Note: Descriptions are shown in the official language in which they were submitted.
CA 02774546 2014-05-01
COMPOSITIONS AND METHODS FOR DETERMINING ALLOYS FOR THERMAL
SPRAY, WELD OVERLAY, THERMAL SPRAY POST PROCESSING
APPLICATIONS, AND CASTINGS
Technical Field
The present invention relates generally to metallurgy. More particularly, some
embodiments relate to: amorphous, nanocrystalline, or microcystalline metals;
and weld overlay
materials.
Description of the Related Art
Amorphous metallic materials made of multiple components with a non-
crystalline
structure are also known as "metallic glass" materials. The materials often
have different
behaviors from corresponding metals with crystalline structures. Notably, an
amorphous
metallic material is usually stronger than a crystalline alloy of the same or
similar composition.
Bulk metallic glasses are a specific type of amorphous materials or metallic
glass made directly
from the liquid state without any crystalline phase. Bulk metallic glasses
typically exhibit slow
critical cooling rates, e.g., less than 100 Kis, high material strength and
high resistance to
corrosion. Bulk metallic glasses may be pioduced by various processes, e.g.,
rapid solidification
of molten alloys at a rate that the atoms of the multiple components do not
have sufficient time to
align and form crystalline structures. Alloys with high amorphous formability
can be cooled at
slower rates and thus be made into larger volumes and can be produced using
common industrial
practices such as thermal spray processing or welding. The determination of an
amorphous
material is commonly made using X-ray di ffractometry. Amorphous materials
lack translational
symmetry, and thus produce X-ray diffraction spectra composed of a single
broad hump as
opposed to the sharp peaks defined over a narrow diffraction angle range
typical to crystalline
materials.
-1.-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
The formation of metallic glasses is very complex as compared to conventional
crystalline materials, and thus modeling efforts designed to understand and
predict production of
metallic glasses are not very accurate. Many modeling criteria have been
developed to predict
certain aspects of metallic glass design. These models typically fail to
include specific
quantifiable components and therefore fail to provide concrete metallic glass
formation ranges.
As a result, metallic glasses are developed primarily through a trial and
error experimental.
process, where many alloys must be produced and evaluated before a metallic
glass composition
is discovered.
Despite the many advantageous properties of metallic glasses, it is often
useful to contain
a level of crystalline in the material ranging from a small fraction to
completely crystalline.
Nanocrystalline or fine-scale grained materials are known to contain higher
hardness and
strength than equivalent larger grained materials. Metallic glasses are known
to form
nanocrystalline precipitates when cooled a slower rate than their glass
forming ability requires.
Even slower cooling produces complete crystallinity ranging from nanometer
sized grains and
up. In general, materials which form metallic glasses have slower
crystallization kinetics and
will thus form smaller grain sized than common materials processed under the
same conditions.
In addition controlling the rate of cooling, it is alien possible to dictate
the crystallinity fraction
and grain size of a material though compositional control. By altering the
composition from its
optimum glass forming concentration, the precipitation of a particular
crystalline phase can be
encouraged under appropriate processing conditions. This technique has been
used to increase
ductility in metallic glasses.
Most materials, even those capable of forming completely amorphous structures
under
thermal spray processing, do not have slow enough crystallization kinetics to
form. an amorphous
material when welded. Nevertheless, the crystallization kinetics are such that
a fine-scale grain
structure is likely to form.
Most hardfacing materials, especially when dealing with weld overlays capable
of
exceeding 60 Rockwell C hardness, suffer from. cracking during the weld
process as well as poor
toughness. In addition to other problems, this cracking limits such a
materials use in any
application where impact occurs. Accordingly, the durability of hardfacing
weld overlays can be
substantial improved by reducing the potential for cracking and increasing the
overall toughness
of the weld.
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
Using tungsten carbide ("WC") as a hard particle reinforcement in the weld
overlay
technique is another typical method of hardfacing. This technique involves
pouring WC into the
molten weld bead as the hardfacing material is being welded onto the
substrate. In many
applications this technique offers a very good hardfacing layer, however it is
difficult to apply a
hardfacing layer of this type using a hard material as the matrix for the WC
particles, particularly
when cracking in the hardfacing layer is not desirable. Extreme wear
applications often demand
improved wear perfoi __ mance beyond that which can be offered using a ductile
matrix with WC
particles, because the matrix itself is likely to wear away at an accelerated
rate leaving the hard
particles exposed to shatter or pull out from the surface. Under conditions of
extreme impact as
.10 well as wear it is important to eliminate cracking in the weld bead.
Hardbanding is a technique used to protect the drill stem during operation in
oil and gas
drilling. The hardbanding is a weld overlay made onto a round tool joint,
typically 6" in
diameter, which is applied in the field. The hardbanding overlay is designed
to be a hard wear
resistant alloy which centers the drill stem within the casing, as well as
protects the drill stern
from wearing itself away on the casing.
Brief Summary of Embodiments of the Invention
According to certain aspects of the present disclosure, weld overlay materials
are
disclosed. In some embodiments, one or more materials of the present
disclosure can be used as
a superior weld overlay material for the protection of tool joints in oil and
gas drilling operations.
In some embodiments, one or more materials of the present disclosure can he
used for other
overlay hardfacing applications.
According to certain aspects of the present disclosure, an iron-based alloy is
provided.
The alloy can have a microstructure comprising a fine-grained ferritic matrix.
The alloy can
have a 60+ Rockwell C surface. The ferritic matrix can comprise <10um Nb and W
carbide
precipitates.
According to certain aspects of the present disclosure, a method of welding is
provided.
The method can comprise forming a crack free hardbanding weld overlay coating
with an iron-
based alloy. The alloy can have a microstructure comprising a fine-grained
ferritic matrix. The
alloy can have a 60+ Rockwell C surface. The terrific matrix can comprise <I
Onm .Nb and W
carbide precipitates.
-3-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
According to certain aspects of the present disclosure, a method of designing
an alloy
capable of forming a crack free hardbanding weld overlay is provided. The
method can
comprise the step of determining an amorphous forming epicenter composition.
The method can
further comprise the step of determining a variant composition having a
predetermined change in
constituent elements from the amorphous forming epicenter composition. The
method can
further comprise forming and analyzing an alloy having the variant
composition.
Other features and aspects of the invention will become apparent from the
following
detailed description, taken in conjunction with the accompanying drawings,
which illustrate, by
way of example, the features in accordance with embodiments of the invention.
The summary is
not intended to limit the scope of the invention, which is defined solely by
the claims attached
hereto.
Brief Description of the Drawings
The present invention, in accordance with one or more various embodiments, is
described
in detail with reference to the following figures. The drawings are provided
for purposes of
illustration only and merely depict typical or example embodiments of the
invention. These
drawings are provided to facilitate the reader's understanding of the
invention and shall not be
considered limiting of the breadth, scope, or applicability of the invention.
It should be noted
that for clarity and ease of illustration these drawings are not necessarily
made to scale.
Figure IA is a table illustrating a variety of atomic radii for some elements
that may serve
as constituents of some embodiments of the invention.
Figures 1B-1.1 are graphs illustrating various characteristics of some
embodiments of the
invention.
Figure 2 is an x-ray diffraction spectrum of an embodiment of the invention.
Figure 3 is an x-ray diffraction spectrum of an embodiment of the invention.
Figure 4 is an x-ray diffraction spectrum of an embodiment of the invention.
Figure 5 is an x-ray diffraction spectrum of an embodiment of the invention.
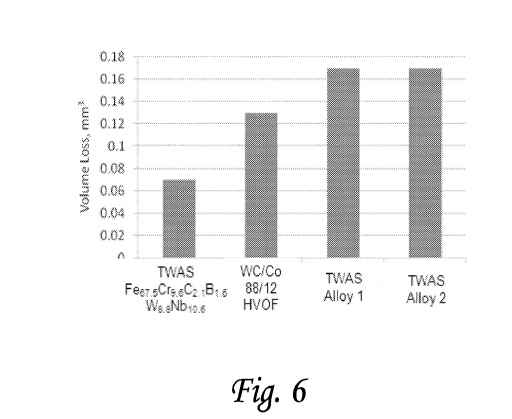
Figure 6 is a wear performance comparison between an embodiment of the
invention and
other materials.
-4-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
Figure 7 is a coefficient of friction comparison between an embodiment of the
invention
and other materials.
Figure 8 is a galvanic potential comparison between an embodiment of the
invention and
another m.ateria1.
Figure 9 is a scanning electron microscope image of an embodiment of the
invention.
