Note: Descriptions are shown in the official language in which they were submitted.
CA 02774430 2013-11-21
CRITICAL LEVELS OF GLYCERIN BALANCED WITH SALT
FINES FOR DE-ICING SALT
FIELD OF INVENTION
This invention relates to de-icing and ice melting compositions in reducing
ice and
snow on surfaces.
BACKGROUND OF THE INVENTION
De-icing compositions are widely used in northern areas of the country,
particularly
in northern climates subject to heavy ice and snow conditions in the winter
months. The
inventor of the present composition has numerous patents on ice melters. See,
for example,
U.S. Patent No. 7,122,127, which relates to liquid ice melters, and U.S.
Patent No.
5,683,619 which relates to solid ice melting compositions which are
environmentally
friendly.
A good ice melter for roads, sidewalks, parking lots, etc. is inexpensive,
easy to
manufacture, effective in melting snow and ice, and easy to apply. The best
ones also
provide reduced corrosion to application equipment while also having
beneficial effects to
vegetation. All of these advantages in one ice melter have been a goal of the
ice melting
industry for some time.
Effective in melting means a product capable of melting below zero F. Ease of
application is also important because labor cost is one of the largest
components of melting
snow and ice. Liquid melters bring ease to the application process.
In Ossian, Inc.'s earlier U.S. Patent 5,683,619 (Ossian & Steinhauser), we
created a
product that melted below zero and could have a positive effect on vegetation.
The major
disadvantages to this earlier invention were the high cost to produce the
product and cost of
application. It used calcium chloride and urea in a dry melter composition.
When calcium
chloride is manufactured for industrial use it starts out as a liquid. The
water is then
evaporated to form a flake or pellet. This manufacturing process uses
considerable energy
adding to the cost of manufacture for the raw material. Some of this cost
could be avoided
if the ice melter were liquid as finished.
1
CA 02774430 2013-11-21
The solid ice melter of U.S. Pat. No. 5,683,619 is advantageous in that it is
an
effective melter, and brings a positive effect on vegetation. It is in content
a combination
of urea and calcium chloride in a solid particle format. In recent times it
has been of
interest to develop liquid ice melters. I n some environments, liquid ice
melters are
preferred to solid ice melters in that they give better coverage, they are
much quicker acting
melters, and they are more economical to prepare.
The liquid ice melter of U.S. Patent No. 7,122,127 is a product that is less
expensive to manufacture, easy to use, melts below zero and can have a
positive effect on
vegetation. In that invention, we used liquid calcium chloride solution
combined with
either dry or liquid urea, in critical ratios to achieve an effective liquid
ice melter.
The present inventor has invented both solid ice melters of the type described
above
and liquid ice melters of the type described above. Both have their useful
approaches
depending on the use, climate and conditions. One particularly preferred ice
melter is the
solid type ice melter of U.S. Patent No. 6,039,890 which relates to a quick
acting ice
melter, its melt value enhanced by the addition of an ice melter compatible
surface active
agent. The present invention represents yet further improvement on the
invention of U.S.
Patent No. 6,039,890 of March 21, 2000 entitled "QUICK ACTING ICE MELTING
COMPOSITION", Ossian et al.
In most general sense, the above-identified U.S. Patent No. 6,039,890 uses a
variety
of different surfactant surface active agents as coatings of solid ice melters
to achieve
enhanced melt values and provide quicker melt action. The application of the
present
Applicant, Ossian, along with another joint inventor, U.S. Patent No.
7,473,379 entitled
"PROCESSED RAFFINATE MATERIAL FOR ENHANCING MELT VALUE OF DE-
ICERS" involves addition of the product known as Raffinate to conventional
liquid or solid
ice-melters in order to further enhance melt value. The present invention may
be used with
the system of the previously incorporated by reference U.S. Patent No.
6,039,890 or with
the system of U.S. Patent No. 7,473,379 to the extent it describes solid
melters with
Raffinate additives.
