Note: Descriptions are shown in the official language in which they were submitted.
CA 02775717 2012-04-27
METHOD AND COMPOSITION FOR POLYMER-REINFORCED
COMPOSITE CE1VIENTITIOUS CONSTRUCTION MATERIAL
15 FIELD OF THE INVENTION
The present invention relates to a method and composition for polymer-
reinforced composite cementitious construction material, such as lightweight
concrete,
reinforced concrete, precast concrete, gypsum wallboards, reinforced gypsum
composite boards, plasters, machinable cementitious materials, joint treatment
compounds, and acoustical tiles, for example. The method and composition is
also
useful for lightweight and strong moldable cementitious products such as
orthopedic
plaster casts and dental models.
BACKGROUND OF THE INVENTION
Cementitious building materials, such as concrete and gypsum products, are
typically prepared by mixing dehydrated inorganic material with water and
casting the
resulting slurry into molds, forms, or sheets where it hydrates, hardens, and
dries. For
example, the gypsum-containing articles are produced by combining calcined
gypsum
powder (calcium sulfate hemihydrate and/or calcium sulfate anhydrite) with
water
(and often a small percentage of a variety of additives), and casting the
mixture into a
desired shaped mold or onto a surface. The resulting hydration reaction
produces an
CA 02775717 2012-04-27
2
interlocking matrix of gypsum crystals (calcium sulfate dihydrate). This is
often followed
by mild heating to drive off the remaining free (unreacted) water to yield a
dry product.
Cementitious materials are used universally, primarily in the construction
industry, for their desirable qualities of ease of casting, high compressive
strength, and
fire-resistance. Cementitious products include concrete, lightweight conacte,
reinforced
concrete, concrete board, gypsum boards, reinforced gypsum composite boards,
plasters,
machinable materials, joint treatment materials, acoustical tiles, plaster
casts, and dental
models. The most notable shortcoming is the weight of the products produced
using
cementitious materials, which results in relatively high production,
installation, and
building costs. Since the strength of a given composition is proportional to
its density,
current cementitious building materials must have relatively high densities in
order to
achieve desired performance requirements. The density of the material, and
thus the
overall weight of the products, can be reduced by introducing air voids or
expanded filler
into the inorganic material but only with a loss in strength that is more than
merely
proportional to the weight loss,
All of the cementitious products described above would benefit from increased
strength-to-weight ratio, which would make them more resistant to the stresses
encountered during use while reducing weight and building costs. Wallboard,
the largest
volume gypsum product would particularly benefit from such an improvement.
Wallboard typically consists of a gypsum core sandwiched between sheets of
cover paper.
In an effort to decrease the weight of the product, producers have steadily
increased the
porosity of the gypsum core by incorporating air voids or lightweight filler.
The core is
thus weak and the majority of current wallboard strength is provided by fiber-
oriented,
multi-ply cover paper. Paper is by far the most expensive component of
wallboard
manufacture, contributing more than 40% to the manufacturing cost. In
addition, the
paper facing of wallboard is subject to mold, which consumes the cellulosic
material,
== CA 02775717 2012-04-27
3
deteriorates the mechanical integrity of the board, and produces foul
smelling, toxic
chemicals.
There is continuing effort to make gypsum-containing products lighter in
weight
by substituting lower density materials (e.g., expanded perlite or air voids)
for part of
their set gypsum matrix. In such compositions, there is a need to increase the
strength of
the set gypsum above normal levels in order to maintain overall product
strength because
there is less set gypsum mass to provide strength in the lower density
product.
A number of additives, such as cellulosic particles and fibers, have been
included
to further improve the mechanical properties of cementitious products. More
expensive
glass fibers are used in place of wood in applications where high fire
resistance is
required. However, conventional fibers, particularly glass, do not adhere well
to the
gypsum matrix and decrease the workability of the gypsum slurry, thus limiting
improvement of the board. Glass fibers are also brittle and can be easily
dislodged during
board handling, installation, or demolition to cause irritation of the skin or
lungs.
More recently, there has been increasing interest in improving the strength
and
wear resistance of construction materials by incorporating synthetic polymers.
Cementitious composites containing water-dispersible polymers having modest
improvement in strength-to-weight have been found by adding latex or other
strengthening polymers to the cementitious materials. However, several unique
challenges have thus far restricted the commercialization of polymer
reinforced
cementitious products to relatively expensive niche products.
U.S. Pat. No. 6,402,832 ("the '832 Patent") describes the use of additives in
quick-drying joint compound. In one example, a water soluble functional
polymer with
either a nitrogen or a sulfonate group, such as poly(vinyl pyrrolidone)
("PVP") at a
molecular weight of between 20,000 and 40,000 (all molecular weights reported
herein
are in Daltons), was combined with a powdered solid bisphenol-A-based epoxy
resin,
== CA 02775717 2012-04-27
4
such as Shell EPONTM 1002F ("Epoxy"), achieving a crack resistance strength
slightly
higher than PVP alone and a slightly faster drying time than PVP alone.
However, the PVP and Epoxy additives of the '832 Patent, either alone or
together, decreased the porosity caused by evaporation of water from the
slurry (the '832
Patent, column 4,11. 46-54). According to the '832 Patent, the decrease in
porosity of the
joint compound was the primary mechanism in the increased crack resistance
(the '832
Patent, column 1,11. 50-59), which was based on the load required for crack
initiation in
the joint between two pieces of wallboard.
In other examples, the '832 Patent taught that a range of molecular weight of
between 40,000 and 80,000 for PVP produced significantly improved crack
resistance
compared to higher molecular weight PVP (the '832 Patent, column 6, 11. 3-5).
At this
molecular weight, a concentration of between 3 wt% and 6 wt% of PVP with
between 2
wt% and 4 wt% Epoxy was disclosed as an optimal, lowest range of concentration
to
achieve an optimally improved combination of both crack resistance and drying
times
(the '832 Patent, column 7, 11. 7-21).
The cost of polymers is typically hundreds of times that of the inorganic
material,
particularly for gypsum products, and additions of strengthening polymers
normally are
restricted to a small percentage of the mixture (e.g., less than 1 % of weight
of stucco for
wallboard applications) to be successful commercially. However, because high
strength
polymers typically have a low adhesion to inorganic materials and tend to
coagulate in
aqueous solution, large amounts of polymer (or compatibilizers, such as
surfactants) are
needed to improve the strength to weight ratio of the composites.
Alternatively, hydrophilic polymers adhere well to gypsum crystals but tend to
either: (1) have low intrinsic film strengths; (2) bind so well to gypsum
crystals that
hydration and crystal growth, and thus composite strength, are severely
retarded; or (3)
CA 02775717 2012-04-27
show a greater affinity to water than the inorganic material and migrate to
the edges of the
sample with the evaporating moisture leaving the core without reinforcement
and weak.
In U.S. Application Serial No. 10/094,572, filed March 7, 2002, ("the '572
Application"), now U.S. Patent No. 6,743,830, polymers
5 overcoat the inorganic, filler particles, providing adhesion between the
particles and
cohesion (thus mechanical/dimensional stability) of the overall core
composite. In
addition to bridging the particles, the polymeric binder offers viscoelastic
damping (thus
acoustic energy absorption), leading to superior noise reduction. The overall
system is
lightweight and possesses fire/flame retardancy similar to conventional gypsum
boards.
Furthermore, the high insulation efficiency afforded by the large void
fraction protects the
framing structure (2" x 4" studs) from becoming overheated in the event of an
actual fire.
The strengthening of the gypsum wallboard products made with the low-density
cores of
the '572 Application is primarily attributed to the strength of cover paper or
other higher
density layers formed at the surfaces of the wallboard, and the core itself is
reduced in
weight.
A longstanding need exists in the industry to substantially enhance the
strength-to-weight ratio of cementitious materials, including cement and
wallboard
products, to produce lightweight products or stronger, wear resistant
products. In
addition, eliminating or reducing other additives, such as wallboard cover
paper and glass
fiber can reduce board and construction costs, environmental degradation and
hazards to
human health. Furthermore, a need exists to improve the thermal and sound
insulation
properties of high strength cementitious building materials.
SUMMARY OF THE INVENTION
One embodiment of the present invention is a method for production of a
polymer-reinforced composite cementitious construction material. Another
embodiment
CA 02775717 2012-04-27
6
is a composition of matter comprising a polymer-reinforced composite
cementitious
construction material that incorporates porosity within the composite building
material.