Figure 10 is a dry sand wear test comparison between an embodiment of
invention and
other materials.
Figure 11 is a scanning electron microscope image of the results of a Vickers
indentation
test on an embodiment of the invention.
Figure 12 is a scanning electron microscope image of an embodiment of the
invention.
Figures 13A and 13B are scanning electron microscope images of an embodiment
of the
invention.
Figure 14 is a scanning electron microscope image of an embodiment of the
invention.
Figure 15 is a scanning electron microscope image of an embodiment of the
invention.
Figure 16 is a scanning electron microscope image of an embodiment of the
invention.
Figure 17 is a scanning electron microscope image of an embodiment of the
invention.
Figure 18 is M1G weld head of alloy on 4140 steel 6" diameter pipe showing no
cracking
or cross-checking as measured using liquid dye penetrant.
Figure 19 is a diagram depicting an alloy desip process according to certain
aspects of
the present disclosure.
Figure 20 is a graph illustrating an amorphous forming composition epicenter
and an
associated amorphous forming composition range according to certain aspects of
the present
disclosure.
Figure 21 shows an exemplary arc melter that can be used to melt an
homogeneous alloy
ingot for solidification analysis.
-5-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
Figure 22 is a phase diagram that is used for predicting behavior of an alloy
when
specific alloying elements are either added or subtracted from an amorphous
forming epicenter
composition according to certain aspects of the present disclosure..
Figure 23 is a diagram illustrating an exemplary alloy formation and analysis
procedure.
Figure 24 is a diagram depicting liquid composition versus cooling curves for
various
constituent compositions.
The figures are not intended to be exhaustive or to limit the invention to the
precise form
disclosed. It should be understood that the invention can be practiced with
modification and
alteration, and that the invention be limited only by the claim.s and the
equivalents thereof
Detailed Description of the Embodiments of the Invention
Some embodiments are described herein in terms of structural sites. In these
embodiments sonic components occupy solvent sites and others occupy primary
solute sites. In
further alloys, further components occupy secondary solute sites and in some
cases components
occupy tertiary solute sites. In many embodiments, the primary solute elements
are defined as
the solute which are larger than the solvent. elements. For example, the
primary solute elements
may he approximately as at least 5% larger than the solvent elements. Figure 1
A is a table
illustrating atomic radii of various elements that may serve as components in
various alloys
according to some embodiments of the invention.
In some embodiments of the invention, a class or group of compositions is
determined
using two criteria. In these embodiments, the first criteria is that the
primary solute elements are
larger than the solvent element, and the second criteria is that the
thermodynamic properties of
the compositions vary from those that would be predicted from the constituent
elements alone.
As an example of the first criteria, the primary solute element may comprise
an element that is at
least approximately 10% larger than the solvent element,
In one embodiment, a first class of alloys that satisfy these criteria may be
formed when
the solvent elements comprise transition metals ranging in atomic sizes from
approximately 1.27
to 1.34 A.. A.s illustrated in Figure IA. some of these candidate elements may
comprise V. Cr,
Mn, Fe, Co, Ni, or Cu, for example. In this embodiment, some bulk metallic
glasses may be
formed by the addition of a larger primary solute element having an atomic
size at least
-6-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
approximately 10% larger than the size of the solvent element. In this
embodiment, these
primary solute elements range from elements having atomic radii of at least
about 1.41 A for
solvent elements having atomic radii of approximately 1.27 A to elements
having atomic radii of
at least about 1.47 A for solvent elements having atomic radii of 1.34 A. For
example, for Cu as
a solvent, some possible candidate primary solutes comprise Mo. Pd, W, A.g,
Al, Ti, or larger
elements. Compositions formed according to this embodiment may further
accommodate
secondary or tertiary solute elements comprising metalloids or nonmetal
elements. For example,
such elements might comprise C, B, Si, P, N, or S. In further embodiments,
this range of
compositions may be more precisely defined according to certain filet Eno-
dynamic properties.
In these embodiments, the second criteria for the class of compositions is
satisfied when
the alloys have a low liquid energetic state in comparison with the energy of
the solid.-state. For
example, deep eutectics may be used as an experimental measure of the
theruodynamic strength
of the liquid in relation to the potential solid phases which it can loon. in
some embodiments,
these energy comparisons may be performed by quantifying the eutectics of the
compositions
using a comparison between the actual melting or liquidus temperature of a
specific. alloy is
compared to a calculated predicted liquidus temperature of the alloy. In these
embodiments, the
calculated liquidus temperature may he determined using a rule of mixtures
type equation using
the atomic percentages of the component elements and their respective pure
melting temperature.
For example, the calculated liquidus temperature Te is determined according to
the equation
x17 where x, is the at.% of the component i and Ti is its pure melting
temperature. For
example, alloys within the compositional ranges of some embodiments of the
invention may
have calculated liquidus temperatures, T.?, that are at least approximately 5%
greater than the
actual melting temperatures of the alloys. In further embodiments, different
ratios between
actual and calculated melting temperatures may he used. For example, as
described herein some
embodiments may comprise alloys having some degree of crystallinity, for
example some alloys
may comprise a micro or nanocrystal line alloys. Alloys within these
embodiments may have
calculated temperatures that are, for example, at least approximately 2% or 3%
greater than the
actual melting temperatures of the alloys. In still further embodiments, even
deeper eutectics
might be desirable for some applications, such as situations where molten
alloys will experience
lower than typical cooling rates. Alloys within these embodiments may have
calculated
temperatures that are, for example, at least approximately 7% or 8% greater
than the actual
melting temperatures of the alloys.
-7..
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
In some embodiments, the components of alloys may occupy distinct topological
sites
within the alloy. For example, a larger primary solute element may act as a
centralized, cluster
site for solvent atoms to bind to during cooling. hi these embodiments, these
clusters allow the
formation of a non-translational atomic packing scheme which resists
crystallization.
Furthermore, these larger solute atoms may generate elastic strain energy in
an emerging
crystalline embryo lattice composed. of solvent elements and increase the
likelihood for such an.
embryo to re-dissolve instead of acting as a seed for crystallization. in
som.e instances, the
topologies of these embodiments further allow secondary and tertiary solute
elements to occupy
interstitial sites that occur between the dense packing clusters. In some
cases, these secondary or
tertiary solute elements may create strong chemical interactions with the
solvent elements.
In one embodiment, a class of metallic glass forming alloys comprises
transition metal
solvents with atomic radius sizes ranging from 1.27 to 134 A. in this
embodiment, primary
solute sites may make up between 3 to 20 at.% of the alloy composition. These
primary solute
sites may be occupied by elements with atomic radii that are at least
approximately 10% larger
than those of the solvent. This embodiment may further comprise secondary
solute sites that
comprise approximately 10 to 25 at.% of the alloy composition. These secondary
solute sites
may be occupied by metalloid or nonmetal elements., for example C, B, Si, P,
N, or S. The
alloys within this embodiment further comprise alloys having melting
temperatures that are at
least approximately 5% less than a theoretical melting temperature calculated
using a sum of the
pure melting temperature of the components of the alloy weighted by their
atomic percentages.
Figures 1B through II illustrates some characteristics of examples of such
alloys. In these
xiT
-figures the alpha parameter is determined according to the formula a = ,
where xi is
atomic percent of the ith element, Ti is the melting temperature of ith
element, and Ti is the
liquid-us temperature of the alloy. As these figures illustrate, alloys that
form amorphous
structures tend to occur in ranges described herein.
In some alloys within this class, the number of available -- or occupied ¨
solute sites may
vary according to various characteristics of the components. For example, the
available
secondary solute site may be somewhat dependent on characteristics of the
primary solute or the
solvent. For example, a primary solute that has a radius approximately 15%
larger than that of
the solvent may allow different secondary solutes or different amounts of
secondary solutes to be
used while still retaining metallic glass forming characteristics_
-8-
CA 02774546 2012-03-16
WO 2011/035193
PCT/US2010/049381
In a further embodiment of the invention, a second class of alloys may
comprise alloys
having solvent elements with atomic sizes in the range of 1.39 to 1.58 A. For
example, solvent
elements within this second class may comprise Al, Ti, Zr, Nb, or Mo. In some
instances, alloys
within this class can accommodate a tertiary solute element in addition to
primary and secondary
solute elements. In this embodiment, primary solute sites may make up
approximately 10 to 30
at.% of the alloy composition. These primary solute sites may be occupied by
metallic elements
having atomic radii that are at least approximately 5% smaller than the
solvent elements. These
alloys may further comprise elements making up 2 to 10 at.% of the alloy
composition and
occupying secondary solute sites. In some embodiments, these secondary solute
elements may
comprise elements having atomic radii that are at least approximately 5%
larger than the solvent
elements. In further embodiments, these alloys may further comprise elements
making up 5-20
at.% of the alloy composition and occupying tertiary solute sites. These
elements occupying
tertiary solute sites may comprise metalloid or nonmetal elements such as C,
B, Si, P, N, or S.