The most common and therefore least expensive solid ice melters are those
based
upon chemical salts that gradually dissolve and form a salt solution (brine)
which lowers
2
CA 02774430 2012-04-12
freezing point. Salts used are chloride or acetates salts of Group I or Group
II metals, such
as sodium, potassium, calcium and magnesium. These salts can then be combined
with
environmentally friendly organic materials to enhance their melt value such as
urea.
In instances where chemical salts are used, these are generally white in color
which
blends completely in color with snow. It is at times desirable to add dyes to
these chemical
salts so that the person applying the deicer can easily distinguish areas
where the deicer has
been spread and areas where it has not. Typically used dyes are
environmentally friendly
water soluble, visible dyes of distinctly different colors than white.
The dye material must not be introduced into the deicer with water because the
chemical salts as above described tend to be hygroscopic and the contact with
contact with
moisture will cause them to bridge or cake in the packages. This is of course
undesirable.
It affects their ability to spread, the amount of surface upon which they can
effectively be
used, and make them difficult to handle.
It has further been found and, indeed, my previous Patent No. 6,039,890
addresses
the speed of melting as a critical component for an effective deicer. The
reason for this is
quite simple. Ice on surfaces represents a risk and the quicker the ice is
removed, the more
effective the ice melter and thus the higher the value it has to the consumer.
Accordingly, it is a primary objective of the present invention to enhance
melt value
of conventional chemical salt deicers.
It is another objective of the present invention to enhance melt value using
an
environmentally friendly, water soluble material which will dissolve water
soluble dyes.
Yet a further objective of the present invention is to provide a coating
material on
conventional chemical salt deicers which can apply not only ice melt value
enhancement
but also anti-caking and anti-bridging properties to allow for easier
packaging and
spreading of the deicer.
A further objective of the present invention is to find effective uses for
industrial
waste stream glycerin which is currently a glut on the market due to the
current high
popularity of bio-diesel fuel.
Another objective of the present invention is to provide the correct balance
of salt
fines and glycerin to achieve the correct anti-caking and anti-bridging
properties.
3
CA 02774430 2013-11-21
A method and means for accomplishing each of the above objectives as well as
others will become apparent from the detailed description of the invention
which follows
hereinafter.
BRIEF SUMMARY OF THE INVENTION
Solid ice melters are improved with an exterior coating of glycerin balanced
to the
correct critical level in comparison to salt fines which prevents caking and
bridging and
enhances melt value.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a graph showing melt value increase with a glycerin coating on the
solid
ice melt composition surface.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
It goes without saying that the ice melters of the present invention may be
used
alone or in combination with abrasives and absorbents, for example as
described in the
earlier U.S. Patent No. 6,039,890.
The deicer composition of the present invention is normally solid and is
formed
from a mixture by way of example of metal salts of alkaline and alkaline earth
metals.
Preferably with metal chloride salts and most preferably, the alkaline and
alkaline earth
metals, such as sodium chloride, potassium chloride, magnesium chloride and
calcium
chloride. Acetate salts may also be employed.
In its broadest process sense, these materials are mixed, then ground,
screened for
size and blended with any coating material, mixed and are then discharged into
a packaging
bin.
Ice melters work by attracting moisture from their surrounding environment and
creating a liquid brine. This brine lowers the freezing point of water, and
effectively
dissolves ice and snow on contact until it becomes so diluted to a
concentration where its
freezing point is raised nearly to that of water. At this point in time, its
effectiveness is
gone. As is well known, ice melters work because the ice melting composition
or brine
lowers the temperature at which water will freeze. In ice melter compositions
that do not
4
CA 02774430 2012-04-12
contain surface active agents at the interface of the ice melter brine and the
packed snow or
ice, the molecules are attracted inward in accordance with natural principles
of adhesion.
However, it has now been discovered with the addition of surface active
molecules as
herein described, the adhesion attraction of like molecules of a liquid
substance is quickly
absorbed into the ice or packed snow, increasing significantly its melting
speed. This is the
invention of the õ890 patent.
