Note: Descriptions are shown in the official language in which they were submitted.
CA 02777324 2012-05-17
Serie 8915
PROCESS FOR RECOVERING HYDROGEN AND CARBON DIOXIDE
Cross-Reference to Related Applications
This application claims priority to U.S. Provisional Patent Application No.
61/487,490,
filed May 18, 2011, and U.S. Patent Application No. 13/169,191, filed June 27,
2011, the
entire content of each incorporated herein by reference.
Field of the Invention
The present invention relates to a process for recovering hydrogen and carbon
dioxide
from a process stream utilizing a carbon dioxide separation unit and two
membrane
separation units. The present invention further provides for a process within
a hydrogen
generation plant to increase recovery of hydrogen and capture equal to or
greater than
80% of the carbon dioxide in the syngas stream utilizing a carbon dioxide
separation unit
and two membrane separation units.
Background
Hydrogen is an important feedstock for many chemical and petrochemical
processes.
However, hydrogen production is associated with large amounts of carbon
dioxide (C02)
emissions. Accordingly, it is desirable to not only provide a means to produce
hydrogen
but also a means to recover the carbon dioxide associated with the hydrogen
production.
With increased emission regulations and a possible future CO2 tax there is a
need to
develop carbon dioxide capture solutions. The cost of capture can impact the
cost of
hydrogen production. Therefore, it is important to develop a solution with
lower cost of
capture and improved efficiency of hydrogen production plant.
Physical or chemical solvents such as rectisol, selexol, amines, potassium
carbonate etc.
have been used traditionally for many decades to absorb carbon dioxide from
syngas in
hydrogen plants, HYCO (hydrogen and carbon monoxide co-production) plants.
However, the process of solvent absorption requires absorber and stripper
columns with
very high capital costs. The scrubbing solvent needs to be regenerated by
temperature
swing or pressure swing in order to release the absorbed carbon dioxide. The
regeneration
CA 02777324 2012-05-17
Serie 8915
process can involve large amounts of steam or compression energy resulting in
high
operating costs. Another disadvantage of using the solvent absorber is that
the purity of
the recovered carbon dioxide may not be very high and further processing using
liquefaction and partial condensation may be needed. Example U.S. Patent No.
6,500,241 describes the use of acid gas removal unit and auto refrigeration
unit for
removing carbon dioxide from syngas and PSA off-gas. U.S. Patent No. 4,553,981
and
U.S. Patent No. 7,682,597 describe the use of a carbon dioxide scrubber
downstream of
the shift reactor to remove carbon dioxide from syngas.
Carbon dioxide can be captured from a hydrogen plant by using cryogenic
processing viz,
partial liquefaction or distillation at low temperatures. Such a process is
favorable at
higher carbon dioxide concentrations. In hydrogen plants, carbon dioxide can
be
captured from several different locations in the process train including
process syngas or
flue gas. Flue gas processing can pose several challenges because of many new
impurities that therefore make the capture process very expensive. Carbon
dioxide from
process gas can be captured from high pressure syngas before pressure swing
adsorption
unit or from pressure swing adsorption off-gas. The concentration of carbon
dioxide in
the gas before pressure swing adsorption is much lower than in the pressure
swing
adsorption off-gas and hence the pressure swing adsorption off-gas is more
suitable for
cryogenic separation. U.S. Patent No. 6,301,927 provides an example of an auto
refrigeration process that employs the use of a compression and expansion
turbine to
liquefy carbon dioxide and further separate it from other gases. Another
patent, FR
Patent No. 2877939 provides a way to remove carbon dioxide from pressure swing
adsorption off-gas by using successive steps of compression and cooling to
remove
carbon dioxide by partial liquefaction and/or distillation. This patent
describes the use of
a membrane on the non-condensable gas to permeate hydrogen and recycle
hydrogen
back to the pressure swing adsorption unit in order to increase hydrogen
recovery.
However, carbon dioxide recovery for the unit is not very high. In U.S. Patent
No.
4,639,257, provides the use of carbon dioxide selective membrane in
combination with
distillation column for a carbon dioxide containing gas mixture in order to
increase the
recovery of carbon dioxide. A carbon dioxide selective membrane is used on the
2
CA 02777324 2012-05-17
Serie 8915
overhead of the distillation column with carbon dioxide rich permeate recycled
back to
feed or to the distillation column itself. Another carbon dioxide selective
membrane is
proposed for the feed gas before the distillation column in case the
concentration of
carbon dioxide is below equilibrium concentration at the freezing temperature
of the
mixture. However, this patent is suitable for a gas mixture containing carbon
dioxide,
nitrogen, methane and hydrocarbon. U.S. Patent Publication No. 2010/0129284,
describes the use of a hydrogen selective membrane, a carbon dioxide selective
membrane in combination with carbon dioxide liquefier in order to increase the
recovery
of hydrogen and carbon dioxide. However, carbon dioxide selective membrane is
always
located upstream of the liquefier requiring additional compression of the
carbon dioxide
permeate from the membrane feeding to the liquefier.
Hydrogen plants can emit large quantities of carbon dioxide into the
atmosphere. Carbon
dioxide capture solutions have been proposed in the past using several
different
separation techniques like absorption, cryogenic, adsorption or membrane.
There is
always some hydrogen loss from pressure swing adsorption processes. In
addition, there
is some carbon dioxide loss from the capture process which can be recovered by
improving the carbon dioxide capture process. If additional hydrogen and
carbon dioxide
can be recovered from the capture process there can be significant savings
with regards to
the size of reformer, natural gas consumption, carbon dioxide tax etc. for the
same size
hydrogen plant.
Accordingly, there still exists a need for a process to recover both hydrogen
and carbon
dioxide with a carbon dioxide recovery rate of at least 50% from syngas, as
well as a
process for producing hydrogen in a hydrogen generation plant that allows for
the overall
capture of at least 80% of the carbon dioxide, in the hydrogen production
process.
Summary
This present invention provides a method to more efficiently recover hydrogen
and
carbon dioxide, preferably at least 50%, even more preferably at least 75% and
most
preferably at least 90% of the carbon dioxide. The present invention further
provides the
3
CA 02777324 2012-05-17
Serie 8915
design for capture of at least 80%, carbon dioxide from syngas that allows for
the
simultaneous production of medium to high amounts of hydrogen in the syngas as
a part
of the production of hydrogen in a hydrogen generation plant. By using the
process of the
present invention, especially in terms of a hydrogen generation plant, it is
possible to
increase recovery of hydrogen and capture of the carbon dioxide in the syngas
stream by
balancing the recycle of the hydrogen rich permeate from the hydrogen membrane
separation unit to the process unit and/or the water gas shift as capacity
allows when a
carbon dioxide separation unit, a carbon dioxide membrane separation unit and
a
hydrogen membrane separation unit are utilized. The proposed use combines
hydrogen
selective membranes and carbon dioxide selective membranes together with
carbon
dioxide separation units such that hydrogen and carbon dioxide are produced
with
increased recoveries and improved process efficiency, especially with regard
to a
hydrogen generation plant. Increased hydrogen recovery by using hydrogen
selective
membranes can reduce the size of the feed gas producing unit, natural gas
consumption
for feed and fuel etc for the same size hydrogen plant. Increased carbon
dioxide recovery
will reduce the emissions of carbon dioxide into the atmosphere and will
result in cost
savings in case of a carbon tax.
Detailed Description of the Drawings
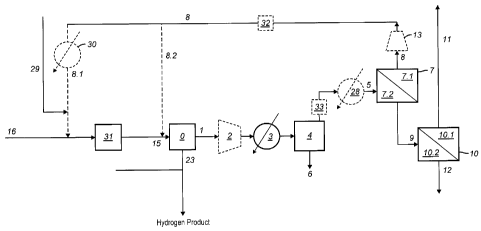
Figure 1 provides a schematic of one embodiment of the present process.
Figure 2 provides an expanded view of one variation of the carbon dioxide
separation
unit of Figure 1.
Figure 3 provides an expanded view of another variation of the carbon dioxide
separation
unit of Figure 1.
Figure 4 provides an expanded view of still a further variation of the carbon
dioxide
separation unit of Figure 1.
Figure 5 provides a schematic of a second embodiment of the present process.
Figure 6 provides an alternative to the schematic of Figure 5.
Figure 7 provides an alternative to the schematic of Figure 1.
Detailed Description of the Invention
4
CA 02777324 2012-05-17
Serie 8915
It is possible to efficiently recover hydrogen and carbon dioxide from process
streams
obtained from process units which have a purification step that provides a
hydrogen rich
fraction which can be utilized downstream (more specifically, in the
production of
electricity) as in the present process. While overcoming many of the
disadvantages of the
prior art systems that deal with the recovery of hydrogen and carbon dioxide
from such
streams, this can be accomplished by integrating a carbon dioxide separation
unit, a
hydrogen selective membrane separation unit and a carbon dioxide selective
membrane
separation unit into the process for treating streams taken from such process
units in the
manner noted herein. In addition, increased production of hydrogen and carbon
dioxide
capture of equal to or greater than 80% from syngas in hydrogen generation
plants may
also be accomplished by integrating a carbon dioxide separation unit, a
hydrogen
selective membrane separation unit and a carbon dioxide selective membrane
separation
unit into the schematic of a hydrogen generation plant. Accordingly, two main
processes
are proposed herein.
With regard to the first noted process, the proposed schematic includes a
process unit, an
optional compressor, a heat exchanger, a carbon dioxide separation unit, a
hydrogen
selective membrane separation unit and a carbon dioxide selective membrane
separation
unit. With regard to the second noted process, the proposed schematic includes
a feed
gas producing unit, a pressure swing adsorption unit, an optional compressor,
a heat
exchanger, a carbon dioxide separation unit, a hydrogen selective membrane
separation
unit and a carbon dioxide selective membrane separation unit.
