Note: Descriptions are shown in the official language in which they were submitted.
CA 02778865 2012-05-25
1
Alloys of the Type Fe3AlTa(Ru) And Use Thereof as Electrode Material
for the Synthesis of Sodium Chlorate
FIELD OF INVENTION
The present invention relates to new catalytic alloys based on Fe, Al, Ta and
catalytic
species such as Ru.
The present invention also relates to the use of such catalytic alloys as
electrode material for
the synthesis of sodium chlorate.
TECHNOLOGICAL BACKGROUND
Nanocrystalline alloys of the formula
1-xMyTz
wherein
M represents at least one catalytic specie selected from the group consisting
of Ru, Jr. Pd, Pt,
Rh, Os, Re and Ag;
T represents at least one element selected from the group consisting of Mo,
Co, Cr, V, Cu,
Zn, Nb, W, Zr, Y, Mn, Cd, Si, B, C, 0, N, P, F. S, Cl and Na;
x is a number higher than -1 and smaller than or equal to +1
y is a number higher than 0 and smaller than or equal to +1
z is a number ranging between 0 and +1
have been disclosed recently as efficient cathodic materials for the synthesis
of sodium
chlorate (see CA 2,687,129 and the corresponding international application WO
2008/138148). These catalytic materials, when use as cathode for the
eleetrosynthesis of
sodium chlorate show very low cathodic overpotentials and they do not absorb
hydrogen
when the H2 evolution reaction takes place on their surfaces. These materials
also show good
CA 02778865 2012-05-25
2
corrosion resistance in the sodium chlorate electrolyte under typical
industrial operating
conditions (NaC103: 550g/1; NaCI: 110 g/1; NaCr207: 3g/I; NaCIO: 1 g/I; pH=
6.5 and
temperatures around 70 C).
Although corrosion resistance of these Fe3A11+,MTz alloys is quite good at pH
6.5, it is not
the case in acidic conditions. Standard industrial practises use acid wash
from time to time to
clean electrochemical cells and electrodes. To do so, MCI solutions at
concentrations varying
between 3% (¨ 1M) and 9% (¨ 3M) are often used. When the above mentioned
alloys are
put in contact with such concentrated acidic solutions, they can be severely
damaged.
Indeed, the corrosion resistance of these new catalytic alloys in HC1
solutions at low pH is
not so good.
SUMMARY OF THE INVENTION
To solve this problem, the inventors of record have search new formulations
and have
discover surprisingly that the addition of a small amount of Ta to these
materials could make
these new alloys not only highly resistant to corrosion in chlorate
electrolyte but also in
acidic (HC1) solutions without loosing any performance regarding the
electrochemical
synthesis of sodium chlorate.
Fe3A1(Ru) alloys are often single phase solid solutions usually prepared in a
nanocrystalline
form by mecanosynthesis. A powder mixture of ruthenium and iron aluminide is
milled
intensively for several hours until the Ru catalytic element enters and gets
highly dispersed
into the cubic crystalline structure of iron aluminide (Fe3A1). The
nanocrystalline Fe3A1(Ru)
alloy thus formed is highly active thanks to its high surface area and highly
dispersed
electrocatalytic element.
The present inventors have actually found by investigating various ternary
phase diagrams
that Ta (tantalum) which is known to be a good corrosion resistant element, is
quite soluble
CA 02778865 2012-05-25
3
in Fe-Al alloys. Therefore, Fe3A1(Ru)Tat with various Ta concentration "t" can
be prepared
as a single phase material by mechanosynthesis very easily and these new
alloys show not
only good electrocatalytic activity towards the electrosynthesis of sodium
chlorate but also
good corrosion resistance in the sodium chlorate electrolyte as well as in
concentrated HC1
solutions.
Therefore, the first object of the present invention is an alloy characterized
by the following
formula:
Fe3_,A11+,MyT,Tat
wherein:
M represents at least one catalytic specie selected from the group consisting
of Ru, 1r, Pd, Pt,
Rh, Os, Re and Ag;
T represents at least one element selected from the group consisting of Mo,
Co, Cr, V, Cu.
Zn, Nb, W, Zr, Y, Mn, Cd, Si, B, C, 0, N, P, F, S, Cl, Na and Ti;
x is a number higher than -1 and smaller than or equal to +1
y is a number higher than 0 and smaller than or equal to +1
z is a number ranging between 0 and +I
and t is a number higher than 0 and smaller than or equal to +1, preferably
lower than 0.4
and more preferably lower than or equal to 0.2
The alloy of the invention is preferably in a nanocrystalline state. If
nanocrystalline, the
crystallites are smaller than 100nm. The alloy is also preferably a single
phase material with
a cubic crystallographic structure but can also be multiphase depending on the
x, y, z and t
composition. Most of the time, these alloys are metastable. In other words,
they decompose
or transform into a different state when heated at high temperatures. But
again, they can also
be thermodynamically stable depending on the x, y, z and t composition.