The critical component in the õ890 patent ice melter invention is the surface
active
agent that reduces the surface tension in the melting brine produced by the
various ice
melting salts. This surface active agent must be able to reduce surface
tension in high salt
solution concentrations at temperatures below the freezing point of water.
This is referred
to as being ice melter compatible.
The complexity of measuring surface active agent's effects on surface tension
when
used with ice melting agents to increase melting volume and melting speed can
be
overwhelming at best. The surface tension will change with each salt, the
concentration of
that salt in the solution, and the temperature of the solution. In the melting
process, the
concentration of the salt is constantly changing because the melting process
is one of
constant dilution. Also, temperature could and often changes with each
application. The
colder the temperature, the greater the surface tension becomes. It becomes
even more
complex when combinations of various ice melting salts are used.
There are several agents that can be used to reduce surface tension. Some of
the
various possibilities include nonionic, anionic, cationic and amphoteric
surfactants. For
these surface active agents to be successful, they would exhibit superior
wetting properties
in a high salt solution of sodium chloride, calcium chloride, magnesium
chloride,
potassium chloride or urea, either individually or in combination.
The overall objective is to reduce the surface tension in a high salt brine
solution.
This will allow the dry salt to convert to a liquid melting brine faster to
increase the melt
volume and the melting speed of the ice melting salt.
In the past, chemical salt based solid deicers often incorporated a dye for
visibility.
This was done by adding it to propylene glycol which was mixed with the other
materials.
Propylene glycol was used because one would normally not want to add water to
a
chemical salt based deicers, since the water would accentuate and accelerate
caking and
5
CA 02774430 2012-04-12
bridging. It has now been discovered that if the herein described levels of
glycerin are used
to dissolve the dye, then the dye and glycerin mixture or even glycerin alone
can be used as
coating on the solid mix with surprising results. Among its advantages, it is
cheaper than
propylene glycol; it has the advantage of being an anti-caking agent; and
surprisingly it also
enhances melt value.
Ice melting salts such as sodium chloride or blends of sodium chloride,
calcium
chloride, and magnesium chloride by their nature will congeal and cake. This
is a major
safety issue with salt storage in larger storage areas. The salt may bridge,
only to give away
later potentially causing injury or even death to someone who could be buried
by the
landslide effect of the salt pile. Such salt piles are common at city storage
areas.
Caking and congealing of salt or salt blends is also a major problem in
packaged
containers. Over time the material packs from the pressure of being piled on
top of other
bags. Add to this that salt is slightly hygroscopic and this drawing moisture
action will
lead to caking and bridging not only in bulk storage but as well in bag
storage, such as at
stores.
Glycerin added in the herein described percentage as an exterior coating will
decrease the caking of salt in either bulk storage or bag storage and will
increase melt
value.
Increasing melt value of salt is a goal of users in the field. Increasing the
melt value
of salt will reduce the quantity of salt being used. Reducing the amount of
salt usage has
long been a goal of users in the industry. In Patent No. 6,039,890, we
introduced the
concept of coating salt with surfactants to increase melt value. This has been
very effective
and millions of pounds of product have been used employing this technique.
This
percentage level glycerin coating improves it further.
Glycerin may be coated alone or coated on top of a surfactant coated material
and
may be coated as glycerin in pure form or by product glycerin and may be mixed
with
visible dye or not mixed with visible dye, as the applicator wishes. An
important feature is
the percentage level of glycerin used. Generally speaking, the amount of
glycerin can be
from 0.5% to 4.0% by the total weight of the ice melt composition. The volume
of glycerin
used will largely be determined by the quantity of salt fines. Fines are
defined here by the
percentage of the total salt mixture that passes through #10 U.S. Screen. The
more fines in
6
CA 02774430 2012-04-12
the salt mixture the more exposed surface area would be expected. Salt fines
are a major
contributor to caking and bridging of salt in bulk piles and packaged. For
glycerin to
provide anti-caking value it is helpful to coat the available surface area of
the salt. Coarse
salt containing minimal amount of fines would require 0.5% to 1.0%. Coarse
salt would be
defined here as that salt mixture that would not pass through #10 U.S. Screen.
