Note: Descriptions are shown in the official language in which they were submitted.
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 HUMANIZED FcyR MICE
2
3 FIELD OF INVENTION
4 [0001] The field of invention is genetically modified non-human
animals that lack
endogenous murine FcyR genes, including genetically modified animals that
comprise a
6 replacement of endogenous FcyR genes with human FcyR genes, and including
mice that are
7 capable of expressing at least two, three, four, or five functional human
low affinity FcyR genes,
8 and including genetically modified mice comprising immune cells that do
not express
9 endogenous low affinity FcyR genes.
11 BACKGROUND
12 [0002] Fc receptors (FcRs) are proteins found on the surface of
cells of the immune system
13 that carry out a variety of functions of the immune system in mammals.
FcRs exist in a variety
14 of types, on a variety of cells, and mediate a variety of immune
functions such as, for example,
binding to antibodies that are attached to infected cells or invading
pathogens, stimulating
16 phagocytic or cytotoxic cells to destroy microbes, or infected cells by
antibody-mediated
17 phagocytosis or antibody-dependent cell-mediated cytotoxicity (ADCC).
18 [0003] ADCC is a process whereby effector cells of the immune
system lyse a target cell
19 bound by antibodies. This process depends on prior exposure to a foreign
antigen or cell,
resulting in an antibody response. ADCC can be mediated through effector cells
such as, for
21 example, natural killer (NK) cells, by binding of FcR expressed on the
surface of the effector cell
22 to the Fc portion of the antibody which itself is bound to the foreign
antigen or cell. Because of
23 the central role that FcRs play in the immune response, useful non-human
animals that co-
24 express multiple human FcRs are needed, including non-human animals that
co-express
multiple human low affinity FcRs. There exists a need for non-human animal
models of human
26 FcR function and human processes of ADCC for the study and elucidation
of human disease
27 therapies, in particular anti-tumor therapies and therapies for treating
autoimmune diseases,
28 and pharmaceutical drug development, in particular in the development,
design, and testing of
29 human antibody pharmaceuticals.
1
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 BRIEF DESCRIPTION OF THE FIGURES
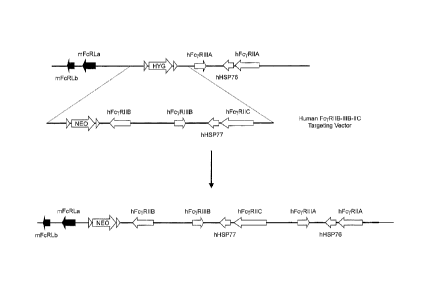
2 [0004] Figure 1 is a schematic depiction of a wild type low
affinity FcyR locus in a mouse,
3 showing mouse FcyRIIB, FcyRIV and Fc7R111 genes and a mouse FcyR
targeting vector used for
4 a targeted deletion of these genes, which includes a neomycin cassette
flanked by site-specific
recombination sites.
6 [0005] Figure 2 shows histograms of splenocytes gated for B cells
(anti-CD19), NK cells
7 (anti-NKp46) and macrophages (anti-F4/80) including expression of
endogenous mFcyRII and
8 mFcyRIII genes for wild type and low affinity FcyR cc-chain gene-
deficient mice (mFcyR KO).
9 [0006] Figures 3A-3D show in vivo depletion of B cells with a
human anti-human CD20
antibody with mouse Fc (Ab 168) or human Fc (Ab 735) in humanized CD20 mice
(hCD20) and
11 humanized CD20 mice bred to FcyR knockout mice (hCD20/FcyR KO) in
several lymphocyte
12 compartments: bone marrow (Figure 3A), blood (Figure 3B), lymph node
(Figure 3C) and spleen
13 (Figure 3D). For each graph, the y-axis shows the percent of gated B
cells (B220-11gM+ or
14 B220+/CD19+) and the x-axis shows the antibody dose for each animal
group: 10 mg/kg Control
antibody (C), 2 mg/kg human anti-human CD20 antibody (2 Ab) and 10 mg/kg human
anti-
16 human CD20 antibody (10 Ab).
17 [0007] Figure 4 is a schematic depiction of a neomycin-targeted
deletion of the low-affinity
18 mouse FcyR locus and a second targeting vector for inserting two human
low affinity FcyR
19 genes (hFcyRIIIA and hFcyRIIA) into the deleted mouse locus, which
includes a hygromycin
cassette flanked by site-specific recombination sites. For expression of
hFcyRIIA on platelets,
21 an extended promoter region operably linked to the hFcyRIIA gene of the
Human FcyRIIIA-IIA
22 Targeting Vector is employed; to prevent expression of hFcyRIIA on
platelets, the promoter
23 region is omitted or substantially omitted.
24 [0008] Figure 5A shows histograms of splenocytes gated for NK
cells (anti-NKp46) and
macrophages (anti-F4/80) including expression of human FcyRIIIA for wild type
and human
26 FcyRIIIA-IIA homozygote mice (Human FcyRIIIA/FcyRIIA HO).
27 [0009] Figure 5B shows histograms of splenocytes gated for
neutrophils (anti-Ly6G) and
28 macrophages (anti-F4/80) including expression of human FcyRIIA for wild
type and human
29 FcyRIIIA-IIA homozygote mice (Human FcyRIIIA/FcyRIIA HO).
[0010] Figure 6 is a schematic depiction of a hygromycin-targeted deletion
of the low affinity
31 mouse FcyR locus including an insertion of two low affinity human FoiR
genes (hFcyRIIIA and
32 hFcyRIIA) and a third targeting vector for inserting three additional
low affinity human FcyR
2
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 genes (hFcyRIIB, hFcyRIIIB and hFc7RIIC) and a neomycin cassette flanked
by site-specific
2 recombination sites.
3 [0011] Figure 7 shows histograms of splenocytes gated for B cells
(anti-CD19) and
4 neutrophils (anti-Ly6G) including expression of human Fc7RIIB and human
Fc7RIIIB for wild
type and human Fc7RIIIA-11113-11A-11B-11C homozygote mice (Human
6 FcyRIIIA/Fc7R111B/Fc7R11A/Fc7RIIB/Fc7RIIC HO).
7
8 SUMMARY
9 [0012] Genetically modified cells, non-human embryos, non-human
animals and methods
and compositions for making and using them are provided. In various aspects,
the non-human
11 animals comprise a human Fc7R receptor, a deletion of an endogenous low
affinity FcyR
12 receptor, and/or a replacement of an endogenous FcyR receptor with a
human Fc7R receptor at
13 an endogenous mouse low affinity Fc7R locus.
14 [0013] In one aspect, genetically modified cells, non-human
embryos, and non-human
animals are provided that comprise a functional FcR 7¨chain, wherein the
cells, embryos, and
16 animals comprise a further modification comprising a replacement of the
low affinity
17 endogenous non-human FcyR gene sequences (e.g., Fc7RIIB, Fc7RIV and
Fc7RIII) with one or
18 more low affinity human Fc7R gene sequences (e.g., selected from
Fc7RIIA, Fc7RIIB, Fc7RIIC,
19 Fc7RIIIA, Fc7RIIIB, and a combination thereof).
[0014] In one embodiment, the cells, non-human embryos, and non-human
animals are
21 murine. In one embodiment, the functional FcR 7-chain is a mouse FcR 7-
chain. In one
22 embodiment, the mouse FcR 7-chain is an FcR 7-chain endogenous to the
mouse, the cell, or
23 the embryo.
24 [0015] In one embodiment, the cells, embryos, and animals are
mice, and the mice express
a functional a-chain of a human low affinity Fc7R receptor and a functional
endogenous mouse
26 7-chain.
27 [0016] In one aspect, a genetically modified mouse is provided,
wherein the mouse does
28 not express an endogenous a-chain selected from an Fc7RIIB a-chain, an
FcyRIV a-chain, an
29 FcyRIII a-chain, and a combination thereof; wherein the mouse expresses
a functional
endogenous mouse -/-chain.
3
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 [0017] In a specific embodiment, the mouse does not express a
functional FcyRIIB a-chain,
2 does not express a functional FcyRIV a-chain, and does not express a
functional FcyRIII
3 chain.
4 [0018] In one embodiment, the mouse genome comprises a deletion of
an endogenous
FcyRIIB a-chain, a deletion of an endogenous FcyRIV a-chain, and a deletion of
an endogenous
6 FcyRIII a-chain.
7 [0019] In one embodiment, the mouse comprises a deletion of an
endogenous Fc7RIIB a-
8 chain, a deletion of an endogenous FcyRIV a-chain, and a deletion of an
endogenous Fc7R111
9 chain, and further comprises a reduced ability to make an immune response
to an antigen as
compared with a wild type mouse's ability with respect to the same antigen. In
one
11 embodiment, the reduced immune response includes a decreased antibody-
dependent cell-
12 mediated cytotoxicity (ADCC). In one embodiment, the reduced immune
response includes a
13 reduced ability in a cell killing assay to achieve antibody-dependent NK
cell killing. In specific
14 embodiments, the reduction in ADCC or antibody-dependent NK cell killing
is at least 50%, in
one embodiment at least 75%, in one embodiment at least 90%.
16 [0020] In one embodiment, the mouse comprises a deletion of an
endogenous FcyRIIB a-
17 chain, a deletion of an endogenous FcyRIV a-chain, and a deletion of an
endogenous Fc7R111 a-
18 chain, and further comprises an increased humoral antibody response upon
immunization with
19 an antigen as compared to a wild type mouse, e.g., a mouse of the same
or similar strain that
does not comprise the deletion. In one embodiment, the increased humoral
antibody response
21 is 2-fold as compared to a wild type mouse. In one embodiment, the
increased humoral
22 antibody response is 3-fold as compared to a wild type mouse. In one
embodiment, the
23 increased humoral antibody response is 5-fold as compared to a wild type
mouse. In one
24 embodiment, the increased humoral antibody response is 7-fold as
compared to a wild type
mouse. In one embodiment, the increased humoral antibody response is 10-fold
as compared
26 to a wild type mouse. In a specific embodiment, humoral antibody
response is measured by
27 micrograms of antibody that specifically binds an antigen (with which
the mouse has been
28 immunized) per microgram of serum protein from the mouse. In one
embodiment, the
29 increased humoral antibody response is with respect to an antigen to
which a wild type mouse
exhibits tolerance, or to an antigen which in a wild type mouse exhibits a
poor or minimal
31 humoral immune response. In a specific embodiment, the antigen is a
mouse antigen. In a
4
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 specific embodiment, the antigen is a human antigen that exhibits an
identity with a mouse
2 protein of at least about 95%, 96%, 97%, 98%, or 99%.
3 [0021] In one aspect, a genetically modified mouse is provided,
comprising a replacement
4 of a low affinity mouse Fc7R a-chain gene with a low affinity human Fc7R
a-chain gene, wherein
the replacement is at the endogenous mouse Fc7R a-chain gene locus. In one
embodiment,
6 the low affinity mouse Fc7R a-chain gene is selected from an Fc7R11B,
Fc7RIV and an Fc7R111 a-
7 chain gene. In a specific embodiment, a genetically modified mouse is
provided, wherein the
8 mouse expresses an endogenous FcR 7-chain, and wherein the low affinity
human Fc7R a-
9 chain gene is Fc7RIIIA a-chain. In another specific embodiment, the
genetically modified
mouse expresses an endogenous FcR 7-chain and a functional human Fc7RIIIA a-
chain on NK
11 cells. In a specific embodiment, the functionality of Fc7RIIIA a-chain
on NK cells is reflected by
12 human antibody-mediated NK killing (e.g., ADCC mediated by a human
antibody).
