Note: Descriptions are shown in the official language in which they were submitted.
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
1
PHARMACEUTICAL COMPOSITION COMPRISING VITAMIN D ANALOGUE AND
COSOLVENT-SURFACTANT MIXTURE
FIELD OF THE INVENTION
The present invention relates to a topical pharmaceutical composition for
cutaneous
application comprising a pharmacologically active agent, a surfactant, a co-
solvent and an
aqueous phase.
BACKGROUND OF THE INVENTION
Psoriasis is a chronic inflammatory skin disease that manifests as
erythematous, dry, scaling
plaques resulting from hyperkeratosis. The plaques are most often found on the
elbows,
knees and scalp, though more extensive lesions may appear on other parts of
the body,
notably the lumbosacral region. The most common treatment of mild to moderate
psoriasis
involves topical application of a composition containing a corticosteroid as
the active
ingredient. While efficacious, corticosteroids have the disadvantage of a
number of adverse
effects such as skin atrophy, striae, acneiform eruptions, perioral
dermatitis, overgrowth of
skin fungus and bacteria, hypopigmentation of pigmented skin and rosacea.
For many years, however, an advantageous non-steroidal treatment of psoriasis
has consisted
in topical treatment with the vitamin D analogue compound, calcipotriol,
formulated in an
ointment composition (marketed as Daivonex(@ or Dovonex ointment by LEO
Pharma) in
which the calcipotriol is present in solution or a cream composition (marketed
as Daivonex
or Dovonex cream by LEO Pharma). The solvent in the ointment composition is
propylene
glycol which has the advantage of enhancing penetration of the active
ingredient into the
skin, leading to an improved efficacy, but which is also known to act as a
skin irritant. Thus,
it has been reported that the inclusion of propylene glycol in topical
compositions frequently
causes patients to develop contact dermatitis (one study reported a number of
irritant
reactions to propylene glycol of 12.5%, cf. M. Hannuksela et al., Contact
Dermatitis 1, 1975,
pp. 112-116) , and the number of irritant reactions increases when propylene
glycol is used in
high concentrations (as reviewed by J. Catanzaro and J. Graham Smith, J. Am.
Acad.
Dermatol. 24, 1991, pp. 90-95). Due to the improved penetration of
calcipotriol into the skin
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
2
resulting, inter alia, from the presence of propylene glycol, Daivonex
ointment has been
found to be more efficacious in the treatment of psoriatic lesions than
Daivonex cream, but
has also caused skin irritation in a significant proportion of psoriasis
patients.
It is therefore an object of the invention to provide a topical composition
comprising a
vitamin D derivative or analogue as the active ingredient, which has skin
penetration and
biological activity properties comparable to those of Daivonex ointment, but
which does not
contain propylene glycol as the solvent.
SUMMARY OF THE INVENTION
Human skin, in particular the outer layer, the stratum corneum, provides an
effective barrier
against penetration of microbial pathogens and toxic chemicals. While this
property of skin is
generally beneficial, it complicates the dermal administration of
pharmaceuticals in that a
large quantity, if not most, of the active ingredient applied on the skin of a
patient suffering
from a dermal disease may not penetrate into the viable layers of the skin
where it exerts its
activity. To ensure an adequate penetration of the active ingredient to the
dermis and
epidermis, it is generally preferred to include the active ingredient in a
dissolved state,
typically in the presence of a solvent in the form of an alcohol, e.g.
ethanol, or diol, e.g.
propylene glycol. Propylene glycol is a well-known penetration enhancer, i.e.
a substance
which is capable of penetrating the stratum corneum and "draw" low-molecular
components
such as therapeutically active components in the vehicle into the epidermis.
Propylene glycol
may in itself give rise to significant skin irritation, and it is also capable
of "drawing" low-
molecular and potentially irritative components of the vehicle into the
epidermis, leading to
an overall irritative effect of conventional vehicles including propylene
glycol. For this
reason, the presence of propylene glycol as a solvent in compositions intended
for the
treatment of inflammatory skin diseases may exacerbate the inflammatory
response.
In the research leading to the present invention, it was an object to identify
a solvent
combination which is more effective to dissolve a sparingly soluble compound
such as a
vitamin D analogue than low-molecular alcohols or diols when used on their own
as co-
solvents in admixture with an aqueous phase, and which in addition comprises a
significantly
lower amount of the low-molecular alcohol co-solvent. It has surprisingly been
found that
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
3
mixing certain surfactants with certain lower alkanols provides mixtures with
an
exceptionally high solubilization capacity. The resulting composition where
the individual
solvent components act synergistically resulting in a satifactory penetration
of the vitamin D
derivative or analogue thereof into the viable layers of the skin at a lower
concentration of
co-solvent than when an alcohol or diol alone is used as the co-solvent.
Furthermore,
compositions of the invention exhibit a comparable or higher biological
activity to that of
Daivonex ointment as determined in the target gene activation assay described
in Example 4
below. In addition, the composition is physically stable, and the vitamin D
analogue is
chemically stable therein.
