Note: Descriptions are shown in the official language in which they were submitted.
CA 02783519 2012-07-23
MTC-070-013-CA1
1 TITLE
2 METHOD FOR SIMULTANEOUS ELIMINATION OF ORTHOPHOSPHATE AND
3 AMMONIUM USING ELECTROLYTIC PROCESS
4
FIELD OF THE INVENTION
6 This invention relates to a method for the simultaneous elimination of
orthophospate
7 and ammonium (NH4) from a nitrogen-rich effluent using an electrolytic
process and
8 thereby electro-synthesis of struvite.
9
BACKGROUND OF THE INVENTION
11 Electrocoagulation was already proposed in the late 19th and early 20th
century. The
12 use of electrocoagulation with aluminum and iron was patented in 1909 in
the United
13 States (Robinson, Australian Water & Wastewater Association, Joint NSW
and
14 Victoria State Conference in Wodonga, 22-24 November 1999
(www.electropure.com.au/paper.htm); Vik etal. WaterResearch, volume 18, Issue
1,
16 1984, pages 1355-1360).
17 Coagulation is essentially to neutralize, or reduce, the electric charge
of colloids and
18 hence promote the aggregation of colloidal particles. To destabilize a
suspension it is
19 necessary that the attractive forces between particles are greater than
the repulsive
forces thereof. Attractive forces are mainly van der Weals forces, which act
at a short
21 distance thereof. In general, the total energy that controls the
stability of the energy
22 dispersion comprises attractive van der Weals energy of repulsion at
short distance,
23 the electrostatic energy and energy due to the steric effect of
molecules solvent.
24 Coagulation can be done by chemical or electrical means. Alun, lime
and/or polymers
have been used as chemical coagulants. Chemical coagulation is becoming less
1
CA 02783519 2012-07-23
MTC-070-013-CAI =
1 popular today because of high costs associated with the chemical
treatments of a
2 significant volume of sludge and hazardous heavy metals such as metal
hydroxides
3 generated thereof in addition to the cost of chemical products needed for
coagulation
4 itself. Chemical coagulation has been used for decades.
Although the electrocoagulation mechanism resembles chemical coagulation,
6 although, some differences benefit electrocoagulation. Indeed,
electrocoagulated
7 flocs differ from those generated by chemical coagulation. Flocs created
with the
8 electrocoagulation process tend to contain less bound water, are more
resistant to
9 shearing and are more easily filterable.
Flocs are created during the electrocoagulation water treatment with oxydo-
reduction
11 reactions. Currents of ions and charged particles, created by the
electric field,
12 increase the probability of collisions between ions and particles of
opposite signs that
13 migrate in opposite directions. This phenomenon allows the aggregation
of
14 suspended solids to form flocs.
The electrolytic reactions that take place at the electrodes are accompanied
by
16 production of micro bubbles of hydrogen (at the cathode) and oxygen (at
the anode).
17 These micro bubbles heading up will result in an upward movement of the
flocs
18 formed thereof that are recovered at the surface (this mechanism is
named flotation).
19 The complexity of the mechanisms involved in the process of
electrocoagulation in
the treatment of water is not well scientifically elucidated (Yousuf et aL,
Journal of
21 Hazardous Material B84, 2001). There are various features of the
mechanism of the
22 process and the geometry, or design, of the reactor in the literature.
The different
23 physico-chemical treatment, the shape of the reactor and the shape and
size of
24 electrodes affect the performance of the treatment. The wide variety of
processing
. parameters reported in the literature and the lack of scientific data for
efficient model
26 processing and optimal processing conditions translate into a lack of
development in
27 this field. At this time, electrocoagulation is still problematic and
therefore not popular
2
CA 02783519 2012-07-23
MTC-070-013-CA1
1 (Holt et al., Colloids and Surfaces A: Physicochem. Eng. Aspects
211(2002); Holt et
2 al., Chemosphere 59(2005) 355-367).
3 The existence of an electric current in a body of water implicitly
requires Faraday
4 reactions surrounding the electrodes. The formation of chemical gradients
depends
on the electrolytic magnitude. The consequences of chemical reactions become
more
6 pronounced and significant in the prolonged application of
electrokinetic. The effects
7 include electrolytic of water with the simultaneous development of pH
gradients and
8 the transfer of electrolytic dissolution of the anode producing metal
ions (Fe3+, Al3+,
9 Mg, etc..) or cations of the electrolyte from the anode to the cathode.
Chemical
reactions can, in ion exchange or precipitation, form new mineral phases for
cleaning
11 water for instance.
