 145, a wireless transmitter or transceiver 135 and an
antenna 140. Transceiver 135 can be used to communicate with a transceiver 150
located external to the patient. Signals received by transceiver 150 can be
communicated to and stored at a workstation 160, which may be a personal
computer, for example.
[41] As the WCE capsule must be swallowed by the patient, each of the elements
of WCE capsule 110 must be limited in size to enable the WCE capsule to be
compact. In particular, battery 145 must be kept small. Generally, battery 145
is
operable to supply power to WCE capsule 110 for approximately 8 to 10 hours,
in
some cases longer, which can be sufficient for the WCE capsule 110 to traverse
the
GI tract of a patient. The size constraint of battery 145 and the need to
supply power
for the entirety of the WCE capsule's passage through the GI tract imposes a
trade-
off. In particular, the image processing and wireless transmission performed
by WCE
capsule 110 must be size and energy efficient, while maintaining sufficient
image
quality to enable accurate medical diagnosis.
[42] Light source 125 can be a light emitting diode (LED), for example. When
capturing endoscopic images, several changeable light modes can be used, such
as
white-band imaging (WBI), narrow-band imaging (NBI), and, in some cases, auto-
fluorescence imaging (AFI). In WBI, broad spectrum light (e.g., white light)
is used to
illuminate the GI surface. In NBI, two or more discrete bands of light can be
used.
For example, in GI imaging, one blue and one green wavelength of light can be
used
(e.g., with center wavelengths at 415 nm and at 540 nm). Narrow band blue
light can
be suitable for displaying superficial capillary networks, while narrow band
green
light can be suitable for displaying subepithelial vessels. When the two are
combined, a high contrast image of the tissue surface can be produced.
[43] In some cases, light source 125 may comprise a plurality of light
sources,
which can be controlled remotely (e.g., from workstation 160) to switch
between
narrow and wide band imaging. For example, a remotely controlled switch may be
provided to selectively switch a WBI light source and a NBI light source on
and off,
as needed.
¨7--
CA 02773795 2012-04-11
. . .
[44] In some other cases, a WBI light source can be used, and filters can be
positioned in front of image sensor 120 to effect NBI lighting conditions. For
example, the filters may be mechanically moved in and out of position using
actuators provided at the WOE capsule (not shown). Alternatively, different
color
filters may cover different portions of image sensor 120, which may be
activated or
read from as needed.
[45] To enable remote switching between WBI and NBI modes, transceiver 140
and transceiver 150 may be configured to provide duplex communication.
[46] A WOE capsule generally traverses the GI tract via peristaltic
contraction.
Accordingly, images of clinically relevant and important tissues may be missed
as
the capsule is propelled over them. However, one or more techniques can be
employed to ensure that relevant tissues are not missed. For example, a high
sample rate (e.g., number of images per second) can be maintained, multiple
imaging sensors can be used (e.g., oriented in different directions), and
motility
devices (e.g., miniature robotic arms) can be used. Each of these approaches
increases the size and power requirements for the WCE capsule. Accordingly,
the
importance of efficient image processing and wireless transmission may be
further
increased.
[47] Described herein are methods and apparatus for image processing suitable
for use in a WOE capsule that may support one or both of the WBI and NBI
modes.
[48] The image processing methods and apparatus can be interfaced directly
with
commercially-available RGB image sensors that support the digital video port
(DVP)
interface, without the need for an intermediate buffer memory to fill blocks
or frames
(DVP devices generally output pixels in a raster scan fashion). The described
image
processing methods and apparatus can also be applied in other applications.
[49] In contrast, conventional image processing and image compression
techniques generally work on a block-by-block basis. For example, in image
compression based on the Discrete Cosine Transform (DOT), 4x4 or 8x8 pixel
blocks need to be accessed from the image sensor. Since commercial CMOS image
sensors send pixels in a row-by-row fashion (and do not provide their own
buffer
memory), buffer memory needs to be provided to support such DOT-based
algorithms.