A mixture
of 50% coarse and 50% fines would require 2.0% glycerin. The objective is to
thoroughly
coat the salt mixture based on the screen sizing of the salt. To increase
levels beyond those
listed would likely lead to run-off and leaching issues. But, the minimum
level here
described is needed to be effective. Generally the finer the salt material,
the more glycerin
is needed.
A summary of glycerin requirements based on salt screening follows:
Coarse Salt ¨ 1/2 to 1 gallon Glycerin coating (0.5% to 1.0% by weight) per
1000 lbs. of
salt.
Typical Screen Range
U.S. Screen 1/2" 0 to 24 % percentage cumulative
U.S. Screen 3/8" 3 to 45 % percentage cumulative
U.S. Screen 1/4" 16 to 45% percentage cumulative
U.S. Screen # 4 38 to 85% percentage cumulative
U.S. Screen # 7 63 to 95% percentage cumulative
U.S. Screen # 10 83 to 99% percentage cumulative
U.S. Screen # 18 95 to 100% percentage cumulative
Medium Salt ¨ 1 to 2 gallon Glycerin coating (1.0% to 2.0% by weight) per 1000
lbs. of
salt.
Typical Range
U.S. Screen # 6 10 to 40% percentage cumulative
U.S. Screen # 8 10 to 40% percentage cumulative
U.S. Screen # 10 10 to 40% percentage cumulative
Pan 0 to 10% percentage cumulative
7
= CA 02774430 2012-04-12
Fine Salt ¨ 2 to 4 gallon Glycerin coating (2.0% to 4.0% by weight) per 1000
lbs. of salt.
Typical Range
U.S. Screen # 12 10 to 40% percentage cumulative
U.S. Screen # 14 10 to 40% percentage cumulative
U.S. Screen # 18 10 to 40% percentage cumulative
Pan 0 to 20% percentage cumulative
The following are typical industrial specifications for preparing a product
which is
both the '890 patent and the present improvement all in the same ice melter.
Production Specifications ¨ Procedures for Blue Dye plus Surfactant mixes:
1. Begin with an empty tank;
2. Add 160 gallons (1,648 lbs.) Glycerin;
3. Add 4500 grams of blue polymeric colorant
4. Mix with mixer blades
5. Add 40 gallons (350 lbs.) 8 mol nonionic surfactant
6. Mix with mixer blades
7. Mix with prop prior to adding to the blender/mixer.
One gallon of dye will weigh approximately 9.5 lbs. per gallon.
The above mix could be repeated using just the glycerin. The ratio would be
4500
grams of polymeric colorant to 200 gallons of glycerin.
The dye mixture is added to salt as a coating process in a
blender/mixer/coating
auger. The dye mixture ratio may vary per product. The glycerin and nonionic
surfactant
may vary per product. A typical production example follows:
Product Specifications ¨ Procedures for dye coating
1. Begin with an empty and clean blender;
2. Add 5000 lbs. of screened medium salt (through # 4 and on # 10)
3. Add 3 1/2 gallons Blue Dye Surfactant mix;
4. Add 5000 lbs. of screened medium salt (through # 4 and on #
10)