A systematic method of microstructure engineering produces a construction
material of controlled microstructure, having improved strength to weight as
well as
superior sound isolation and thermal insulative properties. The microstructure
includes
both organic and inorganic components in a controlled morphology. Pores or
voids are
dispersed in the microstructure that, in combination with the organic
component, tend to
dampen acoustic vibrations and improve thermal insulation properties. Also,
the porosity
reduces the density of the construction material. The inorganic component
provides for
compressive strength and imparts fire/flame inhibition and retardancy and may
be
spherical, irregular, faceted, fibrous, needle-like and/or flakes, for
example. Additional
flame retardancy may be added by inclusion of conventional flame retardant
materials,
such as alumina trihydrate, antimony oxides, aluminum sulfamate, bismuth
oxide, tin
oxide, ferric oxide, molybdenum oxide, bismuth carbonate, boric acid, sodium
borate
(borax) and phosphonium salts.
The porosity may form either a co-continuous, tortuous phase, intertwining
with
the organic and inorganic components or as a collection of dispersed voids.
The void
phase may be designed to impart an impedance mismatch of vibrational/acoustic
transmission and destructive interference of either or both of transmitted and
reflected
waves, causing sound attenuation. Specifically, microphase-separated domains
on the
order of 100 nanometers (nn) may advantageously attenuate audible sound waves.
The
porosity may be formed by the evaporation of water during rehydration, by
introducing
foam, for example by rapid stirring or introduction of a surfactant foaming
agent or by
adding an ingredient that evolves a gas phase (e.g. nitrogen or carbon
dioxide), for
example a blowing agent or decomposition reaction. The porous microstructure
may
appear as Fig. 5, which has percolation network of coated inorganic particles
CA 02775717 2012-04-27
7
interpenetrated by porosity. In a preferred embodiment, two or more components
are
added to form the porosity, such as emulsifiers (surfactants) and expanding
agents
(propellants). Emulsifiers are amphiphilic compounds possessing both
hydrophilic and
hydrophobic moieties. Examples of emulsifiers include lower molecular weight
TM TM TM TM
polyvinylalcohols, block coiiolymers, Span, Tween, AOT and WetAid, which are
known
in the art. The selection is partially based on empiricism and partially based
on guidelines
known as the hydrophilic-lipophilic balance (HLB) method. The emulsifier
system
stabilizes the porous microstructure, providing an oil-in-water or water-in-
oil type
dispersion, and the solid phases can be in either or both of the other phases.
The blowing
agent may be, for example, physical agents, such as pentane, isopentane,
cyclopentane,
hexane isomers, cyclohexane and hptane isomers, and/or chemical agents, such
as sodium
bicarbonate, dinitrosopentamethylenetetramine, sulfonyl hydrazides,
azodicarbonamide,
p-toluenesulfonyl semicarbazide, 5-phenyltetrazole, di-isopropyl hydrazo-
dicarboxylate,
5-phenyl-3,6-dihydro-1,3,4-oxadiazin-2-one, and sodium borohydride. Physical
agents
volatilize and chemical agents decompose, for example upon heating.
Alternatively, expanded or expandable fillers may be added to the composite to
introduce porosity, or microspheres, for example expandable microspheres such
as
Expancel) may be added, ensuring that closed cell porosity is introduced into
the
composite building material.
The organic phase may be a binder, such as a polymer binder. For example,
naturally occurring materials such as bitumens and asphalts and/or synthetics
such as
thermoplastic and/or thermosetting polymers may be used. Acrylonitrile-
Butadeine-
Styrene (ABS), acrylics, methacrylics, cellulosics, nylons, polycarbonates,
polyolefins,
vynyls, styrenics, epoxies, formaldehydes, polyesters, polyurethanes and
silicons are some
examples, but the invention is limited thereto. A polymer binder may be pre-
polymerized
before mixing all the components of the composite construction material or may
be
= CA 02775717 2012-04-27
8
polymerized in-situ, for example to form a crosslinked network. Some examples
are
epoxies, polyketones/diamines, polyurethanes, poly(sodiurn styrene sulfonate)
and
poly(maleic anhydride alt-l-octadecene)/diamine.
In one specific embodiment, hydration of calcined gypsum in the presence of a
synergistic combination of strengthening component and a crosslinking
component
causes the set gypsum produced by such hydration to achieve a surprising and
unexpected
improvement in nail pull resistance, flexural strength and other properties,
satisfying a
longstanding and unresolved need for cost-effective, lightweight building
materials.
In one embodiment of the method, the method produces a building material with
a
microstructural morphology of the composite cementitious construction material
that
provides superior material properties compared to known building materials,
including
flexural strength, nail-pull resistance, hardness, thermal/sound insulation,
resistance to
moisture/fungus/microbial deterioration, and reduced environmental impact on
human
health while reducing overall construction costs compared to other methods of
producing
building materials.
For example, the composition of matter of one embodiment of the present
invention comprises an organic, water-dispersible polymer strengthening
component and
a crosslinking component that creates a molecularly crosslinked network that
interpenetrates an inorganic cementitious matrix material. The inorganic
matrix provides
mechanical and dimensional stability as well as fire/flame retardancy. In
specific
embodiments, the strengthening and crosslinking components combine
synergistically
and are effective at very low concentration.
In alternative embodiments the formulation enhances gypsum hydration and
crystal growth, contributing to superior adhesion between an inorganic
cementitious
material and a crosslinked organic strengthening component. It is believed by
the
inventors, without limiting the present invention in anyway, that the superior
adhesion
= CA 02775717 2012-04-27
9
results in a surprising and unexpected improvement in the strength-to-weight
ratio of the
composite material, because the crosslinking of the organic water-dispersible
strengthening polymer causes gelation of the strengthening polymer, which
prevents the
migration of the strengthening polymer to the surfaces of the composite that,
otherwise,
has been observed to occur during drying. Therefore, an interpenetrating
organic polymer
network and inorganic matrix material imparts superior strength to the
cementitious
building materials compared to previously known methods. Thus, a reduction in
weight
and/or an increase in strength of products produced using the composition of
matter are
achieved.
In alternative embodiments, the cost of both of the strengthening and
crosslinking
components is more than offset by the reduction or removal of other
strengthening agents.
In one specific alternative embodiment, the number of layers and/or thickness
of the
- paper facings can be reduced or eliminated. In another specific
alternative embodiment,
the weight of the composition of matter is reduced compared to known
compositions of
matter having the same strength, size and thickness by adding porosity (air
voids), which
significantly enhances the insulating properties (for example, both thermal
insulation
value and high-frequency sound attenuation) of the cementitious building
material.
The polymer-reinforced composite cementitious construction materials of the
present invention are ideally suited for use as lightweight building
materials, including
applications normally using concrete, high strength concrete, reinforced
concrete,
concrete board, wallboards, sheathing board, gypsum glass mat board, paperless
wallboard, ceiling board, plasters, machinable cementitious materials,
wallboard joint
treatment materials, spackling or repair materials, and acoustical tiles. In
addition,
specific embodiments of the method are useful for preparing lightweight and/or
high
strength plaster casts and dental models, for example. Furthermore, decorative
tiles,
roofing tiles, plumbing fixtures, and countertops benefit from the high
strength and/or
CA 02775717 2012-04-27
lightweight polymer-reinforced composite cementitious construction materials
of the
present invention.
In yet another alternative embodiment of the present invention, the polymer-
reinforced composite cementitious construction materials may be filled between
any two
5 surfaces, such as wood veneers and fiber reinforced polymer panels, to
fabricate fire
resistant, lightweight panels and other structures, for example doors. The low
density and
high strength of building materials made from polymer-reinforced composite
cementitious construction materials of the present invention also
synergistically reduce
the costs of surrounding structures, the cost of shipping, and the time and
labor required
10 for installation, reducing overall construction costs compared to
conventional, known
building materials.
DESCRIPTION OF THE DRAWINGS
For the purpose of illustrating the invention, representative embodiments are
shown in the accompanying figures, it being understood that the invention is
not intended
to be limited to the precise arrangements and instrumentalities shown. The
data discussed
in Figures 1-4 are derived from paperless samples.