12 At the cathode, the main reaction is:
13 4H20 + 4e" 2H2 + 40H" (Equation 1)
14 The increase in hydroxyl ions can increase the precipitation of metal
hydroxide. The
pH of the cathode's region is basic. The following equations describe the
chemical
16 reactions at the anode:
17 2H20 -- 02 + 4H+ + 4e- (Equation 2)
18 If the anode is made of magnesium:
19 Mg Mg2+ + 2e" (Equation 3)
It is noted that twice as many water molecules are electrolysed at the cathode
21 compared to the anode for the same quantity of electricity.
22 The struvite is a compound with a little solubility and used as a
fertilizer in agricultural
23 fields. This compound is of the formula NH4MgPO4, 6H20 and comprised
P043" and
24 NH4+ ions, both essential to plants growth. Struvite is known as a
fertilizer and have
been proved potent in soils having a pH between 5.5 and 6.5.
3
CA 02783519 2012-07-23
MTC-070-013-CA1
1 Precipitation of struvite in a wasted water allows the elimination of the
ortho-
2 phosphate, NH4+ and magnesium present in the wasted water. Currently,
processes
3 for precipitating struvite use fluidized beds, or contained tanks
reactors. In Japan, the
4 precipitation of struvite has been tested in a sludge treatment reactor.
To obtain a
good performance, it is essential to optimized both nucleation and
precipitation by
6 optimizing the treatment time in the reactor and the nature of the
support particules
7 for the precipitation.
8 Precipation of struvite is controlled by the pH, the supersaturation, the
temperature
9 and the presence of impurities such as calcium and can occur when the
concentration in magnesium, ammonium and phosphore ions exceed the solubility
11 product of the complex as per the following expression:
12 Ksp = [ Mg2+ ][NH41[P043] pKs = 13.26
13 The presence of organic matter impact on the nucleation and growth of
struvite
14 crystals and reduce the precipitation rate. In a wasted water to be
treated, NH4 + and
P043" are among the components to be eliminated. While adding Mg2+ in the
solution
16 with a basic pH, the precipitate is formed. Several conditions are
required for the
17 reaction to occur:
18 a phosphorous concentration higher than 5Oppm
19 a pH value between 7 and 11, preferably between 8 and 9.2
a molar ratio Mg/P of 0,9 to 1,5
21 a strong agitation
22 a simultaneous increase in pH and temperature to reduce time of
precipitation
23 Mg2+ + NH4 + + P043" + 6 H20 MgNH4PO4.6H20
24 Many patent applications have been filed for the synthesis of the
struvite. WO
01/19735 discloses a process for the treatment of manure. WO 95/05347
discloses
4
CA 02783519 2012-07-23
MTC-070-013-CA1
1 an
electrolytic system using a series of electrodes. WO 2007/009749
14
17 by addressing one or more of the existing needs in the art.
18 Accordingly, the present invention provides for a method for the
treatment of nitrogen-
19 rich effluent and production of struvite comprising the steps of
introducing the effluent
20 in an electrolytic system comprising a first electrolytic reactor having
at least one
21 cathode and at least one anode adapted to perform electrolytic treatment
of the
22 effluent in the first electrolytic reactor; and a second electrolytic
reactor comprising at
23 least one cathode and at least one magnesium anode adapted to perform
electrolytic
24 of the effluent in the second electrolytic reactor; performing a first
electrolytic
25 treatment to the effluent in the first electrolytic reactor, thereby
eliminating organic
26 matter that impact on nucleation of struvite; and performing a second
electrolytic
27 treatment to the effluent in the second electrolytic reactor, thereby
injecting Mg ions
CA 02783519 2012-07-23
MTC-070-013-CA1
1 which react with ammonium and orthophosphates from the effluent to form a
struvite
2 precipitate.
3 In one embodiment of the present invention, the method further comprises
a
4 conditioning step prior to the first electrolytic treatment, the
conditioning step
comprising adjusting the stoechiometric ratio of orthophosphate in the
effluent and
6 determining, based on initial concentration of NH4, orthophosphate and
calcium
7 comprised in the effluent, the current intensity and treatment time
needed to be
8 applied.