[50] For example, in order to start processing of the first 8x8 block of a
256x256
size image, the compressor must wait until the first 8x8 block is available,
which
¨8¨
CA 02773795 2012-04-11
=
. . .
means that seven full rows of the image, plus the first eight pixels of the
eighth row
must be received and stored (256 x 7 + 8 = 1800 pixels, assuming progressive
scan). Hence, a 5.3kB buffer memory may seem enough (assuming 24 bits per
pixel
for a color image). However, without a full size buffer memory (i.e., 192kB to
store
the entirety of the 256x256 image), the image sensor output would need to be
stopped (or paused) until the stored pixels are processed, otherwise no
additional
memory would be available to store new pixels. Alternatively, two parallel
buffer
memories of size 5.3kB can be used, so that while the compressor works with
pixels
of one buffer, the new pixels continuously received from the image sensor can
be
stored in the other buffer. However, such an approach introduces timing
challenges,
juggling between the compression and input. Moreover, buffer memory can occupy
significant area and consume power.
[51] Beyond memory, the computational cost associated in such DCT-based
algorithms, which involve multiplication, addition, data scheduling and other
operations, can result in high area and power consumption.
[52] Other compression algorithms such as LZW may require content addressable
memory (CAM) to operate, as well as memory to store a coder dictionary.
[53] The described methods and apparatus overcome many of the disadvantages
of conventional image processing techniques when applied to wireless capsule
endoscopy.
[54] The described image processing is low complexity and thus consumes low
power, conserving limited battery life while it travels through the GI tract.
This
enables the image resolution and frame rate (in frames per second or FPS) of
the
image sensor to be increased, for example. Other features, such as multi-
camera
imaging can also be enabled using the conserved power. Both WBI and NBI are
supported, and the resulting processed images are sufficiently compressed to
fit
within the limited bandwidth of a medically implantable wireless transceiver.
For
example, the Zarlink ZL70081 is one wireless transmitter compatible with
medical
implant communication service (MICS), and supports a maximum data rate of 2.7
Mbps. The described image processing techniques can produce a target frame
rate
of at least 2-5 FPS.
[55] Quality of the reconstructed image can be ensured, as the described image
processing results in reconstructed images with a minimum peak signal-to-noise
ratio (PSNR) of at least 35 dB. Similar satisfactory results occur with other
evaluation
¨9¨
CA 02773795 2012-04-11
criteria, such as structural similarity index (SSIM), visual information
fidelity (VIF),
and visual signal-to-noise ratio (VSNR).
[56] Analysis of WBI and NBI images reveals endoscopic images generally
exhibit
dominance in red color components, and a relatively lower amount of green and
blue
components. Accordingly, a color conversion can be employed to take advantage
of
this characteristic by consolidating image information common to all three
color
channels in a single color channel.
[57] In conventional image processing, luminance-chrominance (or luma-chroma)
color spaces are used to take advantage of the human visual acuity system's
sensitivity to changes in brightness and relative lack of sensitivity to
changes in
color.
[58] In particular, due to the presence of relatively more rod cells (which
are
sensitive to brightness) than cone cells (sensitive to color), the human eye
is more
sensitive to small changes in brightness than to changes in color. Thus, the
loss of
information relating to changes in color (e.g., chrominance components) can be
tolerated without dramatically degrading image quality.
[59] One example of such a color space is the YUV color space, in which Y
represents luminance and two chrominance components are represented by U and
V. U represents the difference between the blue channel and luminance, while V
represents the difference between the red channel and luminance.
[60] However, conversion from the RGB color space, typically output by image
sensors, and the YUV color space generally requires multiplication and the use
of
constants that are not powers of two. The use of such constants, and the
resulting
multiplication, necessitates the implementation of additional processing
hardware,
which in turn requires area and power.
[61] Described herein is a color space conversion that does not rely on
arbitrary
constants or multiplication operations.
[62] The described color space can be defined as YEF, in which a luminance
component (i.e., luma) is represented by Y, a first chrominance component
(chroma)
is represented by E, and a second chrominance component is represented by F.
[63] Generally, E may represent the difference between luminance and the green
component (in short, chroma-E), and F may primarily represent the difference
between luminance and the blue component (in short, chroma-F). These
relationships are shown in Equations (1), (2) and (3).
