Note: Descriptions are shown in the official language in which they were submitted.
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
MAILLARD FLAVOR COMPOSITIONS WITH POLAR SOLVENTS DIFFERENT FROM WATER
AND METHODS FOR MAKING SUCH COMPOSITIONS
CROSS REFERENCE TO RELATED APPLICATIONS
BACKGROUND OF THE INVENTION
Field of the Invention
[0001] The invention relates generally to flavor compositions and methods for
making flavor
compositions and particularly to Maillard flavor compositions, methods for
making Maillard
compositions, and their use for enhancing palatability of comestible
compositions.
Description of the Related Art
[0002] It is well-known that many flavors, colors, and aromas associated with
cooking processes
result from nonenzymatic, or nonenzymic, browning. Generally, nonenzymic
browning comprises
pyrolysis, carmelization, and Maillard reactions. Of these, the Maillard
reaction may be the most
significant. Discovered in 1912, the Maillard reaction is actually a group of
complex chemical reactions
between available carbonyl groups and available amino groups. In food systems,
reducing groups can
be found on reducing sugars and amino groups can be found on free amino acids,
peptides, and proteins.
Initially, a reactive carbonyl group of a reducing sugar condenses with a free
amino group, with a
concomitant loss of a water molecule. The resultant N-substituted
glycoaldosylamine is not stable. The
aldosylamine compound rearranges, through an Amadori rearrangement, to form a
ketosamine.
Ketosamines that are so-formed may further react through any of the following
three pathways: (a)
further dehydration to form reductones and dehydroreductones; (b) hydrolytic
fission to form short
chain products, such as diacetyl, acetol, pyruvaldehyde, and the like, which
can, in turn, undergo
Strecker degradation with additional amino groups to form aldehydes, and
condensation, to form aldols;
and (c) loss of water molecules, followed by reaction with additional amino
groups and water, followed
by condensation and/or polymerization into melanoids. Factors that affect the
rate and/or extent of
Maillard reactions include among others the temperature, water activity (A,),
and pH. The Maillard
reaction is enhanced by high temperature, low moisture levels (e.g., Aw from
about 0.6 to about 0.7),
and alkaline pH. The skilled artisan will appreciate that Maillard reactions
are thus very complex and a
great variety of reaction products can be generated. At each stage of the
Maillard reaction, and under
specified conditions, the reaction may generate compounds that contribute to
the palatability of a food
or to a unique flavor profile associated with that food cooked in a particular
way. WO/03/051139 Al
discloses the method for producing a composition for chicken flavors which
includes making the
Maillard reaction in a water phase and adding oil after start of the chemical
reactions. This is very
different from the present invention where the complete reaction occurs in the
presence of oil, of an
emulsifier and of at least one polar solvent, which is different from water.
[0003] Emulsions in food systems are also well known. Both oil-in-water (e.g.,
salad dressings,
milk) and water-in-oil (e.g., butter, margarine) emulsions are common.
W09962357 discloses
1
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
emulsions used for various purposes in the food industry, including delivery
of flavor compositions.
US20080038428 proposes using emulsions with an aqueous continuous phase as a
means of conducting
Maillard reactions. W02007060177 discloses an oil-in-water emulsion wherein
the oil droplets are
structured using emulsifiers that can be useful for performing a Maillard
reaction. W000033671
discloses processes for producing Maillard reaction aroma products in an
emulsifier and water mixture.
However, no oil and no polar solvent different from water is used. With these
2 parameters, the man
skilled in the art cannot predict the efficiency of the Maillard reaction in
the particular system of the
present invention which is clearly demonstrated in example 1. These systems
are useful but inefficient
for conducting Maillard reactions and delivering Maillard compositions useful
for enhancing
palatability. There is, therefore, a need for new and efficient methods for
producing Maillard reaction
products and Maillard compositions that are useful for enhancing palatability.
SUMMARY OF THE INVENTION
[0004] It is, therefore, an object of the present invention to provide
Maillard flavor compositions
useful for enhancing palatability.
[0005] It is another object of the invention to provide Maillard flavor
compositions that can be
easily introduced into food and petfood products.
[0006] It is another object of the invention to provide methods for making
Maillard flavor
compositions useful for enhancing palatability.
[0007] It is another object of the invention to provide foods, dietary
supplements, medicaments, or
other comestible materials comprising at least one Maillard flavor
composition.
[0008] It is a further object of the invention to provide compositions and
methods for enhancing
palatability of foods, dietary supplements, medicaments, or other comestible
materials.
[0009] It is another object of the invention to provide comestible
compositions that contain one or
more structured lipid phases that produce Maillard reaction products during
preparation, e.g., when
heated.
[0010] One or more of these and other objects are achieved using novel
Maillard flavor
compositions that enhance palatability of foods, dietary supplements,
medicaments, or other comestible
materials. The compositions comprise a structured lipid phase comprising a
continuous lipid phase
comprising a lipid and a dispersed polar solvent. In the following
specifications, it is understood that a
polar solvent is a polar solvent different from water or a mixture of polar
solvents where at least one of
the polar solvents is different form water. The composition comprises at least
a first reactant having a
free carbonyl group and a second reactant having an amino group available for
reaction with the free
carbonyl on the first reactant. Upon incubation under suitable conditions, a
Maillard reaction occurs
between the first reactant and the second reactant. This reaction produces at
least one Maillard reaction
product. These Maillard flavor compositions are useful for enhancing the
palatability of products to an
animal, e.g., food compositions.
2
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
[0011] These and other and further objects, features, and advantages of the
present invention will be
readily apparent to those skilled in the art.
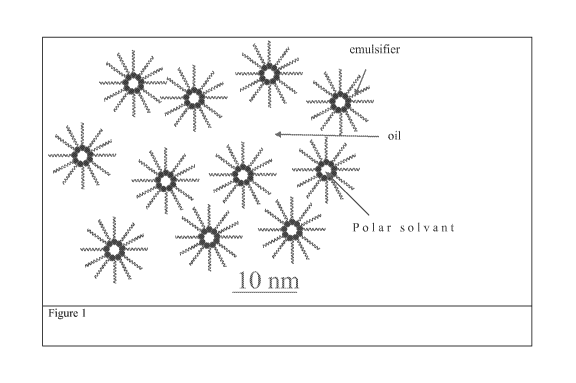
BRIEF DESCRIPTION OF THE FIGURES
These figures illustrate possible structure of structured lipids phase. Other
structures, not shown in these
figures are possible and are protected by the present invention.
[0012] FIG. 1 illustrates a polar solvent-in-oil microemulsion. Polar solvent
may comprize water or
not. The continuous phase is an oil wherein the typical size of the polar
solvent domain is between 0.5
and 100 nm and an emulsifier is used to obtain this structure. There might be
one type of emulsifiers or
several types of emulsifiers.
[0013] FIG. 2 illustrates a polar solvent -in-oil emulsion. Polar solvent may
comprize water or not.
The continuous phase is an oil wherein the typical size of the polar solvent
domain is between 50 nm
and 1 mm and an emulsifier might be used to obtain this structure. There might
be one type of
emulsifiers or several types of emulsifiers.
[0014] FIG. 3 illustrates a mixture between a polar solvent-in-oil emulsion
and a polar solvent-in-oil
microemulsion. It is composed of polar solvent-in-oil emulsion droplets and
polar solvent-in-oil
microemulsion droplets. The two types of droplets define polar solvent domains
which are surrounded
by emulsifiers. There might be one type of emulsifiers or several types of
emulsifiers. The size of the
polar solvent domains are typically the sizes of a polar solvent-in-oil
emulsion droplet or of polar
solvent microemulsion droplet.[\L,aurent, Send drawing\]
DETAILED DESCRIPTION OF THE INVENTION
Definitions
[0015] The term "structured lipid" or "structured lipid phase" means a polar
solvent-in-lipid
dispersion comprising a continuous lipid phase made of oil, lipid or
emulsifiers (also called lipohilic
additives), and a dispersed polar solvent featuring polar solvent domains that
are dispersed, emulsified,
or microemulsified within the lipidic phase. The polar solvent may be a
mixture of various solvents
including at least one polar solvent different from water. The term polar
solvent includes low molecular
weight glycols, alkane glycols and mixtures thereof, as well as mixtures of
these polar solvents with
water. In particular, low molecular weight glycols include glycerol
(glycerine), propylene glycol and di
propylene glycol. Any low molecular weight glycol can be used. In particular,
alkane polyols
correspond to the formula R-CH2-(CHOH)n-CH2OH, wherein n is a whole number
from 0 to 4 and R
corresponds to H or OH. The polar solvent can also be methanol, propanol, iso-
propanol, n-butanol and
ethanol. A preferred polar solvent is glycerol or a mixture glycerol/water. In
the following specification,
it is understood that a polar solvent is a polar solvent different from water
or a mixture of polar solvents
where at least one of the polar solvents is different form water. Preferred
embodiments of the structured
lipid further comprise one or more lipophilic additives (also called
emulsifiers) that emulsify or
3
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
stabilize the structured lipid phase by reducing the surface tension between
the continuous and
dispersed phases. Structured lipids may be present on their own or coexist
with a product or with an
excess water or with an excess of polar solvent or with an excess of any other
food constituant. It is
understood by an excess of polar solvent, any polar solvent which is not
solubilized or dispersed and
therefore forming domains having a diameter larger than 1 micron, preferably
larger than 10 microns
and even more preferably domains larger than 100 microns. Structured lipids
encompass lipids with or
without art-recognized structures such as polar solvent-in-oil emulsions,
polar solvent-in-oil
microemulsions, reversed microemulsions, liquid crystalline structures (e.g.,
reversed micellar cubic,
reversed bicontinuous cubic, or reversed hexagonal structures), lamellar
liquid crystalline structures,
sponge phases (L3) or the like, or any combinations thereof. A reversed
structure is defined as a
structure in which the stabilizing film is curved towards the polar solvent.