The processes of the present invention will be further described with regard
to the figures
contained herein. These figures are included merely for illustration purposes
and are not
intended in any way to limit the processes of the present invention. The first
process of
the present invention as depicted in Figure 1 involves the recovery of
hydrogen and
carbon dioxide from a process stream (1) that is obtained from a process unit
(0). As
used herein, the phrase "process unit" refers to any unit which includes a
purification step
that results in the production of a hydrogen rich fraction that can be used
downstream.
More specifically, the "process unit" is a unit in which as one step of the
process,
CA 02777324 2012-05-17
Serie 8915
hydrogen is removed from a feed stream in such a manner that allows for the
recovery of
hydrogen in a more concentrated form than presented in the original noted feed
stream-
a hydrogen rich fraction that is the product stream (23)-and a tail gas stream
that is the
process stream (1).
The feed gas (15) that is supplied to the process unit (0) can be any feed
stream that will
typically be subjected to treatment for the removal of hydrogen. For example,
the feed
gas (15) may be a feed gas (15) produced in a feed gas producing unit (31),
for example a
feed gas (15) from a reformer unit/water gas shift unit, a partial oxidation
unit (POx), an
autothermal reformer unit (ATR), syngas from a coal gasification unit,
refinery off gas or
any other gas mixture that contains hydrogen, carbon monoxide and carbon
dioxide as
components in the gas mixture. In the more typical situation, the feed gas
(15) will be the
product of a hydrocarbon containing feed stream (16) that has been subjected
to at least
steam hydrocarbon reforming (preferably steam methane reforming)(not shown in
Figure
1). In a further embodiment, the feed gas (15) will be the product of a
hydrocarbon feed
stream (16) that has been subjected to a reformer unit/water gas shift unit,
an ATR unit, a
Pox unit or a gasification unit. In the more preferred situation, the feed gas
(15) will be
the product of a hydrocarbon containing feed stream (16) that has been
subjected to at
least steam hydrocarbon reforming and water gas shift (not shown in Figure 1).
In a still
further embodiment, the feed gas (15) will be the product of a gas stream that
has been
subjected to pre-reforming and steam hydrocarbon reforming and finally, the
product of a
gas stream that has been subjected to pre-reforming, steam hydrocarbon
reforming and
water gas shift (not shown in Figure 1). Each of these is described more
specifically
below with regard to the second process. In addition, those of ordinary skill
in the art
will recognize that the present invention is not meant to be limited by the
hydrocarbon
feed stream (16) which will ultimately form the feed gas (15) utilized in the
present
invention. Depending upon the source of the hydrocarbon feed streams (16),
those of
ordinary skill in the art will recognize that there will likely be small
amounts of other
components present in the ultimate feed gas (15), e.g. inerts such as
nitrogen.
Accordingly, while reference is made herein in more general terms to the major
components (such as hydrogen, carbon monoxide, carbon dioxide, methane and
water
6
CA 02777324 2012-05-17
Serie 8915
vapor) of the hydrocarbon feed streams (16) and feed gas (15), those skilled
in the art will
recognize that inerts such as nitrogen are also present and make up part of
the stream.
Preferably, the process unit (0) utilized will be a pressure swing adsorption
unit that is
used to recovery and purify hydrogen, although those of ordinary skill in the
art will
recognize that any other unit that functions to carry out hydrogen
purification may is also
contemplated to be within the scope of the present invention. The pressure
swing
adsorption unit utilized can be any pressure swing adsorption unit known in
the art and
can comprise anywhere from two to twelve adsorption beds (not shown) although
more
adsorption beds may be utilized. During the process of hydrogen purification,
each of the
adsorption beds (not shown) will individually under go a cycle that comprises:
a)
pressurization with pure hydrogen product, b) constant feed and hydrogen
product
release; c) pressure equalization to transfer high pressure hydrogen-rich void
gas to
another bed at low pressure, the other bed being about to commence product
pressurization; d) depressurization to slightly above atmospheric pressure; e)
purge using
product hydrogen; and f) pressure equalization with another bed at higher
pressure to
accept hydrogen-rich void gas. Preferably the adsorbents used in the pressure
swing
adsorption unit (0) include, but are not limited to, activated alumina,
activated carbon,
zeolite and their combinations. As a result of hydrogen purification, two
separate gas
streams are obtained-one that is a gaseous medium to very high purity hydrogen
stream
that is withdrawn and used as a hydrogen product (23) and the other which is
often
referred to as a pressure swing adsorption tail gas (referred to hereinafter
as the "process
stream") which is withdrawn after desorption of the adsorption bed as process
stream (1).
The process stream (1) is withdrawn from the adsorption beds of the pressure
swing
adsorption unit during the depressurization and purge steps. As used herein,
the phrase
"medium to very high purity hydrogen stream" refers to greater than 99%
hydrogen.
Furthermore, as used herein, the phrase "high purity hydrogen stream" refers
to greater than
99.9 % Hydrogen.
The removal of hydrogen product (23) from the feed gas (15) in the process
unit (0)
results in a process stream (1) that is purged from the process unit (0). This
process stream
7
CA 02777324 2012-05-17
Serie 8915
(1) contains at least carbon dioxide, hydrogen and methane. Typically, the
process stream
contains at least methane, carbon monoxide, carbon dioxide, water, and any
unrecovered
hydrogen.
In the process of the present invention as depicted in Figure 1, the process
stream (1)
obtained from the process unit (0) is further treated to remove additional
hydrogen and
carbon dioxide by passing the process stream (0) through a carbon dioxide
separation unit
(4), a hydrogen selective membrane unit (7) and a carbon dioxide selective
membrane
unit (10).
Prior to being introduced into the carbon dioxide separation unit (4), the
process stream
(1) obtained is optionally compressed in a first compressor (2). As used
throughout, the
term "compressor" is meant to include not only a compressor that has a single
stage for
compression but also a compressor that includes multiple stages for
compression
(typically from two to eight stages) with means for cooling between the
various stages of
the compressor. Note that the number of stages necessary to achieve the
desired level of
compression (pressure) depends on the inlet/outlet pressure ratio. Such
determinations
are readily apparent (determinable) to those skilled in the art. The degree of
compression
at this stage of the process (prior to the cooling of the stream) will depend
in part upon
the configuration of the carbon dioxide separation unit (4). More
specifically, when the
carbon dioxide separation unit (4) does not include a compressor, the process
stream (1)
will be compressed to a pressure equal to or greater than 35 bar prior to the
cooling in the
heat exchanger (3) of the present process as depicted in Figure 1. However,
when the
carbon dioxide separation unit (4) does include a compressor as a component of
the
carbon dioxide separation unit (4) which allows for the process stream (1) to
be
compressed either prior to or as a part of the actual separation and
purification steps
within the carbon dioxide separation unit (4), then only partial compression
or no
compression will take place prior to the cooling step in the heat exchange (3)
of the
present process (thereby making the compression of the process stream (1)
optional
before being introduced into the carbon dioxide separation unit (4). The
intent is to have
a process steam (1) that is at a pressure equal to or greater than 35 bar
while being treated
8
CA 02777324 2012-05-17
Serie 8915
in the carbon dioxide separation unit (4). More specifically, in order to
accomplish this
degree of compression, the process stream (1) may be compressed in a variety
of
manners. For example, the process stream (1) may be compressed in whole (to
equal to
or greater than 35 bar) or in part (compression to a pressure less than 35 bar
in
compressor (2) but when further compressed downstream (in a compressor that is
a
component of the carbon dioxide separation unit (4)) achieves a level of
compression that
is equal to or greater than 35 bar) provided that the final pressure of the
process stream
(1) is equal to or greater than 35 bar. For example, for a process stream (1)
that is at a
pressure of 20 bar, it may be possible to increase the pressure in the
compressor (2) to 30
bar prior to the cooling of the stream in the heat exchanger (3) and then
raise the pressure
to 37 bar in the compressor that is a component of the carbon dioxide
separation unit (4).
Preferably, the process stream (1) is compressed to above 50 bar while being
treated in
the carbon dioxide separation unit (4). Most of the compression, if not all,
is preferably
accomplished in the compressor (2) prior to cooling (before being introduced
into the
carbon dioxide separation unit (4)). Those skilled in the art will recognize
that the
addition compressor (not shown) while being a part of the carbon dioxide
separation unit
(4) will for practical reasons, typically be positioned outside of the cold
box of the carbon
dioxide separation unit (4) (separated from those components that are
typically at less
than ambient temperature). In addition to the options of the process stream
(1) being
compressed to the desired pressure, or being partially compressed to the
desired pressure
(and further compressed in the carbon dioxide separation unit (4)), or not
being
compressed (and being fully compressed in the carbon dioxide separation unit
(4)), those
skilled in the art will recognize that in certain instances, it may be
desirable to utilize/treat
a portion or fraction of the process stream (1) while in other instances it
may be desirable
to utilize/treat the entire process stream (1). Accordingly, when compression
takes place,
only that fraction that will be utilized/treated will typically be compressed.
Prior to being optionally compressed, the process stream (1) may optionally be
passed
through one or more filters, including a series of filters (not shown) in
order to remove
any adsorbent that may have passed through from the process unit (0). Those
skilled in
the art will recognize that a variety of different types of filters may be
utilized to filter the
9
CA 02777324 2012-05-17
Serie 8915
process stream, including, but not limited to, ceramic filters, baghouses,
metallic filters
etc.
The optionally compressed process stream (1) (or portion thereof) is then
subjected to
cooling to a temperature that is equal to or less than -10 C by subjecting the
process
stream (1) to heat exchange in a heat exchanger (3). Those skilled in the art
will
recognize that while the heat exchanger (3) of Figure 1 is positioned outside
of the carbon
dioxide separation unit (4), this heat exchanger (3) for all practical
purposes is considered
to be a part of the carbon dioxide separation unit (4). In a preferred
embodiment, the
process stream (1) is cooled to a temperature that is equal to or less than -
30 C. Any type
of heat exchanger (3) that is known in the art may be utilized to cool the
process stream
(1) to the desired temperature.