¨ 10 ¨
CA 02773795 2012-04-11
,
. . .
R G B
Y=++-
(1)
4 2 4
Y G R G B
E =---+ 128 =---+-+ 128
(2)
2 2 8 4 8
(3)
2 8 8 8 8 4
[64] From Equations (1), (2), and (3), it can be seen that the conversion
between
RGB and YEF color spaces involves only a few additions, negations and shift
operations using the RGB components.
[65] Referring now to FIGS. 2A and 2B, there are illustrated 3D plots of RGB
component values (i.e., red, green and blue) for the pixel positions of an
image of
both WBI and NBI endoscopic images. The x- and y-axes correspond to row and
column of each pixel, while the z-axis corresponds to the pixel value for each
color.
[66] It can be observed that, in the RGB color space, the changes in pixel
values
are high, and there is comparable information content in all three color
components.
[67] Referring now to FIGS. 2C and 2D, there are illustrated corresponding 3D
plots of the same image of FIGS. 2A and 2B, with the equivalent YEF component
values.
[68] It can be observed that, in the YEF color space, there is less change in
pixel
values in the two chrominance components (E and F), which indicates that the E
and
F components contain less information.
[69] Through experimentation, it has been determined that the intensity
distribution
of the green component in RGB endoscopic images is very similar to that of the
blue
component (as can be seen in FIGS. 2A and 2B). It has been further determined
that
the intensity distribution of luminance (Y) is similar to the intensity
distribution of
green and blue RGB components. Accordingly, subtracting green and blue
components from the luminance component can produce differential pixel values
that
are generally small and exhibit little entropy.
Color space WBI StdDev NBI StdDev WBI Entropy NBI Entropy
RGB R 46.6 44.1 7.1 7.2
-
G 39.4 39.6 7.0 7.0
B 34.7 36.1 6.7 6.9 -
YUV Y 34.3 34.6 6.8 6.8
U 7.0 3.0 4.4 3.2
_
/ 9.6 5.6 4.9 4.1
YCoCg Y 38.8 39.5 7.0 7.0
Co 13.9 7.0 5.5 4.5
-11-
CA 02773795 2012-04-11
Cg 5.3 3.3 4.1 3.6
YEF Y 38.8 39.5 TO 7.0
2.7 1.7 3.2 2.6
4.7 2.1 3.9 2.8
Table 1
[70] Table 1 illustrates the average standard deviation and entropy for each
of the
components of several color spaces, for a plurality of both WBI and NBI
exemplary
endoscopic images. It can be observed that the YEF color space has the lowest
standard deviation and entropy in its chroma components. The low entropy
suggests
the YEF color space should be subject to good compression.
[71] Referring now to FIGS. 3A and 3B, there are illustrated the histograms
for a
WBI and an NBI endoscopic image, respectively, following conversion into the
YEF
color space.
[72] It can be observed that the variations of the E and F are relatively
narrow, due
in part to the color homogeneity of the endoscopic images. Accordingly, in
some
embodiments, subsampling of the chroma components can be employed to reduce
the amount of information that is to be compressed and transmitted.
[73] Subsampling can be performed, for example, by selecting one out of every
nth
pixel to be encoded. For example, in one subsampling scheme, defined as
YEF811,
for every eight Y component samples, one E component sample and one F
component sample are used. In another example scheme, defined as YEF812, for
every eight Y component samples, one E component sample and two F component
samples are used. The YEF812 subsampling scheme is illustrated in FIG. 4.
[74] The effect of various subsampling schemes on the quality of exemplary
reconstructed WBI endoscopic images, as determined by various quality metrics,
is
shown in Table 2.
Luminance Chroma
SSIM VIF PSNR VSNR PSNR(E) PSNR(F)
YEF888 0.999 0.988 57.3 58.0 60.2 60.5
YEF422 0.998 0.986 57.0 55.5 57.8 58.9
YEF412 0.998 0.984 56.5 54.7 54.6 58.8
YEF814 0.998 0.977 55.3 52.5 51.0 58.3
YEF822 0.998 0.978 55.9 53.2 54.6 55.0
YEF812 0.998 0.972 54.7 51.4 51.0 54.8
YEF811 0.998 0.957 53.6 46.1 51.0 50.4
YEF16.1.2 0.997 0.952 52.3 43.9 47.9 50.4
Table 2
- 12 -
CA 02773795 2012-04-11
[75] The effect of various subsampling schemes on the quality of exemplary
reconstructed NBI endoscopic images, as determined by various quality metrics,
is
shown in Table 3.