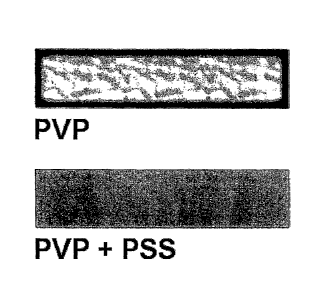
Fig. 1 illustrates an iodine staining technique showing, by shading, the
location of
the strengthening component, PVP, in a cementitious composite, wherein the
image
labeled PVP used no crosslinking component and the image labeled PVP + PSS
used
poly(sodium 4-styrenesulfonate (PSS) as a crosslinking component.
Fig. 2 shows the actual nail pull resistance load with respect to density of
embodiments of the invention containing PVP in combination with PSS (squares),
and
the nail pull resistance as a function of density for control samples created
without any
strengthening or crosslinking components added (solid line with triangles).
CA 02775717 2012-04-27
11
Fig. 3 shows the percentage improvement in nail pull resistance of several
embodiments having PVP only (dashed line with circles), PSS only (dotted line
with
triangles) and PVP in combination with PSS at a molecular weight of 1 million
(solid line
with squares) as a function of the molecular weight of PVP (except for PSS
only, which
is a function of the molecular weight of the PSS).
Fig. 4 shows the normalized flexural strength of several embodiments having
PVP
only (dashed line with circles) and PVP in combination with PSS (solid line
with squares)
as a function of the molecular weight of PVP.
Fig. 5 illustrates the microstructure of an expanded foam composite building
material having an organic binder, inorganic particles and porosity (shown by
air
pockets).
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention will now be described in detail for specific embodiments
of
the invention. These embodiments are intended only as illustrative examples
and the
invention is not to be limited thereto.
In one embodiment, the polymer-reinforced composite cementitious construction
material comprises a cementitious material, such as a gypsum or a cement, for
example,
and a polymer composition formed by combining a strengthening component and a
crosslinking component. It is believed, without limiting the invention in any
way, that the
crosslinking component acts upon the strengthening component to crosslink the
strengthening component in situ, causing a superior distribution of the
strengthening
component and establishing an intimate interaction between the strengthening
component
and the inorganic particles.
In one embodiment, small additions of strengthening polymers act
synergistically
with a crosslinking component, achieving a surprising and unexpected increase
in the
= CA 02775717 2012-04-27
12
strength-to-weight of the polymer-reinforced composite cementitious
construction
material compared to conventional additions of polymers without the synergy of
a
crosslinking component, Furthermore, the inventors believe, without limiting
the
invention in any way, that high molecular weight components form an extended
molecular network throughout the cementitious construction material, which
significantly
improves properties compared to the low molecular weight polymers used
conventionally.
In one specific embodiment, the strengthening component is a water-dispersible
nitrogenous polymer. For example, a water soluble and highly polar polymer
provides for
strong interaction between the strengthening component and the inorganic
matrix
particles, helping to bind particles and strengthening the composite
cementitious
construction material.
It is believed that the crosslinked polymer network also causes a change in
the
morphology of the calcium sulfate dihydrate crystals. Micrographs of the
microstructure
of the specimens of one embodiment revealed crystals that appeared both longer
and
thicker than the crystals in specimens prepared according to the known art.
This effect is
greatly influenced by the process chosen for mixing, hydration, forming, and
drying of the
resulting polymer-reinforced cementitious material.
In addition, it is believed that the crosslinked molecular network acts as a
binder
to promote adhesion between the crystals, enhancing the strength of the
composite. The
molecularly crosslinked polymer network does not coagulate, agglomerate, or
migrate to
the surface as shown in Fig. 1. Instead, it has a high affinity for the
inorganic crystals,
perhaps coating them with a fine layer. Therefore, strength is enhanced even
at low
polymer loading with reduced cost compared to known strengthening additions.
Also,
low polymer loading allows much higher molecular weight polymers to be used as
the
CA 02775717 2012-04-27
13
strengthening component without undesirably increasing the viscosity of the
slurry that
could otherwise lead to processing difficulties.
Fig. 1 shows that the addition of PSS, which acts as a crosslinking component
to
PVP, which acts as a strengthening gomponent, substantially reduces the
migration of the
water-soluble PVP to the surface of the slurry that would otherwise occur
during drying.
Migration of water-dispersible polymers and additives such as starch and borax
is well
known in the art, as the evaporating water carries water-dispersible and water-
soluble
components to the surface during the drying process. Using a technique of
iodine
staining, Fig. 1 shows that without a crosslinking component, PVP migrates to
the
surface; however, very small additions of a crosslinking component, for
example 1 part of
PSS to 4 parts of PVP, substantially reduces migration of the strengthening
component to
the surface during drying.
Migration of the strengthening component to the surface of a specimen enriches
the concentration of strengthening component in the facing layers, which
experience the
highest bending stress. This may lead to enhanced flexural strength, but
leaves the core
of the specimen devoid of the strengthening component. By preventing migration
of the
strengthening component, even small additions of a crosslinking component
synergistically enhance the resistance of the material to the pull-out of
nails.. Poor nail
pull resistance is a primary shortcoming of known reduced density cementitious
building
materials, such as lightweight wallboard. The low-density core of conventional
reduced
density cementitious building materials is weakened by migration of the
strengthening
component to the surface of the lightweight wallboard, and the low-density
core tends to
fail easily under a load, for example by crushing, densifying or deforming,
leaving only
the surface layers to resist pull-out of the nails.. In contrast, in one
embodiment of the
present invention, a small addition of a crosslinking component, which reduces
migration
of the strengthening component to the surface during drying, greatly enhances
the nail
CA 02775717 2012-04-27
14
pull resistance of the core region. Therefore, the overall nail pull
resistance of the
wallboard is enhanced significantly compared to conventional lightweight
wallboard.
The solid line in Fig. 4 shows the nail pull resistance (in lbs.) as a
function of the
density of unreinforced control specimens (in Wee), which was calculated based
on the
experimental control specimens (black triangles) and known strength-density
relationships. Points A and B illustrate that the current invention provides
for a weight
reduction greater than 20% for a nail pull resistance of 50 lbs.
In Fig. 2, the improvement in the nail pull resistance is the percentage
improvement in the nail pull resistance of the strengthened composition that
is
normalized by dividing the measured nail pull resistance of a specimen by the
nail pull
resistance of an unreinforced specimen at the same density. The nail pull
resistance of an
unreinforced specimen at the same density is determined from the relationship
between
the nail pull resistance and density as shown by the solid line in Fig. 4. The
improvement
in the flexural strength of Fig. 3 was calculated using a similar method,
using the known
relationship for the flexural strength versus density of an unreinforced
specimen to
normalize the flexural strength data. Thus, a nail pull improvement of 0% in
Fig. 2
reflects a nail pull resistance that is equal to that expected for a control
specimen of the
same density as the specimen tested, and a flexural strength improvement of 0%
in Fig. 3
reflects a flexural strength that is equal to that of a control specimen with
the same
density. An improvement of 100% means that the value is twice the value
expected for a
control specimen of the same density as the specimen tested.
In one specific embodiment, the strengthening component comprises a vinyl
pyrrolidone, wherein the vinyl pyrrolidone comprises homopolymers, copolymers
or both
homopolymers and copolymers. The strengthening component is used in
combination
with a crosslinking component that has the ability to crosslink the vinyl
pyrrolidone.
Results show a dramatic increase in the nail-pull resistance and flexural
strength of the
CA 02775717 2012-04-27
4
composite cementitious construction material compared to control specimens
(for
examples see Table I and Figs. 2-4). As can be seen for the control specimens
in Fig. 4 a
decrease in the density of a specimen tends to cause a decrease in the
flexural strength
and nail-pull resistance of the specimen. This is as expected given the
relationship
5 between relative strengths and stiffn.esses, and relative density, for
example as shown in
Cellular Solids Structure and Properties, 2d Ed., L. Gibson and M. Ashby,
Cambridge
University Press, New York (1997) pp. 192-198. This tendency is overcome in
some
specific embodiments according to the present invention.