13 [0022] In one aspect, a genetically modified cell, non-human
embryo, or non-human animal
14 is provided, wherein the genetic modification comprises a replacement of
at least one
endogenous low affinity Fc7R a-chain gene with a human Fc7R a-chain gene, and
the cell,
16 embryo, or animal expresses a functional FcR 7-chain. In one embodiment,
the functional FcRy-
17 chain is an endogenous FcR 7-chain. In one embodiment, the low affinity
human Fc7R a-chain
18 gene is selected from an Fc7RIIA a-chain gene, an Fc7RIIIA a-chain gene,
and a combination
19 thereof. In a specific embodiment, the human Fc7RIIA gene comprises a
polymorphism,
wherein the polymorphism is selected from a 131His low responder polymorphism
and a 131Arg
21 high responder polymorphism. In a specific embodiment, the Fc7RIIA
polymorphism is the
22 131 His low responder polymorphism. In one embodiment, the Fc7RIIIA gene
is a specific allelic
23 variant, wherein the allelic variant is selected from a 158Val variant
and a 158Phe variant. In a
24 specific embodiment, the Fc7RIIIA allelic variant is the 158Val variant.
[0023] In one embodiment the low affinity human Fc7R gene is selected from
an Fc7RI1B,
26 FcyRIIC, an Fc7R111B gene, and a combination thereof. In a specific
embodiment, the human
27 Fc7RIIB gene comprises an amino acid substitution, wherein the
substitution is selected from an
28 2321Ie or a 232Thr substitution. In another specific embodiment, amino
acid substitution is a
29 23211e substitution. In a specific embodiment, the Fc7RIIIB gene is a
specific allelic variant,
wherein the allelic variant is selected from a NA1 variant and a NA2 variant.
In another specific
31 embodiment, the Fc7RIIIB allelic variant is a NA2 variant.
5
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 [0024] In one embodiment the low-affinity human FcyR a-chain gene
is selected from a
2 FcyRIIA, Fc7RIIB, FcyRIIC, Fc7R111A, Fc7RIIIB a-chain gene, and a
combination thereof.
3 [0025] In one embodiment, the low affinity mouse Fc7RIV a-chain
gene and the FeyRIII a-
4 chain gene are replaced with at least one low affinity human FcyR a-chain
gene. In one
embodiment, the low affinity mouse FcyRIV a-chain gene and the Fc7RIIB a-chain
gene are
6 replaced with at least one low affinity human Fc7R a-chain gene. In one
embodiment, the low
7 affinity mouse Fc7RIIB a-chain gene and the Fc7RlIl a-chain gene are
replaced with at least one
8 low affinity human FcyR a-chain gene. In a specific embodiment, the at
least one low affinity
9 human FcyR a-chain gene is selected from an Fc7RIIA, FcyRI1B, FcyRIIC,
Fc7RIIIA , FcyRII1B
chain gene, and a combination thereof. In another specific embodiment, the at
least one low
11 affinity human Fc7R a-chain gene is selected from an Fc7RIIA a-chain
gene, an Fc7RIIIA a-
12 chain gene, and a combination thereof. In another specific embodiment,
the at least one low
13 affinity human FcyR a-chain gene is selected from an Fc7RIIB, FcyRIIC,
Fc7RIIIB a-chain gene,
14 and a combination thereof. In another specific embodiment, the low
affinity mouse FcyR genes
are replaced with a human Fc7RIIA a-chain gene and a human FcyRIIIA a-chain
gene. In
16 another specific embodiment, the low affinity human Fc7RIIA and Fc7RIIIA
a-chain genes
17 comprise variants, wherein the Fc7RIIA a-chain gene comprises a 131His
variant and the
18 Fc7RIIIA a-chain gene comprises a 158Val variant. In another specific
embodiment, the low
19 affinity mouse Fc7R a-chain genes are replaced with the following low
affinity human Fc7R a-
chain genes: Fc7RIIB, FcyRIIC and FcyRIIIB. In another specific embodiment,
the low affinity
21 human FcyRIIB a-chain gene and FcyRIIIB a-chain gene comprise variants,
wherein the
22 Fc7RIIB a-chain gene comprises a 23211e variant and the Fc-fRIIIB a-
chain gene comprises an
23 NA2 variant.
24 [0026] In one embodiment, the genetic modifications comprise a
replacement of syntenic
genomic sequences of mouse and human chromosome 1. In a specific embodiment,
the
26 genetic modifications comprise a replacement of a genomic fragment
comprising endogenous
27 low affinity mouse FcyR genes with a genomic fragment comprising low
affinity human Fc7R
28 genes. In another specific embodiment, the mouse genome from chromosome
1:172,889,983
29 to chromosome1:172,989,911 is replaced with a human genomic fragment
comprising human
chromosome 1:161,474,729 to chromosome1:161,620,458.
6
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 [0027] In one aspect, a genetically modified cell, non-human
embryo, or non-human animal
2 is provided, wherein the genetic modification comprises a knockout of one
or more endogenous
3 low affinity receptor a-chain genes, and the presence of an episome
comprising one or more
4 human Fc7R a-chain genes. In a specific embodiment, the cell, embryo, or
animal expresses a
functional FcR 7-chain. In a specific embodiment, the episome is a mini
chromosome. In one
6 embodiment, the functional FcR 7-chain is endogenous to the cell, embryo,
or animal.
7 [0028] In one aspect, a genetically modified mouse is provided,
comprising a replacement
8 of a low affinity mouse Fc7R a-chain gene with a low affinity human Fc7R
a-chain gene, the
9 mouse comprises a mouse FcR7-chain gene, and the mouse expresses a
functional human low
affinity Fc7R receptor. In one embodiment, the functional low affinity Fc7R
receptor is
11 expressed on a cell type in which the low affinity Fc7R receptor is
expressed in humans. In a
12 specific embodiment, the functional human low affinity Fc7R receptor is
Fc7RIIIA and the
13 Fc7RIIIA is expressed on NK cells.
14 [0029] In one embodiment, the mouse comprises a deletion of two
mouse Fc7R a-chain
genes. In another embodiment, the mouse comprises a deletion of three mouse
FcR a-chain
16 genes.
17 [0030] In one embodiment, the mouse comprises a replacement of
three mouse Fc7R a-
18 chain genes with at least one human FcyR a-chain gene. In another
embodiment, the mouse
19 comprises a replacement of two mouse Fc7R a-chain genes with at least
one human Fc7R a-
chain gene. In a specific embodiment, the mouse comprises a replacement of
three mouse
21 Fc7R a-chain genes with at least two human Fc7R u-chain genes. In
another specific
22 embodiment, the three mouse Fc7R a-chain genes are replaced with three
human Fc7R a-chain
23 genes. In another specific embodiment, the mouse comprises a replacement
of two mouse
24 Fc7R a-chain genes with at least two human FcyR a-chain genes. In yet
another specific
embodiment, the two mouse Fc7R a-chain genes are replaced with at least three
human Fc7R
26 a-chain genes.
27 [0031] In one embodiment, the low affinity mouse Fc7R a-chain gene
is selected from an
28 Fc7RIIB, Fc7RIV, Fc7RlIl a-chain gene, and a combination thereof.
29 [0032] In one embodiment, the low affinity human Fc7R a-chain gene
is selected from an
Fc7RIIA, Fc7RIIB, Fc7RIIC, Fc7RIIIA, Fc7RIIIB a-chain gene, and a combination
thereof. In one
31 embodiment, the low affinity human Fc7R a-chain gene is selected from an
FcyRIIA, an
7
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 Fc7RIIIA a-chain gene, and a combination thereof. In one embodiment, the
low affinity human
2 Fc7R a-chain gene is selected from an Fc7RIIB, Fc7R11C, an Fc7R111B a-
chain gene, and a
3 combination thereof.
4 [0033] In one embodiment, the low affinity mouse Fc7R1V a-chain
gene and the Fc7RIII
chain gene are replaced with at least one human Fc7R a-chain gene. In one
embodiment, the
6 low-affinity mouse Fc7RIV a-chain gene and the Fc7RIIB a-chain gene are
replaced with at least
7 one human FcyR a-chain gene. In one embodiment, the low affinity mouse
FcyRIIB a-chain
8 gene and the FcyRII1B a-chain gene are replaced with at least one human
FcyR a-chain gene.
9 In a specific embodiment, the at least one human FcyR a-chain gene is
selected from an
Fc7RIIA, Fc7RIIB, Fc7RIIC, FORMA, Fc7R111B a-chain gene, and a combination
thereof. In
11 another specific embodiment, the at least one human FcyR a-chain gene is
selected from an
12 Fc7RIIA, an Fc7RIIIA a-chain gene, and a combination thereof. In another
specific embodiment,
13 the at least one human FcyR a-chain gene is selected from an Fc7RIIB,
FcyRIIC, Fc7RIIIB
14 chain gene, and a combination thereof. In another specific embodiment,
the mouse a-chain
genes are replaced with the following human Fc7R a-chain genes: Fc7RIIA and
Fc7RIIIA. In yet
16 another specific embodiment, the mouse a-chain genes are replaced with
the following human
17 FcyR a-chain genes: FcyRIIB, Fc7RIIC and Fc7R111B.
18 [0034] In one aspect, a genetically modified mouse is provided,
comprising a low affinity
19 human FcyR a-chain and a mouse FcR 7-chain subunit, wherein the mouse
expresses the
human FcyR a-chain on a cell selected from a neutrophil, an eosinophil, a
basophil, a
21 monocyte, a macrophage, a platelet, a Langerhans cell, a dendritic cell,
an NK cell, a mast cell,
22 a B cell, a T cell, and a combination thereof. In one embodiment, the
mouse expresses a
23 human Fc7RIIA a-chain on a cell selected from a neturophil, a
macrophage, an eosinophil, a
24 platelet, a dendritic cell, a Langerhans cell, and a combination
thereof. In one embodiment, the
mouse is capable of phagocytosis, ADCC and cellular activation initiated or
mediated through
26 the expressed human FcyRI1A a-chain. In one embodiment the mouse
expresses a human
27 Fc7RIIIA a-chain on a cell selected from a macrophage, an NK cell, a
monocyte, a mast cell, an
28 eosinophil, a dendritic cell, a Langerhans cell, at least one T cell
type, and a combination
29 thereof. In one embodiment, the mouse is capable of ADCC mediated
through the human
FoyRIIIA a-chain expressed on NK cells. In a specific embodiment, the mouse
exhibits
31 hFcyR111A-mediated ADCC in response to an antibody comprising a human
Fc.
8
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 [0035] In one embodiment, the mouse expresses both a human Fc7RIIA
a-chain and a
2 human Fc7RIIIA a-chain. In one embodiment, the human FcyRIIA a-chain is
expressed on
3 platelets and the human Fc7RIIIA a-chain is expressed on NK cells. In one
embodiment, the
4 mouse is capable of ADCC mediated by an antibody comprising a human Fc,
wherein the
mediation is through either the human Fc7RIIA a-chain or through the human
Fc7RIIIA a-chain
6 expressed on the surface of accessory cells. In one embodiment, the human
Fc7RIIA a-chain is
7 not expressed on platelets. In a specific embodiment wherein the human
Fc7RIIA a-chain is not
8 expressed on platelets, the mouse lacks or substantially lacks a human
promoter sequence that
9 operably linked to the human FcyRIIA a-chain in a human genome.
[0036] In one embodiment, the mouse expresses a human Fc7RIIB a-chain on a
cell
11 selected from a B cell, a mast cell, a basophil, a macrophage, an
eosinophil, a neutrophil, a
12 dendritic cell, a Langerhans cell, and a combination thereof. In a
specific embodiment, the
13 mouse expresses a human Fc7RIIB a-chain on a B cell and a mast cell. In
another specific
14 embodiment, the mouse is capable of endocytosis of immune complexes
mediated through the
expressed human Fc7RIIB a-chain. In one embodiment, the mouse expresses a
human
16 FcyRIIC a-chain on a cell selected from a neutrophil, a macrophage, an
eosinophil, a platelet, a
17 dendritic cell, a Langerhans cell, and a combination thereof. In a
specific embodiment, the
18 mouse is capable of phagocytosis, ADCC and cellular activation initiated
through the expressed
19 human Fc7RIIC a-chain.
