Note: Descriptions are shown in the official language in which they were submitted.
(
CA 02780895 2014-07-10
- I -
FLUID FILTRATION MEDIUM
Technical Field
The present application relates to improved filtration of fluids.
Description of the Prior Art
Water filtration processes have been developed over the history of mankind to
enhance clarity and to remove impurities from mediums, such as recreation and
potable
water. For example, water transported by aqueducts built by Roman engineers
for the
purposes of filling city fountains for drinking and bathing were passed
through beds of
sand to clarify the water.
Brief Description of the Drawings
The novel features believed characteristic of the application are set forth in
the
appended claims, However, the application itself, as well as a preferred mode
of use,
and further objectives and advantages thereof, will best be understood with
reference to
the following detailed description when read in conjunction with the
accompanying
drawings, wherein:
Figure 1 is a flowchart of an example fluid filtration process according to
the
present disclosure.
While the filtration process of the present application is susceptible to
various
modifications and alternative forms, specific embodiments thereof have been
shown by
way of example in the drawings and are herein described in detail. It should
be
understood, however, that the description herein of specific embodiments is
not
intended to limit the invention to the particular embodiment disclosed.
CA 02780895 2014-07-10
- 2 -
The scope of the claims should not be limited by the preferred embodiments
set forth in the examples, but should be given the broadest interpretation
consistent
with the description as a whole.
Description of the Preferred Embodiment
The present disclosure describes modified, natural surfactant-treated zeolite
products (interchangeably referred to as "zeolite products"); processes for
producing the
zeolite products, including the bonding of cation surfactants to zeolite;
methods for
activating the zeolite products; and use of the zeolite products for enhanced
turbidity
removal from fluids. In some instances, one or more of the zeolite products
may be in a
granular form. An advantage of the zeolite products is that the zeolite
products provide
for improved fluid filtration, For example, in some instances, an example
zeolite product
may be used in the turbidity reduction of water.
The present disclosure encompasses turbidity-removing zeolites along with
methods of use and production thereof. One aspect encompasses a turbidity-
removing
zeolite product including a granulized zeolite and a quantity of cationic
surfactant to
cover from 20 percent to 100 percent of External Cation Exchange ("ECEC")
sites of the
granulized zeolite.
Another aspect encompasses a method of forming a treated granulized zeolite
product that can include applying a cationic surfactant to a granulized
zeolite material
such that an amount applied to the granulized zeolite material covers at least
20 percent
of the ECEC sites of the granulized zeolite material.
A further aspect encompasses a method for removing turbid particles from a
fluid
that can include installing a quantity of surfactant-treated zeolite in a
filter vessel,
activating the surfactant-treated zeolite, passing a volume of fluid
containing turbid
particles through the activated surfactant-treated zeolite, and removing an
amount of
the turbid particles from the fluid with the activated, surfactant-treated
zeolite.
CA 02780895 2014-07-10
- 2a -
In accordance with one aspect of the present invention, there is provided a
turbidity-removing zeolite product, comprising, a granulized zeolite, having:
a zeolite that
includes a silica-to-alumina ratio equal to or greater than about 2.5; and a
quantity of
cationic surfactant to cover from about 20 percent to about 100 percent of
External
Cation Exchange- ("ECEC") sites of the granulized zeolite.
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 3 -
The various aspects can include one or more of the following features. The
granulized zeolite can include a zeolite having a silica-to-alumina ratio
equal to or
greater than 2.5. The granulized zeolite can include at least one of
clinoptilolite,
mordenite, phillipsite, erionite, chabazite, or faujasite. The granulized
zeolite can
include at least one of feldspar, mica, or polymorphs of silica. The
granulized zeolite
can include a cation exchange capacity equal to or greater than 0,5
milliequivalents per
gram. The granulized zeolite can include a BET surface area greater than 10
m2/g.
The granulized zeolite can include a d10 in the range of 0.3 mm to 0.7 mm and
a d60 in
the range of 0.6 mm to 1.5 mm. The granulized zeolite can include a Uniformity
Coefficient equal to or less than 2.2. The granulized zeolite can include a
dry, bulk
density in the range of 44 to 56 lbs./ft,2, The turbidity-removing zeolite
product can
include a total un-bound moisture content in the range of 12 to 18 percent,
The
turbidity-removing zeolite product is free-flowing. The cationic surfactant
can include at
least one of polyamines, quaternary amines, alkylamines, or organo-silane
quats.
The various aspects can also include one or more of the following features. A
zeolite product may be formed from of a granulized zeolite material. A zeolite
material
can be crushed, and the crushed zeolite material can be sieved with series of
sieves,
the series of sieves including sieves having sieve sizes ranging from a minus
12 mesh
(1.7 mm) to a plus 50 mesh (0.3 mm). The crushed zeolite material can have a
d10 in
the range of 0.3 mm to 0.7 mm and a d60 in the range of 0.6 mm to 1.5 mm. The
granulized zeolite material can include an un-bound moisture content of six
percent.
Applying a cationic surfactant to a granulized zeolite material such that an
amount
applied to the granulized zeolite material covers at least 20 percent of the
ECEC sites of
the granulized zeolite material can include applying an aqueous solution of
cationic
surfactant to the granulized zeolite.