Fig. 2 shows that the addition of one specific crosslinking component (PSS),
by
10 itself, decreases the value of the nail pull resistance. However, the
combination of one or
more specific strengthening components with one or more specific crosslinking
components in a method according to an embodiment of the present invention has
a
surprising and unexpected synergistic effect on the strength-to-weight of the
composite
cementitious construction material, which is reflected in Figs. 2-4.
Specifically, Fig. 2
15 shows a synergistic nail pull resistance improvement by combining PVP
with PSS as a
crosslinking component.
The nail pull resistance is a key mechanical property for wallboard. The
typical
way of fastening wallboard to studs is to insert a nail or screw through the
wallboard and
into a supporting stud within a wall. Lightweight wallboard has difficulty
meeting the
standards for nail-pull resistance especially if the core density is reduced
by adding
porosity. By using a water-dispersible strengthening component in combination
with a
crosslinking component as additives to gypsum wallboard, embodiments of the
present
invention meet or exceed the nail-pull resistance standards at substantially
reduced weight
for a standard size wallboard, which solves a longstanding and unresolved
need.
Increasing the molecular weight of the strengthening component modestly
strengthens the composite even in the absence of a crosslinking component as
shown in
CA 02775717 2012-04-27
16
Figs. 2 and 3. For example, PVP was added at a concentration of 2 g per 100 g
of
inorganic cementitious powder, as shown in Table I. In this case, PVP modestly
enhanced the nail pull resistance and flexural strength of the composite.
Adding a
crosslinking component has a synergistic effect that exceeds the effect of
merely adding
more of the strengthening component for PVP having molecular weights greater
than
about 100,000. This surprising result solves the longstanding problem of cost
effectively
enhancing the strength to weight of cementitious building materials, by
permitting the use
of small additions, for example only 1% or less, of a strengthening component
in a
lightweight product that meets or exceeds standards used in the construction
industry,
even if only very small amounts of a high molecular weight crosslinking
component is
added to crosslink the PVP.
Indeed, the nail pull resistance of a composite with PVP as a strengthening
component and PSS as a crosslinking component (at lower polymer loading than
the
specimens containing PVP alone) greatly exceeds that with PVP alone (no
crosslinking
component) as shown in the results of Table I and Figs. 2 and 3. Fig. 2 shows
that the
nail pull resistance of composites of the invention is substantially improved
for molecular
weights of PVP that are about 100,000 or greater. Herein, substantially
improved is used
to define an improvement in nail pull resistance of at least about 10%. The
term "about
100,000" is used to indicate that differing processing parameters and
selection of
differing strengthening and crosslinking components cause the value of the
molecular
weight that causes substantially improved nail pull resistance to vary. The
normalized
flexural strength also increases at molecular weights greater than 100,000.
Therefore, a
high-molecular-weight strengthening component is defined as one that has a
molecular
weight of at least about 100,000.
Substantially improved nail pull resistance begins at a molecular weight of
80,000
for some compositions. Others require a molecular weight of 120,000 before
achieving a
=
CA 02775717 2012-04-27
17
substantial improvement in nail pull resistance. Therefore, the term "about
100,000" is
meant to encompass variations of plus or minus 20%, depending on variations in
manufacturing tolerances caused, for example, by specific processing
conditions or
specific compositions of the strengthening components and crosslinking
components.
Preferably, the strengthening component has a molecular weight of at least
about
100,000. More preferably, the strengthening component has a molecular weight
of at
least 100,000 but no greater than 1.3 million. The results in Figs. 2 and 3
show that
above 1.3 million, increasing molecular weight no longer increases the nail
pull resistance
and decreases the improvement in flexural strength.
Specific examples of a strengthening component include a vinyl pyrrolidone
including, but not limited to, poly(vinyl pyrrolidone), poly(vinyl pyrrolidone-
co-vinyl
caprolactam), poly(vinyl pyrrolidone-co-diethylaminoacrylate), poly(vinyl
pyrrolidone-co-vinyl acetate), poly(vinyl pyrrolidone-co-styrene), poly(vinyl
pyrrolidone-co-imidazole), poly(vinyl pyrrolidone-co-vinyl
caprolactam-co-diethylaminoacrylate), poly(vinyl pyrrolidone-co-vinyl
caprolactam-co-vinyl acetate), and mixtures thereof.
The inventors believe, without being limited thereto, that a high molecular
weight
strengthening component is desirable, because the gel that forms by adding a
crosslinking
component binds the strengthening component and prevents its migration to the
surface
of the composition of matter during drying by evaporation of water. The
inventors believe
that an embodiment of the present invention forms a crosslinked molecular
network co-
continuous with the inorganic matrix phase.
For example, the crosslinking component may bind the strengthening component
through ionic interactions, hydrogen bonding, covalent bonding, or physical
interactions,
and combinations thereof. In one specific preferred embodiment the inventors
believe
= CA 02775717 2012-04-27
18
that the crosslinking component binds the strengthening component with ionic
bonding,
hydrogen bonding or both ionic and hydrogen binding.
In one specific embodiment, the crosslinking component is a substance
different
than the strengthening component, for example a polymer that is different than
the
strengthening component. In an alternative embodiment, the strengthening and
crosslinking components are regions within the same polymer macromolecule,
such as a
block or random copolymer containing pendant groups capable of pendant ionic
or
hydrogen bonding that interact with one another to form a crosslinked network.
In yet another embodiment, the crosslinking component is a catalyst that
initiates the
formation of covalent bonds between polymer chains of the strengthening
component
forming a crosslinked network.
In an alternative embodiment, the inventors believe, without restricting the
invention thereto, that the crosslinking component has a physical, rather than
specific
chemical interaction, with the strengthening component. For example, the
crosslinking
component comprises additives that form a gel in water, which physically
restricts the
migration of the strengthening component and/or aids in physically tangling
the polymer
chains of the strengthening component.
In alternative embodiments, combinations of other high molecular weight,
water-dispersible nitrogenous homopolymers, copolymers and combinations of
homopolymers and copolymers have been found to enhance the strength-to-weight
ratio
of the composite cementitious construction material, when synergistically
combined with
a crosslinldng component compared to control specimens and specimens adding
water-
dispersible strengthening component in the absence of a crosslinking
component.
Although experimental results are too numerous to list herein, some specific
examples of
alternative embodiments include, but are not limited to, polyacrylamide,
poly(acrylamide-2-methyl-1-propane sulfonic acid), poly(vinyl caprolactam),
CA 02775717 2012-04-27
19
poly(2-ethyl-2-oxazoline), poly(vinyl pyridine), poly(vinyl imidazole),
acrylamide
copolymers, 2-ethyl-2-oxazoline copolymers, vinyl caprolactam copolymers,
vinyl
pyridine copolymers, vinyl imidazole copolymers, and mixtures thereof. These
specific
embodiments also tend to show a synergistic effect at high molecular weight of
the
strengthening component with a component that acts to crosslink the
strengthening
component, whether by physical or chemical crosslinkage.
Some examples of ionic crosslinking components include polysulfonates,
polycarboxylates, and polyphosphates. For example, polysulfonates are
preferred when
used with some specific strengthening components. Examples of polysulfonates
include
metal (e.g., alkali and alkaline earth cations) and ammonium salts of
poly(styrene sulfonic
acid), poly(vinyl sulfonic acid), poly(2-aerylamido-2-methyl- 1-
propanesulfonic acid),
naphthalene sulfonate condensates, melamine sulfate condensates, lignin
sulfonate, and
copolymers containing salts of styrene sulfonic acid, vinyl sulfonic acid,
propane sulfonic
acid, and 2-acrylamido-2-methyl-1- propanesulfonic acid, and mixtures thereof.
Other
ionomers were also found to act synergistically with strengthening components
include
polyphosphates, such as ammonium polyphosphate, polyphosphonates, and
polycarboxylates, such as salts of copolymers of acrylic acid.
The improvement in strength to weight may depend not only on the choice of the
particular crosslinking component, but also may depend on the molecular weight
of the
crosslinking component, especially if the crosslinking component is an ionic
crosslinking
component. For example, the effect of PSS used in combination with PVP having
a
molecular weight of about 1.3 million, on the strength-to-weight ratios of
gypsum
composites increases steadily with molecular weight. PSS with a high molecular
weight,
of at least 70,000, more preferably at least about 100,000, and even more
preferably
about 1,000,000, is used in specific embodiments of the invention (where
"about"
continues to mean plus or minus 20%).