A second object of the present invention is the use of such alloys as
electrode material for
the synthesis of sodium chlorate. In order to prepare an electrode of these
alloys, several
CA 02778865 2012-05-25
4
methods can be used. A preferred one is thermal spray such as the high
velocity oxyfuel
(HVOF) technique using the alloy in powder form as feedstock for the spray
gun. If the
method of preparation involves a rapid quenching process, the alloy can be
prepared in a
nanocrystalline state.
Even though, the preferred application of these new materials is sodium
chlorate, several
other electrochemical processes can take advantage of these alloys such as
industrial and
swimming pool water treatment.
Moreover, since the corrosion resistance of these new alloys is very good in
various
conditions, a third object of the present invention is the use of these alloys
as coating for the
protection against corrosion. If the targeted application is a coating for
protection against
corrosion. there may be no advantage of adding a large amount of expensive
catalytic
element to the alloy. In these cases, the molar content "y" can be chosen
small to reduce
costs. Moreover, it may be advantageous to add some Ti (titanium) to the alloy
since Ti is
also known for its good corrosion resistance and the inventors of the present
invention found
that Ti like Ta is quite soluble in iron-aluminium alloys.
The invention and its associated advantages will be better understood upon
reading the
following more detailed but not limitative description of preferred modes of
achievement of
it, made with reference to the enclosed drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
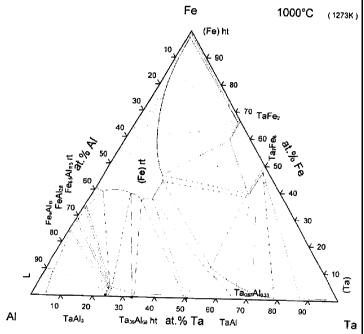
Fig. 1 shows an equilibrium ternary phase diagram of the Fe, Al and Ta at 1000
C.
Fig. 2 shows pictures of a corrosion test in 5% HC1 solution for a sample
containing Ta
according to the invention (right end side) and a similar sample not
containing Ta (left end
side).
CA 02778865 2012-05-25
Fig. 3 represents the hydrogen released as a function of time during corrosion
tests in a 5%
HC1 solution for samples according to the invention containing Ta with
composition t of 0.1,
0.2, 0.3 and 0.4 and a similar sample not containing Ta.
Fig. 4 represents a figure similar to Fig. 3 where in addition to the
previously presented
results, the hydrogen released as a function of time is shown for a sample of
the invention
Fe3_,A.11+,MyTzTat containing both Nb (element T) and Ta with a respective
molar content of
z = 0.1 and t = 0.2.
Fig. 5 shows X-ray diffraction spectra of a mixture of a powder of the prior
art Fe3_
,A11,MyT, and a powder of Ta at an equivalent molar content of t = 0.2 as a
function of
milling time during a mecanosynthesis process.
Fig. 6 shows a picture of a ball milled powder of the invention containing Nb
and Ta at a
respective molar content of z = 0.1 and t = 0.2.
Fig. 7 a) and b) show pictures of a coating according to the invention made
from the powder
of Fig. 6 using a thermal spray technique at two different magnifications 120
and 5000x.
Fig. 8 show samples of coated electrodes after lhour immersion in 5% HC1
solution. The left
end side is an electrode of the prior art while the right end side is an
electrode according to
the present invention containing Ta.
Fig. 9 shows cyclic voltametric curves (current ¨ voltage curves) in a
chlorate solution taken
at a rate of 5mV/sec at 20 C and pH=6.5 for a sample of the invention and a
standard
stainless steel 316 sample.
Fig. 10 shows an equilibrium ternary phase diagram of the Fe, Al and Ti at
1200 C.
CA 02778865 2012-05-25
6
Fig. 11 shows an electrochemical test in a standard chlorate solution using an
electrode
according to the invention containing Ta at a molar content oft = 0.1.
DETAILED DECRIPTION OF THE INVENTION
As it can be seen, Fig. 1 shows a ternary phase diagram of Fe, Al and Ta at
1000 C. Ta is
quite soluble in FexAli_x alloys especially near the equiatomic composition (x
= 0,5). By
inserting Ta into the cubic FeAl alloy at high temperature, a single phase
FeAlTat material
can be prepared at room temperature using a rapid quenching process. If the Ta
content is
high, the single phase obtain at room temperature will most likely be
metastable.
Fig. 2 represents a corrosion test of an alloy containing Ta according to the
invention in
comparison with a similar alloy not containing Ta. The samples are immerged in
a 5% HC1
solution. On the left end part of the picture, we see for the alloy not
containing Ta, a lot of
hydrogen bubbles indicating severe corrosion in the acidic solution. On the
contrary, for the
alloy containing Ta in the picture on the right end side, very little bubble
formation is
observed indicating a much better corrosion resistance in the HC1 solution.