Preferred structured lipids
include reversed polar solvent-in-oil microemulsions, polar solvent-in-oil
structures or emulsions, or
combinations thereof. Reversed microemulsions are preferably of the L2 or
bicontinuous type.
Preferred polar solvent-in-oil reversed microemulsions show a phase separation
when diluted with polar
solvent, and dilution with polar solvent or with an aqueous phase results in a
two phase or in a multi-
phase system: reversed microemulsion plus polar solvent or aqueous phase or
other phases. The
structured lipid includes any structure that has the characteristic of a polar
solvent-in-oil emulsion, polar
solvent-in-oil microemulsion, reversed microemulsion, liquid crystalline
structure (e.g., reversed
micellar cubic, reversed bicontinuous cubic, or reversed hexagonal
structures), lamellar liquid
crystalline structure, sponge phase (L3) or the like, or any combinations
thereof at storage temperatures
or at temperatures at which the Maillard reaction occurs or at any
temperatures between storage
temperatures and temperatures at which the Maillard reaction occurs.
[0016] The term "lipophilic additive" or "emulsifier" means a compound or
composition that
comprises one or more molecules, compounds, or ingredients for emulsifying or
stabilizing a water-in-
oil emulsion or a water-in-oil microemulsion. The lipophilic additive or
emulsifier can also be defined
using its hydrophilic-hydrophobic balance (HLB). Suitable emulsifiers or
emulsifier mixtures have a
HLB preferably lower than 8, preferably lower than 7. Emulsifiers include
monoglycerides, including
saturated and unsaturated monoglycerides, diglycerides, phospholipids,
lecithins, polyglycerol esters of
fatty acids, propylene glycerol esters of fatty acids, polyglycerol
polyricinoleates, stearoyl lactylates,
sorbitan esters of fatty acids, derivatives of the foregoing, salts of the
foregoing, particularly sodium
and/or calcium salts, or any combinations the foregoing. Also useful as
emulsifiers are mono- or di-
glyceride esters of fatty acids, for example, esters of tartaric acid, acetic
acid, citric acid, lactic acid,
sorbic acid, or other edible, food-grade, or food-compatible acids,
monoglyceride phosphates, and other
derivatives or salts of mono- or diglycerides. Other useful lipophilic
additives (emulsifier) are long-
chain alcohols, fatty acids, pegylated fatty acids, glycerol fatty acid
esters, derivatives of mono-
diglycerides, pegylated vegetable oils, sorbitan esters, polyoxyethylene
sorbitan esters, propylene
glycol mono- or diesters, phosphatides, cerebrosides, gangliosides, cephalins,
lipids, glycolipids,
4
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
sulfatides, sugar esters, sugar ethers, sucrose esters, sterols, polyglycerol
esters, myristic acid, oleic acid,
lauric acid, stearic acid, palmitic acid, PEG 1-4 stearate, PEG 2-4 oleate,
PEG-4 dilaurate, PEG-4
dioleate, PEG-4 distearate, PEG-6 dioleate, PEG-6 distearate, PEG-8-dioleate,
PEG-3-16 castor oil,
PEG 5-10 hydrogenated castor oil, PEG 6-20 corn oil, PEG 6-20 almond oil, PEG-
6 olive oil, PEG-6
peanut oil, PEG-6 palm kernel oil, PEG-6 hydrogenated palm kernel oil, PEG-4
capric/caprylic
triglyceride, mono, di, tri, tetraesters of vegetable oil and sorbitol,
pentaerythrityl di, tetra stearate,
isostearate, oleate, caprylate or caprate, polyglyceryl-3 dioleate, stearate,
or isostearate, plyglyceryl 4-
pentaoleate, polyglyceryl 2-4 oleate, stearate, or isostearate, polyglyceryl 4-
10 pentaoleate,
polyglycewryl-3 dioleate, polyglyceryl-6 dioleate, polyglyceryl-10 trioleate,
polyglyceryl-3 distearate
propylene glycol mono- or diesters of C6 to C20 fatty acid, monoglycerides of
C6 to C20 fatty acid,
lactic acid derivatives of monoglycerides, lactic acid derivatives of
diglycerides, diacetyl tartaric ester
of monoglycerides, triglycerol monostearate cholesterol, phytosterol, PEG 5-20
soya sterol, PEG-6
sorbitan tetra, hexasterarate, PEG-6 sorbitan tetraoleate, sorbitan
monolaurate, sorbitan monopalmitate,
sorbitan mono trioleate, sorbitan mono and tristearate, sorbitan
monoisostearate, sorbitan sesquioleate,
sorbitan sesquistearate, PEG-2-5 oleyl ether, POE 2-4 lauryl ether, PEG-2
cetyl ether, PEG-2 stearyl
ether, sucrose distearate, sucrose dipalmitate, ethyl oleate, isopropyl
myristate, isopropyl palmitate,
ethyl linoleate, isopropyl linoleate, poloxamers, phospolipids, lecithins,
lyzo-lecithins, polysorbates,
cephalins, oat lipids and lipophilic amphiphilic lipids from other plants; and
mixtures thereof. Other
molecules or combination of molecules are possible as long as they provide
water-in-oil emulsion, polar
solvent-in-oil emulsion, water-in-oil microemulsion, polar solvent-in-oil
microemulsion, liquid
crystalline phase where the continuous phase is lipid, self assembly structure
where the continuous
phase is lipid, or combination of them. Examples of commercial products that
may be useful as
emulsifiers herein include Dimodan Distilled Monoglycerides, Panodan DATEM
(Diacetyl Tartaric
Acid Esters), GrindstedTM ACETEM (Acetic Acid Esters of Monoglycerides),
GrindstedTM CITREM
(Citric Acid Esters of Monoglycerides), GrindstedTM LACTEM (Lactic Acid Esters
of Monoglycerides),
GrindstedTM Mono-Di (Mono and Diglycerides), GrindstedTM PGE or PGPR
(Polyglycerol Esters of
Fatty Acids, Polyglycerol Polyricinoleate), GrindstedTM PGMS (Propylene
Glycerol Esters of Fatty
Acids), and GrindstedTM SMS or STS (Sorbitan Monostearate, Sorbitan
Tristearate) (all, Danisco,
Denmark). In some embodiments, one or more proteins with emulsifying
properties may also be useful
as emulsifiers, alone, or more preferably, in combination with any other
emulsifier or combination
thereof. Presently preferred emulsifiers comprise saturated or unsaturated
monoglycerides, lecithins,
phospholipids, or any combination thereof.
[0017] The term "micro emulsion" means an immiscible lipid-polar solvent
system in which a
dispersed phase is dispersed within a continuous phase and wherein the
droplets, domains, or channels
of the dispersed phase are of an average nominal size on the order of less
than about 300 nm in
diameter. More preferably they average 100 nm, 80 nm, 50 nm, or less. In one
embodiment, the
microemulsion contains micelles, droplets, domains, or channels that range in
size from about 0.5 to
5
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
about 300 nm. In other embodiments, the polar solvent domains ranges in size
from 2 to about 200 nm,
or 10 to 100 nm. Microemulsions are generally thermodynamically stable and can
be clear or nearly
clear. When an immiscible lipid-polar solvent system has been prepared so as
to form a microemulsion,
it is sometimes referred to herein as "microemulsified." Presently preferred
structure lipids encompass
microemulsions having an L2 structure. In a preferred embodiment, the polar
solventsolvent droplet
size is about 100 times smaller than in a normal water-in-oil emulsion or
polar solventsolvent-in-oil-
emulsion. In standard microemulsions, the dispersed phase droplets are known
as "micelles."
[0018] A normal or standard "emulsion" refers to an immiscible lipid-polar
solvent system where a
dispersed phase is dispersed within a continuous phase, and wherein the
dispersed phase includes
droplets, domains, or channels of nominal size larger than about 250 nm in
diameter, or in some
embodiments, larger than 300 nm to about 1 m. These emulsions are generally
thermodynamically
unstable and at least slightly turbid. The immiscible phases will generally
separate given time,
depending on temperature and other factors. The skilled artisan will
appreciate that many emulsions
contain at least some droplets, domains, or channels of less than 200, 100,
50, or even 10 nm.
Emulsions are nonetheless generally differentiated from microemulsions, which
exclude such large
droplets, domains and channels. When an immiscible lipid-polar solvent system
has been prepared so as
to form an emulsion, it is sometimes referred to herein as "emulsified." The
term "emulsion" also
means emulsions like oil-in-polar solvent-in-oil double emulsion.
[0019] The term "polar solvent-in-oil" emulsion or microemulsion means that
the continuous phase
is lipid and the dispersed phase contains the polar solvent. In the following
specification, it is
understood that a polar solvent is a polar solvent different from water or a
mixture of polar solvents
where at least one of the polar solvents is different form water. The skilled
artisan will appreciate that
emulsions and microemulsions may be solid, semi-solid or liquid. As used
herein, a polar solvent
dispersed phase can comprise any manner, variety, or combination of micelles,
droplets, domains, or
channels. The polar solvent can contain any polar solvent with at least one
polar solvent different from
water, and any solutes or combination of solutes may be dissolved therein to
the limit of their solubility,
including reducing reactants, amino reactants, catalysts, salts, buffers,
acids, and the like. In preferred
embodiments, the polar solvent phase predominantly contains one or more
reducing sugars and amino
acids or proteins dissolved therein. In other embodiments, the polar solvent
phase contains phosphate-
containing or carboxylate-containing compounds, such as salts, acids, or
buffers. Such compounds are
useful for adjusting the pH, buffering against pH changes, and catalyzing
Maillard reactions.