Similarly to the first class of alloys, the alloys of these embodiments may be
further defined
according to their melting temperatures, wherein their melting temperature is
below a
predetermined percentage of a theoretical integer calculated using a weighted
sum of the pure
melting temperatures of the alloy's components. For example, in some
embodiments the alloys
may be defined according to a melting temperature that is at least
approximately 5% less than
such a theoretical temperature. In some embodiments, the addition of this
tertiary solute element
may increase packing density and thereby further increase viscosity of the
alloy.
In further embodiments of the invention, the described alloys may be modified
to produce
alloys forming micro or nanocrystalline structures. For example, the relative
sizes or amounts of
the solvents or solutes may be varied to promote such formations. For example,
the use of 1-2%
more of a solvent may result in an alloys that forms a nanocrystalline or fine-
grained structure
instead of an amorphous structure. Additionally, the temperature requirements
of some
embodiments may be relaxed so that alloys having slightly higher melting
temperatures, such as
2% less than the theoretical melting temperature, may be investigated for
micro or
nanocrystalline properties. In still further embodiments, bulk metallic glass
alloys may be used
to form micro, nanocrystalline or partially crystalline alloys without
modification. For example,
alloys within the above classes may be cooled at different rates or under
different conditions to
allow at least some crystallinity in the alloy.
-9-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
Figures 2 through 5 are x-ray diffraction spectrograms of alloys determined
according to
an embodiment of the invention. As discussed herein, in some applications may
be desirable to
provide compositions that are partially amorphous and partially
nan.ocrystalline. For example,
these coatings may be useful in wear and corrosion resistant twin wire arc
spray coatings. In
some applications, th.e coating may benefit from. having some limited amount
of crystallinity in
the coating to act as a hinder phase for the remaining hard amorphous
particles.
Figure 2 is an x-ray diffraction spectrogram illustrating a twin wire arc
spray coating
having the following composition:
Element Fe Cr Mo C B W Ni
wt percent (atomic 62 13 12 2.2 2.2 3.8 4.8
percent) (56.3) (12.7) (6.3) (9.3) (10.3) (1)
(4.1)
hi the illustrated coating, an amorphous phase fraction of approximately 75-
85% was
formed in the composition. In this composition, the Fe, Cr, and Ni occupy
solvent sites, the Mo
and W occupy primary solute sites, and the C and B occupy secondary solute
sites. Accordingly,
in the illustrated embodiment, elements occupying solvent sites comprise
approximately 73 at.%
of the alloy; elements occupying primary solute sites comprise approximately
7.3 at.% of the
alloy; and elements occupying secondary solute sites comprise approximately
19.6 at.% of the
alloy. In further embodiments, the specific elements occupying the topological
sites may vary
without significantly changing the atomic percentages of elements occupying
those top logical
sites. For example, in a further embodiment, an alloy may be formed by
reducing the percentage
of chromium while increasing the percentage of nickel to form an alloy having
a melting
temperature that is approximately 5% less than the calculated rule-of-mixtures
melting
temperature. In still further embodiments, the percentage of occupied sites
may vary. For
example, the atomic percentages of elements occupying the secondary solute
sites may be
increased at the expense of the elements occupying the solvent sites to form
an alloy having a
melting temperature that is approximately 3% less than the calculated rule-of-
mixtures melting
temperature.
Figure 3 is an x-ray diffraction spectrogram illustrating a twin wire are
spray coating
having the following composition:
Element Fe Cr Nb B Ni Si
Mu
wt percent (atomic 65.6 14.5 8.6 4.2 4.8 1.1
1.2
percent) (56.5) (13.4) (4.5) (18.7) (3.9) (1.9)
(1.1)
CA 02774546 2012-03-16
WO 2011/035193
PCT/US2010/049381
The illustrated composition has an amorphous phase fraction of approximately
45-55 %.
In this composition, the Fe, Cr, Ni, and Mn occupy solvent sites, the Nb
occupies primary solute
sites, and the Si and B occupy secondary solute sites. Accordingly, in the
illustrated
embodiment, the elements occupying the solvent sites make up approximately
74.9 at.% of the
composition; elements occupying the primary solute sites make up approximately
4.5 at.% of the
composition; and elements occupying secondary solute sites comprise
approximately 20.6 at.%
of the composition. As described herein, some variations of this alloy might
comprise
substituting similarly sized elements at appropriate topological sites, such
as a substituting Ga.
for Ni; other variations of th.i.s alloy might comprise increasing or
decreasing the atomic
percentages of the various sites, such as decreasing or increasing the atomic
percent of primary
solute site elements by 1-5% and increasing or decreasing the atomic percent
of secondary solute
site elements by a corresponding amount.
Figure 4 is an x-ray diffraction spectrogram illustrating a twin wire arc
spray coating
having the following composition:
Element Fe Cr Nb B Si Mn
wt. percent (atomic 65.9 24.6 4.6 2.2 1.5 1.2
percent) (59) (23.9) (3) (10.3) (2.7) (1.1)
The illustrated composition has an amorphous phase fraction of approximately
3545 %.
In this composition, the Fe, Cr, and Mn occupy solvent sites, the Nb occupies
primary solute
sites, and the Si and 13 occupy secondary solute sites. Accordingly, in the
illustrated
embodiment, the elements occupying the solvent sites make up approximately 84
at.% of the
composition; elements occupying the primary solute sites make up approximately
3 at.% of the
composition; and elements occupying secondary solute sites comprise
approximately 14 at.% of
the composition.
Figure 5 is an x-ray diffraction spectrogram illustrating a twin wire arc
spray coating
having the following composition:
Element Fe Cr
wt percent (atomic 67.3 9.6 2.1. 1.6 8.8
10.6
percent) (64.3) (9.8) (9.3) (7.9) (2.6) (6.1)
In this composition, the Fe and Cr occupy solvent sites, the Nb and W occupy
primary
solute sites, and the C and B occupy secondary solute sites. The illustrated
composition has an
amorphous phase fraction of approximately 0-20 %.
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
in general, amorphous phase fraction and coating hardness will vary according
to varying
spray parameters. In the embodiments illustrated in Figures 2-5, the coating
hardness as range
from approximately between 800 and 1100 Vickers hardness. The particle
hardness as our
functions of the material compositions and not the coating porosity or inter
particle adhesion.
Typical embodiments of amorphous or n.anocrystalline alloys formed within
these classes have
hardnesses that exceed 1200 Vickers.
Figures 6 and 7 are figures comparing known materials to the performance of
an. alloy
according to an embodiment of the invention. In these figures,
Fe67.5Cr9.6C2.IB I .611418.8Nb 1 0.6 was
compared to a tungsten carbide/cobalt (WC/C0) having 88% al. ,4) WC and 12
at.% Co; a first
Fe-based fine grain coating comprising FebõlanceC .04-.06S i ,6-1,5Cr25-30Ni5-
7Mn1.2-2.4B3. 2-3 .7 (Alloy 1);
and a second Fe-based fine grain coating comprising
FebaianceCr.,.:25Mo<A5B.,5W.,5C,2Mn<2Si<2
(Alloy 2). hi these comparisons, Fe67.5Cr9.6C2.1131.6%.sNb10,6 and the two
other Fe-based alloys
were deposited on a surface as under a twin wire arc spray coating. Due to the
properties of
WC/Co, this material was deposited using a high velocity oxygen fuel thermal
spray process. As
these results demonstrate, embodiments of this invention may serve as superior
materials for a
variety of applications requiring hardness and wear resistance. For example,
some embodiments
of this invention may serve as superior materials for bearing coatings, or for
bearings themselves.
Figure 6 demonstrates the results of a volume loss comparison using the ASTM
G77
metal sliding wear test. As the figure demonstrates,
Fe67,5Cr9.6CBi.6W8.8Nbio.6 had about a
0.07 min3 volume loss in the test, while WC/Co had about a 0.13 nur0 volume
loss and Alloy 1
and 2 each demonstrated about a 0.17 min3 volume loss. As these results
demonstrate,
Fe67,5Cr9.6C21131.6W8.8Nb10,6 demonstrated about an 86% improvement over WC/Co
and about an
142% improvement over Alloys 1 and 2. As described herein, similarity in
structures between
this embodiment and other embodiments of the invention are expected to result
in similar
improvements.