Accordingly, the present invention relates to a topical composition for
cutaneous application
which is an oil-in-water-in-oil emulsion comprising an aqueous phase
containing, dispersed
therein, a lipophilic phase comprising
(a) a vitamin D derivative or analogue in dissolved form;
(b) a non-ionic surfactant selected from the group consisting of polyoxyl
glycerides,
polyoxyethylene castor oil derivatives, polyoxyethylene alkyl ethers,
polysorbates or
poloxamers; and
(c) a lower alkanol co-solvent;
said aqueous phase being dispersed in a pharmaceutically acceptable anhydrous
lipophilic
carrier or vehicle.
In another aspect, the invention relates to a topical composition as described
herein for use in
the prevention or treatment of dermal diseases or conditions.
BRIEF DESCRIPTION OF THE DRAWINGS
Figs. 1-4 are graphs showing the solubility of calcipotriol monohydrate in co-
solvent-
surfactant mixtures included in the present composition compared to the
solubility of
calcipotriol monohydrate in either the co-solvent or the surfactant alone.
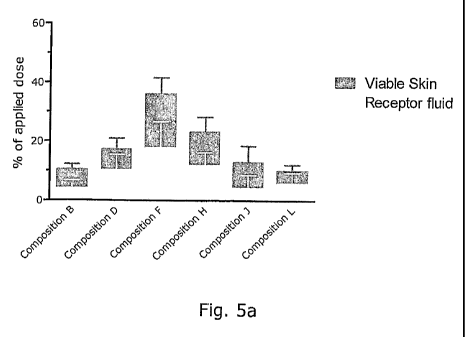
Fig. 5a and 5b are graphs showing the penetration into the skin of composition
of the
invention.
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
4
Fig. 6 is a schematic representation of the activation of the gene encoding
cathelicidin by
vitamin D3 in human keratinocytes. The mechanism of cathelicidin gene
activation is used in
a biological assay using reconstructed human epidermis (human keratinocytes
cultured so as
to form the epidermal layers characteristic of human skin) on which
calcipotriol-containing
compositions of the invention are applied to activate cathelicidin as
described in detail in
Example 4 below.
DETAILED DESCRIPTION OF THE INVENTION
Definitions
In the present context, the term "non-ionic surfactant" is intended to
indicate a surfactant
comprising a hydrophilic and a hydrophobic portion in which the hydrophilic
portion carries
no charge but derives its surface activity from highly polar groups such as
polyoxyethylene
groups. For the present purpose, the surfactant is an oil-in-water surfactant
with an HLB
value of 9-18.
The term "lower alkanol co-solvent" is intended to indicate a solvent
consisting essentially of
a C1_6 straight or branched alkanol, e.g. methanol, ethanol, propanol,
isopropanol or butanol.
The term "vitamin D derivative" is intended to indicate a biologically active
metabolite of
vitamin D3, such as calcitriol, or a precursor to such a metabolite, such as
alfacalcidol.
The term "vitamin D analogue" is intended to indicate a synthetic compound
comprising a
vitamin D scaffold with sidechain modifications and/or modifications of the
scaffold itself.
The analogue exhibits a biological activity on the vitamin D receptor
comparable to that of
naturally occurring vitamin D compounds.
"Calcipotriol" is a vitamin D analogue of the formula
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
OH
H
IH
HO' OH
Calcipotriol has been found to exist in two crystalline forms, an anhydrate
and a
monohydrate. Calcipotriol monohydrate and its preparation are disclosed in WO
94/15912.
5 The term "storage stability" is intended to indicate that the composition
exhibits chemical
and physical stability characteristics that permit storage of the composition,
at refrigeration
or, preferably, room temperature for a sufficient period of time to make the
composition
commercially viable, such as at least 12 months, in particular at least 18
months, and
preferably at least 2 years.
The term "chemical stability" or "chemically stable" is intended to indicate
that no more than
10%, preferably no more than 5%, of the vitamin D derivative or analogue
degrades over the
shelf-life of the product, typically 2 years. An approximation of chemical
stability at room
temperature is obtained by subjecting the composition to accelerated stability
studies at 40 C.
If less than about 10% of the substance has degraded after 3 months at 40 C,
this is usually
taken to correspond to a shelf-life of 2 years at room temperature. In
particular with respect
to calcipotriol, "chemical stability" is intended to mean that the
calcipotriol does not degrade
significantly over time to 24-epi calcipotriol or other degradation products
of calcipotriol in
the finished pharmaceutical product.
The term "physical stability" or "physically stable" is intended to mean that
the composition
retains its macroscopic and microscopic appearance over the shelf-life of the
product, e.g.
that the vitamin D derivative or analogue does not precipitate from the
solvent phase or that
there is no visible phase separation of the solvent phase and the carrier
phase.
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
6
The term "substantially anhydrous" is intended to mean that the content of
free water in the
lipophilic carrier or vehicle does not exceed about 2% by weight, preferably
not about I% by
weight, of the carrier or vehicle.