[0020] The term "reducing reactant' 'means a reactant that comprises a
reactive aldehyde (-CHO) or
keto (-CO-) group, e.g., a reactant with a free or available carbonyl group,
such that the carbonyl group
is available to react with an amino group on a reactant in a Maillard
reaction. In preferred embodiments,
the reducing reactant is a reducing sugar, e.g., a sugar that can reduce a
test reagent, e.g., can reduce
Cu2+ to Cu+, or can be oxidized by such reagents. Monosaccharides,
disaccharides, oligosaccharides,
polysaccharides (e.g., dextrins, starches, and edible gums) and their
hydrolysis products are suitable
6
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
reducing reactants if they have at least one reducing group that can
participate in a Maillard reaction.
Reducing sugars include aldoses or ketoses such as glucose, fructose, maltose,
lactose, glyceraldehyde,
dihydoxyacetone, arabinose, xylose, ribose, mannose, erythrose, threose, and
galactose. Other reducing
reactants include uronic acids (e.g., glucuronic acid and galacturonic acid)
or Maillard reaction
intermediates bearing at least one carbonyl group such as aldehydes, ketones,
alpha-hydroxycarbonyl or
dicarbonyl compounds.
[0021] The term "amino reactant" means a reactant having a free amino group
that is available to
react with a reducing reactant in a Maillard reaction. Amino reactants include
amino acids, peptides
(including dipeptides, tripeptides, and oligopeptides), proteins, proteolytic
or nonenzymatic digests
thereof, and other compounds that react with reducing sugars and similar
compounds in a Maillard
reaction. In some embodiments, the amino reactant also provides one or more
sulfur-containing groups.
[0022] The term "Maillard reaction product" means any compound produced by a
Maillard reaction.
In preferred embodiments, the Maillard reaction product is a compound that
provides flavor ("Maillard
flavor"), color ("Maillard color"), or a combination thereof. The term
"flavor" includes "odor" and
"taste."
[0023] The term "Maillard flavor composition" means a composition comprising a
structured lipid,
a first reducing reactant, a second amino reactant, and optionally Maillard
reaction products produced
by a Maillard reaction between the first and second reactants.
[0024] The term "animal" means any animal that could benefit from enhanced
palatability resulting
from Maillard compositions, including human, avian, bovine, canine, equine,
feline, hicrine, lupine,
murine, ovine, or porcine animals.
[0025] The term "companion animal" means domesticated animals such as cats,
dogs, rabbits,
guinea pigs, ferrets, hamsters, mice, gerbils, horses, cows, goats, sheep,
donkeys, pigs, and the like.
[0026] The term "palatability" refer to a quality of a food, food supplement,
food additive, dietary
supplement, medicament, or the like, that makes it appealing or pleasing to
one or more of an animal's
senses, particularly the senses of taste and smell. Accordingly, palatability
is determined subjectively.
As used herein, whenever an animal shows a preference for one of two or more
foods, the preferred
food has greater or enhanced palatability. For companion animals and other non-
human species, the
relative palatability of one food compared to one or more other foods can be
determined, for example,
in side-by-side, free-choice comparisons, e.g., by relative consumption of the
foods, or other
appropriate measures of preference indicative of palatability. The skilled
artisan will appreciate that
various aspects or phases of "palatability" can be considered both
independently and interdependently.
For example, "initial appeal," "continued consumption palatability," and
"repeated presentation
palatability" can all be considered. "Initial appeal" is an aspect of
palatability that induces an animal to
initially taste or try a food, dietary supplement, or medicament. "Continued
consumption palatability' is
an aspect of palatability that induces an animal to continue consuming a
product that has been initially
only tasted or tried. "Repeated presentation palatability" or "repeated
feeding palatability" is an aspect
7
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
of palatability evident when a food composition, dietary supplement, or
medicament, which has
previously been both tasted and consumed, is presented repeatedly to the
animal for consumption over
time. For example, a complete and nutritionally-balanced food composition that
is fed daily to an
animal will hopefully provide palatability for each repeated presentation of
feeding, such that the
animal continues to consume adequate quantities of the food.
[0027] The term "palatability enhancer" means any compound, composition,
formulation, or other
material useful for enhancing the palatability of a comestible composition
such as a food composition,
supplement, medicament, or the like. Palatability enhancers enhance
palatability at any one or more of
the aspects of palatability. Thus, such palatability enhancers may contribute
to initial appeal, continued
consumption, or repeated presentation aspects of palatability, or any
combination thereof. Examples of
palatability enhancers include fats (e.g., tallow), flavors, aromas, extracts,
digests, and the like.
[0028] The term "animal digest" means a material that results from chemical
and/or enzymatic
hydrolysis of clean, undecomposed animal tissue. In certain embodiments,
"animal digest" as used
herein, is fully consistent with the definition of animal digest promulgated
by the Association of
American Feed Control Officials, Inc. (AAFCO). Animal digest is preferably
derived from animal
tissues, including cold-blooded marine animals, excluding hair, horns, teeth,
hooves, and feathers. The
skilled artisan will appreciate that while such tissues are not preferred,
trace amounts might be found
unavoidably even under good manufacturing practices. Also not included are
visceral contents or
foreign or fecal matter, although trace contaminant amounts are sometimes
present. When an animal
digest is dried, it may be referred to as "dried animal digest." Animal
digests in accordance herewith are
suitable for use in food or feed compositions. Specifically included are (1)
Digest of Beef (or Poultry,
Pork, Lamb, Fish, etc): material from beef (poultry, pork, etc.) which results
from chemical and/or
enzymatic hydrolysis of clean and undecomposed tissue; (2) Digest of Beef (or
Pork, Lamb, etc) By-
Products: material from beef (poultry, pork, etc.) which results from chemical
and/or enzymatic
hydrolysis of clean and undecomposed tissue from non-rendered clean parts from
cattle (pigs, lambs,
fish, etc), other than meat, for example lungs, spleen, kidneys, brain,
livers, blood, bone, partially-
defatted low-temperature fatty tissue, and stomachs and intestines, freed of
their contents; and (3)
Digest of Poultry By-Products: material which results from chemical and/or
enzymatic hydrolysis of
clean and undecomposed tissue from non-rendered clean parts of carcasses of
slaughtered poultry such
as heads, feet, and viscera. As used herein "poultry" encompasses any species
or kind of bird,
preferably chicken, turkey, duck, or other food species.
[0029] The term "effective amount" means an amount of a compound, material,
composition,
medicament, or other material that is effective to achieve a particular
desired result. Such results
include, but are not limited to, one or more of the following: (a) enhancing
palatability; (b) inducing an
animal to consume more of a particular food or other material than the animal
otherwise would, in
either a single feeding or over the course of multiple feedings; or (c)
inducing an animal to consume a
medicament or a food or dietary supplement that the animal might not otherwise
voluntarily consume.
8
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
[0030] The term "food" or "food composition" means a product or composition
that is intended for
ingestion by an animal, including a human, and provides at least one nutrient
or comestible ingredient
to the animal. The term "food" includes any food, feed, snack, food
supplement, treat, meal substitute,
or meal replacement, whether intended for a human or another animal. "Food"
encompasses such
products in any form, solids, liquids, gels, or mixtures or combinations
thereof Thus, beverages of any
type are clearly encompassed within the term "food." The skilled artisan will
appreciate that the
ingredients or components of a food composition are comestible or edible by an
animal in the normal
course, and such ingredients or components do not include compounds that are
toxic or otherwise
deleterious to health in the amounts used in the food composition.
[0031] The term "pet food" or "pet food composition" or the like, means a
composition intended for
consumption by a non-human animal, preferably by a companion animal.
Nutritionally-balanced pet
food compositions are widely known and used in the art.
[0032] A "nutritionally-complete," "nutritionally-balanced," or "complete and
nutritionally-
balanced" food is one that contains all known required nutrients for the
intended recipient or consumer
of the food, in appropriate amounts and proportions, based, for example, on
recommendations of
recognized or competent authorities in the field of companion animal
nutrition. Such foods are therefore
capable of serving as a sole source of dietary intake to maintain life or
promote production, without the
addition of supplemental nutritional sources. The terms include any food,
feed, snack, food supplement,
treat, meal substitute, or meal replacement, whether intended for a human or
another animal, in any
form, including solids, liquids, gels and the like. Such foods, when intended
for companion animals, are
frequently in the form of extruded pet foods, such as kibble-type foods for
dogs and/or cats.
[0033] The term "dietary supplement' 'means a product that is intended to be
ingested in addition to
the normal animal diet. Dietary supplements may be in any form, e.g., solid,
liquid, gel, tablets,
capsules, powder, and the like. Preferably they are provided in convenient
dosage forms. In some
embodiments, dietary supplements are provided in bulk consumer packages such
as bulk powders,
liquids, gels, or oils. In other embodiments, supplements are provided in bulk
quantities to be included
in other food items such as snacks, treats, supplement bars, beverages, and
the like.