Figure 7 demonstrates the results of a coefficient of friction comparison
using the ASTM
G'77 metal sliding wear test. As the figure demonstrates,
Fe67.5Cr9,6C2.1B1.6W8.8Nb 10.6 has a
coefficient of friction of about 0.53, while WC/Co and Alloy 1 each have a
coefficient of friction
of about 0.61, and Alloy 2 has a coefficient of friction of about 0.65. As
these results
demonstrate, Fe67.5Cr9,6C2.1B1.6-W8.8Nb1l6 demonstrated about a 15%
improvement over WC/Co
and Alloy 1, and a 23% improvement over Alloy 2. As described herein,
similarity in structures
-12-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
and properties between this embodiment and other embodiments of the invention
are expected to
result in similar improvements.
Figure 8 shows the results of a galvanic potential comparison between an
embodiment of
the invention arid a comparison alloy. In this test,
Fe65.9Cr24.6Nb4.6B2.2Si1.5Mn1 .2 was compared to
FetralanecC:04-.(xiSi..6-1:5Cr25-30Nk7Mn1.2-2.4B3.2-3.7 in a seawater galvanic
cell with 316 stainless steel
serving as a reference electrode. The alloy according to an embodiment of the
invention
demonstrated a galvanic potential of about -275 mV as compared to about -375
mV for
FebaianceC.o4-ooSi.6-1.50.25-30N15-7Mni 2-1483.2-3.7. These results
demonstrate the superiority of some
embodiments of the invention in corrosive environments, such as seawater. The
results
demonstrate that some embodiments of the invention have potentials similar to
that of 400 series
stainless steels. Accordingly, embodiments of the invention may serve as
superior wear resistant
coatings in applications such as ship hulls where traditional Fe-based
coatings, even corrosive
resistant coatings such as FebalanceC.04-.06Si.6-1.5Cr25-30Ni5-7Mn1.2-2.4B3.2-
3.7, degrade too rapidly.
In various embodiments, many different materials may be formed using the
methods
described herein. For example, bulk metallic glass forming materials may be
determined
according to the formula Fe62-66Cr13-25(Vlo,Nb)4_12(C,B)2.74.4Ni0.4.8Si0-
1.5Mno-1 2W0-3.8, and
particularly according to the formulae
Fe62_66Cr14_16N138.10B44,4Ni3.4.8Si0_i.1Mn0_i:2 and Fe60-oCr20-
25N134-513 i-3Si .5Mn1 _2. In further embodiments, composite materials may be
formed by
combining components that are formed according to these formulae.
As described herein, adjusting some parameters may result in materials that
fotin
nanocrystalline or fine grained structures. For example, such nanocrystalline
or fine grained
structure may comprise materials de-fined by the formula
Fe67.69Cr9.6_10.9(Mo,Nb)9.2-1 0.6C 1 .4-".1B1.6-
1.8Sio-oiTio-o.2W73-9, and more particularly according to the formulae Fe67-
69Cr9.6-10.9C1.4-2.1 B1.6-
1.13W7.3-9Nb9.2-1 0_6 and Fe67-69Cr9.6-10.9M04-3C t I .8W7.3-9Nb4-.5.3. In
further embodiments,
composite materials may be formed by combining components that are formed
according to
these formulae. In still further embodiments, other amorphous forming
materials may be
similarly modified to result in materials that form nanocrystalline or fine
grained structures.
In additional embodiments, composite materials may be made that tend to form
partially
amorphous and partially nanocrystalline or fine grained structures. For
example, one or more
components defined by the above formulae for amorphous structured materials
may be combined
with one or more components defined by the above formulae for nanocrystalline
or fine grained
-13-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
structured materials. In a specific embodiment, such a material may comprise a
mixture of
components selected from the group comprising:
(1) Fe62Cr13Mo12C2.2B2.2W38Ni4.8,
(2) Fe65.9Cr24.6Nb4.682.2Sit.5Mn1.2,
(3) Fe65.6Cr14.5Nh8.6B4.2Ni4.8Si
(4) Fe67.5Cr9fIC2.1B I W 8.8Nb 1 0.6i and
(5) Fe63.4Cr9.4M012.5C2.5131.aW 10.4-
As described herein, some alloys that have a tendency to form amorphous or
partially
amorphous structures in some conditions are likely to form fine-grained weld
overlays.
Accordingly, some embodiments of the invention demonstrate improved hardness
and toughness
in hardface welding applications. Figure 9 is a scanning election microscope
(SEM) image of an
alloy according to an embodiment of the invention,
Fe67.5er9X2_1%.6W8.8.Nb10.6, demonstrating
this fine grain structure in a weld overlay coating. Figure 10 illustrates the
results of a test
comparing the alloy of Figure 9 to a first Fe-based fine grain coating
comprising
FebaranceCtwo6Si-6-1-5Cr25-3oNifoMik),1.4B3.2-3.2 (Alloy 1) and a second Fe-
based fine gain coating
comprising FebalanceCr.(25Mo<15L5W,5G2Mn<2Si<2 (Alloy 2). As illustrated, the
alloy according
the embodiment of the invention demonstrates a mass loss of about 0.07 G,
compared to about
0.14(3 for Alloy 1 and about 0.26 G for Alloy 2. Accordingly, the alloy of the
embodiment of
the invention demonstrates around a 100% to 200% improvement over Alloys 1 and
2.
In some hardfacing applications, WC or other hard particles are used as
reinforcing the
weld overlay. For example, coarse hard carbide particles may be introduced
into the weld bead
as it is being deposited. Some embodiments of the invention allow enable
hardfacing weld
overlays to be formed using WC or other hard particle reinforcement without
significant
cracking or decreased toughness. Furthermore, when these embodiments are used
for the matrix
of such reinforced weld overlays, they retain the hardness and wear resistance
described herein.
Figure 11 is an SEM image demonstrating the results of 1000 kg load Vickers
indentation on a
hardfacing weld overlay comprising a matrix of an alloy formed according to an
embodiment of
the invention and coarse carbide particles. In this test,
Fe75_1Cr1oNb1o84.65Tir25 was used as a
matrix for coarse carbide particles where the coarse carbide particles
constituted 50% by volume
of the weld overlay. These test results demonstrate the toughness of some
embodiments of the
invention; even under a 1000 kg load Vickers indentation, there was no
cracking at the interface
between the carbide particles and the matrix. Figure 12 is an SEM showing a
portion of this
-14-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
interface. As this figure illustrates, the hard carbide particles
reprecipitate at the interface as
opposed to dissolving into the matrix, which would otherwise introduce
brittleness into the
matrix. Figure 1.3 is a further illustration of the toughness of a carbide
reinforced weld overlay
according to an embodiment of the invention. Figure 13A demonstrates the fine
gain structure
of a carbide reinforced weld overlay comprising Fe751CrioNbtoR.I.65Ti.25.
Figure 1 3B illustrates
a further 1000 kg load Vickers indentation test, again demonstrating an
absence of cracking at
the interface between the carbide particles and the matrix.
Further embodiments of the invention comprise ranges of alloys that
demonstrate
precipitation of a substantial fraction of hard carbide particles in carbide
reinforced weld
overlays. inane of the embodiments, a range of alloys is defined by the
formula: Fe67_7
9.70V10,N08.8-1o.6C18-2.2131.4-1.6W74_8.8. For example, the alloy discussed
with respect to Figures 9
and 10 is an alloy within this embodiment. In a further embodiment, the range
of alloys
comprises alloys defined by the formula Fe67_71 Cr9,6_9,7M 0 g.8_10.5C)
s_2.2B1.4_1.6W7A-8.8. In further
embodiments, an alloy may be made up of a plurality of components, wherein one
or more of the
components comprises alloys defined by these formulae. Materials formed
according to these
embodiments have typical hardnesses of 1300-1450 Vickers hardness throughout
the entire
microstructures.