The term "solubilization capacity" is intended to indicate the ability of a
solvent or mixture
of solvents to dissolve a given substance, expressed as the amount required to
effect complete
solubilization of the substance.
The term "synergistic(ally)" is intended to imply that the solubility of the
vitamin D
derivative or analogue is significantly higher, in some instances several fold
higher, when a
combination of co-solvent and surfactant is present in the aqueous phase than
the sum of
solubilities in either the co-solvent or the surfactant when these are added
individually to the
aqueous phase.
The term "skin penetration" is intended to mean the diffusion of the active
ingredient into the
different layers of the skin, i.e. the stratum corneum, epidermis and dermis.
The term "skin permeation" is intended to mean the flux of the active
ingredient through the
skin into the systemic circulation or, in case of in vitro studies such as
those reported in
Example 3 below, the receptor fluid of the Franz cell apparatus used in the
experiment.
The term "biological activity" is intended to mean the activity of a vitamin D
derivative or
analogue when applied to skin in a composition of the invention. The
biological activity of
compositions is determined in an in vitro assay measuring the activation of a
target gene
encoding cathelicidin in a reconstructed human epidermis model involving
cultured human
keratinocytes, as described in detail in Example 5 below.
Embodiments of the invention
In an embodiment of the invention, the composition comprises a vitamin D
derivative or
analogue selected from the group consisting of calcipotriol, calcitriol,
tacalcitol,
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
7
maxacalcitol, paricalcitol and alfacalcidol. In a currently favoured
embodiment, the
composition comprises calcipotriol or calcipotriol monohydrate as the vitamin
D analogue.
In the present composition, the surfactant is generally present in a
concentration of from
about 0.5% by weight to about 5% by weight, or from about 1% by weight to
about 3% by
weight, or from about 1.2% by weight to about 2% by weight, such as about 1.5%
by weight,
of the composition.
According to the invention, the non-ionic surfactant is preferably selected
from the group
consisting of polyethylene glycol 8 caprylic/capric glyceride (a polyethylene
glycol
derivative of a mixture of mono-, di- and triglycerides of caprylic and capric
acids with an
average of 8 moles of ethylene oxide) or polyethylene glycol 6 caprylic/capric
glyceride (a
polyethylene glycol derivative of a mixture of mono-, di- and triglycerides of
caprylic and
capric acids with an average of 6 moles of ethylene oxide). The non-ionic
surfactant is
favourably polyethylene glycol 8 caprylic/capric glyceride, e.g. available
from Gattefosse
under the trade name Labrasol or from Condea under the trade name Softigen
767.
The non-ionic surfactant may also preferably be a polyethylene glycol C6_20
fatty acid
glyceride selected from the group consisting of caprylocaproyl PEG glyceride,
lauroyl PEG
glyceride, linoeoyl PEG glyceride, oleoyl PEG glyceride and stearoyl PEG
glyceride, a
polyoxyethylene C8_20 alkyl ether selected from the group consisting of PEG
monocetyl ether,
PEG monolauryl ether, PEG monooleyl ether and PEG monostearyl ether, a
polysorbate
selected from the group consisting of polysorbate 20, 40, 60 and 80, a
poloxamer selected
from the group consisting of poloxamer 124, 237, 338 and 407, or a
polyoxyethylene castor
oil derivative such as polyoxyl castor oil or hydrogenated polyoxyl castor
oil.
As indicated above, the composition further comprises a lower alkanol co-
solvent which may
favourably be selected from the group consisting of methanol, ethanol, n-
propanol,
isopropanol, n-butanol or 2-butanol. It has surprisingly been found that the
amount of lower
alkanol required to completely dissolve the vitamin D derivative or analogue
may be
substantially reduced (e.g. 2-5 fold reduced) in the presence of the
surfactant compared to the
amount required when the lower alkanol is used alone as a solvent. The lower
alkanol co-
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
8
solvent may favourably be present in a concentration of about 0.5-5 %, in
particular about 1-
3%, or about 2%, by weight of the composition.
In a currently favoured embodiment, the co-solvent is ethanol and the non-
ionic surfactant is
polyethylene glycol 8 caprylic/capric glyceride, polysorbate 80 or PEG
monocetyl ether, or
the co-solvent is isopropanol and the non-ionic surfactant is polyoxyl castor
oil, polyethylene
glycol 8 caprylic/capric glyceride or polysorbate 80.
The ointment carrier may be a hydrocarbon or mixture of hydrocarbons with
chain lengths
ranging from C5 to C60. A frequently used ointment carrier is petrolatum, or
white soft
paraffin, which is composed of hydrocarbons of different chain lengths peaking
at about C40-
44, or a mixture of petrolatum and liquid paraffin (consisting of hydrocarbons
of different
chain lengths peaking at C2840). While petrolatum provides occlusion of the
treated skin
surface, reducing transdermal loss of water and potentiating the therapeutic
effect of the
active ingredient in the composition, it tends to have a greasy and/or tacky
feel which persists
for quite some time after application, and it is not easily spreadable. It may
therefore be
preferred to employ paraffins consisting of hydrocarbons of a somewhat lower
chain length,
such as paraffins consisting of hydrocarbons with chain lengths peaking at C14-
16, C18-22, C20-
22, 020.26 or mixtures thereof (the hydrocarbon composition of the paraffins
has been
determined by gas chromatography). It has been found that such paraffins are
more
cosmetically acceptable in that they are less tacky and/or greasy on
application and more
easily spreadable. They are therefore expected to result in improved patient
compliance.