[0037] In one embodiment, the mouse expresses a human Fc7RIIIB a-chain on
neutrophils
21 and eosinophils. In a specific embodiment, the mouse is capable of
cellular activation,
22 phagocytosis, ADCC and degranulation, wherein the activation,
phagocytosis, ADCC, and
23 degranulation are mediated through the expressed human FcyRIIIB a-chain.
24 [0038] In one aspect, a mouse is provided that comprises a
deletion of the endogenous
Fc7RIIB, FcyRIV and FcyRIII genes and insertion of human Fc7RIIA, Fc7RIIB,
FcyRIIC, Fc7RIIIA,
26 and Fc7RIIIB genes, and wherein the mouse comprises a functional mouse
FcR 7¨chain gene.
27 [0039] In one embodiment, the mouse comprises a deletion of the a-
chains encoded by
28 endogenous Fc7RIIB, FcyRIV and Fc/R111 genes and insertion of the a-
chains encoded by
29 human FcyRIIA, Fc7RIIB, Fc71:111C, Fc7RIIIA, and Fc7RIIIB genes.
[0040] In one embodiment, the insertion of the human FcyRIIA, Fc7RIIB,
FeyRIIC, Fc7RIIIA,
31 and FcyRIIIB a-chain genes is at a random location within the mouse
genome.
9
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 [0041] In one
embodiment, the insertion of the human FcyRIIA, FcyRIIC, FcyRIIIA,
2 and FcyRIIIB a-chain genes is at the endogenous mouse low affinity FOR a-
chain locus.
3 [0042] In one embodiment, the mouse expresses human FcyRIIIA on NK
cells and
4 macrophages. In a specific embodiment, all or substantially all NK cells
from a splenocyte
sample of the mouse express human Fc7R111A. In a specific embodiment, all or
substantially all
6 macrophages from a splenocyte sample of the mouse express human FcyRIIIA.
7 [0043] In one embodiment, the mouse expresses a human FcyR
selected from human
8 FcyRIIA, human FcyRIIIA, and a combination thereof, on a cell type
selected from neutrophils,
9 macrophages, and a combination thereof. In a specific embodiment, the
mouse expresses
human FcyRIIA and human FcyRIIIA on all or substantially all neutrophils and
macrophages of a
11 splenocyte sample from the mouse.
12 [0044] In one embodiment, the mouse expresses human FcyRIIB and
human FcyRIIIB on B
13 cells and neutrophils of B cells from a B cell-gated splenocyte sample
from the mouse. In a
14 specific embodiment, the mouse expresses FcyRIIIB and FcyRIIB on all or
substantially all B
cells and neutrophils from a B cell-gated splenocyte sample from the mouse.
16 [0045] In one embodiment, the mouse further comprises a humanized
CD20 gene. In one
17 embodiment, the mouse that further comprises the humanized CD20 gene
following treatment
18 with an anti-CD20 binding protein that comprises an Fc exhibits
depletion (in vivo) of B cells. In
19 one embodiment, the depletion is in a compartment selected from bone
marrow, blood, lymph
node, spleen, and a combination thereof. In one embodiment, the Fc is a human
Fc. In one
21 embodiment, the Fc is a mouse Fc. In one embodiment, the anti-CD20
binding protein is an
22 anti-CD20 antibody.
23 [0046] In one aspect, a cell is provided comprising a genetic
modification as described
24 herein. In one embodiment, the cell is selected from an embryonic stem
(ES) cell, a pluripotent
cell, an induced pluripotent cell, and a totipotent cell. In one embodiment,
the cell is selected
26 from a mouse cell and a rat cell. In a specific embodiment, the cell is
an ES cell. In a more
27 specific embodiment, the cell is a mouse ES cell.
28 [0047] In one aspect, a non-human embryo is provided, comprising a
genetic modification
29 as described herein. In one embodiment, the non-human embryo is selected
from a mouse
embryo and a rat embryo.
31 [0048] In one aspect, a method is provided for determining
efficacy of a therapeutic. In one
32 embodiment, the therapeutic is an antibody (e.g., mono-, bi-, tri-,
multispecific) comprising a
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 human Fc. In one embodiment, the therapeutic is a human antibody. In one
embodiment, the
2 efficacy is efficacy of therapeutic-mediated cell killing (e.g., ADCC).
In a specific embodiment,
3 the human therapeutic is a fusion protein comprising an Fc of a human
immunoglobulin heavy
4 chain. In one embodiment, the therapeutic is administered to a mouse as
described herein and
a level of therapeutic-dependent ADCC is measured. In one embodiment, the
mouse is used to
6 assess the ADCC activity of a therapeutic by administering the
therapeutic to the mouse and
7 then detecting (e.g., in vitro from a sample (e.g., blood) taken from the
animal) binding of the
8 therapeutic to a human low affinity FcyR on an FOR-expressing cell. In a
specific embodiment,
9 accessory cells of the mouse are isolated from the mouse and tested for
the ability, in the
presence and absence of the therapeutic, to mediate therapeutic-dependent
ADCC.
11 [0049] In one aspect, a method is provided for determining whether
a low affinity FOR is
12 associated with a human disease or disorder, comprising a step of
determining a trait
13 associated with the human disease or disorder in a mouse according to
the invention. In one
14 embodiment, the trait is a phenotype associated with the absence or loss
of a function of one or
more low affinity FcyRs. In a specific embodiment, the disease or disorder is
an autoimmune
16 disease or disorder. In a specific embodiment, the autoimmune disease or
disorder is selected
17 from Rheumatoid Arthritis (RA), Systemic Lupus Erythematosus (SLE), type
I diabetes, Guillain-
18 Barre syndrome, sclerosis, multiple sclerosis, Goodpasture's syndrome,
Wegener's
19 Granulomatosis and experimental autoimmune encephalomyelitis (EAE). In a
specific
embodiment, the mouse comprises a polymorphism in a low affinity FOR, and the
trait is
21 selected from an enhanced ability to mediate ADCC in comparison to the
majority of the human
22 population that does not bear the polymorphism, and a reduced ability to
mediate ADCC in
23 comparison to the majority of the human population that does not bear
the polymorphism.
24 [0050] In one aspect, a method for making an anti-human FcR a-
chain antibody in a mouse
is provided, comprising exposing a mouse according to the invention to a human
FcR as
26 described herein. In one embodiment, an antibody that recognizes the
human FcR is isolated
27 from the mouse. In another embodiment, a nucleic acid sequence that
encodes all or part of a
28 variable region of an antibody that recognizes the human FcR is
identified and cloned.
29 [0051] In one aspect, a method for determining ability of anti-
human FcR antibodies to
target molecules to FcR-expressing cells for phagocytosis of the target
molecule is provided,
31 comprising exposing a mouse as described herein to an agent comprising
an anti-human FcR
32 antibody, and measuring' phagocytosis of the target molecule.
11
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 [0052] In one aspect, a method is provided for making an antibody,
in a mouse, to an
2 antigen that is poorly immunogenic in a mouse that is wild type with
respect to one or more
3 FeyRs, comprising exposing a mouse as described herein that lacks a mouse
low affinity FcR
4 but expresses an FcyR 7-chain to the antigen that is poorly immunogenic
in the mouse that is
wild type with respect to one or more Fc7Rs, and identifying an antibody that
recognizes the
6 poorly antigenic antigen. In one embodiment, the method comprises
isolating the antibody from
7 the mouse. In another embodiment, a nucleic acid sequence that encodes
all or part of a
=
8 variable region of the antibody is identified and cloned.
9 [0053] In one aspect, a method for making a mouse capable of making
antibodies
comprising human variable regions is provided, comprising a step of breeding a
first mouse as
11 described herein with a second mouse that comprises (a) one or more
human immunoglobulin
12 variable region gene segments and one or more human constant region
genes; or, (b) one or
13 more human immunoglobulin variable region gene segments operably linked
to a mouse
14 constant region gene, wherein the human gene segments replace variable
region gene
segments at the mouse variable region gene segment locus.
16 [0054] In one embodiment, the second mouse (a) comprises a
transgene that comprises
17 one or more human immunoglobulin light chain variable region gene
segments and a human
18 light chain constant gene, and a transgene that comprises one or more
human immunoglobulin
19 heavy chain variable region gene segments and one or more human heavy
chain constant
genes. In one embodiment, the transgene that comprises one or more human
immunoglobulin
21 heavy chain variable region gene segments comprises two or more heavy
chain constant genes
22 and is capable of class switching. In a specific embodiment, the mouse
comprises an
23 inactivated endogenous light chain locus and/or an inactivated
endogenous heavy chain locus.
24 In a specific embodiment, the mouse comprises a deletion of an
endogenous light chain locus
and/or a deletion of an endogenous heavy chain locus.
26 [0055] In one embodiment, the second mouse (b) comprises human
heavy and human light
27 variable region gene segments, at the heavy an light mouse loci,
respectively.
28 [0056] In one aspect, a method is provided for selecting an anti-
tumor antibody, comprising
29 a step of determining the ability of an antibody to mediate ADCC,
wherein the ability of the
antibody to mediate ADCC is tested by determining ADCC mediated by a cell of a
mouse as
31 described herein, and the antibody is selected if it mediates ADCC
employing a cell of a
32 genetically modified mouse as described herein. In a specific
embodiment, binding of the
12
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 antibody to the cell of the genetically modified mouse is determined, and
the anti-tumor antibody
2 is selected for its ability to bind a human Fc7R on the cell. In a
specific embodiment, the human
3 Fc7R is a low affinity Fc7R.
4 [0057] In one embodiment, the anti-tumor antibody is identified by
its enhanced ability to
mediate ADCC through a cell of the mouse as compared to ability of the anti-
tumor antibody to
6 mediate ADCC through a cell of a wild type mouse. In a specific
embodiment, the anti-tumor
7 antibody is identified by its ability to mediate ADCC through NK cells.
In a specific embodiment,
8 the NK cells express human Fc7RIIIA.
9 [0058] In one embodiment, a method is provided for selecting an
anti-tumor agent,
comprising a step of administering an agent comprising a human Fc or a
modified human Fc to
11 a first non-human animal wherein the first non-human animal is
genetically modified in
12 accordance with the invention and comprises a human tumor; a step of
administering the agent
13 to a second non-human animal comprising the tumor; and determining the
ability of the first non-
14 human animal and the second non-human animal to retard growth of the
human tumor following
administration of the agent, wherein the agent is selected as an anti-tumor
agent if it exhibits an
16 enhanced ability to retard growth of the human tumor in the first non-
human animal but not in
17 the second non-human animal.
18 [0059] In one embodiment, the first non-human animal is modified
to comprise a deletion of
19 an endogenous FcR a-subunit, and is modified to comprise a human FcR a-
subunit selected
from the group consisting of an FcyRIIA a-subunit, an FcyRIIB a-subunit, an
FcyRIIC a-subunit,
21 an Fc7RIIIA a-subunit, an Fc7RIIIB a-subunit, and a combination thereof.
In one embodiment,
22 the second animal is a wild type animal. In one embodiment, the first
non-human animal
23 expresses an endogenous FcR 7-chain.
24 [0060] In one embodiment, the first non-human animal expresses a
functional endogenous
FOR!.
26 [0061] In one aspect, a method is provided for making a mouse that
lacks a low affinity
27 mouse FOR, expresses a functional FcR 7-chain, and comprises genes
encoding a-chains of
28 the human FcyRIIA, FcyRIIB, FcyRIIC, Fc7RIIIA, and Fc7RIIIB, comprising
a step of replacing
29 the low affinity mouse Fc7R a-chains with human Fc7R a-chains, at the
mouse Fc7R a-chain
locus.
31 [0062] In one embodiment, a first step comprises deleting the a-
chains of the endogenous
32 Fc7RIIB, Fc7RIV and FcyRlIl genes and inserting the a-chains of the
human Fc7RIIA and
13
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 Fc7RIIIA genes; a second step comprises inserting the a-chains of the
human Fc7RIIB, FcyRIIC
2 and Fc7RIIIB genes into the mouse genome that results from the first
step; wherein the mouse
3 comprises a functional mouse FcR 7¨chain gene. In a specific embodiment,
the a-chains of the
4 human Fc7RI1B, Fc7RIIC and Fc7RIIIB genes of the second step are inserted
5' relative to the a-
chains of the human Fc7RIIA and Fc7RIIIA genes of the first step.