Luminance Chroma
SSIM VIF PSNR VSNR PSNR(E) PSNR(F)
YEF888 0.998 0.989 57.1 69.2 60.7 60.0
YEF422 0.998 0.989 57.1 66.2 58.3 59.2
YEF412 0.998 0.988 57.0 66.2 55.1 59.2
YEF814 0.998 0.987 56.9 66.2 51.8 59.1
YEF822 0.998 0.986 56.7 66.4 55.1 56.7
YEF812 0.998 0.985 56.8 66.5 51.8 56.7
YEF811 0.998 0.980 56.5 59.4 51.8 53.5
YEF16.1.2 0.998 0.978 56.5 59.3 49.4 53.5
YEF16.1.1 0.998 0.973 56.2 52.1 49.4 50.9
Table 3
[76] It can be seen from Table 2 and Table 3 that the YEF888 scheme yields the
best performance, which is to be expected as no sub-sampling is performed. For
WBI images, the YEF16.1.2 scheme (in which, for every 16 Y components, one E
and two F components are taken) produces the poorest result, due to heavier
sub-
sampling. In the case of NBI images, it is YEF16.1.1 that exhibits the poorest
results
in the above table.
[77] In the examples above, the YEF814, YEF822, and YEF812 schemes may
offer a suitable trade-off between compression ratio and image quality for the
WBI
mode, for example.
[78] A further characteristic of endoscopic images is that changes between
adjacent pixel values are generally small. In general, component values tend
to
change gradually and slowly, as sharp edges are rare in endoscopic images. The
change in component values (dX) with respect to its adjacent left pixel in any
row
can be expressed by Equation (4):
dXr,c = Xr,c - Xnc_i
(4)
where, X,,, is the pixel value at row r and column c, and Xrc_, is its
adjacent left pixel
value. In this example, X can refer to Y, E, or F component values.
[79] Referring now to FIG. 5, there is illustrated the change in dX for an
endoscopic image, in all three YEF components.
[80] As noted above, the difference in pixel (dX) with respect to the adjacent
left
pixel is found to be small in endoscopic images. As a result, a form of
differential
pulse code modulation (DPCM) may be used in some embodiments to encode the
- 13 -
CA 02773795 2012-04-11
. . .
pixel values efficiently. DPCM is a lossless encoding scheme with little
computational
complexity.
[81] Referring now to FIG. 6, there is illustrated a block diagram of an
exemplary
DPCM encoder. DPCM encoder 600 comprises a prediction module 605, an
addition/subtraction module 610 and a symbol encoder 615.
[82] Prediction module 605 algorithmically selects a predicted next pixel
value
based on an input pixel (and, optionally, one or more previous input pixels).
The
predicted pixel value is subtracted from the input pixel value at 610 and the
difference dX is encoded for transmission by symbol encoder 615.
[83] One form of DPCM that may be used in some embodiments is a JPEG
lossless prediction mode. In particular, JPEG lossless prediction mode-1
(e.g., left
pixel prediction) can be used, as it can be efficiently implemented in
hardware and is
suitable for processing raster scanned pixels without the requirement for a
buffer
memory.
[84] Further compression of DPCM-encoded data can be performed through the
use of a suitable variable-length encoding scheme. Such variable-length
encoding
may also incorporate error correction or detection aspects.
[85] Referring now to FIGS. 7A and 7B, there are illustrated histograms of dY,
dE
and dF of exemplary WBI and NBI endoscopic images, respectively. The
histograms
demonstrate what is generally a two-sided geometric distribution.
[86] For geometric distributions, Golomb coding can be used to provide an
optimum code length.