9 In one embodiment of the present invention, the method further comprises
a
conditioning step prior to the second electrolytic treatment, the conditioning
step
11 comprising adjusting stoechiometric ratio of orthophosphate in the
effluent and
12 determining, based on initial concentration of NH4, orthophosphate and
calcium
13 comprised in the effluent, the current intensity and treatment time
needed to be
14 applied.
The present invention provides for a method wherein at least one anode of the
first
16 electrolytic reactor is made of a material selected from the group
consisting of
17 magnesium, aluminium, iron and an other inert material.
18 In one embodiment of the present invention, at least one anode of at
least one of the
19 first and second electrolytic reactor is tubular. Preferably, at least
one of the first and
second electrolytic reactors comprise 9 tubular anodes disposed circularly and
21 parallel to the central axis of the reactor.
22 In an alternative embodiment of the present invention, at least one of
said first and
23 second electrolytic reactors comprises one cylindrical anode disposed
along the
24 central axis of the reactor.
The present invention provides for a method wherein the at least one cathode
of the
26 first and second electrolytic reactors consists in a central cathode or
a peripheral
27 cathode. In one embodiment of the present invention, at least one of the
first and
6
CA 02783519 2012-07-23
MTC-070-013-CA1
1 second electrolytic reactors comprise both a central and a peripheral
cathode. It is
2 provided that the cathodes used in the present invention are made of a
material
3 selected from the group consisting of stainless, galvanized steel and a
material
4 having a potential close to the one of the material of anodes. It is also
provided that
the cathode can be of the same material as the anode provided that in the
second
6 electrolytic reactor the cathode and anode are made of magnesium.
7 The present invention provides for a method wherein the effluent is
treated at a pH
8 between 7.0 and 9.5, preferably between 8.0 and 9.5 and most preferably
at a pH of
9 9.2.
In a preferred embodiment of the present invention, the amount of
orthophosphate in
11 the effluent is adjusted to be about five time the amount of NH4.
12 In a preferred embodiment of the present invention, the concentration of
13 orthophosphate in the effluent is adjusted to be between 50 and 300 ppm.
14 In a preferred embodiment of the present invention, the effluent is
agitated while
being treated in the electrolytic reactors.
16 In a preferred embodiment of the present invention, the second
electrolytic treatment
17 generates Mg2+ ions in a quantity such to obtain a molar ratio Mg/P
between 0.9 and
18 1.5.
19 In a preferred embodiment of the present invention, the electrical
current intensity
used in the electrolytic treatments is between 1 and 120 A.
21 The present invention is suitable for any nitrogen-rich effluent, but
most particularly
22 for wasted water from industrial source, wasted water from agricultural
source and
23 manure.
24 In the present invention, the electrolytic treatment used can be
electrocoagulation,
electrofloatation or a combination of both.
7
CA 02783519 2012-07-23
MTC-070-013-CA1
1 Other objects and further scope of applicability of the present invention
will become
2 apparent from the detailed description given hereinafter. However, it
should be
3 understood that the detailed description and specific examples, while
indicating
4 preferred embodiments of the invention, are given by way of illustration
only, since
various changes and modifications within the spirit and scope of the invention
will
6 become apparent to those skilled in the art from this detailed
description.
7 Additional and/or alternative advantages and salient features of the
invention will
8 become apparent from the following detailed description, which, taken in
conjunction
9 with the annexed drawings, disclose preferred embodiments of the
invention
11 BRIEF DESCRIPTION OF THE DRAWINGS
12 Referring now to the drawings which form a part of this original
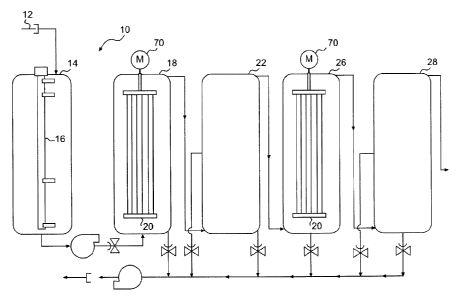
disclosure:
13 Figure 1 is a schematic illustration of the electrolytic system with at
least one
14 embodiment of the invention; and
Figure 2 is a schematic illustration of a modular electrolytic apparatus in
accordance
16 with at least one embodiment of the invention.