Suitable paraffins of this type, termed petrolatum jelly, are manufactured by
Sonneborn and
marketed under the trade name Sonnecone, e.g. Sonnecone CM, Sonnecone DM I,
Sonnecone
DM2 and Sonnecone HV. These paraffins are further disclosed and characterized
in WO
2008/141078 which is incorporated herein by reference.
To impart a desired viscosity to the present composition, it may suitably
include a lipophilic
viscosity-increasing ingredient such as a wax. The wax may be a mineral wax
composed of a
mixture of high molecular weight hydrocarbons, e.g. saturated C35_70 alkanes,
such as
microcrystalline wax. Alternatively, the wax may be a vegetable or animal wax,
e.g. esters of
014.32 fatty acids and C14-32 fatty alcohols, such as beeswax. The amount of
viscosity-
increasing ingredient may vary according to the viscosifying power of the
ingredient, but
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
9
may typically be in the range of about 1-20% by weight of the composition.
When the
viscosity-increasing ingredient is microcrystalline wax it is typically
present in an amount in
the range of about 5-15% by weight, e.g. about 10% by weight, of the
composition.
The composition may additionally comprise an emollient which may act to soften
the
thickened epidermis of the psoriatic plaques. A suitable emollient for
inclusion in the present
composition may be a silicone wax or a volatile silicone oil as the presence
of silicone has
additionally been found to aid penetration of calcipotriol into the skin.
Compositions
including a silicone have also been found to result in less skin irritation.
Suitable silicone oils
for inclusion in the present composition may be selected from cyclomethicone,
dimethicone.
The amount of silicone oil included in the present composition is typically in
the range of
from about 1 to about 10% by weight, e.g. about 5% by weight, of the
composition.
In Daivonex ointment, the presence of propylene glycol is believed to be a
major
contributor to the skin irritation experienced by many patients. However, it
has been found
that calcipotriol may in itself be mildly irritative in some patients (A.
Fullerton and J. Serup,
Br. J. Dermatol. 137, 1997, pp. 234-240 and A. Fullerton et al., Br. J.
Dermatol. 138, 1998,
pp. 259-265). It may therefore be advantageous to include an anti-irritant
compound in the
present composition, such as glycerol, butylene glycol, sorbitol, sucrose,
saccharin, menthol
or nicotinamide. Glycerol has been described as a substance that is capable of
protecting the
skin against irritative substances (J. Bettinger et al., Dermatology 197,
1998, pp. 18-24) and
has been found by us to reduce the release of IL-la in a dose-dependent
manner: thus, it has
been found that the presence of 15% by weight of glycerol in a calcipotriol
ointment results
in a significantly lower level of release of IL-1 a than does the inclusion of
10% by weight of
glycerol which, in turn, results in a significantly lower level of IL-1 a
release than does the
inclusion of 5% by weight of glycerol.
However, in addition to the anti-irritative effect, it has surprisingly been
found that glycerol
is capable of potentiating the biological activity of calcipotriol in that the
expression of
cathelicidin (in the assay described in Example 4 below) has been found to be
increased with
decreasing amounts of glycerol in the composition (i.e. more cathelicidin is
expressed when
the amount of glycerol is 5% by weight than when the amount of glycerol is 10%
or 15%,
respectively): this implies that with respect to inclusion of glycerol a
balance has to be struck
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
between a favourable anti-irritative effect and a favourable potentiating
effect. We have
found that the inclusion of about 5-10% by weight of glycerol in the present
composition
results in a significant anti-irritative effect as well as a significant
potentiation of the
biological activity of calcipotriol.
5
Calcipotriol is known to be a substance which is extremely sensitive to acid
conditions (pH
below about 7.0 in aqueous compositions or acidic reacting substances in non-
aqueous
compositions) which contribute to the rapid degradation of calcipotriol. To
ensure an
adequate chemical stability of the substance throughout the shelf-life of the
composition, it
10 may be advisable to include a compound capable of neutralizing acidic
impurities which may
be present in one or more of the excipients of the composition and which are
detrimental to
the chemical stability of calcipotriol. The acid neutralizing compound may
favourably be
selected from a buffer such as a phosphate buffer which may be included in an
amount of
about 0.025-0.1% by weight of the composition. The acid neutralizing compound
may also
be an amine such as triethanolamine, trometamol, monoethanolamine or
diethanolamine,
which may be included in the composition in an amount of about 0.1-2% by
weight.