The various aspects can also include one or more of the following features.
Activating the surfactant-treated zeolite can include saturating the
surfactant-treated
zeolite in water to cause surfactant contained within the surfactant-treated
zeolite to
SUBSTITUTE SHEET (RULE 261)
= CA 02780895 2014-07-10
- 4 -
bond to ECEC sites of the zeolite, backwashing the surfactant-treated zeolite,
and
rinsing the surfactant-treated zeolite. The surfactant-treated zeolite can
include a
granulized zeolite formed from at least one of a clinoptilolite, mordenite,
phillipsite,
erionite, chabazite, or faujasite combined with an amount of cationic
surfactant to cover
from 20 percent to 100 percent of ECEC sites of the granulized zeolite. The
cationic
surfactant can include at least one of polyamines, quaternary amines,
alkylamines, or
organo-silane quats. Removing an amount of the turbid particles from the fluid
with the
activated surfactant-treated zeolite satisfies criteria defined ANSI/NSF
Standard 50 ¨
2009a, Annex B.4.
In the present disclosure, aspects of the zeolite products are discussed with
respect to the filtration of water.
Thus, the examples discussed below are provided merely
as examples and are not meant to limit the applicability of the zeolite
products to such
examples in any way.
In one example implementation, a natural zeolite utilized for producing one or
more of the zeolite products may have a silica-to-alumina ratio equal or
greater than
2.5. Examples of some of the zeolite minerals in this group may include
clinoptilolite,
mordenite, phillipsite, erionite, chabazite, and faujasite. However, many
other natural
zeolites could be used. In some instances, the zeolite mineral ore may contain
greater
than 50 percent zeolite. In some instances, gangue minerals of clay,
evaporates, and
calcium carbonate may be avoided as such materials may be detrimental to the
performance of the zeolite product.
In other instances, the zeolite used in the production of the zeolite products
may
include feldspar, mica, and polymorphs of silica, Such materials may take up
space in
the zeolite ore but may not be detrimental to the resulting zeolite product.
In some
implementations, the natural zeolite may have a total cation exchange capacity
equal to
or greater than 0.5 milliequivalents per gram and a BET surface area (i.e., a
surface
area determined using the Brunauer, Emmett and Teller method) of greater than
10
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 5 -
square meters per gram (m2/g). In some implementations, zeolite ore having
such
properties may be crushed, screened, and, in some instances, dried to an un-
bound
moisture content of less than 10 percent The crushed zeolite ore may also be
subjected to a screen analysis of approximately minus 12 mesh (1.7 mm) to plus
50
mesh (0.3 mm).
Un-bound moisture refers to water associated with zeolite that may be
liberated
by raising the temperature of zeolite to not more than 212 C. In some
implementations,
the crushed or granulated zeolite may have a d10 (i.e., a tenth percentile
distribution in
particle size of the granular zeolite material) in the range of 0.3 mm to 0.7
mm and a d60
(i.e., a 60th percentile distribution in particle size of the granular zeolite
material) in the
range of 0.6 mm to 1.5 mm. In some instances, the Uniformity Coefficient of
the
granular zeolite material, a ratio of dlo to d60, may be less than 2.2. In
still other
instances, the bulk density of the dry, granular zeolite material may be in
the range of
44 to 56 pounds per cubic foot (lbs./ft.2).
As explained above, implementations of the zeolite products of the present
disclosure may include one or more of the material properties described herein
while
one or more other properties may vary from those values and/or ranges
described.
However, the scope of the present disclosure is intended to encompass zeolite
products
having variations of the property values and/or ranges from those described
herein.
Thus, the property values and/or ranges described herein are provided merely
as
examples and are not intended to limit the scope of the present disclosure.
In some instances, a zeolite product according to the present disclosure may
provide backwash performance, for example backwash up to 20 gpm per square
foot,
as well as head pressure loss performance, for example head pressure loss of
less than
12 psi, that is comparable to media used in most pressure filtration vessels
designed
and standardized for the pool and spa industry. Additionally, in some
instances, a
zeolite product according to the present disclosure may also provide suitable
flow rates,
bed cleaning, and head pressure for gravity filtration systems utilized in
pool, industrial,
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 6 -
and municipal applications. In still other instances, example zeolite products
may be
used in gravity-flow filtration systems favored for use in some potable water,
wastewater, pre-treatment streams, irrigation, industrial, or a myriad of
other
applications.
The surface of zeolite granules utilized for the production of the zeolite
products
may be modified with a chemical of the cationic surfactant group (referred to
interchangeably as "surfactant").
Example surfactants may include polyamines,
quaternary amines, alkylamines, or organo-silane quats.
The surfactant bonds
chemically with the zeolite forming a surfactant-modified zeolite granule
(interchangeably referred to as "surfactant-treated zeolite") that contains
negative ionic
charges within the internal crystalline surfaces of the zeolite granule and a
positive ionic
charge at locations where the surfactant has bonded to the external
crystalline surface
of the zeolite granule. Consequently, the modified zeolite provides a
crystalline entity
with an active electrochemical surface that is amphoteric.