[0034] The term "in conjunction" in certain contexts means that a Maillard
flavor composition, e.g.,
for enhancing palatability of a food composition or the like, and that food
composition or the like
whose palatability is to be enhanced, are administered to an animal (1)
together in a food composition,
or the like (e.g., dietary supplement, or medicament), or (2) separately, at
the same or different
frequency, using the same or different administration routes, at about the
same time, or periodically.
"Periodically" means that the Maillard flavor composition is administered on a
dosage schedule
acceptable for that specific palatability enhancer and that the food, dietary
supplement, or medicament,
is provided to an animal routinely as appropriate for the particular animal.
"About the same time"
generally means that the food, dietary supplement, or medicament, and the
Maillard flavor composition
are administered at the same time or within about 72 hours of each other. "In
conjunction" specifically
9
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
includes administration schemes wherein a palatability enhancer is
administered for a predetermined,
prescribed, or desired period, and the compositions disclosed herein are
administered within a defined
window of time before, during, or after providing the food, dietary
supplement, or medicament whose
palatability is to be enhanced, the window being between from about 0 to about
240 minutes before the
start of, and after the completion of, e.g., the animal's normal feeding time,
supplement time, or
medicament administration time.
[0035] The term "single package" means that the components of a kit are
physically associated, in or
with one or more containers, and considered a unit for manufacture,
distribution, sale, or use.
Containers include, but are not limited to, bags, boxes or cartons, bottles,
packages of any type or
design or material, over-wrap, shrink-wrap, affixed components (e.g., stapled,
adhered, or the like), or
combinations thereof. A single package may be containers of individual
Maillard flavor compositions
and comestible compositions, e.g., food ingredients or food compositions,
physically associated such
that they are considered a unit for manufacture, distribution, sale, or use.
[0036] The term "virtual package" means that the components of a kit are
associated by directions
on one or more physical or virtual kit components instructing the user how to
obtain the other
components, e.g., in a bag or other container containing one component and
directions instructing the
user to go to a website, contact a recorded message or a fax-back service,
view a visual message, or
contact a caregiver or instructor to obtain instructions on how to use the
kit, or safety or technical
information about one or more components of a kit. Examples of information
that can be provided as
part of a virtual kit include instructions for use; safety information such as
material safety data sheets;
poison control information; information on potential adverse reactions;
clinical study results; dietary
information such as food composition or caloric composition; general
information on improving
palatability in the diet, or Maillard reaction products for such us, or
increasing appetite in an animal in
need thereof; health consequences stemming from a decrease in nutrient intake,
or from inadequate
nutrient intake; or general information on nutrition or providing optimal
nutrition; self-help relating to
nutrition and appetite; caregiver information for those caring for animals
with nutritional challenges,
and diseases that result in decreased body weight, wasting, or the like, or
other loss of appetite
challenges; improving acceptance of orally-administered dietary supplements or
medicaments, and use,
benefits, and potential side-effects or counter-indications, if any, for the
compositions described herein,
e.g., palatability enhancers.
[0037] All percentages expressed herein are by weight of the total
composition, including any water
content ("wet weight"), unless indicated otherwise.
[0038] As used throughout, ranges herein are stated in shorthand, so as to
avoid having to set out at
length and describe each and every value within the range. Any appropriate
value within the range can
be selected, where appropriate, as the upper value, lower value, or the
terminus of the range. For
example, a range of 0.1 to 1.0 represents the terminal values of 0.1 and 1.0
and the intermediate values
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
of 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and all intermediate ranges
encompassed within 0.1 to 1.0, such
as 0.2 to 0.5, 0.2 to 0.8, 0.7 to 1.0, and so on.
[0039] As used herein and in the appended claims, the singular form of a word
includes the plural,
and vice versa, unless the context clearly dictates otherwise. Thus, the
references "a", "an", and "the"
are generally inclusive of the plurals of the respective terms. For example,
reference to "a palatability
enhancer", "a method", or "a food" includes a plurality of such "palatability
enhancers", "methods", or
"foods." Reference herein, for example to "an antioxidant" includes a
plurality of such antioxidants,
whereas reference to "pieces" includes a single piece. Similarly, the words
"comprise", "comprises",
and "comprising" are to be interpreted inclusively rather than exclusively.
Likewise the terms "include",
"including" and "or" should all be construed to be inclusive, unless such a
construction is clearly
prohibited from the context. Where used herein the term "examples,"
particularly when followed by a
listing of terms is merely exemplary and illustrative, and should not be
deemed to be exclusive or
comprehensive.
[0040] The methods and compositions and other advances disclosed here are not
limited to
particular methodology, protocols, and reagents described herein because, as
the skilled artisan will
appreciate, they may vary. Further, the terminology used herein is for the
purpose of describing
particular embodiments only, and is not intended to, and does not, limit the
scope of that which is
disclosed or claimed.
[0041] Unless defined otherwise, all technical and scientific terms, terms of
art, and acronyms used
herein have the meanings commonly understood by one of ordinary skill in the
art in the field(s) of the
invention, or in the field(s) where the term is used. Although any
compositions, methods, articles of
manufacture, or other means or materials similar or equivalent to those
described herein can be used in
the practice of the present invention, the preferred compositions, methods,
articles of manufacture, or
other means or materials are described herein.
[0042] All patents, patent applications, publications, technical and/or
scholarly articles, and other
references cited or referred to herein are in their entirety incorporated
herein by reference to the extent
allowed by law. The discussion of those references is intended merely to
summarize the assertions
made therein. No admission is made that any such patents, patent applications,
publications or
references, or any portion thereof, are relevant, material, or prior art. The
right to challenge the
accuracy and pertinence of any assertion of such patents, patent applications,
publications, and other
references as relevant, material, or prior art is specifically reserved.
[0043] The methods and compositions and other advances disclosed here are not
limited to
particular methodology, protocols, and reagents described herein because, as
the skilled artisan will
appreciate, they may vary. Further, the terminology used herein is for the
purpose of describing
particular embodiments only, and is not intended to and does not limit the
scope of that which is
disclosed or claimed.
11
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
The Invention
[0044] In one aspect, the invention provides methods for making Maillard
flavor compositions
suitable for enhancing the palatability of foods, dietary supplements,
medicaments, or other comestible
materials. The methods comprise (a) making a structured lipid phase comprising
a continuous lipid
phase comprising a lipid and a dispersed polar solvent, different from water,
or a mixture of polar
solvents, with at least one polar solvent different from water and which
contains at least a first reactant
having a free carbonyl group, and a second reactant having an amino group
available for reaction with the
free carbonyl on the first reactant; and optionally (b) incubating the
structured lipid phase under conditions
of time and temperature sufficient for a Maillard reaction to occur between
the first and second reactants,
such that at least one Maillard reaction product is formed. The Maillard
flavor compositions comprise one
or more Maillard reaction products, including Maillard flavors, Maillard
colors, and Maillard aromas.
The Maillard reaction products are generally present or produced in structured
lipids, e.g., polar
solvent-in-oil emulsions, a polar solvent-in-oil microemulsions, a liquid
crystalline phase where the
lipid is the continuous phase or a self-assembly structure where the lipid is
the continuous phase.
[0045] The emulsions, microemulsions, the liquid crystalline phase where the
lipid is the
continuous phase and the self-assembly structure where the lipid is the
continuous phase comprise a
structured lipid phase having a continuous lipid phase and a dispersed polar
solvent phase. In certain
case the structure can be bicontinuous; the lipid forms a continuous phase and
the polar solvent form
also a continuous phase. The structure lipids or the emulsion or the
microemulsion contains at least a
first reactant having a free carbonyl group, and a second reactant having an
amino group available for
reaction with the free carbonyl on the first reactant, such that, upon
incubating the structured lipid phase
at a suitable temperature for a suitable time, a Maillard reaction occurs
between the first and second
reactants, and at least one Maillard reaction product is formed. The invention
also provides the Maillard
flavor compositions produced using these methods.
[0046] Without being bound by theory, it appears that the Maillard reaction
that occurs within the
structured lipid occurs within the micelles, dispersed droplets, domains,
and/or channels of the polar
solvent phase. The polar solvent soluble reactants are concentrated in the
polar solvent phase, and
perhaps with the interfacial areas between the continuous and dispersed phases
of the structured lipid
phase, e.g., polar solvent-in-oil emulsions and microemulsions. The first, or
reducing reactant, the second,
or amino reactant, and other Maillard reactants are polar solvent-soluble and
cannot migrate out of the
micelles, polar solvent domains, droplets, and/or channels of the dispersed
polar solvent phase. In prior
Maillard reaction systems, including bulk aqueous reactions and reactions in
emulsions with an aqueous
continuous phase, the hydrophilic reactants (e.g., sugars and amino acids) are
dispersed and not restricted
or concentrated in the micelles, droplets, and/or channels. In the present
invention, the hydrophilic reactants
do not migrate out of polar solvent domains into the oil; they remain
concentrated in the hydrophilic
micelles, droplets, and/or channels. This keeps their concentration relatively
high and therefore increases
the Maillard reaction rate. Further, many reaction products resulting from the
Maillard reaction are
12
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
hydrophobic. In prior systems, the reaction products accumulate and gradually
shift the equilibrium away
from product formation. This decreases the reaction rate or decreases the
extent of conversion of reactant to
product. In the present invention, the hydrophobic Maillard reaction products
migrate out of the micelle
into the continuous lipid phase (e.g., oil). This migration removes the
Maillard reaction products from the
micelles, droplets, and/or channels and shifts the equilibrium of the Maillard
reaction to product formation.