In another embodiment of the invention, a material that is suitable for hard
particle
reinforcement weld overlays comprises a component defined by the formula Fe43-
54Cr5.7-
7.2(Mo,N1:)6.6-15.5C38i_islA9,8_28Til _7. In further embodiments, such a
component may be
defined by the formula Fe50.5-532Cr6-7.2M06.6-7.9C1.3-LoBi-L9W25-76.6Ti3_5. In
additional.
embodiments, a component of a material may be defined partially by the first
formula and
partially by the second. For example, a component might comprise
Fe52Cr5.7M08.9C1.1 W262Ti6.1 =
In still further embodiments, an alloy comprises a plurality of components,
wherein the
components are each defined by one of the above formulae. Embodiments of the
invention
formed according to these formulae may demonstrate substantial precipitation
of hard particles in.
reinforced weld overlay applications. For example, Figure 14 is a SE.M
demonstrating the
precipitation of WC particles in a slow quenched ingot having a matrix
comprising
Fe43.2Cr5.7Mo 53C1.8B13W17.5Ti5. Figure 14 further demonstrates that alloys
formed according to
these embodiments retain a .fine-grained microstructure even under slow
cooling conditions.
Materials formed according to these embodiments have typical hardnesses of
13004450 Vickers
hardness throughout the entire microstru.ctures.
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
In one embodiment of the invention, a material that demonstrates hard particle
precipitation comprises a component defined by the formula
Fes4_75Cr9_14.4Ni.04.8(M.o,NbI7.9-19.7C1.6-2.1B1.3-4_6W0-9.98110.25-7Sio- .!
Mn0.1.1. Figure 15
demonstrates that a component comprising Fe54.6Cr7.2M019.7C2.1B1.1W9.5Ti5
demonstrates
precipitation of a high fraction of embedded hard particles during slow
cooling. Figure 15
further demonstrates the fine-grained nature of these embodiments that occur
in non-amorphous
phase forming conditions. In a further embodiment of the invention, materials
may be formed
having a component that is defined by the formula Fe70-7509-10Nb7-10B4-
4.6Ti.25-7, F e54-63Cr7.2-
9.6M08.6-19.7C I ,6-2. B1.1_1_7%.5_9.5Ti3_7. Additionally, in some
embodiments, materials may be
formed having components that comprise combinations of these formulae.
Materials formed
according to these embodiments have typical hardnesses of 1300-1450 Vickers
hardness
throughout the entire microstructures.
In further embodiments of the invention, materials may be foi tiled that
comprise mixtures
of alloys formed according to the formulae described herein. For example, a
material may be
formed comprising a plurality of components that are defined by the foitnulae
Fe54-75C1-0-14.4Ni.0-4.8(MO,N07.9.19.7C1.6.7.181.3-4.6W0-9.98Ti0.25-7Sio-
1.11V1110-1./ and Fe43_54Cr5.7-
7.2(M0,Nb)6.6.15.5C1-13B1-1.8W9.98-28Tii In a specific embodiment, a
material comprises a
mixture of one or more of the following components:
(I) Fe67.5Cr9,6M 05.3C2. I Bi_6W8.8Nbs.3,
(2) Fe69Cr1o.9Nh,.131.8C-1.4W7.3Si0.2Ti0.2,
(3) Fe67_5C,r9.6Mo1 0.5C2.2B
(4) Fe70,9Cr9.7M08,8C l3 W74,
(5) Fe43.1Cr5.7M015.5C1.8B1.3W)7.5Ti5,
(6) Fe50.5Cr7.2Mo7.9C1.6B1./W26.6Ti5,
75 (7) Fe53.7C r7,3M06.6C 1 .3B1W25.61).57
(8) Fe50.3Cr7.2Nb7.9C2.6B1.2W25.8Ti5,
(9) Fe57.2Cr73m06.6c1 W25.61.11,
(10) FC51,2Cr7.31\406.6C1.3B W25.61n7,
(11) Fe54.6Cr7.21V.016.7C2.1BI.IW9.5Tis,
(12) Fe67.4Cr9.6M09.4C1 sB W8.8 Ti
(13) Fe63Cr9.6M.08.6C1.6B .71A18.5Ti73
( 1 4) Fe70.2Cr96Mo8.7C1.7B1.4W7.4Ti ,
- I 6-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
(15) Fe66Cr9M08.2C .6131.3W6.9M,
(16) Fe75.1Cr1oNb OB4.65TiO.25
(17) Fe74.6Cr9.9Nh9.9134.6Ti1,
(18) Fe70Cr9.3Nb9.3B4.4Ti77
(19) Fe64.9Cr144Nb8.5B4,8 Ni4.1Tii Si1. Mill .2/
(20) FeoCr135Nb79B4.5
(21) Fe63.8Cr9,6Nb11,7C2ABI.5W10ri1,
(22) Fe62.3Cr9.6Nb9,6C1.9131.5W8,1Ti7.
In a process according to one embodiment of the invention, coarse hard
particles are
combined with a hard matrix material comprising components described herein.
This process
comprises melting a component as described herein over a layer of coarse
particles. For
example, an arc metier may be used to melt a matrix material over a bed of
coarse WC particles.
In sonic embodiments, although melted WC may have a tendency to reprecipitate,
it is desirable
to minimize the amount of WC that dissolves into the melted matrix. In one
embodiment, the
coarse particles are disposed on a cooling body, such as a grooved hearth. For
example, a water-
cooled grooved copper hearth may be used. In this embodiment, the coarse
particles are kept at a
lower temperature to increase their resistance to dissolution. Accordingly, in
these
embodiments, the WC particles are allowed to metallurgically bind to the
matrix without
substantially dissolving into the matrix.
hi particular experiments, this procedure was conducted using 30%, 40%, and
50% by
weight coarse tungsten carbide particles, wherein the remaining weight
percentages comprised a
matrix material comprising components described herein. Figures 16 and 17 are
SEM. images
demonstrating typical results of these experiments. Figure 16 illustrates a
typical result where 4-
8 mesh 80-20 WC/Co was used as the hard particle, forming a composite
material:
Fe37oCr5Nb5C1.8B2_4W42.2C06. As this figure indicates, the matrix forms a
metallurgical bond
with the WC/Co particle without substantially dissolving the particle into the
matrix. Figure 17
illustrates a typical result where 4-8 mesh 88-12 WC/Co particles served as
the hard particle,
forming a composite material: Fe52.7Cr71\1b7C1B1.3W23C06. This figure also
demonstrates a
metallurgical bond between the WC/Co particle and the matrix, without
substantial dissolution of
the particle into the matrix. The materials formed according to this
embodiment are Fe and W
based compositions comprising composite materials of WC hard particles
embedded in a hard
matrix. In these experiments, the Vickers hardness of the WC is approximately
1400, while the
-17-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
matrix demonstrated Vickers hardnesses of approximately 1200 due to some
dissolution of the
coarse particles into the matrix. The materials also demonstrate resistance to
cracking at the
interface between the particles and the matrix. Accordingly, these material
are well-suited for
applications where both extreme impact and extreme abrasive wear occur. In
some
embodiments, these materials may be pre-formed for use as components in other
applications.
As the materials cool, they may contract. Accordingly, the cooling surfaces,
such as the grooved
hearth, will typically be adjusted for such contractions.
In some embodiments, these composite materials may be formed using components
defined by the formula (Fe54.6-75.3Cr7.2-24.6M00- I 9.7C0-2.3B t .54.7 W0-
9.5Nb0-10Ii.0-7Si0-1.5Mn0-
1 0 1.2)"76.S-84.5C32-3,5C011-20)loo.x where x-50-70. In these embodiments,
a matrix material may
therefore be defined by the formula Fe54.6-75-3Cr7.2-24.6M00-19.7C0-2.3B1.5-
4.7W0-9.5Nb01017i.0-7Si0-
1.5Mno-1 .2- In some specific embodiments, a composite material comprises one
or more
components defined by the formulae:
(1) Fe)8.2Cr5M.o13.8C2.7B1.2W31113.5CO3.6
(2) Fe27.2Cr3.6M09.9C2.913o.9W47112.5C-06
(3) Fe38.2C,r5Mot 3.3C2.7B .21vV32Ti3.5CO3.6
(4) Fe32Cr4.6Mo5C).6B0.sW42.5112.5Coui
(5) Fe52,7Cr7Nb7C1B3.3W23Co6
(6) Fe46.20717.2Nb3.2C1.1B1..5W25.31I32Si i .1 .Mn0.8CO3.6
(7) Fe49Cr(i.5Nb6.5C1.1B3.1W15.3C06Ti.4.9Co3.6
(8) Fe37.6Cr5Nb5Ci,5B2.4W42.2C06
In further embodiments, other materials disclosed herein may serve as suitable
matrix
materials. For example, the compounds described above with respect to weld
overlay
applications may serve as suitable matrix materials for these composite
materials.
As described herein, a variety of elements may occupy solvent sites in various
embodiments of the invention. For example, both Fe and Ni have an atomic
radius of 128 A.