17
18 DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
19 A preferred embodiment of the present invention is described bellow with
reference to
the drawings.
21 The electrolytic system 10, as illustrated in Figure 1, comprises a
prefilter 12 that
22 retains particules and allows the colloidal fraction to access a
conditioning tank 14. In
23 the conditioning tank 14, there is a level captor 16 measuring and
controlling the level
24 of fluid in the tanks. Also, they are sensors (not shown in Figure 1)
that allow for the
measurement of conductivity, pH, initial concentrations in NH4, calcium and
8
CA 02783519 2012-07-23
MTC-070-013-CA1
1 orthophosphates as well as initial organic content. Those measured values
allow the
2 continuous evaluation of the conductivity of the affluent, its pH and
allows the
3 adjustment of the quantity of orthophosphate in solution with respect to
the NH4
4 concentration in order to respect the stoechiometry of the reaction
desired.
Conductivity and pH probes are well known in the art and are easily available.
The
6 measure of NH4 + can be made, for example, with a ISE WTW probe coupled
with a
7 VARION PLUS 700IQ sensor. Phosphate analysis can be made using
colorimetric
8 devices such as PHOS200 and TOPHO. The organic charge can be evaluated
using
9 a CSS70 sensor. Also, UV sensors allow for the measurement of absorbance
at
254nm, which can be easily correlated with the chemical demand in oxygen.
11 The measurement of the NH4 + concentration in solution also allows for
the
12 determination of the Mg concentration needed to precipitate the
struvite. The second
13 law of Faraday is used to convert the Mg concentration into current
intensity and
14 treatment time in order to maximized the production of struvite.
Once conditioned, the effluent is pumped in a first electrolytic reactor 18
comprising a
16 fixed electrocoagulation module 20. For the purpose of the present
invention, the
17 electrocoagulation could be interchanged with an electrofloatation
module. The first
18 electrolytic treatment reduce of about 85% the organic charge of the
effluent and the
19 treated effluent is brought in a first decantor 22 to separate the solid-
liquid fractions.
An automatic dosing device (not shown in Figure 1) is placed between the exit
of the
21 first decantor 22 and the entry of a second electrolytic reactor 26.
This automatic
22 dosing device allows the adjustment of the quantity of orthophosphate in
the effluent
23 needed to react with all the NH4 + in solution. After this second
conditioning step, the
24 effluent is introduced in a second electrolytic reactor 26, which also
comprises a fixed
electrocoagulation module 20. The second electrolytic reactor 26 comprises in
its
26 fixed electrocoagulation module 20 at least one soluble anode made of
magnesium.
27 The ions Mg2+ generated while applying the electrical current react with
the NH4 + and
28 orthophosphate in solution and therefore produce a struvite precipitate.
Both first and
29 second electrolytic reactors 18 and 26 optionally comprises a motor 70
allowing the
9
CA 02783519 2012-07-23
MTC-070-013-CA1
1 rotation of the electrocoagulation module 20, providing for an additional
agitation of
2 the fluid in the reactors 18 and 26.
3 After this second electrolytic treatment, the effluent is brought in a
second decantor
4 28 for isolating the struvite precipitate.
An exemplary electrocoagulation module 20 is illustrated in Figure 2 with a
section
6 view allowing a better view of its construction. The electrocoagulation
module 20
7 comprises an anode module 30 and a cathode module 32 adapted to interact
in an
8 electrolytic process producing electrocoagulation. The electrocoagulation
module 20
9 of the present embodiment includes an inlet 34 and an outlet 36
configured to
respectively receive and extract the fluid to and from the electrocoagulation
module
11 20. The fluid, once introduced in the electrocoagulation module 20,
follows a path or a
12 fluidic circuit configured to put the fluid in communication with the
electrolytic process
13 that is produced in the electrocoagulation module 20. In the present
example, the fluid
14 follows a path identified by a series of arrows 38 defined by internal
walls 40. A pump,
which is not illustrated in Figure 1, pushes the fluid through the
electrocoagulation
16 module 20. An opening 42 disposed on a bottom portion 44 of the
electrocoagulation
17 module 20 is normally closed with a plug (not illustrated) to prevent
the fluid to exit the
18 electrocoagulation module 20. The opening 42 can be opened to remove the
fluid
19 from the electrocoagulation module 20 to purge the electrocoagulation
module 20 for
maintenance purposes, for instance. The electrocoagulation module 20 can also
be
21 purged to remove particles and debris. A larger closure member 46 is
used to close
22 the bottom portion of the electrocoagulation module 20 lower body 48.