It has been shown that the cationic surfactant bonds to particular locations
of the
zeolite crystalline structure called the External Cation Exchange sites
(referred to
hereinafter as "ECEC" or "ECEC sites"). The surfactants include a carbon chain
that is
too large to enter the three to 10 angstrom molecular pore spaces within the
various
zeolite crystalline structures. The result is a surface-modified zeolite
(referred to
interchangeably as "modified zeolite") with the positive charge moity of the
cationic
surfactant bonded by cation exchange to the crystalline surface. At the same
time, the
zeolite retains a natural negative charge at Internal Cation Exchange sites
(referred to
hereinafter as "ICEC" or "ICEC sites"). The negative charge is available for
exchange
with metal cations that fit within the zeolite's crystalline lattice.
The surfactant, for example, in the form of an aqueous solution, may be
applied
to the granulated zeolite to form a zeolite product. An amount of aqueous
solution may
be applied to the granulated zeolite so as to introduce enough surfactant to
cover 20 to
100 percent of the ECEC sites of the zeolite. In some instances, the
surfactant may be
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 7 -
sprayed or pumped onto the zeolite granules in a mixer to wet the surface of
the
granules without saturating the granules. Application of an amount of
surfactant in
excess of an amount to cover 100 percent of the ECEC sites may create a double
surfactant layer or possibly a micelle layer that is detrimental to turbidity
reduction.
In the context of fluid filtration, a modified zeolite in which 20 percent of
the
ECEC sites is bonded with surfactant may perform as well or substantially as
well as a
modified zeolite in which 50 to 100 percent of the ECEC sites is bonded with
surfactant.
Further, cationic surfactants in which the molecular weight varies from 120 up
to over
500 may perform similarly with respect to turbidity reduction, independent of
whether
the carbon chain of the surfactant is C8 up to or exceeding C16.
According to some implementations, granulated zeolite having six percent un-
bound moisture may be metered into a continuous-flow paddle mixer. A
surfactant
solution may be pumped onto the granulated zeolite to result in an eight
percent added
moisture. In some instances, the aqueous surfactant solution may contain three
and a
half percent active ingredient diluted in 96.5 percent water. In still further
instances, the
granulated zeolite product may exit the paddle mixer with an amount of
surfactant to
satisfy 50 percent of the ECEC sites. As a result, the outputted zeolite
product may
contain a total un-bound water of 14 percent.
Because zeolite granules are micro-porous and hydrophilic, many, if not most,
natural zeolite mineral ores can hold up to, and in some cases exceed, 18
percent un-
bound moisture while remaining free-flowing. Consequently, granulated zeolite
to which
enough surfactant has been added to satisfy up to 100 percent of the ECEC
sites may
remain a volume of free-flowing granules that may be processed accordingly. As
such,
the treated zeolite granules described herein may be processed and transported
in
manners similar to other dry bulk materials. Further, because the surfactant-
treated
zeolite retains a free-flowing condition, additional processing, such as
processing to dry
out the zeolite material is not required, leading to processing time and cost
savings.
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 8 -
Decreasing the un-bound moisture content in the surfactant-treated zeolite may
cause the zeolite granules to become difficult to wet when introduced into a
filtration
vessel. For example, the surfactant on a dry zeolite granule forms a
hydrophobic
surface that is difficult to wet when placed into a filtration vessel. As
such, according to
some implementations, moisture in the surfactant-treated zeolite granules may
not be
removed. In some instances, surfactant-treated zeolite granules having a
moisture
content of between 12 and 18 percent may be easily wetted when introduced into
a
filtration vessel.
As explained above, the granulated zeolite is free-flowing and, thus, may be
handled in ways similar to other bulk materials. As such, once the surfactant
is applied
(as well as at other times during the processing of the zeolite), the
granulated zeolite
may be handled by standard package equipment for dry, free-flow products and
stored
in bins, bagged, or otherwise packaged. In some instances, the surfactant-
treated
zeolite granules may be packaged in paper or poly film. In this manner, the
surfactant-
treated zeolite granules are highly stable and may enjoy a shelf life in
excess of several
years. Activation for complete ion-exchange of the cationic surfactant onto
the zeolite
granules may be completed at a later stage.
Activation may be completed when the pre-sorbed zeolite granules, i.e.,
surfactant-treated zeolite granules in which the surfactant has not been
bonded to the
zeolite particles, are placed into a vessel and saturated with water. For
example, the
pre-sorbed zeolite granules may be activated by introducing the zeolite
granules into a
filter vessel for use in a desired application and saturating the zeolite
granules with
water. Saturation of the zeolite granules causes the cationic surfactant to re-
solubilize
and come in contact with the crystalline surfaces of the zeolite in the
aqueous phase.
Consequently, the ion exchange between the zeolite and the surfactant occurs.
In excess of 80 percent of the chemical bonding of the surfactant to the
zeolite is
typically complete within 20 minutes at ambient temperature. Once activation
is
complete or substantially complete, the modified zeolite may be used as a
filtration
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 9 -
medium. In some instances, activation may be allowed to proceed for 24 hours
before
use, while, in other instances, activation may be allowed to proceed for 24
hours prior to
use of the filtration medium.