Accordingly, Ni may be substituted for some or all of an amount of Fe in the
materials and
components described herein. For example, in the material described with
respect to Figure 3,
Fe6.5.6Cr 145Nb8B42.Ni48Si11Mi. 2, arbitrary amounts of Ni may be substituted
for arbitrary
amounts of Fe, such that the melting temperature of the resultant alloy
remains at least
approximately 5% less than the melting temperature predicted by a rule of
mixtures. In some
-18-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
embodiments, these materials containing Ni may be particularly well-suited for
brazing
applications. In particular embodiments, these brazing alloys comprise alloys
having
components defined by the formula (Ni,Fe)50_95(Si,B,P)o-2oCro_35. In further
embodiments the
relationship between Ni and Fe may be further defined according to the methods
and processes
described herein, such as inspection of melting temperatures compared to rule
of mixture melting
temperatures. In a specific embodiment, such a braze material comprises at
least one component
selected from the group comprising Ni52B17Si3Fe28, Ni551318Cr4.Fe24,
Nie4B14SLI, Cr4Fe24,
Ni521370Fe28, and Fe43Cr33Ni10B14,
In additional embodiments, the alloys may further contain additives to enhance
or
introduce various features. For example, small amounts of Al, Ca, Y, mi.sch
metal, or other
materials may be added as oxygen getters. In the above formula, the addition
of these oxygen
getters results in the formula (Ni,Fe)50.95(Si,B,P)0_10Cr0.301,CA,Y,misch)0_1,
or more particularly
(Ni.,Fe)50-95(Si,13,P)0-2oCro_301.,CA,Y,misch)0_0.2.
In further embodiments, binder materials such as Al may be added to the
compositions
1.5 described herein. For example, the materials employed in twin wire arc
spray methods described
herein may be wrapped with a sheath such as mild steel, stainless steel,
nickel, nickel chrome, or
aluminum such that the resultant coating shows an increase in bond strength.
In some
embodiments, an amorphous or natiocrystalline coating produced using the twin
wire arc spray
method manufactured using a mild steel, stainless steel, nickel, or nickel
chrome sheath resulted
in bond strengths exceeding 8000 psi as measured by A.ST.M C 633. In further
embodiments,
wrapping an Al sheath around a solid or cored wire containing Ni-base
materials described
herein also may result in increased bond strengths. In additional embodiments,
AI may be added
to any material described herein in a range of concentrations. In these
embodiments, the other
elements of the material will typically be reduced by a proportional amount so
maintain their
relative concentrations. For example, Al may be added in concentrations of 0.5-
10% to form.
materials having components defined by:
(1) (Fe62-66Cr1 3-140V10,N43-12(C,B)4.2-4.4Ni4,8Sial . M110- I .2W0-3.8)1
00..xAlx
(2) (Fe67_69Cr9.6-to.9(M0,Nh)9.o.6C I .4-2. I 131.6-1.8S10-).2Ti0-0.2W7.3-
9)100-xAlx
(3) (Fe67-71Cr9.6-9.7M08.8-10.5C I.8-2.2B1.4-1 .6W7.4-8.8)100-xAlx
(4) ( Fe43-53Crs.74.2(M0,Nb)6.6-15.5C1-1 ..3B .3-1 .8W9.9S-28Ti I -7)1 00-
xAlx
(5) (Fe61-75Cr9.144 Ni0_4,8(M.O,Nb)7.9-1 I .7C1.6-2.1 B1.3-
4.6W0-9.98Ti0.254Sio-1.1.Mno-
.1)loo-Alx
-19-
CA 02774546 2012-03-16
WO 2011/035193
PCT/US2010/049381
where x ranges from 0.5% to 10%. In a specific embodiment, increased bond
strengths occur in
some applications where components are defined by the formula Fe65-67Cr1 i-
i3Nb4-6B4-51s114..6Sio.
1.5Mn0.1.5A11.3. In particular, the composition FeeCruNb6B4NisS11MniAl2
demonstrated a bond
strength exceeding 10,000 psi. Accordingly, the addition of Al to materials
described herein
may further increase the materials' utilities in applications requiring high
coating bond strength
and abrasion resistance.
Certain materials disclosed in the present disclosure can be directed toward
weld overlay
materials. In some embodiments, although they are suitable for other weld
overlay hardfacing
applications, the materials serve as a superior weld overlay material for the
protection of tool
joints in oil and gas drilling operations.
Some embodiments comprise an iron-based alloy capable of forming a crack free
hardbanding weld overlay coating on a curved substrate of 6" or smaller
without any pre-heating
or slow cooling methods, resulting in a 60+ Rockwell C surface. In further
embodiments, when
welded, the alloy has a welded microstructure comprising a fine-gained
ferritic matrix
containing <I Own Nb and W carbide precipitates. In still further embodiments,
the alloys may
be magnetic or non-magnetic in nature.
Particular embodiments comprise alloys falling within the range of alloys
defined by the
formula (in weight percent): Fe67.3-77.05Cr3-7N47C0.5-1.4130.6-1.75W9.5-
15A5Ti0_05Si0-0.5Mn0-2Nio-2.
Other embodiments comprise alloys falling within the range of alloys defined
by the formula (in
weight percent): Fe67.3-77.05Cr3-7Nb4-7C0.5-1.4B0 6-
1.75W9.5.15.45110_0.5Sio_05Mno_fiNio_3. A specific
embodiment comprises the alloy given by the formula (in weight percent):
Fe74.35Cr5Nb4V2B1Co.sW12.45Sio.isTio,25. Other embodiments comprise the alloy
given by the
formula (in weight percent): B I .15-1.25C1 .0-1.1Cr4.11-5.0FebalMn<1.0Nb.4.0-
4.2S i<1 .01.10.2-0.3 V 1 .95-2.05W 2.4 -
12.5. Other embodiments comprise the alloy given by the formula (in weight
percent): B1.15_
1.25C1.o-i.iCr4.8-5.oFeboMn<1.0Nb.4.0-4.2Si1.0Ti0.20.3V0.40-0.60%.8-9.2.
Figure 18 illustrates a metal inert gas (MIG) weld bead of an alloy
implemented in
accordance with an embodiment of the invention. Here, alloy 1800 comprised the
alloy defined
by the formula (in weight percent): Fe67.3-77.05Cr3.7Nb4-7C0.5- 1.4B0.6- 1
.75W9.5-15.45Ti0-0.5%0-0.5 Mn0-
-20-
CA 02774546 2012-03-16
WO 2011/035193
PCT/US2010/049381
6Nio-3. The weld bead 1800 was applied to a 4140 steel 6" diameter pipe 1801.
As measured
using a liquid dye penetrant, the weld bead shoed no cracking or cross-
checking.
In one embodiment, a microstructure of an alloy by the formula (in weight
percent):
Fe74.35Cr5Nb4V2B1 CosW i2.45Sio.15Tio.25 is provided. The microstructure of
this alloy includes an
optimized microstructure with a ferrite matrix having fine-grained niobium and
tungsten based
precipitates. These precipitates are less than about 10um on. average and
produce an alloy
having a unique hardness and toughness. The matrix is a fine-gained
ferriticiaustentic matrix
which is fully interconnected. The matrix is able to blunt cracking and
provides toughness to
the overall material The secondary phases and are extremely hard and are
plentiful in the
microstructure, forming up to 30% by volume fraction, but are isolated from
each other by the
interconnected matrix.
Three alloy compositions have been determined for manufacture into welding
wires for
hardbanding testing. The alloys have been determined from experimental results
as part of an
ongoing project to design hardbanding alloys, and subsequent laboratory
analysis of potential
alloys compositions. Initial laboratory results suggested these alloys as
ideal candidates and the
experimental welding trials have been conducted.
Th.c alloy presented in this disclosure, namely,
Fe74_35Cr,N14\72BiC0.5W12.45Si0a5Ti0.25,
immediately showed promise as the alloy formed a crack free weld overlay on a
6" round pipe
without the use of a pre-heating step. Further analysis, including independent
verification of a
crack-free weld, and wear performance, indicated that the weld alloy
represented a technological
advance to currently used alloys and materials for use in oil and gas
drilling.
The alloys presented in this disclosure offer many unique advantages to
currently
available weld overlay alloys, which when simultaneously utilized provide
substantial benefit to
the oil and gas drilling operation. Previously, no other single alloy could
offer all these benefits
to the hardbanding process and operation. Some of the advantages that
embodiments of the
invention present include the following.