The closure
23 member 46 can be optionally removed to provide a larger access in the
24 electrocoagulation module 20. The lower body 48 can threadedly engage
the upper
body 56 and be removed from the upper body 56, if desirable.
26 Still referring to Figure 2, the closure member 46 is located at the
lower portion of the
27 electrocoagulation module 20 to receive particles therein. The cathode
module 32 is
28 bottomless and allows the particles to drop in the closure member 46
acting as a
29 particles-receiving member 46. The removable particles-receiving member
46 is
CA 02783519 2012-07-23
MTC-070-013-CA1
1 preferably disposed in the center of the cathode module 32 as illustrated
in the
2 present embodiment and is used for removing decanted particles from the
cathode
3 module 32. The opening 42 in the closure member 46 can alternatively be
used to
4 inject gas, like air, or liquids for further conditioning the liquid in
the electrocoagulation
module 20 and/or influence the electrocoagulation process inside the cathode
module
6 32.
7 The electrocoagulation module 20 further includes body portions 48, 56
that can
8 optionally include insulating material to prevent heat transfer with the
environment.
9 Conversely, the electrocoagulation module 20 might be equipped with
heating/cooling
elements 58 to keep the electrocoagulation apparatus 20 at a predetermined
11 operating temperature. The upper body 56 of an embodiment can be made of
an
12 insulating material preventing heat transfer between the inside of the
13 electrocoagulation module 20 and the outside of the electrocoagulation
module 20.
14 The lower body 48 of the embodiment illustrated in Figure 2 is made of a
material that
is less insulating the electrocoagulation module 20. Heating or cooling
elements 58
16 are disposed, for example, in a spiral around the lower body 48 to
either heat or cool
17 the lower body 48. The heating or cooling elements 58 can use a fluid
circulating in a
18 tubular system or electric elements in contact with, or nearby, the
lower body 48.
19 Another embodiment is using the upper body 56 to transfer heat to/from
the
electrocoagulation module 20 in cooperation or not with the lower body 48.
21 Still referring to the embodiment of Figure 2, the anode module 30 is
secured to the
22 upper body 56 and extends above the upper body 56 to allow electrical
connection 62
23 thereto. The cathode module 32 of the present embodiment is also secured
to the
24 upper body 56 and extends therefrom 60 to allow electrical connection
thereto. A
power supply (not illustrated) is connected to the cathode module 32 to
provide
26 negative power thereof. Electrical polarity reversal is provided when
desired to avoid
27 passivation of the anode module 30 and the anodes 68 secured thereon.
Insulators
28 may be placed between two adjacent electrodes to prevent short circuits
thereof. The
29 cathode 32 and the anodes 68 are subjected to DC current. One skilled in
the art can
11
CA 02783519 2012-07-23
MTC-070-013-CA1
1 also appreciate that the upper body 56 is made of an insulating material
to prevent
2 establishing an electrical connection between the cathode 32 and the
anode module
3 30.
4 The anode module 30 can be made of soluble or inert materials. The
cathode module
32 can be made of steel, aluminium, stainless steel, galvanized steel, brass
or other
6 materials that can be of the same nature as the anode module 30 material
or having
7 an electrolytic potential close to the electrolytic potential of the
anode 68. The cathode
8 module 32 of the present embodiment has a hollowed cylindrical shape,
fabricated of
9 sheet material, and can be equipped with an optional lower frustoconical
portion (not
illustrated in Figure 2). The inter electrode distance of an embodiment of the
invention
11 is about between 8-25 mm and preferably 10 mm for electro floatation and
20 mm for
12 electrocoagulation. The interior of the cathode module 32 electrically
interacts with
13 the outside of the anode module 30. The electrocoagulation module 20
internal wall
14 includes non-conductive material, like polymer, in an embodiment of the
invention.
The cathode module 32 could alternatively serve as a reservoir, or reactor, at
the
16 same time thus holding the liquid to treat therein in other embodiments.
The cathode
17 module 32 can be made of a material different from the anode material 30
or can
18 alternatively be made of the same material, like, for instance,
magnesium.