In some instances, "backwash" procedures and "rinse to waste" cycles may be
performed once the media has been activated or substantially activated and
prior to
using the activated zeolite filtration medium in filtration mode. However, in
other
instances, backwashing and/or "rinse to waste" cycling may not be required or
desired
prior to utilizing the activated zeolite filtration medium in filtration mode.
The
backwashing and "rinse to waste" cycles may be utilized to wash out any
excess, un-
bonded surfactant as well as detritus that would interfere with the filtration
process. In
some implementations, a backwash may include at least three bed volumes of
flush
water, while a "rinse to waste" cycle may include at least one bed volume of
water.
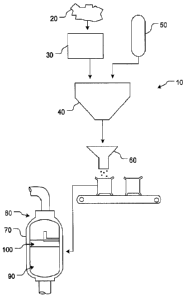
Figure 1 shows a flowchart of an example production process 10 for a
surfactant-
treated zeolite. At 20, a zeolite material, such as one or more of the zeolite
materials
described above, may be extracted from the earth. At 30, the extracted zeolite
material
may be subjected to one or more operations or processes granulize the zeolite
material.
For example, the zeolite material may be subjected to crushing and/or
screening. As
explained above, in some instances, the zeolite may be crushed and subjected
to a
minus 12 mesh (1.7 mm) to plus 50 mesh (0.3 mm) screening analysis. In other
implementations, a screening analysis may encompass a broader or narrower
range.
For example, an application of the granular zeolite may define one or more of
the
crushing or screening operations to which the zeolite material is subjected.
Further, in
some implementations, a granular zeolite having a d10 within the range of 0.3
mm to 0.7
mm and a d60 within the range of 0.6 mm to 1.5 mm.
In some instances, the zeolite material may be subjected to one or more drying
operations. For example, a desired un-bound moisture content of six to ten
percent
water may be desired. The initial moisture content of the zeolite material may
determine whether or not one or more drying operations are required.
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 10 -
At 40, the granular zeolite material may be introduced into a mixing apparatus
along with an amount of surfactant, such as one or more of the surfactants
described
above. Dispensation of one or more of the granular zeolite or surfactant may
be
metered. For example, one or more of the granular zeolite or surfactant may be
metered such that a surfactant-treated granular zeolite material in which 20
to 100
percent of the zeolite's ECEC sites are bonded with the surfactant results. At
50, the
surfactant may be formed into an aqueous solution of a desired concentration.
For
example, in some instances, the aqueous solution may be 3.5 percent surfactant
and
96.5 percent water. However, such solution is merely an example. Thus, other
surfactant solution concentrations may be used.
In some instances, the surfactant and the granular zeolite may be combined
such
that the un-bound moisture content does not exceed 18 percent. In other
instances, the
un-bound moisture content may be lower or higher than 18 percent. In some
instances,
a fluid, in addition to the surfactant solution, may be introduced into the
mix to control
the un-bound moisture content, while, in other implementations, additional
fluids may
not be used. Further, controlling the un-bound moisture content so as not to
exceed 18
percent allows the treated zeolite granules to remain free-flowing,
facilitating the ease of
subsequent processing of the material.
The surfactant-treated zeolite material may be transferred to a packaging
process at 60. The treated zeolite material may be packaged in any desired
manner.
For example, in some instances, the treated zeolite may be packaged into paper
or poly
film containers. The containers may be of any desired size. In other
instances,
packaging may be avoided and the material may be distributed, for example, in
bulk via
rail cars, trucks, etc.
At 70, the surfactant-treated zeolite material may be utilized for filtration
of fluids.
For example, the treated zeolite material may be used in the filtration of
water sources.
Particularly, the treated zeolite may be used to remove turbid particles from
a water
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 11 -
source. In some instances, feature 70 may represent a filtration vessel 80
having a bed
of the surfactant-treated zeolite 90 with a water layer 100 above the zeolite
bed 90.
Further, in some instances, the treated zeolite material may be activated at
70.
For example, during activation, the surfactant-treated zeolite may be soaked
in an
excess of water (e,g., saturated) in the filtration vessel 80 for a desired
period of time.
In some implementations, the treated zeolite material may be soaked for 20
minutes at
ambient temperature to complete activation, In other examples, activation may
be
permitted to occur for longer or shorter periods. For example, the treated
zeolite may
be allowed to soak in excess water for 24 hours or more prior to use. In other
examples, the treated zeolite material may be soaked for between 20 minutes
and 24
hours prior to use.
The activated surfactant-treated zeolite may be backwashed prior to use.
Backwashing may be performed, for example, to remove excess surfactant and/or
detritus. One or more cycles of "rinse to waste" may also be performed prior
to use of
the surfactant-treated zeolite in filtering a fluid source.
While the above description provides examples with regard to the processing
and/or production of the surfactant-treated zeolite, such are only examples
and are not
meant to limit or otherwise define the scope of the present disclosure.
Consequently,
production and/or use of the surfactant-treated zeolite may vary from those
provided
herein while remaining within the scope of the present disclosure.