The next step of the process involves the separation and purification of the
cooled process
stream (1) in a carbon dioxide separation unit (4) to produce a carbon dioxide
rich liquid
stream (6) and a carbon dioxide lean non-condensable stream (5). The carbon
dioxide
separation unit (4) may be any unit which is capable of separating/purifying
carbon
dioxide from a stream that contains carbon dioxide at a temperature that is
equal to or less
than -10 C, preferably equal to or less than -40 C. In other words, the carbon
dioxide
separation occurs at sub-ambient temperatures and conditions. Those of
ordinary skill in
the art recognize that such sub-ambient separation/purification is known in
the art.
Accordingly, the present process is not meant to be limited by the carbon
dioxide
separation unit (4) or the process for carrying out the
separation/purification in the carbon
dioxide separation unit (4). As used throughout with regard to the present
invention, the
phrase "carbon dioxide separation unit" refers not only to the liquefaction
units and/or
distillation columns included therein, but also to all of the additional
components that
typically are considered to make up a carbon dioxide separation unit (4),
including, but
not limited to, one or more components selected from additional heat
exchangers,
additional compressors, dryers, etc. With regard to the present carbon dioxide
separation
unit (4), the separation/purification is typically carried out utilizing
single or multi-step
partial liquefaction as depicted in Figure 2 which includes one liquefaction
unit (14);
CA 02777324 2012-05-17
Serie 8915
single or multi-step partial liquefaction in combination with at least one
distillation
column as depicted in Figure 3 which includes two liquefaction units (a first
liquefaction
unit 14.1 and a second liquefaction unit 14.2) and one distillation column
(24); and single
or multi-step partial liquefaction in combination with at least one
distillation column and
at least one compressor and/or heat exchanger as depicted in Figure 4 which
includes two
liquefaction units (a first liquefaction unit 14.1 and a second liquefaction
unit 14.2), one
distillation column (24), one compressor (25) and one heat exchanger (26).
When two or
more liquefaction units (14) are included in the carbon dioxide separation
unit (4), those
skilled in the art will recognize that liquefaction within each of these units
may take place
at the same temperature (with different pressures) or at different
temperatures (with the
same pressure). In any event, the temperature for such liquefaction will
generally be
between about -10 C and -57 C, preferably between about -30 C and -57 C. In
addition,
note that with regard to Figure 4, while the compressor (25) and heat
exchanger (26) are
outside of the box (4) which denotes the carbon dioxide separation unit (4),
they are still
considered to be a part of the carbon dioxide separation unit (4) and are
simply included
where they are for feasibility purposes (outside of the cold box).
As a result of the separation/purification that takes place in the carbon
dioxide separation
unit (4), there is produced a carbon dioxide lean non-condensable stream (5)
and a carbon
dioxide rich liquid stream (6). The carbon dioxide rich liquid stream (6) is
withdrawn
from the carbon dioxide separation unit (4) as a product stream and directed
for further
use. In addition, note that while cooling in the heat exchanger (26) of the
carbon dioxide
separation unit (4) can be accomplished utilizing an external coolant such as
ammonia,
the carbon dioxide rich liquid stream (6) may also be used, prior to the
stream being
withdrawn from the carbon dioxide separation unit (4), to provide cooling
within the heat
exchanger (26) of the carbon dioxide separation unit (4). Those of ordinary
skill in the
art will recognize that such streams (6) will typically include from about 90%
to more
than 99.9% carbon dioxide and may be used for enhanced oil recovery,
industrial uses,
sequestration in geological formations, etc. This carbon dioxide rich liquid
stream (6)
can be utilized as a liquid or may be vaporized to produce a carbon dioxide
rich gas
stream.
11
CA 02777324 2012-05-17
Serie 8915
The carbon dioxide non-condensable stream (5) that is withdrawn from the
carbon
dioxide separation unit is typically at a high or medium pressure since the
process stream
(1) treated in the carbon dioxide separation unit (4) will be at a pressure
that is equal to or
greater than 35 bar. As used herein with regard to the carbon dioxide non-
condensable
stream (5), the phrase "high pressure" refers to a pressure that ranges from
about 50 bar
to about 100 bar, preferably from about 50 bar to about 80 bar. As used herein
with
regard to the carbon dioxide non-condensable stream (5), the phrase "medium
pressure"
refers to a pressure that ranges from about 10 bar to about 49 bar, preferably
from about
25 bar to about 49 bar.
Once the carbon dioxide lean non-condensable stream (5) is withdrawn from the
carbon
dioxide separation unit (4), it is passed through a hydrogen selective
membrane
separation unit (7) where the hydrogen passes through the hydrogen selective
membrane
to form a hydrogen rich permeate stream (8). As used herein with regard to the
hydrogen
rich permeate stream (8), the phrase "hydrogen rich" refers to the permeate
stream having
a percentage of hydrogen that is greater than the percentage of the other
components in
the hydrogen rich permeate stream (8). The hydrogen selective membrane
preferentially
permeates hydrogen over carbon monoxide, carbon dioxide and methane as well as
any
other components in the stream being subjected to the hydrogen selective
membrane. In
the preferred embodiment of the present process, the hydrogen selective
membrane
utilized has a hydrogen permeability that is at least 1.25, preferably 5, more
preferably 8
and even more preferably 12, times that of the gas or gases from which the
hydrogen is
separated under the chosen operating conditions. Fluid permeation through a
polymeric
membrane can be described as the overall mass transport of a fluid species
across the
membrane, where the fluid species is introduced as feed at a higher pressure
than the
pressure on the opposite of the membrane, which is commonly referred to as the
permeate side of the membrane. Typically in a separation process, the fluid
species is a
mixture of several components, at a minimum two, with the membrane exhibiting
a
higher selectivity for one component (for example "component A") over the
other
component (for example "component B"). Component A permeates faster than
12
CA 02777324 2012-05-17
Serie 8915
component B, therefore relative to the feed, the permeate is enriched in
component A and
the portion of the feed that does not permeate, commonly referred to as the
retentate or
residue is enriched in component B. With regard to this particular invention,
the fluid is
in a gaseous form and the polymeric continuous phase of the active membrane
layer is
nonporous. By "nonporous" it is meant that the continuous phase is
substantially free of
cavities or pores formed in a network through which migrating components of
the gas
mixture may flow from the feed to the permeate side of the membrane.
Transmembrane rate of transport of migrating components through the polymeric
continuous phase is commonly referred to as flux and is driven primarily by
molecular
solution/diffusion mechanisms. Preferably, the polymer is selectively gas
permeable to
the components, meaning that the gases to be separated from each other
permeate the
membrane at different rates. That is, a highly permeable gas will travel a
distance
through the continuous phase faster than will a less permeable gas. The
selectivity of a
gas permeable polymer is the ratio of the permeabilities of the individual
component
gases, e.g. Permeability of component A to permeability of component B. Hence,
the
greater the difference between transmembrane fluxes of individual components,
the
larger will be the component pair selectivity of a particular polymeric
membrane.
With regard to the present process, the permeate stream that is obtained will
generally
contain from about 40 % to about 90 % hydrogen with the remaining part of the
permeate
stream comprising the other components contained in the carbon dioxide non-
condensable stream (5). Accordingly, a "hydrogen rich" permeate stream will
generally
contain greater than or equal to 40% hydrogen, preferably up to or greater
than 90%
hydrogen. In an alternative embodiment of the present process, the carbon
dioxide non-
condensable stream (5) can be treated in the hydrogen selective membrane
separation unit
(7) at low pressure in order to increase the recovery of hydrogen. As used
herein with
regard to the carbon dioxide non-condensable stream (5), the phrase "low
pressure" refers
to a pressure that is equal to or less than 10 bar, preferably from about
equal to or less
than I bar absolute to less than 10 bar. Note that when the carbon dioxide non-
condensable stream (5) is permeated at low pressure, the carbon dioxide non-
condensable
13
CA 02777324 2012-05-17
Serie 8915
stream (5) pressure is reduced (as it will be at high to medium pressure) by
any method
known in the art such as one or more valves, a turbine, etc. (not shown). In a
still further
embodiment, the hydrogen rich permeate stream (8) is permeated at the same
pressure as
the feed gas (15) of the process unit (0).
The remaining components in the carbon dioxide lean non-condensable stream (5)
form a
hydrogen lean residue stream (9). As used herein with regard to the hydrogen
lean
residue stream (9), the phrase "hydrogen lean" refers to the residue stream
having a
percentage of hydrogen that is less than that in the carbon dioxide non-
condensable sream
(5).
The hydrogen selective membrane separation unit (7) utilized in the process of
the
present invention contains at least one membrane that is selective for
hydrogen over the
other components in the carbon dioxide lean non-condensable stream (5). Note
that the
target molecule, in this case hydrogen, determines how the permeate stream is
used. With
regard to each of the membranes utilized in the present process, each membrane
has a
permeate side (7.1) and a residue side (7.2). Since the membrane is selective
for
hydrogen, it allows for the passing of hydrogen through the membrane to the
permeate
side (7.1) of the membrane. While a variety of different types of membranes
may be
utilized in the hydrogen selective membrane separation unit (7) of the process
of the
present invention, the preferred membrane is a polymeric membrane that is
selective for
hydrogen that is selected from one or more polyamides, polyaramides,
polybenzimidazoles, polybenzimidazole blends with polyimides,
polyamides/imides.