19 The size and the available active surface area of the cathode module 32
can be
adapted to various conditions without departing from the scope of the present
21 invention. The surface ration of the cathode/anode can be identical or
vary to about
22 1.5. The cathode module 32 of other embodiments can alternatively be
oval or
23 conical; its diameter expending upward or downward. The
electrocoagulation module
24 20 can include therein an optional fluid agitator module 64 adapted to
apply kinetic
energy to the fluid contained in the electrocoagulation module 20 by moving or
26 vibrating the fluid in the electrocoagulation module 20 as it is
illustrated in the
27 embodiment depicted in Figure 2.
28 As mentioned above, the movement of the fluid increases the kinetic
energy
29 contained therein to destabilize the colloidal solution. This can be
achieved by
12
CA 02783519 2012-07-23
MTC-070-013-CA1
1 turbulently injecting the fluid in the electrolytic module (the speed and
tangential
2 injection of the fluid are possible ways to create turbulences in the
fluid). The fluid
3 agitator module 64 in this embodiment is a spiral shaped protrusion
member 64 that
4 is secured to the anode module 30. The movement of the fluid between the
anode
module 30 and the cathode module 32 is intensified by the protrusion member
64,
6 which influences the electrolytic process. The anode module 30 of an
alternate
7 embodiment that is not illustrated in Figure 2 could be rotatably secured
to the upper
8 body 56 of the electrocoagulation module 20 and be rotated by an external
motor to
9 rotate the anode and the protrusion members secured thereon to apply
additional
kinetic energy to the fluid as it will be discussed below. As it is
illustrated in Figure 2,
11 the anode module 30 is preferably centred inside the electrocoagulation
module 20
12 and preferably located at equal distance from the cathode module 32.
13 The electrocoagulation module 20 of Figure 2 further comprises a pair of
14 electrocoagulation module connectors 66 adapted to operatively install
the
electrocoagulation module 20 in a larger fluid treatment process if desired.
The
16 electrocoagulation module 20 can removably be mounted in series, or in
parallel, in
17 the fluid treatment process. This way, the electrocoagulation module 20
can easily be
18 added, maintained, replaced and/or removed from the fluid treatment
process.
19 Example 1
An effluent from the agri-food industry has been treated using the method and
21 process of the present invention. This effluent was providing from a
pork
22 transformation plant and was charged in urine, feces and blood with a pH
of 6.8. The
23 effluent has been treated with the process of the present invention
using a 2 reactors
24 and decantors process, with a variable tension generator (0-30V)
offering current
between 1 and 120A. The anodes of the reactors were in magnesium and the
26 measures of the chemical oxygen demand, orthophosphate concentration,
NH4
27 concentration, calcium concentration and magnesium concentration made
using
28 HACH chemicals.
13
CA 02783519 2012-07-23
MTC-070-013-CA1
1 Table 1
Analysis
Sample Brut effluent Conditioned Treated sample Treated
sample
effluent
Time 10:00 am 11:00 am 1:30 pm 2:30 pm
Temperature (C) 28 28 43 43
pH 7.02 1.02 9.03 8.85
M.E.S (mg/I) 1700 1900 0 8.85
Turbidity (NTU) 817 1100 2 9
P043" (mg/I) 43 135 0.4 0.4
NH4+ (mg/I) 55 55 26 13
2
3
4
It is shown in Table 1 that the brut effluent has an initial concentration of
6 orthophosphate of 43 ppm and ammonium concentration of 55 ppm. To
eliminate
7 these two elements, the stoechiometric ratio has to be respected. An
initial
8 concentration in orthophosphates of 55 x5 = 275 ppm should have been
needed
9 according to the initial data. However, the effluent has been conditioned
to have an
orthophosphate concentration of 135 ppm, which allowed a reduction in NH4 + of
135:5
11 = 27 ppm corresponding to the results obtained (26 ppm). This example
shows the
12 importance of respecting the stoechiometric ratio to allow an optimal
reduction of
13 NH4 + as well as maintaining a pH of about 9.2.
14
14
CA 02783519 2012-07-23
MTC-070-013-CA1
1 Table 2
NH4 + P043" P043" P043" P043" NH 4+
NH4+
(mg/I) (mg/I) (mg/I) (mg/I) (mg/I) final eliminati
initial initial theory added final on
(%)
1 2 3 4 5 6
9:00 am 68 57 340 0 0 55 19
12:30 pm 70 73 350 174 0 28 60
1:30 pm 55 43 275 152 0.4 26 52
2:30 pm 55 43 275 230 0.4 13 77
3:00 pm 50 51 250 235 0 7 86
2
3
4 Table 2 illustrates that the ions ortho phosphate are needed to eliminate
NH4 + and
that the closer the ratio orthophosphate/NH4 + is closer to 5:1, the better is
the NH4+
6 elimination.