Several examples involving the use of the modified granular zeolite material
are
described below.
Example No. 1
In Example No. 1, several filtration media were tested in a 12 inch-long
polycarbonate column having a 2.5-inch inner diameter ("ID") (hereinafter
referred to as
"column"). The column was prepared with 0-ring-fitted stoppers on both ends. A
pump
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 12 -
having a ball valve control was plumbed to pull water at various flow rates
from a 20 liter
reservoir with down-flow, through the column, and back to the reservoir. The
filtration
media were pre-washed or prepared and placed in the column such that the
filtration
medium formed a bed height of six inches, A 50 mesh stainless steel screen was
disposed at a lower end of the filtration medium. A perforated plastic disc
was placed
on an upper end of the filtration medium, and a three-inch head of water was
maintained above the upper end of the filtration medium to prevent scouring
from the
high-volume water flow entering the column.
Flow rate of the water was determined by measuring the rate at which the water
was discharged from the column, Flow rate was adjusted with an in-line ball
valve.
Each filtration medium was backwashed after being placed into the column with
about a
30 percent bed lift until clear water resulted. The bed was further cleaned by
a brief
down-flow rinse. For each filtration medium, the column was then capped and
fitted
with an inlet and outlet hose. Water flow rate was regulated between 15 and 20
gpm
per foot squared of media surface area (gpm/ft2).
The 20 liter reservoir was filled with tap water and adjusted to about 50 NTU
(Nephelometric Turbidity Unit) with U.S. Silica ground silica (Sil-co-sil #
106), produced
by U.S. Silica Company of Berkeley Springs, West Virginia, to make up the
challenge
water. A LaMotte Model 2020 turbidimeter, produced by LaMotte Company of 802
Washington Avenue, Chestertown, Maryland, was used for all of the turbidity
testing.
Circulating water in the clean bed was measured at less than 2 NTU in all
trials before
the challenge water was introduced to the columns. Operational procedures and
calculations follow NSF/ANSI Standard 50 ¨ 2009a, part B5.
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 13 -
Table 1. Experimental Results of Example 1.
Media Treatment ECEC Starting End NTU after
Percent
Coverage NTU Five (5)
Turbidity
Percent Turnovers
Reduction
Silica Sand None 60 25 58
Zeolite #1 None 50 13 74
Zeolite #2 None 54 24 55
Zeolite #3 None 53 30 43
Treated #1 HDTMA 100 49 1.1 98
Treated #2 HDTMA 50 52 2.1 96
Treated #3 DADMA 100 60 8.0 86
Treated #4 DADMA 100 50 4.0 92
With respect to Table 1, above, Silica Sand is filter sand obtained from
Leslie's
Swimming Pool Supply of 2740 Wyoming Blvd NE, Albuquerque, NM 87111. Zeolite
#1
is Clinoptilolite produced by St. Cloud Mining Co., of Winston Millsite
Winston, New
Mexico 87943. Zeolite #2 is Clinoptilolite produced by Zeotech Corporation of
115 West
7th Street, Suite 1400, Fort Worth, Texas 76102. Zeolite #3 is Clinoptilolite
produced by
Bear River Zeolite Co., of 4323 East Glendale Road, Preston, Idaho 83263.
Treated #1
is a surfactant-treated zeolite formed from Hexadecyltrimethyl ammonium
chloride
(interchangeably referred to as "HDTMA") on Zeolite #1 soaked for 3 hours.
Treated #2
is a surfactant-treated zeolite formed from Hexadecyltrimethyl ammonium
chloride on
Zeolite #1 soaked for 30 minutes. Treated #3 is a surfactant-treated zeolite
formed from
Dimethyldialyl ammonium chloride (interchangeably referred to as "DADMA") on
Zeolite
#1 soaked for 40minutes. Treated #4 is a surfactant-treated zeolite formed
from
Dimethyldialyl ammonium chloride on Zeolite #1 soaked for 8 minutes.
The turbidity reduction for Silica Sand and three untreated, commercially
available zeolites (Zeolites #1, #2, and #3, respectively) commonly used in
water
filtration systems varied from 43 to 74 percent removal after five turnovers.
It is noted
that a turnover is the time in which the water volume in the reservoir,
flowing at a given
flow rate, would have completely passed through the filtration medium bed.
Thus, five
turnovers is the amount of time, at a given flow rate, the amount of water in
the reservoir
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2014-07-10
=
- 14 -
would have passed through the filtration medium bed five times. Testing in
columns
and in commercial applications over the last several years suggest that the
removal
rates determined and listed in Table 1 are typical removal rates for these
products, i.e.,
the Silica Sand and Zeolites #1, #2, and #3.
On the other hand, the results of turbidity reduction for all of the
surfactant-
treated zeolites show a marked improvement. HDTMA-treated zeolites or DADMA-
treated zeolites had a turbidity reduction of 86 to 98 percent after five
turnovers.