Hydrogen selective membranes will have a H2/CO2 selectivity given by the ratio
of H2
permeance to the CO2 permeance at the operating conditions that is greater
than 1.25,
preferably greater than 5, more preferably greater than 8. . In a preferred
embodiment of
the present invention, the polymeric membranes of the first hydrogen selective
membrane
separation unit (4) and the second hydrogen selective membrane separation unit
(11) will
be made of the same polymeric materials.
14
CA 02777324 2012-05-17
Serie 8915
The hydrogen selective membranes of the present invention can be fabricated
into any
membrane form by any appropriate conventional method. For example, the
hydrogen
selective membranes may be cast as a sheet at the desired thickness onto a
flat support
layer (for flat sheet membranes), or extruded through a conventional hollow
fiber
spinneret (for hollow fiber membranes). Processes for preparing uniformly
dense
membranes or asymmetric membranes are also available and known to those
skilled in
the art. In addition, it is possible to prepare composite membranes by casting
or
extruding the membrane over a porous support of another material in either
flat film or
hollow fiber form. The separating layer of the composite membrane can be a
dense ultra-
thin or asymmetric film. In the preferred embodiment of the present process,
the
hydrogen selective membranes are in the form of modules comprising membranes
formed as either hollow fibers or spiral wound asymmetric flat sheets.
The hydrogen selective membrane separation unit (7) includes at least one of
the above
noted membranes. With regard to the actual configuration of the hydrogen
selective
membrane separation unit (7), the hydrogen selective membrane separation unit
(7) can
take on any number of configurations. In one embodiment, there is only one
membrane
element in the hydrogen selective membrane separation unit (7). In an
alternative
embodiment, the hydrogen selective membrane separation unit (7) comprises a
series of
hydrogen selective membrane elements within a single membrane housing (not
shown).
With regard to this embodiment, the series of hydrogen selective membranes can
be made
up of hydrogen selective membranes of the same type selected from the hydrogen
selective membranes detailed above or of two or more different hydrogen
selective
membranes selected from the hydrogen selective membranes detailed above. In
the
embodiment where there are two or more hydrogen selective membranes, the
hydrogen
selective membranes will preferably be of the same type and the same
fabrication (for
example, sheets or fibers). In a still further embodiment concerning the
configuration of
the hydrogen selective membrane separation unit (7), the hydrogen selective
membrane
separation unit (7) comprises two or more membrane housings with each of the
housings
having one or more hydrogen selective membranes as described hereinbefore.
More
specifically, in this embodiment, there can be two or more membrane housings,
with each
CA 02777324 2012-05-17
Serie 8915
of the housings having either one hydrogen selective membrane or two or more
hydrogen
selective membranes of the same type or two or more hydrogen selective
membranes of
two of more different types. The resulting hydrogen selective membranes may be
mounted in any convenient type of housing or vessel adapted to provide a
supply of the
carbon dioxide non-condensable stream (5), and removal of the permeate stream
(7.1)
and residue stream (7.2). The housing also provides a high-pressure side (for
the carbon
dioxide non-condensable stream (5) and the residue stream) and a low-pressure
side of
the hydrogen selective membrane (for the permeate stream). As an example of
configurations contemplated to be within the present invention, flat-sheet
membranes can
be stacked in plate-and-frame modules or wound in spiral-wound modules. Hollow-
fiber
membranes can be potted with a thermoset resin in cylindrical housings. The
final
hydrogen selective membrane separation unit (7) comprises one or more membrane
modules or housings, which may be housed individually in pressure vessels or
multiple
elements may be mounted together in a sealed housing of appropriate diameter
and
length.
As noted above, as a result of passing the carbon dioxide non-condensable
stream (5)
through the hydrogen selective membrane separation unit (7), two separate
streams are
formed--a hydrogen rich permeate stream (8) and a hydrogen lean residue stream
(9).
The hydrogen rich permeate stream (8) is optionally compressed in a second
compressor
(13) before being recycled for use as a supplemental feed stream for the
process unit (0)
or as a supplemental feed stream for other processes upstream. More
specifically, in the
preferred embodiment, the hydrogen rich permeate stream (8) is utilized as two
separate
fractions-as a first hydrogen rich permeate fraction (8.1) to be used as a
supplemental
feed stream for processes that are upstream of the process unit (0) (not
shown) and as a
second hydrogen rich permeate fraction (8.2) to be used as a supplemental feed
stream in
the process unit (0) (not shown) with the objective being to optimize the use
of the
recycle stream (8) in order to maximize the conversion of carbon monoxide to
carbon
dioxide and hydrogen. With regard to this particular embodiment, the
proportion of each
fraction recycled to the corresponding devices (0, and what ever device is
upstream)
depends upon the percentage of production (the load) from the feed gas
producing unit.
16
CA 02777324 2012-05-17
Serie 8915
Those of ordinary skill in the art will recognize that a number of different
factors can
contribute to the determination of the load including, but not limited to, the
design of the
plant and the size of the various components of the feed gas producing unit,
the process
unit (0), heat exchangers, carbon dioxide removal unit, etc. Preferably the
conversion of
carbon monoxide to carbon dioxide and hydrogen is maximized utilizing a
portion of the
recycle stream (8.1) while the remaining portion of the recycle stream (8.2)
is sent to the
process unit (0). This is accomplished by first directing the flow of the
hydrogen rich
permeate stream (8) to be added to the stream that is to be fed into the feed
gas producing
unit. The optimum solution is to split the hydrogen rich permeate stream (8)
with one
part or fraction going to the feed gas producing unit and the other part or
fraction going to
the stream to be introduced into the process unit.
In a still further embodiment of the present invention, a water gas shift
reactor (32) may
be installed along the line transporting the hydrogen rich permeate stream (8)
in order to
reduce the carbon monoxide that may be present in the stream (8). It is
especially
preferred to reduce the level of carbon monoxide to such a low level that
there is no
further incentive to convert the carbon monoxide contained in the stream (8),
In the
preferred embodiment, this water gas shift reactor (32) would be a low
temperature water
gas shift reactor. As used herein, the phrase "low temperature water gas shift
reactor"
refers to a water gas shift reaction that generally occurs in the 180 to 240 C
range to
further reduce carbon monoxide levels compared to the higher temperature water
gas
shift reactor which generally operates in a higher temperature range and is
utilized to
convert bulk (higher percentages) of carbon monoxide. Low temperature water
gas shift
reactors of the type contemplated in the present invention are known in the
art and
accordingly will not be described in great detail herein other than to not
that such reactors
require proper heating means (not shown) and steam injection (not shown).
Furthermore,
the size of the low temperature water gas shift reactor (32) will depend upon
the quantity
of hydrogen rich permeate (8) processed. Typically, this stream (8) will be
smaller in
size than the quantity of the feed stream (15) processed.
17
CA 02777324 2012-05-17
Serie 8915
With regard to the additional streams, while the hydrogen product stream (23)
is
recovered as product, as in the previous embodiments, a portion of this stream
(23) can
be used for hydrogen fueling of the feed gas producing unit (31). In a still
further
modification to this embodiment, it is advantageous to further heat the first
hydrogen rich
permeate fraction (8.1) prior to this stream being added as a supplemental
feed to the feed
gas producing unit.
In the next step of the present process, the hydrogen lean residue stream (9)
is passed
through a carbon dioxide selective membrane separation unit (10) in order to
form a
carbon dioxide enriched permeate stream (11). As used herein with regard to
the carbon
dioxide enriched permeate stream (11), the phrase "carbon dioxide enrich"
refers to the
permeate stream having a percentage of carbon dioxide that is greater than the
percentage
of the other components in the carbon dioxide enriched permeate stream (11).
The
carbon dioxide selective membrane of the carbon dioxide selective membrane
separation
unit (10) is used to preferentially permeate carbon dioxide over carbon
monoxide,
methane and nitrogen as well as any other components in the stream being
subjected to
the carbon dioxide selective membrane. In the preferred embodiment of the
present
process, the carbon dioxide selective membrane utilized had a carbon dioxide
permeability that is more than 5 times, preferably greater than 10 times and
even more
preferably greater than 20 times that of the gas or gases from which the
carbon dioxide is
separated under the chosen operating conditions, with the exception of
hydrogen.
The remaining components in the hydrogen lean residue stream (9) form a carbon
dioxide
depleted residue stream (12). As used herein with regard to the carbon dioxide
depleted
residue stream (12), the phrase "carbon dioxide depleted" refers to the
residue stream
having a percentage of carbon dioxide that is less than that in the stream
introduced into
the carbon dioxide membrane separation unit (10) (the hydrogen lean residue
stream (9)).
The carbon dioxide selective membrane separation unit (10) utilized in the
process of the
present invention contains at least one membrane that is selective for carbon
dioxide over
the other components in the hydrogen lean residue stream (9). Note that the
target
18
CA 02777324 2012-05-17
Serie 8915
molecule, in this case carbon dioxide, determines how the permeate stream is
used. With
regard to each of the membranes utilized in the present process, each carbon
dioxide
selective membrane has a permeate side (10.1) and a residue side (10.2). Since
the
membrane is selective for carbon dioxide, it allows for the passing of carbon
dioxide
through the membrane to the permeate side (10.1) of the membrane.
While a variety of different types of membranes may be utilized in the carbon
dioxide
selective membrane separation unit (10) of the process of the present
invention, the
preferred membrane is a polymeric membrane that is selective for carbon
dioxide that is
selected from one or more polyimides, polyetherimides polysulfone,
polyethersulfones,
polyarylsulfone, polycarbonate, tetrabromo- bisphenol A polycarbonate,
tetrachloro-
bisphenol A polycarbonate, polydimethylsiloxane, natural rubber, cellulose
actetate,
cellulose triacetate, ethyl cellulose, PDD-TFE and polytriazole.