First, crack-free as deposited welds: The alloys disclosed can he welded onto
curved
surfaces without the use of pre-heating or slow cooling techniques, and form a
continuous crack
free weld bead. The lack of pre-heating required is very advantageous not only
because it
eliminates an extra step in the process, but it prevents the possible
deterioration of the inner
polymer coating which is commonly used in drill pipes and is subject to
failure when the pipe is
-21-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
pre-heated. Slow cooling is also a step which is generally unavailable to
hardbanding done in the
field, and it is advantageous if it is not required. Previously, these
capabilities could be achieved
only with weld overlay alloys that had substantially lower surface hardness
levels.
Second, ability to be welded over itself and other weld beads without
cracking: Weld
overlays metallurgically bon.d to the substrate material and form a novel
diluted alloy which is
partially the original welding alloy and partially the substrate base material
alloy. This dilution
effect create different weld compositions depending upon the base metal that
it is being welded
onto. In the practice of oil drilling hardbanding it is common to re-weld over
the top of a weld
bead once it has partially worn away. hi the case of many hardbanding alloys,
this re-welding
creates enough of a compositional shift in the weld when compared to welding
atop of the
original un-welded part, that cracking occurs in the re-weld whereas none
occurred in the
original weld. The alloys presented in this patent are have sufficient crack
resistant such that
they can be welded atop previous welds and experience no cracking. Previously,
this capability
could be achieved only with weld overlay alloys that result in substantially
lower surface
hardness levels.
Third, high hardness: The alloys described in this patent contain hardness
levels of 60
Rockwell C or higher in the diluted condition when welded onto 4140 steel pipe
under
conditions similar to those used in the field application of hardbanding
alloys for tool joints.
Typical hardbanding alloys report 60+ Rockwell C values only when measured in
the undiluted
condition. However, in actual single pass weld overlays with significant
dilution, which is the
condition used for these applications, these alloys experience lower hardness
values.
Fourth, improved wear resistance: The alloys described in this patent possess
improved
wear resistance compared to the previously most advanced hardbanding alloys
used in oil and
gas drilling operations. The wear resistance is measured using the ASTM Ci65
dry sand wear
test. The wear loss of this alloy in the diluted condition (the condition
typically used in the
actual oil and gas drilling operations) was 0.1092 grams lost, significantly
better than previous
technologies which report un-diluted (condition resulting in lower wear
losses, and not a
condition used in the field) 0.12 g lost.
Fifth, the ability to absorb excess carbon with no or limited cracking: The
alloys
described in this patent arc compositionally designed to form a high fraction
of finely grained
carbide precipitates. The thermodynamics inherent to these alloys allow for
excess carbon to he
-22-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
absorbed into the weld without altering the advantageous microstructure,
resultin.g in no or
minimal cracking. This effect is advantageous in the hardbanding industry as a
MIG carbide
process is typically used to create hardbanding weld beads. in this process,
WC/Co particles 1
mm in size) are fed into the weld bead as the weld is being made. This process
creates a very
difficult substrate to weld atop as there is a large concentration of W and C
which will be
introduced into the re-welded composition and microstructure. Previously used
hardbanding
alloys would experience drastic changes in microstructure and properties as a
result of being re-
welded onto this particular substrate. However, the designed chemistry of the
alloy presented in
this patent are flexible enough to absorb the excess carbon and tungsten and
see relatively small
changes in microstructure and properties. Thus, even when welded onto a un-
worn MIG-carbide
weld bead, they will experience only slight cross checking.
Sixth, optimal microstructure for limited casing wear: In drilling operations,
the
hardbanding weld bead.s constantly rub and wear against the outer casing. It
is very critical that
the hardbanding weld bead not wear away sufficiently against the casing so as
to cause casing
failure, Alloys which do not result in. extreme casing wear are termed,
'casing friendly'. Early
hardbanding techniques such as MIG carbide, where coarse carbide grains were
introduced into
the weld, resulted in extreme casing wear and proved unacceptable in drilling
operations where a
casing was a requirement. The MIG carbide welds are formed of a soil steel
matrix containing
large carbide grains. The wear behavior is such that the steel quickly wears a
way leaving the
sharp carbide particles to gouge away at the casing. In the alloys presented
in this patent, the
carbide particles are very fine and evenly distributed so as not to cause
highly localized regions
of wear on the casing. Furthermore, the matrix is a hardened fine-grained
structure, which
exhibits hardening according to the Hall-Petch relationship. Thus, the casing
will be in contact
with a relatively smooth surface as opposed to a weld bead with sharp hard
particles which
locally wear and cause casing failure.
Some embodiments of hardbanding materials comprise alloys falling within the
range of
alloys defined, by the formula (in weight percent): Fe65.3-79.95Cr3_7Nio4)Mno-
oNb3.5-7V0-2.05C0.5-
1.5B0.6-1,75W8.5-15.45SiO-1oTio_i Alo..4. Particular embodiments comprise
alloy defined by the formula
(in weight percent): Fe653_79.95Cr5Ni0_6 Mnce6Nb3.s-6 V0-2C0.8-1.5[30.8-
1.4W8.5-13,5 SiO. 5Ti0,54A10.4.
Weight percents of various constituent elements in some exemplary embodiments
falling within
the range are listed, in the following table:
-23-
CA 02774546 2012-03-16
WO 2011/035193 PCT/US2010/049381
---------------------------------------------- -r
13
. . .
_.....
Fe Cr Ni Mn Nb V C W
Si Ti Al
,
Alloy ID ,
, .
_
115A 71.35 5 0 2 4 2 0.8 1 13.45 0.15
. 0.25 0
, .
-
11513 67.3 5 0 6 4 2 0.85 1 13.45 0.15
0.25 0
H5C 71.3 5 2 0 4 2 0.85 1 13.45 0.15 0.25 0
_ h
115D 67.3 5 6 04 2 0.85 1 13.45 0.15
0.25 0
J . .. _ .
H5E 65.3 5 2 6 4 2 0.85 1 13.45 0.15 0.25 0
. _ - _
115F 65.3 5 6 2 4. 2 0.85 1 13.45 0.15
0.25 0
. _
1I5G 72.65 5 0 0 4 2 1.3 1.2 13.45 0.15 0.25 0
= - _ .
11511 72.65 . 5 0 0 4 2 1.5 1 ,. 13.45 0.15 .
0.25 0
_
1151 72.85 5 0 0 4 2 1.5 0.8 13.45 0.15
0.25 0
. _
H5.1 72.15 5 0 0.5 4 2 1.1 1.4 13.45 0.15
0.25 0
. _ _
115K 77.3 5 0 0 4 2_ 0.8 1 9.5 0.15 0.25
0
H51. 75.5 5 0 . 0 4 2 1 . 1.1 11 . 0.15 ,
0.25 0
H5M 73.25 5
2 0 4 2 0.85 1 11.5 0.15 0.25 0
. -
115N 75.25 5
0 0 6 2 0.85 1 9.5 0.15 0.25 0
- - - .
H50 75.25 5 2 0 4 2 0.85 1 9.5 .._ 0.15
0.25 0
-
H5P , 75.3 5 2 0 4 _ 2 1 0.8 9.5 0.15 0.25
0
_
115Q 70.8 5 2 0.5 4 2 0.85 1 13.45 0.15
0.25 0 .
_ _
H5R 73.3 5
2 0 6 2 1 0.8 9.5 0.15 0.25 0
... , _ .
H5S . 73.8 5 . 3 , 0.5 4 2 1 0.8 9.5 0.15
0.25 0
115T 72.8 . 5 4 0.5 4 2 1 0.8 9.5 0.15 0.25
0
-
115U 66.9 5 , 6 0 4 2 _1.4 ... 0.8 13.5 0.15
, 0.25 0 _
H5V 72.3 5 . 3 2 4 . 2 1 0.8 9.5 0.15
0.25 0
- _
. 115W 73.3 . 5 3 . 1 4 , 2 1 0.8 9.5 0.15
_._ 0.25 0
H5X . 72.1 5 3 2 4 2_ 1.2 0.8 9.5 0.15
0.25 0
- _
H5Y 71.9 5 3 2 4 2 1.4 0.8 9.5 0.15 0.25
0
, - .