To maintain good physical stability of the composition, in particular to avoid
separation of
the aqueous and lipid phases therein, it may be advantageous to include a
water-in-oil
emulsifier with an HLB value of 3-8. Examples of such emulsifiers are
polyoxyethylene C8_22
alkyl ethers, e.g. polyoxyethylene stearyl ether, polyoxyethylene cetyl ether
or
polyoxyethylene lauryl ether.
The amount of water in the composition may range from about 1% to about 15% by
weight,
e.g. from about 5% to about 10% by weight, of the composition.
In a specific embodiment, the present composition comprises
0.003-0.008% w/w calcipotriol (as monohydrate)
1-3% w/w polyethylene glycol 8 caprylic/capric glyceride
1-3% w/w ethanol
3-8% w/w polyoxyethylene stearyl ether
5-10% w/w water
80-93% w/w paraffin carrier.
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
11
In another embodiment, the present composition comprises
0.003-0.008% w/w calcipotriol (as monohydrate)
1-3% w/w polysorbate 80
1-3% w/w ethanol
3-8% w/w polyoxyethylene stearyl ether
5-10% w/w water
80-93% w/w paraffin carrier.
In a further embodiment, the composition comprises
0.003-0.008% w/w calcipotriol (as monohydrate)
1-3% w/w polyethylene glycol monocetyl ether
1-3% w/w ethanol
3-8% w/w polyoxyethylene stearyl ether
5-10% w/w water
80-93% w/w paraffin carrier.
In a still further embodiment, the present composition comprises
0.003-0.008% w/w calcipotriol (as monohydrate)
1-3% w/w polyethylene glycol 8 caprylic/capric glyceride
1-3% w/w isopropanol
3-8% w/w polyoxyethylene stearyl ether
5-10% w/w water
80-93% w/w paraffin carrier.
In a still further embodiment, the present composition comprises
0.003-0.008% w/w calcipotriol (as monohydrate)
1-3% w/w polyoxyl castor oil
1-3% w/w isopropanol
3-8% w/w polyoxyethylene stearyl ether
5-10% w/w water
80-93% w/w paraffin carrier.
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
12
In a still further embodiment, the present composition comprises
0.003-0.008% w/w calcipotriol (as monohydrate)
1-3% w/w polysorbate 80
1-3% w/w isopropanol
3-8% w/w polyoxyethylene stearyl ether
5-10% w/w water
80-93% w/w paraffin carrier.
The present composition may also comprise other components commonly used in
dermal
formulations, e.g. antioxidants (e.g. alpha-tocopherol), preservatives, sodium
edetate,
pigments, skin soothing agents, skin healing agents and skin conditioning
agents such as
urea, allantoin or bisabolol, cf. CTFA Cosmetic Ingredients Handbook, 2"d Ed.,
1992.
The composition of the invention may be used in the treatment of psoriasis,
sebopsoriasis,
pustulosis palmoplantaris, dermatitis, ichtyosis, rosacea and acne and related
skin diseases by
topically administering an effective amount of a composition according to the
invention to a
patient in need of such treatment. Said method preferably comprises topical
administration
once or twice a day of a therapeutically sufficient dosage of said
composition. To that end,
the composition according to the invention preferably contains about 0.001-0.5
mg/g,
preferably about 0.002-0.25 mg/g, in particular 0.005-0.05 mg/g, of the
vitamin D derivative
or analogue. It is envisaged that the present composition may advantageously
been used for
maintenance treatment of these dermal diseases, i.e. continued treatment after
the
diseappearance of visible symptoms to delay the recurrence of symptoms.
To provide a more effective treatment of psoriasis and other dermal conditions
in the acute
phase, it may be desirable to include one or more additional therapeutically
active ingredients
in the composition. Examples of such additional active ingredients include,
but are not
limited to, anti-inflammatory drugs such as corticosteroids, such as
betamethasone and esters
thereof, e.g. the valerate or dipropionate ester, clobetasol or esters
thereof, such as the
propionate, hydrocortisone or esters thereof, such as the acetate; non-
steroidal anti-
inflammatory drugs such as naproxen, indomethacin, diclofenac, ibuprofen,
dexibuprofen,
ketoprofen, flurbiprofen, piroxicam, tenoxicam, lornoxicam or nabumeton,
phosphodiesterase
4 inhibitors or p38 MAP kinase inhibitors.
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
13
The invention is further illustrated by the following examples which are not
in any way
intended to limit the scope of the invention as claimed.
Example 1
Compositions of the invention
The compositions shown in Tables 1 a and 1 b below were prepared by initially
mixing the
surfactant (Cremophor EL, Labrasol, polysorbate 80 or cetomacrogol 1000) with
the co-
solvent (ethanol or isopropanol), dissolving calcipotriol monohydrate in the
mixture and
finally adding the mixture to the aqueous buffer solution adjusted to pH 8.0
and glycerol
(when included). The resulting dispersion was then mixed with a mixture of
paraffins,
emulsifier (polyoxyethylene stearyl ether), DL-a-tocopherol and sodium
edetate.