This results in an increase in the reaction rate and ultimately the extent of
the conversion from reactants to
products, i.e., the production of Maillard reaction products and Maillard
compositions. Thus, by conducting
the reaction according to the disclosed methods, the reactants remain
concentrated within the hydrophilic
micelles, droplets, domains, and/or channels while the hydrophobic reaction
products migrate out into the
lipophilic environment of the continuous lipid phase. In WO 00033671, the oil
was not present which
makes this migration less efficient and leads to a lower Maillard reaction
yield.
[0047] The resultant shift in equilibrium increases both the reaction rate and
the amount of Maillard
reactants converted to Maillard reaction products. Surprisingly, and quite
unexpectedly, almost all of the
Maillard reactants are converted to Maillard reaction products. In contrast,
in prior art systems, Maillard
reactants are converted to Maillard reaction products in amounts of less than
50%, typically in the range of
10% to 30%.
[0048] In addition to increasing the amount of Maillard reactants converted to
Maillard reaction
products, the Maillard reaction products produced by the methods of the
invention have a different flavor
profile and different concentrations as compared to control reactions
conducted in water, normal oil-in-
water emulsions, structured oil-in-water emulsions, other bulk aqueous phase
systems, or other reported
Maillard reaction environments. Also, the Maillard reactions products and
compositions obtained herein
are easier to make, more economical to make, easier to store, easier to
maintain, easier to use, and easier to
introduce in products, particularly foods and related compositions.
[0049] In various embodiments, the structured lipid phase comprises from about
0.1% to about
99.7% lipid and from about 0.3% to about 95% polar solvent phase. The skilled
artisan will appreciate
that the structured lipid phase can comprise any relative proportions of lipid
to polar solvent phase
provided that the structured lipid phase can be prepared, e.g., as a polar
solvent-in-oil emulsion or
microemulsion. In preferred embodiments of the structured lipid phase, the
lipid is an oil, afat, an
emulsifier (also called lipophilic additive) or mixture of thereof. In various
embodiments, the structured
lipid phase comprises from about 0.5% to about 99.5% lipid, preferably from
about 1% to about 99.5%
lipid, more preferably from about 5% to about 95% lipid, and from about 0.5%
to about 90% polar
solvent phase, preferably from about 1% to about 85% polar solvent phase, more
preferably from about
1% to about 80% polar solvent phase.
[0050] Oil is used in the broad sense. An oil can be liquid, solid (fat),
crystallized, or amorphous at
room temperature. Possible oils for making the structured lipid are mineral
oils, hydrocarbons,
vegetable oils, animal oils, waxes, alcohols, fatty acids, mono-, di-, tri-
acylglycerols, essential oils,
13
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
flavoring oils, lipophilic vitamins, esters, nutraceuticals, terpins, terpenes
and mixtures thereof Possible
oils for making the structured lipids also comprise oils, such as those
described above, which have been
partially hydrolyzed. These oils may be hydrolyzed by any suitable hydrolysis
procedure, such as
alkaline hydrolysis, steam stripping or enzymatic hydrolysis.
[0051] In one embodiment, the first reactant is a reducing reactant such as an
aldose, ketose, uronic
acid, or Maillard reaction intermediates bearing at least one carbonyl group,
particularly a
monosaccharide, a disaccharide, an oligosaccharide, a polysaccharide, or their
hydrolysis products,
provided that it has at least one reducing group. The saccharide can have any
number of carbon atoms,
and thus may be a triose, a tetrose, a pentose, a hexose, a heptose, and so
on, or any combination
thereof. In preferred embodiments, the first reactant is a reducing sugar.
Preferred reducing sugars for
use herein are glucose, fructose, mannose, maltose, lactose, xylose,
arabinose, or any combination
thereof. Preferred reducing sugars are readily-available reducing sugars that
are food-derived, or
generally regarded as safe (GRAS) ingredients.
[0052] The second reactant is any amino reactant with an available amino group
that can participate
in a Maillard reaction. In preferred embodiments, the second reactant is an
amino acid, peptide,
hydrolyzed protein, polypeptide, or any combination thereof.
[0053] In the method, the step of making the structured lipid phase comprises
mixing the lipid and
the polar solvent, to generate a structured lipid phase wherein the mixing
step is sufficient to form a
polar solvent-in-oil emulsion, a polar solvent-in-oil microemulsion, or other
structured lipid phase.
Mixing as used herein is a very broad term intended to encompass any act of
combining the lipid and
polar solvent into the form of an emulsion or microemulsion. The skilled
artisan has available a large
number of methods, and devices for forming structured lipid phases. Any such
methods or devices
known in the art for forming an emulsion or microemulsion are useful herein.
In some embodiments,
the microemulsion may be a fully- or partially-self-assembling microemulsion.
[0054] The Maillard reactants tend to be polar solvent-soluble. Therefore, the
polar solvent soluble
reactants are dissolved or dispersed within the polar solvent phase before the
mixing step. In one
embodiment, at least the first and second reactants are dissolved in the polar
solvent before the mixing.
In other embodiments, additional polar solvent-soluble compounds are dissolved
in the polar solvent.
Such compounds may include additional Maillard reactants, catalysts, buffers,
compounds for adjusting
pH such as acids, buffers, or salts, emulsifiers, and stabilizers. In various
embodiments, the polar
solvent comprises from about 0.001% to about 50% reducing reactants, about
0.001% to about 50%
amino reactants, and from about 0.001% to about 50% other solutes or
additives.
[0055] The step of making the structured lipid phase generally comprises
adding one or more
emulsifiers before or during the mixing step. The emulsifiers are useful for
emulsifying or stabilizing,
or both, the structured lipid phase. In one presently preferred embodiment,
the emulsifiers have a
hydrophilic lipophilic balance (HLB) of less than about 8, preferably less
than 7.
14
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
[0056] The structured lipid phase comprises from about 0.1% to about 99.6%
emulsifier. The
emulsifier can comprise any one or more emulsifying compounds, and preferably,
the emulsifier is
suitable for use in a food system, or as a food additive, or is GRAS. In
presently preferred embodiments,
the emulsifier is a monoglyceride, a diglyceride, a polyglycerol ester, or a
phospholipid, a lecithin, or
any combination thereof. The emulsifier can encompass a saturated or
unsaturated molecule, such as
mono- or di-glycerides.
[0057] The lipid phase preferably comprises a lipid derived from a plant or
animal that is an edible
or comestible lipid. The lipid comprises beef tallow, lamb tallow, lard,
poultry fat, chicken fat, soy oil,
sunflower oil, palm oil, cotton seed oil, rapeseed oil, coconut oil, corn oil,
canola oil, olive oil, or any
combination thereof in various embodiments. In some embodiments, the lipid
phase comprises lipids
such as those described above that have been partially hydrolyzed. These
lipids may be hydrolyzed by
any suitable hydrolysis procedure, such as alkaline hydrolysis, steam
stripping, or enzymatic hydrolysis.
It will be appreciated that the hydrolyzed lipid phase produced by these
processes is unlikely to be
completely hydrolyzed in that amounts of mono-, di- and/or triglycerides will
be present in the
hydrolyzed lipid phase. If desired, these glycerides may be removed by
conventional separation
techniques, but this is not necessary.
[0058] In certain embodiments, the method comprises a further step of adding
at least a portion of
the structured lipid to at least one comestible ingredient, food composition,
dietary supplement,
medicament, or other material. The adding step is conducted before, during, or
after the incubating step,
or a combination thereof. In one embodiment, the adding step is conducted
before the incubation step,
or before the conclusion of the incubation. In such embodiments, the
incubation step is conducted at
least in part, in conjunction with a further step of processing the comestible
ingredient, food
composition, dietary supplement, or medicament. The skilled artisan will
recognize that in such
embodiments, at least a portion of the Maillard reaction products will be
formed in situ in, e.g., the food.
In other embodiments, the incubation step is conducted, and thus further
Maillard reaction products
form, at least in part, during storage, or during shipment of the comestible
ingredient, food composition,
dietary supplement, or medicament.
[0059] In other embodiments, the adding step is conducted prior to the
incubating step, and
preferably the incubating step is conducted, at least in part, in conjunction
with a thermal process
applied to the food composition, dietary supplement, or medicament. Any type
or kind of thermal
process used the arts of food processing or pharmaceutical processing may be
useful for the methods
herein. Preferred thermal process comprises extrusion, retorting, baking, or
pasteurization.
[0060] In other embodiments, the adding step comprises adding at least one
additional composition
that provides or enhances palatability of the comestible ingredient, food
composition, dietary
supplement, medicament, or other material. The skilled artisan will appreciate
that many compounds
useful for enhancing palatability are known in the art, and all are suitable
for use herein. Exemplary
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
compounds include flavors, aromas, and the like, as well as fats or oils,
sweeteners, salt, and the like. In
one embodiment, the additional palatability enhancer is an animal digest.
[0061] As discussed above, the method can be conducted in a variety of manners
to produce the
structure lipid phase. In one embodiment, the making step comprises dissolving
at least the first and
second reactants in the polar solvent; mixing the polar solvent with one or
more lipids and one or more
emulsifiers; and forming a polar solvent-in-oil emulsion or microemulsion.
Energy input in the form of
mixing, agitating, emulsifying, blending, micronizing, and the like is
preferably used in the making step.