With regard to each of the carbon dioxide selective membranes utilized in the
carbon
dioxide selective membrane separation unit (10) of the present process, each
carbon
dioxide selective membrane has a permeate side (10.1) and a residue side
(10.2). Since
the membrane is selective for carbon dioxide, it allows for the passing of
carbon dioxide
through the membrane to the permeate side (10.1) of the membrane. While the
membrane is selective for carbon dioxide, those skilled in the art will
recognize that a
minor portion of the other components in the hydrogen lean residue stream (9)
will also
pass through the carbon dioxide selective membrane to become a part of the
permeate.
Accordingly, with regard to the present process, the permeate stream that is
obtained will
generally contain from about 40 % to about 90 % carbon dioxide with the
remaining part
of the permeate stream comprising the other components contained in the
hydrogen lean
residue stream (9). As a result of passing the hydrogen lean residue stream
(9) into the
carbon dioxide selective membrane separation unit (10) and through the
membrane, this
stream is separated into two streams-one which is considered to be carbon
dioxide
enriched and one which is considered to be carbon dioxide depleted.
While a variety of different types of membranes may be utilized in the carbon
dioxide
19
CA 02777324 2012-05-17
Serie 8915
selective membrane separation unit (10) of the process of the present
invention, the
preferred membrane is made of any number of polymers that are suitable as
membrane
materials. With regard to the membranes of the present invention, these
polymers
include, but are not limited to, substituted or unsubstituted polymers
selected from
polysiloxane, polycarbonates, silicone-containing polycarbonates, brominated
polycarbonates, polysulfones, polyether sulfones, sulfonated polysulfones,
sulfonated
polyether sulfones, polyimides and aryl polyimides, polyether imides,
polyketones,
polyether ketones, polyamides including aryl polyamides, poly(esteramide-
diisocyanate),
polyamide/imides, polyolefins such as polyethylene, polypropylene,
polybutylene, poly-
4-methyl pentene, polyacetylenes, polytrimethysilylpropyne, fluorinated
polymers such
as those formed from tetrafluoroethylene and perfluorodioxoles,
poly(styrenes), including
styrene-containing copolymers such as acrylonitrile-styrene copolymers,
styrene-
butadiene copolymers and styrene-vinylbenzylhalide copolymers, cellulosic
polymers,
such as cellulose acetate-butyrate, cellulose propionate, ethyl cellulose,
methyl cellulose,
cellulose triacetate, and nitrocellulose, polyethers, poly(arylene oxides)
such as
poly(phenylene oxide) and poly(xylene oxide), polyurethanes, polyesters
(including
polyarylates), such as poly(ethylene terephthalate), and poly(phenylene
terephthalate),
poly(alkyl methacrylates), poly(acrylates), polysulfides, polyvinyls, e.g.,
poly(vinyl
chloride), poly(vinyl fluoride), poly(vinylidene chloride), poly(vinylidene
fluoride),
poly(vinyl alcohol), poly(vinyl esters) such as poly(vinyl acetate) and
poly(vinyl
propionate), poly(vinyl pyridines), poly(vinyl pyrrolidones), poly(vinyl
ketones),
poly(vinyl ethers), poly(vinyl aldehydes) such as poly(vinyl formal) and
poly(vinyl
butyral), poly(vinyl amides), poly(vinyl amines), poly(vinyl urethanes),
poly(vinyl
ureas), poly(vinyl phosphates), and poly(vinyl sulfates), polyallyls,
poly(benzobenzimidazole), polyhydrazides, polyoxadiazoles, polytriazoles:
poly(benzimidazole), polycarbodiimides, polyphosphazines, and interpolymers,
including
block interpolymers containing repeating units from the above such as
terpolymers of
acrylonitrile-vinyl bromide-sodium salt of para-sulfophenylmethallyl ethers,
and grafts
and blends containing any of the foregoing. The polymer suitable for use is
intended to
also encompass copolymers of two or more monomers utilized to obtain any of
the
homopolymers or copolymers named above. Typical substituents providing
substituted
CA 02777324 2012-05-17
Serie 8915
polymers include halogens such as fluorine, chlorine and bromine, hydroxyl
groups,
lower alkyl groups, lower alkoxy groups, monocyclic aryl, lower acyl groups
and the
like.
With regard to one embodiment of the present invention, the preferred polymers
include,
but are not limited to, polysiloxane, polycarbonates, silicone-containing
polycarbonates,
brominated polycarbonates, polysulfones, polyether sulfones, sulfonated
polysulfones,
sulfonated polyether sulfones, polyimides, polyetherimides, polyketones,
polyether
ketones, polyamides, polyamide/imides, polyolefins such as poly-4-methyl
pentene,
polyacetylenes such as polytrimethysilylpropyne, and fluoropolymers including
fluorinated polymers and copolymers of fluorinated monomers such as
fluorinated olefins
and fluorodioxoles, and cellulosic polymers, such as cellulose diacetate and
cellulose
triacetate. Examples of preferred polyimides are Ultem 1000, P84 and P84-HT
polymers, and Matrimid 5218.
Of the above noted polymeric membranes, the most preferred membranes are those
made
of polyimides. More specifically, polyimides of the type disclosed in U.S.
Patent No.
7,018,445 and U.S. Patent No. 7,025,804, each incorporated herein in their
entirety by
reference. With regard to these types of membranes, the process of the present
invention
preferably utilizes a membrane comprising a blend of at least one polymer of a
Type 1
copolyimide and at least one polymer of a Type 2 copolyimide in which the Type
1
copolyimide comprises repeating units of formula I
00
K
-R, -N~rR -
~_Y
O O
(I)
in which R2 is a moiety having a composition selected from the group
consisting of
formula A, formula B, formula C and a mixture thereof,
21
CA 02777324 2012-05-17
Serie 8915
O 0 0 O Z O
(A) (B) (C)
Z is a moiety having a composition selected from the group consisting of
formula L,
formula M, formula N and a mixture thereof, and
0 0
II II
/cam O -S-
II
0
(L) (M) (N)
R, is a moiety having a composition selected from the group consisting of
formula Q,
formula S, formula T, and a mixture thereof,
- 0-CH, O
41--- O
CH3
CH3
(Q) (S) (T)
in which the Type 2 copolyimide comprises the repeating units of formulas IIa
and Ilb
O 0 0 0
II II II II
-Ar-N
C/Ra C N -Ar-N C/ C
RbN -
O O O O
(IIa) (IIb)
in which Ar is a moiety having a composition selected from the group
consisting of
formula U, formula V, and a mixture thereof,
22
CA 02777324 2012-05-17
Serie 8915
X
0 X 0 X,
X, X3 X11 X3
XZ
(U) (V)
in which
X, XI, X2, X3 independently are hydrogen or an alkyl group having 1 to 6
carbon atoms,
provided that at least two of X, XI, X2, or X3 on each of U and V are an alkyl
group,
Ar' is any aromatic moiety,
Ra and Rb each independently have composition of formulas A, B, C, D or a
mixture
thereof, and
)a -b-d- -b-Z-6
(A) (B) (C)
F3C CF3
0 0
(D)
Z is a moiety having composition selected from the group consisting of formula
L,
formula M, formula N and a mixture thereof
0 0
11 11
C N-11 0 -s-
II
0
(L) (M) (N)
23
CA 02777324 2012-05-17
Serie 8915
The material of the membrane consists essentially of the blend of these
copolyimides.
Provided that they do not significantly adversely affect the separation
performance of the
membrane, other components can be present in the blend such as, processing
aids,
chemical and thermal stabilizers and the like.
In a preferred embodiment, the repeating units of the Type 1 copolyimide have
the
composition of formula Ia.
O o
1
-R, -N O O N-
C
0 p O
(Ia)
Wherein R1 is as defined hereinbefore. A preferred polymer of this composition
in which
it is understood that R1 is formula Q in about 16 % of the repeating units,
formula S in
about 64 % of the repeating units and formula T in about 20 % of the repeating
units is
available from HP Polymer GmbH under the tradename P84
In another preferred embodiment, the Type 1 copolyimide comprises repeating
units of
formula lb.
O O
1
-R1 -N O N-
O O
(lb)
Wherein R1 is as defined hereinbefore. Preference is given to using the Type 1
copolyimide of formula lb in which R1 is a composition of formula Q in about 1-
99 % of
the repeating units, and of formula s in a complementary amount totaling 100 %
of the
repeating units.
24
CA 02777324 2012-05-17
Serie 8915
In yet another preferred embodiment, the Type 1 copolyimide is a copolymer
comprising
repeating units of both formula la and lb in which units of formula lb
constitute about 1 -
99 % of the total repeating units of formulas la and lb. A polymer of this
structure is
available from HP Polymer GmbH under the tradename P84-HT325.
In the Type 2 polyimide, the repeating unit of formula IIa should be at least
about 25%,
and preferably at least about 50% of the total repeating units of formula IIa
and formula
IIb. Ar' can be the same as or different from Ar.
The polyimides utilized to form the membranes of the present process will
typically have
a weight average molecular weight within the range of about 23,000 to about
400,000 and
preferably about 50,000 to about 280,000.
The carbon dioxide selective membranes of the present process can be
fabricated into any
membrane form by any appropriate conventional method as noted hereinbefore
with
regard to the hydrogen selective membranes (i.e., flat sheet membranes or
hollow fiber
membranes). While the carbon dioxide selective membranes do not have to be in
the
same form as the hydrogen selective membranes, in one preferred embodiment,
the form
of the carbon dioxide selective membranes is in the hollow fiber form and the
hydrogen
selective membranes are in the same form.
As with the hydrogen selective membrane separation unit (7), the carbon
dioxide
membrane separation unit (10) includes at least one of the above noted
membranes. With
regard to the actual configuration of the carbon dioxide selective membrane
separation
unit (10), the carbon dioxide selective membrane separation unit (10) can take
on any
number of different configurations as discussed hereinbefore with regard to
the hydrogen
selective membrane separation unit (7).