CA 02775717 2012-04-27
In specific examples of alternative embodiments, polymers with pendant groups
suitable for hydrogen bonding interactions with PVP show increased strength to
weight,
including, but not limited to, poly(vinyl alcohol), poly(acrylic acid),
copolymers of acrylic
acid, copolymers of methacrylic acid, copolymers of styrene sulfonic acid,
copolymers
5 containing salts of styrene sulfonic acid, copolymers of acrylamido-2-
methyl-
1-propane-sulfonic acid, and copolymers and mixtures thereof.
The molecular weight threshold for improved strength-to-weight ratio varies
depending on the structure of the crosslinking component and optimal ranges
must be
independently determined. The increase in strength-to-weight ratio attributed
to the
10 crosslinking component may depend on molecular weight. For example,
commonly used
TM
dispersants for use in the manufacture of wallboard such as Daxad (naphthalene
sulfonate
condensate; MW 7,000 to 10,000) and lignin sulfonate (MW = 10,000 to 20,000)
have
- little or no synergistic effect when used in combination with PVP, even
if the PVP has a
high molecular weight. In some instances, an organic crosslinking component
with a
15- molecular weight of about 50,000 or greater is sufficient, depending on
processing
conditions, molecular weight of at least about 100,000 being more preferred in
general.
More preferably, an organic, ionic crosslinking agent with a molecular weight
of at least
70,000 may be selected.
In specific examples of alternative embodiments, the strengthening component
and
20 crosslinking component are different regions within the same polymer
macromolecule
including, but not limited to, random or block copolymers of vinyl pyrrolidone
and
styrene sulfonate, random or block copolymers of vinyl caprolactam and vinyl
sulfonate,
random or block copolymers of vinyl pyridine and acrylamido-2-methylpropane
sulfonic
acid), random or block copolymers of acrylamide and acrylic acid (or its
salts), and
mixtures thereof.
= CA 02775717 2012-04-27
21
In one specific embodiment, the high molecular weight strengthening component
is self-crosslinked in water using a catalytic crosslinking component. In this
case, the
molecular weight of the catalytic crosslinking component is not critical,
because the high
molecular weight strengthening component is self-crosslinked. As one example
of a
method of manufacture, a strengthening component, poly(vinyl pyrrolidone), is
heated
with a combination of aqueous hydrogen peroxide and copper(II) chloride (the
combination being the crosslinking component) to covalently crosslink P'VP
into a gel,
which is then immediately combined with an inorganic cementitious material,
such as
gypsum. In an alternative embodiment, the crosslinking reaction is conducted
by heating
after mixing together the inorganic cementitious material, the strengthening
component,
and the crosslinking component. In each case the desired strengthening is
observed.
In contrast, the addition of commercially available lightly cross-linked
poly(vinyl
pyrrolidone) (PVPP), which is provided as a powder that is insoluble in water,
to a
gypsum slurry negatively affects the strength of the resulting composite. The
inventors
believe without being limited thereto, that some embodiments of the present
invention
create a crosslinked molecular network of P'VP co-continous with the inorganic
matrix
phase by diffusion or migration of the PVP molecules, molecular crosslinking,
and/or
gypsum crystal growth, but PVPP is insoluble particulate matter, with little
affinity for
inorganic materials typically used in construction. Therefore, PVPP does not
form a
crosslinked molecular network co-continuous with the inorganic material.
In one preferred embodiment, a mixture comprising the strengthening component,
the crosslinking component and the inorganic material are mixed together prior
to adding
water to the mixture, which forms an inorganic matrix interpenetrated by a
polymeric
network that dramatically improves the strength to weight ratio upon drying of
the
polymer-reinforced cementitious composite construction material.
= = CA 02775717 2012-04-27
22
In an alternative embodiment, the individual components and inorganic material
were
mixed with water individually. For example, the enhancing polymer and cross-
linking
agent can be dissolved individually at low concentrations in separate
solutions with
slightly elevated viscosities. In a specific embodiment, these two solutions
were then
combined, forming a gel of a much higher viscosity than the individual
solutions at the
same concentration. The inorganic material, for example a calcined mineral
such as
calcium sulfate hemi-hydrate (stucco), is then added to the gelled solution to
form a
slurry. At increased polymer concentrations, the slurry separates into an
aqueous phase
and a slurry phase of stucco and polymer gel, which demonstrates the positive
interaction
between the hydrating inorganic material and the cross-linked polymeric
network.
In another embodiment, the inorganic material is added to the strengthening
component and water to form a slurry prior to mixing with the crosslinking
component.
The crosslinking component is either added dry to the slurry or premixed with
water.
Alternatively, the crosslinking component can be a liquid component.
In yet another embodiment, the inorganic material is added to the crosslinking
component and water to form a slurry, and then the strengthening component is
added to
the slurry in either a dry or liquid form.
As yet another alternative, the chemical reaction that causes the crosslinking
component to react with the strengthening component is temperature sensitive,
and the
reaction occurs within a preferred temperature range.
Specimens were cast and mechanically tested to compare the flexural strength
and
nail-pull resistance of various specific embodiments of the present invention
to various
control specimens, for example containing no additives other than set
accelerator, and to
comparative samples containing sodium trimetaphosphate (STMP), a commercially
available enhancing material for resistance to permanent deformation. STMP is
used
primarily for improving sag resistance. STMP has been shown to improve nail
pull
CA 02775717 2012-04-27
23
resistance by about 15%, but it adversely affects flexural strength. For these
test
purposes, beta calcium sulfate hemihydrate (stucco) and finely ground calcium
sulfate
dihydrate set accelerator were used. PVP and vinyl pyrrolidone copolymers were
obtained from Aldrich Chemical Co., BASF corp., and ISP corp. PSS was obtained
from
Alco Chemical Co. and Aldrich Chemical Co. Sodium trimetaphosphate (STMP) was
TM
obtained from Aldrich Chemical Co. Daxad (naphthalene sulfonate condensate, MW
=
7,000 - 10,000) was obtained from Dow Chemical Co. Lignin sulfonate (MW =
10,000 -
20,000) was obtained from Borregaard Chemical Co.
The order of adding the various components is not thought to be critical to
the
success of the method, but may limit the processing time and equipment that
can be used
to perform the various processing steps, which may affect the cost and/or
quality of the
the polymer-reinforced composite material produced according to the specific
processing
steps chosen. Nevertheless, the mechanical behavior of the specimens created
by
combining a high molecular weight strengthening component, a crosslinking
component,
an inorganic cementitious material and water was similar so long as the
materials were
well mixed and allowed to set while still in the form of a slurry. The
inventors believe,
without being limited thereto, that the process should allow sufficient time
for the
forming of a crosslinked molecular network that is co-continuous with the
inorganic
matrix phase. For example, the slurry should be allowed to set before the end
of a
chemical reaction causing chemical crosslinking, but after the chemical
crosslinking has
proceeded to bind the strengthening agent enough to reduce migration during
drying.
In a specific embodiment of the method used to produce test specimens, samples
were cast by dry mixing 150 g of stucco and 0.2 g of set accelerator with a
strengthening
component, such as PVP, and a crosslinking component, for example PSS. Then,
for
example, the resulting dry powder mixture was added at room temperature, to a
500
TM
milliliter Waring blender containing a sufficient amount of water, for example
tap water,
CA 02775717 2012-04-27
24
to obtain the desired water to plaster ratio. The water to plaster ratio
affects the density of
the specimens. Alternatively, liquid additions or additions in solution were
first mixed
with the water prior to dry ingredients being added to the water, for example
a liquid or
solution crosslinking component may be added to the water prior to adding the
remaining
premixed dry powder mixture.
Then, optionally, the stucco mixture was allowed to soak, for example for
several
seconds, before blending. In one specific embodiment blending occurred at the
low speed
setting of the Waring blender. Comparable results were obtained when using the
high
speed setting. Alternatively, it may be desirable to introduce air into the
mixture or to
produce frothing by rapid stirring, for example, to reduce the density of the
final product.
The low speed setting of the Waring blender caused some frothing and reduced
density,
regardless of the ratio of water to inorganic material (WR) and regardless of
the amount
of strengthening component and crosslinking component added to the mixture.