6 [0063] In one aspect, a method for determining cell killing by a
human therapeutic in a non-
7 primate is provided, comprising a step of exposing a cell, non-human
embryo, or non-human
8 animal to a human therapeutic that comprises a human Fc, wherein the
cell, embryo, or animal
9 comprises a functional FcR 7-chain and comprises a replacement of one or
more endogenous
low affinity Fc7R a-chain genes with one or more human Fc7R a-chains, and
determining the
11 ability of the human therapeutic to mediate cell killing through a low
affinity human Fc7R of the
12 cell, embryo, or animal.
13 [0064] In one embodiment, the non-primate is a mouse. In a
specific embodiment,
14 endogenous mouse FeyR a-chain genes FcyRIIB, Fc7RIV and FcyRIII are
replaced with human
FaiR a-chain genes Fc7RIIA, Fc7RIIB, FcyRIIC, Fc7RIIIA, and Fc7RIIIB.
16 [0065] In one embodiment, the cell is selected from a B cell, a
mast cell, a basophil, a
17 macrophage, an eosinophil, a neutrophil, a dendritic cell, a Langerhans
cell, and a combination
18 thereof. In a specific embodiment, the cell is an NK cell and NK cell-
mediated ADCC by a
19 human or a humanized antibody is determined. In a specific embodiment,
the low affinity
human Fc7111 is a human Fc7RIIIA.
21 [0066] In one aspect, a method for determining therapeutic-
dependent thrombosis is
22 provided, comprising exposing a first non-human animal that expresses a
human Fc7FillA on a
23 platelet to a therapeutic; exposing a second non-human animal that does
not express the
24 human Fc7RIIA on a platelet to said therapeutic; measuring in the first
non-human animal and in
the second non-human animal an amount of therapeutic-dependent thrombosis;
and,
26 determining a difference in therapeutic-dependent thrombosis.
27 [0067] In one embodiment, the non-human animal is selected from a
mouse and a rat.
28 [0068] In one embodiment, the determined difference in therapeutic-
dependent thrombosis
29 is employed to identify a risk associated with administering the
therapeutic to a human. In one
embodiment, the determined difference results in a change of administration of
the therapeutic
31 to a human patient in need thereof.
32
14
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 DETAILED DESCRIPTION
2 [0069] The invention is not limited to particular methods, and
experimental conditions
3 described, as such methods and conditions may vary. The terminology used
herein is for the
4 purpose of describing particular embodiments only, and is not intended to
be limiting, since the
scope of the present invention will be limited only by the claims.
6 [0070] Unless defined otherwise, all technical and scientific
terms used herein have the
7 same meaning as commonly understood by those of ordinary skill in the art
to which this
8 invention belongs. Although any methods and materials similar or
equivalent to those described
9 herein can be used in the practice or testing of the present invention,
particular methods and
materials are now described.
11 [0071] The phrase "targeting construct" includes a polynucleotide
molecule that comprises a
12 targeting region. A targeting region comprises a sequence that is
substantially homologous to a
13 sequence in a target cell, tissue or animal and provides for integration
of the targeting construct
14 into a position within the genome of the cell, tissue or animal. In a
specific embodiment, the
targeting construct further comprises a nucleic acid sequence or gene of
particular interest, a
16 selectable marker, control and or regulatory sequences, and other
nucleic acid sequences that
17 allow for recombination mediated through the exogenous addition of
proteins that aid in or
18 facilitate recombination involving such sequences. In another specific
embodiment, the
19 targeting construct further comprises a gene of interest, wherein the
gene of interest is a
heterologous gene that encodes a protein that has a similar function as a
protein encoded by
21 the endogenous sequence.
22 [0072] The term "replacement" includes wherein a DNA sequence is
placed into a genome
23 of a cell in such a way as to replace a sequence within a genome, at the
locus of the genomic
24 sequence, with a heterologous sequence (e.g., a human sequence in a
mouse), unless
otherwise indicated. The DNA sequence so placed may include one or more
regulatory
26 sequences that are part of source DNA used to obtain the sequence so
placed (e.g., promoters,
27 enhancers, 5'- or 3'-untranslated regions, etc.). For example, in
various embodiments, the
28 replacement is a substitution of an endogenous sequence for a
heterologous sequence that
29 results in the production of a gene product from the DNA sequence so
placed (comprising the
heterologous sequence), but not expression of the endogenous sequence; the
replacement is of
31 an endogenous genomic sequence with a DNA sequence that encodes a
protein that has a
32 similar function as a protein encoded by the endogenous genomic sequence
(e.g., the
33 endogenous genomic sequence encodes a low affinity mouse Fc7F1 receptor,
and the DNA
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 fragment encodes one or more human low affinity Fc7R receptors, such as,
e.g., a human
2 Fc7RI IC and/or an Fc7RI II B).
3 [0073] The term "FcyR" includes a receptor for an Fc, e.g., an Fc
portion of an IgG
4 immunoglobulin. The FcyR genes include an a-chain that is expressed on
the surface of the
cell and serves as a ligand-binding domain, and associates with either a
homodimer of the FcR
6 7¨chain or a heterodimer of the FcR 7¨chain and the 6-chain. There are
several different Fc7R
7 genes and they can be categorized into low affinity and high affinity
types according to
8 preferential binding to IgG in immune complexes. Low affinity Fc7R genes
in humans include
9 FcyRIIA, FcyRIIB, FcyRIIC, Fc7RIIIA and Fc7RIIIB and within most of these
genes naturally
occurring genetic differences, or polymorphisms, have been described in human
subjects with
11 autoimmune diseases. Persons of skill upon reading this disclosure will
recognize that one or
12 more endogenous low affinity FcyR genes in a genome (or all) can be
replaced by one or more
13 heterologous low affinity FcyR genes (e.g., variants or polymorphisms
such as allelic forms,
14 genes from another species, chimeric forms, etc.).
[0074] The phrase "allelic variants" includes variations of a normal
sequence of a gene
16 resulting in a series of different forms of the same gene. The different
forms may comprise
17 differences of up to, e.g., 20 amino acids in the sequence of a protein
from a gene. For
18 example, alleles can be understood to be alternative DNA sequences at
the same physical gene
19 locus, which may or may not result in different traits (e.g., heritable
phenotypic characteristics)
such as susceptibility to certain diseases or conditions that do not result in
other alleles for the
21 same gene or result in varying degrees in the other alleles.
22 [0075] An "accessory cell" includes an immune cell that is involved
in the effector functions
23 of the immune response. Exemplary immune cells include a cell of
lymphoid or myeloid origin,
24 e.g., lymphocytes, natural killer (NK) cells, monocytes, macrophages,
neutrophils, eosinophils,
basophils, platelets, Langerhans cells, dendritic cells, mast cells etc.
Accessory cells carry out
26 specific functions of the immune system through receptors, e.g., FcRs,
expressed on their
27 surfaces. In a specific embodiment, an accessory cell is capable of
triggering ADCC mediated
28 through an FcR, e.g., a low affinity Fc7R, expressed on the cell
surface. For example,
29 macrophages expressing FcRs are involved in phagocytosis and destruction
of antibody-coated
bacteria. Accessory cells might also be capable of releasing an agent that
mediates other
31 immune processes. For example, mast cells can be activated by antibody
bound to FcRs to
32 release granules, e.g., inflammatory molecules (e.g., cytokines) at a
site of infection. In various
16
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 other embodiments, the expression of FcRs on accessory cells can be
regulated by other
2 factors (e.g., cytokines). For example, FcyRI and FcyRIII expression can
be inducted by
3 stimulation with interferon-y (IFN-y).
4
Mouse and Human FcRs
6 [0076] The receptors for the Fc (i.e., constant) regions of
immunoglobulins (FcRs) play an
7 important role in the regulation of the immune response. FcRs are present
on accessory cells
8 of the host's immune system to effectively dispose of foreign antigens
bound by an antibody.
9 FcRs also play important roles in balancing both activating and
inhibitory responses of the
accessory cells of the immune system. FcRs are involved in phagocytosis by
macrophages,
11 degranulation of mast cells, uptake of antibody-antigen complexes and
modulation of the
12 immune response, as well as other immune system processes.
13 [0077] In mice and humans, distinct FcRs are differentially
expressed on the surface of
14 different accessory cells that are each specific for the immunoglobulin
isotypes present in the
expressed antibody repertoire. For example, immunoglobulin G (IgG) antibodies
mediate
16 effector functions through IgG receptors (FcyRs). FcyRs have been
classified into three groups:
17 high affinity activating Fc7R1 (CD64), low affinity inhibitory FcyRII
(CD32) and low affinity
18 activating FcyRIII (CD16). Although each group is present in both mice
and humans, the
19 number of isoforms and subsets of immune cells on which they are present
are different. For
example, FcyRIIA and FcyRIIIB are expressed on accessory cells in humans but
are reportedly
21 absent from mice. Further, affinities of the different IgG isotypes
(e.g., IgG1) for each FcyR is
22 different in mice and humans.
23 [0078] Activation or inhibition of cell signaling through FcyRs
and the effector functions
24 associated with antibody binding to FcyRs are believed to be mediated by
specific sequence
motifs of intracellular domains of FcyRs, or of the subunits of co-receptors.
Activating receptors
26 are most commonly associated with the common y-chain (FcR y¨chain) which
contains an
27 immunoreceptor tyrosine-based activation motif (ITAM). ITAMs contain a
specific sequence of
28 about 9-12 amino acids that include tyrosine residues that are
phosphorlyated in response to
29 antibody binding to an FcR. Phosphorylation leads to a signal
transduction cascade. Mice that
lack a gene encoding an FcR y¨chain (FcR y-chain KO) have been reported (e.g.,
see Takai et
31 al. (1994) FcR y Chain Depletion Results in Pleiotrophic Effector Cell
Defects, Cell 76:519-529;
32 van Vugt etal. (1996) FcR 7-Chain Is Essential for Both Surface
Expression and Function of
17
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 Human Fc7RI (CD64) In Vivo, Blood 87(9):3593-3599; and Park etal. (1998)
Resistance of Fc
2 Receptor-deficient Mice to Fatal Glomerulonephritis, J. Clin. Invest.
102(6):1229-1238). The
3 FcR 7¨chain is reportedly essential for proper surface expression and
function (e.g., signal
4 transduction, phagocytosis, etc.) of most of the FcRs; FcR 7-chain KO
mice lack Fc7RI
according to some reports. However, other reports reveal that FcR 7-chain KO
mice indeed
6 express Fc7RI on the surface of certain accessory cells, and the Fc7RI
expressed reportedly
7 appears functional in that it binds IgG in mice in the absence of
expressed FcR 7¨chain (Barnes
8 et al. (2002) Fc7RI-Deficient Mice Show Multiple Alterations to
Inflammatory and Immune
9 Responses, Immunity 16:379-389).
[0079] In contrast, Fc7RIIB is an inhibitory receptor that contains an
immunoreceptor
11 tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain. Like
ITAMs, ITIMs are
12 sequence motifs that include phosphorylatable tyrosine residues.
However, downstream events
13 following phosphorylation of an ITM lead to inhibition, not activation,
of immune cell functions.
14 Mice deficient in Fc7RIIB reportedly exhibit an increased antibody
response in comparison to
wild type mice (Takai etal. (1996) Augmented humoral and anaphylactic
responses in FcgRII-
16 deficient mice, Nature 379:346-349), an observation that supports the
role of FcyRIIB as a
17 downregulator of the B cell antibody response.
18 [0080] In humans, FcyRIIA, Fc7RIIB, Fc7RI IC, Fc7RIIIA and
Fc7RIIIB are considered the
19 classical low affinity Fc7R genes and are located together on the same
chromosome (Su et al.