[0062] The incubating step comprises allowing the reactants to interact at any
temperature
conductive for conducting a Maillard reaction, e.g., room temperature or lower
depending on the
reactants. In preferred embodiments, incubating step comprises heating to a
temperature of from about
60 C to about 180 C. In various embodiments, the temperatures for incubating
or heating are from
about 80 C to 150 C, or preferably, the temperatures are from about 90 C to
120 C. The time for the
incubating step is from about 1 minute to about 12 hours. Preferably, the
incubation time is from about
1 minute to about 640 minutes. Other preferred times for incubation are from
about 5 minutes to about
300 minutes, preferably from about 10 minutes to about 180 minutes. For both
temperature and time,
the only firm requirements are that the time and temperature combination are
sufficient for a Maillard
reaction to occur within the polar solvent-in-oil system. In some systems, the
Maillard reaction occurs
during a retorting process. Because of the effective concentration of
reactants within micelles, droplets,
domains, and channels, and potentially at the interfaces between the
continuous and dispersed phases,
the required times and temperature may differ substantially from those
required in bulk aqueous
Maillard reactions, or even other complex food systems. Accordingly, the time
and temperature for the
nonenzymic reactions can be readily determined by observing or measuring an
increase in reaction
product(s) or a decrease in reactants. Incubation temperatures can be obtained
using any suitable
heating method such as microwave heating or can be obtained in any suitable
process such as baking or
retorting.
[0063] In some embodiments, the polar solvent further comprises one or more of
a catalyst suitable
for enhancing the rate of Maillard reactions, or a compound for adjusting the
pH of the polar solvent.
The catalyst preferably comprises a compound having a phosphate or a
carboxylate group, or other
known Maillard reaction catalyst or enhancer.
[0064] In various embodiments, the structured lipid phase comprises more than
0.3% polar solvent,
more than 0.1% lipid. Preferably, the structured lipid phase comprises from
about 0.5% to about 90%
polar solvent and from about 10% to about 99.5% lipid. More preferably, the
structured lipid phase
comprises from about 0.5% to about 69% polar solvent, and from about 31% to
about 99.5% lipid.
Even more preferably, the structured lipid phase comprises from about 0.5% to
about 25% polar solvent,
and from about 25% to about 99.5% lipid. As above, the HLB of the emulsifier
is preferably less than
about 8, preferably less than about 7.
16
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
[0065] The skilled artisan will appreciate the emulsions and microemulsions
feature micelles,
droplets, domain, channels of varied size and varied average size as defined
herein.
[0066] The methods for making the Maillard flavor compositions have proven to
provide enhanced
conversions of Maillard reactants into Maillard reaction products, including
Maillard flavors and
Maillard colors. In one embodiment, the methods provide a conversion of
Maillard reactants into
Maillard reaction products in the structured lipid phase that exceeds the
conversion of Maillard
reactants into Maillard reaction products in a control Maillard reaction
conducted under the same
conditions with the same reactants in an aqueous system, or in a structured
lipid phase containing only
water as a polar solvent or in pure polar solvent phase. In one embodiment,
the conversion of Maillard
reactants is at least 10% greater than the conversion in the control reaction
resulting in an enhanced
formation of Maillard reaction products, particularly in some key compounds
like furfuryl thiol (FFT)
or methyl furyl thiol (MFT). In another, the conversion of Maillard reactants
is at least 50% higher than
in the control reaction. In yet other embodiments, the reaction is nearly
complete, providing a
conversion of reactants of at least 80, 85, 90, 95%, or more.
[0067] In another aspect, the invention provides products made using the
methods of the invention.
[0068] In another aspect, the invention provides a Maillard flavor composition
comprising a
structured lipid phase, a polar solvent, different from water, and at least
one Maillard reaction product.
[0069] . The structure lipid phase comprises any amounts or proportions of
lipid, emulsifier, and
polar solvent that can form a polar solvent-in-oil emulsion or microemulsion.
Preferably, the structured
lipid phase comprises from about 0.3% to about 95% polar solvent and from
about 5% to about 99.7%
lipid plus emulsifier. More preferably, the structured lipid phase comprises
from about 0.5% to about
75% polar solvent, most preferably from about 0.5 to about 25%. Preferably,
the emulsifier has a HLB
less than 8 and the lipid comprises a comestible oil or fat. The Maillard
reaction product is produced
within and is within the structured lipid phase.
[0070] The Maillard flavor compositions are produced by the methods of the
invention. In one
embodiment the Maillard flavor composition is produced by a method comprising
(a) making a
structured lipid phase comprising a continuous lipid phase comprising a lipid,
and a dispersed phase
comprising a polar solvent, wherein the polar solvent phase contains at least
a first reactant the reactant
having a free carbonyl group, and a second reactant having an amino group
available for reaction with
the free carbonyl on the first reactant; and (b) incubating the structured
lipid phase under conditions of
time and temperature sufficient for a Maillard reaction to occur between the
first and second reactants,
such that at least one Maillard reaction product is formed. In an other
embodiement, the Maillard flavor
composition is produced by a method comprising making a structured lipid phase
comprising a
continuous lipid phase comprising a lipid, and a dispersed phase comprising a
polar solvent, wherein
the polar solvent phase contains at least a first reactant the reactant having
a free carbonyl group, and a
second reactant having an amino group available for reaction with the free
carbonyl on the first reactant.
In this last embodiment, the Maillard reaction may be added to a product. The
Maillard reaction may
17
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
occur at a further stage. This further stage may include thermal processing,
extrusion, retorting, home
preparation such as cooking, frying, heating, pan heating, oven heating, oven
baking, microwave
heating, steam heating of the product.
[0071] In one preferred embodiment, the structured lipid phase is a
microemulsion. The
microemulsion can exist at suitable temperature. Preferably, microemulsion has
a temperature lower
than 50 C, more preferably lower than 40 C. The emulsifier comprises a
saturated or unsaturated
monoglyceride in certain embodiments. The composition can further comprise at
least one catalyst of a
Maillard reaction, at least one additional palatability enhancer, or both.
[0072] In another aspect, the invention provides comestible compositions
comprising at least one
comestible ingredient and at least one Maillard flavor composition. In
preferred embodiments, the
comestible composition comprises from about 0.001% to about 50% Maillard
flavor composition.
Preferably, the comestible composition is a food, dietary supplement,
medicament, or other comestible
material, most preferably a food composition.
[0073] In other embodiments, the comestible composition further comprises at
least one additional
palatability enhancer such as an animal digest. Preferably, the comestible
composition with the added
Maillard flavor composition has measurably enhanced palatability compared to a
control comestible
composition that does not contain the Maillard flavor composition. In some
embodiments, the
comestible composition is preferred by at least a factor of 10% more than the
control comestible
composition. It other embodiments, an improvement of 20, 30, 40, or 50% is
observed. In other
embodiments, the comestible composition is preferred up to 2:1, 3:1 or more
over the control
comestible composition. In one embodiment, the comestible composition is a
food composition. In
another, the food composition is formulated as an animal food such as a pet
food or companion animal
food.
[0074] In another aspect, the invention provides methods for enhancing
palatability of a comestible
composition. The methods comprise adding to a comestible composition at least
one Maillard flavor
composition in an amount effective for enhancing palatability of the
comestible composition compared
to a control that does not have the Maillard flavor composition added. The
amount of Maillard flavor
composition added is preferably from about 0.001% to about 50% of the
comestible composition. The
invention also provides the comestible compositions produced using these
methods.
[0075] In another aspect, the invention provides food compositions comprising
at least one
comestible ingredient and a polar solvent-in-oil emulsion, microemulsion, or
another reversed
structured phase comprising a continuous lipid phase comprising a comestible
fat or oil and a dispersed
polar solvent phase. The polar solvent has dissolved therein at least a
comestible reducing reactant
having a free carbonyl, and a comestible second reactant containing an amino
group, and an emulsifier
having an HLB less than 8. The reducing reactant and the second reactant can
undergo a Maillard
reaction to form at least one Maillard reaction product under suitable
conditions. Preferably, the
emulsion or microemulsion comprises from about 0.3% to about 95% polar solvent
and from about 5%
18
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
to about 99.7% lipid plus emulsifier. More preferably, the structured lipid
phase comprises from about
0.5% to about 75% polar solvent, most preferably from about 0.5 to about 25%.
Preferred emulsifier
include saturated and unsaturated monoglycerides.
[0076] In one embodiment, the food composition has been subjected to a thermal
processing step or
storage conditions under which at least one Maillard reaction product is
formed from the reducing
reactant and the second reactant. Any thermal processing step above ambient
temperature at which a
Maillard reaction product can form is useful herein. The food composition is a
pet food composition in
one embodiment. In presently preferred embodiments, the composition comprises
at least one
additional palatability enhancer.
[0077] In another aspect, the invention provides comestible compositions
comprising (1) one or
more comestible ingredients and (2) one or more structured lipids comprising a
continuous lipid phase
comprising a lipid and a dispersed polar solvent which at least a first
reactant having a free carbonyl
group, and a second reactant having an amino group available for reaction with
the free carbonyl on the
first reactant.
[0078] The comestible ingredients are any comestible ingredients compatible
with the structured
lipids. Preferably, the comestible ingredients are ones that require or are
made more palatable by
heating, e.g., by warming or by cooking.
[0079] The comestible compositions are made by combining one or more
comestible ingredients
with one or more structured lipids. The compositions can be stored or
otherwise retained until needed,
e.g., for consumption or for further preparation and subsequent consumption.
[0080] These compositions can be consumed as made but are preferably heated
before consumption.