The bulk
of any density change between specimens prepared with a lower ratio of water
to
inorganic materials and with an increased ratio of water to inorganic material
was as a
result of increased evaporation of water from specimens with an increased
ratio of water
to inorganic material. In this specific embodiment, the blending at low speed
was
continued for 15 to 25 seconds; however, the length of blending depends on the
size of
the batch, the type of process used for blending, and the viscosity.
Generally, blending
should be continued until the slurry is well mixed but not so long that the
gypsum begins
to set.
Also, the blending container was optionally hand shaken about halfway through
blending to ensure even mixing throughout the container. Following blending,
the
resulting slurry was cast into a rectangular mold approximately 2 in x 7 in x
0.5 in. After
i the composition hardened (for example 15 - 30 minutes), the sample was
removed from
the mold and dried in a convection oven at 40 C until dry, for example until
the sample
CA 02775717 2012-04-27
remained at a constant weight for a predetermined time, for example one hour.
Typically,
a sample the size of the one prepared by this specific embodiment requires at
least 24
hours at 40 C to completely dry. Drying time depends on the constituent
components and
additives and would be significantly decreased using higher temperatures
and/or
5 multizone ovens.
Then, the samples were cut into two inch by five inch rectangular test
specimens
and accurately dimensioned and weighed to determine density. The test
specimens were
TM
mechanically tested on an Instron model 4466 bench-top mechanical testing
system
equipped with data acquisition software. Both three-point-bend flexural
strength and
10 nail-pull resistance were determined for each specimen.
The flexural strength measurements were conducted in a fashion similar to the
flexural strength test described in ASTM C473, method B. The support span was
decreased to four inches to accommodate the sample size with the standard size
support
radius of 0.125". The loading rate was decreased to from 1 to 0.1 inch/minute
to attain
15 more accurate readings due to the stiffness of the gypsum samples.
The nail-pull resistance of the specimens was determined for specimen halves
remaining from flexural strength testing in a manner similar to the nail pull
resistance test
of ASTM C473, method B. The nail was machined according to the size and shape
standards specified in ASTM C473. The specimen support plate hole diameter was
20 decreased from 3 to 1.375 inches to accommodate specimen size. The
resulting densities
and mechanical strength values were catalogued along with the composition of
the
sample formulation. Power functions constructed from control data were used to
normalize experimental data to provide a quick comparison amongst samples of
differing
densities and the results are reported in Table I.
25 The synergistic interaction between strengthening and crosslinldng
components
dramatically improves the strength-to-weight ratio of cementitious composites
with very
=
CA 02775717 2012-04-27
26
low additions of polymers. Also, the low weight percent of polymer additions
keeps the
viscosity of the slurry within acceptable parameters for production of polymer-
reinforced
composite cementitious construction materials.
For example, one embodiment is particularly applicable for the production of
gypsum products, such as wallboard, because the core density can be reduced
25% by
increased inclusion of air voids (porosity) without sacrificing wallboard
strength while
only adding modestly to the cost of the materials.
In an alternative embodiment, the wallboard product produced according to one
embodiment of a method of producing a polymer-reinforced cementitious
wallboard
maintained strength and weight of standard wallboard without a paper facing.
Yet
another embodiment replaced the multi-ply paper reinforcement on each surface
with a
segregated polymer skim layer without loss of strength or increase in weight
compared to
standard wallboard. The elimination of the paper in these methods increase the
resistance
of the wallboard to deterioration while simultaneously reducing the cost of
production.
Variations of this method are disclosed in the '572 Application,
In another embodiment, a comparatively dense skim
layer was used in addition to a paper facing, providing better binding between
the paper
facing and a lower density core. In this case the skim layer may be added
first to the
paper or first to the core, depending on the process chosen for fabrication of
the
composite wallboard structure.
These and other embodiments having improved strength-to-weight ratio provide
for a lighter construction material, allowing easier installation, reducing
shipping costs,
which are a significant portion of wallboard costs, and/or eliminating other
strengthening
additives that have negative environmental impact, such as frangible fibers,
dust, and
cover paper, which is subject to mold that causes deterioration of the
wallboard and may
CA 02775717 2012-04-27
27
be toxic to some people. Furthermore, high molecular weight ionomers may
provide
excellent paper bond and foaming properties.
Addition of 2 g of either low molecular weight PVP or PSS alone (not in
combination) to 100 g of stucco reduces the density, for example due to air
void
incorporation, but also reduces the nail pull resistance of test specimens 4-
10, regardless
of the molecular weight of PSS and for low molecular weight PVP additions
(where low
molecular weight is defined as less than about 100,000). Only a modest
improvement in
normalized nail pull resistance was found for high molecular weight PVP
additions
(where high molecular weight is defined as about 100,000 or greater).
The results, for example in Table I, show that high molecular weight PVP
additions significantly increase the flexural strength compared to control
samples with no
PVP, and low molecular weight PVP additions decrease the flexural strength.
The
addition of only a crosslinking component (not in combination with a
strengthening
component), for example, PSS, either reduced or caused merely a slight
increase. Figs. 2
and 3 show that the synergistic combination of high molecular weight PVP and
PSS
enhances the nail pull resistance and flexural strength to a greater extent
than one would
expect based on the individual effects of high molecular weight PVP and PSS
alone (not
in combination). In alternative embodiments, a high molecular weight
crosslinking
component in combination with a strengthening component also having high
molecular
weight improves both the flexural strength and the nail-pull resistance and/or
allows for
weight reduction of the composite, cementitious building material compared to
unreinforced materials, materials with only a strengthening component or a
crosslinking
component (not in combination), and materials with a low molecular weight
strengthening component in combination with a crosslinking component.
In one preferred embodiment, a molecular weight of PVP of about 400,000 is
combined with PSS at a molecular weight of about 70,000 to achieve excellent
nail pull
CA 02 7 7 5 7 17 2 0 12 - 0 4 - 2 7
. .
28
resistance and flexural strength, which synergistically exceeds the additive
effect that
would be expected from results of experiments testing PVP and PSS separately.
In embodiments shown in Table I as items 27 an 28, the addition of only 1 g of
a
combined strengthening component (0.75 g at a high molecular weight) and
crosslinking
component (0.25 g at high molecular weight) per 100 g of stucco increases both
the
flexural strength and the nail pull resistance of composites having about the
same weight
as the customary commercial board weight (1700 lb/1000 ft2).
Table I
Nail-Put
Strengthening 9/1009 CrosslInking g/100 g W/P ratio Density Flexural
Resistance Noma! Normal
Example component stucco Component stucco (g/g)
(g/cc) Strength (psi) , (lbs) Rex Nall
1 control 1.5 0.629 242.43
46.31 1.07 1.01
- ,
2 control 1.7 0.571 164.88
35.87 0.87 0.99
3 control 2 0.490 147.84
. 25.10 1.04 1.00 ,
4 PVP, MW 2M 2 1.5 0.471 198.55
31.57 1.51 1.39
5 PVP, MW 1.3M 2 1.5 0.492 275.01
33.21 1.92 1.32
6 PVP, MW 400k 2 1.3 0.529 304.03
36.32 1.85 1.21
7 PVP, MW 55k 2 1.5 0.430 165.64
19.19 1.50 1,06
8 PVP, MW 10k 2 1.5 0.448 146.07
19.89 1.22 0.99
9 PSS, MW 1M 2 1.5 0.536 227.54
23.14 1.35 0,74
..