As noted, as a result of passing the hydrogen lean residue stream (9) through
the carbon
dioxide selective membrane separation unit (10), two separate streams are
formed--a
carbon dioxide enriched permeate stream (11) and a carbon dioxide depleted
residue
CA 02777324 2012-05-17
Serie 8915
stream (12) wherein the enrichment and depletion of carbon dioxide is with
reference to
the feed stream fed to the carbon dioxide selective membrane separation unit
(10). The
carbon dioxide enriched permeate stream (11) may be further utilized in a
variety of
manners. More specifically, the carbon dioxide enriched permeate stream (11)
may be
recycled to the process stream (1) from the process unit (0) where is it added
to the
process stream (1) prior to the compressor (2) (as shown in Figure 1) or
within the
compressor (2) between two of the stages of compression (not shown in Figure
1) or
optionally compressing the carbon dioxide enriched permeate stream (11) and
recycling
the optionally compressed carbon dioxide enriched permeate stream (11) to be
used as a
supplemental feed stream in other processes such as a supplemental feed stream
for a
water gas shift reactor in a hydrogen production plant. The carbon dioxide
enriched
permeate stream (11) may also be recycled directly back to the carbon dioxide
separation
unit (4) for further processing.
The carbon dioxide depleted residue stream (12) that is obtained from the
carbon dioxide
selective membrane separation unit (10) can be withdrawn for further use. For
example,
the carbon dioxide depleted residue stream (12) can be used as a fuel (for
example as a
steam methane reformer fuel), as a feed stream (for example as a steam methane
reformer
feed stream) or as both a fuel and a feed stream in other processes such as in
a hydrogen
generation plant. In addition, the carbon dioxide depleted residue stream (12)
can be
used to regenerate any dryers that may be positioned within the process
schematic of the
present invention to remove moisture, thereby increasing the efficiency of
carbon dioxide
removal in the carbon dioxide separation unit (4) at lower temperatures.
The operating temperatures for the hydrogen selective membranes and the carbon
dioxide
selective membranes are each independently selected based on the physical
properties of
each membrane such that it is mechanically stable and a sufficient gas flux
can be
maintained across the membrane. Typically, the stream being fed to each of the
membrane separation units (7, 10) will be heated or cooled, if necessary, to a
temperature
which ranges from about -55 C to about 150 C. In other words, the process of
membrane
separation in each of these units (7, 10) typically operates at the noted
temperature. In
26
CA 02777324 2012-05-17
Serie 8915
one alternative, the hydrogen lean reside stream (9) is fed into the carbon
dioxide
selective membrane unit (10) at low to sub-ambient temperatures, preferably
from -55 C
to about 30 C, preferably from -55 C to about 10 C. In such cases, the carbon
dioxide
selective membranes are cold membranes. In still another alternative, the
carbon dioxide
lean non-condensable stream (5) from the carbon dioxide separation unit (4) is
fed to the
hydrogen selective membrane separation unit (7) after being heated to a
temperature from
about 50 C to about 150 C in an optional heat exchanger 28. With regard to
this
particular alternative, the heat brought to the carbon dioxide lean non-
condensable stream
(5) is taken from the process stream (1) after the step of compression.
In an even further still embodiment of the present invention, it is also
possible to
incorporate an optional water gas shift reactor (33) just prior to the
hydrogen and carbon
dioxide membrane units (7, 10) in order to further convert any carbon monoxide
presenting the carbon dioxide lean non-condensable stream (5). As with the
optional
water gas shift reactor located along the hydrogen rich permeate stream (8),
this water
gas shift reactor (33) would also preferably be a low temperature water gas
shift reactor
as described hereinbefore. The size of the low temperature water gas shift
reactor (33)
would depend upon the amount of the carbon dioxide lean non-condensable stream
(5)
being processed. Note that when this option is utilized, the membranes
utilized in the
hydrogen and carbon dioxide membrane units (7, 10) will be designed to address
wet
syngas.
While the preferred embodiments would be to place the optional water gas shift
reactor
(32 or 33) along the permeate line (8) or after the carbon dioxide separation
unit (4) and
just prior to the hydrogen and carbon dioxide membranes (7, 10) respectively,
in a still
further embodiment, it would be possible to place a low temperature water gas
shift
reactor (not shown) just prior to the process unit (0) to treat the feed gas
(15).
Additional embodiments of the present invention are depicted in Figures 5 to
7. These
embodiments relate to a process for producing hydrogen in a hydrogen
generation plant
from a hydrocarbon containing feed stream (16) (preferably natural gas) and
capturing at
27
CA 02777324 2012-05-17
Serie 8915
least 80%, preferably at least 90%, even more preferably at least 99%, and
further still
approaching or obtaining 100% capture, of the overall emissions of carbon
dioxide of the
feed gas producing unit (31) utilizing a carbon dioxide separation unit and
two membrane
separation units. More specifically, in the process of the present invention,
the process
can be executed in a variety of manners including utilizing a feed gas
producing unit (31),
a pressure swing adsorption unit (0), a carbon dioxide separation unit (4), a
hydrogen
selective membrane separation unit (7) and a carbon dioxide selective membrane
separation unit (10). As noted previously herein, the phrase "feed gas
producing unit"
refers to any unit which produces a feed gas that can be subjected to
treatment for the
removal of hydrogen. More specifically, the feed gas (15) may be a feed gas
(15) from a
reformer unit/water gas shift unit, a POx unit, an ATR unit, syngas from a
coal
gasification unit, refinery off gas or any other gas mixture that contains
hydrogen, carbon
monoxide and carbon dioxide as components in the gas mixture. The present
process is
preferably executed in a variety of manners including: A) using one or more
pre-
reformers (17), a steam methane reformer (19), a water gas shift reactor (21),
a pressure
swing adsorption unit (0), a carbon dioxide separation unit (4), a hydrogen
selective
membrane separation unit (7) and a carbon dioxide selective membrane
separation unit
(10) or B) a steam methane reformer (19) , a water gas shift reactor (21), a
pressure swing
adsorption unit (0), a carbon dioxide separation unit (4), a hydrogen
selective membrane
separation unit (7) and a carbon dioxide selective membrane separation unit
(10). With
regard to these preferred embodiments, a hydrocarbon containing feed stream
(16) is
optionally pre-reformed in at least one pre-reformer (17) to form a pre-
reformed gas
stream (18). Pre-reforming is carried out in those cases where it is
considered to be
advantageous to reform the heavier hydrocarbons in the hydrocarbon containing
feed
stream (16) thereby reducing cracking on the catalyst in the main steam
methane
reformer (19) and preventing excessive heat rise in the main reformer. The
present
process is not meant to be limited by the type of pre-reformer (17) utilized
for carrying
out the process of the present invention. Accordingly, any pre-reformer (17)
that is
known in the art may be used in the process of the present invention. The pre-
reformer
(17) can be a single high pressure (typically from about 25 to about 30 bar)
adiabatic
vessel where desulfurized natural gas preheated to around 600 C is fed to a
bed filled
28
CA 02777324 2012-05-17
Serie 8915
with pre-reforming catalyst (typically catalyst with a high nickel content).
Such vessels
typically have an outlet temperature around 400 C. The pre-reformer (17) can
also be a
series of at least two adiabatic pre-reformers (17) with heating in between
the vessels in
order to provide additional benefits by minimizing the amount of fuel required
and thus
the amount of hydrogen to fuel. The advantage of such pre-reformers (17) is
that the
overall need for fuel to provide direct heat to the reforming reaction is
reduced, hence
naturally decreasing carbon dioxide production in the plant (leading to the
high overall
carbon dioxide recovery). In addition, the pre-reformer (17) may be operated
in the same
manner that is known in the art utilizing general conditions, including
temperatures and
pressures.
The next step of the preferred process involves reforming the pre-reformed gas
stream
(18) (or in the case where there is no pre-reforming, the hydrocarbon
containing gas
stream (16)) in a steam methane reformer unit (19) in order to obtain a syngas
stream
(20). As with the pre-reformer (17), the present invention is not meant to be
limited by
the steam methane reformer unit (19) or the process for carrying out the
reaction in the
steam methane reformer unit (19). Accordingly, any steam methane reformer unit
(19)
known in the art may be used in the process of the present invention. By way
of general
description, with regard to the steam methane reformer unit (19) of Figure 5,
the pre-
reformed gas stream (18) (or hydrocarbon containing gas stream (16)) will be
combined
with high pressure steam (not shown in Figure 5) before entering the steam
methane
reforming unit (19). Such steam methane reformer units (19) typically contain
tubes (not
shown) packed with catalyst (typically a nickel catalyst) through which the
steam and gas
stream (18) mixtures passes. An elevated temperature of about 860 C is
typically
maintained to drive the reaction which is endothermic. As used throughout with
regard
to the present invention, the phrase "steam methane reformer unit" refers not
only to the
actual reformer units, but also to all of the additional components that
typically are
considered to make up a steam methane reformer, including, but not limited to,
one or
more components selected from heat exchangers, the reformer, tubes with one or
more
types of catalyst, etc. Prior to be introduced into the actual reformer of the
steam
methane reformer (19), the stream to be treated will typically be compressed,
e.g. to
29
CA 02777324 2012-05-17
Serie 8915
about 200 to 600 psig, and combined with the steam as described hereinbefore.
In those
instances where pre-reforming is utilized, the stream to be pre-reformed will
typically be
compressed to e.g., about 200 to 600 psig, thereby resulting in a pre-reformed
gas stream
(18) which does not require further compression before being introduced into
the steam
methane reformer (19). The reaction product from the steam methane reformer
unit (19)
is principally a hydrogen rich effluent that contains hydrogen, carbon
monoxide,
methane, water vapor and carbon dioxide in proportions close to equilibrium
amounts at
the elevated temperature and pressure. This effluent is referred to as the
syngas stream
(20) in the present process.