Results also indicate that a DADMA-treated zeolite in which the DADMA was
allowed to
soak into the zeolite granules for eight minutes performed as well as a DADMA-
treated
zeolite in which the DADMA was allowed to soak into the zeolite granules for
40
minutes. Similarly, results indicated that HDTMA-treated zeolite in which the
HDTMA
was allowed to soak into the zeolite granules for 30 minutes performed as well
as a
HDTMA-treated zeolite in which the HDTMA was allowed to soak into the zeolite
granules for three hours.
Example No. 2
In Example No. 2, a series of four consecutive filtration runs and backwashes
were made with a surfactant-treated zeolite medium to determine attenuation of
turbidity
removal over time and use. Zeolite #1 was sorbed at a level of 25 percent of
the ECEC
with an organo-silane compound. Dry zeolite granules were sprayed with a 2.5
percent
solution of the organo-silane compound (interchangeably referred to as "silane
solution"). Particularly, the organo-silane compound used was Zycrobial
produced by
=
ZydeTIndustries of 25-A Gandhi Oil Mill Compound, Gorwa, Vadodara-390016,
Gujarat,
India. Six hundred cubic centimeters of filter media were sprayed with 60
milliliters of
the silane solution. The treated zeolite that resulted was free-flowing.
A twelve-inch column similar to the one described in Example No. 1, above, was
loaded with the surfactant-treated Zeolite # 1 and soaked with enough tap
water to
cover the media for 30 minutes. The column was then backwashed and rinsed.
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 15 -
Challenge water containing 60 NTU of Sil-co-sil 106 was flowed through the
column at a
rate of 20 gpm per foot squared of media surface area.
Turbidity measurements were taken at the end of each turnover on a 20 liter
reservoir for five turnovers and results were recorded. At the end of the five
turnovers,
the column was backwashed and rinsed prior to performing a subsequent
filtration test
with new challenge water. This procedure was repeated twice using Sil-co-sil
106 as
the water contaminant and twice using ISO 12103-1 A3 Medium Test Dust as the
water
contaminate. The results are shown below in Table 2.
Table 2. Experimental Results of Example 2.
Run Contaminant Initial NTU at NTU at
Percent
No. NTU First
Fifth Turnover Removal after
Turnover
Fifth Turnover
1 Sil-co-sil 106 60 5.0 1.0 98
_ 2 Sil-co-sil 106 55 4.5 0.3 99
3 A3 Medium Test 60 5.3 1,0 98
Dust
4 A3 Medium Test 95 22 2.6 97
Dust
The results demonstrate continued efficacy for turbidity reduction of the
surfactant-treated zeolite through four column runs of five turnovers, each
run being
followed by rigorous backwash. The results of Run No. 1 show that the
surfactant-
treated zeolite removed 98.3 percent of turbidity from a 60 NTU challenge
water after
five turnovers, while the results of Run No. 4 show that the surfactant-
treated zeolite
removed 97.3 percent of the turbidity from a 95 NTU challenge water on the
fifth
turnover. Based on the test results, it is believed that the organo-silane is
bonded to the
zeolite and remains effective after considerable "break-in" use.
Example No. 3
Example No. 3 addresses whether salinity content of a challenge water affects
the turbidity reduction properties of a surfactant-modified zeolite. Many
modern
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 16 -
swimming pools as well as some industrial applications require filtration of
saline or
brackish water. Saline swimming pools generally regulate salt content at 1,500
to 3,000
ppm sodium chloride ("NaCI"). A concern is that a high sodium ion
concentration in
contact with the surfactant-modified zeolite may degrade the zeolite by
exchange of
and/or displacement of the cationic surfactant bonded to the zeolite
crystalline surface
at the ECEC sites by the sodium ions.
In Example No. 3, a column was set up in a manner similar to that described
above in Example No. 1 with Zeolite #1 sorbed with DADMA so as to cover 20
percent
of the ECEC sites. A recirculating body of water was prepared containing 3,000
ppm of
NaCI. Clear recirculating water had a turbidity level of 0.4 NTU. Sil-co-sil
106 was
utilized to form a challenge water having turbidity level of 45 NTU. Results
of Example
No. 3 are shown in Table 3.
Table 3. Experimental Results of Example No. 3.
Turnover NTU Turbidity Removal
Percentage
1 21 53
2 5.5 88
3 2.8 94
4 1.8 96
5 Water Spilled
The results demonstrate that the surfactant-modified zeolite produces a
superior
turbidity reduction despite a high salinity content of the challenge water.
The
anomalous reading in turnover No. 1 may have been from a "bumping" of the
column
resulting in an upset. Data from turnover 5 was lost due to a spillage.
Nevertheless,
the data associated with turnovers 2 through 4 are consistent with having the
surfactant
fully functional and remaining on the zeolite media in spite of the high
sodium content of
the challenge water.
Example No. 4
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 17 -
Example No. 4 tested the cleanability of a surfactant-modified zeolite filter
medium using a test procedure defined in ANSI/NSF Standard 50 ¨ 2009a, Annex
B.4.
A 24-inch diameter, high-rate sand filter vessel was used. One hundred fifty
pounds of
Zeolite #2 was sorbed with enough DADMA to cover 50% of the ECEC sites. The
surfactant-modified zeolite and loaded into the filter vessel. After backwash
and rinse,
clean water was run through the filter, and an initial static head loss of 9.2
psi at a flow
of 20 gpm/ft2 was measured across the filter vessel.