(2002) Genomic organization of classical human low-affinity Fey receptor
genes, Genes and
21 Immunity 3 (Supple 1):S51-S56). These genes exhibit several
polymorphisms associated with
22 distinct phenotypes, e.g., an alteration of ligand binding and function
of the receptor. Some
23 polymorphisms are associated with autoimmune diseases, e.g., systemic
lupus erythematosus
24 (SLE), rheumatoid arthritis (RA), and multiple sclerosis (MS).
Transgenic mice for different
human Fc7Rs (hFc7Rs) have been developed and used as disease models,
generating high
26 affinity antibodies, testing therapeutic antibodies for ability to
elicit specific cellular responses,
27 screening compounds that ameliorate aberrant immune responses, etc.
(e.g., see Heijnen et al.
28 (1996) A Human FcgRI/CD64 Transgenic Model for In Vivo Analysis of
(Bispecific) Antibody
29 Therapeutics, J. Hematother. 4:351-356; Heijnen and van de Winkel (1996)
Antigen Targeting
to Myeloid-specific Human FcgRI/CD64 Triggers Enhanced Antibody Responses in
Transgenic,
31 J. Clin. Invest. 97(2):331-338; US Pat Nos. 6,111,166, 6,676,927,
7,351,875, 7,402,728, and
32 7,416,726).
18
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 [0081] Despite the significant roles of the FcRs in providing the
bridge between antibodies
2 and accessory cells of the immune system, no model system currently
exists in which all the low
3 affinity hFcyRs are expressed. A mouse in which all the low-affinity
hFcyRs are co-expressed-
4 including mice that lack endogenous mouse FeyRs¨in various embodiments
could be used to
accurately reflect effects of a human antibody therapeutic, including ADCC-
mediated effects.
6 Such a mouse would serve as a vital tool in the engineering, analysis and
evaluation of
7 therapeutic antibodies for treatment of human diseases such as, e.g., RA,
type I diabetes, SLE,
8 and autoimmunity, by providing an animal model capable of achieving a
more accurate
9 assessment of immunological processes in humans, particularly in the
context of testing human
antibody therapeutics. The mouse will also be a valuable source of cells
bearing the low affinity
11 receptors, which cells can be used in in vitro assays for assessing
therapeutic-dependent cell
12 killing for therapeutics that bind the low affinity receptors, and thus
for identifying useful human
13 therapeutics.
14
Endogenous Low Affinity FcyR Gene Deficient Mice
16 [0082] Genetically modified non-human animals are provided that do
not express
17 endogenous low affinity mouse FcyR genes, but that express an endogenous
mouse FcR y-
18 chain. In various embodiments, the FcR 7-chain is expressed in a
distribution (i.e., in cell types)
19 and at a level in the mouse that is the same or substantially the same
as in a wild type mouse.
Endogenous low affinity FcyR genes can be expressed either on the surface of
immune cells or
21 in a soluble manner in the periphery of the animals. Genetic
modifications for making a non-
22 human animal that does not express endogenous low affinity mouse FcyR
genes are
23 conveniently described by using the mouse as an illustration. A
genetically modified mouse
24 according to the invention can be made in a variety of ways, particular
embodiments of which
are discussed herein.
26 [0083] A schematic illustration (not to scale) of low affinity
mouse Fc-õ,R gene locus is
27 provided in Figure 1 (top) to show Fc7R gene arrangement at the
endogenous locus. As
28 illustrated, low affinity mouse FcyR genes FcyRilB, Fc7RIV and Fc7RlIl
are present together in
29 close proximity on one chromosome. Each of these genes comprise the u-
chain or ligand
binding domain responsible for the binding the Fc portion of an antibody
molecule.
31 [0084] A genetically modified mouse lacking a nucleotide sequence
encoding an u-chain of
32 the endogenous low affinity FcyR genes can be made by any method known
in the art. For
19
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 example, a targeting vector can be made that deletes the low affinity
mouse FcyR a-chain
2 genes with selectable marker gene. Figure 1 illustrates a mouse genome
(bottom) targeted by
3 a targeting construct having a 5' homology arm containing sequence
upstream of the
4 endogenous low affinity FcyR a-chain locus, followed by a drug selection
cassette (e.g. a
neomycin resistance gene flanked by loxP sequences), and a 3' homology arm
containing
6 sequence downstream of the endogenous low affinity FcyR a-chain locus.
Upon homologous
7 recombination at the locus, the endogenous low affinity FcyR a-chain
locus is replaced by a
8 drug selection cassette (bottom of Figure 1). The endogenous low affinity
FcyR a-chain gene
9 locus is thereby deleted resulting in a cell or non-human animal that
does not express
endogenous low-affinity mouse FcyR a-chain genes. The drug selection cassette
may
11 optionally be removed by the subsequent addition of a recombinase (e.g.,
by Cre treatment).
12 [0085] Genetically modifying a mouse to render an endogenous low-
affinity mouse FcyR
13 chain gene or genes nonfunctional, in various embodiments, results in a
mouse that exhibits
14 defects in immune responses, making the mouse useful for evaluating
cooperative, as well as
individual, roles of the endogenous low-affinity mouse Fc7R genes in normal
and disordered
16 immune function, IgG-mediated processes, and autoimmune disease. In
various embodiments,
17 modifying the a-chains of the endogenous low-affinity mouse FcyR genes,
but not the FcR
18 7¨chain, avoids a potential reduction of other endogenous FcR genes
(e.g., high affinity Fc7RI)
19 that require the FcR y¨chain for surface expression and function, thus
maintaining various other
immunological functions and processes mediated through y-chain-dependent
processes.
21 [0086] According to some reports, FcR 7¨chain deficient mice lack
surface expression of
22 FcyRIII and FcyRI. However, Fc7R1 has reportedly been detected on the
cell surface in FcR
23 y¨chain deficient mice and is reportedly at least partially functional.
In contrast, mice according
24 to the present invention contain unmodified endogenous FcR y¨chain,
which preserves natural
cell surface expression patterns and cellular functions of other FcR genes
that require FcR y-
26 chain.
27 [0087] In various embodiments, mice of the present invention
present an advantage over
28 other FcyR gene-deficient mice in that the genetic modifications that
they bear result in the
29 maintenance of other genes necessary for other immunological functions
not entirely devoted to
low affinity FcyR genes. For example, with a functional FcR 7¨chain, other y-
chain-dependent
31 proteins (e.g., Fcy1:31) will be able to associate with the FcR 7¨chain
and participate in effector
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 cell functions in the immune response. In various genetically modified
mice in accordance with
2 the invention, it is believed that maintaining such functions (due to the
presence of a functional
3 FcRy¨chain) while deleting endogenous low affinity Fc7R genes (one or
more a-subunits)
4 enables a more precise elucidation of the roles of FcRs in autoimmunity.
6 Low Affinity FcyR Humanized Mice
7 [0088] Genetically modified non-human animals are provided that
express low-affinity
8 human FcyR genes. Low affinity human FcyR genes can be expressed either
on the surface of
9 accessory cells of the animal's immune system or in a soluble manner in
the periphery of the
animals.
11 [0089] The genetic modification, in various embodiments, comprises
a deletion of a
12 functional a-chain of one or more low-affinity mouse Fc-yR genes, and in
some embodiments a
13 further modification comprising a replacement with two or more, with
three or more, with four or
14 more, or with five low-affinity human Fo(Fi a-subunit genes, wherein the
non-human animal
expresses a functional mouse FcR 7¨chain gene. Genetically modified non-human
embryos,
16 cells, and targeting constructs for making the non-human animals, non-
human embryos, and
17 cells are also provided.
18 [0090] Compositions and methods for making a mouse that expresses
a human FcyR gene,
19 including specific polymorphic forms or allelic variants (e.g., single
amino acid differences), are
provided, including compositions and method for making a mouse that expresses
such genes
21 from a human promoter and a human regulatory sequence. The methods
include selectively
22 rendering an endogenous low affinity mouse Fc7R gene nonfunctional
(e.g., by a deletion of its
23 a-chain), and employing an a-chain of a low affinity human FcyR gene at
the endogenous low
24 affinity mouse Fd.,,R gene locus to express a low affinity human FcyR a-
subunit gene in a
mouse. The deletion of the low affinity mouse FcyR gene is made by deletion of
one or more a-
26 chain genes, but not an FoRy-chain gene. The approach selectively
renders one or more
27 endogenous low affinity FcyR a-chain genes nonfunctional while retaining
a functional
28 endogenous FcRy-chain.
29 [0091] The endogenous Fc7R u-chain replacement approach employs a
relatively minimal
disruption in natural FcyR-mediated signal transduction in the animal, in
various embodiments,
31 because the genomic sequence of the FcyR a-chains are replaced in a
single fragment and
32 therefore retain normal functionality by including necessary regulatory
sequences. Thus, in
21
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 such embodiments, the FcyR a-chain modification does not affect other
endogenous FcRs
2 dependent upon functional FcRy-chain molecules. Further, in various
embodiments, the
3 modification does not affect the assembly of a functional receptor
complex involving an FcyR
4 chain and the endogenous FcR 7-chain, which is believed to be required
for proper expression
of some FeyR a-chains on the cell surface and for downstream signaling
resulting from an
6 activated receptor. Because the FcR 7-chain is not deleted, in various
embodiments animals
7 containing a replacement of endogenous FcyR a-chain genes with human FayR
a-chain genes
8 should be able to process normal effector functions from antibodies
through binding of the Fc
9 portion of IgG immunoglobulins to the human FcyR a-chains present on the
surface of
accessory cells.
11 [0092] A schematic illustration (not to scale) of a deleted
endogenous low affinity mouse
12 FcyR gene is provided in Figure 4 (top). As illustrated, low affinity
human FcyR genes FcyRIIA
13 and FcyRIIIA are inserted into the deleted endogenous low affinity mouse
FcyR gene locus by a
14 targeting construct (Human Fc7R111A-IIA Targeting Vector) with a genomic
fragment containing
the human low affinity human FcyRI1A and Fc7RIIIA genes. Each of these genes
comprise the
16 a-chain or ligand-binding domain of the human FcR genes responsible for
the binding the Fc
17 portion of an antibody molecule.
18 [0093] A genetically modified mouse that expresses low affinity
human FcyR genes at the
19 endogenous low affinity mouse FcyR locus can be made by any method known
in the art. For
example, a targeting vector can be made that introduces low affinity human
FcyR genes (e.g.,
21 Fc7RIIA and FcyRIIIA) with a selectable marker gene. Figure 4
illustrates a mouse genome
22 comprising a deletion of the endogenous low affinity FcgR locus (top).
As illustrated, the
23 targeting construct contains a 5' homology arm containing sequence
upstream of the
24 endogenous low affinity mouse FcyR locus, followed by a drug selection
cassette (e.g., a
hygromycin resistance gene flanked on both sides by loxP sequences), a genomic
fragment
26 containing a human FcyRIIA gene, human HSP76 gene and human Fc7RIIIA
gene, and a 3'
27 homology arm containing sequence downstream of the endogenous low
affinity mouse FcyR
28 locus. Upon homologous recombination at the deleted locus, the drug
selection cassette is
29 replaced by the sequence contained in the targeting vector (bottom of
Figure 4). The
endogenous low affinity FcyR gene locus is thus replaced with low affinity
human FcyR genes
31 resulting in a cell or animal that expresses low-affinity human FcyR
genes. The drug selection
22
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 cassette may optionally be removed by the subsequent addition of a
recombinase (e.g., by Cre
2 treatment).
3 [0094] For expression of hFcyRIIA on platelets, the targeting
construct Human hFcyRIIA -IIA
4 Targeting Vector comprises an extended sequence that includes, e.g., all
or substantially all of
the human promoter region operably linked to the hFcgRIIA gene in a human
genome. For
6 preventing expression of hFcyRIIA on platelets, the targeting construct
lacks all or substantially
7 all of the human promoter region operably linked to the hFcyRIIA gene in
a human.