Fig. 3 represents corrosion tests similar to the ones of Fig. 2 showing the
amount of
hydrogen released during the test as a function of time. The sample of the
prior art not
containing Ta, Fe3Ali+,M,T, releases 11m1 of hydrogen in about 7,5min while
the sample
of the present invention Fe3_xA11+\M)TzTa0,2 containing Ta at a molar content
of y= 0.2
releases only 3.9 ml in 340min. Fig. 3 also shows that, even with a small
concentration of Ta
of t = 0.1, significant improvement in the corrosion resistance of the alloy
can be achieved.
This very large improvement in the corrosion resistance of the alloy of the
prior art with very
small additions of Ta was unexpected.
Fig. 4 represents corrosion tests similar to the ones of Fig. 3 where, in
addition to the curves
presented in Fig. 3, the results of a sample containing both Nb and Ta are
presented. When
CA 02778865 2012-05-25
7
Ta is added at a composition t = 0.2 to a sample of the prior art already
containing Nb
(Fe3_,A11+,M),T, where T is Nb at a molar content of z = 0.1) a synergetic
effect takes place
and a huge improvement in the corrosion resistance is achieved. The sample
Fe3_,A11,,M),Nbo iTa0,2 released only 1,5m1 of hydrogen in 500min. This
synergetic effect
when both Nb and Ta are present in the alloy and which gives incredible
improvement in the
corrosion resistance of the alloy in HCI solution was also unexpected.
Fig. 5 shows X-ray diffraction spectra of a mixture of an alloy powder of the
prior art with a
powder of Ta at a molar content of t = 0.2 as a function of the milling time
during a
mecanosynthesis process. We see the characteristic X-ray peak of the iron
aluminide powder
(Fe3A1) cubic structure around 44 and a peak corresponding to Ta at about
38.4 . By
increasing the milling time from 1 h to 12h, we observe that the intensity of
the Ta peak
decreases and vanishes after about 12h of milling. This indicates that all of
the Ta has
penetrated into the crystalline structure of iron aluminide to form a
metastable solid solution.
Fig. 6 shows a scanning electron micrograph taken at a magnification of 2000x
of a ball
milled powder of the invention Fe3.,A11+,MyTzTat which comprises both Nb and
Ta at a
molar content of Nbo 1Ta0,2 (T is Nb, z = 0.1 and t = 0.2). The average
particle size of this
nanocrystalline powder is around 10 microns.
Fig. 7a) and b) represent scanning electron micrographs at 120x and 5000x
magnification
respectively of the surface of a coating according to the invention made by
FIVOF thermal
spray using the powder shown in Fig. 6. Thus, the material of the coating
contains both Nb
and Ta elements. This electrocatalytic coating is not only corrosion resistant
in the chlorate
electrolytic but also in hydrochloric acid solutions.
Fig. 8 represents images of electrodes after immersion in a 5% HC1 solution
for one hour.
The left side is a picture of an electrode of the prior art (Fe3,Ali,õMyTz)
while the right side
shows a picture of an electrode of the present invention (Fe3_,,Ali+xMyTzTat).
The electrode
CA 02778865 2012-05-25
8
of the prior art has been destroyed by the acid wash treatment. The catalytic
coating has
peeled off from the substrate. On the contrary. the electrode of the present
invention shown
on the right end side is intact and shows no damage.
Fig. 9 shows current versus voltage curves taken at a scan rate of 5mV/sec in
a highly
corrosive environment (chlorate solution at 20 C and pH=6.5) for a coating of
the invention
containing both Ta and Nb and a standard stainless steel 316 sample. The
breakdown
potentials on the anodic side (positive voltages) are almost the same for the
two samples
indicating that under these conditions, the coating material of the invention
is a corrosion
resistant material as good as stainless steel 316.
Fig. 10 represents a ternary phase diagram of Fe, Al and Ti at 1200 C. One can
see that, on
the Fe rich side of the Fe-Al system, Ti is quite soluble in the alloys.
Therefore, for
applications as corrosion resistant coatings, it may be advantageous of adding
not only Ta
but also Ti to the alloys since Ti is known to be a good corrosion resistant
element especially
in chlorine environment. However, the addition of Ti is not recommended in
applications as
electrode for the hydrogen evolution reaction since Ti is known to form stable
hydrides as
discussed in CA 2,687,129 mentioned hereinabove.
Fig. 11 shows an electrochemical test conducted in a standard chlorate
solution using a DSA
as anode and an electrode material of the invention as cathode. The material
according to the
invention contains Ta at a molar content of t = 0.1. The anodic and cathodic
voltages are
measured with respect to a Ag/AgC1 reference electrode. 1.3 volt has been
substracted from
the Anode-Cathode voltage difference in order to show the three traces on the
same figure.
Open circuit (OC) events for durations of 30sec. lmin and 2min have been
conducted during
the test. Has it can be seen, the voltage of the cell remains stable in spite
of these events.