Once the reforming is carried out, the resulting syngas stream (20) is
subjected to a shift
reaction in a water gas shift reactor (21) in order to obtain a feed gas (15).
The syngas
stream (20) is subjected to a shift reaction due to the high amount of carbon
monoxide
that is often present due to the steam methane reforming (the amount of carbon
monoxide
actually depends upon the composition of the initial stream injected into the
steam
methane reformer unit (19)). The water gas shift reactor (21) functions to
form additional
hydrogen and carbon dioxide by further reacting or treating the syngas stream
(20) in
order to obtain a feed gas (15) for the process unit (0). The syngas stream
(20) is
introduced into the water gas shift reactor (21) (which can contain a variety
of stages or
one stage; various stages not shown) along with steam (not shown) to form
additional
hydrogen and carbon dioxide. The water gas shift reactor (21) converts the
carbon
monoxide to carbon dioxide with the liberation of additional hydrogen by
reaction at high
temperature in the presence of the additional steam. Such reactors (21)
typically operate at
a temperature from about 200 C to about 500 C. In some cases the stream from
the steam
methane reformer (19) will be at a higher temperature so optionally the stream
may first be
cooled with a heat exchanger (typically a steam generator - not shown) before
being passed
through the water gas shift reactor (21). In a preferred alternative, the
water gas shift
reactor (21) is a multiple stage water gas shift reactor which includes high
temperature
shift (typically about 371 C or above), medium temperature shift (typically
around
288 C), low temperature shift (typically about 177 C to 204 C) or any
combination
thereof. Such multiple stage water gas shift reactors are known and are used
to
CA 02777324 2012-05-17
Serie 8915
concentrate the amount of carbon dioxide in the resulting gas stream by the
manner in
which the shifts are arranged (with the high temperature shift resulting in
less carbon
monoxide reaction and the low temperature shift resulting in more carbon
monoxide
reaction).
The feed gas (15) from the water gas shift reactor (21) is then subjected to
the process as
described hereinbefore involving a process unit (0), a carbon dioxide
separation unit (4),
a hydrogen selective membrane separation unit (7), and a carbon dioxide
membrane
separation unit (10), each as described hereinbefore.
In this particular embodiment, the feed gas (15) is introduced into the
process unit (0) (in
this particular case a pressure swing adsorption unit) where it undergoes
pressure swing
adsorption to produce a hydrogen product stream (23) and a process stream (1).
While
the hydrogen product stream (23) is recovered as product, a portion of this
stream (23)
can be used for hydrogen fueling of the steam methane reformer (19). The
process
stream (1) is further treated in the carbon dioxide separation unit as
described
hereinbefore. As noted previously, the process stream (1) may optionally be
completely
compressed in the compressor (2) or partially compressed in the compressor (2)
or
completely compressed in an additional compressor that forms a part of the
carbon
dioxide separation unit (4) or partially compressed in an additional
compressor that forms
a part of the carbon dioxide separation unit (4) as described hereinbefore.
The process
stream (1) is cooled in the heat exchanger (3) prior to the
separation/purification steps of
the carbon dioxide separation unit (4). As a result of treating the process
stream (1) in the
carbon dioxide separation unit (4), a carbon dioxide rich liquid stream (which
can be
vaporized) is produced. This stream is withdrawn from the carbon dioxide
separation
unit (4) where it can be used as product. The remaining components from the
process
stream (1) form a carbon dioxide lean non-condensable stream (5) which is then
passed
through a hydrogen selective membrane separation unit (7) thereby forming a
hydrogen
rich permeate stream (8) and a hydrogen lean residue stream (9). As noted in
the
previously described process of Figure 1, the hydrogen rich permeate stream
(8) may be
31
CA 02777324 2012-05-17
Serie 8915
optionally compressed in a compressor (13) and recycled to be used as
supplemental feed
for the process unit (0).
However, when the present embodiment is utilized in a hydrogen generation
plant as
shown in Figure 5, in addition to being recycled for use as a supplemental
feed for the
process unit (0), the hydrogen rich permeate stream (8) may also be used as a
supplemental feed stream for a steam methane reformer (19) and/or for a water
gas shift
reactor (21) after optionally compressing the stream (8). Accordingly, with
regard to the
preferred embodiment, the hydrogen rich permeate stream (8) may be recycled as
a
supplemental feed for one or more of 1) the process unit (0), 2) the steam
methane
reformer (19) and 3) the water gas shift reactor (21). Also, the hydrogen rich
permeate
stream (8) may be utilized as a supplemental fuel for a steam methane reformer
(19). In
one embodiment, it is especially preferable to use the hydrogen rich permeate
stream (8)
as a fuel to the steam methane reformer (19) since doing so can boost the
percentage of
carbon dioxide capture. In addition, by doing so, it is possible to eliminate
or reduce the
carbon dioxide emissions from the steam methane reformer (19) as the natural
gas fuel
has been eliminated/minimized.
In another embodiment, the hydrogen rich permeate stream (8) can be used as a
supplemental feed for the process unit (0) with the objective of increasing
hydrogen
production. In an alternative embodiment, the hydrogen rich permeate stream
(8) can be
used as a supplemental feed for the water gas shift reactor (21) with the
objective of
driving the reaction towards the production of more carbon dioxide and
hydrogen
(converting more of the carbon monoxide into carbon dioxide and hydrogen). In
a still
further embodiment as depicted in Figure 6, the process unit (0) is a pressure
swing
adsorption unit (0) and the hydrogen rich permeate stream (8) is utilized as
two separate
fractions-as a first hydrogen rich permeate fraction (8.1) to be used as a
supplemental
feed stream in the water gas shift reactor (21) and as a second hydrogen rich
permeate
fraction (8.2) to be used as a supplemental feed stream in the pressure swing
adsorption
unit (0). For purposes of Figure 6, the remaining recycle streams that are
noted in Figure
have been omitted in order to concentrate more specifically upon the recycle
of the
32
CA 02777324 2012-05-17
Serie 8915
fractions (8.1, 8.2) of the hydrogen rich permeate stream (8). With regard to
this
particular embodiment, the objective is to optimize the use of the recycle
stream (8) in
order to maximize the conversion of carbon monoxide to carbon dioxide and
hydrogen
while at the same time maximizing the production of hydrogen product.
With regard to this particular embodiment, the proportion of each fraction
recycled to the
corresponding devices (0, 21) depends upon the percentage of production (the
load) from
the steam methane reformer (19). Those of ordinary skill in the art will
recognize that a
number of factors can contribute to the determination of the load for the
steam methane
reformer (19) including, but not limited to, the design of the plant, the size
of the various
components such as the steam methane reformer (19), water gas shift reactor
(21), the
pressure swing adsorption unit (0), heat exchangers, carbon dioxide removal
unit, etc.
With regard to this particular embodiment, the shift reaction is maximized
utilizing a
portion of the recycle stream (8.1) while the remaining portion of the recycle
stream (8.2)
is sent to the pressure swing adsorption unit (0). This is accomplished by
first directing
the flow of the hydrogen rich permeate stream (8) to be added to the syngas
stream (20)
that is to be fed into the water gas shift reactor (21). As noted, the
quantity of this first
fraction (8.1) is determined by the load of the steam methane reformer unit
(19). More
specifically, when the steam methane reformer unit (19) is running at full
load or
capacity, a much higher flow is being sent to the water gas shift unit (21)
and
consequently, a much higher flow is being sent further downstream.
Accordingly, for
plants that are retrofitted and not specifically designed to handle this
degree of flow of
recycle, there may exist limitations on shift capacity, heat exchanger duties,
etc.
Therefore, in some instances, there may be limitations when the entire recycle
(8) is
added prior to the water gas shift unit (21). However, it is desirable to
recycle to the
water gas shift reactor (21) as an ultimate increase in hydrogen production
can be seen
(an increase of up to 15% or more). In those instances where the recycle (8)
is simply
sent to the stream (15) before the pressure swing adsorption unit (0), there
may still be
capacity issues with regard to the actual pressure swing adsorption unit (0)
and the
downstream compressors. Even so, this option is also desirable as an increase
in
hydrogen production can also be obtained with this option.
33
CA 02777324 2012-05-17
Serie 8915
The optimum solution is to split the hydrogen rich permeate stream (8) with
one part or
fraction going to the water gas shift reactor (19) and the other part or
fraction going to the
pressure swing adsorption unit (21). With regard to this embodiment, it is
preferable to
first direct as much as possible of the flow of the recycle (8.1) to the
syngas stream (20)
before the water gas shift reactor (21) until the water gas shift reactor (21)
reaches it
maximum capacity (being determined in part by the steam methane reformer (19)
load)
or unit 100% of the recycle stream (8.1) is recycled to the water gas shift
reactor (21) and
then directing the remaining fraction (8.2), if any, to the pressure swing
adsorption unit
(0).
As used herein the phrase "steam methane reformer (19) load" refers to the
actual volume
of the gas stream processed in the steam methane reformer (19) compared to the
volume
that the steam methane reformer (19) is capable of processing. For example, if
the steam
methane reformer is capable of processing 50,000 standard cubic meters of
natural gas
but only processes 45,000 standard cubic meters of natural gas, then the load
would be
considered to be 90%. When the load from the steam methane reformer unit (19)
is
considered to be relatively low, a larger proportion of the recycle will go to
the water gas
shift reactor (21) rather than to the pressure swing adsorption unit (0).