8 [0095] Further modifications to the chimeric locus (bottom of
Figure 4) can be achieved
9 using similar techniques as described for replacement with two human FcyR
genes. The
modification to replace the endogenous low affinity FcyR gene locus with two
human FcyR
11 genes can further provide a starting point for incorporation of other
low affinity human FcyR
12 genes. For example, a schematic illustration (not to scale) of an
endogenous low affinity FcyR
13 locus replaced with two human low affinity FcyR genes is provided in
Figure 6 (top). As
14 illustrated, low affinity human FcyR genes FcyRI1B, FcyRIIC and FcyRIIIB
are inserted into the
modified endogenous low affinity mouse FcyR gene locus by another targeting
construct
16 (Human FcyRIIB-IIIB-IIC Targeting Vector) with a genomic fragment
containing the low affinity
17 human FcyRIIB, FcyRIIC and FcyRIIIB genes. Each of these genes comprise
the a-chain or
18 ligand-binding domain of the human FcyR genes responsible for the
binding the Fc portion of an
19 antibody molecule.
[0096] A genetically modified mouse that expresses five low affinity human
Fc7R genes at
21 the endogenous low affinity mouse FcyR locus can be made by any method
known in the art.
22 For example, a targeting vector can be made that introduces low affinity
human Fc7R genes
23 (e.g., FcyRIIB, FcyRIIC and FcyRIIIB) with a selectable marker gene.
Figure 6 illustrates a
24 mouse genome comprising a replacement of the endogenous low affinity
FcyR locus with two
low affinity human FcyR genes (top). As illustrated, the targeting construct
contains a 5'
26 homology arm containing sequence upstream of the endogenous low affinity
mouse FcyR locus,
27 followed by a drug selection cassette (e.g., a neomycin resistance gene
flanked on both sides
28 by loxP sequences), a genomic fragment containing a human FcyRIIB gene,
a human Fc7R111B,
29 a human HSP77 gene, a human FcyRIIC gene, followed by a 3' homology arm
containing
sequence upstream of the low affinity human FcyRIIIA gene present at the
endogenous locus.
31 Upon homologous recombination at the modified locus, a human Fc7R11B,
FcyRIIIB and FcyRIIC
23
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 gene are inserted 5' to the human FcyRIIIA and FcyRIIA genes previously
present at the
2 endogenous low affinity FcyR gene locus by the sequence contained in the
targeting vector
3 (bottom of Figure 6). The modified endogenous low affinity FcyR gene
locus is thus further
4 modified to incorporate three additional low affinity human FcyR genes
resulting in a cell or
animal that expresses five low-affinity human FcyR genes. The drug selection
cassette may
6 optionally be removed by the subsequent addition of a recombinase (e.g.,
by Cre treatment).
7 Figure 6 (bottom) shows the structure of the resulting locus, which will
express five low affinity
8 human Fc7R genes that can be detected on the surface of accessory cells
of the animal's
9 immune system and independently associate, as appropriate, with an
endogenous FcRy-chain.
11 Experimental Models of FcyR Deficient Mice and FcyR Humanized Mice
12 [0097] Genetically modified non-human animals that do not express
endogenous low affinity
13 mouse FcyR genes are useful, e.g., to elucidate the various functions of
the individual low
14 affinity FcyR genes in the immune response, to measure the efficacy of a
human therapeutic
antibody via cell-mediated immunity (e.g., ADCC), to determine an Fc7R's role
in immune
16 diseases or disorder, to serve as models of immune diseases or
disorders, to generate
17 antibodies against one or more FcyR proteins, and to serve as breeding
mates to generate
18 other genetically modified mice of interest.
19 [0098] In one embodiment, a mouse according to the invention can
be used to determine a
cytotoxic effect lost (in comparison to a wild type mouse) by a mouse that
does not express low
21 affinity FcyR genes by administering an agent to such a mouse, where the
agent is known to
22 trigger an FcyR-dependent cytotoxic effect in wild type mice. In one
embodiment, a mouse of
23 the present invention is implanted with tumor cells and, after a
subsequent period of time,
24 injected with an antibody specific for an antigen expressed on the
surface of the tumor cells.
The isotype of the antibody is known prior to injection and the animals are
analyzed for
26 impairment of FcyR.-dependent ADCC by comparison to ADCC observed in
wild type animals.
27 [0099] In another aspect, mice deficient in endogenous low
affinity receptors could be
28 combined (e.g., by breeding) with other immune deficient mice to develop
in .vivo models of
29 autoimmune disease. For example, Severe Combined Immunodeficiency (SCID)
mice are
routinely used in the art as model organisms for studying the immune system.
SCID mice have
31 an impaired ability to make T or B lymphocytes, or activate some
components of the
32 complement system, and cannot efficiently fight infections, reject
tumors, and reject transplants.
24
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 Low affinity FcyR a-subunit gene-deficient mice of the present invention
may be bred to SCID
2 mice to ascertain cell depletion in a host animal in response to
administration of an antibody
3 therapeutic (e.g., an anti-tumor antibody), which wouid determine the
roles of ADCC and
4 complement-dependent cytotoxicity (CDC) in tumor cell depletion in vivo.
[00100] In another aspect, genetically modified non-human animals
comprising a
6 replacement of the endogenous low affinity FcyR genes with low-affinity
human FcyR genes are
7 provided. Such animals are useful for studying the pharmacokinetics of
fully human antibodies
8 and hFcyR-mediated ADCC. In addition, human FcyR genes have been shown to
exhibit
9 polymorphisms or allelic variants associated with disease (e.g., SLE, RA,
Wegener's
granulomatosis, Guillain-Barre syndrome and Multiple Sclerosis). Thus,
genetically modified
11 non-human animals that comprise a replacement of the endogenous low
affinity FcyR genes
12 with specific allelic or polymorphic forms of human Fc7IR genes can be
used to study human
13 autoimmune diseases, and traits associated with the polymorphisms, in
the animal. In a specific
14 embodiment, the allelic forms of human FayR genes are associated with
enhanced efficacy for
human IgG.
16 [00101] In another specific embodiment, the affect of a human low
affinity FcyR
17 polymorphism on the efficacy of a human antibody therapeutic is
determined. In a specific
18 embodiment, an anti-tumor antibody is administered to a first humanized
mouse comprising a
19 first polymorphism of a human FcyR and also to a second humanized mouse
comprising a
second polymorphism of a human Fc7R, wherein the first and the second mice
each comprise a
21 human tumor cell; and the anti-tumor activity of the anti-tumor antibody
is assessed in the first
22 mouse and in the second mouse. In a specific embodiment, a treatment
option is selected by a
23 physician with respect to treating a human haying the first or the
second polymorphism and
24 having a tumor corresponding to the human tumor cell, based on the
assessment of efficacy of
the anti-tumor antibody in the first mouse and in the second mouse.
26 [00102] Suitable polymorphisms of human FcyR genes include all
those known in the art.
27 For the human FayRIIA gene, polymorphisms include, e.g., the high
responder and low
28 responder phenotype reported by the ability of T cells to proliferate in
response to IgG. The
29 high responder polymorphism is characterized by an arginine residue at
position 131 (131Arg)
while the low responder is characterized by a histidine residue at position
131 (131His). In a =
31 specific embodiment, the human FcyRI IA sequence comprises the 131His
polymorphism. A
32 representative protein sequence of the human FcyRIIA u.-chain is shown
in SEQ ID NO:32.
22836950 1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 [00103] Single-nucleotide substitutions of the human Fc7RI1B gene
result in mis-sense
2 substitutions in the ligand-binding domain (a-chain) and putatively
affect the binding ability of an
3 Fc portion of an IgG to bind to the a-chain of Fc7R11B on the cell
surface. For example,
4 substitution of a threonine residue for an isoleucine at position 232
(11e232Thr) within the
transmembrane domain of the FcyRIIB gene in mice has been shown to impair the
signaling
6 ability of the receptor. In a specific embodiment, the human Fc/RI1B gene
comprises the
7 isoleucine variant (23211e). A representative protein sequence of the
human Fc7RI1B a-chain is
8 shown in SEQ ID NO:33.
9 [00104] Allelic variants of the human Fc7RII1A gene are proposed
to be involved in
susceptibility to SLE and RA. This allelic variant includes a phenylalanine
substitution for valine
11 at position 158 (Va1158Phe). The valine allelic variant (158Val) is
characterized to have a
12 higher affinity for IgG1 and IgG3 than the phenylalanine allelic variant
(158Phe). The 158Phe
13 allelic variant has been proposed to lead to a reduced clearance of
immune complexes. In a
14 specific embodiment, the human FORMA gene comprises the 158Val allelic
variant. A
representative protein sequence of the human Fe7RII1A a-chain is shown in SEQ
ID NO:35.
16 [00105] Allelic variants of the human FcyRIIIB gene include the
neutrophil antigen 1 (NA1)
17 and neutrophil antigen 2 (NA2) alleles. These allelic variants have been
proposed to be
18 involved in blood-transfusion reactions, alloimmune neutropaenia, SLE
and Wegener's
19 granulomatosis. The NA2 allelic variant is characterized by a diminished
ability to mediate
phagocytosis. In a specific embodiment, the human Fc7R111B gene comprises the
NA2 allelic
21 variant. A representative protein sequence of the human FcyRIIIB a-chain
is shown in SEQ ID
22 NO:36.
23 [00106] In one aspect, the genetically modified non-human animals
are useful for optimizing
24 FcyR-mediated functions triggered by the Fc portion of therapeutic
antibodies. The Fc regions
of antibodies can be modified by any method known in the art. For example,
amino acid
26 residues within the Fc portion (e.g., CH2 and CH3 domains) can be
modified to selectively
27 enhance the binding affinity to human Fc7RII1A. Thus, the resulting
antibody should have
28 enhanced Fen/FHA-dependent ADCC. In a specific embodiment, an animal
expressing human
29 FcyRII1A of the present invention is used to evaluate the enhanced ADCC
ability of a modified
human antibody by administering a modified human antibody to the animal,
detecting (e.g., in
31 vitro) antibody binding to FORMA-expressing cells and comparing the ADCC
activity observed
32 to the ADCC activity observed from that determined in a wild type
animal.
26
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1
2 EXAMPLES
3
4 Example 1: Generation of Low Affinity FcyR Gene Deficient Mice
[00107] A targeting construct for introducing a deletion of the endogenous
low affinity mouse
6 FcyR locus (described below) was constructed (Figure 1).
7 [00108] The targeting construct was made using VELOCIGENEO technology
(see, e.g., US
8 Pat. No. 6,586,251 and Valenzuela etal. (2003) High-throughput
engineering of the mouse
9 genome coupled with high-resolution expression analysis, Nature Biotech.
21(6):652-659) to
modify the Bacterial Artificial Chromosome (BAG) RP23-395f6 (Invitrogen). RP23-
395f6 BAG
11 DNA was modified to delete the endogenous low affinity FcyRIIB, FcyRIV
and FcyRIII genes
12 comprising the a-chain of each of the FcyRs.
13 [00109] Briefly, upstream and downstream homology arms were made
employing primers
14 mFcR 5-up-1 (5'-ACCAGGATAT GACCTGTAGA G; SEQ ID NO:1) and mFcR 3-up-1a
(GTCCATGGGT AAGTAGAAAC A; SEQ ID NO:2), and mFcR 5-DN (ATGCGAGCTC
16 ATGCATCTATG TCGGGTGCGG AGAAAGAGGT AATGCATTCT TGCCCAATAC TTAC; SEQ
17 ID NO:3) and mFcR 3-DN (ACTCATGGAG CCTCAACAGG A; SEQ ID NO:4),
respectively.
18 These homology arms were used to make a cassette that deleted the a-
chains of the
19 endogenous low affinity FcyRIIB, FcyRIV and FcyRIII genes. The targeting
construct included a
loxed neomycin resistance gene comprising homology arms comprising sequence
homologous
21 to a 5' and a 3' region with respect to the endogenous locus. Genes
and/or sequences
22 upstream of the endogenous FcyRIIB gene and downstream of the endogenous
Fc7R111 gene
23 (see Figure 1) were unmodified by the targeting construct.