Table 1 a
Compositions A-F according to the invention
Composition A B C D E F
mg/g
Calcipotriol monohydrate 0.050 0.050 0.050 0.050 0.050 0.050
Disodium hydrogen phosphate 0.65 0.65 0.65 0.65 0.26 0.26
Paraffin, liquid 50 50 50 50 50 50
Polyoxyethylene stearyl ether 50 50 50 50 50 50
Water, purified 65 65 65 65 65 65
Cetom acrogo l 1000 15 15
Cremophor EL 15 15
Labrasol 15 15
Ethanol 20 20 20 20
Isopropanol 20 20
Glycerol 85% 65 65 65
Sodium edetate 0.065 0.065 0.065 0.065 0.065 0.065
DL-a-tocopherol 0.02 0.02 0.02 0.02 0.02 0.02
Paraffin, white soft up to 1 g up to 1 g up to I g up to 1 g up to 1 g up to I
g
Table 1 b
Compositions G-L of the invention
Composition G H I J K L
mg/g
Calcipotriol monohydrate 0.050 0.050 0.050 0.050 0.050 0.050
Disodium hydrogen phosphate 0.65 0.65 0.65 0.65 0.65 0.65
Paraffin, liquid 50 50 50 50 50 50
Polyoxyethylene stearyl ether 50 50 50 50 50 50
Water, purified 65 65 65 65 65 65
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
14
Labrasol 15 15
Polysorbate 80 15 15 15 15
Ethanol 20 20
Isopropanol 20 20 20 20
Glycerol 85% 65 65 65
Sodium edetate 0.065 0.065 0.065 0.065 0.065 0.065
DL-a-tocopherol 0.02 0.02 0.02 0.02 0.02 0.02
Paraffin, white soft up to I g up to I g up to I g up to I g up to 1 g up to 1
g
Table 2
Compositions M and N of the invention
Composition mg/g M N
Calcipotriol monohydrate 0.050 0.050
Di sod iumhydrogenphosphate 0.65
Triethanolamine 1
Paraffin, liquid 50 50
Polyoxyethylene stearyl ether 50 50
Water, purified 65 65
Polysorbate 80 15
Labrasol 15
Ethanol 20 20
Glycerol 85% 65
Sodium edetate 0.065
DL-a-tocopherol 0.02 0.02
Petrolatum Jelly White 699.2 634.2
(Sonnecone DM 1)
Microcrystalline wax (Multiwax 100 100
180 MH)
Compositions M and N were prepared essentially as described above for
compositions A-L
with the exception that a mixture of Petrolatum Jelly White (Sonnecone DM 1)
and
microcrystalline wax was used instead of white soft paraffin and, as regards
composition N,
triethanolamine was added to the aqueous phase instead of disodium hydrogen
phosphate.
Example 2
Solubility of calcipotriol in solvent/surfactant mixtures in aqueous buffer
Cetomacrogol 1000 in combination with ethanol
The solubility, at 25 C, of calcipotriol monohydrate, determined as
calcipotriol, in aqueous
buffer pH 7.4 containing cetomacrogol 1000 as surfactant and ethanol as co-
solvent is shown
in Table 3 below. It appears from Table 3 that there is a small synergistic
effect of the
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
combination of cetomacrogol 1000 and ethanol on the solubility of calcipotriol
in the
aqueous phosphate buffer solution, i.e. the observed solubility of
calcipotriol in aqueous
phosphate buffer containing 15 % cetomacrogol 1000 and 20 % ethanol is 1468
p.g/g, which
is approximately 1.4 times higher than the sum of the solubility of
calcipotriol in aqueous
5 phosphate buffer containing either 15 % cetomacrogol 1000 or 20 % ethanol
(1049 g/g +
2.33 tg/g = 1051 g/g), see Figure 1.
Table 3: Solubility of calcipotriol (as pg/g) in aqueous vehicles using
cetomacrogol 1000 as
surfactant and ethanol as co-solvent at 25 C
Vehicle Solubility g/g
Phosphate buffer pH 7.4:ethanol (80:20) 2.33
Phosphate buffer pH 7.4: cetomacrogol 1000 (85:15) 1049
Phosphate buffer pH 7.4: cetomacrogol 1000: ethanol (65:15:20) 1468
Labrasol in combination with ethanol
The solubility, at 25 C, of calcipotriol monohydrate, determined as
calcipotriol, in aqueous
buffer pH 7.4 containing Labrasol as surfactant and ethanol as co-solvent is
shown in Table 4
below. It appears from Table 4 that there is a pronounced synergistic effect
of the
combination of Labrasol and ethanol on the solubility of calcipotriol in the
aqueous
phosphate buffer solution, i.e. the observed solubility of calcipotriol in
aqueous phosphate
buffer containing 15 % Labrasol and 20 % ethanol is 1059 p.g/g, which is
approximately 3.6
times higher than the sum of the solubility of calcipotriol in aqueous
phosphate buffer
containing either 15 % Labrasol or 20 % ethanol (292 g/g + 2.33 .tg/g = 294
p.g/g), see
Figure 2.