[87] For the purposes of hardware efficiency and ease of implementation,
Golomb-
Rice coding may be used as an alternative to Golomb coding, as the former
exhibits
similar compression efficiency to the latter.
[88] Golomb-Rice coding uses positive integers, however dX can be positive or
negative. Accordingly, the values of dX can be mapped to m_dX using Equation
(5):
1 2dX, when dX 0 1
m_dX =
t2IdX1¨ 1, when dX < 01
(5)
[89] Experimental verification indicates that the values of dY generally fall
in the
range between +127 and -128, in part due to the absence of sharp transitions
between two consecutive pixels in endoscopic images. Moreover, dE and dF
values
generally fall within an even narrower range. Accordingly, integers in m_dX
can be
¨14¨
CA 02773795 2012-04-11
=
mapped to the range between 0 and 255, which can be expressed in binary form
using eight bits.
[90] An optimized Golomb-Rice coding scheme can be defined as follows:
= 28 = 256
(6)
M = 2k (7)
[91] Where M is a predefined integer and a power of 2, then m_dX can divided
by
M as follows:
q = Integer (mm_dX)
(8)
r = m_dx mod M
(9)
[92] The quotient q can be expressed in unary in q+1 bits. The remainder r can
be
concated with the unary code, and r expressed in binary in k bits.
[93] Generally, length of the Golomb-Rice code can be limited by using a
parameter gfimit. In particular, if:
q gumit ¨ log2 I ¨ 1
(10)
then the unary code of gimut¨ log2 / ¨ 1 can be prepared. This can be used as
an
"escape code" for the decoder, and can be followed by the binary
representation of
m_dX in log2 / bits.
[94] The length of a golomb-rice code can be calculated using (11) and (12):
I = giimit ¨ log2 / ¨ 1
(11)
grien =fq + 1 + k, when q <
(12)
t gut, when q j
[95] The maximum length of the Golomb-Rice code (giimit) can be selected to
be,
for example, 32.
[96] Referring now to FIG. 8, there is illustrated a plot showing the length
of
Golomb-Rice codes for various integer values, as a function of k. From the
histograms of FIGS. 7A and 7B, it can be observed that the most frequently
occurring value of dE and dF is 0, followed by other values close to zero.
Generally,
smaller length codes for zero and near-zero values can be assigned to
facilitate
good compression. Accordingly, k = 1 can be selected for encoding the mapped
integers for dE and dF.
[97] Since dY generally spans a wider range of values in NBI images than in
WBI
images (e.g., due to the presence of sharper edges), the k parameter can be
¨ 15 ¨
CA 02773795 2012-04-11
. . =
selected differently depending on use case. Exemplary k parameters are
illustrated
in Table 4.
m_dY m_dE m_dF
WBI 2 1 1
NBI 3 1 1
Table 4
[98] Generally, in wireless endoscopy, the image sensor may be encased in a
capsule-shaped tube. Due to the generally rounded shape of the capsule, corner
areas in a captured image may be distorted (e.g., stretched) and thus less
reliable
for diagnostic purposes. When such areas can be safely disregarded, additional
compression can be gained by clipping the distorted areas.
[99] From the implementation point of view, it is generally easier to clip
four
corners of an image along a straight diagonal line, rather than applying a
radial cut.
The diagonal line can be chosen to encompass the radial line that might
otherwise
be desired, and some small additional number of pixels. Clipping is not
limited to one
or more corners. In other embodiments, other areas of the image may be
discarded
(e.g., center portion of the image) according to various geometries.
[100] However, if corner clipping is applied, once the horizontal and vertical
length
of the corner cut is determined, a clipping technique can be implemented with
relatively few combinational logic blocks.
[101] For example, column and row pixel positions can be identified to
determine
whether they fall within a desired viewing region. If the pixel is within the
viewing
region, it can be sampled, otherwise, the pixel may be ignored or discard
(e.g., not
sampled).
[102] FIG. 9 illustrates an example of an image with corner clipping applied,
in
which the areas shaded in black represent clipped pixels.
[103] Once L is determined as shown in Fig. 9, it is simple to implement the
clipping
algorithm in hardware with just a few combinational logic blocks. As seen from
the
pseudo code below, the column and row pixel positions are checked to see
whether
they fall into the desired visual region; if the position is inside, it is
sampled; if not, the
pixel is ignored (i.e., not sampled).