Those skilled in
the art will recognize that the load for the steam methane reformer (19) will
depend upon
any number of a variety of variables such as the size of the plant, the size
of the steam
methane reformer (19), the size of the equipment utilized downstream, and the
composition of the natural gas stream. Accordingly, the phrase "relatively
low" when
used in terms of the steam methane reformer (19) load operated under standard
conditions that are known to those skilled in the art, refers to those
instances where the
load with regard to the steam methane reformer (19) is, for example, less than
or equal to
85%. In such instances, often the first hydrogen rich permeate fraction (8.1)
will be
greater than the second hydrogen rich permeate fraction (8.2) in terms of
quantity. In
other words, the first hydrogen rich permeate will comprise greater than 50%
of the total
amount of the hydrogen rich permeate stream (8). Otherwise, in those instances
where
the steam methane reformer (19) load is greater than 85%, preferably greater
than 90%,
34
CA 02777324 2012-05-17
Serie 8915
the second hydrogen rich permeate stream will range from greater than 50% of
the
hydrogen rich permeate stream (8) up to 100% of the hydrogen rich permeate
stream (8).
In a still further modification to this embodiment, it is advantageous to
further heat the
first hydrogen rich permeate fraction (8.1) prior to this stream being added
as a
supplemental feed to the syngas stream of line (20). This combined fraction
(8.1) and
syngas stream (20) are then fed into the water gas shift reactor (21). While
this heating
may be carried out in any manner known in the art, preferably the first
hydrogen rich
permeate fraction (8.1) is heated utilizing a heat exchanger (30) specifically
for this
permeate fraction (8.1). In addition to heating this first hydrogen rich
permeate fraction
(8.1) before adding the fraction to the syngas stream (20), steam can be
injected into this
fraction (8.1) via line (29) just prior to the fraction (8.1) being mixed with
the syngas
stream (20). The heating of this first hydrogen rich permeate fraction (8.1)
further
improves the efficiency of the recycle. By injecting steam into this fraction
(8.1), it is
possible to avoid steam condensation (which is detrimental to the catalyst in
the water gas
shift reactor (21)) when mixed with the syngas stream (20). In addition, by
injecting
steam at this point, it will be possible to further drive the carbon monoxide
shift.
Note that with regard to the hydrogen production and carbon dioxide capture
embodiments, an optional water gas shift reactor may also be utilized to shift
away
carbon monoxide in the hydrogen permeate stream (8) as discussed hereinbefore.
Preferably such a water gas shift unit (32) would be a low temperature water
gas shift
unit as defined hereinbefore. In addition with regard to these embodiments, an
optional
water gas shift reactor (33) may also be utilized prior to the hydrogen and
carbon dioxide
membrane units (7, 10) as defined hereinbefore. Finally, a low temperature
water gas
shift reactor (not shown) may also be considered to be placed just prior to
the process unit
(0) to treat the feed gas (15).
The hydrogen lean residue stream (9) is passed through the carbon dioxide
selective
membrane separation unit (10) thereby forming a carbon dioxide enrich permeate
stream
(11) and a carbon dioxide depleted residue stream (12) as described
hereinbefore. The
CA 02777324 2012-05-17
Serie 8915
carbon dioxide enriched permeate stream (11) can be recycled in a variety of
manners
including 1) to the process stream (1) from the process unit (0) where is it
added to the
process stream (1) just prior to the compressor (2) (as shown in Figure 5) or
within the
compressor (2) between two of the stages of compression (not shown in Figure
5); 2)
optionally compressing the carbon dioxide enriched permeate stream (11) and
recycling
the optionally compressed carbon dioxide enriched permeate stream (11) to be
used as a
supplemental feed stream processes other than the present process; or recycled
directly
back to the carbon dioxide separation unit (4) for further processing. The
carbon dioxide
depleted residue stream (12) that is recovered, after optionally being turbo
expanded in a
turbo expander (22) (in order to recover compressed gas energy and use this
energy to
drive other components of the process) can be used as a supplemental feed for
the pre-
reformer (17) or the steam methane reformer (19). While it is possible to also
use the
carbon dioxide depleted residue stream (12) as a supplemental fuel for the
steam methane
reformer (19), when higher levels of capture are desirable, the amount of
residue stream
(12) used as fuel will need to be minimized (when levels approaching 90% are
desired) or
eliminated (when levels of carbon dioxide capture approaching 100% are
desired).
With regard to this particular process, it is possible to achieve an overall
capture rate of
carbon dioxide that is equal to or greater than 80%, even more preferably
equal to or
greater than 90%, and even still more preferably equal to or greater than 99%,
and further
still approaching or achieving 100% capture, when hydrogen fueling is
utilized. Those of
ordinary skill in the art will recognize that in order to eliminate possible
issues such as
build up of inerts (e.g., nitrogen) downstream of the pressure swing
adsorption unit in the
system, it may be desirable to configure the pressure swing adsorption unit to
allow for
selectivity for those inerts thereby creating a hydrogen stream which is rich
in inerts, this
hydrogen stream that is rich in inerts to be used as fuel for the steam
methane reformer
unit (19).
In a broader aspect, the process for producing hydrogen and capturing carbon
dioxide
from a hydrocarbon containing feed stream (16) in a hydrogen generation plant
may be
carried out utilizing any feed gas producing unit (31)(see Figure 7). As noted
above, the
36
CA 02777324 2012-05-17
Serie 8915
preferred method is carried out using a steam methane reformer (19) with a
water gas
shift reactor (21) and an optional pre-reformer (17). However, as depicted in
the
embodiment set forth in Figure 7, such feed gas producing units (31) may also
include
POx units, ATR units, coal gasification units or refinery process units (where
the feed gas
(15) results from refinery processing; a refinery off gas) or any other feed
gas producing
unit that produces a gas mixture that contains at least hydrogen, carbon
monoxide and
carbon dioxide. With regard to the embodiments which contain a gasification
unit or a
refinery process unit which produces a feed gas, in addition to the hydrogen
rich
permeate stream (8.2) being recycled for use as a supplemental feed for the
process unit
(0), the hydrogen rich permeate stream (8.1) may also be recycled back to the
source
(feed gas producing unit 31) that produces the feed gas (15) that is supplied
to the process
unit (0) (feed gas producing unit 31) to be used as a supplemental feed
stream. More
specifically, in addition to the hydrogen permeate stream (8) being used in a
schematic
where it can be used as a supplemental feed stream for the steam hydrocarbon
reformer
unit/water gas shift unit, this stream (8) may also be used as a supplemental
feed stream
for a POx unit, an ATR unit, a coal gasification unit or refinery process unit
or other unit
which produces a feed gas stream (15). As in the previous embodiment, it may
be
advantageous to further heat the first hydrogen rich permeate fraction (8.1)
prior to this
stream being added as a supplemental feed to the stream of line (16). This
combined
fraction (8.1) and stream (16) are then fed into the feed gas producing unit
(31). While
this heating may be carried out in any manner known in the art, preferably the
first
hydrogen rich permeate fraction (8.1) is heated utilizing a heat exchanger
(30)
specifically for this permeate fraction (8.1). In addition to heating this
first hydrogen rich
permeate fraction (8.1) before adding the fraction to the stream (16), steam
can be
injected into this fraction (8.1) via line (29) just prior to the fraction
(8.1) being mixed
with the stream (16). The heating of this first hydrogen rich permeate
fraction (8.1)
further improves the efficiency of the recycle.
With regard to the additional streams, while the hydrogen product stream (23)
is
recovered as product, as in the previous embodiments, a portion of this stream
(23) can
be used for hydrogen fueling of the feed gas producing unit (31). In addition,
as with the
37
CA 02777324 2012-05-17
Serie 8915
previous embodiments, the carbon dioxide enriched permeate stream (11) may be
recycled to the process stream (1) from the process unit (0) where is it added
to the
process stream (1) prior to the compressor (2) or within the compressor (2)
between two
of the stages of compression or optionally compressing the carbon dioxide
enriched
permeate stream (11) and recycling the optionally compressed carbon dioxide
enriched
permeate stream (11) to be used as a supplemental feed stream in other
processes. The
carbon dioxide enriched permeate stream (11) may also be recycled directly
back to the
carbon dioxide separation unit (4) for further processing.
Note that the use of hydrogen selective membrane and carbon dioxide selective
membrane is in order to increase the recovery of hydrogen and carbon dioxide.
This can
boost hydrogen production and reduce carbon dioxide emissions for the existing
plants. It
can also reduce the size of reformer, natural gas consumption for the same
size new
plants with reduced carbon dioxide emissions.
LIST OF ELEMENTS
0 process unit
1 process stream
2 first compressor
3 heat exchanger
4 carbon dioxide separation unit
carbon dioxide lean non-condensable stream
6 carbon dioxide rich liquid stream
7 hydrogen selective membrane separation unit
7.1 permeate side of hydrogen selective membrane
7.2 residue side of hydrogen selective membrane
8 hydrogen rich permeate stream
8.1 first hydrogen rich permeate fraction
8.2 second hydrogen rich permeate fraction
9 hydrogen lean residue stream
carbon dioxide selective membrane separation unit
38
CA 02777324 2012-05-17
Serie 8915
10.1 permeate side of carbon dioxide selective membrane
10.2 residue side of carbon dioxide selective membrane
l 1 carbon dioxide enrich permeate stream
12 carbon dioxide depleted residue stream
13 second compressor
14 liquefaction unit
14.1 first liquefaction unit
14.2 second liquefaction unit
15 feed gas
16 hydrocarbon containing feed stream
17 pre-reformer
18 pre-reformed gas stream
19 steam methane reformer unit
20 syngas stream
21 water gas shift reactor
22 turbo expander
23 hydrogen product stream from pressure swing adsorption unit
24 distillation column
25 additional compressor
26 additional heat exchanger
27 cooling element for additional compressor
28 optional heat exchanger
29 line for injecting steam into the first hydrogen rich permeate fraction
30 heat exchanger for recycle
31 feed gas producing unit
32 optional second water gas shift reactor
33 optional third water gas shift reactor
39