Table 4: Solubility of calcipotriol (as p.g/g) in aqueous vehicles using
Labrasol as surfactant
and ethanol as co-solvent at 25 C
Vehicle Solubility g/g
Phosphate buffer pH 7.4:ethanol (80:20) 2.33
Phosphate buffer pH 7.4: Labrasol (85:15) 292
Phosphate buffer pH 7.4: Labrasol: ethanol (65:15:20) 1059
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
16
Labrasol in combination with isopropanol
The solubility, at 25 C, of calcipotriol monohydrate, determined as
calcipotriol, in aqueous
buffer pH 7.4 containing Labrasol as surfactant and isopropanol as co-solvent
is shown in
Table 5 below. It appears from Table 5 that there is a pronounced synergistic
effect of the
combination of Labrasol and isopropanol on the solubility of calcipotriol in
the aqueous
phosphate buffer solution, i.e. the observed solubility of calcipotriol in
aqueous phosphate
buffer containing 15 % Labrasol and 20 % isopropanol is 1508 g/g, which is
approximately
4.9 times higher than the sum of the solubility of calcipotriol in aqueous
phosphate buffer
containing either 15 % Labrasol or 20 % isopropanol (292 pg/g + 17.2 g/g =
309 ltg/g), see
Figure 3.
Table 5: Solubility of calcipotriol (as g/g) in aqueous vehicles using
Labrasol as surfactant
and isopropanol as co-solvent at 25 C
Vehicle Solubility pg/g
Phosphate buffer pH 7.4: isopropanol (80:20) 17.2
Phosphate buffer pH 7.4: Labrasol (85:15) 292
Phosphate buffer pH 7.4: Labrasol: isopropanol (65:15:20) 1508
Polysorbate 80 in combination with ethanol
The solubility, at 25 C, of calcipotriol monohydrate, determined as
calcipotriol, in aqueous
buffer pH 7.4 containing polysorbate (Tween) 80 as surfactant and ethanol as
co-solvent is
shown in Table 6 below. It appears from Table 6 that there is a synergistic
effect of the
combination of polysorbate 80 and ethanol on the solubility of calcipotriol in
the aqueous
phosphate buffer solution, i.e. the observed solubility of calcipotriol in
aqueous phosphate
buffer containing 15 % polysorbate 80 and 20 % ethanol is 740 tg/g, which is
approximately
2.0 times higher than the sum of the solubility of calcipotriol in aqueous
phosphate buffer
containing either 15 % polysorbate 80 or 20 % ethanol (360 pg/g + 2.33 .tg/g =
362 pg/g),
see Figure 4.
Table 6: Solubility of calcipotriol (as pg/g) in aqueous vehicles using
polysorbate 80 as
surfactant and ethanol as co-solvent at 25 C
Vehicle Solubility pg/g
Phosphate buffer pH 7.4:ethanol (80:20) 2.33
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
17
Phosphate buffer pH 7.4: polysorbate 80 (85:15) 360
Phosphate buffer pH 7.4: polysorbate 80: ethanol (65:15:20) 740
The results presented in Tables 3-6 below show that a higher solubility of
calcipotriol
monohydrate may be achieved in an aqueous solution by the synergistic action
of a co-
solvent combined with a surfactant instead of using either the co-solvent
alone or the
surfactant alone. This implies that a lower amount of the combination may be
used to achieve
the same solubility of calcipotriol monohydrate than when either solvent is
used on its own.
Example 3
Penetration studies
To investigate the skin penetration and permeation of calcipotriol from
compositions of the
invention, a skin diffusion experiment was conducted. Full thickness skin from
pig ears was
used in the study. The ears were kept frozen at -18 C before use. On the day
prior to the
experiment the ears were placed in a refrigerator (5 3 C) for slow defrosting.
On the day of
the experiment, the hairs were removed using a veterinary hair trimmer. The
skin was
cleaned for subcutaneous fat using a scalpel and two pieces of skin were cut
from each ear
and mounted on Franz diffusion cells in a balanced order.
Static Franz-type diffusion cells with an available diffusion area of 3.14 cm2
and receptor
volumes ranging from 8.6 to 11.1 ml were used in substantially the manner
described by T.J.
Franz, "The finite dose technique as a valid in vitro model for the study of
percutaneous
absorption in man", in Current Problems in Dermatology, 1978, J.W.H. Mall
(Ed.), Karger,
Basel, pp. 58-68. The specific volume was measured and registered for each
cell. A magnetic
bar was placed in the receptor compartment of each cell. After mounting the
skin,
physiological saline (35 C) was filled into each receptor chamber for
hydration of the skin.
The cells were placed in a thermally controlled water bath which was placed on
a magnetic
stirrer set at 400 rpm. The circulating water in the water baths was kept at
35 1 C resulting in
a temperature of about 32 C on the skin surface. After one hour the saline was
replaced by
receptor medium, 0.04 M isotonic phosphate buffer, pH 7.4 (35 C), containing
4% bovine
serum albumin. Sink conditions were maintained at all times during the period
of the study,
i.e. the concentration of the active compounds in the receptor medium was
below 10% of the
solubility of the compounds in the medium.