24 [00110] The targeted deletion was confirmed by polymerase chain
reaction (PCR) using
primers outside the deleted region and within the targeting construct. The
upstream region of
26 the deleted locus was confirmed by PCR using primers to mFcR-up-detect
(ATCCTGAGTA
27 TACTATGACA AGA; SEQ ID NO:5) and PGK-up-detect (ACTAGTGAGA CGTGCTACTT C;
28 SEQ ID NO:6), whereas the downstream region of the deleted locus was
confirmed using
29 primers pA-DN-detect (CTCCCACTCA TGATCTATAG A; SEQ ID NO:7) and mFcR-DN-
detect
(TGGAGCCTCA ACAGGACTCC A; SEQ ID NO:8). The nucleotide sequence across the
31 upstream deletion point included the following, which indicates
endogenous mouse sequence
32 downstream of the FcyRIIB gene (contained within the parentheses below)
linked contiguously
27
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 to cassette sequence present at the deletion point: (GTCCATGGGT
AAGTAGAAAC
2 A)TTCGCTACC TTAGGACCGT TA (SEQ ID NO:9). The nucleotide sequence across
the
3 downstream deletion point included the following, which indicates
cassette sequence
4 contiguous with endogenous mouse sequence upstream of the FORIII gene
(contained within
the parentheses below): CGGGTGCGGA GAAAGAGGTA AT(GCATTCTT GCCCAATACT TA)
6 (SEQ ID NO:10).
7 [00111] Mice deficient in FcyRIIB, FORIII and FcyRIV were
generated through
8 electroporation of a targeted BAG DNA (described above) into mouse ES
cells. Positive ES
9 cells clones are confirmed by Taqman TM screening and karyotyping.
Positive ES cell clones
were then used to implant female mice to give rise to a litter of pups
deficient in low affinity FOR
11 genes.
12
13 Example 2: Characterization of Low Affinity FcyR Gene Deficient Mice
14 [00112] Spleens were harvested from FcyR deficient and wild type
mice and perfused with 10
mL Collagenase-D in sterile disposable bags. Each bag containing a single
spleen was then
16 placed into a Stomacher (Seward) and homogenized at a medium setting
for 30 seconds.
17 Homogenized spleens were transferred to 10 cm petri dishes and incubated
for 25 minutes at
18 37 C. Cells were separated with a pipette using a 1:50 dilution of 0.5 M
EDTA, followed by
19 another incubation for five minutes at 37 C. Cells were then pelleted
with a centrifuge (1000
rpm for 10 minutes) and red blood cells were lysed in 4 mL ACK buffer
(Invitrogen) for three
21 minutes. Splenocytes were diluted with RPMI-1640 (Sigma) and centrifuged
again. Pelleted
22 cells were resuspended in 10mL RPMI-1640 and filtered with a 0.2 pm cell
strainer.
23 [00113] Flow Cytometry. Lymphocyte cell populations were
identified by FACs on the BD
24 LSR II System (BD Bioscience) with the following flourochrome conjugated
cell surface markers:
anti-CD19 (B cells), anti-CD3 (T cells), anti-NKp46 (NK cells) and anti-F4/80
(macrophages).
26 Lymphocytes were gated for specific cell lineages and analyzed for
expression of endogenous
27 FcyRIII and FcyfRIIB with a rat anti-mouse Fo(RIII/II antibody (clone
2.4G2, BD Biosciences).
28 Clone 2.4G2 recognizes a common polymorphic epitope on the extracellular
domains of murine
29 FORM and Fc71:111. The results show that there was no detectable murine
low affinity FcyRIII or
FORII on B-cells, NK cells and macrophages in mFoiR KO mice (Figure 2).
31 [00114] ADCC Assay. Splenocytes isolated from FOR gene deficient and
wild type mice
32 were analyzed for their ability to perform ADCC in a cell-killing assay.
Cell populations were
28
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 isolated and separated using MACS Technology (Miltenyi Biotec). Briefly,
T-cells were
2 depleted from splenocytes using magnetically labeled anti-mouse CD3
beads. The T-cell
3 depleted splenocytes were then enriched for NK cells using magnetically
labeled anti-mouse
4 CD49B beads. Separately, Raji cells (expressing human CD20) were coated
with varying
concentrations (ranging from 0.1 to 10 pg/mL) of mouse anti-human CO20
antibody (Clone Bl;
6 Beckman Coulter) for 30 minutes at 4 C. The antibody-coated Raji cells
were incubated with
7 the enriched NK cells at ratios (NK:Raji) of 100:1 and 50:1 for four
hours at 37 C. Cell death
8 was measured using the CytoTox-Glo TM Cytotoxicity Assay (Promega).
Luminescence signal is
9 derived from lysed cells and proportional to the number of dead cells.
Luminescence from
controls (no anti-CD20 antibody) was determined for background dead cell count
for each ratio
11 and subtracted from measurements for wild type and KO mice. Average cell
death was
12 calculated and percent decrease in cell killing (% ADCC) was determined
by comparison to wild
13 type. Results are shown in Table 1.
14
Table 1
% ADCC
mFcyR KO 10 pg/mL 1 pg/mL 0.1 pg/mL
B1 Antibody B1 Antibody B1 Antibody
100:1 42 53 35
NK cell:Raji cell __________________________________________________
50:1 15 0 0
16
17 Example 3: In Vivo Depletion of B cells in Low Affinity Fcyll Gene
Deficient Mice
18 [00115] The effect of human or murine Fc isotypes on B cell
depletion through the ADCC
19 pathway was determined for various B cell compartments in low affinity
Fc-IR gene deficient
mice engineered to express human CD20 using a human anti-human CD20 antibody.
Mice
21 expressing human CD20 were separately engineered using techniques known
in the art. Mice
22 that express human CD20 on B cells and deficient in low affinity FcyR
genes (described in
23 Example 1) were made by standard breeding techniques of the two
engineered strains.
24 [00116] Separate groups of mice that expressed human CD20 and had a full
complement of
endogenous low affinity Fc7R genes were each administered one of the
following: (1) 10 mg/kg
26 control antibody (N=4; human antibody not specific for human CD20 having
a mouse IgG2a);
27 (2) 2 mg/kg Ab 168 (N=3; human anti-hCD20 antibody with a mouse IgG2a;
heavy and light
28 chain variable region sequences found in SEQ ID NOs: 339 and 347,
respectively, of US Patent
29
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 Publication No. 2009/0035322); (3) 10 mg/kg Ab 168; (4) 2 mg/kg Ab 735
(N=3; Ab 168 with
2 human IgG1); (5) 10 mg/kg Ab 735. In a similar set of experiments, groups
of mice that
3 expressed human CD20 and had a deletion of the endogenous low affinity
FcyR genes were
4 administered the control and human anti-hCD20 antibodies (described
above).
[00117] Mice in each group were administered the antibodies by intra-
peritoneal injections.
6 Seven days post-injection, animals were euthanized and the remaining B
cell contents of bone
7 marrow (B220+/IgM+), peripheral blood (B220+/CD19-), lymph node
(B220+/CD19+) and spleen
8 (B220+/CD19') were identified by multi-color FACS performed on a LSR-
11flow cytometer and
9 analyzed using Flow-Jo software (as described above). The results of the
B cell depletion
experiments are shown in Figures 3A-3D.
11 [00118] As shown in Figures 3A-3D, Ab 735 depleted B cells with a
lower efficiency than Ab
12 168 in mice containing a complete complement of low affinity FcyR genes.
Further, for both
13 antibodies (mouse and human Fc), B cell depletion was significantly
reduced in mice lacking a
14 complete complement of low affinity FcyR genes. This Example shows that
the ability to deplete
B cells through the ADCC pathway requires low affinity FcyRs and demonstrate
that measuring
16 ADCC efficiency for antibodies containing human constant regions in mice
is more suitable by
17 the use of genetically engineered mice containing a full complement of
human low affinity FcyR
18 genes.
19
Example 4: Generation of FcyRIIIA/FcyRIIA Humanized Mice
21 [00119] A targeting construct for introducing two low affinity
human FcyR genes into a
22 deleted endogenous low affinity mouse FcyR locus (described below) was
constructed (Figure
23 4).
24 [00120] A targeting construct comprising human FcyRIIA and FcyRIIIA
genes was made
using similar methods (see Example 1) through modification of BAG RP23-395f6
and CTD-
26 2514j12 (Invitrogen). BAG DNA of both BACs was modified to introduce a
deletion of the CI:-
27 chains of the low affinity human FcyRIIA and Fc7RIIIA genes into the
deleted endogenous low
28 affinity FcyR locus.
29 [00121] In a similar fashion, upstream and downstream homology
arms were made
employing primers h14 (GCCAGCCACA AAGGAGATAA TC; SEQ ID NO:11) and h15
31 (GCAACATTTA GGACAACTCG GG; SEQ ID NO:12), and h4 (GATTTCCTAA CCACCTACCC
32 C; SEQ ID NO:13) and h5 (TCTTTTCCAA TGGCAGTTG; SEQ ID NO:14),
respectively. These
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 homology arms were used to make a cassette that introduced the a-chains
of low affinity human
2 FcyRIIA and FORMA genes into the endogenous mouse low affinity FcyR
locus. The targeting
3 construct included a 5' homology arm including sequence 5' to the deleted
endogenous low
4 affinity FcyR locus, a FRT'ed hygromycin resistance gene, followed by a
human genomic
fragment from BAG CTD-2514j12 comprising low affinity human FcyRIIA and Fe-
J=1111A a-chain
6 genes, and a 3' homology arm comprising mouse sequence 3' to the deleted
endogenous low
7 affinity FcyR locus (middle of Figure 4). For a mouse that expresses
FcyRIIA on mouse
8 platelets, a targeting construct was made in a similar manner (using the
same BACs) except
9 that the construct comprises an extended promoter sequence operably
linked to the human
FcyRIIA gene in the human genome, e.g., up to about 18 kb or more, using a
hygromycin
11 cassette that is flanked on both sides by 1ox2372 sites, wherein the
junction of the promoter
12 region and the first lox 2372 site is ATCGGGGATA GAGATGTTTG (CC)GCGATCGC
13 GGTACCGGGC (SEQ ID NO:37 human/lox2372 junction in parentheses) and
wherein the
14 junction of the second 1ox2372 site and mouse sequence is TTATACGAAG
TTATACCGG(T
G)CATTCTTGC CCAATACTTA (SEQ ID NO:38 1ox2372/mouse junction in parentheses).
16 Suitable primers were used to genotype the humanization comprising the
promoter region.
17 [00122] Targeted insertion of the human FcyRIIA and Fcy19111A a-
chain genes was confirmed
18 by FOR (as described above). The upstream region of the partially
humanized locus was
19 confirmed by FOR using primers h16 (CCCAGGTAAG TCGTGATGAA ACAG; SEQ ID
NO:15)
and pA-ON-detect (CTCCCACTCA TGATCTATAG A; SEQ ID NO:16), whereas the
21 downstream region of the partially humanized locus was confirmed using
primers mFcR ON-
22 detect-9 (TGGAGCCTCA ACAGGACTCC A; SEQ ID NO:17) and h6 (CACACATCTC
23 CTGGTGACTT G; SEQ ID NO:18). The nucleotide sequence across the
downstream junction
24 included the following, which indicates a novel insertion point of
endogenous human sequence
upstream of the hFcyRIIA gene (contained within the parentheses below)
contiguous with
26 endogenous mouse sequence 3' of the deleted low affinity FcyR locus:
(CAACTGCCAT
27 TGGAAAAGA)C TCGAGTGCCA TTTCATTACC TO (SEQ ID NO:19). The upstream
junction
28 includes two novel sequences. One point of the upstream junction
includes the following, which
29 indicates nucleotide sequence of the hygromycin cassette contiguous with
human genomic
sequence (contained within the parentheses below) that comprises the upstream
region of the
31 inserted hFcyRIIIA gene: TAAACCCGCG GTGGAGCTC(G CCAGCCACAA AGGAGATAAT
32 CA) (SEQ ID NO:20). The second point of the upstream junction includes
the following, which
31
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 indicates a nucleotide sequence of an endogenous mouse sequence
(contained within the
2 parentheses below) from the upstream region of the deleted low affinity
FcyR locus contiguous
3 with a nucleotide sequence within the hygromycin cassette: (CCATGGGTAA
GTAGAAAC)TC
4 TAGACCCCCG GGCTCGATAA CT (SEQ ID NO:21).
[00123] Mice containing two low affinity human FcyR genes (hFcyRIIA,
lacking extended
6 promoter region, and hFcyRIIIA) in place of the endogenous low affinity
mouse FcyR locus were
7 generated through electroporation of the targeted BAG DNA (described
above) into mouse ES
8 cells. Positive ES cells clones were confirmed by Taqman TM screening and
karyotyping.