When consumed as made, the first and second reactants react to produce
Maillard reaction products that
increase the palatability of the comestible compositions. Although the
reaction occurs, it is generally slower
than optimal. When heated, the compositions are heated to temperatures useful
to prepare the comestible
ingredients for consumption, generally by cooking or otherwise heating the
compositions. Upon heating,
the first and second reactants react to produce one or more Maillard reaction
products. Heating
facilitates the reaction process and produces more Maillard reaction products
than would not have been
produced without heating. Such Maillard reaction products increase the
palatability of the comestible
compositions, particularly when produced in amounts made by heating.
[0081] Any temperature suitable for preparing the comestible compositions and
for causing a
Maillard reaction is suitable. Typically, the compositions are heated to
temperatures of from about 60 C
to about 400 C, or from about 60 C to 350 C, or from about 60 C to 300 C or
from about 60 C to
250 C, or from about 60 C to 233 C, or from about 60 C to 220 C, or from about
70 C to 180 C, or
from about 80 C to 120 C, or from about 80 C to 100 C. Heating the
compositions containing the
structured lipids causes the first and second reactants react and form
Maillard reaction products that
increase the palatability of the compositions. The comestible compositions can
be heated by any
19
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
suitable means. Typically, the compositions are baked or cooked in an oven;
heated on a stove or by a
fire, e.g., in a pan, pot, or other suitable container; steam heated; or
heated using a microwave oven.
[0082] The first and second reactants can be any such reactant compatible with
the comestible
ingredients in the composition. In various embodiments, the first and second
reactants are (1) one or
more reducing sugars and one or more amino acids or (2) one or more reducing
sugars and one or more
proteins.
[0083] In preferred embodiments, the structured lipids are mixed with the
comestible ingredients,
topically applied to the comestible ingredients, added onto or into preferred
locations or sections in or
on the ingredients, or otherwise distributed evenly or unevenly in or on the
ingredients.
[0084] In one embodiment, food compositions that will be heated for serving,
e.g., a product to be
baked, comprise the product ingredients and one or more one or more structured
lipids. The product is
placed in an oven and heated to a temperature suitable for baking the product.
As the product bakes, the
heat induces a reaction involving the first and second reactants. The reaction
produces Maillard reaction
products that enhance the palatability of the comestible composition.
[0085] In preferred embodiments, the comestible compositions are food
compositions suitable for
consumption by an animal, more preferably food compositions suitable for
consumption by a
companion animal, most preferably food compositions suitable for consumption
by pets. In an
embodiment, the comestible composition is a pet food suitable for warming in a
microwave oven. The
pet food is heated sufficiently to produce Maillard reaction products in the
food and served to the pet.
[0086] In another aspect, the invention provides compositions made by heating
comestible
compositions comprising (1) one or more comestible ingredients and (2) one or
more structured lipids
comprising a continuous lipid phase comprising a lipid and a dispersed polar
solvent which contains at
least a first reactant having a free carbonyl group, and a second reactant
having an amino group available
for reaction with the free carbonyl on the first reactant. The compositions
have an enhanced palatability
due to the presence of Maillard reaction products resulting from heating the
compositions as described
herein.
[0087] In another aspect, the invention provides kits suitable for enhancing
palatability of a
comestible composition. The kits comprise in separate containers in a single
package or in separate
containers in a virtual package, as appropriate for the kit component, one or
more Maillard flavor
composition and one or more of (1) one or more ingredients suitable for
consumption by an animal, (2)
one or more palatability enhancers, (3) instructions for combining kit
components to produce a
composition useful for enhancing palatability of a food composition, (4)
instructions for using Maillard
reaction products, Maillard flavor compositions, or other components of the
kit for the benefit of the
animal, (5) a vessel for preparing or combining the kit components to produce
a composition for
administration to an animal, such as bowl, container, bag, or the like, (6) a
means for admixing one or
more kit components, such as a spoon, a spatula, or other suitable utensil, or
(7) a means for
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
administering combined or prepared kit components to an animal, such as a
bowl, a spoon, a bottle, a
cup, or the like.
[0088] In one embodiment, the Maillard flavor composition comprises at least
one Maillard reaction
product and a structured lipid phase comprising, for example, at least 0.1%
polar solvent, and at least
50% lipid plus emulsifier. Preferably, the emulsifier has a HLB less than 8,
and the lipid is a comestible
oil or fat. In preferred embodiments, the Maillard reaction product is
produced within the structured
lipid phase.
[0089] Other kits provided herein include kits suitable for enhancing
palatability of a food
composition comprising, in separate containers in a single package, or in
separate containers in a virtual
package, a polar solvent-in-oil emulsion or microemulsion comprising a
continuous lipid phase
comprising a comestible fat or oil and a dispersed polar solvent which
contains at least a comestible
reducing reactant having a free carbonyl, and a comestible second reactant
containing an amino group,
and an emulsifier. Preferably, the emulsifier has an HLB less than 8. The
reducing reactant and the
second reactant can preferably undergo a Maillard reaction to form at least
one Maillard reaction
product under suitable conditions. The emulsion or microemulsion, in preferred
embodiments,
comprises from about 0.5% to about 25% polar solvent, and from about 75% to
about 99.5% lipid plus
emulsifier. The kits further comprise one or more of (1) one or more
ingredients suitable for
consumption by an animal, (2) one or more palatability enhancers, (3)
instructions for combining kit
components to produce a composition useful for enhancing palatability of a
food composition, (4)
instructions on applying a thermal processing step to combined or uncombined
kit components to
produce one or more Maillard reaction products (5) instructions for using
Maillard reaction products,
Maillard flavor compositions, and other components of the kit for the benefit
of the animal, (6) a vessel
for preparing or combining the kit components to produce a composition for
administration to an
animal, such as a bowl, container, bag, box or the like, (7) a means for
admixing one or more kit
components, such as a spoon, spatula, or other utensil, or (8) a means for
administering combined or
prepared kit components to an animal, such as a plate, bowl, spoon, bottle,
glass, or the like.
[0090] In a further aspect, the invention provides means for communicating
information about, or
instruction for use of, a Maillard flavor composition comprising at least one
Maillard reaction product
and a structured lipid phase comprising at least 0.1% polar solvent, and at
least 50% lipid plus
emulsifier; wherein the emulsifier has a HLB less than 8, the lipid comprising
a comestible oil or fat,
wherein the Maillard reaction product is produced within the structured lipid
phase, wherein the
information is about, or the instructions are for, one or more of. (1)
instructions for administering the
composition to an animal in conjunction with at least one comestible
ingredient; (2) instructions for one
or more methods of using the composition for enhancing palatability of a food
composition; (3)
information on providing proper nutrition, including the use of the
composition, to an animal in need of
foods having enhanced palatability, or an animal having a diminished appetite
due to a disease or other
health condition; (4) information about palatability, and appetite; (5)
information regarding physical,
21
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
cellular and biochemical results of under-nutrition, conditions causing loss
of appetite, or wasting
diseases, or recovery from, or prevention or treatment of the same, or (6)
comparative information or
test results regarding the composition, or regarding the palatability of food
compositions to which it is
added.
[0091] In various embodiments, the means of communicating comprises a physical
or electronic
document, digital storage media, optical storage media, audio presentation,
audiovisual display, or
visual display containing the information or instructions. The means can be a
displayed web site, visual
display kiosk, brochure, product label, package insert, advertisement,
handout, public announcement,
audiotape, videotape, DVD, CD-ROM, computer readable chip, computer readable
card, computer
readable disk, USB device, FireWire device, computer memory, or any
combination thereof.
[0092] In another aspect, the invention provides packages comprising a
Maillard flavor composition
generally comprising at least one Maillard reaction product and a structured
lipid phase comprising at
least 0.1% polar solvent, and at least 50% lipid plus emulsifier; wherein the
emulsifier has a HLB less
than 8, the lipid comprising a comestible oil or fat, wherein the Maillard
reaction product is produced
within the structured lipid phase. The package contains a word or words,
picture, design, logo, graphic,
symbol, acronym, slogan, phrase, or other device, or combination thereof,
either directly on the package
or on a label affixed thereto, indicating that the composition is useful for
enhancing palatability of a
food composition. In one embodiment, the Maillard flavor composition in the
package is a component
of a comestible composition. In another, the Maillard flavor composition in
the package is a component
of a food composition.
EXAMPLES
[0093] The invention can be further illustrated by the following examples,
although it will be
understood that the examples are included merely for purposes of illustration
and is not intended to
limit the scope of the invention unless otherwise specifically indicated.
Materials and Methods
[0094] The following method ("Method 1") was used to prepare the compositions
used in some of
the Examples. Reducing sugars, amino acids, catalysts (where used), and acids
or bases (where used)
were added to glycerol and agitated until dissolved, resulting in a glycerol
solution. The glycerol
solution was mixed with fat or oil and lipophilic additives (emulsifiers). The
resulting mixture was
agitated at 500 to 3000 rpm, for 1 to 5 minutes, to generate a glycerol-in-oil
emulsion comprising a
continuous structured lipid phase, having dispersed glycerol phase featuring
glycerol domains that are
emulsified or microemulsified within the lipidic phase. Such glycerol-in-oil
emulsions are referred to
herein as "structured lipid phase."
[0095] To promote the Maillard reaction, the structured lipid phase was heated
to about 85 C to
180 C for 5 to 180 minutes. Agitation was continued during heating. The
temperature was then lowered
to about 45 C to 60 C, with agitation to ensure homogenous cooling.