Is inside visual area := False
_ _ _
¨ 16 ¨
CA 02773795 2012-04-11
= . .
If ( cY < L ) {
If cX >=(L-cY) And cX <(W-(L-cY))
Is inside visual area := True 1
¨ ¨
Else if cY >= (W - L) {
If cX >=((cY-(W-L))+1) And cX<(W-((cY-(W-
L))+1))
Is inside visual area := True 1
¨ ¨
Else
Is inside visual area := True
_ _ _
[104] Referring now to FIG. 10, there is illustrated a simplified block
diagram of an
exemplary image processor in accordance with at least some embodiments.
[105] Image processor 1000 generally comprises a color conversion module 1020,
a clipper 1030, a subsampler 1040, a predictive encoder 1050 and a variable
length
coder 1060. Image processor 1000 may be implemented as a hardware processor,
such as an application specific integrated circuit (ASIC), in a field
programmable
gate array (FPGA) device, or the like. In some cases, image processor 1000 may
be
implemented as software instructions stored on a non-transitory computer
readable
medium, wherein the software instructions, when executed by a general purpose
processor, cause the processor to perform the described functions. In some
embodiments, portions of image processor 1000 may be implemented in software
and other portions implemented in hardware.
[106] Color conversion module 1020 may receive image data, for example from an
image sensor of a WCE capsule, and convert from a first color space to a
second
color space. For example, color conversion module 1020 may convert RGB pixel
data to the YEF color space, according to Equations (1), (2) and (3).
Accordingly,
color conversion module 1020 may be configured to generate a luma channel
based
on the plurality of RGB color channels, generate a first chroma channel based
on a
difference between the luma channel and a first color channel in the plurality
of color
channels (e.g., green component), and generate a second chroma channel based
on
a difference between the luma channel and a second color channel in the
plurality of
color channels (e.g., blue).
¨ 17 ¨
CA 02773795 2012-04-11
. . .
[107] Each sample value of the luma channel may be generated using addition
and
shift operations. Similarly, each sample value of the chroma channels may be
generated using addition, negation and shift operations, as described herein.
[108] Moreover, each sample value of the luma channel may be generated based
on a summation of corresponding sample values in the plurality of color
channels, as
described herein. The summation may be of: a first color channel sample value
bitshifted once to divide by two; a second color channel sample value
bitshifted twice
to divide by four; and a remaining color channel sample value bitshifted twice
to
divide by four.
[109] Correspondingly, the difference between the luma channel and the first
color
channel may be computed based on: the luma sample value bitshifted once to
divide
by two; and the first color channel sample value bitshifted once to divide by
two.
Similarly, the difference between the luma channel and the second color
channel
may be computed by: computing a sum of the first color channel sample value
and
the remaining color channel sample value, bitshifting the sum three times to
divide
by eight, and subtracting from the bitshifted sum the second color channel
sample
value bitshifted twice to divide by four.
[110] The first and second color channels can be selected to correspond to
colors
other than a dominant color in the image (e.g., red, for endoscopic images).
[111] Optionally, YEF image data may be provided to clipper 1030, which may
clip
at least one portion of the image according to a clipping algorithm, as
described
herein. In some embodiments, clipping may be performed on RGB image data,
prior
to conversion into the YEF color space.
[112] Optionally, the YEF image data may be provided to subsampler 1040, which
may subsample YEF image data according to one or more selected subsannpling
schemes. For example, subsannpler 1040 may be configured to subsample the YEF
image data according to a YEF812 scheme. Accordingly, subsannpler 1040 may
subsample the first chroma channel relative to the luma channel, or subsample
the
second chroma channel relative to the luma channel.
[113] YEF image data may further be provided to predictive encoder 1050, which
may be a DPCM encoder, a JPEG lossless predictive coder. In some cases,
predictive encoder 1050 may employ left pixel prediction based on JPEG
lossless
predictive coding mode-1.
¨ 18 ¨
CA 02773795 2012-04-11
'
. . .