9 Positive ES cell clones were then used to implant female mice using the
VELOCIMOUSECD
method (described below) to generate a litter of pups containing a replacement
of the
11 endogenous low affinity FcyR genes with the two human low affinity FcyR
genes.
12 [00124] Targeted ES cells described above were used as donor ES cells
and introduced into
13 an 8-cell stage mouse embryo by the VELOCIMOUSEO method (see, e.g., US
Pat. No.
14 7,294,754 and Poueymirou etal. (2007) FO generation mice that are
essentially fully derived
from the donor gene-targeted ES cells allowing immediate phenotypic analyses
Nature Biotech.
16 25(1):91-99. VELOCIMICEO (FO mice fully derived from the donor ES cell)
bearing hFcyRIIA
17 and hFcyRIIIA were identified by genotyping using a modification of
allele assay (Valenzuela at
18 al., supra) that detected the presence of the hFcyR genes.
19 [00125] Mice bearing the hFcyR genes can be bred to a Cre deleter
mouse strain (see, e.g.,
International Patent Application Publication No. WO 2009/114400) in order to
remove any loxed
21 neo cassette introduced by the targeting construct that is not removed,
e.g., at the ES cell stage
22 or in the embryo. Optionally, the neomycin cassette is retained in the
mice.
23 [00126] Pups are genotyped and a pup heterozygous for the hFcyR
genes is selected for
24 characterizing FcyRIIA and FcyRIIIA humanizations.
26 Example 5: Characterization of FcyRIIIA/FcylRilAl-lumanized Mice
27 [00127] Spleens were harvested from humanized FcyRIIIA/FcyRIIA
(heterozygotes, lacking
28 the extended FcyRIIA promoter region) and wild type mice and prepared
for FACs (as described
29 above).
[00128] Flow Cytometry. Lymphocytes were gated for specific cell lineages
and analyzed
31 for expression of hFc7R11 and hFcyR111 using a mouse anti-human Fc7R11
antibody (Clone
32 FLI8.26; BD Biosciences) and a mouse anti-human FcyR III antibody (Clone
3G8; BD
32
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 Biosciences), respectively. Relative expression (++, +) or no expression
(-) observed for each
2 lymphocyte subpopulation is shown in Table 2.
3
4 Table 2
Lymphocyte Lineage hFcyR111 hFcyRII
B cells
NK cells ++
Macrophages
Neutrophils
6 [00129] In a similar experiment, spleens were harvested from
humanized FcyRIIIA/FcyRIIA
7 (homozygotes, lacking the extended FcyRIIA promoter region) and wild type
mice and prepared
8 for FACs (as described above). Results are shown in Figures 5A and 5B.
Percent of separate
9 lymphocyte cell populations expressing human FcyRIIIA, FcyRIIA or both in
FcyRIIIA/FcyRIIA
homozygote mice is shown in Table 3.
11
12 Table 3
Lymphocyte Lineage hFcyRIII hFc7R11 hFcyRII/hFcyR111
NK cells 97
Macrophages 26 14 39
Neutrophils 94
13
14 [00130] As shown in this Example, genetically modified mice (both
heterozygote and
homozygote genotypes) generated in accordance with Example 3 expressed human
FcyRIIIA
16 on NK cells and macrophages; and human FORMA on neutrophils and
macrophages, but not
17 platelets. Human FcyRIIIA was highly expressed on NK cells. The
expression pattern of human
18 FcyR genes shown in this Example is consistent with the expression
patterns of these genes in
19 human accessory cells.
33
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 Example 6: Generation of Low Affinity FcyR Humanized Mice
2 [00131] A targeting construct for introducing three additional low
affinity human Fc7R genes
3 into a partially humanized endogenous low affinity Fc7R locus (described
below) was
4 constructed (Figure 6).
[00132] A targeting construct comprising human Fc7RIIB, Fc7RIIIB and FcyRIIC
genes was
6 made using similar methods (see Example 1) through modification of BAG RP-
23 395f6 and
7 RP-11 697e5 (Invitrogen). BAG DNA of both BACs was modified to introduce
the a-chains of
8 the low affinity human FcyRIIB, Fc7RIIIB and Fc/RIIC genes into the
partially humanized
9 endogenous low affinity Fc^/IR locus containing two human low affinity
FcyR genes.
[00133] In a similar fashion, upstream and downstream homology arms were
made
11 employing primers mFcR up-1 (ACCAGGATAT GACCTGTAGA G; SEQ ID NO:22) and
12 mFcR2b Nhel-2 (GTTTCTACTT ACCCATGGAC; SEQ ID NO:23), and h10 (AAATACACAC
13 TGCCACAGAC AG; SEQ ID NO:24) and h11 (CCTCTTTTGT GAGTTTCCTG TG; SEQ ID
14 NO:25), respectively. These homology arms were used to make a cassette
that introduced
DNA sequences encoding the a-chains of low affinity human Fc-yRIIB, Fc7RIIIB
and Fc7RIIC.
16 The targeting construct included a 5' homology arm including mouse
sequence 5' to the deleted
17 endogenous low affinity FcyR locus, a loxed neomycin resistance gene,
followed by a human
18 genomic fragment from BAG RP-11 697e5 comprising low affinity human
Fc7RIIB, FcyRIIIB and
19 FcyRIIC a-chain genes, and a 3' homology arm comprising human sequence
5' to the low
affinity human FcyRIIIA a-chain gene (middle of Figure 6).
21 [00134] Targeted insertion of three additional low affinity human
FOR genes was confirmed
22 by PCR (as described above). The upstream region of the fully humanized
locus was confirmed
23 by PCR using primers mFcR up-detect-3 (GAGTATACTA TGACAAGAGC ATC; SEQ ID
NO:26)
24 and PGK up-detect (ACTAGTGAGA CGTGCTACTT C; SEQ ID NO:27), whereas the
downstream region of the fully humanized locus was confirmed using primers neo
detect
26 (CTCCCACTCA TGATCTATAG A; SEQ ID NO:28) and h12 (CTTTTTATGG TCCCACAATC
27 AG; SEQ ID NO:29). The nucleotide sequence across the downstream
junction included the
28 same human genomic sequence upstream of the hFc-,,RIIA a-chain gene (see
Example 3; SEQ
29 ID NO:19). The nucleotide sequence across the upstream junction included
the following, which
indicates two novel junctions of mouse and cassette sequences and cassette and
human
31 genomic sequences at the insertion point. The junction of genomic mouse
sequence (contained
32 within the parentheses below) and the upstream region of the neo
cassette sequence is:
34
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 (GTCCATGGGT AAGTAGAAAC A)TTCGCTACC TTAGGACCGT TA (SEQ ID NO:30). The
2 second novel junction includes the joining of the 3' end of neo cassette
(contained within the
3 parentheses below) and a human genomic sequence downstream of the
hFcgRIIB a-chain
4 gene: (GCTTATCGAT ACCGTCGAC)A AATACACACT GCCACAGACA GG; SEQ ID NO:31).
These junctions are show in Figure 6 (middle) within the targeting construct.
The resulting
6 modified genome of the fully humanized low affinity FcyR locus is shown
in Figure 6 (bottom).
7 [00135] Mice containing five low affinity human Fc7R genes in
place of the endogenous low
8 affinity mouse Fc7R locus were generated through electroporation of the
targeted BAG DNA
9 (described above) into mouse ES cells. Positive ES cells clones were
confirmed by Tadman TM
screening and karyotyping. Positive ES cell clones were then used to implant
female mice (as
11 described above) to give rise to a litter of pups containing a
replacement of the endogenous low
12 affinity FayR genes for five human low affinity Fc7R genes.
13
14 Example 7: Characterization of Low Affinity Fcyi-71 Humanized Mice
[00136] Spleens were harvested from fully humanized Fc7R (heterozygotes)
and wild type
16 mice and prepared for FACs (as described above).
1/ [00137] Flow Cytometry. Lymphocytes were gated for specific cell
lineages and analyzed
18 for expression of human Fc7RIIA and FcyRIIIA using a mouse anti-human
Fc7RII antibody
19 (Clone FLI8.26; BD Biosciences) and a mouse anti-human FcTRIII antibody
(Clone 3G8; BD
Biosciences), respectively. Relative expression (++, +) or no expression (-)
observed for each
21 lymphocyte subpopulation is shown in Table 4.
22
23 Table 4
Lymphocyte Lineage hFcyRill hFcyRil
B cells
NK cells
Macrophages
Neutrophils +
24
[00138] In a similar experiment, spleens were harvested from fully
humanized Fc-,,R
26 (homozygotes) and wild type mice and prepared for FACs (as described
above). Results are
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 shown in Figure 7. Percent of separate lymphocyte cell populations
expressing human
2 FcyRIIIA, human Fc/RIIIB, human FcyRIIA, human FcyRIIB, human Fc/RIIC or
a combination
3 thereof in fully humanized FcyR homozygote mice is shown in Table 5.
4
Table 5
Lymphocyte Lineage hFcyRIII hFcyR11 hFcyRII/hFcyR111
B cells 100
NK cells 30
Macrophages <1 55 26
Neutrophils 100
6
7 [00139] As shown in this Example, genetically modified mice (both
heterzyogote and
8 homozygote genotypes) generated in accordance with Example 5 expressed
human FcyRIIIA
9 on NK cells and macrophages, human FcyRIIIB on neutrophils, human Fc/RIIA
on neutrophils
and macrophages, human FcyRIIB on B cells, and human Fc:RIIC on NK cells. The
expression
11 pattern of human FcyR genes shown in this Example is consistent with the
expression patterns
12 of these genes in human accessory cells.
13
14 Example 8: ADCC in Humanized FcyR Mice
[00140] Splenocytes isolated from FcyR gene deficient (i.e. knockout),
Fc/R111A/FcyRIIA
16 (homozygotes), Fc7R111A/Fc7R111B/FcyRIIA/FcyRIIB/FayRIIC (homozygotes)
and wild type mice
17 were analyzed for their ability to perform ADCC in a cell-killing assay
(as described above in
18 Example 2).
19 [00141] Briefly, cell populations were isolated and separated
using MACS Technology
(Miltenyi Biotec). Briefly, T and B cell depleted splenocytes were cultured
for two weeks in the
21 presence of mouse IL-2 (500 U/mL). The resulting expanded NK cells were
used as effector
22 cells in the ADCC assays at a ratio of 50:1 (NK:Raji). Raji cells were
coated with 10 ug/mL of
23 Ab 168 or Ab 735 (as described above in Example 3). Results are shown in
Table 6.
24
36
22836950.1
CA 02784953 2016-01-13
CA 2,784,953
Blakes Ref: 68271/00042
1 Table e,
% ADCC
pg/mL 10 pg/mL
NK Cell Genotype
Ab 168 Ab 735
Wild Type 89 72
Mouse FcyR KO 13 14
Human FeyRIIIA-11A HO 78 85
Human FcyRIIIA-111B-11A-11B-11C HO 81 59
2
3 [00142] The present invention is not to be limited in scope by the
specific embodiments
4 describe herein. Indeed, various modifications of the invention in
addition to those described
5 herein will become apparent to those skilled in the art from the
foregoing description and the
6 accompanying figures. Such modifications are intended to fall within the
scope of the appended
7 claims.
8
37
22836950.1