8
CA 02774430 2012-04-12
5. Add 3 1/2 gallons Blue Dye Surfactant mix;
6. Let mixer run approximately two minutes, discharge into packaging bin.
The small weight percentages of the glycerin, surfactant and coating used in
the
above 10,000 pound batch are as follows:
Glycerin content: .005768
Nonionic surfactant content: .001225
Polymeric colorant: .000035
The typical ranges of the above will vary depending on the specific product
and the
fines content of the salt in the mixture, but for the most part will fall into
the following
ranges:
Glycerin content: from .005 to .04
Nonionic surfactant: from .001 to .002
Polymeric colorant: from .00001 to .0001
The polymeric colorant can be any suitable water soluble environmentally
friendly
dye. It can for example be Liquitint (trade mark) Brilliant Orange, Liquitinit
(trademark)
Pink AL, Liquitinit (trade mark) Green HMC, and Liquitinit (trade mark) Patent
Blue. The
dyes can be purchased from Milliken Chemical, 1440 Campton Road, Inman, South
Carolina 29349.
The following example and test are offered to further illustrate but not limit
the
process and product of the present invention.
9
= CA 02774430 2012-04-12
EXAMPLE
To test the value of glycerin, glycerin from bio-diesel production was tested
as
below described:
Sample A and Sample B
Dried Solar Salt was screened through a # 4 U.S. screen to eliminate large
particles
and balance was kept on a # 10 U.S. screen to eliminate fines. Fines tend to
increase
caking issues. Sample A was weighted out to 1000 grams and water was added to
equal 1
percent. Sample B was weighted out to 1000 grams and glycerin was added to
equal 1
percent. After the glycerin coating to Sample B water was added to equal 1
percent. This
was the same sample amount of water that was added to Sample A. Both samples
were
placed in a lab oven at 120 degrees F for ten days to accelerate caking issues
from summer
heat.
Results
After ten days the two samples were removed from the lab oven with the
following
results:
Sample A ¨ hard cake ¨ would not flow
Sample B ¨ free flowing
From the results observed above, we concluded percentages of glycerin either
pure
or from bio-diesel fuel production waste stream can significantly reduce
caking of sodium
chloride in storage. This knowledge was used to set up melt value tests.
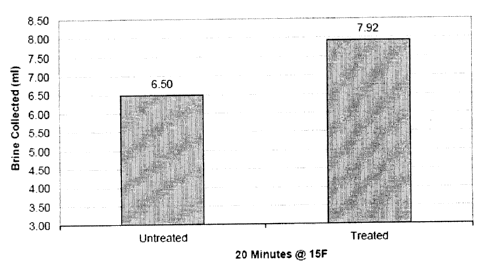
Using the Strategic Highway Research Program (SHRP) H-205.1 standards for
testing ice melters the following samples were placed in a laboratory
controlled freezer and
melt values established for 20 minutes at 15 degrees F. The test was repeated
three times
for the untreated sample and three times for the treated sample with averages
plotted on the
graph of Figure 1.
- CA 02774430 2012-04-12
,
..-
All samples were tightly screened through # 8 US Screen and on # 6 US Screen.
No
fines were present in the samples tested. One set of three samples was not
treated as a
control and the other three samples were coated with 1 per cent glycerin.
Samples # 1 through # 4 ¨ sodium chloride
Samples # 5 through # 6 ¨ sodium chloride coated with 1 per cent glycerin
From the above example, it is seen that the present invention has demonstrated
that
glycerin from bio-diesel production and used in percentages from 0.5% to 4.0%
will reduce
caking and bridging in storage of sodium chloride. The ice melting tests
(Figure 1) have
shown glycerin coated sodium chloride or blends of sodium chloride with
additional ice
melting agents in small percentages will increase melt value. In addition,
glycerin can
enhance the dye mix process commonly used in ice melters without causing
caking and
bridging. Glycerin replaces the water and/or propylene glycol that are added
as the carrier
for dye mixes that are used to coat ice melting salts. Glycerin dye mixes may
also be
blended with surfactants prior to coating the ice melting salts, as discussed
above.
Further as can be seen as a general trend, the more finer, the more glycerin
is
required. Correspondingly, the more coarse and less finer composition requires
less
glycerin. Fines tend to increase caking issues that need to be dealt with to
maintain caking
and bridging properties within the range of acceptable de-icer qualities.
11