A mixture of 1.89 lbs. of ball clay, 0.03 lbs. of baby oil, and 1.89 lbs. of
diatomaceous earth was mixed into a slurry. The slurry was added to the re-
circulating
water until the pressure across the filter vessel increased to 24.05 psi. The
filtration
mode was stopped and backwash mode was initiated.
Backwash continued for five minutes at 63 gpm (20 gpm/ft2) followed by a
filter
rinse of one minute. The vessel was then turned back on to filtration mode.
The head
loss measured across the filter vessel was 9.1 psi. The results show slightly
less head
loss pressure than the starting value of 9.2 psi. Consequently, the results of
this
example demonstrate excellent cleanability of the surfactant-modified zeolite
filtration
medium.
Example No. 5
In Example No. 5, a turbidity reduction test was run on two surfactant-
modified
zeolite media in accordance with the procedure outlined in ANSI/NSF Standard
50 ¨
2009a, Annex B. Tests were performed on 150 lbs. of media placed into a 24-
inch
diameter, Sta-Rite pressure filter. A 1,000 gallon test tank was used with a
cross-
sectional flow-rate of 20 gpm/ft2 of media. The challenge water was prepared
by adding
ground silica (Sil-co-sil 106) until the challenge water reached a turbidity
of 45 10
NTU. A sample of the challenge water was measured for turbidity (NTU) at the
end of
each reservoir turnover. Turnover of the reservoir at a total flow rate =of 63
gpm was 9
minutes, 39 seconds.
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 18 -
Two different filtration media were tested, a surfactant-modified Zeolite #2
and a
surfactant-modified Zeolite #1. Results are presented below in Tables 4 and 5,
respectively. For the surfactant-modified Zeolite #2, the water used to form
the
challenge water had a turbidity level of 0.88 NTU, while the challenge water
had a
turbidity level of 49.3 NTU. For the surfactant-modified Zeolite #1, the water
used to
form the challenge water had a turbidity of 0.53, while the challenge water
had a
turbidity level of 40.8 NTU.
Table 4. Experimental Results for Surfactant-Modified Zeolite #2 with 50% ECEC
DADMA.
Turnover Effluent Turbidity Percent
Removal
1 12.00 75.7
2 7.02 85.8
3 4.46 90.9
4 2.51 94.9
5 1.39 97.2
Notes: Initial Turbidity ¨ 0.88 NTU; Challenge Water Turbidity ¨ 49.3 NTU
Table 5. Experimental Results for Surfactant-Modified Zeolite #1 with 50% ECEC
DADMA.
Turnover Effluent Turbidity Percent
Removal
1 4.36 89.3
2 0.59 98.5
3 0.44 98.9
4 0.34 99.2
5 0.43 98.9
Notes: Initial Turbidity ¨ 0.53 NTU; Challenge Water Turbidity ¨ 40.8 NTU
For the test defined by ANSI/NSF Standard 50 ¨ 2009a, Annex B, the NSF
requires at least a 70 percent turbidity reduction by the fifth turnover in
order to pass the
test. Both filtration media surpassed a 70 percent turbidity reduction by the
first
turnover. Accordingly, the surfactant-modified zeolites demonstrate a superior
ability for
reducing turbidity.
Example No. 6
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
- 19 -
Both significant reduction in turbidity particles ("TSS") as well as a
determination
of the "fineness" of filtration are important aspects in fluid filtration,
particularly in the
filtration of potable water supplies. The "fineness" of filtration is
sometimes referred to
as "micron reduction" or "nominal particle reduction". These terms relate to
the smallest
particle size effectively removed by a given filter medium.
To obtain an approximation of relative particle removal from filtered water,
several columns were prepared with sand and various zeolite media. The media
were
placed in 2.5-inch ID polycarbonate columns. The bed depth for the filtration
media was
eight inches. The media were backwashed, rinsed, and flooded with tap water
containing 30 NTU of ISO 12103-1 A3 Medium Test Dust at a flow rate of 15
gpm/ft2.
The influent, i.e., the challenge water containing the test dust prior to
being passed
through the filtration media, included particles from 0.7 to 70 microns. The
85th
percentile of particles measured 22 microns. A particle count of the control
water
reservoir was taken from samples of the challenge water at the beginning and
end of
the trial, as shown in Table 6.
The challenge water was pumped into the columns containing the media with
approximately three inches of water above the upper end of the media bed. For
the
filtration media used for testing, an eight-inch bed depth within the columns
contains
approximately 350 ml of open pore space, also referred to as pore volume. Five
liters of
challenge water (14 pore volumes) were passed through each column at a rate of
15
gpm/ft2. Immediately after the passing five liters of challenge water through
the
columns, a 250 ml aliquot sample of effluent, i.e., challenge water after
having passed
through the filtration media, was taken and analyzed for particles. The
particle content
data are reported in Table 6, below,
Table 6 contains particle size data for various samples, showing the reduction
in
particle sizes of particles contained in the challenge water before and after
passage
through the filtration media. Particularly, Table 6 includes sample data for
the 50th
percentile and 85th percentile particle sizes. The percentile values were
obtained from
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2014-07-10
- 20 -
a particle size distribution curve plotted on semi-log paper for each of the
samples. The
analyses were run on a MicrotraTC1X100, produced by Microtrac, Inc., of 44
Hokes Mill
Road, York, PA 17404, with a run time of 30 seconds, done in triplicate.