22
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
[0096] Method 1 produces a flavor composition (a "Maillard flavor
composition") containing
Maillard reaction products, e.g., Maillard flavors. The Maillard flavor
composition is stored at 10 C to
60 C until use.
[0097] When preparing a food composition using a Maillard flavor compositions
prepared according
to Method 1, the Maillard flavor composition can be conveniently added to a
fat or oil that is sprayed
onto, or added to the food composition in amounts of from about 0.001% to
about 9%, by weight, based
on total food composition. When used with other flavors, the other flavors,
including flavors prepared
by using hydrolytic enzymes to clean animal tissue, including liver and/or
viscera, e.g., animal digests,
can be added to or applied to the food composition.
[0098] The following method ("Method 2") was used to prepare the compositions
used in some of
the Examples. The steps of Method 1 were repeated except that a mixture of
glycerol and water was
used. Maillard flavor compositions prepared according to Method 2 can be
conveniently added to a fat
or oil that is sprayed onto, or added to the food composition in amounts of
from about 0.00 1% to about
9% by weight based on total food composition. When used with other flavors,
the other flavors,
including flavors prepared by using hydrolytic enzymes to clean animal tissue,
including liver and/or
viscera, e.g., animal digests, can be added to or applied onto the food
composition.
Example 1
[0099] A first Maillard flavor composition was made according to the invention
using Method 1.
Glucose, cysteine and thiamine (Vitamin B 1) were used as reactants for the
Maillard reaction. A second
Maillard flavor composition was made by mixing glucose, cysteine and thiamine
in glycerol to produce
a glycerol solution. A third Maillard flavor composition was made by mixing
glucose, cysteine and
thiamine in glycerol and mixing this glycerol solution to fat or oil without
lipophilic additives to
produce a biphasic glycerol / fat or oil solution. A fourth Maillard flavor
composition was made using
the steps of Method 1 except that water was used instead of glycerol. Glucose,
cysteine and thiamine
were also used as reactants. A fifth Maillard flavor composition was made by
mixing glucose, cysteine
and thiamine in water to produce an aqueous solution. Each compositions
contained 1.5% glucose,
0.9% cysteine and 0.7% thiamine by weight. The detailed composition of these
five Maillard flavor
compositions are given in Table 1.
Table 1
Maillard 1st 2nd 3rd 4th 5th
flavor composition composition composition composition composition
compositions (% by weight) (% by weight) (% by weight) (% by (% by weight)
according to for for weight) for for comparison
the invention comparison comparison comparison
Glucose 1.5 1.5 1.5 1.5 1.5
Cysteine 0.9 0.9 0.9 0.9 0.9
Thiamine 0.7 0.7 0.7 0.7 0.7
Water - - - 6.9 96.9
23
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
Glycerol 6.9 96.9 6.9 - -
Dimodan 54.0 - - 54 -
U/J
Sunflower 36.0 - 90 36 -
oil
TOTAL 100 100 100 100 100
[00100] All five compositions were heated to the same temperature and for the
same time, i.e., at
90 C for 40 minutes, to cause a Maillard reaction using glucose. After 40
minutes, the samples were
tested for residual glucose and for their color (visual inspection) as a
measure of the extent of the
Maillard reaction. The results are given in Table 2.
Table 2
Maillard flavor composition Residual glucose Color
(in % of initial
amount)
1st composition, according to the 10.2 brown
invention
2nd composition, for comparison 31.1 orange
3rd composition, for comparison 48.2 orange
4th composition, for comparison 60.9 light yellow
5th composition, for comparison 99.5 uncolored
[00101] These results surprisingly show a strong increase of Maillard reactant
(sugar) conversion into
Maillard reaction products (89.8% of glucose degraded) in the first
composition as compared to the
other compositions. The substantial improvement in the sugar conversion was
unexpected. The present
invention produces Maillard flavor compositions containing substantially more
Maillard reaction
products, and thus, more flavor, for a given amount of reactants.
Example 2
[00102] A structured lipid phase was prepared using Method 2 and using the
components shown in
Table 3. Reducing sugars and amino acids were added to glycerol and agitated
until dissolved, resulting
in a glycerol solution. The glycerol solution was mixed with fat or oil and
lipophilic additives. The
resulting mixture was agitated at 500 to 3000 rpm, for 1 to 5 minutes, to
generate a glycerol-in-oil
emulsion according to the invention, referred to herein as "structured lipid
phase."
Table 3
Ingredients % in Formula
Glucose 0.60
24
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
Rhamnose 0.02
Fructose 0.72
Cysteine 0.36
Proline 2.40
Glycerol 5.90
Rapeseed oil 30.00
Unsaturated 60.00
Total 100.00
[00103] The structured lipid phase was coated externally on a chilled bread
dough (at 1.0% based on
the weight of the food product). The coated bread dough (test food product)
was then stored overnight
at +4 C.
[00104] A control sample was prepared as follows: glucose (6.0%), rhamnose
(0.2%), fructose
(7.2%), cysteine (3.6%) and proline (24.0%) were added to glycerol (59.0%) and
agitated until
dissolved, resulting in a glycerol solution. This glycerol solution was then
coated externally on a chilled
bread dough (at 0.1 % based on the weight of the food product, to ensure
similar reducing sugars and
amino acids levels between the control and test food products). The coated
bread dough (control food
product) was then stored overnight at +4 C.
[00105] For sensory evaluation, the test food product and the control food
product were heated in a
microwave oven (1 min 30 s, 750 W). The aroma perceived in the room during
microwave heating and
the flavor of the microwave heated food products were evaluated by a selected
panel. The panel found
the aroma and flavor from the control food product to be almost
indistinguishable from those of a non-
coated chilled bread dough (yeast-leavened bread aroma/flavor), whereas the
test food product gave a
rich, freshly baked bread aroma/flavor impression.
Example 3
[00106] A structured lipid phase was prepared using Method 2 and using the
components shown in
Table 4. Reducing sugars and amino acids were added to glycerol and water and
agitated until dissolved,
resulting in a wet glycerol solution. The wet glycerol solution was mixed with
fat or oil and lipophilic
additives. The resulting mixture was agitated at 500 to 3000 rpm, for 1 to 5
minutes, to generate a wet
glycerol-in-oil emulsion according to the invention, referred to herein as
"structured lipid phase."
Table 4
Ingredients % in Formula
Glucose 0.95
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
Rhamnose 0.25
Proline 3.80
Water 6.00
Glycerol 9.00
Palm olein 32.00
Unsaturated 48.00
Total 100.00
[00107] The structured lipid phase was coated externally on a chilled bread
dough (at 1.5% based on
the weight of the food product). The coated bread dough (test food product)
was then stored overnight
at +4 C.
[00108] A control sample was prepared as follows: glucose (4.75%), rhamnose
(1.25%) and proline
(19.0%) were added to water (30.0%) and glycerol (45.0%) and agitated until
dissolved, resulting in a
glycerol solution. This glycerol solution was then coated externally on a
chilled bread dough (at 0.3%
based on the weight of the food product, to ensure similar reducing sugars and
amino acids levels
between the control and test food products). The coated bread dough (control
food product) was then
stored overnight at +4 C.
[00109] For sensory evaluation, the test food product and the control food
product were heated in a
microwave oven (1 min 30 s, 750 W). The aroma perceived in the room during
microwave heating and
the flavor of the microwave heated food products were evaluated by a selected
panel. The panel found
the aroma and flavor from the control food product to be almost
indistinguishable from those of a non-
coated chilled bread dough (yeast-leavened bread aroma/flavor), whereas the
test food product gave a
rich, freshly baked bread aroma/flavor impression.
Example 4
[00110] A structured lipid phase was prepared using Method 2 and using the
components shown in
Table 5. Reducing sugars and amino acids were added to glycerol and water and
agitated until dissolved,
resulting in a wet glycerol solution. The wet glycerol solution was mixed with
fat or oil and lipophilic
additives. The resulting mixture was agitated at 500 to 3000 rpm, for 1 to 5
minutes, to generate a wet
glycerol-in-oil emulsion according to the invention, referred to herein as
"structured lipid phase."
Table 5
Ingredients % in Formula
Glucose 2.20
Rhamnose 2.00
26
CA 02784520 2012-06-14
WO 2011/073035 PCT/EP2010/068688
Cysteine 2.10
Proline 13.95
Water 12.15
Glycerol 37.10
Palm olein 28.00
PGPR 90 2.00
Carrageenan 0.50
Total 100.00
[00111] The structured lipid phase was coated externally on a chilled bread
dough (at 1.0% based on
the weight of the food product). The coated bread dough (test food product)
was then stored overnight
at +4 C.
[00112] A control sample was prepared as follows: glucose (3.2%), rhamnose
(2.9%), cysteine
(3.0%) and proline (20.1%) were added to water (17.5%) and glycerol (53.3%)
and agitated until
dissolved, resulting in a wet glycerol solution. This wet glycerol solution
was then coated externally on
a chilled bread dough (at 0.7% based on the weight of the food product, to
ensure similar reducing
sugars and amino acids levels between the control and test food products). The
coated bread dough
(control food product) was then stored overnight at +4 C.
[00113] For sensory evaluation, the test food product and the control food
product were heated in a
microwave oven (1 min 30 s, 750 W). The aroma perceived in the room during
microwave heating and
the flavor of the microwave heated food products were evaluated by a selected
panel. The panel found
the aroma and flavor from the control food product to be almost
indistinguishable from those of a non-
coated chilled bread dough (yeast-leavened bread aroma/flavor), whereas the
test food product gave a
rich, freshly baked bread aroma/flavor impression.
27