PSS, MW 70k 2 1.5 0,522 212.15 24.94 1.33 0.86
11 PVP, MW 1 .3M 2 PSS, MW 1M 2 1.5 0.631
332.65 64.08 1.45 1.38
12 PVP, MW 1.3M ' 2 PSS, MW 1M - 1 2
0,497 306.68 , 46.45 2.10 , 1.80
13 PVP, MW 1.3M 2 PSS, MW 1M 0.5 1.5 0.605
620.40 79.14 2.93 1.89
14 PVP, MW 400k 2 PSS, MW 1M 0.5 1.5 0.537
402.40 50.63 ' 2.38 1.62
-
18 PVP, MW 1.3M 1.5 PSS, MINIM 0.75 1.5
0.629 366.78 63.14, 1.61 1.37
- ,
16 PVP, MW 1.3M 1 PSS, MW 1M 1 1.5 0.621
452.17 65.56 2.04 1.47
17 PVP, MW 1,3M 1 PSS, MW 1M 1 2 0.495
310,41 44.43 2.15 1.73 ,
18 PVP, MW 1.3M 1 PSS, MW 1M 0.5 1.5 0.550
234,28 45.08 1.33 1.36
19 PVP, MO/ 1.3M 1 PSS, MW 1M 0,25 1.5 0.569
298,70 53.13 1.59 1.48
PVP, MW 55k 1 PSS, MW 1M 1 1.5 0.626 248.16 42.34
1.10 0.93
21 PVP, MW 10k 1 PSS, MW 1M , 1 1.3 0.525
210.40 25.72 1.30 0.87
22 PVP, MW 10k 1 PSS, MW 1M 1 1.5 0.452
188.56 , 20.95 , 1.55 0,99
23 PVP, MW 1.3M 1 PSS, MW 70k 1 1.5 0,566
236.45 41.72 1.27 1.17
24 PVP, MW 55k ' 1 PSS, MW 70k 1 ', 1.3 ' 0.482
199.92 25.61 1.45 1.07
PVP, MW 55k 1 Fss, mw 70k 1 1.5 0.540 212.20,
30.58 1.24 ' 0.97
26 PVP, MW 1.3M 1 Lignosulfonate 1 1.5
0.507 204.02 30.21 1.35 1.11
27 PVP, MW 1.3M 1 Daxad ., 1 . 1.5 0.614j
, 365.55 47.09 ' 1.68 1.09
28 PVP, MW 2M 0.75 PSS, MW 1M 0.25 1.5 0495
335.87 64.78 1.64 1.61
29 PVP, MW 1.314A 0.75 PSS, MW 1M 0.25 1.5
0,540 299.81 53.48 1.76 1.69
STMP 4 , 1.5 0.624 , 209.53 51.14 0.93
1.13
31 STMP - 2 1.5 0,600 221.06
47.90 õ 1.043 1.17
-
0.5 1.5 0.624 209.53
51.14 0.94 1.14
- 0.25 1.5 0.618 183.44
53.21 0.83 1.21
= CA 02775717 2012-04-27
29
In another embodiment, the addition of only 1 g of a combination strengthening
component and crosslinking component per 100 g of stucco allows the reduction
of board
weight by more than 25% (to 1250 lb/1000 ft2) compared to customary commercial
board
weight (1700 lb/1000 ft2) with no loss in the flexural strength or the nail
pull resistance.
In yet another embodiment, for example item 13 in Table I, the addition of
only
2.5 g of a combination of strengthening component (2 g at high molecular
weight) and
crosslinking component (0.5 g at high molecular weight) per 100 g of stucco
produces a
pap erless composite wallboard having a density equivalent to a board weight
of about
1600 lb/1000 ft2. which exhibits about the same flexural strength and nail
pull resistance
of current commercial, multi-ply paper-faced wallboard. Herein the term about
is used to
suggest that the values determined for board weight, flexural strength and
nail pulling
resistance are subject to variability, for example based on sources and
quality of raw
materials, milling, stirring, and other processing variations and
manufacturing tolerances,
as is known to one of ordinary skill in the art of manufacturing wallboard and
other
building materials.
Furthermore, comparative specimens in Table I clearly demonstrate the
surprising
and unexpected increase in flexural strength and nail pull resistance of the
embodiments
combining a strengthening component at high molecular weight with a
crosslinking
component at a high molecular weight compared to the control specimens and
specimens
without such synergistic combination.
One embodiment of a composition of matter according to the present invention
comprises a network of an organic strengthening component and an organic
crosslinking
component in a hydrated, inorganic cementitious material. In one specific
embodiment,
the inorganic cementitious material is calcium sulfate hemihydrate. In an
alternative
embodiment the inorganic cementitious material is calcium sulfate anhydrite.
In another
specific embodiment, the organic strengthening component is PVP, and the
organic
= CA 02775717 2012-04-27
crosslinking component is selected to chemically or physically crosslink the
PVP. For
example, in one specific embodiment the organic crosslinking component is PSS.
One embodiment of a cementitious building material according to the present
invention comprises a composition of matter including a network of an organic
5 strengthening component and an organic crosslinking component in a
hydrated, inorganic
cementitious material in the form of a sheet, for example a flat sheet. In one
specific
embodiment, the cementitious building material is wallboard, and in an
alternative
embodiment the wallboard further comprises at least one layer of paper applied
to at least
one surface of the sheet. In another alternative embodiment, the wallboard
further
10 comprises an additive that segregates to a stratified layer on at least
one surface of the
sheet.
One embodiment of a composite wallboard comprises a stratified structure
having
a thin skim layer of a high density gypsum with a high proportion of PVP/PSS
on the
surface of a foamed gypsum core containing a low proportion of PVP/PSS. The
resulting
15 composite thus maximizes the advantages of PVP/PSS for flexural strength
and paper
bond, while minimizing cost.
Another embodiment of a composite wallboard comprises a skim layer that has a
proportion of a surface modifying additive, for example a polyurethane
dispersion, that is
either not present in the core or is present in the core at a lower
concentration than at the
20 surface. This surface modifying addition, for example a polymer
addition, may be
present as a stratified layer that segregates to the surface of the slurry
during processing
and drying of the composite wallboard. In one embodiment, the surface
modifying
addition has a lower concentration in the PVP/PSS reinforced core of the
material, such
that the concentration at the surface is higher than the concentration of
PVP/PSS at the
25 surface, but the concentration in the core is less than the
concentration of PVP/PSS in the
core.
CA 02775717 2012-04-27
31
For example, such a stratified structure may impart a high mechanical strength
to
the wallboard and/or to the surface of the wallboard, allowing the composite
wallboard to
meet standards for building construction with no paper added to the surface of
the
wallboard. Alternatively, the stratified structure may reduce the number of
paper plies
required to meet the nail pull resistance and flexural strength standards for
building
construction or may improve the paint absorption properties of the wallboard.
For
example, the composite wallboard may then be paperless or covered with a
single-ply
paper facing for decorative purposes.
In an embodiment having only a single facing sheet on one side of the
composite
wallboard, the other side (open surface) can be textured with holes, fissures,
grooves or
ridges for enhanced sound attenuation/absorption. For example, reflection of
sound
waves impinging on the textured surface can be greatly reduced. In addition,
polymeric
materials may be chosen for sound attenuation purposes that undergo
viscoelastic
relaxation (transitions), for example within a range of frequencies between 20
Hertz (Hz)
and 20 kHz and at room temperature.
Another embodiment of a composition of matter comprising a surface layer
including a high concentration of fibers, for example glass, wood, or
cellulose fibers, that
sandwiches a PVP/PSS reinforced core is suitable for sheathing board
applications and/or
applications requiring high impact strength.
In another alternative embodiment, a gradient structure is obtained through
modification of the relative ratio of PVP to PSS and/or the molecular weight
of the PVP
component. By using a higher ratio of PVP to PSS, only a fraction of the PVP
interacts
with the PSS to form a gel. The remainder of the PVP tends to migrate towards
the
surface of the material carried by the evaporating water. The result is a
composite that
has a reinforced core, with a higher percentage of PVP near the drying
surfaces of the
composite structure.
CA 02775717 2012-04-27
32
In one specific embodiment, a small amount of strengthening component, wherein
a small amount is defined as a mass less than 2% of the mass of the inorganic
cementitious material, is combined with a trace amount of a crosslinking
component,
wherein a trace amount is as little as 1% of the mass of the strengthening
agent. In this
specific embodiment, a facing layer having a comparatively high concentration
of the
strengthening component is present at the drying surfaces of the composite
structure
(compared to the concentration in the core).
In an alternative embodiment, a highly active crosslinking component, for
example a catalytic crosslinking component, could be used with a high
molecular weight,
water-soluble strengthening component even at a trace amount to form a
composite
structure with a comparatively uniform concentration of the strengthening
component
throughout the composite structure. It is possible for one of ordinary skill
to predict the
activity of the crosslinking component by empirical trial and error or by
calculating the
reaction rates and the extent of the reaction that leads to a crosslinked
network. Therefore,
the amount of strengthening component available for diffusion to the surface
may be
quantified for preparation of a specific embodiment of a structural composite.
Furthermore, the reaction between the catalytic crosslinking component and the
strengthening component can be delayed, for example by using a temperature
sensitive
catalytic crosslinking component or by using a commercially available
retardant.