7 Example 2
8 A lixiviat has been treated using the method and process of the present
invention.
9 The effluent has been treated with the process of the present invention
using a 2
reactors and decantors process, with a variable tension generator (0-30V)
offering
11 current between 1 and 120A. The anodes of the reactors were in magnesium
and the
12 measures of the chemical oxygen demand, orthophosphate concentration,
NH4+
13 concentration, calcium concentration and magnesium concentration made
using
14 HACH chemicals.
The effluent was treated with a tension of 27.3V and a current of 100A.
16
CA 02783519 2012-07-23
MTC-070-013-CA1
1 Table 3
Analysis
Sample Brut lixiviat Conditioned lixiviat Treated
lixiviat
Temperature 0 0 27
pH 7.19 3.75 9.09
M.E.S (mg/I) 234 352 27
Turbidity (NTU) 276 390 45
P043" (mg/I) 29 225 0.5
NH4+ (mg/I) 190 190 140
2
3
4 In this example, it is demonstrated again that the reduction of the NH4 +
is in
accordance with the stoechiometric ratio. To eliminate the residual NH4, an
total
6 amount of 950ppm of orthophosphate should have been in the conditioned
lixiviat.
7 Example 3
8 An combined effluent from landfill sites has been treated using the
method and
9 process of the present invention. The effluent has been treated with the
process of
the present invention using a 2 reactors and decantors process, with a
variable
11 tension generator (0-30V) offering current between 1 and 120A. The
anodes of the
12 reactors were in magnesium and the measures of the chemical oxygen
demand,
13 orthophosphate concentration, NH4 + concentration, calcium concentration
and
14 magnesium concentration made using HACH chemicals. Several batches (A-H)
of
the initial effluent have been treated and the results are shown in Table 4.
16
16
CA 02783519 2012-07-23
MTC-070-013-CA1
1 Table 4
Sample Time T(C) pH Conductivity MES (mg/I)
P043" NH4+
(mS/cm)
(mg/I) (m9/1)
initial 0 min 22 7.84 5.94 1140 90 310
A 5 min 42 8.82 3.90 24 6.2
220
B 4 min 42 8.77 3.98 39 7.4
280
C 5 min 43 8.66 3.74 21 4.3
120
D 4 min 42 8.58 3.84 29 6.2
280
E 5 min 43 8.63 3.75 20 5.4
270
F 4 min 42 8.62 3.95 22 4.8
290
G 5 min 43 8.56 3.60 18 8.6
290
H 4 min 41 8.51 3.72 25 10.6
150
2
3
4
The results shown in Table 4 demonstrate that both the stoechiometric ratio
and the
6 time of treatment need to be sufficient for allowing a satisfactory
elimination of NH4.
7 If the stoechiometric ratio is not respected, the complete, or at least
satisfactory
8 elimination of NH4 + is impossible. Also, the treatment needs to be
performed for a
9 time sufficient to allow the production of a minimal quantity of Mg2+
ions, otherwise the
reaction cannot be optimal.
11 While the invention has been described in connection with what is
presently
12 considered to be the most practical and preferred embodiments, it is to
be understood
13 that the invention is not to be limited to the disclosed embodiments and
elements, but,
14 to the contrary, is intended to cover various modifications,
combinations of features,
equivalent arrangements, and equivalent elements included within the spirit
and
16 scope of the appended claims. Furthermore, the dimensions of features of
various
17 components that may appear on the drawings are not meant to be limiting,
and the
18 size of the components therein can vary from the size that may be
portrayed in the
17
CA 02783519 2012-07-23
MTC-070-013-CA1
1 figures herein. Thus, it is intended that the present invention covers
the modifications
2 and variations of the invention, provided they come within the scope of
the appended
3 claims and their equivalents.
4
6
7
8
9
18