[114] Image data encoded by predictive encoder 1050 may further be provided to
variable length coder 1010, which may be a Golomb-Rice encoder, for example,
as
described herein.
[115] Following variable length coding, the image data may be output to
produce a
processed image, e.g., for transmission by a WCE capsule transceiver.
[116] Some components have been omitted so as not to obscure description of
the
exemplary embodiments. For example, a parallel to serial converter (P2S) may
be
provided at the output of image processor 1000, to format output data into
serial data
suitable for wireless transmission.
[117] Experimental implementation and verification of image processor 1000
indicates that an average compression ratio (using a YEF812 sub-sampling
scheme)
is 80.4% for a WBI image and 79.2% for a NBI image, with an average PSNR of
43.7dB. Accordingly, resultant images of a QVGA resolution can be transmitted
at a
frame rate of 5 FPS in the experimental implementation.
[118] Referring now to FIG. 11, there is illustrated a flow diagram for an
exemplary
method of processing an image captured using a color image sensor, in
accordance
with at least some embodiments.
[119] Method 1100 begins with receiving image data from an image sensor at
1105.
In some cases, the image sensor may be an RGB image sensor and the image data
may be RGB image data.
[120] Optionally, at 1110, the image data may be clipped, for example by
clipper
1030. Alternatively, image data may be clipped following conversion to the YEF
color
space.
[121] At 1115, a luma channel in the YEF color space may be generated from the
RGB image data, for example by color conversion module 1020, as described
herein.
[122] At 1120, a first chroma channel may be generated from the RGB image
data,
for example by color conversion module 1020, as described herein. Similarly, a
second chroma channel may be generated from the RGB image data at 1125.
[123] Optionally, the first chroma channel image data may be subsampled at
1130,
for example by subsampler 1040, as described herein. Similarly, the second
chroma
channel image data optionally may be subsampled at 1135.
¨ 19 ¨
CA 02773795 2012-04-11
[124] At 1140, a plurality of difference values between the plurality of
predicted
sample values and the respective generated sample values may be computed, for
example by predictive encoder 1050, as described herein.
[125] At 1150, the plurality of difference values may be variable length
encoded, for
example by variable length coder 1060, as described herein.
[126] Image data may be output at 1160 to be transmitted wirelessly, for
example.
[127] Referring now to FIG. 12, there is illustrated a flow diagram for an
exemplary
method of generating an endoscopic image for wireless transmission, in
accordance
with at least some embodiments.
[128] Method 1200 begins at 1210, by illuminating a diagnostic area, for
example a
GI tract of a patient, using a light source, such as light source 125. In
particular, the
light source may be a wide spectrum light source, producing white light.
[129] At 1220, an image of the diagnostic area is captured using an image
sensor,
such as image sensor 120.
[130] Upon capturing the image, method 1200 may process the image, for example
by proceeding to 1105 of method 1100.
[131] Referring now to FIG. 13, there is illustrated a flow diagram for
another
exemplary method of generating an endoscopic image for wireless transmission,
in
accordance with at least some embodiments.
[132] Method 1300 begins at 1310, by illuminating a diagnostic area, for
example a
GI tract of a patient, using a light source, such as light source 125. In
particular, the
light source may comprise at least one narrow band light source, as described
herein. The narrow band light source may, for example, produce green or blue
light.
[133] At 1320, an image of the diagnostic area is captured using an image
sensor,
such as image sensor 120.
[134] Upon capturing the image, method 1300 may process the image, for example
by proceeding to 1105 of method 1100.
[135] The present invention has been described here by way of example only,
while
numerous specific details are set forth herein in order to provide a thorough
understanding of the exemplary embodiments described herein. However, it will
be
understood by those of ordinary skill in the art that these embodiments may,
in some
cases, be practiced without these specific details. In other instances, well-
known
methods, procedures and components have not been described in detail so as not
to
obscure the description of the embodiments. Various modification and
variations
¨ 20 ¨
CA 02773795 2012-04-11
may be made to these exemplary embodiments without departing from the spirit
and
scope of the invention, which is limited only by the appended claims.
¨ 21 ¨