H5&, 70.1 5 3 2 4 2 1.2 0.8 _ 11.5 0.15
40.25 0
H5Z 70.9 5 6 0 4 2 1.4 0.8 _ 9.5 ... 0.15
0.25 0
H7A 78.75 5
0 0 4 0.25 1 1.1 9.5 0.15 0.25 0
_ _
117B 79 , 5 0 0 4 0 1 1.1 _ 9.5 0.15 0.25
0
-
H7C 76.75 5 0 0 4 0.25 1 1.1 9.5 0.15
. 0.25 2
_
117D 74.75 5 0 0 4 0.25 1 1.1 , 9.5 0.15
_ 0.25 4
H7E 78.75 5 0 0 4 0 1 1.1 9.5 , 0.15
0.5 0
1171; 78.25 5 0 0 4 0 1 1.1 9.5 0.15 1
0
. _ _ -
i
H7Ci 78.5 5 0 0 4 0.5 1 1.1 9.5 _ 0.15
0.25 0
11711 78.55 5 0 0 4 0 1.2 1.1 9.5 _ 0.15
0.5 0
H71 78.85 5 0 0
4 0.25 1.2 0.8 9.5 0.15 0.25 0
_ .
H7.1 78.75 5 0 0 4 0 0.8 1.3 9.5 0.15 0.5
0 1
. _
H7K 78.95 5 . 0 0 4 0.25 1 0.9 9.5 0.15
0.25 0 i
- _ _
.
H71, 78.55 5 0 0 4 0.5 0.9 1.4 90.15
0.5 0
_
H7M
77.95 5 0 0 4 0.5 1 1.4 9.5 0.15 0.5 0 ,
H7N 79.65 5 , 0 0 . 4 0 1.1 1.1 8.5 0.15
0.5 0 .:
1170 79.3 5 0
0 4 0 1 0.8 9.5 0.15 0.25 0
-24-
CA 02774546 2014-11-25
- .
WO 2011/035193 PCUUS2010/049381
________________________ _ - - =
___ _
Fe Cr Ni Mn Nb V C B W . Si Ti
Al ..
_
117P . 79.9 5 0 0 4 0 , 1 1.2 8.5 0.15
0.25 0
....... . _ ,
H7Q 79.95 5 0 0 4 0.25 1 0.9 . 8.5 0.15 0.25
0
H7R 79.75 5 , 0 0 4 0.25 1.1 1 8.5
0.15 0.25 0
H7S 79.85 , 5 0 0 3.5 0.25 1.1 0.9 9 .
0.15 0.25 0
H TT 78.65 5 0 0 4.5 0.25 1.1 1.1 9
0.15 0.25 0
11713 78 5 0 0 4.5 0.5 1 1.1 9.5 0.15
0.25 0 -
MN/ 78.75 5 0 0 4.5 0.25 1 1.1 9
0.15 0.25 0.
117W 79 5 0 , 0 3.5 0.5 1 1.1 9.5 0.15
0.25 0
._._ .
H7X 78.75 5 0 () 4.5 0.25 1.1 1 9 0.15
. 0.25 0
-
1-17Y , 78.3 5 0 0 4.5 0.25 1.1 1.2 9
0.15 0.5 ._ 0 ,
H7Z 78.25 5 0 __ 0 4.5 0.25 0.9 1.2 9.5
0.15 0.25 0 ...
Figure 19 is a diagram depicting an alloy desip process 1900 according to
certain
aspects of the present disclosure. The alloy design process comprises a 4-
component metallic
glass modeling technique based on topology, liquidus temperature, chemical
short range order
and elastic, strain to determine an amorphous forming epicenter composition.
An amorphous
forming composition epicenter 2010 and an associated amorphous forming
composition range
2020 are shown in diagram 2000 of Figure 20. Various aspects of such a 4-
component metallic
glass modeling technique are described above in the present disclosure and
also in University of
California, San Diego Ph.D dissertation "Modeling the Glass Forming Ability of
Metals" by
Justin Lee Cheney, . The modeling
technique can be used to maximize the potential for amorphous forming ability
for the design of
an amorphous material 1920 having a metallic glass epicenter composition.
After determining an amorphous forming epicenter composition, a variant
composition
. having a predetermined change in constituent elements from the amorphous
forming epicenter
composition is determined, and an alloy having the variant composition is
formed and analyzed.
For example, a first or second variant technique 1930 or 1940 may be employed
to design
a thermal spray material (e.g., glass/crystal composite 1950) for use as a
thermal spray wire or a
fine-grained.crystalline material (e.g., um-structured crystalline 1960) for
use as a weld overlay
material, respectively.
-25-
= _ CA 02774546 2014-11-25
=
WO 2011/035193
PCUUS2010/049381
A. Design of a Thermal Spray Material
The first variant technique 1930 for designing a thermal spray material (e.g.,
glass/crystal
composite structures 1950) involves vitrification potential determination 1932
and solidification
analysis 1934.
With regard to the vitrification potential determination 1932, in order to
design
glass/crystal composites, one or more variant compositions ranging from
between about 5 and
10% atomic percent offset in constituent elements from an amorphous forming
composition
epicenter 2010 are chosen. As used herein, the term "about" means within
normal manufacturing
tolerances. This range is termed nanocrystalline/glass composite zone 2030 in
diagram 2000
shown in Figure 20. A variant composition in this nanocrystalling/glass
composite zone can
include one or more additional components that are not present in the
amorphous forming
epicenter composition. In certain embodiments, the variant composition
includes between about
0.1 and 1.0% additional constituent that is not present in the amorphous
forming epicenter
composition.
The solidification analysis 1934 can be performed through a lab-based
technique to
simulate the cooling rate in thermal spray materials thus determined. In an
exemplary setup
2108, an homogeneous alloy ingot is melted within an arc melter such as the
one shown in.
Figure 21 in a water cooled copper cavity 2107. When a fully molten copper
plate 2105, termed
the splat block, is dropped onto the liquid alloy ingot 2106, the liquid alloy
ingot is rapidly
cooled in quench chamber 2109 in the form of a thin sheet (between about 0.25
and lmm) in thickness. The resulting
composite nanocrystalline/glass microstructure can be evaluated using any
known structural =
analysis methods including, but not limited to, XRD and SEM analysis. Those
variant =
compositions that satisfy certain conditions (e.g., hardness and structural
integrity) are selected.
Variant compositions designed and selected through the processes described
above can be
produced as thermal spray wires, for instance.
B. Design of a irm Crystalline Structure (Weld Overlay Material)
The second variant technique 1940 for designing a fine-grained crystalline
material (e.g.,
um-structured crystalline 1960) can involve a phase diagram prediction 1942
and a phase
chemistry prediction 1944.
=
-26-
=
CA 02774546 2014-11-25 =
=
In the phase diagram prediction 1942, specific alloying elements are either
a.dd.ed or
subtracted to encourage an. evolution of desired crystalline phases in the
microstructure as
illustrated by phase diagram 2200 shown in Figure 22. The phase chemistry
prediction 1944 can
be used to model any shifts in elemental concentration of the liquid as
primary crystallites
nucleate,
Analysis of composition behavior is completed using specially designed
experimental
lab-based techniques to simulate the cooling rate of the weld. Figure 23
illustrates an exemplary
alloy formation and analysis Procedure. In the procedure, an homogeneous alloy
ingot is melted 2310,
e.g., within an arc melter in a water cooled copper cavity. Size of the
homogenous alloy ingot .
being melted ("melt") is preferably between about 10 and 20g to ensure that
the cooling rate
closely matches that experienced in MIG welding. As the alloy cools, particles
can be formed as shown in
2320 and 2330. Figure 24 is a diagram 2400 depicting liquid composition versus
cooling curves for various
constituent compositions. Certain variant compositions designed, analyzed and
selected through the processes
described above can be produced as welding wires, for instance.
While various embodiments of the present invention have been described above,
it should
be understood that they have been presented by way of example only, and not of
limitation.
Likewise, the various diagrams may depict an example architectural or other
configuration for
the invention, which is done to aid in understanding the features and
functionality that can be
included in the invention. The invention is not restricted to the illustrated
example architectures
or configurations, but the desired features can be implemented using a variety
of alternative
architectures and configurations. Indeed, it will be apparent to one of skill
in the art how
alternative functional, logical or physical partitioning and configurations
can be implemented to
implement the desired features of the present invention. Also, a multitude of
different
constituent module names other than those, depicted herein can be applied to
the various
partitions. Additionally, with regard to flow diagrams, operational
descriptions and method
claims, the order in which the steps are presented herein shall not mandate
that various
embodiments be implemented to perform the recited functionality in the same
order unless the
context dictates otherwise.
-27-
CA 02774546 2014-05-01
While embodiments of the invention have been described in the detailed
description,
the scope of the claims should not be limited by the preferred embodiments set
forth
in the examples, but should be given the broadest interpretation consistent
with the
description as a whole.
10
IS
25
-28-