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
18
The in vitro skin permeation of each test composition was tested in 6
replicates (i.e. n=6).
Each test composition was applied to the skin membrane at 0 hours in an
intended dose of 4
mg/cm2. A glass spatula was used for the application, and the residual amount
of the
composition was determined so as to give the amount of the composition
actually applied on
the skin.
The skin penetration experiment was allowed to proceed for 21 hours. Samples
were then
collected from the following compartments:
The stratum corneum was collected by tape stripping 10 times using D-Squame
tape
(diameter 22 mm, CuDerm Corp., Dallas, Texas, USA). Each tape strip is applied
to the test
area using a standard pressure for 5 seconds and removed from the test area in
one gentle,
continuous move. For each repeated strop, the direction of tearing off was
varied. The viable
epidermis and dermis was then sampled from the skin in a similar fashion.
Samples (1 ml) of the receptor fluid remaining in the diffusion cell were
collected and
analysed.
The concentration of calcipotriol in the samples were determined by LC mass
spectrometry.
The results appear from Figure 5a and 5b below which show the amount of
calcipotriol found
in viable skin (dermis and epidermis) and receptor fluid in % of the applied
dose. An
excellent penetration and permeation profile was found for compositions of the
invention, in
particular those containing Labrasol as the surfactant component.
Example 4
Biological activity of the compositions
As shown in figure 6 below, cathelicidin is an antimicrobial peptide expressed
in human
keratinocytes. The expression of cathelicidin is strongly induced on infection
of the skin or
disruption of the skin barrier. In psoriasis, the level of cathelicidin is
increased in lesional
skin of psoriasis patients. It has been found that the expression of the gene
encoding
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
19
cathelicidin may be induced by vitamin D3 or vitamin D analogues such as
calcipotriol (cf.
TT Wang et al, J. Immunol. 173(5), 2004, pp. 2909-2912; J Schauber et al.,
Immunology
118(4), 2006, pp. 509-519; Schauber and Gallo, J. Allergy Clin Immunol 122,
2008, pp. 261-
266; M. Peric et al., PloS One 4(7), 22 July 2009, e6340) through binding to
the vitamin D
receptor. This finding has been utilized to develop an assay in which the
uptake and
biological activity of calcipotriol in human keratinocytes from the tested
compositions has
been determined by measuring the level of induction of the gene encoding
cathelicidin.
In the assay, compositions A, B, C, F, G, I, J, K, L prepared as described in
Example 1 above
were applied topically in triplicate on reconstructed human epidermis
consisting of normal
human keratinocytes cultured for 12 days on 0.5 cm2 polycarbonate filters
(available from
SkinEthic Laboratories, Nice, France) in an amount of 10 l. The tissue was
treated for two
days followed by separation of the epidermis from the polycarbonate filter and
snap-frozen in
liquid nitrogen. RNA was extracted from the cells and cDNA synthesized by
conventional
procedures. Quantitative real-time PCR (qPCR) was then performed using the
following
assays from Applied Biosystems: CAMP Hs0018038_ml and GAPDH Hs99999905_m1.
The expression levels of cathelicidin were normalized to GAPDH and a relative
quantification was made by comparison with Daivonex ointment.
The results appear from Table 7 below.
Table 7
Composition Fold activation
Daivonex ointment 1.0
composition A 1.9
composition B 9.4/6.4
composition C 3.5
composition F 2.1
composition G 10.9
composition I 4.1
composition J 5.2
composition K 6.6
CA 02785249 2012-06-21
WO 2011/076206 PCT/DK2009/000266
composition L 1.9
relative to Daivonex ointment
The results presented in Table 7 show that the compositions of the invention
result in higher
activation of the target gene, i.e. they may have a higher biological activity
in vivo than the
5 marketed ointment.
Example 5
Local tolerance study in minipigs
10 The local tolerability of compositions B, G, M and N of Example 1 was
assessed when
administered daily by dermal application to minipigs for 4 weeks. Daivonex
ointment was
used for comparison. Each day the animals were exposed to the test items for 8
hours.
The study was conducted in 10 female Gottingen SPF minipigs. Each animal had 6
15 application sites and received a volume of 250 mg test formulation per
application site.
Clinical signs were recorded daily and skin reactions at the application sites
were scored once
daily prior to start of dosing and, furthermore, on the day of necropsy in
relation to erythema
and oedema. Food consumption was recorded daily and the body weight weekly. At
the end
of the treatment period a gross necropsy was performed on all animals and skin
samples were
20 collected from histopathological examination.
The results show that no adverse treatment-related clinical signs were
observed during the
study though grade 1-2 skin reactions (erythema) were observed. Except for
composition G,
the erythemas were less pronounced than those observed for Daivonex ointment.
The results
imply that compositions of the invention may be better tolerated in human
patients than
Daivonex ointment.