In another specific embodiment, the amount of the strengthening component is
about the same as the amount of the crosslinking component, whereby the
crosslinking
component and the strengthening component are both present as crosslinked
chains in a
polymeric network with the inorganic cementitious material.
In alternative embodiments, the amount of the strengthening component and the
amount of the crosslinking component may be selected with a ratio of between
100:1 to
1:10. For physical crosslinkages the ratio is more preferably between 10:1 and
1:2. For
chemically crosslinked networks a range of between 5:1 and 1:1 has shown good
results.
=
CA 02775717 2012-04-27
33
Nevertheless, the invention is not limited to any specific ratio of
strengthening
component to crosslinking component, because the preferred ratio depends on
the choice
of strengthening component, crosslinking component, and the type of
crosslinking
achieved. Indeed, a broad range of strengthening components and crosslinking
cOmponents can be used to produce a gel that creates a crosslinked polymer
network with
long, crosslinked polymer chains, which the inventors believe creates the
synergistic
strengthening mechanism described and claimed herein.
The present invention is compatible with all common additives to cementitious
products including inorganic fillers (such as perlite, expanded perlite, mica,
clay,
vermiculite), wood or glass fibers, starch, sodium trimetaphosphate,
surfactants, foaming
agents, borates (such as boric acid and sodium borate), and asphalt. In
addition, while
TM
low molecular weight ionomers (e.g. Daxad) are not suitable crosslinking
components, it
may be desirable to also include a minor portion of lower molecular weight
ionomers
with the present invention to perform a different function, such as a
dispersant or water
reducer.
The invention may also be used in combination with other additives to improve
moisture resistance or to further enhance the strength or crack resistance of
the
composite. Examples include wax emulsions, poly(vinyl acetate emulsions),
poly(vinyl
alcohol)/borate crosslinked systems, poly(vinyl acetate)/borate crosslinked
systems,
acrylate emulsions, polyurethane emulsions, epoxies, and melamine.
The invention may also be used in combination with monomers or oligomers that
can then be further reacted in situ. In this embodiment, the network formed by
the
strengthening and crosslinking components serves to confine added monomers or
oligomers within the core of the sample preventing migration. The monomer or
oligomer
is then post-polymerized or chain-extended to provide a further reinforced
network. For
example, an epoxy dispersion and polyamine curing agent are added in
combination with
poly(vinyl pyri-olidone) and sodium poly(styrene sulfonate) to the gypsum
slurry. The
CA 02775717 2012-04-27
34
gelation of the strengthening and crosslinking components constrains the epoxy
and
curing agent to the core of the sample. The epoxy then cures after water has
evaporated
to give a further reinforced composite.
In one specific embodiment, finely ground and calcined gypsum is mixed with
water, hydrogenated polybutadiene terminated with hydroxyl groups, low
molecular
TM
weight polyacrylic acid (less than 250,000 MW), emulsifying agent (WetAid),
sodium
hypophosphite and cyclohexane. In an optional alternative, digested paper
fibers from
recycled sources were added. The ingredients were well-mixed, creating a
slurry, which
was heated in an extruder to 350 degrees Fahrenheit (transit time
approximately one
minute) and discharged into the feed zone of two parallel calender rolls set
at a 1/2 inch
gap. Output from the calender rolls was picked up by two sheets of continuous
paper,
sandwiching the foamed core.
In another specific embodiment, a Portland cement mix was added to a mixture
of
maleic acid substituted styrene-butadiene-styrene block copolymer of roughly
50:50
composition (styrene:butadiene), ethoxylated trimethalolpropane triacrylate,
AlBN (a
TM
thermal initiator), methyl ethyl ketone, AOT, water and sodium bicarbonate.
The
ingredients were well-mixed, creating a slurry, which was fed through a
heating tube
under pressure. The outlet was fitted with a multi-orifice nozzle whereby the
expanded
slurry was broken into small droplets (10-100 microns) that continued to
expand and
solidify (set). The resulting foamed granules are fused into a layer of foam.
For example,
the foamed granules are further mixed with a hot melt adhesive and injected
into a
preformed door cavity, providing reinforcement, sound attenuation, thermal
insulation
and fire/flame retardancy.
In another specific embodiment, calcined gypsum is mixed with antimony oxides
in the presence of water. Caboxymethylcellulose (CMC) and polyacrylic acid are
added.
In an optional alternative, dimethylol-dihydroxyethylene-urea (DMDHEU)
replaced
TM
polyacrylic acid. Then, the pH is adjusted to the range of 4 to 5. Pre-
expanded Expanel
CA 02775717 2012-04-27
(10% by weight) is introduced into the mixture, which is fed from the mixing
tank to a
holding tank, whereby a porous felt mat (web) is passed through the holding
tank and
entrains a quantity of the mixture with one or more passes through the holding
tank. A
pad roller line heats the loaded felt, causing expansion and curing of the
slurry. Then, the
5 composite is bonded to a paper facing sheet (or sheets), for example in a
continuous
operation.
In another specific embodiment, the previous embodiment is modified by first
exposing the calcined gypsum to an aqueous solution of ethylene diamine or,
alternatively, polyethylene-imine (PEI). The treated calcined gypsum is next
mixed with
TM
10 CMC and maleic anhydride substituted polybutadiene (from Ricon). The
latter
component is solvated in pentane and emulsified by addition of a surfactant,
for example
either or both of Tween and Span. The pentane volatilizes upon drying and
curing,
. producing porosity. The carboxyl groups of the CMC form strong bonds with
the coated
amines, creating a microstructure similar to that shown in Fig. 5 with gypsum
particles in
15 a bonded, interconnected bridge-like network.
In another specific embodiment, calcined gypsum is mixed with water and an
organic phase containing urethane precursors, blowing agents and surfactants.
The
urethane is formed from polyols, which are lightly branched having a long
chain
architecture, and a di-isocyanate, such as toluene diisocyanate. Blowing
agents are
20 chosen from at least one of trychlorofluoromethane or methylene
chloride. Vigorous
TM TM
agitation and addition of Tween/Span, as a surfactant, create a slurry of
creamy viscosity.
Upon heating (up to 100 degrees Celsius) the gypsum rehydrates and the
urethane is
blown by the at least one of trychlorofluoromethane or methylene chloride,
adhering
strongly to the inorganic particles, creating an open celled foam, such as the
one
25 illustrated in Fig. 5. The foamed mixture is laid upon a continuous face
sheet on one
side, while the opposite side is perforated by an embossed roller to create
surface featers
that absorb audible sound waves.
CA 02775717 2012-04-27
36
In yet another specific embodiment, a rubbery latex is formed from at least
one of the
following elastomeric materials: natural rubber, SBR, nitrile rubber,
polychloroprene,
chlorosulfonated polyethylene-ethylene-propylene terpolymer, butyl rubber,
polyacrylate or
combinations thereof. To the rubbery latex is added a decomposable blowing
agent, for
example sodium bicarbonate, azodicarbonamide, dinitrosopentamethyletetramine,
2,2'-azobis-
isobutyronitrile, and/or 4,4'-oxybix(benzenesulfonyl hydrazide), which is
stabilized by
AOTTm. One part of this mixture is added to two parts of Plaster of Paris
slurry. Then, this
mixture is heated while being poured over a plywood board, allowing the
mixture to expand
and set into a one-inch layer, which is sandwiched between the plywood
underlayer and
another layer, for example another plywood layer or a decorative paper
topcoat.
According to one embodiment, there is provided a composition of matter
comprising a
strengthening component selected from the group of strengthening components
consisting of
PVP, a vinyl pyrrolidone copolymer, a vinyl caprolactam copolymer, and
mixtures thereof;
PSS; and a hydrated inorganic cementitious material, wherein the strengthening
component is
crosslinked by the PSS after mixing the strengthening component with the
hydrated inorganic
cementitious material, and wherein the strengthening component has a molecular
weight of at
least about 100,000 and PSS has a molecular weight of at least 70,000.
According to another
embodiment, there is provided an adhesive incorporating the composition of
matter.
Additional variations and permutations, including mixing and processing steps,
choice
of strengthening and crosslinking components, and use of conventional
additives are within
the knowledge of one of ordinary skill in the art and fall within the scope of
the present
invention.
=