Table 6. Particle Size Reduction in Microns.
Sample Media
Influent Particle Particle Size Effluent
No. Turbidity Size @ Size @ Range
Turbidity
NTU 50% 85% Of NTU
Particles
Control A3 Med Test Dust 30 9.0 22 0.7 - 70 NA
9909-1 Silica Sand, #20 30 8.0 15 0.7 - 20 30
9909-2 Zeolite #3 30 6.0 12 0.7 - 30 25
9909-3 Zeolite #1 30 8.0 12 0.7 - 30 27
9909-4 Zeolite #2 30 7.0 15 0.7 - 30
9909- Zeolite #1 with 30 2.5 3.5 0.7 ¨ 5.0 2.5
11 DADMA
9909-6 Zeolite #2 with 30 2.5 3.0 0.7 ¨ 5.0 10
DADMA
Control A3 Med Test Dust 30 8.0 18 0.7 ¨ 70 NA
The tests represent severe conditions in that the filtration media are limited
to an eight-
inch bed depth, the challenge water flow rate was 15 gpm/f12, and the samples
were
collected after only 5 liters, or 14 pore volumes, of challenge water had been
passed
through the columns. These results may not reflect data that would be
collected
through strict ANSI/NSF 42 protocol. However, the test results for each
filtration
medium can be compared relative to each other.
Challenge water with A3 Medium Test Dust has an 85th percentile particle size
of
22 microns. The results for silica sand, Zeolite #1, Zeolite #2, and Zeolite
#3 were
similar to each other with the 50th percentile from 6 to 8 microns and 85th
percentile
from 12 to 15 microns. Surfactant-treated Zeolite #1 with DADMA and Zeolite #2
with
DADMA removed turbidity effectively, as demonstrated in prior examples. The
ECEC
coverage of the treated zeolites was approximately 50 percent. However the
particle
CA 02780895 2012 05 14
WO 2011/060348
PCT/US2010/056682
-21 -
analysis of the remaining turbid particles shows particle size reduction to
the range of
2,5 to 3.5 microns, This is excellent for a granular media and far superior to
the
untreated samples of Zeolite #1 and Zeolite #2.
Example No. 7
Example No. 7 addressed the effect of chlorine concentrations on the
surfactant-
modified zeolites. Water in pools, spas, and water attractions routinely
require "chlorine
shock" or similar severe disinfectant treatment in order to reduce the level
of pathogens.
The oxidation potential of a typical chlorine shock of 20 to 30 milligrams per
liter of "free-
chlorine" is a concern for the longevity of a cation surface-modified zeolite
medium.
A test was performed to determine any degradation of the surfactant-modified
zeolite filtration media caused by a 35 milligram per liter chlorine shock. A
sample of
Zeolite #1 was prepared with DADMA at 80 percent ECEC loading. A filtration
column
was prepared in a manner similar to that described above in Example No. 1.
Results
are provided in Table 7.
Table 7. Experimental Results of Example No. 7.
Run Chlorine Initial NTU after NTU after Fifth
Percentage
No. Level in PPM NTU First Turnover Removal
after Fifth
Turnover Turnover
1 2 81 5.5 3.8
95.3
2 35 67.3 38.2 23.6
64.9
3 2 86 3.0 0.8
99.1
4 2 80 7.8 3.1
96.1
5 2 85 9.7 3.9
95.4
The results of Table 7 show removal of ISO 12103-1 A3 Medium Test Dust of
greater than 90 percent at five turnovers. The water flow for all five runs
was 12
gpm/ft.2. Run No. 1 was performed to establish baseline performance. In Run
No. 2,
35 milligrams per liter of free chlorine in the form of sodium hypochlorite
was added to
the reservoir. The high chlorine level considerably reduced the turbidity
reduction.
Particularly, the turbidity reduction obtained from the surfactant-modified
zeolite
SUBSTITUTE SHEET (RULE 261)
CA 02780895 2014-07-10
-22 -
decreased from the 90 percent and greater turbidity reduction observed in
previous
tests to about a 65 percent reduction. Three subsequent runs were made with
the
same media after backwash and rinse. Run No. 3, immediately following the high
chlorine shock, gave the best results for turbidity removal¨a 99.1 percent
turbidity
reduction. Consequently, it is believed that the bonded surfactant, the DADMA
in this
particular example, was not removed, degraded, or oxidized by the high-
chlorine level.
Rather, the performance of the surfactant-modified zeolite was improved. Run
Nos. 4
and 5 continue to show the DADMA sorbed onto the zeolite being effective in
subsequent filtration runs after backwash.
Although the present disclosure has been described with several
implementations, various changes and modifications may be suggested to one
skilled in
the art. It is intended that the present disclosure encompass such changes and
modifications as fall within the scope of the appended claims